- 1College of Plant Protection, Gansu Agricultural University, Lanzhou, China
- 2Ecology and Nature Conservation Institute, Chinese Academy of Forestry, Beijing, China
- 3College of Biodiversity Conservation, Southwest Forestry University, Kunming, China
- 4College of Pharmacy and Life Sciences, Jiu Jiang University, Jiujiang, China
Introduction: Cortinariaceae, which belongs to the Agaricales order, is a globally recognized family, known for its high species diversity.
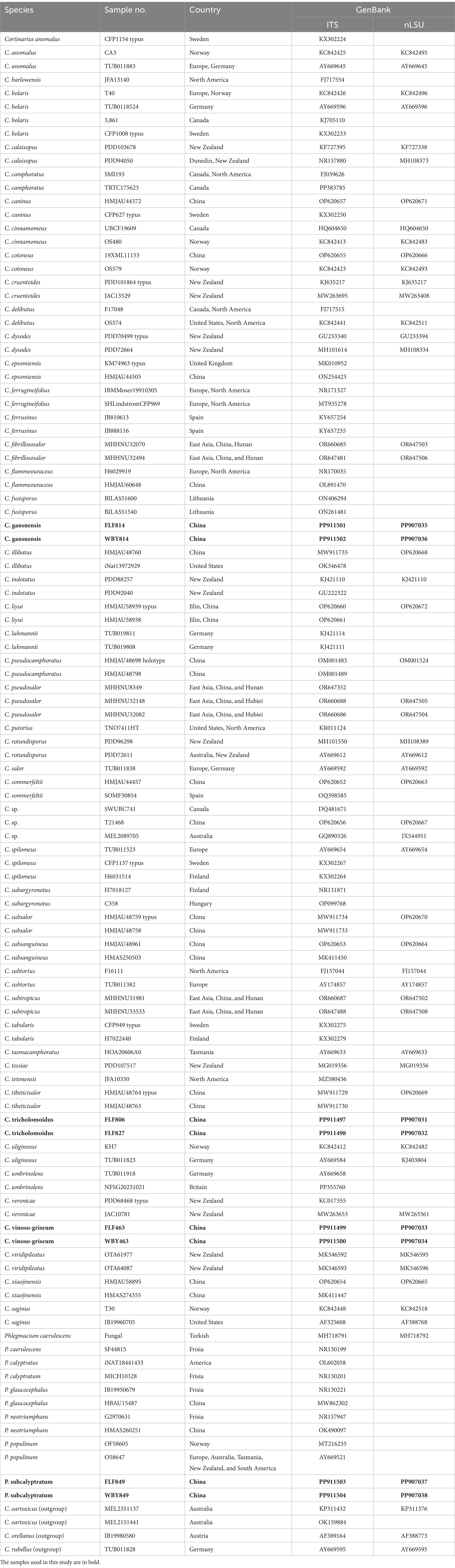
Methods: Eight internal transcribed spacer (ITS) and nuclear large ribosomal subunit (LSU) sequences were newly generated, and phylogenetic analyses were performed by combining ITS and LSU datasets. Four species were identified as forming four independent lineages with robust support in phylogenies based on both datasets.
Results: These new species in the taxa, Cortinarius gansuensis, Cortinarius tricholomoidus, Cortinarius vinoso-griseum, and Phlegmacium subcalyptratum from Northwestern China are described and illustrated based on morphological and molecular evidence. Cortinarius gansuensis is characterized by a convex and brownish vinaceous pileus, generative hyphae with clamp connections, and ellipsoid basidiospores (8.5–10.6 μm × 5.4–6.8 μm); Cortinarius tricholomoidus is characterized by a broadly umbonate and snuff brown pileus, generative hyphae with clamp connections, and broadly ellipsoid to subglobose basidiospores (7.4–8.5 μm × 6.2–7.3 μm); Cortinarius vinoso-griseum is characterized by a violaceous gray pileus, generative hyphae with clamp connections, and smaller basidiospores (7.5–9.7 μm × 5.6–7.8 μm); and Phlegmacium subcalyptratum is characterized by a small and apricot-orange pileus, generative hyphae with clamp connections, and fusiform basidiospores (10.0–12.7 μm × 5.6–6.8 μm).
Discussion: Full descriptions, illustrations, and results of phylogenetic analyses of the four species along with discussions on related species are provided.
1 Introduction
The species within the family Cortinariaceae are crucial ectomycorrhizal fungi associated with various plants, including the gymnosperms, rosid angiosperms, and some shrubs. The genus is typically characterized by mostly brown and dark-spored basidiospores (Matheny et al., 2015; Dentinger et al., 2016).
The family Cortinariaceae includes 10 genera, Aureonarius Niskanen & Liimat., Austro-cortinarius Niskanen & Liimat., Calonarius Niskanen & Liimat., Cortinarius (Pers.) Gray, Cystinarius Niskanen & Liimat., Hygronarius Niskanen & Liimat., Mystinarius Niskanen & Liimat., Phlegmacium (Fr.) Wünsche, Thaxterogaster Singer, and Volvanarius Niskanen & Liimat. (Liimatainen et al., 2022). Among these genera, Cortinarius is the largest genus in the Agaricales with a worldwide distribution. However, the taxonomic status of this genus has long been a subject of controversy owing to the overlapping morphological characteristics. Numerous sections or clades have been proposed to address this issue (Brandrud, 1998; Niskanen et al., 2016; Soop et al., 2019; Zhang et al., 2023; Long et al., 2024). The currently recognized subgenera include Cortinarius, Camphorati, Dermocybe, Illumini, Infracti, Iodolentes, Leprocybe, Myxacium, Orellani, Paramyxacium, and Telamonia (Liimatainen et al., 2022). Cortinarius s.l. is a cosmopolitan genus with 313 accepted taxa, including 23 taxa originally described from Northwestern China. In addition, there are at least over 5,000 accepted species in Cortinarius s.s.
The species of Leprocybe are distributed in both the Northern and Southern Hemispheres, and this subgenus is characterized by small-to medium-sized basidiomes, mostly agaricoid or sequestrate. A morpho-genetic revision of the Northern Hemispheric Leprocybe was conducted by Ammirati et al. (2021) and Bidaud et al. (2021). Currently, seven sections are included in the genus Leprocybe: Leprocybe, Fuscotomentosi, Melanoti, Persplendidi, Squamiveneti, Veneti, and Veronicae.
Phlegmacium (Fr.) (Liimatainen et al., 2022), distributed in the Northern Hemisphere, is characterized by medium-to large-sized, predominantly stipitocarpic, yellow agaricoid (phlegmacioid) basidiomes. Four subgenera were recognized: Phlegmacium, Bulbopodium, Carbonella, and Cyanicium.
In this study, we focused on Cortinariaceae represented by eight specimens from Gansu, China. Phylogenetic analyses based on the internal transcribed spacer (ITS) and nuclear large ribosomal subunit (LSU) rDNA sequences were carried out, and four new species were recognized. The current study aimed to further explore the species diversity of Cortinariaceae in the Gansu province of Northwestern China and confirm the taxonomic position of Phlegmacium within the Cortinariaceae family.
2 Materials and methods
2.1 Morphological studies
All specimens were deposited in the Fungal Herbarium of Gansu Agricultural University (MHGAU, China) and the National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control (NIOHP, China CDC). The size of the basidiomes, as determined by the pileus width, was described as small (<5.0 cm), medium (5.0–9.0 cm), and large (> 9.0 cm). The samples were recorded in the growth environment and geographical location and photographed while fresh; then, they were dried using a food dehydrator (30–35°C for several hours till dry) and stored in a refrigerator (−20°C) for morphological studies. The morphological descriptions were based on field notes and dried specimens. The microscopic features were examined and described in 5% KOH, 1% Phloxine B (C20H4Br4Cl2K2O5), or Melzer’s reagent and observed under a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) with a magnification of up to ×1,000 following Fan et al. (2021) and Long et al. (2024). Thirty basidiospores were measured per collection (excluding apiculus and ornamentation), and the averages (av. X) and quotients (av. Q = L/B) were calculated. L = mean spore length, B = mean spore width, and Q = variation in the ratios of L/B between the specimens studied. Color terms were cited from Royal Botanic Garden Edinburgh (1969) and Kornerup and Wanscher (1978).
2.2 Molecular phylogeny
A Phire® Plant Direct PCR Kit was used to obtain PCR products from the dried specimens according to the manufacturer’s instructions and as described previously by Long et al. (2024), with some modifications. The primer pairs ITS5 and ITS4 for ITS and LR0R and LR7 for nrLSU were used to amplify the internal transcribed spacer (ITS) and large ribosomal subunit (LSU), respectively (White et al., 1990). The PCR procedure for the ITS was as follows: initial denaturation at 98°C for 5 min, followed by 35 cycles at 94°C for 5 s, 54°C for 5 s, and 72°C for 5 s, and a final extension of 72°C for 10 min. The PCR procedure for the nrLSU was as follows: initial denaturation at 94°C for 1 min, followed by 35 cycles at 94°C for 30 s, 50°C for 1 min, and 72°C for 1.5 min, and a final extension of 72°C for 10 min (Fan et al., 2021). The PCR products were purified and sequenced by Engine Biotech, China. The newly generated sequences from this study have been deposited in GenBank and are listed in Table 1.
The new sequences generated and additional sequences retrieved from GenBank (Table 1) were aligned using BioEdit 7.0.5.3 and ClustalX 1.83, followed by manual adjustments. The sequences of the Orellana clade including Cortinarius rubellus Cooke, C. orellanus Fr., and C. eartoxicus Gasparini were utilized as outgroups (Dima et al., 2021). The phylogenetic analysis was conducted based on the maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) methods. The best-fit model was selected using ModelFinder (Kalyaanamoorthy et al., 2017), with the Akaike information criterion (AIC) guiding the process.
The MP, ML, and BI analyses were performed using PAUP on XSEDE (4.a165), RAxML 8.2.12, and MrBayes 3.2.5, respectively (Ronquist et al., 2012). Four Markov chains were run for two independent runs from random starting trees for 10 million generations, with the trees sampled every 1,000 generations. The burn-in was set at 25% to discard initial trees. The sequence alignment was deposited in TreeBase (submission ID: 31486). The branches with bootstrap support for the MP, ML (BP), and Bayesian posterior probabilities (BPPs) greater than or equal to 50% (BP) and 0.90 (BPP) were considered significantly supported.
3 Results
The ITS+LSU dataset comprised 112 fungal collections representing approximately 65 taxa of the genus Cortinarius. ModelFinder suggested that GTR + I + G was the best-fit model of nucleotide evolution for the BI. The Bayesian analysis resulted in a concordant topology with an average standard deviation of split frequencies of 0.000067. The MP, ML, and BI analyses resulted in nearly identical topologies, and thus, only the ML tree was presented with the bootstrap supports for BP and BPP when they were greater than or equal to 50% and 0.90, respectively.
Our phylogeny, which was inferred from the ITS+LSU sequences (Figure 1), was similar to the research by Zhang et al. (2023) and Long et al. (2024). The phylogenetic analysis showed 12 sections, sect. Delibuti, Phlegmacium, Anomali, Spilomei, Bolares, Camphorati, Dermocybe, Fusispori, Leprocybe, Subtorti, Liyuorum, and Orellani, and each section formed separate monophyletic lineages with strong statistical support.
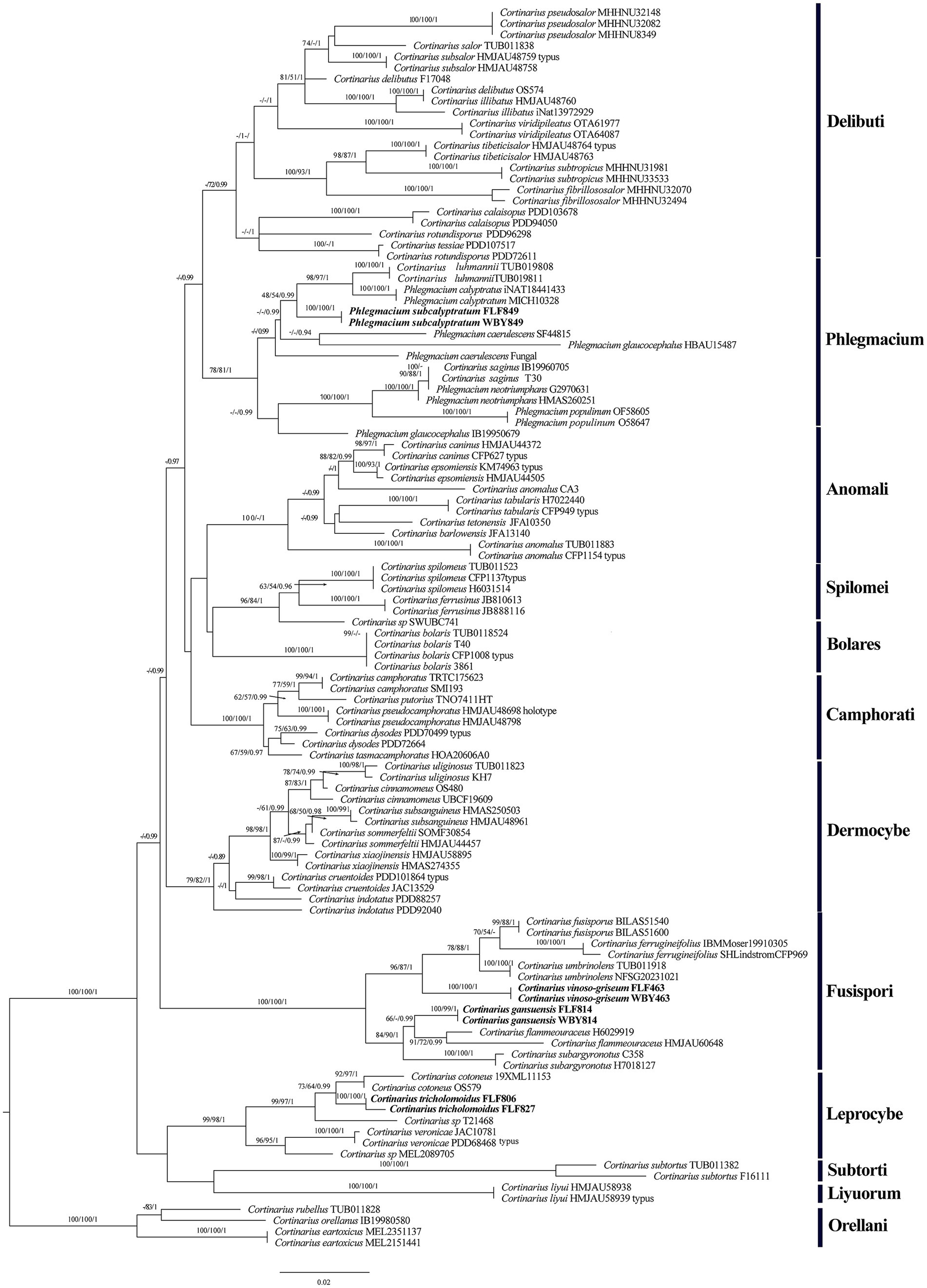
Figure 1. Phylogeny of Cortinarius s. l. by ML analysis based on the ITS + LSU dataset. The branches are labeled with parsimony bootstrap proportions, maximum likelihood bootstrap >50%, and Bayesian posterior probabilities >0.90. The new species are in bold.
The section Fusispori formed a distinct, high-supported clade (BP = 100 and BPP = 1) and was separated from other sections. Two new species, namely Cortinarius gansuensis and Cortinarius vinoso-griseum, nested within the sect. Fusispori clade. Cortinarius tricholomoidus, nested within the sect. Leprocybe clade, formed an independent lineage with high statistical support (100/100/1). It is worth noting that the collections of Phlegmacium subcalyptratum were nested within the sect. Phlegmacium clade (Figure 1).
4 Taxonomy
Cortinarius gansuensis B.Y. Wang, T.F. Ma & L.F. Fan, sp. nov. (Figures 2, 3).
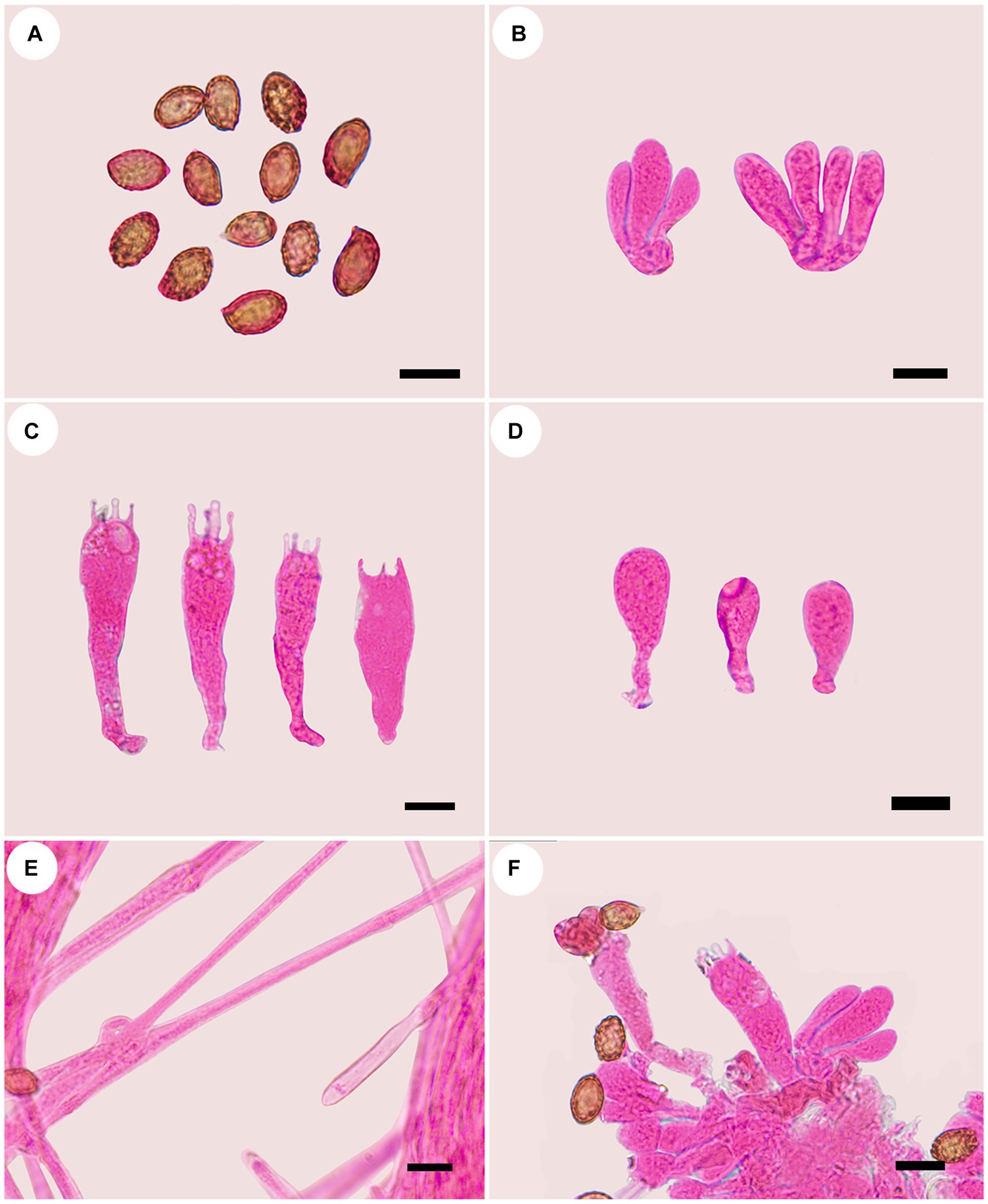
Figure 3. Microscopic structures of Cortinarius gansuensis (holotype, FLF 814). (A) Basidiospores; (B) Basidioles; (C) Basidia; (D) Cystidia; (E) Hyphae with clamp connections; (F) A section of the hymenium. Scales: 10 μm.
MycoBank No: 854910.
Etymology: Gansuensis (Latin) refers to the species found in Gansu Province.
Holotype: CHINA. Gansu Province, Zhuoni County, Dayu Valley, July 2023, FLF 814 (MHGAU).
Diagnosis: Differs from C. flammeouraceus by having longer basidiospores.
Description: Basidiomes small-to medium-sized. Pileus 1.6–3.6 cm, conical to hemispherical, convex to plane with an umbo, broadly umbonate at the center, margin incurved; at first brownish vinaceous, tinged darker brown at the center, with brown universal veil remains at the margin; surface silky when dry or glutinous when wet. Context thin, brown, soft. Lamellae adnate to adnexed, lilac, moderately distant, sometimes margin wavy. Stipe cylindrical to clavate, 3.8–4.5 cm long, 4.0–6.0 mm wide, brown, leaving a brownish ring on the upper stem, hollow. Odor indistinct.
Basidiospores [100/5/5] (8.1–)8.5–10.6(−11.3) × 5.4–6.8(−7.2) μm, av. 9.6 × 6.1 μm, Q = 1.6, ellipsoid, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia (28.0–)45.8–49.6 × (8.2–)8.6–10.7 μm, 4-spored, sterigmata up to 4.0 μm, clavate to subcylindrical, colorless or with granules. Pileipellis duplex, hyphae 58.0 μm wide, epicutis gelatinous, 35.0–50.0 μm thick, composed of colorless or amber yellow, irregularly arranged and strongly interwoven hyphae, hypocuits 25.0–40.0 μm thick, composed of colorless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia absent. Lamellar trama regular, 40.0–80.0 μm thick, composed of parallel arranged hyphae, hyphae 4.0–8.0 μm wide, with clamp connections. Stipitipellis gelatinous, stipe hyphae 9.0–11.0 μm wide, thin-walled, cylindrical, interwoven.
Habitat, ecology, and distribution: Solitary on mixed coniferous and broad-leaved forestland, known from Gansu, China, July to September. Additional specimens examined. China, Gansu Province: Zhuoni County, Taohe National Nature Reserve, at 29.769154°N, 110.086577°E, alt. 1,405 m, 14 September 2023, L.F. Fan and B.Y. Wang, (WBY 814, MHGAU).
Notes: Cortinarius gansuensis can be differentiated from other species of section Fusisori for its conical pileus, usually under mixed coniferous and broad-leaved forestland at 1,405–1,500 m. In addition, basidiospores ellipsoid, rarely subglobose, while other members in this section usually subglobose to broadly ellipsoid.
Cortinarius tricholomoidus B.Y. Wang, T.F. Ma & L.F. Fan, sp. nov. (Figures 4, 5).
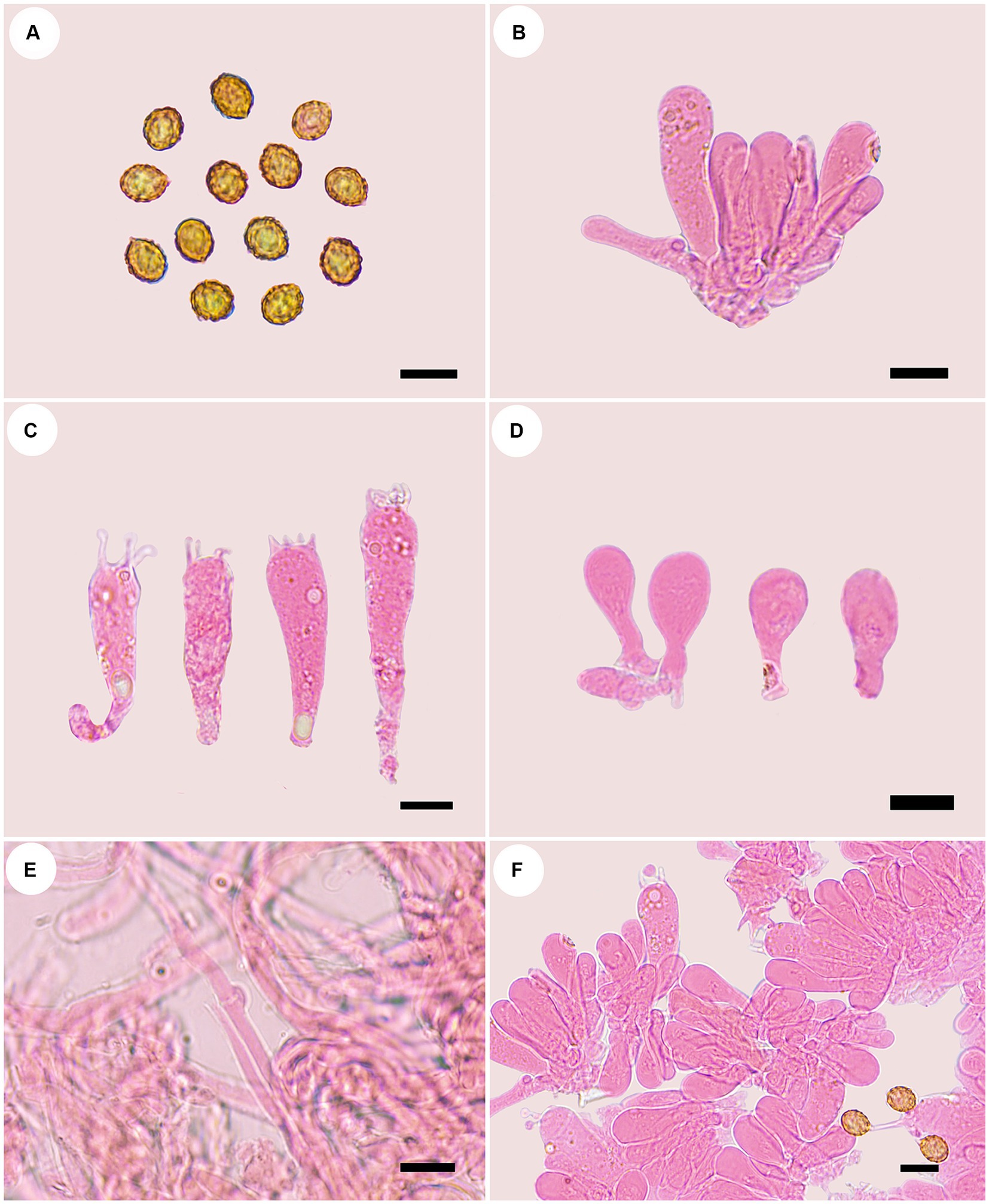
Figure 5. Microscopic structures of Cortinarius tricholomoidus (holotype, FLF 806). (A) Basidiospores; (B) Basidioles; (C) Basidia; (D) Cystidia; (E) Hyphae with clamp connections; (F) A section of the hymenium. Scales 10 μm.
MycoBank No: 854911.
Etymology: Tricholomoidus (Latin) refers to the stipe or the shape of this species being similar to Tricholoma spp.
Holotype: CHINA. Gansu Province, Zhuoni County, Boyu Valley, September 2023, FLF 806 (MHGAU).
Diagnosis: Differs from the C. gansuensis by its shorter stipe.
Description: Basidiomes small-to medium-sized. Pileus 3.0–6.0 cm, broadly umbonate at the center, margin incurved; at first snuff brown, tinged umber at the center, with brown universal veil remains at margin; surface finely felty. Context brown, soft. Lamellae adnate to adnexed, lilac, moderately distant. Stipe cylindrical to clavate, 6.1–7.6 cm long, 1.3–1.6 mm wide, brown, leaving a brownish ring on the upper stem, hollow. Odor indistinct.
Basidiospores [100/5/5] (7.1–)7.4–8.5(−8.7) × (5.9–)6.2–7.3(−7.4) μm, av. 7.98 × 6.78 μm, Q = 1.18, broadly ellipsoid to subglobose, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia 32.0–47.0 μm × 9.0–11.0 μm, four-spored, sterigmata up to 4.0 μm, clavate to subcylindrical, colorless or with granules. Pileipellis duplex, hyphae 4.0–8.0 μm wide, epicutis strongly gelatinous, 88.0–120.0 μm thick, composed of colorless or amber yellow, irregularly arranged and strongly interwoven hyphae, hypocuits 20.0–30.0 μm thick, composed of colorless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia absent. Lamellar trama regular, 50.0–60.0 μm thick, composed of parallel arranged hyphae, hyphae 4.0–8.0 μm wide, with clamp connections. Stipitipellis gelatinous, stipe hyphae 3.0–6.0 μm wide, thin-walled, cylindrical, interwoven.
Habitat, ecology and distribution: Usually solitary on coniferous forestland, from Gansu, China, July to September. Additional specimens examined. China, Gansu Province: Zhuoni County, Taohe National Nature Reserve, at 34.563761°N, 103.553064°E, alt. 2,845 m, 13 September 2023, L.F. Fan and B.Y. Wang, (FLF 827, MHGAU).
Notes: Cortinarius tricholomoidus can be differentiated from other species of section Leprocybe for its finely felty pileus, usually under coniferous forestland at 2,845 m. In addition, basidiospores broadly globose to subglobose.
Cortinarius vinoso-griseum B.Y. Wang, T. F. Ma & L.F. Fan sp. nov. (Figures 6, 7).
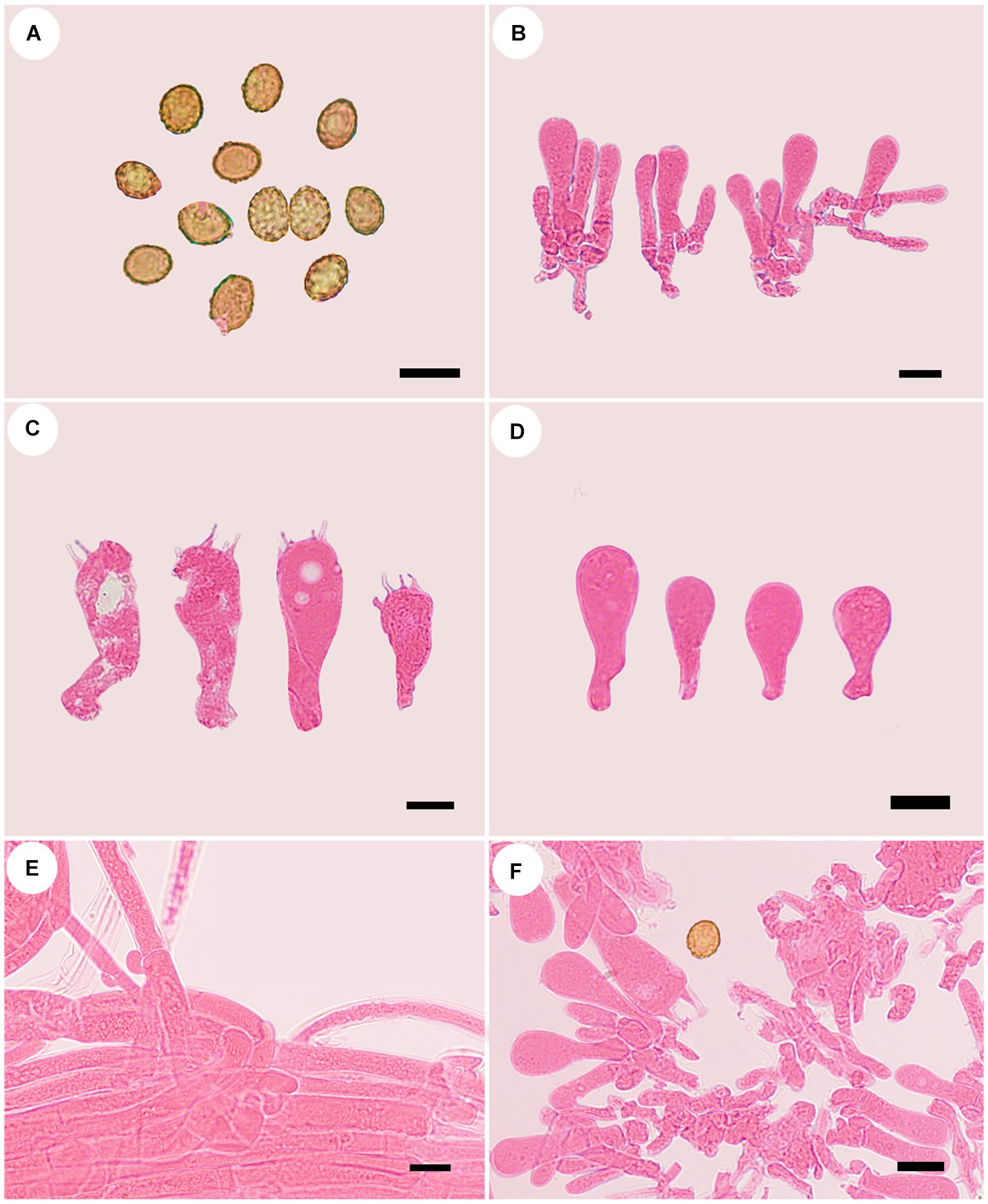
Figure 7. Microscopic structures of Cortinarius vinoso-griseum (holotype, FLF 463). (A) Basid-iospores; (B) Basidioles; (C) Basidia; (D) Basidioles; (E) Hyphae; (F) A section of the hymenium. Scales 10 μm.
MycoBank No: 854912.
Etymology: Vinoso-griseum (Latin) refers to the violaceous gray pileus.
Holotype: CHINA. Gansu Province, Zhuoni County, Dayu Valley, July 2023, FLF 463 (MHGAU).
Diagnosis: Differs from C. ferrugineifolius by its wider basidiospores.
Description: Basidiomes small-to medium-sized. Pileus 1.5–3.8 cm, at first broadly convex, then lower convex to plane, broadly umbonate at the center, margin incurved; at first violaceous gray, tinged cigar brown at the center, finely fibrillose, with brown universal veil remains at the margin; surface silky when dry or glutinous when wet. Context thin, creamy white, soft. Lamellae adnate to adnexed, lilac, moderately distant, sometimes margin wavy. Stipe cylindrical to clavate, gradually slender toward the apex, 3.2–4.9 cm long, 1.9–3.6 cm wide, violaceous buff when young then fading to white tint, leaving an ochraceous ring on the upper stem, hollow. Odor indistinct.
Basidiospores [100/5/5] 7.5–9.7× 5.6–7.8 μm, av. 9.7 × 7.8 μm, Q = 1.24, ellipsoid to broadly ellipsoid to subglobose, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia (15.0–) 25.0–40.0 × (6.2–)7.0–13.6(−14.5) μm, 4-spored, sterigmata up to 2.0–5.0 μm, clavate to subcylindrical, colorless or with granules. Pileipellis duplex, hyphae 7.0–14.0 μm wide, epicutis gelatinous, 58.0–108.0 μm thick, composed of colorless, irregularly arranged and strongly interwoven hyphae, hypocuits 22.0–35.0 μm thick, composed of colorless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia absent. Lamellar trama uniform, 45.0–60.0 μm thick, composed of parallel arranged hyphae, hyphae 6.0–8.0 μm wide. Stipitipellis gelatinous, stipe hyphae 11.0–13.0 μm wide, thin-walled, cylindrical, interwoven.
Habitat, ecology and distribution: Solitary to gregarious on mixed coniferous and broad-leaved forestland, from Gansu, China, July to September. Additional specimens examined. China, Gansu Province: Zhuoni County, Taohe National Nature Reserve, at 34.1175°N, 103.6293°E, alt. 2,800 m, 28 July 2022, L.F. Fan and B.Y. Wang, (WBY463, MHGAU).
Notes: Cortinarius vinoso-griseum can be differentiated from other species of section Fusispori for its violaceous gray pileus, usually distributed under mixed coniferous and broad-leaved forestland at 2,800 m. In addition, basidiospores broadly globose.
Phlegmacium subcalyptratum B.Y. Wang, T.F. Ma & L.F. Fan sp. nov. (Figures 8, 9).
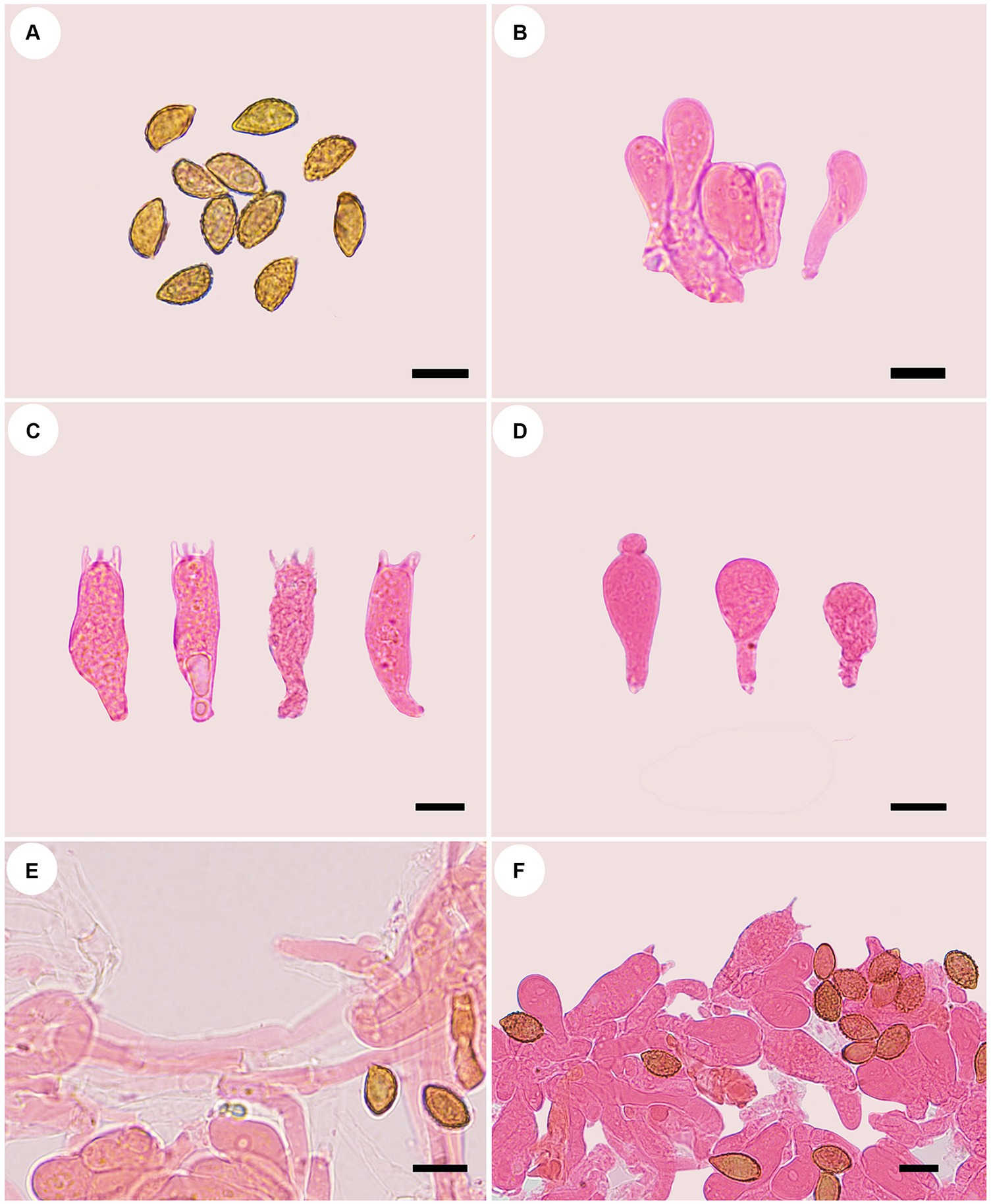
Figure 9. Microscopic structures of Phlegmacium subcalyptratum (holotype, FLF 849). (A) Ba-sidiospores; (B) Basidioles; (C) Basidia; (D) Cystidia; (E) Hyphae with clamp connections; (F) A section of the hymenium. Scales 10 μm.
MycoBank No: 854913.
Etymology: Subcalyptratum (Latin) refers to the species being similar to Phlegmacium calyptratum.
Holotype: CHINA. Gansu Province, Zhuoni County, Cheba Valley, September 2023, FLF 849 (MHGAU).
Diagnosis: Differs from P. calyptratum by its fibrillose pileus.
Description: Basidiomes small-to medium-sized. Pileus 3.8–4.0 cm, broadly convex, broadly umbonate at the center, margin incurved or decurved to upturned; at first apricot-orange, tinged coral at the center, smooth, with brown universal veil remains at the margin; surface silky when dry or glutinous when wet. Context thin, white, soft, lavender when bruised. Lamellae adnexed, fawn, moderately distant, sometimes margin wavy. Stipe cylindrical to clavate, slightly bent, gradually slender toward the apex, up to 6.0 cm long, 1.8 cm wide, cinnamon when young then fading to saffron tint, leaving an ochraceous ring on the upper stem, hollow. Odor indistinct.
Basidiospores [100/5/5] (9.7–) 10.0–12.7 (−13.6) × (4.6–) 5.6–6.8 (−7.3) μm, av. 11.3 × 6.2 μm, Qm = 1.82, fusiform, rarely subglobose, yellowish brown, moderately verrucose, without amyloid and dextrinoid reaction. Basidia (25.9–) 29.0–36.0 × 8.0–10.6(−11.5) μm, four-spored, sterigmata up to 2.6–5.0 μm, clavate to subcylindrical, colorless or with amber yellow oily inclusions or granules. Pileipellis duplex, hyphae 8.0–14.0 μm wide, with clamp connections, epicutis strongly gelatinous, 60.0–85.0 μm thick, composed of colorless or amber yellow, irregularly arranged and strongly interwoven hyphae, hypocuits 25.0–40.0 μm thick, composed of colorless or amber yellow, nearly parallel cylindrical hyphae. Lamellar edges fertile. Cystidia clavate, 14.3–19.4 × 9.6–11.7 μm. Lamellar trama regular, 45.0–80.0 μm thick, composed of parallel arranged hyphae, hyphae 5.0–7.0 μm wide. Stipitipellis gelatinous, stipe hyphae 5.0–11.0 μm wide, thin-walled, cylindrical, interwoven.
Habitat, ecology and distribution: Usually solitary on coniferous forest land, from Gansu, China, July to September. Additional specimens examined. China, Gansu Province: Zhuoni County, Taohe National Nature Reserve, at 34.737533 °N, 103.485595 °E, alt. 2,870 m, 31 July 2022, L.F. Fan and B.Y. Wang, (WBY 849, MHGAU).
Notes: Phlegmacium subcalyptratum can be differentiated from other species for its apricot-orange pileus, usually under coniferous forest land at 2,870 m. In addition, basidiospores fusiform.
5 Discussion
The main aim of this study was to carry out a molecular revision of Cortinarius s. l. species from Northwestern China and to define characteristics useful for delimiting and redefining species. The phylogenetic analysis showed 12 sections, sect. Delibuti, Phlegmacium, Anomali, Spilomei, Bolares, Camphorati, Dermocybe, Leprocybe, Subtorti, Liyuorum, Orellani, and Fusispori, with strong statistical support. However, the phylogenetic position of Cortinarius fusisporus and other taxa is still unclear as no supported sister relationship was revealed in the phylogenetic analysis, and the new clade, Fusispori clade, was proposed.
Cortinarius flammeouraceus and C. subargyronotus resemble C. gansuensis by having brown pilei, but C. flammeouraceus is different from C. gansuensis by having shorter basidiospores (7.5–8.5 μm vs. 8.5–10.6 μm, Niskanen, 2020); C. subargyronotus is different from C. gansuensis by having ellipsoid basidiospores (Liimatainen, 2014). Cortinarius evernius (Fr.) Fr. resembles C. gansuensis by having brown pilei and long stipe, but the latter has a white stipe and broader pilei (Malloch et al., 2024).
Cortinarius tricholomoidus is related to C. cotoneus, but C. cotoneus is different from C. tricholomoidus due to its olivaceus pileus (Liimatainen et al., 2022). Cortinarius tricholomoidus is similar to C. caninoide by having small-to-medium brown basidiomes with a bulbous stipe, but the latter has smaller basidiospores (6.1–7.5 × 3.8–4.7 μm vs. 7.4–8.5 × 6.2–7.3 μm, Malloch et al., 2024). Cortinarius hemitrichus resembles Cortinarius tricholomoidus by having a felty pileus, but the former has narrower basidiospores (4.2–5.8 μm vs. 6.2–7.3 μm, Xie, 2018).
According to our phylogenetic analysis, Cortinarius vinoso-griseum sisters to C. ferrugineifolius, C. fusisporus, and C. umbrinolens. However, C. ferrugineifolius is different from C. vinoso-griseum by having a violaceous gray pileus and shorter basidiospores (7.3–11.3 × 4.3–6.3 μm vs. 7.5–9.7 × 5.6–7.8 μm, Moser, 1993); C. fusisporus is different from C. vinoso-griseum by having larger basidiospores (9.5–11.5 μm vs. 7.5–9.7 μm, Kühner, 1955); C. umbrinolens has narrower basidiospores (5.6–7.8 vs. 4.3–4.8 μm, Xie, 2022). Cortinarius vinoso-griseum is easily confused with C. pseudobiformis in dark brown basidiomes, but the latter has strongly dextrinoid basidiospores (Malloch et al., 2024).
Phlegmacium subcalyptratum resembles Cortinarius armillatus and Cortinarius gentilis (Fr.) Fr. by having an orange pileus, but the latter has a bigger pileus (5–11 cm vs. 3.8–4 cm, Fries, 1838) and shorter basidiospores (7.8–9.7 μm vs. 10.0–12.7 μm).
Northwestern China boasts of the most significant virgin forests in the country, which serve as vital habitats for a variety of unique macrofungi. These forests create ideal environments for these specialized fungi to thrive in and play a crucial role in maintaining the delicate balance of the ecosystem (Xie, 2018; Zhou et al., 2022; Xie et al., 2022).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
LF: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. TM: Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing. HZ: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. BW: Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing. XJ: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Research Foundation of Gansu Agricultural University for Advanced Talents, grant number GAU-KYQD-2021-26, the Gansu Provincial University Foundation for Youth Doctors, grant number 2022QB-080, and the National Natural Science Foundation of China, Project Nos. 32460005.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1454736/full#supplementary-material
References
Ammirati, J. F., Liimatainen, K., Bojantchev, D., Peintner, U., Kuhnert-Finkernagel, R., Cripps, C., et al. (2021). Cortinarius subgenus Leprocybe, unexpected diversity and signifcant diferences in species compositions between western and eastern North America. Persoonia 46, 216–239. doi: 10.3767/persoonia.2021.46.08
Royal Botanic Garden Edinburgh . (1969). Flora of British Fungi. Colour Identification Chart. London: Her Majesty’s Stationery Office.
Bidaud, A., Loizides, M., Armada, F., de Dios Reyes, J., Carteret, X., Corriol, G., et al. (2021). Cortinarius subgenus Leprocybe in Europe: expanded sanger and next generation sequencing unveil unexpected diversity in the med-iterranean. Persoonia 46, 188–215. doi: 10.3767/persoonia.2021.46.07
Brandrud, T. E. (1998). Cortinarius subgenus Phlegmacium section Phlegmacioides (= Variecolores) in Europe. Edinb. J. Bot. 55, 65–156. doi: 10.1017/S0960428600004364
Dentinger, B. T., Gaya, E., O'Brien, H., Suz, L. M., Lachlan, R., Díaz-Valderrama, J. R., et al. (2016). Tales from the crypt: genome mining from fungarium specimens improves resolution of the mushroom tree of life. Biol. J. Linn. Soc. 117, 11–32. doi: 10.1111/bij.12553
Dima, B., Liimatainen, K., Niskanen, T., Bojantchev, D., Harrower, E., Papp, V., et al. (2021). Type studies and fourteen new North American species of Cortinarius section Anomali reveal high continental species diversity. Mycol. Prog. 20, 1399–1439. doi: 10.1007/s11557-021-01738-0
Fan, L. F., Alvarenga, R. L. M., Gibertoni, T. B., Wu, F., and Dai, Y. C. (2021). Four new species in the Tremella fibulifera complex (Tremellales, Basidiomycota). MycoKeys 82, 33–56. doi: 10.3897/mycokeys.82.63241
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Von Haeseler, A., and Jermiin, L. S. (2017). Model finder: fast model selec-tion for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Kornerup, A., and Wanscher, J. H. (1978) in Methuen Handbook of Color. ed. E. Methuen . 3rd ed (London: Methuen and Co., Ltd).
Kühner, R. (1955). Complements à la Flore Analytique IV. Espèces nouvelles ou critiques des Cortinaires. Bull. Mens. Soc. Linn. Lyon. 24, 39–54.
Liimatainen, K., Kim, J. T., Pokorny, L., Kirk, P. M., Dentinger, B., and Niskanen, T. (2022). Taming the beast: a revised classification of Cortinariaceae based on genomic data. Fungal Divers. 112, 89–170. doi: 10.1007/s13225-022-00499-9
Long, P., Zhou, S. Y., Li, S. N., Liu, F. F., and Chen, Z. H. (2024). Three new species of Cortinarius section Delibuti (Cortinariaceae, Agari-cales) from China. MycoKeys 101, 143–162. doi: 10.3897/mycokeys.101.114705
Malloch, D., Justo, A., and Ammirati, J. (2024). Recent records of telamonioid species of Cortinarius (Agaricales: Cortinariaceae) in New Brunswick, Canada. Can. Field Nat. 137, 13–25. doi: 10.22621/cfn.v137i1.3047
Matheny, P. B., Moreau, P. A., Vizzini, A., Harrower, E., De Haan, A., Contu, M., et al. (2015). Crassisporium and Romagnesiella: two new genera of dark-spored Agaricales. Syst. Biodivers. 13, 28–41. doi: 10.1080/14772000.2014.967823
Moser, M. (1993). Studies on north American Cortinarii III. The Cortinarius of dwarf and shrubby Salix associations in the alpine zone of the Windriver Mountains, Wyoming, USA. Sydowia 45, 275–306.
Niskanen, T., Liimatainen, K., Kytövuori, I., Lindström, H., Dentinger, B., and Ammirati, J. F. (2016). Cortinarius subgenus Callistei in North America and Europe-type studies, diversity, and distribution of species. Mycologia 108, 1018–1027. doi: 10.3852/16-033
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice, across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Soop, K., Dima, B., Cooper, J. A., Park, D., and Oertel, B. (2019). A phylogenetic approach to a global supraspecific taxonomy of Cortinarius (Agaricales) with an emphasis on the southern mycota. Persoonia 42, 261–290. doi: 10.3767/persoonia.2019.42.10
White, T. J., Bruns, T. D., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics” in PCR Protocols: A Guide to Methods and Applications. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (US: New York Academic Press), 315–322.
Xie, M.L. (2018). Resources and taxonomy of Cortinarius in northeast of China. Master’s thesis. Jilin Agricultural University, Jilin.
Xie, M.L. (2022). Taxonomic, molecular phylogenetic and biogeographic studies of Cortinarius in China. Dissertation thesis. Northeast Normal University, Jilin.
Xie, M. L., Phukhamsakda, C., Wei, T. Z., Li, J. P., Wang, K., and Wang, Y. (2022). Morphological and phylogenetic evidence reveal five new telamonioid species of (Agaricales) from East Asia. J. Fungi. 8:257. doi: 10.3390/jof8030257
Zhang, Q. Y., Jin, C., Zhou, H. M., Ma, Z. Y., Zhang, Y. Z., Liang, J. Q., et al. (2023). Enlargement of the knowledge of Cortinarius section Anomali (Agaricales, Basidiomycota): introducing three new species from China. Front. Cell. Infect. Mi. 13:1215579. doi: 10.3389/fcimb.2023.1215579
Keywords: Cortinarius, ITS + LSU, morphology, phylogeny, taxonomy
Citation: Fan L, Zhong X, Ma T, Zhou H, Wang B and Ji X (2024) Four new species of Cortinariaceae (Agaricales) from Northwestern China. Front. Microbiol. 15:1454736. doi: 10.3389/fmicb.2024.1454736
Edited by:
Zhou Shi, Gladstone Institutes, United StatesReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaJing Si, Beijing Forestry University, China
Copyright © 2024 Fan, Zhong, Ma, Zhou, Wang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longfei Fan, ZmFubGZAZ3NhdS5lZHUuY24=; Xiaohong Ji, eGhqaTIwMTBAMTYzLmNvbQ==
 Longfei Fan
Longfei Fan Xue Zhong
Xue Zhong Tianfu Ma1
Tianfu Ma1