
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 19 February 2024
Sec. Extreme Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1328083
This article is part of the Research Topic Exploring Microbial Mat Communities in Extreme Environments View all 9 articles
 Jessica Lumian1
Jessica Lumian1 Dawn Y. Sumner2
Dawn Y. Sumner2 Christen L. Grettenberger2,3
Christen L. Grettenberger2,3 Anne D. Jungblut4
Anne D. Jungblut4 Luiz Irber5
Luiz Irber5 N. Tessa Pierce-Ward5
N. Tessa Pierce-Ward5 C. Titus Brown5*
C. Titus Brown5*Cyanobacteria form diverse communities and are important primary producers in Antarctic freshwater environments, but their geographic distribution patterns in Antarctica and globally are still unresolved. There are however few genomes of cultured cyanobacteria from Antarctica available and therefore metagenome-assembled genomes (MAGs) from Antarctic cyanobacteria microbial mats provide an opportunity to explore distribution of uncultured taxa. These MAGs also allow comparison with metagenomes of cyanobacteria enriched communities from a range of habitats, geographic locations, and climates. However, most MAGs do not contain 16S rRNA gene sequences, making a 16S rRNA gene-based biogeography comparison difficult. An alternative technique is to use large-scale k-mer searching to find genomes of interest in public metagenomes. This paper presents the results of k-mer based searches for 5 Antarctic cyanobacteria MAGs from Lake Fryxell and Lake Vanda, assigned the names Phormidium pseudopriestleyi FRX01, Microcoleus sp. MP8IB2.171, Leptolyngbya sp. BulkMat.35, Pseudanabaenaceae cyanobacterium MP8IB2.15, and Leptolyngbyaceae cyanobacterium MP9P1.79 in 498,942 unassembled metagenomes from the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA). The Microcoleus sp. MP8IB2.171 MAG was found in a wide variety of environments, the P. pseudopriestleyi MAG was found in environments with challenging conditions, the Leptolyngbyaceae cyanobacterium MP9P1.79 MAG was only found in Antarctica, and the Leptolyngbya sp. BulkMat.35 and Pseudanabaenaceae cyanobacterium MP8IB2.15 MAGs were found in Antarctic and other cold environments. The findings based on metagenome matches and global comparisons suggest that these Antarctic cyanobacteria have distinct distribution patterns ranging from locally restricted to global distribution across the cold biosphere and other climatic zones.
Cyanobacteria are a diverse group of oxygenic photosynthetic bacteria that are prevalent in a wide range of environments. In terrestrial polar environments, such as lakes, ephemeral streams, and soils, cyanobacteria play an important part in supporting local ecosystems because of their role as primary producers (Stal, 2007; Quesada and Vincent, 2012; Chrismas et al., 2016). Cyanobacteria that thrive in Antarctica face many challenges including variable light availability, cold temperatures, and freeze-drying conditions. To withstand these conditions, cyanobacteria may have tolerance mechanisms encoded in their genomes (Chrismas et al., 2015, 2016). However, the presence of tolerance genes in their genomes may make it more difficult for polar cyanobacteria to compete with other cyanobacteria in non-polar environments. Consequently, some polar cyanobacteria may only occur in the extremes of polar environments, while others may also be present in environments that share similar conditions to the stresses they face in Antarctica, such as cold temperatures or light stress (Jungblut et al., 2016; Chrismas et al., 2018; Lumian et al., 2021).
Currently, polar cyanobacteria are underrepresented in genomic databases, despite the important role they play in primary productivity. Recent research has focused on characterizing cyanobacteria in benthic biofilms in perennially ice-covered lakes in the McMurdo Dry Valleys in Antarctica (Sumner et al., 2015; Zhang et al., 2015; Jungblut et al., 2016; Dillon et al., 2020; Grettenberger et al., 2020; Lumian et al., 2021). Due to a lack of grazers and limited water mixing in these lakes, vast microbial mats prosper and sustain complex geochemical gradients (Jungblut et al., 2016; Sumner et al., 2016; Lumian et al., 2021). These geochemical gradients structure competition within communities, which are also dealing with challenging environmental conditions, such as highly seasonal irradiance, nutrient limitation, cold temperatures, and in some cases sulfidic water (Jungblut et al., 2016; Dillon et al., 2020; Lumian et al., 2021).
The question of why Antarctic cyanobacteria can survive in challenging conditions and what other environments they grow in can be addressed by biogeography studies (Whitaker et al., 2003; Martiny et al., 2006; Fierer, 2008; Green et al., 2008). Previous 16S rRNA gene surveys based on amplicon sequencing provided support for the longstanding theory that microbes have unlimited dispersal and community distribution is selected by the environment (Baas-Becking, 1934; Jungblut et al., 2010; Harding et al., 2011). However, studies from other environments and climatic zones have shown that 16S rRNA gene and single gene markers might not provide sufficient information to resolve genotypes and populations. Yet, most biogeography studies on polar microbiomes and cyanobacteria to date are based on 16S rRNA gene amplicon sequencing in the context of local environmental conditions of sampling sites or pole-to-pole comparisons using clone library surveys and high throughput sequencing approaches (Taton et al., 2006; Namsaraev et al., 2010; Bahl et al., 2011; Jungblut et al., 2010; Moreira et al., 2013; Harke et al., 2016; Kleinteich et al., 2017; Ribeiro et al., 2018). Although 16S rRNA gene sequences are computationally easier to compare to each other, there are limitations to 16S rRNA gene-based biogeography studies. The 16S rRNA gene is conserved and therefore likely leads to an under estimation of genotype level richness. Furthermore, the short read length of high throughput sequencing only allows the coverage of a few variable regions which further reduces phylogenetic resolution. While recent genomic work has provided advances in biogeography of polar microbes (Chrismas et al., 2015), the 16S rRNA gene sequence may not assemble and bin well from metagenomes, which can prohibit MAGs from being incorporated into 16S rRNA gene-based biogeographical distributions.
An alternative to 16S rRNA gene-based biogeography is to apply comparative genomic approaches, but this is computationally more complicated because of the size and scale of metagenome datasets. One option is to use an alignment-based approach in which the reads are aligned to reference genomes, which has been done for large-scale viral genome discovery with Serratus (Edgar et al., 2022). Another option is to apply large-scale k-mer matching to unassembled metagenomes, which can be implemented using software like sourmash and its specialized implementation branchwater (Brown and Irber, 2016; Irber, 2020a,b; Brown, 2021; Irber et al., 2022a,b). These techniques open the possibility of using metagenomic data for biogeography studies by searching all publicly available metagenomes on the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (Leinonen et al., 2011) for MAGs of interest. In this paper, branchwater was used to search 498,942 unassembled metagenomes from the NCBI SRA for the presence of five Antarctic cyanobacteria MAGs that lack the 16S rRNA gene. Identifying global matches in the metagenomes allowed widespread biogeographical analyses. These findings provide new insights based on comparative genomic analyses into the distribution patterns of cyanobacteria in cold biospheres: some cyanobacteria MAGs were only found in cold or polar regions, while others were found in a variety of environmental conditions.
Phormidium pseudopriestleyi FRX01 is a well characterized cyanobacteria in Lake Fryxell, Antarctica (Lumian et al., 2021). Lake Fryxell is a perennially ice-covered lake located at 77.36° S, 162.6° E in the McMurdo Dry Valleys. The lake floor is covered with microbial mats to depths of almost 10 m, with P. pseudopriestleyi FRX01 dominating the mats at 9.8 m in depth in 2012, where light levels are low (1–2 μmol photons m-2 s-1) and sulfide is present in the water column (0.091 mg L−1) (Lumian et al., 2021). P. pseudopriestleyi FRX01 performs oxygenic photosynthesis in the presence of hydrogen sulfide, even though sulfide inhibits oxygenic photosynthesis (Sumner et al., 2015; Lumian et al., 2021). Lake conditions and sampling have been described in Jungblut et al. (2016), Dillon et al. (2020), and Lumian et al. (2021).
The Leptolyngbyaceae cyanobacterium MP9P1.79, Leptolyngbya sp. BulkMat.35, Microcoleus sp. MP8IB2.171, and Pseudanabaenaceae cyanobacterium MP8IB2.15 MAGs are from microbial mats sampled from Lake Vanda, McMurdo Dry Valleys. Lake Vanda is also a perennially ice-covered lake and is located at 77.53° S, 161.58° E. Microbial mats in Lake Vanda contain pinnacles that range from millimeters to centimeters tall. Unlike Lake Fryxell, there is no sulfide where we sampled, and it is better illuminated at the sampled location than Lake Fryxell, though samples from the inside of pinnacles receive little light (Sumner et al., 2016). Sampling methods and lake conditions have previously been described in Sumner et al. (2016).
The methods to obtain the P. pseudopriestleyi FRX01 MAG (ASM1731333v1) has been previously described in Lumian et al. (2021). Briefly, the P. pseudopriestleyi FRX01 MAG was obtained from a microbial mat sample sequenced on an Illumina HiSeq 2,500 PE250 platform and a laboratory culture was sequenced on an Illumina 2000 PE100 platform. The microbial mat sample was quality filtered, and forward and reverse reads were joined using PEAR v0.9.6 (Zhang et al., 2014). For the isolated strain, trimmomatic v0.36 (Bolger et al., 2014) was used to trim sequencing adapters, and the interleave-reads.py script in khmer v2.1.2 (Crusoe et al., 2015) was used to interleave the reads. Both samples were assembled separately and together as a co-assembly by MEGAHIT v1.1.2 (Li et al., 2015) and mapped with bwa v2.3 (Li, 2013) and samtools v1.9 (Li et al., 2009). A single cyanobacteria bin was obtained using the CONCOCT binning algorithm in anvi’o and identified using CheckM (Eren et al., 2015; Parks et al., 2015; Delmont and Eren, 2018). The P. pseudopriestleyi FRX01 bin was refined with spacegraphcats to extract additional content from the metagenomes with a k-mer size of 21 and a radius of 1 (Brown et al., 2020).
Methods to obtain the Microcoleus sp. MP8IB2.171, P. cyanobacterium MP8IB2.15, Leptolyngbya sp. BulkMat.35, and Leptolyngbyaceae cyanobacterium MP9P1.79 MAGs from Lake Vanda were described in (Lumian et al., 2024). Filtered and quality controlled raw data was retrieved from the NCBI Sequence Read Archive under the accession numbers SRR6448204 - SRR6448219 and SRR 6831528. MEGAHIT v1.9.6 was used to assemble metagenomes with a minimum contig length of 1,500 bp and a paired end setting. Bowtie2 v1.2.2 and samtools v1.7 were used to map reads back to the assembly. A depth file was generated using jgi_summarize_bam_contig_depths from MetaBAT v2.12.1 (Kang et al., 2015), which was also used to generate bins with a minimum contig length of 2,500 bp. The completeness and contamination of the bins were calculated with CheckM v1.0.7 (Parks, D.H., et al., 2014). Bins that were contained within the phylum Cyanobacteriota in the phylogenetic tree generated by CheckM were retained for further analysis. 139 single copy marker genes (Campbell et al., 2013) were collected using the anvi-run-hmms command in anvi’o v6.2 (Eren et al., 2021) and a phylogenetic tree was constructed using the anvi-gen-phylogenomic-tree command. Genome similarity between bins was computed using the anvi-compute-genome-similarity command. Bins were grouped into taxa if they shared more than 98% average nucleotide similarity and were phylogenetically cohesive. When a taxon was found in multiple metagenomes, the most complete bin with the lowest level of contamination for that taxon was selected for additional analysis and was referred to as the MAG for that taxon. Taxa were classified using GTDB-tk v.2.1.0 (Chaumeil et al., 2020). MAGs for each taxon are available in the NCBI sequence read archive under the accession numbers: ASM1731333v1, JARCMA000000000.1, JARCMB000000000.1, JARCMC000000000.1, JARCMD000000000.1.
The branchwater software used large-scale k-mer searching to search metagenomes in the NCBI SRA for matches with genomes of interest (Brown and Irber, 2016; Pierce et al., 2019). Signature files of the genomes of interest were generated using sourmash v3.5.0 (Brown and Irber, 2016) with k-mer sizes of 21, 31, 51, the scaled parameter set to 1,000, and abundance tracking. This generated a unique signature file specific to each of the five Antarctic MAGs. These signature files were searched against signature files previously generated for 498,942 publicly available unassembled metagenome sets on the September 2020 branchwater SRA database using exact k-mer matching. Results are organized by containment, which is the proportion of the query MAG k-mers found in the metagenome. Branchwater also provides Average Nucleotide Identity (ANI) values estimated from k-mer containment; the use of k = 31 as a k-mer size enables detection of matches to ~91% ANI at 5% containment and ~ 96% ANI at 30% containment (Irber et al., 2022a,b; Hera et al., 2023). The size of the Antarctic query MAGs ranged from 2.7 Mbp – 6.07 Mbp, so a match with containment value of 5% implies 135,000–303,500 matching k-mers with k = 31 and 4,185,000 – 9,408,500 matching base pairs, which indicates significant shared genomic material between MAGs and metagenome matches. The number of matching bases pairs also depends on the depth of metagenomic sequencing and sample community characteristics, including the ANI similarity of organisms to the query MAG, their abundance in the community, and the diversity of the community. Thus, low containment does not demonstrate the absence of an organism. However, high containment requires ANI similarity of an organism that has sufficient abundance to have its genome content well represented in the metagenome.
Validation of k-mer results from branchwater was done by mapping the Antarctic MAGs back to the metagenomes from the SRA using minimap2 v2.24 in genome-grist v0.8.4 (Li, 2018; Irber et al., 2022a,b). Environmental metadata for the top hits of all MAGs with hits above 5% were recorded, except for Microcoleus sp. MP8IB2.171, which had over 1,000 matches above that threshold (Table 1; Supplementary Tables S1, S2).
In an effort to generate MAGs of our five taxa of interest in metagenomes with >5% containment, we retrieved the relevant unassembled metagenomes. Unassembled metagenomes from geographically distinct environments were assembled with MEGAHIT v1.9.6, mapped with bowtie2 v1.2.2 and samtools v1.7, and binned with MetaBAT v.2.12.1. However, none of the assemblies were high enough quality to yield bins (Supplementary Table S3). The code from this project is available at: https://github.com/dib-lab/2022-pipeline-antarctic-biogeography
The five polar cyanobacteria MAGs used as search queries were found in a variety of non-polar metagenomic data sets in a range of environmental conditions (Table 1; Figure 1). The metagenome data sets with >5% containment of the MAGs described in Supplementary Tables S1, S2. Information about additional environments where the Microcoleus sp. MP8IB2.171 MAG was found with over 20% containment is displayed in Supplementary Table S2. Validation mapping data are available in Table 2. The SRA accession numbers of additional hits are available in Supplementary Tables S4–S8.
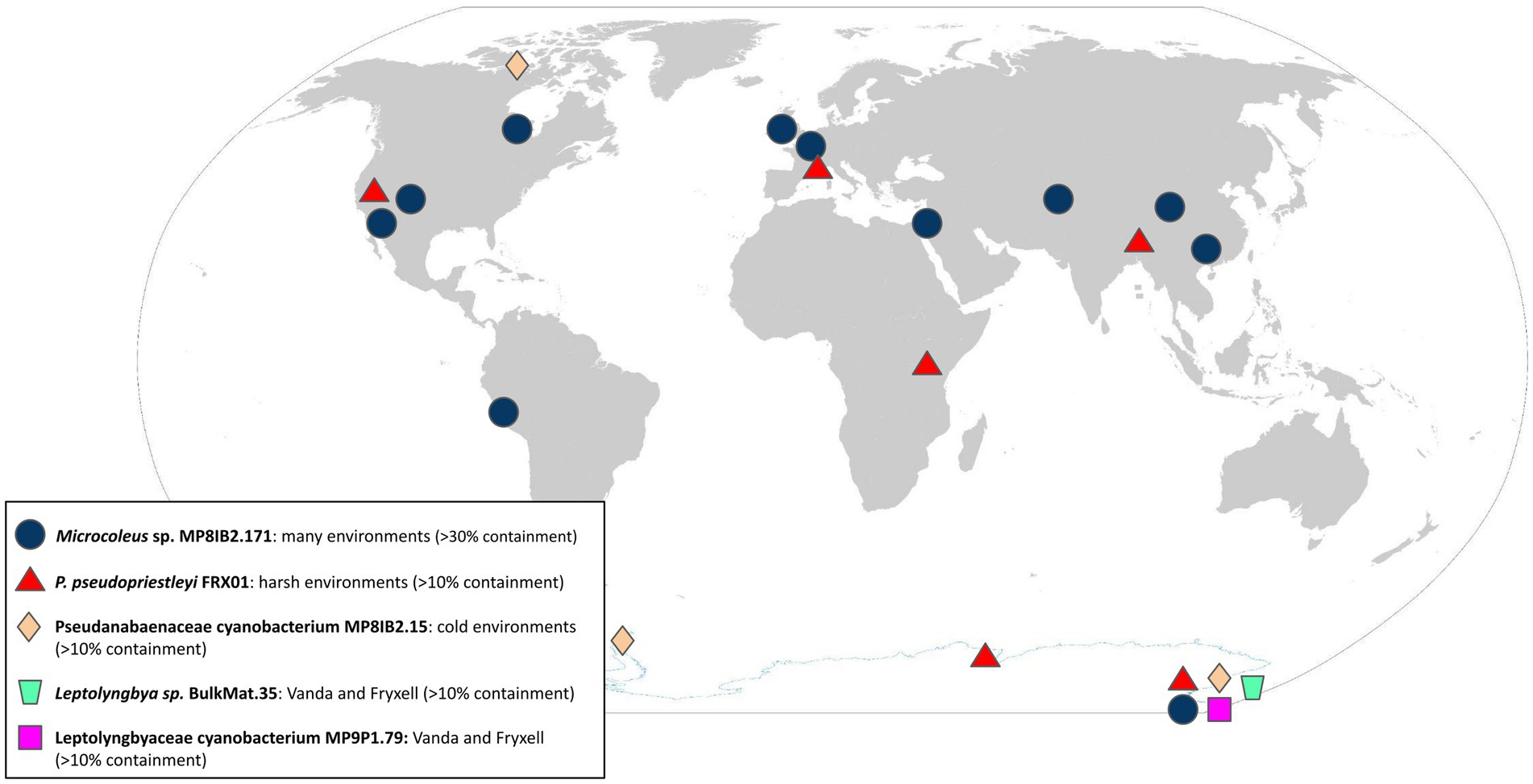
Figure 1. Global distribution of query MAGs. Environments with >10% containment are marked for P. pseudopriestleyi FRX01, Pseudoanabaenaceae cyanobacterium MP8IB2.15, and Leptolyngbya sp. BulkMat.35. (File:WorldMap.svg, 2022).
The purpose of applying branchwater was to find shared genomic data between Antarctic MAGs and SRA metagenomes from different habitats, geographic locations, and climate zones. Matches of our selected Antarctic cyanobacteria MAGs in these metagenomes may indicate the occurrence of Antarctic cyanobacteria or closely related taxa in environments across the globe. A k-mer size of 31 with at least 5% containment indicates a ~ 91% ANI between matched sequences; at 30% containment, this value increases to ~96% ANI (Hera et al., 2023). Thus, a high containment value indicates the presence in the metagenome of genomic DNA similar to the MAG and supports the presence of a closely related organism in the sampling location of that metagenome. Low k-mer containment values may represent smaller regions of shared genomic material or the presence of a related species but cannot definitively support the presence of the same species in that environment. Containment, particularly at low values, can be affected by factors such as plasmids, low population size relative to metagenome sequencing depth, or small portions of shared contamination between the MAG and metagenome.
The number of metagenome samples with containment for the 5 MAGs depends both on the distribution of available metagenomes and on the distribution of the MAG within ecosystems. Only about 3% of the SRA metagenomes contained matches to any of the k-mers in the query MAGs. Many of the metagenomes available in the SRA were from dark environments that are not expected to support growth of cyanobacteria; only about 5,000 metagenomes contain “photic” within their metadata. Significantly more research needs to be done to understand how to interpret the proportion of total samples with containment for MAGs of different types of organisms.
Even though the larger context of the low proportion of metagenomes containing our MAGs is poorly constrained, variations in relative containment for the cyanobacteria represented by the 5 query MAGs are robust because all 5 were searched for in the same way across the same dataset. Interpretations of their geographic distribution must be contextualized relative to available metagenomes, which are biased by prior sampling. Biases in the SRA metagenome data set also raise questions concerning environmental interpretations. In many cases, the metadata associated with metagenomes do not provide sufficient environmental context (e.g., irradiance, pH, abundance of important nutrients, and other geochemical parameters) for robust comparisons among environments. In some cases, the environment of sampling appears inconsistent with cyanobacterial growth (e.g., infant gut; Supplementary Table S1), raising questions about the cause of the detection (e.g., Kennedy et al., 2023). We choose to include these environments in our discussion for completeness and moderate our interpretations of environmental context based on available data.
The Microcoleus sp. MP8IB2.171 MAG was the most widely distributed MAG with 27 globally distinct locations above 25% containment (Table 1). The Microcoleus sp. MP8IB2.171 and P. pseudopriestleyi FRX01 MAGs were present in the most time series and subsamples from the same environmental location, which resulted in 1,121 hits above 5% for the Microcoleus sp. MP8IB2.171 MAG and 131 hits for P. pseudopriestleyi FRX01 MAG (Table 1). The Pseudanabaenaceae cyanobacterium MP8IB2.15 and P. pseudopriestleyi FRX01 MAGs were found in three distinct locations above 25% containment while the Leptolyngbyaceae cyanobacterium MP9P1.79 and Leptolyngbya sp. BulkMat.35 MAGs were only found in one location each above 25% containment (Table 1).
The Microcoleus sp. MP8IB2.171 MAG was found in diverse environments with conditions ranging from hot to cold climates and including both arid and wet locations (Supplementary Tables S1, S2). Some environments are cold year-round such as Puca Glacier in Peru (36.30% containment), glacier snow in China (39.54% containment), and the ice-covered Lake Vanda, whereas others are temperate, like Wisconsin, USA (37.04% containment), or Southwest Germany (33.58% containment). P. pseudopriestleyi FRX01 MAG was found in three Antarctic metagenome data sets: Lake Fryxell mat samples (98.49% containment), Ace Lake (55.8% containment) and the Rauer Islands (23.54% containment). The highest 30 hits for the P. pseudopriestleyi FRX01 MAG, including the three samples used to create the MAG, were from Lake Fryxell. This search revealed that P. pseudopriestleyi FRX01 is likely present in other depths of Lake Fryxell than 9.8 m despite not being prevalent at those depths based on 16S sequencing (Jungblut et al., 2010). Besides Antarctica, the P. pseudopriestleyi FRX01 MAG was found in a bird reserve next to a lagoon in France called Les Salins du Lion (20.63% containment) as well as a hydrocarbon polluted saline lagoon called Étang de Berre (18.25% containment), which were part of a study on the effects of hydrocarbon pollution on microbial communities (Aubé et al., 2016). The P. pseudopriestleyi FRX01 MAG was also found in the Salar del Huasco salt flat in Chile (8.98% containment) and antimicrobial treated sewage collected in Nairobi, Kenya (11.99% containment). All these environments represent extreme conditions for cyanobacteria. This MAG was also found in an infant gut fecal sample (7.61% containment). This is likely due to contamination of the sample or from ingestion. However, non-photosynthetic Cyanobacteria (Vampirovibronia or Melainabacteria) are interpreted as living in human guts (e.g., Di Rienzi et al., 2013), and the relatively low containment might indicate the presence of an organism with genetic material shared with the P. pseudopriestleyi FRX01 MAG.
Although the Microcoleus sp. MP8IB2.171, P. cyanobacterium MP8IB2.15, Leptolyngbyaceae cyanobacterium MP9P1.79, and Leptolyngbya sp. BulkMat.35 MAGs were obtained from microbial mat pinnacles in Lake Vanda, they were all present in high containment (>97%) in mat lift-off samples from Lake Fryxell where the P. pseudopriestleyi FRX01 MAG was not detected. The Pseudanabaenaceae cyanobacterium MP8IB2.15 MAG was also found in a dry sand community in the McMurdo Dry Valleys (37.45% containment), where lakes Vanda and Fryxell are located, as well as Whaler’s Bay on Deception Island in Antarctic (18.31% containment) and the Canadian High Arctic such as Nunavut, Canada (33.54% containment), which is cold but geographically distant from Antarctica.
Metagenomes representing geographically distinct locations were selected for further analysis to compare genomic data from different environments to the Antarctic MAGs. These data sets were run through an assembly and binning pipeline to obtain bins that could be compared to the Antarctic MAGs. However, metagenome assemblies were poor quality with the majority of the N50s under 1,000 base pairs, which is the minimum contig length required to bin with MetaBAT. Thus, bins were not generated likely due to insufficient sequencing depth, and it would not have been possible to identify the presence of the MAGs in these metagenomes without using an assembly-independent technique. Validation of the branchwater results was done by mapping the MAGs to metagenomes (Table 2). The percentage of the MAG detected in metagenome and average MAG coverage confirm the results of branchwater independent of k-mer comparisons, with all but one sample exhibiting higher mapping-based detection in the metagenome than k-mer containment.
The presence of the Microcoleus sp. MP8IB2.171 MAG in diverse environments indicates that it can survive in a range of different ecological conditions and climatic zones. The findings agree with previous biogeographic assessments of cultured cyanobacteria belonging to the species Microcoleus vaginatus and the Microcoleus spp. based on the 16S rRNA gene (Dvořák et al., 2012; Strunecký et al., 2013). To survive cold temperatures in Lake Vanda, Microcoleus sp. MP8IB2.171 must deal with cellular membranes becoming brittle and slowed metabolism. However, some environments where the Microcoleus sp. MP8IB2.171 was found are only cold for part of the year (Moab Green Butte Desert; Ningxia, China; Southwest Germany; Milwaukee, Wisconsin; and the United Kingdom) while other environments are cold year-round (Puca Glacier, Peru, and glacial snow in China). In contrast to cold conditions, hot temperatures can cause proteins to denature, and prolonged exposure to sunlight can cause high light and UV stress. These conditions occur in the Moab Green Butte Desert, the Sonoran Desert, and the Negev Desert. Furthermore, the Moab Desert and Sonoran Desert experience extreme temperature changes between morning and night (Turnage and Hinckley, 1938; Balling et al., 1998; McCann et al., 2018), forcing the Microcoleus sp. MP8IB2.171 to persist through both conditions on a 24-h cycle.
In addition to temperature range, the Microcoleus sp. MP8IB2.171 MAG was found in metagenomes from environments with different levels of water availability and habitat types. Locations included arid desert soil crusts (Moab Desert, USA and Negev Desert, Israel), mine tailings (Shaoyang, China; the United Kingdom; Milwaukee, USA), freshwater rivers (Qing River, China), saline lakes (Ace Lake, Antarctica), and plant microbiomes (wild Arabidopsis, Germany). The Microcoleus sp. MP8IB2.171 MAG was also found in data from both high and low elevation environments (5,800 m elevation in glacial snow in China and 0 m elevation in the Negev Desert). Overall, the variety of conditions where the Microcoleus sp. MP8IB2.171 MAG was found indicates that it may live in an impressive range of environments spanning moderate climates to extreme heat or cold.
P. pseudopriestleyi FRX01 is a sulfide-tolerant cyanobacteria found in a low light environment in Lake Fryxell, Antarctica. Our study identified the P. pseudopriestleyi FRX01 MAG in metagenomes from additional locations in Antarctica such as the saline Ace Lake (Vestfold Hills) and lakes on the Rauer Islands, which agrees with previous 16S rRNA gene sequencing where the species was documented from Salt Pond and Fresh Pond on McMurdo Ice Shelf (Jungblut et al., 2005; Lumian et al., 2021) as well as Ace Lake (Taton et al., 2006). Interestingly, P. pseudopriestleyi FRX01 or a close relative is present also at low abundance in a pond at Les Salins du Lion, a bird reserve (20.63% containment, 95% cANI), and Étang de Berre, a hydrocarbon polluted saline lagoon (18.25% containment, 94% cANI), both in southern France (Aubé et al., 2016). Four environmental conditions can be compared in these locations: irradiance, salinity, temperature, and sulfide concentrations. The irradiance at Les Salins du Lion pond and Étang de Berre lagoon was not measured when environmental sampling occurred, but the elevation of the lagoon was recorded to be at 0 m, and we infer that irradiance was higher at the surface of the pond than the low irradiance at the depth of sampling in Lake Fryxell (1–2 μmol/photon m−2 s−1) (Sumner et al., 2015). Furthermore, Salt Pond and Fresh Pond have high illumination levels in the summer (Roos and Vincent, 1998; Jungblut et al., 2005), indicating that P. pseudopriestleyi FRX01 may have the capability to overcome high irradiation and UV fluxes for prolonged periods. Les Salins du Lion (14 g L−1 NaCl) and Étang de Berre (20 g L−1 NaCl) have a lower salinity than 9.8 m in Lake Fryxell (70.13 g L−1 NaCl) and Salt Pond (~990 g L−1 NaCl), which is hypersaline (Jungblut et al., 2005; Aubé et al., 2016; Lumian et al., 2021). Previous work has showed that a close relative of P. pseudopriestleyi FRX01 (Oscillatoria acuminata Jungblut et al., 2016) increases the thickness of its extracellular polymeric substance layer in response to saline stress (Agrawal and Singh, 1999). Sulfide is also present in Les Salins du Lion, with a concentration of ~0.24 g L−1 at the time of sampling (Aubé et al., 2016), which was the highest value at any location or time sampled included in the study. This indicates a higher sulfide tolerance than what was previously recorded in the Lake Fryxell sampling site, which was 9.8 × 10–5 g L−1 (Lumian et al., 2021).
In addition to Les Salins du Lion and Étang de Berre, P. pseudopriestleyi FRX01 MAG genome content was found in globally distributed challenging environments such as a salt flat in Chile, antimicrobial treated sewage in Kenya, and infant gut, where it may be ingested material or contamination. The fact that P. pseudopriestleyi FRX01 thrives in environments with harsh conditions suggests that it has capabilities to overcome diverse environmental stresses. In Lake Fryxell, P. pseudopriestleyi FRX01 dominates microbial mats at 9.8 m depth in low light and sulfidic conditions but it is less abundant at shallower depths, even though there is more light availability and no sulfide (Jungblut et al., 2016; Dillon et al., 2020). Thus, P. pseudopriestleyi FRX01 may grow slowly and find ecological success in environments that are too harsh for faster growing cyanobacteria, which is consistent with the slow growth rate of P. pseudopriestleyi FRX01 seen in unpublished laboratory observations. The other environments where genomes similar to P. pseudopriestleyi FRX01 were found may provide challenges that prohibit many other cyanobacteria from growing (polar environments, alkaline lake Big Soda Lake, antimicrobial treated sewage in Nairobi, Kenya), allowing P. pseudopriestleyi FRX01 to become sufficiently abundant to be represented in metagenomes from nonpolar environments.
The top matches for the Pseudanabaenaceae cyanobacterium MP8IB2.15, Leptolyngbyaceae cyanobacterium MP9P1.79, and Leptolyngbya sp. BulkMat.35 MAGs showed that they were also present in Lake Fryxell and that the Pseudanabaenaceae cyanobacterium MP8IB2.15 MAG was in sediment in the McMurdo Dry Valleys. The Leptolyngbyaceae cyanobacterium MP9P1.79 MAG was only present in the McMurdo Dry Valleys, however the presence of the Leptolyngbya sp. BulkMat.35 and Pseudanabaenaceae cyanobacterium MP8IB2.15 MAGs in geographically distant locations in the Arctic (Norway and Canada respectively) suggests that the cyanobacteria forming these MAGs have a global distribution in cold environments and might have undergone long range dispersal. The mechanism of long-range distribution could be wind; atmospheric studies show bacteria from the Saharan desert are transported by wind throughout the Atlantic (Griffin et al., 2002; Gorbushina et al., 2007; Jungblut et al., 2010). A similar process is expected to allow Antarctic cyanobacteria to cross large distances and populate diverse geographic regions. However, the lack of non-polar locations in metagenomes may suggest that they are not as successful at integrating into non-polar environments. Thus, these cyanobacteria may be specific to polar environments even though they may be transported globally, which agrees with 16S rRNA gene analysis that proposed the presence of cosmopolitan cold ecotypes (Jungblut et al., 2010).
The perceived distributions of organisms in biogeography studies are affected by sampling and publishing biases. Sampling in remote locations is logistically difficult and is often centered around established sampling locations which may be near research stations and infrastructure. This results in many studies and publications from established sampling locations and a deeper understanding of local ecology and geochemical processes in these environments. Biogeography studies, however, benefit from widespread sampling in many locations. Conducting widespread ecological sampling is expensive and can be impractical, so it is advantageous to search existing datasets for as much information as possible. Using branchwater to search public metagenomes makes the most out of data from remote areas by revealing previously unknown locations of organisms of interest. Furthermore, results from this analysis included remote areas, including various sites in Antarctica, which may not have otherwise been identified as locations of the query MAGs. Finally, the rapid rate of metagenome additions to the SRA database suggests that this technique will become increasingly valuable. For example, the number of metagenomes nearly doubled between construction of our dataset in September 2020 and final revisions in January 2024. Reanalysis would likely identify additional locations for globally distributed organisms whereas it may not for endemic organisms.
Despite being affected by sampling bias like all biogeography studies, the results showed that the Microcoleus sp. MP8IB2.171 MAG was globally distributed over a wide variety of environments, the P. pseudopriestleyi FRX01 MAG was found in predominantly in harsh environments, the Leptolyngbyaceae cyanobacterium MP9P1.79 was only in the Antarctic, and the Leptolyngbya sp. BulkMat.35 MAG and the Pseudanabaenaceae cyanobacterium MP8IB2.15 MAGs were in geographically separated polar environments. The numerous sites containing the Microcoleus sp. MP8IB2.171 MAG imply that this species has the genetic capacity to adapt to many types of environments. It may also have a faster growth rate than an extreme conditions specialist, like P. pseudopriestleyi FRX01, which would allow it to compete in a variety of ecological communities, some of which experience stressful conditions. Previous work has shown Microcoleus sensu stricto to be a cosmopolitan genus (Garcia-Pichel et al., 1996, 2001).
Although the Microcoleus sp. MP8IB2.171 MAG is by far the most globally diverse cyanobacterial genome in this study, there is variety in the distributions of the other four MAGs. The prevalence of the P. pseudopriestleyi FRX01 MAG in harsh environments indicates that it finds ecological success in stressful conditions, and it is likely outperformed by other organisms in moderate environments. The Pseudanabaenaceae cyanobacterium MP8IB2.15, Leptolyngbyaceae cyanobacterium MP9P1.79, and Leptolyngbya sp. BulkMat.35 MAGs were only found in polar environments, indicating they may be outcompeted in moderate environments. Diving deeper into the metabolic potential of each organism and interactions between metagenome community members may offer insights as to how and why some organisms are prevalent in a multitude of environments while others are prevalent in only certain conditions.
This paper presents the first biogeography study using a large-scale k-mer-based approach and characterizes the global distribution of five distinct Antarctic cyanobacteria based on public data. We show that the Microcoleus sp. MP8IB2.171 MAG has cosmopolitan distribution and presence in a variety of environments, whereas the P. pseudopriestleyi FRX01 MAG is also globally distributed but mostly present in harsh environments. Leptolyngbya sp. BulkMat.35, and Pseudanabaenaceae cyanobacterium MP8IB2.15 MAGs were only found in polar environments from Arctic to Antarctica suggesting the existence of cosmopolitan cold ecotypes. The Leptolyngbyaceae cyanobacterium MP9P1.79 MAG was only detected in Antarctica and provides support for more restricted distribution patterns and potential endemicity. Further in situ transcriptomic studies of these MAGs may reveal adaptation mechanisms including why the Microcoleus sp. MP8IB2.171 is so pervasive compared to the other cyanobacteria in this study.
Branchwater can search ~500,000 metagenomes with a query genome in under 24 h on commodity hardware (Irber et al., 2022a,b). The ability to quickly find genomes similar to query MAGs in publicly available unassembled metagenomic data sets has important implications for biogeography studies, which have been predominantly based on 16S rRNA gene sequencing due to the prevalence of data and ease of comparison. Branchwater greatly increases the amount of data that can be used for biogeography studies. This technique is especially helpful for organisms that are in remote locations and underrepresented in genomic data, such as polar cyanobacteria, by providing a much larger number of known environments than would be possible with targeted field studies. Additionally, branchwater can be used to identify accessible sampling locations of organisms from remote environments, such as the Microcoleus sp. MP8IB2.171 being identified in the Moab Green Butte Desert in Colorado, USA at 41.10% containment. As more metagenome datasets are made publicly available on the NCBI SRA, more information about the distribution of cryosphere cyanobacteria can be attained. The results further demonstrate the potential of metagenomics and k-mer based MAG approaches in investigating biogeography and ecology of cyanobacteria and environmental microbiology in the polar regions.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.ncbi.nlm.nih.gov/, SRR6448204-SRR6448219 and SRR6831528, GCF_017313335.1, JARCMA000000000.1, JARCMB000000000.1, JARCMC000000000.1, JARCMD000000000.1.
JL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. DYS: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. CLG: Conceptualization, Investigation, Validation, Writing – review & editing. ADJ: Methodology, Supervision, Writing – review & editing. LI: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Validation, Writing – review & editing. NT-W: Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – review & editing. CT: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is funded in part by the Gordon and Betty Moore Foundation’s Data-Driven Discovery Initiative through Grant GBMF4551, as well as US National Science Foundation grant BIO-2018911, both to C. Titus Brown. Support was also provided by US National Science Foundation grant OPP-1937748 to Sumner.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1328083/full#supplementary-material
Agrawal, S. C., and Singh, V. (1999). Viability of dried vegetative trichomes, formation of akinetes and heterocysts and akinete germination in some blue-green algae under water stress. Folia Microbiol. 44, 411–418. doi: 10.1007/BF02903715
Aubé, J., Senin, P., Pringault, O., Bonin, P., Deflandre, B., Bouchez, O., et al. (2016). The impact of long-term hydrocarbon exposure on the structure, activity, and biogeochemical functioning of microbial mats. Mar. Pollut. Bull. 111, 115–125. doi: 10.1016/j.marpolbul.2016.07.023
Baas-Becking, L. G. M. (1934). Geobiologie; of inleiding tot de milieukunde. The Hague, the Netherlands: WP Van Stockum & Zoon NV.
Bahl, J., Lau, M. C. Y., Smith, G. J. D., Vijaykrishna, D., Cary, S. C., Lacap, D. C., et al. (2011). Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nat. Commun. 2:163. doi: 10.1038/ncomms1167
Balling, R. C., Klopatek, J. M., Hildebrandt, M. L., Moritz, C. K., and Watts, C. J. (1998). Impacts of land degradation on historical temperature records from the Sonoran Desert. Clim. Chang. 40, 669–681. doi: 10.1023/A:1005370115396
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Brown, C. T. (2021). Searching all public metagenomes with sourmash. Living Ivory Basement Available at: http://ivory.idyll.org/blog/2021-MAGsearch.html
Brown, C. T., and Irber, L. (2016). Sourmash: a library for MinHash sketching of DNA. J. Open Source Soft. 1:27. doi: 10.21105/joss.00027
Brown, C. T., Moritz, D., O’Brien, M. P., Reidl, F., Reiter, T., and Sullivan, B. D. (2020). Exploring neighborhoods in large metagenome assembly graphs using spacegraphcats reveals hidden sequence diversity. Genome Biol. 21:164. doi: 10.1186/s13059-020-02066-4
Campbell, J. H., O’Donoghue, P., Campbell, A. G., Schwientek, P., Sczyrba, A., Woyke, T., et al. (2013). UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proc. Natl. Acad. Sci. 110, 5540–5545. doi: 10.1073/pnas.1303090110
Chaumeil, P.-A., Mussig, A. J., Hugenholtz, P., and Parks, D. H. (2020). GTDB-Tk: a toolkit to classify genomes with the genome taxonomy database. Bioinformatics 36, 1925–1927. doi: 10.1093/bioinformatics/btz848
Chrismas, N. A. M., Anesio, A. M., and Sánchez-Baracaldo, P. (2015). Multiple adaptations to polar and alpine environments within cyanobacteria: a phylogenomic and Bayesian approach. Front. Microbiol. 6:1070. doi: 10.3389/fmicb.2015.01070
Chrismas, N. A. M., Barker, G., Anesio, A. M., and Sánchez-Baracaldo, P. (2016). Genomic mechanisms for cold tolerance and production of exopolysaccharides in the Arctic cyanobacterium Phormidesmis priestleyi BC1401. BMC Genomics 17:533. doi: 10.1186/s12864-016-2846-4
Chrismas, N. A. M., Williamson, C. J., Yallop, M. L., Anesio, A. M., and Sánchez-Baracaldo, P. (2018). Photoecology of the Antarctic cyanobacterium Leptolyngbya sp. BC1307 brought to light through community analysis, comparative genomics and in vitro photophysiology. Mol. Ecol. 27, 5279–5293. doi: 10.1111/mec.14953
Crusoe, M. R., Alameldin, H. F., Awad, S., Boucher, E., Caldwell, A., Cartwright, R., et al. (2015). The khmer software package: enabling efficient nucleotide sequence analysis. F1000Research :4. doi: 10.12688/f1000research.6924.1
Delmont, T. O., and Eren, A. M. (2018). Linking pangenomes and metagenomes: the Prochlorococcus metapangenome. PeerJ 6:e4320. doi: 10.7717/peerj.4320
Di Rienzi, S. C., Sharon, I., Wrighton, K. C., Koren, O., Hug, L. A., Thomas, B. C., et al. (2013). The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to cyanobacteria. elife 2:e01102. doi: 10.7554/eLife.01102
Dillon, M. L., Hawes, I., Jungblut, A. D., Mackey, T. J., Eisen, J. A., Doran, P. T., et al. (2020). Energetic and environmental constraints on the community structure of benthic microbial mats in Lake Fryxell, Antarctica. FEMS Microbiol. Ecol. 96:fiz207. doi: 10.1093/femsec/fiz207
Dvořák, P., Hašler, P., and Poulíčková, A. (2012). Phylogeography of the Microcoleus vaginatus (cyanobacteria) from three continents – a spatial and temporal characterization. PLoS One 7:e40153. doi: 10.1371/journal.pone.0040153
Edgar, R. C., Taylor, J., Lin, V., Altman, T., Barbera, P., Meleshko, D., et al. (2022). Petabase-scale sequence alignment catalyses viral discovery. Nature 602, 142–147. doi: 10.1038/s41586-021-04332-2
Eren, A. M., Esen, Ö. C., Quince, C., Vineis, J. H., Morrison, H. G., Sogin, M. L., et al. (2015). Anvi’o: an advanced analysis and visualization platform for ‘omics data. PeerJ 3:e1319. doi: 10.7717/peerj.1319
Eren, A. M., Kiefl, E., Shaiber, A., Veseli, I., Miller, S. E., Schechter, M. S., et al. (2021). Community-led, integrated, reproducible multi-omics with anvi’o. Nat. Microbiol. 6, 3–6. doi: 10.1038/s41564-020-00834-3
Fierer, N. (2008). “Microbial biogeography: patterns in microbial diversity across space and time” in K. Zengler (Ed.), Accessing uncultivated microorganisms. (John Wiley & Sons, Ltd.), 95–115.
File:WorldMap.svg. (2022). Paul Lachapelle and Don Albrecht: Wikimedia commons Available at: https://commons.wikimedia.org/w/index.php?title=File:WorldMap.svg&oldid=715663460
Garcia-Pichel, F., López-Cortés, A., and Nübel, U. (2001). Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado plateau. Appl. Environ. Microbiol. 67, 1902–1910. doi: 10.1128/AEM.67.4.1902-1910.2001
Garcia-Pichel, F., Prufert-Bebout, L., and Muyzer, G. (1996). Phenotypic and phylogenetic analyses show Microcoleus chthonoplastes to be a cosmopolitan cyanobacterium. Appl. Environ. Microbiol. 62, 3284–3291. doi: 10.1128/aem.62.9.3284-3291.1996
Gorbushina, A. A., Kort, R., Schulte, A., Lazarus, D., Schnetger, B., Brumsack, H.-J., et al. (2007). Life in Darwin’s dust: intercontinental transport and survival of microbes in the nineteenth century. Environ. Microbiol. 9, 2911–2922. doi: 10.1111/j.1462-2920.2007.01461.x
Green, J. L., Bohannan, B. J. M., and Whitaker, R. J. (2008). Microbial biogeography: from taxonomy to traits. Science 320, 1039–1043. doi: 10.1126/science.1153475
Grettenberger, C. L., Sumner, D. Y., Wall, K., Brown, C. T., Eisen, J. A., Mackey, T. J., et al. (2020). A phylogenetically novel cyanobacterium most closely related to Gloeobacter. ISME J. 14, 2142–2152. doi: 10.1038/s41396-020-0668-5
Griffin, D. W., Kellogg, C. A., Garrison, V. H., and Shinn, E. A. (2002). The global transport of dust: an intercontinental river of dust, microorganisms and toxic chemicals flows through the Earth’s atmosphere. Am. Sci. 90, 228–235. doi: 10.1511/2002.9.228
Harding, T., Jungblut, A. D., Lovejoy, C., and Vincent, W. F. (2011). Microbes in high Arctic snow and implications for the cold biosphere. Appl. Environ. Microbiol. 77, 3234–3243. doi: 10.1128/AEM.02611-10
Harke, M. J., Steffen, M. M., Gobler, C. J., Otten, T. G., Wilhelm, S. W., Wood, S. A., et al. (2016). A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54, 4–20. doi: 10.1016/j.hal.2015.12.007
Hera, M. R., Pierce-Ward, N. T., and Koslicki, D. (2023). Deriving confidence intervals for mutation rates across a wide range of evolutionary distances using FracMinHash. Genome Res. 33, 1061–1068. doi: 10.1101/gr.277651.123
Irber, L. C. (2020a). Decentralizing indices for genomic data [Ph.D., University of California, Davis]. ProQuest dissertations and theses Available at: https://www.proquest.com/docview/2503641751/abstract/7B8543548D284D81PQ/1
Irber, L. C. (2020b). MinHashing all the things: a quick analysis of MAG search results. Gabbleblotchits. Available at: https://blog.luizirber.org/2020/07/24/mag-results/
Irber, L., Brooks, P. T., Reiter, T. E., Pierce-Ward, N. T., Hera, M. R., Koslicki, D., et al. (2022a). Lightweight compositional analysis of metagenomes with FracMinHash and minimum metagenome covers. BioRxiv. [Epub ahead of preprint] doi: 10.1101/2022.01.11.475838
Irber, L., Pierce-Ward, N. T., and Brown, C. T. (2022b). Sourmash branchwater enables lightweight petabyte-scale sequence search. bioRxiv Available at: https://www.biorxiv.org/content/10.1101/2022.11.02.514947v1
Jungblut, A. D., Hawes, I., Mackey, T. J., Krusor, M., Doran, P. T., Sumner, D. Y., et al. (2016). Microbial mat communities along an oxygen gradient in a perennially ice-covered Antarctic lake. Appl. Environ. Microbiol. 82, 620–630. doi: 10.1128/AEM.02699-15
Jungblut, A.-D., Hawes, I., Mountfort, D., Hitzfeld, B., Dietrich, D. R., Burns, B. P., et al. (2005). Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo ice shelf, Antarctica. Environ. Microbiol. 7, 519–529. doi: 10.1111/j.1462-2920.2005.00717.x
Jungblut, A. D., Lovejoy, C., and Vincent, W. F. (2010). Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J. 4, 191–202. doi: 10.1038/ismej.2009.113
Kang, D. D., Froula, J., Egan, R., and Wang, Z. (2015). MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3:e1165. doi: 10.7717/peerj.1165
Kennedy, K. M., de Goffau, M. C., Perez-Muñoz, M. E., Arrieta, M.-C., Bäckhed, F., Bork, P., et al. (2023). Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 613, 639–649. doi: 10.1038/s41586-022-05546-8
Kleinteich, J., Hildebrand, F., Bahram, M., Voigt, A. Y., Wood, S. A., Jungblut, A. D., et al. (2017). Pole-to-Pole connections: Similarities between Arctic and Antarctic microbiomes and their vulnerability to environmental change. Front. Ecol. Evol. 5:137. doi: 10.3389/fevo.2017.00137
Leinonen, R., Sugawara, H., and Shumway, M.on behalf of the International Nucleotide Sequence Database Collaboration (2011). The sequence read archive. Nucleic Acids Res. 39, D19–D21. doi: 10.1093/nar/gkq1019
Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Available at: http://arxiv.org/abs/1303.3997
Li, H. (2018). Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100. doi: 10.1093/bioinformatics/bty191
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, D., Liu, C.-M., Luo, R., Sadakane, K., and Lam, T.-W. (2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676. doi: 10.1093/bioinformatics/btv033
Lumian, J., Grettenberger, C., Jungblut, A. D., Mackey, T. J., Hawes, I., Alatorre-Acevedo, E., et al. (2024). Genomic profiles of four novel cyanobacteria MAGs from Lake Vanda, Antarctica: insights into photosynthesis, cold tolerance, and the circadian clock. Front. Microbiol. 14:1330602. doi: 10.3389/fmicb.2023.1330602
Lumian, J. E., Jungblut, A. D., Dillion, M. L., Hawes, I., Doran, P. T., Mackey, T. J., et al. (2021). Metabolic capacity of the Antarctic cyanobacterium Phormidium pseudopriestleyi that sustains oxygenic photosynthesis in the presence of hydrogen sulfide. Genes 12:426. doi: 10.3390/genes12030426
Martiny, J. B. H., Bohannan, B. J. M., Brown, J. H., Colwell, R. K., Fuhrman, J. A., Green, J. L., et al. (2006). Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112. doi: 10.1038/nrmicro1341
McCann, R. B., Lynch, J., and Adams, J. (2018). “Mitigating projected impacts of climate change and building resiliency through permaculture” in Paul Lachapelle and Don (Ed.), Addressing climate change at the community level in the United States (Oxfordshire, England, UK: Routledge)
Moreira, C., Vasconcelos, V., and Antunes, A. (2013). Phylogeny and biogeography of cyanobacteria and their produced toxins. Mar. Drugs 11, 4350–4369. doi: 10.3390/md11114350
Namsaraev, Z., Mano, M.-J., Fernandez, R., and Wilmotte, A. (2010). Biogeography of terrestrial cyanobacteria from Antarctic ice-free areas. Ann. Glaciol. 51, 171–177. doi: 10.3189/172756411795931930
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P., and Tyson, G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055. doi: 10.1101/gr.186072.114
Pierce, N. T., Irber, L., Reiter, T., Brooks, P., and Brown, C. T. (2019). Large-scale sequence comparisons with sourmash 1006. F1000Research 8:1006. doi: 10.12688/f1000research.19675.1
Quesada, A., and Vincent, W. F. (2012). “Cyanobacteria in the cryosphere: snow, ice and extreme cold” in Ecology of cyanobacteria II: Their diversity in space and time. ed. B. A. Whitton (Netherlands: Springer), 387–399.
Ribeiro, K. F., Duarte, L., and Crossetti, L. O. (2018). Everything is not everywhere: a tale on the biogeography of cyanobacteria. Hydrobiologia 820, 23–48. doi: 10.1007/s10750-018-3669-x
Roos, J. C., and Vincent, W. F. (1998). Temperature dependence of UV radiation effects on Antarctic cyanobacteria. J. Phycol. 34, 118–125. doi: 10.1046/j.1529-8817.1998.340118.x
Stal, L. J. (2007). “Cyanobacteria” in Algae and cyanobacteria in extreme environments. ed. J. Seckbach (Netherlands: Springer), 659–680.
Strunecký, O., Komárek, J., Johansen, J., Lukešová, A., and Elster, J. (2013). Molecular and morphological criteria for revision of the genus microcoleus (Oscillatoriales, cyanobacteria). J. Phycol. 49, 1167–1180. doi: 10.1111/jpy.12128
Sumner, D. Y., Hawes, I., Mackey, T. J., Jungblut, A. D., and Doran, P. T. (2015). Antarctic microbial mats: a modern analog for Archean lacustrine oxygen oases. Geology 43, 887–890. doi: 10.1130/G36966.1
Sumner, D. Y., Jungblut, A. D., Hawes, I., Andersen, D. T., Mackey, T. J., and Wall, K. (2016). Growth of elaborate microbial pinnacles in Lake Vanda, Antarctica. Geobiology 14, 556–574. doi: 10.1111/gbi.12188
Taton, A., Grubisic, S., Balthasart, P., Hodgson, D. A., Laybourn-Parry, J., and Wilmotte, A. (2006). Biogeographical distribution and ecological ranges of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol. Ecol. 57, 272–289. doi: 10.1111/j.1574-6941.2006.00110.x
Turnage, W. V., and Hinckley, A. L. (1938). Freezing weather in relation to plant distribution in the Sonoran Desert. Ecol. Monogr. 8, 529–550. doi: 10.2307/1943083
Whitaker, R. J., Grogan, D. W., and Taylor, J. W. (2003). Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301, 976–978. doi: 10.1126/science.1086909
Zhang, L., Jungblut, A. D., Hawes, I., Andersen, D. T., Sumner, D. Y., and Mackey, T. J. (2015). Cyanobacterial diversity in benthic mats of the McMurdo Dry Valley lakes, Antarctica. Polar Biol. 38, 1097–1110. doi: 10.1007/s00300-015-1669-0
Keywords: biogeography, bioinformatics, cyrosphere, polar cyanobacteria, metagenomics
Citation: Lumian J, Sumner DY, Grettenberger CL, Jungblut AD, Irber L, Pierce-Ward NT and Brown CT (2024) Biogeographic distribution of five Antarctic cyanobacteria using large-scale k-mer searching with sourmash branchwater. Front. Microbiol. 15:1328083. doi: 10.3389/fmicb.2024.1328083
Received: 26 October 2023; Accepted: 06 February 2024;
Published: 19 February 2024.
Edited by:
Luisa I. Falcon, National Autonomous University of Mexico, MexicoReviewed by:
Aharon Oren, Hebrew University of Jerusalem, IsraelCopyright © 2024 Lumian, Sumner, Grettenberger, Jungblut, Irber, Pierce-Ward and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Titus Brown, Y3Ricm93bkB1Y2RhdmlzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.