- 1Key Laboratory of Soil Resource Sustainable Utilization for Jilin Province Commodity Grain Bases, College of Resource and Environmental Science, Jilin Agricultural University, Changchun, China
- 2Institute of Agricultural Environment and Resources Research, Jilin Academy of Agricultural Sciences, Changchun, China
Ammonia oxidation is the rate-limiting step in nitrification and the key step in the nitrogen (N) cycle. Most soil nutrients and biological indicators are extremely sensitive to irrigation systems, from the perspective of improving soil fertility and soil ecological environment, the evaluation of different irrigation systems and suitability of selection, promote crop production and soil quality, study the influence of the soil microenvironment contribute to accurate evaluation of irrigation farmland soil health. Based on the amoA gene, the abundance and community diversity of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) and their responses to soil physicochemical factors and enzyme activities were studied in semi-arid areas of Northeast China. The study consisted of three irrigation systems: flood irrigation (FP), shallow buried drip irrigation (DI), and mulched drip irrigation (MF). The results showed that DI and MF significantly increased the contents of alkaline hydrolyzed nitrogen (AN), nitrate nitrogen (NO3–-N), soil moisture, and the activities of ammonia monooxygenase (AMO) and hydroxylamine oxidase (HAO). Compared with FP, DI significantly increased the abundance of soil AOA and AOB, while MF significantly increased the abundance of soil AOB. Irrigation systems significantly affected the community composition of ammonia-oxidizing microorganisms (AOM). Also, AN and soil moisture had the greatest influence on the community composition of AOA and AOB, respectively. The AOB community had better stability and stress resistance. Moreover, the symbiotic network of AOB in the three irrigation systems was more complex than that of AOA. Compared with FP, the AOA community under treatment DI had higher complexity and stability, maintaining the versatility and sustainability of the ecosystem, while the AOB community under treatment MF had higher transfer efficiency in terms of matter and energy. In conclusion, DI and MF were more conducive to the propagation of soil AOM in the semi-arid area of Northeast China, which can provide a scientific basis for rational irrigation and N regulation from the perspective of microbiology.
1 Introduction
Nitrogen (N) is an important nutrient for plant growth and plays an indispensable role in agro-ecosystems. Nitrification and denitrification processes are the main processes of the N cycle, both of which are driven by microorganisms. The composition and function of microbial communities in the N cycle can affect the bioavailability of N and thus regulate the growth characteristics of crops (Yu et al., 2019). The nitrification process plays a key role in the global N cycle, where ammonia nitrogen oxides into nitrite and nitrite oxides into nitrate. The nitrite oxidation is a two-step process that is respectively catalyzed by ammonia-oxidizing microorganisms (AOM) and nitrite oxidizing bacteria (NOB). The AOM includes ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) and both AOB and AOA contain amoA genes which are often used as molecular markers to study the diversity and abundance of AOM in the natural environment (Tolar et al., 2017; Yao et al., 2022). The amoA gene catalyzes the conversion of NH3 to NO2–. It is an intracellular enzyme, including three subunits amoA, amoB, and amoC. AOB and AOA are widely distributed, and their relative abundance is closely related to the concentration of ammonia nitrogen. Petersen et al. (2012) found that the abundance of functional genes of AOM is the most important variable in predicting the potential rate of nitrification in soil. AOA and AOB coexist in most soils, and AOA is usually more abundant than AOB, hence AOA is considered to be the dominant microorganism in the soil ammonia oxidation process (Xiao et al., 2021). However, some studies have contradictorily indicated that the change in ammoxylation activity is only related to the abundance and composition of the AOB community, but not to the AOA community (Ouyang et al., 2016). With the continuous development of molecular biology, several studies have focused on understanding AOA and AOB communities (Xiao et al., 2021). At present, the niche differentiation and functional complementarity between AOA and AOB have been concluded (Lehtovirta-Morley, 2018). In addition, most studies have paid attention to the effects of different environmental conditions on AOA, AOB abundance, and community structure composition, as well as the relative contributions of AOA and AOB to nitrification (Ouyang et al., 2017; Szukics et al., 2019). Environmental factors such as pH, temperature, and N will all have a certain impact on the main role of AOA and AOB in the ammonia oxidation process, hence it is of great practical significance to further analyze the relative roles of AOA and AOB combined with environmental factors (Kou et al., 2023). Based on the previous research results, we found that different water management led to changes in the abundance and community structure of soil nitrifying microorganisms. However, under different environmental conditions, the response of these functional microorganisms to different water management varies (Guo et al., 2021; Wang and Huang, 2021). Therefore, studying the effects of different irrigation modes on soil AOM can help enrich our understanding of soil N cycling and key microorganisms, and is of great significance for targeted regulation of soil N transformation processes. However, the diversity of AOA and AOB communities under different irrigation modes and their response mechanisms to soil physicochemical factors and enzyme activities remain bleak.
Farmland in the western part of Northeast China is widely distributed and is one of the major grain production areas. However, the damaged ecological environment, semi-arid climate, and interaction of human factors in this region restrict the sustainability of food production in this region to a certain extent (Gu et al., 2018; Xin et al., 2020). Some studies have confirmed that crop yield in semi-arid areas is especially dependent on flood irrigation (Zhang et al., 2017). Heavy irrigation with low water efficiency can cause nitrate to enter deeper soil and groundwater, or sometimes it is permanently lost (Beaudoin et al., 2021). Therefore, since the 1990s, various agricultural measures have been adopted to reduce flood irrigation and N fertilizer consumption and maintain high crop yields (Zhang et al., 2019a). Drip irrigation is an effective irrigation and fertilization measure that helps to improve the water and N utilization efficiency of crops (Fan et al., 2020). China continuously promotes and optimizes water-saving irrigation technology in agricultural production. Drip irrigation, with its remarkable effect of saving water and increasing production and its eco-friendly features, has been rapidly promoted and applied as a model of efficient water-saving irrigation in semi-arid planting areas of northeast and western China. At present, common drip irrigation methods include under-film drip irrigation and shallow-buried drip irrigation (Li H. et al., 2021). With the characteristics of water retention, fertilizer saving, and high light efficiency, drip irrigation under film can achieve efficient water saving and effectively increase the accumulation of dry matter (Li et al., 2023). The shallow-buried drip irrigation method lacks film coverage, resulting in a decrease in soil water retention and a rapid decrease in soil temperature, culminating in an insignificant increase in production. However, the shallow-buried drip irrigation method can overcome the problems of premature aging, residual film pollution, and high cost of maintenance in the later growth stage of drip irrigation (Wang Z. et al., 2019). Irrigation is a key factor affecting agricultural ecosystems and it can shape the microbial community structure closely related to the N cycle in soil by changing the soil microenvironment. The growth of AOM is affected by the soil environment. Therefore, a reasonable irrigation mode can be determined by exploring the characterization of AOM in soil under different irrigation methods.
At present, the effects of different irrigation methods on soil ammonia-oxidizing microbial community structure are still unclear. Therefore, in this study, real-time qPCR and Illumina Miseqsequencing techniques were used to analyze the abundance, community composition, and diversity of soil AOM. This will help to identify the main driving factors of ammonia-oxidizing microbial community changes and clarify the mutual response of ammonia-oxidizing microbial community with soil nutrients and enzyme activities under different irrigation methods. The findings of this study will provide an important reference and theoretical basis for the application and popularization of drip irrigation technology in the semi-arid area of Northeast China.
2 Materials and methods
2.1 Experimental design
The test site was located in Minle Village, Ningjiang District, Songyuan City, Jilin Province (45° 26′ N,125° 88′ E). The rainfall period is normally from May to September. The test soil is the meadow soil and the field trial started in 2017, with three treatments set up. Treatment 1: traditional flooding irrigation (FP, all fertilizers were applied as base fertilizer at one time); Treatment 2: shallow buried drip irrigation (DI, 30% N fertilizer and 50% phosphate and potassium fertilizer were applied as the base fertilizer, and the remaining fertilizer was applied with water for 4 times in the later stage); Treatment 3: mulched film drip irrigation (MF, white transparent plastic film was covered on the corn and drip irrigation belt, 30% N fertilizer and 50% phosphorus and potassium fertilizer were applied as the base fertilizer, and the remaining fertilizer was applied with water for 4 times in the later stage). The N application rates for treatments DI and MF were pre-seeding (30%), jointing stage (30%), flowering stage (20%), silking stage (10%) and filling stage (10%), and the application rates for phosphate (P) and potassium (K) fertilizer were pre-seeding (50%), jointing stage (20%), flowering stage (15%), silking stage (10%) and filling stage (5%). The experimental maize variety was Xiangyu 998, and the planting density was 70 thousand hm–2. The same rates of N, P, and K were applied in all treatments, and the amount of irrigation was the same. The fertilizer dosage of N-P2O5-K2O was 210-90-90 kg⋅hm–2, the base fertilizer was urea (N 46%), diammonium phosphate (N-P2O5-K2O: 18-46-0), potassium chloride (K2O 60%). Topdressing was done using urea (N 46%), water-soluble fertilizer mono-ammonium phosphate (N-P2O5-K2O: 12-61-0), and water-soluble potassium chloride (K2O 60%). The size of each experimental plot was 10 m long, 3.6 m wide, and 36 m2 in area, and three repeated random plots were arranged. The experiment adopted a large-ridge double-row cultivation mode, with a narrow row spacing of 40 cm and a wide row spacing of 80 cm. The drip irrigation belt was laid in the middle of the narrow row during seeding, and the inner diameter of the drip irrigation belt was 16 mm with a drop head spacing of 30 cm. The source of water for the experiment was underground well water, and each district was connected to an independent fertilizer tank. During the fertilization process, the fertilizer tank was filled with the amount of fertilizer required by each district and thoroughly stirred for the fertilizer to be fully dissolved. Before fertilizing, the fertilizer tank valve was opened and the completely dissolved fertilizer was dropped. After fertilization, the water was allowed to continue dropping for 30 min.
2.2 Sample collection
In this study, soil samples were collected from the 0∼20 cm layer during the harvest period in 2021, and the soil samples from 5 randomly selected points were collected for each plot and mixed evenly to form a composite soil sample. After collection, the samples were returned to the laboratory in an ice box, and impurities such as roots and stones were removed, followed by passing the samples through a 2 mm sieve. Fresh soil samples were used for the determination of soil enzyme activity. Part of the soil samples were stored in a −80°C refrigerator at low temperature for the extraction of soil DNA, and the remaining soil samples were air-dried at normal temperature for the determination of soil physical and chemical properties.
2.3 Determination of physical and chemical properties of soil
Soil organic matter (SOM) was determined the by potassium dichromate oxidation and ferrous sulfate titration method; soil moisture was determined by the drying method; pH was measured by pH meter (water-to-soil ratio was 2.5:1); soil total nitrogen (TN) was determined by Kjeldahl method; soil alkali-hydrolyzed nitrogen (AN) was determined by diffusion method; soil ammonium nitrogen (NH4+-N) and soil nitrate nitrogen (NO3–-N) were determined by the continuous flow analyzer (Lu, 1999).
2.4 Determination of soil enzyme activities
Fresh soil samples (2 g) were extracted by ultrasonic wave for 10 min in 5 mL cell lysis solution (50 mmolL–1 Tris HCl (pH7.5), 10% (m/v) sucrose solution, 300 mmolL–1 sodium chloride and 90 mmolL–2 EDTA disodium salt). The extraction volume was adjusted to 25 mL with a buffer solution (pH 7.8) consisting of 50 mmolL–1 TrishCl and 5 mmolL–1 magnesium chloride. The extracts were centrifuged at 12000 r/min for 20 min, and enzyme activity was measured with supernatant. AMO activity was determined using the AMOELISA kit (SaintBio, Shanghai) according to the manufacturer’s instructions. The activity of HAO was measured by Abramyan et al. (1989).
2.5 Soil DNA extraction and fluorescence quantitative PCR
The total DNA was extracted from 0.5 g fresh Soil according to the FastDNA® SPIN Kit for Soil (MP Biomedicals, USA) kit. The DNA concentration was determined by NanoDrop 2000 UV-VIS spectrophotometer (Thermo Scientific, USA), and the DNA quality was determined by 1% agarose gel electrophoresis. The primer sequences for the soil nitrification microbial amoA gene were A26F (5′-GACTACATMTTCTAYACWGAYTGGGC-3′) and A416R (5′-GGKGTCATRTATGG WGGYAAYGTTGG-3′) (Francis et al., 2005). The AOA amoA and AOB amoA genes were amplified with primer sequences amoA-1F (5′-GGGGTTTCTACTGGTGGT-3′) and amoA-1R (5′-CCCCTCKGSAAAGCCTTCTTC-3′), respectively (Chang et al., 2017). The reaction system was as follows: 10 μM upstream primer and downstream primer 1 μL, 1 μL SYBR®PremixExTaq™II (TliRNaseHPlus), ROXplusL, 25 μL 2 × TaqMasterMix, and 22 μL water, making a total of 50 μL. The PCR amplification conditions of the amoA gene were: predenaturation at 94°C for 5 min, 94°C 30 s, 55°C 30 s, 72°C 30 s, 30 cycles, and then extended at 72°C for 10 min after the last cycle was completed. Each sample was repeated 3 times, and the standard curve was established by soil ammonia-oxidizing microorganisms with known copy numbers.
2.6 Illumina Miseq sequencing and data analysis
The amoA gene was amplified using the total DNA extracted from soil microorganisms as a template. An 8 bp barcode sequence was added to the upstream 5′ end and downstream 3′ end of the primer in AOA amoA and AOB amoA to distinguish different samples. PCR reaction system (total system 25 μL): 12.5 μL 2× TaqPlusMasterMix, 3 μL BSA (2 ng μL–1), 1 μL ForwardPrimer (5 μM), 1 μL ReversePrimer (5 μM), 2 μL DNA (total added DNA was 30 ng), finally, 5.5 μL ddH2O was added to 25 μL. Reaction parameters: predenaturation at 95°C for 5 min, denatured at 95°C for 45 s, annealed at 55°C for 50 s, extended at 72°C for 45 s, 28 cycles, extended at 72°C for 10 min. The PCR products were detected by 1% agarose gel electrophoresis and purified by EncourAGee TampureXP nucleic acid purification kit. The PCR products were used to construct the microbial diversity sequencing library and were sequenced at Beijing Ovison Gene Technology Co., LTD.
The disembarkation data is segmented by QIME (V1.8.0) software according to the barcode sequence, and Pear (V0.9.6) software was used to filter and concatenate the data. Scores that were lower than 20, containing ambiguous base and primer mismatches were removed. The minimum overlap during splicing is set to 10 bp and the mismatch rate was 0.1. The Denovo method of Vsearch (V2.7.1) software was used to remove short sequences and chimeric sequences. The Vsearch UPARSE algorithm (V2.7.1) software was used to process the classification unit OTU (Operational Taxonomic Units) for high-quality sequence clustering, and the similarity threshold was 97%. The Blast algorithm was used to compare GenBank non-redundant nucleotide databases (nt) and a threshold of 1e−5 was set to obtain a species taxonomic information corresponding to each OTU. All raw sequences generated in this study have been deposited in NCBI under the accession SRP479978.
2.7 Statistical analysis methods
Using SPSS 23.0, F test in one-way ANOVA, multiple comparison of means (P < 0.05) was conducted with a Fisher’s protected least significant difference (LSD) in statistical analysis in soil physical and chemical properties, soil enzyme activities, and microbial diversities of ammonia oxidation among the different treatments. Pearson correlation analysis among soil physicochemical properties, soil enzyme activities, and abundance was performed using SPSS (V23.0). Canoco (V5.0) software was used for redundancy analysis (RDA) to elucidate the relationship between soil enzyme activities and physicochemical properties. Heatmap was done using R (V3.4.2) ‘pheatmap’ and ‘ggplot2’ packages (R Core Team, 2016). Principal component analysis (PCoA) and Anosim were performed using the Bray-Curtis algorithm in R software (V3.4.2). The ternary graph was created using “ggplot2” in R (V3.4.2). The structural equation model (SEM) was constructed by Amos (V23), chi-square test (P > 0.05), degree of freedom chi-square ratio (CMIN) / DF < 3, fitness index (CFI > 0.9). The visualization of cooccurrence network analysis was done by using the “Phych” packet of R (V3.4.2) software to generate the species correlation matrix. In addition, Gephi (V0.9.2) was used to draw the microbial network map and calculate the topological parameters. The function of nodes in a co-occurrence network was determined by inter-module connectivity (Pi) and intra-module connectivity (Zi) representation. According to inter-module connectivity (Pi) and intra-module connectivity (Zi), nodes were classified into peripheral nodes (Zi < 2.5 and Pi < 0.62), connectors (Zi < 2.5 and Pi > 0.62), module hubs (Zi > 2.5 and Pi < 0.62) and network hubs (Zi > 2.5 and Pi > 0.62).
3 Results
3.1 Soil physicochemical properties and AMO and HAO activities
Compared with FP, DI, and MF significantly increased the content of AN, NO3–-N, and soil moisture, and significantly decreased the content of NH4+-N. Compared with DI, MF significantly increased Moisture content, while pH and TN had no significant difference between different treatments (Table 1). The irrigation methods significantly changed soil enzyme activity. Compared with FP, DI and MF significantly increased the activities of AMO and HAO, where DI increased AMO and HAO activities by 1.64 and 1.28 times while MF increased by 1.26 and 1.18 times, respectively. Compared with DI, MF significantly reduced the activity of AMO and HAO by 1.3 and 1.03 times, respectively (Figure 1). The correlation between enzyme activities and soil physicochemical properties showed that the activity of HAO was positively correlated with AN (r = 0.862, P < 0.01), SOM (r = 0.723, P < 0.05) and NH4+-N (r = 0.783, P < 0.05). AMO was significantly positively correlated with AN (r = 0.810, P < 0.01), and SOM (r = 0.854, P < 0.01) (Supplementary Figure 1).

Figure 1. The soil AMO (A) and HAO activities (B) under different irrigation methods. Bars indicate SD, different letters indicate significant differences among samples (P < 0.05, ANOVA). AMO, ammonia monooxygenase; HAO, hydroxylamine oxidase; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
3.2 Abundance of soil ammonia-oxidizing microorganisms
Among the three irrigation methods, the abundance of AOA ranged from 1.53 to 2.60 × 106 g–1 soil, and the abundance of AOB ranged from 1.26 to 4.26 × 104g–1 soil, and the abundance of AOA was significantly higher than that of AOB (Figure 2). Compared with FP, DI significantly increased the abundance of AOA, 1.63 times than that of FP (P < 0.05) (Figure 2A). Compared with DI, MF significantly decreased the abundance of AOA, and DI was 1.60 times higher than that of MF (P < 0.05). Compared with FP, DI, and MF significantly increased the abundance of AOB by 3.4 times and 2.3 times, respectively (P < 0.05). Compared with DI, MF significantly decreased the abundance of AOB, and DI was about 1.48 times higher than that of MF (P < 0.05) (Figure 2B). The abundance ratio of AOA amoA and AOB amoA genes ranged from 58.64 to 121.16, which was not similar to the trend of AOA amoA and AOB amoA gene abundance. Compared with FP, DI and MF treatments significantly reduced the abundance ratio of AOA amoA and AOB amoA genes (Figure 2C). The correlation analysis of AOA and AOB abundance and soil physicochemical properties under three irrigation treatments showed that AOA abundance was significantly positively correlated with AN (r = 0.668, P < 0.05), SOM (r = 0.682, P < 0.05), and AOB abundance was significantly positively correlated with SOM (r = 0.710, P < 0.05), NH4+-N (r = 0.795, P < 0.05), AN (r = 0.911, P < 0.001) (Supplementary Figure 1). Correlation analysis of AOA and AOB abundance and enzyme activities under the three irrigation methods showed that AOA abundance was significantly positively correlated with AMO (r = 0.821, P < 0.01) and HAO (r = 0.737, P < 0.05), AOB abundance was significantly positively correlated with AMO (r = 0.931, P < 0.001) and HAO (r = 0.963, P < 0.001) (Supplementary Figure 1).
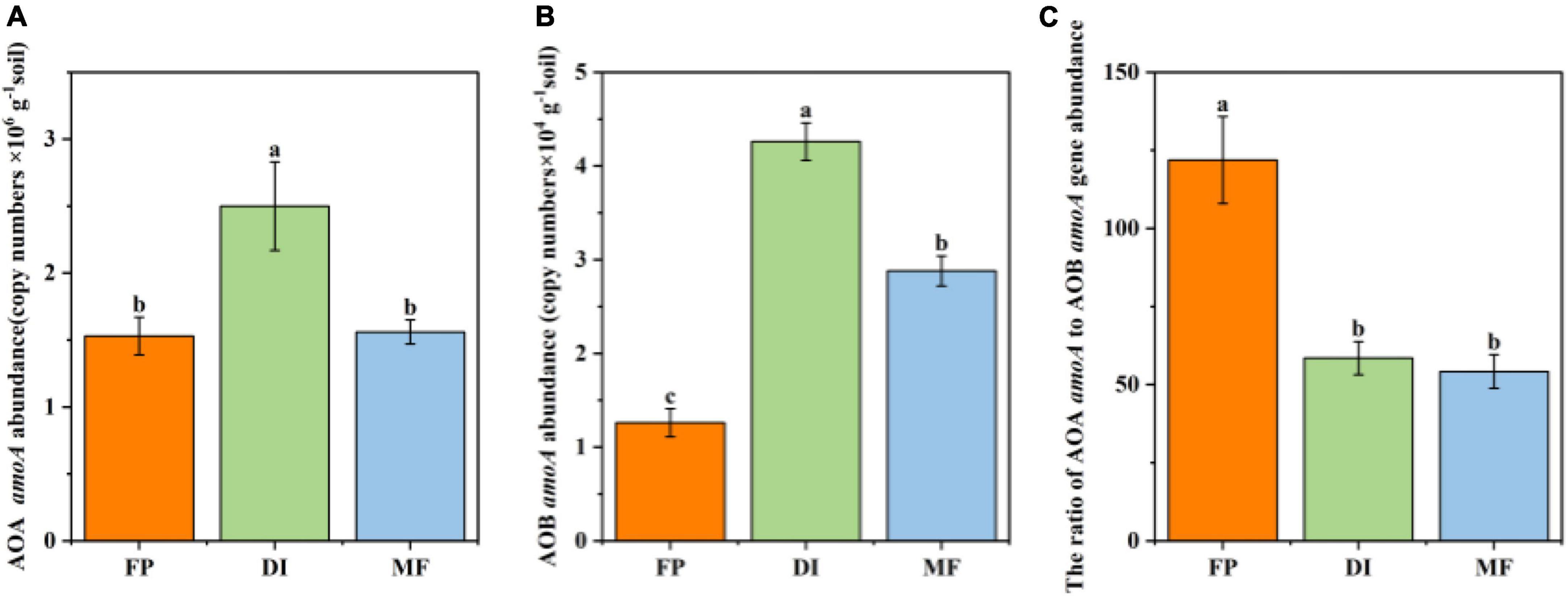
Figure 2. AOA (A), AOB (B), and AOA/AOB (C) amoA gene abundance under different irrigation methods. Bars indicate SD, different letters indicate significant differences among samples (P < 0.05, ANOVA). FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
3.3 Community composition of soil ammonia-oxidizing microorganisms
Sequencing from Illumina MiSeq, in order to analyze the microbial communities at the same sequencing depth, the lowest sequencing number of 73,184 sequences for AOA amoA gene and 30,160 sequences for AOB amoA gene were for randomly selected per sample. 799,214 high-quality sequences were obtained from all samples of the AOA amoA gene, each sample had 75,159–115,274 high-quality sequences, and the sequence length was optimized from 200 to 540 bp (Supplementary Table 1). Moreover, 385,327 optimized sequences were obtained from all samples of the AOB amoA gene, with 33,440 to 56,157 high-quality sequences per sample, and optimized sequence lengths of 200 to 540 bp (Supplementary Table 2). The OTU distribution among the samples revealed the OTU sharing of the microbiome in different irrigation treatments. The ternary phase diagram analysis of AOA and AOB based on three irrigation methods. In the ternary phase diagram of AOA, species were evenly distributed in the three treatments. The center of the triplet diagram is the influence of soil microbiome selection on the three irrigation treatments, and the core microbiome spans FP, DI, and MF treatments. FP recorded the highest relative abundance of Thaumarchaeota (80%), MF recorded the highest relative abundance of Proteobacteria (60%), and DI recorded the highest relative abundance of Crenarchaeota (80%) (Figure 3A). In the ternary phase diagram of AOB, the distribution of species in the three treatments was not very uniform. The relative abundance of Proteobacteria, Gemmatimonadetes, and Actinobacteria was higher in FP, DI, and MF treatments respectively (Figure 3B). Venn analysis revealed that FP, DI, and MF had 128 AOA OTUs and 988 AOB OTUs (Supplementary Figure 2). A total of 20 different OTUs were selected under AOA. Among them, OTU42 was the dominant bacterium with the relative abundance of 6.11–11.33%. Moreover, MF recorded a significant increase in the relative abundance of OTU42 (Figure 4A). BLAST analysis indicated that OTU42 (KM595440.1) might be an uncultured Nitrososphaerota (Supplementary Table 3). A total of 20 different OTUs were selected by AOB, among which OTU100 (MH589282.1) and OTU95 (KP212533.1) were the dominant bacteria, and their relative abundances were 10.64–14.75% and 7.81–11.87%, respectively. BLAST analysis showed that OTU100 (MH589282.1) could be uncultivated Nitrososphaerota and OTU95 (KP212533.1) may be uncultured Nitrosospira sp. (Supplementary Table 4). The heatmap showed that in AOA, compared with FP, DI significantly increased the relative abundance of OTU174, OTU99, OTU142, OTU9, OTU69 and OTU245 while MF significantly increased the relative abundance of OTU208, OTU68, OTU28, OTU7, OTU42, OTU42, and OTU149 (Figure 4A). In AOB, compared with FP, DI significantly increased the relative abundance of OTU139, OTU491, OTU499, OTU99, OTU58, OTU747, OTU707, OTU103, OTU144, OTU435, OTU887, OTU116 and OTU830, while MF significantly increased the relative abundance of OTU134, OTU949, OTU48, OTU808, OTU250, OTU125, OTU18, OTU119, and OTU738 (Figure 4B).
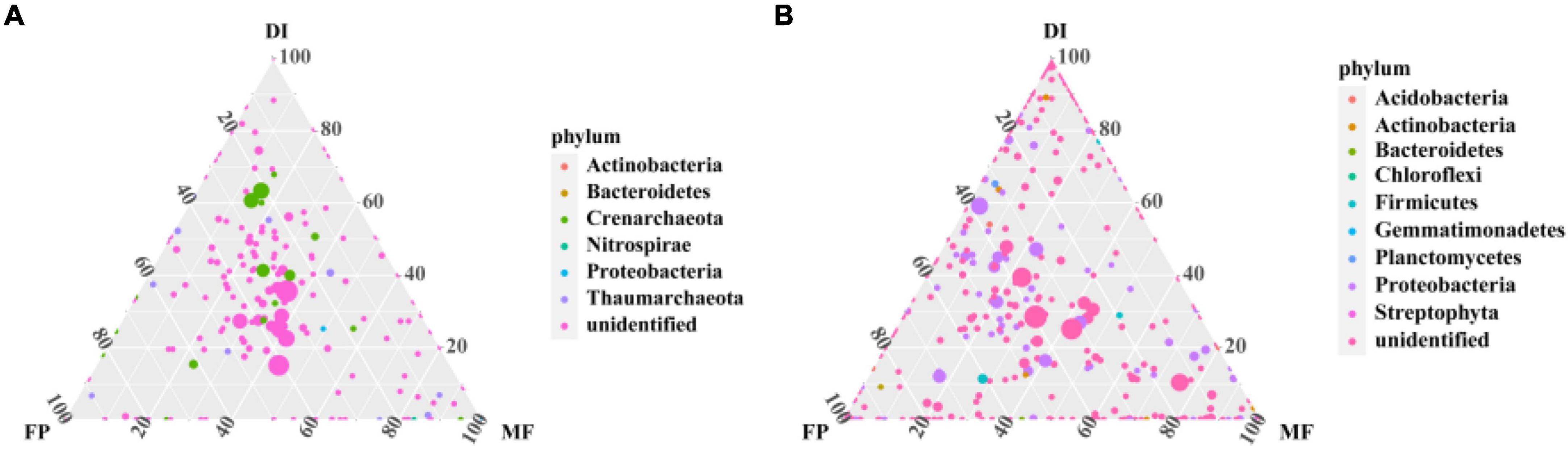
Figure 3. Phylum-level taxa of AOM communities uniquely associated with different irrigation methods. (A) Ternary phase diagram of AOA community at phylum level, (B) ternary phase diagram of AOB community at phylum level. The different colors in panels (A,B) represent different species, the dot size is proportional to relative abundance, and the closer the dot is to a vertex, the more abundant the species is in the group, with dotted grid intervals corresponding to a 20 percent increment. AOM, ammonia oxidizing microorganisms; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
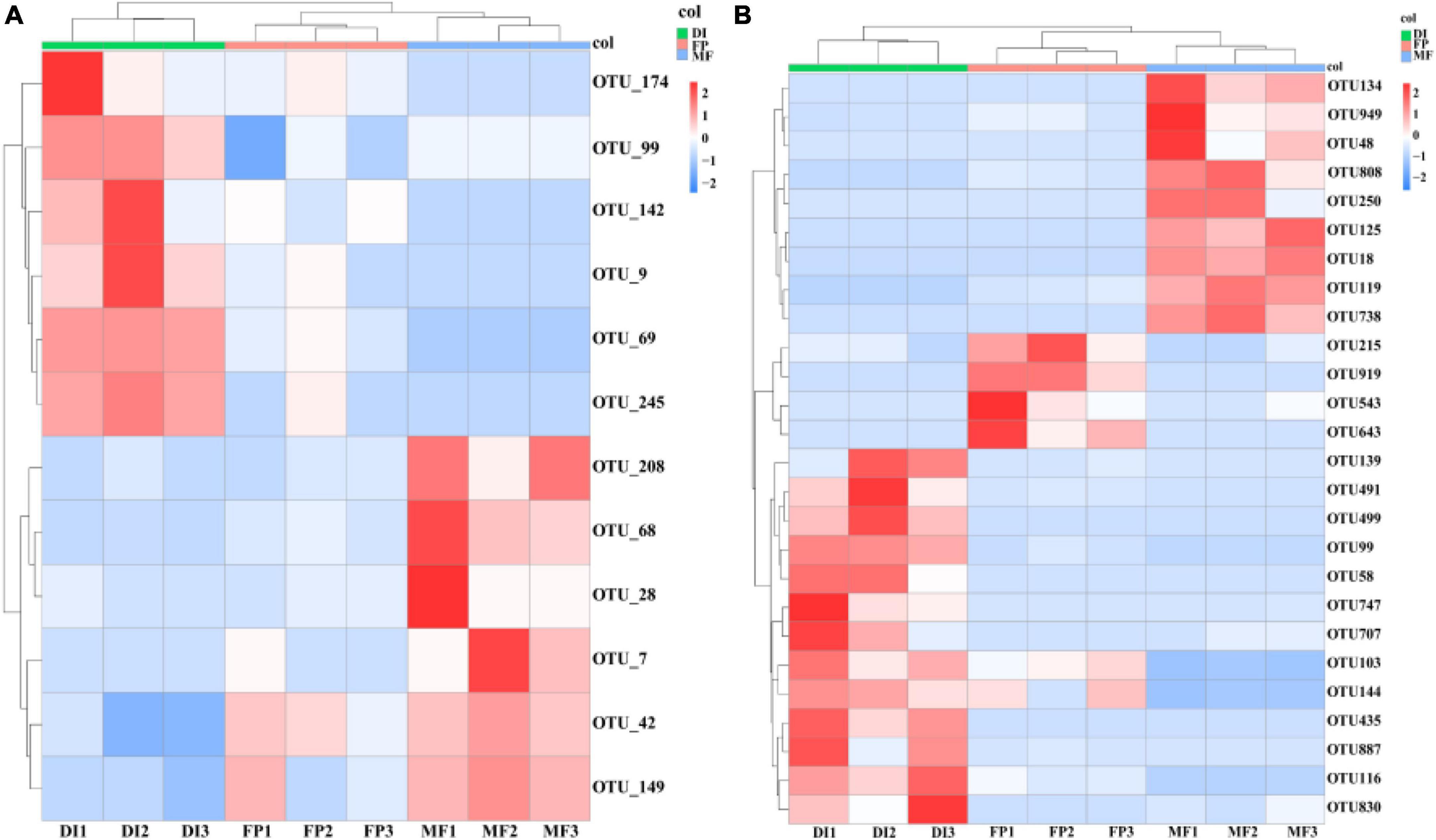
Figure 4. The relative abundance of dominant OTUs of soil AOM communities (A is AOA, B is AOB) under different irrigation methods. AOM, ammonia oxidizing microorganisms; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
3.4 Diversity of soil ammonia-oxidizing microorganisms
Compared with FP and DI, MF significantly reduced the Shannon index and Chao1 index of AOA (Figures 5A, B). In AOB, the Shannon index and Chao1 index showed no significant difference in the different irrigation treatments (Figures 5C, D). The PCoA of soil AOM showed that for the PCA results of AOA, the contribution values of the first principal component and the second principal component were 77.30 and 12.40%, respectively, totaling 89.70%, reflecting the main reason for the difference among the three irrigation treatments. The relative distance between FP and MF treatment was relatively close, which was different from that of DI treatment (Figure 6A). The PCA results of AOB showed that the contribution values of the first principal component and the second principal component were 37.89 and 17.80%, respectively, totaling 55.69%, which was also the main reason for the difference among the three different irrigation treatments. The samples of FP and DI treatments were relatively clustered, and there was a difference between them and MF treatment (Figure 6B). In order to further determine whether the difference in soil AOA and AOB between groups and within groups was significant, Anosim analysis was performed on soil microbial colony structure, and the results showed that the difference between groups of the three irrigation treatments was significantly greater than the difference within groups (P < 0.05) (Figures 6C, D). In conclusion, irrigation methods significantly affected the community structure of AOA and AOB.
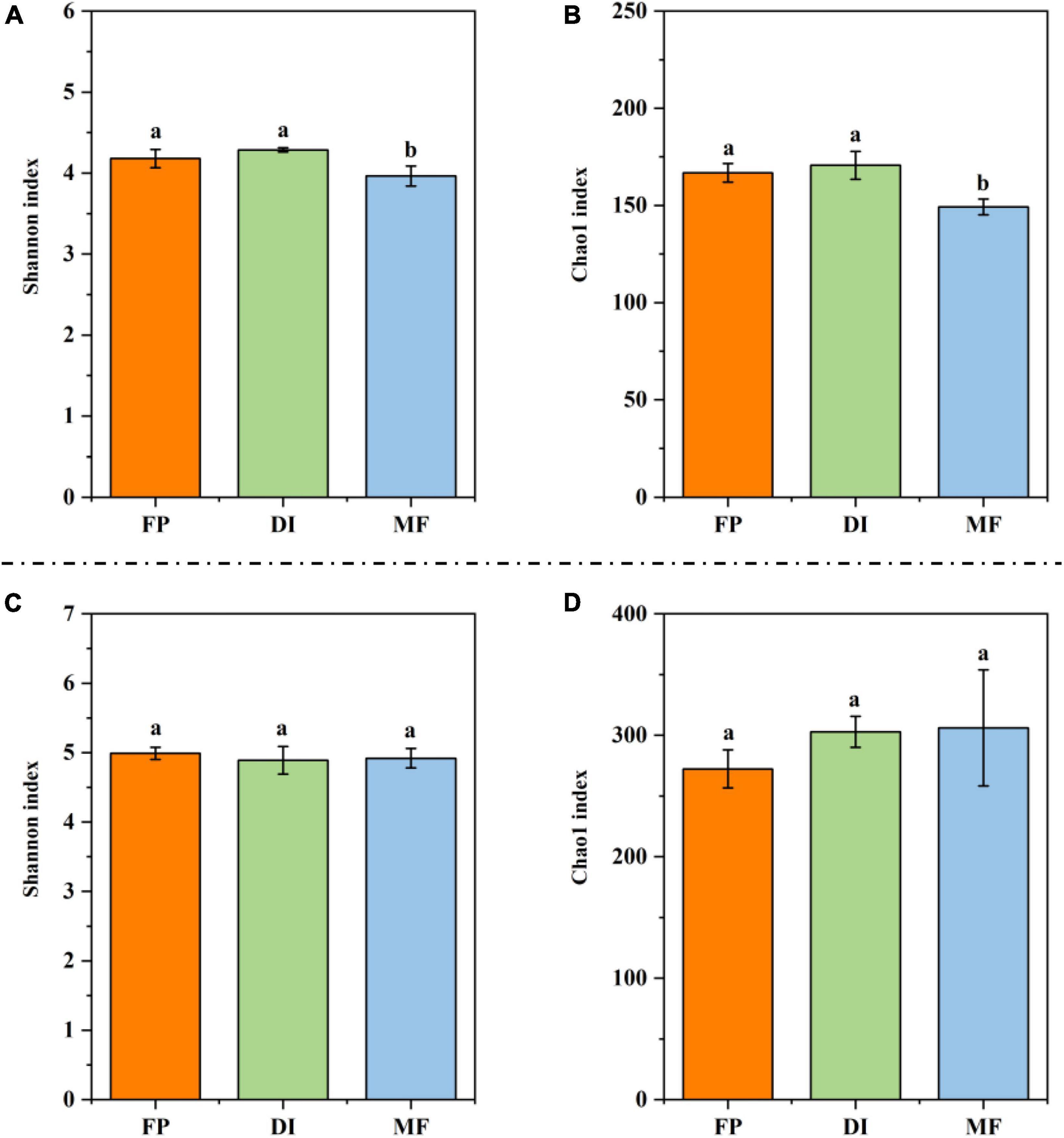
Figure 5. The alpha diversity for AOM communities under different irrigation methods. (A) Shannon index for AOA amoA gene; (B) Chao1 index for AOA amoA gene; (C) Shannon index for AOB amoA gene; (D) Chao1 index for AOB amoA gene. Bars indicate SD; different letters indicate significant differences among samples (P < 0.05, ANOVA). AOM, ammonia oxidizing microorganisms; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
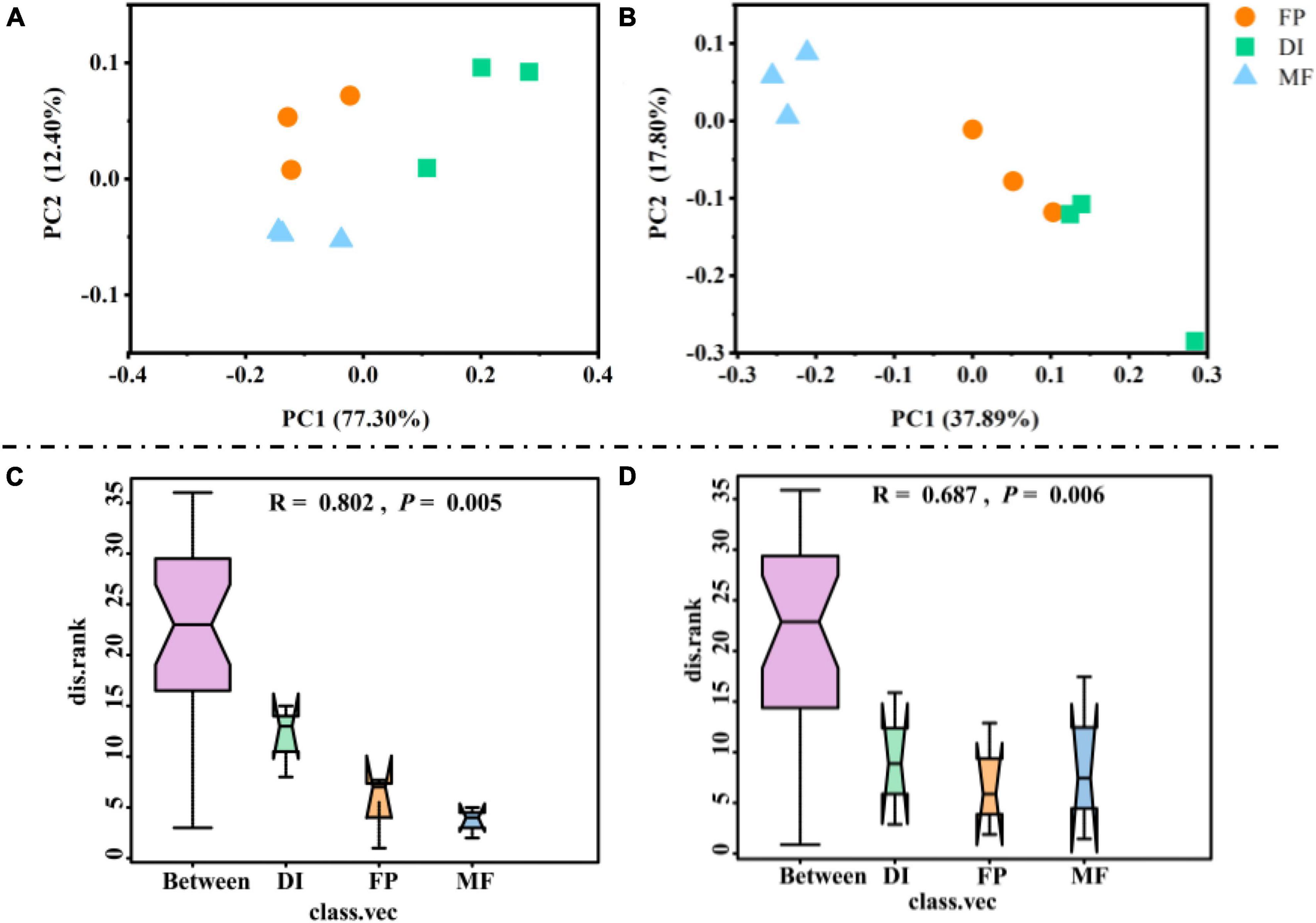
Figure 6. The beta diversity and Anosim analysis for AOM communities under different irrigation methods. PCoA of AOA (A), AOB (B) communities based on OTU. Anosim analysis of AOA (C) and AOB (D) based on Bray-Curtis. AOM, ammonia oxidizing microorganisms; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
3.5 Environmental factors affecting soil ammonia-oxidizing microbial communities
RDA showed that in AOA, AN was the main driver of AOA community structural changes (Supplementary Table 5), and SOM, TN, AN, NH4+-N were positively correlated with DI. Moreover, pH, NO3–-N, and soil moisture were positively correlated with MF (Figure 7A). In AOB, soil moisture was the main driving factor of AOB community structural changes (Supplementary Table 6). The pH, NO3–-N and soil moisture were positively correlated with MF, while AN, SOM, TN, and NH4+-N were positively correlated with DI (Figure 7B). For AOA, SEM perfectly fitted the experimental data (χ2/df = 0.450, P = 0.77, CFI = 1.00), and the equation model explained 85% of the variation in AMO, 84% of the variation in HAO, and 80% of the variation in AOA community (Figure 7C). TN, AN, NO3–-N, NH4+-N and Moisture had direct effects on AMO, HAO and AOA communities, among which AN had the greatest effect on AMO (λ = 0.86, P < 0.001), HAO (λ = 1.56, P < 0.01) and AOA (λ = 1.046, P < 0.001) community. AMO and HAO had direct effects on AOA community, and AMO (λ = 0.96, P < 0.001) had the greatest effect on AOA community. For AOB, SEM fully fitted the experimental data (χ2/df = 1.119, P = 3.26, CFI = 0.998), and the equation model could explain 83% of the variation in the AMO, 81% of the variation in HAO, and 86% of the variation in AOB community (Figure 7D). TN, AN, NO3–-N, NH4+-N and Moisture had direct effects on AMO, HAO and AOB community, among which AN had the greatest effect on AMO (λ = 0.86, P < 0.001) and HAO (λ = 1.56, P < 0.01), Moisture (λ = 1.103, P < 0.05) had the greatest effect on AOB community. AMO and HAO had direct effects on AOB community, and HAO (λ = 1.57, P < 0.01) had the greatest effect on AOB community.
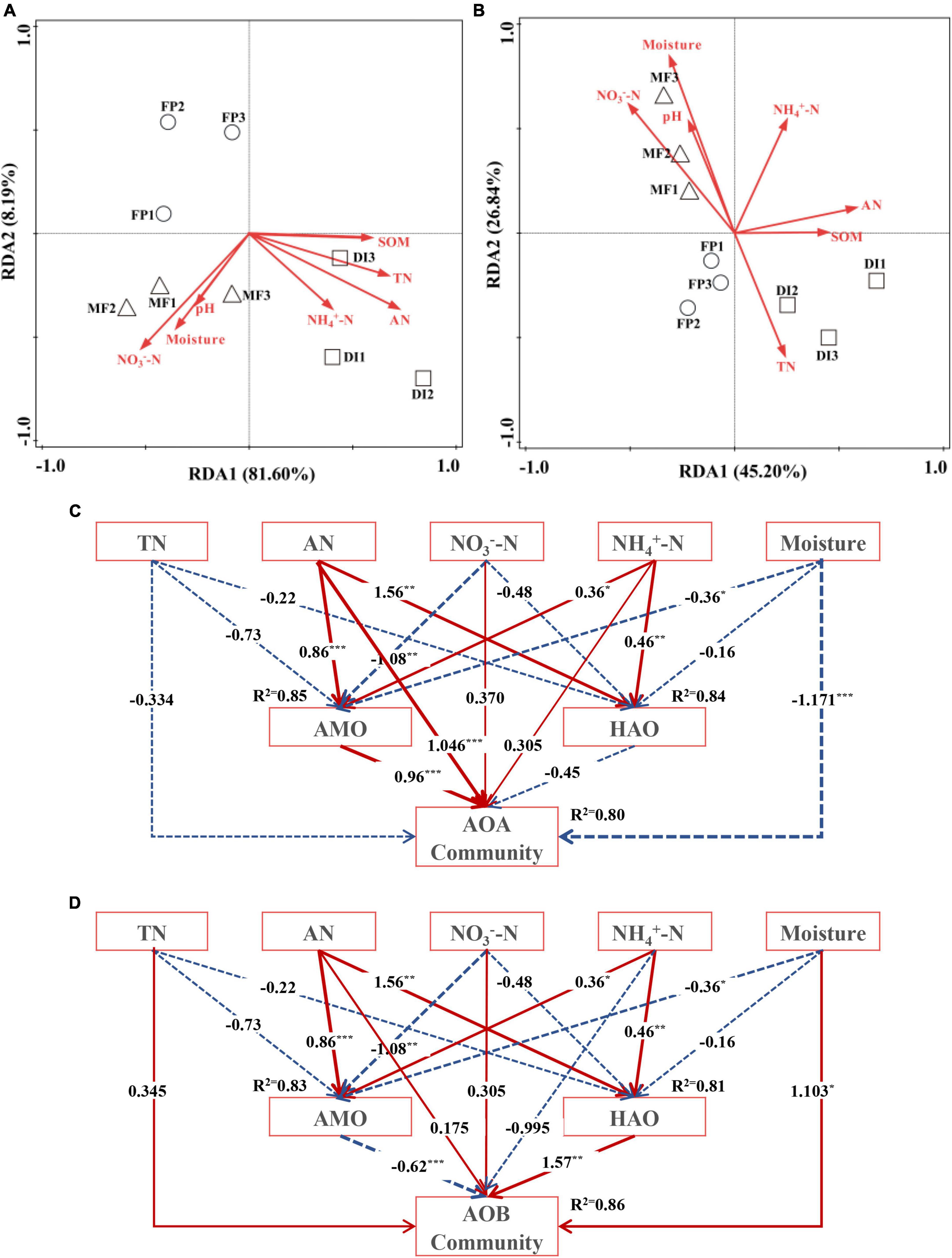
Figure 7. The RDA between AOM communities (A is AOA, B is AOB) and soil properties. The structural equation model (SEM) between the soil nutrient content, AMO, HAO, and AOA community (C), AOB community (D). The arrow width is proportional to the strength of the path co-efficients. Solid red arrows indicate a positive correlation, while blue dotted arrows indicate a negative relationship. *, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001, respectively. SOM, soil organic matter; TN, total nitrogen; AN, alkaline hydrolyzed nitrogen; NH4+-N, ammonium nitrogen; NO3––N, nitrate nitrogen; AMO, ammonia monooxygenase; HAO, hydroxylamine oxidase; AOM, ammonia oxidizing microorganisms; FP, traditional flooding irrigation; DI, shallow drip irrigation; MF, mulching drip irrigation.
3.6 Symbiotic network analysis
The AOA and AOB symbiotic networks of the three irrigation treatments were used to test whether irrigation methods showed unique topological properties that affect microbial communities. Through the analysis of the average degree, clustering coefficient, average path length, complexity, density, and modularity characteristics of AOM networks, it was revealed that each network has some typical personalized modularity characteristics (Table 2). The AOA symbiotic network consisted of 153 (FP), 157 (DI), 144 (MF) nodes and 961 (FP), 1137 (DI), and 849 (MF) edges. These results showed that DI increased the number of nodes in the network compared to FP and MF. Also, DI increased the edge of the interaction of AOA species, the complexity of soil AOA networks, and enhanced the interactions between AOA species (Figures 8A–C). The AOB symbiotic network consisted of 241 (FP), 249 (DI), 276 (MF) nodes and 3034 (FP), 4442 (DI), 5514 (MF) edges. Compared with FP and DI, MF increases the number of nodes in the network and also increases the interaction edges of AOB species. MF increased the complexity of the soil AOB network and enhanced the interaction between AOB species (Figures 8D–F). Compared with AOA networks, AOB networks had the highest average degree, the shortest average path length, and the highest average clustering coefficient, indicating that AOB networks had the highest interspecific interaction degree and the highest connection tightness with closer OTUs connections.
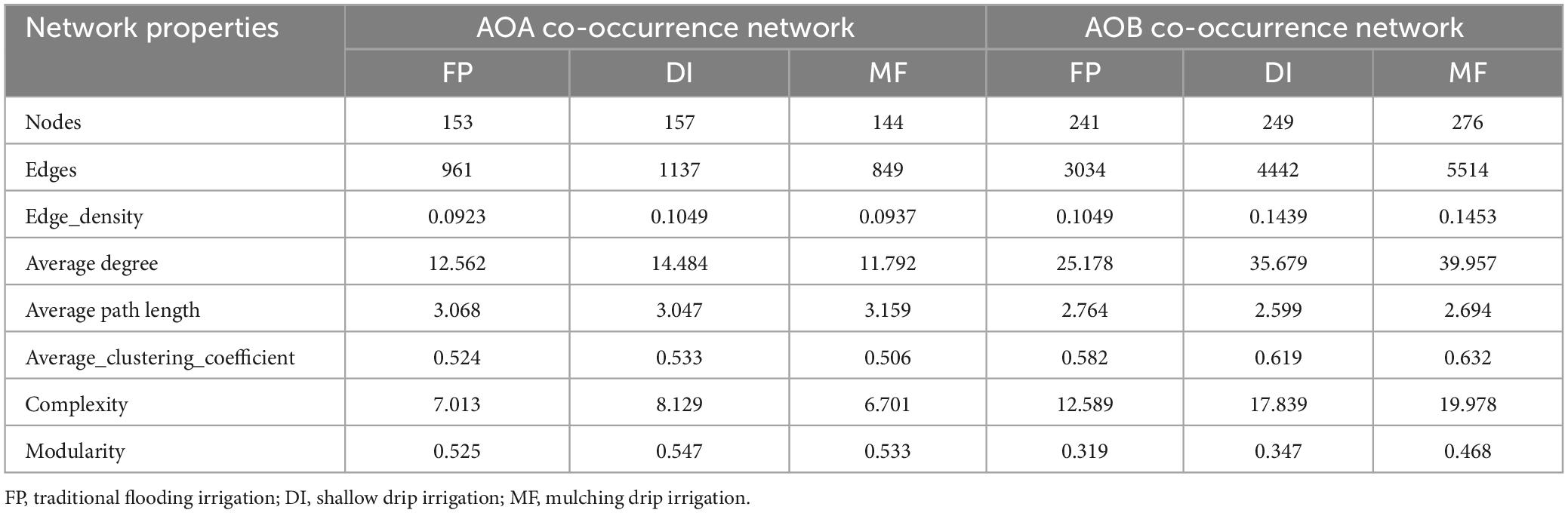
Table 2. Network topological parameters of soil AOA and AOB communities under different irrigation methods.
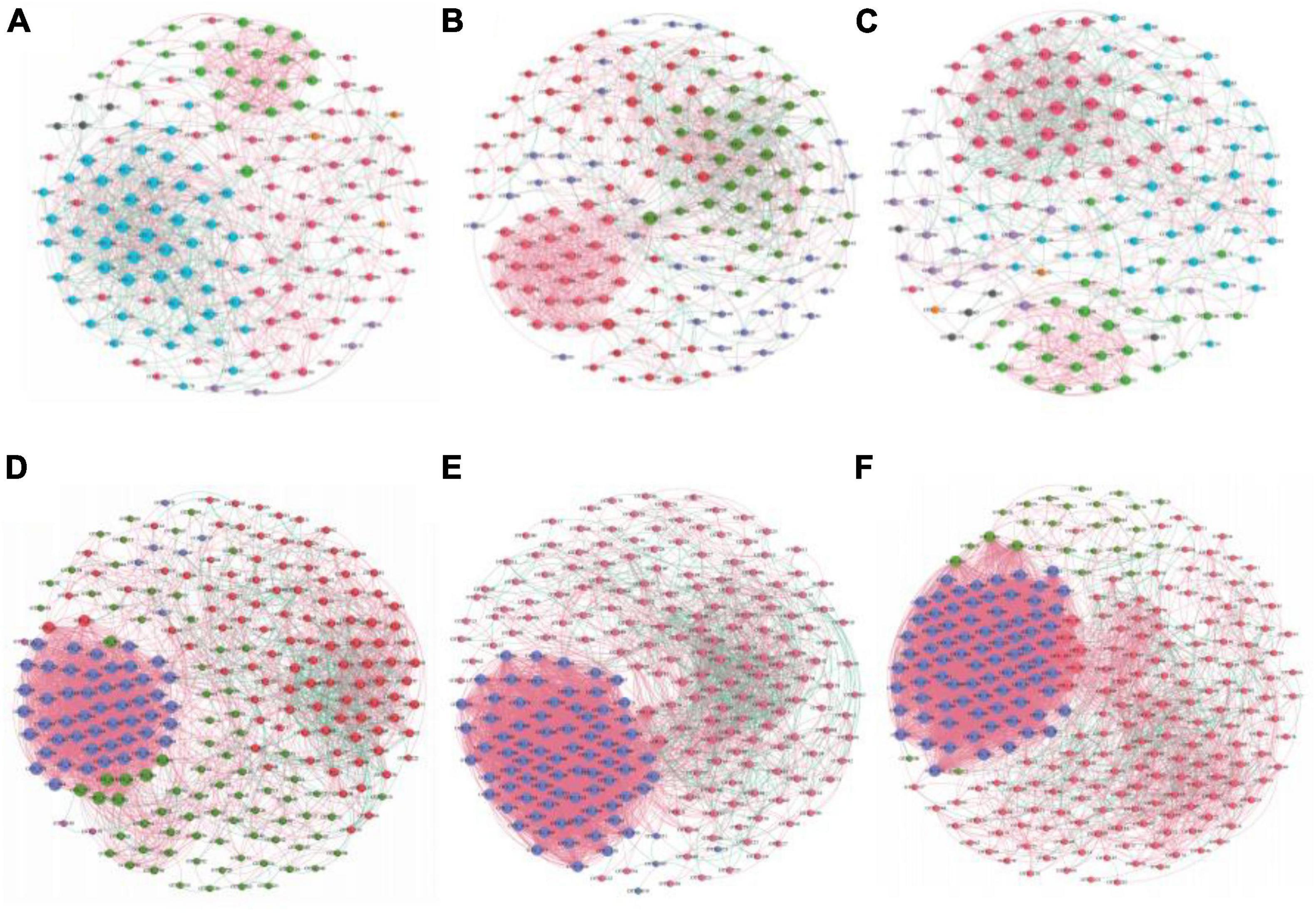
Figure 8. Co-occurrence network analysis of AOM communities under different irrigation methods. (A) AOA of FP, (B) AOA of DI, (C) AOA of MF, (D) AOB of FP, (E) AOB of DI, (F) AOB of MF. A connection represented a strong (Spearman’s correlation coefficient ρ > 0.7) and significant (P < 0.01) correlation. The thickness of each connection between two nodes, that is edge, is proportional to the value of Spearman’s correlation coefficients, the size of each node is proportional to the number of connections (degree).
Most of the nodes in the AOA and AOB symbiotic networks under the three irrigation modes were peripheral nodes, and none of the nodes in the AOA and AOB networks fell within the network hub. In the AOA symbiotic network: FP had 1 node in the module hub and 12 nodes in the connector; DI had 1 node in the module hub and 5 nodes in the connector; MF had 1 node in the module hub and 3 nodes in the connector (Figures 9A–C). In the AOB symbiotic network: FP had 6 nodes in the module hub and 3 nodes in the connector; DI had 1 node in the module hub; MF had 5 nodes in the module hub and 1 node in the connector (Figures 9D–F).
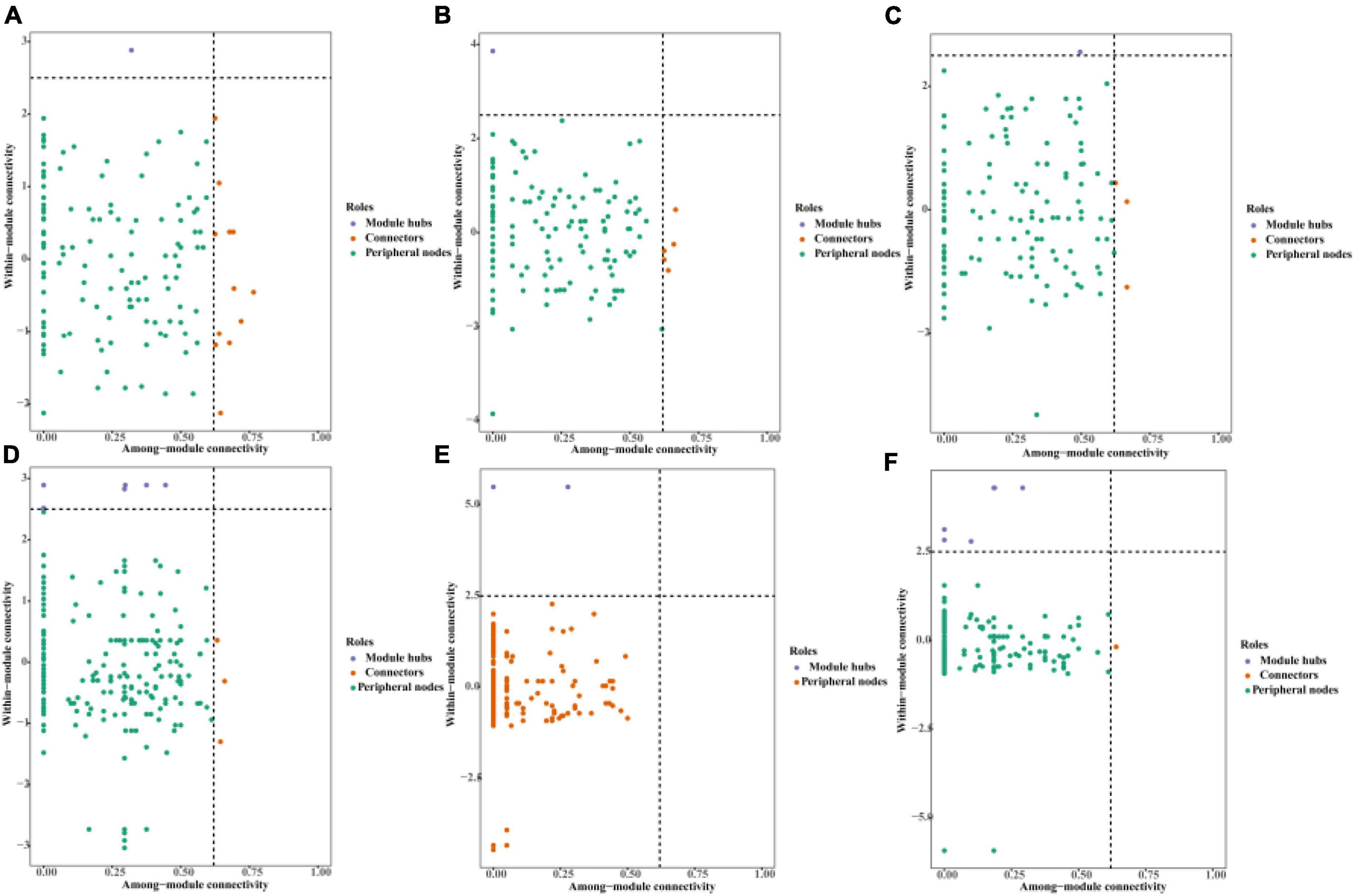
Figure 9. Topological role of AOM communities in different irrigation methods. The horizontal lines indicate 2.5 intra-module connectivity (Zi) and vertical lines indicate 0.62 inter-module connectivity (Pi). (A) AOA community of traditional irrigation; (B) AOA community of shallow drip irrigation; (C) AOA community of covered drip irrigation; (D) AOB community of traditional irrigation; (E) AOA community of shallow drip irrigation; (F) AOB community of covered drip irrigation.
4 Discussion
4.1 Irrigation methods on soil physical and chemical properties
Compared with FP, DI and MF significantly increased soil water content. Qu et al. (2019) also indicated that mulching film significantly increased soil water content in arid and semi-arid areas. This may be due to the slow and uniform immersion of soil water into the soil during drip irrigation, which keeps the soil water-holding capacity relatively suitable and stable while not destroying the soil structure, thereby reducing the evaporation of soil water (Liu and Xu, 2023). To a certain extent, mulching film mitigates the impact of air convection, increases temperature, and preserves soil moisture, thus inhibiting the evaporation of surface water. This improves soil hydrothermal conditions and promotes the early growth of crops while saving water and fertilizer, thereby improving water use efficiency (Yu et al., 2018). There was no significant difference in SOM under different irrigation modes, which was consistent with the study by Núez et al. (2022). In this study, there was no significant difference in TN content among the three irrigation methods, which may be due to the dynamic balance of N in the soil (Lu et al., 2021). Compared with FP, DI, and MF significantly increased the content of AN in soil, which may be because the content of AN in the soil is not stable enough and is easily affected by soil hydrothermal and biological activities. Mulching increased soil temperature, intensified microbial activity, promoted nutrient release, and increased the content of AN (Wang et al., 2021a; Araujo et al., 2023). Compared with FP, DI and MF significantly increased NO3–-N content, while decreasing soil NH4+-N content, which may be due to faster evaporation of soil water after drip irrigation, better soil aeration, and soil surface N accumulation under film mulching treatment (Chen et al., 2020). Moreover, the film coating can slow down the downward migration of NO3–-N, leading to an increase in NO3–-N content under mulched drip irrigation (Wang et al., 2022). With the increase in soil aeration and soil temperature, the activity of soil nitrifying bacteria was enhanced, and the nitrification reaction was intensified, resulting in decreases in NH4+-N content (Li et al., 2022).
4.2 Influence of irrigation methods on the abundance of ammonia-oxidizing microorganisms
This study showed that the abundance of AOA amoA gene was significantly higher than that of AOB amoA gene, which was consistent with earlier studies that indicated that AOA amoA gene abundance dominates in some weakly acidic soils (Sun et al., 2019; Yao et al., 2022). Compared with FP, DI significantly increased the abundance of AOA amoA and AOB amoA genes, and MF significantly increased the abundance of AOB amoA gene. Drip irrigation promotes the nitrification of soil such that under drip irrigation conditions, water droplets slowly enter the soil and distribute around crop roots under the action of capillary force and gravity. In this region, the nitrification effect is higher than the denitrification effect (Sarula et al., 2023), thus increasing the abundance of soil AOA amoA and AOB amoA genes. In this study, the correlation between soil physicochemical properties and the abundance of AOA amoA and AOB amoA genes was studied by Pearson correlation analysis. The results showed that the soil AOA amoA abundance was positively correlated with AN and SOM, and the soil AOB amoA abundance was positively correlated with AN, SOM, and NH4+-N. Studies have shown that AOA is more adaptable to environmental conditions with low available ammonium N concentration, and plays a leading role in the nitrification process than AOB (Lin et al., 2021). On the contrary, AOB is more dominant in the nitrification process of neutral soils with high alkaline and N abundance (Wang et al., 2021b). The results indicated that the change of NH4+-N content had a more obvious effect on AOB abundance due to the different irrigation methods.
4.3 Effects of irrigation methods on the composition of ammonia-oxidizing microbial communities
At the phylum level, soil AOB community composition was significantly affected by irrigation methods, while soil AOA community changes were less affected. This may be due to the fact that the ecological niche of soil AOA is smaller than that of AOB, that is, it occupies a narrower range of resources and space in the soil, while soil AOB has a wider ecological niche and can survive under different conditions such as temperature, pH and N elements (Guo et al., 2023). Compared with FP, DI, and MF significantly changed the community structure and abundance of AOM in soil. The dominant microorganisms are relatively abundant in soil and play an important role in the regulation of ecological functions. The results of this study indicated that Thaumarchaeota, Crenarchaeota, and Proteobacteria are the dominant phyla in AOA. Thaumarchaeota is a typical ammonia-oxidizing archaea, and some studies have suggested that Thaumarchaeota plays an important role in the ammonia-oxidizing process (Yang et al., 2018; Wang N. et al., 2019). The significant increase in the relative abundance of Thaumarchaeota in FP may be due to the application of N fertilizer. Araujo et al. (2023) showed that Thaumarchaeota is the dominant archaea under N application systems. The research of Luo et al. (2023) also showed that Thaumarchaeota is significantly positively correlated with soil NH4+-N content and other related indicators, suggesting that Thaumarchaeota can accelerate catalytic ammonia oxidation to obtain energy for autotrophic growth (Wu et al., 2020). Studies have shown that most OTUs in AOA belong to Crenarchaeota (Wang and Huang, 2021), and an increase in the relative abundance of Crenarchaeota is conducive to promoting the rate of soil ammonia oxidation. An appropriate combination of fertilization and irrigation frequency can regulate soil AOA community composition, and the physicochemical properties also have different effects on Crenarchaeota. The highly relative abundance of Crenarchaeota in DI treatment may be due to the high SOM content and better soil fertility. Proteobacteria have a wide ecological range and strong suitability and can form a relatively stable ecological niche in different environments (Jiang et al., 2021). The highly relative abundance of Proteobacteria in MF may be due to the difference in soil water content. The findings of this study are consistent with Chen et al. (2020) who reported that Proteobacteria propagated in large numbers under high soil-water conditions.
In AOB, Firmicutes, Gemmatimonadetes and Actinobacteria are dominant bacteria. The Bacillus in Firmicutes produces spores that can resist drying, dehydration, and extreme environments. In the case of drought, spores can prolong the hydration of cells and provide nutrients to cells, so that cells can resist dehydration and other extreme environments (Li et al., 2020). The highly relative abundance of Firmicutes in FP treatment may be due to the fluctuations in moisture and the proliferation of these bacteria in low water content environments (Veach and Zeglin, 2020). Gemmatimonadetes was the dominant phylum of DI. Gemmatimonadetes can adapt to low-humidity environments, but cannot resist moisture fluctuations caused by dry and wet cycles. Moreover, studies have shown that Gemmatimonadetes is negatively correlated with soil organic matter (Zhang et al., 2019b). The highly relative abundance of Actinobacteria in MF may be due to the influence of SOM content. Javed et al. (2021) confirmed that the abundance of Actinobacteria bacteria can promote the formation of humus and decomposition of organic matter in soil, and also have a certain effect on the soil N cycle. As Actinobacteria tends to grow in soil with low SOM content, film mulching reduces the organic carbon content of rhizosphere soil, which is conducive to promoting the growth and propagation of Actinobacteria (Chen et al., 2017). According to Blast analysis, Nitrososphaerota is an important strain of AOA, and Nitrososphaera belongs to Nitrososphaerota. Previous studies have shown that the main AOA group in the black soil of Northeast China is Nitrososphaera (Ding et al., 2020), and it may be the main driving factor for nitrification of acidic soil in China (Li W. et al., 2021). Nitrosospira sp. was the dominant genus of AOB, which is consistent with previous studies on agricultural soil (Zhang et al., 2019c; Hu et al., 2021). Nitrosospira sp. produces nitrite by oxidizing soil nitrifying bacteria, which plays a crucial role in the nitrification process, making nitrogen easily absorbed by crops.
4.4 Environmental factors affecting the changes of ammonia-oxidizing microbial communities
In this study, the RDA of environmental factors and AOA community structure showed that AN was the main driving factor affecting change in the AOA community. The results of this study are consistent with the results of Sarula et al. (2023), who similarly reported that AOA community structure mainly depends on AN and pH. On the contrary, soil pH did not have much influence on the AOA community probably because there was little change in soil pH in this study. Sun et al. (2019) showed that pH plays an important role in shaping AOA community structure as similarly reported by other studies (Gubry-Rangin et al., 2011; Hu et al., 2013). Moreover, the RDA of environmental factors and AOB community structure showed that water content was the main driving factor affecting the change of the AOB community, which may be caused by the response of AOB to oxygen conditions. Similarly, Yang et al. (2018) showed that there was a significant correlation between the AOB community and soil water which indicates that soil water content significantly affects the community structure of nitrifying microorganisms in soil (Cong et al., 2023).
4.5 Influence of irrigation mode on the symbiotic network
In general, the higher the microbial community diversity index, the more complex the community structure and the better the stability (Jiang et al., 2020). The AOB symbiotic network was more complex than the AOA symbiotic network in the three irrigation methods, indicating that the three irrigation methods had better stability and stress resistance effects on the soil AOB community. This may be due to the significant increase in some key species in AMO in our study, thereby improving the network stability and connectivity of species in soil (Xiang et al., 2020). At the same time, the high stability of microbial communities is an important guarantee for the realization of ecological functions. Therefore, AOB plays an important role in enhancing the function of farmland soil under different irrigation methods. The study also identified key species of microbial communities by analyzing the topological structure of symbiotic networks, which change the composition of microbial communities (Zhou et al., 2021). In a microbial symbiotic network, nodes represent microbiota species in the biome, and the topological characteristics of different nodes can be used to determine the key species. Generally, node attributes are divided into four types; peripheral nodes (Zi < 2.5 and Pi < 0.62), connectors (Zi < 2.5 and Pi > 0.62), module hubs (Zi > 2.5 and Pi < 0.62), and network hubs (Zi > 2.5 and Pi > 0.62), where all nodes of the network hub are key species in the network. Nodes that fall within connectors and module hubs play an important role between and within modules (Ya et al., 2021). DI recorded the highest number of network edges with more complex structures and the interaction between AOA communities was closer. The high connectivity within the AOA community in DI results in higher complexity and stability, thereby maintaining the versatility and sustainability of the ecosystem. In addition, the interaction between AOB communities was closer under MF due to the high number of network edges which made the structure more complex. This indicated that MF had better connectivity and higher energy transfer efficiency between AOB communities.
5 Conclusion
This study focused on characterizing soil physicochemical properties, soil ammoxidation-related enzyme activities, AOA and AOB abundance, and community structure under three different irrigation methods, and also explored the response of AOA and AOB communities to the three irrigation methods using symbiotic networks. The results showed that DI and MF significantly increased the contents of AN, NO3–-N, moisture, and the activities of AMO and HAO in soil. Compared with FP, both water-saving irrigation methods (DI and MF) could increase the abundance of AOM, and DI had a more significant effect on the abundance of soil AOA and AOB. Different irrigation methods also significantly affected the community structure of AOM. Moreover, AN and moisture were the main driving factors affecting the changes in AOA and AOB communities, respectively, and the sensitivity of the AOB community to irrigation methods was higher than that of the AOA community. The network complexity of AOB was higher than that of AOA, indicating that the bacteria in the AOB community had a closer synergistic relationship and better-improved resource utilization efficiency and biological metabolic rate. The AOB community under MF had higher complexity and stability, and MF had a positive impact on the community structure of AOB.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SRP479978.
Author contributions
RQ: Data curation, Writing – original draft, Methodology. MW: Funding acquisition, Writing – review and editing, Project administration. QL: Software, Writing – original draft. YL: Data curation, Writing – original draft. CL: Formal analysis, Writing – review and editing. JZ: Validation, Writing – review and editing. HL: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Capital Construction Funds in the budget of Jilin Province in 2023 (2023C036-2) and the Jilin Province Science and Technology Development Program (grant numbers 20220101176JC, 20240304026SF, 20220203022SF, and 20210101028JC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1374618/full#supplementary-material
References
Abramyan, S., Araksyan, S., and Galstyan, A. (1989). Determination of hydroxylamine oxidase activity of soil. Soviet Agric. Sci. 54, 18–21.
Araujo, A. S. F., Rocha, S. M. B., Pereira, A. P. A., Melo, V. M. M., Oliveira, F. A. S., Neto, F. A., et al. (2023). Responses of bacterial and archaeal communities to nitrogen fertilization in a compost-amended soil. Pedobiologia 101:150915. doi: 10.1016/j.pedobi.2023.150915
Beaudoin, N., Venet, E., Maucorps, J., Vandenberghe, C., Pugeaux, N., Viennot, P., et al. (2021). Long term response of water and nitrogen fluxes to good agricultural practices at field and catchment scales. Sci. Total Environ. 776:145954. doi: 10.1016/j.scitotenv.2021.145954
Chang, Y., Fan, J., Su, J., Ming, H., Zhao, W., Shi, Y., et al. (2017). Spatial abundance, diversity, and activity of ammonia-oxidizing bacteria in coastal sediments of the Liaohe estuary. Curr. Microbiol. 74, 632–640. doi: 10.1007/s00284-017-1226-x
Chen, D., Yuan, L., Liu, Y., Ji, J., and Hou, H. (2017). Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 90, 34–42. doi: 10.1016/j.eja.2017.07.007
Chen, N., Li, X., Šimůnek, J., Shi, H., Ding, Z., and Zhang, Y. (2020). The effects of biodegradable and plastic film mulching on nitrogen uptake, distribution, and leaching in a drip-irrigated sandy field. Agric. Ecosyst. Environ. 292:106817. doi: 10.1016/j.agee.2020.106817
Cong, J. J., Ma, J. L., Li, S. H., Gao, W., Bao, X. L., Zhu, X. F., et al. (2023). Variation of nitrification and denitrification microbial community structures in black soil under different moisture content. Plant Nutr. Fert. Sci. 29, 1400–1410. doi: 10.11674/zwyf.2022712
Ding, J., Ma, M., Jiang, X., Liu, Y., Zhang, J., Suo, L., et al. (2020). Effects of applying inorganic fertilizer and organic manure for 35 years on the structure and diversity of ammonia-oxidizing archaea communities in a Chinese Mollisols field. Microbiologyopen 9:e00942. doi: 10.1002/mbo3.942
Fan, J., Lu, X., Gu, S., and Guo, X. (2020). Improving nutrient and water use efficiencies using water-drip irrigation and fertilization technology in Northeast China. Agric. Water Manag. 241:106352. doi: 10.1016/j.agwat.2020.106352
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E., and Oakley, B. B. (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U.S.A. 102, 14683–14688. doi: 10.1073/pnas.0506625102
Gu, Q., Wei, J., Luo, S., Ma, M., and Tang, X. (2018). Potential and environmental control of carbon sequestration in major ecosystems across arid and semi-arid regions in China. Sci. Total Environ. 645, 796–805. doi: 10.1016/j.scitotenv.2018.07.139
Gubry-Rangin, C., Hai, B., Quince, C., Engel, M., Thomson, B. C., James, P., et al. (2011). Niche specialization of terrestrial archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. U.S.A. 108, 21206–21211. doi: 10.1073/pnas.1109000108
Guo, H., Ma, L., Liang, Y., Hou, Z., and Min, W. (2021). Response of ammonia-oxidizing Bacteria and Archaea to long-term saline water irrigation in alluvial grey desert soils. Sci. Rep. 10:489. doi: 10.1038/s41598-019-57402-x
Guo, X., Du, S., Guo, H., and Min, W. (2023). Long-term saline water drip irrigation alters soil physicochemical properties, bacterial community structure, and nitrogen transformations in cotton. Appl. Soil Ecol. 182:104719. doi: 10.1016/j.apsoil.2022.104719
Hu, H. W., Zhang, L. M., Dai, Y., Di, H. J., and He, J. Z. (2013). pH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by high-throughput pyrosequencing. J. Soil Sediment. 13, 1439–1449. doi: 10.1007/s11368-013-0726-y
Hu, J., Zhao, Y., Yao, X., Wang, J., Zheng, P., Xi, C., et al. (2021). Dominance of comammox Nitrospira in soil nitrification. Sci. Total Environ. 780:146558. doi: 10.1016/j.scitotenv.2021.146558
Javed, Z., Tripathi, G. D., Mishra, M., and Dashora, K. (2021). Actinomycetes–the microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal. Agric. Biotechnol. 31:101893. doi: 10.1016/j.bcab.2020.101893
Jiang, R., Wang, M., Chen, W., Li, X., and Balseiro-Romero, M. (2020). Changes in the integrated functional stability of microbial community under chemical stresses and the impacting factors in field soils. Ecol. Indic. 110:105919. doi: 10.1016/j.ecolind.2019.105919
Jiang, S., Xing, Y., Liu, G., Hu, C., Wang, X., Yan, G., et al. (2021). Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 161:108393. doi: 10.1016/j.soilbio.2021.108393
Kou, Y., Li, C., Tu, B., Li, J., and Li, X. (2023). The responses of ammonia-oxidizing microorganisms to different environmental factors determine their elevational distribution and assembly patterns. Microb. Ecol. 86, 485–496. doi: 10.1007/s00248-022-02076-8
Lehtovirta-Morley, L. E. (2018). Ammonia oxidation: Ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol. Lett. 365:fny058. doi: 10.1093/femsle/fny058
Li, H., Mei, X., Wang, J., Huang, F., Hao, W., and Li, B. (2021). Drip fertigation significantly increased crop yield, water productivity and nitrogen use efficiency with respect to traditional irrigation and fertilization practices: A meta-analysis in China. Agric. Water Manag. 244:106534. doi: 10.1016/j.agwat.2020.106534
Li, W., Zheng, M., Wang, C., and Shen, R. (2021). Nitrososphaera may be a major driver of nitrification in acidic soils. Soils 53, 13–20.
Li, S., Chen, Y., Li, T., Yu, F., Zhang, Y., Liu, K., et al. (2022). Alternate wetting and moderate soil drying irrigation counteracts the negative effects of lower nitrogen levels on rice yield. Plant Soil. 481, 367–384. doi: 10.1007/s11104-022-05644-6
Li, X., Sun, P., Zhang, Y., Jin, C., and Guan, C. (2020). A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 174:104023. doi: 10.1016/j.envexpbot.2020.104023
Li, Z., Wan, S., Chen, G., Han, Y., Lei, Y., Ma, Y., et al. (2023). Effects of irrigation regime on soil hydrothermal microenvironment, cotton biomass, and yield under non-film drip irrigation system in cotton fields in southern Xinjiang. China. Ind Crops Prod. 198:116738. doi: 10.1016/j.indcrop.2023.116738
Lin, Y., Hu, H. W., Ye, G., Fan, J., Ding, W., He, Z. Y., et al. (2021). Ammonia-oxidizing bacteria play an important role in nitrification of acidic soils: A meta-analysis. Geoderma 404:115395. doi: 10.1016/j.geoderma.2021.115395
Liu, X., and Xu, Y. (2023). Effect of integration of water and fertilizer on soil water–nitrogen transport characteristics in bubbled-root irrigation. Irrigation Sci. 41, 379–393. doi: 10.1007/s00271-023-00851-0
Lu, J., Hu, T., Zhang, B., Wang, L., Yang, S., Fan, J., et al. (2021). Nitrogen fertilizer management effects on soil nitrate leaching, grain yield and economic benefit of summer maize in Northwest China. Agric. Water Manag. 247:106739. doi: 10.1016/j.agwat.2021.106739
Lu, R. K. (1999). Methods for agrochemical analysis of soil. Beijing: China Agricultural Science and Technology Press.
Luo, J. R., Song, W. J., Liu, W., Zhang, D. H., Jiang, X., and Lu, B. L. (2023). Effects of straw returning and nitrogen fertilizer application on rice (Oryza sativa) growth, soil properties and microbial diversity in the early stage. J. Agric. Biotechnol. 31, 2019–2034. doi: 10.3969/j.issn.1674-7968.2023.10.003
Núez, A., Cotrufo, F., and Schipanski, M. (2022). Irrigation effects on the formation of soil organic matter from aboveground plant litter inputs in semiarid agricultural systems. Geoderma 416:115804. doi: 10.1016/j.geoderma.2022.115804
Ouyang, Y., Norton, J. M., and Stark, J. M. (2017). Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and Archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 113, 161–172. doi: 10.1016/j.soilbio.2017.06.010
Ouyang, Y., Norton, J. M., Stark, J. M., Reeve, J. R., and Habteselassie, M. Y. (2016). Ammonia-oxidizing bacteria are more responsive than Archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 96, 4–15. doi: 10.1016/j.soilbio.2016.01.012
Petersen, D. G., Blazewicz, S. J., Firestone, M., Herman, D. J., Turetsky, M., and Waldrop, M. (2012). Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 14, 993–1008. doi: 10.1111/j.1462-2920.2011.02679.x
Qu, J. C., Jiang, A. N., Yan, J. Q., Lu, H. B., Zhao, H. C., and Huang, Z. H. (2019). Effects of mulching methods on soil water condition and yield of spring maize in cold and dry region. J. Hebei North Univ. 35, 42–47. doi: 10.3969/j.issn.1673-1492.2019.03.010
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Sarula, Hengshan, Y., Zhang, R., and Li, Y. (2023). Shallow-buried drip irrigation promoted the enrichment of beneficial microorganisms in surface soil. Rhizosphere 28:100776. doi: 10.1016/j.rhisph.2023.100776
Sun, R., Myrold, D. D., Wang, D., Guo, X., and Chu, H. (2019). AOA and AOB communities respond differently to changes of soil pH under long-term fertilization. Soil Ecol. Lett. 1, 126–135. doi: 10.1007/s42832-019-0016-8
Szukics, U., Grigulis, K., Legay, N., Kastl, E. M., Baxendale, C., Bardgett, R. D., et al. (2019). Management versus site effects on the abundance of nitrifiers and denitrifiers in European mountain grasslands. Sci. Total Environ. 648, 745–753. doi: 10.1016/j.scitotenv.2018.08.039
Tolar, B. B., Herrmann, J., Bargar, J. R., Bedem, H., Wakatsuki, S., and Francis, C. A. (2017). Integrated structural biology and molecular ecology of N-cycling enzymes from ammonia-oxidizing Archaea. Environ. Microbiol. Rep. 9, 484–491. doi: 10.1111/1758-2229.12567
Veach, A. M., and Zeglin, L. H. (2020). Historical drought affects microbial population dynamics and activity during soil drying and re-wet. Microb. Ecol. 79, 662–674. doi: 10.1007/s00248-019-01432-5
Wang, D., Mo, Y., Li, G., Wilkerson, C. J., and Hoogenboom, G. (2021a). Improving maize production and decreasing nitrogen residue in soil using mulched drip fertigation. Agric. Water Manag. 251:106871. doi: 10.1016/j.agwat.2021.106871
Wang, D., Sheng, K., Zhao, W., Li, L., Zhang, Q., and Wang, Y. (2021b). Dominance of archaeal ammonia-oxidizers in soil nitrification across different soil types and fertilities in North China plain. Eur. J. Soil Biol. 106:103354. doi: 10.1016/j.ejsobi.2021.103354
Wang, L., and Huang, D. (2021). Soil ammonia-oxidizing Archaea in a paddy field with different irrigation and fertilization managements. Sci. Rep. 11:14563. doi: 10.1038/s41598-021-93898-y
Wang, N., Zhao, Y. H., Ai, Y. C., Zhang, Y. C., Wang, J. D., and Yu, J. G. (2019). Responses of archaeal community composition in paddy soils to straw return. J. Agric. Environ. Sci. 38, 374–382. doi: 10.11654/jaes.2018-0508
Wang, Z., Wu, Q., Fan, B., Zheng, X., Zhang, J., Li, W., et al. (2019). Effects of mulching biodegradable films under drip irrigation on soil hydrothermal conditions and cotton (Gossypium hirsutum L.) yield. Agric. Water Manag. 213, 477–485. doi: 10.1016/j.agwat.2018.10.036
Wang, S., Xia, G., Zheng, J., Wang, Y., Chen, T., Chi, D., et al. (2022). Mulched drip irrigation and biochar application reduce gaseous nitrogen emissions, but increase nitrogen uptake and peanut yield. Sci. Total Environ. 830:154753. doi: 10.1016/j.scitotenv.2022.154753
Wu, L., Chen, X., Wei, W., Liu, Y., Wang, D., and Ni, B. J. A. (2020). Critical review on nitrous oxide production by ammonia-oxidizing Archaea. Environ. Sci. Technol. 54, 9175–9190. doi: 10.1021/acs.est.0c03948
Xiang, X., Liu, J., Zhang, J., Li, D., Xu, C., and Kuzyakov, Y. (2020). Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Till. Res. 196:104491. doi: 10.1016/j.still.2019.104491
Xiao, R., Ran, W., Hu, S., and Guo, H. (2021). The response of ammonia oxidizing archaea and bacteria in relation to heterotrophs under different carbon and nitrogen amendments in two agricultural soils. Appl. Soil Ecol. 158:103812. doi: 10.1016/j.apsoil.2020.103812
Xin, F., Xiao, X., Dong, J., Zhang, G., Zhang, Y., Wu, X., et al. (2020). Large increases of paddy rice area, gross primary production, and grain production in Northeast China during 2000–2017. Sci. Total Environ 711:135183. doi: 10.1016/j.scitotenv.2019.135183
Ya, T., Du, S., Li, Z., Liu, S., Zhu, M., Liu, X., et al. (2021). Successional dynamics of molecular ecological network of anammox microbial communities under elevated salinity. Water Res. 188:116540. doi: 10.1016/j.watres.2020.116540
Yang, Y. D., Song, R. K., Zhao, J., Wang, P. X., Xu, X. L., and Ceng, Z. H. (2018). Effects of long-term different fertilization regimes on the abundance and community structure of ammonia oxidizers in paddy soils. Chin. J. Appl. Ecol. 29, 3829–3837. doi: 10.13287/j.1001-9332.201811.031
Yao, R., Li, H., Yang, J., Zhu, W., Yin, C., Wang, X., et al. (2022). Combined application of biochar and N fertilizer shifted nitrification rate and amoA gene abundance of ammonia-oxidizing microorganisms in salt-affected anthropogenic-alluvial soil. Appl. Soil Ecol. 171:104348. doi: 10.1016/j.apsoil.2021.104348
Yu, L., Tang, Y., Wang, Z., Gou, Y., and Wang, J. (2019). Nitrogen-cycling genes and rhizosphere microbial community with reduced nitrogen application in maize/soybean strip intercropping. Nutr. Cycl. Agroecosyst. 113, 35–49. doi: 10.1007/s10705-018-9960-4
Yu, Y. Y., Turner, N. C., Gong, Y. H., Li, F. M., Fang, C., Ge, L. J., et al. (2018). Benefits and limitations to straw-and plastic-film mulch on maize yield and water use efficiency: A meta-analysis across hydrothermal gradients. Eur. J. Agron. 99, 138–147. doi: 10.1016/j.eja.2018.07.005
Zhang, X., Bol, R., Rahn, C., Xiao, G., Meng, F., and Wu, W. (2017). Agricultural sustainable intensification improved nitrogen use efficiency and maintained high crop yield during 1980–2014 in Northern China. Sci. Total Environ. 596, 61–68. doi: 10.1016/j.scitotenv.2017.04.064
Zhang, Q., Li, Y., He, Y., Liu, H., Dumont, M. G., Brookes, P. C., et al. (2019c). Nitrosospira cluster 3-like bacterial ammonia oxidizers and Nitrospira-like nitrite oxidizers dominate nitrification activity in acidic terrace paddy soils. Soil Biol. Biochem. 131, 229–237. doi: 10.1016/j.soilbio.2019.01.006
Zhang, X., Meng, F., Li, H., Wang, L., Wu, S., Xiao, G., et al. (2019a). Optimized fertigation maintains high yield and mitigates N2O and NO emissions in an intensified wheat–maize cropping system. Agric. Water Manag. 211, 26–36. doi: 10.1016/j.agwat.2018.09.045
Zhang, X., Zhang, H. Y., Lu, C., Pang, H. C., Jin, C. W., Gao, X., et al. (2019b). Effects of the different autumn irrigation years on soil bacterial community in Hetao irrigation district. Sci. Agric. Sin. 52, 3380–3392. doi: 10.3864/j.issn.0578-1752.2019.19.009
Keywords: mulched drip irrigation, AOA, AOB, community composition, symbiotic network
Citation: Qiang R, Wang M, Li Q, Li Y, Li C, Zhang J and Liu H (2024) The different responses of AOA and AOB communities to irrigation systems in the semi-arid region of Northeast China. Front. Microbiol. 15:1374618. doi: 10.3389/fmicb.2024.1374618
Received: 19 February 2024; Accepted: 05 April 2024;
Published: 07 May 2024.
Edited by:
Xuebin Qi, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Wenliang Wei, Qingdao Agricultural University, ChinaXiaojing Hu, Chinese Academy of Sciences (CAS), China
Xiancan Zhu, Anhui Normal University, China
Copyright © 2024 Qiang, Wang, Li, Li, Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hang Liu, aGFuZ2xAamxhdS5lZHUuY24=
†These authors have contributed equally to this work
 Ruowen Qiang1†
Ruowen Qiang1† Meng Wang
Meng Wang Jinjing Zhang
Jinjing Zhang Hang Liu
Hang Liu