- Department of Gastroenterology, Beijing Key Laboratory of Functional Gastrointestinal Disorders Diagnosis and Treatment of Traditional Chinese Medicine, Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background and aims: Functional dyspepsia (FD) is a common gastrointestinal disorder associated with brain–gut interaction disturbances. In recent years, accumulating evidence points to the duodenum as a key integrator in dyspepsia symptom generation. Investigations into the pathological changes in the duodenum of FD patients have begun to focus on the role of duodenal microbiota dysbiosis. This review summarizes duodenal microbiota changes in FD patients and explores their relationship with gut-brain interaction dysregulation.
Methods: Ten databases, including PubMed, MEDLINE, and the Cochrane Library, were searched from inception to 10th October 2023 for clinical interventional and observational studies comparing the duodenal microbiota of FD patients with controls. We extracted and qualitatively summarized the alpha diversity, beta diversity, microbiota composition, and dysbiosis-related factors.
Results: A total of nine studies, consisting of 391 FD patients and 132 non-FD controls, were included. The findings reveal that the alpha diversity of the duodenal microbiota in FD patients does not exhibit a significant difference compared to non-FD controls, although an upward trend is observed. Furthermore, alterations in the duodenal microbiota of FD patients are associated with the symptom burden, which, in turn, impacts their quality of life. In FD patients, a considerable number of duodenal microbiota demonstrate a marked ascending trend in relative abundance, including taxa such as the phylum Fusobacteria, the genera Alloprevotella, Corynebacterium, Peptostreptococcus, Staphylococcus, Clostridium, and Streptococcus. A more pronounced declining trend is observed in the populations of the genera Actinomyces, Gemella, Haemophilus, Megasphaera, Mogibacterium, and Selenomonas within FD patients. A negative correlation in the relative abundance changes between Streptococcus and Prevotella is identified, which correlates with the severity of symptom burden in FD patients. Moreover, the alterations in specific microbial communities in FD patients and their potential interactions with the gut–brain axis merit significant attention.
Conclusion: Microbial dysbiosis in FD patients is linked to the onset and exacerbation of symptoms and is related to the disorder of gut–brain interaction. Larger-scale, higher-quality studies, along with comprehensive meta-omics research, are essential to further elucidate the characteristics of the duodenal microbiota in FD patients and its role in FD pathogenesis.
Systematic review registration: CRD42023470279, URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023470279.
1 Introduction
Functional dyspepsia (FD) is one of the most prevalent functional gastrointestinal (GI) disorders of gastroduodenal origin (Stanghellini et al., 2016). The global prevalence of FD is approximately 16% and may vary substantially according to different countries and the definition criteria of the disease (Ford et al., 2020). FD is defined as the presence of one or more of four symptoms: postprandial fullness, early satiation, epigastric pain, and epigastric burning, and cannot be explained by structural or biochemical abnormalities identified in routine clinical settings (Drossman, 2016; Ford et al., 2020). Based on the predominant symptom pattern, Rome IV provides for two functional dyspepsia subtypes of FD: epigastric pain syndrome (EPS) with epigastric pain and/or epigastric burning and postprandial distress syndrome (PDS) with postprandial fullness and/or early satiety (Drossman, 2016). Although FD does not affect survival, the symptoms of FD can be quite troublesome and difficult to treat, and patients usually have a natural history of recurrence and remission. FD is believed to affect the diet (quantity and quality of meals) and quality of life of patients, reduce the productivity of patients, lead to emotional disorders and somatization, and may result in high medical costs, which seriously burden individuals and society (Lacy et al., 2013; Gracie et al., 2015; Aziz et al., 2018; Esterita et al., 2021).
Due to the multifactorial and heterogeneous nature of functional dyspepsia symptoms, many factors influence the pathogenesis of FD. At present, the mainstream theory believes that the key to the pathogenesis of FD is related to the disorder of gut–brain interaction (DGBI) (Zhou et al., 2022). In addition, emerging research in recent years has begun to point toward the duodenum as a key player in the pathogenesis of FD.
The gastric sensorimotor dysfunction observed in FD patients may be attributed to the activation of duodeno-gastric reflexes, which transmit noxious stimuli from the duodenal mucosa via afferent nerves, leading to abnormal gastric motility and hypersensitivity (Vanheel and Ricard, 2013; Miwa et al., 2019; Wauters et al., 2020a). As a pathogenic epicenter, the duodenum induces upper gastrointestinal symptoms in FD patients primarily due to the stimulation of duodenal contents, low-grade inflammation, and increased mucosal permeability in the duodenum. Alterations in the duodenal microbiota, or “dysbiosis,” are posited to be a significant factor contributing to the emergence of these duodenal pathologies (Miwa et al., 2019; Wauters et al., 2020a). Recent research has focused on the presence of small intestinal bacterial overgrowth (SIBO) in FD patients (Gurusamy et al., 2021a). Relevant studies have uncovered a dysbiotic state within the duodenal mucosa of FD patients, characterized by increased bacterial load and diversity. This microbial imbalance has been found to be associated with the manifestation of gastrointestinal symptoms in FD patients (Zhong et al., 2017). The gut microbiota may communicate with the central nervous system via neural, endocrine, and immune pathways, thereby influencing brain function. In FD patients, dysbiosis of the gut microbiota is known to affect the gut–brain interactive functions, with the microbiota-gut–brain axis being recognized as playing a significant role in FD (Rupp and Andreas, 2022).
However, in previous studies, due to the difficulty in obtaining duodenal microbiota samples, most studies on the gastrointestinal microbiota of FD patients focused on the microbiota of the stomach, large intestine, and feces; the understanding of duodenal microbiota was relatively limited (Tziatzios et al., 2020; Zhou et al., 2022). The dysbiosis of the duodenal microbiota and its implications for the pathogenesis of FD warrant further exploration.
Drawing from the aforementioned perspectives, we postulate that alterations in the duodenal microbiota represent a crucial yet underappreciated nexus influencing the pathogenesis of functional dyspepsia (FD) while also engaging in the gut–brain axis of FD patients. Recent observational studies have provided pertinent insights into the involvement of the duodenal microbiota in FD onset. Building upon these foundations, this study synthesizes the characteristic changes in duodenal microbiota among FD patients, endeavors to elucidate the mechanisms by which duodenal microbiota participate in gut–brain interactions, explores the role of duodenal microbiota alterations in FD pathogenesis, and endeavors to identify bacterial targets pertinent to FD diagnosis or treatment.
2 Methods
2.1 Protocol and registration
This systematic review followed the recommended approach described in the Preferred Reporting Items for Systematic Review and Meta-Analyses Protocols (PRISMA-P) 2015 Statement Guidelines (PRISMA-P Group et al., 2015; Page et al., 2020).
The protocol has been registered with the International Prospective Register of Systematic Reviews, and the PROSPERO registration number is CRD42023470279.
2.2 Search strategy
We performed a systematic search of 10 electronic databases, including PubMed, MEDLINE (Medical Literature Analysis and Retrieval System Online), Cochrane Library, ClinicalTrials.gov, Excerpta Medica Database (EMBASE), Web of Science (Wos), Wanfang Data, China National Knowledge Infrastructure (CNKI), VIP Information Resource Integration Service Platform (CQVIP), and Chinese Biomedical Literature Database (SinoMed), from inception to 10th October 2023, with the keywords “duodenum,” “microbiota,” and “functional dyspepsia.” We also manually searched for other relevant literature based on the references to the identified articles.
2.3 Study selection and patient population
This review selected observational and interventional studies (i.e., case–control, cohort, and randomized and non-randomized clinical trial studies) that compared the composition of the duodenal microbiota, microbial diversity, or microbial richness between FD patients and non-FD controls. FD patients must have a presumed diagnosis of FD based on the clinical assessment, questionnaire data, or specific symptom-based criteria, including the Rome criteria, and patients must not show any evidence of gastric/duodenal mucosal abnormalities, lesions, or structural changes (based on endoscopic and clinical histology findings). The control group (CG) comprises individuals without functional dyspepsia, including healthy controls, and is required to possess demographic data comparable to that of the FD group. There are no restrictions on the age and gender of the population. We excluded studies in which the experimental group included patients with functional gastrointestinal disorders or FD combined with other diseases. Case reports, expert opinions, and reviews were also excluded.
2.4 Data extraction
Data were extracted by two independent authors (XPZ and LC) and confirmed by a third (XLS). The following data were extracted: (1) study design, including the name of the first author, year of publication, journal, country, study design, inclusion and exclusion criteria, and criterion for FD diagnosis; (2) population characteristics, including age, sex, sample size, body mass index (BMI), follow-up duration, usage of proton pump inhibitor (PPI), and subtype(s) of FD if specified; (3) outcome measures: specimen processing (collection, storage, and DNA extraction), sequencing platforms, method of gut microbiota estimation, alpha diversity, beta diversity index, duodenal microbiota profile, and relative abundance. The primary outcome was the difference in individual duodenal bacterial species taxonomic classification reported in FD patients compared to non-FD controls.
2.5 Quality of studies
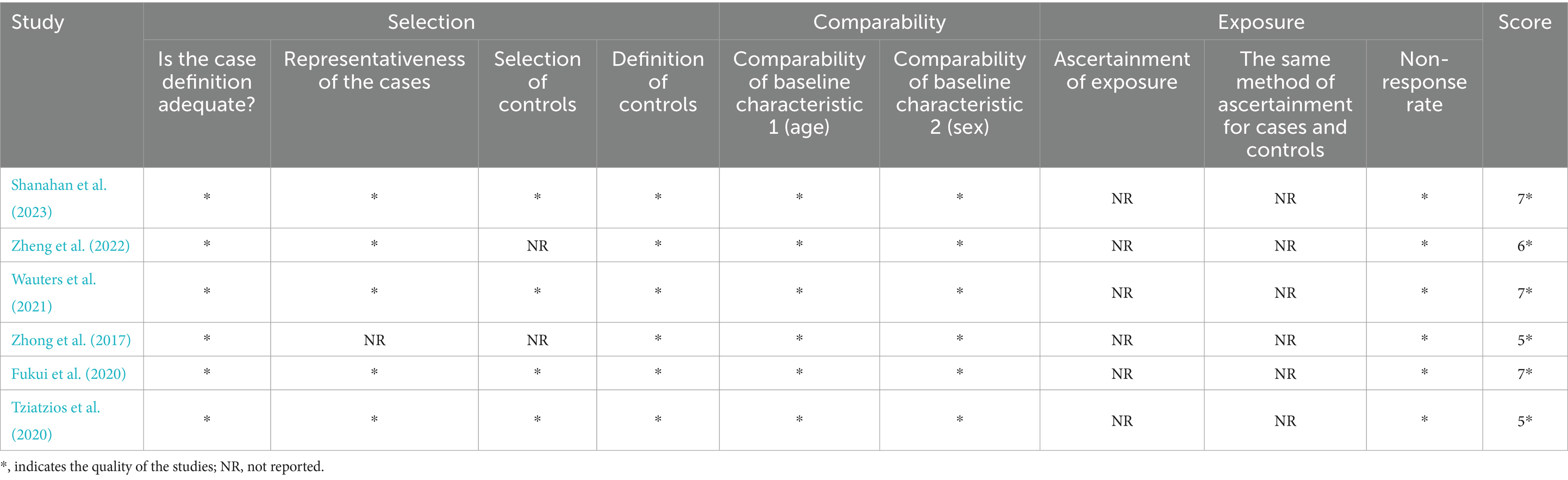
Two independent researchers (QYW and ZTZ) evaluated the risk of research bias separately. Study quality (risk of bias) has been assessed by the Newcastle-Ottawa scale (NOS) tool, which evaluates research through population selection, comparability, exposure, or outcomes (Stang, 2010). Any differences between the two researchers will be resolved through discussion or negotiation with the third reviewer (TZ).
3 Results
3.1 Study selection
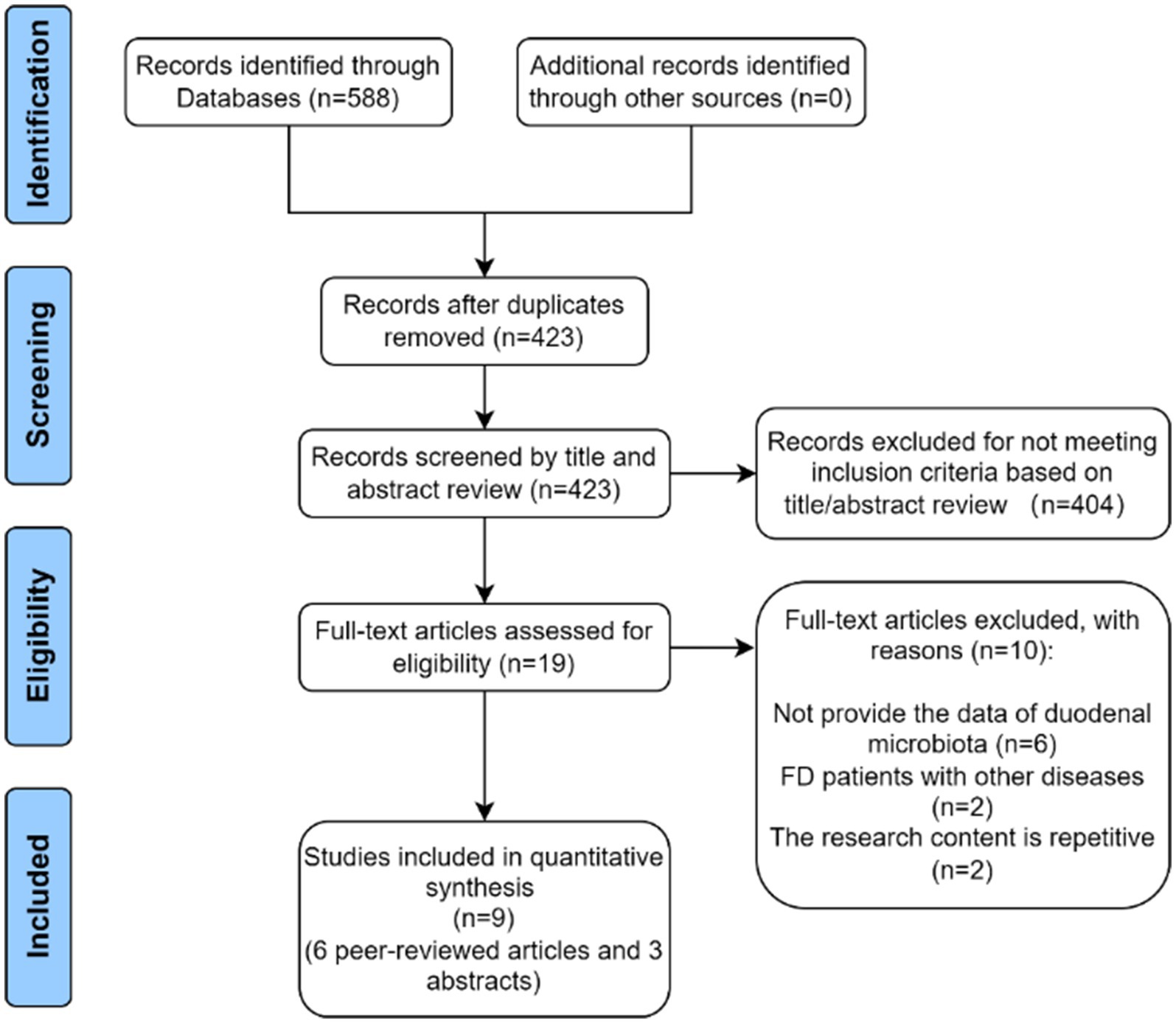
A total of 588 studies were identified in the initial literature search. Then, 165 duplicates were identified and excluded. Afterward, 404 studies were rejected based on relevance to the title abstract. After full-text screening, 10 articles were excluded, and six full-text articles and three abstract studies were finally included (Figure 1).
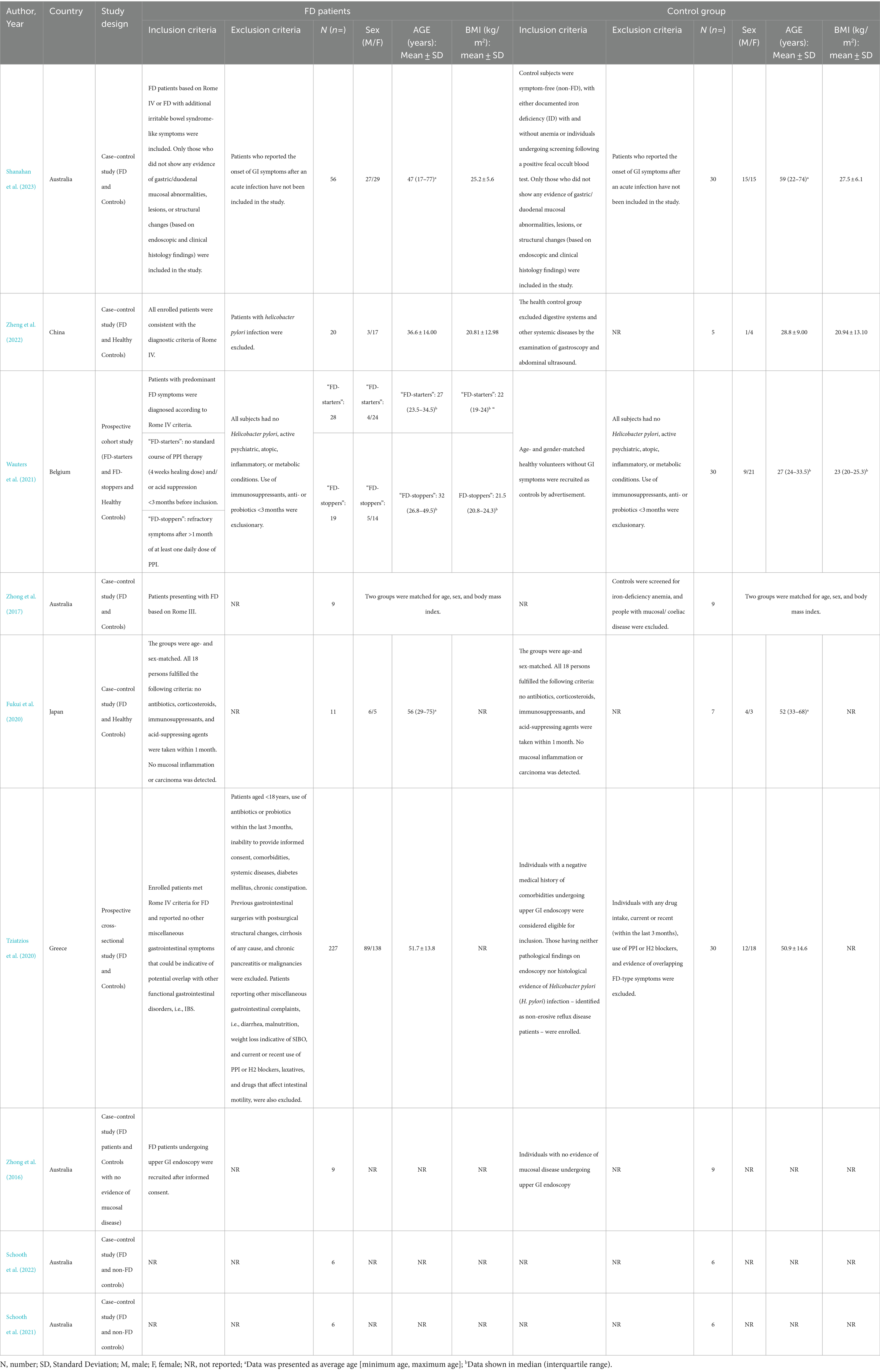
3.2 Characteristics of reviewed studies
Table 1 provides an overview of the study’s characteristics. The nine studies consist of 391 FD patients and 132 non-FD controls. Out of all the studies included, only one had a sample size greater than 100, with 257 cases (Tziatzios et al., 2021). The remaining studies had sample sizes below 100. Five studies were conducted in Australia (Zhong et al., 2016, 2017; Schooth et al., 2021, 2022; Shanahan et al., 2023), while others were conducted in Belgium (Wauters et al., 2021), China (Zheng et al., 2022), Greece (Tziatzios et al., 2021), and Japan (Fukui et al., 2020). This review contains one prospective cohort study (Wauters et al., 2021), one prospective cross-sectional study (Tziatzios et al., 2021), and seven case–control studies (Zhong et al., 2016, 2017; Fukui et al., 2020; Schooth et al., 2021, 2022; Zheng et al., 2022; Shanahan et al., 2023). The search did not include interventional studies carried out on FD patients. Out of all the studies included, three were explicitly presented as FD patients compared to healthy controls (Fukui et al., 2020; Wauters et al., 2021; Zheng et al., 2022), while the rest compared FD patients to non-FD patients (Zhong et al., 2016, 2017; Schooth et al., 2021, 2022; Tziatzios et al., 2021; Shanahan et al., 2023). Among the studies in which FD patients were compared with healthy controls, one study (Wauters et al., 2021) compared FD patients who had not been treated with PPI therapy (4-week healing dose) or other acid suppression < 3 months before inclusion (“FD-starters”) with FD patients who had persistent symptoms after >1 month of at least one daily dose of PPI (“FD-stoppers”) and healthy controls (Table 1).
3.3 Microbiome assessment methods
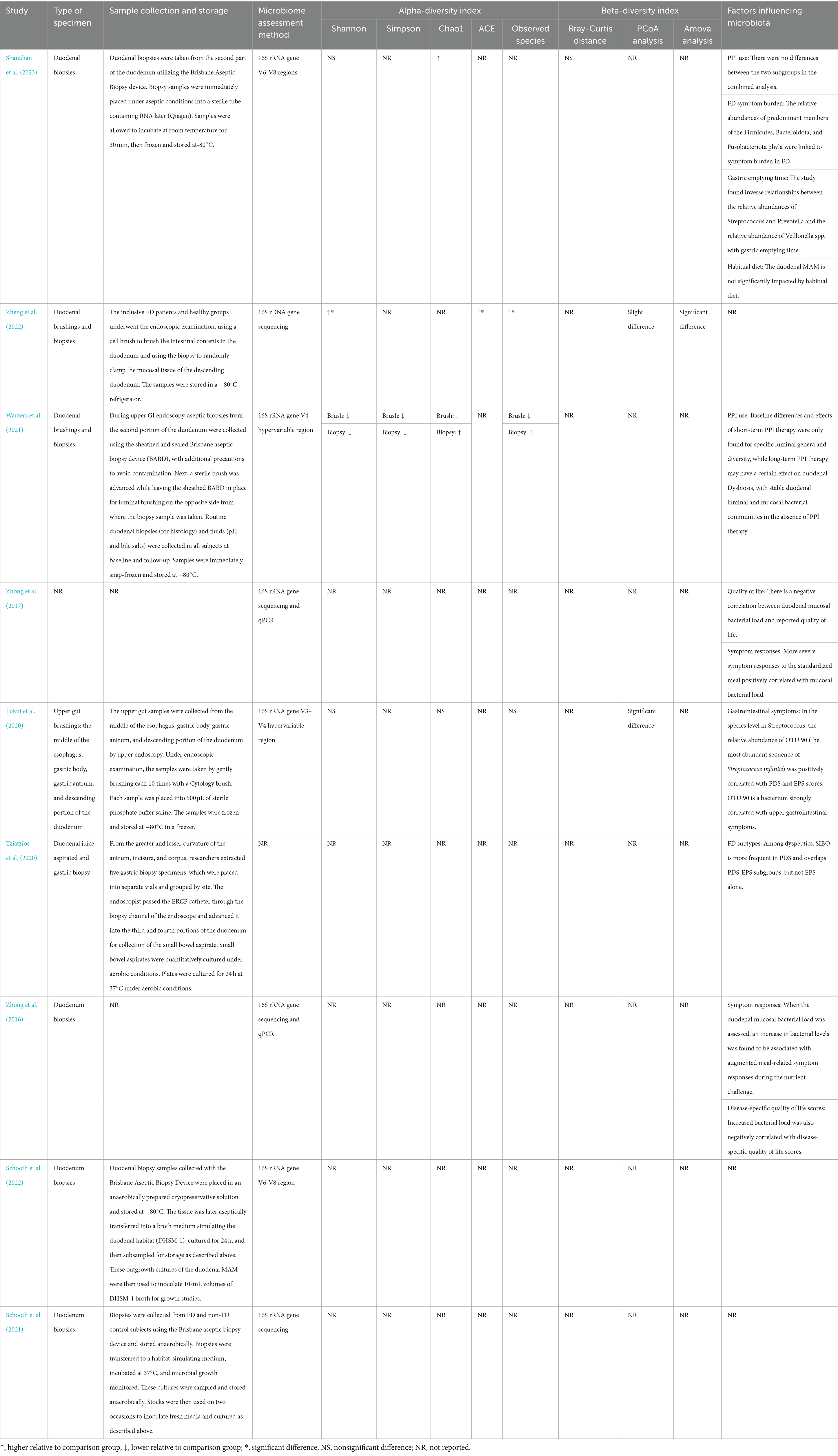
Of the nine studies included in this systematic review, four studies (Zhong et al., 2016; Schooth et al., 2021, 2022; Shanahan et al., 2023) collected samples through duodenal biopsy only, and one (Fukui et al., 2020) performed upper gut brushing, while two others (Wauters et al., 2021; Zheng et al., 2022) used both duodenal biopsy and brushing. One study (Wauters et al., 2021) collected gastric and duodenal fluids, and another (Tziatzios et al., 2021) used duodenal fluid aspiration along with gastric biopsy. In terms of microbial assessment, 16S rRNA gene sequencing was used by most studies (n = 8) (Zhong et al., 2016, 2017; Fukui et al., 2020; Schooth et al., 2021, 2022; Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023). There were differences in the variable region sequenced among the eight studies that used bacterial 16S rRNA gene sequencing. One study (Wauters et al., 2021) used the V4 hypervariable region, one study (Fukui et al., 2020) used the V3-V4 hypervariable region, and two studies sequenced V6–V8 (Schooth et al., 2022; Shanahan et al., 2023), which was the commonly studied variable region (Table 2).
3.4 Alterations in the duodenal microbiota composition of FD patients
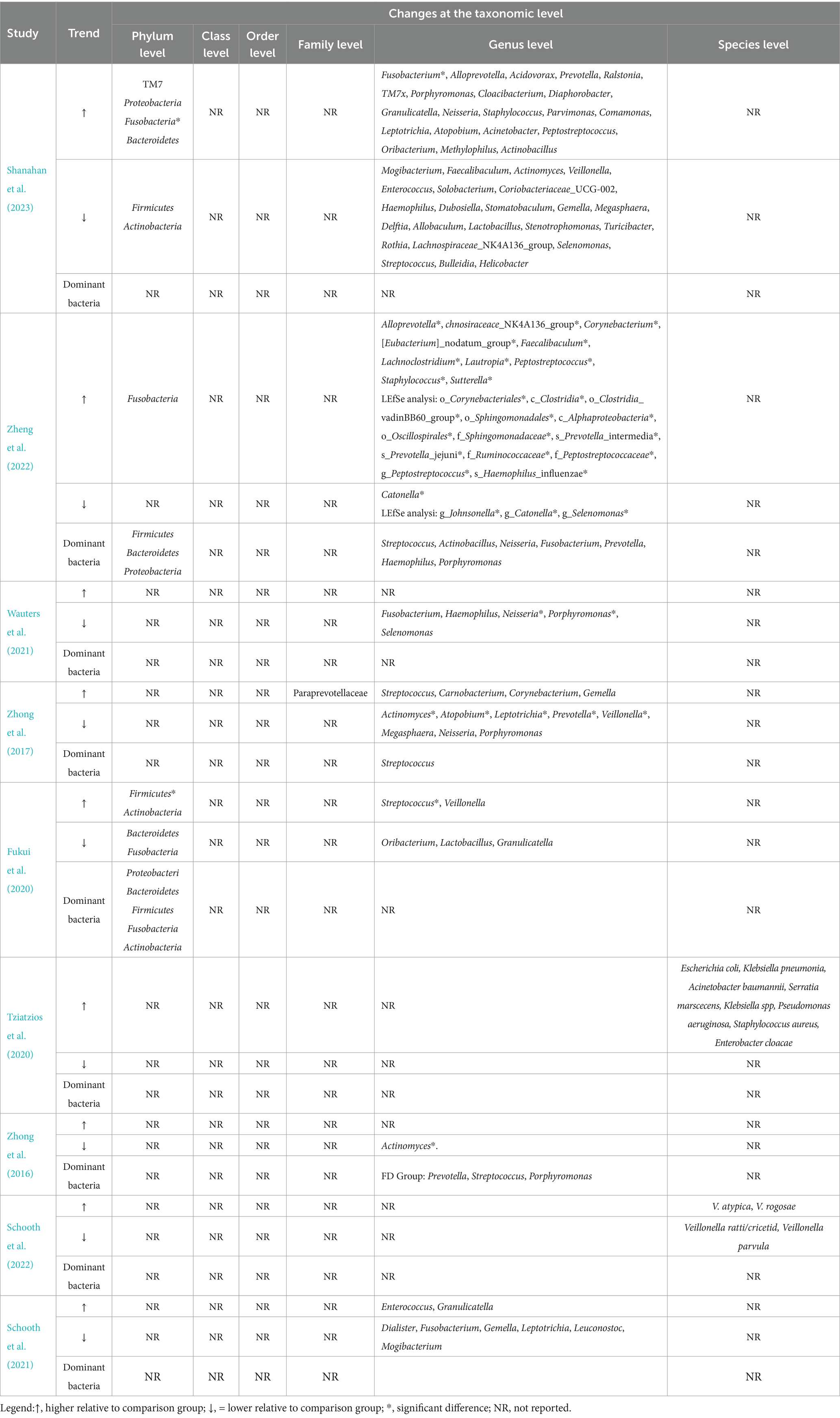
Three studies (Fukui et al., 2020; Zheng et al., 2022; Shanahan et al., 2023) have delineated variations in the duodenal microbiota at the phylum level. Two studies (Zheng et al., 2022; Shanahan et al., 2023) found an increase in the amounts of Fusobacteria in FD patients, whereas a third study (Fukui et al., 2020) showed no difference compared to healthy controls. Findings from one study (Zheng et al., 2022) suggest that the phylum Firmicutes constitutes a substantial proportion of the total sequences in both FD patients and healthy individuals and represents the dominant bacteria of the duodenal flora. One study (Fukui et al., 2020) demonstrated a significant increase in the phylum Firmicutes in FD patients. While another study (Shanahan et al., 2023) did not observe a marked difference in the relative abundance of Firmicutes between FD patients and healthy controls, it did discern a correlation between the abundance of this phylum and the severity of FD symptoms, with an escalation in Firmicutes accompanying an increased symptom burden in FD patients. In one study (Zheng et al., 2022), the phylum Bacteroidetes and Proteobacteria were identified as the dominant species of duodenal flora. However, three investigations (Fukui et al., 2020; Zheng et al., 2022; Shanahan et al., 2023) collectively found no significant differences in the abundance of Bacteroidetes and Proteobacteria between FD patients and the control group. Additionally, one study (Zheng et al., 2022) remarked that there was no significant difference in the composition of duodenal flora at the phylum level between FD patients and healthy controls.
At the genus level, there were three Australian studies (Zhong et al., 2016, 2017; Shanahan et al., 2023) demonstrating a significantly decreased number of bacteria in the genus Actinomyces. The genus Selenomonas was evaluated in three studies (Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023). Of these, one study (Zheng et al., 2022) showed a significant decrease in the amount of the genus Selenomonas in FD patients, while the other two studies (Wauters et al., 2021; Shanahan et al., 2023) showed a non-significant trend toward decreased amounts. The results of a study in China (Zheng et al., 2022) showed significantly increased numbers of the genera Staphylococcus and Peptostreptococcus in FD patients. In contrast, an Australian study (Shanahan et al., 2023) showed a non-significant trend of increase for these genera. Another study identified the genus Staphylococcus as a predominant component of the duodenal microbiota in FD patients (Zhong et al., 2016). Two studies evaluated the genus Alloprevotella in 76 FD patients (35 healthy controls) and showed a significant increase in one study (Zheng et al., 2022) and a non-significant increase in another (Shanahan et al., 2023). Genus Haemophilus showed a decreasing trend in two studies (Wauters et al., 2021; Shanahan et al., 2023), whereas in one study of 9 FD patients compared to 9 healthy controls (Zhong et al., 2017), the relative abundance of the genus Haemophilus was not found to be significantly different. Another study from China (Zheng et al., 2022) found that the genus Haemophilus was one of the most important microorganisms in the duodenal flora of both FD patients and healthy controls. Furthermore, two studies (Zhong et al., 2017; Shanahan et al., 2023) compared a total of 65 FD patients with 39 healthy controls and concluded that the microbial counts of genus Megasphaera showed a decreasing trend in FD patients.
At the genus level, there were conflicting results of cumulative evidence for several significant microbiota. Three studies (Zhong et al., 2017; Zheng et al., 2022; Shanahan et al., 2023) have identified the genus Streptococcus as the dominant organism in the duodenal flora of both FD patients and healthy controls. A Japanese study (Fukui et al., 2020) showed a significant increase in the duodenal genus Streptococcus counts in FD patients compared to controls, and the results of another study (Zhong et al., 2017) also suggested a non-significant trend toward an increase, while the result of an Australian study (Shanahan et al., 2023) showed a non-significant decreasing trend of this genus. Genus Prevotella was also identified as a dominant species within the duodenal microbiota in three studies (Zhong et al., 2016; Zheng et al., 2022; Shanahan et al., 2023). However, a significant decrease in the genus Prevotella was found in one study (Zhong et al., 2017), and a non-significant increase was found in another study (Shanahan et al., 2023). A study conducted in China (Zheng et al., 2022) found that the genus Fusobacterium, genus Neisseria, and genus Porphyromonas were significant microbiota in the duodenum of both healthy individuals and FD patients at the genus level. Another study (Zhong et al., 2016) also identified the genus Porphyromonas as an important component of the duodenal flora in FD patients. Both the genera Neisseria and Porphyromonas had the same trend of alteration in three articles (Zhong et al., 2017; Wauters et al., 2021; Shanahan et al., 2023). In two of the articles (Zhong et al., 2017; Wauters et al., 2021), the relative abundance of these two genera in FD patients showed a decreasing trend, whereas in another article (Shanahan et al., 2023), their relative abundance exhibited an increasing trend. Four studies (Zhong et al., 2017; Schooth et al., 2021; Wauters et al., 2021; Shanahan et al., 2023) assessed the genus Fusobacterium. One study found a significantly increased amount of this genus in FD patients (Shanahan et al., 2023), while two studies (Schooth et al., 2021; Wauters et al., 2021)showed a trend for a decreased amount, and the other (Zhong et al., 2017) revealed a non-significant difference.
Summarizing the abundance changes of genera reported in two or more studies and categorizing them at the phylum level, it can be observed that genera under the phylum Firmicutes exhibit the highest number of changes between FD and non-FD control groups, followed by those under the phylum Actinobacteria (Figure 2; Table 3).
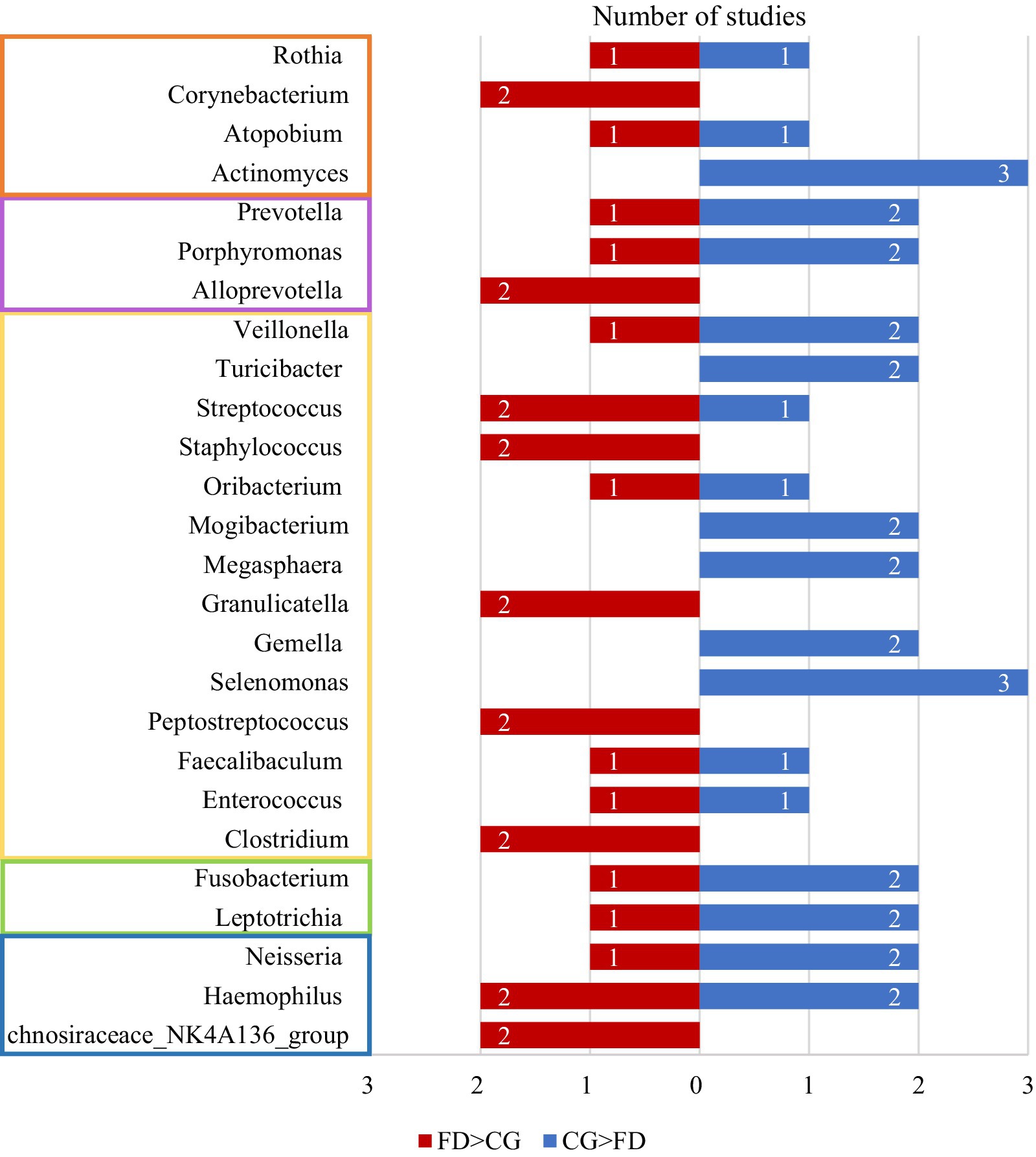
Figure 2. The number of studies reporting differences in bacterial genera between the FD group and the CG group across two or more studies. The blue bars represent the number of studies where the abundance of bacterial genera was higher in the CG group compared to the FD group, while the red bars indicate the number of studies where the abundance was higher in the FD group than in the CG group. Genera within the orange frame belong to the Actinobacteria, those in the purple frame to the Bacteroidetes, those in the yellow frame to the Firmicutes, those in the green frame to the Fusobacteria, and those in the blue frame to the Proteobacteria. CG, control group; FD, functional dyspepsia.
3.5 Duodenal microbiota diversity in FD patients
Four articles (Fukui et al., 2020; Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023) compared the alpha diversity of FD patients and controls by using different indices and methods. Four studies (Fukui et al., 2020; Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023) reported the Shannon index, three reported the Chao1 index (Fukui et al., 2020; Wauters et al., 2021; Shanahan et al., 2023), three reported observed species (Fukui et al., 2020; Wauters et al., 2021; Zheng et al., 2022), one reported the ACE index (Zheng et al., 2022), and one reported the Simpson index (Wauters et al., 2021). Only one study (Zheng et al., 2022) showed a significant increase in alpha diversity in FD patients, while three other studies (Fukui et al., 2020; Wauters et al., 2021; Shanahan et al., 2023) found no significant difference in alpha diversity between FD patients and the controls.
Three studies analyzed the β diversity of duodenal microbiota in FD patients and their control groups (Fukui et al., 2020; Zheng et al., 2022; Shanahan et al., 2023). Two studies showed the result through the β diversity principal coordinates analysis (PCoA) (Fukui et al., 2020; Zheng et al., 2022). One of the studies (Fukui et al., 2020) showed that there were microbial structural differences between FD and healthy subjects when PCoA was used. The other study (Zheng et al., 2022) based on the results of PCoA showed that the duodenal flora of FD patients and healthy people did not show a clear separate trend, but further Amova analysis of the research revealed that there was a significant difference between the two groups, indicating that the structure of duodenal flora changed in FD patients. One study (Shanahan et al., 2023) independently demonstrated an insignificant difference in β diversity (Bray-Curtis distance; Table 2).
3.6 Factors associated with duodenal microbiota alterations
Two studies have described the relationship between changes in the duodenal microbiota and the use of PPI in FD patients (Wauters et al., 2021; Shanahan et al., 2023). One of the studies showed that (Shanahan et al., 2023) the impacts of PPI use on the duodenal mucosa-associated microbiota (MAM) can be variable but overall limited. The other study (Wauters et al., 2021) showed that baseline differences and effects of short-term PPI therapy were only found for specific luminal genera and diversity, while long-term PPI therapy may have a certain effect on duodenal dysbiosis. Four studies (Zhong et al., 2016, 2017; Fukui et al., 2020; Shanahan et al., 2023) investigated the correlation between duodenal flora changes and FD symptoms burden. A study (Shanahan et al., 2023) suggested that the relative abundances of predominant members of the phylum Firmicutes and Bacteroidota were linked to symptom burden in FD, in which the relative abundance of taxa affiliated with Firmicutes increased with FD symptom burden, whereas taxa affiliated with Bacteroidota decreased. The other two studies (Zhong et al., 2016, 2017) suggested that more severe symptom responses to the standardized meal positively correlated with mucosal bacterial load. These two studies (Zhong et al., 2016, 2017)also explored the relationship between duodenal microbiota dysbiosis and the quality of life in FD patients and found a negative correlation exists between duodenal mucosal bacterial load and reported quality of life (Table 2).
3.7 Quality of the evidence
The quality of evidence was assessed by the Newcastle-Ottawa Scale for the six full-text articles, with two of the six articles scoring 7, one scoring 6, and three scoring 5, giving an overall moderately high level of article quality. Among the six full-text articles reviewed, all studies had clear definitions of the study and control populations. Regarding the study population, four studies (Tziatzios et al., 2021; Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023) explicitly diagnosed and included FD patients based on the Rome IV criteria; one study used the Rome III criteria; and only one study did not specify the exact diagnostic criteria for FD patients but established standards for endoscopic findings in FD patients. Additionally, five studies (Fukui et al., 2020; Tziatzios et al., 2021; Wauters et al., 2021; Zheng et al., 2022; Shanahan et al., 2023) mentioned that both FD and control groups underwent endoscopic examination to rule out gastric/duodenal mucosal abnormalities, lesions, or structural changes, with only one study (Zhong et al., 2017) not explicitly addressing this point. For the control groups, three studies (Fukui et al., 2020; Wauters et al., 2021; Zheng et al., 2022) used healthy controls, and the other three (Zhong et al., 2017; Tziatzios et al., 2021; Shanahan et al., 2023) used non-FD controls who did not exhibit FD symptoms and were confirmed to have no upper gastrointestinal mucosal lesions via endoscopy. All studies assessed whether baseline data, mainly age and gender, were statistically different between the two groups, with only one study (Shanahan et al., 2023) having a significantly younger group of FD patients than the control group. None of the trials reported whether blinding was used (Table 4).
4 Discussion
The duodenum is currently postulated to play an important role in the pathogenesis of FD. The increased permeability and microinflammation of the duodenal mucosa implicated in the etiology of FD are thought to be associated with alterations in the microbial community (Miwa et al., 2019). FD has been recognized as a disorder of brain-gut interaction, implicating the duodenal microbiota as a potentially significant yet understudied player in the brain-gut dialog. We present the first comprehensive review of existing studies on the duodenal microbiota in FD, attempting to elucidate the composition and characteristics of the duodenal microbiota in FD patients and establish a correspondence between symptom production and disease pathogenesis. The results of this study showed that the diversity of the duodenal microbiota in FD patients was not significantly different from that of non-FD controls but tended to increase and that changes in the duodenal microbiota of FD patients correlated with patient symptom burden and affected patient quality of life. In FD patients, a number of duodenal microbiota have a relatively significant upward trend, and we consider this type of microorganism to be potentially harmful, including the phylum Fusobacteria, the genera Alloprevotella, Corynebacterium, Peptostreptococcus, Staphylococcus, Clostridium, Streptococcus, and others. Flora with a more pronounced downward trend in FD patients include the genera Actinomyces, Gemella, Haemophilus, Megasphaera, Mogibacterium, Selenomonas, and others, and we believe that there may be potentially beneficial bacteria for FD patients in these microbiotas. Furthermore, alterations in specific microbial taxonomic groups and their implications for the brain-gut axis interactions in patients with functional dyspepsia warrant attention.
4.1 Correlation of Streptococcus and Prevotella abundance with FD symptoms
The results of a Japanese study (Fukui et al., 2020) observed a significant increase in the abundance of Firmicutes in FD compared to healthy controls in all sites of the upper gut, and the evaluation of taxonomic changes of each genus in the Firmicutes revealed that only genus Streptococcus was significantly increased in all sites in the upper gut in FD compared to healthy controls, and this study also found a positive correlation between the relative abundance of genus Streptococcus and upper gastrointestinal symptoms in FD patients. This finding is consistent with another included Australian study (Shanahan et al., 2023), which also found that the relative abundance of the Firmicutes was significantly higher in FD patients and the relative abundance of taxa affiliated with the Firmicutes increased with FD symptom burden, and this positive correlation trend continued when the dominant genus of this phylum (i.e., Streptococcus) was examined. This suggests that Streptococcus spp. may be the main suspected genus closely associated with symptoms in FD patients. In contrast, the results of the study also showed that the relative abundance of Prevotella spp. was negatively correlated with the severity of FD symptom burden. In addition, both experiments (Zhong et al., 2017; Shanahan et al., 2023) indicated that the relative abundance of genus Streptococcus and genus Prevotella in FD patients was inversely related.
The genera Streptococcus and Prevotella are the main bacteria of the upper gastrointestinal tract. Interestingly, one study found that the changes in the relative abundance of the genera Streptococcus and Prevotella are thought to correlate significantly with changes in duodenal pH, and the two genera show an opposite trend in correlation with duodenal pH, with Streptococcus predominantly exhibiting a positive correlation and Prevotella demonstrating a negative association with the changes in duodenal pH (Seekatz et al., 2019). This seems to confirm that the genera Streptococcus and Prevotella are inversely characterized in the duodenum of FD patients and that changes in the relative abundance of the two genera correlate with the changes in duodenal pH in FD patients. There is a strong correlation and an interactive relationship between the duodenal microbiota and the pH of the duodenal contents. Large fluctuations in environmental pH may select genera such as Streptococcus, which can also regulate intracellular pH (Abuhelwa et al., 2017; Seekatz et al., 2019).
The growth of microorganisms, including the genera Streptococcus and Prevotella, can lead to pH changes by mediating the metabolism of short-chain fatty acids (SCFA; Zoetendal et al., 2012). Streptococcus has been demonstrated to elevate concentrations of lactate and butyrate within the duodenum, consequently lowering the pH of the duodenal environment (Zoetendal et al., 2012). In vitro studies (Chen et al., 2017) have corroborated that Prevotella exhibits a heightened capacity for fiber utilization and serves as an efficient producer of propionate from arabinoxylan and oligofructose. The enhanced capacity for fiber degradation makes the genus Prevotella potentially beneficial for glucose homeostasis and host metabolism (Tett et al., 2021). Furthermore, Prevotella has been shown to augment bile acid metabolism (Péan et al., 2020), thereby exerting influence on the duodenal pH. Alterations in duodenal pH are posited to contribute to the pathogenesis of FD, with FD patients demonstrating evidence of increased duodenal acid exposure (Bratten and Jones, 2009). Additionally, the sensitivity of the duodenal mucosa to acid is thought to be associated with FD, and acid exposure at the duodenal site in FD patients is believed to worsen FD symptoms (Bratten and Jones, 2009; Ishii et al., 2010).
Based on the included studies, we observed that the duodenal microbiota of FD patients exhibited negatively correlated changes in the genera Streptococcus and Prevotella. These variations in the two genera may be closely associated with the onset of FD symptoms. The change in duodenal pH may play a significant role in this process.
4.2 Staphylococcal enterotoxin may induce duodenal immune inflammation leading to functional dyspepsia
According to the results from the included studies, the genus Staphylococcus exhibits a notably increased trend in the duodenal microbiota of FD patients. Furthermore, one study (Tziatzios et al., 2021) indicated that the proportion of Staphylococcus aureus in small intestinal aspirates is higher in FD patients with small intestinal bacterial overgrowth (SIBO) compared to non-FD patients. Duodenal inflammation is considered a novel target in the pathogenesis of FD, and microinflammation in the form of local immune cell infiltration plays an important role in the pathogenesis of FD (du et al., 2018; Wauters et al., 2020b). The increase in the genus Staphylococcus presence observed in this study may provide a new avenue of investigation into the mechanisms underlying FD. Previous studies have demonstrated a correlation between the overgrowth of Staphylococcus species and inflammatory bowel disease (IBD), which is attributed to the inflammation induced by staphylococcal superantigens (Jun et al., 2003; Collado et al., 2008). Staphylococcus aureus is a member of the genus Staphylococcus, and the staphylococcal enterotoxin (SE) produced by S. aureus is considered to be the causative agent of human food poisoning as well as a potent immune superantigen that readily crosses the intact intestinal epithelium, inducing the proliferation of activated T cells and the release of large amounts of pro-inflammatory cytokines (White et al., 1989; Moretó and Pérez-Bosque, 2009; Principato and Qian, 2014). Staphylococcal enterotoxin A (SEA) produced by Staphylococcus aureus has been shown to elicit a rapid immunological response in the stomach and duodenum of rats, and the duodenum displayed a greater leukocytic response than the stomach to SEA, while studies have shown that SEA does not induce gastrointestinal mucous membrane damage encompassing edema, cytolysis, tissue sloughing, luminal necrotic tissue, or alternations in epithelial mitotic (Beery et al., 1984). Liu et al. (2022) reported that SEA significantly upregulated the expression of NLRP3 inflammasome-associated proteins and downregulated the expression of tight junction (TJ) proteins, which triggered the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) signaling pathways in jejunal tissue, inducing intestinal barrier dysfunction and small bowel injury in mice. Staphylococcal enterotoxin B (SEB) was found to evoke significant increases in myeloperoxidase (MPO), macrophage infiltration, T-cell activation, and perturbed epithelialion transport and cause low-grade inflammation of the colonic lumen (Jun et al., 2003). Studies (Moretó and Pérez-Bosque, 2009; Pérez-Bosque and Miquel, 2010) have also shown that SEB exposure decreases the expression of mucosal tight-junction and adherent-junction proteins, leading to increased mucosal permeability and intestinal secretion.
In conclusion, although the definitive studies elucidating the mechanistic role of duodenal Staphylococcus aureus in FD are lacking, SE can damage the intestinal mucosal barrier, activate duodenal immunity, and induce an inflammatory response, and its potential inducing effect on the pathogenesis of FD may be worthy of attention.
4.3 Selenomonas as a potential “beneficial bacterium” in the duodenum of functional dyspepsia patients
Several studies have found (Ricke et al., 1996; Jordan and Kurt, 2010) that Selenomonas have multiple carbon flow routes for carbohydrate catabolism and ATP generation and that catalytically efficientβ-D-xylosidase from Selenomonas can hydrolyze cellulose, and Selenomonas is thought to promote nutrient digestibility. In addition, recent research has found that (Kim et al., 2022) treatment during the asthma sensitization period with Selenomonas sputigena resulted in a significant reduction in airway hyperresponsiveness and a decrease in inflammatory cells present in bronchoalveolar lavage fluid, suggesting that the Selenomonas may be able to mitigate the severity of asthmatic phenotypes. In terms of gastrointestinal diseases, the researchers found that the symptoms of the patients with functional constipation (FC) and comorbid depression and anxiety were improved after fecal microbiota transplantation. An increase in the abundance of Selenomonas within FC patients’ gut microbiota has been noted, leading to the hypothesis that changes in the prevalence of this bacterial genus may be involved in the pathogenesis of constipation presenting with psychiatric symptoms (Yang et al., 2023).
The above results suggest that Selenomonas may have a potential “protective” effect on the human body. Current research on Selenomonas predominantly focuses on ruminant animals, with a relative paucity of studies elucidating its mechanisms of action in humans. However, in the present study, the genus Selenomonas exhibited a relatively significant downward trend in the duodenal microbiota of FD patients. Although it is unclear whether this is the cause or the result of FD, considering the characteristics of this genus, Selenomonas may represent a potentially beneficial bacterial genus for FD patients.
4.4 The role of duodenal microbial dysbiosis in gut–brain interactions in functional dyspepsia
Functional GI disorders are classified by the Rome IV criteria as disorders of gut–brain interaction with contributions of both altered brain processing and luminal changes, including dysbiosis (Drossman and William, 2016). Emerging data increasingly points to the duodenum as a key integrator in the generation of dyspepsia symptoms. In patients with dyspepsia, irritation of the duodenum is thought to cause disturbances in duodenal-gastric feedback, leading to gastric motility dysfunction, which in turn causes dyspeptic symptoms (Walker and Nicholas, 2017; Miwa et al., 2019). A study from Leuven (Cirillo et al., 2015) demonstrated a correlation between duodenal eosinophils or mast cells and the Ca+ transient amplitude under high-K+ depolarization or electrical pulses, suggesting a role for duodenal inflammation in FD neuronal signal transmission.
Current evidence (Fukui et al., 2020) suggests that dysbiosis of the duodenal MAM is considered to play a pivotal role in the development of symptoms in FD patients. The results of a systematic review and meta-analysis (Gurusamy et al., 2021b) further corroborate a link between FD and small intestinal bacterial overgrowth. Additionally, relevant preclinical studies (de Palma et al., 2015) have indicated that stress can induce dysregulation of the gut microbiota, thereby impacting the function and behavior of the central nervous system. Thus, consideration must be given to the potential stimulation of the duodenal mucosa and its neuronal signal transmission by duodenal microbiota and their metabolic products, which may consequently contribute to the pathogenesis of FD through gastrointestinal dysmotility.
The composition and abundance fluctuations of the gut microbiota have been associated with compromised mucosal surface integrity (Zhou et al., 2022). In FD patients, the duodenal mucosa exhibits compromised mucosal integrity and increased permeability, which is believed to permit luminal triggers to initiate local and systemic immune cascades, leading to alterations in neuronal signal transduction and the consequent manifestation of dyspeptic symptoms (Nojkov et al., 2020; Wauters et al., 2020a). Enterotoxins released by Staphylococcus are recognized to disrupt small intestinal mucosal barrier function, invade the intestinal mucosa, and elicit immune and inflammatory responses. Genus Alloprevotella has also been identified as a key bacterium capable of inducing colonic mucosal damage (Wang et al., 2021). Studies by Zhao et al. (2021) have demonstrated that sodium caprylate (SC) treatment increased the populations of Prevotella_9 in the ileum and Lachnoclostridium and Roseburia in the colon but decreased the abundances of Streptococcus and Enterococcus in the ileum and Lactobacillus and Clostridium_sensu_stricto_1 in the colon, thereby ameliorating intestinal barrier function and maintaining gut health.
The gut microbiota exerts a pivotal influence on the nervous, neuroendocrine, and metabolic systems, producing microbial metabolites, signaling molecules, and hormones with potential implications for alterations in brain signaling (Cowan et al., 2020; Vanuytsel et al., 2023). Short-chain fatty acids (SCFAs) represent metabolites of gastrointestinal microbial communities and are often considered key mediators of communication between the central nervous system and the gut. SCFAs can induce the secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), γ-aminobutyric acid (GABA), and other hormones capable of transmitting stimuli to the central nervous system via the circulatory system or the vagus nerve pathway (Silva et al., 2020). SCFAs are primarily produced by bacteria belonging to genera such as Prevotella, Streptococcus, Lactobacillus, and Bifidobacterium, utilizing dietary fibers, resistant starch, oligosaccharides, and other intestinal compounds as substrates (Markowiak-Kopeć and Katarzyna, 2020). Among the taxa exhibiting a declining trend in this study, genus Prevotella demonstrates robust SCFA production capability and high fiber utilization efficiency, serving as proficient propionate producers from arabinoxylan and fructooligosaccharides (Chen et al., 2017). Furthermore, studies (Zhao et al., 2018) have revealed positive correlations between the increased abundance of genera Prevotella, Lactobacillus, and Alistipes and concentrations of saturated long-chain fatty acids (SLCFAs) in the gut, where elevated SLCFA levels promote intestinal smooth muscle contraction, leading to increased gut motility; however, the precise mechanisms through which SLCFAs enhance gastrointestinal motility remain unclear. The gastrointestinal microbiota also modulates various facets of the gut–brain axis (GBA) through serotonin metabolism. Serotonin, derived solely from tryptophan, serves as a critical monoaminergic neurotransmitter involved in central nervous transmission and intestinal physiological functions (Gao et al., 2020). Numerous bacterial strains, including those from Lactococcus, Lactobacillus, Streptococcus, Escherichia coli, and Klebsiella genera, have been documented to possess the capability of serotonin synthesis through the expression of tryptophan synthetase (O’Mahony et al., 2015).
Psychosomatic comorbidities play a significant role in the generation of symptoms in functional dyspepsia (Ford et al., 2020). Longitudinal studies examining bidirectional effects between the gut and the brain (Koloski et al., 2012, 2016) have revealed that individuals with functional dyspepsia at baseline are more prone to experiencing anxiety or depression during follow-up compared to those without functional dyspepsia, while individuals with anxiety or depression at baseline are more likely to develop functional dyspepsia than those without anxiety or depression. The gut microbiota can engage in bottom-up signaling through the production of metabolites, interacting with the central nervous system via neural pathways or by crossing the intestinal barrier, and modulating neurotransmitter levels, thereby influencing mental wellbeing (Evrensel and Mehmet-Emin, 2015; Barandouzi et al., 2020). In this study, bacterial genera such as Corynebacterium, Clostridium, and Streptococcus, which showed a significant upward trend in two or more studies, exhibiting significant increases in abundance in the duodenum of FD patients, were also found to significantly increase in the gut microbiota of individuals with depression. Conversely, bacterial genera such as Lactobacillus and Prevotella, which were found to have decreased relative abundance in two or more included studies, were also found to significantly decrease the gut microbiota of individuals with depression (Barandouzi et al., 2020; Park et al., 2023). Furthermore, ingestion of lactobacilli is believed to regulate central GABA receptor expression via the vagus nerve and reduce stress-induced corticosterone as well as anxiety and depression-related behaviors (Bravo et al., 2011).
In summary, the duodenal microbiota plays a role in the gut-brain axis in FD patients. The duodenal microbiota may influence the crucial duodenal functional status implicated in FD pathogenesis through endocrine, neural signaling, and immune-inflammatory responses. Additionally, it participates in the bidirectional brain-gut interactions in FD patients, thus constituting a dysregulated pathogenesis of FD based on the microbiota-gut–brain axis, ultimately leading to the onset of FD (Figure 3).
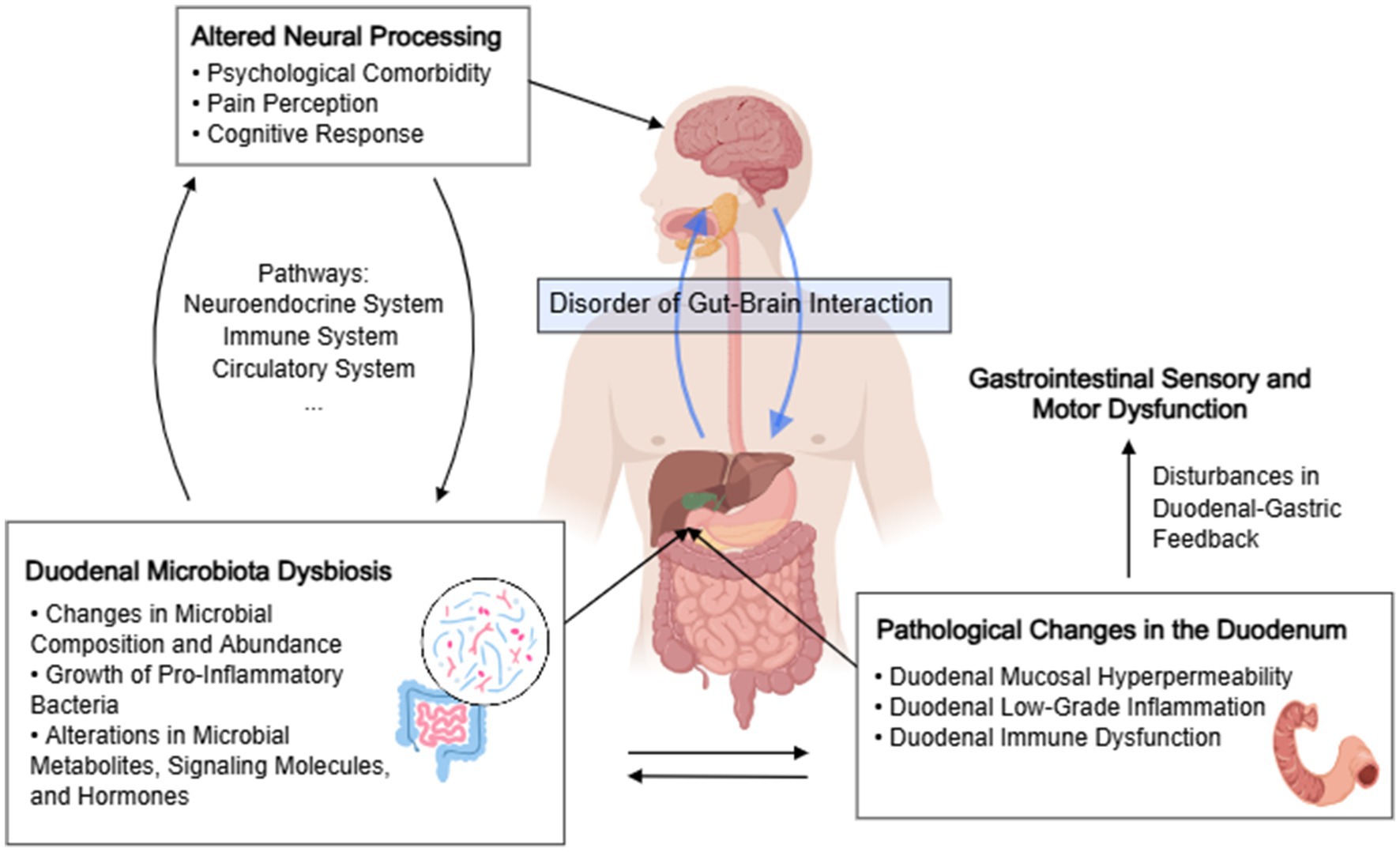
Figure 3. The role of duodenal microbial dysbiosis in disorders of gut–brain interactions in functional dyspepsia. The chart was created by MedPeer.
5 Conclusion and outlook
In summary, while the overall diversity of the duodenal microbiota does not significantly differ between FD patients and healthy individuals, certain taxa—including Selenomonas, Streptococcus, Prevotella, and Staphylococcus—exhibit notable variations that warrant further investigation. Our study suggests that alterations in the duodenal microbiota may be crucial for the onset and manifestation of FD symptoms. The microbial species significantly altered in this study are known to influence the bidirectional interactions between the brain and gut in FD patients by modulating endocrine, neural signaling, and immune-inflammatory responses, thereby contributing to FD pathogenesis. This highlights the significant role of the duodenal microbiota in the brain-gut interactions of FD patients.
The duodenum is increasingly recognized as pivotal in FD pathogenesis, and the gut microbiota’s role has garnered considerable attention. However, due to technological limitations in previous studies, research on the duodenal microbiota of FD patients remains insufficient. The limited number of studies, small sample sizes, and inconsistent quality of the articles result in contradictory findings, insufficient data for meta-analyses, and challenges in standardized data processing. Consequently, only a general trend of dysbiosis in the duodenal microbiota of FD patients can be discerned, with insufficient evidence to pinpoint key microbial communities.
Future research urgently needs to prioritize higher-quality, larger sample-size studies across diverse populations. Efforts should focus on eliminating bias and incorporating findings from multi-omics studies to further elucidate the characteristics and pathogenic mechanisms of the duodenal microbiota in FD patients. Additionally, attention should be given to confounding factors affecting duodenal microbiota changes in FD patients, such as dietary habits and proton pump inhibitor use. These in-depth studies could provide potential strategic references for the prevention and treatment of FD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Visualization, Writing – review & editing. TZ: Conceptualization, Methodology, Writing – review & editing. RG: Formal analysis, Software, Validation, Writing – review & editing. QW: Data curation, Project administration, Writing – review & editing. ZZ: Methodology, Supervision, Writing – review & editing. MY: Data curation, Writing – review & editing. WW: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. XS: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work received support from the following funding sources: National Key Research and Development Program of China (No.: 2022YFC3500503) and the Special Project for Clinical Evidence-Based Research in Traditional Chinese Medicine under the High-Level Traditional Chinese Medicine Hospital Construction Project of Wangjing Hospital, China Academy of Chinese Medical Sciences (No.: WJYY-XZKT-2023-22).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abuhelwa, A.-Y., Williams, D. B., Upton, R. N., and Foster, D. J. R. (2017). Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 112, 234–248. doi: 10.1016/j.ejpb.2016.11.034
Aziz, I., Palsson, O. S., Törnblom, H., Sperber, A. D., Whitehead, W. E., and Simrén, M. (2018). Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 3, 252–262. doi: 10.1016/S2468-1253(18)30003-7
Barandouzi, Z.-A., Starkweather, A. R., Henderson, W. A., Gyamfi, A., and Cong, X. S. (2020). Altered composition of gut microbiota in depression: a systematic review. Front. Psych. 11:541. doi: 10.3389/fpsyt.2020.00541
Beery, J.-T., Taylor, S.-L., Schlunz, L.-R., Freed, R. C., and Bergdoll, M. S. (1984). Effects of staphylococcal enterotoxin a on the rat gastrointestinal tract. Infect. Immun. 44, 234–240. doi: 10.1128/iai.44.2.234-240.1984
Bratten, J., and Jones, M.-P. (2009). Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J. Clin. Gastroenterol. 43, 527–533. doi: 10.1097/MCG.0b013e31818e37ab
Bravo, J.-A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion ofLactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Chen, T., Long, W., Zhang, C., Liu, S., Zhao, L., and Hamaker, B. R. (2017). Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 7:4. doi: 10.1038/s41598-017-02995-4
Cirillo, C., Bessissow, T., Desmet, A.-S., Vanheel, H., Tack, J., and vanden Berghe, P. (2015). Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am. J. Gastroenterol. 110, 1205–1215. doi: 10.1038/ajg.2015.158
Collado, M. C., Isolauri, E., Laitinen, K., and Salminen, S. (2008). Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899. doi: 10.1093/ajcn/88.4.894
Cowan, C. S. M., Dinan, T. G., and Cryan, J. F. (2020). Annual research review: critical windows—the microbiota-gut–brain axis in neurocognitive development. J. Child Psychol. Psychiatry 61, 353–371. doi: 10.1111/jcpp.13156
de Palma, G., Blennerhassett, P., Lu, J., Deng, Y., Park, A. J., Green, W., et al. (2015). Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 6:7735. doi: 10.1038/ncomms8735
Drossman, D. A. (2016). Functional gastrointestinal disorder and the Rome IV process[M]. Raleigh, N.C: Rome Foundation.
Drossman, D.-A., and William, H. (2016). Rome IV-functional GI disorders: disorders of gut–brain interaction. Gastroenterology 150, 1257–1261. doi: 10.1053/j.gastro.2016.03.035
du, L., Chen, B., Kim, J.-J., Chen, X., and Dai, N. (2018). Micro-inflammation in functional dyspepsia: a systematic review and meta-analysis. Neurogastroenterol. Motil. 30:e13304. doi: 10.1111/nmo.13304
Esterita, T., Dewi, S., Suryatenggara, F. G., and Glenardi, G. (2021). Association of Functional Dyspepsia with depression and anxiety: a systematic review. J. Gastrointestin. Liver Dis. 30, 259–266. doi: 10.15403/jgld-3325
Evrensel, A., and Mehmet-Emin, C. (2015). The gut–brain Axis: The missing link in depression. Clin Psychopharmacol Neurosci 13, 239–244. doi: 10.9758/cpn.2015.13.3.239
Ford, A.-C., Mahadeva, S., Carbone, M. F., Lacy, B. E., and Talley, N. J. (2020). Functional dyspepsia. Lancet 396, 1689–1702. doi: 10.1016/S0140-6736(20)30469-4
Fukui, A., Takagi, T., Naito, Y., Inoue, R., Kashiwagi, S., Mizushima, K., et al. (2020). Higher levels of Streptococcus in upper gastrointestinal mucosa associated with symptoms in patients with functional dyspepsia. Digestion 101, 38–45. doi: 10.1159/000504090
Gao, K., Mu, C. L., Farzi, A., and Zhu, W. Y. (2020). Tryptophan metabolism: a link between the gut microbiota and brain. Adv. Nutr. 11, 709–723. doi: 10.1093/advances/nmz127
Gracie, D.-J., Bercik, P., Morgan, D.-G., Bolino, C., Pintos-Sanchez, M. I., Moayyedi, P., et al. (2015). No increase in prevalence of somatization in functional vs organic dyspepsia: a cross-sectional survey. Neurogastroenterol. Motil. 27, 1024–1031. doi: 10.1111/nmo.12578
Gurusamy, S.-R., Shah, A., Talley, N. J., Koloski, N. A., Jones, M. P., Walker, M. M., et al. (2021a). Sa389 Small Intestinal Bacterial Overgrowth In Functional Dyspepsia: A Systematic Review And Meta-Analysis. Gastroenterology 160:S437. doi: 10.1016/S0016-5085(21)01871-0
Gurusamy, S.-R., Shah, A., Talley, N.-J., Koloski, N., Jones, M. P., Walker, M. M., et al. (2021b). Small intestinal bacterial overgrowth in functional dyspepsia: a systematic review and Meta-analysis. Am. J. Gastroenterol. 116, 935–942. doi: 10.14309/ajg.0000000000001197
Ishii, M., Kusunoki, H., Manabe, N., Kamada, T., Sato, M., Imamura, H., et al. (2010). Evaluation of duodenal hypersensitivity induced by duodenal acidification using transnasal endoscopy. J. Gastroenterol. Hepatol. 25, 913–918. doi: 10.1111/j.1440-1746.2009.06143.x
Jordan, D.-B., and Kurt, W. (2010). Properties and applications of microbial β-D-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl. Microbiol. Biotechnol. 86, 1647–1658. doi: 10.1007/s00253-010-2538-y
Jun, L., Arthur, W., Sara, A., Hershberg, R. M., and DM, M. K. (2003). Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology 125, 1785–1795. doi: 10.1053/j.gastro.2003.09.020
Kim, Y.-C., Choi, S., Sohn, K.-H., Bang, J.-Y., Kim, Y., Jung, J.-W., et al. (2022). Selenomonas: a marker of asthma severity with the potential therapeutic effect. Allergy 77, 317–320. doi: 10.1111/all.15114
Koloski, N.-A., Jones, M., Kalantar, J., Weltman, M., Zaguirre, J., and Talley, N. J. (2012). The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 61, 1284–1290. doi: 10.1136/gutjnl-2011-300474
Koloski, N.-A., Jones, M., and Talley, N.-J. (2016). Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment. Pharmacol. Ther. 44, 592–600. doi: 10.1111/apt.13738
Lacy, B.-E., Weiser, K.-T., Kennedy, A.-T., Crowell, M. D., and Talley, N. J. (2013). Functional dyspepsia: the economic impact to patients. Aliment. Pharmacol. Ther. 38, 170–177. doi: 10.1111/apt.12355
Liu, C., Chi, K., Yang, M., and Guo, N. (2022). Staphylococcal enterotoxin a induces intestinal barrier dysfunction and activates NLRP3 Inflammasome via NF-κB/MAPK signaling pathways in mice. Toxins 14:29. doi: 10.3390/toxins14010029
Markowiak-Kopeć, P., and Katarzyna, Ś. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 12:1107. doi: 10.3390/nu12041107
Miwa, H., Oshima, T., Tomita, T., Fukui, H., Kondo, T., Yamasaki, T., et al. (2019). Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J. Gastroenterol. 54, 305–311. doi: 10.1007/s00535-019-01550-4
Moretó, M., and Pérez-Bosque, A. (2009). Dietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosa1. J. Anim. Sci. 87, E92–E100. doi: 10.2527/jas.2008-1381
Nojkov, B., Zhou, S.-Y., Dolan, R. D., Davis, E. M., Appelman, H. D., Guo, X., et al. (2020). Evidence of duodenal epithelial barrier impairment and increased Pyroptosis in patients with functional dyspepsia on confocal laser Endomicroscopy and “ex vivo” mucosa analysis. Am. J. Gastroenterol. 115, 1891–1901. doi: 10.14309/ajg.0000000000000827
O’Mahony, S. M., Clarke, G., Borre, Y.-E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi: 10.1016/j.bbr.2014.07.027
Page, M.-J., Joanne-E, M. K., Patrick-M, B., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2020). Statement: an updated guideline for reporting systematic reviews. BMJ 2021:n71. doi: 10.1136/bmj.n71
Park, S., Li, C., Wu, X., and Zhang, T. (2023). Gut microbiota alterations and their functional differences in depression according to Enterotypes in Asian individuals. Int. J. Mol. Sci. 24:13329. doi: 10.3390/ijms241713329
Péan, N., le Lay, A., Brial, F., Wasserscheid, J., Rouch, C., Vincent, M., et al. (2020). Dominant gut Prevotella copri in gastrectomised non-obese diabetic Goto–Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia 63, 1223–1235. doi: 10.1007/s00125-020-05122-7
Pérez-Bosque, A., and Miquel, M. (2010). A rat model of mild intestinal inflammation induced by Staphylococcus aureus enterotoxin B. Proc. Nutr. Soc. 69, 447–453. doi: 10.1017/S0029665110001849
Principato, M., and Qian, B.-F. (2014). Staphylococcal enterotoxins in the etiopathogenesis of mucosal autoimmunity within the gastrointestinal tract. Toxins 6, 1471–1489. doi: 10.3390/toxins6051471
PRISMA-P GroupMoher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4:1. doi: 10.1186/2046-4053-4-1
Ricke, S.-C., Martin, S.-A., and Nisbet, D.-J. (1996). Ecology, metabolism, and genetics of ruminal selenomonads. Crit. Rev. Microbiol. 22, 27–65. doi: 10.3109/10408419609106455
Rupp, S.-K., and Andreas, S. (2022). Bi-directionality of the microbiota-gut–brain Axis in patients with functional dyspepsia: relevance of psychotherapy and probiotics. Front. Neurosci. 16:564. doi: 10.3389/fnins.2022.844564
Schooth, L., Kang, S., Lim, Y., Shah, A., Fairlie, T., Teh, J. J., et al. (2021). Differentiation and capture of the human duodenal mucosa-associated microbiota by a novel ex vivo combination of microbe culture and metagenomic sequencing. J. Gastroenterol. Hepatol. 36:134.
Schooth, L., Sid-Ahmed, A., Kang, S., Lim, Y., Fairlie, T., Shah, A., et al. (2022). Diversity of Veillonella in cultures of the duodenal mucosa-associated microbiota suggests species-level differences between people with and without functional gastrointestinal symptoms. J. Gastroenterol. Hepatol. 37, 108–109.
Seekatz, A.-M., Schnizlein, M.-K., Koenigsknecht, M.-J., Baker, J. R., Hasler, W. L., Bleske, B. E., et al. (2019). Spatial and temporal analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere 4:19. doi: 10.1128/mSphere.00126-19
Shanahan, E. R., Kang, S., Staudacher, H., Shah, A., do, A., Burns, G., et al. (2023). Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut 72, 929–938. doi: 10.1136/gutjnl-2021-326158
Silva, Y.-P., Andressa, B., and Rudimar-Luiz, F. (2020). The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front. Endocrinol. 11:25. doi: 10.3389/fendo.2020.00025
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Stanghellini, V., Chan, F. K. L., Hasler, W. L., Malagelada, J. R., Suzuki, H., Tack, J., et al. (2016). Gastroduodenal Disorders. Gastroenterology 150, 1380–1392. doi: 10.1053/j.gastro.2016.02.011
Tett, A., Pasolli, E., Masetti, G., Ercolini, D., and Segata, N. (2021). Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 19, 585–599. doi: 10.1038/s41579-021-00559-y
Tziatzios, G., Gkolfakis, P., Papanikolaou, I. S., Mathur, R., Pimentel, M., Damoraki, G., et al. (2021). High prevalence of small intestinal bacterial overgrowth among functional dyspepsia patients. Dig. Dis. 39, 382–390. doi: 10.1159/000511944
Tziatzios, G., Gkolfakis, P., Papanikolaou, I. S., Mathur, R., Pimentel, M., Giamarellos-Bourboulis, E. J., et al. (2020). Gut microbiota Dysbiosis in functional dyspepsia. Microorganisms. 8:691. doi: 10.3390/microorganisms8050691
Vanheel, H., and Ricard, F. (2013). Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 142–149. doi: 10.1038/nrgastro.2012.255
Vanuytsel, T., Bercik, P., and Boeckxstaens, G. (2023). Understanding neuroimmune interactions in disorders of gut–brain interaction: from functional to immune-mediated disorders. Gut 72, 787–798. doi: 10.1136/gutjnl-2020-320633
Walker, M.-M., and Nicholas, T. (2017). The role of duodenal inflammation in functional dyspepsia. J. Clin. Gastroenterol. 51, 12–18. doi: 10.1097/MCG.0000000000000740
Wang, K., Xu, X., Maimaiti, A., Hao, M., Sang, X., Shan, Q., et al. (2021). Gut microbiota disorder caused by diterpenoids extracted from Euphorbia pekinensis aggravates intestinal mucosal damage. Pharmacol. Res. Perspect. 9:765. doi: 10.1002/prp2.765
Wauters, L., Burns, G., Ceulemans, M., Walker, M. M., Vanuytsel, T., Keely, S., et al. (2020b). Duodenal inflammation: an emerging target for functional dyspepsia? Expert Opin. Ther. Targets 24, 511–523. doi: 10.1080/14728222.2020.1752181
Wauters, L., Talley, N. J., Walker, M. M., Tack, J., and Vanuytsel, T. (2020a). Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 69, 591–600. doi: 10.1136/gutjnl-2019-318536
Wauters, L., Tito, R. Y., Ceulemans, M., Lambaerts, M., Accarie, A., Rymenans, L., et al. (2021). Duodenal dysbiosis and relation to the efficacy of proton pump inhibitors in functional dyspepsia. Int. J. Mol. Sci. 22:13609. doi: 10.3390/ijms222413609
White, J., Herman, A., Pullen, A. M., Kubo, R., Kappler, J. W., and Marrack, P. (1989). The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 56, 27–35. doi: 10.1016/0092-8674(89)90980-X
Yang, C., Hu, T., Xue, X., Su, X., Zhang, X., Fan, Y., et al. (2023). Multi-omics analysis of fecal microbiota transplantation’s impact on functional constipation and comorbid depression and anxiety. BMC Microbiol. 23:1. doi: 10.1186/s12866-023-03123-1
Zhao, J., Hu, J., and Ma, X. (2021). Sodium caprylate improves intestinal mucosal barrier function and antioxidant capacity by altering gut microbial metabolism. Food Funct. 12, 9750–9762. doi: 10.1039/D1FO01975A
Zhao, L., Huang, Y., Lu, L., Yang, W., Huang, T., Lin, Z., et al. (2018). Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome 6:107. doi: 10.1186/s40168-018-0492-6
Zheng, Y. F., Liang, S. P., Zhong, Z. S., Zhang, W., Wu, Y. Y., Liu, J. B., et al. (2022). Duodenal microbiota makes an important impact in functional dyspepsia. Microb. Pathog. 162:105297. doi: 10.1016/j.micpath.2021.105297
Zhong, L., Shanahan, E. R., Raj, A. S., Koloski, N. A., Fletcher, L., Morrison, M., et al. (2016). Su1948 Duodenal Microbiota and Response to a Nutrient Challenge in Functional Dyspepsia[J]. Gastroenterol. 4:S596. doi: 10.1016/S0016-5085(16)32047-9
Zhong, L., Shanahan, E. R., Raj, A., Koloski, N. A., Fletcher, L., Morrison, M., et al. (2017). Dyspepsia and the microbiome: time to focus on the small intestine. Gut 66, 1168–1169. doi: 10.1136/gutjnl-2016-312574
Zhou, L., Zeng, Y., Zhang, H., and Ma, Y. (2022). The role of gastrointestinal microbiota in functional dyspepsia: a review. Front. Physiol. 13:910568. doi: 10.3389/fphys.2022.910568
Keywords: microbiota, functional dyspepsia, duodenum, systematic review, gut-brain interaction
Citation: Zhang X, Chen L, Zhang T, Gabo R, Wang Q, Zhong Z, Yao M, Wei W and Su X (2024) Duodenal microbiota dysbiosis in functional dyspepsia and its potential role of the duodenal microbiota in gut–brain axis interaction: a systematic review. Front. Microbiol. 15:1409280. doi: 10.3389/fmicb.2024.1409280
Edited by:
Jin Song, Capital Medical University, ChinaReviewed by:
Diego Armando Esquivel Hernandez, Metropolitan Autonomous University, MexicoLaura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2024 Zhang, Chen, Zhang, Gabo, Wang, Zhong, Yao, Wei and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wei, c3h4dHl5QHNpbmEuY29t; Xiaolan Su, c3V4aWFvbGFuMTk4MkAxMjYuY29t
 Xueping Zhang
Xueping Zhang Lei Chen
Lei Chen Tao Zhang
Tao Zhang Ryu Gabo
Ryu Gabo Wei Wei
Wei Wei Xiaolan Su
Xiaolan Su