
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 September 2024
Sec. Terrestrial Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1413973
This article is part of the Research TopicExploring the Effects of Human Activities and Climate Change on Soil Microorganisms in GrasslandsView all 9 articles
Land conversion to agriculture is an important factor affecting soil ecological processes in the desert grasslands of northern China. However, soil fungal-community structure and function in response to Land conversion remain unclear. In this study, desert grassland, artificial shrubland, and land conversion were investigated in the western part of the Mu Us Sandland (Yanchi, Ningxia; Dingbian, Shaanxi). We found that land conversion significantly increased soil total carbon, nitrogen, and phosphorus, and available phosphorous and potassium contents. In the early stage of conversion to agricultural (April), soil fungal operational taxonomic units and abundance-based coverage estimator were lower than those of dessert grasslands and shrubland plots and had significant correlations with pH, electric conductivity, and available phosphorus and potassium. The dominant phyla strongly correlated with soil physicochemical properties. Concomitantly, the relative abundance of Glomeromycota was significantly lower, and the complexity of the network in the land conversion plots was lower than that in the shrubland plots. In the late stage of land conversion (September), soil fungal operational taxonomic units and abundance-based coverage estimator were lower in the conversion plots than in the desert grassland plots, with more complex network relationships compared to the desert grassland or shrubland plots. Symbiotrophic groups, a functional group of desert grassland soil fungi, can be used as a predictor of environmental change; in addition, land conversion decreases the relative abundance of arbuscular mycorrhizal functional groups. Our study highlights the response of soil fungal communities and functions to human disturbances in desert grasslands. Considering the potential of land conversion to agriculture to influence soil secondary salinization, there is a need for continued observation of soil ecological health over the time continuum of land conversion to agriculture.
Global climate change profoundly affects the structure and function of desert grassland (DG) ecosystems, where limited water availability has become a major barrier to restoration and reconstruction (Yang et al., 2021; Sun et al., 2022). In addition, human disturbances have increased the vulnerability of desert grassland ecosystems by, for example, the reduction of vegetation cover associated with land conversion to agriculture (spring clearing and fall harvesting) (Xu et al., 2020), or through further salinization of the soil by groundwater extraction for irrigation (Hu et al., 2021). The additional water and nutrient inputs to the soils of arid areas associated with land conversion to agriculture not only alter the soil’s physical properties but break the elemental balance of the soils in this region as well, thereby accelerating the irreversible impacts of the loss of soil nutrients such as soil organic matter and nitrogen (Li et al., 2020; Wang et al., 2023). Hence, there is a need for research on farmland conversion in the desert grasslands of northwestern China, as such farmland conversion is widespread in this region.
Soil fungal communities play an important role in regulating the operation of terrestrial ecosystems and researchers have extensively reported on the effects of land-use changes, such as afforestation and land conversion (Qiang et al., 2020; Luo S. et al., 2023; Luo X. et al., 2023). Thus, while afforestation increases fungal diversity, land conversion alters soil fungal community structure. Specifically, the richness of the fungal community increases with increasing time of conversion, and the diversity of the fungal community changes (Qiang et al., 2020). Further, it is worth noting that soil fungal communities are sensitive to changing climatic factors, especially precipitation and temperature. Seasonal variation-driven environmental changes in these variables profoundly affect soil fungal-community structure and function (Wang et al., 2020). For example, the functional groups of saprophytic fungi explain the effects of land use on soil physical and chemical properties in desert grasslands (Kang et al., 2023). The relationship between soil fungi and plant communities, and the drivers of soil fungal communities that differ between deserts and grasslands constitute another example (Wang et al., 2018). However, the effects of land conversion to agriculture on the community structure and function of soil fungi have been largely ignored.
While studying desert grasslands in northern China, to date researchers have not only focused on the interrelationship between soil fungal communities and plant communities (Wang et al., 2018), additionally, they have clarified the response of soil fungal communities to environmental changes in these ecosystems (Cui et al., 2018). In an increasing number of studies, the strength of network relationships has been used to prove the response of fungal taxa to climate and soil environment changes. However, most studies focus on the changes of nodes and edges in the network, because the degree of nodes often reflects the importance of microbial taxa in the network relationship, just like a transportation hub. Positive links and negative edges can also intuitively represent the relationship between nodes. In recent studies, the calculation and extraction of subnetwork data not only deepened our understanding of network complexity, but also clarified the important role that the network complexity index (NCI) plays in explaining environmental change, which represents the complex interactions between microbes, including symbiosis, competition, predation, and other relationships (Luo S. et al., 2023; Luo X. et al., 2023). Therefore, it is reasonable to assume that elucidating the response characteristics of fungal network NCI to soil environmental changes in the context of land conversion in desert grasslands is also of positive significance.
In addition, the fungal functional guild is not only a way to analyze the lifestyle of identified taxa (Nguyen et al., 2016), but also serves as a class of groups for evaluating the response of fungal taxa to soil environmental changes (Kang et al., 2023; Liu et al., 2023). Further, the nutrient acquisition strategies and ecological functions of the fungal taxa can be represented in functional guilds (Nguyen et al., 2016). Especially in the west of the Mu Us Sandland in northern China (Yanchi, Ningxia; Dingbian, Shaanxi), the dynamic response of the soil fungal-community structure and functional changes to land conversion in desert grassland ecosystems, remain unclear. Therefore, this study investigated desert grassland, shrubland, and converted land soils, and analyzed soil physicochemical properties and fungal ITS high-throughput sequencing in April and September, respectively, to address the following scientific questions: (1) which soil environmental factors have a positive response to soil fungal community structures under land conversion; (2) what are the changes of soil fungal functional guilds under conversion to agriculture?
The study area is located in the west of Ordos and the Mu Us sandy land in the east, which belongs to the desert grassland ecosystem. The predominant plants in the study area are Sophora alopecuroides, Peganum harmala, Caragana korshinkii, Tamarix chinensis, and Nitraria tangutorum, most of which are xerophytes. The average annual temperature in the past 5 years was 7.9°C and the average annual precipitation was 255.35 mm. Maize cultivation has become a major approach to improve the economic income of local farmers within the region. However, arable land is limited and conversion of desert grassland for maize cultivation is widespread. The desert grassland is converted and then fertilized to increase nitrogen, phosphorus, and potassium inputs (CH4N2O: 450 kg/ha year−1; N-P2O5-K2O: 300 kg/ha year−1); this is followed by another application of fertilizers at the same concentration as the first time in June and August, and approximately three times throughout the year. The corn field is watered mainly by pumping groundwater approximately 4–5 times according to the actual precipitation of the year.
This study was conducted on desert grassland (DG, S. alopecuroides community) and artificial shrub land (SL, C. korshinkii community) that had been maintained for over 8 years, and land conversion to agricultural land (RL, a former S. alopecuroides community converted to agriculture in March 2021 and planted with maize). Among the studied areas, the DG and SL each cover an area exceeding 10 km2, while the RL is approximately 5 km2. Within each study area, comprising DG, SL, and RL, nine sampling sites were selected, with the distance between each sampling point exceeding 200 meters (Figure 1). To clarify the effect on soil physicochemical properties under seasonal variations, we obtained samples in April (average temperature (MMT), 10.1°C and average precipitation (MMP), 29.3 mm) and September (average temperature, 17.9°C and average precipitation, 55.5 mm) 2021. In each sampling site, 100 m2 was randomly divided and 0–10 cm of soil was collected by the 5-point sampling method as a representative of that sampling site (Pan et al., 2021). After surface impurities were removed, two soil samples were collected at each sample point and transferred to the lab using an ice box. One of the soil samples was used for physical and chemical characterization after being sieved through a 2-mm sieve. The other was used for ITS high-throughput sequencing of fungi. The latter process was carried out in a sterile environment. PBS buffer was added to the soil sample and shaken to remove roots and other impurities. Then, the soil sample was centrifuged at 6000 g for ten minutes at 4°C, and the supernatant was removed to obtain the desired soil sample.
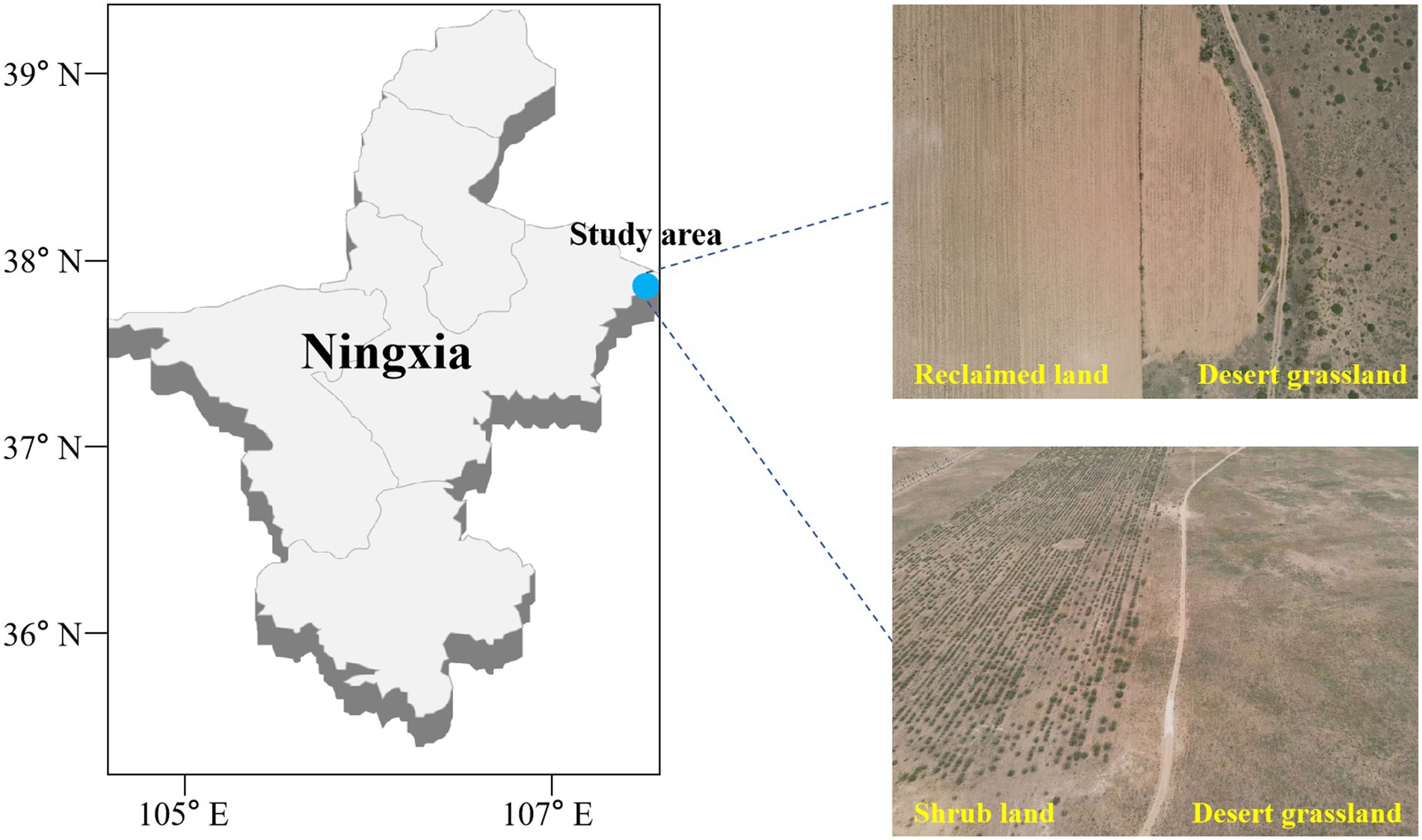
Figure 1. Sampling sites in the western part of the Mu Us Sandland at the junction of Yanchi County, Ningxia and Dingbian County, Shaanxi.
The soil water content (SWC) was determined using the weighing method, and soil pH and electrical conductivity (EC) were determined at a soil-water ratio of 1:2.5 (Bao, 2000). The sieved soil was introduced into a carbon and nitrogen analyzer for the determination of total carbon (TC) and nitrogen (TN) (CN 802 Carbon Nitrogen Elemental Analyzer, Shanghai, China). The content of total phosphorus (TP) in the soil was determined using the sulfate-molybdenum dysprosium resistance colorimetric method. Soil total organic carbon (TOC) content was determined through potassium dichromate oxidation spectrophotometry (Jiang et al., 2024). The content of soil alkali-hydrolysable nitrogen (AN) was determined using the zinc-ferrous sulfate reduction method (Wu et al., 2021). The soil available phosphorus (AP) was determined using the sodium hydrogen carbonate-molybdenum-dysprosium anti spectrophotometric method, and the soil available potassium (AK) was determined using ammonium acetate flame spectrophotometry (Bao, 2000).
DNA was extracted from screened soils using a 16-alkyltrimethylammonium bromide kit, and the ITS1-2 gene region of 54 eligible samples was amplified with primers ITS1 (5’-CTTG GTCA TTTA GAGG AAGT AA-3′) and ITS2 (5’-GCTG CGTT CTTC ATCG ATGC-3′) (Cheng et al., 2022). After PCR amplification, Illumina NovaSeq PE250 platform was used for sequencing. An average of 86,200 tags were measured per sample, and an average of 84,713 valid data were obtained for quality control. Subsequently, we standardized the data from each sample by subsampling based on the sample which has a minimum sequence depth. All subsequent analyses are based on this program. Clustering sequences into operational taxonomic units (OTUs) with 97% identity yields 5,893 OTUs. Fungal raw data for 54 soil samples from this study were submitted to the NCBI database (PRJNA1064665).
Soil physicochemical characteristics of the three sampling sites in April and September were statistically analyzed using ANOVA (Supplementary Figure S1). The OTUs, and Shannon and ACE indices for the fungal communities were calculated using QIIME (V1.9.1) software (Caporaso et al., 2012). Bray-Curtis was used to calculate the beta diversity index of fungal communities (Kang et al., 2022). The Mantel test, implemented via the linkET package in R software (v 4.1), was employed to assess the Spearman correlation between the matrix of fungal alpha diversity (e.g., OTUs, and Shannon and ACE indices) and soil physicochemical properties. During this process, the default mode was selected for other parameters (Sunagawa et al., 2015) (Figure 2). The dominant fungal phyla (average relative abundance >1%) between the groups were presented by the “circlize” package (Pan et al., 2021). In addition, the differences in the relative abundance of dominant phyla were also analyzed by ANOVA and shown using the “ggplot2” software package (Figure 3). To clarify the response of soil fungal phyla to soil environmental changes in the three sampling plots, we determined the relationship between dominant phyla and environmental factors by Spearman’s analysis (Pan et al., 2022). After the fungal community was standardized by the Hellinger method, a non-metric multidimensional scaling (NMDS) analysis based on Bray-Curtis distance was performed. Meanwhile, the redundancy analysis (RDA) analysis further demonstrated the relationship between dominant fungal phyla and environmental factors at the OTU level in desert grassland (Supplementary Figure S2).
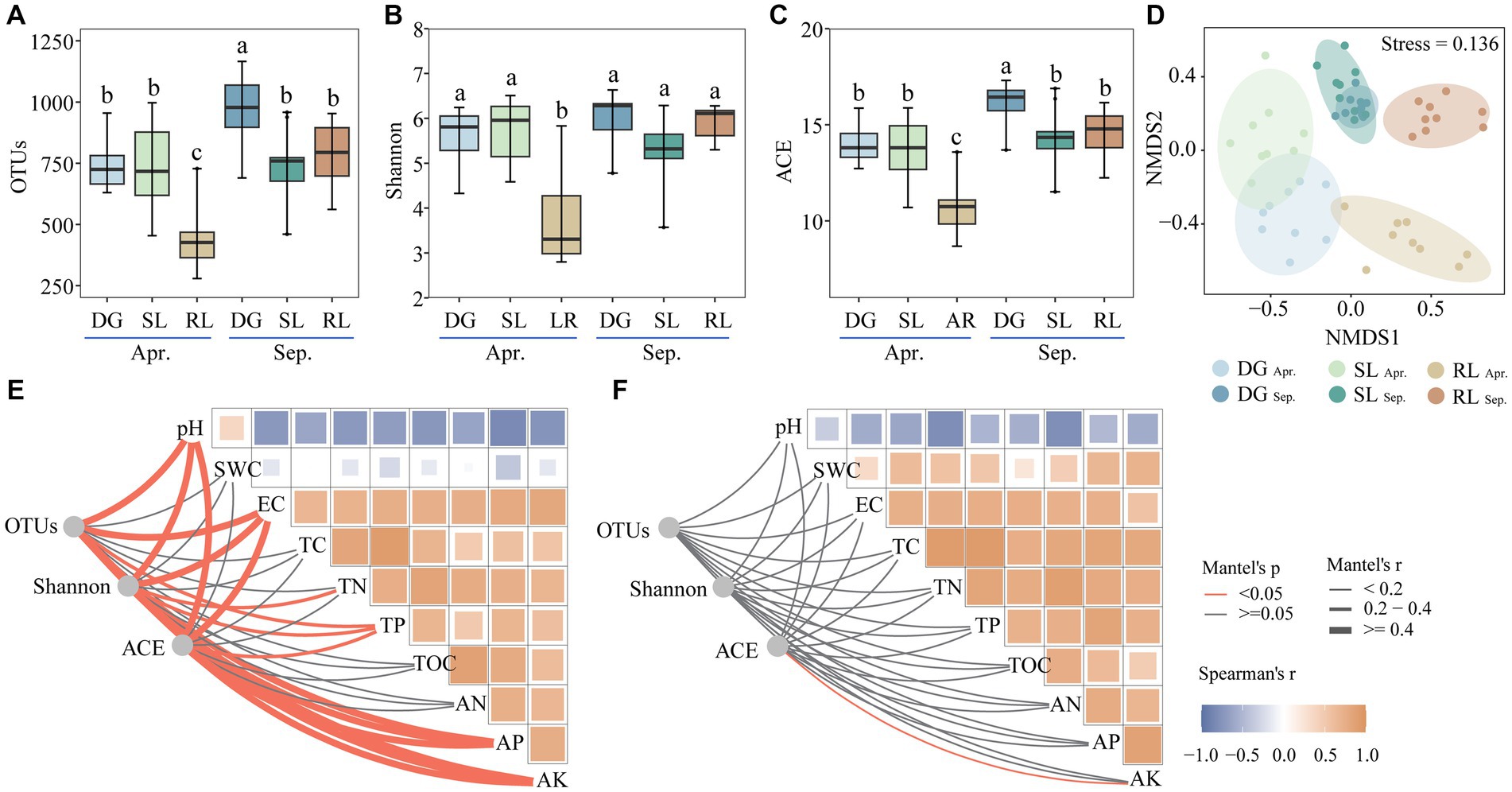
Figure 2. Alpha diversity (A–C), NMDS (D) and Mantel’s test (E) in April, (F) in September under seasonal variation and land conversion in desert grassland. DG, desert grasslands plots; SL, shrub land plots; RL, land conversion plots. Apr, April; Sep, September.
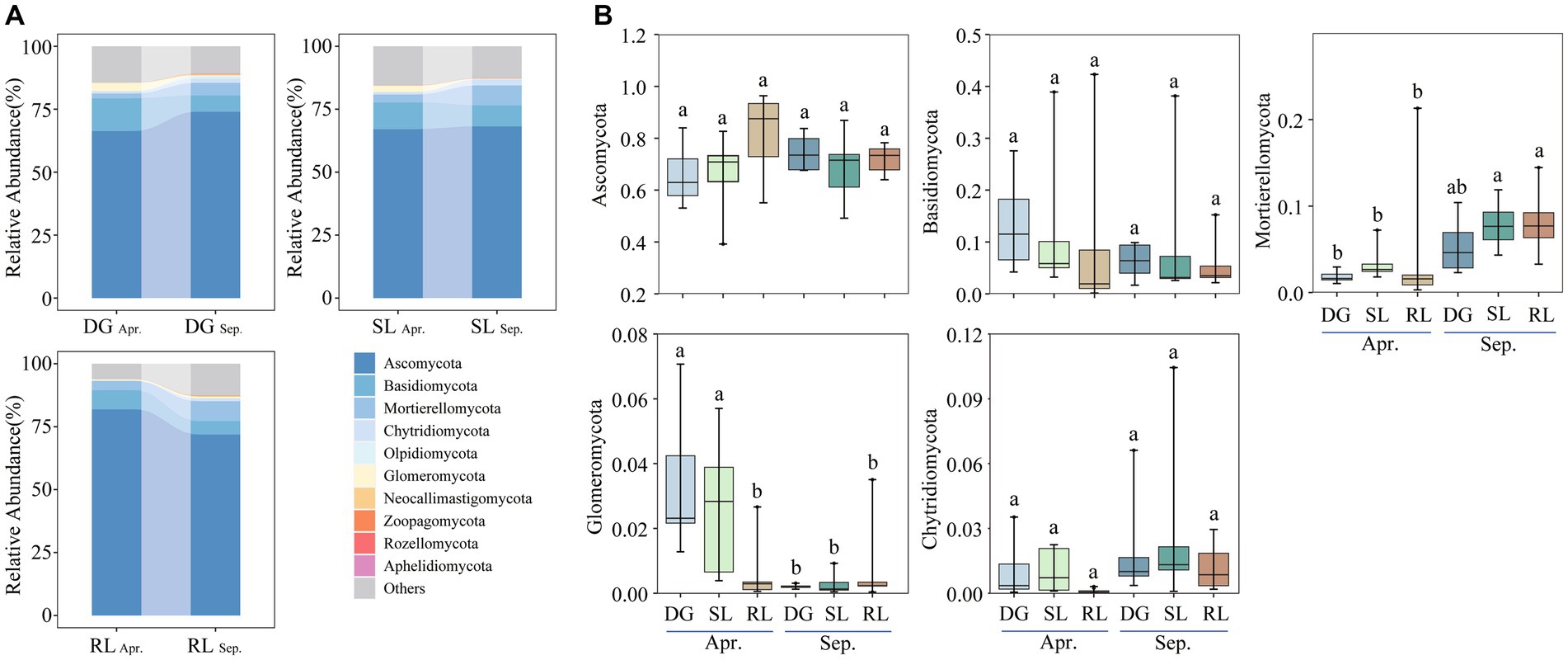
Figure 3. Relative abundance of dominant fungal phyla (A) top10 phyla; (B) top5 phyla under seasonal variation and land conversion in desert grassland. DG: desert grasslands plots; SL: shrub land plots; RL: land conversion plots. Apr: April; Sep: September.
In this study, the correlation of the absolute abundance table of fungi at the OTU level in April and September in the three sampling sites was subjected to network analysis (with a correlation threshold of |r| > 0.9). In detail, using the Molecular Ecological Network Analysis Pipeline (MENAP, ieg4.rccc.ou.edu/mena/) based on Random Matrix Theory (RMT), we calculated the Spearman correlation for OTUs that had a detection rate exceeding 77% and underwent centered log-ratio transformation, provided they were present in at least 7 out of 9 replicates, and the p-value of the chi-square test for Poisson distribution was specified to be greater than 0.05. After obtaining the node and edge data, the information was visualized using Cytoscape software (v 3.7.1) (Figure 4). After that, sub-network topological features such as node, edge, average density, transitivity, diameter, and average path length were generated for each sample from the global microbial network using the subgraph() function in the “igraph” package (Ma et al., 2016; Manning et al., 2018). The PCA1 values were further obtained by principal component analysis of the sub-network data to characterize the complexity of the fungal network (NCI) (Zhang et al., 2018; Qiu et al., 2021); where diameter and average path length were calculated by inverse form (X-1). Spearman’s correlation analysis of NCI, subnetwork data, and soil physicochemical properties was also demonstrated for each sample plot. We further identified environmental factors with high explanatory rates for NCI by random forest analysis (Jiao et al., 2022) (Figure 5).
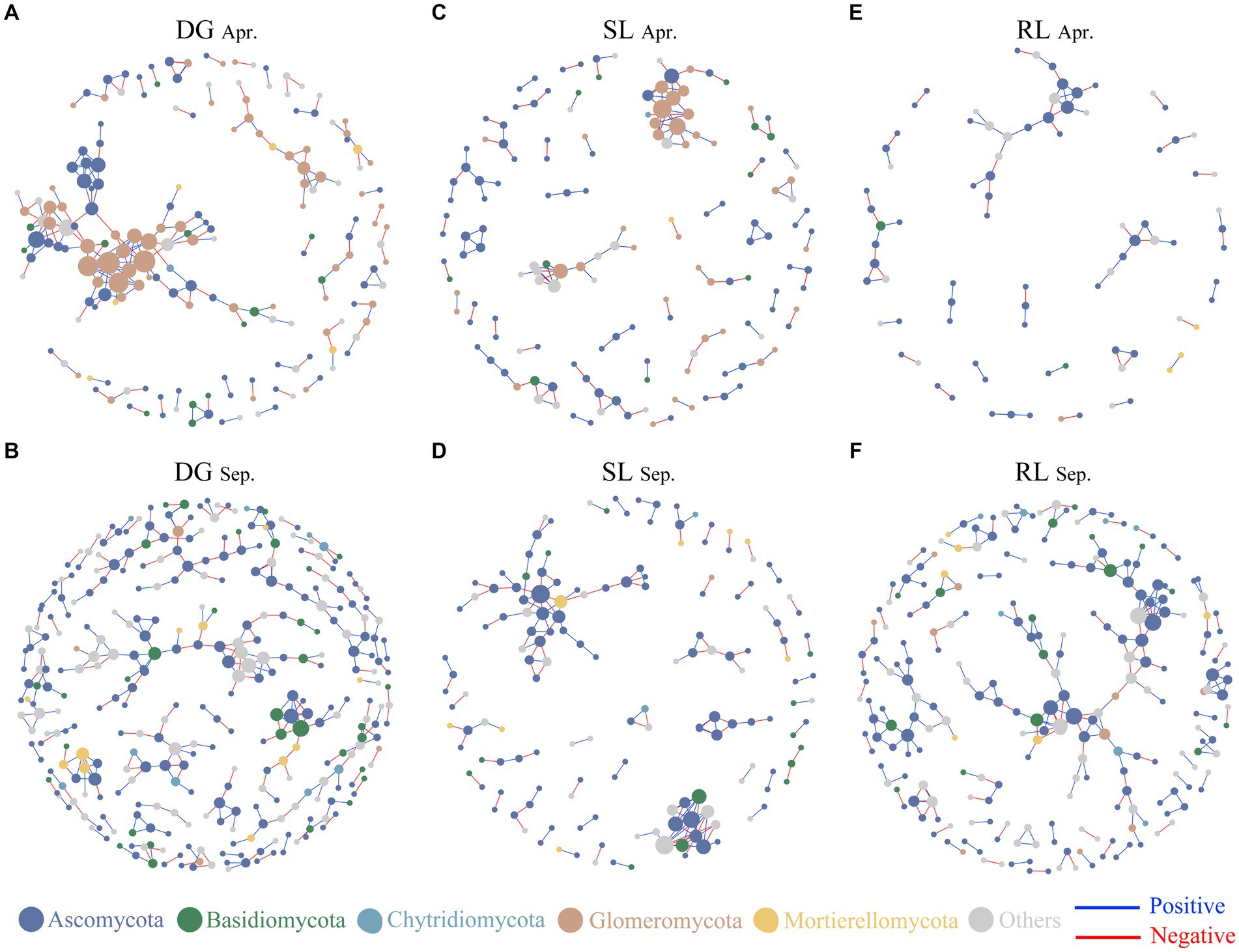
Figure 4. Soil fungal networks under seasonal variation and land conversion in desert grassland. The color of the nodes indicates different fungal phyla. DG, desert grasslands plots (A, B); SL, shrub land plots (C, D); RL, land conversion plots (E, F). Apr, April; Sep, September.
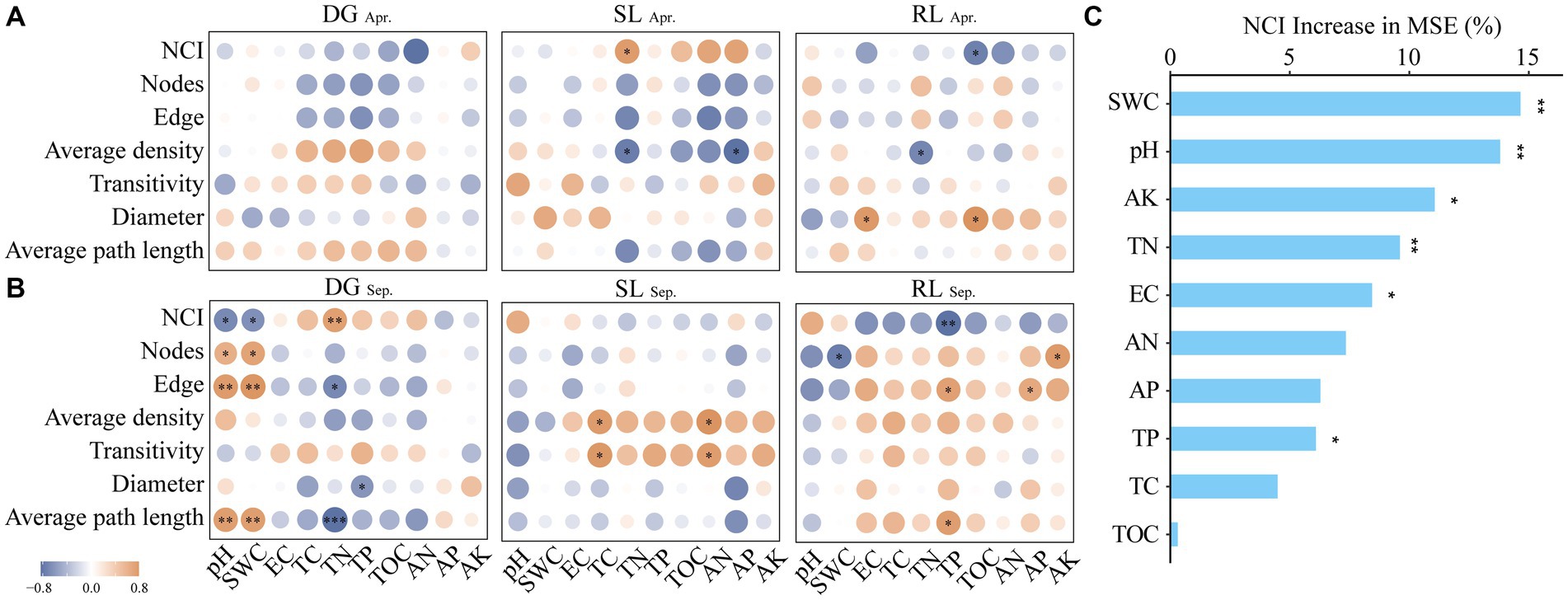
Figure 5. Correlation analysis between NCI and environmental factors (A) Apr; (B) Sep and random forest analysis (C) under seasonal variation and land conversion in desert grassland. DG, desert grasslands plots; SL, shrub land plots; RL, land conversion plots. Apr, April; Sep, September.
Based on Funguild fungal function prediction, we classified fungal functional guilds into symbiotrophic, saprotrophic, pathotrophic, arbuscular mycorrhizal (AM) and ectomycorrhizal (EcM); and the differences in fungal functional guilds among the sample plot was analyzed using ANOVA. Finally, the interrelationships between fungal functional guild groups and environmental factors in the DG, SL and RL samples were determined by RDA at OTU level, following transformation by Hellinger method (Figure 6).
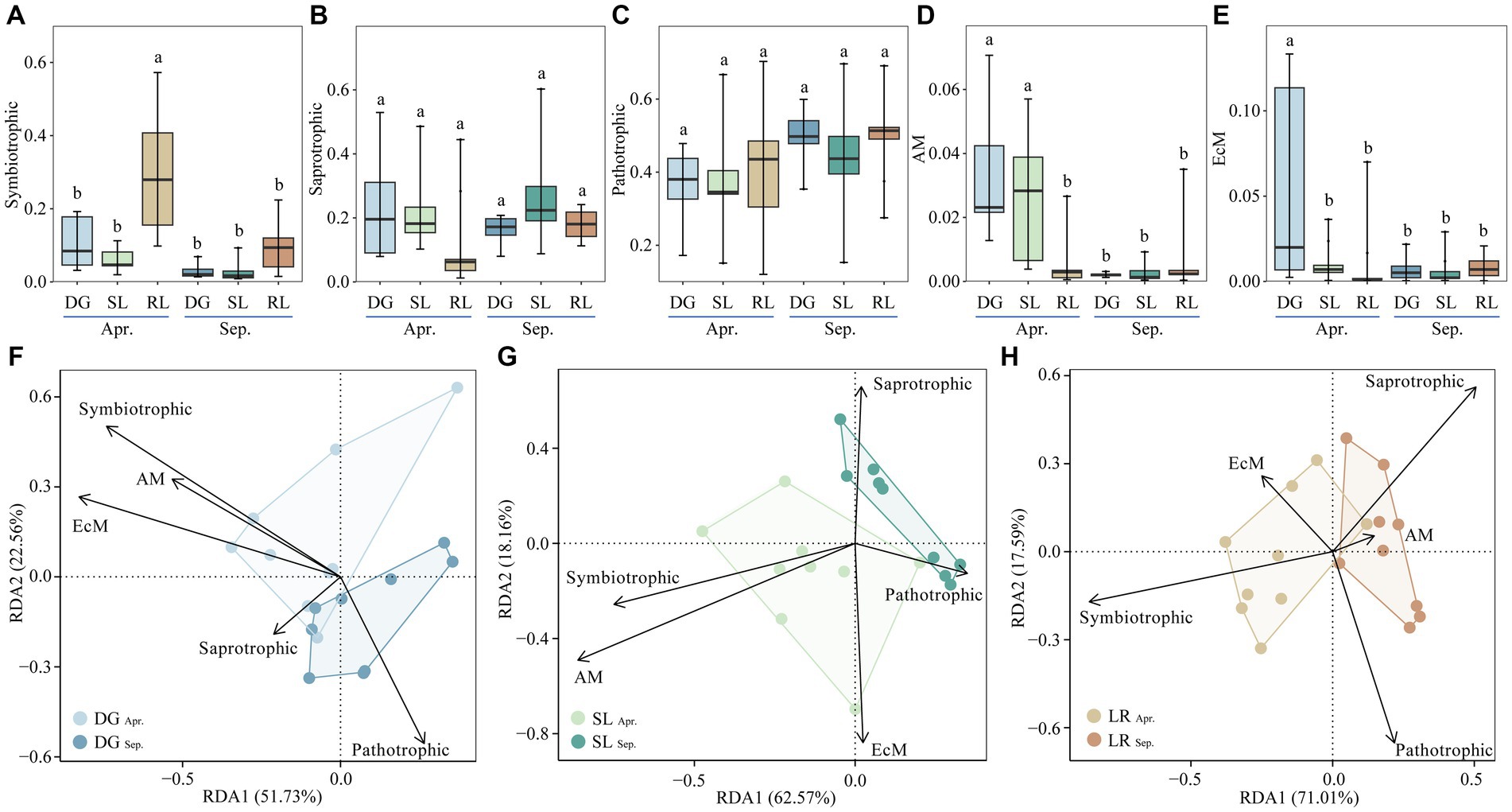
Figure 6. Relative abundance of soil fungal functional guild groups (A–E) and RDA analysis (F–H) under seasonal variation and land conversion in desert grassland. DG, desert grasslands plots; SL, shrub land plots; RL, land conversion plots. Apr, April; Sep, September.
Conversion to agriculture profoundly affected soil pH, EC, total carbon (TC), total nitrogen (TN), total phosphorus (TP), total organic carbon (TOC), available nitrogen (AN), available phosphorus (AP), and available potassium (AK) contents; among these, soil electric conductivity (EC) increased significantly at the beginning of land conversion. With seasonal succession, soil pH and soil water content (SWC) also increased. Compared to desert grassland (DG) plots, both soil TN and AN content in shrubland (SL) and conversion (RL) plots significantly (p < 0.05) increased by seasonal succession (Supplementary Figure S1).
Operational Taxonomic Units (OTUs), Shannon, and ACE indices of the fungal community were significantly reduced at the beginning of conversion to agriculture. In September, OTUs and ACE diversity indices in SL and RL plots were significantly lower than those in DG plots. In particular, seasonal succession significantly (p < 0.05) increased the number of OTUs and the ACE index values in DG plots, while reducing those in RL plots (Figures 2A–C). In addition, NMDS analysis revealed that the fungal communities in the experimental plots differed among groups in April and September (stress value <0.2) (Figure 2D).
Further, Mantel’s test showed that pH correlated negatively with EC, TC, TN, TP, TOC, AN, AP, and AK both in April and September. Further, in April, the alpha diversity index of the fungal community significantly correlated with pH, EC, TN, TP, AP, and AK. In contrast, in September, only the ACE diversity index significantly (p < 0.05) correlated with AK (Figures 2E,F).
Ascomycota, Basidiomycota, Mortierellomycota, Glomeromycota, and Chytridiomycota were the dominant phyla of soil fungi in the study area (Figure 3A). The relative abundance of Glomeromycota significantly decreased during the early stages of land conversion. Further, seasonal succession had a significant effect on the relative abundances of Mortierellomycota and Glomeromycota in the soil. Particularly, the relative abundance of Mortierellomycota was higher in September than that in April, whereas Glomeromycota showed the opposite trend (Figure 3B).
Further analysis revealed that the dominant phyla of soil fungi showed different degrees of correlation with soil physicochemical properties in the early stages of land conversion (Supplementary Figure S2A), whereas, in September, only the relative abundance of Mortierellomycota was positively correlated with SWC (Supplementary Figure S2B). Moreover, redundancy analysis (RDA) revealed that SWC correlated significantly with Mortierellomycota, while EC significantly correlated with Ascomycota (Supplementary Figure S2C). Further analysis of the RDA of soil land patterns revealed that, in DG, the soil microbial in April was positively correlated with TC, TN, and TP. However, in SL, the soil microbial was negatively correlated with physicochemical properties while EC was positively with soil microbial in RL (Supplementary Figure S3A–C). In September, factors such as SWC, pH, AN, AK, and TOC were all positively correlated with the soil microbial community (Supplementary Figure S3).
Based on Spearman’s analysis, we constructed a co-occurrence network among fungal OTUs. The results showed that the nodes and edges of the fungal networks in RL plots were lower than those in DG and SL plots at the early stage of land conversion (Figures 4A–C). In contrast, in September, the nodes and edges of the networks in RL plots were higher than those in SL plots but lower than those in DG plots (Table 1, Figures 4D–F).

Table 1. Topological features of fungal networks under seasonal variation and land conversion in desert grassland.
The analysis of fungal network complexity revealed that SL plots had more complex fungal-network relationships in April, whereas in September, the fungal network complexity in RL plots was higher than that in SL or DG plots (Supplementary Figure S4). In the correlation analysis between NCI and environmental factors, at DG and SL plots in September, NCI had negative correlations with pH and SWC and positive correlations with TN, respectively. And in the RL plots, NCI was negatively correlated with TP (Figures 5A,B). Random forest analysis found that SWC had a high explanatory rate for NCI, followed by environmental factors such as AK, TN, and pH (Figure 5C).
Among the five functional guilds, the relative abundance of symbiotrophic was higher at the RL plots than at the other plots in April (Figure 6A). The relative abundance of saprotrophic and pathotrophic were not significantly different among the plots (Figures 6B,C). In April, the relative abundance of AM in the DG plot was the highest, followed by that in the SL plot, and all of them were higher than other plots (Figure 6D). Similarly, the relative abundance of EcM was highest at the DG plots in April and was also higher than at the other plots (Figure 6E). Further analysis showed that symbiotrophic, AM and EcM had higher interpretation of the soil environment in the DG plots. In the SL plots, symbiotrophic and AM were more pronounced in indicating the soil environment. In the RL plots, symbiotrophic and Saprotrophic responded more positively to changes in the environment than the other functional guild groups (Figures 6F–H).
Conversion to agriculture profoundly affects soil physical properties, chemical structure, and soil microbial community (Hu et al., 2021; Kang et al., 2023). In this study, there were no significant differences in OTUs, Shannon, or ACE indices between DG and SL plots at the early stage of land conversion, whereas OTUs, Shannon, and ACE indices in RL plots were significantly lower than those in DG and SL plots. It has been shown that land conversion significantly reduces the diversity and richness indices of the soil fungal community, and it has also been suggested that desert grasslands are fragility and highly susceptible to human activities (Xu et al., 2017), largely because of the reduction in vegetation cover, which adversely affects the diversity of the soil fungal community (Cao et al., 2017). In the later stages of land conversion, there was no significant difference in the Shannon index of the soil fungal communities among the three treatment plots under study herein. More recent studies have suggested that, in general, land conversion has less of an effect on alpha diversity of fungal community than it does in the case of undisturbed desert soils (Hu et al., 2023). In addition, beta diversity analysis of fungal community further demonstrated the profound effects of different land-use practices on fungal community composition, consistent with previous studies (Li et al., 2017; Kang et al., 2023).
Interestingly, we found that the alpha diversity indices of fungal communities were significantly correlated with soil pH, EC, TN, TP, AP, and AK in April. However, seasonal variations (in September) reduced the significance of the relationship between soil fungal-community diversity and environmental changes. Soil fungal community diversity is highly influenced by environmental factors, such as pH and nutrients, and is subject to seasonal variation (Birgander et al., 2014; Hu et al., 2021). It can be speculated that, on one hand, soil fungal communities were more sensitive to environmental changes due to less precipitation in April, which likely strengthened their connections (Matulich et al., 2015), whereas, on the other hand, the increase in precipitation due to seasonal variations (including irrigation for conversion to agriculture), disrupted the dependence of fungal communities on environmental changes (Xu et al., 2017). In particular, the correlation between the ACE index and AK in September further indicated the importance of the effect of AK on the fungal community after land conversion (Slabbert et al., 2022). In summary, the land conversion from DG and SL to RL decreased the alpha diversity of fungi, with the fungal community structure experiencing alterations from a range of soil physicochemical properties in April, particularly being profoundly impacted by AK in September.
Soil fungal community composition in desert grasslands responds differently to land conversion and is primarily driven by changes in soil moisture and chemical properties (Hu et al., 2021). In particular, Ascomycota is an important group among soil fungi, and our RDA analysis revealed a strong correlation between Ascomycota, EC, and AP. Indeed, EC is the main factor influencing the relative abundance of Ascomycota (Yang et al., 2022). In turn, AP content in the case of disturbed grassland is also one of the major factors influencing the relative abundance of Ascomycota, whereby, it is not uncommon to find that the soil fungal-community structure under seasonal succession responds positively to changes in soil physical and chemical properties (Vargas-Gastélum et al., 2015). In addition, the relative abundance of the dominant phylum Mortierellomycota showed an upward trend influenced by seasonal succession. Consistently, a recent study pointed out that Mortierellomycota was significantly affected by seasonal changes, especially in SWC, which correlated positively with the relative abundance of Mortierellomycota (Yang et al., 2022). Hence, in this study, together with a gradual increase in SWC under seasonal succession, the relative abundance of Mortierellomycota might be used as an ecological indicator of soil moisture changes in desert grasslands.
Additionally, we found that the relative abundance of Glomeromycota decreased with seasonal succession. This might be explained by the fact that most members of the Glomeromycota (including AM) are profoundly affected by changes in the plant–soil system. In general, seasonal succession induces the input of plant litter into the soil, thereby increasing the number of Glomeromycota members (Ji et al., 2021; Kemmelmeier et al., 2022). We hypothesized that the increase in soil water content and available nutrients due to seasonal succession might have reduced plant dependence on soil nutrients, which in turn led to a decrease in the relative abundance of Glomeromycota (Zhang et al., 2023a,b). This result can be observed from the significant negative correlation between Glomeromycota members and TOC, AN, AP, and AK contents in April, indicating that soil fungal communities are regulated by seasonal changes.
The complexity of soil fungal communities usually reflects the interrelationships among plants, environments, and the structure of the fungal community (Zhao et al., 2024). In this study, the number of edges and nodes in the RL plot network decreased during the early stage of land conversion, presumably because fungal communities are more susceptible to human disturbances, making changes in their network complexity more pronounced (Manning et al., 2023). We observed that the complexity of the RL plot network was significantly lower than that of SL plots; this finding agreed well with studies reporting that, when land is converted, the complexity of microbial networks is reduced (Cui et al., 2016; Chen et al., 2023). In September, the network complexity of RL plots was significantly higher than that of SL or DG plots. Soil nitrogen and phosphorus enrichment after land conversion significantly enhances the complexity of soil microbial community networks (Zhang et al., 2023a,b). In addition, the fungal network complexity in RL plots increased with seasonal succession, which supported previous findings suggesting that fungal network complexity increases with conversion time (Ma et al., 2021). These results indicated that in the initial stages of land conversion to agriculture, the soil fungal community was significantly disrupted. However, as cultivation continued and water and nutrients were introduced to the soil in later stages, the fungal community began to show signs of recovery and stabilization.
It is worth mentioning that seasonal changes have a strong effect on microbial network structure (Lan et al., 2022). However, in this study, the soil fungal network complexity in DG and SL plots did not significantly differ with seasonal succession. While on one hand, this finding indicates that soil fungal-network interrelationships in desert grasslands and shrublands are less affected by seasonal changes, on the other hand, it indicates that land conversion affected soil fungal-network interrelationships, because land conversion affects the physical and chemical properties of the soil, which in turn may affect soil microorganisms and, consequently, fungal network relationships (Lienhard et al., 2013). These results indicated the fragility of fungal communities in desert grasslands. Additionally, in our study, fungal network complexity in SL plots was consistently higher than that in DG plots, which further reflects the effect of desert grassland afforestation on soil fungal-network relationships. Complex network relationships generally indicate high stability of soil fungal communities, and contribute to the maintenance of soil ecosystem functions (Kang et al., 2023). Combined with previous studies, it is easy to find that additional water and nutrient inputs from agricultural land conversion have a positive effect on fungal network complexity. Meanwhile, the random forest analysis pointed out that SWC and AK were important environmental factors explaining the changes in NCI. Therefore, it further confirmed the previous studies and pointed out that the interrelationships among desert grassland fungi were influenced by SWC. The seasonal changes observed in the results validated that following land conversion to agriculture, the introduction of nutrients and water contributes to an increase in the network complexity of soil fungi. This means that additional resource investment may be one way to restore the ecosystem function of desert grasslands.
Land use change is one of the most important factors affecting the ecological function of soil fungi (Liu et al., 2023). The results showed that symbiotrophic was the functional guild indicative of environmental change in the three plots. It has been noted that symbiotrophic is more sensitive to changes in the land environment, including soil water content, carbon, and nitrogen nutrients are the main factors affecting changes in this group (Ren et al., 2023). Notably, symbiotrophic groups were significantly higher in the RL plots than in the other plots in April. This may be owing to the input of organic fertilizer (manure) during the conversion to agriculture process, which increased the relative abundance of symbiotrophic groups (Qiao et al., 2021). It has been suggested that the aggregation of saprotrophic groups is more sensitive to the transformation of land properties (Peng et al., 2019; Kang et al., 2023). The present study similarly found that saprotrophic groups were more closely related to soil physicochemical properties in the RL plots. In addition, the relative abundance of arbuscular mycorrhizal was higher in both DG and SL plots than in RL plots in April. On the one hand, we hypothesize that the DG plots served as the initial habitat for the colonization of AM groups and that habitat changes such as afforestation and land conversion have altered the original group aggregation (Rao et al., 2020). On the other hand, it has also been demonstrated that the response of AM functional groups to environmental changes such as soil SWC and pH can also serve as an important factor for predicting biodiversity in desert grasslands (Lüneberg et al., 2019; Wang et al., 2020). Different from previous studies, we found that the response of AM functional groups to environmental changes was not significant in the RL plots. A study showed that disruption of soil microbial communities by land conversion reduces AM response to environmental change (Holland et al., 2016). More studies have also concluded that the aggregation of AM functional groups is more sensitive to changes in plant diversity (Bahram et al., 2020). This study further confirms that changes in water and nutrient inputs into desert grasslands reportedly affect soil fungal communities and their functions in response to changes in soil ecological processes. The response exhibited by the functional groups of soil fungi during soil transformation suggests that resource investment may play vital roles in promoting the restoration of desert grasslands.
Conversion to agriculture has profound effects on the structure and function of fungal soil communities in desert grassland ecosystems. Land conversion significantly increased soil TC, TN, TP, AP, and AK contents. In the early stage of land conversion (in April), soil fungal OTUs and ACE indices were lower than those in DG and SL plots and significantly correlated with pH, EC, AP, and AK; moreover, the dominant phyla had strong correlations with soil physicochemical properties; concomitantly, the relative abundance of Glomeromycota was significantly lower, and the complexity of the fungal network in RL plots was lower than that in SL plots. Meanwhile, in the late stage of land conversion (in September), soil fungal OTUs and ACE indices were lower in RL plots than in DG plots, with more complex network relationships, compared to those in DG and SL plots. Symbiotrophic groups, a functional group of desert grassland soil fungi, can be used as a predictor of environmental change; in addition, land conversion decreases the relative abundance of AM functional groups. This study highlights the response of soil fungal communities and functions to human disturbances in desert grasslands. Considering the potential for land conversion to affect the secondary salinization of soils, there is a need for continued observation of soil ecological health under the time continuum of land conversion to agriculture.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
PK: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Data curation, Conceptualization. JH: Writing – review & editing. YP: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. XQ: Writing – original draft, Investigation, Formal analysis. YR: Writing – original draft, Investigation, Formal analysis. CY: Writing – original draft, Investigation, Formal analysis. BL: Writing – original draft, Investigation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported financially by Fundamental Research Funds for the Central Universities (2024XYYZSK02), and National Natural Science Foundation of China (32360426, U22A20504).
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1413973/full#supplementary-material
Bahram, M., Netherway, T., Hildebrand, F., Pritsch, K., Drenkhan, R., Loit, K., et al. (2020). Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol. 227, 1189–1199. doi: 10.1111/nph.16598
Birgander, J., Rousk, J., and Olsson, P. A. (2014). Comparison of fertility and seasonal effects on grassland microbial communities. Soil Biol. Biochem. 76, 80–89. doi: 10.1016/j.soilbio.2014.05.007
Cao, C., Zhang, Y. Q., Cui, Z., Feng, S., Wang, T., and Ren, Q. (2017). Soil bacterial community responses to revegetation of moving sand dune in semi-arid grassland. Appl. Microbiol. Biotechnol. 101, 6217–6228. doi: 10.1007/s00253-017-8336-z
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Chen, C., Yin, G., Hou, L., Jiang, Y., Sun, D., Liang, X., et al. (2023). Reclamation of tidal flats to paddy soils reshuffles the soil microbiomes along a 53-year reclamation chronosequence: evidence from assembly processes, co-occurrence patterns and multifunctionality. Environ. Int. 179:108151. doi: 10.1016/j.envint.2023.108151
Cheng, H., Yuan, M., Tang, L., Shen, Y., Yu, Q., and Li, S. (2022). Integrated microbiology and metabolomics analysis reveal responses of soil microorganisms and metabolic functions to phosphorus fertilizer on semiarid farm. Sci. Total Environ. 817:152878. doi: 10.1016/j.scitotenv.2021.152878
Cui, Y., Fang, L., Guo, X., Wang, X., Wang, Y., Li, P., et al. (2018). Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 642, 45–55. doi: 10.1016/j.scitotenv.2018.06.033
Cui, X., Hu, J., Wang, J., Yang, J., and Lin, X. (2016). Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 98, 140–149. doi: 10.1016/j.apsoil.2015.10.008
Holland, T. C., Bowen, P. A., Bogdanoff, C. P., Lowery, T. D., Shaposhnikova, O., Smith, S., et al. (2016). Evaluating the diversity of soil microbial communities in vineyards relative to adjacent native ecosystems. Appl. Soil Ecol. 100, 91–103. doi: 10.1016/j.apsoil.2015.12.001
Hu, Y., He, Z., Wang, Y., Liu, W., Shi, Y., Pan, C., et al. (2023). Soil microbial biomass and community structure in response to agricultural use of deserts across northern China. Eur. J. Soil Biol. 119:103544. doi: 10.1016/j.ejsobi.2023.103544
Hu, Y., Wang, Z., Zhang, Z., Song, N., Zhou, H., Li, Y., et al. (2021). Alteration of desert soil microbial community structure in response to agricultural reclamation and abandonment. Catena 207:105678. doi: 10.1016/j.catena.2021.105678
Ji, L., Yang, Y., Yang, N., Khan, A., and Yang, L. (2021). Seasonal variation of diversity and co-occurrence patterns of arbuscular mycorrhizal fungal communities in mixed broadleaf-conifer forests. Appl. Soil Ecol. 158:103782. doi: 10.1016/j.apsoil.2020.103782
Jiang, R., Sui, Y., Zhang, X., Lin, N., Zheng, X., Li, B., et al. (2024). Estimation of soil organic carbon by combining hyperspectral and radar remote sensing to reduce coupling effects of soil surface moisture and roughness. Geoderma 444:116874. doi: 10.1016/j.geoderma.2024.116874
Jiao, S., Lu, Y., and Wei, G. (2022). Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Chang. Biol. 28, 140–153. doi: 10.1111/gcb.15917
Kang, P., Pan, Y., Ran, Y., Li, W., Shao, M., Zhang, Y., et al. (2023). Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 150:110224. doi: 10.1016/j.ecolind.2023.110224
Kang, P., Pan, Y., Yang, P., Hu, J., Zhao, T., Zhang, Y., et al. (2022). A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches. Front. Microbiol. 13:1018077. doi: 10.3389/fmicb.2022.1018077
Kemmelmeier, K., dos Santos, D. A., Grittz, G. S., and Stürmer, S. L. (2022). Composition and seasonal variation of the arbuscular mycorrhizal fungi spore community in litter, root mat, and soil from a subtropical rain forest. Mycorrhiza 32, 409–423. doi: 10.1007/s00572-022-01084-3
Lan, G., Yang, C., Wu, Z., Sun, R., Chen, B., and Zhang, X. (2022). Network complexity of rubber plantations is lower than tropical forests for soil bacteria but not for fungi. Soil 8, 149–161. doi: 10.5194/soil-8-149-2022
Li, Y., Li, J., Jiao, S., Li, Y., Xu, Z., and Kong, B. (2020). Ecosystem-scale carbon allocation among different land uses: implications for carbon stocks in the Yellow River Delta. Ecosphere 11:e03125. doi: 10.1002/ecs2.3125
Li, H., Zhang, J., Hu, H., Chen, L., Zhu, Y., Shen, H., et al. (2017). Shift in soil microbial communities with shrub encroachment in Inner Mongolia grasslands, China. Eur. J. Soil Biol. 79, 40–47. doi: 10.1016/j.ejsobi.2017.02.004
Lienhard, P., Tivet, F., Chabanne, A., Dequiedt, S., Lelièvre, M., Sayphoummie, S., et al. (2013). No-till and cover crops shift soil microbial abundance and diversity in Laos tropical grasslands. Agron. Sustain. Dev. 33, 375–384. doi: 10.1007/s13593-012-0099-4
Liu, C., Jin, Y., Lin, F., Jiang, C., Zeng, X., Feng, D., et al. (2023). Land use change alters carbon and nitrogen dynamics mediated by fungal functional guilds within soil aggregates. Sci. Total Environ. 902:166080. doi: 10.1016/j.scitotenv.2023.166080
Lüneberg, K., Schneider, D., Brinkmann, N., Siebe, C., and Daniel, R. (2019). Land use change and water quality use for irrigation alters drylands soil fungal community in the Mezquital Valley, Mexico. Front. Microbiol. 10:1220. doi: 10.3389/fmicb.2019.01220
Luo, X., Hou, E., Zhang, L., Kuang, Y., and Wen, D. (2023). Altered soil microbial properties and functions after afforestation increase soil carbon and nitrogen but not phosphorus accumulation. Biol. Fertil. Soils 59, 645–658. doi: 10.1007/s00374-023-01726-4
Luo, S., Png, G. K., Ostle, N. J., Zhou, H., Hou, X., Luo, C., et al. (2023). Grassland degradation-induced declines in soil fungal complexity reduce fungal community stability and ecosystem multifunctionality. Soil Biol. Biochem. 176:108865. doi: 10.1016/j.soilbio.2022.108865
Ma, J., Gonzalez-Ollauri, A., Zhang, Q., Xiao, D., and Chen, F. (2021). Ecological network analysis to assess the restoration success of disturbed mine soil in Zoucheng, China. Land Degrad. Dev. 32, 5393–5411. doi: 10.1002/ldr.4116
Ma, B., Wang, H., Dsouza, M., Lou, J., He, Y., Dai, Z., et al. (2016). Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 10, 1891–1901. doi: 10.1038/ismej.2015.261
Manning, P., van der Plas, F., Soliveres, S., Allan, E., Maestre, F. T., Mace, G., et al. (2018). Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2, 427–436. doi: 10.1038/s41559-017-0461-7
Manning, P., van der Plas, F., Soliveres, S., Allan, E., Maestre, F. T., Mace, G., et al. (2023). Changes in assembly processes and differential responses of soil microbial communities during mining disturbance in mining reclamation and surrounding grassland. Catena 231:107332. doi: 10.1016/j.catena.2023.107332
Matulich, K. L., Weihe, C., Allison, S. D., Amend, A. S., Berlemont, R., Goulden, M. L., et al. (2015). Temporal variation overshadows the response of leaf litter microbial communities to simulated global change. ISME J. 9, 2477–2489. doi: 10.1038/ismej.2015.58
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., et al. (2016). FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/j.funeco.2015.06.006
Pan, Y., Kang, P., Hu, J., and Song, N. (2021). Bacterial community demonstrates stronger network connectivity than fungal community in desert-grassland salt marsh. Sci. Total Environ. 798:149118. doi: 10.1016/j.scitotenv.2021.149118
Pan, Y., Kang, P., Tan, M., Hu, J., Zhang, Y., Zhang, J., et al. (2022). Root exudates and rhizosphere soil bacterial relationships of Nitraria tangutorum are linked to k-strategists bacterial community under salt stress. Front. Plant Sci. 13:997292. doi: 10.3389/fpls.2022.997292
Peng, J., Wang, K., Yin, X., Yin, X., Du, M., Gao, Y., et al. (2019). Trophic mode and organics metabolic characteristic of fungal community in swine manure composting. Front. Environ. Sci. Eng. 13:93. doi: 10.1007/s11783-019-1177-5
Qiang, W., Yang, B., Liu, Y., Qi, K., Yang, T., and Pang, X. (2020). Effects of reclamation age on soil microbial communities and enzymatic activities in the sloping citrus orchards of southwestern China. Appl. Soil Ecol. 152:103566. doi: 10.1016/j.apsoil.2020.103566
Qiao, C., Penton, C. R., Liu, C., Tao, C., Deng, X., Ou, Y., et al. (2021). Patterns of fungal community succession triggered by C/N ratios during composting. J. Hazard. Mater. 401:123344. doi: 10.1016/j.jhazmat.2020.123344
Qiu, L., Zhang, Q., Zhu, H., Reich, P. B., Banerjee, S., van der Heijden, M. G. A., et al. (2021). Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 15, 2474–2489. doi: 10.1038/s41396-021-00913-1
Rao, M. V., Rice, R. A., Fleischer, R. C., and Muletz-Wolz, C. R. (2020). Soil fungal communities differ between shaded and sun-intensive coffee plantations in El Salvador. PLoS One 15:e0231875. doi: 10.1371/journal.pone.0231875
Ren, Y., Ge, W., Dong, C., Wang, H., Zhao, S., Li, C., et al. (2023). Specialist species of fungi and bacteria are more important than the intermediate and generalist species in near-urban agricultural soils. Appl. Soil Ecol. 188:104894. doi: 10.1016/j.apsoil.2023.104894
Slabbert, E. L., Knight, T. M., Wubet, T., Kautzner, A., Baessler, C., Auge, H., et al. (2022). Abiotic factors are more important than land management and biotic interactions in shaping vascular plant and soil fungal communities. Glob. Ecol. Conserv. 33:e01960. doi: 10.1016/j.gecco.2021.e01960
Sun, H., Chen, J., Yang, Y., Yan, D., Xue, J., Wang, J., et al. (2022). Assessment of long-term water stress for ecosystems across China using the maximum entropy production theory-based evapotranspiration product. J. Clean. Prod. 349:131414. doi: 10.1016/j.jclepro.2022.131414
Sunagawa, S., Coelho, L. P., Chaffron, S., Kultima, J. R., Labadie, K., Salazar, G., et al. (2015). Ocean plankton. Structure and function of the global ocean microbiome. Science 348:1261359. doi: 10.1126/science.1261359
Vargas-Gastélum, L., Romero-Olivares, A. L., Escalante, A. E., Rocha-Olivares, A., Brizuela, C., and Riquelme, M. (2015). Impact of seasonal changes on fungal diversity of a semi-arid ecosystem revealed by 454 pyrosequencing. FEMS Microbiol. Ecol. 91:fiv044. doi: 10.1093/femsec/fiv044
Wang, J., Chen, C., Ye, Z., Li, J., Feng, Y., and Lu, Q. (2018). Relationships between fungal and plant communities differ between desert and grassland in a typical dryland region of Northwest China. Front. Microbiol. 9:2327. doi: 10.3389/fmicb.2018.02327
Wang, H., Ta, N., Jin, K., Ji, B., Schellenberg, M. P., Wei, Z., et al. (2020). Interactive effects of nitrogen fertilizer and altered precipitation on fungal communities in arid grasslands of northern China. J. Soils Sediments 20, 1344–1356. doi: 10.1007/s11368-019-02512-2
Wang, F., Wang, T., Gustave, W., Wang, J., Zhou, Y., and Chen, J. (2023). Spatial-temporal patterns of organic carbon sequestration capacity after long-term coastal wetland reclamation. Agric. Ecosyst. Environ. 341:108209. doi: 10.1016/j.agee.2022.108209
Wu, Y., Chen, W., Li, Q., Guo, Z., Li, Y., Zhao, Z., et al. (2021). Ecoenzymatic stoichiometry and nutrient limitation under a natural secondary succession of vegetation on the loess plateau, China. Land Degrad. Dev. 32, 399–409. doi: 10.1002/ldr.3723
Xu, F., Cai, T., Yang, X., and Sui, W. (2017). Soil fungal community variation by large-scale reclamation in Sanjiang plain, China. Ann. Microbiol. 67, 679–689. doi: 10.1007/s13213-017-1296-9
Xu, E., Zhang, H., and Xu, Y. (2020). Exploring land reclamation history: soil organic carbon sequestration due to dramatic oasis agriculture expansion in arid region of Northwest China. Ecol. Indic. 108:105746. doi: 10.1016/j.ecolind.2019.105746
Yang, Y., Kim, J., Chung, J. O., Cho, D., Roh, J. H., Hong, Y. D., et al. (2022). Variations in the composition of tea leaves and soil microbial community. Biol. Fertil. Soils 58, 167–179. doi: 10.1007/s00374-021-01615-8
Yang, X., Tian, H., Feng, J., Zang, J., Ji, B., Wang, Z., et al. (2021). Inter-annual precipitation variability alters the effects of soil resource enrichment and mycorrhizal suppression on plant communities in desert steppe. J. Veg. Sci. 32:e13082. doi: 10.1111/jvs.13082
Zhang, L., Ge, A. H., Tóth, T., Yang, F., Wang, Z., and An, F. (2023a). Enrichment of keystone fungal taxa after flue gas desulphurization gypsum application drives reclamation of the saline-sodic soil. Land Degrad. Dev. 34, 2276–2287. doi: 10.1002/ldr.4607
Zhang, L., Ren, G., and Chu, G. (2023b). Land reclamation increased labile and moderately labile P fractions and strengthened co-occurrence network of gcd community in calcareous soils. Land Degrad. Dev. 34, 5542–5555. doi: 10.1002/ldr.4863
Zhang, T., Zhao, Y., Wang, Y., Liu, Z., and Gao, C. (2018). Comprehensive analysis of MYB gene family and their expressions under abiotic stresses and hormone treatments in tamarix hispida. Front. Plant Sci. 9:1303. doi: 10.3389/fpls.2018.01303
Keywords: desert grassland, land conversion, fungal community, network, functional groups
Citation: Kang P, Hu J, Pan Y, Qu X, Ran Y, Yang C and Liu B (2024) Response of soil fungal-community structure and function to land conversion to agriculture in desert grassland. Front. Microbiol. 15:1413973. doi: 10.3389/fmicb.2024.1413973
Received: 08 April 2024; Accepted: 06 August 2024;
Published: 09 September 2024.
Edited by:
Paul Christiaan Struik, Wageningen University and Research, NetherlandsReviewed by:
Christopher Blackwood, Michigan State University, United StatesCopyright © 2024 Kang, Hu, Pan, Qu, Ran, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaqing Pan, cGFueWFxaW5nQG5pZWVyLmFjLmNu; Bingru Liu, YmluZ3J1LmxpdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.