
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 27 January 2025
Sec. Infectious Agents and Disease
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1514684
This article is part of the Research TopicWomen in Infectious Agents and Disease: 2024View all 10 articles
 Insaf Bel Hadj Ali1
Insaf Bel Hadj Ali1 Yusr Saadi-Ben Aoun1
Yusr Saadi-Ben Aoun1 Imen Khammeri2
Imen Khammeri2 Hejer Souguir1
Hejer Souguir1 Emna Harigua-Souiai1
Emna Harigua-Souiai1 Hamed Chouaieb2
Hamed Chouaieb2 Ahmed S. Chakroun1
Ahmed S. Chakroun1 Meryem Lemrani3
Meryem Lemrani3 Aicha Kallel4
Aicha Kallel4 Kalthoum Kallel4
Kalthoum Kallel4 Nabil Haddad5
Nabil Haddad5 Oussaima El Dbouni6
Oussaima El Dbouni6 Rhea N. Coler7,8,9
Rhea N. Coler7,8,9 Steven G. Reed10
Steven G. Reed10 Akila Fathallah-Mili1,2
Akila Fathallah-Mili1,2 Ikram Guizani1*
Ikram Guizani1*Introduction: Cutaneous leishmaniases (CL), a wide range of cutaneous diseases caused by diverse species of Leishmania genus parasites, are among the most neglected infectious diseases. While they are non-fatal, CL are highly morbid with disfiguring lesions, which could be chronic, leaving lifelong unsightly scars; they are combined with psychological distress and social stigma. The efficiency of treatment highly depends on the infecting Leishmania species. Diagnosis is mainly based on microscopic direct examination (DE) of Giemsa-stained smears needing experienced microscopists. It can be laborious and time-consuming when the parasite load is low. DE is poorly sensitive and does not identify Leishmania species. So far, only DNA assays accurately identify the species. Despite their wide use for generic detection, PCR methods also require equipment and additional steps to identify causal Leishmania species. L. major is hyperendemic in many countries in Africa, the Middle East, and Asia, where other species co-occur with different endemicity levels according to the situations. This complicates disease management and treatment, particularly as distribution and epidemiology of leishmaniases remain poorly understood. Here, we aimed for a simple and rapid molecular diagnostic test to detect and identify L. major, a predominant CL causal species, which could be prone to become a control tool at the point of care, in endemic areas, using isothermal recombinase DNA amplification (recombinase polymerase amplification, RPA, or recombinase aided amplification, RAA) coupled to detection by the lateral flow (LF) chromatography on a PCRD cassette.
Methods: To develop an L. major species-specific RPA-LF assay, computational analysis of 70 Leishmania DNA targets, identified through bibliography and database searches, selected five targets. We designed and tested 7 primer pairs/probe sets to specifically amplify L. major DNAs. First, the primers were tested for species specificity and sensitivity using basic RPA chemistry. Then, to develop RPA-coupled LF detection, we shifted to the nfo chemistry.
Results: This way, we retained one set for further investigation, which confirmed it is L. major species-specific. Tested on 86 human cutaneous samples, this selected set was able to detect 100% of L. major infections in confirmed CL patients. We did not observe any cross-reactivity with lesions due to L. infantum or L. tropica.
Cutaneous leishmaniases (CL) constitute a wide range of cutaneous vector-borne diseases caused by diverse species of Leishmania genus parasites. This group represents one of the most neglected infectious diseases. In Old World, more than 1 million CL cases are reported each year, of which 80% occur in the Middle East and North Africa (MENA) region. Moreover, the MENA is at increased risk of emergence and epidemics due to the changes in epidemiological trends related to environmental or climate changes, migrations, or conflicts (reviewed by Tabbabi, 2019). Indeed, CL in MENA, caused by four species (L. major, L. tropica, L. infantum, and L. donovani), have complex epidemiology due to species diversity and co-endemicity and even co-sympatry, their different levels of prevalence, microfocal distribution, and many unknowns in transmission cycles.
Zoonotic cutaneous leishmaniasis (ZCL) due to L. major is endemic in all countries in the MENA region (reviewed by Tabbabi, 2019). It has diverse clinical manifestations and is known to be epidemic to hyperendemic in these countries while the transmission of other species encountered is less frequent to sporadic, with exceptions of Syria or Sudan where L. tropica and L. donovani are highly endemic respectively, and Morocco where L. tropica is increasingly prevalent (El Idrissi Saik et al., 2022). Furthermore, while several Sub-Saharan African countries face gaps in identifying the etiology of Leishmania species responsible for CL, others, such as Burkina Faso, Nigeria, Niger, and Cameroon, have reported L. major as the sole species causing CL in these regions (reviewed by Blaizot et al., 2024), a statement that may be revised by the increasing use of DNA assays for species detection and identification.
While CL is not fatal, it is highly morbid with disfiguring and/or lifelong scars leading to social stigma and psychological distress (Chahed et al., 2016; Elfaki et al., 2024). Furthermore, CL was recently called “the great imitator” (Gurel et al., 2020) as it could mimic a wide range of skin diseases, which makes it difficult to diagnose on the sole base of clinical manifestation. CL diagnosis is mainly done by microscopic direct examination (DE) of smears of Giemsa-stained lesion that requires experienced microscopists, time-consuming, and laborious when parasite loads are low. DE is 100% specific but is poorly sensitive (43%) (Mouttaki et al., 2014) and does not identify Leishmania species given the similar morphology of their amastigote forms. Only molecular DNA assays accurately identify the species. Etiologic diagnosis of CL is crucial as it is known that treatment protocols depend on the species (Arevalo et al., 2007; Mosimann et al., 2013); efficiency of CL treatment is also highly dependent on Leishmania species (Madusanka et al., 2022).
Several PCR-based methods have been developed for the specific and sensitive detection of Leishmania parasites responsible for CL (Table 1). These include conventional PCR assays (Rostami et al., 2020), real-time PCR, PCR-RFLP, and PCR sequencing, among others. While these methods can detect Leishmania parasites, species identification often requires complementary techniques, such as RFLP (Schönian et al., 2003; Bel Hadj Ali et al., 2021) or sequencing (Stevenson et al., 2010), to enable species identification. Real-time PCR is among the most highly sensitive methods for detecting Leishmania DNA (Stevenson et al., 2010; Nath-Chowdhury et al., 2016). However, the requirement for specialized equipment, such as real-time PCR machines, can limit its accessibility in resource-limited settings.
In low-resource or remote settings where there is a lack of equipped laboratories, molecular diagnostics with a point-of-care (POC) format would guarantee an accurate diagnosis and equitable and better access of patients to health care wherever they are. Actually, healthcare challenges in such settings mainly relate to infrastructure and budget (Maphumulo and Bhengu, 2019). This emphasizes the importance of point-of-care molecular diagnostics for prevention, timely patient management, and disease control.
Over the last decade, the POC format has been given a special attention, and a broad spectrum of technologies, reagents, test kits, and equipment were developed (Kozel and Burnham-Marusich, 2017). With the advent of PCR as the gold standard technology in molecular diagnostics for pathogen detection, a range of DNA amplification technologies have been developed to expand their use at the POC, thus allowing to address the need for POC CL testing, requiring minimal equipment while being fast in delivering DNA products. The most commonly used are the loop-mediated isothermal amplification (LAMP) (Erber et al., 2022; Soares et al., 2024; Taye et al., 2024) and the recombinase-based amplification [recombinase polymerase amplification (RPA) or recombinase-aided amplification (RAA)] (Bharadwaj et al., 2021; Khan et al., 2021; Travi et al., 2021; Mondal et al., 2016). RPA/RAA is an isothermal DNA amplification technology with a simple experimental design, by contrast to LAMP that requires four to six primer pairs, to amplify one DNA target. Indeed, RPA operates at low temperatures (37°C–42°C) and requires only two primers or two primers and a probe depending on the used chemistry (Piepenburg et al., 2006). RAA is another designation for this technology (Xue et al., 2020). Using RPA or RAA (according to the kit supplier) to develop molecular diagnostics potentially overcomes current limitations of cost, complexity, and resources while ensuring rapidity, efficiency, and accuracy of the results (Ghosh et al., 2021).
In this study, we aimed to develop a simple, rapid sensitive, and cost-effective L. major-specific DNA-based assay. The test based on an isothermal recombinase-based amplification coupled to lateral flow immuno-assay detection would contribute to diagnosing L. major-infected patients or their screening in remote areas.
The study was conducted after obtaining the ethical approval from Ethic Committees of: Institut Pasteur de Tunis (Ref:2016/24/I/LRIPT04), Rafic Hariri University Hospital Lebanon (Ref: INV-2017-324) and Faculty of Medicine and Pharmacy University Mohamed V Rabat, Morocco Faculty of Medical Sciences (Ref: 51/17). All study participants provided signed informed consent. The collected samples and data were codified and treated anonymously.
Sequencing surveys and RPA assays were set up and optimized using characterized strains belonging to L. major (N = 14), L. infantum (N = 11), L. donovani (N = 5) L. tropica (N = 11), and other Old World Leishmania species including L. aethiopica (N = 1), L. arabica (N = 1), L. turanica (N = 1), and the Sauroleishmania L. tarentolae (N = 1). The corresponding strains were isolated from a range of hosts including human cases, sandflies, and reservoirs from various geographical origins in Africa, the Middle East, or Asia. Tunisian and Sudanese strains were obtained from field studies or health centers in Tunisia and Sudan. All the other strains were obtained from reference centers in Montpellier, London, or Rome. Details describing these strains are shown in Table 2.
The sample and data collection were undertaken in different study sites, including Tunisia, Lebanon, and Morocco. In Tunisia, we collected, in total, 55 samples from informed and consented patients referred for routine diagnosis to the parasitology departments of Farhat Hached Hospital in Sousse (N = 49) and La Rabta Hospital in Tunis (N = 6). Patient recruitment in these departments mainly reflect the different levels of CL endemicity and distribution of parasites in the different regions of Tunisia (Central vs. Northern Tunisia). In Lebanon, we collected eight CL samples from consented patients having consulted at the Rafic Hariri Hospital; two of whom were Lebanese, and the six others were Syrian refugees. In Morocco, we conducted a field study in the Azilal Province and Ouarzazate/Zagora where we collected samples from 23 consented patients. Patients were sampled by dermal scraping of the lesions in Tunisia and Morocco, and by swabs of lesions following lesion scrapping in Lebanon. Demographic and clinical data of the collected samples are provided in Supplementary Table S1. It also includes the country or city of patient recruitment. The nucleic acid extraction method was harmonized by using the QIAamp DNA Kit (Qiagen, Germany) at the different study sites. We used microscopic direct examination as the reference method for parasitological diagnosis by the visual detection of amastigotes. We followed the criteria suggested by the WHO (WHO, 2010) for the parasitic load classification reported in Supplementary Table S1. Detection and identification of etiological agents in lesions were carried out by ITS1 PCR-HaeIII RFLP as described by Schönian et al. (2003). Epidemiological and clinical data were collected using a harmonized questionnaire that was digitalized as described by Preprint Harigua et al. (2020).
We used different approaches to identify species-specific targets for the recombinase amplification-based assays. First, we used a set of Leishmania coding sequences (CDS) in a range of species, available in our laboratory, as potential targets. Then, a second screen included a bibliography search of known targets described by other studies for their species specificity (Rogers et al., 2011) and species differentiation ability (Zelazny et al., 2005). The third screen included a computational screening of all known CDS in the L. major genome against the whole genomes of L. infantum and L. tropica available in the public databases selecting those that presented less than an 80% similarity rate. Within all the studied targets, those presenting high interspecies polymorphism rates or sequence gaps were retained for the design of RPA primers.
We designed RPA primers according to the TwistDx guidelines [twistamp-assay-design-manual_rev1_v3.pdf (twistdx.co.uk)]. They are 30–35 bases long with 30–70% of GC content. The primers were designed to produce 100-bp to 400-bp L. major-specific amplicons. A fragment of at least 52 bp was maintained between the primer pairs to allow for internal probe design for lateral flow detection. The specificity of primer pairs was tested using RPA basic chemistry for the amplification and detection of products by the agarose gel electrophoresis.
In order to verify sequence conservation within Leishmania species and validate the designed RPA primers, for each selected target, we designed external PCR primers to survey conservation of the priming and probing sites through sequence analyzes of the targeted fragments across strains and species (Supplementary Table S2). Sequencing was performed on both strands using the BigDye Terminator v3.1 Cycle Sequencing Kit and an ABI 3500 sequencer (Applied Biosystems). The chromatograms were visualized and manually adjusted using the DNA Baser sequence assembler v4 program (Heracle BioSoft, www.DnaBaser.com). Multiple sequence alignment was performed using ClustalW, and the UPGMA tree was constructed using the Tree Builder tool, both integrated into Geneious 3.6.3 software.
Reactions were set up following the TwistDx™ Basic RPA protocol; each reaction contained 29.5 μL rehydration buffer, 2.4 μL of each forward and reverse primers (10 μM), 11.2 μL dH20, and 2 μL of Leishmania genomic DNA (20 ng/μl) for each reaction mix. A negative control (no template) reaction was included in each set of reactions. The reaction mix was then added to the dried RPA pellets containing a mix of enzymes and dNTPs, supplied as strips. Then, 2.5 μL of magnesium acetate (280 mM stock) was added to each lid making a total reaction volume of 50 μL. The strips were spun down and immediately placed into a thermoshaker (Neobiotech, France) for reaction initiation. Incubation was performed at 40°C for 30 min and under 600 rpm agitation, instead of manual shaking to ensure experiment reproducibility. Amplification products were next purified using the QIAquick PCR Purification Kit (Qiagen, Germany) and run on a 2% agarose gel. The primer pairs that showed positive L. major-specific amplification and negative results with L. tropica and L. infantum/L. donovani species were selected for further investigations.
Multiple sequence alignment and experimental validation of the primers/probe sets were used to set up RPA under nfo chemistry to be coupled with LF detection assay. RPA reactions were performed in a 50 μL volume using an RAA amplification Kit—test strip method (Jiangsu Qitian Gene Biotechnology Co., Ltd. Wuxi, China). A master mix containing 420 nM of each forward and biotinylated reverse RPA primer, 120 nM FAM-tagged RPA probe, 25 μL of rehydration buffer, and DNase-free water was prepared and distributed into each reaction tube containing the dried enzyme pellet. Sample DNA (2 μL) was then added into each tube, and finally, a volume of magnesium acetate (280 mM) was pipetted into the tube lids and then centrifuged to allow initiation of the isothermal amplification reactions. Optimizations of RPA reaction were performed in order to determine the optimal conditions of reactions. Different magnesium acetate final concentrations ranging from 14 mM to 30 mM were tested. We also assessed different reaction times (10, 15, 20, 30, 35, and 40 min) and incubation temperatures (37, 39, 40, and 42°C).
Lateral flow detection was performed using generic 2 test lines PCRD cassettes (Abingdon Health, UK). Different dilution factors of the RPA products were tested involving 6, 10, 15, and 20 μL in a final volume of 90 μL with the supplied PCRD extraction buffer (provided with the kit by Abingdon Health). A volume of 75 μL was pipetted into the sample well of the PCRD test cassette and left for 5–10 min before it was imaged using a smartphone camera.
We performed the RPA and LF reactions in a unidirectional workflow using separated laboratory work areas for each step to prevent amplicon carryover contamination.
To determine the analytical sensitivity, we used 10-fold serial dilutions of L. major DNA (R44) ranging from 20 ng/μl to 2 × 10−5 ng/μl where 2 μL was added in the reaction. Three independent experiments were performed to define the limit of detection of our assay.
A total of 86 DNAs extracted from skin lesion samples of 86 patients from the different study sites described above were tested blindly in order to evaluate our test performances. Sample status (positive/negative) was elaborated using the microscopy direct examination (DE) and ITS1 PCR (Schönian et al., 2003). In the case of samples with negative ITS1 PCR and positive RPA, DNA quality and/or the presence of PCR inhibitors was assessed by amplifying the human beta-globin gene (β-globin) as described by Bauer et al. (1991). In case of discordant results between the two reference tests (DE and ITS1 PCR), Lei70 PCR (Spanakos et al., 2002; Haouas et al., 2017) was used for confirmation of results. Species assignment was assessed using the ITS1 PCR-HaeIII RFLP (Schönian et al., 2003). Results were kept unknown from the user in order to eliminate the bias due to human behavior influenced by what is already known (Day and Altman, 2000).
In total, we worked on 63 nuclear and 7 kinetoplast DNA targets presenting a percentage of pairwise similarity ranging between 94.7 and 76.3% among the different species of relevance: L. major, L. infantum, L. donovani, and L. tropica. These targets were also analyzed considering multiple sequence alignments to identify L. major-specific fragments as potential priming and probing sites, taking into account the recommendations for the design of primers and probes (size of segments and spacing), and the number of mismatches in these sites occurring between the different Leishmania species. In fact, available sequences of each target were retrieved from our data and the public databases aligned and the SNPs were identified for each species (L. major, L. infantum/L. donovani, and L. tropica) and manually screened for the criteria of specificity to L. major established above. We selected five DNA markers to target RPA development as the most interesting in terms of sequence specificity to L. major. Criteria for their selection were the presence of no less than four mismatches in at least one of the identified primers/probe sites when compared to the sequences of L. tropica and L. infantum/L. donovani strains and no more than one mismatch at the intraspecific level in each primer/probe site identified. We refined manually the design of the primers and probe sets. Positions of polymorphisms of the designed primers and probes are presented in Supplementary Table S3.
Given the number of targets investigated, to be cost-effective and productive, we have elaborated a decision-making tree for the selection of targets and primers and their validation through an iterative approach (Figure 1). Experimental validation of the primers was first done using the basic amplification kit for the detection of agarose gels. Then, the most interesting primers were labeled and tested for amplification including in the reaction mix the labeled internal probe (that acted as a semi-nested amplification primer) for more specific amplification, and sensitive and specific detection of the amplified products by lateral flow chromatography using a PCRD cassette.
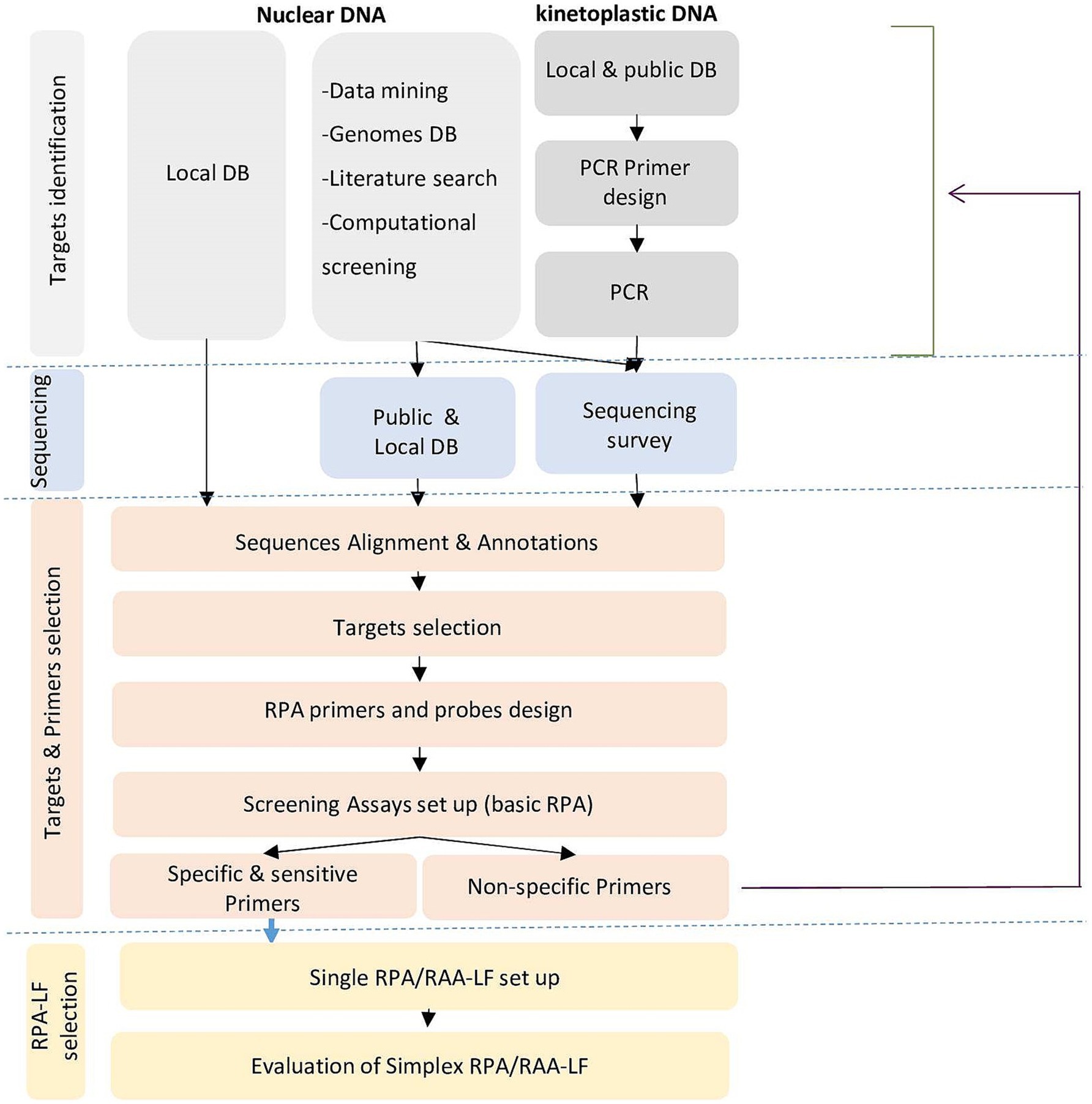
Figure 1. Decision tree for selection of targets and primers and their validation through an iterative approach for species-specific RPA/LF assays.
To validate the primers and probe design, we surveyed the sequences selected for the occurrence of interspecies and intraspecies polymorphisms for five targeted markers (m30 (Genbank accession numbers PQ472465-PQ472485), m22 (Genbank accession numbers PQ538791-PQ538551 & PQ538541-538551), m27, m02 and m31) for which this information was missing for the studied Leishmania species (L. major, L. infantum/L. donovani, and L. tropica). The primers that showed intraspecies polymorphisms were rejected and other primers were designed using these sequence data, in regions conserved for L. major, but variable for the other Leishmania species. In total, we designed seven primer pairs and seven probes in five targets. Description of designed primers and probes is shown in Supplementary Table S4.
As a first step, we screened the primers for specific amplification of L. major using RPA basic chemistry and agarose gel electrophoresis detection. Among the designed primers, we selected four primer pairs (m30_1, m30_2, m27, and m22) that gave specific amplifications of DNAs of L. major strains (Figure 2). The remaining primer sets showed cross-reactivity with other Leishmania species DNAs (m_02) or did not show amplification (m31 and m_30_3) with L. major DNA.
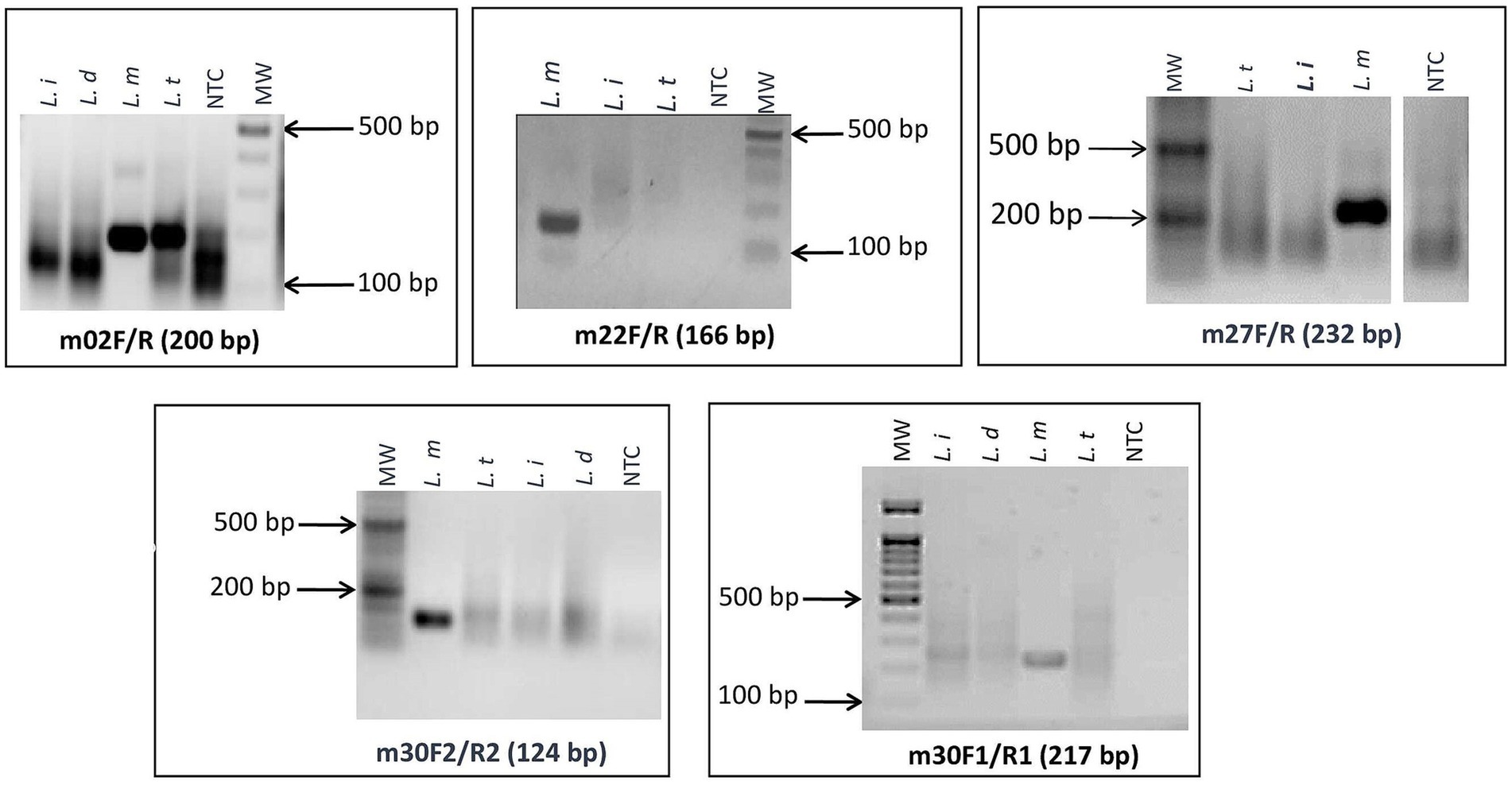
Figure 2. Screening assays and selection of L. major specific targets: agarose gel electrophoresis of targets that gave specific amplifications of DNA of L. major strains. L. i: L. infantum; L. d: L. donovani; L. t: L. tropica; L. m: L. major; NTC: no-template control; MW: 100-bp molecular weight.
As a second step, the retained primers and their corresponding probes were tested using the RPA nfo chemistry Kit (TwistDx, UK), and when not anymore available on the market, the RAA test strip Kit (Qitian, China) was used to confirm the reproducibility of the results obtained. Amplified products were visualized on PCRD cassettes (Abingdon, UK). The selection criteria at this step were the specificity of results with the primers/probe sets used, and the absence of background noise with the no-template control in the PCRD test. Among the tested primers, only one (m22) gave the aimed result. Optimizations with this set allowed us to select incubation temperature of 40°C for 40 min and an Mg2+ concentration of 14 mM as optimal conditions for RPA or RAA (Figure 3). We selected 6 μL as amplification product input for the PCRD detection as higher reaction volumes have led to the appearance of background noise in the negative control tests.
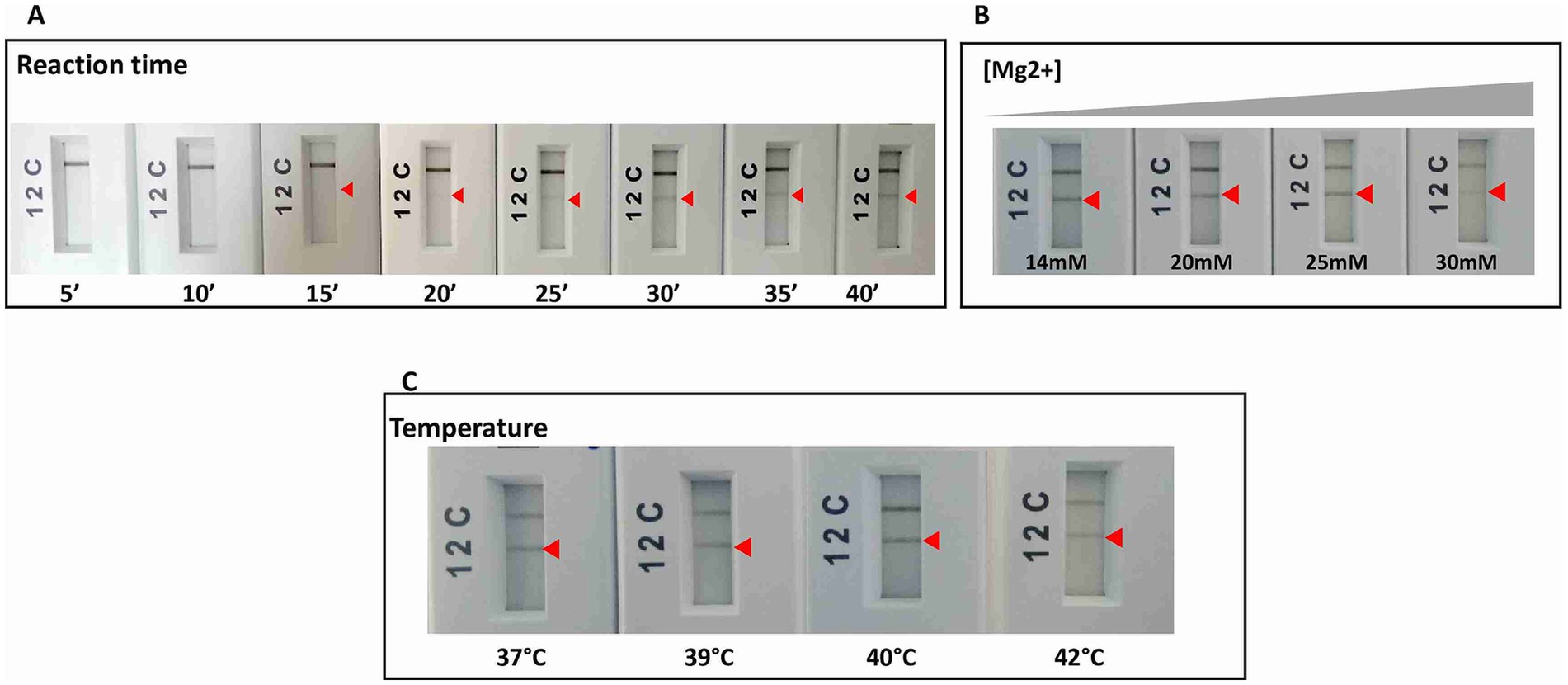
Figure 3. RPA reaction optimizations for the specific and sensitive detection of L. major species by varying: (A) time of reaction. (B) Magnesium concentration. (C) Temperature of reaction. Arrow: positive test line.
Consistent reactivity of the m22 primer set was assessed using a panel of DNAs of Leishmania species and strains. We have observed that our test yielded specific amplification of Leishmania major DNAs, consistently reacting with all the L. major representative strains DNA tested (N = 14) having different host and geographical origins. There was no cross-reactivity of the primers with L. tropica strains DNA (N = 8) nor with L. infantum strains DNA (N = 10) (Figure 4A) as demonstrated by the lack of amplified products for the DNAs of these species. Other species, including L. donovani, L. aethiopica, L. turanica, L. arabica, and the Sauroleishmania L. tarentolae, were also tested and showed no reaction with the m22 target (Figure 4B). This result is supported by the sequence alignment and analysis showing a pairwise similarity of less than 80% (Figure 5) and the UPGMA phylogenetic analysis (Figure 6).
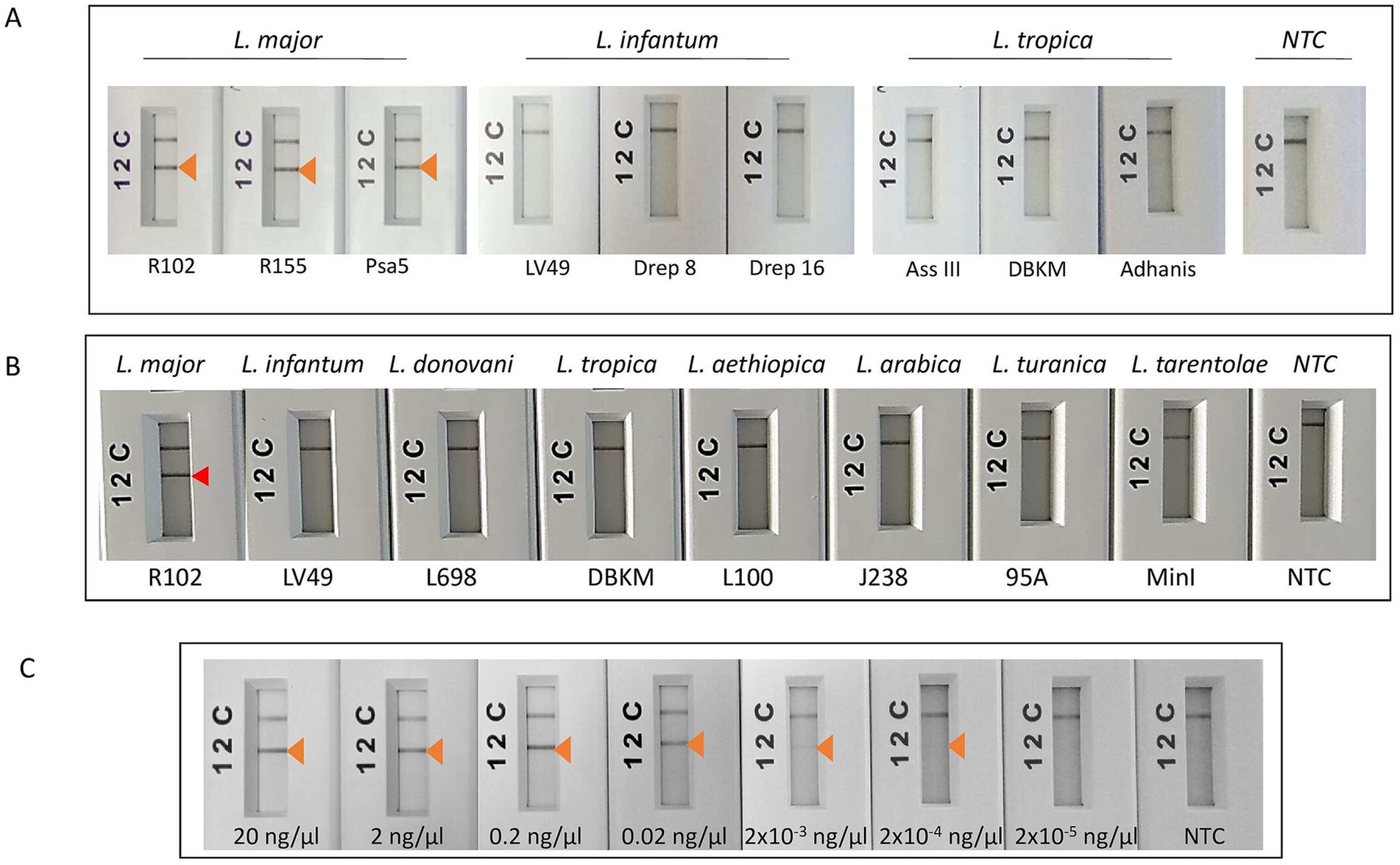
Figure 4. Test of the analytical specificity and sensitivity of m22-RPA/LF assays. (A) Test of species specificity of the selected m22-RPA/LF L. major-specific assay using different strains belonging to L. major, L. infantum, and L. tropica species. (B) Test of the reactivity of the m22-RPA/LF with other species. No cross-reactivity was observed with any of the species tested. The arrowhead indicates the positive test line. (C) Test of the analytical sensitivity of the selected m22-RPA/LF L. major-specific assay: We used serial dilutions of L. major strain DNA starting from 20 ng/μl to 2 × 10−5 ng/μl, using 2 μL as input for each reaction and 6 μL of the amplification product for LF detection. Arrow: Positive test line, NTC: no-template control. The test line corresponding to a DNA concentration of 2 × 10−4 ng/μL is visible to the naked eye but does not clearly appear in the smartphone photograph.
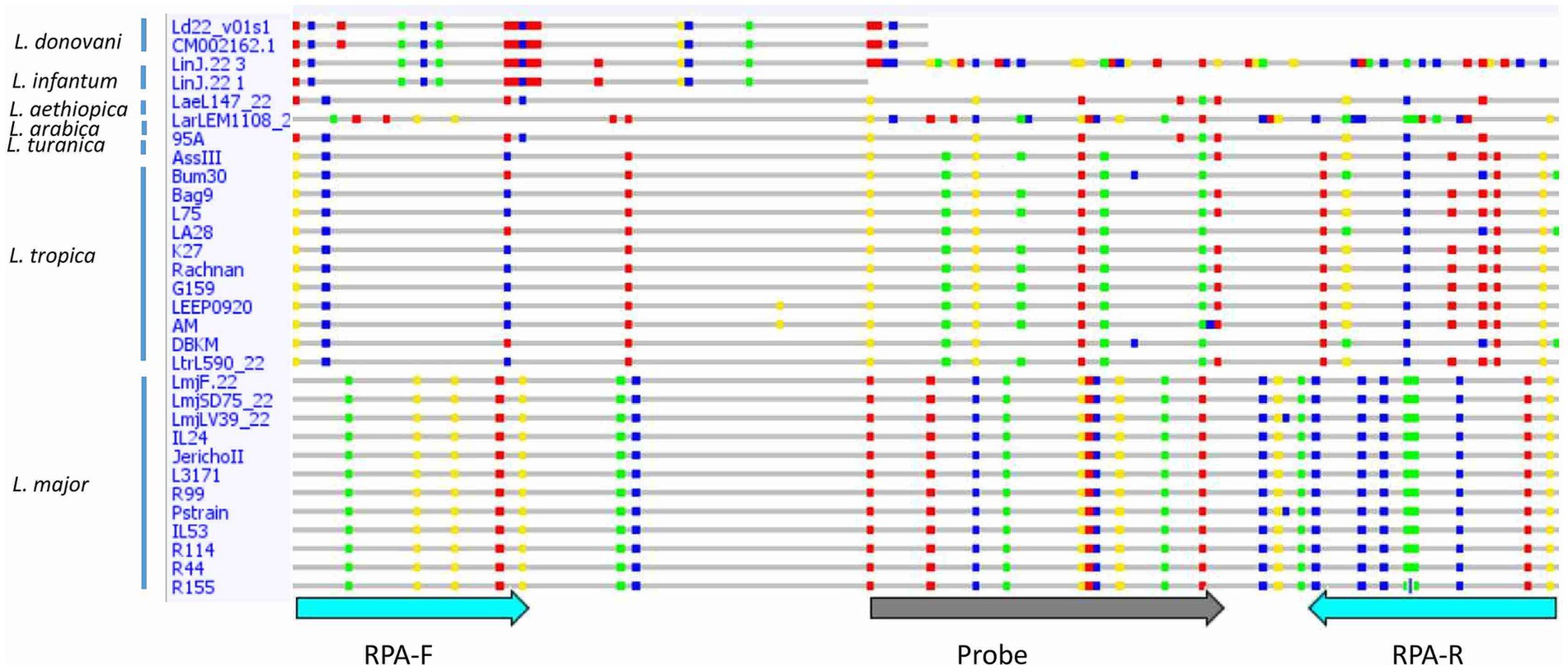
Figure 5. Sequence alignment of the selected target showing differences between Leishmania species/strains. Single nucleotide polymorphisms (SNPs) are shown in colored dots. RPA-F, Forward RPA primer; RPA-R, Reverse RPA primer.
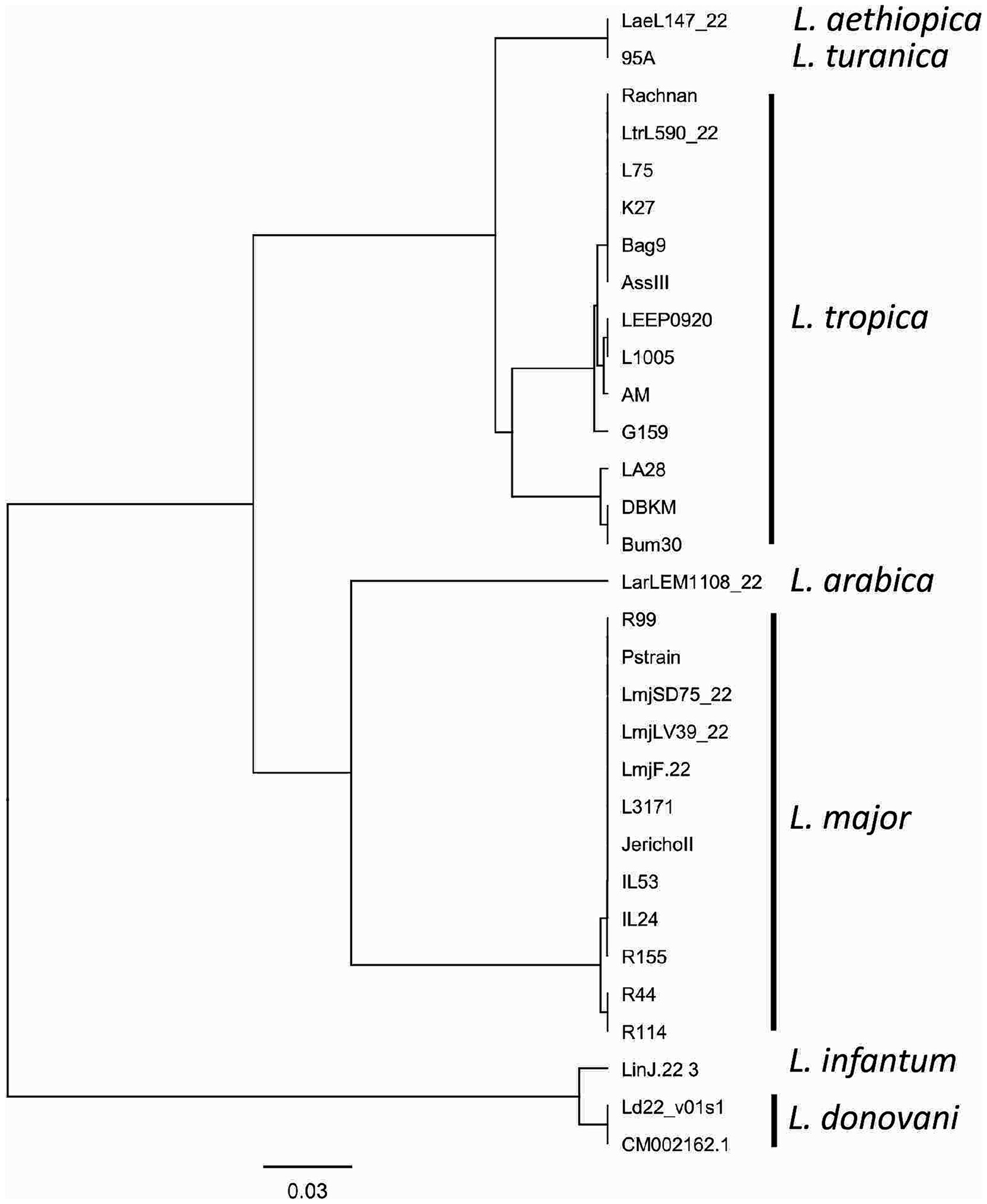
Figure 6. UPGMA phylogenetic tree based on the Jukes–Cantor genetic distance model. The tree illustrates the evolutionary relationships between the analyzed sequences, with branch lengths proportional to genetic distances. The Jukes–Cantor model was used to correct for multiple substitutions, ensuring an accurate representation of sequence divergence.
The analytical limit of detection of the m22 set of primers was also tested using serial dilution of one L. major strain DNA starting from 20 ng/μl to 2 × 10−5 ng/μl, using 2 μL as input for each reaction and 6 μL of the amplification product for LF detection. We noticed that we were able to detect up to an input concentration of 2 × 10−4 ng/μl when we used RPA/LF analysis using the kits supplied from TwistDx (UK) or Qiagen (China) (Figure 4C), which here (4 × 10−4 ng) could correspond to 2.5 parasites considering that the average of diploid genome mass is 80 fg (Tupperwar et al., 2008).
To evaluate the use of the m22-RPA/LF assay for L. major detection in clinical samples, we tested 86 DNAs extracted from cutaneous scrapings and swabs taken from patients seeking diagnosis of cutaneous lesions. We used microscopic direct examination and ITS1 PCR for Leishmania detection and ITS1 PCR-HaeIII RFLP for identification of parasites as the classical methods. The samples provided blindly to the experimenter include infections due to L. major (N = 38), infections due to L. infantum (N = 6), infections due to L. tropica (N = 10), infections due to unknown species with positive direct examination and negative ITS1 PCR (N = 3) and negatives for Leishmania infections (N = 29) (Table 3).
Using our assays, we were also able to detect 100% of L. major infections (38/38) within L. major CL patients having different parasite loads (Table 3; Figure 7), with 22 of them (58%) having a low parasite load (1–10 amastigotes/1,000 fields) (Table 3; Supplementary Table S1). The three patients with positive direct examination and negative ITS1 PCR results (PER340204, PER340223, and PER340286) were assigned to L. major when tested by our m22-RPA/LF tool. They were also positive when tested with the Lei70 PCR. No cross-species reactivity was seen when CL patients infected with L. tropica and L. infantum were tested. Among the 29 negative samples (DE-, ITS1-), seven gave positive results with our RPA/LF test. They were also tested with the generic Lei70 PCR (Spanakos et al., 2002; Haouas et al., 2017) to confirm their positivity. Among the seven samples, four were also positive with the Lei70 PCR (PER340295, PER340337, PER570110, and PER570107) (Supplementary Figure S1A). The remaining three samples were negative (PER340294, PER570106, and PER570104), with two showing a faint β-globin amplification band on upon agarose gel electrophoresis (Supplementary Figure S1B).

Figure 7. Test of L. major-specific target on CL samples using the m22-RPA/LF improved protocol. All L. major-infected patients were positive with our assay. PC: positive control, NTC: no-template control.
Cutaneous leishmaniases represent a wide range of cutaneous diseases characterized by their diverse clinical manifestations. These diseases are not fatal, but they are highly morbid and have a negative psychological impact on patients due to the lesions, the disfiguring scars, and subsequent stigma (Chahed et al., 2016; Pires et al., 2019).
CL are poverty-related infections that occur mainly in low-income and middle-income countries (Moya-Salazar et al., 2021) where health centers lack essential equipment for their diagnosis. Routinely Leishmania parasites from CL lesions are detected by direct microscopy examination, a laborious technique that lacks sensitivity. In addition, this technique is not able to identify Leishmania species, an important step that should be taken into consideration to guide CL treatment and patient management (Arevalo et al., 2007; Mosimann et al., 2013). This highlights the need for POC CL diagnostics to ensure healthcare equity for patients wherever they are.
The World Health Organization’s (WHO) roadmap to 2030, in terms of Research and Development on diagnostics needs to cover all NTDs, pointed to inadequate existing tools. Concurrently, in this study, we aimed to enhance access to CL diagnosis in endemic areas by developing an isothermal amplification coupled with lateral flow detection of L. major-infecting parasites. This test intends to improve CL diagnosis in low-resource settings where infections due to L. major are predominant like, for instance, in North African countries, or highly endemic like in other countries in Africa, the Middle East, and Asia. According to WHO, an ideal POC test should be affordable, sensitive, specific, user-friendly, and equipment-free and delivered to patients (ASSURED) (Kosack et al., 2017). In this study, we provided a proof of concept in a laboratory environment that RPA/LF assays are suitable for the detection and identification of L. major in clinical samples. The method has several inherent advantages; it is simple, rapidly processed, and needs low-cost basic laboratory equipment. The time to result delivery is 1 h, and it includes amplification, detection, and identification of L. major parasites. The RPA ready-to-use lyophilized reagents provided in a single tube make the reactions simple and rapid to perform without any expertise needed. In addition, the design of the assay is simpler than LAMP, an isothermal amplification technology that requires four to six primer pairs. It is also known that RPA tolerates PCR inhibitors (Kersting et al., 2014; Krõlov et al., 2014; Rosser et al., 2015; Tan et al., 2022), so it could be coupled to minimally processed and simplified methods for DNA extraction (Saldarriaga et al., 2016; Moore and Jaykus, 2017; Munawar et al., 2020). All these advantages are favoring factors for the widespread adoption of the RPA/LF platform to support POC diagnosis of cutaneous leishmaniases.
RPA technology was used for the detection of diverse pathogens including bacteria, viruses, and parasites (Kersting et al., 2014; Krõlov et al., 2014; Rosser et al., 2015; Munawar et al., 2020). During the last decade, many studies reporting RPA assays for the detection of Leishmania parasites were published (Mondal et al., 2016; Saldarriaga et al., 2016; Gunaratna et al., 2018; Cossio et al., 2021; Ghosh et al., 2021; Khan et al., 2021; Mesa et al., 2021). These assays use protocols with different end-point readouts including LF (Saldarriaga et al., 2016) or real-time portable tubes scanner (Mondal et al., 2016) to detect, namely, L. donovani (Mondal et al., 2016) and L. viannia parasites (Cossio et al., 2021; Travi et al., 2021; Saldarriaga et al., 2016). A recent study evaluated a mobile laboratory suitcase that includes an RPA method for the detection of L. donovani parasites in VL and PKDL cases in a multi-country phase 2 study and demonstrated the accuracy of this test for VL and PKDL diagnosis (Ghosh et al., 2021). However, investigations using RPA technology for CL diagnosis were lacking. Thus, we intended to develop a CL RPA test that could be used as a screening assay to complement other DNA methods in countries/regions where L. major is highly prevalent. To our knowledge, this is the first report describing an RPA test that specifically detects L. major parasites. It is known that L. major infections occur mostly in rural arid areas of North Africa and the Middle East where it frequently causes epidemics (Gradoni, 2017). L. major is also a widespread species across Africa and Asia. All these transmission areas typically lack healthcare infrastructure; point-of-care diagnostics that could be deployed as portable solutions, relying on basic equipment, is therefore needed for timely patient management and early alert on emergence. However, it is clear that a cost-effectiveness analysis of the RPA/LF assays is requested as it is another crucial factor for diagnostics scalability and implementation in resource-limited settings (Aerts et al., 2019).
In this study, we report on the development of an L. major RPA/LF assay in which we encountered two major challenges. The first one was the design of primers/probes that selectively detect L. major strains. RPA is known to tolerate mismatches in priming sites (Boyle et al., 2013; Daher et al., 2015). However, Boyle et al. (2013) were unable to amplify one HIV variant using an RPA reaction because of the presence of six nucleotide changes and one insertion in the primer/probe binding sites (Boyle et al., 2013). Thus, our strategy has relied on selecting an extensive number of mismatches between L. major and other Leishmania DNAs, flanking our target fragment, to maximize the chances to amplify selectively L. major DNA. In fact, the finally retained assay, m22-RPA, targeted a non-coding fragment in L. major, originally identified as an L. infantum-specific coding gene. In L. major, the selected primers/probe sites include five mismatches in the forward primer, nine in the reverse one, and nine in the probe when compared to L. infantum or L. tropica DNA sequences. These mismatches are specific to L. major strains and are present in all sequenced L. major DNA fragments amplified covering strains from North Africa, Asia, and the Middle East and in the sequences extracted from the database of two Middle Eastern (Friedlin and LV39) and one African strains (SD75). This confirms the relevance of the primers and probe set for a consistent reactivity within the species. In addition, in the cases of L. aethiopica and L. donovani, the priming site for the reverse primer was absent, while for the other species, the priming sites exhibited significant divergence from the L. major sequence, with less than 80% similarity. The UPGMA tree is consistent with these results, further highlighting the taxonomic potential of the selected m22 target. It was further confirmed experimentally with the panel of Leishmania species and strains tested. In addition, our results corroborated that efficient RPA reaction leading to species-specific amplification may depend on the number and positions of mismatches (Daher et al., 2015). The type of nucleotide involved in the mismatch was also shown to significantly impact RPA reaction efficiency, with primers’ terminal cytosine–thymine and guanine–adenine mismatches having the most detrimental effects (Higgins et al., 2022).
The second challenge was the formation of primers and probe/primer dimers during the RPA reaction. Indeed, the length of the primers/probes together with the low reaction temperature contribute to primer dimers noise that could appear in the LF-negative tests and lead to confusing results. Some studies opted for a post-RPA heating step (Liu et al., 2017) or used self-avoiding molecular recognition systems (SAMRS) oligonucleotides (Sharma et al., 2014) to overcome this noise issue. In our case, we have aimed at delivering simple assays performing at a single and low temperature. Therefore, to resolve this problem, we have designed and tested many primers to produce reliable assays and clear negative results in the LF device with the negative samples and the control samples without template DNA. We also carefully selected the oligonucleotides after checking their in silico properties (secondary structure, GC content, and repeats) but also their specificity, by performing a blast analysis with all DNA sequences available in NCBI and TritrypDB databases. It was reported that the non-specific background DNA could interfere with RPA reactions and diminish its efficiency depending on the primers/probes used and their level of interaction with the background DNA (Rohrman and Richards-Kortum, 2015). In this study, we used CL samples during the whole process of development in order to verify potential cross-reactivity of the tests under development with the human DNA and other pathogens that could be present in cutaneous lesions. Then, we extended the study by evaluating our assays on a larger number of DNAs extracted from cutaneous samples from different geographic origins in countries from MENA, including Tunisia, Morocco, and Lebanon. We used harmonized protocols for sampling. Tunisian, and Moroccan teams succeeded in using lesions scrapping for the detection and identification of parasites. However, the Lebanese team performed DNA extraction on swabs taken on the scrapped lesions. As standard techniques for RPA assay validation, we used the combination of direct examination and ITS1 PCR for the detection of Leishmania parasites, and ITS1 PCR-HaeIII RFLP for parasites identification. Our L. major RPA-/LF-specific test was able to detect 100% of L. major infections within CL patients, independently of the parasite load estimated by microscopy on the slides. Importantly, the test performed well in all cases where the parasite load was low, highlighting the relevance of the assay to support diagnosis as a complement or alternative to microscopy. Among the negative samples, seven showed positive results. These samples belong to patients with CL-like clinical features.
Four of them (PER340295, PER340337, PER570110, and PER570107) showed positive results with the generic Lei70 PCR. These samples are considered true positive. The three remaining samples (PER340294, PER570106, and PER570104) were negative with the generic Lei70 PCR. Two of these patients (PER570106 and PER570104) exhibited a faint β-globin amplification band, suggesting poor DNA quality or the presence of PCR inhibitors in the extracted DNA. Unlike PCR, however, it is well documented that a wide range of inhibitors in the reaction can be tolerated, making it a more robust method for challenging samples (Kersting et al., 2014; Krõlov et al., 2014; Rosser et al., 2015; Tan et al., 2022). For patient PER340294, the parasite load in the lesion sampled may be very low and therefore not detectable by the standard molecular tests used nor by microscopic direct examination. Two hypotheses could be stated in this case. The first one is that these samples are false positives, and then, our test is not 100% specific (Sp = 88%). The second hypothesis is that these samples are true positive, and then, our test is more sensitive than the used reference diagnosis tests.
Given the ongoing climatic change impacting the complex epidemiology of Leishmaniases, particularly in Africa and the Middle East, there is a growing risk of the emergence of new Leishmania species in areas where L. major is prevalent, which may lead to co-sympatry, mixed infections, and generation of interspecific hybrids. Expansion of L. major into other areas so far free of leishmaniases could also occur. Accurate diagnosis of leishmaniases, L. major cases in particular, is therefore increasingly important. In instances where the developed test is negative for L. major, additional tests should be considered to confirm whether the sample is truly negative or is infected with another Leishmania species. It was shown that using multiple diagnostics tests achieves a greater specificity than any single test (Gass, 2020). In addition, establishing an algorithm to guide diagnosis should be envisaged as it may depend on the tested population, disease prevalence, and specimens (Hurt et al., 2017). Developing a simple and rapid molecular test to screen L. major CL infections in regions where this species is predominant is in line with such a purpose.
Expanding the RPA-LF platform to encompass CL cases due to other Leishmania species of relevance is in our perspectives, permitting us to detect and identify all CL cases including those that are caused by mixed infections or by natural interspecific hybrids such as the ones recently reported in Ethiopia (Hadermann et al., 2023). As a Leishmania infantum RPA/LF assay has already been developed and validated for the diagnosis of visceral leishmaniasis (Castellanos-Gonzalez et al., 2015), it would be worthwhile to explore its potential application for cutaneous leishmaniasis caused by this species, and the closely related L. donovani. Furthermore, as a future direction, the development of L. tropica and L. aethiopica species-specific RPA/LF assays could address a critical diagnostics gap, particularly in the African and Middle Eastern region, where L. major, L. tropica, L. aethiopica, and L. infantum/L. donovani are the most prevalent species causing cutaneous leishmaniases. Expanding the RPA/LF platform to cover these key species would greatly enhance diagnostics capacity in these endemic areas. Moreover, incorporating into the assays an internal control for human DNA compatible with an LF detection will enhance the reliability of the test by verifying its validity and confirming negative results.
Additional developments to our current RPA/LF assay and future platform should be considered, bringing them closer to the point of care by shortening further the timeline of the workflow, reducing the costs, and automating the readouts. Indeed, the current DNA extraction step presents a challenge for implementing the test in resource-limited settings and its scalability. Therefore, the integration of an upstream simple DNA extraction and a less costly method within the workflow, as described by Mondal et al. (2016), will enhance the feasibility and usability of the test at the point of care. Moreover, developing and using a lateral flow smartphone reader application could significantly enhance test interpretation and minimize observer bias. This technology is particularly valuable for resolving ambiguities associated with faint bands and overcoming challenges like the one we encountered during our evaluation of the analytical limit of detection of assay.
We have developed a platform of an L. major-specific and sensitive RPA/LF assays. Through this proof-of-concept study, we have provided evidence of the efficacy of our RPA-LF assays for the detection and identification of L. major species in CL lesions. Our tests could be expanded/used to complement other recombinase-based amplifications to detect and identify Leishmania parasites in a point-of-need context.
The datasets presented in this study can be found in the manuscript. The DNA sequences can be found in online databases with the names of the database and accession number(s) found in the article.
The study was conducted after obtaining the ethical approval from Ethic Committees of: Institut Pasteur de Tunis (Ref:2016/24/I/LRIPT04), Rafic Hariri University Hospital Lebanon (Ref: INV-2017-324) and Faculty of Medicine and Pharmacy University Mohamed V Rabat, Morocco Faculty of Medical Sciences (Ref: 51/17). All study participants (or their legal guardian/next of kin) provided written informed consent. The collected samples and data were codified, shared and treated anonymously.
IBHA: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft. YS: Conceptualization, Methodology, Writing – review & editing, Formal analysis, Investigation, Validation. IK: Investigation, Validation, Writing – review & editing, Project administration. HS: Formal analysis, Investigation, Validation, Writing – review & editing. EH-S: Data curation, Software, Writing – review & editing, Investigation, Methodology. HC: Investigation, Resources, Validation, Writing – review & editing. AC: Methodology, Resources, Writing – review & editing. ML: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. AK: Investigation, Resources, Writing – review & editing. KK: Methodology, Project administration, Supervision, Writing – review & editing. NH: Investigation, Methodology, Supervision, Validation, Writing – review & editing. OD: Writing – review & editing, Investigation, Resources. RC: Supervision, Writing – review & editing. SR: Supervision, Writing – review & editing. AF-M: Formal analysis, Methodology, Resources, Supervision, Writing – review & editing. IG: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ministry of Higher Education and Research of Tunisia through the Research laboratory contract program (LR16IPT04, PI: IG) and the Clinical Investigation Center of Institut Pasteur de Tunis (CIC2016/IPT02, PI:IG) and by the National Academy of Sciences and the USAID through the Partnership for Enhanced Engagement in Research (PEER) program (cooperative agreement number No. AIDOAA-A-11-00012-PEER 518; PI:IG). This research was funded in part by Science for Africa Foundation to the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme (Del-22-005; PI:IG) with support from Wellcome Trust and the UK Foreign, Commonwealth & Development Office and is part of the EDCTP2 programme supported by the European Union. For purposes of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors acknowledge all the staff who contributed to data and sample collection. We would like to thank all participants for their cooperation.
SR was employed by HDT Bio.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1514684/full#supplementary-material
Aerts, C., Vink, M., Pashtoon, S. J., Nahzat, S., Picado, A., Cruz, I., et al. (2019). Cost effectiveness of new diagnostic tools for cutaneous leishmaniasis in Afghanistan. Appl. Health Econ. Health Policy 17, 213–230. doi: 10.1007/s40258-018-0449-8
Al-Rashed, A. S., Al Jindan, R., Al Jaroodi, S., Al Mohanna, A., Abdelhady, A., and El-Badry, A. A. (2022). Genotypic and phylogenic analyses of cutaneous leishmaniasis in Al Ahsa, eastern Saudi Arabia during the coronavirus disease 2019 pandemic: first cases of Leishmania tropica with the predominance of Leishmania major. Sci. Rep. 12:10753. doi: 10.1038/s41598-022-14702-z
Arevalo, J., Ramirez, L., Adaui, V., Zimic, M., Tulliano, G., Miranda-Verástegui, C., et al. (2007). Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195, 1846–1851. doi: 10.1086/518041
Bauer, H. M., Ting, Y., Greer, C. E., Chambers, J. C., Tashiro, C. J., Chimera, J., et al. (1991). Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 265, 472–477. doi: 10.1001/jama.1991.03460040048027
Bel Hadj Ali, I., Chouaieb, H., Saadi Ben Aoun, Y., Harigua-Souiai, S., Souguir, H., Yakoub, A., et al. (2021). Dipeptidyl peptidase III as a DNA marker to investigate epidemiology and taxonomy of Old World Leishmania species. PLoS Negl. Trop. Dis. 15:e0009530. doi: 10.1371/journal.pntd.0009530
Bharadwaj, M., Bengtson, M., Golverdingen, M., Waling, L., and Dekker, C. (2021). Diagnosing point-of-care diagnostics for neglected tropical diseases. PLoS Negl. Trop. Dis. 15:e0009405. doi: 10.1371/journal.pntd.0009405
Blaizot, R., Pasquier, G., Kone, A. K., Duvignaud, A., and Demar, M. (2024). Cutaneous leishmaniasis in sub-Saharan Africa: a systematic review of Leishmania species, vectors and reservoirs. Parasit. Vectors 17:318. doi: 10.1186/s13071-024-06381-8
Boyle, D. S., Lehman, D. A., Lillis, L., Peterson, D., Singhal, M., Armes, N., et al. (2013). Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. MBio 4, e00135–e00113. doi: 10.1128/mBio.00135-13
Castellanos-Gonzalez, A., Saldarriaga, O. A., Tartaglino, L., Gacek, R., Temple, E., Sparks, H., et al. (2015). A novel molecular test to diagnose canine visceral Leishmaniasis at the point of care. Am. J. Trop. Med. Hyg. 93, 970–975. doi: 10.4269/ajtmh.15-0145
Chahed, M. K., Bellali, H., Jemaa, S. B., and Bellaj, T. (2016). Psychological and psychosocial consequences of zoonotic cutaneous Leishmaniasis among women in Tunisia: preliminary findings from an exploratory study. PLoS Negl. Trop. Dis. 10:e0005090. doi: 10.1371/journal.pntd.0005090
Cossio, A., Jojoa, J., Castro, M. D., Castillo, R. M., Osorio, L., Shelite, T. R., et al. (2021). Diagnostic performance of a recombinant polymerase amplification test—lateral flow (RPA-LF) for cutaneous leishmaniasis in an endemic setting of Colombia. PLoS Negl. Trop. Dis. 15:e0009291. doi: 10.1371/journal.pntd.0009291
Daher, R. K., Stewart, G., Boissinot, M., Boudreau, D. K., and Bergeron, M. G. (2015). Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol. Cell. Probes 29, 116–121. doi: 10.1016/j.mcp.2014.11.005
Day, S. J., and Altman, D. G. (2000). Blinding in clinical trials and other studies. BMJ 321:504. doi: 10.1136/bmj.321.7259.504
El Idrissi Saik, I., Benlabsir, C., Fellah, H., Lemrani, M., and Riyad, M. (2022). Transmission patterns of Leishmania tropica around the Mediterranean basin: could Morocco be impacted by a zoonotic spillover? PLoS Negl. Trop. Dis. 16:e0010009. doi: 10.1371/journal.pntd.0010009
Elfaki, N. K., Alzahrani, M. J., Abdalla, Y. H. A., Adeh, A. I., Abdalla, A. M. A. O., Alkhadher, M. A., et al. (2024). Perceived social stigma of cutaneous Leishmaniasis in Hubuna, Saudi Arabia. J. Multidiscip. Healthc. 17, 867–876. doi: 10.2147/JMDH.S454135
Erber, A. C., Sandler, P. J., de Avelar, D. M., Swoboda, I., Cota, G., and Walochnik, J. (2022). Diagnosis of visceral and cutaneous leishmaniasis using loop-mediated isothermal amplification (LAMP) protocols: a systematic review and meta-analysis. Parasit. Vectors 15:34. doi: 10.1186/s13071-021-05133-2
Filgueira, C. P. B., Moreira, O. C., Cantanhêde, L. M., De Farias, H. M. T., Porrozzi, R., Britto, C., et al. (2020). Comparison and clinical validation of qPCR assays targeting Leishmania 18S rDNA and HSP70 genes in patients with American Tegumentary Leishmaniasis. PLoS Negl. Trop. Dis. 14:e0008750. doi: 10.1371/journal.pntd.0008750
Gass, K. (2020). Time for a diagnostic sea-change: rethinking neglected tropical disease diagnostics to achieve elimination. PLoS Negl. Trop. Dis. 14:e0008933. doi: 10.1371/journal.pntd.0008933
Ghosh, P., Sharma, A., Bhattarai, N. R., Abhishek, K., Nisansala, T., Kumar, A., et al. (2021). A Multi-Country, Single-Blinded, Phase 2 Study to Evaluate a Point-of-Need System for Rapid Detection of Leishmaniasis and Its Implementation in Endemic Settings. Microorganisms 9:588. doi: 10.3390/microorganisms9030588
Gradoni, L. (2017). The Leishmaniases of the Mediterranean region. Curr. Trop. Med. Rep. 4, 21–26. doi: 10.1007/s40475-017-0099-1
Gunaratna, G., Manamperi, A., Böhlken-Fascher, S., Wickremasinge, R., Gunawardena, K., Yapa, B., et al. (2018). Evaluation of rapid extraction and isothermal amplification techniques for the detection of Leishmania donovani DNA from skin lesions of suspected cases at the point of need in Sri Lanka. Parasit. Vectors 11:665. doi: 10.1186/s13071-018-3238-1
Gurel, M., Tekin, B., and Uzun, S. (2020). Cutaneous leishmaniasis: a great imitator. Clin. Dermatol. 38, 140–151. doi: 10.1016/j.clindermatol.2019.10.008
Hadermann, A., Heeren, S., Maes, I., Dujardin, J.-C., Domagalska, M. A., and Van den Broeck, F. (2023). Genome diversity of Leishmania aethiopica. Front. Cell. Infect. Microbiol. 13:1147998. doi: 10.3389/fcimb.2023.1147998
Haouas, N., Amer, O., Alshammri, F. F., Al-Shammari, S., Remadi, L., and Ashankyty, I. (2017). Cutaneous leishmaniasis in northwestern Saudi Arabia: identification of sand fly fauna and parasites. Parasit. Vectors 10:544. doi: 10.1186/s13071-017-2497-6
Harigua, E., Ben Salem, Y., Hariga, M., Saadi, Y., Souguir, H., Chouaieb, H., et al. (2020). Lesionia: a digital data management system to enhance epidemiological and clinical data management of patients with cutaneous diseases. doi: 10.2139/ssrn.3965285
Higgins, M., Stringer, O. W., Ward, D., Andrews, J. M., Forrest, M. S., Campino, S., et al. (2022). Characterizing the impact of primer-template mismatches on recombinase polymerase amplification. J. Mol. Diagn. 24, 1207–1216. doi: 10.1016/j.jmoldx.2022.08.005
Hurt, C. B., Nelson, J. A. E., Hightow-Weidman, L. B., and Miller, W. C. (2017). Selecting an HIV test: a narrative review for clinicians and researchers. Sex. Transm. Dis. 44, 739–746. doi: 10.1097/OLQ.0000000000000719
Kersting, S., Rausch, V., Bier, F. F., and von Nickisch-Rosenegk, M. (2014). Rapid detection of plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar. J. 13:99. doi: 10.1186/1475-2875-13-99
Khan, M. A. A., Faisal, K., Chowdhury, R., Ghosh, P., Hossain, F., Weidmann, M., et al. (2021). Development of quantitative rapid isothermal amplification assay for Leishmania donovani. Diagn. Basel Switz. 11:1963. doi: 10.3390/diagnostics11111963
Kosack, C. S., Page, A.-L., and Klatser, P. R. (2017). A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 95, 639–645. doi: 10.2471/BLT.16.187468
Kozel, T. R., and Burnham-Marusich, A. R. (2017). Point-of-care testing for infectious diseases: past, present, and future. J. Clin. Microbiol. 55, 2313–2320. doi: 10.1128/JCM.00476-17
Krõlov, K., Frolova, J., Tudoran, O., Suhorutsenko, J., Lehto, T., Sibul, H., et al. (2014). Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. J. Mol. Diagn. 16, 127–135. doi: 10.1016/j.jmoldx.2013.08.003
Liu, H., Zang, Y.-X., Du, X., Li, P., and Wang, S. (2017). Development of an isothermal amplification-based assay for the rapid visual detection of Salmonella bacteria. J. Dairy Sci. 100, 7016–7025. doi: 10.3168/jds.2017-12566
Madusanka, R. K., Silva, H., and Karunaweera, N. D. (2022). Treatment of cutaneous Leishmaniasis and insights into species-specific responses: a narrative review. Infect. Dis. Ther. 11, 695–711. doi: 10.1007/s40121-022-00602-2
Maphumulo, W. T., and Bhengu, B. R. (2019). Challenges of quality improvement in the healthcare of South Africa post-apartheid: a critical review. Curationis 42, e1–e9. doi: 10.4102/curationis.v42i1.1901
Mesa, L. E., Manrique, R., Robledo, S. M., Tabares, J., Pineda, T., and Muskus, C. (2021). The performance of the recombinase polymerase amplification test for detecting Leishmania deoxyribonucleic acid from skin lesions of patients with clinical or epidemiological suspicion of cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 115, 1427–1433. doi: 10.1093/trstmh/trab073
Mondal, D., Ghosh, P., Khan, M. A. A., Hossain, F., Böhlken-Fascher, S., Matlashewski, G., et al. (2016). Mobile suitcase laboratory for rapid detection of Leishmania donovani using recombinase polymerase amplification assay. Parasit. Vectors 9:281. doi: 10.1186/s13071-016-1572-8
Moore, M. D., and Jaykus, L.-A. (2017). Development of a recombinase polymerase amplification assay for detection of epidemic human noroviruses. Sci. Rep. 7:40244. doi: 10.1038/srep40244
Mosimann, V., Neumayr, A., Hatz, C., and Blum, J. A. (2013). Cutaneous leishmaniasis in Switzerland: first experience with species-specific treatment. Infection 41, 1177–1182. doi: 10.1007/s15010-013-0500-5
Mouttaki, T., Morales-Yuste, M., Merino-Espinosa, G., Chiheb, S., Fellah, H., Martin-Sanchez, J., et al. (2014). Molecular diagnosis of cutaneous leishmaniasis and identification of the causative Leishmania species in Morocco by using three PCR-based assays. Parasit. Vectors 7:420. doi: 10.1186/1756-3305-7-420
Moya-Salazar, J., Pasco, I. A., Cañari, B., and Contreras-Pulache, H. (2021). Cutaneous Leishmaniasis associated with the level of poverty of the Andean rural population: a five-year single-center study. Electron. J. Gen. Med. 18:em335. doi: 10.29333/ejgm/11335
Munawar, M. A., Toljamo, A., Martin, F., Oksanen, E., and Kokko, H. (2020). Development and evaluation of a recombinase polymerase amplification assay for rapid detection of strawberry red stele pathogen. Phytopathol. Res. 2:26. doi: 10.1186/s42483-020-00069-4
Nath-Chowdhury, M., Sangaralingam, M., Bastien, P., Ravel, C., Pratlong, F., Mendez, J., et al. (2016). Real-time PCR using FRET technology for Old World cutaneous leishmaniasis species differentiation. Parasit. Vectors 9:255. doi: 10.1186/s13071-016-1531-4
Piepenburg, O., Williams, C. H., Stemple, D. L., and Armes, N. A. (2006). DNA detection using recombination proteins. PLoS Biol. 4:e204. doi: 10.1371/journal.pbio.0040204
Pires, M., Wright, B., Kaye, P. M., Da Conceição, V., and Churchill, R. C. (2019). The impact of leishmaniasis on mental health and psychosocial well-being: a systematic review. PLoS One 14:e0223313. doi: 10.1371/journal.pone.0223313
Rogers, M. B., Hilley, J. D., Dickens, N. J., Wilkes, J., Bates, P. A., Depledge, D. P., et al. (2011). Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 21:2129. doi: 10.1101/gr.122945.111
Rohrman, B., and Richards-Kortum, R. (2015). Inhibition of recombinase polymerase amplification by background DNA: a lateral flow-based method for enriching target DNA. Anal. Chem. 87, 1963–1967. doi: 10.1021/ac504365v
Rosser, A., Rollinson, D., Forrest, M., and Webster, B. L. (2015). Isothermal recombinase polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasit. Vectors 8:446. doi: 10.1186/s13071-015-1055-3
Rostami, N. M., Darzi, F., Farahmand, M., Aghaei, M., and Parvizi, P. (2020). Performance of a universal PCR assay to identify different Leishmania species causative of Old World cutaneous leishmaniasis. Parasit. Vectors 13:431. doi: 10.1186/s13071-020-04261-5
Saldarriaga, O. A., Castellanos-Gonzalez, A., Porrozzi, R., Baldeviano, G. C., Lescano, A. G., de Los Santos, M. B., et al. (2016). An Innovative Field-Applicable Molecular Test to Diagnose Cutaneous Leishmania Viannia spp. Infections. PLoS Negl. Trop. Dis. 10:e0004638. doi: 10.1371/journal.pntd.0004638
Schönian, G., Nasereddin, A., Dinse, N., Schweynoch, C., Schallig, H. D. F. H., Presber, W., et al. (2003). PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 47, 349–358. doi: 10.1016/S0732-8893(03)00093-2
Sharma, N., Hoshika, S., Hutter, D., Bradley, K. M., and Benner, S. A. (2014). Recombinase-based isothermal amplification of nucleic acids with self-avoiding molecular recognition systems (SAMRS). Chembiochem. Eur. J. Chem. Biol. 15, 2268–2274. doi: 10.1002/cbic.201402250
Soares, A. R. C., De Faria, V. C. S., and De Avelar, D. M. (2024). Development and accuracy evaluation of a new loop-mediated isothermal amplification assay targeting the HSP70 gene for the diagnosis of cutaneous leishmaniasis. PLoS One 19:e0306967. doi: 10.1371/journal.pone.0306967
Spanakos, G., Patsoula, E., Kremastinou, T., Saroglou, G., and Vakalis, N. (2002). Development of a PCR-based method for diagnosis of Leishmania in blood samples. Mol. Cell. Probes 16, 415–420. doi: 10.1006/mcpr.2002.0436
Stevenson, L. G., Fedorko, D. P., and Zelazny, A. M. (2010). An enhanced method for the identification of Leishmania spp. using real-time polymerase chain reaction and sequence analysis of the 7SL RNA gene region. Diagn. Microbiol. Infect. Dis. 66, 432–435. doi: 10.1016/j.diagmicrobio.2009.11.005
Tabbabi, A. (2019). Review of Leishmaniasis in the Middle East and North Africa. Afr. Health Sci. 19, 1329–1337. doi: 10.4314/ahs.v19i1.4
Tan, M., Liao, C., Liang, L., Yi, X., Zhou, Z., and Wei, G. (2022). Recent advances in recombinase polymerase amplification: principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 12:1019071. doi: 10.3389/fcimb.2022.1019071
Taye, B., Melkamu, R., Tajebe, F., Ibarra-Meneses, A. V., Adane, D., Atnafu, S., et al. (2024). Evaluation of Loopamp Leishmania detection kit for the diagnosis of cutaneous leishmaniasis in Ethiopia. Parasit. Vectors 17:431. doi: 10.1186/s13071-024-06475-3
Travi, B. L., Delos Santos, M. B., Shelite, T. R., Santos, R. P., Rosales, L. A., Castellanos-Gonzalez, A., et al. (2021). Diagnostic efficacy of recombinase-polymerase-amplification coupled with lateral flow strip Reading in patients with cutaneous Leishmaniasis from the Amazonas rainforest of Perú. Vector Borne Zoonotic Dis. 21, 941–947. doi: 10.1089/vbz.2021.0038
Tupperwar, N., Vineeth, V., Rath, S., and Vaidya, T. (2008). Development of a real-time polymerase chain reaction assay for the quantification of Leishmania species and the monitoring of systemic distribution of the pathogen. Diagn. Microbiol. Infect. Dis. 61, 23–30. doi: 10.1016/j.diagmicrobio.2007.12.013
WHO (2010). Control of the leishmaniases WHO TRS n° 949. Available at: https://www.who.int/publications/i/item/WHO-TRS-949 (Accessed December 20, 2024).
Xue, G., Li, S., Zhao, H., Yan, C., Feng, Y., Cui, J., et al. (2020). Use of a rapid recombinase-aided amplification assay for Mycoplasma pneumoniae detection. BMC Infect. Dis. 20:79. doi: 10.1186/s12879-019-4750-4
Keywords: cutaneous leishmaniases, molecular diagnosis, RPA/RAA, Leishmania major, lateral flow chromatography, point of care diagnosis
Citation: Bel Hadj Ali I, Saadi-Ben Aoun Y, Khammeri I, Souguir H, Harigua-Souiai E, Chouaieb H, Chakroun AS, Lemrani M, Kallel A, Kallel K, Haddad N, El Dbouni O, Coler RN, Reed SG, Fathallah-Mili A and Guizani I (2025) Recombinase-based amplification coupled with lateral flow chromatography for the specific and sensitive detection and identification of Leishmania major in cutaneous leishmaniasis patients. Front. Microbiol. 15:1514684. doi: 10.3389/fmicb.2024.1514684
Received: 21 October 2024; Accepted: 16 December 2024;
Published: 27 January 2025.
Edited by:
Ze Chen, Hebei Normal University, ChinaReviewed by:
Sueli Fumie Yamada-Ogatta, State University of Londrina, BrazilCopyright © 2025 Bel Hadj Ali, Saadi-Ben Aoun, Khammeri, Souguir, Harigua-Souiai, Chouaieb, Chakroun, Lemrani, Kallel, Kallel, Haddad, El Dbouni, Coler, Reed, Fathallah-Mili and Guizani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ikram Guizani, aWtyYW0uZ3VpemFuaUBwYXN0ZXVyLnV0bS50bg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.