- 1Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun, China
- 2College of Mycology, Jilin Agricultural University, Changchun, China
- 3Institute of Agricultural Applied Microbiology, Jiangxi Academy of Agricultural Sciences, Nanchang, China
- 4School of Life Science, Zhejiang Normal University, Jinhua, China
- 5Council for Scientific and Industrial Research (CSIR), Oil Palm Research Institute, Coconut Research Programme, Sekondi, Ghana
- 6Industrial Development Institute for Plants, Animals, and Fungi Integration of Biyang County, Zhumadian, China
Diachea is an important genus of myxomycetes, recognized for its ecological role and wide distribution. This study aimed to expand knowledge of species diversity within this genus in China. We collected Diachea specimens from various locations in Shaanxi and Sichuan provinces and characterized them through morphological analysis and phylogenetic analysis using four genetic markers: small subunit ribosomal RNA (nSSU), translation elongation factor 1-alpha (EF-1α), mitochondrial small subunit (mtSSU), and alpha-tubulin gene (α-Tub). Based on these analyses, we describe two new species, namely, Diachea plectophylla and D. sichuanensis, discovered in the Shaanxi and Sichuan provinces, respectively. Diachea plectophylla is distinguished by its dense, rigid capillitium, spore warts, and distinct separation of capillitium ends from the peridium. Diachea sichuanensis, closely related to D. leucopodia, is identified by its blunt-headed columella, clustered spore warts, and robust stalks. In addition to these new species, we recorded five previously documented species, including D. bulbillosa in Gansu province, D. leucopodia in Yunnan and Sichuan provinces, and D. subsessilis in Sichuan province. Detailed descriptions, micrographs, taxonomic comparisons, and an identification key are provided to aid in accurate identification. The discovery of these new species not only enhances the known diversity of slime molds in the region but also provides valuable information for future studies on their geographical distribution and ecological relationships.
1 Introduction
Myxogastria, commonly known as true slime molds, are fungal-like eukaryotes classified within the kingdom Protozoa, forming a monophyletic group within the Amoebozoa (Adl et al., 2012; Kang et al., 2017). These organisms typically thrive in damp, cool environments, obtaining nutrients from decaying substrates such as rotting wood, dead leaves, branches, and moss. While myxomycetes can adapt to various vegetation types and environmental conditions, they show a preference for these specific habitats. According to Lado (2005/2024), over 1,100 species of myxomycetes are currently recognized.
The genus Diachea Fr., within the order Physarales, includes only 18 known species worldwide, seven of which have been reported in China. Species within this genus are primarily distinguished by the glittering gold, bronze, or bluish iridescence of their peridium. The capillitium, a network of threads inside the sporotheca, lacks limestone and is dark brown. Both the stalk and columella, a prominent structure at the base of the sporotheca or plasmodiocarp, contain granular or crystalline limestone (Martin and Alexopoulos, 1969; Keller et al., 2004; Kirk et al., 2008; Poulain et al., 2011).
Diachea was established by Fries in 1825 based on D. leucopodia (Bull.) Rostaf. (Martin and Alexopoulos, 1969). Historically, researchers have focused on species within Diachea that featured a halo-peridium, capillitium without limestone, and a well-developed columella—traits resembling those of the Stemonitidales. As a result, Diachea was initially classified in the family Stemonitaceae, suggesting a close relationship with Lamproderma (Fries, 1829; Martin and Alexopoulos, 1969). However, resemblances to species in the Physarales prompted Rostafiński (1874) and Alexopoulos and Saenz (1975) to reclassify it into the family Didymiaceae within the order Physarales (Alexopoulos and Saenz, 1975). Subsequent research by Cavalcanti et al. (2006) supported the placement of D. leucopodia within Didymiaceae and Physarales.
The classification of Diachea has fluctuated between the Physarales and Stemonitidales (Alexopoulos, 1982; Kalyanasundaram and Mubarak, 1989; Lado, 2005/2024). Recent studies by García-García-Martín et al. (2023) and Prikhodko et al. (2023), using multigene phylogenetic analyses, confirmed that Diachea belongs to Didymiaceae within Physarales. This taxonomic uncertainty may stem from variations in capillitium features during different developmental stages. A comprehensive phylogenetic analysis of Diachea remains incomplete due to limited sequence data, leaving its classification unresolved.
China’s diverse natural environment contributes to a rich myxomycetes species diversity. However, research on this group in China remains limited. In this study, we describe two new Diachea species and confirm the presence of five previously recorded species. We employed four genetic markers (nSSU, EF-1α, mtSSU, and α-Tub) for our molecular analyses. The discovery of these new and newly recorded species enhances our understanding of myxomycetes diversity and provides essential data for future systematic studies of the genus Diachea.
2 Materials and methods
2.1 Morphological studies
Specimens were collected from eight locations across China over a 12-year period (2010–2021). Samples, including dead bark, leaves, branches, and litter, were gathered in the field, air-dried in a ventilated area, and stored in collection boxes. For species description, photographs of mature sporocarps were taken using a Zeiss dissecting microscope (Axio Zoom V16, Carl Zeiss Microscopy GmbH, Göttingen, Germany) and a Leica stereoscopic dissector (Leica M165, Wetzlar, Germany). Light micrographs were captured with a Lab A1 microscope (Carl Zeiss AG, Jena, Germany) and processed using ZEN 2.3 software (Carl Zeiss AG). Dried specimens were mounted in 3% KOH for spore examination under oil immersion, and the diameters of 20 spores were measured. Spore and capillitium dimensions are reported as (minimum–) 25th percentile – 75th percentile (−maximum) (Leontyev et al., 2023). Specimens were also examined using a scanning electron microscope (SEM), and photomicrographs were taken using a Hitachi S-4800 SEM (Japan) at 10–15 kV. All analyzed specimens were deposited in the myxomycete collection of the Herbarium of Mycology at Jilin Agriculture University (HMJAU), China.
2.2 DNA extraction, amplification, and sequencing
Genomic DNA from the putative new species was extracted from dried materials using the TIANamp Micro DNA Kit (TianGen Biotech Co., Ltd., Beijing, China). Four genetic markers were used: nSSU (S2F/SR4DarkR), EF-1α (PB1F/PB1R), mtSSU (Kmit-F/Kmit-R), and α-Tub (kTub-F2/KTub-R1) (García-Martín et al., 2023; Novozhilov et al., 2013). These markers are commonly used for barcoding in protists (Adl et al., 2014) and myxomycetes (García-Cunchillos et al., 2022; Leontyev and Schnittler, 2022; García-Martín et al., 2023). The PCR mixture composition, sample preparation, and sequencing protocols followed Prikhodko et al. (2023) and García-Martín et al. (2023). PCR products were analyzed via gel electrophoresis and purified using the Universal DNA Purification Kit (TianGen Biotech Co., Ltd., Beijing, China). Purified PCR products were sequenced by Kumei Biotechnology Co., Ltd. (Changchun City, China) using the Sanger method. The resulting sequences were deposited in the National Center for Biotechnology Information (NCBI) GenBank database (Table 1).1
2.3 Phylogenetic analysis
For phylogenetic analysis, we used 214 sequences derived from 33 species to construct a concatenated dataset. Raw sequence editing and assembly were performed using BioEdit 7.1.3 (Hall, 1999). Sequences for nSSU, EF-1α, mtSSU, and α-Tub from GenBank were retrieved for other Diachea species, as well as representatives from the families Didymiaceae and Physaraceae. Single-gene alignments were generated using MAFFT V7.490 (Katoh and Standley, 2013), and manual adjustments were made in BioEdit (Hall, 1999). Phylogenetic analyses were performed separately for each gene and for the concatenated dataset using PhyloSuite v1.2.2 (Zhang et al., 2020; Xiang et al., 2023).
Maximum likelihood (ML) and Bayesian inference (BI) methods were employed for phylogenetic tree construction. ML analyses were conducted using IQ-TREE 1.6.12 with 1,000 non-parametric replicates (Nguyen et al., 2015). BI analyses were performed with MrBayes 3.2.6 (Ronquist and Huelsenbeck, 2003), running two simultaneous chains for 10 million generations; trees were sampled every 1,000 generations, and the first 25% were discarded as burn-in. Convergence of the Markov chain Monte Carlo (MCMC) was assessed with TRACER 1.7.2 (Rambaut et al., 2018). Phylogenetic trees were visualized using ITOL version 6.9 (Bork, 2007) and further edited using Adobe Illustrator (San Jose, CA, United States).
3 Results
3.1 Phylogenetic analyses
We analyzed 167 sequences representing 33 species in this study, including 47 newly acquired sequences from the specimens examined: 15 sequences each for nSSU, EF-1α, and mtSSU and 2 sequences for α-Tub. The dataset consisted of 72 sequences with 7,606 characters for nSSU, 57 sequences with 2,505 characters for EF-1α, 55 sequences with 477 characters for mtSSU, and 29 sequences with 1,007 characters for α-Tub, including gaps.
The phylogenetic trees generated using both maximum likelihood (ML) and Bayesian inference (BI) analyses showed consistent topologies across the single-gene datasets. For clarity, only the ML tree is presented here. In the multigene phylogenetic tree, which combines the alignments of nSSU, EF-1α, mtSSU, and α-Tub sequences, all 11 specimens of the genus Diachea formed a cohesive and well-supported clade (PP = 1, BS = 99.3%). Within this group, Diachea plectophylla and D. sichuanensis emerged as distinct, strongly supported clades (PP = 1, BS = 100%). These results, along with unique morphological traits, confirm the discovery of these two previously undocumented species, identifying D. plectophylla and D. sichuanensis as distinct lineages (Figure 1).
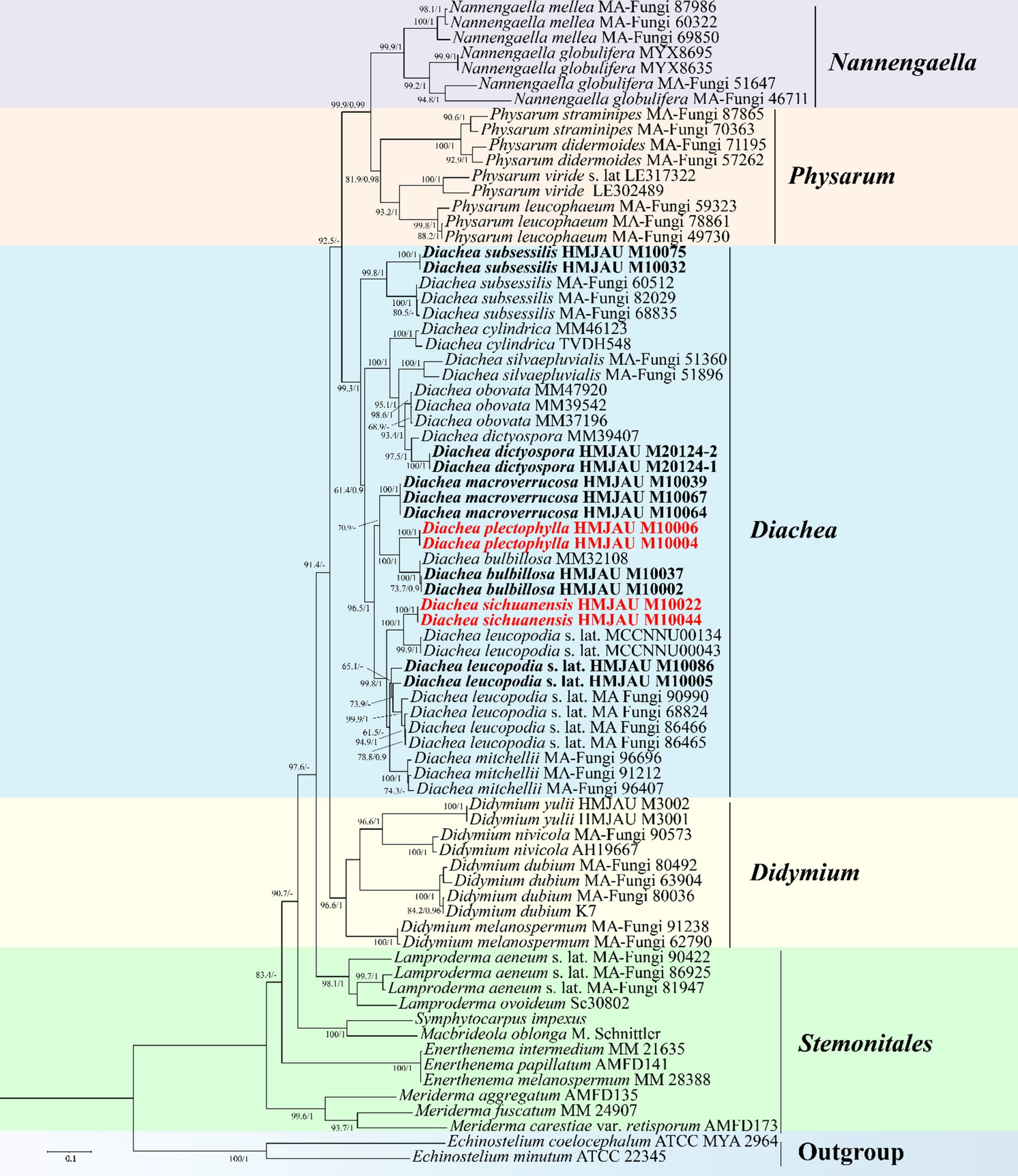
Figure 1. Maximum likelihood phylogenetic tree generated from the nSSU, EF-1α, mt SSU, and α-Tub datasets of the target clade containing the new species from Diachea. The two values of internal nodes, respectively, represent the maximum likelihood bootstrap (BS)/Bayesian posterior probability (PP). This study species is in bold and red font.
Taxonomy
Diachea plectophylla X.F. Li, D. Dai, B. Zhang & Y. Li, sp. nov.
MycoBank: MB854209.
(Figure 2)
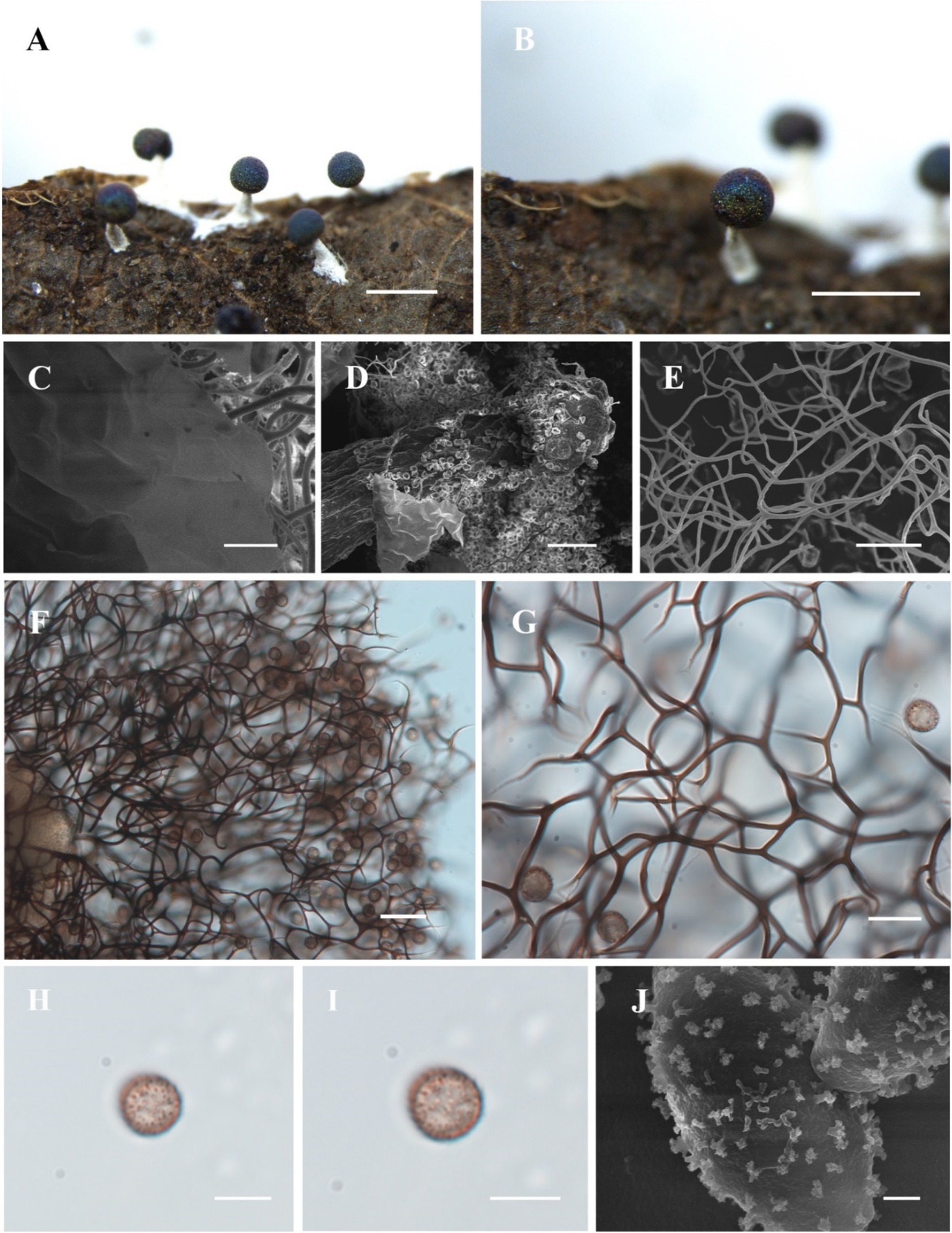
Figure 2. Habitat and microstructure of Diachea plectophylla (HMJAU M10004). (A,B) Sporocarps; (C) peridium by SEM; (D) columella by SEM; (E–G) capillitium by SEM and TL; (H–J) spores with cluster warts by TL and SEM. Scale bars: (A), B = 1 mm, C = 10 μm, (D), F = 40 μm, (E), G = 20 μm, H, I = 10 μm, J = 1 μm.
Diagnosis. Capillitium dense, hard, and straight; ends not connected to the peridium.
Holotype. China, Shaanxi province, Niubeiliang National Nature Reserve, on the rotten leaves, 23 July 2014, B. Zhang, HMJAU M10004.
Etymology. The epithet “plectophylla” refers to crude the dense capillitium.
Description. Sporocarp, gregarious, stipitate, spherical, with blue-purple halo, (0.3–) 0.35–0.5 (−0.55) mm in diameter, (0.5–) 0.6–1.2 (−1.3) mm in tall. Stalk calcareous, white, with enlarged at the base, tapering upward, with numerous calcareous particles. Peridium single-layered, thin, halo, persistent, irregular dehiscence. Hypothallus inconspicuous. Columella white, conical, blunt-headed, tapering upward, reaching approximately half of the sporotheca, an extension of the stalk. Capillitium dense, thick, hard, black brown, branched, and connected, radiating from columella, not connected to the peridium. Spores dark in mass, dark brown by transmitted light, with sparse warts, sometimes warts connect to form ridges, 8–9 (−9.5) μm in diameter.
Habitat. On the rotten leaves and branches.
Distribution in China. Shaanxi province.
Distribution in the World. China.
Additional specimens examined. China, Shaanxi province, Niubeiliang National Nature Reserve, on the rotten leaves, 23 July 2014, B. Zhang, HMJAU M10006.
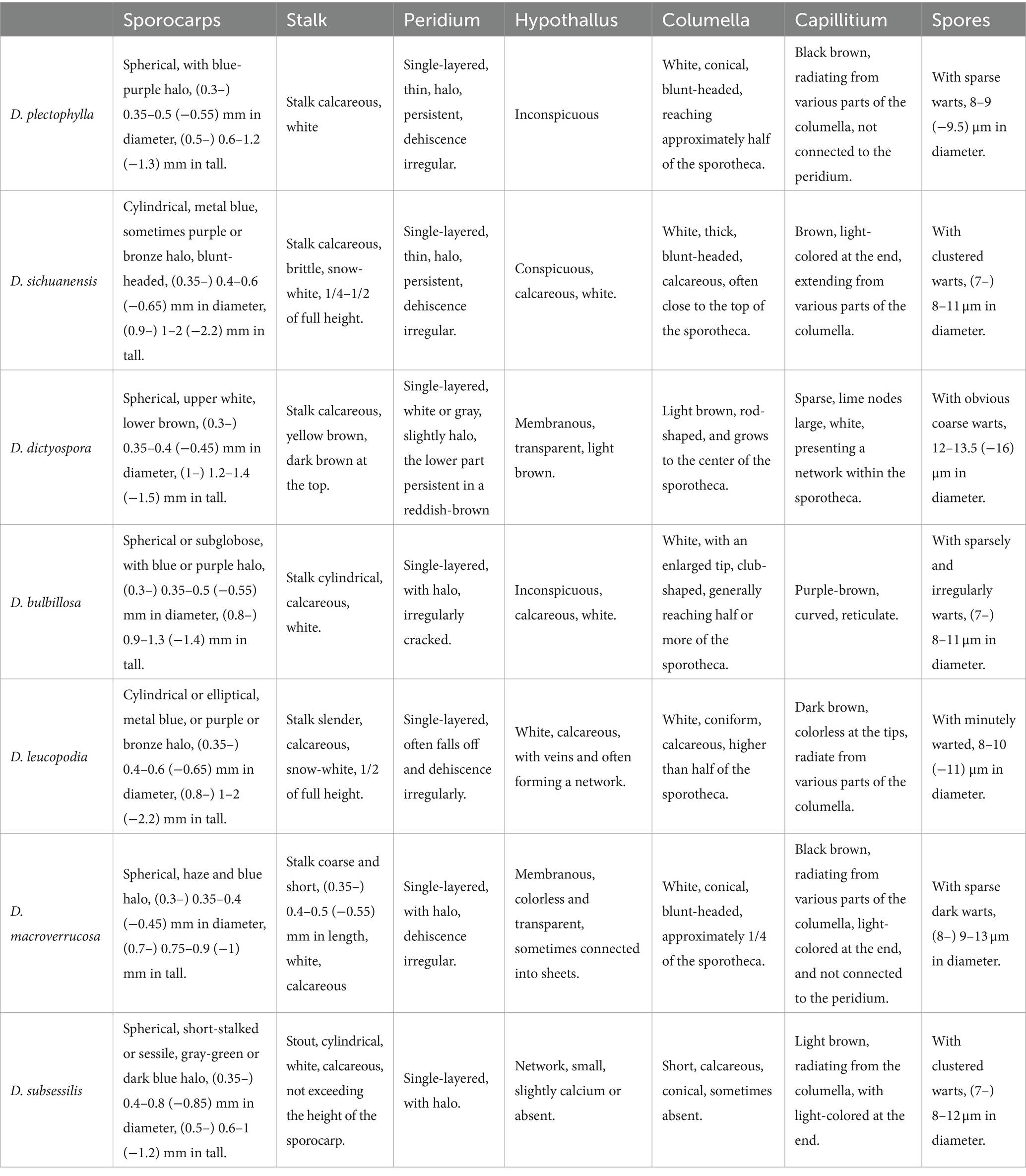
Notes. Diachea plectophylla is morphologically similar to D. bulbillosa, D. macroverrucosa, and D. splendens due to its blue halo, subglobose sporocarp, white stalk, and obvious columella (Table 2). However, D. plectophylla differs from D. macroverrucosa in having relatively large columella and small spores. Diachea plectophylla differs from D. bulbillosa in its dense capillitium and small spores (Table 2). Diachea plectophylla can be distinguished from D. splendens by its dense capillitium, where warts connect to form ridges, whereas D. splendens has sparse reticular capillitium, and spores have rough nodules and ridges, arranged in a network pattern. From the phylogenetic tree, D. plectophylla is similar to D. bulbillosa but has a separate branch.
Diachea sichuanensis X.F. Li, D. Dai, B. Zhang & Y. Li, sp. nov.
MycoBank: 854210.
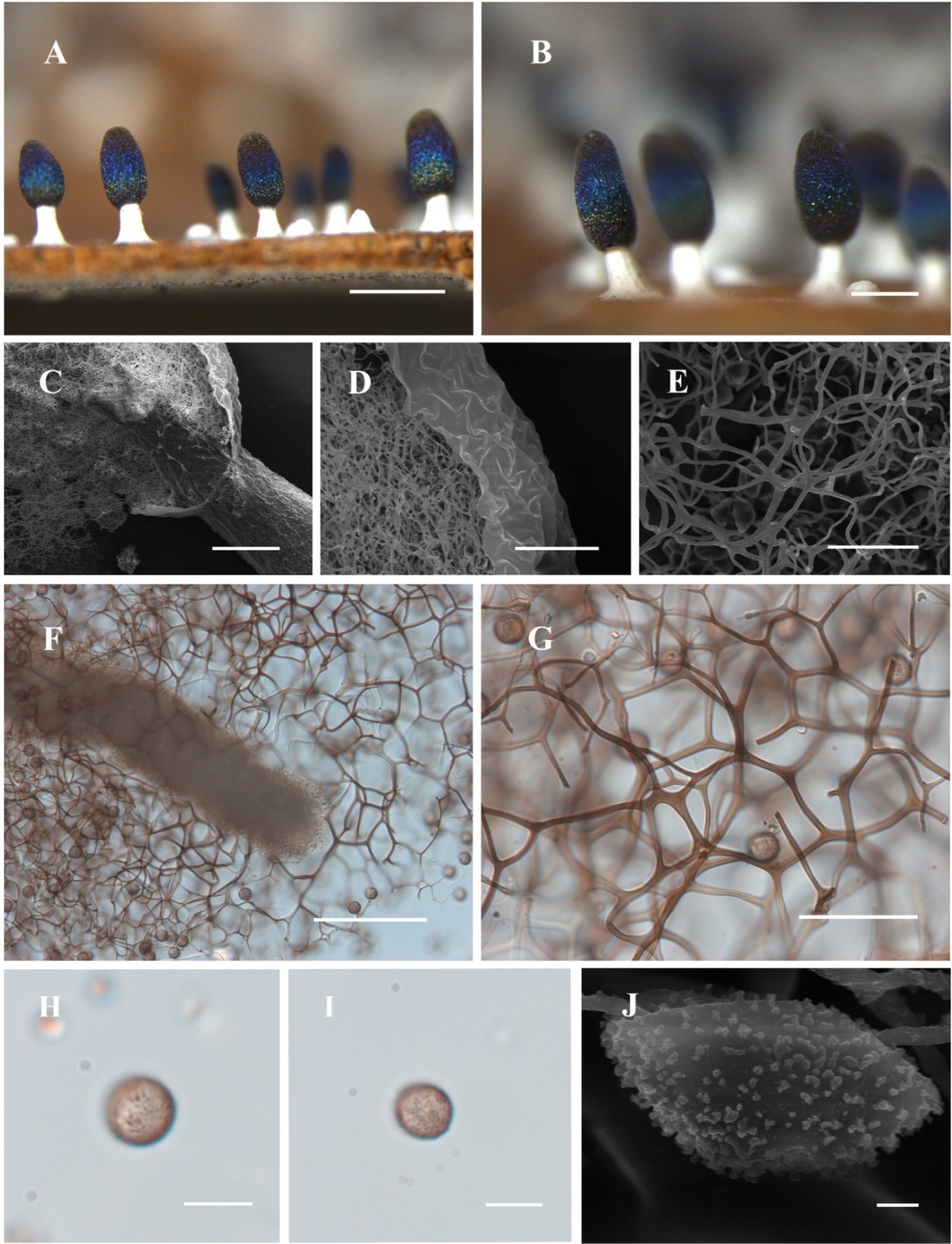
Figure 3. Habitat and microstructure of Diachea sichuanensis (HMJAU M10022). (A,B) Sporocarps; C. columella by SEM; (D,E) peridium and capillitium by SEM; (F) columella and capillitium by TL; (G) capillitium and spores by TL; (H–J) Spores with clustered warts by TL and SEM. Scale bars: A = 1 mm, B = 500 μm, C, F = 100 μm, D, G = 40 μm, E = 20 μm, H, I = 10 μm, J = 1 μm.
Diagnosis. Clustered warts are present on the surface of spores, and the capillitium is connected to the peridium.
Holotype. China, Sichuan province, Liangshan Yi Autonomous Prefecture, Mianning County, Lingshan Temple, on the branches, rotten leaves, and living plants, 12 July 2013, B. Zhang, HMJAU M10022.
Etymology. The epithet “sichuanensis” refers to Sichuan, the location of the holotype.
Description. Sporocarp dense, gregarious, stipitate, metal blue, sometimes with a purple or bronze halo, cylindrical or rarely subspherical, blunt-headed, (0.35–) 0.4–0.6 (−0.65) mm in diameter, (0.9–) 1–2 (−2.2) mm in tall. Stalk robust, calcareous, brittle, snow-white, 1/4–1/2 of full height, tapering upward. Peridium single-layered, thin, halo, persistent, with irregular dehiscence. Hypothallus conspicuous, white, calcareous, with a network of veins. Columella thick, gradually tapering upward, blunt-headed, white, calcareous, higher than half of the sporotheca, often close to the top of the sporotheca. Capillitium curved, branched, and connected, brown, light-colored at the end, radiating from various parts of the columella. Spore dark in mass, light purple under transmitted light, (7–) 8–11 μm in diameter, rough, with clustered warts.
Habitat. On the branches, rotten leaves, and living plants.
Distribution in China. Sichuan province.
Distribution in the World. China.
Additional specimens examined. China, Sichuan province, Liangshan Yi Autonomous Prefecture, Mianning County, Lingshan Temple, on the branches, rotten leaves, and living plants, 12 July 2013, B. Zhang, HMJAU M10044.
Notes. The primary characteristic of this species is the clustered warts on the surface of spores, with capillitium connected to the peridium. Diachea sichuanensis shares similarities with D. leucopodia in terms of sporocarp size and stalk color. However, D. sichuanensis can be distinguished from D. leucopodia by its blunt-headed columella, with spores of cluster warts and robust stalks. Diachea sichuanensis can be distinguished from D. bulbillosa by its spores with clustered warts, while the latter has sparse and large warts (Table 2). From the phylogenetic tree, D. sichuanensis is similar to D. leucopodia, but the genetic distance between the two is relatively far.
Diachea dictyospora (Rostaf.) J.M. García-Martín, J.C. Zamora & Lado, Persoonia 51:106 (2023).

Figure 4. Habitat and microstructure of Diachea dictyospora (HMJAU M20124). (A,B). Sporocarps; (C) stalk by SEM; (D) peridium by SEM; (E) lime nodes by TL; (E–G) capillitium, lime nodes, and spores by SEM; (H–I) spores with sparse and large ridges by TL and SEM. Scale bars: A = 2 mm, B = 1 mm, C, F = 100 μm, D = 20 μm, E, G = 40 μm, H = 10 μm, I = 5 μm.
Description. Sporocarp, gregarious or densely crowded, erect, stipitate, spherical, upper white, lower brown, (0.3–) 0.35–0.4 (−0.45) mm in diameter, (1–) 1.2–1.4 (−1.5) mm in tall. Stalk slender, gradually tapering upward, calcareous, yellow brown, dark brown at the top, with longitudinal grooves, lighter, (0.7–) 0.8–1 (−1.1) mm in length. Peridium thin, membranous, irregularly cracked, covered with a layer of small calcareous particles on the surface, white or gray, slightly halo, with the upper part gray, the lower part persistent in a reddish-brown cup-shaped shape. Hypothallus membranous, transparent, light brown. Columella rod-shaped, light brown, and reaching the center of the sporotheca. Capillitium sparse, with large lime nodes, white, forming a network within the sporotheca. Spores dark in mass, red-brown by transmitted light, with coarse warts, sometimes forming an incomplete net of warts fused into shout ridge, 12–13.5 (−16) μm in diameter.
Habitat. On the dead branches.
Distribution in China. Jilin province and Heilongjiang province.
Distribution in the World. China, United States, Spain, France, Portugal, Germany, Italy, Japan.
Specimens examined. China, Jilin province, Baishan City, Fusong County, Lushuihe Town National Forest Park Hunting Ground, on the dead branches, 23 July 2018, B. Zhang, HMJAU M20124, HMJAU M20125, HMJAU M20126; China, Jilin province, Jian City, Wunvfeng National Forest Park, on the dead branches, 19 July 2018, B. Zhang, HMJAU M20127, HMJAU M20128.
Notes. The main characteristics of this species are red-brown or gray-brown sporocarp, large lime nodes, and dark, coarsely warted spores. Diachea dictyospora is similar to D. muscorum in terms of its sporocarp shape and spore color and size. However, some morphological features of D. dictyospora differ from D. muscorum, such as a short stalk and spores that have loosely reticulate ridges approximately 1 μm high. In contrast, D. dictyospora has a long stalk, with spores marked by an incomplete net of broad fused into short ridges. These two species previously belonged to the genus Craterium Trentep.; Lado et al. redefined the genus Diachea in 2023. Diachea dictyospora, D. muscorum, and D. obovata were transferred to this genus and classified based on their morphological characteristics (García-Martín et al., 2023).
Diachea bulbillosa (Berk. & Broome) Lister, in Penzig, Myxomyc. Fl. Buitenzorg 47 (1898).
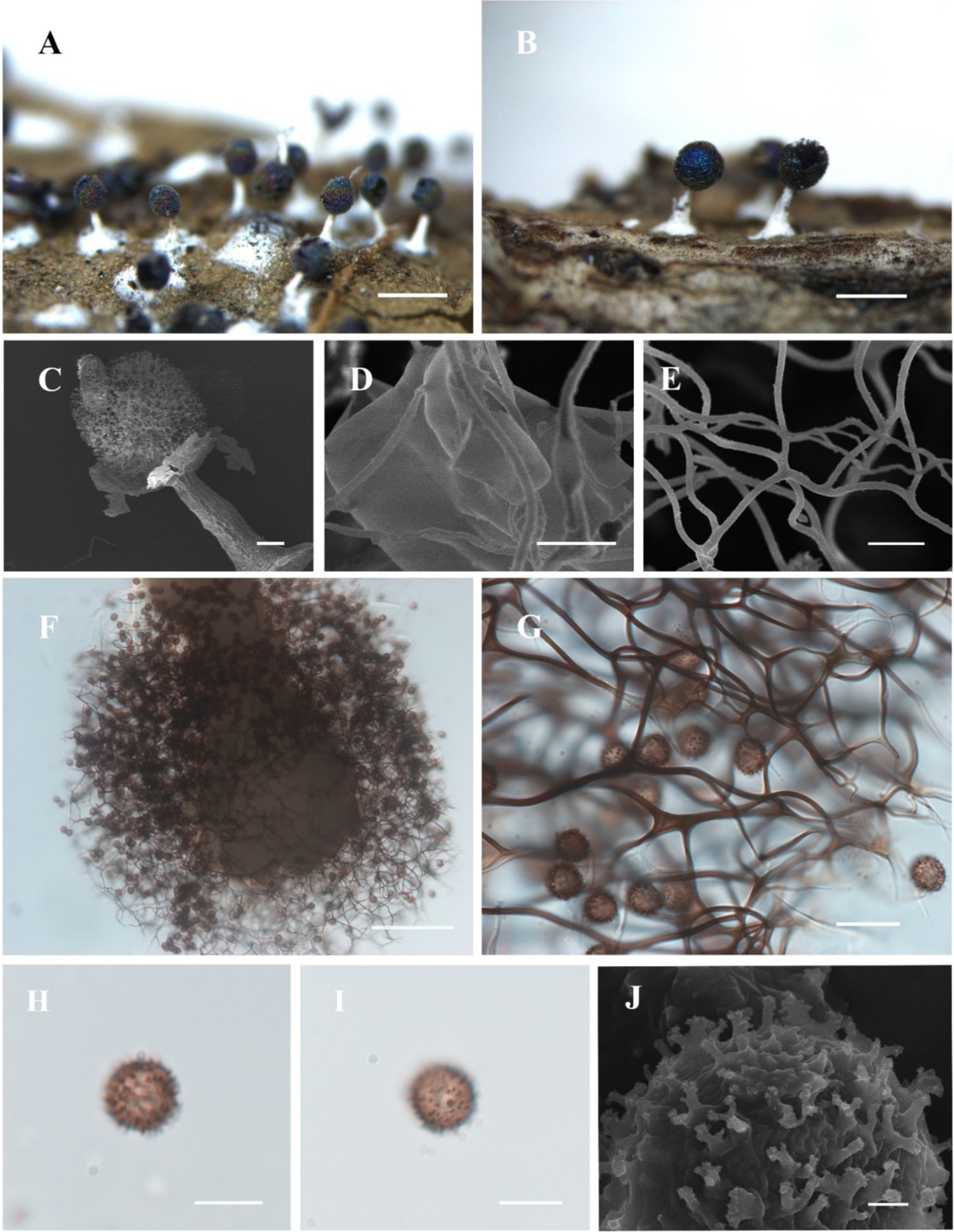
Figure 5. Habitat and microstructure of Diachea bulbillosa (HMJAU M10002). (A,B) Sporocarps; (C) sporocarp by SEM; (D) peridium and capillitium connected by SEM; (E) capillitium by SEM; (F) columella and capillitium by TL; (G) capillitium and spores by TL; H-J. Spores with sparse and large warts by TL. Scale bars: (A), B = 1 mm, (C), F = 100 μm, D, E = 5 μm, G = 20 μm, (H), I = 10 μm, J = 1 μm.
Description. Sporocarp, gregarious, stipitate, spherical or subglobose, with blue or purple halo, darkening with age, (0.3–) 0.35–0.5 (−0.55) mm in diameter, (0.8–) 0.9–1.3 (−1.4) mm in tall. Stalk cylindrical, gradually tapering upward, calcareous, white with an enlarged base. Peridium thin, membranous, with halo, irregularly cracked. Hypothallus inconspicuous, white. Columella obvious, white, calcareous, with an enlarged tip, club-shaped, reaching half or more of the sporocyst. Capillitium sparse, purple-brown, curved, reticulate, with membranous enlargement. Spores dark in mass, violet-gray by transmitted light, (7–) 8–11 μm in diameter, large, with sparsely and irregularly warted.
Habitat. On the dead branches and fallen leaves.
Distribution in China. Jilin province, Liaoning province, Gansu province, Beijing City, Hebei province, Inner Mongolia Autonomous Region, Anhui province, Guangdong province, Heilongjiang province, Taiwan, Henan province, Jiangsu province.
Distribution in the World. Widely distributed.
Specimens examined. China, Jilin province, Jiaohe City, Lafashan National Forest Park, on the dead branches and fallen leaves, 6 September 2018, B. Zhang, HMJAU M10037. China, Liaoning province, Fuxin City, Haitangshan National Nature Reserve, on the branches and leaves, 1 September 2012, B. Zhang, HMJAU M10007. China, Gansu province, Tianshui City, Dangchuan Town forest farm, Maiji District, on the dead leaves and branches, 16 August 2010, B. Zhang, HMJAU M10002.
Notes. Diachea bulbillosa is morphologically similar to D. splendens, sharing features such as a subglobose sporocarp with a halo, long stalk, and conspicuous columella. However, D. bulbillosa differs from D. splendens by having larger, sparsely, and irregularly distributed warts, while D. splendens possesses thick tumorous processes, often connected into irregular lines or incomplete mesh.
Diachea leucopodia (Bull.) Rostaf., Sluzowce monogr. 190 (1874).
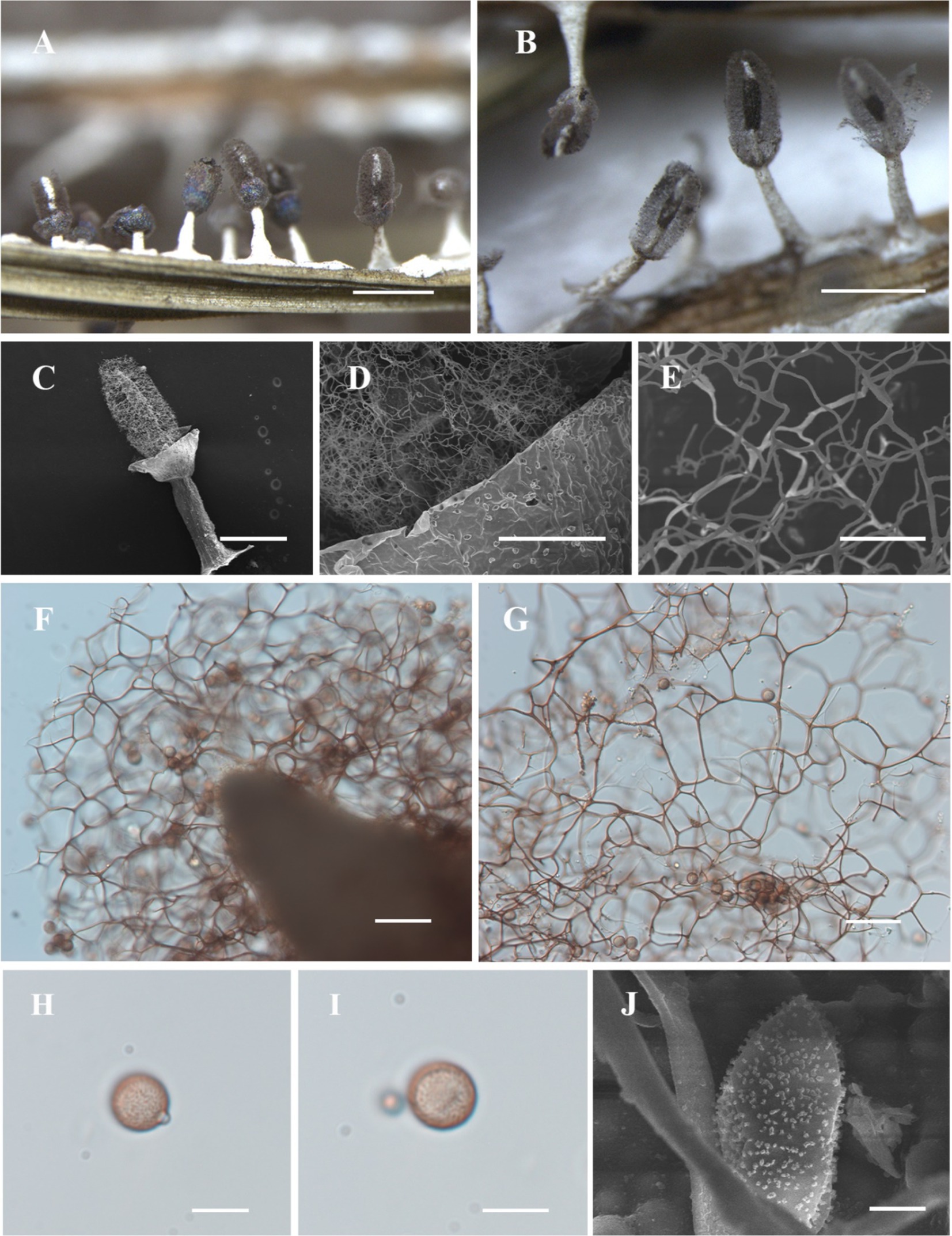
Figure 6. Habitat and microstructure of Diachea leucopodia (HMJAU M10005). (A,B) Sporocarps; (C) sporocarp by SEM; (D) capillitium and peridium by SEM; (E) capillitium by SEM; (F,G) columella and capillitium by TL; (H–J) spores with cluster warts by TL and SEM. Scale bars: (A), B = 1 mm, C = 400 μm, D = 100 μm, E = 20 μm, (F), G = 40 μm, (H), I = 10 μm, J = 2 μm.
Description. Sporocarp, gregarious or densely crowded, stipitate, metallic blue, or with a purple or bronze halo, cylindrical or elliptical, occasionally subspherical, with slightly concave navel below, (0.35–) 0.4–0.6 (−0.65) mm in diameter, (0.8–) 1–2 (−2.2) mm in tall. Stalk slender, calcareous, brittle, snow-white, approximately 1/2 the full height, tapering upward. Peridium often dehiscencing irregularly and falling off. Hypothallus white, calcareous, with veins and often forming a network. Columella thick, conical, gradually tapering upward, white, calcareous, extending more than half the height of the sporotheca, often reaching near the top of the sporotheca. Capillitium curved, branched, and interconnected, dark brown, colorless at the tips, radiating from various points of the columella. Spores dark in mass, light purple by transmitted light, rough, with minutely warted, 8–10 (−11) μm in diameter.
Habitat. On the dead branches and fallen leaves.
Distribution in China. Guangdong province, Guangxi Zhuang Autonomous Region, Yunnan province, Jilin province, Sichuan Province, Hebei province, Shanxi province, Inner Mongolia Autonomous Region, Jiangsu province, Fujian province, Shandong province, Hubei province, Shaanxi province, Gansu province, Henan province, Hunan province, Liaoning province, Taiwan, Hong Kong, Heilongjiang province, Beijing City, Ningxia Hui Autonomous Region, Anhui province.
Distribution in the World. Widely distributed.
Specimens examined. China, Guangdong province, Shaoguan City, Zhenjiang District, on the dead branches and fallen leaves, 13 May 2019, D. Dai, HMJAU M10086; China, Guangxi Zhuang Autonomous Region, Nanning City, Liang Feng Jiang National Forest Park, on the dead branches and fallen leaves, 15 August 2020, D. Dai, HMJAU M10087; China, Yunnan province, Lijiang City, on the dead branches and fallen leaves, 20 August 2012, B. Zhang, HMJAU M10005; China, Jilin province, Lushuihe Town, Changbai Mountain International Hunting Resort, on the dead branches and fallen leaves, 7 August 2014, B. Zhang, HMJAU M10015, HMJAU M10016; China, Jilin province, Jilin Agricultural University campus, on the dead branches and fallen leaves, 28 May 2013, B. Zhang, HMJAU M10018; China, Sichuan province, Ganzi Tibetan Autonomous Prefecture, Yajiang County, Gexigou Nature Reserve, on the dead leaves and branches, 5 August 2015, B. Zhang, HMJAU M10033.
Notes. Diachea leucopodia characterized by its white, calcareous hypothallus, stalk, and columella. The conical columella and cylindrical to oval sporocarps, along with light-color spores, and distinguish this species from others.
Diachea macroverrucosa D. Dai & B. Zhang, Mycology: 51 (2024).
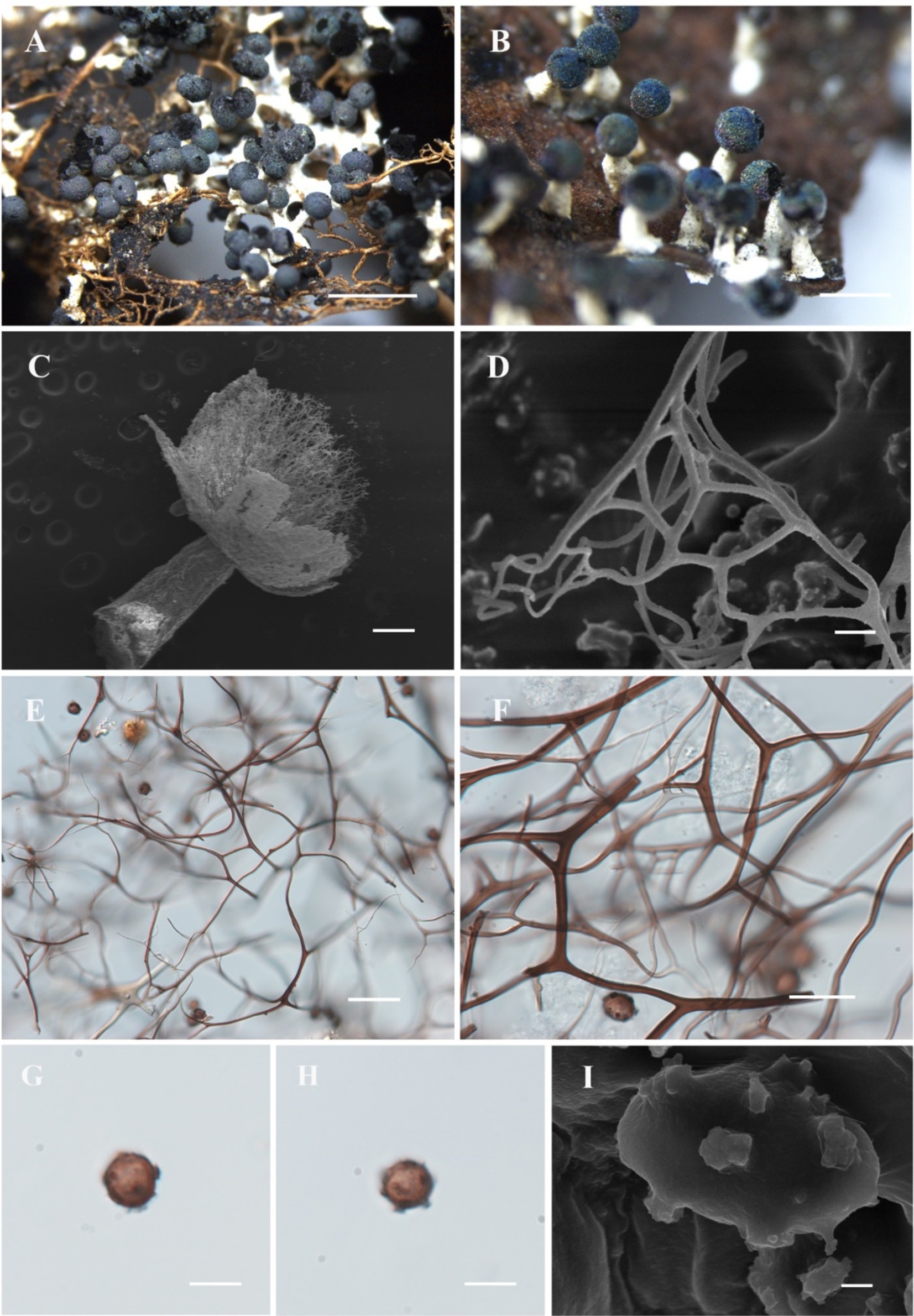
Figure 7. Habitat and microstructure of Diachea macroverrucosa (HMJAU M10064). (A,B) Sporocarps; (C) sporocarp by SEM; (D–F) capillitium by SEM and TL; (G–I) spores with sparse and large warts by TL and SEM. Scale bars: A = 2 mm, B = 1 mm, C = 100 μm, D = 5 μm, E = 40 μm, F = 20 μm, G, H = 10 μm, I = 1 μm.
Description. Sporocarp, gregarious to clusterous, stipitate, spherical, with haze and blue halo, (0.3–) 0.35–0.4 (−0.45) mm in diameter, (0.7–) 0.75–0.9 (−1) mm in tall. Stalk coarse and short, (0.35–) 0.4–0.5 (−0.55) mm in length, white, tapering upward, calcareous, containing numerous calcareous particles. Peridium single-layered, with a halo, dehiscencing irregularly. Hypothallus membranous, colorless, and transparent, sometimes forming sheets. Columella white, conical, blunt-headed, approximately 1/4 of the sporotheca. Capillitium dense, thin, hard, dark brown, branched, and interconnected, radiating from various points of the columella, lighter at the end, and not connected to the peridium. Spores black in mass, dark brown by transmitted light (TL), (8–) 9–13 μm in diameter, sparsely covered with large dark warts.
Habitat. On the branches and rotten leaves.
Distribution in China. Shaanxi province, Jilin Province, Liaoning Province.
Distribution in the World. China.
Specimens examined, China, Shaanxi province, Niubeiliang National Nature Reserve, on the rotten leaves, 21 July 2014, B. Zhang, HMJAU M10001; China, Jilin province, Yanbian Korean Autonomous Prefecture, Antu County, Changbai Mountain National Nature Reserve, on the rotten branches and leaves, 25 August 2013, B. Zhang, HMJAU M10027; China, Jilin province, Yanbian Korean Autonomous Prefecture, Longjing City, Tianfo Zhishan National Nature Reserve, on the rotten branches and leaves, 4 September 2011, B. Zhang, HMJAU M10009; China, Liaoning province, Fuxin City, Haitangshan National Nature Reserve, on the rotten branches and leaves, 1 September 2012, B. Zhang, HMJAU M10039, HMJAU M10057, HMJAU M10064, HMJAU M10067, HMJAU M10069, HMJAU M10073.
Notes. Diachea macroverrucosa is characterized by its large warts resembling an inflorescence on the spore surface. The columella is conical and does not exceed 1/2 the height of the sporocarp. Morphologically, D. macroverrucosa resembles D. bulbillosa, sharing characteristics such as sporangium shape, color, and spore diameter. Both species possess white columellae and stalks. However, D. macroverrucosa can be differentiated from D. bulbillosa by its shorter overall height and shorter columella, and sparse warts (Table 2).
Diachea subsessilis Peck, Annual Rep. New York State Mus. 31:41 (1878).
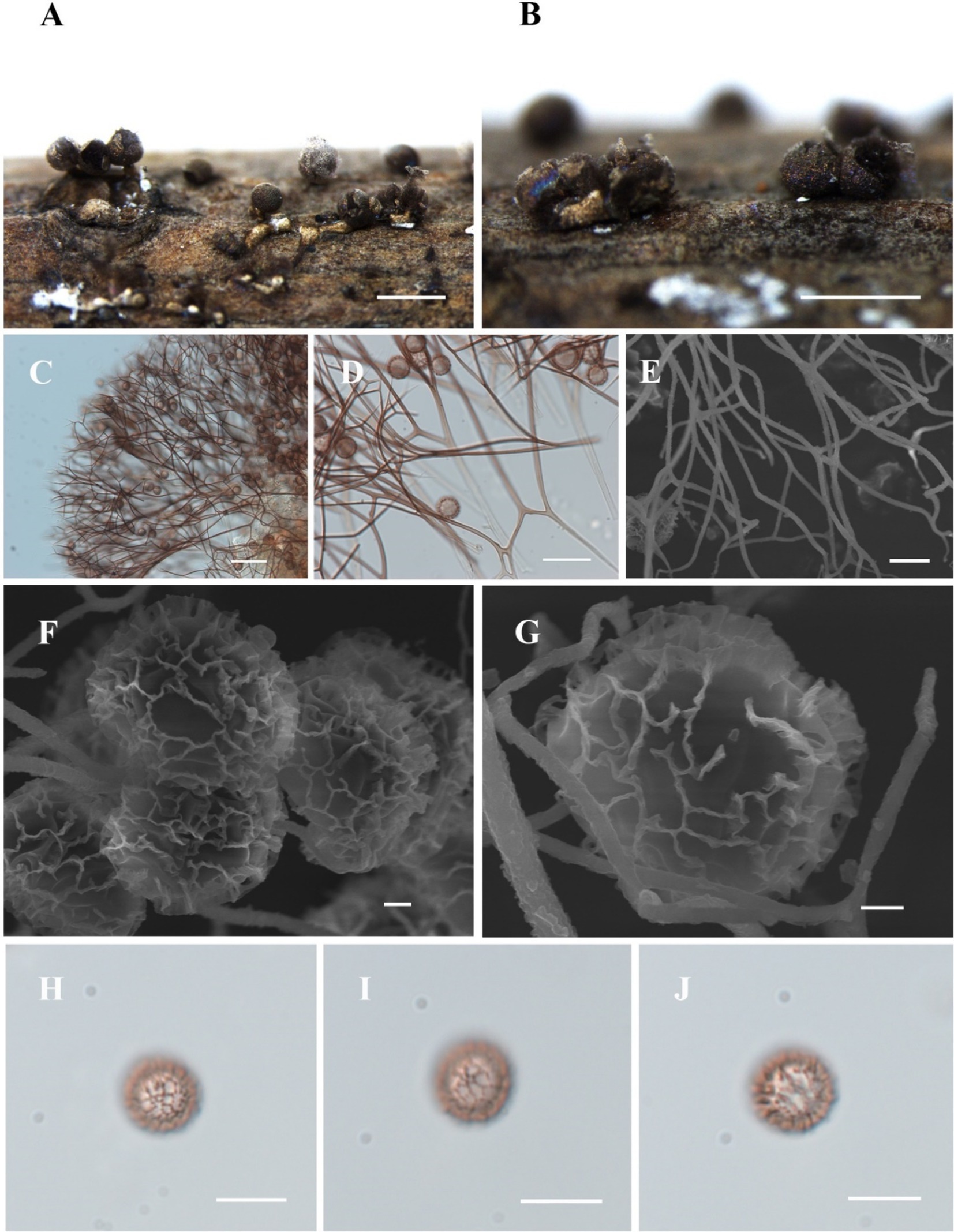
Figure 8. Habitat and microstructure of Diachea subsessilis (HMJAU M10075). (A,B) Sporocarps; (C–E) capillitium by TL and SEM; (F–J) Spores with clustered and connected into a network wart by SEM and TL. Scale bars: A, B = 1 mm, C = 40 μm, D = 20 μm, E = 5 μm, F, G = 1 μm, H-J = 10 μm.
Description. Sporocarp, gregarious or densely crowded, short-stalked or sessile, rarely plasmodiocarp, with gray-green or dark blue halo, (0.35–) 0.4–0.8 (−0.85) mm in diameter, (0.5–) 0.6–1 (−1.2) mm in tall. Stalk, when present, stout, cylindrical, white, calcareous, not exceeding the height of the sporocarp. Peridium single-layered, with halo. Hypothallus small, networked, slightly calcareous, or absent. Columella short, calcareous, conical, and sometimes absent. Capillitium thin, radiating from the columella, light brown, branched and connected, light-colored at the ends. Spores dark in mass, light brown by transmitted light, with clustered warts and lines connected into a network, (7–) 8–12 μm in diameter.
Habitat. On the branches and rotten leaves.
Distribution in China. Sichuan province, Guangxi Zhuang Autonomous Region, Jiangsu province.
Distribution in the World. China, North America, Europe, and Southeast Asia.
Specimens examined. China, Sichuan province, Chengdu city, Tazishan Park, on the branches and rotten leaves, 15 July 2013, B. Zhang, HMJAU M10031, HMJAU M10075.
Notes. Diachea subsessilis is characterized by its short-stalked or sessile sporocarp with a gray-green or dark blue halo. It shares morphological similarities with D. radiata including a subglobose sporocarp, similar spore size, white columella, and stalk. However, D. subsessilis can be differentiated by its short columella. The spores have a spinulose-reticulate surface, often forming a complete net on one side, whereas D. radiata has small spinulose warts.
Taxonomic key to the species of Diachea.
1. Capillitium with limestone………………………………………………………Diachea dictyospora.
1. Capillitium without limestone…………………………………………….…………………………2.
2. Hypothallus, stalk, and columella are orange……………………………..… Diachea silvaepluvialis.
2. Hypothallus, stalk, and columella are white………………………………………………..….……3.
3. Sporocarp generally cylindrical or oval, rarely subglobose…………………………..…..….…….4.
3. Sporocarp spherical…………………………………………………………………………….…….5.
4. Spores are light in color and nearly smooth………………..………………..….Diachea leucopodia.
4. Spores are light in color, with warts and dark clusters…….……………….…Diachea sichuanensis.
5. Spores with reticular patterns and capillitium are soft and slender………………Diachea subsessilis.
5. Spores with warts or nodular protrusion…………………….………………………………………6.
6. Spores with less nodular protrusion, large…………………………………Diachea macroverrucosa.
6. Spores with more nodular protrusion, small…………………………………………………………7.
7. Capillitium are sparse and curly………………………………………………..…Diachea bulbillosa.
7. Capillitium dense, hard, and straight………………….……………………..….Diachea plectophylla.
4 Discussion
This study significantly advances our understanding of Diachea by providing detailed descriptions of two newly identified species, namely, D. plectophylla and D. sichuanensis, supported by robust phylogenetic analysis. In addition, we report new distribution records for five previously known species: D. bulbillosa in Gansu province, D. leucopodia in Yunnan and Sichuan provinces, and D. subsessilis in Sichuan province, China. Morphologically, D. plectophylla resembles D. bulbillosa with its sparse warts, iridescent sporocarps, white stalk, and prominent columella. However, it differs in having smaller spores and occasionally interconnected warts forming ridges. D. sichuanensis can be distinguished from D. leucopodia by its blunt-headed columella, spores with clustered warts, and thicker stalks. A detailed comparison of species characteristics is provided in Table 2.
Traditionally, species identification in myxomycete has relied on morphological traits. However, advances in molecular biology have greatly improved the accuracy of species identification, helping resolve discrepancies caused by morphology alone. Our morphological distinctions are further validated by the unique placements of these species, which confirms their taxonomic status. In our phylogenetic analysis, Diachea species formed a distinct branch within the family Physaraceae, contrasting with some recent research (Prikhodko et al., 2023). Studies by Fiore-Donno et al. (2012) and Cainelli et al. (2020) suggested that Diachea and Didymium are sister branches, albeit with weak support. Our results, however, suggest that Diachea, Nannengaella, and Physarum are more closely related sister branches.
Lado et al. (2022), who used the 18S rRNA, 17S rRNA, and EF-1α genes, faced challenges with incomplete data, leading to weak support in the ML analysis for six species. Discrepancies between our study and previous research may arise from the use of different gene markers. For instance, García-Martín et al. (2023) redefined Diachea to include species previously classified in Physaraceae, such as Craterium spp., but placed Diachea within Didymiaceae, divided into two groups, which diverges from our findings. Similarly, Prikhodko et al. (2023) used three gene markers to analyze Didymiaceae, resulting in some reclassifications. We believe that these differences stem from the varying gene markers, sample size, and available sequences used in each study. In addition, factors such as collection area, climate, and vegetation type may have contributed to the variations observed.
The taxonomic classification of the genus Diachea has historically been contentious. Despite its wide geographical distribution and the growing number of reported species, the limited availability of specimens and genetic sequences has hindered accurate classification. The scarcity of known Diachea sequences, along with unidentified sequences uploaded to NCBI, may lead to misinterpretations when using BLAST search. In recent studies, Diachea has been classified within the order Physarales; but its placement has oscillated between the families Physaraceae (Lister, 1925) and Didymiaceae (Keller and Braun, 1999; García-Martín et al., 2023; Prikhodko et al., 2023).
Our research identified five species forming a distinct evolutionary branch, which we tentatively assigned to the family Didymiaceae based on recent systematic research on Physarales (García-Martín et al., 2023). However, due to the limited number of specimens, we did not conduct a comprehensive analysis of the entire genus, focusing instead on the discovery of two new species. The limited data leave their taxonomic status somewhat uncertain. Nonetheless, our findings provide valuable sequences and preliminary insights for future systematic studies of this genus.
The recent discovery of new Diachea species, such as D. mitchellii (Lado et al., 2022), D. racemosa (Novozhilov et al., 2023), and D. macroverrucosa (Liu et al., 2024), underscores the greater species richness within the genus than previously documented. Looking ahead, we plan to expand our sampling range, enhance morphological observations, increase the number of sequences, and employ additional gene markers to deepen our understanding of the relationships among Diachea, Didymiaceae, and Physaraceae. Future research should incorporate more gene markers (nSSU, EF-1α, mtSSU, COI, and α-Tub) and aim to obtain complete sequences of these genes for a more comprehensive study. To minimize variations caused by regional and environmental factors, collaboration with international researchers to broaden the sampling area is recommended. In addition, increasing specimen collection from extreme environments will facilitate a more thorough and systematic study of this genus and its relatives, ultimately clarifying its taxonomic status.
5 Conclusion
This study identifies two newly discovered species of Diachea and provides a detailed morphological description of these species, along with five previously known ones. An identification key for these species is also included. Given the limited research and sequence data available for this genus, our findings offer valuable foundational information to support future systematic research. Furthermore, the discovery of these new and newly recorded species enhances our understanding of Diachea diversity, contributing to broader research on the community composition ecological roles, and spatiotemporal distribution of myxomycetes and their substrates.
Data availability statement
The datasets presented in this study can be found in https://www.ncbi.nlm.nih.gov/nuccore. The accession number(s) can be found in the article/supplementary material.
Author contributions
XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DD: Methodology, Visualization, Writing – original draft. YT: Formal analysis, Writing – review & editing. YoL: Investigation, Writing – review & editing. JH: Formal analysis, Investigation, Methodology, Writing – review & editing. FS: Writing – review & editing. BZ: Project administration, Resources, Supervision, Writing – review & editing. XL: Project administration, Resources, Supervision, Writing – review & editing. YuL: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by several programs: the Jilin Provincial Key Research and Development Program (nos. 20230202121NC and 20240303101NC), the National Key R & D of Ministry of Science and Technology (no. 2023YFD1201601-2), the Modern Agroindustry Technology Research System (CARS20), and the 111 Program (no. D17014).
Acknowledgments
We gratefully acknowledge Xiaolan He from the Sichuan Edible Fungi Research Institute and Haixia Ma from the Chinese Academy of Tropical Agricultural Sciences for their invaluable assistance with fieldwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Adl, S. M., Habura, A., and Eglit, Y. (2014). Amplification primers of SSU rDNA for soil protists. Soil Biol. Biochem. 69, 328–342. doi: 10.1016/j.soilbio.2013.10.024
Adl, S. M., Simpson, A. G., Lane, C. E., Lukeš, J., Bass, D., Bowser, S. S., et al. (2012). The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59, 429–514. doi: 10.1111/j.1550-7408.2012.00644.x
Alexopoulos, C. J. (1982). Cell biology of Physarum and Didymium V1: Organisms, nucleus, and cell cycle: 3–21. New York, USA: Academic Press.
Bork, P. (2007). Interactive tree of life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23, 127–128. doi: 10.1093/bioinformatics/btl529
Cainelli, R., Haan, M. D., Meyer, M., Bonkowski, M., and Fiore-Donno, A. M. (2020). Phylogeny of Physarida (Amoebozoa, Myxogastria) based on the small-subunit ribosomal RNA gene, redefinition of Physarum pusillum s. str. And reinstatement of P. Gravidum Morgan. J. Eukaryot. Microbiol. 67, 327–336. doi: 10.1111/jeu.12783
Cavalcanti, L. H., Ponte, M. P. M. P., and Mobin, M. (2006). Myxomycetes, state of Piauí, Northeast Brazil. Check List. 2, 70–74. doi: 10.15560/2.2.70
Fiore-Donno, A. M., Berney, C., Pawlowski, J., and Baldauf, S. L. (2005). Higher-order phylogeny of plasmodial slime molds (Myxogastria) based on elongation factor 1-a and small subunit rRNA gene sequences. J. Eukaryot. Microbiol. 52, 201–210. doi: 10.1111/j.1550-7408.2005.00032.x
Fiore-Donno, A. M., Kamono, A., Meyer, M., Schnittler, M., Fukui, M., and Cavalier-Smith, T. (2012). 18S rDNA phylogeny of Lamproderma and allied genera (Stemonitales, Myxomycetes, Amoebozoa). PLoS One 7:e35359. doi: 10.1371/journal.pone.0035359
Fiore-Donno, A. M., Meyer, M., Baldauf, S. L., and Pawlowski, J. (2008). Evolution of dark-spored Myxomycetes (slime-molds): molecules versus morphology. Mol. Phylogenet. Evol. 46, 878–889. doi: 10.1016/j.ympev.2007.12.011
Fries, E. M. (1829). Systema mycologicum, sistens fungorum ordines, genera et species. Germany: Greifswald.
García-Cunchillos, I., Zamora, J. C., Ryberg, M., and Lado, C. (2022). Phylogeny and evolution of morphological structures in a highly diverse lineage of fruiting-body-forming amoebae, order Trichiales (Myxomycetes, Amoebozoa). Mol. Phylogenet. Evol. 177:107609. doi: 10.1016/j.ympev.2022.107609
García-Martín, J. M., Mosquera, J., and Lado, C. (2018). Morphological and molecular characterization of a new succulenticolous Physarum (Myxomycetes, Amoebozoa) with unique polygonal spores linked in chains. Eur. J. Protistol. 63, 13–25. doi: 10.1016/j.ejop.2017.12.004
García-Martín, J. M., Zamora, J. C., and Lado, C. (2023). Multigene phylogeny of the order Physarales (Myxomycetes, Amoebozoa): shedding light on the dark-spored clade. Persoonia 51, 89–124. doi: 10.3767/persoonia.2023.51.02
Hall, T. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 734, 95–98. doi: 10.1021/bk-1999-0734.ch008
Janik, P., Lado, C., and Ronikier, A. (2020). Range-wide Phylogeography of a nivicolous protist Didymium nivicola Meyl. (Myxomycetes, Amoebozoa): striking contrasts between the northern and the southern hemisphere. Protist 171:125771. doi: 10.1016/j.protis.2020.125771
Kalyanasundaram, I., and Mubarak, A. N. (1989). Taxonomic note on the myxomycete genus Diachea. Mycol. Res. 93, 233–235. doi: 10.1016/S0953-7562(89)80124-8
Kang, S., Tice, A. K., Spiegel, F. W., Silberman, J. D., Pánek, T., Cepicka, I., et al. (2017). Between a pod and a hard test: the deep evolution of amoebae. Mol. Biol. Evol. 34, 2258–2270. doi: 10.1093/molbev/msx162
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Keller, H. W., and Braun, K. L. (1999). Myxomycetes of Ohio: their systematics, biology, and use in teaching. Ohio Biol. Surv. Bull. New Series 13, 1–182.
Keller, H. W., Skrabal, M., Eliasson, U. H., and Gaither, T. W. (2004). Tree canopy biodiversity in the Great Smoky Mountains National Park: ecological and developmental observations of a new myxomycete species of Diachea. Mycologia 96, 537–547. doi: 10.1080/15572536.2005.11832952
Kirk, P. M., Cannon, P. F., Minter, D. W., and Stalpers, J. A. (2008). Dictionary of the fungi. 10th Edn. Wallingford, CT: CAB International, 759–771.
Lado, C. (2005/2024). An on line nomenclatural information system of Eumycezotozoa. Available at: http://www.nomen.eumycetozoa.com.
Lado, C., Treviño-Zevallos, I., García-Martín, J. M., and Wrigley, B. D. (2022). Diachea mitchellii: a new myxomycete species from high elevation forests in the tropical Andes of Peru. Mycologia 114, 798–811. doi: 10.1080/00275514.2022.2072140
Leontyev, D., Ishchenko, Y., and Schnittler, M. (2023). Fifteen new species from the myxomycete genus Lycogala. Mycologia 115, 524–560. doi: 10.1080/00275514.2023.2199109
Leontyev, D. V., and Schnittler, M. (2022). The phylogeny and phylogenetically based classification of myxomycetes. Myxomycetes 2022, 97–124. doi: 10.1016/B978-0-12-824281-0.00008-7
Lister, A. (1925). A monograph of the Mycetozoa: A descriptive catalogue of the species in the herbarium of the British museum. 3rd Edn. London, UK: Printed by order of the Trustees.
Liu, S. L., Zhao, P., Cai, L., Shen, S., Wei, H. W., Na, Q., et al. (2024). Catalogue of fungi in China 1. New taxa of plant-inhabiting fungi. Mycology., 1–58. doi: 10.1080/21501203.2024.2316066
Nguyen, L. T., Schmidt, H. A., Haeseler, A. V., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. doi: 10.1093/molbev/msu300
Novozhilov, Y. K., Okun, M. V., Erastova, D. A., Shchepin, O. N., Zemlyanskaya, I. V., García-Carvajal, E., et al. (2013). Description, culture and phylogenetic position of a new xerotolerant species of Physarum. Mycologia 105, 1535–1546. doi: 10.3852/12-284
Novozhilov, Y. K., Prikhodko, I. S., Bortnikov, F. M., Shchepin, O. N., Luptakova, A. D., Dobriakova, K. D., et al. (2023). Diachea racemosa (Myxomycetes = Myxogastrea): a new species with cespitose sporocarps from southern Vietnam and its position within the phylogenetic clade Diachea sensu lato (Physarales). Protistology 17, 189–204. doi: 10.21685/1680-0826-2023-17-4-1
Poulain, M., Meyer, M., and Bozonnet, J. (2011). Les Myxomycetes. Fédération mycologique et botanique Dauphiné-Savoie, Sévrier.
Prikhodko, I. S., Shchepin, O. N., Bortnikova, N. A., Novozhilov, Y. K., Gmoshinskiy, V. I., Moreno, G., et al. (2023). A three-gene phylogeny supports taxonomic rearrangements in the family Didymiaceae (Myxomycetes). Mycol. Prog. 22:11. doi: 10.1007/s11557-022-01858-1
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., and Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxford, England) 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Rostafiński, J. T. (1874). Śluzowce (Mycetozoa) Monografia. Pamiętnik Towarzystwa Nauk Ścisłych Paryżu 5, 1–215.
Shchepin, O. N., López, V. Á., Inoue, M., Prikhodko, I. S., Erastova, D. A., Okun, M. V., et al. (2024). DNA barcodes reliably differentiate between nivicolous species of Diderma (Myxomycetes, Amoebozoa) and reveal regional differences within Eurasia. Protist 175:126023. doi: 10.1016/j.protis.2024.126023
Shchepin, O., Novozhilov, Y., Woyzichovski, J., Bog, M., Prikhodko, I., Fedorova, N., et al. (2022). Genetic structure of the protist Physarum albescens (Amoebozoa) revealed by multiple markers and genotyping by sequencing. Mol. Ecol. 31, 372–390. doi: 10.1111/mec.16239
Walkera, G., Silbermanb, J. D., Karpovc, S. A., Preisfeld, A., Foster, P., Frolov, A. O., et al. (2003). An ultrastructural and molecular study of Hyperamoeba dachnaya, n. sp. and its relationship to the mycetozoan slime moulds - ScienceDirect. Eur. J. Protistol. 39, 319–336. doi: 10.1078/0932-4739-00906
Wikmark, O. G., Haugen, P., Lundblad, E. W., Haugli, K., and Johansen, S. D. (2007). The molecular evolution and structural organization of group I introns at position 1389 in nuclear small subunit rDNA of myxomycetes. J. Eukaryot. Microbiol. 54, 49–56. doi: 10.1111/j.1550-7408.2006.00145.x
Xiang, C. Y., Gao, F. L., Jakovlić, I., Lei, H. P., Hu, Y., Zhang, H., et al. (2023). Using PhyloSuite for molecular phylogeny and tree-based analyses. Imeta 2:e87. doi: 10.1002/imt2.87
Zhang, D., Gao, F., Jakovlić, I., Zou, H., Zhang, J., Li, W. X., et al. (2020). PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 20, 348–355. doi: 10.1111/1755-0998.13096
Keywords: diversity, morphology, multigene phylogeny, new taxa, taxonomy
Citation: Li X, Dai D, Tuo Y, Li Y, Hu J, Sossah FL, Zhang B, Li X and Li Y (2024) Two new species and three new records of Diachea (Physarales) from China based on morphological and molecular evidence. Front. Microbiol. 15:1458944. doi: 10.3389/fmicb.2024.1458944
Edited by:
Muhammad Zahid Mumtaz, Gansu Agricultural University, ChinaReviewed by:
Dalia A. Gaber Mahmoud, University of Applied Sciences Erfurt, GermanyGuohong Liu, Fujian Academy of Agricultural Sciences, China
Rajeshwari Negi, Eternal University, India
Muhammad Waqar Alam, University of Okara, Pakistan
Copyright © 2024 Li, Dai, Tuo, Li, Hu, Sossah, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, emhhbmdib2Z1bmdpQDEyNi5jb20=; Xiao Li, bHhtb2d1QDE2My5jb20=; Yu Li, eXVsaTk2NkAxMjYuY29t
†These authors have contributed equally to this work
 Xuefei Li
Xuefei Li Dan Dai
Dan Dai Yonglan Tuo1,2
Yonglan Tuo1,2 Jiajun Hu
Jiajun Hu Frederick Leo Sossah
Frederick Leo Sossah Bo Zhang
Bo Zhang Xiao Li
Xiao Li Yu Li
Yu Li