
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 17 April 2024
Sec. Systems Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1364373
Escherichia coli (E. coli) is closely associated with the occurrence of puerperal metritis in dairy cows. E. coli carries some the virulence and multi-drug resistant genes, which pose a serious threat to the health of postpartum cows. In this study, E. coli was isolated and identified from the uterine contents of postpartum cows with puerperal metritis in the Ningxia region of China, and its phylogenetic subgroups were determined. Meanwhile, virulence and drug resistance genes carried by E. coli and drug sensitivity were detected, and the characteristics of virulence and drug resistance genes distribution in E. coli phylogroups were further analyzed. The results showed that the isolation rate of E. coli in puerperal metritis samples was 95.2%. E. coli was mainly divided into phylogroups B2 and D, followed by groups A and B1, and was more connected to O157:H7, O169:H4, and ECC-1470 type strains. The virulence genes were mainly dominated by ompF (100%), traT (100%), fimH (97%), papC (96%), csgA (95%), Ang43 (93.9%), and ompC (93%), and the resistance genes were dominated by TEM (99%), tetA (71.7%), aac(3)II (66.7%), and cmlA (53.5%). Additionally, it was observed that the virulence and resistance gene phenotypes could be divided into two subgroups, with subgroup B2 and D having the highest distributions. Drug sensitivity tests also revealed that the E. coli was most sensitive to the fluoroquinolones enrofloxacin, followed by macrolides, aminoglycosides, tetracyclines, β-lactams, peptides and sulfonamides, and least sensitive to lincosamides. These results imply that pathogenic E. coli, which induces puerperal metritis of dairy cows in the Ningxia region of China, primarily belongs to the group B2 and D, contains multiple virulence and drug resistance genes, Moreover, E. coli has evolved resistance to several drugs including penicillin, lincomycin, cotrimoxazole, and streptomycin. It will offer specific guidelines reference for the prevention and treatment of puerperal metritis in dairy cows with E. coli infections in the Ningxia region of China.
Puerperal metritis is a frequent reproductive disorder in dairy cows following parturition, which can result in substantial uterine mucosa damage and incomplete uterine involution, lowering the reproduction and production performance while raising treatment costs and culling rates. Studies have demonstrated that dairy cows with puerperal metritis can delay next pregnancy for up to 95 days, and their failure rate to conceive is 3.8 times higher than that of healthy cows (Piccardi et al., 2016; Ehsanollah et al., 2021). Bacteria are the main cause of puerperal metritis in dairy cows, of which E. coli, Cryptobacterium septicum, Clostridium necrophorum and Prevotella melanogaster have been considered to be the main pathogens and might impact the endometrium and ovarian function (Bicalho et al., 2012). It was reported that E. coli appeared in the uterus within a few days of postpartum cows, followed by C. necrophorum and Cryptobacterium septicum (Jeon and Galvao, 2018). Numerous studies have revealed that E. coli was one of the most important pathogenic bacteria causing postpartum uterine infections in dairy cows (Ma et al., 2018; Schouten et al., 2023). Though the random amplification of polymorphic DNA and 16S rRNA gene sequencing of uterine contents and vaginal secretions of dairy cows with puerperal metritis, Wang et al. (2013) demonstrated that E. coli was predominant in both uterus and vagina. Further studies have revealed that differentially expressed proteins of E. coli induced inflammatory responses in the uterus via various pathways involved in cell proliferation, apoptosis, oxidative stress, and cell metabolism (Piras et al., 2017; Najafi et al., 2022).
Escherichia coli populations are typically subdivided into four main phylogenetic taxa including A, B1, B2, and D groups (Shamsi et al., 2017; Aguirre-Sanchez et al., 2022). Based on different virulence determinants and pathogenic characteristics, E. coli strains can be classified into commensal E. coli, enteropathogenic E. coli (EPEC), and extraintestinal pathogenic E. coli (ExPEC) (Biran and Ron, 2018). ExPEC, mainly belonging to taxa B2 and D, are associated with mucosal infections in multiple tissues and promote inflammatory responses (Van et al., 2008; Yamamura et al., 2022). Among the six species of ExPEC that have been identified, endometrial pathogenic E. coli (EnPEC) is the primary parenteral pathogenic E. coli causing uterine infections in postpartum cows (Bicalho et al., 2010; Sarowska et al., 2019). Additionally, the pathogenicity of E. coli is primarily dependent on the strain serotype and the kinds of virulence factors (VFs) carried by E. coli (Raeispour and Ranjbar, 2018). Recent studies have demonstrated that the development of metritis is mainly associated with the gene expression of ExPEC virulence factors (Kasse et al., 2016; Raheel et al., 2020). The main virulence factors of E. coli associated with puerperal metritis in dairy cows are fimH, astA, kpsMII, and hlyA. These factors are primarily carried on phages, plasmids, or pathogenic islands (PAIs), and they can be transferred horizontally between different microbial strains (Huddleston, 2014; Pakbin et al., 2021). Malahlela et al. (2022) found that the virulence genes of E. coli type O157 were mainly stx1, stx2, eaeA and hlyA, which usually encoded toxins and adhesins causing mild or bloody diarrhea and hemorrhagic colitis in animals. Furthermore, Ban et al. (2015) observed that the virulence genes of E. coli type O169:H41 were mainly dominated by CFs, CS6, and CS8 colonization factors, which mediated the inflammatory response.
Furthermore, the inefficient treatment of uterine infections caused by ExPEC and the emergence of multi-drug resistance in pathogenic bacteria are associated with the type of drug-resistance genes carried by the strains. Existing strains carrying TEM-type β-lactams are relatively resistant to penicillin and broad-spectrum cephalosporins. Furthermore, most resistance genes can be transferred between different serotype strains of E. coli by plasmids, transposons, and integrons, which induce the emergence of multi-drug resistant strains (Ban et al., 2015). However, the drug resistance genes carried by E. coli strains may vary considerably attributed to geographical differences.
The Ningxia region is a highly concentrated dairy farming area in China, which is also an area with a high incidence of puerperal metritis. Our previous study demonstrated that the pathogenic bacteria causing puerperal metritis in dairy cows in the Ningxia region were mainly E. coli (unpublished data). However, to date, the distribution of E. coli populations in the uterine contents of dairy cows with puerperal metritis in this region remains elusive. Meanwhile, the virulence factors and drug resistance genes carried by E. coli are also unknown, which poses many challenges to for the prevention and treatment of puerperal metritis induced by E. coli in dairy cows. Therefore, in this study, we isolated and identified E. coli from the uterine contents of puerperal metritis in dairy cows in the Ningxia region of China and further performed the phylogenetic subgroup and homology analyses of E. coli in the region. Furthermore, the major virulence genes and drug resistance genes carried by E. coli were detected, and the distribution pattern of virulence factors and drug resistance genes of E. coli in its phylogenetic subgroups were analyzed. Additionally, the drug susceptibility analysis of E. coli in this region was conducted. This may provide some references for the prevention and control of puerperal metritis in dairy cows in this region.
All animal experiments in this study were reviewed and approved by the Animal Welfare Committee of Ningxia University (License No. NXUC20220906). All experimental methods were performed in accordance with the Regulations on the Management of Laboratory Animals (Revised 2021) regarding animal care and animal experiments.
Forty-two samples were collected from the uterine contents of cows suffering from metritis at 7–10 d postpartum from June to September 2022 in five different areas of Ningxia, including Wuzhong City (Field A), Yinchuan City (Field B), Shizuishan City (Field C), Pingluo County (Field D) and Lingwu City (Field E). Neosporin was used to clean the vulva of dairy cows before the samples were collected by a homemade sterile sampling device, which ensures effective collection of uterine contents and prevents microbial contamination of the vulva and vagina. The extracted samples were injected into sterile EP tubes and stored in an incubator at 4°C and then transported back to the laboratory for subsequent experiments.
The diagnosis of puerperal metritis was conducted by a skilled veterinarian. Depressed, dehydrated, anorexic (fasting), shortness of breath, decreased milk production, tachycardia, decreased rumination, enlarged uterus on rectal examination. Cows with body temperature above 39.5°C and foul-smelling uterine contents were diagnosed with puerperal metritis (Lomb et al., 2018; Figueiredo et al., 2021).
The collected uterine contents were inoculated on EMB solid medium after 10-fold dilution with saline. Subsequently, monoclonal colonies were selected to be inoculated in TSB liquid medium. Next, the cultured bacterial fluid was inoculated again on EMB solid medium for purification culture. After numerous purifications, homogenous colony matching the colony’s morphology, size, and color formed on the solid medium. Further, typical colonies were picked and smeared for Gram staining followed by preliminary identification by morphology. Furthermore,single colonies were inoculated into TSB liquid medium for expanded culture. Then, bacterial DNA was extracted using the bacterial genomic DNA extraction kit, and bacterial 16S rDNA PCR amplification was conducted using bacterial universal primers. Amplification products recovered and purified after electrophoresis were sent to Shanghai Bioengineering Company for sequencing. Finally, the sequences were subjected to BLAST with those of the NCBI database, and the final determination of bacterial species was made. Universal primers for PCR amplification: F: AGAGTTTGATCCTGGCTCAG; R: TACGGCTACCTTGTTACGACTT. PCR reaction system comprised the following: 12.5 μL Premix TaqTM (TaKaRa TaqTM Version 2.0 plus dye), 1 μL template and 1 μL upstream and downstream primers, the concentration of each primer was 0.08 mΜ, and 9.5 μL ddH2O. PCR conditions were as follows: pre-denaturation at 94°C for 5 min; 30 cycles of denaturation 94°C for 30s, annealing at 55°C for 45 s, and extension at 72°Cfor 90s; extension at 72°C for 10 min.
Following 16S rDNA PCR amplification and sequencing, the obtained sequences were compared in the NCBI database, and the sequences of identified E. coli strains originating from different regions were selected as references. The homology relationship of strains was analyzed by using MEGA11 software.
Phylogenetic clustering was performed using multiple PCR targeting DNA fragments of genes chuA, yjaA, and TspE4-C2 to distinguish the four phylogenetic clusters A, B1, B2, and D. The specific phylogenetic clustering methods are indicated in Table 1 (Clermont et al., 2013; Nowrouzian et al., 2019). The primers of related gene fragments are depicted in Table 2. The 50 μL PCR reaction system comprised the following: 25 μL Premix TaqTM (TaKaRa TaqTM Version 2.0 plus dye), 2 μL template, 1 μL upstream and downstream primers, add ddH2O to 50 μL. Amplification conditions was as follows: pre-denaturation at 94°C for 5 min; 30 cycles of denaturation 94°C for 30s, annealing at 58°C for 45 s, and extension at 72°Cfor 90s; extension at 72°C for 10 min. Subsequently, E. coli was categorized based on the band size after electrophoresis.
The virulence factors carried by E. coli in the uterus of dairy cows in the Ningxia region were determined by amplifying virulence factor genes encoding ExPEC. PCR was used to detect virulence factor genes of each strain, and the reaction procedure and conditions were the same as those described in Method 2.4. PCR amplification primers and annealing temperatures are indicated in Table 3.
Drug resistance analysis was performed by amplifying the resistance genes. PCR was used to detect virulence factor genes of each strain, and the reaction procedure and conditions were the same as those described in Method 2.4. PCR amplification primers and annealing temperatures are indicated in Table 4.
The susceptibility test of E. coli was done with 16 kinds of antibiotic susceptibility test papers commonly used in the clinic tests. Following diluting the concentration of bacteria solution with TSB liquid medium, absorbance was detected using a spectrophotometer. Absorbance value was in the range 0.08–0.1 by adjusting the concentration of bacteria solution, indicating that the number of bacteria in 0.5 McGallowi standard bacteria solution was about 1 × 108 ~ 2 × 108 (McLaughlin et al., 2012). After adjusting the concentration, the bacteria solution was evenly distributed on the plate with a disposable sterilized cotton swab. When the Agar surface was slightly dry, we used sterile forceps to attach the sensitive paper to the Agar surface, with four sheets of sensitive paper per plate. The petri dishes were then placed upside down in the incubator for ≥18 h. The results of drug susceptibility tests were analyzed per the standards of the American Committee for Clinical Laboratory Standardization (NCCLS). The results were divided into sensitive type (sensitive type, S), intermediate type (I) and resistant type (R) based on the diameter of the inhibition zone. The test was conducted in triplicate.
The phylogenetic evolutionary tree of E. coli strains was created using MEGA11 software to examine the homology relationship of the E. coli stains from each region. Origin software was used to construct stacked histograms and matrix plots to analyze the phylogenetic taxon subgroups of E. coli strains in each region and the distribution of E. coli virulence genes and drug resistance genes in the phylogenetic taxa.
Following the isolation and identification of 42 samples, 99 strains of E. coli were finally obtained. E. coli colonies exhibited round, raised, and smooth surface colonies with metallic luster or black pigment production on EMB agar. Preliminary identification of bacteria was done using Gram staining, which revealed that Gram-negative bacteria were red-stained with short rods or rods with blunt rounded ends. PCR results revealed that the fragment size of amplification products was 1,500 bp (partly presented in Figure 1). Eventually, 99 strains were identified as E. coli following comparison of sequencing results with NCBI sequences.
SeqMan and EditSeq were used for sequence analysis and splicing of 99 E. coli strains, and phylogenetic tree was constructed using these sequences with MEGA11. According to the findings, all strains developed two main branches, as presented in Figure 2. Among these, 99 target sequences were on the same large branch as that of human CU656137.1 and nine typical representative strains of different types of E. coli including CP024223.1, CP010344.1, CP062901 (both types), CP060946.1, CP047094.1, CP067426 (both types), and CP097884.1. Specifically, the E. coli strain from farm A in the Wuzhong area was on the same branch as that of CP062901.1. The E. coli strain from farm B in Yinchuan was on the same branch as that of CP067426.1 and CU656137.1 of human origin. The strain of E. coli from farm C in Huinong District was on the same branch as that of CP097784.1. The strain of E. coli from farm D in the Pingluo area was on the same branch as that of CP067426.1, CP047094.1, CP010344.1, and CP024223.1. The E. coli strain from farm E in Lingwu was on the same branch as that of CP060946.1.
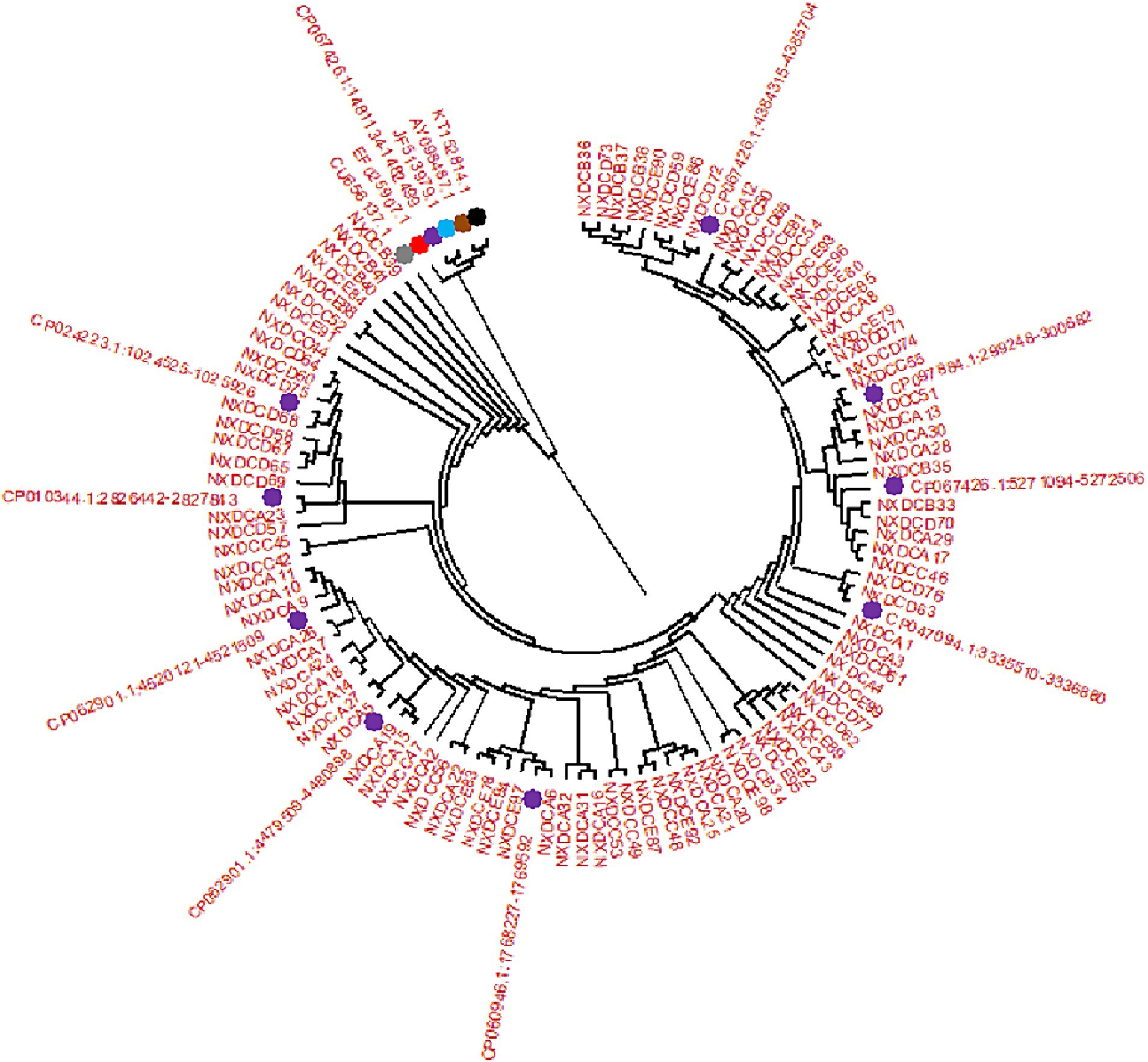
Figure 2. Phylogenetic tree created by E. coli 16S rDNA PCR amplification and sequencing. There are 99 isolate samples of 16S rDNA target sequences, corresponding to the sequences of (NXDCA1-A32), (NXDCB33-B41), (NXDCC42-C56), (NXDCD57-D77), and (NXDCE78-E99). There are 15 16S rDNA reference sequences, and the different color represent the reference sequences of different origins and types, including bovine uterus: KT152814.1, avian origin: AY098487.1, porcine origin: JF513979.1,sheep origin: EF025907.1, human origin: CU656137.1, and 10 classics strains of different types of E. coli CP067426.1 (O22:H8), CP024223.1 (O169:H41), CP010344.1 (ECC-1470), two types of CP062901 (O152:H23), CP060946.1 (O157:H7), CP047094.1 (Salmonella sp), two types of CP067426 (O22:H8), and CP097884.1 (K-12).
The four phylogenetic subgroups A, B1, B2, and D were distinguished using multiplex PCR (Figure 3). The results revealed that the Wuzhong (Farm A) area, which has four phylogenetic subgroups A, B1, B2 and D, was the most abundant in E. coli group categories. E. coli isolates from Yinchuan (Farm B) were mainly divided into subgroups A, B1, and B2. E. coli isolated from Huinong District (Farm C) and Pingluo (Farm D) were mainly distributed in subgroups A, B2, and D. Furthermore, E. coli isolated from Lingwu (Farm E) were distributed only in subgroups B2 and D. These results revealed that pathogenic E. coli from dairy cows with puerperal metritis in Ningxia primarily belonged to subgroups B2 (41.4%) and D (45.5%) followed by subgroups A (10.1%) and B1 (3.03%).
The findings of the E. coli virulence factor analysis are displayed in Table 5. The highest detection rate of virulence genes was 100% for both ompF and traT, and followed by fimH (97%), papC (96%), csgA (95%), Ang43 (93.9%), ompC (93%), iutA (87.9%), ECs3703 (81.8%), irp2 (78.8%), Afa (64.5%), fyuA (59.6%), stx2 (38.4%), F17A (27.3%), KpsMTII (21.2%), and hlyA (19.2%). However, the detection rates of F14, F5, sfa, eae, astA, LT1, and Cnf1 were all zero. These results indicated the comparatively high abundance of virulence genes carried by ExPEC in this region, which were mainly dominated by ompF, traT, fimH, papC, acsgA, Ang43, and ompC.
The findings of the E. coli drug resistance gene analysis are displayed in Table 6. TEM exhibited the highest detection rate of 99%, followed by tetA (71.7%), aac(3)II (66.7%), cmlA (53.5%), qnrA (48.5%), and sul1 (26.3%) whereas CITM and dfrA1 were detected at a detection rate of zero. There were significant differences in the detection rates of cmlA, sul1, qnrA, and tetA genes among the five regions, with the highest detection rate of 72.7% in farm E and the lowest of 37.5% in farm A for the cmlA gene. The highest detection rate of the sul1 gene in Farm B and the lowest in Farm D was 9.5%. The highest detection rate of the qnrA gene was 100% in farm E, and the lowest detection rate of the qnrA gene was 12.5% in farm A. The highest detection rate of the tetA gene was 94% in farm A whereas that of the tetA gene in farm D was 57.1%. These results suggested that ExPEC was generally resistant to Beta-lactams and has varying degrees of resistance to other antibiotic drugs in the Ningxia region of China.
The relationship between the virulence factors of the strains and their phylogenetic subgroups was analyzed, and the findings are displayed in Figure 4. The distributions of the genes csgA, F17A, Afa, irp2, stx2, hlyA, KpsMTII, ECs3703, and fyuA on the four phylogenetic taxa were significantly different. The detection rate of irp2 was significantly lower in subgroup A than in subgroups B1, B2, and D. The detection rate of fyuA was significantly higher in subgroup D than in subgroups A, B1, and B2. Conclusively, the detection rates of virulence factors in subgroups B2 and D were significantly higher than those in subgroups A and B1. Additionally, the same virulence factors were observed in all of the closely associated isolates, and each strain contained at least four different virulence factors. These results indicated that the virulence factors were highly abundant in E. coli strains, which were mainly distributed in subgroups B2 and D in this region.
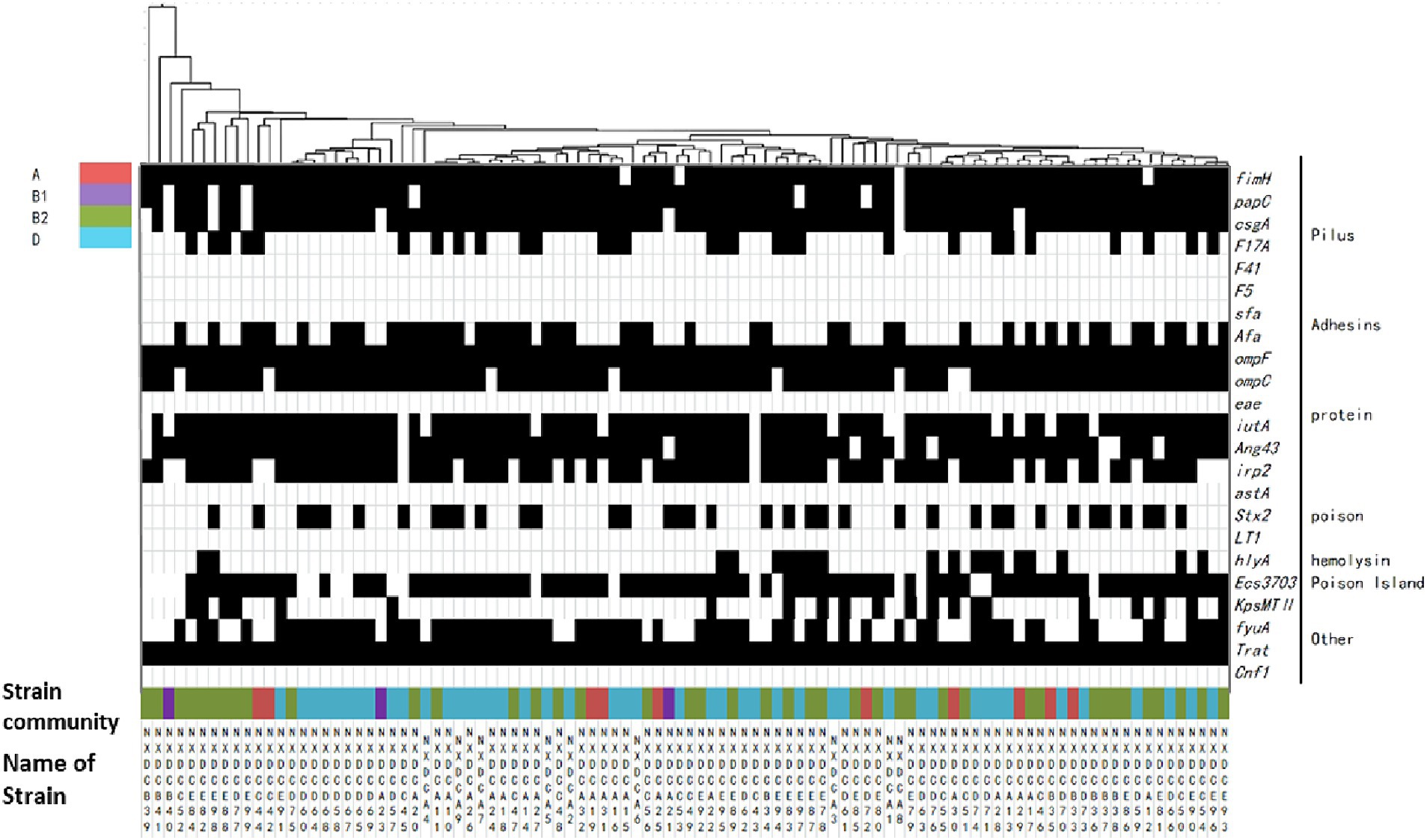
Figure 4. Matrix of distribution patterns of virulence factors in E. coli phylum taxa. Classification and clustering of 99 E. coli strains based on the association between virulence factors and phylogroups. The given genetic phenotypes are represented by black rectangles, and virulence factors and types are located on the right side of the graph. Systematic taxa are represented by different color codes, as indicated by the color labeling in the upper left corner of the figure above. The branches and names of the genes of the strains are indicated above and below, respectively.
The distribution of drug resistance genes in E. coli systemic subgroups are displayed in Figure 5. The distribution of cmlA, sul1, aac(3)II, and qnrA resistance genes on the four developmental taxa varied significantly. The detection rates of cmlA and qnrA in subgroup B1 were zero, which were significantly lower than those in subgroups A, B2, and D. The detection rate of qnrA was significantly higher in subgroup B2 than in subgroups B1, D, and A. The detection rate of sul1 was significantly lower in subgroup D than in subgroups A, B1, and B2. The detection rate of aac(3)II was higher in subgroups A and D than in subgroups B1 and B2. Additionally, closely related strains exhibited nearly identical drug-resistance genes. These results suggested that the drug resistance genes carried by E. coli strains of different systemic groups were different, and the types of drug resistance genes carried by E. coli strains of groups B2 and D were much higher than those of groups A and B1.
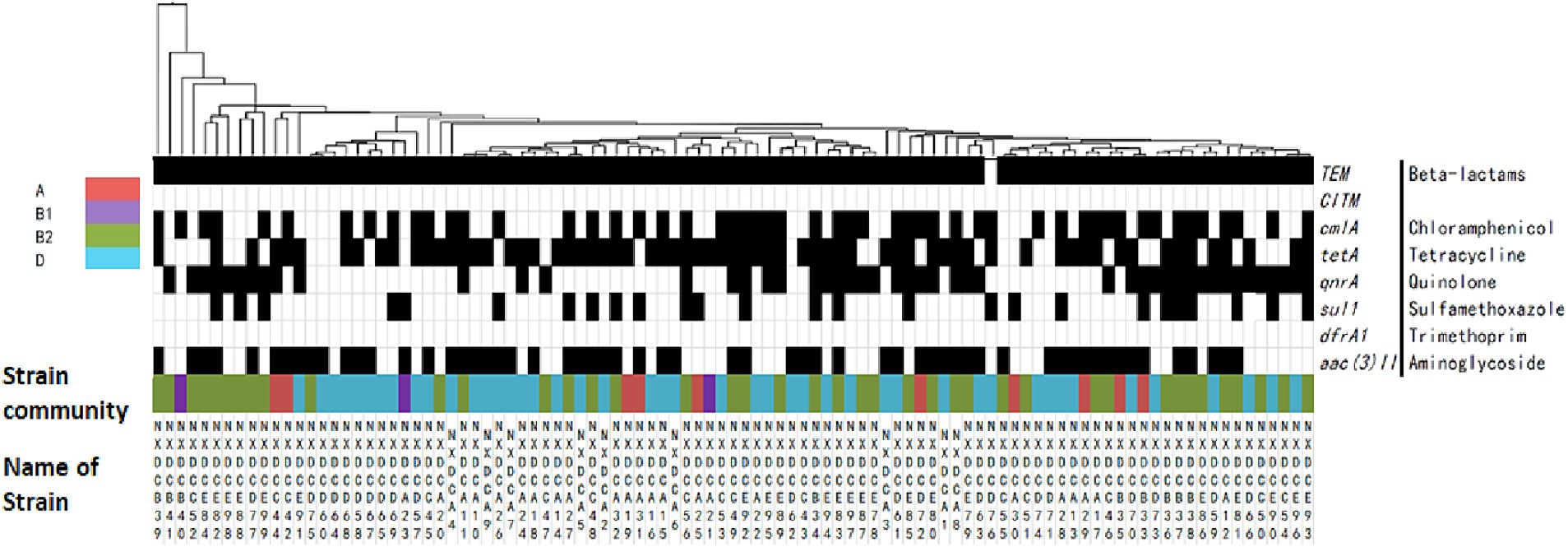
Figure 5. Matrix of the distribution patterns of drug resistance genes in E. coli phylogroups. Classification and clustering of 99 E. coli strains based on their drug resistance gene phenotypes in association with phylogroups and evolution. The identified genetic phenotypes are represented by black rectangles, and drug-resistance genes are located on the right side of the graph. Systematic taxa are indicated by different color coding, as depicted by the colors presented in the upper left corner of the figure above. The branches and names of the genes of the strains are displayed above and below, respectively.
Table 7 displays the drug susceptibility test results of 16 commonly used antibiotics for E. coli. Significant differences were observed in the sensitivity of E. coli to various drugs. The strains were most sensitive to fluoroquinolones enrofloxacin, followed by macrolides, aminoglycosides, tetracycline, β-lactams, polypeptides, and sulfonamides, and least sensitive to lincomycin. E. coli was resistant to penicillin, streptomycin, cotrimoxazole, and lincomycin but sensitive to cefotaxime, ofloxacin, and enrofloxacin.
Escherichia coli has been considered as the most prevalent bacterial species in the cervix and vagina (Saez-Lopez et al., 2016). However, some studies on reproductive tract microbes have highlighted different perspectives on E. coli-induced puerperal metritis. Previous research has revealed that E. coli was nearly non-existent in the vaginal and uterine pathogenic microbial communities of dairy cows (Jeon et al., 2015; Bicalho et al., 2017; Jeon and Galvao, 2018). However, other studies have confirmed that E. coli played a decisive role in the development of puerperal metritis in dairy cows (Miller et al., 2007; Goldstone et al., 2014; Genis et al., 2016; Gonzalez et al., 2020). Escherichia coli of the uterus not only damaged the uterine mucosa but also increased the likelihood of subsequent uterine infection induced by Cryptobacterium septicum, Bacillus necrophorum, and other conditionally pathogenic bacteria in the early postpartum period, which was closely associated with the development of puerperal metritis in dairy cows (Bicalho et al., 2012). This study demonstrated that the isolation rate of E. coli strains from sample species was as high as 95.2%, making it the primary pathogen causing puerperal metritis of dairy cows in the Ningxia region of China.
Escherichia coli O157:H7 and E. coli O169:H41 have been reported to be highly pathogenic (Yang et al., 2017). Further study has observed that two strains belonged to enterohemorrhagic E. coli and caused bloody diarrhea and the potentially fatal hemolytic uremic syndrome (Razmi et al., 2020). Furthermore, E. coli O157:H7 and O169:H41, as important intestinal highly infectious pathogens, were strongly linked to the development of gastroenteritis (Jarocki et al., 2021). Meanwhile, the two E. coli induced an inflammatory response via the release of a Shiga-like toxin (Ten et al., 2017). Surprisingly, we also isolated strains with close affinity to O157:H7 and O169:H41 from the uterine fluid of dairy cows with puerperal metritis. It was speculated that intestinal pathogenic E. coli breaks through the intestinal barrier under certain conditions and reaches the uterus via blood or lymphatic circulation, causing uterine inflammation. Our hypothesis is substantially identical to the identified endogenous “intestinal-mammary gland” pathway transfer mechanism of the microorganisms (Luo et al., 2022; Zhao et al., 2022). Furthermore, certain bacteria that were first detected in the mammary gland have been discovered in animal digestive and reproductive tracts (Fernandez and Rodriguez, 2020). Additionally, research has demonstrated that some common pathogens causing mastitis in dairy cows, such as Staphylococcus and Streptococcus, were predisposed to infecting the mucosal layer of the digestive tract (Rodriguez, 2014). This further adds to the evidence that gastrointestinal and uterine microorganisms in dairy cows are related. Additionally, we discovered that E. coli ECC-1470 accounted for a larger proportion of isolated strains. A study has revealed that E. coli ECC-1470 isolated from the uterus of dairy cows with chronic puerperal metritis had become a frequent strain responsible for inflaming the uterus (Dogan et al., 2006). This is consistent with our findings.
Previous studies have revealed that ExPEC strains typically belonged to groups B2 and D of the phylogenetic taxa, which comprised numerous virulence determinants involved in parenteral infections (Van et al., 2008; Paniagua-Contreras et al., 2017). Another study has reported that strains from groups A and B1 were rarely associated with parenteral infections (Restieri et al., 2007). Our study discovered that subgroup B2 and D strains accounted for 41.4 and 45.5% in all strains isolated from dairy cows with puerperal metritis respectively, which carried numerous virulence genes associated with extraintestinal infections. However, subgroup A and B1 strains accounted for 10.1 and 3.03%, respectively, albeit the detection rates of virulence genes were low. These findings depict that the pathogenic E. coli strains were predominant in dairy cows with puerperal metritis in this region. However, Khawaskar et al. (2022) demonstrated that ExPEC strains of bovine origin were mainly abundant in subgroups A and C. This differed from our results. It was speculated that the different serotypes of the strains caused different distributions of the strains in the phylogenetic subgroups.
It is prominent that the pathogenicity of E. coli depends mainly on the type and amount of virulence factors. The common virulence factors are fimH, ompF, and papC, among which fimH and papC encode for type 1 hair genes (Schembri et al., 2000). It has been reported that the strains of E. coli carrying fimH increased the incidence of puerperal metritis in dairy cows by 4.6 times (Bicalho et al., 2012). Furthermore, fimH promotes bacterial colonization, invasion and biofilm formation, and enhances its pathogenicity (Floyd et al., 2015). The virulence factor ompF, a membrane protein of ExPEC, adheres to and invades host cells (de Pace et al., 2010). Also, the expression of ompF in E. coli strains has been reported to affect the susceptibility of the strain to antibiotic drugs (Huguet et al., 2013; Zhang et al., 2018). Khalifeh and Obaidat (2022) observed that 97 and 32% of the bovine strains carried fimH and papC, respectively, and these strains typically carried two or more virulence genes. Additionally, Mihailovskaya et al. (2022) reported that fimH (91.8%), afa (61.2%), iutA (44.9%), and papC (20.4%) were predominant in the virulence genes carried by ExPEC. In this study, we observed that the main virulence genes carried by E. coli were ompF (100%), traT (100%), fimH (97%), and papC (96%), and each strain contained multiple virulence genes. TraT has been reported to be more prevalent in E. coli isolated from urine and blood samples (Abd et al., 2020). Further studies have revealed that traT encodes resistance genes and increases the risk of infection via ColV plasmid transfer (Cha et al., 2016). In this study, we observed the detection rate of traT to be 100%. It was speculated that E. coli carrying the traT virulence gene migrates to the uterine via blood circulation, and traT is further horizontally transferred between strains with the help of plasmids or phages (Huddleston, 2014), indicating that uterine pathogenic E. coli also carries traT gene.
The long-term use of antibiotics has caused the emergence of multi-drug resistance in pathogenic bacteria. Taylor et al. (2021) demonstrated that triple cephalosporins had low susceptibility to E. coli strains in dairy cows with puerperal metritis. The emergence of pathogenic bacterial resistance genes seriously affects the effectiveness of antibiotic therapy. Furthermore, Goudarztalejerdi et al. (2022) observed that tetA had the highest detection rate of 68%, followed by sul1 (63%), dfrA1 (51%), and TEM (30%) in avian pathogenic E. coli strains. In this study, drug resistance genes analysis revealed that the highest detection rate of β-lactam-resistant TEM genes was 99%, followed by tetA (71.7%), sul1 (26.3%), and dfrA1 (0). The detection rates of TEM and dfrA1 gene were significantly higher than those reported above. It was discovered that the types of resistance genes carried by E. coli strains were distinct since some resistance genes were transferred horizontally between strains with the help of plasmids, transposons, and integrons, among which TEM was the most prevalent (Styles et al., 2022). In this study, we observed that the TEM gene was present in almost all strains and the detection rate was 99%, which is consistent with aforementioned findings. Furthermore, previous studies have reported that strains carrying TEM-type β-lactams were typically resistant to both penicillin and broad-spectrum cephalosporins (Poirel et al., 2004; Jeong et al., 2018). We also discovered that strains carrying TEM-type β-lactams had low susceptibility to penicillin and significantly lower susceptibility to broad-spectrum cephalosporins such as cefotaxime and cefadroxil compared to other types of drugs, which was consistent with aforementioned findings.
Additionally, there is an association between phylogroups of E. coli strains and their distribution of virulence and drug resistance genes. Halaji et al. (2022) discovered that 28 Vfs of avian pathogenic E. coli strains belonged to subgroups B2 and D, and ecA, fyuA, irp2, and kspMTII were abundant, which was significantly higher than those in subgroups A and B1. Futhermore, Halaji et al. (2022) demonstrated that subgroups B2 and D were the major phylogenetic taxa of UPEC strains, and UPEC strains in subgroups B2 and D were more resistant than those in subgroups A and B1. Furthermore, both APEC and UPEC are subtypes of ExPEC, which indicates that ExPEC strains are almost entirely distributed in subgroups B2 and D. In this study, E. coli strains belonging to subgroups A and B1 harbored significantly fewer virulence factors and drug resistance genes than those belonging to phylogenetic taxa B2 and D, which is consistent with the findings above.
In this study, although we discovered pathogenic E. coli to be the predominant bacteria of dairy cows with puerperal metritis in the Ningxia region of China, we also observed that E. coli mainly belonged to groups B2 and D and carried several virulence genes and drug resistance genes in this region. However, further research is required to understand the processes by which virulence and drug resistance genes carried by E. coli regulate the development of puerperal metritis in dairy cows and the development of drug resistance in E. coli.
In the Ningxia region of China, the infection rate of puerperal metritis induced by E. coli in dairy cows was 95.2%, and the strains were mainly distributed in groups B2 and D, with close affinity to (O157:H7), (O169:H4), and (ECC-1470) type strains. Additionally, these E. coli mainly carried seven virulence genes including ompF (100%), traT (100%), fimH (97%), papC (96%), csgA (95%), Ang43 (93.9%), and ompC (93%) and five drug resistance genes containing TEM (99%), tetA (71.7%), aac(3)II (66.7%), and cmlA (53.5%). Additionally, E. coli is resistant to penicillin, streptomycin, cotrimoxazole, and lincomycin, but responsive to cefadroxil, ofloxacin, and enrofloxacin. However, there is a further need to analyze the reasons for the development of drug resistance in these pathogens as well as to investigate the molecular mechanisms by which these bacteria develop drug resistance.
The data presented in the study are deposited in the NCBI repository, accession numbers PP455648 - PP455745.
The animal study was approved by Animal Welfare Committee of Ningxia University. The study was conducted in accordance with the local legislation and institutional requirements.
SW: Writing – original draft, Conceptualization, Data curation, Formal analysis, Visualization. BD: Writing – original draft, Investigation, Methodology, Validation. GW: Writing – review & editing, Project administration. SL: Writing – original draft, Software. HZ: Writing – review & editing, Resources. XD: Writing – review & editing, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the key research project of Ningxia Autonomous Region and the earmarked fund for CARS (No. 2022BBF03023, 2021BBF02037, CARS-36).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd, E. R., Ibrahim, R. A., Mohamed, D. S., Ahmed, E. F., and Hashem, Z. S. (2020). Prevalence of virulence genes and their association with antimicrobial resistance among pathogenic E. coli isolated from egyptian patients with different clinical infections. Infect Drug Resist. 13, 1221–1236. doi: 10.2147/IDR.S241073
Aguirre-Sanchez, J. R., Valdez-Torres, J. B., Del, C. N., Martinez-Urtaza, J., Del, C. N., Lee, B. G., et al. (2022). Phylogenetic group and virulence profile classification in Escherichia coli from distinct isolation sources in mexico. Infect. Genet. Evol. 106:105380. doi: 10.1016/j.meegid.2022.105380
Ban, E., Yoshida, Y., Wakushima, M., Wajima, T., Hamabata, T., Ichikawa, N., et al. (2015). Characterization of unstable pentyn10 from enterotoxigenic Escherichia coli (etec) o169:h41. Virulence 6, 735–744. doi: 10.1080/21505594.2015.1094606
Bertin, Y., Martin, C., Oswald, E., and Girardeau, J. P. (1996). Rapid and specific detection of f17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex pcr. J. Clin. Microbiol. 34, 2921–2928. doi: 10.1128/jcm.34.12.2921-2928.1996
Bicalho, R. C., Machado, V. S., Bicalho, M. L., Gilbert, R. O., Teixeira, A. G., Caixeta, L. S., et al. (2010). Molecular and epidemiological characterization of bovine intrauterine Escherichia coli. J. Dairy Sci. 93, 5818–5830. doi: 10.3168/jds.2010-3550
Bicalho, M. L., Machado, V. S., Oikonomou, G., Gilbert, R. O., and Bicalho, R. C. (2012). Association between virulence factors of Escherichia coli, fusobacterium necrophorum, and arcanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 157, 125–131. doi: 10.1016/j.vetmic.2011.11.034
Bicalho, M., Santin, T., Rodrigues, M. X., Marques, C. E., Lima, S. F., and Bicalho, R. C. (2017). Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: associations with uterine diseases and reproductive outcome. J. Dairy Sci. 100, 3043–3058. doi: 10.3168/jds.2016-11623
Biran, D., and Ron, E. Z. (2018). Extraintestinal pathogenic Escherichia coli. Curr. Top. Microbiol. Immunol. 416, 149–161. doi: 10.1007/82_2018_108
Cha, M. K., Kang, C. I., Kim, S. H., Cho, S. Y., Ha, Y. E., Wi, Y. M., et al. (2016). Comparison of the microbiological characteristics and virulence factors of st131 and non-st131 clones among extended-spectrum beta-lactamase-producing Escherichia coli causing bacteremia. Diagn. Microbiol. Infect. Dis. 84, 102–104. doi: 10.1016/j.diagmicrobio.2015.10.015
Clermont, O., Christenson, J. K., Denamur, E., and Gordon, D. M. (2013). The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 5, 58–65. doi: 10.1111/1758-2229.12019
de Pace, F., Nakazato, G., Pacheco, A., de Paiva, J. B., Sperandio, V., and Da, S. W. (2010). The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect. Immun. 78, 4990–4998. doi: 10.1128/IAI.00531-10
Dogan, B., Klaessig, S., Rishniw, M., Almeida, R. A., Oliver, S. P., Simpson, K., et al. (2006). Adherent and invasive Escherichia coli are associated with persistent bovine mastitis. Vet. Microbiol. 116, 270–282. doi: 10.1016/j.vetmic.2006.04.023
Ehsanollah, S., Pouya, R., Risco, C. A., and Hernandez, J. A. (2021). Observed and expected combined effects of metritis and other postpartum diseases on time to conception and rate of conception failure in first lactation cows in Iran. Theriogenology 164, 36–41. doi: 10.1016/j.theriogenology.2021.01.016
Fernandez, L., and Rodriguez, J. M. (2020). Human milk microbiota: origin and potential uses. Nestle Nutr. Inst. Workshop Ser. 94, 75–85. doi: 10.1159/000505031
Figueiredo, C. C., Merenda, V. R., de Oliveira, E. B., Lima, F. S., Chebel, R. C., Galvao, K. N., et al. (2021). Failure of clinical cure in dairy cows treated for metritis is associated with reduced productive and reproductive performance. J. Dairy Sci. 104, 7056–7070. doi: 10.3168/jds.2020-19661
Floyd, K. A., Moore, J. L., Eberly, A. R., Good, J. A., Shaffer, C. L., Zaver, H., et al. (2015). Adhesive fiber stratification in uropathogenic Escherichia coli biofilms unveils oxygen-mediated control of type 1 pili. PLoS Pathog. 11:e1004697. doi: 10.1371/journal.ppat.1004697
Genis, S., Bach, A., Fabregas, F., and Aris, A. (2016). Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology 85, 625–637. doi: 10.1016/j.theriogenology.2015.09.054
Goldstone, R. J., Talbot, R., Schuberth, H. J., Sandra, O., Sheldon, I. M., and Smith, D. G. (2014). Draft genome sequence of Escherichia coli ms499, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc. 2:e00217-14. doi: 10.1128/genomeA.00217-14
Gonzalez, M. C., Torres, L. A., Oliszewski, R., Rosa, R. J., and Otero, M. C. (2020). Characterization of native Escherichia coli populations from bovine vagina of healthy heifers and cows with postpartum uterine disease. PLoS One 15:e228294:e0228294. doi: 10.1371/journal.pone.0228294
Goudarztalejerdi, A., Mohammadzadeh, A., Niazi, K., and Mohammad, M. M. (2022). High prevalence of multidrug resistance and biofilm-formation ability among avian Escherichia coli isolated from broilers in Iran. Microb. Drug Resist. 28, 244–254. doi: 10.1089/mdr.2021.0091
Halaji, M., Fayyazi, A., Rajabnia, M., Zare, D., Pournajaf, A., and Ranjbar, R. (2022). Phylogenetic group distribution of uropathogenic Escherichia coli and related antimicrobial resistance pattern: a meta-analysis and systematic review. Front. Cell. Infect. Microbiol. 12:790184. doi: 10.3389/fcimb.2022.790184
Huddleston, J. R. (2014). Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect Drug Resist. 7, 167–176. doi: 10.2147/IDR.S48820
Huguet, A., Pensec, J., and Soumet, C. (2013). Resistance in Escherichia coli: variable contribution of efflux pumps with respect to different fluoroquinolones. J. Appl. Microbiol. 114, 1294–1299. doi: 10.1111/jam.12156
Jarocki, V. M., Hess, S., Anantanawat, K., Berendonk, T. U., and Djordjevic, S. P. (2021). Multidrug-resistant lineage of enterotoxigenic Escherichia coli st182 with serotype o169:h41 in airline waste. Front. Microbiol. 12:731050. doi: 10.3389/fmicb.2021.731050
Jeon, S. J., and Galvao, K. N. (2018). An advanced understanding of uterine microbial ecology associated with metritis in dairy cows. Genomics Inform. 16:e21. doi: 10.5808/GI.2018.16.4.e21
Jeon, S. J., Vieira-Neto, A., Gobikrushanth, M., Daetz, R., Mingoti, R. D., Parize, A. C., et al. (2015). Uterine microbiota progression from calving until establishment of metritis in dairy cows. Appl. Environ. Microbiol. 81, 6324–6332. doi: 10.1128/AEM.01753-15
Jeong, S., Kim, J. O., Yoon, E. J., Bae, I. K., Lee, W., Lee, H., et al. (2018). Extensively drug-resistant Escherichia coli sequence type 1642 carrying an incx3 plasmid containing the blakpc-2 gene associated with transposon tn4401a. Ann. Lab. Med. 38, 17–22. doi: 10.3343/alm.2018.38.1.17
Kasse, F. N., Fairbrother, J. M., and Dubuc, J. (2016). Relationship between Escherichia coli virulence factors and postpartum metritis in dairy cows. J. Dairy Sci. 99, 4656–4667. doi: 10.3168/jds.2015-10094
Khalifeh, O. M., and Obaidat, M. M. (2022). Urinary tract virulence genes in extended-spectrum beta-lactamase E. coli from dairy cows, beef cattle, and small ruminants. Acta Trop. 234:106611. doi: 10.1016/j.actatropica.2022.106611
Khawaskar, D., Subbaiyan, A., Balusamy, D., Inbaraj, S., Abhishek,, Vinodh, K. O., et al. (2022). A comparative genomics approach for identifying genetic factors in Escherichia coli isolates associated with bovine diseases. J. Appl. Microbiol. 133, 3490–3501. doi: 10.1111/jam.15781
Lomb, J., Weary, D. M., Mills, K. E., and von Keyserlingk, M. (2018). Effects of metritis on stall use and social behavior at the lying stall. J. Dairy Sci. 101, 7471–7479. doi: 10.3168/jds.2017-14149
Luo, S., Wang, Y., Kang, X., Liu, P., and Wang, G. (2022). Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 173:105809. doi: 10.1016/j.micpath.2022.105809
Ma, Z., Ginn, A., Kang, M., Galvao, K. N., and Jeong, K. C. (2018). Genomic and virulence characterization of intrauterine pathogenic Escherichia coli with multi-drug resistance isolated from cow uteri with metritis. Front. Microbiol. 9:3137. doi: 10.3389/fmicb.2018.03137
Malahlela, M. N., Cenci-Goga, B. T., Marufu, M. C., Fonkui, T. Y., Grispoldi, L., Etter, E., et al. (2022). Occurrence, serotypes and virulence characteristics of Shiga-toxin-producing Escherichia coli isolates from goats on communal rangeland in South Africa. Toxins (Basel) 14:353. doi: 10.3390/toxins14050353
McLaughlin, C. L., Stanisiewski, E., Lucas, M. J., Cornell, C. P., Watkins, J., Bryson, L., et al. (2012). Evaluation of two doses of ceftiofur crystalline free acid sterile suspension for treatment of metritis in lactating dairy cows. J. Dairy Sci. 95, 4363–4371. doi: 10.3168/jds.2011-5111
Mihailovskaya, V. S., Remezovskaya, N. B., Zhdanova, I. N., Starcic, E. M., and Kuznetsova, M. V. (2022). Virulence potential of faecal Escherichia coli strains isolated from healthy cows and calves on farms in perm krai. Vavilovskii Zhurnal Genet Selektsii 26, 486–494. doi: 10.18699/VJGB-22-59
Miller, A. N., Williams, E. J., Sibley, K., Herath, S., Lane, E. A., Fishwick, J., et al. (2007). The effects of arcanobacterium pyogenes on endometrial function in vitro, and on uterine and ovarian function in vivo. Theriogenology 68, 972–980. doi: 10.1016/j.theriogenology.2007.07.013
Monroy-Perez, E., Ceron, A. B., Garcia, C. L., Alonso, N. N., Dominguez-Trejo, P., Hernandez-Jaimes, T., et al. (2020). Virulence gene transcription, phylogroups, and antibiotic resistance of cervico-vaginal pathogenice. Coli in mexico. PLoS One 15:e234730. doi: 10.1371/journal.pone.0234730
Najafi, M., Guo, Y., Andersson, G., Humblot, P., and Bongcam-Rudloff, E. (2022). Gene networks and pathways involved in lps-induced proliferative response of bovine endometrial epithelial cells. Genes (Basel) 13:2342. doi: 10.3390/genes13122342
Nowrouzian, F. L., Clermont, O., Edin, M., Ostblom, A., Denamur, E., Wold, A. E., et al. (2019). Escherichia coli b2 phylogenetic subgroups in the infant gut microbiota: predominance of uropathogenic lineages in swedish infants and enteropathogenic lineages in pakistani infants. Appl. Environ. Microbiol. 85:e01681-19. doi: 10.1128/AEM.01681-19
Pakbin, B., Bruck, W. M., and Rossen, J. (2021). Virulence factors of enteric pathogenic Escherichia coli: a review. Int. J. Mol. Sci. 22:9922. doi: 10.3390/ijms22189922
Paniagua-Contreras, G. L., Hernandez-Jaimes, T., Monroy-Perez, E., Vaca-Paniagua, F., Diaz-Velasquez, C., Uribe-Garcia, A., et al. (2017). Comprehensive expression analysis of pathogenicity genes in uropathogenic Escherichia coli strains. Microb. Pathog. 103, 1–7. doi: 10.1016/j.micpath.2016.12.008
Paton, A. W., and Paton, J. C. (1998). Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex pcr assays for stx1, stx2, eaea, enterohemorrhagic e. coli hlya, rfbo111, and rfbo157. J. Clin. Microbiol. 36, 598–602. doi: 10.1128/JCM.36.2.598-602.1998
Piccardi, M., Romero, G., Veneranda, G., Castello, E., Romero, D., Balzarini, M., et al. (2016). Effect of puerperal metritis on reproductive and productive performance in dairy cows in argentina. Theriogenology 85, 887–893. doi: 10.1016/j.theriogenology.2015.10.038
Piras, C., Guo, Y., Soggiu, A., Chanrot, M., Greco, V., Urbani, A., et al. (2017). Changes in protein expression profiles in bovine endometrial epithelial cells exposed to E. coli LPS challenge. Mol. BioSyst. 13, 392–405. doi: 10.1039/c6mb00723f
Poirel, L., Mammeri, H., and Nordmann, P. (2004). Tem-121, a novel complex mutant of tem-type beta-lactamase from enterobacter aerogenes. Antimicrob. Agents Chemother. 48, 4528–4531. doi: 10.1128/AAC.48.12.4528-4531.2004
Raeispour, M., and Ranjbar, R. (2018). Antibiotic resistance, virulence factors and genotyping of uropathogenic Escherichia coli strains. Antimicrob. Resist. Infect. Control 7:118. doi: 10.1186/s13756-018-0411-4
Raheel, I., Hassan, W. H., Salem, S., and Salam, H. (2020). Biofilm forming potentiality of Escherichia coli isolated from bovine endometritis and their antibiotic resistance profiles. J. Adv. Vet. Anim. Res. 7, 442–451. doi: 10.5455/javar.2020.g440
Razmi, N., Hasanzadeh, M., Willander, M., and Nur, O. (2020). Recent progress on the electrochemical biosensing of Escherichia coli O157:H7: material and methods overview. Biosensors (Basel) 10:54. doi: 10.3390/bios10050054
Restieri, C., Garriss, G., Locas, M. C., and Dozois, C. M. (2007). Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 73, 1553–1562. doi: 10.1128/AEM.01542-06
Rodriguez, J. M. (2014). The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 5, 779–784. doi: 10.3945/an.114.007229
Saez-Lopez, E., Cossa, A., Benmessaoud, R., Madrid, L., Moraleda, C., Villanueva, S., et al. (2016). Characterization of vaginal Escherichia coli isolated from pregnant women in two different african sites. PLoS One 11:e158695. doi: 10.1371/journal.pone.0158695
Sarowska, J., Futoma-Koloch, B., Jama-Kmiecik, A., Frej-Madrzak, M., Ksiazczyk, M., Bugla-Ploskonska, G., et al. (2019). Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 11:10. doi: 10.1186/s13099-019-0290-0
Schembri, M. A., Sokurenko, E. V., and Klemm, P. (2000). Functional flexibility of the fimh adhesin: insights from a random mutant library. Infect. Immun. 68, 2638–2646. doi: 10.1128/IAI.68.5.2638-2646.2000
Schouten, I., Bernys-Karolys, A., Schneider, P., Dror, T., Ofer, L., Shimoni, C., et al. (2023). Mesenchymal stromal cells modulate infection and inflammation in the uterus and mammary gland. BMC Vet. Res. 19:64. doi: 10.1186/s12917-023-03616-1
Shamsi, H., Mardani, K., and Ownagh, A. (2017). Phylogenetic analysis of Escherichia coli isolated from broilers with colibacillosis based on gyra gene sequences. Can. J. Vet. Res. 81, 28–32.
Styles, K. M., Locke, R. K., Cowley, L. A., Brown, A. T., and Sagona, A. P. (2022). Transposable element insertions into the Escherichia coli polysialic acid gene cluster result in resistance to the k1f bacteriophage. Microbiol. Spectr. 10:e211221. doi: 10.1128/spectrum.02112-21
Taylor, E. A., Ossa-Trujillo, C., Vinasco, J., Jordan, E. R., Garcia, B. J., Hagevoort, R., et al. (2021). Use of critically important antimicrobial classes early in life may adversely impact bacterial resistance profiles during adult years: potential co-selection for plasmid-borne fluoroquinolone and macrolide resistance via extended-spectrum beta-lactam use in dairy cattle. Lett. Appl. Microbiol. 72, 220–224. doi: 10.1111/lam.13419
Ten, S. T., Hashim, U., Gopinath, S., Liu, W. W., Foo, K. L., Sam, S. T., et al. (2017). Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 93, 146–154. doi: 10.1016/j.bios.2016.09.035
Van, T. T., Chin, J., Chapman, T., Tran, L. T., and Coloe, P. J. (2008). Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli solations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 124, 217–223. doi: 10.1016/j.ijfoodmicro.2008.03.029
Wang, Y., Ametaj, B. N., Ambrose, D. J., and Ganzle, M. G. (2013). Characterisation of the bacterial microbiota of the vagina of dairy cows and isolation of pediocin-producing pediococcus acidilactici. BMC Microbiol. 13:19. doi: 10.1186/1471-2180-13-19
Washizaki, A., Yonesaki, T., and Otsuka, Y. (2016). Characterization of the interactions between escherichia coli receptors, LPS and OmpC, and bacteriophage T4 long tail fibers. Microbiology 5, 1003–1015. doi: 10.1002/mbo3.384
Yamamura, F., Sugiura, T., Munby, M., Shiokura, Y., Murata, R., Nakamura, T., et al. (2022). Relationship between Escherichia coli virulence factors, notably kpsmtii, and symptoms of clinical metritis and endometritis in dairy cows. J. Vet. Med. Sci. 84, 420–428. doi: 10.1292/jvms.21-0586
Yang, S. C., Lin, C. H., Aljuffali, I. A., and Fang, J. Y. (2017). Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 199, 811–825. doi: 10.1007/s00203-017-1393-y
Zhang, B., Wang, H., Zhao, W., Shan, C., Liu, C., Gao, L., et al. (2021). New insights into the construction of wild-type saba pig-derived Escherichia coli irp2 gene deletion strains. 3 Biotech 11:408. doi: 10.1007/s13205-021-02951-0
Zhang, D., Zhang, Z., Huang, C., Gao, X., Wang, Z., Liu, Y., et al. (2018). The phylogenetic group, antimicrobial susceptibility, and virulence genes of Escherichia coli from clinical bovine mastitis. J. Dairy Sci. 101, 572–580. doi: 10.3168/jds.2017-13159
Keywords: dairy cows, drug resistance gene, drug sensitivity, Escherichia coli , puerperal metritis, virulence gene
Citation: Wei S, Ding B, Wang G, Luo S, Zhao H and Dan X (2024) Population characteristics of pathogenic Escherichia coli in puerperal metritis of dairy cows in Ningxia region of China: a systemic taxa distribution of virulence factors and drug resistance genes. Front. Microbiol. 15:1364373. doi: 10.3389/fmicb.2024.1364373
Received: 02 January 2024; Accepted: 28 March 2024;
Published: 17 April 2024.
Edited by:
Neeta Agarwal, Indian Veterinary Research Institute (IVRI), IndiaReviewed by:
Sachin Kumar, National Dairy Research Institute (ICAR), IndiaCopyright © 2024 Wei, Ding, Wang, Luo, Zhao and Dan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxi Zhao, emhhb2hvbmd4aTIwMDZAMTYzLmNvbQ==, Xingang Dan, eGdkYW5Abnh1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.