- Institute of Microbiome Frontiers and One Health, School of Public Health, Lanzhou University, Lanzhou, Gansu, China
Introduction: Tick-borne pathogens especially viruses are continuously appearing worldwide, which have caused severe public health threats. Understanding the species, distribution and epidemiological trends of tick-borne viruses (TBVs) is essential for disease surveillance and control.
Methods: In this study, the data on TBVs and the distribution of ticks in China were collected from databases and literature. The geographic distribution of TBVs in China was mapped based on geographic locations of viruses where they were prevalent or they were detected in vector ticks. TBVs sequences were collected from The National Center for Biotechnology Information and used to structure the phylogenetic tree.
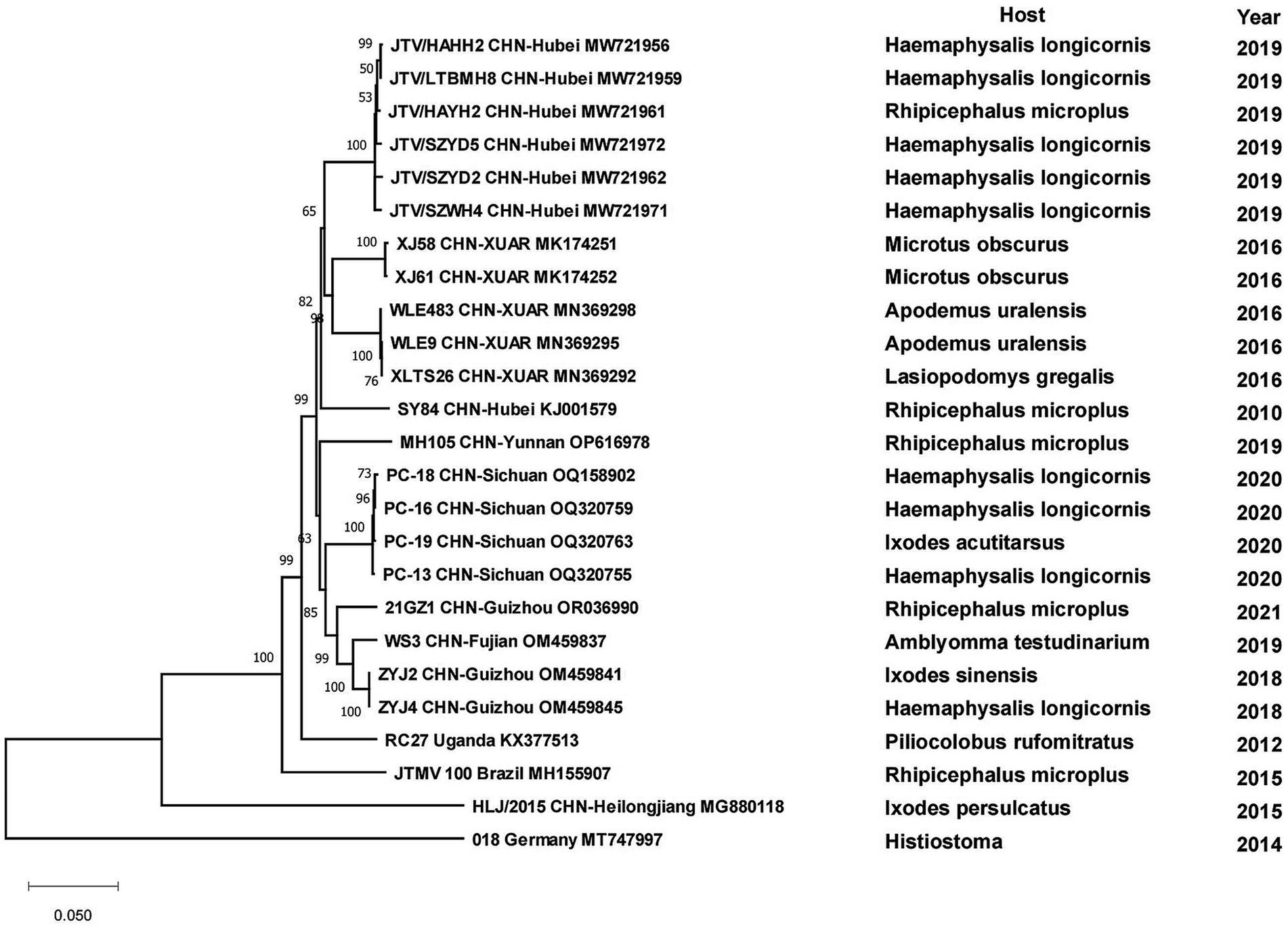
Results: Eighteen TBVs from eight genera of five families were prevalent in China. Five genera of ticks played an important role in the transmission of TBVs in China. According to phylogenetic analysis, some new viral genotypes, such as the Dabieshan tick virus (DTV) strain detected in Liaoning Province and the JMTV strain detected in Heilongjiang Province existed in China.
Discussion: TBVs were widely distributed but the specific ranges of viruses from different families still varied in China. Seven TBVs belonging to the genus Orthonairovirus of the family Nairoviridae such as Nairobi sheep disease virus (NSDV) clustered in the Xinjiang Uygur Autonomous Region (XUAR) and northeastern areas of China. All viruses of the family Phenuiviridae except Severe fever with thrombocytopenia syndrome virus (SFTSV) were novel viruses that appeared in the last few years, such as Guertu virus (GTV) and Tacheng tick virus 2 (TcTV-2). They were mainly distributed in the central plains of China. Jingmen tick virus (JMTV) was distributed in at least fourteen provinces and had been detected in more than ten species of tick such as Rhipicephalus microplus and Haemaphysalis longicornis, which had the widest distribution and the largest number of vector ticks among all TBVs. Parainfluenza virus 5 (PIV5) and Lymphatic choriomeningitis virus (LCMV) were two potential TBVs in Northeast China that could cause serious diseases in humans or animals. Ixodes persulcatus carried the highest number of TBVs, followed by Dermacentor nuttalli and H. longicornis. They could carry as many as ten TBVs. Three strains of Tick-borne encephalitis (TBEV) from Inner Mongolia Province clustered with ones from Russia, Japan and Heilongjiang Province, respectively. Several SFTSV strains from Zhejiang Province clustered with strains from Korea and Japan. Specific surveillance of dominant TBVs should be established in different areas in China.
1 Introduction
With the ever-changing global climate and ecology, the rapid spread of arthropod-borne diseases around the world has become a severe public health problem. Ticks are carriers of a wide range of pathogens including viruses, bacteria and parasites (Fang et al., 2015). The majority of ticks have to change 2 or 3 hosts throughout their whole life cycles, known as two-host ticks or three-host ticks, respectively. The pathogens are transmitted to hosts when ticks suck blood for energy and nutrients to grow (Simo et al., 2017; Boulanger et al., 2019). A variety of severe diseases to humans and animals are transmitted by ticks, such as Crimean Congo hemorrhagic fever, Tick-borne encephalitis, Severe fever with thrombocytopenia syndrome, Babesiosis and Anaplasmosis (Eisen et al., 2017; Tsao et al., 2021; El-Alfy et al., 2023). The transmission of tick-borne diseases is influenced by many factors such as animal reservoir ecology, environment and vector ecology, among which the environment plays an important role. Different species of ticks require different living environments, and their developmental stages, population distribution and abundance are also influenced by the climate (Gilbert, 2021; Couret et al., 2022). The species and health status of the animal hosts, the presence of symbionts, and tick-host interactions also modulate the tick microbiome. Due to the diversity of factors, tick-borne diseases are very difficult to prevent.
The viruses carried and transmitted by ticks are called tick-borne viruses which are very important among tick-borne pathogens. TBVs from at least two orders, nine families, and twelve genera are distributed in many countries around the world, such as Heartland virus, Bourbon virus and Powassan virus in the United States, Omsk hemorrhagic fever virus in Europe and Crimean Congo hemorrhagic fever virus (CCHFV)in Africa. Once transmitted to their susceptible hosts, these viruses rapidly begin to replicate and invade target organs or cells, resulting in serious diseases in humans or animals (Owen et al., 2021). With the development of pathogen detection and high-throughput sequencing technologies, more and more novel TBVs or novel genotypes of TBVs have been discovered through either isolating in patients after tick bites or assembling and annotating by metagenomic next-generation sequencing (Damian et al., 2020; Madison-Antenucci et al., 2020; Piantadosi and Kanjilal, 2020).
The emergence of novel TBVs deserves more attention (Park et al., 2020). In recent years, there have been numerous incidents of people being bitten by ticks and being infected with pathogens that have led to serious illnesses and even deaths (Dragonjic et al., 2022; Jumpertz et al., 2023). China covers various lands with large environmental differences between regions. The widespread distribution of ticks makes China a “natural greenhouse” for the spread and evolution of TBVs. However, most of the previous reviews on tick-borne pathogens in China focused on bacteria, parasites, and viruses together, with viruses only having a small portion (Wu et al., 2013; Luan et al., 2023). Although some studies only focus on TBVs in China, they were not comprehensive due to geographical limitations (Bai et al., 2023; Cai et al., 2023). The overall distribution of TBVs in China is not clear. Due to the rapid evolution, mutation, and recombination of viruses, the database of TBVs needed to be updated continuously even though TBVs were considered to be slower evolving among the arboviruses. Therefore, this study aimed to analyze the prevalence of TBVs in China, including the species of virus vectors and their geographic distribution.
2 Materials and methods
2.1 Data collection
Firstly, data on TBVs in China was from a database named “global dataset of sequence, diversity and biosafety recommendation of arbovirus and arthropod-specific virus” recently published by Huang et al. This database contains taxonomic, genomic, sequence, host, biosafety, and isolation source information for arboviruses and arthropod-specific viruses (Huang Y et al., 2023). The literature on TBVs in China was searched in PubMed, Web of Science, and CNKI with the keywords “China” and “tick-borne viruses,” “TBVs” or specific virus names such as “Crimean Congo hemorrhagic fever virus” or “CCHFV” to collect more data. All records of viruses in this study were detected by PCR, nested PCR, RT-PCR or isolated and cultured. Those that only were identified in high-throughput sequencing but not detected in human or animal hosts were excluded. The distribution of ticks in this study was from a dataset of distribution and diversity of ticks in China and published literature on tick species (Chen et al., 2010; Zhang G et al., 2019; Zhang Y et al., 2019; Yang et al., 2020; Zhao et al., 2021).
2.2 Mapping the distribution of TBVs
The geographic distribution of TBVs in China was mapped based on geographic locations of viruses where they were prevalent or they were detected in vector ticks. In addition to the general distribution map of TBVs, the distribution of different virus families was mapped separately. A land-cover map of China was downloaded through the National Earth System Science Data Center, National Science & Technology Infrastructure of China1 as a base map for the geographic distribution of the TBVs (Wang et al., 2019). There are ten major categories of land cover information including Cultivated land, Forest, Bush, Grassland, Water, Wetland, Glacier, Artificial land, Bare soil and Tundra. The distribution of all ticks that are carriers of a particular virus was mapped and compared with the geographic distribution of the virus. All maps were drawn using ArcGIS 10.7 software.
2.3 Phylogenetic analysis of TBVs
TBVs sequences were collected from The National Center for Biotechnology Information (NCBI). The S-segment of CCHFV, NSDV, SFTSV, as well as DTV, the first segment of JMTV, and the whole genomes TBEV were aligned using MUSCLE version 3.8.31 software. Phylogenetic trees of NJ were constructed using the MEGA 11 software with the Kimura two-parameter model and 1,000 Bootstrap replications.
3 Results
Eighteen viruses from eight genera of five families were identified in China (Table 1). Some of these viruses were also widely distributed in other areas of the world, some were only found in China. More than sixteen species of ticks from six genera were found to carry or transmit TBVs in China, most of which were from the family Ixodidae. TBVs found and reported only by high-throughput sequencing were listed in Supplementary Table S1.
3.1 Nairoviridae
Seven TBVs are belonging to the genus Orthonairovirus of the family Nairoviridae in China. CCHFV, one of the most notable tick-borne viruses of the Nairoviridae, is widely distributed in the world and has been reported in Asia, Africa and Europe (Serretiello et al., 2020). The earliest record of CCHFV reported in China could be traced back to an outbreak of hemorrhagic fever in the southern XUAR in 1968, which was the reason that CCHFV was named Xinjiang hemorrhagic fever in China (Saijo, 2007). A study reported that more than 200 cases of CCHFV had occurred in China with more than 50 deaths as of 2003 (Fereidouni et al., 2023). A population serologic study for CCHFV reported that seropositive cases could be found in northeastern, northwestern, and southern China, but no cases of human infection had been detected outside of XUAR (Teng et al., 2022). Hyalomma spp. particularly Hyalomma asiaticum are the main vectors for the transmission of CCHFV. NSDV could cause zoonotic disease and was first identified in 1910 in sheep and goats in Kenya. In the past, East Africa was the main endemic area, but it was gradually being reported in Asia, including India and Sri Lanka (Krasteva et al., 2020). The first detection of NSDV in ticks in China was in Haemaphysalis longicornis collected from cattle and sheep in Jilin and Liaoning Provinces in 2015. Its genotype was different from other strains in Africa and Asia (Gong S et al., 2015). NSDV was also found in Hubei Province (Yang et al., 2019). Subsequently, NSDV was found by high-throughput sequencing in Liaoning Province with the same strain as previously reported in Hubei Province (Bai et al., 2023). The current records of NSDV in China were all found in H. longicornis.
According to the taxonomy of the order Bunyavirales updated by the International Committee on Taxonomy of Viruses (ICTV) in 2019, Tamdy virus (TAMV) and Tacheng tick virus 1 (TcTV-1) were both members of the species Tamdy orthonairovirus (Abudurexiti et al., 2019). TAMV has appeared frequently in Asia since it was first detected in Uzbekistan in 1971. It was not reported in China until it was detected in 2019 in hard ticks collected from Bactrian Camels in XUAR (Lvov et al., 1976b; Zhou et al., 2019). Evidence of human infection of TAMV was provided by the presence of TAMV IgG in humans from XUAR (Moming et al., 2021). TcTV-1 was first detected by high-throughput sequencing of Dermacentor marginatus in XUAR in 2015 (Li et al., 2015). Researchers isolated TcTV-1 in the cerebrospinal fluid, throat swabs, and urine of a woman engaged in agricultural activities in the XUAR who developed fever and rash symptoms after being bitten by a tick (Liu et al., 2020). The seroprevalence of TcTV-1 in the local population was as high as 15% and the serum samples from cattle and sheep were also partially positive by RT-PCR. TCTV-1 was found in 4 native tick species, of which D. marginatus had the highest positive rate. A study reported a man who developed clinical symptoms after a tick bite in XUAR and TcTV-1 and Rickettsia raoultii were detected in his blood samples by RT-PCR, he was finally diagnosed with severe acute meningitis co-infected with Rickettsia raoultii and TcTV-1 (Zhang et al., 2021). TcTV-1 infection in animals has been detected in giant gerbils from XUAR, and Songling virus (SGLV) was also detected (Ji et al., 2023). SGLV was a new Orthonairovirus first identified in 2021 in a male farmer bitten by a tick in Heilongjiang Province, with genomic and morphological characteristics similar to other Orthonairovirus. It was phylogenetically related to members of Tamdy orthonairovirus and was positive in 6.38% of local patients hospitalized for tick bites. SGLV was also found in local Ixodes crenulatus, Ixodes persulcatus, Haemaphysalis concinna, and H. longicornis. Subsequently, some researchers annotated the sequence of SGLV in the metagenomic sequencing of ticks from northeastern China (Liu et al., 2022; Cai et al., 2023; Ji et al., 2023). Beiji nairovirus (BJNV), also a new nairo-like virus, was first mentioned in high-throughput sequencing, and then it was detected in a febrile patient in Inner Mongolia Province. Researchers identified BJNV infections in several tick-bitten patients in Heilongjiang and Jilin Provinces, and BJNV was also detected in local ticks, including I. persulcatus, I. crenulatus, Demacentor silvarum, D. nuttalli, H. concinna and H. longicornis (Wang YC et al., 2021; Liu et al., 2022). Yezo virus (YEZV) is a new Orthonairovirus first detected in Japan in 2019 and is currently only reported in Japan and China (Kodama et al., 2021). The first detection in China was in a man bitten by an I. persulcatus tick from Inner Mongolia Province. YEZV was also detected in I. persulcatus collected in Heilongjiang, Inner Mongolia, and Jilin Provinces. YEZV was genetically related to Sulina virus found in Ixodes ricinus from Romania in phylogenetic analysis, but YEZV was in a unique cluster of the phylogenetic tree with TAMV, SGLV, TcTV-1 (Lv et al., 2023). Notably, a novel virus strain was detected from D. silvarum in Jilin Province recently. Phylogenetic analyses showed that it was in the same branch as SGLV, but homology analyses with the SGLV strain showed that this strain might be a novel Orthonairovirus, and researchers have named it Antu virus (Li et al., 2023).
3.2 Phenuiviridae
The family Phenuiviridae in this study included four TBVs, of which the most widely distributed in China was SFTSV of the genus Bandavirus. SFTSV is a new virus of the order Bunyavirus discovered in China in 2011. The first strain was isolated from a man in Henan Province, and researchers subsequently detected SFTSV in several febrile patients, as well as in H. longicornis collected from domestic animals in the area where the patients lived. SFTSV was also reported in South Korea, Japan, Vietnam and Pakistan in recent years (Yu et al., 2011; Han et al., 2018; Kim et al., 2018; Zohaib et al., 2020; Fujikawa et al., 2021; Tran et al., 2022). H. longicornis was the most common vector of SFTSV, and it was found that SFTSV could be transmitted vertically in H. longicornis. SFTSV could be detected in eggs laid by SFTSV-carrying females as well as in mature larvae, nymphs, and adults after transstadial transmission (Zhuang et al., 2018). From literature published since 2011, it could be found that SFTSV had a very wide geographical distribution in China and its vectors also included Ixodes spp. and Rhipicephalus spp. (Pan et al., 2013; Sun Y et al., 2017). In contrast to other TBVs, SFTSV could not only be transmitted by ticks but also be transmitted directly from person to person through direct contact with the infected blood (Chen et al., 2013; Gong et al., 2018; Fang et al., 2021). Aerosol transmission has also been suggested as a potential human-to-human transmission route of SFTSV (Gong Z et al., 2015). As another member of the genus Bandavirus, GTV is a new tick-borne Bandavirus detected in 2018 from D. nuttalli and human serum samples in XUAR. It is genetically related to SFTSV and Heartland virus by phylogenetic analyses and may have a similar RNA replication mechanism to SFTSV (Shen et al., 2018). Mukawa virus (MKWV) was first detected in I. persulcatus in Japan in 2018, and researchers found that I. persulcatus and H. concinna in Heilongjiang Province carried MKWV and were capable of infecting mammals, but it had not yet been reported in other countries or regions (Wang et al., 2022; Qin et al., 2023). It was curious that MKWV was not genetically similar to any other tick-borne Phenuiviridae viruses on the phylogenetic tree, instead, it was related to viruses transmitted by mosquitoes and sandflies (Matsuno et al., 2018). DTV was initially identified in high-throughput sequencing of H. longicornis in Hubei Province. It exists in several provinces in southern China. DTV genetically clustered into a branch of the Yongjia tick virus (Li et al., 2015; Shao et al., 2020; Zhu et al., 2020; Wang A et al., 2021; Ma et al., 2022). However, there was no evidence of DTV infection in humans. All DTV strains in China were found in ticks, except a report of detection in sheep serum in Shandong Province (Shao L et al., 2021). TcTV-2 was detected by high-throughput sequencing and classified into the family of Phenuiviridae, without being assigned to a specific genus. There was some evidence that humans and several ticks could be infected with TcTV-2. It also appeared in regions outside of China including Turkey and Kazakhstan (Li et al., 2015; Dong et al., 2021; Dincer et al., 2022; Jia et al., 2022).
3.3 Flaviviridae
The family Flaviviridae includes many species of arboviruses, such as Dengue virus, a mosquito-borne virus that poses a serious threat to humans. Those tick-borne flaviviruses of the family Flaviviridae made a group called TBEV serocomplex group, which includes TBEV, Omsk haemorrhagic fever virus (OHFV), Powassan virus (POWV) and Karshi virus (KSIV) (Grard et al., 2007). TBEV was one of the most widespread viruses of the TBEV serocomplex group in the world, especially in Europe. People in Japan, Korea and China were also at risk of TBEV infection (Lindquist and Vapalahti, 2008). There were three subtypes of TBEV including the Far Eastern (TBEV-FE) which was the most prevalent in China, the Siberian (TBEV-Sib) and the European (TBEV-EU), but a new subtype named the Himalayan TBEV was found in China (Phipps and Johnson, 2022). At present, the forested areas of northeastern China have a high prevalence of TBEV, so previous studies on TBEV in China mainly focused on this region (Chen et al., 2019; Li XH et al., 2022). Karshi virus (KSIV) was first identified in Ornithodoros papillipes in Uzbekistan. KSIV was detected in Hy. dromedraii, Hy. asiaticum, and D. nuttalli from 14 sampling sites in XURA with a minimum infection rate of 4.96%. Researchers successfully isolated virus particles from Hy. asiaticum and then found KSIV in local domesticated sheep and wild-caught greater gerbils (Lvov et al., 1976a; Bai et al., 2022). They also demonstrated the existence of serological cross-reactivity between TBEV and KSIV, with the antibodies against these two viruses that could recognize each other’s antigens. JMTV was a novel segmented tick-borne flavivirus that had been reported in several provinces in China, it was first identified by high-throughput sequencing of ticks in Hubei Province in 2014 (Qin et al., 2014; Guo et al., 2020; Yu et al., 2020; Pang et al., 2022; Zhang et al., 2022; Qu et al., 2023; Wu et al., 2023). JMTV was also found in several countries around the world. In addition to ticks, mosquitoes were also reported to be infected with JMTV (Ladner et al., 2016). There already had proof of JMTV infection in humans in China, as a study detected JMTV in a skin biopsy sample taken from a patient’s tick bite site (Jia et al., 2019). Recently, a new type of JMTV was discovered by high-throughput sequencing in ticks in Sichuan Province and named Sichuan tick virus (Huang L et al., 2023). Alongshan virus (ALSV) was also a novel segmented tick-borne virus isolated from a patient presenting with unexplained fever in Inner Mongolia Province in 2019 and was classified in the Jingmenvirus group because of its genetic similarity to JMTV (Wang et al., 2019a). Other local febrile patients with a history of tick bites were also confirmed to be infected with ALSV. Besides, the prevalence of ALSV positivity in ticks collected at the workplace of these patients or at the location where they were bitten was 6.5% (Wang et al., 2019b). ALSV has been reported in Europe (Kuivanen et al., 2019; Kholodilov et al., 2020; Stegmueller et al., 2023). Bovine hepacivirus (BovHepV) was a genus of hepatitis viruses found in R. microplus in Guangdong and Heilongjiang Provinces, which mainly infected cattle with severe impacts on the farming industry. But no human infections were documented (Shao JW et al., 2021; Guo et al., 2022; Yuan et al., 2023).
3.4 Potential tick-borne viruses of other virus families
PIV5 of the family Paramyxoviridae could cause disease in humans and animals (Wang et al., 2023). The detection of PIV5 in arthropods had not been documented until it was found in ticks from Jilin Province (Yang et al., 2022). LCMV of the family Arenaviridae was a group of rodent-borne viruses that could cause neurological disorders in humans (Barton et al., 2002). LCMV was isolated from ticks in northeastern China which was the first report of LCMV in China. It has been predicted that LCMV in ticks and transmitted among ticks in Jilin Province might have occurred in the 1970s or 1980s (Zhang L et al., 2018). Both PIV5 and LCMV were considered zoonotic viruses, they only were detected or isolated from ticks in China. There were no documented cases of ticks as a vector to infect humans, so they should be treated as potential tick-borne viruses.
3.5 Geographical distribution of tick-borne viruses in China
All the TBVs mentioned in this paper were widely distributed in China including northeastern forested areas, coastal areas, and northwestern interior. XUAR currently has the largest number of TBVs, containing eight viruses from the family Nairoviridae (CCHFV, TAMV, TcTV-1, and SLTV), the family Flaviviridae (TBEV, KSTV, and JMTV), and the family Phenuiviridae (GTV). The next richest group of TBVs was found in northeastern Inner Mongolia and Heilongjiang Provinces, where all five virus families were present, with the family Flaviviridae and the family Nairoviridae predominating. Another area of viruses affected was central China, where most of the viruses were from the family Phenuiviride. TBVs in China were less studied focusing on the Tibetan Plateau, northwestern China outside of XUAR, such as Gansu Province and western Inner Mongolia Province, and southern China (Figure 1). TBVs of the family Nairoviridae were distributed in most parts of XUAR and northeastern China (Figure 2A). Many provinces in China had human serologic evidence suggesting the presence of CCHFV, but the provinces where CCHFV was detected in ticks was only XUAR, Yunnan and Inner Mongolia Provinces (Teng et al., 2022). CCHFV was mainly distributed in the grass-covered areas around the Tarim Basin and the Tianshan Mountains in XUAR. In addition, it was reported to be detected in ticks and camels from the Alashan League of Inner Mongolia Province (Xia et al., 2011; Kong et al., 2022). Four species of ticks could carry or transmit CCHFV including Hy. dromedraii, Hy. asiaticum, Hy. marginatum and D. nuttalli which were mainly found in northwestern China (Supplementary Figure S1A). TCTV-1 was only in the Tacheng and Altay regions of XUAR, and geographically clustered in the Tianshan Mountains. TCTV-1 could be transmitted by D. marginatus, D. nuttalli, D. silvarum, and Hy. asiaticum. Ticks of the Dermacentor spp. were the main carriers of TCTV-1 with a higher rate. These ticks were widely distributed in northern China, but there were no records of TCTV-1 except in XUAR (Supplementary Figure S1B). TAMV appeared sporadically in the Kashi, Aksu, and Tacheng regions, with three reported sites around the Tarim Basin and one at the junction of the TianShan Mountains and the Junggar Basin (Supplementary Figure S1C). SLGV was also reported from the Tianshan Mountains in XUAR, although most SLGV in China were now reported from northeastern China, clustering in the forest of the Greater Khingan Mountains. Ticks of Ixodes spp. were the main vectors of SLGV, and all records of SGLV in China were in the I. persulcatus distribution area, with partial overlap of the I. crenulatus or the H. longicornis. Distribution area (Supplementary Figure S1D). NSDV was mainly clustered in Liaoning and Jilin Provinces, except for one sampling site reported in Suizhou City in Hubei Province (Supplementary Figure S1E). BJNV was distributed in northeastern Inner Mongolia Province and eastern Heilongjiang Province (Supplementary Figure S1F).
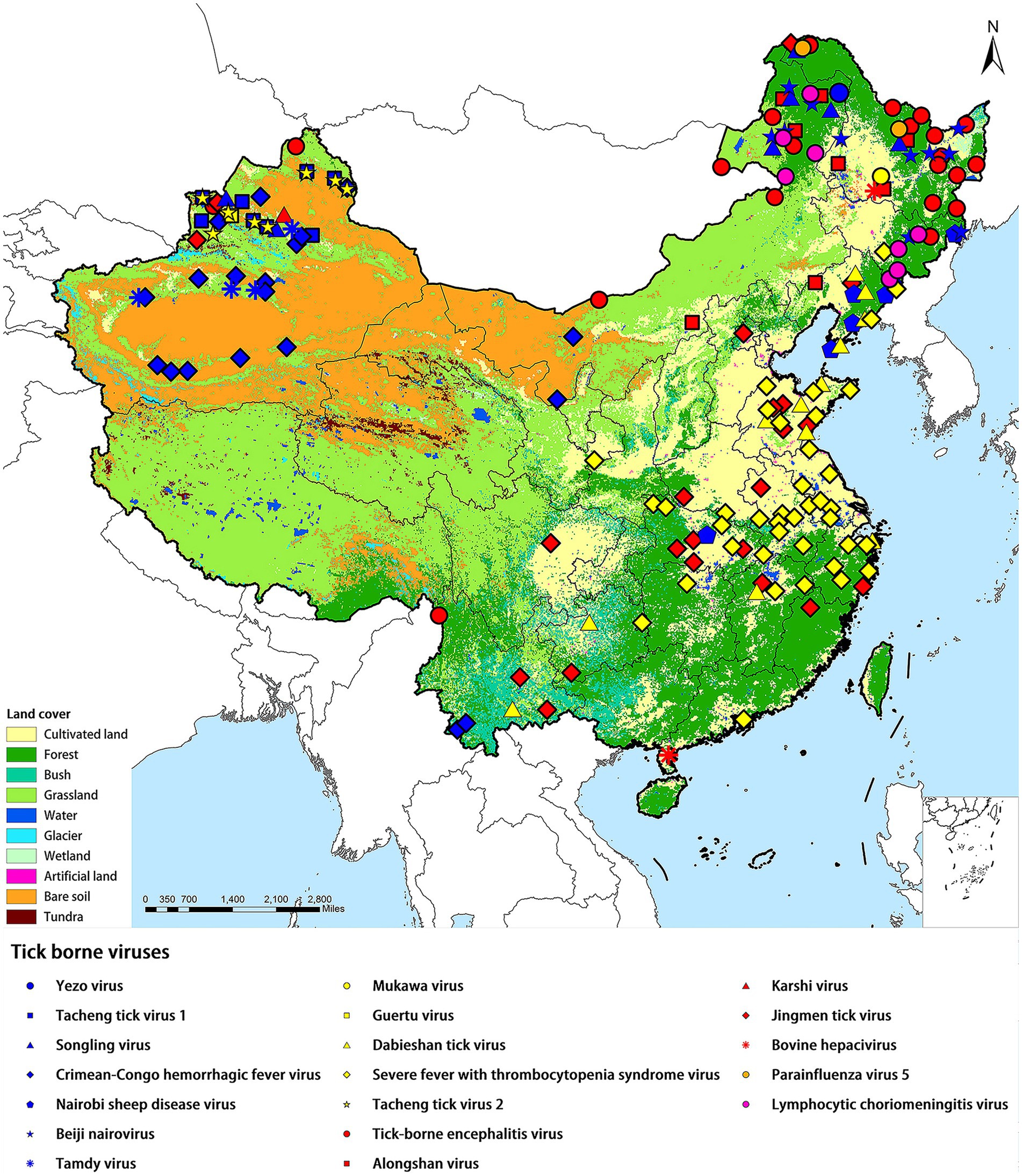
Figure 1. Distribution of tick-borne viruses in China. China’s land cover map was used as a background map. Blue squares represented the Nairoviridae family, yellow squares represented the Phenuiviridae family, and red represented the Flaviviridae family.
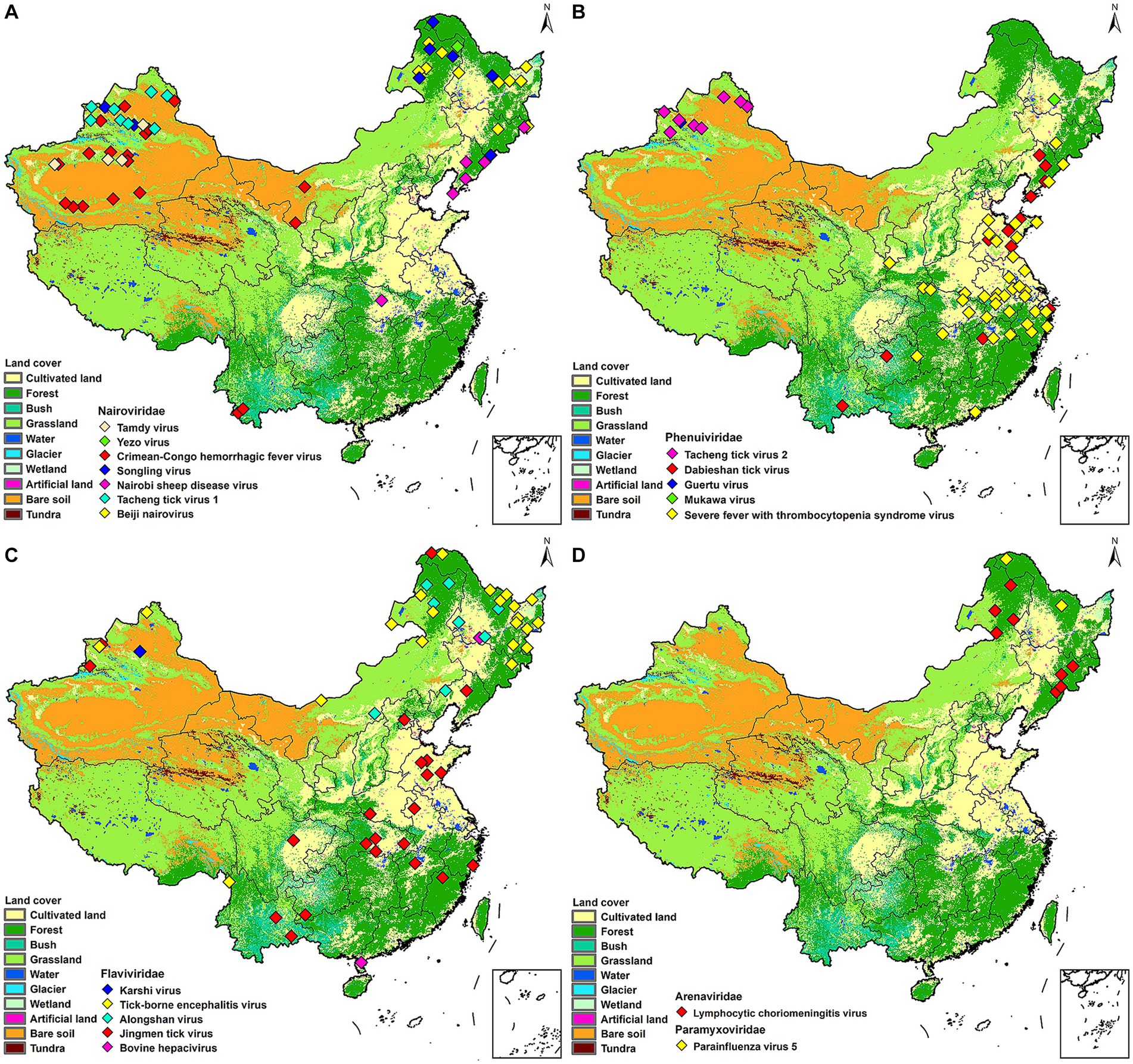
Figure 2. Mapping of tick-borne viruses by family. China’s land cover map was used as a background map. (A) Nairoviridae, (B) Phenuiviridae, (C) Flaviviridae, (D) Paramyxoviridae and Arenaviridae. Different colors were used in each figure to denote different viruses.
Both GTV and MKWV were only recorded once in Usu City and Heilongjiang Province, respectively (Figure 2B). SFTSV were reported in 12 provinces, with Xinyang City in Henan Province having the highest number of cases, and Hubei, Anhui, Zhejiang, and Shandong Provinces all having high prevalence of SFTSV (Miao et al., 2020). The vectors of SFTSV included five species of ticks, H. longicornis was its confirmed vector, but SFTSV were also detected in H. conicinna, H. japonica, I. persulcatus, and R. microplus. However, most of the SFTSVs were still clustered in the H. longicornis distribution areas (Figure 2B; Supplementary Figure S2A). DTV is now distributed in six provinces of China, with a high prevalence in Shandong and Liaoning Provinces, partially overlapping with SFTSV. It was also found in forested and shrub-covered areas of Yunnan and Guizhou Provinces. Two ticks of Rhipicephalus spp. and H. longicornis were the vectors for DTV (Figure 2B; Supplementary Figure S2B). TcTV-2 is currently distributed in XUAR, clustered around the Tianshan Mountains, and has the same vectors and distribution characteristics as TcTV-1, although they are not in the same family (Figure 2B; Supplementary Figure S2C).
JMTV was the most widely distributed virus in our study. It was dispersed in 14 provinces of China, from Mohe City in Heilongjiang Province in the north to Chabuchar Xibe and Bole counties in XUAR in the west, to the coastal areas of Fujian in the east, and Wenshan County in Yunnan Province in the south. However, the areas were mainly in central China, with Hubei and Shandong Provinces reporting more than others (Figure 2C; Supplementary Figure S3A). In addition to the wide geographic distribution, JMTV had the widest variety of vectors. As the dominant tick species varied among regions, the ticks that carried JMTV varied from different regions, including at least 5 genera and 11 species, which were distributed throughout China. TBEV was clustered in Heilongjiang and northeastern Inner Mongolia Provinces, mostly in forest-covered areas. The vector of most TBEV was I. persulcatus, but D. nuttalli and D. silvarum could also carry it in some studies. In addition to the large number of I. persulcatus in the forested areas of Northeast China, it was also found in the Karshi region of XUAR, but there were fewer cases of TBEV in XUAR (Figure 2C; Supplementary Figure S3B). ALSV was also a young virus detected recently, with I. persulcatus and H. conicinna were its vectors, and the only areas where both ticks were distributed were northern XUAR and southern Heilongjiang Province (Figure 2C; Supplementary Figure S3C).
PIV5 was detected only in Mohe City and Yichun City in Heilongjiang Province (Figure 2D; Supplementary Figure S4A). LCMV could be carried by 4 species of ticks, which were densely distributed in the XUAR and Shaanxi Provinces. LCMVs were detected only in the forested areas of the Greater Khingan Mountains and the Changbai Mountains (Figure 2D; Supplementary Figure S4B).
3.6 Phylogenetic analysis of tick-borne viruses
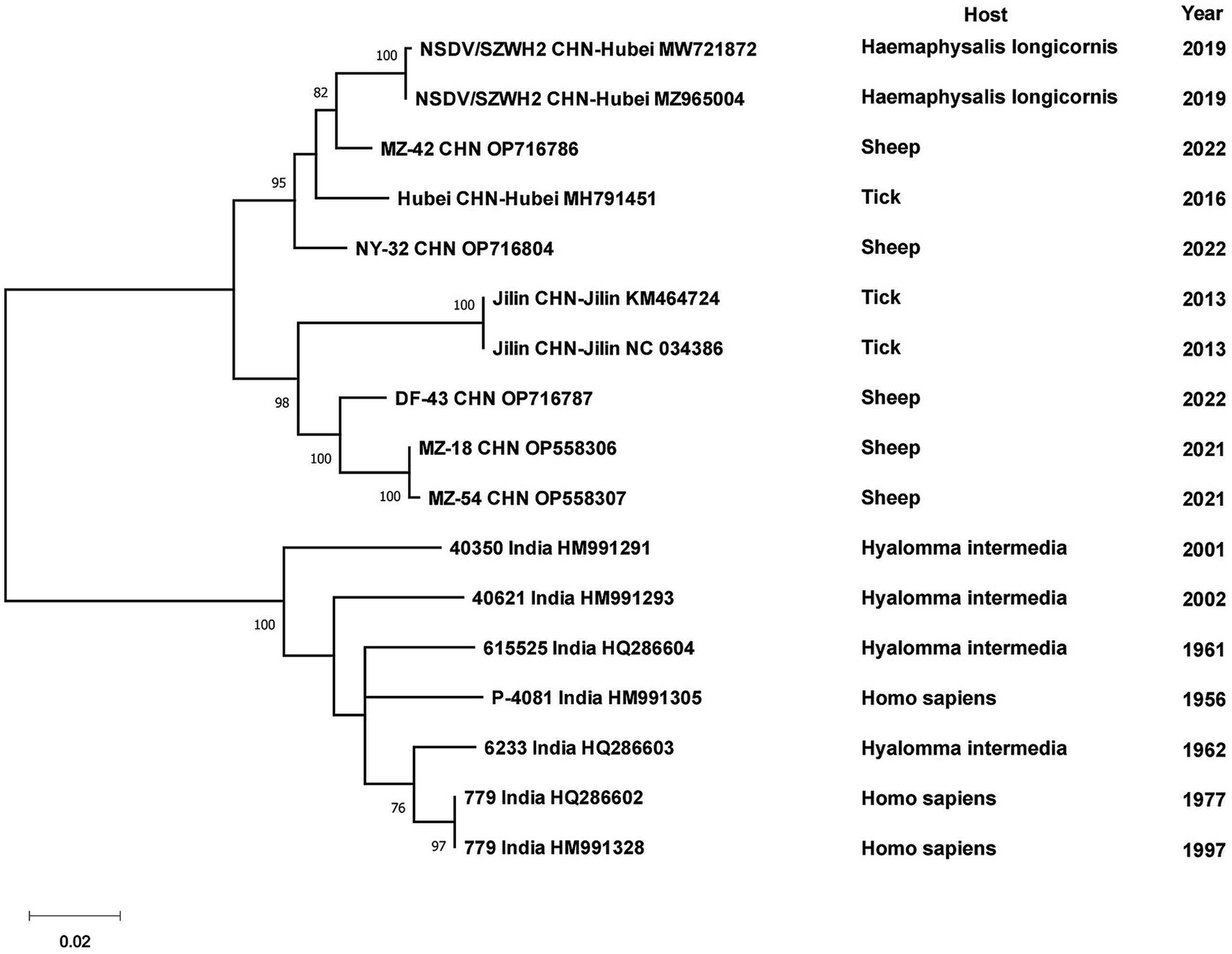
Phylogenetic analyses of the sequences collected in NCBI from different areas of China were performed to analyze the genetic relationships among the viruses. CCHFV was discovered early and classified into three genotypes: Asian, European and African (Serretiello et al., 2020). CCHFV in China was divided into two Asian aggregation branches. Strains from XUAR clustered together with two strains detected from Hy. asiaticum in Inner Mongolia Province. Although the strains found in XUAR had various host species, they remained genetically clustered. The other branch was a cluster of two strains detected in Hy. asiaticum in XUAR and strains from Pakistan (Figure 3). There were not many strains of NSDV in China, but they formed two discrete branches from the Indian strains (Figure 4).
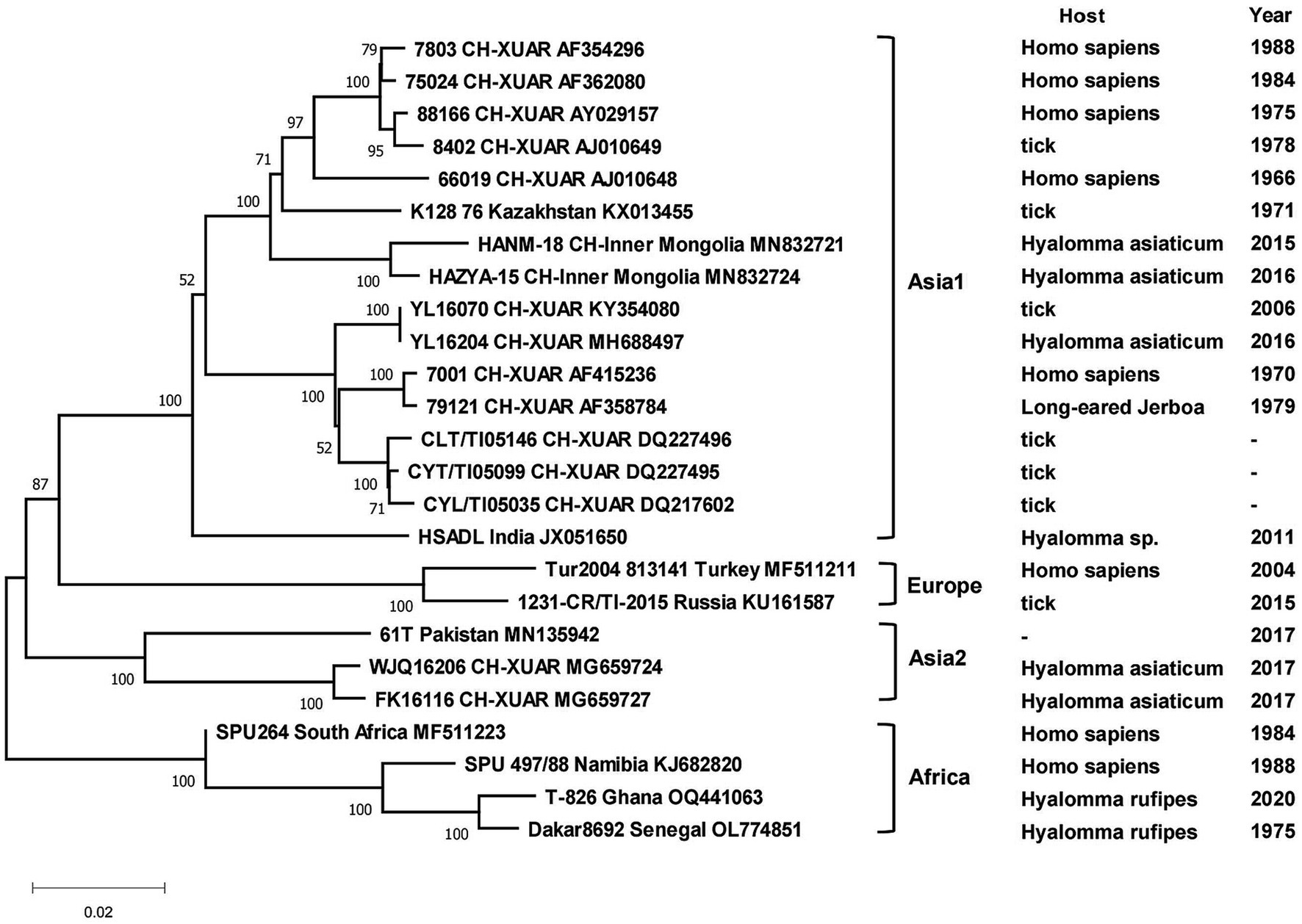
Figure 3. Phylogenetic tree of the S segment of Crimean Congo hemorrhagic fever virus. Each sequence was labeled with its host and sampling time (same for the later figures). The missing data in the figure were not recorded in NCBI.
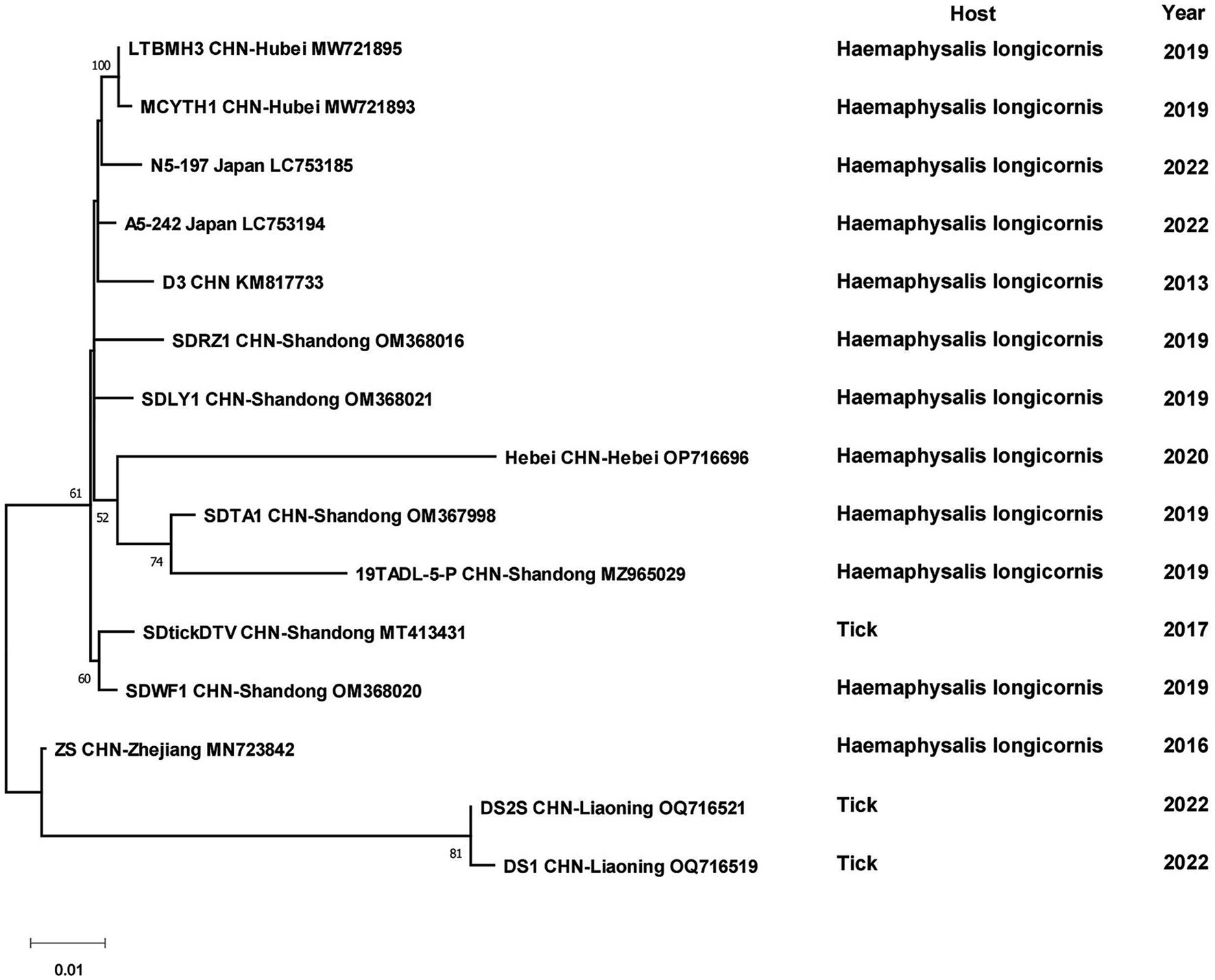
The sequences of 131 SFTSV strains from China and 6 strains from other countries formed two separate branches, one branch was full of Chinese strains, while the other one had some Chinese strains branching with Japanese and South Korean strains. The first cluster could be viewed as five separate branches, I included isolates from Shandong, Henan and Hubei Provinces, II included strains from Zhejiang Province, III included several strains from Zhejiang, Jilin and Liaoning Provinces, IV and V were clusters of strains from Jiangsu and Henan Provinces, respectively. The other branch included strains from Shandong, Henan and Zhejiang Provinces with strains from Japan, Korea and Thailand, in which four strains from Zhejiang Province were clustered with foreign strains. Although sampling times or hosts were different, strains geographically from the same province always clustered together. Some strains from Henan and Shandong Provinces were scattered in the phylogenetic tree (Figure 5). Among the DTV strains, those found from Shandong and Hubei Provinces and Japan formed a larger cluster. DTV found in Liaoning Province in 2022 and Zhejiang Province in 2016 form a distinct branch and did not cluster with the sequences of the strain that was first reported in 2013, especially the Liaoning strains, which were genetically far from the rest strains (Figure 6).
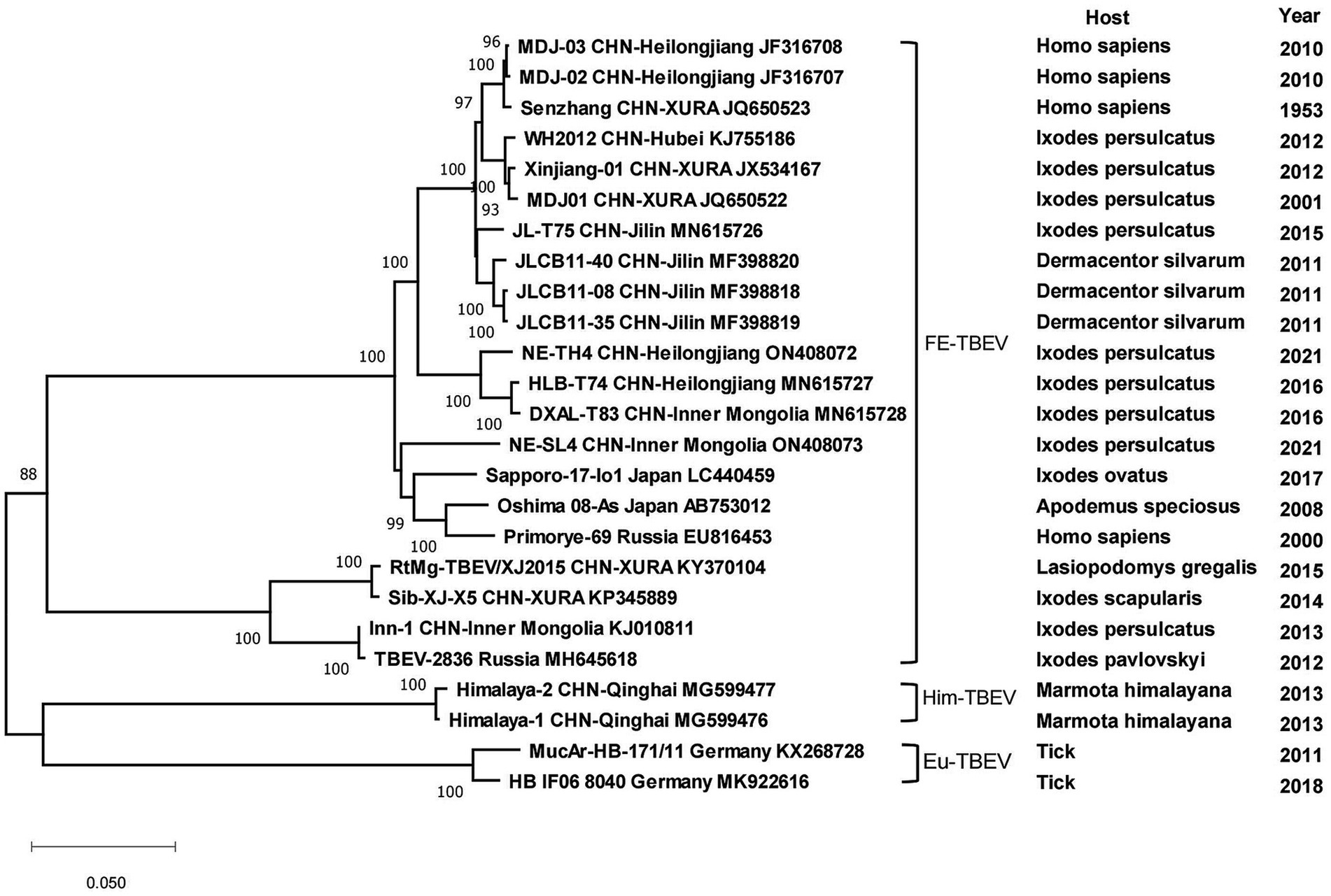
All strains of tick-borne encephalitis viruses in China belonged to the TBEV-FE, except for two Himalayan TBEV strains detected in Qinghai Province in 2013. Three strains collected from I. persulcatus in Inner Mongolia Province with sampling dates of 2013, 2016 and 2021 formed clusters with strain TBEV-2836 isolated from I. pavlovskyi in Russia in 2012, strain HLB-T74 from Heilongjiang Province and strains from Japan and Russia, respectively, and they were in three clusters of different branches. Strains detected from human samples in 2010 in Heilongjiang Province clustered with strains from human samples in XUAR, whereas strains detected in I. persulcatus in 2016 were genetically closely related to strains detected in I. persulcatus in Inner Mongolia in the same year, and the two types of sequences from Heilongjiang Province were genetically distantly related. As for strains from XUAR, strains detected in I. persulcatus in 2001 and 2012 clustered with WH2012 from Hubei Province, but the strains detected in I. scapularis and Lasiopodomys gregalls formed a cluster with Inn-1 and TBEV-2836 and were distant from several other strains (Figure 7). The sequences of JMTV in China were divided into two branches, one was the sequences of XUAR and Hubei Province, which were clustered together regardless of their hosts, and the other was the sequences of Yunnan, Guizhou and Sichuan Provinces, and other places in southwesten China. However, the JMTV sequence found in I. persulcatus from Heilongjiang Province strayed outside the centralized parts of the phylogenetic tree and did not cluster with other strains in China (Figure 8).
4 Discussion
The diversity of TBVs has increased in the twenty-first century with the emergence of new viruses. Heartland virus (HRTV), Bourbon virus, Powassan virus and Colorado tick fever virus are emerging as hotspots for arboviruses in the United States (Rodino et al., 2020). In Europe, in addition to TBEV and CCHFV, several TBVs pose a threat to human health, although few cases have been reported of them, such as Eyach virus, Louping ill virus, Pustyn virus and Sulina virus (Charrel et al., 2004). TBVs were also diverse and widely distributed in China.
According to our statistics, there were at least 8 genera of viruses in 5 families in China, and most of them first appeared in China in the last few years and were detected only in some countries around China. The species of TBVs of the genus Orthonairovirus were increasing, such as Tamdy orthonairovirus, which had similar clinical symptoms. GTV of the family Phenuiviridae and ALSV of the family Flaviviridae were both new tick-borne viruses in recent years. Most of these emerging tick-borne viruses were detected based on surveillance for tick-borne diseases in China, and when researchers were able to isolate a virus in patients, examination of ticks collected in the patient’s place of residence, as well as animals such as cows and sheep, were also partially positive for the virus, suggesting that the virus were spreading in the local ecosystem, which would make the local population at risk of exposure to viral infections. In addition to those newly identified viruses, many TBVs existed but have not been reported in China previously, such as NSDV, TAMV, YEZV, MKWV, and KSIV. NSDV was previously thought to be endemic only in East Africa, but some studies have shown that the Ganjam virus is an Asian variant of NSDV and has been found in India and Sri Lanka. This demonstrated that Asia was also becoming endemic for NSDV. It is notable that the earliest strain of NSDV in China was different from strains from other Asian areas and Africa, and was considered to be a new strain (Marczinke and Nichol, 2002; Gong S et al., 2015; Gong Z et al., 2015). Alternatively, some TBVs could be imported exogenously from other countries and regions. Transmission of tick-borne pathogens across regions was not unusual, with migratory birds being one of the main contributing factors (Hasle, 2013; Hornok et al., 2016). In focusing on emerging TBVs, it is important to identify them from closely related viruses of the same viral family. Bai et al. found that the epidemic of KSIV can lead to misinterpretation of the high seroprevalence of TBEVs, which can cause misjudgment of the prevalence of TBVs and the degree of risk to humans (Bai et al., 2022). In addition to the proliferation of virus species, the vectors of some TBVs also changed such as CCHFV, TBEV and SFTSV, especially the species of ticks carrying SFTSV increased significantly. TBEV was the predominant TBV in Europe and was distributed in at least twenty-five European countries. In contrast to China, Ixodes ricinus was the most important vector for the transmission of TBEV in Europe. While I. persulcatus mostly played its role in transmitting TBEV in the forests of Siberia and Russia. The increasing number of tick-borne virus species and hosts in China has led to a rapid epidemic of tick-borne viral diseases in humans.
In this study, the distribution of TBVs was predominantly in the North of China, especially in the northeastern region and XUAR, which is similar to the general distribution of tick-borne diseases in China by Wu et al. (2013). The geographic distribution of TBVs for each virus family and the distribution of vectors of each virus were mapped. Something was interesting when combining them. The number of Hy. asiaticum was the most dominant factor influencing the distribution of CCHFVs in the previous study (Teng et al., 2022). Moreover, among the environmental factors predicting the distribution of Hy. asiaticum, desert grassland cover and rainfed farmland cover were the main factors (Zhao et al., 2021). Predictions in some articles indicated that Gansu, Ningxia, Shaanxi, and Yunnan Provinces were risk areas for the occurrence of CCHFV, and Yunnan Province had found CCHFV in ticks, whereas Gansu and western Inner Mongolia Provinces were neighbors to the XUAR, with similar landscapes and climates, more desert or bare land, and people engaged in farming, but there had no reports of tick-borne CCHFV or human infections with CCHFV. The reasons need to be studied in the future or the surveillance of tick-borne CCHFV and unidentified febrile illnesses should be strengthened in these regions. Although TCTV-1 and TCTV-2 were in different virus families, they had the common characteristics of higher positivity in ticks of the Dermacentor spp. than the Hyalomma spp. (Zhao et al., 2021). Among the environmental factors affecting distribution, both grass cover and seasonal precipitation had a greater impact on the Dermacentor spp., which might explain why most of TCTV-1 and TCTV-2 were detected in XUAR around the Tianshan Mountains. SGLV was mainly transmitted by I. persulcatus and I. crenulatus distributed in Northeast China and XUAR. The potential for I. persulcatus and I. crenulatus in XUAR to carry SGLV and transmit it to humans was noteworthy because SGLV had been found in local rodents. In a prediction model of SFTSV, the number of H. longicornis and altitude had a greater effect, with lower altitude resulting in more SFTSV cases, while the distribution of H. longicornis was related to precipitation and mean annual temperature. This might be related to the fact that SFTSV cases were mainly in central China, which had a lower elevation and higher precipitation than the northwestern and southwestern China (Miao et al., 2020). H. longicornis was distributed throughout Shaanxi Province where the precipitation and climate were also similar to central China, the reason for fewer reports of SFTSV in Shaanxi Province might be the high altitude. The occurrence of TBEV was closely related to meteorological factors, such as the presence of I. persulcatus and mixed broadleaf-conifer forest (Sun RX et al., 2017). In agreement with previous studies, the majority of TBEV in China was distributed in the forested areas of the Great Khingan Mountain, the Lesser Khingan Mountains, the Changbai Mountains and the Tianshan Mountains in XUAR, which was consistent with the distribution of I. persulcatus. However, when preventing SFTSV and TBEV infections in populations, the specificity that they can be transmitted directly from person to person without insect vectors should be emphasized. TBEV can be transmitted to humans by drinking raw milk from infected animals, and it has also been found that TBEV may be transmitted from infected mothers who have not been immunized with the TBE vaccine to their offspring through breast milk (Kerlik et al., 2022). It suggested that preventive measures cannot be taken against its vectors alone. The majority of JMTV was distributed in the plains of central China, which might be related to its higher detection in H. longicornis. There were no studies on the prediction of JMTV distribution at present. Most of the TBVs had some characteristics in their distribution areas or vector tick species, but JMTV had no specificity probably because of the large number of tick species that could transmit it, so it could not be analyzed what factors had a greater impact on its distribution. PIV5 and LCMV, which appeared in China for the first time, were carried by the I. persulcatus, D. nuttalli and D. silvarum. Both of these two viruses could be found in the forested areas of the Northeast and the Tianshan Mountains, which was also a cross-distribution area for their vectors. These areas might be potential regions of infection for the PIV5 and LCMV (Vilibic-Cavlek et al., 2021). LCMV was a natural teratogen that could be transmitted to the fetus via vertical transmission. It was more dangerous for a pregnant woman if infecting LCMV, which could result in fetal miscarriage, intrauterine death, or severe central nervous system disease. The risk of human infection of LCMV should be assessed by monitoring the prevalence of LCMV in natural rodents and ticks in northeastern China. Animal reservoirs should not be ignored when exploring the factors influencing the distribution of TBVs. The abundance of animal reservoirs had a strong effect on the abundance of ticks. Moreover, the migration of animal reservoirs, either active or passive, led to the geographical expansion of tick populations, which has important implications for the transmission of TBVs between different areas.
All CCHFV strains included in this paper also clustered into some geographically related branches, which was constant with previous phylogenetic analyses (Zhou et al., 2013a). Two of the strains in XUAR that were both detected in 2017 formed a separate Asia branch with the Pakistan strain. It could be concluded that these two strains in XUAR might be imported from Pakistan or spread from XUAR to Pakistan and that they might share a common ancestor with the clustered European strains. At least two different types of CCHFV genotypes currently exist in China. A new strain of NSDV, which was different from other strains in Asia, has only been detected in ticks and sheep in China, but its potential hazards to humans cannot be ignored because of the unknown pathogenicity. A previous study divided the SFTSV strains in China into five genotypes, whereas in this study all strains were divided into two different clusters, suggesting that there might be two types of SFTSV genotypes with large differences in China currently (Yoshikawa et al., 2015). Several strains from Shandong and Zhejiang Provinces were clustered with Japanese and Korean ones, as was shown in other studies. The reason might be that Shandong Province and Japan were located closely together. HRTV from the genus Bandavirus, which was detected only in the United States, was clinically similar and phylogenetically related to SFTSV. It was not considered in the phylogenetic analysis of SFTSV in this paper, but the association between them cannot be ignored in the differential diagnosis of SFTSV. The two branches of the phylogenetic tree indicated that the DTV in China could be divided into two genotypes. The phylogenetic tree of JMTV in China was geographically clustered except for the strain from Heilongjiang Province. As fewer studies were conducted on JMTV in northeast China, there were no other strains for reference to identify whether there are new genotypes or not. One strain of TBEV from Inner Mongolia Province was genetically related to the Russian strain and clustered with two strains from XUAR, both of which had different source hosts, and it could be hypothesized that it was transmitted from Russia to Inner Mongolia Province and then spread to XUAR since they border each other and the Russian strains were sampled the earliest.
The limitation of this study is that only published records of TBVs in the literature are available, some areas where TBVs were endemic but not reported may be missed. In addition, the data analyzed in this article was from different literature and the homogeneity of the samples was not ensured when compared them together.
5 Conclusion
Eighteen TBVs that could cause serious diseases or potential harm to humans or animals existed in China. XUAR and Northeastern China had more TBVs which should be paid more attention to. There were at least five genera of ticks that could carry or transmit TBVs in China. In addition, several strains of DTV and JMTV that appeared in recent years were new genotypes. The complex situation of TBVs in China brought great challenges to public health. It is vital to strengthen TBV surveillance and build a comprehensive surveillance network in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Formal analysis, Visualization, Writing – original draft. QZ: Data curation, Writing – original draft. MM: Investigation, Writing – original draft. HC: Data curation, Writing – original draft. RQ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the “Double first-class” construction and scientific research start-up fund of Lanzhou University.
Acknowledgments
Acknowledgment for the data support from “National Earth System Science Data Center, National Science and Technology Infrastructure of China (http://www.geodata.cn).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1309698/full#supplementary-material
Footnotes
References
Abudurexiti, A., Adkins, S., Alioto, D., Alkhovsky, S. V., Avsic-Zupanc, T., Ballinger, M. J., et al. (2019). Taxonomy of the order Bunyavirales: update 2019. Arch. Virol. 164, 1949–1965. doi: 10.1007/s00705-019-04253-6
Bai, Y., Li, Y., Liu, W., Li, J., Tian, F., Liu, L., et al. (2023). Analysis of the diversity of tick-borne viruses at the border areas in Liaoning Province, China. Front. Microbiol. 14:1179156. doi: 10.3389/fmicb.2023.1179156
Bai, Y., Zhang, Y., Su, Z., Tang, S., Wang, J., Wu, Q., et al. (2022). Discovery of tick-borne Karshi virus implies misinterpretation of the tick-borne encephalitis virus Seroprevalence in Northwest China. Front. Microbiol. 13:872067. doi: 10.3389/fmicb.2022.872067
Barton, L. L., Mets, M. B., and Beauchamp, C. L. (2002). Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187, 1715–1716. doi: 10.1067/mob.2002.126297
Boulanger, N., Boyer, P., Talagrand-Reboul, E., and Hansmann, Y. (2019). Ticks and tick-borne diseases. Medeci Mal Infect 49, 87–97. doi: 10.1016/j.medmal.2019.01.007
Cai, X., Cai, X., Xu, Y., Shao, Y., Fu, L., Men, X., et al. (2023). Virome analysis of ticks and tick-borne viruses in Heilongjiang and Jilin provinces, China. Virus Res. 323:199006. doi: 10.1016/j.virusres.2022.199006
Charrel, R. N., Attoui, H., Butenko, A. M., Clegg, J. C., Deubel, V., Frolova, T. V., et al. (2004). Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. 10, 1040–1055. doi: 10.1111/j.1469-0691.2004.01022.x
Chen, H., Hu, K., Zou, J., and Xiao, J. (2013). A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int. J. Infect. Dis. 17, E206–E208. doi: 10.1016/j.ijid.2012.11.006
Chen, X. J., Li, F., Yin, Q. K., Liu, W. J., Fu, S. H., He, Y., et al. (2019). Epidemiology of tick-borne encephalitis in China, 2007-2018. PLoS One 14:e0226712. doi: 10.1371/journal.pone.0226712
Chen, Z., Yang, X., Bu, F., Yang, X., Yang, X., and Liu, J. (2010). Ticks (acari: ixodoidea: argasidae, ixodidae) of China. Exp. Appl. Acarol. 51, 393–404. doi: 10.1007/s10493-010-9335-2
Couret, J., Schofield, S., and Narasimhan, S. (2022). The environment, the tick, and the pathogen - it is an ensemble. Front. Cell. Infect. Microbiol. 12:1049646. doi: 10.3389/fcimb.2022.1049646
Damian, D., Maghembe, R., Damas, M., Wensman, J. J., and Berg, M. (2020). Application of viral metagenomics for study of emerging and reemerging tick-borne viruses. Vector Borne Zoonotic Dis 20, 557–565. doi: 10.1089/vbz.2019.2579
Dincer, E., Timurkan, M. O., Oguz, B., Sahindokuyucu, I., Sahan, A., Ekinci, M., et al. (2022). Several tick-borne pathogenic viruses in circulation in Anatolia, Turkey. Vector Borne Zoonotic Dis 22, 148–158. doi: 10.1089/vbz.2021.0082
Dincer, E., Timurkan, M. O., Yalcinkaya, D., Hekimoglu, O., Nayir, M. B., Sertkaya, T. Z., et al. (2023). Molecular detection of Tacheng tick Virus-1 (TcTV-1) and Jingmen tick virus in ticks collected from wildlife and livestock in Turkey: first indication of TcTV-1 beyond China. Vector Borne Zoonotic Dis 23, 419–427. doi: 10.1089/vbz.2023.0029
Dong, Z., Yang, M., Wang, Z., Zhao, S., Xie, S., Yang, Y., et al. (2021). Human Tacheng tick virus 2 infection, China, 2019. Emerg. Infect. Dis. 27, 594–598. doi: 10.3201/eid2702.191486
Dragonjic, L. P., Vrbic, M., Tasic, A., Simin, V., Bogdan, I., Mijatovic, D., et al. (2022). Fatal case of imported tick-borne encephalitis in South Serbia. Trop Med Infect Dis 7:7120434. doi: 10.3390/tropicalmed7120434
Eisen, R. J., Kugeler, K. J., Eisen, L., Beard, C. B., and Paddock, C. D. (2017). Tick-borne Zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 58, 319–335. doi: 10.1093/ilar/ilx0005
El-Alfy, E.-S., Abbas, I., Elseadawy, R., El-Sayed, S. A. E.-S., and Rizk, M. A. (2023). Genetic diversity of Merozoite surface antigens in global Babesia bovis populations. Genes. 14:14101936. doi: 10.3390/genes14101936
Fang, X., Hu, J., Peng, Z., Dai, Q., Liu, W., Liang, S., et al. (2021). Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl. Trop. Dis. 15:9037. doi: 10.1371/journal.pntd.0009037
Fang, L. Q., Liu, K., Li, X. L., Liang, S., Yang, Y., Yao, H. W., et al. (2015). Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect. Dis. 15, 1467–1479. doi: 10.1016/s1473-3099(15)00177-2
Fereidouni, M., Apanaskevich, D. A., Pecor, D. B., Pshenichnaya, N. Y., Abuova, G. N., Tishkova, F. H., et al. (2023). Crimean-Congo hemorrhagic fever virus in central, eastern, and South-Eastern Asia. Virol. Sin. 38, 171–183. doi: 10.1016/j.virs.2023.01.001
Fujikawa, T., Yoshikawa, T., Kurosu, T., Shimojima, M., Saijo, M., and Yokota, K. (2021). Co-infection with severe fever with thrombocytopenia syndrome virus and Rickettsia japonica after tick bite, Japan. Emerging Infectious Diseases. 27, 1247–1249. doi: 10.3201/eid2704.203610
Gilbert, L. (2021). The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol. 66, 373–388. doi: 10.1146/annurev-ento-052720-094533
Gong, Z., Gu, S., Zhang, Y., Sun, J., Wu, X., Ling, F., et al. (2015). Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin. Microbiol. Infect. 21, 1115–1120. doi: 10.1016/j.cmi.2015.07.024
Gong, S., He, B., Wang, Z., Shang, L., Wei, F., Liu, Q., et al. (2015). Nairobi sheep disease virus RNA in Ixodid ticks, China, 2013. Emerg. Infect. Dis. 21, 718–720. doi: 10.3201/eid2104.141602
Gong, L., Song, D. D., Wu, J. B., Cao, M. H., Su, B., Sun, Y., et al. (2018). Human-to-human transmissions of severe fever with thrombocytopenia syndrome virus in Anhui province, 2010-2017. Clin. Microbiol. Infect. 24, 920–922. doi: 10.1016/j.cmi.2018.03.014
Grard, G., Moureau, G., Charrel, R. N., Lemasson, J.-J., Gonzalez, J.-P., Gallian, P., et al. (2007). Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 361, 80–92. doi: 10.1016/j.virol.2006.09.015
Guo, J. J., Lin, X. D., Chen, Y. M., Hao, Z. Y., Wang, Z. X., Yu, Z. M., et al. (2020). Diversity and circulation of Jingmen tick virus in ticks and mammals. Virus Evol. 6:veaa051. doi: 10.1093/ve/veaa051
Guo, L., Ma, J., Lin, J., Chen, M., Liu, W., Zha, J., et al. (2022). Virome of Rhipicephalus ticks by metagenomic analysis in Guangdong, southern China. Front. Microbiol. 13:966735. doi: 10.3389/fmicb.2022.966735
Guo, R., Shen, S., Zhang, Y., Shi, J., Su, Z., Liu, D., et al. (2017). A new strain of Crimean-Congo hemorrhagic fever virus isolated from Xinjiang, China. Virol. Sin 32, 80–88. doi: 10.1007/s12250-016-3936-9
Han, M. A., Kim, C. M., Kim, D. M., Yun, N. R., Park, S. W., Han, M. G., et al. (2018). Seroprevalence of severe fever with thrombocytopenia syndrome virus antibodies in rural areas, South Korea. Emerg. Infect. Dis. 24, 872–874. doi: 10.3201/eid2405.152104
Hasle, G. (2013). Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 3:48. doi: 10.3389/fcimb.2013.00048
Holding, M., Dowall, S., and Hewson, R. (2020). Detection of tick-borne encephalitis virus in the UK. Lancet 395:411. doi: 10.1016/S0140-6736(20)30040-4
Hornok, S., Flaisz, B., Takács, N., Kontschán, J., Csörgő, T., Csipak, Á., et al. (2016). Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasit. Vectors 9:101. doi: 10.1186/s13071-016-1365-0
Huang, L., Liu, S., Chen, L., Wang, F., Ye, P., Xia, L., et al. (2023). Identification of novel Jingmen tick virus from parasitic ticks fed on a giant panda and goats in Sichuan Province, southwestern China. Front. Microbiol. 14:1179173. doi: 10.3389/fmicb.2023.1179173
Huang, Y., Wang, S., Liu, H., Atoni, E., Wang, F., Chen, W., et al. (2023). A global dataset of sequence, diversity and biosafety recommendation of arbovirus and arthropod-specific virus. Sci. Data. 10:305. doi: 10.1038/s41597-023-02226-8
Jahfari, S., de Vries, A., Rijks, J. M., Van Gucht, S., Vennema, H., Sprong, H., et al. (2017). Tick-borne encephalitis virus in ticks and roe deer, the Netherlands. Emerg. Infect. Dis. 23, 1028–1030. doi: 10.3201/eid2306.161247
Ji, N., Wang, N., Liu, G., Zhao, S., Liu, Z., Tan, W., et al. (2023). Tacheng tick virus 1 and Songling virus infection in great gerbils (Rhombomys opimus) in northwestern China. J. Wildl. Dis. 59, 138–142. doi: 10.7589/jwd-d-21-00137
Jia, N., Liu, H. B., Ni, X. B., Bell-Sakyi, L., Zheng, Y. C., Song, J. L., et al. (2019). Emergence of human infection with Jingmen tick virus in China: a retrospective study. EBioMedicine 43, 317–324. doi: 10.1016/j.ebiom.2019.04.004
Jia, Y., Wang, S., Yang, M., Ulzhan, N., Omarova, K., Liu, Z., et al. (2022). First detection of Tacheng tick virus 2 in hard ticks from southeastern Kazakhstan. Kafkas Universitesi Veteriner Fakultesi Dergisi. 28, 139–142. doi: 10.9775/kvfd.2021.26453
Jumpertz, M., Sevestre, J., Luciani, L., Houhamdi, L., Fournier, P. E., and Parola, P. (2023). Bacterial agents detected in 418 ticks removed from humans during 2014-2021, France. Emerg. Infect. Dis. 29, 701–710. doi: 10.3201/eid2904.221572
Kerlik, J., Avdicova, M., Musilova, M., Beresova, J., and Mezencev, R. (2022). Breast Milk as route of tick-borne encephalitis virus transmission from mother to infant. Emerg. Infect. Dis. 28, 1060–1061. doi: 10.3201/eid2805.212457
Kholodilov, I. S., Litov, A. G., Klimentov, A. S., Belova, O. A., Polienko, A. E., Nikitin, N. A., et al. (2020). Isolation and characterisation of Alongshan virus in Russia. Viruses 12:362. doi: 10.3390/v12040362
Kim, Y. R., Yun, Y., Bae, S. G., Park, D., Kim, S., Lee, J. M., et al. (2018). Severe fever with thrombocytopenia syndrome virus infection, South Korea, 2010. Emerg. Infect. Dis. 24, 2103–2105. doi: 10.3201/eid2411.170756
Kobayashi, D., Kuwata, R., Kimura, T., Shimoda, H., Fujita, R., Faizah, A. N., et al. (2021). Detection of Jingmenviruses in Japan with evidence of vertical transmission in ticks. Viruses 13:2547. doi: 10.3390/v13122547
Kodama, F., Yamaguchi, H., Park, E., Tatemoto, K., Sashika, M., Nakao, R., et al. (2021). A novel nairovirus associated with acute febrile illness in Hokkaido, Japan. Nat. Commun. 12:5539. doi: 10.1038/s41467-021-25857-0
Kong, Y., Liu, D., Jiang, L., Zhang, G., He, B., and Li, Y. (2022). Phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in inner Mongolia, China. Ticks Tick Borne Dis. 13:101856. doi: 10.1016/j.ttbdis.2021.101856
Krasteva, S., Jara, M., Frias-De-Diego, A., and Machado, G. (2020). Nairobi sheep disease virus: a historical and epidemiological perspective. Front. Vet. Sci. 7:419. doi: 10.3389/fvets.2020.00419
Kuivanen, S., Levanov, L., Karelnen, L., Sironen, T., Jaaskelainen, A. J., Plyusnin, I., et al. (2019). Detection of novel tick-borne pathogen, Alongshan virus, in Ixodes ricinus ticks, South-Eastern Finland, 2019. Eur. Secur. 24, 9–16. doi: 10.2807/1560-7917.Es.2019.24.27.1900394
Ladner, J. T., Wiley, M. R., Beitzel, B., Auguste, A. J., Dupuis, A. P. II, Lindquist, M. E., et al. (2016). A multicomponent animal virus isolated from mosquitoes. Cell Host Microbe 20, 357–367. doi: 10.1016/j.chom.2016.07.011
Li, X. H., Ji, H. W., Wang, D., Che, L. H., Zhang, L., Li, L., et al. (2022). Molecular detection and phylogenetic analysis of tick-borne encephalitis virus in ticks in northeastern China. J. Med. Virol. 94, 507–513. doi: 10.1002/jmv.27303
Li, F., Li, J. X., Song, J. D., Yin, Q. K., Nie, K., Xu, S. T., et al. (2023). Novel orthonairovirus isolated from ticks near China-North Korea border. Emerg. Infect. Dis. 29, 1254–1257. doi: 10.3201/eid2906.230056
Li, C. X., Shi, M., Tian, J. H., Lin, X. D., Kang, Y. J., Chen, L. J., et al. (2015). Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 4:5378. doi: 10.7554/eLife.05378
Li, Y., Yan, C., Liu, D., He, B., and Tu, C. (2020). Seroepidemiological investigation of Crimean-Congo hemorrhagic fever virus in sheep and camels of Inner Mongolia of China. Vector Borne Zoonotic Dis. 20, 461–467. doi: 10.1089/vbz.2019.2529
Li, J., Zhang, S., Liang, W., Zhao, S., Wang, Z., Li, H., et al. (2022). Survey of tick species and molecular detection of selected tick-borne pathogens in Yanbian, China. Parasite 29:38. doi: 10.1051/parasite/2022039
Lindquist, L., and Vapalahti, O. (2008). Tick-borne encephalitis. Lancet 371, 1861–1871. doi: 10.1016/S0140-6736(08)60800-4
Liu, Z., Li, L., Xu, W., Yuan, Y., Liang, X., Zhang, L., et al. (2022). Extensive diversity of RNA viruses in ticks revealed by metagenomics in northeastern China. PLoS Negl. Trop. Dis. 16:e0011017. doi: 10.1371/journal.pntd.0011017
Liu, X., Zhang, X., Wang, Z., Dong, Z., Xie, S., Jiang, M., et al. (2020). A tentative Tamdy orthonairovirus related to febrile illness in northwestern China. Clin. Infect. Dis. 70, 2155–2160. doi: 10.1093/cid/ciz602
Luan, Y., Gou, J., Zhong, D., Ma, L., Yin, C., Shu, M., et al. (2023). The tick-borne pathogens: an overview of China’s situation. Acta Parasitol. 68, –20. doi: 10.1007/s11686-023-00658-1
Lv, X., Liu, Z., Li, L., Xu, W., Yuan, Y., Liang, X., et al. (2023). Yezo virus infection in tick-bitten patient and ticks, Northeastern China. Emerg. Infect. Dis. 29, 797–800. doi: 10.3201/eid2904.220885
Lvov, D. K., Neronov, V. M., Gromashevsky, V. L., Skvortsova, T. M., Berezina, L. K., Sidorova, G. A., et al. (1976a). "Karshi" virus, a new flavivirus (Togaviridae) isolated from Ornithodoros papillipes (Birula, 1895) ticks in Uzbek S.S.R. Arch. Virol. 50, 29–36. doi: 10.1007/bf01317998
Lvov, D. K., Sidorova, G. A., Gromashevsky, V. L., Kurbanov, M., Skvoztsova, L. M., Gofman, Y. P., et al. (1976b). Virus "Tamdy"--a new arbovirus, isolated in the Uzbee S.S.R. And Turkmen S.S.R. From ticks Hyalomma asiaticum asiaticum Schulee et Schlottke, 1929, and Hyalomma plumbeum plumbeum panzer, 1796. Arch. Virol. 51, 15–21. doi: 10.1007/bf01317830
Ma, J., Lv, X. L., Zhang, X., Han, S. Z., Wang, Z. D., Li, L., et al. (2021). Identification of a new orthonairovirus associated with human febrile illness in China. Nat. Med. 27, 434–439. doi: 10.1038/s41591-020-01228-y
Ma, C., Zhang, R., Zhou, H., Yu, G., Yu, L., Li, J., et al. (2022). Prevalence and genetic diversity of Dabieshan tick virus in Shandong Province, China. J. Infect. 85, 90–122. doi: 10.1016/j.jinf.2022.04.002
Madison-Antenucci, S., Kramer, L. D., Gebhardt, L. L., and Kauffman, E. (2020). Emerging tick-borne diseases. Clin. Microbiol. Rev. 33:e00083-18. doi: 10.1128/cmr.00083-18
Marczinke, B. I., and Nichol, S. T. (2002). Nairobi sheep disease virus, an important tick-borne pathogen of sheep and goats in Africa, is also present in Asia. Virology 303, 146–151. doi: 10.1006/viro.2002.1514
Matsuno, K., Kajihara, M., Nakao, R., Nao, N., Mori-Kajihara, A., Muramatsu, M., et al. (2018). The unique phylogenetic position of a novel tick-borne phlebovirus ensures an ixodid origin of the genus Phlebovirus. mSphere 3:e00239-18. doi: 10.1128/mSphere.00239-18
Mekata, H., Kobayashi, I., and Okabayashi, T. (2023). Detection and phylogenetic analysis of Dabieshan tick virus and Okutama tick virus in ticks collected from Cape Toi, Japan. Ticks and Tick-borne Diseases 14:102237. doi: 10.1016/j.ttbdis.2023.102237
Miao, D., Dai, K., Zhao, G. P., Li, X. L., Shi, W. Q., Zhang, J. S., et al. (2020). Mapping the global potential transmission hotspots for severe fever with thrombocytopenia syndrome by machine learning methods. Emerg. Microbes Infect. 9, 817–826. doi: 10.1080/22221751.2020.1748521
Moming, A., Shen, S., Fang, Y., Zhang, J., Zhang, Y., Tang, S., et al. (2021). Evidence of human exposure to Tamdy virus, Northwest China. Emerg. Infect. Dis. 27, 3166–3170. doi: 10.3201/eid2712.203532
Moming, A., Yue, X., Shen, S., Chang, C., Wang, C., Luo, T., et al. (2018). Prevalence and phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in ticks from different ecosystems in Xinjiang, China. Virol. Sin. 33, 67–73. doi: 10.1007/s12250-018-0016-3
Ogola, E. O., Kopp, A., Bastos, A. D. S., Slothouwer, I., Marklewitz, M., Omoga, D., et al. (2022). Jingmen tick virus in ticks from Kenya. Viruses 14:1041. doi: 10.3390/v14051041
Owen, S., Gaffney, C., Grado, L., Woodson, M. L., King, J. A., Balaraman, R. P., et al. (2021). Colorado tick fever virus induces apoptosis in human endothelial cells to facilitate viral replication. Ticks Tick Borne Dis. 12:101775. doi: 10.1016/j.ttbdis.2021.101775
Pan, H., Hu, J., Liu, S., Shen, H., Zhu, Y., Wu, J., et al. (2013). A reported death case of a novel bunyavirus in Shanghai, China. Virol. J. 10, 1–5. doi: 10.1186/1743-422x-10-187
Pang, Z., Jin, Y., Pan, M., Zhang, Y., Wu, Z., Liu, L., et al. (2022). Geographical distribution and phylogenetic analysis of Jingmen tick virus in China. iScience 25:105007. doi: 10.1016/j.isci.2022.105007
Park, K. B., Jo, Y. H., Kim, N. Y., Lee, W. G., Lee, H. I., Cho, S. H., et al. (2020). Tick-borne viruses: current trends in large-scale viral surveillance. Entomol. Res. 50, 379–392. doi: 10.1111/1748-5967.12435
Phipps, L. P., and Johnson, N. (2022). Tick-borne encephalitis virus. J. Med. Microbiol. 71:001492. doi: 10.1099/jmm.0.001492
Piantadosi, A., and Kanjilal, S. (2020). Diagnostic approach for arboviral infections in the United States. J. Clin. Microbiol. 58:e01926-19. doi: 10.1128/jcm.01926-19
Qin, X. C., Shi, M., Tian, J. H., Lin, X. D., Gao, D. Y., He, J. R., et al. (2014). A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc. Natl. Acad. Sci. USA 111, 6744–6749. doi: 10.1073/pnas.1324194111
Qin, T., Shi, M., Zhang, M., Liu, Z., Feng, H., and Sun, Y. (2023). Diversity of RNA viruses of three dominant tick species in North China. Front. Vet. Sci. 9:1057977. doi: 10.3389/fvets.2022.1057977
Qu, L., Li, X., Huang, B., Liu, Y., Li, Q., Shah, T., et al. (2023). Identification and characterization of Jingmen tick virus in ticks from Yunnan imported cattle. Vector Borne Zoonotic Dis. 23, 298–302. doi: 10.1089/vbz.2022.0076
Rodino, K. G., Theel, E. S., and Pritt, B. S. (2020). Tick-borne diseases in the United States. Clin. Chem. 66, 537–548. doi: 10.1093/clinchem/hvaa040
Saijo, M. (2007). “Crimean-Congo hemorrhagic fever in the Xinjiang Uygur autonomous region of western China” in Crimean-Congo Hemorrhagic Fever: A global Perspective. ed. O. Ergonul (White House, CA: Springer, Dordrecht), 115–130.
Serretiello, E., Astorri, R., Chianese, A., Stelitano, D., Zannella, C., Folliero, V., et al. (2020). The emerging tick-borne Crimean-Congo haemorrhagic fever virus: a narrative review. Travel Med. Infect. Dis. 37:101871. doi: 10.1016/j.tmaid.2020.101871
Shao, L., Chang, R., Liu, L., Wang, Y., Gao, Y., Wang, S., et al. (2021). Detection and phylogenetic analysis of a novel tick-borne virus in Haemaphysalis longicornis ticks and sheep from Shandong, China. Virol. J. 18:233. doi: 10.1186/s12985-021-01704-y
Shao, J. W., Guo, L. Y., Yuan, Y. X., Ma, J., Chen, J. M., and Liu, Q. (2021). A novel subtype of bovine hepacivirus identified in ticks reveals the genetic diversity and evolution of bovine hepacivirus. Viruses 13:2206. doi: 10.3390/v13112206
Shao, L., Pang, Z., Fu, H., Chang, R., Lin, Z., Lv, A., et al. (2020). Identification of recently identified tick-borne viruses (Dabieshan tick virus and SFTSV) by metagenomic analysis in ticks from Shandong Province, China. J. Infect. 81, 973–978. doi: 10.1016/j.jinf.2020.10.022
Shen, S., Duan, X. M., Wang, B., Zhu, L. Y., Zhang, Y. F., Zhang, J. Y., et al. (2018). A novel tick-borne phlebovirus, closely related to severe fever with thrombocytopenia syndrome virus and heartland virus, is a potential pathogen. Emerg. Microbes Infect. 7, 1–14. doi: 10.1038/s41426-018-0093-2
Simo, L., Kazimirova, M., Richardson, J., and Bonnet, S. I. (2017). The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 7:281. doi: 10.3389/fcimb.2017.00281
Stegmueller, S., Fraefel, C., and Kubacki, J. (2023). Genome sequence of Alongshan virus from Ixodes ricinus ticks collected in Switzerland. Microbiol. Resour. Announc. 12:e0128722. doi: 10.1128/mra.01287-22
Sun, S., Dai, X., Aishan, M., Wang, X., Meng, W., Feng, C., et al. (2009). Epidemiology and phylogenetic analysis of Crimean-Congo hemorrhagic fever viruses in Xinjiang, China. J. Clin. Microbiol. 47, 2536–2543. doi: 10.1128/jcm.00265-09
Sun, R. X., Lai, S. J., Yang, Y., Li, X. L., Liu, K., Yao, H. W., et al. (2017). Mapping the distribution of tick-borne encephalitis in mainland China. Ticks Tick Borne Dis. 8, 631–639. doi: 10.1016/j.ttbdis.2017.04.009
Sun, Y., Liu, M., Luo, L., Zhao, L., Wen, H., Zhang, Z., et al. (2017). Seroprevalence of severe fever with thrombocytopenia syndrome virus in hedgehog from China. Vector Borne Zoonotic Dis. 17, 347–350. doi: 10.1089/vbz.2016.2019
Takahashi, Y., Kobayashi, S., Ishizuka, M., Hirano, M., Muto, M., Nishiyama, S., et al. (2020). Characterization of tick-borne encephalitis virus isolated from a tick in Central Hokkaido in 2017. J. Gen. Virol. 101, 497–509. doi: 10.1099/jgv.0.001400
Tao, M., Liu, Y., Ling, F., Chen, Y., Zhang, R., Ren, J., et al. (2021). Severe fever with thrombocytopenia syndrome in southeastern China, 2011-2019. Front. Public Health 9:803660. doi: 10.3389/fpubh.2021.803660
Teng, A. Y., Che, T. L., Zhang, A. R., Zhang, Y. Y., Xu, Q., Wang, T., et al. (2022). Mapping the viruses belonging to the order Bunyavirales in China. Infect. Dis. Poverty 11:81. doi: 10.1186/s40249-022-00993-x
Tian, H., Yu, P., Chowell, G., Li, S., Wei, J., Tian, H., et al. (2017). Severe fever with thrombocytopenia syndrome virus in humans, domesticated animals, ticks, and mosquitoes, Shaanxi Province, China. Am. J. Trop. Med. Hyg. 96, 1346–1349. doi: 10.4269/ajtmh.16-0333
Torii, S., Matsuno, K., Qiu, Y., Mori-Kajihara, A., Kajihara, M., Nakao, R., et al. (2019). Infection of newly identified phleboviruses in ticks and wild animals in Hokkaido, Japan indicating tick-borne life cycles. Ticks Tick Borne Dis. 10, 328–335. doi: 10.1016/j.ttbdis.2018.11.012
Tran, X. C., Kim, S. H., Lee, J. E., Kim, S. H., Kang, S. Y., Binh, N. D., et al. (2022). Serological evidence of severe fever with thrombocytopenia syndrome virus and IgM positivity were identified in healthy residents in Vietnam. Viruses 14:2280. doi: 10.3390/v14102280
Tsao, J. I., Hamer, S. A., Han, S., Sidge, J. L., and Hickling, G. J. (2021). The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 58, 1565–1587. doi: 10.1093/jme/tjab047
Vilibic-Cavlek, T., Savic, V., Ferenc, T., Mrzljak, A., Barbic, L., Bogdanic, M., et al. (2021). Lymphocytic choriomeningitis-emerging trends of a neglected virus: a narrative review. Trop. Med. Infect. Dis. 6:88. doi: 10.3390/tropicalmed6020088
Wang, Y.-N., Jiang, R.-R., Ding, H., Zhang, X.-L., Wang, N., Zhang, Y.-F., et al. (2022). First detection of Mukawa virus in Ixodes persulcatus and Haemaphysalis concinna in China. Front. Microbiol. 13:791563. doi: 10.3389/fmicb.2022.791563
Wang, A., Pang, Z., Liu, L., Ma, Q., Han, Y., Guan, Z., et al. (2021). Detection and phylogenetic analysis of a novel tick-borne virus in Yunnan and Guizhou provinces, southwestern China. Pathogens 10:1143. doi: 10.3390/pathogens10091143
Wang, Z. D., Wang, W., Wang, N. N., Qiu, K., Zhang, X., Tana, G., et al. (2019b). Prevalence of the emerging novel Alongshan virus infection in sheep and cattle in Inner Mongolia, northeastern China. Parasit. Vectors 12:450. doi: 10.1186/s13071-019-3707-1
Wang, Z. D., Wang, B., Wei, F., Han, S. Z., Zhang, L., Yang, Z. T., et al. (2019a). A new segmented virus associated with human febrile illness in China. N. Engl. J. Med. 380, 2116–2125. doi: 10.1056/NEJMoa1805068
Wang, Y. C., Wei, Z. K., Lv, X. L., Han, S. Z., Wang, Z. D., Fan, C. F., et al. (2021). A new nairo-like virus associated with human febrile illness in China. Emerg. Microbes Infect. 10, 1200–1208. doi: 10.1080/22221751.2021.1936197
Wang, H., Zhao, X., Zhang, X., Wu, D., and Du, X. (2019). Long time series land cover classification in China from 1982 to 2015 based on Bi-LSTM deep learning. Remote Sens. 11:1639. doi: 10.3390/rs11141639
Wang, T., Zheng, L., Zhao, Q., Yao, Y., Zhou, F., Wei, F., et al. (2023). Parainfluenza virus 5 is a next-generation vaccine vector for human infectious pathogens. J. Med. Virol. 95:e28622. doi: 10.1002/jmv.28622
Wen, H.-L., Zhao, L., Zhai, S., Chi, Y., Cui, F., Wang, D., et al. (2014). Severe fever with thrombocytopenia syndrome, Shandong Province, China, 2011. Emerg. Infect. Dis. 20, 1–5. doi: 10.3201/eid2001.120532
Wu, Z., Chen, J., Zhang, L., Zhang, Y., Liu, L., and Niu, G. (2023). Molecular evidence for potential transovarial transmission of Jingmen tick virus in Haemaphysalis longicornis fed on cattle from Yunnan Province, China. J. Med. Virol. 95:e28357. doi: 10.1002/jmv.28357
Wu, X.-B., Na, R.-H., Wei, S.-S., Zhu, J.-S., and Peng, H.-J. (2013). Distribution of tick-borne diseases in China. Parasit. Vectors 6:119. doi: 10.1186/1756-3305-6-119
Xia, H., Li, P., Yang, J., Pan, L., Zhao, J., Wang, Z., et al. (2011). Epidemiological survey of Crimean-Congo hemorrhagic fever virus in Yunnan, China, 2008. Int. J. Infect. Dis. 15, E459–E463. doi: 10.1016/j.ijid.2011.03.013
Yang, X., Gao, Z., Zhou, T. L., Zhang, J., Wang, L. Q., Xiao, L. J., et al. (2020). Mapping the potential distribution of major tick species in China. Int. J. Environ. Res. Public Health 17:5145. doi: 10.3390/ijerph17145145
Yang, M. F., Ma, Y. N., Jiang, Q., Song, M. X., Kang, H. T., Liu, J. S., et al. (2022). Isolation, identification and pathogenic characteristics of tick-derived parainfluenza virus 5 in Northeast China. Transbound. Emerg. Dis. 69, 3300–3316. doi: 10.1111/tbed.14681
Yang, L. E., Zhao, Z., Hou, G., Zhang, C., Liu, J., Xu, L., et al. (2019). Genomes and seroprevalence of severe fever with thrombocytopenia syndrome virus and Nairobi sheep disease virus in Haemaphysalis longicornis ticks and goats in Hubei, China. Virology 529, 234–245. doi: 10.1016/j.virol.2019.01.026
Yoshikawa, T., Shimojima, M., Fukushi, S., Tani, H., Fukuma, A., Taniguchi, S., et al. (2015). Phylogenetic and geographic relationships of severe fever with thrombocytopenia syndrome virus in China, South Korea, and Japan. J. Infect. Dis. 212, 889–898. doi: 10.1093/infdis/jiv144
Yu, Z.-M., Chen, J.-T., Qin, J., Guo, J.-J., Li, K., Xu, Q.-Y., et al. (2020). Identification and characterization of Jingmen tick virus in rodents from Xinjiang, China. Infect. Genet. Evol. 84:104411. doi: 10.1016/j.meegid.2020.104411
Yu, X. J., Liang, M. F., Zhang, S. Y., Liu, Y., Li, J. D., Sun, Y. L., et al. (2011). Fever with thrombocytopenia associated with a novel Bunyavirus in China. N. Engl. J. Med. 364, 1523–1532. doi: 10.1056/NEJMoa1010095
Yuan, S., Yao, X.-Y., Lian, C.-Y., Kong, S., Shao, J.-W., and Zhang, X.-L. (2023). Molecular detection and genetic characterization of bovine hepacivirus identified in ticks collected from cattle in Harbin, northeastern China. Front. Vet. Sci. 10:1093898. doi: 10.3389/fvets.2023.1093898
Zhang, Y., Jiang, L., Yang, Y., Xie, S., Yuan, W., and Wang, Y. (2021). A tick bite patient with fever and meningitis co-infected with rickettsia raoultii and Tacheng tick virus 1: a case report. BMC Infect. Dis. 21:1187. doi: 10.1186/s12879-021-06877-z
Zhang, L., Li, S., Huang, S. J., Wang, Z. D., Wei, F., Feng, X. M., et al. (2018). Isolation and genomic characterization of lymphocytic choriomeningitis virus in ticks from northeastern China. Transbound. Emerg. Dis. 65, 1733–1739. doi: 10.1111/tbed.12946
Zhang, Y., Li, Z., Pang, Z., Wu, Z., Lin, Z., and Niu, G. (2022). Identification of Jingmen tick virus (JMTV) in Amblyomma testudinarium from Fujian Province, southeastern China. Parasit. Vectors 15:339. doi: 10.1186/s13071-022-05478-2
Zhang, Y., Shen, S., Fang, Y., Liu, J., Su, Z., Liang, J., et al. (2018). Isolation, characterization, and phylogenetic analysis of two new Crimean-Congo hemorrhagic fever virus strains from the northern region of Xinjiang Province, China. Virol. Sin. 33, 74–86. doi: 10.1007/s12250-018-0020-7
Zhang, L., Sun, J., Yan, J., Lv, H., Chai, C., Sun, Y., et al. (2014). Antibodies against severe fever with thrombocytopenia syndrome virus in healthy persons, China, 2013. Emerg. Infect. Dis. 20, 1355–1357. doi: 10.3201/eid2008.131796
Zhang, Y., Zhang, X., and Liu, J.-Z. (2019). Ticks (Acari: Ixodoidea) in China: geographical distribution, host diversity, and specificity. Arch. Insect Biochem. Physiol. 102:e21544. doi: 10.1002/arch.21544
Zhang, G., Zheng, D., Tian, Y., and Li, S. (2019). A dataset of distribution and diversity of ticks in China. Sci. Data. 6:105. doi: 10.1038/s41597-019-0115-5
Zhao, G.-P., Wang, Y.-X., Fan, Z.-W., Ji, Y., Liu, M.-J., Zhang, W.-H., et al. (2021). Mapping ticks and tick-borne pathogens in China. Nat. Commun. 12:1075. doi: 10.1038/s41467-021-21375-1
Zhao, L., Zhai, S., Wen, H., Cui, F., Chi, Y., Wang, L., et al. (2012). Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg. Infect. Dis. 18, 963–965. doi: 10.3201/eid1806.111345
Zhou, Z., Deng, F., Han, N., Wang, H., Sun, S., Zhang, Y., et al. (2013a). Reassortment and migration analysis of Crimean-Congo haemorrhagic fever virus. J. Gen. Virol. 94, 2536–2548. doi: 10.1099/vir.0.056374-0
Zhou, H., Ma, Z., Hu, T., Bi, Y., Mamuti, A., Yu, R., et al. (2019). Tamdy virus in Ixodid ticks infesting Bactrian camels, Xinjiang, China, 2018. Emerg. Infect. Dis. 25, 2136–2138. doi: 10.3201/eid2511.190512
Zhou, Z., Meng, W., Deng, F., Xia, H., Li, T., Sun, S., et al. (2013b). Complete genome sequences of two Crimean-Congo hemorrhagic fever viruses isolated in China. Genome Announc. 1:e00571-13. doi: 10.1128/genomeA.00571-13
Zhu, C., He, T., Wu, T., Ai, L., Hu, D., Yang, X., et al. (2020). Distribution and phylogenetic analysis of Dabieshan tick virus in ticks collected from Zhoushan, China. J. Vet. Med. Sci. 82, 1226–1230. doi: 10.1292/jvms.20-0081
Zhuang, L., Sun, Y., Cui, X.-M., Tang, F., Hu, J.-G., Wang, L.-Y., et al. (2018). Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerg. Infect. Dis. 24, 868–871. doi: 10.3201/eid2405.151435
Keywords: tick-borne virus, tick, distribution, phylogenetic analysis, diversity
Citation: Wu Y, Zhou Q, Mao M, Chen H and Qi R (2024) Diversity of species and geographic distribution of tick-borne viruses in China. Front. Microbiol. 15:1309698. doi: 10.3389/fmicb.2024.1309698
Edited by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Sylvie Lecollinet, UMR ASTRE - CIRAD, FranceMohamed Abdo Rizk, Mansoura University, Egypt
Copyright © 2024 Wu, Zhou, Mao, Chen and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Qi, cWlyQGx6dS5lZHUuY24=; cWlydWlnaEAxNjMuY29t
 Yi Wu
Yi Wu Qian Zhou
Qian Zhou Rui Qi
Rui Qi