- 1Pest Integrated Management Key Laboratory of China Tobacco, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China
- 2Guizhou Academy of Tobacco Science, Guiyang, China
- 3Institute and Enterprise Joint Creation of Tobacco Technology Center, Sichuan Provincial Tobacco Company Liangshanzhou Company, Liangshanzhou, China
- 4Shandong Hezhong Kangyuan Biotechnology Co., Ltd, Zibo, Shandong, China
- 5Department of Vector Biology and Contro, Jinan Center for Disease Control and Prevention, Jinan, China
Introduction: Long-term use of chemical fertilizers (CFs) can cause soil compaction and acidification. In recent years, bio-organic fertilizers (BOFs) have begun to replace CFs in some vegetables and cash crops, but the application of CFs or BOFs has resulted in crop quality and disease occurrence.
Methods: This study aimed to analyze the microbial mechanism of differences between CFs and BOFs in root disease, quality, and yield of tuber Chinese herbal medicine. We studied the effects of CFs, organic fertilizers, commercial BOFs, biocontrol bacteria BOFs, and biocontrol fungi BOFs on rhizosphere microbial community structure and function, root rot, quality, and yield of Codonopsis pilosula at different periods after application and analyzed the correlation.
Results and discussion: Compared to CFs, the emergence rate and yield in BOF treatments were increased by 21.12 and 33.65%, respectively, and the ash content, water content, and disease index in the BOF treatments were decreased by 17.87, 8.19, and 76.60%, respectively. The structural equation model showed that CFs promoted the quality and yield of C. pilosula by influencing soil environmental factors, while BOFs directly drove soil bacterial community to reduce disease index and improve the quality and yield of C. pilosula. There was a stronger interaction and stability of soil microbial networks after BOF treatments. Microlunatus, Rubrobacter, Luteitalea, Nakamurella, and Pedomicrobium were identified as effector bacteria, which were related to disease prevention and yield and quality increase of C. pilosula. Microbial functional analysis indicated that the signal transduction and amino acid metabolism of soil bacteria might play a major role in improving the quality and yield of C. pilosula in the early and middle growth stages. In conclusion, compared to CFs, BOFs obtained a lower disease index of root rot and a higher quality and yield of C. pilosula by changing the structure and function of the rhizosphere bacterial community.
1 Introduction
Codonopsis pilosula is a perennial herbaceous plant and an important Chinese herbal medicine. Its fleshy roots are widely used in the field of medicine, and they can be used as both medicine and food (Lodi et al., 2023). Perennial continuous cropping can lead to the accumulation of pathogenic bacteria, and this can result in the severe root rot of C. pilosula. One of the main goals of current research in the production of Chinese herbal medicines, including C. pilosula, is to develop approaches for enhancing yield and quality and the control of plant diseases without compromising soil health.
Bio-organic fertilizers (BOF) are environmentally friendly fertilizers that can be used for the control of pest populations and for enhancing soil properties. Some of the advantages of BOFs include their positive effects on soil microbial activities and their ability to enhance soil properties and prevent soil-borne diseases, as well as some of the problems caused by CF application, including soil compaction and acidification and ecological imbalances (Rani et al., 2021). BOF application has been shown to be effective in enhancing the production of agricultural crops. Wang et al. (2022a) reported that Bacillus subtilis BOF can promote cabbage growth, with plant height and biomass being 1.20 and 1.93 times greater in the BOF group than in the CF group on the 30th day of the experiment. The sugar–acid ratio of apples was greater under BOF application than under organic fertilizer (OF) application, and this is associated with differences in sucrose accumulation and citric acid degradation (Wang et al., 2022b). The efficacy of different crop protection practices has been studied for several crops, including tobacco, bananas, and tomatoes. BOFs have been shown to be more effective than CFs or OFs in minimizing the deleterious effects of tobacco black shank (Chen et al., 2023), banana wilt (Tao et al., 2020), and tomato bacterial wilt (Shen et al., 2021). However, compared to CFs, the mechanism by which BOFs enhances the yield and quality of root herbs at the microbiological level has not yet been elucidated.
Soil microbial structure and function are likely key for mediating differences in the positive effects of BOFs and CFs on the quality and yield of crops or Chinese herbs. The application of CFs is considered an important source of crop nutrient elements, including macroelements and trace elements, which play key roles in diverse processes, including protein synthesis (Khan et al., 2021), energy conversion processes (Wang et al., 2017), water regulation (Hartmann et al., 2021), and regulation of enzyme activity and hormone synthesis (Li et al., 2021). The application of BOFs has various effects on soil microorganisms, including their ability to respond to abiotic and biotic stresses. BOFs have thus received a lot of research attention. BOF application after fumigation has been shown to increase soil pH and the abundance of 13 functional genes related to amino acid metabolism, suggesting that soil ecological health was improved following the application of BOF (Huang et al., 2023). The BOF SQR9 has been shown to increase pear yield by enhancing the abundance and functional diversity of the rhizosphere microbiome. Specifically, the abundance and functional diversity of the rhizosphere were 21 and 8% higher following BOF application compared to CF and OF application, respectively (Wang et al., 2023). BOFs have been shown to have indirect and lagged effects (Chen et al., 2023), with fruit quality improving more significantly following BOF application than the application of traditional OFs (Wang et al., 2022a; Wang et al., 2022b; Wang et al., 2022c). This improvement may stem from the diverse functions of soil microorganisms.
In this study, the roots of C. pilosula, a Chinese herbal medicine, were explored by conducting an experiment in which plants were subjected to treatments with CFs, OFs, and different BOFs. We (1) measured the emergence rate, incidence rate, yield, and quality of C. pilosula; (2) determined soil physicochemical properties at different periods, α and β diversity of soil microorganisms; (3) analyzed the factors leading to the differences between CFs and BOFs using a structural equation model (SEM); (4) identified differential microorganisms and core microorganisms in the rhizosphere soil through network analysis as key microorganisms affecting C. pilosula; and (5) employed ternary analysis, correlation analysis, and the Mantel test of microbial function to reveal the potential mechanisms by which BOFs influence the quality and yield of C. pilosula through microbial reshaping at different times. This study provides new insights into using different fertilizers to improve the quality and yield of tuber Chinese herbal medicine, highlighting the feasibility of BOFs as an alternative to chemical fertilizers in Chinese herbal medicine cultivation.
2 Materials and methods
2.1 Field experimental design
The experimental site was in Mianliuping Village, Dingxi City, Gansu Province (35.13675°N, 104.215467°E). C. pilosula has been continuously cultivated at the experimental site for several years; the incidence of root rot in the field plot was 20–30%. C. pilosula seedlings with good growth and no disease were transplanted from the seedling nursery on 20 April 2022. Fertilizers were applied the day before C. pilosula transplanting. The experiment comprised five fertilization treatments: CK: 600 kg/ha of conventional chemical fertilizer (total nutrients ≥40%, N–P2O5–K2O = 15–15–10%); T1: 1500 kg/ha of prickly ash seed oil meal OF (without biocontrol bacteria); T2: 1500 kg/ha of commercial BOF (Bacillus complex); T3: 1500 kg/ha of Trichoderma BOF (prickly ash seed meal OF + Trichoderma asperellum); and T4: 1500 kg/ha of Bacillus BOF (prickly ash seed meal OF + Bacillus amyloliquefaciens + B. subtilis). The size of each plot was 200 m2, and a total of 12,000 plants of C. pilosula were transplanted. The plots were randomly arranged, and three replications of each treatment were performed. All plants were cultivated in an open field and cultivated per standard local practices. C. pilosula was harvested on 23 October 2024, 5 months after transplanting. The compound chemical fertilizers were obtained from Su Di Fertilizer Co., Ltd. in Gansu Province. The commercial BOFs were obtained from Hebei Rundong Fertilizer Co. Ltd. Trichoderma and Bacillus BOFs were provided by the Tobacco Research Institute of the Chinese Academy of Agricultural Sciences (Huang et al., 2021).
2.2 Determination of emergence rate, disease index, quality, and yield of C. pilosula
The emergence rate of C. pilosula in each plot was determined at the seedling stage on 25 May 2024. and C. pilosula were considered to have emerged if they were approximately 2 cm above the ground. The disease index of root rot of C. pilosula was measured at the time of harvesting. The disease index was determined using the following four-graded scale: Grade 0, disease spots are absent along the entire root system; Grade 1, the area with lesions is less than one-third of the entire area of the roots; Grade 2, the area with lesions accounts for one-third to two-thirds of the entire root area; and Grade 3, the area with lesions is greater than two-thirds of the entire root area. The formula for the disease index was based on a previous study (Carlos, 2022). During the harvest period, a total of 30\u00B0C. pilosula were randomly excavated from each plot, and branches and large pieces of soil were removed. Samples were dried at 50°C to maintain low moisture content. After drying, the yield of C. pilosula was weighed and counted for each treatment. The water content of C. pilosula samples was determined according to General Rule 0832 from the 2015 edition of the Pharmacopoeia of the People’s Republic of China 2015 (Part 4). The total ash content of C. pilosula samples was determined according to General Rule 2302 of the Pharmacopoeia of the People’s Republic of China (National Pharmacopoeia Commission, 2015). Three parallel experiments were performed. The quality of C. pilosula is mainly determined by its ash and water content. According to the first edition of the Pharmacopoeia of the People’s Republic of China (2015), the water content of C. pilosula should not exceed 16%, with approximately 10% being optimal. Additionally, the total ash content should not exceed 5%; lower total ash content indicates better quality of C. pilosula.
2.3 Collection of soil samples and determination of physicochemical properties
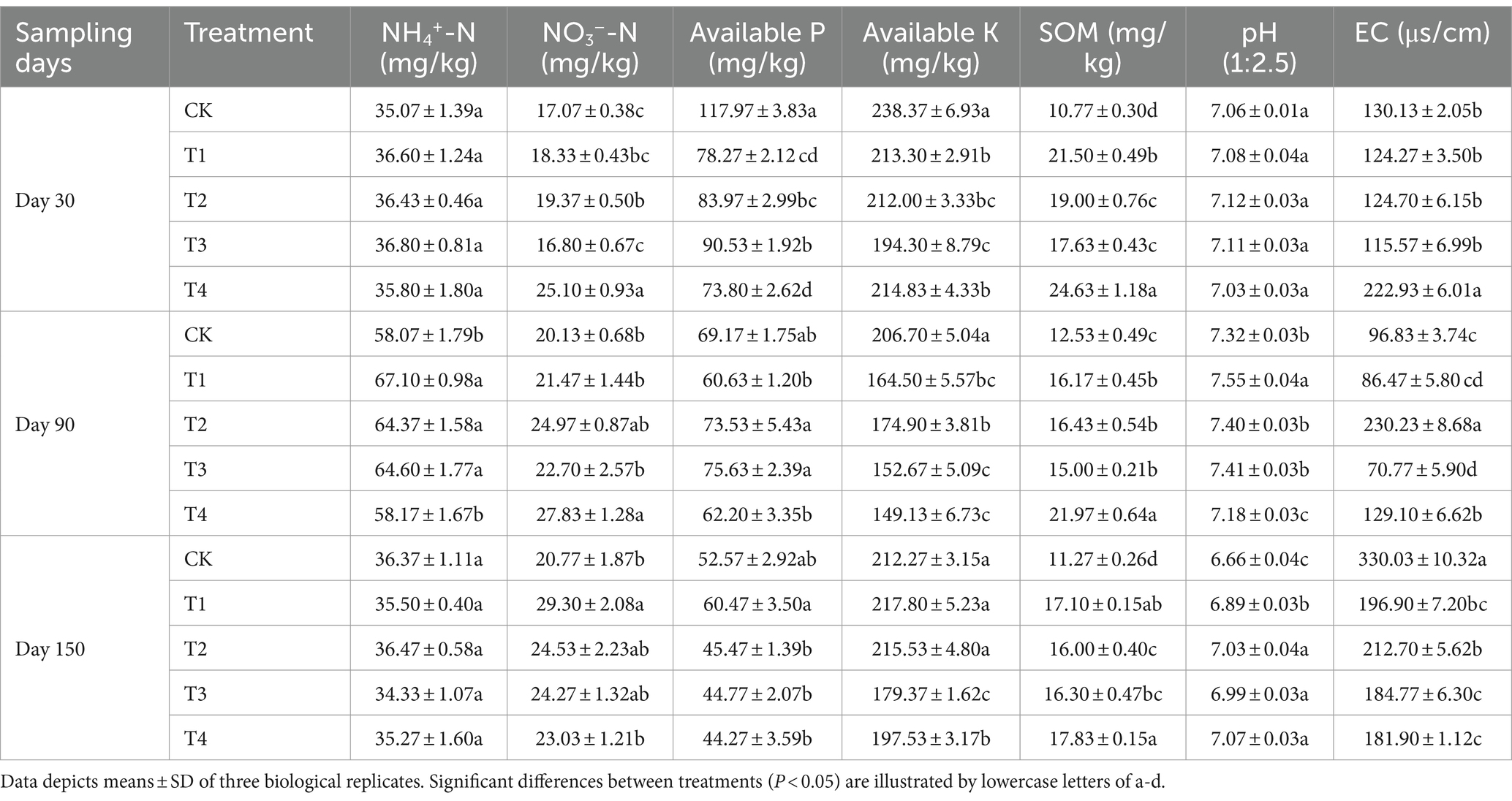
Soil samples were collected on the 30th, 90th, and 150th days after fertilization. The C. pilosula was uprooted, and most of the soil was shaken off. The soil remaining on the surface of the roots was collected with a brush, which was the rhizosphere soil. A total of three replicate soil samples were taken. The obtained soil samples were stored at −20°C for DNA extraction and physical and chemical analyses. Soil ammonium N (NH4+-N) and nitrate nitrogen (NO3−-N) were extracted using 2 M KCl and then determined using a Flow Analytical System (Yan et al., 2017). A sodium bicarbonate extraction agent was used to extract soil-available phosphorus (AP), and the molybdenum antimony resistance colorimetric method was used to measure AP (Olsen, 1954). Soil-available potassium (AK) was extracted from neutral 1 mol/L ammonium acetate solution and determined using a Model 410 flame photometer (Franz et al., 1996). The soil organic matter (OM) was determined using the potassium dichromate method (Xu et al., 2022). Soil pH and water content were measured in a 1:2.5 soil/water suspension.
2.4 High-throughput sequencing
An E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA) was used to extract the total genomic DNA of the rhizosphere soil samples per the manufacturer’s instructions. The V3–V4 variable region of the 16S rRNA gene was sequenced using the forward primer 338F and the reverse primer 806R (Xu et al., 2016) carrying barcode sequences and the extracted DNA as a template. PCR was conducted in 20 μL reactions, and the reaction system and thermal cycling conditions referred to our previous studies (Huang et al., 2023). The samples were then preserved at 4°C. A total of three replicate reactions were performed per sample. PCR products from the same sample were mixed, and 2% agarose gel electrophoresis was performed to recover the samples. The products were purified, recovered, detected, and quantified, and then the PCR library was constructed. Illumina’s MiSeq PE300/NovaSeq PE250 platform (Shanghai Meiji Biomedical Technology Co., Ltd.) was used to sequence the libraries.
2.5 Bioinformation and statistical analysis
FLASH software (v1.2.11) was used to splice the sequences derived from the offline raw data. The QIIME (v1.9.1) platform was used for quality control of the original sequences, and the UCHIME algorithm was used to detect and remove chimeric sequences. The Uparse (v11) pipeline was used to cluster operational taxonomic units (OTUs) with at least 97% similarity (Adams et al., 2013). The taxonomic identity of the bacterial and fungal sequences was determined using the SILVA (v138) database for bacteria with the Mothur algorithm and the UNITE (v8.0) database for fungi (Edgar et al., 2011). The abundance of OTUs was standardized relative to the least abundant sequence in the samples; diversity analysis was conducted using standardized OTU abundance data. The α-diversity of the Simpson and Chao1 indices of microbial communities was analyzed using Mothur (v.1.30.0) software (Schloss et al., 2009). Principal coordinate analysis (PCoA) was performed using Bray–Curtis distances. The relative abundances of species were analyzed using Python (v.2.7.0).
The pheatmap package and vegan package in R were used to generate heatmaps and determine statistical correlations. SEM in IBM SPSS Amos 26 was used to evaluate the effects of soil environmental factors and microorganisms on the quality and yield of C. pilosula (Chu et al., 2022). Environmental factors with significant effects on soil microbial communities were determined using the Mantel tests (Li et al., 2022). Visual maps for correlation analysis of environmental factors (Pheatmap), Ternary maps for three temporal soil microbial functions (Ternary), and pie maps for contribution of species functions (vegan) were drawn using R (4.3.2). The soil microbial co-occurrence network was constructed using the Hmisc package and visualized with Gephi (0.10.0). PICRUSt was used to predict the microbial function of bacterial 16S rRNA sequencing data (Huang et al., 2021). After OTUs standardization, the KEGG database annotation was performed to analyze microbial metabolic pathways. The significance of differences in emergence rate, disease index, quality, yield, and soil physicochemical properties was determined using a one-way analysis of variance and Duncan’s multiple range test.
3 Results and discussion
3.1 Effects of each treatment on the emergence rate, disease index, quality, and yield of C. pilosula
The emergence rate was the lowest in the chemical fertilizer (CK, 67.62%) and the highest in T3 (81.90%). The emergence rate was significantly higher in T2, T3, and T4 than in the CK (Figure 1A). The exogenous application of BOFs has been shown to promote the metabolic activities of soil microorganisms, including respiration, and the water generated might play a key role in inducing the emergence of C. pilosula (Carlos, 2022; Zhao et al., 2019). The disease index was the highest in the CK (11.11) and the lowest in T4 (2.60). The disease index was significantly lower in T1, T2, T3, and T4 than in the CK, and the disease index was significantly lower in T2, T3, and T4 than in T1 (Figure 1B), suggesting that BOFs could effectively control the root rot of C. pilosula.
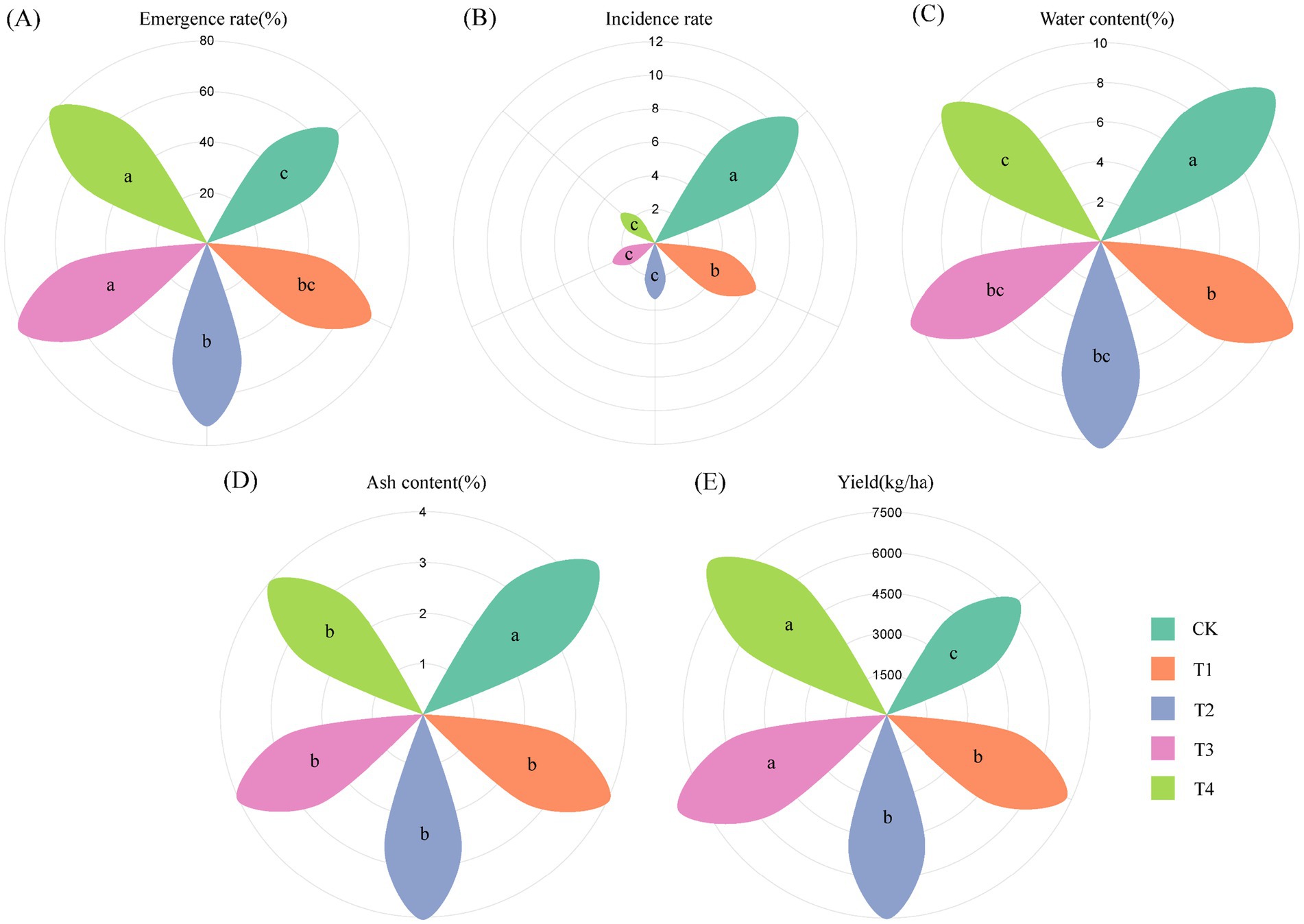
Figure 1. Emergence rate, disease index, yield, and quality of C. pilosula following treatment with CF, OF, and BOF. (A) Emergence rate of C. pilosula; (B) incidence of root rot; (C) moisture content of C. pilosula; (D) ash content of C. pilosula; (E) yield of C. pilosula. Specific information on each indicator is provided in Supplementary Table S1. Different letters (a, b, c) indicate significant differences between treatments.
The water content was significantly higher in the CK (11.23%) than in the other four treatments, and the water content was the lowest in T4 (10.31%). The ash content was significantly higher in the CK (4.87%) than in the other four treatments, and the total ash content was the lowest in T4 (4.00%). According to the first edition of the Pharmacopoeia of the People’s Republic of China (2020), the water content of C. pilosula should not exceed 16%, and the total ash content should not exceed 5%. A previous study has shown that severe worm infestations can occur when the water content of C. pilosula is >15%, and worm infestations can be prevented when the water content is <13% (Leng et al., 2023). The quality and ash content of C. pilosula are inversely related. The fresh root yield was significantly higher in T3 and T4 than in the other three treatments (Figures 1C–E; Supplementary Table S1).
3.2 Effects of fertilizations on the soil physicochemical properties
At 90 days after treatment, the NH4+-N was significantly higher in T1, T2, and T3 than in the CK. The soil NO3−-N content was significantly higher in T4 than in the CK at 30 days and 90 days after treatment. BOF application has been shown to promote the N fixation of microbes in soil and reduce the loss of N (Rahimi et al., 2021) (Table 1).
The content of AP was significantly lower in all OF treatments than in the CK at 30 days after treatment. The content of AP in all soil samples decreased over time. The content of soil AK was significantly lower in all OF treatments than in the CK at 30 d after treatment; the content of soil AK was the lowest in T3, and it was 18.49% lower in T3 than in the CK. The AK content was the lowest at 90 days after treatment in the CK, and the content of AK was 14.56 and 35.39% higher in T2 and T3 than in the CK, respectively. The AK content was 15.49 and 6.94% lower in T3 and T4 than in the CK at 150 days after treatment, and these differences were significant. Compared to chemical fertilizers, BOF treatments were more slow-release and helped to supplement the AK in the middle and late growth of C. pilosula (Yang et al., 2017). The content of OM significantly increased in the OF and BOF treatments during the three periods, and this was driven by the input of exogenous organic carbon (Table 1).
The soil pH was significantly higher in all BOF treatments than in the CK at 150 days after treatment. The excessive application of CF can result in the acidification of soil, which stems from the fact that ammonium sulfate and calcium carbonate make CF acidic (Kakihara and Ogura, 2022). The mixed application of cow manure and different OFs has been shown to increase soil pH to different degrees, which is consistent with the results of our study (Chen et al., 2023). Tobacco black shank is more common in acidic soil, and alterations in soil acidity can alter the abundance of the pathogens that cause tobacco black shank (Ma et al., 2023). Soil acidification also decreases the number of ammoniating bacteria and N-fixing bacteria in soil and the ammoniating and nitrification capabilities of soil microorganisms (Liu et al., 2023). The EC was significantly higher in T4 than in the other treatments at 30 days after treatment, and the EC was significantly higher in the CK than in the other four treatments at 150 days after treatment (Table 1).
3.3 Variation in the α- and β-diversity of soil microbial communities
The Shannon index of rhizosphere soil bacteria was significantly lower in T1, T2, and T3 than in the CK at 30 days after treatment. The Shannon index of bacteria was significantly higher in the OF and BOF treatments than in the CK at 90 days after treatment (Figure 2A). At 30 days after treatment, the Shannon index of soil fungi was the highest in the CK. At 150 days after treatment, the Shannon index of soil fungi was higher in T2, T3, and T4 than in the CK (Figure 2B). Thus, the diversity of soil microbes in BOF treatments was increased in the middle and later stages of the experiment. It may promote the stability and functional diversity of soil ecosystems (Sharma et al., 2022). The Chao1 index of soil bacteria was significantly higher in T3 and T4 than in the CK at 90 days after treatment (Figure 2C). The Chao1 index of soil fungi was significantly higher in the CK than in T4 at 30 days after treatment (Figure 2D).
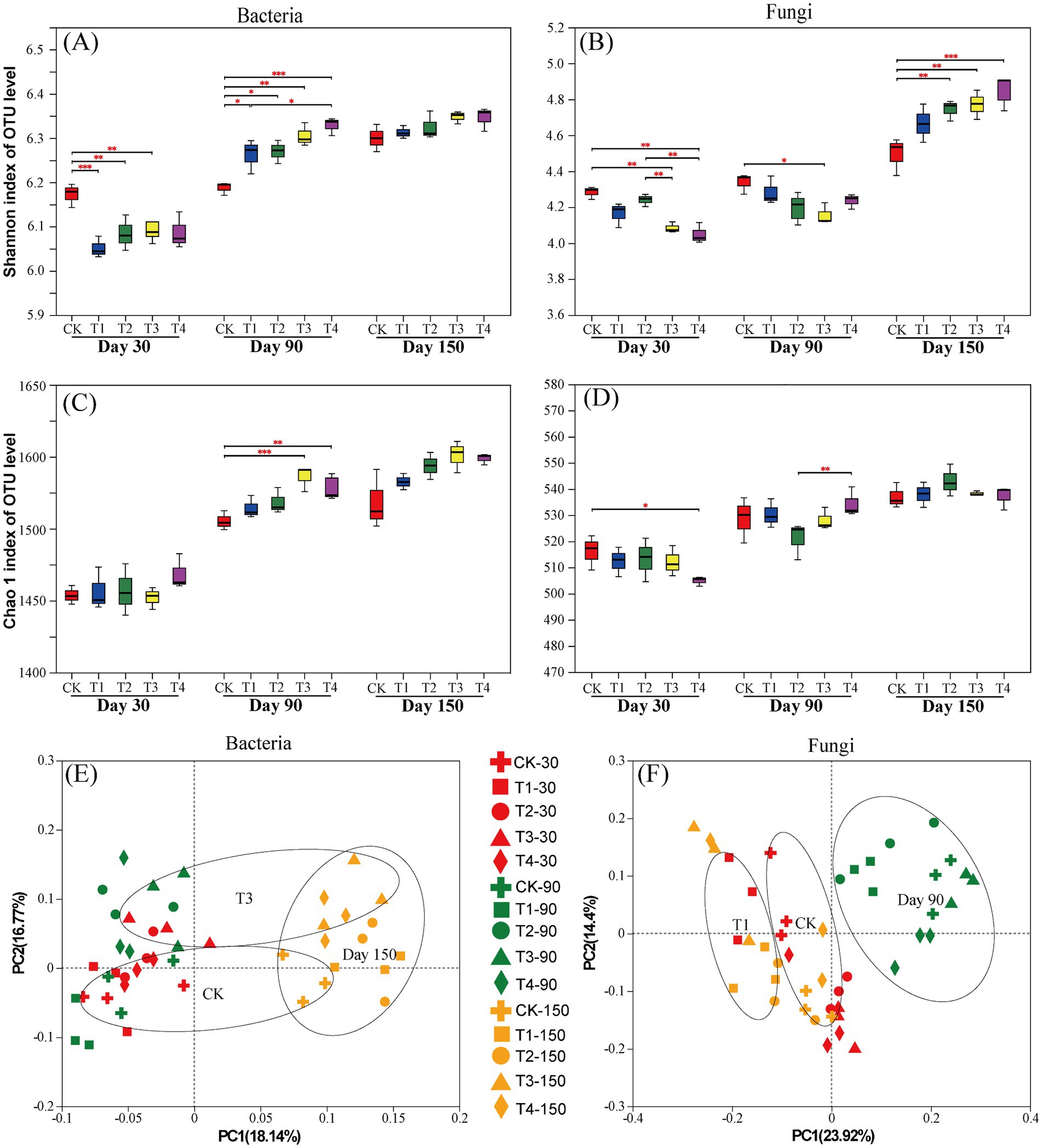
Figure 2. α- and β-diversity of rhizosphere soil microorganisms. (A) Shannon index of the soil bacterial community; (B) Shannon index of the soil fungal community; (C) Chao1 index of the soil bacterial community; (D) Chao1 index of the soil fungal community; (E) PCoA of the soil bacterial community; (F) PCoA of the soil fungal community.
The PCoA results indicated that the contribution of PC1 and PC2 to differences in the composition of species among treatments was 18.14%/16.77% (bacteria) and 23.92%/14.4% (fungi), respectively (Figures 2E,F). The community composition of soil bacteria at 150 days after treatment differed from that at 30 days and 90 days after treatment along PC1. The community composition of soil fungi at 90 days after treatment differed from that at 30 days and 150 days after treatment along PC1, which indicates that soil fungi were more vulnerable to the effects of BOFs in the middle of the experimental period compared to conventional fertilizers.
3.4 The driving factors affecting the emergence, disease prevention, yield, and quality of C. pilosula
To clarify the direct and indirect effects of soil environmental factors (NH4+-N, NO3−-N, AP, AK, OM, pH, and EC) and soil microorganisms on the emergence, disease prevention, yield, and quality of C. pilosula, we established a SEM for the CF (CK; Figure 3A) and BOF treatments (T2, T3, and T4; Figure 3B). Soil environmental properties accounted for the majority of the variation in the soil bacterial community (95.4%) in the CF treatment. Soil environmental properties were significantly negatively correlated with soil bacteria and yield quality and significantly positively correlated with emergence rate (path coefficient of 1.008). The soil fungal community was significantly negatively correlated with the incidence rate (path coefficient of −0.770).
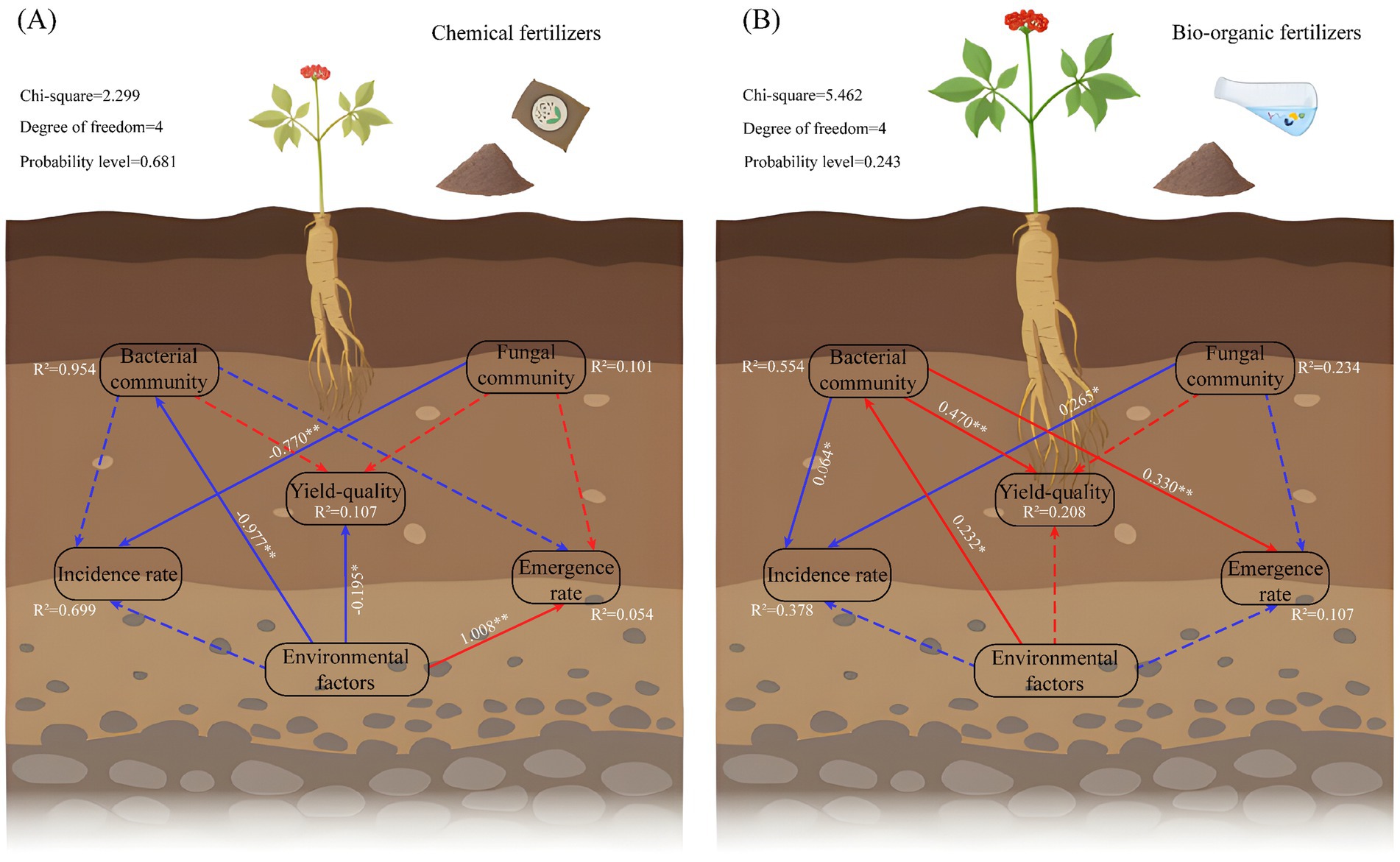
Figure 3. SEM of the effects of soil microbial community structure and environmental factors on the emergence rate, disease prevention, yield, and quality of C. pilosula. (A) SEM for the CF treatment; (B) SEM for the BOF treatment. Continuous and dashed arrows indicate significant and non-significant relationships, respectively. The numbers adjacent to the arrows indicate the path coefficients, and the red and blue arrows indicate negative and positive relationships, respectively. The R2 value indicates the proportion of the explained variance for each variable. The significance level was expressed as *p < 0.05, **p < 0.01, and ***p < 0.001. The total normalized effect of the structural equation model calculations is shown in the upper left. “Yield-quality” represents the yield, ash, and water content of C. pilosula; “Environmental factors” represents the soil physicochemical properties; “Bacterial community” represents the rhizosphere bacterial community structure; and “Fungal community” represents the rhizosphere fungal community structure.
Soil environmental properties explained the most variation in the soil bacteria community (55.4%) under BOF treatment. Soil environmental factors were significantly positively correlated with the soil bacterial community (path coefficient of 0.232). The soil bacterial and fungal communities were significantly positively correlated with incidence rate. The soil bacteria community showed a significant positive correlation with quality, yield, and emergence rate. Under the CF treatment system, soil environmental factors had a significant direct effect on the yield and quality of C. pilosula. However, under the BOF treatment, soil environmental factors directly affected the soil bacterial community, which directly drove the yield and quality of C. pilosula. CFs had a rapid effect and provided abundant nutrients; these nutrients are key for crops over short time intervals, given that CFs directly determine the yield and quality of crops. BOFs can promote N fixation, P solubilization, and K solubilization through the life processes of microbes; BOFs can also mediate reductions in molecular N in the atmosphere, the decomposition and destruction of mineral crystals through the production of various organic acids, the release of fixed P and K in the soil, and the full utilization of nutrients, which enhances the yield and quality of crops (Peng et al., 2022).
3.5 Differential microorganisms in soil bacterial communities
BOFs affect the incidence rate, quality, and yield of C. pilosula by influencing the soil bacterial community. Therefore, we focused on the changes in the soil bacterial community. Linear discriminant analysis effect size (LEFSe) was conducted to compare CF and BOF treatments (T2, T3, and T4) using data from the phylum to genus level, with an LDA threshold set at 2 (Supplementary Figure S1). In the soil bacterial community, p_unclassified_k_norank_d_Bacteria was significantly enriched in the BOF treatments, and no significant phylum was significantly enriched in the CK treatment. The orders Bryobacterales, Solibacterales, Cyanobacteriales, Acetobacterales, Reyranellales, and Sphingomonadaceae and the genera Bryobacter, Reyranella, and Sphingomonas were significantly enriched in the CK treatments. Solibacterales, Cyanobacteriales, and Sphingomonadaceae are all primitive microorganisms (Supplementary Figure S1). These archaea and bacteria inhabit swamps and volcanoes and prefer using simple nutrients to complete their life processes (Gao et al., 2018), which indicates that they would grow optimally with the direct nutrient supply provided by CFs but grow poorly with the indirect nutrient supply provided by BOFs. The orders Streptosporangiales and Rubrobacterales, and the genera Microlunatus and Rubrobacter were significantly enriched in BOF treatment. Many genera in Streptosporangiales produce broad-spectrum antibiotics, including Streptospora rosetta and Streptospora virescini. They can produce polymycin and sporomycin, respectively, and inhibit most plant pathogens. Streptosporangiales may be the key differentiating bacteria in the prevention of the root rot of C. pilosula. SG1 strain of Streptosporangiales showed significant positive results in its control effect on cucumber fusarium wilt and its growth promotion effect on durum wheat. Among them, SG1 can reduce the disease index of cucumber wilt from 77.8 to 16% and significantly increase wheat growth and yield, thus having a wide application prospect (Nassira et al., 2018). Microlunatus and Rubrobacter were isolated from Marine ascidians with obvious antibacterial and anti-inflammatory activities. The former has anti-Candida albicans activity, and the ethyl acetate extract of the latter has good cytotoxic activity (Yuan et al., 2014; Chen et al., 2018). They may play a key role in the prevention of the root rot of C. pilosula.
3.6 Co-occurrence network analysis of soil bacteria community
Co-occurrence networks for the bacterial communities in the CF (CK), OF (T1), and BOF (T4) treatments were constructed to clarify the effects of these fertilizers on the interactions between soil microorganisms. In the soil bacterial co-occurrence network, the CK network included 73 nodes and 56 edges; the OF network included 95 nodes and 102 edges; and the BOF (T4) network included 193 nodes and 506 edges (Supplementary Table S2). The number of nodes and connections in the co-occurrence network of bacteria increased following the application of OFs and BOFs (T4), and the number of nodes and connections was the highest in the BOF (T4) treatment, suggesting that the soil microbial network was more complex and interactions were stronger in the BOF treatment than in the CF/OF treatment. Nodes were classified by their within-module connectivity (Zi) and among-module connectivity (Pi), and their roles in the network were deduced. In the bacterial network, all nodes of CK and OF were identified as peripherals, with no critical nodes. Under the treatment of BOF, Luteitalea, Nakamurella, Pedomicrobium, norank_f__Acetobacteraceae, unclassified_o__Saccharimonadales, norank_f__norank_o__S085, and norank_f__norank_o__norank_c__Subgroup_11 were identified as the key class groups (Figure 4). Of these key groups, one is a Module hub, which is Luteitalea. The remaining six groups are connectors, which are highly connected nodes between modules and can combine different modules to achieve network functions; therefore, the realization of BOF to reduce the incidence of C. pilosula and improve the emergence rate, quality, and yield of C. pilosula may require the joint action of different modules to complete.

Figure 4. Analysis of the co-occurrence network of soil bacteria community induced by CK, OF, and BOF. (A) Co-occurrence network of soil bacterial community after CK (chemical fertilizer). (B) Co-occurrence network of soil bacterial community after organic fertilizer (OF, T1). (C) Co-occurrence network of soil bacterial community after BOF (T4). (D) Distributions of keystone taxa according to the topological properties of CK. (E) Distributions of keystone taxa according to the topological properties of OF. (F) Distributions of keystone taxa according to the topological properties of BOF (T4). The colors of the different nodes indicate different microbial genera, with red edges indicating positive correlations between two nodes and blue edges indicating negative correlations.
Luteitalea, as a phosphorus-solving bacterium in soil, contains ppgC gene and can produce organic acid and reduce calcium-bound phosphorus, thereby improving the utilization efficiency of phosphorus by plants (Ding et al., 2022). Nakamurella is also sensitive to the antibiotic ciprofloxacin and can be used as a marker of environmental ecological health (Kim et al., 2020). Pedomicrobium has been shown to degrade some of the microplastics in soil and repair soil pollution (Rong et al., 2021). Current studies believe that the complexity of the microbial co-occurrence network is a key factor reflecting the interrelation and coexistence of microbes in an ecological environment (Kishore et al., 2023).
3.7 Microbial function analysis of driving emergence, disease prevention, yield, and quality change of C. pilosula at different times
The differential microorganisms obtained from the LEFSe analysis and the key microbial class identified in network analysis after BOF treatment may be effective microorganisms for reducing root rot of C. pilosula and improving yield quality, which we defined as key microorganisms. The relative abundance matrix of their OTU level and the physicochemical properties of soil were used for the Mantel detection (Figure 5B) to characterize the correlation between key microorganisms and soil environmental factors. The results showed that key microorganisms were strongly correlated with NO3−-N (p < 0.01) and AK (p < 0.05), but not strongly correlated with other soil environmental factors. At the same time, there was a significant positive correlation between soil pH and NH4+-N (r = 0.79) and a significant negative correlation between pH and EC (r = −0.68), AK and NH4+-N (r = −0.60), AP and NO3−-N (r = −0.64).
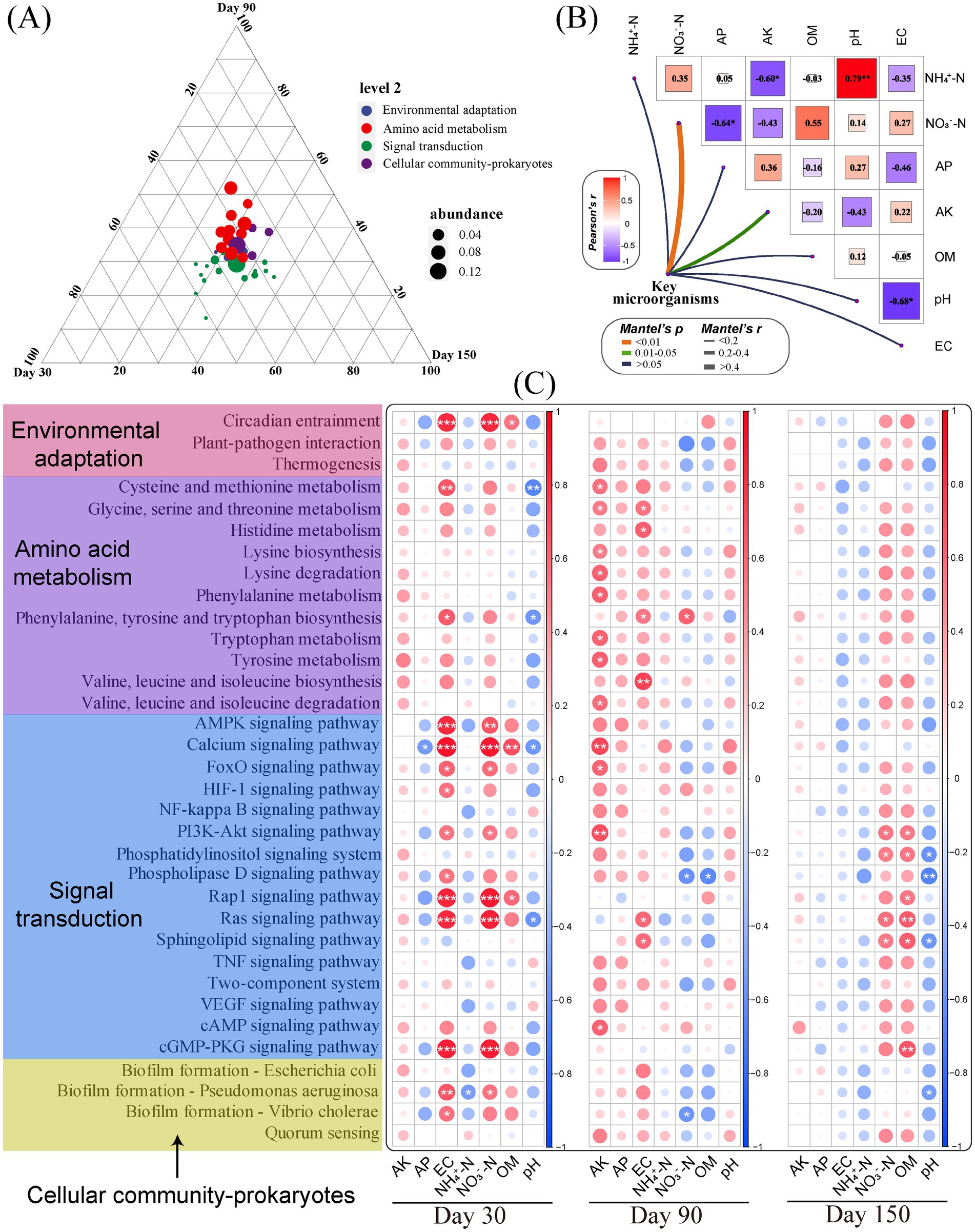
Figure 5. Soil microbial function analysis at different times. (A) Ternary phase analysis of the relative abundance of KEGG level 2 functional genes; (B) Mantel detection of key microorganisms and soil environmental factors; (C) Correlation analysis of the relative abundance of KEGG level 3 functional genes and soil environmental factors in three periods. The significance level was expressed as *p < 0.05, **p < 0.01, and ***p < 0.001.
In addition, we constructed a ternary plot of functional genes at level 3 over three time points (Figure 5A) and a correlation analysis between the relative abundance of functional genes and environmental factors (Figure 5C) to explore how different times and functions affect the yield and quality of C. pilosula. The results showed that the signal transduction was closer to day 30 and day 150, and the amino acid metabolism was closer to day 90. The results of correlation analysis showed that the function of key microorganisms was positively correlated with EC on day 30, AK on day 90, and OM on day 150. After that, we selected 10 functional genes with strong correlation at level 3 (5 on day 30, 3 on day 90, and 2 on day 150) for further analysis, and the results showed that, on day 30, the relative abundance of calcium signaling pathway, Rap1 signaling pathway, Ras signaling pathway, and cGMP-PKG signaling pathway in T4 was the highest. Both were significantly higher than the CK treatment (Supplementary Figure S2). During the initial application of the BOF treatment (especially bacteria), a large number of Bacillus as “invaders” broke the balance of the soil ecosystem, and the soil native bacterial community maintained the balance of the ecosystem by strengthening signal transduction. Calcium, Rap1, Ras, Cgmp-PKG, and other signal pathways are important intracellular signal transmission systems. They are involved in conducting many hormone signaling pathways (inducing or inhibiting the synthesis of hormones within plants to affect crop yield and quality) (Zhang et al., 2022), metabolite signaling pathways (secreting enzymes directly affect the nutrient absorption and metabolic processes of crops) (Wang et al., 2023), and immune system signaling pathways (regulating the plant immune system to affect crop yield and quality) (Joseph et al., 2019). On day 90, glycine, serine, and threonine metabolism in T2 and T3 were significantly higher than those in CK, and valine, leucine, and isoleucine biosynthesis in T2 were significantly higher than those in CK. The biosynthesis of these amino acids is one of the functions of metabolites and plants to drive the change of diseased soil, and their metabolism may play a certain role in the weakening of the root rot of C. pilosula (Li et al., 2018; Wen et al., 2022). On day 150, the sphingolipid signaling pathway in T4 was significantly higher than that in CK. Sphingolipids, as one of the important components of the cell membrane, participate in various physiological processes (Sánchez-Rangel et al., 2015), and play an important role in the stability of the rhizosphere bacterial community at the later stage of the experiment.
Based on the species and functional relative abundance of the samples, the relationship between key microorganisms’ abundance and functional abundance was analyzed to explore the specific microorganisms that performed major functions during the three periods (Supplementary Figure S3). The results show that Nakamurell contributed most to the calcium signaling pathway, phenylalanine, tyrosine, and tryptophan biosynthesis, Rap1 signaling pathway, cGMP-PKG signaling pathway, and Ras signaling pathway, which were 54.03, 51.17, 50.62, 50.92, and 26.03%, respectively. Pedomicrobium contributed most to glycine, serine, and threonine metabolism, phosphatidylinositol signaling system, and sphingolipid signaling pathway, which were 43.85, 33.74, and 27.19%, respectively. Microlunatus contributed most to biofilm formation—Pseudomonas aeruginosa (32.78%). Rubrobacter contributed most to the biosynthesis of valine, leucine, and isoleucine (33.23%).
4 Conclusion
In this study, the yield and quality of C. pilosula were higher, and the disease incidence was lower in the BOF treatment than in the CF treatment. These findings indicate that soil ecology was enhanced by the application of BOFs. The addition of BOFs promoted the diversity and richness of rhizosphere bacteria in the middle growth stage of C. pilosula. The SEM showed that the rhizosphere bacterial community directly drove the yield and quality of C. pilosula after BOF application, while the chemical fertilizer increased the yield and quality by influencing soil environmental factors. LEFSe and network analysis showed that Microlunatus, Rubrobacter, Luteitalea, Nakamurella, and Pedomicrobium as key microorganisms, may play a key role in improving the soil of C. pilosula. At the same time, the microbial co-occurrence network revealed stronger interactions between soil microorganisms after the BOF treatment, which are key to soil biodiversity and ecosystem stability. Key microorganisms increase the signal transduction and amino acid metabolism functions of the rhizosphere bacterial community in the early and middle growth period of C. pilosula. Nakamurell and Pedomicrobium made a major contribution to the change in microbial function. These results concluded that BOFs and CFs have different mechanisms for improving the yield and quality of Chinese herbal medicine. BOFs are more beneficial to soil ecological health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
BH: Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. YuC: Methodology, Software, Visualization, Writing – original draft. YiC: Investigation, Visualization, Writing – review & editing. DL: Resources, Writing – review & editing. HF: Funding acquisition, Resources, Writing – review & editing. CZ: Investigation, Writing – review & editing. DW: Supervision, Visualization, Writing – review & editing. JW: Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation Project of China (32102257), the Science and Technology Cooperation Project of Shandong and Gansu (YDZX2021098), the Science and Technology Project of Guizhou [110202201019 (LS-03)] and Sichuan (SCYC202312), and the Youth Foundation for the Key Open Laboratory of Tobacco Pest Monitoring and Integrated Management (KLTPMIMT2022-10).
Acknowledgments
We thank TopEdit for linguistic assistance during the preparation of this manuscript.
Conflict of interest
DL were employed by the Sichuan Provincial Tobacco Company Liangshanzhou Company. HF and CZ were employed by the Shandong Hezhong Kangyuan Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1484727/full#supplementary-material
References
Adams, R., Miletto, M., Taylor, J., and Bruns, T. (2013). Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 7, 1262–1273. doi: 10.1038/ismej.2013.28
Carlos, J. (2022). Soil microbial inoculant has no effect on plant growth, fruit yield, fruit disorders, and soilborne diseases in bell pepper. J. Veg. Sci. 28, 409–416. doi: 10.1080/19315260.2022.2036888
Chen, Y., Lv, X., Qin, Y., Zhang, D., Zhang, C., Song, Z., et al. (2023). Effects of different botanical oil meal mixed with cow manure organic fertilizers on soil microbial community and function and tobacco yield and quality. Front. Microbiol. 14:1191059. doi: 10.3389/fmicb.2023.1191059
Chen, R., Wang, K., Wang, F., He, Y., Long, L., and Tian, X. (2018). Rubrobacter indicoceani sp. nov., a new marine actinobacterium isolated from Indian Ocean sediment. Int. J. Syst. Evol. Microbiol. 68, 3487–3493. doi: 10.1099/ijsem.0.003018
Chu, X., Bai, N., Zheng, X., Wang, Q., Pan, X., Li, S., et al. (2022). Effects of straw returning combined with earthworm addition on nitrification and ammonia oxidizers in paddy soil. Front. Microbiol. 13:1069554. doi: 10.3389/fmicb.2022.1069554
Ding, Y., Wang, H., Zhang, Q., Chai, B., Lei, X., Ye, M., et al. (2022). Effects of dissolved oxygen on phosphorus transformation in reservoir sediments: novel insights on bacterial community and functional genes. J. Soils Sediments 22, 2094–2104. doi: 10.1007/s11368-022-03233-9
Edgar, R., Haas, B., Clemente, J., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Gao, C., Guo, Z., Lu, X., Chen, H., Liu, L., Yu, Z., et al. (2018). Hexaricins, Pradimicin-like polyketides from a marine sediment-derived Streptosporangium sp. and their antioxidant effects. J. Nat. Prod. 81, 2069–2074. doi: 10.1021/acs.jnatprod.8b00397
Hartmann, H., Link, R., and Schuldt, B. (2021). A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiol. 41, 901–905. doi: 10.1093/treephys/tpab011
Huang, B., Chen, Y., Pei, Z., Jiang, L., Zhang, Y., Wang, J., et al. (2023). Application of microbial organic fertilizers promotes the utilization of nutrients and restoration of microbial community structure and function in rhizosphere soils after dazomet fumigation. Front. Microbiol. 13:1122611. doi: 10.3389/fmicb.2022.1122611
Huang, B., Jia, H., Han, X., Gou, J., Huang, C., Wang, J., et al. (2021). Effects of biocontrol Bacillus and fermentation bacteria additions on the microbial community, functions and antibiotic resistance genes of prickly ash seed oil meal-biochar compost. Bioresour. Technol. 340:125668. doi: 10.1016/j.biortech.2021.125668
Joseph, O., Hassan, K., Kathy, L., Joseph, K., and Henry, F. (2019). A soil bacterium can shape belowground interactions between maize, herbivores and entomopathogenic nematodes. Plant Soil 437, 83–92. doi: 10.1007/s11104-019-03957-7
Kakihara, H., and Ogura, S. (2022). Effect of soil acidification on regrowth of orchardgrass (Dactylis glomerata) under application of grazing cattle dung, cattle manure compost, and chemical fertilizer. Grassl. Sci. 68, 255–262. doi: 10.1111/grs.12361
Khan, M., Jalil, S., Chopra, P., Chhillar, H., and Ansari, M. (2021). Role of GABA in plant growth, development and senescence. Plant Gene 26:100283. doi: 10.1016/j.plgene.2021.100283
Kim, D., Nguyen, L., and Oh, S. (2020). Ecological impact of the antibiotic ciprofloxacin on microbial community of aerobic activated sludge. Environ. Geochem. Health 42, 1531–1541. doi: 10.1007/s10653-019-00392-6
Kishore, D., Birzu, G., Hu, Z., DeLisi, C., Korolev, K., and Segrè, D. (2023). Inferring microbial co-occurrence networks from amplicon data: a systematic evaluation. MSystems. 8:e0096122. doi: 10.1128/msystems.00961-22
Leng, F., Zhang, B., Zhu, X., Kong, Z., Wang, X., and Wang, Y. (2023). A microecological research reveals seasonal variation in rhizosphere-endophytic bacteria and growth and development of Codonopsis pilosula root. Rhizosphere 28:100805. doi: 10.1016/j.rhisph.2023.100805
Li, X., Han, G., Eller, F., Hui, D., Zhu, L., Chen, L., et al. (2021). Acclimation of coastal wetland vegetation to salinization results in the asymmetric response of soil respiration along an experimental precipitation gradient. Agric. For. Meteorol. 310:108626. doi: 10.1016/j.agrformet.2021.108626
Li, S., Li, Y., and Smolke, C. (2018). Strategies for microbial synthesis of high-value phytochemicals. Nat. Chem. 10, 395–404. doi: 10.1038/s41557-018-0013-z
Li, Y., Shi, C., Wei, D., Gu, X., Wang, Y., Sun, L., et al. (2022). Soybean continuous cropping affects yield by changing soil chemical properties and microbial community richness. Front. Microbiol. 13:1083736. doi: 10.3389/fmicb.2022.1083736
Liu, Y., Zhang, M., Li, Y., Zhang, Y., Huang, X., Yang, Y., et al. (2023). Influence of nitrogen fertilizer application on soil acidification characteristics of tea plantations in karst areas of Southwest China. Agriculture 13:849. doi: 10.3390/agriculture13040849
Lodi, R., Dong, X., Jiang, C., Sun, Z., Deng, P., and Sun, S. (2023). Antimicrobial activity and enzymatic analysis of endophytes isolated from Codonopsis pilosula. FEMS Microbiol. Ecol. 99:fiad071. doi: 10.1093/femsec/fiad071
Ma, Y., Gu, Y., Liu, J., Zhang, Y., Wang, X., Xia, Z., et al. (2023). Deciphering the rhizosphere bacteriome associated with biological control of tobacco black shank disease. Front. Plant Sci. 14:1152639. doi: 10.3389/fpls.2023.1152639
Nassira, B., Yacine, G., Miyada, Z., Fawzia, C., Nasserdine, S., Florence, M., et al. (2018). Biocontrol and plant-growth-promoting capacities of actinobacterial strains from the Algerian Sahara and characterisation of Streptosporangium becharense SG1 as a promising biocontrol agent. Bio Sci. Tec. 28, 858–873. doi: 10.1080/09583157.2018.1501466
National Pharmacopoeia Commission (2015). Pharmacopoeia of the People's Republic of China, edition, part 4. Beijing: China Medical Science Press.
Olsen, S. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. New York, NY: US Department of Agriculture. No, 939.
Peng, L., Zhang, Y., Druzhinina, I., Kubicek, C., Wang, Y., Zhu, Z., et al. (2022). A facultative ectomycorrhizal association is triggered by organic nitrogen. Curr. Biol. 32, 5235–5249. doi: 10.1016/j.cub.2022.10.054
Rahimi, A., Amirnia, R., Siavash, M., El, E., Hanapi, S., and Sayyed, R. (2021). Effect of different biological and organic fertilizer sources on the quantitative and qualitative traits of Cephalaria syriaca. Horticulturae 7:397. doi: 10.3390/horticulturae7100397
Rani, R., Raj, K., and Surindra, S. (2021). Production of compost with biopesticide property from toxic weed Lantana: quantification of alkaloids in compost and bacterial pathogen suppression. J. Hazard. Mater. 401:123332. doi: 10.1016/j.jhazmat.2020.123332
Rong, L., Zhao, L., Zhao, L., Cheng, Z., Yao, Y., Yuan, C., et al. (2021). LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 773:145640. doi: 10.1016/j.scitotenv.2021.145640
Sánchez-Rangel, D., Rivas-San, V., De, L., Nájera-Martínez, M., and Plasencia, J. (2015). Deciphering the link between salicylic acid signaling and sphingolipid metabolism. Front. Plant Sci. 6:00125. doi: 10.3389/fpls.2015.00125
Schloss, P., Westcott, S., Ryabin, T., Hall, J., Hartmann, M., Hollister, E., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Sharma, D., Shukla, A., and Gupta, M. (2022). Evaluation of bio-control agents against fusarium wilt of cucumber. Internat J. Eco. Plants. 9, 334–339. doi: 10.23910/2/2022.0495
Shen, T., Lei, Y., Pu, X., Zhang, S., and Du, Y. (2021). Identification and application of Streptomyces microflavus G33 in compost to suppress tomato bacterial wilt disease. Appl. Soil Ecol. 157:103724. doi: 10.1016/j.apsoil.2020.103724
Tao, C., Li, R., Xiong, W., Shen, Z., Liu, S., Wang, B., et al. (2020). Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 8:137. doi: 10.1186/s40168-020-00892-z
Wang, T., Cheng, K., Huo, X., Meng, P., Cai, Z., Wang, Z., et al. (2022a). Bioorganic fertilizer promotes pakchoi growth and shapes the soil microbial structure. Front. Plant Sci. 13:1040437. doi: 10.3389/fpls.2022.1040437
Wang, Y., Xu, C., Zhang, B., Wu, M., and Chen, G. (2017). Physiological and proteomic analysis of rice (Oryza sativa L.) in flag leaf during flowering stage and milk stage under drought stress. Plant Growth Regul. 82, 201–218. doi: 10.1007/s10725-017-0252-9
Wang, Z., Yang, H., Ma, Y., Jiang, G., Mei, X., Li, X., et al. (2022b). WGCNA analysis revealing molecular mechanism that bio-organic fertilizer improves pear fruit quality by increasing sucrose accumulation and reducing citric acid metabolism. Front. Plant Sci. 13:1039671. doi: 10.3389/fpls.2022.1039671
Wang, Z., Yang, T., Mei, X., Wang, N., Li, X., Yang, Q., et al. (2022c). Bio-organic fertilizer promotes pear yield by shaping the rhizosphere microbiome composition and functions. Microbiol. Spectr. 10:e0357222. doi: 10.1128/spectrum.03572-22
Wang, C., Yu, Q., Ji, N., Zheng, Y., Taylor, J., Guo, L., et al. (2023). Bacterial genome size and gene functional diversity negatively correlate with taxonomic diversity along a pH gradient. Nat. Commun. 14:7437. doi: 10.1038/s41467-023-43297-w
Wen, T., Xie, P., Penton, C., Hale, L., Thomashow, L., Yang, S., et al. (2022). Specific metabolites drive the deterministic assembly of diseased rhizosphere microbiome through weakening microbial degradation of autotoxin. Microbiome 10:177. doi: 10.1186/s40168-022-01375-z
Xu, Y., Bi, Z., Zhang, Y., Wu, H., Zhou, L., and Zhang, H. (2022). Impact of wine grape pomace on humification performance and microbial dynamics during pig manurecomposting. Bioresour. Technol. 358:127380. doi: 10.1016/j.biortech.2022.127380
Xu, N., Tan, G., Wang, H., and Gai, X. (2016). Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 74, 1–8. doi: 10.1016/j.ejsobi.2016.02.004
Yan, D., Wang, Q., Li, Y., Ouyang, C., Guo, M., and Cao, A. (2017). Analysis of the inhibitory effects of chloropicrin fumigation on nitrification in various soil types. Chemosphere 175, 459–464. doi: 10.1016/j.chemosphere.2017.02.075
Yang, Y., Li, X., Liu, J., Zhou, Z., Zhang, T., and Wang, X. (2017). Bacterial diversity as affected by application of manure in red soils of subtropical China. Biol. Fertil. Soils 53, 639–649. doi: 10.1007/s00374-017-1209-x
Yuan, M., Yu, Y., Li, H., Dong, N., and Zhang, X. (2014). Phylogenetic diversity and biological activity of Actinobacteria isolated from the Chukchi shelf marine sediments in the Arctic Ocean. Mar. Drugs 12, 1281–1297. doi: 10.3390/md12031281
Zhang, Q., Yang, J., Zhou, X., Ding, Y., Wang, Y., Zhu, X., et al. (2022). Soil-borne bacterium Klebsiella pneumoniae promotes cluster root formation in white lupin through ethylene mediation. New Phytol. 237, 1320–1332. doi: 10.1111/nph.18600
Keywords: chemical and bio-organic fertilizers, Codonopsis pilosula , co-occurrence network, microbial community, yield and quality
Citation: Huang B, Chen Y, Cao Y, Liu D, Fang H, Zhou C, Wang D and Wang J (2024) The structure and function of rhizosphere bacterial communities: impact of chemical vs. bio-organic fertilizers on root disease, quality, and yield of Codonopsis pilosula. Front. Microbiol. 15:1484727. doi: 10.3389/fmicb.2024.1484727
Edited by:
Dongdong Yan, Institute of Plant Protection, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Zhoubin Liu, Hunan Agricultural University, ChinaHaitao Yu, Gansu Academy of Agricultural Sciences (CAAS), China
Copyright © 2024 Huang, Chen, Cao, Liu, Fang, Zhou, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Cao, eWljYW8xMDAxQDE2My5jb20=; Dong Wang, OTgzMDEyOUAxNjMuY29t; Jie Wang, d2FuZ2ppZUBjYWFzLmNu
†These authors have contributed equally to this work
 Bin Huang
Bin Huang Yuxuan Chen
Yuxuan Chen Yi Cao
Yi Cao Dongyang Liu
Dongyang Liu Hua Fang4
Hua Fang4 Jie Wang
Jie Wang