- 1Beijing Key Laboratory for Forest Pest Control, Beijing Forestry University, Beijing, China
- 2Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
- 3Sino-French Joint Laboratory for Invasive Forest Pests in Eurasia, INRAE-Beijing Forestry University, Beijing, China
- 4Guangdong Provincial Key Laboratory of Silviculture, Protection and Utilization, Guangdong Academy of Forestry, Guangzhou, China
- 5Department of Forest Protection, College of Forestry, Hebei Agricultural University, Baoding, China
In northeast China, the invasive woodwasp., Sirex noctilio, attacks Pinus sylvestris var. mongolica Litv and often shares habitat with native Sirex nitobei. Previous research showed that S. noctilio can utilize the volatiles from its symbiotic fungus (A. areolatum IGS-BD) to locate host trees. Consequently, symbiotic fungi (A. areolatum IGS-D and A. chailletii) carried by S. nitobei may influence the behavioral selection of S. noctilio. This study aimed to investigate the impact of fungal odor sources on S. noctilio’s behavior in laboratory and field experiments. Our observations revealed that female woodwasps exhibited greater attraction toward the fungal volatiles of 14-day-old Amylostereum IGS-D in a “Y”-tube olfactometer and wind tunnel. When woodwasps were released into bolts inoculated separately with three strains in the field, females of S. noctilio exhibited a preference for those bolts pre-inoculated with A. areolatum IGS-BD. Gas chromatography–mass spectrometry (GC–MS) analysis revealed that the volatiles emitted by the two genotypes of A. areolatum were similar yet significantly distinct from those of Ampelopsis chailletii. Hence, we postulate that the existence of native A. areolatum IGS-D could potentially facilitate the colonization of S. noctilio in scenarios with minimal or no A. areolatum IGS-BD present in the host.
1 Introduction
Sirex noctilio Fabricius (Hymenoptera: Siricidae), native to Europe and northern Africa, is a major pest in pine forests (Spradbery, 1973). It has accidentally invaded Oceania, Africa, and South America in the past century, and more recently, North America and northeast China through the plantations of exotic pines and human activities (Talbot, 1977; Spradbery and Kirk, 1978; Madden, 1988; Li et al., 2015; Sun et al., 2016). Host species S. noctilio include Pinus, Abies, Larix and Picea species etc. (Madden, 1988). Sirex noctilio has colonized only P. sylvestris var. mongolica Litv in northeast China (Li et al., 2015; Sun et al., 2016). Wasps deposit a combination of eggs, venom gland secretions, and the mutualist fungus Amylostereum via drills into the sapwood (Kobayashi et al., 1978; Fukuda and Hijii, 1996; Cooperband et al., 2012; Ryan et al., 2012b; Wang et al., 2017; Gao et al., 2022). The fungal mutualists can grow in host trees when the wasps are absent, although they usually depend on wasps for long-range dispersal (Wermelinger and Thomsen, 2012; Wang et al., 2020).
The process of oviposition by insect herbivores typically involves host location, followed by acceptance. Host location is primarily mediated by olfaction and vision, starting from a distance. Adult woodwasps exhibit a brief lifespan, necessitating the execution of mating and spawning behaviors within a distinctly limited timeframe (approximately 7 ± 4 days for males and 7 ± 3 days for females) (Fukuda et al., 1993; Slippers et al., 2012). Any delay in host location by 1–2 days significantly impacts their reproduction (Hajek et al., 2021). Consequently, it is hypothesized that S. noctilio exhibits a potent selection ability for promptly identifying hosts. Host plant volatiles, particularly monoterpenes, play a crucial role in attracting woodwasps to host trees over long distances (Madden, 1988; Slippers et al., 2015; Xu et al., 2019b). Monomers or blends of monoterpenes are commonly utilized in field pest monitoring to attract females (Erbilgin et al., 2002; Bashford, 2008; Batista et al., 2018). Additionally, fungal volatiles provide reliable cues for S. noctilio in assessing suitable hosts (Titze, 1965; Ryan et al., 2012a; Sarvary et al., 2016). Volatiles emitted by fungal symbionts seem to exert a stronger attractant effect compared to those produced by the host (Fernandez Ajo et al., 2015; Sarvary et al., 2016). A strong synergistic effect was observed between the volatiles emitted by the symbiotic fungi and those emitted by the host trees (Faal et al., 2021; Masagué et al., 2023).
Research has shown that at low population densities, S. noctilio preferentially selects relatively weak host trees, as these hosts are more conducive to the growth and development of their offspring (Slippers et al., 2012). Meanwhile, other bark beetles and wood borers are likely to infest weakened hosts, which, along with their fungal mutualists, can negatively impact both the availability and suitability of host trees for S. noctilio (Ryan et al., 2012a; Foelker, 2016; Wang et al., 2018). To avoid nutrient competition between populations, S. noctilio colonizes by avoiding locations where other insects lay their eggs (Wang, 2017; Xu et al., 2019b). Ryan discovered that S. noctilio avoided drilling into wood inoculated with the ophiostomatoid fungus Leptographium wingfieldii, a fungus vectored by bark beetles (Ryan and Hurley, 2012; Ryan et al., 2012a), confirming that symbiotic fungi associated with other insects can impact the selection of oviposition sites by S. noctilio. On the other hand, the endophytic fungi of host plants can also impact the selection of oviposition sites by S. noctilio. Infection of the host tree by Ophiostoma inhibits the growth of A. areolatum, thereby indirectly impacting the development of woodwasp larvae (Erbilgin et al., 2002; Phillips, 2002; Carnegie and Loch, 2010; Yousuf et al., 2014; Wang et al., 2018, 2019). Host tree endophytic fungi such as Trichoderma harzianum, T. viride, T. atroviride, and Phlebiopsis gigantea can effectively antagonize A. areolatum, reducing its competitive ability compared to other endophytic fungi (Wang et al., 2018). Ophiostoma minus and Aspergillus niger have been reported to exhibit strong repellent activity against unmated females of S. noctilio (Wang et al., 2018). These results investigate the interactions between the native woodwasp and the now sympatric invasive S. noctilio based on the signals from fungal volatiles and volatile pheromones in the host location.
Sirex nitobei Matsumura is the only native woodwasp that co-habits with the same hosts as S. noctilio in some parts of China (Wang et al., 2017). Both wasps attack stressed pines and may compete for habitat. Each wasp carries only one species of symbiotic fungus. Sirex nitobei was found to carry either Amylostereum chailletii or A. areolatum IGS-D (Wang et al., 2021). In China, S. noctilio consistently carries A. areolatum IGS-BD (Wang et al., 2021). However, when S. nitobei and S. noctilio shared the same host (Wang et al., 2017), a small proportion of S. nitobei individuals carried the invasive A. areolatum IGS-BD, a horizontal transmission from S. noctilio (Wang et al., 2021). This is because S. nitobei acquires the fungus from the host tree, where the symbiotic fungus of S. noctilio grows, rather than from the parent during the larval stage (Wang et al., 2021). This suggests that the presence of A. areolatum IGS-BD, already established in trees, would not hinder S. nitobei from colonizing the same tree. Hence, it is worth exploring whether the established symbiotic fungi of S. nitobei in the tree affect the selection behavior of S. noctilio. Based on the symbiotic relationship between siricid woodwasps and Amylostereum (Tabata et al., 2012; Sarvary et al., 2016), we speculate that the locations of oviposition strongly influence the horizontal acquisition of these symbionts. The objectives of this study were to assess the influence of different genotypes and species of fungi on the behavioral responses of S. noctilio in laboratory and field assays during the overlapping flight period. Here, we performed behavioral assays in the laboratory to test the directional movement of female S. noctilio and to identify volatile compounds among different genotypes and species of Amylostereum. In addition, we examined the oviposition behavior of invasive S. noctilio females in pine bolts (sections of wood), inoculated with the aforementioned fungi in the field.
2 Materials and methods
2.1 Insect rearing
Sirex noctilio adults were collected from four forest stands of damaged Pinus sylvestris var. mongolica in May (before the adult flight period) from Yushu City (YS), Jilin Province. Trees (16–30 cm in diameter at breast height, DBH) were felled, cut into bolts (1 m long), and sent to the quarantine laboratory of the Beijing Forestry University, China. The bolt ends were coated with wax to conserve moisture and to prevent contamination by other fungi, then moved to individual mesh bags at room temperature. Emerged woodwasps were collected daily. They were stored separately in clear plastic cups at 4°C until use. Mating trials were conducted outdoors: woodwasps were put into a square net cage based on a sex ratio of 15:5 (♂: ♀) for observation, and the mated woodwasps were marked for subsequent experiments (Supplementary Figure S1) (Fukuda and Hijii, 1996; Bao et al., 2018). Female insects used for the bioassay were 1–3 days old. The average forewing length was 23.14 ± 5.22 mm.
2.2 Fungal culture
Cultures of Amylostereum were obtained from mycangia of female adults [according to Thomsen and Harding (2011)]. The arthrospores were then transferred to Petri dishes containing an artificial medium made of potato dextrose agar (PDA) (40 g potato, 4 g glucose, and 3 g agar). Cultures maintained in the dark at ambient temperature (PGX-250A, Beijing, China, 25 ± 1°C) for the duration of each treatment [14 days, studies show that S. noctilio is attracted to its volatiles (Martínez et al., 2006; Jofré et al., 2016; Sarvary et al., 2016; Faal et al., 2021)]. Fungal strains were identified according to their morphological features and molecular tools (A. areolatum IGS-BD introduced by S. noctilio, A. areolatum IGS-D, or A. chailletii carried by native S. nitobei; YS, China) (Thomsen and Harding, 2011; Wang et al., 2021). The source of the odor was 64 cm2 of mycelium and culture medium (an amount that elicits a behavioral response) Fernandez Ajo et al., 2015; Sarvary et al., 2016).
2.3 Olfactometer assays
Sirex noctilio (149 mated and 177 unmated females) were exposed to volatiles in a Y-tube olfactometer (base: 30 cm, arms: 25 cm, diameter: 3 cm, angle between arms: 60°) between 9:00 a.m. and 15:00 p.m. The airflow was 0.1 ± 0.02 L min−1 and successively filtered through activated charcoal and distilled water. Petri dishes containing uninoculated PDA stored in the incubator were used as control groups. Two odor sources were enclosed within separate, sealed glass containers. All glassware were thoroughly cleaned using 75% alcohol and distilled water and then dried in an oven at 120°C (Yi Heng, DHG-9140A, Shanghai, China) after repeating each experiment (Wermelinger and Thomsen, 2012). To avoid visual asymmetries in the olfactometer arms and differences in the lighting conditions that could affect woodwasp selection, the arms were switched after each round of five female wasps. If the woodwasp moves to an olfactory arm more than 12.5 cm for at least 10s, it is identified as making a choice. The woodwasp that did not enter an arm after 10 min was considered “No response.” Binomial tests were performed on each responding wasp to assess biases toward fungal volatile vs. clean air.
2.4 Wind tunnel and video tracking
The fungi that attracted females in 2.3 assays have been tested in a wind tunnel to assess their attractiveness at long range under semi-field conditions. Both ends of the wind tunnel are covered with black epoxy metal mesh (160 cm long × 60 cm tall × 60 cm deep, operated at 29 ± 2°C and 70 ± 3% R. H., between 9.00 a.m. and 15.00 p.m.). Petri dishes containing PDA or fungus were used as an odor plume upwind (Figure 1). Woodwasps were released individually from the center of the downwind screen and recorded with two monochrome CCD video cameras (Cohu, San Diego, CA, United States). A software package of EthoVision 3.1, “Track 3D” (Noldus Information Technology, Wageningen, Netherlands), was used for recording 3D track data (30 frames / s). The air velocity was set at 200 mm / s in the positive x-axis (Cooperband et al., 2012; Sarvary et al., 2015). Woodwasps failed to appear on both cameras within 15 min were marked as “No response.” The recording stopped after the woodwasp had been in the camera’s view for more than 10 min. The woodwasp responses were categorized as landing on the upwind screen, the source, or elsewhere in the arena (Figure 1). The odor source (as above mentioned) was changed randomly on different dates.
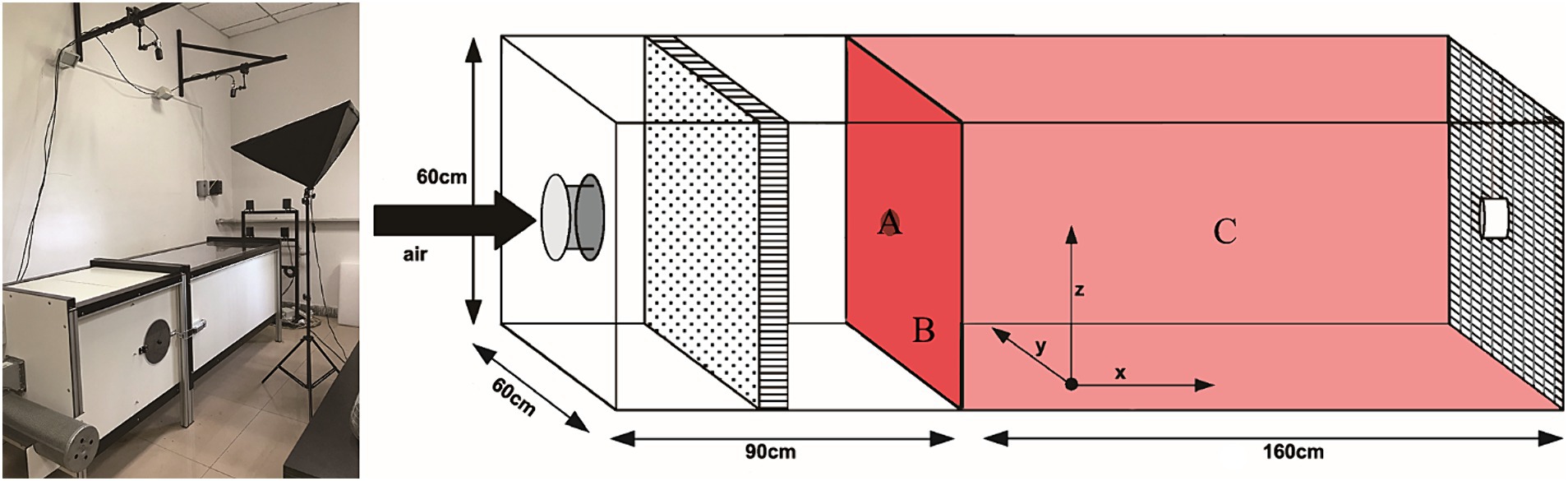
Figure 1. Schematic diagram of wind tunnel. (A) “the source,” (B) “upwind screen,” or (C) “elsewhere” in the arena.
2.5 Ovipositor drilling trials
2.5.1 Fungal inoculation of the experimental bolts
The fungi were cultured in 25°C darkness on PDA for more than 14 days. Circular plugs of different genotype fungi used for inoculating the bolts were cut from the mature cultures based on previous reports (Madden, 1974; Fukuda, 1997). Pinus sylvestris var. mongolica (with no sign of Sirex oviposition) was harvested from a nursery in Jianping County, Liaoning Province. Trees (DBH): 21.97 ± 0.28 cm (Wang et al., 2017) were cut into 100 cm long bolts. Two weeks prior to the experiment, all the bolts were randomly inoculated with the plugs of uninoculated PDA or fungi (A. areolatum IGS-BD, A. areolatum IGS-D, or A. chailletii). A 6-mm diameter electric drill (Lomvum-1201, China) was used to remove 16 “plugs” (approximately 10 mm deep) from the surface of the bolts. Plugs of uninoculated PDA and fungal plugs were placed into holes and then covered with sterilized sawdust (approximately 4 mm thick). There are 16 “plugs” on each bolt, 10 cm apart and 35 cm away from the cross-section of logs, aiming to reduce the potential impact of truncation (Supplementary Figure S2). The unused sawdust is dried in an oven at 80°C for 48 h to weigh and calculate the percentage moisture of the test logs ([% moisture = (wet weight - dry weight) / wet weight × 100]).
2.5.2 Oviposition behavior
The bolts inoculated with fungus or PDA were randomly placed in an outdoor 3 × 3 m mesh tent. Ten S. noctilio females were released in each replicate and observed for 8 h each day (between 9:00 a.m. and 17:00 p.m., except for rainy days, for a total of 30 females (15 mated)). We observed and recorded the spawning points and marked them in time by using colored markers. The cylindrical coordinate system was used to evaluate the surface of each bolt, and unique spatial coordinates were assigned to each spawning point on the bolts. This was accomplished by measuring the distance and direction from the closest “fungal plug.”
2.6 Volatile sampling and chemical analysis
The volatile organic compounds (VOCs) were collected from 14-day-old Amylostereum species (A. areolatum IGS-BD, IGS-D, and A. chailletii) by headspace solid phase microextraction (HS-SPME) (Wang et al., 2018), using a SPME fiber (65 μm, Supelco, Inc., Bellefonte, PA). The experiment involved three replicates for each strain. The silicon-based compounds present in the volatiles were removed. The preliminary identification of the volatiles was performed by combining the retention index(RI) and the National Institute of Standards and Technology database (NIST). To obtain the relative content of each volatile compound, the peak area was calculated through normalization.
2.7 Statistical analysis
One-way analysis of variance (ANOVA) was utilized to compare the response percentages of woodwasps to various odor sources from different fungal species. Tukey’s honestly significant difference (HSD) test was subsequently used to identify statistically significant differences between the fungi. The percentage of S. noctilio responding to different target stimuli was analyzed using Pearson’s chi-square test. We used a generalized linear model (GLM), assuming a binomial distribution of residuals, to assess the influence of mating status on selections. Additionally, we included the variable of target stimulus in the analysis, as experiments were conducted with varying target stimuli. The data were fitted to the model: “chosen source = mating status (either mated or unmated) * target stimulus.” All data analyses were performed using IBM SPSS Statistics 26 (Chicago, IL, United States).
Fisher’s exact test was used to assess variations in the percentage of responsive female S. noctilio landing on the upwind source, screen, or elsewhere. The track duration of woodwasps in response to different target stimuli was compared using a one-way ANOVA followed by Tukey’s HSD test. Differences in flight parameters (Supplementary Table S1) among treatments were assessed using a GLM, Tukey (T), or Games Howell test, depending on the equality of variances (SPSS 26 for Windows).
In the fungal inoculation experiment, differences in the average distance of spawning points between treatments were evaluated using the non-parametric Kruskal–Wallis H-test followed by Dunn’s test for multiple comparisons. The number of spawning points of woodwasps in response to different target stimuli was compared using a one-way ANOVA followed by Tukey’s HSD test (SPSS 26 for Windows). Log10 is used to convert the minimum distance (cm) for standardizing the data. This calculation was performed in R (version 4.0.3 for Windows). We recorded the distribution of drills in each bolt, treated the drills as spatial points, and estimated the spatial distribution using the maptools and spatstat packages in R (Baddeley et al., 2017). We assess the statistical significance of empirical estimates of k functions for a Monte Carlo simulation of 200 complete spatial randomness (CSR).
One-way ANOVA and Tukey’s HSD tests were performed to examine quantitative differences in concentrations of VOCs emitted by various fungal species. VOCs emitted by different fungi were grouped using principal component analysis (PCA) and cluster analysis. PCA was used to create a plot based on their overall volatile profiles. Hierarchical cluster analysis of each sample was conducted using the between-group linkage method and Euclidean distance. All data were analyzed using IBM SPSS Statistics version 26 for Windows.
3 Results
3.1 Olfactory responses to compounds of A. areolatum IGS-D and A. chailletii
Varied response levels were observed in female woodwasps to VOCs emitted by different fungi (F = 9.301, df = 5, p < 0.001; Figure 2). Mated woodwasps exhibited higher response levels when exposed to VOCs emitted by A. areolatum IGS-D and A. chailletii (65–71%). The lowest response level was observed in mated woodwasps exposed to the control group and A. chailletii (46%). Comparisons of the choices made by the responding wasps showed no bias toward any of the olfactometer arms when clean air was presented (p > 0.05). As shown in Figure 3, virgin females were attracted to A. areolatum IGS-D (χ2 = 5.488, p < 0.05) and A. chailletii (χ2 = 0.8, p = 0.37). There were no statistically significant differences in the proportion of unmated wasps that responded to the target stimulus arms (A. areolatum IGS-D vs. A. chailletii; χ2 = 0.29, p = 0.59). Mated females of S. noctilio displayed a strong response to the 14-day-old fungal culture; they responded positively to A. areolatum IGS-D (χ2 = 10.796, p < 0.001) (Figure 3). The target stimulus had the greater impact on the chosen source (GLM, χ2 = 29.816, p < 0.001), particularly evident for mated females (GLM, χ2 = 8.675, p < 0.05).

Figure 2. Percentage of Sirex noctilio responding to olfactometer stimuli (response) vs. showing no response to any arms (no response). Six independent experiments were conducted using various combinations of odor stimuli, and the percentage of woodwasps responding to these stimuli is presented. Red columns represent mated females, while blue represents unmated females. Numbers in parentheses represent the total number of woodwasps tested in each experiment. Different letters above the bars indicate statistical differences between experiments (p < 0.05).
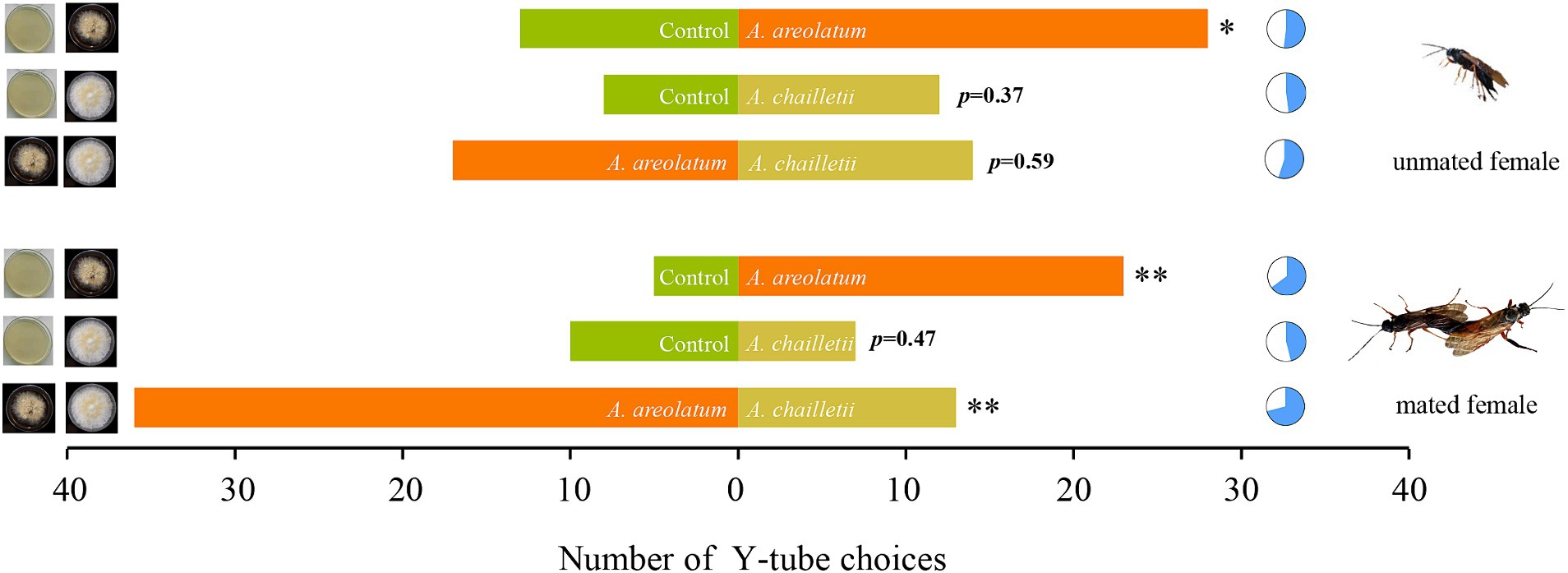
Figure 3. Number of Sirex noctilio responding to different treatments. Six independent experiments were conducted for mated and unmated female wasps landing on Amylostereum areolatum_D vs. A. chailletii cultures. The pie charts indicate the percentage of wasps responding to each treatment and the significant difference by marking with an asterisk (p-values are based on Pearson’s chi-square test, *p < 0.05; **p < 0.01).
Out of the 235 woodwasps tested, 215 (90%) responded by flying upwind. The upwind flight response differed significantly between treatments (χ2 = 11.388, p < 0.05). The distribution of landing areas exhibited statistically significant variation when exposed to A. areolatum IGS-D vs. PDA (χ2 = 9.519, p < 0.05). When exposed to the A. areolatum IGS-D, the percentage of landings on the upwind screen reached up to 23% (Figure 4: screen, n = 94). Volatiles from A. areolatum IGS-D caused 7% of the woodwasps that responded to land on the source center, a phenomenon not observed in other treatments (Figure 4: source). The track duration was greater for virgin females that landed on A. chailletii (Figure 5; t = 306.9 s, F = 13.01, p < 0.01) than for those exposed to A. areolatum IGS-D. The trajectories were reconstructed into 3D images, as shown in Supplementary Figure S3. The trajectories revealed that woodwasps tended to fly upwind along the x-axis in all treatments. The average flight speed exposed to A. areolatum IGS-D volatiles was 88.06 ± 19.94 mm/s. Woodwasps were more rapidly activated in the presence of a plume of A. areolatum IGS-D compared to those exposed to PDA (Table 1) (GLM, p < 0.05). Woodwasps exposed to A. areolatum IGS-D and A. chailletii exhibited significant differences in Path 3D and Path x-z (Path 3D: GLM, p < 0.05; Path x-z: GLM, p < 0.05). No statistical differences were observed among the other flight parameters (Table 1, p > 0.05).
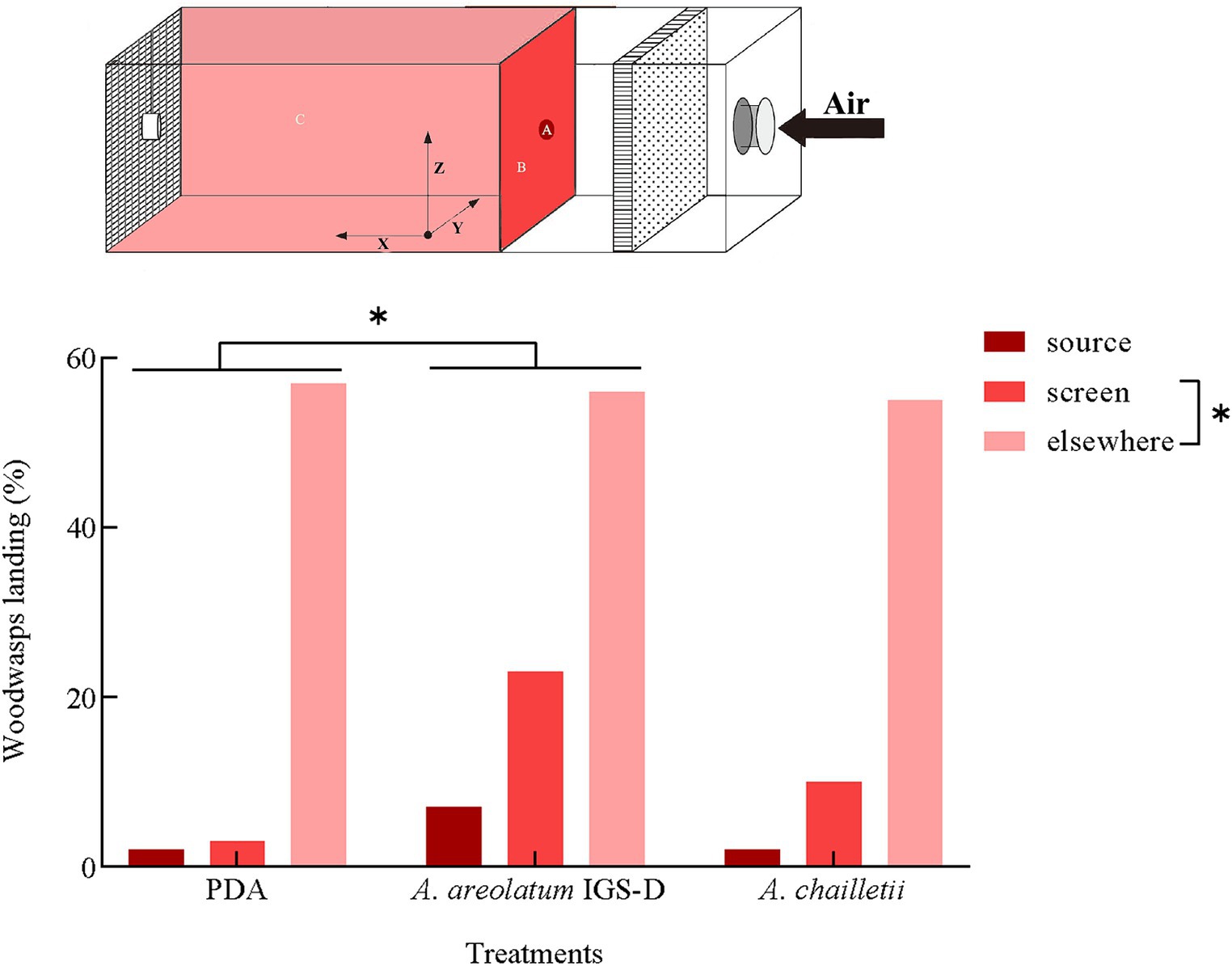
Figure 4. Percentage of responding Sirex noctilio females landing on the upwind source, screen, or elsewhere in the arena per treatment. Percentage (%) = percentage of woodwasps leaving the release site within 5 min (Pearson’s chi-square test, p < 0.05).
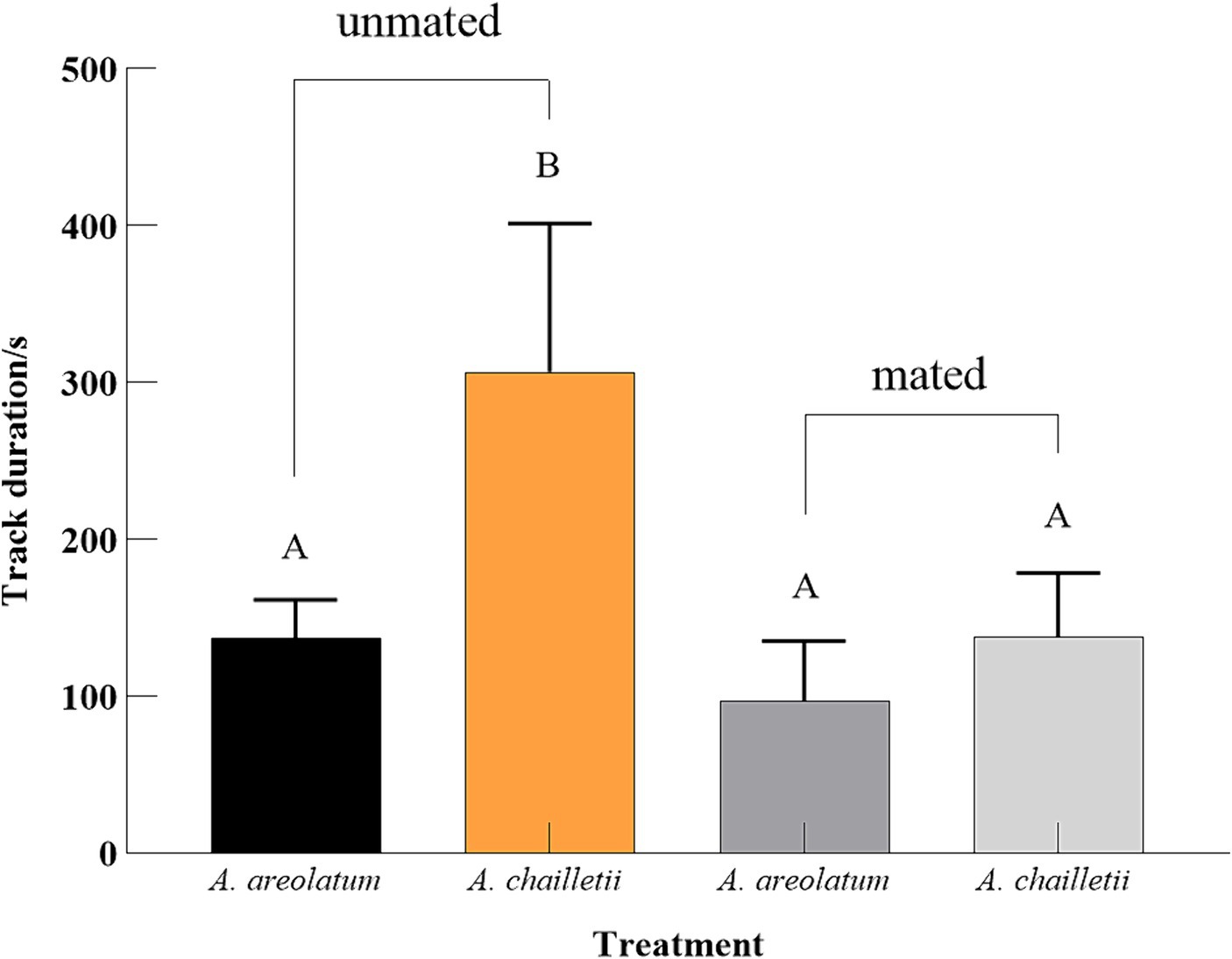
Figure 5. Mean track duration with four different treatments. Responding woodwasps landed on the upwind screen (one-way ANOVA followed by Tukey’s HSD test, p < 0.001).

Table 1. Summary of parameter values (mean ± SE) obtained from 3D flight tracks of Sirex noctilio responding to three treatments: uninoculated PDA (ck), Amylostereum areolatum IGS-D, and A. chailletii.
3.2 Symbiont-mediated oviposition choice
The origin of the coordinate represents the inoculation points, and the points of different colors represent the spawning points of S. noctilio females under different treatments (Supplementary Figure S4). All the bolts had moisture levels ranging from 43.3 to 47.5%, indicating a medium moisture content environment (Madden, 1968; Hajek et al., 2018). Inoculated fungi were isolated from most of the bolts. We observed significant spatial aggregation in the location of the spawning points when the spatial scale >3.5 cm (Figure 6), except for the control treatment. The K-function estimates of fungal-treated bolts were similar but different from those of the control (CSR) (Figure 6).
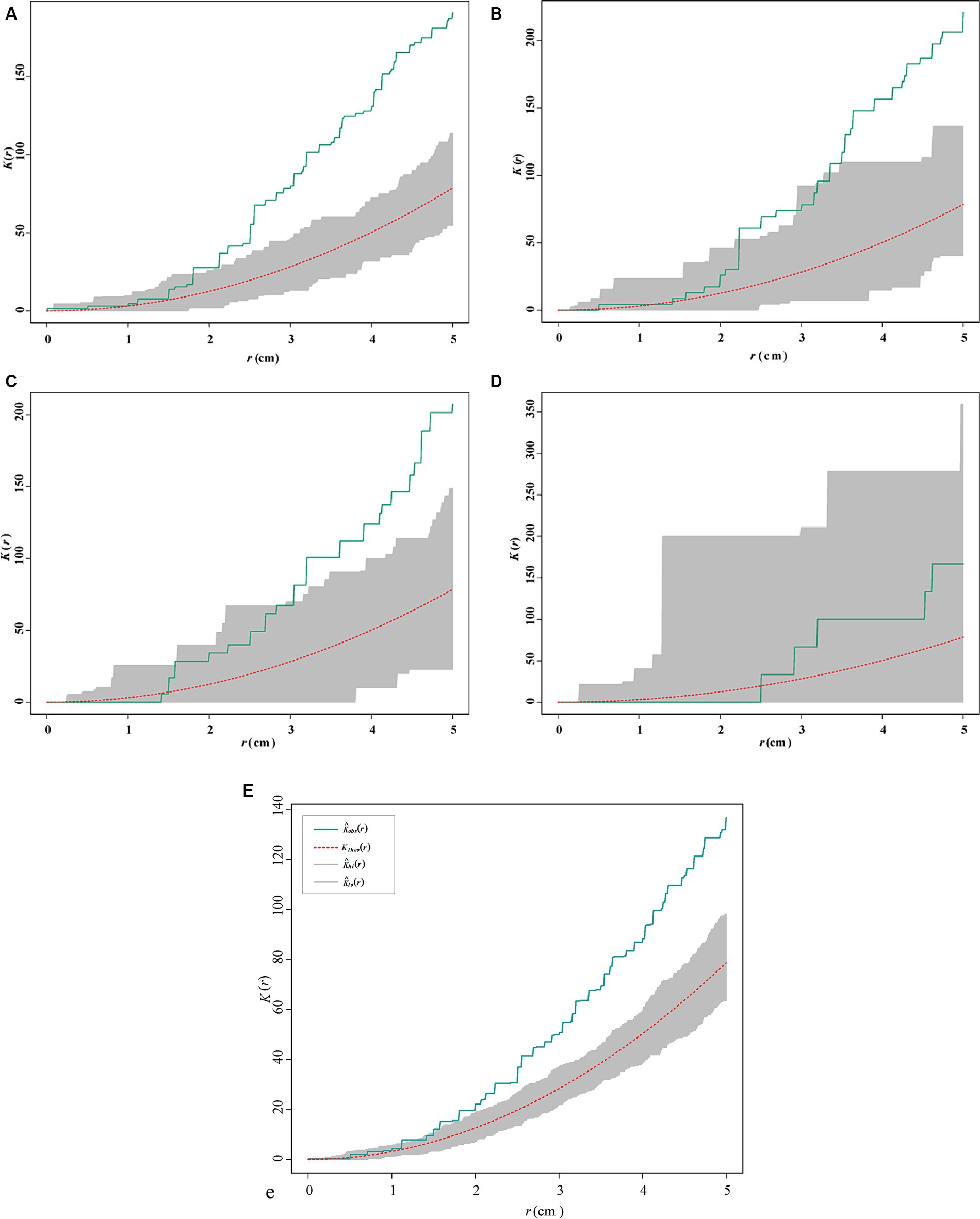
Figure 6. Ripley K-function estimates (solid green line) of the spatial location of the spawning points for Sirex noctilio in 2019 (A) Amylostereum areolatum_BD, (B) A. areolatum_D, (C) A. chailletii, (D) ck (uninoculated PDA), and (E) all treatments. The red dotted lines indicate the expected values under complete spatial randomness (CSR) based on 200 simulations.
In terms of spawning points per bolt, we found significant differences among treatments (Figure 7). Selection tests displayed that S. noctilio drilled more into bolts with the invasive A. areolatum IGS-BD (n = 43, p < 0.05) and the least drilling into control bolts (n = 8). Mean distances between spawning points and “fungal plus” or control were presented in Figure 7. We observed a significant treatment effect (p < 0.001). Sirex noctilio females drilled closest to A. areolatum IGS-BD (11.15 ± 1.19 cm) and PDA, and furthest to A. chailletii (21.55 ± 1.75 cm).
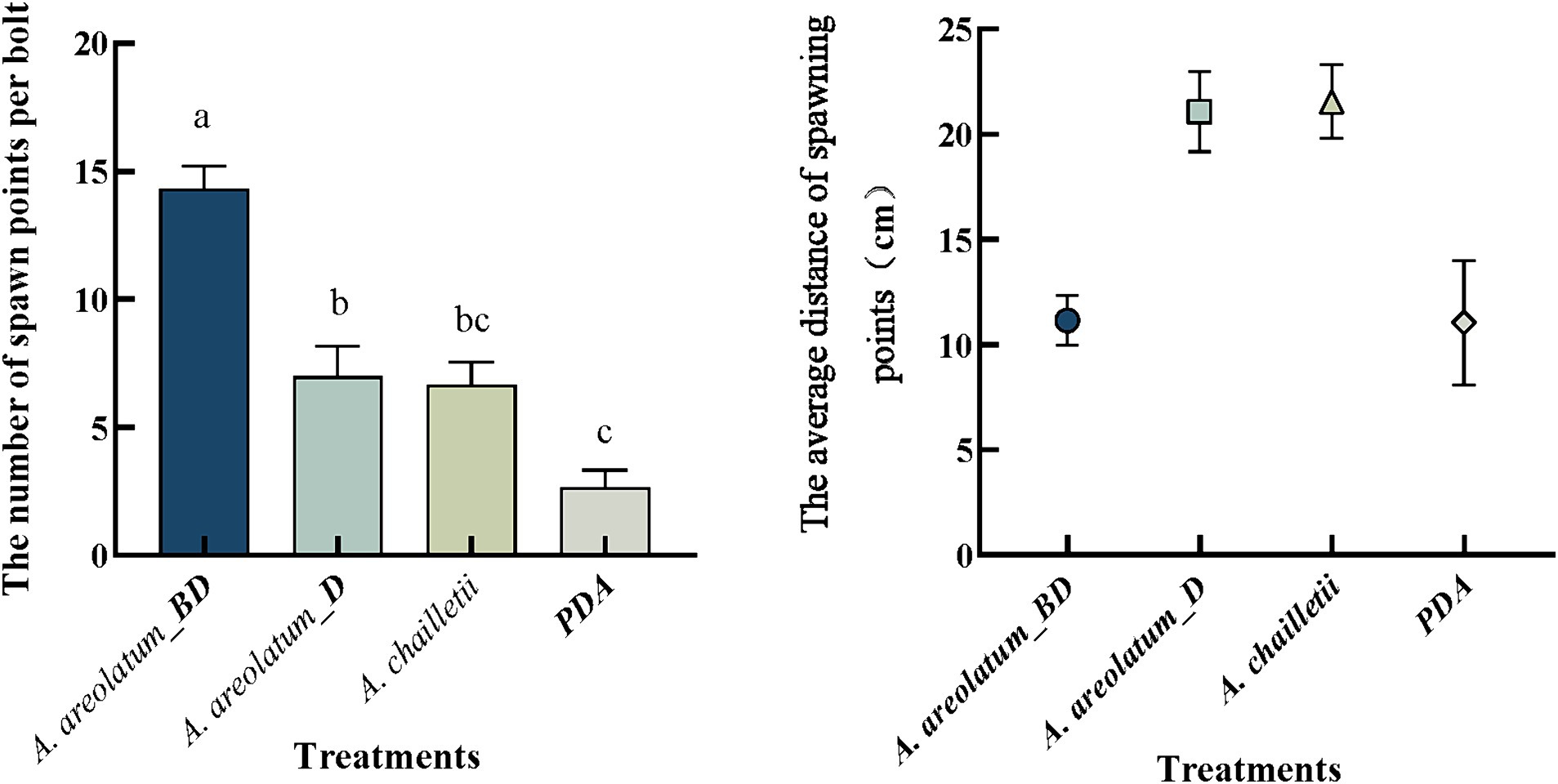
Figure 7. Number of spawning points per bolt and the average distance between spawning points and control or fungal plus. Different uppercase letters represent significant differences among the treatments (p < 0.05).
3.3 Composition of the fungal volatiles
The basic chemical composition of A. areolatum (IGS-BD or D) and A. chailletii were different. Forty-one VOCs were identified in fungal samples grown for 14 days. The major component of fungi volatiles was 1-octen-3-ol (59.06% ~ 71.93%) (Supplementary Figure S5; Table 2). The extracted volatiles mainly include alcohol (75.34% ~ 85.87%), aldehyde, ketone, and terpene (Figure 8).
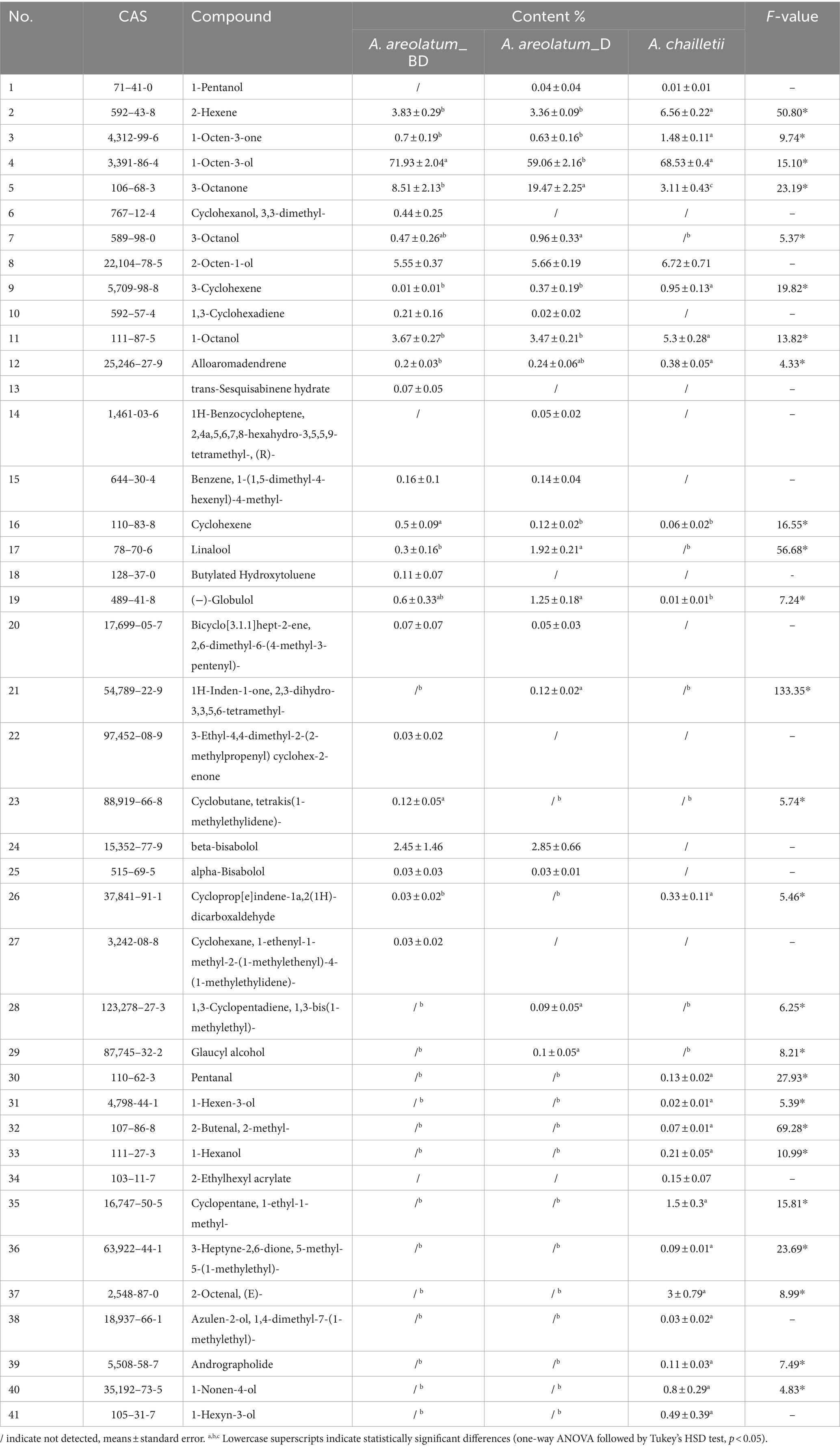
Table 2. Comparison of main volatile components between Amylostereum areolatum_BD, A. areolatum_D, and A. chailletii.
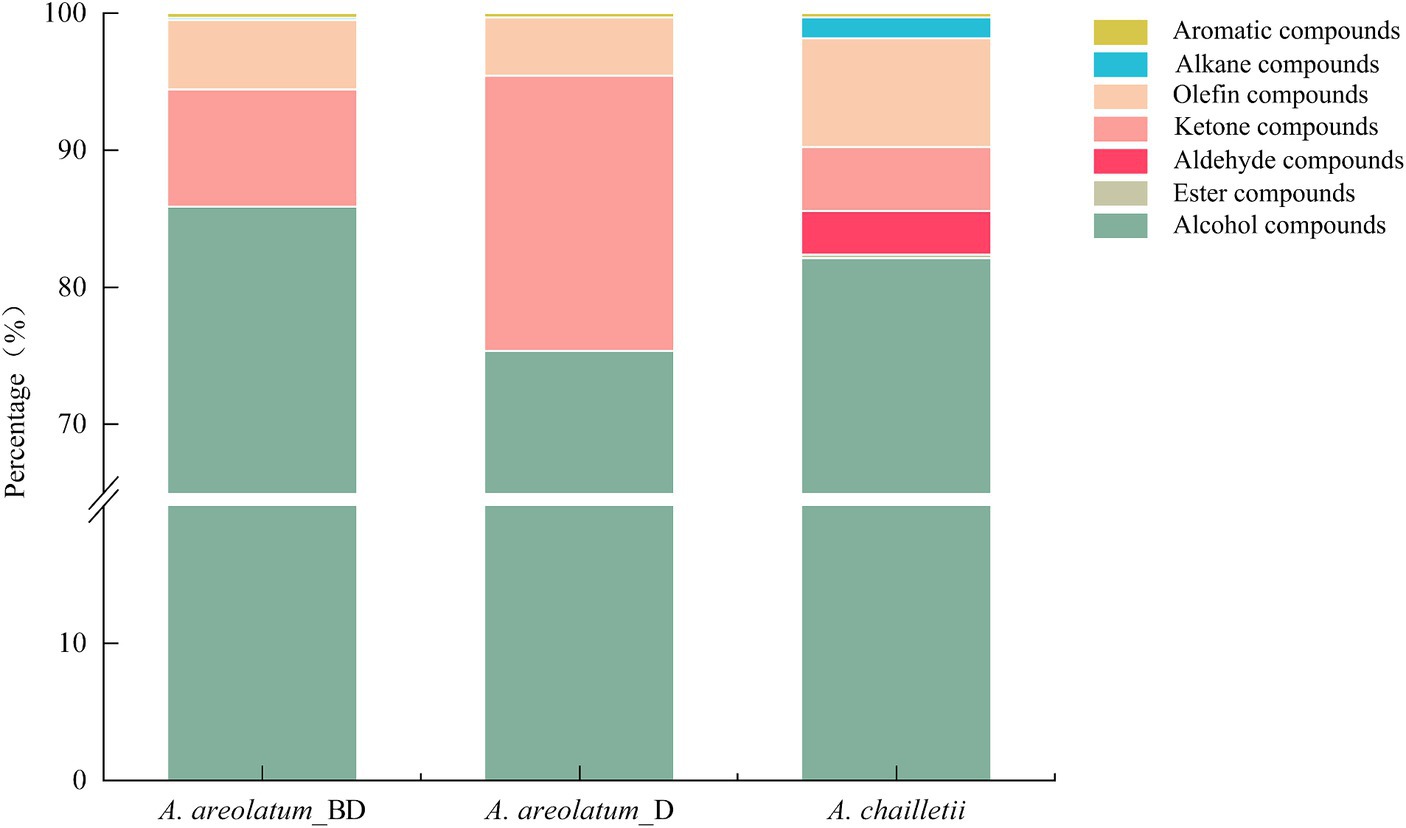
Figure 8. Relative content of volatile compound groups of Amylostereum areolatum (IGS_BD, D) and A. chailletii.
Figure 9 shows the PCA score plot (A) and loading plot (B) of volatile compounds. The PCs explained 39.2 and 15.9% of the variance (PC1 and PC2, respectively) (Figure 9). PC1 was strongly influenced by 2-butenal,2-methyl -, 3-heptyne-2,6-dione,5-methyl-5-(1-methylethyl)-, 2-hexene and 1-hexanol (2, 32, 33, 36) on the positive axis. As for PC2 on the positive side, 1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl-, linalool, 1,3-cyclopentadiene, 1,3-bis(1-methyl)- and glaucyl alcohol (17, 21, 28, 29) showed high loading (Figure 9B). Based on the hierarchical cluster analysis between-groups linkage (squared Euclidean distance), at a distance >7.2 and < 9.9, the samples were clustered into two groups. Amylostereum areolatum IGS-BD and D were grouped into one group, which means that the samples share similar characteristics. The main difference between the two clusters is that there are aldehydes and alkanes in the volatile compounds of A. chailletii (Figure 10).
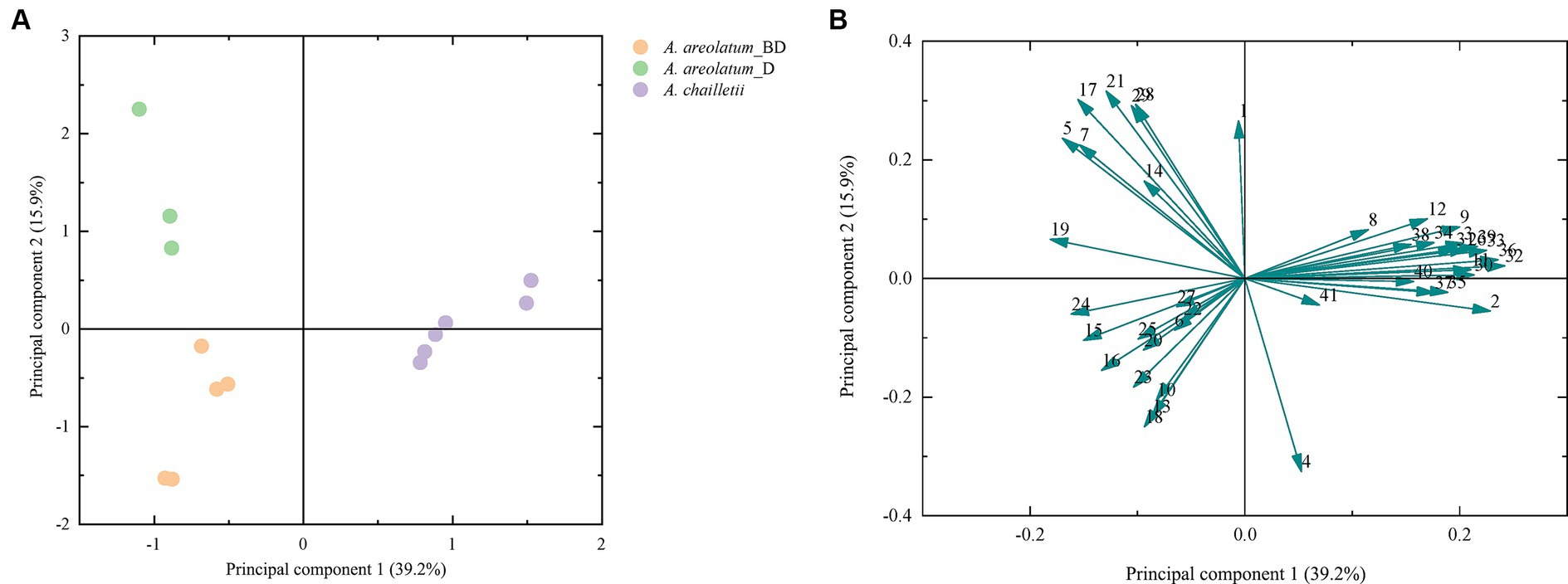
Figure 9. Score plot (A) and loading plot (B) from PCA of volatile compounds of Amylostereum areolatum (IGS_BD, D) and A. chailletii. Each number corresponds to Table 2.
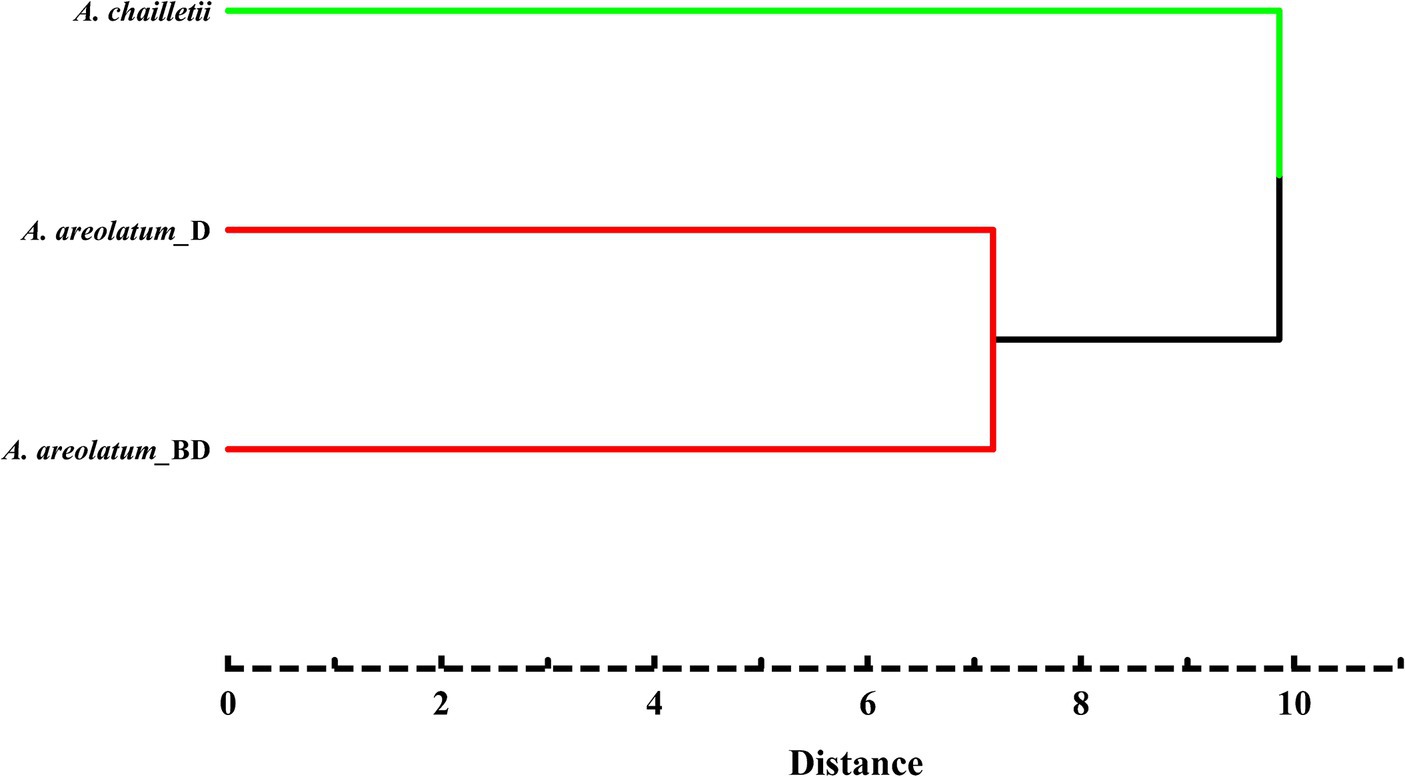
Figure 10. Cluster analysis diagram of volatiles of Amylostereum areolatum (IGS_BD, D) and A. chailletii based on Euclidean distance.
4 Discussion
4.1 Behavioral responses of Sirex noctilio to the symbiotic fungi that are in association with S. nitobei
Sirex noctilio females were attracted to Amylostereum areolatum IGS-D and A. chailletii, and mated females were significantly more active. This phenomenon is similar to that observed in South America and North America (Fernandez Ajo et al., 2015; Sarvary et al., 2015). It was also shown that there were significant differences in flight behavior between treatment groups during long-range directional upwind flight, especially in the odor source region. The results suggest that the overall response level is enhanced by fungal volatiles, implying that a heavily infested tree emits substantial amounts of attractive material. Sirex noctilio females drilled most into bolts inoculated with A. areolatum IGS-BD rather than into control bolts (Madden, 1974; Fernandez Ajo et al., 2015). Similarities between the volatile profiles of A. areolatum and those of Eurasian origin may be due to innate responses (Madden, 1988; Sarvary et al., 2016). Drillings in bolts inoculated with symbiotic fungi of S. nitobei were at an intermediate level. Consistent with these findings, females can detect A. areolatum volatiles in a semi-field setting (Fernandez Ajo et al., 2015; Sarvary et al., 2016; Wang et al., 2018).
It was observed that S. noctilio drilled the furthest distance (21.55 ± 1.75 cm) toward A. chailletii. Wooding et al. (2013) reported that very few S. noctilio individuals can carry A. chailletii. This phenomenon has not been found in China (Wang et al., 2021). Amylostereum areolatum and A. chailletii were only somatically incompatible, which is consistent with past results (Hajek et al., 2013; Castrillo et al., 2015). Amylostereum chailletii did not completely inhibit different genotypes of A. areolatum. Therefore, that may be a strategy to improve S. noctilio adaptability and potentially reduce the competitive antagonism with A. chailletii being pre-inoculated (Margrete Thomsen and Koch, 1999; Vasiliauskas and Stenlid, 1999).
A relatively uniform distribution of spawning sites of S. noctilio in Pinus radiata was noted by Madden (1974) in Tasmania. However, drilling was clustered in more resistant trees (Madden, 1974). This occurs because S. noctilio injects symbiotic fungi and toxins during oviposition, creating a conducive microenvironment for offspring development (Slippers et al., 2012). However, drill aggregation enhances the delivery of toxins and fungi, which synergistically resist tree defenses. Our findings are consistent with the phenomenon of S. noctilio in North America and the Southern Hemisphere making more drills to overcome tree defenses (Coutts and Dolezal, 1969; Madden, 1974; Thompson et al., 2014; Hajek et al., 2018). The drills of the control group were relatively random, suggesting that the woodwasp chose to inject venom and symbiotic fungi into the host to pre-condition wood (“pre-infection”) (Madden, 1974; Xu et al., 2019a).
Hajek et al. (2018) observed that females preferred wood without prior fungal emplacement (A. areolatum IGS-BD carried by S. noctilio or A. areolatum IGS-BE carried by S. nitidus) inconsistent with the report of attraction to fungal volatiles (A. areolatum IGS-BD) and our results, it is possible that the effects observed in those studies are mediated by chemicals operating at different distance scales (Sarvary et al., 2016; Hajek et al., 2018). Studies have shown that both the species and physiological conditions of host trees can affect the drills’ distribution (Hajek et al., 2018; Xu et al., 2019a). It has been hypothesized that differences in host species (North America: P. sylvestris or P. resinosa), invasion duration, and habitat in newly colonized areas may ultimately lead to different oviposition patterns. (Madden, 1974; Hoebeke et al., 2005; Hajek et al., 2018; Xu et al., 2019a).
4.2 The influence of symbiotic fungi volatiles on Sirex noctilio’s behavior and biological control
A total of 41 volatile components were identified by SPME-GC–MS, of which alcohol compounds were the most common. Some VOCs differ from those previously reported, possibly due to differences in the method and duration of volatile extraction (Bryant, 2010; Jofré et al., 2016; Sarvary et al., 2016; Faal et al., 2021; Masagué et al., 2023). Previous studies suggest that 2-hexene (6.56 ± 0.22%), Cycloprop[e] indene1a, 2(1H)-dicarboxaldehyde, and (−) -Globulol emitted by A. chailletii were attractant compounds (Wang et al., 2018). These compounds with a molecular weight of approximately 200 demonstrate sufficient volatility to act as airborne attractants at long distances (Nation, 2008; Wang et al., 2018). However, some alkane compounds and aldehyde compounds unique to A. chailletii may contain antifungal substances, which lead to the antagonistic effect on A. areolatum. Earlier findings revealed that A. chailletii had an obvious repelling effect on A. areolatum, but did not kill it (Hajek et al., 2013; Castrillo et al., 2015). This may also be the reason why S. noctilio drills farthest from A. chailletii.
The successful invasion of Dendroctonus valens (its associated fungus enhances the production and release of 3-carene) and Bursaphelenchus xylophilus (using chemicals already present at the invasion area) showed the role of semiochemicals in enhancing the biological invasion (Yan et al., 2005; Zhao, 2006; Lu et al., 2010). The volatiles of native A. areolatum IGS-D were similar to those of invasive A. areolatum IGS-BD, both of which could attract S. noctilio drilling aggregate. It may be more advantageous for females to quickly find a host tree that has been inoculated with symbiotic fungi that provide nutrients to their offspring.
It was speculated that the synergistic effect of the volatiles of native symbiotic fungi and host trees would be conducive to the colonization of S. noctilio. According to the bioassay results of our study, the volatiles of the symbiotic fungi can be mixed to prepare an effective attractant. The lures may be adjusted by optimizing the release rates of attractive different genotypes of A. areolatum fungal VOCs in the future (Faal et al., 2021).
The natural enemy, Ibalia leucospoides (“double-edged sword”) can locate the wasp larvae by symbiotic fungus VOCs (Madden, 1968; Spradbery, 1970; Martínez et al., 2006; Bryant, 2010; Pietrantuono et al., 2012; Robertson, 2014; Jofré et al., 2016; Faal et al., 2021). It was speculated that S. noctilio carrying different symbiotic fungi in this study can be a reliable signal for native parasitoids (I. leucospoides (Hochenw) (Wang, 2017)) as linalool can attract I. leucospoides females (Faal et al., 2021). However, linalool was not detected in A. chailletii, which could protect its host, S. nitobei (which carries A. chailletii), to a certain extent. In northeastern China, Sirex noctilio has often been present at low population levels, making it difficult to obtain individuals to conduct further field experiments. We suggest that further studies can be carried out to investigate the differences in volatile compounds between hosts after inoculation with different genotypes of symbiotic fungi and to assess their attractiveness to both woodwasps and their parasitoids in laboratory and field assays.
5 Conclusion
The current findings lead us to assume that females of S. noctilio can detect trees previously attacked by S. nitobei with fungal volatiles. Different genotypes of A. areolatum (IGS-BD and IGS-D) and A. chailletii were able to detect these compounds, which are attractive to S. noctilio, 2-hexene (6.56 ± 0.22%), cycloprop[e] indene-1a, 2 (1H)-dicarboxaldehyde, and (−) -globulol. Sirex noctilio is preferred to its symbiotic fungi (A. areolatum IGS-BD) but is furthest from A. chailletii. That may be a strategy to increase adult fitness and lessen the volatile-mediated antagonistic effects of A. chailletii. Studying the response of female woodwasps to these native fungi will help to understand the spatial distribution of woodwasps. Different species of siricids can co-infest the same trees; accompanied by fungal horizontal transmission, which could make pest management more difficult. The role of fungal volatiles may provide improved tools for surveying and dynamic monitoring of pests.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MW: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. CG: Resources, Writing – review & editing. QX: Resources, Writing – review & editing. NF: Resources, Writing – review & editing. JL: Resources, Writing – review & editing. LR: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. YL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Key R&D Program of China (2022YFD1401000) and Beijing’s Science and Technology Planning Project “Z201100008020001.” The authors express gratitude to TopEdit for their English language editing of this study.
Acknowledgments
The authors appreciate the help of Ciyuan Gao and Zhengtong Wang in collecting samples, as well as the workers at the Forest Pest Control and Quarantine Station of Yushu City for their assistance during fieldwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1341646/full#supplementary-material
References
Baddeley, A., Rubak, E., and Turner, R. (2017). Spatial point patterns: methodology and applications with R. Math. Geosci. 75, 1–6.doi: 10.18637/jss.v075.b02
Bao, M., Ren, L., Liu, X., Liu, R., Ao, T., Bai, S., et al. (2018). Mating behavior and attractiveness of male cuticle extracts based on electroantennogram and behavioral assay in Sirex noctilio Fabricius. J. Environ. Entomol. 40, 324–332. doi: 10.3969/j.issn.1674-0858.2018.02.12
Bashford, R. (2008). The development of static trapping systems to monitor for wood-boring insects in forestry plantations. Aust. For. 71, 236–241. doi: 10.1080/00049158.2008.10675041
Batista, E. S. P., Redak, R. A., Busoli, A. C., Camargo, M. B., and Allison, J. D. (2018). Trapping for Sirex Woodwasp in Brazilian pine plantations: lure, trap type and height of deployment. J. Insect Behav. 31, 210–221. doi: 10.1007/s10905-018-9674-0
Bryant, P. W. (2010). Kairomonal attraction of the parasitoid Ibalia leucospoides (Hymenoptera: Ibaliidae) to volatiles of the fungus Amylostereum areolatum, an obligate symbiont of the European woodwasp, Sirex noctilio. Syracuse, New York: State University of New York, College of Environmental Science and Forestry.
Carnegie, A. J., and Loch, A. D. (2010). Is Ips grandicollis disrupting the biological control of Sirex noctilio in Australia? In USDA Research Forum on Invasive Species. pp. 8–10.
Castrillo, L. A., Hajek, A. E., Pajares, J. A., Thomsen, I. M., Csóka, G., Kenaley, S. C., et al. (2015). Multilocus genotyping of Amylostereum spp. associated with Sirex noctilio and other woodwasps from Europe reveal clonal lineage introduced to the US. Fungal Biol. 119, 595–604. doi: 10.1016/j.funbio.2015.03.004
Cooperband, M. F., Böröczky, K., Hartness, A., Jones, T. H., Zylstra, K. E., Tumlinson, J. H., et al. (2012). Male-produced pheromone in the European Woodwasp, Sirex noctilio. J. Chem. Ecol. 38, 52–62. doi: 10.1007/s10886-012-0060-7
Coutts, M. P., and Dolezal, J. E. (1969). Emplacement of fungal spores by the Woodwasp, Sirex noctilio, during oviposition. For. Sci. 15, 412–416. doi: 10.1093/forestscience/15.4.412
Erbilgin, N., Nordheim, E. V., Aukema, B. H., and Raffa, K. F. (2002). Population dynamics ofIps Piniandips grandicollisin red pine plantations in Wisconsin: within- and between-year associations with predators, competitors, and habitat quality. Environ. Entomol. 31, 1043–1051. doi: 10.1603/0046-225X-31.6.1043
Faal, H., Cha, D. H., Hajek, A. E., and Teale, S. A. (2021). A double-edged sword: Amylostereum areolatum odors attract both Sirex noctilio (Hymenoptera: Siricidae) and its parasitoid. Ibalia leucospoidesFungal Ecol. 54:101108. doi: 10.1016/j.funeco.2021.101108
Fernandez Ajo, A. A., Martínez, A. S., Villacide, J. M., and Corley, J. C. (2015). Behavioural response of the woodwaspSirex noctilioto volatile emissions of its fungal symbiont. J. Appl. Entomol. 139, 654–659. doi: 10.1111/jen.12211
Foelker, C. J. (2016). Beneath the bark: associations amongSirex noctiliodevelopment, bluestain fungi, and pine host species inNorthAmerica. Ecol. Entomol. 41, 676–684. doi: 10.1111/een.12342
Fukuda, H. (1997). Resource utilization and reproductive strategy of three woodwasp species (Hymenoptera: Siricidae). Bull. Nagoya Univ. For. 16, 23–73.
Fukuda, H., and Hijii, N. (1996). Host-tree conditions affecting the oviposition activities of the Woodwasp, Sirex nitobei Matsumura (Hymenoptera: Siricidae). J. For. Res. Jpn. 1, 177–181. doi: 10.1007/BF02348198
Fukuda, H., Kajimura, H., and Hijii, N. (1993). Fecundity of the woodwasp, Sirex nitobei Matsumura, in relation to its body size. J. Jpn. For. Soc. 75, 405–408. doi: 10.11519/jjfs1953.75.5_405
Gao, C., Ren, L., Wang, M., Wang, Z., Fu, N., Wang, H., et al. (2022). Full-length transcriptome sequencing-based analysis of Pinus sylvestris var. mongolica in response to Sirex noctilio venom. Insects 13:13. doi: 10.3390/insects13040338
Hajek, A. E., Haavik, L. J., and Stephen, F. M. (Eds.) (2021). Biology and ecology of Sirex noctilio in North America. Morgantown, West Virginia: USDA, Forest Service, US Department of Argiculture, Forest Health Assessment and Applied Sciences Team.
Hajek, A. E., Nielsen, C., Kepler, R. M., Long, S. J., and Castrillo, L. (2013). Fidelity among Sirex Woodwasps and their fungal symbionts. Microb. Ecol. 65, 753–762. doi: 10.1007/s00248-013-0218-z
Hajek, A. E., Tobin, P. C., Kroll, S. A., and Long, S. J. (2018). Symbionts mediate oviposition behaviour in invasive and native woodwasps. Agric. For. Entomol. 20, 442–450. doi: 10.1111/afe.12276
Hoebeke, E. R., Haugen, D. A., and Haack, R. A. (2005). Sirex noctilio: discovery of a Palearctic Siricid Woodwasp in New York. The Great Lakes Entomologist, 1/2 Suppl.1, 50.
Jofré, N., Pildain, M. B., Cirigliano, A. M., Cabrera, G. M., Corley, J. C., and Martínez, A. S. (2016). Host selection Byibalia leucospoidesbased on temporal variations of volatiles from the hosts’ fungal symbiont. J. Appl. Entomol. 140, 736–743. doi: 10.1111/jen.12313
Kobayashi, T., Sasaki, K., and Enda, N. (1978). Correlation between Sirex nitobei and Amylostereum areolatum, associated with the death of Japanese pine trees during winter season. J. Jpn. For. Soc. 60, 405–411. doi: 10.11519/jjfs1953.60.11_405
Li, D., Shi, J., Lu, M., Ren, L., Zhen, C., and Luo, Y. (2015). Detection and identification of the invasive Sirex noctilio (Hymenoptera: Siricidae) fungal symbiont, Amylostereum areolatum (Russulales: Amylostereacea), in China and the stimulating effect of insect venom on laccase production by A. areolatum YQL03. J. Econ. Entomol. 108, 1136–1147. doi: 10.1093/jee/tov072
Lu, M., Wingfield, M., Gillette, N., Mori, S., and Sun, J. H. (2010). Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol. 187, 859–866. doi: 10.1111/j.1469-8137.2010.03316.x
Madden, J. L. (1968). Behavioural responses of parasites to the symbiotic fungus associated with Sirex noctilio F. Nature 218, 189–190. doi: 10.1038/218189a0
Madden, J. L. (1974). Oviposition behaviour of the Woodwasp, Sirex noctilio F. Aust. J. Zool. 22, 341–351. doi: 10.1071/zo9740341
Madden, J. L. (1988). “Sirex in Australasia” in Dynamics of Forest insect populations: Patterns, causes, implications population ecology. ed. A. A. Berryman (Boston, MA: Springer US), 407–429.
Margrete Thomsen, I., and Koch, J. (1999). Somatic compatibility in Amylostereum areolatum and A. Chailletii as a consequence of symbiosis with siricid woodwasps. Mycol. Res. 103, 817–823. doi: 10.1017/S0953756298007783
Martínez, A. S., Fernández-Arhex, V., and Corley, J. C. (2006). Chemical information from the fungus Amylostereum areolatum and host-foraging behaviour in the parasitoid Ibalia leucospoides. Physiol. Entomol. 31, 336–340. doi: 10.1111/j.1365-3032.2006.00523.x
Masagué, S., Fernández, P. C., Devescovi, F., Segura, D. F., de la Vega, G. J., Corley, J. C., et al. (2023). Oviposition substrate location by the invasive woodwasp Sirex noctilio: the combined effect of chemical cues emitted by its obligate symbiont Amylostereum areolatum and different host-tree species. Pest Manag. Sci. 79, 3959–3969. doi: 10.1002/ps.7596
Phillips, C. (2002). Learning to live with Sirex and Ips. Austr. For. Grower 25:19. doi: 10.3316/informit.525927967756984
Pietrantuono, A. L., Fernández-Arhex, V., Jofré, N., and Corley, J. C. (2012). Food and host searching decisions made by Ibalia leucospoides (Hymenoptera: Ibaliidae), a parasitoid of Sirex noctilio (Hymenoptera:Siricidae). J. Insect Behav. 25, 320–327. doi: 10.1007/s10905-011-9301-9
Robertson, D. J. (2014). Chemical ecology of Ibalia leucospoides ensiger, University of Georgia. 99. doi: 10.13140/RG.2.1.2799.8886
Ryan, K., de Groot, P., Davis, C., and Smith, S. M. (2012a). Effect of two bark beetle-vectored Fungi on the on-host search and oviposition behavior of the introduced Woodwasp Sirex noctilio (Hymenoptera: Siricidae) on Pinus sylvestris trees and logs. J. Insect Behav. 25, 453–466. doi: 10.1007/s10905-011-9313-5
Ryan, K., and Hurley, B. P. (2012). “Life history and biology of Sirex noctilio,” in The Sirex Woodwasp and its fungal symbiont: Research and Management of a Worldwide Invasive Pest, eds. B. Slippers, P. Grootde, and M. J. Wingfield (Dordrecht: Springer Netherlands), 15–30.
Ryan, K., de Groot, P., and Smith, S. M. (2012b). Evidence of interaction between Sirex noctilio and other species inhabiting the bole of Pinus. Agric. For. Entomol. 14, 187–195. doi: 10.1111/j.1461-9563.2011.00558.x
Sarvary, M. A., Cooperband, M. F., and Hajek, A. E. (2015). The importance of olfactory and visual cues in developing better monitoring tools forSirex noctilio(Hymenoptera: Siricidae). Agric. For. Entomol. 17, 29–35. doi: 10.1111/afe.12077
Sarvary, M., Hajek, A., Böröczky, K., Raguso, R., and Cooperband, M. (2016). Investigating the effects of symbiotic fungi on the flight behaviour of Sirex noctilio (Hymenoptera: Siricidae). Can. Entomol. 148, 543–551. doi: 10.4039/tce.2016.10
Slippers, B., Groot, P. D., and Wingfield, M. J. (2012). The Sirex woodwasp and its fungal symbiont. Netherlands: Springer.
Slippers, B., Hurley, B. P., and Wingfield, M. J. (2015). Sirex Woodwasp: a model for evolving management paradigms of invasive Forest pests. Annu. Rev. Entomol. 60, 601–619. doi: 10.1146/annurev-ento-010814-021118
Spradbery, J. P. (1970). Host finding by Rhyssa persuasoria (L.), an ichneumonid parasite of siricid woodwasps. Anim. Behav. 18, 103–114. doi: 10.1016/0003-3472(70)90077-1
Spradbery, J. P. (1973). A comparative study of the phytotoxic effects of siricid woodwasps on conifers. Ann. Appl. Biol. 75, 309–320. doi: 10.1111/j.1744-7348.1973.tb07980.x
Spradbery, J. P., and Kirk, A. A. (1978). Aspects of the ecology of siricid woodwasps (Hymenoptera: Siricidae) in Europe, North Africa and Turkey with special reference to the biological control of Sirex noctilio F. in Australia. Bull. Entomol Res. 68, 341–359. doi: 10.1017/S0007485300009330
Sun, X., Tao, J., Ren, L., Shi, J., and Luo, Y. (2016). Identification of Sirex noctilio (Hymenoptera: Siricidae) using a species-specific cytochrome C oxidase subunit I PCR assay. J. Econ. Entomol. 109, 1424–1430. doi: 10.1093/jee/tow060
Tabata, M., Miyata, H., and Maeto, K. (2012). “Siricid Woodwasps and their fungal symbionts in Asia, specifically those occurring in Japan,” in The Sirex Woodwasp and its fungal symbiont: Research and Management of a Worldwide Invasive Pest, eds. B. Slippers, P. Grootde, and M. J. Wingfield (Dordrecht: Springer Netherlands), 95–102.
Talbot, P. H. B. (1977). The Sirex-Amylostereum-Pinus association. Annu. Rev. Phytopathol. 15, 41–54. doi: 10.1146/annurev.py.15.090177.000353
Thompson, B. M., Bodart, J., McEwen, C., and Gruner, D. S. (2014). Adaptations for symbiont-mediated external digestion in Sirex noctilio (Hymenoptera: Siricidae). Ann. Entomol. Soc. Am. 107, 453–460. doi: 10.1603/AN13128
Thomsen, I. M., and Harding, S. (2011). Fungal symbionts of siricid woodwasps: isolation techniques and identification. For. Pathol. 41, 325–333. doi: 10.1111/j.1439-0329.2010.00677.x
Titze, J. (1965). Physiological suppression in Pinus radiata and its susceptibility to Sirex. Austr. For. Res. 1, 51–55.
Vasiliauskas, R., and Stenlid, J. (1999). Vegetative compatibility groups of Amylostereum areolatum and A. Chailletii from Sweden and Lithuania. Mycol. Res. 103, 824–829. doi: 10.1017/S0953756298007862
Wang, H. (2017). Study on natural enemies of Sirex noctilio and biology of its dominant parasitic wasp. Beijing Forestry University. doi: 10.26949/d.cnki.gblyu.2017.001040
Wang, M., Bao, M., Ao, T., Ren, L., and Luo, Y. (2017). Population distribution patterns and ecological niches of two Sirex species damaging Pinus sylvestris var. mongolica. Chin. J. Appl. Entomol. 54, 924–932. doi: 10.7679/j.issn.2095-1353.2017.110
Wang, M., Fu, N., Gao, C., Wang, L., Ren, L., and Luo, Y. (2021). Multilocus genotyping and intergenic spacer single nucleotide polymorphisms of Amylostereum areolatum (Russulales: Amylostereacea) symbionts of native and non-native Sirex species. J. Fungi 7:1065. doi: 10.3390/jof7121065
Wang, L., Li, C., Shi, J., Li, C., Li, J., Ren, L., et al. (2019). Incidental Fungi in host trees disrupt the development of Sirex noctilio (Hymenoptera: Siricidae) symbiotic fungus and larvae. J. Econ. Entomol. 113, 832–838. doi: 10.1093/jee/toz314
Wang, L., Ren, L., Liu, X.-B., Shi, J., Wang, J., and Luo, Y. (2018). Effects of endophytic fungi in Mongolian pine on the selection behavior of woodwasp (Sirex noctilio) and the growth of its fungal symbiont. Pest Manag. Sci. 75, 492–505. doi: 10.1002/ps.5146
Wang, M., Wang, L., Li, D., Fu, N., Li, C., Luo, Y., et al. (2020). Advances in the study of mutualism relationship between Amylostereum areolatum and Sirex noctilio. J. Temp. For. Res. 3, 1–11. doi: 10.3969/j.issn.2096-4900.2020.02.001
Wermelinger, B., and Thomsen, I. M. (2012). “The Woodwasp Sirex noctilio and its associated fungus Amylostereum areolatum in Europe,” in The Sirex Woodwasp and its fungal symbiont: Research and Management of a Worldwide Invasive Pest, eds. B. Slippers, P. Grootde, and M. J. Wingfield (Dordrecht: Springer Netherlands), 65–80.
Wooding, A. L., Wingfield, M. J., Hurley, B. P., Garnas, J. R., de Groot, P., and Slippers, B. (2013). Lack of fidelity revealed in an insect-fungal mutualism after invasion. Biol. Lett. 9:20130342. doi: 10.1098/rsbl.2013.0342
Xu, Q., Lv, H., Wu, C., Mao, Y., Song, G., Shi, J., et al. (2019a). Oviposition behavior of Sirex noctilio (Hymenoptera: Siricidae). Acta Entomol. Sin. 62, 468–474. doi: 10.16380/j.kcxb.2019.04.009
Xu, Q., Sun, X., Lu, P., Luo, Y., and Shi, J. (2019b). Volatile profiles of three tree species in the northeastern China and associated effects on Sirex noctilio activity. J. Plant Interact. 14, 334–339. doi: 10.1080/17429145.2019.1629035
Yan, Z., Sun, J., Don, O., and Zhang, Z. (2005). The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): an exotic invasive pest of pine in China. Biodivers. Conserv. 14, 1735–1760. doi: 10.1007/s10531-004-0697-9
Yousuf, F., Gurr, G. M., Carnegie, A. J., Bedding, R. A., Bashford, R., Gitau, C. W., et al. (2014). The bark beetle, Ips grandicollis, disrupts biological control of the woodwasp, Sirex noctilio, via fungal symbiont interactions. FEMS Microbiol. Ecol. 88, 38–47. doi: 10.1111/1574-6941.12267
Keywords: Sirex woodwasp, volatiles, olfactory assays, oviposition, symbiotic fungi
Citation: Wang M, Gao C, Xu Q, Fu N, Li J, Ren L and Luo Y (2024) Different genotypes and species of symbiotic fungi mediate the behavioral response of invasive Sirex noctilio fabricius (Hymenoptera: Siricidae). Front. Microbiol. 15:1341646. doi: 10.3389/fmicb.2024.1341646
Edited by:
Xiao-Li Bing, Nanjing Agricultural University, ChinaReviewed by:
Jinping Shu, Chinese Academy of Forestry, ChinaSakineh Abbasi, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), France
Lixiang Wang, Gansu Agricultural University, China
Copyright © 2024 Wang, Gao, Xu, Fu, Li, Ren and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: LiLi Ren, bGlseV9yZW5AYmpmdS5lZHUuY24=; YouQing Luo, eW91cWluZ2x1b0AxMjYuY29t
 Ming Wang
Ming Wang ChengLong Gao
ChengLong Gao QinWang Xu
QinWang Xu NingNing Fu
NingNing Fu JiaLe Li
JiaLe Li LiLi Ren
LiLi Ren YouQing Luo
YouQing Luo