- 1State Key Laboratory of Black Soils Conservation and Utilization, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 2University of Chinese Academy of Sciences, Beijing, China
Freshwater wetlands are the wetland ecosystems surrounded by freshwater, which are at the interface of terrestrial and freshwater ecosystems, and are rich in ecological composition and function. Biodiversity in freshwater wetlands plays a key role in maintaining the stability of their habitat functions. Due to anthropogenic interference and global change, the biodiversity of freshwater wetlands decreases, which in turn destroys the habitat function of freshwater wetlands and leads to serious degradation of wetlands. An in-depth understanding of the effects of biodiversity on the stability of habitat function and its regulation in freshwater wetlands is crucial for wetland conservation. Therefore, this paper reviews the environmental drivers of habitat function stability in freshwater wetlands, explores the effects of plant diversity and microbial diversity on habitat function stability, reveals the impacts and mechanisms of habitat changes on biodiversity, and further proposes an outlook for freshwater wetland research. This paper provides an important reference for freshwater wetland conservation and its habitat function enhancement.
1 Introduction
Freshwater wetlands (FWs) are ecosystems formed by the interaction between freshwater rivers, lakes and land, mainly including riverine wetlands, lakes, marshes and floodplains. FWs not only provide suitable habitats for many plants and animals (McKown et al., 2021), but also play an important role in nutrient cycling, water purification and biodiversity maintenance (Li C. et al., 2022; Yu et al., 2023; Li et al., 2024). FWs have four the ecological services categories: provisioning, regulating, cultural and supporting services (Keddy et al., 2009). However, FWs have been severely damaged due to the increase in global population and economic development, resulting in a decrease in the global wetland area (Davidson, 2014), and a consequent severe destruction of wetland functions and biodiversity (Herbert et al., 2015; Ndehedehe et al., 2020).
Biodiversity is a complex system formed by the interaction between organisms and the external environment, expressing in genetic diversity, species diversity, and ecosystem diversity (Song, 2017; Liang et al., 2023). Habitat function refers to the specific functions and conditions providing for organisms, and many studies have shown that biodiversity plays a crucial role in habitat function and its stability (Weisser et al., 2017; Yao et al., 2017). FWs are complex ecosystems composed of special environmental conditions and organisms, and their functional stability is affected by many factors (Rideout et al., 2022). In FWs, high biodiversity can enhance the stability of wetland functions, such as nutrient cycling, water purification, and biodiversity maintenance (Thomaz, 2023). Rich diversity can alleviate competitive pressures among organisms by providing more ecological niches through complementary effects, allowing different species in FWs to fully utilize resources such as water, nutrients and sunlight (Steudel et al., 2011). In addition, biodiversity can also improve the stability and disturbance resistance of food chains, mitigating external disturbances in wetlands by building complex foodweb structures (Peel et al., 2019; Hatton et al., 2024).
Although many studies showed that the biodiversity of FWs has an important impact on the functional stability of the habitats in which they exist, few literatures have been reviewed and summarized. Therefore, the objectives of this study are to (1) analyze the effects of biodiversity on the functional stability of freshwater wetland habitats; (2) illuminate the impacts and mechanisms of habitat change on biodiversity; and (3) propose future research directions and perspectives. This paper synthesizes the environmental drivers of functional stability in FWs, the effects of plant and microbial diversity on the functional stability of FWs, and further discusses the effects and mechanisms of habitat change on biodiversity.
2 Environmental drivers of functional stability in freshwater wetlands
Freshwater wetlands provide numerous functions such as biodiversity maintenance, freshwater supply, carbon storage, etc., and at the same time they are one of the most fragile ecosystems (Zedler and Kercher, 2005). Changes in environmental drivers such as hydrological factors, climatic factors, water quality, and soil physicochemical properties have led to serious functional degradation of some wetlands (Xue et al., 2018; Xiu et al., 2019). Therefore, understanding the effects of these environmental drivers on freshwater wetland ecosystems (Table 1) is important for improving the functional stability of FWs and optimizing wetland management options.
2.1 Hydrology
Water plays a crucial role in the formation, development, succession, and extinction of wetlands, directly affecting their structure, function, and ecosystem stability (Wang et al., 2015). Human activities and climate change cause changes in precipitation, evapotranspiration, and temperature, which lead to changes in hydrological conditions such as water-holding capacity, water level, and inundation duration of wetlands (Karim et al., 2015). Changes in these hydrological characteristics in turn affect the structure, distribution (Todd et al., 2010; Maietta et al., 2020a) and biogeochemical cycling (Chen et al., 2013) of biological communities in FWs, leading to degradation of wetland ecosystem functions.
An increase in water loss from FWs leads to hydrological conditions variation and a decrease in available water resources, which can disrupt their freshwater supply (Zhao and Liu, 2016). Hydrological changes can also affect the structure, distribution and biogeochemical cycling of freshwater wetland biological communities, which in turn can degrade wetland ecosystems (Chen et al., 2013; Maietta et al., 2020a). Large fluctuations in water level can affect the structure and diversity of biological communities (Luo, 2009). During periods of low water levels in the Paraná River delta, the beta diversity and individual biomass of zooplankton decreases, leading to a simplification of the functional diversity (Gutierrez et al., 2022) and a degradation of the wetland environment that sustains aquatic vegetation in Lake Michigan-Lake Huron (DeVries-Zimmerman et al., 2021), whereas high water levels have led to a decrease in vegetation cover in Lake Ontario (Smith et al., 2021), resulting in habitat loss and the frustration of the supply functions of FWs. Overall, water level with too low or high is not conducive to wetland ecosystems. Soil water content, aeration conditions and redox potential also change with fluctuations in wetland water level, affecting the ecological processes and metabolic activities of microbial communities (Ma et al., 2018). Therefore, the relative stability of water level plays an important role in maintaining the functional stability of FWs.
2.2 Water quality and soil properties
Humans production and life discharge heavy metals (Li et al., 2021), pesticides and nutrient salts (Sremacki et al., 2020; Ding et al., 2021) into freshwater wetland ecosystems, directly and indirectly leading to changes in water quality and soil physicochemical properties of wetlands, which in turn cause wetland degradation (Wei et al., 2019). Relevant studies have shown that increased loading of nutrients such as nitrogen and phosphorus in water will deteriorate water quality, and cause eutrophication of the water body, leading to significant changes in the structure and function of wetland ecosystems (Khan and Ansari, 2005; Bano et al., 2022). It has been found that increased loading of nitrogen and phosphorus in FWs may affect the rates of nitrification, denitrification, and methane production, which in turn affects the nutrient cycling (Herbert et al., 2020). Soil physicochemical properties are key factors in shaping microbial community structure, composition, and metabolic activity (Ou et al., 2019). Changes in soil physicochemical properties caused by human disturbances and natural processes likewise have serious impacts on freshwater wetland biological communities (Lai, 2010).
2.3 Temperature
Temperature is recognized as one of the key climatic factors influencing the functional stability of FWs (Bano et al., 2022). Changes in temperature can have pervasive effects on the structure and function of freshwater wetland ecosystems (Hamilton, 2010). Wetland plant growth and photosynthesis efficiency increase with increasing temperatures within a certain range, increasing nutrient uptake and conversion (Zou et al., 2014). However, excessively high temperatures may reduce the germination of plant seeds and incubation of animals, which can have serious effects on wetland plant and microbial communities, disrupting wetland biodiversity (Nielsen et al., 2015). Temperature changes can also have an impact on microbial metabolism, for example, the role of iron-reducing bacteria in inhibiting methane production may diminish as the global average temperature increases, thus affecting greenhouse gas emissions from FWs. In addition, temperature changes may also lead to species migration and range shifts (Chen X. et al., 2023).
The hydrological conditions of wetlands are closely related to temperature changes, and global warming will lead to changes in evaporation and precipitation, which may alter the hydrological cycle of wetlands and thus indirectly affect the functional stability of wetlands (Luo, 2009; You et al., 2015). A previous study showed that a 10% decrease in rainfall will lead to changes in the redox conditions of the soil in the Everglades, thus affecting its biogeochemical processes; whereas the elemental load of the wetland ecosystem may increase when rainfall increases by 10%, which helps to maintain suitable redox conditions and promotes biogeochemical elemental cycling (Orem et al., 2015).
3 Impact of plant diversity on functional stability of freshwater wetlands
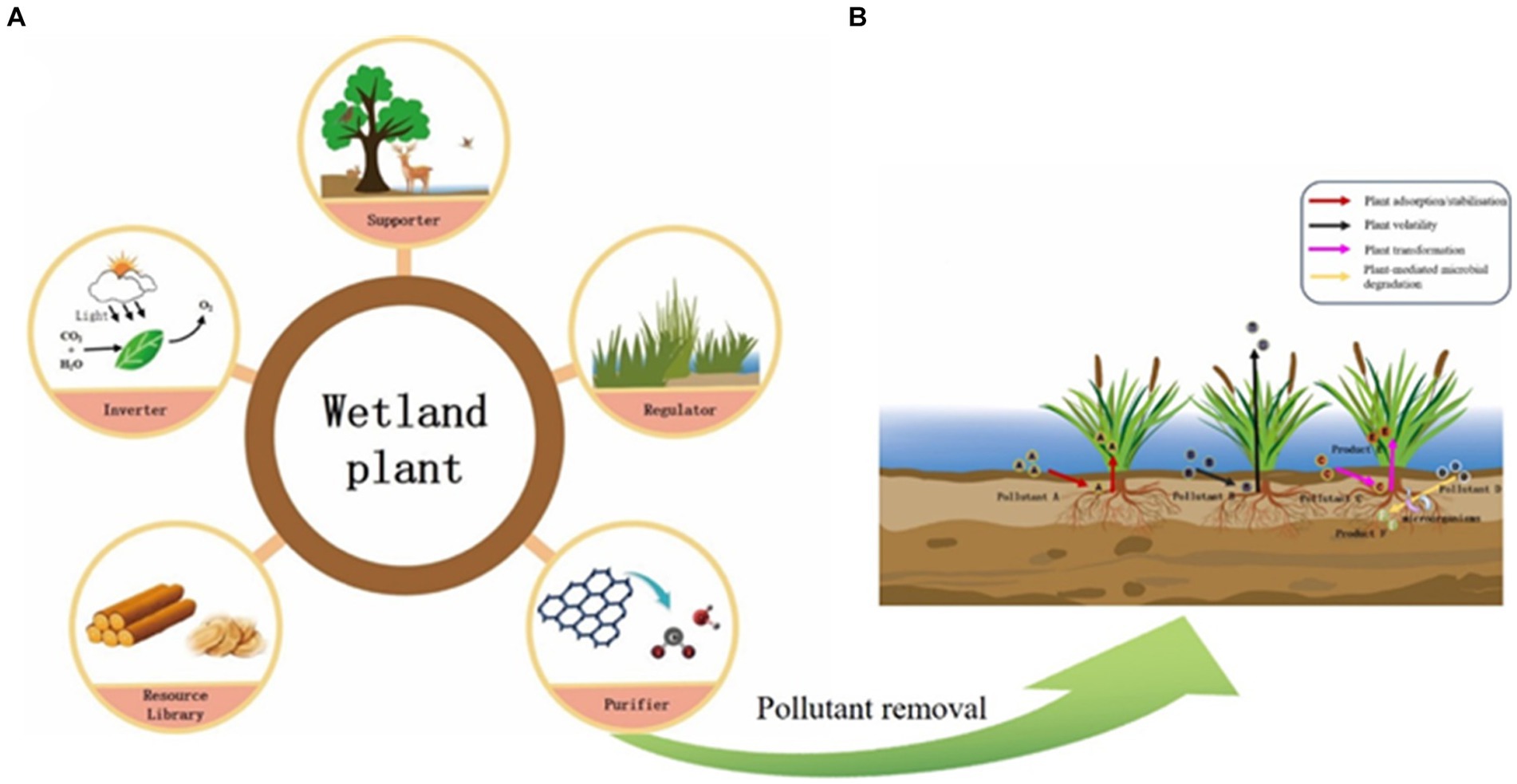
Freshwater wetlands are rich in plant species, which play multiple roles in wetland ecosystems (Figure 1A). Different types of wetlands have different dominant vegetation, and diverse plants play an important role in maintaining the stability of wetland habitat functions (e.g., water purification, carbon storage, biodiversity maintenance, etc.) (Zhang et al., 2014).
3.1 Water purification
Removal of pollutants by wetlands plants is one of the main ways of water quality purification, mainly through two main pathways involving in direct pollutants removal and microbial processes mediating (Figure 1B; Stottmeister et al., 2003). The uptake of nutrients and heavy metals varies among different plant species (Adhikari et al., 2011; Abbasi et al., 2018). The study showed that the nitrogen uptake and fixation capacity of Rhododendron ilfescens Siberianum was higher, and the remediation of nitrogen pollution in wetlands was more effective (Weragoda et al., 2012). In addition, the dissolved oxygen in the water were affected by the abundance of submerged plant species (Qian, 2019), and different plants had different inter-roots, physiological processes, and growth modes, which might affect the community structure and activity of microorganisms, and further affect water quality purification (Zhang et al., 2010; Pang et al., 2016). Resource complementarity between plant species may also play a positive role in nutrient uptake and water purification (Choudhury et al., 2018). Therefore, maintaining high plant diversity can help to improve pollutants removal from water (Brisson et al., 2020).
3.2 Carbon storage
Freshwater wetlands are one of the valuable carbon storage sites, covering about 6% of the land area, and contain more than 30% of the soil carbon pool (Stewart et al., 2024). Plants play an important role in wetland carbon storage (Sheng et al., 2021). Wetland plants can convert atmospheric carbon dioxide into biomass through photosynthesis, and plant residues and leaves are deposited at wetland after death, which is one of the main mechanisms of carbon storage in wetlands (Adhikari et al., 2009). Previous studies have shown that the plants vary in nutrient and light utilization (Abbasi et al., 2018). Plant diversity has an important effect on freshwater wetland productivity (Isbell et al., 2013; Chaturvedi and Raghubanshi, 2015). Means et al. (2016) found a positive correlation between plant diversity and productivity in freshwater artificial wetlands. Cardinale et al. (2011) found that high diversity plant communities can use more ecological niches and increase the efficiency of nutrient utilization, which in turn increases primary productivity. An increase in wetland productivity can increase the capacity and total amount of carbon input from plants to the soil, which in turn increases carbon storage (Zhang et al., 2022).
In addition, the decomposition mode (humification and mineralization) and rate of plant apoplasts are particularly important for wetland carbon storage (Prescott and Vesterdal, 2021). Litter from different types of plants has different chemical compositions (Yan et al., 2018) and decomposition rates (Xi et al., 2023). It has been shown that the litter of freshwater wetland vegetation has the ability to alter the nutrient content of soil nitrogen and carbon, thus leading to the construction of different dominant microorganisms (Bonetti et al., 2021). Some plant litter leads to the production of microbial communities of humification, while others lead to the construction of microbial communities of carbon dioxide or methane production (Lin et al., 2015). Increased plant diversity can provide a wider variety of little, and this little can lead to the construction of more stable and resilient microbial communities, affecting the carbon storage capacity of the wetland (Maietta et al., 2020b).
3.3 Biodiversity maintenance
Plants can create unique microhabitat structures and provide suitable conditions for many animals and microorganisms (Choi et al., 2014; Weilhoefer et al., 2017). Freshwater wetland plants serve as the basis of the food chain in this ecosystem, and rich wetland plant communities provide a more complex and stable food web that supports the nutrient needs of many animals and microorganisms, thus contributing to the maintenance of biodiversity (Peel et al., 2019). In addition, higher plant diversity improves the resistance of wetland ecosystems to invasive alien species and better defends against invasive alien species, thus maintaining the stability of other organisms within the wetland (Peter and Burdick, 2010). Therefore, the protection and maintenance of plant diversity in FWs is essential for maintaining wetland biodiversity.
4 Impact of microbial diversity on functional stability of freshwater wetlands
Microorganisms in FWs are rich and diverse, with some differences in microbial composition among different wetland types, which can be mainly categorized into bacteria, archaea, fungi and protozoa (Cao et al., 2017). Microorganisms play an irreplaceable role in maintaining the stability of freshwater wetland habitat functions (e.g., water purification and biogeochemical cycles, etc.) (De Mandal et al., 2020; Chen M. et al., 2023; Qiao et al., 2023; Chen et al., 2024).
4.1 Water purification
Microorganisms can participate in various water purification processes through a series of metabolic and interaction processes, especially some functional microorganisms play a crucial role in wetland water purification (Wang et al., 2022). For example, some inter-root microorganisms such as Pseudomonas and Flavobacterium can effectively remove micropollutants (Brunhoferova et al., 2022). Fusobacterium, Rhizobium and Erythrobacterium have significant removal effects on organic pollutants such as petroleum in wetlands, and their removal rates are positively correlated with the abundance of bacterial species (Xiang et al., 2020). Burkholderia, Hydrophilus, and Thiobacillus play important roles in the remediation of arsenic and antimony pollution in wetlands (Deng et al., 2022).
The areas riched in wetland microbial diversity usually have higher degradation capacity of organic pollutants, and different microbial communities can co-operate together to decompose complex organic matter and convert it into harmless products (Berrier et al., 2022). Studies have shown that hydrocarbon-degrading microorganisms (e.g., Pseudomonas, Rhodococcus, and Nocardia) in FWs can form microbial aggregates, improving the removal efficiency of n-alkanes and polycyclic aromatic hydrocarbons (PAHs) (Liu et al., 2021). Anaerobic ammonia-oxidizing bacteria in wetlands can cooperate with certain archaea (e.g., nitrate archaea and sulfate-dependent archaea) to complete the denitrification process in wetlands (Wang et al., 2019). In addition, some microorganisms can remove multiple pollutants simultaneously. For example, Flavobacterium and Chryseobacterium can simultaneously degrade nitrogen and organic matter in wetlands (Shen et al., 2018). Sulfate-reducing bacteria, such as Desulfovibrio, Desulfobacter, and Desulfobulbus, also play dual roles in wetland restoration: (1) participating in the sulfate reduction process, producing hydrogen sulfide; (2) hydrogen sulfide reacts with heavy metals to form precipitation, which promotes the passivation of heavy metals (Chen et al., 2021).
4.2 Biogeochemical cycles
Wetland microorganisms are involved in the process of storage, transformation and release of C, N and other elements, and are the dominant driver of the biogeochemical cycle in FWs (Hussain et al., 2023).
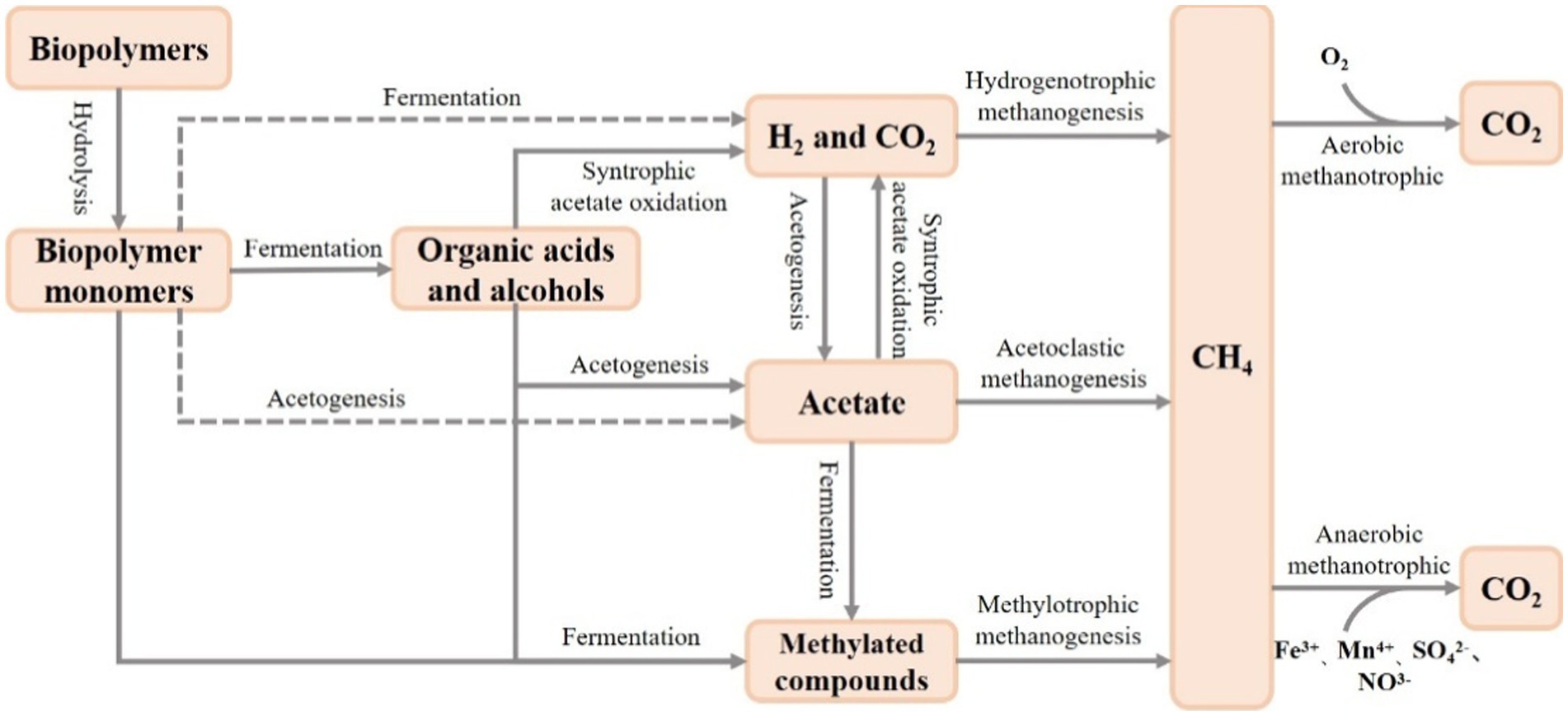
The biogeochemical cycle of carbon in FWs has received much attention (Zou et al., 2022; Bao et al., 2023; Qian et al., 2023), and microorganisms are mainly involved in the carbon cycle through the processes of respiration, methane production and conversion, and decomposition of organic matter (Bardgett et al., 2008). Microorganisms play an important role in methane production and transformation of FWs (Figure 2). It is now widely accepted that methanogenic bacteria are distributed in seven orders of the phylum Euryarchaeota (Methanopyrales, Methanococcales, Methanobacteriales, Methanomicrobiales, Methanomassiliicoccales, Methanosarcinales, and Methanocellales) (Dean et al., 2018). Among them, Methanomicrobiales, Methanosarcinales, Methanomassiliicoccales, and Methanobacteriaceae methanogenic bacteria have widely found in wetland ecosystems (Horn Marcus et al., 2003; Zhang et al., 2008; Söllinger et al., 2016). There are three main pathways of freshwater wetland methanogens involved in methanogenesis: acetate fermentation, hydrogenotrophic and methylotrophic methanogenesis (Narrowe et al., 2019), whereas wetland methane oxidation is of two types: aerobic and anaerobic oxidation. The diverse microorganisms can adapt to the different environmental conditions and can better maintain the balance of wetland methane production and conversion. It was found that the microbial community can change the methanogenic pathway by adjusting the composition and activity of the microbial community under the fluctuation of nutrients, and then maintaining the stability of carbon cycle (Holmes et al., 2014). In addition, the richness of microbial diversity in FWs is closely related to the rate of mineralization of organic matter, and an active microbial community can increase organic matter degradation and mineralization (Li et al., 2015).
Microorganisms in FWs are also critical for maintaining the relative stability of the nitrogen cycle, and diverse microorganisms are an important player in driving nitrogen conversion and its cycling processes (Mellado and Vera, 2021; Sheng et al., 2023). Microorganisms such as nitrogen-fixing bacteria and cyanobacteria can convert atmospheric N2 into bioavailable forms such as ammonia and nitrate, supplying the wetland ecosystem with available nitrogen (Bae et al., 2018). It has been found that the efficiency and rate of nitrogen fixation are usually positively correlated with the number and diversity of microorganisms such as nitrogen-fixing bacteria (Li H. et al., 2022). On the other hand, some microorganisms (e.g., anaerobic ammonia-oxidizing bacteria, ammonia-oxidizing archaea, and denitrifying anaerobic methane-oxidizing bacteria) are also present in FWs, involved in key nitrogen transformation processes such as ammonia oxidation, nitrification and denitrification (Chen et al., 2020). These microorganisms differ in their tolerance and sensitivity to environmental factors, and a high diversity of microorganisms can provide different kinds of microbial functional groups, improving the adaptability and stability of FWs to environmental changes and maintaining the relative stability of the nitrogen cycle (Hu et al., 2017).
5 Impacts and mechanisms of habitat change on biodiversity
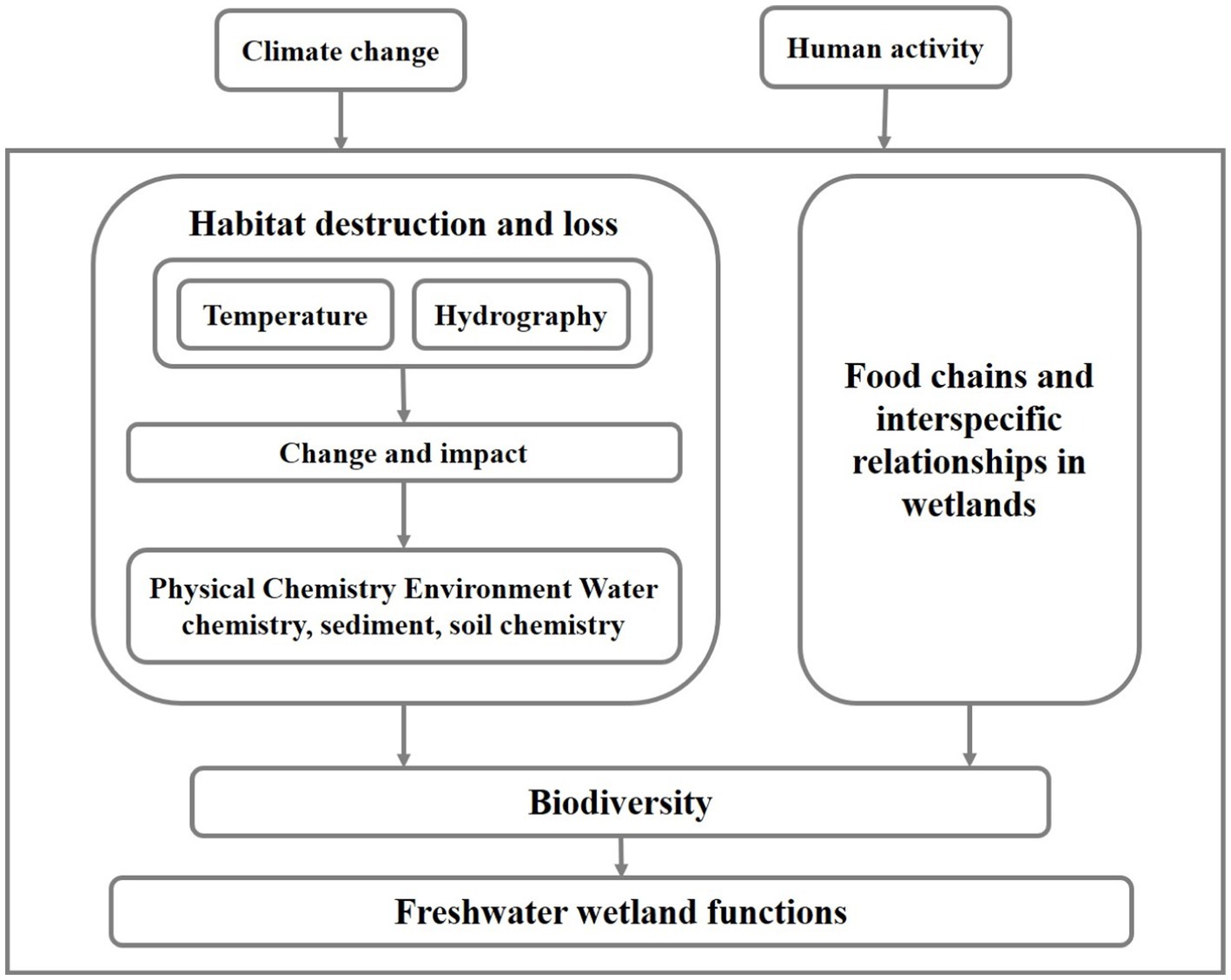
Wetlands provide habitat for nearly 20% of the world’s species and are one of the most biodiversity-rich systems, however, they are under great pressure from human activities and climate change (Fang et al., 2006). This is causing a large degree of degradation of FWs and affecting the biodiversity of ecosystems (Al-Obaid et al., 2017). Habitat changes have important effects on wetlands (Figure 3). Among these, habitat changes and alterations in food chains and interspecific relationships are the two main factors (Ohba et al., 2019; Wang et al., 2021).
Habitat loss and fragmentation can result in the reduction and fragmentation of freshwater wetland areas, weakening the available area and connectivity of habitats for species, and these can directly lead to the reduction of the number and distribution range of some species, and consequently the decline of biodiversity (Jamin et al., 2020). For example, the size and connectivity of wetlands in Xin Jiang Wan Town, Shanghai, decreased with the accelerated urbanization of the area, leading to habitat loss and diversity reduction of wetland birds (Xu et al., 2018). Vascular plants in the wetlands of the canton of Zurich in eastern Switzerland became extinct as a result of the reduction of wetland connectivity and patch size under human activities (Jamin et al., 2020). In addition, the movement and migration of amphibians are limited when wetlands are fragmented, which may lead to the delayed extinction of these species (Gimmi et al., 2011).
Habitat change also affects wetland biodiversity by altering wetland food chains and interspecific relationships (Araújo et al., 2014). Previous studies have found that species richness of insectivorous birds in the Lampertheimer Altrhein area has decreased, due to the reducing food resources for insectivorous birds under agricultural intensification (Schrauth and Wink, 2018). The reduction in species richness and cover of plant communities during the degradation of the Ruoerge wetland has led to changes in the trophic structure of omnivores and algae, which in turn had a serious impact on the diversity of nematode communities (Wu et al., 2017). In addition, biological invasions are recognized as one of the main drivers of biodiversity loss (Mazor et al., 2018). Habitat changes can promote the invasion and spread of non-native species (e.g., Spartina alterniflora), and these invasive species can disrupt the original food chains and interspecific relationships of ecosystems, thus leading to biodiversity reduction (Wang et al., 2021).
In addition, changes in environmental factors such as wetland water level and pollution have significant impacts on biodiversity. For example, during the degradation of wet marshes to meadows in the Sanjiang Plain, changes in wetland water level alter the living conditions of organisms, which in turn affects the diversity and community composition of plants and microorganisms (Sui et al., 2017; Liping et al., 2020). The overuse of herbicides and pesticides in agricultural production activities has caused severe pollution of the Infranz wetlands in north-west Ethiopia, adversely affecting their biodiversity (Eneyew and Assefa, 2021).
6 Future prospects
Freshwater wetlands with high biodiversity play an extremely important role in maintaining the functional stability of wetland habitats. Many environmental drivers such as water level, water quality, soil properties, temperature, and biological drivers (e.g., plant/microbial diversity) have important impacts on the functional stability of freshwater wetland ecosystems, but many in-depth studies are needed in the following aspects in the future:
1. Changes in biodiversity can directly or indirectly regulate ecosystem processes, and biodiversity is the main determinant of maintaining ecosystem functional stability. Therefore, it is of great significance to investigate the relationship between biodiversity and functional stability. Nowadays, most studies on the functional stability and biodiversity of freshwater wetland have focused on small-scale scales and homogeneous habitats, ignoring the effects of spatial and temporal scales and environmental heterogeneity. Therefore, the study on the multi-scale integration and relationship between biodiversity and functional stability at different scales is important. This will help maintain the stability of freshwater ecosystems and provide theoretical support for the conservation of FWs.
2. Many studies are about the response of habitat function to environmental and biological elements in the context of global change. Most studies agreed that high levels of biodiversity can better maintain the stability of habitat function. In addition, changes in environmental factors can indirectly affect ecosystem habitat function through biodiversity. Therefore, future research needs to focus on the mechanisms by which environmental and biological factors drive habitat function enhancement through community composition, species diversity, environmental heterogeneity and biological interactions.
7 Conclusion
Freshwater wetlands are one of the most biodiverse ecosystems, and abundant species has a significant impact on the habitat function of FWs. Many environmental factors are changing under global change and human activities, and these changes can either directly affect the stability of wetland habitat functions or indirectly affect habitat functions by altering the biodiversity of FWs. Our study analyzes the roles of environmental drivers maintaining the stability of wetland habitat functions, such as hydrology, temperature, and water quality, discusses the impacts of plant and microbial diversity on the functional stability of FWs, and further reveals the impacts and mechanisms of habitat changes on biodiversity. In general, biodiversity can promote the stability of habitat functions in FWs. However, most studies focus on small-scale scales and homogeneous habitats. Therefore, future studies on biodiversity and stability of habitat functions in FWs at large scales and non-homogeneous habitats still need to be further explored.
Author contributions
AS: Writing – original draft. SL: Data curation, Software, Validation, Writing – review & editing. HL: Conceptualization, Project administration, Supervision, Writing – review & editing. BY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Key R&D Program of China (no. 2022YFF1300901), National Natural Science Foundation of China (no. 42077353), and Natural Science Foundation of Jilin Province (no. 20230101100JC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, H. N., Xie, J., Vymazal, J., and Lu, X. (2018). Kinetics of nutrient uptake by economical vegetable species grown in constructed wetlands. J. Anim. Plant Sci. 28:726.
Adhikari, A. R., Acharya, K., Shanahan, S. A., and Zhou, X. (2011). Removal of nutrients and metals by constructed and naturally created wetlands in the Las Vegas Valley, Nevada. Environ. Monit. Assess. 180, 97–113. doi: 10.1007/s10661-010-1775-y
Adhikari, S., Bajracharaya, R. M., and Sitaula, B. K. (2009). A review of carbon dynamics and sequestration in wetlands. J. Wetl. Ecol. 2, 42–46. doi: 10.3126/jowe.v2i1.1855
Al-Obaid, S., Samraoui, B., Thomas, J., El-Serehy, H. A., Alfarhan, A. H., Schneider, W., et al. (2017). An overview of wetlands of Saudi Arabia: values, threats, and perspectives. Ambio 46, 98–108. doi: 10.1007/s13280-016-0807-4
Araújo, M. S., Langerhans, R. B., Giery, S. T., and Layman, C. A. (2014). Ecosystem fragmentation drives increased diet variation in an endemic livebearing fish of the Bahamas. Ecol. Evol. 4, 3298–3308. doi: 10.1002/ece3.1140
Bae, H.-S., Morrison, E., Chanton, J. P., and Ogram, A. (2018). Methanogens are major contributors to nitrogen fixation in soils of the Florida Everglades. Appl. Environ. Microbiol. 84, e02222–e02217. doi: 10.1128/AEM.02222-17
Bano, H., Rather, R. A., Malik, S., Bhat, M. A., Khan, A. H., Americo-Pinheiro, J. H. P., et al. (2022). Effect of seasonal variation on pollution load of water of Hokersar wetland: a case study of queen wetland of Kashmir, J & K, India. Water Air Soil Pollut. 233, 1–25. doi: 10.1007/s11270-022-05988-w
Bao, T., Jia, G., and Xu, X. (2023). Weakening greenhouse gas sink of pristine wetlands under warming. Nat. Clim. Chang. 13, 462–469. doi: 10.1038/s41558-023-01637-0
Bardgett, R. D., Freeman, C., and Ostle, N. J. (2008). Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2, 805–814. doi: 10.1038/ismej.2008.58
Berrier, D. J., Neubauer, S. C., and Franklin, R. B. (2022). Cooperative microbial interactions mediate community biogeochemical responses to saltwater intrusion in wetland soils. FEMS Microbiol. Ecol. 98, 1–12. doi: 10.1093/femsec/fiac019
Bonetti, G., Trevathan-Tackett, S. M., Carnell, P. E., Treby, S., and Macreadie, P. I. (2021). Local vegetation and hydroperiod influence spatial and temporal patterns of carbon and microbe response to wetland rehabilitation. Appl. Soil Ecol. 163:103917. doi: 10.1016/j.apsoil.2021.103917
Brisson, J., Rodriguez, M., Martin, C. A., and Proulx, R. (2020). Plant diversity effect on water quality in wetlands: a meta-analysis based on experimental systems. Ecol. Appl. 30:e02074. doi: 10.1002/eap.2074
Brunhoferova, H., Venditti, S., Laczny, C. C., Lebrun, L., and Hansen, J. (2022). Bioremediation of 27 micropollutants by symbiotic microorganisms of wetland Macrophytes. Sustain. For. 14:3944. doi: 10.3390/su14073944
Cao, Q., Wang, H., Chen, X., Wang, R., and Liu, J. (2017). Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecol. Eng. 98, 40–48. doi: 10.1016/j.ecoleng.2016.10.063
Cardinale, B. J., Matulich, K. L., Hooper, D. U., Byrnes, J. E., Duffy, E., Gamfeldt, L., et al. (2011). The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. doi: 10.3732/ajb.1000364
Chaturvedi, R. K., and Raghubanshi, A. S. (2015). Assessment of carbon density and accumulation in mono-and multi-specific stands in teak and Sal forests of a tropical dry region in India. For. Ecol. Manag. 339, 11–21. doi: 10.1016/j.foreco.2014.12.002
Chen, J., Li, X., Jia, W., Shen, S., Deng, S., Ji, B., et al. (2021). Promotion of bioremediation performance in constructed wetland microcosms for acid mine drainage treatment by using organic substrates and supplementing domestic wastewater and plant litter broth. J. Hazard. Mater. 404:124125. doi: 10.1016/j.jhazmat.2020.124125
Chen, X., Sheng, Y., Wang, G., Zhou, P., Liao, F., Mao, H., et al. (2024). Spatiotemporal successions of N, S, C, Fe, and as cycling genes in groundwater of a wetland ecosystem: enhanced heterogeneity in wet season. Water Res. 251:121105. doi: 10.1016/j.watres.2024.121105
Chen, X., Wang, G., Sheng, Y., Liao, F., Mao, H., Li, B., et al. (2023). Nitrogen species and microbial community coevolution along groundwater flowpath in the southwest of Poyang Lake area, China. Chemosphere 329:138627. doi: 10.1016/j.chemosphere.2023.138627
Chen, H., Wang, H., Wu, M., Yu, G., Chen, J., and Liu, D. (2020). Recent advances in microbe-driven nitrogen transformation in freshwater wetland ecosystems. J. Hydraul. Eng. 51, 158–168. doi: 10.13243/j.cnki.slxb.20190592
Chen, M., Zeng, S., Jiang, B., Wen, Z., Wu, J., and Xia, J. (2023). The comprehensive evaluation of how water level fluctuation and temperature change affect vegetation cover variations at a Lake of ecological importance (Poyang Lake), China. Ecol. Indic. 148:110041. doi: 10.1016/j.ecolind.2023.110041
Chen, H., Zhu, Q., Peng, C., Wu, N., Wang, Y., Fang, X., et al. (2013). The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan plateau. Glob. Chang. Biol. 19, 2940–2955. doi: 10.1111/gcb.12277
Choi, J.-Y., Jeong, K.-S., La, G.-H., and Joo, G.-J. (2014). Effect of removal of free-floating macrophytes on zooplankton habitat in shallow wetland. Knowl. Manag. Aquat. Ecosyst. 414:11. doi: 10.1051/kmae/2014023
Choudhury, M. I., McKie, B. G., Hallin, S., and Ecke, F. (2018). Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Sci. Total Environ. 635, 1436–1443. doi: 10.1016/j.scitotenv.2018.04.193
Davidson, N. (2014). How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 65, 934–941. doi: 10.1071/MF14173
De Mandal, S., Laskar, F., Panda, A. K., and Mishra, R. (2020). Chapter 12—Microbial Diversity and Functional Potential in Wetland Ecosystems, Recent Advancements in Microbial Diversity Academic Press, 289–314.
Dean, J. F., Middelburg, J. J., Röckmann, T., Aerts, R., Blauw, L. G., Egger, M., et al. (2018). Methane feedbacks to the global climate system in a warmer world. Rev. Geophys. 56, 207–250. doi: 10.1002/2017RG000559
Deng, J., Xiao, T., Fan, W., Ning, Z., and Xiao, E. (2022). Relevance of the microbial community to Sb and as biogeochemical cycling in natural wetlands. Sci. Total Environ. 818:151826. doi: 10.1016/j.scitotenv.2021.151826
DeVries-Zimmerman, S. J., Yurk, B., Fast, K. M., Donaldson, A., and Hansen, E. C. (2021). Waxing and waning slacks: the changing ecohydrology of interdunal wetlands/slacks in a Lake Michigan coastal dune complex during rising Lake Michigan-Huron levels. J. Great Lakes Res. 47, 1565–1580. doi: 10.1016/j.jglr.2021.09.001
Ding, Y. D., Song, C. C., Chen, G. J., Zhang, X. H., and Mao, R. (2021). Effects of long-term nitrogen addition on dissolved organic matter characteristics in a temperate wetland of Northeast China. Ecotoxicol. Environ. Saf. 226:112822. doi: 10.1016/j.ecoenv.2021.112822
Donato, M., Johnson, O., Steven, B., and Lawrence, B. A. (2020). Nitrogen enrichment stimulates wetland plant responses whereas salt amendments alter sediment microbial communities and biogeochemical responses. PLoS One 15:e0235225. doi: 10.1371/journal.pone.0235225
Eneyew, B. G., and Assefa, W. W. (2021). Anthropogenic effect on wetland biodiversity in Lake Tana region: a case of Infranz wetland, Northwestern Ethiopia. Environ. Sustain. Indicators 12:100158. doi: 10.1016/j.indic.2021.100158
Epele, L. B., Grech, M. G., Williams-Subiza, E. A., Stenert, C., McLean, K., Greig, H. S., et al. (2022). Perils of life on the edge: climatic threats to global diversity patterns of wetland macroinvertebrates. Sci. Total Environ. 820:153052. doi: 10.1016/j.scitotenv.2022.153052
Fang, J., Wang, Z., Zhao, S., Li, Y., Tang, Z., Yu, D., et al. (2006). Biodiversity changes in the lakes of the Central Yangtze. Front. Ecol. Environ. 4, 369–377. doi: 10.1890/1540-9295(2006)004[0369,BCITLO]2.0.CO;2
Gimmi, U., Lachat, T., and Bürgi, M. (2011). Reconstructing the collapse of wetland networks in the Swiss lowlands 1850–2000. Landsc. Ecol. 26, 1071–1083. doi: 10.1007/s10980-011-9633-z
Gutierrez, M. F., Epele, L. B., Mayora, G., Aquino, D., Mora, C., Quintana, R., et al. (2022). Hydro-climatic changes promote shifts in zooplankton composition and diversity in wetlands of the lower Paraná River Delta. Hydrobiologia 849, 3463–3480. doi: 10.1007/s10750-022-04955-0
Hamilton, S. K. (2010). Biogeochemical implications of climate change for tropical rivers and floodplains. Hydrobiologia 657, 19–35. doi: 10.1007/s10750-009-0086-1
Hatton, I. A., Mazzarisi, O., Altieri, A., and Smerlak, M. (2024). Diversity begets stability: sublinear growth and competitive coexistence across ecosystems. Science 383:eadg8488. doi: 10.1126/science.adg8488
Herbert, E. R., Boon, P., Burgin, A. J., Neubauer, S. C., Franklin, R. B., Ardon, M., et al. (2015). A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 1–43. doi: 10.1890/es14-00534.1
Herbert, E. R., Schubauer-Berigan, J. P., and Craft, C. B. (2020). Effects of 10 yr of nitrogen and phosphorus fertilization on carbon and nutrient cycling in a tidal freshwater marsh. Limnol. Oceanogr. 65, 1669–1687. doi: 10.1002/lno.11411
Holmes, M. E., Chanton, J. P., Bae, H.-S., and Ogram, A. (2014). Effect of nutrient enrichment on δ13CH4 and the methane production pathway in the Florida Everglades. J. Geophys. Res. Biogeosci. 119, 1267–1280. doi: 10.1002/jgrg.20122
Horn Marcus, A., Matthies, C., Küsel, K., Schramm, A., and Drake Harold, L. (2003). Hydrogenotrophic Methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat. Appl. Environ. Microbiol. 69, 74–83. doi: 10.1128/AEM.69.1.74-83.2003
Hu, Q., Zheng, P., and Kang, D. (2017). Taxonomy, characteristics, and biotechniques used for the analysis of anaerobic ammonium oxidation bacteria. Chin. J. Appl. Environ. Biol. 23, 384–391. doi: 10.3724/SP.J.1145.2016.04022
Hussain, S., Chen, M., Liu, Y., Mustafa, G., Wang, X., Liu, J., et al. (2023). Composition and assembly mechanisms of prokaryotic communities in wetlands, and their relationships with different vegetation and reclamation methods. Sci. Total Environ. 897:166190. doi: 10.1016/j.scitotenv.2023.166190
Isbell, F., Reich, P. B., Tilman, D., Hobbie, S. E., Polasky, S., and Binder, S. (2013). Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl. Acad. Sci. 110, 11911–11916. doi: 10.1073/pnas.1310880110
Jamin, A., Peintinger, M., Gimmi, U., Holderegger, R., and Bergamini, A. (2020). Evidence for a possible extinction debt in Swiss wetland specialist plants. Ecol. Evol. 10, 1264–1277. doi: 10.1002/ece3.5980
Jolly, I. D., McEwan, K. L., and Holland, K. L. (2008). A review of groundwater-surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1, 43–58. doi: 10.1002/eco.6
Karim, F., Dutta, D., Marvanek, S., Petheram, C., Ticehurst, C., Lerat, J., et al. (2015). Assessing the impacts of climate change and dams on floodplain inundation and wetland connectivity in the wet–dry tropics of northern Australia. J. Hydrol. 522, 80–94. doi: 10.1016/j.jhydrol.2014.12.005
Keddy, P. A., Fraser, L. H., Solomeshch, A. I., Junk, W. J., Campbell, D. R., Arroyo, M. T. K., et al. (2009). Wet and wonderful: the world's largest wetlands are conservation priorities. Bioscience 59, 39–51. doi: 10.1525/bio.2009.59.1.8
Khan, F. A., and Ansari, A. A. (2005). Eutrophication: An ecological vision. Bot. Rev. 71, 449–482. doi: 10.1663/0006-8101(2005)071[0449:Eaev]2.0.Co;2
Lai, D. Y. F. (2010). Biogeochemistry of wetlands: science and applications. Ecol. Eng. 36, 607–608. doi: 10.1016/j.ecoleng.2010.01.001
Li, X., Hou, L., Liu, M., Lin, X., Li, Y., and Li, S. (2015). Primary effects of extracellular enzyme activity and microbial community on carbon and nitrogen mineralization in estuarine and tidal wetlands. Appl. Microbiol. Biotechnol. 99, 2895–2909. doi: 10.1007/s00253-014-6187-4
Li, C., Li, X., Yang, Y., Shi, Y., and Li, H. (2022). Degradation reduces the diversity of nitrogen-fixing bacteria in the alpine wetland on the Qinghai-Tibet plateau. Front. Plant Sci. 13:939762. doi: 10.3389/fpls.2022.939762
Li, H., Liang, S., Chi, Z., Wu, H., and Yan, B. (2022). Unveiling microbial community and function involved in anammox in paddy vadose under groundwater irrigation. Sci. Total Environ. 849:157876. doi: 10.1016/j.scitotenv.2022.157876
Li, Y., Shi, K. Y., Yuan, J., and Kuang, Q. Y. (2021). Evaluation of heavy metal pollutants from plateau mines in wetland surface deposits. Front. Environ. Sci. 8:557302. doi: 10.3389/fenvs.2020.557302
Li, H., Song, A., and Chi, Z. (2024). Deep groundwater irrigation altered microbial community and increased anammox and methane oxidation in paddy wetlands of Sanjiang plain, China. Front. Microbiol. 15:1354279. doi: 10.3389/fmicb.2024.1354279
Liang, S., Li, H., and Wu, H. (2023). Microorganisms in coastal wetland sediments: a review on microbial community structure, functional gene, and environmental potential. Front. Microbiol. 14:1163896. doi: 10.3389/fmicb.2023.1163896
Lin, Y., Liu, D., Ding, W., Kang, H., Freeman, C., Yuan, J., et al. (2015). Substrate sources regulate spatial variation of metabolically active methanogens from two contrasting freshwater wetlands. Appl. Microbiol. Biotechnol. 99, 10779–10791. doi: 10.1007/s00253-015-6912-7
Lindborg, R., Ermold, M., Kuglerová, L., Jansson, R., Larson, K. W., Milbau, A., et al. (2021). How does a wetland plant respond to increasing temperature along a latitudinal gradient? Ecol. Evol. 11, 16228–16238. doi: 10.1002/ece3.8303
Liping, S., Song, C., Zhang, X., Wang, X., and Luan, Z. (2020). Responses of above-ground biomass, plant diversity, and dominant species to habitat change in a freshwater wetland of Northeast China. Russ. J. Ecol. 51, 57–63. doi: 10.1134/S1067413620010051
Liu, H., Yang, G., Jia, H., and Yao, J. (2021). Impact of long-term cultivation with crude oil on wetland microbial community shifts and the hydrocarbon degradation potential. Energy Sour. A. Recov. Utilization Environ. Effects 1-13, 1–13. doi: 10.1080/15567036.2021.1896609
Luo, W. (2009). Growth and morphological responses to water level and nutrient supply in three emergent macrophyte species. Hydrobiologia 624, 151–160. doi: 10.1007/s10750-008-9689-1
Ma, Y., Li, J., Wu, J., Kong, Z., Feinstein, L. M., Ding, X., et al. (2018). Bacterial and fungal community composition and functional activity associated with Lake wetland water level gradients. Sci. Rep. 8:760. doi: 10.1038/s41598-018-19153-z
Maietta, C. E., Hondula, K. L., Jones, C. N., and Palmer, M. A. (2020a). Hydrological conditions influence soil and methane-cycling microbial populations in seasonally saturated wetlands. Front. Environ. Sci. 8:593942. doi: 10.3389/fenvs.2020.593942
Maietta, C. E., Monsaint-Queeney, V., Wood, L., Baldwin, A. H., and Yarwood, S. A. (2020b). Plant litter amendments in restored wetland soils altered microbial communities more than clay additions. Soil Biol. Biochem. 147:107846. doi: 10.1016/j.soilbio.2020.107846
Mazor, T., Doropoulos, C., Schwarzmueller, F., Gladish, D. W., Kumaran, N., Merkel, K., et al. (2018). Global mismatch of policy and research on drivers of biodiversity loss. Nat. Ecol. Evol. 2, 1071–1074. doi: 10.1038/s41559-018-0563-x
McKown, J. G., Moore, G. E., Payne, A. R., White, N. A., and Gibson, J. L. (2021). Successional dynamics of a 35 year old freshwater mitigation wetland in southeastern New Hampshire. PLoS One 16:e0251748. doi: 10.1371/journal.pone.0251748
Means, M. M., Ahn, C., Korol, A. R., and Williams, L. D. (2016). Carbon storage potential by four macrophytes as affected by planting diversity in a created wetland. J. Environ. Manag. 165, 133–139. doi: 10.1016/j.jenvman.2015.09.016
Mellado, M., and Vera, J. (2021). Microorganisms that participate in biochemical cycles in wetlands. Can. J. Microbiol. 67, 771–788. doi: 10.1139/cjm-2020-0336
Morrissey, E. M., Berrier, D. J., Neubauer, S. C., and Franklin, R. B. (2014). Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 117, 473–490. doi: 10.1007/s10533-013-9894-5
Mu, S. J., Yang, G. S., Xu, X. B., Wan, R. R., and Li, B. (2022). Assessing the inundation dynamics and its impacts on habitat suitability in Poyang Lake based on integrating Landsat and MODIS observations. Sci. Total Environ. 834:154936. doi: 10.1016/j.scitotenv.2022.154936
Narrowe, A. B., Borton, M. A., Hoyt, D. W., Smith, G. J., Daly, R. A., Angle, J. C., et al. (2019). Uncovering the diversity and activity of methylotrophic methanogens in freshwater wetland soils. mSystems 4:e00320-19. doi: 10.1128/mSystems.00320-19
Ndehedehe, C. E., Burford, M. A., Stewart-Koster, B., and Bunn, S. E. (2020). Satellite-derived changes in floodplain productivity and freshwater habitats in northern Australia (1991–2019). Ecol. Indic. 114:106320. doi: 10.1016/j.ecolind.2020.106320
Nielsen, D. L., Jasper, E. W., Ning, N., and Lawler, S. (2015). High sediment temperatures influence the emergence of dormant aquatic biota. Mar. Freshw. Res. 66, 1138–1146. doi: 10.1071/MF14272
Ohba, S.-Y., Suzuki, K., Sakai, Y., Shibata, J.-Y., and Okuda, N. (2019). Effects of irrigation system alterations on the trophic position of a threatened top predator in rice-field ecosystems. Freshw. Biol. 64, 1737–1746. doi: 10.1111/fwb.13365
Ojdanič, N., Holcar, M., Golob, A., and Gaberščik, A. (2023). Environmental extremes affect productivity and habitus of common reed in intermittent wetland. Ecol. Eng. 189:106911. doi: 10.1016/j.ecoleng.2023.106911
Orem, W., Newman, S., Osborne, T. Z., and Reddy, K. R. (2015). Projecting changes in Everglades soil biogeochemistry for carbon and other key elements, to possible 2060 climate and hydrologic scenarios. Environ. Manag. 55, 776–798. doi: 10.1007/s00267-014-0381-0
Ou, Y., Rousseau, A. N., Wang, L., Yan, B., Gumiere, T., and Zhu, H. (2019). Identification of the alteration of riparian wetland on soil properties, enzyme activities and microbial communities following extreme flooding. Geoderma 337, 825–833. doi: 10.1016/j.geoderma.2018.10.032
Pang, S., Zhang, S., Lv, X., Han, B., Liu, K., Qiu, C., et al. (2016). Characterization of bacterial community in biofilm and sediments of wetlands dominated by aquatic macrophytes. Ecol. Eng. 97, 242–250. doi: 10.1016/j.ecoleng.2016.10.011
Peel, R., Hill, J., Taylor, G., and Weyl, O. (2019). Food web structure and trophic dynamics of a fish Community in an Ephemeral Floodplain Lake. Front. Environ. Sci. 7:192. doi: 10.3389/fenvs.2019.00192
Peter, C. R., and Burdick, D. M. (2010). Can plant competition and diversity reduce the growth and survival of exotic Phragmites australis invading a tidal marsh? Estuar. Coasts 33, 1225–1236. doi: 10.1007/s12237-010-9328-8
Prescott, C. E., and Vesterdal, L. (2021). Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 498:119522. doi: 10.1016/j.foreco.2021.119522
Qian, Z. (2019). Does species richness affect the growth and water quality of submerged macrophyte assemblages? Aquat. Bot. 153, 51–57. doi: 10.1016/j.aquabot.2018.11.006
Qian, X., Mao, S., Jiang, Y., Ye, C., Lu, B., Shan, N., et al. (2023). Research progress of wetland carbon cycle in China based on bibliometrics. J. Environ. Eng. Technol. 13, 742–752. doi: 10.12153/j.issn.1674-991X.20220029
Qiao, Z., Sheng, Y., Wang, G., Chen, X., Liao, F., Mao, H., et al. (2023). Deterministic factors modulating assembly of groundwater microbial community in a nitrogen-contaminated and hydraulically-connected river-lake-floodplain ecosystem. J. Environ. Manag. 347:119210. doi: 10.1016/j.jenvman.2023.119210
Rideout, N. K., Compson, Z. G., Monk, W. A., Bruce, M. R., Hajibabaei, M., Porter, T. M., et al. (2022). Environmental filtering of macroinvertebrate traits influences ecosystem functioning in a large river floodplain. Funct. Ecol. 36, 2791–2805. doi: 10.1111/1365-2435.14168
Schrauth, F. E., and Wink, M. (2018). Changes in species composition of birds and declining number of breeding territories over 40 years in a nature conservation area in Southwest Germany. Diversity 10:97. doi: 10.3390/d10030097
Shen, Y., Zheng, Y., Wang, X., Jia, C., and Zhao, M. (2018). Mechanism of different scales subsurface flow constructed wetlands for purifying polluted river water. Chin. J. Environ. Eng. 12, 1667–1675. doi: 10.12030/j.cjee.201711009
Sheng, Y., Baars, O., Guo, D., Whitham, J., Srivastava, S., and Dong, H. (2023). Mineral-bound trace metals as cofactors for anaerobic biological nitrogen fixation. Environ. Sci. Technol. 57, 7206–7216. doi: 10.1021/acs.est.3c01371
Sheng, Y., Dong, H., Kukkadapu, R. K., Ni, S., Zeng, Q., Hu, J., et al. (2021). Lignin-enhanced reduction of structural Fe (III) in nontronite: dual roles of lignin as electron shuttle and donor. Geochim. Cosmochim. Acta 307, 1–21. doi: 10.1016/j.gca.2021.05.037
Smith, I. M., Fiorino, G. E., Grabas, G. P., and Wilcox, D. A. (2021). Wetland vegetation response to record-high Lake Ontario water levels. J. Great Lakes Res. 47, 160–167. doi: 10.1016/j.jglr.2020.10.013
Söllinger, A., Schwab, C., Weinmaier, T., Loy, A., Tveit, A. T., Schleper, C., et al. (2016). Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol. Ecol. 92:fiv149. doi: 10.1093/femsec/fiv149
Song, G. (2017). What determines species diversity? Chin. Sci. Bull. 62, 2033–2041. doi: 10.1360/N972017-00125
Sremacki, M., Obrovski, B., Petrovic, M., Mihajlovic, I., Dragicevic, P., Radic, J., et al. (2020). Comprehensive environmental monitoring and assessment of protected wetland and lake water quality in Croatia and Serbia. Environ. Monit. Assess. 192:187. doi: 10.1007/s10661-020-8141-5
Steudel, B., Hautier, Y., Hector, A., and Kessler, M. (2011). Diverse marsh plant communities are more consistently productive across a range of different environmental conditions through functional complementarity. J. Appl. Ecol. 48, 1117–1124. doi: 10.1111/j.1365-2664.2011.01986.x
Stewart, A. J., Halabisky, M., Babcock, C., Butman, D. E., D’Amore, D. V., and Moskal, L. M. (2024). Revealing the hidden carbon in forested wetland soils. Nat. Commun. 15:726. doi: 10.1038/s41467-024-44888-x
Stottmeister, U., Wiessner, A., Kuschk, P., Kappelmeyer, U., Kästner, M., Bederski, O., et al. (2003). Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 22, 93–117. doi: 10.1016/j.biotechadv.2003.08.010
Sui, X., Zhang, R., Yang, L., Li, M., Xu, N., Liu, Y., et al. (2017). Differences in the microbial population associated with three wetland types in the Sanjiang plain, Northeast China. Appl. Ecol. Environ. Res. 15, 79–92. doi: 10.15666/aeer/1501_079092
Thomaz, S. M. (2023). Ecosystem services provided by freshwater macrophytes. Hydrobiologia 850, 2757–2777. doi: 10.1007/s10750-021-04739-y
Todd, M. J., Muneepeerakul, R., Pumo, D., Azaele, S., Miralles-Wilhelm, F., Rinaldo, A., et al. (2010). Hydrological drivers of wetland vegetation community distribution within Everglades National Park, Florida. Adv. Water Resour. 33, 1279–1289. doi: 10.1016/j.advwatres.2010.04.003
Wang, Y.-F., Dick, R. P., Lorenz, N., and Lee, N. (2019). Interactions and responses of n-damo archaea, n-damo bacteria and anammox bacteria to various electron acceptors in natural and constructed wetland sediments. Int. Biodeterior. Biodegradation 144:104749. doi: 10.1016/j.ibiod.2019.104749
Wang, J., Long, Y., Yu, G., Wang, G., Zhou, Z., Li, P., et al. (2022). A review on microorganisms in constructed wetlands for typical pollutant removal: species, function, and diversity. Front. Microbiol. 13:845725. doi: 10.3389/fmicb.2022.845725
Wang, Y., Tan, W., Li, B., Wen, L., and Lei, G. (2021). Habitat alteration facilitates the dominance of invasive species through disrupting niche partitioning in floodplain wetlands. Divers. Distrib. 27, 1861–1871. doi: 10.1111/ddi.13376
Wang, C., Zhao, H., and Wang, G. (2015). Vegetation development and water level changes in Shenjiadian peatland in Sanjiang plain, Northeast China. Chin. Geogr. Sci. 25, 451–461. doi: 10.1007/s11769-015-0768-8
Wei, J., Gao, J., Wang, N., Liu, Y., Wang, Y., Bai, Z., et al. (2019). Differences in soil microbial response to anthropogenic disturbances in Sanjiang and Momoge wetlands, China. FEMS Microbiol. Ecol. 95:fiz110. doi: 10.1093/femsec/fiz110
Weilhoefer, C. L., Williams, D., Nguyen, I., Jakstis, K., and Fischer, C. (2017). The effects of reed canary grass (Phalaris arundinacea L.) on wetland habitat and arthropod community composition in an urban freshwater wetland. Wetl. Ecol. Manag. 25, 159–175. doi: 10.1007/s11273-016-9507-x
Weisser, W. W., Roscher, C., Meyer, S. T., Ebeling, A., Luo, G., Allan, E., et al. (2017). Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: patterns, mechanisms, and open questions. Basic Appl. Ecol. 23, 1–73. doi: 10.1016/j.baae.2017.06.002
Weragoda, S. K., Jinadasa, K. B. S. N., Zhang, D. Q., Gersberg, R. M., Tan, S. K., Tanaka, N., et al. (2012). Tropical application of floating treatment wetlands. Wetlands 32, 955–961. doi: 10.1007/s13157-012-0333-5
Wu, P., Zhang, H., Cui, L., Wickings, K., Fu, S., and Wang, C. (2017). Impacts of alpine wetland degradation on the composition, diversity and trophic structure of soil nematodes on the Qinghai-Tibetan plateau. Sci. Rep. 7:837. doi: 10.1038/s41598-017-00805-5
Xi, L., Chen, S., Bian, H., Peng, Z., Niu, Y., and Li, Y. (2023). Organic carbon release from litter decomposition of woody and herbaceous plants in the Dongting Lake wetlands: a comparative study. Ecohydrol. Hydrobiol. 23, 408–419. doi: 10.1016/j.ecohyd.2023.06.003
Xiang, W., Xiao, X., and Xue, J. (2020). Purification effect and microorganisms diversity in an Acorus calamus constructed wetland on petroleum-containing wastewater. Environ. Pollut. Bioavailab. 32, 19–25. doi: 10.1080/26395940.2019.1711200
Xiu, L., Yan, C., Li, X., Qian, D., and Feng, K. (2019). Changes in wetlands and surrounding land cover in a desert area under the influences of human and climatic factors: a case study of the Hongjian Nur region. Ecol. Indic. 101, 261–273. doi: 10.1016/j.ecolind.2019.01.025
Xu, X., Xie, Y., Qi, K., Luo, Z., and Wang, X. (2018). Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Sci. Total Environ. 624, 1561–1576. doi: 10.1016/j.scitotenv.2017.12.143
Xue, Z., Lyu, X., Chen, Z., Zhang, Z., Jiang, M., Zhang, K., et al. (2018). Spatial and temporal changes of wetlands on the Qinghai-Tibetan plateau from the 1970s to 2010s. Chin. Geogr. Sci. 28, 935–945. doi: 10.1007/s11769-018-1003-1
Yan, J., Wang, L., Hu, Y., Tsang, Y. F., Zhang, Y., Wu, J., et al. (2018). Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 319, 194–203. doi: 10.1016/j.geoderma.2018.01.009
Yang, J. S., Liu, J. S., Hu, X. J., Li, X. X., Wang, Y., and Li, H. Y. (2013). Effect of water table level on CO2, CH4 and N2O emissions in a freshwater marsh of Northeast China. Soil Biol. Biochem. 61, 52–60. doi: 10.1016/j.soilbio.2013.02.009
Yao, J., Sánchez-Pérez, J. M., Sauvage, S., Teissier, S., Attard, E., Lauga, B., et al. (2017). Biodiversity and ecosystem purification service in an alluvial wetland. Ecol. Eng. 103, 359–371. doi: 10.1016/j.ecoleng.2016.02.019
You, H., Xu, L., Liu, G., Wang, X., Wu, Y., and Jiang, J. (2015). Effects of inter-annual water level fluctuations on vegetation evolution in typical wetlands of Poyang Lake, China. Wetlands 35, 931–943. doi: 10.1007/s13157-015-0684-9
Yu, Z., Jiang, M., and Chen, F. (2023). Wetland science, ecosystem services and protection actions in China. Fund. Res. 3, 831–832. doi: 10.1016/j.fmre.2023.09.001
Zedler, J. B., and Kercher, S. (2005). Wetland resources: status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 30, 39–74. doi: 10.1146/annurev.energy.30.050504.144248
Zhang, G., Tian, J., Jiang, N., Guo, X., Wang, Y., and Dong, X. (2008). Methanogen community in Zoige wetland of Tibetan plateau and phenotypic characterization of a dominant uncultured methanogen cluster ZC-I. Environ. Microbiol. 10, 1850–1860. doi: 10.1111/j.1462-2920.2008.01606.x
Zhang, C.-B., Wang, J., Liu, W.-L., Zhu, S.-X., Ge, H.-L., Chang, S. X., et al. (2010). Effects of plant diversity on microbial biomass and community metabolic profiles in a full-scale constructed wetland. Ecol. Eng. 36, 62–68. doi: 10.1016/j.ecoleng.2009.09.010
Zhang, Q., Wang, Z., Xia, S., Zhang, G., Li, S., Yu, D., et al. (2022). Hydrologic-induced concentrated soil nutrients and improved plant growth increased carbon storage in a floodplain wetland over wet-dry alternating zones. Sci. Total Environ. 822:153512. doi: 10.1016/j.scitotenv.2022.153512
Zhang, Y., Xu, H., Chen, H., Wang, F., and Huai, H. (2014). Diversity of wetland plants used traditionally in China: a literature review. J. Ethnobiol. Ethnomed. 10, 1–19. doi: 10.1186/1746-4269-10-72
Zhao, Q. Q., Bai, J. H., Jia, J., Zhang, G. L., Wang, J. N., and Gao, Y. C. (2022). The effects of drainage on the soil fungal Community in Freshwater Wetlands. Front. Ecol. Evol. 10:837747. doi: 10.3389/fevo.2022.837747
Zhao, X. S., and Liu, Y. B. (2016). Evapotranspiration partitioning and response to abnormally low water levels in a floodplain wetland in China. Adv. Meteorol. 2016, 1–11. doi: 10.1155/2016/3695427
Zou, Y., Wang, G., Grace, M., Lou, X., Yu, X., and Lu, X. (2014). Response of two dominant boreal freshwater wetland plants to manipulated warming and altered precipitation. PLoS One 9:e104454. doi: 10.1371/journal.pone.0104454
Keywords: biodiversity, habitat functional stability, freshwater wetlands, habitat change, impact mechanisms
Citation: Song A, Liang S, Li H and Yan B (2024) Effects of biodiversity on functional stability of freshwater wetlands: a systematic review. Front. Microbiol. 15:1397683. doi: 10.3389/fmicb.2024.1397683
Edited by:
Yizhi Sheng, China University of Geosciences, ChinaReviewed by:
Jia Meng, Harbin Institute of Technology, ChinaZhaorui Chu, Guangzhou University, China
Copyright © 2024 Song, Liang, Li and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huai Li, bGlodWFpQGlnYS5hYy5jbg==
 Aiwen Song1,2
Aiwen Song1,2 Huai Li
Huai Li