- 1Department of Agricultural, Food, and Nutritional Science, Faculty of Agricultural, Life, and Environmental Sciences, University of Alberta, Edmonton, AB, Canada
- 2Department of Large Animal Clinical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK, Canada
- 3HEAT-AMR (Human-Environment-Animal Transdisciplinary AMR) Research Group, School of Public Health, University of Alberta, Edmonton, AB, Canada
- 4Healthy Environments Thematic Area Lead, Centre for Healthy Communities, School of Public Health, University of Alberta, Edmonton, AB, Canada
- 5Department of Animal and Poultry Science, College of Agriculture and Bioresources, University of Saskatchewan, Saskatoon, SK, Canada
- 6Department of Computer Science, College of Arts and Science, University of Saskatchewan, Saskatoon, SK, Canada
Introduction: Bovine respiratory disease (BRD) is one of the most important animal health problems in the beef industry. While bacterial culture and antimicrobial susceptibility testing have been used for diagnostic testing, the common practice of examining one isolate per species does not fully reflect the bacterial population in the sample. In contrast, a recent study with metagenomic sequencing of nasal swabs from feedlot cattle is promising in terms of bacterial pathogen identification and detection of antimicrobial resistance genes (ARGs). However, the sensitivity of metagenomic sequencing was impeded by the high proportion of host biomass in the nasal swab samples.
Methods: This pilot study employed a non-selective bacterial enrichment step before nucleic acid extraction to increase the relative proportion of bacterial DNA for sequencing.
Results: Non-selective bacterial enrichment increased the proportion of bacteria relative to host sequence data, allowing increased detection of BRD pathogens compared with unenriched samples. This process also allowed for enhanced detection of ARGs with species-level resolution, including detection of ARGs for bacterial species of interest that were not targeted for culture and susceptibility testing. The long-read sequencing approach enabled ARG detection on individual bacterial reads without the need for assembly. Metagenomics following non-selective bacterial enrichment resulted in substantial agreement for four of six comparisons with culture for respiratory bacteria and substantial or better correlation with qPCR. Comparison between isolate susceptibility results and detection of ARGs was best for macrolide ARGs in Mannheimia haemolytica reads but was also substantial for sulfonamide ARGs within M. haemolytica and Pasteurella multocida reads and tetracycline ARGs in Histophilus somni reads.
Discussion: By increasing the proportion of bacterial DNA relative to host DNA through non-selective enrichment, we demonstrated a corresponding increase in the proportion of sequencing data identifying BRD-associated pathogens and ARGs in deep nasopharyngeal swabs from feedlot cattle using long-read metagenomic sequencing. This method shows promise as a detection strategy for BRD pathogens and ARGs and strikes a balance between processing time, input costs, and generation of on-target data. This approach could serve as a valuable tool to inform antimicrobial management for BRD and support antimicrobial stewardship.
1 Introduction
Bovine respiratory disease (BRD) is an important cause of morbidity and mortality and is responsible for most of the injectable antimicrobial use in feedlot cattle in western Canada (Brault et al., 2019). Respiratory disease is complex and multifactorial, often involving a combination of bacterial and viral pathogens exacerbated in animals stressed by weaning, transportation, and comingling (Griffin et al., 2010). Antimicrobial use (AMU) is necessary for managing the impacts of BRD on animal health and welfare. Recent WHO guidelines recommended antimicrobial prescriptions for livestock on diagnostic test data (Aidara-Kane et al., 2018).
Laboratory diagnostics to inform AMU for BRD have traditionally relied on bacterial culture, with or without antimicrobial susceptibility testing (AST). Culture-based diagnostic strategies can take up to a week to provide actionable information and, therefore, have limited utility to guide rapid therapeutic decisions that are critical for ensuring BRD treatment success (Wolfger et al., 2015). Typically, culture-based methods test susceptibility of a single isolate per sample and might not represent the susceptibility status of the population of all bacteria of interest within the sample. Additionally, multi-drug-resistant strains of BRD pathogens have emerged (Michael et al., 2012; Lubbers and Hanzlicek, 2013; Klima et al., 2016, 2020; Rainbolt et al., 2016; Snyder et al., 2017), highlighting the importance of identifying bacterial BRD pathogens and characterizing antimicrobial resistance (AMR) to inform prudent AMU.
Molecular methods have been used in veterinary diagnostic testing for decades and provide comparatively faster and potentially more sensitive results than traditional culture (Loy, 2020). Quantitative polymerase chain reaction (qPCR) is widely used in BRD diagnostic testing in the form of commercially available kits (Pneumo4, DNA Diagnostic A/S, Risskov, Denmark) and has been used for detection and quantitation of antimicrobial resistance genes (ARG) in the nasopharyngeal microbiota of Canadian feedlot cattle (Holman et al., 2018; Guo et al., 2020). While qPCR can be superior to classical culture-based methods in terms of turnaround time, it is inherently limited in scope to the known assay targets for which primers have been developed and tested. Typically, multiple reactions are required to test for the presence of multiple pathogens and ARGs.
In contrast, whole genome sequencing (WGS) produces high resolution genomic information for outbreak and AMR surveillance and management (Harrison et al., 2013; Besser et al., 2019; Delgado-Suárez et al., 2021). However, WGS requires culture, isolation, and nucleic acid extraction prior to sequencing.
A shotgun metagenomic sequencing approach combines the rapidness of using DNA extracted directly from clinical samples with a broad, untargeted view of all genetic information in a sample. Metagenomics has the potential to find multiple pathogens and ARGs in a single sequencing run without the need for pathogen isolation or specifying a known genetic target (Adewusi et al., 2024).
Previous proof-of-concept work using third generation Oxford Nanopore Technology (ONT) for long-read metagenomic sequencing of nasal swabs collected from feedlot cattle has shown multiple advantages compared with traditional laboratory methods (Freeman et al., 2022). Bacterial BRD pathogens, including the difficult-to-culture Mycoplasma bovis, were reliably identified. Not only did long-read metagenomic sequencing detect BRD pathogens more frequently than bacterial culture but it was also faster and produced some information about the presence of ARGs in the sample. However, concordance between ARGs detected by long-read metagenomic sequencing and phenotypic resistance detected by AST was limited likely due to relatively low sequence coverage of target BRD organisms and excess of bovine-derived host sequence, even with extensive host-depletion. Among 25 samples, the average proportion of non-host-derived sequence was 6%. An excessive host to non-host ratio in sequencing output reduced the ability to characterize the sample for the presence of pathogen and ARG. Analyses with short-read sequencing have shown that high ratios of host to non-host data reduced the sensitivity of microbiome and resistome profiling (Zaheer et al., 2018; Pereira-Marques et al., 2019).
Developing a reliable and cost-effective detection strategy for BRD pathogens and ARGs based on long-read metagenomic sequencing of samples requires more bacterial sequencing coverage than was previously achieved. This additional coverage could be facilitated by increasing the relative amount of non-host to host in the sequenced DNA. Our objective was to evaluate the use of a low-cost non-selective bacterial enrichment of bovine nasopharyngeal swabs as a means of increasing the proportion of target species of interest relative to host biomass. In this study we demonstrate that bacterial enrichment enhances the detection of BRD pathogens (Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni) and species-associated ARGs, in addition to allowing us to detect M. bovis and Bibersteinia trehalosi. Our approach of using non-selective enrichment increased the proportion of sequenced on-target DNA, resulting in the reliability and utility of long-read metagenomic sequencing of nasal swab samples for diagnostics and surveillance. Additionally, using previously collected frozen swabs provided an opportunity to evaluate the potential of this method to assess archived samples.
2 Methods
2.1 Sample selection
We tested frozen swab heads from deep nasopharyngeal (DNP) swabs collected from 20 beef calves stored at −80°C as part of a larger sample collection. The samples used in this experiment were selected for a range of culture and phenotypic AST outcomes. Overall, 10 of the 20 samples were collected in 2020 from a single pen of calves sampled at 6 days on feed (DOF). Six samples were collected from calves sampled at 13 DOF from other pens from the same study, where calves arrived from October to early December 2020. Four samples were collected in 2021 from different early-, mid-, and late-filled feedlot pens (Supplementary Table S1A). The research protocols and procedures for this study were approved by the University of Saskatchewan Animal Care Committee (AUP 20190069).
2.2 Animals and sample collection
Samples were collected at a research feedlot operated by the University of Saskatchewan from 1,600 recently weaned mixed-breed steers purchased from a western Canadian auction market in the fall of 2020 and 2021. Calves were restrained in a hydraulic chute with a neck-extender, and three DNP swabs were collected from each calf from alternating nostrils (collection protocol details in Supplementary material). All three DNP swabs per calf were pooled in the same vial.
2.3 Initial sample processing
Samples were transported to the University of Saskatchewan for processing. The pooled samples (three swabs from each calf) were vortexed for 1 min to release biomass from the swab to the transport medium, and an aliquot was submitted to Prairie Diagnostic Services, Inc. (PDS; Saskatoon, Saskatchewan, Canada) for culture and AST.
2.4 Culture and antimicrobial susceptibility testing
In total, 10 μL inoculation loop of sample was cultured on 5% Columbia sheep blood (BA) and chocolate agar (CHOC) plates and incubated at 35°C for 18 h in an environment of 5% CO2 to isolate M. haemolytica, P. multocida, and H. somni. Bacterial colonies were incubated for 18 h and 42 h. Isolates of interest were identified using the MALDI-TOF MS Microflex LT instrument (Bruker Daltonik, Bremen, Germany) and the MALDI-TOF MS Biotyper Microflex LT Compass version 1.4 software with MSP library, according to the manufacturer’s guidelines. Isolate abundance was scored using a semi-quantitative scale (trace, 1+ to 4+) based on counts of visible colonies following streaking plates by quadrants (identification and quality control details in Supplementary material).
Susceptibility was measured by a commercially available serial broth microdilution panel using the Sensititre™ platform and the commercially available bovine BOPO7F Plate (ThermoFisher Scientific™, Thermo Fisher Scientific, Waltham, Massachusetts, United States), following the manufacturer’s instructions and recommendations for testing and quality control (antimicrobials and concentration ranges in Supplementary material). E. coli ATCC 25922, Staphylococcus aureus ATCC 29213, and H. somni ATCC 700025 were used to evaluate the performance. The minimum inhibitory concentration (MIC) plate was placed and read on the BIOMIC® V3 microplate reader. The MIC value was considered equal to the lowest concentration of antimicrobial that inhibited visible growth.
The MICs were compared against breakpoints designated by the Clinical and Laboratory Standards Institute (CLSI) for the pathogens of interest. Isolates with MIC values considered intermediate were categorized as “susceptible” for all analyses (CLSI, 2023).
2.5 Sample processing for molecular and genomic testing
For samples collected in 2020, 2 mL of the remaining transport medium was centrifuged at 4,000 × g for 10 min to pellet biomass. Then, 900 μL of supernatant was decanted, and the pellet was resuspended in the remaining transport medium and host-depleted using the HostZERO™ Microbial DNA Kit (Zymo Research, Irvine, California, United States), according to the manufacturer’s recommended protocol and extracted using the QIAGEN DNEasy Blood and Tissue Vacuum Kit (QIAGEN, Hilden, Germany). The dry, swab heads were then stored in cryovials at −80°C without media or cryoprotectant.
Dry swab heads for all samples/years were removed from −80°C for enrichment. The swab head was thawed briefly at room temperature and placed aseptically into sterile glass vials with 14 mL of BHI medium containing 1% glucose and a stir bar. Glass vials were sealed with air permeable Air ‘o Top membranes (Thomson Instrument Company, Oceanside, California, United States). Cultures were grown with vigorous aeration at 35°C for 22 h. During this period, 1 mL of each sample was retrieved at 0 h and 8 h and, subsequently, every 2 h until the sample had reached the stationary phase of growth. Stationary phase was determined by optical density (OD) that indicated culture saturation at three consecutive timepoints. Each 1 mL sample was pelleted at 4,000 × g for 10 min, and the medium was discarded. Bacterial pellets were stored at −20°C overnight. Nucleic acid extraction of the resulting enriched bacterial pellets was performed using the Gentra Puregene Buccal Cell Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions.
DNA concentration was determined using Qubit™ 1x dsDNA Broad Range (BR) or High Sensitivity (HS) Assay Kits (Invitrogen, Carlsbad, California, United States), according to the manufacturer’s specifications. The extracted DNA was stored at 4°C for <1 month in TE-buffer until library preparation and qPCR.
2.6 qPCR
To identify M. haemolytica, P. multocida, and H. somni, qPCR was performed in triplicate on the Aria MX (Agilent Technologies, Santa Clara, California, United States) using Taqman™ Fast Advanced MasterMix (Invitrogen, Carlsbad, California, United States). All samples were normalized to 10 ng/μl for amplification and compared against a standard curve. Total microbial load was quantified by targeting the bacterial 16S rRNA gene (Nadkarni et al., 2002), and the abundance of organisms of interest was quantified by targeting species-specific marker genes (Kishimoto et al., 2017). The results from qPCR were examined to identify time point(s) with the highest concentrations among all BRD organisms of interest for comparative testing after the stationary phase of growth was attained.
2.7 Library preparation and sequencing
Two candidate optimal enrichment timepoints were identified based on qPCR results for M. haemolytica, P. multocida, and H. somni. DNA from the optimal timepoints and an aliquot of DNA that was extracted without enrichment were selected for further testing. Sequencing libraries were prepared using the ONT ligation kit SQK-LSK109 and native barcoding kit (EXP-NBD104 and EXP-NBD114), according to the manufacturer’s instructions, with the following reductions to reaction volumes: repair and end prep reactions were scaled to 15 μL, and barcode ligation was scaled to 20 μL. Eight barcoded samples were normalized and pooled into each library, resulting in seven sequencing runs, each run containing eight samples. Sequencing libraries were quantified with the Qubit™ 1x High Sensitivity (HS) Assay Kit (Invitrogen, Carlsbad, California, United States). Overall, 200 ng of each prepared library was loaded onto an FLO-MIN106 flow cell and sequenced on an ONT GridION device for 72 h.
2.8 Bioinformatic analysis
ONT GridION default run parameters and high-accuracy Guppy (v4.0) real-time base calling were used to process raw signal data and remove reads with an average quality < Q7, after which terminal and internal adapters in split reads were removed with Porechop v0.2.4 (Wick et al., 2017). Reads shorter than 100 bp were removed using NanoFilt v2.6.0, and sequence statistics were calculated using NanoStat v1.5.0 (De Coster et al., 2018).
Kraken2 v2.0.8-beta (Wood et al., 2019) was used to classify host and non-host reads with a custom database. The database included all complete genomes in NCBI RefSeq for the bacterial, viral, and archaeal domains on 17 October 2020, as well as the Bos taurus reference genome assembly ARS-UCD1.2_Btau5.0.1Y, which consists of the ARS-UCD1.2 genome assembly (Elsik et al., 2009; Rosen et al., 2020). Following classification, reads were divided into two groups using the KrakenTools v1.0 utility extract_kraken_reads.py: those assigned to the B. taurus genome were placed into the “host” dataset and those were classified as any other taxa or were unclassified and placed into the “non-host” dataset. Chimeric reads were retrieved from the host dataset as described by Freeman et al. (2022). Taxonomic abundance estimates for organisms of interest were computed by Bracken v2.5 (Lu et al., 2017) from the “non-host” dataset after removing host-like sequences using the KrakenTools script filter_bracken_out.py. The number of reads and total base pairs were reported for BRD bacteria, such as M. haemolytica, P. multocida, H. somni, M. bovis, and B. trehalosi.
Antimicrobial resistance genes were identified in non-host reads using ABRicate v.1.0.1 (Seemann, n.d.) and AMRFinderPlus v3.9.8 (Feldgarden et al., 2019), both with the NCBI Bacterial Antimicrobial Resistance Reference Gene Database (PRJNA313047, version 2020-12-17). ABRicate was also run using the Comprehensive Antimicrobial Resistance Database (CARD) (Alcock et al., 2019) and MEGARes 2.0 database (Doster et al., 2019). For AMRFinderPlus, the minimum percent identity and percent coverage thresholds were set to 60%, as it is more stringent in reporting ARGs, and the -plus option was used to direct the program to search for genes involved in virulence, biocide, heat, metal, and acid resistance. Default parameters were used for ABRicate (80% minimum percent identity and percent coverage).
Theoretical genome coverage was calculated as the sum of the lengths of reads classified as a particular BRD pathogen divided by the size of its reference genome (M. haemolytica: 2.8 Mb [NCBI GCF_002285575.1], P. multocida: 2.3 Mb [NCBI GCF_002073255.2], H. somni: 2.2 Mb [NCBI GCF_000019405.1], M. bovis: 0.9 Mb [NCBI GCF_001930225.1], and B. trehalosi: 2.3 Mb [NCBI GCF_000521725.1]).
2.9 Statistical analysis
Sequence statistics (number of reads, total base pairs, and theoretical genome coverage) and qPCR results for organism detection were summarized as medians. qPCR copy numbers and sequence statistics were compared between culture-positive and culture-negative samples for no enrichment and 10h and 14h enrichment using the Wilcoxon rank sum test for each organism (StataSE ver 18.0, StataCorp, College Station, TX). The Wilcoxon signed-rank test was used to compare the total base pairs detected for each organism among matched pairs of samples that were unenriched to samples enriched for 10h and 14h. The number of total base pairs was also compared with qPCR concentration for the 10h and 14h enrichment scenarios using Spearman’s correlation coefficient.
Concordance was assessed between genomic detection, defined by surpassing a threshold of sequencing reads per organism, and the traditional culture results (either positive or negative) using the kappa statistic for each organism and enrichment duration. In the present study, the cutoff representing species detection with metagenomics was estimated based on the distribution of read counts to optimize the distinction between culture positive and negative samples, leveraging previously reported metagenomic sequencing examples from the literature that used cutoffs of 100 and 1,000 reads (Zhang et al., 2022; Liu et al., 2023). Kappa was interpreted as 0.81–1.0 almost perfect agreement; 0.61–0.80 substantial agreement, 0.40–0.60 moderate agreement, 0.21–0.40 fair agreement, and 0.01–0.20 none to slight agreement (Dohoo et al., 2009).
For AMR and ARG detection for antimicrobials of interest in managing BRD, statistical analyses included kappa to assess agreement between MIC-based AST results and detection of ARGs, Wilcoxon rank sum test to assess differences in ARG numbers between isolates with and without phenotypic AMR, and the Wilcoxon singed-rank test to assess differences in ARGs among unenriched samples and samples enriched for 10h and 14 h. p-values ≤ 0.05 were considered significant.
3 Results
3.1 Trajectory of bacterial growth from frozen swabs
Sequential qPCR testing was used initially to screen samples from all time periods and select candidate enrichment time points for metagenomic sequencing. Total bacterial abundance continued to increase up to 10 h of incubation, as determined by qPCR targeting the 16S rRNA gene (Figures 1A,B). M. haemolytica plateaued after 8 h. P. multocida and H. somni were highest at 14 h. Samples from 10 and 14 h were selected for metagenomic sequencing; 10 h was the first time point after total bacterial abundance peaked and 14 h represented as the highest joint concentrations of M. haemolytica, P. multocida, and H. somni.
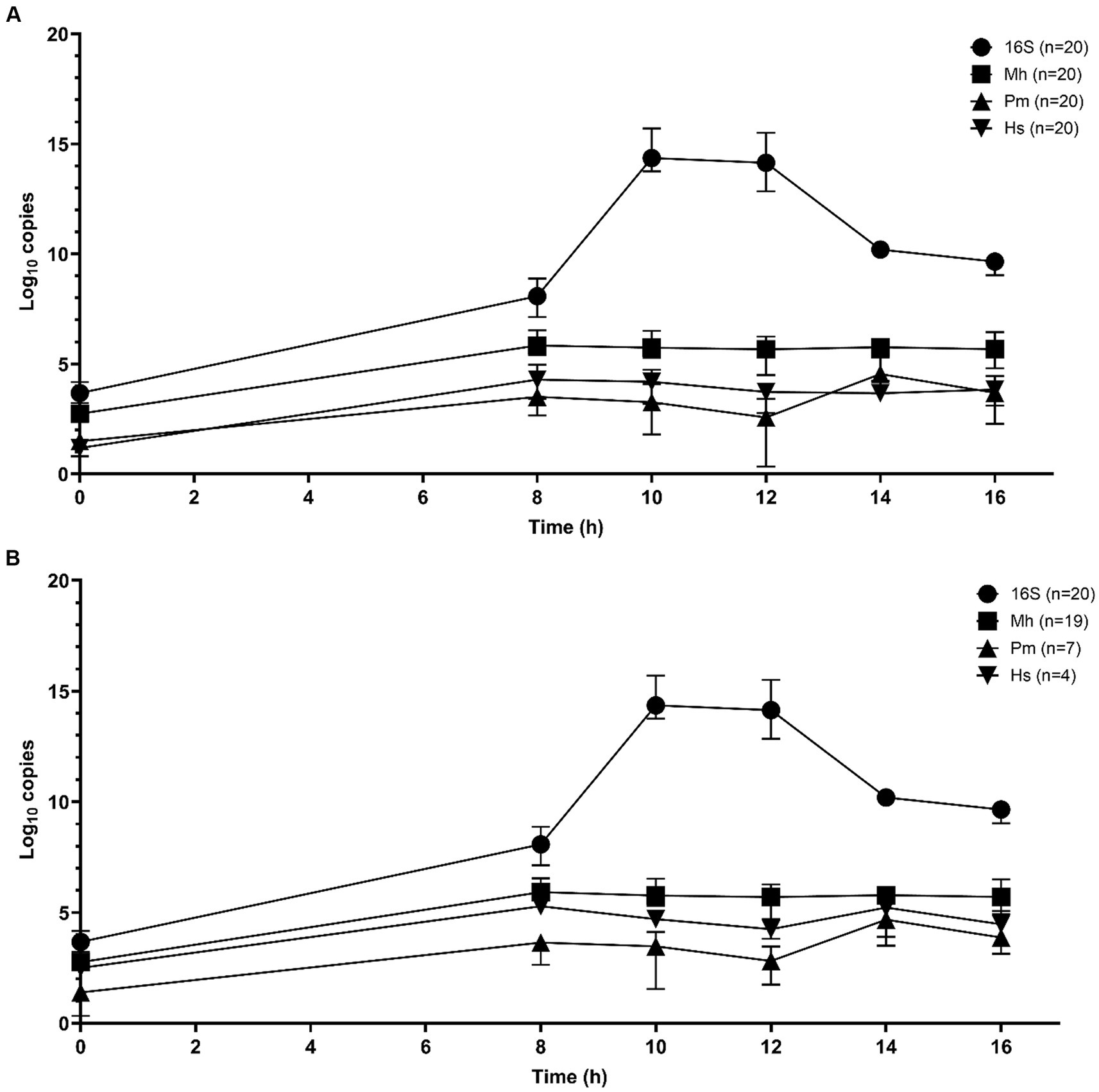
Figure 1. Mean qPCR copy numbers (three repeats per n samples—median value and interquartile range) of bacterial Bovine Respiratory Disease pathogens for DNA samples extracted from frozen swabs from 0 h to 16 h of O2 incubation in BHI broth with 1% glucose (n = 20 samples) for total 16S rRNA gene copies, M. haemolytica (Mh), P. multocida (Pm), and H. somni (Hs) (Nadkarni et al., 2002; Kishimoto et al., 2017). (A) All samples and (B) the results restricted to samples that were culture-positive for M. haemolytica (19 samples), P. multocida (7 samples), and H. somni (4 samples).
3.2 Sequence statistics
After removal of short (<100 bp) and low-quality (<Q7) sequences, the median total base pairs of data available for analyses, number of total reads, read lengths, and read quality were summarized for unenriched, 10- and 14-h enriched samples (Supplementary Tables S1A,B). The median read lengths were longer for enriched than unenriched samples. Non-selective enrichment increased the percentage of non-host DNA in most samples (Table 1). The percentage of non-host sequence measured by total base pairs and total non-host reads was less than 7% for all non-enriched samples. The median percentage of non-host sequence was higher for enriched samples; 54% (range 7–93%) for 10 h and 61% (range 38–94%) for 14 h (Table 1).
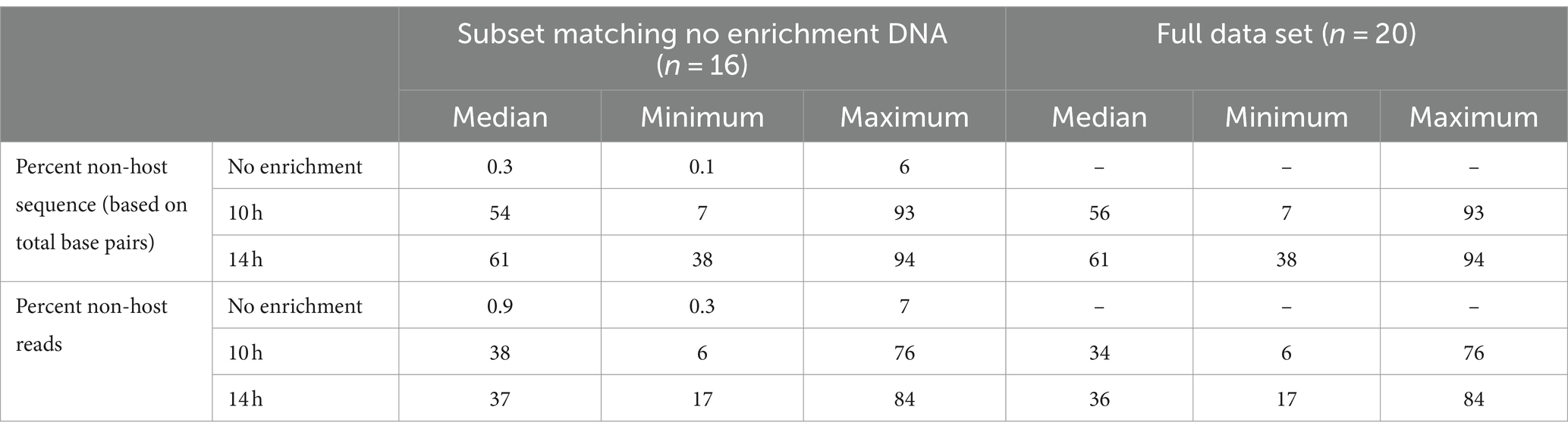
Table 1. Percentage non-host sequence based on total base pairs and reads for samples undergoing bacterial enrichment for 10h and 14h compared with DNA from samples that were not enriched.
Bacterial enrichment increased the percentage of sequence data from BRD pathogens relative to unenriched samples. The increase was two to almost four orders of magnitude higher for read numbers and total sequence length (bp) for M. haemolytica and two orders of magnitude for P. multocida (Table 2, Figure 2, Supplementary Tables S2A,B). There were no comparable culture-positive unenriched samples for H. somni (Supplementary Table S2C).
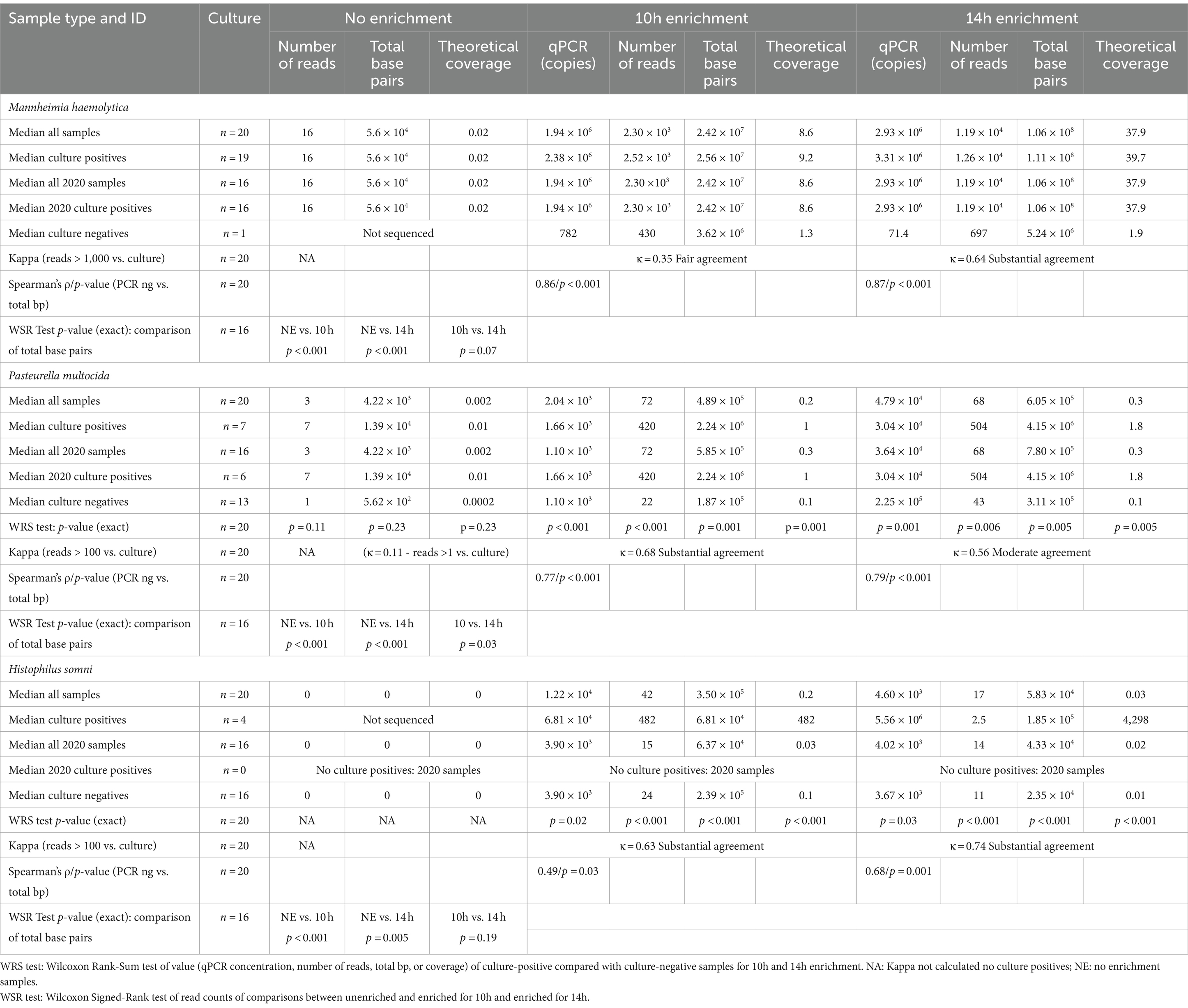
Table 2. Taxonomic classification statistics for Bovine Respiratory Disease pathogens: culture results, number of reads, amount of sequence (total base pairs—bp), and the theoretical genomic coverage of that sequence for different sample enrichment treatments.
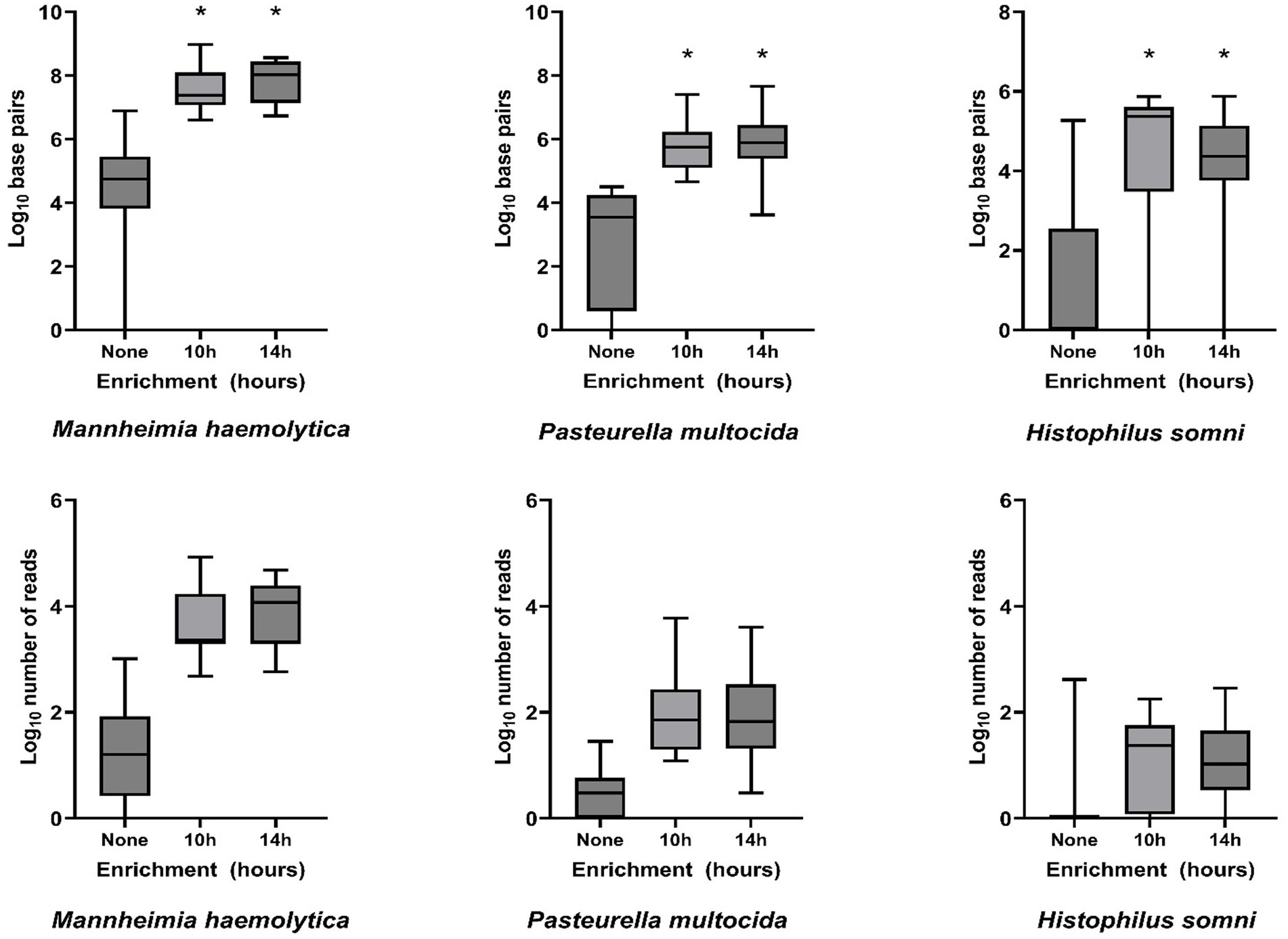
Figure 2. Log10-transformed total DNA base pairs and total number of DNA read counts for each enrichment treatment (none, 10 h, and 14 h, n = 16 for each) of frozen swabs to detect bacterial Bovine Respiratory Disease pathogens by metagenomic sequencing. Box and whisker plots: boxes include the median and upper and lower quartiles; whiskers include the minimum and maximum values. *p < 0.01 on Wilcoxon signed-rank test of the total base pairs for enrichment treatment compared with no enrichment.
In the absence of enrichment, only one sample produced >1x theoretical genome coverage for any organism (Supplementary Tables S2A–C). This sample had 2.8x coverage for M. haemolytica (Supplementary Table S2A) and was the only sample where a culture was semi-quantitatively scored as 3+ out of a possible 4+ abundance of colony growth on the culture plate (Table 2). In contrast, at 14 h, M. haemolytica was detected at >30x theoretical coverage in 11 of 19 culture-positive samples (all samples >1.8x coverage) (Supplementary Table S2A). At 14 h, P. multocida was detected at >1.6x theoretical coverage in five of seven culture-positive samples (Supplementary Table S2B), and H. somni was detected at >8x theoretical coverage in all four culture-positive samples (Supplementary Table S2C). The median theoretical coverage was ≤0.1 for culture negative samples of P. multocida and H. somni at 10h and 14 h (Supplementary Tables S2B,C). There was only one sample culture negative for M. haemolytica with a theoretical coverage of 1.3 for 10 h and 1.9 for 14 h (Supplementary Table S2A). Notably, this sample was also qPCR positive.
M. bovis was only detected in enriched samples (6/20 at 10 h and 2/20 at 14 h) and B. trehalosi was detected in more enriched (12/20 at 10 h and 18/20 at 14 h) than unenriched samples (3/16) (p < 0.05) (additional details are included in Supplementary material).
3.3 Concordance for species detection
M. haemolytica had been cultured from 19 of 20 samples, followed by P. multocida (n = 7) and H. somni (n = 4). Two BRD pathogens were co-isolated from 10 of 20 samples (Supplementary Tables S2A–C).
Culture detection for each of the three organisms of interest was compared with taxonomic classification of sequencing data (Table 2). All 16 unenriched samples (Table 2, Supplementary Table S2A) were M. haemolytica positive, and kappa could not be calculated. One unenriched sample with a 3+ abundance culture score had >1,000 M. haemolytica reads and the rest had <250 reads. In total, 6 of the 16 culture-positive unenriched samples from 2020 had <10 reads for M. haemolytica.
As there was only one culture-negative sample, the estimation of kappa for M. haemolytica was limited for the 20 enriched samples. However, there was substantial agreement (κ = 0.64) between culture positivity and samples with >1,000 reads for 14h. There was very good correlation between the qPCR results and total base pairs sequenced for M. haemolytica at 10 h (ρ = 0.86) and 14h (ρ = 0.87) (Table 2, Supplementary Table S2A).
There were no samples with >100 P. multocida reads in the unenriched group (Table 2, Supplementary Table S2B). If samples were considered positive at ≥1 P. multocida reads, agreement of unenriched sequencing with culture results was poor (κ = 0.11). Agreement between detection of >100 reads and culture positivity was substantial (κ = 0.68) at 14 h and moderate (κ = 0.56) at 10 h (Table 2). At 14 h, two P. multocida culture-negative samples had >100 reads; one was strong positive for P. multocida on qPCR suggesting a false-negative culture (Supplementary Table S2B). At 10h and 14h, culture-positive-enriched samples had higher (p < 0.01) numbers of P. multocida reads, total base pairs, and theoretical genome coverage than culture-negative samples; whereas, there were no significant differences without enrichment (Table 2). There was also good correlation between qPCR results and total base pairs for P. multocida at 10h (ρ = 0.77) and 14 h (ρ = 0.79) (Table 2).
For H. somni (Table 2, Supplementary Table S2C), all 2020 samples were culture-negative. In the 10h- and 14h-enriched samples, the agreement between total H. somni reads >100 and culture-positive samples was substantial (κ = 0.63, κ = 0.74). Correlation was satisfactory to good between qPCR results and total base pairs of H. somni at 10 (ρ = 0.49) and 14 h (ρ = 0.68) (Table 2). The 10h- and 14h-enriched samples had higher (p < 0.001) numbers of H. somni reads, total base pairs, and theoretical genome coverage in culture-positive versus culture-negative samples (Table 2).
3.4 ARG detection and concordance for enriched samples
Concordance was summarized between culture of target BRD bacteria with phenotypic AST based on MICs (Supplementary Table S3) and identification of known ARGs (Supplementary Tables S4, S5) for unenriched samples, where ARGs were detected and for all samples enriched for 10 h and 14 h (Tables 3–5). The most common types of phenotypic resistance based on CLSI breakpoints were for the macrolides gamithromycin and tulathromycin, with only one tested sample with a tetracycline resistance isolate; all three antimicrobials are used in BRD management (Brault et al., 2019) (Tables 3–5, Supplementary Table S3). Samples with sulfadimethoxine MICs >256 μg/mL were also considered in the analysis based on the prevalence (Supplementary Table S3) and the use of trimethoprim-sulfamethoxazole in the treatment of BRD (Brault et al., 2019).
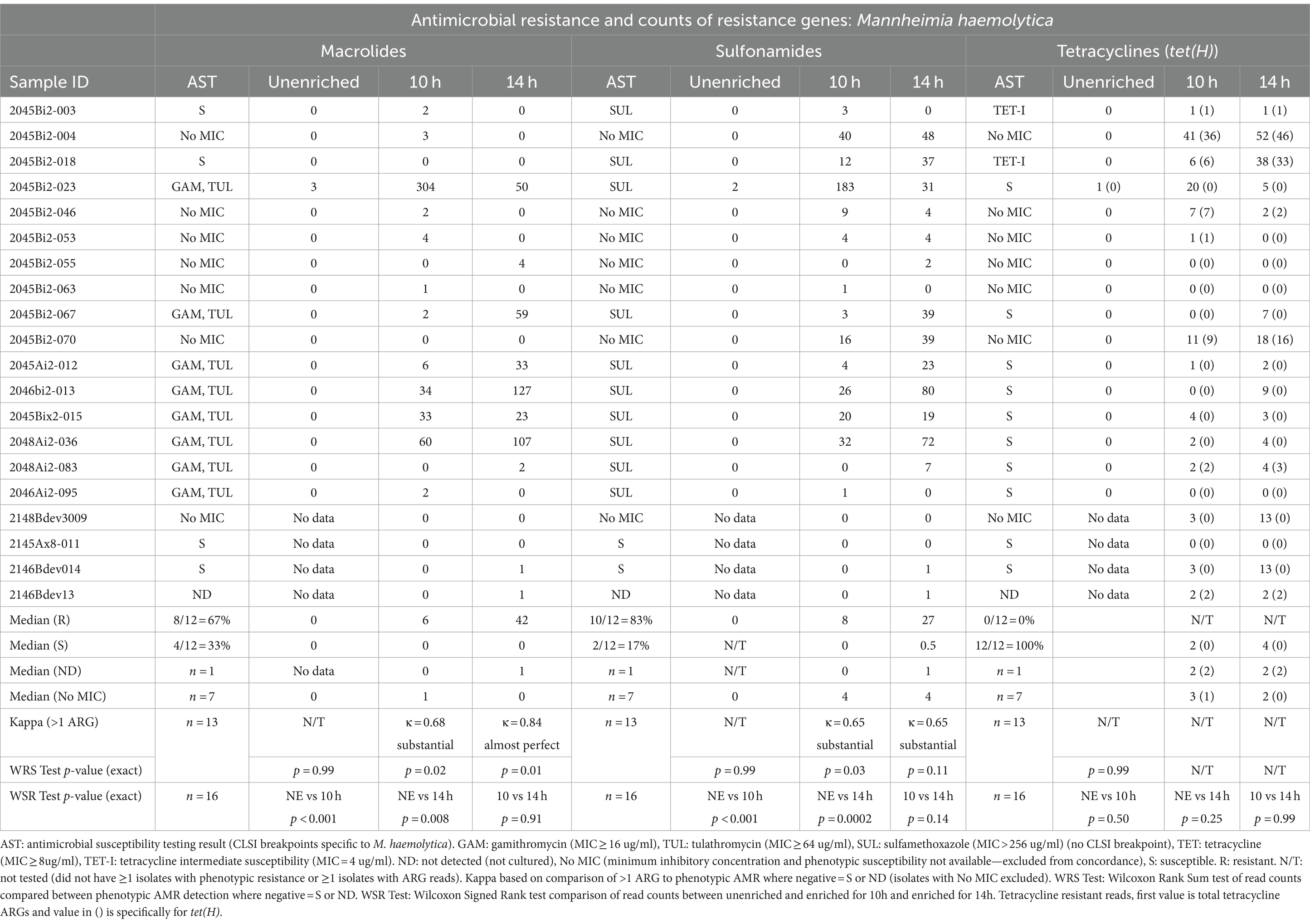
Table 3. Concordance between phenotypic antimicrobial resistance and number of reads with antimicrobial resistance genes (ARGs) within Mannheimia haemolytica reads.
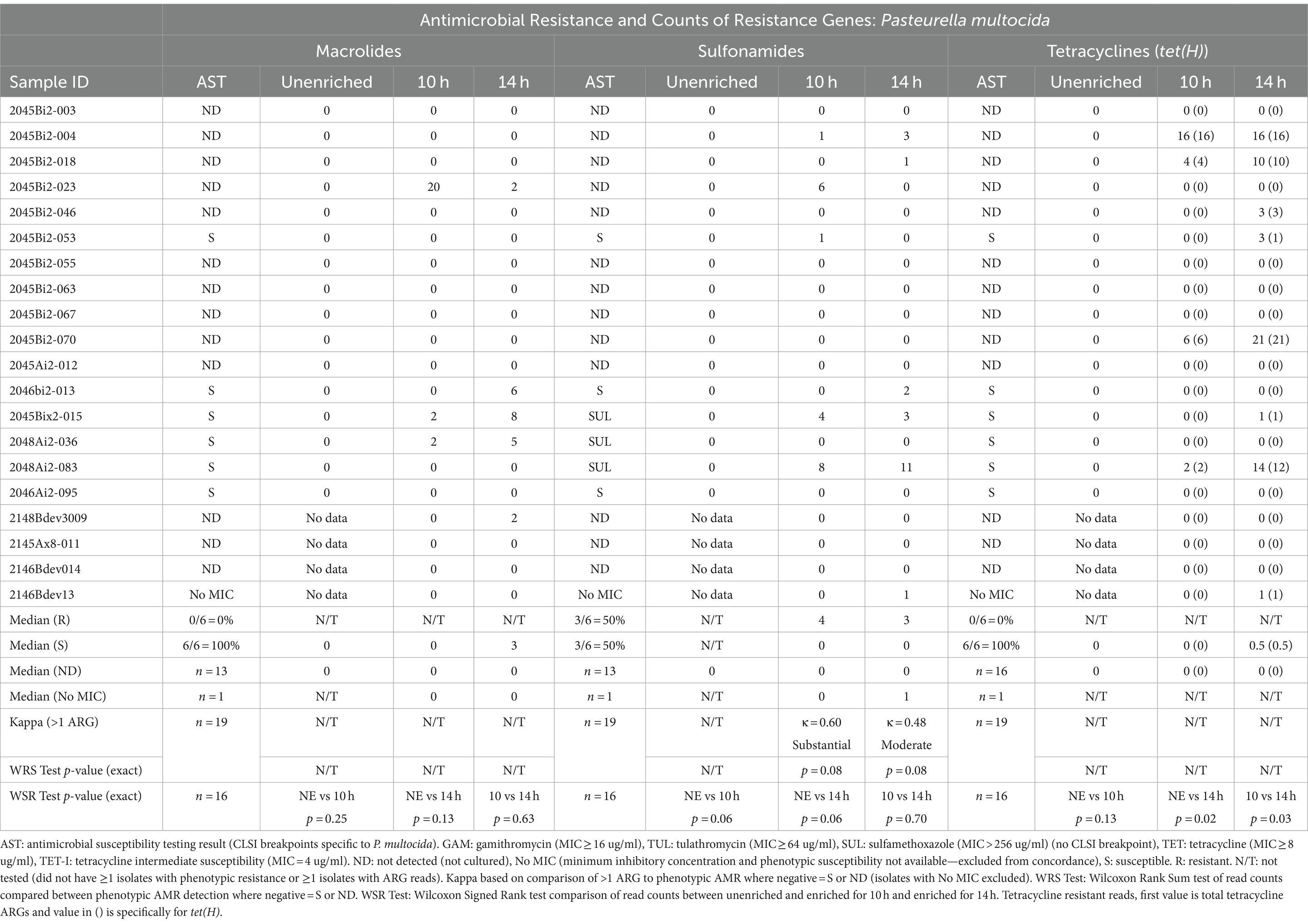
Table 4. Concordance between phenotypic antimicrobial resistance and number of reads with antimicrobial resistance genes (ARGs) within Pasteurella multocida reads.
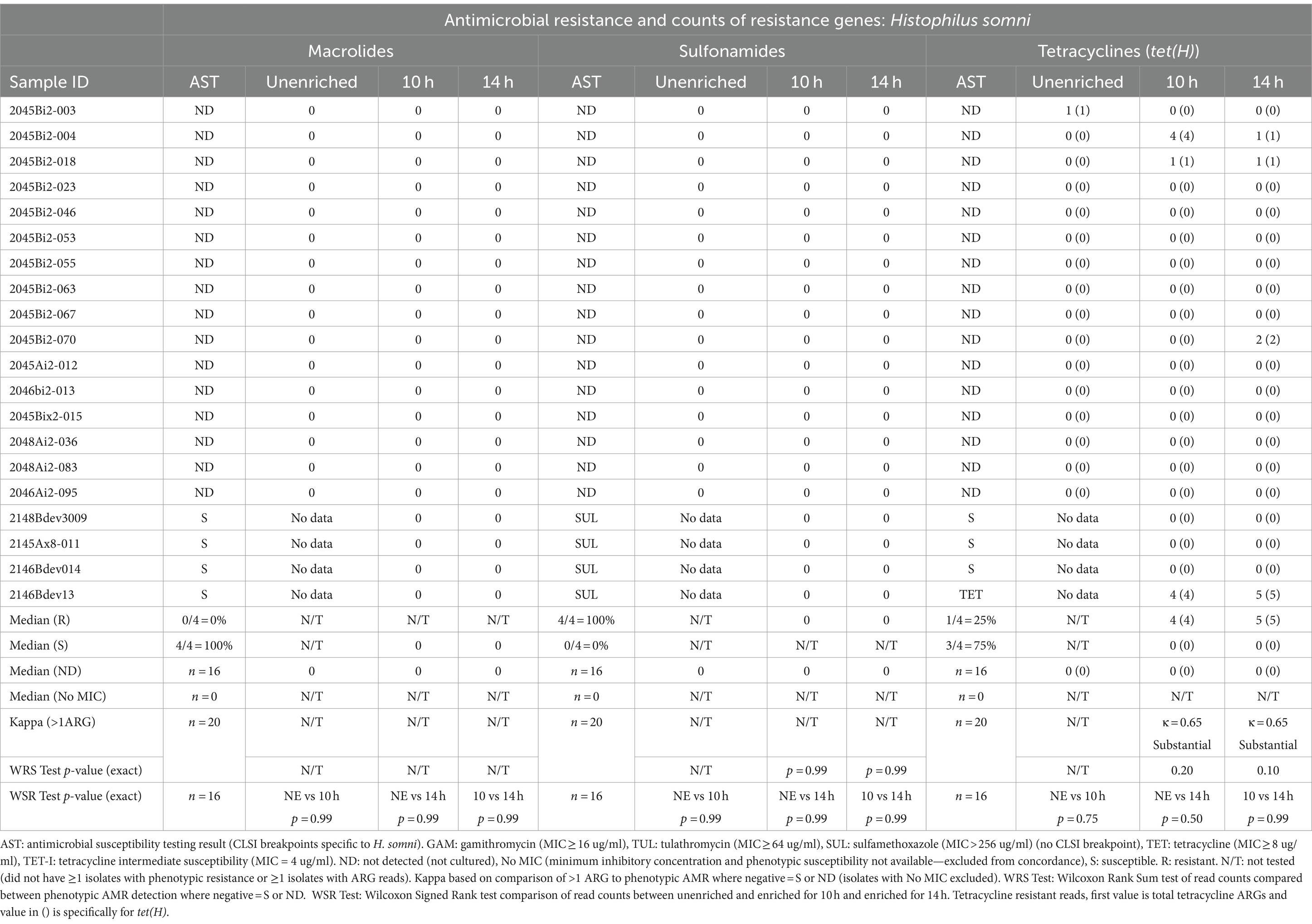
Table 5. Concordance between phenotypic antimicrobial resistance and number of reads with antimicrobial resistance genes (ARGs) within Histophilus somni reads.
3.5 Antimicrobial resistance genes in unenriched samples
Detection of ARGs in unenriched samples was limited to ≤3 reads in two samples (Tables 3–5). In one sample, where the unenriched theoretical coverage of M. haemolytica was 2.8 (Supplementary Table S2A, sample 2045Bi2-023), and AST phenotypes included macrolide resistance and sulfadimethoxine MICs >256 μg/mL, 2 sul2, 2 mphE, 1 msrE, and 1 tet(34) genes were detected in the unenriched sequence data (Table 3, Supplementary Tables S4, S5A). In the second sample (2045Bi2-003), which was culture-positive for H. somni (Supplementary Table S2C) with no detected phenotypic resistance (Table 5), a single tet(H) gene was identified in the unenriched sequence data (Table 5, Supplementary Tables S4, S5C).
Resistance genes were detected more frequently in enriched samples than in unenriched samples, particularly where phenotypic resistance was most prevalent (Tables 3–5). The differences between unenriched and enriched samples were significant for macrolide and sulfonamide resistance genes within M. haemolytica reads (Table 3) and for tetracycline resistance genes within P. multocida reads (Table 4). Furthermore, ARGs were more likely to be detected (> 1 ARG) regardless of enrichment status with increased total number of base pairs for M. haemolytica (p < 0.001), P. multocida (p < 0.001), or H. somni (p = 0.03).
3.6 Detection of ARGs coding for macrolide resistance from enriched samples
Macrolide ARGs were identified within at least two M. haemolytica reads (Table 3, Supplementary Tables S4, S5A) at 10h (11/20 samples) and 14 h (8/20 samples). Agreement between detection of at least two M. haemolytica reads with macrolide ARGs with phenotypic AMR for gamithromycin and tulathromycin was substantial (κ = 0.68) for 10 h and almost perfect (κ = 0.83) for 14 h (Table 3). The number of M. haemolytica reads with macrolide ARGs was also higher at 10h (p = 0.02) and 14 h (p = 0.01) in samples where M. haemolytica isolates displayed phenotypic macrolide resistance compared with samples susceptible M. haemolytica isolates.
Genes coding for macrolide resistance were identified in 463 unique M. haemolytica reads sequenced in the enriched samples by at least one of the NCBI, CARD, or MEGARes databases (Supplementary Tables S4, S5A). The 859 macrolide genes on M. haemolytica reads had a median identity of 96% (IQR, 93–97%) and a median coverage of 98% (IQR, 97–99%) (Supplemental Table S5A). The macrolide resistance-associated genes identified on M. haemolytica reads in these samples were mphE (53%, 457/859) and msrE (47%, 402/859). Both mphE and msrE were identified on 81% of the 463 reads that contained at least one of these genes. Both genes were also identified on M. haemolytica reads in all eight samples with macrolide-resistant isolates and five of the remaining eight samples with susceptible isolates.
Macrolide ARGs were also identified within at least two P. multocida reads (Table 4, Supplementary Tables S4, S5B) from samples at 10h (3/20 samples) and 14 h (5/20 samples). However, there were no samples with cultured macrolide-resistant P. multocida (Table 4, Supplementary Table S3). Macrolide ARGs (53% msrE (25/47) and 47% mphE (22/47)) were identified on 24 P. multocida reads in enriched samples by at least one database. The median gene identity was 96% (IQR, 89–99%) and the median coverage was 98% (IQR, 95–100%) (Supplementary Table S5B).
No macrolide resistance-associated genes were identified within the H. somni reads from the 20 enriched samples (Table 5, Supplementary Tables S4, S5C). None of the samples had H. somni isolates that displayed phenotypic resistance to macrolides (Table 5, Supplementary Table S3).
Macrolide ARGs (msrE, mphE) were identified in 46 B. trehalosi reads from four samples enriched for 10 h and the same four samples plus two more enriched for 14 h. Both genes had been identified in M. haemolytica reads from the same six samples.
3.7 Detection of ARGs coding for sulfonamide resistance for enriched samples
M. haemolytica reads containing sulfonamide ARGs (Table 3, Supplementary Tables S4, S5A) were identified at least twice in samples for 10 h (12/20 samples) and 14 h (13/20 samples). Agreement for detection of at least two M. haemolytica reads containing sulfonamide ARGs (sul2 gene) and samples culture-positive for M. haemolytica with MIC >256 μg/mL for sulfadimethoxine were substantial (κ = 0.65) for 10 and 14 h (Table 3, Supplementary Table S3). The number of sul2 genes detected in M. haemolytica reads was higher for samples with M. haemolytica isolates with sulfadimethoxine for MICs >256 μg/mL than those without sulfadimethoxine in 10 h-enriched samples (p = 0.03) but not in 14-h-enriched samples (p = 0.11). The sul2 gene (n = 761) was identified in 735 M. haemolytica reads from enriched samples. The median sul2 gene identity was 95% (IQR, 92–97%) and the median coverage was 98% (IQR, 97–99%).
Sulfonamide ARGs within P. multocida reads (Table 4, Supplementary Tables S4, S5B) were identified in at least two reads for samples for 10h (3/20) and 14 h (4/20). Agreement between detection of sul2 genes and phenotypic MICs >256 μg/mL was moderate for 10h- (κ = 0.60) and 14h-enriched samples (κ = 0.48) (Table 4). There was no significant difference in the number of sul2 genes detected for samples with P. multocida isolates with MICs for sulfonamides >256 μg/mL and those without MICs (Table 4). Forty sul2 genes were identified in 28 P. multocida reads from enriched samples; median identity was 95% (IQR, 93–97%) and median coverage was 98% (IQR, 97–99%).
No sulfonamide ARGs were detected within H. somni sequence data from samples enriched for either 10h or 14 h (Table 5, Supplementary Tables S4, S5C). However, the sulfadimethoxine MIC for all four H. somni isolates was >256 μg/mL.
A sulfonamide ARG (sul2) was identified within B. trehalosi in 56 reads from four samples enriched for 10 h and the same four samples plus five additional samples enriched for 14 h.
3.8 Detection of ARGs coding for tetracycline resistance for enriched samples
Tetracycline ARGs were detected within at least two M. haemolytica reads (Table 3, Supplementary Tables S4, S5A) in 10h (11/20) and 14h-enriched (14/20) samples; however, there was no detected phenotypic resistance in culture-positive isolates (Table 3, Supplementary Table S3). In all enriched samples, 277 tetracycline ARGs were detected in 275 M. haemolytica reads. The primary tetracycline ARGs included tet(H) (60%, 167/277) and tet(34) (39%, 109/277). The median identity for the tet(H) genes was 95% (IQR, 93–97%) and median coverage was 98% (IQR, 97–99%) but median identity for the tet(34) genes was only 62% (IQR, 61–62%) and median coverage was 77% (IQR, 67–84%). The tet(34) genes were not detected by the Abricate option and MEGARes databases likely due to the higher cutoff of 80% identity. The tet(H) genes were detected in the same six samples for 10h and 14 h.
Tetracycline ARGs were detected within at least two P. multocida reads (Supplementary Tables S4, S5B) for 10h (4/20) and 14 h (6/20) (Table 4). The tet(H) gene was detected in at least two P. multocida reads in four samples for 10 h and five samples for 14 h. One sample contained two tet(34) genes with low identity scores (62%) as reported for the same gene from M. haemolytica. No P. multocida isolates were phenotypically resistant to tetracycline (Table 4, Supplementary Table S3).
The tet(H) genes were detected in at least two H. somni reads for two samples for 10h and 14 h. The tet(H) gene was identified in one of four H. somni culture-positive samples with tetracycline-resistant isolates (Table 5, Supplementary Tables S3, S4, S5C).
The tetracycline resistance gene (tet(H)) was identified within B. trehalosi in eight reads from one sample for 10 h and the same sample enriched for 14 h.
3.9 Other resistance genes
The only other ARGs reported at least twice in enriched samples conferred aminoglycoside resistance. The most common was APH(3′). There were 282 APH(3′)-Ia genes identified on 278 M. haemolytica reads from 14 samples and 13 genes on 12 P. multocida reads from 4 samples (Supplementary Tables S4A,B, S5A,B). There were also 232 APH(3′)-Ib genes identified on 230 M. haemolytica reads from 14 samples, and 20 genes were identified on 18 P. multocida reads from eight samples.
Another identified aminoglycoside resistance gene was APH(6)-Id, with 245 APH(6)-Id genes identified on 240 M. haemolytica reads from 14 samples and 13 genes on 12 P. multocida reads from 4 samples (Supplementary Tables S4A,B, S5A,B).
No aminoglycoside ARGs were identified in H. somni reads (Supplementary Tables S4A,B, S5C) or B. trehalosi reads.
3.10 Detection of multiple ARGs on single reads
Multiple ARGs were detected on 739 long individual reads for 31 metagenomic analyses of 19 unique samples (Supplementary Table S6). In total, 2 of the 739 reads with multiple ARGs were from unenriched samples.
The median number of unique ARGs on each read was three (5th percentile = two, 95th percentile = four). The median length of individual reads on which multiple ARGs were found was 21,941 bp (5th percentile 7,971, 95th percentile 53,678 bp). Most reads with multiple ARGs were M. haemolytica (n = 694), with smaller numbers of P. multocida (n = 42) and only one H. somni read. The most common pattern of multiple ARGs per read was msrE, mphE, and sul2 in 349 reads followed by APH(3″)-Ib, APH(6)-Id, APH(3′)-Ia, and sul2 in 188 reads. Both macrolide ARGs and sulfonamide ARGs were detected in 45% of the 774 M. haemolytica reads with at least one of these genes and in at least one M. haemolytica read from 78% (14/18) of samples with at least one of these genes.
4 Discussion
Non-selective bacterial enrichment increased the amount of on-target data available from metagenomic sequencing of nasal samples from feedlot cattle enabling previously unreported robust detection of ARGs on species-specific respiratory bacterial reads in complex respiratory samples from healthy animals. Incubating the sample in growth medium for 10h and 14 h increased bacterial numbers for three important BRD pathogens of interest relative to unenriched samples and for B. trehalosi. In the case of M. haemolytica and B. trehalosi, enrichment also increased the read detections of ARGs for macrolide and sulfonamide resistance, and tetracycline ARGs for P. multocida.
Host DNA is a major impediment in metagenome analysis, particularly in nasal swab samples, where >90% of sequencing reads can be host-derived (Marotz et al., 2018; Chen and Xu, 2023; Ring et al., 2023). High proportions of host DNA reduce the sensitivity of metagenomic sequencing, especially for detecting low-abundance bacteria (Pereira-Marques et al., 2019). In this study, all 20 enriched samples resulted in >93% non-host DNA for both the 10h and 14h protocols. For the three BRD bacterial pathogens of most interest, M. haemolytica, P. multocida, and H. somni, non-selective enrichment increased the detection of targeted sequence by several orders of magnitude, particularly in culture-positive samples. Increased coverage of the pathogen genomes was directly associated with the detections of ARGs within identified bacterial reads for antimicrobials of interest such as macrolides and tetracyclines. Another recent study of metagenomic identification of pathogens in blood cultures also described increased ARG detection with increased total base pairs reported for target organisms of interest (Liu et al., 2023), confirming the clinical relevance of additional on-target bacterial sequence data.
At least some reads for all three BRD pathogens were detected in sequence data from samples with negative culture results, particularly for enriched samples. This finding could partially be explained by the ability of sequence-based approaches to detect DNA from growth-inhibited or dead pathogens (Shao et al., 2022). This discrepancy could also be due to the inherent sampling bias of streak plate-based identification of pathogens, wherein only 10 μL loop of the original sample is used for analysis, which could miss low-abundance organisms. In previous studies comparing detection of BRD pathogens, sequencing demonstrated positive results more frequently than culture, and the concordance between these techniques varied by organism (Bell et al., 2014; Freeman et al., 2022). However, in the present study, the sequence and qPCR data generated for enriched culture-positive samples were significantly higher than culture-negative samples.
In the present study, one advantage of metagenomic sequencing for BRD pathogen detection is that it can provide a snapshot of the nasal bacteria beyond the detection of specific organisms targeted by routine culture, such as M. bovis and B. trehalosi. However, non-selective bacterial enrichment can modify the relative quantities of identified organisms, as the community structure can be altered by variation in replication rate and overgrowth of some organisms (Jarvis et al., 2015; Leonard et al., 2015). In the present study, differences in the absolute read numbers and relative depth of sequence data were recognized for target organisms of interest. There was a higher chance of detecting ARGs of interest in samples that had higher reads and more base pairs for BRD organisms. However, the protocol was optimized to include 14h enrichment based on qPCR detection of the three primary organisms of interest. The long-read metagenomic sequencing of our samples had good agreement with culture and reflected what we expected to observe in calves when they arrive at the feedlot; initial recovery of M. haemolytica and P. multocida with increasing frequency of H. somni and M. bovis in the feeding period (Alhamami et al., 2021; Andrés-Lasheras et al., 2021; Younes et al., 2022).
While the impact of non-selective enrichment on description of the microbiome requires further research, this study demonstrates that this approach was successful in identifying primary bacteria of interest for managing BRD and enhancing detection of ARGs in these organisms. While not all organisms were expected to benefit equally from the enrichment protocol, culture times were relatively short; DNA from unculturable (i.e., dead) or difficult to culture bacteria was unlikely to be lost with enrichment, and therefore, the risk of losing taxonomic breadth was minimal (Lennon et al., 2018; Shao et al., 2022). Even M. bovis, which replicates slowly and requires additional nutrients not provided by the enrichment medium used in this study (McVey et al., 2013), was detected following bacterial enrichment. Furthermore, M. bovis was identified in calves sampled early in the feeding period when we did not expect a high prevalence (Freeman et al., 2022). However, the resulting sequencing data were limited with 0.23 as the highest observed theoretical coverage of M. bovis in a single sample.
In a proof-of-concept study (Freeman et al., 2022), metagenomic sequencing without bacterial enrichment detected BRD pathogens of interest more frequently than did culture, but ARGs from relevant taxa were not reliably detected due to high levels of host DNA. Our findings show that with enrichment and the resulting increased sequencing coverage of target organisms, hundreds of ARGs were detected with taxonomic resolution to the species level, with moderate and better concordance with phenotypic resistance.
Samples with M. haemolytica isolates displaying phenotypic resistance to the 15-member macrolides, tulathromycin and gamithromycin, were selected for this pilot study. Agreement between AST results for macrolides in M. haemolytica and the detection of ARGs on M. haemolytica reads was in agreement with 14h enrichment. The genes mphE and msrE encode a macrolide phosphotransferase protein and an ABC transporter protein, respectively (Kadlec et al., 2011), and have been consistently associated with macrolide resistance in Pasteurellaceae isolates derived from feedlot cattle (Alhamami et al., 2021; Andrés-Lasheras et al., 2021; Younes et al., 2022). These two genes were recovered together on 81% of individual M. haemolytica reads with any macrolide resistance and all of the samples with phenotypic macrolide-resistant M. haemolytica. The colocation of these two genes was expected as was found in previous reports using PCR and assemblies based on whole genome sequencing (Clawson et al., 2016; Snyder et al., 2019; Klima et al., 2020; Stanford et al., 2020) but has not been previously described on individual raw reads from metagenomic data.
The erm42 gene is often present in isolates exhibiting resistance to gamithromycin, tulathromycin, and tilmicosin (Desmolaize et al., 2011; Rose et al., 2012; Snyder et al., 2019) but was not detected in these samples. Although macrolide resistance can emerge from point mutations in the 23S rRNA gene (Olsen et al., 2015); the phenotypic resistance in this study was explained by the presence of previously characterized macrolide ARGs. Macrolide ARGs were also detected on reads of BRD organisms where the specific organisms were absent on culture or AST reported for the tested isolate from the sample was susceptible to macrolides. However, smaller number of macrolide ARGs were present in the absence of phenotypic macrolide-resistant M. haemolytica. Metagenomics could have detected ARG reads in samples with isolates that were either not detected or not selected for MIC testing.
In this study, high MICs for sulfadimethoxine (MIC >256 μg/mL) were observed for isolates of all three BRD pathogens. Agreement between the sul2 gene and high sulfadimethoxine MICs was at least moderate for M. haemolytica and P. multocida. However, in the four samples where sulfadimethoxine-resistant H. somni isolates were recovered, no sul2 genes were detected suggesting another potential, undetected resistance mechanism. Furthermore, almost half of all M. haemolytica reads and four out of five samples with either macrolide or sulfonamide ARGs had both macrolide and sulfonamide ARGs. This suggests that these genes are frequently collocated potentially on integrative and conjugative elements (ICE) (Clawson et al., 2016; Beker et al., 2018). Colocation of macrolide and sulfonamide ARGs was also observed in B. trehalosi reads.
In this study, tetracycline phenotypic resistance was only reported in one H. somni isolate. The tetracycline resistance gene tet(H) was identified in this sample and encoded a tetracycline efflux protein. Other studies have found variable concordance with phenotypic resistance to tetracycline and tet genes (Owen et al., 2017; Snyder et al., 2020). The tet(34) gene was also identified in several samples; however, given the lack of concordance with phenotypic resistance and the low percentage identity of the tet(34) genes, these results were unlikely to be clinically relevant.
Concordance between genotypic ARG detection and phenotypic AST varied, and there were sufficient samples with phenotype positive and negative isolates to generate reliable metrics. However, most calculations suggested substantial agreement and significant differences in read numbers between samples with AST-positive and AST-negative isolates. Concordance can vary depending on the organism and antimicrobial, level of transcriptional expression, and sequence quality. In some previous reports, phenotypic resistance was highly correlated with known resistance determinants. Concordance between AST and WGS for M. haemolytica from 20 stocker calves calculated from raw data was very good for tilmicosin (κ = 0.96), tulathromycin (κ = 0.96), and tetracycline (κ = 1.0) (Snyder et al., 2020). Agreement was lower between oxytetracycline resistance and detection of tet(H) (κ = 0.66) and sulfonamide resistance and detection of sul2 (κ = 0.38) for 64 WGSs for M. haemolytica, P. multocida, and H. somni isolates from beef and dairy calves (Owen et al., 2017).
This study demonstrated the use of long-read metagenomic sequencing for the detection of ARGs linked directly to bacterial species without the need for genome assembly. The detection of ARGs has been reported from long-read metagenomic sequencing for one study of aspirates from human ventilator-associated pneumonia (Chen et al., 2023) but more typically from samples with substantially less host DNA, such as those recovered from positive blood cultures (Taxt et al., 2020; Liu et al., 2023), bile cultures (Whittle et al., 2022), milk cultures (Ahmadi et al., 2023), and urine cultures (Zhang et al., 2022; Ring et al., 2023).
There were no previous reports of ARG detection within identified pathogen reads using long-read metagenomic sequencing in human or veterinary upper respiratory tract samples (Chen and Xu, 2023; Adewusi et al., 2024) with the exception of the limited success in earlier study published by our group (Freeman et al., 2022). Resistance genes were not recovered in a veterinary study of skin infections in dogs due to the high percentage of host DNA (Ring et al., 2023). None of the metagenomic studies reporting ARGs explicitly reported either individual or multiple ARGs on single long reads of the organisms of interest at the species level as described in the present study, potentially increasing the clinical relevance of the ARG detection.
Short-read data can be used for read-based detection of ARGs; however, it typically requires higher genome coverage and more computing resources compared with long-read detection (Gupta et al., 2020), making long-read metagenomic options such as our method attractive as genomics moves toward rapid diagnostic solutions to inform antimicrobial stewardship. Long-read methods such as ONT also offer access to taxonomic real-time data during the sequencing run, which may also speed up the time for results and is not currently available for short-read sequencing methods (Votintseva et al., 2017; Charalampous et al., 2019; Chan et al., 2020; Ring et al., 2023).
Although bacterial enrichment of samples has improved the sensitivity of both taxonomic and ARG detection, this method is not without drawbacks. Indeed, it adds time and complexity to sample preparation for sequencing, and it does not offer the same unbiased insight into the microbial community structure for those whose objective might be traditional microbiome research as does metagenomic sequencing of unenriched samples. However, in our case, the goal was to detect reads of specific BRD pathogens with ARGs to inform clinical decisions and not to produce a general description of the nasal microbiome. Larger studies including more animals will further validate this tool against alternative testing methods and assess whether this method might be a cost-effective option to help inform antimicrobial stewardship.
5 Conclusion
Long-read metagenomic sequencing of enriched DNP samples from feedlot cattle to detect BRD pathogens and ARGs shows promise as a diagnostic testing strategy for feedlot cattle production. Agreement between pathogen detection and traditional culture-based methods was improved by the enrichment step. While this step adds time and makes the process less portable, the relative benefits of improving sequence quantity for non-host reads and ARG detection outweighed these costs. This method provides additional promise for the characterization of species not specifically targeted by routine culture and susceptibility protocols with no additional diagnostic costs.
Data availability statement
The data presented in this study were deposited to the Sequence Read Archive as submission SUB14263813 and as BioProject PRJNA1096931.
Ethics statement
The animal study was approved by University of Saskatchewan Animal Care Committee (AUP 20190069). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EH: Methodology, Writing – original draft, Software. SL: Methodology, Conceptualization, Project administration, Writing – review & editing. CF: Methodology, Writing – original draft. SO: Funding acquisition, Writing – review & editing. EM: Writing – review & editing, Data curation, Software. ML: Data curation, Software, Formal analysis, Writing – review & editing. PS: Software, Writing – review & editing. CW: Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research is being conducted as part of the “Genomic ASSETS (Antimicrobial Stewardship Systems from Evidence-based Treatment Strategies) for Livestock” project funded by Genome Canada with support from Genome Prairie, Genome Alberta, and the Saskatchewan Agriculture Development Fund, as well as support from the University of Saskatchewan and the University of Alberta.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1386319/full#supplementary-material
References
Adewusi, O. O., Waldner, C. L., Hanington, P. C., Hill, J. E., Freeman, C. N., and Otto, S. J. G. (2024). Laboratory tools for the direct detection of bacterial respiratory infections and antimicrobial resistance: a scoping review. J. Vet. Diagn. Invest. :10406387241235968. doi: 10.1177/10406387241235968
Ahmadi, A., Khezri, A., Nørstebø, H., and Ahmad, R. (2023). A culture-, amplification-independent, and rapid method for identification of pathogens and antibiotic resistance profile in bovine mastitis milk. Front. Microbiol. 13:1104701. doi: 10.3389/fmicb.2022.1104701
Aidara-Kane, A. A. F., Conly, J. M., Minato, Y., Silbergeld, E. K., SA, M. E., et al. (2018). World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 7:7. doi: 10.1186/s13756-017-0294-9
Alcock, B. P., Raphenya, A. R., Lau, T. T. Y., Tsang, K. K., Bouchard, M., Edalatmand, A., et al. (2019). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48, D517–D525. doi: 10.1093/nar/gkz935
Alhamami, T., Chowdhury, P., Gomes, N., Carr, M., Veltman, T., Khazandi, M., et al. (2021). First emergence of resistance to macrolides and tetracycline identified in Mannheimia haemolytica and Pasteurella multocida isolates from beef feedlots in Australia. Microorganisms 9:1322. doi: 10.3390/microorganisms9061322
Andrés-Lasheras, S., Ha, R., Zaheer, R., Lee, C., Booker, C. W., Dorin, C., et al. (2021). Prevalence and risk factors associated with antimicrobial resistance in Bacteria related to bovine respiratory disease—a broad cross-sectional study of beef cattle at entry into Canadian feedlots. Front. Vet. Sci. 8:692646. doi: 10.3389/fvets.2021.692646
Beker, M., Rose, S., Lykkebo, C. A., and Douthwaite, S. (2018). Integrative and conjugative elements (ICEs) in Pasteurellaceae species and their detection by multiplex PCR. Front. Microbiol. 9:1329. doi: 10.3389/fmicb.2018.01329
Bell, C. J., Blackburn, P., Elliott, M., Patterson, T. I. A. P., Ellison, S., Lahuerta-Marin, A., et al. (2014). Investigation of polymerase chain reaction assays to improve detection of bacterial involvement in bovine respiratory disease. J. Vet. Diagn. Invest. 26, 631–634. doi: 10.1177/1040638714540166
Besser, J. M., Carleton, H. A., Trees, E., Stroika, S. G., Hise, K., Wise, M., et al. (2019). Interpretation of whole-genome sequencing for enteric disease surveillance and outbreak investigation. Foodborne Pathog. Dis. 16, 504–512. doi: 10.1089/fpd.2019.2650
Brault, S. A., Hannon, S. J., Gow, S. P., Warr, B. N., Withell, J., Song, J., et al. (2019). Antimicrobial use on 36 beef feedlots in Western Canada: 2008–2012. Front. Vet. Sci. 6:329. doi: 10.3389/fvets.2019.00329
Chan, W. S., Au, C. H., Chung, Y., Leung, H. C. M., Ho, D. N., Wong, E. Y. L., et al. (2020). Rapid and economical drug resistance profiling with Nanopore MinION for clinical specimens with low bacillary burden of Mycobacterium tuberculosis. BMC. Res. Notes 13:444. doi: 10.1186/s13104-020-05287-9
Charalampous, T., Kay, G. L., Richardson, H., Aydin, A., Baldan, R., Jeanes, C., et al. (2019). Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat. Biotechnol. 37, 783–792. doi: 10.1038/s41587-019-0156-5
Chen, J., and Xu, F. (2023). Application of Nanopore sequencing in the diagnosis and treatment of pulmonary infections. Mol. Diagn. Ther. 27, 685–701. doi: 10.1007/s40291-023-00669-8
Chen, T., Zhang, L., Huang, W., Zong, H., Li, Q., Zheng, Y., et al. (2023). Detection of pathogens and antimicrobial resistance genes in ventilator-associated pneumonia by metagenomic next-generation sequencing approach. Infect. Drug Resist. 16, 923–936. doi: 10.2147/idr.S397755
Clawson, M. L., Murray, R. W., Sweeney, M. T., Apley, M. D., DeDonder, K. D., Capik, S. F., et al. (2016). Genomic signatures of Mannheimia haemolytica that associate with the lungs of cattle with respiratory disease, an integrative conjugative element, and antibiotic resistance genes. BMC Genomics 17:982. doi: 10.1186/s12864-016-3316-8
CLSI (2023). Performance standards for antimicrobial disk and dilution susceptibility tests for bacterial isolated from animals. 6th ed. CLSI Supplement VET01S ed. Wayne, PA: The Clinical and Laboratory Standards Institute.
De Coster, W., D’Hert, S., Schultz, D. T., Cruts, M., and Van Broeckhoven, C. (2018). NanoPack: visualizing and processing long-read sequencing data. Bioinformatics 34, 2666–2669. doi: 10.1093/bioinformatics/bty149
Delgado-Suárez, E. J., Palós-Guitérrez, T., Ruíz-López, F. A., Hernández Pérez, C. F., Ballesteros-Nova, N. E., Soberanis-Ramos, O., et al. (2021). Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant non-typhoidal Salmonella in Mexico. PLoS One 16:e0243681. doi: 10.1371/journal.pone.0243681
Desmolaize, B., Rose, S., Warrass, R., and Douthwaite, S. (2011). A novel Erm monomethyltransferase in antibiotic-resistant isolates of Mannheimia haemolytica and Pasteurella multocida. Mol. Microbiol. 80, 184–194. doi: 10.1111/j.1365-2958.2011.07567.x
Dohoo, I. R., Martin, S. W., and Stryhn, H. (2009). Veterinary epidemiologic research. Charlottetown: P.E.I: VER, Inc.
Doster, E., Lakin, S. M., Dean, C. J., Wolfe, C., Young, J. G., Boucher, C., et al. (2019). MEGARes 2.0: a database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 48, D561–D569. doi: 10.1093/nar/gkz1010
Elsik, C. G., Tellam, R. L., Worley, K. C., Gibbs, R. A., Muzny, D. M., Weinstock, G. M., et al. (2009). The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science 324, 522–528. doi: 10.1126/science.1169588
Feldgarden, M., Brover, V., Haft, D. H., Prasad, A. B., Slotta, D. J., Tolstoy, I., et al. (2019). Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 63. doi: 10.1128/AAC.00483-19
Freeman, C. N., Herman, E. K., Abi Younes, J., Ramsay, D. E., Erikson, N., Stothard, P., et al. (2022). Evaluating the potential of third generation metagenomic sequencing for the detection of BRD pathogens and genetic determinants of antimicrobial resistance in chronically ill feedlot cattle. BMC Vet. Res. 18:211. doi: 10.1186/s12917-022-03269-6
Griffin, D., Chengappa, M. M., Kuszak, J., and McVey, D. S. (2010). Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 26, 381–394. doi: 10.1016/j.cvfa.2010.04.004
Guo, Y., McMullen, C., Timsit, E., Hallewell, J., Orsel, K., van der Meer, F., et al. (2020). Genetic relatedness and antimicrobial resistance in respiratory bacteria from beef calves sampled from spring processing to 40 days after feedlot entry. Vet. Microbiol. 240:108478. doi: 10.1016/j.vetmic.2019.108478
Gupta, C. L., Tiwari, R. K., and Cytryn, E. (2020). Platforms for elucidating antibiotic resistance in single genomes and complex metagenomes. Environ. Int. 138:105667. doi: 10.1016/j.envint.2020.105667
Harrison, E. M., Paterson, G. K., Holden, M. T. G., Larsen, J., Stegger, M., Larsen, A. R., et al. (2013). Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol. Med. 5, 509–515. doi: 10.1002/emmm.201202413
Holman, D. B., Timsit, E., Booker, C. W., and Alexander, T. W. (2018). Injectable antimicrobials in commercial feedlot cattle and their effect on the nasopharyngeal microbiota and antimicrobial resistance. Vet. Microbiol. 214, 140–147. doi: 10.1016/j.vetmic.2017.12.015
Jarvis, K. G., White, J. R., Grim, C. J., Ewing, L., Ottesen, A. R., Beaubrun, J. J.-G., et al. (2015). Cilantro microbiome before and after nonselective pre-enrichment for Salmonella using 16S rRNA and metagenomic sequencing. BMC Microbiol. 15:160. doi: 10.1186/s12866-015-0497-2
Kadlec, K., Brenner Michael, G., Sweeney, M. T., Brzuszkiewicz, E., Liesegang, H., Daniel, R., et al. (2011). Molecular basis of macrolide, triamilide, and lincosamide resistance in Pasteurella multocida from bovine respiratory disease. Antimicrob. Agents Chemother. 55, 2475–2477. doi: 10.1128/AAC.00092-11
Kishimoto, M., Tsuchiaka, S., Rahpaya, S. S., Hasebe, A., Otsu, K., Sugimura, S., et al. (2017). Development of a one-run real-time PCR detection system for pathogens associated with bovine respiratory disease complex. J. Vet. Med. Sci. 79, 517–523. doi: 10.1292/jvms.16-0489
Klima, C. L., Cook, S. R., Zaheer, R., Laing, C., Gannon, V. P., Xu, Y., et al. (2016). Comparative genomic analysis of Mannheimia haemolytica from bovine sources. PLoS One 11, –e0149520. doi: 10.1371/journal.pone.0149520
Klima, C. L., Holman, D. B., Cook, S. R., Conrad, C. C., Ralston, B. J., Allan, N., et al. (2020). Multidrug resistance in Pasteurellaceae associated with bovine respiratory disease mortalities in North America from 2011 to 2016. Front. Microbiol. 11:606438. doi: 10.3389/fmicb.2020.606438
Lennon, J. T., Muscarella, M. E., Placella, S. A., and Lehmkuhl, B. K. (2018). How, when, and where relic DNA affects microbial diversity. mBio 9:e00637-18. doi: 10.1128/mBio.00637-18
Leonard, S. R., Mammel, M. K., Lacher, D. W., and Elkins, C. A. (2015). Application of metagenomic sequencing to food safety: detection of Shiga toxin-producing Escherichia coli on fresh bagged spinach. Appl. Environ. Microbiol. 81, 8183–8191. doi: 10.1128/AEM.02601-15
Liu, Y., Xu, Y., Xu, X., Chen, X., Chen, H., Zhang, J., et al. (2023). Metagenomic identification of pathogens and antimicrobial-resistant genes in bacterial positive blood cultures by nanopore sequencing. Front. Cell. Infect. Microbiol. 13:1283094. doi: 10.3389/fcimb.2023.1283094
Loy, J. D. (2020). Development and application of molecular diagnostics and proteomics to bovine respiratory disease (BRD). Anim. Health Res. Rev. 21, 164–167. doi: 10.1017/S1466252320000092
Lu, J., Breitwieser, F. P., Thielen, P., and Salzberg, S. L. (2017). Bracken: estimating species abundance in metagenomics data. PeerJ. Comput. Sci. 3:e104. doi: 10.7717/peerj-cs.104
Lubbers, B. V., and Hanzlicek, G. A. (2013). Antimicrobial multidrug resistance and coresistance patterns of Mannheimia haemolytica isolated from bovine respiratory disease cases—a three-year (2009–2011) retrospective analysis. J. Vet. Diagn. Invest. 25, 413–417. doi: 10.1177/1040638713485227
Marotz, C. A., Sanders, J. G., Zuniga, C., Zaramela, L. S., Knight, R., and Zengler, K. (2018). Improving saliva shotgun metagenomics by chemical host DNA depletion. Microbiome 6, 42–49. doi: 10.1186/s40168-018-0426-3
McVey, S., Kennedy, M., and Chengappa, M. M. (2013). Veterinary microbiology, New Jersey: Wiley Blackwell.
Michael, G. B., Kadlec, K., Sweeney, M. T., Brzuszkiewicz, E., Liesegang, H., Daniel, R., et al. (2012). ICEPmu1, an integrative conjugative element (ICE) of Pasteurella multocida: analysis of the regions that comprise 12 antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 84–90. doi: 10.1093/jac/dkr406
Nadkarni, M. A., Martin, F. E., Jacques, N. A., and Hunter, N. (2002). Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148, 257–266. doi: 10.1099/00221287-148-1-257
Olsen, A. S., Warrass, R., and Douthwaite, S. (2015). Macrolide resistance conferred by rRNA mutations in field isolates of Mannheimia haemolytica and Pasteurella multocida. J. Antimicrob. Chemother. 70, 420–423. doi: 10.1093/jac/dku385
Owen, J. R., Noyes, N., Young, A. E., Prince, D. J., Blanchard, P. C., Lehenbauer, T. W., et al. (2017). Whole-genome sequencing and concordance between antimicrobial susceptibility genotypes and phenotypes of bacterial isolates associated with bovine respiratory disease. G3 7, 3059–3071. doi: 10.1534/g3.117.1137
Pereira-Marques, J., Hout, A., Ferreira, R. M., Weber, M., Pinto-Ribeiro, I., van Doorn, L.-J., et al. (2019). Impact of host DNA and sequencing depth on the taxonomic resolution of whole metagenome sequencing for microbiome analysis. Front. Microbiol. 10:1277. doi: 10.3389/fmicb.2019.01277
Rainbolt, S., Pillai, D. K., Lubbers, B. V., Moore, M., Davis, R., Amrine, D., et al. (2016). Comparison of Mannheimia haemolytica isolates from an outbreak of bovine respiratory disease. Vet. Microbiol. 182, 82–86. doi: 10.1016/j.vetmic.2015.10.020
Ring, N., Low, A. S., Wee, B., Paterson, G. K., Nuttall, T., Gally, D., et al. (2023). Rapid metagenomic sequencing for diagnosis and antimicrobial sensitivity prediction of canine bacterial infections. Microb. Genom. 9:mgen001066. doi: 10.1099/mgen.0.001066
Rose, S., Desmolaize, B., Jaju, P., Wilhelm, C., Warrass, R., and Douthwaite, S. (2012). Multiplex PCR to identify macrolide resistance determinants in Mannheimia haemolytica and Pasteurella multocida. Antimicrob. Agents Chemother. 56, 3664–3669. doi: 10.1128/AAC.00266-12
Rosen, B. D., Bickhart, D. M., Schnabel, R. D., Koren, S., Elsik, C. G., Tseng, E., et al. (2020). De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience 9:giaa021. doi: 10.1093/gigascience/giaa021
Seemann, T. Abricate. Available at: https://github.com/tseemann/abricate.
Shao, J., Hassouna, A., Wang, Y., Zhang, R., Zhen, L., Li, R., et al. (2022). Next-generation sequencing as an advanced supplementary tool for the diagnosis of pathogens in lower respiratory tract infections: an observational trial in Xi'an, China. Biomed. Rep. 16:14. doi: 10.3892/br.2021.1497
Snyder, E. R., Alvarez-Narvaez, S., and Credille, B. C. (2019). Genetic characterization of susceptible and multi-drug resistant Mannheimia haemolytica isolated from high-risk stocker calves prior to and after antimicrobial metaphylaxis. Vet. Microbiol. 235, 110–117. doi: 10.1016/j.vetmic.2019.06.012
Snyder, E., Credille, B., Berghaus, R., and Giguere, S. (2017). Prevalence of multi drug antimicrobial resistance in isolated from high-risk stocker cattle at arrival and two weeks after processing. J. Anim. Sci. 95, 1124–1131. doi: 10.2527/jas.2016.1110
Snyder, E. R., Savitske, B. J., and Credille, B. C. (2020). Concordance of disk diffusion, broth microdilution, and whole-genome sequencing for determination of in vitro antimicrobial susceptibility of Mannheimia haemolytica. J. Vet. Intern. Med. 34, 2158–2168. doi: 10.1111/jvim.15883
Stanford, K., Zaheer, R., Klima, C., McAllister, T., Peters, D., Niu, Y. D., et al. (2020). Antimicrobial resistance in members of the bacterial bovine respiratory disease complex isolated from lung tissue of cattle mortalities managed with or without the use of antimicrobials. Microorganisms 8:288. doi: 10.3390/microorganisms8020288
Taxt, A. M., Avershina, E., Frye, S. A., Naseer, U., and Ahmad, R. (2020). Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 10:7622. doi: 10.1038/s41598-020-64616-x
Votintseva, A. A., Bradley, P., Pankhurst, L., del Ojo Elias, C., Loose, M., Nilgiriwala, K., et al. (2017). Same-day diagnostic and surveillance data for tuberculosis via whole-genome sequencing of direct respiratory samples. J. Clin. Microbiol. 55, 1285–1298. doi: 10.1128/JCM.02483-16
Whittle, E., Yonkus, J. A., Jeraldo, P., Alva-Ruiz, R., Nelson, H., Kendrick, M. L., et al. (2022). Optimizing Nanopore sequencing for rapid detection of microbial species and antimicrobial resistance in patients at risk of surgical site infections. mSphere 7, e0096421–e0000921. doi: 10.1128/msphere.00964-21
Wick, R. R., Judd, L. M., Gorrie, C. L., and Holt, K. E. (2017). Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genom. 3:e000132. doi: 10.1099/mgen.0.000132
Wolfger, B., Timsit, E., White, B. J., and Orsel, K. (2015). A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. The Veterinary clinics of North America. Food Anim. Practi. 31, 351–365. doi: 10.1016/j.cvfa.2015.05.005
Wood, D. E., Lu, J., and Langmead, B. (2019). Improved metagenomic analysis with kraken 2. Genome Biol. 20:257. doi: 10.1186/s13059-019-1891-0
Younes, J. A., Ramsay, D. E., Lacoste, S., Deschner, D., Hill, J. E., Campbell, J., et al. (2022). Changes in the phenotypic susceptibility of Mannheimia haemolytica isolates to macrolide antimicrobials during the early feeding period following metaphylactic tulathromycin use in western Canadian feedlot calves. Can. Vet. J. 63, 920–928.
Zaheer, R., Noyes, N., Ortega Polo, R., Cook, S. R., Marinier, E., Van Domselaar, G., et al. (2018). Impact of sequencing depth on the characterization of the microbiome and resistome. Sci. Rep. 8:5890. doi: 10.1038/s41598-018-24280-8
Zhang, L., Huang, W., Zhang, S., Li, Q., Wang, Y., Chen, T., et al. (2022). Rapid detection of bacterial pathogens and antimicrobial resistance genes in clinical urine samples with urinary tract infection by metagenomic Nanopore sequencing. Front. Microbiol. 13:858777. doi: 10.3389/fmicb.2022.858777
Keywords: long-read metagenomic sequencing, bovine respiratory disease, antimicrobial resistance, feedlot cattle, antimicrobial resistance genes
Citation: Herman EK, Lacoste SR, Freeman CN, Otto SJG, McCarthy EL, Links MG, Stothard P and Waldner CL (2024) Bacterial enrichment prior to third-generation metagenomic sequencing improves detection of BRD pathogens and genetic determinants of antimicrobial resistance in feedlot cattle. Front. Microbiol. 15:1386319. doi: 10.3389/fmicb.2024.1386319
Edited by:
Sebastien Olivier Leclercq, INRA Centre Val de Loire, FranceReviewed by:
Saria Otani, Technical University of Denmark, DenmarkRobert Valeris-Chacin, Texas A&M University, United States
Copyright © 2024 Herman, Lacoste, Freeman, Otto, McCarthy, Links, Stothard and Waldner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheryl L. Waldner, Y2hlcnlsLndhbGRuZXJAdXNhc2suY2E=; Matthew G. Links, bWF0dGhldy5saW5rc0B1c2Fzay5jYQ==
 Emily K. Herman1
Emily K. Herman1 Simon J. G. Otto
Simon J. G. Otto Matthew G. Links
Matthew G. Links Paul Stothard
Paul Stothard Cheryl L. Waldner
Cheryl L. Waldner