
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 May 2024
Sec. Infectious Agents and Disease
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1384392
 Sena Alkan1
Sena Alkan1 Ilker Inanc Balkan1
Ilker Inanc Balkan1 Serkan Surme2
Serkan Surme2 Osman Faruk Bayramlar3
Osman Faruk Bayramlar3 Sibel Yildiz Kaya1
Sibel Yildiz Kaya1 Ridvan Karaali1
Ridvan Karaali1 Bilgul Mete1
Bilgul Mete1 Gokhan Aygun1
Gokhan Aygun1 Fehmi Tabak1
Fehmi Tabak1 Nese Saltoglu1*
Nese Saltoglu1*Objective: Urinary tract infections (UTIs) due to extended-spectrum beta-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae are among the leading causes of morbidity and mortality in older adults. Identifying associated factors for ESBL production may contribute to more appropriate empirical treatment.
Materials and methods: This was a prospective observational study. Hospitalized patients of age > 65 with community-onset or hospital-acquired upper UTI due to E. coli or Klebsiella pneumoniae were included. A multivariate analysis was performed.
Results: A total of 97 patients were included. ESBL prevalence among UTIs with E. coli or Klebsiella pneumoniae was 69.1% (n = 67). CRP values at the time of UTI diagnosis were found to be significantly higher in the ESBL-producing group (p = 0.004). The multivariate analysis revealed that male gender (OR: 2.72, CI: 1.02–7.25), prior recurrent UTI (OR: 3.14, CI: 1.21–8.14), and the development of secondary bacteremia (OR: 4.95, CI: 1.03–23.89) were major associated factors for UTI in older adults due to ESBL-producing E. coli and Klebsiella pneumoniae.
Conclusion: Severe UTI in older men with a history of recurrent UTI may be a warning to the clinician for ESBL production in the setting of high ESBL prevalence. Carbapenems may be prioritized in the empirical treatment of patients with known risk factors for ESBL.
Urinary tract infections (UTIs) are among the most common infectious diseases in the geriatric population and account for 25% of all infections (Curns et al., 2005). The most frequently isolated microorganisms in UTI are Escherichia coli (E. coli) (75–95%), followed by Klebsiella pneumoniae. Among these species, extended-spectrum beta-lactamase (ESBL)-producing strains are increasing worldwide, causing infections not only in the hospital but also in the community setting (Saltoglu et al., 2015; Lob et al., 2016).
ESBL-producing isolates are resistant to most beta-lactams as well as to other classes of antibiotics (Schwaber et al., 2005). As a result, more patients face the risk of receiving inadequate empirical therapy, leading to an increase in morbidity, mortality, and healthcare costs, as well as longer hospital stays and readmissions (Mac Vane et al., 2014; Bischoff et al., 2018; Vallejo-Torres et al., 2018). On the other hand, overuse of antimicrobial agents, particularly carbapenems, such as meropenem and imipenem, perpetuates the antimicrobial resistance problem by promoting the selection of multidrug-resistant pathogens.
Several studies have been conducted to investigate the associated factors for ESBL-producing E. coli and Klebsiella pneumoniae UTI. However, there are a limited number of studies demonstrating associated factors for ESBL in older adults with UTIs. In this study, we aimed to determine associated factors for ESBL production in UTIs with E. coli and Klebsiella pneumoniae among older adults. By doing so, we aim to contribute to antimicrobial stewardship practices by determining the prevalence and associated factors for ESBL-producing E. coli and Klebsiella pneumoniae in hospitalized older adults with upper UTIs.
This study was conducted in a tertiary-care university hospital, which has 676 active adult beds. A prospective observational study of consecutive hospitalized patients with the diagnosis of community-onset or hospital-acquired UTI was conducted in a tertiary university hospital over a 1-year period from October 2019 to September 2020. All adult patients who were consulted by the Infectious Diseases and Clinical Microbiology Department with the diagnosis of complicated UTI in the emergency department and hospital wards were recorded. Patients >65 years were included in the study if their urinary specimen culture showed monomicrobial isolation of E.coli or Klebsiella pneumoniae with a colony-forming unit count of ≥10,000/ml. Diagnosis of acute pyelonephritis or sepsis, severe sepsis, or septic shock of urinary origin was based on symptoms and abnormal urinalysis in patients with no other apparent source of concomitant infection.
In 1 year, 393 adult patients were followed up with the diagnosis of complicated UTIs. A total of 97 of the 393 patients were finally included in the study (Figure 1). All included patients were aged >65 years with E. coli or Klebsiella pneumoniae upper UTIs.
Demographic data such as age, gender, and comorbidities, predisposing and immunosuppressive conditions, symptoms and physical examination findings, laboratory tests, culture results, antimicrobial treatments, and response to treatment of the patients diagnosed with UTIs were recorded. We verified the past medical histories of the patients through the electronic system of our hospital (ISHOP), the “e-nabiz” electronic system of the Ministry of Health, and the “medeczane” electronic database of the National Reimbursement Agency of our country.
In order to determine the clinical factors predicting ESBL production, cases with E. coli and Klebsiella pneumoniae isolated in urine cultures were classified according to their ESBL positivity.
Possible associated factors analyzed as independent variables included age, gender, previous recurrent UTIs, comorbidities, clinical findings (fever, vital signs, and acute phase parameters), and clinical severity (bacteremia, acute renal failure, and septic shock). Outcome variables were inappropriate empirical antibiotic treatment (IEAT), recurrence of UTIs, and in-hospital mortality. The cases were followed up for 3 months in terms of relapse/reinfection.
The diagnosis of UTI was established by evaluating the clinical and laboratory findings of the patients according to the IDSA guidelines. Acute pyelonephritis/upper UTI was defined as the involvement of the bladder and kidneys with fever, flank pain, and costovertebral angle tenderness. Urosepsis/severe UTI was defined as an uncontrolled immune response in the host against urinary infection, leading to life-threatening organ failure. Organ failure was evaluated according to the “Sequential Organ Failure Assessment (SOFA)” scoring. In a patient with sepsis, the persistence of hypotension despite vasopressor treatment and serum lactate level > 2 millimoles/liter (mmol/L) despite adequate fluid resuscitation was defined as septic shock.
Secondary bacteremia was defined as the isolation of the same microorganism in both urine and blood cultures with the same antibiotic susceptibility profile.
A UTI was classified as hospital-acquired if the infection onset was either 48 h after hospitalization or within 3 days after discharge.
A history of recurrent UTIs was defined as ≥2 episodes of UTIs within 6 months or ≥ 3 episodes within 12 months, as documented in the patients’ past medical history.
An empirical antimicrobial in vitro resistance against the causative microorganism was regarded as IEAT.
Urine and blood culture results were reported in the laboratory of the Infectious Diseases and Clinical Microbiology Department. Urine samples were collected sterile as midstream clean catch or from a urinary catheter. The samples were inoculated onto chromogenic (HiCrome Urinary Tract Infection Agar) media. The blood cultures were performed using the BACTEC (Becton Dickinson, Franklin Lakes, NI, USA) automated system. Isolated organisms were identified according to conventional procedures. Antimicrobial susceptibility of organisms was determined using the Kirby–Bauer disk diffusion method on Mueller–Hinton agar according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations. Isolates with “intermediate” susceptibility were classified as “resistant” in the final analysis. Confirmation of ESBL positivity (generally characterized by reduced susceptibility to one or more of ceftazidime, cefotaxime, and ceftriaxone) was performed using a double disk synergy test (DDST). The increased inhibitor activity of third-generation cephalosporins in the presence of vicinal clavulanic acid (synergy) indicates the production of ESBL in Gram-negative bacilli.
The chi-square test was used for categorical variables. The Mann–Whitney U-test was used to compare continuous variables. Univariate regression analysis was performed. All variables detected as significant in a univariate analysis were included in the multivariate analysis. Results were evaluated at a 95% confidence interval, and the statistical significance level was defined as a p-value of <0.05. The analyses were performed using the IBM Statistical Package for Social Sciences 25 (SPSS-25, Chicago, IL, USA).
Of 97 patients with UTIs, 54.6% were women, and the mean age was 75.8 ± 7.9 years. Most of the patients had one or more comorbidities. Of the urine samples, 66 were collected via urine clean catch and 31 were obtained via urine catheters. Almost half of the cases were hospital-acquired UTIs. Urosepsis was determined in 73.2% of cases, and septic shock developed in 8.2%. The main demographic and clinical characteristics of the patients are shown in Table 1.
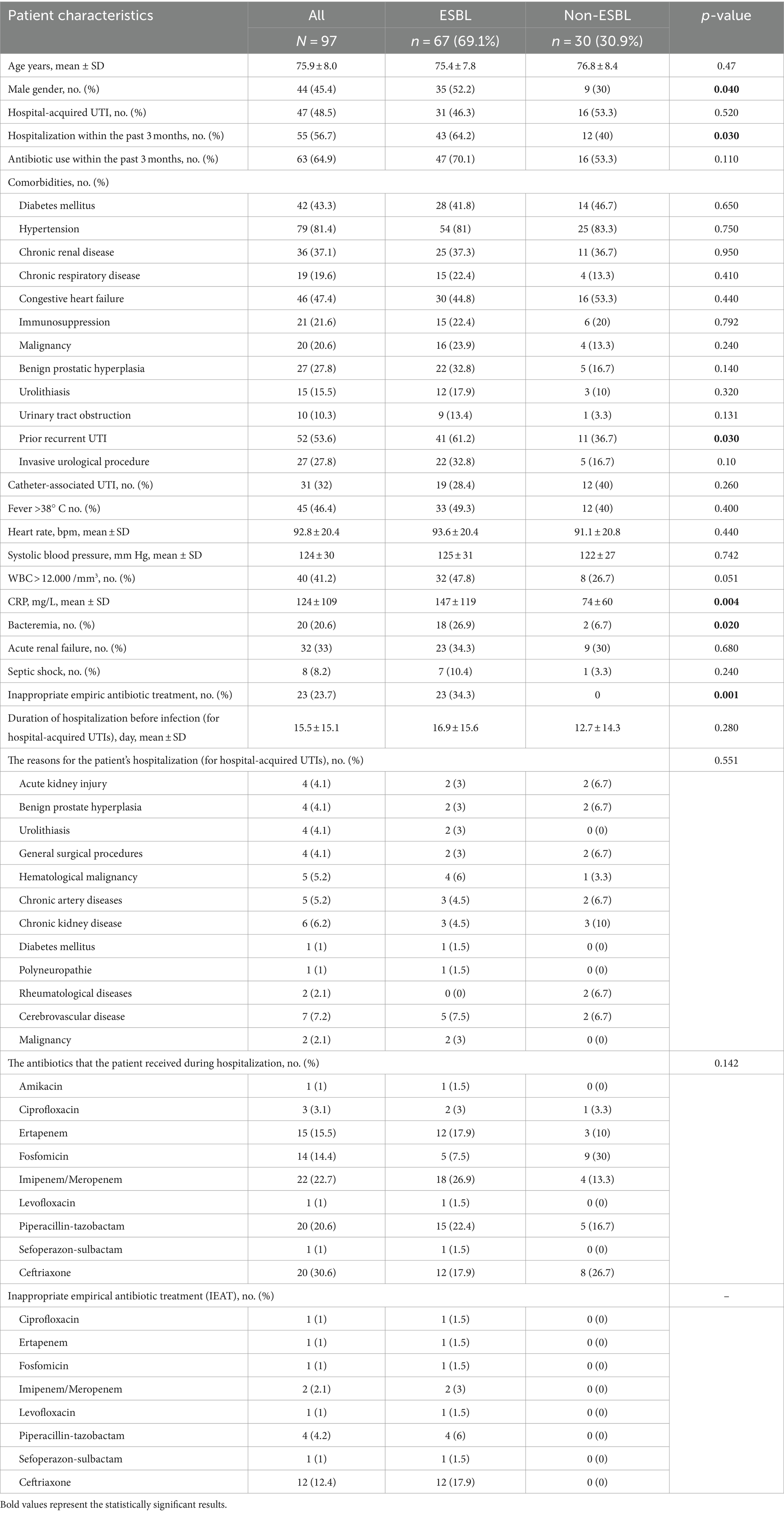
Table 1. Characteristics of patients with urinary tract infections caused by extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae and non-ESBL-producing isolates.
The antibiotics given for the prior infections were ciprofloxacin (n = 20, 20.6%), cefixime (n = 12, 12.4%), cefuroxime (n = 11, 11.3%), ceftriaxone (n = 14, 14.4%), and amoxicillin/clavulanate (n = 6, 6.2%).
E. coli was present in 64 patients, and Klebsiella pneumoniae was present in 33 patients. ESBL rates (64% vs. 78.8%, p = 0.137) and demographic characteristics of patients with E. coli and Klebsiella pneumoniae were similar.
ESBL prevalence among UTIs with E. coli or Klebsiella pneumoniae was 69.1% (n = 67). Male gender (52.2% vs. 30%), hospitalization within the past 3 months (64.2% vs. 40%), prior recurrent UTIs (61.2% vs. 36.7%), and secondary bacteremia (26.9% vs. 6.7%) in the follow-up were more prevalent in the ESBL-producing group than in the non-ESBL-producing group. Leukocytosis (leukocyte count >12.000/mm3) was detected more frequently in the ESBL-producing group (47.8% vs. 26.7%, p = 0.051). CRP values at the time of UTI diagnosis were found to be significantly higher in the ESBL-producing group. The mean CRP was 146.8 mg/L in the ESBL-producing group and 73.5 mg/L in the non-ESBL-producing group (p = 0.004). The IEAT rate was observed as 34.3% in the ESBL-producing group and none in the non-ESBL-producing group. The rate of in-hospital mortality was 3.1%. All three deaths were in the ESBL-producing group. The difference between the rates of post-treatment recurrence in ESBL producers (38.8%) and non-ESBL producers (46.7%) was not statistically significant (p = 0.470).
The empirical antibiotics used for treatment were: meropenem in 22 (22.7%) cases, with 4.5% being IEAT; ceftriaxone in 20 (20.6%), with 60% being IEAT; piperacillin-tazobactam in 20 (20.6%), with 25% being IEAT; ertapenem in 14 (14.4%), with 7.1% being IEAT; fosfomycin in 15 (15.5%), with 6.7% being IEAT; ciprofloxacin/levofloxacin in 4 (4.1%), with 50% being IEAT; amikacin in 1 (1%), which was appropriate; and cefoperazone sulbactam in 1 (1%), which was IEAT.
Antimicrobial resistance profiles of E. coli and Klebsiella pneumoniae isolates in accordance with major epidemiological variables are provided in Table 2. Ertapenem and meropenem were the two antibiotics with resistance rates of less than 20% in the whole cohort. Piperacillin-tazobactam and amikacin resistance rates were 21.9 and 22.6%, respectively.
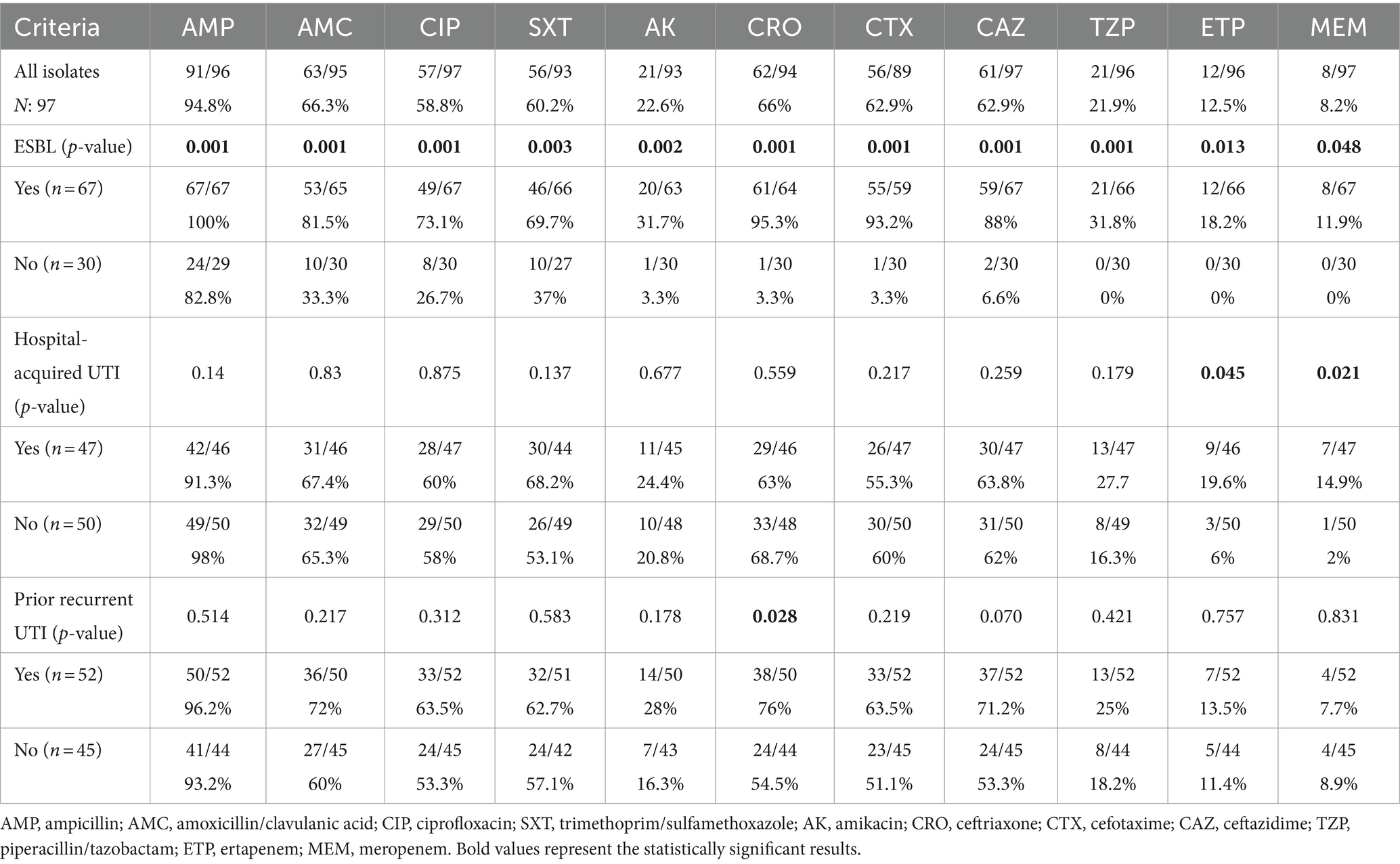
Table 2. Antimicrobial resistance profiles of urinary E. coli and Klebsiella spp. isolates in accordance with major epidemiological groups.
The multivariate analysis revealed that male gender (OR: 2.72, CI: 1.02–7.25), prior recurrent UTI (OR: 3.14, CI: 1.21–8.14), and development of secondary bacteremia (OR 4.95, CI 1.03–23.89) were major associated factors for UTIs in the older adults due to ESBL-producing E. coli and Klebsiella pneumoniae.
In this study, we found that male gender, recurrent UTIs, and secondary bacteremia were independent associated factors for acquiring an ESBL resistance profile in hospitalized older adults with E. coli and Klebsiella pneumoniae UTIs. Prior hospitalization was significant in the univariate analysis, but the multivariate analysis did not reveal it as an associated factor for ESBL UTIs.
In a recent study on hospitalized older adults, Artero et al. identified recurrent UTIs and healthcare-associated UTIs (hospitalization within 3 months, antibiotic use within 3 months, or nursing home residence) as associated factors for ESBL-positive UTIs (Artero et al., 2017).
Many previous studies have similarly documented being male as a predictor of ESBL urinary infection in adults (Nakai et al., 2016; Lee et al., 2018). A recent retrospective study from China, which included a case series where 56% of the participants were over 60 years of age, showed that male gender, older age, hospital stay within the preceding 3 months, invasive urological procedure, and antibiotic use within the previous 3 months were independent factors associated with the development of an ESBL-positive Enterobacteriaceae UTI (Liu et al., 2022). Similar risk factors, such as diabetes mellitus, recurrent UTIs, age over 60, and male gender, were also described by Rodríguez-Bano and Pascual (2004).
Despite many studies identifying a history of antibiotic exposure (IV or oral) as an associated factor for ESBL-positive UTI, we were not able to document recent exposure to antibiotics as an associated factor for infection with ESBL in our study, similar to a recently published study (Hertz et al., 2016). One reason for this may be the high rates of antibiotic use in both ESBL-positive and non-ESBL groups.
The prevalence of ESBL organisms varies by geographical region, ranging from 5% in Japan to 31% in China and 36% in some European countries, as well as approximately 40% in Egypt and Pakistan (Hanberger et al., 1999; Al-Zarouni et al., 2008; Nakai et al., 2016; Abrar et al., 2018; Ranjan Dash et al., 2018; Shash et al., 2019). In the United States, a multicenter prospective study found the prevalence of ESBL-producing Enterobacterales among UTI patients to be 17.2% (Lee et al., 2018).
According to the data of our country’s National Antimicrobial Resistance Surveillance System, the rates of ESBL E. coli were 44.54, 44.61, and 54.53%, and ESBL Klebsiella pneumoniae rates were 52.13, 50.24, and 66% in healthcare-associated infections in Turkey in 2019, 2020, and 2021, respectively (National Antimicrobial Resistance Surveillance System, 2021). Accordingly, in our study, we found an alarmingly high percentage of ESBL-producing E. coli and Klebsiella pneumoniae in hospitalized older adults with UTIs (69.1%). Tüzün T. et al. from Turkey also reported that 50.5% of the cases were infected with ESBL-producing E. coli in their prospective cohort study of community-onset UTIs caused by E. coli between 2012 and 2014 (Tüzün et al., 2019).
Similar to a very recently published study, we found a high rate of ESBL positivity and resistance to other antibiotic classes (fluoroquinolones, sulfonamides, and aminoglycosides) in both community-onset and hospital-acquired UTI cases (Herbawi et al., 2024). Carbapenem resistance was also detected in 11.9% of cases with ESBL positivity. We assume that this is due to the clinical complexity of our cases as well as the fact that we are a high ESBL prevalence country, with similar rates reported from India (Tankhiwale et al., 2004).
ESBL production has been identified as a significant risk factor associated with mortality in systemic infections due to Enterobacterales (Kang et al., 2005; Tumbarello et al., 2006). In their study, Nam Su Ku et al. found that previous antimicrobial therapy and a high initial SOFA score were independent risk factors for 30-day mortality in older adults infected with ESBL-positive agents, but the urinary source of infection was an independent determinant for non-mortality (Ku et al., 2014). In our study, although the mortality rate in the ESBL group (4.5%) was higher compared to the non-ESBL group, we did not find it statistically significant.
We found that the rates of post-treatment recurrent UTIs were also similar in both ESBL and non-ESBL groups. One reason may be that the patients were followed prospectively and carbapenem treatment was initiated promptly in cases of sepsis. In a multicenter study conducted in Sweden, recurrent infection, diabetes mellitus, and urogenital disease were associated with relapse in UTIs caused by ESBL-producing Enterobacterales (Montelin et al., 2024).
In our cases, we found secondary bacteremia significantly higher in the ESBL producers. Switching directly to carbapenem therapy should be considered when secondary bacteremia is detected.
This study has several limitations. First, the sample size was small to include more variables in the multivariate analysis. Second, only inpatients were recruited. Therefore, our study group may not be representative of the greater population.
In conclusion, severe UTIs in older men with a history of recurrent UTIs may serve as a warning to clinicians regarding the potential for ESBL production, especially in settings with a high prevalence of ESBL. These risk factors should be taken into account in the management of empirical treatment. Carbapenems may be prioritized in the empirical treatment of patients with known risk factors for ESBL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Clinical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SA: Conceptualization, Formal analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Data curation, Funding acquisition, Investigation, Resources, Software, Visualization. IB: Supervision, Validation, Writing – original draft, Writing – review & editing. SS: Formal analysis, Validation, Writing – original draft, Writing – review & editing. OB: Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing. RK: Supervision, Writing – original draft, Writing – review & editing. BM: Supervision, Writing – original draft, Writing – review & editing. GA: Supervision, Writing – original draft, Writing – review & editing. FT: Supervision, Writing – original draft, Writing – review & editing. NS: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrar, S., Hussain, S., Khan, R. A., Ain, N. U., Haider, H., and Saba, R. S. (2018). Prevalence of extended-spectrum-β-lactamase producing Enterobacteriaceae: first systematic meta-analysis report from Pakistan. Antimicrob. Resist. Infect. Control 7:26. doi: 10.1186/s13756-018-0309-1
Al-Zarouni, M., Senok, A., Rashid, F., Al-Jesmi, S. M., and Panigrahi, D. (2008). Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Med. Princ. Pract. 17, 32–36. doi: 10.1159/000109587
Artero, A., Esparcia, A., Alberola, J., Madrazo, M., Nogueira, J. M., and Eiros, J. M. (2017). Prospective cohort study of risk factors for extended-spectrum ß-lactamase-producing Escherichia coli urinary tract infections in elderly patients admitted to hospital. Int. J. Clin. Pract. 71:e13001. doi: 10.1111/ijcp.13001
Bischoff, S., Walter, T., Gerigk, M., Ebert, M., and Vogelmann, R. (2018). Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect. Dis. 18:56. doi: 10.1186/s12879-018-2960-9
Curns, A. T., Holman, R. C., Sejvar, J. J., Owings, M. F., and Schonberger, L. B. (2005). Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch. Intern. Med. 165, 2514–2520. doi: 10.1001/archinte.165.21.2514
Hanberger, H., Garcia-Rodriguez, J. A., Gobernado, M., Goossens, H., Nilsson, L. E., and Struelens, M. J. (1999). Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU study groups. JAMA 281, 67–71. doi: 10.1001/jama.281.1.67
Herbawi, A., Abu Taha, A., Aiesh, B. M., Sabateen, A., and Zyoud, S. H. (2024). Spectrum and antibiotic resistance in community-and hospital-acquired urinary tract infections among adults: experience from a large tertiary care center in a developing country. Urologia :3915603241236361. doi: 10.1177/03915603241236361
Hertz, F. B., Schønning, K., Rasmussen, S. C., Littauer, P., Knudsen, J. D., Løbner-Olesen, A., et al. (2016). Epidemiological factors associated with ESBL-and non ESBL-producing E. coli causing urinary tract infection in general practice. Infect Dis. 48, 241–245. doi: 10.3109/23744235.2015.1103895
Kang, C. I., Kim, S. H., Park, W. B., Lee, K. D., Kim, H. B., Kim, E. C., et al. (2005). Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 49, 760–766. doi: 10.1128/AAC.49.2.760-766.2005
Ku, N. S., Kim, Y. C., Kim, M. H., Song, J. E., Oh, D. H., Ahn, J. Y., et al. (2014). Risk factors for 28-day mortality in elderly patients with extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae bacteremia. Arch. Gerontol. Geriatr. 58, 105–109. doi: 10.1016/j.archger.2013.07.002
Lee, H., Han, S. B., Kim, J. H., Kang, S., and Durey, A. (2018). Risk factors of urinary tract infection caused by extended spectrum β-lactamase-producing Escherichia coli in emergency department. Am. J. Emerg. Med. 36, 1608–1612. doi: 10.1016/j.ajem.2018.01.046
Liu, H., Qiu, S., Chen, M., Lyu, J., Yu, G., and Xue, L. (2022). A clinical prediction tool for extended-spectrum β-lactamase-producing Enterobacteriaceae urinary tract infection. BMC Infect. Dis. 22:50. doi: 10.1186/s12879-022-07040-y
Lob, S. H., Nicolle, L. E., Hoban, D. J., Kazmierczak, K. M., Badal, R. E., and Sahm, D. F. (2016). Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn. Microbiol. Infect. Dis. 85, 459–465. doi: 10.1016/j.diagmicrobio.2016.04.022
Mac Vane, S. H., Tuttle, L. O., and Nicolau, D. P. (2014). Impact of extended-spectrum β-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J. Hosp. Med. 9, 232–238. doi: 10.1002/jhm.2157
Montelin, H., Camporeale, A., Hallgren, A., Angelin, M., Hogvall, J., Östholm Balkhed, Å., et al. (2024). Treatment, outcomes and characterization of pathogens in urinary tract infections caused by ESBL-producing Enterobacterales: a prospective multicentre study. J. Antimicrob. Chemother. 79, 531–538. doi: 10.1093/jac/dkad402
Nakai, H., Hagihara, M., Kato, H., Hirai, J., Nishiyama, N., Koizumi, Y., et al. (2016). Prevalence and risk factors of infections caused by extended-spectrum b-lactamase (ESBL)-producing Enterobacteriaceae. J. Infect. Chemother. 22, 319–326. doi: 10.1016/j.jiac.2016.02.004
National Antimicrobial Resistance Surveillance System. (2021) Ulusal Antimikrobiyal Direnç Sürveyans Sistemi. Ulusal Sağlık Hizmeti İlişkili Enfeksiyonlar Sürveyans Ağı Özet Raporu 2021. Available at: https://hsgm.saglik.gov.tr/tr/dokumanlar-bulasicihastaliklar.html (Accessed April 29, 2024).
Ranjan Dash, N., Albataineh, M. T., Alhourani, N., Khoudeir, A. M., Ghanim, M., Wasim, M., et al. (2018). Community-acquired urinary tract infections due to extended-spectrum β-lactamase-producing organisms in United Arab Emirates. Travel Med. Infect. Dis. 22, 46–50. doi: 10.1016/j.tmaid.2018.01.007
Rodríguez-Bano, J., and Pascual, A. (2004). Multiresistant bacteria, nosocomially or community acquired? Enferm. Infecc. Microbiol. Clin. 22, 505–506. doi: 10.1157/13067616
Saltoglu, N., Karali, R., Yemisen, M., Ozaras, R., Balkan, I. I., Mete, B., et al. (2015). Comparison of community-onset healthcare-associated and hospital-acquired urinary infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and antimicrobial activities. Int. J. Clin. Pract. 69, 766–770. doi: 10.1111/ijcp.12608
Schwaber, M. J., Navon-Venezia, S., Schwartz, D., and Carmeli, Y. (2005). High levels of antimicrobial coresistance among extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 49, 2137–2139. doi: 10.1128/AAC.49.5.2137-2139.2005
Shash, R. Y., Elshimy, A. A., Soliman, M. Y., and Mosharafa, A. A. (2019). Molecular characterization of extended-Spectrumβ-lactamase Enterobacteriaceae isolated from Egyptian patients with community-and hospital-acquired urinary tract infection. Am. J. Trop. Med. Hyg. 100, 522–528. doi: 10.4269/ajtmh.18-0396
Tankhiwale, S. S., Jalgaonkar, S. V., Ahamad, S., and Hassani, U. (2004). Evaluation of extended spectrum beta lactamase in urinary isolates. Indian J. Med. Res. 120, 553–556.
Tumbarello, M., Spanu, T., Sanguinetti, M., Citton, R., Montuori, E., Leone, F., et al. (2006). Bloodstream infections caused by extended-spectrum-beta-lactamase producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob. Agents Chemother. 50, 498–504. doi: 10.1128/AAC.50.2.498-504.2006
Tüzün, T., Sayın Kutlu, S., Kutlu, M., and Kaleli, İ. (2019). Risk factors for community-onset urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Turk. J. Med. Sci. 49, 1206–1211. doi: 10.3906/sag-1902-24
Vallejo-Torres, L., Pujol, M., Shaw, E., Wiegand, I., Vigo, J. M., Stoddart, M., et al. (2018). RESCUING study group and study sites. Cost of hospitalised patients due to complicated urinary tract infections: a retrospective observational study in countries with high prevalence of multidrug-resistant gram-negative bacteria: the COMBACTE-MAGNET, RESCUING study. BMJ Open 8:e020251. doi: 10.1136/bmjopen-2017-020251
Keywords: urinary tract infection, extended-spectrum beta-lactamase, Escherichia coli , Klebsiella pneumoniae , associated factors
Citation: Alkan S, Balkan II, Surme S, Bayramlar OF, Kaya SY, Karaali R, Mete B, Aygun G, Tabak F and Saltoglu N (2024) Urinary tract infections in older adults: associated factors for extended-spectrum beta-lactamase production. Front. Microbiol. 15:1384392. doi: 10.3389/fmicb.2024.1384392
Received: 09 February 2024; Accepted: 17 April 2024;
Published: 09 May 2024.
Edited by:
Jens Andre Hammerl, Bundesinstitut für Risikobewertung, GermanyReviewed by:
Fereshteh Jabalameli, Tehran University of Medical Sciences, IranCopyright © 2024 Alkan, Balkan, Surme, Bayramlar, Kaya, Karaali, Mete, Aygun, Tabak and Saltoglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nese Saltoglu, c2FsdG9nbHVAaXVjLmVkdS50cg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.