- 1The Third Affiliated Hospital of Shanghai University, Wenzhou People’s Hospital, Wenzhou, China
- 2Department of Gastroenterology, Sir Run Run Shaw Hospital Affiliated to Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3School of Medicine, Shanghai University, Shanghai, China
- 4The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 5School of Pharmacy, Collaborative Innovation Center of Advanced Drug Delivery System and Biotech Drugs in Universities of Shandong, Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University), Ministry of Education, Yantai University, Yantai, China
- 6The Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou, China
The resistance of Helicobacter pylori (H. pylori) has increased in recent years, prompting a trend in the research and development of new drugs. In our study, three derivatives (JF-1, JF-2, and JF-3) were synthesized using 6-gingerol as the main component, while JF-4, containing both 6-gingerol and 6-shogaol as the main components, was extracted from dried ginger. The minimum inhibitory concentrations (MICs), determined using the ratio dilution method, were 80 μg/mL for JF-1, 40 μg/mL for JF-2, 30 μg/mL for JF-3, 40 μg/mL for JF-4, 60 μg/mL for 6-gingerol standard (SS), and 0.03 μg/mL for amoxicillin (AMX). After treating H. pylori-infected mice, the inflammation of the gastric mucosa was suppressed. The eradication rate of H. pylori was 16.7% of JF-3 low-dose treatment (LDT), 25.0% of JF-3 high-dose treatment (HDT), 16.7% of JF-4 LDT, 16.7% of JF-4 HDT, 30% of SS LDT, 50% of SS HDT, and 36.4% of the positive control group (PCG). The levels of gastrin, somatostatin (SST), IFN-γ, IL-4, and IL-8 were significantly recovered in the JF-3 and JF-4 administration groups, but the effect was stronger in the high-dose group. These results demonstrate that 6-gingerol and its derivatives have significant anti-Helicobacter pylori effects and are promising potential treatments for H. pylori infection.
1 Introduction
Helicobacter pylori (H. pylori) is a common human pathogen that selectively colonizes human gastric epithelial cells (Sjomina et al., 2018). The average prevalence of H. pylori in China is 44.2%, which means that approximately 589 million people are infected (McColl, 2010). The H. pylori infection can cause varying degrees of intragastric disorders, including dyspepsia (5–10%), chronic gastritis (90%), peptic ulcers (15–20%), and gastric malignancies (1%) (Ding et al., 2021). This infection is also associated with extragastric disorders, such as inflammatory bowel disease, gastroesophageal reflux disease, non-alcoholic fatty liver disease, hepatocellular carcinoma, cholelithiasis, and cholecystitis (Santos et al., 2020).
The International Agency for Research on Cancer (IARC) classified H. pylori as a class I carcinogen in 1994, and an early intervention to eradicate H. pylori can reverse chronic active gastritis. Currently, the first-line treatment regimen for the eradication of H. pylori infection is the quadruple therapy consisting of bismuth+proton-pump inhibitors (PPI) + 2 antimicrobial drugs. There are other treatments, which include standard triple therapies, high-dose dual therapies, sequential therapies, traditional Chinese medicine (TCM) treatments, the use of probiotics, and H. pylori vaccine development (Sugano et al., 2015; Sulo and Šipková, 2021; Yang and Hu, 2021). However, H. pylori resistance to antibiotics has been increasing globally due to their widespread use, with primary and secondary resistance rates of clarithromycin, metronidazole, and levofloxacin being more than 15% in all regions monitored by the World Health Organization (WHO) (Yoshii et al., 2020). In addition, there are common side effects of standard quadruple therapy, which include abdominal pain, nausea and vomiting, rash, abdominal distension, and dizziness (Yan et al., 2024). These side effects also cause significant discomfort for patients during treatment. Therefore, developing new drugs is an effective way to alleviate the challenge of microbial resistance.
Ginger is a perennial herbaceous plant of the genus Zingiber in the family Zingiberaceae. It is a traditional Chinese medicinal and food plant. Gingerol is the main active ingredient in dried ginger, which also contains shogaol, gingerone, and other spicy substances (Liu et al., 2019). In 1969, Connell and Sutherland isolated gingerol and confirmed that the content of 6-gingerol (6-G) accounted for approximately 75% of the gingerol. Gingerol has a variety of biological properties, such as antitumor, anti-inflammatory, antioxidant, anticoagulant, analgesic, antiemetic, hypoglycemic, hypolipidemic, cardiovascular, and neuroprotective effects (Wang et al., 2015), and has been widely used in the alleviation and treatment of many diseases (Chey et al., 2017; Liu et al., 2018). Several studies have shown that 6-gingerol also has antimicrobial (Fallone et al., 2016), antiviral (Haikh and Fallone, 2016; Ko S. W. et al., 2019), and antiparasitic activities (Graham et al., 2018; Li et al., 2019), which may be related to the inhibition of bacterial ATP synthase binding, thereby reducing bacterial biofilm formation and virulence (Sun, 2011; Smith et al., 2017).
There are no studies on gingerols and their derivatives against H. pylori. To explore the resistance of 6-gingerol to H. pylori and to develop new natural product drugs, in the early stage of this study, self-made dried ginger slices were optimized according to the orthogonal test method, and 6-gingerol purified (JF-4) was obtained after isolation and purification. The main components of JF-4 are 6-gingerol and 6-shogaol, as described in the LC–MS database. However, 6-gingerol is chemically unstable and prone to degradation and dehydration (Hu et al., 2017). To solve the instability of 6-gingerol and maintain good properties, three 6-gingerol derivatives, JF-1, JF-2, and JF-3, were synthesized based on the retention of the active group of 6-gingerol. This synthesis improved the stability, solubility, and potency of 6-gingerol and helped it play its active role better. The results showed that 6-gingerol oil purification and derivatives had anti-H. pylori infection in vitro and in vivo (Figure 1).
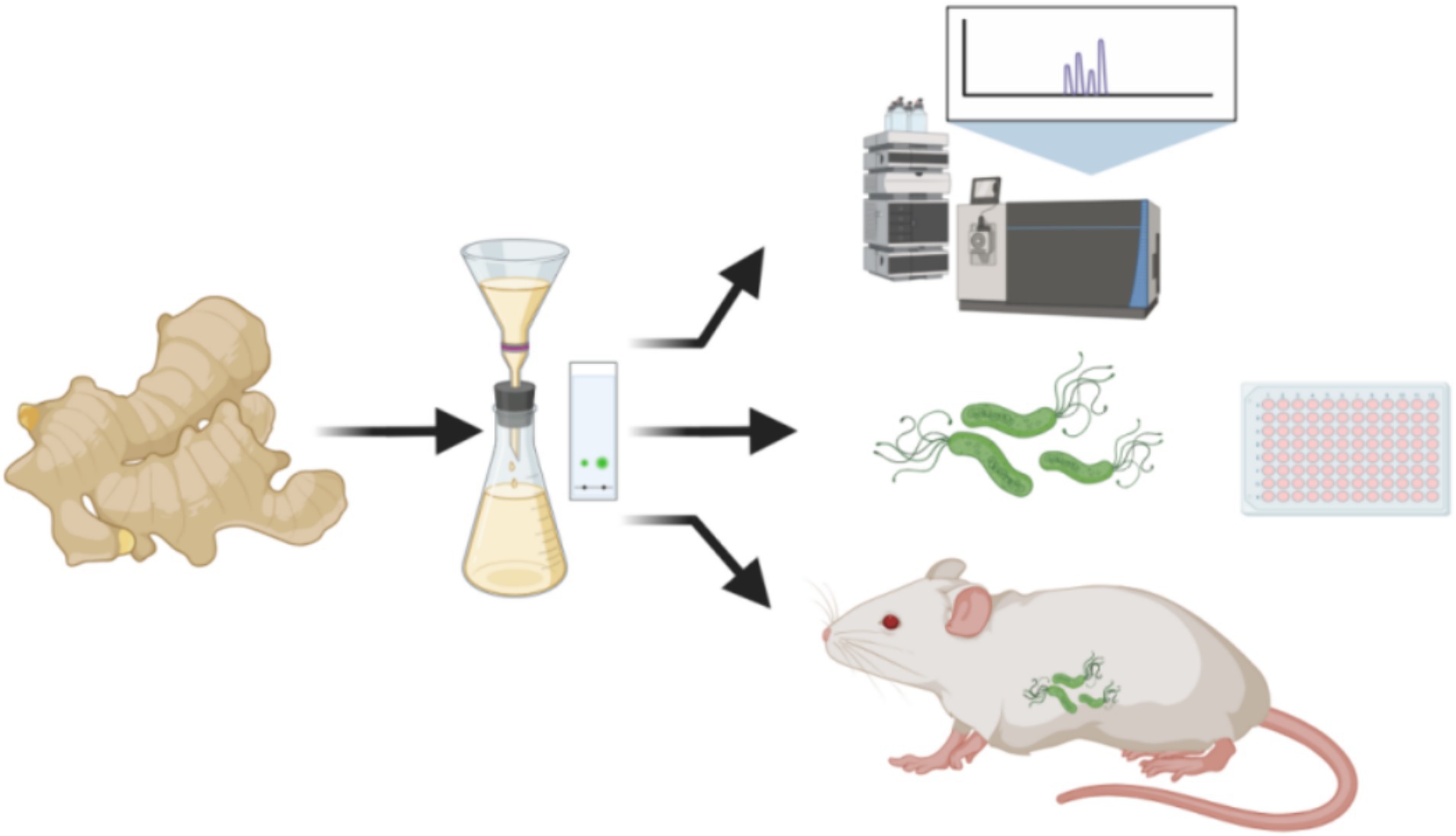
Figure 1. Dried ginger oil was extracted and purified for the LC–MS analysis, H. pylori antibacterial assay, and in vivo animal treatment experiment. (Images produced by https://www.biorender.com/).
2 Materials and methods
2.1 Chemicals
Analytical grade chemicals including 4% paraformaldehyde, dimethyl sulfoxide (DMSO), 6-gingerol standard (SS), clarithromycin, amoxicillin, omeprazole, bismuth potassium citrate, and anhydrous ethanol were obtained from Beijing Solarbio Science & Technology Co., Ltd., Shanghai Macklin Biochemical Co. Ltd., and Shanghai Aladdin Bio-Chem Technology Co., LTD. Tianjin Beichen Fangzheng Reagent Factory was the source of solvents and reagents.
2.2 Purification and synthesis of 6-gingerol derivatives
Fresh ginger slices were steamed for 30 min and were baked in an electric blast drying oven for 60 min at a temperature of 50°C. Then, the slices were dried and ground into a powder, followed by ultrasonic extraction using 10 times the volume of 80% ethanol for 30 min at a temperature of 60°C. The extraction was filtered using a 0.22-μm microporous filter membrane and then was freeze-dried to obtain the gingerol extract. Furthermore, 400 g of 100–200 mesh silica gel was used for chromatography, being eluted with a gradient mixture of petroleum ether and ethyl acetate at a ratio of 5:1. Afterward, the mixture underwent rotary evaporation and concentration to obtain the purified 6-gingerol.
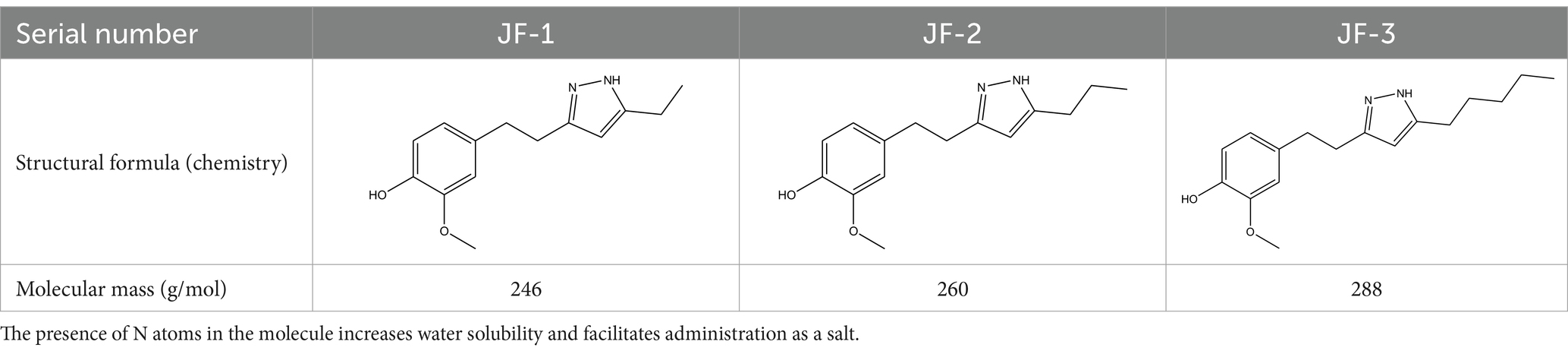
JF-1, JF-2, and JF-3 were designed and synthesized by Dr. Huang Xianfeng’s team at Changzhou University, with the structural formula as shown in Table 1.
2.3 Minimum anti-Helicobacter pylori concentration of dried ginger extracts
Helicobacter pylori GDMCC 1.1820 was purchased from Guangzhou Di Jing Microbiology Co. The ratio dilution method was as follows: Three novel compounds (JF-1, JF-2, and JF-3), 6-gingerol purifier (JF-4), control drugs (amoxicillin and AMX), and SS were prepared as a masterbatch in dimethylsulfoxide (DMSO) at a concentration of 3,200 μg/mL, and the masterbatch was filtered and sterilized using a 0.22-μm filter. Then, 160 μL of an H. pylori culture solution was added to the wells of column 1 of a 96-well plate, followed by the addition of 40 μL of the masterbatch of drugs. Furthermore, 100 μL of the H. pylori culture solution was added to the wells of columns 2 through 12. Then, 100 μL of the mixture from column 1 was pipetted into column 2, 100 μL of the mixture from column 2 was pipetted into column 3, and finally, the mixture was doubled and diluted to column 12 in this order. The mixture from column 12, amounting to 100 μL, was discarded, and 100 μL of the abovementioned H. pylori bacterial solution was added to columns 1 through 12. The final concentrations of the drug in the wells of columns 1–12 were 320 μg/mL, 160 μg/mL, 80 μg/mL, 40 μg/mL, 20 μg/mL, 10 μg/mL, 5 μg/mL, 2.5 μg/mL, 1.25 μg/mL, 0.625 μg/mL, 0.3125 μg/mL, and 0.15625 μg/mL. In addition, the negative control (with or without the bacteria solution) and the positive control (with or without the bacteria solution) were set up. The 96-well plate was incubated at 37°C in a microaerobic environment within a constant temperature incubator for 48 to 96 h. No precipitation at the bottom and a clear solution without reddening indicated that bacteria did not grow. The lowest drug concentration was the minimum inhibitory concentration (MIC).
2.4 Animal grouping and modeling
Male SPF BALB/c mice, 6–8 weeks old and weighing 18–22 g, were purchased from Changzhou Cavins Laboratory Animal Co. Ltd. The mice were acclimatized and fed for 1 week before the formal experiments, and during this period, the mice were free to eat and move around. The animal experiments were approved by the Ethics Committee of the Animal Experimentation Center of Yantai University as required.
In this study, the mice were randomly divided into nine groups of 10–12 mice each: normal control group (NCG), H. pylori model group (HMG), H. pylori model group + JF-3 low-dose treatment (LDT) group (50 mg/kg, JF-3 LDT), H. pylori model group + JF-3 high-dose treatment (HDT) group (200 mg/kg, JF-3 HDT), H. pylori model group + JF-4 low-dose treatment group (50 mg/kg, JF-4 LDT), H. pylori model group + JF-4 high-dose treatment group (200 mg/kg, JF-4 HDT), H. pylori model group +6-G standard low-dose treatment group (50 mg/kg, SS LDT), H. pylori model group +6-G standard high-dose treatment group (200 mg/kg, SS HDT), and positive control group (PCG, standard quadruple-drug treatment).
The mice model of the H. pylori-infected gastritis group was constructed as follows: After 1 week of acclimatization, the mice were randomly divided into normal control and H. pylori-infected groups. The H. pylori-infected group was given 0.3 mL of H. pylori suspension (9 × 108 CFU/mL) by gavage every other day, and 0.3 mL of saline was given to the control group by gavage. Before the gavage, a period of 12 h of fasting, including water fasting, was imposed, and 4 h of free feeding was performed after the gavage. A total of five inoculations were performed (0.3 mL of 5% NaHCO3 was given to the mice 0.5 h before the H. pylori gavage to increase the rate of H. pylori infection). After 4 weeks of continued feeding, eight mice in the infected group were randomly executed and subjected to a rapid urease test and staining of pathological sections (Warthin-Starry silver staining + HE staining) to detect whether they were infected with H. pylori or not. A positive rate of ≥75% was considered successful.
After confirming the success of the model, the mice infected with H. pylori were randomly divided into eight groups and treated with corresponding drugs for 2 weeks: the H. pylori model group: 0.3 mL/ sterile saline was given twice a day; the standard quadruple-drug treatment group: amoxicillin 150 mg/kg + clarithromycin 75 mg/kg + omeprazole 6 mg/kg + bismuth potassium citrate 33 mg/kg was given twice a day; the high-dose and low-dose groups: the mice were treated with three drugs, JF-3, JF-4, and 6-G standard, with corresponding doses, a low dose of 50 mg/kg and a high dose of 200 mg/kg, by gavage twice a day; and the normal control group: 0.3 mL/ sterile saline was given by gavage twice a day. All mice were killed 4 weeks after the withdrawal of drugs.
2.5 Evaluation of urease activity
The appropriate amount of urease reaction solution was taken in Eppendorf (EP) tubes. Then, a small piece of mucosa from the gastric sinus and gastric body of the mice was clipped and placed in the reaction solution. The tubes were sealed and left to sit for 10 min to observe for any color change. If the color change was minimal, the tubes were placed in a 37°C incubator and the color change was observed after 12 h. A 0.2-mL suspension of H. pylori was taken for comparison in the same way.
2.6 ELISA (double antibody sandwich assay) for inflammation-related factors
For the serum, blood was collected from the eyeballs of the mice immediately after execution, allowed to sit at room temperature for 2 h, and then centrifuged at 1000 rpm for 15 min at 2–8°C. A portion of the supernatant was used for the test. For the gastric mucosa, a portion of the gastric mucosa tissue of the mice was collected, washed with PBS to remove blood stains, cut into small pieces, and put into a tissue grinder. Then, 1 mL of PBS was added to create a homogenate, which was then centrifuged at 5000 rpm for 5 min at 2–8°C. The supernatant was used for the experiment. Standard wells and sample wells were set up separately. Then, 100 μL of the standard or sample to be tested was added to each well, fully mixed and covered with a plate sticker, and was incubated at 37°C for 2 h. The liquid was discarded, and the wells were shaken dry; 100 μL of biotin-labeled antibody was added to each well, covered with a new plate sticker, and incubated at 37°C for 1 h. Then, the liquid in the wells was discarded, the wells were shaken dry, and the plate was washed three times for 2–3 min each. Furthermore, 100 μL of horseradish peroxidase-labeled working solution was added to each well, covered with a new plate seal, and incubated at 37°C for 1 h. The liquid in the wells was discarded, the wells were shaken dry, and the plate was washed five times for 2–3 min. A volume of 100 μL of the substrate solution (TMB) was added to each well, and the reaction was terminated by adding 50 μL of the termination solution to each reaction well after it was made to stand for 20 min at 37°C in a dark environment. The optical density (OD) of each well was measured with an enzyme counter at 450 nm within 5 min after the reaction termination. The concentration fitting curve was calculated according to the OD value of the standard, and the actual concentration was calculated according to the fitting curve.
2.7 Statistical methods
The data were statistically analyzed using SPSS 26.0 statistical software and presented as mean ± standard. The data between two groups were analyzed by performing an independent samples t-test, comparisons between multiple groups were analyzed using a one-way ANOVA, two-by-two comparisons between groups were analyzed by performing Dunnett’s t-test, and variance disagreement was analyzed by performing Tamhane’s T2 test. A p-value of <0.05 indicated that the difference was statistically significant.
3 Results
3.1 Effect of 6-gingerol purification
A 5:1 gradient of petroleum ether to ethyl acetate was used as the mobile phase for elution. The light brown oily liquid obtained by column chromatography (Figure 2A) was 6-gingerol oil. Kim J. et al. (2022) found the main components of dried ginger extract, including substances suchas 6-gingerol (Figure 2B), 6-shogal(Figure 2C), 8-gingerol, and 10-gingerol, and verified that dried ginger extract can enhance immunity after influenza vaccination. Other studies have shown (Mustafa et al., 2019; Kim J. Y. et al., 2022; Mustafa and Chin, 2023) that different extraction methods, freshness, species, and parts of the plant have an effect on the extraction results, but most of the constituents are gingerols, shogaols, and some alkenes. Ginger extracts have been reported in the research fields for their anti-inflammatory (Ballester et al., 2022), antioxidant (Mustafa and Chin, 2023), antimicrobial (He et al., 2023), and anticancer properties (Mahomoodally et al., 2019), and 6-gingerol can be converted to 6-gingerol by subcritical water extraction (Ko M. J. et al., 2019). Some studies have shown that 6-gingerol inhibits adipocyte hypertrophy and proliferation, improves metabolic disorders (Cheng et al., 2022), and ameliorates fatty liver degeneration, inflammation, and oxidative stress in mice by activating the LKB1/AMPK signaling pathway (Liu et al., 2023). It also ameliorates multiple disorders, such as brain injury (Tang et al., 2022), lung injury (Li et al., 2024), myocardial ischemia (Lv et al., 2021), and other multiple diseases.
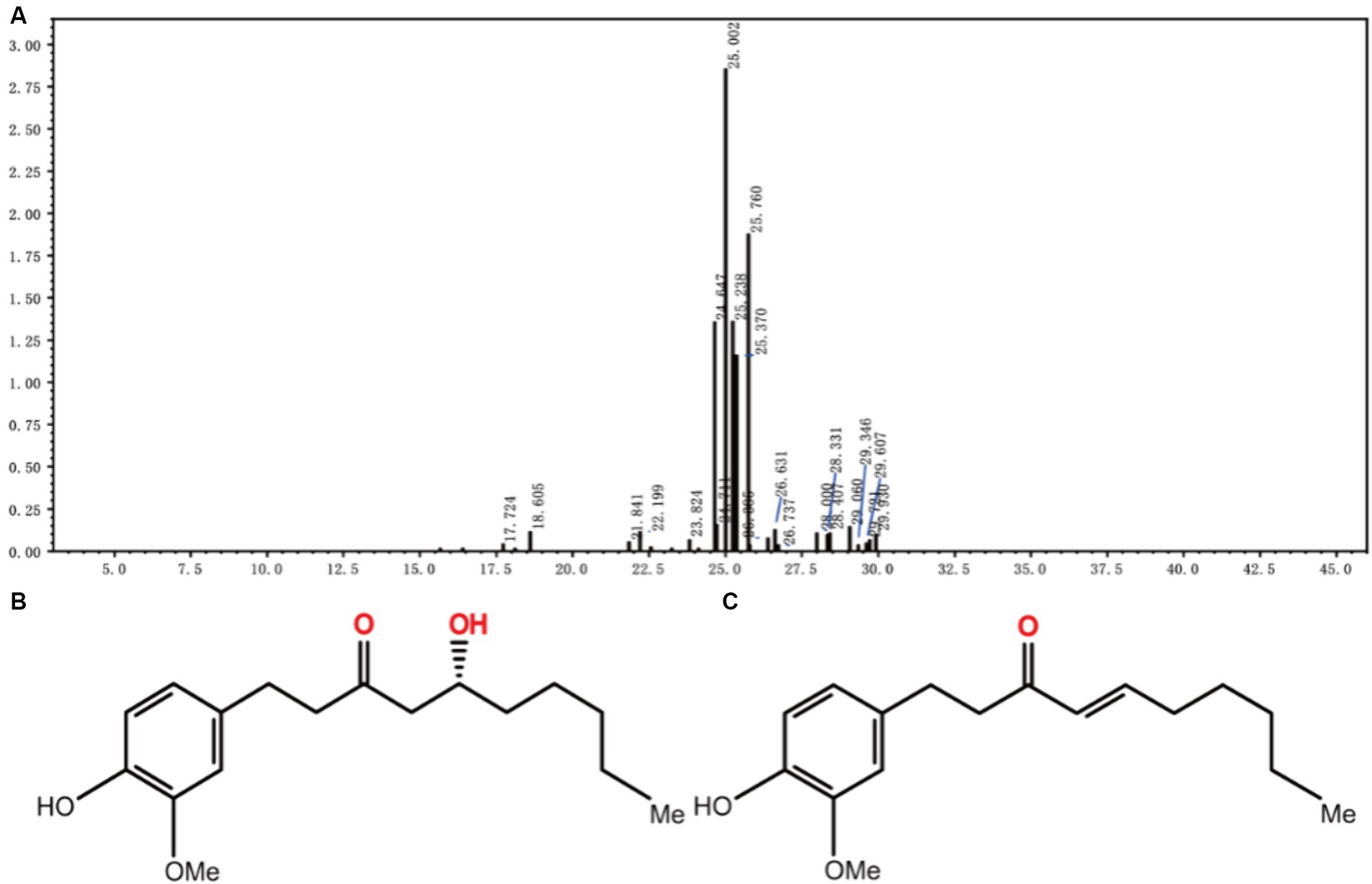
Figure 2. LC–MS mass spectra of JF-4 (A). Schematic representation of the molecular structure of 6-gingerol (B). Schematic representation of the molecular structure of 6-shogaol (C).
3.2 In vitro minimum inhibitory concentration (MIC)
The results of the doubled dilution method were as follows: three novel compounds, JF-1, JF-2, and JF-3, and the purified product (JF-4) were found to be bacteriostatic against H. pylori in vitro, with average minimum inhibitory concentrations of 80 μg/mL, 40 μg/mL, 20 μg/mL, and 40 μg/mL, respectively. The minimum inhibitory concentration of the SS was 40 μg/mL, and the minimum inhibitory concentration of amoxicillin was 0.03 μg/mL. The antibacterial effects of JF-1 and JF-2 were weak, and no animal experiments were performed (Table 2).
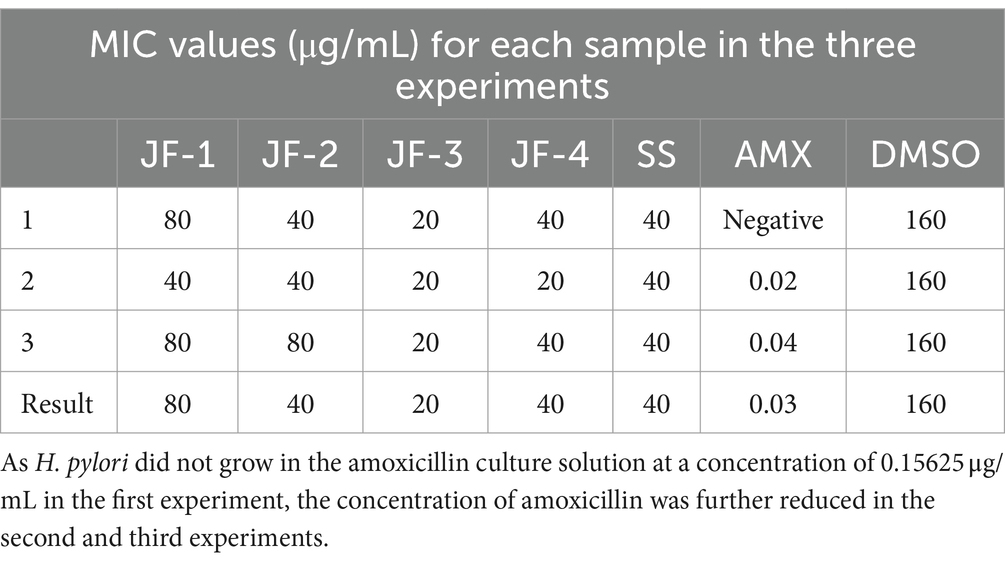
Table 2. MIC values (μg/mL) of in vitro anti-H. pylori for each sample of the multiplicative dilution method.
The present study demonstrated that dried ginger extract has good anti-H. pylori properties and has potential therapeutic effects against H. pylori infection. In gingerol antimicrobial-related studies, dried ginger extract inhibited the biofilm formation of Candida albicans, reduced its virulence, and prevented mycelial growth and aggregation (Lee et al., 2018). Hughes et al. (2021) reported that it had an ATP synthase inhibitory effect on E. coli. According to Wang et al. (2020) 6-gingerol was found to affect the composition of the intestinal microbiota and was able to significantly increase the abundance of probiotics, such as bifidobacteria and enterococci. Finally, 6-gingerol was found to be a potential prebiotic (Wang et al., 2020).
3.3 Modeling
There were 10 mice in the normal control group with good growth activities, and there were 98 mice in the H. pylori-infected group, some of which showed loss of appetite, depression, and unresponsiveness during the feeding process. Microscopic examination of the gastric mucosa in mice revealed infiltration of neutrophils, lymphocytes, and other inflammatory cells. H. pylori colonization was also evident in the pathological sections (Figure 3).
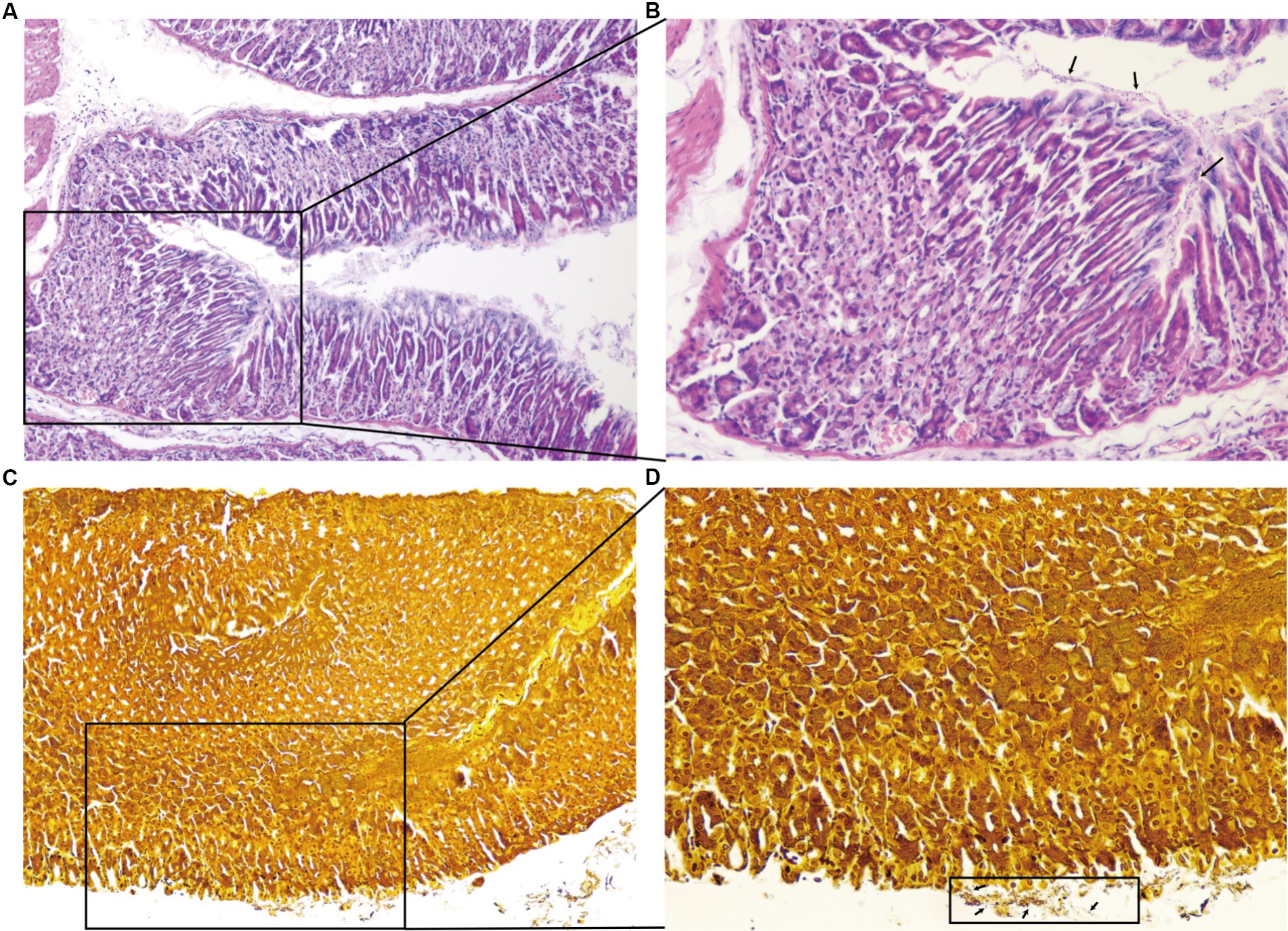
Figure 3. Results of the pathological sections of the gastric antrum tissue. HE staining showing neutrophilic infiltration, lymphocytes, and other inflammatory cell infiltration (A ×100,B ×200). Warthin–Starry silver staining ×200, a large number of H. pylori colonization on the surface of gastric mucosal epithelial cells and the bottom layer of gastric mucus (C ×100,D ×200).
The H. pylori infection causes intense lymphocyte infiltration in the stomach, and CD8 + T cells infiltrate the gastric mucosa soon after the infection, playing a crucial role in controlling the pathogen by performing antigen-specific effector properties (Koch et al., 2023). Moreover, it has been demonstrated that H. pylori cleaves the epithelial cell attachment adhesion molecule to disrupt gastric epithelial integrity (Marques et al., 2021).
3.4 Helicobacter pylori decontamination rate results
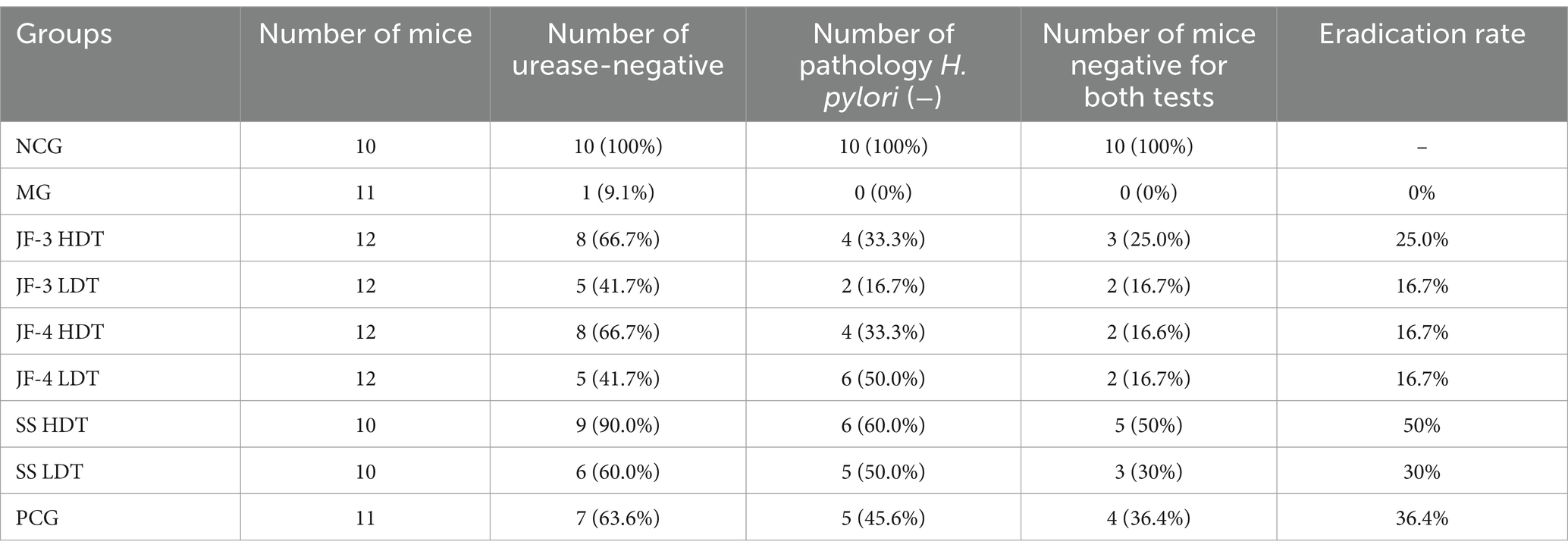
As shown in Table 3, it was observed that all mice in the normal control group tested negative for gastric mucosal urease and showed no abnormalities during the pathological examination. On the other hand, 90.9% of mice in the H. pylori model group tested positive for gastric mucosal urease, all of which exhibited abnormalities during the pathological examination and were infected with H. pylori. The eradication rate was calculated as the number of mice with both tests negative in each group divided by the total number of mice in each group, and the eradication rates for each drug were 25.0% (JF-3 HDT), 16.7% (JF-3 LDT), 16.7% (JF-4 HDT), 16.7% (JF-4 LDT), 50% (SS HDT), 30% (SS LDT), and 36.4% (PCG).
3.5 Pathological findings
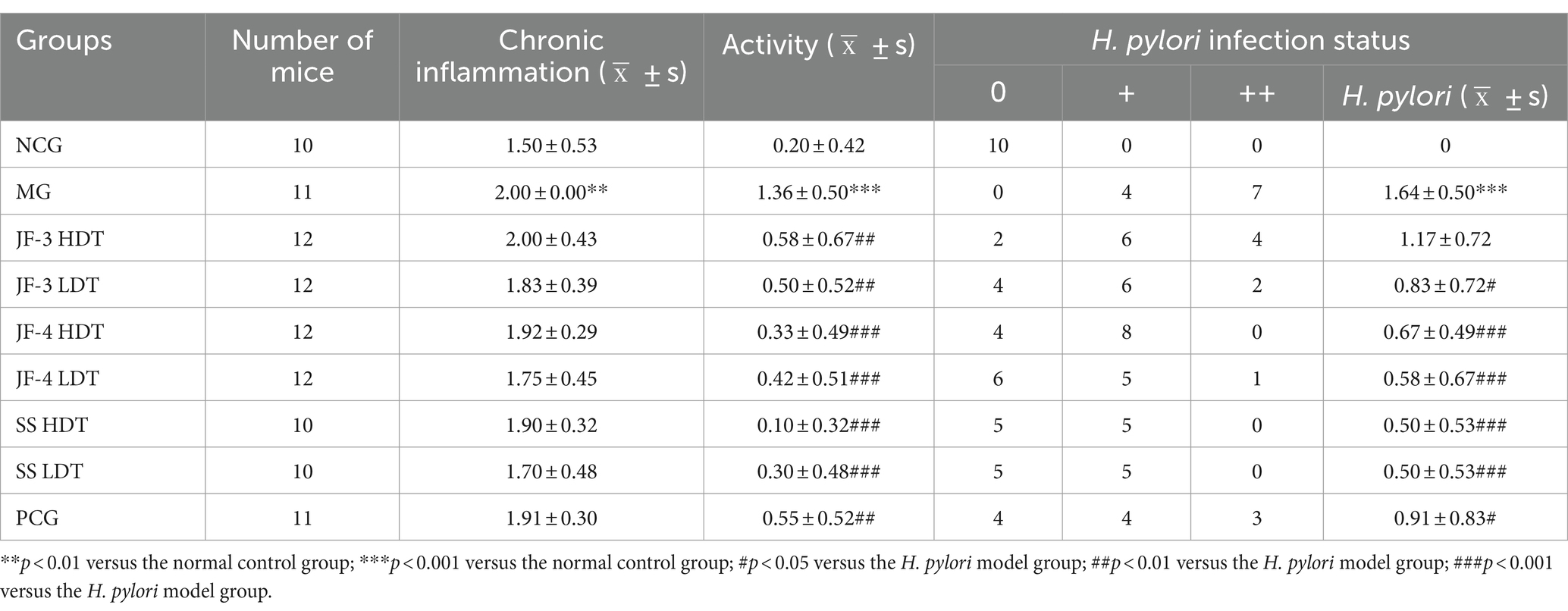
As shown in Figure 4 and Table 4, compared to the normal control group, chronic inflammation of the gastric mucosa in mice from the H. pylori model group was more severe; the activity was obvious and the difference was statistically significant (p < 0.01; p < 0.001). The H. pylori infection was observed in all of the H. pylori model groups. Compared with the HMG, chronic inflammation of the gastric mucosa in mice was partially improved after drug treatment, but none of the differences were statistically significant. Compared with the HMG, the inflammatory activity and H. pylori infection of the gastric mucosa in mice were significantly improved (neutrophil infiltration was reduced) after 14 days of treatment with JF-3, JF-4, and SS, as well as after treatment with the PCG (p < 0.01). It was shown (Tian et al., 2022) that 6-gingerol attenuates Dextran Sulfate Sodium Salt (DSS)-induced colitis, mainly by interfering with antioxidant and anti-inflammatory pathways and the ratio of the thick-walled bacillus phylum/anabolic bacillus phylum. It also has the ability to inhibit the activity of Staphylococcus aureus and Escherichia coli, which suggests a good antimicrobial effect (Ahad et al., 2023). 6-gingerol has also been shown to regulate the structure and metabolites of digestive flora (Shen et al., 2022). Li et al. (2017) demonstrated that 6-gingerol could protect against intestinal barrier damage by inhibiting p38 MAPK to NF-κB signaling.
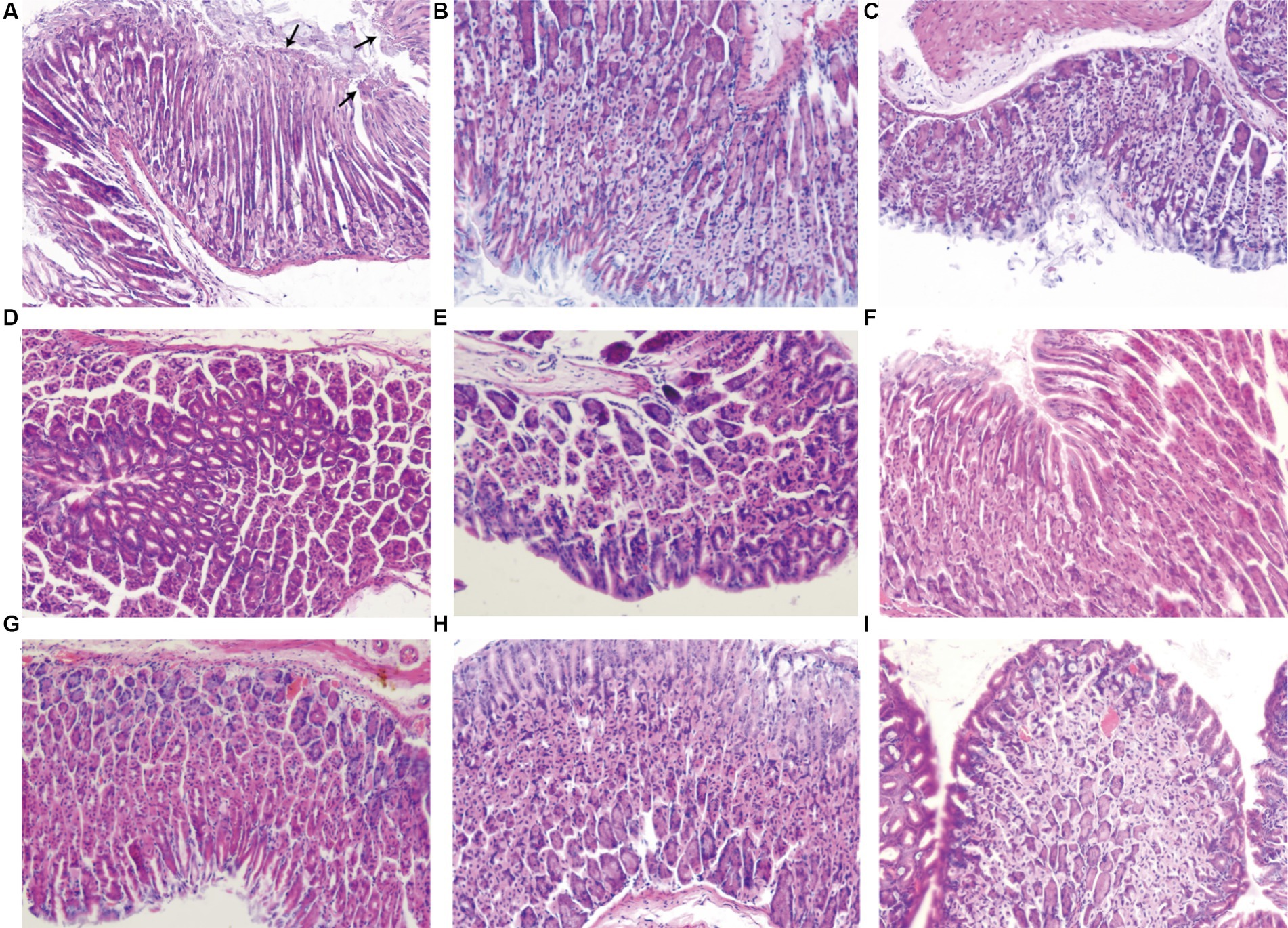
Figure 4. Results of the pathological sections of the gastric antrum tissue. In the H. pylori model group (A), neutrophil infiltration was observed in the background of chronic inflammation, and short rod-shaped H. pylori fixation was observed at the arrow under the microscope. Normal control group (B). Positive control group (C). JF-3 high-dose group (D) and low-dose group (G). JF-4 high-dose group (E) and low-dose group (H). SS high-dose group (F) and low-dose group (I).
3.6 Expression results of gastrin and somatostatin in serum
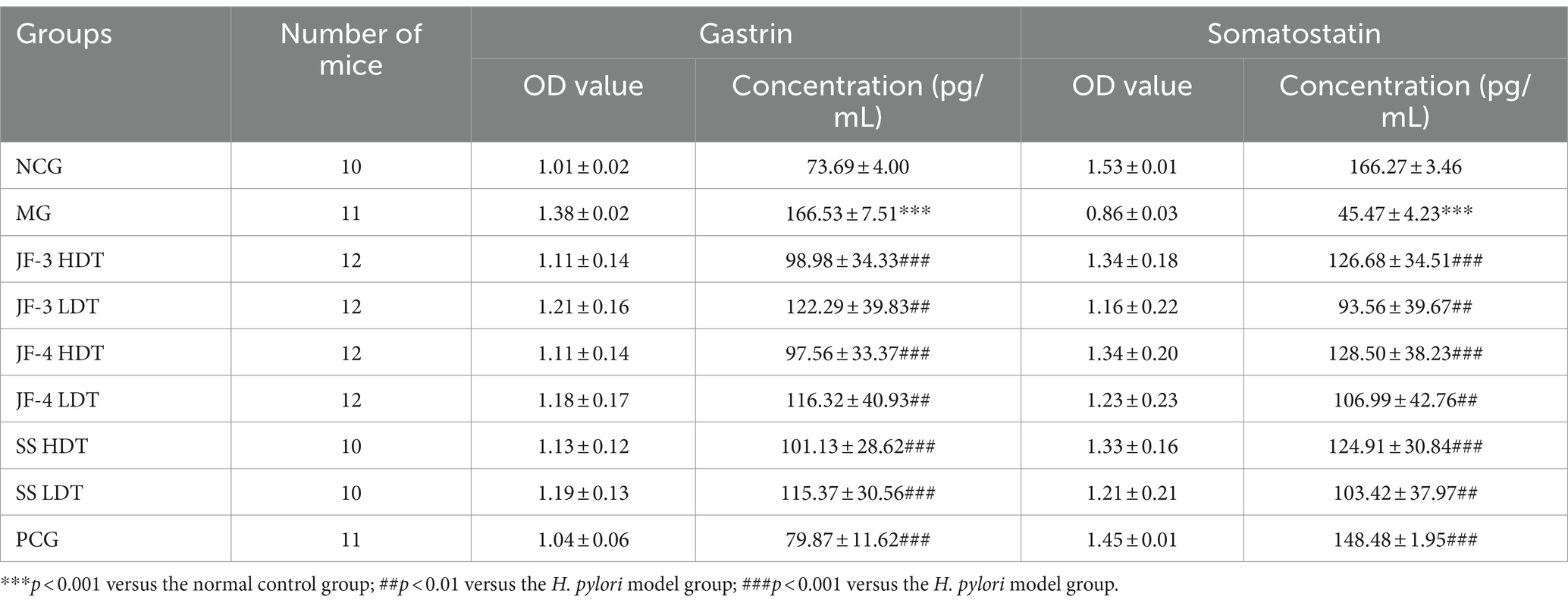
Serum gastrin and somatostatin (SST) concentrations were significantly changed in the H. pylori-infected mice compared with the normal control. After 14 days of treatment with JF-3, JF-4, and SS, it was observed that the treatment with gastrin and somatostatin resulted in varying degrees of recovery. When comparing different doses of the same therapeutic drug, the changes caused by the high dose were found to be more significant; in particular, the high dose of JF-3 had a statistically significant effect relative to the low dose of JF-4 (Figure 5 and Table 5).
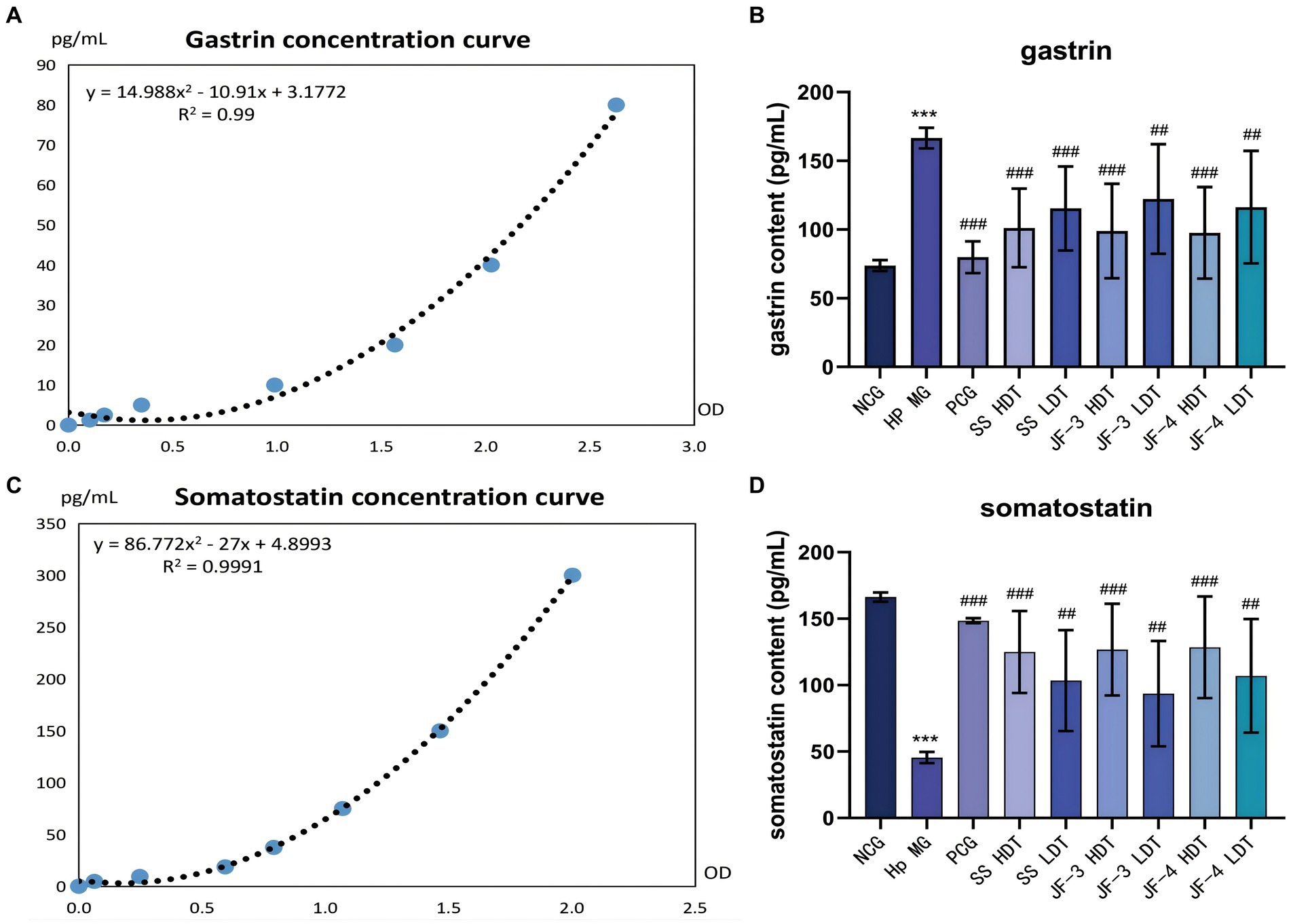
Figure 5. Expression results of gastrin and somatostatin in the serum. Fitting curve of gastrin concentration (dilution of 10 times) (A). Concentration of gastrin in the serum of different groups of mice (B). Fitting curve of somatostatin concentration (C). Concentration of somatostatin in the serum of different groups of mice (D). *P < 0.05 vs normal control group; **P < 0.01 vs normal control group; ***P < 0.001 vs normal control group; #P < 0.05 vs Hp model group; ##P < 0.01 vs Hp model group; ###P < 0.001 vs Hp model group.
Gastrin primarily regulates gastric acid secretion and cell proliferation in the acid-secreting mucosa. Chronic gastritis caused by the H. pylori infection usually shows high levels of gastrin expression (Rehfeld, 2021; Veysey-Smith et al., 2021). Somatostatin (SST) is a major inhibitor of gastric acid secretion, which can inhibit the gastrin secretion of G cells and the gastric acid secretion of parietal cells (Schubert and Rehfeld, 2019). SST also has anti-proliferative and pro-apoptotic properties and has been listed as a first-line anticancer drug in clinical practice (Shamsi et al., 2021). The imbalance between somatostatin and gastrin further leads to chronic inflammation and the occurrence of gastric cancer. The metabolites of H. pylori increase the pH value of the gastric epithelial surface, and hence, the feedback effect of gastric acid on gastrin is disturbed. Furthermore, TNF α, IFN - gamma, and cytokine stimulation, such as IL-8 G cells, secrete gastric elements (Maciorkowska et al., 2006). In addition, there was a decrease in the number of D cells and in the expression level of somatostatin (Kim et al., 2020; Kunovsky et al., 2021).
The study’s findings indicated that the serum gastrin content increased and the somatostatin content decreased in the mice infected with H. pylori. However, after treatment with 6-gingerol and its derivatives, the serum gastrin and somatostatin concentrations significantly recovered. The H. pylori infection causes an imbalance between somatostatin and gastrin, which promotes chronic inflammation and the development of gastric cancer. Gingerol treatment significantly enhances the secretion of gastrin and somatostatin, reducing the risk of peptic ulcer. This confirmed that gingerol, especially in the high-dose group, could be a potential agent for repairing gastric injury caused by H. pylori.
3.7 Results of IFN-γ, IL-4, and IL-8 expression levels in gastric mucosa tissues
In the mice model of H. pylori infection, significantly changes were observed in the expression levels of IFN-γ, IL-4, and IL-8 in the gastric mucosa of the mice compared to those in the normal control group. Following the infection, there was a notable increase in the expression of IFN-γ, IL-4, and IL-8. After 14 days of treatment with JF-3, JF-4, and SS, the expression levels of IFN-γ, IL-4, and IL-8 were all reversed to varying degrees. During the comparison of different drug doses, it was observed that the expression levels of IFN-γ, IL-4, and IL-8 decreased more significantly after high-dose treatment as compared to low-dose treatment, although the difference was not statistically significant. After PCG treatment, the expression levels of IFN-γ, IL-4, and IL-8 decreased significantly (Figure 6 and Table 6).

Figure 6. Expression results of IFN-γ, IL-4, and IL-8 in gastric mucosa tissues. IFN-γ concentration curves (A), expression levels of IFN-γ in the gastric mucosa of different groups of mice (B). IL-4 concentration curves (C), expression levels of IL-4 in the gastric mucosa of different groups of mice (D). IL-8 concentration curves (E), expression levels of IL-8 in the gastric mucosa of different groups of mice (F).
The results of several studies (Bagheri et al., 2018; Yang et al., 2020) have shown that, after H. pylori infection, the host’s Th1 immune response is enhanced and dominant, and the expression levels of inflammatory factors, such as INF-γ, IL-2, and TNF-α, are increased. The phosphorylation of AKT and STAT3 promotes differentiation and increases IFN-γ-producing T cells. ADM also induces macrophages to produce IL-12 and aggravates T-cell response (Kong et al., 2020), which further promotes the occurrence of H. pylori-associated gastritis. Blocking or knocking out IFN-γ can reduce the inflammatory response of gastric mucosa in mice infected with H. pylori. Multiple studies, carried out both domestically and internationally, have shown that CagA, which is produced by highly virulent strains of H. pylori, can trigger the NF-κB and AP-1 pathways in gastric epithelial cells and the nuclear heptose biosynthesis pathway in Lipopolysaccharide (LPS). This activation results in the expression of IL-8 (Stein et al., 2017; Zhang et al., 2019; Yu et al., 2020).
After treatment with 6-gingerol and JF-3, the expression levels of IFN-γ, IL-4, and IL-8 decreased significantly. This suggests that 6-gingerol and its derivatives may help restore the expression levels of INF-γ and IL-4, ultimately balancing the Th1/Th2 immune response. Consequently, this effect leads to an improvement in gastric mucosa inflammation.
4 Discussion
Helicobacter pylori is a unipolar, multi-flagellated, helical, and Gram-stain-negative bacterium, which was first identified in the human gastric mucosa by Marshall and Warren (1984). H. pylori releases various virulence factors, such as CagA and VacA, which further exacerbate the inflammatory response and cause sustained damage to the gastric mucosa (Kao et al., 2016). H. pylori can also form biofilms, which help reduce its susceptibility to antibiotics and develop resistance (Fauzia et al., 2020). The CagA protein, a major virulence factor produced by highly virulent strains of H. pylori, not only causes a strong inflammatory response but also induces cellular alterations. It also reduces cell viability, causes cells to undergo proliferation or apoptosis, and alters tumor suppressor mechanisms in the gastric epithelium (Sharndama and Mba, 2022). This is a significant factor leading to atrophic gastritis and gastric cancer. Another major virulence factor is VacA, which can disrupt intracellular lysosomal vesicular transport and impair the autophagy pathway (Abdullah et al., 2019), causing vacuolization of host cells, which leads to cell necrosis. It can also induce apoptosis in gastric epithelial cells via the mitochondrial pathway.
The in-vitro experiments showed that the eradication rate of the quadruple therapy was slightly higher than that of the three compounds (JF-1, 2, and 3) and the mixture (JF-4). However, the highest eradication rate was observed with the high-dose 6-gingerol standardized product (SS). The best improvement, as evidenced by serum and gastric pathology tests, was observed with the positive drug, while the effect of high-dose 6-gingerol and its derivatives was comparable to that of the positive drug. 6-gingerol and its derivatives can effectively inhibit the growth of H. pylori in vitro. It is hypothesized that the eradication of H. pylori may be dependent on high-dose 6-gingerol and its derivatives. However, the safety of these compounds needs to be verified in both animals and human studies. 6-Gingerol and its derivatives are effective in inhibiting the growth of H. pylori in vitro.
Helicobacter pylori infection induces a certain degree of immune response in the host, releasing numerous inflammatory mediators, exacerbating gastric mucosal damage, and leading to gastroduodenal-related diseases. There is abundant evidence (Laky et al., 2015; Bagheri et al., 2016; Xie et al., 2020) that CD4+ T helper cells are activated in the immune response against H. pylori infection, differentiating into various effector cells (including Th1, Th2, Th17, and Treg) and producing characteristic cytokines. Among them, Th1 cells characteristically secrete IFN-γ, IL-2, and TNF-α, which mediate cellular immune responses, while Th2 cells secrete IL-4, IL-11, and IL-13, which mediate humoral immunity (Yang et al., 2021). There are reciprocal inhibition and transformation among them.
The expression of IL-8 increases in the host after the H. pylori infection (Zhang et al., 2019; Yu et al., 2020). Several studies (Aktan et al., 2006; Saha et al., 2016) have shown that ginger extracts, including 6-gingerol, inhibit the activation of NF-κB induced by many drugs and downregulate gene products involved in the NF-κB regulation of cell proliferation and angiogenesis, including IL-8. It is suggested that H. pylori infection may trigger the secretion of IL-8 from gastric epithelial cells by activating the NF-κB pathway, leading to the recruitment of inflammatory cells, and these compounds may block this pathway (Figure 7).
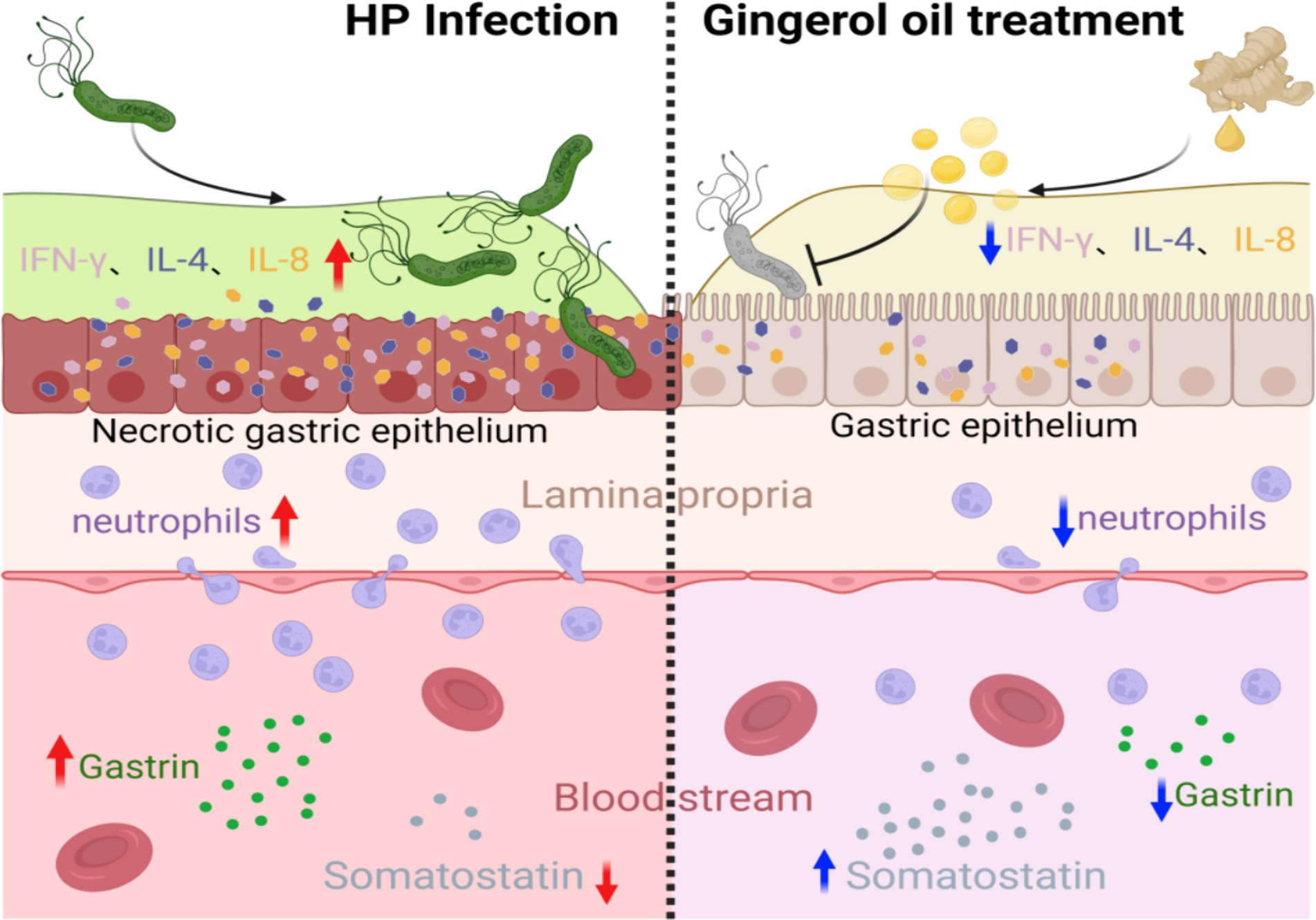
Figure 7. 6-Gingerol treatment alleviates gastric epithelial inflammation after H. pylori infection, promotes the secretion of somatostatin, and inhibits gastrin. (Image produced by https://www.biorender.com/).
5 Conclusion
In summary, 6-gingerol and its derivatives could effectively inhibit the growth of H. pylori in vitro. In light of the high MIC values obtained, it is speculated that the eradication of H. pylori may depend on high doses of 6-gingerol and its derivatives. The safety of these compounds must be confirmed in animal and human studies. When used alone, 6-ginger hydroxybenzene or its derivatives have a low eradication rate of H. pylori, but they can still effectively suppress H. pylori proliferation, improve gastric mucosa inflammation, reduce inflammatory cytokines, balance the Th1/Th2 immune response, and restore gastric secretion elements and somatostatin levels. Therefore, 6-gingerol and its derivatives as a new auxiliary for H. pylori infection gastritis treatment strategies help to improve gastritis and reduce the occurrence of tumors. In addition, the combination of antibiotics and PPI can improve the eradication rate of H. pylori and reduce side effects.
This study has certain limitations. First, our study was only confined to the macro-level analysis of drug inhibition of H. pylori. The specific inhibition mechanism needs to be further studied, and the identification method of H. pylori infection needs to be further improved. Second, among the many phenotypes in gastric inflammation, the study only detected Th1/Th2-related characteristic indicators, rendering the analysis incomplete to a certain extent. Based on the findings of this study, it is suggested that future studies should explore the following aspects: the specific mechanism of 6-gingerol and its derivatives to inhibit H. pylori and whether these compounds can improve other stomach diseases, such as gastric cancer, in vivo. Exploring these research avenues not only complement the shortcomings of existing research but also lead to more valuable discoveries.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by the Ethics Committee of the Animal Experimentation Center of Yantai University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JQ: Conceptualization, Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. ZL: Data curation, Formal analysis, Project administration, Software, Writing – original draft, Writing – review & editing. JW: Data curation, Formal analysis, Writing – review & editing. YL: Conceptualization, Formal analysis, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. YY: Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Wenzhou Traditional Chinese Medicine construction fund subsidy project TD-ZY2020001.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, M., Greenfield, L. K., Bronte-Tinkew, D., Capurro, M. I., Rizzuti, D., and Jones, N. L. (2019). VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection. Sci. Rep. 9:38. doi: 10.1038/s41598-018-37095-4
Ahad, T., Gull, A., Masoodi, F. A., Gani, A., Nissar, J., Ganaie, T. A., et al. (2023). Protein and polysaccharide based encapsulation of ginger oleoresin: impact of wall materials on powder stability, release rate and antimicrobial characteristics. Int. J. Biol. Macromol. 240:124331. doi: 10.1016/j.ijbiomac.2023.124331
Aktan, F., Henness, S., Tran, V., Duke, C., Roufogalis, B., and Ammit, A. (2006). Gingerol metabolite and a synthetic analogue Capsarol inhibit macrophage NF-kappaB-mediated iNOS gene expression and enzyme activity. Planta Med. 72, 727–734. doi: 10.1055/s-2006-931588
Bagheri, N., Azadegan-Dehkordi, F., Rahimian, G., Rafieian-Kopaei, M., and Shirzad, H. (2016). Role of regulatory T-cells in different clinical expressions of Helicobacter pylori infection. Arch. Med. Res. 47, 245–254. doi: 10.1016/j.arcmed.2016.07.013
Bagheri, N., Salimzadeh, L., and Shirzad, H. (2018). The role of T helper 1-cell response in Helicobacter pylori-infection. Microb. Pathog. 123, 1–8. doi: 10.1016/j.micpath.2018.06.033
Ballester, P., Cerdá, B., Arcusa, R., Marhuenda, J., Yamedjeu, K., and Zafrilla, P. (2022). Effect of ginger on inflammatory diseases. Molecules 27:7223. doi: 10.3390/molecules27217223
Cheng, Z., Xiong, X., Zhou, Y., Wu, F., Shao, Q., Dong, R., et al. (2022). 6-gingerol ameliorates metabolic disorders by inhibiting hypertrophy and hyperplasia of adipocytes in high-fat-diet induced obese mice. Biomed. Pharmacother. 146:112491. doi: 10.1016/j.biopha.2021.112491
Chey, W. D., Leontiadis, G. I., Howden, C. W., and Moss, S. F. (2017). ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 112, 212–239. doi: 10.1038/ajg.2016.563
Ding, S. Z., du, Y. Q., Lu, H., Wang, W. H., Cheng, H., Chen, S. Y., et al. (2021). Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 edition). Gut 71, 238–253. doi: 10.1136/gutjnl-2021-325630
Fallone, C. A., Chiba, N., van Zanten, S. V., Fischbach, L., Gisbert, J. P., Hunt, R. H., et al. (2016). The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151, 51–69.e14. doi: 10.1053/j.gastro.2016.04.006
Fauzia, K. A., Miftahussurur, M., Syam, A. F., Waskito, L. A., Doohan, D., Rezkitha, Y. A. A., et al. (2020). Biofilm formation and antibiotic resistance phenotype of Helicobacter pylori clinical isolates. Toxins 12:473. doi: 10.3390/toxins12080473
Graham, D. Y., Dore, M. P., and Lu, H. (2018). Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev. Anti-Infect. Ther. 16, 679–687. doi: 10.1080/14787210.2018.1511427
Haikh, T., and Fallone, C. A. (2016). Effectiveness of second through sixth line salvage Helicobacter pylori treatment: bismuth quadruple therapy is almost always a reasonable choice. Can. J. Gastroenterol. Hepatol. 2016:7321574. doi: 10.1155/2016/7321574
He, J., Hadidi, M., Yang, S., Khan, M. R., Zhang, W., and Cong, X. (2023). Natural food preservation with ginger essential oil: biological properties and delivery systems. Food Res. Int. 173:113221. doi: 10.1016/j.foodres.2023.113221
Hu, Y., Zhu, Y., and Lu, N. H. (2017). Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front. Cell. Infect. Microbiol. 7:168. doi: 10.3389/fcimb.2017.00168
Hughes, T., Azim, S., and Ahmad, Z. (2021). Inhibition of Escherichia coli ATP synthase by dietary ginger phenolics. Int. J. Biol. Macromol. 182, 2130–2143. doi: 10.1016/j.ijbiomac.2021.05.168
Kao, C. Y., Sheu, B. S., and Wu, J. J. (2016). Helicobacter pylori infection: an overview of bacterial virulence factors and pathogenesis. Biom. J. 39, 14–23. doi: 10.1016/j.bj.2015.06.002
Kim, J., Lee, H., and You, S. (2022). Dried ginger extract restores the T helper type 1/T helper type 2 balance and antibody production in cyclophosphamide-induced immunocompromised mice after flu vaccination. Nutrients 14:1984. doi: 10.3390/nu14091984
Kim, D. U., Moon, J. H., Lee, Y. H., Paik, S. S., Kim, Y., and Kim, Y. J. (2020). Analysis of somatostatin-secreting gastric delta cells according to upper abdominal symptoms and Helicobacter pylori infection in children. Pediatr. Gastroenterol. Hepatol. Nutr. 23, 243–250. doi: 10.5223/pghn.2020.23.3.243
Kim, J. Y., Park, S. E., Kim, E. J., Seo, S. H., Whon, T. W., Cho, K. M., et al. (2022). Long-term population dynamics of viable microbes in a closed ecosystem of fermented vegetables. Food Res. Int. 154:111044. doi: 10.1016/j.foodres.2022.111044
Ko, S. W., Kim, Y. J., Chung, W. C., and Lee, S. J. (2019). Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter 24:e12565. doi: 10.1111/hel.12565
Ko, M. J., Nam, H. H., and Chung, M. S. (2019). Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 270, 149–155. doi: 10.1016/j.foodchem.2018.07.078
Koch, M. R. A., Gong, R., Friedrich, V., Engelsberger, V., Kretschmer, L., Wanisch, A., et al. (2023). CagA-specific gastric CD8+ tissue-resident T cells control Helicobacter pylori during the early infection phase. Gastroenterology 164, 550–566. doi: 10.1053/j.gastro.2022.12.016
Kong, H., You, N., Chen, H., Teng, Y. S., Liu, Y. G., Lv, Y. P., et al. (2020). Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis. 11:189. doi: 10.1038/s41419-020-2391-6
Kunovsky, L., Dite, P., Jabandziev, P., Dolina, J., Vaculova, J., Blaho, M., et al. (2021). Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 13, 835–844. doi: 10.4251/wjgo.v13.i8.835
Laky, K., Evans, S., Perez-Diez, A., and Fowlkes, B. J. (2015). Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity 42, 80–94. doi: 10.1016/j.immuni.2014.12.027
Lee, J. H., Kim, Y. G., Choi, P., Ham, J., Park, J. G., and Lee, J. (2018). Antibiofilm and Antivirulence activities of 6-Gingerol and 6-Shogaol against Candida albicans due to hyphal inhibition. Front. Cell. Infect. Microbiol. 8:299. doi: 10.3389/fcimb.2018.00299
Li, H., Wang, R., and Sun, H. (2019). Systems approaches for unveiling the mechanism of action of bismuth drugs: new medicinal applications beyond Helicobacter pylori infection. Acc. Chem. Res. 52, 216–227. doi: 10.1021/acs.accounts.8b00439
Li, Q., Xiao, C., Gu, J., Chen, X., Yuan, J., Li, S., et al. (2024). 6-Gingerol ameliorates alveolar hypercoagulation and fibrinolytic inhibition in LPS-provoked ARDS via RUNX1/NF-κB signaling pathway. Int. Immunopharmacol. 128:111459. doi: 10.1016/j.intimp.2023.111459
Li, Y., Xu, B., Xu, M., Chen, D., Xiong, Y., Lian, M., et al. (2017). 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol. Res. 119, 137–148. doi: 10.1016/j.phrs.2017.01.026
Liu, Y., Li, D., Wang, S., Peng, Z., Tan, Q., He, Q., et al. (2023). 6-Gingerol ameliorates hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice through activating LKB1/AMPK signaling. Int. J. Mol. Sci. 24:6285. doi: 10.3390/ijms24076285
Liu, X., Shi, Y., and Ding, S. (2019). Relationship between Helicobacter pylori infection and chronic gastritis. Clin. Rev. 2019 34:389–393.
Liu, W. Z., Xie, Y., Lu, H., Cheng, H., Zeng, Z. R., Zhou, L. Y., et al. (2018). Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. The fifth national consensus report on Helicobacter pylori infection management. Helicobacter 23:e12475. doi: 10.1111/hel.12475
Lv, X. W., Wang, M. J., Qin, Q. Y., Lu, P., and Qin, G. W. (2021). 6-Gingerol relieves myocardial ischaemia/reperfusion injury by regulating lncRNA H19/miR-143/ATG7 signaling axis-mediated autophagy. Lab. Investig. 101, 865–877. doi: 10.1038/s41374-021-00575-9
Maciorkowska, E., Panasiuk, A., Kondej-Muszyńska, K., Kaczmarski, M., and Kemona, A. (2006). Mucosal gastrin cells and serum gastrin levels in children with Helicobacter pylori infection. Adv. Med. Sci. 51, 137–141
Mahomoodally, M. F., Aumeeruddy, M. Z., Rengasamy, K. R. R., Roshan, S., Hammad, S., Pandohee, J., et al. (2019). Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Semin. Cancer Biol. 69, 140–149. doi: 10.1016/j.semcancer.2019.08.009
Marques, M. S., Costa, A. C., Osório, H., Pinto, M. L., Relvas, S., Dinis-Ribeiro, M., et al. (2021). Helicobacter pylori PqqE is a new virulence factor that cleaves junctional adhesion molecule a and disrupts gastric epithelial integrity. Gut Microbes 13, 1–21. doi: 10.1080/19490976.2021.1921928
Marshall, B. J., and Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315
McColl, K. E. (2010). Helicobacter pylori infection. N. Engl. J. Med. 363, 595–596. doi: 10.1056/NEJMc1006158
Mustafa, I., and Chin, N. L. (2023). Antioxidant properties of dried ginger (Zingiber officinale roscoe) var. Bentong. Food Secur. 12:178. doi: 10.3390/foods12010178
Mustafa, I., Chin, N. L., Fakurazi, S., and Palanisamy, A. (2019). Comparison of phytochemicals, antioxidant and anti-inflammatory properties of Sun-, oven- and freeze-dried ginger extracts. Food Secur. 8:456. doi: 10.3390/foods8100456
Rehfeld, J. F. (2021). Gastrin and the moderate Hypergastrinemias. Int. J. Mol. Sci. 22:6977. doi: 10.3390/ijms22136977
Saha, P., Katarkar, A., das, B., Bhattacharyya, A., and Chaudhuri, K. (2016). 6-Gingerol inhibits Vibrio cholerae-induced proinflammatory cytokines in intestinal epithelial cells via modulation of NF-κB. Pharm. Biol. 54, 1606–1615. doi: 10.3109/13880209.2015.1110598
Santos, M. L. C., Brito, B. B., Silva, F. A. F., Sampaio, M. M., Marques, H. S., Silva,, et al. (2020). Helicobacter pylori infection: beyond gastric manifestations. World J. Gastroenterol. 26, 4076–4093. doi: 10.3748/wjg.v26.i28.4076
Schubert, M. L., and Rehfeld, J. F. (2019). Gastric peptides-gastrin and somatostatin. Compr. Physiol. 10, 197–228. doi: 10.1002/cphy.c180035
Shamsi, B. H., Chatoo, M., Xu, X. K., Xu, X., and Chen, X. Q. (2021). Versatile functions of somatostatin and somatostatin receptors in the gastrointestinal system. Front. Endocrinol. 12:652363. doi: 10.3389/fendo.2021.652363
Sharndama, H. C., and Mba, I. E. (2022). Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 53, 33–50. doi: 10.1007/s42770-021-00675-0
Shen, C. L., Wang, R., Ji, G., Elmassry, M. M., Zabet-Moghaddam, M., Vellers, H., et al. (2022). Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 100:108904. doi: 10.1016/j.jnutbio.2021.108904
Sjomina, O., Pavlova, J., Niv, Y., and Leja, M. (2018). Epidemiology of Helicobacter pylori infection. Helicobacter 23:e12514. doi: 10.1111/hel.12514
Smith, S., Boyle, B., Brennan, D., Buckley, M., Crotty, P., Doyle, M., et al. (2017). The Irish Helicobacter pylori working group consensus for the diagnosis and treatment of H.Pylori infection in adult patients in Ireland. Eur. J. Gastroenterol. Hepatol. 29, 552–559. doi: 10.1097/MEG.0000000000000822
Stein, S. C., Faber, E., Bats, S. H., Murillo, T., Speidel, Y., Coombs, N., et al. (2017). Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog. 13:e1006514. doi: 10.1371/journal.ppat.1006514
Sugano, K., Tack, J., Kuipers, E. J., Graham, D. Y., el-Omar, E. M., Miura, S., et al. (2015). Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367. doi: 10.1136/gutjnl-2015-309252
Sulo, P., and Šipková, B. (2021). DNA diagnostics for reliable and universal identification of Helicobacter pylori. World J. Gastroenterol. 27, 7100–7112. doi: 10.3748/wjg.v27.i41.7100
Tang, H., Shao, C., Wang, X., Cao, Y., Li, Z., Luo, X., et al. (2022). 6-Gingerol attenuates subarachnoid hemorrhage-induced early brain injury via GBP2/PI3K/AKT pathway in the rat model. Front. Pharmacol. 13:882121. doi: 10.3389/fphar.2022.882121
Tian, W., Wang, H., Zhu, Y., Wang, Q., Song, M., Cao, Y., et al. (2022). Intervention effects of delivery vehicles on the therapeutic efficacy of 6-gingerol on colitis. J. Control. Release 349, 51–66. doi: 10.1016/j.jconrel.2022.06.058
Veysey-Smith, R., Moore, A. R., Murugesan, S. V., Tiszlavicz, L., Dockray, G. J., Varro, A., et al. (2021). Effects of proton pump inhibitor therapy, H. pylori infection and gastric preneoplastic pathology on fasting serum gastrin concentrations. Front. Endocrinol. 12:741887. doi: 10.3389/fendo.2021.741887
Wang, J., Chen, Y., Hu, X., Feng, F., Cai, L., and Chen, F. (2020). Assessing the effects of ginger extract on polyphenol profiles and the subsequent impact on the fecal microbiota by simulating digestion and fermentation in vitro. Nutrients 12:3194. doi: 10.3390/nu12103194
Wang, Y. K., Kuo, F. C., Liu, C. J., Wu, M. C., Shih, H. Y., Wang, S. S., et al. (2015). Diagnosis of Helicobacter pylori infection: current options and developments. World J. Gastroenterol. 21, 11221–11235. doi: 10.3748/wjg.v21.i40.11221
Xie, J., Wen, J., Chen, C., Luo, M., Hu, B., Wu, D., et al. (2020). Notch 1 is involved in CD4+ T cell differentiation into Th1 subtype during Helicobacter pylori infection. Front. Cell. Infect. Microbiol. 10:575271. doi: 10.3389/fcimb.2020.575271
Yan, T. L., Wang, J. H., He, X. J., Zhu, Y. B., Lu, L. J., Wang, Y. J., et al. (2024). Ten-day Vonoprazan-amoxicillin dual therapy vs standard 14-day bismuth-based quadruple therapy for first-line Helicobacter pylori eradication: a multicenter randomized clinical trial. Am. J. Gastroenterol. 119, 655–661. doi: 10.14309/ajg.0000000000002592
Yang, E., Chua, W., Ng, W., and Roberts, T. L. (2021). Peripheral cytokine levels as a prognostic indicator in gastric cancer: a review of existing literature. Biomedicines 9:1916. doi: 10.3390/biomedicines9121916
Yang, H., and Hu, B. (2021). Diagnosis of Helicobacter pylori infection and recent advances. Diagnostics 11:1305. doi: 10.3390/diagnostics11081305
Yang, T., Wang, R., Zhang, J., Bao, C., Zhang, J., Li, R., et al. (2020). Mechanism of berberine in treating Helicobacter pylori induced chronic atrophic gastritis through IRF8-IFN-γ signaling axis suppressing. Life Sci. 248:117456. doi: 10.1016/j.lfs.2020.117456
Yoshii, S., Mabe, K., Watano, K., Ohno, M., Matsumoto, M., Ono, S., et al. (2020). Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig. Endosc. 32, 74–83. doi: 10.1111/den.13486
Yu, X., Feng, D., Wang, G., Dong, Z., Zhou, Q., and Zhang, Y. (2020). Correlation analysis of Helicobacter pylori infection and digestive tract symptoms in children and related factors of infection. Iran. J. Public Health 49, 1912–1920. doi: 10.18502/ijph.v49i10.4694
Keywords: Helicobacter pylori, 6-gingerol, MIC, gastrin, somatostatin, IFN-γ, IL-4, IL-8
Citation: Qian J, Li Z, Wang J, Lin Y and Yu Y (2024) 6-gingerol and its derivatives inhibit Helicobacter pylori-induced gastric mucosal inflammation and improve gastrin and somatostatin secretion. Front. Microbiol. 15:1451563. doi: 10.3389/fmicb.2024.1451563
Edited by:
Nagendran Tharmalingam, Houston Methodist Research Institute, United StatesReviewed by:
Sasikala Muthusamy, Brigham and Women’s Hospital and Harvard Medical School, United StatesVikram Sawant, Texas Tech University, United States
Copyright © 2024 Qian, Li, Wang, Lin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingcong Yu, Nzg0MDM4NjA4QHFxLmNvbQ==; Yuxian Lin, bGlueXV4aWFuMDgxNUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Jiali Qian1,2†
Jiali Qian1,2† Zhennan Li
Zhennan Li Yuxian Lin
Yuxian Lin