- 1State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu, China
- 2Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Operative Dentistry and Endodontics, West China School of Stomatology, Sichuan University, Chengdu, China
Streptococcus mutans (S. mutans) is the main pathogenic bacterium causing dental caries, and the modes in which its traits, such as acid production, acid tolerance, and adhesion that contribute to the dental caries process, has been clarified. However, a growing number of animal experiments and clinical revelations signify that these traits of S. mutans are not restricted to the detriment of dental tissues. These traits can assist S. mutans in evading the immune system within body fluids; they empower S. mutans to adhere not merely to the surface of teeth but also to other tissues such as vascular endothelium; they can additionally trigger inflammatory reactions and inflict damage on various organs, thereby leading to the occurrence of systemic diseases. These traits mostly originate from some correlative findings, lacking a comprehensive evaluation of the impact of S. mutans on systemic diseases. Therefore, this review mainly centers on the dissemination route of S. mutans: “Entering the blood circulation - Occurrence of tissue adhesion - Extensive possible proinflammatory mechanisms - Concentration in individual organs” and analyses the specific effects and possible mechanisms of S. mutans in systemic diseases such as cerebral hemorrhage, inflammatory bowel disease, tumors, and infective endocarditis that have been identified hitherto.
1 Introduction
Streptococcus is the most abundant genus in the mouth, and as a member of this genus, Streptococcus mutans (S. mutans) plays a central role in the development of caries. It can synthesize extracellular polysaccharides through glucose transferase and promote bacterial adhesion and aggregation, leading to the formation of plaque biofilm and colonization in the oral cavity. Subsequently, S. mutans can metabolize carbohydrates to produce acids that act on dental tissues to demineralize and form cavities. The glucose transferase of S. mutans is highly compatible with the ideal adherent surfaces and sufficient carbohydrates in the oral cavity, thus playing a unique advantage.
However, when there are adherent surfaces and suitable carbohydrates in other parts of the body, the glucose transferase of S. mutans may still be functioning, and its other biological structures are also likely to be so. Furthermore, many studies have found that S. mutans can indeed enter the bloodstream with other oral microorganisms during dental extraction, root canal treatment, or even during daily toothbrushing (Li et al., 2000; Sonbol et al., 2009). Depending on the special biological structure, some special types of S. mutans that enter the circulation may not be recognized and eliminated by the immune system, but adhere to various sites of the circulation pathway, leading to inflammatory responses and tissue damage. In such cases, clinical practice may consider S. mutans as an important therapeutic target. Therefore, this review focuses on some of the pathogenic biological structures, possible pro-inflammatory mechanisms, and the complex and important pathogenic process, mechanisms, and factors of S. mutans-induced systemic diseases, with the hope of enabling clinical practice and fundamental research to have a more holistic understanding and recognition of the role of S. mutans in systemic diseases.
2 Pathogenic factors of S. mutans
Streptococcus mutans has many different surface biological structures that play a major role in the pathogenesis of oral and systemic diseases. For example, Rhamnose glucose polymer (RGP) is responsible for different serotypes of S. mutans (Yamashita et al., 1999), while glucosyltransferase, protein antigens, and polysaccharide-binding protease are the main surface protein antigens of S. mutans, which are essential for certain steps in the pathogenic process (Fujita et al., 2007). Additionally, S. mutans has many adhesins, and in the oral cavity, the adhesion of S. mutans is a key factor in the development of caries (Li et al., 2021), while in systemic diseases, adhesins represent an important advantage for host colonization.
2.1 Rhamnose glucose polymer and glucose side chain
Streptococcus mutans is classified into four serotypes (c, e, f, and k) based on the composition and structure of the cell wall-associated rhamnose polysaccharides. Serotype c is predominant in the oral cavity, with a detection rate of over 70% in the mouths of healthy subjects; serotype e constitutes approximately 20%, and serotype k constitutes less than 5% (Nakano et al., 2004; Nakano et al., 2007c).
In systemic diseases, RGP contributes to the resistance of S. mutans against phagocytosis by human polymorphonuclear leukocytes (Tsuda et al., 2000), and is capable of binding to fibronectin, type I collagen, and laminin. This enables it to aggregate at the endocardium, thereby enhancing the virulence of infectious endocarditis (IE) strains. In the IE rat model, the virulence level of the strain containing intact RGP is significantly higher than that of the RGP- mutant, and RGP can trigger platelet aggregation, which may result in vascular stenosis at the site of S. mutans aggregation (Nagata et al., 2006). Additionally, the glucose side chains also exert varying degrees of influence on pathogenicity. For instance, the serotype k strain of Streptococcus with a defective glucose side chain has been demonstrated to be less prone to phagocytosis by polymorphonuclear leukocytes, leading to prolonged bacteremia.
2.2 Glucosyltransferases (GTFs) and glucan-binding proteases (GBPs)
Glucosyltransferases have been implicated in sucrose-dependent adhesion of S. mutans to tooth surfaces. S. mutans strains produce up to three glycosyltransferases, Gtf-B, -C, and -D. From infected heart valve tissue, S. mutans strains lacking expression of each of the three GTFs could be isolated (Munro and Macrina, 1993; Nomura et al., 2006). Mice infected with isogenic mutant strains lacking all three GTFs had significantly reduced survival (Shun et al., 2005). Nucleotides with high homology to erythromycin and macromycin resistance genes were found in the gtfB and gtfD genes respectively. The insertion of these resistance genes resulted in loss of GTFs expression and acquisition of bacterial resistance, possibly contributing to the high virulence of the strains against cardiovascular diseases (Nemoto et al., 2008).
GBPs, owing to their glucan-binding attributes, constitute significant virulence factors in dental caries, jointly functioning with GTFs to facilitate caries. However, within the bloodstream, only a deficiency in GBPs has been discovered to extend the duration of S. mutans bacteremia (Nomura et al., 2004).
2.3 Protein antigens (PA)
In the oral cavity, this structurally complex, multifunctional adhesin mediates bacterial attachment to the salivary membrane of teeth through interactions with host scavenger receptors glycoprotein gp340 or protein DMBT-1 (Brady et al., 2010). In a rat bacteremia model, PA- S. mutants showed the strongest resistance to phagocytosis, exhibited stronger cell hydrophobicity, significantly prolonged the duration of bacteremia, and had significantly higher inflammatory markers than rats infected with the parental strain (Nakano et al., 2006b; Nakano et al., 2008; Matsumoto-Nakano et al., 2009).
2.4 Collagen-binding proteins (CBPs)
Collagen-binding proteins primarily consists of Cnm and Cbm, and CBP + S. mutans is more commonly isolated from dental plaque of patients with bacteremia and infective endocarditis. Approximately 15% of S. mutans isolates contain Cnm, while Cbm is rarely detected (Abranches et al., 2011; Nomura et al., 2012). The genes encoding CBPs are not uniformly distributed among serotypes and are mainly concentrated in strains e, f, and k (Lapirattanakul et al., 2011).
Cnm is a 120 kDa protein encoded by the cnm gene (Sato et al., 2004), which is found in 10–20% of oral bacterial strains and is most commonly found in serotypes f and k (Nakano et al., 2007a; Nomura et al., 2009). Because Cnm can also bind to type I collagen and laminin, it may produce similar effects to RGP. Therefore, Cnm is highly associated with bacterial attachment to blood vessels, and studies have shown that Cnm is a necessary condition for S. mutans to invade endothelial cells and subsequently cause cardiovascular disease.
Cbm is another CBP that is encoded by the cbm gene. Its hypothesized collagen-binding domain has 80% identity and 90% similarity with the homologous region of Cnm. Cbm is found in less than 3% of oral strains, most commonly in serotype k, where most lack PA expression (Nomura et al., 2012; Nomura et al., 2013). The type I collagen binding level of Cbm is higher than that of Cnm and can bind fibrinogen. Nomura et al. (2014) found that in the presence of fibrinogen, Cbm + /PA- strains had higher platelet aggregation induction and fibrinogen binding activity than Cbm- mutants. In the absence of fibrinogen, the platelet aggregation induction activity of Cbm + strains was relatively weak, as most CBP + S. mutans cells have a negative charge on their surface, which inhibits the aggregation of similarly charged platelets (Nakano et al., 2011). Therefore, for Cbm + mutant Streptococcus, the presence of fibrinogen is essential, and it may act as a bridge molecule to allow Cbm + mutant Streptococcus to bind to platelets.
2.5 Fibronectin-binding proteins
Fibronectin-binding proteins are cell envelope-associated proteins of S. mutans, including AtlA, RgpG, BrpA, and Psr (Lemos et al., 2019). Fibronectin-binding proteins mediate specific adhesion of S. mutans to fibronectin and endothelial cells (Chia et al., 2000). For instance, BrpA- S. mutants were more resistant to polymorphonuclear leukocyte phagocytosis and aggregation of platelets than the parental strain. The duration of bacteremia induced by this defective strain was longer in rats (Nakano et al., 2005).
The recently identified fibronectin-binding protein AtlA plays a role in S. mutans resistance to phagocytosis and survival in the bloodstream, and its deficiency results in reduced autolysin, prolonged chain length, and significantly reduced biofilm formation. Furthermore, physiological serum calcium concentrations promote its maturation. It is found to be associated with S. mutans survival and virulence to IE in a rat model (Jung et al., 2009).
3 Proinflammatory mechanisms
Inflammation is characterized by leukocyte migration from the vascular system to damaged tissue, regulated by proinflammatory cytokines. S. mutans infection can induce a series of inflammatory reactions, explaining systemic diseases caused by S. mutans. S. mutans surface PA has potential to stimulate TNF-α, IL-1β, and IL-6 production (Paik et al., 2003). TNF-α and IL-1β are proinflammatory mediators. And IL-6 leads to increased cell adhesion molecule and chemokine expression, attracting macrophages and neutrophils for microbial elimination as first-line defense.
3.1 Induction of TNF-α and IL-1β
In macrophages, S. mutans induces TNF-α and IL-1β production by activating signaling pathway ERK/p38/JNK and NF-κB through TLR2 (Toll-like receptor 2) and TLR4(Toll-like receptor 4), respectively (Kim et al., 2012). NF-êB activates a transcriptional response, leading to pro-IL-1β synthesis, and a second signaling pathway activates caspase-1-dependent IL-1β maturation and secretion via inflammasomes (Schroder and Tschopp, 2010).
TNF-α initiates a cytokine cascade and increases vascular permeability, recruiting macrophages and neutrophils to a site of infection. IL-1b is a cytokine produced mainly by activated mononuclear phagocytes, mediating host inflammatory responses in innate immunity. It has several biological effects, including phagocyte activation, antibody production, and T-cell polarization.
3.2 Induction of IL-6
IL-6 and its soluble receptors are key mediators regulating neutrophils and monocytes recruitment (Kaplanski et al., 2003). One study showed GTFs didn’t promote infectivity without glucan but affected inflammation. In acute inflammation, GTFs were the main regulatory protein inducing IL-6 (Chia et al., 2001). GTFs may stimulate IL-6 during systemic infection in the spleen and surrounding vegetations (Chia et al., 2002). Clinical investigation showed serum IL-6 levels significantly increased in all endocarditis stages, while other cytokines didn’t significantly increase (Rawczynska-Englert et al., 2000; Alter et al., 2002). Therefore, sustained IL-6 activation may promote myocardial injury. Additionally, as a gram + bacterium, S. mutans collagen combined with adhesive matrix molecules can inhibit the classical complement pathway (Kang et al., 2013).
4 Role in systemic diseases
4.1 Role in cardiovascular disease
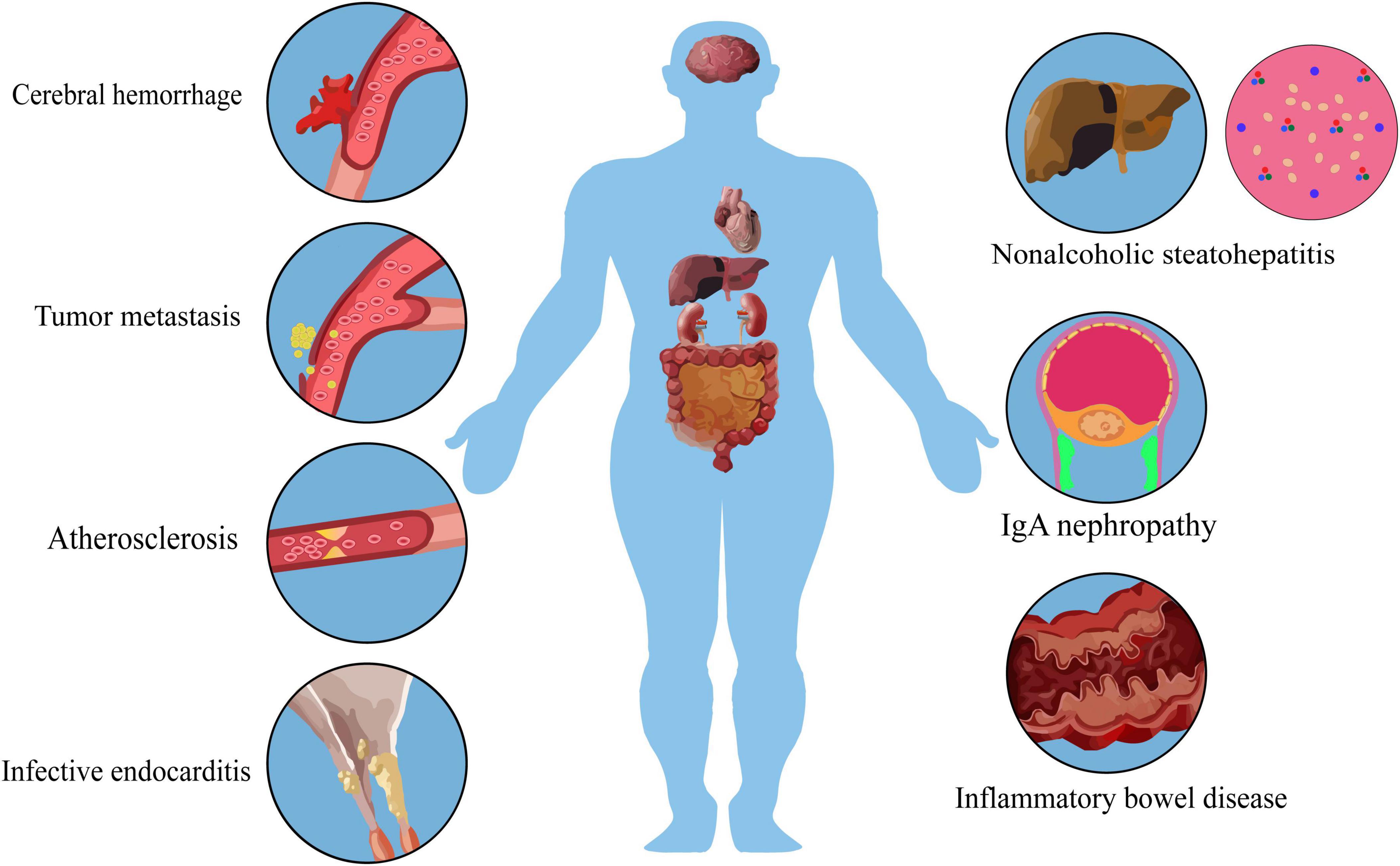
Streptococcus mutans can cause damage to various organs and systems after entering the blood. At present, the mechanism of research on cardiovascular system damage is relatively clear. CBPs can bind with type I collagen to damage blood vessels, and cause cardiovascular diseases such as cerebral hemorrhage and infectious endocarditis, as well as inflammatory bowel disease, IgA nephropathy and tumor metastasis.
In various clinical and animal studies, S. mutans is frequently detected in heart valve and atherosclerotic plaque samples, and its role in causing bacteremia and vascular damage has been observed (Lockhart et al., 2004; Nakano et al., 2006a; Nakano et al., 2007b; Nakano et al., 2009; Oliveira et al., 2015; Nomura et al., 2020a).
Compared to healthy subjects, the detection rate of non-serotype c strains, such as serotype k, is significantly higher in the heart valves of patients with cardiovascular disease. Due to changes in cell surface antigens of serotype k strains, including high-frequency loss of PA expression and GbpA (Glucan-binding protease A) expression, as well as reduced GbpC expression, these strains can resist leukocyte phagocytosis and survive for extended periods in the bloodstream (Nakano et al., 2002). Importantly, serotype k strains highly express Cnm or Cbm, which are critical virulence factors for adhesion to vascular endothelial cells and heart valves, contributing to cardiovascular disease.
4.1.1 Atherosclerosis (AS)
Atherosclerosis is a disease where lipid patchy deposits form in the walls of medium or large arteries, resulting in reduced or blocked blood flow. S mutans accelerated AS induction in mice through its damaging effects on vascular endothelial cells, which was more pronounced in the injured endothelium (Kesavalu et al., 2012).
It is now believed S. mutans infection of Human Aortic Endothelial Cells (HAECs) significantly upregulates intracellular TLR2 and NOD2 (nucleotide-binding oligomerization domain protein) expression, leading to greater cardiovascular disease-associated pro-inflammatory cytokine (IL-6, IL-8, MCP-1) production (Nagata and Oho, 2017). In vitro studies found upregulated cellular DMBT1(deleted in malignant brain tumor 1, proposed to be a tumor suppressor gene) expression in HAECs after S. mutans infection. S. mutans was more invasive to DMBT1 knockdown HAECs, resulting in higher cytokine production. This suggests DMBT1 inhibits bacterial adhesion to HAECs and provides protection against S. mutans infection (Oho and Nagata, 2019).
4.1.2 Infective endocarditis
After S. mutans reaches the damaged cardiac endothelium, it binds to the exposed collagen, where activated platelets also bind via the factor vWF. When the Cbm + /PA- strain binds to the Glycoprotein IIb/IIIa receptor on platelets, it may interact with fibrinogen, accelerating the formation of redundant organisms, leading to the occurrence of IE (Koga et al., 1990).
Recently, Nomura et al. (2020b) suggested the type IV collagen-Cnm-ARHGEF 38 pathway plays an important role in IE pathogenesis, explaining IE occurrence without heart valve injury. Activation and inactivation of small G proteins (A class of signal transduction proteins featuring guanylate binding sites and GTPase activity, and having a molecular weight of merely 20–30 kDa) involve cytoskeletal rearrangements in human cells (Cherfils and Zeghouf, 2013). Many bacteria invade human cells by expressing Rho guanine nucleotide exchange factors, activating small G proteins, and Rho GTPase-activating proteins (A protein involved in cellular signal transduction), inactivating small G proteins, to rearrange the cytoskeleton (Patel and Galán, 2006; Handa et al., 2007; Roppenser et al., 2009). Conversely, GTPase-activating protein may prevent Cnm + mutant streptococcal invasion by inhibiting type IV collagen binding.
4.1.3 Cerebral hemorrhage
Eishi et al. (1995) showed in a mouse model that Cnm + S. mutans were also associated with worsening cerebral hemorrhage, a complication of IE occurring in about 10% of cases. A cross-sectional study showed Cnm + S. mutans increased cerebral microhemorrhages (CMBs) (Miyatani et al., 2015). A later clinical longitudinal study also showed Cnm + S. mutans associated with hypertensive cerebral hemorrhage and deep CMBs, two major hemorrhagic phenotypes of penetrating atherosclerosis (Hosoki et al., 2020).
Possible mechanisms of cerebral hemorrhage from Cnm + S. mutans are: blood-brain barrier permeability increases with age due to endothelial integrity decrease. Chronic hypertension also affects small vessel structure, leading not only to blood-brain barrier disruption, but also type I collagen accumulation. In addition, vascular changes from aging and hypertension release vascular factors attracting Cnm + S. mutans binding, promoting attachment to exposed collagen of small cerebral perforating arteries. Subsequent neutrophil infiltration and local inflammation exacerbation increase blood-brain barrier permeability and enzyme release (myeloperoxidase MPO-16 and matrix metalloproteinase MMP-9) accelerating endothelial damage (Nakano et al., 2011; Tonomura et al., 2016). Additionally, bacterial surface negative charge prevents infiltration of negatively charged platelets with collagen, promoting focal hemorrhage. Endothelial injury from aging and hypertension is more pronounced deeper, further progressing to cerebral hemorrhage and hemorrhagic stroke (Lammie, 2000).
4.2 IgA nephropathy (IgAN)
IgAN is characterized by predominant IgA1 deposition in the mesangial region, with extracellular matrix proteins composed of type IV collagen, fibronectin, and more (Kokubo et al., 1998; Narita and Gejyo, 2008; Abrahamson et al., 2013). In IgA nephropathy, the relationship between tonsil immunity and glomerulonephritis has been established. However, certain pathogens may impact tonsil immunity, resulting in IgAN (Komatsu et al., 2008). Mucosal changes like infection can activate the innate immune system, exacerbate existing IgAN, and promote disease manifestations like hematuria (Floege and Feehally, 2016). Higher type IV collagen concentrations could promote more pronounced aggregation of S. mutans. Some reports suggest that S. mutans can induce severe nephritis involving the glomeruli, tubules in rabbits, as well as IgAN-like glomerulonephritis in rats (Albini et al., 1985; Naka et al., 2021). Cnm + S. mutans strains are associated with elevated urine protein and severe IgAN, while the Cnm protein itself has no direct effect on the kidneys (Misaki et al., 2016; Ito et al., 2019). Immune reactions with IgA in Cnm + mucosal tissue may lead to glycosylation defects in serum IgA1 molecules, with glycosylation defense in IgA1 molecules as the primary pathogenesis of IgAN (Tomana et al., 1999; Rodrigues et al., 2017). This plays an important role in IgAN pathogenesis.
Hotta et al. (2001) proposed tonsillectomy and steroid pulse therapy for IgAN patients can not only reduce hematuria/proteinuria but also improve remission rates. This may support the significance of tonsillectomy in IgAN patient treatment and the importance of oral care in preventing kidney disease.
4.3 Non-alcoholic steatohepatitis (NASH)
”Two hit theory” is widely believed to be the mechanism for NASH development, where insulin resistance caused by metabolic syndrome from excessive nutrition leads to simple steatosis as the first hit. The second hit is oxidative stress, lipid peroxidation, and mitochondrial failure, accelerating liver inflammation and fibrosis, leading to NASH (Day and James, 1998). Compared to Cnm + /PA- variant S. mutans strain, Cnm + /PA + strain showed high virulence in aggravating NASH in mice (Naka et al., 2014; Naka et al., 2016). Cnm helps S. mutans adhere to liver cells without fatty acid accumulation. Cnm and PA may facilitate adhesion of S. mutans cells to hepatocytes without and with fatty acids, respectively. S. mutans expressing both PA and Cnm on the bacterial cell surface localizes in the liver and attaches to hepatocytes, leading to increased inflammatory cytokines related to oxidative stress, such as IFN-γ and metallothionein. This results in a second hit on liver cells, aggravating NASH (Naka et al., 2014). Increasing evidence supports clinical interaction between type IV collagen and Cnm + S. mutans.
4.4 Inflammatory bowel disease
Ulcerative colitis and Crohn’s disease are major inflammatory bowel diseases, which are chronic and recurrent intestinal diseases. Available data show that proinflammatory cytokines (TNF-α, IL-1β, and IL-6), and immunosuppressive IL-10 deficiency are markers of intestinal inflammation (Vasovic et al., 2016). IL-6 is the main cytokine in the inflammatory region of ulcerative colitis patients, and its concentration is related to the score of disease severity under endoscopy (Zdravkovic et al., 2014).
The aggravation of colitis may result from S mutans infection of the liver, IFN-γ released from the liver during S mutans infection is the first step for various inflammatory cascade reactions (Kojima et al., 2012; Kojima et al., 2014).
4.5 Tumor related mechanisms
Streptococcus mutans infection contributes to tumor invasiveness and is associated with poor disease control. In an oral squamous cell carcinoma mouse model, S. mutans promoted oral cancer development and progression by increasing IL-6 production (Tsai et al., 2022). S. mutans has the ability to induce vascular inflammation and disrupt the vascular barrier by upregulating IL-8, reducing vascular endothelial-cadherin expression, and enhancing intercellular adhesion molecule 1 signaling, thereby facilitating tumor cell extravasation and migration toward the endothelium (Yu et al., 2009; Yu et al., 2022; Figure 1).
The impairment of the vascular endothelium lead to cerebral hemorrhage, infective endocarditis and atherosclerosis. S. mutans can induce an upregulation of interleukin, which, in conjunction with its vascular damage, may contribute to the vascular metastasis of primary tumors.
During serotype k infection, the liver releases IFN-γ and triggers inflammation. This, combined with the interaction of type IV collagen, a basal membrane protein in parahepatic sinus cells, with S. mutans, can result in tissue fibrosis and non-alcoholic steatohepatitis. Simultaneously, IFN-γ released by the liver may be a primary factor in the exacerbation of colitis.
IgA immune reactions in Cnm + mucosal tissue may cause glycosylation defects in serum IgA1 molecules, with impaired glycosylation being the primary pathogenesis of IgAN. Immunofluorescence reveals IgA deposition in the mesangial region.
5 Conclusions and perspectives
The oral cavity is one of the most crucial interaction interfaces between the human body and the external environment. Oral microorganisms not only directly influence the disease status of dental caries and periodontal diseases but also exert crucial pathogenic effects in multiple organs. With the continuous advancement of metagenomics and high-throughput sequencing technologies, the relationship between oral microorganisms and systemic diseases has become increasingly explicit.
At present, frequent diseases caused by oral microorganisms in clinical practice include cardiovascular diseases, strokes, premature birth, diabetes, and pneumonia, among others. These diseases are frequently the outcomes of the concerted actions of several bacteria that inflict damage on the host. To precisely define the strict correlation between systemic diseases and oral microorganisms, it might be necessary to clarify the specific roles played by each type of oral microbe. S. mutans has its own distinctive features compared to other oral microorganisms, which enables it to cause not only the aforementioned diseases but also different systemic diseases such as IgAN and NASH.
However, precisely due to the unique features of each distinct species, the task of clarifying specific pathogenic mechanisms is colossal. In previous studies on S. mutans, many have remained at the stage of discovering correlations, lacking well-conducted clinical trials. Only a small portion, such as studies on cardiovascular diseases caused by S. mutans, have identified clear pathogenic mechanisms and perhaps intervention targets. On the whole, our understanding of the association between S. mutans and systemic diseases might still be in its initial stage.
In the future, it might be possible to carry out large-scale studies on diverse populations and carefully analyze the risk factors of different individuals, such as genetic background and lifestyle. Simultaneously, the future goal should not only focus on identifying more unidentified pathogenic mechanisms of S. mutans causing systemic diseases but also on the existing pathogenic factors of S. mutans. Measures to improve clinical treatment or other strategies should be adopted to address the adverse effects of S. mutans, such as maintaining good oral hygiene habits, undergoing regular dental examinations, and prophylactic use of antibiotics before treatment. A more comprehensive understanding and more rational responses to S. mutans will have an indelible significance for the prevention, diagnosis, and treatment of human systemic diseases.
Author contributions
YF: Writing – original draft, Conceptualization. XC: Writing – original draft. CC: Supervision, Writing – review and editing. OY: Supervision, Writing – review and editing. JH: Supervision, Writing – review and editing. ML: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This review manuscript was supported by the Sichuan Science and Technology Program Nos. 2021YFH0188 (ML) and 2020YJ0240 (JH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrahamson, D. R., St John, P. L., Stroganova, L., Zelenchuk, A., and Steenhard, B. M. (2013). Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J. Histochem. Cytochem. 61, 706–718. doi: 10.1369/0022155413501677
Abranches, J., Miller, J. H., Martinez, A. R., Simpson-Haidaris, P. J., Burne, R. A., and Lemos, J. A. (2011). The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun. 79, 2277–2284. doi: 10.1128/IAI.00767-10
Albini, B., Nisengard, R. J., Glurich, I., Neiders, M. E., and Stinson, M. W. (1985). Streptococcus mutans-induced nephritis in rabbits. Am. J. Pathol. 118, 408–418.
Alter, P., Hoeschen, J., Ritter, M., and Maisch, B. (2002). Usefulness of cytokines interleukin-6 and interleukin-2R concentrations in diagnosing active infective endocarditis involving native valves. Am. J. Cardiol. 89, 1400–1404. doi: 10.1016/s0002-9149(02)02353-6
Brady, L. J., Maddocks, S. E., Larson, M. R., Forsgren, N., Persson, K., Deivanayagam, C. C., et al. (2010). The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 77, 276–286.
Cherfils, J., and Zeghouf, M. (2013). Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309.
Chia, J. S., Lien, H. T., Hsueh, P. R., Chen, P. M., Sun, A., and Chen, J. Y. (2002). Induction of cytokines by glucosyltransferases of streptococcus mutans. Clin. Diagn. Lab. Immunol. 9, 892–897.
Chia, J. S., Yeh, C. Y., and Chen, J. Y. (2000). Identification of a fibronectin binding protein from Streptococcus mutans. Infect. Immun. 68, 1864–1870.
Chia, J. S., You, C. M., Hu, C. Y., Chiang, B. L., and Chen, J. Y. (2001). Human T-cell responses to the glucosyltransferases of Streptococcus mutans. Clin. Diagn. Lab. Immunol. 8, 441–445. doi: 10.1128/CDLI.8.2.441-445.2001
Day, C. P., and James, O. F. (1998). Steatohepatitis: a tale of two “hits”? Gastroenterology. 114, 842–845. doi: 10.1016/s0016-5085(98)70599-2
Eishi, K., Kawazoe, K., Kuriyama, Y., Kitoh, Y., Kawashima, Y., and Omae, T. (1995). Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J. Thorac. Cardiovasc. Surg. 110, 1745–1755. doi: 10.1016/S0022-5223(95)70038-2
Floege, J., and Feehally, J. (2016). The mucosa-kidney axis in IgA nephropathy. Nat. Rev. Nephrol. 12, 147–156.
Fujita, K., Matsumoto-Nakano, M., Inagaki, S., and Ooshima, T. (2007). Biological functions of glucan-binding protein B of Streptococcus mutans. Oral Microbiol. Immunol. 22, 289–292.
Handa, Y., Suzuki, M., Ohya, K., Iwai, H., Ishijima, N., Koleske, A. J., et al. (2007). Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat. Cell Biol. 9, 121–128. doi: 10.1038/ncb1526
Hosoki, S., Saito, S., Tonomura, S., Ishiyama, H., Yoshimoto, T., Ikeda, S., et al. (2020). Oral carriage of Streptococcus mutans Harboring the cnm gene relates to an increased incidence of cerebral microbleeds. Stroke 51, 3632–3639. doi: 10.1161/STROKEAHA.120.029607
Hotta, O., Miyazaki, M., Furuta, T., Tomioka, S., Chiba, S., Horigome, I., et al. (2001). Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am. J. Kidney Dis. 38, 736–743.
Ito, S., Misaki, T., Naka, S., Wato, K., Nagasawa, Y., Nomura, R., et al. (2019). Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci. Rep. 9:20130. doi: 10.1038/s41598-019-56679-2
Jung, C. J., Zheng, Q. H., Shieh, Y. H., Lin, C. S., and Chia, J. S. (2009). Streptococcus mutans autolysin AtlA is a fibronectin-binding protein and contributes to bacterial survival in the bloodstream and virulence for infective endocarditis. Mol. Microbiol. 74, 888–902. doi: 10.1111/j.1365-2958.2009.06903.x
Kang, M., Ko, Y. P., Liang, X., Ross, C. L., Liu, Q., Murray, B. E., et al. (2013). Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J. Biol. Chem. 288, 20520–20531. doi: 10.1074/jbc.M113.454462
Kaplanski, G., Marin, V., Montero-Julian, F., Mantovani, A., and Farnarier, C. (2003). IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24, 25–29.
Kesavalu, L., Lucas, A. R., Verma, R. K., Liu, L., Dai, E., Sampson, E., et al. (2012). Increased atherogenesis during Streptococcus mutans infection in ApoE-null mice. J. Dent. Res. 91, 255–260. doi: 10.1177/0022034511435101
Kim, J. S., Kim, K. D., Na, H. S., Jeong, S. Y., Park, H. R., Kim, S., et al. (2012). Tumor necrosis factor-α and interleukin-1β expression pathway induced by Streptococcus mutans in macrophage cell line RAW 264.7. Mol. Oral Microbiol. 27, 149–159.
Koga, T., Okahashi, N., Takahashi, I., Kanamoto, T., Asakawa, H., and Iwaki, M. (1990). Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect. Immun. 58, 289–296.
Kojima, A., Nakano, K., Wada, K., Takahashi, H., Katayama, K., Yoneda, M., et al. (2012). Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2:332. doi: 10.1038/srep00332
Kojima, A., Nomura, R., Naka, S., Okawa, R., Ooshima, T., and Nakano, K. (2014). Aggravation of inflammatory bowel diseases by oral streptococci. Oral Dis. 20, 359–366. doi: 10.1111/odi.12125
Kokubo, T., Hiki, Y., Iwase, H., Tanaka, A., Toma, K., Hotta, K., et al. (1998). Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J. Am. Soc. Nephrol. 9, 2048–2054. doi: 10.1681/ASN.V9112048
Komatsu, H., Fujimoto, S., Hara, S., Sato, Y., Yamada, K., and Kitamura, K. (2008). Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: a controlled study. Clin. J. Am. Soc. Nephrol. 3, 1301–1307. doi: 10.2215/CJN.00310108
Lapirattanakul, J., Nakano, K., Nomura, R., Leelataweewud, P., Chalermsarp, N., Klaophimai, A., et al. (2011). Multilocus sequence typing analysis of Streptococcus mutans strains with the cnm gene encoding collagen-binding adhesin. J. Med. Microbiol. 60(Pt. 11), 1677–1684.
Lemos, J. A., Palmer, S. R., Zeng, L., Wen, Z. T., Kajfasz, J. K., Freires, I. A., et al. (2019). The biology of Streptococcus mutans. Microbiol Spectr. 7, doi: 10.1128/microbiolspec.gpp3-0051-2018
Li, Q., Shen, J., Qin, T., Zhou, G., Li, Y., Chen, Z., et al. (2021). A qualitative and comprehensive analysis of caries susceptibility for dental fluorosis patients. Antibiotics (Basel) 10:1047. doi: 10.3390/antibiotics10091047
Li, X., Kolltveit, K. M., Tronstad, L., and Olsen, I. (2000). Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13, 547–558.
Lockhart, P. B., Brennan, M. T., Kent, M. L., Norton, H. J., and Weinrib, D. A. (2004). Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation 109, 2878–2884. doi: 10.1161/01.CIR.0000129303.90488.29
Matsumoto-Nakano, M., Tsuji, M., Inagaki, S., Fujita, K., Nagayama, K., Nomura, R., et al. (2009). Contribution of cell surface protein antigen c of Streptococcus mutans to platelet aggregation. Oral Microbiol. Immunol. 24, 427–430. doi: 10.1111/j.1399-302X.2009.00521.x
Misaki, T., Naka, S., Hatakeyama, R., Fukunaga, A., Nomura, R., Isozaki, T., et al. (2016). Presence of Streptococcus mutans strains harbouring the cnm gene correlates with dental caries status and IgA nephropathy conditions. Sci. Rep. 6:36455. doi: 10.1038/srep36455
Miyatani, F., Kuriyama, N., Watanabe, I., Nomura, R., Nakano, K., Matsui, D., et al. (2015). Relationship between Cnm-positive Streptococcus mutans and cerebral microbleeds in humans. Oral Dis. 21, 886–893. doi: 10.1111/odi.12360
Munro, C. L., and Macrina, F. L. (1993). Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol. Microbiol. 8, 133–142. doi: 10.1111/j.1365-2958.1993.tb01210.x
Nagata, E., and Oho, T. (2017). Invasive Streptococcus mutans induces inflammatory cytokine production in human aortic endothelial cells via regulation of intracellular toll-like receptor 2 and nucleotide-binding oligomerization domain 2. Mol. Oral Microbiol. 32, 131–141. doi: 10.1111/omi.12159
Nagata, E., Okayama, H., Ito, H. O., Yamashita, Y., Inoue, M., and Oho, T. (2006). Serotype-specific polysaccharide of Streptococcus mutans contributes to infectivity in endocarditis. Oral Microbiol. Immunol. 21, 420–423. doi: 10.1111/j.1399-302X.2006.00317.x
Naka, S., Hatakeyama, R., Takashima, Y., Matsumoto-Nakano, M., Nomura, R., and Nakano, K. (2016). Contributions of Streptococcus mutans Cnm and PA antigens to aggravation of non-alcoholic steatohepatitis in mice. Sci. Rep. 6:36886. doi: 10.1038/srep36886
Naka, S., Nomura, R., Takashima, Y., Okawa, R., Ooshima, T., and Nakano, K. (2014). A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis. 20, 700–706. doi: 10.1111/odi.12191
Naka, S., Wato, K., Misaki, T., Ito, S., Matsuoka, D., Nagasawa, Y., et al. (2021). Streptococcus mutans induces IgA nephropathy-like glomerulonephritis in rats with severe dental caries. Sci. Rep. 11:5784. doi: 10.1038/s41598-021-85196-4
Nakano, K., Fujita, K., Nishimura, K., Nomura, R., and Ooshima, T. (2005). Contribution of biofilm regulatory protein A of Streptococcus mutans, to systemic virulence. Microbes Infect. 7, 1246–1255. doi: 10.1016/j.micinf.2005.04.012
Nakano, K., Hokamura, K., Taniguchi, N., Wada, K., Kudo, C., Nomura, R., et al. (2011). The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2:485. doi: 10.1038/ncomms1491
Nakano, K., Inaba, H., Nomura, R., Nemoto, H., Takeda, M., Yoshioka, H., et al. (2006a). Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol. 44, 3313–3317. doi: 10.1128/JCM.00377-06
Nakano, K., Lapirattanakul, J., Nomura, R., Nemoto, H., Alaluusua, S., Grönroos, L., et al. (2007a). Streptococcus mutans clonal variation revealed by multilocus sequence typing. J. Clin. Microbiol. 45, 2616–2625. doi: 10.1128/JCM.02343-06
Nakano, K., Matsumura, M., Kawaguchi, M., Fujiwara, T., Sobue, S., Nakagawa, I., et al. (2002). Attenuation of glucan-binding protein C reduces the cariogenicity of Streptococcus mutans: analysis of strains isolated from human blood. J. Dent. Res. 81, 376–379. doi: 10.1177/0810376
Nakano, K., Nemoto, H., Nomura, R., Homma, H., Yoshioka, H., Shudo, Y., et al. (2007b). Serotype distribution of Streptococcus mutans a pathogen of dental caries in cardiovascular specimens from Japanese patients. J. Med. Microbiol. 56(Pt. 4), 551–556. doi: 10.1099/jmm.0.47051-0
Nakano, K., Nemoto, H., Nomura, R., Inaba, H., Yoshioka, H., Taniguchi, K., et al. (2009). Detection of oral bacteria in cardiovascular specimens. Oral Microbiol. Immunol. 24, 64–68.
Nakano, K., Nomura, R., Nakagawa, I., Hamada, S., and Ooshima, T. (2004). Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J. Clin. Microbiol. 42, 198–202. doi: 10.1128/JCM.42.1.198-202.2004
Nakano, K., Nomura, R., Nemoto, H., Lapirattanakul, J., Taniguchi, N., Grönroos, L., et al. (2008). Protein antigen in serotype k Streptococcus mutans clinical isolates. J. Dent. Res. 87, 964–968.
Nakano, K., Nomura, R., Nemoto, H., Mukai, T., Yoshioka, H., Shudo, Y., et al. (2007c). Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J. Med. Microbiol. 56(Pt. 10), 1413–1415.
Nakano, K., Tsuji, M., Nishimura, K., Nomura, R., and Ooshima, T. (2006b). Contribution of cell surface protein antigen PAc of Streptococcus mutans to bacteremia. Microbes Infect. 8, 114–121.
Narita, I., and Gejyo, F. (2008). Pathogenetic significance of aberrant glycosylation of IgA1 in IgA nephropathy. Clin. Exp. Nephrol. 12, 332–338.
Nemoto, H., Nakano, K., Nomura, R., and Ooshima, T. (2008). Molecular characterization of Streptococcus mutans strains isolated from the heart valve of an infective endocarditis patient. J. Med. Microbiol. 57(Pt. 7), 891–895.
Nomura, R., Matayoshi, S., Otsugu, M., Kitamura, T., Teramoto, N., and Nakano, K. (2020a). Contribution of severe dental caries induced by Streptococcus mutans to the pathogenicity of infective endocarditis. Infect. Immun. 88:e00897–19. doi: 10.1128/IAI.00897-19
Nomura, R., Naka, S., Nemoto, H., Otsugu, M., Nakamura, S., Ooshima, T., et al. (2013). Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch. Oral Biol. 58, 1627–1634. doi: 10.1016/j.archoralbio.2013.06.008
Nomura, R., Nakano, K., and Ooshima, T. (2004). Contribution of glucan-binding protein C of Streptococcus mutans to bacteremia occurrence. Arch. Oral Biol. 49, 783–788. doi: 10.1016/j.archoralbio.2004.04.001
Nomura, R., Nakano, K., Naka, S., Nemoto, H., Masuda, K., Lapirattanakul, J., et al. (2012). Identification and characterization of a collagen-binding protein, Cbm, in Streptococcus mutans. Mol. Oral Microbiol. 27, 308–323. doi: 10.1111/j.2041-1014.2012.00649.x
Nomura, R., Nakano, K., Nemoto, H., Fujita, K., Inagaki, S., Takahashi, T., et al. (2006). Isolation and characterization of Streptococcus mutans in heart valve and dental plaque specimens from a patient with infective endocarditis. J. Med. Microbiol. 55(Pt. 8), 1135–1140. doi: 10.1099/jmm.0.46609-0
Nomura, R., Nakano, K., Taniguchi, N., Lapirattanakul, J., Nemoto, H., Grönroos, L., et al. (2009). Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J. Med. Microbiol. 58(Pt. 4), 469–475.
Nomura, R., Otsugu, M., Hamada, M., Matayoshi, S., Teramoto, N., Iwashita, N., et al. (2020b). Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci. Rep. 10:19118. doi: 10.1038/s41598-020-75933-6
Nomura, R., Otsugu, M., Naka, S., Teramoto, N., Kojima, A., Muranaka, Y., et al. (2014). Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis. Infect. Immun. 82, 5223–5234. doi: 10.1128/IAI.02164-14
Oho, T., and Nagata, E. (2019). DMBT1 involvement in the human aortic endothelial cell response to Streptococcus mutans. Mol. Oral Microbiol. 34, 108–117. doi: 10.1111/omi.12257
Oliveira, F. A. F., Forte, C. P. F., Silva, P. G. B., Lopes, C. B., Montenegro, R. C., Santos, ÂK., et al. (2015). Molecular analysis of oral bacteria in heart valve of patients with cardiovascular disease by real-time polymerase chain reaction. Medicine 94:e2067.
Paik, S., Brown, A., Munro, C. L., Cornelissen, C. N., and Kitten, T. (2003). The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J. Bacteriol. 185, 5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003
Patel, J. C., and Galán, J. E. (2006). Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 175, 453–463. doi: 10.1083/jcb.200605144
Rawczynska-Englert, I., Hryniewiecki, T., and Dzierzanowska, D. (2000). Evaluation of serum cytokine concentrations in patients with infective endocarditis. J. Heart Valve Dis. 9, 705–709.
Rodrigues, J. C., Haas, M., and Reich, H. N. (2017). IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12, 677–686.
Roppenser, B., Röder, A., Hentschke, M., Ruckdeschel, K., and Aepfelbacher, M. (2009). Yersinia enterocolitica differentially modulates RhoG activity in host cells. J. Cell Sci. 122(Pt. 5), 696–705. doi: 10.1242/jcs.040345
Sato, Y., Okamoto, K., Kagami, A., Yamamoto, Y., Igarashi, T., and Kizaki, H. (2004). Streptococcus mutans strains harboring collagen-binding adhesin. J. Dent. Res. 83, 534–539.
Schroder, K., and Tschopp, J. (2010). The inflammasomes. Cell 140, 821–832. doi: 10.3390/jfb15020032
Shun, C. T., Lu, S. Y., Yeh, C. Y., Chiang, C. P., Chia, J. S., and Chen, J. Y. (2005). Glucosyltransferases of viridans streptococci are modulins of interleukin-6 induction in infective endocarditis. Infect. Immun. 73, 3261–3270.
Sonbol, H., Spratt, D., Roberts, G. J., and Lucas, V. S. (2009). Prevalence, intensity and identity of bacteraemia following conservative dental procedures in children. Oral Microbiol. Immunol. 24, 177–182. doi: 10.1111/j.1399-302X.2008.00492.x
Tomana, M., Novak, J., Julian, B. A., Matousovic, K., Konecny, K., and Mestecky, J. (1999). Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Invest. 104, 73–81. doi: 10.1172/JCI5535
Tonomura, S., Ihara, M., Kawano, T., Tanaka, T., Okuno, Y., Saito, S., et al. (2016). Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci. Rep. 6:20074.
Tsai, M. S., Chen, Y. Y., Chen, W. C., and Chen, M. F. (2022). Streptococcus mutans promotes tumor progression in oral squamous cell carcinoma. J. Cancer 13, 3358–3367.
Tsuda, H., Yamashita, Y., Toyoshima, K., Yamaguchi, N., Oho, T., Nakano, Y., et al. (2000). Role of serotype-specific polysaccharide in the resistance of Streptococcus mutans to phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 68, 644–650. doi: 10.1128/IAI.68.2.644-650.2000
Vasovic, M., Gajovic, N., Brajkovic, D., Jovanovic, M., Zdravkovaic, N., and Kanjevac, T. (2016). The relationship between the immune system and oral manifestations of inflammatory bowel disease: a review. Central Eur. J. Immunol. 41, 302–310.
Yamashita, Y., Shibata, Y., Nakano, Y., Tsuda, H., Kido, N., Ohta, M., et al. (1999). A novel gene required for rhamnose-glucose polysaccharide synthesis in Streptococcus mutans. J. Bacteriol. 181, 6556–6559.
Yu, H., Pardoll, D., and Jove, R. (2009). STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809.
Yu, L., Maishi, N., Akahori, E., Hasebe, A., Takeda, R., Matsuda, A. Y., et al. (2022). The oral bacterium Streptococcus mutans promotes tumor metastasis by inducing vascular inflammation. Cancer Sci. 113, 3980–3994.
Keywords: dental caries, Streptococcus mutans, pathogenic factors, proinflammatory mechanisms, systemic diseases
Citation: Fang Y, Chen X, Chu CH, Yu OY, He J and Li M (2024) Roles of Streptococcus mutans in human health: beyond dental caries. Front. Microbiol. 15:1503657. doi: 10.3389/fmicb.2024.1503657
Received: 29 September 2024; Accepted: 09 December 2024;
Published: 19 December 2024.
Edited by:
Xiaojing Huang, Fujian Medical University, ChinaReviewed by:
Shujie Cheng, China Pharmaceutical University, ChinaYaling Jiang, Academic Centre for Dentistry Amsterdam, Netherlands
Zhu Chen, Affiliated Stomatological Hospital of Zunyi Medical University, China
Copyright © 2024 Fang, Chen, Chu, Yu, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyun Li, bGltaW5neXVuQHNjdS5lZHUuY24=; Jinzhi He, aGVqaW56aGlAc2N1LmVkdS5jbg==
 Yanke Fang
Yanke Fang Xin Chen1
Xin Chen1 Chun Hung Chu
Chun Hung Chu Ollie Yiru Yu
Ollie Yiru Yu Jinzhi He
Jinzhi He Mingyun Li
Mingyun Li