- 1School of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia
- 2School of Medical Laboratory Sciences, College Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 3Department of Medical Laboratory Sciences, College of Health Sciences, Ethiopian Police University, Addis Ababa, Ethiopia
Background: The permanence of HIV patients in healthcare provision centers exposes their weak immunity to various nosocomial microorganisms that migrate into and out of the hospital environment. The incidence of bacterial infections, including urinary tract infection, was inversely correlated with CD4+ T cells. Urinary tract infection (UTI) is one of the clinical problems among HIV patients. There was scarcity of published data on the relationship between viral load, CD4+ level, and UTI. This study aimed to assess the relationship between viral load and CD4 with bacterial UTI among HIV patients.
Methods: The cross-sectional study was conducted in the Wolaita Sodo Town Health Center ART clinic. The socio-demographic data were collected using a pre-designed questionnaire. Patients' charts were reviewed to collect the current CD4 and viral load. Urine specimens were inoculated on blood agar, cysteine lactose electrolyte deficient (CLED) agar, and MacConkey agar, and bacterial species were finally identified using various biochemical methods. Antimicrobial sensitivity testing was conducted using standard microbiological tests. Bivariate and multivariate analyses were employed to describe the association between pairs of variables and to examine the relationship between independent variables and dependent variables.
Results: In this study, the overall prevalence of urinary tract infection (UTI) was 13.7%. Escherichia coli, Staphylococcus aureus, Pseudomonas aeroginosa, Staphylococcus saprophyticus, Proteus mirabilis, and Klebsiella pneumoniae were bacterial uropathogens detected in this study. E.coli (45.7%) was the predominant isolate followed by S. aureus (14.3%). Positive correlation between CD4+ count and urinary tract infection was detected and found statistically significant (r = 0.288 p > 0.01), whereas the viral load and urinary tract infection negatively correlated and showed statistically significant association (p < 0.01). The resistance rate of E.coli was 94%, 75%, and 69% to ciprofloxacin, norfloxacin, and cefepime, respectively. This study revealed that E.coli exhibited 94% and 75% resistance to amoxicillin-clavulanic acid and tetracycline, respectively. K. pneumoniae demonstrated complete resistance (100%) to amoxicillin-clavulanic acid, tetracycline, and trimethoprim-sulfamethoxazole, while showing 100% susceptibility to ciprofloxacin and nitrofurantoin. In the present study, the magnitude of the multi-drug resistance (MDR) was found to be 80%. CD4+ count, combination of antiretroviral therapy (ART) drugs, and a history of hospitalization were risk factors for urinary tract infection.
Conclusion: In the current study, urinary tract infection emerged as a significant health concern among people living with HIV following their ART. The occurrence of urinary tract infection among HIV patients could be influenced by multifactorial factors that require further study. The CD4+ count was positively correlated with the prevalence of UTI, whereas the viral load was negatively correlated. The CD4+ count, combination of ART, and history of hospitalization were independent risk factors for UTI. The prevalence of MDR bacterial pathogens were notably high. Therefore, the treatment of UTI in HIV patients should be prescribed based on antibacterial susceptibility testing results.
Introduction
The destruction of CD4+ cells caused by human immunodeficiency virus (HIV) leads to acquired immunodeficiency syndrome (AIDS), resulting in progressive weakening of the immune system leading to the development of serious opportunistic infections (Schönwald et al., 1999). HIV, the virus with unique pathogenesis involving the gradual decline of immunity, reduces body's ability to fight off invading commensal organisms in people living with HIV (PLHIV) (Debalke et al., 2014; Skrzat-Klapaczyńska et al., 2018; Adhanom et al., 2019). HIV patients with low CD4 counts due to advanced AIDS are at the risk of developing renal syndromes and neurological complications such as hyperreflexia and hyporeflexia, which can lead to urinary stasis and ultimately infection (Rashmi et al., 2013). Urinary tract infection (UTI), which could be symptomatic or asymptomatic, is a notable opportunistic infection among HIV patients. People living with HIV (PLHIV) are at the high risk of developing UTIs that can progress eventually to bacteremia, sepsis, and potentially lead to death due to weakened immunity (Ifeanyichukwu et al., 2013; Barnie et al., 2019). Bacterial UTIs are more common and can progress to severe form under certain underlying conditions in individuals with HIV (Olowe et al., 2015). The permanence of HIV patients in hospitals increases the risk of nosocomial transmission of virulent microorganisms, which can migrate into and out of the hospital environment (Panis et al., 2009). E.coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. aureus, and S. saprophyticus are the most common bacterial etiologies of UTI (Panis et al., 2009; Marami et al., 2019; Simeneh et al., 2022).
Expanding resistance to available antibiotics used to treat UTIs is another concern, which may contribute to the emergence of MDR bacterial uropathogen strains in HIV patients (Debalke et al., 2014; Fenta et al., 2016). The burden associated with MDR pathogens is high in developing countries due to lack of advanced microbiological techniques, resulting in incorrect diagnosis, irrational use of antibiotics, and inadequate infection prevention strategies (Murugesh et al., 2014; Tuem et al., 2019; Simeneh et al., 2022). UTI is one of the public healthcare problems that decreases the quality of life and leads to work absence. Various studies conducted in Ethiopia, including those involving HIV patients, have reported prevalence rates ranging from 4% to 25% (Debalke et al., 2014; Fenta et al., 2016; Getu et al., 2017; Ayelign et al., 2018; Marami et al., 2019). Due to antimicrobial-resistant uropathogens, UTI is continuing as public health challenge, indicating that further research in this area is required with an objective to develop effective control mechanism of antibiotics usage and enhance infection prevention programs (Ifeanyichukwu et al., 2013; Fenta et al., 2016). The incidence of UTIs among HIV patients is higher in individuals with CD4 counts of <500cells/mm3, indicating an association between CD4 counts and bacterial UTI (Fenta et al., 2016). In Africa, it has also been revealed that PLHIV with CD4 cell counts <200 cells/mm3 are more likely to develop UTIs (Chaula et al., 2017; Tessema et al., 2020). There is scarcity of available data on correlation of bacterial UTI with CD4 cell counts, viral loads, and other predictive factors in the study area. Therefore, the current study aimed to detect antimicrobial-resistant bacterial uropathogens among PLHIV and their correlation with viral load and CD4 counts among HIV patients.
Methods
Study area
Health institution-based cross-sectional study was conducted in Wolaita Sodo Town Health Center (STHC) located in Wolaita Sodo town, South Ethiopia. STHC provides ART services for the Sodo town and neighboring woredas. The town has a latitude and longitude of 6°54′N 37°45′E with an elevation between 1600 and 2100 m above sea level. The mean annual temperature and rainfall of Wolaita Sodo ranges from 15.1 to 20°C and 1201–1600 mm, respectively. According Southern Nations, Nationalities and People Region bureau of Finance and Economic Development report, the town has a total population of 2,542,595, of whom 125,855 are men and 128,440 women. Wolaita Sodo town has three health centers (Wadu Health Center, Arada Health Center, and Sodo Town Health Center); among them, only STHC provides ART services. The total number of for were 650 in STHC provides ART follow-up for a total of 650 HIV-infected patients. Every working day, individuals living with HIV attend the ART Clinic for their regular follow-up.
Study design
An institutional-based cross-sectional study was conducted among people living with HIV who were receiving healthcare at Wolaita Sodo town health centers from April to July 2021.
Source population
The source population included all HIV-positive adults attending the WSHC ART clinic.
Study population
The study population included all HIV-positive adults attending the ART clinic during the study period.
Inclusion criteria and exclusion criteria
Adult patients who were not taking antibiotics for a current UTI were included in the study, while critically ill patients and those receiving any antibiotics treatment were excluded from the study.
Sample size and sampling technique
The required sample size was determined using a single population formula, considering the following assumptions:
Prevalence = 11.3% prevalence of culture proven UTI among HIV patients in Ethiopia (Fenta et al., 2016)
where
no = the required sample size, Z = Z score for 95 % confidence interval = 1.96,
p = prevalence; 11.3% (0.113), d = tolerable error =3 %.
1-p = Q = negative proportion.
Hence, n = [(1.96)2 × 0.113 × (1–0.113)]/(0.03)2 = 423.
If the total population was <10,000, then the following correction formula was applied:
The study participants were selected using the systematic sampling technique, with a predetermined Kth value, where Kth value = total population/sample size = 650/256 = 3. After randomly selecting the first participant among the first three early arrivals at the ART clinic, subsequent participants were selected at the intervals of three individuals until the required sample size was reached.
Data collection and laboratory processing
The structured questionnaire was employed to collect socio-demographic characteristics and associated factors. Secondary data regarding ART and clinical history were retrieved from patient's charts. Participants' current CD4 value and viral load were collected from their medical records.
Sample collection, handling, and transport
A sterile screw-capped container was used to collect mid-stream urine (MSU) samples. All urine specimens were promptly placed in a cold box and transported to the Central laboratory within 30 min of collection (Cheesbrough, 2005).
Culture and identification
Cysteine lactose electrolyte deficient (CLED), medium, blood agar, MacConkey agar (MAC), and mannitol salt agar (MSA) were used to culture urine samples. A colony count ≥105CFU/ml of urine was defined as a positive urine culture. All positive cultures were further identified by their colony characteristics. Gram staining was conducted to differentiate between gram positives and gram negatives. Pure isolate was sub-cultured onto a nutrient broth medium and incubated aerobically at 37°C for 12–24 h for biochemical testing (Cheesbrough, 2009). Additionally, a single isolated bacterium was inoculated onto a nutrient agar slant and stored in a refrigerator following 24 h of incubation for maintenance. A series of biochemical tests, including Kligler iron agar, Simmons citrate agar, lysine iron agar, urea, glucose, and lactose fermentation, lysine decarboxylation, gas and H2S production, motility tests, and indole (Cheesbrough, 2009), were used to identify gram-negative bacteria. Gram staining, catalase, coagulase, and novobiocin were used to identify gram-positive bacteria.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was conducted using the Kirby-Bauer disk diffusion method on Mueller–Hinton agar plates (Oxoid Ltd.) prepared to a 4 mm thickness. Selected antimicrobial disks, including ampicillin (10 μg), amoxicillin-clavulanic acid (20/10 μg), cefoxitin (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), tetracycline (10 μg), norfloxacin (10 μg), cefepime (30 μg), ceftriaxone (30 μg), nitrofurantoin (F) 300 μg, amikacin (10 μg), and azithromycin (30 μg) (Oxoid Ltd.), were used for susceptibility testing. After measuring the zone of inhibition diameter of bacterial isolates, it was compared with interpretative standards as in the CLSI guideline to classify as sensitive (S), intermediate (I), or resistance (R) (Sahu et al., 2018).
Data processing and data analysis
The data were coded and entered into SPSS version 24 for analysis. After cleaning the data were cleaned using frequencies and cross-tabulation before regression analysis. Results were presented using tables, figures and charts after analysis of descriptive statistics and presented for continuous and discrete data in terms of mean and standard deviation for those normally distributed data. The binary logistic regression model was used to test the association between dependent and independent variables. All variables with a p < 0.25 in the binary regression analysis were included in the multivariable regression analysis. The degree of the association was interpreted using the adjusted odds ratio with 95% confidence intervals, and the significance level was declared at a p < 0.05.
Results
Socio-demographic characteristics
The data were collected from a total of 255 study participants; among them, 153 (60%) were female individuals and 102 (40%) were male individuals. Of the 255 total study participants, 43.9% belonged to the 28–37 age group. In terms of marital status, ~123 (43.9%) participants were married, and 77.25% of study participants were urban residents (Table 1).
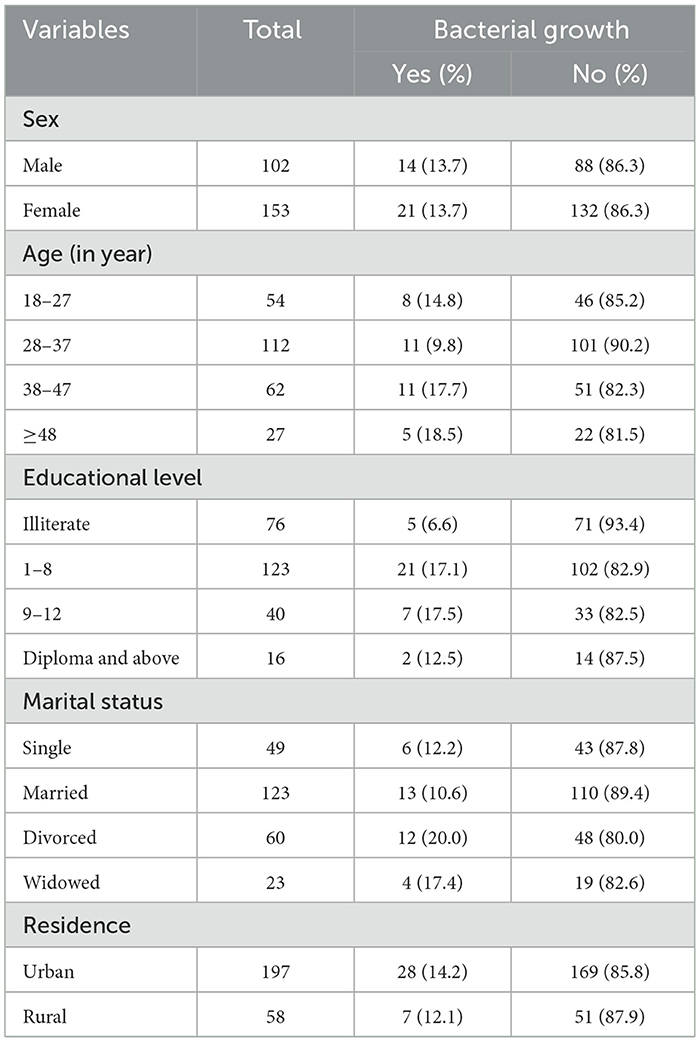
Table 1. Bacteriuria and socio-demographic characteristics among ART followers in Wolaita Sodo, Ethiopia, 2021.
ART and clinical related characteristics
Out of the total 255 study participants, 48.2% of the total study participants had been HIV-positive for 5 years and above and 38.4% of those had following their therapy for 2–5 years. The majority of the study participants (82.4%) had CD4 count value of > 200 cell/mm3. Among the total 255 study participants, 74.1% of study participants had <1.7log current viral load. TDF-3TC-EFV (60%) and AZT-3TC-NVP (26.7%) were the most common ART combinations used to treat HIV patients (Table 2).
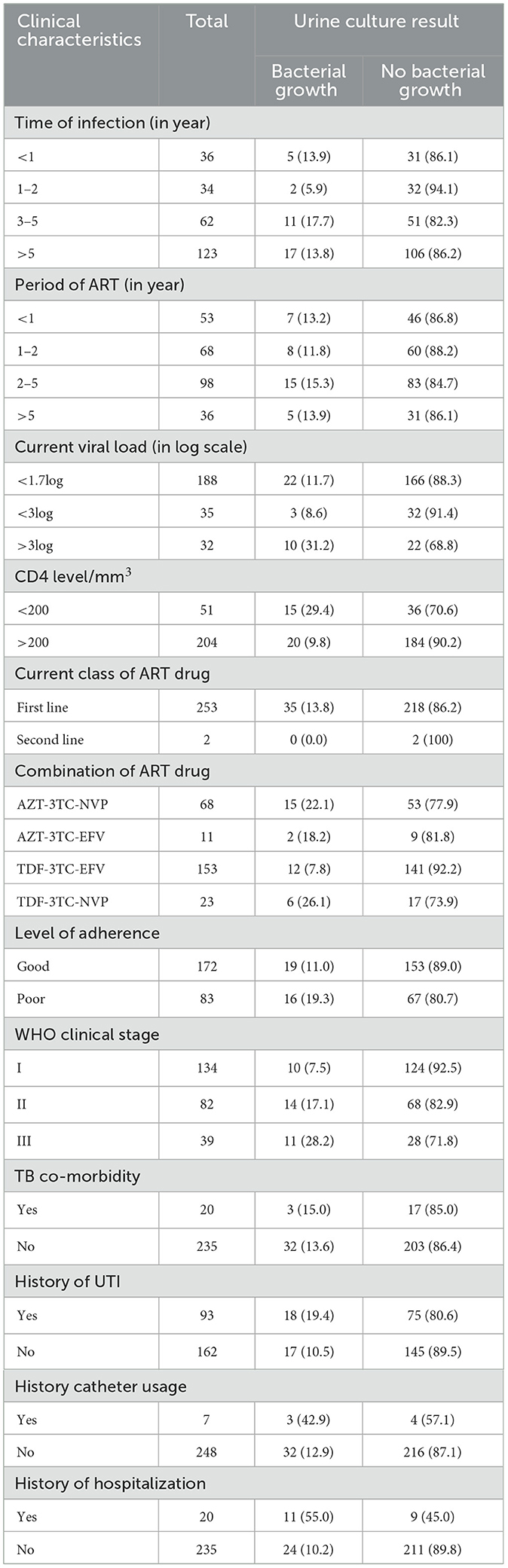
Table 2. Bacteriuria and clinical characteristics among ART followers in Wolaita Sodo, Ethiopia, 2021.
Prevalence of urinary tract infection
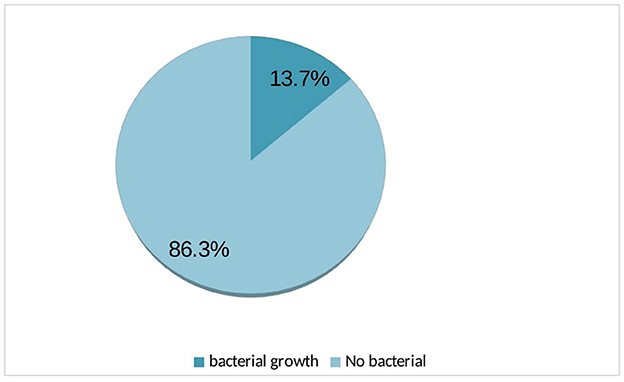
Urine cultures from 35 participants exhibited bacterial growth, resulting in the overall prevalence of 13.7% (Figure 1).
Urinary tract infection with socio-demographic characteristics
The bacterial detection rate was similar in both sexes; however, among the total positive cases, female participants were more likely to be infected by UTI (Table 1).
Bacterial etiologies of UTI
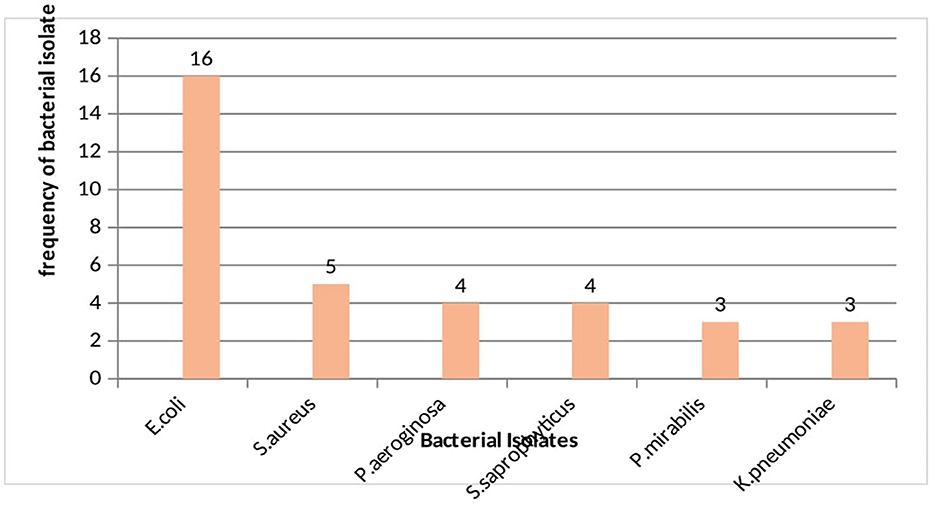
Among the total of 35 uropathogenic bacterial isolates, 74.3% were gram negative bacteria and E. coli became the predominant isolate (45.7%) followed by S. aureus (14.3%) (Figure 2).
Correlation of UTI with CD4 counts and viral load
As determined using Pearson's product correlation analysis, CD4 and bacterial growth were found to be low positive and statistically significant (r = 0.288 p > 0.01). This result showed that an increase in CD4 could lead to an increase in the prevalence of bacterial UTI in people living with HIV. Whereas, the viral load and bacterial growth negatively correlated and statistically significant (r = −0.153 p > 0.05). Pearson's product correlation analysis revealed a positive association between viral load and duration of ART with correlation coefficient (r = 0.262) and statistically significant (p < 0.01) as shown in the Table 3.
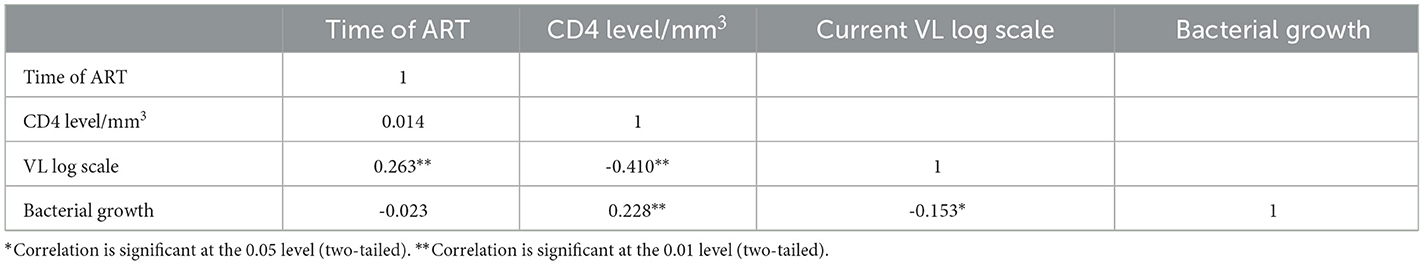
Table 3. Correlation analysis of UTI, CD4+ count, and viral load among ART followers in Wolaita Sodo, Ethiopia, 2021.
Predictive factors of urinary tract infections
The bivariate regression analysis was initially conducted, and variables with a p ≥0.25 were transferred to the multivariate regression analysis. CD4+ count [(AOR = 0.322, 95% CI = 0.108, 0.956)], current UTI [(AOR = 0.113, 95% CI = 0.041, 0.309)], and history of hospitalization [(AOR = 0.163, 95% CI = 0.041, 0.640)] were predictive factors of UTI. The likelihood of acquiring UTI in participants with high load was 2.147 times higher than in those with lower viral load (Table 4).
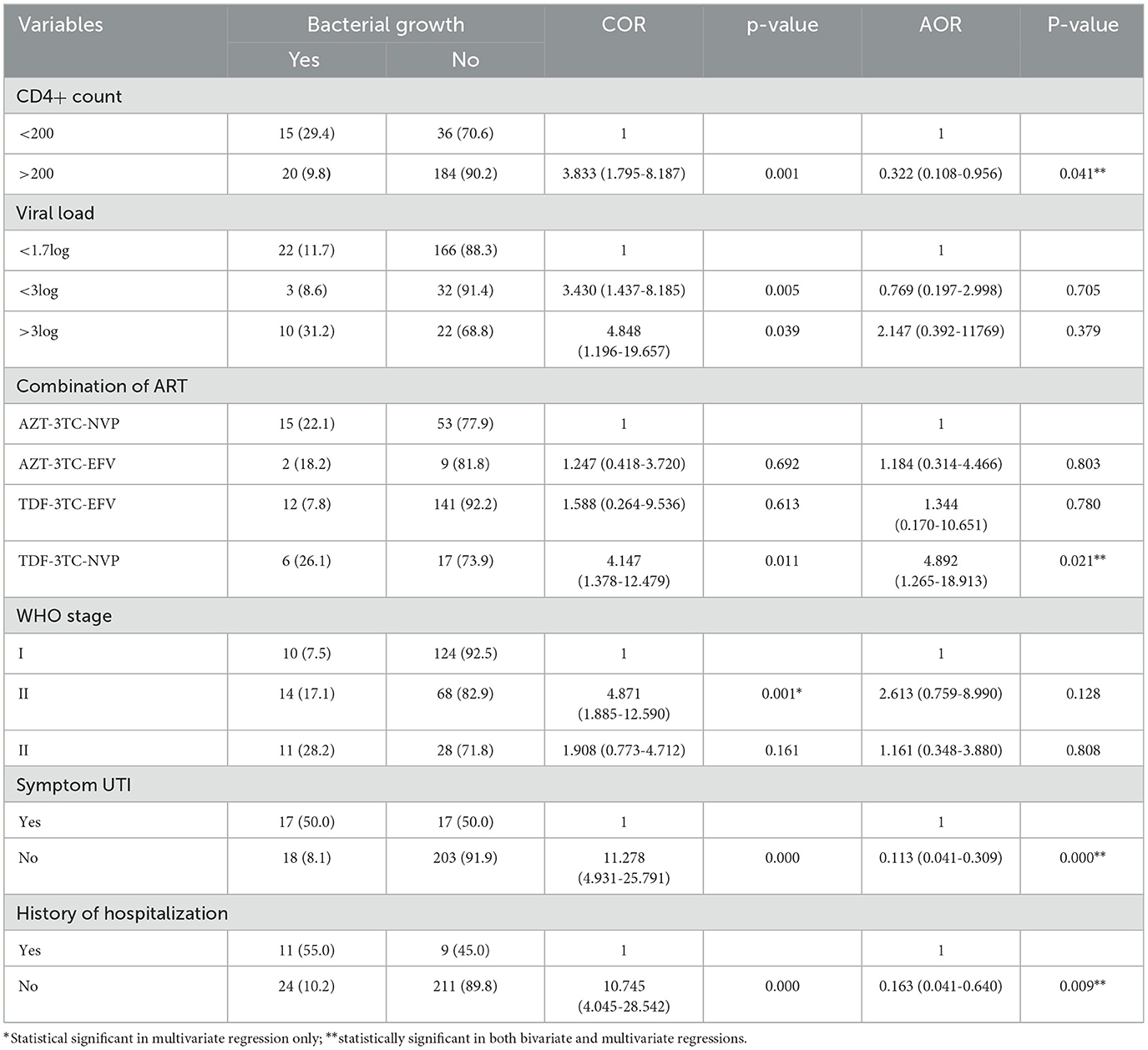
Table 4. Bivariate and multivariate analyses of risk factors for UTI among ART followers in Wolaita Sodo, Ethiopia, 2021.
Antimicrobial resistance profiles of uropathogenic bacteria: gram-negative bacteria
As shown in Table 5, antimicrobial resistance patterns of E. coli to amoxicillin-clavulanic acid, tetracycline, and trimethoprim-sulfomethoxazole were 94%, 75%, and 50%, respectively. E. coli showed susceptibility rates of 94%, 75%, 69%, 62.5%, 50% and 50% to ciprofloxacin, norfloxacin, cefepime, ceftriaxone, and trimethoprim-sulphamethazole (SXT), respectively. P. aeruginosa showed complete resistance (100%) to amoxicillin-clavulanic acid, cefepime, trimethoprim-sulphamethazole, and ciprofloxacin. P. mirabilis isolates were 60% sensitive to all antibiotics with complete sensitivity to norfloxacin, tetracycline, and ciprofloxacin. K. pneumoniae showed the higher rate of resistance to amoxicillin-clavulanic acid (100%), tetracycline (100%), and trimethoprim-sulphamethazole (100%). K. pneumoniae was 100% susceptible to nitrofurantoin, cefepime, and ciprofloxacin.
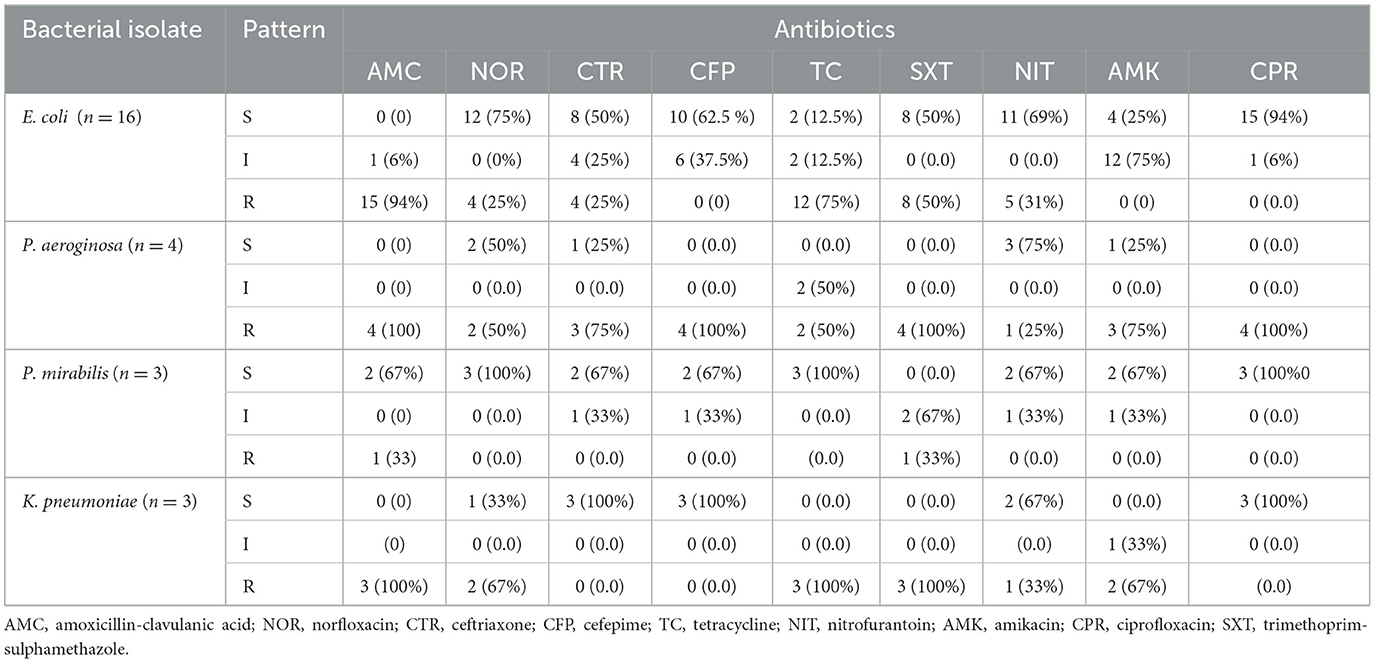
Table 5. Antimicrobial resistance profiles of gram-negative bacterial isolates (n = 26) ART followers in Wolaita Sodo, Ethiopia, 2021.
Gram-positive bacterial isolates
S. aureus exhibited susceptibility rates of 100%, 80%, 80%, and 60% to nitrofurantoin, ciprofloxacin, gentamicin, and azithromycin, respectively, whereas it showed 100%, 100%, and 80% resistance to ampicillin, cefoxitin, and tetracycline, respectively. S. saprophyticus showed complete resistance (100%) to ampicillin, amoxicillin-clavulanic acid, cefoxitin, and azithromycin, whereas it became 100% susceptible to gentamicin and nitrofurantoin (Table 6).
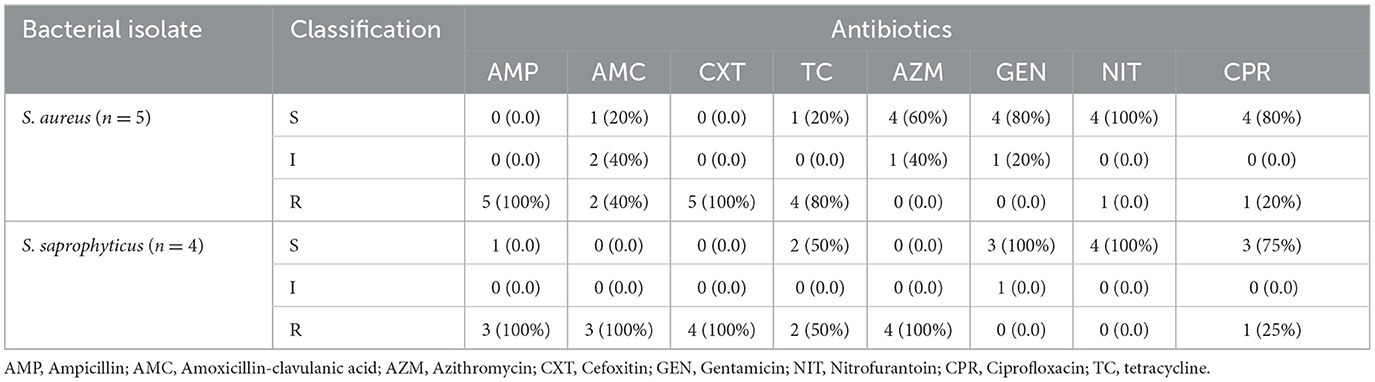
Table 6. Antimicrobial resistance profiles of gram-positive isolates (n = 9) among ART followers in Wolaita Sodo, Ethiopia, 2021.
Multiple drug resistance patterns of uropathogenic bacterial isolates
A total of 28 (80%) multi-drug-resistant (MDR) bacterial pathogens were detected from the total 35 bacterial isolates. As shown in Table 7, gram-negative bacteria accounts for 21 (75%) and gram positive 7 (25%). Among the gram-negative isolates, all P. aeroginosa and K. pneumoniae became MDR bacterial pathogens. Out of 28 MDR isolates, 15 (53.6%) were resistant to four classes of antibiotics.
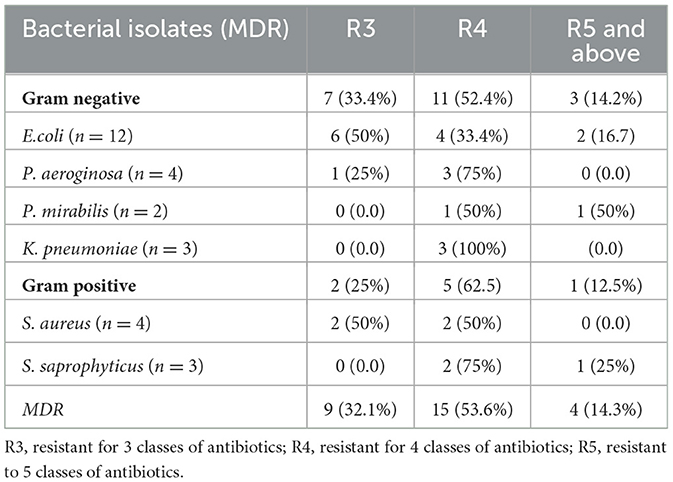
Table 7. Multidrug resistance patterns of bacterial isolates among ART followers in Wolaita Sodo, Ethiopia, 2021.
Discussion
This study aimed at detecting bacterial UTI and antibacterial resistance and their correlation with CD4+ count and viral load among HIV patients. This study revealed that the overall culture-confirmed UTI prevalence was 13.7%. The finding was compared with previous studies conducted in Northeastern Ethiopia 11.6% (Alemu et al., 2020), Hawassa 13.6% (Nigussie and Amsalu, 2017), and Romania 12% (Chiţǎ et al., 2016). However, this finding was relatively lower than that of previous studies conducted in Addis Ababa 14.9% (Woldemariam et al., 2019), Gondar 17.8% (Yismaw et al., 2012), Nekemet 16.5%, and Sudan 19.5% (Hamdan et al., 2015). This finding was lower than that of other studies conducted in Nigeria that reported the range of UTI as 23.5–57.3% (Omoregie and Eghafona, 2009; Bigwan and Wakjissa, 2013; Kanu et al., 2016; Kemajou et al., 2016). This finding was relatively higher than that of other studies on UTI conducted in Addis Ababa 10.9% (Yeshitela et al., 2012) and Debre Tabor 10.9% (Worku et al., 2017). The variation might be due to by differences in socio-demographic characteristics, the host factor, and practices such as the social habits of the community, standards of personal hygiene, health education practices, and study population.
The study detected correlation between immune status and UTI. While many infections occur in immune-compromised people, UTIs are less common among this population compared to general population, with the exception of patients who have undergone renal transplantation (Hoepelman et al., 1992; Tolkoff-Rubin and Rubin, 1997). In current study, bacterial uropathogens were mainly isolated from HIV patients with a CD4 count of >200 cells/ml. This finding was corroborated with other study conducted in Chad (Abderrazzack et al., 2015). There was a significant relationship between CD4+ cell count and UTI (p < 0.05). This report was in line with other study conducted in the Netherlands to assess the relationship between bacteriuria and immune status (Hoepelman et al., 1992). In the current study, the prevalence of UTI was negatively correlated with viral load in HIV patients. Many studies reported numerous risk factors for UTI (Widmer et al., 2010; Ezechi et al., 2013; Skrzat-Klapaczyńska et al., 2018). In the present study, current CD4+ count, history of hospitalization, current UTI, and combination ART drugs were the predictive factors of UTI. This study demonstrated that 15 out of 35 participants with a CD4 count of <200/mm3 were infected by uropathogens. This finding was supported by other studies conducted in Tanzania (Chaula et al., 2017), India (Chandwani et al., 2022), and Brazil (Back-Brito et al., 2011). The most commonly used combination of ART drug was TDF-3TC-EFV to treat UTI. Bacterial growth was higher among TDF-3TC-NVP users (26.1%) followed by AZT-3TC-NVP users (22.1%). The bacterial growth was significantly higher among participants with high viral load (>3log) (31.5%). The bacterial profiling revealed that both gram-negative (E. coli, K. pneumoniae, P. mirabilis and P. aeroginosa) and gram-positive bacteria (S. aureus and S. saprophyticus) were involved. Gram negative bacteria (74.3%) were predominant causative agents of UTI. This is corroborated with other studies in which they were dominant bacterial uropathogens somewhere else including Ethiopia (Tolkoff-Rubin and Rubin, 1997; Omoregie and Eghafona, 2009; Bigwan and Wakjissa, 2013; Hamdan et al., 2015; Abate et al., 2017; Pragash et al., 2017).
In this study, E. coli became a predominant isolate (45.7%). This finding was in line with that of other studies conducted in Nigeria 50% (Ibadin et al., 2006). This finding was opposed with that of the study conducted in Nigeria, which reported that S. aureus was the most common uropathogen (Omoregie and Eghafona, 2009). Other study conducted in Nigeria (Kemajou et al., 2016) also reported comparable prevalence of bacterial pathogens, including S. aureus (29.7%), E. coli (28.5%), and P. aeruginosa (27.9%), which contradicted our study finding. This variation in bacterial etiology of UTI could be due to difference in pathogens from place to place, as well as variations in their susceptibility and resistance patterns. The criteria for choosing antibacterial agents should be based on the local availability of antibiotics and its expected susceptibility pattern to most likely pathogens because UTI are mostly treated empirically (Prakash and Saxena, 2013). Antimicrobial resistance pattern profiling was conducted on all isolated uropathogens using the Kirby-Bauer disk diffusion method, and the identified isolates showed varying patterns of antimicrobial resistance and susceptibility. A higher rate of resistance to amoxicillin-clavulanic acid, tetracycline, and trimethoprim-sulfamethoxazole was detected in gram-negative bacterial uropathogens, which was comparable with other studies conducted in Ethiopia that reported high resistance rate of antimicrobial agents (Kebamo et al., 2017; Nigussie and Amsalu, 2017). This aspect might be due to uncontrolled and easy access of antibiotics and overuse of the antimicrobial agents for long period. However, high susceptibility of gram-negative bacteria was detected to norfloxacin, ciprofloxacin, nitrofurantoin, and cefepime in the present study. According to the current study, the magnitude of MDR bacterial uropathogens was 80%. Comparable finding was reported from the previous study conducted in South Ethiopia 79.3% (Haile Hantalo et al., 2020) and Addis Ababa 78.4% (Fenta et al., 2016). This finding was absolutely higher than that of the study conducted in Ethiopia that reported 14.3% (Simeneh et al., 2022). However, this finding was relatively higher than that of other studies conducted in Ethiopia that revealed MDR rates of <50%−60% (Biset et al., 2020; Kasew et al., 2022). In contrast to our study, higher prevalence of MDR was reported from previous studies conducted in Gondar 91.7% (Yismaw et al., 2012) and north Ethiopia 95% (Alemu et al., 2012). This change in MDR patterns in the study area and across the world could be attributed to variations in antibiotic usage practices, including the prescription of antimicrobials, high frequency of antibiotic use, and lack of standard microbiological facilities.
Conclusion
UTI is a significant cause of morbidity among HIV patients in the area. The CD4+ count was positively correlated with the prevalence of UTI, whereas viral load was negatively correlated. CD4+ count, combination of ART, current UTI, and history of hospitalization were independent risk factors for UTI. The prevalence of MDR bacterial pathogens was notably high. Therefore, the treatment of UTI in HIV patients should be prescribed based on antibacterial susceptibility testing results.
Limitations of the study
Being a cross-sectional study, cause-effect relationship of CD4+ count and HIV load with bacterial UTI cannot be established. So, further study aimed at detecting cause-effect relationship of CD4+ cell count and HIV viral load and urinary tract infection is needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Wolaita Sodo University Research Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TM: Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research was funded by the Wolaita Sodo University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors would like to thank Wolaita Sodo University Faculty of Medicine and Health Sciences for sponsoring the research. We also extend profound gratitude to the study participants for their voluntary in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abate, D., Kabew, G., Urgessa, F., and Meaza, D. (2017). Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr. J. Health Biomed. Sci. 1, 11–20.
Abderrazzack, A. F., Salou, M., Patassi, A., Yehadji, D., and Ameyapoh, Y. (2015). Correlation between asymptomatic bacteriuria and HIV-1 viral load level and CD4 count in pregnant women on antiretroviral therapy in N'djamena (Chad). World J. AIDS 5:308. doi: 10.4236/wja.2015.54033
Adhanom, G., Gebreegziabiher, D., Weldu, Y., Gebreyesus Wasihun, A., Araya, T., Legese, H., et al. (2019). Species, risk factors, and antimicrobial susceptibility profiles of bacterial isolates from HIV-infected patients suspected to have pneumonia in Mekelle zone, Tigray, northern Ethiopia. BioMed Res. Int. doi: 10.1155/2019/8768439
Alemu, A., Moges, F., Shiferaw, Y., Tafess, K., Kassu, A., Anagaw, B., et al. (2012). Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res. Notes 5, 1–7. doi: 10.1186/1756-0500-5-197
Alemu, M., Belete, M. A., Gebreselassie, S., Belay, A., and Gebretsadik, D. (2020). Bacterial profiles and their associated factors of urinary tract infection and detection of extended spectrum beta-lactamase producing gram-negative uropathogens among patients with diabetes mellitus at Dessie Referral Hospital, Northeastern Ethiopia. Diab. Metabolic Syndr. Obesity 13, 2935–2948. doi: 10.2147/DMSO.S262760
Ayelign, B., Abebe, B., Shibeshi, A., Meshesha, S., Shibabaw, T., Addis, Z., et al. (2018). Bacterial isolates and their antimicrobial susceptibility patterns among pediatric patients with urinary tract infections. Turkish J. Urol. 44:62. doi: 10.5152/tud.2017.33678
Back-Brito, G. N., El Ackhar, V. N. R., Querido, S. M. R., dos Santos, S. S. F., Jorge, A. O. C., de Macedo, R., et al. (2011). Staphylococcus spp., Enterobacteriaceae and Pseudomonadaceae oral isolates from Brazilian HIV-positive patients. Correlation with CD4 cell counts and viral load. Arch. Oral Biol. 56, 1041–1046. doi: 10.1016/j.archoralbio.2011.02.016
Barnie, P. A., Akwetey, S., Swallah, M. H., Acheampong, D. O., and Kwakye-Nuako, G. (2019). Occurrence and distribution of bacterial uropathogens among antiretroviral therapy users and non-users, Cape Coast Teaching Hospital. Am. J. Multidiscip. Res. 8, 1–14.
Bigwan, E. I., and Wakjissa, F. D. (2013). Prevalence of urinary tract infections among HIV patients attending a non-governmental health facility in Jos, Plateau State, Nigeria. Int. J. Biomed. Adv. Res. 4, 528–533. doi: 10.7439/ijbar.v4i8.440
Biset, S., Moges, F., Endalamaw, D., and Eshetie, S. (2020). Multi-drug resistant and extended-spectrum β-lactamases producing bacterial uropathogens among pregnant women in Northwest Ethiopia. Annal. Clin. Microbiol. Antimicrobials 19, 1–9. doi: 10.1186/s12941-020-00365-z
Chandwani, J., Meena, P., Mathur, S., and Parihar, G. (2022). Bacterial urinary tract infections and its relation with CD4+ T lymphocyte cell count among people living with HIV in Ajmer City, Center of Rajasthan. J. Family Med. Primary Care 11, 7378–7382. doi: 10.4103/jfmpc.jfmpc_1304_22
Chaula, T., Seni, J., Ng'walida, N., Kajura, A., Mirambo, M. M., DeVinney, R., and Mshana, S. E. (2017). Urinary tract infections among HIV-positive pregnant women in Mwanza city, Tanzania, are high and predicted by low CD4+ Count. Int. J. Microbiol. doi: 10.1155/2017/4042686
Cheesbrough, M. (2005). District Laboratory Practice in Tropical Countries, Part 2. Cambridge: Cambridge University Press.
Cheesbrough, M. (2009). District Laboratory Practice in Tropical Countries. Part 1, 2nd Edn. Cambridge: Cambridge University Press.
Chiţǎ, T., Timar, B., Muntean, D., Bǎdiţoiu, L., Horhat, F., Hogea, E., and Licker, M. (2016). Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Therapeutics Clin. Risk Manage. 18, 1–7. doi: 10.2147/TCRM.S123226
Debalke, S., Cheneke, W., Tassew, H., and Awol, M. (2014). Urinary tract infection among antiretroviral therapy users and nonusers in Jimma University Specialized Hospital, Jimma, Ethiopia. Int. J. Microbiol. doi: 10.1155/2014/968716
Ezechi, O. C., Gab-Okafor, C. V., Oladele, D. A., Kalejaiye, O. O., Oke, B. O., Ekama, S. O., et al. (2013). Prevalence and risk factors of asymptomatic bacteriuria among pregnant Nigerians infected with HIV. The J. Mat. -Fetal Neonatal Med. 26, 402–406. doi: 10.3109/14767058.2012.733782
Fenta, G. M., Legese, M., and Weldearegay, G. (2016). Bacteriuria and their antibiotic susceptibility patterns among people living with HIV attending Tikur Anbessa Specialized and Zewditu Memorial Hospital ART Clinics, Addis Ababa, Ethiopia. J. Bacteriol. Parasitol. 7:292. doi: 10.4172/2155-9597.1000292
Getu, Y., Ali, I., Lema, T., Belay, H., and Yeshetela, B. (2017). Bacteriuria and antimicrobial susceptibility pattern among HIV patients attending ALERT Center, Addis Ababa, Ethiopia. Am. J. Health Res. 5, 76–82. doi: 10.11648/j.ajhr.20170503.14
Haile Hantalo, A., Haile Taassaw, K., Solomon Bisetegen, F., and Woldeamanuel Mulate, Y. (2020). Isolation and antibiotic susceptibility pattern of bacterial uropathogens and associated factors among adult people living with HIV/AIDS attending the HIV Center at Wolaita Sodo University Teaching Referral Hospital, South Ethiopia. HIV/AIDS-Res. Palliative Care12, 799–808. doi: 10.2147/HIV.S244619
Hamdan, H. Z., Kubbara, E., Adam, A. M., Hassan, O. S., Suliman, S. O., Adam, I., et al. (2015). Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Annal. Clin. Microbiol. Antimicrobials 14, 1–6. doi: 10.1186/s12941-015-0082-4
Hoepelman, A. I., van Buren, M., van den Broek, J., and Borleffs, J. C. (1992). Bacteriuria in men infected with HIV-1 is related to their immune status (CD4+ cell count). Aids 6, 179–184. doi: 10.1097/00002030-199202000-00006
Ibadin, O. M., Onunu, A., and Ukoh, G. (2006). Urinary tract infection in adolescent/young adult Nigerians with acquired human immuno deficiency disease in Benin city. J. Med. Biomed. Res. 5, 55–60.
Ifeanyichukwu, I., Emmanuel, N., Chika, E., Anthonia, O., Esther, U. I., Ngozi, A., et al. (2013). Frequency and antibiogram of uropathogens isolated from urine samples of HIV infected patients on antiretroviral therapy. Am. J. BioScience 1, 50–53. doi: 10.11648/j.ajbio.20130103.11
Kanu, A. M., Mgbajiaka, N., and Abadom, N. (2016). Prevalence of urinary tract infection among HIV patients in Aba, Nigeria. Int. J. Inf. Dis. 45:229. doi: 10.1016/j.ijid.2016.02.517
Kasew, D., Desalegn, B., Aynalem, M., Tila, S., Diriba, D., Afework, B., et al. (2022). Antimicrobial resistance trend of bacterial uropathogens at the university of Gondar comprehensive specialized hospital, northwest Ethiopia: a 10 years retrospective study. PloS ONE 17:e0266878. doi: 10.1371/journal.pone.0266878
Kebamo, S., Dabso, R., Deressa, A., and Gebrie, M. (2017). Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending Nekemte Referral Hospital, Ethiopia. Am J Curr Microbiol 5, 19–31.
Kemajou, T. S., Ajugwo, A. O., Oshoma, C. E., and Oi, E. (2016). Antibiotic resistance of bacterial isolates from HIV positive patients with Urinary tract infection (UTI) in Portharcourt, Nigeria. J. AIDS Clin. Res. 7, 8–11. doi: 10.4172/2155-6113.1000594
Marami, D., Balakrishnan, S., and Seyoum, B. (2019). Prevalence, antimicrobial susceptibility pattern of bacterial isolates, and associated factors of urinary tract infections among hiv-positive patients at hiwot fana specialized university hospital, eastern Ethiopia. Can. J. Inf. Dis. Med. Microbiol. doi: 10.1155/2019/6780354
Murugesh, K., Deepa, S., Ravindranath, C., and Venkatesha, D. (2014). Multi drug resistant uropathogens in HIV: are they a threat to community. Int. J. Sci. Study 2, 38–42.
Nigussie, D., and Amsalu, A. (2017). Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turkish J. Urol. 43:85. doi: 10.5152/tud.2016.86155
Olowe, O., Ojo-Johnson, B., Makanjuola, O., Olowe, R., and Mabayoje, V. (2015). Detection of bacteriuria among human immunodeficiency virus seropositive individuals in Osogbo, south-western Nigeria. Eur. J. Microbiol. Immunol. 5, 126–130. doi: 10.1556/EuJMI-D-14-00036
Omoregie, R., and Eghafona, N. O. (2009). Urinary tract infection among asymptomatic HIV patients in Benin City, Nigeria. Br. J. Biomed. Sci. 66, 190–193. doi: 10.1080/09674845.2009.11730272
Panis, C., Matsuo, T., and Reiche, E. M. V. (2009). Nosocomial infections in human immunodeficiency virus type 1 (HIV-1) infected and AIDS patients: major microorganisms and immunological profile. Braz. J. Microbiol. 40, 155–162. doi: 10.1590/S1517-83822009000100027
Pragash, D. S., Girija, S., Sekar, U., Rayapu, V., and Sheriff, D. (2017). Uropathogens and diabetes mellitus-a perspective. IOSR J. Dent. Med. Sci. 16, 29–32. doi: 10.9790/0853-1605052932
Prakash, D., and Saxena, R. S. (2013). Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut city, India. Int. Scholarly Res. Notices. doi: 10.1155/2013/749629
Rashmi, K. S., RaviKumar, K. L., and Bhagyashree, H. N. (2013). Asymptomatic bacteriuria in HIV/AIDS patients: occurrence and risk associated with low CD4 counts. J. Evol. Med. Dental Sci. 2, 3358–3367. doi: 10.14260/jemds/705
Sahu, C., Jain, V., Mishra, P., and Prasad, K. N. (2018). Clinical and laboratory standards institute versus European committee for antimicrobial susceptibility testing guidelines for interpretation of carbapenem antimicrobial susceptibility results for Escherichia coli in urinary tract infection (UTI). J. Lab. Phys. 10, 289–293. doi: 10.4103/JLP.JLP_176_17
Schönwald, S., Begovac, J., and Skerk, V. (1999). Urinary tract infections in HIV disease. Int. J. Antimicrob. Agents 11, 309–311. doi: 10.1016/S0924-8579(99)00036-9
Simeneh, E., Gezimu, T., Woldemariam, M., and Alelign, D. (2022). Magnitude of multidrug-resistant bacterial uropathogens and associated factors in urinary tract infection suspected adult HIV-positive patients in southern Ethiopia. The Open Microbiol. J. 16:180. doi: 10.2174/18742858-v16-e2208180
Skrzat-Klapaczyńska, A., Matłosz, B., Bednarska, A., Paciorek, M., Firlag-Burkacka, E., Horban, A., et al. (2018). Factors associated with urinary tract infections among HIV-1 infected patients. PLoS ONE 13:e0190564. doi: 10.1371/journal.pone.0190564
Tessema, N. N., Ali, M. M., and Zenebe, M. H. (2020). Bacterial associated urinary tract infection, risk factors, and drug susceptibility profile among adult people living with HIV at Haswassa University Comprehensive Specialized Hospital, Hawassa, Southern Esthiopia. Sci. Rep. 10:0790. doi: 10.1038/s41598-020-67840-7
Tolkoff-Rubin, N. E., and Rubin, R. H. (1997). Urinary tract infection in the immunocompromised host: Lessons from kidney transplantation and the AIDS epidemic. Inf. Dis. Clin. North Am. 11, 707–717. doi: 10.1016/S0891-5520(05)70381-0
Tuem, K. B., Desta, R., Bitew, H., Ibrahim, S., and Hishe, H. Z. (2019). Antimicrobial resistance patterns of uropathogens isolated between 2012 and 2017 from a tertiary hospital in Northern Ethiopia. J. Glob. Antimicrob. Resist. 18, 109–114. doi: 10.1016/j.jgar.2019.01.022
Widmer, T. A., Theron, G., and Grove, D. (2010). Prevalence and risks of asymptomatic bacteriuria among HIV-positive pregnant women. Southern Afr. J. Epidemiol. Inf. 25, 28–32. doi: 10.1080/10158782.2010.11441374
Woldemariam, H. K., Geleta, D. A., Tulu, K. D., Aber, N. A., Legese, M. H., Fenta, G. M., et al. (2019). Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Inf. Dis. 19, 1–10. doi: 10.1186/s12879-018-3669-5
Worku, S., Derbie, A., Sinishaw, M. A., Adem, Y., and Biadglegne, F. (2017). Prevalence of bacteriuria and antimicrobial susceptibility patterns among diabetic and nondiabetic patients attending at Debre Tabor Hospital, Northwest Ethiopia. Int. J. Microbiol. doi: 10.1155/2017/5809494
Yeshitela, B., Gebre-Selassie, S., and Feleke, Y. (2012). Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in Tikur Anbessa Specialized University Hospital, Addis Ababa, Ethiopia. Ethiopian Med. J. 50, 239–249.
Keywords: urinary tract infection, correlation, CD4 count, viral load, antimicrobial resistance, Ethiopia
Citation: Hantalo AH, Shano AK and Meja TI (2024) Correlation of CD4+ count and viral load with urinary tract infection and antimicrobial resistance pattern of bacterial uropathogens among HIV patients in Wolaita Sodo, South Ethiopia. Front. Microbiol. 15:1363287. doi: 10.3389/fmicb.2024.1363287
Received: 30 December 2023; Accepted: 16 February 2024;
Published: 30 April 2024.
Edited by:
Santi M. Mandal, Indian Institute of Technology Kharagpur, IndiaReviewed by:
Samuel Sunday Taiwo, Ladoke Akintola University of Technology, NigeriaAhmed Abduljabbar Jaloob Aljanaby, University of Kufa, Iraq
Copyright © 2024 Hantalo, Shano and Meja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Admasu Haile Hantalo, YWRtYXN1LndrdS5lZHUuZXRAZ21haWwuY29t
 Admasu Haile Hantalo
Admasu Haile Hantalo Abera Kumalo Shano
Abera Kumalo Shano Tekilu Israel Meja3
Tekilu Israel Meja3