
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 17 April 2024
Sec. Infectious Agents and Disease
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1376141
Objective: This study aimed to evaluate the prevalence of human papillomavirus (HPV) infection and presence of licensed HPV vaccine genotypes among patients with genital warts in Foshan, China from 2015 to 2022, to provide useful references for the detection, prevention and control of genital warts in Foshan.
Methods: The present study retrospectively analyzed the HPV detection rates in patients with genital warts. A total of 1,625 patients were seen at the Second People’s Hospital of Foshan, Guangdong Province, China, from 2015 to 2022. Samples were collected from various lesions and genotyped for 21 genotypes of HPV by infusion hybridization. The classification principle of HPV genotypes in this study: (1) Based on the relationship between HPV and carcinogenicity; (2) Based on the number of HPV genotypes infected; (3) Based on the HPV genotypes of licensed HPV vaccines.
Results: The detection rate of any HPV in patients with genital warts was 80.37% (1,306/1,625). The detection rates of HPV for low-risk infection, co-infection and high-risk infection were 49.48% (804/1,625), 24.92% (405/1,625) and 5.97% (97/1,625), respectively. Single infection was the predominant type (51.94%, 844/1625). HPV-6 and HPV-11 were the predominant types of single infection; HPV-6 and HPV-52 were the predominant types of paired combinations of multiple infection. 82.22% (1,336/1,625) of the cases had an age distribution of ≤ 24, 25–34, and 35–44. The distribution of some HPV genotypes had age specificity, annual specificity and gender specificity. The genotype detection rates of 2v, 4v and 9v showed a decreasing trend with ages (all P < 0.05). The genotype detection rates of 4v and 9v showed a decreasing trend over the 8-year period (both P < 0.05). The genotype detection rates of 4v and 9v in the male group were higher than those in the female group (both P < 0.05). The genotype detection rate of 9v was significantly higher than that of 2v and 4v in the female group (both P < 0.05).
Conclusion: Our study demonstrated that low-risk infection and single infection were the main types of HPV infection in patients with genital warts, mainly among young patients. Our study provides epidemiological data for the detection, prevention and control of genital warts in China.
Human papillomavirus (HPV) is a round, non-enveloped, double-stranded DNA virus belonging to the family papillomaviridae, whose genome consists of approximately 8,000 bases (Owczarek et al., 2021). It is epithelialized. Based on their oncogenic potential, they are mainly classified into 2 major groups: high-risk HPV (HR-HPV) and low-risk HPV (LR-HPV) (Stanley, 2007). HR-HPV such as HPV-16 and HPV-18, are the main causes of cervical precancerous lesions and cervical cancer (Crow, 2012). LR-HPV such as HPV-6 and HPV-11 are responsible for over 90 percent of the genital warts (Steben and Garland, 2014). Genital warts also known as condyloma acuminatum (CA) or venereal warts, is a sexually transmitted disease caused by human papillomavirus, which mainly occurs in the genital area of the skin mucosa, and is harmful to human health. LR-HPV can cause benign genital warts, such as condyloma acuminatum, and the probability of causing malignant transformation is relatively low. HR-HPV causes hyperproliferation of keratinocytes in lesions, which can lead to cancer in severe cases (Medda et al., 2021). Recent studies highlight the complex interplay between high-risk and low-risk HPV types, suggesting that this interaction may significantly elevate the risk of genital warts development. This nuanced understanding of HPV type interactions underscores the importance of comprehensive HPV typing in clinical and epidemiological investigations of HPV-related diseases (Hasanzadeh et al., 2019; Malagutti et al., 2020).
The attention to HPV vaccine is showing an increasing trend year by year (Bhagavathula and Massey, 2022). Genital warts can be prevented by HPV vaccination, and 2 licensed vaccines related to low-risk types of HPV, the quadrivalent HPV vaccine (4vHPV, Gardasil-4, HPV-6/11/16/18) and the nine-valent HPV vaccine (9vHPV, Gardasil-9, HPV-6/11/16/18/31/33/45/52/58), can effectively prevent HPV-6 and HPV-11 infections (Geretti et al., 2016). There are five types of HPV preventive vaccines that have completed relevant clinical trials in China and have been proven to have good tolerance and immunogenicity: bivalent HPV vaccine (Cervarix, approved in 2016, WHO), quadrivalent HPV vaccine (Gardasil, approved in 2017, WHO), nine-valent HPV vaccine (Gardasil-9, approved in 2018, WHO), homemade Escherichia coli produced HPV bivalent vaccine (Cecolin, approved in 2019, China), and Pichia pastoris produced HPV bivalent vaccine (Walrinvax, approved in 2022, China) (Li et al., 2023). A summary analysis of 1.7 million ordinary women in China showed that the overall prevalence of HPV infection in the country was 15.54% (95% CI: 13.83–17.24%). The top 5 common HPV types detected in the general population were HPV-16 (3.52%, 95% CI: 3.18–3.86%), HPV-52 (2.20%, 95% CI: 1.93–2.46%), HPV-58 (2.10%, 95% CI: 1.88–2.32%), HPV-18 (1.20%, 95% CI: 1.05–1.35%), and HPV-33 (1.02%, 95% CI: 0.89–1.14%) (Zhu B. et al., 2019). Countries with high HPV vaccination coverage have reported a decrease in the burden of genital warts in young women (Smith et al., 2015). However, only 11 countries have included men (World Health Organization [WHO], 2017). According to recent studies, 1/3 of men worldwide are infected with at least one type of genital HPV. Sexually active men, regardless of age, are important hosts for genital HPV infections (Bruni et al., 2023). Prevention and treatment of human papillomavirus in men benefits both men and women (Zou et al., 2022).
At present, research on HPV infection mainly focuses on female, and HPV vaccination mainly prevents cervical cancer in female. The development of licensed vaccines is mainly based on epidemiological data from western countries, and they cannot prevent all prevalent HPV genotypes. Therefore, this study evaluated the epidemiological data of human papillomavirus infection and presence of licensed HPV vaccine genotypes among genital warts patients (including males) in Foshan, providing useful references for the detection, prevention, and control of related genital warts in Foshan.
This study was conducted from January 2015 to December 2022 in the Second People’s Hospital of Foshan. The study enrolled a total of 1,625 patients, comprising 48.06% males and 51.94% females. The age of the participants ranged from 15 to 87 years, with a median age of 31 years. This demographic information is crucial for contextualizing our findings within the broader scope of HPV prevalence and vaccine coverage discussions. The study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of the Second People’s Hospital of Foshan. The inclusion criteria were as follows: (1) All cases met the clinical diagnostic criteria in the Chinese Guidelines for the Diagnosis and Treatment of genital warts (2014); (2) All cases had an epidemiologic history with typical clinical manifestations; (3) All cases had resided in Foshan, Guangdong Province; (4) All cases had signed an informed consent form and voluntarily participated in this study. The exclusion criteria were as follows: (1) Cases with abnormal sample collection or undetected internal standards; (2) Cases with incomplete medical information, such as name, age, gender, visit time, and clinical diagnosis; (3) Cases with genital or vulvar cancer.
Patients were clinically examined by experienced gynecologists or dermatologists at the time of the visit to determine the presence of genital warts lesions in the cervix, vagina, vulva, anus, and perianal region of women, and genital warts lesions in the external genitalia, anus, and perianal region of men. If genital warts lesions were found, a sample preservation tube was used to collect biopsy tissue from the genital warts lesion and stored at 4°C until nucleic acid extraction.
The viral genome extraction kit and human papilloma typing kit (HybriMax) were purchased from Guangdong Kaipu Biotechnology Co., Ltd. 21 HPV genotypes could be detected by the human papilloma typing kit (HybriMax), including 6 low-risk types (HPV-6, HPV-11, HPV-42, HPV-43, HPV-44, HPV-81) and 15 high-risk types (HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-53, HPV-56, HPV-58, HPV-59, HPV-66, HPV-68). The main instruments included: TC-96/G/H(b) gene amplifier, which was produced by Hangzhou BORI Technology Co., Ltd; medical nucleic acid molecular rapid hybridizer HB-2012A and automatic nucleic acid extractor HBNP-4801A, which were produced by Guangdong Kaipu Biotechnology Co., Ltd.
(1) DNA was extracted from the samples by an automated nucleic acid extractor using a nucleic acid extraction kit (Magnetic Bead Method DR-4801-KZ). The quality of the extracted DNA was confirmed by amplifying the β-bead protein gene as an internal control. (2) 24 μL of prepared amplification reagent was added to the set PCR reaction tubes separately. 1 μL each of treated sample DNA, blank control, and positive control were added, respectively. The PCR amplification protocol was executed as follows: an initial hold at 20°C for 10 min, followed by a primary denaturation at 95°C for 9 min. This was succeeded by 40 cycles, each consisting of denaturation at 95°C for 20 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Upon completion of these cycles, a final extension was conducted at 72°C for 5 min, with the reaction subsequently maintained at 4°C. (3) After completion of the amplification of the L region of the HPV genome, the membrane strips were labeled with the patient’s number and then fixed into the hybridizer, and hybridization and color development were carried out in accordance with the instruction manual. (4) Judgment of results: naked eye observation, the positive points and quality control points of the test results were clearly visible blue purple biotin staining points.
The classification and statistics of HPV in this study were based on the following principles: (1) Based on the relationship between HPV and carcinogenicity: high-risk infection, low-risk infection, and co-infection (Infection caused by both HR-HPV and LR-HPV). (2) Based on the number of HPV genotypes infected: single infection and multiple infection. (3) Based on the HPV genotypes of licensed HPV vaccines: bivalent genotypes (2v) (HPV-16/18), quadrivalent genotypes (4v) (HPV-6/11/16/18), nine-valent genotypes (9v) (HPV-6/11/16/18/31/33/45/52/58), and non-9v genotypes (Non-9v) (HPV-42/43/44/81/35/39/51/53/56/59/66/68).
Participants grouping principles: (1) Participants were categorized into five groups according to their age (≤ 24, 25–34, 35–44, 45–54, and ≥ 55). (2) Participants were categorized into 8 groups based on the year of their visitation (2015, 2016, 2017, 2018, 2019, 2020, 2021, 2022). (3) Participants were categorized into 2 groups based on their gender (male and female). This study was conducted using WPS Office (2023) for data processing and analysis, and SPSS 25.0 statistical software was used to count the data. Count data were expressed as percentages (%) using the chi-square test. P < 0.05 was considered to indicate a statistically significant difference. The Goodman Kruskal Gamma method was used to analyze the correlation between HPV detection rate and age/year (ordered categorical variables), and calculated the Gamma coefficient. The Gamma coefficient ranges from −1 to 1, where G = 0 indicated two variables were uncorrelated, G > 0 indicated two variables were positively correlated, and G < 0 indicated two variables were negatively correlated. Chord plot, heat maps and bidirectional bar chart were plotted using the bioladder tools. Chord plot was used to display paired combinations of different types of HPV in patients with multiple infection. Heatmaps were used to visually display the differences in HPV genotype detection rates among different groups. Before drawing a heatmap, the data such as age and year were converted into detection rates. The data file was saved and imported into the heatmap tool of Bioloader, and the grouping information was imported into the Y-axis. Logarithmic processing, row clustering, and column clustering were not performed. After submission, a heatmap containing Z-values could be obtained. Using the Z-score method to normalize data with large differences by row could make the heatmap more vividly reflect the detection rate differences between different age and year groups of the same HPV type. Bidirectional bar chart was used to display the differences in HPV detection rates among different gender groups.
Of the 1,625 patients with genital warts, 1,306 were detected as HPV positive, with a detection rate of 80.37% (1,306/1,625). The detection rates of HPV for low-risk infection, co-infection and high-risk infection were 49.48% (804/1,625), 24.92% (405/1,625) and 5.97% (97/1,625), respectively.
Of the 1,306 HPV infections, 844 (51.94%, 844/1,625) were caused by infection with a single HPV type, especially HPV-6 (23.75%, 386/1,625) and HPV-11 (14.46%, 235/1,625). Of the 462 (28.43%, 462/1,625) multiple infections, 280 (17.23%, 280/1,625) were double infections, 118 (7.26%, 118/1,625) were triple infections, 41 (2.52%, 41/1,625) were quadruple infections, and 23 (1.42%, 23/1,625) were quintuple or more infections. The detection rate of single infection was significantly higher than that of multiple infection (P < 0.05). Pairwise combinations of different HPV types in multiple infection could be clearly observed by chord plot (Figure 1), the most common combinations included HPV-6 and HPV-52 (47 times); HPV-52 and HPV-81 (38 times), and HPV-6 and HPV-16 (36 times).
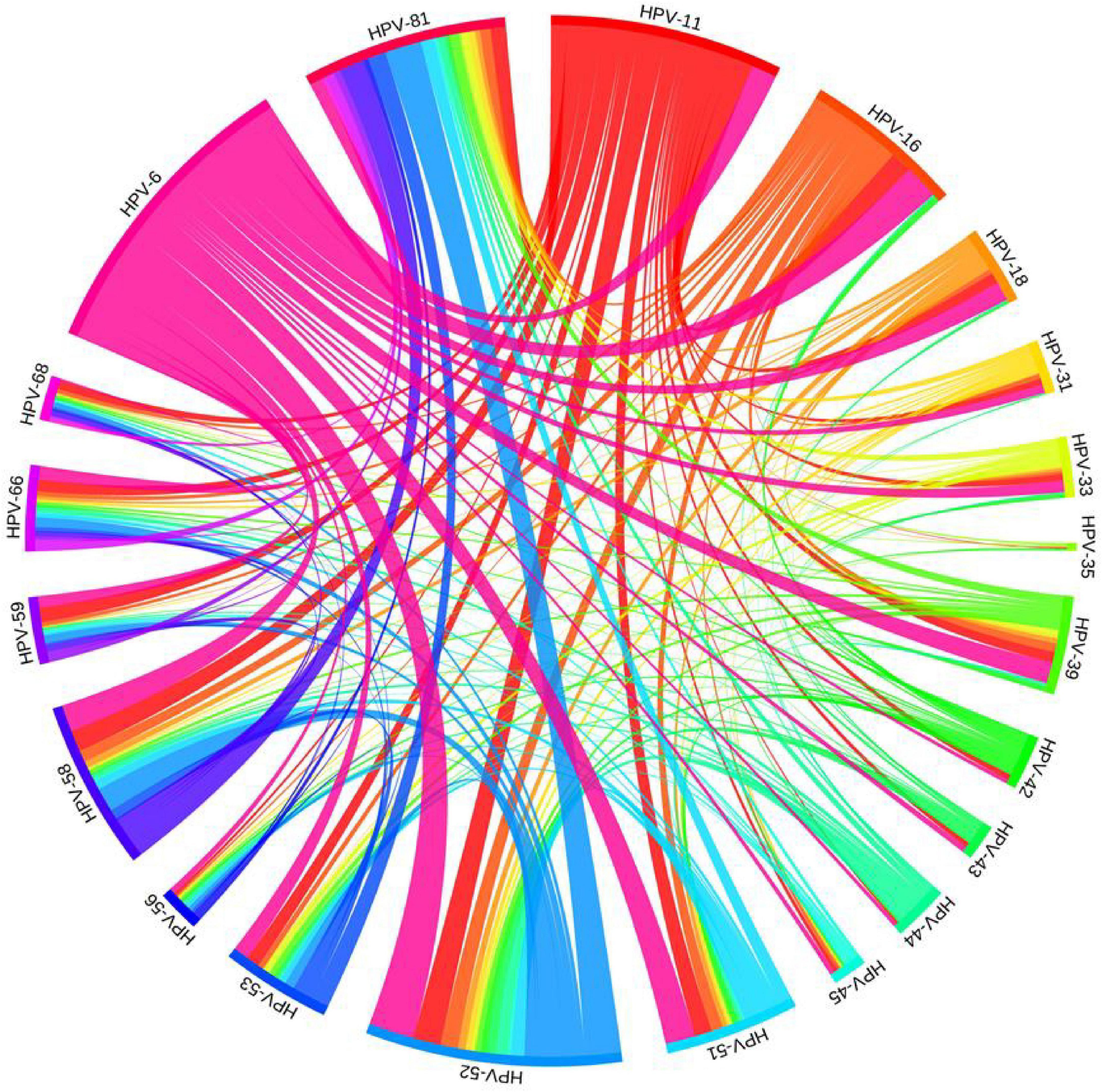
Figure 1. Chord plot of pairwise combinations of different HPV types in patients with multiple infection of genital warts in Foshan, China.
The detection rates of any HPV within the age groups ≤ 24, 25–34, 35–44, 45–54, and ≥ 55 were 82.11% (303/369), 79.69% (506/635), 81.33% (270/332), 82.86% (145/175) and 71.93% (82/114), respectively (Table 1). There were no statistically significant differences in the detection rates of any HPV, low-risk infection, co-infection, single infection and multiple infection among different age groups (all P > 0.05). The differences in detection rates of 3 types of LR-HPV (HPV-11, HPV-44, HPV-81) and 4 types of HR-HPV (HPV-16, HPV-33, HPV-39, HPV-51) were statistically significant (all P < 0.05). The detection rates of HPV-44 and HPV-81 tended to increase with ages (both P < 0.05), and the detection rates of high-risk infection, HPV-11, HPV-16, HPV-33, HPV-51, and HPV-58 trended to decrease with ages (all P < 0.05). The distribution of detection rates of HPV genotypes in different age groups can be seen by heat map (Figure 2).
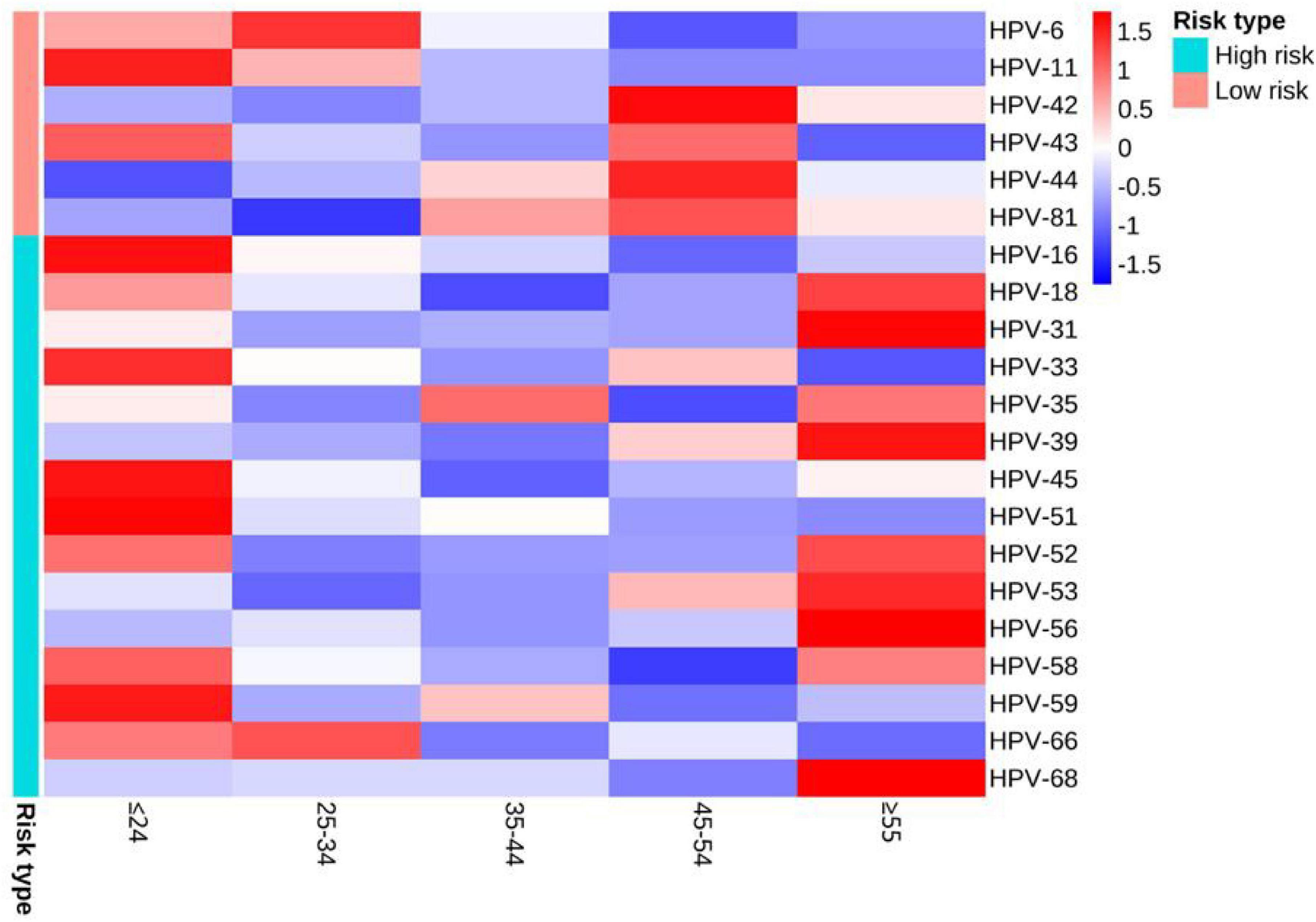
Figure 2. Heat map of the distribution of detection rates of HPV genotypes in different age groups. When the Z-value in the legend is lower than the average value, Z is negative, otherwise it is positive.
The detection rates of any HPV in 2015, 2016, 2017, 2018, 2019, 2020, 2011, and 2022 were 86.29% (107/124), 60.69% (88/145), 76.76% (109/142), 93.65% (118/126), 83.15% (153/184), 80.09% (189/236), 81.58% (248/304) and 80.77% (294/364), respectively (Table 2). There were no statistically significant differences in the detection rates of any HPV, high-risk infection, low-risk infection and single infection among different year groups (all P > 0.05). However, the differences in detection rates of co-infection and multiple infection were statistically significant (both P < 0.05). The differences in detection rates of 4 types of LR-HPV (HPV-11, HPV-42, HPV-43, HPV-44) and 1 type of HR-HPV (HPV-16) were statistically significant (all P < 0.05). The detection rates of co-infection, multiple infection, HPV-42, HPV-43, HPV-44 and HPV-16 tended to increase with years (all P < 0.05), and the detection rates of HPV-6 and HPV-11 trended to decrease with years (both P < 0.05). The distribution of detection rates of HPV genotypes in different year groups can be seen by heat map (Figure 3).
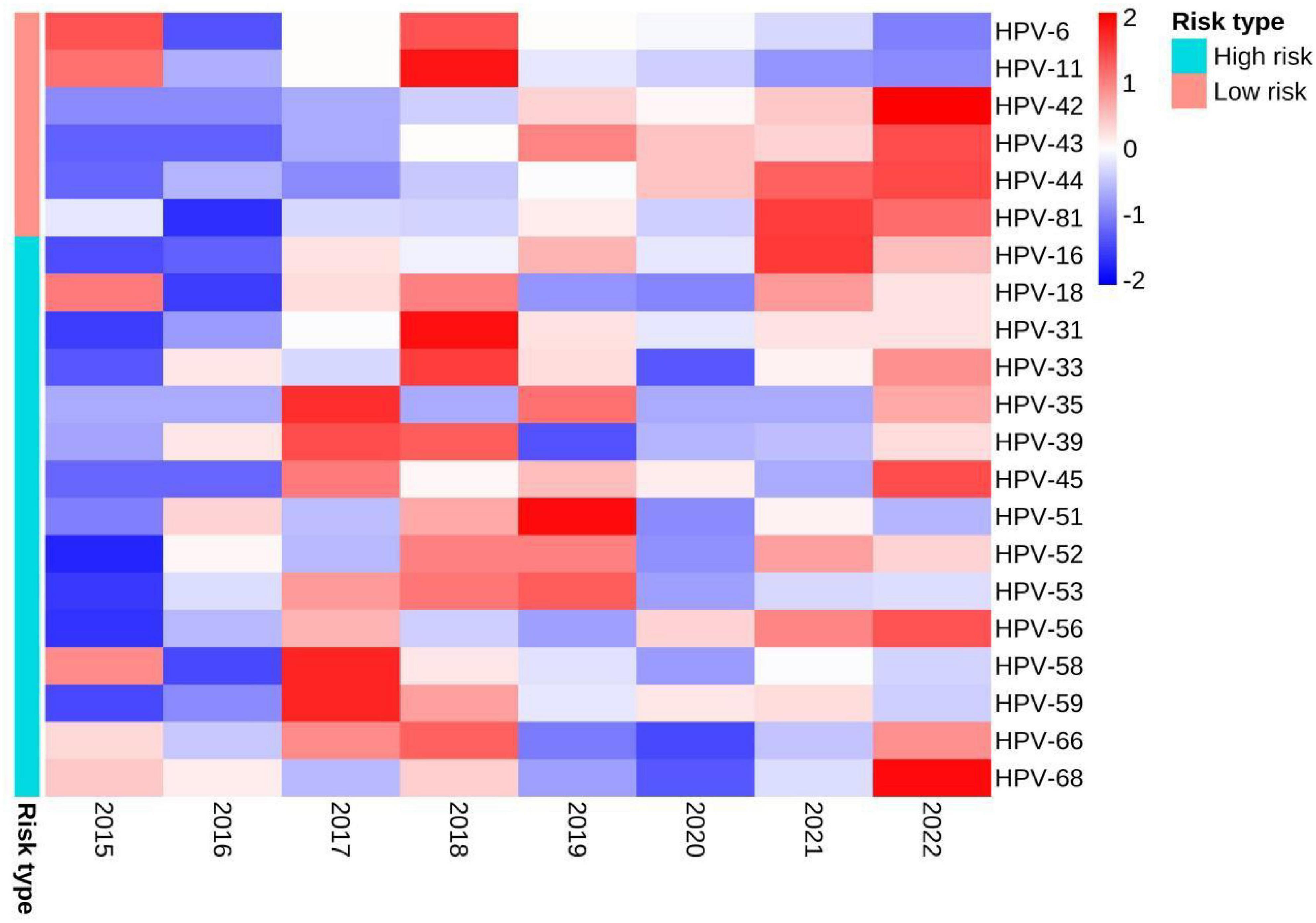
Figure 3. Heat map of the distribution of detection rates of HPV genotypes in different year groups. When the Z-value in the legend is lower than the average value, Z is negative, otherwise it is positive.
The detection rates of any HPV in the male and the female groups were 76.70% (599/781) and 83.77% (707/844), respectively (Table 3). There were no statistically significant differences in the detection rates of high-risk infection, HPV-16, HPV-18, HPV-31, HPV-35, HPV-45, HPV-59 and HPV-66 between the two groups (all P > 0.05). The detection rates of low-risk infection, single infection, HPV-6 and HPV-11 in the male group were higher than that in the female group (all P < 0.05). The detection rates of any HPV, co-infection, multiple infection, HPV-42, HPV-43, HPV-44, HPV-81, HPV-33, HPV-39, HPV-51, HPV-52, HPV-53, HPV-56, HPV-58 and HPV-68 in the female group were higher than that in the male group (all P < 0.05). The distribution of the detection rates of HPV genotypes in different gender groups can be seen by the bidirectional bar chart (Figure 4).
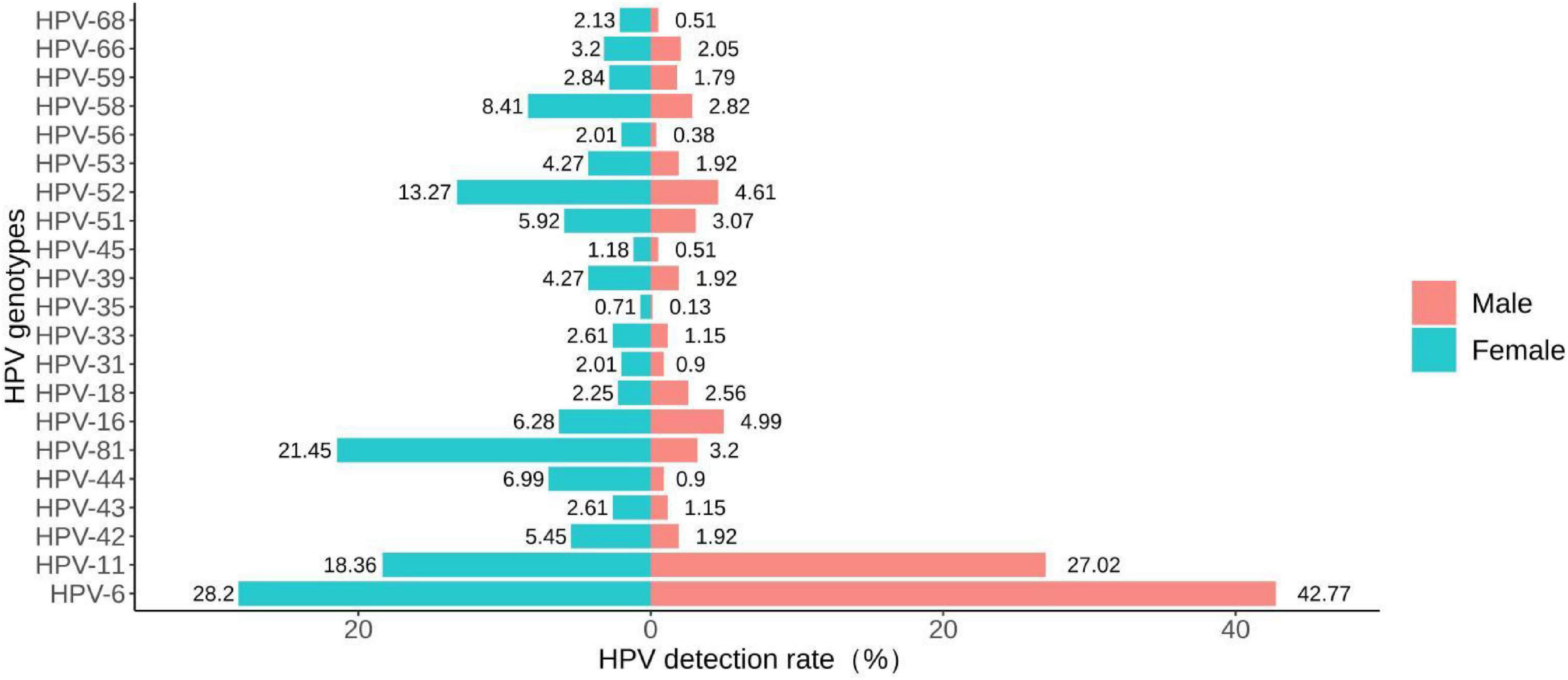
Figure 4. Bidirectional bar chart of the distribution of detection rates of HPV genotypes in different gender groups.
The detection rates of 2v, 4v, 9v, and Non-9v genotypes were 7.75% (126/1,625), 59.08% (960/1,625), 66.65% (1,083/1,625), and 13.72% (223/1,625), respectively (Table 3). There were statistically significant differences in genotype detection rates of 2v, 4v, 9v, and Non-9v among different age groups (all P < 0.05) (Table 1). The genotype detection rates of 2v, 4v and 9v showed a decreasing trend with ages (all P < 0.05), while the genotype detection rate of Non-9v showed an increasing trend with ages (P < 0.05) (Figure 5).
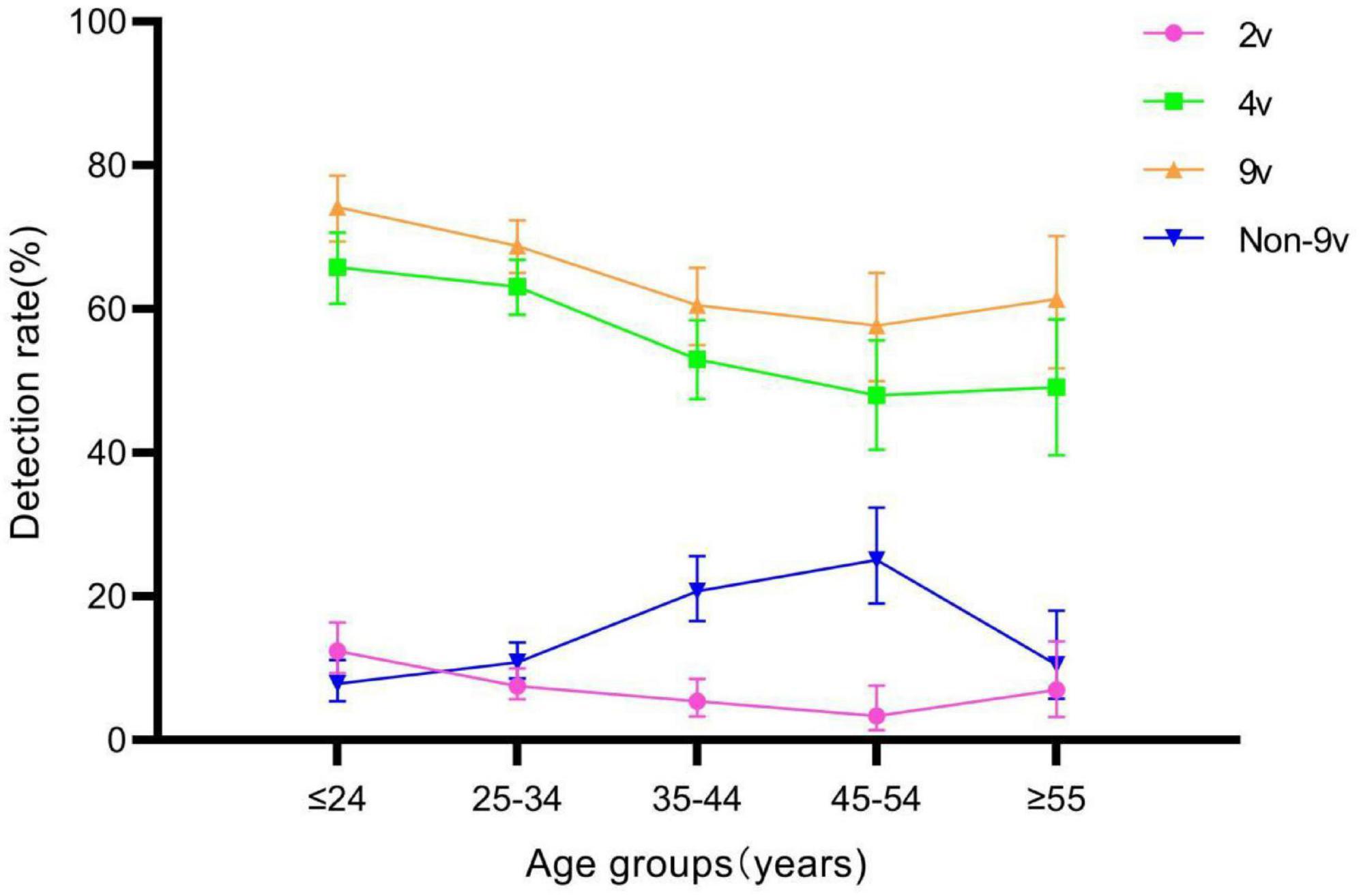
Figure 5. Genotype detection rates of 2v, 4v, 9v, and Non-9v among different age groups. Error bars represent 95% confidence intervals.
There were statistically significant differences in genotype detection rates of 2v, 4v, 9v, and Non-9v among different year groups (all P < 0.05) (Table 2). The genotype detection rates of 2v and Non-9v showed an increasing trend from 2015 to 2022 (both P < 0.05), while the genotype detection rates of 4v and 9v showed a decreasing trend from 2015 to 2022 (both P < 0.05) (Figure 6).
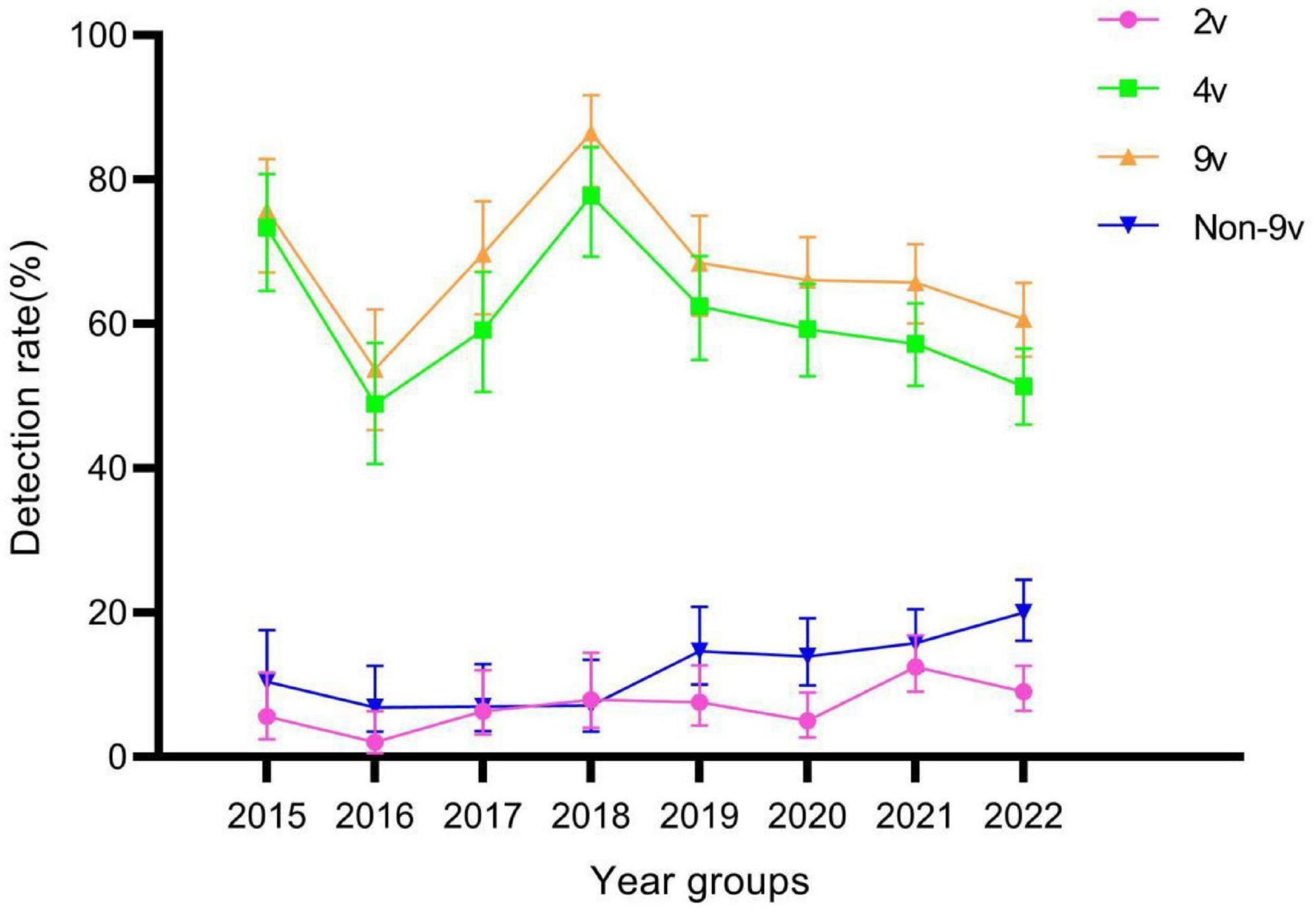
Figure 6. Genotype detection rates of 2v, 4v, 9v, and Non-9v among different year groups. Error bars represent 95% confidence intervals.
We compared the differences in genotype detection rates of 2v, 4v, 9v and Non-9v between genders. The genotype detection rates of 4v and 9v in the male group were higher than those in the female group (both P < 0.05). The genotype detection rate of Non-9v in the female group was higher than that in the male group (P < 0.05) (Table 3). We also compared the differences in genotype detection rates among 2v, 4v and 9v of the same gender (Table 4). The genotype detection rates of 4v and 9v in the male group were significantly higher than that of 2v (both P < 0.05). However, there was no statistically significant difference in the genotype detection rate between 4v and 9v (P > 0.05) in the male group. The genotype detection rates among 2v, 4v, and 9v in the female group showed statistically significant differences (all P < 0.05).
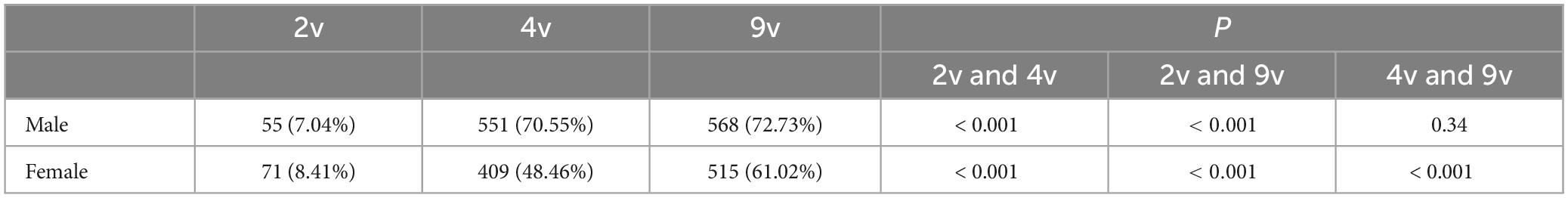
Table 4. Genotype detection rates among 2v, 4v and 9v in patients with genital warts of the same gender.
Comparative studies of the HPV distribution in patients with genital warts of both sexes in the Asia-Pacific region are limited, with even fewer reports from China. At present, researches on the distribution of HPV in China mainly focus on the female population (Chen et al., 2023; Wei et al., 2023; Zhang et al., 2023); Research on the distribution of HPV among general warts is commonly found in northern China (Zhu C. et al., 2019). HPV infection is mainly transmitted between male and female, and the distribution of HPV also has regional characteristics. On the other hand, based on the HPV infection status in patients with genital warts in both genders, research on the genotype detection rate of licensed HPV vaccines is also very rare. This long-term study of 1,625 patients with genital warts in southern China, provided information about HPV infection in Chinese patients with genital warts by age groups, year groups, gender groups, and different genotypes of licensed HPV vaccine groups. The aim was to evaluate the prevalence of human papillomavirus (HPV) infection and presence of licensed HPV vaccine genotypes among genital wart patients. We acknowledge that a high HPV DNA load may indeed reflect elevated viral DNA replication activity, which could correlate with more severe infection states. However, we also highlight the inherent challenges associated with quantifying HPV DNA load due to the heterogenous nature of sampling. Unlike plasma, genital wart samples present difficulties in achieving precise quantification, which influenced our decision to employ qualitative PCR for sample detection. Our study employed infusion hybridization for HPV typing, a method known for its high sensitivity and specificity. This choice was based on the method’s established reliability for detecting a wide range of HPV genotypes. Given the large sample size and the long-term periodic analysis conducted, we opted not to use DNA sequencing for additional genotype verification, considering the strengths of the chosen method. While our study based on hybridization methods has provided valuable information on the epidemiological characteristics of HPV, we acknowledge that more effective methods such as next-generation sequencing (NGS) could offer more detailed HPV genotype information, including co-infections and minor genetic variations, enabling us to more accurately describe the associations between HPV genotypes and various factors. Moreover, to prevent nucleic acid contamination that could affect the outcomes of multiple infections, we handled samples under stringent anti-contamination conditions, used quality control samples, repeated all dubious results, and conducted regular environmental cleaning to ensure the consistency and accuracy of our results.
The detection rate of any HPV was 80.37%. To clarify, the overall undetected rate of HPV in our study was 19.63%. This undetection may be attributed to several factors including the nature of the sample type (e.g., samples from the cervix, vagina, vulva, anus, and perianal region in women, and from lesions in the external genitalia, anus, and perianal region in men), the methodology employed for HPV detection, and the limitations inherent to the sampling process itself. Additionally, the range of HPV types that can be detected by our methodology may also contribute to this outcome. It’s noteworthy that similar studies in the past have reported undetection rates within this range (Ingles et al., 2015; Yuan et al., 2023), suggesting that our findings are consistent with the broader body of research. To mitigate the undetected rate, future research should consider employing other more sensitive and extensive methods to verify HPV negative results. As seen from the chord plot, 21 HPV genotypes were detected in multiple infection. The number of samples with high-risk HPV was quite high in patients with genital warts, which is consistent with the results of a previous study (Zare-Bidaki et al., 2022). Some studies have reported that high-risk HPV genotypes may also be associated with warts (Garland et al., 2009; Hasanzadeh et al., 2019). In this study, co-infections and multiple infections were analyzed separately because, according to the HPV genotype classification criteria in this paper, co-infection is caused by a combination of high-risk and low-risk infections. The presence of high-risk types brings a risk of carcinogenesis. Multiple infections, on the other hand, focus on the number of HPV genotypes, without distinguishing between low-risk and high-risk types.
Our study found that 82.22% (1,336/1,625) of the cases had an age distribution of ≤ 24, 25–34, and 35–44, which may be related to the level of sexual activity. This study found no significant difference in HPV detection rates among different age groups, which is different from a previous study of high-risk male HPV infected individuals (Yin et al., 2020), This may be related to the fact that the research subjects of this study are male and female patients with genital warts. In addition, long-term and persistent HPV infection may pose a risk of cancer transformation, so it is necessary to conduct comprehensive and regular screening for elderly people infected with HPV (Sudenga et al., 2017). Our study also showed that the detection rates of some HPV genotypes were age-specific. Due to cost and technical difficulties, licensed HPV vaccines cannot cover all HPV genotypes. But if different HPV vaccines are designed for different age groups, it may be possible to solve the above problem.
Current literature studies on annual trends in HPV have focused on HPV infection in women (Li et al., 2022), and there are fewer studies on annual trends in patients with genital warts. Our study found no significant difference in the detection rate of any HPV among patients with genital warts from 2015 to 2022. However, the detection rates of co-infection, multiple infection, HPV-42, HPV-43, HPV-44, and HPV-16 showed a gradual increased over time. This differs from another study on the annual distribution of HPV genotypes in male genital warts patients in Shanghai (Li et al., 2021). This suggests that there might be other factors influencing the annual detection rates of HPV infections, including but not limited to trends in the prevalence of each HPV subtype, the impact of HPV vaccination, patients’ sexual behavior habits, and other environmental or societal factors not directly considered in our study. It is worth noting that the currently approved HPV vaccines do not include the low-risk HPV-81 as well as HPV-42, HPV-43, and HPV-44 with increasing detection rates year by year.
Our study found that the detection rate of any HPV was higher in the female group than in the male group. A similar study from Xi’an found that the detection rate of any HPV was higher in males than in females. However, the detection rate of LR-HPV was significantly higher in men than in women (52.3 vs. 35.7%, p < 0.01), which was consistent with this study (Zhu C. et al., 2019). A study suggested that the incidence or clearance of HPV infection may be higher in females than in males due to differences in the biological structure between males and females (Widdice et al., 2010). Our study found that the most common genotypes for males are HPV-6 (334/781, 42.77%) and HPV-11 (211/781, 27.02%), while the most common genotypes for females are HPV-6 (238/844, 28.20%) and HPV-81 (181/844, 21.45%). In contrast, research results from Shandong Province showed that the common genotypes for both males and females in patients with genital warts were HPV-6 and HPV-11 (Yuan et al., 2023).
The human papillomavirus vaccine was originally developed to reduce the number of HPV-related cancers and precancerous lesions in women. Related studies have also confirmed that the incidence of genital warts in young heterosexuals could be reduced by HPV vaccination (Read et al., 2011; Chow et al., 2015). Based on the three main vaccine types currently produced globally (2v, 4v, and 9v) (Luckett and Feldman, 2016), we statistically analyzed the HPV detection rates of their HPV genotypes. The genotype detection rates of 2v, 4v and 9v showed a decreasing trend with ages. From 2015 to 2022, the genotypes detection rates of 4v and 9v in patients with genital warts showed a decreasing trend. However, the genotype detection rate of 2v tended to increase. This may be due to the fact that 2v vaccine does not include HPV-6 and HPV-11, which cause genital warts. Therefore, we can hypothesize that as vaccination with the 4v and 9v vaccines becomes more prevalent over time, leading to a decreasing detection rate of HPV-6 and HPV-11 in the population with genital warts. On the other hand, the increase in HPV-16 detection rate in 2v vaccine is not mainly due to genital warts, but may coexist with low-risk HPV types. We also found that the genotype detection rates of 4v and 9v in the male group were higher than those in the female group, which was opposite to the genotype detection rate of any HPV. The reason was that the genotype detection rate of Non-9v in the female group was higher than that in the male group. The higher detection rate of Non-9v genotypes in females may reflect the protective effect of vaccination against specific HPV types, though it’s unclear if all females tested had received the vaccine. Future research could further collect patient vaccination information, allowing for a more accurate interpretation of these findings. It indicated that females with genital warts were more likely to be infected with more types of HPV. In addition, the genotype detection rate of 9v was significantly higher than that of 2v and 4v in the female group. However, as in the United States (Hirth, 2019), the existing HPV vaccination policy only allows girls aged 13–15 years to receive free vaccinations in specific areas in China (Zhang et al., 2022). The insufficient supply of HPV 9v vaccine, coupled with the high cost of the vaccine (Chen et al., 2021), have led to difficulties in promoting the HPV vaccine in China. Similar to previous studies (Schlecht et al., 2021; Yuan et al., 2023), HPV-42, HPV-44, and HPV-81, as the high prevalence genotypes in this study, have not been included in any licensed HPV vaccines. Therefore, it is necessary to develop a wider range of HPV vaccine (Castle and Maza, 2016). In developed countries that adopt male HPV vaccination, cost-effectiveness models indicate the superiority of incorporating male vaccination (Lin et al., 2017).
In conclusion, our study reveals that low-risk HPV infections and single HPV infections predominate among patients with genital warts, highlighting the significant burden of these infections within the age group of 15 to 44 years. The prevention and diagnosis of genital warts should fully consider factors such as age, year and gender. These findings underscore the urgent need for the development and implementation of broader-spectrum HPV vaccines. Additionally, our data suggest the importance of expanding HPV vaccination programs to include males, as a strategy to reduce the incidence of genital warts. This approach not only targets the prevention of HPV-related diseases in women but also addresses the transmission dynamics of HPV infections, thereby contributing to the overall reduction of HPV prevalence in the population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Second People’s Hospital of Foshan. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
ZH: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. SY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LZ: Formal analysis, Writing – original draft, Writing – review & editing. WX: Formal analysis, Writing – original draft, Writing – review & editing. DX: Formal analysis, Writing – original draft, Writing – review & editing. WL: Formal analysis, Writing – original draft, Writing – review & editing. DT: Data curation, Writing – original draft, Writing – review & editing. JS: Data curation, Writing – original draft, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foshan Medical Science and Technology Research Project (No. 2016AB001591) and Foshan Medical Key Specialized Training Project (No. FSPY3-2015022).
We would like to acknowledge the hard and dedicated work of all the staff who carried out the research and evaluation work of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bhagavathula, A. S., and Massey, P. M. (2022). Google trends on human papillomavirus vaccine searches in the United States From 2010 to 2021: Infodemiology study. JMIR Public Health Surveill. 8:e37656. doi: 10.2196/37656
Bruni, L., Albero, G., Rowley, J., Alemany, L., Arbyn, M., Giuliano, A. R., et al. (2023). Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 11, e1345–e1362. doi: 10.1016/S2214-109X(23)00305-4
Castle, P. E., and Maza, M. (2016). Prophylactic HPV vaccination: Past, present, and future. Epidemiol. Infect. 144, 449–468. doi: 10.1017/S0950268815002198
Chen, G., Wu, B., Dai, X., Zhang, M., Liu, Y., Huang, H., et al. (2021). Gender differences in knowledge and attitude towards HPV and HPV vaccine among college students in Wenzhou, China. Vaccines 10:10. doi: 10.3390/vaccines10010010
Chen, Q., Qu, W., Zhao, Y., Shu, L., Wang, Y., and Chen, X. (2023). The prevalence of HPV among 164,137 women in China exhibited some unique epidemiological characteristics. Infect. Agents Cancer 18:72. doi: 10.1186/s13027-023-00553-4
Chow, E. P., Read, T. R., Wigan, R., Donovan, B., Chen, M. Y., Bradshaw, C. S., et al. (2015). Ongoing decline in genital warts among young heterosexuals 7 years after the Australian human papillomavirus (HPV) vaccination programme. Sex. Transm. Infect. 91, 214–219. doi: 10.1136/sextrans-2014-051813
Garland, S. M., Steben, M., Sings, H. L., James, M., Lu, S., Railkar, R., et al. (2009). Natural history of genital warts: Analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 199, 805–814. doi: 10.1086/597071
Geretti, A. M., Brook, G., Cameron, C., Chadwick, D., French, N., Heyderman, R., et al. (2016). British HIV association guidelines on the use of vaccines in HIV-positive adults 2015. HIV Med. 17(Suppl. 3), s2–s81. doi: 10.1111/hiv.12424
Hasanzadeh, M., Rejali, M., Mehramiz, M., Akbari, M., Mousavi Seresht, L., Yazdandoost, Y., et al. (2019). The interaction of high and low-risk human papillomavirus genotypes increases the risk of developing genital warts: A population-based cohort study. J. Cell. Biochem. 120, 12870–12874. doi: 10.1002/jcb.28557
Hirth, J. (2019). Disparities in HPV vaccination rates and HPV prevalence in the United States: A review of the literature. Hum. Vaccin. Immunother. 15, 146–155. doi: 10.1080/21645515.2018.1512453
Ingles, D. J., Pierce Campbell, C. M., Messina, J. A., Stoler, M. H., Lin, H. Y., Fulp, W. J., et al. (2015). Human papillomavirus virus (HPV) genotype- and age-specific analyses of external genital lesions among men in the HPV Infection in Men (HIM) Study. J. Infect. Dis. 211, 1060–1067. doi: 10.1093/infdis/jiu587
Li, M., Zhao, C., Zhao, Y., Li, J., and Wei, L. (2023). Immunogenicity, efficacy, and safety of human papillomavirus vaccine: Data from China. Front. Immunol. 14:1112750. doi: 10.3389/fimmu.2023.1112750
Li, X., Xiang, F., Chen, Z., Zhang, T., Zhu, Z., Zhang, M., et al. (2021). Genital Human Papillomavirus Prevalence and Genotyping Among Males in Putuo District of Shanghai, China 2015-2019. MEDICAL SCIENCE MONITOR 27, e932093. doi: 10.12659/MSM.932093
Li, X., Xiang, F., Dai, J., Zhang, T., Chen, Z., Zhang, M., et al. (2022). Prevalence of cervicovaginal human papillomavirus infection and genotype distribution in Shanghai, China. Virol. J. 19:146. doi: 10.1186/s12985-022-01879-y
Lin, A., Ong, K. J., Hobbelen, P., King, E., Mesher, D., Edmunds, W. J., et al. (2017). Impact and cost-effectiveness of selective human papillomavirus vaccination of men who have sex with men. Clin. Infect. Dis. 64, 580–588. doi: 10.1093/cid/ciw845
Luckett, R., and Feldman, S. (2016). Impact of 2-, 4- and 9-valent HPV vaccines on morbidity and mortality from cervical cancer. Hum. Vaccin. Immunother. 12, 1332–1342. doi: 10.1080/21645515.2015.1108500
Malagutti, N., Rotondo, J. C., Cerritelli, L., Melchiorri, C., De Mattei, M., Selvatici, R., et al. (2020). High human papillomavirus DNA loads in inflammatory middle ear diseases. Pathogens 9:224. doi: 10.3390/pathogens9030224
Medda, A., Duca, D., and Chiocca, S. (2021). Human papillomavirus and cellular pathways: Hits and targets. Pathogens 10:262. doi: 10.3390/pathogens10030262
Owczarek, W., Slowinska, M., Walecka, I., Ciazynska, M., Nowicka, D., Walczak, L., et al. (2021). Correlation of the ALA-PDT treatment efficacy and the HPV genotype profile of genital warts after cryotherapy failure and podophyllotoxin therapy in male patients. Life 11:146. doi: 10.3390/life11020146
Read, T. R., Hocking, J. S., Chen, M. Y., Donovan, B., Bradshaw, C. S., and Fairley, C. K. (2011). The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex. Transm. Infect. 87, 544–547. doi: 10.1136/sextrans-2011-050234
Schlecht, N. F., Diaz, A., Nucci-Sack, A., Shyhalla, K., Shankar, V., Guillot, M., et al. (2021). Incidence and types of human papillomavirus infections in adolescent girls and young women immunized with the human papillomavirus vaccine. JAMA Netw. Open 4:e2121893. doi: 10.1001/jamanetworkopen.2021.21893
Smith, M. A., Liu, B., McIntyre, P., Menzies, R., Dey, A., and Canfell, K. (2015). Fall in genital warts diagnoses in the general and indigenous Australian population following implementation of a national human papillomavirus vaccination program: Analysis of routinely collected national hospital data. J. Infect. Dis. 211, 91–99. doi: 10.1093/infdis/jiu370
Stanley, M. (2007). Prophylactic HPV vaccines: Prospects for eliminating ano-genital cancer. Br. J. Cancer 96, 1320–1323. doi: 10.1038/sj.bjc.6603695
Steben, M., and Garland, S. M. (2014). Genital warts. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 1063–1073. doi: 10.1016/j.bpobgyn.2014.07.002
Sudenga, S. L., Torres, B. N., Silva, R., Villa, L. L., Lazcano-Ponce, E., Abrahamsen, M., et al. (2017). Comparison of the natural history of genital HPV infection among men by country: Brazil, Mexico, and the United States. Cancer Epidemiol. Biomark. Prev. 26, 1043–1052. doi: 10.1158/1055-9965.EPI-17-0040
Wei, X., Zhang, J., Mei, Y., Dai, Q., Yang, X., and Wang, X. (2023). Prevalence and genotype distribution of HPV6/11/16/18 infections among 180,276 outpatient females from a Women’s and Children’s Central Hospital, 2015-2021, Chengdu, China. Sci. Rep. 13:22249. doi: 10.1038/s41598-023-48222-1
Widdice, L. E., Breland, D. J., Jonte, J., Farhat, S., Ma, Y., Leonard, A. C., et al. (2010). Human papillomavirus concordance in heterosexual couples. J. Adolesc. Health 47, 151–159. doi: 10.1016/j.jadohealth.2010.01.006
World Health Organization [WHO] (2017). Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine 35, 5753–5755. doi: 10.1016/j.vaccine.2017.05.069
Yin, W. G., Yang, M., Peng, L., Liu, Y. M., Cheng, B., Xuan, S. X., et al. (2020). Male papillomavirus infection and genotyping in the Qingyuan area. Virol. J. 17:155. doi: 10.1186/s12985-020-01423-w
Yuan, H., Li, R., Lv, J., Yi, G., Sun, X., Zhao, N., et al. (2023). Epidemiology of human papillomavirus on condyloma acuminatum in Shandong Province, China. Hum. Vaccin. Immunother. 19:2170662. doi: 10.1080/21645515.2023.2170662
Zare-Bidaki, M., Zardast, M., Nadjafi-Semnani, A., Nadjafi-Semnani, M., Javanmard, D., Ghafari, S., et al. (2022). Investigation of frequency and typing of human papillomavirus among genital warts using a reverse dot blot hybridization approach. BMC Infect. Dis. 22:278. doi: 10.1186/s12879-022-07276-8
Zhang, W., Guo, N., Li, B., Shang, E., Wang, J., Zhang, M., et al. (2023). Prevalence and genotype distribution of human papillomavirus infections in Beijing, China between 2016 and 2020. Virol. J. 20:11. doi: 10.1186/s12985-023-01959-7
Zhang, X. X., Wang, W., Song, Y. F., Zhang, Z. N., and Yu, W. Z. (2022). [Expert recommendations on human papillomavirus vaccine immunization strategies in China]. Zhonghua Yu Fang Yi Xue Za Zhi 56, 1165–1174. doi: 10.3760/cma.j.cn112150-20220505-00443
Zhu, B., Liu, Y., Zuo, T., Cui, X., Li, M., Zhang, J., et al. (2019). The prevalence, trends, and geographical distribution of human papillomavirus infection in China: The pooled analysis of 1.7 million women. Cancer Med. 8, 5373–5385. doi: 10.1002/cam4.2017
Zhu, C., Wang, Y., Mao, W., Zhang, H., and Ma, J. (2019). Prevalence and distribution of HPV types in genital warts in Xi’an, China: A prospective study. BMJ Open 9:e023897. doi: 10.1136/bmjopen-2018-023897
Keywords: human papillomavirus, infection, HPV vaccine, genotype, genital warts
Citation: Huang Z, Yao S, Zou L, Xie W, Xie D, Li W, Tan D and Shuai J (2024) Evaluation of HPV infection and presence of licensed HPV vaccine genotypes among genital warts in Foshan, China. Front. Microbiol. 15:1376141. doi: 10.3389/fmicb.2024.1376141
Received: 25 January 2024; Accepted: 01 April 2024;
Published: 17 April 2024.
Edited by:
Toshana Foster, University of Nottingham, United KingdomReviewed by:
Leabaneng Tawe, Botswana-UPenn Partnership (BUP), BotswanaCopyright © 2024 Huang, Yao, Zou, Xie, Xie, Li, Tan and Shuai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeqi Huang, aHVhbmd6ZXFpQDEzOS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.