- 1Laboratory of Microbiology, Department of Clinical Laboratory, Children’s Hospital of Fudan University, National Children's Medical Center, Shanghai, China
- 2Nosocomial Infection Control Department, Children’s Hospital of Fudan University, National Children's Medical Center, Shanghai, China
- 3CAS Key Laboratory of Molecular Virology and Immunology, The Center for Microbes, Development and Health, Shanghai Institute of Immunity and Infection, Chinese Academy of Sciences, Shanghai, China
Background: Pertussis is a highly contagious respiratory illness mainly caused by Bordetella pertussis (BP). Bordetella parapertussis (BPP) can induce symptoms compatible with pertussis, but has been underdiagnosed and underreported. The current pertussis vaccines offer low protection against BPP. Herein, we aim to reveal the epidemiology and genomic evolution of BPP in Shanghai, China.
Methods: Children diagnosed with BPP infection from January 2017 to December 2022 in Shanghai, China were enrolled. We performed antimicrobial susceptibility testing (AST), multiple locus variable-number tandem repeat analysis (MLVA), and whole genome sequencing (WGS) analysis. A total of 260 international BPP genomes were chosen for comparison to investigate the genomic diversity and phylogenetic characteristics of Chinese strains within a global context.
Results: Sixty patients were diagnosed with BPP infection by culture, with the positive ratio of 3.5‰ (60/17337) for BPP in nasopharyngeal swap samples. The average age of patients was 4.5 ± 0.3 years. BPPs contained four MLVA types including MT6 (65.0%), MT4 (26.7%), untype-1 (6.7%) and MT5 (1.7%), and none of strains showed resistance to macrolides. All strains carried virulence genotype of ptxP37/ptxA13/ptxB3/ptxC3/ptxD3/ptxE3/fim2-2/fim3-10. MT4 and MT5 strains carried prn54, whereas MT6 and untype-1 BPPs expressed prn101. We identified two outbreaks after 2020 caused by MT4 and MT6 strains, each corresponding to distinct WGS-based phylogenetic lineages. The MT4-lineage is estimated to have originated around 1991 and has since spread globally, being introduced to China between 2005 and 2010. In contrast, the MT6-lineage was exclusively identified in China and is inferred to have originated around 2002.
Conclusion: We revealed the genomic diversity of BPPs circulating in Shanghai, China, and reported the outbreaks of MT6 and MT4 BPPs after 2020. This is the first report on the emergence and regional outbreak of MT6 BPPs in the world, indicating that continuous surveillance on BPPs are thus required.
Introduction
Whooping cough (pertussis) is a highly contagious respiratory disease of humans, which is mainly caused by Bordetella pertussis (BP; Feng et al., 2021; Gorgojo et al., 2023). Compared to BP, B. parapertussis (BPP) causes a milder whooping cough-like syndrome and is responsible for a smaller proportion (2%~20%) of pertussis (Toubiana et al., 2019). However, BPP infection has been poorly recognized in the world. Pertussis was previously thought to mainly occur in infants (Fu et al., 2019). However, more studies reveal that the prevalence and re-emergence of pertussis has been increasing in older children, adolescents and adults, making a great public threaten in the world (Moore et al., 2019; Zhang et al., 2022).
There are many differences between BBP and BP. For example, BPP lacks the production of the pertussis toxin (Ptx) due to a mutation in the promoter region of the genes encoding this toxin (Arico and Rappuoli, 1987). The World Health Organization (WHO) recommended two types of approach to diagnosis, including direct diagnosis [culture, real-time polymerase chain reaction (RT-PCR)] and indirect diagnosis (serology). These two species can be distinguished based on a number of biochemical characteristics: BPP grow faster and appear grayish; the oxidase test was positive in BP but negative in BPP, etc. Moreover, a series of targets including IS481, IS1001, IS1002, etc. were used to distinguish different Bordetella species. For example, IS1001 which presented in all BPPs but was absent in BPs, was widely used to identify BPP (Riffelmann et al., 2005).
BPP might vary from an unrecognized infection to a mild illness or typical pertussis presentation; it is increasingly recognized and reported to public health agencies (Liko et al., 2017). Pertussis vaccines are produced as combination vaccines with diphtheria and tetanus toxoids. In China, a routine immunization schedule of diphtheria, tetanus, whole-cell pertussis vaccine (DTwP) was implemented in the 1960s. Starting in 2005, both DTwP and diphtheria-tetanus-acellular pertussis vaccine (DTaP) were used in China, with DTaP gradually replacing DTwP by 2010 (Wu et al., 2023). Although DTap vaccine significantly reduced the incidence of pertussis, many studies have shown that pertussis vaccination is irrelevant to or just partially protect against BPP infection (Liko et al., 2017). The rodent model showed that aP vaccination, by priming the host response against BP clearance, confers an advantage to BPP by interfering with optimal immune clearance and resulting in increased lung colony-forming units (Long et al., 2010).
Until now, a series of studies on BP strains were reported in China, including Zhejiang province (Lin et al., 2022), Shanghai (Fu et al., 2023b), Shenzhen (Wu et al., 2021), and Beijing (Zhou et al., 2024). However, systematic studies or reports on BPP strains are very scarce in the world. National surveillance on BPP strains are largely lacking in China. In this study, we performed a continuous surveillance on Bordertella spp. based on culture, and collected a total of 60 BPP strains from January 2017 to December 2022 in Shanghai, China. We systematically analyzed the clinical and epidemiology features, the antimicrobial resistance (AMR) profiles, and the genomic evolution of those strains.
Materials and methods
Enrollment of pertussis cases
From January 2017 to December 2022, there were 740 children diagnosed as pertussis by bacterial culture in Shanghai, China. Their nasopharyngeal swab (NP) samples were collected for Bordetella spp. culture and antimicrobial resistance testing. Their basic information, clinical diagnosis, and X-ray imaging were collected based on the electronic medical records. The laboratory testing results were collected and analyzed in this study, including white blood cell counts (WBC, ×109/L) and C-reactive protein (CRP, mg/L). All data collection and analysis were anonymous. This study was approved by the Ethics Committee of the Children’s Hospital of Fudan University (no. 2022-66).
Culture and antimicrobial susceptibility testing of BPP strains
NP samples were delivered to clinical microbiology laboratory and immediately spread onto charcoal agar (OXOID, United Kingdom) plates supplemented with 10% defibrinated sheep blood and cephalexin (40 mg/L). The plates were incubated in a humidified incubator at 35°C for 3 to 5 days. Different Bordetella species were verified by Gram staining, biochemical tests, and Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, Bruker, Germany).
The BPP isolates were suspended equivalent to a 0.5 McFarland standard and inoculated onto charcoal agar containing 10% sheep blood without cephalexin. The minimum inhibitory concentrations of four antimicrobial agents, including erythromycin, azithromycin, clarithromycin, and sulfamethoxazole/trimethoprim, were determined by the E-test after 72 h of incubation at 35°C. The standardized interpretation criteria are based on our previous report (Fu et al., 2019).
Whole genome sequencing and analysis
Genomic DNA of BPP strains were extracted using QIAamp DNA mini kit (QIAGEN) and whole-genome sequencing were performed on Illumina NovaSeq platform. Sequencing data were analyzed as previously described (Yang et al., 2022; Fu et al., 2023b). Briefly, species identification was performed using Kraken 2 based on sequencing data (Lu et al., 2022).1 Genome assembly was performed using shovill pipeline. The genome characteristics of newly sequenced data were calculated using Quast v5.0.2 (Gurevich et al., 2013). The prevalence of insertion sequence (IS) IS1001 were detected by searching against assembled genome sequences using BLASTN. It was considered present if the BLASTN hit coverage and identity were at least 90%. Core-genome single-nucleotide-polymorphisms (SNPs) were identified using the Snippy pipeline,2 with strain 12822 [accession number: (NC_002928.3)] as the reference genome. Maximum-likelihood phylogenetic trees were constructed using RAxML-NG based on core-genome SNPs (Kozlov et al., 2019). The maximum-likelihood tree was rooted using the midpoint method. The dated phylogenetic trees were automatically rooted based on temporal signal using a root-to-tip linear regression with BactDating (Didelot et al., 2018). New sequencing data have been deposited in NCBI Sequence Read Archive (SRA) under accession number PRJNA1060880.
A total of 260 publicly available international genomes were downloaded from NCBI GenBank or SRA database, with accession numbers listed in the appendix (Supplementary Table S1). The international BPP strains included France (118), USA (91), Spain (29), Germany (3), Austria (2), United Kingdom (1), Australia (1), Japan (1), Iran (1), and unknow (13).
Multiple locus variable-number tandem repeat analysis, multilocus sequence typing, and Bordetella spp. virulence genotyping analysis
Genomic DNA of BPP isolates was prepared by a QIAamp DNA mini kit (QIAGEN). Multiple locus variable-number tandem repeat analysis (MLVA) was performed following the procedures according to the report of Kamachi et al. (2019). Four loci (VNTR4, VNTR13, VNTR14, and VNTR15) were amplified by PCR. The number of repeats at each VNTR locus was calculated from the DNA fragment length. The assignment of an MLVA type (MT) was based on the combination of repeat counts for VNTR4, VNTR13, VNTR14, and VNTR15 according to previous reports (Kamachi et al., 2019).
Multilocus sequence typing (MLST) were analyzed by seven housekeeping genes (adk, fumC, glyA, tyrB, icd, pepA, and pgm). BPP genomes data were matched on the website.3 The alleles at each of the seven loci defined the allelic profile or sequence type (ST).
Assembled BPP genome sequences were used for virulence genotyping by searching against BIGSdb-Pasteur genomic platform for Bordetella.20. The virulence-related genes included pertussis toxin (PTX) promoter (ptxp), five ptx genes (ptxA, ptxB, ptxC, ptxD, ptxE), pertactin (prn), filamentous hemagglutinin B (fhaB), and fimbrial proteins (fim2, fim3).
Statistical analysis
All statistical analyses were performed using the GraphPad Prism software version 8.0. The t test and Bonferroni correction were performed to compare the differences of clinical characteristics and laboratory testing results between two groups. A p-value of less than 0.05 was considered statistically significant.
Results
Distributions and detection of BPP cases from 2017 to 2022
As shown in Figure 1A, there were 740 children diagnosed as pertussis by bacterial culture, and 91.4% (676 patients), 8.1% (60 patients) and 0.5% (4 patients) of the pertussis were caused by BP, BPP, and Bordetella bronchitis (BB), respectively.
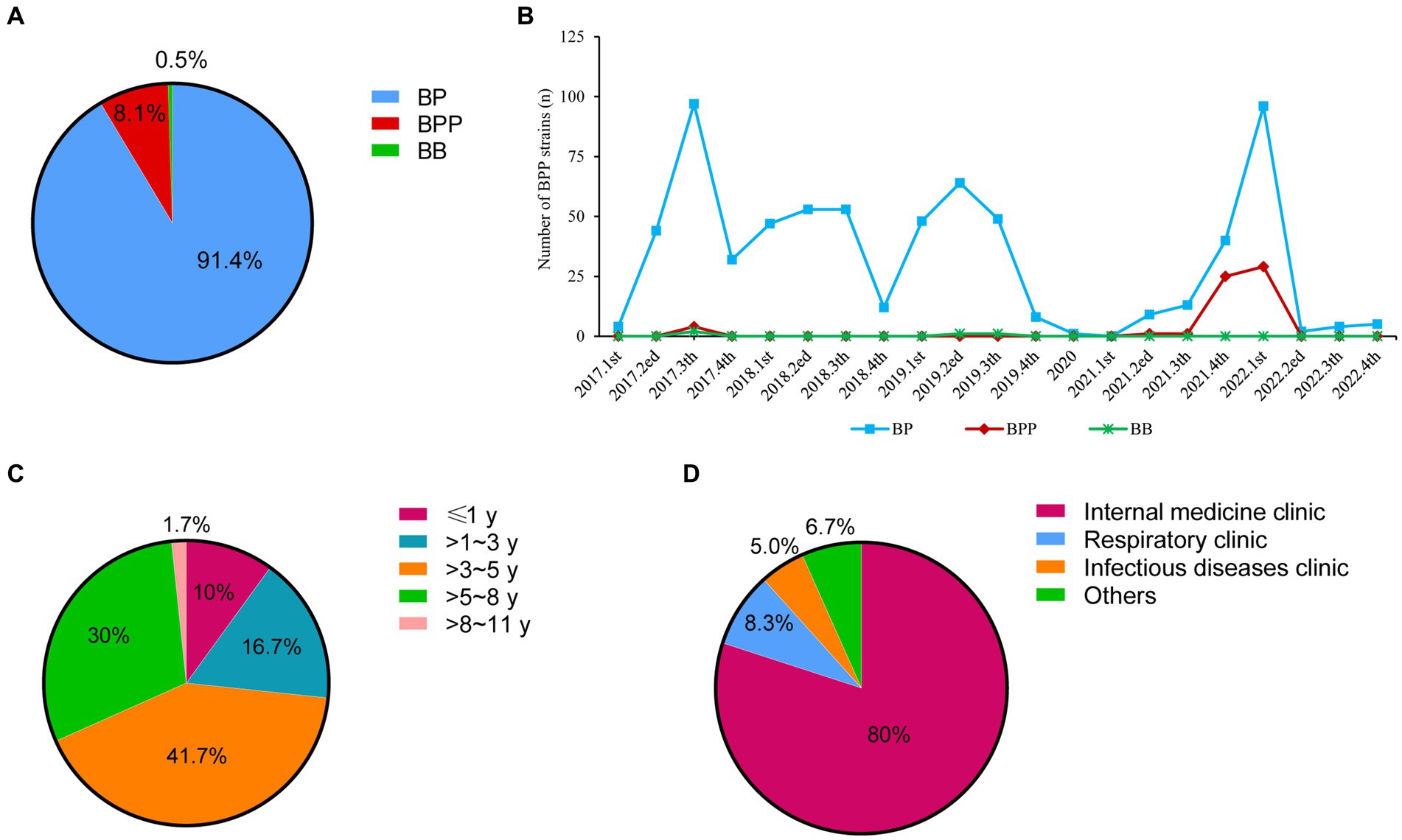
Figure 1. Distributions of Bordetella species and basic information of BPP infection cases from 2017 to 2022. (A) Distributions of different Bordetella species; (B) Detection ratios of three Bordetella species from 2017 to 2022; (C) Age distributions of BPP infection cases; (D) Department distributions of BPP infection cases. BP, Bordetella pertussis; BPP, Bordetella parapertussis; BB, Bordetella bronchitis.
BP strains were continuously isolated from 2017 to 2019 (ranging from 4 strains to 97 strains per quarter), but only one BP strain was collected in 2020. Notably, BP infection was re-emerged from 2021 to 2022, and there were two peaks of BP infection at 4th quarter of 2021 (40 strains) and 1st quarter of 2022 (96 strains). Compared to BP cases, BPP infection was quite scarce before 2021. Only four BPP strains were identified at 3rd quarter of 2017. Concurrent with the BP re-emergence time, BPP infection had outbreaks at 4th quarter of 2021 (25 strains) and 1st quarter of 2022 (29 strains; Figure 1B).
Among 17,337 patients who received nasopharyngeal swap samples culture, there were 60 children diagnosed with BPP infection, with the positive ratio of 3.5‰ (60/17337). The average ages were (4.5 ± 0.3) years old. The infants (≤1 year), toddler (>1~3 years), preschool (>3~5 years), school age (>5~8 years) and adolescents (>8~11 years) accounted for 10% (6), 16.7% (10), 41.7% (25), 30% (18), and 1.7% (1), respectively (Figure 1C). Most of patients came from the internal medicine clinic (80%, 48; Figure 1D).
Clinical and laboratory characteristics of BPP cases
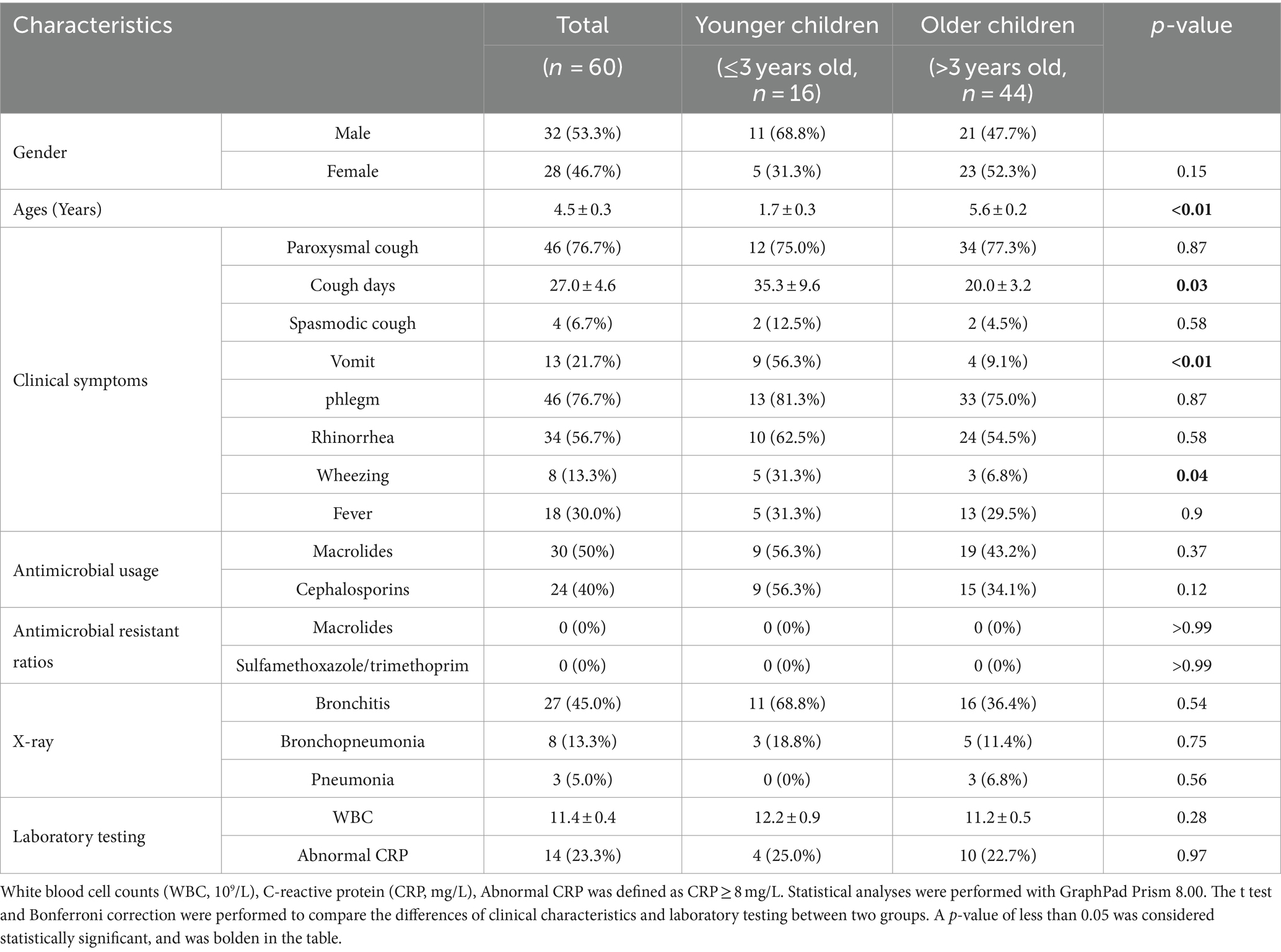
As shown in Table 1, most of the patients (76.7%, 46 patients) presented paroxysmal cough and phlegm, and the average cough period was (27.0 ± 4.6) days. Moreover, there were 56.7% (34 patients), 30% (18 patients), 21.7% (13 patients), 13.3% (8 patients) and 6.7% (4 patients) of BPP cases presented rhinorrhea, fever, vomiting, wheezing and spasmodic cough, respectively. We further compared the difference of younger children (1.7 ± 0.3 years old, 2 months to 3 years) and older children (5.6 ± 0.2 years old, >3 years to 11 years). It is noted that younger children presented more severe clinical symptoms than older children, including longer cough periods [(35.3 ± 9.6) days vs. (20.0 ± 3.2) days, p = 0.03), vomit (56.3% vs.9.1%, p < 0.01), and wheezing (31.3% vs.6.8%, p = 0.04].
There were 50% (30 patients) and 40% (24 patients) of BPP cases treated by macrolides and cephalosporins, respectively. However, no BPP strains isolated from the patients showed resistance to macrolides, and all strains presented sensitive to sulfamethoxazole/trimethoprim. The chest X-ray of BPP cases presented either bronchitis (45.0%, 27), bronchopneumonia (13.3%, 8) or pneumonia (5.0%, 3), and there was no difference between younger children and older children. WBC was slightly increased to (11.4 ± 0.4) × 109/L, and 23.3% (14) of patients presented abnormal CRP (>8 mg/L; Table 1).
Prevalence of different BPP types from 2017 to 2022
All 60 BPP strains belonged to one MLST type 19 (ST19, 100%), whereas MLVA analysis further revealed four different BPP subtypes in this study. MLVA type 6 (MT6) with the VNTR profiles of 4-5-13-5 was the major subtype (65.0%, 39 strains) of BPPs in this study, followed by MT4 (VNTRs: 3-7-18-4, 26.7%, 16 strains). Other subtype including MT5 (VNTRs: 3-7-21-4) and untype-1 (VNTRs: 4-5-10-4) were less frequently detected, with the ratios of 1.7% (1 strain) and 6.7% (4 strains), respectively (Figure 2A).
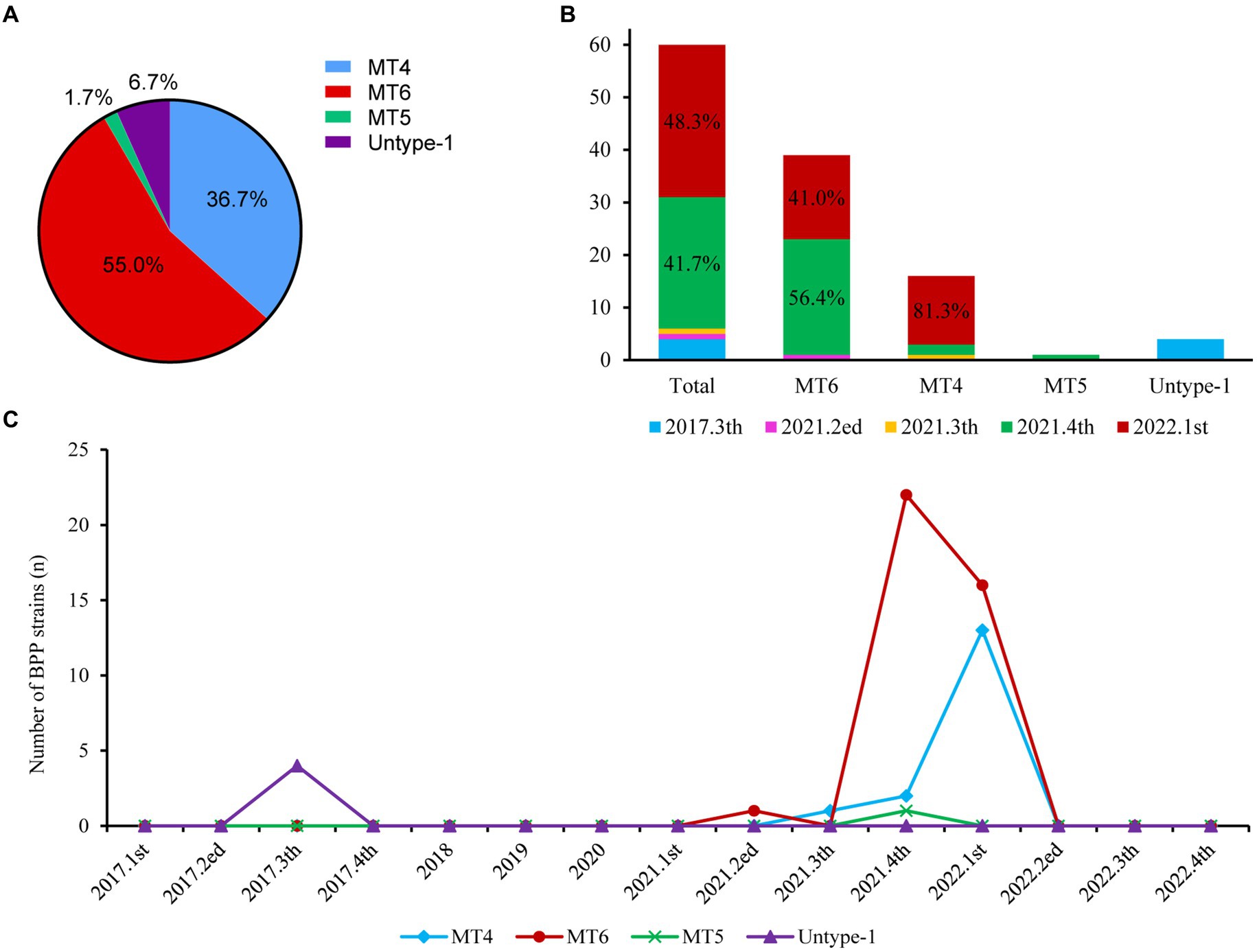
Figure 2. Prevalence of different BPP subtypes from 2017 to 2022. (A). MT subtypes of all BPPs; (B). Distributions of different MLVA types over time; (C). Detection of different BPP subtypes per quarter from 2017 to 2022.
MT6 and MT4 were mostly isolated at 4th quarter, 2021 (56.4% and 12.5%) and 1st quarter, 2022 (41.0% and 81.3%), respectively (Figure 2B). There were MT6-BPP outbreaks at 4th quarter, 2021 (22 strains) and 1st quarter, 2022 (16 strains), and MT4-BPP outbreak at 1st quarter, 2022 (13), respectively (Figure 2C).
Genomic characteristics and evolution of Shanghai BPP strains
For the newly sequenced strains, the average GC content, number of contigs and size of assemblies were 68.17% (68.16–68.17%), 80 (70–92) and 4.72 Mb (4.72–4.73), with an average of 92-fold (87–141) depth for each genome. The marker sequence of BPP, IS1001, was found in all Chinese BPP strains.
We compared 60 Shanghai BPPs with 260 public genomes of global BPPs to reveal the phylogenetic relationship of those strains. After integrated the MLVA subtypes of 60 BPPs with WGS analysis, we defined two lineages: MT4-lineage and MT6-lineage, corresponding to MT4 and MT6 strains that caused disease outbreaks. As shown in Figure 3A, MT4-lineage isolates in Shanghai were closely related to those isolated from the France, United States, Austria, and Spain. MT6-lineage strains isolated after 2020 in Shanghai were quite different to other strains. Figure 3B showed that MT4-lineage was estimated to have originated at 1991 [95% confidence interval (CI): 1985~1996] probably from USA, and has spread to multiple regions, including Europe (France and Spain), Australia and China. This lineage was inferred to have been introduced to China around 2005 to 2010, much earlier than our first MT4 strain isolated in 2021. MT6-lineage was different to either the MT4-lineage strains or other international strains. Notably, this lineage was estimated to have originated in China at 2002 (95% CI: 1970~2013) and was only identified in Shanghai, China (Figure 3C).
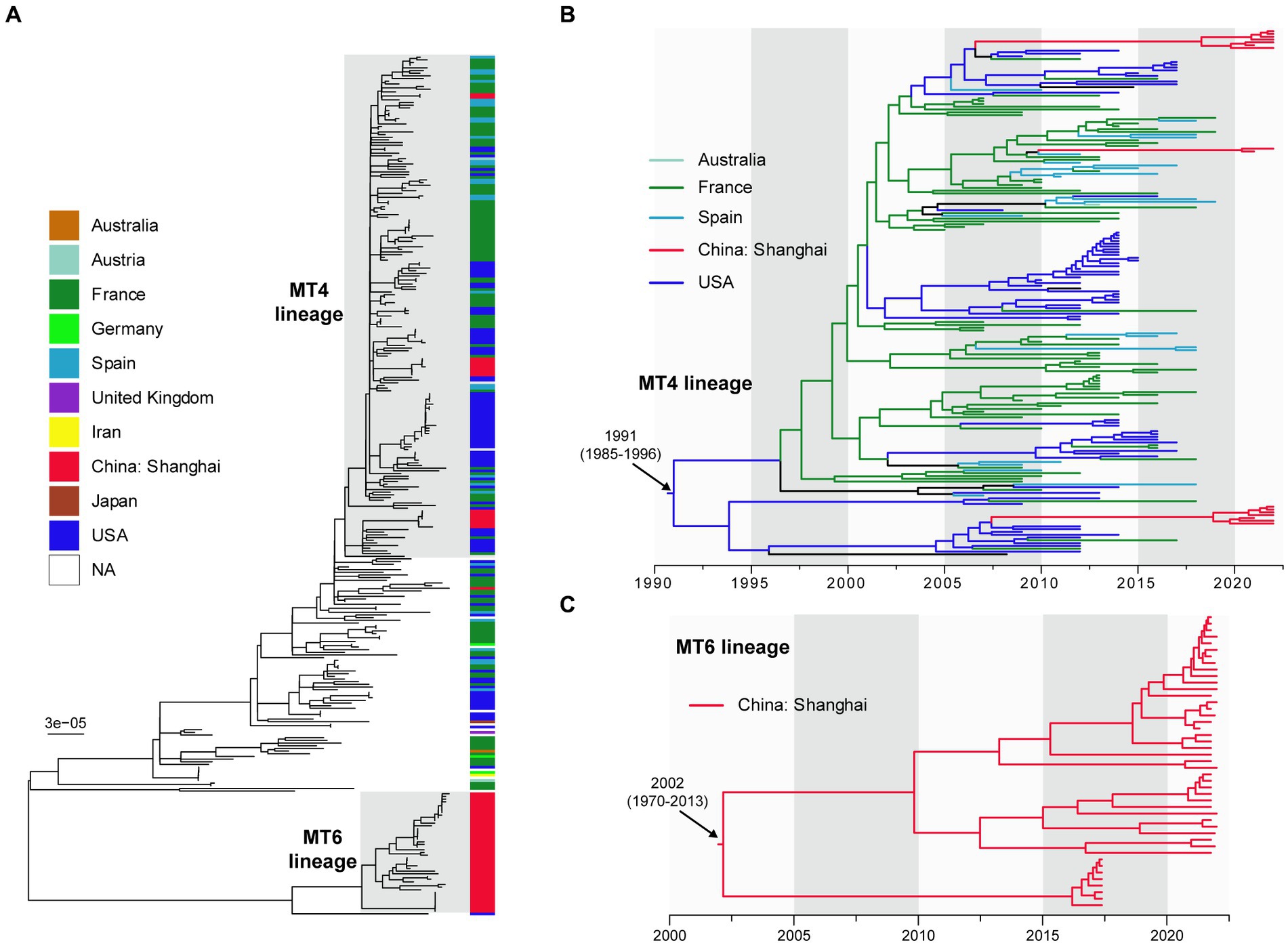
Figure 3. The dated maximum-likelihood phylogenetic tree of 60 Shanghai, China and 260 international BPP strains (A). Different colors presented different geographic areas and red color indicates Shanghai strains. MT4-lineage (B) and MT6 lineage (C) are enlarged for visualization. NA, not applicable. The maximum-likelihood tree (A) was rooted using the midpoint method. The dated phylogenetic trees (B,C) were automatically rooted based on temporal signal.
We further identified 74 SNP sites that can be used to distinguish MT4-lineage and MT6-lineage, with alleles that are completely different in the two lineage strains. Among these SNPs, 64 located within gene regions (64 genes, each with one SNP), and an additional 10 SNPs located in intergenic regions (Supplementary Table S3). SNP sites can be used to distinguish MT4- and MT6-lineages, exhibiting alleles that are completely different in the two lineage strains. For example, the SNP at position 130,756 has two alleles: A and C. All MT4-lineage strains carried allele A, while all MT6-lineage strains carried allele C.
In this study, BPPs expressed a series of Bordetella virulence factors, with the profiles of ptxP37/ptxA13/ptxB3/ptxC3/ptxD3/ptxE3/ fim2-2/fim3-10. All of MT4 and MT5 strains carried prn54, whereas MT6 and untype-1 BPs expressed prn101 (Supplementary Table S2). The fhaB genes included four different alleles: fhaB-45 (48.3%), fhaB-3 (18.3%), fhaB-22 (18.3%), and fhaB-44 (15.1%), all of which were not related to any MT subtypes.
We further compared the clinical and laboratory characteristics of MT4-lineage and MT6-lineage BPPs (Supplementary Table S4). There were no big differences between those two BPP lineages in Shanghai (p > 0.05), including the clinical symptoms, the inflammatory factors, X-rays, antimicrobial treatment history and AMR profiles. However, MT6-lineage BPP cases presented longer cough periods of (31.2 ± 5.6) days, which was two or more times as long as MT4-lineage (14.2 ± 2.7 days, p = 0.04).
Discussion
BPP can cause whooping cough in human, but the epidemiology of respiratory illness caused by BPP strains has been poorly recognized in the world (Mastrantonio et al., 1998). Herein, we collected a total of 60 BPPs from 2017 to 2022 in Shanghai, China, and systematically analyzed the clinical and epidemiologic features and the genomic evolution of those strains. BPP accounted for 8.1% of whooping cough in this study, and caused more severe clinical symptoms in younger children (0~3 year). BPPs circulating in Shanghai contained four different MT types. It is noted that there was the outbreak of BPP infection after 2020, and the major MLVA types were MT4 and MT6. These two subtypes have evolved independently: MT4-lineage was highly homogeneous to international BPPs which were estimated to originate at USA and introduced to China around 2005 to 2010, whereas MT6-lineage was estimated to originate in China and was only identified in Shanghai, China.
After aP vaccine replaced wP vaccine by 2010 in China, there are two types of DTaP formulations licensed in China: one is the two-component DTaP containing Ptx and Fha, and another is the three-component DTaP containing Ptx, Fha and Prn. The current vaccine in Shanghai contains Ptx and Fha. However, BP vaccines fail to or partially induce protection against BPPs and the incidence of this species has been rising over the years (Long et al., 2010; Liko et al., 2017). BPP has been circulating worldwide and causes outbreaks despite high pertussis vaccine coverage of young children. Unlike BP infections which were primarily identified in infants before 2020 in Shanghai, China (Fu et al., 2019, 2023b), the average ages of BPP cases were (4.5 ± 0.3) years, and only 10% patients aged less than 1-year-old. The average cough period of BPP infection was much longer than our previous report of BP infection (27.0 ± 4.6 days vs. 15.5 ± 0.8 days; Fu et al., 2023a). Moreover, younger children (0–3 years) presented more severe clinical symptoms than those aged more than 3-years-old. Therefore, we must pay attention to BPP infections among children, especially the younger children.
Previously, erythromycin has been the mainstay of antibiotic therapy for pertussis as it decreases the transmission of infection and ameliorates symptoms particularly in younger and more severely affected infants (Mortensen and Rodgers, 2000). However, after the erythromycin-resistant BPs in China was firstly isolated in Shandong Province, China in 2011, more macrolides-resistant BP (MRBP) strains were reported in China, making macrolides less effective against BP infection (Zhang et al., 2013). Antimicrobials such as macrolides and sulfamethoxazole/trimethoprim recommended for BP infection have also been used for treating and preventing BPP infection (Mortensen and Rodgers, 2000). This study revealed a high proportion of macrolides treatment (50%) against BPP infection, but none of the BPPs were resistant to macrolides. This is quite different with high macrolides resistance of BPs in China (Feng et al., 2021; Wu et al., 2022; Fu et al., 2023b), indicating that macrolides are still effective against BPP infection. The resistance mechanism in BPs and BPPs are different: The 23S rRNA A2047G mutation is considered the major mechanism of resistance to macrolides in BP strains. However, the macrolides resistance mechanism in BPP is still unclear. Lately, Fong et al. (2022) reported that the macrolides resistance in BPP was probably related to the upregulation of an efflux pump mechanism, but it still needs further investigation. Therefore, we hypothesize that the macrolides resistance due to A2047G mutation was stable and has the potential spread capability than any other resistance mechanisms.
BPP can caused regional or national outbreaks. For example, Koepke et al. (2015) reported the concurrent outbreak of BPPs and BPs during 2011 to 2012 in Wisconsin, USA, and the BPPs accounted for nearly 6.0% of pertussis cases. In this study, we identified BPP outbreak after 2020 in Shanghai, China, which is greatly consistent with BP re-emergence time after 2020. We hypothesized that the potential re-emerging of BPPs and BPs in this study was related to the suppressed spread or circulation of respiratory pathogens during COVID-19. There were two major MLVA types including MT6 and MT4, the VNTR profiles of MT6 and MT4 were quite heterogenous, revealing the independent spread and genomic evolution of these two subtypes. Recently, Kamachi et al. (2019) constructed the MLVA analysis method of BPP strains, and identified one MT6 strain isolated at 2010 in Taiwan, China, and two MT4 strains isolated at 2010 in Taiwan, China and at 1988 in France, respectively.
BPP and BP share the same virulence factors including Prn, dermonecrotic toxin, Fha and adenylate cyclase (Mastrantonio et al., 1998). However, the Ptx as one of the major virulence factors is only expressed in BP since the ptx operon in BPP is dysfunctional (Arico and Rappuoli, 1987). In this study, many of the virulence factors characterized in BP strains are commonly expressed in BPPs. BPPs in Shanghai carried a series of virulence factors, including ptxP37/ptxA13/ptxB3/ptxC3/ptxD3/ptxE3/fim2-2/fim3-10. It is noted that although BPPs carry ptx genes in the genomes, they cannot express and secrete the pertussis toxin due to mutation in Ptx promoter region. Prn allele was diverse among different BPP subtypes: MT4 and MT5 strains all carried prn54, whereas MT6 and untype-1 BPs expressed prn101, revealing the heterogeneity of virulence genes among different BPP subtypes. Prn-deficient BPs have widely been reported in countries using aP vaccines, such as the United States (85%), Australia (>80%), Sweden (69%), and Italy (55%; Byrne and Slack, 2006; Martin et al., 2015; Zomer et al., 2018; Weigand et al., 2019; Ma et al., 2021). Herein, all BPPs expressed prn gene without any mutation or disruption, and none of prn-negative BPP was identified. The aP vaccines containing Prn as an immunogen was thought to be the selection pressure for prn-negative BP strains (Ma et al., 2021). The current ACVs in Shanghai only contain Ptx and Fha, so we hypothesized that prn expression was not influenced by vaccine pressure because the current ACVs used in Shanghai contain no Prn antigen.
We collected 260 international BPPs for comparison, revealing the different genomic characteristics and molecular evolution of MT4-lineage and MT6-lineage. Firstly, 64 SNPs in gene regions and 10 SNPs in intergenic regions were identified among these two lineages. Secondly, the dated phylogenetic trees further revealed evolutionary differences between MT4- and MT6-lineage: MT4-lineage which originated at 1991 from USA was introduced to China around 2005~2010; MT6-lineage presented genomic heterogeneity to any other BPP strains, and was exclusively identified in Shanghai, China. We further compared the clinical and laboratory features of MT4-lineage and MT6-lineage BPPs. Although most of the clinical manifestations and inflammatory factors between these two lineages showed no significant differences, MT6-lineage cases presented the longer cough period (31.2 ± 5.6 days), which was about two or more times as long as MT4-linage cases. Therefore, it is thus important to keep continuous surveillance of MT-6 lineage BPP strains.
In summary, we revealed the genomic diversity and molecular evolution of different BPP subtypes circulating in China, and reported the emergence and outbreak of MT6 and MT4 BPPs after 2020 in Shanghai, China. To the best of our knowledge, it is the first report on the emergence and regional outbreak of MT6-lineage BPP in the world, highlighting that continuous surveillance and effective detection on BPP strains are thus required.
Data availability statement
The original contributions presented in the study are publicly available. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Children’s Hospital of Fudan University (no. 2022-66). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PF: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. YL: Data curation, Investigation, Methodology, Writing – review & editing. JQ: Investigation, Methodology, Writing – review & editing. LX: Methodology, Writing – review & editing. CY: Supervision, Writing – original draft, Writing – review & editing. CW: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant no. 82202567) and the Shanghai municipal three-year action plan for strengthening the construction of the public health system (2023–2025) GWVI-2.1.2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1428766/full#supplementary-material
Footnotes
References
Arico, B., and Rappuoli, R. (1987). Bordetella parapertussis and bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J. Bacteriol. 169, 2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987
Byrne, S., and Slack, A. T. (2006). Analysis of bordetella pertussis pertactin and pertussis toxin types from Queensland, Australia, 1999-2003. BMC Infect. Dis. 6:53. doi: 10.1186/1471-2334-6-53
Didelot, X., Croucher, N. J., Bentley, S. D., Harris, S. R., and Wilson, D. J. (2018). Bayesian inference of ancestral dates on bacterial phylogenetic trees. Nucleic Acids Res. 46:e134. doi: 10.1093/nar/gky783
Feng, Y., Chiu, C. H., Heininger, U., Hozbor, D. F., Tan, T. Q., and von Konig, C. W. (2021). Emerging macrolide resistance in bordetella pertussis in mainland China: findings and warning from the global pertussis initiative. Lancet Reg Health West Pac. 8:100098. doi: 10.1016/j.lanwpc.2021.100098
Fong, W., Timms, V., Sim, E., Pey, K., Nguyen, T., and Sintchenko, V. (2022). Genomic and transcriptomic variation in bordetella spp. following induction of erythromycin resistance. J. Antimicrob. Chemother. 77, 3016–3025. doi: 10.1093/jac/dkac272
Fu, P., Wang, C., Tian, H., Kang, Z., and Zeng, M. (2019). Bordetella pertussis infection in infants and young children in shanghai, China, 2016-2017: clinical features, genotype variations of antigenic genes and macrolides resistance. Pediatr. Infect. Dis. J. 38, 370–376. doi: 10.1097/INF.0000000000002160
Fu, P., Zhou, J., Meng, J., Liu, Z., Nijiati, Y., He, L., et al. (2023a). Emergence and spread of mt28 ptxp3 allele macrolide-resistant bordetella pertussis from 2021 to 2022 in China. Int. J. Infect. Dis. 128, 205–211. doi: 10.1016/j.ijid.2023.01.005
Fu, P., Zhou, J., Yang, C., Nijiati, Y., Zhou, L., Yan, G., et al. (2023b). Molecular evolution and increasing macrolide resistance of bordetella pertussis, shanghai, China, 2016-2022. Emerg. Infect. Dis. 30, 29–38. doi: 10.3201/eid3001.221588
Gorgojo, J. P., Carrica, M., Baroli, C. M., Valdez, H. A., Alvarez, H. J., and Rodriguez, M. E. (2023). Adenylate cyclase toxin of bordetella parapertussis disrupts the epithelial barrier granting the bacterial access to the intracellular space of epithelial cells. PLoS One 18:e291331. doi: 10.1371/journal.pone.0291331
Gurevich, A., Saveliev, V., Vyahhi, N., and Tesler, G. (2013). Quast: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. doi: 10.1093/bioinformatics/btt086
Kamachi, K., Otsuka, N., Fumimoto, R., Ozawa, K., Yao, S. M., Chiang, C. S., et al. (2019). A novel multilocus variable-number tandem repeat analysis for bordetella parapertussis. J. Med. Microbiol. 68, 1671–1676. doi: 10.1099/jmm.0.001095
Koepke, R., Bartholomew, M. L., Eickhoff, J. C., Ayele, R. A., Rodd, D., Kuennen, J., et al. (2015). Widespread bordetella parapertussis infections-Wisconsin, 2011-2012: clinical and epidemiologic features and antibiotic use for treatment and prevention. Clin. Infect. Dis. 61, 1421–1431. doi: 10.1093/cid/civ514
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B., and Stamatakis, A. (2019). Raxml-ng: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455. doi: 10.1093/bioinformatics/btz305
Liko, J., Robison, S. G., and Cieslak, P. R. (2017). Do pertussis vaccines protect against bordetella parapertussis? Clin. Infect. Dis. 64, 1795–1797. doi: 10.1093/cid/cix221
Lin, L. N., Zhou, J. S., Hua, C. Z., Bai, G. N., Mi, Y. M., and Zhou, M. M. (2022). Epidemiological and clinical characteristics of pertussis in children and their close contacts in households: a cross-sectional survey in Zhejiang province, China. Front. Pediatr. 10:976796. doi: 10.3389/fped.2022.976796
Long, G. H., Karanikas, A. T., Harvill, E. T., Read, A. F., and Hudson, P. J. (2010). Acellular pertussis vaccination facilitates bordetella parapertussis infection in a rodent model of bordetellosis. Proc. Biol. Sci. 277, 2017–2025. doi: 10.1098/rspb.2010.0010
Lu, J., Rincon, N., Wood, D. E., Breitwieser, F. P., Pockrandt, C., Langmead, B., et al. (2022). Metagenome analysis using the kraken software suite. Nat. Protoc. 17, 2815–2839. doi: 10.1038/s41596-022-00738-y
Ma, L., Caulfield, A., Dewan, K. K., and Harvill, E. T. (2021). Pertactin-deficient bordetella pertussis, vaccine-driven evolution, and reemergence of pertussis. Emerg. Infect. Dis. 27, 1561–1566. doi: 10.3201/eid2706.203850
Martin, S. W., Pawloski, L., Williams, M., Weening, K., DeBolt, C., Qin, X., et al. (2015). Pertactin-negative bordetella pertussis strains: evidence for a possible selective advantage. Clin. Infect. Dis. 60, 223–227. doi: 10.1093/cid/ciu788
Mastrantonio, P., Stefanelli, P., Giuliano, M., Herrera, R. Y., Ciofi, D. A. M., Anemona, A., et al. (1998). Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J. Clin. Microbiol. 36, 999–1002. doi: 10.1128/JCM.36.4.999-1002.1998
Moore, A., Harnden, A., Grant, C. C., Patel, S., and Irwin, R. S. (2019). Clinically diagnosing pertussis-associated cough in adults and children: chest guideline and expert panel report. Chest 155, 147–154. doi: 10.1016/j.chest.2018.09.027
Mortensen, J. E., and Rodgers, G. L. (2000). In vitro activity of gemifloxacin and other antimicrobial agents against isolates of bordetella pertussis and bordetella parapertussis. J. Antimicrob. Chemother. 45, 47–49. doi: 10.1093/jac/45.suppl_3.47
Riffelmann, M., Wirsing, V. K. C., Caro, V., and Guiso, N. (2005). Nucleic acid amplification tests for diagnosis of bordetella infections. J. Clin. Microbiol. 43, 4925–4929. doi: 10.1128/JCM.43.10.4925-4929.2005
Toubiana, J., Azarnoush, S., Bouchez, V., Landier, A., Guillot, S., Matczak, S., et al. (2019). Bordetella parapertussis bacteremia: clinical expression and bacterial genomics. Open Forum Infect. Dis. 6:z122. doi: 10.1093/ofid/ofz122
Weigand, M. R., Williams, M. M., Peng, Y., Kania, D., Pawloski, L. C., and Tondella, M. L. (2019). Genomic survey of bordetella pertussis diversity, United States, 2000-2013. Emerg. Infect. Dis. 25, 780–783. doi: 10.3201/eid2504.180812
Wu, X., Du, Q., Li, D., Yuan, L., Meng, Q., Fu, Z., et al. (2022). A cross-sectional study revealing the emergence of erythromycin-resistant bordetella pertussis carrying ptxp3 alleles in China. Front. Microbiol. 13:901617. doi: 10.3389/fmicb.2022.901617
Wu, S., Hu, Q., Yang, C., Zhou, H., Chen, H., Zhang, Y., et al. (2021). Molecular epidemiology of bordetella pertussis and analysis of vaccine antigen genes from clinical isolates from Shenzhen, China. Ann. Clin. Microbiol. Antimicrob. 20:53. doi: 10.1186/s12941-021-00458-3
Wu, D., Jing, R., Zheng, H., He, K., Li, Y., Yu, W., et al. (2023). Health and economic evaluation of vaccination against pertussis in China: a 40-year analysis. Value Health 26, 666–675. doi: 10.1016/j.jval.2022.10.011
Yang, C., Li, Y., Jiang, M., Wang, L., Jiang, Y., Hu, L., et al. (2022). Outbreak dynamics of foodborne pathogen vibrio parahaemolyticus over a seventeen year period implies hidden reservoirs. Nat. Microbiol. 7, 1221–1229. doi: 10.1038/s41564-022-01182-0
Zhang, J., Deng, J., and Yang, Y. (2022). Pertussis vaccination in chinese children with increasing reported pertussis cases. Lancet Infect. Dis. 22, 21–22. doi: 10.1016/S1473-3099(21)00752-0
Zhang, Q., Li, M., Wang, L., Xin, T., and He, Q. (2013). High-resolution melting analysis for the detection of two erythromycin-resistant bordetella pertussis strains carried by healthy schoolchildren in China. Clin. Microbiol. Infect. 19, E260–E262. doi: 10.1111/1469-0691.12161
Zhou, G., Li, Y., Wang, H., Wang, Y., Gao, Y., Xu, J., et al. (2024). Emergence of erythromycin-resistant and pertactin- and filamentous hemagglutinin-deficient bordetella pertussis strains—Beijing, China, 2022-2023. China CDC Wkly. 6, 437–441. doi: 10.46234/ccdcw2024.085
Keywords: Bordetella parapertussis, MT6, MT4, children, China
Citation: Fu P, Li Y, Qin J, Xie L, Yang C and Wang C (2024) Molecular epidemiology and genomic features of Bordetella parapertussis in Shanghai, China, 2017–2022. Front. Microbiol. 15:1428766. doi: 10.3389/fmicb.2024.1428766
Edited by:
Xuanyu Tao, University of Oklahoma, United StatesReviewed by:
Daniela Hozbor, Institute of Biotechnology and Molecular Biology (IBBM), ArgentinaMichael Payne, University of New South Wales, Australia
Azadeh Safarchi, Health and Biosecurity (CSIRO), Australia
Copyright © 2024 Fu, Li, Qin, Xie, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Yang, Y3lhbmdAc2lpaS5jYXMuY24=; Chuanqing Wang, Y2h1YW5xaW5nNTIzQDE2My5jb20=
†These authors have contributed equally to this work
 Pan Fu
Pan Fu Yijia Li1†
Yijia Li1† Jie Qin
Jie Qin Chao Yang
Chao Yang Chuanqing Wang
Chuanqing Wang