- 1Department of Biology, College of Science, University of Ha’il, Ha’il, Saudi Arabia
- 2Department of Biology, College of Science, Qassim University, Qassim, Saudi Arabia
- 3Faculty of Health and Life Sciences, INTI International University, Nilai, Malaysia
- 4Department of Physics, College of Science, Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia
- 5Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia
- 6College of Education, University of Ha’il, Ha’il, Saudi Arabia
Background: Rumex vesicarius is a wild leafy plant belonging to the family Polygonaceae, renowned for its therapeutic benefits. The genus Rumex comprises approximately 150 species distributed globally.
Objective: The study aimed to investigate the biological activities of R. vesicarius using in vitro and in silico methods.
Methods: Rumex vesicarius was collected from the mountains in Hail and extracted with methanol. The phytochemical composition was qualitatively determined using colorimetric detection methods. Additional analyses included elemental analysis, in silico docking, antioxidant, antibacterial, and anti-biofilm properties.
Results: The extract contained various classes of phytochemicals, including flavonoids, phenolics, tannins, terpenes, and saponins. Sixteen constituents were identified through molecular docking, revealing inhibition against the filamentous temperature-sensitive protein Z (FtsZ), a crucial factor in bacterial cell division. Six compounds exhibited low binding scores ranging from −8.3 to −5.0 kcal/mol, indicating efficient interaction at the active site. Elemental analysis identified 15 elements, with potassium being the most abundant, followed by calcium, aluminum, silicon, iron, phosphorus, sulfur, magnesium, titanium, strontium, zinc, manganese, bromine, and chromium. Antioxidant analysis revealed significant properties at lower concentrations compared to ascorbic acid, butylated hydroxytoluene, and β-carotene. Antibacterial analysis demonstrated inhibitory effects on Bacillus subtilis MTCC121 and Pseudomonas aeruginosa MTCC 741, with inhibition zones of 13.67 ± 1.0 mm and 11.50 ± 1.0 mm, respectively. The MIC and MBC values ranged from 250 to 500 μg/mL. R. vesicarius also exhibited anti-biofilm activity.
Conclusion: Wild-grown R. vesicarius from the mountains of Hail is rich in bioactive phytochemicals and essential minerals, exhibiting notable antioxidant and antibacterial properties.
Introduction
Presently, a diverse array of microbial pathogens represents a pervasive global menace. As per the World Health Organization (WHO), antimicrobial-resistant infections have attained the status of the third-leading cause of mortality on a worldwide scale (primarily bacteria) (Salam et al., 2023). Moreover, studies conducted in 2019 have documented that antimicrobial resistance accounted for approximately 4.95 million fatalities globally. The predominant involvement of multidrug-resistant pathogens, notably Gram-negative bacteria, is evident in most infectious cases (Oliva et al., 2023).
Ironically, microorganisms have evolved ingenious strategies to acquire resistance to drugs, such as forming biofilms. The EPS matrix, known as Extracellular Polymeric Substance, is a physical obstacle that restricts the entry of antibiotics and inhibits the efficient delivery of medications to microbial cells. In addition, microbes that live in biofilms have phenotypic alterations, including decreased rates of growth and modified metabolic pathways, which might make them less vulnerable to antibiotics. In addition, biofilms include persistent cells that are quiescent and physiologically inactive. These cells may avoid the effects of antibiotics and function as reservoirs for recurring infections (Rather et al., 2021a,b). Medicinal plants may serve as crucial agents in preventing the production of biofilms, as they utilize mechanisms distinct from antibiotics (Al-Mijalli et al., 2023a). Green nanotechnology has attracted considerable attention in the development of modern pharmaceuticals. Therefore, nanomaterials (NMs) have been considered “a wonder of contemporary medicine” and are promoted as powerful agents for fighting against biofilms (Rather et al., 2022; Rather and Mandal, 2023).
With an increasing global emphasis on health consciousness, there is a rising demand for herbal medicines due to their multiple advantages, including being safer, more affordable, and having fewer side effects than artificial modern drugs (Zhao et al., 2023). Medicinal plants are experiencing substantial demand in worldwide markets. As per the Food and Agriculture Organization (FAO), the international trade of herbal plants exhibited significant growth, escalating from 1.3 billion USD in 1996 to 120 billion USD in 2019 (Bhatkar and Shirkole, 2023). Considering that approximately 80% of the world’s population relies on medicinal plants for their primary healthcare, a promising future is seen for maintaining health using medicinal plants (Riasat et al., 2023).
Rumex vesicarius L. (R. vesicarius) is a member of the Polygonaceae family. This family contains 50 genera and approximately 1200 species of flowering weeds. Noteworthy among these are the genus Eriogonum, with 2410 species; Rumex, with 200 species; Cocoloba, with 120 species; and Persicaria, with 100 species, among others (Tonny et al., 2017). R. vesicarius is a consumable green leafy plant. Indigenous to the desert and semi-desert areas in Northern Africa and Southwest Asia. This weed exhibits an annual growth pattern, thriving particularly during the fall and spring rainy seasons (Beddou et al., 2015). In traditional medicine, R. vesicarius is employed by traditional healers to address a range of health concerns, including hepatic diseases, constipation, heart issues, indigestion, pain, spleen disorders, flatulence, dyspepsia, asthma, bronchitis, vomiting, toothache, piles, scabies, and leucoderma, as well as for its claimed properties as an appetizer, analgesic diuretic, stomachic, tonic, and laxative (El-Hawary et al., 2011; Mostafa et al., 2011; Salama et al., 2022).
The Hail region is in the northern, central part of Saudi Arabia. The natural plant life in the Hail area covers a significant expanse, coexisting with a diverse array of animals and plants. It is the home of various plant species, which Bedouins have used for multifaceted purposes, including as a source of food, herbal medicine, and nutrition for grazing animals (Sulieman et al., 2023a,b).
Local people use this plant for its many uses, including as an appetite stimulant, diuretic, astringent, remedy for snake and scorpion bites, and diarrhea; they consume roasted seeds as an antidote. Therefore, the current study aimed to explore the biological activities of this plant by investigating the chemical constituents of R. vesicarius grown wildly in harsh conditions of the mountainous area of Hail province, evaluating its antimicrobial properties, assessing antioxidant activity, and conducting docking analysis to predict molecular interactions.
Materials and methods
Sample collection and the study area
The botanical specimens of R. vesicarius, locally referred to as “Humaid,” were systematically gathered from Aja Mountain in the Hail region of Saudi Arabia during the spring season, spanning from March to May 2022, with temperature ranging from 27.9 to 33.2°C. We collected aerial parts from at least five individuals of the same plant. The collection site’s geographic coordinates are from 41°45′ E to 43°00′ E longitudes and from 27°25′ N to 30°00′ N latitudes. The study area, situated in the northern middle part of Saudi Arabia, encompasses an extensive expanse of 118,322 square kilometers, equivalent to 6% of the entire landmass of Saudi Arabia. This region contains an exposed complex of Precambrian igneous and metamorphic rocks, which form an integral part of the extensive Phanerozoic formations, overlaying the northern and eastern peripheries of the Arabian Shield. Aja Mountain, positioned between Hail town and the An-Nafud desert, is the largest mountain in this region. R. vesicarius is used by some Bedouins in the Hail region to treat digestive problems (personal communication). The vegetation of these mountains is notably influenced by the flora of Mediterranean countries, contributing to a distinctive ecological landscape. Most of the Hail region’s plant species are annual (Figure 1).

Figure 1. Map of Saudi Arabia showing the collection area of Rumex vesicarius. (A) Rumex vesicarius, (B) Location of Hail region in green.
Chemicals
All the chemicals, including methanol, ethanol, and chloroform, employed in the study were of analytical grade. These chemicals and indicators were in their original state without any purification processes. They procured from Merck (Merck®, USA). Folin–Ciocalteu’s phenol reagent (ACS reagent grade), aluminum chloride hexahydrate (reagent grade), quercetin [≥ 95% (HPLC), solid], H2SO4 (analytical grade ≥ 95%). Standard vitamin E (USP grade) and DPPH (2,2-Diphenyl-1-picrylhydrazyl) reagents (ACS reagent grade) were obtained from Sigma-Aldrich (St. Louis, MI, USA). Bacterial media were bought from Oxoid, UK.
Bacterial strains
Bacterial strains, namely B. subtilis (MTCC121) and P. aeruginosa (MTCC741), were obtained from the Microbial Type Culture Collection (MTCC) in Chandigarh, India, and cultured in our laboratory on Muller-Hinton agar. A singular bacterial colony was then transferred to fresh media and allowed to incubate overnight at 37°C, facilitating the development of bacterial cultures. The turbidity of the culture was adjusted to confirm the 0.5 McFarland standard (106 CFU/mL) using a sterile typical saline solution (0.9%).
Preparation of plant extract
Fresh samples of R. vesicarius aerial parts (whole plant) were collected from the mountains and identified by the Department of Biology, College of Science, University of Ha’il, Saudi Arabia. A specimen (voucher specimen no. HAU002021/2) was deposited. We dried the obtained R. vesicarius in the shade for up to a week. Subsequently, we ground the entire plant material using a mechanical grinder (Electric Grinder, OMCG2145, Olsenmark, New York, NY, USA) to achieve a finer particle size, optimizing solvent extraction efficiency. 100 g of the plant’s powder was macerated in 1,000 mL of 80% methanol for up to three days in darkness at room temperature (approximately 35–37°C) with regular agitation. Previous studies have shown that methanol is the optimal solvent for extracting plant compounds, as it yields the highest extraction efficiency, particularly with an 80% methanol solution (Anwar and Przybylski, 2012; Razali et al., 2012). The methanol was then evaporated, and the resulting extract was concentrated using a rotary vacuum evaporator (8 kW, 50 L, Henan Lanphan Industry Co., Ltd., Zhengzhou, China) (Sulieman et al., 2023a). Furthermore, the unfiltered extracts were dissolved in hexane and micro-filtered before injected into the GC-MS.
Qualitative phytochemical profile
The determination of major phytochemical compounds in the crude methanol extract was conducted using standard qualitative methods to detect saponins, terpenes, flavonoids, phenols, tannins, alkaloids, and cardiac glycosides, as reported by Banso and Adeyemo (2006) and Raaman (2006).
Quantitative tests for total phenolic, flavonoid and tannin
Determination of total phenolic content
The total phenolic content (TPC) of various extracts was measured using the Folin-Ciocalteu reagent, following the procedure outlined by Kumar et al. (2013). The samples were examined at a concentration of 1 mg/mL. Subsequently, the extract underwent a ten-fold dilution with deionized water and was placed in a test tube with 0.75 mL of Folin-Ciocalteu reagent, where it was stirred. The resultant mixture was allowed to stand at 25°C for 5 min. The liquid was gently mixed after adding 0.75 mL of a saturated sodium carbonate solution. The absorbance at 725 nm was then measured after 90 min at 25°C using a UV-Vis spectrophotometer. A calibration curve was developed using gallic acid, and the total phenolic content was quantified in terms of gallic acid equivalents (GAE) in mg of gallic acid/mg of dry weight.
Determination of total flavonoid content
The extracts’ total flavonoid concentration (TFC) was quantitatively determined following a previously published methodology with minor modifications (Zhao et al., 2018). A volume of 1.5 mL (1 mg/mL) of the extract was combined with an equal amount of 2% AlCl3–6H2O. After 10 min of incubation, the mixture underwent vigorous agitation, and the absorbance at 367 nm was recorded. A quercetin calibration line measured the total flavonoid content in milligrams of quercetin (mg QE) per gram of dry weight (mg QE/g). Each sample underwent three tests.
Determination of total tannin content
Tannins were quantified using a colorimetric method involving a modified vanillin test (Selvi et al., 2012). A volume of 1.5 mL of concentrated H2SO4 and 3 mL of a 4% methanolic vanillin solution was added to 50 mL of the extract (1 mg/mL). After allowing the mixture to stand for 15 min, the absorbance at 500 nm was measured using methanol or water as a reference. Total tannin content was determined as mg catechin/g of dry weight or mg CE/mg. Each sample underwent three replication analyses.
The DPPH assay
DPPH of extracts and standard vitamin E were assessed using Chakraborty and Paulraj’s (2010) technique. Different concentrations of extracts (20 mg/mL) and standard (1 mg/mL) were pipetted into tiny tubes. A 0.5 mL amount of DPPH∙ methanolic solution was added to each sample and standard. For 30 min, the blend was left at 25°C in the dark. The solution’s absorbance was measured at 520 nm using a spectrophotometer. 0.5 mL of DPPH solution and 0.5 mL of methanol were mixed for the control.
The blank was pure methanol. The DPPH inhibition (PI%) is determined using the equation (Equation 1): PI (%) = 100 x (Acontrol—Asample) /Acontrol, where Acontrol and Asample are the absorbances of the control solution and test sample or standard, respectively.
ABTS radical scavenging assay
ABTS cation scavenging activity test, also known as 2,2′-casino-bis (3-ethylbenzthiazoline-6-sulphonic acid), was employed for an antiradical assay (Chakraborty and Paulraj, 2010). Radical monocation was achieved by adding 2.45 mM K2S2O8 to ABTS solution (7 mM). The combination was left at room temperature for 15 h in the dark. Samples were dissolved in methanol and distilled water. Variable extract quantities and tocopherol (vitamin E). The test was conducted and compared to the standard. The antioxidant activity was evaluated by mixing 800 mL of diluted ABTS+ with 200 mL of each standard and sample. After 30 min, we measured absorbance at 734 nm using spectrophotometry. Each measurement was tripled. Percent (%) inhibition was utilized to reflect the antioxidant capacity of test samples and reference material.
The % scavenging of ABTS+ radicals was estimated using Equation 2:
Acontrol and Asample represent the absorbance of the control and test sample or standard, respectively.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
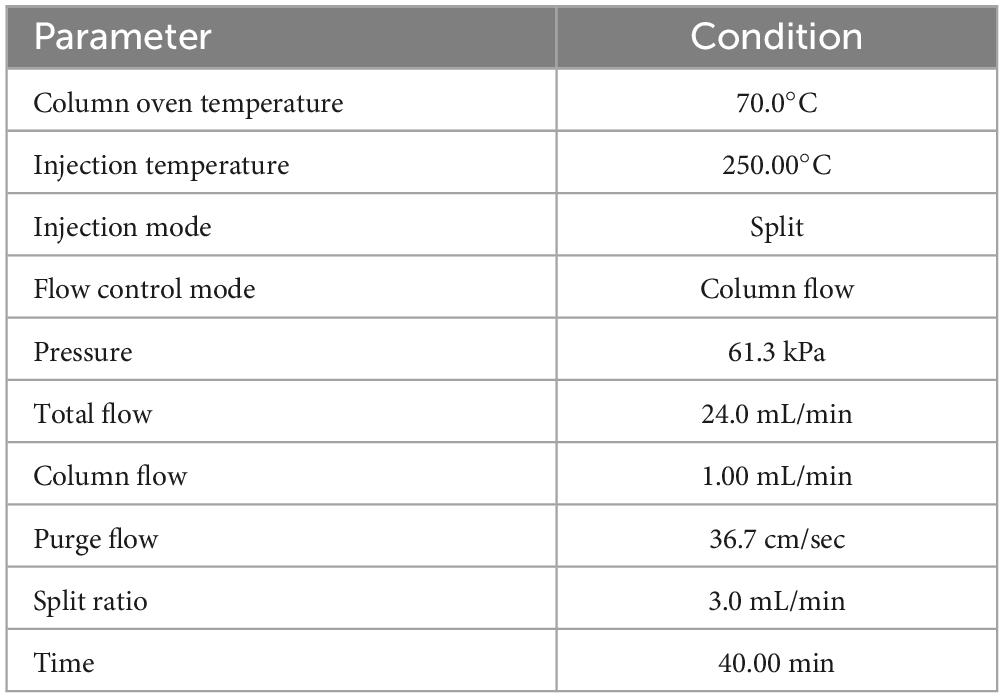
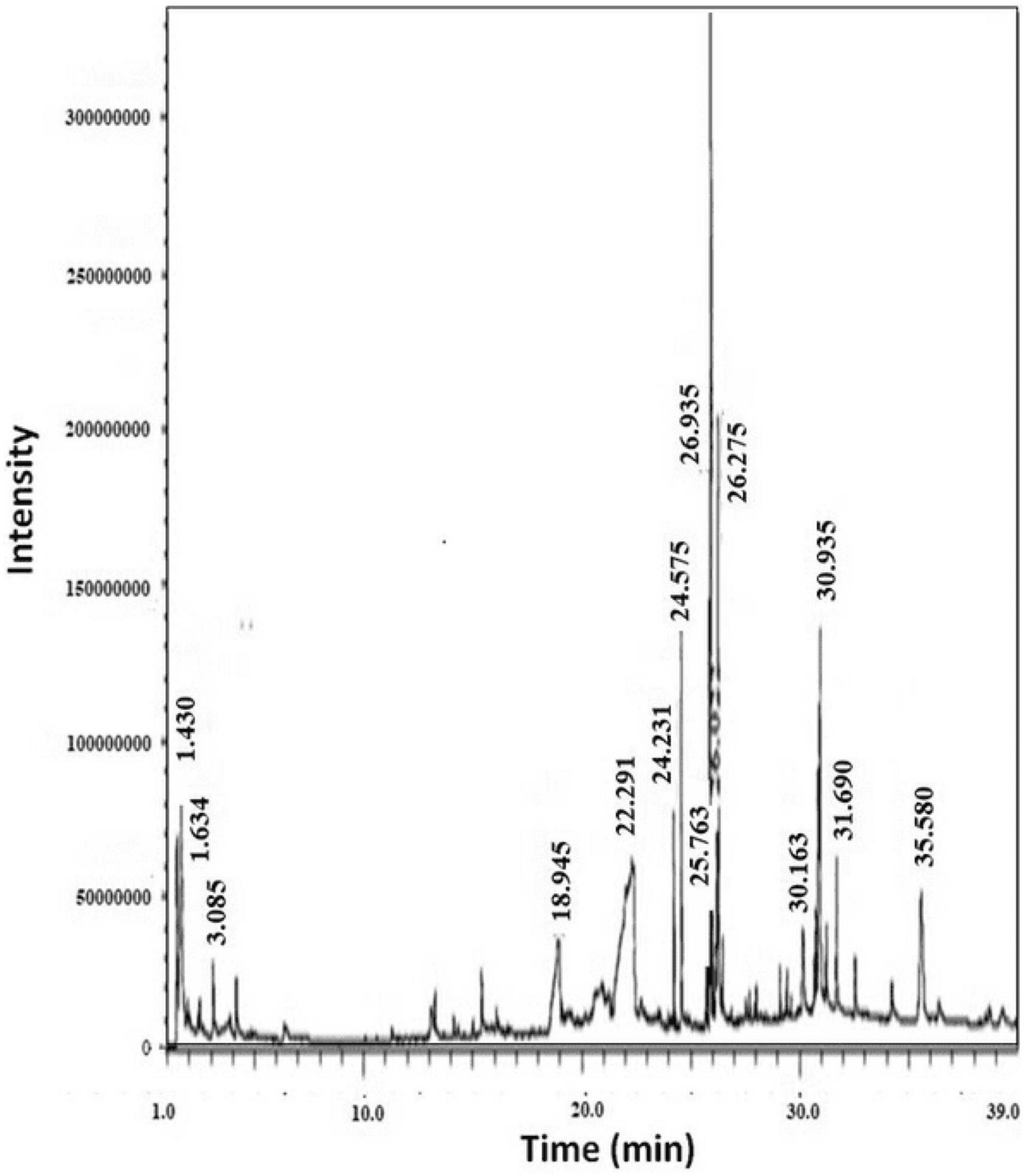
The GC-MS from Perkin Elmer Clarus 600 was utilized to analyze the crude extract of R. vesicarius. The GC-MS consisted of a Rtx 5MS capillary column (30 m length, 0.25 mm inner diameter, and 0.25 m film thickness). The system was capable of reaching a maximum temperature of 350°C. Furthermore, the GC system was coupled to a Perkin Elmer Clarus 600C MS. The carrier gas employed in the present investigation was ultra-high-quality helium (99.9999%), maintained at a constant 1.0 mL/min flow rate. The operational temperatures of the ion source, transfer line, and injector were recorded as 280°C, 270°C, and 270°C, respectively. We measured the ionizing energy of the system to be 70 electron volts (eV). The electron multiplier (EM) voltage was acquired by using auto-tune. We collected the data by conducting comprehensive full-scan mass spectrometry within 40 to 550 atomic mass units (AMU). The compound fragmentation patterns from the mass spectra were compared to those stored in the spectrometer database utilizing the National Institute of Standards and Technology’s (NIST) Mass Spectral Library (Lalitha et al., 2021). Table 1 and Figure 2 show the experimental parameters employed for the GC-MS system analysis.
Molecular docking
Molecular docking was performed using AutoDock Vina software (Ver. 1.2.5) (Löwe and Amos, 1999), targeting the filamentous temperature-sensitive Z (FtsZ). The 3D crystal structure for FtsZ was obtained from the RCSB protein data bank website (PDB ID: 1OFU). The structure of the ligands and PC190723 was obtained from the PubChem database. Regarding the non-availability of 3D, the obtained 2D was converted to 3D via the OpenBabel online converter (Kusuma et al., 2019). Their energies were minimized using UCSF Chimera, which was used with the Discovery Studio visualizer for imaging and viewing ligand-protein interactions (Vanjare et al., 2023).
Element analysis
The elemental quantities of the R. vesicarius sample were determined by energy dispersive X-ray fluorescence (EDXRF) analysis conducted by Thermo Fisher Scientific® (USA). The device is built with a cutting-edge silicon drift detector (SDD) that effectively removes spectrum interference and provides a quick response. The 30 mm2 active area facilitates a broad hard angle, enhancing X-ray collection efficiency. The high-flux rhodium anode tube has been specifically engineered to enable direct excitation of X-ray tubes or customized excitation through various filters, thereby enhancing the sensitivity to different elements (Shaltout and Hassan, 2021).
Antioxidant activity
DPPH radical scavenging method
The free radical scavenging activity of the extracts was assessed using the DPPH radical scavenging method, with vitamin E as the standard (Sulieman et al., 2023b). The stock solutions and standard concentration were 20 mg/mL and 1 mg/mL, respectively. Each sample and the standard were combined with an equal volume (0.5 mL) of DPPH methanolic solution. Following vigorous stirring, the mixture was allowed to stand for 30 min at a temperature of 25°C in the dark. The absorbance of the resulting solution at 520 nm was measured using a spectrophotometer, and measurement was performed thrice. The control solution was prepared by combining 0.5 mL of the DPPH solution with 0.5 mL of methanol, while pure methanol served as the blank. The percentage (PI%) of free radical DPPH inhibition was calculated using the equation:
Where, AControl and ASample are the absorbance of the control solution and a test sample or standard.
ABTS radical scavenging activity method
The antiradical assay was conducted utilizing the ABTS cation scavenging activity test involving 2,20-casino-bis (3-ethylbenzthiazoline-6-sulphonic acid), commonly known as ABTS (Chakraborty and Paulraj, 2010). The ABTS radical mono-cation was generated by reacting to a 7 mM ABTS solution with 2.45 mM potassium persulfate (K2S2O8), allowing the combination to stand at room temperature and in the dark for 15 h. Samples of the organic extracts were dissolved in methanol. The tocopherol (vitamin E) standard and the extract concentration were evaluated against the benchmark. The antioxidant activity was determined by mixing 800 mL of diluted ABTS with 200 mL of each standard and sample, and the absorbance was measured spectrophotometrically at 734 nm after 30 min. Each measurement was conducted three times. The antioxidant capacity of the test sample and the standard was expressed as a percentage (%) of inhibition. The proportion of ABTS scavenging was calculated using the following equation:
Where, AControl and ASample are the absorbance of the control solution and a test sample or standard.
β-carotene/linoleic acid method
The extracts’ ability to inhibit the bleaching of β-carotene was evaluated using a previously described method (Ikram et al., 2009). When linoleic acid is heated with β-carotene and linoleic acid compounds, it produces a free radical. 20 mL of linoleic acid and 200 mL of Tween-20 were combined with 2 mL of the β-carotene solution (1.5 mg β-carotene/2.5 mL chloroform). Using a rotary evaporator, the chloroform was evaporated at 40°C under vacuum. The resulting dried material was then mixed with 50 mL of distilled water to form a β-carotene-linoleic acid emulsion. The capacity of each extract to bleach β-carotene was examined by adding 0.800 mL of the emulsion to 0.200 mL of extracts at varied concentrations (stock solution 20 mg/mL). The absorbance at 470 nm was measured before and after the combinations were incubated for 120 min at 50°C in a water bath. Each test was performed three times. The antioxidant activity of the extracts was calculated using the following formula:
Where, the absorbance values of the test sample, standard, or control recorded at zero time are represented by A0 and Ac0, respectively. In contrast, the corresponding absorbance values measured after 120 min are denoted by At and Act at zero time, respectively, and At and Act are the corresponding absorbance values measured after incubation for 120 min, respectively.
Antibacterial activity
Disk-diffusion test
Following a previously published method, we used the disk-diffusion test to assess the antibacterial efficacy of the methanolic extract from R. vesicarius (Sulieman et al., 2023a). In brief, suspensions of the tested bacteria, B. subtilis MTCC121 and P. aeruginosa MTCC741, brought from our microbiology lab in broth culture, and the bacteria were sub-cultured on Nutrient agar (Sigma Aldrich®, USA), and routinely examined using Gram staining and appropriate biochemical tests. Then, we adjusted bacteria to McFarland turbidity to attain approximately 106 CFU/mL. These suspensions were then transferred onto sterile plates containing 20 mL of Mueller-Hinton agar. Under aseptic conditions, two sterile filter disks with a diameter of 6 mm (Whatman No. 1) were placed onto agar plates previously inoculated with bacteria after being saturated with 1,000 μg/ml of the adjusted methanolic crude extract (the 6 mm disk absorbs 10 μl). Disks solely impregnated with 80% methanol were employed as a negative control, while ampicillin, at 10 μg/disk, served as a positive control (standard drug). The plates were incubated at room temperature for 24 h. Subsequently, we calculated the mean value based on three individual repetitions.
Determination of MIC and MBC
The microdilution method was employed on 96-well microplates to estimate the methanolic extract’s minimum inhibitory concentration (MIC) from R. vesicarius. In summary, serial two-fold dilutions of the extract were prepared, ranging from 500 to 31.25 μg/mL, using 5% DMSO for each row of microplates. Subsequently, the microplates were incubated with 20 μL of bacterial suspensions adjusted to 0.5 McFarland and 160 μL of Mueller-Hinton broth for 24 h at 37°C. We assessed the bacterial growth by incubating 40 μL of 2,3,5-triphenyl tetrazolium chloride (TTC) at a 0.2 g/mL concentration for 30 min at 37°C. TTC identified wells with bacterial growth by staining bacterial cells with a red dye. The MIC was determined in the microplate wells with the least amount of extract and no discernible bacterial growth. On the other side, the minimum bactericidal concentration (MBC) of the methanol extract from R. vesicarius against the tested bacteria was determined using the agar diffusion test. Mueller-Hinton agar plates were inoculated with 50 μL from the tubes of the MIC test that exhibited no apparent growth with B. subtilis and P. aeruginosa. These plates were incubated for up to 24 h at 35–37°C, and we examined the bacterial growth after incubation. The lowest concentration preventing the growth of even a single bacterial colony on the Mueller-Hinton agar plate was defined as the MBC value. The MBC/MIC ratio was also calculated (Al-Mijalli et al., 2023b).
Antibiofilm method
The impact of the methanol extract from R. vesicarius on the formation of biofilms by B. subtilis and P. aeruginosa was evaluated through the crystal violet staining assay (Venkatramanan et al., 2020). The assay used 96-well microplates in Mueller-Hinton broth supplemented with 1% glucose. Sub-inhibitory concentrations (sub-MIC) of R. vesicarius extract (1/2 MIC and 1/4 MIC) were introduced, with bacterial strains adjusted to approximately 106 cells/mL loaded into the wells. Positive controls included Mueller-Hinton broth and 80% methanol (as the solvent for the crude extract) without the extract. Incubation occurred at 30°C for 24 h in the 96-well microplates. After incubation, planktonic cells were meticulously aspirated and removed from the wells, followed by three washes with phosphate-buffered saline (PBS, pH 7.4). The microplate was air-dried for 24 h, and the wells containing the formed biofilms were stained with a 0.3% (w/v) crystal violet solution (200 μL) for 15 min at room temperature. After staining, wells were subjected to three PBS washes to eliminate unabsorbed dye. Subsequently, glacial acetic acid (30% v/v) was employed to dissolve crystal violet, and the microplate was read at 570 nm. The percentage of biofilm inhibition was calculated using the provided equation:
Statistical analysis
Data analysis was performed using the SPSS application for Windows, version 20 (SPSS Inc., Chicago, IL, USA). Differences between groups were determined through a one-way ANOVA, and further insights were obtained using Duncan’s multiple-range test. The results were presented as means and standard error of means (Std. Err.) or standard deviation, with statistical significance set at p ≤ 0.05.
Results
Phytochemical properties of R. vesicarius
The plant extract of R. vesicarius exhibited notable positive phytochemical outcomes, as evidenced by significant color changes observed through qualitative assessment, as shown in Table 2. The prevalent chemicals identified in the plant following the screening included flavonoids, phenolics, tannins, terpenes, and saponins. Figure 2 displays the GC-MS chromatogram of the methanol extract obtained from R. vesicarius crude extract. The chromatograms were analyzed by comparing various parameters, such as peak retention duration, peak area (%), height percentage, and mass spectrum fragmentation characteristics, with the corresponding values for standard substances available in the National Institute of Standards and Technology (NIST) library. GC-MS analysis of the extract identified sixteen chemical compounds. Table 3 displays the chemical compounds’ respective percentages in the R. vesicarius crude extract. The identified chemical compound concentrations were in the order of 6,9,12,15-Docosatetraenoic acid, methyl ester (17.63%) > Glycidyl isobutyl ether (11.35%)> Dichloroacetic acid, tridec-2-ynyl ester (10.31) > Rockogenin (9.28) > 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (7.15%) > n-Hexadecanoic acid (6.62%) > Glyceryl linolenate (6.37) > Isoamyl nitrite (6.12%) > S-Methyl methanethiosulphonate (3.97) > D-Amygdalin (3.87) > Phytol (3.62%) > 13-Docosenamide, (Z)-(3.57%) > Methyl stearidonate (Methyl isohexadecanoate) (3.45%) > Glyceraldehyde (3.33%) > Alpha.-d-Mannofuranoside, isopropyl- (1.34%).
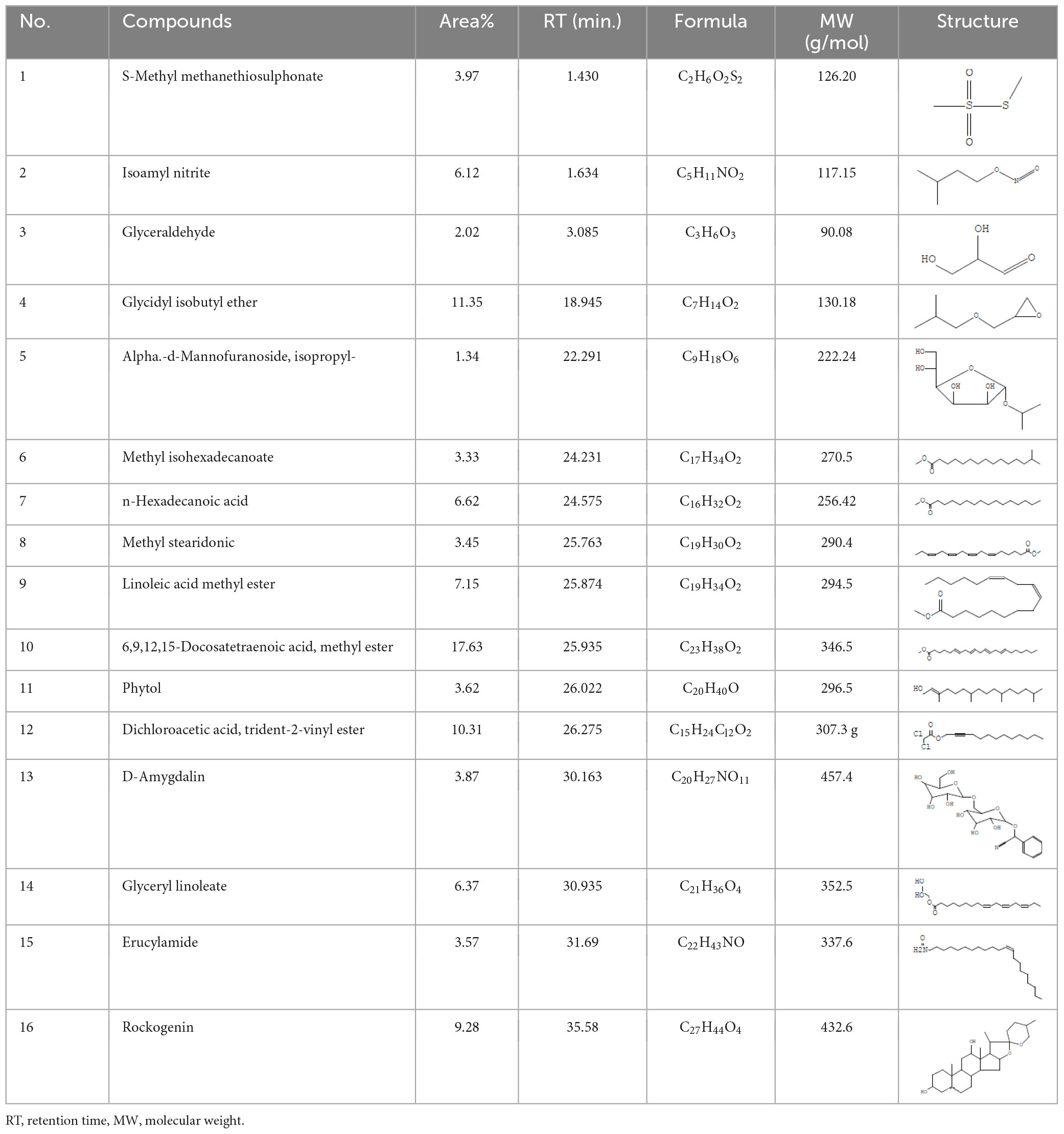
Table 3. Major compounds were identified via GC-MS analysis from the crude extract of R. vesicarius.
Docking analysis of compounds from R. vesicarius
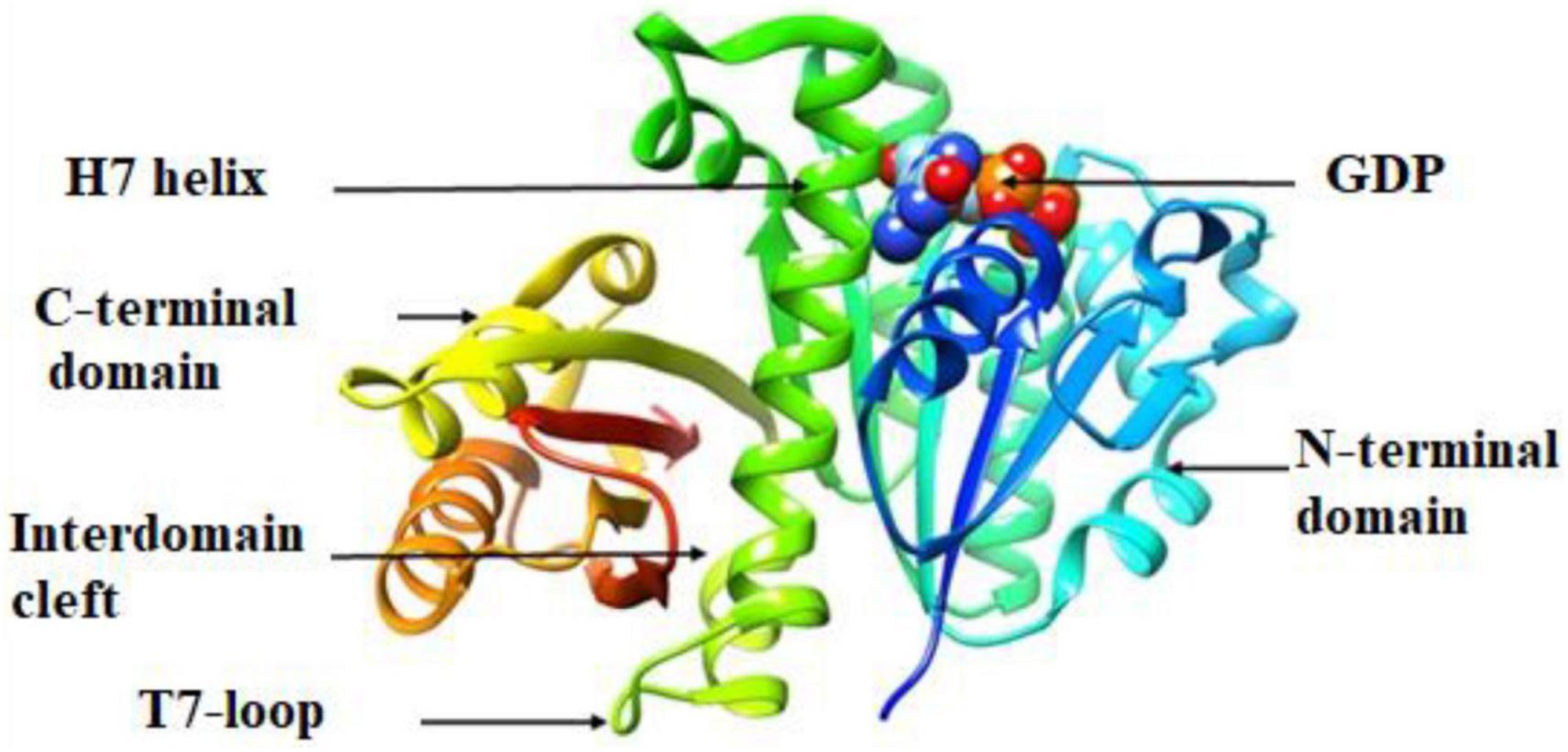
The molecular docking results (in silico) are represented in Figures 3–6 and Table 4. We have detected sixteen constituents, and molecular docking analysis unveiled their inhibitory potential against the filamentous temperature-sensitive protein Z (FtsZ), a critical regulator in bacterial cell division. Notably, six of these compounds demonstrated notable efficiency in interacting with the active site, as evidenced by their low binding scores falling within the range of −8.3 to −5.0 kcal/mol. The filamentous temperature-sensitive Z (FtsZ) is a cytoplasmic guanosine triphosphate (GTP) protein (Figure 3). It is highly conserved in most prokaryotic organisms and is a homolog of the eukaryotic cytoskeletal tubulin. GTP binding and hydrolysis are involved in FtsZ polymerizing into the Z-ring form, which divides the mother cell into two new cells.
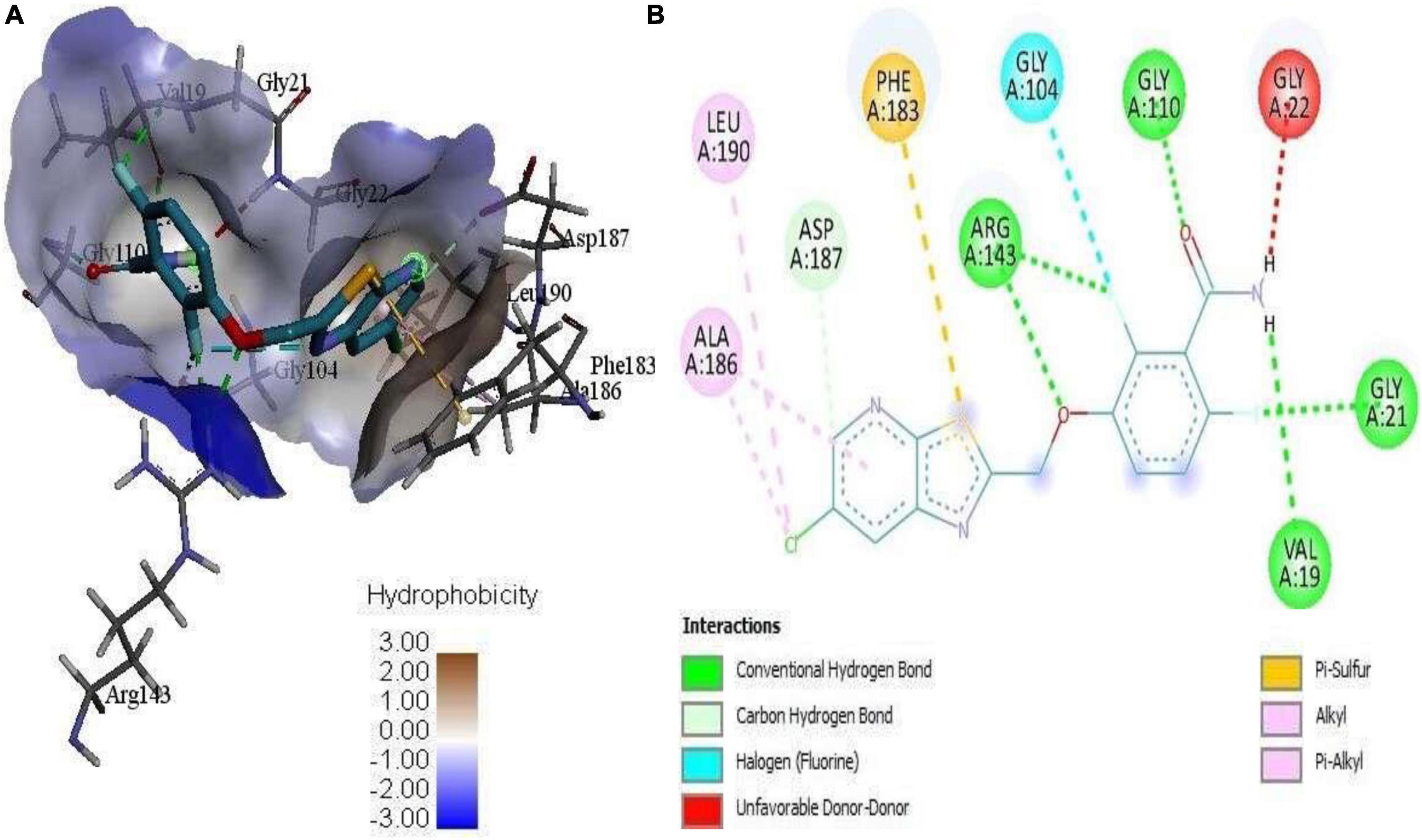
Figure 4. (A) 3D complex form of the reference PC190723 with FtsZ docked to the GPT site pocket and (B) bond interactions in 2D.
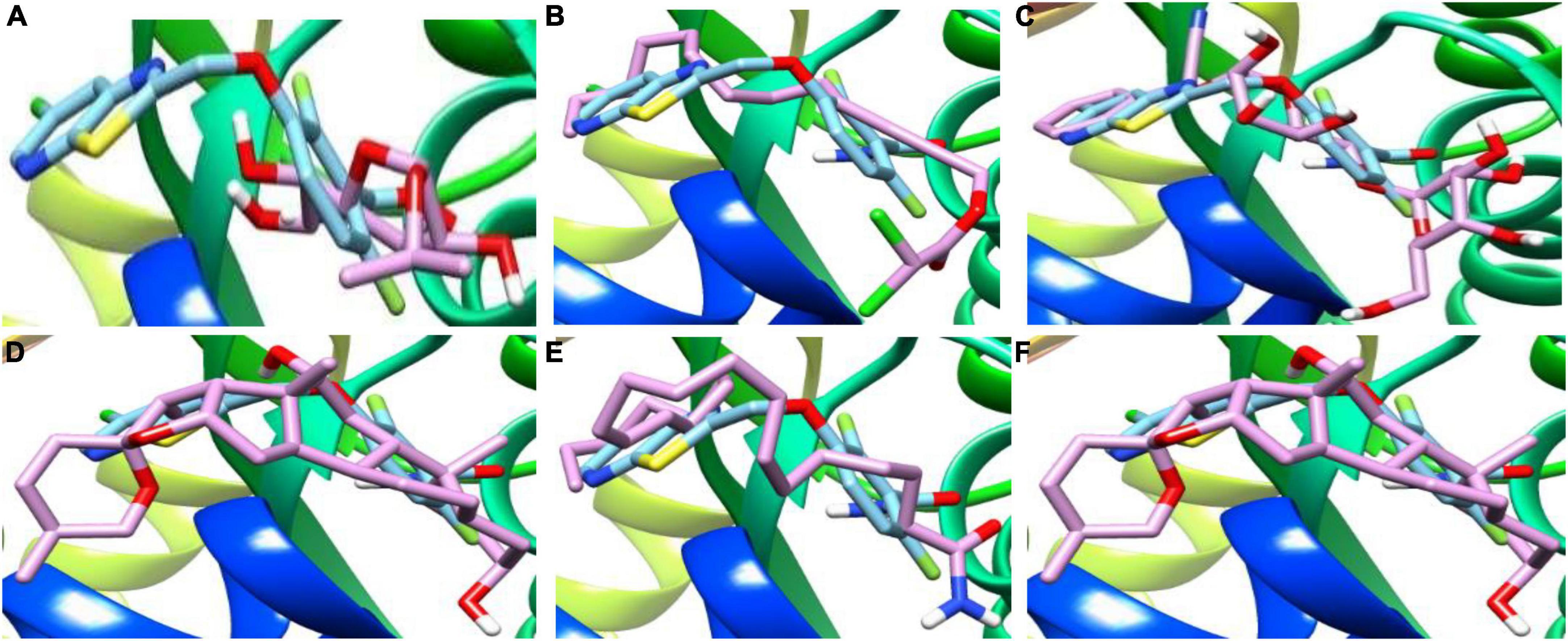
Figure 5. 3D complex of the reference PC190723 (with hydrocarbon cyan) at FtsZ GPT. site superimposed with (A) Alpha-d-Mannofuranoside, isopropyl-, (B) Dichloroacetic acid, tridec-2-ynyl ester, (C) D-Amygdalin, (D) Glyceryl linolenate, (E) Erucylamide, (F) Rockogenin.
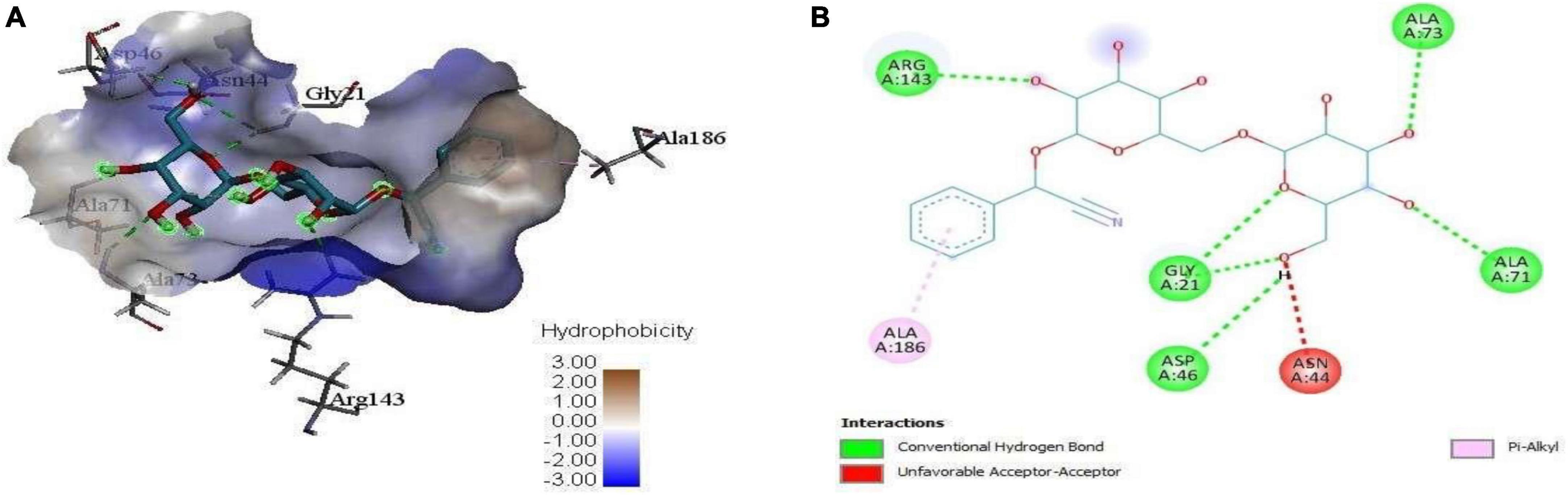
Figure 6. (A) 3D complex form of D-Amygdalin with FtsZ docked to the GPT site pocket and (B) bond interactions in 2D.
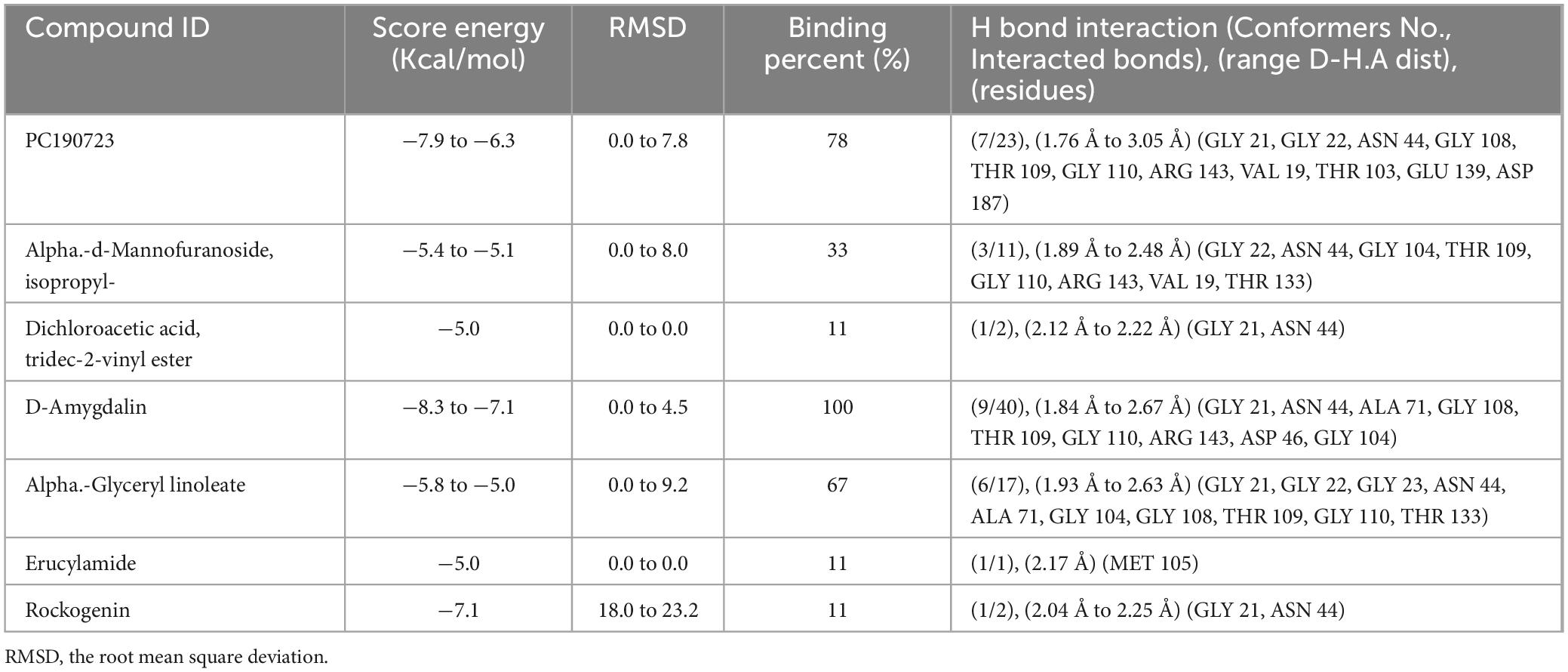
Table 4. The binding affinity of the identified via GC-MS analysis from the crude extract of R. vesicarius with FtsZ protein.
Element contents of R. vesicarius
In the current study, the aerial parts of R. vesicarius underwent quantitative elemental analysis. As shown in Table 5, the elemental analysis of R. vesicarius resulted in the detection of 15 elements. From the table, it can be noticed that the mineral concentrations were in the order of K > Ca > Cl > Al > Si > Fe > P > S > Mg > Ti > Sr > Zn > Mn > Br > Cr. The elements present in the highest quantities were K (29.02 ± 0.20 m/m%), Ca (21.13 ± 0.16 m/m%), and Cl (10.62 ± 0.15 m/m%). In contrast, Si, Fe, P, S, and Mg were found in relatively lower amounts, ranging from 4.14 ± 0.10 m/m% to 0.89 ± 0.12 m/m%. The trace elements, namely Ti, Sr, Zn, Mn, Br, and Cr, were detected in minimal quantities, ranging from 0.260 ± 0.013 to 0.0159 ± 0.0057 m/m%. Mineral elements play distinct and indispensable roles in plant metabolism, categorized into macronutrient elements (N, P, S, K, Mg, Ca), micronutrient elements (Fe, Mn, Zn, Cu, B, Mo, Cl, Ni), and beneficial elements (Na, Si, Co, Al)37. Al, Co, Na, Se, and Si are beneficial plant elements.
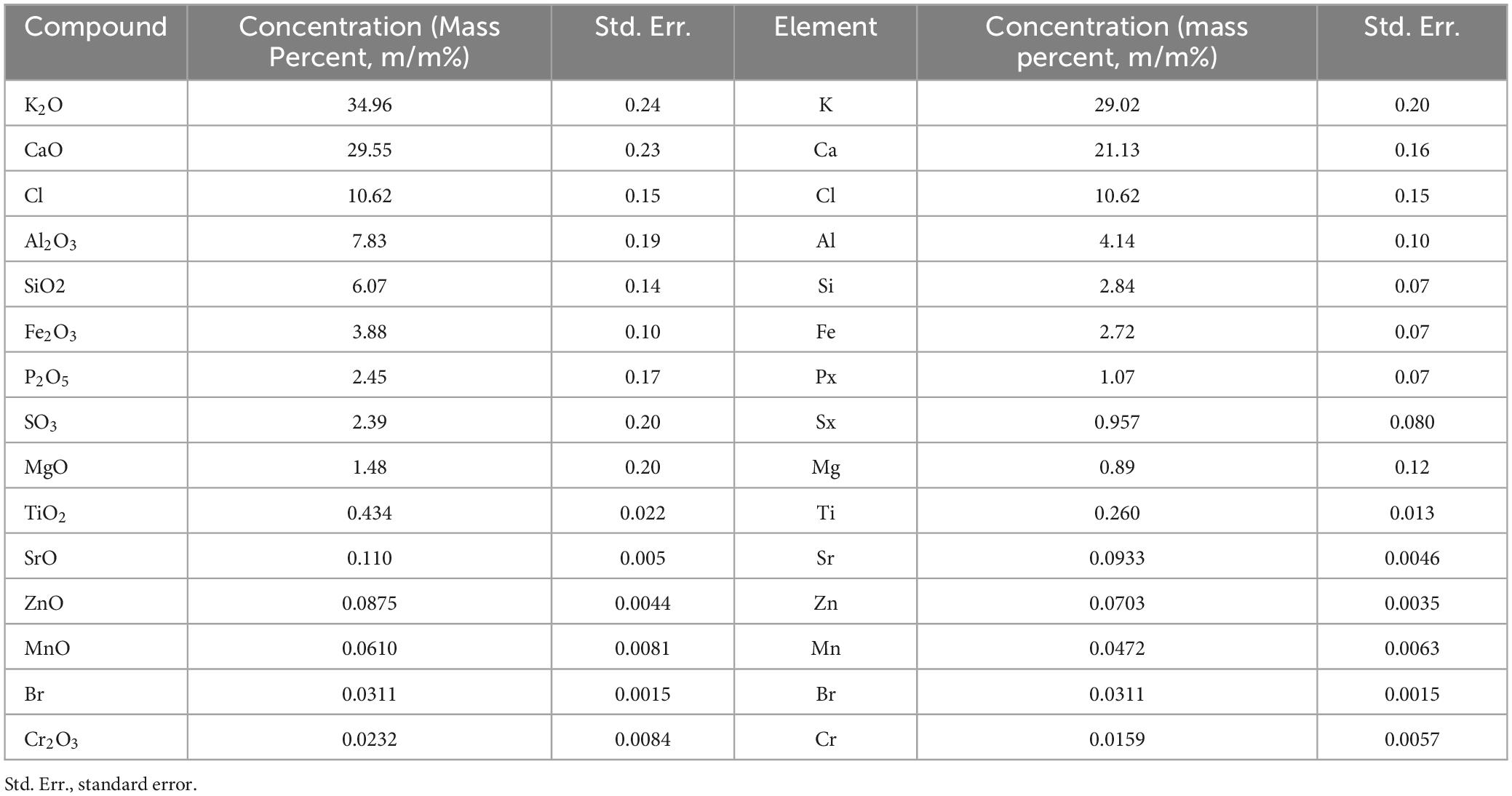
Table 5. Mineral composition of R. vesicarius (aereal parts) using energy dispersive X-ray fluorescence analysis.
Antioxidant properties of R. vesicarius
Table 6 shows the evaluation results of R. vesicarius’s antioxidant properties, which included measurements of total tannins, flavonoids, DPPH scavenging activity, β-carotene, and ABTS IC50 (mg/mL). The results show that the compounds have antioxidant activities, even at lower concentrations than the typical molecules like β-carotene, butylated hydroxytoluene (BHT), and ascorbic acid (AA). Analyzing its antioxidant properties revealed that it was more effective than ascorbic acid, butylated hydroxytoluene, and β-carotene at lower doses (Table 6). The total tannins, total flavonoids, and total phenols in R. vesicarius were measured at 28.08 + 0.20 mg TAE/g, 153.58 + 0.87 mg QE/g, and 14.56 + 0.29 mg GAE/g, respectively. Examining how an antioxidant affected the DPPH free radical’s stability was the study’s primary goal. Finding the sample concentrations that inhibited DPPH radical scavenging activity by 50% (IC50) was the specific goal of the investigation. With an average IC50 value of 0.08 + 0.002 mg/mL, the R. vesicarius methanol extract showed vigorous antioxidant activity.
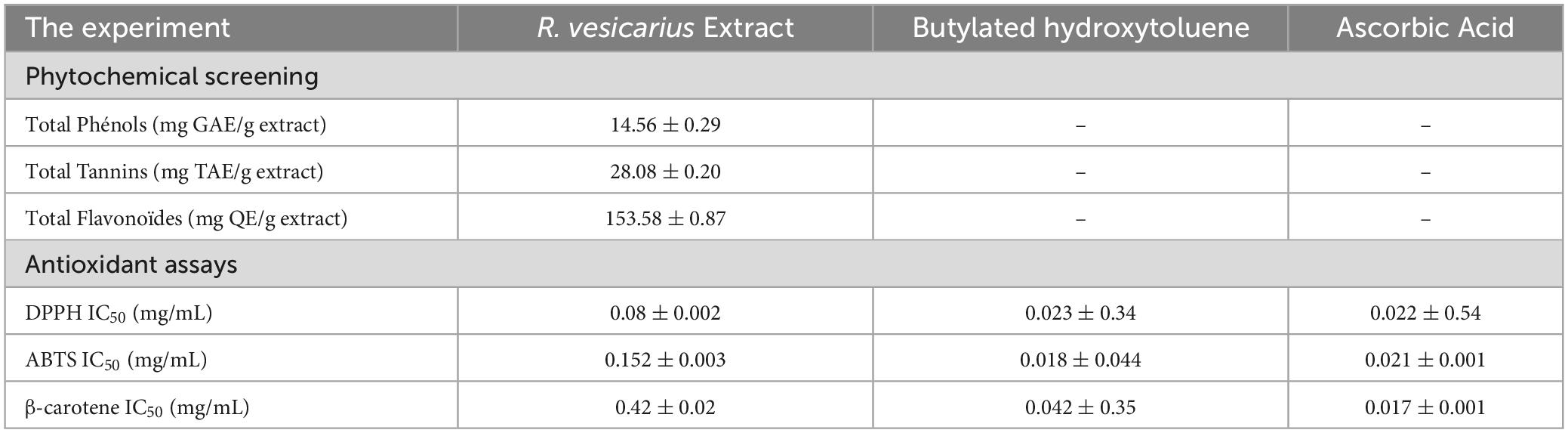
Table 6. Antioxidant activities of R. vesicarius methanol extract (80% v/v) compared to known drugs.
Antibacterial properties of R. vesicarius
We evaluated the overall antibacterial efficacy of the R. vesicarius extract through a disk diffusion assay, and the results are presented in Table 7. The data in the table indicates that the highest susceptibility to the R. vesicarius extract was exhibited by B. subtilis, as evidenced by the most expansive mean zone of inhibition measuring 13.67 ± 1.5 mm, followed by P. aeruginosa, with a zone of inhibition measuring 11.50 ± 1.0 mm. The findings were consistent with the positive control tested (ampicillin 10 μg/disk) and statistically significant at p ≤ 0.05. The Gram-positive bacterium (B. subtilis) generally exhibited heightened susceptibility compared to the Gram-negative bacterium (P. aeruginosa). The MIC assay was carried out to determine the minimum concentration of essential oil necessary for effectively inhibiting the growth of the tested bacterium.
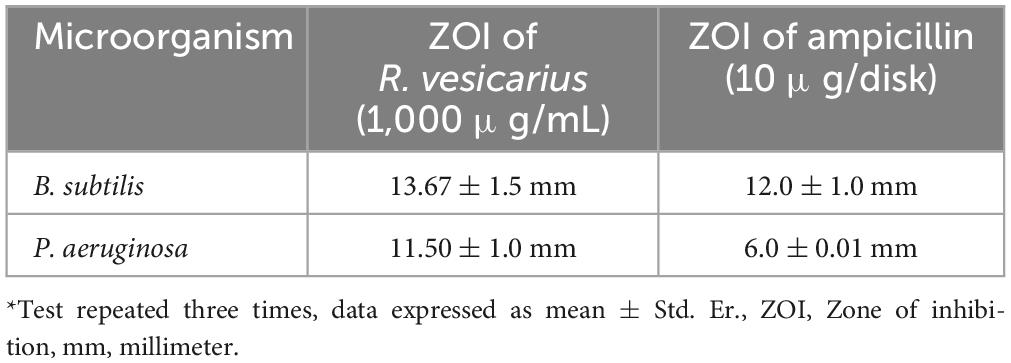
Table 7. Antibacterial activity of R. vesicarius methanol extract (80% v/v) using disk-diffusion method*.
Additionally, the MBC was conducted to establish the essential oil concentration required to completely eradicate a specific bacterium. The results of these assays and the MBC/MIC ratios, are presented in Table 8. As depicted in the table, variations in MIC and MBC values are observed when assessed across the two different bacterial strains, thereby supporting the findings of the disk-diffusion test. Notably, the Gram-positive strain (B. subtilis) demonstrated lower MIC values than its Gram-negative counterparts (P. aeruginosa), indicating that the Gram-positives are more susceptible to the plant extract. The MBC of the extract against both bacteria, B. subtilis and P. aeruginosa is identical at 500 μg/mL, indicating that this concentration of the tested substance is equally effective in completely eradicating both bacterial strains. This emphasizes the possible broad-spectrum potency of the antibacterial principles of R. vesicarius. The outcomes of the antibiofilm assessment using a crystal violet assay at sub-MIC concentrations for R. vesicarius methanol extract against B. subtilis and P. aeruginosa are illustrated in Figure 7. The crude extract demonstrated an effective inhibition of biofilm formation in a dose-dependent manner. Specifically, at sub-MIC concentrations, the inhibition of biofilm formation was observed to be 40.19% (1/2 MIC) and 21.60% (1/4 MIC) for B. subtilis, and 33.69% (1/2 MIC) and 17.77% (1/4 MIC) for P. aeruginosa. Limited information is available on the antibiofilm properties of R. vesicarius.
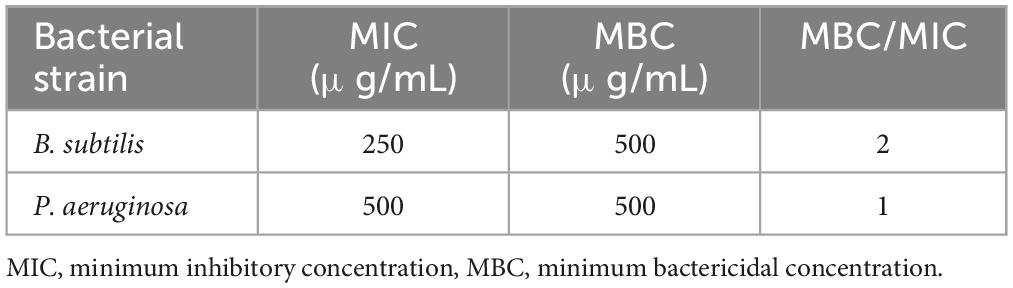
Table 8. The MIC, MBC, and MBC/MIC ratios of R. vesicarius methanol extract (80% v/v) against the chosen bacteria.
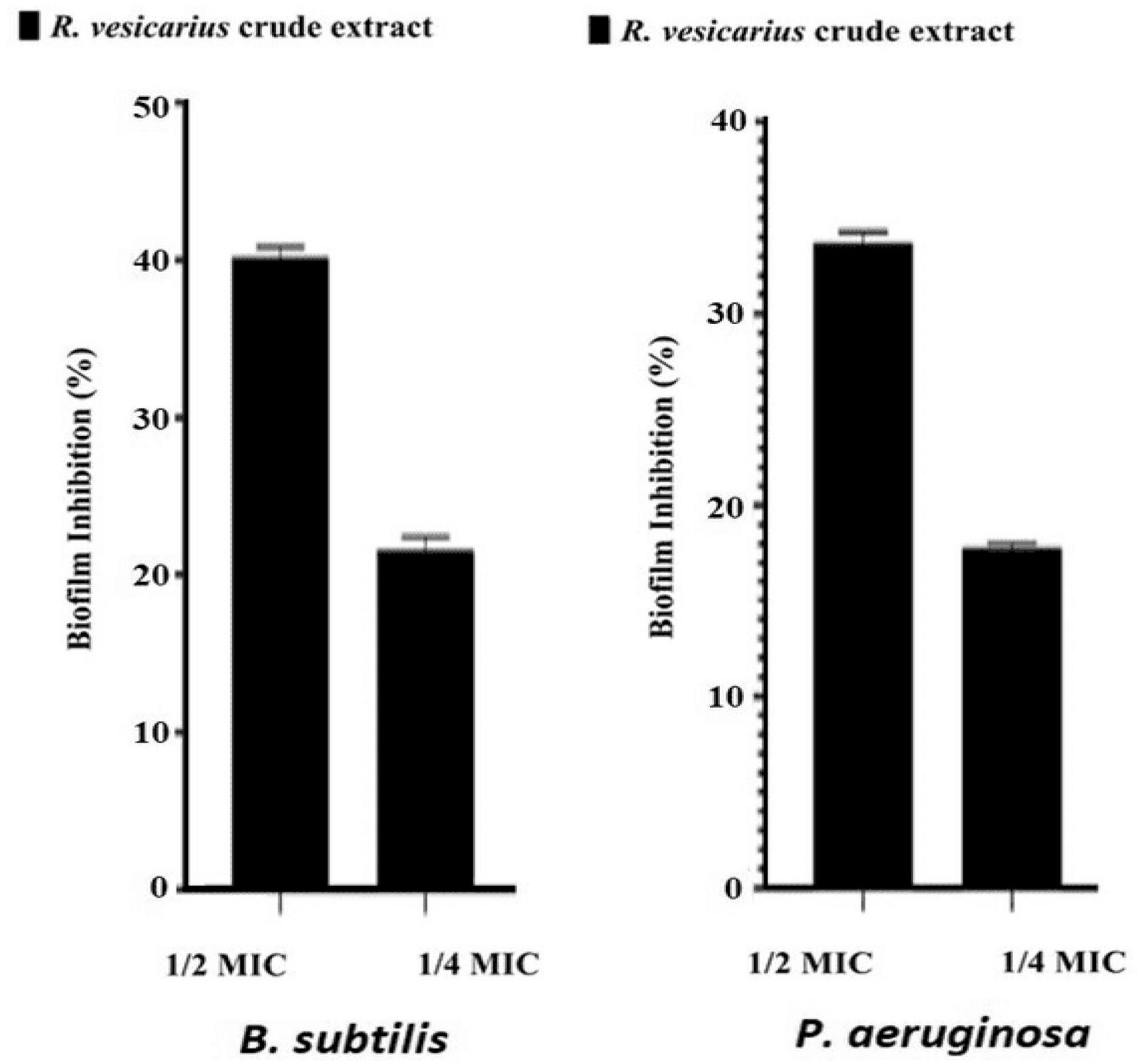
Figure 7. Antibiofilm potential of R. vesicarius methanol extract against B. subtilis and P. aeruginosa. Values are represented as the mean ± SD of three independent experiments.
Discussion
The current investigations revealed various phytochemical constituents of biological importance (Table 2). Phytochemical substances refer to secondary metabolic products synthesized by plant cells to perform specific actions crucial for their continued existence in the surrounding ecosystem. Additionally, these substances enhance the plant’s ability to withstand biotic and abiotic challenges (Costa et al., 2023). These compounds exhibit various functional effects on either or indirectly on eukaryotic cells, prokaryotic cells, and viruses. However, they do not play a role in the growth or development of vegetation (Lin et al., 2023). For instance, it has been postulated that polyphenols and flavonoids may mitigate inflammation by impeding the synthesis of pro-inflammatory molecules.
Moreover, polyphenols possess antibacterial properties. Given that numerous pathogenic microorganisms have acquired the capacity to withstand existing antimicrobial drugs, eliminating these germs could potentially be facilitated by utilizing the antibacterial properties of phytochemical molecules (Lin et al., 2023). Furthermore, a prior study aligns in part with our findings. This study reported qualitative phytochemical screening, indicating the presence of flavonoids, saponins, alkaloids, and tannins in 10 to 30-day-old seedlings of R. vesicarius (El-Bakry et al., 2011).
The article identified variations in the presence and quantity of specific biologically active constituents in different germination periods (El-Bakry et al., 2011). The Gas Chromatography-Mass Spectrometry (GC-MS) results are represented in Figure 2 and Tables 3, 4. The integration of gas chromatography with mass spectroscopy is an indispensable technique in the analysis of chemical compounds. It is recognized as one of the most widely employed techniques for separating natural products derived from plants (Lalitha et al., 2021).
Mass spectrometry enables the identification of compounds, serving as a valuable source of qualitative data regarding the chemical constituents (Urban, 2016). Based on our research results, we identified sixteen constituents, among which molecular docking analysis indicated inhibition targeting the filamentous temperature-sensitive protein Z (FtsZ), a pivotal component in bacterial cell division. Molecular docking was performed using AutoDock Vina software. Notably, six of these compounds displayed notable efficiency in active site interaction, as evidenced by their low binding scores ranging from −8.3 to −5.0 kcal/mol. This pivotal role of FtsZ in cell division has made it an appealing target for antibiotic medication research. In addition to the GTP binding site, FtsZ features a distinct inter-domain cleft and T7-loop that serve as inhibitory active pockets (Haydon et al., 2008). The FtsZ-inhibitor PC190723 has been used for docking accuracy as reference material (Haydon et al., 2008). PC190723 was docked to the GTP pocket with a score binding energy ranging from −6.3 to 7.9 Kcal/mol and a binding percent 89. Figure 5 shows the 2D and 3D interactions between PC190723 and the active site of the FtsZ protein. The docked poses a high degree of hydrogen bond formation capability. Six conformers make eight bonds with the ARG 143 residue out of the seven conformers docked to the active pocket. The residues VAL 19, THR 103, GLU 139, and ASP 187 were found to be important in hydrogen formation as acceptors. This finding may highlight the importance of the ARG 143 residue in inhibiting the FtsZ protein. Except for Phytol, which did not dock to the FtsZ active site, all of our hits interacted with GTP pockets. The full results are tabulated in Table 4 after the candidates and conformers were ignored, and a score binding energy was lower than 5.0 Kcal/mol. The best hits superimposed with the PC190723 reference are shown in Figure 4. D-amygdalin has demonstrated potential activity superior to PC190723 in terms of binding energy, binding percent, and hydrogen bond formation power. According to an analysis of the literature, the lactic acid bacteria strains separated from fermented rice noodles and containing D-amygdalin exhibited high inhibitory action against Helicobacter pylori (Techo et al., 2019). Also, amygdalin has stronger anti-HepG2 activity (Zhou et al., 2012). The best conformer of D-amygdalin that interacted with the FtsZ GPT site is shown in Figure 6. The significant hits involved in the extracts of R. vesicarius’s inhibitory action against B. subtilis and P. aeruginosa have been revealed by this docking data.
Our investigation also revealed the presence of 15 elements, as detected by elemental analysis, arranged in the following order: K > Ca > Cl > Al > Si > Fe > P > S > Mg > Ti > Sr > Zn > Mn > Br > Cr. While not required by all plant species, these elements have been reported to be essential in promoting plant growth and improving resilience to biotic stresses, such as pathogens and herbivory. Furthermore, they have demonstrated the ability to alleviate abiotic stresses, including salinity, drought, and nutrient toxicity or deficiency (Pilon-Smits et al., 2009; Pandey, 2015). Interestingly, our results showed that toxic heavy metals such as Cd and Pb were not found in the R. vesicarius sample from the Hail region, indicating that the soil there is pure and unpolluted. In recent years, heavy metals such as Cd, Co, Pb, and Cu in agricultural soil and cultivated medicinal plants due to the accumulation of residues and pollutants has become an issue of interest and research. This is particularly concerning as medicinal plants are freely sold in open-air markets without chemical or biological analysis (Sarma et al., 2011; Shen et al., 2017). Our research aligns with a prior investigation that identified R. vesicarius as a valuable reservoir of essential and health-promoting minerals, including but not limited to calcium, copper, iron, magnesium, potassium, sodium, and zinc (Alfawaz, 2006).
In our investigation, antioxidant evaluation unveiled notable antioxidant attributes at reduced concentrations when juxtaposed with those of ascorbic acid, butylated hydroxytoluene, and β-carotene. Our results are consistent with a prior investigation that demonstrated the antioxidant activity of the methanolic extract of R. vesicarius (Lobo et al., 2010; Khan et al., 2014). Antioxidants neutralize harmful free radicals. Free radicals emerge as natural byproducts of the body’s regular metabolic activities within biological systems. Antioxidants play a crucial role in safeguarding against various ailments by neutralizing these free radicals (Lobo et al., 2010; Snoussi et al., 2023).
The antioxidant potential of R. vesicarius was assessed using parameters such as total phenols, total tannins, flavonoids, DPPH scavenging activity, beta-carotene, and ABTS IC50 (mg/mL). We compared these measurements to established standard molecules; the results are represented in Table 4. Upon comparing the results with those of ascorbic acid (AA), butylated hydroxytoluene (BHT), and β-carotene, it is evident that R. vesicarius exhibits promising antioxidant properties at lower concentrations. It was published that the DPPH scavenging activity test revealed that R. vesicarius extract derived from leaves (at the early vegetative stage) exhibited the lowest IC50 value, signifying the highest effectiveness compared to the other plant parts, with an IC50 value of 0.345 ± 0.005 mg/mL. It varies with the vegetative growth stage (El-Bakry et al., 2012). Moreover, the presence of an antioxidant significantly reduced the stability of the DPPH free radical, as evidenced by strong and visible UV-Vis absorption at 517 nm.
The decrease in the ability of methanol solution to absorb the DPPH radical at 517 nm correlated with increased antioxidant activity. Experimentally determined sample concentrations resulting in a 50% inhibition (IC50) of DPPH radical scavenging activity were compared to evaluate both DPPH scavenging action and the efficiency of extraction solvents. A lower IC50 value denotes higher antioxidant activity (Brighente et al., 2007). Our study also revealed the presence of tannins, flavonoids, and total phenolic content in R. vesicarius extract. Different extracts demonstrated the ability to quench stable ABTS and O2 free radicals, allowing for the assessment of their free radical scavenging capacity. However, the extracts exhibited significantly lower capacities to neutralize O2 and ABTS radicals compared to standard compounds (Schinella et al., 2010). Polyphenols, encompassing phenolic acids, tannins, coumarins, anthraquinones, and flavonoids, were identified as significant antioxidants isolated from higher plants. Their redox properties enable them to act as reducing agents, singlet oxygen quenchers, hydrogen donors, and potential metal ion chelators (Noumi et al., 2023).
Utilizing the Folin–Ciocalteu phenol reagent, the total polyphenol content of R. vesicarius was quantified at 3.72 ± 0.05 mg gallic acid equivalent per gram of dry plant weight. Finally, the development of an innovative drug, such as an antibiotic, must incorporate an antioxidant agent to mitigate side effects, as suggested by insights from gentamicin, a potent agent targeting Gram-negative bacteria. However, it leads to nephrotoxicity as a significant complication associated with gentamicin administration, attributed to oxygen metabolites and reactive oxygen species (ROS) (Al-Amer et al., 2023).
Phytomedicines offer potent therapeutic effects due to their various endogenous antioxidant compounds. These include phenolics, tannins, flavones, flavonols, flavonoids, alkaloids, saponins, terpenoids, and reducing Sugars. Investigation of medicinal plants is a subject of great interest because botanicals are known to contain compound active antioxidant substances. This concern has awakened curiosity around the globe for a medicinal plant as a credible supplier of potent antioxidants.
The antioxidant activities of R. vesicarius extract can be associated to the presence of many metabolites like n-Hexadecanoic acid (Kalpana et al., 2012), Phytol (Pejin et al., 2014), methyl stearidonic (Jitendra et al., 2015), Methyl isohexadecanoate (Mohamed et al., 2018), linoleic acid methyl ester (Gıdık, 2021), and amygdalin (Barakat et al., 2022). In fact, previous researchers have reported that n-Hexadecanoic acid has antioxidant activity as follows: DPPH (30.19–89.13% at 100–500 μg/ml), ABTS (42.18–83.86% at 100–500 μg/ml), reducing power (0.02–0.16% at 100–500 μg/ml), nitric oxide (18.65–73.17% at 100–500 μg/ml), and superoxide (17.18–81.21% at 100–500 μg/ml), respectively (Kalpana et al., 2012). It has been reported that the diterpene member (Phytol) reduced the production of many radicals by 56, 50, and 48% for carbon-dioxide anion radical, methoxy radical, and DPPH radicals, respectively (Pejin et al., 2014).
On the other side, our results indicated that in vitro antibacterial analysis displayed inhibitory effects against B. subtilis MTCC121 and P. aeruginosa MTCC 741, with inhibition zones measuring 13.67 ± 1.0 mm and 11.50 ± 1.0 mm, respectively. The MIC and MBC values ranged from 250 to 500 μg/mL. Furthermore, R. vesicarius demonstrated antibiofilm activity. Our claim finds support in existing literature, exemplified by a study indicating the potent antibacterial activities of various alcoholic extracts of R. vesicarius against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus. Furthermore, numerous additional studies corroborate our findings and validate the presence of antibacterial agents in R. vesicarius (El-Bakry et al., 2012; Shah et al., 2015; Chaity et al., 2020). Our findings were relatively in concordance with a previously published article, which reported that for different bacterial strains, the highest MIC value of R. vesicarius was 250 mg/mL, and the lowest MIC value was 31.25 mg/mL (Tukappa and Londonkar, 2013). Furthermore, the MBC/MIC ratio was computed as detailed in Table 6. Our observations reveal a range of 2.0 to 1.0 for this ratio across the examined bacterial strains, underscoring the evident bactericidal efficacy of the methanol extract derived from R. vesicarius against these bacterial species. The classification of a tested compound as possessing bactericidal properties occurs when the MBC/MIC ratio is at or below 4.0, while a ratio exceeding 4.0 indicates the compound exhibits bacteriostatic attributes (Abdallah, 2016). The assessment of the MBC/MIC ratio serves as a critical parameter for evaluating a substance’s ability to eliminate bacteria. The primary objective in determining the MBC/MIC ratio was to enhance the understanding of the mode of action associated with the tested extract (Jeddi et al., 2023). It was stated that the MBC/MIC ratios of various R. vesicarius extracts against three bacterial strains were less than 4, indicating that all R. vesicarius extracts exhibit bactericidal properties (Tukappa and Londonkar, 2013). Further investigations are recommended to comprehensively elucidate the growth kinetics and mode of action of microorganisms when exposed to treatment with R. vesicarius extract, both in vitro and in vivo.
Nevertheless, a published paper indicates that R. vesicarius exhibits promise as an antibiofilm agent against various bacteria, including Staphylococcus epidermidis, Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Moreover, it has demonstrated efficacy in addressing infections associated with indwelling medical devices (Fady et al., 2023). Another study demonstrated that the anti-biofilm attribute of another species within the genus Rumex, Rumex dentatus, is directly proportional to the concentration of extracts observed at both 48 and 72 h (Najafabadi et al., 2020). Biofilms are believed to be linked to 80% of microbial infections, and it is widely acknowledged that the growth of microorganisms within biofilms can significantly bolster their resistance to antimicrobial agents. Consequently, conventional antimicrobial therapies frequently prove ineffective in eradicating biofilms from infection sites. This underscores the imperative need for innovative anti-biofilm agents that target novel mechanisms and exhibit unique modes of action (Brackman and Coenye, 2015; Akova et al., 2021).
Conclusion
In conclusion, the investigation of R. vesicarius from the Hail highlands in Saudi Arabia revealed a diverse array of phytochemical constituents, identifying 16 compounds. Docking was performed between the identified organic compounds and the filamentous temperature-sensitive protein Z (FtsZ), essential for bacterial cell division. Following the formation of single-stranded filaments, FtsZ constructs the exceedingly dynamic Z-ring scaffold. The docking results showed that 6 out of 16 substances inhibited FtsZ, with binding scores ranging from −8.3 to −5.0 kcal/mol. The docked poses a high degree of hydrogen bond formation capability to ARG 143 residue in the active pocket.
Meanwhile, VAL 19, THR 103, GLU 139, and ASP 187 contribute to hydrogen acceptor formation. Elemental analysis revealed the presence of 15 elements, excluding heavy metals, with potassium (K), calcium (Ca), and chlorine (Cl) prevailing—recognized as essential nutrients and health-promoting elements. The antioxidant analysis demonstrated notable properties at lower concentrations than commonly known antioxidants, potentially offering cell protection against free radicals implicated in various chronic diseases. The antibacterial assessment showcased significant inhibitory effects against Bacillus subtilis and P. aeruginosa, with promising MIC and MBC values. The MBC/MIC ratio suggested potential bactericidal efficacy.
Additionally, the study underscored the antibiofilm formation activity of R. vesicarius. While B. subtilis may play positive roles in specific food processes, P. aeruginosa is generally regarded as harmful and undesirable in food due to its potential pathogenicity and spoilage effects. These findings provide valuable insights into the phytochemical composition and biological activities of the edible plant R. vesicarius, supporting its potential application in pharmaceutical and medicinal contexts. However, further research is recommended to elucidate specific mechanisms of action and validate its therapeutic potential through comprehensive in vitro and in vivo studies. More comprehensive experimental evidence, such as in vitro and in vivo assays, is recommended for future investigations to enhance the potential impact of biological activity and contribution to the field.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AMES: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. EA: Methodology, Resources, Writing – original draft. NA: Conceptualization, Resources, Writing – review & editing. HI: Formal analysis, Supervision, Writing – original draft. MA: Methodology, Validation, Visualization, Writing – original draft. AJ: Methodology, Resources, Software, Writing – original draft. SS: Methodology, Resources, Writing – review & editing. MS: Conceptualization, Writing – original draft.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the University of Ha’il; grant number R.G. 23-0-35 and the APC was funded by the University of Ha’il.
Acknowledgments
We express gratitude to the technicians, employees, and administrators at Ha’il University for their cooperation, facilitated the research process and financing this project (R.G. 23-0-35).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, E. M. (2016). Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug-resistant Acinetobacter baumannii. J. Acute Dis. 5, 512–516. doi: 10.1016/j.joad.2016.08.024
Akova, B., Kivanç, S., and Kivanç, M. (2021). Antibiofilm effect of probiotic lactic acid bacteria against Bacillus spp obtained from the ocular surface. Eur. Rev. Med. Pharmacol. Sci. 25, 7799–7805. doi: 10.26355/eurrev_202112_27626
Al-Amer, H., Al-Sowayan, N., Alfheeaid, H., Althwab, S., Alrobaish, S., Hamad, E., et al. (2023). Oral administration of naringenin and a mixture of coconut water and Arabic gum attenuate oxidative stress and lipid peroxidation in gentamicin-induced nephrotoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 27, 10427–10437. doi: 10.26355/eurrev_202402_35454
Alfawaz, M. A. (2006). Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J. Food Comp. Anal. 19, 552–555. doi: 10.1007/s11356-021-16335-7
Al-Mijalli, S. H., El Hachlafi, N., Abdallah, E. M., Jeddi, M., Assaggaf, H., and Qasem, A. (2023a). Exploring the antibacterial mechanisms of chemically characterized essential oils from leaves and buds of Syzygium aromaticum (L.) Merr. et Perry against Staphylococcus aureus and Pseudomonas aeruginosa. Ind. Crops Products 205:117561. doi: 10.1016/j.indcrop.2023.117561
Al-Mijalli, S. H., Mrabti, H. N., El Hachlafi, N., El Kamili, T., Elbouzidi, A., Abdallah, E. M., et al. (2023b). Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: a comprehensive In vitro and In silico study. Biochem. Syst. Ecol. 110:104700. doi: 10.1016/j.heliyon.2023.e23084
Anwar, F., and Przybylski, R. (2012). Effect of solvent extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 11, 293–302.
Banso, A., and Adeyemo, S. (2006). Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera, and Datura stramonium. Biokemistri 18:1. doi: 10.4314/biokem.v18i1.56390
Barakat, H., Aljutaily, T., Almujaydil, M., Algheshairy, R., Alhomaid, R., Almutairi, A., et al. (2022). Amygdalin: a review on its characteristics, antioxidant potential, gastrointestinal microbiota intervention, anticancer therapeutic and mechanisms, toxicity, and encapsulation. Biomolecules 12:1514.
Beddou, F., Bekhechi, C., Ksouri, R., Chabane, S. D., and Atik, B. F. (2015). Potential assessment of Rumex vesicarius L. as a source of natural antioxidants and bioactive compounds. J. Food Sci. Tech. 52, 3549–3560. doi: 10.1007/s13197-014-1420-9
Bhatkar, N. S., and Shirkole, S. S. (2023). “Future prospect and global market demand for dried herbs, spices and medicinal plants,” in Drying of Herbs, Spices, and Medicinal Plants, eds C. L. Hii and S. S. Shirkole (Boca Raton, FL: CRC Press), 217–229.
Brackman, G., and Coenye, T. (2015). Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 21, 5–11. doi: 10.2174/1381612820666140905114627
Brighente, I., Dias, M., Verdi, L., and Pizzolatti, M. (2007). Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 45, 156–161. doi: 10.1080/13880200601113131
Chaity, A. S., Islam, M. A., Nasrin, T., Sarker, S. R., Dutta, A. K., Sikdar, B., et al. (2020). Screening of in vitro antibacterial activity of Rumex vesicarius (L.) leaves extract against twelve pathogenic bacteria. J. Adv. Microbiol. 19, 1–6. doi: 10.9734/jamb/2019/v19i430199
Chakraborty, K., and Paulraj, R. (2010). Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 122, 31–41. doi: 10.1016/j.foodchem.2010.02.012
Costa, S. P. D., Schuenck-Rodrigues, R. A., Cardoso, V. S., Valverde, S. S., Beatriz, V. A., and Ricci-Júnior, E. (2023). Phytochemical analysis of Brugmansia suaveolens Bercht. & J. Presl and its therapeutic potential for topical use. Nat. Prod. Res. 37, 3177–3183. doi: 10.1080/14786419.2022.2147930
El-Bakry, A., Mostafa, H., and Alam, E. (2012). Antioxidant activity of Rumex vesicarius L. at the vegetative stage of growth. Asian J. Pharm. Clin. Res. 5, 111–117.
El-Bakry, A., Mostafa, H., and Eman, A. A. (2011). Evaluation of some growth parameters and chemical composition of in vitro grown seedlings of Rumex vesicarius L.(Polygonaceae). J. Am. Sci. 7, 170–179. doi: 10.1016/j.fochx.2022.100306
El-Hawary, S. A., Sokkar, N. M., Ali, Z. Y., and Yehia, M. M. (2011). A profile of bioactive compounds of Rumex vesicarius L. J. Food Sci. 76, C1195–C1202. doi: 10.1111/j.1750-3841.2011.02370.x
Fady, M., Rizwana, H., Alarjani, K. M., Alghamdi, M. A., Ibrahim, S. S., Geyer, J., et al. (2023). Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract. Open Chem. 21, 20220286. doi: 10.1515/chem-2022-0286
Gıdık, B. (2021). Antioxidant, antimicrobial activities and fatty acid compositions of wild berberis spp. by different techniques combined with chemometrics (PCA and HCA). Molecules 26:7448.
Haydon, D. J., Stokes, N. R., Ure, R., Galbraith, G., Bennett, J. M., Brown, D. R., et al. (2008). An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 321, 1673–1675. doi: 10.1126/science.1159961
Ikram, E. H. K., Eng, K. H., Jalil, A. M. M., Ismail, A., Idris, S., Azlan, A., et al. (2009). Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Comp. Anal. 22, 388–393. doi: 10.1016/j.jfca.2009.04.001
Jeddi, M., El Hachlafi, N., El Fadili, M., Benkhaira, N., Al-Mijalli, S. H., Kandsi, F., et al. (2023). Antimicrobial, antioxidant, α-amylase and α-glucosidase inhibitory activities of a chemically characterized essential oil from Lavandula angustifolia Mill.: in vitro and in silico investigations. Biochem. Syst. Ecol. 111:104731. doi: 10.1016/j.bse.2023.104731
Jitendra, R., Sonu, R., and Narayan, S. R. (2015). Chemical study of fatty acids in mimusops elengiseeds by GC-MS and comparison of its antibacterial activity with trimethoprim. World J. Pharm. Res. 4, 1022–1026.
Kalpana, D., Shanmugasundaram, R., and Mohan, V. (2012). G.C.–M.S. analysis of ethanol extract of Entada pursueth D.C. seed. Biosci. Disc. 3, 30–33.
Khan, T. H., Ganaie, M. A., Siddiqui, N. A., Alam, A., and Ansari, M. N. (2014). Antioxidant potential of Rumex vesicarius L.: in vitro approach. Asian Pac. J. Trop. Biomed. 4, 538–544. doi: 10.12980/APJTB.4.2014C1168
Kumar, V. D. R., Jayanthi, G., Gurusamy, K., and Gowri, S. (2013). Antioxidant and anticancer activities of ethanolic extract of Ficus glomerata roxb. In dmba induced rats. Int. J. Pharma. Sci. Res. 4:3087. doi: 10.13040/IJPSR.0975-8232
Kusuma, K. D., Payne, M., Ung, A. T., Bottomley, A. L., and Harry, E. J. (2019). FtsZ as an antibacterial target: status and guidelines for progressing this avenue. ACS Infect. Dis. 5, 1279–1294. doi: 10.1021/acsinfecdis.9b00055
Lalitha, P., Parthiban, A., Sachithanandam, V., Purvaja, R., and Ramesh, R. (2021). Antibacterial and antioxidant potential of GC-MS analysis of crude ethyl acetate extract from the tropical mangrove plant Avicennia officinalis L. S. Afr. J. Bot. 142, 149–155. doi: 10.1016/j.sajb.2021.06.023
Lin, J., Yin, X., Zeng, Y., Hong, X., Zhang, S., Cui, B., et al. (2023). Progress and prospect: biosynthesis of plant natural products based on plant chassis. Biotech. Adv. 69:108266. doi: 10.1016/j.biotechadv.2023.108266
Lobo, V., Patil, A., Phatak, A., and Chandra, N. (2010). Free radicals, antioxidants, and functional foods: impact on human health. Pharmacogn. Rev. 4:118. doi: 10.4103/0973-7847.70902
Löwe, J., and Amos, L. A. (1999). Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 18, 2364–2371. doi: 10.1093/emboj/18.9.2364
Mohamed, H. I., El-Beltagi, H. S., Aly, A. A., and Latif, H. H. (2018). The role of systemic and non systemic fungicides on the physiological and biochemical parameters in Gossypium hirsutum plant, implications for defense responses. Fresen. Environ. Bull. 27, 8585–8593.
Mostafa, H., Elbakry, A., and Eman, A. A. (2011). Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L.(Polygonaceae). Int. J. Pharm. Pharm. Sci. 3, 109–118.
Najafabadi, M. P., Mohammadi-Sichani, M., Kazemi, M. J., Shirsalimian, M. S., and Tavakoli, M. (2020). Antibiofilm activity of methanol extract of Rumex dentatus against Pseudomonas aeruginosa. Biosis. Biol. Syst. 1, 25–32. doi: 10.37819/biosis.001.01.0044
Noumi, E., Ahmad, I., Bouali, N., Patel, H., Ghannay, S., ALrashidi, A. A., et al. (2023). Thymus musilii velen. methanolic extract: in vitro and in silico screening of its antimicrobial, antioxidant, anti-quorum sensing, antibiofilm, and anticancer activities. Life 13:62.
Oliva, A., Pallecchi, L., Rossolini, G., Travaglino, F., and Zanatta, P. (2023). Rationale and evidence for the adjunctive use of N-acetylcysteine in multidrug-resistant infections. Eur. Rev. Med. Pharmacol. Sci. 27, 4316–4325. doi: 10.26355/eurrev_202305_32342
Pandey, R. (2015). “Mineral nutrition of plants,” in Plant Biol Biotech, Vol I: Plant Diversity, Organization, Function. and Improvement, eds B. Bahadur, M. V. Rajam, and L. Sahijram (Berlin: Springer).
Pejin, B., Savic, A., Sokovic, M., Glamoclija, J., Ciric, A., Nikolic, M., et al. (2014). Further in vitro evaluation of antiradical and antimicrobial activities of Phytol. Nat. Prod. Res. 28, 372–376.
Pilon-Smits, E. A., Quinn, C. F., Tapken, W., Malagoli, M., and Schiavon, M. (2009). Physiological functions of beneficial elements. Curr. Opin. Plant. Biol. 12, 267–274. doi: 10.1016/j.pbi.2009.04.009
Rather, M. A., Deori, P. J., Gupta, K., Daimary, N., Deka, D., and Qureshi, A. (2022). Ecofriendly phytofabrication of silver nanoparticles using aqueous extract of Cuphea carthagenensis and their antioxidant potential and antibacterial activity against clinically significant human pathogens. Chemosphere 300:134497. doi: 10.1016/j.chemosphere.2022.134497
Rather, M. A., Gupta, K., Bardhan, P., Borah, M., Sarkar, A., and Eldiehy, K. S. (2021a). Microbial biofilm: a matter of grave concern for human health and food industry. J. Basic Microbiol. 61, 380–395. doi: 10.1002/jobm.202000678
Rather, M. A., Gupta, K., and Mandal, M. (2021b). Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 52, 1701–1718. doi: 10.1007/s42770-021-00624-x
Rather, M. A., and Mandal, M. (2023). Attenuation of biofilm and quorum sensing regulated virulence factors of an opportunistic pathogen Pseudomonas aeruginosa by phytofabricated silver nanoparticles. Microb. Pathogenesis 185:106433. doi: 10.1016/j.micpath.2023.106433
Razali, N., Mat-Junit, S., Abdul-Muthalib, A. F., Subramaniam, S., and Abdul-Aziz, A. (2012). Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins, and skins of Tamarindus indica L. Food Chem. 131, 441–448. doi: 10.1016/j.foodchem.2011.09.001
Riasat, A., Jahangeer, M., Sarwar, A., Saleem, Y., Shahzad, K., Rahma, S., et al. (2023). Scrutinizing the therapeutic response of Phyllanthus exmblica’s different doses to restore the immunomodulation potential in immunosuppressed female albino rats. Eur. Rev. Med. Pharmacol. Sci. 27, 9854–9865.
Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., et al. (2023). In antimicrobial resistance: a severe growing threat for global public health. Healthcare 11:1946. doi: 10.3390/healthcare11131946
Salama, S. A., Al-Faifi, Z. E., Masood, M. F., and El-Amier, Y. A. (2022). Investigation and biological assessment of Rumex vesicarius L. extract: characterization of the chemical components and antioxidant, antimicrobial, cytotoxic, and anti-dengue vector activity. Molecules 27:3177. doi: 10.3390/molecules27103177
Sarma, H., Deka, S., Deka, H., and Saikia, R. R. (2011). Accumulation of heavy metals in selected medicinal plants. Rev. Env. Cont. Tox. 2011, 63–86. doi: 10.1007/978-1-4614-0668-6_4
Schinella, G., Mosca, S., Cienfuegos-Jovellanos, E., Pasamar, M. Á, Muguerza, B., Ramón, D., et al. (2010). Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 43, 1614–1623. doi: 10.1016/j.foodres.2010.04.032
Selvi, A. T., Anjugam, E., Devi, R. A., Madhan, B., Kannappan, S., and Chandrasekaran, B. (2012). Isolation and characterization of bacteria from tannery effluent treatment plant and their tolerance to heavy metals and antibiotics. Asian J. Exp. Biol. Sci. 3, 34–41. doi: 10.1007/s40201-022-00791-5
Shah, A., Sharma, P., and Vijayvergia, R. (2015). Antimicrobial properties of different solvents extract of Rumex Vesicarius Linn. on some selected bacterial and fungal isolates. Int. J. Pharm. Sci. Res. 6:1107. doi: 10.13040/IJPSR.0975-8232.6(3).1107-14
Shaltout, A. A., and Hassan, F. A. (2021). Seasonal variability of elemental composition and phytochemical analysis of Moringa oleifera leaves using energy-dispersive X-ray fluorescence and other related methods. Biol. Trace Elem. Res. 199, 4319–4329. doi: 10.1007/s12011-020-02523-y
Shen, Z. J., Chen, Y. S., and Zhang, Z. (2017). Heavy metals translocation and accumulation from the rhizosphere soils to the edible parts of the medicinal plant Fengdan (Paeonia ostii) grown in a metal mining area in China. Ecotoxicol. Environ. Saf. 143, 19–27. doi: 10.1016/j.ecoenv.2017.04.042
Snoussi, M., Lajimi, R. H., Badraoui, R., Al-Reshidi, M., Abdulhakeem, M. A., Patel, M., et al. (2023). Chemical composition of Ducrosia flabellifolia L. methanolic extract and volatile oil: ADME properties, in vitro and in silico screening of antimicrobial, antioxidant and anticancer activities. Metabolites 13:64. doi: 10.3390/metabo13010064
Sulieman, A. M. E., Al-Anaizy, E. S. S., Al-Alanaizy, N. A., Abdallah, E. M., Idriss, H., and Salih, Z. A. (2023a). Unveiling chemical, antioxidant, and antibacterial properties of Fagonia indica Grown in the hail mountains, Saudi Arabia. Plants 12:1354. doi: 10.3390/plants12061354
Sulieman, A. M. E., Al-Anaizy, E. S. S., Al-Anaizy, N. A., Abdulhakeem, M. A., and Snoussi, M. (2023b). Assessment of antimicrobial and antioxidant activity of methanolic extract from Arnebia decumbens aerial parts growing wild in Aja Mountain. Adv. Life Sci. 10, 84–92.
Techo, S., Visessanguan, W., Vilaichone, R., and Tanasupawat, S. (2019). Characterization and antibacterial activity against Helicobacter pylori of lactic acid bacteria isolated from Thai fermented rice noodles. Probio. Antimicro. Prot. 11, 92–102. doi: 10.1038/s41598-023-47687-4
Tonny, T. S., Sultana, S., and Siddika, F. (2017). Study on medicinal uses of persicaria and rumex species of Polygonaceae family. J. Pharma. Phytochem. 6, 587–589.
Tukappa, A., and Londonkar, R. L. (2013). Evaluation of antibacterial and antioxidant activities of different methanol extracts of Rumex vesicarius L. Am. J. Drug Discov. Dev. 3, 72–83. doi: 10.3923/ajdd.2013.72.83
Urban, P. L. (2016). Quantitative spectrometry: an overview. Philos. Trans. Math. Phys. Eng. Sci. 374:20150382. doi: 10.1098/rsta.2015.0382
Vanjare, B. D., Choi, N. G., Eom, Y. S., Raza, H., Hassan, M., Lee, K. H., et al. (2023). Synthesis, carbonic anhydrase inhibition, anticancer activity, and molecular docking studies of 1, 3, 4-oxadiazole derivatives. Mol. Divers. 27, 193–208. doi: 10.1007/s11030-022-10416-6
Venkatramanan, M., Sankar, G. P., Senthil, R., Akshay, J., Veera, R. A., Langeswaran, K., et al. (2020). Inhibition of quorum sensing and biofilm formation in Chromobacterium violaceum by fruit extracts of Passiflora edulis. ACS. Omega 5, 25605–25616. doi: 10.1021/acsomega.0c02483
Zhao, J., Hu, S., Fan, L., Zeng, Y., Yang, Y., Zhao, Y., et al. (2023). Does higher demand for medicinal plants lead to more harvest? Evidence from the dual trade of Nardostachy jatamansi and Fritillaria cirrhosa and Tibetan people’s harvesting behavior. Front. Ecol. Evol. 11:1145928. doi: 10.3389/fevo.2023.1145928
Zhao, L., Liu, W., Xiong, S., Tang, J., Lou, Z., Xie, M., et al. (2018). Determination of total flavonoid contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Analyt. Chem. 2018:8195784. doi: 10.1155/2018/8195784
Keywords: plants, phytochemical analysis, molecular docking, elemental analysis, antioxidant, antibacterial activity
Citation: Sulieman AME, Abdallah EM, Alanazi NA, Idriss H, Adnan M, Jamal A, Shommo SAM and Snoussi M (2024) Bioactive profiling of Rumex vesicarius L. from the Hail region of Saudi Arabia: a study on its phytochemical and elemental analysis, antibiofilm, antibacterial, antioxidant properties, and molecular docking analysis. Front. Microbiol. 15:1421143. doi: 10.3389/fmicb.2024.1421143
Received: 21 April 2024; Accepted: 25 June 2024;
Published: 29 July 2024.
Edited by:
Thamir A. Alandijany, King Abdulaziz University, Saudi ArabiaReviewed by:
Malgorzata Ziarno, Warsaw University of Life Sciences, PolandMuzamil Rather, Tezpur University, India
Nikhil Malhotra, Indian Council of Agricultural Research (ICAR), India
Kanika Sharma, Central Institute for Research on Cotton Technology (CIRCOT), India
Copyright © 2024 Sulieman, Abdallah, Alanazi, Idriss, Adnan, Jamal, Shommo and Snoussi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mejdi Snoussi, c25tZWpkaUB5YWhvby5mcg==
 Abdel Moneim Elhadi Sulieman
Abdel Moneim Elhadi Sulieman Emad M. Abdallah2,3
Emad M. Abdallah2,3 Mohd Adnan
Mohd Adnan Arshad Jamal
Arshad Jamal Sohair A. M. Shommo
Sohair A. M. Shommo Mejdi Snoussi
Mejdi Snoussi