- 1Department of Digestive Diseases, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 2The First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
Introduction: Observational studies have discovered a contradictory phenomenon between Helicobacter pylori (H. pylori) infection and inflammatory bowel disease (IBD). The study aimed to confirm the causal association between H. pylori and IBD, including ulcerative colitis (UC) and Crohn's disease (CD).
Methods: We conducted a Mendelian randomization (MR) study with two sample Genome-Wide Association Studies (GWAS) to determine whether there is a causal relationship between H. pylori infection and IBD, as well as the possible pathogenic factors that may be involved. The reliability of the main MR assumptions was examined through a series of sensitivity analyses.
Results: Two genetic variants (SNPs) previously identified were employed as instrumental variables (IVs) for H. pylori infection. GWAS data for IBD, UC, and CD were obtained from the recent DF10 release10 of the FinnGen study. Our findings indicated a significant association between H. pylori seropositivity and an increased risk of IBD and UC (IBD: OR: 1.16, 95% CI, 1.03–1.31, P < 0.05; UC: OR: 1.22, 95% CI, 1.08–1.37, P < 0.001) while no causal relationship with CD (P > 0.05). Analysis of the main virulence pathogenic factors revealed a causal relationship between cytotoxin-associated protein A (CagA) and IBD and UC (IBD: OR: 1. 06, 95% CI, 1.001–1.11, P < 0.05; UC: OR: 1.07, 95% CI, 1.004–1.14, P < 0.05), while no correlation was found for vacuolar cytotoxin A (VacA) (P > 0.05). After applying the False Discovery Rate (FDR) correction, the causal relationship between CagA and the risk of IBD or UC was no longer statistically significant.
Conclusion: This study suggests a potential causal relationship between H. pylori infection and IBD, particularly UC. The effect may be more pronounced in individuals with previous H. pylori infections.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, clinically encompassing conditions such as Crohn's disease (CD) and ulcerative colitis (UC) (Bruner et al., 2023). IBD represents a globally prevalent chronic condition significantly impacting patients' quality of life. Epidemiological data indicate a rapid increase in incidence rates in emerging nations with historically low prevalence, while rates remain stable or are rapidly rising in Western countries (Kaplan and Windsor, 2021). The pathogenesis of IBD is thought to involve inappropriate and sustained inflammatory responses to commensal microorganisms in genetically predisposed individuals, although the precise mechanisms remain unclear (Khor et al., 2011).
Helicobacter pylori (H. pylori) infection is highly prevalent, affecting ~60% of the global population (Hooi et al., 2017). H. pylori is a major risk factor for the development of gastric cancer, and recent research has increasingly implicated H. pylori in the pathogenesis of other diseases. Cytotoxin-associated antigen A (CagA) and vacuolar cytotoxin A (VacA) are among the important virulence factors associated with H. pylori (Nejati et al., 2018).
Current evidence suggested that H. pylori infection may induce exacerbation of inflammatory responses, potentially contributing to the pathogenesis of IBD. However, some argued that H. pylori infection may have a protective effect against IBD. This controversial topic has led scholars worldwide to conduct numerous clinical studies, yet the clinical data remain contradictory, lacking consensus (Papamichael et al., 2014). Mendelian randomization (MR), which uses genetic variations to investigate causal relationships between exposures and outcomes, has emerged as a powerful tool to address the limitations and confounding factors present in observational studies (Emdin et al., 2017; Cai et al., 2023). This study applied MR analysis to elucidate causal relationships and guide further research into the pathogenesis of IBD.
Methods
Mendelian randomization design
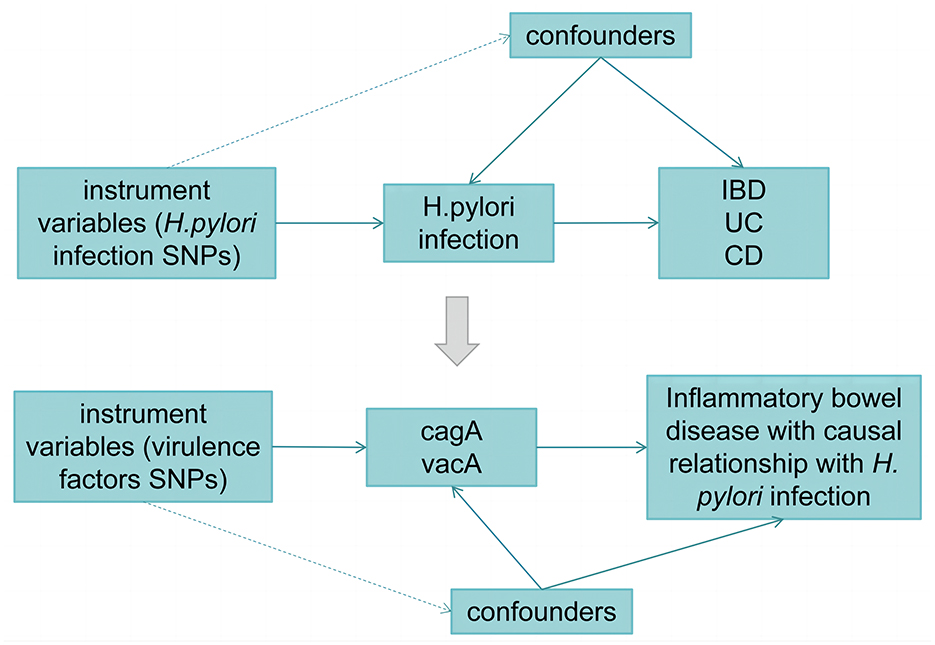
Figure 1 illustrates the workflow of the current Mendelian randomization (MR) study. In our study, we utilized genetic variants as instrumental variables (IVs) for MR analysis. The validity of our MR study relies on three core assumptions:
(1) The relevance assumption: genetic variants are closely associated with the exposure.
(2) The independence assumption: genetic variants are unrelated to any confounding factors that may mediate the pathway from exposure to outcome.
(3) The exclusion-restriction assumption: genetic variants affect the outcome only through the exposure.
The effectiveness of our MR analysis hinges upon these fundamental assumptions.
Data sources
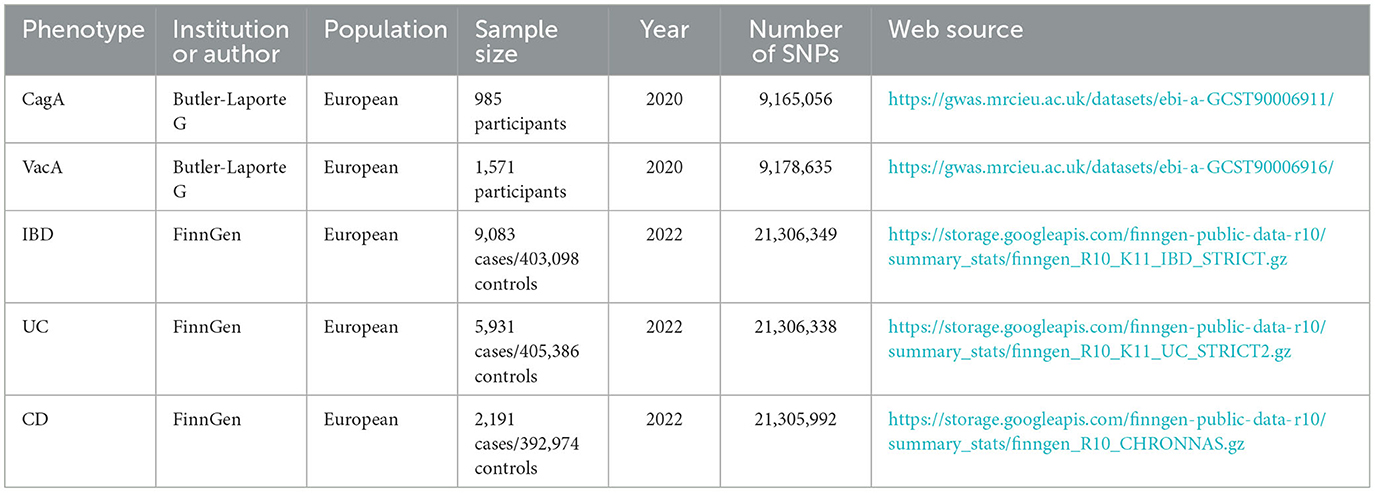
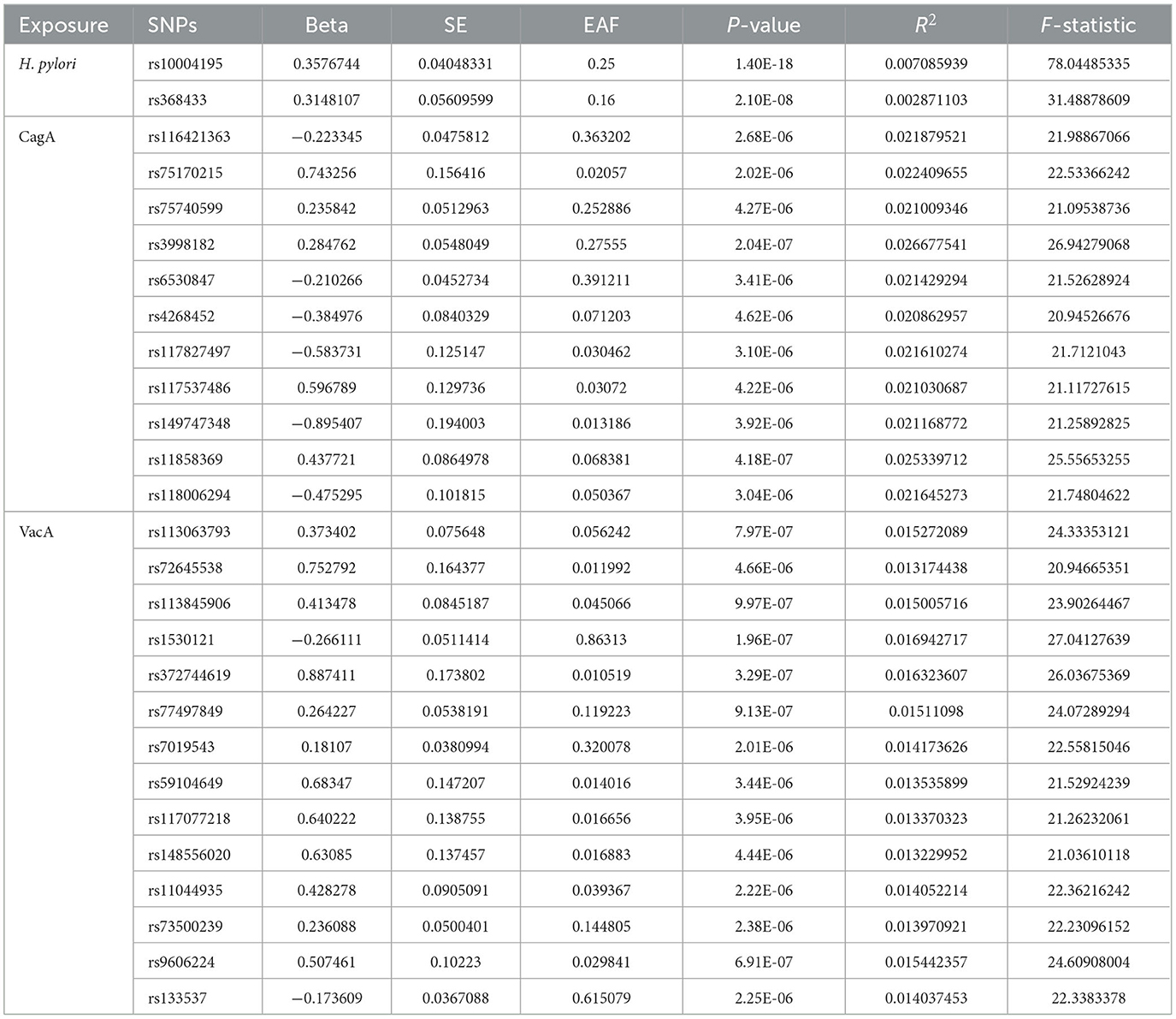
Genetic instrumental variables (IVs) can be obtained through two main approaches: from previous literature or directly from summary statistics of Genome-Wide Association Studies (GWAS). The genetic IVs for Helicobacter pylori infection were abtained from a prior study by Mayerle et al. (2013). Urea breath test, H. pylori fecal antigen detection, and serological testing are commonly used diagnostic methods for Helicobacter pylori in clinical practice. This study utilized the detection of anti-H. pylori IgG titers to determine serum positivity, which served as an indicator of current or past infections. A positive fecal H. pylori antigen detection was considered indicative of an active infection. The study cohort consisted of 10,938 participants, all of European descent. Two single nucleotide polymorphisms (SNPs), rs10004195 (P = 1.4 × 10−8) and rs368433 (P = 2.1 × 10−8, were identified as the strongest genetic variants associated with H. pylori seropositivity, with both SNPs exhibiting P-values below the genome-wide significance threshold of 5 × 10−8. IVs related to CagA were obtained from publicly available data collected from the European Bioinformatics Institute (EBI) database (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90006911/), comprising 985 samples with 9,165,056 SNPs, among which 15 SNPs with a P-value < 5 × 10−6 were considered associated with CagA. IVs related to VacA were also acquired from the EBI database (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90006916/), consisting of 1,571 samples with 9,178,635 SNPs, where 15 SNPs with a P-value < 5 × 10−6 were deemed associated with VacA.
A linkage disequilibrium (LD) clumping algorithm with an R2 threshold < 0.001, window size = 10,000 kb, and a significance threshold of P < 5 × 10−8 was utilized to remove SNPs in strong LD. Subsequently, to ensure that the effect alleles belonged to the same allele, a harmonization of exposure and outcome datasets was performed to eliminate SNPs with intermediate allele frequencies and SNPs with ambiguous alleles. Following these stringent selection criteria, these SNPs were used as IVs for subsequent analysis.
GWAS data for IBD, UC, and CD were obtained from the recent DF10 release (Kurki et al., 2023) of the FinnGen study (https://www.finngen.fi/en), with cases strictly defined through rigorous screening. Details of the GWAS included in this study are provided in Table 1.
Statistical analyses
The inverse-variance weighted (IVW) meta-analysis with multiplicative random model was used as the major analysis for causal estimation. Cochran's Q-test was performed to detect heterogeneity among the genetic variants. The MR–Egger regression model and leave-one-out test were performed as sensitivity analyses and to examine the existence of horizontal pleiotropy that violated the main MR assumptions. MR–Egger regression were applied to detect and correct for pleiotropic effects (Verbanck et al., 2018).
Analyses were performed in R (v.4.3.2) statistical software. The two-sample univariable analyses were performed using the package “TwoSampleMR” (0.5.8). The P-values in this study were 2-sided, and values < 0.05 were deemed significant.
Results
Causal effect of H. pylori infection on IBD, UC, and CD
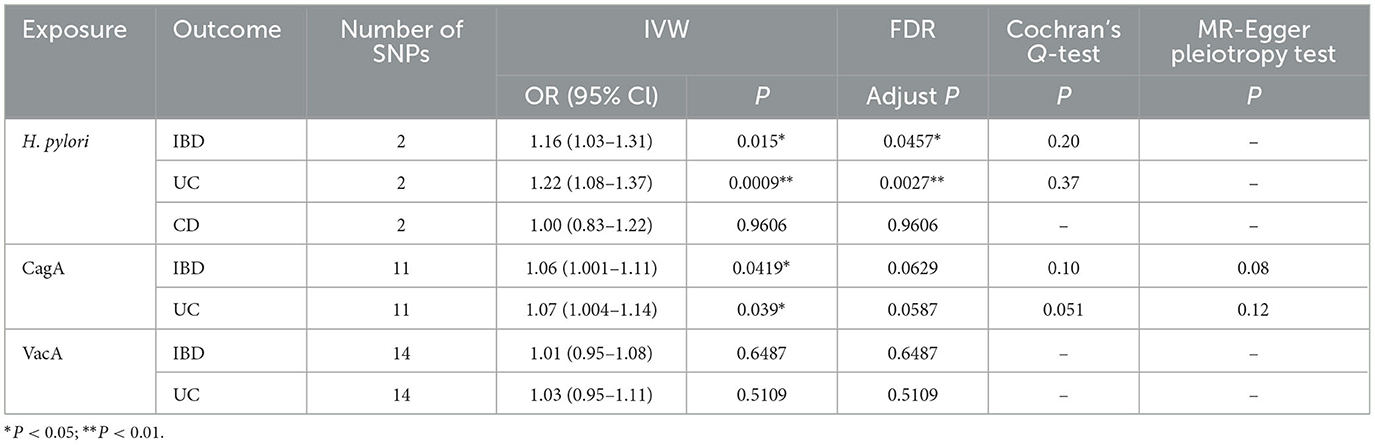
After setting the independence of the exposed data through clumping, and merging the exposure and outcome datasets, the SNP rs10004195 (T > A) and the SNP rs368433 (T > C) were used in the MR analysis working as IVs. The F statistics were were both above 20. All genetic associations were aligned to the allele that increases the H. pylori seropositivity. Genetically predicted H. pylori infection showed causally association with an increased risk of IBD in the FinnGen GWAS under the IVW method [odds ratio (OR): 1.16, P < 0.05; 95% confidence interval (CI), 1.03–1.31; value of P = 0.015]. And causally association with an increased risk of UC in the FinnGen GWAS under the IVW method (OR: 1.22, 95% CI, 1.08–1.37, P = 0.00090) (Figures 2A, B). While no causal relationship showed on CD (P = 0.96).
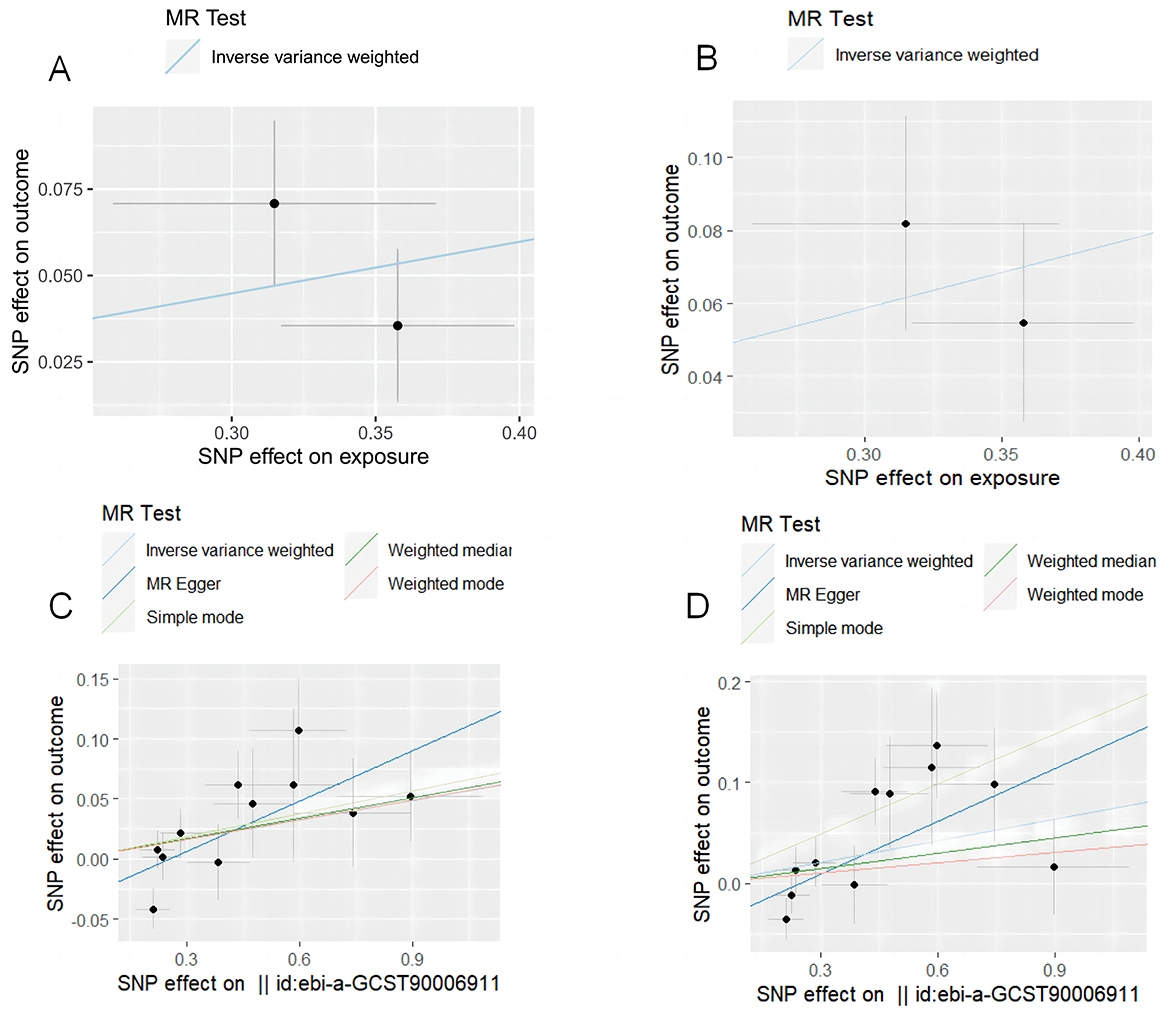
Figure 2. MR test plot. (A) H. pylori infection on IBD. (B) H. pylori infection on UC. (C) CagA on IBD. (D) CagA on UC.
Causal effect of virulence pathogenic factors of H. pylori infection on IBD and UC
After setting the independence of the exposed data through clumping, and merging the exposure and outcome datasets, merging the exposure and outcome datasets, 11 IVs were used for the analysis of CagA, and 14 IVs were used for the analysis of VacA. The F statistics of the selected variables were all above 20, suggesting that there was no strong evidence for weak instrument bias (details of the IVs included in this study are provided in Table 2). Genetically predicted CagA showed causally association with an increased risk of IBD and UC under the IVW method (OR: 1.16, P < 0.05; 95% CI, 1.03–1.31; value of P = 0.015). And causally association with an increased risk of UC in the FinnGen GWAS under the IVW method (IBD: OR: 1. 06, 95% CI, 1.001–1.11, P = 0.042; UC: OR: 1.07, 95% CI, 1.004–1.14, P = 0.039) (Figures 2C, D). While VacA showed no causal relationship on IBD and UC (IBD: P = 0.65; UC: P = 0.51).
After all analyses were completed, FDR (False Discovery Rate) correction was applied to the P-values of the same disease to reduce the probability of false positives, and ultimately the causal relationship between CagA and the risk of IBD or UC was no longer significant (Table 3).
Sensitivity analyses
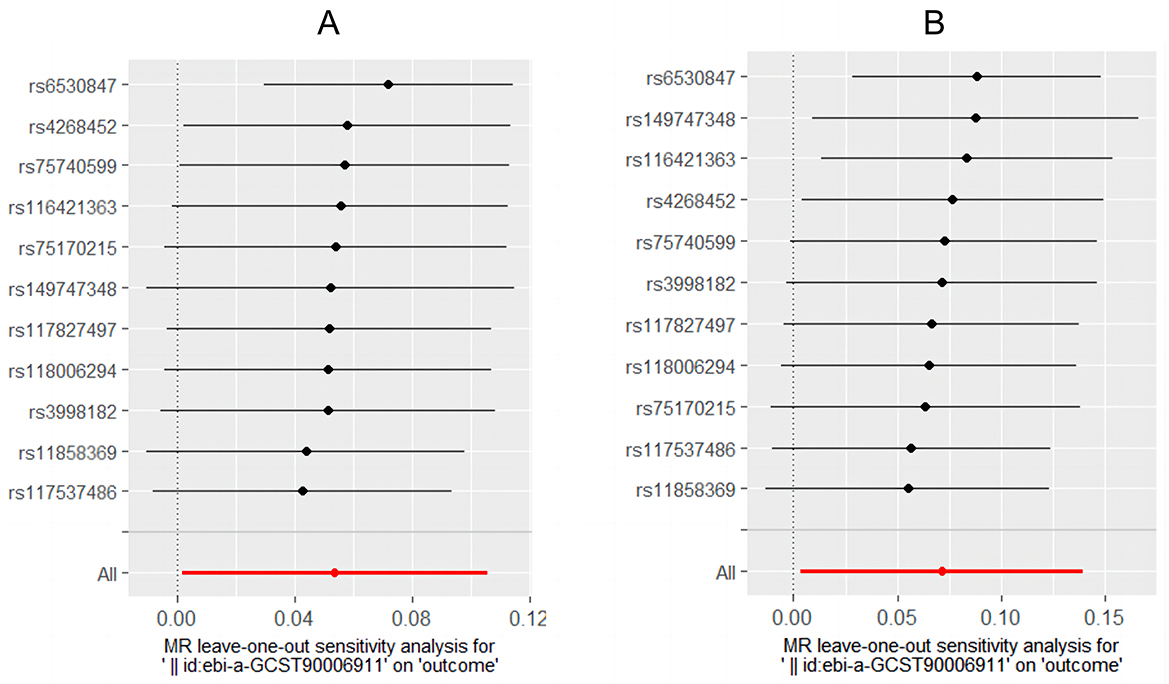
Cochran's Q-test showed that the main estimates of H. pylori infection and CagA on IBD and UC were hardly affected by any heterogeneity (P > 0.05). MR Egger regression model and leave-one-out analyses all showed that the main estimates of CagA on IBD and UC were hardly affected by any pleiotropy (P > 0.05) (Table 3). The leaveoneout plot was used to visualize the sensitivity (Figure 3).
Discussion
In this study, we found an association between H. pylori infection and an increased risk of IBD, primarily linked to the risk of UC. Currently, the association between H. pylori infection and the risk of IBD diseases has been extensively studied, with some indicating that H. pylori infection may induce exacerbation of inflammatory responses, potentially being related to the pathogenesis of IBD.
Due to its microaerobic metabolism, helical shape, and unique motility, H. pylori easily colonizes the surface of the gastrointestinal tract (Sonnenberg, 2013). In inflammatory bowel disease, dysregulation of the host's immune response to commensal bacteria is considered a significant potential pathogenic mechanism. When the gastric mucosa mounts an immune response to H. pylori, products of the local immune reaction may disseminate to extragastric sites. In animal models, enterohepatic Helicobacter species such as H. hepaticus and H. bilis have been shown to induce sustained inflammation in the colon and cecum of immunodeficient rodents (Kullberg et al., 1998; Shomer et al., 1997). Some studies also suggested that infection may exert a protective effect on IBD by altering the diversity of the intestinal microbiota, subsequently modifying the local chemical characteristics of the intestine and the pattern of intestinal immune response (Feilstrecker Balani et al., 2023).
Regarding this controversial topic, scholars from various countries have conducted numerous clinical studies, yet their clinical data remain contradictory, lacking unified results. In a study involving 160 Chinese IBD patients (10%) and 80 control subjects (6.3%), there was no significant difference in the detection rate of Helicobacter genus in intestinal biopsy specimens (Zhang et al., 2011). Some studies had found a lower prevalence of H. pylori infection in IBD patients compared to control groups, suggesting a protective effect of H. pylori infection on the development of IBD (Jin et al., 2013). However, In one study, the PCR positivity rate for Helicobacter genus in UC patients was significantly higher compared to the control group (Thomson et al., 2011). Another study found that the H. pylori genus in the intestinal mucosa of CD patients was significantly higher than that in patients without inflammatory bowel disease through PCR (Oliveira et al., 2006). Clinical studies on H. pylori infection are inherently observational and lack randomization, prospective design, and blinding, leading to significant limitations and confounding factors such as differences in patient medication history and detection methods. Given this contradictory phenomenon, our study aimed to elucidate the potential causal relationship between H. pylori and IBD, the results of MR showed a causal relationship between H. pylori infection and increased risk of UC, but no causal relationship with CD. It is worth mentioning that, given the genetic IV sources of H. pylori infections included in this MR study, H. pylori infections here include both current and past infections. However, the current clinical trials have mainly focused on current H. pylori infections, which may also be the reason for the differences in results.
This study further explored the pathogenic virulence factors and identified an association between CagA and an increased risk of IBD, while VacA was not found to have a related effect. However, after FDR correction, there was no causal relationship between CagA and IBD (P = 0.0629) or between CagA and UC (P = 0.0587). Therefore, the correlation between H. pylori infection and increased risk of IBD may not be related to virulence factors. At the same time, two antibodies related to virulence factors, H. pylori VacA antibody and H. pylori CagA antibody, also represent some current H. pylori infections.
To assess the reliability of our study results, we conducted a series of sensitivity analyses. Cochran's Q-test, MR Egger, and leave-one-out analyses were employed, yielding robust results. These findings suggest that patients with H. pylori infection and associated intestinal symptoms may require screening for IBD. Furthermore, they provide insights for further exploration into the pathogenesis of UC. Perhaps further clinical or laboratory studies are needed to compare current H. pylori infections with previous infections. Further research is needed on the pathogenic factors involved. Meanwhile, it once again indicates the possible differences in the pathogenesis between UC and CD.
Although UC and CD have historically been studied together because they share common features, it is clear that they represent two distinct pathophysiological entities (de Souza and Fiocchi, 2016). Widespread activation of humoral immune response was observed in both diseases, resulting in various changes in immunoglobulin subclasses (MacDermott et al., 1989; Scott et al., 1986). A reported human epithelial colon self antigen recognized by tissue bound IgG antibodies in UC has not been recognized by CD mucosa (Takahashi and Das, 1985). This self antigen also exists in typical sites of extraintestinal manifestations of UC, suggesting that antibody mediated immune responses may be related to the intestinal and extraintestinal pathology of UC patients (Halstensen et al., 1993).
In addition, we found that a Mendelian randomization study on the same topic was published in February of this year (Yang et al., 2024), drawing a conclusion of no causal relationship. After comparing with the content of this article, the main reason for the different conclusions drawn is the different sources of H. pylori infection exposure data. Our genetic IVs were derived from a study in JAMA that identified genetic loci associated with susceptibility to H. pylori (Mayerle et al., 2013). Its diagnosis comes from serum anti Helicobacter pylori IgG and H. pylori antigen in feces, including current and previous infections. The final determined SNPs have P-values < 5 × 10−8. The H. pylori related genetic IVs obtained by Yang et al. were sourced from the EBI database, which includes Anti-H. pylori IgG Phenotype data from previous infections. There were no SNPs in this database with P-values < 5 × 10−8. When the conditions were relaxed to P-values < 5 × 10−6, 11 SNPs were selected for analysis. Therefore, this may not conflict with our research findings.
The current clinical research mainly focused on grouping analysis based on whether H. pylori is currently infected, and inconsistent results have been obtained from each group. In this MR analysis, after FDR correction, it was found that there is no causal relationship between virulence factor related IVs and IBD in some current infections, while genetic IVs determined based on current and past H. pylori infections have a causal relationship with IBD, especially UC. Currently, some studies had reported a suspected increase in the probability of IBD after H. pylori sterilization (Homolak et al., 2021; Chiba et al., 2016). A retrospective cohort study evaluated more than 5 million patients receiving H. pylori eradication treatment, and found that the incidence of IBD increased, and the incidence rate of UC in patients ≥ 30 years old increased more significantly (Mizukami et al., 2023). Therefore, based on current clinical reports and MR results, we speculate that a history of H. pylori infection is associated with an increased risk of IBD (especially UC). The reason for the increase in the incidence rate of IBD after H. pylori eradication may include factors of past H. pylori infection (antibody mediated immune response, changes in flora, etc.) in addition to antibiotics. However, this speculation cannot be validated through MR analysis and may require further clinical or laboratory research.
Conclusion
Our study suggests a potential causal relationship between H. pylori infection and IBD, especially UC. The effect may be more pronounced in individuals with previous H. pylori infections. Further research is needed on the pathogenesis of IBD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YC: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. JLi: Writing – review & editing, Validation. BZ: Software, Writing – review & editing. JLiu: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Henan Province Traditional Chinese Medicine Inheritance and Innovation talent Project Chinese medicine discipline top talent project and Postdoctoral research funding project of Henan Province (HN2022073).
Acknowledgments
Chatgpt 3.5 was used to polish the language of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CagA, cytotoxin-associated protein A; CD, Crohn's disease; CI, confidence interval; EBI, European Bioinformatics Institute; FDR, False Discovery Rate; GWAS, Genome-Wide Association Studies; H. pylori, Helicobacter pylori; IBD, inflammatory bowel disease; IVs, instrumental variables; IVW, inverse-variance weighted; MR, Mendelian randomization; OR, odds ratio; SNP, single nucleotide polymorphism; UC, ulcerative colitis; VacA, vacuolar cytotoxin A.
References
Bruner, L. P., White, A. M., and Proksell, S. (2023). Inflammatory bowel disease. Prim. Care 50, 411–427. doi: 10.1016/j.pop.2023.03.009
Cai, Y., Jia, X., Xu, L., Chen, H., Xie, S., and Cai, J. (2023). Interleukin-17 and inflammatory bowel disease: a 2-sample Mendelian randomization study. Front. Immunol. 14:1238457. doi: 10.3389/fimmu.2023.1238457
Chiba, M., Tsuji, T., Takahashi, K., Komatsu, M., and Sugawara, T. (2016). Ono I. onset of ulcerative colitis after Helicobacter Pylori eradication therapy: a case report. Perm. J. 20, e115–e118. doi: 10.7812/TPP/15-085
de Souza, H. S., and Fiocchi, C. (2016). Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27. doi: 10.1038/nrgastro.2015.186
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian randomization. JAMA 318, 1925–1926. doi: 10.1001/jama.2017.17219
Feilstrecker Balani, G., Dos Santos Cortez, M., Picasky da Silveira Freitas, J. E., Freire de Melo, F., Zarpelon-Schutz, A. C., and Teixeira, K. N. (2023). Immune response modulation in inflammatory bowel diseases by Helicobacter pylori infection. World J. Gastroenterol. 29, 4604–4615. doi: 10.3748/wjg.v29.i30.4604
Halstensen, T. S., Das, K. M., and Brandtzaeg, P. (1993). Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut 34, 650–657. doi: 10.1136/gut.34.5.650
Homolak, J., Nikolić, M., Potoč, D., Živković, M., Bakula, D., Budimir, I., et al. (2021). The onset of ulcerative colitis upon Helicobacter pylori eradication in a 72-year-old woman: report of a rare case with a 3-year follow-up. BMC Gastroenterol. 21:303. doi: 10.1186/s12876-021-01876-5
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153, 420–429. doi: 10.1053/j.gastro.2017.04.022
Jin, X., Chen, Y. P., Chen, S. H., and Xiang, Z. (2013). Association between Helicobacter pylori infection and ulcerative colitis–a case control study from China. Int. J. Med. Sci. 10, 1479–1484. doi: 10.7150/ijms.6934
Kaplan, G. G., and Windsor, J. W. (2021). The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66. doi: 10.1038/s41575-020-00360-x
Khor, B., Gardet, A., and Xavier, R. J. (2011). Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317. doi: 10.1038/nature10209
Kullberg, M. C., Ward, J. M., Gorelick, P. L., Caspar, P., Hieny, S., Cheever, A., et al. (1998). Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 66, 5157–5166. doi: 10.1128/IAI.66.11.5157-5166.1998
Kurki, M. I., Karjalainen, J., Palta, P., Sipilä, T. P., Kristiansson, K., and Donner, K. M. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518. doi: 10.1038/s41586-022-05473-8
MacDermott, R. P., Nash, G. S., and Nahm, M. H. (1989). Antibody secretion by human intestinal mononuclear cells from normal controls and inflammatory bowel disease patients. Immunol. Invest. 18, 449–457. doi: 10.3109/08820138909112255
Mayerle, J., den Hoed, C. M., Schurmann, C., Stolk, L., Homuth, G., Peters, M. J., et al. (2013). Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 309, 1912–1920. doi: 10.1001/jama.2013.4350
Mizukami, K., Sugano, K., Takeshima, T., and Murakami, K. (2023). Disease trends after Helicobacter pylori eradication based on Japanese nationwide claims and the health check-up database. World J. Gastroenterol. 29, 692–705. doi: 10.3748/wjg.v29.i4.692
Nejati, S., Karkhah, A., Darvish, H., Validi, M., Ebrahimpour, S., and Nouri, H. R. (2018). Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb. Pathog. 117, 43–48. doi: 10.1016/j.micpath.2018.02.016
Oliveira, A. G., Rocha, G. A., Rocha, A. M., Sanna, M., d, Moura, S. B., Dani, R., et al. (2006). Isolation of Helicobacter pylori from the intestinal mucosa of patients with Crohn's disease. Helicobacter 11, 2–9. doi: 10.1111/j.0083-8703.2006.00368.x
Papamichael, K., Konstantopoulos, P., and Mantzaris, G. J. (2014). Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J. Gastroenterol. 20, 6374–6385. doi: 10.3748/wjg.v20.i21.6374
Scott, M. G., Nahm, M. H., Macke, K., Nash, G. S., Bertovich, M. J., and MacDermott, R. P. (1986). Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn's disease, and controls. Clin. Exp. Immunol. 66, 209–215.
Shomer, N. H., Dangler, C. A., Schrenzel, M. D., and Fox, J. G. (1997). Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect. Immun. 65, 4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997
Sonnenberg, A. (2013). Review article: historic changes of Helicobacter pylori-associated diseases. Aliment. Pharmacol. Ther. 38, 329–342. doi: 10.1111/apt.12380
Takahashi, F., and Das, K. M. (1985). Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J. Clin. Invest. 76, 311–318. doi: 10.1172/JCI111963
Thomson, J. M., Hansen, R., Berry, S. H., Hope, M. E., Murray, G. I., Mukhopadhya, I., et al. (2011). Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS ONE 6:e17184. doi: 10.1371/journal.pone.0017184
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi: 10.1038/s41588-018-0099-7
Yang, K., Ding, Y., Chen, J., and Sun, X. (2024). No potential causal link between HP infection and IBD: a 2way Mendelian randomization study. Medicine 103:e37175. doi: 10.1097/MD.0000000000037175
Keywords: Crohn's disease, Helicobacter pylori, inflammatory bowel disease, Mendelian randomization, ulcerative colitis
Citation: Cui Y, Li J, Zhao B and Liu J (2024) Helicobacter pylori infection and inflammatory bowel disease: a 2-sample Mendelian randomization study. Front. Microbiol. 15:1384285. doi: 10.3389/fmicb.2024.1384285
Received: 16 February 2024; Accepted: 07 October 2024;
Published: 21 October 2024.
Edited by:
Maria Oana Sasaran, “George Emil Palade” University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Vasiliki Bourika, National and Kapodistrian University of Athens, GreeceMarcos Edgar Herkenhoff, University of São Paulo, Brazil
Shi Xue Dai, Guangdong Provincial People's Hospital, China
Copyright © 2024 Cui, Li, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junying Liu, bGl1anVueWluZzExMUAxNjMuY29t
 Yurong Cui1
Yurong Cui1 Junying Liu
Junying Liu