- 1Faculty of Geography, Yunnan Normal University, Kunming, China
- 2CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences (CAS), Kunming, China
- 3Yunnan Key Laboratory for Fungal Diversity and Green Development, Kunming, China
Introduction: The genus Laccaria has been reported from temperate and tropical areas and is an important constituent in forest ecosystems. However, the species diversity of Laccaria in Southwest China (Yunnan) has been underestimated.
Methods: In this paper, descriptions based on morphological and multi-gene sequence data from internal transcribed spacer (ITS) region, large subunit ribosomal RNA gene (nrLSU), translation elongation factor 1-α (TEF1α) and the polymerase II second largest subunit (RPB2) of three new Laccaria species from Southwest China (Yunnan) are reported.
Results: Two of these were characterized by orange pileus and globose to subglobose basidiospores: L. cinnabarina and L. spinulosa. While L. cinnabarina has orange red colored basidiocarps with conspicuously pellucid-striate pattern, and a fibrillose stipe with longitudinally striations, L. spinulosa has a brownish orange to brown fruiting body with light white pruinae and 2-spored basidia. Laccaria longistriata is characterized by brown to flesh-colored basidioma, prominently striate to sulcate pileus and globose to subglobose basidiospores.
Discussion: The three new species were described, illustrated and compared with closely related species in morphology and phylogeny.
1 Introduction
Laccaria Berk. & Broome (Hydnangiaceae) is an important ectomycorrhizal (ECM) genus with significant ecological and economic value and a cosmopolitan distribution (Mueller, 1992; Kropp and Mueller, 1999; Wu et al., 2019). Species in this genus, which range from host generalists to host specialists, play a crucial role as symbionts with trees (Mueller, 1992; Roy et al., 2008; Wilson et al., 2017; Herrera et al., 2022). Additionally, Laccaria species are known to be pioneers in difficult environments and they are advantageous for both primary and secondary succession in forest ecosystems (Nara et al., 2003). Several Laccaria species possess therapeutic properties that are vital for human health while also being edible and tasty (Dai et al., 2009; Wu et al., 2019).
Due to its mostly orange or violet-colored basidioma and echinulate basidiospores, the genus Laccaria is morphologically distinct from other genera in the Hydnangiaceae family (Berkeley and Broome, 1883; Mueller, 1984, 1991, 1992; Vellinga, 1986; Wang et al., 2004). This genus has been the focus of several taxonomic studies, mostly in North America or Europe (Heinemann, 1964; Singer, 1967, 1986; Besson and Kühner, 1971; Bon, 1983; Ballero and Contu, 1989; May, 1991; Pázmány, 1994; Kropp and Mueller, 1999; Osmundson et al., 2005; Dovana et al., 2021). However, species delimitation has long been problematic due to phenotypic plasticity and morphological stasis.
In China, approximately 32 Laccaria taxa have been identified based on morphological and limited molecular phylogenetic data. These taxa are distributed across more than 20 provinces and regions of China, mostly in Northeast China, East China, South China, and southwest China (Wang et al., 2004; Wilson et al., 2013; Popa et al., 2014; Luo et al., 2016; Vincenot et al., 2017; Corrales et al., 2020; Li, 2020; Cui et al., 2021; Wang et al., 2022; Zhang et al., 2023). However, most of these species bear European and North American names, and their identities are based solely on their morphological and limited molecular characteristics.
The understanding of Laccaria species diversity in China remains limited. Therefore, a broader taxon sampling, coupled with both molecular and morphological data, is needed to completely understand the species diversity in China.
In this study, three new species, namely L. cinnabarina, L. longistriata, and L. spinulosa, from Southwest China (Yunnan) have been described with both molecular and morphological data as evidence. These species are generally found in subtropical coniferous and broad-leaved mixed forests.
2 Materials and methods
2.1 Specimen collection
The 16 specimens examined in this study were collected from Yunnan, China, during the years 2011–2019. They are deposited in the Herbarium of Cryptogams, Kunming Institute of Botany, Chinese Academy of Sciences (KUN-HKAS). Among them, 15 specimens were collected from subtropical broad-leaved forests, while one specimen (KUN-HKAS129615) was collected from a subalpine forest.
2.2 DNA extraction, PCR amplification, and DNA sequencing
Genomic DNA was extracted from silica-gel dried or herbarium material using the cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle, 1987). The universal primer pairs ITS1F/ITS4 (White et al., 1990), LR0R/LR5 (Vilgalys and Hester, 1990), EF1-altertative-3f/EF1-altertative-3r (Stielow et al., 2015), f RPB2-4F/b RPB2-7R, bRPB2-7R2, and bRPB2-7.1R (Liu et al., 1999; Matheny, 2005) were used to amplify the internal transcribed spacer (ITS) region, large subunit ribosomal RNA gene (nrLSU), translation elongation factor 1-α (TEF1α), and the polymerase II second largest subunit (RPB2), respectively. However, we were unable to obtain PCR products from many specimens using RPB2 primers. Instead, new primers, LRPB2_F_new (5′-TTWSGWATGCTWTTCCGAAA-3′)/LRPB2_R_new (5′-GGGAAAGGWATWATGCTGGCW-3′), were designed using Primer 3 (version 0.4.0) (Rozen and Skaletsky, 2000) based on sequences available in GenBank and the sequences newly generated in this study.
The PCR reaction was conducted on a SimpliAmp™ Thermal Cycler (Applied Biosystems, Foster City, CA, United States) under the following conditions: 94°C for 4 min, followed by 35 cycles of 94°C for 40 s, 52°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 8 min. The PCR products were purified using a Gel Extraction and PCR Purification Combo Kit (Spin-Column; Bioteke Corporation, Beijing, China). The purified products were sent to Sangon Biotech (Shanghai, China) for sequencing on an ABI-3730-XL sequence analyzer (Applied Biosystems, Foster City, CA, United States) using the same primer combination as for the PCR.
2.3 Phylogenetic analyses
Sequences newly generated and retrieved from GenBank are listed in Table 1. Mythicomyces corneipes (Fr.) Redhead & A.H. Sm. was chosen as an outgroup according to recent phylogenetic studies by Wilson et al. (2017). The datasets were then aligned using MAFFT v7.130b (Katoh and Standley, 2013) and manually optimized in Bioedit v7.0.9 (Hall, 1999). Phylogenetic trees for multi-loci and single-locus datasets were conducted using maximum likelihood (ML) and Bayesian inference (BI) analyses, which are based on RAxML v. 7.2.6 (Stamatakis, 2006) and MrBayes v. 3.1.2 (Ronquist and Huelsenbeck, 2003), respectively. The most appropriate substitution models for the four datasets were chosen using MrModeltest v. 2.3 (Nylander, 2004) under the Akaike information criterion (AIC).
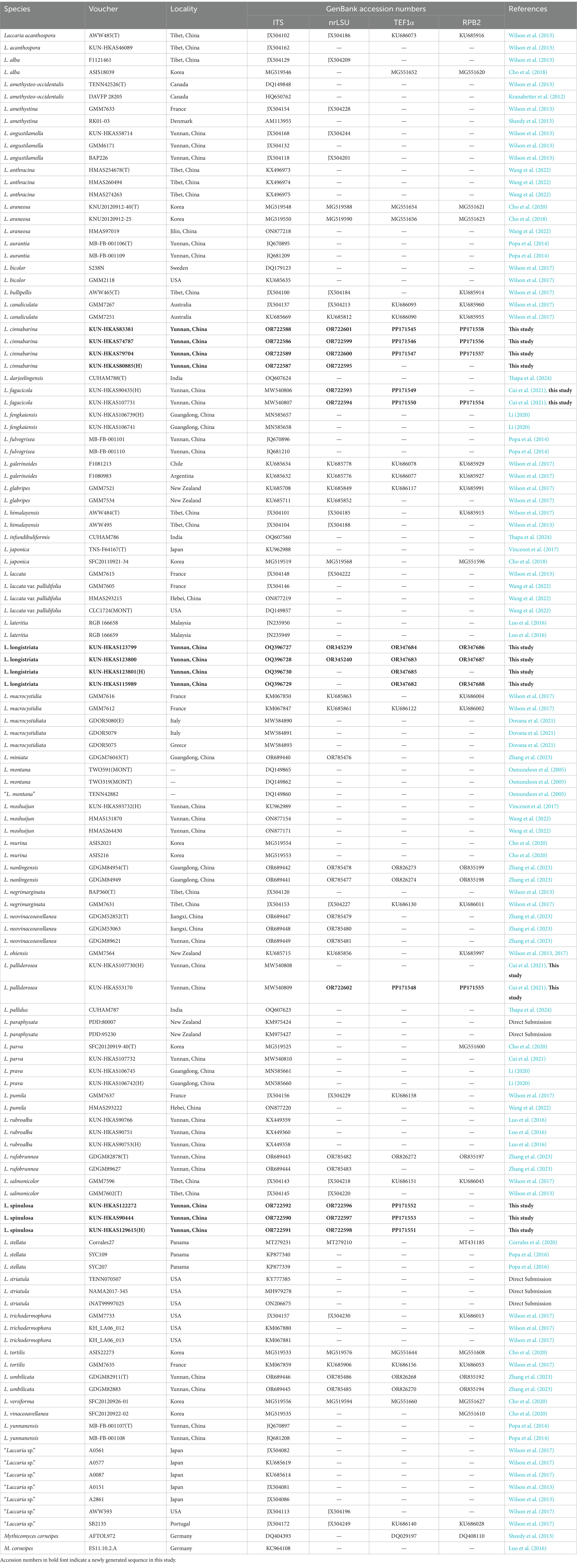
Table 1. Taxa included in molecular phylogenetic analyses and their GenBank accession numbers for ITS, nrLSU, TEF1α, and RPB2 sequences.
Statistical support for the phylogenetic trees was calculated using non-parametric bootstrapping with 1,000 replicates for ML analysis (ML bootstrapping: MLB). For BI analyses, the selected models and four chains were used, and the analysis was stopped when the standard deviation of the split frequencies fell below 0.01 and the effective sample size (ESS) values were >200. Tracer v 1.7 (Rambaut et al., 2018) was used to monitor chain convergence. Trees were sampled every 100 generations. Subsequently, the trees were summarized, and statistical support was obtained using the “sump” and “sumt” commands in MrBayes by discarding the first 25% of generations as burn-ins. Bayesian posterior probabilities (BPP) for internodes were estimated based on the majority rule consensus with the remaining trees.
2.4 Morphological studies
Field notes and digital photos were used to describe macroscopic features, with color codes based on the study by Kornerup and Wanscher (1981). Microscopic characteristics were observed on dried specimens mounted in 5% KOH and stained with Congo red when necessary, under a microscope. The notation “[n/m/p]” denotes the measurement of n basidiospores from m basidiomata of p collections. The size of basidiospores is represented as “(a–) b–c (−d),” where at least 90% of the measured values fall within the range b–c.
Parentheses are used to indicate extreme values in a and d. The symbol represents the average length of basidiospores ± sample standard deviation × average width of basidiospores ± sample standard deviation. Q is the length-to-width ratio of a basidiospore in the side view. Qm indicates the average Q of all measured basidiospores ± sample standard deviation. SigmaPlot 10.0 was used to calculate these measurements (Systat Software, San Jose, CA).
With a phase contrast objective (× 1,000), line drawings of the new species were illustrated by placing a hand under the microscope. The scanning electron microscopy (SEM) was applied to observe the characters of basidiospores.
3 Results
3.1 Phylogenetic analyses
In total, 44 sequences generated in this study were included in the single-locus and multi-loci datasets. For the four single-locus datasets, the GTR + GAMMA + I model was identified as the most appropriate substitution model for ITS, nrLSU, and RPB2, while the SYM + GAMMA + I model was found to be the most suitable one for TEF1α. The aligned four-gene matrix contained a total of 126 samples, with 3,693 aligned bases per sample.
The phylogenetic trees of the Laccaria species complex were constructed based on these single-locus and four-gene matrices. Taking into consideration that the topology of the phylogenetic trees based on the four-gene dataset generated from ML and BI analyses was almost identical, with only slight differences in statistical support, only the tree inferred from the ML analysis is displayed (Figure 1).
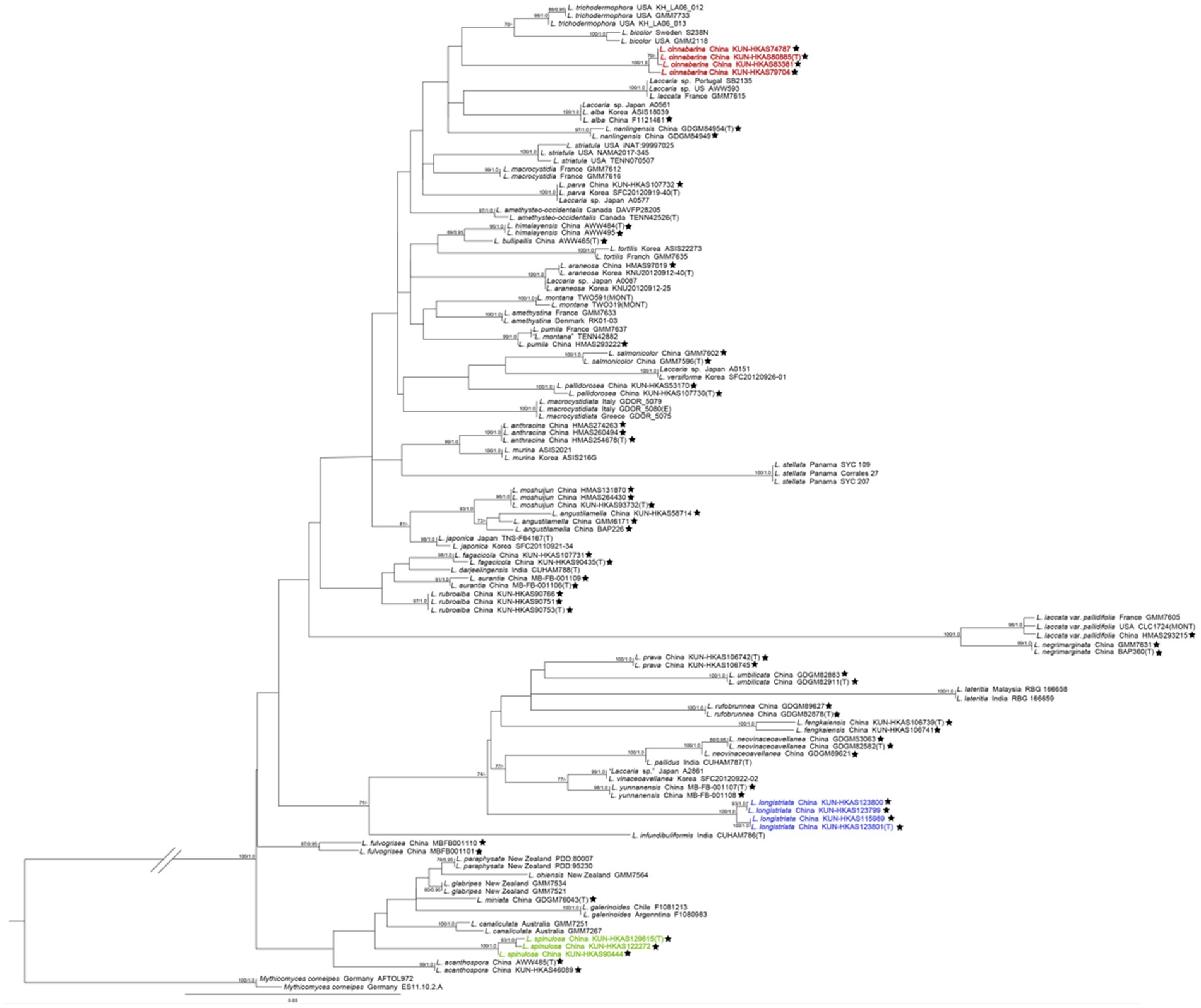
Figure 1. The maximum likelihood (ML) tree of Laccaria was inferred from the multi-locus dataset (ITS, nrLSU, TEF1α, and RPB2). ML bootstrap values over 70% (MLB ≥70%) and Bayesian posterior probabilities over 0.90 (BPP ≥0.90) are shown above or beneath individual branches. Sequences from type, holotype, or epitype specimens are marked with (T), (H), or (E), respectively; the three new species are indicated in red, purple, and green boldface, and species identified in China are labelled with a black pentagram. The double slash was used to optimize the phylogenetic tree, which has a long branch between Laccaria and Mythicomyces.
In our multi-loci phylogenetic analysis, the three new species occupied independent positions in Laccaria, each with high support (MLB/BPP = 100%/1.0). Laccaria cinnabarina is related to Laccaria trichodermophora G. M. Mueller and Laccaria bicolor (Maire) P. D. Orton. Laccaria longistriata clustered together with Laccaria yunnanensis Popa et al., Laccaria vinaceoavellanea Hongo, and Laccaria pallidus A. Thapa and K. Acharya, Laccaria neovinaceoavellanea Ming Zhang and X. L. Gao, Laccaria fengkaiensis Fang Li, Laccaria rufobrunnea Ming Zhang and X. L. Gao, Laccaria lateritia Malençon, Laccaria umblilicata Ming Zhang, and Laccaria prava Fang Li. Three specimens of L. spinulosa formed a monophyletic clade close to Laccaria acanthospora A. W. Wilson and G. M. Mueller, Laccaria canaliculata (Sacc.) Massee, Laccaria galerinoides Singer, Laccaria miniata Ming Zhang, Laccaria glabripes McNabb, Laccaria ohiensis (Mont.) Singer, and Laccaria paraphysata (McNabb) J. A. Cooper at the base of the genus.
3.2 Morphological observations
Sixteen specimens representing three new species (L. cinnabarina, L. longistriata, and L. spinulosa) were morphologically examined (Figure 2). The SEM images of basidiospores are presented in Figure 3. The line drawings of the three new species from the type specimen are provided in Figures 4–6.

Figure 2. Fresh basidiomata of Laccaria cinnabarina, Laccaria longistriata, and Laccaria spinulosa. (A,B) Laccaria cinnabarina (a holotype, KUN-HKAS80885, b KUN-HKAS83302). (C,D) Laccaria longistriata (C holotype, KUN-HKAS123801, D KUN-HKAS123317). (E,F) Laccaria spinulosa (E holotype, KUN-HKAS129615, F KUN-HKAS90444). Bars: 2 cm.
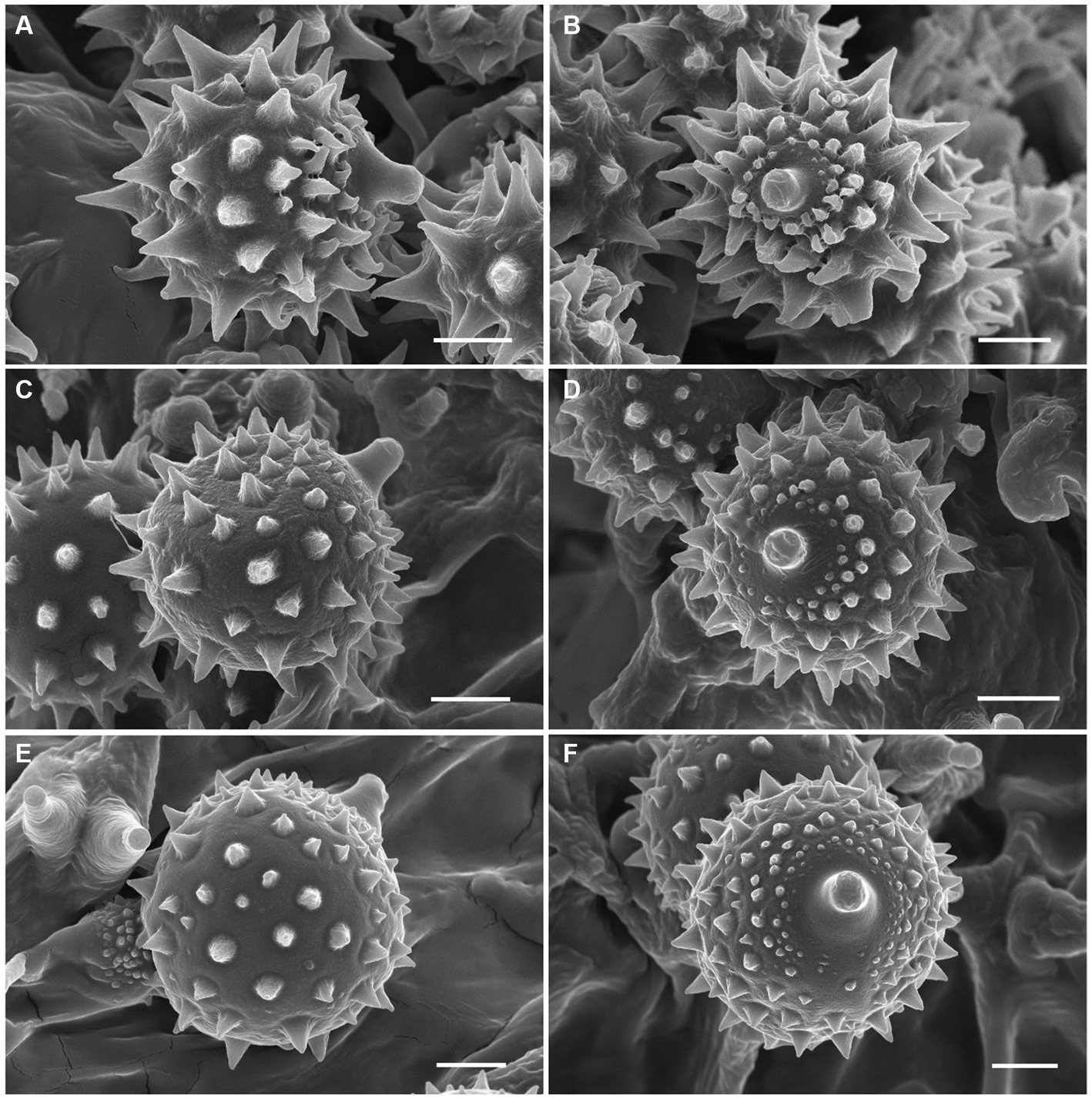
Figure 3. Basidiospores of Laccaria cinnabarina, Laccaria longistriata, and Laccaria spinulosa under SEM. (A,B) Laccaria cinnabarina (holotype, KUN-HKAS80885). (C,D) Laccaria longistriata (holotype, KUN-HKAS123801). (E,F) Laccaria spinulosa (holotype, KUN-HKAS129615). Bars: 2 μm.
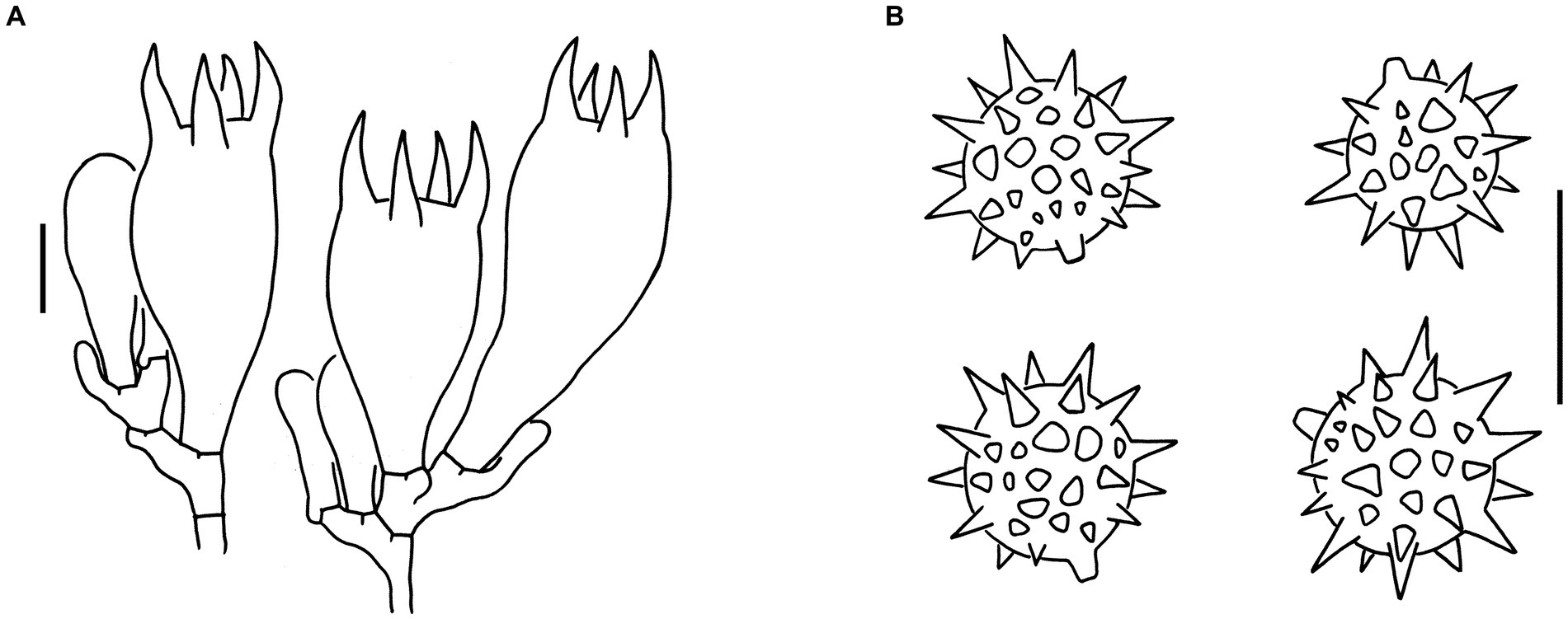
Figure 4. Microscopic features of Laccaria cinnabarina (holotype, KUN-HKAS80885). (A) Hymenium and subhymenium. (B) Basidiospores. Bars: A,B = 10 μm.
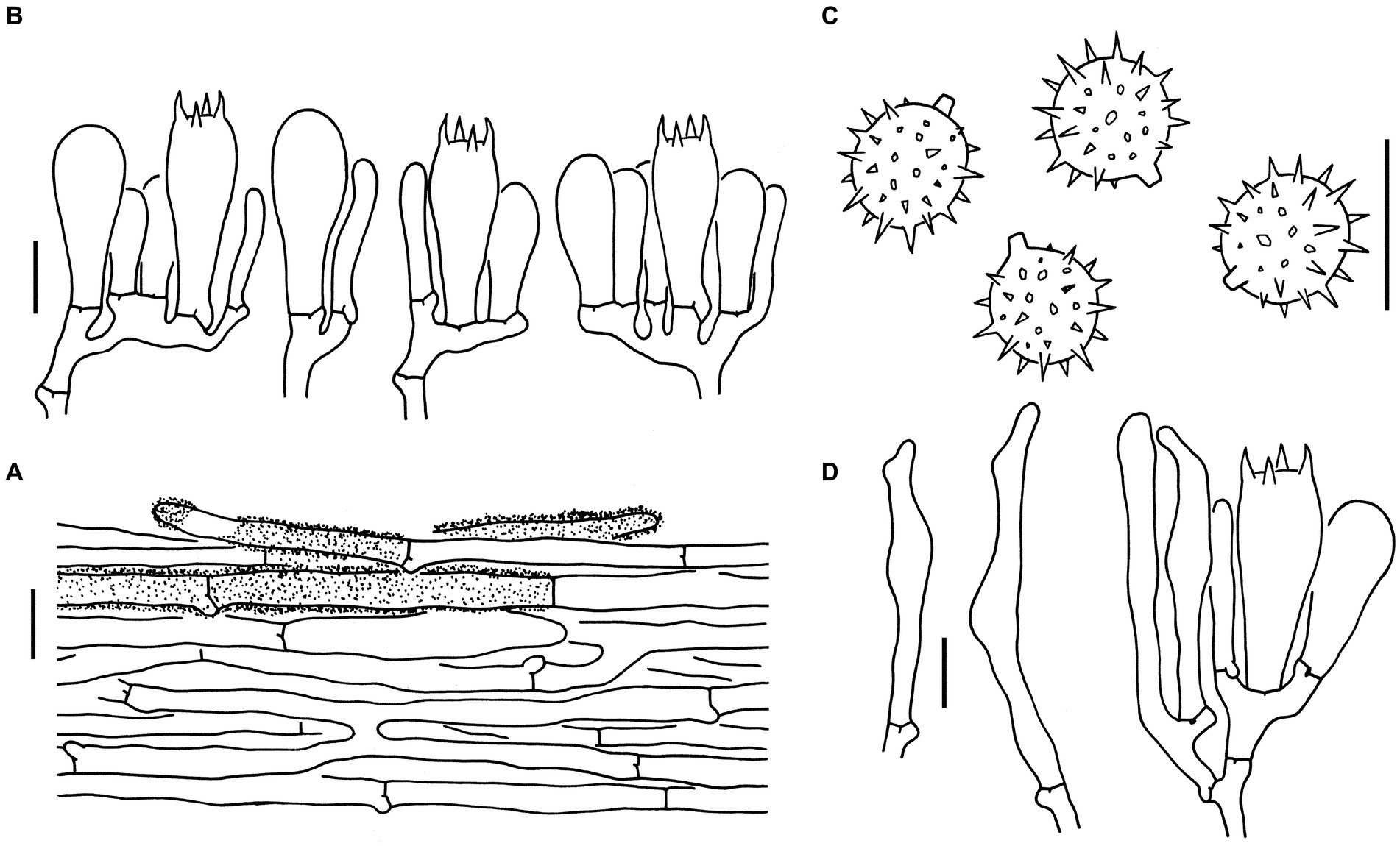
Figure 5. Microscopic features of Laccaria longistriata (holotype, KUN-HKAS123801). (A) Pileipellis. (B) Hymenium and subhymenium. (C) Basidiospores. (D) Marginal cells in the lamellar edge. Bars: A = 20 μm, B–D = 10 μm.
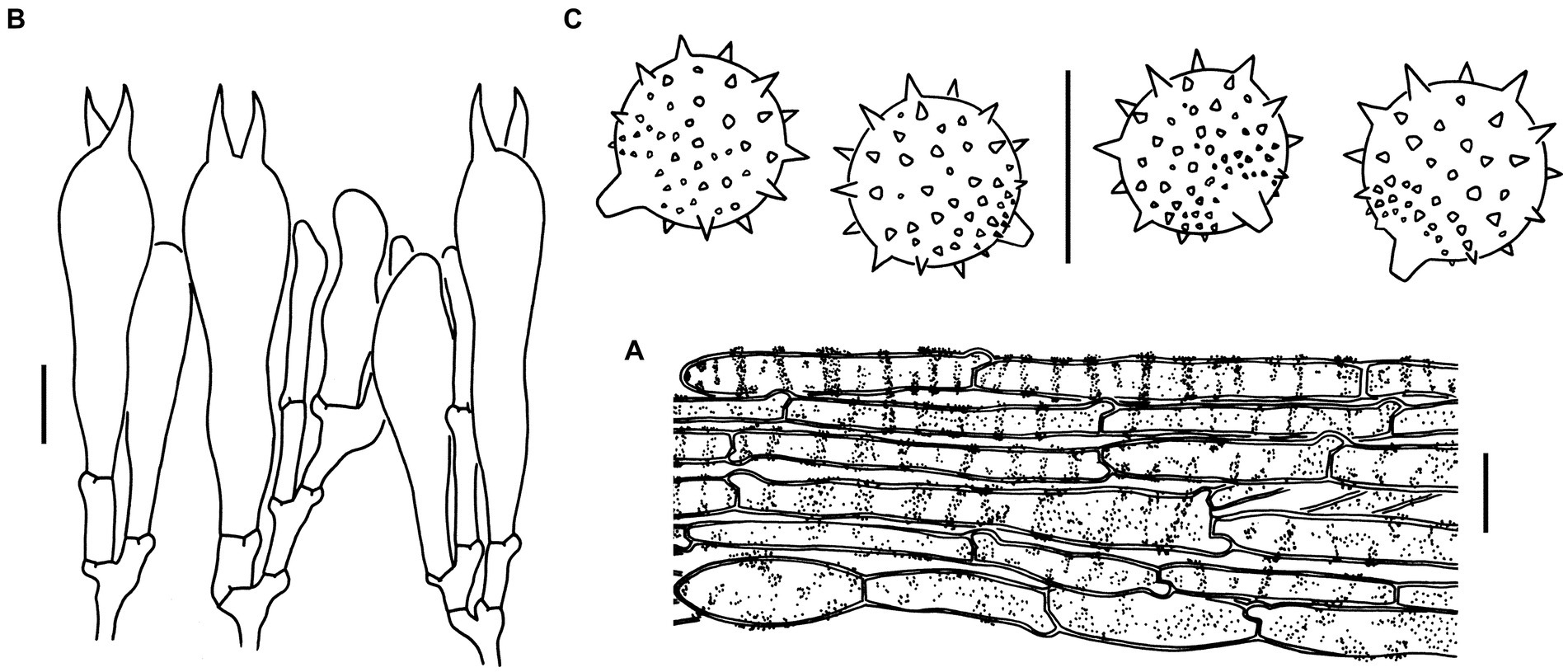
Figure 6. Microscopic features of Laccaria spinulosa (holotype, KUN-HKAS129615). (A) Pileipellis. (B) Hymenium and subhymenium. (C) Basidiospores. Bars: A = 20 μm, B,C = 10 μm.
3.3 Taxonomy
Laccaria cinnabarina J. Li and Y. Y. Cui, sp. nov.
Figures 2A,B, 3A,B, 4.
MycoBank No.: MB 850637.
Etymology: The species name cinnabarina refers to its orange-red pileus.
Diagnosis: Similar to Laccaria himalayensis A. W. Wilson and G. M. Mueller but differs by its very small to medium-sized pileus, dark reddish-brown stipe, and globose to subglobose basidiospores.
Holotype: China. Yunnan Province: Lvchun County, 22°30′18″ N, 102°03′59″ E, altitude 2,300 m, in a broad-leaved forest with trees of Fagaceae, 26 May 2013, Qi Zhao 1778 (KUN-HKAS80885).
Description:
• Basidioma: Very small to medium-sized.
• Pileus: 1–9 cm in diameter; convex to applanate, with an obvious depressed at the center; dark brown (6E6–8) at the center and orange-red (6B6–8) toward the margin; conspicuously pellucid-striate, slightly sulcate; context cream (1A2) to orange (6A2–5).
• Lamellae: Sinuate to adnate; slightly distant, sometimes furcate and intervenose at the margin; pinkish (5A2–4) to pale orange (6A2); lamellulae attenuate.
• Stipe: 3–9 × 0.2–1 cm, cylindrical, fibrillose, longitudinally striate, dark reddish brown (6E4–8); basal mycelium white (1A1).
• Pileipellis: Composed of ± radially arranged, slightly thick-walled (ca. 0.5 μm), orange-yellow filamentous hyphae that are 2–9 μm wide.
• Basidia: 30–45 × 14–16 μm, clavate, hyaline, 4-spored; sterigmata 6–10 μm long.
• Basidiospores: (Excluding ornamentation) [100/5/5] (6.5–) 7–9.5 (−10.5) × (6–) 7–9 (−9.5) μm, = 8.3 ± 0.7 × 8 ± 0.7, Q = 1–1.11 (−1.25), Qm = 1.05 ± 0.04, globose to subglobose, hyaline, and echinate; spines ca. 2 μm long, up to 2 μm wide at the base and crowded; hilar appendix that is 1.0–1.5 μm long and prominent.
• Pleurocystidia and cheilocystidia: Lacking.
• Subhymenium: Composed of filamentous hyphae that are 2–3 μm wide.
• Lamellar trama: Regular, composed of a slightly thick-walled (ca. 0.5 μm) filamentous hyphae that are 6–12 μm wide.
• Stipitipellis: Composed of appressed, parallel, simply septate, thin to slightly thick-walled (ca. 0.5 μm), colorless to pale yellow hyphae that are 2–11 μm wide.
• Caulocystidia: Lacking.
• Clamps: Present in all parts of the basidioma.
Habitat and distribution: Found singly, scattered, or in groups on soil in subtropical broad-leaved forests with trees of Fagaceae. Basidioma occurs from summer to autumn. It is known from southwest China (Yunnan).
Additional specimens studied: China
Yunnan Province:
• Longling County, 24°07′52″ N, 98°25′46″ E, altitude 2,500 m, in a coniferous and broad-leaved mixed forest, 16 June 2014, Jiao Qin 885 (KUN-HKAS83302)
• Longling County, altitude 2,480 m, in a coniferous and broad-leaved mixed forest, 29 July 2014, Jiao Qin 964 (KUN-HKAS83381)
• Fugong County, 26°54′06″ N, 98°52′40″ E, altitude 1,370 m, in a coniferous and broad-leaved mixed forest, 2 August 2011, Gang Wu 476 (KUN-HKAS74787)
• Jingdong Yi Autonomous County, 24°23′06″ N, 100°50′44″ E, altitude 2,491 m, in a coniferous and broad-leaved mixed forest, 22 July 2013, Yang-Yang Cui 24 (KUN-HKAS79704).
Laccaria longistriata J. Li and Y.Y. Cui, sp. nov.
Figures 2C,D, 3C,D, 5.
MycoBank No.: MB 847530.
Etymology: The species longistriata refers to its conspicuous striations on the pileus.
Diagnosis: Similar to Laccaria yunnanensis but differs by its very small to small basidioma, slightly larger basidiospores, and occurrence in the Ailao Shan Mountains in Yunnan at an altitude of approximately 2,500 m.
Holotype: China. Yunnan Province: Mengla County, 21°45′52″ N, 101°56′46″ E, altitude 960 m, in a broad-leaved forest with trees of Fagaceae, 27 August 2019, Liu-Kun Jia 481 (KUN-HKAS123801).
Description:
• Basidioma: Very small to small.
• Pileus: 0.5–4.5 cm in diameter, plano-convex to applanate and slightly depressed at the center; brownish (6C4–7) to brown (6D5–7) at the center and cream (1A2), flesh-colored (7A5) to brownish (6C4–7) toward the margin; conspicuously striate, often sulcate; context flesh-colored (7A5) to brownish (6C6–8).
• Lamellae: Subdecurrent, obviously distant; flesh-colored (7A5), brownish (6C6–8) to brown (6D5–7); lamellulae attenuate.
• Stipe: 4–6 × 0.3–0.8 cm, cylindrical, glabrous, brownish (6C3–6) to brown (6D5–7); basal mycelium white (1A1).
• Pileipellis: Composed of ± radially arranged, slightly thin-walled (ca. 0.5 μm), colorless to brownish filamentous hyphae that are 4–12 μm wide.
• Basidia: 27–42 × 10–13 μm, clavate, hyaline, 4-spored; sterigmata 4–6 μm long.
• Basidiospores: (Excluding ornamentation) [90/5/5] 6.5–8 × 6–8 μm, = 7.3 ± 0.4 × 7 ± 0.4, Q = 1–1.07 (−1.17), Qm = 1.02 ± 0.04, globose to subglobose, and hyaline, echinate; spines 0.5–2 μm long, ca. 0.5 μm wide at the base and crowded; hilar appendix ca. 1 μm long and prominent.
• Pleurocystidia: Lacking.
• Marginal cells in lamellar edge: Fertile.
• Cheilocystidia: Present, 30–60 × 4–8 μm, filamentous, narrowly clavate to subcapitate, thin-walled, colorless, hyaline.
• Subhymenium: Composed of filamentous hyphae that are 2–5 μm wide. Lamellar trama regular, composed of thin-walled filamentous hyphae that are 2–7 μm wide.
• Stipitipellis: Composed of appressed, parallel, simply septate, thin-walled, colorless to brownish hyphae that are 4–7 μm wide.
• Caulocystidia 30–35 × 6–12 μm, clavate, slightly thick-walled (up to 1 μm), colorless, clustered in small groups.
• Clamps: Present in all parts of basidioma.
Habitat and distribution: Found singly, scattered, or in groups on soil in subtropical broad-leaved forests with trees of Fagaceae. Basidioma occurs from summer to autumn. Known from southwest China (Yunnan).
Additional specimens studied: China:
Yunnan Province:
• Mengla County, 21°45′52″ N, 101°56′46″ E, altitude 960 m, in a broad-leaved forest with trees of Fagaceae, 27 August 2019, Geng-Shen Wang 761 (KUN-HKAS121527).
• Same county, altitude 1,000 m, in a broad-leaved forest with trees of Fagaceae, 29 August 2019, Liu-Kun Jia 547 (KUN-HKAS127112).
• Jinghong, 22°01′00″ N, 100°46′17″ E, altitude 1,200 m, in a broad-leaved forest with trees of Fagaceae, 22 August 2019, Geng-Shen Wang 684 (KUN-HKAS123317).
• Same city, altitude 820 m, in a broad-leaved forest with trees of Fagaceae, 21 August 2019, Liu-Kun Jia 349 (KUN-HKAS123800).
• Jiangcheng Hani-Yi Autonomous County, 22°35′06″ N, 101°51′44″ E, altitude 1,000 m, in a broad-leaved forest with trees of Fagaceae, 25 June 2019, Geng-Shen Wang 243 (KUN-HKAS109631).
• Same county, altitude 1,100 m, in a broad-leaved forest with trees of Fagaceae, 28 June 2019, Geng-Shen Wang 288 (KUN-HKAS115989).
• Same county, altitude 1,101 m, in a broad-leaved forest with trees of Fagaceae, 21 September 2019, Sai Gong 912 (KUN-HKAS123799).
Laccaria spinulosa J. Li and Y. Y. Cui, sp. nov.
Figures 2E,F, 3E,F, 6.
MycoBank No.: MB 850641.
Etymology: The species name spinulosa refers to its spinulose basidiospores.
Diagnosis: Similar to Laccaria pumila Fayod but differs by its pinkish flesh lamellae, pileus, and stipe, both with light white pruinae, and smaller and globose to subglobose basidiospores.
Holotype: China. Yunnan Province: Yulong Naxi Autonomous County, 26°59′48″ N, 100°12′02″ E, altitude 2,880 m, in a coniferous and broad-leaved mixed forest with trees of Pinaceae and Fagaceae, 5 August 2019, Jian-Wei Liu 1,696 (KUN-HKAS129615).
Description:
• Basidioma: Very small.
• Pileus: 0.8–2.5 cm in diameter; convex to applanate, slightly depressed at the center, and sometimes slightly uplifted at the margin when mature; brownish orange (6A5–7) to brown (6E6–8) in color, sometimes flesh-colored (6A3–6) to brownish (6D4–7) at the margin; glabrous or sometimes with light white (1A1) pruinae; striate; context brownish orange (6A5–7) to brownish (6D4–7).
• Lamellae: Sinuate to adnate; distant; brownish orange (6A5–7) to brownish (6D4–7) in color; lamellulae attenuate.
• Stipe: 1.5–3 × 0.2–0.5 cm, cylindrical, hollow, brownish orange (6C7) to brown (6E6–8), with light white pruinae; basal mycelium white (1A1).
• Pileipellis: Composed of ± radially arranged, thick-walled (ca. 1 μm), orange-yellow filamentous hyphae 5–23 μm wide.
• Basidia: 43–53 × 10–14 μm, clavate, hyaline, 2-spored; sterigmata 7–14 μm long.
• Basidiospores: (excluding ornamentation) [60/3/3] 9–11 (−12) × (8.5–) 9–10.5 (−11.5) μm, = 9.8 ± 0.6 × 9.5 ± 0.5, Q = 1–1.06 (−1.11), Qm = 1.03 ± 0.03, globose to subglobose, hyaline, echinate, crowded; spines 0.5–2 μm long, up to 1.5 μm wide at the base; hilar appendix ca. 2 μm long, prominent, subtruncate.
• Pleurocystidia and cheilocystidia: Lacking.
• Subhymenium: Composed of filamentous hyphae that are 2–11 μm wide.
• Lamellar trama: Subregular, composed of thick-walled (ca. 0.5 μm), pale yellow filamentous hyphae that are 6–23 μm wide.
• Stipitipellis: Composed of appressed, parallel, simply septate, thick-walled (ca. 0.5 μm), colorless to pale grey hyphae that are 3–9 μm wide.
• Caulocystidia: Lacking.
• Clamps: Prominent and present in all parts of the basidioma.
Habitat and distribution: Found singly, scattered, or in groups on soil in subtropical broad-leaved forests with trees of the Fagaceae family. Basidioma occurs from summer to autumn. Known from southwest China (Yunnan).
Additional specimens studied: China:
Yunnan Province:
• Jingdong Yi Autonomous County, 24°27′02″ N, 100°12′46″ E, altitude 1,300 m, in a coniferous and broad-leaved mixed forest with trees of Pinaceae and Fagaceae, 30 July 2015, Qi Zhao 2570 (KUN-HKAS90444).
• Panlong District, 25°08′37″ N, 102°44′33″ E, altitude 1,953 m, in a coniferous and broad-leaved mixed forest with trees of Pinaceae and Fagaceae, 2 July 2021, Zhu-Liang Yang 6500 (KUN-HKAS122272).
4 Discussion
Only a few morphological characters of Laccaria species are available for taxonomy, and the color of the fruiting body varies widely (Mueller, 1992). Therefore, the application of molecular methods is necessary for the classification and identification of the Laccaria species. To date, the discovery of new species in Laccaria is rapidly increasing through molecular analyses (Popa et al., 2014, 2016; Vincenot et al., 2017; Cho et al., 2018; Cui et al., 2021), especially the multi-loci phylogenetic analysis (Sheedy et al., 2013; Wilson et al., 2017; Cho et al., 2020; Zhang et al., 2023). In this study, we determined three species as new through both molecular phylogenetic data and morphological features.
Laccaria cinnabarina is characterized by orange-red-colored basidiocarps with a conspicuously pellucid-striate pattern, fibrillose stipe, globose to subglobose, and echinulate basidiospores. According to our phylogenetic analysis, this species is related to L. bicolor and L. trichodermophora. However, L. bicolor can be distinguished from L. cinnabarina by its pinkish, fresh-colored, and non-striate pileus (Mueller, 1992). L. trichodermophora has a brownish orange non-striate and strongly pruinose to fibrillose pileus, subglobose to broadly ellipsoid basidiospores (Mueller, 1992).
On the other hand, L. cinnabarina is morphologically similar to L. himalayensis, L. acanthospora, Laccaria aurantia Popa et al., L. fengkaiensis, Laccaria nanlingensis Ming Zhang, and L. umblilicata, which are characterized by an orange pileus with striations. However, L. himalayensis differs by having characteristics such as large to very large basidioma, pastel red to grayish red-brown at the disk to orange-pink toward the margin of the pileus, globose basidiospores, and growing in mixed temperate alpine conifer forests (Wilson et al., 2013). L. acanthospora differs from L. cinnabarina by its orange, pink stipe, obellipsoid to globose basidiospores, and growing on sandy banks in mixed temperate alpine forests (Wilson et al., 2013).
L. aurantia differs from L. cinnabarina by its vividly orange pileus, stipe with a brownish-colored covering, and larger basidiospores (9–10 × 8–10 μm) (Popa et al., 2014). Laccaria fengkaiensis differs by its medium-sized to large basidioma, pastel red to grayish red lamellae, and smaller globose to obellipsoid basidiospores (5.2–6.3 × 5.1–6.3 μm) (Li, 2020). Laccaria nanlingensis differs by its pale red to grayish red lamellae, smaller basidiospores (6.5–7.5 × 6–7 μm), shorter spines (ca. 0.5–1 μm), and the presence of caulocystidia (Zhang et al., 2023). Laccaria umblilicata is characterized by its pale orange to light orange pileus, shorter, yellowish-white to orange-white stipe, and the presence of caulocystidia (Zhang et al., 2023).
Laccaria longistriata has a very small to small, brown to fresh-colored basidioma, a conspicuously striate to sulcate pileus, and globose to subglobose, echinate basidiospores (6.5–8 × 6–8 μm, Q = 1–1.07, Qm = 1.02 ± 0.04) with spines that are 0.5–2 μm long and 0.5 μm wide at the base. Our phylogenetic analysis revealed that L. longistriata is related to L. yunnanensis, L. vinaceoavellanea, L. pallidus, L. neovinaceoavellanea, L. fengkaiensis, L. rufobrunnea, L. lateritia, L. umblilicata, and L. prava.
Indeed, L. yunnanensis, L. vinaceoavellanea, L. pallidus, L. rufobrunnea, and L. prava all have reddish to brownish basidiomata with prominent striate to sulcate pilei (Li, 2020; Zhang et al., 2023; Thapa et al., 2024). However, L. yunnanensis can be distinguished from L. longistriata by its medium-sized to large basidioma (6–10 diam.), slightly larger basidiospores (8–9 × 8–10 μm), and its occurrence in the Ailao Shan Mountains in Yunnan at approximately 2,500 m altitude, which is associated with the mid-montane humid evergreen broad-leaved forest (Popa et al., 2014). Laccaria vinaceoavellanea has a brownish vinaceous pileus, globose to subglobose basidiospores with relatively longer spines, which are 1.4–2.8 μm long (Hongo, 1971; Mueller, 1992; Wang et al., 2004). Laccaria pallidus is characterized by its medium-sized basidiocarps, globose, and smaller basidiospores (5.88–6.17 × 5.88–6.17 μm) (Thapa et al., 2024). Laccaria rufobrunnea is distinctive with its brownish orange to brownish red pileus and white to pinkish-white stipe (Zhang et al., 2023). Laccaria lateritia may be separated from L. longistriata by its 2-spored basidia (Popa et al., 2016). Laccaria prava differs from L. longistriata by its medium-sized to large basidioma, an orange stipe covered with brownish squamules, and basidiospores (7–8 × 6.5–7.5 μm) with relatively shorter spines, which are 0.5–1 μm long (Li, 2020). In addition, Laccaria prava is currently only known from Heishiding at approximately 100 m altitude, which is dominated by the monsoon evergreen broad-leaved forest in the south subtropical area of Guangdong Province (Li, 2020). Although L. fengkaiensis and L. umblilicata may be close to L. longistriata, these two species share orange-colored basidiomata (Li, 2020; Zhang et al., 2023). Laccaria neovinaceoavellanea is a unique species with a pastel pink to pale violet pileus in the lineage (Zhang et al., 2023).
Morphologically, Laccaria rubroalba Luo et al. and Laccaria fulvogrisea Popa et al. can be confused with L. longistriata due to their strongly striate to sulcate pileus and associations with broad-leaved trees (Popa et al., 2014; Luo et al., 2016). However, L. rubroalba has medium-sized, globose and subglobose and broadly ellipsoid basidiospores (6–9 × 5–7 μm) (Luo et al., 2016). L. fulvogrisea can be characterized by its grey to brownish basidioma with violet tinges and echinulate basidiospores with longer spines (1.7–2.5 μm long) (Popa et al., 2014). In addition, Laccaria longistriata also resembles Laccaria striatula (Peck) Peck (Bon, 1983), but the latter has a larger basidioma, has echinulate basidiospores with longer spines (1.4–2.8 μm long), and is abundant in wet mossy areas in eastern North America (Mueller, 1992; Popa et al., 2016).
Laccaria spinulosa is characterized by a brownish-orange basidiocarp and stipe with light white pruinae and globose to subglobose basidiospores. Our phylogenetic analysis revealed that specimens identified as L. spinulosa form a monophyletic lineage close to L. acanthospora, L. canaliculata, L. galerinoides, L. miniata, L. glabripes, L. ohiensis, and L. paraphysata and could be distinguished from the other known Asian Laccaria species that are currently recognized (Figure 1). Laccaria acanthospora differs from L. spinulosa by its small to large basidiocarps, orange pileus, obellipsoid to globose basidiospores, and its occurrence on sandy banks in mixed temperate alpine forests (Wilson et al., 2013).
Laccaria canaliculate differs from its submembranous, velvety, light brown pileus, which is slightly crenulate, and a pallid stipe (Mueller, 1992). Laccaria galerinoides differs by having a light ochraceous-brown to golden-ochraceous pileus, a longer and tubular stipe, and ellipsoid basidiospores (Mueller, 1992). Laccaria miniata differs by its red pileus, subglabrous to fibrillose stipe, and occurrence in north-subtropical habitats (Zhang et al., 2023). All three of the latter species have a reddish-brown pileus. However, L. ohiensis, originally reported from North America, differs by having a glabrous to finely fibrillose stipe with a striate to slightly longitudinally striate and smaller basidiospores (7.7–9.4 × 7–9 μm) (McNabb, 1972). The other two species were originally reported from New Zealand.
Laccaria spinulosa can be distinguished from other small, brownish-orange, plicate-striate Laccaria taxa, such as L. fagacicola Y. Y. Cui et al., L. araneosa H. J. Cho and Y. W. Lim, L. parva H. J. Cho and Y. W. Lim, L. torosa H. J. Cho and Y. W. Lim, L. rufobrunnea, by its 2-sterigmate basidia that bear large basidiospores with long and broad echinulae (Cho et al., 2018; Cui et al., 2021).
In addition, the macroscopic features of L. spinulosa are similar to those of Laccaria species with two-spored basidia, namely L. pumila, Laccaria tortilis (Bolton) Cooke, and Laccaria nigra Hongo. However, L. spinulosa can be differentiated from these three taxa in the following ways:
• Basidiospores: Laccaria spinulosa has a relatively smaller diameter (9–11 × 9–10.5 μm) and globose to subglobose basidiospores compared to L. pumila and L. tortilis (Mueller, 1992).
• Pileus: L. nigra has a grayish pileus and globose basidiospores (Mueller, 1992), whereas L. spinulosa does not.
It is essential to include more Laccaria species from different regions of China, combining morphological characters, molecular data, and ecological information, to understand the species diversity of Laccaria in China in future studies. In terms of morphology, the size of basidia, the presence of two- or four-spored basidia, and the length and density of spines on the spore surface can provide useful directions for species identification.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found in the article/supplementary material.
Author contributions
JL: Writing – original draft, Conceptualization, Software. N-JC: Formal analysis, Writing – original draft. Y-YC: Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Yunnan Revitalization Talent Support Program “Young Talent” Project (XDYC-QNRC-2022-0509) and the Applied Basic Research Programs of Yunnan Province (No. 202101AU070051).
Acknowledgments
The authors are grateful to Jiao Qin, Qi Zhao, Liu-Kun Jia, Zhu L. Yang, Gang Wu, Jian-Wei Liu, Geng-Shen Wang, and Sai Gong for providing valuable specimens. The authors thank Zhi-Jia Gu for providing SEM images. The authors also thank reviewers for their professional suggestions and comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ballero, M., and Contu, M. (1989). Inquadramento tassonomico delle specie europee del genere Laccaria Berk. & Br. Candollea 44, 119–127.
Berkeley, M. J., and Broome, C. E. (1883). Notices of British fungi (1989–2027). Ann. Mag. Nat. Hist. 12, 370–374. doi: 10.1080/00222938309459647
Besson, M., and Kühner, R. (1971). Ultrastructure de la paroi sporique des Laccaria Berk. et Br. (Agaricales). C. R. Hebd. Séances Acad. Sci. 272, 1078–1081.
Bon, M. (1983). Tricholomataceae de France et d’Europe occidentale (6 ème partie: Tribu Clitocybeae Fay.). Doc. Mycol. 13, 1–53.
Cho, H. J., Lee, H., Park, M. S., Park, K. H., Park, J. H., Cho, Y., et al. (2020). Two new species of Laccaria (Agaricales, Basidiomycota) from Korea. Mycobiology 48, 288–295. doi: 10.1080/12298093.2020.1786961
Cho, H. J., Park, M. S., Lee, H., Oh, S. Y., Wilson, A. W., Mueller, G. M., et al. (2018). A systematic revision of the ectomycorrhizal genus Laccaria from Korea. Mycologia 110, 948–961. doi: 10.1080/00275514.2018.1507542
Corrales, A., Wilson, A. W., Mueller, G., and Ovrebo, M. (2020). Novel Laccaria species from Juglandaceae forest in Panama with notes on their ecology. Front. Microbiol. 11:1597. doi: 10.3389/fmicb.2020.01597
Cui, Y. Y., Cai, Q., Li, J., and Yang, Z. L. (2021). Two new Laccaria species from China based on molecular and morphological evidence. Mycol. Prog. 20, 567–576. doi: 10.1007/s11557-021-01698-5
Dai, Y. C., Yang, Z. L., Cui, B. K., Yu, C. J., and Zhou, L. W. (2009). Species diversity and utilization of medicinal mushrooms and fungi in China (review). Int. J. Med. Mushrooms 11, 287–302. doi: 10.1615/IntJMedMushr.v11.i3.80
Dovana, F., Moreno, G., Para, R., Lavorato, C., and Mucciarelli, M. (2021). Phylogenetic reappraisal and epitypification of Laccaria macrocystidiata (Hydnangiaceae, Basidiomycota). Phytotaxa 514, 129–139. doi: 10.11646/phytotaxa.514.2.4
Doyle, J., and Doyle, L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bullet. 19, 11–15.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Heinemann, P. (1964). Champignons récoltés au Congo par Madame M. Goossens-Fontana: VI. Laccaria. Bull. Jard. Bot. État Bruxelles 34, 309–312.
Herrera, M., Yu, F. Q., Ramos-Rendon, D., Martinez-Reyes, M., Hernandez-Santiago, F., Chater, C. C. C., et al. (2022). Morphoanatomical and phylogenetic characterization of the ectomycorrhiza between Laccaria squarrosa with Pinus pseudostrobus and ITS relevance for reforestation programs. Bot. Sci 100, 397–411. doi: 10.17129/botsci.2830
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben. 3rd Edn. Zürich und Göttingen: Muster-Schmidt Verlag, 240.
Kranabetter, J. M., Stoehr, M. U., and O’Neill, G. A. (2012). Divergence in ectomycorrhizal communities with foreign Douglas-fir populations and implications for assisted migration. Ecol. Appl. 22, 550–560. doi: 10.1890/11-1514.1
Kropp, B. R., and Mueller, G. M. (1999). “Laccaria” in Ectomycorrhizal fungi: Key genera in profile. eds. J. W. G. Cairney and S. M. Chambers (Berlin: Springer Press), 65–88.
Li, F. (2020). Two new species of Laccaria from South China, with a note on Hodophilus glaberipes. Mycol. Prog. 19, 525–539. doi: 10.1007/s11557-020-01573-9
Liu, Y. L., Whelen, S., and Hall, B. D. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 16, 1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092
Luo, X., Ye, L., Chen, J., Karunarathna, S. C., Xu, J. C., Hyde, K. D., et al. (2016). Laccaria rubroalba sp. nov. (Hydnangiaceae, Agaricales) from southwestern China. Phytotaxa 284, 041–050. doi: 10.11646/phytotaxa.284.1.4
Matheny, B. P. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 35, 1–20. doi: 10.1016/j.ympev.2004.11.014
May, T. W. (1991). A taxonomic study of the Australian species of Laccaria [dissertation]. Australia: Monash University.
McNabb, R. F. R. (1972). The tricholomataceae of New Zealand 1. Laccaria Berk. and Br. New Zeal. J. Bot. 10, 461–484.
Mueller, G. M. (1991). Laccaria longipes, a new North America species of the Laccaria laccata complex. Mycotaxon 40, 145–150.
Mueller, G. M. (1992). Systematics of Laccaria (Agaricales) in the continental United States and Canada, with discussions on extralimital taxa and descriptions of extant types. Fieldiana Bot. New Ser. 30, 1–158. doi: 10.5962/bhl.title.2598
Nara, K., Nakaya, H., Wu, B., Zhou, Z., and Hogetsu, T. (2003). Underground primary succession of ectomycorrhizal fungi in a volcano desert on Mount Fuji. New Phytol. 159, 743–756. doi: 10.1046/j.1469-8137.2003.00844.x
Nylander, J. A. A. (2004). MrModeltest v2.4. Program dstributed by the author. Evolutionary biology Centre, Uppsala University, Uppsala. Available at: http://github.com/nylander/MrModeltest2/tree/v.2.4 (Accessed September 11, 2018)
Osmundson, T. W., Cripps, C. L., and Mueller, G. M. (2005). Morphological and molecular systematics of Rocky Mountain alpine Laccaria. Mycologia 97, 949–972. doi: 10.1080/15572536.2006.11832746
Popa, F., Jimenéz, S. Y. C., Weisenborn, J., Donges, K., Rexer, K. H., and Piepenbring, M. (2016). A new Laccaria species from cloud forest of Fortuna, Panama. Mycol. Prog. 15:19. doi: 10.1007/s11557-015-1139-7
Popa, F., Rexer, K. H., Donges, K., Yang, Z. L., and Kost, G. (2014). Three new Laccaria species from Southwest China (Yunnan). Mycol. Prog. 13, 1105–1117. doi: 10.1007/s11557-014-0998-7
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., and Suchard, M. A. (2018). Posterior summarisation in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Roy, M., Dubois, M. P., Proffit, M., Vincenot, L., Desmarais, E., and Selosse, M. A. (2008). Evidence from population genetics that theectomycorrhizal basidiomycete Laccaria amethystine is an actual multihost symbiont. Mol. Ecol. 17, 2825–2838. doi: 10.1111/j.1365-294X.2008.03790.x
Rozen, S., and Skaletsky, H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386. doi: 10.1385/1-59259-192-2:365
Sheedy, E. M., Van de Wouw, A. P., Howlett, B. J., and May, T. W. (2013). Multigene sequence data reveal morphologically cryptic phylogenetic species within the genus Laccaria in southern Australia. Mycologia. 105, 547–563.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Stielow, J. B., Lévesque, C. A., Seifert, K. A., Meyer, W., Irinyi, L., Smits, D., et al. (2015). One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35, 242–263. doi: 10.3767/003158515X689135
Thapa, A., Tamang, J., and Acharya, K. (2024). Three new species of Laccaria (Hydnangiaceae) from India (Darjeeling Hills) based on molecular and morphological evidence. Curr. Microbiol. 81:79. doi: 10.1007/s00284-023-03598-1
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Vincenot, L., Popa, F., Laso, F., Donges, K., Rexer, K. H., Kost, G., et al. (2017). Out of Asia: biogeography of fungal populations reveals Asian origin of diversification of the Laccaria amethystina complex, and two new species of violet Laccaria. Fungal Biol. 121, 939–955. doi: 10.1016/j.funbio.2017.08.001
Wang, K., Liu, D. M., Li, G. J., Liu, T. Z., Xie, M. L., Du, Z., et al. (2022). Taxonomy of Laccaria in China based on the specimens collected in the HMAS. J. Fungal Res. 20, 271–284. doi: 10.13348/j.jfr.2022.1566
Wang, L., Yang, Z. L., and Liu, J. H. (2004). Two new species of Laccaria (Basidiomycetes) from China. Nova Hedwigia 79, 511–517. doi: 10.1127/0029-5035/2004/0079-0511
White, T. J., Bruns, T. D., Lee, S. B., and Taylor, J. W. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics” in PCR protocols: a guide to methods and applications. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (London: Academic Press), 315–322.
Wilson, A. W., Hosaka, K., and Mueller, G. (2017). Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol. 213, 1862–1873. doi: 10.1111/nph.14270
Wilson, A. W., Hosaka, K., Perry, B. A., and Mueller, G. M. (2013). Laccaria (Agaricomycetes, Basidiomycota) from Tibet (Xizang Autonomous Region, China). Mycoscience 54, 406–419. doi: 10.1016/j.myc.2013.01.006
Wu, F., Zhou, L. W., Yang, Z. L., Bau, T., Li, T. H., and Dai, Y. C. (2019). Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Divers. 98, 1–76. doi: 10.1007/s13225-019-00432-7
Keywords: morphological characters, multi-gene sequence, novel species, Laccaria, taxonomy
Citation: Li J, Che N-J and Cui Y-Y (2024) Three new species of Laccaria (Agaricales, Basidiomycota) from Southwest China (Yunnan) based on morphological and multi-gene sequence data. Front. Microbiol. 15:1411488. doi: 10.3389/fmicb.2024.1411488
Edited by:
Dipesh Dhakal, University of Florida, United StatesReviewed by:
Yifei Sun, Beijing Forestry University, ChinaShun Liu, Beijing Forestry University, China
Copyright © 2024 Li, Che and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cui Yang-Yang, Y3VpeWFuZ3lhbmdAbWFpbC5raWIuYWMuY24=
 Jing Li
Jing Li Nian-Jie Che2
Nian-Jie Che2