
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 05 August 2024
Sec. Food Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1406971
Specialty mushrooms have been implicated in foodborne illness outbreaks in the U.S. in recent years. These mushrooms are available to consumers in both their fresh and dried states. Dehydrating mushrooms is a convenient way to increase shelf life. The dehydration process results in a lowered water activity (aw) of the commodity, creating an environment where both spoilage and pathogenic bacteria cannot proliferate. Prior to food preparation and consumption, these mushrooms are typically rehydrated and possibly stored for later use which could lead to increased levels of pathogens. This study examined the survival and growth of Listeria monocytogenes and Salmonella enterica on dehydrated enoki and wood ear mushrooms during rehydration and subsequent storage. Mushrooms were heat dehydrated, inoculated at 3 log CFU/g, and rehydrated at either 5 or 25°C for 2 h. Rehydrated mushrooms were stored at 5, 10, or 25°C for up to 14 d. L. monocytogenes and S. enterica survived on enoki and wood ear mushroom types during rehydration at 5 and 25°C, with populations often <2.39 log CFU/g. During subsequent storage, no growth was observed on wood ear mushrooms, regardless of the rehydration or storage temperature, with populations remaining <2.39 log CFU/g for both pathogens. When stored at 5°C, no growth was observed for either pathogen on enoki mushrooms. During storage at 10 and 25°C, pathogen growth rates and populations after 14 d were generally significantly higher on the enoki mushrooms rehydrated at 25°C; the highest growth rate (3.56 ± 0.75 log CFU/g/d) and population (9.48 ± 0.62 log CFU/g) after 14 d for either pathogen was observed by S. enterica at 25°C storage temperature. Results indicate a marked difference in pathogen survival and proliferation on the two specialty mushrooms examined in this study and highlight the need for individual product assessments. Data can be used to assist in informing guidelines for time and temperature control for the safety of rehydrated mushrooms.
Edible specialty mushrooms are common in the cuisines of Japan, China, and the Republic of Korea and are often consumed in soups, hot pots, and stir fry dishes. These specialty mushrooms have grown in popularity in the United States as more global cuisines are embraced by consumers. However, recent multistate foodborne illness outbreaks have occurred in the U.S. associated with two types of imported specialty mushrooms, enoki and wood ear (CDC, 2020a,b; FDA, 2020a,b; CDC, 2023). Firstly, fresh enoki mushrooms were the subject of two listeriosis outbreaks in 2020 and 2023 (CDC, 2020a; FDA, 2020a; CDC, 2023). The former outbreak resulted in 36 illnesses across 17 states, 31 hospitalizations, and four deaths (CDC, 2020a; FDA, 2020a), and the latter outbreak resulted in five illnesses and hospitalizations across four states (CDC, 2023). The enoki mushrooms implicated in these outbreaks were imported from Asian countries, including the Republic of Korea and China, and sold to consumers via retail establishments. Secondly, dried wood ear mushrooms were the subject of a salmonellosis outbreak in 2020, resulting in 55 illnesses across 12 states and six hospitalizations (CDC, 2020b; FDA, 2020b). The implicated dried wood ear mushrooms were sold directly to restaurants and, at least in some instances, were used as an ingredient in ramen soup (CDC, 2020b).
Specialty mushrooms, including enoki and wood ear, are commercially available to consumers in both their fresh and dried states. The shelf life of fresh mushrooms, like other fresh fruits and vegetables, is approximately 3–12 days depending on the packaging and storage conditions (Diamantopoulou and Philippoussis, 2015). One method commonly used to prolong the shelf life of mushrooms is drying or dehydration. Dried mushrooms have water activities (aw) <0.70 which prevents the proliferation of foodborne pathogens, if present. Previous research has determined that both L. monocytogenes and S. enterica can survive on dehydrated enoki and wood ear mushrooms stored for 180 d at 25°C (Fay et al., 2023). With an initial inoculation level of 6 log CFU/g, populations were reduced by approximately 2 and 4 log CFU/g on wood ear and enoki mushrooms during storage, respectively; however, both pathogens remained detectable via enrichment on the mushrooms after 180 d of storage. Similarly, L. monocytogenes and S. enterica are known to persist for long periods of time in other food products, including nuts and seeds, spices, flour, and confectionaries, often with no population reductions (Komitopoulou and Penaloza, 2009; Blessington et al., 2012; Farakos et al., 2017; Nascimento et al., 2018; Taylor et al., 2018).
If L. monocytogenes or S. enterica are present on the dried mushrooms prior to rehydration or incorporation into another food product with high aw, it is possible that the pathogens could survive or proliferate in the more favorable environments. For example, previous research has determined that L. monocytognees and S. enterica could survive on dehydrated vegetables, including carrot, corn, onion, pepper, and potato, during rehydration at 5 or 25°C and subsequent storage at 5, 10, or 25°C (Fay et al., 2023). In some cases, the growth rates during storage were as high as 2.37 and 1.63 log CFU/g/d for L. monocytogenes on potato and S. enterica on carrot, respectively, when the vegetables were rehydrated at 5°C and stored at 25°C. Lower pathogen growth rates were generally observed when the dehydrated vegetables were rehydrated at 5°C and then stored at 5°C. Results highlight the importance of holding rehydrated vegetables at refrigeration temperatures to reduce the potential for pathogen proliferation.
The objective of this study was to examine the survival of L. monocytogenes and S. enterica on dehydrated enoki and wood ear mushrooms during both rehydration and subsequent storage at different temperatures. Two different rehydration temperatures (5 and 25°C) and three different storage temperatures (5, 10, and 25°C) were employed in the study. The FDA Food Code currently lists certain fresh cut produce, including cut leafy greens, cut tomatoes, and cut melon, as foods requiring time and temperature control for safety (TCS foods) (FDA, 2022). However, there currently are no time and temperature guidelines for safety for the rehydration and storage of previously-dehydrated foods, including mushrooms. The results from this study will provide information on the time and temperature combinations for rehydration and storage for the safety of these two specialty mushrooms.
Fresh raw enoki and wood ear mushrooms were purchased from local grocers in Illinois (United States) and stored at 5°C for up to 24 h prior to dehydration. For enoki mushrooms, the bottom 3 cm containing the substrate was discarded and mushrooms were chopped into 2.5 cm long pieces. For wood ear, mushrooms were chopped into 2.5 × 2.5 cm pieces. Chopped mushrooms were arranged in a single layer on wax paper and dehydrated at 60°C for up to 24 h in a commercial food dehydrator (Excalibur model EXC10EL, The Legacy Companies, Weston, FL, United States). Triplicate 10-g mushroom samples were removed from the dehydrator after 0, 1, 2, 3, 4, 6, and 24 h for moisture content analysis (see section 2.5). After dehydration, mushrooms were stored in sealed bags for up to 24 h.
A four-strain cocktail of either Salmonella enterica or Listeria monocytogenes was used in this study. The S. enterica strains consisted of Enteritidis PT30 (ATCC BAA-1045), Agona 447967, Typhimurium 46249 (cantaloupe outbreak isolate), and Newport 36796 (CFSAN046260, tomato outbreak isolate). For L. monocytogenes, the strains used were LS806 (isolated from hummus), LS3132 (isolated from avocado), LS1863 (FDA1142659-C001-001, enoki mushroom outbreak isolate), and ScottA. All of the L. monocytogenes strains used were rifampicin resistant (100 μg/mL); spontaneous rifampicin resistant variants were obtained by successive culturing with increasing concentrations of rifampicin, up to 100 μg/mL. Strains were cultured individually in Tryptic Soy Broth (TSB; Becton, Dickinson and Co., Sparks, MD) for 16–18 h at 37°C. Cultures were washed twice with Butterfields’s Phosphate Buffer (BPB, pH 7.2) and combined in equal volumes to create a four-strain cocktail of either S. enterica or L. monocytogenes (9 log CFU/mL). The cocktails were serially diluted in BPB and plated onto Tryptic Soy Agar (TSA; Becton, Dickinson and Co.) to verify the initial population levels; plates were incubated at 37°C for 24 h prior to enumeration.
Dehydrated mushrooms (200 g) were inoculated with a 5 mL of a diluted cocktail of either S. enterica or L. monocytogenes in a 3-L stomacher bag, resulting in approximately 3 log CFU/g. The bag was shaken by hand for 5 min to evenly distribute the inoculum. Inoculated mushrooms were spread into a single layer on a foil tray and dried at ambient temperature in a biosafety cabinet for 1 h with the blower on. Triplicate 10-g mushroom samples after inoculation and after 1 h drying were used for moisture content analysis (see section 2.5) and pathogen enumeration (see section 2.6).
Dehydrated mushrooms (approximately 170 g) were rehydrated 1:20 with water in sterile metal bowls for 2 h. The water and air temperature during rehydration was maintained at either 5 or 25°C. After 0, 5, 15, 30, 60, 90, and 120 min of rehydration, approximately 50-g of mushrooms were removed from the water and strained for 10 min. Triplicate 10-g samples were used for pathogen enumeration (see section 2.6) and duplicate 10-g samples were used for moisture content and water activity analysis (see section 2.5). After 2 h of rehydration, the remaining mushrooms were strained for 10 min using a colander. After draining, mushrooms were portioned into 8-oz. deli containers with lids (40 g each) and stored at 5, 10, or 25°C for up to 14 d. After 0, 1, 3, 6, 9, and 14 d, both L. monocytogenes and S. enterica were enumerated from triplicate 10-g samples (see section 2.6). Three independent trials for each pathogen-mushroom-rehydration temperature combination were conducted in triplicate (n = 9).
To measure aw, a 1-g sample of inoculated mushroom was measured using an aw meter (Aqualab 4TE, Meter Group, Pullman, WA, United States). For moisture content, a 10-g sample of inoculation mushroom was placed into an oven at 100°C for 24 h. The solid weight after 24 h was measured and the moisture content (wet basis) was then calculated based on the initial weight.
Mushroom samples (10 g) were combined 1:10 with either Buffered Listeria Enrichment Broth (BLEB; Becton, Dickinson and Co., Sparks, MD, United States) or BPB for L. monocytogenes or S. enterica, respectively, and homogenized using a stomacher (Stomacher 400C Circulator, Seward Laboratory Systems Inc., Bohemia, NY) for 1 min. Homogenates were serially diluted and plated onto Brain Heart Infusion Agar (BHIA; Becton, Dickinson and Co., Sparks, MD, United States) with 100 μg/mL of rifampicin (BHIArif) or onto TSA with Xylose Lysine Deoxycholate (XLD; Becton, Dickinson and Co., Sparks, MD, United States) agar overlay for enumeration of L. monocytogenes or S. enterica, respectively. Agar plates were incubated at 37°C for 24–48 h. Data were expressed as log CFU/g. The lower limit of enumeration of the plate count assay (i.e., 25 CFU/plate) was 2.39 log CFU/g. When pathogen populations were expected to be below the limit of enumeration, samples were enriched according to the FDA Bacteriological Analytical Manual (FDA, 2017).
The growth kinetics (growth rates and lag phases) of both L. monocytogenes and S. enterica on rehydrated enoki mushrooms during 14 d storage at 5, 10, or 25°C were determined using the DMFit v 3.0 add-in for Excel using the Baranyi and Roberts primary model (Baranyi and Roberts, 1994). Differences between growth rates were statistically analyzed using ANCOVA with Tukey’s post hoc test. Differences in aw, moisture contents, and populations were statistically analyzed using ANOVA with Tukey’s post hoc test. Differences in the number of wood ear mushroom samples where L. monocytogenes or S. enterica was detected were determined using Fisher’s Exact test. p ≤ 0.05 was considered significant.
The aw and moisture contents (wet basis) of the fresh enoki and wood ear mushrooms have been previously reported (Fay et al., 2023). The aw values were 0.974 ± 0.012 and 0.976 ± 0.008 and the moisture contents were 89.34 ± 0.98 and 88.60 ± 0.55%, respectively. The aw and moisture contents of both mushroom types after dehydration, after inoculation, and after drying of the inoculum were determined in this study (Table 1). After dehydration at 60°C for 24 h, the aw and moisture contents of both mushroom types significantly decreased; the aw of the dehydrated enoki and wood ear mushrooms were 0.209 ± 0.002 and 0.186 ± 0.026 and the moisture contents were 4.50 ± 2.00 and 4.91 ± 2.73%, respectively. No difference was determined between the two mushroom types. After inoculation with either the L. monocytogenes or S. enterica cocktail, the aw of both mushroom types significantly increased, while no significant differences in the moisture contents was observed. After inoculation, the aw of the wood ear mushrooms (0.537 ± 0.099) was higher than that of enoki (0.302 ± 0.066). However, after the inoculum was allowed to dry on the mushrooms for 1 h, no significant difference in values was observed between the enoki and wood ear aw values. The aw of both the wood ear and enoki mushrooms were significantly different after drying (0.287 ± 0.062 and 0.313 ± 0.024, respectively) compared to pre-inoculation (0.186 ± 0.026 and 0.209 ± 0.002, respectively).
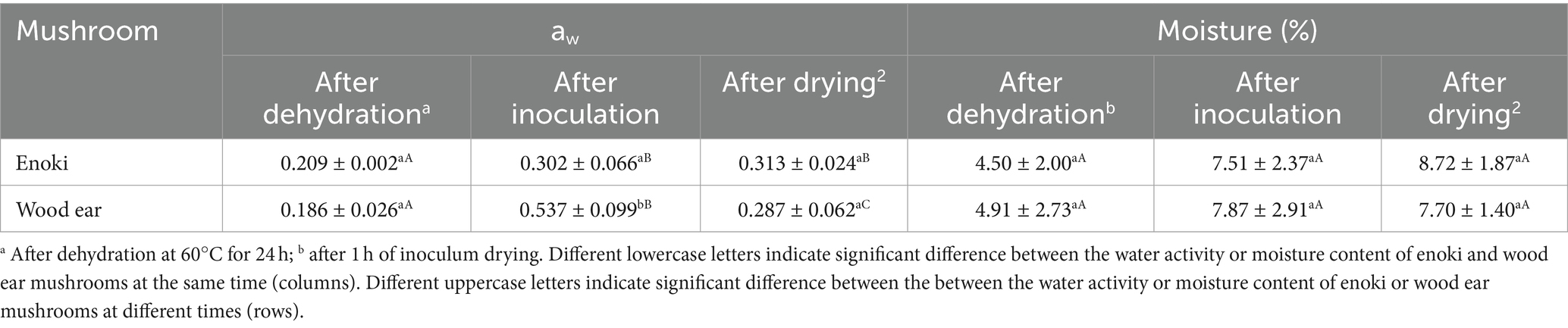
Table 1. The water activity (aw) and moisture content (%) of the enoki and wood ear mushrooms after dehydration, after inoculation, and after drying.
The moisture content of both mushroom types was monitored during rehydration at either 5 or 25°C for 2 h (Figure 1). Prior to rehydration (after drying the inoculum for 1 h), the moisture contents of the enoki and wood ear mushrooms were 8.72 ± 1.87 and 7.70 ± 1.40%, respectively. A significant increase in moisture content for both mushroom types occurred after only 5 min of rehydration: for enoki mushrooms, moisture contents were 79.96 ± 3.79 and 71.73 ± 2.39% at 5 and 25°C, respectively, which were significantly different; the moisture contents for the wood ear mushrooms were 77.50 ± 4.10 and 74.25 ± 3.59%, respectively.
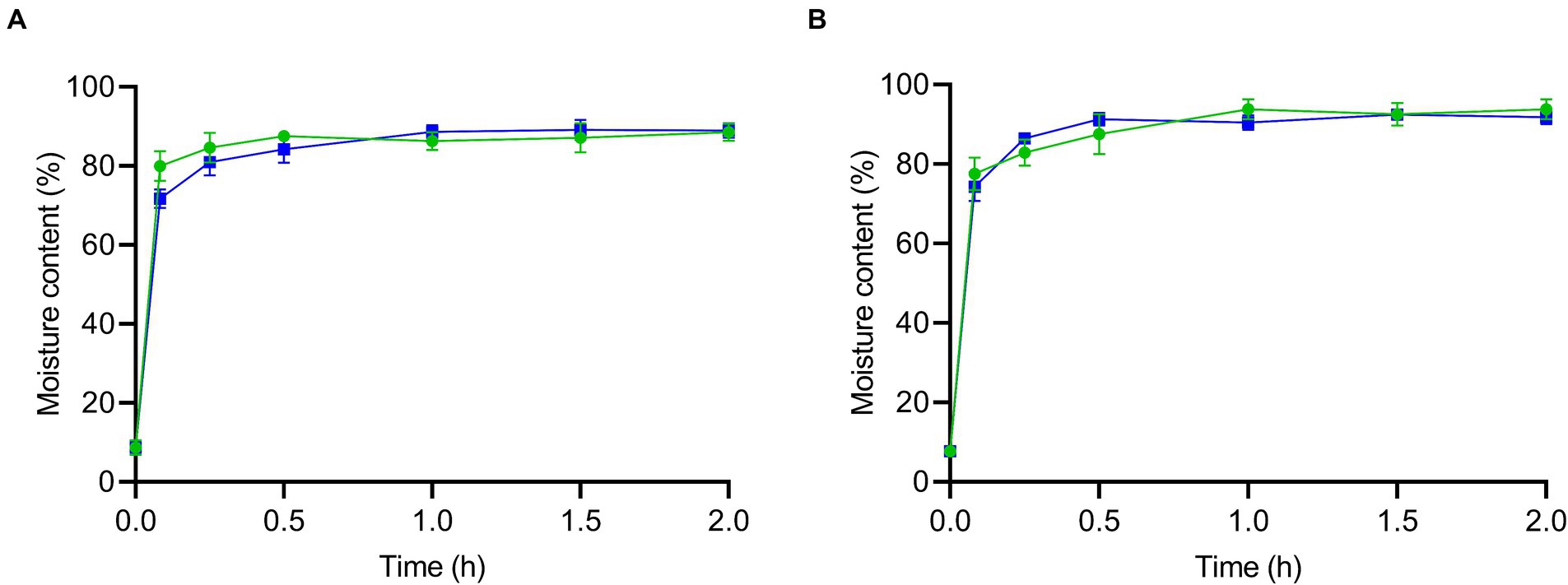
Figure 1. The moisture content (%) of the enoki (A) and wood ear (B) mushrooms during rehydration at 5 (green circles) or 25°C (blue squares) for 2 h. Data are mean values ± standard deviation (n = 9).
The populations of L. monocytogenes and S. enterica after inoculation, after drying of the inoculum for 1 h, and after rehydration at 5 or 25°C for 2 h are presented in Table 2. The initial inoculation levels of both pathogens on both mushroom types were not significantly different and ranged from 2.73 ± 0.02 to 3.27 ± 0.27 log CFU/g. No significant difference in populations were observed on the mushrooms after drying the inocula for 1 h. However, a significantly lower population of L. monocytogenes was observed on the wood ear mushrooms (2.58 ± 0.15 log CFU/g) after drying compared to on the enoki (3.22 ± 0.26 log CFU/g). After inoculum drying, both mushroom types were rehydrated at either 5 or 25°C for 2 h. Both pathogens survived on both mushroom types after rehydration regardless of the temperature. After rehydration at 5°C, both pathogen populations were < 2.39 log CFU/g on both mushroom types; both pathogens remained detectable on both mushroom types via enrichments. After rehydration at 25°C, pathogen populations on enoki mushrooms were 2.65 (8/9 values <2.39 log CFU/g) and 2.78 ± 0.27 log CFU/g for L. monocytogenes and S. enterica, respectively. On wood ear mushrooms, the populations of both pathogens were < 2.39 log CFU/g but remained detectable via enrichments.
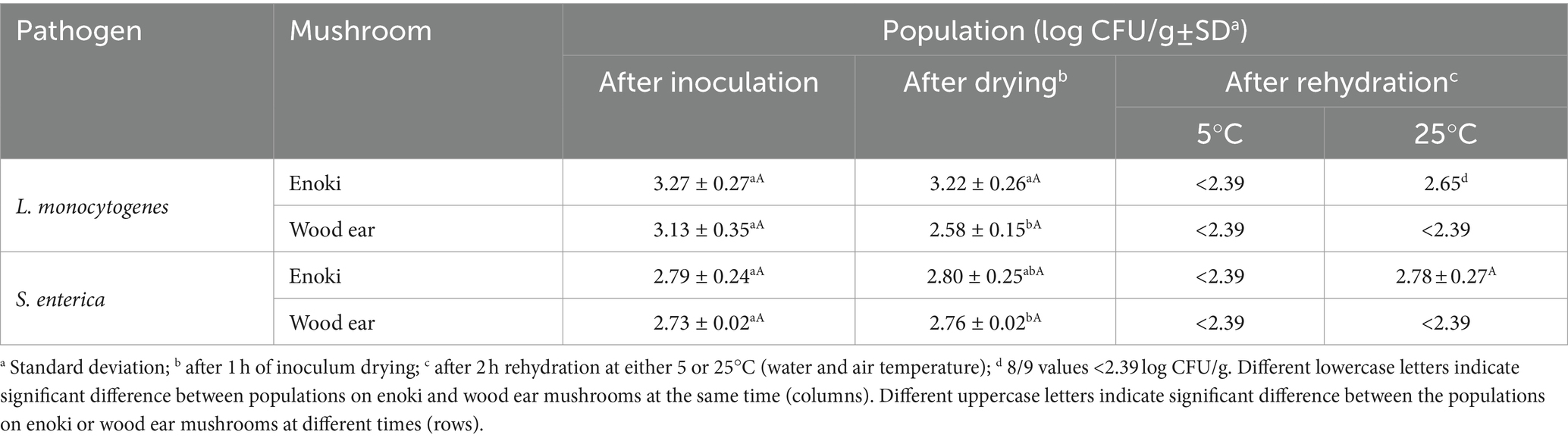
Table 2. Populations of L. monocytogenes and S. enterica on dehydrated enoki and wood ear mushrooms after inoculation, after drying, and after rehydration at 5 or 25°C.
After the enoki mushrooms were rehydrated at either 5 or 25°C for 2 h, the mushrooms were stored at 5, 10, or 25°C for up to 14 d. The population dynamics of L. monocytogenes and S. enterica on rehydrated enoki mushrooms during storage are displayed in Figure 2. Specific growth kinetics, including growth rates, lag phases, and times for a 1 log CFU/g population increase are presented in Table 3. When enoki mushrooms were rehydrated at 5°C and stored at 5°C, L. monocytogenes survived but did not increase in population during storage; the population after 14 d was <2.39 log CFU/g. When rehydrated at 5°C and stored at the higher temperatures, 10 and 25°C, L. monocytogenes proliferated on the enoki mushrooms with growth rates of 0.17 ± 0.11 and 1.22 ± 0.55 log CFU/g/d, respectively, resulting in 1 log CFU/g increases in 5.88 and 0.82 d. While no lag phases were predicted for L. monocytogenes on enoki mushrooms rehydrated at 5°C, a lag phase of 5.67 ± 1.39 d was predicted when rehydrated at 25°C and stored at 10°C. Both the growth rate and population of L. monocytogenes (0.42 ± 0.08 log CFU/g/d and 6.47 ± 0.76 log CFU/g, respectively) on the enoki mushrooms rehydrated at 25°C and stored at 10°C were significantly higher compared to when rehydration occurred at 5°C. The growth rate of L. monocytogenes on the enoki mushrooms rehydrated at 25°C and stored at 25°C was 1.75 ± 0.84 log CFU/g/d, resulting in a 1 log CFU/g population increase in only 0.57 d. The population after 14 d was 7.21 ± 1.05 log CFU/g, which was the highest population attained by L. monocytogenes.
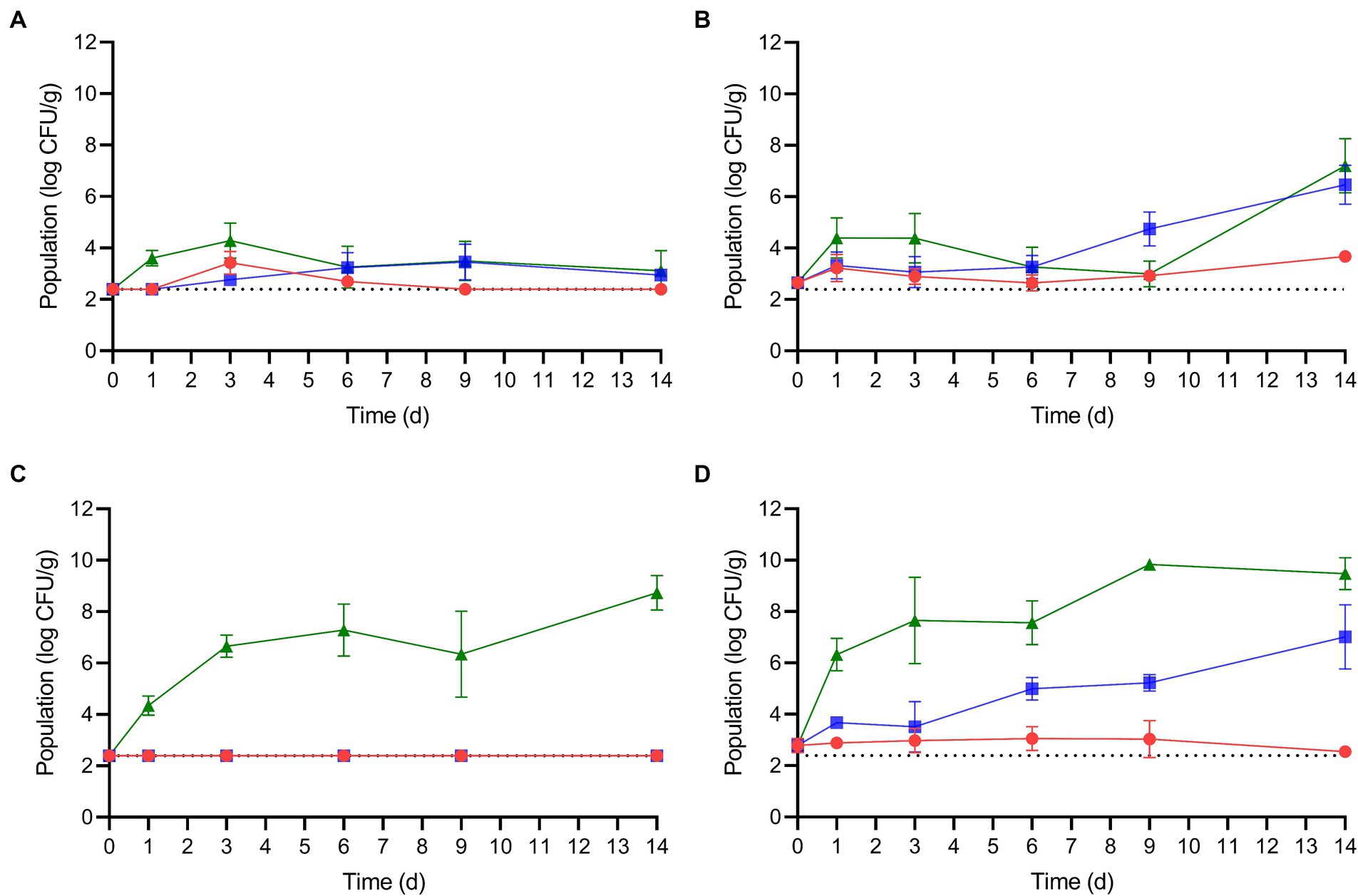
Figure 2. The population dynamics of L. monocytogenes (A,B) and S. enterica (C,D) on rehydrated enoki mushrooms during storage at 5 (red circles), 10 (blue squares) or 25°C (green triangles) for 14 days. Mushrooms were rehydrated at either 5 (A,C) or 25°C (B,D). Data are mean values ± standard deviation (n = 9). The dotted horizontal line indicates the lower level of enumeration (2.39 log CFU/g).
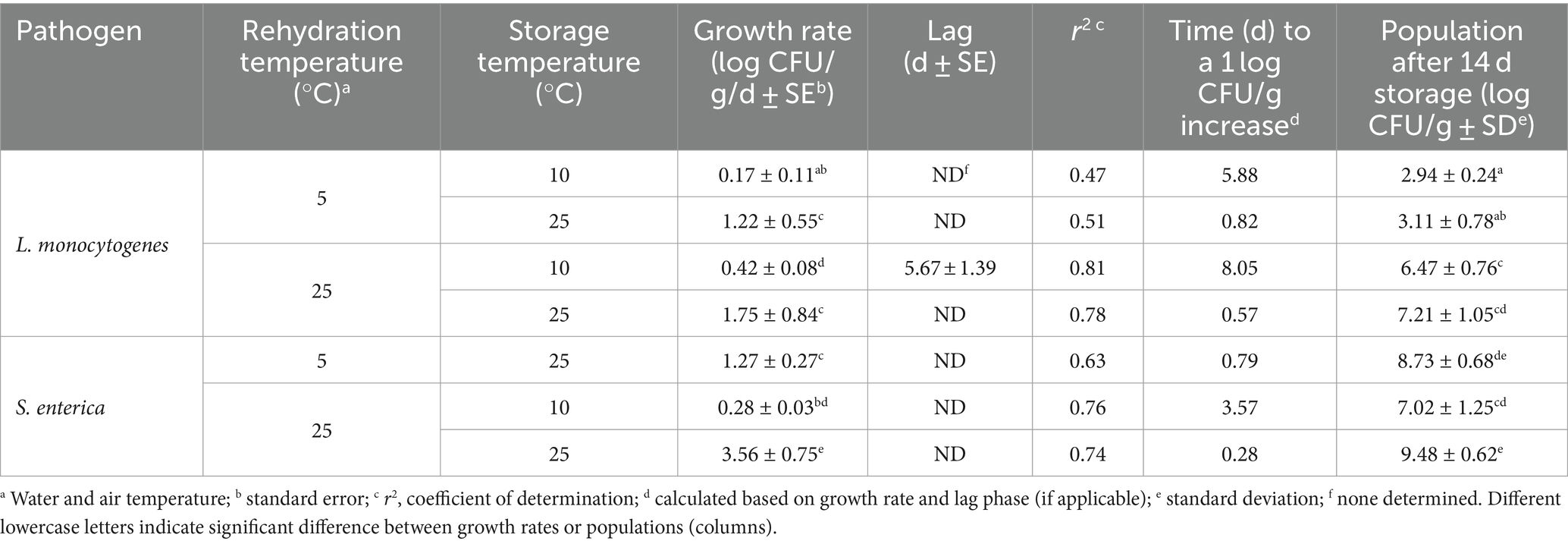
Table 3. Growth kinetics of L. monocytogenes and S. enterica on rehydrated enoki mushrooms during subsequent storage at 5, 10, or 25°C for 14 days.
When enoki mushrooms were rehydrated at 5°C and stored at 5 or 10°C, S. enterica survived but remained <2.39 log CFU/g during storage. When rehydrated at 5°C and stored at 25°C, S. enterica proliferated on the enoki mushrooms with a growth rate of 1.27 ± 0.27 log CFU/g/d, resulting in a 1 log CFU/g population increase in only 0.79 d. The population after 14 d of storage at 25°C was 8.73 ± 0.68 log CFU/g. S. enterica survived but did not grow on enoki mushrooms rehydrated at 25°C and stored at 5°C, with a population after 14 d of 2.54 log CFU/g (8/9 values <2.39 log CFU/g). The pathogen proliferated on enoki mushrooms rehydrated at 25°C and stored at 10°C, with a growth rate of 0.28 ± 0.03 log CFU/g/d, resulting in 1 log CFU/g population increases in 3.57 d, respectively. The highest growth rate of either pathogen on enoki mushrooms was observed by S. enterica at 25°C, 3.57 ± 0.75 log CFU/g/d, resulting in a 1 log CFU/g population increase in only 0.28 d. The population of S. enterica after 14 d storage at 25°C was 9.48 ± 0.62 log CFU/g, which was the highest population attained by either pathogen on enoki mushrooms.
After the wood ear mushrooms were rehydrated at either 5 or 25°C for 2 h, the mushrooms were stored at 5, 10, or 25°C for up to 14 d. Both L. monocytogenes and S. enterica populations remained <2.39 log CFU/g during storage, regardless of the rehydration or storage temperature. Table 4 displays the number of wood ear mushroom samples where L. monocytogenes or S. enterica were detected (n/9) via enrichment after 1, 7, and 14 d of storage at 5, 10, or 25°C. After 1 d of storage, L. monocytogenes was detected in 100% (9/9) of samples regardless of the rehydration or storage temperature. S. enterica was detected in 100% (9/9) of samples at all storage temperatures when wood ear mushrooms were rehydrated at 25°C, however, was detected in 78% (7/9) of samples when rehydrated at 5°C and stored at 10 or 25°C. After 7 d of storage, no significant difference in detection occurred with either pathogen, ranging from 100% (9/9) of samples for L monocytogenes on wood ear mushrooms rehydrated at 25°C and stored at 5 or 10°C to 56% (5/9) of samples for S. enterica on mushrooms rehydrated at 5°C and stored at 10°C. After 14 d of storage, L. monocytogenes detection was lowest on wood ear mushrooms stored at 25°C (33% (3/9) and 56% (5/9) of samples when mushrooms were rehydrated at 5 and 25°C, respectively). Detection of S. enterica was lowest on wood ear mushrooms rehydrated at 25°C and stored at 10 or 25°C (44% (4/9) of samples).
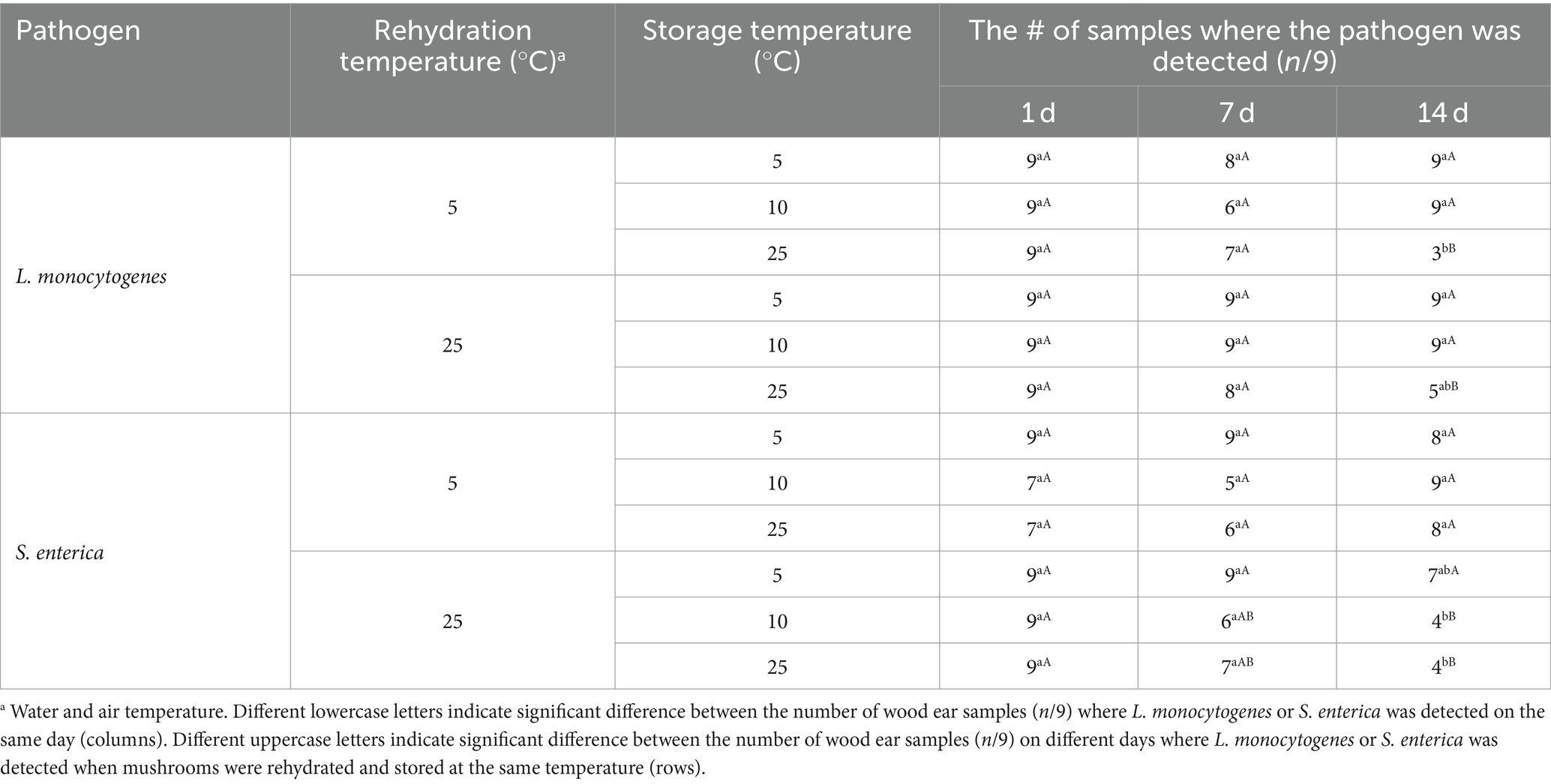
Table 4. Detection of L. monocytogenes and S. enterica on rehydrated wood ear mushrooms during subsequent storage at 5, 10, or 25°C for 14 days.
Recent outbreaks associated with enoki and wood ear mushrooms in the U.S. have prompted research to understand the fate of L. monocytogenes and S. enterica on these food products. Since the shelf life of fresh mushrooms is relatively short, mushrooms are often dried to extend their shelf life, reducing their aw. Prior to consumption, dried mushrooms could be rehydrated and stored for later use. Once dried mushrooms are rehydrated, the high moisture content and relatively neutral pH of these foods would provide an environment suitable for the proliferation of foodborne pathogens, and hence product assessments are needed. Therefore, this study examined the survival of L. monocytogenes and S. enterica on dehydrated enoki and wood ear mushrooms during rehydration and subsequent storage.
Two rehydration temperatures were incorporated in this study to understand if the temperature of rehydration influenced the survival or proliferation of either pathogen. Since consumers, retail establishments, and restaurants may rehydrate the dehydrated mushrooms in the refrigerator or at room temperature, these two conditions (5 and 25°C) were mimicked in this study. Both L. monocytogenes and S. enterica survived on both mushroom types during rehydration, while populations were often below quantification limits (<2.39 log CFU/g). Similar L. monocytogenes populations were observed in a previous study when dehydrated vegetables, including carrot, corn, onion, pepper, and potato, were rehydrated at 5 or 25°C (Fay et al., 2023). Similar S. enterica populations were also observed on the vegetables during rehydration at 5°C, although much higher populations (4–6 log CFU/g) were attained on certain vegetables (carrot, pepper, and potato) when rehydration occurred at 25°C. It is noted that a 24-h rehydration was used compared to the 2-h rehydration in this study, and results may be different if rehydration continued for longer than 2 h. The survival and/or proliferation of pathogens during rehydration at 5 or 25°C appears to be food matrix dependent.
After the dehydrated mushrooms were rehydrated, they were stored at 5, 10, or 25°C, mimicking refrigeration, temperature abuse, and room temperature, respectively. Striking differences were observed in the survival of both pathogens on the two mushroom types during storage with enoki mushrooms appearing to be a much more favorable environment. While both pathogens survived on enoki mushrooms stored at 5°C regardless of the rehydration temperature, both pathogens proliferated at 10 and 25°C with 1 log CFU/g population increases in 0.28 to 8.05 d. However, in the case of wood ear mushrooms, both pathogens survived during the 14-d storage period but did not grow, even at the higher storage temperatures. This difference may be attributed to antimicrobial compounds present in wood ear mushrooms, including polysaccharides, melanin, and polyphenols; these compounds have been shown to inhibit growth and biofilm formation of bacterial pathogens (Cai et al., 2015; Yu and Oh, 2016; Liu et al., 2021). While the antimicrobial aspect of wood ear mushrooms resulted in hindered growth of both pathogens, both pathogens remained detectable during storage.
The antimicrobial nature of the wood ear mushrooms may have also contributed to the significantly lower population of L. monocytogenes on these mushrooms when compared to the enoki mushrooms after drying of the inocula in this study. Interestingly, the antimicrobial nature of wood ear mushrooms may be more pronounced when the mushrooms are in their dehydrated state or rehydrated from dried, as previous research has shown that both L. monocytogenes and S. enterica can proliferate on fresh whole and chopped wood ear mushrooms (Fay et al., 2023). For example, L. monocytogenes grew on whole and chopped wood ear mushrooms at both 10 and 25°C, with 1 log CFU/g population increases in 33.33 to 1.82 d. S. enterica also proliferated on whole and chopped wood ear mushrooms, but only at 25°C, with a 1 log CFU/g population increase in 0.80 to 2.08 d. A similar phenomenon has been shown to occur with onions, also known to have antimicrobial properties (de Niederhäusern et al., 2021), where the growth rates of L. monocytogenes were significantly higher on fresh onion compared to dehydrated onion that had been rehydrated and stored at 10 or 25°C (Salazar et al., 2017; Fay et al., 2023).
The survival and growth of L. monocytogenes on the rehydrated enoki mushrooms in this study is comparable to that on fresh whole and chopped enoki mushrooms stored at both 10 and 25°C, with no significant difference observed in growth rates (Fay et al., 2023). For S. enterica, growth rates were generally similar at all storage temperatures on the fresh enoki and the enoki rehydrated at 25°C (Fay et al., 2023). However, S. enterica did not grow on the enoki rehydrated at 5°C and stored 10°C in this study, whereas 1 log CFU/g population increases were observed in 7.69 to 8.33 d on the fresh enoki stored at 10°C. These findings indicate that pathogen survival may be different on the same commodity depending on if it is fresh or rehydrated from a dehydrated state, further highlighting the need for specific product assessments.
This study evaluated the survival of L. monocytogenes and S. enterica on dehydrated enoki and wood ear mushrooms during rehydration and storage. Marked differences were observed in pathogen survival on these two rehydrated specialty mushrooms, emphasizing the need for individual assessments. Rehydrated enoki mushrooms appeared to be a more favorable environment for both pathogens and growth was hindered when rehydration occurred at 5°C and the mushrooms were stored at 5°C. The results therefore highlight the importance of storing rehydrated mushrooms at refrigeration temperatures. Data from this study can be used to inform regulatory decisions surrounding time and temperature control for safety for these food products.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MF: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DS: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. DI: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by grant U19FD005322 from the U.S. Food and Drug Administration to the Illinois Institute of Technology. MF was supported by the Oak Ridge Institute for Science and Education Research Participation Program to the U.S. Food and Drug Administration. The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Karl Reineke for obtaining the mushrooms used in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baranyi, J., and Roberts, T. A. (1994). A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23, 277–294. doi: 10.1016/0168-1605(94)90157-0
Blessington, T., Mitcham, E. J., and Harris, L. J. (2012). Survival of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes on inoculated walnut kernels during storage. J. Food Prot. 75, 245–254. doi: 10.4315/0362-028X.JFP-11-278
Cai, M., Lin, Y. H., Luo, Y. L., Liang, H. H., and Sun, P. (2015). Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the wood ear medicinal mushroom Auricularia auricula-judae (higher basidiomycetes). Int. J Med. Mushrooms. 17, 591–600. doi: 10.1615/IntJMedMushrooms.v17.i6.90
CDC (2020a). Outbreak of Listeria infections linked to enoki mushrooms (final update). Available at: https://www.cdc.gov/listeria/outbreaks/enoki-mushrooms-03-20/index.html
CDC (2020b). Outbreak of Salmonella Stanley infections linked to wood ear mushrooms. Available at: https://www.cdc.gov/salmonella/stanley-09-20/index.html
CDC (2023). Listeria outbreak linked to enoki mushrooms. Available at: https://www.cdc.gov/listeria/outbreaks/enoki-11-22/index.html
de Niederhäusern, S., Bondi, M., Camellini, S., Sabia, C., Messi, P., and Iseppi, R. (2021). Plant extracts for the control of Listeria monocytogenes in meat products. Appl. Sci. 11:10820. doi: 10.3390/app112210820
Diamantopoulou, P., and Philippoussis, A. (2015). Cultivated mushrooms: Preservation and processing. Handbook of vegetable preservation and processing. Boca Raton, FL: CRC Press.
Farakos, S. M., Pouillot, R., and Keller, S. E. (2017). Salmonella survival kinetics on pecans, hazelnuts, and pine nuts at various water activities and temperatures. J. Food Prot. 80, 879–886. doi: 10.4315/0362-028X.JFP-16-392
Fay, M. L., Salazar, J. K., Chavda, N. J., Patil, G. R., and Ingram, D. T. (2023). Survival kinetics of listeria monocytogenes and Salmonella enterica on dehydrated enoki and wood ear mushrooms during long-term storage. Food Microbiol. 114:104304. doi: 10.1016/j.fm.2023.104304
Fay, M. L., Salazar, J. K., George, J., Chavda, N. J., Lingareddygari, P., Patil, G. R., et al. (2023). Modeling the fate of listeria monocytogenes and Salmonella enterica on fresh whole and chopped wood ear and enoki mushrooms. J. Food Prot. 86:100075. doi: 10.1016/j.jfp.2023.100075
Fay, M. L., Salazar, J. K., Ren, Y., Wu, Z., Mate, M., Khouja, B. A., et al. (2023). Growth kinetics of listeria monocytogenes and Salmonella enterica on dehydrated vegetables during rehydration and subsequent storage. Food Secur. 12:2561. doi: 10.3390/foods12132561
FDA (2017). Bacteriological Analytical Manual (BAM). Available at: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm2006949.htm
FDA (2020a). Outbreak investigation of Listeria monocytogenes: enoki mushrooms. Available at: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-enoki-mushrooms-march-2020
FDA (2020b). Outbreak investigation of Salmonella Stanley: wood ear mushrooms - dried fungus Available at: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-stanley-wood-ear-mushrooms-dried-fungus-september-2020
FDA (2022). FDA Food Code. Available at: https://www.fda.gov/food/retail-food-protection/fda-food-code
Komitopoulou, E., and Penaloza, W. (2009). Fate of salmonella in dry confectionery raw materials. J. Appl. Microbiol. 106, 1892–1900. doi: 10.1111/j.1365-2672.2009.04144.x
Liu, E., Ji, Y., Zhang, F., Liu, B., and Meng, X. (2021). Review on Auricularia auricula-judae as a functional food: growth, chemical composition, and biological activities. J. Agric. Food Chem. 69, 1739–1750. doi: 10.1021/acs.jafc.0c05934
Nascimento, M. S., Carminati, J. A., Morishita, K. N., Amorim Neto, D. P., Pinheiro, H. P., and Maia, R. P. (2018). Long-term kinetics of Salmonella Typhimurium ATCC 14028 survival on peanuts and peanut confectionery products. PLoS One 13:e0192457. doi: 10.1371/journal.pone.0192457
Salazar, J. K., Sahu, S. N., Hildebrandt, I. M., Zhang, L., Qi, Y., Liggans, G., et al. (2017). Growth kinetics of Listeria monocytogenes in cut produce. J. Food Prot. 80, 1328–1336. doi: 10.4315/0362-028X.JFP-16-516
Taylor, M. H., Tsai, H. C., Rasco, B., Tang, J., and Zhu, M. J. (2018). Stability of Listeria monocytogenes in wheat flour during extended storage and isothermal treatment. Food Control 91, 434–439. doi: 10.1016/j.foodcont.2018.04.008
Keywords: dried mushrooms, growth rates, Listeria, salmonella, survival
Citation: Salazar JK, George J, Fay ML, Stewart DS and Ingram DT (2024) Comparative growth kinetics of Listeria monocytogenes and Salmonella enterica on dehydrated enoki and wood ear mushrooms during rehydration and storage. Front. Microbiol. 15:1406971. doi: 10.3389/fmicb.2024.1406971
Received: 25 March 2024; Accepted: 25 July 2024;
Published: 05 August 2024.
Edited by:
Aldo Corsetti, University of Teramo, ItalyReviewed by:
Donald W. Schaffner, Rutgers, The State University of New Jersey, United StatesCopyright © 2024 Salazar, George, Fay, Stewart and Ingram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joelle K. Salazar, am9lbGxlLnNhbGF6YXJAZmRhLmhocy5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.