- 1Medical Laboratory Science Department, Sodo Christian General Hospital, Sodo, Ethiopia
- 2School of Medical Laboratory Sciences, College of Health Sciences and Medicine, Wolaita Sodo University, Sodo, Ethiopia
- 3Department of Medical Laboratory Science, Hosanna Health Science College, Hosanna, Ethiopia
Background: Extended-spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae are an increasing problem for patients today. Data on clinical samples for ESBL and carbapenemase-producing Enterobacteriaceae for surgical site infection patients in developing countries are limited, including Ethiopia, mainly due to resource constraints. Hence, this study aimed to determine the prevalence of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae among patients suspected to have surgical site infection at Hospital in Southern Ethiopia.
Materials and methods: A hospital-based cross-sectional study was conducted on 422 suspected surgical site infections from June 1, 2022 to August 30, 2022 at Hospitals in Southern Ethiopia. Sociodemographic and clinical data were obtained by using a structured questionnaire. Clinical samples (pus, pus aspirates, and wound swabs) were collected aseptically and processed within 30 min by placing the swabs in sterile test tubes containing sterile normal saline (0.5 mL). Samples were cultured on blood and MacConkey agar plates. All positive cultures were characterized by colony morphology, Gram staining, and standard biochemical tests. Antimicrobial sensitivity tests were performed using Kirby Baur disk diffusion on Mueller–Hinton agar. ESBL production was confirmed using a double-disc synergy test (DDST) method. Carbapenemase production was assessed using the modified Hodge test. Logistic regression analysis was used to determine associated factors. A P-value < 0.05 were considered statistically significant.
Result: Bacteria belonging to the order Enterobacterales were cultured in 23.7% out of 422 patients with suspected surgical wound infection. Of all the isolates, Enterobacteriaceae (69 isolates) were the most frequent, with E. coli (29/69) followed by K. pneumoniae (14/69). Of 69 Enterobacteriaceae isolates, 66.6 % (46/69) were positive for ESBL production, and 21.7 (15/69) were positive for carbapenemase-producing Enterobacteriaceae. The majority of Enterobacteriaceae isolates showed sensitivity to meropenem (72.1%); however revealed 63.9% and 70.5% were resistant to gentamicin and ciprofloxacin, respectively. Similarly, a higher resistance rate to cefepime (91.8%), amoxicillin-clavulanic acid (98.4%), ceftriaxone (95.1%), and ceftazidime (91.8%). MDR rate of Enterobacteriaceae isolates was 25/61 (41%) among patients suspected for surgical site infection. The Multivariable analysis revealed that length of hospital stay in hospital [AOR = 3.81 (95% CI 2.08–6.95)] remained statistically significant factor associated with surgical site infection due to ESBL producing Enterobacteriaceae.
Conclusion: Study results showed the severity of ESBL-producing Enterobacteriaceae is critical and CPE is alarming. Meropenem is the most effective antibiotic against the ESBL-producing Enterobacteriaceae. MDR rate of Enterobacteriaceae isolates was 61 (61%) among patients suspected for surgical site infection. Therefore, antibiotic selection should be based on the results of the culture and sensitivity tests.
Introduction
A surgical site infection (SSI) is an infection that develops in the part of the body where the surgery is performed (Jolivet et al., 2018). Surgical site infections are among the most frequent hospital-acquired infections among surgical patients, increasing morbidity, mortality, and expense. Infections from the surgical site may be superficial infections confined to the skin. More serious infections that arise from the surgical site can affect tissues beneath the skin, organs, or implanted materials (CDC et al., 2022).
Members of the Enterobacteriaceae family of gram-negative bacteria produce a class of enzymes known as Extended-spectrum β-lactamases, they are complex, varied and are located on plasmids that facilitated their dissemination (Jolivet et al., 2018; CDC et al., 2022). Conversely, all Enterobacteriaceae that are resistant to carbapenem antibiotics (apart from those with intermediate resistance) are referred to as carbapenemase-producing Enterobacteriaceae (CPE). These bacteria can acquire resistance genes to tetracycline, aminoglycosides, trimethoprim, sulfonamides, and chloramphenicol through plasmids and transposons, which are essential for the development of multidrug resistance (MDR) in these bacteria (Tilahun, 2022; Ghafourian et al., 2014; Cantón et al., 2008).
According to the Health Care-Associated Infection (HAI) prevalence survey conducted by the Centers for Disease Control (CDC), there were an estimated 110,800 surgical site infections (SSIs) in 2015 that were linked to inpatient surgeries. In 2020, all National Healthcare Safety Network (NHSN) categories for surgical procedures reported a 5% decrease in the surgical site infection standardized infection ratio (SIR) compared to the year before, according to the results of the 2020 HAI data presented in the National Healthcare Safety Networks progress report (Batchelor et al., 2008).
While there have been advancements in infection prevention and control practices, such as better ventilation of surgical sites, surgical techniques, barriers, and the availability of antimicrobials or antibiotics used for prophylaxis, surgical site infections (SSIs) still account for a major cause of prolonged hospital stays, morbidity, and mortality; a study report indicates that 20% of all HAIs are caused by SSI and are associated with a 2- to 11-fold increased risk of mortality, with 75% of SSI-associated deaths being directly related to SSI (Van Duin and Doi, 2017).
On the other hand, Enterobacteriaceae that produce ESBL have been recognized as major bacteria that led to multidrug resistant can causing serious community-acquired and hospital-acquired infections worldwide (Ban et al., 2017). Advanced drug resistance studies recently have publicized that the emergence of ESBL-producing Enterobacteriaceae has increased over time. As β-lactamase-producing Enterobacteriaceae are not always detected in routine antimicrobial susceptibility testing, they typically demonstrate multidrug resistance. Members of the Enterobacteriaceae family, such as Escherichia coli, Klebsiella pneumoniae and other lactose non-fermenting gram-negative rods, such as Pseudomonas species and Acinetobacter spp. are important pathogens that can cause surgical site infections (Van Duin, 2017).
Global studies indicate that between 2009 and 2010, K. pneumoniae and E. coli that produce ESBL in Europe was 38.9 and 17.6%, respectively (Moges et al., 2019). In North America, the similar frequency has been estimated at 8.8 and 8.5%, respectively (Sangare et al., 2015), whereas, the prevalence in China ranged from 61 to 67% respectively. The prevalence of ESBLs in Africa, varies widely: Sudan, 30.2% (Hoban et al., 2012), Tanzania 77.1% (Saravanan et al., 2018), Algeria 47.6% (Nelson et al., 2014), Nigeria 10.3–27.5% (Nedjai et al., 2013), and from South Africa 8.8–13.1% (Hello et al., 2010), and 37.4% of isolates were ESBL positive in various samples collected from hospitals and communities in Kenya (Storberg, 2014).
Several studies in Ethiopia, have documented the range of ESBL patterns of bacteria causing site infection, there are substantial changes in the resistance profiles and etiology of bacterial pathogens across geographical areas and time (Kumalo et al., 2023). A recent report from Addis Ababa, Ethiopia: (84.1%; Beyene et al., 2019), Gondar, northwestern Ethiopia: (83.9%; Dessie et al., 2016), and Jimma southwest Ethiopia: (87.3%; Mama et al., 2014) confirmed that there is a high prevalence of ESBL producing Enterobacteriaceae.
However, the extensive use of antibiotics, together with the length of time over which the drugs are available in the market, has led to major problems with the emergence of resistant organisms (Mohammed et al., 2017; Temesgen et al., 2023). The issue of ESBL in developing countries has been made severe by the overuse of antibiotics, overdosing, self-medication, prescribing medications without conducting the proper susceptibility testing, and extended hospital stays. Yet, research on ESBL and CPE for SSI is lacking in developing nations like Ethiopia, and no study has previously examined the prevalence of ESBL and CPE with SSI in the southern Ethiopian region of Wolaita. Therefore, this study was aimed to determine the prevalence of extended-spectrum beta-lactamase- and Carbapenemase-producing Enterobacteriaceae among patients suspected to have surgical site infection at Hospital in Southern Ethiopia.
Methods and materials
Study design, period, and area
A hospital-based cross-sectional study was conducted from June 1, 2022 to August 30, 2022. The study was conducted at Wolaita Sodo Comprehensive Specialized Hospital (WSUCSH) and Sodo Christian General Hospital (SCGH) of the Sodo Town, Southern Ethiopia. Sodo Town is found in Wolaita zone and it is the regional cities of the Southern Ethiopia Region. The town is 329 km from Addis Ababa, the capital city of Ethiopia, and 154 km from Hawassa which is the former capital city of the region South Nation Nationalities and People Region. The WSCSH is the one of the largest hospitals in the area, which was established in 1928. It grew from District Hospital to Zonal Hospital in Wolaita Zone, town of Sodo, formerly named Wolaita Sodo Zone Hospital, until June 30, 2004, and then incorporated Wolaita Sodo University, currently serving more than 5 million people per year around the area, and also it is taught under graduating and post-graduating students. Also Sodo Christian General Hospital was established in 1997 as a primary hospital and it serves more than 4.2 million people per year including the surrounding zones and regions. Generally both hospitals have its own main mission, including quality service and providing teaching, preventive, curative care and other services in in different outpatient and inpatient departments.
Study population
Patients who were clinically suspected of having surgical site infection.
Sample size determination and sampling technique
Sample size determination
The sample size was calculated by using single population proportion formula so, considering 95% confidence interval (CI), 5% margin of error, and 50% proportion, and observed for evidence of infection on a daily basis. The clinical criteria for surgical site infection development (deep incisional surgical site infection, organ/space any surgical site infection, and superficial incisional surgical site infection) from any surgical site infection categorization system from the CDC guidelines were used (Khan and Khalil, 2011).
By Using a 95% confidence level, the Z value was 1.96, 5% margin of error (d).
Therefore, by considering a 10% contingency the final sample size was: n = 422.
Sampling technique
The study participants were enrolled using a systematic sampling technique until a sample size of 422 was achieved. The first participant was selected using the lottery method and the formula K = N/n. Thus, the Kth value for the total sample size of 422 was 1,398/422= 3. The final 422 study participants were allocated proportionally to the size of each health facility based on patient flow, as shown below. The patient flow rates for both hospitals during the previous year, 2021, were presented below.
• nWSCSH = number of sample required from Wolaita Sodo Comprehensive Specialized Hospital.
• NWSCSH = is number of SSI Patient who came to WSUCH from June 1, 2021 to August 30, 2021 GC were 728.
○ nSCGH = number of sample required from Sodo Christian General hospital.
○ NSCGH = is number of SSI Patient who came to Sodo Christian General hospital from June1, 2021 to August 30, 2021GC were 670.
▪ n = is the total sample size of the study was 422.
NTotal = the sum of SSI patients who came for service from both hospitals from June 1, 2021, to August 30, 2021, which was 1,398.
Therefore,
• nWSCSH (from Wolaita Sodo Comprehensive Specialized Hospital) = (n/NTotal) * NWSCSH.
○ nWSCSH = (422/1,398)*728 = 220, sample size were allocated to WSUCH.
Whereas
• nSCGH (from Sodo Christian General Hospital) = (n/NTotal) * NSCGH.
○ nSCGH= (422/1,398) × 670 = 202 was allocated to SCGH.
Methods and tools of data collection
The participants provided with written consent, and their socio demographic and clinical data were collected using a pre-tested structured questionnaire. To ensure consistency, the questionnaire was first created in English, translated into Amharic, and then back into English. The patients' socio demographic information (age, sex, marital status, educational attainment, and employment status) and clinical information (such as antibiotic use, history of chronic illnesses, and duration of hospital stay, steroid treatment, and surgical history) were gathered via a questionnaire. Additionally, wound swabs and aspirates were obtained for laboratory analysis. Nurses and laboratory technologists from WSCSH and SCGH trained the data collectors. An extensive review of the literature was carried out (Beyene et al., 2019; Dessie et al., 2016; Mama et al., 2014).
Specimen collection procedure and transportation
Swabs and aspirates were collected from study participants suspected of having SSI by nurses (for swabs) and clinicians (for aspirates if suspected to be related to SSI). Sterile cotton swabs were rotated on a 1 cm2 area of clean granulation tissue for a period of 5 s using gentle pressure to release tissue exudate (Miller et al., 2018; National and Pillars, 2010). The fluid aspirates were collected using a 5 ml syringe. The collected specimens were transported to the Sodo Christian General Hospital Microbiology Laboratory for processing in sterile test tubes within 30 min. During the delay, Ameis transport medium was used. For carbapenemase-producing organisms, the stored organisms were transported to the Ethiopia Public Health Institute using a triple packaging system for confirmation after preserving the organisms for long-term storage (Farzana et al., 2013).
Identification of bacterial isolates
All collected specimens were inoculated onto primary isolation culture media like blood agar (Oxoid Ltd, UK) and MacConkey agar (Oxoid Ltd, UK) found in Figure 1. Both blood agar (Oxoid Ltd, UK) and MacConkey agar (Oxoid Ltd, UK) were incubated at 37°C for 24–48 h. Positive cultures were inspected for growth characteristics and Gram staining was performed. Biochemical tests, such as the oxidase test, lysine and ornithine decarboxylase test, hydrogen sulfide, indole production, Vogues Proskauer (VP), urease, phenylalanine deaminase, citrate utilization, sugar fermentation from glucose and gas production, adonitol, lactose, arabinose, sorbitol, and dulcitol fermentation, were performed on colonies from primary cultures for the final identification of the isolates.
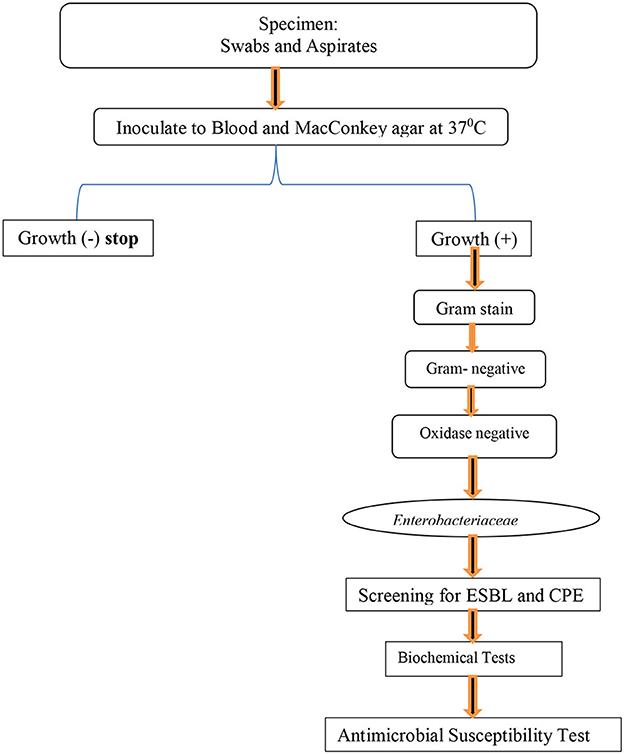
Figure 1. Flow chart for identification, AST, ESBL detection, and carbapenemase formation test for isolated bacterial at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022.
Antimicrobial susceptibility of the isolated bacteria
Antimicrobial susceptibility testing was performed on Muller-Hinton agar (Oxoid Ltd, UK) using Modified Kirby-Bauer disc diffusion. Pure bacterial colonies were resuspended in 3–4 ml of saline solution until reaching a turbidity equivalent to 0.5 McFarland. This bacterial suspension was inoculated onto Mueller-Hinton agar plates using cotton swabs (Weinstein, 2021). The following antibacterial agents were selected based on local prescription habits and CLSI recommendations. The standard antibiotic disks (Thermo Fisher Scientific, USA) and their concentrations were as follows: Gentamicin GEN 10 μg, Chloramphenicol CHO 30 μg, Ciprofloxacin CIP 5 μg, Amoxicillin-clavulanic acid AUG (25+30) μg), Meropenem MER 10 μg, Cefepime CEF 30 μg, Ceftazidime CAZ 30 μg, ceftriaxone CRO 30 μg, and Cefixime CFM 5 μg. The diameter of the inhibition zone around the disc was measured with a ruler in millimeters and interpreted as sensitive, intermediate and resistant according to Clinical Laboratory Standards Institute (Weinstein, 2021; Carvalhaes et al., 2009).
Detection of ESBL-producing Enterobacteriaceae
Double-disc synergy test method
Disks containing ceftazidime were applied next to those containing clavulanic acid and amoxicillin. Positive results were obtained when the inhibition zones around any of the cephalosporin disks was enlarged in the direction of the disc containing the clavulanic acid. The distance between the disks is critical and a 20 mm center-to-center has been found to be optimal for cephalosporin 30 μg disks (Weinstein, 2021).
Methods for confirmation of carbapenemase-producing Enterobacteriaceae
Modified Hodge test
The Modified Hodge Test (MHT) is a simple phenotypic test and has been suggested as a method used for screening carbapenemases in bacteria. It is based on the inactivation of carbapenem by carbapenemase-producing strains, that is, test isolates that enable a carbapenem-susceptible indicator strain E. coli ATCC 25922 to extend growth toward a carbapenem-containing disc along with the streak of the inoculum of the test strain. A positive test result indicated a clover leaf-like indentation (Cury et al., 2012; Date, 2018; Pierce et al., 2017; Reumert et al., 2003; Giske and Cantón, 2013).
Enterobacteriaceae suspected to be producers of carbapenemase were confirmed 10 μg meropenem disc (MRP) and by evaluating the synergistic effects when combined with the following inhibitors: phenylboronic acid, DPA/EDTA and Cloxacilline (Giske et al., 2011; Tsakris et al., 2010). The inhibition zone around the meropenem disc combined with the inhibitor was compared with the zone around the disc (Alebel et al., 2021).
Laboratory quality assurance
The sample quality was examined and handled in accordance with each hospital's quality control protocols. Using sterile cotton, skilled staff nurses aseptically removed pus swabs representative of the infected tissue from the affected area. The pus swabs were then cleaned with an antiseptic solution. As soon as possible, the gathered specimens were moved to the Sodo Christian General Hospital Microbiology Laboratory, and the specimens that were delayed were put in the Amies transport medium. Standard operating procedures were followed when performing laboratory analyses (SOPs). In order to confirm the sterility of the culture media, 5% of the prepared media were incubated overnight before being inoculated with the standard reference strains of E. coli (ATCC 700682) used to check of ESBLs (ATCC 25922), used as negative control of ESBLs, K. pneumoniae (ATCC 13883 and Klebsiella quasipneumoniae formerly known as K. pneumoniae (ATCC 700603) were used for the positive and negative control, respectively.
Data processing, analysis, and interpretation
Data were entered, checked, cleaned, coded for completeness in Epi 4.6.0.2, and imported into the Statistical Package for Social Sciences (SPSS) software version 25 for analysis. Descriptive statistics were computed, summarized, and presented using graphs and tables. Binary logistic regression was used to examine the relationship between dependent and independent variables. Statistical significance was set at P < 0.05.
Results
The study participants' socio-demographic characteristics
With a 100% response rate, 422 patients across all age groups who had a suspicion of SSI were included in this study found in Table 1. Two-hundred and thirty-five (55.7%) of the study groups' members were female. With an age range of 5–96 years, the participants with the highest number, 224 (53.1%), were between the ages of 31 and 44. The majority of participants, or 303 (71.8%), were from urban areas; 123 (52.1%) were students, 69 (16.4%) were government workers, 31 (98.3%) were merchants, 84 (19.9%) were college graduates or above, and 277 (67.1%) were married.
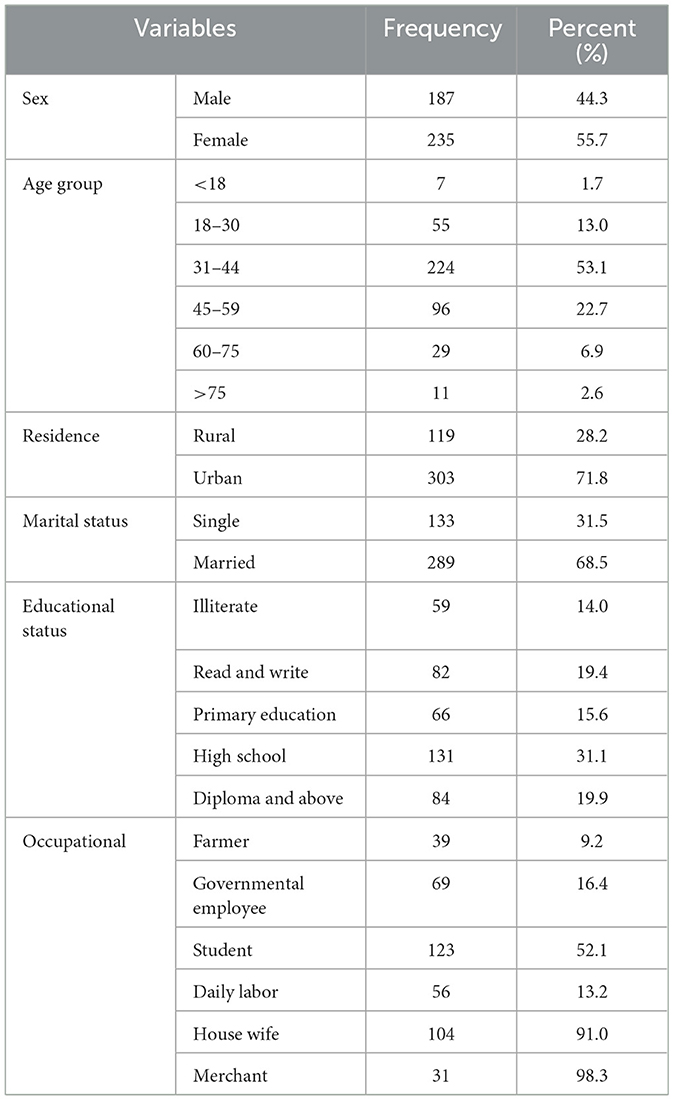
Table 1. Socio-demographic characteristics of patients with suspicion of SSI at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 422).
Clinical and medical status of the study participants
A total of 422 participants were admitted to the study. Of these, 221 (52.4%) were from the orthopedics ward, 112 (26.5%) from the surgery ward, and 89 (21.1%) from the Obstetrics and Gynecology Unit. A significant percentage of the participants (44.3%) had clean wounds. The largest number of days spent in the hospital was 8–14 days (34.8%), and there were 27 (6.4%) diabetics overall (Table 2).
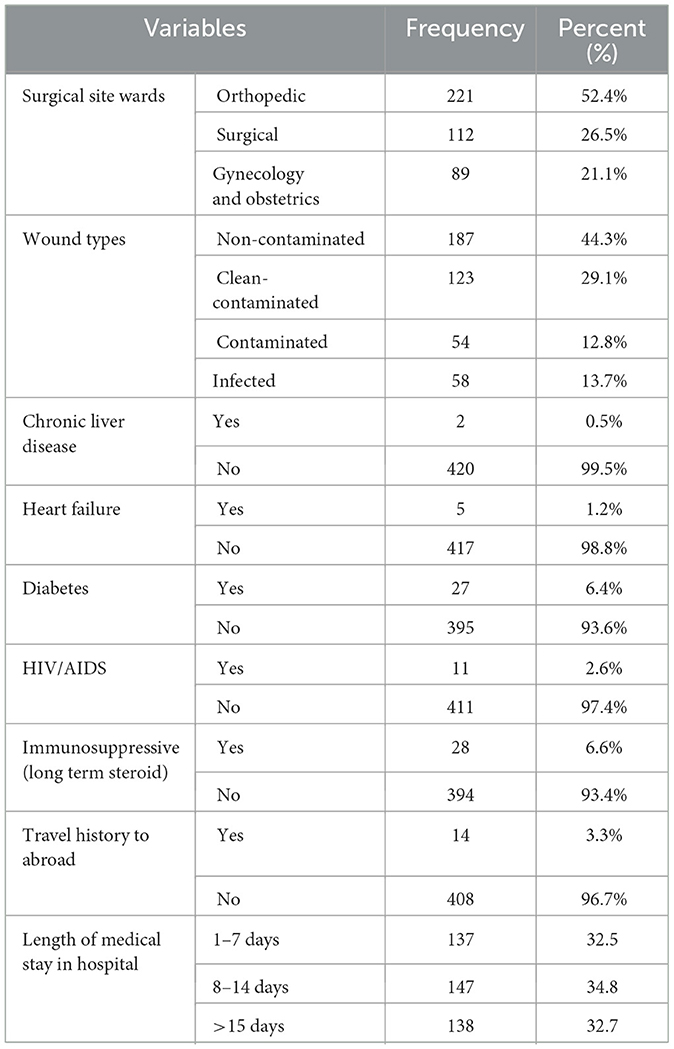
Table 2. Clinical and medical status among patients with suspicion of SSI at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 422).
Distribution of Enterobacterales isolates among patients suspected for surgical site infection
Four hundred and twenty-two wound swabs in total were used to cultivate Enterobacterales (family Enterobacteriaceae and Morganellaceae). One hundred out of the suspects exhibited both Enterobacteriaceae and Morganellaceae growth. Significant Enterobacterales infections were present in 23.7% (100/422) of patients suspected of having surgical site infections. From the total isolates, 69 (69%) strains belong to the family Enterobacteriaceae includes E. coli 29 (29%), followed by K. pneumoniae 14 (14%), was the most common isolate. And also, 31(31%) strains considered to be the family Morganellaceae (M. morganii, Providence spp., and P. mirabilis) were isolated (Table 3).
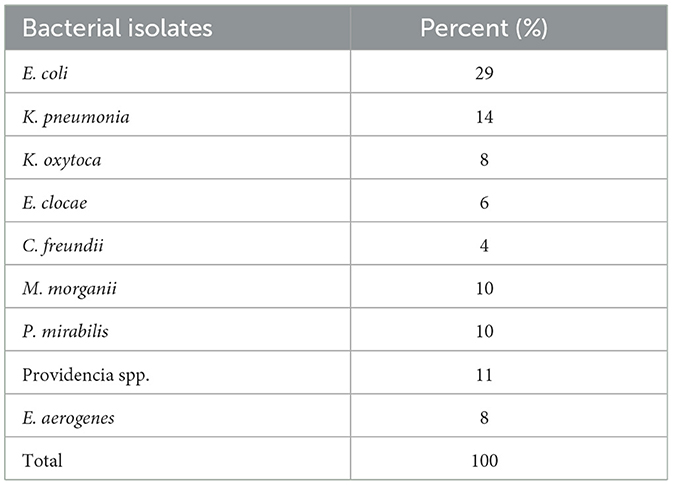
Table 3. Identified Enterobacterales isolates among patients suspected for surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 100).
Antimicrobial susceptibility pattern of extended-spectrum β-lactamase and carbapenemase producing Enterobacterales
In present study (Table 4), the higher resistance rate to cefepime reported by the majority of Enterobacteriaceae isolates (91.8%), ceftriaxone (95.1%), ceftazidime (91.8%), and amoxicillin-clavulanic acid (98.4%). However, Enterobacteriaceae isolates showed relatively high sensitivity to Meropenem 44 (72.1%). On the other hand, high percentages of resistance were obtained, gentamicin: 39 (63.9%) and ciprofloxacin: 43 (70.5%), respectively. The isolates of K. pneumoniae is resistant to cefepime, Ceftriaxone, Ceftazidime, Amoxicillin-clavulanic acid, Gentamicin, ciprofloxacin and meropenem; 14 (100%), 14 (100%), 14 (100%), 14 (100%), 12 (85.7%), 11 (78.6%), and 9 (64.3%), respectively. And also, isolates of C. freundii resistant to cefepime, ceftriaxone, ceftazidime, amoxicillin-clavulanic acid was 4 (100%) by each, and gentamicin and chloramphenicol was 3 (75%) by each, similarly, the resistance of E.coli species to cefepime, Ceftriaxone, Ceftazidime, Amoxicillin-clavulanic acid and ciprofloxacin, 26 (90%), 27 (93.2%), 26 (90%), 28 (96.6%), and 22 (75.9%), respectively. On the other hand, sensitivity rates for chloramphenicol 7(87.5%) and meropenem 6(75%) were highest for K.oxytoca. The susceptibility patterns of K. pneumoniae to gentamicin was 2(14.4%), ciprofloxacin 3 (21.4%), chloramphenicol 6 (42.8%), and meropenem 5 (35.7%) that was < 50%.
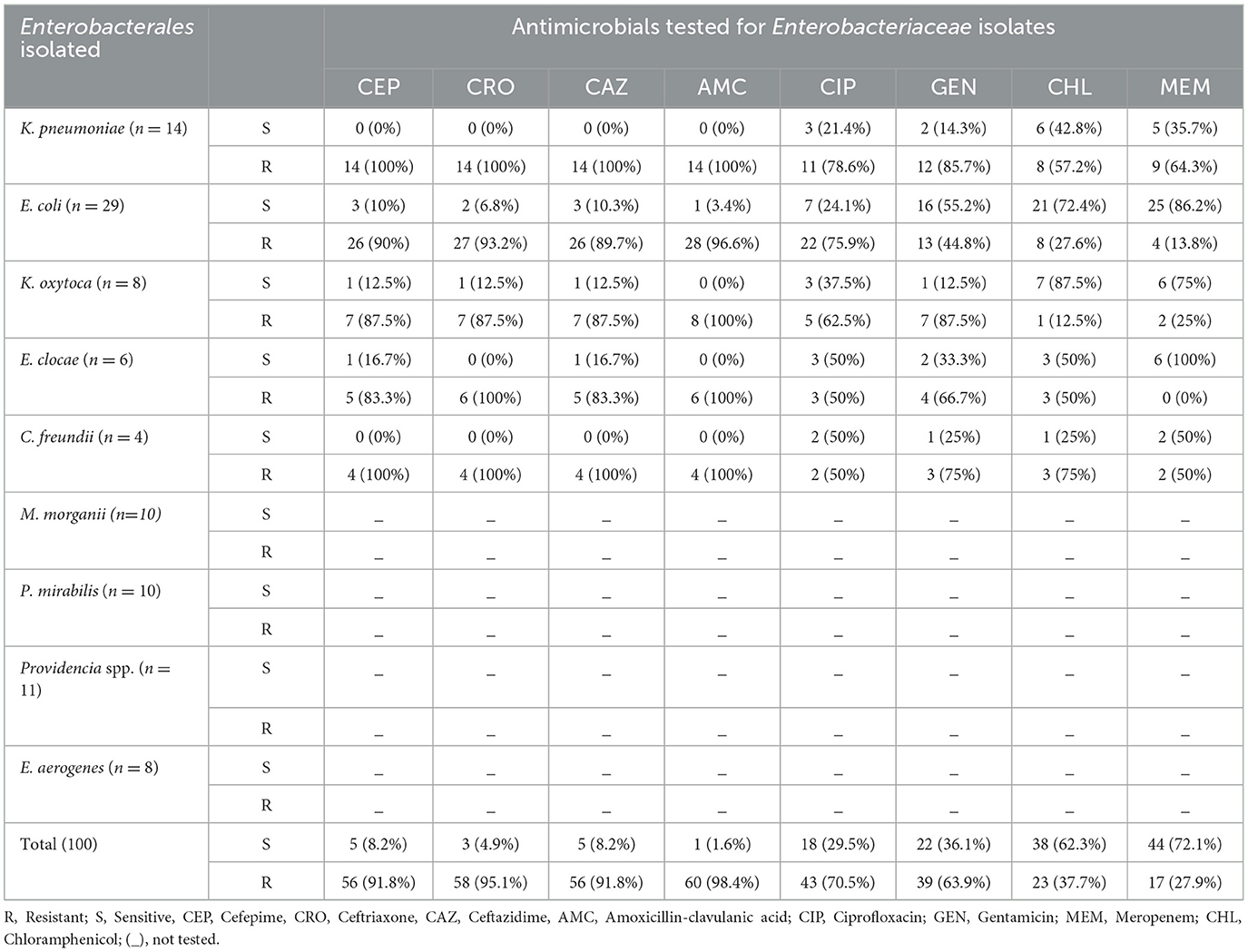
Table 4. Antimicrobial susceptibility pattern of extended-spectrum β-lactamase and carbapenemase producing Enterobacterales isolates (n = 100) among surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 100).
Prevalence of extended-spectrum β-lactamase and carbapenemase producing Enterobacterales
From wound swabs, 100 and 61 different Enterobacterales and Enterobacteriaceae were identified, respectively. Forty-six (66.6%), out of the 69 Enterobacteriaceae isolates had their ESBL production confirmed phenotypically. From all isolates of Enterobacteriaceae that were suspected producing ESBLs, three Enterobacteriaceae isolates were ESBLs tested positive: E. coli (54.35%, n = 25/46), K. pneumoniae (15.2%, n = 7/46), K. oxytoca (13.04%, n = 6/46), C. freundii (4.35%, n = 2/46), E.clocae (13.04%, n = 6/46), and E. aerogenes (0%, n = 0/46). The proportion of ESBL Enterobacterales isolates overall was 46% (46/100; Figure 2).
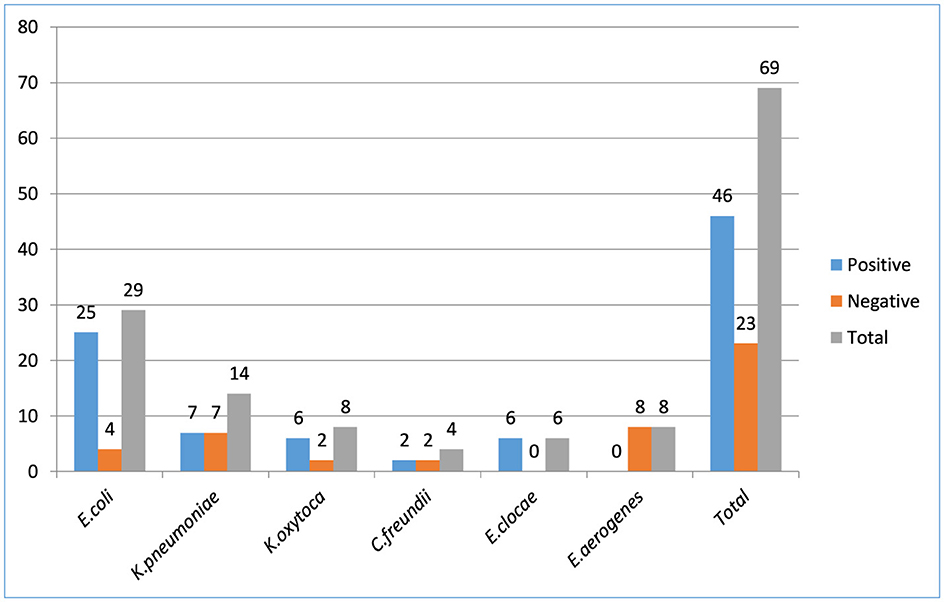
Figure 2. Enterobacteriaceae bacterial isolates producing extended-spectrum β-lactamase among patients suspected for surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (N = 422).
Carbapenemase production was tested against all Enterobacterales, regardless of the ESBL results, and 21.7 % (15/69) isolates had carbapenemase production confirmed. Among tested isolates resistant to carbapenem, K. pneumoniae; 7/15 (46.7%), E. coli; 4/15 (26.7%), K. oxytoca; 2/15 (13.3%), and C. freundii; 2/15 (13.3%) were the most common Enterobacteriaceae. The overall proportion of Enterobacterales isolates that producing carbapenemase were 15% (15/100) (Figure 3).
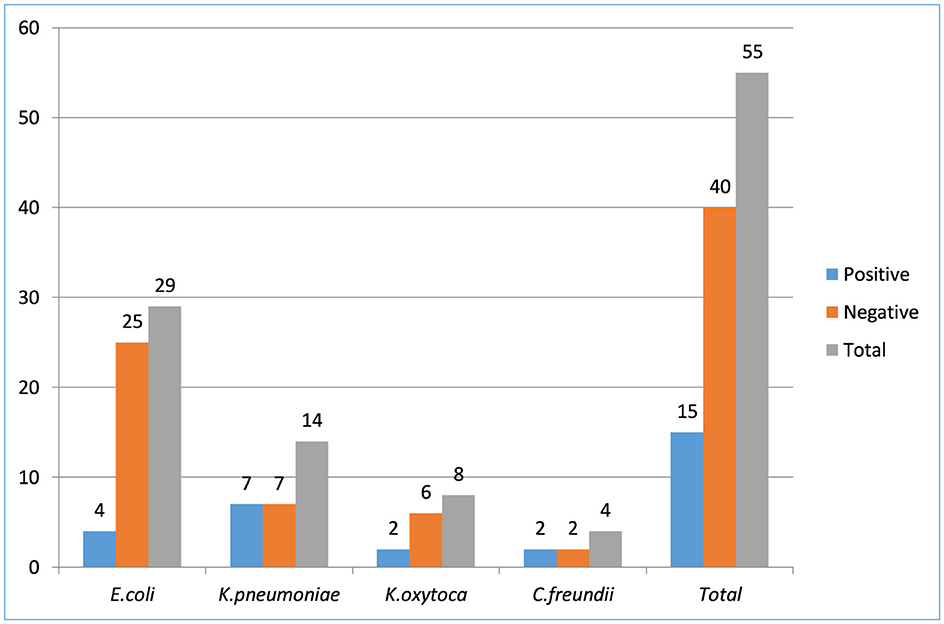
Figure 3. Enterobacteriaceae bacterial isolates producing carbapenemase among patients suspected for surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (N = 422).
Class carbapenemase producing Enterobacterales
Out of fifteen distinct Enterobacterales isolates that were thoroughly examined and were thought to be non-repeats, seven (K. pneumoniae), four (E. coli), two (C. freundii), and two (K. oxytoca) tested positive for carbapenemase production. Specifically, carbapenemases were not detected in 80% of the carbapenem-resistant strains. In this group, AmpC plus porin loss was detected. Only in 3 strains were these enzymes detected (2 NDM and 1 KPC). AmpC plus porin loss 12 (80%), KPC plus MBL 1 (6.7%), and MBL 2 (13.3%) were the results of the isolates.
Multidrug-resistant pattern of extended-spectrum β-lactamase and carbapenemase producing Enterobacterales
The total Enterobacterales isolated from patients suspected for surgical site infections, MDR rate were 25/61 (41%) found in Table 5. Approximately 41.0% of Enterobacterales isolates were MDR, 47.5% XDR, and 4.9% PDR isolates. Among the Enterobacterales isolates, E. coli (48.3% MDR, 37.9% XDR, and 3.4% PDR), K. pneumoniae (21.4% MDR, 71.4% XDR, and 7.1% of PDR), K.oxytoca (37.5% MDR, 50% XDR, and 12.5% PDR), E. clocae (66.7% MDR, 16.7% XDR), and C. freundii (25% MDR, 75% XDR) were developed multidrug resistant.
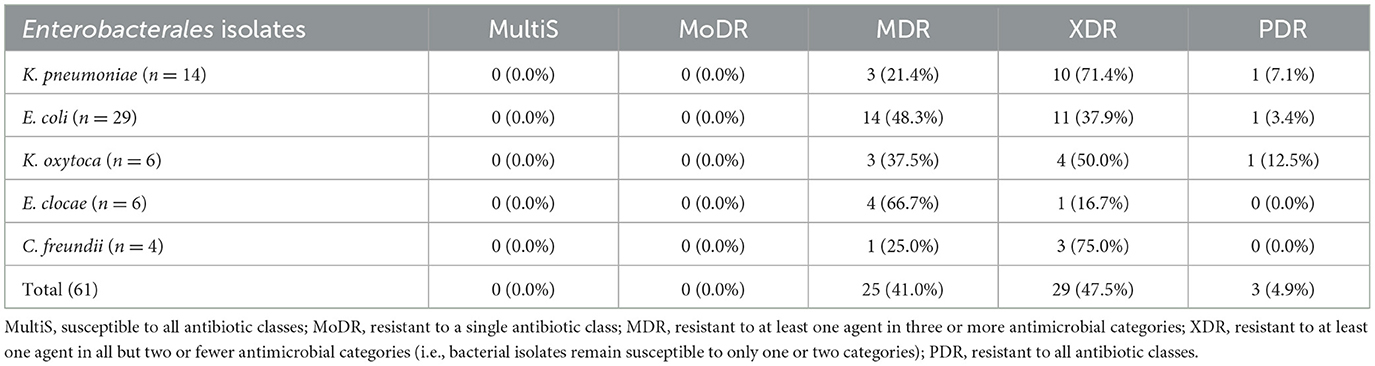
Table 5. Multidrug-resistant pattern of extended-spectrum β-lactamase and carbapenemase producing Enterobacteriaceae isolates among surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 61).
Factors association with extended-spectrum β-lactamase and carbapenemase producing Enterobacteriaceae among patients suspected for surgical site infection
Four variables with p-values < 0.25 in univariate analysis suggested using the multivariate model. Age ranges of 31–44 and 45–96 years, respectively, had AORs of 1.51 and 2.88 and 0.92 and 0.45–1.85, 95% confidence intervals. Individuals admitted to the orthopedic and surgical wards had AORs of 1.15 and 0.64–2.05 and 1.94 and 95% CI: 1.04–3.65, respectively, and their length of hospital stay were 8.14 days for the former and >15 days for the latter (AOR = 3.81 and 95% CI: 2.08–6.95). Candidates for multivariate logistic regression analysis were patients with diabetes and those without (AOR = 1.91 and 95% CI: 0.77–4.76), respectively. A similar independent association between the candidate variables and surgical site infection caused by Enterobacteriaceae that produce ESBLs and CPEs was further evaluated at a cutoff point of P-value < 0.05 in multivariable logistic regression analysis. Out of all of them, the multivariate logistic regression analysis revealed a significant association with only the length of hospital stay being independently associated. Length of medical stay in hospital AOR = 3.81 (95% CI 2.08–6.95) was found to be a statistically significant factor in surgical site infection caused by Enterobacteriaceae that produce CPE and ESBL in the multivariable analysis. Due to Enterobacteriaceae that produce CPE and ESBL, patients hospitalized for 8–14 days had a 6-fold higher risk of surgical site infection than patients who stayed for >15 days; the other variables were not significantly associated with this risk.
Discussion
The main aim of this study was to address three key objectives. All these objectives determined the prevalence, antibiotic susceptibility patterns, and factors associated with Extended-spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae among patients suspected with surgical site infection.
In the present study, the total prevalence of Enterobacteriaceae growth among surgical site infections was 23.7%. This finding is consistent with the previous reports in Addis Ababa, Ethiopia (25.13%) (Beyene et al., 2019), Bahir Dar, Ethiopia (24.8%) (Alebel et al., 2021), France (25%) (Baron et al., 2013), Nepal (28.2%) (Kayastha et al., 2020), Qatar (26%) (Sid Ahmed et al., 2016), and India (21.4%) (Gupta et al., 2016). However, this was lower than the research conducted in Debra Markos, Ethiopia (72.6%) (Shimekaw et al., 2022), Gondar (83.9%) (Mohammed et al., 2017), Jimma (87.3%) (Mama et al., 2014), Addis Ababa (84.1%) (Beyene et al., 2019), Nigeria (64.8%) (Egbe et al., 2011), India (68 %) (Vasundhara et al., 2017), and Nepal (65%) (Rijal et al., 2017) and higher than the study done in Mexico (19%) (Uc-Cachón et al., 2019), India (19.5%) (Singh et al., 2016), and Nepal (12.6%) (Kayastha et al., 2020). These variations among different studies could be due to variations in the study population, bad or good antibiotic stewardship policies, level of wound care, and specimen type.
In the present study, the prevalence of ESBL-producing Enterobacterales was 46%, which is in line with the institution-based based cross-sectional study conducted in Algeria (47.6%) (Nedjai et al., 2013), and Philippines (43%) (Bell et al., 2003). However, it was higher than that reported in Bahir Dar Ethiopia 24.8% (Alebel et al., 2021), India 35.2%(Oberoi et al., 2013), France (25%) (Baron et al., 2013), Nepal (28.2%) (Kayastha et al., 2020), Qatar (26%) (Sid Ahmed et al., 2016), and India (21.4%) (Gupta et al., 2016). In some of this study since compared to the aforementioned studies or for ESBL-producing Enterobacteriaceae, colistin was an alternative treatment only in the study area, however carbapenemase are not used as treatment (Katip et al., 2021).
In this study among Enterobacteriaceae, E. coli 29/100 (29.0%) was the predominant isolate associated with surgical site infection, followed by K. pneumoniae 14/100 (14%), K. oxytoca 8/100(8%), E. clocae 6/100 (6%) and C. freundii 4/100 (4%). This is supported by various findings in Africa, Uganda, Algeria, Brazil (Iabadene et al., 2008; Andrew et al., 2017; Ibrahim et al., 2013; Pollack and Srinivasan, 2014) and Mexico (Uc-Cachón et al., 2019). These findings revealed that Enterobacteriaceae isolates were the most common hospital-acquired infections.
In our study, the prevalence of carbapenemase producing Enterobacteriaceae isolates was 15%, which agreed with the research conducted in Taiwan, (15.4%) (Chang et al., 2015) Indonesia (13.7%) (Saharman and Lestari, 2013). This finding is higher than that study conducted in Bahir Dar, Ethiopia (5.2%) (Alebel et al., 2021), Addis Ababa Ethiopia (2%) (Beyene et al., 2019), and CDC reports from (2001) (1.2%) and 2011 (4.2%) (Ramana et al., 2013); however, our findings were lower than those reported in Nigeria (36%) (Pollack and Srinivasan, 2014), India (34.5%) (Oberoi et al., 2013), and 44.1% (Makharita et al., 2020). This may be attributed to the rapid expansion of CPE-producing organisms or misuse of antibiotics.
The most common carbapenemase producing Enterobacteriaceae isolates in our study were K. pneumoniae (46.7 %), followed by E. coli (26.7%) and K. oxytoca (13.3%). A similar finding in Nigeria E. coli 15.4% and K. pneumoniae 40.9% (Pollack and Srinivasan, 2014), and Tanzania E. coli (14%), followed by K. pneumoniae (10.57%) are carbapenemase-producing Enterobacteriaceae (Mushi et al., 2014). In this study, the most common carbapenemases were MBL 2 (13.3%), KPC plus MBL 1 (6.7%) and on the other side, AmpC plus porin loss 12 (80%). These findings are in line those study conducted on USA isolates produced KPC 36 (28%), MBL 31 (24.2%), KPC plus MBL 4 (3.1%), ESBL 19 (14.8), AmpC 10 (7.8%) (van Dijk et al., 2014). A similar study in India showed MBL producers were 10.98% similar to this study (Kumar et al., 2012), while AmpC production was observed in 5.4% (Oberoi et al., 2013), which is much less than our findings. This shows that in our study, plasmid and transposon-mediated gene transfer was more common in our isolates than in the Indian study. This is may be due to variation of geographical location and use of sample size amount. The coexistence of different classes of β-lactamases in a single bacterial isolate may pose diagnostic and treatment challenges, although high-level expression of AmpC β-lactamases may mask the recognition of ESBLs, which may result in more difficult antimicrobial therapy (Oberoi et al., 2013).
The current study showed that Enterobacteriaceae had higher antibiotic resistance rates of for cefepime (91.8%), ceftriaxone (95.1%), ceftazidime (91.8%), and amoxicillin-clavulanic acid (98.4%). However, Enterobacteriaceae isolates showed relatively high sensitivity to Meropenem 44 (72.1%). On the other hand, high percentages of resistance were obtained, gentamicin: 39 (63.9%) and ciprofloxacin: 43 (70.5%), respectively. This finding is consistent with that study conducted in Nepal on cefepime (86.2%), ceftriaxone (100%), and ceftazidime (100%) were resistance (Beyene et al., 2019). Similarly to studies conducted in Ethiopia, amoxicillin/clavulanic acid 75% and cefotaxime (73.7%) were found to be resistant to Enterobacteriaceae isolates (Dessie et al., 2016).
In the current study, MDR rate of Enterobacteriaceae isolates was 41% (showing resistance to at least one agent in ≥3 antimicrobial categories) among patients suspected of surgical site infection, 47.5% (showing resistance at least to one agent in all but two or fewer antimicrobial categories) XDR, and 4.9% (showing resistance to all agents in all antimicrobial categories) PDR were resistant, respectively. Among the Enterobacteriaceae isolates, E. coli, 48.3% MDR, 37.9% XDR, 3.4% PDR, K. pneumoniae 21.4% MDR, 71.4% XDR, 7.1% of PDR, K. oxytoca 37.5% MDR, 50% XDR, 12.5% PDR, E. clocae 66.7% MDR, 16.7% XDR, C. freundii 25% MDR, 75% XDR were developed resistance, while the study in Jimma, Ethiopia showed MDR 30.16%, XDR 41.27%, and PDR 19.0% were found (Mama et al., 2014; Gashaw et al., 2018), there are few previous reports from Ethiopia on XDR and PDR Enterobacteriaceae to compare with this finding, which has been associated with lack of AMR surveillance and stewardship programs at WSUCSH and SCGH and also in southern Ethiopia.
Factors associated with surgical site infection with ESBL and CPE-producing Enterobacteriaceae included indicators of contact with the health care system (length of hospital stay, immunosuppressive drug (long-term steroid; Livermore et al., 2011), patient admitted wards, educational level, and the presence of comorbidities). Four variables of associated factors were independently predictive of ESBL positivity as showed P-value of < 0.25 by univariate analysis: age in group 31–45 years, immunosuppressive drug use (long-term steroid), length of hospital stay, and diabetes. Among them, only the length of hospital stay was independently associated with the multivariate logistic regression analysis, which showed a significant association. This study was supported by a previous study conducted in Israel (Mendelson et al., 2005). The increased odds of our finding was that the presence of predisposing conditions such as surgical intervention was significantly associated with ESBL producers (P < 0.05) compared with a short length of hospital stay, which is similar to a study conducted in Debra Markos, Ethiopia (Alebel et al., 2021). In this study, the remaining variables were not significantly associated with surgical site infections caused by ESBL-producing Enterobacteriaceae (P >0.05; Table 6).
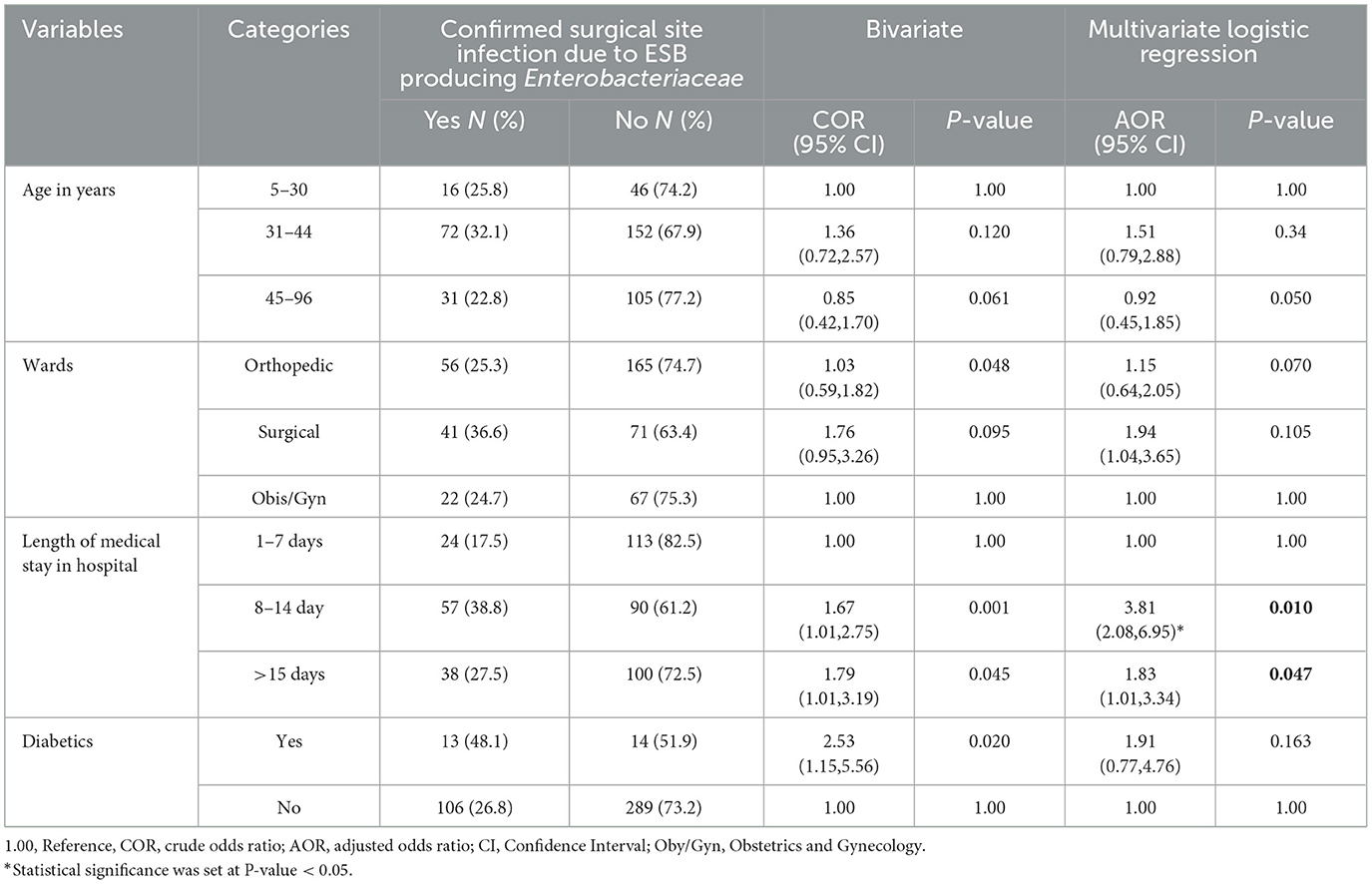
Table 6. Bivariate and multivariate logistic regression analysis on socio demographic and clinical condition of the patients suspected for surgical site infection at Hospitals in Southern Ethiopia, from Jun 1, 2022 to August 30, 2022 (n = 422).
Strength and limitation of the study
This study has several advantages. It provides useful information about the prevalence and antimicrobial susceptibility trends of potentially pathogenic Enterobacteriaceae in hospital settings, especially in SSI cases. This study also found the presence of multidrug-resistant bacteria, including ESBLs and carbapenemase-producing strains, which are extremely dangerous in healthcare settings. Drug susceptibility testing for nine antibiotics including extended-spectrum cephalosporins; 3rd and 4th generation cephalosporins, carbapenemase plus combination disks like Meropenem 10 μg, Meropenem+Phenolboroic acid, Meropenem+Dipicolonic acid, Meropenem +Cloxacillin, and Temocillin 30 μg was tested. This study had a drawback, although it was conducted in a two hospital, which might not be an accurate representation of other hospitals in the country level. Anaerobic and slow-growing bacteria were not considered in this study. Ultimately, the results of the study were a low number of Enterobacteriaceae isolates, which resulted in a low number of ESBL and CPE that might have affected the findings of the risk factors. Additionally, the study was limited by the available resources and time frame for research that makes identification of ESBL- and carbapenemase-producing strains by phenotypically only even if it is recommended to perform detection by molecular biology techniques for identification of ESBL- and carbapenemase-producing strains.
Conclusion and recommendation
Enterobacteriaceae were present in all SSIs at a rate of 23.7% (100/422). E. coli predominated, followed by K. pneumoniae, with a total prevalence of 46% for ESBL and 15% for CPE. It was concerning to see the high rates of MDR and XDR ESBL and carbapenem resistance. According to the study's findings, a hospital stay's duration significantly affects the risk of site infection. When making decisions about the high prevalence of MDR Enterobacteriaceae in relation to antibiotics like cefepime, ceftriaxone, ceftazidime, and amoxicillin-clavulanic acid, empirical treatment should be taken into account. On the other hand, thorough instruction, referral, and standard hospital procedures help avoid or manage surgical site infections. When choosing the optimal antimicrobial agents for patients with infections caused by ESBL Enterobacteriaceae and CRE in southern Ethiopia, physicians can make informed decisions about public health thanks to the documented data presented in this study. Therefore, in order to address this public health issue, stakeholders involved in Ethiopia's health system must work cooperatively. And also, appropriate antibiotic prescribing practices and avoidance of broad spectrum antibiotics can prevent long-term emergence of resistance. Additionally, more investigation is required to fully understand the implications of Enterobacteriaceae that produce CPE and ESBL and their effects on patients who may be at risk for surgical site infections in different hospital with different level.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethical Review Committee (ERC) of the College of Health Science and Medicine, Wolaita Sodo University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
DO: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. TD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. TM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. MT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Wolaita Sodo University Specialized Hospital and Sodo Christian General Hospital for providing permission to conduct the study. We would also like to thanks Ethiopian Public Health Institutes for providing control organisms, culture media, reagents, and antimicrobial disks. The authors especially thank to Tilahun (2022), doi: 10.20944/preprints202305.2212.v1 and Temesgen et al. (2023) due to we followed their methods in order to receiving some necessary information for our study. Finally, all study participants, data collectors, and supervisors were acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alebel, M., Mekonnen, F., and Mulu, W. (2021). Extended-spectrum β-lactamase and carbapenemase producing gram-negative bacilli infections among patients in intensive care units of felegehiwot referral hospital: a prospective cross-sectional study. Infect. Drug Resist. 14, 391–405. doi: 10.2147/IDR.S292246
Andrew, B., Kagirita, A., and Bazira, J. (2017). Prevalence of extended-spectrum beta-lactamases-producing microorganisms in patients admitted at KRRH, Southwestern Uganda. Int. J. Microbiol. 2017:3183076. doi: 10.1155/2017/3183076
Ban, K. A., Minei, J. P., Laronga, C., Harbrecht, B. G., Jensen, E. H., Fry, D. E., et al. (2017). American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 224, 59–74. doi: 10.1016/j.jamcollsurg.2016.10.029
Baron, E. J., Miller, J. M., Weinstein, M. P., Richter, S. S., Gilligan, P. H., Thomson, R. B., et al. (2013). A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the infectious diseases society of America (IDSA) and the American Society for Microbiology (ASM). Clin. Infect. Dis. 57:cit441. doi: 10.1093/cid/cit441
Batchelor, M., Hopkins, K. L., Liebana, E., Slickers, P., Ehricht, R., Mafura, M., et al. (2008). Development of a miniaturised microarray-based assay for the rapid identification of antimicrobial resistance genes in Gram-negative bacteria. Int. J. Antimicrob. Agents 31, 440–451. doi: 10.1016/j.ijantimicag.2007.11.017
Bell, J. M., Turnidge, J. D., and Jones, R. N. (2003). Prevalence of extended-spectrum β-lactamase-producing enterobacter cloacae in the Asia-Pacific Region: results from the SENTRY Antimicrobial Surveillance Program, 1998 to 2001. Antimicrob. Agents Chemother. 47, 3989–3993. doi: 10.1128/AAC.47.12.3989-3993.2003
Beyene, D., Bitew, A., Fantew, S., Mihret, A., and Evans, M. (2019). Multidrug-resistant profile and prevalence of extended spectrum β-lactamase and carbapenemase production in fermentative Gram-negative bacilli recovered from patients and specimens referred to National Reference Laboratory, Addis Ababa, Ethiopia. PLoS ONE 14:e0222911. doi: 10.1371/journal.pone.0222911
Cantón, R., Novais, A., Valverde, A., Machado, E., Peixe, L., Baquero, F., et al. (2008). Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl.1), 144–153. doi: 10.1111/j.1469-0691.2007.01850.x
Carvalhaes, C. G., Picão, R. C., Nicoletti, A. G., Xavier, D. E., and Gales, A. C. (2009). Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother. 65, 249–251. doi: 10.1093/jac/dkp431
CDC, T., Report, H. A. I. P., Care, S., and Project, I. (2022). Surgical Site Infection Event (SSI) Introduction: Settings: Requirements. National center for emergency and zoonotic infectious diseases. 1–39.
Chang, Y. Y., Chuang, Y. C., Siu, L. K., Wu, T. L., Lin, J. C., Lu, P. L., et al. (2015). Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J. Microbiol. Immunol. Infect. 48, 219–225. doi: 10.1016/j.jmii.2014.05.010
Cury, A. P., Andreazzi, D., Maffucci, M., Caiaffa-Junior, H. H., and Rossi, F. (2012). The modified Hodge test is a useful tool for ruling out Klebsiella pneumoniae carbapenemase. Clinics 67, 1427–1431. doi: 10.6061/clinics/2012(12)13
Date, A. (2018). Modified Hodge Test for Suspected Carbapenemase Production in Enterobacterales. Clinical and Laboratory Standards Institute Archived Methods, 2–4.
Dessie, W., Mulugeta, G., Fentaw, S., Mihret, A., Hassen, M., Abebe, E., et al. (2016). Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals-Ethiopia. Int. J. Microbiol. 2016:2418902. doi: 10.1155/2016/2418902
Egbe, C. A., Omoregie, R., Igbarumah, I. O., and Onemu, S. (2011). Microbiology of wound infections and its associated risk factors among patients of a Tertiary hospital in Benin City, Nigeria. JRHS 11, 109–113.
Farzana, R., Shamsuzzaman, S. M., Mamun, K. Z., and Shears, P. (2013). Antimicrobial susceptibility pattern of extended spectrum β-lactamase producing gram-negative bacteria isolated from wound and urine in a tertiary care hospital, Dhaka City, Bangladesh. Southeast Asian J. Trop. Med. Publ. Health 44, 96–103.
Gashaw, M., Berhane, M., Bekele, S., Kibru, G., Teshager, L., Yilma, Y., et al. (2018). Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: a cross sectional study. Antimicrob. Resist. Infect. Control 18, 1–8. doi: 10.1186/s13756-018-0431-0
Ghafourian, S., Sadeghifard, N., Soheili, S., and Sekawi, Z. (2014). Extended spectrum beta-lactamases: definition, classification and epidemiology. Curr. Iss. Mol. Biol. 17, 11–22.
Giske, C. G., and Cantón, R. (2013). European Committee on Antimicrobial Susceptibility Testing Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance, Version 1. 1–40.
Giske, C. G., Gezelius, L., Samuelsen, A., Warner, M., Sundsfjord, A., and Woodford, N. (2011). A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17, 552–556. doi: 10.1111/j.1469-0691.2010.03294.x
Gupta, R., Malik, A., Rizvi, M., and Ahmed, M. (2016). Presence of metallo-beta-lactamases (MBL), extended-spectrum beta-lactamase (ESBL) & AmpC positive non-fermenting gram-negative bacilli among intensive care unit patients with special reference to molecular detection of blaCTX-M & blaAmpC genes. Indian J. Med. Res. 144, 271–275. doi: 10.4103/0971-5916.195043
Hello, M., Caroff, N., Jacqueline, C., Caillon, J., Potel, G., Batard, E., et al. (2010). Influence of the AtlE autolysin on the activity of cell wall-active agents against Staphylococcus epidermidis. Int. J. Antimicrob. Agents 35, 204–206. doi: 10.1016/j.ijantimicag.2009.09.026
Hoban, D. J., Lascols, C., Nicolle, L. E., Badal, R., Bouchillon, S., Hackel, M., et al. (2012). Antimicrobial susceptibility of Enterobacteriaceae, including molecular characterization of extended-spectrum beta-lactamase-producing species, in urinary tract isolates from hospitalized patients in North America and Europe: results from the SMART study. Diagn. Microbiol. Infect. Dis. 74:627. doi: 10.1016/j.diagmicrobio.2012.05.024
Iabadene, H., Messai, Y., Ammari, H., Ramdani-Bouguessa, N., Lounes, S., Bakour, R., et al. (2008). Dissemination of ESBL and Qnr determinants in Enterobacter cloacae in Algeria. J. Antimicrob. Chemother. 62, 133–136. doi: 10.1093/jac/dkn145
Ibrahim, M. E., Bilal, N. E., Magzoub, M. A., and Hamid, M. E. (2013). Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from hospitals in Khartoum State, Sudan. Oman. Med. J. 28, 116–120. doi: 10.5001/omj.2013.30
Jolivet, S., Lescure, F. X., Armand-Lefevre, L., Raffoul, R., Dilly, M. P., Ghodbane, W., et al. (2018). Surgical site infection with extended-spectrum β-lactamase-producing Enterobacteriaceae after cardiac surgery: incidence and risk factors. Clin. Microbiol. Infect. 24, 283–288. doi: 10.1016/j.cmi.2017.07.004
Katip, W., Yoodee, J., Uitrakul, S., and Oberdorfer, P. (2021). Efficacy of loading dose colistin versus carbapenems for treatment of extended spectrum beta lactamase producing Enterobacteriaceae. Sci. Rep. 11, 1–8. doi: 10.1038/s41598-020-78098-4
Kayastha, K., Dhungel, B., Karki, S., Adhikari, B., Banjara, M. R., Rijal, K. R., et al. (2020). Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children's Hospital, Kathmandu, Nepal. Infect. Dis. Res. Treat. 13:117863372090979. doi: 10.1177/1178633720909798
Khan, M., and Khalil, J. (2011). Rooh-ul-Muqim, Zarin M, Touseef Ul Hassan, Ahmed N, et al. Rate and risk factors for surgical site infection at a tertiary care facility in Peshawar, Pakistan. J. Ayub. Med. Coll. Abbottabad. 23, 15–18.
Kumalo, A., Gebre, B., Shiferaw, S., Wolde, W., and Shonde, T. (2023). Group B Streptococci recto-vaginal colonization, antimicrobial susceptibility pattern, and associated factors among pregnant women at selected health facilities of Wolaita Sodo Town, Southern Ethiopia. Front. Microbiol. 14:1277928. doi: 10.3389/fmicb.2023.1277928
Kumar, S. H., De, A. S., Baveja, S. M., and Gore, M. A. (2012). Prevalence and risk factors of metallo β-lactamase producing Pseudomonas aeruginosa and Acinetobacter species in burns and surgical wards in a Tertiary Care Hospital. J. Lab. Physicians 4, 039–42. doi: 10.4103/0974-2727.98670
Livermore, D. M., Warner, M., Mushtaq, S., Doumith, M., Zhang, J., Woodford, N., et al. (2011). What remains against carbapenem-resistant Enterobacteriaceae? evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37, 415–419. doi: 10.1016/j.ijantimicag.2011.01.012
Makharita, R. R., El-Kholy, I., Hetta, H. F., Abdelaziz, M. H., Hagagy, F. I., Ahmed, A. A., et al. (2020). Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect. Drug Resist. 13, 3991–4002. doi: 10.2147/IDR.S276975
Mama, M., Abdissa, A., and Sewunet, T. (2014). Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann. Clin. Microbiol. Antimicrob. 13, 1–10. doi: 10.1186/1476-0711-13-14
Mendelson, G., Hait, V., Ben-Israel, J., Gronich, D., Granot, E., Raz, R., et al. (2005). Prevalence and risk factors of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an Israeli long-term care facility. Eur. J. Clin. Microbiol. Infect. Dis. 24, 17–22. doi: 10.1007/s10096-004-1264-8
Miller, J. M., Binnicker, M. J., Campbell, S., Carroll, K. C., Chapin, K. C., Gilligan, P. H., et al. (2018). A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 67, e1–94. doi: 10.1093/cid/ciy584
Moges, F., Eshetie, S., Abebe, W., Mekonnen, F., Dagnew, M., Endale, A., et al. (2019). High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS ONE 14, 1–13. doi: 10.1371/journal.pone.0215177
Mohammed, A., Seid, M. E., Gebrecherkos, T., Tiruneh, M., and Moges, F. (2017). Bacterial isolates and their antimicrobial susceptibility patterns of wound infections among inpatients and outpatients attending the University of Gondar Referral Hospital, Northwest Ethiopia. Int. J. Microbiol. 2017:8953829. doi: 10.1155/2017/8953829
Mushi, M. F., Mshana, S. E., Imirzalioglu, C., and Bwanga, F. (2014). Carbapenemase genes among multidrug resistant gram negative clinical isolates from a tertiary hospital in Mwanza, Tanzania. Biomed. Res. Int. 2014, 3–8. doi: 10.1155/2014/303104
National, G., and Pillars, H. (2010). Cheesbrough Monica: District Laboratory Practice in Tropical Countries Volume II: Microbiology. Cambridge: Cambridge University Press, 51, 82–25, 38–39.
Nedjai, S., Barguigua, A., Djahmi, N., Jamali, L., Zerouali, K., Dekhil, M., et al. (2013). Prevalence and characterization of extended spectrum beta-lactamase-producing Enterobacter cloacae strains in Algeria. J. Infect. Dev. Ctries 7, 804–811. doi: 10.3855/jidc.3127
Nelson, E., Kayega, J., Seni, J., Mushi, M. F., Kidenya, B. R., Hokororo, A., et al. (2014). Evaluation of existence and transmission of extended spectrum beta lactamase producing bacteria from post-delivery women to neonates at Bugando Medical Center, Mwanza-Tanzania. BMC Res. Not. 7, 1–6. doi: 10.1186/1756-0500-7-279
Oberoi, L., Singh, N., Sharma, P., and Aggarwal, A. (2013). ESBL, MBL and Ampc β lactamases producing superbugs—Havoc in the intensive care units of Punjab India. J. Clin. Diagnost. Res. 7, 70–73. doi: 10.7860/JCDR/2012/5016.2673
Pierce, V. M., Simner, P. J., Lonsway, D. R., Roe-Carpenter, D. E., Johnson, J. K., Brasso, W. B., et al. (2017). Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J. Clin. Microbiol. 55, 2321–2333. doi: 10.1128/JCM.00193-17
Pollack, L. A., and Srinivasan, A. (2014). Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 59, S97–S100. doi: 10.1093/cid/ciu542
Ramana, K. V., Rao, R., Sharada, C. V., Kareem, M. A., Rajashekar Reddy, L., Ratna Mani, M. S., et al. (2013). Modified Hodge test: a useful and the low-cost phenotypic method for detection of carbapenemase producers in Enterobacteriaceae members. J. Nat. Sci. Biol. Med. 4, 346–348. doi: 10.4103/0976-9668.117009
Reumert, P., Nielsen, S., Diagnostica, M. R., Diatabs, G., Diatabs, I., Diatabs, K., et al. (2003). Rosco Diagnostica Rosco Diagnostica. Stensmosevej, 1–9.
Rijal, B. P., Satyal, D., and Parajuli, N. P. (2017). High burden of antimicrobial resistance among bacteria causing pyogenic wound infections at a Tertiary Care Hospital in Kathmandu, Nepal. J. Pathog. 2017, 1–7. doi: 10.1155/2017/9458218
Saharman, Y. R., and Lestari, D. C. (2013). Phenotype characterization of beta-lactamase producing Enterobacteriaceae in the intensive care unit (ICU) of Cipto Mangunkusumo Hospital in 2011. Acta Med. Indones 45, 11–16.
Sangare, S. A., Maiga, A. I., Guindo, I., Maiga, A., Camara, N., Savadogo, S., et al. (2015). Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated from blood cultures in Africa. Med. Mal. Infect. 45, 374–382. doi: 10.1016/j.medmal.2015.08.003
Saravanan, M., Ramachandran, B., and Barabadi, H. (2018). The prevalence and drug resistance pattern of extended spectrum β-lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 114:18092. doi: 10.1016/j.micpath.2017.11.061
Shimekaw, M., Tigabu, A., and Tessema, B. (2022). Bacterial profile, antimicrobial susceptibility pattern, and associated risk factors among patients with wound infections at Debre Markos Referral Hospital, Northwest, Ethiopia. Int. J. Low Extrem. Wounds 21, 182–192. doi: 10.1177/1534734620933731
Sid Ahmed, M. A., Bansal, D., Acharya, A., Elmi, A. A., Hamid, J. M., Sid Ahmed, A. M., et al. (2016). Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad medical corporation, Qatar. Antimicrob. Resist. Infect. Control 5, 1–6. doi: 10.1186/s13756-016-0103-x
Singh, N., Pattnaik, D., Neogi, D. K., Jena, J., and Mallick, B. (2016). Prevalence of ESBL in Escherichia coli isolates among ICU patients in a tertiary care hospital. J. Clin. Diagnost. Res. 10, DC19–DC22. doi: 10.7860/JCDR/2016/21260.8544
Storberg, V. (2014). ESBL-producing Enterobacteriaceae in Africa - a non-systematic literature review of research published 2008-2012. Infect. Ecol. Epidemiol. 4:20342. doi: 10.3402/iee.v4.20342
Temesgen, M., Kumalo, A., Teklu, T., Alemu, G., and Odoko, D. (2023). Bacterial profile and their antimicrobial susceptibility pattern of isolates recovered from intensive care unit environments at Wachemo University Nigist Ellen Mohammed Memorial Comprehensive Specialized Hospital, Southern Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2023:1216553. doi: 10.1155/2023/1216553
Tilahun, M. (2022). Multi-drug resistance profile, prevalence of extended-spectrum beta-lactamase and carbapenemase-producing gram negative bacilli among admitted patients after surgery with suspected of surgical site nosocomial infection North East Ethiopia. Infect. Drug Resist. 2022, 3949–3965. doi: 10.2147/IDR.S376622
Tsakris, A., Poulou, A., Pournaras, S., Voulgari, E., Vrioni, G., Themeli-Digalaki, K., et al. (2010). A simple phenotypic method for the differentiation of metallo-β-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J. Antimicrob. Chemother. 65, 1664–1671. doi: 10.1093/jac/dkq210
Uc-Cachón, A. H., Gracida-Osorno, C., Luna-Chi, I. G., Jiménez-Guillermo, J. G., and Molina-Salinas, G. M. (2019). High prevalence of antimicrobial resistance among gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Med. 55:588. doi: 10.3390/medicina55090588
van Dijk, K., Scharringa, J., Voets, G., Voskuil, S., Fluit, A., Rottier, W., et al. (2014). A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin. Microbiol. Infect. 20, 345–349. doi: 10.1111/1469-0691.12322
Van Duin, D. (2017). Carbapenem-resistant Enterobacteriaceae: what we know and what we need to know. Virulence 8, 379–382. doi: 10.1080/21505594.2017.1306621
Van Duin, D., and Doi, Y. (2017). The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8, 460–469. doi: 10.1080/21505594.2016.1222343
Vasundhara, P., Sreenivasuslu, P., and Shabnaum, M. (2017). Microbial profile and antibiotic susceptibility pattern of orthopedic infections in a tertiary care hospital: a study from South India. Int. J. Med. Sci. Publ. Heal. 6:1. doi: 10.5455/ijmsph.2017.1165105122016
Keywords: ESBL, carbapenemase, Enterobacteriaceae, SSI, southern Ethiopia
Citation: Odoko D, Kumalo A, Alemu G, Demisse T, Mulugeta T and Temesgen M (2024) Extended-spectrum β-lactamase and carbapenemase producing Enterobacteriaceae among patients suspected with surgical site infection at Hospitals in Southern Ethiopia. Front. Microbiol. 15:1417425. doi: 10.3389/fmicb.2024.1417425
Received: 14 April 2024; Accepted: 14 October 2024;
Published: 11 November 2024.
Edited by:
João Pedro Rueda Furlan, Federal University of São Paulo, BrazilReviewed by:
Gamal Mohamed El-Said El-Sherbiny, Al-Azhar University, EgyptHelia Magaly Bello-Toledo, University of Concepcion, Chile
Copyright © 2024 Odoko, Kumalo, Alemu, Demisse, Mulugeta and Temesgen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abera Kumalo, YWJlcmFrMjAwMEBnbWFpbC5jb20=
 Desta Odoko
Desta Odoko Abera Kumalo
Abera Kumalo Getachew Alemu2
Getachew Alemu2