- 1School of Pharmaceutical Sciences, Sun Yat-sen University, Shenzhen, China
- 2State Key Laboratory of Integration and Innovation of Classic Formula and Modern Chinese Medicine, Lunan Pharmaceutical Group Co., Ltd., Linyi, China
- 3International Pharmaceutical Engineering Laboratory in Shandong Province, Shandong New Time Pharmaceutical Co., Ltd., Linyi, China
- 4Nanchang Research Institute, Sun Yat-sen University, Jiangxi, China
Background: Helicobacter pylori (H. pylori) is thought to primarily colonize the human stomach and lead to various gastrointestinal disorders, such as gastritis and gastric cancer. Currently, main eradication treatment is triple or quadruple therapy centered on antibiotics. Due to antibiotic resistance, the eradication rate of H. pylori is decreasing gradually. Therefore, searching for anti-H. pylori drugs from herbal sources has become a strategy for the treatment. Our team proposed a Hezi Qingyou Formula (HZQYF), composed of Chebulae Fructus, Ficus hirta Vahl and Cloves, and studied its anti-H. pylori activity and mechanism.
Methods: Chemical components of HZQYF were studied using UHPLC–MS/MS and HPLC. Broth microdilution method and agar dilution method were used to evaluate HZQYF’s antibacterial activity. The effects of HZQYF on expression of adhesion genes (alpA, alpB, babA), urease genes (ureE, ureF), and flagellar genes (flaA, flaB) were explored using Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) technology. Effects on morphology and permeability of the extracellular membrane were studied using scanning electron microscopy (SEM) and N-phenylnaphthalen-1-amine (NPN) uptake. Effect on urease activity was studied using a urease kinetics analysis in vitro. Immunofluorescence staining method was used to examine the effect on adhesion. Western blot was used to examine the effect on cagA protein.
Results: Minimum inhibitory concentration (MIC) values of the formula against H. pylori clinical strains and standard strains were 80–160 μg/mL, and minimum bactericidal concentration (MBC) values were 160–320 μg/mL. The formula could down-regulate the expression of adhesion genes (alpA, alpB, babA), urease genes (ureE, ureF) and flagellar genes (flaA, flaB), change the morphology of H. pylori, increase its extracellular membrane permeability, and decrease its urease activity.
Conclusion: Present studies confirmed that HZQYF had promising in vitro anti-H. pylori activities and demonstrated its possible mechanism of action by down-regulating the bacterial adhesion, urease, and flagellar gene expression, which provided scientific bases for further clinical investigations.
1 Introduction
Helicobacter pylori (H. pylori) is a Gram-negative microaerobic bacterium with a flagellum and multiple virulence factors. Currently, H. pylori infects more than half of the world’s population, with an infection rate of 56.22% in China (Nagy et al., 2016). H. pylori infection has been linked to a variety of gastrointestinal and extra-gastrointestinal diseases, including adverse metabolic characteristics in obese people, type II diabetes, ischemic heart disease, anemia, gastric ulcers, etc. (Bravo et al., 2018). Research indicated that eradicating H. pylori infection can prevent the development of various gastric diseases (Hu et al., 2017), such as peptic ulcers (Liu et al., 2017) and gastric cancer (Li et al., 2021). Currently, antibiotics are used in the majority of H. pylori infection treatment regimens, including standard triple and bismuth quadruple therapy (Malfertheiner et al., 2016). However, the worldwide abuse of antibiotics has led to the growth of H. pylori’s resistance toward commonly used antibiotics, resulting in a significant obstacle in the successful treatment of H. pylori infection (Thung et al., 2016). Previous studies indicated that a significant proportion of treatments, ranging from 10 to 30 percent of the initial and subsequent treatment plans proposed in global treatment guidelines have been proven ineffective over the past decade (Li et al., 2015; Malfertheiner et al., 2016). In instances where the antibiotics-centered treatment fails to achieve a cure of H. pylori infection, the absence of any reliable therapeutic alternatives poses considerable challenges for patients in terms of their treatment options (Shiotani et al., 2017; Fallone et al., 2019). Searching for anti-H. pylori drugs from herbal medicines has been recommended as an effective strategy (Ghasemian et al., 2019). There are various herbal medicines, such as Coptis chinensis Franch (Huanglian) and Scutellaria baicalensis Georgi (Huangqin), showing potent anti-H. pylori activity (Ma, 2010). However, their primary antimicrobial ability lies in direct bactericidal action, which is similar to the action of antibiotics. Therefore, despite their strong bactericidal capabilities, prolonged use of these medicines can also lead to toxic side effects such as diarrhea. Consequently, it is more beneficial to look for Chinese herbal medications with improved anti-H. pylori activities and decreased side effects.
In addition, the integration of traditional Chinese medicine with antibiotics has emerged as a novel approach in antimicrobial therapy. Extensive research has demonstrated that the concurrent administration of traditional Chinese medicine and antibiotics can significantly enhance the eradication efficacy of H. pylori when compared to the conventional clinical antibiotic triple or quadruple therapy (Xue et al., 2023).
We have discovered a Chinese medicine formula from clinical practice, which had effectively reduced the load of H. pylori in the stomach of infected individuals. Furthermore, it was found in long-term clinical observations that patients using this formula indeed experienced very few side effects such as diarrhea. The formula is composed of Chebulae Fructus, Ficus hirta Vahl, and Cloves, which is called “Hezi Qingyou Formula (HZQYF).”
Chebulae Fructus, also known as Shijunzi or Rongmao Chebulae Fructus, refers to the ripe dried fruit of the plant belonging to the Rutaceae family. Chebulae Fructus is listed in various editions of the “Pharmacopoeia of the People’s Republic of China.” Our team has discovered that Chebulae Fructus exhibits a good anti-H. pylori effect in vitro (Ou et al., 2023), with an MIC for antibacterial activity at approximately 20–160 μg/mL.
Ficus hirta Vahl, also known as Five Fingered Gourd, is the root of Ficus simplicissima Lour., a plant of the mulberry family (Moraceae). Research indicates that the water extract of Ficus hirta Vahl inhibits the growth of some common bacteria, such as Escherichia coli, Bacillus subtilis, and Staphylococcus aureus. Modern pharmacology indicates that Ficus hirta Vahl has several beneficial effects, including enhancing the immune system, antibacterial properties, protecting the gastric mucosa, improving microcirculation, and acting as an antioxidant (Au, 2009; Zeng et al., 2012; Ma et al., 2017). Therefore, in the formulation of HZQYF, the role of Ficus hirta Vahl in terms of antibacterial action is not the primary focus, and enhancing immune function should be the main consideration.
Cloves refer to the dried flower buds of the plant Eugenia caryophyllata Thunb. from the Myrtaceae family. As a traditional Chinese medicine, cloves are used to alleviate symptoms such as spleen and stomach ailments, belching, and vomiting. Recent research has shown that cloves have various potential therapeutic properties, including antiviral, antifungal, anticancer, antioxidant, and anti-inflammatory effects (Zaidi et al., 2012). Our previous research indicated that cloves exhibit promising anti-H. pylori activity (Peng et al., 2022). They also show potential for alleviating inflammation caused by H. pylori infection and enhancing the innate immune system’s function (Peng et al., 2023). Cloves in the HZQYF may possess both antibacterial effects and the ability to ease gastrointestinal symptoms, as well as enhance host innate immunity.
Among the three herbs in the HZQYF, Chebulae Fructus not only has a strong bactericidal effect but also exhibits excellent anti-diarrheal properties. Cloves have certain antibacterial effect and immune enhancement, while Ficus hirta Vahl plays a significant role in enhancing physical fitness and immune function. Therefore, the combination of them should indeed be effective in terms of antibacterial activity. At the same time, it can reduce some side effects by regulating the intestinal flora. As a traditional Chinese medicine formula for treating H. pylori infection, its antibacterial ability remains the focus of this study, and research on immune enhancement will be published separately.
The purpose of this study was to investigate the anti-H. pylori activity of the water extract of HZQYF and explore its potential mechanism of action.
2 Materials and methods
2.1 Chemicals and reagents
The reagents and materials were obtained as follows.
Sterile-defibrinated sheep blood (Hongquan Biotechnology Co., Ltd., Guangzhou, Guangdong, China). Fetal bovine serum (FBS) (Inner Mongolia Jin Yuan Kang Bioengineering Co., Ltd., Inner Mongolia, China). Brain heart infusion (BHI), MUELLER-HINTON (MH)AGAR and Columbia agar base (Oxiod Ltd. in Basingstoke, Hampshire, UK). Clarithromycin (CLR) and metronidazole (MET) (Macklin Biochemical Co., Ltd., Shanghai, China). Amoxicillin (AMO) (National Institutes for Food and Drug Control, Beijing, China). Levofloxacin (LEF) (Target Molecule Corp., Boston, MA, USA). Phosphate buffered saline (PBS) (Cytiva, Shanghai, China). Purelink RNA kit and BCA protein assay kit (Thermo Fisher Scientific, USA). SYBR Premix Ex Tap™ kit and PrimeScript RT reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Minamikusatsu, Japan). Water (Sangon Biotech, Shanghai, China). Urea and acetohydroxamic acid (Shanghai McLean Company, Shanghai, China). N-Phenyl-1-naphthylamine (Aladdin Bio-Chem Technology Co., LTD., Shanghai, China). Tween-20 (Biotopped, Beijing, China). Phenol red (Sigma). Electron microscope fixative (Servicebio, Beijing, China). Chebulic acid, chebulanin, and ellagic acid (Shandong Bokang Jingxi Chemical Co., Ltd., Liaocheng, China). Gallic acid and corilagin (National Institutes for Food and Drug Control, Beijing, China). 4% Paraformaldehyde Fix Solution, DAPI Staining Solution, Antifade Mounting Medium, RIPA reagent, PMSF, 1% protease inhibitor cocktail, SDS-PAGE Gel Preparation Kit, BeyoColor™ Prestained Color Protein Marker, BeyoECL Plus Kit, and the second antibodies (1:2500) against mouse (A0216) (Beyotime Institute of Biotechnology, Shanghai, China). FITC (Biotopped Life sciences, Beijing, China). Roswell Park Memorial Institute (RPMI) 1640 (Gibco-Life Technologies LLC., Rockville, USA). Anti-H. pylori CagA (sc-28368) (Santa Cruz Biotechnology, Texas, USA).
2.2 Plant materials and sample preparation
Chebulae Fructus and Ficus hirta Vahl (Root) were purchased from Guangzhou Zhining Pharmaceutical Co., Ltd. Cloves (flower bud) was purchased from Guangzhou Junyuan Chenxiangshan Traditional Chinese Medicine Herbal Pieces Co., Ltd.
The preparation of HZQYF follows the water extraction method. Firstly, Chebulae Fructus, Ficus hirta Vahl, and Cloves were separately ground and passed through an 80-mesh sieve. 10 g of HZQYF (Ficus hirta Vahl:Chebulae Fructus:Cloves = 6:3:1) were weighed precisely and 10–12 times of water in volume was added. Then the mixture was extracted three times at 90°C for 1 h each time, and the suspension was filtered to obtain the supernatant. The supernatant was subjected to rotary evaporation (N-1300, EYELA, Japan) at 0.08 MPa and 55°C. Afterwards, the concentrate was freeze-dried into a powdered form, and stored at −20°C for future use.
2.3 Composition of the herbal extract
Components in the extracts of HZQYF were identified by UHPLC–MS/MS (Thermo scientific). 1 mg/mL of HZQYF solution was prepared in methanol–water (15:85, v/v) and injected into LC–MS system after filtered through a 0.22-μm membrane filter (Sartorius). The LC–MS analysis was performed on an ACQUITY UPLC BEH C18 column (Waters, 2.1*100 mm,1.7 μm) at 30°C. The mobile phase was water containing 0.2% acetic acid (solvent A) and methanol (solvent B) and pumped at a flow rate of 0.2 mL/min. The gradient elution was as follows: 0–5 min, 5–15% mobile phase B; 5–10 min, 15–25% mobile phase B; 10–20 min, 25–60% mobile phase B; 20–35 min, 60–90% mobile phase B; 35–45 min, 90% mobile phase B; 45.1–60 min, 5% mobile phase B. Mass spectrum was performed via an ESI source. Master scan was Full MS monitoring, scan range of mass-to-charge ratio was 100–1,500. The spray voltage was set at 3.5 kV in positive ion mode and 3.0 kV in negative ion mode, ion transfer tube temperature at 325°C and vaporizer temperature at 300°C.
Three batches of the HZQYF were ultrasonically dissolved in ultrapure water to obtain solutions with a concentration of 200 μg/mL individually. A mixed standard containing 0.2 mg/mL of chebulic acid, 0.2 mg/mL of gallic Acid, 0.2 mg/mL of corilagin, 0.08 mg/mL of chebulanin, 0.6 mg/mL of ellagic acid was prepared. The standard and samples were filtered through a 0.22-μm membrane filter before injected into HPLC. The HPLC analysis was performed with a Chromeleon Workstation (UltiMate 3000, USA). Reverse phase chromatography was used under gradient conditions with an Accalim C18 column (Thermo, 4.6 mm × 250 mm, 5-μm) at 25°C. The wavelength of 270 nm was chosen as the detection wavelength according to the UV absorption curve. The mobile phase was water containing 0.1% trifluoroacetic acid (solvent A) and acetonitrile (solvent B). The gradient was composed of 3 to 30% (B) from 0 to 40 min. The flow rate was 1.0 mL/min and the volume injected was 20 μL. The detected compounds were identified by comparing their retention time with those of the standard. The contents of the compounds were expressed as micrograms per gram of extracts by correlating the peak areas with those of the reference substances.
2.4 Helicobacter pylori strains and growth conditions
The standard strains of H. pylori, ATCC 43504, ATCC 700392, and SS1, were obtained from the American Type Culture Collection (ATCC) in Manassas, Virginia, USA. Clinical isolated strains, ICDC111001 and CSO1, were obtained from Guangzhou University of Chinese Medicine and University of Shanghai for Science and Technology, respectively. Clinical isolated strains, QYZ-001, QYZ-003, and QYZ-004, were obtained from Qingyuan Hospital of Traditional Chinese Medicine in Guangzhou.
All clinical strains were maintained at −80°C in a combination of 65% BHI, 25% glycerol and 10% FBS (v/v/v), and confirmed by the providers through morphological inspection, Gram staining, and biochemical reaction. The European Committee on Antimicrobial Susceptibility Testing’s (EUCAST 2019) breakpoint values for antibiotic resistance have been developed. For the current study, all strains were cultured in Columbia agar base supplemented with 5% sterile defibrinated sheep blood at 37°C in a tri-gas incubator (CB170, BINDER, Germany) containing 10% CO2, 5% O2, and 85% N2. As a result of prior whole-genome random sequencing, annotation, and re-annotation of the H. pylori ATCC 700392 strain genomes, it was chosen for RT-qPCR detection and transcriptome analysis. Additionally, it is a reference strain that is resistant to none of the tested antibiotics, hence it was also utilized in checkerboard analysis and drug resistance studies.
2.5 Minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC)
The most frequently used method micro-broth dilution and agar dilution method were used in this study to detect the MIC.
Ninety-six-well (96-well) microtiter plates with 50 μL in each well were prepared with twofold serial dilutions of the test substances. In order to achieve a final concentration of 1 × 106 CFU/mL, a 2-day-old H. pylori solid culture was obtained in BHI with 20% FBS and inserted into the 96-well microtiter plates with 50 μL in each well. The plates were incubated for 3 days at 37°C in microaerophilic environment with constant 150 rpm shaking. The plates were examined optically after incubation, and the lowest level that produced no turbidity was defined as MIC. Each batch of HZQYF was also tested with clarithromycin as a quality control measure. Additionally, growth controls without any test chemicals and negative controls using HZQYF dissolved in blank medium without microbes were required. The experiments were performed three times. Following the measurement of MIC values using the broth microdilution method, the MBC was established. In short, 100 μL of a solution containing 1, 2, or 4 times the MIC of HZQYF were withdrawn from the 96-well microtiter plate and cultivated for 3 days at 37°C in a microaerophilic environment. A 99.9% decline in viability when compared to the control group was defined as the MBC value. This experiment was conducted three times.
Different H. pylori strains were adjusted to 2 McF using DensiCHEK Plus densicheck (BioMerieux, French) and then 100 μL of them were added to MH blood plate that contain different concentrations of HZQYF (80, 160, 320 μg/mL). After 72 h’s cuture, the MICs were defined by the concentration of HZQYF when there was no colony growth on the surface of the blood plate or there was a significant decrease in the number of colonies. And clarithromycin was used as the positive control in each experiment.
2.6 Inhibiting kinetics and killing kinetics assays
Assays for killing kinetics and inhibiting kinetics were carried out as follows. H. pylori ATCC 43504 and ATCC 700392 were subjected to water (control), CLR (for ATCC 43504 is 0.016 μg/mL and for ATCC 700392 is 0.004 μg/mL) and the HZQYF extracts (40, 80, 160 μg/mL) in BHI broth containing 10% FBS while being shaken at 150 rpm in the tri-gas incubator for the inhibitory kinetics experiments. Then, 100 μL of each sample was pipetted for absorbance measurement at 600 nm using POLARstar Omega (BGM Labtech) at 0, 8, 24, 32, 46, 48, 50, 52, 56, and 72 h. This experiment was repeated three times.
Helicobacter pylori ATCC 43504 and ATCC 700392 were inoculated in BHI broth with 10% FBS as part of the killing kinetics experiments. Each sample had 50 μL withdrawn for a series of 10-fold dilutions after being exposed to water (control) or the HZQYF extracts (320, 640, 1,280 μg/mL) for 0, 12, 24, 36, 48, 60, or 72 h. Following that, single colonies were created by plating 100 μL dilutions for 5 days on solid agar. Automatic Colony Counter (count 60, China) was used to count the colonies, and the findings were represented as Log (CFU/mL). This experiment was repeated twice.
2.7 Combination with antibiotics
According to a known approach with minor modifications, the effects of HZQYF extracts coupled with antibiotics were assessed. To figure out if HZQYF extracts and antibiotics work together synergistically to treat H. pylori infection, six serial, two-fold dilutions of HZQYF extracts were made. The antibiotics included were clarithromycin, metronidazole, amoxicillin, and levofloxacin. A total of 60 μL of H. pylori ATCC 700392 bacterium suspension was placed in each well of a 96-well plate, along with 30 μL of HZQYF (0, 160, 320, 640, 1,280, 2,560 μg/mL) extracts and 30 μL of antibiotics to achieve a final bacteria concentration of around 1 × 106 CFU/mL. The concentration ranges of CLR, LEF, MTZ and AMO were from 0 to 0.008 μg/mL, 0 to 0.16 μg/mL, 0 to 12.8 μg/mL, and 0 to 0.4 μg/mL, respectively. The plates were then incubated for 3 days at 37°C, in the same gas atmosphere as described before and shaken at 150 rpm. The fractional inhibitory concentration index (FICI) was created to assess the interactions between HZQYF and antibiotics.
FICI = MIC (A combined)/MIC (A alone) + MIC (B combined)/MIC (B alone).
FICI ≤0.5 was considered synergistic, 0.5 < FICI ≤1 was additive, 1 < FICI ≤2 was indifferent, and FICI >2 was antagonistic. This experiment was repeated three times.
2.8 RT-qPCR for detection of virulence gene expression
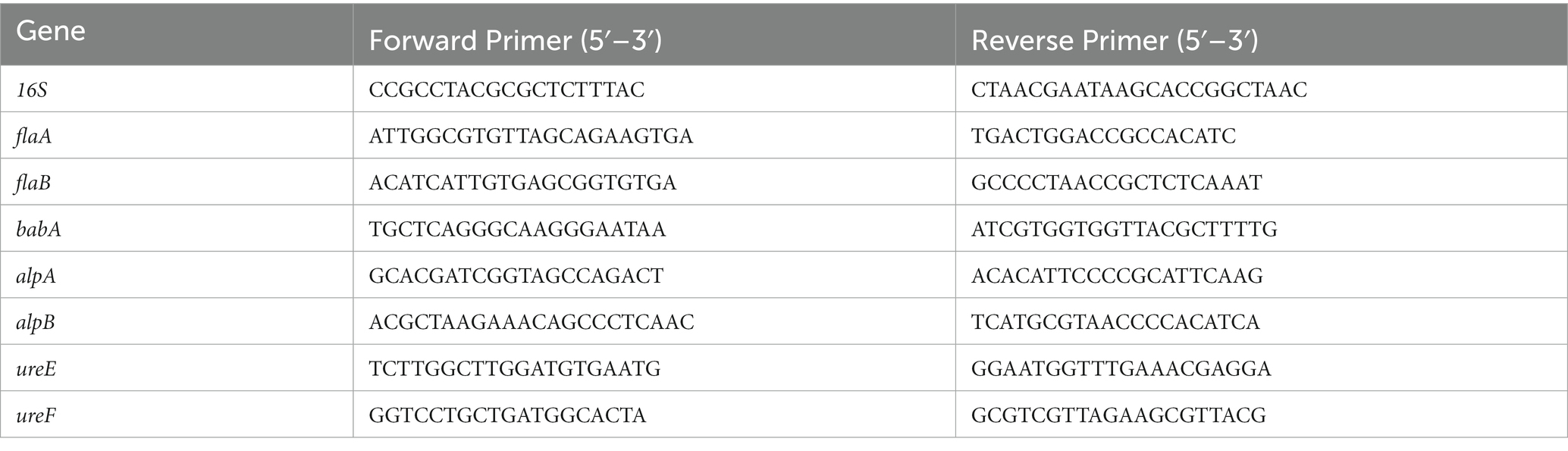
To collect enough bacteria, H. pylori ATCC 700392 was adjusted to 1 McF using DensiCHEK Plus densicheck (BioMerieux, French) and cultured for 2 days. In a second experiment, 1 mL of bacterial solution (OD600 = 0.5–0.6) was diluted 50-fold in BHI broth adding 10% FBS with or without the MIC of HZQYF for 24 h. The sample’s total RNA was extracted using Purelink RNA kit. Their concentrations were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). On a Thermo Scientific 7,500 Fast Real-Time PCR System (Quantitative Real-Time PCR System), RT-qPCR was carried out using the SYBR Premix Ex Tap™ kit (Takara) and following the manufacturer’s instructions. The 2−ΔΔCt method was used to examine the data. Table 1 lists the particular primers used (Shu et al., 2016) to amplify mRNA fragments. The expression regulation of these genes was assessed in comparison to bacteria from the control group following normalization with the reference gene 16S.
2.9 Scanning electron microscope (SEM)
The impacts of HZQYF on H. pylori morphology and ultrastructure were studied using SEM, as previously described (Shen et al., 2021). Initially, in order to grow enough bacteria, H. pylori ATCC 700392 was diluted to 1 McF in BHI including 10% FBS and cultured for 24 h under microaerophilic conditions with shaking at 150 rpm. In a second experiment, 1 mL of bacterial solution (OD600 = 0.5–0.6) was diluted 50-fold in BHI broth adding 10% FBS with or without the MIC dosage of HZQYF extract for 24 h. After centrifuging the bacteria for 10 min at 6000 rpm, they underwent two PBS washes. They were then left to cure for the night at 4°C in 2.5% glutaraldehyde. Before being lyophilized and fixed, the materials underwent a graded ethanol sequence of dehydration. The specimens were examined using a Sigma500 scanning electron microscope (ZEISS, Germany) after metal spraying.
2.10 The impact of cell membrane permeability
The determination of the penetration ability of HZQYF on the outer membrane of H. pylori was done by NPN uptake method. H. pylori was scraped from blood agar plates and adjusted to an optical density of 1 McF using DensiCHEK Plus densicheck (BioMerieux, French). Different concentrations of HZQYF extracts (80 and 160 μg/mL) were added, and a growth control was set. After incubation in a tri-gas incubator for 24 h, the samples were centrifuged at 6000 rpm for 10 min and washed twice with PBS buffer. Then, they were resuspended in PBS. The bacterial solution and 40 μM NPN were added into a 96-well plate, and incubated at 37°C for 30 min before conducting fluorescence measurements. The fluorescence measurements are performed using a multifunctional microplate reader with an excitation wavelength of 350 nm, an emission wavelength of 420 nm, and an excitation bandwidth of 5 nm. All measurements are repeated three times.
2.11 Measurement of urease enzymatic activity
The method of urease determination in this study were based on a previous study with slight modification (Debowski et al., 2017). The strains cultured on blood plates for 48 h were scraped, the turbidity was adjusted to 1 McF using DensiCHEK Plus densicheck (BioMerieux, French), and the same volume of 80 μg/mL and 160 μg/mL aqueous extracts of HZQYF and the bacterial solution were added 1:1 in 6-well plates. A growth control group and a positive control group (40 μg/mL and 80 μg/mL of acetohydroxamic acid) were set up and placed into the triple-air incubator for 24 h at 150 rpm. The collected bacterial solution was centrifuged at 6000 rpm for 10 min, washed twice with PBS, resuspended in 1 mL of PBS after centrifugation, and adjusted to OD600 = 0.2. Then, 50 μL of the bacterial suspension was combined with 50 μL of buffer B (pH 6.8, 25 mM phosphate buffer, 0.2% Tween-20). The diluted suspension was placed into a 96-well plate and diluted with 150 μL of buffer C (pH 6.8, 25 mM phosphate buffer, 250 M phenol red), which was then incubated at 37°C for 5 min. A POLARstar Omega (BGM Labtech) plate equipment was used to measure the absorbance at 560 nm every 72 s for 75 cycles after adding 75 μL of a urea solution (0.5 mM) to the wells. The activity was measured as a percentage of the urease activity of the growth control strain and was reported as the rate of change in absorbance over time. The experiment was performed at least three times, and all urease activity measurements were made in duplicate.
2.12 Effect on cagA protein expression
In order to detect the expression of the H. pylori virulence protein cagA after drug treatment, 1 McF H. pylori strains (ATCC 43504 &ATCC 700392) were added into 6-well plates and exposed to water (control) and the HZQYF extracts (80, 160, 320 μg/mL) in BHI broth containing 10% FBS, shaking at 150 rpm in the tri-gas incubator for 24 h. Then, the bacteria of each well were collected after centrifuged at 6000 rpm for 10 min using centrifuging (HERMLE, Germany) and washed twice with PBS. Next, each sample was lysed using 120 μL RIPA reagent containing 1 mM PMSF and 1% protease inhibitor cocktail for bacterial extracts. The protein concentration of the lysate was determined utilizing a BCA protein assay kit. Subsequently, 30 μg of the proteins were loaded onto SDS-PAGE gels for separation and transferred to PVDF membranes. Thereafter, membranes were blocked using 5% skim milk powder for 1 h at room temperature and then blotted with anti-H. pylori CagA primary antibodies at 4°C overnight. After that, the membranes were incubated with second antibodies (1:2500) against mouse for 1.5 h at room temperature. Finally, the protein bands were visualized by BeyoECL Plus Kit and exposed with a ChemiScope 6200 (Bio-Rad, Hercules, CA, USA). The IntDen values of the bands were analyzed by ImageJ software.
2.13 Effect on adhesion ability
This method refers to Jia’s previous studies (Jia et al., 2022). The GES-1 cells (4 × 105 /well) were seeded into 6-well plates for 24 h and then they were treated with different concentrations of HZQYF (0, 80, 160 and 320 μg/mL). 4 h later, they were infected with ATCC 700392 which was stained with 1% FITC, at a MOI of 100 for 8 h. Next, the mixture was fixed with 4% Paraformaldehyde Fix Solution for 20 min after unadhered H. pylori were removed. Subsequently, the 4% paraformaldehyde was discarded. And DAPI Staining Solution and Antifade Mounting Medium were added to stain the cells. Finally, fluorescence signals were analyzed using IM-5FLD Fluorescent inverted microscope (OPTIKA, Italy).
2.14 Statistics analysis
Experimental results were analyzed with GraphPad 9.0.0 software and presented as mean ± standard deviation (SD). For the statistical analysis, a one-way ANOVA method based on the Kruskal-Wallis test was applied, and a post-hoc test was then conducted. A statistically significant difference was shown by p < 0.05.
3 Results
3.1 Composition of the herbal extract
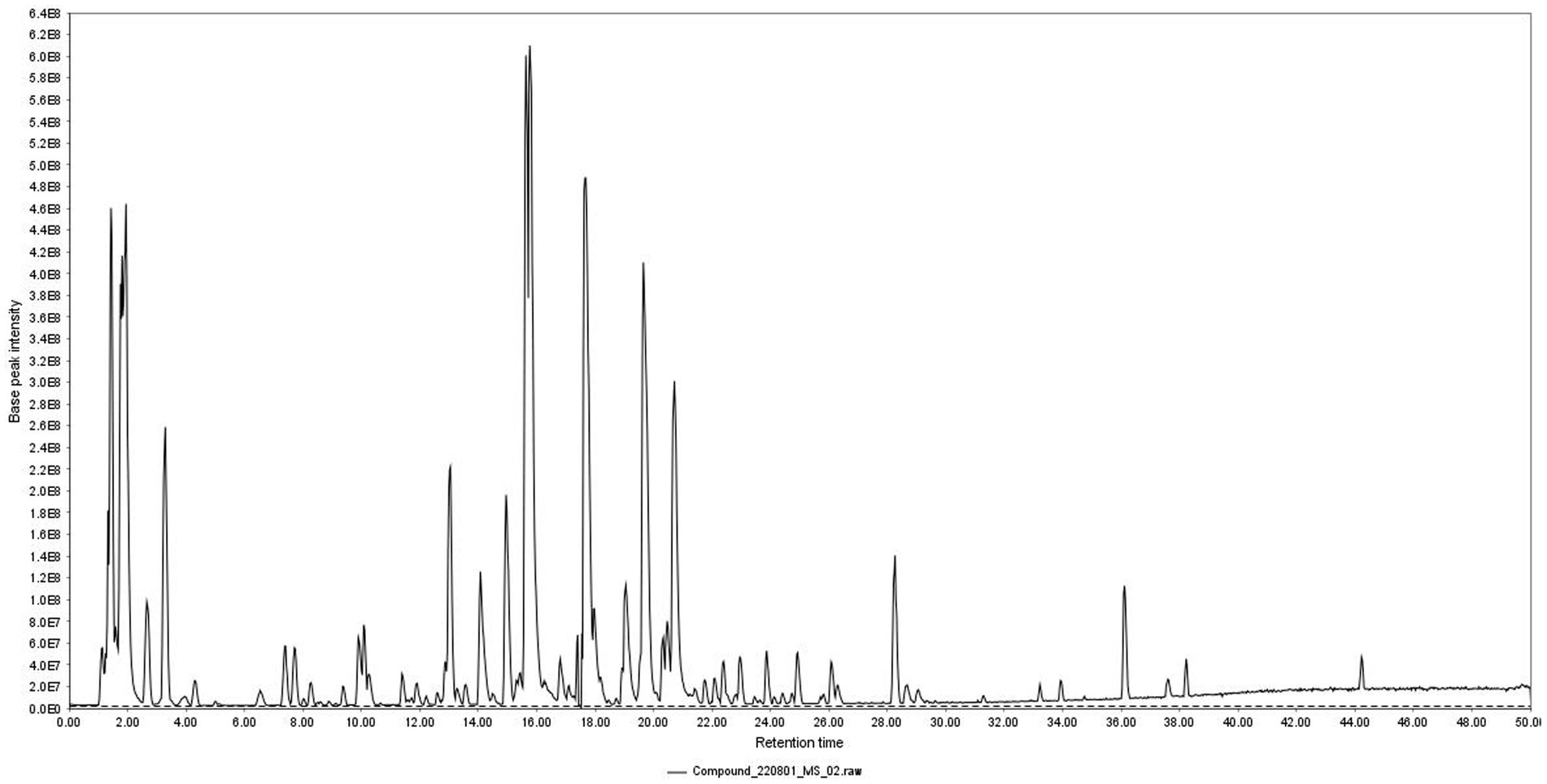
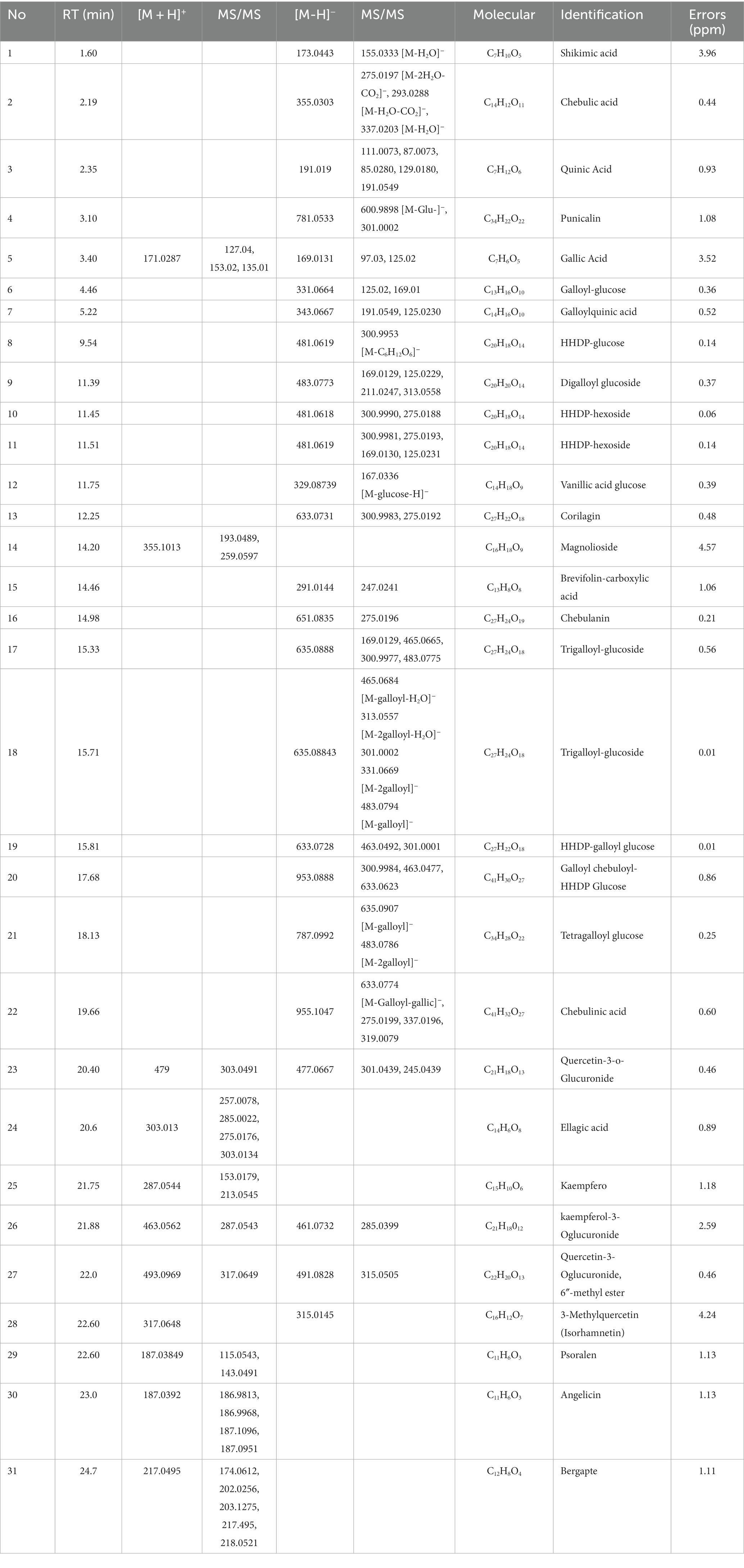
Total ion chromatogram of HZQYF was displayed in Figure 1. 31 primary components were identified by UHPLC–MS/MS and listed in Table 2.
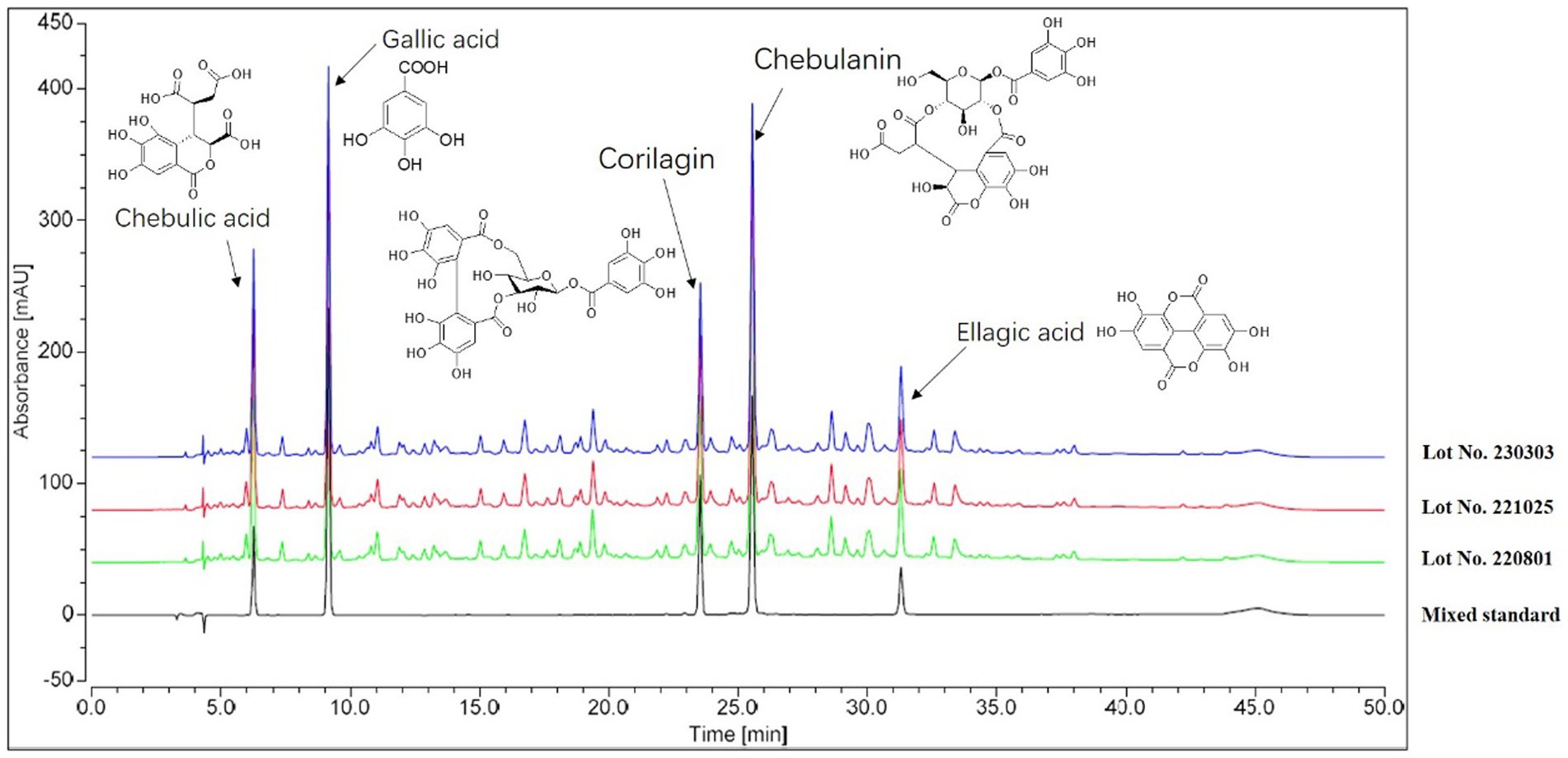
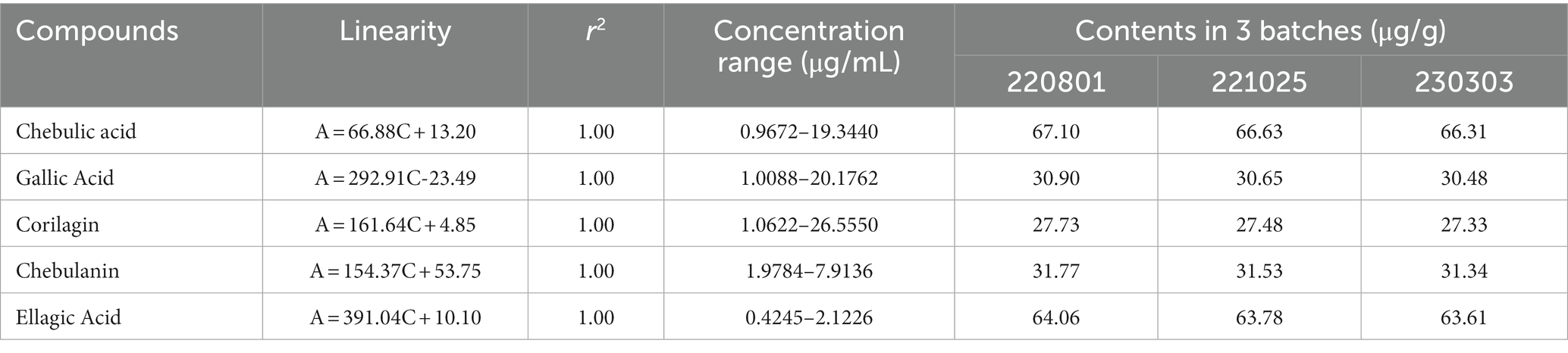
In order to ensure the consistency of the components in the extracts, three independent batches of HZQYF were separately prepared and then analyzed by HPLC. The HPLC chromatograms of the three separate lots of HZQYF are showed in Figure 2, and the quantitative results are listed in Table 3. The results indicate the composition of the five major components were consistent across various HZQYF batches.
3.2 MIC and MBC
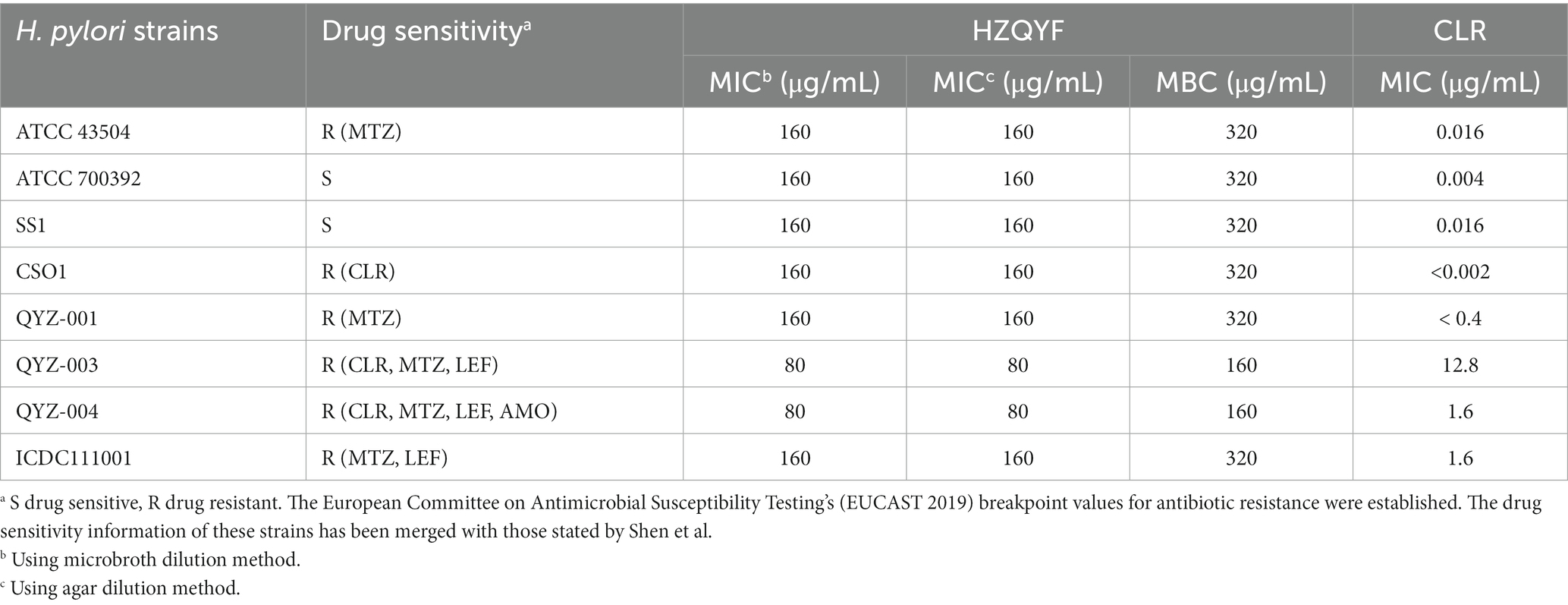
The antibacterial properties of HZQYF against multiple strains of H. pylori were assessed through the determination of their MIC and MBC. The MIC of HZQYF against three standard H. pylori strains and five clinically isolated strains ranged from 80 to 160 μg/mL (Table 4). The MBC of HZQYF against multiple H. pylori strains ranged from 160 to 320 μg/mL, indicating that HZQYF exhibited both bacteriostatic and bactericidal effects. In conclusion, these findings demonstrated that HZQYF has the ability to inhibit various strains of H. pylori, irrespective of their antibiotic resistance.
3.3 Inhibiting kinetics and killing kinetics assays
Based on the findings of the Figures 3A,B, the inhibitory activity of HZQYF on the ATCC 43504 and ATCC 700392 strains exhibited a dose-dependent relationship. In the case of ATCC 43504, a concentration of ½ MIC demonstrated a significant ability to impede bacterial growth, whereas a concentration of ¼ MIC exhibited a mild antibacterial effect on H. pylori, primarily characterized by a reduction in the final bacterial count. Regarding ATCC 700392, concentrations of ½ MIC and ¼ MIC displayed slight inhibitory effects on the bacterial strain. However, both MICs can significantly inhibit the growth of the above two bacterial strains, and their inhibitory effects were comparable to clarithromycin.
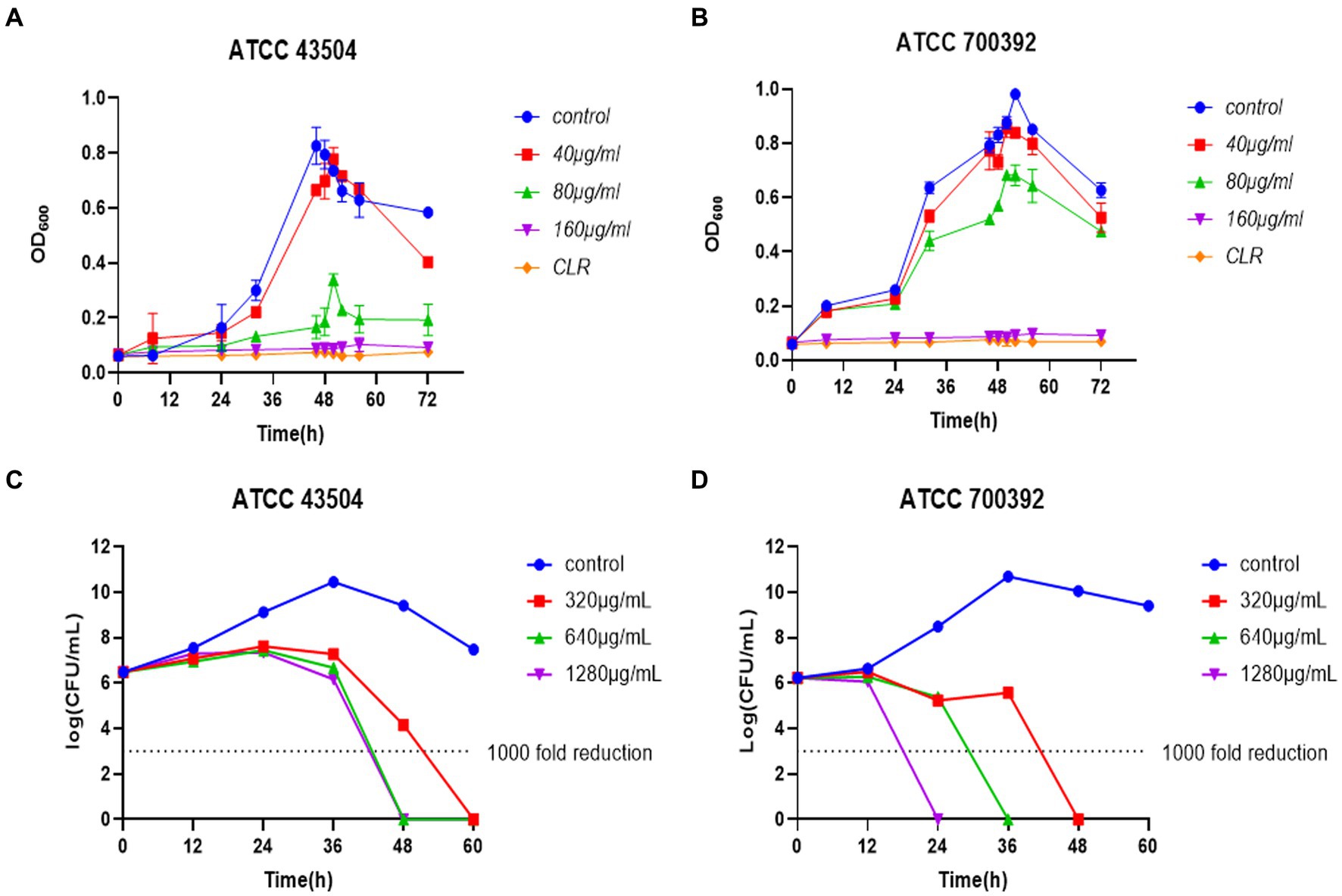
Figure 3. (A) Inhibiting kinetics curves of HZQYF on ATCC 43504. Concentration of CLR was 0.016 μg/mL. (B) Inhibiting kinetics curves of HZQYF on ATCC 700392. Concentration of CLR was 0.004 μg/mL. (C) Killing kinetics curves of HZQYF on ATCC 43504. (D) Killing kinetics curves of HZQYF on ATCC 700392.
A time-killing curve was created by counting bacterial colonies at different times. As shown in Figures 3C,D, a concentration of 320 μg/mL (MBC) of HZQYF can completely kill H. pylori within 60 h for ATCC 43504, and higher concentrations of 640 μg/mL (2MBC) and 1,280 μg/mL (4MBC) can kill the H. pylori within 48 h. HZQYF had a greater bactericidal effect on ATCC 700392 than on ATCC 43504. In the case of ATCC 700392, a concentration of 1,280 μg/mL (4MBC) of HZQYF can eliminate the H. pylori within 24 h, 640 μg/mL can eradicate the H. pylori within 36 h, and 320 μg/mL (MBC) can eliminate the H. pylori within 48 h. The treatments yielded a reduction of over 1,000-fold in the bacterial count at the conclusion of the experiments, in comparison to the initial inoculation.
3.4 Effects of HZQYF combined with antibiotics on Helicobacter pylori
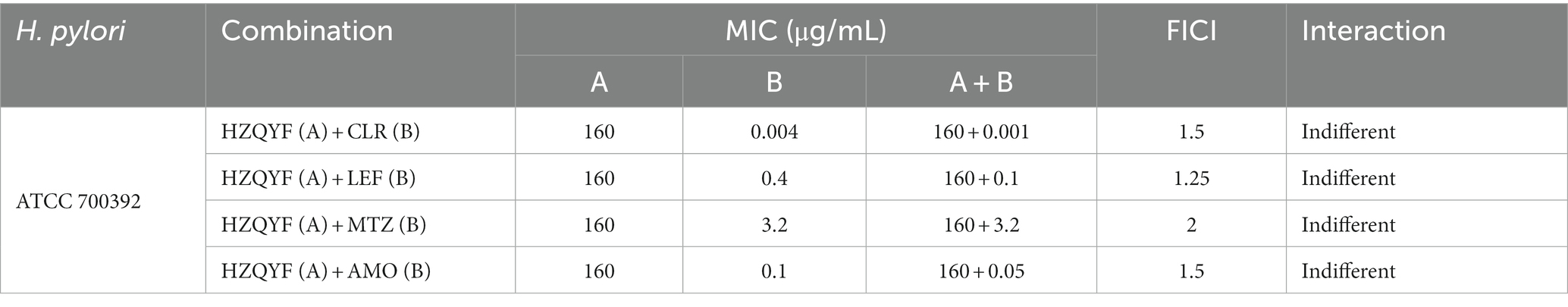
Using checkerboard determination, the interactions between HZQYF and four antibiotics commonly used in clinical practice for the treatment of H. pylori infection were evaluated. As shown in Table 5, the combination of HZQYF with these four antibiotics was not synergistic but also not antagonistic.
3.5 Effects of HZQYF on the virulence genes expression of Helicobacter pylori
As demonstrated in Figure 4, for the ATCC 700392 strain, incubation at ½ MIC for 24 h upregulated the expression of flagellar genes (flaA, flaB), adhesion genes (alpA, alpB, babA), and urease genes (ureE, ureF). However, at MIC, there was a significant downregulation of flagellar genes (flaA, flaB), adhesion genes (alpA, alpB, babA), and urease genes (ureE, ureF) after 24 h of incubation.
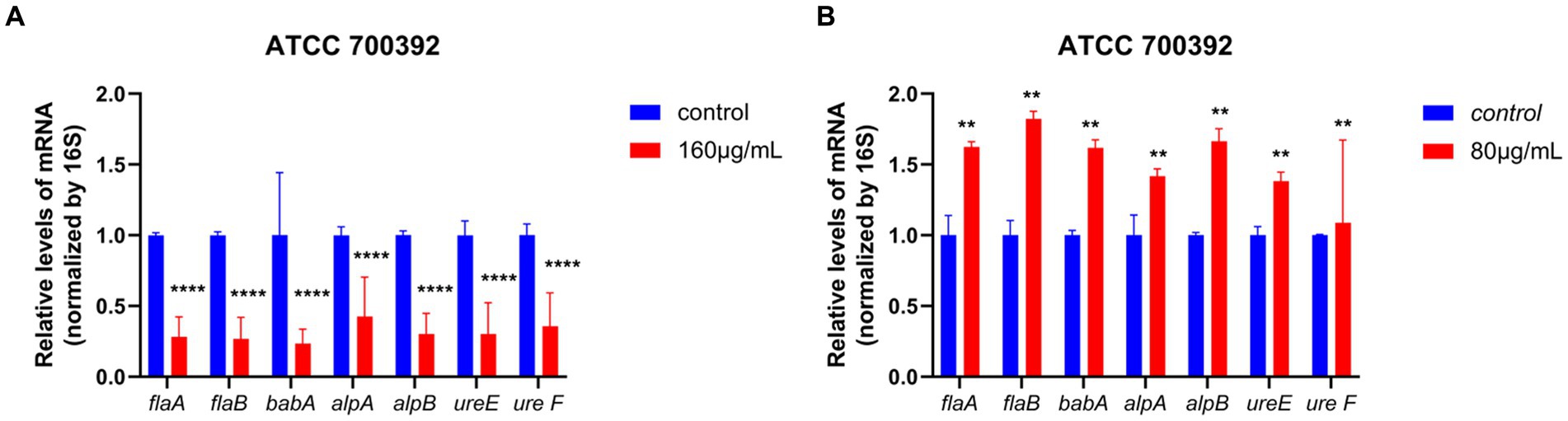
Figure 4. RT-qPCR analysis results showed the effects of HZQYF on the mRNA levels of H. pylori virulence genes. The bacteria strain was ATCC 700392, and the concentration of HZQYF were 160 μg/mL (A) and 80 μg/mL (B). And the incubation time of HZQYF was all 24 h. p < 0.05, vs. control group. **Stands for p < 0.01, and ****Stands for p < 0.0001, compared with control group.
3.6 Effect of HZQYF on Helicobacter pylori morphology
Differences in the morphology of H. pylori with or without HZQYF under MIC incubation conditions were observed using scanning electron microscopy (SEM). From Figure 5, compared to the control group, ATCC 700392 showed multiple surface damages after 24 h of incubation at MIC. Therefore, HZQYF may disrupt the morphology of bacteria and consequently impact the survival of H. pylori.
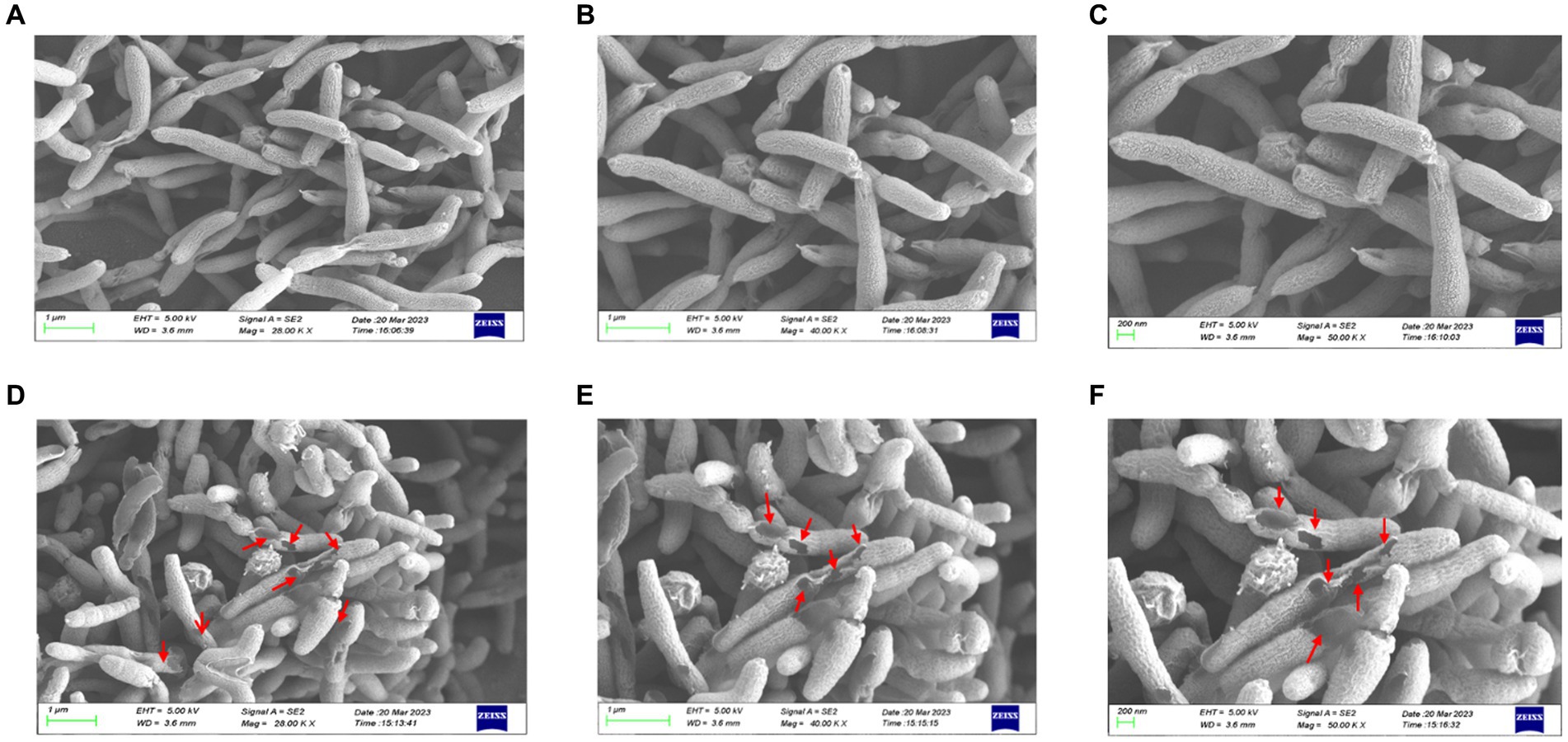
Figure 5. SEM images of ATCC 700392. Morphological images of H. pylori cells on SEM (magnification of 20.0, 40.0, and 50.0 kx) of control (A–C) and HZQYF treatment (D–F) at MIC (160 μg/mL) for 24 h.
3.7 The impact of cell membrane permeability
The results of the impact of 1/2 MIC and MIC of HZQYF on the cell membrane permeability of H. pylori are shown in Figures 6A,B. Gram-negative bacteria are enveloped by two layers of membranes, the inner membrane and the outer membrane, with the intact outer membrane acting as a permeation barrier. NPN has weak fluorescence in aqueous environments, but strong fluorescence in non-polar or hydrophobic environments. Once the outer membrane structure is damaged, NPN can enter the phospholipid layer, resulting in significant fluorescence. Thus, the ability of H. pylori to uptake NPN corresponds to the changes in the structure and permeability of the extracellular membrane. As shown in Figures 6A,B, the fluorescence intensity of intracellular NPN increased proportionally after both ATCC 43504 and ATCC 700392 were treated with HZQYF at ½ MIC and MICs. The fluorescence intensity of the sample-treated group was significantly higher than that of the control group, indicating that the permeability of the outer membrane of H. pylori was enhanced after treatment with the HZQYF extracts.
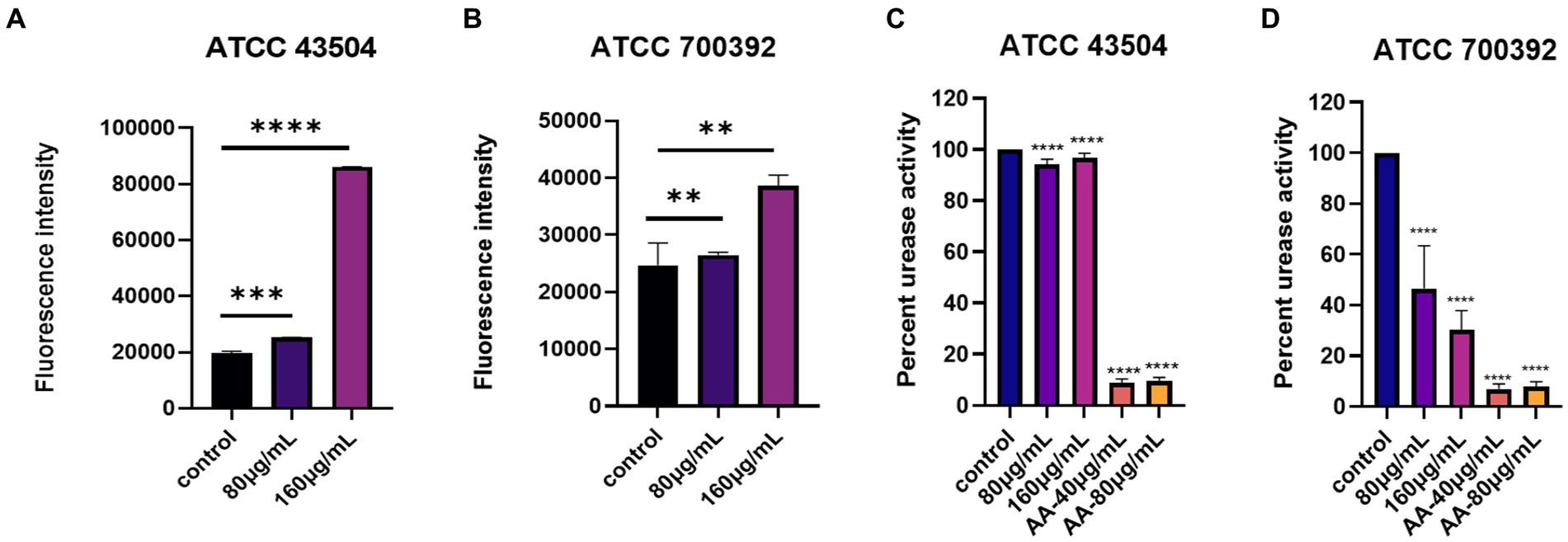
Figure 6. (A) The impact of cell membrane permeability of HZQYF on ATCC 43504. (B) The impact of cell membrane permeability of HZQYF on ATCC 700392. (C) The change in urease activity of ATCC 43504. (D) The change in urease activity of ATCC 700392. **Stands for p < 0.01, ***Stands for p < 0.001, and ****Stands for p < 0.0001, compared with control group.
3.8 Measurement of urease activity
The change in urease activity of H. pylori with different HZQYF concentrations is shown in Figures 6C,D. Urease is one of the key factors for the successful colonization of H. pylori in the stomach. Under highly acidic conditions, H. pylori can produce a large amount of urease. This urease can hydrolyze urea into ammonia, which can neutralize gastric acid and create an environment suitable for the growth of H. pylori. Therefore, inhibiting the activity of urease is an indication of inhibiting the growth of H. pylori. As shown in Figure 6, the urease inhibitor acetyl oxime acid (AA, worked as positive control) can significantly inhibit urease activity at 40 μg/mL and 80 μg/mL concentrations for both ATCC 43504 and ATCC 700392 strains. Compared to the control group, HZQYF had an inhibitory effect on urease activity for both ATCC 43504 and ATCC 700392 strains, but the urease activity for ATCC 43504 strain was still 90%, while for ATCC 700392 strain, the urease activity had already decreased to below 50%.
3.9 Effect of HZQYF on cagA protein expression using western blot
As shown in Figure 7, the expression of CagA proteins of ATCC 43504 and ATCC 700392 were significantly reduced after treatment with HZQYF (80,160, 320 μg/mL) compared with control group.
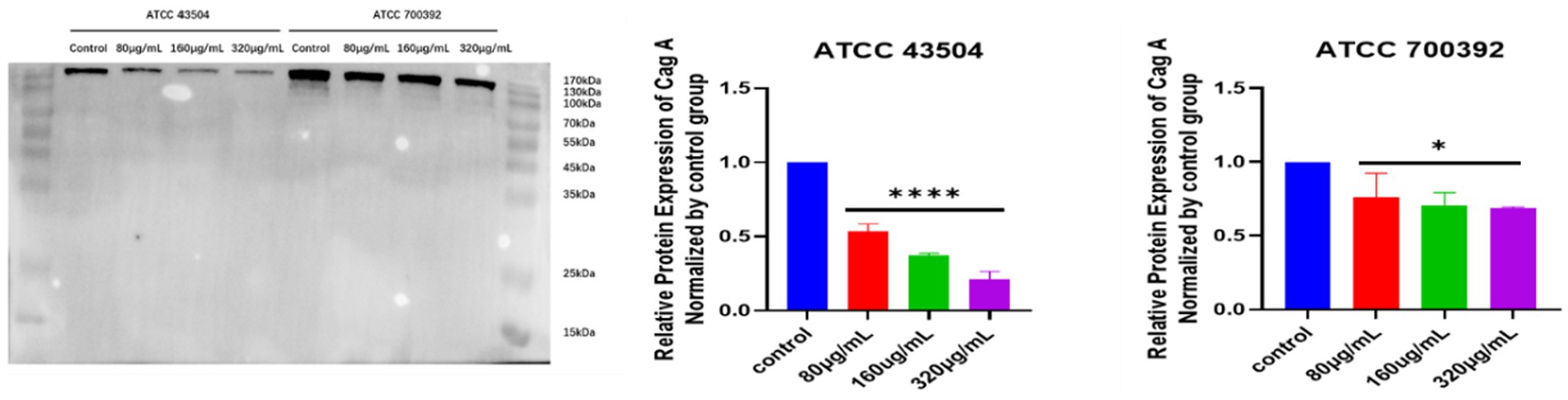
Figure 7. CagA protein expression in H. pylori ATCC 43504 and ATCC 700392 in different groups. *Stands for p < 0.05, and ****Stands for p < 0.0001, compared with control group.
3.10 Effect of HZQYF on adhesion ability
As shown in Figure 8, blue fluorescence indicates GES-1 cell nuclei and green fluorescence indicates H. pylori. The number of H. pylori adhered to GES-1 cells decreased significantly after the administration of HZQYF, and adhesion ability is inhibited gradually with the increase of the dosing concentration.
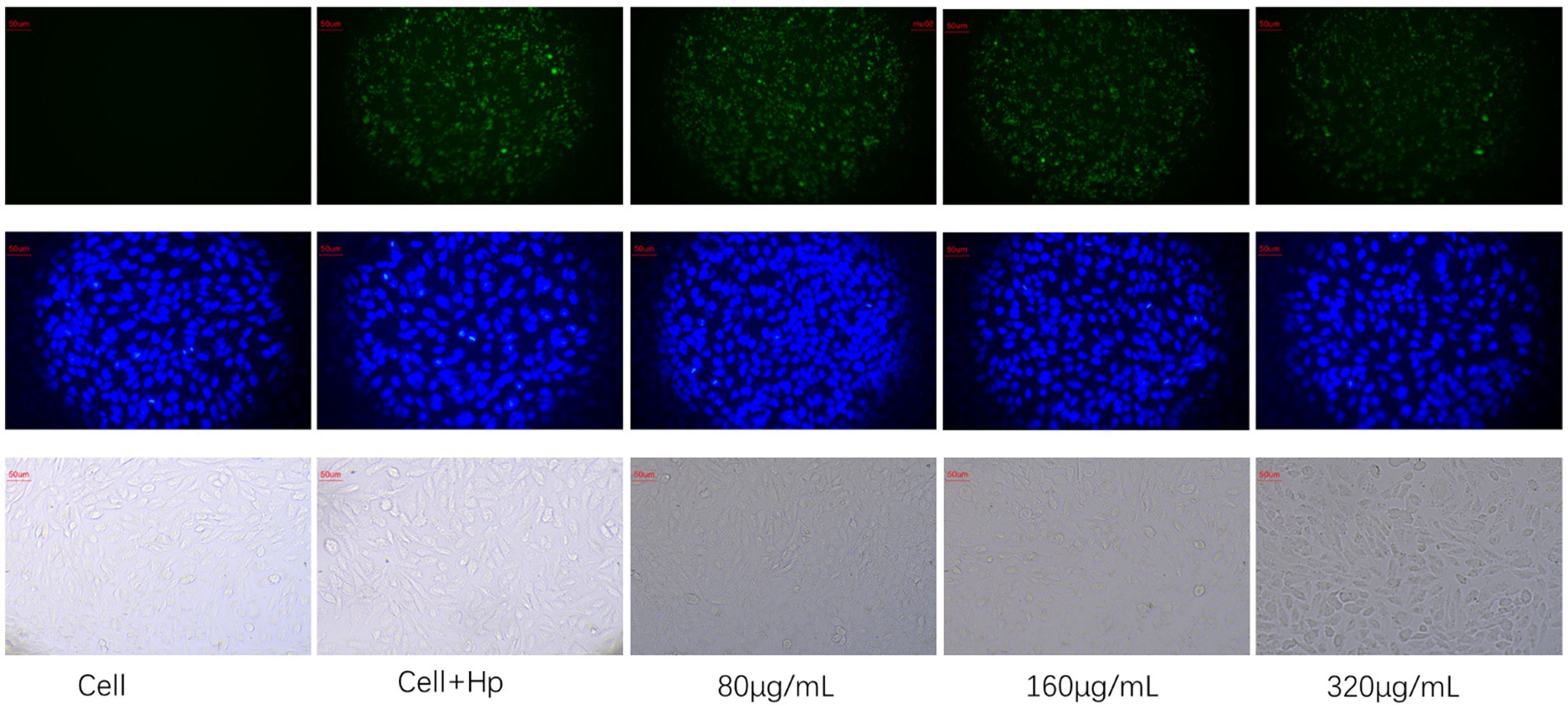
Figure 8. Effect of HZQYF (80,160, 320 μg/mL) on adhesion of ATCC 700392. The first row was marked with 1% FITC for H. pylori, and the second row was marked with DAPI, and all groups except cell group were added to H. pylori at MOI = 100:1.
4 Discussion
In this study, a formulation containing Chebulae Fructus, Ficus hirta Vahl, and Cloves was found to have anti-H. pylori activity; in vitro anti-H. pylori activity studies were conducted as well as the preliminary mechanism of action was revealed.
The composition of HZQYF was studied using HPLC. The results showed that the components in three batches of HZQYF were relatively consistent. Among them, the contents of chebulic and ellagic acid were the highest, followed by chebulanin, gallic acid, and corilatin.
The bacteriostatic activity of the HZQYF was confirmed. The MIC and MBC of HZQYF against three standard strains of H. pylori and five clinical isolates were studied. The results showed that HZQYF inhibited and killed both standard strains and clinical strains with MICs of 80–160 μg/mL and MBCs of 160–320 μg/mL. The time-inhibition curves showed that HZQYF inhibited H. pylori in a dose-dependent manner. The 40 μg/mL-80 μg/mL had a slight inhibitory effect on H. pylori, but 160 μg/mL had a strong inhibitory effect on H. pylori, which was almost the same as that of the positive control group after clarithromycin treatment. The time-bactericidal curve showed that HZQYF at MIC could control H. pylori below 1,000 times of the starting bacterial load at 60 h. If the dose was increased to 640–1,280 μg/mL, H. pylori could be controlled below 1,000 times of the starting bacterial load at 48 h.
The effect of HZQYF in combination with antibiotics on H. pylori was then investigated. It was found that HZQYF could be combined with clarithromycin, metronidazole, levofloxacin and amoxicillin with neither antagonism nor synergistic.
The mechanism of killing H. pylori involved inducing alterations in the visual morphology of the bacterium as well as disrupting the integrity of its cell membrane (Bittencourt et al., 2020). For morphological observations, after 24 h of treatment at the MIC, the SEM results indicated a significant alteration in the morphology of H. pylori and interference with the integrity of its cell membrane caused by HZQYF, which might lead to alternations of osmotic pressure and thereby induce bleb formation and cell lysis (Yan et al., 2022).
Previous studies have demonstrated that gastric epithelial adhesion plays a crucial role in enhancing inflammation caused by H. pylori (Yonezawa et al., 2016). Specifically, the alpAB locus has been identified as encoding an adhesion that is associated with the ability of H. pylori to adhere to human gastric tissues (Odenbreit et al., 1999; de Jonge et al., 2004a,b; Lu et al., 2007). In their research, Olga A. Senkovich et al. observed that the deletion of H. pylori alpA and alpB resulted in a reduction in H. pylori binding to laminin. Conversely, the expression of plasmid-borne alpA or alpB enabled E. coli to bind to laminin. Furthermore, H. pylori strains lacking alpB were also found to lack the ability to bind to laminin. Both alpA and alpB have been found to play a role in H. pylori laminin binding, as demonstrated by the absence of laminin binding in H. pylori strains lacking only alpB. Furthermore, fucosylated ABO blood group antigens and sialyl-Lewis x/a antigens are believed to function as receptors for H. pylori adherence (Boren et al., 1993; Oberhuber et al., 1997). The ABO antigens are specifically recognized by the blood group antigen binding adhesion (babA) (Aspholm-Hurtig et al., 2004), while the sialyl-Lewis x/a antigens are recognized by the H. pylori sialic acid binding adhesion (Fujimoto et al., 2007).
In our study, we investigated the expression of alpA, alpB, and babA genes using RT-qPCR. The findings revealed a down-regulation in the expression of these genes following the administration of HZQYF. And fluorescent staining was used to detect the effect of H. pylori on the adhesion ability on GES-1 cells, and the results showed that HZQYF could inhibit the adhesion ability of H. pylori. These may lead to a reduction in long-term infection and the associated inflammatory response.
The inhibition of urease activity is crucial for the eradication of H. pylori, as well H. pylori as for mitigating excessive oxidative stress and potential cancer development following H. pylori infection. Bacterial urease proteins encompass both structural proteins (ureA, ureB, and ureC) and auxiliary proteins (ureD, ureH, ureE, ureF, and ureG), each playing distinct roles in the activation of urease. The structural proteins form the active center of the urease, while the auxiliary proteins primarily facilitate the delivery of nickel ions (Li et al., 2019). Numerous studies have demonstrated the involvement of urease in the pathogenesis induced by H. pylori (Baj et al., 2020), such as overactivation of oxidative stress (Shi et al., 2019), which ultimately induces carcinogenesis (Yang et al., 2021). Therefore, attenuating oxidative stress is particularly important in anti-H. pylori therapy. In the current study, the RT-qPCR assay aimed at detecting virulence factors. It was observed that the expression of urease genes ureE and ureF were down-regulated by HZQYF. These genes primarily contribute to urease activation (Moncrief and Hausinger, 1997; Song et al., 2001; Biagi et al., 2013; Fong et al., 2013; Masetti et al., 2021), leading to the hypothesis that HZQYF might impede the activation process of the urease enzyme, thereby diminishing its activity. In the urease activity assay experiment, it was found that HZQYF could reduce the urease activity of H. pylori, but the inhibitory effect on urease varied for different strains of the bacteria. We hypothesized that this might be related to the single nucleotide polymorphisms of different strains of the bacteria. CagA is a Cytotoxin-associated protein which is considered the most important and widely studied virulence factor of H. pylori, and associated with gastric mucosal inflammation (Miftahussurur et al., 2020). At present, it has been shown a crucial role in the development of stomach cancer, which may promote the enhancement of drug resistance, proliferation and metastasis, and tumor stemness formation of gastric cancer cells make a difference (Zhang et al., 2012; El-Shouny et al., 2020). It can reduce the expression of cagA protein, which provides a direction for us for further study about effect of HZQYF on related inflammation after H. pylori infection.
5 Conclusion
In summary, HZQYF has a good bacteriostatic and bactericidal effect on H. pylori. There is not antagonistic effect in the use of HZQYF combined with antibiotics. Through scanning electron microscopy, it was observed that after administration, the morphology of H. pylori was disrupted, causing damage to its surface. Using the NPN uptake method, it was found that the outer membrane permeability of H. pylori was significantly enhanced after administration of the HZQYF. Through RT-qPCR technology, we detected changes in the expression of relevant virulence factor genes. The results showed that after 24 h of cultivation at the MIC, the HZQYF can downregulate the expression of flagellar genes (flaA, flaB), urease genes (ureE, ureF), and adhesion genes (alpA, alpB, babA). It was found through urease activity assays that HZQYF can reduce the urease activity of H. pylori and decrease the colonization of H. pylori. Based on these results, HZQYF could be an important anti-H. pylori agent.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZF: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft. HL: Data curation, Formal analysis, Visualization, Writing – original draft. YH: Visualization, Writing – original draft, Writing – review & editing. CP: Data curation, Writing – review & editing. LO: Visualization, Writing – review & editing. JJ: Formal analysis, Writing – review & editing. MX: Data curation, Formal analysis, Writing – review & editing. YZ: Visualization, Writing – review & editing. MC: Visualization, Writing – review & editing. GZ: Conceptualization, Funding acquisition, Writing – review & editing. MY: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work has been supported by the National Natural Science Foundation of China (grants 81973552) and the Key R&D Program of Shandong Province, China (2020CXGC010506).
Acknowledgments
Thanks to other staffs of Lunan Pharmaceutical Group Co., Ltd. that provide assistance in this study, including Xiaoyan Shi, Yanhua Wang, Zhixiang Zhu, Qingbang Hou, et al.
Conflict of interest
ZF, HL, YH, JJ, MX, and GZ were employed by Lunan Pharmaceutical Group Co., Ltd., and Shandong New Time Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aspholm-Hurtig, M., Dailide, G., Lahmann, M., Kalia, A., Ilver, D., Roche, N., et al. (2004). Functional adaptation of baba, the h-pylori abo blood group antigen binding adhesin. Science 305, 519–522. doi: 10.1126/science.1098801
Au, C.T.D. (2009). Pharmacognostical studies on hakka herbal medicine wuzhimaotao. HKBU Institutional Repository 2009.
Baj, J., Forma, A., Sitarz, M., Portincasa, P., Garruti, G., Krasowska, D., et al. (2020). Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cell 10:27. doi: 10.3390/cells10010027
Biagi, F., Musiani, F., and Ciurli, S. (2013). Structure of the ured-uref-ureg-uree complex in helicobacter pylori: a model study. J. Biol. Inorg. Chem. 18, 571–577. doi: 10.1007/s00775-013-1002-8
Bittencourt, M., Rodrigues, R. P., Kitagawa, R. R., and Gonçalves, R. (2020). The gastroprotective potential of silibinin against helicobacter pylori infection and gastric tumor cells. Life Sci. 256:117977. doi: 10.1016/j.lfs.2020.117977
Boren, T., Falk, P., Roth, K. A., Larson, G., and Normark, S. (1993). Attachment of helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895. doi: 10.1126/science.8018146
Bravo, D., Hoare, A., Soto, C., Valenzuela, M. A., and Quest, A. F. G. (2018). Helicobacter pylori in human health and disease: mechanisms for local gastric and systemic effects. World J. Gastroenterol. 24, 3071–3089. doi: 10.3748/wjg.v24.i28.3071
de Jonge, R., Durrani, Z., Rijpkema, S. G., Kuipers, E. J., van Vliet, A., and Kusters, J. G. (2004a). Role of the helicobacter pylori outer-membrane proteins alpa and alpb in colonization of the guinea pig stomach. J. Med. Microbiol. 53, 375–379. doi: 10.1099/jmm.0.45551-0
de Jonge, R., Durrani, Z., Rijpkema, S. G., Kuipers, E. J., van Vliet, A., and Kusters, J. G. (2004b). The role of the helicobacter pylori outer membrane proteins alpa and alpb in the colonisation of the guinea pig stomach. Gastroenterology 126, A125–A126.
Debowski, A. W., Walton, S. M., Chua, E., Tay, A. C., Liao, T., Lamichhane, B., et al. (2017). Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection. PLoS Pathog. 13:e1006464. doi: 10.1371/journal.ppat.1006464
El-Shouny, W. A., Ali, S. S., Hegazy, H. M., Abd Elnabi, M. K., Ali, A., and Sun, J. (2020). Syzygium aromaticum l.: traditional herbal medicine against caga and vaca toxin genes-producing drug resistant helicobacter pylori. J. Tradit. Complement. Med. 10, 366–377. doi: 10.1016/j.jtcme.2019.05.002
Fallone, C. A., Moss, S. F., and Malfertheiner, P. (2019). Reconciliation of recent helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 157, 44–53. doi: 10.1053/j.gastro.2019.04.011
Fong, Y. H., Wong, H. C., Yuen, M. H., Lau, P. H., Chen, Y. W., and Wong, K. (2013). Structure of ureg/uref/ureh complex reveals how urease accessory proteins facilitate maturation of helicobacter pylori urease. PLoS Biol. 11:e1001678. doi: 10.1371/journal.pbio.1001678
Fujimoto, S., Olaniyi Ojo, O., Arnqvist, A., Yih Wu, J., Odenbreit, S., Haas, R., et al. (2007). Helicobacter pylori baba expression, gastric mucosal injury, and clinical outcome. Clin. Gastroenterol. Hepatol. 5, 49–58. doi: 10.1016/j.cgh.2006.09.015
Ghasemian, A., Fattahi, A., Shokouhi Mostafavi, S. K., Almarzoqi, A. H., Memariani, M., Ben Braiek, O., et al. (2019). Herbal medicine as an auspicious therapeutic approach for the eradication of helicobacter pylori infection: a concise review. J. Cell. Physiol. 234, 16847–16860. doi: 10.1002/jcp.28363
Hu, Y., Wan, J. H., Li, X. Y., Zhu, Y., Graham, D. Y., and Lu, N. H. (2017). Systematic review with meta-analysis: the global recurrence rate of helicobacter pylori. Aliment. Pharmacol. Ther. 46, 773–779. doi: 10.1111/apt.14319
Jia, X., Huang, Q., Lin, M., Chu, Y., Shi, Z., Zhang, X., et al. (2022). Revealing the novel effect of jinghua weikang capsule against the antibiotic resistance of helicobacter pylori. Front. Microbiol. 13:962354. doi: 10.3389/fmicb.2022.962354
Li, B. Z., Threapleton, D. E., Wang, J. Y., Xu, J. M., Yuan, J. Q., Zhang, C., et al. (2015). Comparative effectiveness and tolerance of treatments for helicobacter pylori: systematic review and network meta-analysis. BMJ 351:h4052. doi: 10.1136/bmj.h4052
Li, R., Zhang, P., Hu, Z., Yi, Y., Chen, L., and Zhang, H. (2021). Helicobacter pylori reinfection and its risk factors after initial eradication a protocol for systematic review and meta-analysis. Medicine 100:e25949. doi: 10.1097/MD.0000000000025949
Li, X., Zhao, S., Zheng, N., Cheng, J., and Wang, J. (2019). Progress in bacterial urease complexes and their activation mechanisms. Sheng Wu Gong Cheng Xue Bao 35, 204–215. doi: 10.13345/j.cjb.180239
Liu, G., Xie, J., Lu, Z. R., Cheng, L. Y., Zeng, Y., Zhou, J. B., et al. (2017). Fifth chinese national consensus report on the management of helicobacter pylori infection. Zhonghua Nei Ke Za Zhi 56, 532–545. doi: 10.3760/cma.j.issn.0578-1426.2017.07.014
Lu, H., Wu, J. Y., Beswick, E. J., Ohno, T., Odenbreit, S., Haas, R., et al. (2007). Functional and intracellular signaling differences associated with the helicobacter pylori alpab adhesin from western and east asian strains. J. Biol. Chem. 282, 6242–6254. doi: 10.1074/jbc.M611178200
Ma, F. (2010). Screening test for anti-helicobacter pylori activity of traditional chinese herbal medicines. World J. Gastroenterol. 16, 5629–5634. doi: 10.3748/wjg.v16.i44.5629
Ma, Y., Liu, H., Shi, J., Shang, M., Li, Y., Liu, G., et al. (2017). Study on quality standard of fici hirtae radix. Chin. Tradit. Herb. Drug 48, 782–791.
Malfertheiner, P., Megraud, F., O'Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2016). Management ofhelicobacter pylori infection—the Maastricht v/Florence consensus report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Masetti, M., Bertazzo, M., Recanatini, M., Ciurli, S., and Musiani, F. (2021). Probing the transport of ni(ii) ions through the internal tunnels of the helicobacter pylori uredfg multimeric protein complex. J. Inorg. Biochem. 223:111554. doi: 10.1016/j.jinorgbio.2021.111554
Miftahussurur, M., Doohan, D., Syam, A. F., Nusi, I. A., Waskito, L. A., Fauzia, K. A., et al. (2020). The validation of the helicobacter pylori caga typing by immunohistochemistry: nationwide application in Indonesia. Acta Histochem. 122:151594. doi: 10.1016/j.acthis.2020.151594
Moncrief, M. B., and Hausinger, R. P. (1997). Characterization of ureg, identification of a ured-uref-ureg complex, and evidence suggesting that a nucleotide-binding site in ureg is required for in vivo metallocenter assembly of klebsiella aerogenes urease. J. Bacteriol. 179, 4081–4086. doi: 10.1128/jb.179.13.4081-4086.1997
Nagy, P., Johansson, S., and Molloy-Bland, M. (2016). Systematic review of time trends in the prevalence of helicobacter pylori infection in China and the USA. Gut Pathogens 8:8. doi: 10.1186/s13099-016-0091-7
Oberhuber, G., Kranz, A., Dejaco, C., Dragosics, B., Mosberger, I., Mayr, W., et al. (1997). Blood groups Lewis(b) and abh expression in gastric mucosa: lack of inter-relation with helicobacter pylori colonisation and occurrence of gastric malt lymphoma. Gut 41, 37–42. doi: 10.1136/gut.41.1.37
Odenbreit, S., Till, M., Hofreuter, D., Faller, G., and Haas, R. (1999). Genetic and functional characterization of the alpab gene locus essential for the adhesion of helicobacter pylori to human gastric tissue. Mol. Microbiol. 31, 1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x
Ou, L., Liu, H., Shi, X., Peng, C., Zou, Y., Jia, J., et al. (2023). Terminalia chebula retz. Aqueous extract inhibits the helicobacter pylori-induced inflammatory response by regulating the inflammasome signaling and er-stress pathway. J. Ethnopharmacol. 320:117428. doi: 10.1016/j.jep.2023.117428
Peng, C., Feng, Z., Ou, L., Zou, Y., Sang, S., Liu, H., et al. (2023). Syzygium aromaticum enhances innate immunity by triggering macrophage m1 polarization and alleviates helicobacter pylori-induced inflammation. J. Funct. Food. 107:105626. doi: 10.1016/j.jff.2023.105626
Peng, C., Sang, S., Shen, X., Zhang, W., Yan, J., Chen, P., et al. (2022). In vitro anti-helicobacter pylori activity of syzygium aromaticum and the preliminary mechanism of action. J. Ethnopharmacol. 288:114995. doi: 10.1016/j.jep.2022.114995
Shen, X., Zhang, W., Peng, C., Yan, J., Chen, P., Jiang, C., et al. (2021). In vitro anti-bacterial activity and network pharmacology analysis of sanguisorba officinalis l. against helicobacter pylori infection. Chin. Med. 16:33. doi: 10.1186/s13020-021-00442-1
Shi, Y., Wang, P., Guo, Y., Liang, X., Li, Y., and Ding, S. (2019). Helicobacter pylori-induced dna damage is a potential driver for human gastric cancer ags cells. DNA Cell Biol. 38, 272–280. doi: 10.1089/dna.2018.4487
Shiotani, A., Lu, H., Dore, M. P., and Graham, D. Y. (2017). Treating helicobacter pylori effectively while minimizing misuse of antibiotics. Clevel. Clin. J. Med. 84, 310–318. doi: 10.3949/ccjm.84a.14110
Shu, Z., Zhao-Xun, L., Fei, M. O., Yun, H. E., Chao-Qin, S., Wan-Ying, Z., et al. (2016). Influence of polygonum capitatum on the adherency and colonization of helicobacter pylori. Chinese J. Zoonoses 8, 734–740.
Song, H. K., Mulrooney, S. B., Huber, R., and Hausinger, R. P. (2001). Crystal structure of klebsiella aerogenes uree, a nickel-binding metallochaperone for urease activation. J. Biol. Chem. 276, 49359–49364. doi: 10.1074/jbc.M108619200
Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J. Y., Crowe, S. E., et al. (2016). Review article: the global emergence of helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 43, 514–533. doi: 10.1111/apt.13497
Xue, P., Sang, R., Li, N., Du, S., Kong, X., Tai, M., et al. (2023). A new approach to overcoming antibiotic-resistant bacteria: traditional chinese medicine therapy based on the gut microbiota. Front. Cell. Infect. Microbiol. 13:1119037. doi: 10.3389/fcimb.2023.1119037
Yan, J., Peng, C., Chen, P., Zhang, W., Jiang, C., Sang, S., et al. (2022). In-vitro anti-helicobacter pylori activity and preliminary mechanism of action of canarium album raeusch. Fruit extracts. J. Ethnopharmacol. 283:114578. doi: 10.1016/j.jep.2021.114578
Yang, H., Wei, B., and Hu, B. (2021). Chronic inflammation and long-lasting changes in the gastric mucosa after helicobacter pylori infection involved in gastric cancer. Inflamm. Res. 70, 1015–1026. doi: 10.1007/s00011-021-01501-x
Yonezawa, H., Osaki, T., Hojo, F., and Kamiya, S. (2016). Critical role of alpa and alpb for biofilm formation and cell adhesion of helicobacter pylori. Helicobacter 21:118.
Zaidi, S. F., Muhammad, J. S., Shahryar, S., Usmanghani, K., Gilani, A., Jafri, W., et al. (2012). Anti-inflammatory and cytoprotective effects of selected pakistani medicinal plants in helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 141, 403–410. doi: 10.1016/j.jep.2012.03.001
Zeng, Y. W., Liu, X. Z., Lv, Z. C., and Peng, Y. H. (2012). Effects of ficus hirta vahl. (Wuzhimaotao) extracts on growth inhibition of hela cells. Exp. Toxicol. Pathol. 64, 743–749. doi: 10.1016/j.etp.2011.01.009
Zhang, C., Xu, S., and Xu, D. (2012). Risk assessment of gastric cancer caused by helicobacter pylori using caga sequence markers. PLoS One 7:e36844. doi: 10.1371/journal.pone.0036844
Glossary
Keywords: Helicobacter pylori, Hezi Qingyou Formula, antibacterial activity, mechanism of action, in vitro
Citation: Feng Z, Li H, Hao Y, Peng C, Ou L, Jia J, Xun M, Zou Y, Chen M, Zhang G and Yao M (2024) In vitro anti-Helicobacter pylori activity and the underlining mechanism of an empirical herbal formula – Hezi Qingyou. Front. Microbiol. 15:1355460. doi: 10.3389/fmicb.2024.1355460
Edited by:
Alain Pierre Gobert, Vanderbilt University Medical Center, United StatesReviewed by:
Ghalia Khoder, University of Sharjah, United Arab EmiratesSinem Oktem Okullu, Acibadem Mehmet Ali Aydinlar University, Türkiye
Copyright © 2024 Feng, Li, Hao, Peng, Ou, Jia, Xun, Zou, Chen, Zhang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meicun Yao, bHNzeW1jQG1haWwuc3lzdS5jbg==; Guimin Zhang, bHVuYW56aGFuZ2d1aW1pbkAxNjMuY29t
†These authors have contributed equally to this work
 Zhong Feng
Zhong Feng Hui Li2,3†
Hui Li2,3† Meiyun Chen
Meiyun Chen Meicun Yao
Meicun Yao