- Institute of Biopharmaceutical Research, Liaocheng University, Liaocheng, Shandong, China
The infection of the central nervous system (CNS) with neurotropic viruses induces neuroinflammation and an immune response, which is associated with the development of neuroinflammatory and neurodegenerative diseases, including multiple sclerosis (MS). The activation of both innate and adaptive immune responses, involving microglia, macrophages, and T and B cells, while required for efficient viral control within the CNS, is also associated with neuropathology. Under pathological events, such as CNS viral infection, microglia/macrophage undergo a reactive response, leading to the infiltration of immune cells from the periphery into the brain, disrupting CNS homeostasis and contributing to the pathogenesis of disease. The Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelination disease (TMEV-IDD), which serves as a mouse model of MS. This murine model made significant contributions to our understanding of the pathophysiology of MS following subsequent to infection. Microglia/macrophages could be activated into two different states, classic activated state (M1 state) and alternative activated state (M2 state) during TMEV infection. M1 possesses the capacity to initiate inflammatory response and secretes pro-inflammatory cytokines, and M2-liked microglia/macrophages are anti-inflammatory characterized by the secretion of anti-inflammatory cytokines. This review aims to discuss the roles of microglia/macrophages M1/M2-liked polarization during TMEV infection, and explore the potential therapeutic effect of balancing M1/M2-liked polarization of microglia/macrophages on MS.
1 Introduction
Theiler’s murine encephalomyelitis virus (TMEV) belongs to the genus cardiovirus of the picornaviridae family (Gerhauser et al., 2019). The pathogenesis of TMEV involves a complex interaction between viral infection and the host immune response, particularly the activation of glial cells and the immune response to the central nervous system (CNS) (DePaula-Silva et al., 2021). Microglia and macrophagesare highly engaged in the neuroinflammatory process during viral encephalitis and are key contributors to the initiation of the innate and adaptive immune response (Bosco et al., 2020; Filgueira et al., 2021). TMEV-induced demyelinating disease (TMEV-IDD) represents a well-established animal model for demyelinating diseases in humans, especially resembling significant characteristics of the progressive forms of MS (Pike et al., 2022). This review will focus on the role of microglia and infiltrating peripheral macrophages TMEV-IDD model, highlighting the contribution of M1/M2-liked phenotypic changes in microglia /macrophages to disease progression during TMEV infection.
2 Overview of TMEV
TMEV can be classified into two subgroups, based on neurovirulence: highly neurovirulent (George Davis 7–GDVII) and low neurovirulent (Theiler’s original—TO). The strains of GDVII and FA are contained within the GDVII subgroup. Among the strains in the TO subgroup are the DA, BeAn 8,386 (BeAn), TO4, Yale, WW, and 4,727 (Jarousse et al., 1998; Pavelko et al., 2012; Brinkmeyer-Langford et al., 2017; Sato et al., 2017). The highly neurovirulent strains can cause fatal encephalomyelitis in mice or mice die within 1–2 weeks of infection (Jarousse et al., 1998; Pavelko et al., 2012; Brinkmeyer-Langford et al., 2017; Sato et al., 2017). In contrast, the low neurovirulent strains cause different diseases depending on the mouse strain (DePaula-Silva et al., 2017). The intracerebral (i.c.) infection of Swiss Jim Lambert (SJL) mice with low neurovirulent strains, such as DA or BeAn, causes encephalitis during the acute stage of infection and demyelinating disease during the chronic stage of infection develops a MS-like disease termed TMEV-induced demyelinating disease (TMEV-IDD) (Daniels et al., 1952; Lipton, 1975; Libbey et al., 2008; Tsunoda and Fujinami, 2010), while the C57BL/6J mouse strain, when i.c. infected with TMEV, develops acute seizures that progress to epilepsy (Libbey et al., 2008). The difference between the mouse strains seems to be partially explained by the strong antiviral innate immune response, for example type I interferon (IFN) in C57BL/6J mice (Rodriguez et al., 1995).
The TMEV model is an important tool for the study of neuroinflammatory and neurodegenerative diseases, providing a key platform for investigating the pathophysiological mechanisms of the diseases and developing potential therapeutic strategies. The following sections will focus primarily on the TMEV-IDD model by describing the roles of microglia and macrophages.
3 MS
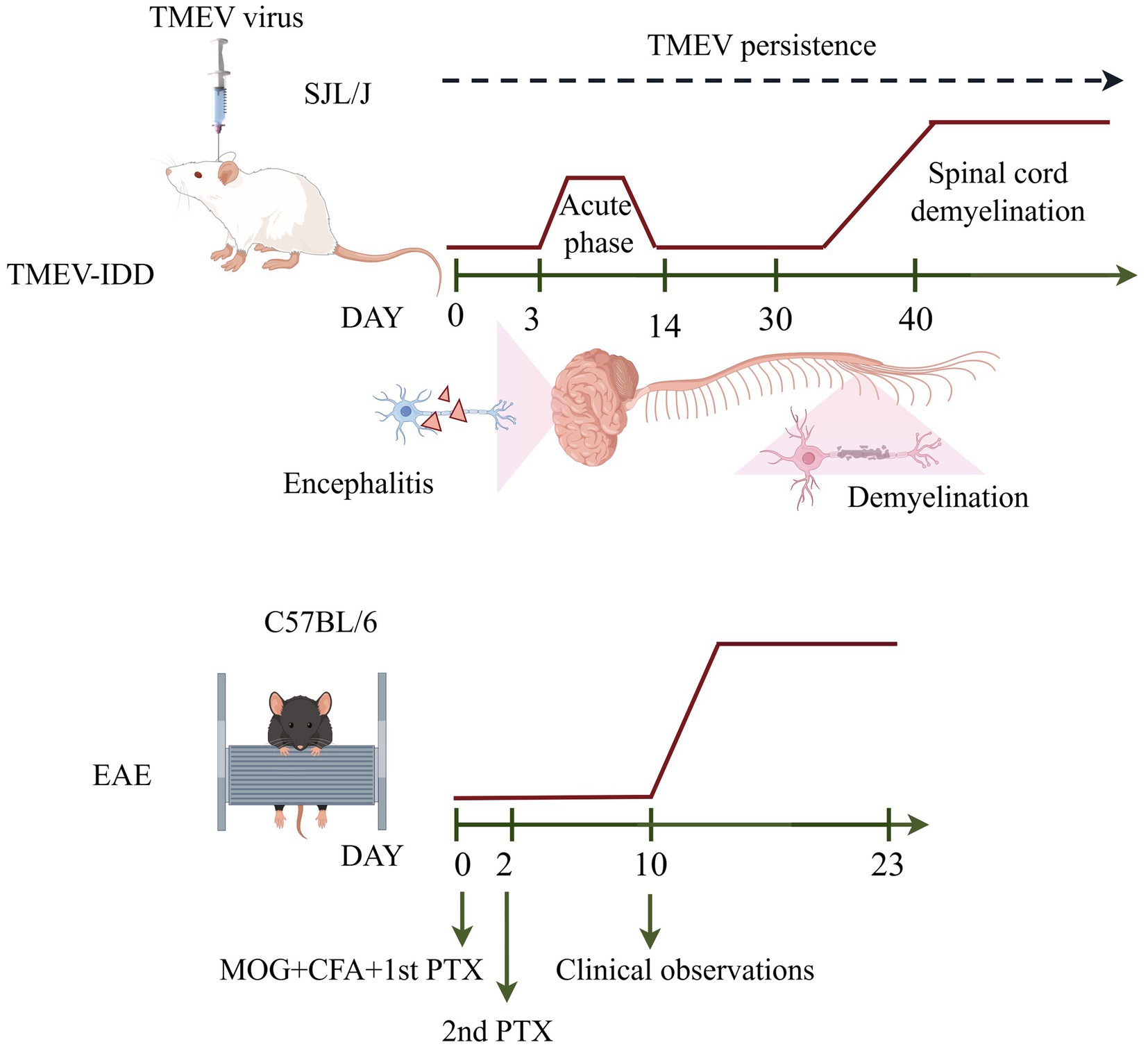
MS is a cell-mediated chronic progressive neuroinflammatory and neurodegenerative autoimmune disease of the CNS characterized by inflammatory demyelination, axonal damage and progressive neurological dysfunction (DePaula-Silva, 2024). MS shows clear geographic variations, with higher rates in Europe and North America and lower rates in sub-Saharan Africa and East Asia, but its overall prevalence is increasing globally (Walton et al., 2020). MS usually develops between the age of 20 and 50 and is more frequently diagnosed in women (Rolak, 2003). As an autoimmune disease, the host immune system attacks its own myelin proteins. In individuals with MS, due to the myelin destruction, the saltatory conduction is impaired resulting in inefficient (Zhang et al., 2022). Signs and symptoms of the disease include cognitive and motor impairment, vertigo, loss of vision, weakness and dementia (Magyari, 2016; Psenicka et al., 2021). The pathogenesis of MS is not yet clear, but genetic and environmental factors may be strongly associated with the development of the disease (Davis et al., 2013). The experimental autoimmune encephalomyelitis (EAE) animal model and TMEV-IDD animal model are the most commonly used animal models for studying MS. The choice of the most precise model is predominantly influenced by the particular research and/or experimental question to be addressed. The main stages in the pathogenesis of EAE and TMEV-IDD are shown in Table 1, and schematic representation of mouse models of EAE and the TMEV-IDD are given in Figure 1.
Recent research has revealed several promising strategies to improve the process of MS. For example, Bernardo-Faura et al. found that TAK1 inhibitors, in combination with existing MS drugs, significantly improved MS in an animal model of the disease, based on network-based modelling (Bernardo-Faura et al., 2021). Similarly, Kerstetter et al. discovered that the blockade of chemokine receptors, such as CXCR2, promotes the regeneration of myelin sheaths and enhances functional recovery in MS models (Kerstetter et al., 2009). Furthermore, mTOR inhibitors like rapamycin have demonstrated efficacy in reducing disease severity in MS models by balancing the immune response and promoting oligodendrocyte survival (Vakrakou et al., 2022). Another crucial pathway, the Keap1/Nrf2/ARE pathway, is essential for the regulation of oxidative stress and inflammation in MS (Michaličková et al., 2020), offering potential therapeutic targets for future interventions.
3.1 TMEV-IDD, an animal model of progressive MS
TMEV-IDD, which is a model for progressive forms of MS (Pike et al., 2022). TMEV-IDD in SJL/J mice is characterized by an acute phase, that occurs first week post-infection, which have high viral replication in neurons, and a chronic phase, which begins within 1 month after TMEV inoculation, marked by persistent infection in glial cells and chronic demyelination (Tsunoda and Fujinami, 2010). During the chronic phase of the disease, SJL/J mice infected with TMEV exhibit progressive weakness in their hind limbs, leading to severe spastic paralysis with no observed recovery, similar to what is observed in patients with the primary progressive multiple form of MS (Tsunoda and Fujinami, 2002; DePaula-Silva et al., 2017). In this disease model, the presence of demyelination in the CNS is associated with a prolonged inflammatory immune reaction caused by the persistence of the virus.
Toll Like Receptors (TLR) recognize pathogens through pathogen-associated molecular patterns (PAMPs), triggering the innate immune response and inducing pro-inflammatory chemokines and cytokines to recruit immune cells. Viral infections lead to the induction of type I IFN and IFN-γ cytokines. Activation of the type I IFN pathway increases levels of IFN-α and IFN-β and the type II IFN-γ. Activation of the type I IFN pathway leads to increased levels of expression of interferon-stimulated genes (ISGs), which can exert an antiviral effect (Biron, 2001; Olson and Miller, 2004; Olson, 2014; Bolívar et al., 2018).
In the acute phase, neurons within the hippocampus and cerebral cortex are predominantly infected (Gerhauser et al., 2019). Elevated levels of CD4+ and CD8+ T cells, monocytes, and a small number of B cells and plasma cells have been detected in the grey matter of the brain, indicative of CNS inflammation (encephalitis) (Frischer et al., 2009; Michel et al., 2015; DePaula-Silva et al., 2017; Faissner et al., 2019). Following the initial acute phase, a reduction in viral load occurs. However, the immune response is insufficient to fully eradicate the viral infection, resulting in progression to the chronic phase. During this chronic phase, inflammatory cells persist in the white matter while demyelination in the spinal cord and axonal damage are also observed (Martinat et al., 1999; Kummerfeld et al., 2012). Meanwhile, TMEV-infected SJL/J mice exibit a notable decrease in the quantity of neuronal progenitor cells and early postmitotic neurons in hippocampus at the chronic phase. Deficits in hippocampal neurogenesis observed following TMEV-infected SJL/J mice have mirrored the learning and memory impairments (Jafari et al., 2012). Unlike the acute phase, TMEV is not localized in neurons but instead remains present in oligodendrocytes, astrocytes, and microglia/macrophages, as evidenced by immunohistochemistry and in situ hybridization techniques (Clatch et al., 1990; Girard et al., 2002; Misra et al., 2008). Therefore, while TMEV persistence is important to induce demyelination, the trigger mechanism for demyelination is inflammation and the induction of autoimmunity.
4 Profile of microglia and macrophages
Microglia are the innate immune cells in the CNS, accounting for approximately 10–15% of all brain cells (Onose et al., 2022). They originate from myeloid lineage in the yolk sac and infiltrate into the CNS during embryonic development (Ginhoux et al., 2010). In contrast, peripheral macrophages/monocytes are derived from adult bone marrow hematopoietic stem cells, under pathological conditions, monocytes can infiltrate the CNS and differentiate into macrophages. Both microglia and macrophages can drive neuroinflammation and promote the development of MS (Zaslona et al., 2012; Jiang et al., 2022).
In vitro experiments have demonstrated that microglia can sustain a persistent infection with TMEV, enabling them to induce a pro-inflammatory state in distal, uninfected cells through the secretion of viral RNA-laden exosomes. These exosomes subsequently activate microglia, enhancing their ability to process viral and myelin epitopes and to prime memory CD4+ Th1 cells. Intriguingly, direct infection of microglia with TMEV proves almost as effective as high levels of IFN-γ stimulation in imparting antigen-presenting cell (APC) functionality (Olson et al., 2001; Luong and Olson, 2021). Highly purified macrophages, isolated from mice infected with TMEV, exhibit exceptionally potent APC capabilities, akin to those of microglia, particularly in the context of chronically induced demyelinating disease (Chastain et al., 2011).
Microglia exhibit many functional and phenotypic similarities to peripheral macrophage due to their common myeloid lineage, including the presence of similar surface molecules, which makes it difficult to distinguish the two populations of cells in the CNS during an immune response to TMEV, such as CD 11b. However, microglia can be distinguished from macrophage by their lower expression level of CD45 (Aloisi, 2001). In addition, DePaula-Silva et al. studied the functional distinctions and commonalities in the gene expression profiles of microglial and macrophage immune responses during neurotropic viral infection. Among these genes, 43 were found to be expressed exclusively by infiltrating macrophages, 43 were uniquely expressed by reactive microglia, and 50 were identified as being commonly expressed in both infiltrating macrophages and reactive microglia. They suggested the identified and validated genes that are uniquely expressed at the cell surface of microglia or macrophages, which can be used to distinguish between these two cell populations (DePaula-Silva et al., 2019).
4.1 The role of microglia and infiltrating macrophage in demyelination following TMEV CNS infection
The role of microglia/macrophages in TMEV-IDD is partially related to the ability of virus to persist in these cells. During the acute period, the numbers of activated microglia were increased in the brains of SJL/J mice after TMEV infection, which inhibited the survival of neural progenitor cells and impeded neuronal differentiation, ultimately leading to hippocampal neuropathy (Ekdahl et al., 2003; Monje et al., 2003; Jafari et al., 2012). Moreover, during demyelination, microglia/macrophages are found near the lesion site, which contain TMEV virus antigens. Microglia/macrophages are capable of efficiently engulfing viral particles through phagocytosis. Although studies have demonstrated persistent infection of microglia/macrophages by TMEV in SJL mice, these cells exhibit low permissiveness for viral replication, and produced few viral particles during TMEV infection (Rodriguez et al., 1983; Clatch et al., 1990; Lipton et al., 1995; Rossi et al., 1997; Trottier et al., 2001). Microglia are capable of detecting a viral infection or cell-associated damage signals through pattern recognition receptors (PRRs) and damage-associated molecular patterns (DAMPs), respectively, leading to their activation. Reactive inflammatory microglia trigger the expression of Type-I IFN (IFN-I), and NF-κB, thus up-regulating the expression of inflammatory mediators including IL-6, IL-12, tumor necrosis factor (TNF)α, CCL2, CCL3, and CCL5 (Olson et al., 2001; Olson and Miller, 2009; Kim, 2021; Luong and Olson, 2021). In addition, excessive or continuous activation of microglia/macrophages leads to local tissue damage/immunopathology in CNS (DePaula-Silva et al., 2017). Activated microglia increase the expression of MHC-I and MHC-II, and costimulatory molecules, enhancing their ability to present antigens to T cells (Schetters et al., 2017; Moseman et al., 2020; Goddery et al., 2021). Presentation of viral antigens to Th1 CD4+ T cells results in the secretion of chemokines by these T cells, thereby enhancing the recruitment of peripheral macrophages. The excess of CNS inflammation causes bystander myelin damage. In TMEV-infected mice, macrophages and microglia have the ability to uptake myelin antigens, then presenting them to autoreactive myelin-specific CD4+ T cells (DePaula-Silva et al., 2017).
Microglia and macrophage activation is an initial occurrence in MS/TMEV and persists throughout the course of the disease (Gentile et al., 2016; Bell et al., 2020). Sustained expression of M1-liked related genes in microglia/macrophage leads to persistent inflammation and hindered myelin regeneration (Herder et al., 2015). Furthermore, M1-liked microglia are known to secrete proteolytic enzymes, including matrix metalloproteinases (MMPs), which contribute to the degradation of myelin basic protein (MBP) and subsequent direct damage to myelin (Liuzzi et al., 1995; Ulrich et al., 2006). Hansmann et al. indicates that deficiency in MMP12 is linked to decreased activation of M1-liked microglia during the TMEV demyelinating phase, suggesting a significant involvement of MMP12 in the activation and polarization of microglia (Hansmann et al., 2019). Additionally, TMEV can trigger the activation of NLRP3 inflammasome through the Toll-like receptor (TLR) signaling pathway. NLRP3 inflammasome, function as signaling platforms for caspase-1-driven activation of IL-1-type cytokines (IL-1β and IL-18), which contributed to the neuroinflammatory response in the CNS (Tsunoda and Fujinami, 2010; Gerhauser et al., 2012; Jeong et al., 2022). Activation of the NLRP3 inflammasome and the downstream PGE2 promote the pathogenesis of TMEV-induced demyelinating disease by enhancing the production of IL-17 (Kim et al., 2017). Moreover, NLRP3 polarizes microglia/macrophage toward a pro-inflammatory M1-liked phenotype, promote microglia/macrophage-mediated neuroinflammation and contribute to neuroprogression (Wang et al., 2020; Yang et al., 2022). As discussed previously, M1-liked microglia/macrophage have detrimental effects in the course of MS/TMEV (Mikita et al., 2011; Guo et al., 2022). Nevertheless, it does not mean that pro-inflammatory mediators such as M1-liked microglia /macrophage and Th1 cells only contribute adversely to the pathogenesis of MS/EAE. Differentiation of appropriate numbers of M1-liked microglia have been shown to promote neurogenesis and oligogenesis, whereas excessive activation has been found to be inhibitory (Butovsky et al., 2006a,b). In contrast, M2-liked microglia/macrophage are regulatory/anti-inflammatory and secrete regulatory cytokines such as IL-10 and TGF-β, promoting tissue repair and resolving inflammation within the CNS by downregulating M1 and Th1 immune responses (Laskin, 2009; Herder et al., 2015). Park HJ et al. demonstrated the important contribution of M2-liked microglia/macrophage in the clearance of debris and support of neuronal survival by releasing neurotrophic factors and participating in phagocytosis (Oñate et al., 2016; Park et al., 2016).
It has been revealed that an imbalance of M1-liked cells in the spinal cord of infected mice (Miron et al., 2013; Colombo et al., 2014; Hansmann et al., 2019). In addition, TMEV preferentially infects activated myeloid cells that exhibit pro-inflammatory functions M1-like, which expressed CD16/32 and IFN-γ, in vitro (Jelachich and Lipton, 1999). It is also tempting to speculate that prolonged M1-liked polarization contributes to viral persistence in susceptible mouse strains by creating an environment conducive to the virus. With disease progression an accumulation of neuroprotective M2-liked cells was found. Despite mounting M2-liked polarization and the expression of regeneration promoting factors, such as Tgfb1, CNS recovery restricted and only insufficient remyelination attempts by oligodendrocytes and Schwann cells were found in the spinal cord during the late chronic TME phase (Herder et al., 2015). M2-liked microglia/macrophages were not reverse the long-term damage to the CNS caused by M1-liked microglia/macrophages in TMEV-IDD.
5 Drugs targeting microglia/macrophages
Various pharmacological interventions aimed at altering the behavior of microglia have demonstrated potential in the treatment of TMEV infection and MS by modulating immune responses, reducing inflammation, and promoting remyelination. Qie et al. discovered that candesartan, an AT1 receptor blocker (ARB), effectively regulated the neuroinflammatory response, reversed neurotoxic effects, and induced a shift in microglia from the M1-liked to M2-liked phenotype, at least in part through the inhibition of the TLR4/NF-κB signaling pathway (Qie et al., 2020). Edetomidine, an agonist of the alpha2 adrenoceptor, induces M2-liked microglia polarization through the inhibition of ERK1/2 signaling (Qiu et al., 2020). Shen et al. have engineered neutrophil-derived nanovesicles (NNV) to modulate neuroinflammation by enhancing the clearance of myelin debris in microglia (Shen et al., 2022). Additionally, Zheng et al. have developed a formulation called resveratrol-containing RAW-Exo (RSV&Exo) that targets microglia to mitigate neurodegeneration (Zheng et al., 2023). Mecha et al. found that the administration of the endogenous cannabinoid 2-AG selectively targets microglia, improving their capacity to clear myelin and promoting the differentiation of oligodendrocyte progenitor cells, thereby enhancing myelin regeneration (Mecha et al., 2019). In a MS animal model of EAE, lipoic acid was reported to reduce microglia/macrophages activation and the occurrence of MS disease (Kamma et al., 2022). It seems the mechanisms underlying microglia/macrophages polarization are complex, and combining different mechanisms together may have a helpful effect in the therapy of MS. Relationship between the pathogenesis of MS and microglia/macrophages polarization remains to be studied.
Author contributions
QZ: Funding acquisition, Visualization, Writing – original draft, Writing – review & editing, Data curation. WS: Data curation, Visualization, Writing – review & editing, Formal analysis. MZ: Data curation, Formal analysis, Visualization, Writing – review & editing. NZ: Visualization, Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Open Project of Liaocheng University Animal husbandry discipline (No. 319312105-21), Guangyue Young Scholar Innovation Team of Liaocheng University (No. LCUGYTD2023-03). This work was also technically supported by Shandong Collaborative Innovation Center for Antibody Drugs and Engineering Research Center for Nanomedicine and Drug Delivery Systems.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhazzani, K., Ahmad, S. F., Al-Harbi, N. O., Attia, S. M., Bakheet, S. A., Sarawi, W., et al. (2021). Pharmacological inhibition of STAT3 by Stattic ameliorates clinical symptoms and reduces autoinflammation in myeloid, lymphoid, and neuronal tissue compartments in relapsing-remitting model of experimental autoimmune encephalomyelitis in SJL/J mice. Pharmaceutics 13. doi: 10.3390/pharmaceutics13070925
Bell, L. A., Wallis, G. J., and Wilcox, K. S. (2020). Reactivity and increased proliferation of NG2 cells following central nervous system infection with Theiler's murine encephalomyelitis virus. J. Neuroinflammation 17:369. doi: 10.1186/s12974-020-02043-5
Berard, J. L., Wolak, K., Fournier, S., and David, S. (2010). Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia 58, 434–445. doi: 10.1002/glia.20935
Bernardo-Faura, M., Rinas, M., Wirbel, J., Pertsovskaya, I., Pliaka, V., Messinis, D. E., et al. (2021). Prediction of combination therapies based on topological modeling of the immune signaling network in multiple sclerosis. Genome Med. 13:117. doi: 10.1186/s13073-021-00925-8
Biron, C. A. (2001). Interferons alpha and beta as immune regulators--a new look. Immunity 14, 661–664. doi: 10.1016/s1074-7613(01)00154-6
Bolívar, S., Anfossi, R., Humeres, C., Vivar, R., Boza, P., Muñoz, C., et al. (2018). IFN-β plays both pro- and anti-inflammatory roles in the rat cardiac fibroblast through differential STAT protein activation. Front. Pharmacol. 9:1368. doi: 10.3389/fphar.2018.01368
Bosco, D. B., Tian, D. S., and Wu, L. J. (2020). Neuroimmune interaction in seizures and epilepsy: focusing on monocyte infiltration. FEBS J. 287, 4822–4837. doi: 10.1111/febs.15428
Brinkmeyer-Langford, C. L., Rech, R., Amstalden, K., Kochan, K. J., Hillhouse, A. E., Young, C., et al. (2017). Host genetic background influences diverse neurological responses to viral infection in mice. Sci. Rep. 7:12194. doi: 10.1038/s41598-017-12477-2
Bullard, D. C., Hu, X., Adams, J. E., Schoeb, T. R., and Barnum, S. R. (2007). p150/95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. Am. J. Pathol. 170, 2001–2008. doi: 10.2353/ajpath.2007.061016
Butovsky, O., Landa, G., Kunis, G., Ziv, Y., Avidan, H., Greenberg, N., et al. (2006a). Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J. Clin. Invest. 116, 905–915. doi: 10.1172/jci26836
Butovsky, O., Ziv, Y., Schwartz, A., Landa, G., Talpalar, A. E., Pluchino, S., et al. (2006b). Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 31, 149–160. doi: 10.1016/j.mcn.2005.10.006
Chastain, E. M., Duncan, D. S., Rodgers, J. M., and Miller, S. D. (2011). The role of antigen presenting cells in multiple sclerosis. Biochim. Biophys. Acta 1812, 265–274. doi: 10.1016/j.bbadis.2010.07.008
Clatch, R. J., Miller, S. D., Metzner, R., Dal Canto, M. C., and Lipton, H. L. (1990). Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV). Virology 176, 244–254. doi: 10.1016/0042-6822(90)90249-q
Colombo, L., Parravicini, C., Lecca, D., Dossi, E., Heine, C., Cimino, M., et al. (2014). Ventral tegmental area/substantia nigra and prefrontal cortex rodent organotypic brain slices as an integrated model to study the cellular changes induced by oxygen/glucose deprivation and reperfusion: effect of neuroprotective agents. Neurochem. Int. 66, 43–54. doi: 10.1016/j.neuint.2014.01.008
Constantinescu, C. S., Farooqi, N., O'Brien, K., and Gran, B. (2011). Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x
Daniels, J. B., Pappenheimer, A. M., and Richardson, S. (1952). Observations on encephalomyelitis of mice (DA strain). J. Exp. Med. 96, 517–530. doi: 10.1084/jem.96.6.517
Davis, M. F., Sriram, S., Bush, W. S., Denny, J. C., and Haines, J. L. (2013). Automated extraction of clinical traits of multiple sclerosis in electronic medical records. J. Am. Med. Inform. Assoc. 20, e334–e340. doi: 10.1136/amiajnl-2013-001999
DePaula-Silva, A. B. (2024). The contribution of microglia and brain-infiltrating macrophages to the pathogenesis of neuroinflammatory and neurodegenerative diseases during TMEV infection of the central nervous system. Viruses 16. doi: 10.3390/v16010119
DePaula-Silva, A. B., Bell, L. A., Wallis, G. J., and Wilcox, K. S. (2021). Inflammation unleashed in viral-induced Epileptogenesis. Epilepsy Curr. 21, 433–440. doi: 10.1177/15357597211040939
DePaula-Silva, A. B., Gorbea, C., Doty, D. J., Libbey, J. E., Sanchez, J. M. S., Hanak, T. J., et al. (2019). Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J. Neuroinflammation 16:152. doi: 10.1186/s12974-019-1545-x
DePaula-Silva, A. B., Hanak, T. J., Libbey, J. E., and Fujinami, R. S. (2017). Theiler's murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: models for multiple sclerosis and epilepsy. J. Neuroimmunol. 308, 30–42. doi: 10.1016/j.jneuroim.2017.02.012
Ekdahl, C. T., Claasen, J. H., Bonde, S., Kokaia, Z., and Lindvall, O. (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 100, 13632–13637. doi: 10.1073/pnas.2234031100
Faissner, S., Plemel, J. R., Gold, R., and Yong, V. W. (2019). Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922. doi: 10.1038/s41573-019-0035-2
Filgueira, L., Larionov, A., and Lannes, N. (2021). The influence of virus infection on microglia and accelerated brain aging. Cells 10. doi: 10.3390/cells10071836
Frischer, J. M., Bramow, S., Dal-Bianco, A., Lucchinetti, C. F., Rauschka, H., Schmidbauer, M., et al. (2009). The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189. doi: 10.1093/brain/awp070
Gentile, A., Musella, A., Bullitta, S., Fresegna, D., De Vito, F., Fantozzi, R., et al. (2016). Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflammation 13:207. doi: 10.1186/s12974-016-0686-4
Gerhauser, I., Hansmann, F., Ciurkiewicz, M., Löscher, W., and Beineke, A. (2019). Facets of Theiler's murine encephalomyelitis virus-induced diseases: an update. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20020448
Gerhauser, I., Hansmann, F., Puff, C., Kumnok, J., Schaudien, D., Wewetzer, K., et al. (2012). Theiler's murine encephalomyelitis virus induced phenotype switch of microglia in vitro. J. Neuroimmunol. 252, 49–55. doi: 10.1016/j.jneuroim.2012.07.018
Gilli, F., Li, L., and Pachner, A. R. (2016). The immune response in the CNS in Theiler's virus induced demyelinating disease switches from an early adaptive response to a chronic innate-like response. J. Neurovirol. 22, 66–79. doi: 10.1007/s13365-015-0369-4
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. doi: 10.1126/science.1194637
Girard, S., Gosselin, A. S., Pelletier, I., Colbère-Garapin, F., Couderc, T., and Blondel, B. (2002). Restriction of poliovirus RNA replication in persistently infected nerve cells. J. Gen. Virol. 83, 1087–1093. doi: 10.1099/0022-1317-83-5-1087
Goddery, E. N., Fain, C. E., Lipovsky, C. G., Ayasoufi, K., Yokanovich, L. T., Malo, C. S., et al. (2021). Microglia and perivascular macrophages act as antigen presenting cells to promote CD8 T cell infiltration of the brain. Front. Immunol. 12:726421. doi: 10.3389/fimmu.2021.726421
Guo, S., Wang, H., and Yin, Y. (2022). Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 14:815347. doi: 10.3389/fnagi.2022.815347
Hansmann, F., Zhang, N., Herder, V., Leitzen, E., and Baumgärtner, W. (2019). Delayed astrogliosis associated with reduced M1 microglia activation in matrix metalloproteinase 12 knockout mice during Theiler's murine encephalomyelitis. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20071702
Herder, V., Iskandar, C. D., Kegler, K., Hansmann, F., Elmarabet, S. A., Khan, M. A., et al. (2015). Dynamic changes of microglia/macrophage M1 and M2 polarization in Theiler's murine encephalomyelitis. Brain Pathol. 25, 712–723. doi: 10.1111/bpa.12238
Jafari, M., Haist, V., Baumgärtner, W., Wagner, S., Stein, V. M., Tipold, A., et al. (2012). Impact of Theiler's virus infection on hippocampal neuronal progenitor cells: differential effects in two mouse strains. Neuropathol. Appl. Neurobiol. 38, 647–664. doi: 10.1111/j.1365-2990.2012.01256.x
Jarousse, N., Syan, S., Martinat, C., and Brahic, M. (1998). The neurovirulence of the DA and GDVII strains of Theiler's virus correlates with their ability to infect cultured neurons. J. Virol. 72, 7213–7220. doi: 10.1128/jvi.72.9.7213-7220.1998
Jelachich, M. L., and Lipton, H. L. (1999). Restricted Theiler's murine encephalomyelitis virus infection in murine macrophages induces apoptosis. J. Gen. Virol. 80, 1701–1705. doi: 10.1099/0022-1317-80-7-1701
Jeong, G. U., Lyu, J., Kim, K. D., Chung, Y. C., Yoon, G. Y., Lee, S., et al. (2022). SARS-CoV-2 infection of microglia elicits Proinflammatory activation and apoptotic cell death. Microbiol. Spectr. 10:e0109122. doi: 10.1128/spectrum.01091-22
Jiang, J., Li, Y., Sun, Z., Gong, L., Li, X., Shi, F., et al. (2022). LncNSPL facilitates influenza a viral immune escape by restricting TRIM25-mediated K63-linked RIG-I ubiquitination. iScience 25:104607. doi: 10.1016/j.isci.2022.104607
Kamma, E., Lasisi, W., Libner, C., Ng, H. S., and Plemel, J. R. (2022). Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics. J. Neuroinflammation 19:45. doi: 10.1186/s12974-022-02408-y
Kerstetter, A. E., Padovani-Claudio, D. A., Bai, L., and Miller, R. H. (2009). Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp. Neurol. 220, 44–56. doi: 10.1016/j.expneurol.2009.07.010
Kim, B. S. (2021). Excessive innate immunity steers pathogenic adaptive immunity in the development of Theiler's virus-induced demyelinating disease. Int. J. Mol. Sci. 22. doi: 10.3390/ijms22105254
Kim, S. J., Jin, Y. H., and Kim, B. S. (2017). Prostaglandin E2 produced following infection with Theiler's virus promotes the pathogenesis of demyelinating disease. PLoS One 12:e0176406. doi: 10.1371/journal.pone.0176406
Kim, B. S., Palma, J. P., Kwon, D., and Fuller, A. C. (2005). Innate immune response induced by Theiler's murine encephalomyelitis virus infection. Immunol. Res. 31, 01–12. doi: 10.1385/ir:31:1:01
Kummerfeld, M., Seehusen, F., Klein, S., Ulrich, R., Kreutzer, R., Gerhauser, I., et al. (2012). Periventricular demyelination and axonal pathology is associated with subependymal virus spread in a murine model for multiple sclerosis. Intervirology 55, 401–416. doi: 10.1159/000336563
Laskin, D. L. (2009). Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 22, 1376–1385. doi: 10.1021/tx900086v
Libbey, J. E., Kirkman, N. J., Smith, M. C., Tanaka, T., Wilcox, K. S., White, H. S., et al. (2008). Seizures following picornavirus infection. Epilepsia 49, 1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x
Lipton, H. L. (1975). Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 11, 1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975
Lipton, H. L., Twaddle, G., and Jelachich, M. L. (1995). The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 69, 2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995
Liuzzi, G. M., Riccio, P., and Dal Canto, M. C. (1995). Release of myelin basic protein-degrading proteolytic activity from microglia and macrophages after infection with Theiler's murine encephalomyelitis virus: comparison between susceptible and resistant mice. J. Neuroimmunol. 62, 91–102. doi: 10.1016/0165-5728(95)00110-n
Luong, N., and Olson, J. K. (2021). Exosomes secreted by microglia during virus infection in the central nervous system activate an inflammatory response in bystander cells. Front. Cell Dev. Biol. 9:661935. doi: 10.3389/fcell.2021.661935
Magyari, M. (2016). Gender differences in multiple sclerosis epidemiology and treatment response. Dan. Med. J. 63.
Martinat, C., Jarousse, N., Prévost, M. C., and Brahic, M. (1999). The GDVII strain of Theiler's virus spreads via axonal transport. J. Virol. 73, 6093–6098. doi: 10.1128/jvi.73.7.6093-6098.1999
McRae, B. L., Vanderlugt, C. L., Dal Canto, M. C., and Miller, S. D. (1995). Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 182, 75–85. doi: 10.1084/jem.182.1.75
Mecha, M., Yanguas-Casás, N., Feliú, A., Mestre, L., Carrillo-Salinas, F., Azcoitia, I., et al. (2019). The endocannabinoid 2-AG enhances spontaneous remyelination by targeting microglia. Brain Behav. Immun. 77, 110–126. doi: 10.1016/j.bbi.2018.12.013
Mendel, I., Kerlero de Rosbo, N., and Ben-Nun, A. (1995). A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur. J. Immunol. 25, 1951–1959. doi: 10.1002/eji.1830250723
Michaličková, D., Hrnčíř, T., Canová, N. K., and Slanař, O. (2020). Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 873:172973. doi: 10.1016/j.ejphar.2020.172973
Michel, L., Touil, H., Pikor, N. B., Gommerman, J. L., Prat, A., and Bar-Or, A. (2015). B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front. Immunol. 6:636. doi: 10.3389/fimmu.2015.00636
Mikita, J., Dubourdieu-Cassagno, N., Deloire, M. S., Vekris, A., Biran, M., Raffard, G., et al. (2011). Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult. Scler. 17, 2–15. doi: 10.1177/1352458510379243
Miron, V. E., Boyd, A., Zhao, J. W., Yuen, T. J., Ruckh, J. M., Shadrach, J. L., et al. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218. doi: 10.1038/nn.3469
Misra, U. K., Tan, C. T., and Kalita, J. (2008). Viral encephalitis and epilepsy. Epilepsia 49, 13–18. doi: 10.1111/j.1528-1167.2008.01751.x
Miyagawa, F., Gutermuth, J., Zhang, H., and Katz, S. I. (2010). The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J. Autoimmun. 35, 192–198. doi: 10.1016/j.jaut.2010.06.007
Monje, M. L., Toda, H., and Palmer, T. D. (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765. doi: 10.1126/science.1088417
Montilla, A., Zabala, A., Er-Lukowiak, M., Rissiek, B., Magnus, T., Rodriguez-Iglesias, N., et al. (2023). Microglia and meningeal macrophages depletion delays the onset of experimental autoimmune encephalomyelitis. Cell Death Dis. 14:16. doi: 10.1038/s41419-023-05551-3
Moseman, E. A., Blanchard, A. C., Nayak, D., and McGavern, D. B. (2020). T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci Immunol 5. doi: 10.1126/sciimmunol.abb1817
Olson, J. K. (2014). Effect of the innate immune response on development of Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Neurovirol. 20, 427–436. doi: 10.1007/s13365-014-0262-6
Olson, J. K., Girvin, A. M., and Miller, S. D. (2001). Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 75, 9780–9789. doi: 10.1128/jvi.75.20.9780-9789.2001
Olson, J. K., and Miller, S. D. (2004). Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173, 3916–3924. doi: 10.4049/jimmunol.173.6.3916
Olson, J. K., and Miller, S. D. (2009). The innate immune response affects the development of the autoimmune response in Theiler's virus-induced demyelinating disease. J. Immunol. 182, 5712–5722. doi: 10.4049/jimmunol.0801940
Oñate, M., Catenaccio, A., Martínez, G., Armentano, D., Parsons, G., Kerr, B., et al. (2016). Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep. 6:21709. doi: 10.1038/srep21709
Onose, G., Anghelescu, A., Blendea, D., Ciobanu, V., Daia, C., Firan, F. C., et al. (2022). Cellular and molecular targets for non-invasive, non-pharmacological therapeutic/rehabilitative interventions in acute ischemic stroke. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23020907
Park, H. J., Oh, S. H., Kim, H. N., Jung, Y. J., and Lee, P. H. (2016). Mesenchymal stem cells enhance α-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 132, 685–701. doi: 10.1007/s00401-016-1605-6
Pavelko, K. D., Mendez-Fernandez, Y., Bell, M. P., Hansen, M. J., Johnson, A. J., David, C. S., et al. (2012). Nonequivalence of classical MHC class I loci in ability to direct effective antiviral immunity. PLoS Pathog. 8:e1002541. doi: 10.1371/journal.ppat.1002541
Pike, S. C., Welsh, N., Linzey, M., and Gilli, F. (2022). Theiler's virus-induced demyelinating disease as an infectious model of progressive multiple sclerosis. Front. Mol. Neurosci. 15:1019799. doi: 10.3389/fnmol.2022.1019799
Psenicka, M. W., Smith, B. C., Tinkey, R. A., and Williams, J. L. (2021). Connecting neuroinflammation and neurodegeneration in multiple sclerosis: are oligodendrocyte precursor cells a Nexus of disease? Front. Cell. Neurosci. 15:654284. doi: 10.3389/fncel.2021.654284
Qie, S., Ran, Y., Lu, X., Su, W., Li, W., Xi, J., et al. (2020). Candesartan modulates microglia activation and polarization via NF-κB signaling pathway. Int. J. Immunopathol. Pharmacol. 34:2058738420974900. doi: 10.1177/2058738420974900
Qiu, Z., Lu, P., Wang, K., Zhao, X., Li, Q., Wen, J., et al. (2020). Dexmedetomidine inhibits Neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem. Res. 45, 345–353. doi: 10.1007/s11064-019-02922-1
Rodriguez, M., Leibowitz, J. L., and Lampert, P. W. (1983). Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann. Neurol. 13, 426–433. doi: 10.1002/ana.410130409
Rodriguez, M., Pavelko, K., and Coffman, R. L. (1995). Gamma interferon is critical for resistance to Theiler's virus-induced demyelination. J. Virol. 69, 7286–7290. doi: 10.1128/jvi.69.11.7286-7290.1995
Rolak, L. A. (2003). Multiple sclerosis: it's not the disease you thought it was. Clin. Med. Res. 1, 57–60. doi: 10.3121/cmr.1.1.57
Rossi, C. P., Delcroix, M., Huitinga, I., McAllister, A., van Rooijen, N., Claassen, E., et al. (1997). Role of macrophages during Theiler's virus infection. J. Virol. 71, 3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997
Sato, F., Kawai, E., Martinez, N. E., Omura, S., Park, A. M., Takahashi, S., et al. (2017). T-bet, but not Gata3, overexpression is detrimental in a neurotropic viral infection. Sci. Rep. 7:10496. doi: 10.1038/s41598-017-10980-0
Sato, F., Tanaka, H., Hasanovic, F., and Tsunoda, I. (2011). Theiler's virus infection: pathophysiology of demyelination and neurodegeneration. Pathophysiology 18, 31–41. doi: 10.1016/j.pathophys.2010.04.011
Schetters, S. T. T., Gomez-Nicola, D., Garcia-Vallejo, J. J., and Van Kooyk, Y. (2017). Neuroinflammation: microglia and T cells get ready to tango. Front. Immunol. 8:1905. doi: 10.3389/fimmu.2017.01905
Shen, S., Cheng, X., Zhou, L., Zhao, Y., Wang, H., Zhang, J., et al. (2022). Neutrophil Nanovesicle protects against experimental autoimmune encephalomyelitis through enhancing myelin clearance by microglia. ACS Nano 16, 18886–18897. doi: 10.1021/acsnano.2c07798
Trottier, M., Kallio, P., Wang, W., and Lipton, H. L. (2001). High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler's virus. J. Virol. 75, 7420–7428. doi: 10.1128/jvi.75.16.7420-7428.2001
Tsunoda, I., and Fujinami, R. S. (2002). Inside-out versus outside-in models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin. Immunopathol. 24, 105–125. doi: 10.1007/s00281-002-0105-z
Tsunoda, I., and Fujinami, R. S. (2010). Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J. Neuroimmune Pharmacol. 5, 355–369. doi: 10.1007/s11481-009-9179-x
Ulrich, R., Baumgärtner, W., Gerhauser, I., Seeliger, F., Haist, V., Deschl, U., et al. (2006). MMP-12, MMP-3, and TIMP-1 are markedly upregulated in chronic demyelinating theiler murine encephalomyelitis. J. Neuropathol. Exp. Neurol. 65, 783–793. doi: 10.1097/01.jnen.0000229990.32795.0d
Vakrakou, A. G., Alexaki, A., Brinia, M. E., Anagnostouli, M., Stefanis, L., and Stathopoulos, P. (2022). The mTOR signaling pathway in multiple sclerosis; from animal models to human data. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23158077
Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R. A., et al. (2020). Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS. Mult. Scler. 26, 1816–1821. doi: 10.1177/1352458520970841
Wang, L., Li, Y., Wang, X., Wang, P., Essandoh, K., Cui, S., et al. (2020). GDF3 protects mice against Sepsis-induced cardiac dysfunction and mortality by suppression of macrophage pro-inflammatory phenotype. Cells 9. doi: 10.3390/cells9010120
Whitham, R. H., Bourdette, D. N., Hashim, G. A., Herndon, R. M., Ilg, R. C., Vandenbark, A. A., et al. (1991). Lymphocytes from SJL/J mice immunized with spinal cord respond selectively to a peptide of proteolipid protein and transfer relapsing demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 146, 101–107. doi: 10.4049/jimmunol.146.1.101
Yang, C. J., Li, X., Feng, X. Q., Chen, Y., Feng, J. G., Jia, J., et al. (2022). Activation of LRP1 ameliorates cerebral ischemia/reperfusion injury and cognitive decline by suppressing Neuroinflammation and oxidative stress through TXNIP/NLRP3 signaling pathway in mice. Oxidative Med. Cell. Longev. 2022, 8729398–8729323. doi: 10.1155/2022/8729398
Zaslona, Z., Serezani, C. H., Okunishi, K., Aronoff, D. M., and Peters-Golden, M. (2012). Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase a signaling. Blood 119, 2358–2367. doi: 10.1182/blood-2011-08-374207
Zhang, Z., Li, X., Zhou, H., and Zhou, J. (2022). NG2-glia crosstalk with microglia in health and disease. CNS Neurosci. Ther. 28, 1663–1674. doi: 10.1111/cns.13948
Keywords: Theiler’s murine encephalomyelitis virus (TMEV), multiple sclerosis (MS), microglia/macrophage, M1/M2, neuroinflammation
Citation: Zhang Q, Sun W, Zheng M and Zhang N (2024) Contribution of microglia/macrophage to the pathogenesis of TMEV infection in the central nervous system. Front. Microbiol. 15:1452390. doi: 10.3389/fmicb.2024.1452390
Edited by:
Yimin Wang, Henan Institute of Science and Technology, ChinaReviewed by:
Sourish Ghosh, Indian Institute of Chemical Biology (CSIR), IndiaMinshu Li, Tianjin Medical University General Hospital, China
Copyright © 2024 Zhang, Sun, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhang, emhhbmduaW5nMTExMUAxMjYuY29t
 Qianye Zhang
Qianye Zhang Wei Sun
Wei Sun Ning Zhang
Ning Zhang