- 1Interdisciplinary Program in Smart Agriculture, Kangwon National University, Chuncheon-si, Republic of Korea
- 2Plant Medicine Program, Division of Bioresource Sciences, College of Agriculture and Life Sciences, Kangwon National University, Chuncheon-si, Republic of Korea
Persister cell and viable but non-culturable (VBNC) state of bacteria are survival strategies against antibiotics and various environmental stresses, respectively, but they tend to be ignored in agriculture fields, even though bacteria can regain their abilities to survive and produce disease once those stresses disappear. This study was carried out to determine whether persister cell and VBNC state in Erwinia amylovora are present after exposures to streptomycin, the length of their persistence, and the steps needed to decrease the inoculum. Persister cells were observed using biphasic killed growth curve for 4–8 h when the late stationary phase cells of E. amylovora were cultured in liquid medium containing streptomycin. This state was maintained for up to 12 h based on the colony forming units (CFUs) of the colonies that grew on the mannitol glutamate yeast extract (MGY) medium after streptomycin was removed. The CFUs on the MGY medium were lower than the total count determined using the LIVE/DEAD Kit, suggesting that persister cells and VBNC state might co-exist for up to 12 h after exposure to streptomycin. However, after 12 h, E. amylovora cells did not continue to grow on the medium for 9 days, suggesting that they entered a VBNC state at that time and remained in a persistent state. In addition, based on the Redox Sensor Green staining method, the presence of both states was confirmed for up to 12 h, and only then did the VBNC state became apparent. Furthermore, persister cells were observed for up to 24 h, and damaged cells reduced when E. amylovora cells were culture in distilled water with streptomycin, indicating that the uptake of lower nutrients in E. amylovora led to prolonged persister cells and VBNC state, which are more likely to survive after streptomycin treatments. The addition of sucrose and oxytetracycline to distilled water containing streptomycin reduced persister cells than other sources did. Thus, to inhibit the spread of fire blight, management techniques must consider the hazards of using streptomycin treatments that induce dormancy, such as persister cells and VBNC state, beyond the development of resistant strain.
Introduction
Fire blight is caused by the Gram-negative bacterium Erwinia amylovora. It was first detected in 2015 (Myung et al., 2016; Park et al., 2016) and has since spread to 28 cities/districts in Republic of Korea in 2022, making it the most destructive disease in pome fruits, including apple and pear trees in Republic of Korea (Ham et al., 2020a,b; Choi et al., 2022; Jik Lee et al., 2022). Thus, chemical control strategy based on copper compounds and agricultural antibiotics was primarily adopted as a management technique against continual fire blight situation in Republic of Korea. Among the chemical control agents, agricultural antibiotics such as streptomycin, oxytetracycline, oxolinic acid, and validamycin have been registered by the Rural Development Administration of Korea (RDA) and used intensively twice during the blooming season by spraying directly into flowers (Park et al., 2017; Choi et al., 2022). However, the European Union, the United States, and Canada have prohibited the use of antibiotics because of several adverse effects such as increased disease resistance to antibiotics and subsequent public health issues and ecosystem disconnection caused by alteration of microbial diversity in soils and waters (Fried et al., 2014; USDA, 2019; Mikiciński et al., 2020). The concerned authorities in Republic of Korea is aware of these issues, and there are several policies currently in place to reduce the use of antibiotics. However, agricultural antibiotics are still used in agriculture fields across the country to treat agricultural disease, especially fire blight, because there are no alternative control agents. E. amylovora is a quarantine pathogen in Republic of Korea; hence, efficacy test must be conducted in small and insulated green houses. Streptomycin had been reported to show the strongest control effect among tested antibiotics (Norelli and Gilpatrick, 1982; McManus and Jones, 1994) but its effects varied depending on environmental conditions and disease severity between experimental plots (personal communication with Dr. Lee, Y. H. in RDA). Streptomycin was the first aminoglycoside antibiotic isolated in 1943 from Streptomyces griseus (Schatz et al., 1944) and has been used in agricultural fields since 1955 (McManus et al., 2002). Streptomycin, like many other aminoglycoside antibiotics, is also composed of an inositol derivative linked to at least one amino sugar, containing hydroxyl and amino groups, and streptamine (Cunha, 2006; Becker and Cooper, 2013). These amino sugars are the key binding elements that interact with the 16S rRNA and the ribosomal protein S12 of the 30S ribosome, interfering with initial tRNA selection in protein synthesis (Carter et al., 2000). Thus, streptomycin causes bacterial death by destabilizing membranes, inhibiting ribosomal activity, and disrupting metabolism and respiration due to lack of translational fidelity (Davis et al., 1986; Davis, 1987; Kohanski et al., 2007). Hence, its killing effect, low phytotoxicity, and cost effectiveness have made it the most widely used antibiotic against fire blight since the 1960s in USA and 2015 in Republic of Korea (Moller et al., 1981; Park et al., 2017). However, molecular mechanisms such as enzymatic inactivation (strAB) and spontaneous mutation (rpsL) have been reported as key factors of streptomycin resistance in E. amylovora (Chiou and Jones, 1995; Sundin and Bender, 1996), and the streptomycin resistant strains that mutated in those genes are present worldwide, including the USA, Canada, New Zealand, Israel, Lebanon, and Mexico (McManus et al., 2002; De León Door et al., 2013; Tancos et al., 2016; Sundin and Wang, 2018), suggesting that the use of streptomycin may no longer be the best strategy to control fire blight. Thus, streptomycin resistance in E. amylovora is seems to be a major obstacle to its use; therefore, its incidence is monitored in areas where fire blight is produced. In Republic of Korea, governors, researchers, and growers are aware of the problem caused by the emergence of resistant strains, fortunately, despite nationwide surveillance and routine monitoring, no reports of streptomycin-resistant strains have been made to date (Lee et al., 2018). In addition to the significance of streptomycin resistance, low efficacy during field treatment of streptomycin is also important. Because this phenomenon may arise from rebounding of pathogen via dormancy of pathogenic bacteria following streptomycin treatment at inappropriate concentrations, interval, and timing.
Dormancy has been reported to be one of the survival strategies used by non-sporulating bacteria in their surrounding environments, and it can be divided into two phenomena: persistence and viable but non-culturable (VBNC) states (Ayrapetyan et al., 2015, 2018; Martins et al., 2018; Zou et al., 2021). Persister cells have been found to reduce killing rate, which represents the biphasic kill curve after the bacterial cells are exposed to stresses such as antibiotics and then re-grown on media after the stresses are removed (Orman and Brynildsen, 2013). In contrast, VBNC cells, which are also described to be alive, lose their ability to grow on media with induced stresses such as antibiotics, but their re-growth requires some triggers such as carbon sources or in case of pathogens with natural hosts (Ayrapetyan et al., 2018). According to the mentioned concept between these two states, there seems to be a different strategy for bacteria to survive under unfavorable conditions. However, both persister and VBNC cells were formed under similar environmental stresses and stochastically (Fisher et al., 2017; Ayrapetyan et al., 2018), and no statistical differences were found between these two states of dormancy based on antibiotic tolerance, recovery rates, morphology, and metabolic activity (Kim et al., 2018a). Therefore, persister cells and VBNC state are likely to be part of a continuum of dormancy according to physiological relatedness (Ayrapetyan et al., 2015, 2018).
Although studies on plant pathogenic bacteria have focused more on VBNC than on persister cells, a few studies have shown that VBNC may survive under unfavorable conditions such as copper or chlorine exposure, low temperature, oxidative stress, and starvation (Ghezzi and Steck, 1999; Grey and Steck, 2001; Venisse et al., 2001; Ordax et al., 2006; Rodrigues et al., 2008; Álvarez et al., 2008; Santander et al., 2014; Postnikova et al., 2015; Santander and Biosca, 2017), and persister cells have only been reported for two bacteria (Muranaka et al., 2012; Peng et al., 2021). Thus, in this study, we examined the two dormancy states in E. amylovora (persistence and VBNC) after treating it with streptomycin, which is the most widely used bactericide for fire blight control in Republic of Korea. We also examined the relationships between these two states in terms of composition rates and periods, phenotypic properties, and appropriate treatment to reduce the survival strategies of E. amylovora cells that are in the persistent and VBNC state.
Materials and methods
Streptomycin-degradation test
Streptomycin-degradation tests were conducted using both hydrolysis and photolysis. In the hydrolysis test, streptomycin solutions were prepared in 50 mL conical tubes containing each 50 mg/mL of laboratory streptomycin (Streptomycin Sulfate, Fujifilm Wako Pure Chemical Co., Ltd. Osaka, Japan) and 250, 500, 1,000, and 2,500 μg/mL of bactericidal pesticide with streptomycin as 20% of active gradient (Agrepto, Kyungnong Co., Ltd, Seoul, Republic of Korea) and maintained at 28°C for 7 days in hydrolysis test. After 7 days, 10 μL of each streptomycin solution at 7 days was spot-inoculated on the center of mannitol glutamate yeast extract (MGY) medium that had been pre-plated with 100 μL of E. amylovora TS3128 bacterial suspension (Kang et al., 2021) at an optical density (OD) at 600 nm of 0.1 (approximately 3.1 × 108 CFU/mL) and incubated at 28°C for 2 days. The effect of streptomycin degradation via hydrolysis was considered as an inhibitory zone against TS3128 by comparing the reduced size of the inhibitory zone with day 0 solution. In the photolysis test, 50 and 500 μg/mL of laboratory and bactericidal pesticide streptomycin solutions in 50 mL conical tubes, respectively, were exposed to an OD of 302 nm (UVB) using Ultraviolet Transilluminator (MaestroGen Inc., Hsinchu City, Taiwan) for 2 days, and TS3128 was then inoculated at a concentration of OD600 nm = 0.3. The effect of UVB on streptomycin degradation was assessed by counting the colony forming unit (CFU/mL) of colonies grown on MGY medium without streptomycin at daily intervals until 5 days. All experiments were performed twice.
Killing curve after treatment with streptomycin
To confirm whether E. amylovora TS3128 cells entered the persistent state, which represents the biphasic kill curve after streptomycin treatment, each single colony of TS3128 was cultured in King’s B broth at 28°C for 20 h and then sub-cultured in the same liquid medium at 28°C for 5, 20, and 96 h to correspond to lag, early stationary, and late stationary phase, respectively. At the sub-culture phase, 50 and 500 μg/mL of laboratory and pesticide streptomycin were added to each phase-culture, respectively, and was shaken at 28°C until 5 and 8 h for lag and early and late stationary phases, respectively. To remove residents of streptomycin, 1 mL of samples were collected twice an hour, re-suspended in 10 mM MgCl2 twice, and colonies were counted after 8 h on MGY agar medium. To date, a streptomycin resistant isolate of E. amylovora has not been found in Republic of Korea; therefore, a streptomycin resistant isolate of Pseudomonas syringae pv. actinidiae biovar 2, which was isolated in Republic of Korea, was used as a negative control (Psa2, Lee et al., 2020). Psa2 was cultured in King’s B broth with or without streptomycin for 20 h, and 1 mL of samples were collected and re-suspended with same procedures for TS3128. Colonies were then counted after 0, 12, and 24 h on King’s B medium with or without supplemented laboratory streptomycin (50 μg/mL).
To determine the end point of the persistence state induced by streptomycin, sub-cultivation was conducted in King’s B broth and distilled water, respectively. The CFUs of growing colonies were counted several times up to 72 h following previously described procedures. The experiments were conducted two times.
Cells staining and flow cytometry
To prepare the early stationary phase during staining, E. amylovora cells were cultivated in King’s B broth at 28°C for 20 h as the nutrient rich case. The cultures were then centrifuged at 4,000 rpm for 6 min, re-suspended in 10 mM MgCl2 twice, and the pellets were suspended in same volume of distilled water as lack of nutrient case. Following the preparation of the two case samples, 500 μg/mL of pesticide streptomycin was added to two samples, and 1 mL of samples were taken at 3 days intervals till 9 and 15 days for nutrient rich and deficient status, respectively, and rinsed in 10 mM MgCl2 twice to remove streptomycin residue.
E. amylovora cells were stained with SYTO9 and propidium iodide (PI) using a BacLight Live/Dead viability kit (Invitrogen, CA, USA) to count total, live, and culturable cells according to previous reports (Santander et al., 2014; Kim et al., 2023). This was performed to produce a biphasic kill curve within the first few hours of cultivation, which showed a declining growth curve and identified the VBNC state after streptomycin treatment. To determine the proportions of each different physiological state in VBNC, staining with RedoxSensor Green (RSG) and PI was also conducted using the BacLight RSG Green vitality kit (Invitrogen, CA, USA) in accordance with a recommended protocol and previous report (Patel et al., 2021). Each 1 mL of the two case samples contained 1 μL of SYTO9 (3.34 μM) and PI (20 mM) or each of 1 μL RSG (1 mM) and PI and were incubated for 15 min in the dark. Subsequently, 200 μL of each stained samples were added to a FACS test tube and exposed for 1 min to FACSCalibur (BD Biosciences) and FACSymphony (BD Biosciences) for SYTO9 and PI and RSG and PI, respectively. FL1 530/30 bandpass filter for SYTO9 and FL3 670LP filter for PI in FACSCalibur and FITC (fluorescein isothiocyanate) 530/30 filter for RSG and PE 575/30 filter for PI in FACSymphony were used to capture fluorescence. Data analysis was conducted using the BD CellQuest Pro and BD FACSDiva software for Live/Dead and RSG kit, respectively. A total of nine fields were captured for fluorescence in three repeats. Four locations were identified among the recorded events (Supplementary Figure 1): PI-red (Q1), RSG and PI merge-yellow (Q2), no signal (Q3), and RSG-green signal (Q4). These zones were designated by 70% ethanol-killing, persistence and VBNC state, unstaining, and live cells, respectively. The CFUs of the culturable cells grown on MGY medium were counted as culturable cells in both staining.
Secondary treatment experiments
To find alternative reagents that can shorten the duration of the persistence and VBNC state of E. amylovora cells induced by streptomycin, early stationary phase cells in distilled water were treated with 500 μg/mL of pesticide streptomycin and 10 mM of carbon sources such as glucose, sucrose, fructose, and sorbitol. One day after the first treatment, the second treatment with registered pesticides such as streptomycin (Agrepto), oxytetracycline (Sungbocycline, Sungbo Chemicals Co., Ltd., Republic of Korea), and tri-basic copper sulfate (Saebinna, Syngenta Co., Ltd., Republic of Korea), was carried out. The same procedures were used to assess the impact of secondary treatment on the reduction of the persistence and VBNC state induced by treated streptomycin alone.
Antibiotic uptake assay
Streptomycin absorption was measured as the diameter of the growth inhibition zone of normal E. amylovora cells after they were extracted using E. amylovora persister cells (Chen et al., 2019). Briefly, 1 mL of persister cells induced by streptomycin with 10 mM carbon sources or second treatments with antibiotics were centrifuged at 4,000 rpm for 6 min, washed twice with 10 mM MgCl2 buffer, resuspended in 100 μL of cell wall digestion buffer [30 mM Tris–HCl (pH 8.0), 1 mM EDTA, 1 mg/mL lysozyme], and incubated at ambient temperature for 2 h. The pellets were frozen by treating them three times with liquid nitrogen. Subsequently, they were denatured for 10 min at 90°C using heat block and centrifuged at 12,000 rpm for 3 min to separate the debris and supernatant. A 10 μL supernatant was used to inoculate a spot in the middle of the pre-inoculated MGY medium with normal E. amylovora cells (100 μL of OD600 nm = 0.1) and incubated at 28°C for 24 h. The diameter of cell growth inhibition zone was measured in two repeats.
Recovery assays
An in vitro test was used to confirm that the persister and VBNC cells had recovered after the second antibiotic treatment following streptomycin treatment. Following the second treatment, 1 mL of cells were centrifuged at 4,000 rpm for 6 min and then washed twice in 10 mM MgCl2 buffer. Thereafter, 50 μL of cells was injected into 5 mL of M9 minimal broth supplemented with 40 mg/mL of asparagine, aspartate, glutamine, tryptophan, thiamine, threonine, histidine, and nicotinic acid and 1.5 mg/mL of succinate and pyruvate. The mixture was then culture at 28°C for 24 h in a shaking incubator. After plating 10 μL of the cultured cells onto MGY medium and incubating them at 28°C for 24 h, the number of bacterial colonies were counted as the number of recovered cells. Three plating cycles were used and three repetitions of recovery test were conducted. King’s B broth served as a positive control and was used in place of the M9 minimal broth supplemented nutrients.
Statistical analysis
All experiments were independently repeated at least two times. Data were analyzed using t-test and analysis of variance, and the means were compared using Duncan’s least significant range test at p < 0.05. The analysis was performed using the Jamovi project, 2021 (ver. 2.3.21.0).
Results
Subpopulation of E. amylovora is not related to streptomycin degradation
The degradation rates of different concentration of streptomycin solutions in water at day 7 were not significantly affected, indicating that streptomycin degradation by hydrolysis was not significant (Supplementary Figure 1A). However, the inhibitory zone of 2,500 μg/mL of bactericidal pesticide at day 7 was significantly reduced compared to that at day 0. This result demonstrated that the inhibitory zones of bactericidal pesticide against E. amylovora emerged in a dosage-dependent manner, such that high concentrations (e.g., 2,500 μg/mL) might be degraded to reduce to inhibition size in water under normal light during the 28°C test periods. Streptomycin degradation by UVB, which is a strong factor in photolysis, was not exhibited under the tested conditions in this study (Supplementary Figure 1B). Therefore, we believe that the experimental system has the potential to determine whether E. amylovora can enter dormancy states, including persistence and VBNC, when treated with streptomycin.
Streptomycin treatments produce E. amylovora persister cells
Kinetic killing curve assays were used to describe the subpopulation of E. amylovora TS3128 in the presence of 50 and 500 mg/mL concentrations of the laboratory and pesticide streptomycin. In the lag phase, the population declined within 3 h and most of them died (Figure 1A). In contrast, the number of survivors of early and late stationary phase reduced at 4 h and then remained the same until 8 h in both laboratory and pesticide streptomycin treatments (Figures 1B, C). These results suggested that both streptomycin treatments caused TS3128 cells in the stationary phase to enter the culturable persistence state. However, populations of streptomycin-resistant isolate (Psa2) did not decline when treated with streptomycin, and they showed a growth curve that was comparable to King’s B broth without streptomycin (Supplementary Figures 2A, B), indicating that Psa2 was not persistent. The end points of persistence in the kinetic killing curve of King’s B broth and distilled water were determined to be 24 and 48 h, respectively (Figures 1D, E), indicating that the combination of streptomycin treatment and lack of nutrient treatment may induce a longer period of persistence than just streptomycin treatment only.
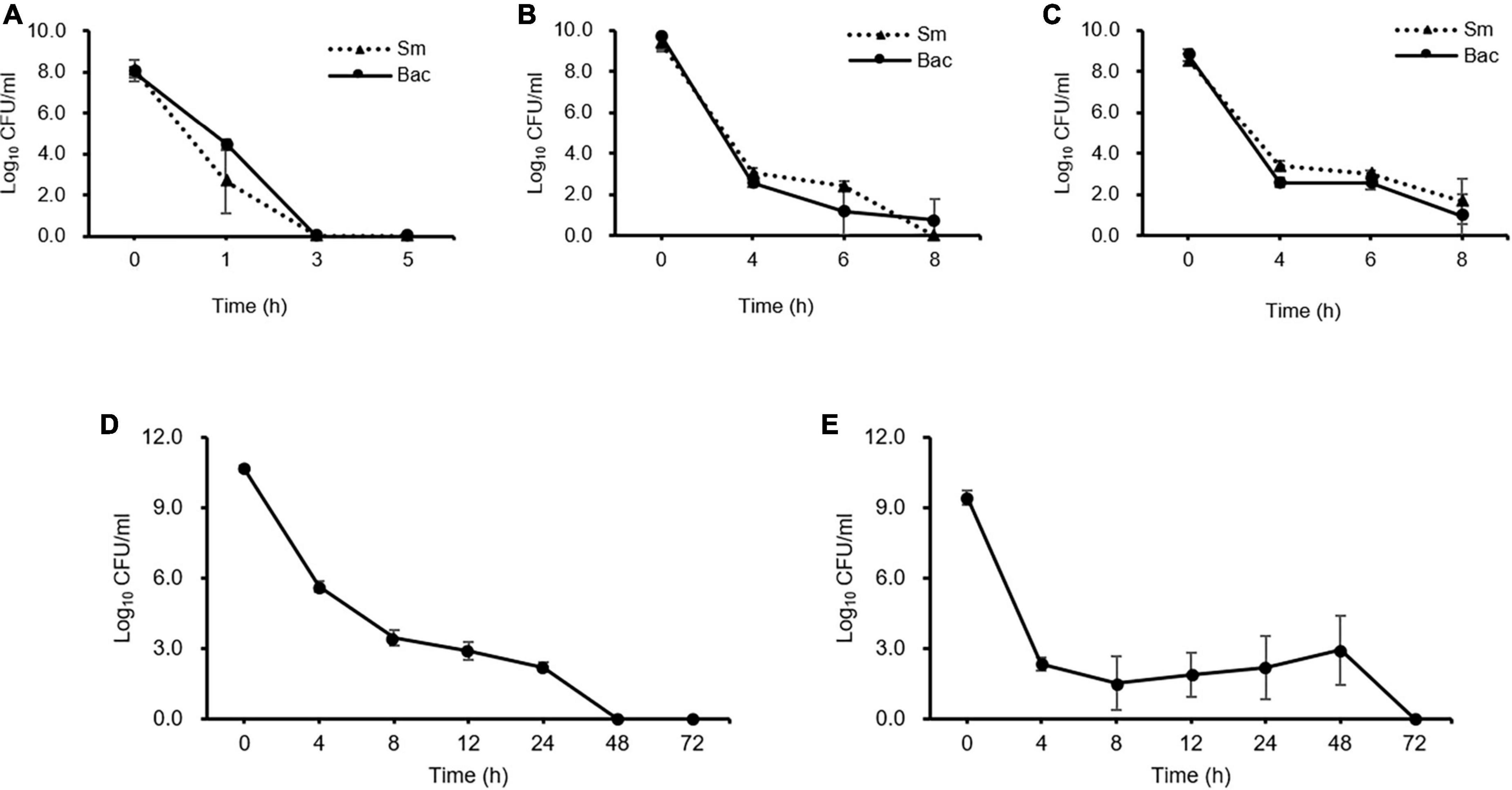
Figure 1. Comparison of persister cells after streptomycin treatments in Erwinia amylovora at 5 h for lag (A), 20 h for early stationary (B), and 96 h for late stationary (C) phases. E. amylovora cultures were treated with 50 and 100 mg/mL of laboratory and pesticide streptomycin, respectively, when the cells were inoculated in all three phases. The colony forming units were counted immediately after both streptomycin treatments and at 2 h intervals. Determination of the end point of the persister cells in E. amylovora in King’s B broth (D) and distilled water (E). Data represent means of two replicates performed twice; error bars represent the standard deviation.
VBNC state is induced after inducing the persistence state by streptomycin treatment
During the counting of total, live, and culturable cells, culturable cells were observed until days 1 and 2 in King’s B broth and distilled water, respectively (Figures 2A, B). These patterns were linked to those of the kinetic killing curves, indicating that the persistence state occurred early in cells that had contact with streptomycin and would last longer under severe stress condition than in single stress. However, unculturable cells that were considered to be in the VBNC state after the persistence state were analyzed by staining each individual cell according to its physiological characteristics. We were unable to get the five categories that Patel et al. (2021) defined, because in this study, only two fluorescent dyes (the green vitality indicator RSG and red membrane permeability indicator PI) and one mixture fluorescence of them were used. Instead, we identified three classes (Figure 2C) and quadrant (Supplementary Figure 3): class 1, redox-active cells with intact membranes being regarded VBNC cells in this experiment (green, Q4 in FITC-A image); class 2, which are redox-active cells with damaged membranes and considered to be true VBNC cells (yellow, Q2 in FITC-A image); class 3, redox-inactive cells with damaged membranes (red, Q1 in FITC-A image); and no defined class, unstaining cells (white, Q3 in FITC-A image).
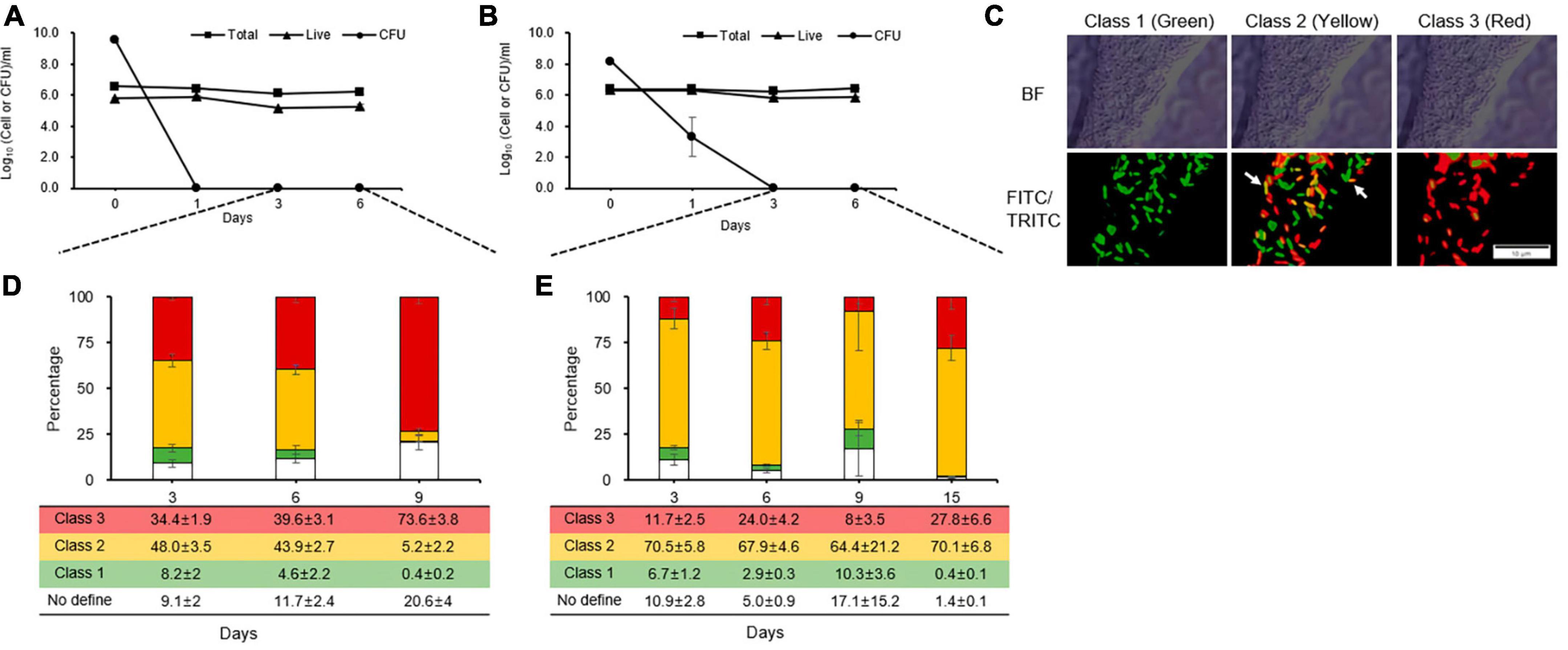
Figure 2. Distinct physiological states of unculturable Erwinia amylovora cells, presumable viable but non-culturable, after streptomycin treatment in King’s B broth (A) and distilled water (B). Closed squares, triangles, and circles represent total, viable, and culturable cell count from early stationary phase with streptomycin treatment, respectively. (C) Representative cells depicting E. amylovora stained with RSG and PI classes used in this study. The ratio represents the total proportion of all cells in a class at King’s B broth (D) and distilled water (E) at different time point, as counted across nine images collected from three repetitions. Error bars represent the standard deviation across three independent experiments. BF, bright field; FITC/TRITC, merged captures between 488 and 532 nm wavelength for GFP and RFP, respectively. Scale bar = 10 mm.
Up to 3 and 6 days after being treated with streptomycin in King’s B broth, an average of 34% and 39% of the populations were composed of class 3. After exposure to streptomycin at day 9, class 3 increased by an average of 73%, indicating that many populations changed to damaged membrane cells, which eventually became dead cells (Figure 2D). Unlike in King’s B broth, the populations VBNC cells in class 1 and 2 were the highest, with an average of 71, 70, 74, and 70% for 3, 6, 9, and 15 days, respectively, in streptomycin treated cells in distilled water (Figure 2E). However, class 3 populations did not significantly increase during the long exposure time to streptomycin. Thus, E. amylovora cells were maintained for longer periods as VBNC state in nutrient-deficient condition than in nutrient-rich one.
These findings allowed E. amylovora to experience periods of persistence and VBNC states after exposure to streptomycin, as shown in Figure 3. The persister cells were visible until day 1 before losing their culturability and became VBNC state during days 1–9, and they may have become dead in nutrient-rich condition such as King’s B broth after that time. The persister cells could be seen for up to 2 days in nutrient-deficient conditions such as the distilled water used in this study. After 2 days, they varnished and seemed to be sustained to VBNC cells for an additional 15 days, and they may begin to die after this time. In both conditions, both the persister and VBNC cells were found at those duration times, suggesting that the persistence and VBNC states represent a continuum of dormancy that aids in preventing target activation of streptomycin.
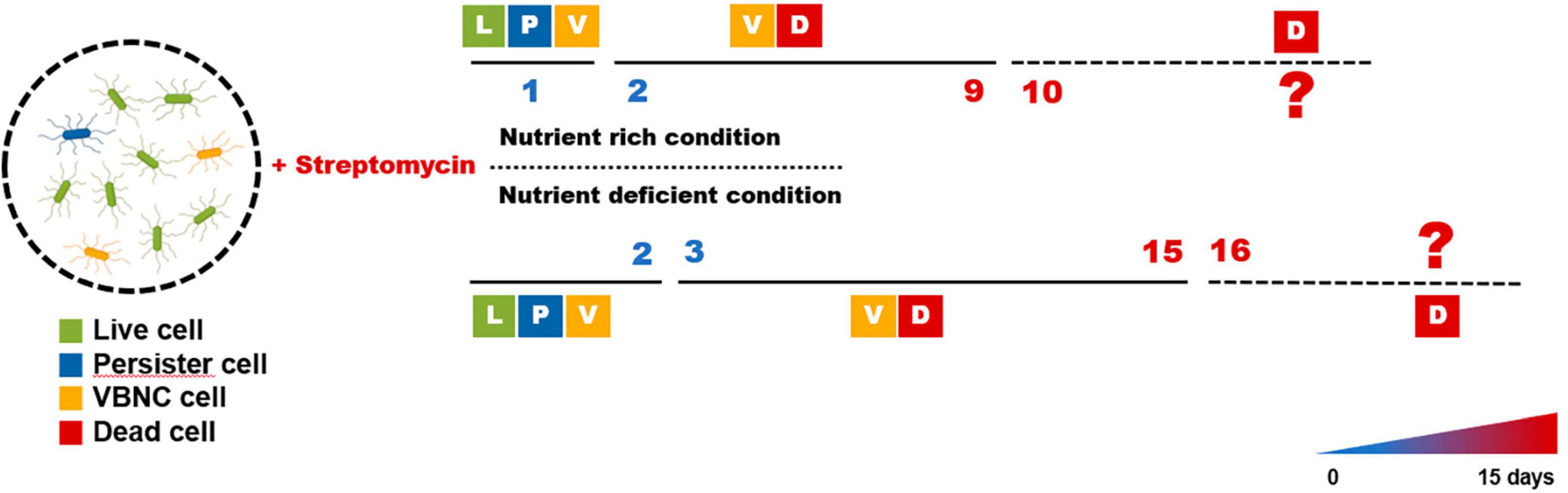
Figure 3. Predicted progression of the persister and viable but non-culturable (VBNC) cell formation in Erwinia amylovora after streptomycin treatment. Majority of the cells remained culturable after streptomycin treatment (called persister) for 1 and 2 days in King’s B broth and distilled water, respectively; however, few cells were presented as both live and VBNC state during those times. After each time point, bacterial cells were found to be in VBNC state, indicating that they lost culturability after continuous exposure to streptomycin. The membrane of all cells were affected after 10 and 15 days in nutrient rich and nutrient deficient conditions, respectively, to be considered dead cells.
Sucrose and copper reduce streptomycin-induced persistence and VBNC state in E. amylovora
The reduction rates of the persistence and VBNC states induced by streptomycin were evaluated through co-treatment cultivation with carbon sources and second treatment with chemical control agents for fire blight. When rates of stained cells were compared between streptomycin treatment and co-treatments with carbon sources, class 2 rate, which represents the persistence and VBNC state, decreased from 67% (Figure 2E) to 47% in sucrose co-treatment (Figure 4A). Conversely, the rates of class 3, which represents the dead cell caused by membrane damage, increased from 24% to a maximum of 51%, suggesting that sucrose is utilized to activate metabolism-related normal growth. The rates of stained cell types did not change significantly for glucose, fructose, and sorbitol (Figures 4B–D). However, in co-treatments of fructose and sorbitol, the rates of class 1 increased to a maximum of 27–34% at day 7, and populations of culturable cells increased significantly (data not shown), suggesting that these two carbon sources may have initiated re-growth. Thus, we concluded that glucose, fructose, and sorbitol should not be used in formulation of additive composites.
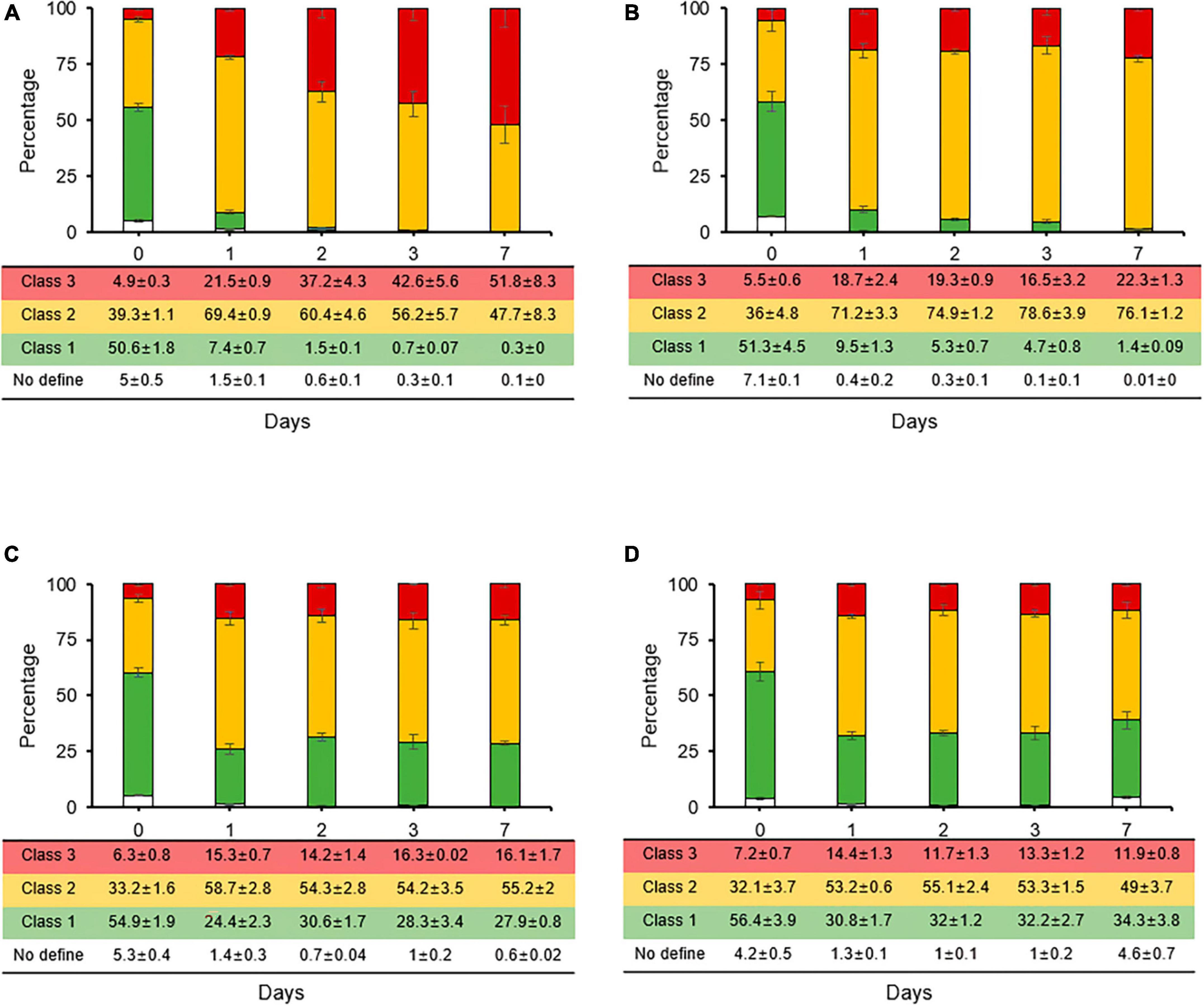
Figure 4. Relative proportions of Erwinia amylovora individuals in three physiological classes after streptomycin treatment with carbon sources such as sucrose (A), glucose (B), fructose (C), and sorbitol (D). Each ratio was calculated from nine fields with three repetitions. Error bars represent the standard deviation across three independent experiments.
The rates of the three classes in the second treatments of registered pesticides (streptomycin and oxytetracycline) all had the same ratios with streptomycin alone (Figures 5A–C), and the CFUs of culturable cells disappeared at day 3 (data not shown). These results suggest that the two most popular agriculture-antibiotics did not affect the persistence and VBNC states induced by first streptomycin treatment. However, all the types of stained cells quickly changed to class 3 in the second treatment with copper (Figure 5D); thus, we concluded that unculturable cells after 3 days with copper were not in the VBNC state but were all dead cells under in vitro condition. However, two or three treatments with different agricultural antibiotics are recommended as a control technique for fire blight under field conditions. Therefore, according to our results, copper should be used as a treatment once every three treatments.
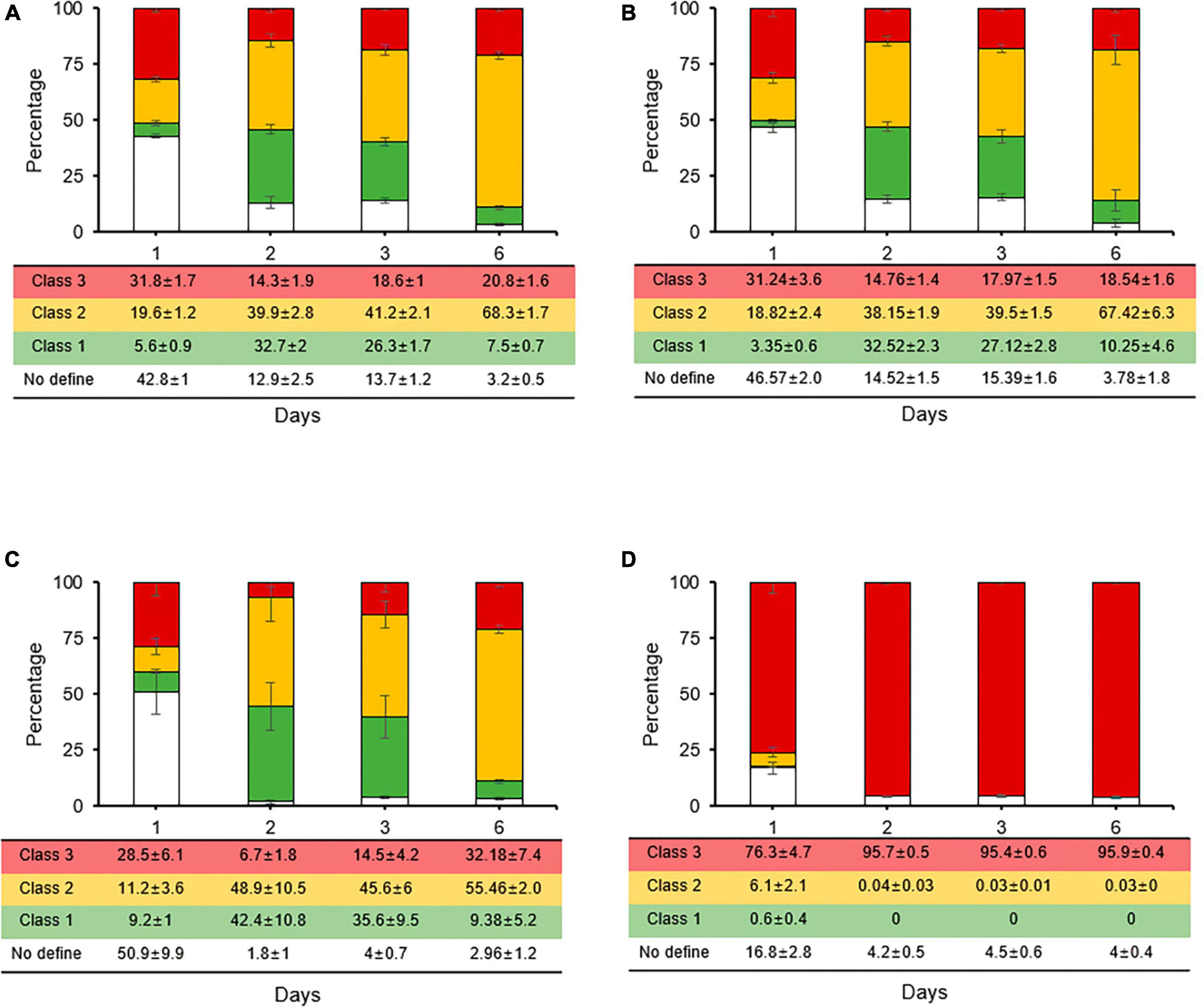
Figure 5. Relative proportions of Erwinia amylovora individuals in three physiological classes after single streptomycin treatment (A) following second treatment with streptomycin (B), tetracycline (C), and copper (D). Each ratio was calculated from nine fields with three repeats. Error bars represent the standard deviation across three independent experiments.
In the streptomycin absorption experiment, co-treatment of the persister cells with sucrose showed larger inhibitory zone against normal E. amylovora growth than co-treatment of the persister cells with glucose, fructose, and sorbitol (Table 1), and this corresponded to the changed ratios of classes 2 and 3. However, the inhibitory zones of the second treatment of antibiotics were significantly smaller in size than those of the carbon source treatment (Table 1), indicating that antibiotics were not activated by proton motive force.
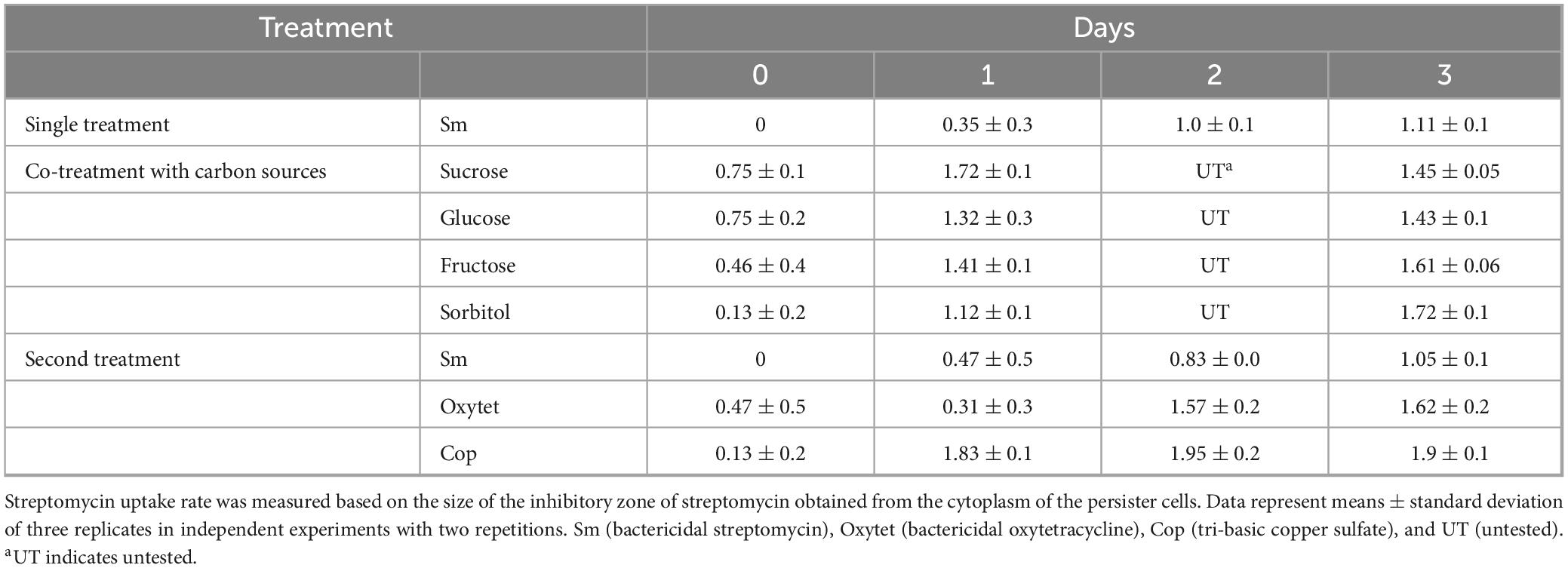
Table 1. Carbonsources enhance the uptake of streptomycin in streptomycin-induced Erwinia amylovora persister cells but not after the second antibiotic treatment.
Oxytetracycline eradicates streptomycin-induced persistence and VBNC state in E. amylovora
No change in the ratios of classes 2 and 3 in the second antibiotic treatment was observed, and persister cells of the secondary oxytetracycline treatment did not recover after 2 days (Figure 6A) compared to bactericidal streptomycin treatment alone (Figure 6B) and secondary bactericidal streptomycin treatment (Figure 6C), regardless of the types of amino acid and metabolites. Conversely, the recovered cells from the second streptomycin treatment showed comparable CFUs, indicating that the persister cells induced by single streptomycin treatment were not eliminated by second streptomycin treatment (data now shown). Thus, we concluded that streptomycin persister cells did not survive in the presence of oxytetracycline and oxytetracycline treatment because the second application was highly effective in eliminating culturable streptomycin persister cells.
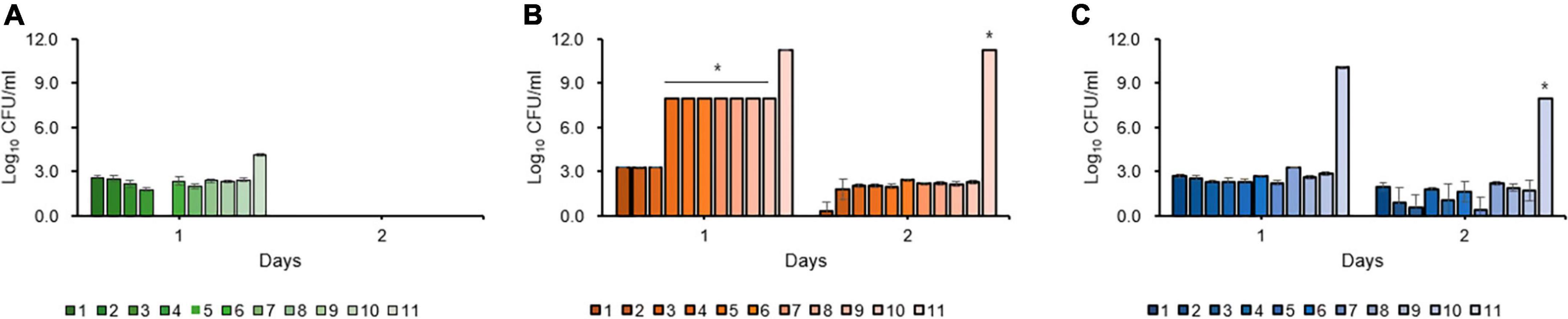
Figure 6. Resuscitation of persister and viable but non-culturable (VBNC) cells after single streptomycin treatment following second treatment with oxytetracycline (A), single bactericidal streptomycin (B), and second bactericidal streptomycin treatment (C). Oxytetracycline inhibits the formation and maintenance of the persister and VBNC cells of Erwinia amylovora induced by streptomycin in all tested amino acids and metabolites. Error bars represent the standard deviation across three independent experiments. *Indicates recovered colonies with each amino acids and metabolites; however, CFUs were not counted due to countless numbers.
Discussion
Regardless of the two- or three-times treatment strategies in pre- or post-blooming seasons, streptomycin remains an important component for controlling fire blight in Republic of Korea (RDA’s standard operating procedure (SOP) for fire blight control practice in Republic of Korea). According to the SOP, streptomycin and oxolinic acid should be applied using a two times strategy, and oxytetracycline, streptomycin, and oxolinic acid should be applied using a three times strategy. This suggest that streptomycin is a key component of chemical control strategy. However, because fire blight is still classified as a quarantine disease, any efficacy tests against this disease must be conducted by people certified to handle quarantine pathogens in a confined and insulated greenhouse called plant pest containment facility (Level 3), which we do not yet have. Thus, the effectiveness of their consecutive applications could not be guaranteed owing to the small scale of experimental designs and repetitions and the possibility of excessive subjectivity in evaluation of each test. Furthermore, in a few cases with copper (Ordax et al., 2006) but not in a majority of cases with antibiotics, results of the fire blight pathogen E. amylovora varied by dormancy, including the induction of the persister and VBNC cells by chemical control agents. Recently, Patel et al. (2021) reported that P. syringae pv. phaseolicola has the persistence to survive under streptomycin treatment. However, there is no study on the persistence and VBNC states in fire blight pathogen treated with streptomycin. Therefore, the present study aimed to identify the durations and ratios of the persistence and VBNC states that streptomycin induces in E. amylovora and the agents that eliminate these two states.
To achieve these, we first verified whether streptomycin was degraded by hydrolysis and photolysis during the test period. In a previous report, streptomycin lost its antibacterial activity due to degradation, and it was faster under light exposure water than it did in the dark water (Shen et al., 2017). However, in this study, throughout the measured durations for 7 and 5 days in water and UVB exposure, respectively, neither laboratory nor pesticide streptomycin was degraded in water under normal light at 28°C. This result showed that photo-degradation of kasugamycin and oxytetracycline had negative effects on their activities, but that of streptomycin did not have any negative impact on its activity (Slack et al., 2021). Thus, without the use of artificial streptomycin-degradable components, we could evaluate subsequent experiments, in which E. amylovora is exposed to streptomycin, to induce dormancy states.
Several important evidence for persistence differentiation between resistance and tolerance against antibiotics is the biphasic killing curve have been reported in the literature (Brauner et al., 2016; Renbarger et al., 2017; Balaban et al., 2019). In the early and late stationary phases, E. amylovora cells exhibited the two phases of the decline curve, that is, a rapid decline at first and a gradual decline at second with general concentrations of both laboratory and pesticide streptomycin, but this was not observed in the lag phase. Previous reports have shown that persistence is not logarithmic phase, and stationary phase cells have significantly higher numbers of the persistence cells than log phase cells (Ayrapetyan et al., 2018; Patel et al., 2021). Hence, early stationary phase studies were conducted in subsequent experiments. In addition, Psa2, the streptomycin-resistant strain that was isolated in Republic of Korea (Lee et al., 2018) and used as the negative control in this study, was able to replicate and survive, but E. amylovora was not able to replicate and survive. This means that E. amylovora cells were exposed to stress from streptomycin and were able to persist under stressful conditions.
The culturable cells were counted until 24 and 48 h in King’s B broth and distilled water using extended biphasic kill curve experiments and a BacLight Live/Dead viability kit. It means that persistent and living culturable cells would be damage or die from continuous exposure to streptomycin. Thus, the persister cells, which are culturable on solid media, seemed to change to VBNC as a result of continuous exposure to streptomycin until they are doomed to die. This suggests that persistence is pre-status before changing to VBNC and a continuum phenotype against stress such as streptomycin treatment. The VBNC cells are generally known to be in a deeper state than the persister cells (Ayrapetyan et al., 2018). In addition, following streptomycin treatment, E. amylovora cells were shown to exhibit persistence rather than tolerance because single cell staining ratios revealed three classes of subpopulations based on redox-activation and membrane stability. Generally, persistence affects only a subpopulation of cells under stress (Balaban et al., 2019); thus, it is clearly demonstrated that streptomycin treatment caused E. amylovora cells to become persistence rather than developing resistance to the antibiotics. Actually, single cell staining using PI and RSG, which are membrane permeability and redox indicators related to metabolic activity, respectively (Baert et al., 2016), in the Live/Dead viability kit and RSG Green vitality kit are very powerful tools to show status at single cell. Single cell observation using flow cytometry after staining indicate that all distinct cell statuses survive until culturable stage, and the administration of streptomycin causes a prolonged increase in membrane-damaged cells. These findings are consistent with previous reports that staining can confirm the presence of persister and VBNC cells in Escherichia coli (Kim et al., 2018b) and that the number of the VBNC cells is higher than that of the persister cells after antibiotic treatment (Bamford et al., 2017).
In addition, cell types and their up and down variations can be quantified using single cell staining with flow cytometry to identify second treatment agents that reduce the persister and VBNC cells after the initial streptomycin treatment. In previous reports involving environmentally relevant bacteria, glucose, mannitol, fructose, and pyruvate enabled the gentamicin killing of E. coli and Vibrio cholera persister cells (Allison et al., 2011; Paranjape and Shashidhar, 2020). E. coli persister cells use glycerol mostly as carbon sources (Amato et al., 2014), suggesting that increased antibiotic killing effects are stimulated by proton motive force after increased antibiotic uptake that was responsible for the persister cell killing (Orman and Brynildsen, 2013). Among the carbon sources investigated in this study, glucose and sucrose showed the greatest killing effect on E. amylovora persister cells induced by streptomycin. In addition, streptomycin potentiation by carbon sources in E. amylovora revealed an increase in inhibitory areas after carbon source treatment in comparison to single streptomycin treatment. This suggests that the increased uptake was responsible for the killing of the persister cells in E. amylovora. Hence, E. amylovora persister cells might be mostly eradicated in a more practical way using sucrose and then glucose in a metabolism-dependent manner.
To determine the most effective strategy for eradicating streptomycin persister cells using single cell observations in a real-world control system with antibiotics, we discovered that copper can completely eliminate persister cells that exhibit classes 1 and 2 that changed to class 3. This pattern was also described in Xanthomonas citri persister cells induced by antibiotics, high temperature, and metals (Martins et al., 2021). However, coppers were known agricultural agents with negative effects, such as injury on immature shoots and fruits on apple trees; hence, farmers prefer not to use them at any growing stage of apple trees. Thus, practical approaches are needed to define the usage of coppers in preventing injury and eliminating persister cells. In addition to the copper effect, second treatments with either streptomycin or tetracycline did not change the classes deduced by flow cytometry when compared to a single streptomycin treatment, indicating that neither of the antibiotic treatments were effective in eliminating the persister cells induced by streptomycin. Additionally, the diameters of the inhibitory zones were almost similar between the single streptomycin and second streptomycin or tetracycline treatments. This indicates that combined treatments of antibiotics, particularly aminoglycoside and tetracycline, did not increase the absorption of antibiotic by stimulating proton motive force. It is believed that antibiotics never used nutrients and growth factors to increase uptake of streptomycin. However, in the recovery experiments, E. amylovora persister cells completely lost their culturability on solid medium, suggesting that tetracycline has the strongest effect in eliminating the persister cells induced by streptomycin in a manner that is different from the metabolism-independent method. Hence, streptomycin treatment, with sucrose as supplement or additive agent in the final formulation, is recommended in orchards to have optimal result in field settings. Tetracycline should also be used in combination with other treatments rather than streptomycin alone to treat fire blight more effectively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YK: Writing – original draft, Conceptualization, Data curation, Formal Analysis, Methodology, Validation. HC: Formal Analysis, Data curation, Methodology, Writing – original draft. DP: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Rural Development Administration (PJ015302012023), Republic of Korea.
Acknowledgments
We would like to thank Prof. Gyoung Hee Kim at Sunchon National University, Republic of Korea for sharing Psa2 strain.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1346300/full#supplementary-material
References
Allison, K. R., Brynildsen, M. P., and Collins, J. J. (2011). Metabolite-enabled eradication of bacterial persisters by Aminoglycosides. Nature 473, 216–220.
Álvarez, B., López, M. M., and Biosca, E. G. (2008). Survival strategies and pathogenicity of Ralstonia solanacearum phylotype II subjected to prolonged starvation in environmental water microcosms. Microbiology 154, 3590– 3598.
Amato, S. M., Fazen, C. H., Henry, T. C., Mok, W. W., Orman, M. A., Sandvik, E. L., et al. (2014). The role of metabolism in bacterial persistence. Front. Microbiol. 5:70. doi: 10.3389/fmicb.2014.00070
Ayrapetyan, M., Williams, T. C., and Oliver, J. D. (2015). Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 23, 7–13. doi: 10.1016/j.tim.2014.09.004
Ayrapetyan, M., Williams, T. C., and Oliver, J. D. (2018). Relationship between the viable but nonculturable state and antibiotic persister cells. J. Bacteriol. 200:e00249-18. doi: 10.1128/JB.00249-18
Baert, J., Delepierre, A., Telek, S., Fickers, P., Toye, D., Delamotte, A., et al. (2016). Microbial population heterogeneity versus bioreactor heterogeneity: evaluation of Redox Sensor Green as an exogenous metabolic biosensor. Eng. Life Sci. 16, 643–651. doi: 10.1002/elsc.201500149
Balaban, N. Q., Helaine, S., Lewis, K., Ackermann, M., Aldridge, B., Andersson, D. I., et al. (2019). Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 17, 441–448. doi: 10.1038/s41579-019-0196-3
Bamford, R. A., Smith, A., Metz, J., Glover, G., Titball, R. W., and Pagliara, S. (2017). Investigating the physiology of viable but non-culturable bacteria by microfluidics and time-lapse microscopy. BMC Biol. 15:121. doi: 10.1186/s12915-017-0465-4
Becker, B., and Cooper, M. A. (2013). Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 8, 105–115. doi: 10.1021/cb3005116
Brauner, A., Fridman, O., Gefen, O., and Balaban, N. Q. (2016). Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330. doi: 10.1038/nrmicro.2016.34
Carter, A. P., Clemons, W. M., Brodersen, D. E., Morgan-Warren, R. J., Wimberly, B. T., and Ramakrishnan, V. (2000). Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348. doi: 10.1038/35030019
Chen, Z., Gao, Y., Lv, B., Sun, F., Yao, W., Wang, Y., et al. (2019). Hypoionic shock facilitates aminoglycoside killing of both nutrient shift- and starvation-induced bacterial persister cells by rapidly enhancing aminoglycoside uptake. Front. Microbiol. 10:2028. doi: 10.3389/fmicb.2019.02028
Chiou, C. S., and Jones, A. L. (1995). Molecular analysis of high-level streptomycin resistance in Erwinia amylovora. Phytopathology 85, 324–328. doi: 10.1094/Phyto-85-324
Choi, J. H., Kim, J. Y., and Park, D. H. (2022). Evidence of greater competitive fitness of Erwinia amylovora over E. pyrifoliae in Korean isolates. Plant Pathol. J. 38, 355–365. doi: 10.5423/PPJ.OA.04.2022.0056
Cunha, B. A. (2006). New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited. Med. Clin. N. Am. 90, 1089–1107. doi: 10.1016/j.mcna.2006.07.006
Davis, B. D. (1987). Mechanism of bactericidal action of Aminoglycosides. Microbiol. Rev. 51, 341–350. doi: 10.1128/mr.51.3.341-350.1987
Davis, B. D., Chen, L. L., and Tai, P. C. (1986). Misread protein creates membrane channels: an essential step in the bactericidal action of Aminoglycosides. Proc. Natl Acad. Sci. U. S. A. 83, 6164–6168. doi: 10.1073/pnas.83.16.6164
De León Door, A. P., Romo Chacón, A., and Acosta Muñiz, C. (2013). Detection of streptomycin resistance in Erwinia amylovora strains isolated from apple orchards in Chihuahua. Mexico. Eur. J. Plant Pathol. 137, 223–229. doi: 10.1007/s10658-013-0241-4
Fisher, R. A., Gollan, B., and Helaine, S. (2017). Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15, 453–464. doi: 10.1038/nrmicro.2017.42
Fried, A., Schell, E., Moltmann, E., and Wensing, A. (2014). Control of fire blight in Baden-Württemberg at the end of the streptomycin era. Acta Hortic. 1056, 55–56. doi: 10.17660/ActaHortic.2014.1056.5
Ghezzi, J. I., and Steck, T. R. (1999). Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol. Ecol. 30, 203–208. doi: 10.1111/j.1574-6941.1999.tb00648.x
Grey, B. E., and Steck, T. R. (2001). The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection. Appl. Environ. Microbiol. 67, 3866–3872. doi: 10.1128/AEM.67.9.3866-3872.2001
Ham, H., Lee, K. J., Hong, S. J., Kong, H. G., Lee, M.-H., Kim, H.-R., et al. (2020a). Outbreak of fire blight of apple and pear and its characteristics in Korea in 2019. Res. Plant Dis. 26, 239–249.
Ham, H., Lee, Y.-K., Kong, H. G., Hong, S. J., Lee, K. J., Oh, G.-R., et al. (2020b). Outbreak of fire blight of apple and Asian pear in 2015–2019 in Korea. Res. Plant Dis. 26, 222–228.
Jik Lee, H., Woo Lee, S., Suh, S., and Hyun, I. (2022). Recent spread and potential pathways for fire blight in South Korea. EPPO Bull. 52, 135–140.
Kang, I. J., Park, D. H., Lee, Y. K., Han, S. W., Kwak, Y. S., and Oh, C. S. (2021). Complete genome sequence of Erwinia amylovora strain TS3128, a Korean strain isolated in an Asian pear orchard in 2015. Microbiol. Resour. Announc. 10:e0069421. doi: 10.1128/MRA.00694-21
Kim, J. S., Chowdhury, N., Yamasaki, R., and Wood, T. K. (2018a). Viable but non-culturable and persistence describe the same bacterial stress state. Environ. Microbiol. 20, 2038–2048. doi: 10.1111/1462-2920.14075
Kim, J. S., Yamasaki, R., Song, S., Zhang, W., and Wood, T. K. (2018b). Single cell observations show persister cells wake based on ribosome content. Environ. Microbiol. 20, 2085–2098. doi: 10.1111/1462-2920.14093
Kim, Y. J., Choi, D. H., Choi, H. J., and Park, D. H. (2023). Risk of Erwinia amylovora transmission in viable but nonculturable (VBNC) state via contaminated pruning shears. Eur. J. Plant Pathol. 165, 433–445. doi: 10.1007/s10658-022-02615-6
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A., and Collins, J. J. (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810. doi: 10.1016/j.cell.2007.06.049
Lee, M. S., Lee, I., Kim, S. K., Oh, C.-S., and Park, D. H. (2018). In vitro screening of antibacterial agents for suppression of fire blight disease in Korea. Res. Plant Dis. 24, 41–51.
Lee, Y. S., Kim, G. H., Song, Y.-R., Oh, C.-S., Koh, Y. J., and Jung, J. S. (2020). Streptomycin resistant isolates of Pseudomonas syringae pv. actinidiae in Korea. Res. Plant Dis. 26, 44–47.
Martins, P. M. M., Merfa, M. V., Takita, M. A., and De Souza, A. A. (2018). Persistence in phytopathogenic bacteria: do we know enough? Front. Microbiol. 9:1099. doi: 10.3389/fmicb.2018.01099
Martins, P. M. M., Wood, T. K., and de Souza, A. A. (2021). Persister cells form in the plant pathogen Xanthomonas citri subsp. citri under different stress conditions. Microorganisms 9:384.
McManus, P. S., and Jones, A. L. (1994). Epidemiology and genetic analysis of streptomycin-resistant Erwinia amylovora from Michigan and evaluation of oxytetracycline for control. Phytopathology 84, 627–633. doi: 10.1094/Phyto-84-627
McManus, P. S., Stockwell, V. O., Sundin, G. W., and Jones, A. L. (2002). Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 40, 443–465.
Mikiciński, A., Puławska, J., Molzhigitova, A., and Sobiczewski, P. (2020). Bacterial species recognized for the first time for its biocontrol activity against fire blight (Erwinia amylovora). Eur. J. Plant Pathol. 156, 257–272.
Moller, W. J., Schroth, M. N., and Thomson, S. V. (1981). The scenario of fire blight and streptomycin resistance. Plant Dis. 65, 563–568.
Muranaka, L. S., Takita, M. A., Olivato, J. C., Kishi, L. T., and de Souza, A. A. (2012). Global expression profile of biofilm resistance to antimicrobial compounds in the plant-pathogenic bacterium Xylella fastidiosa reveals evidence of persister cells. J. Bacteriol. 194, 4561–4569. doi: 10.1128/JB.00436-12
Myung, I.-S., Lee, J.-Y., Yun, M.-J., Lee, Y.-H., Lee, Y.-K., Park, D.-H., et al. (2016). Fire blight of apple, caused by Erwinia amylovora, a new disease in Korea. Plant Dis. 100:1774.
Norelli, J. L., and Gilpatrick, J. D. (1982). Techniques for screening chemicals for fire blight control. Plant Dis. 66, 1162–1165.
Ordax, M., Marco-Noales, E., López, M. M., and Biosca, E. G. (2006). Survival strategy of Erwinia amylovora against copper: induction of the viable-but-nonculturable state. Appl. Environ. Microbiol. 72, 3482–3488.
Orman, M. A., and Brynildsen, M. P. (2013). Establishment of a method to rapidly assay bacterial persister metabolism. Antimicrob. Agents Chemother. 57, 4398–4409.
Paranjape, S. S., and Shashidhar, R. (2020). Glucose sensitizes the stationary and persistent population of Vibrio cholerae to ciprofloxacin. Arch. Microbiol. 202, 343–349.
Park, D. H., Lee, Y. G., Kim, J. S., Cha, J. S., and Oh, C.-S. (2017). Current status of fire blight caused by Erwinia amylovora and action for its management in Korea. J. Plant Pathol. 99, 59–63.
Park, D. H., Yu, J.-G., Oh, E.-J., Han, K.-S., Yea, M. C., Lee, S. J., et al. (2016). First report of fire blight disease on Asian pear caused by Erwinia amylovora in Korea. Plant Dis. 100:1946.
Patel, R. R., Kandel, P. P., Traverso, E., Hockett, K. L., and Triplett, L. R. (2021). Pseudomonas syringae pv. phaseolicola uses distinct modes of stationary-phase persistence to survive bacteriocin and streptomycin treatments. mBio 12:e00161-21.
Peng, J., Triplett, L. R., and Sundin, G. (2021). Activation of metabolic and stress responses during subtoxic expression of the type I toxin hok in Erwinia amylovora. BMC Genomics 22:74. doi: 10.1186/s12864-021-07376-w
Postnikova, O. A., Shao, J., Mock, N. M., Baker, C. J., and Nemchinov, L. G. (2015). Gene expression profiling in viable but nonculturable (VBNC) cells of Pseudomonas syringae pv. syringae. Front. Microbiol. 6:1419. doi: 10.3389/fmicb.2015.01419
Renbarger, T. L., Baker, J. M., and Sattley, W. M. (2017). Slow and steady wins the race: an examination of bacterial persistence. AIMS Microbiol. 3, 171–185.
Rodrigues, C. M., Takita, M. A., Coletta-Filho, H. D., Olivato, J. C., Caserta, R., Machado, M. A., et al. (2008). Copper resistance of biofilm cells of the plant pathogen Xylella fastidiosa. Appl. Microbiol. Biotechnol. 77, 1145–1157.
Santander, R. D., and Biosca, E. G. (2017). Erwinia amylovora psychrotrophic adaptations: evidence of pathogenic potential and survival at temperate and low environmental temperatures. PeerJ. 5:e3931. doi: 10.7717/peerj.3931
Santander, R. D., Oliver, J. D., and Biosca, E. G. (2014). Cellular, physiological, and molecular adaptive responses of Erwinia amylovora to starvation. FEMS Microbiol. Ecol. 88, 258–271. doi: 10.1111/1574-6941.12290
Schatz, A., Bugle, E., and Waksman, S. A. (1944). Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69. doi: 10.3181/00379727-55-14461
Shen, Y., Zhao, W., Zhang, C., Shan, Y., and Shi, J. (2017). Degradation of streptomycin in aquatic environment: kinetics, pathway, and antibacterial activity analysis. Environ. Sci. Pollut. Res. Int. 24, 14337–14345. doi: 10.1007/s11356-017-8978-5
Slack, S. M., Walters, K. J., Outwater, C. A., and Sundin, G. W. (2021). Effect of kasugamycin, oxytetracycline, and streptomycin on in-orchard population dynamics of Erwinia amylovora on apple flower stigmas. Plant Dis. 105, 1843–1850.
Sundin, G. W., and Bender, C. L. (1996). Dissemination of the strA-strB streptomycin-resistance genes among commensal and pathogenic bacteria from humans, animals, and plants. Mol. Ecol. 5, 133–143.
Sundin, G. W., and Wang, N. (2018). Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 56, 161–180.
Tancos, K. A., Villani, S., Kuehne, S., Borejsza-Wysocka, E., Breth, D., Carol, J., et al. (2016). Prevalence of streptomycin-resistant Erwinia amylovora in New York apple orchards. Plant Dis. 100, 802–809.
USDA (2019). Agricultural Chemical Use Program. Washington, DC: United States Department of Agriculture.
Venisse, J. S., Gullner, G., and Brisset, M. N. (2001). Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol. 125, 2164–2172.
Keywords: antibiotics, Erwinia amylovora, fire blight, persister cell, streptomycin, viable but non-culturable state
Citation: Kim YJ, Choi HS and Park DH (2024) Persistence and viable but non-culturable state induced by streptomycin in Erwinia amylovora. Front. Microbiol. 15:1346300. doi: 10.3389/fmicb.2024.1346300
Received: 29 November 2023; Accepted: 08 February 2024;
Published: 21 February 2024.
Edited by:
Takashi Azuma, Osaka Medical College, JapanReviewed by:
Alexandro Rodríguez-Rojas, University of Veterinary Medicine Vienna, AustriaJesús Muñoz-Rojas, Meritorious Autonomous University of Puebla, Mexico
Copyright © 2024 Kim, Choi and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duck Hwan Park, ZGhwQGthbmd3b24uYWMua3I=
 Yeon Ju Kim
Yeon Ju Kim Hyun Seo Choi
Hyun Seo Choi Duck Hwan Park
Duck Hwan Park