- 1Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, China
- 2Peking University Health Science Center, Beijing, China
- 3Library, Peking University Third Hospital, Beijing, China
- 4Department of Gastroenterology, Peking University Third Hospital, Beijing, China
Background: Inflammatory bowel diseases (IBD) are chronic inflammation conditions affecting the gastrointestinal tract. Studies point out an association between Helicobacter pylori (H. pylori) infection and IBD. This study aims to visually assess the research trends and hotspots in the field of H. pylori infection and IBD, review mainstream perspectives in this field, and provide a foundation for future research and treatment.
Methods: We searched the Web of Science Core Collection Database for literature related to H. pylori and IBD, using VOS viewer to generate visual charts.
Results: A total of 246 publications were included, with articles being the predominant type of document. A significant increase in the number of publications was observed after 2011. China contributed the most of researches. Keyword clusters revealed that the researches primarily focused on immune mechanism, gut microbiome, diagnosis and treatment of IBD. Time trend results indicated that current researches centered on gut microbiota and immune mechanisms.
Conclusion: H. pylori infection may have a protective effect on IBD. The exact mechanisms remain unclear and may involve immunomodulation and changes of gut microbiota. Further researches are necessary for better understanding this relationship and its implications for clinical practice. Further researches and clinical practice should pay attention to this topic.
1 Introduction
Inflammatory bowel diseases (IBD), including two main types-Crohn’s disease (CD) and ulcerative colitis (UC) (Singh et al., 2017), are a group of chronic conditions affecting the gastrointestinal tract, which are characterized by episodes of abdominal pain, diarrhea, hematochezia, and weight loss (Chang, 2020). In CD, any segment of the gastrointestinal tract can be affected, and the most affected areas are distal ileum, cecum, perianal region, and colon. In UC, the inflammation is confined exclusively to the colon and rectum. Regarding histology, in CD the intestinal pathologies present submucosal thickening, transmural inflammation, ulceration, and non-caseous granuloma. In UC, the inflammation is limited to the mucosal layer, with recesses and abscesses (James et al., 2014; Mak et al., 2020).
More than six million people suffer from IBD worldwide (Imawana et al., 2020). Once in the 20th century, IBD was predominantly regarded as a disease prevalent in Western nations, particularly in Europe and North America (Molodecky et al., 2012; Kaplan and Windsor, 2021). However, the 21st century has witnessed a notable increase in the onset of IBD worldwide, particularly in developing countries in Asia, including China and India (Kaplan, 2015). IBD can not only influence individuals’ quality of life, but also induce other diseases such as malnutrition or even gastrointestinal cancer (Quaglio et al., 2022; Nadeem et al., 2020). The rising prevalence of IBD imposes an escalating socioeconomic burden (Kaplan, 2015). The pathogenesis of IBD involves a combination of host genetic predisposition (Graham and Xavier, 2020), intestinal flora (Glassner et al., 2020; Guo et al., 2022; Haneishi et al., 2023), environmental factors (Mak et al., 2020; Adolph et al., 2022), and immunological abnormalities (Monteleone et al., 2006), but the specific mechanisms is unclear (Mak et al., 2020; Guo et al., 2022). The intestinal flora is crucial to the homeostasis of the gastrointestinal tract, which contributes to immune tolerance process and the development or differentiation of the local and systemic immune system. It may also protect the host from pathogenic intestinal infections. Thus, the dysbiosis of gut microbiota triggers deregulated immune responses, acting as a significant contributor to the development of IBD (Balani et al., 2023).
Helicobacter pylori (H. pylori) is a Gram-negative, spiral bacterium that infects the stomach. H. pylori infection generally occurs in childhood via person-to-person transmission (Li et al., 2023). It is estimated that approximately 50% of the global population is infected with this bacterium (Ren et al., 2022). H. pylori infection is a risk factor for various gastrointestinal diseases, including gastritis, peptic ulcers, and gastric cancer. Additionally, some studies report that H. pylori infection can increase the risk of autoimmune diseases (Yang et al., 2023). However, other studies suggest that H. pylori infection may confer a protective effect against the development of IBD (Fialho et al., 2019; Castaño-Rodríguez et al., 2017). In addition to hygiene hypothesis using to explain the epidemiological correlation between H. pylori infection and IBD (Tanner et al., 2023) another potential explanation involves the alteration of gut microbiota composition resulting from H. pylori colonization (Bai et al., 2022; Pellicano et al., 2020). However, contrasting evidence exists, with some studies suggesting that H. pylori infection is linked to an increased risk of IBD during childhood (Kong et al., 2023; Man et al., 2008; Zhong et al., 2021; Sayar et al., 2019).
In 1969, Alan Pritchard articulated the concept of bibliometrics as “the application of mathematical and statistical methods to books and other media of communication,” using this approach for literature analysis. Bibliometric analysis relies on various publication parameters (such as countries, institutions, and authors) and integrates mathematical and statistical techniques to quantitatively describe the current status, research hotspots, and research trends (Shi et al., 2022). It can process extensive datasets and assess outputs within specific fields (da Cruz et al., 2022). Our study employs bibliometric analysis to examine data pertaining to H. pylori infection and IBD.
2 Materials and methods
2.1 Data sources and screening strategies
We utilized Web of Science Core collection (WOSCC), the most used database for bibliometric analysis to collect literature. This database encompasses a diverse array of prominent international academic journals in the world (Yang et al., 2022). All studies included were identified in WOSCC on February 20, 2024. We employed MeSH database and free text words to improve retrieval accuracy. The search strategy incorporated the following terms include: TS = (“Inflammatory Bowel Disease” OR “inflammatory Bowel Diseases” OR “Crohn’s Enteritis” OR “Regional Enteritis” OR “Crohn’s Disease” OR “Crohns Disease” OR “Inflammatory Bowel Disease 1” OR “Granulomatous Enteritis” OR “Ileocolitis” OR “Granulomatous Colitis” OR “Terminal Ileitis” OR “Regional Ileitides” OR “Regional Ileitis” OR “Idiopathic Proctocolitis” OR “Ulcerative Colitis” OR “Colitis Gravis”) AND TS = (“Helicobacter nemestrinae” OR “Helicobacter pylori” OR “H. pylori” OR “Campylobacter pylori” OR “Campylobacter pylori subsp. Pylori” OR “Campylobacter pyloridis” OR “Campylobacter” OR “C. pylori”).
2.2 Screening criteria and results
The inclusion criteria are as follows:
1. The literature pertains to topics of IBD and H. pylori, and their relationship.
2. The literature was written in English.
The exclusion criteria as follows:
1. Incomplete, corrected or retracted publication.
The complete text was evaluated by two authors independently according to the inclusion and exclusion criteria.
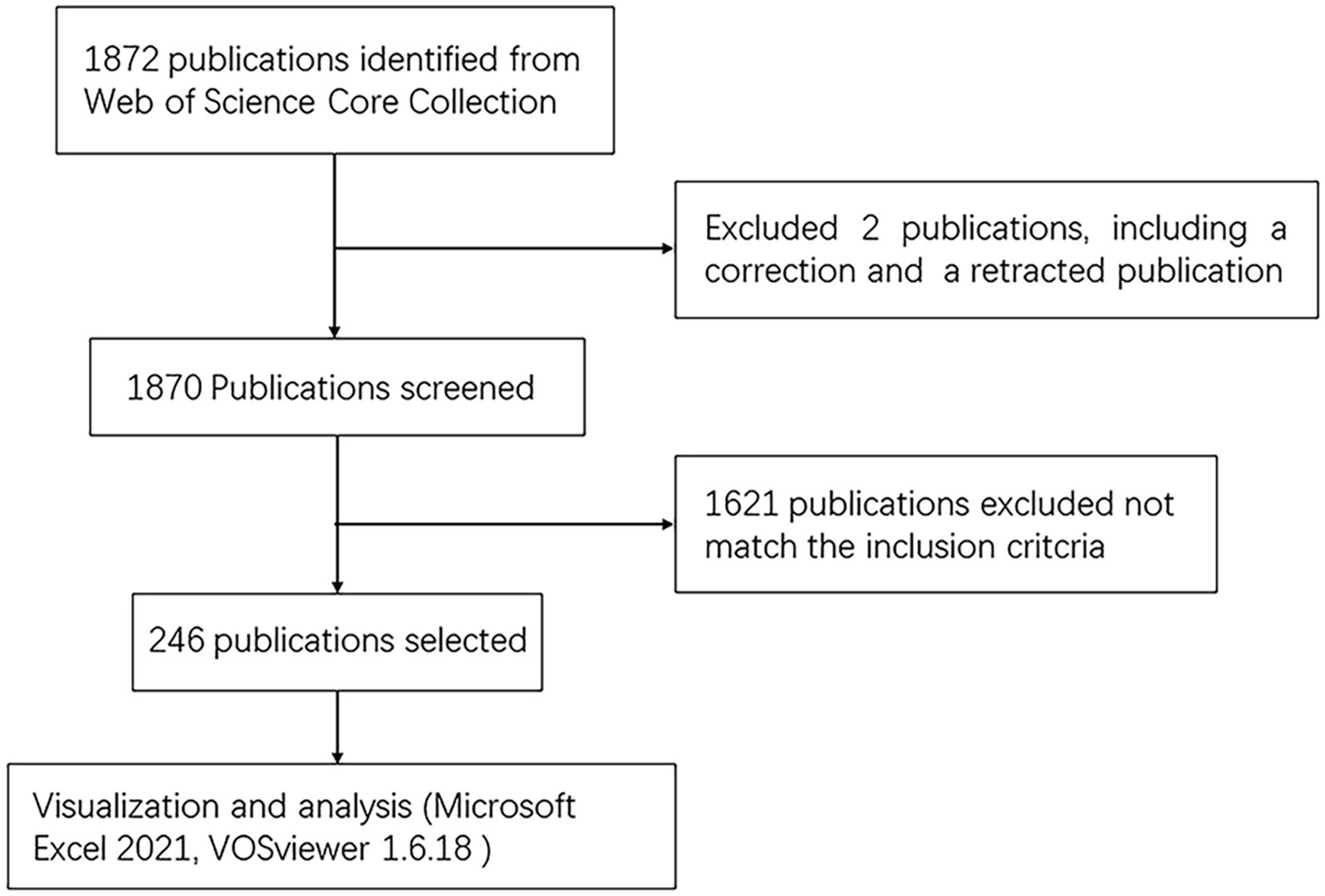
As illustrated in Figure 1, 1,872 articles were identified from the WOSCC database, of which two were excluded due to corrected and retracted. The selection of the remaining papers was conducted by two authors. At last, 246 articles were eventually included.
2.3 Data extraction and statistical analysis
Two authors (ZW and SZ) independently evaluated the abstracts and titles of all pertinent publications. Any discrepancy in evaluation was addressed by the third author (YSh). The data from the WOSCC database were collected by Microsoft Office Excel 2021. The software VOSviewer 1.6.18 was used to analyze the data like countries/regions, institutions, authors, keywords, journals, co-authorships, co-occurrences, citations and to generate visual charts (Waltman et al., 2010). In these visualization results, different nodes represent different parameters such as countries/regions, institutions, authors and keywords. Nodes are classified into different clusters according to the degree of linking (distinguished by color). The size of each node refers to the frequency of occurrence of associated parameters and the thickness of the connections between nodes illustrates the strength of linking.
3 Results
3.1 Publication output
The 246 documents can be categorized into two periods by time trend of publication, the years 1994 to 2010, and the years 2011 to 2023. The publication counts for each period illustrate the evolving trend of research within this domain (Figure 2A). From 1994 to 2010, a total of 80 publications were recorded. From 2011, the number of publications is increasing, culminating in 22 publications in 2022 alone. From 2011 to 2023, 166 publications were published, accounting for about 67% of the total.
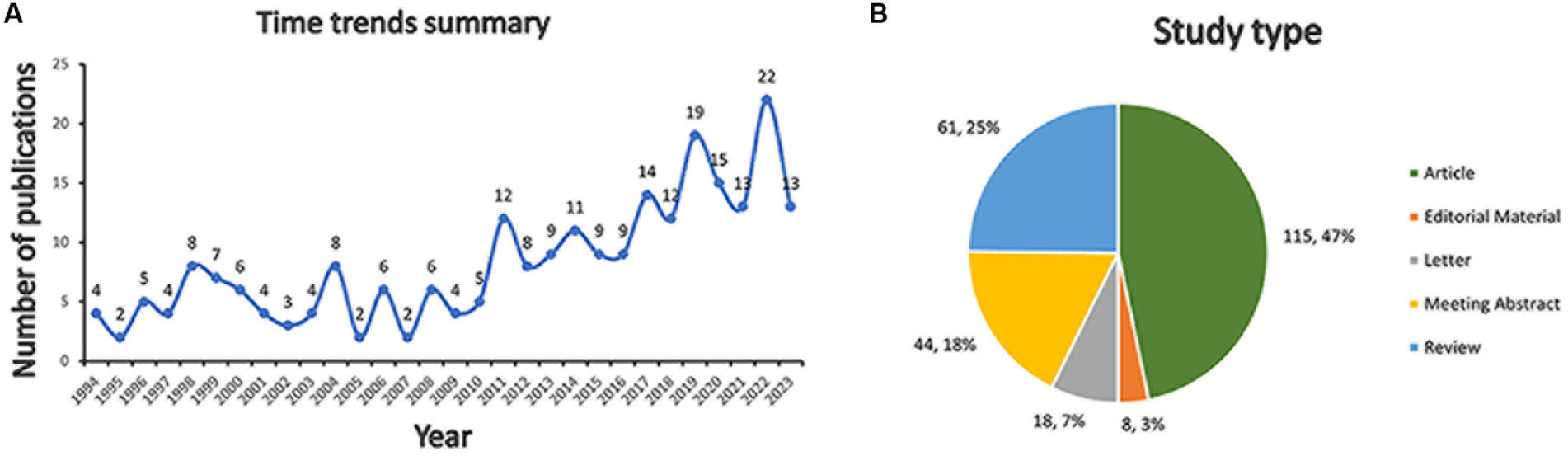
Figure 2. Bibliometric analysis of H. pylori and IBD. (A) Time trends summary. (B) Study type composition.
There were 115 (47%) articles, 61 (25%) reviews, 44 (18%) meeting abstracts, 18 (7%) letters, and 8 (3%) editorial materials (Figure 2B).
3.2 Country and institution analysis
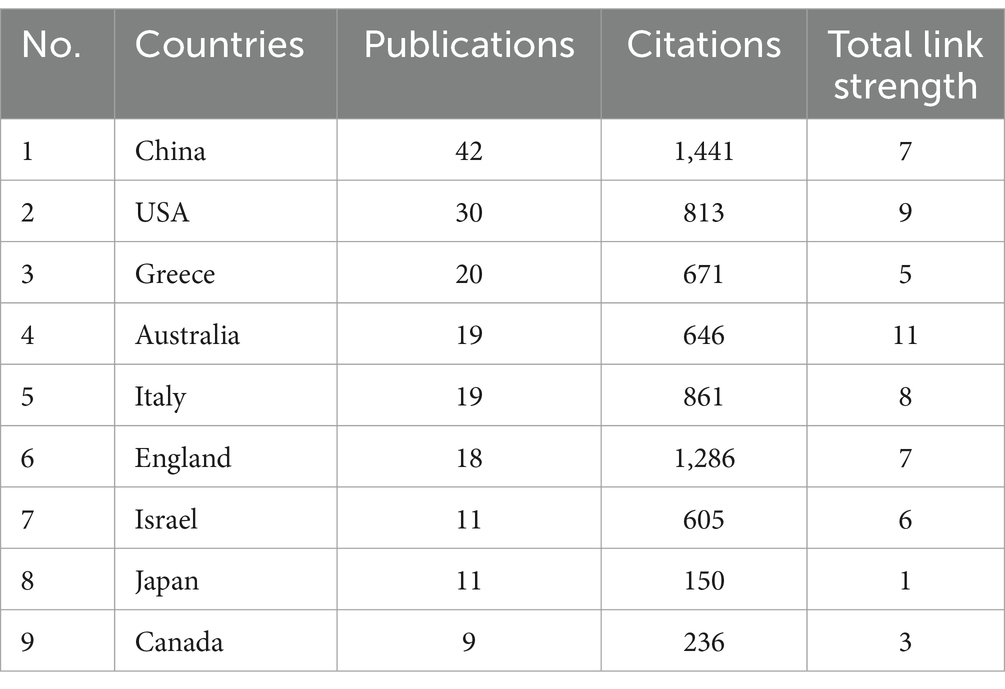
The top 9 countries with the most publications (above 9 records) are listed in Table 1. China published most (42 records), followed by USA (30 records), Greece (20 records) and Australia (19 records). As shown in Figure 3A, China has established cooperative relations with four countries, including Australia, England, USA and Israel. Australia is the most cooperative country with a total link strength (TLS) of 11, collaborating with China, New Zealand, England and Malaysia (Figure 3A).
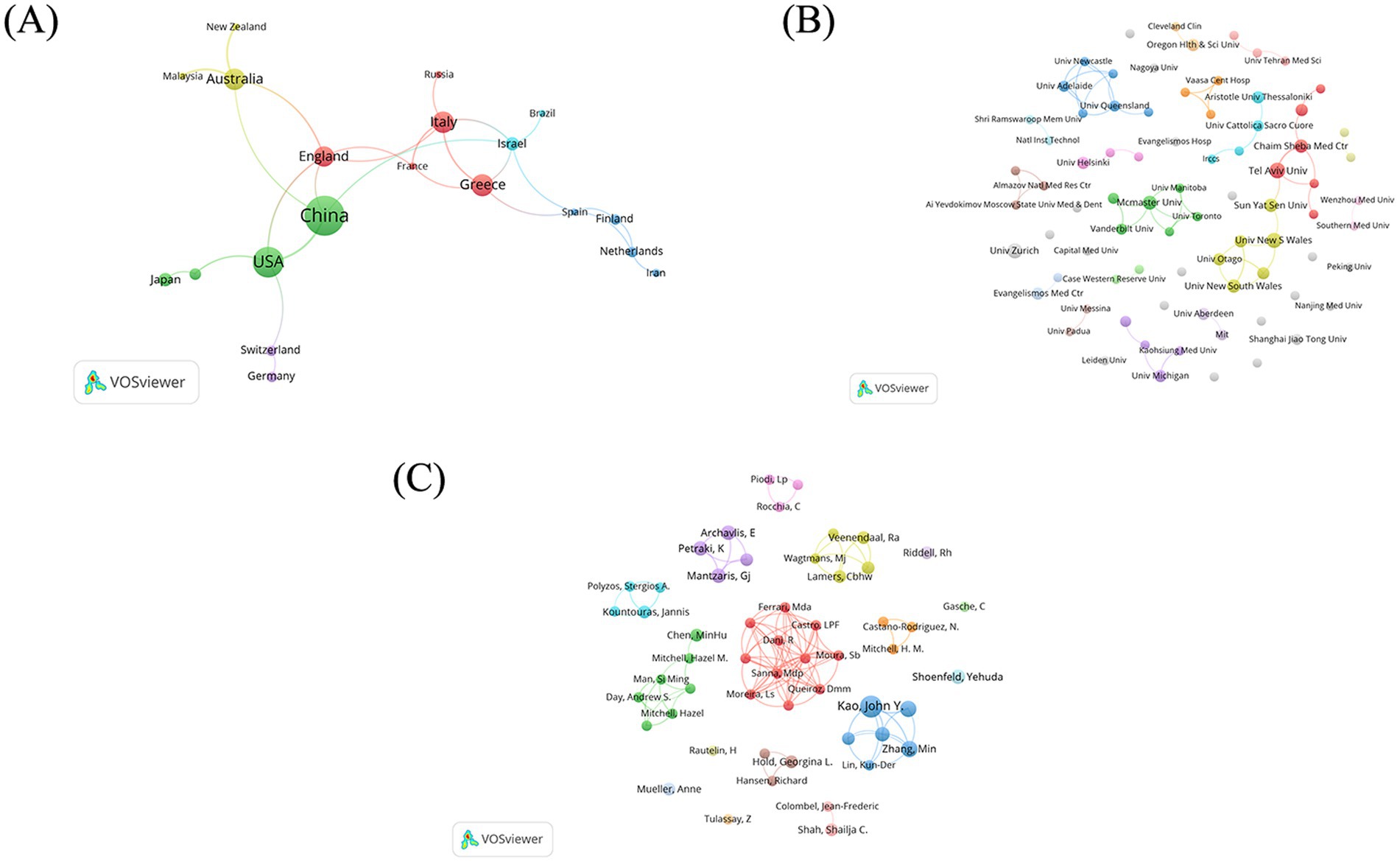
Figure 3. Bibliometric analysis of co-occurrence network map. (A) Countries. (B) Institutions. (C) Authors.
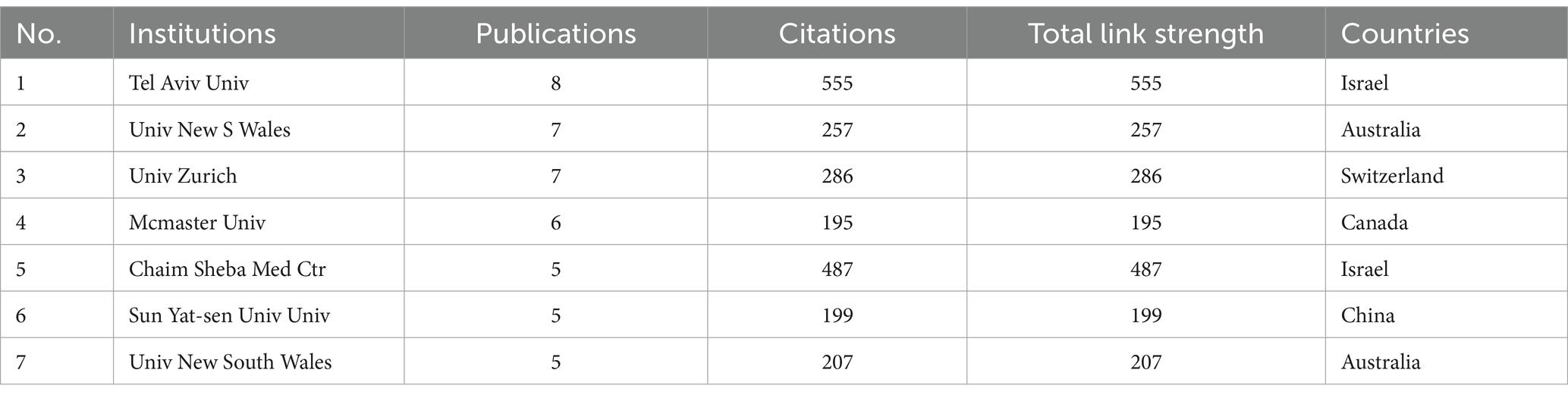
A total of 341 institutions participated in the aforementioned 246 studies (Figure 3B). Among these, 79 institutions published more than 2 documents. The top 9 institutions with the most publications (above 5 records) are detailed in Table 2. Tel Aviv University (Tel Aviv Univ) emerges as the most prolific institution, with 8 related papers, followed by The University of New South Wales (Univ New S Wales) (7 records) in Australia, University of Zurich (Univ Zurich) (7 records) in Switzerland and McMaster University (McMaster Univ) (6 records) in Canada. Tel Aviv University (Tel Aviv Univ) and Chaim Sheba Medical Center (Chaim Sheba Med Ctr) from Israel are the main collaborators (link strength 4). There is a cooperation between Sun Yat-sen University (Sun Yat-sen Univ) in China, the University of New South Wales (Univ New S Wales) in Australia and Tel Aviv University (Tel Aviv Univ) in Israel (link strength 1).
3.3 Author and journal analysis
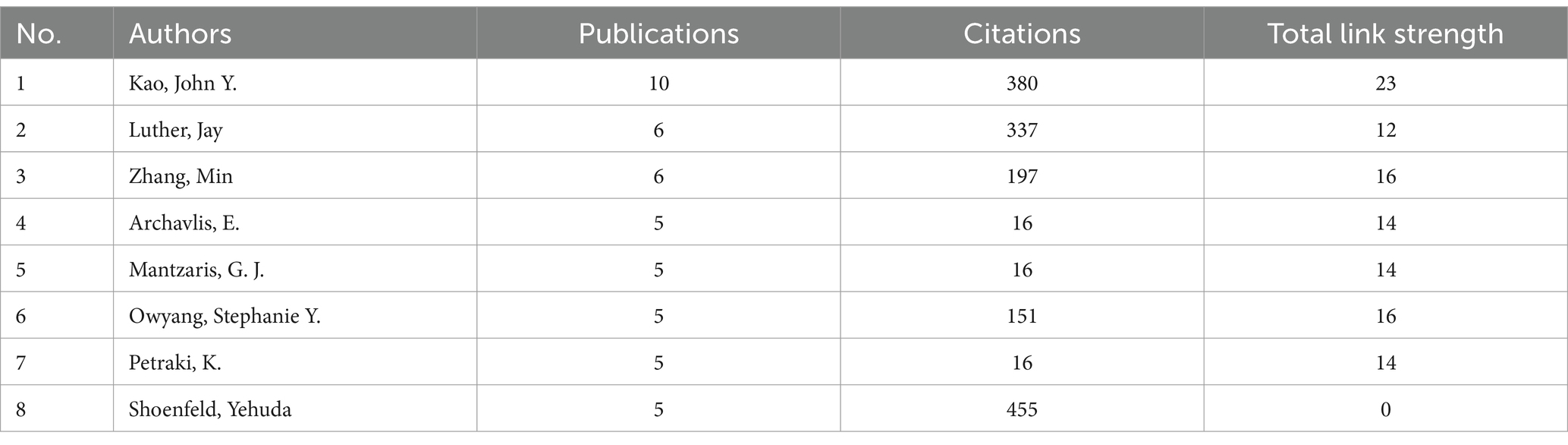
A total of 1,106 authors published papers on the topic of H. pylori and IBD, 54 of whom have published more than 3 papers on the subject. The clusters of authors are illustrated in Figure 3C. Among them, 11 authors form the most closely collaborative cluster (link strength 3). The top eight authors with the most publications are shown in Table 3. Prof. Kao, John Y. published the most documents (10 records), followed by Prof. Luther, Jay (6 records) and Prof. Zhang, Min (6 records).
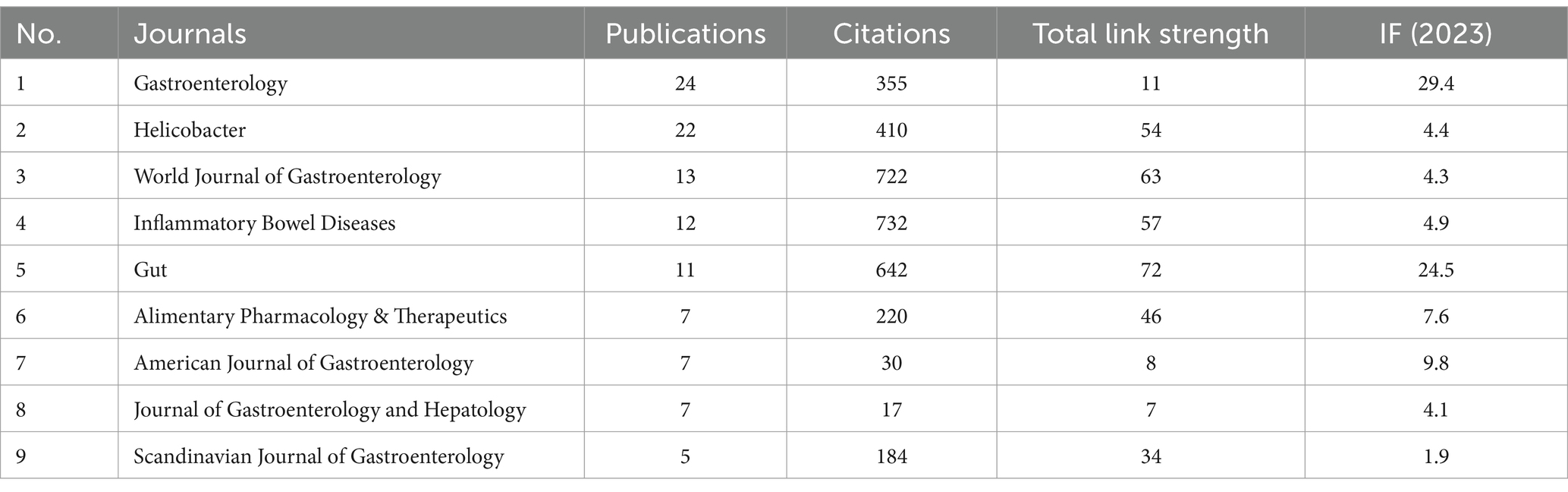
A total of 112 journals published the pertinent papers, of which 25 journals published more than 5 papers. The two most prolific journals were Gastroenterology (n = 24), followed by Helicobacter (n = 22), collectively representing 19% of the publications in the WOSCC database. Table 4 presents the top 9 most active journals.
3.4 Keyword visualization and co-occurrence analysis
We manually standardized the keywords, merged and replaced similar keywords before constructing the cluster maps. The analysis involved a total of 65 keywords, selected from 854 keywords in total (occurrence >5). The four most popular keywords were “inflammatory-bowel-disease” (TLS: 795, occurrences: 146), “Helicobacter pylori” (TLS: 760, occurrences: 141), “Crohn’s Disease” (TLS: 481, occurrences: 83), and “ulcerative colitis” (TLS: 453, occurrences: 78). The analysis yielded six distinct clusters of keywords (Figure 4). Cluster 1 comprised 21 items that represented diseases and immunity (shown in red) including keywords such as vacuolating cytotoxin, regulatory T-cells, NF-kappa-B, dendritic cells, immune tolerance and molecular mimicry. Cluster 2 included 16 items associated with intestinal microbiota (shown in green) including keywords such as Campylobacter concisus, Escherichia coli, Faecalibacterium prausnitzii and fecal microbiota. Cluster 3 comprised 9 items that represented intestinal symptoms (shown in blue) with keywords including Crohn’s disease, gastritis, lesions and ulcer. Cluster 4 contained 9 items that related to the diagnosis of bacterial infections and experimental techniques (shown in yellow) and included keywords such as DNA, PCR, and Cluster 5 comprised 7 items that described clinical features and status of diseases (shown in purple) and included keywords such as cytokine production, diagnosis and prevalence. Cluster 6 consisted of 3 items pertaining to therapeutic treatments for IBD (shown in orange), including keywords such as nonsteroidal anti-inflammatory drugs, seroprevalence, sulfasalazine.
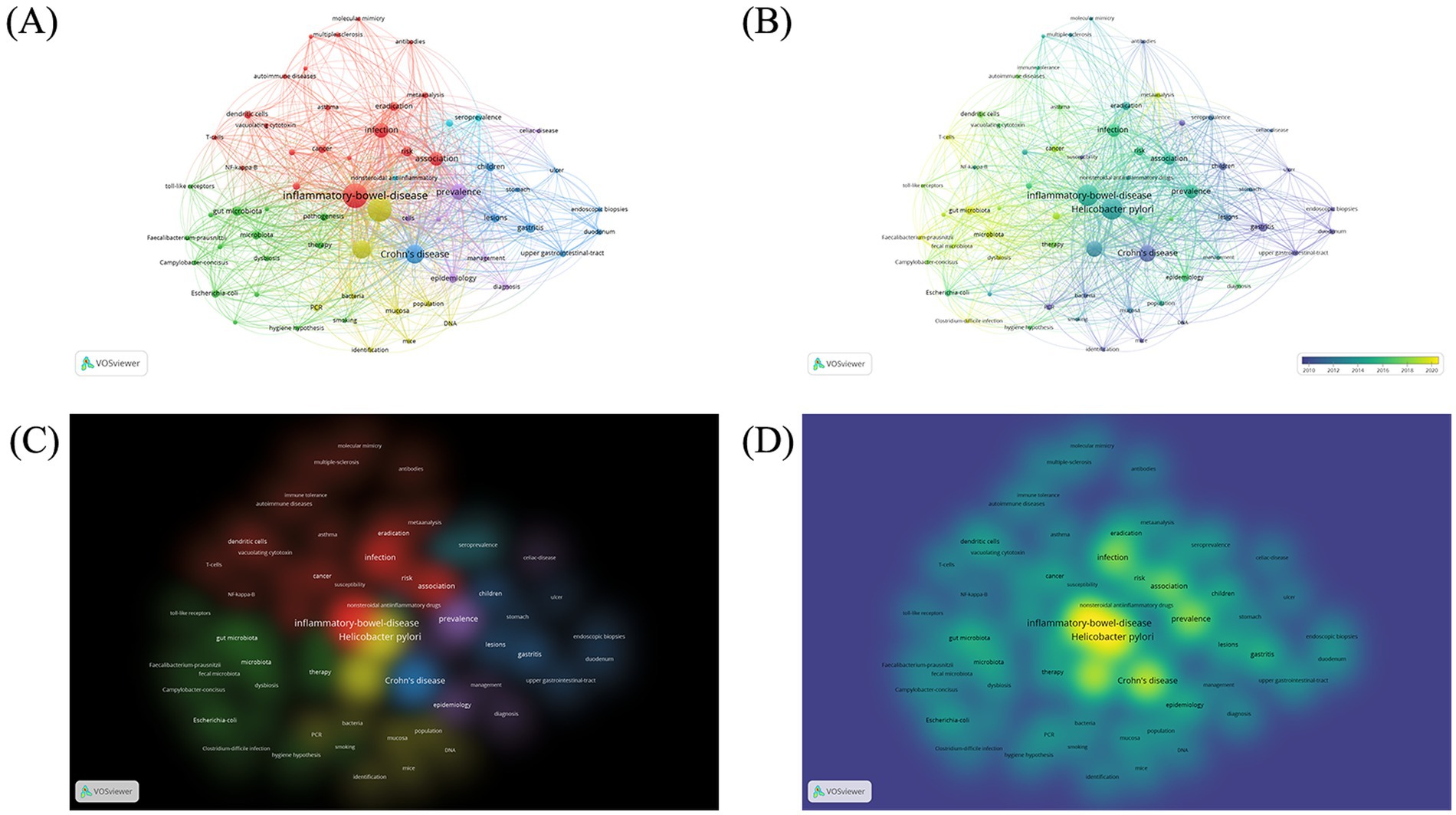
Figure 4. Keyword co-occurrence bibliometric analyses. (A) Network visualization. (B) Overlay visualization. (C) Item density visualization. (D) Cluster density visualization.
The time trend analysis revealed that early research concerning the association between H. pylori infection and IBD predominantly concentrated on the characterization and diagnosis of clinical features and related symptoms. Currently, there is a switch in focus towards elucidating the mechanisms of H. pylori infection on IBD, including gut microbiota and immunological mechanisms.
4 Discussion
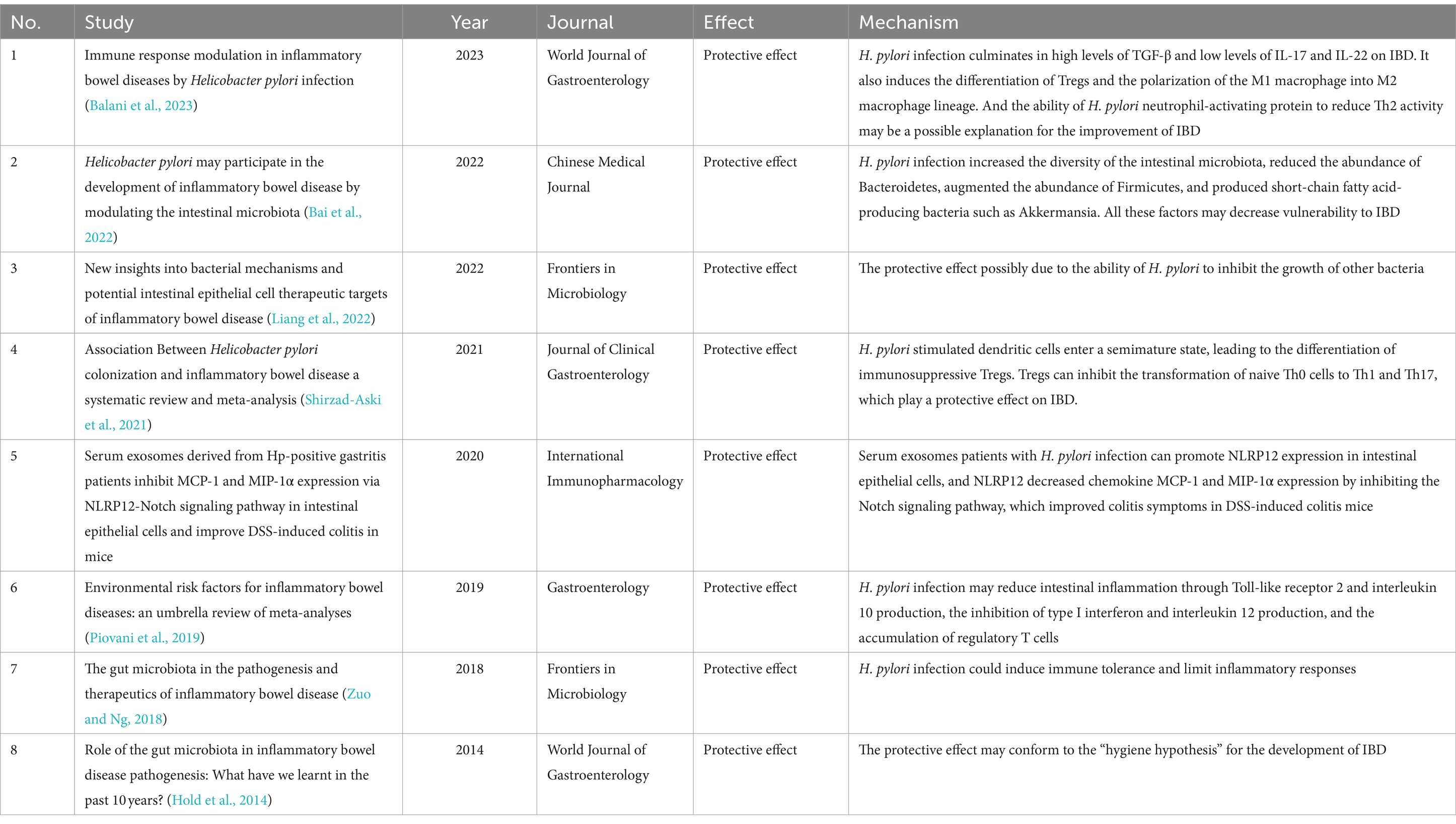
Utilizing bibliometric analysis, we analyzed 246 articles published from 1994 to February 20, 2024. Most studies find a negative relationship between H. pylori infection and IBD. This relationship is usually demonstrated from the following three perspectives: individuals with H. pylori infection are less likely develop IBD (Elghannam et al., 2024; Ravikumara, 2023; Kahlam et al., 2023; Kayali et al., 2018); the prevalence of H. pylori infection is diminished in patients with IBD (Mizukami et al., 2023); and the eradication of H. pylori is associated with an increased risk of IBD (Taskin, 2023). Animal studies demonstrated that colonization by H. pylori reduces colonic inflammation and lessens the damage to the colonic mucosal induced by dextran sulfate sodium (DSS) administration (Hu et al., 2024; Kim et al., 2013). However, some researchers posit that there is no significant correlation between H. pylori infection and the onset of IBD (Kong et al., 2023; Pokrotnieks and Sitkin, 2023). For example, researchers explain the association between H. pylori infection and IBD by the fucosylation status of host proteoglycan in gastrointestinal tract, that is, the two are non-causal (Pokrotnieks and Sitkin, 2023). We summarized the critical publications that elucidate potential mechanisms of the protective effect on IBD in Table 5. The mechanisms of H. pylori infection focus on the regulation of immune system (Shirzad-Aski et al., 2021; Setshedi and Watermeyer, 2022; Wang et al., 2022) and alterations in the composition of the intestinal flora when infected (Bai et al., 2022; Liang et al., 2022) (Figure 5).
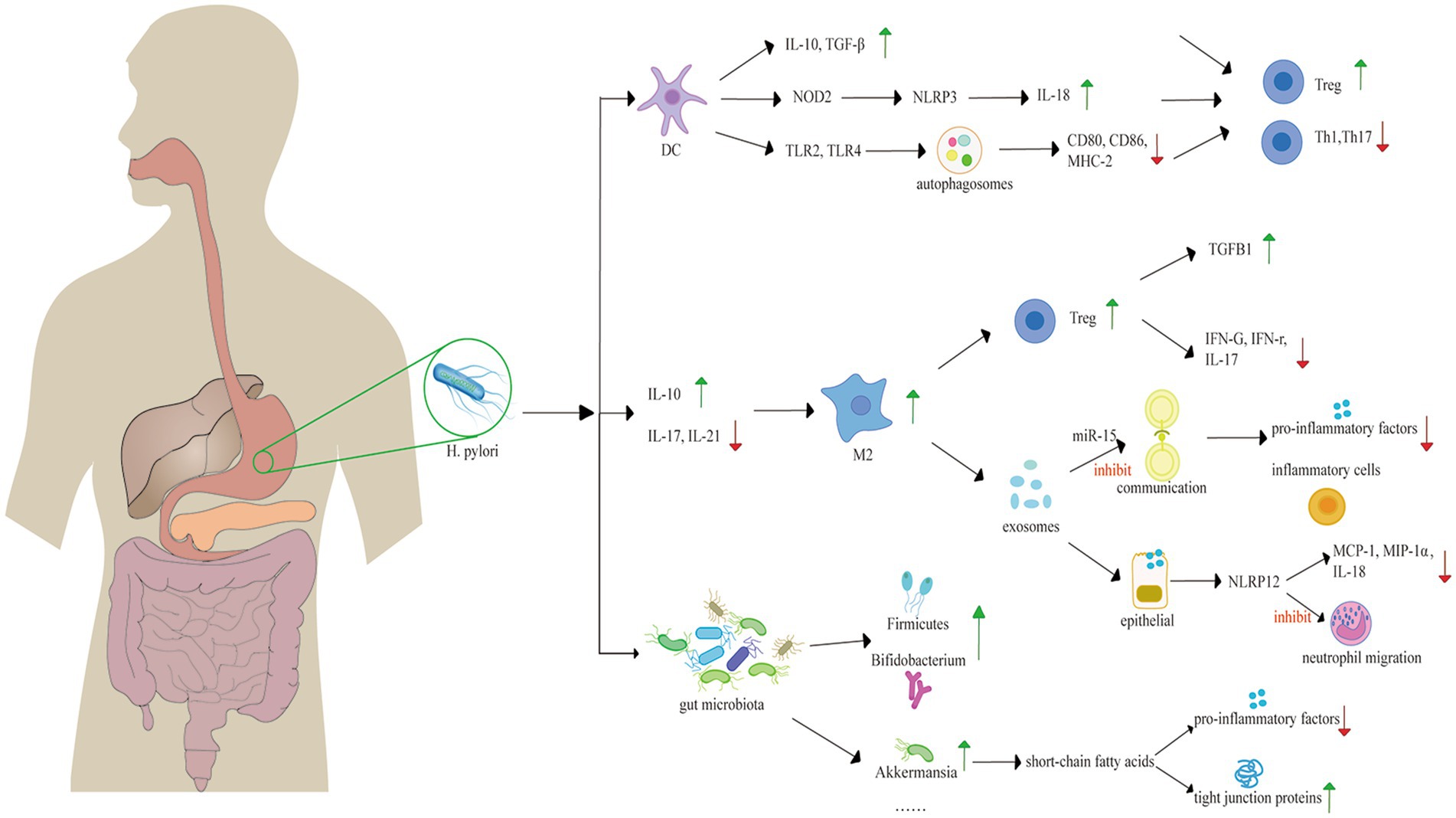
Figure 5. Mechanisms underlying the protective role of H. pylori against IBD, including the change of gut microbiota and immune system regulation. H. pylori promotes the transformation of DCs into tolerogenic DCs, expressing high levels of anti-inflammatory factors, such as IL-10 and TGF-β. H. pylori activates NOD2 to activate the NLRP3 inflammasome of DCs, leading to the expression of IL-18. H. pylori triggers autophagosome activation, diminishing the secretion of MHC-2, CD80 and CD86. The secretion of these immune factors induce the differentiation of immunosuppressive Tregs, rather than Th1 or Th17 cells. H. pylori also induces the switch of M2 macrophages with increasing IL-10 and diminishing IL-17 and IL-21. M2 macrophages can induce the proliferation of Treg, conferring an anti-inflammatory effect. Exosomes derived from macrophages can suppress the inflammatory response. H. pylori alters gut microbiota with a healthier diversity, increasing SCFAs production, and protecting the intestinal barrier with increased tight junction proteins. H. pylori, Helicobacter pylori; DC, dendritic cell; IL, interleukin; TLR, Toll-like receptor; Treg, regulatory T cell; M2, M2 macrophage.
4.1 Helicobacter pylori infection affects intestinal immune mechanisms in patients with IBD
Immunosuppression may explain the negative link between H. pylori infection and IBD (Murad, 2016). Tolerogenic H. pylori-specific dendritic cells (DCs) are fundamental in immunosuppression. As the most potent antigen-presenting cells and the unique activators of naive T lymphocytes (Th0), DCs play a key role in modulating adaptive immunity through presenting pathogen antigens and inducing the differentiation of Th0 cells into various lymphocyte subsets (Yu et al., 2018). Tolerogenic DCs induced by H. pylori express less MHC-2, CD80, CD86, and CD40; secrete fewer pro-inflammatory factors and produce increasing anti-inflammatory factors, such as interleukin (IL)-10, IL-18, and TGF-β (Yu et al., 2018). This process fosters a specific immune microenvironment that mediates the differentiation of immunosuppressive Tregs, rather than T helper 1 cells (Th1) or Th17 cells from naive Th0 cells (Papamichael et al., 2014). Th1 cells and Th17 cells secret interferon (IFN)-γ, IL-17, and IL-23, which protect the body from pathogens. However, excessive activation of Th1 and Th17 cells disrupt the immunological balance between helper T cells and regulatory T cells, leading to cytokine dysregulation, which plays a significant role in the development of IBD (Park et al., 2023; Zhang et al., 2020; Stromnes et al., 2008). Furthermore, Tregs inhibit the conversion of Th0 cells into Th1 and Th17 cells and sustain the tolerogenic state of DCs through direct contact and the secretion of IL-10 and TGF-β (Arnold and Müller, 2016). Tregs in the gastric mucosa of H. pylori-infected individuals can migrate to other lymphoid tissues in intestinal tract, exerting an protective effect (Yu et al., 2018).
The induction of tolerogenic DCs by H. pylori involves multiple potential mechanisms. One such mechanism contains the NLR family pyrin domain containing 3 (NLRP3) inflammasome. NLRP3 inflammasome is a multiprotein complex that plays a crucial role in the innate immune response (Balani et al., 2023). H. pylori binds to pattern recognition receptors-Toll like receptor 2 (TLR2) and nucleotide oligomerization domain 2 (NOD2) presented on DCs, activating the inflammasome, thereby culminating in the cleavage/activation of caspase-1, an enzyme responsible for processing pro-IL-1β and pro-IL-18. IL-18 is related to the differentiation of Tregs and the modulation of immune responses mediated by CD4+ T cells (Balani et al., 2023; Engler et al., 2015). Studies demonstrate that the activation of NLRP3 may improve the prognosis of IBDs-mice infected with H. pylori, which exhibits less severity of DSS-induced colitis, and characterized by notably reduced inflammation and less epithelial alterations (Kim et al., 2013). Another mechanism involves the formation of autophagosome in DCs induced by H. pylori via TLR2/TLR4. CD80, CD86, and MHC-2 molecules accumulate within the autophagic vacuoles that contain H. pylori, which down-regulates their surface expression, thereby impairing the function of DC cells (Wang et al., 2010).
The macrophage phenotype switch plays a role in the correlation between H. pylori infection and IBD. Macrophages are categorized into M1 and M2 subtypes, according to their polarization state. M1 macrophages are pro-inflammatory cells, contributing to a tissue-destructive pro-inflammatory response, while M2 macrophages exhibit anti-inflammatory properties and participate in tissue repair after injury. The conversion of the M1 macrophage lineage into M2 will lead to milder inflammatory responses (Mills, 2014). Researches demonstrate that colonization of H. pylori is associated with the phenotypic switch of M1subtype towards M2, which is linked to concomitant increase of CagA-dependent Th2 pattern cytokines and more Treg cells, and TGF-β, as well as the inhibition of TLR-mediated signaling pathways, resulting in decreased levels of IL-17F and IL-21 (Zhang et al., 2018; Marotti et al., 2008). Experiments show that TGF-β is key in the differentiation of Tregs-inducing by M2 macrophages (Schmidt et al., 2016; Tiemessen et al., 2007). Tregs induced by M2 macrophages reduce expression of pro-inflammatory cytokines, including lower levels of IFNG mRNA, IFN-γ, and IL17A mRNA, while express higher level of TGFB1 mRNA. These results suggest that M2-Tregs possess an ability of immunosuppressive (Schmidt et al., 2016).
Exosomes generated from macrophages induced by H. pylori play a role in the immunosuppression. Exosomes that contain microRNA-155, serve as molecular carriers in the communication between immune cells. MiR-155 is a negative regulator, diminishing the secretion of pro-inflammatory factors. Murine experiments demonstrate that the presence of miR-155 within exosomes correlates with a reduction of inflammatory cells, such as neutrophils and monocytes (Wang et al., 2016).
Serum exosomes induced by H. pylori infection can modulate the immune response. Research indicates that the serum exosomes lead to an upregulation of NLRP12 expression in intestinal epithelial cells, which inhibits the expression of chemokine MCP-1 and MIP-1α in these cells, alleviating DSS-induced colitis (Chen et al., 2020). Similar to NLRP3, NLRP12 activates NLRP12 inflammasomes, which activates caspase-1, regulating the release of interleukin like IL-18 (Wang et al., 2002). The persistent activation and excessive recruitment of neutrophils are characteristic features of various inflammatory diseases (Drury et al., 2021), producing high levels of reactive oxygen species (ROS), proteases, pro-inflammatory cytokines and mediators, such as IL-8, TNF-α, and leukotriene B4, leading to damage of the epithelial barrier and recruit monocytes and more neutrophils to the gastrointestinal tract (Biasi et al., 2013; Wéra et al., 2016; Zhou and Liu, 2017; Aviello and Knaus, 2017). However, NLRP12 deregulates the TLR-mediated expression of inflammatory cytokines (Zaki et al., 2011), modulates neutrophil functions and inhibits their migration (Zamoshnikova et al., 2016).
In conclusion, H. pylori infection protects against the development of IBD through interaction with various immune components, including tolerogenic DCs, Tregs, inflammasomes, macrophages, exosomes, and so on. However, this information alone does not comprehensively elucidate the mechanisms underlying the protective effects of H. pylori, warranting further investigation.
4.2 Helicobacter pylori infection alters the gut microbiota in patients with IBD
Dysbiosis of gut microbiota is fundamental pathogenic factor of IBD (Hu et al., 2024). Studies demonstrate that patients with IBD exhibit an altered intestinal microecological environment, characterized by diminished microbial diversity and increased bacterial instability. The microbiota in canines with experimentally induced IBD identifies Campylobacter as the predominant genus (26.12%), followed by Helicobacter (24.53%), Anaerobiospirillum (16.52%), and Fusobacterium (10.16%), collectively constituting 77.33% of the bacterial composition (Mao et al., 2023). At the genus level, there are significant increases in Actinobacteria, Proteobacteria, Roseisolibacter, Rhodoplanes, Collinsella, Pseudarthrobacter, Angelakisella, and Campylobacter, alongside a notable decrease in the relative abundance of Bacteroidetes, Firmicutes, Oscillospira, Alistipes, Barnesiella, Gastranaerophilales, Allobaculum, Subdoligranulum, Phascolarctobacterium, Parabacteroides, Prevotella, Alloprevotella, Ruminococcus torques group, Faecalibacterium, Sutterella, and Desulfovibrio, and bacteria producing short-chain fatty acid (SCFA) (Mao et al., 2023; Caruso et al., 2020; Andoh and Nishida, 2023). In individuals infected with H. pylori, study indicates a decrease in the levels of Bacteroidetes (Fujimori, 2021). However, H. pylori infection also results in an increase in the abundance of Firmicutes, and probiotic bacteria such as Bifidobacterium, Lactobacillus, and short-chain fatty acid-producing bacteria including Akkermansia and members of the Muribaculaceae family within the Bacteroidetes phylum (Balani et al., 2023; Bai et al., 2022; Hu et al., 2024). These alterations contribute to a more diverse and healthier alpha diversity of the intestinal microbiota (Hu et al., 2024). Therefore, the alterations in the intestinal flora induced by H. pylori may be linked to the protective effects against IBD.
SCFA may be involved in molecular patterns that cause the alteration of microbiota. SCFA may have a protective effect on intestinal tract (Liang et al., 2022). It interacts with G protein-coupled receptors, modulate immune cell activity, and diminish the expression of pro-inflammatory factors (Nicolas and Chang, 2019), influencing immune responses (Vieira et al., 2015). Furthermore, SCFA contributes to the maintenance of intestinal barrier integrity and homeostasis (Vieira et al., 2015). Researches show that in the colon of DSS-treated mice, the expression of three tight junction proteins-claudin 1, occludin, and ZO-1, essential for preserving intestinal barrier function, is reduced. However, this decrease is significantly reversed after the colonization by H. pylori, suggesting a function of intestinal barrier repaired by H. pylori colonization (Hu et al., 2024; Chelakkot et al., 2018).
Furthermore, several studies suggest that the protective effect of H. pylori involves the combination of the secretion of gastric acid (Balani et al., 2023), intestinal hormones (Lopetuso et al., 2014), and the presence of an intact type IV secretion system (T4SS) (Heimesaat et al., 2014). Chronic H. pylori infection results in hypochlorhydria, which enables colonization of the distal intestine by acidic pH-sensitive bacteria; H. pylori infection also enhance the secretion of gastrin and leptin, modifying gut metabolism (Balani et al., 2023). Additionally, the presence of an intact T4SS in H. pylori is linked to alterations in the composition of the microbiota of distal gastrointestinal tract. Strains with a cag pathogenicity island are capable of translocating the effector protein CagA into host cells via the T4SS (Wessler and Backert, 2008). Once inside the host cell, CagA gets tyrosine phosphorylated by host kinases and interferes signal transduction pathways that regulate cell polarity, inflammation, proliferation, and apoptosis (Hu et al., 2024; Heimesaat et al., 2014). Despite the pathogenic potential associated with H. pylori CagA+, these strains may positively influence the prognosis of IBD. A meta-analysis indicates a significant reduction in the prevalence of IBD associated with H. pylori CagA+ strains, particularly in CD (Tepler et al., 2019; Bretto et al., 2024). Hypochlorhydria and hypergastrinemia are observed in experiments involving Mongolian gerbils infected with H. pylori T4SS+, which may be an explanation for the alterations in the microbiota within the gastrointestinal tract (Rieder et al., 2005).
4.3 The interaction between Helicobacter pylori therapy and treatment of IBD
H. pylori infection causes chronic gastritis, which can progress to severe gastroduodenal pathologies, such as peptic ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma (Malfertheiner et al., 2023). Thus, the eradication therapy for H. pylori is recommended by all societies (Elghannam et al., 2024). However, researches indicate that patients may be at an increased risk of developing CD or UC after the eradication therapy (Mizukami et al., 2023; Murad, 2016; Tursi, 2006; Jovanovic et al., 2001; Liu et al., 2024). A case study reports that a 34-year-old man experienced rapid onset of CD after H. pylori eradication. Potential explanations are the imbalance between the Th1 and Th2 immune patterns, which may contribute to susceptibility to CD. H. pylori eradication is proven to reduce Th2 pattern cytokines (notably IL-4, IL-5 and IL-6) while increasing Th1 pattern pro-inflammatory cytokines, which may trigger the onset of CD (Murad, 2016; Papamichael et al., 2014). Another hypothesis posits that the antibiotics used to treat H. pylori may disrupt the gut microbiome, leading to decreased diversity, similar to the microbiota composition in patients with IBD (Matsumura et al., 2001; Triantafillidis et al., 2003; Huang et al., 2024). However, studies indicate that the eradication of H. pylori is not linked to the development of IBD (Kong et al., 2023; Yang et al., 2024; Dilaghi et al., 2024). While a large amount of studies investigate the implication of H. pylori eradication on IBD, seldom research exists concerning the effects of antibiotics utilized for H. pylori eradication on patients with IBD. Several investigations report different influences of antibiotics employed in quadruple therapy on the development of IBD, including tetracycline, clarithromycin and metronidazole (Maloy, 2012; Bejcek et al., 2024; Lloyd et al., 2020). One study indicates a positive relationship between the use of oral tetracyclines and the onset of IBD, particularly CD (Maloy, 2012). A retrospective cohort study shows that there is an increased risk of Clostridioides difficile infections in patients treated with metronidazole-class antibiotics (Bejcek et al., 2024). Additionally, researches propose that clarithromycin may serve as an anti-inflammatory therapeutic agent for IBD, through a combination of innovative bioinformatic analyses and laboratory experiments. Clarithromycin inhibits LPS-induced NF-κB (p65) DNA binding in vivo, alleviates DSS-induced colitis in murine models, and suppresses TNF-induced NF-κB (p65) nuclear localization in human ileal organoids, further study is necessary to confirm its effects in patients (Lloyd et al., 2020).
Some meta-analyses suggest that antibiotic therapy may facilitate remission in patients with active CD and UC (Khan et al., 2011; Fedorak and Ismond, 2016), despite the expansion of antibiotic is linked to an increased risk of IBD (Faye et al., 2023; Piovani et al., 2019; Ananthakrishnan et al., 2018) and the converse results from other studies (Huang et al., 2011; Kronman et al., 2012). The underlying mechanisms may be associated with changes in the gut microbiota-different classes of antibiotics induce different patterns of microbiota alteration because of their spectrum and bacterial target. For instance, macrolides, which inhibit protein synthesis, have active coverage against Gram-positive bacteria, including Actinobacteria and Firmicutes (Ianiro et al., 2016). Additionally, certain antibiotics have beneficial impacts on gut microbiota by promoting the growth of probiotic bacteria. Such as nitrofurantoin, a broad-spectrum antibiotic, is proven to temporarily increase the population of the beneficial Bifidobacterium genus (Vervoort et al., 2015).
The medications used to treat IBD include sulfasalazine, mesalazine, corticosteroids and immunosuppressants. Sulfasalazine and mesalazine are classified as 5-aminosalicylic acids, while corticosteroids and immunosuppressants are categorized as immune system inhibitors. A study demonstrates that IBD treatments, such as anti-TNF-α agents, immunosuppressants, or corticosteroids have no relationship with the prevalence of H. pylori infection (Triantafillidis et al., 2014). Additionally, research indicates that there is no significant difference in the prevalence of H. pylori infection between these two classes of medications (Zhong et al., 2021).
Regarding the eradication of H. pylori infection in children, the latest ESPGHAN/NASPGHAN guidelines recommend a discussion with families concerning the advantages and disadvantages of eradication in children diagnosed with H. pylori-associated gastritis and other gastrointestinal diseases (Homan et al., 2024). Our study supports this guideline. Besides, it is important to remember that H. pylori has coevolved with humans over thousands of years. Despite more than half of the global population is infected, clinical disease occurs only in a small proportion of the infected individuals (Ravikumara, 2023). It has been estimated that the H. pylori infection is associated with gastric and duodenal ulcers in 1–10% of infected patients, gastric carcinoma in 0.1–0.3%, and gastric MALT lymphoma in less than 0.01% (McColl, 2010). Therefore, we propose that H. pylori infection should not be eradicated unconditionally in every case, as the bacterium may confer a protective effect in patients with IBD. The purpose of this review is not to dispute the fact that H. pylori is a class 1 human carcinogen, but to highlight potential beneficial effects against IBD. Clinicians should carefully assess the risks and benefits of H. pylori eradication in patients with IBD and comorbid H. pylori infection.
5 Conclusion
In this study, we explore research trends and hotspots regarding the relationship between H. pylori infection and pathogenesis of IBD using bibliometric analysis. To the best of our knowledge, no bibliometrics studies on this specific topic have been published previously. We objectively assess the contributions of various academic institutions and researchers, finding that the number of papers on H. pylori and IBD has increased significantly over the past two decades. China, the United States, and Australia are at the forefront of this field with the most published journals being Gastroenterology and Helicobacter.
The mainstream view is that H. pylori infection may have a protective effect in IBD patients. Recent studies have delved into molecular mechanisms, concentrating on immune cells, cytokines, and changes in microbial composition. We review several well-researched mechanisms, such as regulation of immune system and alterations in intestinal flora, and also examine the interaction between H. pylori treatment and IBD.
Despite notable progress in the last decade, challenges remain. The exact relationship between H. pylori and IBD is still uncertain. Many studies suggest that H. pylori infection may reduce the risk and severity of IBD (Balani et al., 2023; Bretto et al., 2024; Wu et al., 2023), but others present different perspectives (Mukherjee et al., 2024; Desmedt et al., 2024).
Future studies should focus on clarifying the relationship between H. pylori infection and IBD by investigating mechanisms like changes in gut microbiota and regulation of immune. Such research could provide new insights into the treatment of H. pylori infection.
Lastly, our study has some limitations. We relied on the WOSCC database, which, though extensive, excludes publications from other sources that may influence results. Additionally, selection bias and publication bias may affect the analysis due to manual screening process used for paper selection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZW: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization. SZ: Data curation, Investigation, Writing – original draft, Writing – review & editing. XZ: Investigation, Writing – review & editing. YSu: Investigation, Writing – review & editing. YSo: Project administration, Writing – review & editing. JL: Writing – review & editing. YSh: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Beijing Natural Science Foundation of China, Grant No. J230002 and National Natural Science Foundation of China, Grant No. 81700496, International Institute of Population Health, Peking University Health Science Center (JKCJ202305), Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, and the special fund of the Beijing Clinical Key Specialty Construction Program, P. R. China (2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adolph, T. E., Meyer, M., Schwärzler, J., Mayr, L., Grabherr, F., and Tilg, H. (2022). The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 19, 753–767. doi: 10.1038/s41575-022-00658-y
Ananthakrishnan, A. N., Bernstein, C. N., Iliopoulos, D., Macpherson, A., Neurath, M. F., Ali, R. A. R., et al. (2018). Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 15, 39–49. doi: 10.1038/nrgastro.2017.136
Andoh, A., and Nishida, A. (2023). Alteration of the gut microbiome in inflammatory bowel disease. Digestion 104, 16–23. doi: 10.1159/000525925
Arnold, I. C., and Müller, A. (2016). Helicobacter pylori: does gastritis prevent colitis? Inflamm. Intest. Dis. 1, 102–112. doi: 10.1159/000445985
Aviello, G., and Knaus, U. G. (2017). ROS in gastrointestinal inflammation: rescue or sabotage? Br. J. Pharmacol. 174, 1704–1718. doi: 10.1111/bph.13428
Bai, X. Y., Jiang, L. J., Ruan, G. C., Liu, T. T., and Yang, H. (2022). Helicobacter pylori may participate in the development of inflammatory bowel disease by modulating the intestinal microbiota. Chin. Med. J. 135, 634–638. doi: 10.1097/CM9.0000000000002008
Balani, G. F., Cortez, M. D., Freitas, J., de Melo, F. F., Zarpelon-Schutz, A. C., and Teixeira, K. N. (2023). Immune response modulation in inflammatory bowel diseases by Helicobacter pylori infection. World J. Gastroenterol. 29, 4604–4615. doi: 10.3748/wjg.v29.i30.4604
Bejcek, A., Ancha, A., Lewis, M., Beaver, R., Tecson, K., Bomar, J., et al. (2024). Antibiotic use and risk of Clostridioides difficile infection in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. doi: 10.1111/jgh.16720
Biasi, F., Leonarduzzi, G., Oteiza, P. I., and Poli, G. (2013). Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 19, 1711–1747. doi: 10.1089/ars.2012.4530
Bretto, E., Frara, S., Armandi, A., Caviglia, G. P., Saracco, G. M., Bugianesi, E., et al. (2024). Helicobacter pylori in inflammatory bowel diseases: active protagonist or innocent bystander? Antibiotics 13:267. doi: 10.3390/antibiotics13030267
Caruso, R., Lo, B. C., and Núñez, G. (2020). Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 20, 411–426. doi: 10.1038/s41577-019-0268-7
Castaño-Rodríguez, N., Kaakoush, N. O., Lee, W. S., and Mitchell, H. M. (2017). Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut 66, 235–249. doi: 10.1136/gutjnl-2015-310545
Chang, J. T. (2020). Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664. doi: 10.1056/NEJMra2002697
Chelakkot, C., Ghim, J., and Ryu, S. H. (2018). Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 50, 1–9. doi: 10.1038/s12276-018-0126-x
Chen, Y. F., Huang, J. B., Li, H., Li, P., and Xu, C. D. (2020). Serum exosomes derived from Hp-positive gastritis patients inhibit MCP-1 and MIP-1α expression via NLRP12-notch signaling pathway in intestinal epithelial cells and improve DSS-induced colitis in mice. Int. Immunopharmacol. 88:107012. doi: 10.1016/j.intimp.2020.107012
da Cruz, M. F., Magno, M. B., Jural, L. A., Pimentel, T. C., Ferreira, D., Esmerino, E. A., et al. (2022). Probiotics and dairy products in dentistry: a bibliometric and critical review of randomized clinical trials. Food Res. Int. 151:111228. doi: 10.1016/j.foodres.2022.111228
Desmedt, V., Geldof, J., Hoorens, A., and Lobaton, T. (2024). Case report and review of literature: IgG4-gastroduodenitis in upper GI Crohn’s disease: two separate entities or just a marker of disease severity? Front. Med. 11:1388940. doi: 10.3389/fmed.2024.1388940
Dilaghi, E., Felici, E., Lahner, E., Pilozzi, E., Furio, S., Lucchini, L., et al. (2024). Helicobacter pylori infection in children with inflammatory bowel disease: a prospective multicenter study. BMC Pediatr. 24:417. doi: 10.1186/s12887-024-04902-z
Drury, B., Hardisty, G., Gray, R. D., and Ho, G. T. (2021). Neutrophil extracellular traps in inflammatory bowel disease: pathogenic mechanisms and clinical translation. Cell. Mol. Gastroenterol. Hepatol. 12, 321–333. doi: 10.1016/j.jcmgh.2021.03.002
Elghannam, M. T., Hassanien, M. H., Ameen, Y. A., Turky, E. A., Elattar, G. M., ElRay, A. A., et al. (2024). Helicobacter pylori and oral-gut microbiome: clinical implications. Infection 52, 289–300. doi: 10.1007/s15010-023-02115-7
Engler, D. B., Leonardi, I., Hartung, M. L., Kyburz, A., Spath, S., Becher, B., et al. (2015). Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm. Bowel Dis. 21, 854–861. doi: 10.1097/MIB.0000000000000318
Faye, A. S., Allin, K. H., Iversen, A. T., Agrawal, M., Faith, J., Colombel, J.-F., et al. (2023). Antibiotic use as a risk factor for inflammatory bowel disease across the ages: a population-based cohort study. Gut 72, 663–670. doi: 10.1136/gutjnl-2022-327845
Fedorak, R. N., and Ismond, K. P. (2016). Practical considerations and the intestinal microbiome in disease: antibiotics for IBD therapy. Dig. Dis. 34, 112–121. doi: 10.1159/000443014
Fialho, A., Fialho, A., Nassri, A., Muenyi, V., Malespin, M., Shen, B., et al. (2019). Helicobacter pylori is associated with less fistulizing, stricturing, and active colitis in Crohn’s disease patients. Cureus 11:e6226. doi: 10.7759/cureus.6226
Fujimori, S. (2021). Progress in elucidating the relationship between Helicobacter pylori infection and intestinal diseases. World J. Gastroenterol. 27, 8040–8046. doi: 10.3748/wjg.v27.i47.8040
Glassner, K. L., Abraham, B. P., and Quigley, E. M. M. (2020). The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 145, 16–27. doi: 10.1016/j.jaci.2019.11.003
Graham, D. B., and Xavier, R. J. (2020). Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 578, 527–539. doi: 10.1038/s41586-020-2025-2
Guo, X., Huang, C., Xu, J., Xu, H. M., Liu, L., Zhao, H. L., et al. (2022). Gut microbiota is a potential biomarker in inflammatory bowel disease. Front. Nutr. 8:818902. doi: 10.3389/fnut.2021.818902
Haneishi, Y., Furuya, Y., Hasegawa, M., Picarelli, A., Rossi, M., and Miyamoto, J. (2023). Inflammatory bowel diseases and gut microbiota. Int. J. Mol. Sci. 24:3817. doi: 10.3390/ijms24043817
Heimesaat, M. M., Fischer, A., Plickert, R., Wiedemann, T., Loddenkemper, C., Göbel, U. B., et al. (2014). Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS One 9:e100362. doi: 10.1371/journal.pone.0100362
Hold, G. L., Smith, M., Grange, C., Watt, E. R., El-Omar, E. M., and Mukhopadhya, I. (2014). Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J. Gastroenterol. 20, 1192–1210. doi: 10.3748/wjg.v20.i5.1192
Homan, M., Jones, N. L., Bontems, P., Carroll, M. W., Czinn, S. J., Gold, B. D., et al. (2024). Updated joint ESPGHAN/NASPGHAN guidelines for management of Helicobacter pylori infection in children and adolescents (2023). J. Pediatr. Gastroenterol. Nutr. 79, 758–785. doi: 10.1002/jpn3.12314
Hu, W., Zhai, Z. Y., Huang, Z. Y., Chen, Z. M., Zhou, P., Li, X. X., et al. (2024). Dual RNA sequencing of Helicobacter pylori and host cell transcriptomes reveals ontologically distinct host-pathogen interaction. mSystems 9:e0020624. doi: 10.1128/msystems.00206-24
Huang, H., Gan, C. X., Cai, Y., and Wu, L. K. (2024). Immunological relationship between Helicobacter pylori and anti-tumor necrosis factor α agents in inflammatory bowel disease. Cent. Eur. J. Immunol. 49, 70–76. doi: 10.5114/ceji.2024.136376
Huang, J., Liao, C., Wu, L., Cao, Y., and Gao, F. (2011). A meta-analysis of randomized controlled trials comparing antibacterial therapy with placebo in Crohn’s disease. Colorectal Dis. 13, 617–626. doi: 10.1111/j.1463-1318.2009.02164.x
Ianiro, G., Tilg, H., and Gasbarrini, A. (2016). Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65, 1906–1915. doi: 10.1136/gutjnl-2016-312297
Imawana, R. A., Smith, D. R., and Goodson, M. L. (2020). The relationship between inflammatory bowel disease and Helicobacter pylori across East Asian, European and Mediterranean countries: a meta-analysis. Ann. Gastroenterol. 33:485. doi: 10.20524/aog.2020.0507
James, S. D., Wise, P. E., Zuluaga-Toro, T., Schwartz, D. A., Washington, M. K., and Shi, C. (2014). Identification of pathologic features associated with “ulcerative colitis-like” Crohn’s disease. World J. Gastroenterol. 20, 13139–13145. doi: 10.3748/wjg.v20.i36.13139
Jovanovic, I. R., Milosavjevic, T. N., Jankovic, G. P., Micev, M. M., Dugalic, P. D., Saranovic, D., et al. (2001). Clinical onset of the Crohn’s disease after eradication therapy of Helicobacter pylori infection. Does Helicobacter pylori infection interact with natural history of inflammatory bowel diseases? Med. Sci. Monit. 7, 137–141
Kahlam, A., Khrais, A., Khalessi, A., and Ahlawat, S. (2023). Trends and complication rates in ulcerative colitis patients with and without Helicobacter pylori infections. Cureus 15:e37345. doi: 10.7759/cureus.37345
Kaplan, G. G. (2015). The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727. doi: 10.1038/nrgastro.2015.150
Kaplan, G. G., and Windsor, J. W. (2021). The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66. doi: 10.1038/s41575-020-00360-x
Kayali, S., Gaiani, F., Manfredi, M., Minelli, R., Nervi, G., Nouvenne, A., et al. (2018). Inverse association between Helicobacter pylori and inflammatory bowel disease: myth or fact? Acta Biomed. 89, 81–86. doi: 10.23750/abm.v89i9-S.7878
Khan, K. J., Ullman, T. A., Ford, A. C., Abreu, M. T., Abadir, A., Marshall, J. K., et al. (2011). Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 106, 661–673. doi: 10.1038/ajg.2011.72
Kim, D. J., Park, J. H., Franchi, L., Backert, S., and Núñez, G. (2013). The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1β production in Helicobacter pylori infected dendritic cells. Eur. J. Immunol. 43, 2650–2658. doi: 10.1002/eji.201243281
Kong, G. P., Liu, Z. F., Lu, Y., Li, M., and Guo, H. M. (2023). The association between Helicobacter pylori infection and inflammatory bowel disease in children: a systematic review with meta-analysis. Medicine 102:e34882. doi: 10.1097/MD.0000000000034882
Kronman, M. P., Gerber, J. S., Prasad, P. A., Adler, A. L., Bass, J. A., Newland, J. G., et al. (2012). Variation in antibiotic use for children hospitalized with inflammatory bowel disease exacerbation: a multicenter validation study. J. Pediatric Infect. Dis. Soc. 1, 306–313. doi: 10.1093/jpids/pis053
Li, Y. H., Choi, H., Leung, K., Jiang, F., Graham, D. Y., and Leung, W. K. (2023). Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 553–564. doi: 10.1016/S2468-1253(23)00070-5
Liang, B., Wu, C. H., Wang, C., Sun, W. S., Chen, W. J., Hu, X. K., et al. (2022). New insights into bacterial mechanisms and potential intestinal epithelial cell therapeutic targets of inflammatory bowel disease. Front. Microbiol. 13:1065608. doi: 10.3389/fmicb.2022.1065608
Liu, W. D., Jiang, Q., Xue, S. L., Hui, W. J., Kong, W. J., Zhang, M. X., et al. (2024). Clinical characteristics of ulcerative colitis patients with different types of Helicobacter pylori infection. Microbiol. Spectr. 12:e0355423. doi: 10.1128/spectrum.03554-23
Lloyd, K., Papoutsopoulou, S., Smith, E., Stegmaier, P., Bergey, F., Morris, L., et al. (2020). Using systems medicine to identify a therapeutic agent with potential for repurposing in inflammatory bowel disease. Dis. Model. Mech. 13:dmm044040. doi: 10.1242/dmm.044040
Lopetuso, L. R., Scaldaferri, F., Franceschi, F., and Gasbarrini, A. (2014). The gastrointestinal microbiome—functional interference between stomach and intestine. Best Pract. Res. Clin. Gastroenterol. 28, 995–1002. doi: 10.1016/j.bpg.2014.10.004
Mak, W. Y., Zhao, M., Ng, S. C., and Burisch, J. (2020). The epidemiology of inflammatory bowel disease: east meets west. J. Gastroenterol. Hepatol. 35, 380–389. doi: 10.1111/jgh.14872
Malfertheiner, P., Camargo, M. C., El-Omar, E., Liou, J. M., Peek, R., Schulz, C., et al. (2023). Helicobacter pylori infection. Nat. Rev. Dis. Primers 9:19. doi: 10.1038/s41572-023-00431-8
Maloy, KA. (2012). The relationship of oral tetracycline use and isotretinoin therapy to the development of IBD within genetically predisposed individuals. [dissertation/master’s thesis]. New York (NY): Weill Medical College of Cornell University.
Man, S. M., Zhang, L., Day, A. S., Leach, S., and Mitchell, H. (2008). Detection of enterohepatic and gastric Helicobacter species in fecal specimens of children with Crohn’s disease. Helicobacter 13, 234–238. doi: 10.1111/j.1523-5378.2008.00607.x
Mao, A. P., Zhao, W. G., Zhu, Y. H., Kong, F. T., Chen, D. Y., Si, H. Z., et al. (2023). Gut bacterial community determines the therapeutic effect of ginsenoside on canine inflammatory bowel disease by modulating the colonic mucosal barrier. Microorganisms 11:2616. doi: 10.3390/microorganisms11112616
Marotti, B., Rocco, A., De Colibus, P., Compare, D., de Nucci, G., Staibano, S., et al. (2008). Interleukin-13 mucosal production in Helicobacter pylori-related gastric diseases. Dig. Liver Dis. 40, 240–247. doi: 10.1016/j.dld.2007.11.021
Matsumura, M., Matsui, T., Hatakeyama, S., Matake, H., Uno, H., Sakurai, T., et al. (2001). Prevalence of Helicobacter pylori infection and correlation between severity of upper gastrointestinal lesions and H. pylori infection in Japanese patients with Crohn’s disease. J. Gastroenterol. 36, 740–747. doi: 10.1007/s005350170015
McColl, K. E. (2010). Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 362, 1597–1604. doi: 10.1056/NEJMcp1001110
Mills, C. D. (2014). Spatial, temporal, and functional aspects of macrophages during “the good, the bad, and the ugly” phases of inflammation. Front. Immunol. 5:612. doi: 10.3389/fimmu.2014.00612
Mizukami, K., Sugano, K., Takeshima, T., and Murakami, K. (2023). Disease trends after Helicobacter pylori eradication based on Japanese nationwide claims and the health check-up database. World J. Gastroenterol. 29, 692–705. doi: 10.3748/wjg.v29.i4.692
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42. doi: 10.1053/j.gastro.2011.10.001
Monteleone, G., Fina, D., Caruso, R., and Pallone, F. (2006). New mediators of immunity and inflammation in inflammatory bowel disease. Curr. Opin. Gastroenterol. 22, 361–364. doi: 10.1097/01.mog.0000231808.10773.8e
Mukherjee, S., Chopra, A., Karmakar, S., and Bhat, S. G. (2024). Periodontitis increases the risk of gastrointestinal dysfunction: an update on the plausible pathogenic molecuar mechanisms. Crit. Rev. Microbiol., 1–31. doi: 10.1080/1040841X.2024.2339260
Murad, H. A. (2016). Does Helicobacter pylori eradication therapy trigger or protect against Crohn’s disease? Acta Gastro-Enterol. Belg. 79, 349–354.
Nadeem, M. S., Kumar, V., Al-Abbasi, F. A., Kamal, M. A., and Anwar, F. (2020). Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 64, 51–60. doi: 10.1016/j.semcancer.2019.05.001
Nicolas, G. R., and Chang, P. V. (2019). Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol. Sci. 40, 430–445. doi: 10.1016/j.tips.2019.04.006
Papamichael, K., Konstantopoulos, P., and Mantzaris, G. J. (2014). Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J. Gastroenterol. 20, 6374–6385. doi: 10.3748/wjg.v20.i21.6374
Park, J., Son, M. J., Ho, C. C., Lee, S. H., Kim, Y., An, J., et al. (2023). Transcriptional inhibition of STAT1 functions in the nucleus alleviates Th1 and Th17 cell-mediated inflammatory diseases. Front. Immunol. 14:1307575. doi: 10.3389/fimmu.2023.1307575
Pellicano, R., Ianiro, G., Fagoonee, S., Settanni, C. R., and Gasbarrini, A. (2020). Review: extragastric diseases and Helicobacter pylori. Helicobacter 25:e12741. doi: 10.1111/hel.12741
Piovani, D., Danese, S., Peyrin-Biroulet, L., Nikolopoulos, G. K., Lytras, T., and Bonovas, S. (2019). Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4. doi: 10.1053/j.gastro.2019.04.016
Pokrotnieks, J., and Sitkin, S. (2023). Helicobacter pylori and inflammatory bowel disease: an unresolved enigma. Inflamm. Bowel Dis. 29, E5–E6. doi: 10.1093/ibd/izac248
Quaglio, A. E. V., Grillo, T. G., De Oliveira, E. C. S., Di Stasi, L. C., and Sassaki, L. Y. (2022). Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 28, 4053–4060. doi: 10.3748/wjg.v28.i30.4053
Ravikumara, M. (2023). Helicobacter pylori in children: think before you kill the bug! Ther. Adv. Gastroenterol. 16:17562848231177610. doi: 10.1177/17562848231177610
Ren, S., Cai, P. P., Liu, Y. Q., Wang, T. P., Zhang, Y., Li, Q., et al. (2022). Prevalence of Helicobacter pylori infection in China: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 37, 464–470. doi: 10.1111/jgh.15751
Rieder, G., Merchant, J. L., and Haas, R. (2005). Helicobacter pylori cage-type IV secretion systems facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242. doi: 10.1053/j.gastro.2005.02.064
Sayar, R., Shirvani, J. S., Hajian-Tilaki, K., Vosough, Z., and Ranaei, M. (2019). The negative association between inflammatory bowel disease and Helicobacter pylori seropositivity. Caspian J. Intern. Med. 10, 217–222. doi: 10.22088/cjim.10.2.217
Schmidt, A., Zhang, X. M., Joshi, R. N., Iqbal, S., Wahlund, C., Gabrielsson, S., et al. (2016). Human macrophages induce CD4+ Foxp3+ regulatory T cells via binding and re-release of TGF-β. Immunol. Cell Biol. 94, 747–762. doi: 10.1038/icb.2016.34
Setshedi, M., and Watermeyer, G. (2022). The impact of Helicobacter pylori and intestinal helminth infections on gastric adenocarcinoma and inflammatory bowel disease in Sub-Saharan Africa. Front. Med. 9:1013779. doi: 10.3389/fmed.2022.1013779
Shi, Y. Y., Zheng, H. L., Guo, Z. J., Deng, R. Y., Yu, W., Song, Y. H., et al. (2022). Effect of Helicobacter pylori on immunotherapy is gaining more attention. Helicobacter 27:e12925. doi: 10.1111/hel.12925
Shirzad-Aski, H., Besharat, S., Kienesberger, S., Sohrabi, A., Roshandel, G., Amiriani, T., et al. (2021). Association between Helicobacter pylori colonization and inflammatory bowel disease a systematic review and meta-analysis. J. Clin. Gastroenterol. 55, 380–392. doi: 10.1097/MCG.0000000000001415
Singh, P., Ananthakrishnan, A., and Ahuja, V. (2017). Pivot to Asia: inflammatory bowel disease burden. Intest. Res. 15, 138–141. doi: 10.5217/ir.2017.15.1.138
Stromnes, I. M., Cerretti, L. M., Liggitt, D., Harris, R. A., and Goverman, J. M. (2008). Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nat. Med. 14, 337–342. doi: 10.1038/nm1715
Tanner, S., Katz, J., Cominelli, F., Regueiro, M., Cooper, G., and Mansoor, E. (2023). Inflammatory bowel disease and Helicobacter pylori: protective or present? Inflamm. Bowel Dis. 29, 1005–1007. doi: 10.1093/ibd/izac202
Taskin, D. G. (2023). Evaluation of the presence of Helicobacter pylori in inflammatory bowel disease in children. Med. J. Bakirkoy 19, 93–96. doi: 10.4274/BMJ.galenos.2023.2022.10-1
Tepler, A., Narula, N., Peek, R. M., Patel, A., Edelson, C., Colombel, J. F., et al. (2019). Systematic review with meta-analysis: association between Helicobacter pylori CagA seropositivity and odds of inflammatory bowel disease. Aliment. Pharmacol. Ther. 50, 121–131. doi: 10.1111/apt.15306
Tiemessen, M. M., Jagger, A. L., Evans, H. G., van Herwijnen, M. J. C., John, S., and Taams, L. S. (2007). CD4+ CD25+ Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 19446–19451. doi: 10.1073/pnas.0706832104
Triantafillidis, J. K., Gikas, A., Apostolidiss, N., Merikas, E., Mallass, E., and Peros, G. (2003). The low prevalence of Helicobacter infection in patients with inflammatory bowel disease could be attributed to previous antibiotic treatment. Am. J. Gastroenterol. 98, 1213–1214. doi: 10.1111/j.1572-0241.2003.07434.x
Triantafillidis, J. K., Gikas, A., and Merikas, E. (2014). Treatment of inflammatory bowel disease patients with anti-TNF-alpha factors and immunosuppressives does not influence the prevalence of Helicobacter pylori infection. Indian J. Gastroenterol. 33, 383–384. doi: 10.1007/s12664-013-0389-z
Tursi, A. (2006). Onset of Crohn’s disease after Helicobacter pylori eradication. Inflamm. Bowel Dis. 12, 1008–1009. doi: 10.1097/01.mib.0000235100.09231.d7
Vervoort, J., Xavier, B. B., Stewardson, A., Coenen, S., Godycki-Cwirko, M., Adriaenssens, N., et al. (2015). Metagenomic analysis of the impact of nitrofurantoin treatment on the human faecal microbiota. J. Antimicrob. Chemother. 70, 1989–1992. doi: 10.1093/jac/dkv062
Vieira, A. T., Macia, L., Galvão, I., Martins, F. S., Canesso, M. C., Amaral, F. A., et al. (2015). A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. 67, 1646–1656. doi: 10.1002/art.39107
Waltman, L., van Eck, N. J., and Noyons, E. C. M. (2010). A unified approach to mapping and clustering of bibliometric networks. J. Informet. 4, 629–635. doi: 10.1016/j.joi.2010.07.002
Wang, L., Cao, Z. M., Zhang, L. L., Dai, X. C., Liu, Z. J., Zeng, Y. X., et al. (2022). Helicobacter pylori and autoimmune diseases: involving multiple systems. Front. Immunol. 13:833424. doi: 10.3389/fimmu.2022.833424
Wang, J. J., Deng, Z. Y., Wang, Z. Y., Wu, J. H., Gu, T., Jiang, Y. B., et al. (2016). MicroRNA-155 in exosomes secreted from Helicobacter pylori infection macrophages immunomodulates inflammatory response. Am. J. Transl. Res. 8, 3700–3709
Wang, Y. H., Gorvel, J. P., Chu, Y. T., Wu, J. J., and Lei, H. Y. (2010). Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One 5:e10844. doi: 10.1371/journal.pone.0010844
Wang, L., Manji, G. A., Grenier, J. M., Al-Garawi, A., Merriam, S., Lora, J. M., et al. (2002). PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880. doi: 10.1074/jbc.M203915200
Wéra, O., Lancellotti, P., and Oury, C. (2016). The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 5:118. doi: 10.3390/jcm5120118
Wessler, S., and Backert, S. (2008). Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 16, 397–405. doi: 10.1016/j.tim.2008.05.005
Wu, D. X., Li, T., Zhuang, X. J., Zhan, S. K., Liu, C. G., Chen, M. H., et al. (2023). The association between Helicobacter pylori infection and its virulence classification and Crohn’s disease. J. Gastroenterol. Hepatol. 38:93.
Yang, K. Q., Ding, Y. C., Chen, J. L., and Sun, X. J. (2024). No potential causal link between HP infection and IBD: a 2way Mendelian randomization study. Medicine 103:e37175. doi: 10.1097/MD.0000000000037175
Yang, H., Mou, Y., and Hu, B. (2023). Discussion on the common controversies of Helicobacter pylori infection. Helicobacter 28:e12938. doi: 10.1111/hel.12938
Yang, S. S., Zhao, S. Y., Ye, Y. X., Jia, L. Q., and Lou, Y. N. (2022). Global research trends on the links between gut microbiota and cancer immunotherapy: a bibliometric analysis (2012–2021). Front. Immunol. 13:952546. doi: 10.3389/fimmu.2022.952546
Yu, Y., Zhu, S. T., Li, P., Min, L., and Zhang, S. T. (2018). Helicobacter pylori infection and inflammatory bowel disease: a crosstalk between upper and lower digestive tract. Cell Death Dis. 9:961. doi: 10.1038/s41419-018-0982-2
Zaki, M. H., Vogel, P., Malireddi, R. K. S., Body-Malapel, M., Anand, P. K., Bertin, J., et al. (2011). The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20, 649–660. doi: 10.1016/j.ccr.2011.10.022
Zamoshnikova, A., Gross, C. J., Schuster, S., Chen, K. W. W., Wilson, A., Tacchini-Cottier, F., et al. (2016). NLRP12 is a neutrophil-specific, negative regulator of in vitro cell migration but does not modulate LPS-or infection-induced NF-κB or ERK signalling. Immunobiology 221, 341–346. doi: 10.1016/j.imbio.2015.10.001
Zhang, H. C., Dai, Y., Liu, Y., Wu, T., Li, J., Wang, X. L., et al. (2018). Helicobacter pylori colonization protects against chronic experimental colitis by regulating Th17/Treg balance. Inflamm. Bowel Dis. 24, 1481–1492. doi: 10.1093/ibd/izy107
Zhang, X. M., Liu, C. Y., and Shao, Z. H. (2020). Advances in the role of helper T cells in autoimmune diseases. Chin. Med. J. 133, 968–974. doi: 10.1097/CM9.0000000000000748
Zhong, Y. F., Zhang, Z. C., Lin, Y. Y., and Wu, L. L. (2021). The relationship between Helicobacter pylori and inflammatory bowel disease. Arch. Iran. Med. 24, 317–325. doi: 10.34172/aim.2021.44
Zhou, G. X., and Liu, Z. J. (2017). Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 18, 495–503. doi: 10.1111/1751-2980.12540
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, H. pylori, intestinal microbiota, immune modulation
Citation: Wang Z, Zhao S, Zhong X, Su Y, Song Y, Li J and Shi Y (2024) Debate on the relationship between Helicobacter pylori infection and inflammatory bowel disease: a bibliometric analysis. Front. Microbiol. 15:1479941. doi: 10.3389/fmicb.2024.1479941
Edited by:
Honghua Hu, Macquarie University, AustraliaReviewed by:
Ning Sun, Guangzhou Cadre and Talent Health Management Center, ChinaHaoning Gong, Jinhua Institute of Zhejiang University, China
Copyright © 2024 Wang, Zhao, Zhong, Su, Song, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yahan Song, cHVoM19saWJAYmptdS5lZHUuY24=; Jun Li, eWFud3QyMDAzQDE2My5jb20=; Yanyan Shi, MDcyOXhzdEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Yahan Song, orcid.org/0000-0002-3694-3380
 Ziye Wang
Ziye Wang Shiqing Zhao
Shiqing Zhao Xiaotian Zhong
Xiaotian Zhong Yi Su
Yi Su Yahan Song3*‡
Yahan Song3*‡ Jun Li
Jun Li Yanyan Shi
Yanyan Shi