
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 18 October 2024
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1459162
This article is part of the Research TopicOpportunistic pathogens: pathogenesis and multi-drug resistance mechanismsView all 10 articles
Background: Morganella morganii is an emerging nosocomial opportunistic pathogen with increasing multidrug resistance. Antibiotic resistance, driven primarily by the horizontal transfer of resistance genes, has become a global health crisis. Integrons, mobile genetic elements, are now understood to facilitate the transfer of these genes, contributing to the rapid proliferation of resistant strains. Understanding the regulatory role of integrons in drug resistance gene expression is crucial for developing novel strategies to combat this pressing public health issue.
Objective: To investigate the distribution of promoter types in the variable regions of class 1 integrons isolated from clinical isolates of M. morganii and their regulatory role in the expression of downstream drug resistance gene cassettes.
Methods: Ninety seven clinical isolates of M. morganii were screened for the presence of class 1 integrons (intI1) using polymerase chain reaction (PCR). Gene cassettes within the variable regions of positive isolates were characterized, and the gene cassette promoter Pc variants and downstream auxiliary promoter P2 were identified. Enterobacterial repetitive intergenic consensus (ERIC)-PCR was employed for homology analysis. Recombinant plasmids containing different variable region promoters and gene cassettes were constructed to evaluate drug resistance genes and integrase (intI1) expression levels using reverse transcription-quantitative PCR (RT-qPCR) and antimicrobial susceptibility testing.
Results: Of the clinical isolates, 28.9% (n = 28/97) were positive for class 1 integrons. 24.7% (n = 24/97) of these isolates carried gene cassettes encoding resistance to aminoglycosides and trimethoprim. Three Pc promoter types (PcH1, PcS, and PcW) were identified, while all P2 promoters were inactive with a 14-base pair spacing between the −35 and −10 regions. ERIC-PCR analysis classified the integron-positive strains into 6 genotypes, with high consistency in promoter types and gene cassettes within each genotype. RT-qPCR and antimicrobial susceptibility testing demonstrated that strong promoters significantly enhanced the expression of downstream drug resistance gene cassettes compared to weak promoters. Additionally, RT-qPCR revealed a negative correlation between intI1 expression and Pc promoter strength.
Conclusion: Class 1 integrons are prevalent in M. morganii. The promoter types within these integrons are diverse, and promoter strength is closely linked to downstream gene cassette expression. Integron-positive strains exhibit high homology, suggesting horizontal gene transfer and dissemination in clinical settings.
Morganella morganii, a member of the Enterobacteriaceae family, has historically been detected infrequently in clinical settings (Agrawal et al., 2021). While traditionally associated with urinary tract infections, wound infections, and bloodstream infections (Laupland et al., 2022; Shi et al., 2022; Gameiro et al., 2023; Kvopka et al., 2023; Li et al., 2023; Yeşil et al., 2023; Alsaadi et al., 2024), its clinical isolation rate has been rapidly increasing in recent years, leading to severe invasive infections (Zaric et al., 2021; Alelyani et al., 2022; Behera et al., 2023a; Elmi et al., 2024). Due to its high mortality rates in specific patient populations, the World Health Organization has designated M. morganii as a globally prioritized pathogen (Behera et al., 2023b). Some clinically isolated strains of M. morganii have acquired resistance to multiple antibiotics through the carriage of various drug resistance genes, posing serious challenges for clinical infection control (Liu et al., 2016). Horizontal transfer of drug resistance genes, facilitated by mobile genetic elements such as integrons, is a common mechanism for acquiring resistance in bacteria (Leverstein-van Hall et al., 2002; von Wintersdorff et al., 2016; Coluzzi et al., 2023). However, the role of integrons in M. morganii resistance has been largely understudied.
Current evidence suggests that integrons, typically consisting of three main components: a 5′ conserved region, a variable region containing various drug resistance gene cassettes, and a 3′ conserved region that varies with integron type, play a pivotal role in the horizontal transfer of drug resistance genes among bacteria (Hall and Stokes, 1993). They are natural cloning and expression vectors, capturing and disseminating gene cassettes through site-specific recombination (Hall and Collis, 2006). One of the most extensively studied integron types is class 1 integrons, which include the Pc promoter in the 5′ conserved region. As most gene cassettes lack promoters, the Pc promoter plays a crucial role in regulating the expression of downstream gene cassettes within integrons (Hanau-Berçot et al., 2002; Tseng et al., 2014). In some cases, the Pc promoter complements the auxiliary promoter P2, forming a Pc-P2 dual promoter configuration that further influences gene regulation (Stokes et al., 1997). The strength of the promoter, determined by transcriptional dominance, varies among different Pc variants defined by their −35 and −10 hexamer sequences. The most common Pc promoters in clinical and natural settings are Pc strong (PcS), Pc weak (PcW), Pc Hybrid 1 (PcH1), and Pc Hybrid 2 (PcH2) (Fonseca and Vicente, 2022). The transcription level of genes within integrons largely depends on the regulatory role of these promoters (Novačić et al., 2022), influenced by factors such as promoter strength and proximity to the gene cassette (Jacquier et al., 2009). Integron-positive M. morganii can express resistance to relevant drug resistance genes within the integron, and the expression of these genes is primarily dependent on the regulation of the variable region Pc promoter. The horizontal transmission of drug resistance genes between different bacterial strains, facilitated by integron-carrying M. morganii, poses a significant challenge in clinical settings. To investigate the relationship between variable region promoters of class 1 integrons in M. morganii and the regulation of drug resistance gene expression, this study analyzed antibiotic resistance data from non-duplicated clinical isolates collected from November 2015 to August 2021 at the Affiliated LiHuiLi Hospital of Ningbo University. The findings of this study provide valuable insights into the mechanism and expression regulation of drug resistance genes in integron-positive M. morganii, which has significant clinical implications for the prevention and treatment of this rare pathogen.
Ninety seven non-duplicated clinical isolates of M. morganii were collected from urine, bile, and other specimen types at the Affiliated LiHuiLi Hospital of Ningbo University between November 2015 and August 2021. Strains lacking complete clinical data were excluded. This study was approved by the Medical Ethics Committee of the Affiliated LiHuiLi Hospital of Ningbo University. Escherichia coli DH5α served as the integron-negative control strain, Proteus mirabilis 47437 as the class 1 integron-positive control strain, E. coli JM109 as the competent strain, and E. coli ATCC25922 as the control strain for antimicrobial susceptibility testing. Plasmid pACYC184 was used as the cloning and expression vector. All strains and plasmids were maintained in our laboratory.
DNA templates were extracted from experimental strains using the boiling method. PCR amplification was performed using intI1 screening primers (intF & P2R, Table 1) at an annealing temperature of 55°C. PCR products were analyzed by agarose gel electrophoresis to identify positive bands. Sequencing analysis was conducted to confirm the presence of class 1 integrons in the positive strains. Gene cassettes within the variable regions of class 1 integron-positive strains were amplified using specific primers (5CS & 3CS, Table 1) at an annealing temperature of 55°C. Positive bands were visualized by agarose gel electrophoresis, and sequencing of the PCR products was followed by comparison with the BLAST database to determine the composition of variable gene cassettes in these strains.
To identify Pc and P2 promoters in class 1 integron-positive strains, reverse primers were designed based on the sequences of the first gene cassettes at the 5′-conserved sequence (5CS) end, in conjunction with the forward primer intF (Table 1). PCR was conducted with annealing temperatures ranging from 52°C to 56°C for 35 cycles. Amplified products were visualized by agarose gel electrophoresis, and positive bands were sequenced to determine the promoter types. Clinical isolates were categorized according to their integron promoter types. A retrospective analysis of antibiotic resistance data was performed for each group of isolates, followed by a comparative analysis to evaluate differences in antibiotic resistance profiles among the various integron promoter-based groups.
Enterobacterial repetitive intergenic consensus (ERIC) sequences are non-coding and highly conserved regions originally discovered in Enterobacteriaceae. Enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR) involves the design of primers based on the conserved ERIC region for PCR amplification. The number and size of ERIC bands in the bacterial genome are then determined, and the degree of bacterial relatedness is calculated for typing purposes. Compared to other typing methods, ERIC-PCR is a popular choice due to its lower cost and faster operation (Meacham et al., 2003; Banoub et al., 2022). In the present study, integron-positive strains were subjected to ERIC-PCR analysis (using primer ERIC2, Table 1, annealing temperature 40°C, 40 cycles) to assess homology. Positive bands and their positions on agarose gel electrophoresis of PCR products were recorded, photographed, and compiled into a matrix table. NTsys 2.10e software was used to generate a clustering dendrogram. Additionally, integron-positive strains were genotyped, and homology analysis was conducted in conjunction with Pc promoter and drug resistance gene cassettes in the variable region.
Two sets of recombinant plasmids were constructed based on different promoter types and gene cassettes within the variable region. The first set employed various variable region promoters while maintaining the same gene cassette, while the second set utilized the same variable region promoter and gene cassette but varied the distance from the Pc promoter. The pACYC184 plasmid, which confers chloramphenicol resistance, served as the vector. HindIII and AseI restriction enzymes were chosen to digest the plasmid, replacing the tet promoter to avoid interference with the integron promoters in the recombinant gene fragment (Figure 1). The larger fragment of the digested plasmid was then purified. A synthetic gene fragment, encompassing the integrase intI1 to 3CS region (including the variable region promoter), was ligated to this purified plasmid fragment. The resulting recombinant plasmids were confirmed through sequencing and subsequently transformed into competent E. coli JM109 cells.
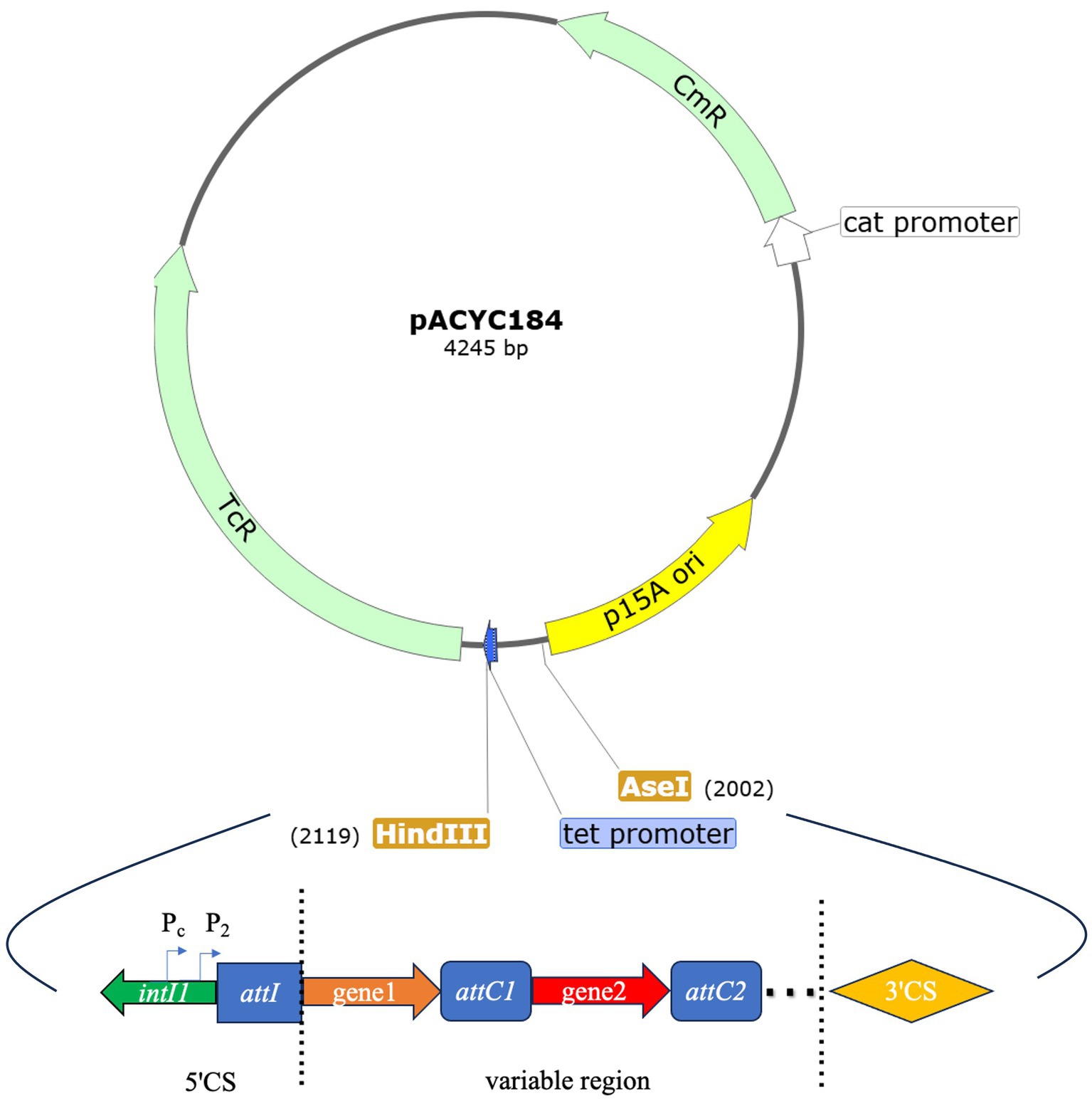
Figure 1. pACYC184 plasmid map and the sites of enzyme digestion HindIII and AseI. The pACYC184 plasmid map shows the positions of HindIII and AseI. The tet promoter has been disrupted by enzymatic cleavage. The map of the recombinant gene fragment that was integrated into the digested plasmid: the map includes the complete sequence from intI1 to 3CS. tet: tetracycline resistance gene, cat: chloramphenicol resistance gene.
Total RNA was extracted from the logarithmic growth phase of the constructed strains. The extracted RNA was purified and subjected to reverse transcription (RT) to obtain cDNA templates, which were then diluted to appropriate concentrations for subsequent quantitative PCR (qPCR) detection. qPCR was performed using specific primers targeting the genes of interest in the constructed strains. The pACYC184 plasmid-encoded cat gene was used as an internal reference gene. Relative expression levels of genes were calculated using the 2−ΔΔCt method. The expression levels of the target gene and the integrase gene intI1 were analyzed to compare transcriptional differences among the various constructed strains.
Antimicrobial susceptibility testing and interpretation of experimental results was conducted following Clinical and Laboratory Standards Institute (CLSI) guidelines (M-100, Ed 2022) (CLSI, 2022). The broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) of antibiotics mediated by drug resistance gene cassettes in the variable region. Chloramphenicol (mediated by the internal reference gene cat) and tetracycline (mediated by gene tet) were also tested in each constructed strain. E. coli ATCC25922 served as the quality control strain for susceptibility testing, and E. coli JM109 was used as the negative control strain. The Kirby-Bauer (K-B) disk diffusion method was employed to validate the results obtained from MIC determination, allowing for a comparison of resistance differences among the constructed strains.
In this study, the comparison of drug resistance rates between different groups was analyzed using Fisher’s exact test (two-sided) with SPSS 25.0 software, considering a significance level of p < 0.05. Gene relative expression levels in qPCR were analyzed using one-way ANOVA for multiple comparisons among strains with GraphPad Prism 8.0 software, using a significance level of p < 0.05.
We identified 28 out of 97 clinical isolates of M. morganii as positive for intI1, resulting in a positivity rate of 28.9% (Supplementary material: Sequence of intI1). Among these intI1-positive isolates, 10 different types of gene cassettes were amplified, while 4 isolates did not amplify any gene cassettes (Supplementary material: Sequence of variable region). The amplified gene cassettes primarily conferred resistance to aminoglycosides and trimethoprim, as shown in Table 2.
Among the 28 strains positive for class 1 integrons, three types of Pc promoters were detected: PcH1 (n = 19), PcS (n = 5), and PcW (n = 4). PcS exhibited stronger promoter activity, PcW showed weaker activity, and PcH1 demonstrated intermediate strength. All downstream P2 promoters were inactive types, spaced 14 base pairs apart from the −35 to −10 regions (Supplementary material: Sequence of promoter). Based on the different types of variable region promoters in the integrons, the positive strains were categorized into three groups. Retrospective analysis of drug resistance data was conducted among strains containing different types of variable region promoters. Although there were no significant differences in resistance rates to cotrimoxazole, gentamicin, or tobramycin among the groups (p > 0.05), strains in the PcS group exhibited higher resistance rates to all antibiotics compared to the PcH1 and PcW groups, while the PcW group demonstrated the lowest resistance rates among the three groups (Figure 2).
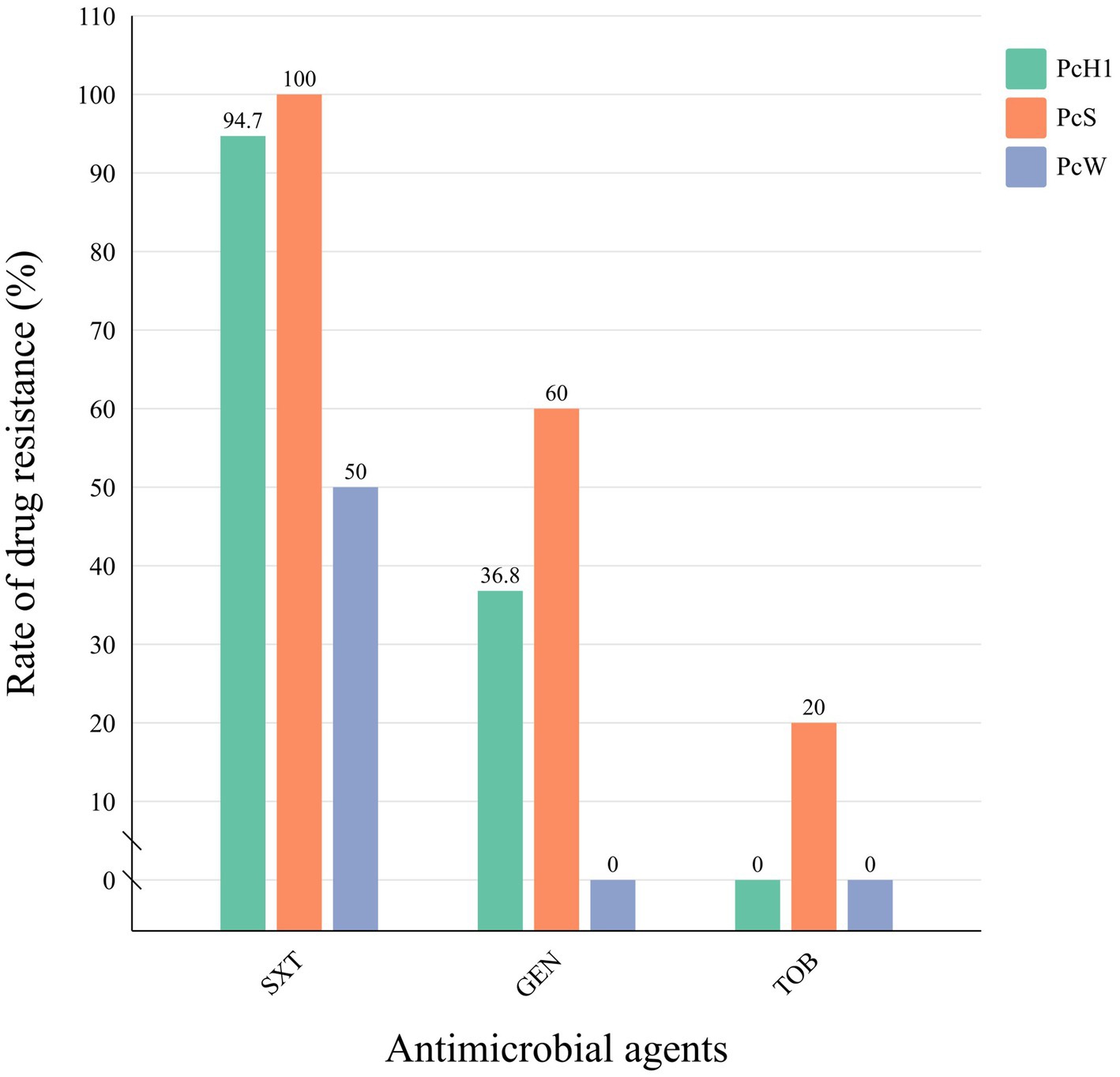
Figure 2. The comparison of antibiotic resistance rates among different promoter groups in Morganella morganii isolates. STX, cotrimoxazole; GEN, gentamicin; TOB: tobramycin.
ERIC-PCR fingerprinting of the 28 class 1 integron-positive strains was encoded and organized into a matrix table (Supplementary Figure S1; ERIC-PCR electrophoretogram). NTsys 2.10e software was used to generate a dendrogram with a reference line set at 75% similarity (Bakhshi et al., 2018). Based on this analysis, the positive strains were classified into 6 distinct genotypes (A, B, C, D, E, F). Genotype A was the most prevalent, with 11 strains, followed by genotype C with 7 strains. The predominant type of variable region promoter Pc was PcH1. Gene cassette dfrA was the dominant gene in the variable region of type A isolates, which exhibited high resistance to trimethoprim. Type B isolates primarily carried the PcH1/dfrA17-aadA5 combination, associated with resistance to aminoglycosides and trimethoprim. Type C isolates predominantly had the PcS promoter and showed relatively high resistance rates to various antibiotics. The distribution of gene cassettes was concentrated, primarily consisting of aminoglycoside and trimethoprim drug resistance gene cassettes. Specific details are illustrated in Figure 3.
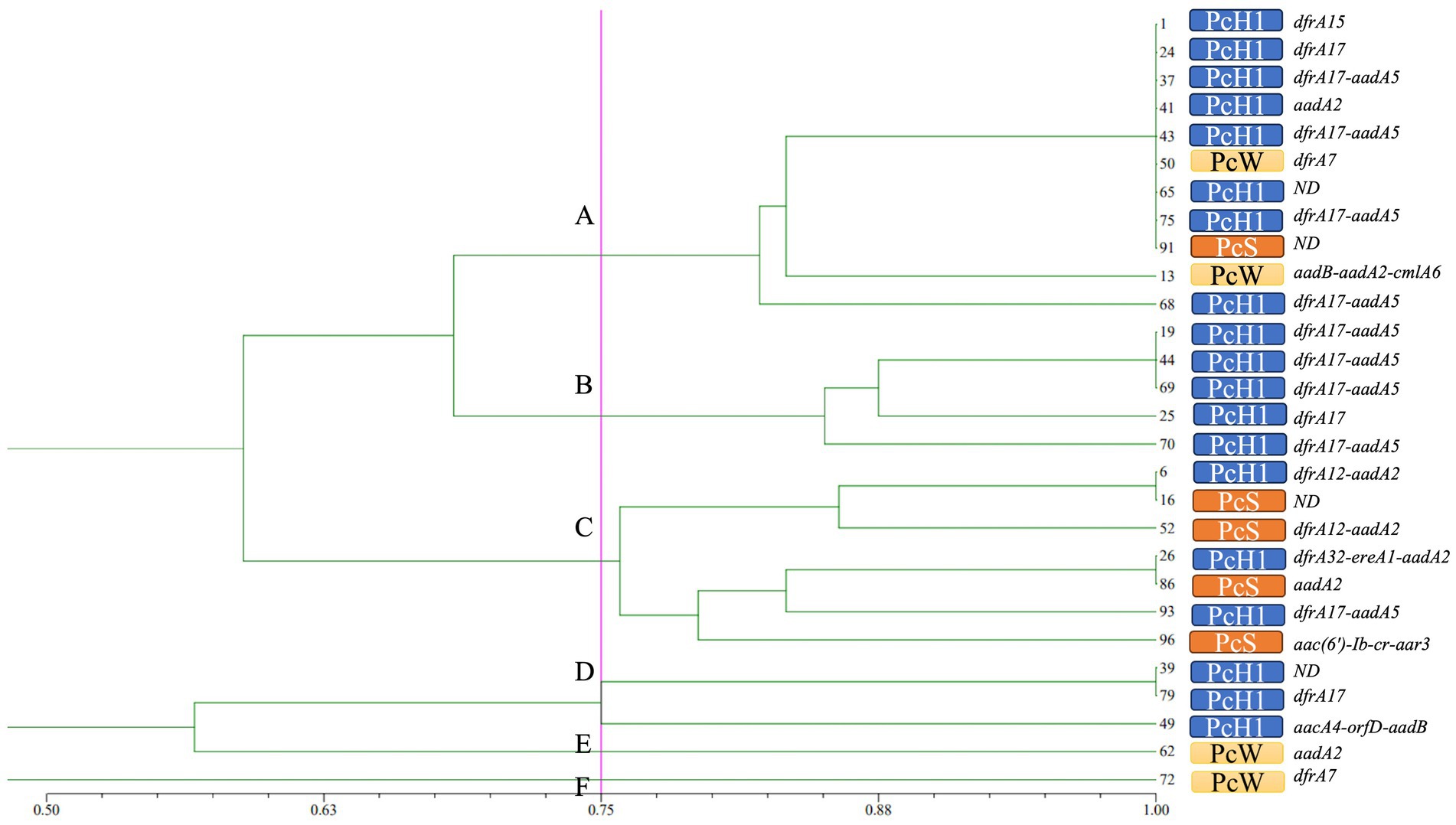
Figure 3. The dendrogram of ERIC-PCR. The solid magenta line represents the 75% homologous gene typing reference line. On the right side of the dendrogram are serial numbers of strains, variable region promoter Pc and gene cassettes of integrons successively.
Based on five class 1 integron-positive strains of M. morganii (MM-6, MM-26, MM-41, MM-62, MM-86), each containing the aadA2 gene cassette in the variable region, the Pc promoters were PcH1, PcH1, PcH1, PcW, and PcS, respectively. The P2 sequences were uniformly inactive, spanning 14 base pairs between the −35 and −10 regions. Gene fragments containing integrase, promoters, variable region gene cassettes, and inter-cassette sequences were synthesized according to actual sequencing results (Supplementary material: The gene synthesis of target segment in recombinant plasmids). These gene fragments were used to recombine plasmids, resulting in the creation of five recombinant strains named JM-6, JM-26, JM-41, JM-62, and JM-86, as listed in Table 3.
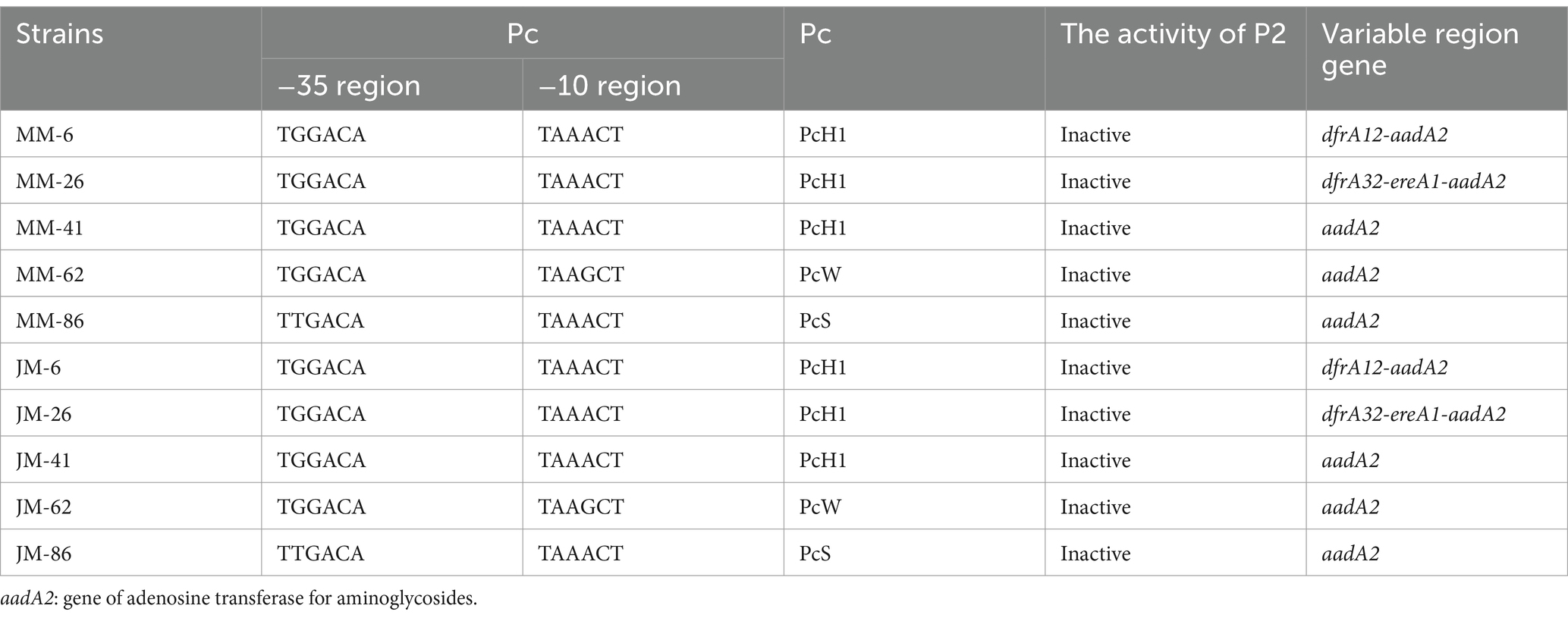
Table 3. Distribution of variable region promoters and gene cassettes of 5 isolates and 5 constructed strains.
The five constructed strains were divided into two groups based on their Pc promoter and aadA2 gene cassette configurations: Group 1 comprised strains JM-41, JM-62, and JM-86, which had different Pc promoters but all carried the aadA2 gene cassette. Group 2 included strains JM-6, JM-26, and JM-41, which had the same Pc promoter but varied in the distance between the aadA2 gene cassette and the Pc promoter. In the qPCR analysis, strain JM-41 served as the reference strain. Multiple comparisons among the strains revealed that JM-86 exhibited the highest relative expression of the aadA2 gene compared to the other four strains, with a significant statistical difference (p < 0.0001). The relative expression in JM-86 was approximately 100 times higher than that in JM-62, which had the lowest expression. The relative expression of the intI1 gene followed the order JM-62 > JM-41 > JM-86, with statistically significant differences among the strains (p < 0.001). JM-62 showed approximately 50 times higher expression than JM-86, which had the lowest expression level. For further details, please refer to Figure 4.
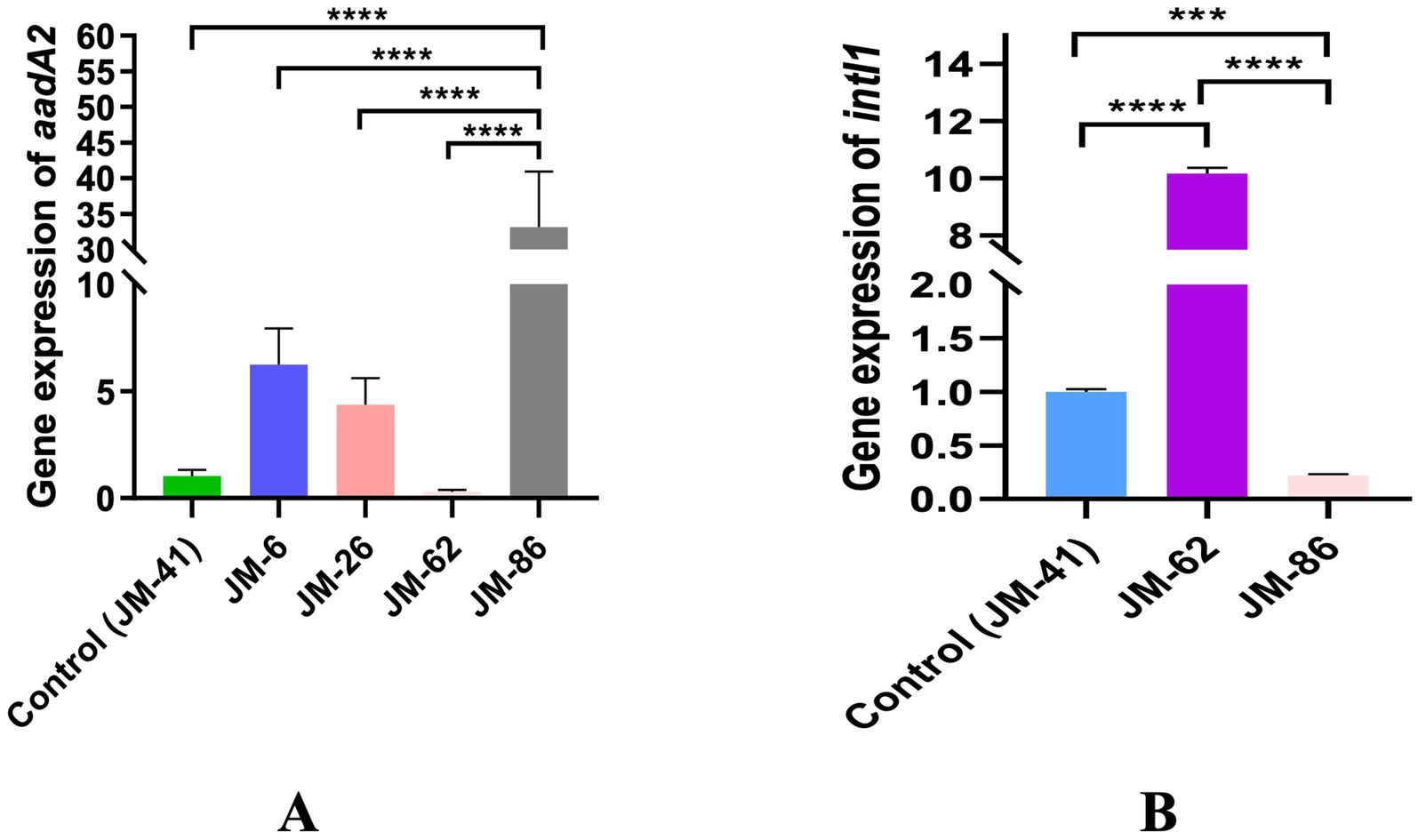
Figure 4. (A) The relative expression of aadA2 in different constructed strains; (B) The relative expression of intI1 in different constructed strains, ****p < 0.0001, ***p < 0.001.
Antibiotic susceptibility testing was conducted on the five constructed strains carrying aminoglycoside and trimethoprim resistance gene cassettes using the microbroth dilution method for streptomycin, amikacin, chloramphenicol, and tetracycline. The results revealed streptomycin MIC values obtained by microbroth dilution were in the order JM-86 > JM-41 > JM-26 > JM-6 > JM-62. The K-B assay confirmed this trend, with inhibition zone diameters for streptomycin aligning closely with the microbroth dilution results (JM-86 < JM-41 < JM-26 < JM-6 = JM-62). Amikacin showed no variation in MIC values or inhibition zone diameters among the constructed strains, indicating susceptibility across all strains. All constructed strains exhibited resistance to chloramphenicol. Only JM-86 demonstrated intermediate resistance to tetracycline in both microbroth dilution and K-B assays, while the other strains were sensitive. The antibiotic sensitivity testing results for the quality control strain E. coli ATCC25922 and the negative control E. coli JM109 fell within the effective range, as shown in Table 4.
M. morganii, the sole species within the genus Morganella, is a widely distributed bacterium that serves as a significant reservoir for the cloning and dissemination of various antibiotic resistance genes. In recent years, antibiotic resistance in M. morganii has been rapidly increasing, primarily driven by exogenous genetic elements such as transposons and integrons (Luo et al., 2022). While previous research on M. morganii has primarily focused on case analyses for treatment, epidemiology, and broad-spectrum β-lactamase analysis (Shrestha et al., 2020; Marado and Guerra, 2022; Alsaadi et al., 2024), studies investigating the correlation between its resistance mechanisms and the expression regulation of drug resistance gene cassettes within integrons are limited. This study employed clinical isolates of M. morganii positive for class 1 integrons collected over the past 6 years from our hospital. We initially screened these strains for drug resistance gene cassettes and promoters in the variable region, then analyzed the correlation between strain resistance and variable region promoters in integrons. Additionally, we constructed strains containing different types of promoters and gene cassettes in the variable region to conduct qPCR and antimicrobial susceptibility tests, thereby analyzing the regulatory role of variable region promoters in class 1 integrons on gene cassette expression.
Studies on class 1 integrons are more prevalent in Enterobacteriaceae bacteria such as E. coli, Klebsiella pneumoniae, and non-fermenting bacteria like Pseudomonas aeruginosa and Acinetobacter baumannii (Han et al., 2008; Wei et al., 2013; Bocharova et al., 2020; Farajzadeh Sheikh et al., 2024). In contrast, few studies have focused on M. morganii and class 1 integrons. Among the 97 non-duplicated clinical isolates of M. morganii in this study, the prevalence of class 1 integron-positive strains was 28.9%, lower than that observed in common Enterobacteriaceae bacteria. This lower prevalence might be attributed to the relatively lower detection rate of M. morganii in clinical settings. Wei et al. reported a detection rate of class 1 integrons in clinical isolates of E. coli as 72% (Wei et al., 2013), and in another study of K. pneumoniae, the positivity rate of class 1 integrons was 31.5% (Farajzadeh Sheikh et al., 2024). The host bacteria in these studies exhibited relatively higher levels of antibiotic resistance compared to the M. morganii isolates in the current study. The detected variable region gene cassettes in class 1 integron-positive strains primarily included aminoglycoside resistance genes (aadA, aacA4, aadB) and the trimethoprim resistance gene (dfrA), consistent with findings from other genera with class 1 integron-positive strains (Xiao et al., 2019; Li et al., 2022). In this study, some integron-positive strains failed to amplify gene cassettes, possibly due to atypical gene cassettes in the variable region. This could be attributed to gene recombination or insertion mutations in transposons, leading to the absence of the 3′ conserved segment or an excessive number of gene cassettes in the variable region. The latter may have exceeded the capability of conventional PCR amplification, resulting in amplification failure. Further validation using inverse PCR (Green and Sambrook, 2019; Figueroa-Bossi et al., 2024) techniques is necessary to address this issue (Odetoyin et al., 2017).
Extensive research has confirmed that promoters are crucial in regulating gene expression (Carrier et al., 2018; Brandis et al., 2021). Consequently, the expression of gene cassettes within integrons is significantly influenced by the variable region promoters. In this study, the predominant Pc promoter type in class 1 integron-positive strains was PcH1, a relatively weak promoter. The detection rates of PcS and PcW were lower than that of PcH1. The detection rate of PcH1 was also relatively high in other class 1 integron-positive Enterobacteriaceae. For instance, a study of class 1 integron-positive isolates of Proteus revealed a detection rate of PcH1 as high as 51%, making it the most common type (Xiao et al., 2019). Another study on the molecular characterization of class 1 integrons in carbapenem-resistant Enterobacteriaceae showed that PcH1 was also the predominant Pc (Wang et al., 2023). All downstream P2 promoters, containing 14 bases between the −35 and −10 regions, were inactive. Retrospective analysis of antibiotic resistance data in class 1 integron-positive strains did not reveal significant statistical differences in resistance rates to aminoglycosides or trimethoprim among strains with different Pc strengths. However, strains with stronger Pc promoters exhibited numerically higher resistance rates to these antibiotics than those with weaker Pc promoters. This observation reflects the fact that integrons contribute only partially to the host bacterium’s drug resistance genes. Furthermore, the expression and level of drug resistance genes may be influenced by various internal and external factors. Most integrons are located on plasmids or transposons (Liebert et al., 1999), which can potentially impact the resistance of the host bacterium, though not necessarily dominantly. To minimize interference and further explore the effect of variable region promoters within integrons on downstream gene expression, we used the low-copy plasmid pACYC184 as the vector. Recombinant plasmids were constructed to include variable region promoters and gene cassettes from class 1 integrons. Gene segments were synthesized based on actual sequencing results from experimental strains, preserving the natural configuration of integrons in these strains to a great extent. Additionally, HindIII and AseI were selected as the sites of enzyme digestion to replace the original tet promoter on the plasmid, positioning them away from the internal cat reference gene. This design allows the inserted gene segments to maximize the expression of drug resistance genes.
The strength of promoters and their distance from gene cassettes are primary factors influencing the transcription of downstream gene cassettes (Fonseca and Vicente, 2022). This project found significant differences in the expression efficiency of the antibiotic resistance gene cassette aadA2 mediated by promoters of different strengths. qPCR results confirmed that stronger Pc promoters in integrons corresponded to higher transcription levels of downstream gene cassettes. In antimicrobial susceptibility tests, only strain JM-86, carrying a strong promoter, exhibited intermediate resistance to tetracycline in both microbroth dilution and K-B methods, whereas other strains remained sensitive. This suggests that the Pc promoter within the recombinant plasmid may regulate the downstream tet gene to some extent, highlighting the substantial impact of promoter strength on regulating gene expression. Interestingly, contrary to expectations based on other studies (Jacquier et al., 2009; Souque et al., 2021), the distance of Pc to downstream gene cassettes did not similarly affect gene expression in our experiment. Under the condition of identical Pc types, strains JM-6 and JM-26, located farther from Pc, exhibited higher relative expression levels of aadA2 compared to the closer strain JM-41. Previous research in class 2 integrons has suggested that functional promoters of gene cassettes, like ereA located at the second position in the array, may enhance the expression of adjacent gene cassettes (Fonseca and Vicente, 2022), potentially explaining the increased expression of aadA2 in strain JM-26 at a distance. Additionally, the copy number of plasmids within engineered bacteria also influences gene cassette expression levels (Stokes and Hall, 1989). Furthermore, the gene segments used in this study were derived from clinical strains containing unknown functional sequences within integrons that might also regulate the expression of gene cassettes in the variable region, warranting further investigation. Notably, the expression levels of intI1 in strains with different Pc strengths exhibited an inverse relationship with aadA2 expression: stronger Pc promoters correlated with higher expression levels of antibiotic resistance genes in the variable region but lower expression levels of intI1. This suggests that the integrase’s ability for integration and excision may decrease, maintaining bacterial stability internally, consistent with the literature (Wei et al., 2011). Differences in streptomycin MICs observed among engineered strains using microbroth dilution indicate varied regulatory effects of different promoter expressions, whereas no differences were observed for other aminoglycoside antibiotics like amikacin. This discrepancy likely stems from the product of aadA2 being an aminoglycoside adenylyl transferase, which confers resistance to streptomycin and spectinomycin but not necessarily to other aminoglycosides (Bito and Susani, 1994; Walker et al., 2001).
The horizontal transfer of drug resistance genes is the fastest and most common way to spread clinical resistant strains (Warnes et al., 2012; Mathers et al., 2015), making homogeneity analysis of integron-positive strains particularly important. Herein, ERIC-PCR was used to analyze the homogeneity of 28 non-repetitive clinical isolates of M. morganii positive for class 1 integrons, using a similarity cutoff of 0.75. The results revealed three main genotypes: A, B, and C, comprising 11, 5, and 7 isolates, respectively. The distribution of promoter types and gene cassettes in the variable region was relatively concentrated within these genotypes. Furthermore, analysis of the strain data indicated that integron-positive M. morganii strains were predominantly found in the ICU and urology departments. This distribution is likely associated with the working environment of these departments. Factors such as surgical procedures in the ICU, critically ill patients, a high frequency of medical devices, and frequent operations increase the risk of cross-infection. Similarly, urological procedures involving catheterization and intravenous administration are invasive, contributing further to this risk. Therefore, it is crucial to enhance disinfection and sterilization of departmental environments and surgical instruments, as well as to standardize medical procedures such as intravenous administration and catheterization to minimize or prevent cross-infection. These measures aim to reduce the potential for clonal spread of integrons within hospitals.
Mobile genetic elements play a role in the horizontal transfer of drug resistance genes. Under antibiotic stress, class 1 integrons can capture gene cassette-like structures from DNA fragments ingested by bacteria and integrate them into attI1 sites through upregulation of integrase expression. Attempted transcription and translation of reading frames present in gene cassettes occur through variable region promoters and the mechanisms of translation termination-reinitiation coupling. Regardless of whether the reading frame in the gene cassette has a promoter or ribosomal binding site, if the expression product of the reading frame can confer resistance to the antibacterial agent, the strain can survive. The captured drug resistance gene cassettes can be disseminated through the proliferation and horizontal transfer of strains, leading to the emergence and dissemination of bacterial resistance (Guerin et al., 2009).
In summary, integron promoters exhibit a diverse distribution, predominantly carrying drug resistance gene cassettes for aminoglycosides and trimethoprim. The strength of the class 1 integron variable region promoters significantly correlates with the expression levels of downstream gene cassettes. It is likely that the clonal spread of class 1 integron-positive M. morganii occurs within our local healthcare setting. This study aimed to investigate the promoter distribution characteristics of class 1 integrons and their regulatory effects on drug resistance genes in M. morganii isolates, providing a theoretical basis for the prevention and treatment of infections with M. morganii in clinical practice. Additionally, this study may offer new insights for addressing other rare clinical bacteria with increasing drug resistance in the future. The effect of antibiotic factors on integrons will be studied in our future work.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
YY: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HZ: Investigation, Supervision, Writing – original draft. RZ: Data curation, Methodology, Writing – review & editing. XQ: Investigation, Project administration, Resources, Supervision, Writing – original draft. JY: Investigation, Supervision, Writing – original draft. WL: Data curation, Investigation, Writing – original draft. QL: Funding acquisition, Methodology, Supervision, Writing – review & editing. GW: Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Medical and Health Research Project of Zhejiang Province (Grant No. 2024KY288), the Key Cultivation Disciplines Foundation of Ningbo Medical Centre Lihuili Hospital (Grant No. 2022-P07), and was partly supported by the Traditional Chinese Medicine Research Project of Zhejiang Province (Grant No. 2024ZL940), and Ningbo Health Science and Technology Plan Project (Grant No. 2022Y03).
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1459162/full#supplementary-material
Agrawal, K. U., Limaye Joshi, K., and Gad, M. (2021). A rare case of fulminant acute postoperative Morganella morganii Endophthalmitis. Ocul. Immunol. Inflamm. 31, 123–126. doi: 10.1080/09273948.2021.1993269
Alelyani, F. M., Almutawif, Y. A., Ali, H. M., Aljohani, R. Z., Almutairi, A. Z., and Murshid, W. R. (2022). A pituitary abscess caused by Morganella morganii: a case report. Am. J. Case Rep. 23:e936743. doi: 10.12659/ajcr.936743
Alsaadi, A., Alghamdi, A. A., Akkielah, L., Alanazi, M., Alghamdi, S., Abanamy, H., et al. (2024). Epidemiology and clinical characteristics of Morganella morganii infections: a multicenter retrospective study. J. Infect. Public Health 17, 430–434. doi: 10.1016/j.jiph.2023.12.013
Bakhshi, B., Afshari, N., and Fallah, F. (2018). Enterobacterial repetitive intergenic consensus (ERIC)-PCR analysis as a reliable evidence for suspected Shigella spp. outbreaks. Braz. J. Microbiol. 49, 529–533. doi: 10.1016/j.bjm.2017.01.014
Banoub, J., Kundu, J., Kansal, S., Rathore, S., Kaundal, M., Angrup, A., et al. (2022). Evaluation of ERIC-PCR and MALDI-TOF as typing tools for multidrug resistant Klebsiella pneumoniae clinical isolates from a tertiary care center in India. PLoS One 17:e0271652. doi: 10.1371/journal.pone.0271652
Behera, D. U., Dixit, S., Gaur, M., Mishra, R., Sahoo, R. K., Sahoo, M., et al. (2023a). Sequencing and characterization of M. morganii strain UM869: a comprehensive comparative genomic analysis of virulence, antibiotic resistance, and functional pathways. Genes 14:1279. doi: 10.3390/genes14061279
Behera, D. U., Ratnajothy, K., Dey, S., Gaur, M., Sahoo, R. K., Sahoo, S., et al. (2023b). In vitro synergistic interaction of colistin and other antimicrobials against intrinsic colistin-resistant Morganella morganii isolates. 3 Biotech 13:127. doi: 10.1007/s13205-023-03551-w
Bito, A., and Susani, M. (1994). Revised analysis of aadA2 gene of plasmid pSa. Antimicrob. Agents Chemother. 38, 1172–1175. doi: 10.1128/aac.38.5.1172
Bocharova, Y., Savinova, T., Lazareva, A., Polikarpova, S., Gordinskaya, N., Mayanskiy, N., et al. (2020). Genotypes, carbapenemase carriage, integron diversity and oprD alterations among carbapenem-resistant Pseudomonas aeruginosa from Russia. Int. J. Antimicrob. Agents 55:105899. doi: 10.1016/j.ijantimicag.2020.105899
Brandis, G., Gockel, J., Garoff, L., Guy, L., and Hughes, D. (2021). Expression of the qepA1 gene is induced under antibiotic exposure. J. Antimicrob. Chemother. 76, 1433–1440. doi: 10.1093/jac/dkab045
Carrier, M.-C., Lalaouna, D., and Massé, E. (2018). Broadening the definition of bacterial small RNAs: characteristics and mechanisms of action. Ann. Rev. Microbiol. 72, 141–161. doi: 10.1146/annurev-micro-090817-062607
CLSI (2022). Performance standards for antimicrobial susceptibility testing. 32nd Edn CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
Coluzzi, C., Guillemet, M., Mazzamurro, F., Touchon, M., Godfroid, M., Achaz, G., et al. (2023). Chance favors the prepared Genomes_ horizontal transfer shapes the emergence of antibiotic resistance mutations in Core genes. Mol. Biol. Evol. 40:msad217. doi: 10.1093/molbev/msad217
Elmi, S. M., Obame, F. L. O., Dokponou, Y. C. H., Yassin, M. R., Attari, S. E., El Asri, A. C. C., et al. (2024). Brain abscess caused by Morganella morganii: a case report and review of the literature. Surg. Neurol. Int. 15:7. doi: 10.25259/sni_759_2023
Farajzadeh Sheikh, A., Abdi, M., and Farshadzadeh, Z. (2024). Molecular detection of class 1, 2, and 3 integrons in hypervirulent and classic Klebsiella pneumoniae isolates: a cross-sectional study. Health Sci. Rep. 7:e1962. doi: 10.1002/hsr2.1962
Figueroa-Bossi, N., Balbontín, R., and Bossi, L. (2024). Mapping transposon insertion sites by inverse polymerase chain reaction and sanger sequencing. Cold Spring Harb. Protoc. 2024:108197. doi: 10.1101/pdb.prot108197
Fonseca, É. L., and Vicente, A. C. (2022). Integron functionality and genome innovation: an update on the subtle and smart strategy of integrase and gene cassette expression regulation. Microorganisms 10:224. doi: 10.3390/microorganisms10020224
Gameiro, I., Botelho, T., Martins, A. I., Henriques, R., and Lapa, P. (2023). Morganella morganii: a rare cause of early-onset neonatal Sepsis. Cureus 15:e45600. doi: 10.7759/cureus.45600
Green, M. R., and Sambrook, J. (2019). Inverse polymerase chain reaction (PCR). Cold Spring Harbor Protoc. 2019, 170–174. doi: 10.1101/pdb.prot095166
Guerin, E., Cambray, G., Sanchez-Alberola, N., Campoy, S., Erill, I., Da Re, S., et al. (2009). The SOS response controls integron recombination. Science 324:1034. doi: 10.1126/science.1172914
Hall, R. M., and Collis, C. M. (2006). Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15, 593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x
Hall, R. M., and Stokes, H. W. (1993). Integrons:novel DNA elements which capture genes by site-specific recombination. Genetica 90, 115–132. doi: 10.1007/BF01435034
Han, H. L., Jang, S. J., Park, G., Kook, J. K., Shin, J. H., Shin, S. H., et al. (2008). Identification of an atypical integron carrying an IS26-disrupted aadA1 gene cassette in Acinetobacter baumannii. Int. J. Antimicrob. Agents 32, 165–169. doi: 10.1016/j.ijantimicag.2008.03.009
Hanau-Berçot, B., Podglajen, I., Casin, I., and Collatz, E. (2002). An intrinsic control element for translational initiation in class 1 integrons. Mol. Microbiol. 44, 119–130. doi: 10.1046/j.1365-2958.2002.02843.x
Jacquier, H., Zaoui, C., Sanson-Le Pors, M. J., Mazel, D., and Berçot, B. (2009). Translation regulation of integrons gene cassette expression by the attC sites. Mol. Microbiol. 72, 1475–1486. doi: 10.1111/j.1365-2958.2009.06736.x
Kvopka, M., Chan, W., Baranage, D., and Sia, D. (2023). Morganella morganii and Enterococcus faecalis endophthalmitis following intravitreal injection. BMC Ophthalmol. 23:450. doi: 10.1186/s12886-023-03198-4
Laupland, K., Paterson, D., Edwards, F., Stewart, A., and Harris, P. (2022). Morganella morganii, an emerging cause of bloodstream infections. Microbiol. Spectr. 10:e0056922. doi: 10.1128/spectrum.00569-22
Leverstein-Van Hall, M., Box, A., Blok, H., Paauw, A., Fluit, A., and Verhoef, J. (2002). Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J. Infect. Dis. 186, 49–56. doi: 10.1086/341078
Li, W., Ma, J., Sun, X., Liu, M., and Wang, H. (2022). Antimicrobial resistance and molecular characterization of gene cassettes from class 1 Integrons in Escherichia coli strains. Microb. Drug Resist. 28, 413–418. doi: 10.1089/mdr.2021.0172
Li, C., Wang, H., Zhang, J., Wang, Z., Wei, Y., and Zhu, Y. (2023). Endocarditis induced by M. morganii in an immunocompetent patient without underlying valvular abnormalities. Heliyon 9:e17069. doi: 10.1016/j.heliyon.2023.e17069
Liebert, C. A., Hall, R. M., and Summers, A. O. (1999). Transposon Tn 21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63, 507–522. doi: 10.1128/mmbr.63.3.507-522.1999
Liu, H., Zhu, J., Hu, Q., and Rao, X. (2016). Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 50, 10–17. doi: 10.1016/j.ijid.2016.07.006
Lu, W., Qiu, X., Chen, K., Zhao, R., Li, Q., and Wu, Q. (2022). Distribution and molecular characterization of functional class 2 Integrons in clinical Proteus mirabilis isolates. Infect. Drug Resist. 15, 465–474. doi: 10.2147/idr.S347119
Luo, X. W., Liu, P. Y., Miao, Q. Q., Han, R. J., Wu, H., Liu, J. H., et al. (2022). Multidrug resistance genes carried by a novel transposon Tn7376 and a Genomic Island named MMGI-4 in a pathogenic Morganella morganii isolate. Microbiol. Spectr. 10:e0026522. doi: 10.1128/spectrum.00265-22
Marado, D., and Guerra, M. (2022). Fulminans Purpura due to Morganella morganii. Eur. J. Case Rep. Internal Med. 9:003670. doi: 10.12890/2022_003670
Mathers, A. J., Peirano, G., and Pitout, J. D. D. (2015). The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 28, 565–591. doi: 10.1128/cmr.00116-14
Meacham, K. J., Zhang, L., Foxman, B., Bauer, R. J., and Marrs, C. F. (2003). Evaluation of genotyping large numbers of Escherichia coli isolates by Enterobacterial repetitive intergenic consensus-PCR. J. Clin. Microbiol. 41, 5224–5226. doi: 10.1128/jcm.41.11.5224-5226.2003
Novačić, A., Menéndez, D., Ljubas, J., Barbarić, S., Stutz, F., Soudet, J., et al. (2022). Antisense non-coding transcription represses the PHO5 model gene at the level of promoter chromatin structure. PLoS Genet. 18:e1010432. doi: 10.1371/journal.pgen.1010432
Odetoyin, B. W., Labar, A. S., Lamikanra, A., Aboderin, A. O., and Okeke, I. N. (2017). Classes 1 and 2 integrons in faecal Escherichia coli strains isolated from mother-child pairs in Nigeria. PLoS One 12:e0183383. doi: 10.1371/journal.pone.0183383
Shi, H., Chen, X., Yao, Y., and Xu, J. (2022). Morganella morganii: an unusual analysis of 11 cases of pediatric urinary tract infections. J. Clin. Lab. Anal. 36:e24399. doi: 10.1002/jcla.24399
Shrestha, S., Tada, T., Sherchan, J. B., Uchida, H., Hishinuma, T., Oshiro, S., et al. (2020). Highly multidrug-resistant Morganella morganii clinical isolates from Nepal co-producing NDM-type metallo-β-lactamases and the 16S rRNA methylase ArmA. J. Med. Microbiol. 69, 572–575. doi: 10.1099/jmm.0.001160
Souque, C., Escudero, J. A., and Maclean, R. C. (2021). Integron activity accelerates the evolution of antibiotic resistance. eLife 10:e62474. doi: 10.7554/eLife.62474
Stokes, H. W., and Hall, R. M. (1989). A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3, 1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x
Stokes, H. W., O'Gorman, D. B., Recchia, G. D., Parsekhian, M., and Hall, R. M. (1997). Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26, 731–745. doi: 10.1046/j.1365-2958.1997.6091980.x
Tseng, C.-S., Yen, Y.-C., Chang, C.-C., and Hsu, Y.-M. (2014). Polymorphism of gene cassette promoter variants of class 1 integron harbored in S. Choleraesuis and typhimurium isolated from Taiwan. Biomedicine 4:20. doi: 10.7603/s40681-014-0020-3
Von Wintersdorff, C. J. H., Penders, J., Van Niekerk, J. M., Mills, N. D., Majumder, S., Van Alphen, L. B., et al. (2016). Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 7:173. doi: 10.3389/fmicb.2016.00173
Wang, T., Zhu, Y., Zhu, W., Cao, M., and Wei, Q. (2023). Molecular characterization of class 1 integrons in carbapenem-resistant Enterobacterales isolates. Microb. Pathog. 177:106051. doi: 10.1016/j.micpath.2023.106051
Walker, R. A., Lindsay, E., Woodward, M. J., Ward, L. R., and Threlfall, E. J. (2001). Variation in clonality and antibiotic-resistance genes among multiresistant Salmonella enterica serotype typhimurium phage-type U302 (MR U302) from humans, animals, and foods. Microb. Drug Resist. 7, 13–21. doi: 10.1089/107662901750152701
Warnes, S. L., Highmore, C. J., and Keevil, C. W. (2012). Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: implications for public health. MBio 3, e00489–e00412. doi: 10.1128/mBio.00489-12
Wei, Q. H. (2010). The research of regulation mechamism for integron capturing and expressing 477 antibiotic resistance gene cassettes in bacteria. Ph.D. Thesis,: Fudan University.
Wei, Q., Jiang, X., Li, M., Chen, X., Li, G., Li, R., et al. (2011). Transcription of integron-harboured gene cassette impacts integration efficiency in class 1 integron. Mol. Microbiol. 80, 1326–1336. doi: 10.1111/j.1365-2958.2011.07648.x
Wei, Q., Jiang, X., Li, M., Li, G., Hu, Q., Lu, H., et al. (2013). Diversity of gene cassette promoter variants of class 1 Integrons in Uropathogenic Escherichia coli. Curr. Microbiol. 67, 543–549. doi: 10.1007/s00284-013-0399-1
Xiao, L., Wang, X., Kong, N., Cao, M., Zhang, L., Wei, Q., et al. (2019). Polymorphisms of gene cassette promoters of the class 1 Integron in clinical Proteus isolates. Front. Microbiol. 10:790. doi: 10.3389/fmicb.2019.00790
Yeşil, M., Özcan, Ö., Karasu, N., and Kağan Yılmaz, B. (2023). Atypical compartment syndrome of the forearm due to mixed infection with Proteus mirabilis and Morganella morganii after a penetrating injury: a limb-saving approach. Joint Dis. Relat. Surg. 34, 752–756. doi: 10.52312/jdrs.2023.1066
Keywords: Morganella morganii, integron, promoter, expression regulation, homology
Citation: Yang Y, Zhang H, Zhao R, Qiu X, Ye J, Lu W, Li Q and Wu G (2024) Distribution diversity and expression regulation of class 1 integron promoters in clinical isolates of Morganella morganii. Front. Microbiol. 15:1459162. doi: 10.3389/fmicb.2024.1459162
Received: 03 July 2024; Accepted: 09 October 2024;
Published: 18 October 2024.
Edited by:
Juan A. Ayala, Autonomous University of Madrid, SpainReviewed by:
Sangita Dixit, Siksha O Anusandhan University, IndiaCopyright © 2024 Yang, Zhang, Zhao, Qiu, Ye, Lu, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingcao Li, lqc_lab@163.com; Guangliang Wu, wuguangliang2024@126.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.