- 1Research Administration Section, Chiang Mai University, Chiang Mai, Thailand
- 2Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
Tuta absoluta, known as the South American tomato leaf miner, significantly impacts tomato plants (Solanum lycopersicum) economically on a global scale. This pest, belonging to the Gelechiidae family, is native to South America and was first identified in Peru in 1917. Since its discovery, T. absoluta has rapidly spread to Europe, Africa, and Asia, severely threatening tomato production in these regions. The widespread application of chemical pesticides against this pest has resulted in significant environmental harm, including contamination of soil and water, and has had negative effects on non-target species such as beneficial insects, birds, and aquatic life. Although substantial research has been conducted, biological control methods for T. absoluta remain insufficient, necessitating further study. This review covers the Biology, Classification, and Entomopathogen-Based Management of T. absoluta (Meyrick) in Asia. It provides essential insights into the pest’s life cycle, ecological impacts, and the potential of entomopathogens as biocontrol agents. The detailed information presented aims to facilitate the development of sustainable pest control strategies, minimizing environmental impact and promoting the use of entomopathogens as viable alternatives to chemical pesticides in controlling T. absoluta insect pest.
1 Introduction
Invasive insect pests pose significant threats to global agricultural food production, exacerbated by factors such as climate change and the international trade of agricultural commodities (Skendžić et al., 2021). Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) exemplifies this challenge as a devastating pest of tomato (S. lycopersicum) and other solanaceous crops. The economic impact of T. absoluta is profound, with substantial global expenditures incurred for its control and the mitigation of crop losses (Vivekanandhan et al., 2024a,b,c). For instance, Turkey spends approximately $183.7 million USD annually on T. absoluta control (Oztemiz, 2014), while Nepal reported crop losses totaling $19.7 million in the initial year of the invasion (Bajracharya et al., 2016). These losses have significant socio-economic repercussions, including a substantial 32% surge in tomato prices (Vivekanandhan et al., 2024a,b,c).
The global spread of T. absoluta has been rapid and extensive, impacting tomato production across continents (Fiaboe et al., 2021; Ndiaye et al., 2021; Vivekanandhan et al., 2024a,b,c). Initially detected in Spain in 2006 (Campos et al., 2017), T. absoluta has since spread to Africa, Eurasia, and Western Africa following its introduction to Niger in 2012 (Biondi et al., 2018). In Asia, the pest was first identified in Turkey in 2009 (Kılıç, 2010) and subsequently reported in Taiwan (2020), Bangladesh, Nepal (2016), Myanmar (2017), and regions of China (2017–2018) (Ramasamy, 2020; Yule et al., 2021; Zhang et al., 2021). The movement of tomato seedlings and fruits through international trade routes has facilitated its dispersal in Asia (Guimapi et al., 2020). To effectively manage the spread of T. absoluta and mitigate its impact on non-infested regions in Asia such as Bhutan, and North Korea stringent quarantine measures and phytosanitary protocols are imperative. Understanding the pest’s biology, climatic preferences, and pathways of human-mediated dispersal are crucial for assessing invasion risks and developing sustainable management strategies (Banks et al., 2015).
Research focused on the biology, ecological impact, spread dynamics, and control tactics against T. absoluta in Asia is essential for mitigating the persistent threat posed by this invasive species. Non-infested countries must prioritize proactive measures to prevent the introduction of T. absoluta and safeguard their agricultural industries from potential disruptions and economic losses. By leveraging scientific knowledge and fostering international cooperation, we can effectively reduce the risk of T. absoluta invasion while promoting sustainable agricultural practices globally.
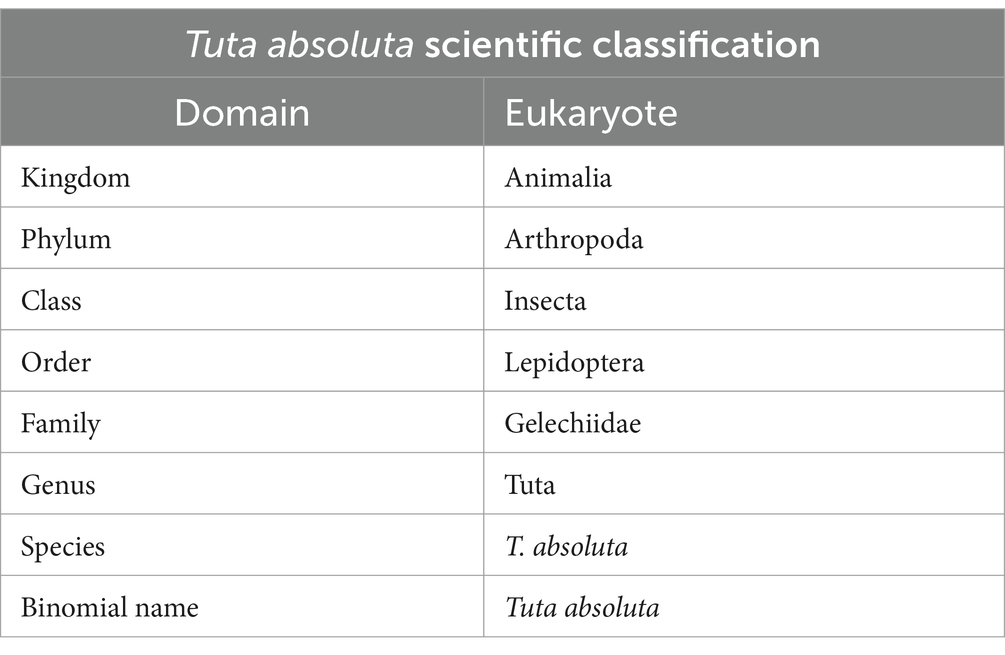
2 Scientific classifications
Tuta absoluta, commonly known as the tomato leafminer, is classified within the domain Eukaryota and kingdom Animalia. It belongs to the phylum Arthropoda and class Insecta. This species is part of the order Lepidoptera and family Gelechiidae (Table 1). Within this family, it is placed in the genus Tuta, with its species designation being T. absoluta. This moth is a significant agricultural pest, particularly affecting Solanaceae crops.
2.1 Tuta absoluta biology
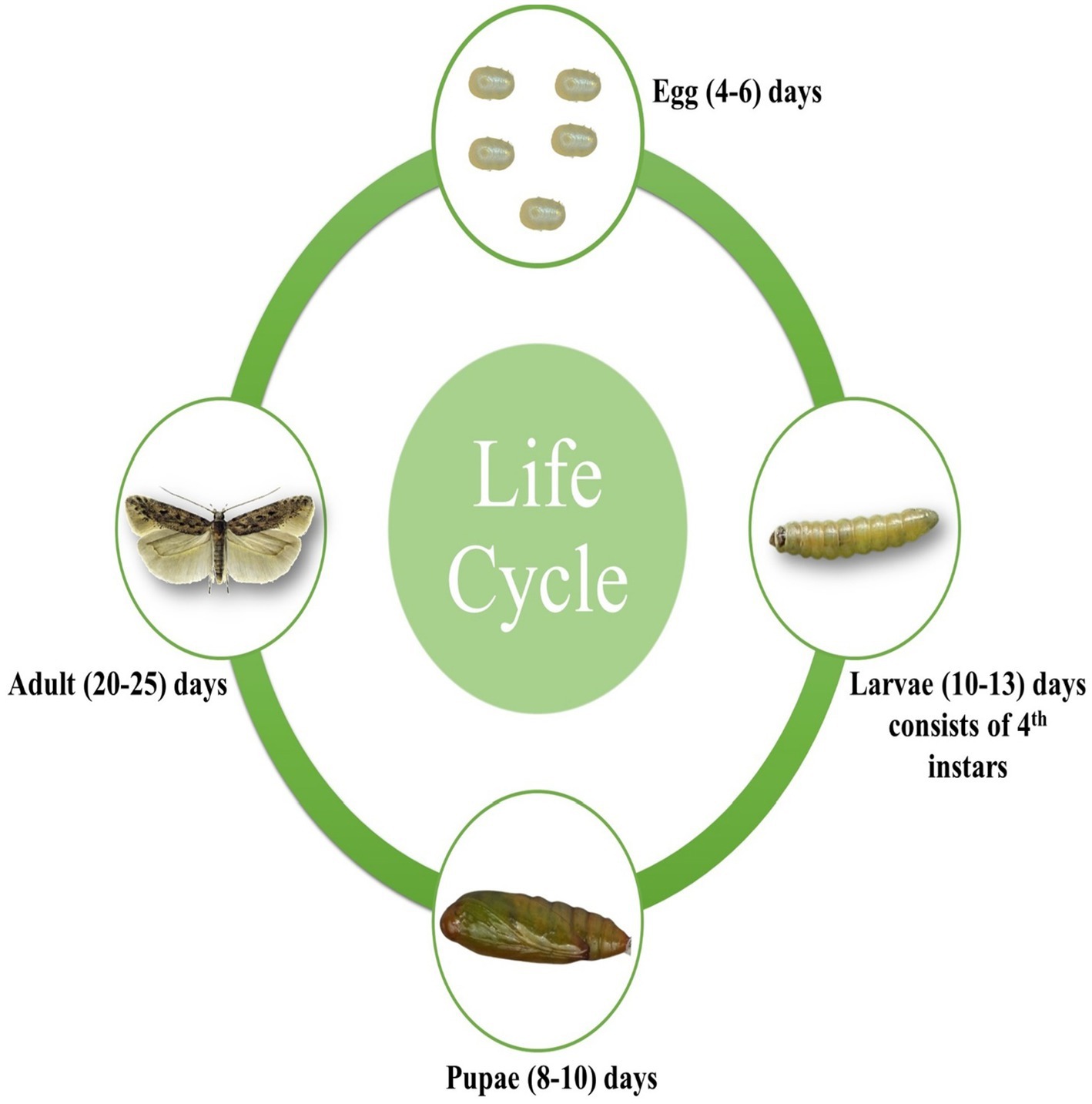
Tuta absoluta, a holometabolous insect, has a complex life cycle encompassing four distinct stages: egg, larva, pupa, and adult (Figure 1). Each stage exhibits unique morphological and behavioral characteristics. Understanding these stages in detail is essential for developing effective and targeted pest management strategies, thereby mitigating the significant economic impact on tomato production.
2.1.1 Egg stage
Adult female T. absoluta deposit yellow, elliptical eggs (0.33 × 0.22 mm) on the upper surfaces of their host plants, such as sepals, young leaves, or stems (Figure 1). Each female can lay approximately 260 eggs during her lifetime (Uchoa-Fernandes et al., 1995). Under favorable conditions, the eggs hatch within 4–6 days in tomato plants.
2.1.2 Larval stage
The larvae of T. absoluta are highly destructive, causing significant damage to plant foliage by mining through the mesophyll layer of leaves and later penetrating auxiliary buds and fruits, resulting in yield losses (Cocco et al., 2015). The larval stage consists of four instars, with body lengths progressively increasing from 2.8 mm to 7.7 mm (Colmenárez et al., 2022). Larvae change color from white in the early instars to light green in later stages. Under favorable conditions, the larval stage lasts 10–13 days in tomato plants.
2.1.3 Pupal stage
After completing their larval development, mature T. absoluta larvae typically drop to the soil to pupate, although pupation can also occur on plant leaves. The pupae are initially green but gradually turn dark brown (Figure 1). Mature pupae measure approximately 4.35 mm in length and 1.1 mm in width (Colmenárez et al., 2022). Under favorable conditions, the pupal stage lasts 8–10 days in tomato plants.
2.1.4 Adult stage
According to Colmenárez et al. (2022), adult T. absoluta moths are approximately 6 mm long, with dark gray coloration and brown and off-white scales. Nocturnal by nature, they hide among leaves during the day (EPPO, 2005). The duration of each life stage varies with environmental conditions (Figure 1). Erdogan and Babaroglu (2014) reported that at 25°C and 65% relative humidity, the egg, larval, and pupal stages last about 4.1, 11.0, and 9.5 days, respectively, resulting in an egg-to-adult lifespan of approximately 30.2 days. Under favorable conditions, the adult stage lasts 20–25 days in tomato plants.
2.2 Life cycle and reproduction
The complete life cycle of T. absoluta typically spans between 29 to 38 days, with variability influenced significantly by environmental conditions such as temperature and humidity (EPPO, 2005). Adult males and females of T. absoluta have relatively short lifespans, with males surviving approximately 15.8 days and females about 18.2 days on average. The oviposition period lasts around 7.9 days, during which females can lay up to 141 eggs each (Erdogan and Babaroglu, 2014; Vivekanandhan et al., 2024a,b,c). However, EPPO (2005) suggests a higher fecundity rate, reporting that females may lay up to 260 eggs over their lifetime. The combination of high reproductive capacity and short generation time enables T. absoluta to undergo rapid population growth and inflict severe damage on tomato and other solanaceous crops. This pest’s ability to complete multiple generations in a single growing season further exacerbates its impact on agricultural productivity.
2.2.1 Effect of hot climatic conditions on Tuta absoluta development
Temperature profoundly influences the growth, development, and behavior of T. absoluta, a significant insect pest impacting tomato and solanaceous crops. Studies have extensively examined how temperature affects various stages of its life cycle, revealing the species’ remarkable adaptability to thermal conditions (Van Damme et al., 2015). Cuthbertson et al. (2013) identified the optimal temperature range for T. absoluta development as 19–23°C, with egg hatching rates peaking at 13°C and adult emergence rates at 19°C. Temperatures below 10°C were found to result in developmental failure, highlighting the pest’s sensitivity to cold conditions. Conversely, Martins et al. (2016) reported an optimal temperature of 30°C for T. absoluta development, with lower and upper thresholds of 14°C and 34.6°C, respectively, indicating considerable variability in thermal preferences.
Tuta absoluta’s ability to undergo multiple generations per year without diapause further underscores its adaptability (EPPO, 2005; Biondi et al., 2018). Overwintering studies in Western Europe, particularly in greenhouses, reveal its persistence during colder months. Research on cold resistance shows larvae, pupae, and adults can withstand temperatures as low as −18.2°C, −16.7°C, and − 17.8°C, respectively (Van Damme et al., 2015). Moreover, LT50 values at 0°C indicate varying cold tolerance among life stages, with adults exhibiting higher resistance compared to larvae and pupae.
Unlike many insects, T. absoluta does not enter reproductive diapause in response to seasonal changes in temperature and day length, enhancing its ability to thrive in temperate climates (Van Damme et al., 2015). These adaptive traits contribute to its widespread distribution and ability to inflict substantial economic losses year-round. Understanding the thermal biology and adaptive mechanisms of T. absoluta is crucial for devising effective integrated pest management strategies tailored to mitigate its impact on tomatoes and other host crops across diverse environmental conditions.
2.2.2 Effect of humidity on Tuta absoluta development
Humidity plays a crucial role in the development and population dynamics of T. absoluta, the tomato leafminer (Kachave et al., 2020; Vivekanandhan et al., 2024a,b,c). This pest thrives in environments with moderate to high humidity levels, which are conducive to its reproductive success and overall lifecycle (Buragohain et al., 2021). High humidity enhances the survival and growth rates of T. absoluta eggs and larvae, facilitating faster development through its various life stages (Kachave et al., 2020; Vivekanandhan et al., 2024a,b,c). However, excessively high humidity levels can also favor the proliferation of fungal pathogens that affect T. absoluta populations. Conversely, low humidity conditions can impede egg hatching and larval development, thereby potentially reducing pest pressure on crops.
2.2.3 Host plants of Tuta absoluta
Tuta absoluta is a polyphagous pest with a broad host range primarily within the Solanaceae family. It significantly impacts economically important crops such as tomato, potato, brinjal, sweet pepper, and tobacco (Mohamed et al., 2015; Abbes et al., 2016; Vivekanandhan et al., 2024a,b,c). Abbes et al. (2016) identified Solanum nigrum (European black nightshade) as particularly susceptible to T. absoluta infestations. Furthermore, this pest has been documented to harm plants from diverse families including Malvaceae, Amaranthaceae, Fabaceae, and Convolvulaceae, indicating its polyphagous behavior and adaptability to various agricultural and weed species (Bawin et al., 2016).
Tuta absoluta is recognized as a highly destructive pest that imposes significant economic losses in tomato farming (Figures 2A–F). In both greenhouse and open field environments, unchecked infestations of T. absoluta can result in yield reductions ranging from 80 to 100% (Figures 2A–F). The pest typically establishes colonies on tomato plants shortly after transplanting and reaches peak infestation levels during flowering and fruiting stages (Figures 2A–F). Diatte et al. (2018) documented the highest rates of T. absoluta infestation during the early fruiting stage, followed by early flowering, vegetative growth, and harvesting stages.
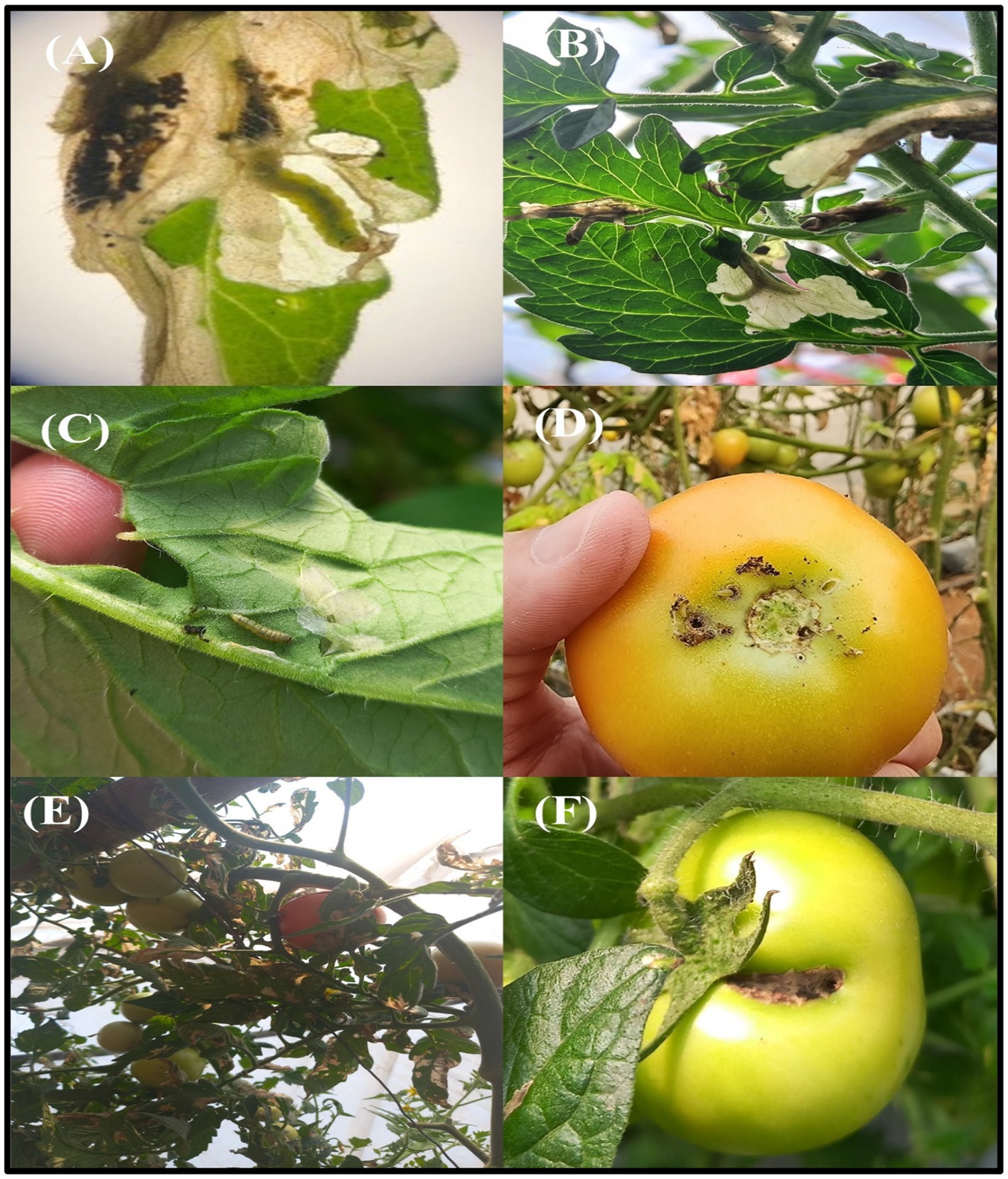
Figure 2. Symptoms of T. absoluta infection in tomato plants. T. absoluta damage in tomato plants and their parts (A–F).
Research in Nepal by Bajracharya et al. (2018) highlighted varying degrees of damage caused by T. absoluta across different tomato varieties. The Karita variety suffered extensive damage ranging from 76 to 100%, while the Samjhana and Srijana varieties exhibited damage levels between 51 and 75%. This variability underscores the importance of understanding host susceptibility and emphasizes the need for selecting resistant or tolerant tomato cultivars as part of integrated pest management strategies. The infestation patterns and damage severity associated with T. absoluta underscore its impact on global tomato production.
3 Invasion in Asian countries
The invasion of T. absoluta in Asian countries has profoundly affected agriculture and economies since its initial appearance. The pest was first detected in Turkey in 2009 and has subsequently spread across a wide swath of Asia, including Iran, Kazakhstan, Afghanistan, Lebanon, Bangladesh, Myanmar, Bahrain, Pakistan, Iraq, Turkmenistan, China, Kuwait, India, Nepal, Israel, Jordan, Kyrgyzstan, Qatar, Saudi Arabia, Syria, Tajikistan, United Arab Emirates, Uzbekistan, and Yemen (Guimapi et al., 2020; EPPO, 2023) (Figure 3).
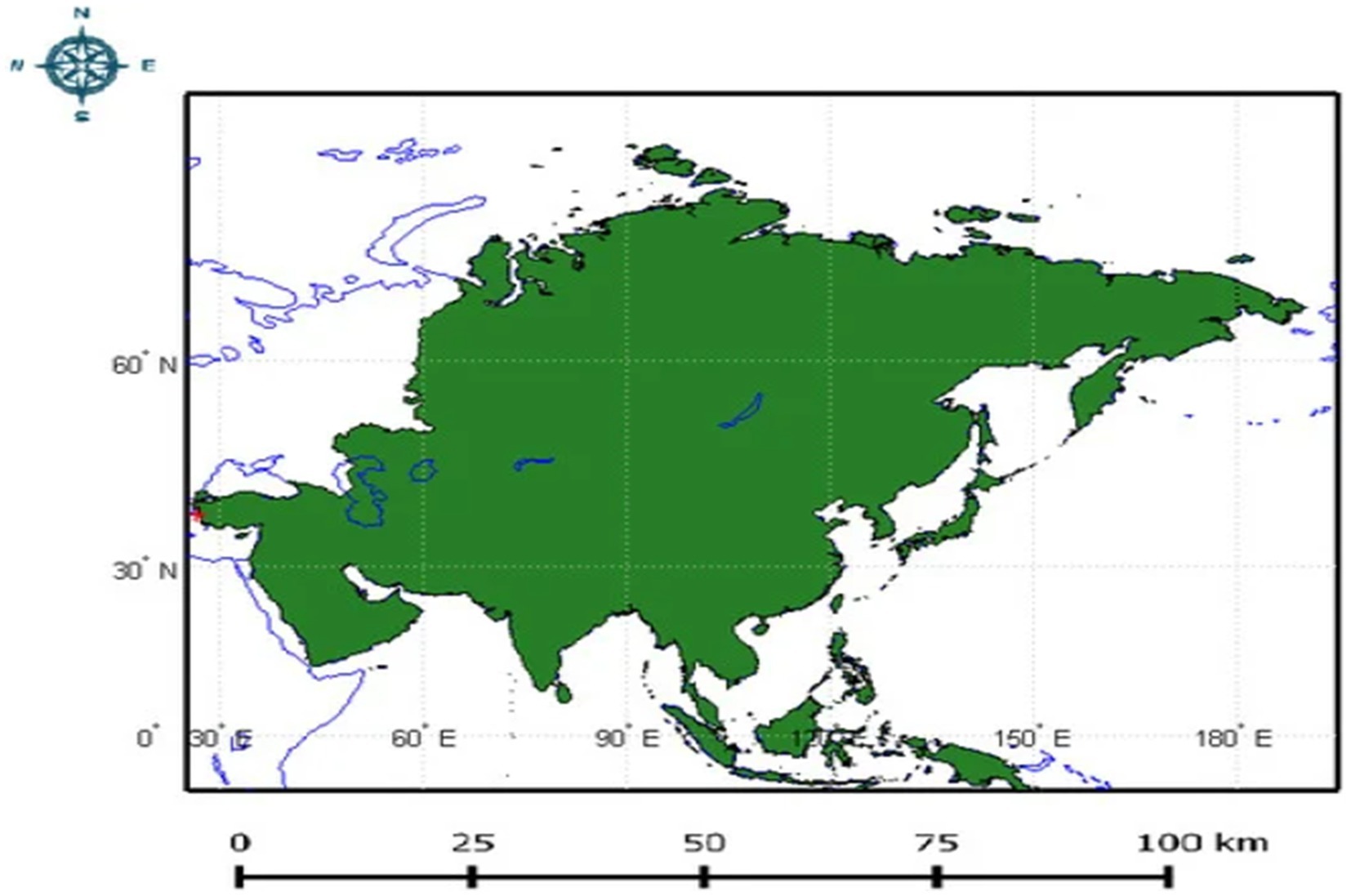
Figure 3. Depicts the Asian continent highlighted in green, with a red dot indicating the location in Turkey where T. absoluta was first discovered in 2009. This location marks the initial entry point of T. absoluta into Asia (Adapted from Guimapi et al., 2020).
In India, T. absoluta was first reported in 2014 in Maharashtra and has since spread to key tomato-growing regions like Karnataka, Tamil Nadu, Gujarat (Ballal et al., 2016), Andhra Pradesh, Telangana (Kumari et al., 2014), New Delhi (Shashank et al., 2016), Madhya Pradesh (Swathi et al., 2017), Punjab (Sidhu et al., 2017), Meghalaya (Sankarganesh et al., 2017), Himachal Pradesh (Sharma and Gavkare, 2017), and Uttarakhand (Singh and Panchbhaiya, 2018). The exact entry route into India remains uncertain, likely facilitated by unrestricted agricultural trade between states and prevailing wind patterns (Shashank et al., 2016). In May 2016, Bangladesh recorded its first instance of T. absoluta in tomato fields in Panchagarh district, swiftly spreading to neighboring districts (Hossain et al., 2016).
China documented infestations in the Ili Kizakg and Ili Xinjiang regions, causing significant damage to tomato, potato, and eggplant crops (Zhang et al., 2020). Taiwan faced invasion by T. absoluta in June 2020 (Ramasamy, 2020), while Myanmar reported varying infestation levels from 10 to 82% (Yule et al., 2021). Southeast Asian and Pacific nations like Indonesia, Korea, Japan, and Australia have not officially reported T. absoluta invasion but remain susceptible due to extensive trade in tomatoes and related crops with affected regions (McNitt et al., 2019; El-Shafie, 2020; Zhang et al., 2021).
4 Tuta absoluta management
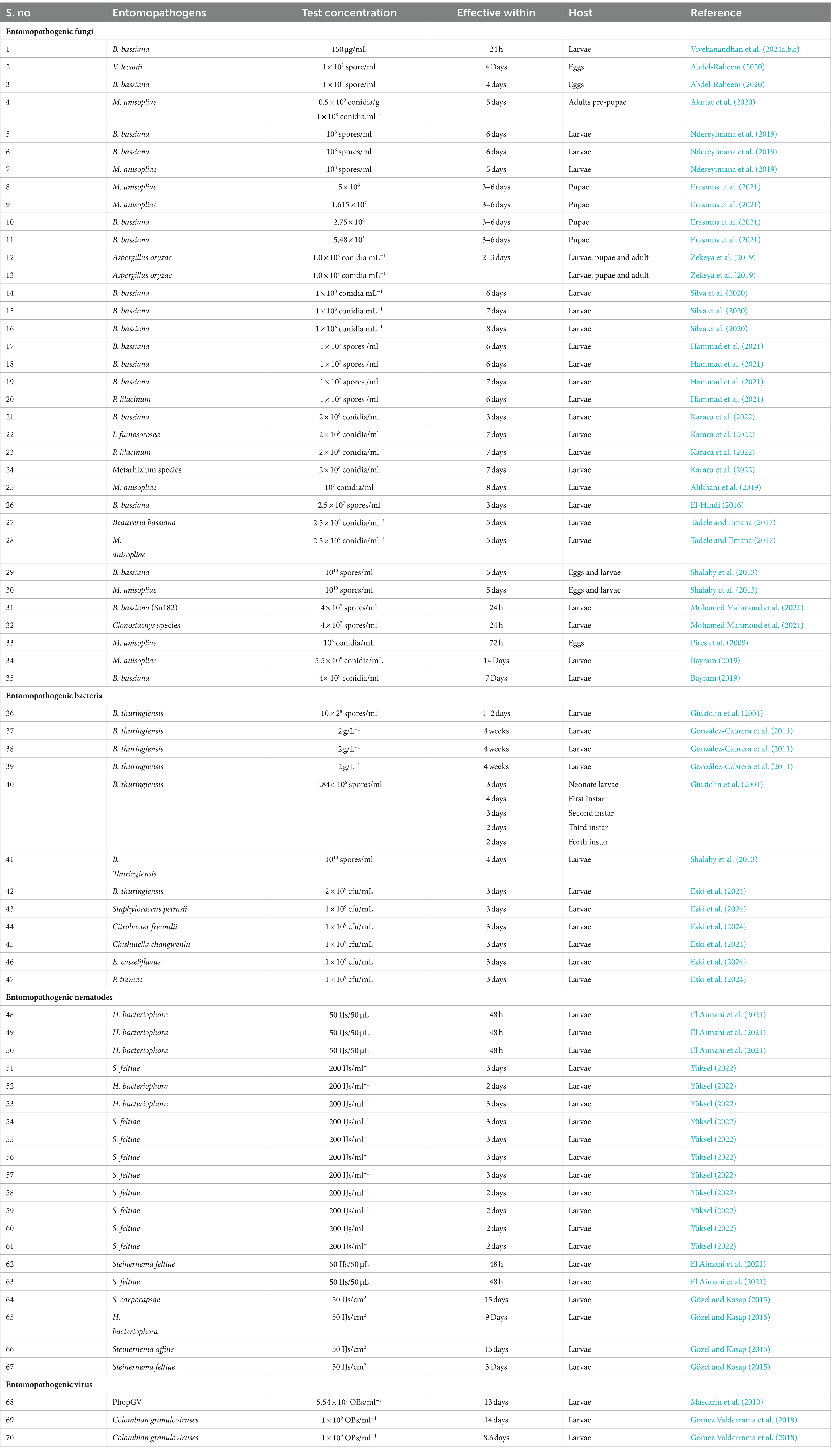
Management strategies for T. absoluta utilizing entomopathogens offer a broad array of effective options, encompassing various biological agents such as entomopathogenic fungi (e.g., Beauveria spp., Metarhizium spp.), bacteria (e.g., Bacillus thuringiensis), viruses (e.g., nucleopolyhedroviruses), and nematodes (e.g., Steinernema spp.). These agents exhibit efficacy against multiple life stages of the T. absoluta insect pest, including eggs, larvae, pupae, and adults (see Table 2). Their application with entomopathogens based management programs provides sustainable alternatives to chemical pesticides, contributing to environmentally friendly and economically viable pest control strategies.
4.1 Entomopathogenic fungi and bacteria
Entomopathogenic fungi (EPF) are heterotrophic, eukaryotic filamentous microorganisms that reproduce conidia either sexually or asexually (Mora et al., 2017; Vivekanandhan et al., 2021, 2024a,b). The majority of EPF, including Beauveria bassiana, Metarhizium anisopliae, Metarhizium acridum, Metarhizium brunneum, Isaria fumosorosea, Hirsutella thompsonii, and Lecanicillium lecanii, are classified as Ascomycetes and highly virulent to a broad range of medical and agricultural insect pests (Dara, 2017; Vivekanandhan et al., 2022a,b, 2023, 2024a,b,c; Swathy et al., 2023, 2024; Krutmuang et al., 2024; Perumal et al., 2024a,b). The fungi are pathogenic to various insect genera, causing muscardine disease in a wide range of hosts with minimal environmental impact and insect resistance (El-Hindi, 2016; Krutmuang et al., 2023; Perumal et al., 2023a,b). Although the efficacy of these entomopathogenic fungi depends on environmental conditions, B. bassiana and M. anisopliae are the most extensively researched and commercialized fungal species (Vivekanandhan et al., 2020; Kannan et al., 2023; Vivekanandhan et al., 2024a,b,c). These EPF demonstrated high larval mortality against several agriculturally important insect pests.
Studies on the effectiveness of B. bassiana and B. thuringiensis against T. absoluta have demonstrated varying levels of vulnerability across larval stages. González-Cabrera et al. (2011) and Alsaedi et al. (2017) found that first instar larvae were the most susceptible to B. thuringiensis, aiding in keeping T. absoluta populations below economic thresholds. In contrast, research indicated that third instar larvae were particularly vulnerable to both B. bassiana and B. thuringiensis.
Additionally, Biondi et al. (2018) reported that Wolbachia bacterial infection might benefit T. absoluta by affecting its reproduction. Spinosad, derived from Saccharopolyspora spinosa, has also been effective in controlling T. absoluta (Baniameri and Cheraghian, 2012; Caparros Megido et al., 2012). Studies by El-Ghany et al. (2016) and Aynalem et al. (2022) highlighted the significant pathogenicity of entomopathogenic fungi and bacteria, such as B. bassiana, M. anisopliae, and B. thuringiensis, against T. absoluta in field conditions.
B. bassiana has demonstrated potential as an epiphytic, endophytic, and insecticidal agent in greenhouse environments (Klieber and Reineke, 2016). It can colonize tomato plants endophytically, providing effective control against the tomato leaf miner (Allegrucci et al., 2017). Ibranhim et al. (2017) suggested that M. anisopliae and B. bassiana conidia are promising for short-term T. absoluta control. Further studies by Tadele and Emana (2017) and Ayele et al. (2020) confirmed the high insecticidal activity of these fungi in Ethiopian laboratories and glasshouses.
Entomopathogenic bacteria, such as B. thuringiensis, can induce diseases in various insect pests. B. thuringiensis (Bt) is a Gram-positive, spore-forming bacterium that produces δ-endotoxin, hemotoxin, and vegetative proteins. Since the 1950s, Bt has been used as a natural insecticide to control specific insect pests. The toxic genes on the Bt plasmid, which encode crystal proteins, are vital for developing pest-resistant genetically modified plants. This makes Bt a significant biopesticide worldwide, with targeted insecticidal activity that minimizes harm to non-target organisms. Researchers have classified numerous crystal protein-coding genes in Bt, grouped based on their sequences. Different Cry genes produce toxins targeting specific insect groups, including lepidopterans, coleopterans, nematodes, and dipterans. Bt strains can carry multiple crystal toxin genes, suggesting a mechanism for gene transfer between strains, enhancing toxin diversity (Aynalem, 2022).
4.2 Entomopathogenic nematode
Entomopathogenic nematodes (EPNs) are cosmopolitan, non-segmented, cylindrical, and elongated organisms playing a crucial role in biological control (Hominick et al., 1996). These nematodes are classified into 23 families, with seven families, including Mermithidae, Tetradonematidae, Allantonematidae, Phaenopsitylenchidae, Sphaerulariidae, Heterorhabditidae, and Steinernematidae, containing the most effective species for insect pest control (Lacey and Georgis, 2012). EPNs have shown high efficacy in controlling T. absoluta larvae, achieving 79–100% mortality under laboratory conditions (Batalla-Carrera et al., 2010). Leaflet bioassays revealed 77–92% larval nematode infection within the galleries, while pot experiments demonstrated an 87–95% reduction in T. absoluta infection (Batalla-Carrera et al., 2010).
Two nematode species, Heterorhabditis bacteriophora and Steinernema carpocapsae, caused 92–96% and 89–91% larval mortality under laboratory conditions, respectively. These species also achieved 48–51% control of T. absoluta in greenhouse conditions (Kamali et al., 2018). Additionally, H. bacteriophora, S. carpocapsae, and Steinernema feltiae showed significant insecticidal activity, with 77–97.4% mortality on T. absoluta larvae (Van Damme et al., 2016). EPNs utilize mutualistic intestinal bacteria to eliminate insect pests (Boemare, 2002; De Waal et al., 2011; Van Damme et al., 2016).
Their use in pest management is widespread and effective across various taxa, including similar Lepidopterans like the false codling moth (Thaumatotibia leucotreta), codling moth (Cydia pomonella), and sugarcane borer (Eldana saccharina) (De Waal et al., 2011; Malan et al., 2011; Nthenga et al., 2014). Recent research has confirmed that S. feltiae, S. carpocapsae, and H. bacteriophora are effective against all larval instars of T. absoluta (Kamali et al., 2018). These findings indicate that EPNs have significant potential in managing T. absoluta and can be integrated into pest management strategies.
Entomopathogenic fungi, such as B. bassiana and M. anisopliae, are often preferred over entomopathogenic bacteria, viruses, and nematodes for controlling T. absoluta due to their broader host range and effective modes of action. These fungi can infect T. absoluta through direct contact or ingestion, providing effective control against both larvae and adults. They are environmentally safe, adaptable to various conditions, and less prone to resistance development compared to other entomopathogens (Aynalem, 2022). Furthermore, fungi offer versatility in formulation and application methods, making them suitable for integrated pest management strategies. Entomopathogenic fungi present promising prospects for sustainable and effective T. absoluta management.
5 Mode of action of entomopathogenic fungi on Tuta absoluta
Entomopathogenic fungi are a group of fungi that specifically infect and kill insect pests. These fungi have evolved intricate strategies to invade, proliferate within, and ultimately cause the death of their insect hosts. The mode of action of entomopathogenic fungi involves several key steps:
5.1 Attachment and adhesion
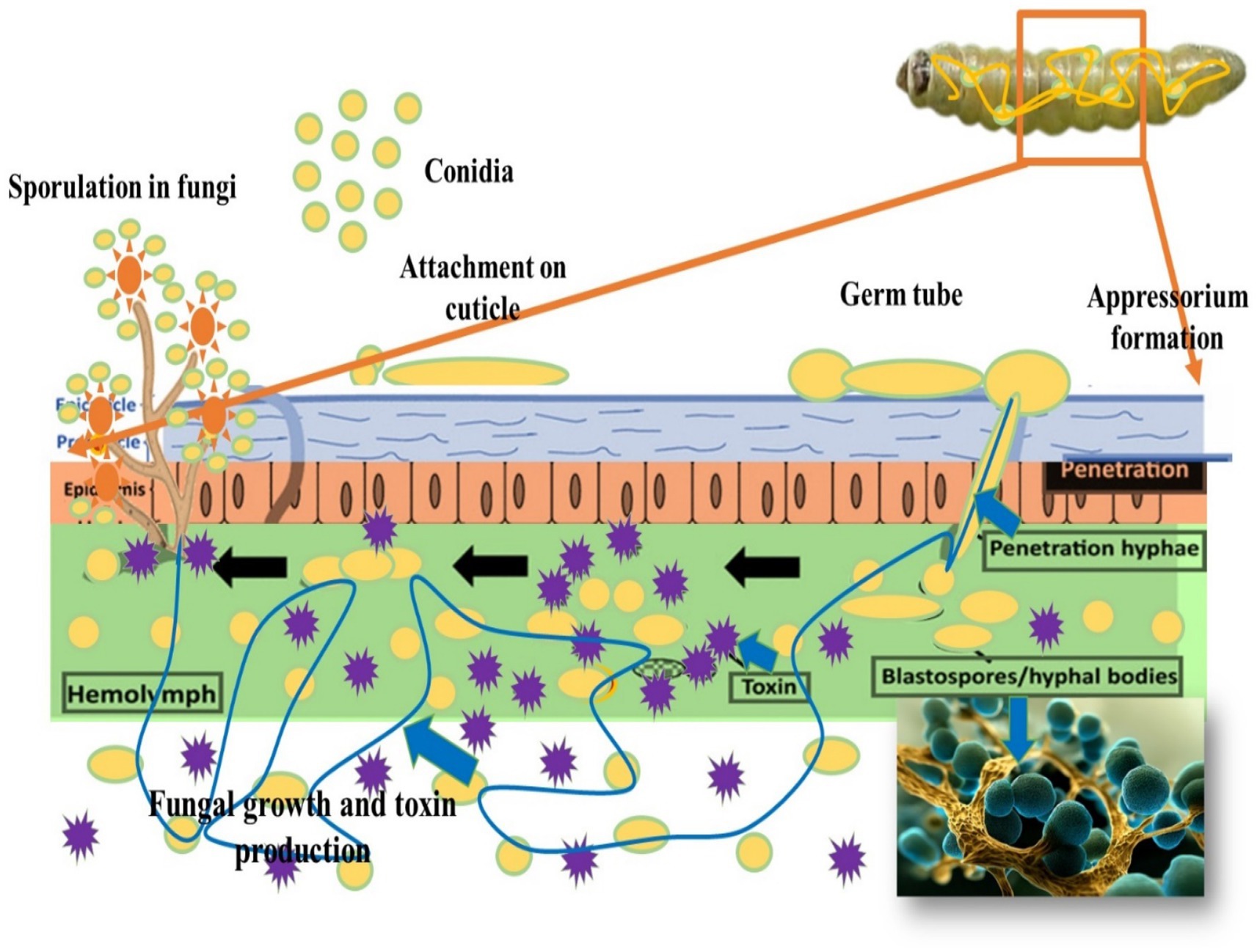
Entomopathogenic fungi possess specialized spores called conidia, which are adapted to attach to the insect’s cuticle. These conidia feature structures such as hydrophobins or other adhesive proteins that facilitate binding to the insect’s exoskeleton (see Figure 4). This attachment is crucial for initiating the infection process and subsequent penetration into the insect’s body. The hydrophobic nature of these structures ensures that the spores adhere firmly to the insect’s surface, even under humid conditions, establishing the fungal infection effectively (Vidhate et al., 2023). This initial adhesion is a critical step in the process through which entomopathogenic fungi infect their insect hosts.
5.2 Penetration
Once attached to the insect’s cuticle, the conidia of entomopathogenic fungi undergo germination, developing specialized structures essential for host penetration. One such structure is the appressorium, a highly specialized cell type that exerts mechanical force and enzymatic activity to breach the insect cuticle. Appressoria are pressure-sensitive cells that apply physical pressure to penetrate the insect cuticle. Additionally, they secrete enzymes, including chitinases and proteases, which degrade the cuticle’s components. Chitinases target chitin, a major component of the cuticle, while proteases break down cuticular proteins. This combined mechanical and enzymatic action allows the fungal hyphae to penetrate the insect’s body, overcoming the protective barrier of the cuticle and establishing infection within the host (Ma et al., 2024).
5.3 Colonization and proliferation
After penetrating the insect’s cuticle, the entomopathogenic fungus enters the hemocoel, the body cavity containing hemolymph. Inside the hemocoel, the fungus undergoes a transformative growth phase, developing filamentous hyphae. These hyphae extend and spread throughout the hemocoel, invading various tissues and organs of the insect host. As the hyphae proliferate, they disrupt normal physiological functions and cause extensive damage to internal structures. The fungal hyphae absorb nutrients from the insect’s tissues, depriving the host of essential resources necessary for survival. This invasive process highlights the pathogenic nature of entomopathogenic fungi and their ability to efficiently colonize and exploit their insect hosts. Ultimately, the fungal infection progresses, consuming vital host tissues and resources, leading to the death of the insect (Ma et al., 2024).
5.4 Nutrient utilization and host tissue degradation
Nutrient utilization and host tissue degradation by entomopathogenic fungi are critical phases in the infection process. Once inside the insect’s body, the invading fungal hyphae secrete various enzymes that facilitate tissue degradation and nutrient acquisition. Proteases and lipases play pivotal roles in this process. Proteases target proteins, cleaving them into smaller peptides and amino acids, which breaks down structural and functional proteins within the host’s body. Lipases hydrolyze lipids, accessing lipid reserves and membrane-bound lipids, which are essential components of cell membranes and storage tissues in insects (Quesada-Moraga et al., 2024).
This enzymatic activity leads to significant degradation of host tissues, disrupting normal physiological functions. Vital organs and structures, such as muscles and fat bodies, are progressively broken down by the fungal hyphae, releasing nutrients required for fungal growth and reproduction. This process exemplifies the parasitic nature of entomopathogenic fungi, as they sustain their growth and propagation by harnessing host-derived nutrients. The disruption of normal physiological functions due to tissue degradation contributes to the progression of the fungal infection and eventual mortality of the insect (Liu et al., 2023).
5.5 Immune evasion
Immune evasion is a critical adaptation employed by entomopathogenic fungi to overcome the insect’s immune defense and establish successful infections. These fungi have evolved sophisticated strategies, including the production of secondary metabolites, to evade or suppress the host’s immune response.
One key mechanism involves the secretion of secondary metabolites that have immunomodulatory effects. These metabolites can disrupt the recognition and activation of immune cells, such as haemocytes, which are the main cellular defense against pathogens. Some metabolites directly inhibit immune responses, such as phagocytosis (the engulfment of pathogens by immune cells) or the production of antimicrobial peptides. By impairing these immune mechanisms, the fungi can proliferate and spread within the insect’s body without encountering effective cellular defense (Ma et al., 2024).
Furthermore, entomopathogenic fungi may secrete compounds that disrupt signaling pathways involved in immune activation, dampening the insect’s ability to mount a robust immune response. This ability to evade or suppress the host’s immune defense is critical for the pathogenicity and successful colonization of the insect host. By manipulating the insect’s immune system through the production of specific secondary metabolites, these fungi can establish infections and exploit host resources for growth and reproduction.
5.6 Systemic effects and death
As entomopathogenic fungi establish and progress through infection within the insect host, they induce systemic effects that ultimately culminate in the death of the host organism. These effects arise from the relentless growth and metabolic activity of the fungal hyphae within the insect’s body (Mahanta et al., 2023).
The fungal hyphae proliferate and extensively colonize the insect’s tissues, actively consuming and depleting host nutrients, including proteins, carbohydrates, and lipids. This nutrient drain deprives the insect of essential resources necessary for sustaining life functions and physiological processes. The invasive growth of fungal hyphae disrupts the integrity and function of vital organs and tissues within the insect’s body, leading to organ failure and impairing critical physiological processes such as digestion, circulation, and metabolism (De Fine Licht et al., 2024).
Entomopathogenic fungi frequently disrupt the insect’s molting process, which is crucial for growth and development. The presence of fungal hyphae can disrupt the synthesis and release of molting hormones, leading to improper or failed molting cycles. This hampers the insect’s ability to shed its exoskeleton and grow, ultimately compromising its survival (Yang et al., 2023).
During the course of infection, entomopathogenic fungi produce various metabolic by-products and toxins. The accumulation of these toxic metabolites within the insect’s body contributes to physiological stress, cellular damage, and an overall decline in health. After killing the insect host, the fungus produces new spores (conidia) on the cadaver. These spores are released into the environment and can infect new susceptible hosts, completing the fungal life cycle (Lima et al., 2024) (see Figure 4).
6 Entomopathogenic fungi: advantages, limitations, and future directions
Entomopathogenic fungi offer several advantages as biocontrol agents for managing insect pests. They are highly specific to insects, exhibiting low toxicity to non-target organisms, including humans and other vertebrates. This specificity makes them suitable for integrated pest management (IPM) strategies, minimizing ecological impact. These fungi are environmentally friendly alternatives to chemical pesticides, as they are naturally occurring organisms that degrade quickly in the environment. They support sustainable pest management approaches that reduce reliance on synthetic chemicals (Sharma et al., 2023; Perumal et al., 2024a).
Entomopathogenic fungi employ multiple modes of action to kill insects, including mechanical penetration, enzymatic degradation, and immune evasion. This multifaceted approach reduces the likelihood of insect resistance development compared to single-mode chemical insecticides (Liu et al., 2023). Some entomopathogenic fungi can persist in the environment for extended periods, providing longer-term pest control benefits. They also demonstrate adaptability to various environmental conditions and host species, enhancing their versatility in pest management programs. Entomopathogenic fungi can be effectively integrated with other pest management tactics, such as cultural practices, biological controls (e.g., predators, parasitoids), and, when necessary, chemical controls. This integration enhances overall pest control efficacy and sustainability (Smagghe et al., 2023).
6.1 Challenges of entomopathogenic fungi in pest management
Entomopathogenic fungi, while promising as biocontrol agents, face several challenges that limit their widespread adoption in pest management strategies. Compared to chemical insecticides, entomopathogenic fungi typically exhibit slower action in controlling insect populations. They require time to infect, colonize, and ultimately kill target insects, which may not provide rapid control needed in some agricultural settings (Vivekanandhan et al., 2023).
Environmental sensitivity poses another challenge. Factors such as temperature and humidity significantly influence the efficacy of entomopathogenic fungi. Optimal environmental conditions are crucial for successful fungal infection and proliferation, limiting their effectiveness under adverse conditions (Perumal et al., 2024a). While entomopathogenic fungi are highly specific to insects, their narrow host range can restrict their utility to certain target pests. Some fungi are effective only against specific insect groups or life stages, which limits their broader applicability across diverse pest populations.
The production and formulation of entomopathogenic fungi for commercial use present technical and economic challenges. Large-scale production requires specialized facilities and technologies, making it costly and technically demanding. Improvements in production methods and formulation technologies are necessary to enhance the practicality and cost-effectiveness of using these fungi in pest management (Jaronski, 2023; Quesada-Moraga et al., 2024). Moreover, regulatory approval for entomopathogenic fungi as biopesticides can be complex and time-consuming. The process involves rigorous evaluation of safety and efficacy data, which adds to the challenges of bringing these products to market and integrating them into agricultural practices. Addressing these challenges through research and innovation will be essential to maximize the potential of entomopathogenic fungi in sustainable agriculture and integrated pest management programs.
6.2 Advancing entomopathogenic fungi in pest management
Entomopathogenic fungi represent a promising avenue for sustainable pest management, yet advancing their application requires addressing several key areas of research and development (Qin et al., 2023). Efforts should prioritize enhancing formulation technologies to improve the stability, shelf-life, and application methods of entomopathogenic fungi (Bhattacharyya et al., 2023). Innovations in encapsulation, adjuvants, and targeted delivery systems are crucial for maximizing efficacy and practicality in diverse environmental conditions. Expanding the host range and efficacy of entomopathogenic fungi through genetic and ecological studies is essential. Genetic engineering can potentially enhance traits such as virulence and environmental tolerance, broadening the spectrum of pests these fungi can effectively control.
Optimizing the integration of entomopathogenic fungi with other pest management tactics, including biological controls and cultural practices, will enhance overall efficacy and sustainability (Smagghe et al., 2023). Continued research is needed to develop integrated pest management strategies that synergistically combine these approaches. Comprehensive environmental monitoring and impact assessments are critical to ensure the safe and sustainable use of entomopathogenic fungi across different ecosystems. Understanding their persistence and ecological interactions is vital for minimizing unintended environmental consequences.
Streamlining production processes, reducing costs, and navigating regulatory pathways are essential for the successful commercialization and widespread adoption of entomopathogenic fungi in agricultural and urban settings (Lankinen et al., 2024). Overcoming these hurdles will facilitate their integration into mainstream pest control practices (Ahmed et al., 2024). Entomopathogenic fungi offer significant potential as effective, environmentally friendly tools for pest management. Addressing current challenges and exploring these future directions will be instrumental in realizing their full potential and promoting sustainable agriculture worldwide.
7 Conclusion and perspectives
Entomopathogenic microorganisms, such as bacteria, fungi, and viruses, present promising prospects for controlling T. absoluta, a notorious pest of tomato crops. Extensive studies have underscored the effectiveness of various entomopathogens, including B. thuringiensis (Bt), B. bassiana, M. anisopliae, and nucleopolyhedroviral viruses (NPVs), against both larvae and adults of T. absoluta. Utilizing entomopathogens offers several advantages in insect pest management. Entomopathogens are highly specific to insects, exerting minimal impact on non-target organisms, which positions them as environmentally friendly alternatives to chemical pesticides. Moreover, entomopathogens employ diverse modes of action such as direct infection, toxin production, and physiological interference with insect hosts. However, the successful application of entomopathogens for T. absoluta control necessitates addressing several challenges. These include optimizing application methods to enhance efficacy under varying environmental conditions, improving formulation stability to prolong shelf-life and efficacy, and comprehensively understanding their interactions with environmental factors.
Future directions in entomopathogens research involve exploring novel strains or combinations of entomopathogens, developing integrated pest management (IPM) strategies that synergize entomopathogens with other pest control methods, and innovating delivery systems to ensure consistent and reliable pest suppression. Entomopathogens hold significant promise as sustainable tools for managing T. absoluta, offering effective alternatives to synthetic pesticides while promoting environmentally friendly agricultural practices. Continued research and innovation are imperative to fully harness the potential of entomopathogens within integrated pest management programs aimed at sustainable agriculture.
Data availability statement
The information supporting this review article is fully contained within the article itself.
Author contributions
PV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. PS: Software, Validation, Writing – original draft, Writing – review & editing. KP: Conceptualization, Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Office of Research Administration at Chiang Mai University, Chiang Mai 50200, Thailand (Grant Numbers EP010219).
Acknowledgments
We thank the Office of Research Administration at Chiang Mai University, Chiang Mai 50200, Thailand, for providing funding for this study (Grant Numbers EP010219). We would also like to express our gratitude to the Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Thailand.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbes, K., Harbi, A., Elimem, M., Hafsi, A., and Chermiti, B. (2016). Bioassay of three Solanaceous weeds as alternative hosts for the invasive tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae) and insights on their carryover potential. Afr. Entomol. 24, 334–342. doi: 10.4001/003.024.0334
Abdel-Raheem, M. (2020). “Isolation, mass production and application of entomopathogenic fungi for insect pests control” in Cottage industry of biocontrol agents and their applications: practical aspects to deal biologically with pests and stresses facing strategic crops, Eds. El-Wakeil, N., Saleh, M., and Abu-hashim, M., (Springer) 231–251.
Ahmed, N., Zhang, B., Deng, L., Bozdar, B., Li, J., Chachar, S., et al. (2024). Advancing horizons in vegetable cultivation: a journey from ageold practices to high-tech greenhouse cultivation—a review. Front. Plant Sci. 15:1357153. doi: 10.3389/fpls.2024.1357153
Akutse, K. S., Subramanian, S., Khamis, F. M., Ekesi, S., and Mohamed, S. A. (2020). Entomopathogenic fungus isolates for adult Tuta absoluta (Lepidoptera: Gelechiidae) management and their compatibility with Tutapheromone. J. Appl. Entomol. 144, 777–787. doi: 10.1111/jen.12812
Alikhani, M., Safavi, S. A., and Iranipour, S. (2019). Effect of the entomopathogenic fungus, Metarhizium anisopliae (Metschnikoff) Sorokin, on demographic fitness of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 29, 1–7. doi: 10.1186/s41938-019-0121-0
Allegrucci, N., Velazquez, M.S., Russo, M.L., Pérez, M.E., and Scorsetti, A.C., (2017). Endophytic colonisation of tomato by the entomopathogenic fungus Beauveria bassiana: the use of different inoculation techniques and their effects on the tomato leafminer Tuta absoluta Lepidoptera: Gelechiidae. (Polish Academy of Sciences, J. Plant Prot. Res) 57, 331–337.
Alsaedi, G., Ashouri, A., and Talaei-Hassanloui, R. (2017). Evaluation of Bacillus thuringiensis to control Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under laboratory conditions. Agric. Sci. 8, 591–599. doi: 10.4236/as.2017.87045
Ayele, B. A., Muleta, D., Venegas, J., and Assefa, F. (2020). Morphological, molecular, and pathogenicity characteristics of the native isolates of Metarhizium anisopliae against the tomato leafminer, Tuta absoluta (Meyrick 1917) (Lepidoptera: Gelechiidae) in Ethiopia. Egypt. J. Biol. Pest Control 30, 1–11. doi: 10.1186/s41938-020-00261-w
Aynalem, B. , (2022). Empirical review of Tuta absoluta meyrick effect on the tomato production and their protection attempts. Adv. agric, 2595470.
Aynalem, B., Muleta, D., Jida, M., Shemekite, F., and Aseffa, F. (2022). Biocontrol competence of Beauveria bassiana, Metarhizium anisopliae and Bacillus thuringiensis against tomato leaf miner, Tuta absoluta Meyrick 1917 under greenhouse and field conditions. Heliyon 8:e09694. doi: 10.1016/j.heliyon.2022.e09694
Bajracharya, A. S., Bhat, B., and Sharma, P. N. (2018). Geographical distribution of south American tomato leaf miner Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), vol. 5, Springer (International Journal of Tropical Insect Science). 203–216.
Bajracharya, A. S. R., Mainali, R. P., Bhat, B., Bista, S., Shashank, P. R., and Meshram, N. M. (2016). The first record of south American tomato leaf miner, Tuta absoluta (Meyrick 1917) (Lepidoptera: Gelechiidae) in Nepal. J. Entomol. Zool. Stud. 4, 1359–1363.
Ballal, C. R., Gupta, A., Mohan, M., Lalitha, Y., and Verghese, A. (2016). The new invasive pest Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in India and its natural enemies along with evaluation of Trichogrammatids for its biological 3X spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present, and future. Annu. Rev. Entomol. 110, 2155–2258. doi: 10.18520/cs/v110/i11/2155-2159
Baniameri, V., and Cheraghian, A. (2012). The first report and control strategies of Tuta absoluta in Iran. EPPO Bullet. 42, 322–324. doi: 10.1111/epp.2577
Banks, N. C., Paini, D. R., Bayliss, K. L., and Hodda, M., (2015). The role of global trade and transport network topology in the human‐mediated dispersal of alien species .Ecol. Lett,18, 188–199.
Batalla-Carrera, L., Morton, A., and García-del-Pino, F. (2010). Efficacy of entomopathogenic nematodes against the tomato leafminer Tuta absoluta in laboratory and greenhouse conditions. BioControl 55, 523–530. doi: 10.1007/s10526-010-9284-z
Bawin, T., Dujeu, D., De Backer, L., Francis, F., and Verheggen, F. J., (2016). Ability of Tuta absoluta (Lepidoptera: Gelechiidae) to develop on alternative host plant species. Can. Entomol. 148, 434–442.
Bayram, Y. (2019). Efficacy of two entomopathogenic fungi and their combination with summer oil against tomato moth, Tuta absoluta. Fresenius Environ. Bull. 28, 3435–3440.
Bhattacharyya, P. N., Sarmah, S. R., Roy, S., Sarma, B., Nath, B. C., and Bhattacharyya, L. H. (2023). Perspectives of Beauveria bassiana, an entomopathogenic fungus for the control of insect-pests in tea [Camellia sinensis (L.) O. Kuntze]: opportunities and challenges. International journal of tropical insect. Science 43, 1–19. doi: 10.1007/s42690-022-00932-1
Biondi, A., Guedes, R. N. C., Wan, F. H., and Desneux, N. (2018). Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present, and future. Annu. Rev. Entomol. 63, 239–258. doi: 10.1146/annurev-ento-031616-034933
Boemare, N. O. E. L. (2002). “Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus” in Entomopathogenic nematology Ed. R. Gaugler (Wallingford UK: CABI Publishing), 35–56.
Buragohain, P., Saikia, D. K., Sotelo-Cardona, P., and Srinivasan, R. (2021). Evaluation of bio-pesticides against the south American tomato leaf miner, Tuta absoluta Meyrick (Lepidoptera: gelechiidae) in India. Horticulturae 7:325. doi: 10.3390/horticulturae7090325
Campos, M. R., Biondi, A., Adiga, A., Guedes, R. N., and Desneux, N. (2017). From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest. Sci. 90, 787–796. doi: 10.1007/s10340-017-0867-7
Caparros Megido, R., Haubruge, E., and Verheggen, F. J. (2012). First evidence of deuterotokous parthenogenesis in the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest. Sci. 85, 409–412. doi: 10.1007/s10340-012-0458-6
Cocco, A., Deliperi, S., Lentini, A., Mannu, R., and Delrio, G. (2015). Seasonal phenology of Tuta absoluta (Lepidoptera: Gelechiidae) in protected and open-field crops under Mediterranean climatic conditions. Phytoparasitica 43, 713–724. doi: 10.1007/s12600-015-0486-x
Colmenárez, Y. C., Vásquez, C., de Freitas Bueno, A., Cantor, F., Hidalgo, E., Corniani, N., et al. (2022). Sustainable management of the invasive Tuta absoluta (Lepidoptera: Gelechiidae): an overview of case studies from Latin American countries participating in plantwise. J. Integrated Pest Manag. 13:15. doi: 10.1093/jipm/pmac012
Cuthbertson, A. G., Mathers, J. J., Blackburn, L. F., Korycinska, A., Luo, W., Jacobson, R. J., et al. (2013). Population development of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under simulated UK glasshouse conditions. Insects 4, 185–197. doi: 10.3390/insects4020185
Dara, S. K. (2017). Entomopathogenic microorganisms: modes of action and role in IPM. UCANR eJ. Entomol Biol., 1–7.
De Fine Licht, H. H., Csontos, Z., Nielsen, P. J. D. N., Langkilde, E. B., Kjaergaard Hansen, A. K., and Shik, J. Z. (2024). Insect hosts are nutritional landscapes navigated by fungal pathogens. bioRxiv, 2024–2001. doi: 10.1101/2024.01.04.574030
De Waal, J. Y., Malan, A. P., and Addison, M. F. (2011). Efficacy of entomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) against codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in temperate regions. Biocontrol Sci. Tech. 21, 1161–1176. doi: 10.1080/09583157.2011.607922
Diatte, M., Brévault, T., Sylla, S., Tendeng, E., Sall-Sy, D., and Diarra, K. (2018). Arthropod pest complex and associated damage in field-grown tomato in Senegal. Int. J. Tropical Insect Sci. 38, 243–253. doi: 10.1017/S1742758418000061
El Aimani, A., Mokrini, F., Houari, A., Laasli, S. E., Sbaghi, M., Mentag, R., et al. (2021). Potential of indigenous entomopathogenic nematodes for controlling tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under laboratory and field conditions in Morocco. Physiol. Mol. Plant Pathol. 116:101710. doi: 10.1016/j.pmpp.2021.101710
El-Ghany, N. M. A., Abdel-Razek, A. S., Ebadah, I. M., and Mahmoud, Y. A. (2016). Evaluation of some microbial agents, natural and chemical compounds for controlling tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Plant Protect. Res. 56, 372–379. doi: 10.1515/jppr-2016-0055
El-Hindi, M. (2016). Safe approach to the biological control of the tomato Leafminer Tuta absoluta by entomopathogenic fungi Beauveria bassiana isolates from Gaza strip. IJAR 2, 351–355.
El-Shafie, H. A. F. (ed.) (2020). “Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): an invasive insect pest threatening the world tomato production” in Invasive species-introduction pathways, economic impact, and possible management options, (BoD – Books on Demand) 1–16. doi: 10.5772/intechopen.93390
EPPO (2005). Data sheets on quarantine pests. EPPO Bull. 122, 43–47. doi: 10.1111/j.1439-0418.1998.tb01459.x
EPPO . (2023) Tuta absoluta. EPPO Global Database; (GNORAB). Available at: https://gd.eppo.int/taxon/GNORAB
Erasmus, R., van den Berg, J., and du Plessis, H. (2021). Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) pupae to soil applied entomopathogenic fungal biopesticides. Insects 12:515. doi: 10.3390/insects12060515
Erdogan, P., and Babaroglu, N. E. (2014). Life table of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Agric. Facult. Gaziosmanpasa Univ. 31:75. doi: 10.13002/jafag723
Eski, A., Erdoğan, P., Demirbağ, Z., and Demir, İ. (2024). Isolation and identification of bacteria from the invasive pest Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and evaluation of their biocontrol potential. Int. Microbiol. 27, 631–643. doi: 10.1007/s10123-023-00418-1
Fiaboe, K. R., Agboka, K., Agboyi, L. K., Koffi, D., Ofoe, R., Kpadonou, G. E., et al. (2021). First report and distribution of the south American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Togo. Phytoparasitica 49, 167–177. doi: 10.1007/s12600-020-00841-4
Giustolin, T. A., Vendramim, J. D., Alves, S. B., Vieira, S. A., and Pereira, R. M. (2001). Susceptibility ofTuta absoluta (Meyrick) (Lep., Gelechiidae) reared on two species of lycopersicon to Bacillus thuringiensis var. kurstaki. J. Appl. Entomol. 125, 551–556. doi: 10.1046/j.1439-0418.2001.00579.x
Gómez Valderrama, J. A., Barrera, G., López‐Ferber, M., Belaich, M., Ghiringhelli, P. D., and Villamizar, L., (2018). Potential of betabaculoviruses to control the tomato leafminer Tuta absoluta (Meyrick). J. Appl. Entomol, 142, 67–77.
González-Cabrera, J., Mollá, O., Montón, H., and Urbaneja, A. (2011). Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). BioControl 56, 71–80. doi: 10.1007/s10526-010-9310-1
Gözel, Ç., and Kasap, I., (2015). Efficacy of entomopathogenic nematodes against the Tomato leafminer, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) in tomato field. TURK ENTOMOL DERG-TU, 39, 229–237.
Guimapi, R. A., Srinivasan, R., Tonnang, H. E., Sotelo-Cardona, P., and Mohamed, A. (2020). Exploring the mechanisms of the spatiotemporal invasion of Tuta absoluta in Asia. Agriculture 10:124. doi: 10.3390/agriculture10040124
Hammad, A. M. A., Bashir, H. A. A. A., Abdelbagi, A. O., Ishag, A. E. S. A., Ali, M. M. Y., Bashir, M. O., et al. (2021). Efficacy of indigenous entomopathogenic fungi for the control of the tomato leafminer Tuta absoluta (Meyrick) in Sudan. Int. J. Tropic. Insect Sci. 42, 1449–1459. doi: 10.1007/s42690-021-00663-9
Hominick, W. M., Reid, A. P., Bohan, D. A., and Briscoe, B. R. (1996). Entomopathogenic nematodes: biodiversity, geographical distribution and the convention on biological diversity. Biocontrol Sci. Tech. 6, 317–332. doi: 10.1080/09583159631307
Hossain, M. S., Mian, M. Y., and Muniappan, R. (2016). First record of Tuta absoluta (Lepidoptera: Gelechiidae) from Bangladesh1. J. Agric. Urban Entomol. 32, 101–105. doi: 10.3954/1523-5475-32.1.101
Ibranhim, L., Dakache, C., El Kreidy, M., Dagher, R., Ezzeddine, N., and Ibrahim, S. (2017). Environmentally sustainable production of Metrhizium anisopliae and Beauveria bassiana for control of Tuta absoluta. Int. J. Agric. Biosyst. Engin. 2, 1–12.
Jaronski, S. T. (2023). Mass production of entomopathogenic fungi—state of the art. Mass Product. Beneficial Organisms, 317–357. doi: 10.1016/B978-0-12-822106-8.00017-8
Kachave, D. R., Sonkamble, M. M., and Patil, S. K. (2020). Population dynamics of major insect pests infesting to tomato, Lycopersicon esculentum (miller). J. Pharmacog. Phytochem. 9, 344–348.
Kamali, S., Karimi, J., and Koppenhöfer, A. M. (2018). New insight into the management of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae) with entomopathogenic nematodes. J. Econ. Entomol. 111, 112–119. doi: 10.1093/jee/tox332
Kannan, S., Perumal, V., Yuvaraj, A., Pittarate, S., Kim, J. S., and Krutmuang, P. (2023). Biodegradation of pesticide in agricultural soil employing entomopathogenic fungi: current state of the art and future perspectives. Heliyon 10:e23406. doi: 10.1016/j.heliyon.2023.e23406
Karaca, G., Erol, A. B., Çığgın, B. A., Acarbulut, H., and Karaca, İ. (2022). Efficacy of some entomopathogenic fungi against tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 32:84. doi: 10.1186/s41938-022-00577-9
Kılıç, T. (2010). First record of Tuta absoluta in Turkey. Phytoparasitica 38, 243–244. doi: 10.1007/s12600-010-0095-7
Klieber, J., and Reineke, A. (2016). The entomopathogen B eauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner T uta absoluta. J. Appl. Entomol. 140, 580–589. doi: 10.1111/jen.12287
Krutmuang, P., Rajula, J., Pittarate, S., Chanbang, Y., Perumal, V., Alford, L., et al. (2023). Biocontrol efficacy of Beauveria bassiana in combination with tobacco short stem and modified lure traps. Int. J. Tropic. Insect Sci. 43, 1591–1600. doi: 10.1007/s42690-023-01063-x
Krutmuang, P., Sanchatthai, M., Rajula, J., Jing, L. W., Wan, P., Mekchay, S., et al. (2024). A comparison of the reproductive systems: a virgin and mated female Spodoptera frugiperda (Lepidoptera: Noctuidae). Int. J. Tropic. Insect Sci. 44, 637–645. doi: 10.1007/s42690-024-01180-1
Kumari, D. A., Anitha, G., Anitha, V., Lakshmi, B. K. M., Vennila, S., and Rao, N. H. P., (2014). New record of leaf miner, Tuta absoluta (Meyrich) in tomato. Insect Environment, Vol. 20.
Lacey, L. A., and Georgis, R. (2012). Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 44, 218–225
Lankinen, Å., Witzell, J., Aleklett, K., Furenhed, S., Karlsson Green, K., Latz, M., et al. (2024). Challenges and opportunities for increasing the use of low-risk plant protection products in sustainable production. Agron. Sustain. Develop. 44:21. doi: 10.1007/s13593-024-00957-5
Lima, V. H., Matugawa, A. T., Mascarin, G. M., and Fernandes, É. K. K. (2024). Complex nitrogen sources from agro-industrial byproducts: impact on production, multi-stress tolerance, virulence, and quality of Beauveria bassiana blastospores. Microbiol. Spectrum 12, e04040–e04023. doi: 10.1128/spectrum.04040-23
Liu, D., Smagghe, G., and Liu, T. X. (2023). Interactions between entomopathogenic fungi and insects and prospects with glycans. J. Fungi 9:575. doi: 10.3390/jof9050575
Ma, M., Luo, J., Li, C., Eleftherianos, I., Zhang, W., and Xu, L. (2024). A life-and-death struggle: interaction of insects with entomopathogenic fungi across various infection stages. Front. Immunol. 14:1329843. doi: 10.3389/fimmu.2023.1329843
Mahanta, D. K., Bhoi, T. K., Komal, J., Samal, I., Nikhil, R. M., Paschapur, A. U., et al. (2023). Insect-pathogen crosstalk and the cellular-molecular mechanisms of insect immunity: uncovering the underlying signaling pathways and immune regulatory function of non-coding RNAs. Front. Immunol. 14:1169152. doi: 10.3389/fimmu.2023.1169152
Malan, A. P., Knoetze, R., and Moore, S. D. (2011). Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. J. Invertebr. Pathol. 108, 115–125. doi: 10.1016/j.jip.2011.07.006
Martins, J. C., Picanço, M. C., Bacci, L., Guedes, R. N. C., Santana, P. A., Ferreira, D. O., et al. (2016). Life table determination of thermal requirements of the tomato borer Tuta absoluta. J. Pest. Sci. 89, 897–908. doi: 10.1007/s10340-016-0729-8
Mascarin, G. M., Alves, S. B., Rampelotti-Ferreira, F. T., Urbano, M. R., Demétrio, C. G. B., and Delalibera, I. (2010). Potential of a granulovirus isolate to control Phthorimaea operculella (Lepidoptera: Gelechiidae). BioControl 55, 657–671. doi: 10.1007/s10526-010-9277-y
McNitt, J., Chungbaek, Y. Y., Mortveit, H., Marathe, M., Campos, M. R., Desneux, N., et al. (2019). Assessing the multi-pathway threat from an invasive agricultural pest: Tuta absoluta in Asia. Proc. R. Soc. B 286:20191159. doi: 10.1098/rspb.2019.1159
Mohamed Mahmoud, F., Bendebbah, R., Benssaci, B., Toudji, F., Tafifet, L., and Krimi, Z. (2021). Entomopathogenic efficacy of the endophytic fungi: Clonostachys sp. and Beauveria bassiana on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae under laboratory and greenhouse conditions. Egypt. J. Biol. Pest Control 31, 1–6. doi: 10.1186/s41938-021-00392-8
Mohamed, E. S. I., Mahmoud, M. E. E., Elhaj, M. A. M., Mohamed, S. A., and Ekesi, S. (2015). Host plants record for tomato leaf miner Tuta absoluta (Meyrick) in Sudan. EPPO Bull. 45, 108–111. doi: 10.1111/epp.12178
Mora, M. A. E., Castilho, A. M. C., and Fraga, M. E. (2017). Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 84:e0552015. doi: 10.1590/1808-1657000552015
Ndereyimana, A., Nyalala, S., Murerwa, P., and Gaidashova, S. (2019). Pathogenicity of some commercial formulations of entomopathogenic fungi on the tomato leaf miner, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 29, 1–5. doi: 10.1186/s41938-019-0184-y
Ndiaye, A., Bal, A. B., Chailleux, A., Garba, M., Brévault, T., and Gauthier, N. (2021). Range-wide mitochondrial genetic homogeneity in the invasive south American tomato pinworm, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), with a focus on Africa. Afr. Entomol. 29, 42–58. doi: 10.4001/003.029.0042
Nthenga, I., Knoetze, R., Berry, S., Tiedt, L. R., and Malan, A. P. (2014). Steinernema sacchari n. sp.(Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology 16, 475–494.
Oztemiz, S. , (2014). Tuta absoluta Povolny (Lepidoptera: Gelechiidae), the exotic pest in Turkey. Romanian Journal of Biology, 59, 47–58.
Perumal, V., Kannan, S., Alford, L., Pittarate, S., Geedi, R., Elangovan, D., et al. (2023a). First report on the enzymatic and immune response of Metarhizium majus bag formulated conidia against Spodoptera frugiperda: an ecofriendly microbial insecticide. Front. Microbiol. 14:1104079. doi: 10.3389/fmicb.2023.1104079
Perumal, V., Kannan, S., Alford, L., Pittarate, S., and Krutmuang, P. (2024b). Study on the virulence of Metarhizium anisopliae against Spodoptera frugiperda (JE Smith, 1797). J. Basic Microbiol. 64:2300599. doi: 10.1002/jobm.202300599
Perumal, V., Kannan, S., Alford, L., Pittarate, S., Mekchay, S., Reddy, G. V., et al. (2023b). Biocontrol effect of entomopathogenic fungi Metarhizium anisopliae ethyl acetate-derived chemical molecules: an eco-friendly anti-malarial drug and insecticide. Arch. Insect Biochem. Physiol. 114, 1–19. doi: 10.1002/arch.22037
Perumal, V., Kannan, S., Pittarate, S., and Krutmuang, P. (2024a). A review of entomopathogenic fungi as a potential tool for mosquito vector control: a cost-effective and environmentally friendly approach. Entomol. Res. 54:e12717. doi: 10.1111/1748-5967.12717
Pires, L. M., Marques, E. J., Wanderley-Teixeira, V., Teixeira, Á. A., Alves, L. C., and Alves, E. S. B. (2009). Ultrastructure of Tuta absoluta parasitized eggs and the reproductive potential of females after parasitism by Metarhizium anisopliae. Micron 40, 255–261. doi: 10.1016/j.micron.2008.07.008
Qin, Y., Liu, X., Peng, G., Xia, Y., and Cao, Y. (2023). Recent advancements in pathogenic mechanisms, applications and strategies for entomopathogenic fungi in mosquito biocontrol. J. Fungi 9:746. doi: 10.3390/jof9070746
Quesada-Moraga, E., González-Mas, N., Yousef-Yousef, M., Garrido-Jurado, I., and Fernández-Bravo, M. (2024). Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest. Sci. 97, 1–15. doi: 10.1007/s10340-023-01622-8
Sankarganesh, E., Firake, D. M., Sharma, B., Verma, V. K., and Behere, G. T. (2017). Invasion of the south American tomato pinworm, Tuta absoluta, in northeastern India: a new challenge and biosecurity concerns. Entomologia Generalis 36, 335–345. doi: 10.1127/entomologia/2017/0489
Shalaby, H. H., Faragalla, F. H., El-Saadany, H. M., and Ibrahim, A. A. (2013). Efficacy of three entomopathogenic agents for control the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Nat. Sci. 11, 63–72.
Sharma, P. L., and Gavkare, O. (2017). New distributional record of invasive pest Tuta absoluta (Meyrick) in North-Western Himalayan region of India. Natl. Acad. Sci. Lett. 40, 217–220. doi: 10.1007/s40009-016-0526-1
Sharma, A., Sharma, S., and Yadav, P. K. (2023). Entomopathogenic fungi and their relevance in sustainable agriculture: a review. Cogent Food Agric. 9:2180857. doi: 10.1080/23311932.2023.2180857
Shashank, P. R., Suroshe, S. S., Singh, P. K., Chandrashekar, K., Nebapure, S. M., and Meshram, N. M. (2016). Report of invasive tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae) from northern India. Indian J. Agric. Sci. 86, 1635–1636. doi: 10.56093/ijas.v86i12.65687
Sidhu, S. K., Sridhar, V., Sharma, A., and Asokan, R., (2017). Report on the occurrence of South American Tomato moth, Tuta absoluta (Meyrick) in Punjab, India as evident from trap catches and molecular diagnosis. Pest Management in Horticultural Ecosystems, 23, 89–91.
Silva, A. C. L., Silva, G. A., Abib, P. H. N., Carolino, A. T., and Samuels, R. I. (2020). Endophytic colonization of tomato plants by the entomopathogenic fungus Beauveria bassiana for controlling the south American tomato pinworm, Tuta absoluta. CABI Agric. Biosci. 1, 1–9. doi: 10.1186/s43170-020-00002-x
Singh, D. K., and Panchbhaiya, A. (2018). First record of tomato leaf miner, an invasive pest in Uttarakhand, India under polyhouse condition. J. Hill Agric. 9, 127–130. doi: 10.5958/2230-7338.2018.00024.1
Skendžić, S., Zovko, M., Pajač Živković, I., Lešić, V., and Lemić, D. (2021). Effect of climate change on introduced and native agricultural invasive insect pests in Europe. Insects 12:985. doi: 10.3390/insects12110985
Smagghe, F., Spooner-Hart, R., Chen, Z. H., and Donovan-Mak, M. (2023). Biological control of arthropod pests in protected cropping by employing entomopathogens: efficiency, production and safety. Biol. Control 186:105337. doi: 10.1016/j.biocontrol.2023.105337
Swathi, P., Swathi, B., Das, S. B., Sridhar, V., Giribabu, O., Snehalatha, G., et al., (2017). First report of South American tomato leaf miner, Tuta absoluta (Meyrick) from Madhya Pradesh, India. Pest Management in Horticultural Ecosystems, 23, 92–93.
Swathy, K., Nisha, V., and Vivekanandhan, P. (2024). Biological control effect of Trichoderma harzianum (Hypocreales: Hypocreaceae) against phytopathogens. Environ. Qual. Manag, 34:e22227.
Swathy, K., Parmar, M. K., and Vivekanandhan, P. (2023). Biocontrol efficacy of entomopathogenic fungi Beauveria bassiana conidia against agricultural insect pests. Environ. Qual. Manag. 34. doi: 10.1002/tqem.22174
Tadele, S., and Emana, G. (2017). Entomopathogenic effect of Beauveria bassiana (Bals.) and Metarrhizium anisopliae (Metschn.) on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae under laboratory and glasshouse conditions in Ethiopia. J. Plant Pathol. Microbiol. 8, 411–414.
Uchoa-Fernandes, M. A., Della Lucia, T. M., and Vilela, E. F. (1995). Mating, oviposition and pupation of Scrobipalpuloides absoluta (Meyr.) (Lepidoptera: Gelechiidae). Anais Soc. Entomol. Brasil 24, 159–164. doi: 10.37486/0301-8059.v24i1.1007
Van Damme, V. M., Beck, B. K., Berckmoes, E., Moerkens, R., Wittemans, L., De Vis, R., et al. (2016). Efficacy of entomopathogenic nematodes against larvae of Tuta absoluta in the laboratory. Pest Manag. Sci. 72, 1702–1709. doi: 10.1002/ps.4195
Van Damme, V., Berkvens, N., Moerkens, R., Berckmoes, E., Wittemans, L., De Vis, R., et al. (2015). Overwintering potential of the invasive leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) as a pest in greenhouse tomato production in Western Europe. J. Pest. Sci. 88, 533–541. doi: 10.1007/s10340-014-0636-9
Vidhate, R. P., Dawkar, V. V., Punekar, S. A., and Giri, A. P. (2023). Genomic determinants of entomopathogenic fungi and their involvement in pathogenesis. Microb. Ecol. 85, 49–60. doi: 10.1007/s00248-021-01936-z
Vivekanandhan, P., Alahmadi, T. A., and Ansari, M. J. (2024a). Pathogenicity of Metarhizium rileyi (Hypocreales: Clavicipitaceae) against Tenebrio molitor (Coleoptera: Tenebrionidae). J. Basic Microbiol. 64:2300744. doi: 10.1002/jobm.202300744
Vivekanandhan, P., Kamaraj, C., Alharbi, S. A., and Ansari, M. J. (2024b). Novel report on soil infection with Metarhizium rileyi against soil-dwelling life stages of insect pests. J. Basic Microbiol. :e2400159. doi: 10.1002/jobm.202400159
Vivekanandhan, P., Swathy, K., Alahmadi, T. A., and Ansari, M. J. (2024c). Biocontrol effects of chemical molecules derived from Beauveria bassiana against larvae of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Front. Microbiol. 15:1336334. doi: 10.3389/fmicb.2024.1336334
Vivekanandhan, P., Swathy, K., Alford, L., Pittarate, S., Subala, S. P. R. R., Mekchay, S., et al. (2022a). Toxicity of Metarhizium flavoviride conidia virulence against Spodoptera litura (Lepidoptera: Noctuidae) and its impact on physiological and biochemical activities. Sci. Rep. 12:16775. doi: 10.1038/s41598-022-20426-x
Vivekanandhan, P., Swathy, K., Lucy, A., Sarayut, P., and Patcharin, K. (2023). Entomopathogenic fungi based microbial insecticides and their physiological and biochemical effects on Spodoptera frugiperda (JE smith). Front. Cell. Infect. Microbiol. 13:1254475. doi: 10.3389/fcimb.2023.1254475
Vivekanandhan, P., Swathy, K., Murugan, A. C., and Krutmuang, P. (2022b). Insecticidal efficacy of Metarhizium anisopliae derived chemical constituents against disease-vector mosquitoes. J. Fungi 8:300. doi: 10.3390/jof8030300
Vivekanandhan, P., Swathy, K., Kalaimurugan, D., Ramachandran, M., Yuvaraj, A., Pittarate, A.N., et al. (2020). Larvicidal toxicity of Metarhizium anisopliae metabolites against three mosquito species and non-targeting organisms. Plos one 15:e0232172.
Vivekanandhan, P., Swathy, K., Thomas, A., Kweka, E. J., Rahman, A., Pittarate, S., et al. (2021). Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int. J. Environ. Res. Public Health 18:10536. doi: 10.3390/ijerph181910536
Yang, L., Li, J., Yang, L., Wang, X., Xiao, S., Xiong, S., et al. (2023). Altered gene expression of the parasitoid Pteromalus puparum after Entomopathogenic fungus Beauveria bassiana infection. Int. J. Mol. Sci. 24:17030. doi: 10.3390/ijms242317030
Yüksel, E. , (2022). Biocontrol potential of endosymbiotic bacteria of entomopathogenic nematodes against the tomato leaf miner, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Egyptian Journal of Biological Pest Control, 32, 135.
Yule, S., Htain, N. N., Oo, A. K., Sotelo-Cardona, P., and Srinivasan, R. (2021). Occurrence of the south American tomato leaf miner, Tuta absoluta (Meyrick) in southern Shan, Myanmar. Insects 12:962. doi: 10.3390/insects12110962
Zekeya, N., Mtambo, M., Ramasamy, S., Chacha, M., Ndakidemi, P. A., and Mbega, E. R. (2019). First record of an entomopathogenic fungus of tomato leafminer, Tuta absoluta (Meyrick) in Tanzania. Biocontrol Sci. Tech. 29, 626–637. doi: 10.1080/09583157.2019.1573972
Zhang, G. F., Ma, D. Y., Wang, Y. S., Gao, Y. H., Liu, W. X., Zhang, R., et al. (2020). First report of the south American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 19, 1912–1917. doi: 10.1016/S2095-3119(20)63165-3
Zhang, G. F., Wang, Y. S., Gao, Y. H., Liu, W. X., Zhang, R., Fu, W. J., et al. (2020). First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J Integr Agric, 19, 1912–1917.
Keywords: invasive insect pest, major pest of tomato, management, south American tomato leaf miner, Tuta absoluta
Citation: Vivekanandhan P, Swathy K, Sarayut P and Patcharin K (2024) Biology, classification, and entomopathogen-based management and their mode of action on Tuta absoluta (Meyrick) in Asia. Front. Microbiol. 15:1429690. doi: 10.3389/fmicb.2024.1429690
Edited by:
Md. Motaher Hossain, Bangabandhu Sheikh Mujibur Rahman Agricultural University, BangladeshReviewed by:
N. Bakthavatsalam, National Bureau of Agricultural Insect Resources, IndiaTange Denis Achiri, University of Buea, Cameroon
Copyright © 2024 Vivekanandhan, Swathy, Sarayut and Patcharin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krutmuang Patcharin, cGF0Y2hhcmluLmtAY211LmFjLnRo
 Perumal Vivekanandhan
Perumal Vivekanandhan Kannan Swathy
Kannan Swathy Pittarate Sarayut
Pittarate Sarayut Krutmuang Patcharin
Krutmuang Patcharin