- 1College of Health Sciences, VinUniversity, Hanoi, Vietnam
- 2Center for Global Health, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 3College of Health and Human Sciences, Charles Darwin University, Casuarina, NT, Australia
More than 75 arboviruses are indigenous to Australia, of which at least 13 are known to cause disease in humans. Alphaviruses are the most common arboviruses, notably including Ross River and Barmah Forest viruses, which contribute a significant public health and economic burden in Australia. Both can cause febrile illness with arthritic symptoms. Each circulates nationally across diverse climates and environments, and has multi-host, multi-vector dynamics. Several medically important flaviviruses also circulate in Australia. Infection with Murray Valley encephalitis or Kunjin viruses is less common but is associated with brain inflammation. Key research priorities for Australian arboviruses aim to understand clinical manifestations, develop timely diagnostics, and identify transmission cycles that permit the maintenance of arboviruses. While these can now be answered for a handful of notifiable alpha- and flaviviruses there are others for which non-human vertebrate hosts and competent arthropod invertebrate vectors are still to be identified and/or whose role in transmission is not well understood. One or more of these ‘neglected’ arboviruses may be the causative agent of a proportion of the many thousands of fever-related illnesses reported annually in Australia that at present remain undiagnosed. Here, what is known about enzootic cycling of viruses between arthropod vectors and mammalian and avian reservoir hosts is summarised. How and to what extent these interactions influence the epidemiology of arbovirus transmission and infection is discussed.
1 Introduction
Arthropod-borne viruses, commonly known as arboviruses, are a polyphyletic group of RNA viruses that circulate between different arthropod vectors (insects, usually mosquitoes, but also midges, sand flies and black flies; and arachnid ticks) and human and non-human vertebrate hosts (Franz et al., 2015). While these are predominantly mammals and birds the possible role of reptiles and amphibians in arbovirus transmission cycles has been discussed (Bosco-Lauth et al., 2018). These viruses are typically transmitted through the bite of an infectious arthropod vector, which acquires the virus by feeding on a viraemic vertebrate host (Lequime et al., 2016).
Arboviruses are significant health threats in tropical and sub-tropical regions with 3.9 billion people at risk, leading to an estimated disease burden of 300,000 to 5 million disability-adjusted life years lost annually (Labeaud et al., 2011). Arboviral infection can cause disease in vertebrates but does not trigger significant pathology in arthropods (Franz et al., 2015). While some human arboviral infections are asymptomatic or present with a mild influenza-like illness, arboviral pathogens causing serious illness ranging from rash and arthritis to encephalitis and haemorrhagic fever are an increasing threat to global health security (Wilder-Smith et al., 2017). The most striking illustration of this is the worldwide advance of dengue over the last 70 years (Gyawali et al., 2016a), while Japanese encephalitis virus (JEV) causes the majority of viral encephalitis cases in Asia (World Health Organization, 2019). Moreover, recent epidemics of chikungunya (CHIKV) and West Nile virus infection, plus the transcontinental spread of Zika virus (ZIKV), exemplify the growing risk posed by previously obscure pathogens and hence justify the concern over such emerging arboviruses (Gyawali et al., 2016b).
The interactions between invertebrate arthropod vectors and vertebrate hosts play a crucial role in the transmission and maintenance of arboviruses in nature (Kuno and Chang, 2005). Arthropod vectors serve as both the primary natural reservoirs and the means of transmission for arboviruses, while vertebrate hosts can act as amplifiers of viral replication (Kuno and Chang, 2005). The transmission cycle between these hosts allows for the persistence and spread of arboviruses in the environment. Arboviruses generally establish lifelong infection in vectors but exhibit transient infection of variable magnitude and duration in vertebrate hosts (Althouse and Hanley, 2015). Host factors such as tissue barriers, immune responses, genetic diversity, and replication dynamics all contribute to the complex dynamics of arbovirus transmission. Important ecological factors include population abundance, vector-host contact rate, and host migratory and other behaviours.
Only by fully understanding interactions between vectors and hosts can arbovirus transmission be controlled and/or prevented. Nowhere is this of more relevance than Australia, where more than 75 arboviruses that are indigenous to the country have been identified (Centers for Disease Control and Prevention, 2024). The alphaviruses Ross River (RRV) and Barmah Forest (BFV), and the flavivirus Murray Valley encephalitis (MVEV), are established as causative agents of debilitating diseases (e.g., Fraser, 1986), each of which may be detected by both antibody-based recognition and nucleic-acid amplification. However, for most of the remaining arboviruses (e.g., Alfuy [ALFV], Edge-Hill [EHV], Kokobera (KOKV], Sindbis [SINV], and Stratford [STRV]), that are or may be associated with pathology in humans, including some undifferentiated febrile illnesses routine tests are not available to diagnose infection (Gyawali et al., 2017a; Gyawali et al., 2019a). Prominent among public health challenges in parts of Australia north of the Tropic of Capricorn, as well as occasionally in more southerly latitudes, are so-called ‘neglected’ Australian arboviruses. Some of these viruses cause acute undifferentiated febrile illness, for which over half of all cases that occur annually in Australia are not diagnosed (Susilawati and McBride, 2014). Instigating a rigorous identification program would reduce the possibility of significant outbreaks of these indigenous arboviruses at a time when population growth accelerates across regional Australia, thereby bringing humans into closer proximity of native wildlife and vectors (Gyawali et al., 2017a).
Until very recently, JEV was limited to Far North Australia (Torres Strait Islands and Tiwi Island). During the hot, wet summers of 2021-22 and 2022-23, however, there was a dramatic geographical expansion of JEV across central and southern Australia (Pendrey and Martin, 2023). Of 45 clinical cases (35 laboratory-confirmed and 10 suggestive epidemiologically and/or symptomatically), there were seven deaths (Yakob et al., 2023). Given that local transmission occurred over two consecutive mosquito seasons, it is likely that JEV is now established endemically on the Australian mainland, placing as much as 750,000 people, 3% of the national population, at risk of JEV (Yakob et al., 2023). While sporadic cases of the closely related MVEV are largely confined to Northern Australia, major outbreaks were reported across southern and eastern regions of the country in 1951 (45 cases), 1974 (58 cases) and 2011 (17 cases) (Selvey et al., 2014a). In early 2023, six cases of MVEV infection were notified in the south-eastern state of Victoria, three of which were fatal (Braddick et al., 2023).
The concurrent (re-)emergence and co-circulation of JEV with MVEV (McGuinness et al., 2023) highlights key knowledge gaps in vector ecology, transmission dynamics and intervention efficacy. Integral to tailoring a control and prevention strategy to suit all Australian arboviruses is a better understanding of the interactions between their arthropod vectors and vertebrate hosts, which underpins their transmission epidemiology. While the number of arthropods from which these viruses have been recovered is considerable, less is known about the non-human vertebrate hosts that may be involved in their environment cycling or in the biting preferences of different mosquito species for these different reservoir hosts (Gyawali et al., 2019b; Gyawali et al., 2020). Information on the epidemiology and ecology of most of the neglected arboviruses is sketchy but they are known, or at least assumed, to be largely maintained in zoonotic cycles rather than exclusively by human-to-human transmission.
2 Transmission cycles of arboviruses
The transmission cycle of an arbovirus is determined by virus-vector host interactions. Arboviruses are transmitted between hosts by their arthropod vectors. The transmission cycle starts when an arthropod feeds on viraemic blood. Arboviruses can establish infection in the midgut epithelial cells of the arthropod vector and subsequently replicate in various tissues, including the salivary glands, enabling transmission to occur subsequently when the vector takes a blood meal (Lequime et al., 2016). Many arboviruses (e.g., MVEV, KUNV and JEV) have complex transmission cycles that include multiple host and vector species in maintenance and spillover (Kuno and Chang, 2005). Some hosts develop sufficiently high viraemias to infect susceptible vectors that feed on them while others do not. Failure to develop a viraemia sufficient to infect a vector does not mean that the host will not develop clinical symptoms. Cycles of transmission may involve only arthropods and humans (e.g., epidemic cycle of dengue virus, DENV, and RRV) or only non-human vertebrates and vectors (e.g., Akabane virus) or there may be transmission of viruses between human and non-human hosts (zoonoses, e.g., BFV, MVE, RRV). Most Australian arboviruses are zoonotic and maintain enzootic cycles involving birds and mammals as reservoir hosts (Go et al., 2014; Russell and Kay, 2004). In this cycle, the virus is continuously maintained in nature and may or may not cause disease in the enzootic host. Infections and epidemics in human populations can arise from direct spill-over of these enzootic and epizootic (exploiting domestic animals, e.g., JEV) cycles when amplification achieves a viraemia high enough for transmission (Weaver and Barrett, 2004).
3 Arboviruses and their vertebrate hosts
3.1 Mammals
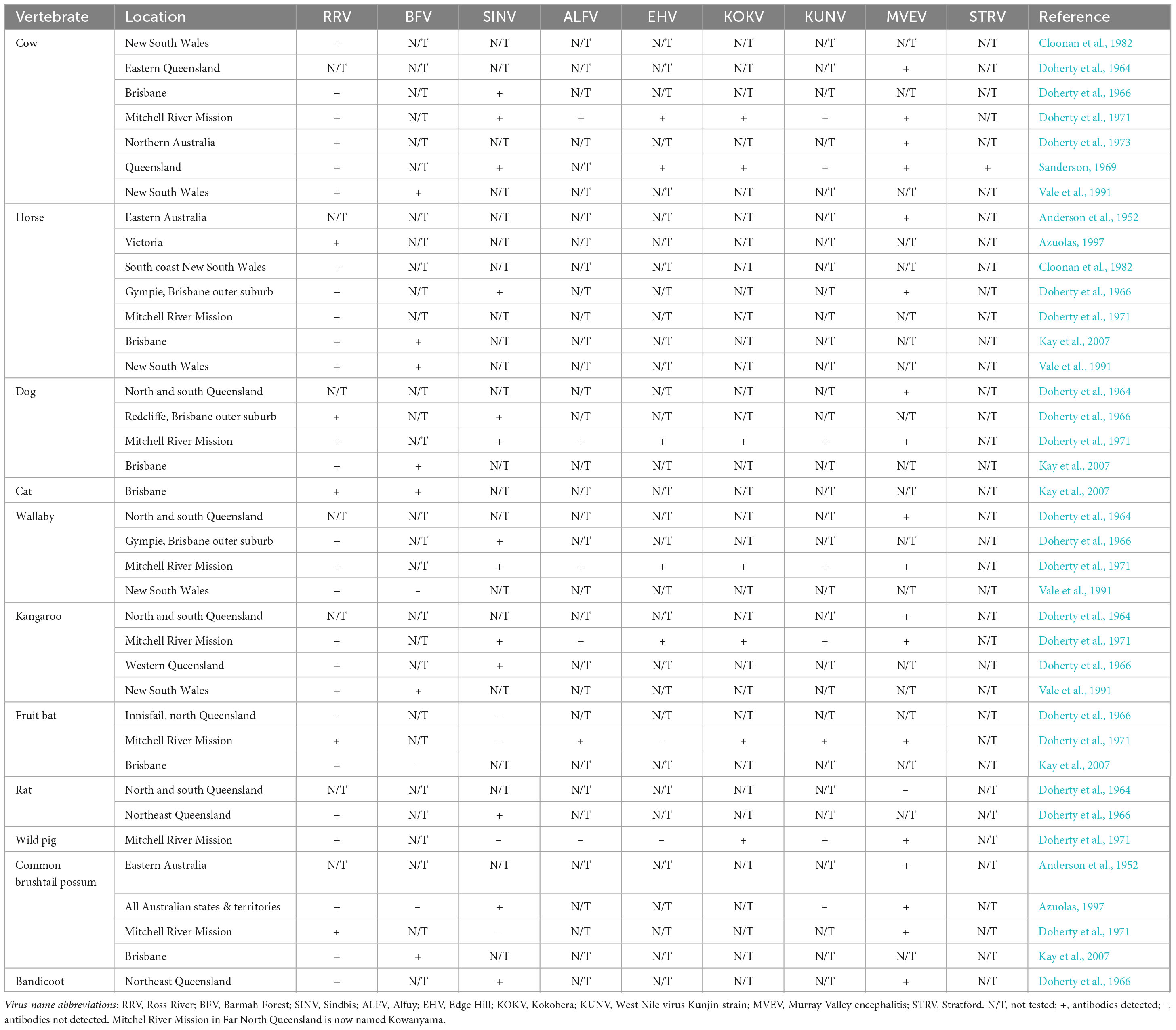
Vertebrates can be considered as a possible reservoir for an arbovirus when a minimum of three commonly used criteria are met: viraemia; virus isolation; and relatively high antibody titres. While the presence of serum antibody against arboviruses in a host does not, per se, prove that an animal has been viraemic or involved in virus transmission as a reservoir, serological surveys do provide information about which animals might be involved in the transmission cycles of arboviruses (Table 1).
3.1.1 Marsupials
Macropods (notably, kangaroos and wallabies) are reservoirs and likely focal hosts of RRV. Laboratory infections and transmission of RRV from different kangaroos species to mosquitoes (Doherty et al., 1964; Doherty et al., 1966; Kay and Aaskov, 1989; Kay et al., 1986; Lindsay et al., 2005; Potter et al., 2014), isolation of RRV from agile wallabies (Macropus agilis) at Mitchell River Mission in Cape York Peninsula, Far North Queensland (Doherty et al., 1971), and viraemias of maximum titre of 4.6–5.6 suckling mouse intracerebral inoculation (SMIC) LD50/mL for between 4 and 6 days after introduction of RRV by the bite of infected mosquitoes (Kay et al., 1986) to Eastern grey kangaroos (Macropus giganteus) and agile wallabies have strongly suggested that marsupials are suitable hosts for RRV. While experimental studies of infection of macropods with all contemporary arboviruses have not been undertaken, one study reported titres of MVEV in eastern grey kangaroos as high as 103 SMIC LD50/mL up to 6 days following the bite of an infected mosquito (Kay et al., 1986). Eastern grey kangaroos are terrestrial and widespread throughout eastern Australia, from Cape York to Victoria (Kay et al., 1985a). In Western Australia, the equivalent macropod is the closely related Western grey kangaroo (Macropus fuliginosus), which recent preliminary research (based on antibody detection) suggests is a common host for RRV and BFV (Gyawali et al., 2020). Common brushtail possums (Trichosurus vulpecula), a semi-arboreal marsupial, abound in the forest and urban areas throughout the eastern, northern, and south-western regions of Australia (How and Hillcox, 2000), and have been found to produce high titre viraemias following the bite of RRV-infected mosquitoes (Boyd et al., 2001), suggesting they may play a role in the urban transmission of RRV. Brushtail possums also generate mild viraemia for JEV (Daniels et al., 2000).
3.1.2 Ungulates
The epidemic spread of RRV infection across the Western Pacific region in 1979–80 (Aaskov et al., 1981; Rosen et al., 1981; Tesh et al., 1981), and presence of historical antibody of RRV in animal sera collected from the south Pacific (Togami et al., 2020) have demonstrated endemic transmission of RRV in the absence of marsupial reservoirs. Serological studies have detected anti-RRV antibodies in domestic ungulates (large mammals with hooves), including cows and horses (Table 1). Comprehensive experimental studies of infection of cattle and horses with all arboviruses have not been undertaken. However, when cattle were infected with MVEV using orally infected Culex annulirostris, no viraemia was detected (Kay et al., 1985b). Similarly, when horses were infected with RRV by intravenous injection or the bite of infected Cx. annulirostris, virus could not be recovered in cell culture when sera from infected horses were cultured. Yet, the viraemia was sufficient to infect laboratory mosquitoes when Ae. vigilax and Cx. annulirostris were fed on the infected horses (Kay et al., 1987; Ryan et al., 1997). The presence of anti-RRV antibodies in horses has led some arbovirologists to propose that they may act as amplifying hosts for RRV and to the further suggestion that viraemic horses could transport RRV from peri-urban to urban or city environments (Doherty et al., 1966; Gard et al., 1977; Cloonan et al., 1982; McManus and Marshall, 1986; Pascoe et al., 1978). Apart from RRV, antibodies to BFV, MVEV, and Sindbis virus (SINV) have also been detected in horses (Table 1). In addition, isolation of West Nile virus Kunjin strain (KUNV) following experimental infection (Badman et al., 1984) and natural infection (Frost et al., 2012) indicate the possible role of horses in the transmission cycle of each of these viruses. KUNV was responsible for a large outbreak of neurological disease in horses in 2011 (Frost et al., 2012; Roche et al., 2013). While neutralising antibodies against neglected Australian arboviruses have been detected in cattle (Table 1), Kay et al. (1985b) were unable to detect viraemia in cows infected by MVEV using Cx. annulirostris. Intensive pig farming and a large feral pig population may have aided to recent Australian JEV outbreaks (Williams et al., 2022). The latter serve as amplifying hosts of JEV and MVEV, and their geographic range and large number (∼ 3.2 million) provide a latent risk of spillover to domestic piggeries and humans (Mackenzie and Smith, 2024). It is unknown how population dynamics and distribution of amplifying hosts influence arbovirus transmission.
3.1.3 Cats and dogs
No viraemia sufficient to infect mosquitoes developed in small domestic animals such as dogs and cats following experimental infection with RRV or BFV through the bite of infected Ae. vigilax (Boyd and Kay, 2002). A relatively low antibody prevalence (∼ 10%) in serological survey of these animals suggested them less likely to be significant reservoirs of RRV, BFV and JEV.
3.1.4 Bats
Bats have been associated with several zoonotic pathogens including viruses causing Ebola (filo virus), Lassa fever (Lassa virus) and COVID-19 (SARS-CoV-2). They are also suggested for having association with arboviruses. Fruit bats, also called flying foxes are found to have low viraemia to an experimental infection with RRV and JEV, but still were capable of infecting susceptible mosquitoes (Ryan et al., 1997; van den Hurk et al., 2009).
3.2 Birds
As early as 70 years ago, Anderson postulated that birds might be the primary reservoir of MVEV. The detection of anti-MVEV antibodies in many Ciconiiformes (storks) and Pelecaniformes (ibises, herons and pelicans) (Anderson, 1953; Anderson, 1954; Anderson et al., 1958; Doherty, 1964; Gard et al., 1976; Liehne et al., 1976; Boyle et al., 1983), and the high prevalence of anti-MVEV antibodies in birds (from 44% in adults to 96% in juveniles) after the MVEV epidemics of 1974–1975 (Marshall et al., 1982a) supported Anderson’s hypothesis. Galahs, sulphur-crested cockatoos, corellas, and black ducks produced MVEV viraemias with titres of 102 to 106 SMIC LD50/mL for 1–9 days following the bite of infected Cx. annulirostris mosquitoes (Kay et al., 1985b). With this viraemia in birds, approximately 10% of recipient Cx. annulirostris acquired virus infection.
Many avian species, particularly members of the family Ardeidae (herons, egrets, and allies), whose distribution overlaps with Culex mosquitoes, exhibit high prevalence of MVEV and JEV (Soman et al., 1977). Ardeids are considered the main vertebrate hosts of MVEV (Selvey et al., 2014b). As these avian species migrate to and across Australia (Guay et al., 2012), they share habitats with numerous other resident host species, such as cattle egrets (Bubulcus ibis) and feral pigs. The current role of migratory birds and feral pigs in the maintenance and transmission of arboviruses is a key health research priority for Australia. Where new wetlands, viraemic birds, and high mosquito densities converged near piggeries, the probability of “spillover” and rapid amplification in domestic pigs increased, causing the 2022 JEV outbreak in southern Australia. However, the mechanism of interaction between feral pigs, wildlife, and domestic animals is not clearly known.
The detection of antibodies to MVEV and KUNV in chickens (Doherty et al., 1968), was followed by isolation of MVEV from a sentinel chicken at Echuca, a town on the banks of the Murray River in northern Victoria, during the MVEV epidemic of 1974 (Campbell and Hore, 1975). Subsequently, the health department of several Australian state governments have employed flocks of sentinel chickens to monitor transmission of MVEV and KUNV as an early warning surveillance system to identify the threat of outbreaks (Doherty et al., 1976; Mackenzie et al., 1992). Besides MVEV, other arboviruses including Alfuy virus (ALFV), KUNV, RRV and SINV were also isolated from wild birds collected at Mitchell River Mission between 1963 and 1967 (Whitehead et al., 1968; Doherty, 1972; Doherty, 1977; Doherty et al., 1971). Ongoing research also suggests that birds may contribute to transmission dynamics of RRV and BFV, although their role in maintaining these viruses is still unclear (Vieira et al., 2023).
Furthermore, the increase in rainfall in southern Australia and the migration of water birds due to flowing inland rivers could lead to heightened activity of MVEV in certain areas (Selvey et al., 2014a). These findings underscore the significance of understanding the association of Australian arboviruses with water birds in the context of public health challenges and disease transmission dynamics.
4 Arboviruses and their arthropod vectors
4.1 Mosquitoes
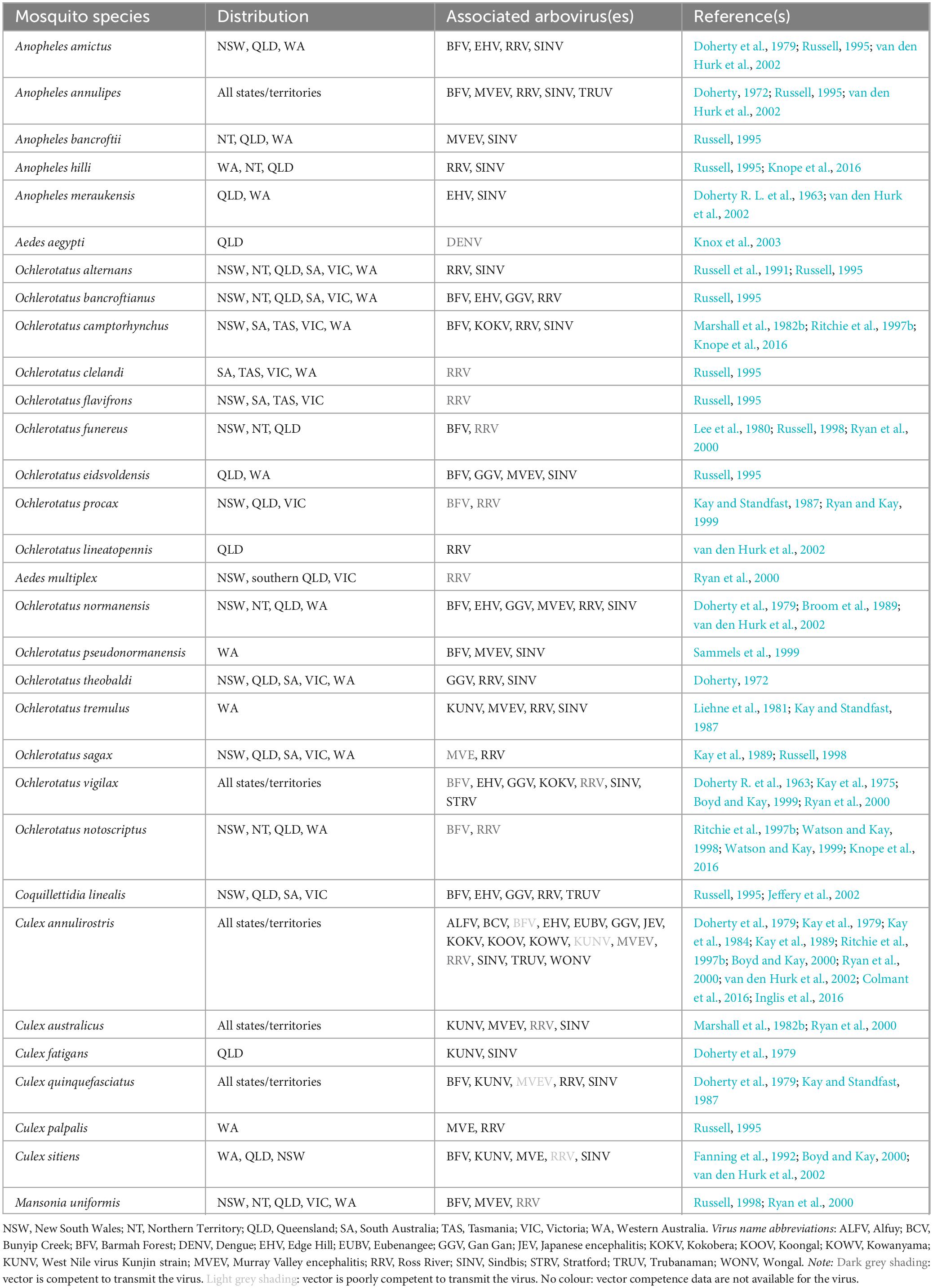
Australia harbours a diverse mosquito fauna of more than 300 species (Webb et al., 2016). However, arboviruses have been recovered from only around 30 of these, mainly from species of Aedes, Anopheles and Culex mosquitoes (Webb et al., 2016). This includes several species and subgenera of Aedes that were reclassified by some authorities as belonging to the Ochlerotatus genus (Reinert, 2000). Isolation of a virus from a mosquito does not imply that it is competent to transmit the virus or that the mosquito plays a significant role in the transmission of that virus (Kain et al., 2022). A mosquito is called a competent vector when an arbovirus is isolated from it in its wild-caught stage; the mosquito can transmit the arbovirus to a host; and the mosquito itself is infected when feeding upon a viraemic host.
Some mosquito species such as Anopheles annulipes, Cx. annulirostris and Cx. australicus are cosmopolitan throughout Australia (Russell, 1998). KOKV, KUNV, MVEV and SINV were each first isolated from Cx. annulirostris collected at Mitchell River Mission in 1960 (Doherty R. L. et al., 1963). BFV was isolated from Cx. annulirostris in northern Victoria in 1974 (Marshall et al., 1982b). Cx. annulirostris is a freshwater mosquito species that is most active from spring to late autumn (Russell, 1995). Females are opportunistic feeders that readily take a blood meal from a wide variety of vertebrates, including humans, mammals, and birds, depending on host availability (Kay et al., 2007; Gyawali et al., 2019b), proliferate under optimal conditions, and are capable of dispersing more than 4 km per day (O’Donnell et al., 1992). Cx. annulirostris is also the primary vector of JEV in Australia. The virus was isolated from this vector during a JE outbreak in 1995 on Badu Island (Ritchie et al., 1997a), and later also on other islands of the Torres Strait (Hanna et al., 1999). The competence of the vector to virus was further demonstrated when JEV infecting a laboratory colony of Cx. annulirostris was transmitted to mice (van den Hurk et al., 2003) and to flying foxes (van den Hurk et al., 2009) via vector bite.
Limited understanding of the spatiotemporal importance of individual Culex species in transmitting endemic MVEV and emerging JEV is attributed to the lack of longitudinal vector and arbovirus surveillance. Other endemic mosquito species that may play a role in JEV maintenance in Australia include two recently established vectors with limited distributions – Culex gelidus has been implicated in previous Australian JEV outbreaks, whereas Culex tritaeniorhynchus is responsible for most of the JEV transmission in Asia (van den Hurk et al., 2022).
Culex quinquefasciatus, Cx. sitiens, Ae. camptorhynchus, Ae. notoscriptus, and Ae. vigilax are other common Australian mosquitoes (Russell, 1995). Culex sitiens is usually found around pools, puddles, ponds, wells, ditches, and rock pools, and often frequents tidal marshes and mangrove swamps. Females are primarily ornithophagic (i.e., feed on birds) but do feed on humans as well (Webb et al., 2016). Culex quinquefasciatus is active only during the warmer months, is generally ornithophagic and feeds on humans during the middle of the night (Webb et al., 2016). Ae. vigilax is a coastal saltmarsh mosquito that breeds in the brackish waters of mangrove swamps and salt marshes. Females are highly active at sunset, very aggressive biters and feed on humans and domestic animals (Belkin, 1962). The first isolate of RRV was taken from Ae. vigilax (Doherty R. et al., 1963). Ae. notoscriptus, a peri-domestic mosquito, is a competent vector of RRV and for these reasons it has been suggested that this species be considered more seriously in the context of urban transmission (Watson and Kay, 1998).
Aedes aegypti, a major global vector of DENV, may have been introduced into Australia in the early or mid-19th century (Mackenzie et al., 1996) and is now widespread throughout urban tropical north Queensland. Although this mosquito was widely distributed across south-east Queensland until the 1950s, it is now limited to an area bounded by Wondai in the south, Goomeri in the south-east and Charleville in the south-west (Queensland Health, 2015; Gyawali et al., 2016c).
Aedes albopictus, the Asian tiger mosquito, is also very able to transmit DENV and is distributed throughout the Torres Strait Islands to the north of Queensland (Ritchie et al., 2006). JEV was isolated from this vector in Malaysia and in Taiwan (Vythilingam et al., 1995; Weng et al., 1999; Su et al., 2014). Laboratory experiments in Australia demonstrated infection to Australian Ae. albopictus by feeding an infectious blood meal with virus titre 103.5 TCID50/mL (Nicholson et al., 2014). The ability of infected Ae. albopictus transmitting JEV to hosts (i.e. laboratory weanling mice) has been demonstrated in Taiwan (Weng et al., 1997). Overall, findings of vector competence within and outside Australia strongly suggest Ae. albopictus as a potential vector for JEV. However, it is not apparent that this mosquito has played a role in any outbreaks in Australia to date.
The Australian arboviruses associated with different mosquito species, whether in terms of competence or evidence of experimental virus transmission, are presented in Table 2.
4.2 Ticks
Very little is known about tick-borne arboviruses in Australia (Dehhaghi et al., 2019). Approximately 70 species of ticks are found in Australia, 16 of which are known to feed on humans (Australian Government Department of Health and Aged Care, 2023). Upolu virus, a bunyavirus, was isolated from the widely distributed soft-bodied tick, Ornithodoros capensis on the Great Barrier Reef, in 1966 (Doherty et al., 1969). Nugget (Orbivirus) and Taggert (Nairovirus) are Kemerovo and Sakhalin group viruses and have been isolated from hard-bodied ticks (Ixodes uriae) from Macquarie Island (Doherty et al., 1975), in the Southern Ocean south-east of Tasmania. Saumarez Reef virus is a flavivirus that was isolated from Or. capensis and Ix. eudyptidis in the Australian region (St George et al., 1977). The transmission cycles and the importance of these viruses in human infection are unresolved.
4.3 Midges
Most of the viruses of the Orbivirus serological group (Bluetongue, Corriparta, Eubenangee, Palyam, Wallal and Warrego viruses) were isolated from biting midges such as Culicoides brevitarsis and C. marksi (Doherty et al., 1977; Standfast et al., 1984). There are reports of the alphavirus, BFV, replicating in, and being isolated from, C. brevitarsis and C. marksi (Standfast et al., 1984). However, it is not known if this vector is competent to transmit BFV. Another virus, Thimiri, from the Simbu sero-group, was isolated from C. histrio collected from northern Australia (Standfast and Dyce, 1982). The previous isolations of Thimiri virus were from birds in India and Egypt (Carey et al., 1971) but the vertebrate host in Australia is uncertain, and the role of these viruses in human infection is yet to be determined.
4.4 Sand flies and black flies
To date, no arbovirus that is indigenous to Australia has been identified to have a transmission cycle involving either a sand fly or a black fly. However, examples do exist of the Phlebotomus and Simulium genera providing competent vectors of arboviruses elsewhere in the world (Blair and Olson, 2015) – for sand fly-borne phleboviruses and black fly-borne rhabdoviruses, respectively (Kuno and Chang, 2005). Hence, these transmission routes should be considered alongside others for those many neglected Australian arboviruses for which our knowledge of their transmission epidemiology is incomplete.
5 Emerging public health threat
There is growing awareness among the Australian healthcare community that indigenous arboviral diseases have a serious impact on national public health (Taylor-Robinson, 2021). More widely, they pose a global epidemic risk (Gyawali et al., 2016d), as exemplified by RRV outbreaks across several Pacific islands (Aaskov et al., 1981; Rosen et al., 1981; Tesh et al., 1981). The projected escalation of human activity in the tropical north of Australia, including economic development and urbanisation, will bring humans into close contact with native reservoir wildlife and vector mosquitoes for Australian indigenous arboviruses (Gyawali and Taylor-Robinson, 2017). The expanded agriculture sector predicted for these locations will change the ecology of these mammals, birds, and insects (Gyawali et al., 2017b). Furthermore, unforeseen climatic and environmental variations (Inglis, 2009), such as increased incidence of cyclones, heavy rainfall, and resultant intensified flooding associated with outbreaks of RRV (Tall et al., 2014) and MVEV (Selvey et al., 2014b), have occurred of late with disconcerting regularity (Knutson et al., 2010), potentially effectuating an ecological change for Australian arboviruses. The projected future climatic suitability of Northern Australia for competent vector mosquito species needs to be evaluated. Moreover, it is worth noting that already this century close relatives of many of these neglected arboviruses have caused regional epidemics and global pandemics (Gyawali et al., 2016b; Mayer et al., 2017).
In recent years, the Australian Government has made significant efforts to develop the regional Australia, focusing northern tropical region of Australia in principle (Australian Government, 2015). As defined by the Northern Australia Infrastructure Facility Act 2016, this aims to harness water resources and improve trade, business, and transport infrastructure in order to stimulate employment and population growth in those historically underinvested area (Australian Government Department of Infrastructure, Transport, Regional Development, Communications and the Arts, 2024). One challenge of the federal government’s commitment to facilitating growth across regional Australia is the potential emergence of unique and poorly understood healthcare threats. With increased human activity in this remote and medically underserved region, there is a major risk of encountering neglected arboviruses that have not been extensively studied (Gyawali and Taylor-Robinson, 2017; Gyawali et al., 2017a).
The economic and social development of the currently sparsely populated tropical north of Australia is set to bring infection-naïve humans into close contact with native reservoir hosts and vector mosquitoes. This convergence of factors may precipitate an increased prevalence of infection with neglected indigenous arboviruses. Moreover, the escalating rate and effects of climate change that are increasingly observed in the tropical north of the country will likely drive a population boom of arbovirus-transmitting mosquitoes. As a commensurate response, continuing assiduous attention to vector monitoring and control is required, harnessing artificial intelligence to rapidly process large volumes of data, thereby improving data analysis, prediction (Sinclair et al., 2019), and decision-making (Taylor-Robinson, 2023). In this overall context, improved epidemiological surveillance and diagnostic screening, including establishing novel, rapid pan-viral tests to facilitate early diagnosis and appropriate treatment of febrile primary care patients, should be considered a public health priority.
6 Future directions
This brief article summarises our current understanding of the complex transmission dynamics between virus, vector and host for neglected Australian arboviruses. It is apparent that there are large knowledge gaps that need closed as a future research priority. Yet, already from the available information, including a deep dive into the sources cited here and detailed elsewhere, public health stakeholders across the nation should be exhorted to consider the complex transmission dynamics between virus, vector, and host for neglected Australian arboviruses.
Key questions to address include:
(1) What are the specific arboviruses indigenous to Australia that are associated with neglected diseases, and what is their prevalence among human populations?
(2) How do arboviruses interact with their arthropod vectors and vertebrate hosts in transmission cycles, and what factors influence the efficiency of transmission between these entities?
(3) Are there alternative modes of transmission for arboviruses, such as vertically, that may impact their circulation and persistence in nature?
(4) How do environmental factors, including climate change, urbanization, and land use changes, influence the transmission dynamics of arboviruses and their vectors in Australia?
(5) What is the role of different vertebrate reservoir hosts in the maintenance and amplification of arboviruses, and how does this impact the risk of spillover to humans?
(6) How do within-host dynamics, such as viraemia levels and host immune responses, affect the transmission success of arboviruses and their ability to establish infection in new hosts?
(7) What are the implications of co-circulation of multiple arboviruses in a given region on transmission dynamics, vector competence, and disease outcomes?
(8) How can advanced technologies, such as artificial intelligence and genomic analyses, be leveraged to enhance surveillance, prediction, and response measures for arbovirus disease outbreaks in Australia?
By shedding light on these interrelated, multifactorial issues researchers can gain a comprehensive understanding of which vector(s) and virus(es) represents a potential threat to Australian public health, and of which geographical location(s), region(s) or state(s) should be targeted for routine vector and virus surveillance and control. Only through unravelling the intricate interactions between viruses, vectors, and hosts in the transmission dynamics of neglected Australian arboviruses will effective strategies for disease control and public health interventions be achieved.
While arbovirus species that are indigenous to Australia provide the focus of this review it should be noted that the same principles broadly apply to invasive species, such as DENV and JEV, and potentially CHIKV and ZIKV, that are mentioned briefly in context herein. As all of these are important human pathogens, given their widening global distribution in recent times there is a growing need for outbreak preparedness. Similar research to that described above is required to determine the capacity for reservoir infections in Australia’s unique native animals and birds as well as the vector competence and ecology of the country’s mosquito species.
7 Conclusion
Extremely little is known about the distribution, epidemiology and transmission ecology of neglected arboviruses that are native to Australia. There is also scant information on the immunopathology and true disease burden, including undiagnosed acute undifferentiated febrile illnesses, for which they are a likely cause. This is despite their predicted emergence as human pathogens in the rapidly developing Northern Australia, thus posing a significant public health threat to that vast region (Gyawali and Taylor-Robinson, 2017), and potentially more so globally (Gyawali et al., 2016d). Consideration of these focal points coupled with improved diagnostic protocols, including the preparation of first-line screening tests for a panel of arboviruses, would help to counter this emerging and hitherto neglected threat to the health of traditionally underserved communities. Moreover, understanding better the vector competence of mosquitoes is crucial for predicting and managing the spread of arboviruses that pose a risk to humans or livestock (Kain et al., 2022). Combatting the vectors that are the most competent, identifying the widest ranging reservoir hosts, assessing the risk of transmission in different locations, and developing targeted control strategies will all directly inform Australian public health efforts as well as contribute to global arbovirus surveillance and control initiatives.
Author contributions
AT-R: Conceptualization, Formal analysis, Writing – original draft, Writing – review and editing.
Funding
The author declares that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Past and present Australian arboviral research colleagues are warmly thanked for unpublished data sharing, expert advice and insightful discussions over several years that have informed the views expressed in this article. These include Narayan Gyawali (Queensland Health), Richard Bradbury (James Cook University), John Aaskov, Francesca Frentiu and Kym Lowry (Queensland University of Technology), Colleen Lau and James Sinclair (University of Queensland), David Huggins (Livingstone Shire Council), Peter Hartt (Rockhampton Regional Council), Wayne Pederick (QML Pathology), Abbey Potter (Murdoch University), Eloise Skinner (Griffith University and Stanford University), Gregor Devine and Leon Hugo (QIMR Berghofer Medical Research Institute), and Tim Inglis (University of Western Australia).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aaskov, J., Mataika, J., Lawrence, G., Rabukawaqa, V., Tucker, M., Miles, J., et al. (1981). An epidemic of Ross River virus infection in Fiji, 1979. Am. J. Trop. Med. Hyg. 30, 1053–1059.
Althouse, B. M., and Hanley, K. A. (2015). The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140299.
Anderson, S. G. (1953). Murray Valley encephalitis: A survey of avian sera, 1951–1952. Med. J. Aust. 1, 573–576.
Anderson, S. G. (1954). Murray Valley encephalitis and Australian X disease. Epidemiol. Infect. 52, 447–468.
Anderson, S., Dobrotworsky, N., and Stevenson, W. (1958). Murray valley encephalitis in the murray Valley, 1956 and 1957. Med. J. Aust. 2, 15–17.
Anderson, S., Donnelley, M., Stevenson, W., Caldwell, N., and Eagle, M. (1952). Murray Valley encephalitis: Surveys of human and animal sera. Med. J. Aust. 1, 110–114.
Australian Government (2015). Our north, our future: White paper on developing Northern Australia. Available online at: https://www.infrastructure.gov.au/sites/default/files/documents/nawp-fullreport.pdf (accessed July 24, 2024).
Australian Government Department of Health and Aged Care (2023). Prevention and management of tick bites in Australia. Available online at: https://www.health.gov.au/resources/publications/prevention-and-management-of-tick-bites-in-australia (accessed July 24, 2024).
Australian Government Department of Infrastructure, Transport, Regional Development, Communications and the Arts (2024). About us. Office of Northern Australia. Available online at: https://www.infrastructure.gov.au/territories-regions-cities/regional-australia/office-northern-australia/about-us (accessed July 24, 2024).
Badman, R. T., Campbell, J., and Aldred, J. (1984). Arbovirus infection in horses — Victoria. Commun. Dis. Intell. 17, 5–6.
Belkin, J. N. (1962). The mosquitoes of the south Pacific (Diptera, Culicidae), Vol. II. Berkeley, CA: University of California Press.
Blair, C. D., and Olson, K. E. (2015). The role of RNA interference (RNAi) in arbovirus-vector interactions. Viruses 2, 820–843.
Bosco-Lauth, A. M., Hartwig, A. E., and Bowen, R. A. (2018). Reptiles and amphibians as potential reservoir hosts of chikungunya virus. Am. J. Trop. Med. Hyg. 98, 841–844. doi: 10.4269/ajtmh.17-0730
Boyd, A. M., and Kay, B. H. (1999). Experimental infection and transmission of Barmah Forest virus by Aedes vigilax (Diptera: Culicidae). J. Med. Entomol. 36, 186–189.
Boyd, A. M., and Kay, B. H. (2000). Vector competence of Aedes aegypti, Culex sitiens, Culex annulirostris, and Culex quinquefasciatus (Diptera: Culicidae) for Barmah Forest virus. J. Med. Entomol. 37, 660–663. doi: 10.1603/0022-2585-37.5.660
Boyd, A. M., and Kay, B. H. (2002). Assessment of the potential of dogs and cats as urban reservoirs of Ross River and Barmah Forest viruses. Aust. Vet. J. 80, 83–86. doi: 10.1111/j.1751-0813.2002.tb12057.x
Boyd, A. M., Hall, R. A., Gemmell, R. T., and Kay, B. H. (2001). Experimental infection of Australian brushtail possums, Trichosurus vulpecula (Phalangeridae: Marsupialia), with Ross River and Barmah Forest viruses by use of a natural mosquito vector system. Am. J. Trop. Med. Hyg. 65, 777–782. doi: 10.4269/ajtmh.2001.65.777
Boyle, D. B., Dickerman, R. W., and Marshall, I. D. (1983). Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust. J. Exp. Biol. Med. Sci. 61, 655–664. doi: 10.1038/icb.1983.62
Braddick, M., O’Brien, H. M., Lim, C. K., Feldman, R., Bunter, C., Neville, P., et al. (2023). An integrated public health response to an outbreak of Murray Valley encephalitis virus infection during the 2022-2023 mosquito season in Victoria. Front. Public Health 11:1256149. doi: 10.3389/fpubh.2023.1256149
Broom, A. K., Wright, A. E., Mackenzie, J. S., Lindsay, M. D., and Robinson, D. (1989). Isolation of murray valley encephalitis and ross river viruses from Aedes normanensis (Diptera: Culicidae) in Western Australia. J. Med. Entomol. 26, 100–103. doi: 10.1093/jmedent/26.2.100
Campbell, J., and Hore, D. E. (1975). Isolation of Murray Valley encephalitis virus from sentinel chickens. Aust. Vet. J. 51, 1–3.
Carey, D., Reuben, R., George, S., Shope, R., and Myers, R. (1971). Kammavanpettai, Kanna-mangalam, Sembalam and Thimiri viruses: Four unrelated new agents isolated from birds in India. Indian J. Med. Res. 59, 1708–1711.
Centers for Disease Control and Prevention (2024). Arbovirus Catalog. Available online at: https://wwwn.cdc.gov/Arbocat/Default.aspx (accessed July 24, 2024).
Cloonan, M. J., O’Neill, B. J., Vale, T. G., Carter, I. W., and Williams, J. E. (1982). Ross River virus activity along the south coast of New South Wales. Aust. J. Exp. Biol. Med. Sci. 60, 701–706.
Colmant, A. M., Bielefeldt-Ohmann, H., Hobson-Peters, J., Suen, W. W., O’Brien, C. A., van den Hurk, A. F., et al. (2016). A newly discovered flavivirus in the yellow fever virus group displays restricted replication in vertebrates. J. Gen. Virol. 97, 1087–1093. doi: 10.1099/jgv.0.000430
Daniels, P. W., Middleton, D., and Lunt, R. (2000). Assessment of the potential of Australian fauna as maintenance or amplifying hosts of Japanese encephalitis (JE) virus. Report to the Northern Australian Quarantine strategy. Geelong, VIC: CSIRO Animal Health Laboratory, 1–3.
Dehhaghi, M., Kazemi Shariat, Panahi, H., Holmes, E. C., Hudson, B. J., Schloeffel, R., et al. (2019). Human tick-borne diseases in Australia. Front. Cell. Infect. Microbiol. 9:3. doi: 10.3389/fcimb.2019.00003
Doherty, R. L. (1964). A review of recent studies of arthropod-borne viruses in Queensland. J. Med. Entomol. 1, 158–165.
Doherty, R. L. (1977). Arthropod-borne viruses in Australia, 1973–1976. Aust. J. Exp. Biol. Med. Sci. 55, 103–130. doi: 10.1038/icb.1977.9
Doherty, R. L., Carley, J. G., and Gorman, B. M. (1964). Studies of arthropod-borne virus infections in Queensland. IV. Further serological investigations of antibodies to Group B arboviruses in man and animals. Aust. J. Exp. Biol. Med. Sci. 42, 149–164. doi: 10.1038/icb.1964.16
Doherty, R. L., Carley, J. G., Filippich, C., Kay, B. H., Gorman, B. M., and Rajapaksa, N. (1977). Isolation of Sindbis (alphavirus) and Leanyer viruses from mosquitoes collected in the Northern Territory of Australia, 1974. Aust. J. Exp. Biol. Med. Sci. 55, 485–489. doi: 10.1038/icb.1977.47
Doherty, R. L., Carley, J. G., Kay, B. H., Filippich, C., and Marks, E. N. (1976). Murray valley encephalitis virus infection in mosquitoes and domestic fowls in Queensland, 1974. Aust. J. Exp. Biol. Med. Sci. 54, 237–243. doi: 10.1038/icb.1976.24
Doherty, R. L., Carley, J. G., Kay, B. H., Filippich, C., Marks, E. N., and Frazier, C. L. (1979). Isolation of virus strains from mosquitoes collected in Queensland, 1972–1976. Aust. J. Exp. Biol. Med. Sci. 57, 509–520. doi: 10.1038/icb.1979.52
Doherty, R. L., Carley, J. G., Murray, M. D., Main, A. J., Kay, B. H., and Domrow, R. (1975). Isolation of arboviruses (Kemerovo group, Sakhalin group) from Ixodes uriae collected at Macquarie Island, Southern Ocean. Am. J. Trop. Med. Hyg. 24, 521–526. doi: 10.4269/ajtmh.1975.24.521
Doherty, R. L., Carley, J., Mackerras, M. J., and Marks, E. N. (1963). Studies of arthropod-borne virus infections in Queensland III. Isolation and characterization of virus strains from wild-caught mosquitoes in North Queensland. Aust. J. Exp. Biol. Med. Sci. 41, 17–39. doi: 10.1038/icb.1963.2
Doherty, R., Whitehead, R., Gorman, B., and O’Gower, A. (1963). The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Sci. 26, 183–184.
Doherty, R. L., Gorman, B. M., Whitehead, R. H., and Carley, J. G. (1966). Studies of arthropod-borne virus infections in Queensland. V. Survey of antibodies to group A arboviruses in man and other animals. Aust. J. Exp. Biol. Med. Sci. 44, 365–377. doi: 10.1038/icb.1966.35
Doherty, R. L., Standfast, H. A., Domrow, R., Wetters, E. J., Whitehead, R. H., and Carley, J. G. (1971). Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland IV. Arbovirus infections of mosquitoes and mammals, 1967–1969. Trans. R. Soc. Trop. Med. Hyg. 65, 504–513. doi: 10.1016/0035-9203(71)90161-1
Doherty, R. L., Whitehead, R. H., Wetters, E. J., and Gorman, B. M. (1968). Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland: II. Arbovirus infections of mosquitoes, man and domestic fowls, 1963–1966. Trans. R. Soc. Trop. Med. Hyg. 62, 430–438. doi: 10.1016/0035-9203(68)90095-3
Doherty, R. L., Whitehead, R. H., Wetters, E. J., and Johnson, H. N. (1969). Isolation of viruses from Ornithodoros capensis Neumann from a tern colony on the Great Barrier Reef. North Queensland. Aust. J. Sci. 31, 363–364.
Doherty, R., St George, T., and Carley, J. (1973). Arbovirus infections of sentinel cattle in Australia and New Guinea. Aust. Vet. J. 49, 574–579. doi: 10.1111/j.1751-0813.1973.tb06737.x
Fanning, I. D., Mottram, P., and Kay, B. H. (1992). Studies on vector competence and autogeny in Culex sitiens Wiedemann (Diptera: Culicidae). Aust. J. Entomol. 31, 249–253.
Franz, A. W. E., Kantor, A. M., Passarelli, A. L., and Clem, R. J. (2015). Tissue barriers to arbovirus infection in mosquitoes. Viruses 7, 3741–3767.
Fraser, J. R. (1986). Epidemic polyarthritis and Ross River virus disease. Clin. Rheum. Dis. 12, 369–388.
Frost, M. J., Zhang, J., Edmonds, J. H., Prow, N. A., Gu, X., Davis, R., et al. (2012). Characterization of virulent West Nile virus Kunjin strain, Australia, 2011. Emerg. Infect. Dis. 18, 792–800.
Gard, G. P., Giles, J. R., Dwyer-Grey, R. J., and Woodroofe, G. M. (1976). Serological evidence of interepidemic infection of feral pigs in New South Wales with Murray Valley encephalitis virus. Aust. J. Exp. Biol. Med. Sci. 54, 297–302. doi: 10.1038/icb.1976.30
Gard, G. P., Marshall, I. D., Walker, K. H., Acland, H. M., and Sarem, W. D. (1977). Association of Australian arboviruses with nervous disease in horses. Aust. Vet. J. 53, 61–66. doi: 10.1111/j.1751-0813.1977.tb14886.x
Go, Y. Y., Balasuriya, U. B., and Lee, C. K. (2014). Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 3, 58–77. doi: 10.7774/cevr.2014.3.1.58
Guay, P.-J., Azuolas, J. K., and Warner, S. (2012). Waterbird movement across the great dividing range and implications for arbovirus irruption into southern Victoria. Aust. Vet. J. 90, 197–198. doi: 10.1111/j.1751-0813.2012.00908.x
Gyawali, N., and Taylor-Robinson, A. W. (2017). Confronting the emerging threat to public health in Northern Australia of neglected indigenous arboviruses. Trop. Med. Infect. Dis. 2:55. doi: 10.3390/tropicalmed2040055
Gyawali, N., Bradbury, R. S., Aaskov, J. G., and Taylor-Robinson, A. W. (2017a). Neglected Australian arboviruses and undifferentiated febrile illness: Addressing public health challenges arising from the ‘Developing Northern Australia’ government policy. Front. Microbiol. 8:2150. doi: 10.3389/fmicb.2017.02150
Gyawali, N., Bradbury, R. S., Aaskov, J. G., and Taylor-Robinson, A. W. (2017b). Neglected Australian arboviruses: Quam gravis? Microbes Infect. 19, 388–401. doi: 10.1016/j.micinf.2017.05.002
Gyawali, N., Bradbury, R. S., and Taylor-Robinson, A. W. (2016a). The epidemiology of dengue infection: Harnessing past experience and current knowledge to support implementation of future control strategies. J. Vector Borne Dis. 53, 293–304.
Gyawali, N., Bradbury, R. S., and Taylor-Robinson, A. W. (2016b). The global spread of Zika virus: Is public and media concern justified in regions currently unaffected? Infect. Dis. Poverty 5:37. doi: 10.1186/s40249-016-0132-y
Gyawali, N., Bradbury, R. S., and Taylor-Robinson, A. W. (2016c). Knowledge, attitude and recommendations for practice regarding dengue among the resident population of Queensland, Australia. Asian Pac. J. Trop. Biomed. 6, 360–366.
Gyawali, N., Bradbury, R. S., and Taylor-Robinson, A. W. (2016d). Do neglected Australian arboviruses pose a global epidemic threat? Aust. N. Z. J. Public Health 40:596. doi: 10.1111/1753-6405.12582
Gyawali, N., Taylor-Robinson, A. W., Bradbury, R. S., Pederick, W., Faddy, H. M., and Aaskov, J. G. (2019a). Neglected Australian arboviruses associated with undifferentiated febrile illnesses. Front. Microbiol. 10:2818. doi: 10.3389/fmicb.2019.02818
Gyawali, N., Taylor-Robinson, A. W., Bradbury, R. S., Huggins, D. W., Hugo, L. E., Lowry, K., et al. (2019b). Identification of the source of blood meals in mosquitoes collected from north-eastern Australia. Parasit. Vectors 12:198. doi: 10.1186/s13071-019-3455-2
Gyawali, N., Taylor-Robinson, A. W., Bradbury, R. S., Potter, A., and Aaskov, J. G. (2020). Infection of western gray kangaroos (Macropus fuliginosus) with Australian arboviruses associated with human infection. Vector Borne Zoon. Dis. 20, 33–39. doi: 10.1089/vbz.2019.2467
Hanna, J. N., Ritchie, S. A., Phillips, D. A., Lee, J. M., Hills, S. L., van den Hurk, A. F., et al. (1999). Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 170, 533–536.
How, R. A., and Hillcox, S. J. (2000). Brushtail possum, Trichosurus vulpecula, populations in south-western Australia: Demography, diet and conservation status. Wildl. Res. 27, 81–89.
Inglis, T. J., Bradbury, R. S., McInnes, R. L., Frances, S. P., Merritt, A. J., Levy, A., et al. (2016). Deployable molecular detection of arboviruses in the Australian outback. Am. J. Trop. Med. Hyg. 95, 633–638. doi: 10.4269/ajtmh.15-0878
Jeffery, J. A., Ryan, P. A., Lyons, S. A., and Kay, B. H. (2002). Vector competence of Coquillettidia linealis (Skuse) (Diptera: Culicidae) for Ross River and Barmah Forest viruses. Aust. J. Entomol. 41, 339–344.
Kain, M. P., Skinner, E. B., Athni, T. S., Ramirez, A. L., Mordecai, E. A., and van den Hurk, A. F. (2022). Not all mosquitoes are created equal: A synthesis of vector competence experiments reinforces virus associations of Australian mosquitoes. PLoS Negl. Trop. Dis. 16:e0010768. doi: 10.1371/journal.pntd.0010768
Kay, B. H., and Aaskov, J. G. (1989). “Ross River virus (epidemic polyarthritis),” in The Arboviruses: Epidemiology and ecology, Vol. 4, ed. T. P. Monath (Boca Raton, FL: CRC Press), 93–112.
Kay, B. H., and Standfast, H. A. (1987). “Ecology of arboviruses and their vectors in Australia,” in Current topics in vector research, Vol. 3, ed. K. F. Harris (New York, NY: Springer-Verlag), 1–36.
Kay, B. H., Barker-Hudson, P., Stallman, N. D., Wiemers, M. A., Marks, E. N., Holt, P. J., et al. (1984). Dengue fever. Reappearance in northern Queensland after 26 years. Med. J. Aust. 140, 264–268. doi: 10.5694/j.1326-5377.1984.tb104033.x
Kay, B. H., Boreham, P. F. L., and Fanning, I. D. (1985a). Host-feeding patterns of Culex annulirostris and other mosquitoes (Diptera: Culicidae) at Charleville, southwestern Queensland, Australia. J. Med. Entomol. 22, 529–535. doi: 10.1093/jmedent/22.5.529
Kay, B. H., Young, P. L., Hall, R. A., and Fanning, I. D. (1985b). Experimental infection with Murray Valley encephalitis virus. Pigs, cattle, sheep, dogs, rabbits, macropods and chickens. Aust. J. Exp. Biol. Med. Sci. 63, 109–126. doi: 10.1038/icb.1985.13
Kay, B. H., Boyd, A. M., Ryan, P. A., and Hall, R. A. (2007). Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am. J. Trop. Med. Hyg. 76, 417–423.
Kay, B. H., Carley, J. G., and Filippich, C. (1975). The multiplication of Queensland and New Guinean arboviruses in Culex annulirostris Skuse and Aedes vigilax (Skuse) (Diptera: Culicidae). J. Med. Entomol. 12, 279–283. doi: 10.1093/jmedent/12.3.279
Kay, B. H., Carley, J. G., Fanning, I. D., and Filippich, C. (1979). Quantitative studies of the vector competence of Aedes aegypti, Culex annulirostris and other mosquitoes (Diptera: Culicidae) with Murray Valley encephalitis and other Queensland arboviruses. J. Med. Entomol. 16, 59–66. doi: 10.1093/jmedent/16.1.59
Kay, B. H., Edman, J. D., Fanning, I. D., and Mottram, P. (1989). Larval diet and the vector competence of Culex annulirostris (Diptera: Culicidae) for Murray valley encephalitis virus. J. Med. Entomol. 26, 487–488. doi: 10.1093/jmedent/26.5.487
Kay, B. H., Hall, R. A., Fanning, I. D., Mottram, P., Young, P. L., and Pollitt, C. C. (1986). “Experimental infection of vertebrates with Murray valley encephalitis and Ross River viruses,” in Arbovirus research in Australia. Proceedings of fourth symposium, eds T. D. St George, B. H. Kay, and J. Blok (Brisbane, QLD: CSIRO-QIMR), 71–75.
Kay, B. H., Pollitt, C. C., Fanning, I. D., and Hall, R. A. (1987). The experimental infection of horses with Murray Valley encephalitis and Ross River viruses. Aust. Vet. J. 64, 52–55.
Knope, K., Doggett, S. L., Jansen, C. C., Johansen, C. A., Kurucz, N., Feldman, R., et al. (2016). Arboviral diseases and malaria in Australia, 2014–15: Annual report of the national arbovirus and Malaria advisory committee. Commun. Dis. Intell. 40, E401–E436. doi: 10.33321/cdi.2019.43.14
Knox, T. B., Kay, B. H., Hall, R. A., and Ryan, P. A. (2003). Enhanced vector competence of Aedes aegypti (Diptera: Culicidae) from the Torres Strait compared with mainland Australia for dengue 2 and 4 viruses. J. Med. Entomol. 40, 950–956. doi: 10.1603/0022-2585-40.6.950
Knutson, T. R., McBride, J. L., Chan, J., Emanuel, K., Holland, G., Landsea, C., et al. (2010). Tropical cyclones and climate change. Nat. Geosci. 3, 157–163.
Kuno, G., and Chang, G.-J. J. (2005). Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 18, 608–637. doi: 10.1128/CMR.18.4.608-637.2005
Labeaud, A. D., Bashir, F., and King, C. H. (2011). Measuring the burden of arboviral diseases: The spectrum of morbidity and mortality from four prevalent infections. Popul. Health Metr. 9:1. doi: 10.1186/1478-7954-9-1
Lee, D. J., Hicks, M., Griffiths, M., Russell, R., and Marks, E. (1980). The Culicidae of the Australasian Region, Vol. I. Canberra, ACT: Australian Government Publishing Service.
Lequime, S., Fontaine, A., Gouilh, M. A., Moltini-Conclois, I., and Lambrechts, L. (2016). Genetic drift, purifying selection and vector genotype shape dengue virus intra-host genetic diversity in mosquitoes. PLoS Genet. 6:e1006111. doi: 10.1371/journal.pgen.1006111
Liehne, C. G., Stanley, N. F., Alpers, M. P., Paul, S., Liehne, P. F., and Chan, K. H. (1976). Ord River arboviruses–serological epidemiology. Aust. J. Exp. Biol. Med. Sci. 54, 505–512. doi: 10.1038/icb.1976.51
Liehne, P. F., Anderson, S., Stanley, N. F., Liehne, C. G., Wright, A. E., Chan, K. H., et al. (1981). Isolation of Murray Valley encephalitis virus and other arboviruses in the Ord River Valley 1972–1976. Aust. J. Exp. Biol. Med. Sci. 59, 347–356. doi: 10.1038/icb.1981.29
Lindsay, M. D. A., Breeze, A. L., Harrington, S. A., Johansen, C. A., Broom, A. K., Gordon, C. J., et al. (2005). Ross River and Barmah Forest viruses in Western Australia, 2000/01–2003/04: Contrasting patterns of disease activity. Arbovirus Res. Aust. 9, 194–201.
Mackenzie, J. S., and Smith, D. W. (2024). Japanese encephalitis virus in Australia: An ecological and epidemiological enigma. Med. J. Aust. 220, 559–560. doi: 10.5694/mja2.52319
Mackenzie, J. S., Broom, A. K., Aldred, J., Hueston, L., and Cunningham, A. L. (1992). Australian encephalitis: Sentinel chicken surveillance programme. Commun. Dis. Intell. Q. Rep. 16, 55–57.
Mackenzie, J. S., la Brooy, J. T., Hueston, L., and Cunningham, A. L. (1996). Dengue in Australia. J. Med. Microbiol. 45, 159–161.
Marshall, I. D., Brown, B. K., Keith, K., Gard, G. P., and Thibos, E. (1982a). Variation in arbovirus infection rates in species of birds sampled in a serological survey during an encephalitis epidemic in the Murray Valley of south-eastern Australia, February 1974. Aust. J. Exp. Biol. Med. Sci. 60, 471–478. doi: 10.1038/icb.1982.52
Marshall, I. D., Woodroofe, G. M., and Hirsch, S. (1982b). Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of south-eastern Australia, February 1974, during an epidemic of encephalitis. Aust. J. Exp. Biol. Med. Sci. 60, 457–470. doi: 10.1038/icb.1982.51
Mayer, S. V., Tesh, R. B., and Vasilakis, N. (2017). The emergence of arthropod-borne viral diseases: A global prospective on dengue, Chikungunya and Zika fevers. Acta Trop. 166, 155–163.
McGuinness, S. L., Lau, C. L., and Leder, K. (2023). Co-circulation of Murray Valley encephalitis virus and Japanese encephalitis virus in south-eastern Australia. J. Travel Med. 30:taad059. doi: 10.1093/jtm/taad059
McManus, T. J., and Marshall, I. D. (1986). Epidemiology of Ross River virus in Tasmania. Arbovirus Res. Aust. 6, 68–72.
Nicholson, J., Ritchie, S. A., and van den Hurk, A. F. (2014). Aedes albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J. Med. Entomol. 51, 661–669. doi: 10.1603/me13204
O’Donnell, M., Berr, I. G., and Carvan, T. (1992). Dispersal of adult females of Culex annulirostris in Griffith, New South Wales, Australia. J. Am. Mosq. Control Assoc. 8, 159–165.
Pascoe, R. R. R., St George, T. D., and Cybinski, D. H. (1978). The isolation of a Ross River virus from a horse. Aust. Vet. J. 54:600.
Pendrey, C. G. A., and Martin, G. E. (2023). Japanese encephalitis clinical update: Changing diseases under a changing climate. Aust. J. Gen. Pract. 52, 275–280. doi: 10.31128/AJGP-07-22-6484
Potter, A., Johansen, C. A., Fenwick, S., Reid, S. A., and Lindsay, M. D. (2014). The seroprevalence and factors associated with Ross River virus infection in western grey kangaroos (Macropus fuliginosus) in Western Australia. Vector Borne Zoonot. Dis. 14, 740–745. doi: 10.1089/vbz.2014.1617
Queensland Health (2015). Queensland dengue management plan 2015–2020. Available online at: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/444433/dengue-mgt-plan.pdf (accessed July 24, 2024).
Reinert, J. F. (2000). New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J. Am. Mosq. Control Assoc. 16, 175–188.
Ritchie, S. A., Phillips, D., Broom, A., Mackenzie, J., Poidinger, M., and van den Hurk, A. (1997a). Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am. J. Trop. Med. Hyg. 56, 80–84. doi: 10.4269/ajtmh.1997.56.80
Ritchie, S. A., Fanning, I. D., Phillips, D. A., Standfast, H. A., McGinn, D., and Kay, B. H. (1997b). Ross River virus in mosquitoes (Diptera: Culicidae) during the 1994 epidemic around Brisbane, Australia. J. Med. Entomol. 34, 156–159. doi: 10.1093/jmedent/34.2.156
Ritchie, S. A., Moore, P., Carruthers, M., Williams, C., Montgomery, B., Foley, P., et al. (2006). Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 22, 358–365. doi: 10.2987/8756-971X(2006)22[358:DOAWIO]2.0.CO;2
Roche, S., Wicks, R., Garner, M., East, I., Paskin, R., Moloney, B., et al. (2013). Descriptive overview of the 2011 epidemic of arboviral disease in horses in Australia. Aust. Vet. J. 91, 5–13. doi: 10.1111/avj.12018
Rosen, L., Gubler, D. J., and Bennett, P. H. (1981). Epidemic polyarthritis (Ross River) virus infection in the Cook Islands. Am. J. Trop. Med. Hyg. 30, 1294–1302.
Russell, R. (1995). Arboviruses and their vectors in Australia: An update on the ecology and epidemiology of some mosquito-borne arboviruses. Rev. Med. Vet. Entomol. 83, 141–158.
Russell, R. C. (1998). Mosquito-borne arboviruses in Australia: The current scene and implications of climate change for human health. Int. J. Parasitol. 28, 955–969. doi: 10.1016/s0020-7519(98)00053-8
Russell, R. C., and Kay, B. H. (2004). Medical entomology: Changes in the spectrum of mosquito-borne disease in Australia and other vector threats and risks, 1972–2004. Aust. J. Entomol. 43, 271–282.
Russell, R. C., Cloonan, M. J., Wells, P. J., and Vale, T. G. (1991). Mosquito (Diptera: Culicidae) and arbovirus activity on the South Coast of New South Wales, Australia, in 1985–1988. J. Med. Entomol. 28, 796–804. doi: 10.1093/jmedent/28.6.796
Ryan, P. A., and Kay, B. H. (1999). Vector competence of mosquitoes (Diptera: Culicidae) from Maroochy Shire, Australia, for Barmah Forest virus. J. Med. Entomol. 36, 856–860.
Ryan, P. A., Do, K. A., and Kay, B. H. (2000). Definition of Ross River virus vectors at Maroochy Shire, Australia. J. Med. Entomol. 37, 146–152. doi: 10.1603/0022-2585-37.1.146
Ryan, P. A., Martin, L., Mackenzie, J. S., and Kay, B. H. (1997). Investigation of gray-headed flying foxes (Pteropus poliocephalus) (Megachiroptera: Pteropodidae) and mosquitoes in the ecology of Ross River virus in Australia. Am. J. Trop. Med. Hyg. 57, 476–482. doi: 10.4269/ajtmh.1997.57.476
Sammels, L. M., Lindsay, M. D., Poidinger, M., Coelen, R. J., and Mackenzie, J. S. (1999). Geographic distribution and evolution of Sindbis virus in Australia. J. Gen. Virol. 80, 739–748.
Sanderson, C. (1969). A serologic survey of Queensland cattle for evidence of arbovirus infection. Am. J. Trop. Med. Hyg. 18, 433–439. doi: 10.4269/ajtmh.1969.18.433
Selvey, L. A., Dailey, L., Lindsay, M., Armstrong, P., Tobin, S., Koehler, A. P., et al. (2014a). The changing epidemiology of Murray Valley encephalitis in Australia: The 2011 outbreak and a review of the literature. PLoS Negl. Trop. Dis. 8:e2656. doi: 10.1371/journal.pntd.0002656
Selvey, L. A., Johansen, C. A., Broom, A. K., Antão, C., Lindsay, M. D., Mackenzie, J. S., et al. (2014b). Rainfall and sentinel chicken seroconversions predict human cases of Murray Valley encephalitis in the north of Western Australia. BMC Infect. Dis. 14:672. doi: 10.1186/s12879-014-0672-3
Sinclair, J. B., Gyawali, N., and Taylor-Robinson, A. W. (2019). Predicting Ross River virus infection by analysis of seroprevalence data. Am. J. Infect. Dis. Microbiol. 7, 1–7.
Soman, R. S., Rodrigues, F. M., Guttikar, S. N., and Guru, P. Y. (1977). Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in ardeid birds. Indian J. Med. Res. 66, 709–718.
St George, T. D., Standeast, H. A., Doherty, R. L., Carley, J. G., Fillipich, C., and Brandsma, J. (1977). The isolation of Saumarez Reef virus, a new flavivirus, from bird ticks Ornithodoros capensis and Ixodes eudyptidis in Australia. Aust. J. Exp. Biol. Med. Sci. 55, 493–499. doi: 10.1038/icb.1977.49
Standfast, H. A., and Dyce, A. L. (1982). Isolation of Thimiri virus from Culicoides histrio (Diptera: Ceratopogonidae) collected in Northern Australia. J. Med. Entomol. 19:212. doi: 10.1093/jmedent/19.2.212
Standfast, H. A., Dyce, A. L., St George, T. D., Muller, M. J., Doherty, R. L., Carley, J. G., et al. (1984). Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974–1976. Aust. J. Biol. Sci. 37, 351–366. doi: 10.1071/bi9840351
Su, C.-L., Yang, C.-F., Teng, H.-J., Lu, L.-C., Lin, C., Tsai, K.-H., et al. (2014). Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 8:e3122. doi: 10.1371/journal.pntd.0003122
Susilawati, T. N., and McBride, W. J. H. (2014). Undiagnosed undifferentiated fever in Far North Queensland, Australia: A retrospective study. Int. J. Infect. Dis. 27, 59–64. doi: 10.1016/j.ijid.2014.05.022
Tall, J. A., Gatton, M. L., and Tong, S. (2014). Ross River virus disease activity associated with naturally occurring nontidal flood events in Australia: A systematic review. J. Med. Entomol. 51, 1097–1108. doi: 10.1603/ME14007
Taylor-Robinson, A. W. (2021). Perfect storm brewing for mosquito-borne viruses. MJA InSight+ View, issue 32, 30 August. Available online at: https://insightplus.mja.com.au/2021/32/perfect-storm-brewing-for-mosquito-borne-viruses/ (accessed July 24, 2024).
Taylor-Robinson, A. W. (2023). Harnessing artificial intelligence to enhance key surveillance and response measures for arbovirus disease outbreaks: The exemplar of Australia. Front. Microbiol. 14:1284838. doi: 10.3389/fmicb.2023.1284838
Tesh, R. B., McLean, R. G., Shroyer, D. A., Calisher, C. H., and Rosen, L. (1981). Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in American Samoa. Trans. R. Soc. Trop. Med. Hyg. 75, 426–431.
Togami, E., Gyawali, N., Ong, O., Kama, M., Cao-Lormeau, V.-M., Aubry, M., et al. (2020). First evidence of concurrent enzootic and endemic transmission of Ross River virus in the absence of marsupial reservoirs in Fiji. Int. J. Infect. Dis. 96, 94–96. doi: 10.1016/j.ijid.2020.02.048
Vale, T. G., Spratt, D. M., and Cloonan, M. J. (1991). Serological evidence of arbovirus infection in native and domesticated mammals on the south coast of New South Wales. Aust. J. Zool. 39, 1–7.
van den Hurk, A. F., Nisbet, D. J., Foley, P. N., Ritchie, S. A., Mackenzie, J. S., and Beebe, N. W. (2002). Isolation of arboviruses from mosquitoes (Diptera: Culicidae) collected from the Gulf Plains region of northwest Queensland, Australia. J. Med. Entomol. 39, 786–792. doi: 10.1603/0022-2585-39.5.786
van den Hurk, A. F., Nisbet, D. J., Hall, R. A., Kay, B. H., Mackenzie, J. S., and Ritchie, S. A. (2003). Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J. Med. Entomol. 40, 82–90.
van den Hurk, A. F., Skinner, E., Ritchie, S. A., and Mackenzie, J. S. (2022). The emergence of Japanese encephalitis virus in Australia in 2022: Existing knowledge of mosquito vectors. Viruses 14:1208.
van den Hurk, A. F., Smith, C. S., Field, H. E., Smith, I. L., Northill, J. A., Taylor, C. T., et al. (2009). Transmission of Japanese encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am. J. Trop. Med. Hyg. 81, 457–462.
Vieira, C., Gyawali, N., Onn, M., Shivas, M., Shearman, D., Darbro, J., et al. (2023). Tracking Ross River virus host diversity using mosquitoes as ‘flying syringes’. Int. J. Infect. Dis. 130:S35.
Vythilingam, I., Oda, K., Chew, T. K., Mahadevan, S., Vijayamalar, B., Morita, K., et al. (1995). Isolation of Japanese encephalitis virus from mosquitoes collected in Sabak Bernam, Selangor, Malaysia in 1992. J. Am. Mosq. Control Assoc. 11, 94–98.
Watson, T. M., and Kay, B. H. (1998). Vector competence of Aedes notoscriptus (Diptera: Culicidae) for Ross River virus in Queensland, Australia. J. Med. Entomol. 35, 104–106. doi: 10.1093/jmedent/35.2.104
Watson, T. M., and Kay, B. H. (1999). Vector competence of Aedes notoscriptus (Diptera: Culicidae) for Barmah Forest virus and of Aedes aegypti (Diptera: Culicidae) for dengue 1–4 viruses in Queensland, Australia. J. Med. Entomol. 36, 508–514. doi: 10.1093/jmedent/36.4.508
Weaver, S. C., and Barrett, A. D. (2004). Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2, 789–801.
Webb, C. E., Doggett, S. L., and Russell, R. C. (2016). A guide to mosquitoes of Australia. Clayton South, VIC: CSIRO Publishing.
Weng, M. H., Lien, J. C., Wang, Y. M., Lin, C. C., Lin, H. C., and Chin, C. (1999). Isolation of Japanese encephalitis virus from mosquitoes collected in Northern Taiwan between 1995 and 1996. J. Microbiol. Immunol. Infect. 32, 9–13.
Weng, M. H., Lien, J. C., Wang, Y. M., Wu, H. L., and Chin, C. (1997). Susceptibility of three laboratory strains of Aedes albopictus (Diptera: Culicidae) to Japanese encephalitis virus from Taiwan. J. Med. Entomol. 34, 745–747. doi: 10.1093/jmedent/34.6.745
Whitehead, R. H., Doherty, R. L., Domrow, R., Standfast, H. A., and Wetters, E. J. (1968). Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland: III. Virus studies of wild birds, 1964–1967. Trans. R. Soc. Trop. Med. Hyg. 62, 439–445. doi: 10.1016/0035-9203(68)90096-5
Wilder-Smith, A., Gubler, D. J., Weaver, S. C., Monath, T. P., Heymann, D. L., and Scott, T. W. (2017). Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 17, e101–e106. doi: 10.1016/S1473-3099(16)30518-7
Williams, C. R., Webb, C. E., Higgs, S., and van den Hurk, A. F. (2022). Japanese encephalitis virus emergence in Australia: Public health importance and iImplications for future surveillance. Vector Borne Zoonot. Dis. 22, 529–534. doi: 10.1089/vbz.2022.0037
Keywords: arbovirus, neglected, transmission, arthropod, vector, reservoir host, enzootic, Australia
Citation: Taylor-Robinson AW (2024) Complex transmission epidemiology of neglected Australian arboviruses: diverse non-human vertebrate hosts and competent arthropod invertebrate vectors. Front. Microbiol. 15:1469710. doi: 10.3389/fmicb.2024.1469710
Received: 24 July 2024; Accepted: 20 August 2024;
Published: 04 September 2024.
Edited by:
Ke Liu, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Sujit Pujhari, University of South Carolina, United StatesJiaxin Ling, Uppsala University, Sweden
Copyright © 2024 Taylor-Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew W. Taylor-Robinson, YW5kcmV3LnRyQHZpbnVuaS5lZHUudm4=
†ORCID: Andrew W. Taylor-Robinson, orcid.org/0000-0001-7342-8348
 Andrew W. Taylor-Robinson
Andrew W. Taylor-Robinson