- 1Clinical Research Center, Chongqing Public Health Medical Center, Chongqing, China
- 2Department of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China
- 3Phase I Clinical Trial Center, Chonggang General Hospital, Chongqing, China
HIV-associated neurocognitive disorder (HAND) is now recognized to be relatively common in people living with HIV (PLWH), and remains a common cause of cognitive impairment. Unfortunately, the fundamental pathogenic processes underlying this specific outcome of HIV infection have not as yet been fully elucidated. With increased interest in research related to the microbiota-gut-brain axis, the gut-brain axis has been shown to play critical roles in regulating central nervous system disorders such as Alzheimer’s disease and Parkinson’s disease. PLWH are characterized by a particular affliction, referred to as gut-associated dysbiosis syndrome, which provokes an alteration in microbial composition and diversity, and of their associated metabolite composition within the gut. Interestingly, the gut microbiota has also been recognized as a key element, which both positively and negatively influences human brain health, including the functioning and development of the central nervous system (CNS). In this review, based on published evidence, we critically discuss the relevant interactions between the microbiota-gut-brain axis and the pathogenesis of HAND in the context of HIV infection. It is likely that HAND manifestation in PLWH mainly results from (i) gut-associated dysbiosis syndrome and a leaky gut on the one hand and (ii) inflammation on the other hand. In other words, the preceding features of HIV infection negatively alter the composition of the gut microbiota (microbes and their associated metabolites) and promote proinflammatory immune responses which singularly or in tandem damage neurons and/or induce inadequate neuronal signaling. Thus, HAND is fairly prevalent in PLWH. This work aims to demonstrate that in the quest to prevent and possibly treat HAND, the gut microbiota may ultimately represent a therapeutically targetable “host factor.”
1 Introduction
Nearly four decades after the emergence of the human immunodeficiency virus (HIV), HIV infection remains a major global public health concern (UNAIDS, 2022). As of 2022, the number of people living with HIV had reached approximately 39 million worldwide, and globally, almost 1.3 million individuals were newly infected by HIV and there were approximately 630,000 deaths in 2022 (UNAIDS, 2022). Fortunately, the development of modern antiretroviral therapy (ART) and widespread implementation thereof has significantly reined in the HIV epidemic, and has reduced its previously inevitable progression to acquired immunodeficiency syndrome (AIDS) (Raubinger et al., 2022). Despite the large number of HIV-infected individuals receiving ART globally [29.8 million (76%)] (The Global HIV and AIDS Epidemic, 2023), HIV (which is known to be a neurotropic virus), continues to affect the brain of people living with HIV (PLWH) (Wang et al., 2020). Indeed, HIV is known to have extensive effects on the neurological system (Mohamed et al., 2020), and for over a decade the term, HIV-associated neurocognitive disorder (HAND), has been used to represent a variety of neurocognitive impairments linked to HIV infection (Eggers et al., 2017).
The 2007 Frascati criteria defines HAND as an acquired cognitive impairment comprising at least two ability domains and, in severe cases, a deterioration in everyday functioning. Asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) are the three stages of cognitive impairment, which are used to characterize the HAND syndrome (Antinori et al., 2007). However, in the present era of modern ART, the Frascati criteria (which were developed in 2007 to aid in the diagnosis of HAND) has been observed to overestimate HAND positivity, particularly when utilized for the diagnosis of the milder forms of the HAND spectrum. Despite this known limitation, the Frascati criteria remain applicable in contemporary times (Meyer, 2022); however, as suggested by Meyer, the criteria require revision and refinement (Meyer, 2022). Some researchers (Goodkin et al., 2014; Hakkers et al., 2018) categorize HAND into two forms, namely mild (ANI and MND) and severe, instead of the three forms presented in the 2007 classification. Other diagnostic tools exist, viz., the international HIV dementia scale (IHDS) and the Montreal cognitive assessment-basic (MoCA-B), and others. It should also be noted here that, similar to the Frascati criteria, the preceding diagnostic methods also possess inadequate psychometric parameter assessment capacity, further limiting their diagnostic accuracy (Mwangala et al., 2018). Although severe neurocognitive impairment has become rare since the introduction of modern highly active antiretroviral therapy (HAART), HAND manifestations, identified by cognitive impairment in HIV-1 infected individuals, persist (Wang et al., 2020). Contemporarily, HAND remains a common cause of cognitive impairment worldwide (Saylor et al., 2016). At the same time, other potential contributing factors for the occurrence of HAND have now been described, including persistent latent HIV-1 reservoirs in the brain, irreversible central nervous system (CNS) insult prior to ART initiation, toxicity associated with antiretroviral drugs, host genetic factors predisposing to the emergence of HAND, deposition of amyloid and tubulin associated unit (Tau) protein, neuroinflammation, as well as damage (at a molecular level) to various neurotransmitter systems (Chang et al., 2008; Nagano-Saito et al., 2009; Hammoud et al., 2010; Sperner-Unterweger et al., 2014; Keegan et al., 2016).
Recently published investigations have highlighted the impact of gut microbiota on the gut-brain axis, and their potential influence on CNS-related diseases and neuropsychiatric disorders. Given that the gut microbiota and their microbial metabolites have been shown to profoundly affect host immunity, cognition, behavior, and metabolism (Marques et al., 2010; Cryan and O’Mahony, 2011; Foster and McVey Neufeld, 2013), increasing attention has been focused on the potential implications of the gut microbiota and microbial metabolites with respect to HAND pathogenesis. The preceding factors suggest that the microbiota-gut-brain axis may represent a thus far unrecognized therapeutic target in the quest to treat HAND. This therapeutic approach may potentially be highly beneficial, as HAND (i) induces a psychological and emotional burden on families, friends, and relatives of HIV positive patients, (ii) provokes difficulties with medication (ART) compliance and follow-up (Sharma, 2021; Zenebe et al., 2021), and (iii) causes HIV positive individuals to engage in high-risk behavior (such as casual unprotected sexual intercourse) favoring HIV transmission (Shrestha and Copenhaver, 2016). Furthermore, the emergence of HAND in HIV-infected individuals places a further burden on already strained resources in hospitals and medical facilities, and also exerts a financial toll on national health resources, even in affluent nations. Therefore, a comprehensive investigation into the role of the microbiota-gut-brain axis in HAND pathogenesis, and also in the treatment and prevention of HAND, may also pave the way toward more robust strategies against HIV infection.
In this review, we comprehensively discuss and highlight the role of the microbiota-gut-brain axis in modulating enteric and central nervous system functions from a clinical perspective. Specifically, we (i) briefly review the prevalence of neurological disorders in HIV-infected individuals, (ii) extensively discuss the mechanisms whereby gut integrity influences the onset of neurological disorders, and (iii) discuss the influence of HIV-associated gut dysbiosis in the onset of neurological outcomes via the consequences of dysbiosis on alterations in the microbiota-gut-brain axis.
2 High neurological disorder prevalence in PLWH
Prior to the present era of modern combination antiretroviral therapy, severe cognitive impairments were reported in up to 50% of people living with HIV (Grant et al., 1987). Although severe neurocognitive impairment secondary to HIV infection has become rare as a result of successful immune reconstitution in this era of modern and potent antiretroviral therapy, milder forms of cognitive impairment remain common in PLWH (Heaton et al., 2010; Clifford and Ances, 2013). To illustrate this point, observations from one recent meta-analysis (of 123 studies conducted in 32 countries) have indicated that the overall prevalence of HAND in HIV-infected adults was calculated to be 42.6%, equating to roughly 16,145,400 cases worldwide. Specifically, the prevalence of asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) were observed to be 23.5, 13.3, and 5.0%, respectively (Wang et al., 2020). Additionally, longitudinal cohort observations have revealed that the presence of ANI, when compared against neurocognitively normal individuals, confers a 2–6-fold increase in risk for earlier development of symptomatic HAND (Grant et al., 2014). These observations are valid even in ART-treated individuals with undetectable HIV viral loads (Grant et al., 2014). Moreover, several observational cohort studies have observed that PLWH on antiretroviral therapy (ART) are relatively more likely to develop dementia compared to people without HIV (Lam et al., 2021, 2022). One of the preceding cohort studies observed that compared to HIV negative individuals, PLWH have a 58% higher risk of developing dementia (Lam et al., 2021). Interestingly, exposure to ART did not reduce this risk. Notably, major depressive disorder (MDD) is the most prevalent psychiatric manifestation associated with HIV infection (Dubé et al., 2005), with a prevalence of up to 36% (Gaynes et al., 2015; Passchier et al., 2018), which is at least twice that observed in healthy community samples (Chibanda et al., 2014; Passchier et al., 2018). Overall, HIV is strongly implicated in the onset of HAND in ART-treated or ART-naïve PLWH. HIV neurotropism in the brain, which is characterized by inflammation resulting from HIV viral protein interactions with endothelial cells (Younas et al., 2016), increased production of reactive oxygen species (ROS) (Cirino et al., 2022), and brain damage resulting from the destruction of astrocytes and pericytes (Ahmed et al., 2018), is possibly responsible for the development of HAND. However, the potential etiological factors for HAND development, which relies solely on the preceding components will be an incomplete description of all the likely causal factors. We believe that the gut may well be seen as a further protagonist in the pathophysiological evolution of HAND. Thus, a picture depicting the influence of HIV on the gut is worth exploring.
3 HIV infection is associated with gut microbiota dysbiosis and related inflammation
The gut microbiota is composed of a community of microorganisms contained within the gastrointestinal (GI) tract, and exists symbiotically with the human host (Thursby and Juge, 2017). A healthy and stable gut microbiota community plays a vital role in maintenance of homeostatic balance of gut barrier integrity, gut function, gut metabolism, and immunity of the gut (Sun and Shen, 2018). It is now well established that profound changes (in microbial composition, metabolites, and immune cells) occur within the gut of an HIV-infected individual.
The GI tract is known to harbor the majority of the body’s complement of immune cells (de Waele, 2021). The vast population of activated memory CD4+ T-cells, with abundant expression of chemokine receptors, provides HIV-1 with an ideal environment to establish infection (Mehandru et al., 2005). Indeed, activated CD4+ T-cells within the gut, in addition to predominantly expressing CXCR4 and CCR5 receptors [which facilitates HIV penetration of these cells (Poles et al., 2001)], are one of the primary targets of HIV (Zaongo et al., 2022). As such, researchers have hypothesized that interventions aimed at reducing the vulnerability of such cells may lower the risk of HIV acquisition (Lajoie et al., 2021). Thus, HIV infection can lead to rapid and substantial depletion of CD4+ T-cells in the lamina propria (Ishizaka et al., 2021). This depletion of CD4+ T-cells is mediated predominantly by apoptosis (Cooper et al., 2013), pyroptosis (Doitsh et al., 2010), and cytotoxic T-cells (Chávez-Galán et al., 2009; Liu et al., 2011). HIV also directly attacks the gut mucosal epithelium, resulting in intercellular tight junction disruption, death of enterocytes, and ultimately a more permeable gut (Younas et al., 2019). The preceding scenario “opens the gate,” so to speak, to gut-associated dysbiosis syndrome, displacement of microbial product into the bloodstream (microbial translocation), and systemic inflammation (Younas et al., 2019). Moreover, researchers have reported that CD4+ T-cell depletion occurs at all stages of HIV disease, and predominantly occurs in the GI tract (Brenchley et al., 2004). Although ART may restore CD4+ T-cells in other anatomical locations, lymphocyte levels within the gut, in the majority of cases, are slow to return to normal levels and restoration of their numbers is most often incomplete (Kotler, 2005). Gut microbiota dysbiosis has the potential to influence HIV disease in various ways throughout all phases in the natural history of HIV disease progression, from transmission to end stage disease (Li et al., 2016).
According to previous research data, gut microbiota dysbiosis in PLWH mainly manifests as alterations in microbial diversity and relative abundance of certain specific gut microorganisms (Dillon et al., 2016; Deusch et al., 2018). The diversity of gut microbiota in HIV-infected people is significantly lower than that of the general population (Lu et al., 2018). One recent meta-analysis examined 22 studies to evaluate alpha (α-) diversity in the gut microbiota of HIV-infected compared to HIV-uninfected individuals, and concluded that HIV status was associated with a decrease in measures of α-diversity (Tuddenham et al., 2020). Apart from changes in diversity, HIV infection is associated with depletion of commensal species and enrichment of opportunistic pathogens (McHardy et al., 2013). Several survey studies in human cohorts have compared the intestinal microbiota composition of HIV-positive patients with that of HIV-uninfected individuals (Lozupone et al., 2013; McHardy et al., 2013; Vujkovic-Cvijin et al., 2013; Dillon et al., 2014; Mutlu et al., 2014; Yu et al., 2014; Dinh et al., 2015; Nowak et al., 2015; Vázquez-Castellanos et al., 2015; Dubourg et al., 2016; Ling et al., 2016; Monaco et al., 2016; Noguera-Julian et al., 2016; Sun et al., 2016; Vesterbacka et al., 2017; Armstrong et al., 2018; Lee et al., 2018; Lu et al., 2018; San-Juan-Vergara et al., 2018; Zhou et al., 2018; Vujkovic-Cvijin and Somsouk, 2019), and these have shown an enrichment of Erysipelotrichaceae, Enterobacteriaceae, Desulfovibrionaceae, and Fusobacteria, and a reduction of Lachnospiraceae (Vujkovic-Cvijin and Somsouk, 2019). Ruminococcaceae and Lachnospiraceae taxa comprise the primary producers of short-chain fatty acids (SCFA) within the gut, meaning that their relative depletion in HIV-infected individuals is also associated with a diminution of SCFA in the gut. Moreover, in HIV-infected individuals, a proliferation of proinflammatory microorganisms such as Candida albicans, and a diminution of anti-inflammatory microorganisms such as Akkermansia muciniphila, is observed (Ouyang et al., 2020; MacCann et al., 2023).
In addition to microbial composition, HIV infection may also cause dysregulation of gut microbiota metabolism (McHardy et al., 2013). SCFAs, which are the fermentation products of intestinal microbiota, is crucial for maintenance of the overall health of intestinal epithelial cells and regulation of local immune responses (Shi et al., 2017). Compared with HIV-negative individuals, circulating levels of butyric acid and valeric acid are reduced in HIV-positive patients (Qing et al., 2019). Moreover, levels of butyric and valeric acids positively correlate with the abundance of species such as Rikenellaceae, Ruminococcaceae, Alistipes, Roseburia, and Lachnospiraceae (Qing et al., 2019). Interestingly, different metabolic functions manifest based on the different microbial communities that are more abundant in HIV-infected subjects. McHardy et al., have observed that imputed metagenomic functions, including amino acid metabolism, vitamin biosynthesis, and siderophore biosynthesis differs significantly between healthy controls and HIV-infected subjects not receiving ART (Kang and Cai, 2019). Gut-resident bacteria with the capacity to metabolize tryptophan (TRP) through the kynurenine (KYN) pathway were observed to be enriched in HIV-infected subjects (Vujkovic-Cvijin et al., 2013), and gut microbiota communities in HIV-infected subjects exhibit an increased capacity to catabolize tryptophan to kynurenine (Serrano-Villar et al., 2016; Vujkovic-Cvijin and Somsouk, 2019). Degradation of TRP via the KYN pathway may result in decreased production of serotonin. Interestingly, several studies have noted low levels of serotonin [5-hydroxytryptamine (5-HT)] in the blood and CSF of patients with HIV-1 infection (Launay et al., 1988; Larsson et al., 1989; Ghare et al., 2022).
Thus, HIV infection is associated with alterations in microbiota composition and microbial metabolites, and physical disruption of the gut endothelial barrier. These changes may contribute to greater microbial translocation and the persistence of a proinflammatory state even subsequent to restoration of circulating CD4+ T-cell counts via ART (Takiishi et al., 2017; MacCann et al., 2023). The preceding repercussions of HIV infection within the gut, together with their consequences, may well influence the gut-brain axis and possibly mediate the subsequent development of HAND.
4 Neurocognitive disorders and their connections to gut microbiota as reported in HIV negative contexts
Cumulative research evidence implicates the gut microbiosis in a variety of psychiatric, neurological, and neurodegenerative diseases (Cryan et al., 2019). Evidence from both clinical and experimental studies show that there is an imbalance of gut microbiota and microbial metabolites present in various CNS diseases (Table 1). For example, clinical studies on the gut microbiota of patients with Alzheimer’s Disease (AD) and the gut microbiota in an AD mouse model have suggested differences in microbial diversity, compared to the control group. Specifically, it has been observed that AD is associated with a decrease in Fusobacteriaceae, Firmicutes, Actinobacteria, and Bifidobacterium, and an increase in Bacteroidetes (Westfall et al., 2017; Megur et al., 2020). In fecal samples from Parkinson’s disease (PD) patients, bacteria more commonly related to anti-inflammatory properties, such as genus Blautia, Coprococcus, and Roseburia, are significantly decreased, while proinflammatory Proteus, Enterococcaceae, and Enterobacteriaceae organisms increased (Keshavarzian et al., 2015; Scheperjans et al., 2015; Bedarf et al., 2017; Quigley, 2017). Similarly, recent studies (Jiang et al., 2015; Aizawa et al., 2016; Sun and Shen, 2018) have suggested that (i) lower Bifidobacterium and Lactobacillus levels are more common in individuals with major depressive disorder, (ii) Faecalibacterium levels are negatively associated with severity of depressive symptoms in patients with major depressive disorder, and (iii) Enterobacteriaceae and Alistipes proportions are increased in people having major depressive disorder, compared to healthy controls. Recently, it has been reported that gut microbiota from patients with HAND showed significantly lower α-diversity compared with that from patients without HAND (Sun et al., 2016; Hamad et al., 2018; Zhang et al., 2018). Notably, the gut microbiota composition in HIV positive patients with neurocognitive impairment is significantly different from those without neurocognitive impairment (Dong et al., 2021). HIV positive individuals with neurocognitive impairment also present a decreased abundance of butyrate-producing bacteria (BPB) and an increased abundance of Klebsiella (Dong et al., 2021).
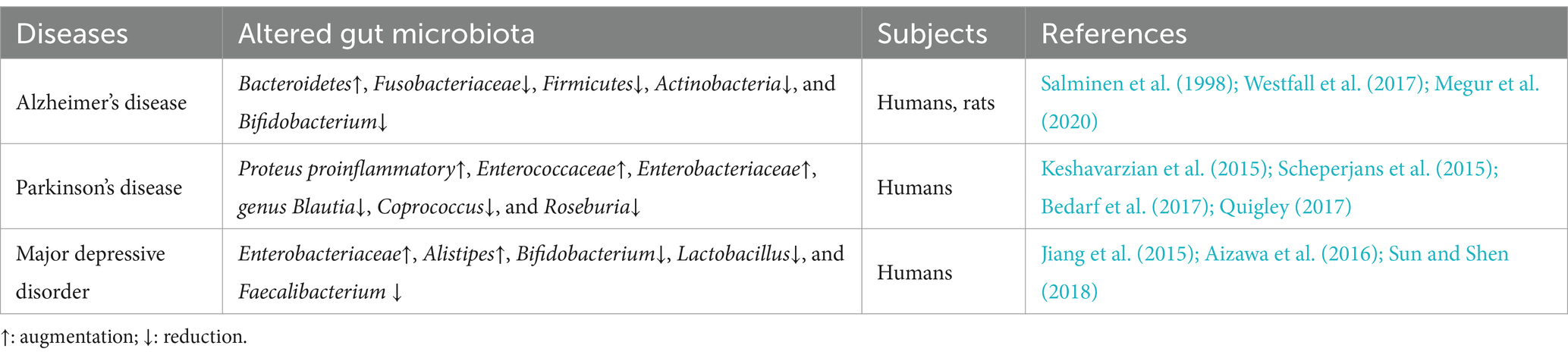
Table 1. Examples of altered gut microbiota composition in HIV-negative patients with CNS diseases or neurocognitive disorders.
Interventions utilizing therapeutic modalities such as antibiotics, probiotics, and fecal microbiota transplantation (FMT) may modify the composition of the gut microbiota and therefore influence the cognitive functioning of the host. Indeed, antibiotics are known to disrupt the gut microbial community, which may have negative consequences on brain function and behavior. In rodents, antibiotic administration induces changes in the gut microbiota, and this has been observed to be associated with subsequent object recognition memory impairment and altered hippocampal function (Desbonnet et al., 2015; Fröhlich et al., 2016; Möhle et al., 2016; Cryan et al., 2019). Additionally, through fecal microbiota transplantation (FMT), it has been demonstrated that transplanted gut microbiota may improve the symptoms of neurological disease by modulating the microbiota-gut-brain axis. For instance, one murine model of AD observed that microbiota transplantation utilizing the gut microbiota from a healthy subject alleviates the formation of amyloid beta (β) plaques and neurofibrillary tangles, as well as improves glial reactivity, and cognitive impairment (Kim et al., 2020). Thus, via the observations gleaned from FMT experiments, it can be seen that gut microbiota may be able to transfer a behavioral phenotype or disease feature to a recipient, providing stronger evidence for a causal relationship between gut microbiota and CNS disease. The utilization of specific probiotics in humans has also demonstrated beneficial effects with respect to cognitive performance in both healthy and unhealthy individuals (Cryan et al., 2019). Significant improvements in various cognitive test scores (inclusive of memory, attention, executive function, and language) were observed in two studies in which PLWH received probiotics as supplements (Ceccarelli et al., 2017). This indicates that the gut-brain axis plays a vital role in the manifestation of neurological disorders. Furthermore, in a recent publication, our team (Zaongo et al., 2023) has published extensive information regarding the elements of the gut, which may influence brain development and functioning. We believe that the gut microbiome may also play critical roles in the development of neurodegenerative disorders (Table 2), inspired from our published article (Zaongo et al., 2023). Notably, according to previously published investigations (Morais et al., 2021; Mayer et al., 2022), it is hypothesized that the specific pathways which potentially define the relationships between the gut-brain axis and neurological disorders are either direct (direct effects on neurons and neuronal signaling), or indirect (via immune responses and/or microbial metabolites).
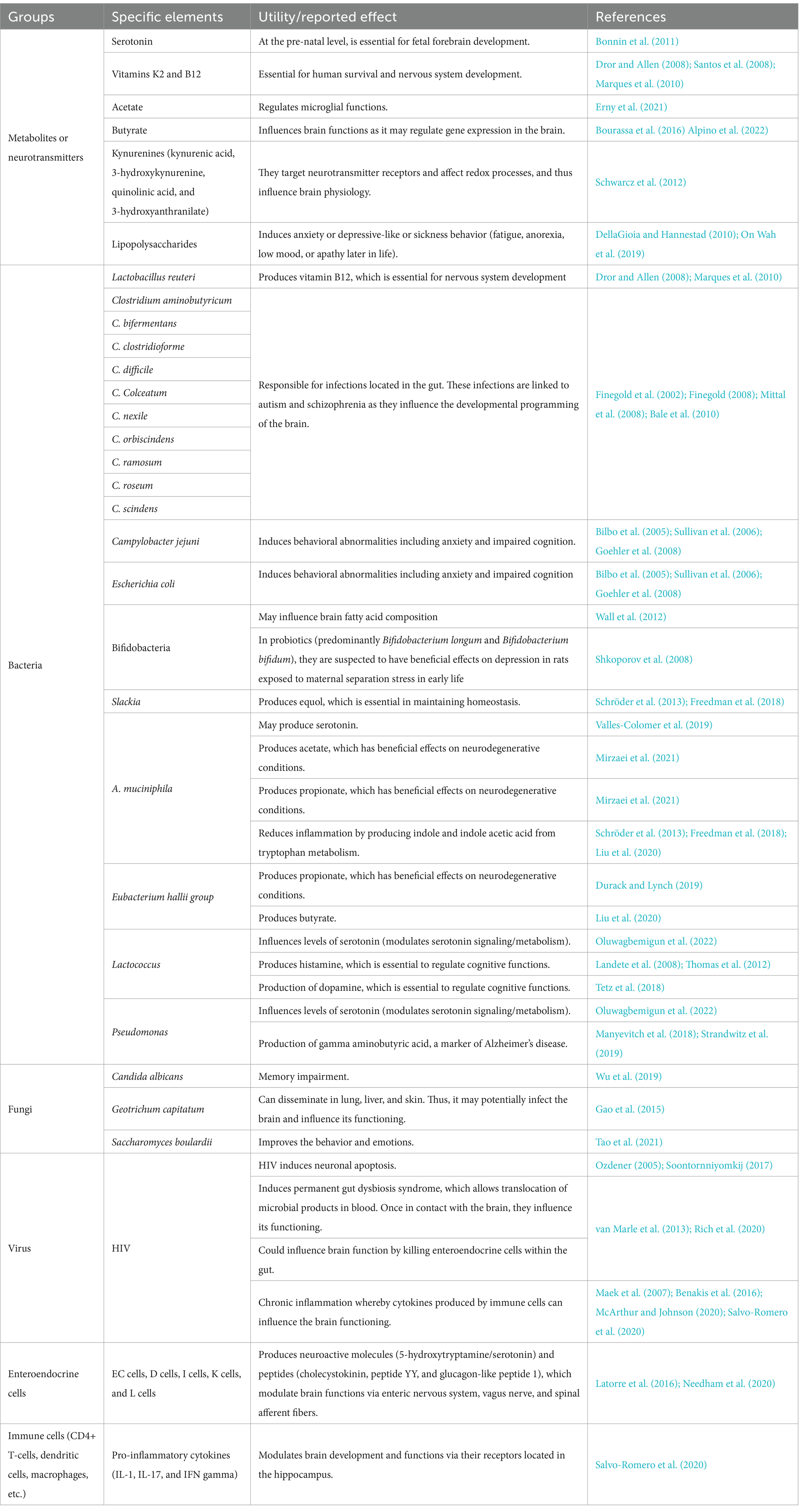
Table 2. Elements of the gut which are potentially involved in the development of neurodegenerative disorders.
4.1 Neurons and neuronal signaling
Two neuronal pathways physically link the gut and the brain (Carabotti et al., 2015). These comprise the direct connections via the vagus nerve between the brain and the gut and the bidirectional exchange between the brain and the gut via the enteric nervous system (ENS) present in the intestine (Gwak and Chang, 2021). The vagus nerve is a significant component of both pathways. Indeed, the vagus nerve (VN) extends from the brainstem as the tenth (and longest) cranial nerve, to innervate the gut and the enteric nervous system (ENS) (Breit et al., 2018). Interestingly, Yoo and Mazmanian have observed that intestinal microbiota may mediate the development and the functional components of the enteric nervous system (ENS) (Yoo and Mazmanian, 2017). Similarly, De Vadder et al. (2018), have demonstrated that neuronal innervation of colonic epithelium is reduced in germ-free (GF) mice, and that this phenotype is reversed 15 days after colonization by gut microbes. Microbes and their products regulate the development and maturation of glial cell networks within the nervous system, and enteric glial cells (EGCs) are the major target of the gut microbiota (Kabouridis et al., 2015). Furthermore, gut microbiota may affect the function of enteric neurons through chemical signaling. Indeed, it has been reported that supplementation with SCFA-producing gut microorganisms may suppress the activation of the neuronal pathway that mediates gut motility (Morais et al., 2021). Additionally, direct neural communication between gut microbiota and the brain is mainly achieved through the VN (Borre et al., 2014). As such, the VN sensory fibers innervate the muscle and mucosal layers of the gastrointestinal tract, detect sensory signals, and subsequently transmit these signals to the CNS (De Vadder et al., 2018). In animal studies, injection of α-synuclein (a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release) into the duodenal and pyloric muscularis layer induces its dissemination along the VN to the middle brain, causing neuronal damage (Kim et al., 2019; Van Den Berge et al., 2019; Benakis et al., 2020). Although the VN is likely to be involved in the pathogenesis of both acute and chronic brain disorders, its role in linking the gut microbiota to the natural history of any particular disease has not as yet been extensively investigated (Benakis et al., 2020).
4.2 Modulation of immune responses
The gut microbiota interacts intimately with the intestinal immune system. Microbial interactions with local immune cells may lead to functional changes that extend beyond the gastrointestinal tract. According to Agirman et al., this scenario may occur through alterations to the release of cytokines into the systemic circulation or through conditioning of immune cells that home to other anatomical sites, including the brain (Agirman and Hsiao, 2021). Notably, proinflammatory cytokines (IL-1, IL-17, and IFN-γ) have been observed to be capable of modulating brain development and functions via their receptors located in the hippocampus (Tetz et al., 2018). In addition to microbial effects on peripheral immune cells, the microbiota is also necessary for healthy development, maturation, and activation of microglia, which are the innate immune cells of the brain (Abdel-Haq et al., 2019; Morais et al., 2021). Moreover, it has been reported that germ-free (GF) mice display global defects in microglia, with altered cell proportions and an immature phenotype, leading to impaired innate immune responses within the murine brain (Erny et al., 2015). Defective microglia are also associated with limited microbiota complexity. Conversely, recolonization of the gut with a complex gut microbiota (particularly enriched with SCFA and bacteria producing SCFA) partially restores normal microglia phenotypic traits (Erny et al., 2015). A separate research group has also demonstrated that short-chain fatty acids and microbiota-derived bacterial fermentation products may restore microglial morphology and function (Erny et al., 2015). Crucially, alterations in microglial function have been linked to stress, behavioral disorders, and neurodegenerative disorders, which suggests that the gut microbiota may influence human neurological diseases through effects mediated by microglia (Morais et al., 2021). The permeability of the blood–brain barrier has been shown to be related to the gut microbiota and microbial metabolites. Some reports show that GF mice have increased blood–brain barrier (BBB) permeability relative to control mice (Braniste et al., 2014). The increased permeability of the gut and the blood–brain barrier induced by gut microbiota derangement may thus potentially mediate or influence the emergence of neurodegenerative disorders (Jiang et al., 2017).
4.3 Microbial metabolites
Microbial products and metabolites, including secondary bile acids, indole-derivatives, and SCFAs may transmit signals through enteroendocrine cells (EECs) and enterochromaffin cells (ECCs) to regulate the secretion of neuropeptides and neuromodulators such as the hormonal neurotransmitter, serotonin. Furthermore, subsets of gut bacteria may directly synthesize and release specific neurotransmitters and neuromodulators (Agirman and Hsiao, 2021). For example, Enterococcus spp., Escherichia spp., Lactobacillus spp., Lactococcus spp., M. morganii, and Streptococcus spp., to list a few, have been observed to produce serotonin (Agirman and Hsiao, 2021).
4.3.1 Short chain fatty acids
Short-chain fatty acids improve gut motility, reduce the release of proinflammatory cytokines, and modulate adaptive immune tolerance as well as the levels of gut hormones and neuropeptides (Dalile et al., 2019; Benakis et al., 2020). SCFAs directly impact brain function, regulating BBB permeability, microglial function, and modulation of neuroinflammatory responses. As an example, Erny et al., have demonstrated that mono-colonization by a butyrate-producing bacterium restores the integrity of the BBB in GF mice, and also plays a critical role in the maturation of microglia (Erny et al., 2015). In addition, SCFAs may affect neuro-inflammation by modulating the production and recruitment of immune cells such as T-cells and neutrophils, and of inflammatory cytokines (Parker et al., 2020). Butyrate has been reported to stimulate memory and synaptic plasticity by inhibition of histone deacetylases (Kaur et al., 2019). SCFAs may also influence health and behavior. For instance, mice exposed to acute exogenous SCFA (sodium butyrate) are observed to have altered production of brain-derived neurotrophic factor (BDNF), which is a neuronal factor that has been associated with depression (Schroeder et al., 2007). In the preceding study, prolonged injection of exogenous sodium butyrate into mice (for 28 days) has been observed to reduce their depressive-like behaviors in a statistically significant manner.
4.3.2 Serotonin
The gut microbiota influences tryptophan (TPH) metabolites, and thus affects the pathogenesis of many neurologic and psychiatric disorders. TPH is the only substrate for serotonin synthesis, which occurs primarily in the distal gastrointestinal tract (90%) and, to a lesser extent, in the central nervous system (10%) (Roth et al., 2021). The commensal gut microbiota has multiple regulatory mechanisms for the peripheral serotonin pool. On the one hand, microbial metabolites such as indole, SCFAs, and secondary bile acids impact the generation and secretion of 5-HT by enteroendocrine cells (EECs) (Gao et al., 2020; Agirman and Hsiao, 2021). On the other hand, the commensal gut microbiota may directly utilize tryptophan to synthesize serotonin. Indeed, it has been reported that specific bacterial strains, such as Lactococcus, Lactobacillus, Streptococcus, Escherichia coli, and several bacteria of the Klebsiella genus, may produce serotonin by expressing tryptophan synthase (O’Mahony et al., 2015; Gao et al., 2020). Apart from changes to the peripheral serotonin pool, modulation of central serotonin metabolism by gut microbiota also occurs in multiple ways. Observations from some studies (Gao et al., 2018, 2019; Lukić et al., 2019) imply that alterations to gut microbial tryptophan metabolism may influence changes in central serotonin metabolism by affecting tryptophan availability. Moreover, the gut microbiota exhibits other pathways, which modulate central serotonin synthesis (Gao et al., 2018, 2019; Lukić et al., 2019). For example, some microbial metabolites (especially butyrate, which may be transported into the systemic circulation) are thought to have neuroprotective effects in stressed mice via an increase in brain serotonin levels and a remediation of BBB impairments (Sun et al., 2016). Additionally, inflammatory stimuli have been observed to decrease serotonin levels in the prefrontal cortices of mice (Zhu et al., 2015). In the CNS, 5-HT is involved in the modulation of a range of mood, behavioral, and cognitive functions (Cryan and Leonard, 2000; Berger et al., 2009; Kennedy et al., 2017), and low serotonin levels have been reported to be associated with depression, fatigue, and impaired cognitive functions (Geldenhuys and Van der Schyf, 2011; Kaur et al., 2019).
4.3.3 Tryptophan metabolism
Approximately 90% of tryptophan is metabolized along the kynurenine pathway. TRP can be catabolized by the heme-dependent enzymes, TRP 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO1), resulting in the production of kynurenine (KYN) and its derivatives (Stone, 1993; Guillemin et al., 2003; Richard et al., 2009). Fluctuating levels of kynurenine pathway metabolites, including kynurenine, kynurenic acid, 3-hydroxyanthranilic acid (3-HAA), 3-hydroxykynurenine (3-HK), and the neurotoxic quinolinic acid are associated with many neurologic and psychiatric disorders (Schwarcz et al., 2012; Kennedy et al., 2017). Kynurenine and quinolinate, for instance, have been proposed as metabolites which are likely to perturb brain functioning and consequently cause depression-like symptoms (Oxenkrug, 2013; Kaur et al., 2019). The preceding observations indicate that the gut microbiota may influence brain functions through modulation of the kynurenine pathway. Studies have reported that the gut microbiota not only regulates the expression of the kynurenine pathway genes in the hippocampus via an microRNA-dependent mechanism, but may also modulate the kynurenine pathway in the brain by directly impacting the activity of its key enzymes (Malmevik et al., 2016; Moloney et al., 2017; Gao et al., 2020). Additionally, circulating SCFAs, such as butyrate, may directly modulate central kynurenine pathways (Agirman et al., 2021; Sanmarco et al., 2021). Notably, the kynurenine pathway plays a primary role in influencing tryptophan availability by the clearance of excess tryptophan. Thus, dysregulation between serotonin synthesis and the kynurenine pathway may incite the emergence of neuropsychiatric disorders, such as depression (Kennedy et al., 2017; Gao et al., 2020). Other than serotonin synthesis and the kynurenine pathway, gut microbiota may directly transform tryptophan into indole derivatives. Subsequently, indoles of bacterial origin may be incorporated into the systemic circulation, may cross the BBB, and may exert neuroprotective effects via aryl hydrocarbon receptor (AhR) signaling (Rothhammer et al., 2016). One recent study observed that the microbial indole metabolites of tryptophan, including indole, indole-3-acetic acid (IAA), and indolic-3-propionic acid (IPA), may activate AhR signaling in astrocytes and hence modulate CNS inflammation (Rothhammer et al., 2016).
4.3.4 Membrane-derived molecules
Other gut microbiota-derived molecules may have significant effects on host immunity and neurological diseases (Benakis et al., 2020). A prominent example is the endotoxin, lipopolysaccharide (LPS). LPS translocation is facilitated by gut permeability, which also causes a potent inflammatory response that may damage/disrupt the BBB and subsequently activate microglia (Banks and Erickson, 2010). For instance, Proteus mirabilis gavage has been observed to replicate PD-like symptoms, and enhances microglia activation via LPS in wild-type mice (Choi et al., 2018).
Three parallel but related communication pathways may be used to send inflammatory signals from the GI tract to the central nervous system (Agirman et al., 2021). Intestinal inflammation (triggered by dysbiosis) induces the release of proinflammatory cytokines and may have dramatic extraintestinal consequences (Parker et al., 2020). Circulating proinflammatory factors may disrupt epithelial tight junctions and compromise both the gut-vascular barrier (GVB) and BBB integrity (Parker et al., 2020), thus “opening the door” for molecules, toxins, and pathogens originating from the gut lumen to enter the brain parenchyma, activating local immune cells, and inducing neuroinflammation. Increased systemic levels of the bacterial wall component, LPS, for instance, has been linked to cognitive decline, microglial activation, neuronal cell death, and cytokine-mediated illness behavior (Zhao et al., 2019). Moreover, the gut microbiota and their byproducts have been shown to have a direct impact on neuroimmunomodulatory functions within the CNS (Agirman et al., 2021), and a lack of immune priming results in an inadequate response to brain insults and inflammatory stimuli (Erny et al., 2015; Rothhammer et al., 2016; Thion et al., 2018; Van Hove et al., 2019). Local immune cells of the CNS may also be programmed by gut-derived cells whose functions can be regulated by the gut microbiota (Agirman et al., 2021; Sanmarco et al., 2021). In the case of gut dysbiosis, intestinal immune cells may directly promote CNS neuroinflammation (Agirman et al., 2021). Multiple sclerosis (MS) is the most prevalent form of inflammatory disease in the CNS, and mounting evidence suggests that other neurological diseases such as AD, PD, and autism spectrum disorder (ASD) may also be significantly associated with inflammatory responses (Heneka et al., 2015; Park and Kim, 2021). Although each neurodegenerative disease has its own unique pathway that ultimately results in neurodegenerative changes, chronic inflammation that originates from and is dependent on the gut microbiota is often a key aspect of the progressive nature of neurodegeneration (Harry, 2013). Immune reactions in the CNS may reportedly have adverse long-term effects, especially in cases of chronic inflammation, and proinflammatory cytokines and oxidative stress have been causally linked to neuronal death (Millán Solano et al., 2023).
From the preceding details, it is fair to reason that the crosstalk between the gut and the brain is a crucial factor to consider when contemplating the fundamental nature of neurocognitive disorders, and this gut-brain crosstalk may involve multiple mechanisms. As such, and based on current evidence, we believe that the gut-brain axis plays a significant role in the pathogenesis of neurocognitive disorders that are encountered in PLWH.
5 The microbiota-gut-brain axis mediates neurological disorders in PLWH: evidence from the literature
Evidence from contemporary literature informs that HIV infection may affect both the brain and the gut. It is known that HIV may overcome BBB protection and penetrate the CNS [via a “trojan horse” (i.e., an infected CD4+ T-cell or a monocyte migrating from the bloodstream into the CNS), or via transcytosis (i.e., infected epithelial cells transporting HIV particles from the systemic circulation into the CNS)], where it may infect different types of cells (macrophages, microglia, and astrocytes), promote inflammation, and provoke neuronal damage (Araínga et al., 2017; Abreu et al., 2019; Wallet et al., 2019; McArthur and Johnson, 2020). The mechanisms (involving neuroinflammatory responses to viral proteins and inflammatory cytokines released by infected microglia and macrophages) whereby HIV exerts deleterious effects on neurons are (i) largely indirect and (ii) triggered by HIV neurotoxicity (Kaul et al., 2001; Ellis et al., 2007; Saylor et al., 2016). As previously described, the proinflammatory reaction in the brain may alter CNS functions (Hsiao et al., 2013; Houser and Tansey, 2017). Thus, neuroinflammation is now considered to be a key factor in the evolution of many neurodegenerative diseases (González et al., 2014; Houser and Tansey, 2017). To illustrate this, observations from recent studies using MRI combined with metabolite spectroscopy have confirmed the presence of persistent neuroinflammation in individuals with HAND (Cysique et al., 2018; Alakkas et al., 2019; McArthur and Johnson, 2020). McArthur and Johnson have also reported that even virologically suppressed HIV-infected individuals display sustained inflammation and neural injury. These investigators have further suggested that a reduction of neuroinflammation and systemic inflammation may protect the CNS from immune-mediated damage (McArthur and Johnson, 2020). In addition to the inflammation resulting from the presence of HIV within the brain, previous studies have also shown that microbial translocation may drive neuroinflammation and thereby contribute to the pathogenesis of HAND (Ancuta et al., 2008; Vera et al., 2015). Indeed, HIV infection leads to gut microbiota imbalance, increases intestinal permeability, and causes persistent release of microbial products into the bloodstream (Lackner et al., 2009). The preceding mechanisms, as observed in past research, significantly contribute to systemic inflammation (Mehraj et al., 2020; Luo et al., 2022). Interestingly, several published articles have demonstrated that probiotic supplementation may positively influence neuronal functions, decrease neuroinflammation, and ameliorate cognitive impairment in PLWH (; Ceccarelli et al., 2017). Additionally, multiple studies have shown that reduced gut microbial diversity and reduction of their associated metabolites contribute to the development of neuroinflammation and cognitive impairment (Magnusson et al., 2015; Beilharz et al., 2016; Li et al., 2019; Saiyasit et al., 2020). Thus, it can be assumed that HIV infection within the gut may indirectly provoke HIV-associated neuronal damage and neurodegenerative diseases in PLWH.
Patients with HIV-1 infection have been shown to have reduced serotonin levels in their blood and CSF (Launay et al., 1988; Keegan et al., 2016; Fu et al., 2023). Gut microbiota may regulate the synthesis of serotonin, and this may be related to an imbalance of gut microbiota composition and the alterations observed in levels of microbiota-associated metabolic products. As reported previously, factors related to the decrease in serotonin levels include a decrease in the abundance of symbiotic gut microorganisms which upregulate serotonin synthesis, an increase in the specific gut microbiota that regulate the kynurenine pathway, and a decrease in the microbial metabolites responsible for serotonin synthesis or release (O’Mahony et al., 2015; Gao et al., 2020; Agirman and Hsiao, 2021). However, it is possible that low levels of serotonin can be therapeutically enhanced, as researchers have demonstrated that specific probiotic supplementation may significantly increase serum serotonin levels in PLWH (Scheri et al., 2017). Serotonin is known as a key neurotransmitter which is involved in a wide range of mood, behavioral and cognitive functions (Cryan and Leonard, 2000; Berger et al., 2009). Low levels of serotonin have been associated with depression, fatigue, and impaired cognitive functions (Geldenhuys and Van der Schyf, 2011; Kaur et al., 2019). Interestingly, several past publications have shown that many HIV-positive patients with depression respond well to oral treatment with selective serotonin-reuptake inhibitors (SSRIs) (Caballero and Nahata, 2005). Accumulated evidence confirms that changes in gut microbiota composition and of their metabolites in PLWH may influence intrinsic serotonin production, and therefore promote the pathogenesis of depression and impaired cognitive functioning.
HIV infection may lead to the enrichment of gut-resident bacteria with the capacity to metabolize tryptophan (TRP) through the kynurenine (KYN) pathway, and these gut-resident bacteria may augment the production of kynurenine metabolites through increased expression of gut IDO1 (Rhee et al., 2005; Atarashi et al., 2011; Vujkovic-Cvijin et al., 2013). Several recent reports have shown that in HIV-infected individuals, serum TRP concentration is markedly decreased while the kynurenine concentration is increased, as reflected in elevated KYN to TRP (KYN/TRP) ratios (Huengsberg et al., 1998; Qi et al., 2018). Furthermore, it has been observed that severity of depression in HIV patients is associated with a decrease in plasma tryptophan concentration and an increase in the KYN/TRP ratio (Martinez et al., 2014). The TRP-KYN pathway may also lead to changes in downstream metabolites (Heyes et al., 1991; Qi et al., 2018). Some of these metabolites may be neurotoxic, such as 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid (QUIN) (Stone, 1993; Guillemin et al., 2003, 2005; Davies et al., 2010; Capuron et al., 2011; Keegan et al., 2016). More importantly, fluctuating levels of kynurenine pathway metabolites have been observed to be associated with several neurologic and psychiatric disorders (Schwarcz et al., 2012; Kennedy et al., 2017). Indeed, cerebrospinal fluid (CSF) levels of quinolinic acid have been shown to increase during HIV infection, and to be associated with HAND severity (Heyes et al., 1989, 1991; Achim et al., 1993; Sei et al., 1995; Valle et al., 2004) Therefore, given the regulatory role of the gut microbiota on kynurenine pathway metabolites during HIV infection and the impact of kynurenine pathway metabolites on neurocognitive impairment, it is reasonable to suggest that HIV infection causes gut microbiota imbalance, promotes the activation of IDO, and favors the continuous accumulation of neurotoxic metabolites, which ultimately fosters the development of neurocognitive disorders.
An overall picture of the potential mechanisms whereby the gut-brain axis may influence HAND development during HIV infection is presented in Figure 1. Furthermore, an illustration of the relationship between HIV, gut microbiota, and HAND is provided in Figure 2.
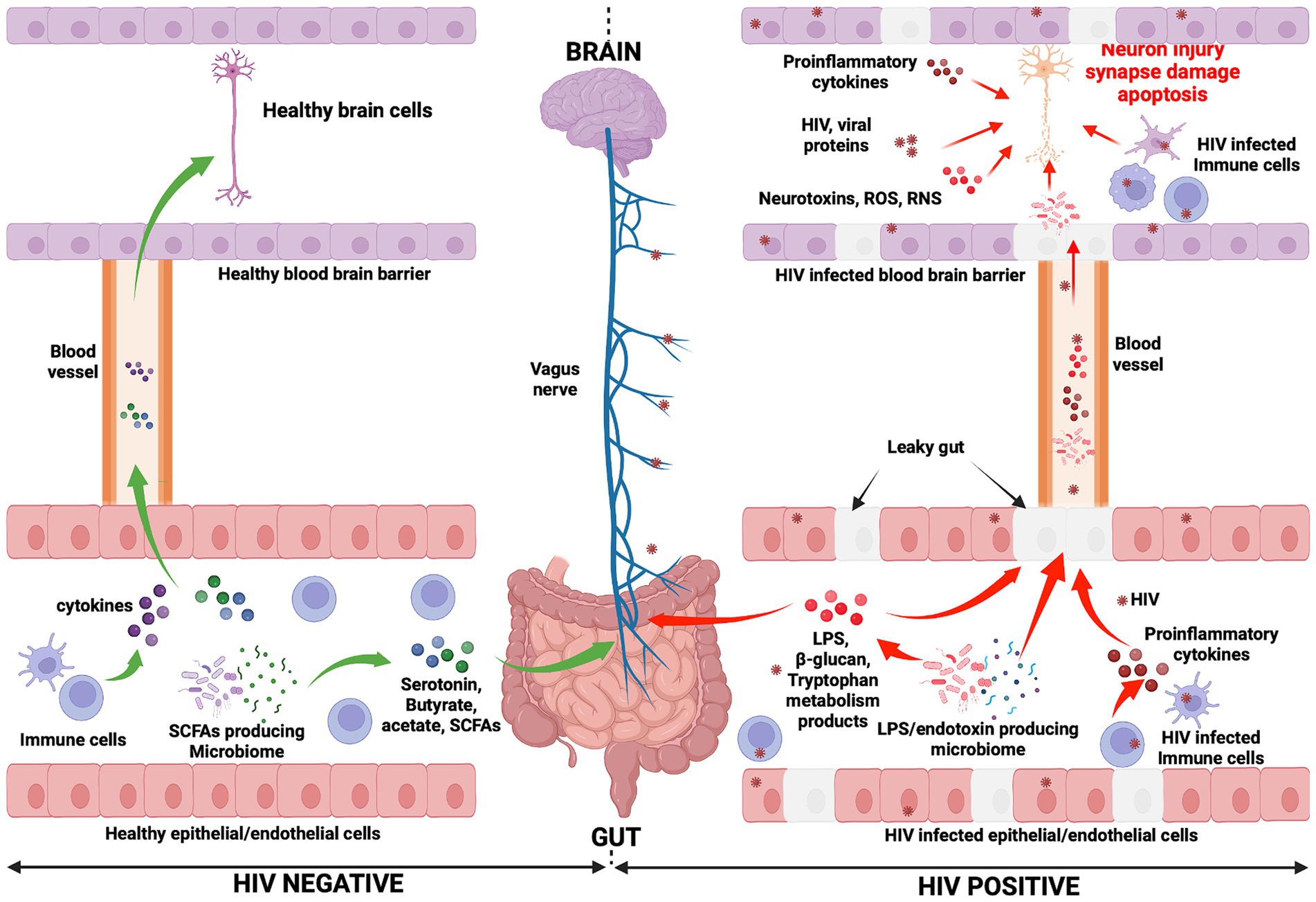
Figure 1. Mechanisms potentially influencing HAND manifestation. In the HIV negative context, immune cells produce cytokines and influence the functioning of the brain (negatively or positively depending on the presence of inflammation or not, respectively) (Haroon et al., 2014, 2016; Haroon and Miller, 2017). They reach the brain through the systemic circulation. Gut microbiota, particularly SCFA-producing microbiota, induce the production of neurotransmitters (serotonin) and metabolites (acetate, butyrate, and SCFAs). Consequently, the vagus nerve is stimulated by these molecules and transmits signals to the brain (Needham et al., 2020). Neurotransmitters and metabolites are able to cross the blood–brain barrier (via the systemic circulation) to subsequently directly affect brain health. Conversely, in the context of HIV infection, the gut epithelial/endothelial barrier and the blood brain barrier are disrupted and consequently are unable to selectively filter elements, which originate in the gut and consequently enter the brain. The production of different dysfunctional elements are also promoted. SCFA-producing microbiota are depleted and replaced by LPS/endotoxin-producing microbiota. The latter are potentially pathogenic microbes, which produce endotoxins. The reduction of SCFA-producing microbiota induces a reduction in the production of serotonin and other beneficial metabolites (butyrate, acetate), while the production of LPS and other endotoxins are promoted by LPS/endotoxin-producing microbiota. In the HIV infection context, immune cells are depleted and produce proinflammatory cytokines, which exert a negative influence on brain cell development and functioning. The preceding elements (LPS, endotoxins, and proinflammatory cytokines) cause abnormal functioning of the vagus nerve, which may induce a sluggish intestinal transit time (constipation) which further promotes the growth of pathogenic bacteria within the gut (George et al., 2014). Furthermore, the preceding toxic elements may then easily reach the brain through the systemic circulation. Within the brain, endotoxins, HIV-infected immune cells (microglia, astrocytes, and macrophages, which produce proinflammatory cytokines, neurotoxins, ROS, and RNS), specific pathogenic microbes (potentially less likely), and HIV viral proteins work together to further compromise neuronal integrity, resulting in neuronal damage, synapse destruction, and ultimately neuronal death (Navarathna et al., 2016; Zhu et al., 2020). The preceding events which occur during HIV infection may well be seen as the potential pathogenic mechanisms whereby the gut-brain axis induces HAND development. ROS, Reactive oxygen species; RNS, Reactive nitrogen species.
6 Conclusion and perspectives
It is important to remember that HIV is a neurotropic virus which has the ability to affect the brain and to disrupt brain functions, and this may well account for the development of HAND. However, cumulative research evidence points, also, to the influential role of the microbiota-gut-brain axis in HAND development. Indeed, HIV-related gut associated dysbiosis syndrome, translocated microbial products, and sustained systemic inflammation are key features of HIV infection, which profoundly disrupts the homeostasis of both the gut and the brain. It is known that the gut and the brain interact via elements such as the autonomic nervous system, microbiota and their metabolites, the immune system, and the endocrine system. Herein, we have considered and discussed how any change that occurs in the composition or functions of the preceding elements as a consequence of HIV infection may significantly dysregulate homeostasis of the gut-brain axis, and thus encourage the pathogenesis of HAND. The information reported in this article represents nuggets of compelling evidence, which exposes the potentially fundamental role played by the microbiota-gut-brain axis in the development of HAND. We have shown that HAND may result from inflammation, HIV-associated gut dysbiosis syndrome, and the leaky gut. As known consequences of HIV infection, these processes induce profound alterations in microbial metabolite composition (SCFA, serotonin, and tryptophan) and favor immune responses which, cumulatively, result in neuronal damage and/or inadequate neuronal signaling (Figure 2).
Despite the preceding observations, further evidence is required to establish the precise role of the microbiota-gut-brain axis during HAND pathogenesis. As such, it is necessary to further explore the correlation between specific gut microbiota components and HAND in future research. Perhaps interventions using antibiotics, probiotics, and fecal microbiota transplantation (FMT) may (i) regulate composition and metabolites of gut microbiota, (ii) regulate the homeostasis of the microbiota-gut-brain axis, and (iii) thereby prevent or alleviate potential neurological diseases in PLWH. Apart from dietary interventions, the administration of cannabinoid based drugs [such as dronabinol, a synthetic version of delta-9-tetrahydrocannabinol (THC)] at low dose may (i) regulate the microbiome-gut-brain axis, (ii) reduce neuroinflammation, and (iii) mitigate HAND pathogenesis in the context of HIV infection, as has been demonstrated recently (McDew-White et al., 2023).
Despite this curated narrative review of the contemporary literature being focused on the relationship between HIV infection, gut microbiota dysbiosis, and HAND, the absence of original data represents a major limitation to this work. Nonetheless, we believe that our work may serve to pave the way toward robust future investigations (particularly clinical trials) in this compelling area of endeavor.
Author contributions
AH: Writing – original draft. SZ: Writing – original draft. VH: Writing – review & editing. XW: Conceptualization, Writing – review & editing. JO: Conceptualization, Writing – review & editing. YC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Medical Research Project (No. CSTB2023TIAD-KPX0063-3) of Chongqing Science & Technology Bureau, the Chongqing Public Health Medical Center Research Initiation Fund (KYLW202321), and the Joint Medical Research Projects of Chongqing Health Committee and Chongqing Science and Technology Bureau (2024ZDXM015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Haq, R., Schlachetzki, J. C. M., Glass, C. K., and Mazmanian, S. K. (2019). Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 216, 41–59. doi: 10.1084/jem.20180794
Abreu, C., Shirk, E. N., Queen, S. E., Beck, S. E., Mangus, L. M., Pate, K. A. M., et al. (2019). Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS 33, S181–S188. doi: 10.1097/qad.0000000000002269
Achim, C. L., Heyes, M. P., and Wiley, C. A. (1993). Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J. Clin. Invest. 91, 2769–2775. doi: 10.1172/jci116518
Agirman, G., and Hsiao, E. Y. (2021). SnapShot: the microbiota-gut-brain axis. Cell 184:2524. doi: 10.1016/j.cell.2021.03.022
Agirman, G., Yu, K. B., and Hsiao, E. Y. (2021). Signaling inflammation across the gut-brain axis. Science 374, 1087–1092. doi: 10.1126/science.abi6087
Ahmed, D., Roy, D., and Cassol, E. (2018). Examining relationships between metabolism and persistent inflammation in HIV patients on antiretroviral therapy. Mediat. Inflamm. 2018:6238978. doi: 10.1155/2018/6238978
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257. doi: 10.1016/j.jad.2016.05.038
Alakkas, A., Ellis, R. J., Watson, C. W., Umlauf, A., Heaton, R. K., Letendre, S., et al. (2019). White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neuro-Oncol. 25, 32–41. doi: 10.1007/s13365-018-0682-9
Alpino, G., Pereira-Sol, G. A., Dias, M. M. E., Aguiar, A. S., and Peluzio, M. (2022). Beneficial effects of butyrate on brain functions: a view of epigenetic. Crit. Rev. Food Sci. Nutr. 64, 3961–3970. doi: 10.1080/10408398.2022.2137776
Ancuta, P., Kamat, A., Kunstman, K. J., Kim, E. Y., Autissier, P., Wurcel, A., et al. (2008). Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 3:e2516. doi: 10.1371/journal.pone.0002516
Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b
Araínga, M., Edagwa, B., Mosley, R. L., Poluektova, L. Y., Gorantla, S., and Gendelman, H. E. (2017). A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology 14:17. doi: 10.1186/s12977-017-0344-7
Armstrong, A. J. S., Shaffer, M., Nusbacher, N. M., Griesmer, C., Fiorillo, S., Schneider, J. M., et al. (2018). An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome 6:198. doi: 10.1186/s40168-018-0580-7
Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y., et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. doi: 10.1126/science.1198469
Bale, T. L., Baram, T. Z., Brown, A. S., Goldstein, J. M., Insel, T. R., McCarthy, M. M., et al. (2010). Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. doi: 10.1016/j.biopsych.2010.05.028
Banks, W. A., and Erickson, M. A. (2010). The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 37, 26–32. doi: 10.1016/j.nbd.2009.07.031
Bedarf, J. R., Hildebrand, F., Coelho, L. P., Sunagawa, S., Bahram, M., Goeser, F., et al. (2017). Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. 9:39. doi: 10.1186/s13073-017-0428-y
Beilharz, J. E., Maniam, J., and Morris, M. J. (2016). Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 306, 1–7. doi: 10.1016/j.bbr.2016.03.018
Benakis, C., Brea, D., Caballero, S., Faraco, G., Moore, J., Murphy, M., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523. doi: 10.1038/nm.4068
Benakis, C., Martin-Gallausiaux, C., Trezzi, J. P., Melton, P., Liesz, A., and Wilmes, P. (2020). The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 61, 1–9. doi: 10.1016/j.conb.2019.11.009
Berger, M., Gray, J. A., and Roth, B. L. (2009). The expanded biology of serotonin. Annu. Rev. Med. 60, 355–366. doi: 10.1146/annurev.med.60.042307.110802
Bilbo, S. D., Levkoff, L. H., Mahoney, J. H., Watkins, L. R., Rudy, J. W., and Maier, S. F. (2005). Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 119, 293–301. doi: 10.1037/0735-7044.119.1.293
Bonnin, A., Goeden, N., Chen, K., Wilson, M. L., King, J., Shih, J. C., et al. (2011). A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350. doi: 10.1038/nature09972
Borre, Y. E., O'Keeffe, G. W., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 20, 509–518. doi: 10.1016/j.molmed.2014.05.002
Bourassa, M. W., Alim, I., Bultman, S. J., and Ratan, R. R. (2016). Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci. Lett. 625, 56–63. doi: 10.1016/j.neulet.2016.02.009
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut Axis in psychiatric and inflammatory disorders. Front. Psychol. 9:44. doi: 10.3389/fpsyt.2018.00044
Brenchley, J. M., Schacker, T. W., Ruff, L. E., Price, D. A., Taylor, J. H., Beilman, G. J., et al. (2004). CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759. doi: 10.1084/jem.20040874
Caballero, J., and Nahata, M. C. (2005). Use of selective serotonin-reuptake inhibitors in the treatment of depression in adults with HIV. Ann. Pharmacother. 39, 141–145. doi: 10.1345/aph.1E248
Capuron, L., Schroecksnadel, S., Féart, C., Aubert, A., Higueret, D., Barberger-Gateau, P., et al. (2011). Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol. Psychiatry 70, 175–182. doi: 10.1016/j.biopsych.2010.12.006
Carabotti, M., Scirocco, A., Maselli, M. A., and Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209
Ceccarelli, G., Brenchley, J. M., Cavallari, E. N., Scheri, G. C., Fratino, M., Pinacchio, C., et al. (2017). Impact of high-dose multi-strain probiotic supplementation on neurocognitive performance and central nervous system immune activation of HIV-1 infected individuals. Nutrients 9:1269. doi: 10.3390/nu9111269
Ceccarelli, G., Fratino, M., Selvaggi, C., Giustini, N., Serafino, S., Schietroma, I., et al. (2017). A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav. 7:e00756. doi: 10.1002/brb3.756
Chang, L., Wang, G. J., Volkow, N. D., Ernst, T., Telang, F., Logan, J., et al. (2008). Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage 42, 869–878. doi: 10.1016/j.neuroimage.2008.05.011
Chávez-Galán, L., Arenas-Del Angel, M. C., Zenteno, E., Chávez, R., and Lascurain, R. (2009). Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 6, 15–25. doi: 10.1038/cmi.2009.3
Chibanda, D., Benjamin, L., Weiss, H. A., and Abas, M. (2014). Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J. Acquir. Immune Defic. Syndr. 67, S54–S67. doi: 10.1097/qai.0000000000000258
Choi, J. G., Kim, N., Ju, I. G., Eo, H., Lim, S. M., Jang, S. E., et al. (2018). Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 8:1275. doi: 10.1038/s41598-018-19646-x
Cirino, T. J., Alleyne, A. R., Duarte, V., Figueroa, A., Simons, C. A., Anceaume, E. M., et al. (2022). Expression of human immunodeficiency virus transactivator of transcription (HIV-tat(1-86)) protein alters nociceptive processing that is sensitive to anti-oxidant and anti-inflammatory interventions. J. NeuroImmune Pharmacol. 17, 152–164. doi: 10.1007/s11481-021-09985-4
Clifford, D. B., and Ances, B. M. (2013). HIV-associated neurocognitive disorder. Lancet Infect. Dis. 13, 976–986. doi: 10.1016/s1473-3099(13)70269-x
Cooper, A., García, M., Petrovas, C., Yamamoto, T., Koup, R. A., and Nabel, G. J. (2013). HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498, 376–379. doi: 10.1038/nature12274
Cryan, J. F., and Leonard, B. E. (2000). 5-HT1A and beyond: the role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol. 15, 113–135. doi: 10.1002/(SICI)1099-1077(200003)15:2<113::AID-HUP150>3.0.CO;2-W
Cryan, J. F., and O’Mahony, S. M. (2011). The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol. Motil. 23, 187–192. doi: 10.1111/j.1365-2982.2010.01664.x
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Cysique, L. A., Jugé, L., Gates, T., Tobia, M., Moffat, K., Brew, B. J., et al. (2018). Covertly active and progressing neurochemical abnormalities in suppressed HIV infection. Neurol. Neuroimmunol. Neuroinflamm. 5:e430. doi: 10.1212/nxi.0000000000000430
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
Davies, N. W., Guillemin, G., and Brew, B. J. (2010). Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int. J. Tryptophan. Res. 3, IJTR.S4321–IJTR.S4140. doi: 10.4137/ijtr.s4321
De Vadder, F., Grasset, E., Mannerås Holm, L., Karsenty, G., Macpherson, A. J., Olofsson, L. E., et al. (2018). Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 115, 6458–6463. doi: 10.1073/pnas.1720017115
de Waele, J. J. (2021). Editorial: the gastrointestinal system in critical care: current insights and perspectives. Curr. Opin. Crit. Care 27, 139–140. doi: 10.1097/mcc.0000000000000807
DellaGioia, N., and Hannestad, J. (2010). A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci. Biobehav. Rev. 34, 130–143. doi: 10.1016/j.neubiorev.2009.07.014
Desbonnet, L., Clarke, G., Traplin, A., O'Sullivan, O., Crispie, F., Moloney, R. D., et al. (2015). Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 48, 165–173. doi: 10.1016/j.bbi.2015.04.004
Deusch, S., Serrano-Villar, S., Rojo, D., Martínez-Martínez, M., Bargiela, R., Vázquez-Castellanos, J. F., et al. (2018). Effects of HIV, antiretroviral therapy and prebiotics on the active fraction of the gut microbiota. AIDS 32, 1229–1237. doi: 10.1097/qad.0000000000001831
Dillon, S. M., Frank, D. N., and Wilson, C. C. (2016). The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS 30, 2737–2751. doi: 10.1097/qad.0000000000001289
Dillon, S. M., Lee, E. J., Kotter, C. V., Austin, G. L., Dong, Z., Hecht, D. K., et al. (2014). An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 7, 983–994. doi: 10.1038/mi.2013.116
Dinh, D. M., Volpe, G. E., Duffalo, C., Bhalchandra, S., Tai, A. K., Kane, A. V., et al. (2015). Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 211, 19–27. doi: 10.1093/infdis/jiu409
Doitsh, G., Cavrois, M., Lassen, K. G., Zepeda, O., Yang, Z., Santiago, M. L., et al. (2010). Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143, 789–801. doi: 10.1016/j.cell.2010.11.001
Dong, R., Lin, H., Chen, X., Shi, R., Yuan, S., Li, J., et al. (2021). Gut microbiota and fecal metabolites associated with neurocognitive impairment in HIV-infected population. Front. Cell. Infect. Microbiol. 11:723840. doi: 10.3389/fcimb.2021.723840
Dror, D. K., and Allen, L. H. (2008). Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr. Rev. 66, 250–255. doi: 10.1111/j.1753-4887.2008.00031.x
Dubé, B., Benton, T., Cruess, D. G., and Evans, D. L. (2005). Neuropsychiatric manifestations of HIV infection and AIDS. J. Psychiatry Neurosci. 30, 237–246.
Dubourg, G., Lagier, J. C., Hüe, S., Surenaud, M., Bachar, D., Robert, C., et al. (2016). Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen. BMJ Open Gastroenterol. 3:e000080. doi: 10.1136/bmjgast-2016-000080
Durack, J., and Lynch, S. V. (2019). The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med. 216, 20–40. doi: 10.1084/jem.20180448
Eggers, C., Arendt, G., Hahn, K., Husstedt, I. W., Maschke, M., Neuen-Jacob, E., et al. (2017). HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 264, 1715–1727. doi: 10.1007/s00415-017-8503-2
Ellis, R., Langford, D., and Masliah, E. (2007). HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 8, 33–44. doi: 10.1038/nrn2040
Erny, D., Dokalis, N., Mezö, C., Castoldi, A., Mossad, O., Staszewski, O., et al. (2021). Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7. doi: 10.1016/j.cmet.2021.10.010
Erny, D., Hrabě de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Finegold, S. M. (2008). Therapy and epidemiology of autism—clostridial spores as key elements. Med. Hypotheses 70, 508–511. doi: 10.1016/j.mehy.2007.07.019
Finegold, S. M., Molitoris, D., Song, Y., Liu, C., Vaisanen, M. L., Bolte, E., et al. (2002). Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 35, S6–S16. doi: 10.1086/341914
Foster, J. A., and McVey Neufeld, K. A. (2013). Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
Freedman, S. N., Shahi, S. K., and Mangalam, A. K. (2018). The "gut feeling": breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 15, 109–125. doi: 10.1007/s13311-017-0588-x
Fröhlich, E. E., Farzi, A., Mayerhofer, R., Reichmann, F., Jačan, A., Wagner, B., et al. (2016). Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav. Immun. 56, 140–155. doi: 10.1016/j.bbi.2016.02.020
Fu, R., Jinnah, H., McKay, J. L., Miller, A. H., Felger, J. C., Farber, E. W., et al. (2023). Cerebrospinal fluid levels of 5-HIAA and dopamine in people with HIV and depression. J. Neuro-Oncol. 29, 440–448. doi: 10.1007/s13365-023-01142-2
Gao, K., Mu, C. L., Farzi, A., and Zhu, W. Y. (2020). Tryptophan metabolism: a link between the gut microbiota and brain. Adv. Nutr. 11, 709–723. doi: 10.1093/advances/nmz127
Gao, K., Pi, Y., Mu, C. L., Farzi, A., Liu, Z., and Zhu, W. Y. (2019). Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota-brain axis. J. Neurochem. 149, 641–659. doi: 10.1111/jnc.14709
Gao, K., Pi, Y., Mu, C. L., Peng, Y., Huang, Z., and Zhu, W. Y. (2018). Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 146, 219–234. doi: 10.1111/jnc.14333
Gao, G. X., Tang, H. L., Zhang, X., Xin, X. L., Feng, J., and Chen, X. Q. (2015). Invasive fungal infection caused by geotrichum capitatum in patients with acute lymphoblastic leukemia: a case study and literature review. Int. J. Clin. Exp. Med. 8, 14228–14235.
Gaynes, B. N., Pence, B. W., Atashili, J., O'Donnell, J. K., Njamnshi, A. K., Tabenyang, M. E., et al. (2015). Changes in HIV outcomes following depression care in a resource-limited setting: results from a pilot study in Bamenda, Cameroon. PLoS One 10:e0140001. doi: 10.1371/journal.pone.0140001
Geldenhuys, W. J., and Van der Schyf, C. J. (2011). Role of serotonin in Alzheimer's disease: a new therapeutic target? CNS Drugs 25, 765–781. doi: 10.2165/11590190-000000000-00000
George, N. S., Sankineni, A., and Parkman, H. P. (2014). Small intestinal bacterial overgrowth in gastroparesis. Dig. Dis. Sci. 59, 645–652. doi: 10.1007/s10620-012-2426-7
Ghare, S., Singhal, R., Bryant, V., Gautam, S., Tirumala, C. C., Srisailam, P. K., et al. (2022). Age-associated gut Dysbiosis, marked by loss of Butyrogenic potential, correlates with altered plasma tryptophan metabolites in older people living with HIV. J. Acquir. Immune Defic. Syndr. 89, S56–s64. doi: 10.1097/qai.0000000000002866
Goehler, L. E., Park, S. M., Opitz, N., Lyte, M., and Gaykema, R. P. (2008). Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 22, 354–366. doi: 10.1016/j.bbi.2007.08.009
González, H., Elgueta, D., Montoya, A., and Pacheco, R. (2014). Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 274, 1–13. doi: 10.1016/j.jneuroim.2014.07.012
Goodkin, K., Hardy, D. J., Singh, D., and Lopez, E. (2014). Diagnostic utility of the international HIV dementia scale for HIV-associated neurocognitive impairment and disorder in South Africa. J. Neuropsychiatr. Clin. Neurosci. 26, 352–358. doi: 10.1176/appi.neuropsych.13080178
Grant, I., Atkinson, J. H., Hesselink, J. R., Kennedy, C. J., Richman, D. D., Spector, S. A., et al. (1987). Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann. Intern. Med. 107, 828–836. doi: 10.7326/0003-4819-107-6-828
Grant, I., Franklin, D. R. Jr., Deutsch, R., Woods, S. P., Vaida, F., Ellis, R. J., et al. (2014). Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82, 2055–2062. doi: 10.1212/wnl.0000000000000492
Guillemin, G. J., Smith, D. G., Smythe, G. A., Armati, P. J., and Brew, B. J. (2003). Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 527, 105–112. doi: 10.1007/978-1-4615-0135-0_12
Guillemin, G. J., Smythe, G., Takikawa, O., and Brew, B. J. (2005). Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49, 15–23. doi: 10.1002/glia.20090
Gwak, M. G., and Chang, S. Y. (2021). Gut-brain connection: microbiome, gut barrier, and environmental sensors. Immune Netw. 21:e20. doi: 10.4110/in.2021.21.e20
Hakkers, C. S., Beunders, A. J. M., Ensing, M. H. M., Barth, R. E., Boelema, S., Devillé, W. L. J., et al. (2018). The Montreal cognitive assessment-basic (MoCA-B) is not a reliable screening tool for cognitive decline in HIV patients receiving combination antiretroviral therapy in rural South Africa. Int. J. Infect. Dis. 67, 36–40. doi: 10.1016/j.ijid.2017.11.024
Hamad, I., Abou Abdallah, R., Ravaux, I., Mokhtari, S., Tissot-Dupont, H., Michelle, C., et al. (2018). Metabarcoding analysis of eukaryotic microbiota in the gut of HIV-infected patients. PLoS One 13:e0191913. doi: 10.1371/journal.pone.0191913
Hammoud, D. A., Endres, C. J., Hammond, E., Uzuner, O., Brown, A., Nath, A., et al. (2010). Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. NeuroImage 49, 2588–2595. doi: 10.1016/j.neuroimage.2009.10.037
Haroon, E., Fleischer, C. C., Felger, J. C., Chen, X., Woolwine, B. J., Patel, T., et al. (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry 21, 1351–1357. doi: 10.1038/mp.2015.206
Haroon, E., and Miller, A. H. (2017). Inflammation effects on brain glutamate in depression: mechanistic considerations and treatment implications. Curr. Top. Behav. Neurosci. 31, 173–198. doi: 10.1007/7854_2016_40
Haroon, E., Woolwine, B. J., Chen, X., Pace, T. W., Parekh, S., Spivey, J. R., et al. (2014). IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39, 1777–1785. doi: 10.1038/npp.2014.25
Harry, G. J. (2013). Microglia during development and aging. Pharmacol. Ther. 139, 313–326. doi: 10.1016/j.pharmthera.2013.04.013
Heaton, R. K., Clifford, D. B., Franklin, D. R. Jr., Woods, S. P., Ake, C., Vaida, F., et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75, 2087–2096. doi: 10.1212/WNL.0b013e318200d727
Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14, 388–405. doi: 10.1016/s1474-4422(15)70016-5
Heyes, M. P., Brew, B. J., Martin, A., Price, R. W., Salazar, A. M., Sidtis, J. J., et al. (1991). Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann. Neurol. 29, 202–209. doi: 10.1002/ana.410290215
Heyes, M. P., Rubinow, D., Lane, C., and Markey, S. P. (1989). Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann. Neurol. 26, 275–277. doi: 10.1002/ana.410260215
Houser, M. C., and Tansey, M. G. (2017). The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. 3:3. doi: 10.1038/s41531-016-0002-0
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
Huengsberg, M., Winer, J. B., Gompels, M., Round, R., Ross, J., and Shahmanesh, M. (1998). Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin. Chem. 44, 858–862. doi: 10.1093/clinchem/44.4.858
Ishizaka, A., Koga, M., Mizutani, T., Parbie, P. K., Prawisuda, D., Yusa, N., et al. (2021). Unique gut microbiome in HIV patients on antiretroviral therapy (ART) suggests association with chronic inflammation. Microbiol. Spectr. 9:e0070821. doi: 10.1128/Spectrum.00708-21
Jiang, C., Li, G., Huang, P., Liu, Z., and Zhao, B. (2017). The gut microbiota and Alzheimer's disease. J. Alzheimers Dis. 58, 1–15. doi: 10.3233/jad-161141
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Kabouridis, P. S., Lasrado, R., McCallum, S., Chng, S. H., Snippert, H. J., Clevers, H., et al. (2015). Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 289–295. doi: 10.1016/j.neuron.2014.12.037
Kang, Y., and Cai, Y. (2019). Altered gut microbiota in HIV infection: future perspective of fecal microbiota transplantation therapy. AIDS Res. Hum. Retrovir. 35, 229–235. doi: 10.1089/aid.2017.0268
Kaul, M., Garden, G. A., and Lipton, S. A. (2001). Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994. doi: 10.1038/35073667
Kaur, H., Bose, C., and Mande, S. S. (2019). Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front. Neurosci. 13:1365. doi: 10.3389/fnins.2019.01365
Keegan, M. R., Chittiprol, S., Letendre, S. L., Winston, A., Fuchs, D., Boasso, A., et al. (2016). Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int. J. Tryptophan. Res. 9, 79–88. doi: 10.4137/ijtr.s36464
Kennedy, P. J., Cryan, J. F., Dinan, T. G., and Clarke, G. (2017). Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412. doi: 10.1016/j.neuropharm.2016.07.002
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson's disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kim, M. S., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., et al. (2020). Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model. Gut 69, 283–294. doi: 10.1136/gutjnl-2018-317431
Kim, S., Kwon, S. H., Kam, T. I., Panicker, N., Karuppagounder, S. S., Lee, S., et al. (2019). Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron 103, 627–641.e7. doi: 10.1016/j.neuron.2019.05.035
Kotler, D. P. (2005). HIV infection and the gastrointestinal tract. AIDS 19, 107–117. doi: 10.1097/00002030-200501280-00002
Lackner, A. A., Mohan, M., and Veazey, R. S. (2009). The gastrointestinal tract and AIDS pathogenesis. Gastroenterology 136, 1965–1978. doi: 10.1053/j.gastro.2008.12.071
Lajoie, J., Kowatsch, M. M., Mwangi, L. W., Boily-Larouche, G., Oyugi, J., Chen, Y., et al. (2021). Low-dose acetylsalicylic acid reduces T cell immune activation: potential implications for HIV prevention. Front. Immunol. 12:778455. doi: 10.3389/fimmu.2021.778455
Lam, J. O., Hou, C. E., Hojilla, J. C., Anderson, A. N., Gilsanz, P., Alexeeff, S. E., et al. (2021). Comparison of dementia risk after age 50 between individuals with and without HIV infection. AIDS 35, 821–828. doi: 10.1097/qad.0000000000002806
Lam, J. O., Lee, C., Gilsanz, P., Hou, C. E., Leyden, W. A., Satre, D. D., et al. (2022). Comparison of dementia incidence and prevalence between individuals with and without HIV infection in primary care from 2000 to 2016. AIDS 36, 437–445. doi: 10.1097/qad.0000000000003134
Landete, J. M., De Las Rivas, B., Marcobal, A., and Muñoz, R. (2008). Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 48, 697–714. doi: 10.1080/10408390701639041
Larsson, M., Hagberg, L., Norkrans, G., and Forsman, A. (1989). Indole amine deficiency in blood and cerebrospinal fluid from patients with human immunodeficiency virus infection. J. Neurosci. Res. 23, 441–446. doi: 10.1002/jnr.490230410
Latorre, R., Sternini, C., De Giorgio, R., and Greenwood-Van Meerveld, B. (2016). Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 28, 620–630. doi: 10.1111/nmo.12754
Launay, J. M., Copel, L., Callebert, J., Corvaïa, N., Lepage, E., Bricaire, F., et al. (1988). Decreased whole blood 5-hydroxytryptamine (serotonin) in AIDS patients. J. Acquir. Immune Defic. Syndr. 1, 324–325
Lee, S. C., Chua, L. L., Yap, S. H., Khang, T. F., Leng, C. Y., Raja Azwa, R. I., et al. (2018). Enrichment of gut-derived Fusobacterium is associated with suboptimal immune recovery in HIV-infected individuals. Sci. Rep. 8:14277. doi: 10.1038/s41598-018-32585-x
Li, S. X., Armstrong, A., Neff, C. P., Shaffer, M., Lozupone, C. A., and Palmer, B. E. (2016). Complexities of gut microbiome dysbiosis in the context of HIV infection and antiretroviral therapy. Clin. Pharmacol. Ther. 99, 600–611. doi: 10.1002/cpt.363
Li, J. M., Yu, R., Zhang, L. P., Wen, S. Y., Wang, S. J., Zhang, X. Y., et al. (2019). Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome 7:98. doi: 10.1186/s40168-019-0713-7
Ling, Z., Jin, C., Xie, T., Cheng, Y., Li, L., and Wu, N. (2016). Alterations in the fecal microbiota of patients with HIV-1 infection: an observational study in a Chinese population. Sci. Rep. 6:30673. doi: 10.1038/srep30673
Liu, S., Gao, J., Zhu, M., Liu, K., and Zhang, H. L. (2020). Gut microbiota and dysbiosis in Alzheimer's disease: implications for pathogenesis and treatment. Mol. Neurobiol. 57, 5026–5043. doi: 10.1007/s12035-020-02073-3
Liu, Y., McNevin, J. P., Holte, S., McElrath, M. J., and Mullins, J. I. (2011). Dynamics of viral evolution and CTL responses in HIV-1 infection. PLoS One 6:e15639. doi: 10.1371/journal.pone.0015639
Lozupone, C. A., Li, M., Campbell, T. B., Flores, S. C., Linderman, D., Gebert, M. J., et al. (2013). Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14, 329–339. doi: 10.1016/j.chom.2013.08.006
Lu, W., Feng, Y., Jing, F., Han, Y., Lyu, N., Liu, F., et al. (2018). Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front. Microbiol. 9:1451. doi: 10.3389/fmicb.2018.01451
Lukić, I., Getselter, D., Koren, O., and Elliott, E. (2019). Role of tryptophan in microbiota-induced depressive-like behavior: evidence from tryptophan depletion study. Front. Behav. Neurosci. 13:123. doi: 10.3389/fnbeh.2019.00123
Luo, Z., Health, S. L., Li, M., Yang, H., Wu, Y., Collins, M., et al. (2022). Variation in blood microbial lipopolysaccharide (LPS) contributes to immune reconstitution in response to suppressive antiretroviral therapy in HIV. EBioMedicine 80:104037. doi: 10.1016/j.ebiom.2022.104037
MacCann, R., Landay, A. L., and Mallon, P. W. G. (2023). HIV and comorbidities—the importance of gut inflammation and the kynurenine pathway. Curr. Opin. HIV AIDS 18, 102–110. doi: 10.1097/coh.0000000000000782
Maek, A. N. W., Buranapraditkun, S., Klaewsongkram, J., and Ruxrungtham, K. (2007). Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 20, 66–75. doi: 10.1089/vim.2006.0063
Magnusson, K. R., Hauck, L., Jeffrey, B. M., Elias, V., Humphrey, A., Nath, R., et al. (2015). Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 300, 128–140. doi: 10.1016/j.neuroscience.2015.05.016
Malmevik, J., Petri, R., Knauff, P., Brattås, P. L., Åkerblom, M., and Jakobsson, J. (2016). Distinct cognitive effects and underlying transcriptome changes upon inhibition of individual miRNAs in hippocampal neurons. Sci. Rep. 6:19879. doi: 10.1038/srep19879
Manyevitch, R., Protas, M., Scarpiello, S., Deliso, M., Bass, B., Nanajian, A., et al. (2018). Evaluation of metabolic and synaptic dysfunction hypotheses of Alzheimer's disease (AD): a meta-analysis of CSF markers. Curr. Alzheimer Res. 15, 164–181. doi: 10.2174/1567205014666170921122458
Marques, T. M., Wall, R., Ross, R. P., Fitzgerald, G. F., Ryan, C. A., and Stanton, C. (2010). Programming infant gut microbiota: influence of dietary and environmental factors. Curr. Opin. Biotechnol. 21, 149–156. doi: 10.1016/j.copbio.2010.03.020
Martinez, P., Tsai, A. C., Muzoora, C., Kembabazi, A., Weiser, S. D., Huang, Y., et al. (2014). Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J. Acquir. Immune Defic. Syndr. 65, 456–462. doi: 10.1097/qai.0000000000000062
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut-brain axis. Annu. Rev. Med. 73, 439–453. doi: 10.1146/annurev-med-042320-014032
McArthur, J. C., and Johnson, T. P. (2020). Chronic inflammation mediates brain injury in HIV infection: relevance for cure strategies. Curr. Opin. Neurol. 33, 397–404. doi: 10.1097/wco.0000000000000807
McDew-White, M., Lee, E., Premadasa, L. S., Alvarez, X., Okeoma, C. M., and Mohan, M. (2023). Cannabinoids modulate the microbiota-gut-brain axis in HIV/SIV infection by reducing neuroinflammation and dysbiosis while concurrently elevating endocannabinoid and indole-3-propionate levels. J. Neuroinflammation 20:62. doi: 10.1186/s12974-023-02729-6
McHardy, I. H., Li, X., Tong, M., Ruegger, P., Jacobs, J., Borneman, J., et al. (2013). HIV infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome 1:26. doi: 10.1186/2049-2618-1-26
Megur, A., Baltriukienė, D., Bukelskienė, V., and Burokas, A. (2020). The microbiota-gut-brain axis and Alzheimer's disease: neuroinflammation is to blame? Nutrients 13:37. doi: 10.3390/nu13010037
Mehandru, S., Tenner-Racz, K., Racz, P., and Markowitz, M. (2005). The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. J. Allergy Clin. Immunol. 116, 419–422. doi: 10.1016/j.jaci.2005.05.040
Mehraj, V., Ramendra, R., Isnard, S., Dupuy, F. P., Ponte, R., Chen, J., et al. (2020). Circulating (1→3)-β-D-glucan is associated with immune activation during human immunodeficiency virus infection. Clin. Infect. Dis. 70, 232–241. doi: 10.1093/cid/ciz212
Meyer, A. L. (2022). Need to revise Frascati criteria for HIV-associated neurocognitive disorders to improve relevance for diverse global populations. Neurol. Clin. Pract. 12, 328–330. doi: 10.1212/cpj.0000000000200063
Millán Solano, M. V., Salinas Lara, C., Sánchez-Garibay, C., Soto-Rojas, L. O., Escobedo-Ávila, I., Tena-Suck, M. L., et al. (2023). Effect of systemic inflammation in the CNS: a silent history of neuronal damage. Int. J. Mol. Sci. 24:11902. doi: 10.3390/ijms241511902
Mirzaei, R., Bouzari, B., Hosseini-Fard, S. R., Mazaheri, M., Ahmadyousefi, Y., Abdi, M., et al. (2021). Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 139:111661. doi: 10.1016/j.biopha.2021.111661
Mittal, V. A., Ellman, L. M., and Cannon, T. D. (2008). Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr. Bull. 34, 1083–1094. doi: 10.1093/schbul/sbn080
Mohamed, A. A., Oduor, C., and Kinyanjui, D. (2020). HIV-associated neurocognitive disorders at Moi teaching and referral hospital, Eldoret, Kenya. BMC Neurol. 20:280. doi: 10.1186/s12883-020-01857-3
Möhle, L., Mattei, D., Heimesaat, M. M., Bereswill, S., Fischer, A., Alutis, M., et al. (2016). Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956. doi: 10.1016/j.celrep.2016.04.074
Moloney, G. M., O'Leary, O. F., Salvo-Romero, E., Desbonnet, L., Shanahan, F., Dinan, T. G., et al. (2017). Microbial regulation of hippocampal miRNA expression: implications for transcription of kynurenine pathway enzymes. Behav. Brain Res. 334, 50–54. doi: 10.1016/j.bbr.2017.07.026
Monaco, C. L., Gootenberg, D. B., Zhao, G., Handley, S. A., Ghebremichael, M. S., Lim, E. S., et al. (2016). Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe 19, 311–322. doi: 10.1016/j.chom.2016.02.011
Morais, L. H., Schreiber, H. L. T., and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Mutlu, E. A., Keshavarzian, A., Losurdo, J., Swanson, G., Siewe, B., Forsyth, C., et al. (2014). A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 10:e1003829. doi: 10.1371/journal.ppat.1003829
Mwangala, P. N., Newton, C. R., Abas, M., and Abubakar, A. (2018). Screening tools for HIV-associated neurocognitive disorders among adults living with HIV in sub-Saharan Africa: a scoping review. AAS Open Res. 1:28. doi: 10.12688/aasopenres.12921.2
Nagano-Saito, A., Liu, J., Doyon, J., and Dagher, A. (2009). Dopamine modulates default mode network deactivation in elderly individuals during the tower of London task. Neurosci. Lett. 458, 1–5. doi: 10.1016/j.neulet.2009.04.025
Navarathna, D. H., Roberts, D. D., Munasinghe, J., and Lizak, M. J. (2016). Imaging Candida infections in the host. Methods Mol. Biol. 1356, 69–78. doi: 10.1007/978-1-4939-3052-4_6
Needham, B. D., Kaddurah-Daouk, R., and Mazmanian, S. K. (2020). Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 21, 717–731. doi: 10.1038/s41583-020-00381-0
Noguera-Julian, M., Rocafort, M., Guillén, Y., Rivera, J., Casadellà, M., Nowak, P., et al. (2016). Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 5, 135–146. doi: 10.1016/j.ebiom.2016.01.032
Nowak, P., Troseid, M., Avershina, E., Barqasho, B., Neogi, U., Holm, K., et al. (2015). Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 29, 2409–2418. doi: 10.1097/qad.0000000000000869
Oluwagbemigun, K., Schnermann, M. E., Schmid, M., Cryan, J. F., and Nöthlings, U. (2022). A prospective investigation into the association between the gut microbiome composition and cognitive performance among healthy young adults. Gut Pathog. 14:15. doi: 10.1186/s13099-022-00487-z
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi: 10.1016/j.bbr.2014.07.027
On Wah, D. T., Kavaliers, M., Bishnoi, I. R., and Ossenkopp, K. P. (2019). Lipopolysaccharide (LPS) induced sickness in early adolescence alters the behavioral effects of the short-chain fatty acid, propionic acid, in late adolescence and adulthood: examining anxiety and startle reactivity. Behav. Brain Res. 360, 312–322. doi: 10.1016/j.bbr.2018.12.003
Ouyang, J., Lin, J., Isnard, S., Fombuena, B., Peng, X., Marette, A., et al. (2020). The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation. Front. Immunol. 11:645. doi: 10.3389/fimmu.2020.00645
Oxenkrug, G. (2013). Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr. Drug Targets 14, 514–521. doi: 10.2174/1389450111314050002
Ozdener, H. (2005). Molecular mechanisms of HIV-1 associated neurodegeneration. J. Biosci. 30, 391–405. doi: 10.1007/bf02703676
Park, J., and Kim, C. H. (2021). Regulation of common neurological disorders by gut microbial metabolites. Exp. Mol. Med. 53, 1821–1833. doi: 10.1038/s12276-021-00703-x
Parker, A., Fonseca, S., and Carding, S. R. (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157. doi: 10.1080/19490976.2019.1638722
Passchier, R. V., Abas, M. A., Ebuenyi, I. D., and Pariante, C. M. (2018). Effectiveness of depression interventions for people living with HIV in sub-Saharan Africa: a systematic review & meta-analysis of psychological & immunological outcomes. Brain Behav. Immun. 73, 261–273. doi: 10.1016/j.bbi.2018.05.010
Poles, M. A., Elliott, J., Taing, P., Anton, P. A., and Chen, I. S. (2001). A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75, 8390–8399. doi: 10.1128/jvi.75.18.8390-8399.2001
Qi, Q., Hua, S., Clish, C. B., Scott, J. M., Hanna, D. B., Wang, T., et al. (2018). Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin. Infect. Dis. 67, 235–242. doi: 10.1093/cid/ciy053
Qing, Y., Xie, H., Su, C., Wang, Y., Yu, Q., Pang, Q., et al. (2019). Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection. Dig. Dis. Sci. 64, 1830–1843. doi: 10.1007/s10620-018-5428-2
Quigley, E. M. M. (2017). Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17:94. doi: 10.1007/s11910-017-0802-6
Raubinger, S., Lee, F. J., and Pinto, A. N. (2022). HIV: the changing paradigm. Intern. Med. J. 52, 542–549. doi: 10.1111/imj.15739
Rhee, S. J., Walker, W. A., and Cherayil, B. J. (2005). Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175, 1127–1136. doi: 10.4049/jimmunol.175.2.1127
Rich, S., Klann, E., Bryant, V., Richards, V., Wijayabahu, A., Bryant, K., et al. (2020). A review of potential microbiome-gut-brain axis mediated neurocognitive conditions in persons living with HIV. Brain Behav. Immun. Health 9:100168. doi: 10.1016/j.bbih.2020.100168
Richard, D. M., Dawes, M. A., Mathias, C. W., Acheson, A., Hill-Kapturczak, N., and Dougherty, D. M. (2009). L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2, IJTR.S2129–IJTR.S2160. doi: 10.4137/ijtr.s2129
Roth, W., Zadeh, K., Vekariya, R., Ge, Y., and Mohamadzadeh, M. (2021). Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 22:2973. doi: 10.3390/ijms22062973
Rothhammer, V., Mascanfroni, I. D., Bunse, L., Takenaka, M. C., Kenison, J. E., Mayo, L., et al. (2016). Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597. doi: 10.1038/nm.4106
Saiyasit, N., Chunchai, T., Prus, D., Suparan, K., Pittayapong, P., Apaijai, N., et al. (2020). Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 69:110576. doi: 10.1016/j.nut.2019.110576
Salminen, S., Bouley, C., Boutron-Ruault, M. C., Cummings, J. H., Franck, A., Gibson, G. R., et al. (1998). Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 80, S147–S171. doi: 10.1079/bjn19980108
Salvo-Romero, E., Stokes, P., and Gareau, M. G. (2020). Microbiota-immune interactions: from gut to brain. LymphoSign J. 7, 1–23. doi: 10.14785/lymphosign-2019-0018
San-Juan-Vergara, H., Zurek, E., Ajami, N. J., Mogollon, C., Peña, M., Portnoy, I., et al. (2018). A Lachnospiraceae-dominated bacterial signature in the fecal microbiota of HIV-infected individuals from Colombia, South America. Sci. Rep. 8:4479. doi: 10.1038/s41598-018-22629-7
Sanmarco, L. M., Wheeler, M. A., Gutiérrez-Vázquez, C., Polonio, C. M., Linnerbauer, M., Pinho-Ribeiro, F. A., et al. (2021). Gut-licensed IFNγ(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature 590, 473–479. doi: 10.1038/s41586-020-03116-4
Santos, F., Wegkamp, A., de Vos, W. M., Smid, E. J., and Hugenholtz, J. (2008). High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 74, 3291–3294. doi: 10.1128/aem.02719-07
Saylor, D., Dickens, A. M., Sacktor, N., Haughey, N., Slusher, B., Pletnikov, M., et al. (2016). HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat. Rev. Neurol. 12, 234–248. doi: 10.1038/nrneurol.2016.27
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Scheri, G. C., Fard, S. N., Schietroma, I., Mastrangelo, A., Pinacchio, C., Giustini, N., et al. (2017). Modulation of tryptophan/serotonin pathway by probiotic supplementation in human immunodeficiency virus-positive patients: preliminary results of a new study approach. Int. J. Tryptophan Res. 10:117864691771066. doi: 10.1177/1178646917710668
Schröder, C., Matthies, A., Engst, W., Blaut, M., and Braune, A. (2013). Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 79, 3494–3502. doi: 10.1128/aem.03693-12
Schroeder, F. A., Lin, C. L., Crusio, W. E., and Akbarian, S. (2007). Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 62, 55–64. doi: 10.1016/j.biopsych.2006.06.036
Schwarcz, R., Bruno, J. P., Muchowski, P. J., and Wu, H. Q. (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477. doi: 10.1038/nrn3257
Sei, S., Saito, K., Stewart, S. K., Crowley, J. S., Brouwers, P., Kleiner, D. E., et al. (1995). Increased human immunodeficiency virus (HIV) type 1 DNA content and quinolinic acid concentration in brain tissues from patients with HIV encephalopathy. J. Infect. Dis. 172, 638–647. doi: 10.1093/infdis/172.3.638
Serrano-Villar, S., Rojo, D., Martínez-Martínez, M., Deusch, S., Vázquez-Castellanos, J. F., Sainz, T., et al. (2016). HIV infection results in metabolic alterations in the gut microbiota different from those induced by other diseases. Sci. Rep. 6:26192. doi: 10.1038/srep26192
Sharma, I. (2021). Interrogating the impact of combination antiretroviral therapies on HIV-associated neurocognitive disorders. HIV Med. 22, 783–790. doi: 10.1111/hiv.13142
Shi, N., Li, N., Duan, X., and Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 4:14. doi: 10.1186/s40779-017-0122-9
Shkoporov, A. N., Khokhlova, E. V., Kulagina, E. V., Smeianov, V. V., Kafarskaia, L. I., and Efimov, B. A. (2008). Application of several molecular techniques to study numerically predominant Bifidobacterium spp. and Bacteroidales order strains in the feces of healthy children. Biosci. Biotechnol. Biochem. 72, 742–748. doi: 10.1271/bbb.70628
Shrestha, R., and Copenhaver, M. (2016). The influence of neurocognitive impairment on HIV risk behaviors and intervention outcomes among high-risk substance users: a systematic review. Front. Public Health 4:16. doi: 10.3389/fpubh.2016.00016
Soontornniyomkij, V. (2017). “Neuropathology of HIV-1 disease” in Global Virology II—HIV and NeuroAIDS (New York: Springer), 143–208.
Sperner-Unterweger, B., Kohl, C., and Fuchs, D. (2014). Immune changes and neurotransmitters: possible interactions in depression? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 48, 268–276. doi: 10.1016/j.pnpbp.2012.10.006
Stone, T. W. (1993). Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 45, 309–379
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403. doi: 10.1038/s41564-018-0307-3
Sullivan, R., Wilson, D. A., Feldon, J., Yee, B. K., Meyer, U., Richter-Levin, G., et al. (2006). The International Society for Developmental Psychobiology annual meeting symposium: impact of early life experiences on brain and behavioral development. Dev. Psychobiol. 48, 583–602. doi: 10.1002/dev.20170
Sun, Y., Ma, Y., Lin, P., Tang, Y. W., Yang, L., Shen, Y., et al. (2016). Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerg. Microbes Infect. 5:e31. doi: 10.1038/emi.2016.25
Sun, M. F., and Shen, Y. Q. (2018). Dysbiosis of gut microbiota and microbial metabolites in Parkinson's disease. Ageing Res. Rev. 45, 53–61. doi: 10.1016/j.arr.2018.04.004
Sun, J., Wang, F., Hong, G., Pang, M., Xu, H., Li, H., et al. (2016). Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 618, 159–166. doi: 10.1016/j.neulet.2016.03.003
Takiishi, T., Fenero, C. I. M., and Câmara, N. O. S. (2017). Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers 5:e1373208. doi: 10.1080/21688370.2017.1373208
Tao, D., Zhong, T., Pang, W., and Li, X. (2021). Saccharomyces boulardii improves the behaviour and emotions of spastic cerebral palsy rats through the gut-brain axis pathway. BMC Neurosci. 22:76. doi: 10.1186/s12868-021-00679-4
Tetz, G., Brown, S. M., Hao, Y., and Tetz, V. (2018). Parkinson's disease and bacteriophages as its overlooked contributors. Sci. Rep. 8:10812. doi: 10.1038/s41598-018-29173-4
The Global HIV and AIDS Epidemic (2023). Global HIV/AIDS Overview. Available at: https://www.hiv.gov/federal-response/pepfar-global-aids/global-hiv-aids-overview/ (Accessed May 6, 2024).
Thion, M. S., Low, D., Silvin, A., Chen, J., Grisel, P., Schulte-Schrepping, J., et al. (2018). Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16. doi: 10.1016/j.cell.2017.11.042
Thomas, C. M., Hong, T., van Pijkeren, J. P., Hemarajata, P., Trinh, D. V., Hu, W., et al. (2012). Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7:e31951. doi: 10.1371/journal.pone.0031951
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836. doi: 10.1042/bcj20160510
Tuddenham, S. A., Koay, W. L. A., Zhao, N., White, J. R., Ghanem, K. G., and Sears, C. L. (2020). The impact of human immunodeficiency virus infection on gut microbiota α-diversity: an individual-level meta-analysis. Clin. Infect. Dis. 70, 615–627. doi: 10.1093/cid/ciz258
UNAIDS (2022). Global Report. Available at: www.unaids.org (Accessed May 6, 2024).
Valle, M., Price, R. W., Nilsson, A., Heyes, M., and Verotta, D. (2004). CSF quinolinic acid levels are determined by local HIV infection: cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain 127, 1047–1060. doi: 10.1093/brain/awh130
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
Van Den Berge, N., Ferreira, N., Gram, H., Mikkelsen, T. W., Alstrup, A. K. O., Casadei, N., et al. (2019). Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 138, 535–550. doi: 10.1007/s00401-019-02040-w
Van Hove, H., Martens, L., Scheyltjens, I., De Vlaminck, K., Pombo Antunes, A. R., De Prijck, S., et al. (2019). A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035. doi: 10.1038/s41593-019-0393-4
van Marle, G., Sharkey, K. A., Gill, M. J., and Church, D. L. (2013). Gastrointestinal viral load and enteroendocrine cell number are associated with altered survival in HIV-1 infected individuals. PLoS One 8:e75967. doi: 10.1371/journal.pone.0075967
Vázquez-Castellanos, J. F., Serrano-Villar, S., Latorre, A., Artacho, A., Ferrús, M. L., Madrid, N., et al. (2015). Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 8, 760–772. doi: 10.1038/mi.2014.107
Vera, J., Guo, Q., Cole, J., Boasso, A., Greathead, L., Kelleher, P., et al. (2015). “Microbial translocation is associated with Neuroinflammation in HIV-infected subjects on ART” in The 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, WA.
Vesterbacka, J., Rivera, J., Noyan, K., Parera, M., Neogi, U., Calle, M., et al. (2017). Richer gut microbiota with distinct metabolic profile in HIV infected elite controllers. Sci. Rep. 7:6269. doi: 10.1038/s41598-017-06675-1
Vujkovic-Cvijin, I., Dunham, R. M., Iwai, S., Maher, M. C., Albright, R. G., Broadhurst, M. J., et al. (2013). Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 5:193ra91. doi: 10.1126/scitranslmed.3006438
Vujkovic-Cvijin, I., and Somsouk, M. (2019). HIV and the gut microbiota: composition, consequences, and avenues for amelioration. Curr. HIV/AIDS Rep. 16, 204–213. doi: 10.1007/s11904-019-00441-w
Wall, R., Marques, T. M., O'Sullivan, O., Ross, R. P., Shanahan, F., Quigley, E. M., et al. (2012). Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 95, 1278–1287. doi: 10.3945/ajcn.111.026435
Wallet, C., De Rovere, M., Van Assche, J., Daouad, F., De Wit, S., Gautier, V., et al. (2019). Microglial cells: the main HIV-1 reservoir in the brain. Front. Cell. Infect. Microbiol. 9:362. doi: 10.3389/fcimb.2019.00362
Wang, Y., Liu, M., Lu, Q., Farrell, M., Lappin, J. M., Shi, J., et al. (2020). Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95, e2610–e2621. doi: 10.1212/wnl.0000000000010752
Westfall, S., Lomis, N., Kahouli, I., Dia, S. Y., Singh, S. P., and Prakash, S. (2017). Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 74, 3769–3787. doi: 10.1007/s00018-017-2550-9
Wu, Y., Du, S., Johnson, J. L., Tung, H. Y., Landers, C. T., Liu, Y., et al. (2019). Microglia and amyloid precursor protein coordinate control of transient Candida cerebritis with memory deficits. Nat. Commun. 10:58. doi: 10.1038/s41467-018-07991-4
Yoo, B. B., and Mazmanian, S. K. (2017). The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46, 910–926. doi: 10.1016/j.immuni.2017.05.011
Younas, M., Psomas, C., Reynes, C., Cezar, R., Kundura, L., Portales, P., et al. (2019). Microbial translocation is linked to a specific immune activation profile in HIV-1-infected adults with suppressed viremia. Front. Immunol. 10:2185. doi: 10.3389/fimmu.2019.02185
Younas, M., Psomas, C., Reynes, J., and Corbeau, P. (2016). Immune activation in the course of HIV-1 infection: causes, phenotypes and persistence under therapy. HIV Med. 17, 89–105. doi: 10.1111/hiv.12310
Yu, G., Fadrosh, D., Ma, B., Ravel, J., and Goedert, J. J. (2014). Anal microbiota profiles in HIV-positive and HIV-negative MSM. AIDS 28, 753–760. doi: 10.1097/qad.0000000000000154
Zaongo, S. D., Harypursat, V., Rashid, F., Dahourou, D. L., Ouedraogo, A. S., and Chen, Y. (2023). Influence of HIV infection on cognition and overall intelligence in HIV-infected individuals: advances and perspectives. Front. Behav. Neurosci. 17:1261784. doi: 10.3389/fnbeh.2023.1261784
Zaongo, S. D., Ouyang, J., Chen, Y., Jiao, Y. M., Wu, H., and Chen, Y. (2022). HIV infection predisposes to increased chances of HBV infection: current understanding of the mechanisms favoring HBV infection at each clinical stage of HIV infection. Front. Immunol. 13:853346. doi: 10.3389/fimmu.2022.853346
Zenebe, Y., Akele, B. M. W. S., and Necho, M. (2021). A systematic review and meta-analysis of HIV associated neurocognitive disorders (HAND) among people with HIV in Ethiopia. AIDS Res. Ther. 18:99. doi: 10.1186/s12981-021-00424-1
Zhang, F., Yang, J., Ji, Y., Sun, M., Shen, J., Sun, J., et al. (2018). Gut microbiota dysbiosis is not independently associated with neurocognitive impairment in people living with HIV. Front. Microbiol. 9:3352. doi: 10.3389/fmicb.2018.03352
Zhao, J., Bi, W., Xiao, S., Lan, X., Cheng, X., Zhang, J., et al. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 9:5790. doi: 10.1038/s41598-019-42286-8
Zhou, Y., Ou, Z., Tang, X., Zhou, Y., Xu, H., Wang, X., et al. (2018). Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J. Cell. Mol. Med. 22, 2263–2271. doi: 10.1111/jcmm.13508
Zhu, N., Liu, W., Prakash, A., Zhang, C., and Kim, K. S. (2020). Targeting E. coli invasion of the blood-brain barrier for investigating the pathogenesis and therapeutic development of E. coli meningitis. Cell. Microbiol. 22:e13231. doi: 10.1111/cmi.13231
Keywords: HIV, gut microbiota, hand, neurocognitive disorder, microbiota-gutbrain axis
Citation: Hu A, Zaongo SD, Harypursat V, Wang X, Ouyang J and Chen Y (2024) HIV-associated neurocognitive disorder: key implications of the microbiota-gut-brain axis. Front. Microbiol. 15:1428239. doi: 10.3389/fmicb.2024.1428239
Edited by:
Yao Liu, Daping Hospital, ChinaReviewed by:
Zhenhua Zeng, Southern Medical University, ChinaYong Zhong Xu, McGill University Health Center, Canada
Copyright © 2024 Hu, Zaongo, Harypursat, Wang, Ouyang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, Mzc0NTQzNjNAcXEuY29t; Jing Ouyang, b3V5YW5namluZzgyMDQyMUAxMjYuY29t; Yaokai Chen, eWFva2FpY2hlbkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Aizhen Hu1†
Aizhen Hu1† Silvere D. Zaongo
Silvere D. Zaongo Vijay Harypursat
Vijay Harypursat Jing Ouyang
Jing Ouyang Yaokai Chen
Yaokai Chen