- 1Department of Crop Science and Horticulture, College of Agriculture, Sokoine University of Agriculture, Morogoro, Tanzania
- 2Department of Soil and Geological Sciences, College of Agriculture, Sokoine University of Agriculture, Morogoro, Tanzania
This systematic review examines the global agricultural relevance and practical environmental implications of arbuscular mycorrhizal fungi (AMF) within the phylum Glomeromycota. Following PRISMA guidelines, ensuring a comprehensive and unbiased literature review, a literature search was conducted, focusing on the functional roles of AMF in enhancing crop productivity, nutrient uptake, and soil health. Key findings reveal that AMF contribute significantly to sustainable agriculture by reducing the need for chemical fertilizers and increasing plant resilience to environmental stressors like drought, salinity, or pest resistance. The review highlights the importance of AMF in forming symbiotic relationships with plants, which enhance nutrient absorption and improve soil structure, showcasing long-term benefits such as reduced erosion or improved water retention. However, the current literature lacks in-depth exploration of the taxonomy and evolutionary aspects of AMF, as well as the specific functional roles they play in different agricultural contexts, e.g., understanding evolution could enhance strain selection for specific crops. This review identifies several urgent research gaps, including a need for a more refined understanding of AMF community dynamics under varying land management practices. For example, there are gaps in and a critical evaluation of advanced molecular techniques. Such techniques are essential for studying these interactions. Addressing these gaps will enhance the integration of AMF into sustainable agricultural systems and improve ecosystem management practices across different geographical regions. Future research should prioritize developing precise molecular imaging techniques and optimizing AMF applications for different crops and soil types to maximize their ecological and agricultural benefits. This could be practical through interdisciplinary collaboration (e.g., involving molecular biologists, agronomists, etc.). In conclusion, this review advances the practical application of AMF in agriculture and its contribution to biodiversity conservation in agroecosystems. Integrating these findings into policy frameworks could encourage sustainable farming practices, promote the adoption of AMF inoculants, and foster incentives for environmentally friendly land management strategies.
Systematic review registration: https://www.bmj.com/content/372/bmj.n71.
1 Introduction
The taxonomy of fungi, particularly those forming arbuscular mycorrhizae in the roots of terrestrial plants, has historically been challenging due to difficulties in isolating spores from soil and maintaining live cultures (Wilkes, 2021; Khaliq et al., 2022), this could be due to the complex soil environment, where spores occur in low abundance and are often entangled with other microorganisms, and because arbuscular mycorrhizal fungi are obligate symbionts, requiring a host plant to complete their life cycle. Early taxonomic efforts based largely on morphological characteristics, grouped fungi with similar structures into broad categories (e.g., spore size, hyphal structure). For instance, the Endogonaceae family, initially associated with vesicular-arbuscular mycorrhizae (VAM), was once thought to belong to the order Mucorales due to its production of non-septate hyphae and zygospores (Morton et al., 1990), a view later revised through molecular studies. However, subsequent studies revealed distinct molecular and morphological features, prompting reclassification (e.g., using gene sequencing) into separate orders and even phyla (Desirò et al., 2017; Yamamoto et al., 2015).
Fungi now classified within Mucoromycotina and Glomeromycota were once grouped under Zygomycota due to shared features like non-septate hyphae and zygospore formation. As molecular techniques, such as DNA sequencing, became more prevalent, these groups were found to have significant genetic differences, justifying their reclassification into separate phyla. Molecular markers, such as ribosomal RNA genes, which are essential in fungal taxonomy due to their conserved nature and variable regions that help distinguish species and trace evolutionary relationships have been pivotal in these taxonomic revisions, highlighting both the diversity and the evolutionary distinctions between these groups (Schüβler et al., 2001; Redecker et al., 2006).
Recent advancements in molecular biology have allowed for a more refined classification of fungi that form arbuscular mycorrhizae. For example, the development of high-throughput sequencing or genomic analysis has revealed genetic signatures unique to AMF, leading to the establishment of the orders Archaeosporales, Diversisporales, Glomerales, and Paraglomerales within the phylum Glomeromycota, each with distinct ecological roles, such as specialization in different habitats or adaptation to varied environmental conditions (Schwarzott et al., 2001; Spatafora et al., 2016; Stürmer and Kemmelmeier, 2020). This molecular approach has provided deeper insights into the evolutionary history of these fungi and clarified their relationships with other fungal groups, enhancing our understanding of their functional roles in ecosystems (e.g., phosphorus uptake, soil aggregation).
Understanding the taxonomy and evolutionary history of AMF is not merely an academic exercise; it has significant practical implications for agriculture and ecosystem management (e.g., improving phosphorus uptake in crops). AMF play a crucial role in plant nutrient uptake, particularly phosphorus, which is often a limiting factor in soil fertility by stabilizing soil aggregates. By forming symbiotic relationships with plant roots, AMF enhance nutrient absorption, promote soil structure, and increase plant resilience to environmental stressors. This makes them invaluable for sustainable agriculture, as through reduction in fertilizer use by a percentage or specific crop improvement (Kumar et al., 2023; Verbruggen and Kiers, 2010). Despite these benefits, there remain significant knowledge gaps in an understanding of AMF. For instance, the diversity of AMF species and their specific interactions with different plant hosts and soil types are not well understood (e.g., to match AMF strains with specific crops for better outcomes). This lack of knowledge hinders the development of targeted strategies for using AMF in agriculture and soil management. Moreover, the evolutionary adaptations that enable AMF to thrive in various ecological niches are not fully understood, limiting the ability to predict their responses to changing environmental conditions (Brundrett, 2002; Li et al., 2021).
This review aims to address these gaps by synthesizing current research on the taxonomy, evolutionary history, and ecological significance of AMF. By focusing on the molecular and morphological features that distinguish AMF from other fungal groups, this review seeks to provide a clearer understanding of their role in ecosystems and their potential applications in sustainable agriculture. Additionally, this review will highlight specific agricultural challenges, such as phosphate limitation, where AMF could provide significant benefits, offering new insights into their practical use in modern farming practices. By narrowing the focus to these areas, this review aims to advance the practical application of AMF in agriculture and contribute to biodiversity conservation in agroecosystems, particularly by supporting plant diversity through enhanced nutrient acquisition and symbiotic relationships.
Based on the existing literature base, this systematic review sought to consolidate existing knowledge on AMF and pinpoint key areas for future research. To achieve this, three primary objectives were established: first, to examine the taxonomic, evolutionary, and ecological dimensions of AMF within the phylum Glomeromycota. It will focus on both historical and contemporary challenges in AMF taxonomy, evolutionary development, and ecological functions. Second, this review aims to evaluate the practical implications of AMF’s roles across various ecosystems, with a particular emphasis on agriculture. It highlights how AMF contribute to soil health, nutrient uptake, and plant resilience, thus underscoring their significance in agricultural practices. Third, to uncover specific knowledge gaps in AMF research and recommend future research directions by assessing the limitations of current methodologies (e.g., molecular, ecological) and enhancing understanding of AMF diversity and functionality across different environmental and agricultural contexts.
2 Literature search
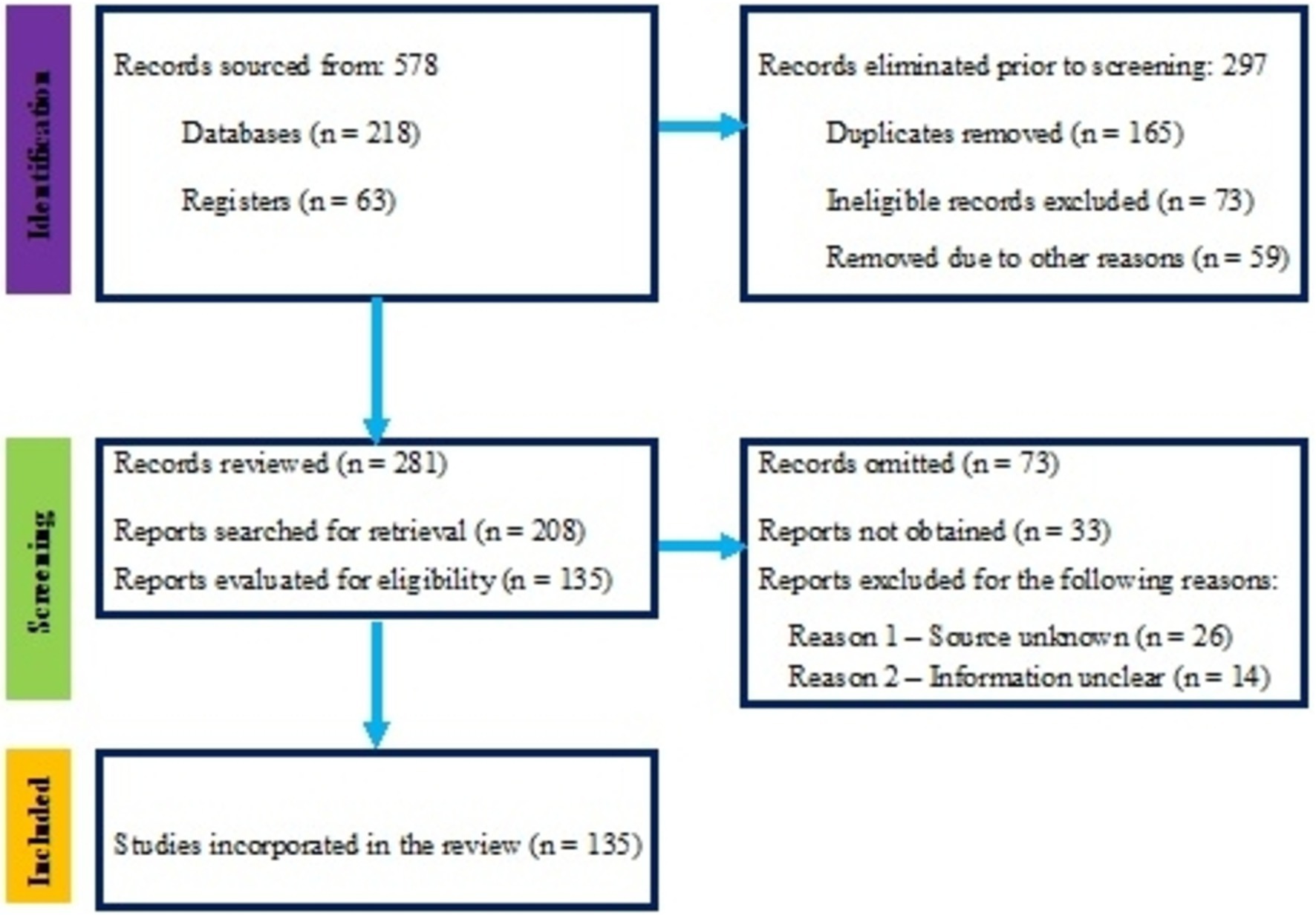
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table 1; Figure 1) to ensure a transparent and comprehensive process as compiled by Page et al. (2021). PRISMA is important for systematic reviews because it provides a standardized framework that enhances the clarity, rigor, and reproducibility of the review process, thereby increasing the credibility and reliability of the findings presented. The PRISMA framework was applied throughout the literature search and selection phases to identify and evaluate relevant studies. Specifically, this review emphasized defining clear inclusion and exclusion criteria, systematically conducting the literature search across multiple databases, and documenting the selection process to ensure transparency and replicability in identifying studies that met the criteria for inclusion. The literature search was conducted using multiple databases, including MEDLINE, Cochrane Library, Scopus, and Web of Science, known for their extensive coverage of health-related, ecological, and evolutionary studies. The Cochrane Library is recognized for high-quality systematic reviews in health sciences, while Scopus offers broad interdisciplinary coverage, especially in social and life sciences. Web of Science excels in indexing high-impact journals and citation analysis. Grey literature sources like Google Scholar were also used to ensure comprehensive coverage. Including grey literature is significant because it often contains valuable research findings, such as thesis, reports, and conference proceedings, that may not be published in peer-reviewed journals. This approach enhances the depth of the review by capturing emerging studies and insights relevant to understanding complex ecological and agricultural dynamics. Search terms included combinations such as “arbuscular mycorrhizal fungi + taxonomic,” “arbuscular mycorrhizal fungi + evolutionary,” “arbuscular mycorrhizal fungi + ecology,” and “Glomeromycota + taxonomy,” among others. These terms were chosen based on prior literature and specific research questions to ensure that the search captured a comprehensive range of relevant studies addressing the taxonomic, evolutionary, and ecological dimensions of arbuscular mycorrhizal fungi. Boolean operators (AND, OR) were utilized to refine the search results. Using “AND” narrowed the results by ensuring that all specified terms appeared in the studies, while “OR” broadened the search to include any of the listed terms. This combination enabled a targeted yet comprehensive retrieval of relevant literature on arbuscular mycorrhizal fungi.
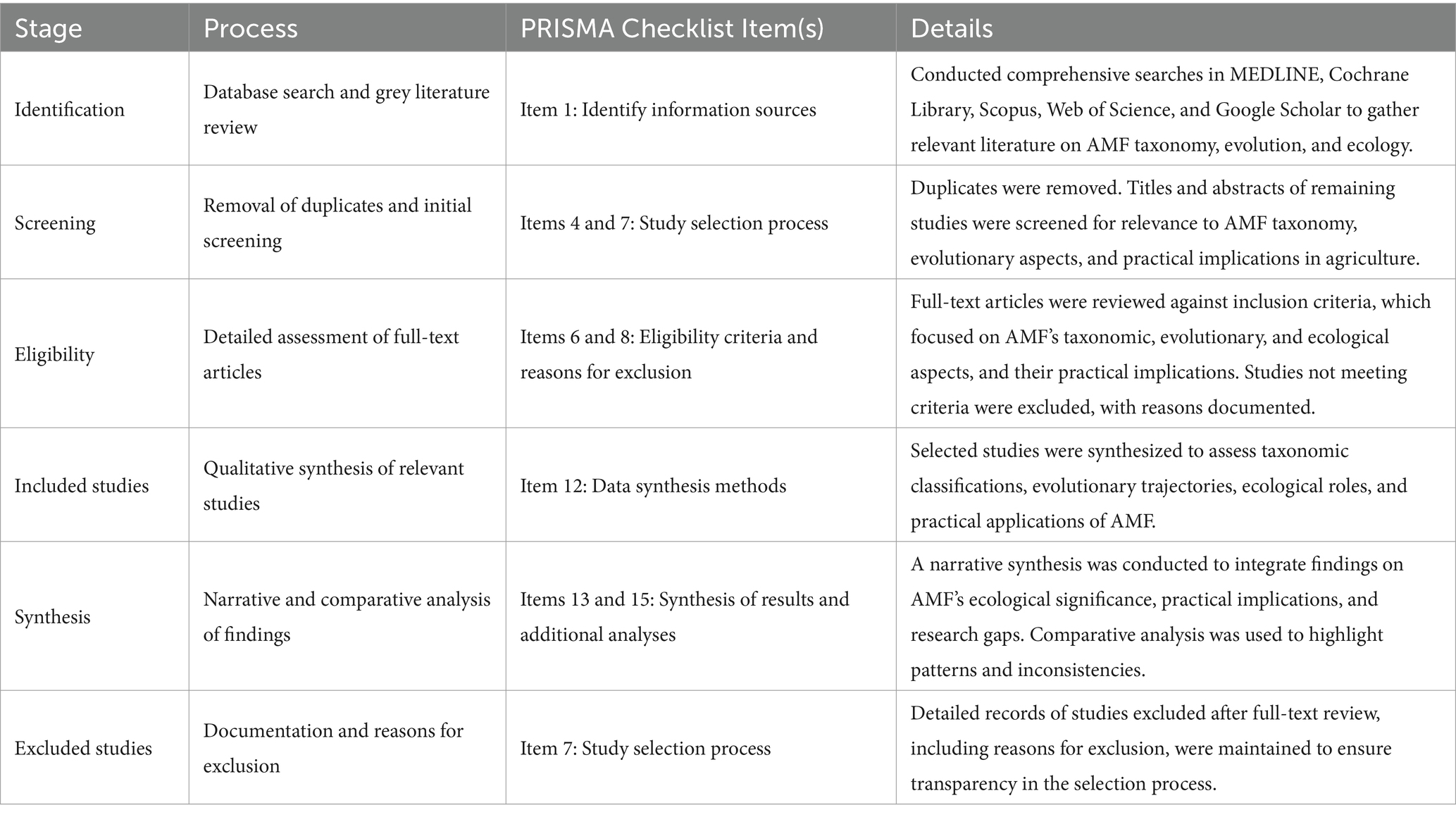
Table 1. Aligning the study search strategy with the PRISMA flowchart items with the checklist of items by Page et al. (2021).
Studies were included based on specific criteria: they had to be peer-reviewed and address taxonomic challenges, evolutionary history, or ecological roles of AMF within the phylum Glomeromycota. The requirement for peer-reviewed studies is crucial as it ensures the quality and reliability of the research, as these studies have undergone rigorous evaluation by experts in the field, thereby enhancing the credibility of the findings included in this review. Articles discussing AMF’s practical applications in agriculture or providing insights into their symbiotic relationships were also considered. The AMF’s ecological functions can inform sustainable agricultural practices, bridging the gap between theoretical knowledge and real-world implementation to enhance soil health and improve crop productivity. Studies not focused on AMF or irrelevant to the review’s objectives were excluded. The screening and selection process involved removing duplicates, reviewing titles and abstracts for relevance, and obtaining full-text articles for those that met the initial criteria. These articles were then evaluated against the inclusion and exclusion criteria. Studies that did not provide clear data on AMF taxonomy, evolution, or ecology were excluded, as were those that did not contribute novel data or perspectives.
Weighting and bias-correction methods were integral to the analysis of included studies in this systematic review. Although formal statistical weighting or bias-correction techniques were not utilized, weighting in this context refers to the process of evaluating the relative importance or contribution of each study based on its quality and relevance to the research questions. This is essential for systematic reviews as it helps ensure that more reliable studies have a greater impact on the overall findings, thus enhancing the credibility and validity of the conclusions drawn from the review. Sensitivity analyses were conducted to examine how variations in study quality might affect the overall findings. Quality and relevance were assessed using criteria such as the clarity of research objectives, robustness of methodology, appropriateness of statistical analyses, and relevance to the taxonomic, evolutionary, and ecological dimensions of arbuscular mycorrhizal fungi. Geographic distribution was carefully considered to provide a comprehensive view of AMF’s ecological impact. Studies from various regions, including those from the North Caucasus (Yurkov et al., 2023) and karst ecosystems (Xiao et al., 2021), were included to capture a broad spectrum of AMF diversity and functions. Geographic distribution was categorized by region and ecosystem type, allowing for an analysis of how different environmental contexts influence the diversity and ecological roles of AMF across various habitats.
This approach aimed to mitigate potential geographic biases by highlighting areas with limited research and suggesting future studies in these underrepresented regions to improve the understanding of AMF’s global ecological roles. The North Caucasus and karst ecosystems were selected due to their unique characteristics, such as diverse climatic conditions and varied soil types, which create distinct ecological niches for AMF. A narrative synthesis was conducted to summarize the findings, identify patterns, and highlight gaps in the literature. Based on this synthesis, future studies will suggest specific research questions and methodologies to address gaps, guiding researchers in underrepresented areas and enhancing the understanding of AMF’s ecological roles.
3 Synthesis of the findings
3.1 Evolutionary history of AMF
Fungi have a rich evolutionary history that spans millions of years. The earliest known fungal fossils, dating back to the Ordovician period around 460 to 455 million years ago, offer glimpses into their early existence (Redecker et al., 2000). These fossils predate the emergence of vascular land plants, which appeared around 425 million years ago (Carris et al., 2012). Molecular data suggest that fungi might have arisen over a billion years ago, challenging traditional views based solely on fossil evidence (Bonneville et al., 2020). Late Cretaceous period mushrooms preserved in amber, approximately 94 million years ago, show that modern-looking mushroom-forming fungi coexisted with dinosaurs (Carris et al., 2012; Hibbett et al., 2003). While fossils offer valuable insights, they present a fragmentary view of fungal evolution, with molecular data helping to fill in the gaps and extend the understanding of fungal history (Bonneville et al., 2019).
The AMF play a critical role in terrestrial ecosystems by forming mutualistic relationships with most land plants. These fungi belong to the phylum Glomeromycota and include a diverse array of families such as Acaulosporaceae, Glomeraceae, and Gigasporaceae. The symbiosis between AMF and plants is ancient, dating back at least 450 million years, and has been essential for the colonization of terrestrial habitats (Lanfranco et al., 2016; Vigneron et al., 2018). As obligate biotrophs, AMF rely on their host plants to complete their life cycles, and their evolution is closely linked to the success and expansion of land plants (Kumar et al., 2023). Understanding the evolutionary history of AMF is crucial for harnessing their benefits in modern agricultural and conservation practices. For example, AMF can enhance soil health, improve plant nutrient uptake, and increase crop resilience to environmental stressors, which is directly informed by knowledge of their evolutionary adaptations (Vigneron et al., 2018). These adaptations, including specialized structures for nutrient exchange and symbiotic relationships with various plant species, enable AMF to facilitate nutrient acquisition and enhance plant stress tolerance. Understanding these traits allows for their strategic application in agriculture, promoting sustainable farming and improving crop productivity in diverse environments.
The classification of AMF has historically been fraught with challenges due to their morphological similarities with other fungi. Early taxonomic approaches often grouped AMF with morphologically similar fungi, leading to inaccurate classifications. For example, the Endogonaceae family was previously categorized under the Mucorales order because of similarities in their non-septate hyphae and zygospores (Morton et al., 1990). Such broad classifications based on morphology alone obscured the true diversity and relationships within the AMF group (Wilkes, 2021). This historical confusion has had implications for the functional application of AMF, as misidentification could lead to suboptimal use of AMF in agricultural or ecological settings. This historical confusion has had significant implications for the functional application of AMF, as misidentification can lead to suboptimal use in agricultural or ecological settings, ultimately hindering the potential benefits that these fungi can provide.
Traditional taxonomic methods, which heavily relied on morphological traits, were limited in their ability to capture the full diversity of AMF. Challenges in isolating and culturing AMF spores contributed to incomplete and sometimes inaccurate classifications (Khaliq et al., 2022). These limitations resulted in many AMF being misclassified under broader fungal categories, which hindered understanding of their true diversity and evolutionary relationships (Desirò et al., 2017; Yamamoto et al., 2015). Accurate taxonomy is essential for identifying AMF species with specific functional traits, such as those beneficial for soil health or plant growth. For instance, correctly identifying AMF species that enhance phosphorus availability can lead to targeted applications in agriculture, improving crop yields and reducing the need for chemical fertilizers, thereby promoting sustainable farming practices.
Recent advances in molecular techniques have significantly improved AMF taxonomy. Molecular markers, such as ribosomal RNA genes, have enabled the reclassification of AMF into more accurate groups, revealing new orders and phyla. For instance, the discovery of the order Archaeosporales through molecular analysis has provided insights into AMF that form symbiotic relationships with a wider range of plant species, including those in nutrient-poor environments. The phylum Glomeromycota has been subdivided into distinct orders, including Archaeosporales, Diversisporales, Glomerales, and Paraglomerales, based on molecular data (Schüβler et al., 2001; Redecker et al., 2006). These advancements have refined understanding of AMF taxonomy and highlight the importance of ongoing molecular research to further clarify fungal classifications (Spatafora et al., 2016; Stürmer and Kemmelmeier, 2020). In practical terms, accurate classification allows for the targeted application of AMF in agriculture and conservation, enabling the selection of specific AMF species or strains that can enhance soil fertility, improves plant health, and support ecosystem restoration efforts.
Fungi engage in a variety of mycorrhizal associations beyond AMF, including ectomycorrhizas (ECM) found in specific plant families such as pines and oaks, and vesicular-arbuscular mycorrhizas (VAM), which are widespread among many plant species (Brundrett, 2002). AMF penetrate plant root cells and form arbuscules to facilitate nutrient exchange, and are prevalent among herbaceous plants and crops (Genre et al., 2005; Wahab et al., 2023), whereas ECM primarily form a protective sheath around root surfaces and typically do not penetrate root cells. The biodiversity of mycorrhizal fungi is vast, influenced by factors such as soil composition, climate, host plant diversity, and ecological interactions (Janowski and Leski, 2022; Martin, 2024). Understanding this biodiversity is crucial for optimizing the functional applications of mycorrhizal fungi. For instance, knowing the specific mycorrhizal types and their ecological roles can guide the use of AMF in enhancing agricultural productivity, restoring degraded ecosystems, and promoting sustainable land management practices.
3.2 The practical implications of AMF’s roles across various agricultural ecosystems
The AMF are vital for soil health and plant productivity in both natural and agricultural ecosystems. These fungi form symbiotic relationships with plant roots, extending their hyphal networks into the soil to enhance water and nutrient uptake (e.g., improved yields, increased soil carbon storage). This interaction improves soil structure, supports nutrient cycling, and contributes to overall plant health, which is crucial for sustainable agricultural practices (Verbruggen and Kiers, 2010; Powell and Rillig, 2018). The extensive networks created by AMF also play a significant role in stabilizing soil and sequestering carbon, thereby aiding in soil fertility and climate change mitigation (Verbruggen et al., 2015; Wang et al., 2016).
AMF functionality in agricultural ecosystems is influenced by several environmental factors and land management practices. Factors such as soil moisture, temperature, pH, and nutrient availability are key determinants of AMF activity and root colonization. Management practices, including tillage and agrochemical use, can significantly impact AMF networks. For example, tillage disrupts hyphal networks, reducing the fungi’s ability to colonize plant roots effectively, while excessive fertilizers and pesticides can negatively affect AMF populations and their interactions with other soil microorganisms (Powell and Rillig, 2018; Wahab et al., 2023).
Crop diversity significantly affects AMF communities, enhancing their resilience and functionality. Diverse cropping systems like cover cropping and crop rotation promote varied AMF networks that facilitate nutrient exchange and support ecosystem functionality by fostering species coexistence and enhancing nutrient cycling. This diversity is crucial for maintaining ecosystem stability and productivity, especially under varying environmental conditions (Ferlian et al., 2018; Lugo et al., 2023). AMF also interact with a wide range of soil microorganisms, forming complex microbial communities that regulate nutrient cycling and soil fertility. These interactions are essential for maintaining soil health, promoting carbon sequestration, and enhancing plant-microbe relationships in agroecosystems (Frąc et al., 2018).
The adaptability of AMF to different environmental conditions and their ability to form diverse communities are crucial for their role in sustainable agriculture (Table 2). Research indicates that AMF community dynamics change with ecological succession, with shifts towards more specialized fungi and different interaction patterns over time (Bennett et al., 2013). Factors such as environmental changes, seasonal variations, and land-use practices also influence AMF community composition and diversity, highlighting their ability to adapt to ecological shifts. For instance, long-term agricultural practices, such as continuous cropping or monoculture, can reduce AMF diversity, emphasizing the need for sustainable land management practices that foster diverse AMF communities (Higo et al., 2019).
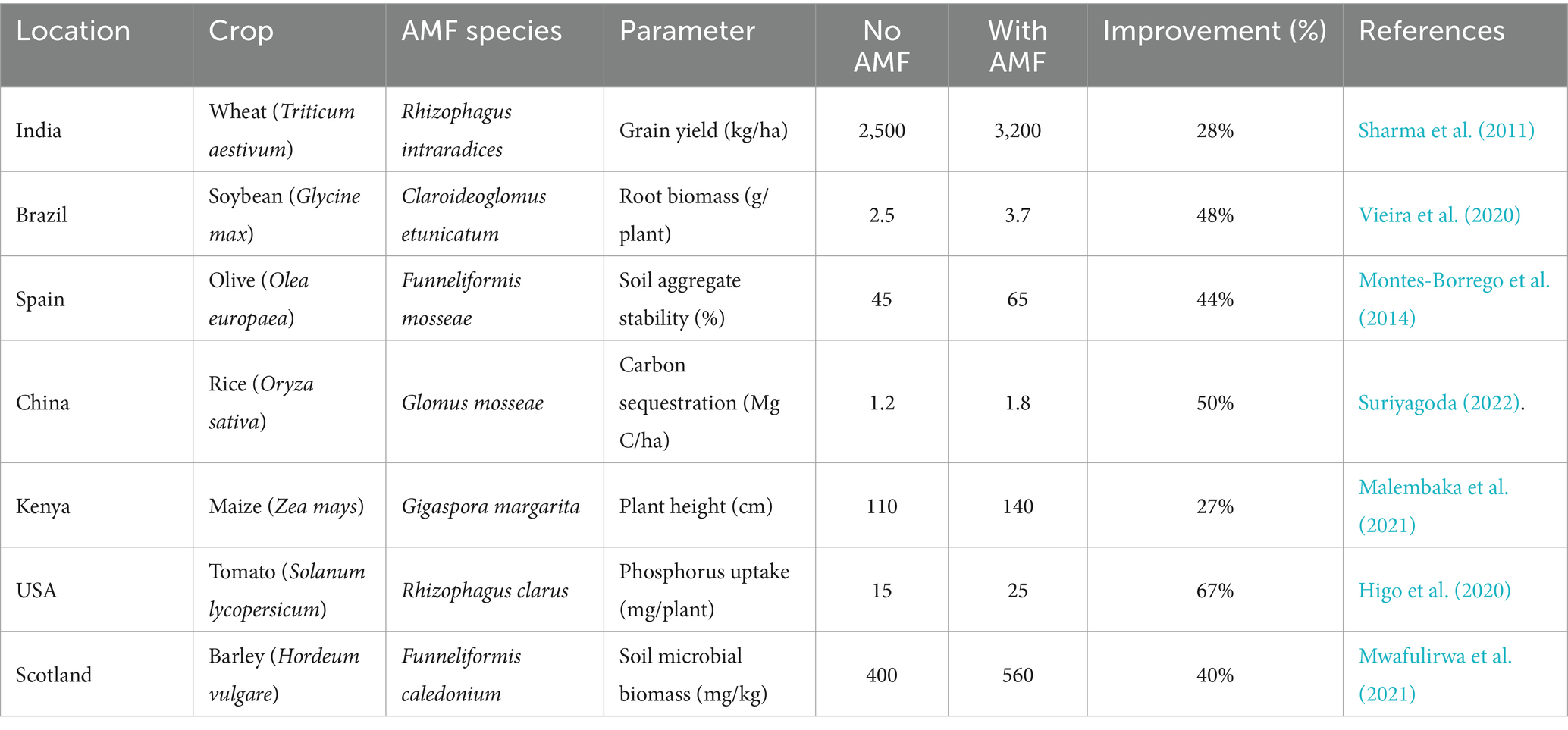
Table 2. Quantitative data on the use of AMF in improving soil health, crop production, and carbon sequestration in different parts of the world.
Beyond nutrient acquisition, AMF play multiple roles in promoting soil health and ecosystem functionality (see Figure 2), particularly in nutrient-poor environments where they enhance plant growth and stress tolerance (Enebe and Erasmus, 2023; Hu et al., 2024). Their main roles include improving nutrient uptake efficiency, as AMF enhance the availability of essential nutrients to plants, particularly phosphorus; reducing dependency on chemical fertilizers by decreasing the need for chemical inputs; enhancing soil health through improved soil structure, which increases water retention and aeration; promoting stress tolerance, helping plants withstand environmental stressors such as drought and salinity; and supporting ecosystem functionality by playing a crucial role in nutrient cycling and overall ecosystem resilience. This makes AMF an integral component of sustainable agricultural practices aimed at enhancing soil fertility, increasing crop yields, and promoting environmental sustainability (Neuenkamp et al., 2019).
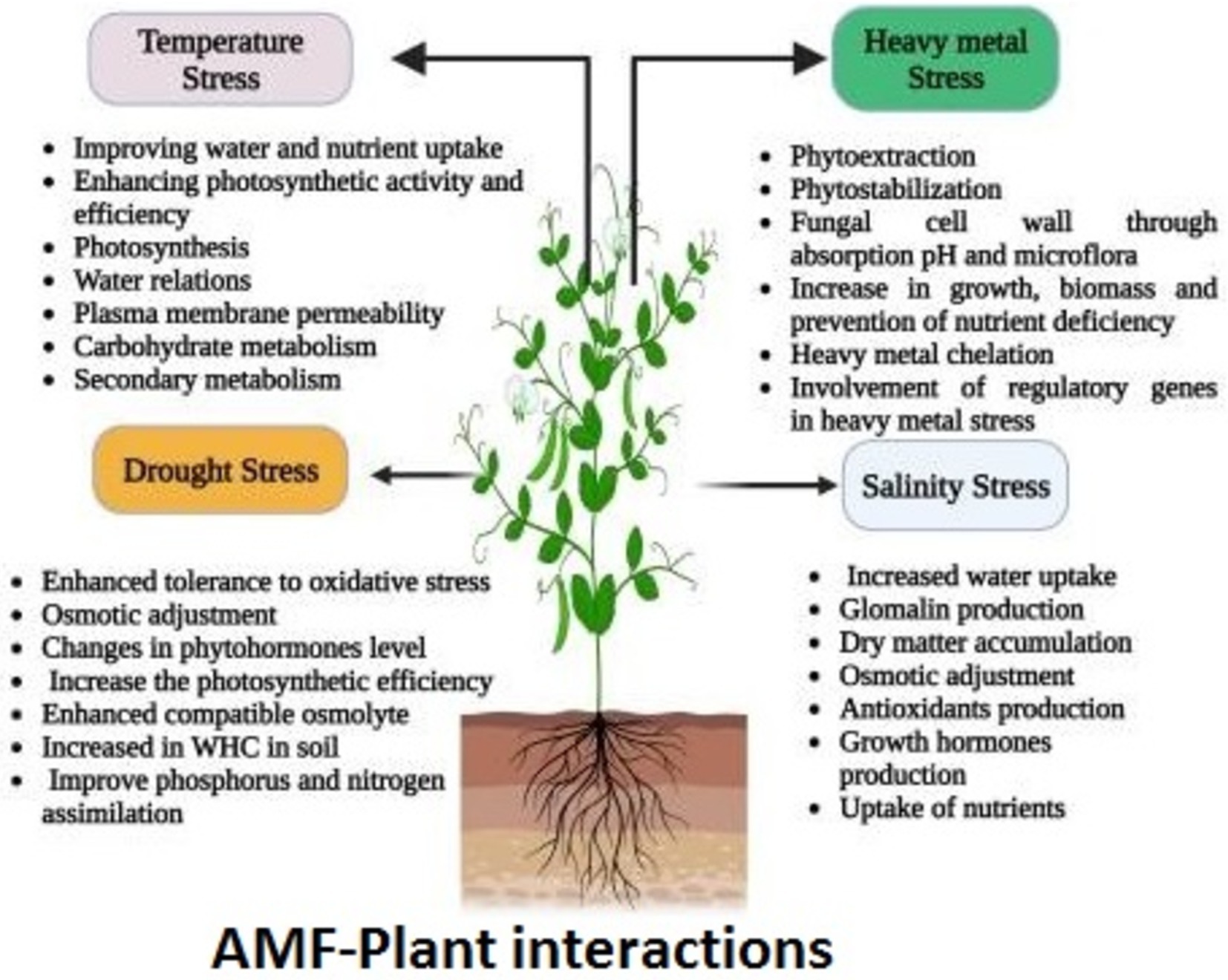
Figure 2. Multi-functionality of AMF in plant growing systems. A visual representation reproduced from Figure 3 of ‘Schematic representation of the different mechanisms imparting abiotic stress tolerance in plants by arbuscular mycorrhiza fungi (AMF)’ by Wahab et al. (2023) under CC BY-NC-ND 4.0 DEED.
Research into indigenous AMF species has demonstrated their potential as effective biofertilizers, improving crop growth and yield across various soil conditions, including rice cultivation (Kalamulla et al., 2021). Findings suggest the importance of leveraging local AMF species that are well-adapted to specific soil and climatic conditions, enhancing their effectiveness in promoting plant growth and soil health. Utilizing native species over introduced ones offers significant benefits, particularly concerning ecosystem resilience and adaptability, as indigenous AMF are better suited to local environments, enabling them to thrive under varying conditions. This adaptability contributes to stronger plant health and improved nutrient cycling, ultimately fostering greater ecosystem stability. Additionally, studies in diverse ecosystems, such as forests and semi-arid regions, reveal the adaptability of AMF communities to different soil properties and seasonal changes, demonstrating their ecological versatility and importance in various environments (Vieira et al., 2020; Djotan et al., 2024).
Understanding the ecological dynamics of AMF is crucial for their role in sustainable agriculture and ecosystem management. Insights into the interactions between AMF, plants, microorganisms, and environmental factors can inform the development of innovative agricultural practices that harness the benefits of AMF. This knowledge is essential for promoting soil health, enhancing crop productivity, and supporting biodiversity, which are vital for sustainable food production and environmental conservation. Integrating AMF into agricultural systems aligns with global sustainability goals, such as improving food security, mitigating climate change, and promoting biodiversity conservation (Enebe and Erasmus, 2023; Hu et al., 2024). Specifically, AMF can help mitigate climate change by enhancing soil carbon sequestration and improving water-use efficiency in crops, making them more resilient to extreme weather conditions. Successful initiatives, such as incorporating indigenous AMF species in agroforestry systems, have demonstrated significant improvements in crop resilience and soil health, addressing both food security and environmental challenges. Ongoing research continues to unveil the complexities of AMF interactions and their ecological roles, underscoring their potential contribution to sustainable agriculture and ecosystem management. Integrating AMF into agricultural systems can promote soil health, increase crop yields, and support environmental sustainability, contributing to a more sustainable and resilient global food system.
The integration of molecular and ecological insights into AMF has profound implications for agricultural productivity, ecosystem management, biotechnology, and soil health monitoring (Table 3). Advances in molecular techniques and ecological research offer valuable tools for enhancing various practices and addressing key environmental challenges. Molecular and ecological insights into AMF directly impact agriculture by improving crop yields and resilience. For example, by understanding the genetic and functional traits of AMF, researchers can develop targeted mycorrhizal inoculants that enhance nutrient uptake, particularly phosphorus, and thus boost plant growth in phosphorus-deficient soils. This targeted approach reduces reliance on chemical fertilizers, fostering more sustainable agricultural practices (Khan et al., 2023; Ghorui et al., 2024; Navarro and Morte, 2024).
Recent molecular techniques, such as high-throughput sequencing and functional genomics, have identified specific AMF strains that improve drought resistance and soil structure (Lahlali et al., 2021). Inoculating crops with these beneficial strains can lead to more resilient agricultural systems capable of withstanding environmental stresses like water scarcity and soil degradation (Breuillard et al., 2020). Ecological insights into AMF also play a crucial role in ecosystem restoration and management. AMF contribute significantly to soil health by promoting nutrient cycling and improving soil structure. In degraded ecosystems—such as deforested areas or mined lands—inoculating the soil with AMF can accelerate restoration by enhancing soil fertility and supporting plant re-establishment (Brundrett and Tedersoo, 2018). Moreover, understanding AMF diversity and community dynamics informs biodiversity-friendly land management practices. Preserving native AMF communities helps maintain ecosystem balance and resilience, supporting biodiversity conservation and enhancing ecosystem services like pollination and water regulation (Augé, 2001).
Molecular research on AMF opens up new possibilities for biotechnology. Genetic engineering can be used to modify AMF strains to enhance their beneficial properties, such as stress tolerance or nutrient uptake. Custom strains with optimized traits can be developed for specific agricultural or environmental applications (Reinhart et al., 2024). Additionally, insights into AMF metabolic pathways and gene functions can lead to innovative biofertilizers and biopesticides. Engineering AMF to produce specific compounds that benefit plant health or protect against pathogens offers environmentally friendly alternatives to traditional chemical fertilizers and pesticides (Gianinazzi et al., 2010; Schüßler et al., 2011). Advanced molecular and imaging technologies enable precise monitoring of soil health and AMF dynamics. Techniques like metagenomics and stable isotope probing (SIP) provide detailed insights into microbial community composition and functional activities (Nicol et al., 2008). This data allows soil scientists and farmers to evaluate the effectiveness of AMF inoculants and track changes in soil health over time.
Integrating molecular data with ecological assessments leads to more accurate soil management strategies. For instance, tracking the impact of AMF on soil nutrient levels and microbial diversity helps optimize fertilization practices, crop rotations, and land use, thereby enhancing soil fertility and productivity while minimizing environmental impacts (van der Heijden et al., 2015). The practical applications of molecular and ecological insights into AMF highlight the need for collaborative and interdisciplinary research. Combining expertise from mycology, genomics, ecology, and agronomy leads to comprehensive solutions for global challenges related to food security, environmental sustainability, and ecosystem health (Fitter et al., 2001; Martin, 2024). Such collaborative efforts drive innovation and facilitate the translation of scientific discoveries into practical applications, benefiting both agricultural and natural ecosystems.
3.3 Knowledge gaps and research priorities on AMF
The AMF research revealed several critical knowledge gaps that need to be addressed to advance both theoretical and practical applications. Despite significant progress with molecular techniques, gaps persist in the taxonomy and diversity of AMF. Many species remain unidentified, and their distribution across various ecosystems is not fully mapped, impeding a comprehensive understanding of their ecological roles and potential uses (Sudová et al., 2020; Yurkov et al., 2023). To address this, advanced genetic tools such as next-generation sequencing and phylogenetic analysis are essential. These methods can uncover the full diversity of AMF species, their evolutionary relationships, and their ecological niches (Bennett et al., 2013). Understanding the functional diversity of AMF is also crucial. Although AMF species exhibit varied traits related to nutrient acquisition, stress tolerance, and symbiotic efficiency, the implications of these variations for ecosystem processes remain underexplored (Bainard et al., 2017). Research should focus on how different AMF species affect plant performance, soil health, and overall ecosystem function, which is vital for developing sustainable agricultural practices and effective ecosystem management strategies (Martin, 2024).
In addition, exploring the mechanisms of AMF symbiosis with plants represents another priority. While basic mechanisms such as nutrient exchange and mycorrhizal signaling are understood, the detailed molecular pathways and gene regulation processes involved are not fully known (Qi et al., 2022; Shen and Duan, 2024). Investigating these molecular interactions is necessary for enhancing plant growth and stress resilience (Boyno et al., 2023; Gao et al., 2023). Interactions between AMF and environmental factors such as soil conditions, climate change, and pollution also require further study. Soil pH, nutrient availability, and moisture levels significantly affect AMF colonization and function, while climate change and pollution can disrupt AMF communities and their interactions with plants (Rillig et al., 2024; Xiao et al., 2021; Boorboori and Zhang, 2022). Understanding these environmental influences is essential for predicting and mitigating the impacts of global changes on AMF and their ecosystem functions (Crișan et al., 2024).
Research into the dynamics and functional significance of common mycorrhizal networks (CMNs) is also critical. These networks facilitate nutrient and carbon transfer between plants and enhance ecosystem stability, yet their formation, stability, and roles in nutrient and carbon cycling require more investigation (Simard et al., 2012; Selosse et al., 2006). Quantifying the benefits of enhanced mycorrhizal connectivity for different ecosystems and crops can offer valuable insights for improving plant productivity and ecosystem health. The role of AMF in agriculture is well-documented (Tian et al., 2024; Slimani et al., 2024), but the specific impacts of various farming techniques on AMF require further clarification. For example, research has shown that organic farming practices, which often involve reduced use of synthetic chemicals and enhanced soil organic matter, can lead to increased AMF colonization and diversity compared to conventional farming (Wang et al., 2023a). In contrast, intensive tillage has been associated with reduced AMF populations due to soil disruption and decreased fungal spore viability (Kuila and Ghosh, 2022). Additionally, case studies such as those by Tian et al. (2024) demonstrate how implementing cover crops in a no-till system can enhance AMF abundance and soil health by providing continuous organic matter inputs.
The application of AMF in sustainable agriculture, ecosystem restoration, and bioremediation holds significant promise. However, further research is needed to identify the most effective AMF species and inoculation methods for various environments and to assess the long-term impacts of AMF on soil health and ecosystem stability (Kalamulla et al., 2021; Wahab et al., 2023). Long-term field studies are essential for capturing the complexities of AMF’s role in natural ecosystems, as short-term studies may not reflect real-world conditions (Johnson et al., 2010; Brundrett and Tedersoo, 2018). The AMF interact with a diverse range of other soil organisms, including bacteria, fungi, and fauna, which significantly affects plant health and ecosystem functioning (Fall et al., 2022; Hnini et al., 2024). Understanding these complex relationships is crucial for a comprehensive view of soil microbial communities and their roles in ecosystem processes (Wagg et al., 2014). Addressing these knowledge gaps will advance the understanding of AMF biology and its implications for sustainable environmental management.
The advancements in molecular techniques have significantly enhanced understanding of AMF’s ecological roles. For instance, next-generation sequencing technologies have revolutionized our ability to identify AMF species with high resolution, even those that are rare or previously uncharacterized (Bainard et al., 2017; Liu et al., 2014). Metagenomic approaches have provided insights into the functional potential of AMF communities by revealing genes involved in nutrient cycling and stress responses (Wahab et al., 2024; Hyde et al., 2024). A notable example is the previous work, which utilized high-throughput sequencing to uncover previously unknown interactions between AMF and soil bacteria that influence plant health and nutrient uptake (Young and Gillung, 2020).
This review offers several novel insights that differentiate it from existing research. Firstly, the findings highlight a previously unrecognized mechanism by which specific AMF species influence plant resilience to drought, a factor that was not thoroughly addressed in earlier studies (Khaliq et al., 2021). This novel aspect is important as it opens up new avenues for agricultural practices aimed at enhancing crop resilience through targeted AMF inoculation. Additionally, this study demonstrates how integrating molecular data with ecological models can refine predictions about AMF community dynamics in response to environmental changes (Santos-González et al., 2007; Sweeney et al., 2022). Practically, these insights can be applied to develop more effective soil management practices that leverage AMF to improve soil health and crop productivity. For example, this review suggests that tailored AMF inoculants could be used in precision agriculture to optimize nutrient uptake in different soil types. This practical application presents the relevance of the findings to both scientific research and agricultural practice. Existing literature underscores the importance of customizing microbial inoculants, such as AMF and rhizobium, to enhance agricultural productivity and sustainability. Marrassini et al. (2024) reveal that AMF isolates show considerable functional variability depending on soil conditions, with certain isolates promoting better nutrient uptake or plant growth based on soil pH and phosphorus levels. Calderon and Dangi (2024) further demonstrate that the effectiveness of AMF and rhizobium inoculants varies by site, with combinations of AMF and rhizobium improving yields in some dryland conditions and AMF enhancing resilience in acidic soils. Additionally, Paravar et al. (2023) review how microbial seed coatings can enhance seed germination and plant stress tolerance, supporting sustainable agriculture by reducing chemical inputs. Together, these studies highlight the need for tailored inoculant strategies and practices to optimize plant health and productivity across different environments.
4 Future directions in AMF research—actionable pathways
The AMF are integral to plant nutrition, soil health, and ecosystem functioning. Despite substantial advancements, significant knowledge gaps and methodological challenges remain, limiting the full utilization of AMF in various applications. This discussion critically assesses these gaps, evaluates past methodologies, and proposes actionable strategies to advance AMF research and applications.
One prominent issue is the taxonomy and diversity of AMF. Although progress has been made, many AMF species remain unidentified, and their distribution across different ecosystems is not fully understood. Traditional morphological methods for identifying AMF are constrained by the high structural similarity among species and an incomplete taxonomic framework. To address these gaps, integrating emerging molecular technologies such as high-throughput sequencing and metagenomics is essential. These technologies offer a comprehensive view of AMF diversity by detecting both known and novel species within complex soil communities (Kalamulla et al., 2021). Conducting global surveys using these advanced methods could map AMF diversity and distribution, leading to the creation of extensive databases that facilitate further research and practical applications.
The functional diversity of AMF is another critical area requiring attention. Although it is acknowledged that AMF species exhibit a range of functional traits, there is insufficient understanding of how these traits vary and their impact on ecosystem processes. Previous research often focused on a limited number of species or traits, missing the broader spectrum of functional diversity within AMF communities (Lee et al., 2013; Albracht et al., 2024). Advanced functional assays and multi-trait analyses utilizing molecular and isotopic techniques can provide insights into how different AMF species contribute to nutrient acquisition, stress tolerance, and soil structure improvement (Wahab et al., 2023). Implementing these studies and integrating ecosystem modeling could predict how variations in AMF functional traits influence nutrient cycling, plant productivity, and soil health, thus informing strategies for optimizing AMF applications in agriculture and conservation (McGale and Sanders, 2022).
Understanding the mechanisms underlying AMF symbiosis with plants is also a significant research priority. While basic mechanisms are known, finer details of the molecular signalling pathways involved in establishing and maintaining symbiotic relationships remain elusive. Research has often concentrated on model species, potentially overlooking the complexity of interactions across diverse plant-AMF pairs (Hu et al., 2022; Hnini et al., 2024). Utilizing transcriptomics and proteomics to map molecular pathways and identify key regulatory genes and proteins is important. Understanding how environmental factors influence these pathways can lead to targeted interventions that enhance symbiosis (Gao et al., 2023).
The AMF interactions with environmental factors such as soil conditions, climate change, and pollution require further exploration. Existing studies often examine these factors in isolation, neglecting the complex interplay between AMF and environmental variables (Rosner and Steinkellner, 2019; Chamard et al., 2024; Kabir et al., 2024). Integrated field and laboratory studies simulating various environmental conditions can provide a more holistic understanding of AMF responses (Feldman et al., 2023; Charakas and Khokhani, 2024; Kozjek et al., 2024). Metagenomic and proteomic approaches to assess adaptation mechanisms can reveal how AMF cope with environmental stresses, which is crucial for developing effective management strategies (Fall et al., 2022).
The dynamics and functional significance of mycorrhizal networks, which connect multiple plants, also warrant deeper investigation. These networks play a vital role in nutrient and carbon cycling, yet their formation, stability, and functions are not fully understood due to past research limitations (Martin, 2024; Ullah et al., 2024). Advanced imaging techniques and network analysis tools can offer insights into the structure and dynamics of mycorrhizal networks (Hammer et al., 2024). Understanding these networks’ roles can help optimize their contribution to ecosystem functions and inform agricultural practices (Hnini et al., 2024).
Applications of AMF in agriculture and ecosystem restoration are increasingly recognized as valuable. Despite their potential benefits, effectively scaling these applications remains a challenge. Long-term field studies are necessary to evaluate the effectiveness of AMF applications in real-world settings (Püschel et al., 2019; Salomon et al., 2021). These studies should focus on the impact of AMF on crop productivity, soil health, and ecosystem restoration, and develop best practices for AMF application based on empirical data. Policy recommendations could support the adoption of AMF-enhanced strategies by providing incentives for farmers and land managers (Gao et al., 2023).
The AMF interactions with a complex array of other soil organisms also merit attention. Previous research may have overlooked these interactions or treated them as secondary to AMF-plant relationships. Holistic approaches that consider the entire soil microbiome, including metagenomic and ecological modeling tools, are needed to understand AMF interactions with other soil microorganisms. Investigating these interactions can reveal their effects on plant health and soil function and inform integrated soil management practices (Kalamulla et al., 2021). Addressing these research priorities and methodological challenges is crucial for advancing the understanding and application of AMF. Leveraging emerging technologies and focusing on actionable research pathways can significantly impact agricultural practices, environmental policies, and ecosystem management. Coordinated efforts among researchers, policymakers, and practitioners will be essential to translate AMF research into tangible benefits for agriculture and conservation.
5 Summary of knowledge gaps and research priorities
A significant number of AMF species remain unidentified, which hinders the understanding of their ecological roles and potential applications. Advanced genetic tools, such as next-generation sequencing, are essential for uncovering AMF diversity and elucidating evolutionary relationships. Furthermore, there is a lack of comprehensive understanding regarding how different AMF species influence nutrient acquisition, plant performance, and broader ecosystem processes. Investigating the variations in AMF traits and their impact on ecosystem health and sustainable agricultural practices is a critical research priority.
While basic mechanisms of nutrient exchange and signaling between plants and AMF are known, detailed insights into the molecular pathways and gene regulation processes governing these interactions remain incomplete. Further exploration of these mechanisms could enhance plant growth and resilience to environmental stressors. Additionally, there is a pressing need for research on how soil conditions, climate change, and pollution affect AMF dynamics, as understanding these factors is vital for predicting and mitigating the impacts of global changes on AMF and the ecosystems they inhabit.
Common mycorrhizal networks (CMNs), which play a crucial role in nutrient and carbon transfer between plants, are still under-explored in terms of their formation and specific functions. Quantifying the benefits of these networks is necessary to improve agricultural productivity and ecosystem stability. Although the significance of AMF in agriculture is acknowledged, further investigation is needed into how various farming techniques, such as organic versus conventional practices, affect AMF populations. Long-term studies are essential for accurately assessing AMF’s roles in natural ecosystems, as short-term investigations may not fully reflect real-world conditions.
Moreover, research is required to identify effective AMF species and optimal inoculation methods for sustainable agriculture, ecosystem restoration, and bioremediation efforts. Understanding AMF interactions with other soil organisms is vital for a holistic perspective on soil health. To advance AMF research effectively, future priorities should include the utilization of advanced technologies, such as high-throughput sequencing and metagenomic approaches, which will facilitate mapping AMF diversity and functional potential. Holistic approaches that integrate field and laboratory studies are necessary to comprehend AMF responses to environmental changes thoroughly. Long-term field studies are essential for evaluating AMF applications in real-world settings and developing best practices for their use. Interdisciplinary collaboration among researchers, policymakers, and practitioners will be vital for translating AMF research into practical benefits for agriculture and conservation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JR: Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. DT: Supervision, Validation, Writing – review & editing. GT: Supervision, Validation, Visualization, Writing – review & editing. LC: Supervision, Validation, Visualization, Writing – review & editing. EN: Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
During the preparation of this work the authors used ChatGPT-OpenAI to improve readability and language. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albracht, C., Solbach, M. D., Hennecke, J., Bassi, L., van der Ploeg, G. R., Eisenhauer, N., et al. (2024). Common soil history is more important than plant history for arbuscular mycorrhizal community assembly in an experimental grassland diversity gradient. Biol. Fertil. Soils 60, 547–562. doi: 10.1007/s00374-024-01821-0
Aparicio Chacón, M. V., Dingenen, J. V., and Goormachtig, S. (2023). Characterization of arbuscular mycorrhizal effector proteins. Int. J. Mol. Sci. 24:9125. doi: 10.3390/ijms24119125
Augé, R. M. (2001). Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11, 3–42. doi: 10.1007/s005720100097
Bainard, L., Chagnon, P., Cade-Menun, B., Lamb, E., LaForge, K., Schellenberg, M., et al. (2017). Plant communities and soil properties mediate agricultural land use impacts on arbuscular mycorrhizal fungi in the mixed prairie ecoregion of the north American Great Plains. Agric. Ecosyst. Environ. 249, 187–195. doi: 10.1016/j.agee.2017.08.010
Belliardo, C., Koutsovoulos, G. D., Rancurel, C., Clément, M., Lipuma, J., and Danchin, E. G. (2022). Improvement of eukaryotic protein predictions from soil metagenomes. Sci. Data 9, 311–314. doi: 10.1038/s41597-022-01420-4
Bennett, A. E., Daniell, T. J., Öpik, M., Davison, J., Moora, M., Zobel, M., et al. (2013). Arbuscular mycorrhizal fungal networks vary throughout the growing season and between successional stages. PLoS One 8:e83241. doi: 10.1371/journal.pone.0083241
Bonneville, S., Delpomdor, F., Préat, A., Chevalier, C., Araki, T., Kazemian, M., et al. (2020). Molecular identification of fungi microfossils in a Neoproterozoic shale rock. Sci. Adv. 6:eaax7599. doi: 10.1126/sciadv.aax7599
Boorboori, M. R., and Zhang, Y. (2022). Arbuscular mycorrhizal fungi are an influential factor in improving the phytoremediation of arsenic, cadmium, lead, and chromium. J. Fungi 8:176. doi: 10.3390/jof8020176
Boyno, G., Danesh, Y. R., Demir, S., Teniz, N., Mulet, J. M., and Porcel, R. (2023). The complex interplay between arbuscular mycorrhizal fungi and strigolactone: mechanisms, sinergies, applications and future directions. Int. J. Mol. Sci. 24:16774. doi: 10.3390/ijms242316774
Breuillard, H., Dupuis, R., Retino, A., Le Contel, O., Amaya, J., and Lapenta, G. (2020). Automatic classification of plasma regions in near-earth space with supervised machine learning: application to magnetospheric multi scale 2016–2019 observations. Front. Astron. Space Sci. 7:55. doi: 10.3389/fspas.2020.00055
Brundrett, M. C. (2002). Coevolution of roots and mycorrhizas of land plants. New Phytol. 154, 275–304. doi: 10.1046/j.1469-8137.2002.00397.x
Brundrett, M. C., and Tedersoo, L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. doi: 10.1111/nph.14976
Calderon, R. B., and Dangi, S. R. (2024). Arbuscular mycorrhizal fungi and rhizobium improve nutrient uptake and microbial diversity relative to dryland site-specific soil conditions. Microorganisms 12:667. doi: 10.3390/microorganisms12040667
Carris, L. M., Little, C. R., and Stiles, C. M. (2012). Introduction to Fungi. Plant Health Instructor. doi: 10.1094/PHI-I-2012-0426-01
Chamard, J., Faticov, M., Blanchet, F. G., Chagnon, L., and Laforest-Lapointe, I. (2024). Interplay of biotic and abiotic factors shapes tree seedling growth and root-associated microbial communities. Commun. Biol. 7:360:360. doi: 10.1038/s42003-024-06042-7
Charakas, C., and Khokhani, D. (2024). Expanded trade: tripartite interactions in the mycorrhizosphere. mSystems 9:e0135223. doi: 10.1128/msystems.01352-23
Chen, E. C., Mathieu, S., Hoffrichter, A., Ropars, J., Dreissig, S., Fuchs, J., et al. (2020). More filtering on SNP calling does not remove evidence of inter-nucleus recombination in dikaryotic arbuscular mycorrhizal fungi. Front. Plant Sci. 11:543611. doi: 10.3389/fpls.2020.00912
Chen, E. C., Mathieu, S., Hoffrichter, A., Sedzielewska-Toro, K., Peart, M., Pelin, A., et al. (2018). Single nucleus sequencing reveals evidence of inter-nucleus recombination in arbuscular mycorrhizal fungi. eLife 7:39813. doi: 10.7554/eLife.39813
Corradi, N., Hijri, M., Fumagalli, L., and Sanders, I. R. (2004). Arbuscular mycorrhizal fungi (Glomeromycota) harbour ancient fungal tubulin genes that resemble those of the chytrids (Chytridiomycota). Fungal Genet. Biol. 41, 1037–1045. doi: 10.1016/j.fgb.2004.08.005
Crișan, I., Balestrini, R., and Pagliarani, C. (2024). The current view on heavy metal remediation: the relevance of the plant interaction with arbuscular mycorrhizal fungi. Plant Stress 12:100439. doi: 10.1016/j.stress.2024.100439
Desirò, A., Rimington, W. R., Jacob, A., Vande-Pol, N., Smith, M. E., and Trappe, J. M. (2017). Multigene phylogeny of Endogonales, an early diverging lineage of fungi associated with plants. IMA Fungus 8, 245–257. doi: 10.5598/imafungus.2017.08.02.03
Djotan, A. K. G., Matsushita, N., and Fukuda, K. (2024). Year-round dynamics of arbuscular mycorrhizal fungi communities in the roots and surrounding soils of Cryptomeria japonica. Mycorrhiza 34, 119–130. doi: 10.1007/s00572-024-01143-x
Dong, Q., Guo, X., Chen, K., Ren, S., Muneer, M. A., Zhang, J., et al. (2021). Phylogenetic correlation and symbiotic network explain the interdependence between plants and arbuscular mycorrhizal fungi in a Tibetan alpine meadow. Front. Plant Sci. 12:804861. doi: 10.3389/fpls.2021.804861
Enebe, M. C., and Erasmus, M. (2023). Symbiosis—a perspective on the effects of host traits and environmental parameters in arbuscular mycorrhizal fungal richness, colonization and ecological functions. Agriculture 13:1899. doi: 10.3390/agriculture13101899
Faghihinia, M., Halverson, L. J., Hršelová, H., Bukovská, P., Rozmoš, M., Kotianová, M., et al. (2024). Nutrient-dependent cross-kingdom interactions in the hyphosphere of an arbuscular mycorrhizal fungus. Front. Microbiol. 14:1284648. doi: 10.3389/fmicb.2023.1284648
Fall, A. F., Nakabonge, G., Ssekandi, J., Apori, S. O., Ndiaye, A., Badji, A., et al. (2022). Roles of arbuscular mycorrhizal fungi on soil fertility: contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 3:723892. doi: 10.3389/ffunb.2022.723892
Feldman, D. R., Aiken, A. C., Boos, W. R., Carroll, R. W. H., Chandrasekar, V., Collis, S., et al. (2023). The surface atmosphere integrated field laboratory (SAIL) campaign. Bull. Am. Meteorol. Soc. 104, E2192–E2222. doi: 10.1175/BAMS-D-22-0049.1
Ferlian, O., Cesarz, S., Craven, D., Hines, J., Barry, K. E., Bruelheide, H., et al. (2018). Mycorrhiza in tree diversity–ecosystem function relationships: conceptual framework and experimental implementation. Ecosphere 9:e02226. doi: 10.1002/ecs2.2226
Fitter, J., Herrmann, R., Dencher, N. A., Blume, A., and Hauss, T. (2001). Activity and stability of a thermostable α-amylase compared to its mesophilic homologue: mechanisms of thermal adaptation. Biochemistry 40, 10723–10731. doi: 10.1021/bi010808b
Frąc, M., Hannula, S. E., Bełka, M., and Jędryczka, M. (2018). Fungal biodiversity and their role in soil health. Front. Microbiol. 9:316246. doi: 10.3389/fmicb.2018.00707
Gao, Y., An, T., Kuang, Q., Wu, Y., Liu, S., Liang, L., et al. (2023). The role of arbuscular mycorrhizal fungi in the alleviation of cadmium stress in cereals: a multilevel meta-analysis. Sci. Total Environ. 902:166091. doi: 10.1016/j.scitotenv.2023.166091
Genre, A., Chabaud, M., Timmers, T., Bonfante, P., and Barker, D. G. (2005). Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17, 3489–3499. doi: 10.1105/tpc.105.035410
Ghorui, M., Chowdhury, S., Balu, P., and Burla, S. (2024). Arbuscular mycorrhizal inoculants and its regulatory landscape. Heliyon 10:e30359. doi: 10.1016/j.heliyon.2024.e30359
Gianinazzi, S., Gollotte, A., Binet, M. N., van Tuinen, D., Redecker, D., and Wipf, D. (2010). Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20, 519–530. doi: 10.1007/s00572-010-0333-3
Hammer, E. C., Arellano-Caicedo, C., Mafla-Endara, P. M., Kiers, E. T., Shimizu, T., Ohlsson, P., et al. (2024). Hyphal exploration strategies and habitat modification of an arbuscular mycorrhizal fungus in microengineered soil chips. Fungal Ecol. 67:101302. doi: 10.1016/j.funeco.2023.101302
Hart, M. M., Aleklett, K., Chagnon, L., Egan, C., Ghignone, S., Helgason, T., et al. (2015). Navigating the labyrinth: a guide to sequence-based, community ecology of arbuscular mycorrhizal fungi. New Phytol. 207, 235–247. doi: 10.1111/nph.13340
Hibbett, D. S., Binder, M., Wang, Z., Goldman, Y., and Hibbett, D. S. (2003). Another fossil agaric from Dominican Amber. Mycologia 95, 685–687. doi: 10.1080/15572536.2004.11833071
Higo, M., Azuma, M., Kamiyoshihara, Y., Kanda, A., Tatewaki, Y., and Isobe, K. (2020). Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 8:178. doi: 10.3390/microorganisms8020178
Higo, M., Kang, D., and Isobe, K. (2019). First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi nuclear disaster in Japan. Sci. Rep. 9, 8240–8210. doi: 10.1038/s41598-019-44665-7
Hnini, M., Rabeh, K., and Oubohssaine, M. (2024). Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: a systematic review. Plant Stress 11:100391. doi: 10.1016/j.stress.2024.100391
Horn, S., Caruso, T., Verbruggen, E., Rillig, M. C., and Hempel, S. (2014). Arbuscular mycorrhizal fungal communities are phylogenetically clustered at small scales. ISME J. 8, 2231–2242. doi: 10.1038/ismej.2014.72
Hu, X., Chen, D., Yan, F., Zheng, X., Fang, X., Bai, Y., et al. (2024). Global research trends on the effects of arbuscular mycorrhizal fungi on the soil carbon cycle: a bibliometric analysis. Ecol. Indic. 158:111543. doi: 10.1016/j.ecolind.2023.111543
Hu, L., Zhang, J., Kaundal, R., Kataria, R., Labbé, J. L., Mitchell, J. C., et al. (2022). Diversity and conservation of plant small secreted proteins associated with arbuscular mycorrhizal symbiosis. Hortic. Res. 9:uhac043. doi: 10.1093/hr/uhac043
Hyde, K. D., Baldrian, P., Chen, Y., Thilini Chethana, K. W., De Hoog, S., Doilom, M., et al. (2024). Current trends, limitations and future research in the fungi? Fungal Divers. 125, 1–71. doi: 10.1007/s13225-023-00532-5
Janowski, D., and Leski, T. (2022). Factors in the distribution of mycorrhizal and soil fungi. Diversity 14:1122. doi: 10.3390/d14121122
Johnson, N. C., Wilson, G. W., Bowker, M. A., Wilson, J. A., and Miller, R. M. (2010). Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. 107, 2093–2098. doi: 10.1073/pnas.0906710107
Kabir, A. H., Baki, M. Z. I., Ahmed, B., and Mostofa, M. G. (2024). Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crops 1:100013. doi: 10.1016/j.ncrops.2024.100013
Kakouridis, A., Yuan, M., Nuccio, E. E., Hagen, J. A., Fossum, C. A., Moore, M. L., et al. (2024). Arbuscular mycorrhiza convey significant plant carbon to a diverse hyphosphere microbial food web and mineral-associated organic matter. New Phytol. 242, 1661–1675. doi: 10.1111/nph.19560
Kalamulla, R., Karunarathna, S. C., Tibpromma, S., Galappaththi, M. C., Suwannarach, N., Stephenson, S. L., et al. (2021). Arbuscular mycorrhizal fungi in sustainable agriculture. Sustain. For. 14:12250. doi: 10.3390/su141912250
Kang, J. E., Ciampi, A., and Hijri, M. (2020a). SeSaMe: metagenome sequence classification of arbuscular mycorrhizal fungi-associated microorganisms. Genom. Proteom. Bioinform. 18, 601–612. doi: 10.1016/j.gpb.2018.07.010
Kang, J. E., Ciampi, A., and Hijri, M. (2020b). SeSaMe PS function: functional analysis of the whole metagenome sequencing data of the arbuscular mycorrhizal fungi. Genom. Proteom. Bioinform. 18, 613–623. doi: 10.1016/j.gpb.2018.07.011
Khaliq, A., Perveen, S., Alamer, K. H., Rafique, Z., Alsudays, I. M., Althobaiti, A. T., et al. (2022). Arbuscular mycorrhizal fungi symbiosis to enhance plant–soil interaction. Sustain. For. 14:7840. doi: 10.3390/su14137840
Khan, F., Siddique, A. B., Shabala, S., Zhou, M., and Zhao, C. (2023). Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plan. Theory 12:2861. doi: 10.3390/plants12152861
Kolaříková, Z., Slavíková, R., Krüger, C., Krüger, M., and Kohout, P. (2021). PacBio sequencing of Glomeromycota rDNA: a novel amplicon covering all widely used ribosomal barcoding regions and its applicability in taxonomy and ecology of arbuscular mycorrhizal fungi. New Phytol. 231, 490–499. doi: 10.1111/nph.17372
Kozjek, K., Kundel, D., Kushwaha, S. K., Olsson, P. A., Ahrén, D., Fliessbach, A., et al. (2024). Long-term agricultural management impacts arbuscular mycorrhizal fungi more than short-term experimental drought. Appl. Soil Ecol. 168:104140. doi: 10.1016/j.apsoil.2021.104140
Krüger, M., Krüger, C., Walker, C., Stockinger, H., and Schüßler, A. (2012). Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 193, 970–984. doi: 10.1111/j.1469-8137.2011.03962.x
Kuila, D., and Ghosh, S. (2022). Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Microbiol. Curr. Res. 3:100107. doi: 10.1016/j.crmicr.2022.100107
Kumar, P., Verma, V., Irfan, M., Sharma, R., and Bhargava, B. (2023). How plants conquered land: evolution of terrestrial adaptation. J. Evol. Biol. 36, 5–14. doi: 10.1111/jeb.14062
Lahlali, R., Ibrahim, D., Belabess, Z., Kadir Roni, M. Z., Radouane, N., Vicente, C. S., et al. (2021). High-throughput molecular technologies for unraveling the mystery of soil microbial community: Challenges and future prospects. Heliyon. 7:e08142. doi: 10.1016/j.heliyon.2021.e08142
Lanfranco, L., and Bonfante, P. (2023). Lessons from arbuscular mycorrhizal fungal genomes. Curr. Opin. Microbiol. 75:102357. doi: 10.1016/j.mib.2023.102357
Lanfranco, L., Bonfante, P., and Genre, A. (2016). The mutualistic interaction between plants and arbuscular mycorrhizal fungi. Microbiol. Spect. 4, 10–1128. doi: 10.1128/microbiolspec.funk-0012-2016
Lee, H., Eo, K., Ka, H., and Eom, H. (2013). Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 41, 121–125. doi: 10.5941/MYCO.2013.41.3.121
Leung, M. L., Wang, Y., Waters, J., and Navin, N. E. (2015). SNES: single nucleus exome sequencing. Genome Biol. 16, 55–10. doi: 10.1186/s13059-015-0616-2
Li, T., Yang, W., Wu, S., Selosse, A., and Gao, J. (2021). Progress and prospects of mycorrhizal fungal diversity in orchids. Front. Plant Sci. 12:646325. doi: 10.3389/fpls.2021.646325
Lin, K., Limpens, E., Zhang, Z., Ivanov, S., Saunders, D. G., Mu, D., et al. (2014). Single nucleus genome sequencing reveals high similarity among nuclei of an endomycorrhizal fungus. PLoS Genet. 10:e1004078. doi: 10.1371/journal.pgen.1004078
Liu, W., Jiang, S., Zhang, Y., Yue, S., Christie, P., Murray, P. J., et al. (2014). Spatiotemporal changes in arbuscular mycorrhizal fungal communities under different nitrogen inputs over a 5-year period in intensive agricultural ecosystems on the North China plain. FEMS Microbiol. Ecol. 90, 436–453. doi: 10.1111/1574-6941.12405
Lugo, M. A., Ontivero, R. E., Iriarte, H. J., Yelikbayev, B., and Pagano, M. C. (2023). The diversity of arbuscular mycorrhizal fungi and their associations in South America: a case study of Argentinean and Brazilian cattle raising productive ecosystems: a review. Diversity 15:1006. doi: 10.3390/d15091006
Luo, Y., Wang, Z., He, Y., Li, G., Lv, X., and Zhuang, L. (2020). High-throughput sequencing analysis of the rhizosphere arbuscular mycorrhizal fungi (AMF) community composition associated with Ferula sinkiangensis. BMC Microbiol. 20, 335–314. doi: 10.1186/s12866-020-02024-x
Malembaka, R., Onwonga, R., Jefwa, J., Ayuke, F., and Nabahungu, L. (2021). Role of native arbuscular mycorrhizal fungi on maize (Zea mays) growth and nutrient uptake in acidic soils under controlled conditions. Int. J. Agron. Agric. Res. 18, 20–32.
Marrassini, V., Ercoli, L., Kuramae, E. E., Kowalchuk, G. A., and Pellegrino, E. (2024). Arbuscular mycorrhizal fungi originated from soils with a fertility gradient highlight a strong intraspecies functional variability. Appl. Soil Ecol. 197:105344. doi: 10.1016/j.apsoil.2024.105344
Martin, F. M. (2024). The mycorrhizal symbiosis: research frontiers in genomics, ecology, and agricultural application. New Phytol. 242, 1486–1506. doi: 10.1111/nph.19541
Masclaux, F. G., Wyss, T., Pagni, M., Rosikiewicz, P., and Sanders, I. R. (2019). Investigating unexplained genetic variation and its expression in the arbuscular mycorrhizal fungus Rhizophagus irregularis: a comparison of whole genome and RAD sequencing data. PLoS One 14:e0226497. doi: 10.1371/journal.pone.0226497
Mateus, I. D., Auxier, B., Ndiaye, S., Cruz, J., Lee, S. J., and Sanders, I. R. (2022). Reciprocal recombination genomic signatures in the symbiotic arbuscular mycorrhizal fungi Rhizophagus irregularis. PLoS One 17:e0270481. doi: 10.1371/journal.pone.0270481
McGale, E., and Sanders, I. R. (2022). Integrating plant and fungal quantitative genetics to improve the ecological and agricultural applications of mycorrhizal symbioses. Curr. Opin. Microbiol. 70:102205. doi: 10.1016/j.mib.2022.102205
Montes-Borrego, M., Metsis, M., and Landa, B. B. (2014). Arbuscular mycorhizal fungi associated with the olive crop across the Andalusian landscape: factors driving community differentiation. PLoS One 9:e96397. doi: 10.1371/journal.pone.0096397
Montoliu-Nerin, M., Sánchez-García Morton, J. B., and Benny, G. L. (1990). Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37, 471–491.
Morton, J. B., Orchard, S., Hilton, S., Bending, G. D., Dickie, I. A., Standish, R. J., et al. (1990). Evolutionary relationships among arbuscular mycorrhizal fungi in the Endogonaceae. Mycologia 82, 192–207. doi: 10.1080/00275514.1990.12025865
Mousa, W. K., Abu-Izneid, T., and Salah-Tantawy, A. (2024). High-throughput sequencing reveals the structure and metabolic resilience of desert microbiome confronting climate change. Front. Plant Sci. 15:1294173. doi: 10.3389/fpls.2024.1294173
Mwafulirwa, L., Baggs, E. M., Russell, J., Hackett, C. A., Morley, N., De la Fuente Cantó, C., et al. (2021). Identification of barley genetic regions influencing plant–microbe interactions and carbon cycling in soil. Plant Soil 468, 165–182. doi: 10.1007/s11104-021-05113-6
Navarro, J. M., and Morte, A. (2024). Arbuscular mycorrhizal fungi as biofertilizers to increase the plant quality of sour-orange seedlings. Agronomy 14:230. doi: 10.3390/agronomy14010230
Neuenkamp, L., Prober, S. M., Price, J. N., Zobel, M., and Standish, R. J. (2019). Benefits of mycorrhizal inoculation to ecological restoration depend on plant functional type, restoration context and time. Fungal Ecol. 40, 140–149. doi: 10.1016/j.funeco.2018.05.004
Nicol, G. W., Leininger, S., Schleper, C., and Prosser, J. I. (2008). The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 10, 2966–2978. doi: 10.1111/j.1462-2920.2008.01701.x
Nuccio, E. E., Blazewicz, S. J., Lafler, M., Campbell, A. N., Kakouridis, A., Kimbrel, J. A., et al. (2022). HT-SIP: a semi-automated stable isotope probing pipeline identifies cross-kingdom interactions in the hyphosphere of arbuscular mycorrhizal fungi. Microbiome 10:199. doi: 10.1186/s40168-022-01391-z
Oyarte Galvez, L., Klein, M., Hink, M. A., Postma, M., Shimizu, T., and Kiers, E. T. (2021). Temporal tracking of quantum-dot apatite across in vitro mycorrhizal networks shows how host demand can influence fungal nutrient transfer strategies. ISME J. 15, 435–449. doi: 10.1038/s41396-020-00786-w
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Paravar, A., Piri, R., Balouchi, H., and Ma, Y. (2023). Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 37:e00781. doi: 10.1016/j.btre.2023.e00781
Powell, J. R., and Rillig, M. C. (2018). Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 220, 1059–1075. doi: 10.1111/nph.15119
Püschel, D., Kolaříková, Z., Šmilauer, P., and Rydlová, J. (2019). Survival and long-term infectivity of arbuscular mycorrhizal fungi in peat-based substrates stored under different temperature regimes. Appl. Soil Ecol. 140, 98–107. doi: 10.1016/j.apsoil.2019.04.020
Qi, S., Wang, J., Wan, L., Dai, Z., Matos, S., Du, D., et al. (2022). Arbuscular mycorrhizal fungi contribute to phosphorous uptake and allocation strategies of Solidago canadensis in a phosphorous-deficient environment. Front. Plant Sci. 13:831654. doi: 10.3389/fpls.2022.831654
Redecker, D., Kodner, R., and Graham, L. E. (2000). Glomalean fungi from the Ordovician. Science 289, 1920–1921. doi: 10.1126/science.289.5486.1920
Redecker, D., Raab, P., Bruns, T. D., Corradi, N., Redecker, D., Taylor, J. W., et al. (2006). Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98, 885–895. doi: 10.1080/15572536.2006.11832618
Reinhart, K. O., Vermeire, L. T., Penn, C. J., and Lekberg, Y. (2024). Experimental evidence that poor soil phosphorus (P) solubility typical of drylands due to calcium co-precipitation favors autonomous plant P acquisition over collaboration with mycorrhizal fungi. Soil Biol. Biochem. 199:109605. doi: 10.1016/j.soilbio.2024.109605
Rillig, M. C., Lehmann, A., Orr, J. A., and Rongstock, R. (2024). Factors of global change affecting plants act at different levels of the ecological hierarchy. Plant J. 117, 1781–1785. doi: 10.1111/tpj.16509
Rosner, K., and Steinkellner, S. (2019). Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 75, 583–590. doi: 10.1002/ps.5220
Salomon, M., Demarmels, R., Watts-Williams, S., McLaughlin, M., Kafle, A., Ketelsen, C., et al. (2021). Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl. Soil Ecol. 169:104225. doi: 10.1016/j.apsoil.2021.104225
Salvioli, A., and Bonfante, P. (2013). Systems biology and “omics” tools: a cooperation for next-generation mycorrhizal studies. Plant Sci. 203-204, 107–114. doi: 10.1016/j.plantsci.2013.01.001
Sandrini, M., Nerva, L., Sillo, F., Balestrini, R., Chitarra, W., and Zampieri, E. (2022). Abiotic stress and belowground microbiome: the potential of omics approaches. Int. J. Mol. Sci. 23:1091. doi: 10.3390/ijms23031091
Santos-González, J. C., Finlay, R. D., and Tehler, A. (2007). Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a seminatural grassland. Appl. Environ. Microbiol. 73, 5613–5623. doi: 10.1128/AEM.00262-07
Santucciu, C., Peruzzu, A., Fara, A. M., Cossu, A., Kronenberg, P. A., Deplazes, P., et al. (2024). Immunohistochemistry as a reliable tool for the diagnosis of cystic echinococcosis in patients from Sardinia, Italy—a confirmatory study. Diseases 12:84. doi: 10.3390/diseases12050084
Schüßler, A., Krüger, M., and Walker, C. (2011). Revealing natural relationships among arbuscular mycorrhizal fungi: culture line BEG47 represents Diversispora epigaea, not Glomus versiforme. PLoS One 6:e23333. doi: 10.1371/journal.pone.0023333
Schüβler, A., Schwarzott, D., and Walker, C. (2001). A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413–1421. doi: 10.1017/S0953756201005196
Schwarzott, D., Walker, C., and Schüßler, A. (2001). Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales), is nonmonophyletic. Mol. Phylogenet. Evol. 21, 190–197. doi: 10.1006/mpev.2001.1007
Selosse, M., Richard, F., He, X., and Simard, S. W. (2006). Mycorrhizal networks: des liaisons dangereuses? Trends Ecol. Evol. 21, 621–628. doi: 10.1016/j.tree.2006.07.003
Shami, A., Jalal, R. S., Ashy, R. A., Abuauf, H. W., Baz, L., Refai, M. Y., et al. (2021). Use of metagenomic whole genome shotgun sequencing data in taxonomic assignment of Dipterygium glaucum rhizosphere and surrounding bulk soil microbiomes, and their response to watering. Sustain. For. 14:8764. doi: 10.3390/su14148764
Sharma, M. P., Reddy, U. G., and Adholeya, A. (2011). Response of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) grown conventionally and on beds in a sandy loam soil. Indian J. Microbiol. 51, 384–389. doi: 10.1007/s12088-011-0134-1
Shen, Y., and Duan, T. (2024). The interaction between arbuscular mycorrhizal fungi (AMF) and grass endophyte (Epichloë) on host plants: a review. J. Fungi 10:174. doi: 10.3390/jof10030174
Shu, J., Wang, H., Shen, H., Wang, R., Fu, Q., Wang, Y., et al. (2021). Phylogenomic analysis reconstructed the order Matoniales from Paleopolyploidy veil. Plan. Theory 11:1529. doi: 10.3390/plants11121529
Simard, S. W., Beiler, K. J., Bingham, M. A., Deslippe, J. R., Philip, L. J., and Teste, F. P. (2012). Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol. Rev. 26, 39–60. doi: 10.1016/j.fbr.2012.01.001
Slimani, A., Boutasknit, A., Anli, M., Abouraicha, E. F., Oufdou, K., Meddich, A., et al. (2024). Molecular and systems biology approaches for harnessing the symbiotic interaction in mycorrhizal symbiosis for grain and oil crop cultivation. Int. J. Mol. Sci. 25:912. doi: 10.3390/ijms25020912
Spatafora, J. W., Chang, Y., Benny, G. L., Lazarus, K., Smith, M. E., Berbee, M. L., et al. (2016). A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046. doi: 10.3852/16-042
Stürmer, S. L., and Kemmelmeier, K. (2020). The glomeromycota in the neotropics. Front. Microbiol. 11:553679. doi: 10.3389/fmicb.2020.553679
Su, D., Yang, L., Shi, X., Ma, X., Zhou, X., Hedges, S. B., et al. (2021). Large-scale phylogenomic analyses reveal the monophyly of bryophytes and Neoproterozoic origin of land plants. Mol. Biol. Evol. 38, 3332–3344. doi: 10.1093/molbev/msab106
Sudová, R., Kohout, P., Rydlová, J., Čtvrtlíková, M., Suda, J., Voříšková, J., et al. (2020). Diverse fungal communities associated with the roots of isoetid plants are structured by host plant identity. Fungal Ecol. 45:100914. doi: 10.1016/j.funeco.2020.100914
Suriyagoda, L. B. D. (2022). Rice production in nutrient-limited soils: strategies for improving crop productivity and land sustainability. J. Natl. Sci. Found. 50:521. doi: 10.4038/jnsfsr.v50i3.10601
Sweeney, C. J., Bottoms, M., Ellis, S., Ernst, G., Kimmel, S., Loutseti, S., et al. (2022). Arbuscular mycorrhizal fungi and the need for a meaningful regulatory plant protection product testing strategy. Environ. Toxicol. Chem. 41, 1808–1823. doi: 10.1002/etc.5400
Tchiechoua, Y. H., Odee, D. W., Ngonkeu, E. L., Kinyua, J., Ngumi, V. W., Machuka, E. M., et al. (2023). DNA sequencing reveals high arbuscular mycorrhizal fungi diversity in the rhizosphere soil of Prunus africana trees in fragmented Afromontane forests. Ann. Microbiol. 73:17. doi: 10.1186/s13213-023-01720-z
Tian, L., Wang, T., Cui, S., Li, Y., Gui, W., Yang, F., et al. (2024). Diversified cover crops and no-till enhanced soil total nitrogen and arbuscular mycorrhizal fungi diversity: A case study from the Karst Area of Southwest China. Agriculture. 14:1103. doi: 10.3390/agriculture14071103
Ullah, A., Gao, D., and Wu, F. (2024). Common mycorrhizal network: the predominant socialist and capitalist responses of possible plant–plant and plant–microbe interactions for sustainable agriculture. Front. Microbiol. 15:11830204. doi: 10.3389/fmicb.2024.1183024
van Der Heijden, M. G., Martin, F. M., Selosse, M. A., and Sanders, I. R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 205, 1406–1423. doi: 10.1111/nph.13288
Verbruggen, E., and Kiers, E. T. (2010). Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol. Appl. 3, 547–560. doi: 10.1111/j.1752-4571.2010.00145.x
Verbruggen, E., Xiang, D., Chen, B., Xu, T., and Rillig, M. C. (2015). Mycorrhizal fungi associated with high soil N: P ratios are more likely to be lost upon conversion from grasslands to arable agriculture. Soil Biol. Biochem. 86, 1–4. doi: 10.1016/j.soilbio.2015.03.008
Victcheckorino, Í. M. M., Berruti, A., Orgiazzi, A., Voyron, S., Bianciotto, V., and Lumini, E. (2020). High-throughput DNA sequence-based analysis of AMF communities. Methods Mol. Biol. 2146, 99–116. doi: 10.1007/978-1-0716-0603-2_9
Vieira, L. C., Silva, D. K., Escobar, I. E., Silva, J. M., Moura, I. A., Oehl, F., et al. (2020). Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plan. Theory 9:52. doi: 10.3390/plants9010052
Vigneron, N., Radhakrishnan, G. V., and Delaux, P. (2018). What have we learnt from studying the evolution of the arbuscular mycorrhizal symbiosis? Curr. Opin. Plant Biol. 44, 49–56. doi: 10.1016/j.pbi.2018.02.004
Wagg, C., Bender, S. F., Widmer, F., and van der Heijden, M. G. (2014). Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. 111, 5266–5270. doi: 10.1073/pnas.1320054111
Wahab, A., Batool, F., Muhammad, M., Zaman, W., Mikhlef, R. M., Qaddoori, S. M., et al. (2024). Unveiling the complex molecular dynamics of arbuscular mycorrhizae: a comprehensive exploration and future perspectives in harnessing phosphate-solubilizing microorganisms for sustainable progress. Environ. Exp. Bot. 219:105633. doi: 10.1016/j.envexpbot.2023.105633
Wahab, A., Muhammad, M., Munir, A., Abdi, G., Zaman, W., Ayaz, A., et al. (2023). Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plan. Theory 12:3102. doi: 10.3390/plants12173102
Wang, M., Li, R., and Zhao, Q. (2023b). Multi-omics techniques in genetic studies and breeding of forest plants. Forests 14:1196. doi: 10.3390/f14061196
Wang, J., Zhou, Z. Y., and Ling, W. T. (2016). Distribution and environmental function of glomalin-related soil protein: a review. Ying Yong sheng tai xue bao= the. J. Appl. Ecol. 27, 634–642
Wang, Y., Zou, Y., Shu, B., and Wu, Q. (2023a). Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 308:111591. doi: 10.1016/j.scienta.2022.111591
Wilkes, T. I. (2021). Arbuscular mycorrhizal fungi in agriculture. Encyclopedia 1, 1132–1154. doi: 10.3390/encyclopedia1040085
Xiao, D., Chen, Y., He, X., Xu, Z., Hosseini Bai, S., Zhang, W., et al. (2021). Temperature and precipitation significantly influence the interactions between arbuscular mycorrhizal fungi and diazotrophs in karst ecosystems. For. Ecol. Manag. 497:119464. doi: 10.1016/j.foreco.2021.119464
Xu, L., Pierroz, G., Wipf, H. M. L., Gao, C., Taylor, J. W., Lemaux, P. G., et al. (2021). Holo-omics for deciphering plant-microbiome interactions. Microbiome 9, 1–11. doi: 10.1186/s40168-021-01014-z
Yamamoto, K., Degawa, Y., Hirose, D., Fukuda, M., and Yamada, A. (2015). Morphology and phylogeny of four Endogone species and Sphaerocreas pubescens collected in Japan. Mycol. Prog. 14, 1–17. doi: 10.1007/s11557-015-1111-6
Young, A. D., and Gillung, J. P. (2020). Phylogenomics — principles, opportunities and pitfalls of big-data phylogenetics. Syst. Entomol. 45, 225–247. doi: 10.1111/syen.12406
Yurkov, A. P., Kryukov, A. A., Gorbunova, A. O., Kudriashova, T. R., Kovalchuk, A. I., Gorenkova, A. I., et al. (2023). Diversity of arbuscular mycorrhizal fungi in distinct ecosystems of the North Caucasus, a temperate biodiversity hotspot. J. Fungi 10:11. doi: 10.3390/jof10010011
Keywords: molecular imaging, nutrient uptake, soil health, sustainable agriculture, symbiotic relationships
Citation: Mwampashi LL, Magubika AJ, Ringo JF, Theonest DJ, Tryphone GM, Chilagane LA and Nassary EK (2024) Exploring agro-ecological significance, knowledge gaps, and research priorities in arbuscular mycorrhizal fungi. Front. Microbiol. 15:1491861. doi: 10.3389/fmicb.2024.1491861
Edited by:
Perumal Vivekanandhan, Chiang Mai University, ThailandReviewed by:
Mohini Prabha Singh, Punjab Agricultural University, IndiaPriyanka Verma, Eternal University, India
Copyright © 2024 Mwampashi, Magubika, Ringo, Theonest, Tryphone, Chilagane and Nassary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lenganji Lackson Mwampashi, bGVuZ2Fuamltd2FtcGFzaGlAZ21haWwuY29t
 Lenganji Lackson Mwampashi
Lenganji Lackson Mwampashi Aneth Japhet Magubika
Aneth Japhet Magubika Job Frank Ringo
Job Frank Ringo Dickson J. Theonest1
Dickson J. Theonest1 Eliakira Kisetu Nassary
Eliakira Kisetu Nassary