
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 20 December 2024
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1499487
Background: Pharmaceuticals are expected to improve human and animal health, but improper management and regulation have led to adverse effects such as reproductive disorders, antibiotic resistance, and biodiversity loss in ecosystems. Their presence in the environment poses significant risks, including a reduction in biodiversity, reproductive issues, and the development of antimicrobial resistance. This review aims to examine the occurrence and sources of pharmaceuticals in the environment and their ecotoxicological and regulatory aspects, with a focus on Ethiopia.
Methods: A narrative review of relevant studies conducted in Ethiopia was undertaken. The review included findings on the occurrence, sources, contributing factors, ecotoxicological impacts, and regulatory concerns related to pharmaceutical residues in the environment. Literature was sourced from Google Scholar, Scopus, PubMed, and institutional repositories.
Result: The findings revealed the detection of pharmaceutical residues in wastewater treatment facilities, aquatic environments (e.g., lakes and rivers), and commercially available animal products. Aquatic samples also showed significant concentrations, with sulfamethoxazole and fluconazole detected at 0.15 μg/L and 0.012 μg/L, respectively. Antimicrobial resistance genes were identified in wastewater and treatment plant samples, which correlate with the presence of pharmaceutical residues. An ecological risk assessment based on the risk quotient (RQ) revealed ciprofloxacin as a major concern, with an RQ of 8.58, indicating high ecological risk. Sulfonamides exhibited moderate risk, with RQ values ranging from 0.1 to 1.
Conclusion: The study highlights the significant presence of pharmaceutical residues in the environment and underscores the inadequacy of regulatory enforcement in addressing this public health issue. Urgent measures are required to prevent environmental contamination and mitigate public health risks, including antimicrobial resistance. Strengthened regulatory measures and proactive interventions by relevant organizations are essential to control and prevent pharmaceutical residues in the environment, offering a critical solution for the country.
Pharmaceuticals for humans and animals play a fundamental role in retarding disease development and related complications, aggregating healthy life, and lowering mortality rates. The increasing and aging global population leads to a rise in the use and production of pharmaceuticals. Given that pharmaceuticals are intended to impact living organisms, it is well known that the lack of proper management of them can negatively affect wildlife and ecosystems (O’Flynn et al., 2021). In addition, their intensified environmental exposure puts pressure on biodiversity, leads to antimicrobial resistance, and risks our long-term health (Ortúzar et al., 2022). According to the study by Lancet (2000–2018), antibiotic consumption among humans increased significantly by 46% (Browne et al., 2021). Comparably, up to 90% of active ingredients in oral dosage formulations were excreted in the urine unchanged, which may be released directly into the environment.
In addition, in husbandry, pharmaceuticals can enter the environment easily, especially when used as a fertilizer. Such entry undergoes degradation, movement, and accumulation, which can result in long-term gloomy environmental consequences. Soil contamination and biomagnification were among the negative impacts, as pharmaceuticals can be absorbed by food crops (Gros et al., 2019). A separate route of the pharmaceutical entrance into the ecosystem is the production of active pharmaceutical ingredients and their formulations. The industrial manufacturing effluents generated while cleaning pharmaceutical ingredients and manufacturing equipment are likely the primary pathways for their release into the environment (Moermond et al., 2022).
Literature indicates that pharmaceuticals are widely present in aquatic ecosystems, where their physicochemical properties and bioactivity can result in ecotoxicological effects, even at low-to-moderate concentrations (Nguyen et al., 2024). The increasing use and subsequent release of these substances into watersheds have emerged as a global concern due to their persistence in the environment and potential adverse impacts on humans, animals, and ecosystems. Factors such as environmental conditions and chemical structure significantly influence their degradation, making many pharmaceuticals non-biodegradable (Moghaddam et al., 2023). Their persistence exacerbates issues such as antibiotic resistance, posing risks to biodiversity and human health (Cherian et al., 2023).
To address this issue, pharmaceutical facilities have adopted various problem-solving methods and technologies. Primarily, these methods focus on eliminating drug residues before pharmaceutical effluents or sludge solids are discharged into water bodies, treatment plants, and, ultimately, the surrounding environment (Samal et al., 2022). Recent advancements in environmental protection have been increasingly embraced by the biotech industry, with a particular focus on the sustainability of drug discovery. This shift represents a critical step toward developing more innovative and effective solutions to mitigate the environmental impact of pharmaceuticals (Wynendaele et al., 2021).
Globally, the occurrence of pharmaceuticals in the surrounding varies by country, which depends on their consumption and monitoring. Contamination due to the reluctant use of pharmaceuticals, poor sanitation, and inadequate wastewater treatment facilities was prevalent in Africa, which resulted in easy occurrence (Waleng and Nomngongo, 2022). Similarly, a portion of medicines can be excreted and enter the aquatic environment easily after municipal treatment. In Ethiopia, such a problem is exacerbated by the lack of waste treatment facilities in the pharmaceutical industries, as only one of them uses a septic tank with limited retention capacity (Wongiel et al., 2018). A recent review puts Ethiopia as the highest rated in Africa for contaminated samples in pharmaceutical residues, followed by Tunisia (Wilkinson et al., 2022). The substantial veterinary residue occurrence in the country can be attributed to the absence of detection facilities and standards for the maximum residual limits for drugs in food (Ame et al., 2022). This review study aimed to bring forward an overarching baseline data assessment on the occurrence, distribution, and effect of pharmaceuticals in the Ethiopian environment, including lakes and seas, by focusing on their sources and finding the contributions to their occurrence. It also provides a record of various pharmaceutical compounds with their concentrations and discusses the ecological and regulatory aspects related to these compounds to better understand the issue of long-term, low-level exposure to pharmaceutical pollution on ecosystems found in the environment.
The review was conducted from May to July 2024 using various online platforms to record relevant information on pharmaceutical residues, their occurrence, ecotoxicological status, and regulatory efforts across the Ethiopian region. Databases such as Google Scholar, Scopus, and PubMed were utilized to retrieve comprehensive scientific information published in English, specifically related to studies conducted in Ethiopia. Institutional repositories were also visited to avoid missing scholarly studies that might not get published in a peer-reviewed journal. Additionally, findings from other regions were included to provide comparative insights into regulatory measures and environmental protection agency activities addressing the release of pharmaceutical residues into the environment.
The literature search employed the following keywords: “Pharmaceutical residue OR Antimicrobial, Environmental health,” “Drug-contaminated wastewater,” “Medicinal wastewater,” “Pharmaceutical wastewater,” “Water safety,” “Contaminants,” “Regulatory agency AND Environmental protection,” “Ecotoxicology OR Aquatic Environmental pollution,” “Wastewater treatment,” “Contaminant removal,” and “Ecotoxicity.”
To ensure the inclusion of relevant studies, specific criteria were established. Studies focusing on the toxicity of pharmaceutical wastewater, its public health impact, and related environmental concerns were included in the review. Boolean operators were employed to refine the search results. The “AND” operator was used to narrow the search by requiring all specified terms to appear in the results, while “OR” broadened the search to include any of the specified terms, such as antimicrobial residue, anti-inflammatory residue, antibiotic residue, or analgesic. The “NOT” operator excluded results containing irrelevant terms. Data extraction and quality assessment were conducted independently by four reviewers (AT, AA, GH, and SS) to ensure methodological validity. Any disagreements were resolved through discussions with AT.
Recent and previous studies have assessed the environmental occurrence of pharmaceuticals in various aquatic bodies, including lakes, rivers (Daniel et al., 2024; Hailu et al., 2024; Gezahegn et al., 2019; Abate, 2022), groundwater, wastewater, and in dairy and poultry products (Bedada et al., 2012; Agmas and Adugna, 2018; Mohammed et al., 2022; Uma and Ashenef, 2023; Abdeta et al., 2024).
A total of 13 papers were reviewed, and over 37 distinct active pharmaceutical ingredients were detected in different samples. Studies were conducted in both target and non-targeted screening of pharmaceutical occurrences. They predominantly focused on antibiotics, especially tetracyclines, ciprofloxacin, and fluoroquinolones. Other categories also include antifungals, antiparasitics, antivirals, analgesics, antipsychotics, and antiepileptics. Notably, oxytetracycline emerged as the most frequently detected active pharmaceutical ingredient, appearing in 8 of 13 included studies, followed by ciprofloxacin, sulfamethoxazole, and carbamazepine. A summary report for the occurrence of pharmaceuticals, their concentration, and the analytical methods used is presented in Table 1.
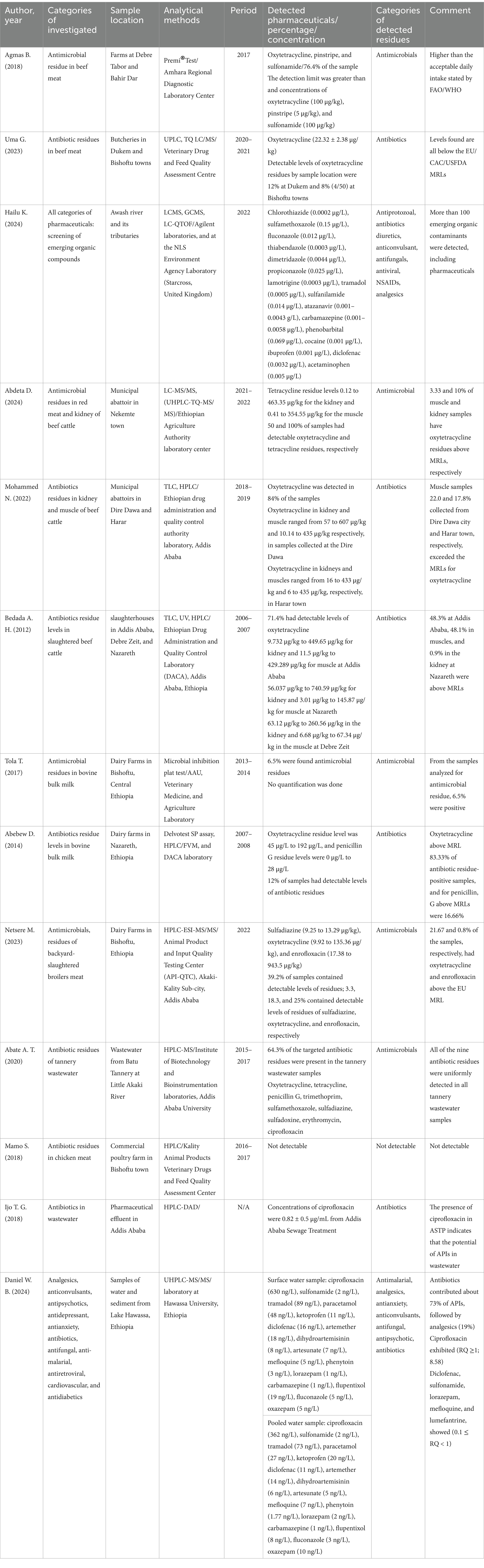
Table 1. Summary of the pharmaceutical residues reported in the environment with respective concentrations, analytical methods, and remark on their detected levels.
Antimicrobial occurrence in aquatic environments is mainly due to the waste generated from municipalities, hospitals, pharmaceutical industries, agriculture, and aquaculture. In addition, antibiotics are well known to be excreted in urine and feces, either as active compounds or their metabolites (Kümmerer, 2009). In Ethiopia, wastewater treatment facilities and waste management systems lack the capacity to effectively remove antibiotic residues effectively (Adugna, 2023). Along this, antibiotic residues have been detected in various aquatic bodies, including rivers, lakes, groundwater, wastewater, and drinking water.
Antibiotics and antifungals (sulfamethoxazole, fluconazole, thiabendazole, dimetridazole, propiconazole, and sulfanilamide) were among the detected antimicrobials in the Awash River basin in Ethiopia (Hailu et al., 2024) (Table 1). In the aquatic samples, sulfamethoxazole is the most abundant antimicrobial detected, with a concentration of 0.15 μg/L, followed by fluconazole at 0.012 μg/L, thiabendazole at 0.0003 μg/L, dimetridazole at 0.0044 μg/L, propiconazole at 0.025 μg/L, and sulfanilamide at 0.014 ng/L (Hailu et al., 2024). It has been shown that the main reason for the increased occurrence level is the disposal of waste from the municipal and industrial sources in Addis Ababa, which is greater than the downstream areas. In Ethiopia, a remarkable portion of tannery wastewater is released directly into the water bodies with minimal or no treatment. On account of this, antibiotic residues of tetracycline, oxytetracycline, erythromycin, penicillin G, trimethoprim, sulfadiazine, sulfadoxine, sulfamethoxazole, and ciprofloxacin have been found in all samples of tannery wastewater from Batu Tannery (Abate, 2022).
A distinct study showed the residence of ciprofloxacin at a concentration of 0.82 ± 0.5 μg/mL from Addis Ababa Sewage Treatment. This might be due to the insufficient treatment facilities for wastewater disposal in Ethiopia, and the study noted that other pharmaceutical pollutants might be found in other wastewater systems (Gezahegn et al., 2019). Additionally, water and sediment samples from Lake Hawassa, Ethiopia, contained ciprofloxacin (630 ng/L) and sulfonamide (2 ng/L), along with other antibiotics and antifungal agents. Ciprofloxacin’s presence at high levels is linked to its heavy use in Ethiopia, which is estimated at 700 tons each year (Daniel et al., 2024).
The studies conducted to assess the presence of antimicrobials in milk and meat also highlighted their use in food-processing animals. Such veterinary pharmaceuticals were commonly used to prevent infections in livestock management. The most detected antibiotics were oxytetracycline, tetracycline, pinstripe, sulfonamide, penicillin G, sulfadiazine, and enrofloxacin from the samples collected at various regions of Ethiopia (Table 1). Oxytetracycline residue concentration was the highest at 607 μg/kg, next to enrofloxacin at 943.5 μg/kg (Mohammed et al., 2022; Netsere, 2023). Interview findings from farmers indicated that the most used pharmaceuticals in the study area were oxytetracycline, sulfonamides, pen-strep, penicillin, procaine penicillin, ivermectin, albendazole, tinidazole, multi-vitamins, and acaricides (Table 2).
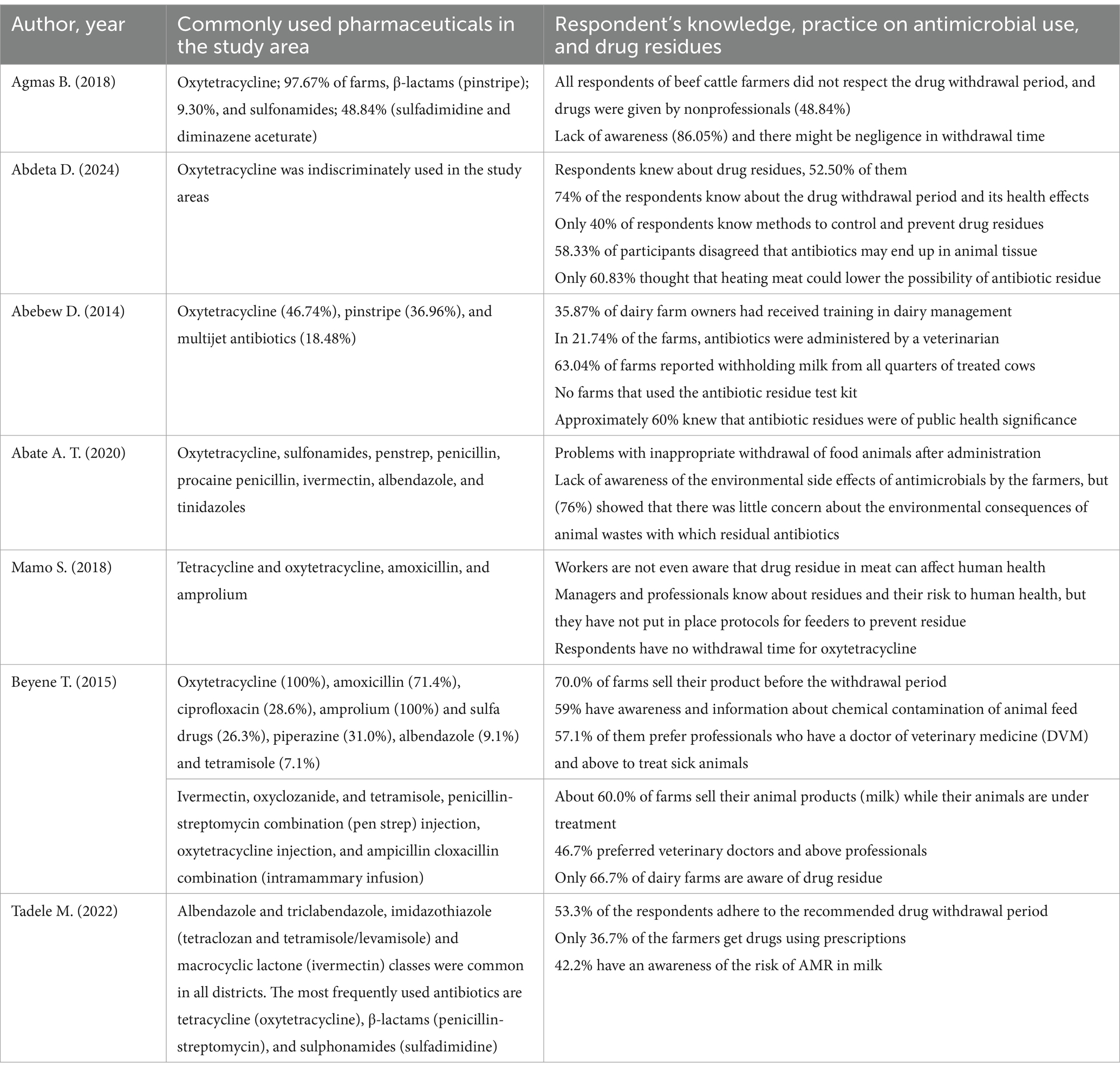
Table 2. Commonly used pharmaceuticals and respondent’s knowledge, practice on antimicrobial use, and drug residues.
Findings indicated that roughly 3.33% of muscle samples and 10% of kidney samples from Nekemte town accommodated oxytetracycline residues above the maximum residual limits. The muscle samples collected from Dire Dawa city showed 22.0%, while those from Harar had 17.8%. In Addis Ababa, 48.3% of muscle samples exceeded the limits; in Nazareth, the figure was 48.1%. Additionally, 83.33% of milk samples from Nazareth and 21.67% of broiler meat in Bishoftu had residues above acceptable levels, which could pose health risks to public health (Table 1).
The studies reveal the presence of analgesics and anti-inflammatory drugs in samples from rivers, wastewater, and industrial effluents. It has been widely acknowledged that non-steroidal anti-inflammatory drugs, even at concentrations below μg/L, are considered hazardous emerging pollutants that can ultimately impact aquatic environments (Tyumina et al., 2020). Analgesic categories of pharmaceuticals detected in the current literature include tramadol, ibuprofen, diclofenac, acetaminophen, and ketoprofen (Daniel et al., 2024; Hailu et al., 2024). Furthermore, atazanavir was identified at concentrations between 0.001 and 0.0043 g/L.
Samples of water and sediment from Lake Hawassa, Ethiopia, showed that various pharmaceutical residues, including anticonvulsants, antipsychotics, antidepressants, antianxiety medications, antimalarials, antiretrovirals, cardiovascular drugs, and antidiabetics, were detected. From the surface water sample, detected residues include artemether (18 ng/L), dihydroartemisinin (8 ng/L), artesunate (7 ng/L), mefloquine (5 ng/L), phenytoin (3 ng/L), lorazepam (1 ng/L), carbamazepine (1 ng/L), flupentixol (19 ng/L), and oxazepam (5 ng/L) (Daniel et al., 2024).
Pharmaceutical residue occurrence in the environment comes at various stages of their product lifecycle, from production and consumption to disposal (O’Flynn et al., 2021). Their sources include wastewater from industries, hospitals, and households, improper disposal of medications, and livestock treatment emissions. Undoubtedly, patients’ utilization of prescription and over-the-counter medications has been taken as a notable source, as they are excreted and easily enter waterways after municipal treatment (Kümmerer, 2009). It has been known that human-consumed medicines reach the environment through direct discharges from municipal wastewater treatment plants (Aydin et al., 2019). In addition, pharmaceuticals also find their way into the environment through agricultural and veterinary practices. In Ethiopia, there is minimal oversight from government authorities, and there is a lack of information regarding rational drug use in veterinary practices. Moreover, animals slaughtered in the country are not tested for drug residues in any slaughterhouses, and the waste generated is not regulated and treated for the benefit of the environment (lack of a greenery approach). Consequently, pharmaceuticals can directly enter the ecosystem during livestock disease management practices by farmers (Wongiel et al., 2018).
Household use of pharmaceuticals can be a cause for their entrance into the environment as a result of home consumption. Evidence shows that in low-income countries, feces and urine with excreted pharmaceuticals often end up in pit latrines, septic tanks, or the environment due to open defecation (Gwenzi et al., 2023). Previous studies showed that household consumption of prescribed and non-prescribed pharmaceuticals is considered the primary source of the environment (Kümmerer, 2009). It is also reported in Ethiopia; currently, beef fattening farms use drugs irrationally in frequent and overdose ways that result in a high burden of veterinary residues. In addition to excreted drugs, a household’s improper disposal of pharmaceuticals can raise drug levels. This happens when people throw away expired or unused medications down sinks, toilets, or with regular trash that goes to landfills. How much these drugs enter the environment depends on how patients dispose of them and how well prescriptions are managed to reduce leftovers (Rogowska and Zimmermann, 2022). In developing countries, including Ethiopia, liquid pharmaceuticals are often flushed down toilets and sewer systems. Among others, in Ethiopia, the community’s knowledge of proper disposal of unused medications was found to be 42.6% (Tegegne et al., 2024). Unused medicines disposed of in garbage bins (63%) are the most common practice in Ethiopia (Kahsay et al., 2020), and the lack of a drug take-back system may exacerbate pharmaceutical pollution in the environment.
Pharmaceuticals were also found in health facilities, as they are known for dispensing and using a wide range of therapeutics, which makes them a source of drugs that enter municipal waterways. It was believed that health facilities have lower pharmaceutical levels than household and industrial waste, and the predominance of rural hospitals in Ethiopia has made the problem more concerning. This is because rural-based hospitals often have smaller, less advanced wastewater treatment plants (WWTPs), which are likely less efficient in retaining pharmaceutical residues (Manderso, 2018). It has been reported in Ethiopia that 68% of respondents from health facilities agreed that current pharmaceutical waste management practices are unsatisfactory and have an impact on the environment. Specifically, 16.3% of pharmaceutical waste was disposed of in open landfills, 63.7% was burned openly, and 20% was treated through incineration (Mohammed et al., 2021). In Ethiopia, there are water bodies in the vicinity of healthcare facilities that can cause easy entrance of the facilities’ effluent. To illustrate, a freshwater lake in Ethiopia, Lake Hawassa, is situated near the Hawassa University Comprehensive Specialized Hospital on its eastern shore. Consequently, this lake receives both wastewater and healthcare waste, which may include active pharmaceutical ingredients (Daniel et al., 2024).
Pharmaceutical production facilities raise significant concerns due to their extremely high discharge concentrations, which contribute to point source pollution (Larsson et al., 2007). Countries such as China, Korea, and India have found that treated wastewater from pharmaceutical industries contains high levels of hygromycin, lincomycin, and fluoroquinolone antibiotics, measured in milligrams per liter (Milaković et al., 2019). Significant water is required during pharmaceutical production, which results in the generation of a substantial amount of wastewater. Additionally, untreated wastewater released into the environment poses a serious risk to ecosystems and human health. This issue is prominent in developing countries with no effective treatment facilities for industrial effluents (Nidheesh et al., 2022). A 2018 survey indicated that the pharmaceutical manufacturing industry in Ethiopia does not utilize high-temperature incineration or waste immobilization methods. As a result, the water used for cleaning during production contains dissolved or suspended medications that are released into the effluent without any prior treatment. Furthermore, nearby vegetable farms that source their water from industrial effluents may expose residents to small quantities of pharmaceuticals by consuming raw vegetables grown in that area (Wongiel et al., 2018).
Veterinary pharmaceuticals enter aquatic ecosystems directly from agriculture and aquaculture activities. Farmers and veterinarians in low-income countries experiencing food insecurity often struggle to obtain effective antibiotics for treating their livestock. However, disruptions in access to these pharmaceuticals lead to environmental contamination, as many individuals lack training and do not adhere to the proper withholding periods after treatment (Grace, 2015). The problem of antibiotic residues in meat is significant and not adequately managed in low- and middle-income countries, including Ethiopia. In Ethiopia, food animals intended for domestic and export markets are not tested for residue presence in any slaughterhouses.
Additionally, it has been reported that oxytetracycline is used indiscriminately in various regions of the country (Abdeta et al., 2024). It is known that aquaculture has been experiencing prominent and rapid growth among food-producing sectors and has become a strong and essential global industry. However, it has also been shown to potentially cause serious environmental harm (Lai et al., 2018). In Ethiopia, despite abundant freshwater and fish resources, favorable climatic conditions, dedicated labor, and high local demand for fish, aquaculture development lags behind other agricultural activities (Chimdo, 2022). The country currently exercises freshwater aquaculture, both in fishponds and fish pens or fish cages.
Inadequate wastewater treatment facilities that allow untreated industrial wastewater to be discharged and runoff from agricultural activities have been linked to the environmental occurrence of pharmaceutical residues. This problem is noteworthy when the aquatic bodies are often located near these industrial wastewater discharges, including those from pharmaceutical manufacturing. The aforementioned issue is particularly acute in Addis Ababa, Ethiopia. Over 90% of industries in Addis Ababa release their waste directly into nearby rivers without proper wastewater treatment (Yohannes and Elias, 2017). This significantly contributes to pharmaceutical contamination in the region and creates serious environmental and public health concerns. Moreover, only one pharmaceutical industry in Ethiopia has a waste treatment facility, despite the mandatory requirement for these industries to prevent the discharge of toxic materials into the sewer system (Wongiel et al., 2018). The study conducted in Hawassa revealed that the direct inflow of untreated wastewater significantly impacted the most polluted areas. Primarily, this wastewater source carries active pharmaceutical ingredients from Hawassa University Comprehensive Specialized Hospital, which currently operates a waste treatment system that is not functioning properly. This, in turn, exacerbates the pharmaceutical pollution issue in the surrounding environment (Daniel et al., 2024).
Pharmaceutical residue presence in animal products may result from the subtherapeutic, prophylactic, or therapeutic use of antibiotics that stems from a lack of awareness and inadequate educational outreach (Mohammed et al., 2022). This, in turn, comes from the misuse and overuse of these drugs, as well as a failure to adhere to withdrawal periods. An Ethiopian study showed that 25.8% of respondents were unaware of the withdrawal period and its potential public health impacts. Additionally, 60% of respondents lack knowledge about different methods to control and prevent drug residues in husbandry (Abdeta et al., 2024). The pharmacokinetics of a drug can be influenced by animals’ health conditions, which, in turn, leads to the accumulation of pharmaceuticals in the affected tissues or organs. During clinical mastitis, for instance, ketoprofen levels in milk rise due to the influx of serum components into the udder (Beyene, 2016).
Moreover, residues can occur due to the indiscriminate use of antibiotics for treating infectious diseases, such as clinical mastitis and viral infections in veterinary practices. Off-label drug use involves using medications for unapproved conditions or administering them at doses higher than recommended, both of which have also been associated with residue occurrence (Ture et al., 2019). Inadequate identification of treated cows is one reason dairy farmers fail to recognize them and withhold milk from the market until the appropriate withdrawal period. Treated animals should be identified, and antibiotic usage must be documented to prevent residues. However, only about 20% of dairy farms in Nazareth, Ethiopia, maintain records of antibiotic treatments. Consequently, according to the study, insufficient record-keeping and a lack of knowledge about withdrawal periods among producers were identified as significant contributors to drug residues in milk (Abebew et al., 2014).
Low-income countries often struggle with insufficient solid waste and wastewater management systems, limited environmental monitoring, and a lack of knowledge about global best practices for the use and disposal of hazardous wastes, such as organized take-back programs for unused and expired medications (Gwenzi et al., 2023). Among the pharmaceutical residues detected at high concentrations, 38% were in a sample collected from a site affected by untreated domestic storm-water runoff (Daniel et al., 2024). This issue may be due to the uncontrolled, open dumpsites practiced in developing nations, including Ethiopia. In addition, a study in Hawassa, Ethiopia, found that expired or unused medication disposal in household garbage bins is a common practice practiced by over two-thirds of the respondents from households (Woldamicael Bekele et al., 2023). This improper disposal of pharmaceuticals in open, non-engineered landfills exposes scavengers to risks and allows pharmaceuticals to leach into water sources.
Pharmaceuticals are active substances that can persist in the environment and are considered to be a threat to ecological balance. The ongoing use of these drugs, even at a small, sub-therapeutic level, can pose risks to public health. In turn, many non-target organisms with similar metabolic processes, receptors, or molecules to humans and animals may unintentionally be exposed to these active substances released into the environment (Isidori et al., 2005).
Pharmaceutical pollution poses a significant threat to both aquatic and terrestrial ecosystems. When improperly disposed of, pharmaceuticals can enter the environment through various pathways, such as wastewater treatment plants, landfills, and agricultural runoff. Once in the environment, these compounds can directly harm organisms, disrupt their endocrine systems, and contribute to the development of antibiotic resistance (Samreen et al., 2021). Additionally, pharmaceuticals can accumulate in the tissues of organisms, leading to biomagnification and posing a greater risk to higher-level predators. The long-term effects of pharmaceutical pollution can include ecosystem disruption, biodiversity loss, and potential health risks to humans. To mitigate these negative impacts, it is crucial to implement effective waste management practices, promote responsible pharmaceutical use, and develop innovative technologies for treating contaminated water and soil (Yohannes and Elias, 2017).
According to a study in Ethiopia, certain pharmaceutical residues were found in a concentration above the maximum residual limits, potentially leading to their accumulation in the aquatic system. Spanning from antibiotics to hormone medicines, it can have acute and chronic toxicity on aquatic life, leading to biodiversity alteration and misfunctioning of ecosystems (Oliveira et al., 2015). Various antibiotics have been detected in surface waters and groundwater in Ethiopia in recent years, including ciprofloxacin, oxytetracycline, tetracycline, erythromycin, penicillin G, trimethoprim, sulfamethoxazole, sulfadoxine, and sulfadiazine.
The ecological risk assessment was based on the risk quotient (RQ) value, calculated from the highest concentrations of detected pharmaceuticals in water and sediment samples from Lake Hawassa. It was ciprofloxacin, among the antibiotics, with an RQ of 8.58, indicating a prominent ecological risk to aquatic life, similar to algae. In addition, sulfonamide also showed a moderate ecological risk, with an RQ of 0.1 to 1 (Daniel et al., 2024). A prior study found that exposure to ciprofloxacin resulted in marked decreases in both the growth rate and cell density of microalgae compared to control groups (Diniz et al., 2021). This review identified oxytetracycline and sulfamethoxazole as detected antibiotics, shown in prior research, to impact the growth of M. aeruginosa and C. microsphaera (Zhou et al., 2021). The antibiotic oxytetracycline functions by disrupting aminoacyl-tRNA binding in ribosomal protein synthesis in bacteria (Chukwudi, 2016). It was also known that it is not effectively removed in wastewater treatment plants, but it is recognized for its fortuitous effect on non-target aquatic organisms (Kovalakova et al., 2020).
Sulfadiazine was another frequently detected antibiotic in the studies reviewed, and it was recognized for its low removal rate in conventional wastewater treatment processes. Recent research indicates that sulfadiazine alone inhibited the growth of microalgae, with the inhibitory effect increasing at higher concentrations (Li et al., 2022). The antiviral drug atazanavir was also detected. Due to their harmful effects on algae, Daphnia, and fish, antiviral drugs have been identified as some of the most dangerous and toxic pharmaceuticals (Zhou et al., 2015). The EC50 values for V. fischeri classify both ciprofloxacin and oxytetracycline as acute aquatic toxicity category 3. The analgesic drug ibuprofen was another detected residue in water bodies, identified primarily for its toxic potential in aquatic living organisms. Ibuprofen’s high lipophilicity and low biodegradability nurture its bioaccumulation in the environment, and its biological effects indicate that it is detrimental to various aquatic species (Oliveira et al., 2015).
A study conducted in Ethiopia revealed that mefloquine, lumefantrine, and lorazepam had an RQ greater than 0.1 in sediment, indicating a moderate risk to the aquatic ecosystem of Lake Hawassa. Lumefantrine is known to have variable effects on the growth of the aquatic plants studied. Low concentrations can induce oxidative stress and affect the growth of chlorophytes and aquatic macrophytes. Previous research demonstrated a decrease in the growth of R. subcapitata and L. minor following exposure to lumefantrine (Chia et al., 2021). Artemether was another antimalarial found to be detectable, which is the most commonly prescribed drug for the treatment of acute uncomplicated malaria. However, it is only slightly soluble in water and may not be easily degradable in the aquatic environment.
Antimicrobial pharmaceutical utilization in animals, even at low doses, is contemporaneous with the development of bacterial resistance and potential cross-resistance with human antibiotics. Thereupon, they can harm terrestrial ecosystems through nitrifying bacteria and inhibit the growth of crops through bioaccumulation. Additionally, antibiotics present in sewage treatment influents can disrupt bacterial treatment processes and lead to toxic effects in terrestrial ecosystems at various levels of the food chain. Furthermore, animals excrete it in their manure, which adversely impacts the survival and reproduction of organisms that break down manure, including dung beetles and entomopathogenic nematodes (Daghrir and Drogui, 2013).
Studies have shown that calculations of the risk quotient (RQ) for oxytetracycline and ciprofloxacin indicate medium to high adverse effects on bacteria and other soil organisms (Parente et al., 2018). In addition, erythromycin was detected in a study conducted in Ethiopia. A prior study indicated that erythromycin induced acute toxic effects on the photosynthesis of Selenastrum capricornutum at a concentration of 0.06 mg/L. It was noted that this antibiotic significantly disrupted key physiological processes, including primary photochemistry, electron transport, photophosphorylation, and carbon assimilation (Liu et al., 2011). A commonly prescribed anticonvulsant, carbamazepine, was also detected in the environment in this review. It is the most researched emerging pharmaceutical in terrestrial systems, primarily because of its low removal efficiency in wastewater treatment plants. It sometimes exhibits negative removal efficiency without seasonal concentration variations (Koba et al., 2016). The non-steroidal anti-inflammatory drug diclofenac has also been found in Ethiopia, as it is recognized for causing significant ecological harm in the sudden decline of vulture populations. Such episodes of decline in vulture populations led to both biological and social consequences for the affected region, as the absence of these scavenging birds allowed harmful bacteria and infections to spread (Ogada et al., 2012).
On average, each person in Addis Ababa consumes approximately 3 kg of chicken meat per year (Dessie et al., 2003; Mesele, 2023). The presence of pharmaceutical residues in animal products (like eggs, meat, and milk) intended for human use is a significant concern for public health. Therefore, foods of animal origin must be safe and nutritious for human consumption. The study revealed that oxytetracycline was found in the kidneys and muscles of slaughtered cattle sold in Nekemte town, with up to 10% of the samples exceeding the recommended maximum residue limits (Abdeta et al., 2024). In samples from Nazareth dairy farms in Ethiopia, 83.33% had oxytetracycline above the maximum residual limits, and 16.66% also exceeded penicillin G (Abebew et al., 2014). It was associated with health risks in humans from consuming chicken tissues containing oxytetracycline residues (Darwish et al., 2013). The possible menace includes the transmission of antibiotic-resistant bacteria that are known foodborne pathogens (such as Salmonella spp., Escherichia coli, and Campylobacter spp.), immunological effects, disruption of intestinal microflora, and inherent carcinogenicity (Lawal et al., 2015).
Slaughtered broiler meat at dairy farms in Bishoftu, Ethiopia, found that 0.8% of samples contained enrofloxacin levels exceeding the EU maximum residue limit (Netsere, 2023). It is known that enrofloxacin disrupts bacterial DNA gyrase and has been previously shown to cause teratogenic effects in rabbit and rat embryos (Guzmán et al., 2003). Comparably, 58.33% of participants in Nekemte town disagreed that antibiotics used in animals end up in their tissues. On the other hand, only 52.50% were aware of drug residues in slaughtered animals (Abdeta et al., 2024). This lack of awareness in Ethiopia could increase the population’s risk of antibiotic residue contamination.
Antimicrobial resistance (AMR) is a pressing global health crisis. It occurs when microorganisms, such as bacteria, viruses, fungi, and parasites, develop resistance to the drugs designed to kill or control them. This resistance can make infections more difficult to treat, leading to increased morbidity, mortality, and healthcare costs. Several factors contribute to AMR, including the overuse and misuse of antibiotics, poor infection prevention and control practices, agricultural use of antibiotics, and the spread of resistant bacteria through travel and trade (Beyene et al., 2023). Although antibiotic residues in food can lead to allergic reactions, teratogenicity, mutagenicity, and carcinogenicity, the most consequential concern is their augmentation of antibiotic resistance. Multi-drug-resistant strains of microbes have been considerably reported in soil and aquatic environments, predominantly due to the poultry and veterinary sectors’ pharmaceutical uses, which increases the prospect of bacteria surviving under antibiotic pressure (Samreen et al., 2021). To address AMR, it is essential to implement strategies that reduce the overuse and misuse of antibiotics, improve infection prevention and control measures, develop new antibiotics, and promote antimicrobial stewardship.
A study conducted at Bishoftu farms in Ethiopia assessed pharmaceutical residues and examined the antimicrobial resistance profiles of 20 Salmonella isolates and 27 E. coli O157:H7 isolates (Netsere, 2023). The results showed that at least one class of antibiotic was present in 19 (95%) of the Salmonella isolates and 26 (96.3%) of the E. coli O157:H7 isolates. Among these, 13 (65%) of the Salmonella isolates and 11 (40.7%) of the E. coli O157:H7 isolates were classified as multidrug-resistant. Additionally, 3 (15%) of the Salmonella isolates were prominently drug-resistant, and 1 (5%) was pan-drug-resistant. Antibiotic residues and resistance genes in wastewater from Batu Tannery, which is released into the Little Akaki River in Ethiopia, were also investigated (Abate, 2022). The specific genes tested for using PCR include tet (A), te t(O), tet (M), tet (Q), erm (B), Sul (I), Sul (II), Otr (A), and qnr (A). Accordingly, the results showed that all targeted genes were present in some wastewater samples except for tet (Q). Therefore, the current wastewater treatment method does not effectively remove antibiotics and resistance genes.
To protect the environment and public health from potentially harmful exposures, it is important to regularly monitor pharmaceutical residues in the environment. Regulatory actions should be initiated when elevated concentrations are detected. Several countries have already provided evidence of the feasibility of monitoring and addressing pharmaceutical residues at various levels. In Ethiopia, pharmaceutical waste production from public and private healthcare facilities, pharmaceutical companies, households, and home care settings has risen sharply. This waste management issue is a significant concern for regulatory authorities. Since 1990, the Ministry of Health has overseen the regulation of healthcare waste in the country (Hygiene and Environmental Health Directorate of the Federal Ministry of Health, 1990). However, much of this waste is frequently mishandled, often ending up in municipal landfills, incinerated at low temperatures, or burned in open areas. This could include expired, unused, and contaminated medications and vaccines, which are classified as pharmaceutical wastes. Waste management in the country is disorganized, unprofessional, and not aligned with established rules and regulations (Abebe et al., 2023).
The Medicines Waste Management and Disposal Directive, enacted by the Ethiopian Food and Drug Authority (EFDA) in August 2011, aims to safeguard public health and the environment from risks associated with improper management and disposal of medicines. However, findings indicate that government oversight of waste generation is limited, with only 43% of respondents from the pharmaceutical industry and import sector reporting that their waste is regulated (Wongiel et al., 2018). Additionally, there is confusion regarding responsibility for waste management, as some stakeholders believe that regulation falls under both the Ethiopian Environmental Protection Agency (EPA) and the EFDA.
Ethiopia’s Federal Environmental Protection Authority (EPA) regulates activities that harm the environment. Its 1997 policy emphasizes community education, public-private partnerships, and modern waste minimization and recycling practices. Moreover, the Hygiene and Environmental Health Directorate of the Federal Ministry of Health created a healthcare waste management manual in 2008, updated in 2021, which outlines methods for managing pharmaceutical waste in healthcare facilities (Hygiene and Environmental Health Directorate of the Federal Ministry of Health, 2021). Recommended practices include returning waste to suppliers, encapsulation, dilution, sewer discharge for small amounts, incineration, and sanitary landfills for non-hazardous waste. It also stresses minimizing antibiotics and pharmaceutical residues in wastewater. Despite federal guidelines, there are no specific rules for managing hazardous and healthcare waste at the micro level. This indicates that sub-city health offices responsible for waste management are not paying enough attention to healthcare waste and lack proper supervision. Additionally, there are challenges, such as the healthcare waste management policy not being reviewed and weak enforcement of laws in healthcare facilities. It also stresses minimizing antibiotics and pharmaceutical residues in wastewater in the manual. However, the agencies overseeing wastewater management, including various ministries and the Environmental Protection Authority (EPA), lack clarity about their roles (Abebe et al., 2023).
Despite Ethiopia having fewer industries, factories still discharge effluents into lakes and rivers, posing significant challenges in balancing industrial development with pollution control For instance, the establishment of the Kilinto Pharmaceutical Industrial Park, aimed at reducing reliance on imports, has underscored the need for comprehensive environmental assessments and effluent treatment systems (Ababa, 2017). The industrial park’s toxic waste of discarded medications and medical waste from the pharmaceutical manufacturing industries particularly impacts the heavily polluted Akaki River in Addis Ababa (Abate, 2022). Fortunately, this indicates that responsible institutions must enforce a strong regulatory framework to ensure compliance with environmental policies (Yohannes and Elias, 2017). This is crucial for fostering socially responsible and eco-friendly sustainable manufacturing industries, which are expected to develop through industrial parks.
In many African countries, including Ethiopia, there is a lack of clear regulations to control antibiotic contamination and residues in husbandry (Darwish et al., 2013). Ethiopia’s Federal Constitutional Decree No. 728-2011 mandates that only veterinarians are authorized to prescribe veterinary medicines; however, diseases are often inadequately treated, with only 36.7% of farmers obtaining prescriptions (Tadele et al., 2022). This highlights challenges in regulatory enforcement, resulting in the irrational use of drugs without proper diagnoses, which ultimately affects the environment. Furthermore, Ethiopia’s current food legislation lacks standards addressing antibiotic residues, despite the widespread use of antibiotics among livestock producers. The country has faced difficulties in establishing effective standards and best practices for safe food production, especially regarding antimicrobial withdrawal times and maximum residue limits. The National Codex Contact Point of Ethiopia (NCCP-Ethiopia) is tasked with adopting standards, such as Ethiopian Standards, and implementing technical regulations in specific areas. Moreover, an integrated national plan, the Integrated National Antimicrobial Resistance and Residue Surveillance Plan in Animal Health, Plant, Food Safety, and Environment Sectors of Ethiopia (2019–2023), aims to address antibiotic waste across various sectors (Food and Agricultural Organization, 2019). However, the lack of sustainable investment, unaccredited quality control tests, and inadequate monitoring of drug residues in animal-derived food are significant limitations to effective regulatory implementation (Beyene et al., 2023). Research has shown that areas with less stringent regulations, particularly in African countries where antibiotics can be purchased over the counter, experience higher levels of pharmaceutical residue in rivers (Wilkinson et al., 2022). This highlights how weak regulation of antibiotic dispensing contributes to improper human and veterinary use, allowing these substances to enter aquatic systems more easily. The Medicines Waste Management and Disposal Directive (MWMDD) seeks to minimize the environmental impact of medicines waste. To achieve this, countries such as Ethiopia and the majority of African countries must implement strategies to prevent and minimize waste generation, establish efficient collection systems, utilize innovative disposal technologies, and monitor and report compliance. Optimizing prescribing practices, educating patients, and improving drug formulations can further reduce medicine waste. Additionally, implementing clear labeling, secure transportation, and proper disposal methods can help prevent the illegal disposal of medicines waste. Regular monitoring and reporting are essential to ensure compliance with the directive and identify areas for improvement. By following these strategies, countries can effectively manage medicine waste and protect the environment.
Pharmaceuticals have been detected in various parts of Ethiopia, including wastewater, effluents from treatment plants, rivers, lakes, groundwater, and the food chain, including dairy and poultry animal products. The easy availability of many drugs without prescriptions and poor regulation of antibiotic use in the country has likely contributed to the increased presence of pharmaceutical residues in the environment. This issue is further exacerbated by the lack of efficient wastewater treatment facilities, inadequate regulatory frameworks, and insufficient political commitment to enforce environmental protection standards. Factors such as failure to adhere to recommended drug withdrawal periods, low awareness of drug residues, and the availability of prescription antibiotics over the counter among veterinary farmers have been cited as contributing to the environmental presence of pharmaceuticals in Ethiopia. Major water bodies have been contaminated by pharmaceutical effluents from manufacturing industries and healthcare facilities. If these residues remain unregulated, as is the current situation, their levels may increase, posing greater risks to the environment and public health. Given the findings of various studies regarding pharmaceutical residues and the associated regulatory challenges in Ethiopia, it is recommended to focus on addressing antibiotic residues, combating antimicrobial resistance, and mitigating the inappropriate use of antibiotics in animal production.
The government and associated veterinary medicine regulatory bodies must strengthen capacity and establish clear procedures and strategies to monitor and control the irrational use of antibiotics, as well as to manage drug residues in dairy and poultry farm products. There is an urgent need for responsible authorities to eliminate antibiotic misuse and enforce restrictions through an approved list of drugs regulated by government authorities. Safety guidelines must be provided to ensure that edible tissues and other animal products intended for human consumption are treated with drugs that are safe for humans. Regulators should make a strong effort to promote responsible use by emphasizing the importance of adhering to maximum residue limits and withdrawal periods. These measures are critical to ensuring food safety and protecting public health.
The Environmental Protection Authority, in collaboration with the national standard body, should prioritize the development and enforcement of guidelines and standards for the treatment and disposal of pharmaceutical manufacturing effluents. The national medicine regulatory authority (EFDA) must also focus on raising awareness about the proper disposal of household medicines and establishing a drug take-back system to prevent improper disposal practices. Moreover, there should be stringent control over wastewater effluents from healthcare facilities. Healthcare facilities must regularly monitor pharmaceutical residue concentrations and implement effective parameters to minimize their environmental impact. These measures are essential to safeguard the environment, protect water resources, and promote public health.
This document calls for the establishment of robust waste management systems, enhanced regulatory frameworks, and community education initiatives to ensure compliance with environmental policies. It also highlights the importance of collaboration among stakeholders to address the challenges of pharmaceutical waste management effectively.
AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. AA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. GH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ababa, A. (2017). Updated environmental and social impact assessment. Addis Ababa: The Federal Democratic Republic of Ethiopia Industrial Parks Development Corporation.
Abate, T. A. (2022). Profiles of physicochemical characteristics, antibiotic residues, antibiotic resistance genes and bacteria community structure of Batu Tannery wastewater released to little Akaki River. Addis Ababa: Addis Ababa University.
Abdeta, D., Tafesse, M., and Bacha, B. (2024). Detection of selected antimicrobial residues in red meat and kidney of beef cattle slaughtered at Nekemte municipal abattoir, Ethiopia. Vet. Med. Sci. 10:e1459. doi: 10.1002/vms3.1459
Abebe, Y., Gashaw, M., Kefale, A., and Brewer, T. (2023). Wastewater governance in the upstream catchment of the Awash Basin, Ethiopia: challenges and opportunities for better accountability. Water Reuse 13, 571–590. doi: 10.2166/wrd.2023.077
Abebew, D., Belihu, K., and Zewde, G. (2014). Detection and determination of oxytetracycline and penicillin G antibiotic residue levels in bovine bulk milk from Nazareth dairy farms, Ethiopia. Ethiop. Vet. J. 18, 1–15.
Adugna, D. (2023). Challenges of sanitation in developing counties-evidenced from a study of fourteen towns, Ethiopia. Heliyon 9:e12932. doi: 10.1016/j.heliyon.2023.e12932
Agmas, B., and Adugna, M. (2018). Antimicrobial residue occurrence and its public health risk of beef meat in Debre Tabor and Bahir Dar, Northwest Ethiopia. Vet. World 11:902. doi: 10.14202/vetworld.2018.902-908
Ame, N. Y., Ame, M. M., Mohammed, C., and Duguma, M. F. (2022). Review on drug residue in foods of animal origin and its public healthy importance and methods of detection in Ethiopia. Acta Entomol Zool 3, 82–93. doi: 10.33545/27080013.2022.v3.i2b.78
Aydin, S., Aydin, M. E., Ulvi, A., and Kilic, H. (2019). Antibiotics in hospital effluents: occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. Res. Int. 26, 544–558. doi: 10.1007/s11356-018-3563-0
Bedada, A. H., Zewde, B. M., and Zewde, B. (2012). Tetracycline residue levels in slaughtered beef cattle from three slaughterhouses in central Ethiopia. Glob. Vet. 8, 546–554.
Beyene, T. (2016). Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J. Vet. Sci. Technol. 7:1000285. doi: 10.4172/2157-7579.1000285
Beyene, A. M., Andualem, T., Dagnaw, G. G., Getahun, M., LeJeune, J., and Ferreira, J. P. (2023). Situational analysis of antimicrobial resistance, laboratory capacities, surveillance systems and containment activities in Ethiopia: a new and one health approach. One Health 16:100527. doi: 10.1016/j.onehlt.2023.100527
Browne, A. J., Chipeta, M. G., Haines-Woodhouse, G., Kumaran, E. P., Hamadani, B. H. K., Zaraa, S., et al. (2021). Global antibiotic consumption and usage in humans, 2000–2018: a spatial modelling study. Lancet Planet. Health 5, e893–e904. doi: 10.1016/S2542-5196(21)00280-1
Cherian, T., Ragavendran, C., Vijayan, S., Kurien, S., and Peijnenburg, W. (2023). A review on the fate, human health and environmental impacts, as well as regulation of antibiotics used in aquaculture. Environ. Adv. 13:100411. doi: 10.1016/j.envadv.2023.100411
Chia, M. A., Ameh, I., Agee, J. T., Otogo, R. A., Shaba, A. F., Bashir, H., et al. (2021). Effects of the antimalarial lumefantrine on Lemna minor, Raphidocelis subcapitata and Chlorella vulgaris. Environ. Toxicol. Pharmacol. 85:103635. doi: 10.1016/j.etap.2021.103635
Chimdo, A. (2022). Review on potential and challenges of aquaculture practice in Ethiopia. Appl. Water Sci. 12:218. doi: 10.1007/s13201-022-01740-1
Chukwudi, C. U. (2016). rRNA binding sites and the molecular mechanism of action of the tetracyclines. Antimicrob. Agents Chemother. 60, 4433–4441. doi: 10.1128/AAC.00594-16
Daghrir, R., and Drogui, P. (2013). Tetracycline antibiotics in the environment: a review. Environ. Chem. Lett. 11, 209–227. doi: 10.1007/s10311-013-0404-8
Daniel, W.-B., Fick, J., Tilahun, G., Dadebo, E., and Gebremariam, Z. (2024). Pharmaceutical pollution in an Ethiopian Rift Valley Lake Hawassa: occurrences and possible ecological risks. Environ. Chall. 15:100901. doi: 10.1016/j.envc.2024.100901
Darwish, W. S., Eldaly, E. A., El-Abbasy, M. T., Ikenaka, Y., Nakayama, S., and Ishizuka, M. (2013). Antibiotic residues in food: the African scenario. Jpn. J. Vet. Res. 61, S13–S22.
Dessie, T., Million, T., Yami, A., and Peters, K. (2003). Village chicken production systems in Ethiopia: 2. Use patterns and performance valuation and chicken products and socio-economic functions of chicken. Livest. Res. Rural Dev. 15, 93–102.
Diniz, V., Rath, G., Rath, S., Rodrigues-Silva, C., Guimarães, J. R., and Cunha, D. G. (2021). Long-term ecotoxicological effects of ciprofloxacin in combination with caffeine on the microalga Raphidocelis subcapitata. Toxicol. Rep. 8, 429–435. doi: 10.1016/j.toxrep.2021.02.020
Food and Agricultural Organization (2019). Integrated national antimicrobial resistance and residue surveillance plan in animal health, plant, food safety and environment sectors of Ethiopia, 2019 to 2023.
Gezahegn, T., Tegegne, B., Zewge, F., and Chandravanshi, B. S. (2019). Salting-out assisted liquid–liquid extraction for the determination of ciprofloxacin residues in water samples by high performance liquid chromatography-diode array detector. BMC Chem. 13:28. doi: 10.1186/s13065-019-0543-5
Grace, D. (2015). Review of evidence on antimicrobial resistance and animal agriculture in developing countries. Evidence on Demand, UK. doi: 10.12774/eod_cr.june2015.graced
Gros, M., Mas-Pla, J., Boy-Roura, M., Geli, I., Domingo, F., and Petrović, M. (2019). Veterinary pharmaceuticals and antibiotics in manure and slurry and their fate in amended agricultural soils: findings from an experimental field site (Baix Empordà, NE Catalonia). Sci. Total Environ. 654, 1337–1349. doi: 10.1016/j.scitotenv.2018.11.061
Guzmán, A., García, C., Marŕna, A.-P., Willoughby, C., and Demestre, I. (2003). Developmental toxicity studies of the quinolone antibacterial agent irloxacin in rats and rabbits. Arzneimittelforschung 53, 121–125. doi: 10.1055/s-0031-1297082
Gwenzi, W., Simbanegavi, T. T., and Rzymski, P. (2023). Household disposal of pharmaceuticals in low-income settings: practices, health hazards, and research needs. Water 15:476. doi: 10.3390/w15030476
Hailu, K., Kebede, S., Birhanu, B., and Lapworth, D. (2024). Tracing contaminants of emerging concern in the Awash River basin, Ethiopia. J. Hydrol. Reg. Stud. 54:101869. doi: 10.1016/j.ejrh.2024.101869
Hygiene and Environmental Health Directorate of the Federal Ministry of Health (1990). Healthcare waste handling and disposal guidance. Addis Ababa, Ethiopia: Hygiene and Environmental Health Directorate of the Federal Ministry of Health.
Hygiene and Environmental Health Directorate of the Federal Ministry of Health (2021). Health-care waste management manual. 2nd Edn. Addis Ababa, Ethiopia: Hygiene and Environmental Health Directorate of the Federal Ministry of Health.
Isidori, M., Lavorgna, M., Nardelli, A., Pascarella, L., and Parrella, A. (2005). Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 346, 87–98. doi: 10.1016/j.scitotenv.2004.11.017
Kahsay, H., Ahmedin, M., Kebede, B., Gebrezihar, K., Araya, H., and Tesfay, D. (2020). Assessment of knowledge, attitude, and disposal practice of unused and expired pharmaceuticals in community of Adigrat City, Northern Ethiopia. J. Environ. Public Health 2020:6725423. doi: 10.1155/2020/6725423
Koba, O., Golovko, O., Kodešová, R., Klement, A., and Grabic, R. (2016). Transformation of atenolol, metoprolol, and carbamazepine in soils: the identification, quantification, and stability of the transformation products and further implications for the environment. Environ. Pollut. 218, 574–585. doi: 10.1016/j.envpol.2016.07.041
Kovalakova, P., Cizmas, L., McDonald, T. J., Marsalek, B., Feng, M., and Sharma, V. K. (2020). Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere 251:126351. doi: 10.1016/j.chemosphere.2020.126351
Kümmerer, K. (2009). The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J. Environ. Manage. 90, 2354–2366. doi: 10.1016/j.jenvman.2009.01.023
Lai, W. W.-P., Lin, Y.-C., Wang, Y.-H., Guo, Y. L., and Lin, A. Y.-C. (2018). Occurrence of emerging contaminants in aquaculture waters: cross-contamination between aquaculture systems and surrounding water. Water Air Soil Pollut. 229:249. doi: 10.1007/s11270-018-3901-3
Larsson, D. J., de Pedro, C., and Paxeus, N. (2007). Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 148, 751–755. doi: 10.1016/j.jhazmat.2007.07.008
Lawal, J. R., Jajere,, Jajere, S. M., Geidam, Y. A., Bello, A. M., Wakil, Y., et al. (2015). Antibiotic residues in edible poultry tissues and products in Nigeria: a potential public health hazard. Int. J. Anim. Vet. Adv. 7, 55–61. doi: 10.19026/ijava.7.5241
Li, Z., Dong, S., Huang, F., Lin, L., Hu, Z., and Zheng, Y. (2022). Toxicological effects of microplastics and sulfadiazine on the microalgae Chlamydomonas reinhardtii. Front. Microbiol. 13:865768. doi: 10.3389/fmicb.2022.865768
Liu, B.-Y., Nie, X.-P., Liu, W.-Q., Snoeijs, P., Guan, C., and Tsui, M. T. (2011). Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 74, 1027–1035. doi: 10.1016/j.ecoenv.2011.01.022
Manderso, T. M. (2018). Overview of existing wastewater management system in case of Debre Markos Town, Ethiopia. Civ. Environ. Res. 2:107.
Mesele, T. L. (2023). Reproduction and production performance of improved chickens, their production constraints, and opportunities under Ethiopian conditions. Trop. Anim. Health Prod. 55:245. doi: 10.1007/s11250-023-03653-w
Milaković, M., Vestergaard, G., González-Plaza, J. J., Petrić, I., Šimatović, A., Senta, I., et al. (2019). Pollution from azithromycin-manufacturing promotes macrolide-resistance gene propagation and induces spatial and seasonal bacterial community shifts in receiving river sediments. Environ. Int. 123, 501–511. doi: 10.1016/j.envint.2018.12.050
Moermond, C. T., Puhlmann, N., Brown, A. R., Owen, S. F., Ryan, J., Snape, J., et al. (2022). GREENER pharmaceuticals for more sustainable healthcare. Environ. Sci. Technol. Lett. 9, 699–705. doi: 10.1021/acs.estlett.2c00446
Moghaddam, A., Khayatan, D., Barzegar, P. E. F., Ranjbar, R., Yazdanian, M., Tahmasebi, E., et al. (2023). Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: a comprehensive overview. Int. J. Environ. Sci. Technol. 20, 5659–5696. doi: 10.1007/s13762-023-04880-2
Mohammed, N., Adare Mengistu, D., Abdurehman, A., Belina, D., and Mengistu, S. (2022). Determination of tetracycline residues in kidney and muscle of beef cattle slaughtered in Dire Dawa and Harar municipal abattoirs, Eastern Ethiopia. Environ. Health Insights 16:11786302221109720. doi: 10.1177/11786302221109720
Mohammed, S. A., Kahissay, M. H., and Hailu, A. D. (2021). Pharmaceuticals wastage and pharmaceuticals waste management in public health facilities of Dessie town, North East Ethiopia. PLoS One 16:e0259160. doi: 10.1371/journal.pone.0259160
Netsere, M. (2023). Investigation of microbial load, selected bacterial pathogens, antimicrobial resistance profile and antibiotic residues of backyard-slaughtered broilers meat from selected farms in Bishoftu. Bishoftu, Ethiopia: Addis Ababa University.
Nguyen, M.-K., Lin, C., Bui, X.-T., Rakib, M. R. J., Nguyen, H.-L., Truong, Q.-M., et al. (2024). Occurrence and fate of pharmaceutical pollutants in wastewater: insights on ecotoxicity, health risk, and state-of-the-art removal. Chemosphere 354:141678. doi: 10.1016/j.chemosphere.2024.141678
Nidheesh, P. V., Ravindran, V., Gopinath, A., and Kumar, M. S. (2022). Emerging technologies for mixed industrial wastewater treatment in developing countries: an overview. Environ. Qual. Manag. 31, 121–141. doi: 10.1002/tqem.21762
O’Flynn, D., Lawler, J., Yusuf, A., Parle-McDermott, A., Harold, D., Mc Cloughlin, T., et al. (2021). A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 13, 575–594. doi: 10.1039/D0AY02098B
Ogada, D. L., Keesing, F., and Virani, M. Z. (2012). Dropping dead: causes and consequences of vulture population declines worldwide. Ann. N Y Acad. Sci. 1249, 57–71. doi: 10.1111/j.1749-6632.2011.06293.x
Oliveira, L. L., Antunes, S. C., Gonçalves, F., Rocha, O., and Nunes, B. (2015). Evaluation of ecotoxicological effects of drugs on Daphnia magna using different enzymatic biomarkers. Ecotoxicol. Environ. Saf. 119, 123–131. doi: 10.1016/j.ecoenv.2015.04.028
Ortúzar, M., Esterhuizen, M., Olicón-Hernández, D. R., González-López, J., and Aranda, E. (2022). Pharmaceutical pollution in aquatic environments: a concise review of environmental impacts and bioremediation systems. Front. Microbiol. 13:869332. doi: 10.3389/fmicb.2022.869332
Parente, E. T., Sierra, J., and Martí, E. (2018). Ecotoxicity and biodegradability of oxytetracycline and ciprofloxacin on terrestrial and aquatic media. Orbital: Electron. J. Chem., 262–271. doi: 10.17807/orbital.v10i4.1063
Rogowska, J., and Zimmermann, A. J. I. (2022). Household pharmaceutical waste disposal as a global problem—a review. Int. J. Environ. Res. Public Health 19:15798. doi: 10.3390/ijerph192315798
Samal, K., Mahapatra, S., and Ali, M. H. (2022). Pharmaceutical wastewater as emerging contaminants (EC): treatment technologies, impact on environment and human health. Energy Nexus 6:100076. doi: 10.1016/j.nexus.2022.100076
Samreen,, Ahmad, I., Malak, H. A., and Abulreesh, H. H. (2021). Environmental antimicrobial resistance and its drivers: a potential threat to public health. J. Glob. Antimicrob. Resist. 27, 101–111. doi: 10.1016/j.jgar.2021.08.001
Tadele, M., Urge, B., Siyoum, T., Kassa, T., Gutema, F., Abera, B., et al. (2022). Veterinary drug utilization behaviour of small-scale dairy farms in three districts of Oromia region. Sci. J. Public Health 10, 21–28. doi: 10.11648/j.sjph.20221001.13
Tegegne, A. A., Genet, G., Workie Limenh, L., Yohannes, L., Mohammed Seid, A., Alemayehu, T. T., et al. (2024). Public awareness, knowledge, and attitude regarding proper disposal of unused medicines and associated factors in Gondar city, northwest Ethiopia. Front. Public Health 12, –1372739. doi: 10.3389/fpubh.2024.1372739
Ture, M., Fentie, T., and Regassa, B. (2019). Veterinary drug residue: the risk, public health significance and its management. J. Dairy Vet. Sci. 13, 555–856. doi: 10.19080/JDVS.2019.13.555856
Tyumina, E., Bazhutin, G., Cartagena Gómez, A. D. P., and Ivshina, I. J. M. (2020). Nonsteroidal anti-inflammatory drugs as emerging contaminants. Microbiology 89, 148–163. doi: 10.1134/S0026261720020125
Uma, G., and Ashenef, A. (2023). Determination of some antibiotic residues (tetracycline, oxytetracycline and penicillin-G) in beef sold for public consumption at Dukem and Bishoftu (Debre Zeyit) towns, central Ethiopia by LC/MS/MS. Cogent Food Agric. 9:2242633. doi: 10.1080/23311932.2023.2242633
Waleng, N. J., and Nomngongo, P. N. (2022). Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environ. Chem. Ecotoxicol. 4, 50–66. doi: 10.1016/j.enceco.2021.11.002
Wilkinson, J. L., Boxall, A. B., Kolpin, D. W., Leung, K. M., Lai, R. W., Galbán-Malagón, C., et al. (2022). Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. U.S.A. 119:e2113947119. doi: 10.1073/pnas.2113947119
Woldamicael Bekele, D., Dadebo, E., Tilahun, G., and Gebremariam, Z. (2023). Awareness and disposal practices of medicines among the community in Hawassa City, Ethiopia. J. Toxicol. 2023:4603993. doi: 10.1155/2023/4603993
Wongiel, S., Kumie, A., and Ashenef, A. (2018). An assessment of pharmaceutical waste management by pharmaceutical industries and importers in and around Addis Ababa, Ethiopia. Ethiop. J. Environ. Stud. Manage. 11, 425–440.
Wynendaele, E., Furman, C., Wielgomas, B., Larsson, P., Hak, E., Block, T., et al. (2021). Sustainability in drug discovery. Med. Drug Discov. 12:100107. doi: 10.1016/j.medidd.2021.100107
Yohannes, H., and Elias, E. (2017). Contamination of rivers and water reservoirs in and around Addis Ababa City and actions to combat it. Environ. Pollut. Clim. Change 1:116. doi: 10.4172/2753-458X.1000116
Zhou, C., Chen, J., Xie, Q., Wei, X., Zhang, Y.-N., and Fu, Z. (2015). Photolysis of three antiviral drugs acyclovir, zidovudine, and lamivudine in surface freshwater and seawater. Chemosphere 138, 792–797. doi: 10.1016/j.chemosphere.2015.08.033
Keywords: pharmaceutical residues, ecosystem disruption, wastewater treatment, drug use, regulatory oversight, sustainable drug practices
Citation: Tegegne AA, Mekasha YT, Ayu AA, Hasen G and Suleman S (2024) A review on emerging pharmaceutical residues in Ethiopia: occurrence, ecotoxicological aspects, and regulatory concerns. Front. Microbiol. 15:1499487. doi: 10.3389/fmicb.2024.1499487
Received: 20 September 2024; Accepted: 06 December 2024;
Published: 20 December 2024.
Edited by:
Takashi Azuma, Osaka Medical College, JapanReviewed by:
Ilunga Kamika, University of South Africa, South AfricaCopyright © 2024 Tegegne, Mekasha, Ayu, Hasen and Suleman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Addisu Afrassa Tegegne, YWRkaXN1YWZyYXNzYTQ2NDhAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.