- 1Department of Forest Sciences, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland
- 2Collaborative Innovation Center of Sustainable Forestry in Southern China, College of Forestry, Nanjing Forestry University, Nanjing, China
Heterobasidion annosum species complex has been regarded as the most destructive disease agent of conifer trees in boreal forests. Tree microbiome can regulate the plant–pathogen interactions by influencing both host resistance and pathogen virulence. Such information would help to improve the future health of forests and explore strategies to enhance ecosystem stability. In this study, using next-generation sequencing technology, we investigated the microbial community in different tree regions (needles, upper stem, and lower stem) of Norway spruce with and without wood decay symptoms. The primary purpose was to uncover signature characteristic microbiome harbored by asymptomatic trees compared to diseased trees. Additionally, the study was to explore the inter-kingdom and intra-kingdom interactions in microbiome (bacteria and fungi) of symptomatic versus asymptomatic trees. The results showed that in upper stem, species richness (Chao1) of fungi and bacteria were both higher in asymptomatic trees than symptomatic trees (P < 0.05). Compared to symptomatic trees, asymptomatic trees harbored a higher abundance of Actinobacteriota, bacterial genera of Methylocella, Conexibacter, Jatrophihabitans, and fungal genera of Mollisia. Fungal communities from the same anatomic region differed between the symptomatic and asymptomatic trees. Bacterial communities from the two stem regions were also distinct between the symptomatic and asymptomatic trees. The symptomatic trees possessed a less stable microbial network with more positive correlations compared to the asymptomatic trees. In the lower stem, at intra-kingdom level, the distribution of correlation numbers was more even in the bacterial network compared to the fungal network. In conclusion, the Heterobasidion attack decreased the microbial community species richness and shifted the community structure and functional structure to varying degrees. The microbial network was enlarged and became more unstable at both inter-kingdom and intra-kingdom level due to the Heterobasidion infection.
Introduction
Forest ecosystems act as one of the most powerful regulators in global climate change yet remain exposed to increasing biotic and abiotic challenges nowadays, including the carbon dioxide increase, warming, drought, and disease attack (Baldrian et al., 2023). In response to these problems, with the mediation of microbiome associations, forest ecosystems could therefore adapt to new environments (Addison et al., 2024). Microbes are ubiquitous and mainly resident in the phyllosphere, rhizosphere, and the internal tissues of plants (Xiong et al., 2023). As the communities associated intimately with plants, microbiome has the potential to act beneficially, commensally, and pathogenically on their host (Chialva et al., 2022).
The complex plant–microbiome interactions provide advantages to the plant host by promoting plant growth, assisting in nutrient uptake, increasing stress tolerance and resisting plant pathogens (Trivedi et al., 2020). In the field of resistance biology, plant microbiome potentially regulates the plant’s immune system and influence the pathogenic colonization as well as pathogenicity, ultimately modulate the whole plant–pathogen interaction (Xiong et al., 2023). Phyllosphere microbiome employs various strategies including competitive exclusion, antibiosis, induction of host immune response to exercise control over the pathogens (Bashir et al., 2022). For example, the phyllosphere bacterium Methylobacterium was shown to facilitate IAA mediated defense response against rot pathogens Aspergillus niger in groundnut (Arachis hypogaea) (Madhaiyan et al., 2006). Endophytes, as an important component of tree microbiome, were reported to suppress pathogens by directly competing with them for resources or by indirectly producing metabolites as antimicrobials (e.g., flavonoids, alkaloids, phenols, and peptides; Pańka et al., 2013; Pavithra et al., 2019). Furthermore, endophytes enhance plant growth through the biosynthesis of advantageous secondary metabolites, such as phytohormones, and by facilitating nutrient acquisition, including essential elements like phosphorus (Terhonen et al., 2018). Another example of microbiome facilitating in the suppression of pathogenic colonization is that the Ampelomyces spp. acting as intracellular parasites, could suppress the sporulation of powdery mildew fungi and kill the host fungal cells (Kiss, 2003). Some endophytic bacteria exhibit the ability to promote the growth of the plant by nitrogen fixation or plant hormone production, for example, Azospirillum, Bacillus subtilis, and Burkholderia vietnamiensis (Steenhoudt and Vanderleyden, 2000; Xin et al., 2009; Hashem et al., 2019), mediating the plant–pathogen interaction in an indirect way.
On the other hand, plant-associated microbial community assembly could be shaped by various factors during the host–pathogen interaction. The leaf epiphytic bacterial species richness was found to decrease when southern leaf blight disease severity increased (Manching et al., 2014). The endophytic bacterial community structures also shifted in response to methyl bacterium-induced protection of plants against pathogens (Ardanov et al., 2012).
The root and butt rot on conifer trees caused by Heterobasidion annosum species complex was considered the most devastating fungal diseases in the northern hemisphere (Asiegbu et al., 2005; Garbelotto and Gonthier, 2013). In Europe, the annual financial loss caused by the species complex was estimated around 800 million euros due to the decay as well as overall reduction in the diameter growth of diseased trees (Asiegbu et al., 2005; Hellgren and Stenlid, 1995; Zeng et al., 2018). Distinct host preference was shown among the three Eurasian intersterile groups of H. annosum species complex, H. annosum s.s. usually attacks pines (Pinus spp.), while Heterobasidion parviporum preferentially infects Norway spruce [Picea abies (L.) Karst.], and Heterobasidion abietinum is commonly associated with silver fir (Abies alba Mill.) (Garbelotto and Gonthier, 2013). H. annosum s.l. primarily uses the basidiospores to infect freshly open wood surfaces and then spread to neighboring hosts by root to root contact (Zaļuma et al., 2019). For decades, the Heterobasidion root rot has been managed by use of the fungal biocontrol agent Phlebiopsis gigantea (Fr.) Julichto which is able to outcompete and out-grow Heterobasidion on stump surfaces (Pratt et al., 2000; Kärhä et al., 2018). Although fewer bacterial biocontrol cases against forest fungal pathogens were reported than those against crop fungal pathogens (Singh et al., 2020), bacteria isolated from healthy Pinus radiata rhizosphere were reported to inhibit the growth of H. annosum in vitro and also protect P. radiata seedlings (Mesanza et al., 2016). Apart from these antagonistic screening studies, with the help of next-generation sequencing, recent studies have focused on the host microbial communities during Heterobasidion infection to further explore the complicated interactions among the microbiome, pathogen, and host. The fungal and bacterial community associated with different anatomic regions (root, bark, down stem, upper stem, and needles) of Norway spruce trees with and without wood decay symptoms were compared respectively to understand the impact of Heterobasidion infection (Kovalchuk et al., 2018; Ren et al., 2019). The microbial community inhabiting Heterobasidion fruiting body and adhering dead wood at different decay stages were also studied, revealing the infection process at a “dynamic” level (Ren et al., 2022, 2023).
Though many studies have focused on microbial communities, not much attention is given to the interactions of microorganisms within the community in situ. However, more evidence showed that priority should be given to microbial network shifts because it could unravel their functions and vulnerability to various disturbances (de Vries et al., 2018). Analyzing microbial networks and identifying core microbes are crucial for understanding the factors that contribute to microbial resilience when subjected to biotic or abiotic disturbances (Astudillo-García et al., 2017; Lemanceau et al., 2017). This analysis also provides opportunity to find promising antagonistic agents against Heterobasidion, since competing relationships with the fungal pathogen could be indicated by the negative correlations between them (Ren et al., 2023). Using co-occurrence microbial network analysis, conclusions were drawn that showed that compared to soil fungal networks, soil bacterial networks represented less stability under drought and linked more tightly to soil functioning during recovery (de Vries et al., 2018). Another study revealed that drought disrupted microbial networks both among bacteria, among fungi, and between bacteria and fungi significantly (Gao et al., 2022).
In this study, a combined taxonomic analysis and network analysis of the phyllosphere and endospheric microbiomes from different regions of Norway spruce trees were conducted. The objectives were to (i) uncover signature characteristic microbiome harbored by asymptomatic trees compared to diseased trees and to (ii) explore the network interaction in microbial community of asymptomatic versus symptomatic Heterobasidion decayed trees.
Materials and methods
Study sites and sample collection
The sampling site was a Norway spruce [P. abies (L.) Karst.] dominated forest with a relatively high incidence of Heterobasidion infection in Myrskylä (Uusimaa region, Southern Finland). The spruce forest is privately owned and targeted for commercial timber production. The spruce trees at the designated locations had undergone natural regeneration and were of the same age around 62 years.
In June 2023, sample collection was carried on trees which were cut down during harvesting. In this way, decayed trees could be distinguished from healthy ones based on the brown stains which represented wood decay symptoms on the stump surfaces (Figure 1). Finally, we sampled 10 decayed trees and 5 healthy trees from the four plots which were located very close to each other: (1) 60°40′04.47″N, 25°45′49.62″E, (2) 60°40′02.78″N, 25°45′49.25″E, (3) 60°40′02.94″N, 25°45′49.07″E, (4) 60°40′05.45″N, 25°45′50.34″E. Needles and stems at both stump base height and breast height from each sampled tree were collected.

Figure 1. The lower stems (stems at stump base height) (a), upper stems (stems at breast height) (c), needles (e) from symptomatic Norway spruce and the lower stems (b), upper stems (d), needles (f) from asymptomatic Norway spruce.
Once collected, the samples from each sample tree were shipped with ice pack and stored at −20°C for later laboratory processing. For the group names described further in the text and figures, the first letter S and A stand for symptomatic trees and asymptomatic trees, respectively. The second letter L, U, and N stands for lower stems (stems at stump base height), upper stems (stems at breast height), and needles, respectively.
DNA extraction, amplification of fungal ITS2, bacterial V3–V4 region, and sequencing
Due to the enormous size of each stem, we firstly cut a wood piece which contained heartwood, sapwood and bark from the stem and then split this piece into small cubes with aid of sterilized tools. The wood cubes from the same stem were then homogenized using mixer mill MM400 (Retsch, Germany) following the manufacturer’s instruction. Needles were homogenized manually with mortars and pestles. The standard cetyltrimethylammonium bromide (CTAB) method (Chang et al., 1993) with modifications (Terhonen et al., 2011) was followed to extract the DNA from samples. NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, USA) was used to measure the quality and concentration of the isolated DNA.
PCR amplification of the fungal ITS2 region and bacterial V3–V4 region and sequencing were performed at Novogene (Cambridge Science Park, UK). The fungal and bacterial amplification was performed using primers ITS3-2024F (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4-2409R (5′-TCCTCCGCTTATTGATATGC-3′) with primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′), respectively. Raw sequences are available from the Sequence Read Archive (SRA) on National Center for Biotechnology Information (NCBI) under project number PRJNA1107117 for bacteria and under project number PRJNA1107041 for fungi.
Sequence processing and data analyses
Paired-end reads were first assigned to samples and truncated by cutting off their unique barcode and primer sequences. Raw tags were generated after merging the paired-end reads using FLASH (V1.2.1.111) (Magoč and Salzberg, 2011). Quality filtering on the raw tags went on using the fastp (Version 0.23.1) software to obtain high-quality clean tags (Bokulich et al., 2013). Based on reference database [Silva database (16S/18S)2 ; Unite database (ITS)3), chimera sequences were detected and removed with the vsearch package (V2.16.04) (Edgar et al., 2011), therefore generated the effective tags. Then denoise was performed with DADA2 in the QIIME2 software (Version QIIME2-202202) to obtain initial ASVs (Amplicon Sequence Variants) which were annotated to species afterward in the same software using Silva database for 16S/18S sequences or United database for ITS sequences. The absolute abundance of ASVs was normalized using a standard of sequence number corresponding to the sample with the least sequences. Subsequent analysis of alpha diversity was performed based on the output of normalized data. Three alpha diversity indices including Chao1, Simpson’s Index of Diversity (1-D), and Pielou’s evenness were selected to reflect the microbial community species richness, microbial community diversity, and microbial community evenness, respectively. In SPSS v26.0 (Chicago, IL, USA), one-way analysis of variance (ANOVA) was adopted to examine the significant differences of the alpha diversity indices while independent-samples T-test was used to examine the significant differences of various parameters between asymptomatic trees and symptomatic trees. GraphPad Prism 8 was adopted to generate the figures.
We performed the network analysis using ASVs with relative abundance over 0.1% (Ren et al., 2023). The Spearman correlations with correlation value over 0.8 and P-value lower than 0.05 between ASVs were calculated and filtered in R. The keystone ASVs were further selected with the top 10% abundance in the network to do the keystone analysis. Then the visualization was done by Gephi (Version 0.9.2) based on the correlation results. The degree distribution and number of edges were calculated in Gephi and visualized further in R.
Results
Microbial community alpha diversity
In upper stems, the species richness of both bacterial community and fungal community was higher in asymptomatic trees than symptomatic trees (P < 0.05) (Figures 2a, d). Additionally, the highest species richness of bacterial community from asymptomatic trees was observed in upper stems compared to needles and lower stems (P < 0.05) (Figure 2a). The highest species richness of fungal community from symptomatic trees was observed in needles, while the fungal community in upper stem and needles from asymptomatic trees exhibited higher species richness than the lower stem (P < 0.05) (Figure 2d). No significant difference of Simpson’s diversity or Pielou’s evenness was found between symptomatic trees and asymptomatic trees (Figures 2b, c, e, f).
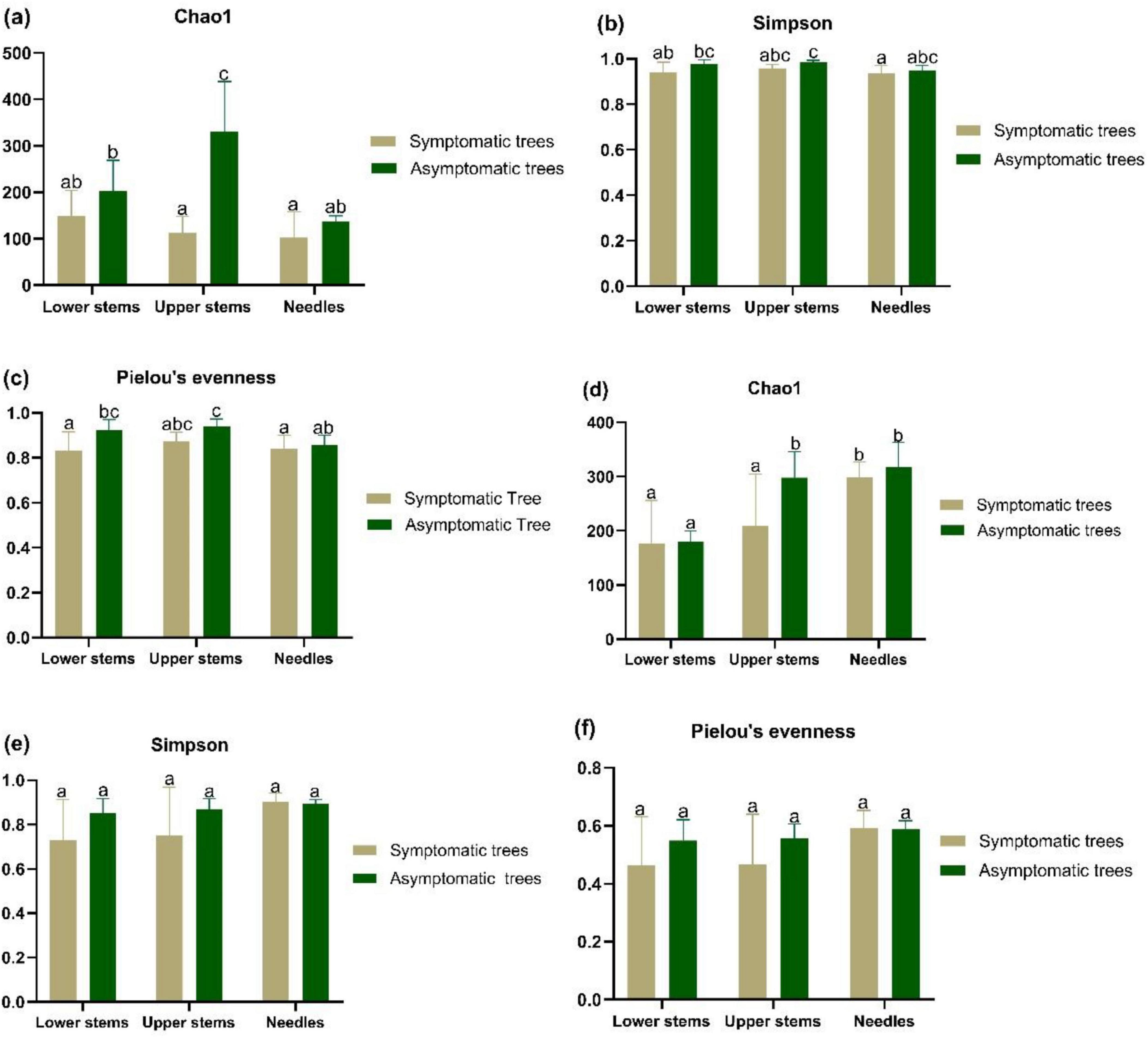
Figure 2. The alpha diversity bacterial community (a–c) and fungal community (d–f) in symptomatic and asymptomatic trees. Bacterial community species richness (Chao1) (a), diversity (Simpson’s diversity) (b), and evenness (Pielou’s evenness) (c) of community. Fungal community species richness (Chao1) (d), diversity (Simpson’s diversity) (e), and evenness (Pielou’s evenness) (f). Letters above the column represent the significant difference (P < 0.05) among groups.
Microbial community composition and structure
Proteobacteria, Acidobacteriota, and Actinobacteriota emerged as the predominant bacterial phyla across all phyla (Figure 3a). Additionally, symptomatic trees exhibited a higher abundance of Proteobacteria and a lower abundance of Actinobacteriota compared to asymptomatic trees (Figure 3a). At bacterial genus level, Endobacter, Bryocella were more abundant in the symptomatic trees while Methylocella, Conexibacter, and Jatrophihabitans were more abundant in the asymptomatic trees (Figure 3b). Ascomycota and Basidiomycota dominated fungal community, and symptomatic trees exhibited higher abundance of Basidiomycota especially in stem regions while asymptomatic trees had more abundant Ascomycota in upper stem and needles (Figure 3c). Genus Heterobasidion was found to be prevalent in symptomatic lower stem (32.6%) and symptomatic upper stem (34.2%), and also present rather infrequently in symptomatic needles (0.07%), asymptomatic lower stem (0.002%), and asymptomatic upper stem (0.004%) (Figure 3d). Mollisia showed more frequently in asymptomatic trees while Fuscidea and Capturomyces exhibited higher abundance along with Heterobasidion in symptomatic trees (Figure 3d).
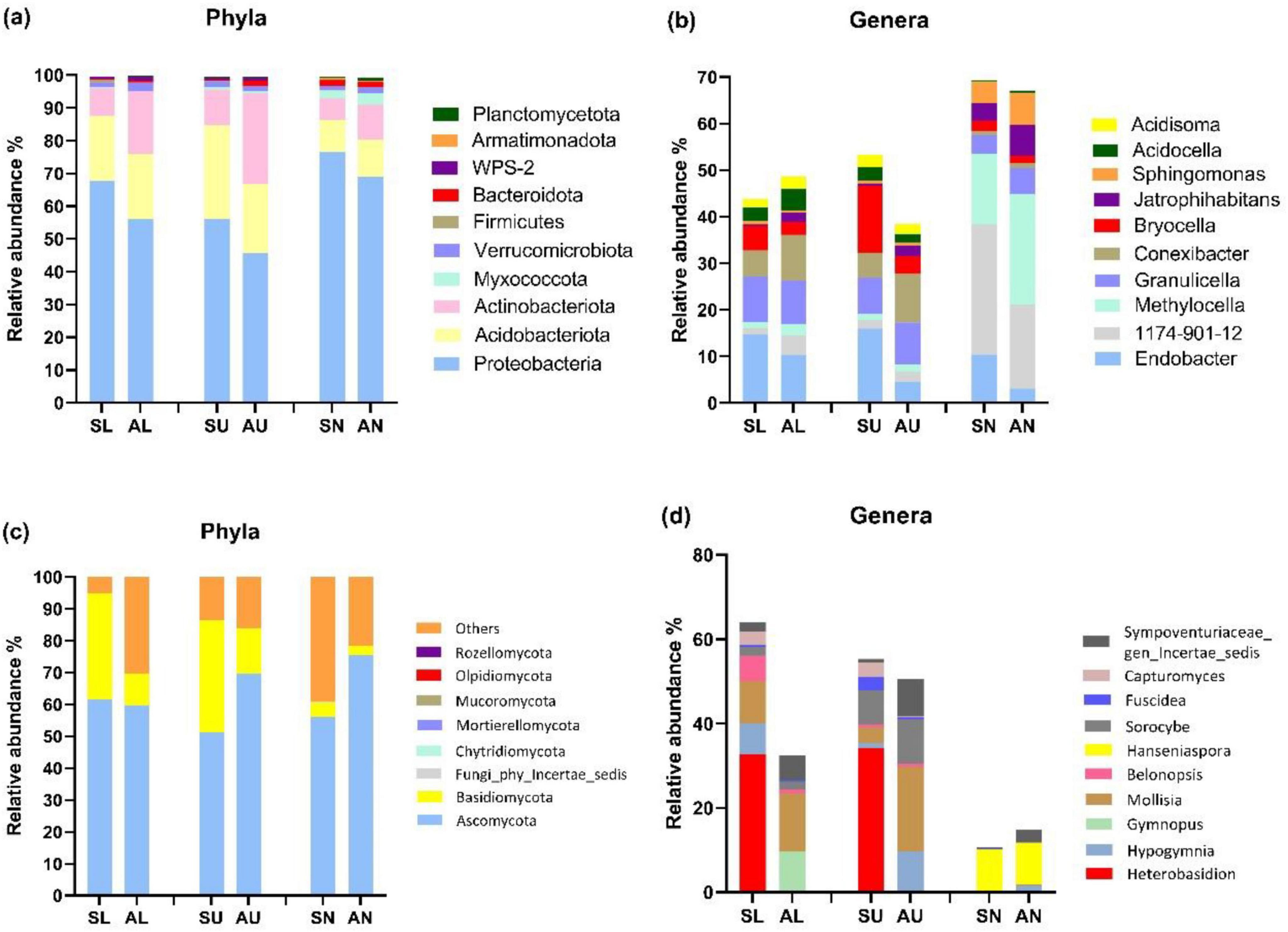
Figure 3. The top 10 most abundant microbial taxa at phylum and genus level in the three regions from symptomatic and asymptomatic Picea abies tree. The bacterial phyla (a), bacterial genera (b), fungal phyla (c), and fungal genera (d). The first letter S and A stand for symptomatic trees and asymptomatic trees, respectively, while the second letter L, U, and N stand for lower stems (stems at stump base height), upper stems (stems at breast height), and needles.
Based on T-test, the bacterial species Actinomycetales bacterium and Acidobacteria bacterium CU2 were more abundant in the upper stem of asymptomatic trees than those of symptomatic trees (P < 0.05) (Table 1). Fungal species Cyphellophora sessilis and Fellomyces horovitziae were more abundant in the needles from asymptomatic trees than those from symptomatic trees (P < 0.05) (Table 1).
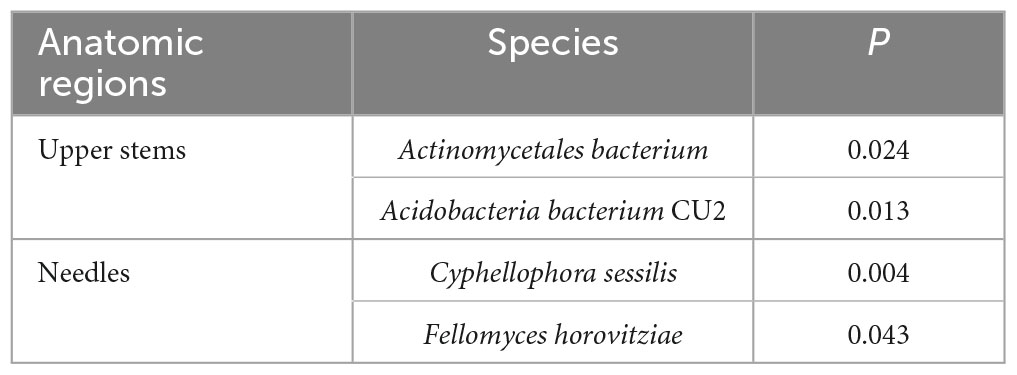
Table 1. The microbial species among the top 50 most abundant fungal and bacterial species showing significant differences in higher relative abundance between asymptomatic and symptomatic trees based on T-test.
The principal co-ordinate analysis (PCoA) and subsequent permutational multivariate analysis of variance (PERMANOVA) revealed that the bacterial community in upper stems and lower stems differed between symptomatic trees and asymptomatic trees, respectively (P < 0.05) (Figure 4a). The fungal communities in the same region were distinct between symptomatic and asymptomatic trees (P < 0.05) (Figure 4b). The lower stem formed distinct bacterial community with the other two regions in both symptomatic and asymptomatic trees (P < 0.05) (Figure 4a). In symptomatic trees, the needles form distinct fungal community with the other two regions (P < 0.05) while each region from asymptomatic trees harbored distinct fungal community (P < 0.05) (Figure 4b).
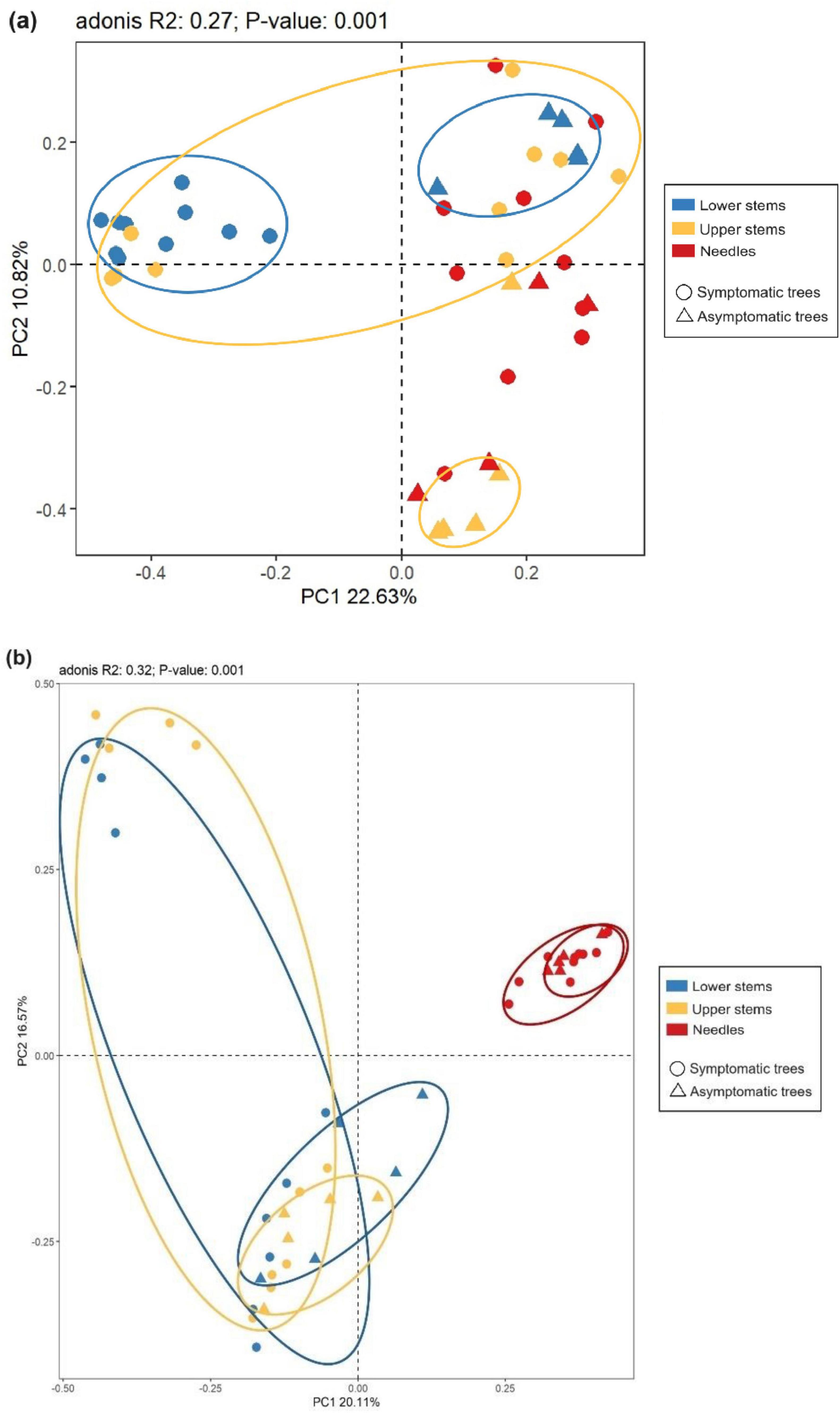
Figure 4. Principal co-ordinates analysis (PCoA) illustrates microbial community structures. Bacterial community structures (a) and fungal community structures (b). The colorful eclipses represent the separation of microbial community from the same region between symptomatic and asymptomatic trees.
Predicted microbial community functional structures
Chemoheterotroph (15.55%) was the most abundant bacterial functional modes in all samples, followed by aerobic chemoheterotroph (11.81%), nitrogen fixation (4.11%), hydrocarbon degradation (3.69%), and methylotrophy (3.68%) (Figure 5a). The bacterial trophic mode of intracellular parasites in needles from symptomatic trees outnumbered that from asymptomatic trees (P < 0.05) (Figure 5a). The composition of bacterial functional modes was quite similar in the stem regions, while the needles seemed to have a different composition pattern which was more even (Figure 5a). Saprotroph (30.59%) dominated in the fungal trophic modes, followed by symbiotroph (8.87%), pathotroph-symbiotroph (7.05%), and pathotroph (2.28%) (Figure 5a). The abundance of saprotroph mode was more abundant in the lower and upper stems from symptomatic trees than that from asymptomatic trees (P < 0.05) (Figure 5b).
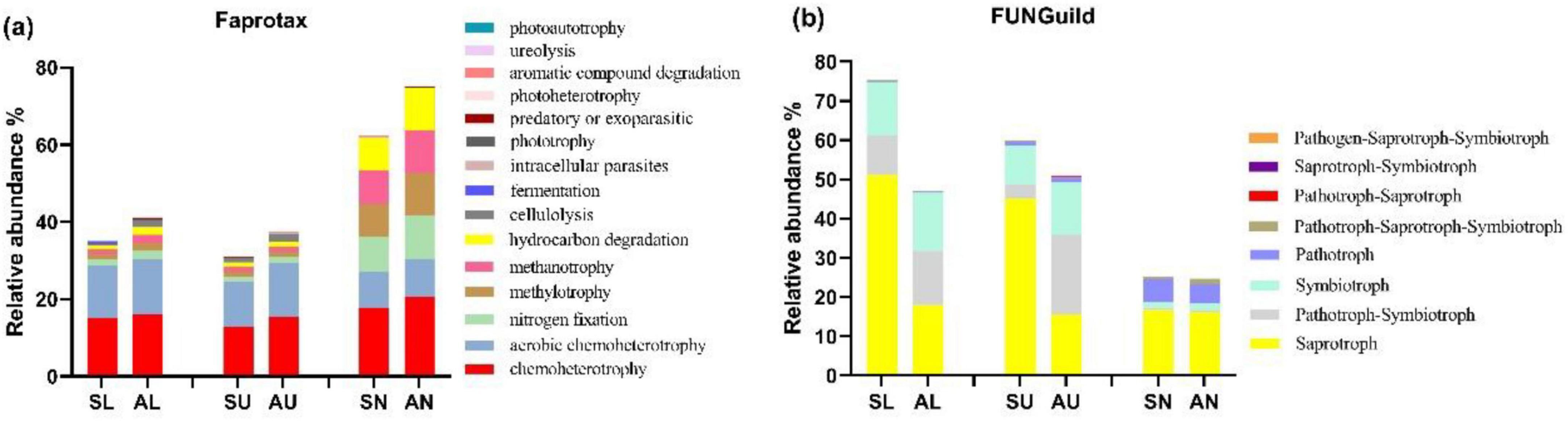
Figure 5. The relative abundance of the top 15 microbial trophic modes based on faprotax analysis for bacteria and FUNGuild analysis for fungi. Bacterial trophic modes (a) based on faprotax analysis, and the fungal trophic modes (b) based on FUNGuild analysis, respectively. For the group names, the first letter S and A stand for symptomatic trees and asymptomatic trees, respectively, while the second letter L, U, and N stand for lower stems (stems at stump base height), upper stems (stems at breast height), and needles.
Functionally, symptomatic trees formed distinct bacterial community with asymptomatic trees in lower stem, and distinct fungal community with asymptomatic trees in upper stem, respectively (P < 0.05) (Figures 6a, b). On symptomatic trees, bacterial functional community in lower stem separated with other two regions (P < 0.05) while each region from asymptomatic trees formed their own bacterial functional community structure (P < 0.05) (Figure 6a). As to fungal functional community, both symptomatic trees and asymptomatic trees, needles formed distinct structures with the other two regions (P < 0.05) (Figure 6b).
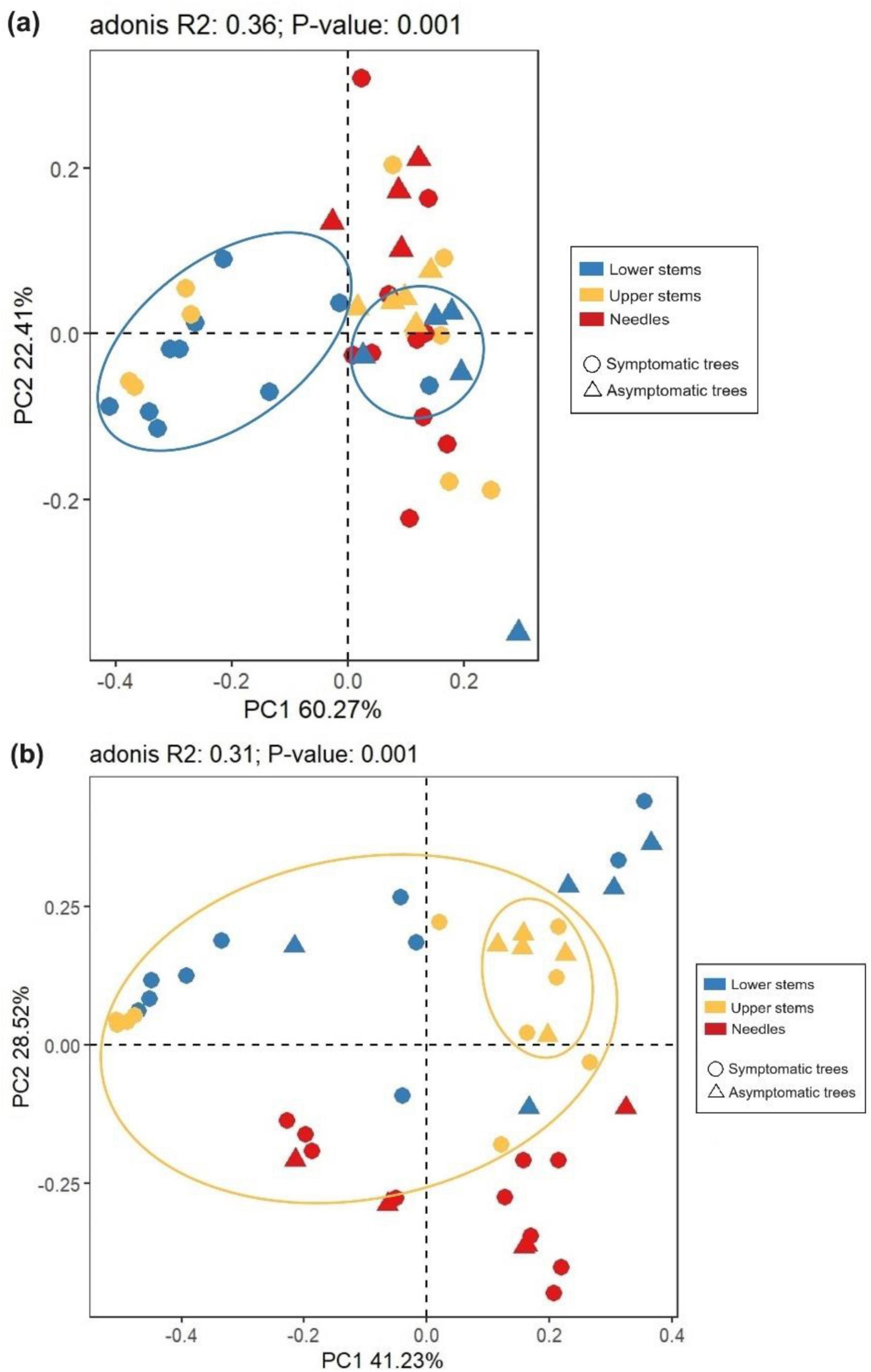
Figure 6. Principal co-ordinates analysis (PCoA) illustrating the microbial community functional structures. Bacterial community functional structures (a) and fungal community functional structures (b). The colorful eclipses represent the separation of microbial community from the same region between symptomatic and asymptomatic trees.
Microbial inter-kingdom and intra-kingdom network analysis
Except for the bacterial ASVs in needles, more microbial ASVs were present in the symptomatic trees than in the asymptomatic trees (Figure 7a). In total, a higher number of edges were observed in each region from symptomatic trees than from asymptomatic trees (Figures 7a, b). The two stem regions possessed higher numbers of ASVs and edges than the needles (Figures 7a, b). Positive edges (77.8%) were more abundant than negative edges in total (22.2%) (Figures 7a, b), however, higher portion of edges were assigned to positive group in the symptomatic trees compared to the asymptomatic trees (Figure 7b). For the intra-kingdom and inter-kingdom correlations, the fungi-fungi edges accounted for the lowest proportion in all regions, while fungi-bacteria edges accounted for the highest proportion in the needle region and bacteria-bacteria edges remained the most in both lower stem and upper stem regions (Figure 7b).
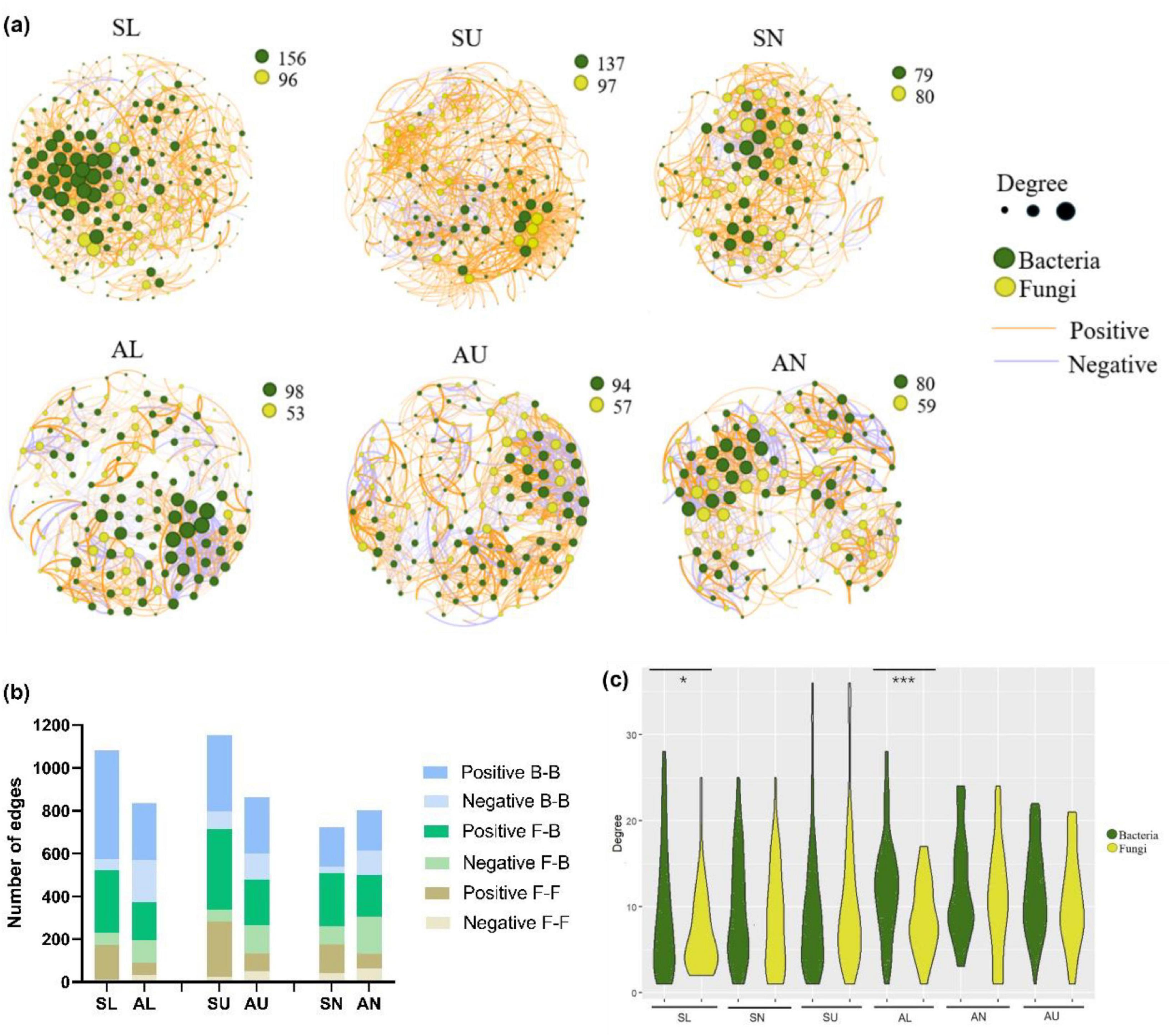
Figure 7. Microbial network analysis in symptomatic trees and asymptomatic trees. Microbial co-occurring network in symptomatic trees and asymptomatic trees (a). Each node represents a bacterial/fungal ASV in the network. The degree stands for the connection amount with a particular node, which is indicated by the node size. The thickness of the edge between two nodes represents their correlation level. The number of positive and negative edges within inter-kingdom and intra-kingdom network (b). F-F, fungi to fungi; F-B, fungi to bacteria; B-B, bacteria to bacteria. The distribution of degrees within bacterial and fungal nodes (c). The symbols ‘*’ and ‘***’ represent a significance difference (P < 0.05) and a significance difference (P < 0.001) respectively.
As to the distribution of degrees, bacterial ASVs appeared to exhibit more even degree pattern than fungal ASVs from the lower stem in both symptomatic trees and asymptomatic trees (P < 0.05) (Figure 7c).
The negative correlations related to Heterobasidion ASVs occupied a higher ratio than the positive correlations in the two stem regions (Figures 8a, b), while only one positive correlation was formed with Heterobasidion ASVs in the needles (Figure 8c). Higher percentage of negative edges were built with bacterial ASVs in the lower stem, around 66.67% (Figure 8a). However, a higher percentage of negative edges were built with fungal ASVs in the upper stem, around 94.74% (Figure 8b). The upper stem harbored the largest microbial network connected to Heterobasidion ASVs among the three regions (Figures 8a–c).
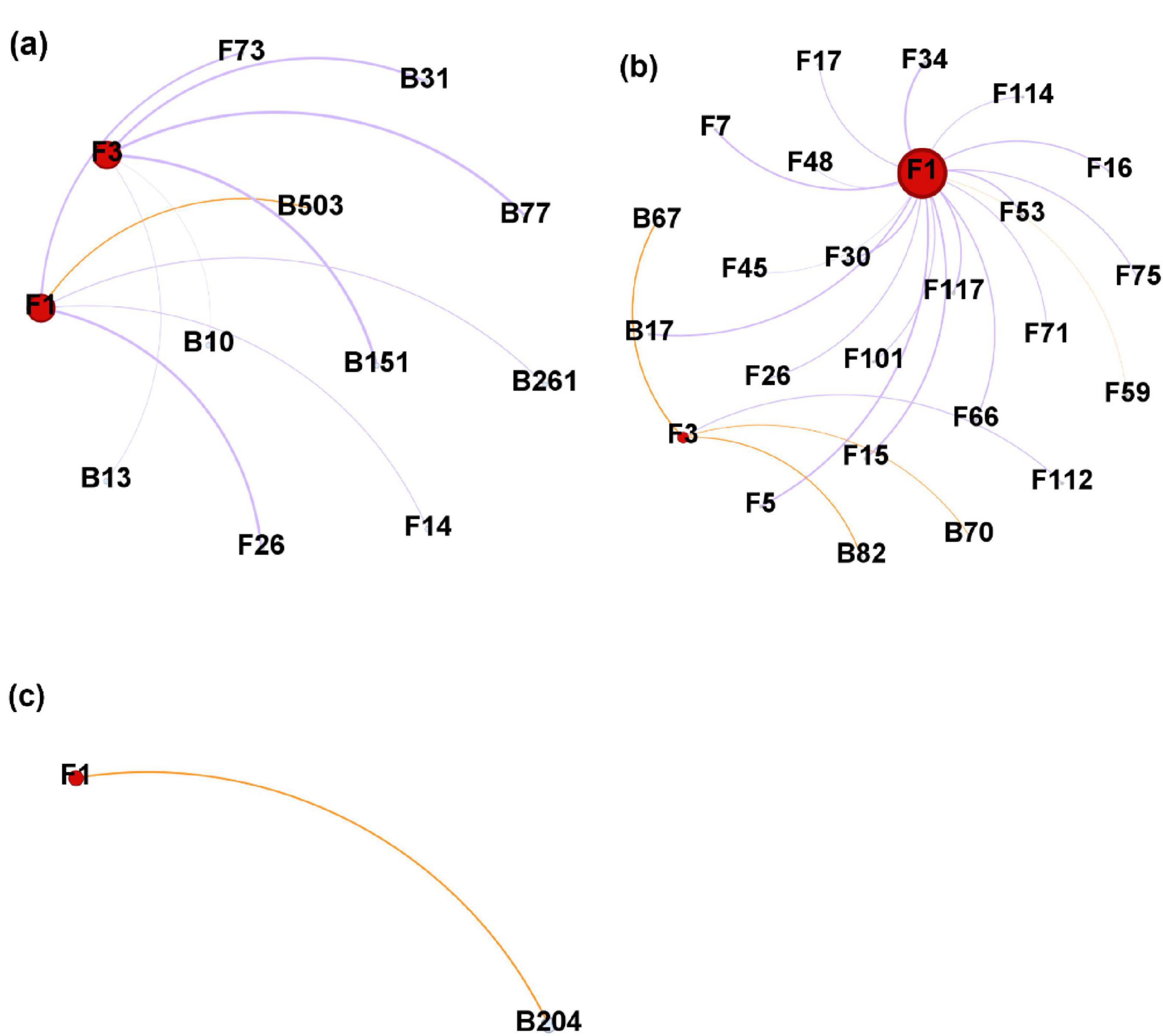
Figure 8. Microbial network generated from Heterobasidion ASVs and their connected ASVs in lower stems (a), upper stems (b), and needles (c) from symptom trees. B, bacterial ASV; F, fungal ASV. Orange edges present positive correlations, purple edges represent negative correlations. F1 and F3 are H. parviporum ASVs.
Based on the network analysis, potential antagonistic fungal agents were proposed by the negative correlated ASVs (Table 2). One fungal ASV Scoliciosporum umbrinum was from the lower stem, while other three fungal ASVs including Hypogymnia physodes, Fuscidea pusilla, and Lecidea nylanderi came from the upper stem (Table 2).
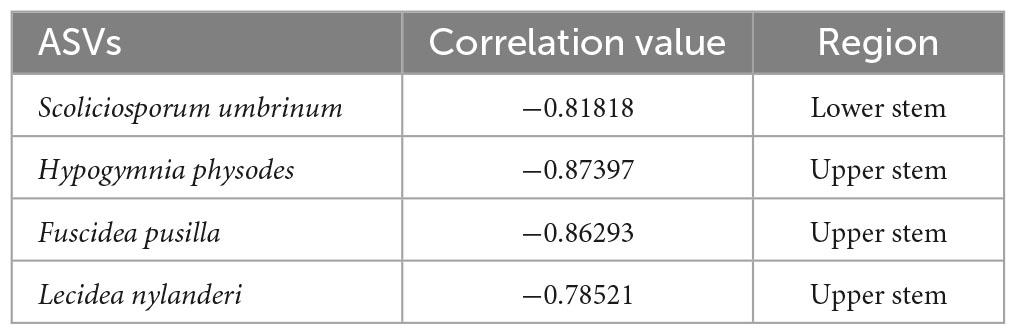
Table 2. The ASVs among the top 10% most abundant ASV within the network showing negative correlation with H. parviporum.
Discussion
It is almost 150 years ago when the first book linking root and butt rot disease of conifers to H. annosum was published by Hartig (1874). Despite the length of time that has elapsed, this disease is still one of the most severe threats to conifer trees especially in Europe (Asiegbu et al., 2005). Consequently, it is still crucial for forest pathologists to keep on conducting and exploring research approaches for the management of the disease with the aid of updated modern tools and techniques. In this study, we investigated the microbial community in different anatomic regions of Norway spruce with and without wood decay symptoms to better understand the role of microbiomes in the tree–pathogen interaction.
Both bacterial and fungal community in the upper stem from the asymptomatic trees harbored higher species richness (Chao1) than those from the symptomatic trees. Other authors observed that that leaf bacterial alpha diversity (Chao1) was lower in pathogen-inoculated maize plots than those without pathogen inoculation (Manching et al., 2014). Soil bacterial species richness (Chao1) was reduced with the increasing severity of bacterial wilt disease caused by Ralstonia (Yang et al., 2017). Fungal alpha diversity of healthy tobacco leaves was also higher than that of diseased leaves (Sun et al., 2023). High proportion of Heterobasidion was found to be prevalent in symptomatic lower stem (32.6%) and symptomatic upper stem (34.2%), and also rather infrequently in symptomatic needles (0.07%), asymptomatic lower stem (0.002%), and asymptomatic upper stem (0.004%). With respect to the prevalence of Heterobasidion spp. in stem regions, it is quite normal because Heterobasidion spreads through root to root contact and up the tree trunk. Obvious decay symptoms would manifest in both lower stems and upper stems depending on the stage of the infection (Asiegbu et al., 2005). Additionally, part of the reason why there was more Heterobasidion in upper stem than lower stem was that in highly decayed lower stem, the host defense system may have been severely weakened consequently making it possible for other saprotrophic fungi to colonize leading to successional changes (Kovalchuk et al., 2018). The presence of Heterobasidion in the needles could be due to aerial spores within the vicinity of the sampled trees.
With respect to variations in the bacteria biota of the sample trees, the bacterial community in the lower stem was more even in the asymptomatic trees than that from symptomatic trees, indicating possible interference of Heterobasidion infection on the bacterial community. Other studies have reported that bacterial leaf blight increased the alpha diversity of bacterial community in rice leaves (Yang et al., 2020). By contrast, no significant change in microbial species richness was induced by the infection of Septoria glycines and Phytophthora sojae (Díaz-Cruz and Cassone, 2022). These results were also expected since the incidence of disease is likely to lead to weakness of host plants thereby increasing the chance for the colonization by other microorganisms (Yang et al., 2020).
Compared to symptomatic trees, asymptomatic trees exhibited a higher abundance of Actinobacteriota phylum which is well known for their outstanding ability to secret antibiotic compounds (Saxena, 2014; Boukhatem et al., 2022). It is most probable that symptomatic trees have reduced ability to harbor many of the antibiotic producing isolates that could have contributed in suppressing Heterobasidion infection. At the genus level, Methylocella, Conexibacter, and Jatrophihabitans were more abundant in asymptomatic trees. Among these genera, the genus Methylocella occupied a considerable proportion (17.69%) in the needles and belonged to the facultatively methanotrophic bacteria (Dedysh et al., 2005). However, in contrast to previous reports, a frequently low appearance of methanotrophs (below 1%) was detected in the phyllosphere bacterial community using metagenomic approaches (Hiroyuki et al., 2015). This genus is able to utilize multi-carbon compounds including acetate, pyruvate, succinate, etc. (Dedysh et al., 2005). The possible difference in abundance of these isolates between symptomatic needles and asymptomatic needles may suggest that the biosynthesis of these compounds was suppressed in Heterobasidion infected Norway spruce. The endophytic bacterial genus Conexibacter (Li et al., 2023), was also reported to be less abundant in Huanglongbing-infested rhizosphere of Citrus than healthy rhizosphere and correlated positively with the available potassium, available phosphorus and organic matter (Lei et al., 2024). It may be inferred that the Heterobasidion infection may have influenced the microbes leading to decrease in the abundance of this Conexibacter in the stem region. The abundance of Heterobasidion was found to be rather infrequent in needles (0.07%) than that in stem regions from symptomatic trees, indicating that the growth of Heterobasidion could barely reach the needles in the infected Norway spruce. Interestingly, Heterobasidion also showed up in stem regions from asymptomatic trees at a very low percentage (0.002% and 0.004% for lower stem and upper stem, respectively), demonstrating that even without wood decay symptom, Norway spruce trees in the field could have small amount of Heterobasidion inoculums. It is possible that infection is at an early stage or that the trees have partial resistance that delays manifestation of symptoms. It is also possible that the accumulation of this fungi have not reached a suitable amount to cause the decay or the asymptomatic trees were able to restrict the Heterobasidion growth by individual genetic resistance background as well as tree microbiome antagonistic agents (Kovalchuk et al., 2018).
In the two genera which showed more frequently in asymptomatic trees, Mollisia is a genus of frequently encountered saprotrophic fungi found on decaying plant tissues in temperate regions (Tanney and Seifert, 2020). Some endophytic species within this genus were reported to produce antifungal secondary metabolites (Fan et al., 2016; Tanney and Seifert, 2020). Furthermore, we could not explain the higher abundance of Endobacter and Bryocella in symptomatic than in asymptomatic trees. However, many Endobacteria have been found associated with ectomycorrhizal fungi whereas many members of Bryocella are commonly isolated from forest soil and methanotrophic environments (Bertaux et al., 2005; García-Fraile et al., 2016). This probably suggests they may have some functional relevance in the ecosystem which deserves to be explored.
Using network analysis, we explored possibility to identify potential biocontrol agents from healthy hosts (Mesanza et al., 2016). Based on T-test, and network analysis, we identified four potential antagonistic agents including fungal species C. sessilis and F. horovitziae, bacterial species A. bacterium and A. bacterium CU2. Among these, A. bacterium belongs to the order Actinomycetales which is known for producing antimicrobial compounds and could be a rich source of antibiotics (Genilloud, 2017; Takahashi and Nakashima, 2018). One Actinomycetales bacteria isolated from a spruce stand was reported to suppress the growth of H. annosum in the coculture experiments (Maier et al., 2004). Acidobacteria are abundant in soil and has potential to become beneficial rhizosphere microbes, but remain difficult to cultivate (Kalam et al., 2020). However, C. sessilis was reported to cause sooty blotch and flyspeck, an economically damaging disease of certain fruit crops, as well as epiphytic colonies on bamboo (Gao et al., 2015). F. horovitziae was reported as a basidiomycetous yeast species isolated from a Xenasmatella basidiocarp (Spaaij et al., 1991).
Between symptomatic trees and asymptomatic trees, bacterial communities differed in the two stem regions while fungal community was distinct in each region. Many studies also reported the endophytic or phyllosphere microbial community shift in response to different diseases (Bulgari et al., 2014; Luo et al., 2019 Yang et al., 2020; Díaz-Cruz and Cassone, 2022; Sun et al., 2023). In the tree–pathogen interaction, Norway spruce trees tend to develop the reaction zone between the decayed heartwood and undecayed sapwood where pH increases to neutrality or alkalinity due to the accumulation of carbonate and phenolic compounds (Shain, 1971; Johansson and Theander, 1974). In addition, inoculation with H. parviporum was found to activate the peroxidases production in Norway spruce trees (Liu M. et al., 2021). The physiochemical response in the stem region of symptomatic Norway spruce trees could explain the variation in microbial community structures. It is interesting that the shift in fungal community structure was observed in the needles. However, even though only two symptomatic trees had yellow needles when the samples were collected. It is possible that defense reactions provoked by the invading Heterobasidion pathogen Norway spruce may have had an influence on the water and nutrient transportation, including producing phenolic compounds in ray parenchyma cells, activating phloem parenchyma cells and forming traumatic resin ducts in the xylem (Krekling et al., 2004), and consequently induce the shift of fungal community in the needles. The bacterial community from the needles, unlike the fungal community, was not distinct between symptomatic and asymptomatic trees which might suggest that fungal communities are more sensitive to various perturbations including plant disease and moisture variation (Kaisermann et al., 2015; Liu L. et al., 2021). The fungal communities in the two stem regions from symptomatic trees did not differ with each other, which is different from the a previous report that each anatomic region formed a distinct fungal community (Kovalchuk et al., 2018). The differences in the observation could be related to variations in the infection level and stages of development of Heterobasidion. Lower stem formed distinct bacterial community in both symptomatic trees and asymptomatic trees, demonstrating that regardless of the Heterobasidion infection, upper stem and needles in Norway spruce shared similar bacterial communities together.
Chemoheterotrophy and aerobic chemoheterotrophy were the top two bacterial functional modes in the stem regions, same with the research in rhizosphere and soil bacterial community (Liu et al., 2022; Sun et al., 2022; Zhang et al., 2023). The dominance of these two modes in the stems indicated that most of the bacteria members were not able to fix carbon and the carbon sources were obtained as energy by the oxidation of organic compounds (Zhang et al., 2018). In needles, nitrogen fixation, methylotrophy, methanotrophy, and hydrocarbon degradation were abundant, demonstrating the ability of bacterial community dwelling in the needles to make use of carbon sources as well as nitrogen. This is likely due to the presence of methanotrophy and nitrogen fixing bacteria in the needles (Fürnkranz et al., 2008; Hiroyuki et al., 2015; Zhu et al., 2023). In the needles, the abundance of intracellular parasitic bacteria in symptomatic Norway spruce trees may have been enhanced by the deteriorating host health status and weakening immune response (Silva and Silva Pestana, 2013). It is however not surprising that the most abundant mode saprotroph was more common in symptomatic trees (Garbelotto and Gonthier, 2013). The invading Heterobasidion could also provide a more favorable living conditions for other saprotrophic fungi (Wen et al., 2019). Between symptomatic and asymptomatic trees, the bacterial functional structures differed only in lower stem. Fungal functional structures differ in upper stem, which is more restrained than microbial community differences. The reason could be that some microbial species share a common trophic mode. It is interesting that each region from symptomatic trees formed distinct bacterial functional structure, indicating that under the Heterobasidion infection, bacteria in different regions of Norway spruce tended to shift to diverse lifestyle modes.
Different from community composition, more complexity could be revealed in resistance of microbial communities by microbial network (Gao et al., 2022). In our study, the network analysis unveiled that the Heterobasidion infection enlarged and complicated the microbial network in the stem regions with more ASVs and correlations, which is in line with the previous study on tobacco rhizosphere and endosphere microbial network with and without wilt disease (Tan et al., 2021). The invasion of pathogens is likely to break the microbalance in the community, create more chances for the colonization of other species and generate more intense relationships (Hu et al., 2020). In addition, a larger proportion of negative correlations was observed in all regions from the asymptomatic trees, demonstrating the higher stability of microbial communities to a deeper level since the microbial competition could dampen cooperative networks and increase stability (Coyte et al., 2015; de Vries et al., 2018). In general, Heterobasidion infection influenced the microbial network at a profound level, by enlarging the network scale and breaking the network stability. Furthermore, it was possible to predict a more balanced microbial network in the needles by the domination of correlations between fungi and bacteria. This is partly because the communication between fungi and bacteria plays a key role in maintaining the balance of microbial community structure (Bamisile et al., 2018; Tan et al., 2021). In the lower stem, the distribution of degrees was more even in bacterial community than fungal community, which is in contrast to the previous study (Ren et al., 2023). The more even bacterial degree distribution was induced by the bacterial ASVs which stayed at higher connections, illustrating that bacterial members rather than fungal members played a decisive role in the network of the lower stem.
To further explore the pattern of network induced by Heterobasidion infection, we visualized the Heterobasidion-centered network. It was obvious that negative correlations dominated in the two stem regions, which clarifies the prevalence of antagonistic relationships built with H. parviporum. The host-associated microorganisms always establish competitive relationships with the pathogen, by nutrient competition, niche occupation, immune regulation, and modulation of virulence factors (Kavita and Howard, 2017; García-Bayona and Comstock, 2018). In addition, the bacteria and fungi played the vital role as antagonistic agents in the lower stem and upper stem, respectively.
The four potential antagonistic agents which were negatively correlated with H. parviporum, however, were four lichen species. H. physodes is a lichenized fungus which can produce secondary compounds with antibacterial activity as well as inhibitory effects on enzymes (Christian et al., 2008; Dymytrova, 2011; Lendemer, 2011; Studzinska-Sroka and Zarabska-Bozjewicz, 2023).
Conclusion
Compared to symptomatic trees, asymptomatic trees harbored microbial community with higher species richness and evenness. Taxonomically, asymptomatic trees exhibited a higher abundance of Actinobacteriota, bacterial genera of Methylocella, Conexibacter, Jatrophihabitans, and fungal genera of Mollisia. A. bacterium and A. bacterium CU2 were identified as potential antagonistic agents. Functionally, chemoheterotrophy and aerobic chemoheterotrophy were the dominant bacterial trophic modes while saprotroph was the dominant fungal trophic mode. Between symptomatic trees and asymptomatic trees, the bacterial community differed in the two stem regions while the fungal community was distinct in each region. At function level, the bacterial community differed in lower stem and fungal community differed in upper stem of symptomatic and asymptomatic trees. Heterobasidion infection enlarged the network scale and decreased the network microbial stability in Norway spruce trees. Negative correlations dominated the Heterobasidion-centered network, where bacterial ASVs and fungal ASVs dominated negative correlations to the lower stem and upper stem, respectively.
Data availability statement
The data sets generated and analyzed during this study are available in the NCBI SRA 621 database number PRJNA1107117 for bacteria and under project number 622 PRJNA1107041 for fungi.
Author contributions
W-JM: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft. Z-LW: Investigation, Writing – review & editing. RK: Investigation, Writing – review & editing. HS: Supervision, Writing – review & editing. FA: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research work was supported by the European Union-Next Generation EU instrument, Research Council of Finland (Grant no. 353365) and Ministry of Agriculture and Forest (Grant no. 4400T-2002). Additionally, China Scholarship Council is acknowledged here for the funding in supporting Wenjing Meng (Student number 202308320045).
Acknowledgments
We are grateful to Abiodun Azeez and Umair Awan for assistance during the field sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ http://ccb.jhu.edu/software/FLASH/
- ^ https://www.arb-silva.de/
- ^ https://unite.ut.ee/
- ^ https://github.com/torognes/vsearch
References
Addison, S. L., Rúa, M. A., Smaill, S. J., Singh, B. K., and Wakelin, S. A. (2024). Partner or perish: Tree microbiomes and climate change. Trends Plant Sci. 29, 1029–1040. doi: 10.1016/j.tplants.2024.03.008
Ardanov, P., Sessitsch, A., Häggman, H., Kozyrovska, N., and Pirttilä, A. M. (2012). Methylobacterium-induced endophyte community changes correspond with protection of plants against pathogen attack. PLoS One 7:e46802. doi: 10.1371/journal.pone.0046802
Asiegbu, F. O., Adomas, A., and Stenlid, J. (2005). Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 6, 395–409. doi: 10.1111/j.1364-3703.2005.00295.x
Astudillo-García, C., Bell, J. J., Webster, N. S., Glasl, B., Jompa, J., Montoya, J. M., et al. (2017). Evaluating the core microbiota in complex communities: A systematic investigation. Environ. Microbiol. 19, 1450–1462. doi: 10.1111/1462-2920.13647
Baldrian, P., López-Mondéjar, R., and Kohout, P. (2023). Forest microbiome and global change. Nat. Rev. Microbiol. 21, 487–501. doi: 10.1038/s41579-023-00876-4
Bamisile, B. S., Dash, C. K., Akutse, K. S., Keppanan, R., and Wang, L. (2018). Fungal endophytes: Beyond herbivore management. Front. Microbiol. 9:544. doi: 10.3389/fmicb.2018.00544
Bashir, I., War, A. F., Rafiq, I., Reshi, Z. A., Rashid, I., and Shouche, Y. S. (2022). Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 254:126888. doi: 10.1016/j.micres.2021.126888
Bertaux, J., Schmid, M., Hutzler, P., Hartmann, A., Garbaye, J., and Frey-Klett, P. (2005). Occurrence and distribution of endobacteria in the plant-associated mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Environ. Microbiol. 7, 1786–1795. doi: 10.1111/j.1462-2920.2005.00867.x
Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., et al. (2013). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Boukhatem, Z. F., Merabet, C., and Tsaki, H. (2022). Plant growth promoting actinobacteria, the most promising candidates as bioinoculants? Front. Agron. 4:849911. doi: 10.3389/fagro.2022.849911
Bulgari, D., Casati, P., Quaglino, F., and Bianco, P. A. (2014). Endophytic bacterial community of grapevine leaves influenced by sampling date and phytoplasma infection process. BMC Microbiol. 14:198. doi: 10.1186/1471-2180-14-198
Chang, S., Puryear, J., and Cairney, J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. doi: 10.1007/BF02670468
Chialva, M., Lanfranco, L., and Bonfante, P. (2022). The plant microbiota: Composition, functions, and engineering. Curr. Opin. Biotechnol. 73, 135–142. doi: 10.1016/j.copbio.2021.07.003
Christian, P., Toby, S., and Tor, T. (2008). Myochroidea, a new genus of corticolous, crustose lichens to accommodate the Lecidea leprosula group [WWW Document]. Available at: https://www.cambridge.org/core/journals/lichenologist/article/abs/myochroidea-a-new-genus-of-corticolous-crustose-lichens-to-accommodate-the-lecidea-leprosula-group/E0DC38E3DB6C2AD2B8A99D892D3F3388 (accessed January 8, 2024).
Coyte, K. Z., Schluter, J., and Foster, K. R. (2015). The ecology of the microbiome: Networks, competition, and stability. Science 350, 663–666. doi: 10.1126/science.aad2602
de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., and Gweon, H. S. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9:3033. doi: 10.1038/s41467-018-05516-7
Dedysh, S. N., Knief, C., and Dunfield, P. F. (2005). Methylocella species are facultatively methanotrophic. J. Bacteriol. 187, 4665–4670. doi: 10.1128/jb.187.13.4665-4670.2005
Díaz-Cruz, G. A., and Cassone, B. J. (2022). Changes in the phyllosphere and rhizosphere microbial communities of soybean in the presence of pathogens. FEMS Microbiol. Ecol. 98:fiac022. doi: 10.1093/femsec/fiac022
Dymytrova, L. (2011). Notes of the genus Scoliciosporum (Lecanorales, Ascomycota) in Ukraine. Polish Bot. J. 56, 61–75.
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Fan, N. W., Chang, H. S., Cheng, M. J., Chan, H. Y., Hsieh, S. Y., Liu, T. W., et al. (2016). New metabolites from the endophytic fungus Mollisia sp. Chem. Nat. Compd. 52, 585–590. doi: 10.1007/s10600-016-1718-0
Fürnkranz, M., Wanek, W., Richter, A., Abell, G., Rasche, F., and Sessitsch, A. (2008). Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2, 561–570. doi: 10.1038/ismej.2008.14
Gao, C., Xu, L., Montoya, L., Madera, M., Hollingsworth, J., Chen, L., et al. (2022). Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 13:3867. doi: 10.1038/s41467-022-31343-y
Gao, L., Ma, Y., Zhao, W., Wei, Z., Gleason, M. L., Chen, H., et al. (2015). Three new species of cyphellophora (Chaetothyriales) associated with sooty blotch and flyspeck. PLoS One 10:e0136857. doi: 10.1371/journal.pone.0136857
Garbelotto, M., and Gonthier, P. (2013). Biology, epidemiology, and control of heterobasidion species worldwide. Ann. Rev. Phytopathol. 51, 39–59. doi: 10.1146/annurev-phyto-082712-102225
García-Bayona, L., and Comstock, L. E. (2018). Bacterial antagonism in host-associated microbial communities. Science 361:aat2456. doi: 10.1126/science.aat2456
García-Fraile, P., Benada, O., Cajthaml, T., Baldrian, P., and Lladó, S. (2016). Terracidiphilus gabretensis gen. nov., sp. nov., an abundant and active forest soil acidobacterium important in organic matter transformation. Appl. Environ. Microbiol. 82, 560–569. doi: 10.1128/AEM.03353-15
Genilloud, O. (2017). Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 34, 1203–1232. doi: 10.1039/C7NP00026J
Hartig, R. (1874). Lehrbuch der Baumkrankheiten (Besprechung). Zeitschrift für Forst Jagdwessen 14, 412–416.
Hashem, A., Tabassum, B., and Fathi Abd_Allah, E. (2019). Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 26, 1291–1297. doi: 10.1016/j.sjbs.2019.05.004
Hellgren, M. B., and Stenlid, J. (1995). Long-term reduction in the diameter growth of butt rot affected Norway spruce. Picea abies. For. Ecol. Manag. 74, 239–243. doi: 10.1016/0378-1127(95)03530-N
Hiroyuki, I., Hiroya, Y., and Yasuyoshi, S. (2015). Interactions of Methylotrophs with Plants and Other Heterotrophic Bacteria [WWW Document]. Available online at: https://www.mdpi.com/2076-2607/3/2/137 (accessed July, 24 2024).
Hu, J., Wei, Z., Kowalchuk, G. A., Xu, Y., Shen, Q., and Jousset, A. (2020). Rhizosphere microbiome functional diversity and pathogen invasion resistance build up during plant development. Environ. Microbiol. 22, 5005–5018. doi: 10.1111/1462-2920.15097
Johansson, M., and Theander, O. (1974). Changes in Sapwood of Roots of Norway Spruce, Attacked by Fomes annosus. Part I [WWW Document]. Available online at: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1399-3054.1974.tb03647.x (accessed July 28, 2024).
Kaisermann, A., Maron, P. A., Beaumelle, L., and Lata, J. C. (2015). Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 86, 158–164. doi: 10.1016/j.apsoil.2014.10.009
Kalam, S., Basu, A., Ahmad, I., Sayyed, R. Z., El-Enshasy, H. A., Dailin, D. J., et al. (2020). Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 11:580024. doi: 10.3389/fmicb.2020.580024
Kärhä, K., Koivusalo, V., Palander, T., and Ronkanen, M. (2018). Treatment of Picea abies and Pinus sylvestris stumps with Urea and Phlebiopsis gigantea for control of heterobasidion. Forests 9:139. doi: 10.3390/f9030139
Kavita, R., and Howard, H. (2017). Biochemical Mechanisms of Pathogen Restriction by Intestinal Bacteria: Trends in Biochemical Sciences [WWW Document]. Available online at: https://www.cell.com/trends/biochemical-sciences/abstract/S0968-0004(17)30158-5?sf175071372=1 (accessed July 31, 2024).
Kiss, L. (2003). A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Manag. Sci. 59, 475–483. doi: 10.1002/ps.689
Kovalchuk, A., Mukrimin, M., Zeng, Z., Raffaello, T., Liu, M., Kasanen, R., et al. (2018). Mycobiome analysis of asymptomatic and symptomatic Norway spruce trees naturally infected by the conifer pathogens Heterobasidion spp. Environ. Microbiol. Rep. 10, 532–541. doi: 10.1111/1758-2229.12654
Krekling, T., Franceschi, V. R., Krokene, P., and Solheim, H. (2004). Differential anatomical response of Norway spruce stem tissues to sterile and fungus infected inoculations. Trees 18, 1–9. doi: 10.1007/s00468-003-0266-y
Lei, M., Rao, W., Hu, J.-F., Yue, Q., Wu, Z., and Fan, G. (2024). Bacterial diversity and structure in rhizosphere soil of citrus infested with huanglongbing. Biotechnol. Bull. 40:266. doi: 10.13560/j.cnki.biotech.bull.1985.2023-0837
Lemanceau, P., Barret, M., Mazurier, S., Mondy, S., Pivato, B., Fort, T., et al. (2017). “Chapter five - plant communication with associated microbiota in the spermosphere, rhizosphere and phyllosphere,” in Advances in Botanical Research, How Plants Communicate with Their Biotic Environment, ed. G. Becard (Cambridge, CA: Academic Press), 101–133.
Lendemer, J. (2011). A review of the morphologically similar species Fuscidea pusilla and Ropalospora viridis in eastern North America. Opuscula Philolichenum 9, 11–20. doi: 10.5962/p.382023
Li, R., Duan, W., Ran, Z., Chen, X., Yu, H., Fang, L., et al. (2023). Diversity and correlation analysis of endophytes and metabolites of Panax quinquefolius L. in various tissues. BMC Plant Biol. 23:275. doi: 10.1186/s12870-023-04282-z
Liu, L., Yan, Y., Ding, H., Zhao, J., Cai, Z., Dai, C., et al. (2021). The fungal community outperforms the bacterial community in predicting plant health status. Appl. Microbiol. Biotechnol. 105, 6499–6513. doi: 10.1007/s00253-021-11486-6
Liu, M., Jaber, E., Zeng, Z., Kovalchuk, A., and Asiegbu, F. O. (2021). Physiochemical and molecular features of the necrotic lesion in the Heterobasidion–Norway spruce pathosystem. Tree Physiol. 41, 791–800. doi: 10.1093/treephys/tpaa141
Liu, Y., Guo, Z., Zhang, P., Du, J., Gao, P., and Zhang, Z. (2022). Diversity and structure of vegetation rhizosphere bacterial community in various habitats of liaohekou coastal wetlands. Sustainability 14:16396. doi: 10.3390/su142416396
Luo, L., Zhang, Z., Wang, P., Han, Y., Jin, D., Su, P., et al. (2019). Variations in phyllosphere microbial community along with the development of angular leaf-spot of cucumber. AMB Exp. 9:76. doi: 10.1186/s13568-019-0800-y
Madhaiyan, M., Suresh Reddy, B. V., Anandham, R., Senthilkumar, M., Poonguzhali, S., Sundaram, S. P., et al. (2006). Plant growth–promoting methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Curr. Microbiol. 53, 270–276. doi: 10.1007/s00284-005-0452-9
Magoč, T., and Salzberg, S. L. (2011). FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Maier, A., Riedlinger, J., Fiedler, H.-P., and Hampp, R. (2004). Actinomycetales bacteria from a spruce stand: Characterization and effects on growth of root symbiotic and plant parasitic soil fungi in dual culture. Mycol. Prog. 3, 129–136. doi: 10.1007/s11557-006-0083-y
Manching, H. C., Balint-Kurti, P. J., and Stapleton, A. E. (2014). Southern leaf blight disease severity is correlated with decreased maize leaf epiphytic bacterial species richness and the phyllosphere bacterial diversity decline is enhanced by nitrogen fertilization. Front. Plant Sci. 5:403. doi: 10.3389/fpls.2014.00403
Mesanza, N., Iturritxa, E., and Patten, C. L. (2016). Native rhizobacteria as biocontrol agents of Heterobasidion annosum s.s. and Armillaria mellea infection of Pinus radiata. Biol. Control 101, 8–16. doi: 10.1016/j.biocontrol.2016.06.003
Pańka, D., Piesik, D., Jeske, M., and Baturo-Cieśniewska, A. (2013). Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J. Plant Physiol. 170, 1010–1019. doi: 10.1016/j.jplph.2013.02.009
Pavithra, G., Bindal, S., Rana, M., and Srivastava, S. (2019). Role of endophytic microbes against plant pathogens: A review. Asian J. Plant Sci. 19, 54–62. doi: 10.3923/ajps.2020.54.62
Pratt, J. E., Niemi, M., and Sierota, Z. H. (2000). Comparison of three products based on Phlebiopsis gigantea for the control of Heterobasidion annosum in Europe. Biocontrol Sci. Technol. 10, 467–477. doi: 10.1080/09583150050115052
Ren, F., Kovalchuk, A., Mukrimin, M., Liu, M., Zeng, Z., Ghimire, R. P., et al. (2019). Tissue microbiome of norway spruce affected by heterobasidion-induced wood decay. Microb. Ecol. 77, 640–650. doi: 10.1007/s00248-018-1240-y
Ren, W., Penttilä, R., Kasanen, R., and Asiegbu, F. O. (2022). Bacteria community inhabiting heterobasidion fruiting body and associated wood of different decay classes. Front. Microbiol. 13:864619. doi: 10.3389/fmicb.2022.864619
Ren, W., Penttilä, R., Kasanen, R., and Asiegbu, F. O. (2023). Interkingdom and intrakingdom interactions in the microbiome of Heterobasidion fruiting body and associated decayed woody tissues. Appl. Environ. Microbiol. 89:e0140623. doi: 10.1128/aem.01406-23
Saxena, S. (2014). Microbial metabolites for development of ecofriendly agrochemicals. Appl. Microbiol. Biotechnol. 55, 395–403. doi: 10.1007/s002530000517
Shain, L. (1971). The response of sapwood of Norway spruce to infection by Fomes annosus. Phytopathology 61:301. doi: 10.1094/Phyto-61-301
Silva, M. T., and Silva Pestana, N. T. (2013). The in vivo extracellular life of facultative intracellular bacterial parasites: Role in pathogenesis. Immunobiology 218, 325–337. doi: 10.1016/j.imbio.2012.05.011
Singh, M., Srivastava, M., Kumar, A., Singh, A. K., and Pandey, K. D. (2020). “4 - Endophytic bacteria in plant disease management,” in Microbial Endophytes, Woodhead Publishing Series in Food Science, Technology and Nutrition, eds A. Kumar and V. K. Singh (Sawston: Woodhead Publishing), 61–89. doi: 10.1016/B978-0-12-818734-0.00004-8
Spaaij, F., Weber, G., Roeijmans, H. J., van Eijk, G. W., and Oberwinkler, F. (1991). Fellomyces horovitziae sp. nov., a new basidiomycetous yeast species isolated from aXenasmatella basidiocarp. Antonie van Leeuwenhoek 59, 293–298. doi: 10.1007/BF00583682
Steenhoudt, O., and Vanderleyden, J. (2000). Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 24, 487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x
Studzinska-Sroka, E., and Zarabska-Bozjewicz, D. (2019). Hypogymnia physodes – a lichen with interesting medicinal potential and ecological properties. J. Herb. Med. 17–18:100287. doi: 10.1016/j.hermed.2019.100287
Sun, M., Shi, C., Huang, Y., Wang, H., Li, J., Cai, L., et al. (2023). Effect of disease severity on the structure and diversity of the phyllosphere microbial community in tobacco. Front. Microbiol. 13:1081576. doi: 10.3389/fmicb.2022.1081576
Sun, W., Li, Z., Lei, J., and Liu, X. (2022). Bacterial communities of forest soils along different elevations: Diversity, structure, and functional composition with potential impacts on CO2 emission. Microorganisms 10:766. doi: 10.3390/microorganisms10040766
Takahashi, Y., and Nakashima, T. (2018). Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics 7:45. doi: 10.3390/antibiotics7020045
Tan, L., Zeng, W., Xiao, Y., Li, P., Gu, S., Wu, S., et al. (2021). Fungi-bacteria associations in wilt diseased Rhizosphere and endosphere by interdomain ecological network analysis. Front. Microbiol. 12:722626. doi: 10.3389/fmicb.2021.722626
Tanney, J. B., and Seifert, K. A. (2020). Mollisiaceae: An overlooked lineage of diverse endophytes. studies in mycology, genera of Hyphomycetes: A tribute to Keith A. Seifert 95, 293–380. doi: 10.1016/j.simyco.2020.02.005
Terhonen, E., Kovalchuk, A., Zarsav, A., and Asiegbu, F. O. (2018). “Biocontrol potential of forest tree endophytes,” in Endophytes of Forest Trees: Biology and Applications, eds A. M. Pirttilä and A. C. Frank (Cham: Springer International Publishing), 283–318.
Terhonen, E., Marc, T., Sun, H., Jalkanen, R., Kasanen, R., Vuorinen, M., et al. (2011). The effect of latitude, season and needle-age on the mycota of scots pine (Pinus sylvestris) in Finland. Silva Fennica 45, 301–307. doi: 10.14214/sf.104
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Wen, Z., Zeng, Z., Ren, F., and Asiegbu, F. O. (2019). The Conifer root and stem rot pathogen (Heterobasidion parviporum): Effectome analysis and roles in interspecific fungal interactions. Microorganisms 7:658. doi: 10.3390/microorganisms7120658
Xin, G., Zhang, G., Kang, J. W., Staley, J. T., and Doty, S. L. (2009). A diazotrophic, indole-3-acetic acid-producing endophyte from wild cottonwood. Biol. Fertil. Soils 45, 669–674. doi: 10.1007/s00374-009-0377-8
Xiong, Q., Yang, J., and Ni, S. (2023). Microbiome-mediated protection against pathogens in woody plants. Int. J. Mol. Sci. 24:16118. doi: 10.3390/ijms242216118
Yang, F., Zhang, J., Zhang, H., Ji, G., Zeng, L., Li, Y., et al. (2020). Bacterial blight induced shifts in endophytic microbiome of rice leaves and the enrichment of specific bacterial strains with pathogen antagonism. Front. Plant Sci. 11:963. doi: 10.3389/fpls.2020.00963
Yang, H., Li, J., Xiao, Y., Gu, Y., Liu, H., Liang, Y., et al. (2017). An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 8:2179. doi: 10.3389/fmicb.2017.02179
Zaļuma, A., Muižnieks, I., Gaitnieks, T., Burn, eviča, N., Jansons, Ā, Jansons, J., et al. (2019). Infection and spread of root rot caused by Heterobasidion spp. in Pinus contorta plantations in Northern Europe: Three case studies. Can. J. For. Res. 49, 969–977. doi: 10.1139/cjfr-2018-0507
Zeng, Z., Sun, H., Vainio, E. J., Raffaello, T., Kovalchuk, A., Morin, E., et al. (2018). Intraspecific comparative genomics of isolates of the Norway spruce pathogen (Heterobasidion parviporum) and identification of its potential virulence factors. BMC Genomics 19:220. doi: 10.1186/s12864-018-4610-4
Zhang, X., Hu, B. X., Ren, H., and Zhang, J. (2018). Composition and functional diversity of microbial community across a mangrove-inhabited mudflat as revealed by 16S rDNA gene sequences. Sci. Total Environ. 633, 518–528. doi: 10.1016/j.scitotenv.2018.03.158
Zhang, X., Zhao, W., Kou, Y., Liu, Y., He, H., and Liu, Q. (2023). Secondary forest succession drives differential responses of bacterial communities and interactions rather than bacterial functional groups in the rhizosphere and bulk soils in a subalpine region. Plant Soil 484, 293–312. doi: 10.1007/s11104-022-05788-5
Keywords: microbial community structure, Heterobasidion, network analysis, microbial interaction, microbial community diversity
Citation: Meng W-j, Wen Z-l, Kasanen R, Sun H and Asiegbu FO (2025) Microbial communities in the phyllosphere and endosphere of Norway spruce under attack by Heterobasidion. Front. Microbiol. 15:1489900. doi: 10.3389/fmicb.2024.1489900
Received: 02 September 2024; Accepted: 19 December 2024;
Published: 08 January 2025.
Edited by:
Daniela Minerdi, University of Turin, ItalyCopyright © 2025 Meng, Wen, Kasanen, Sun and Asiegbu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fred O. Asiegbu, ZnJlZC5hc2llZ2J1QGhlbHNpbmtpLmZp
 Wen-jing Meng
Wen-jing Meng Zi-lan Wen
Zi-lan Wen Risto Kasanen
Risto Kasanen Hui Sun
Hui Sun Fred O. Asiegbu
Fred O. Asiegbu