
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 24 June 2024
Sec. Food Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1430810
Oral health is critical for total body health and well-being; however, little improvement in oral health status has occurred in the U.S. over the past 20 years. Tooth decay and gum disease remain highly prevalent, with more than 90% and 50% of adults suffering from these conditions, respectively. To combat this lack of improvement, alternative approaches to dental care are now being suggested. One such alternative therapy is probiotics for oral care. In the oral cavity, probiotic strains have been shown to reduce levels of oral pathogens, inhibit the formation of dental caries, and reduce the levels of bacteria that cause halitosis. However, as the oral care probiotic market expands, many products contain bacterial species and strains with no documented health benefits leading to confusion and mistrust among consumers and clinicians. This confusion is enhanced by the regulatory status of probiotic products which puts the onus of safety and efficacy on the manufacturer rather than a central regulatory body. The overarching goal of this review is to provide consumers and clinicians with documented evidence supporting (or refuting) the health benefits of oral care probiotics marketed for sale in the United States. This includes defining what constitutes an oral care probiotic product and a strain level analysis of candidate probiotics from the genera Streptococcus, Lactobacillus, Bifidobacterium, and Bacillus. Additionally, prebiotics and postbiotics will be discussed. Finally, a set of considerations for consumers and clinicians is provided to empower probiotic product decision making. Together, this review will improve understanding of oral care probiotics marketed in the US for dental professionals and consumers.
Despite technological and medical advancements, the oral health of American adults has not improved significantly during the past 20 years (National Institute of Dental and Craniofacial Research, 2022). Gum disease and tooth decay continue to affect a majority of the population, with 52% of children diagnosed with at least one cavity by the age of 8 (Centers for Disease Control and Prevention, 2021). Poor oral health is linked to an array of systemic health problems including diabetes, heart disease, and dementia (Centers for Disease Control and Prevention, 2021; National Institute of Dental and Craniofacial Research, 2022). Oral health conditions including cavities, gum disease, and tooth loss also directly affect quality of life, influencing social interactions, employment opportunities, and self-confidence (Kaur et al., 2017; National Institute of Dental and Craniofacial Research, 2022).
Historically, dental care has focused on three preventative therapies: tooth brushing, flossing, and fluoride treatment. However, these recommended therapies have not changed since the introduction of fluoride in drinking water in 1945 (Unde et al., 2018). When preventative methods fail, oral health conditions are treated using reactive therapy, often applied in advanced stages of disease progression (Nock, 2024). Methods including scaling and root planing, fillings, and antibiotic application (among others) have been used in reactive dentistry for more than 50 years (Yilmaz et al., 1994), but these methods have had limited success improving the overall status of oral health in the United States (National Institute of Dental and Craniofacial Research, 2022). Due to the lack of success, alternative proactive approaches to dental care are needed to improve oral health. One such alternative therapy is probiotics.
In 2001, the term “probiotic” was formally defined by the World Health Organization as, “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Food and Agriculture Organization and World Health Organization Expert Consultation, 2001). Probiotics have primarily been marketed to the general public for gastrointestinal disease therapy and, to date, most consumers affiliate probiotic bacteria with gut health. However, the oral cavity is the ideal environment for the application of probiotic therapy because many of the diseases that affect the mouth originate from dysbiosis of the oral microbiome. For example, the primary cause of periodontitis is an increase in key dental pathogens such as Porphyromonas gingivalis (How et al., 2016; Abdulkareem et al., 2023). In a healthy oral cavity, P. gingivalis may be present in the dental biofilm at very low concentrations, but stressors including diet, lifestyle, and individual susceptibility can increase the abundance of this pathogen resulting in disease (How et al., 2016). Cavities also originate from oral dysbiosis, as the primary etiological agent is an overabundance of Streptococcus mutans (Forssten et al., 2010). Other oral conditions, such as halitosis (or bad breath) are also associated with microbial dysbiosis. Individuals suffering from halitosis often have an overabundance of microorganisms that produce volatile sulfur compounds compared to those without halitosis (Haraszthy et al., 2007).
The concept of bacterial probiotic therapy for oral health was first reported in 1985 when Hillman et al. isolated multiple strains of Streptococcus spp. from healthy subgingival plaque. These bacterial strains were capable of inhibiting the growth of periodontal pathogens including Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis (previously Bacteroides gingivalis; Hillman et al., 1985). The method of inhibition was shown to be hydrogen peroxide production, a natural metabolic biproduct of the isolated Streptococcus strains (Hillman et al., 1985). Hillman’s foundational work led to the concept of replacement therapy in the oral cavity. Replacement therapy maintains balance in the oral microbiome by replacing disease causing microorganisms with a higher abundance of beneficial microorganisms through competitive exclusion (Hillman et al., 1987, 2009). This concept continues to serve as the basis for the selection of probiotics for oral health therapy today.
The demand for proactive therapy continues to increase across the dental landscape, resulting in an exponential expansion of the oral-care probiotic market in the United States. The first probiotic product specifically designed for oral-care was marketed in the mid-2000s. Today, more than 25 companies market probiotic products for oral health with over 50 bacterial species and strains included across the products (How and Yeo, 2021). Many of these products contain bacterial species or strains that are “Generally Recognized as Safe” (GRAS), or available for inclusion in food products and dietary supplements based on a history of safe use. However, safety does not necessitate efficacy, and many bacterial strains used in oral care probiotics have no documented health benefit in the oral cavity (Van Holm et al., 2023).
As the market grows, dental professionals and consumers may be overwhelmed by the probiotic products available for use. Conflicting, misleading, or confusing information supplied by competing product manufacturers can overshadow peer-reviewed research and may lead to public distrust of probiotics as a proactive dental therapy. The overarching goal of this review is to demystify the US oral-care probiotic market by providing an in-depth analysis of the science behind bacterial strains currently included in oral-care probiotics. Critical details including the origin of isolation, documented probiotic benefit (s) in the oral cavity (or lack thereof), dosage, efficacy, and safety of the probiotic strains is included. Prebiotics, postbiotics, and the state of the oral health probiotics market is also reviewed. Together, this information will help dental professionals and consumers understand both the science supporting the use of oral-care probiotics and how to sift through marketing messages for a product that delivers targeted, research-backed health benefits.
Developing a probiotic requires careful consideration of multiple factors including (but not limited to): the isolation location of the bacterial strain, ability to survive in the desired body area, safety of the strain, efficacy in the desired body area, ability to ferment on an industrial scale, and potential contraindications in other body areas. Here, the steps used to develop a probiotic product in the United States are outlined, but many of the principles apply globally. The first step for a targeted probiotic is to define the area in which the probiotic effect should occur. For an oral care probiotic, health effects should be expected in the regions covered by the oral cavity, which is defined by the National Institutes of Health as, “refers to the mouth…it includes the lips, the lining inside the cheeks and lips, the front two thirds of the tongue, the upper and lower gums, the floor of the mouth under the tongue, the bony roof of the mouth, and the small area behind the wisdom teeth” (National Cancer Institute, 2024). This area also houses the teeth.
Next, one must consider the location of isolation. Logically, one would propose that a probiotic for oral care should be isolated from the oral cavity; however, the oral cavity contains over 700 species of bacteria (Aas et al., 2005). To narrow down possible probiotic candidates, bacteria routinely found in healthy mouths should be considered first. Sequencing technologies have made this considerably simpler. Bacterial succession from infancy through adulthood follows a common trajectory in a healthy oral cavity. In the first few days of life, members of the genera Streptococcus, Veillonella, and Fusobacteria serve as early colonizers (Dzidic et al., 2018). Members of the genera Rothia and Gemella arrive before the age of one, followed by late colonizers including members of Neisseria and Actinomyces arriving after one year of age (Dzidic et al., 2018; Figure 1). In a healthy adult oral cavity, members of the genera Streptococcus, Rothia, Neisseria, Veillonella, and Actinomyces dominate (Deo and Deshmukh, 2019) while pathogenic species of the genera Tannerella, Bifidobacterium, and Prophyromonas were found in diseased mouths (Aas et al., 2005; Willis and Gabaldón, 2020; Figure 1). While sequencing information can help identify species routinely found in healthy mouths, an estimated 40%–60% of the bacteria in the oral cavity cannot be grown in the lab (Siqueira and Rôças, 2013), further limiting potential probiotic strains. Bacterial strains found in healthy mouths and capable of growth outside the oral cavity move on to the next stage of evaluation to be considered for use as a probiotic.
Following the selection steps above, safety and efficacy of the probiotic strain candidates must be evaluated. Bacteria, as a group, are generally beneficial organisms contributing a wide range of functions essential for life on earth. While only a small proportion of bacteria cause infection and disease (Doron and Gorbach, 2008), serious illness and even death can occur if probiotic candidates are not thoroughly vetted. Extreme caution must be used to verify that bacterial strains considered for probiotic applications are safe for human use, non-pathogenic, and are resistant to at least a portion of commonly available antibiotics (Sanders et al., 2010; Pradhan et al., 2020). Verification of these factors has become markedly simple with advances in sequencing technologies. Entire bacterial genomes, proteomes, and even resistomes can be sequenced and evaluated for relatively low cost at high resolution (Satam et al., 2023). Whole genome sequencing, virulence assessments, antibiotic resistance evaluations, and genetic stability should be the minimum standard for new probiotic strains being considered for the market.
Once a probiotic candidate strain has been isolated from the desired body location, found capable of growing in the laboratory, and deemed safe for use, a thorough analysis of efficacy should be conducted. For oral care probiotics, one must consider what health benefit (s) are desired and can be achieved by a bacterial strain. Probiotics exert health benefits through a variety of mechanisms including competitive exclusion, antimicrobial compound production, bacteriocin production, immune modulation, and interactions with the host endocrine system (Bermudez-Brito et al., 2012; Plaza-Diaz et al., 2019). In the oral cavity, many disease states are the direct result of overgrowth of pathogenic bacteria. Gingivitis, periodontitis, and caries have etiologies of bacterial or microbial origin (Tatakis and Kumar, 2005; Chen et al., 2020); thus, probiotic bacteria that inhibit the growth of or complete for attachment sites with oral pathogens are prime candidates for oral care probiotics. In addition to identifying the potential health benefits of a probiotic candidate strain, a review of interactions between the strain and other oral microorganisms is critical. For example, multiple lactic acid bacteria of the genus Lactobacillus have been proposed as oral care probiotics citing reduced inflammation of oral tissues following use. However, Lactobacillus spp. have been implicated in the formation and progression of dental caries (Caufield et al., 2015; Shimada et al., 2015), suggesting the risk outweighs the potential benefit. This example, explored in more detail in Section 4.2. below, highlights the need for a thorough review of the probiotic interactions within the oral cavity in addition to the mechanism of action.
Following safety and efficacy assessments, marketability and scalability of the probiotic candidate strain must be assessed. Probiotic products sold to consumers must meet multiple manufacturing and consumer requirements that are rarely considered during the strain isolation stage. Manufacturing considerations including strain yield, Good Manufacturing Practices (GMP), absence of contaminants, product consistency, and product stability across varying temperatures and humidity are critical to a successful probiotic product (Fenster et al., 2019) while consumer preferences may include shelf-life, ease-of-use, and sustainability (Siddiqui et al., 2023). Additionally, probiotic candidate strains may be patented for specific uses or in specific combinations, which may limit the use of the probiotic candidate strain (s) to individual companies or designated dosage forms. Businesses considering using probiotic strains in their products must evaluate the strain for propensity to produce a high-yield (>100 Billion colony forming units/g) in an industrial fermentation setting which is not possible for all probiotic candidates (Fenster et al., 2019). Additionally, probiotic stability factors including shelf-life, resistance to contamination, and probiotic viability, are critical for marketed probiotic products.
Probiotic candidate strains that are shown to be safe, effective, and scalable are considered ready for the USA market. In the USA, most probiotic products are marketed as dietary supplements or functional food ingredients which only require pre-market notification, not approval, by the U.S. Food and Drug Administration (FDA). Of special note, the FDA does not approve dietary supplements or functional food ingredients for their safety and efficacy prior to sale; it is considered the responsibility of the manufacturer to ensure the products are safe and labeled following FDA guidelines (United States Food and Drug Administration, 2023). However, new dietary ingredients, or those that that were not marketed in a dietary supplement or as functional food ingredients before 15 October 1994, must notify the FDA at least 75 days prior to sale (U.S. Department of Health and Human Services, 2024). New dietary ingredients include newly isolated probiotic candidate strains being considered for use in probiotic applications. Probiotic candidate strains that have been used historically in food applications, such as starter cultures, may have GRAS status which can serve as the basis of safety for the new dietary ingredient notification. Despite the notification requirement, the exact evidence required to be submitted for safety and efficacy of the new dietary ingredient is not explicitly specified. Following notification, the FDA issues an “acknowledgment of receipt” which is considered a procedural matter, not an attestation of ingredient safety (U.S. Department of Health and Human Services, 2024). This puts the onus of determining product safety on (1) the manufacturer and (2) the consumer (Figure 2). Thus, a thorough understanding of the science behind probiotic strains marketed for oral care is critical.
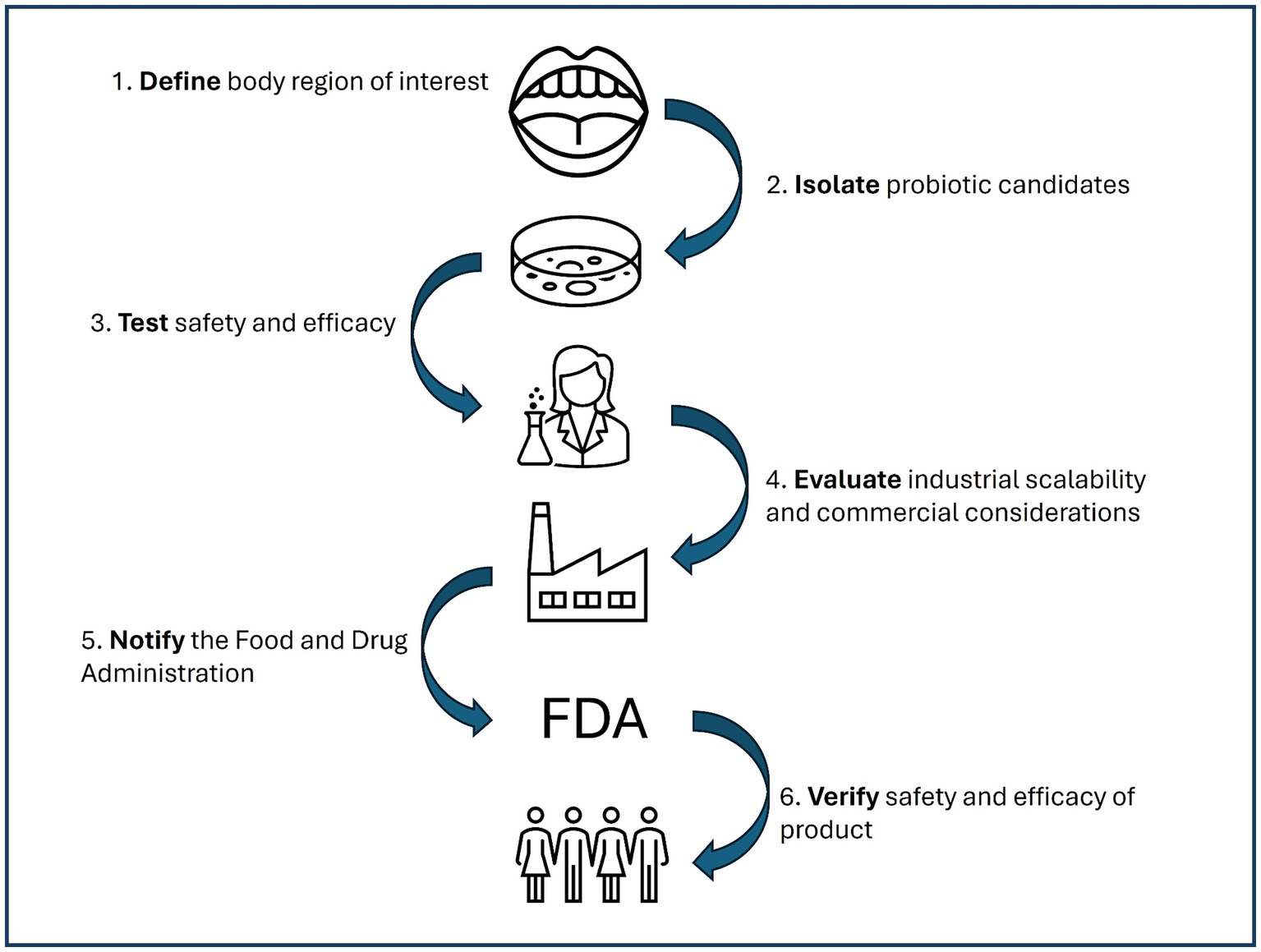
Figure 2. Overview of the probiotic product development process from candidate strain isolation through consumer verification of safety and efficacy.
Many excellent reviews have recently summarized the mechanisms of action of oral probiotics on specific oral diseases including those by Chugh et al. (2020) and Homayouni Rad et al. (2023). In general, these mechanisms fall into one of three categories: (1) competitive species interactions, (2) production of antimicrobials or inhibitory substances, and (3) immune modulation. In the oral cavity, several specific mechanisms of action have been identified and are thoroughly reviewed at the strain level in Section 4 below. In general, the most common mechanism of action of probiotic bacteria in the oral cavity is competitive exclusion (Hibbing et al., 2010). Probiotic bacteria strains directly complete with pathogens for nutrients, resources, and attachment sites. This method is effective against oral pathogens such as Streptococcus mutans, which causes cavities (Kreth et al., 2009), and Tannerella forsythus, which produces the volatile sulfur compounds associated with halitosis (Han et al., 2023). Additionally, many oral probiotic strains produce antimicrobial metabolites such as antibiotics or hydrogen peroxide that inhibit the growth of oral pathogens (Homayouni Rad et al., 2023). This method of inhibition works well against anaerobic periodontal pathogens such as Porphyromonas gingivalis and Tannerella forsythia, which cause periodontitis (Hillman et al., 1985). Additionally, recent research has shown that some oral probiotics may be useful in the identification or treatment of oral cancers via immunomodulation pathways that lead to apoptosis of cancer cells or anti-metastasis activity (Mohd Fuad et al., 2023). While these results are promising, it remains critical to identify the mechanism of action at the strain level for probiotic bacteria.
Members of the genus Streptococcus are Gram-positive, catalase-negative, lactic acid producing bacteria (Jevitz Patterson, 1996; Du Toit et al., 2014). While a few members of the Streptococcus genus are opportunistic pathogens, many streptococci are indigenous commensals in the human microbiome (Abranches et al., 2018; Baty et al., 2022). In the oral cavity, streptococci serve as early colonizers, shaping the oral microbiome and supporting tooth and gum development (Abranches et al., 2018; Sulyanto et al., 2019). Streptococci remain abundant in the oral cavity throughout the transition from childhood to adulthood (Bik et al., 2010; Sulyanto et al., 2019; Ruan et al., 2022) in part due to the production of adhesins. Adhesins produced by streptococci facilitate strong binding teeth and gums. Binding strength is critical for bacterial survival in the oral cavity because saliva flow and food consumption create significant shearing forces capable of displacing bacteria that are more weakly attached (Abranches et al., 2018). Despite their metabolic capacity to produce lactic acid, most commensal oral Streptococcus spp. do not contribute to acidogenic tooth decay due to a negative feedback loop in which acid production reduces oral pH which then inhibits the growth of the commensal (Castillo et al., 2000; Figure 3A); this is in contrast to pathogenic oral streptococci which thrive in a low pH environment (Kreth et al., 2009; Figure 3B). Additionally, many oral streptococci contain the arginine deiminase system (ADS) which converts arginine to ammonia and raises local pH (Baty et al., 2022). Together, these factors support commensal streptococci as probiotic candidates.
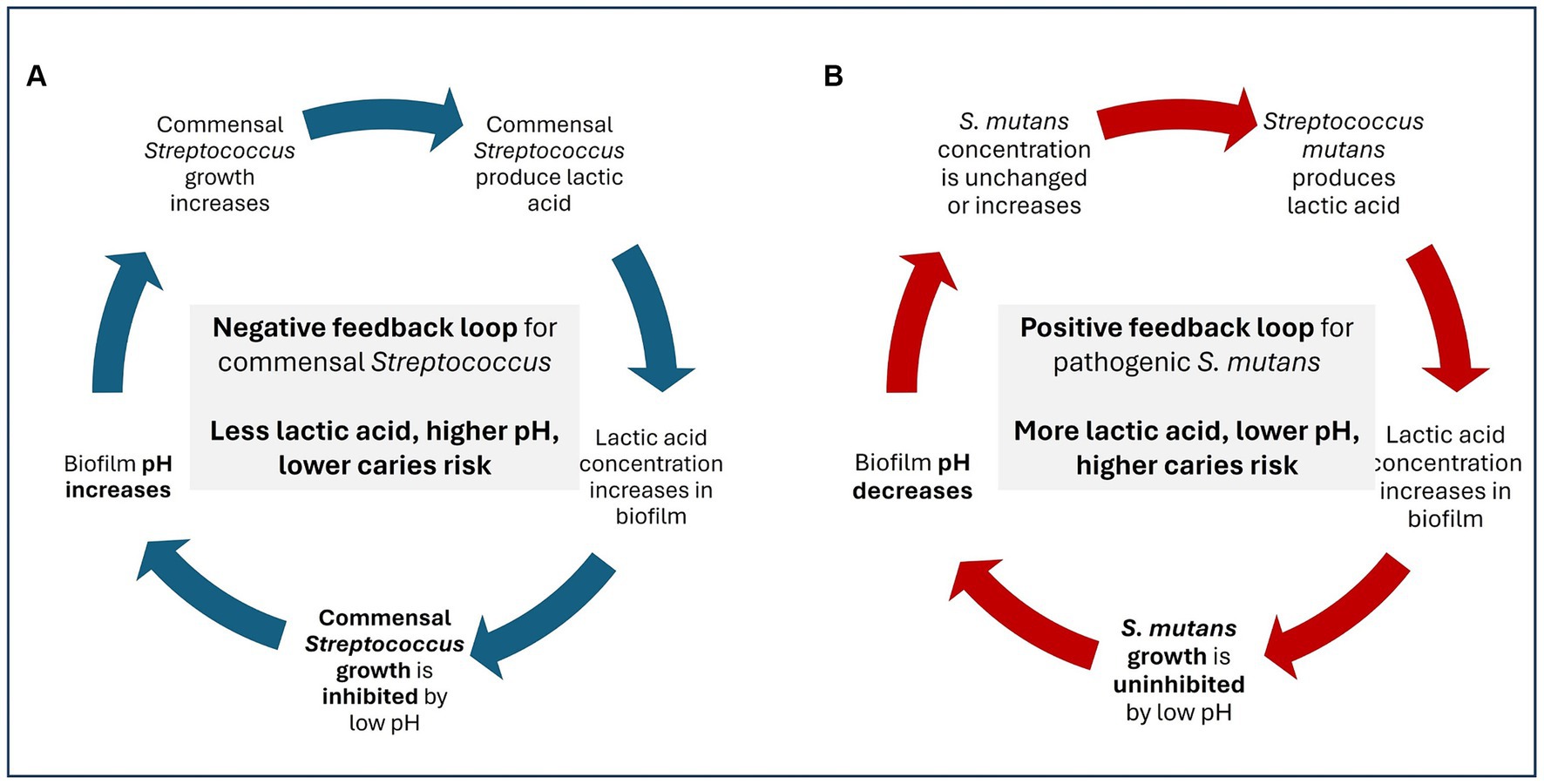
Figure 3. Negative feedback loop (A) and positive feedback loop (B) of lactic acid production by commensal vs. pathogenic Streptococcus spp.
In the oral cavity, Streptococcus spp. supply a health benefit to the host by inhibiting the growth of dental pathogens via metabolic byproducts such as bacteriocins and hydrogen peroxide (Chen et al., 2011; Baty et al., 2022). Oral care probiotic products may contain one or multiple Streptococcus spp., but the potential health benefits are strain specific. The most common Streptococcus strains found in oral care probiotics are explored in more detail in Table 1.
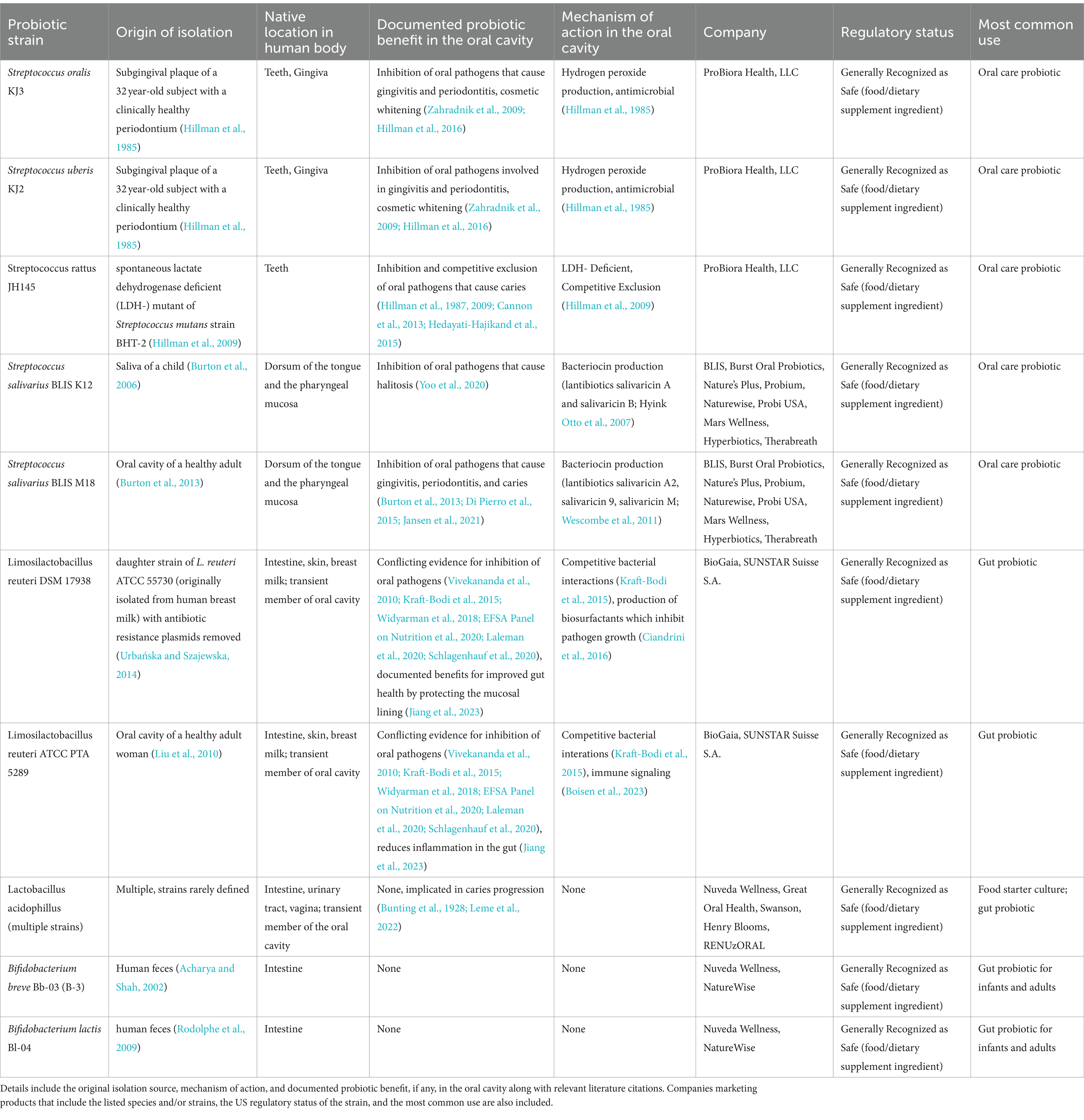
Table 1. Overview of common probiotic candidate species and strains included in oral care probiotics in the United States.
The earliest reported Streptococcus strains displaying a probiotic benefit were isolated from the subgingival plaque of a healthy adult subject in 1985 (Hillman et al., 1985). Two strains, S. oralis strain KJ3 (previously S. sanguis Type II strain KJ3) and S. uberis strain KJ2, were shown to have inhibitory effects on the growth of oral pathogens implicated in periodontitis including Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis (previously Bacteroides gingivalis; Hillman et al., 1985). The production of hydrogen peroxide by S. oralis KJ3 and S. uberis KJ2 was found to be the mechanism of action for pathogen growth inhibition. In addition, S. oralis strains are early colonizers of the tooth surface, binding strongly to the salivary pellicle (Li et al., 2004; Dorkhan et al., 2013). Colonization of the tooth surface by probiotic Streptococcus strains such as S. oralis KJ3 and S. uberis KJ2 has been shown to shift the microbiome of the oral cavity to a healthier state (Zahradnik et al., 2009). Additionally, the low level of hydrogen peroxide produced by S. oralis KJ3 and S. uberis KJ2 provide a whitening effect on the tooth enamel (Hillman et al., 2016). Although dental whitening is frequently cited as a cosmetic benefit, whiter teeth have been shown to have a positive social and psychological effect as well (Estay et al., 2020).
Streptococcus rattus strain JH145 is another probiotic strain in the Streptococcus genus. This bacterium is a spontaneous mutant of a Streptococcus mutans strain isolated from a carious lesion in an adult subject (Hillman et al., 2009). In contrast to S. mutans, S. rattus JH145 is lactate dehydrogenase deficient (LDH-), and thus does not produce lactic acid as part of its metabolism. As an oral care probiotic, S. rattus JH145 provides a health benefit through competitive exclusion by consuming the same resources and inhabiting the same ecological niche as S. mutans strains (Hillman et al., 1987). This effect has been shown in multiple animal and human clinical studies (Hillman et al., 2009; Cannon et al., 2013, 2019; Hedayati-Hajikand et al., 2015).
Streptococcus salivarius strains K12 and M18 also provide probiotic benefits in the oral cavity. These strains were originally isolated from the saliva of a child (S. salivarius K12; Burton et al., 2006) and the oral cavity of a healthy adult (S. salivarius M18; Burton et al., 2013). It should be noted that S. salivarius K12 and M18 are occasionally marketed under the alternative strain identifiers DSM 13084 (strain K12) and DSM 14685 (strain M18), depending on the product. Streptococcus salivarius K12 and M18 produce bacteriocins [lantibiotics (McAuliffe et al., 2001; Hyink Otto et al., 2007; Wescombe et al., 2011)] which act in a similar manner to antimicrobials. Streptococcus salivarius K12 is frequently cited as an ear, nose, throat, and upper respiratory probiotic (Upton et al., 2001; Zupancic et al., 2017; Bertuccioli et al., 2023) while S. salivarius M18 is more often cited as a “true” oral care probiotic inhibiting dental pathogens (Burton et al., 2013; Di Pierro et al., 2015). A combination of the two strains has been shown to reduce immune activation induced by periodontal pathogens (MacDonald et al., 2021), reduce the abundance of periodontal pathogens (Jansen et al., 2021), and reduce the levels of volatile sulfur compounds involved in halitosis (Yoo et al., 2020). In general, these probiotic effects occur due to either (a) inhibition of oral pathogen growth due to bacteriocin production or (b) reducing inflammatory responses by downregulating proinflammatory pathways (MacDonald et al., 2021; Baty et al., 2022). Human clinical trials support the claims that these strains reduce concentrations of caries causing bacteria (Poorni et al., 2022).
Streptococcus thermophilus, a member of the salivarius subgroup, is occasionally included in products marketed for oral care; however, this species is most frequently used as a starter culture for foods including yogurt and some cheeses (Cui et al., 2016). When this strain is included in probiotic products for oral care, it is found in combination with other bacterial species and is, to the best of this author’s knowledge, never identified to the strain level (How and Yeo, 2021). The lack of strain level information for this species suggests that S. thermophilus is not currently a good probiotic candidate for oral care.
Until 2020, the genus Lactobacillus contained more than 250 bacterial species with distinct phenotypes, genotypes, and ecological niches (Zheng et al., 2020). Today, members of the Lactobacillus genus have been reclassified into 25 distinct genera that, despite their new names, continue to comprise a large proportion of the human microbiota (De Angelis and Gobbetti, 2011; Zheng et al., 2020). For simplicity, the name Lactobacillus will be used throughout this section with reference made to the reclassified species and strain names where appropriate. As the name suggests, Lactobacillus species are lactic acid producing, Gram-positive, catalase negative bacteria that are generally considered aerobic but may be able to tolerate low levels of oxygen (Zotta et al., 2017). Many Lactobacillus species are well-known gut probiotics with documented health benefits including immune modulation, competitive exclusion, antimicrobial excretion, and inflammation suppression (for an updated review, see Dempsey and Corr, 2022). However, the safety and efficacy of Lactobacillus spp. for probiotic use in the oral cavity is less well understood.
A few Lactobacillus spp. are found in the oral cavity of newborn infants (Sulyanto et al., 2019), but these populations are no longer measurable after 1 month or after the cessation of breast feeding (Caufield et al., 2015). Lactobacillus spp. are not considered dominant members of the oral cavity, and established populations are often found only in individuals with carious lesions (Caufield et al., 2015). Despite the large body of evidence implicating Lactobacillus spp. in the progression of dental caries (Caufield et al., 2015; Ademe et al., 2020; Sounah and Madfa, 2020; Wen et al., 2022), many oral care products on the market contain Lactobacillus spp. as probiotics. This may appear to parallel the use of commensal Streptococcus spp. as probiotics when S. mutans is a well-known cariogenic bacterium as described in Section 4.1. However, Lactobacillus spp. in the oral cavity are frequently linked to food products in which Lactobacillus strains were used as starter cultures, suggesting they are transient members of the oral microbiota rather than permanent colonizers (Caufield et al., 2015). Research also indicates Lactobacillus spp. are not indigenous to the oral cavity (Caufield et al., 2015) and therefore may not be the preferred choice for an oral care probiotic product, especially if their presence is linked to tooth decay. Regardless, due to their popularity, consumer familiarity with the name, and documented health benefits in gut probiotics (Dempsey and Corr, 2022), Lactobacillus spp. are readily available for use, cheap, and documented as “safe” (Salminen et al., 1998; Rodríguez-Sánchez et al., 2021), leading to incorporation into many marketed oral care probiotic products. The most common Lactobacillus (or former Lactobacillus) species and strains used in oral care probiotics are described in more detail in Table 1.
Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) strain 299 (or DSM 6595) and Lactiplantibacillus plantarum strain 299v (or DSM 9843) were originally isolated from the mucosa of a healthy human intestine (Molin et al., 1993). These two strains are well studied as probiotics for the gut (Nordström et al., 2021). Potential probiotic benefits of these strains in the oral cavity have also been explored with studies showing that L. plantarum 299v can co-aggregate with S. mutans in carious lesions (Twetman et al., 2009) and can inhibit biofilm formation of clinical isolates of S. mutans (Söderling et al., 2011). However, additional research shows that L. plantarum 299v produces significantly more lactic acid than other L. plantarum strains (Haukioja et al., 2008; Keller and Twetman, 2012) which suggests that the acidogenicity of the strain needs to be considered prior to promoting its use as an oral care probiotic. Another L. plantarum strain, HEAL19 (or DSM 15313), has recently been incorporated into oral care probiotic products (Durrell, 2021). However, PubMed has only indexed a total of seven peer-reviewed research papers citing this bacterial strain and none of them support the use of this strain for oral health. Additional strains of L. plantarum have been isolated, and a few have been studied for oral health benefits [such as strains L-137 (Schlagenhauf and Jockel-Schneider, 2021), DSM 32131 (Volgenant et al., 2022), NC8 (Khalaf et al., 2016), and 44048 (Khalaf et al., 2016)]. The results suggest that strains of L. plantarum can survive in the oral cavity, but their inhibition of oral pathogens is strain and pH dependent. In conclusion, not all strains of L. plantarum behave similarly in the oral cavity and more research is needed to confirm a probiotic benefit.
Lacticaseibacillus paracasei strains including 8,700:2 (or DSM 13434), Lpc-37, ET-22, SD1, and adp-1 are also used in oral care probiotics (How and Yeo, 2021). Lacticaseibacillus paracasei strains ET-22 and SD1 do have some support as oral care probiotics in the literature; these strains have been shown to suppress the formation of caries by inhibiting the formation of biofilm (Guo et al., 2023; Zhao et al., 2023) or reducing the concentration of S. mutans in the oral cavity (Teanpaisan et al., 2015). However, limited placebo-controlled, double-blinded clinical studies are available and most of the research on L. paracasei strains remains in gut health.
Limosilactobacillus reuteri strains, especially DSM 17938 and ATCC PTA 5289, are also frequently listed as probiotics for oral care. Limosilactobacillus reuteri DSM 17938 is a daughter strain of L. reuteri ATCC 55730 that was originally isolated from human breast milk (Urbańska and Szajewska, 2014). Limosilactobacillus reuteri ATCC 55730 contained potentially transferable antibiotic resistance plasmids; thus, the daughter strain DSM 17938, in which the antibiotic resistance plasmids were removed, is used in probiotic products (Urbańska and Szajewska, 2014). Limosilactobacillus reuteri ATCC PTA 5289 was isolated from the oral cavity of a healthy adult woman (Liu et al., 2010). Oral outcomes for these strains are mixed; some studies suggest that they improve gingival health (Schlagenhauf et al., 2020) and reduce the concentration of oral pathogens (Kraft-Bodi et al., 2015; Widyarman et al., 2018), however, research also shows that indices such as bleeding on probing may not be improved (Kraft-Bodi et al., 2015) and oral pathogen levels are not always reduced following use (Vivekananda et al., 2010; Laleman et al., 2020). Interestingly, in 2020, the European Food Safety Authority made a definitive statement that current research on L. reuteri strains DSM 17938 and ATCC PTA 5289 is insufficient to determine if they provide a health benefit for the gums (EFSA Panel on Nutrition et al., 2020). Additional research is warranted to determine the efficacy of these strains as oral care probiotics.
The strains detailed above are not exhaustive of the current and former Lactobacillus species included in oral care probiotics; see How and Yeo (2021) for a thorough list. Lactobacillus spp. remain the dominant group of bacteria included in probiotic products, regardless of the body area in which the health benefit should occur. This has led to an abundance of marketed products that do not contain enough information for the consumer to make an informed decision and is exacerbated by a lack of strain level information on probiotic product labels (Weese and Martin, 2011). Together, these factors lead to the scenario described in Section 2; individuals take a probiotic product under the assumption that it will provide a health benefit in the mouth when it may actually be contributing to oral health problems. One such species is Lactobacillus lus, which is often included in probiotics for oral care with no strain identifier (How and Yeo, 2021). As noted previously, strain level information is critical for probiotics as the same bacterial species may vary widely in gene content at the strain level. Lactobacillus acidophilus (formerly Bacillus acidophilus) was one of the first bacteria identified in the progression of dental caries (Bunting et al., 1928; Johnston et al., 1933). More recent research suggests L. acidophilus is frequently found in carious lesions (Leme et al., 2022) and may form dual species biofilms with S. mutans (Mei et al., 2013). Although L. acidophilus ATCC 4356 was able to induce downregulation of glucan production in co-cultured S. mutans (which may reduce biofilm formation), the Lactobacillus strain was still able to incorporate into the oral biofilm (Lee and Kim, 2014) where it can contribute to acid production. Together, these results suggest that L. acidophilus (or any bacterium at the species level only) is not an appropriate candidate for an oral care probiotic, and yet it is still found in many probiotic products targeted for oral health.
Members of the genus Bifidobacterium are Gram-positive anaerobes that are predominantly found in the human gastrointestinal tract. Although the Bifidobacterium genus contains over 90 species (Chen et al., 2021), few are found as indigenous commensals in the oral cavity. For example, although B. dentium and B. longum have been isolated from the mouth (Modesto, 2018), these species are often found associated with carious lesions (Dige et al., 2014; Manome et al., 2019). In the gut, Bifidobacterium spp. are used as probiotics. Milk-based formula may include Bifidobacterium spp. as they have been shown to reduce the risk of gastroenteritis and stimulate the immune system in infants (Lemoine et al., 2023). Additionally, some strains of Bifidobacterium are used in psychological health and may help reduce stress and anxiety (Chen et al., 2021).
In the oral cavity, the probiotic benefits of Bifidobacterium are not well defined. A recent meta-analysis of the role of Bifidobacterium spp. in the oral cavity concluded that limited evidence of a health benefit exists, and additional research is required (Jayachandra et al., 2023). A similar comprehensive review found that Bifidobacterium spp. research in the oral cavity is conflicting, with some studies showing positive reductions in caries causing bacteria and others showing increased acidity and carcinogenicity when Bifidobacterium strains are introduced (Homayouni Rad et al., 2023). Specific Bifidobacterium strains that are included in common oral care probiotics include B. breve strain Bb-03 (or B-3) and B. lactis Bl-04 (How and Yeo, 2021; Table 1). Based on a PubMed and Google Scholar search of both strains, no research exists showing a health effect in the oral cavity following use. However, when Bifidobacterium strains including B. lactis Bb-12 and B. bifidum ATCC 29521 were consumed in food products such as ice cream or yogurt, significant reductions in S. mutans were found (Homayouni Rad et al., 2023). However, these Bifidobacterium strains were often combined with Lactobacillus spp. so the probiotic effect cannot be directly linked to Bifidobacterium spp. alone. As with Lactobacillus spp. many oral care probiotics on the market containing Bifidobacterium do not include the strain identifiers necessary for consumer transparency and confidence (How and Yeo, 2021). Together, these results suggest that Bifidobacterium spp. are not well supported as oral probiotic strains.
Members of the genus Bacillus are gram-positive, spore forming bacteria that can survive in either aerobic or facultative anaerobic environments. As spore formers, Bacillus spp. are resilient to temperature fluctuations, desiccation, and many disinfectants (Turnbull, 1996). This can make Bacillus spp. difficult to kill, and members of the genus are often implicated in food spoilage. In the human body, Bacillus are found in the gut (Lee et al., 2019). Although they are often used as probiotics for crops and livestock (Leistikow et al., 2022), their use as probiotics for human health is less common due to the propensity of Bacillus spp. to transfer antibiotic resistance genes and produce toxic biproducts (Lee et al., 2019).
Only one Bacillus species was found in marketed oral care probiotics: Bacillus coagulans strain Unique IS2 (or ProDura; How and Yeo, 2021). A recent placebo controlled, double-blind clinical trial found that following 14 days of B. coagulans Unique IS2 application, oral levels of S. mutans and Lactobacillus spp. were significantly reduced (Ratna Sudha et al., 2020). However, the mechanism of action was not identified. Additional evidence of efficacy is needed for this oral care probiotic candidate, and caution should be used for any potential Bacillus probiotic based on the factors noted above.
Like probiotics, prebiotics were originally conceived for gut health. Prebiotics were first defined as a, “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health (Gibson and Roberfroid, 1995).” Today, the official definition of prebiotics has expanded in scope to include, “a substrate that is selectively utilized by host microorganisms conferring a health benefit (Gibson et al., 2017),” which includes ingredients that stimulate growth and/or activity of bacteria in the oral cavity. It is important to note that probiotic bacteria, if well-selected for the body area of interest, do not require a prebiotic to confer a health benefit. However, prebiotics may encourage growth of probiotics strains providing an additional benefit.
In the oral cavity, a variety of prebiotics have been investigated. These include some sugars, sugar alcohols, oligosaccharides (complex sugars), amino acids, and nitrogen species (Luo et al., 2024). Prebiotics must be carefully selected to encourage the growth of probiotic strains without stimulating the growth of oral pathogens. Examples of well-researched prebiotics for oral care include xylitol (a sugar alcohol), arginine (an amino acid), and urea (a nitrogen species). Multiple studies have shown xylitol reduces levels of S. mutans in plaque and saliva (Milgrom et al., 2006, 2009; ALHumaid and Bamashmous, 2022) primarily because S. mutans strains cannot ferment xylitol (Nayak et al., 2014). This results in an increase in oral pH. Arginine is a relatively new prebiotic shown to neutralize oral pH (Nascimento, 2018) as a precursor to nitric oxide. The arginine deiminase pathway of many commensal oral bacteria (including S. oralis and S. rattus) produces alkali compounds which inhibits the formation of acidic plaque (Zheng et al., 2017; Nascimento, 2018). Urea works similarly to arginine in the oral cavity, as microbial metabolism converts urea to ammonia, raising the oral pH (Mora and Arioli, 2014).
Although prebiotics for oral health appear promising, additional research is needed to verify if these compounds support the growth of probiotic bacterial species in the mouth. Additionally, most prebiotics are targeted to increase oral pH, which plays a role in caries development and prevention. However, little is known about prebiotics to support other oral health conditions such as gingivitis and halitosis.
Postbiotics are the newest component of microbially derived products that confer a health benefit. Defined in 2019 as, “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host,” postbiotics are compounds that do not require, but must be prepared from, live microorganisms (Salminen et al., 2021). Postbiotics can include any portion of a microbial cell such as the cell wall or cytoplasm or a microbial metabolite. Most often, postbiotics are compounds that are released by live microorganisms that confer a health benefit, such as hydrogen peroxide and bacteriocins produced by members of the Streptococcus genus (Homayouni Rad et al., 2023). Postbiotic research is still in its infancy, but research has shown that the fermentation products of probiotic strains can inhibit the growth of oral pathogens in the absence of the live probiotic strain (Lin et al., 2022). These results are encouraging and provide an alternative avenue for producing the health benefit of probiotic strains without the manufacturing and storage constraints of maintaining a live microorganism. Additionally, the inherent risk of consuming live microbial products is significantly reduced by supplying only the metabolite or cellular component rather than living cells.
Oral health disparity in the United States remains a serious challenge (National Institute of Dental and Craniofacial Research, 2021). Caries, gingivitis, and periodontitis continue to affect a significant portion of the population despite targeted treatment efforts (National Institute of Dental and Craniofacial Research, 2021). Alternative therapies, such as the probiotics described here, are gaining traction. The North American oral probiotic market alone was estimated at a value of more than 100 million USD in 2022 with an expected growth of nearly 10% by 2030 (Grand View Research, 2022). Consumer trends are driving the oral probiotic market, with preferences for more holistic and natural solutions to oral health problems (Grand View Research, 2022). Unfortunately, this has led to an influx of probiotic products marketed for oral care that contain bacterial strains with a history of safe use but no documented oral health benefits. These strains are the easiest to acquire, manufacture, and include in a product despite their lack of efficacy in the oral cavity. This increases consumer confusion regarding strain choice, safety, and potential health benefits of probiotic products.
Although many probiotic strains have been shown to be safe and effective for a variety of oral health conditions, information provided from probiotic companies is not always clear. Consumers are more likely to place trust in information provided by probiotic scientists than from news media or online content; however, science-based information, such as peer-reviewed journal articles, are often inaccessible to consumers (Vijaykumar et al., 2022). Probiotic recommendations for oral care may be more widely accepted from dental clinicians, but clinicians themselves often receive conflicting information and evidence about probiotic products. To help clarify misinformation, the U.S. Department of Health and Human Services prepared a probiotic fact sheet specifically targeted for health professionals; however, probiotics for oral care are not included (National Institutes of Health Office of Dietary Supplements, 2023). This oversight leaves health professionals and consumers to source information themselves from either (a) product labels or (b) the company manufacturing the probiotic products for sale. Due to the presumption of safety rule for probiotic products in the U.S. (meaning products are assumed safe until proven otherwise), consumers are responsible for evaluating the marketing and science messaging probiotic companies provide to determine safety and efficacy. This model is inefficient and may lead to the consumption of probiotic products with no health benefit at best or serious health complications at worst. So how can consumers and dental professionals make informed choices about probiotic products for oral care? A thorough review of the following information is suggested:
1. Does the product label contain the probiotic strain name (s) and active ingredient dosage(s)? Probiotics included in the product should be identified beyond the species level and the specific strain should be easily identifiable.
2. Is there a history of safety and efficacy easily available for the listed probiotic strain? This information should be readily provided by the manufacturer and should include peer-reviewed research articles and summaries accessible to the general public that demonstrate a health benefit in the oral cavity. Clinicians and consumers should also be aware a “familiar” probiotic name (i.e., a species or strain included in multiple products) does not guarantee the probiotic is effective.
3. Does the probiotic strain provide a health benefit for the specific oral condition of concern, such as reducing the bacterial pathogens that contribute to cavity formation or whitening teeth? As a reminder, many common bacterial strains included in oral care probiotics have a history of safe use, usually in food products like yogurt and milk, which makes them simple and quick for manufacturers to include in products to meet consumer demand but does not guarantee a health benefit.
4. Does the product claim to treat, prevent, or cure any disease? These types of claims are not allowed on dietary supplements containing probiotics, as probiotics are not classified as drugs. Instead, the specific health benefit should be described, such as reducing oral pathogens or inhibiting the bacteria that cause bad breath.
As described in detail in this review, probiotic benefits are highly strain specific. Many bacterial species and strains used in probiotic products marketed for oral care have no documented benefit in the oral cavity and are used based on a history of safe use, rather than efficacious use. Of the probiotic genera for oral health reviewed here, Streptococcus spp. have the strongest support for health benefits in the oral cavity including reducing levels of oral pathogens, reducing the incidence of caries, reducing levels of halitosis causing bacteria, improving oral pH, and whitening teeth. Members of the genus Lactobacillus (or former members of Lactobacillus) have mixed support, with some research suggesting they may reduce levels of oral pathogens and inflammation but other studies suggesting the evidence of a health benefit is insufficient. Lactobacillus spp. are frequently included in oral care probiotics at the species level only due to their history of safe use as food starter cultures; however, safety does not guarantee efficacy, especially in the oral cavity. Both Bifidobacterium and Bacillus have little to no documented support for use as oral care probiotics with no specific oral health benefit directly linked to individual strains from these genera.
While the steps listed for consideration above require effort on the part of the consumer and/or clinician, they are critical to ensure health and safety. Additional research is still needed before probiotics are widely accepted as a part of proactive dentistry. Although reported side-effects of probiotic use are minimal to non-existent, there is not yet enough information to provide probiotic dosages for the general population. Regulatory roadblocks in the United States also impact the widespread use of probiotics for oral healthcare. No probiotic strain to date has been approved to treat, prevent, or cure oral health disease. These factors prevent most probiotic products from being covered by insurance providers, increasing the up-front cost to consumers. Future studies investigating health, social, and economic outcomes of long-term probiotic use for proactive dentistry would be beneficial to increase support and widespread adoption.
RB: Writing – review & editing, Writing – original draft, Visualization, Investigation, Data curation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
RB is an employee of ProBiora Health, LLC, the company responsible for the commercial distribution of ProBiora3® oral probiotics.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aas, J. A., Paster, B. J., Stokes, L. N., Ingar, O., and Dewhirst, F. E. (2005). Defining the Normal bacterial Flora of the Oral cavity. J. Clin. Microbiol. 43, 5721–5732. doi: 10.1128/jcm.43.11.5721-5732.2005
Abdulkareem, A. A., Al-Taweel, F. B., Al-Sharqi, A. J. B., Gul, S. S., Sha, A., and Chapple, I. L. C. (2023). Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis. J. Oral Microbiol. 15:2197779. doi: 10.1080/20002297.2023.2197779
Abranches, J., Zeng, L., Kajfasz, J. K., Palmer, S. R., Chakraborty, B., Wen, Z. T., et al. (2018). Biology of Oral streptococci. Microbiol Spectr 6:2018. doi: 10.1128/microbiolspec.GPP3-0042-2018
Acharya, M. R., and Shah, R. K. (2002). Selection of human isolates of bifidobacteria for their use as probiotics. Appl. Biochem. Biotechnol. 102-103, 081–098. doi: 10.1385/ABAB:102-103:1-6:081
Ademe, D., Admassu, D., and Balakrishnan, S. (2020). Analysis of salivary level Lactobacillus spp. and associated factors as determinants of dental caries amongst primary school children in Harar town, eastern Ethiopia. BMC Pediatr. 20:18. doi: 10.1186/s12887-020-1921-9
Alhumaid, J., and Bamashmous, M. (2022). Meta-analysis on the effectiveness of xylitol in caries prevention. J Int Soc Prev Commun Dentist 12:133. doi: 10.4103/jispcd.JISPCD_164_21
Baty, J. J., Stoner, S. N., and Scoffield, J. A. (2022). Oral commensal streptococci: gatekeepers of the Oral cavity. J. Bacteriol. 204:e0025722. doi: 10.1128/jb.00257-22
Bermudez-Brito, M., Plaza-Díaz, J., Muñoz-Quezada, S., Gómez-Llorente, C., and Gil, A. (2012). Probiotic mechanisms of action. Ann. Nutr. Metab. 61, 160–174. doi: 10.1159/000342079
Bertuccioli, A., Gervasi, M., Annibalini, G., Binato, B., Perroni, F., Rocchi, M. B. L., et al. (2023). Use of Streptococcus salivarius K12 in supporting the mucosal immune function of active young subjects: a randomised double-blind study. Front. Immunol. 14:60. doi: 10.3389/fimmu.2023.1129060
Bik, E. M., Long, C. D., Armitage, G. C., Loomer, P., Emerson, J., Mongodin, E. F., et al. (2010). Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 4, 962–974. doi: 10.1038/ismej.2010.30
Boisen, G., Prgomet, Z., Enggren, G., Dahl, H., Mkadmi, C., and Davies, J. R. (2023). Limosilactobacillus reuteri inhibits the acid tolerance response in oral bacteria. Biofilms 6:100136. doi: 10.1016/j.bioflm.2023.100136
Bunting, R. W., Nickerson, G., Hard, D. G., and Crowley, M. (1928). The relation of Bacillus acidophilus to dental caries**from the Laboratories of the School of dentistry, University of Michigan, Ann Arbor, Mich. Read before the section on histology, physiology, pathology, bacteriology and chemistry (research) at the sixty-ninth annual session of the American dental association, Detroit, Mich., Oct. 26, 1927. J. Am. Dent. Assoc. (1922 15, 1230–1233. doi: 10.14219/jada.archive.1928.0202
Burton, J. P., Drummond, B. K., Chilcott, C. N., Tagg, J. R., Thomson, W. M., Hale, J. D. F., et al. (2013). Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 62, 875–884. doi: 10.1099/jmm.0.056663-0
Burton, J. P., Wescombe, P. A., Moore, C. J., Chilcott, C. N., and Tagg, J. R. (2006). Safety assessment of the Oral cavity probiotic Streptococcus salivarius K12. Appl. Environ. Microbiol. 72, 3050–3053. doi: 10.1128/AEM.72.4.3050-3053.2006
Cannon, M., Trent, B., Vorachek, A., Kramer, S., and Esterly, R. (2013). Effectiveness of CRT at measuring the salivary level of Bacteria in caries prone children with probiotic therapy. J. Clin. Pediatr. Dent. 38, 55–60. doi: 10.17796/jcpd.38.1.b481624264142082
Cannon, M. L., Vorachek, A., Le, C., and White, K. (2019). Retrospective review of Oral probiotic therapy. J. Clin. Pediatr. Dent. 43, 367–371. doi: 10.17796/1053-4625-43.6.1
Castillo, A., Rubiano, S., Gutiérrez, J., Hermoso, A., and Liebana, J. (2000). Post-pH effect in oral streptococci. Clin. Microbiol. Infect. 6, 142–146. doi: 10.1046/j.1469-0691.2000.00030.x
Caufield, P. W., Schön, C. N., Saraithong, P., Li, Y., and Argimón, S. (2015). Oral lactobacilli and dental caries: a model for niche adaptation in humans. J. Dent. Res. 94, 110S–118S. doi: 10.1177/0022034515576052
Centers for Disease Control and Prevention (2021). Oral health fast facts. Atlanta, GA: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/oral-health/data-research/facts-stats/index.html
Chen, J., Chen, X., and Ho, C. L. (2021). Recent development of probiotic Bifidobacteria for treating human diseases. Front. Bioeng. Biotechnol. 9:248. doi: 10.3389/fbioe.2021.770248
Chen, X., Daliri, E. B., Kim, N., Kim, J.-R., Yoo, D., and Oh, D.-H. (2020). Microbial etiology and prevention of dental caries: exploiting natural products to inhibit cariogenic biofilms. Pathogens 9:569. doi: 10.3390/pathogens9070569
Chen, L., Ge, X., Dou, Y., Wang, X., Patel, J. R., and Xu, P. (2011). Identification of hydrogen peroxide production-related genes in Streptococcus sanguinis and their functional relationship with pyruvate oxidase. Microbiology 157, 13–20. doi: 10.1099/mic.0.039669-0
Chugh, P., Dutt, R., Sharma, A., Bhagat, N., and Dhar, M. S. (2020). A critical appraisal of the effects of probiotics on oral health. J. Funct. Foods 70:103985. doi: 10.1016/j.jff.2020.103985
Ciandrini, E., Campana, R., Casettari, L., Perinelli, D. R., Fagioli, L., Manti, A., et al. (2016). Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl. Microbiol. Biotechnol. 100, 6767–6777. doi: 10.1007/s00253-016-7531-7
Cui, Y., Xu, T., Qu, X., Hu, T., Jiang, X., and Zhao, C. (2016). New insights into various production characteristics of Streptococcus thermophilus strains. Int. J. Mol. Sci. 17:1701. doi: 10.3390/ijms17101701
De Angelis, M., and Gobbetti, M. (2011). “Lactic acid bacteria | Lactobacillus spp.: General characteristics,” in Encyclopedia of dairy sciences (Second Edition). ed. J. W. Fuquay (Academic Press), 78–90.
Dempsey, E., and Corr, S. C. (2022). Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front. Immunol. 13:245. doi: 10.3389/fimmu.2022.840245
Deo, P. N., and Deshmukh, R. (2019). Oral microbiome: unveiling the fundamentals. J Oral Maxillofacial Pathol 23:122. doi: 10.4103/jomfp.JOMFP_304_18
Di Pierro, F., Zanvit, A., Nobili, P., Risso, P., and Fornaini, C. (2015). Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 7, 107–113. doi: 10.2147/CCIDE.S93066
Dige, I., Grønkjær, L., and Nyvad, B. (2014). Molecular studies of the structural ecology of natural occlusal caries. Caries Res. 48, 451–460. doi: 10.1159/000357920
Dorkhan, M., Svensäter, G., and Davies, J. R. (2013). Salivary pellicles on titanium and their effect on metabolic activity in Streptococcus oralis. BMC Oral Health 13:32. doi: 10.1186/1472-6831-13-32
Doron, S., and Gorbach, S. L. (2008). “Bacterial infections: overview” in International encyclopedia of public health. ed. H. K. K. Heggenhougen (Oxford: Academic Press), 273–282.
Du Toit, M., Huch, M., Cho, G.-S., and Franz, C. M. A. P. (2014). “The genus Streptococcus,” in Lactic acid Bacteria. eds. W. H. Holzapfel and B. J. B. Wood (John Wiley & Sons, Ltd), 457–505.
Durrell, K. (2021). Symrise-Probi partnership yields processed probiotic for oral health. Nutrition Insight. Available at: https://www.nutritioninsight.com/news/symrise-probi-partnership-yields-processed-probiotic-for-oral-health.html (Accessed 1 March 2024).
Dzidic, M., Collado, M. C., Abrahamsson, T., Artacho, A., Stensson, M., Jenmalm, M. C., et al. (2018). Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 12, 2292–2306. doi: 10.1038/s41396-018-0204-z
EFSA Panel on NutritionTurck, D., Castenmiller, J., De Henauw, S., Ildico Hirsch-Ernst, K., Kearney, J., et al. (2020). Orodispersible lozenges containing a combination of Lactobacillus reuteri DSM 17938 and Lactobacillus reuteri ATCC PTA 5289 and normal gum function: evaluation of a health claim pursuant to article 13(5) of regulation (EC) no 1924/2006. EFSA J. 18:e06004. doi: 10.2903/j.efsa.2020.6004
Estay, J., Angel, P., Bersezio, C., Tonetto, M., Jorquera, G., Peña, M., et al. (2020). The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health 20:255. doi: 10.1186/s12903-020-01244-x
Fenster, K., Freeburg, B., Hollard, C., Wong, C., Rønhave Laursen, R., and Ouwehand, A. C. (2019). The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:83. doi: 10.3390/microorganisms7030083
Food and Agriculture Organization and World Health Organization Expert Consultation (2001). Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Cordoba, Argentina: Food and Agriculture Organization and World Health Organization Expert Consultation.
Forssten, S. D., Björklund, M., and Ouwehand, A. C. (2010). Streptococcus mutans, caries and simulation models. Nutrients 2, 290–298. doi: 10.3390/nu2030290
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Gibson, G. R., and Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412. doi: 10.1093/jn/125.6.1401
Grand View Research (2022). North America Oral health probiotics market size, share & trends analysis report by type (probiotics, prebiotics, Postbiotics), by form, by end-user (human, animal), by distribution channel, by region, and segment forecasts, 2023–2030. San Francisco, CA: Grand View Research. Available at: https://www.grandviewresearch.com/industry-analysis/north-america-oral-health-probiotics-market-report#:~:text=b.-,The%20North%20America%20oral%20health%20probiotics%20market%20size%20was%20estimated,USD%20110.9%20million20by202023.
Guo, M., Wu, J., Hung, W., Sun, Z., Zhao, W., Lan, H., et al. (2023). Lactobacillus paracasei ET-22 suppresses dental caries by regulating microbiota of dental plaques and inhibiting biofilm formation. Nutrients 15:316. doi: 10.3390/nu15153316
Han, H., Yum, H., Cho, Y.-D., and Kim, S. (2023). Improvement of halitosis by probiotic bacterium Weissella cibaria CMU: a randomized controlled trial. Front. Microbiol. 14:8762. doi: 10.3389/fmicb.2023.1108762
Haraszthy, V. I., Zambon, J. J., Sreenivasan, P. K., Zambon, M. M., Gerber, D., Rego, R., et al. (2007). Identification of oral bacterial species associated with halitosis. J. Am. Dent. Assoc. 138, 1113–1120. doi: 10.14219/jada.archive.2007.0325
Haukioja, A., Söderling, E., and Tenovuo, J. (2008). Acid production from sugars and sugar alcohols by probiotic lactobacilli and Bifidobacteria in vitro. Caries Res. 42, 449–453. doi: 10.1159/000163020
Hedayati-Hajikand, T., Lundberg, U., Eldh, C., and Twetman, S. (2015). Effect of probiotic chewing tablets on early childhood caries—a randomized controlled trial. BMC Oral Health 15:112. doi: 10.1186/s12903-015-0096-5
Hibbing, M. E., Fuqua, C., Parsek, M. R., and Peterson, S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. doi: 10.1038/nrmicro2259
Hillman, J. D., Dzuback, A. L., and Andrews, S. W. (1987). Colonization of the human Oral cavity by a Streptococcus mutans mutant producing increased Bacteriocin. J. Dent. Res. 66, 1092–1094. doi: 10.1177/00220345870660060101
Hillman, J. D., McDonell, E., Cramm, T., Hillman, C. H., and Zahradnik, R. T. (2009). A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J. Appl. Microbiol. 107, 1551–1558. doi: 10.1111/j.1365-2672.2009.04333.x
Hillman, J. D., McDonell, E., Hillman, C. H., and Handfield, M. (2016). Dental whitening effect of an oral probiotic. Dental Oral Craniofacial Res 2, 202–205. doi: 10.15761/DOCR.1000145
Hillman, J. D., Socransky, S. S., and Shivers, M. (1985). The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Arch. Oral Biol. 30, 791–795. doi: 10.1016/0003-9969(85)90133-5
Homayouni Rad, A., Pourjafar, H., and Mirzakhani, E. (2023). A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 13:1120995. doi: 10.3389/fcimb.2023.1120995
How, K. Y., Song, K. P., and Chan, K. G. (2016). Porphyromonas gingivalis: an overview of Periodontopathic pathogen below the gum line. Front. Microbiol. 7:53. doi: 10.3389/fmicb.2016.00053
How, Y.-H., and Yeo, S.-K. (2021). Oral probiotic and its delivery carriers to improve oral health: a review. Microbiology 167:001076. doi: 10.1099/mic.0.001076
Jansen, P. M., Abdelbary, M. M. H., and Conrads, G. (2021). A concerted probiotic activity to inhibit periodontitis-associated bacteria. PLoS One 16:e0248308. doi: 10.1371/journal.pone.0248308
Jayachandra, M., Gayathiri, R., Aruna, C., Bhat, P., and Arumugam, P. (2023). Clinical effects of Bifidobacterium as a probiotic on oral health: a systematic review. Dental Res J 20:32
Jevitz Patterson, M. (1996). “Streptococcus” in Medical Microbiology (Galveston, TX: University of Texas Medical Branch at Galveston).
Jiang, J., Li, K., Xiao, Y., Zhong, A., Tang, J., Duan, Y., et al. (2023). Limosilactobacillus reuteri regulating intestinal function: a review. Fermentation 9:19. doi: 10.3390/fermentation9010019
Johnston, M. M., Kaake, M. J., and Agnew, M. C. (1933). The relationship of Lactobacillus Acidophilus to dental caries in experimental animals and in human beings**from the research laboratories, Hospital for Sick Children, and the Department of Pediatrics, University of Toronto, under the direction of Alan Brown, M.D. J. Am. Dent. Assoc. (1922 20, 1777–1784. doi: 10.14219/jada.archive.1933.0300
Kaur, P., Singh, S., Mathur, A., Kaur Makkar, D., Pal Aggarwal, V., Batra, M., et al. (2017). Impact of dental disorders and its influence on self esteem levels among adolescents. J. Clin. Diagn. Res. 11, ZC05–ZC08. doi: 10.7860/JCDR/2017/23362.9515
Keller, M. K., and Twetman, S. (2012). Acid production in dental plaque after exposure to probiotic bacteria. BMC Oral Health 12:44. doi: 10.1186/1472-6831-12-44
Khalaf, H., Nakka, S. S., Sandén, C., Svärd, A., Hultenby, K., Scherbak, N., et al. (2016). Antibacterial effects of Lactobacillus and bacteriocin PLNC8 αβ on the periodontal pathogen Porphyromonas gingivalis. BMC Microbiol. 16:188. doi: 10.1186/s12866-016-0810-8
Kraft-Bodi, E., Jørgensen, M. R., Keller, M. K., Kragelund, C., and Twetman, S. (2015). Effect of probiotic Bacteria on Oral Candida in frail elderly. J. Dent. Res. 94, 181S–186S. doi: 10.1177/0022034515595950
Kreth, J., Merritt, J., and Qi, F. (2009). Bacterial and host interactions of Oral streptococci. DNA Cell Biol. 28, 397–403. doi: 10.1089/dna.2009.0868
Laleman, I., Pauwels, M., Quirynen, M., and Teughels, W. (2020). A dual-strain lactobacilli reuteri probiotic improves the treatment of residual pockets: a randomized controlled clinical trial. J. Clin. Periodontol. 47, 43–53. doi: 10.1111/jcpe.13198
Lee, S.-H., and Kim, Y.-J. (2014). A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch. Microbiol. 196, 601–609. doi: 10.1007/s00203-014-0998-7
Lee, N.-K., Kim, W.-S., and Paik, H.-D. (2019). Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 28, 1297–1305. doi: 10.1007/s10068-019-00691-9
Leistikow, K. R., Beattie, R. E., and Hristova, K. R. (2022). Probiotics beyond the farm: benefits, costs, and considerations of using antibiotic alternatives in livestock. Front Antibiot 1:3912. doi: 10.3389/frabi.2022.1003912
Leme, L. A., Rizzardi, K. F., Santos, I. B., and Parisotto, T. M. (2022). Exploring the relationship between salivary levels of TNF-α, Lactobacillus acidophilus, Lactobacillus gasseri, obesity, and caries in early childhood. Pathogens 11:579. doi: 10.3390/pathogens11050579
Lemoine, A., Tounian, P., Adel-Patient, K., and Thomas, M. (2023). Pre-, pro-, syn-, and Postbiotics in infant formulas: what are the immune benefits for infants? Nutrients 15:1231. doi: 10.3390/nu15051231
Li, J., Helmerhorst, E. J., Leone, C. W., Troxler, R. F., Yaskell, T., Haffajee, A. D., et al. (2004). Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 97, 1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x
Lin, C., Chen, Y., Ho, H., Kuo, Y., Lin, W., Chen, J., et al. (2022). Impact of the food grade heat-killed probiotic and postbiotic oral lozenges in oral hygiene. Aging 14, 2221–2238. doi: 10.18632/aging.203923
Liu, Y., Fatheree, N. Y., Mangalat, N., and Rhoads, J. M. (2010). Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1087–G1096. doi: 10.1152/ajpgi.00124.2010
Luo, S.-C., Wei, S.-M., Luo, X.-T., Yang, Q.-Q., Wong, K.-H., Cheung, P. C. K., et al. (2024). How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: an oral microbiota perspective. NPJ Biofilms Microbiomes 10:14. doi: 10.1038/s41522-024-00488-7
MacDonald, K. W., Chanyi, R. M., Macklaim, J. M., Cadieux, P. A., Reid, G., and Burton, J. P. (2021). Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health 21:245. doi: 10.1186/s12903-021-01606-z
Manome, A., Abiko, Y., Kawashima, J., Washio, J., Fukumoto, S., and Takahashi, N. (2019). Acidogenic potential of Oral Bifidobacterium and its high fluoride tolerance. Front. Microbiol. 10:1099. doi: 10.3389/fmicb.2019.01099
McAuliffe, O., Ross, R. P., and Hill, C. (2001). Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25, 285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x
Mei, M., Chu, C., Low, K., Che, C., and Lo, E. (2013). Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. Mutans and L. acidophilus dual-species cariogenic biofilm. Med. Oral Patol. Oral Cir. Bucal 18, e824–e831. doi: 10.4317/medoral.18831
Milgrom, P., Ly, K. A., Roberts, M. C., Rothen, M., Mueller, G., and Yamaguchi, D. K. (2006). Mutans streptococci dose response to xylitol chewing gum. J. Dent. Res. 85, 177–181. doi: 10.1177/154405910608500212
Milgrom, P., Ly, K. A., Tut, O. K., Mancl, L., Roberts, M. C., Briand, K., et al. (2009). Xylitol pediatric topical Oral syrup to prevent dental caries: a double-blind randomized clinical trial of efficacy. Arch. Pediatr. Adolesc. Med. 163, 601–607. doi: 10.1001/archpediatrics.2009.77
Modesto, M. (2018). “Chapter 4—isolation, cultivation, and storage of Bifidobacteria” in The Bifidobacteria and related organisms. eds. P. Mattarelli, B. Biavati, W. H. Holzapfel, and B. J. B. Wood (Academic Press), 67–98.
Mohd Fuad, A. S., Amran, N. A., Nasruddin, N. S., Burhanudin, N. A., Dashper, S., and Arzmi, M. H. (2023). The mechanisms of probiotics, prebiotics, Synbiotics, and Postbiotics in Oral Cancer management. Probiot Antimicrob Proteins 15, 1298–1311. doi: 10.1007/s12602-022-09985-7
Molin, G., Jeppsson, B., Johansson, M.-L., Ahrné, S., Nobaek, S., Ståhl, M., et al. (1993). Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J. Appl. Bacteriol. 74, 314–323. doi: 10.1111/j.1365-2672.1993.tb03031.x
Mora, D., and Arioli, S. (2014). Microbial urease in health and disease. PLoS Pathog. 10:e1004472. doi: 10.1371/journal.ppat.1004472
Nascimento, M. M. (2018). Potential uses of arginine in dentistry. Adv. Dent. Res. 29, 98–103. doi: 10.1177/0022034517735294
National Cancer Institute (2024). “NCI dictionary of Cancer terms,” (National Institutes of Health). Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/oral-cavity (Accessed 1 February 2024).
National Institute of Dental and Craniofacial Research (2021). Oral health in America: Advances and challenges: Executive summary. Bethesda, MD: National Institute of Dental and Craniofacial Research. Available at: https://www.ncbi.nlm.nih.gov/books/NBK576536/
National Institute of Dental and Craniofacial Research (2022). Oral health across the lifespan: Working-age adults. Washington, DC: Natinoal Institutes of Health. Available at: https://www.nidcr.nih.gov/research/oralhealthinamerica/section-3a-summary
National Institutes of Health Office of Dietary Supplements (2023). Probiotics fact sheet for health professionals. Available at: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/
Nayak, P., Nayak, U., and Khandelwal, V. (2014). The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 6, 89–94. doi: 10.2147/CCIDE.S55761
Nock, K. (2024). From reactive to proactive dentistry. Dental Products Report 58. Available at: https://www.dentalproductsreport.com/view/from-reactive-to-proactive-dentistry
Nordström, E. A., Teixeira, C., Montelius, C., Jeppsson, B., and Larsson, N. (2021). Lactiplantibacillus plantarum 299v (LP299V®): three decades of research. Benefic. Microbes 12, 441–465. doi: 10.3920/BM2020.0191
Otto, H., Wescombe, P. A., Mathew, U., Nancy, R., Burton, J. P., and Tagg, J. R. (2007). Salivaricin A2 and the novel Lantibiotic Salivaricin B are encoded at adjacent loci on a 190-Kilobase transmissible Megaplasmid in the Oral probiotic strain Streptococcus salivarius K12. Appl. Environ. Microbiol. 73, 1107–1113. doi: 10.1128/AEM.02265-06
Plaza-Diaz, J., Ruiz-Ojeda, F. J., Gil-Campos, M., and Gil, A. (2019). Mechanisms of action of probiotics. Adv. Nutr. 10, S49–S66. doi: 10.1093/advances/nmy063
Poorni, S., Nivedhitha, M., Srinivasan, M., and Balasubramaniam, A. (2022). Effect of probiotic Streptococcus salivarius K12 and M18 lozenges on the Cariogram parameters of patients with high caries risk: a randomised control trial. Cureus 14:e23282. doi: 10.7759/cureus.23282
Pradhan, D., Mallappa, R. H., and Grover, S. (2020). Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 108:106872. doi: 10.1016/j.foodcont.2019.106872
Ratna Sudha, M., Neelamraju, J., Surendra Reddy, M., and Kumar, M. (2020). Evaluation of the effect of probiotic Bacillus coagulans unique IS2 on Mutans streptococci and lactobacilli levels in saliva and plaque: a double-blind, randomized, placebo-controlled study in children. Int J Dentist 2020:8891708. doi: 10.1155/2020/8891708
Rodolphe, B., Briczinski, E. P., Traeger, L. L., Loquasto, J. R., Melissa, R., Philippe, H., et al. (2009). Comparison of the complete genome sequences of Bifidobacterium animalis subsp. lactis DSM 10140 and Bl-04. J. Bacteriol. 191, 4144–4151. doi: 10.1128/jb.00155-09
Rodríguez-Sánchez, S., Ramos, I. M., Seseña, S., Poveda, J. M., and Palop, M. L. (2021). Potential of Lactobacillus strains for health-promotion and flavouring of fermented dairy foods. LWT 143:111102. doi: 10.1016/j.lwt.2021.111102
Ruan, X., Luo, J., Zhang, P., and Howell, K. (2022). The salivary microbiome shows a high prevalence of core bacterial members yet variability across human populations. NPJ Biofilms Microbiomes 8:85. doi: 10.1038/s41522-022-00343-7
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00440-6
Salminen, S., von Wright, A., Morelli, L., Marteau, P., Brassart, D., de Vos, W. M., et al. (1998). Demonstration of safety of probiotics — a review. Int. J. Food Microbiol. 44, 93–106. doi: 10.1016/S0168-1605(98)00128-7
Sanders, M. E., Akkermans, L. M. A., Haller, D., Hammerman, C., Heimbach, J. T., Hörmannsperger, G., et al. (2010). Safety assessment of probiotics for human use. Gut Microbes 1, 164–185. doi: 10.4161/gmic.1.3.12127
Satam, H., Joshi, K., Mangrolia, U., Waghoo, S., Zaidi, G., Rawool, S., et al. (2023). Next-generation sequencing technology: current trends and advancements. Biology 12:997. doi: 10.3390/biology12070997
Schlagenhauf, U., and Jockel-Schneider, Y. (2021). Probiotics in the Management of Gingivitis and Periodontitis. A review. Front Dental Med 2:8666. doi: 10.3389/fdmed.2021.708666
Schlagenhauf, U., Rehder, J., Gelbrich, G., and Jockel-Schneider, Y. (2020). Consumption of Lactobacillus reuteri-containing lozenges improves periodontal health in navy sailors at sea: a randomized controlled trial. J. Periodontol. 91, 1328–1338. doi: 10.1002/JPER.19-0393
Shimada, A., Noda, M., Matoba, Y., Kumagai, T., Kozai, K., and Sugiyama, M. (2015). Oral lactic acid bacteria related to the occurrence and/or progression of dental caries in Japanese preschool children. Biosci Microbiota Food Health 34, 29–36. doi: 10.12938/bmfh.2014-015
Siddiqui, S. A., Khan, S., Mehdizadeh, M., Nirmal, N. P., Khanashyam, A. C., Fernando, I., et al. (2023). Consumer studies focus on prebiotics, probiotics, and Synbiotics in food packaging: a review. Curr Food Sci Technol Rep 1, 13–29. doi: 10.1007/s43555-023-00003-7
Siqueira, J. F., and Rôças, I. N. (2013). As-yet-uncultivated oral bacteria: breadth and association with oral and extra-oral diseases. J. Oral Microbiol. 5:21077. doi: 10.3402/jom.v5i0.21077
Söderling, E. M., Marttinen, A. M., and Haukioja, A. L. (2011). Probiotic lactobacilli interfere with Streptococcus mutans biofilm formation in vitro. Curr. Microbiol. 62, 618–622. doi: 10.1007/s00284-010-9752-9
Sounah, S. A., and Madfa, A. A. (2020). Correlation between dental caries experience and the level of Streptococcus mutans and lactobacilli in saliva and carious teeth in a Yemeni adult population. BMC. Res. Notes 13:112. doi: 10.1186/s13104-020-04960-3
Sulyanto, R. M., Thompson, Z. A., Beall, C. J., Leys, E. J., and Griffen, A. L. (2019). The predominant Oral microbiota is acquired early in an organized pattern. Sci. Rep. 9:10550. doi: 10.1038/s41598-019-46923-0
Tatakis, D. N., and Kumar, P. S. (2005). Etiology and pathogenesis of periodontal diseases. Dent. Clin. N. Am. 49, 491–516. doi: 10.1016/j.cden.2005.03.001
Teanpaisan, R., Piwat, S., Tianviwat, S., Sophatha, B., and Kampoo, T. (2015). Effect of Long-term consumption of Lactobacillus paracasei SD1 on reducing Mutans streptococci and caries risk: a randomized placebo-controlled trial. Dentist J 3, 43–54. doi: 10.3390/dj3020043
Turnbull, P. C. (1996). “Bacillus” in Medical Microbiology. ed. S. Baron (Glaveston, TX: University of Texas Medical Branch at Galveston).
Twetman, L., Larsen, U., Fiehn, N.-E., Stecksén-Blicks, C., and Twetman, S. (2009). Coaggregation between probiotic bacteria and caries-associated strains: an in vitro study. Acta Odontol. Scand. 67, 284–288. doi: 10.1080/00016350902984237
U.S. Department of Health and Human Services (2024). Dietary supplements: new dietary ingredient notification procedures and timeframes: guidance for industry. Available at: https://www.fda.gov/media/176512/download (Accessed 1 March 2024).
Unde, M. P., Patil, R. U., and Dastoor, P. P. (2018). The untold story of fluoridation: revisiting the changing perspectives. Indian J. Occup. Environ. Med. 22, 121–127. doi: 10.4103/ijoem.IJOEM_124_18
United States Food and Drug Administration (2023). Facts about Dietary Supplements. Available at: https://www.fda.gov/news-events/rumor-control/facts-about-dietary-supplements#:~:text=Before%20you%20decide%20to%20take,are%20safe%20and%20accurately%20labeled (Accessed 2 January 2024).
Upton, M., Tagg, J. R., Wescombe, P., and Jenkinson, H. F. (2001). Intra- and interspecies signaling betweenStreptococcus salivarius and Streptococcus pyogenes mediated by SalA and SalA1 Lantibiotic peptides. J. Bacteriol. 183, 3931–3938. doi: 10.1128/jb.183.13.3931-3938.2001
Urbańska, M., and Szajewska, H. (2014). The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur. J. Pediatr. 173, 1327–1337. doi: 10.1007/s00431-014-2328-0
Van Holm, W., Lauwens, K., De Wever, P., Schuermans, A., Zayed, N., Pamuk, F., et al. (2023). Probiotics for oral health: do they deliver what they promise? Front. Microbiol. 14:692. doi: 10.3389/fmicb.2023.1219692
Vijaykumar, S., McCready, J., Graham, P. L., and Morris, D. (2022). That gut feeling: public perceptions of media coverage and science surrounding probiotic products. Br. Food J. 124, 3428–3446. doi: 10.1108/BFJ-02-2021-0143
Vivekananda, M. R., Vandana, K. L., and Bhat, K. G. (2010). Effect of the probiotic lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. J. Oral Microbiol. 2:5344. doi: 10.3402/jom.v2i0.5344
Volgenant, C. M. C., van der Waal, S. V., Brandt, B. W., Buijs, M. J., van der Veen, M. H., Rosema, N. A. M., et al. (2022). The evaluation of the effects of two probiotic strains on the Oral ecosystem: a randomized clinical trial. Front Oral Health 3:17. doi: 10.3389/froh.2022.825017
Weese, J. S., and Martin, H. (2011). Assessment of commercial probiotic bacterial contents and label accuracy. Can. Vet. J. 52, 43–46.
Wen, Z. T., Huang, X., Ellepola, K., Liao, S., and Li, Y. (2022). Lactobacilli and human dental caries: more than mechanical retention. Microbiology 168:1196. doi: 10.1099/mic.0.001196
Wescombe, P. A., Upton, M., Renault, P., Wirawan, R. E., Power, D., Burton, J. P., et al. (2011). Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology 157, 1290–1299. doi: 10.1099/mic.0.044719-0
Widyarman, A. S., Hartono, V., Marjani, L. I., Irawan, D., Luthfi, L., and Bachtiar, B. M. (2018). Lactobacillus reuteri containing probiotic lozenges consumption reduces Streptococcus mutans, Streptococcus sobrinus, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans in orthodontic patients. J Int Dental Med Res 11, 628–633.
Willis, J. R., and Gabaldón, T. (2020). The human Oral microbiome in health and disease: from sequences to ecosystems. Microorganisms 8:308. doi: 10.3390/microorganisms8020308
Yilmaz, S., Efeoglu, E., Noyan, U., Kuru, B., Kilic, A., and Kuru, L. (1994). The evolution of clinical periodontal therapy. J. Marmara Univ. Dent. Fac. 2, 414–423.
Yoo, H.-J., Jwa, S.-K., Kim, D.-H., and Ji, Y.-J. (2020). Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin Exp Dental Res 6, 207–214. doi: 10.1002/cre2.269
Zahradnik, R. T., Magnusson, I., Walker, C., McDonell, E., Hillman, C. H., and Hillman, J. D. (2009). Preliminary assessment of safety and effectiveness in humans of ProBiora3™, a probiotic mouthwash. J. Appl. Microbiol. 107, 682–690. doi: 10.1111/j.1365-2672.2009.04243.x
Zhao, Z., Wu, J., Sun, Z., Fan, J., Liu, F., Zhao, W., et al. (2023). Postbiotics derived from L. paracasei ET-22 inhibit the formation of S. mutans biofilms and bioactive substances: an analysis. Molecules 28:1236. doi: 10.3390/molecules28031236
Zheng, X., He, J., Wang, L., Zhou, S., Peng, X., Huang, S., et al. (2017). Ecological effect of arginine on Oral microbiota. Sci. Rep. 7:7206. doi: 10.1038/s41598-017-07042-w
Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107
Zotta, T., Parente, E., and Ricciardi, A. (2017). Aerobic metabolism in the genus Lactobacillus: impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 122, 857–869. doi: 10.1111/jam.13399
Keywords: oral care, probiotic, prebiotic, Streptococcus, Lactobacillus, dentistry
Citation: Beattie RE (2024) Probiotics for oral health: a critical evaluation of bacterial strains. Front. Microbiol. 15:1430810. doi: 10.3389/fmicb.2024.1430810
Received: 10 May 2024; Accepted: 11 June 2024;
Published: 24 June 2024.
Edited by:
Valério Monteiro-Neto, Federal University of Maranhão, BrazilCopyright © 2024 Beattie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachelle E. Beattie, cmJlYXR0aWVAcHJvYmlvcmFoZWFsdGguY29t; cmFjaGVsbGUuYmVhdHRpZTIwQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.