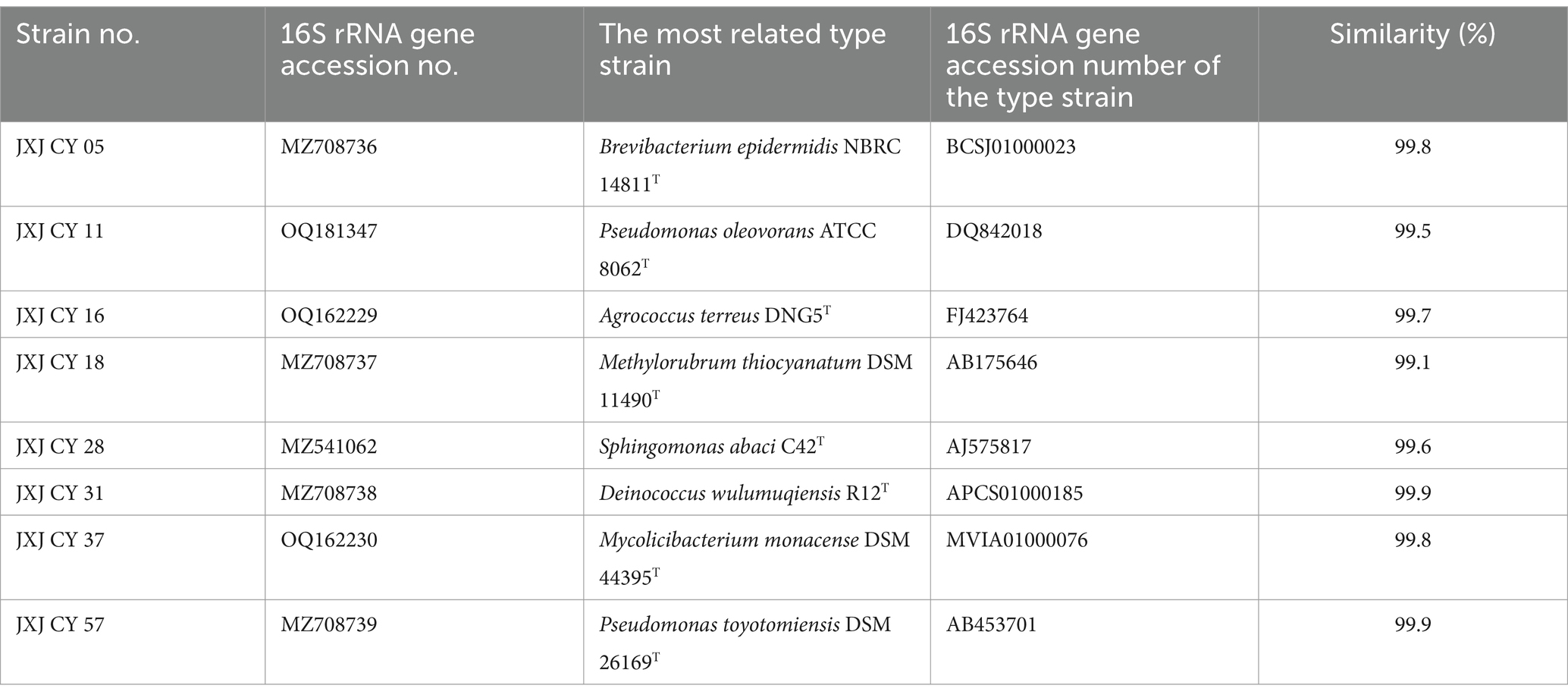
- 1College of Pharmacy and Life Science, Jiujiang University, Jiujiang, China
- 2Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Harmful algal blooms (HABs) in natural waters are of escalating global concern due to their detrimental impact on environmental health. Emerging evidence indicates that algae-bacteria symbionts can affect HAB features, though much about this interplay remains largely unexplored. The current study isolated a new species of Mucilaginibacter (type strain JXJ CY 39T) from culture biomass of the bloom-causing Microcystis aeruginosa FACHB-905 (Maf) from Lake Dianchi, China. Strain JXJ CY 39T was an aerobic, Gram-stain-negative rod bacterium that grew at 5–38°C, pH 4.0–11.0, and 0–3.0% NaCl. Taxonomic evaluation proposed a new species, with Mucilaginibacter lacusdianchii sp. nov., as the species epithet. Experimental results revealed that strain JXJ CY 39T spurred the growth of Maf by supplying soluble phosphorus and nitrogen during cultivation, despite the unavailability of soluble phosphorus and nitrogen. Additionally, by producing the plant hormone indole-3-acetate, strain JXJ CY 39T possibly impacted Maf’s functionality. Results from co-culture experiments with other strains from Maf biomass showed possible effects of strain JXJ CY 39T on the relationship between Maf and other cohabiting bacteria, as well as microcystin toxin production characteristics. Although Maf could foster the growth of strain JXJ CY 39T by supplying organic carbon, the strain’s growth could be regulated via specific chemical compounds based on antibiotic assays. Community composition analysis disclosed that this Mucilaginibacter strain positively affected Maf’s growth and modified densities and types of bacteria linked to Maf. Overall, these results suggest that the interactions between important HAB-causing organisms and their attached bacteria are complex, dynamic, and may influence the growth characteristics of algae.
Highlights
• Harmful algal blooms (HABs) are persistent environmental problems caused by algae.
• A novel bacterium (JXJ CY 39T) was isolated and characterized from the phycosphereof an HAB-alga (Maf).
• JXJ CY 39T modulated the growth and microcystin production of Maf.
• JXJ CY 39T also influenced the attached bacterial assemblages of Maf.
• Bacterial symbionts of HAB-algae should be more carefully considered in treatments.
1 Introduction
Various types of algae cause harmful algal blooms (HABs), which pose an accelerating concern in both oceans and freshwater worldwide owing to their expansion in recent times, primarily due to global climate changes such as warming trends (Glibert et al., 2005). These HABs impair water ecosystems considerably, including posing threats to environment and human health by producing potent toxins (Grattan et al., 2016).
Distinct exudates from different algal species exhibit different chemical characteristics that result in the coexistence of varying bacterial partners with specific algae (Yang et al., 2017), leading to extremely diverse and complex interactions (Shao et al., 2014) that can include nutrient exchange, signal transduction, and gene transfer (Kouzuma and Watanabe, 2015). Among these, nutrient exchange is considered the most common type of interactions (Kouzuma and Watanabe, 2015) and is frequently the basis of algal–bacterial mutualisms (Cooper and Smith, 2015). Specifically, algae provide attached bacteria with a safer habitat and protection from grazing, in addition to dissolved organic matter compounds like polysaccharides, while attached bacteria provide algae with various nutrients like bio-available P, N, CO2, microelements, and vitamins (Yang and Xiao, 2011). The mutualisms between algae and attached bacteria are consequently highly mediated by the provisioning of nutrients from bacteria that may be able to initiate and maintain symbiotic relationships (Cooper and Smith, 2015). Importantly, the various interactions that have co-evolved between algae and their attached bacteria (Kouzuma and Watanabe, 2015; Cirri and Pohnert, 2019) have significant, but distinct impacts on the occurrence, duration and decline of algal blooms (Shao et al., 2014; Zhang et al., 2019). Consequently, the importance of attached bacteria must be considered when identifying treatments to control and mitigate HABs.
Microcystis aeruginosa is a common group of bloom-inducing Cyanobacteria, leading source of hepatotoxic microcystins (MCs) specifically produced in freshwater (Dawson, 1998; Park et al., 2009). Among over 270 different MC compounds (Lin et al., 2021), MC-LR (contains leucine and arginine) is the most prevalent in both natural water blooms and M. aeruginosa cultures in laboratory (Vasconcelos et al., 1996; Liu et al., 2012). M. aeruginosa can also secrete extracellular mucilage primarily composed of polysaccharides that provide habitats for bacteria that attach to Microcystis colonies and interact with the algae (Dziallas and Grossart, 2011; Parveen et al., 2013). Many bacteria attached to M. aeruginosa extracellular matrices have been isolated and identified, including in the genera Erythrobacter (Zhao et al., 2011), Gordonia, Burkholderia (Zhao et al., 2012), Sphingomonas (Guo et al., 2020), Pseudomonas (Yang and Xiao, 2011), and numerous other taxa within the phyla Actinobacteria, Bacteroidetes, Proteobacteria, and Deinococcus-Thermus (Berg et al., 2009; Guo et al., 2020). Indeed, many of these bacterial taxa have evolved with their algal hosts (Ramanan et al., 2016).
In this study, a bacterium JXJ CY 39T was purified from the attached bacterial community of algal M. aeruginosa FACHB-905 (Maf), which was previously obtained from Lake Dianchi. Lake Dianchi is the largest lake on the Yunnan-Guizhou plateau and is one of the most seriously pollution-impacted eutrophic freshwater lakes in China. Cyanobacterial blooms primarily dominated by M. aeruginosa have gradually became a common phenomenon in Lake Dianchi since the 1980’s due to increasing dumping of various wastes into the lake (Liu, 1999). Indeed, HABs occurred almost yearly from 1990 to 2010. Polyphasic taxonomic study revealed that JXJ CY 39T represents a novel species of Mucilaginibacter, belonging to Bacteroidota which are the most common phylum attached to Microcystis HABs (Ndlela et al., 2018). The genus Mucilaginibacter, a large group in Bacteroidota, was first described by Pankratov et al. (2007) and later emended by Urai et al. (2008), Baik et al. (2010), and Chen et al. (2014). Mucilaginibacter currently comprises 80 species with validly published names1 (List of Prokaryotic names with Standing in Nomenclature; December 9, 2023). Here, the interactions between Maf and its attached bacteria, including strain JXJ CY 39T, were investigated with co-culture experiments to understand if and how Maf controls the growth of strain JXJ CY 39T and vice-a-versa. Moreover, the bacterial communities associated with Maf that was co-cultured with and without strain JXJ CY 39T were compared over 35 days with 16S rRNA gene amplicon sequence analysis. Taken together, these experiments were used to more comprehensively understand the interactions among Maf, JXJ CY 39T, and other Maf-attached bacteria. The new insights reported here is to inform the treatment of HABs via consideration of their commensal bacteria.
2 Materials and methods
2.1 Polyphasic taxonomy study on strain JXJ CY 39T
2.1.1 Isolation of the microorganisms
Algal Microcystis aeruginosa FACHB-905 (Maf) was obtained from Lake Dianchi, China, and subsequently incubated in our lab as described previously (Zhang et al., 2016c). Bacteria, including strain JXJ CY 39T and other eight attached strains, were isolated from the culture biomass of Maf using International Streptomyces Project 2 (ISP 2) agar medium according to the described procedure (Xiao et al., 2022a). Isolated bacteria were preserved at 4.0°C using ISP 2 slants and − 80.0°C using glycerol suspensions (30–50%, v/v). Then we tried to purify Maf on BG11 (Blue-Green Medium) agar plate (Allen, 1968) to get rid of the culturable bacteria attached to the Maf. The purified-Maf was confirmed when no bacterial colony was formed by inoculation of the Maf onto newly prepared ISP 2 agar and nutrient agar, respectively. Purified-Maf was cultured using BG11 liquid medium at 25.0°C under illumination of 30 μmol photon/m2/s with a 12 h: 12 h light: dark cycle. In the subsequent experiments, the purified-Maf culture-system without supplement with other attached bacteria served as a basal control group.
2.1.2 Morphological and physiological characterization of strain JXJ CY 39T
Bacterial cellular morphology was observed with transmission electron microscopy (TEM; JEM-2100, JEOL) after growing on ISP 2 agar medium at 28.0°C for four days. Gram stains were conducted using standard procedures. Catalase activity was evaluated from bubble production after addition of a drop of 3% (v/v) H2O2 on cell biomass. Growth at different temperatures (5, 10, 15, 20, 26, 28, 30, 32, 35, 38, 41, and 45°C), pH (2.0–12.0 in 1.0 intervals), and NaCl concentrations (0–10.0% w/v, in 1% intervals) was conducted using ISP 2 agar medium as the basal growth medium. Hydrolysis of starch and Tween (20, 40, and 80) tests were performed according to methods described by Dong and Cai (2001). Other physiological and biochemical characteristics were determined using API systems.
2.1.3 Chemotaxonomic characteristics
Cellular biomass subjected to chemical analysis was obtained from pure cultures grown on ISP 2 agar medium at 28.0°C for four days. Cellular fatty acids were analyzed using the MIDI System (Sherlock version 6.1; MIDI database: TSBA6). Polar lipids were extracted and analyzed by two-dimensional thin-layer chromatography (Minnikin et al., 1979). Further, respiratory quinones were extracted as described by Collins et al. (1977) and analyzed with HPLC (Tamaoka et al., 1983).
2.1.4 Phylogenetic and genome sequence analysis
Genomic DNA was prepared by using Rapid Bacterial Genomic DNA Isolation Kit (Sangon Biotech, Shanghai, China), and subsequently, the 16S rRNA gene sequences were obtained as described previously (Zhang et al., 2016b), and compared against those in the EzBioCloud Database. Phylogenetic trees were reconstructed using neighbor-joining (Saitou and Nei, 1987), maximum-parsimony (Fitch, 1971), and Maximum-Likelihood (Felsenstein, 1981) algorithms in MEGA version 11 (Tamura et al., 2021). The topologies of the phylogenetic trees were evaluated with bootstrap analysis of 1,000 replicates (Felsenstein, 1985).
Whole genome sequencing was conducted on the Illumina HiSeq 4000 platform at Sangon Biotech (Shanghai, China). Sequence read quality was evaluated with FastQC (v.0.11.2) and then trimmed of adapters and low-quality sequence regions with Trimmomatic (v.0.36) (Bolger et al., 2014). The resultant sequence reads were assembled with SPAdes (v.3.5.0) (Bankevich et al., 2012). Gaps in assembled contigs were filled using GapFiller (v.1.11) (Boetzer and Pirovano, 2012) and corrected with PrinSeS-G (v.1.0.0) (Massouras et al., 2010). Genes were predicted using the Prokka annotation program (v.1.10) (Seemann, 2014). Repeat sequences were determined using RepeatModeler (v.2.0.2) and RepeatMasker (v.4.1.0). CRISPR prediction and analysis was conducted with CRT (v.1.2) (Bland et al., 2007). Genomic annotations were further analyzed by searches with the NCBI BLAST+ program (v.2.2.28) using default parameters. The digital DNA–DNA hybridization (dDDH) and average nucleotide identity (ANI) values between the genome of strain JXJ CY 39T and those of other Mucilaginibacter type strains were calculated using the Genome-to-Genome Distance Calculator (GGDC version 3.0) (Meier-Kolthoff et al., 2013) and the JspeciesWS (JSWS) portal, respectively. The genomes of other type strains were downloaded from the NCBI genome database.2 DNA G + C% contents were calculated from genomic sequences. To construct a robust core gene phylogeny, a phylogenomic tree based on the concatenation of protein sequences from strain JXJ CY 39T and the type strains of Mucilaginibacter was inferred with the EasyCGTree software package3 (Xue et al., 2021). Evolutionary distances were calculated with IQ-TREE (v.1.6.1) (Nguyen et al., 2015).
2.2 Co-culture of Maf and the attached bacteria
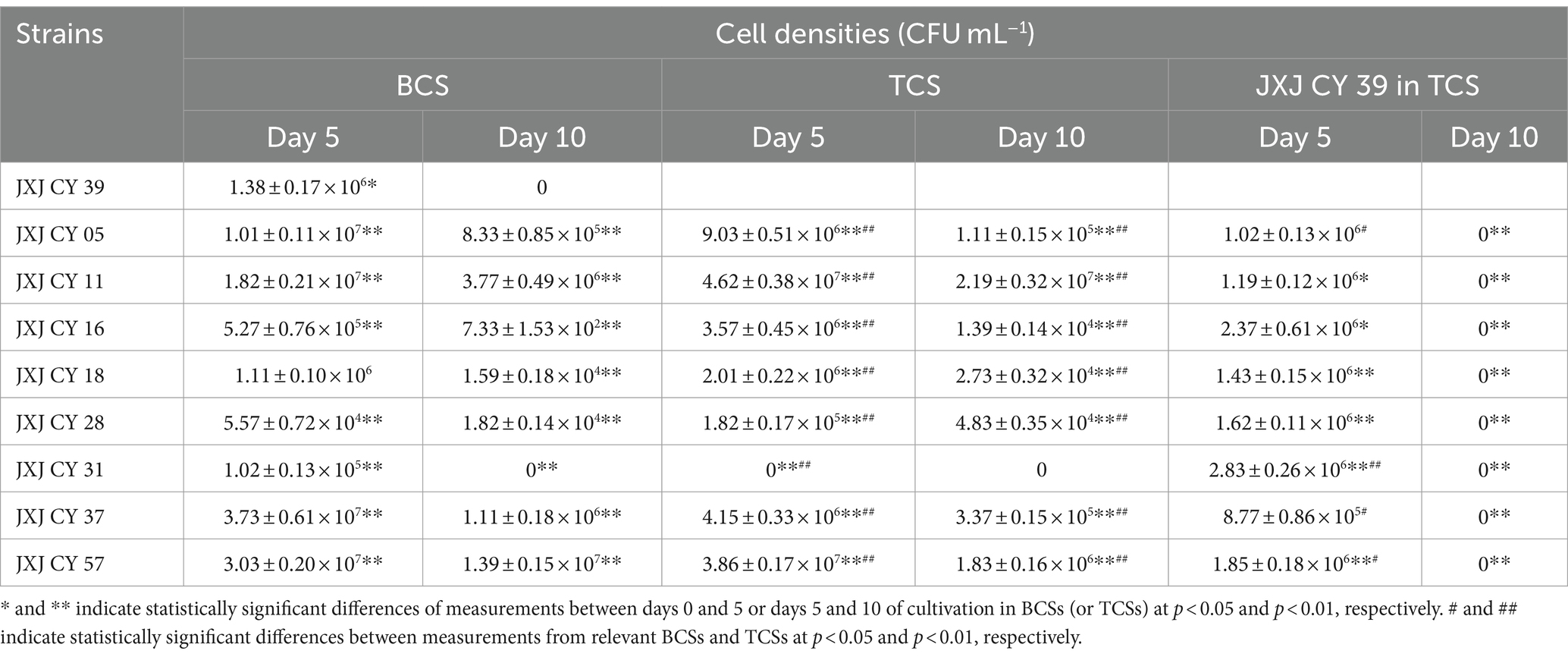
Eight other bacterial strains (Table 1) isolated from Maf cultures were used in the co-culture experiments in addition to strain JXJ CY 39T. Purified Maf (about 1.0 × 106 CFU mL−1) was inoculated with attached bacteria in (i) binary culture systems (BCSs) that comprised Maf and an individual bacterium at a final cellular density of 1 × 106 CFU mL−1 and (ii) ternary culture systems (TCSs) comprising Maf and strain JXJ CY 39T in addition to another bacterium, with final cellular densities of both strains at 1 × 106 CFU mL−1. Here, the purified Maf was cultured without supplement with additional bacteria, which served as the control. Both treatments (BCS and TCS) and controls included triplicate replicates. BCSs, TCSs, and controls were sampled at 5 and 10 days of cultivation. The bacterial cellular densities were determined using spread plate techniques based on the different features of the colors and morphologies of the colonies. Chlorophyll a (chl-a), extracellular microcystin LR (E-MC-LR), and intracellular microcystin LR (I-MC-LR) concentrations were measured using previously described methods (Zhang et al., 2015).
2.3 Nitrogen fixation and dissolution of unavailable phosphate
Nitrogen fixation ability was determined as previously described (Xiao et al., 2022b). The ability to dissolve insoluble phosphorus was evaluated using methods described by Zhang et al. (2016b). Both Ca3(PO4)2 (Damao Chemical Reagent Company, Tianjin, China) and phytin (Aladdin, Shanghai, China) were used as insoluble phosphorus sources at dosages of 1 g L−1. After growing on ISP2 agar plates at 28°C for 3 days, the cell mass of strain JXJ CY 39T was collected and suspended using sterile tap-water, and the cellular suspension was served as the inoculum of nitrogen-free, Ca3(PO4)2 and phytin media.
2.4 Co-culture of Maf and JXJ CY 39T in media with limited availability of N and P
Superfluous available nitrogen and phosphorus in eutrophic water are the critical element triggering cyanobacterial blooms. To assess nutrient provisioning activities, Maf at a density of 3 × 105 CFU mL−1 and strain JXJ CY 39T at a density of 1 × 106 CFU mL−1 were co-cultured using modified BG11 media, where K2HPO4 (Damao Chemical Reagent Company, Tianjin, China) was replaced by Ca3(PO4)2 or NaNO3 (Damao Chemical Reagent Company, Tianjin, China) was removed to represent nitrogen-free media. Controls included purified Maf at a density of 3 × 105 CFU mL−1 and strain JXJ CY 39T at a density of about 1 × 106 CFU mL−1 that were grown in modified BG11 media, respectively. Co-cultures and controls were all established in triplicate replicates. Bacterial cellular densities and the concentrations of chl-a, I-MC-LR, and E-MC-LR were examined as described above on days 7 and 14 for cultures in nitrogen-free medium and on days 9 and 18 for cultures in Ca3(PO4)2 medium. The inoculation of Maf into modified BG11 media was also added 1.86 mg L−1 of K2HPO4 into Ca3(PO4)2 medium and 71.4 mg L−1 of NaNO3 into nitrogen-free medium. The inoculum of strain JXJ CY 39T was prepared as described above.
2.5 Influences of Maf metabolites on the growth of attached bacteria
To assess the influence of Maf metabolites on attached bacteria, Maf cultures (5 L, final cell density of ~2.5 × 107 CFU mL−1) were distilled at 50°C under reduced pressure to remove water. The resultant condensates were extracted using mixture solvents comprising water: methanol: ethanol: ethyl acetate (1,1:1:1, v/v) and extracts were separated by medium pressure liquid chromatography with a C18 column (YMC, ODS-AQ, 50 μm) using stepwise elution of mixed solvents (methanol/water, 0/10 → 1/9 → 2/8 → 3/7 → 4/6 → 5/5 → 6/4 → 7/3 → 8/2 → 9/1 → 10/0, v/v). The resultant eluents were combined into four fractions based on HPLC detection results and were designated as fractions I, II, III, and IV, with quantities of 12.5, 1.75, 0.2, and 0.9 g, respectively. Fractions I, II, and III were water-soluble, while fraction IV was fat-soluble. Only fraction III contained MC-LR at a amount of about 6.2 mg, based on HPLC analyses. The inhibitory activities of these fractions on attached bacteria were investigated using the paper disk method, as previously described (Zhang et al., 2016a). The dosages of fractions I, II, and IV were 4 mg per disk, while disks with fraction III contained 4 μg MC-LR. Total Maf extracts were dissolved with deionized water and solutions were evaluated with antibacterial activities at 4 mg per disk.
2.6 Influence of strain JXJ CY 39T on the growth of non-culturable Maf-attached bacteria
To assess the influences of strain JXJ CY 39T and Maf on other Maf-attached bacteria, Maf and strain JXJ CY 39T at a density of 1 × 106 CFU mL−1 were co-cultured in BG11 medium as described above, followed by sampling at days 5, 10, 15 and 35 of cultivation (corresponding to sample IDs M39_1, M39_2, M39_3, and M39_4, respectively). Purified Maf cultures without strain JXJ CY 39T were sampled at days 5, 10, 15, and 35 of cultivation and were considered the controls (C_1, C_2, C_3, and C_4). All of these samples were spread onto ISP2 plates and incubated at 28.0°C for 7 days to examine whether they were axenic. The V3–V4 hypervariable regions of 16S rRNA genes were amplified using the universal primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The amplicons were sequenced on the Illumina MiSeq platform (Illumina, San Diego, USA) using standard protocols. Sequences were analyzed using the QIIME2 software package (2019.4) and by comparison of sequences to the Greengenes database (DeSantis et al., 2006). In addition, samples were inoculated onto ISP2 plates and cultured at 28.0°C for 5 to 7 days to assess the presence of viable strain JXJ CY 39T.
2.7 Statistics
Data were expressed as means ± standard deviations (n = 3). Comparisons of cellular densities, and concentrations of chl-a and MC-LR between co-cultures of Maf-bacteria and pure cultures of Maf or bacteria were performed using analysis of variance (ANOVA) followed by Tukey’s pairwise comparisons. Significance was set at p values of 0.05.
3 Results and discussion
3.1 Taxonomic study of strain JXJ CY 39T
3.1.1 Morphological and physiological characteristics
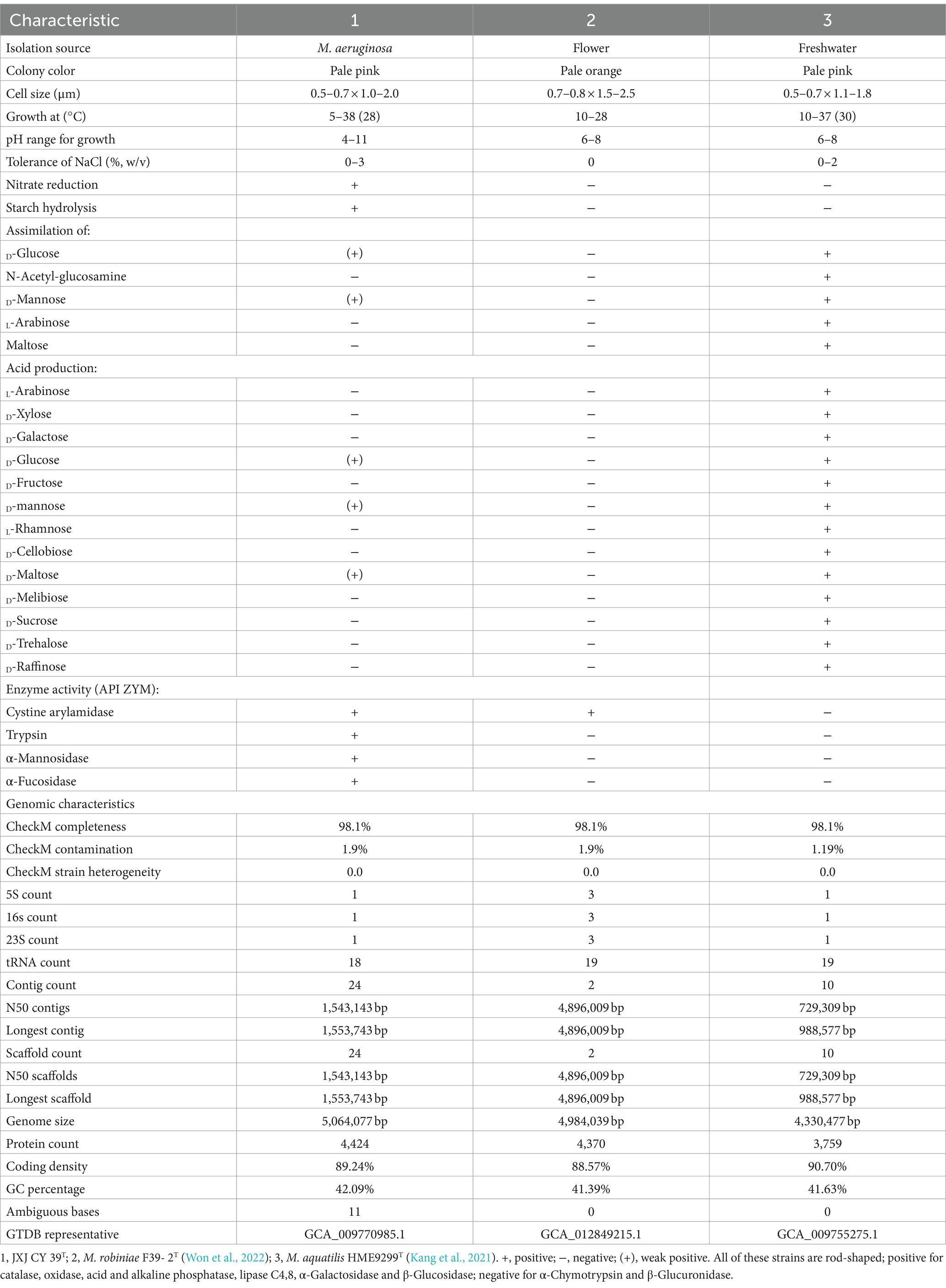
Strain JXJ CY 39T was aerobic, Gram-negative, and rod-shaped (0.5–0.8 × 0.9–2.0 μm) (Supplementary Figure S1). Its colonies were pale pink, smooth, convex, circular, and wet in appearance after 4–5 days of cultivation on IPS 2 agar plates. Growth occurred at 5.0–38.0°C, pH 4.0–11.0, and 0–3.0% NaCl, with optimal growth at 28.0°C, pH 7.0–8.0, and 0% NaCl. Strain JXJ CY 39T was positive for catalase, oxidase, nitrate reduction, hydrolysis of starch, and Tween 40 and 80 tests, but negative for Tween 20. Additional phenotypic characteristics are shown in Table 2, along with the species description.
3.1.2 Chemotaxonomy
The major cellular fatty acids of strain JXJ CY 39T were iso-C15:0 (45.0%) and C16:1ω7c/16:1ω6c (30.3%) (Supplementary Table S1) and the predominant menaquinone was MK-7, while the polar lipids comprised phosphatidylethanolamine (PE), unidentified aminophosphoglycolipid (APGL), unidentified aminoglycolipids (AGL), unidentified phospholipid (PL), and unidentified polar lipids (L1-3) (Supplementary Figure S2). The above characteristics were similar to the chemotaxonomic profile of M. robiniae F39- 2T (Won et al., 2022) and M. aquatilis HME9299T (Kang et al., 2021).
3.1.3 Molecular phylogenetic analysis
A nearly complete 16S rRNA gene sequence (1,509 bp length; GenBank accession MT674523) of strain JXJ CY 39T exhibited close similarities with Mucilaginibacter robiniae F39-2T (97.02%), Mucilaginibacter aquatilis HME9299T (96.69%), M. galii PP-F2F-G47T (96.50%), M. straminoryzae RS28T (96.10%), and M. polytrichastri DSM 26907T (96.05%), respectively, and < 96% similarities with other Mucilaginibacter spp. and these strains formed a distinct clade within the Mucilaginibacter lineage in the phylogenetic trees of 16S rRNA gene sequences (Supplementary Figures S3–S5), which was recapitulated in the Maximum-Likelihood core gene phylogenomic tree (Supplementary Figure S6). The dDDH and ANI values between strain JXJ CY 39T and its phylogenetic neighbors were 18.8–19.9% and 70.43–73.95%, respectively. These values are all much lower than the generally accepted species threshold values (Chun et al., 2018). Therefore, based on the phenotypic, genotypic and phylogenetic properties, strain JXJ CY 39T represents a novel species of the genus Mucilaginibacter, for which the name Mucilaginibacter lacusdianchii sp. nov. is proposed.
3.2 Genomic characteristics
The genome of strain JXJ CY 39T was sequenced and submitted to GenBank under the accession ID WSRW00000000. The genome of strain JXJ CY39T contains six contigs, with a total length of 5,070,224 bp and an N50 length of 1,554,798 bp. The genomic characteristics of the closest reference strains M. robiniae F39- 2T (Won et al., 2022) and M. aquatilis HME9299T (Kang et al., 2021) were included in Table 2.
In the genome of strain JXJ CY 39T, some genes or gene clusters have been identified that are likely to enhance symbiotic interactions with the Maf (Supplementary Tables S2, S3). GO database annotation of genes indicated that strain JXJ CY 39T encoded 19 gene clusters related to symbiotic interactions, immune responses, and their regulation (Supplementary Table S2); 3 gene clusters related to plant growth hormone synthesis (e.g., of indole-3-acetic acid, auxin, and polyamine); 13 gene clusters related to the synthesis of various vitamins (e.g., B1, B2, B6, B12, K2, and biotin) and their derivatives; 4 genes related to ATP-binding cassette (ABC) transporter complexes; 8 genes related to protein secretion; over 26 gene clusters encoding various glycosidases; 76 genes related to carbohydrate catabolism; 44 genes related to phosphatases activity; 185 genes related to organic acid biosynthesis; 22 genes related to organic acid transport; 11 genes related to nodulation; 6 genes related to nitrogen fixation; 3 genes related to catalase activity; and 14 genes related to peroxidases; 7 genes related to carotenoid biosynthetic process (Supplementary Table S3).
The interconnected evolutionary histories of Cyanobacteria and bacteria have led to the formation of various interactions between the two, including through nutrient exchange, signal transduction, gene transfer, and inhibition (Kouzuma and Watanabe, 2015; Cirri and Pohnert, 2019). For example, protein secretion systems and ATP-binding cassette (ABC) transporters are involved in the exchange of substances and signal transduction between cyanobacteria and their attached bacteria (Zhu et al., 2021). Consequently, the above genomic data for strain JXJ CY 39T suggest the potential for exchange of substances and signal transduction between the bacteria and Maf, prompting follow-on experiments to evaluate the presence of such interactions.
3.3 Co-culture growth of Maf and strain JXJ CY 39T
The chl-a concentrations of the control increased from 0.092 mg L−1 to 0.555 and 1.149 mg L−1 after 5 and 10 days of cultivation, respectively. Six of the attached bacteria in the BCSs did not influence the growth of Maf after 5 days of cultivation (p > 0.05). The exceptions were strains JXJ CY 11, 37, and 57 that resulted in decreased chl-a concentrations of 26.1, 11.6, and 24.6% (p < 0.01), respectively (Figure 1A). After 5 days of cultivation, the chl-a concentration of TCS with strains JXJ CY 57 + 39 was 0.466 mg L−1 or 16.1% lower (p < 0.01) than that of the control. The chl-a concentration of TCS with strains JXJ CY 11 + 39 was not different from that of the control (p > 0.05), while the chl-a concentrations of the other six TCSs were 0.580–0.678 mg L−1 or 4.5–22.1% higher (p < 0.05, p < 0.01) than that of the control (Figure 1A). Moreover, all chl-a concentrations of TCSs were 6.7–34.7% higher (p < 0.05, p < 0.01) than those of related BCSs after 5 days of cultivation, while six of the chl-a concentrations of TCSs were 5.6–23.4% higher (p < 0.05, p < 0.01) than that of BCS with JXJ CY 39T (Figure 1A).
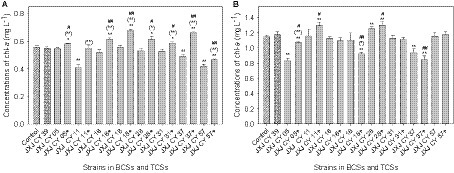
Figure 1. Influence of BCSs and TCSs on Maf growth. (A,B) Represent samples collected at 5 and 10 days of cultivation, respectively. +, indicates addition of strain JXJ CY 39T in the culture. Error bars indicate standard deviation based on measurements of three replicates. * and ** indicate statistically significant differences in measurements between control cultures and BCS (or TCS) at p < 0.05 and p < 0.01, respectively. (*) and (**) indicate statistically significant differences in measurements between relevant BCSs and TCSs at p < 0.05 and p < 0.01, respectively. # and ## indicate statistically significant differences between measurements of BCS with JXJ CY 39T cultures and relevant TCSs at p < 0.05 and p < 0.01, respectively.
After 10 days of culture, the chl-a concentrations of BCSs with strain JXJ CY 05 and 37 were 0.834, and 0.940 mg L−1, representing 27.4 and 18.2% lower (p < 0.01) than that of the control, respectively (Figure 1B). However, the chl-a concentration of BCS with JXJ CY 28 was 1.26 mg L−1 or 9.2% higher (p < 0.01) than that of the control (Figure 1B). In addition, the chl-a concentrations of TCSs with strains JXJ 05 + 39, 18 + 39, and 37 + 39 were 1.069, 0.921, and 0.848 mg L−1 after 10 days of cultivation, representing 7.0, 19.9, and 26.2% lower (p < 0.01) than that of the control. While, the chl-a concentrations of TCSs with strains JXJ CY 11 + 39 and 28 + 39 were 1.292 and 1.300 mg L−1 after 10 days of cultivation, representing 12.4 and 13.1% higher (p < 0.01) than that of the control, respectively. The chl-a concentrations of TCSs with strains JXJ CY 05 + 39 and 18 + 39 were 28.1% higher (p < 0.01) and 16.7% lower (p < 0.05) than those of the relevant BCSs, respectively.
The chl-a concentrations of TCSs with strains JXJ CY 05 + 39, 18 + 39, and 37 + 39 were 9.0, 21.6, and 27.8% lower (p < 0.05, p < 0.01) than that of the BCS with strain JXJ CY 39T, respectively; while the chl-a concentrations of TCSs with strains JXJ CY 11 + 39 and 28 + 39 were 10.0 and 10.7% higher (p < 0.05) than that of BCS with JXJ CY 39T, respectively. These data indicate that various strains may have distinct effects on Maf growth and that all adherent bacteria probably affect the interactions between Maf and other adherent bacteria, corroborating our previous research (Xiao et al., 2022c).
Algal–bacterial mutualisms are largely mediated by nutrition provisioning from bacteria (Cooper and Smith, 2015). Further, algal growth can be promoted by plant hormones, like indole-3-acetic acid (IAA) (Cirri and Pohnert, 2019; Hoke et al., 2021) and auxin (Seyedsayamdost et al., 2011). In addition, Microcystis requires vitamin B12 for methionine biosynthesis and many other vitamins for growth including B1 and B7 (Hoke et al., 2021). Gene annotations (Supplementary Table S3) indicated that strain JXJ CY 39T could potentially provide Maf with plant growth hormones and various vitamins. Such mutualistic behaviors are likely the reason that the chl-a concentrations of some co-cultures were higher than those of the controls grown in BG11 medium (Figure 1). Kim et al. (2019) also reported that attached bacteria can promote Maf growth by decomposing H2O2 under aerobic growth conditions. Strain JXJ CY 39T encodes catalases (Table 2; Supplementary Table S3) and peroxidases (Supplementary Table S3). Consequently, the decomposition of H2O2 produced by Maf could be another mechanism by which strain JXJ CY 39T promotes the growth of Maf. Attached bacteria could provide Microcystis with complementary carotenoid molecules (Pérez-Carrascal et al., 2021). Carotenoids play an important role in protecting chlorophyll molecules against photo-oxidative damage (Young, 1991). Strain JXJ CY 39T has 7 genes related to carotenoid biosynthetic process, indicating that this strain can also potentially protect chlorophyll molecules of Maf against photo-oxidative damage and promote the photosynthetic efficiency of the algae, which is probably another reason of JXJ CY 39T potentially promoting the growth of Maf.
Interactions between algae and attached bacteria are dynamic and can be initiated and ended in response to environmental and developmental cues (Cooper and Smith, 2015). Such dynamism was apparent from the influences of attached bacteria on Maf growth. For example, the influences of attached bacteria on Maf growth in BCSs with strains JXJ 05, 11, 28, and 57, and in TCSs with strains 05 + 39, 11 + 39, 16 + 39, 18 + 39, 31 + 39, 37 + 39, and 57 + 39 changed with cultivation time (Figure 1). Almost the similar phenomena were also observed in previous studies (Zhang et al., 2016b,c, 2017; Xiao et al., 2022a,b,c).
Cellular densities of attached bacteria increased significantly (p < 0.05) after 5 days of cultivation (Table 3), except for those of strains JXJ CY 16, 18, 28, and 31 in BCSs, and strain JXJ CY 39T in TCSs with JXJ CY 05 + 39 and 37 + 39, and strains JXJ CY 28 and 31 in TCSs. The cellular densities then significantly decreased (p < 0.01) with culture time. Further, strain JXJ CY 31 was even not detected on day 10 of BCS cultivation nor on days 5 and 10 of cultivation in TCS, while strain JXJ CY 39T was not detected in both BCS and TCSs on the day 10 of cultivation. The cellular densities of the other eight bacteria in TCSs were significantly influenced (p < 0.01) by cultivation with strain JXJ CY 39T on both days 5 and 10 of cultivation. However, the cellular densities of JXJ CY 39T in TCSs were only influenced by cultivation with strains JXJ CY 05, 16, 31, 37, and 57 on day 5 of cultivation (p < 0.005, p < 0.001), but were not influenced by cultivation with any of the other eight strains on day 10 of cultivation (p > 0.05). Thus, the influences of Maf on attached bacteria likely varies with bacterial strain and cultivation duration. Moreover, all attached bacteria would likely influence the interactions between Maf and other bacterial taxa. These results are consistent with those from our previous study (Xiao et al., 2022c), and also reflect the dynamic interactions between algae and attached bacteria.
BG11 medium is a synthetic medium that lacks sufficient organic carbon sources, such that heterotrophic attached bacteria will not grow due to insufficient carbon and energy sources. Dissolved organic carbon secreted by M. aeruginosa (Casamatta and Wickstrom, 2000) can be used by bacteria for growth. A previous study showed that bacteria attached to Microcystis cells encode higher relative abundances of carbon degradation genes and β-glucosidase activity to enable the use of organic carbon secreted by Microcystis (Yang et al., 2021). Annotation of the strain JXJ CY 39T genome revealed the presence of many genes that would enable use of dissolved organic carbon secreted by Maf (Supplementary Table S3). This likely explains why the attached bacteria grew during the first five days despite the organic carbon-deficient medium used for cultivation.
The analysis of metagenomes, metatranscriptomes and metaproteomes on natural water bloom samples play important roles in the recognition of interactions in phytoplankton communities (Kazamia et al., 2016). However, these technologies can only let us know the overall metabolic capability and ecological state of a community (Kazamia et al., 2016), and many aspects of these interactions, especially specific interactions of the microbes within, are still unknown (Grossart and Simon, 2007; Kazamia et al., 2016; Zhang et al., 2019) because of most of these studies were performed under non-axenic conditions (Grossart and Simon, 2007; Zhang et al., 2019). Specific interactions can only probably be understood through physiological experiments using defined systems such as co-cultures of known and well-characterized partners in the laboratory, which can shed light on the interactions at the molecular and cellular levels (Kazamia et al., 2016). In this study, we have removed other culturable heterotrophic bacteria from Maf, and then inoculated the specific pure cultures of these isolated attached bacteria back to Maf, which can eliminate the distractions of other unknown culturable heterotrophic bacteria on the specific interactions, and let us know more about the interactions between Maf and its attached bacteria like mentioned above and below.
3.4 Influences of co-cultures on MC-LR concentrations
The E-MC-LR concentrations of the control were 37.6 and 172.2 μg mg−1 chl-a on days 5 and 10 of cultivation, respectively. The E-MC-LR concentrations of BCSs with strains JXJ CY 37 and 57, in addition to TCS with strains JXJ CY 57 + 39 on day 5 of cultivation were 44.0, 42.9, and 43.5 μg mg−1 chl-a, respectively, representing 17.0, 14.1 and 15.6% higher (p < 0.05) values compared to controls, respectively. However, the E-MC-LR concentrations of the other seven BCSs did not significantly differ from the control, while the E-MC-LR concentrations of the other seven TCSs were 11.3–30.5% lower (p < 0.05, p < 0.01) than that of the control (Figure 2A). Moreover, the E-MC-LR concentrations of the six TCSs were 11.4–28.2% lower (p < 0.05, p < 0.01) than those of the relevant BCSs (Figure 2A). The E-MC-LR concentrations of the TCSs were 7.6–27.6% lower (p < 0.05, p < 0.01) than that of BCS with strain JXJ CY 39, with the exception of TCS with strain JXJ CY 57 + 39 that was 20.4% higher (p < 0.01) (Figure 2A).

Figure 2. Influence of BCSs and TCSs on E-MC-LR and I-MC-LR concentrations in Maf cultures. (A,C) Represent samples collected at 5 day of cultivation. (B,D) Represent samples collected at 10 day of cultivation. + indicates strain JXJ CY 39T was included in the co-culture. Error bars indicate standard deviations for measurements from three replicates. * and ** indicate statistically significant differences of measurements between control cultures and BCSs (or TCSs) at p < 0.05 and p < 0.01, respectively. (*) and (**) indicate statistically significant differences between measurements from relevant BCSs and TCSs at p < 0.05 and p < 0.01, respectively. # and ## indicate statistically significant differences between measurements of BCS with JXJ CY 39T and relevant TCSs at p < 0.05 and p < 0.01, respectively.
After day 10 of cultivation, the E-MC-LR concentrations of BCSs with strains JXJ CY 11, 18, 28, and 57 were 19.7, 23.6, 31.8, and 40.5% lower (p < 0.01) than that of the control, respectively; while the E-MC-LR concentrations of BCSs with strains JXJ CY 05, 16, 31, 37, and 39 were 60.8, 24.8, 29.7, 51.3, and 27.9% higher (p < 0.01) than that of the control, respectively (Figure 2B). At 10 days of cultivation, the E-MC-LR concentrations of the six TCSs were 21.7–129.8% higher (p < 0.05, p < 0.01) than the control, with the exception of TCSs containing strains JXJ CY 11 + 39 and 57 + 39 (Figure 2B). The E-MC-LR concentrations of the seven TCSs were 24.4–200.9% higher (p < 0.01) than those of relevant BCSs, with the exception of the TCS with strains JXJ CY 05 + 39 (Figure 2B). The E-MC-LR concentrations of TCSs with strains JXJ CY 11 + 39 and 57 + 39 were 21.9–24.3% lower (p < 0.01) than that of BCS with strain JXJ CY 39, while the E-MC-LR concentrations of the other TCSs were 22.2–79.7% higher (p < 0.05) than that of BCS with strain JXJ CY 39, with the exception of the TCS with strain JXJ CY 28 + 39 (Figure 2B). Thus, the different strains exerted different and dynamic influences on the E-MC-LR concentrations of Maf. Moreover, the results suggest that the addition of any strain into a BCS with one attached bacterium would likely influence the E-MC-LR concentrations of Maf. These results are consistent with previous study (Xiao et al., 2022c).
The I-MC-LR concentrations of the controls were 1,019.0 and 900.8 μg mg−1 chl-a on days 5 and 10 of cultivation, respectively (Figures 2C, D). On day 5 of cultivation, the I-MC-LR concentrations of BCSs with strains JXJ CY 11 and 37 were 1,117.8, and 1,130.7 μg mg−1 chl-a, representing 9.7 and 11.0% higher (p < 0.05) than that of the control, respectively. In addition, the I-MC-LR concentrations of BCSs with strains JXJ CY 18 and 39 were 946.9 and 950.1 μg mg−1 chl-a, respectively, representing 7.1 and 6.8% lower (p < 0.05) than that of the control, respectively (Figure 2C). After 5 days of culture, the I-MC-LR concentrations of TCSs with strains JXJ CY 05 + 39 and 57 + 39 were 25.4 and 19.1% higher (p < 0.01) than those of relevant BCSs, respectively. In addition, the I-MC-LR concentrations of TCSs with strains JXJ CY 16 + 39 and 18 + 39 were 17.2 and 7.2% lower (p < 0.05, p < 0.01) than those of the related BCSs, respectively. The I-MC-LR concentrations of TCSs were 4.6–32.1% higher (p < 0.05, p < 0.01) than that of BCS with JXJ CY 39, with the exception of TCSs with strains JXJ CY 16 + 39 and 18 + 39 that exhibited 5.8 and 7.6% lower (p < 0.01, p < 0.05) than that of BCS with strain JXJ CY 39, respectively (Figure 2C).
On day 10 of cultivation, the I-MC-LR concentrations of BCSs with strains JXJ CY 39 and 31 were 25.2 and 16.0% lower (p < 0.01, p < 0.05) than the control, respectively, and the I-MC-LR concentrations of BCSs with strains JXJ CY 37 and 57 were 18.0 and 19.6% higher (p < 0.01) than the control (Figure 2D), respectively. On day 10 of cultivation, the I-MC-LR concentrations of the TCSs were 11.4–43.5% lower (p < 0.05, p < 0.01) than those of the related BCSs, and 19.1–40.6% lower (p < 0.01) than the control, with the exception of TCSs with strains JXJ CY 11 + 39, 37 + 39, and 57 + 39 that were not different from the control (p > 0.05) (Figure 2D). The I-MC-LR concentrations of TCSs with strains JXJ CY 16 + 39 and 18 + 39, in addition to TCSs with strains JXJ CY 11 + 39, 37 + 39, and 57 + 39 were 17.5–20.6% lower (p < 0.05, p < 0.01) and 19.9–24.9% higher (p < 0.01) than that of the BCS with strain JXJ CY 39 (Figure 2D), respectively. While the I-MC-LR concentrations of the TCSs with JXJ 05 + 39 on day10 of cultivation was 24.2% lower (p > 0.01) than the control (Figure 2D) in contrast to that of TCSs with JXJ 05 + 39 on day 5 of cultivation, which was 23.2% higher (p > 0.01) than the control (Figure 2C). These data indicate that different strains probably exert different and dynamic influences on the I-MC-LR concentrations of Maf. Moreover, the addition of any strain into a BCS with one attached bacterium would also likely influence the I-MC-LR concentrations of Maf. These results are consistent with our previous study (Xiao et al., 2022c).
3.5 Capacity for nitrogen fixation and phosphate acquisition
Cellular densities of strain JXJ CY 39T initially increased from 1 × 105 CFU mL−1 to 3.9 × 106 CFU mL−1 after 2 days of cultivation in nitrogen-free medium, indicating that this strain could convert N2 into NH3 to support its growth, which was consistent with annotation of the strain JXJ CY 39T genome with the GO database (Supplementary Table S3). After 2 days of co-cultivation, the concentrations of available phosphorus increased by 10.41 ± 0.44 mg L−1 for Ca3(PO4)2 and 5.68 ± 0.24 mg L−1 for phytin, indicating that strain JXJ CY 39T can dissolve insoluble inorganic and organic phosphate, and release dissoluble phosphorus, consistent with the genomic analyses (Supplementary Table S3). Therefore, strain JXJ CY 39T can potentially provide Maf with available N and P when available N and P are limited in the environment.
3.6 Influences of limited P and N availability on Maf and JXJ CY 39T growth
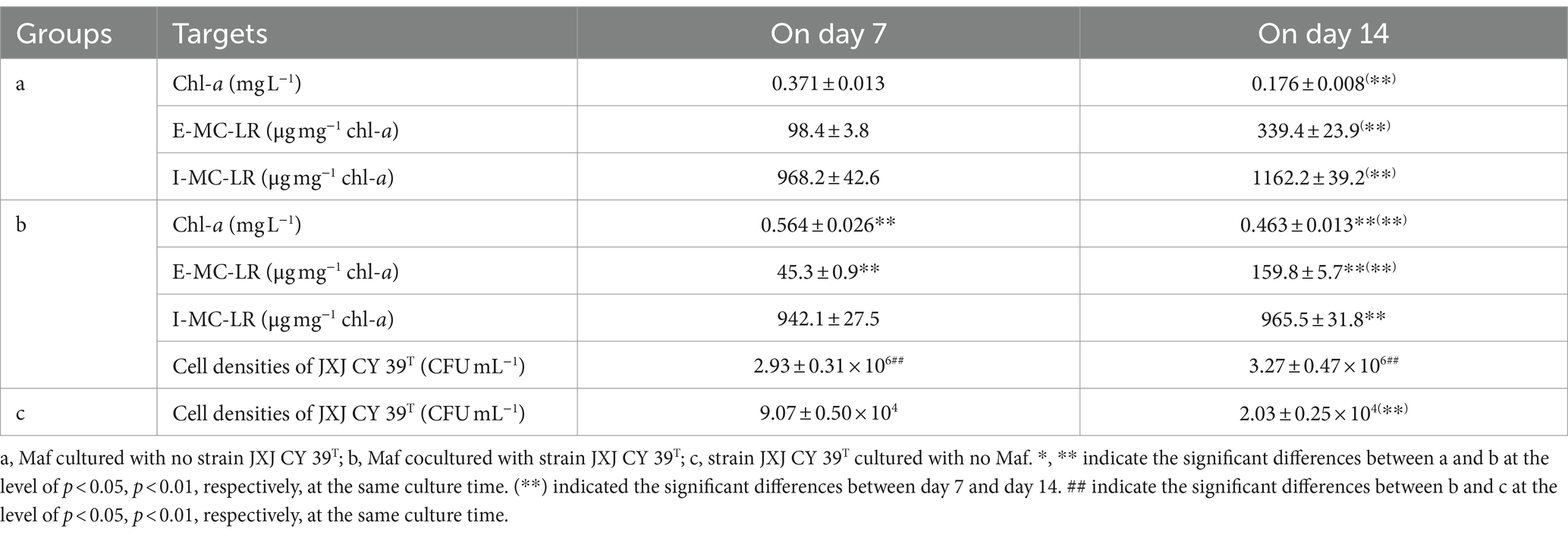
In nitrogen-deficient medium, the color of Maf cultured without JXJ CY39T turned green gradually during the first 7 days, and turned yellow quickly during the second 7 days. Consequently, chl-a concentrations of Maf cultured without JXJ CY39T increased to 0.371 mg L−1 on day 7 from 0.030 mg L−1 on day 0, and then decreased to 0.176 mg L−1 on day 14 (Table 4). However, in nitrogen-deficient medium, the color of Maf co-cultured with JXJ CY39T turned green more quickly during the first 7 days, and turned yellow more slowly during the second 7 days. Consequently, chl-a concentrations of Maf co-cultured with JXJ CY39T increased to 0.564 mg L−1 on day 7, which was 52.0% higher (p < 0.01) than that of the control, and then decreased to 0.463 mg L−1 on day 14, which was 163.1% higher (p < 0.01) than that of the control (Table 4).
As mentioned above, the inoculation of Maf cultured in BG11 imported 71.4 mg L−1 of NaNO3 into nitrogen-free medium, in addition to 0.321 mg L−1 of N as NH4+ in the ferric ammonium citrate. Additionally, degradation of phycobiliproteins of Microcystis can expand biomass by about 50% (Wang et al., 2021). These available nitrogen sources were the reasons of the growth of Maf cultured without JXJ CY39T during the first 7 days in nitrogen-free medium. However, exhausting these available nitrogen sources resulted in mass mortality of Maf, which was the main reason that the color of Maf cultured without JXJ CY39T turned yellow quickly during the second 7 days.
Ammonia is a key component involved in aquatic environment microbial interactions (Cirri and Pohnert, 2019), and can dissolve in water easily and form NH3·H2O, which further ionizes and produces NH4+. Microcystis prefer ammonium (NH4+) because of its attached bacteria often lacking functional genes that mediate nitrification (Yang et al., 2021). Therefore, strain JXJ CY39T can provide Maf with NH4+ since it can convert N2 into NH3, which was the main reason that Maf co-cultured with JXJ CY39T expand more biomass during the first 7 days, and exhaust lesser biomass during the second 7 days (Table 4). Therefore, strain JXJ CY 39T can provide Maf with available N when available N is limited. It is consistent with those of other co-cultures with other attached bacteria and Maf (Xiao et al., 2022a,b,c).
In nitrogen-deficient medium, strain JXJ CY39T also influenced the synthesis of MC-LR (Table 4). The E-MC-LR concentrations of Maf co-cultured with JXJ CY39T were 45.3 and 159.8 μg mg−1 chl-a, which were 54.0, and 52.9% lower (p < 0.01) than those of the controls. The I-MC-LR concentration of Maf co-cultured with strain JXJ CY39T was 1,162.2 μg mg−1 chl-a on day 14 of cultivation, which was 16.9% lower (p < 0.01) than that of the control. Similar phenomena were also found in previous studies (Xiao et al., 2022a,b).
In Ca3(PO4)2 medium, the color of Maf cultured without strain JXJ CY39T turned green gradually during the test time. Consequently, the chl-a concentrations increased to 0.639, and 0.985 mg L−1 after 9 and 18 days of culturation (Table 5), respectively. However, the color of Maf co-cultured with strain JXJ CY39T turned greener during the test time, and the chl-a concentrations increased to 0.745, and 1.163 mg L−1 after 9 and 18 days of culturation, which were 16.6, and 18.1% higher (p < 0.01) than those of the controls (Table 5), respectively.
As mention above, the inoculation of Maf cultured in BG11 imported 1.86 mg L−1 of K2HPO4 into Ca3(PO4)2 medium. And Ca3(PO4)2 is slightly soluble in water and breaks down into Ca2+ and PO43+. Therefore, dissoluble phosphorus is the key element that restricts the growth of Maf in Ca3(PO4)2 medium, in spite of the uptake of PO43+ by Maf would result in more Ca3(PO4)2 being dissolved. These were the reasons that Maf cultured without strain JXJ CY39T turned green more slowly. Secreting phosphatases and organic acids are two of the important mechanisms that microbes degrade insoluble phosphorus into dissoluble phosphorus. Therefore, strain JXJ CY39T can facilitate the dissolving of Ca3(PO4)2 since it has abundant genes related to phosphatase activity, and organic acid biosynthetic process and transmembrane transport (Supplementary Table S3), which would relieve the restriction of lacking dissoluble phosphorus on the growth of Maf in Ca3(PO4)2 medium. This was the main reason that Maf co-cultured with strain JXJ CY39T expanded more biomass than Maf cultured without strain JXJ CY39T in Ca3(PO4)2 medium (Table 5). These results were consistent with previous studied of co-culture of other phosphate-solubilizing attached bacteria and Microcystis (Xiao et al., 2022a,b,c).
Strain JXJ CY39T also significantly influenced MC-LR synthesis of Maf in Ca3(PO4)2 medium (Table 5). The E-MC-LR concentration of Maf co-cultured with strain JXJ CY39T was 19.6 μg mg−1 chl-a on day 9 of cultivation, which was 22.5% higher (p < 0.01) than that of the control. The I-MC-LR concentrations of Maf cultured with strain JXJ CY39T were 1,022.3 and 1,713.1 μg mg−1 chl-a on days 9 and 18 of cultivation, representing 9.8 and 12.1% lower (p < 0.05, p < 0.01) than those of the controls, respectively. Similar phenomena were also found in previous studies (Xiao et al., 2022a,b).
The cellular densities of strain JXJ CY39T cultured without Maf decreased with the cultivation time (p < 0.01) in media with both Ca3(PO4)2 and nitrogen limitation (Tables 4, 5). However, the cellular densities of strain JXJ CY39T co-cultured with Maf in available nitrogen-deficient medium increased to 2.93 × 106 CFU mL−1 after 7 days of cultivation, and maintained about 3 × 106 CFU mL−1during the next 7 days, which were over 32–161-fold higher (p < 0.01) than those cultured without Maf in nitrogen-deficient medium (Table 4). Further, the cellular densities of strain JXJ CY39T co-cultured with Maf in Ca3(PO4)2 medium were 7.27 × 104 and 1.46 × 105 CFU mL−1 on days 9 and 18 of cultivation, which were over 5- and 82-fold increases in densities when cultured without Maf in Ca3(PO4)2 medium (Table 5). As described above, strain JXJ CY39T would disappear from cultures after co-culture with Maf in BG11 medium for 10 days or more. Thus, these results suggested that Maf could adjust interactions with strain JXJ CY39T when available N and P are limited, and further certified that the interactions between algae and attached bacteria are dynamic and can be initiated and ended in response to environmental change.
3.7 Inhibition of attached bacteria by Maf metabolites
Plate-based antibacterial assays (Supplementary Figure S7) revealed that Maf extracts exhibited obvious inhibitory activities on strains JXJ CY 31, 37, and 39, in addition to weak (strains JXJ CY 16 and 28) or no inhibitory activities on other attached bacterial strains. Fraction IV represented the fat-soluble component and did not exhibit inhibitory activity against any of the nine attached bacteria. Fraction II exhibited the strongest inhibitory activity, followed by fraction I. Fraction III contained MC-LR at a concentration of 4 μg per disk and exhibited no or very weak inhibitory activities on any of the nine attached bacteria.
Co-culture analyses (Table 3) revealed that the cell densities of some strains increased at 5 days of cultivation and then decreased at day 10 of cultivation. The reason for this difference was likely because antibacterial compounds secreted by Maf did not achieve concentrations high enough to inhibit bacterial growth after 5 days of cultivation, but did after 10 days of cultivation. However, the cell densities of strains JXJ CY 05, 11, 16, 18, 28, and 57 significantly decreased (p < 0.01) after day 10 of cultivation and even day 5 of cultivation, despite that Maf extracts exhibited no or very weak inhibitory activities on these bacteria grown on plates. It is possible that the synthesis of specific antibacterial compounds required the induction of these bacteria or alternatively that some antibacterial compounds lost their antibacterial activities during chemical extraction and isolation.
Healthy macroalgae can control their attached bacteria to avoid excessive bacterial growth and further competition for nutrients (Kouzuma and Watanabe, 2015). Specifically, healthy M. aeruginosa can apparently control their attached bacteria to avoid competition for nutrients (Zhang et al., 2016b,c; Xiao et al., 2022a,b,c). Likewise, extracts from M. aeruginosa exhibited inhibitory activities on some of their attached bacteria (Casamatta and Wickstrom, 2000) and many other bacterial taxa including Escherichia coli (Ostensvik et al., 1998; Valdor and Aboal, 2007), Streptoverticillium (Valdor and Aboal, 2007), Bacillus subtilis, B. cereus, and Aeromonas hydrophila (Ostensvik et al., 1998). Microcystins including MC-LR, MC-RR, and MC-YR were also evaluated for antibacterial activity. Ostensvik et al. (1998) observed that MCs did not exhibit any inhibitory activities against E. coli, Bacillus subtilis, B. cereus, and Aeromonas hydrophila at a concentration of 1–8 μg mL−1, while Valdor and Aboal (2007) observed that MC-YR inhibited Streptoverticillium at a 12.5 μg mL−1, while MC-RR and MC-LR inhibited Streptoverticillium at a 25 μg mL−1, and MC-LR inhibited E. coli at a 5 μg mL−1. Nevertheless, it has remained unclear whether MCs are the specific chemicals by which Maf control their attached bacteria.
MC-LR accounts for over 57% of the MCs produced in M. aeruginosa laboratory cultures (Liu et al., 2012) and 45.5–99.8% of the MCs in HAB-impacted natural waters (Vasconcelos et al., 1996). The results of this study showed that E-MC-LR, I-MC-LR, and total MC-LR concentrations were 0.017–0.37, 0.44–1.25, and 0.46–1.36 μg mL−1, respectively. Thus, the concentrations of E-MC-LR and even the total MCs from both environmental samples and laboratory culture samples are a small fraction of the concentrations used in previous studies. Consequently, MCs may not be the specific chemicals secreted by Maf that control their attached bacteria. These results were further verified with antibacterial assays (Supplementary Figure S7). Fraction III contained MC-LR at a concentration of 4 μg per disk and exhibited no or very weak inhibitory activities on bacterial strains, while fractions I and II, and especially fraction II, exerted stronger and more broad-spectrum antibacterial activity. Consequently, fraction II likely contains one of the main chemicals that Maf uses to specifically control their attached bacteria.
Co-culture experiments (Table 3) revealed that strain JXJ CY 39T disappeared when co-cultured with Maf in BG11 medium. However, the cellular density of strain JXJ CY 39T on day 18 of cultivation in Ca3(PO4)2 medium was 200% of that on day 9 of cultivation (Table 5). Thus, the concentrations of antibacterial components in Maf cultures did not increase with cultivation time although chl-a concentrations increased by 56.2%. Only enough strain JXJ CY 39T biomass can provide Maf with enough available P in Ca3(PO4)2 medium, probably partially explaining why the cell density of strain JXJ CY 39T increased after 18 days of cultivation. Thus, Maf was apparently able to adjust the synthesis and secretion of specific antibacterial components based on their nutritional requirements. And this further proved that the interactions between algae and attached bacteria are dynamic and can be initiated and ended in response to environmental change.
3.8 Influence of strain JXJ CY 39T on the growth of non-culturable Maf-attached bacteria
No bacteria grew on ISP2 plates after incubation at 28.0°C for 7 days, indicating that strain JXJ CY 39T in the samples had died, and the control cultures remained axenic during the experiments. Community compositional analyses of eight samples of purified Maf co-cultured with strain JXJ CY 39T and purified Maf cultured without strain JXJ CY 39T from days 5, 10, 15, and 35 yielded 820,153 high-quality, partial 16S rRNA gene sequences without singletons, representing 379 amplicon sequence variants (ASVs). Each sample only contained a few unique ASVs (Supplementary Figure S8). Bacterial intra-sample (alpha) diversity was estimated by rarefaction analysis (Supplementary Figure S9) and by calculating the Shannon and Simpson diversity indices, in addition to the observed number of ASVs (Figure 3A). Rarefaction analyses with the Shannon index indicated that the sequencing efforts recovered nearly all diversity expected in these samples. The community diversity of purified Maf co-cultured with strain JXJ CY 39T was higher than that of purified Maf cultured without strain JXJ CY 39T.
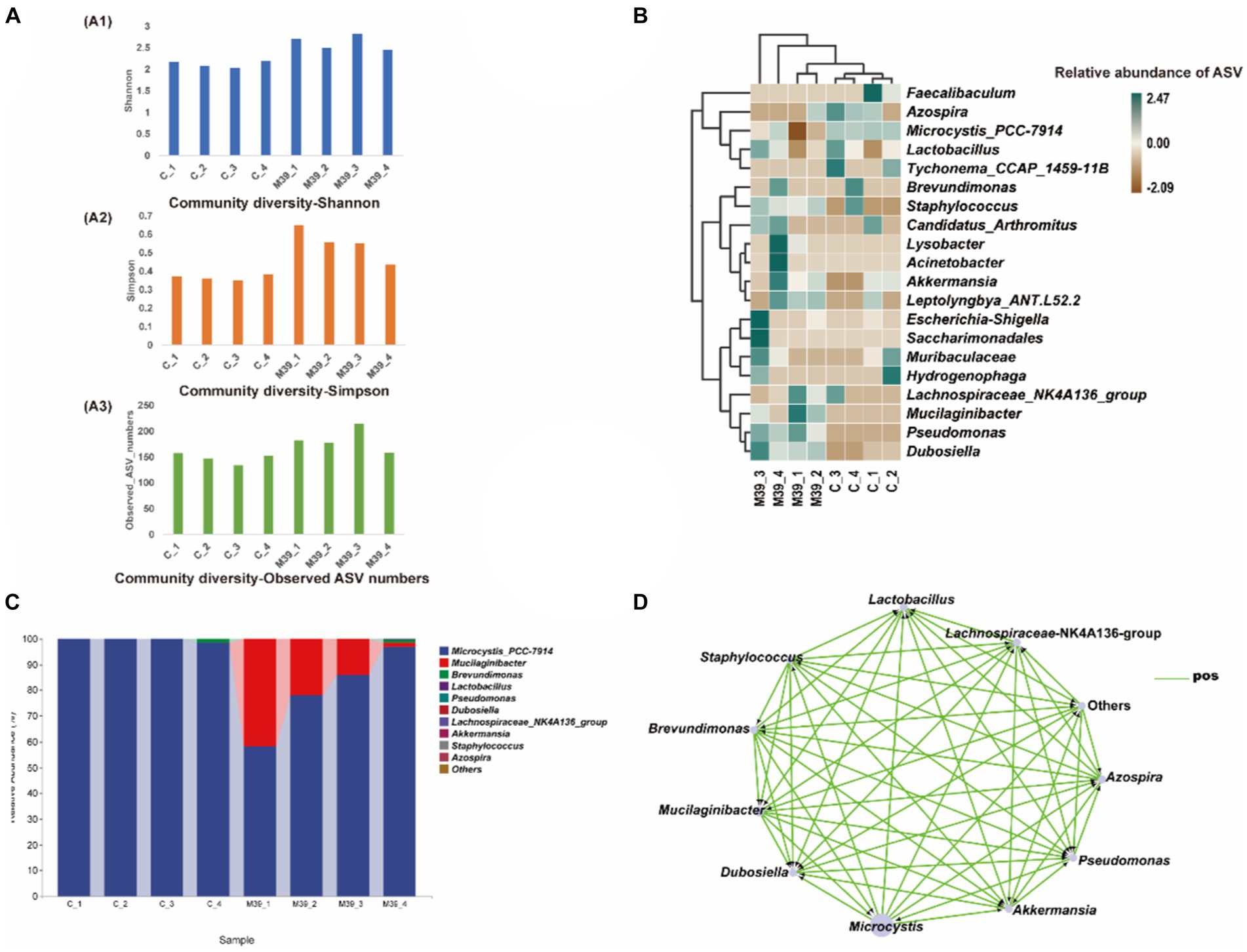
Figure 3. Analysis of bacterial community in samples of purified Maf co-cultured with and without strain JXJ CY 39T on days 5, 10, 15, and 35. (A) Amplicon sequence variant (ASV) richness and diversity indices of culture communities assessed with Shannon diversity, Simpson diversity, and total ASV number indices. (B) Heatmap analysis of enriched bacterial taxa in these culture samples. (C) The relative abundances of the bacterial community at the genus taxon level in purified Maf co-cultured with or without strain JXJ CY 39T on days 5, 10, 15, and 35. The relative abundances of the most abundant genera are shown. (D) Co-occurrence network visualization of dominant genera in the communities. Each node represents a dominant genus and each edge represents two correlated genera based on absolute Spearman’s correlations >0.5 and with a p < 0.05. The edge length is based on Bray-Curtis distances and the node size reflects the relative abundances of genera. C_1, C_2, C_3, and C_4 indicate cultures of Maf without JXJ CY 39T collected on days 5, 10, 15, and 35 of cultivation, respectively. M39_1, M39_2, M39_3, and M39_4 indicate co-cultures of JXJ CY 39T and Maf collected on days 5, 10, 15, and 35 of cultivation, respectively.
ASVs were taxonomically classified using the latest version of the Ribosomal Database Project (RDP) database to assess bacterial taxonomic compositional changes across time in cultures of Maf with and without strain JXJ CY 39T. Differences between culture conditions were evident. In the control groups, there were 159, 150, 134, 154 ASVs detected in purified Maf cultured without strain JXJ CY 39T at days 5, 10, 15, 35 of the culture periods, respectively. While in the experimental groups, higher number of ASVs were detected in bacterial communities, with 183, 178, 217 and 157 ASVs detected in purified Maf co-cultured with strain JXJ CY 39T on day 5, 10, 15, 35, respectively. A total of 9 phyla, 22 orders, and 28 genera were identified in culture samples of purified Maf co-cultured with strain JXJ CY 39T, while 7 phyla, 20 orders, and 27 genera were identified in culture samples of Maf cultured without strain JXJ CY 39T. The relative abundances of Maf cultured without strain JXJ CY 39T were over 98.35% in all samples. Bacteria belonging to several phyla were also detected including Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, and Verrucomicrobia on day 5 of cultivation; Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, Verrucomicrobia, and Deinococcus-Thermus on day 10 of cultivation; Proteobacteria, Bacteroidetes, and Firmicutes on day 15 of cultivation; and Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria on day 35 of cultivation. The relative abundances these phyla ranged between 0.0028–0.05%, except for Proteobacteria on day 35 of cultivation, that exhibited relative abundances of 1.59%. Thus, the relative abundances of non-culturable attached bacteria of Maf changed with cultivation time, while the Proteobacteria, Bacteroidetes, and Firmicutes, and especially Proteobacteria, were the most common phyla associated with Microcystis and were maintained at relatively high abundances. Specifically, on day 5 and 10 of the culture periods, the relative abundance of ASVs belonging to the genus Dubosiella was 0.003%, whereas on days 15 and 35 of the culture periods, it dropped to 0%. Similarly, the abundance of ASVs belonging to the genus Akkermansia decreased from 0.007% on days 5, and 10 of culture to 0% on days 15, and 35 of culture. On the other hand, the relative abundance of ASVs belonging to the genus Lactobacillus was absent (0%) on day 5 of culture, increased to 0.011% on day 10 of culture, 0.026% on day 15 of culture, and decreased to 0.012% on day 35 of the culture period.
The relative abundances of Maf co-cultured with strain JXJ CY 39T were 58.20, 77.94, 85.96, and 96.87% on days 5, 10, 15, and 35 of cultivation, respectively. The relative abundances of strain JXJ CY 39T co-cultured with Maf were 41.72, 21.94, 13.85, and 1.59% on days 5, 10, 15, and 35 of cultivation, respectively. Thus, the relative abundances of Maf increased with cultivation time, in contrast to those of strain JXJ CY 39T. The relative abundances of strain JXJ CY 39T quickly decreased, indicating that its fraction of the community decreased with culture time. In addition, many other bacteria from various phyla were detected in the co-culture samples. Proteobacteria, Firmicutes, and Verrucomicrobia were detected on day 5 of cultivation, while Actinobacteria appeared on day 10 of cultivation along with the three abovementioned phyla. The Epsilonbacteraeota, and Patescibacteria likewise appeared on day 15 of cultivation in addition to the four above phyla, while Deinococcus-Thermus appeared on day 35 of cultivation alongside concomitant disappearances of Epsilonbacteraeota and Patescibacteria. The relative abundances of these phyla ranged between 0.0017–0.075%, except for Proteobacteria on day 35 of cultivation that exhibited a relative abundance of 1.45%. Notably, the Epsilonbacteraeota and Patescibacteria were detected in co-cultures of Maf and strain JXJ CY 39T, unlike Maf cultured without strain JXJ CY 39T.
Linear discriminant analysis (LDA) of effect size (LEfSe) analysis can be used to identify significant enrichment of bacteria among samples (Segata et al., 2011). A cladogram was constructed for LEfSe analysis (Figure 3B) that showed the most differentially abundant bacterial taxa (with default logarithmic, LDA, values >3.0) related to the two groups of samples. The bacterial taxa enriched in culture samples of purified Maf co-cultured with strain JXJ CY 39T were Mucilaginibacter, Dubosiella, and Pseudomonas (Figure 3B). As expected, Microcystis was most abundant in culture samples of purified Maf cultured without JXJ CY 39T. A total of 16 genera were detected only in co-cultures of purified Maf and strain JXJ CY 39T, including Acidovorax, Acinetobacter, Aquabacterium, Arenimonas, Bacteroides, Bifidobacterium, Bosea, Chujaibacter, Helicobacter, Jeotgalicoccus, Lysobacter, Oscillibacter, Pseudomonas, Sphingomonas, Sporosarcina, and Truepera. In addition, a total of 13 genera were found only in the control groups, including Alloprevotella, Azonexus, Blautia, Bradyrhizobium, Cellulomonas, Devosia, Enterorhabdus, Faecalibaculum, Lawsonella, Muribaculum, Quadrisphaera, Romboutsia, and Thermus. The relative abundances of the 11 most abundant genera across all culture samples were specifically investigated (Figure 3C). Brevundimonas was detected in co-culture samples of Maf and strain JXJ CY 39T at day 15 (0.004%), and their relative abundances increased at day 35 (1.401%). In contrast, Brevundimonas was not detected in the samples of purified Maf cultured without JXJ CY 39T until day 35 (1.577%). In addition, the relative abundances of Dubosiella in co-cultures of purified Maf and strain JXJ CY 39T (0.0098, 0.0111, 0.0182, and 0.0087%; on days 5, 10, 15, and 35, respectively) were all higher than in cultures without JXJ CY 39T (0.0028, 0.0031, 0, and 0%, respectively).
Co-occurrence network analysis was used to identify correlations between genera among samples (Figure 3D). Specifically, the relative abundances of the 11 most dominant genera in cultures with purified Maf co-cultured with and without strain JXJ CY 39T were compared at days 5, 10, 15, and 35 of cultivation. Mucilaginibacter (e.g., like strain JXJ CY 39T), Brevundimonas, and Pseudomonas relative abundances were positively associated with Maf growth and Maf was positively correlated to the relative abundances of Akkermansia, Azospira, Dubosiella, Lactobacillus, and Staphylococcus. Consistently, the chl-a concentrations in some co-cultures of purified Maf and strain JXJ CY 39T were higher than control values in BG11 medium. In addition, strains JXJ CY 11 and 57 belonged to the genus Pseudomonas. On day 5 of cultivation, the I-MC-LR concentration of BCS with strain JXJ CY 11 were 9.7% higher (p < 0.05) than that of the control. On day 10 of cultivation, the I-MC-LR concentration of BCS with strain JXJ CY 57 were 19.6% higher (p < 0.01) than that of the control. Thus, Mucilaginibacter and Pseudomonas may positively influence the growth of Maf, consistent with the co-occurrence network analyses. Thus, co-culture with strain JXJ CY 39T may also influence the growth of other attached bacteria of Maf.
3.9 Description of Mucilaginibacter lacusdianchii sp. nov.
Mucilaginibacter lacusdianchii sp. nov. (la.cus.di.a’n.chii L. gen. n. lacus, of a lake; N.L. gen. n. dianchii, of Dianchi; N.L. gen. n. lacusdianchii, of Dianchi lake).
Strain JXJ CY 39T is aerobic, Gram-negative, rod-shaped (0.7–1.0 × 0.9–2.0 μm) and grows well on ISP2 medium. The pH, NaCl content (w/v), and temperature ranges for growth are 4.0–11.0, 0–3.0%, and 5–38°C, respectively, with optimal growth at pH 7.0–8.0, 0% NaCl, and 28.0°C, respectively. The strain is positive for catalase, oxidase, nitrate reduction, hydrolysis of starch, Tween 40 and 80, and negative for Tween 20 hydrolysis. The major cellular fatty acids were iso-C15:0 and C16:1ω7c/16:1ω6c. The predominant respiratory quinone is MK-7. The polar lipids are phosphatidylethanolamine (PE), unidentified aminophosphoglycolipid (APGL), unidentified aminoglycolipids (AGL), an unidentified phospholipid (PL), and unidentified polar lipids (L1-3).
The type strain, JXJ CY 39T (= KCTC 72617T = CGMCC 1.17449T), was isolated from the culture biomass of Microcystis aeruginosa FACHB-905 collected from Lake Dianchi in the Yunnan province of southwestern China. The genome has a G + C content of 42.1%. The GenBank accession numbers for the 16S rRNA gene sequence and draft genome sequence of strain JXJ CY 39T are MT674523 and WSRW00000000, respectively.
4 Conclusion
A novel species (type strain JXJ CY 39T) of the genus Mucilaginibacter was discovered from the phycosphere of Maf and we established Mucilaginibacter lacusdianchii sp. nov. based on the polyphasic taxonomic study. The interplay between the Maf-associated bacteria and their host is intricate and fluctuates with time. Additional research is necessary to determine if manipulating these microbial interactions could serve as a viable strategy for controlling Harmful Algal Blooms (HABs).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
YX: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. MD: Investigation, Validation, Writing – original draft, Writing – review & editing. YD: Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing. QD: Investigation, Validation, Data curation, Resources, Writing – review & editing. XW: Investigation, Validation, Data curation, Writing – review & editing. YY: Investigation, Validation, Writing – review & editing. BZ: Conceptualization, Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing. Y-QZ: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The National Natural Science Foundation of China (32360028; 32170021) supported to catty out the experiments, the Program of Jiujiang University (201511) supported to collect samples and data, and the Beijing Natural Science Foundation (5212018) supported to analyze the data.
Acknowledgments
We thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1295696/full#supplementary-material
Footnotes
References
Allen, M. M. (1968). Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 4, 1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x
Baik, K. S., Park, S. C., Kim, E. M., Lim, C. H., and Seong, C. N. (2010). Mucilaginibacter rigui sp. nov., isolated from wetland freshwater, and emended description of the genus Mucilaginibacter. Int. J. Syst. Evol. Microbiol. 60, 134–139. doi: 10.1099/ijs.0.011130-0
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Berg, K. A., Lyra, C., Sivonen, K., Paulin, L., Suomalainen, S., Tuomi, P., et al. (2009). High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J. 3, 314–325. doi: 10.1038/ismej.2008.110
Bland, C., Ramsey, T. L., Sabree, F., Lowe, M., Brown, K., Kyrpides, N. C., et al. (2007). CRISPR recognition tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics 8:209. doi: 10.1186/1471-2105-8-209
Boetzer, M., and Pirovano, W. (2012). Toward almost closed genomes with GapFiller. Genome Biol. 13:R56. doi: 10.1186/gb-2012-13-6-r56
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Casamatta, D. A., and Wickstrom, C. E. (2000). Sensitivity of two disjunct bacterioplankton communities to exudates from the cyanobacterium Microcystis aeruginosa kützing. Microb. Ecol. 40, 64–73. doi: 10.1007/s002480000035
Chen, X., Zhao, R., Tian, Y., Kong, B., Li, X., Chen, Z., et al. (2014). Mucilaginibacter polytrichastri sp. nov., isolated from a moss (Polytrichastrum formosum), and emended description of the genus Mucilaginibacter. Int. J. Syst. Evol. Microbiol. 64, 1395–1400. doi: 10.1099/ijs.0.055335-0
Chun, J., Oren, A., Ventosa, A., Christensen, H., Arahal, D. R., da Costa, M. S., et al. (2018). Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 68, 461–466. doi: 10.1099/ijsem.0.002516
Cirri, E., and Pohnert, G. (2019). Algae−bacteria interactions that balance the planktonic microbiome. New Phytol. 223, 100–106. doi: 10.1111/nph.15765
Collins, M. D., Pirouz, T., Goodfellow, M., and Minnikin, D. E. (1977). Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 100, 221–230. doi: 10.1099/00221287-100-2-221
Cooper, M. B., and Smith, A. G. (2015). Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 26, 147–153. doi: 10.1016/j.pbi.2015.07.003
Dawson, R. M. (1998). The toxicology of microcystins. Toxicon 36, 953–962. doi: 10.1016/s0041-0101(97)00102-5
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. doi: 10.1128/AEM.03006-05
Dong, X., and Cai, M. (2001). Manual of systematic identification of common bacteria. Science Press: Beijing, pp. 349–389.
Dziallas, C., and Grossart, H. P. (2011). Temperature and biotic factors influence bacterial communities associated with the cyanobacterium Microcystis sp. Environ. Microbiol. 13, 1632–1641. doi: 10.1111/j.1462-2920.2011.02479.x
Felsenstein, J. (1981). Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17, 368–376. doi: 10.1007/bf01734359
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x
Fitch, W. M. (1971). Toward defining the course of evolution: minimum change for a specific tree topology. Syst. Biol. 20, 406–416. doi: 10.1093/sysbio/20.4.406
Glibert, P. M., Anderson, D. M., Gentien, P., Granéli, E., and Sellner, K. G. (2005). The global, complex phenomena of harmful algal blooms. Oceanography 18, 136–147. doi: 10.5670/oceanog.2005.49
Grattan, L. M., Holobaugh, S., and Morris, J. G. Jr. (2016). Harmful algal blooms and public health. Harmful Algae 57, 2–8. doi: 10.1016/j.hal.2016.05.003
Grossart, H. P., and Simon, M. (2007). Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. Aquat. Microb. Ecol. 47, 163–176. doi: 10.3354/ame047163
Guo, Q., Xiong, S., Xiao, Y., Xu, C., and Zhang, B. (2020). The diversity and phosphate solubilization activity of Microcystis aeruginosa-associated bacteria. J. Jiangxi Norm. Univ. Nat. Sci. Ed. 44, 76–81. doi: 10.16357/j.cnki.issn1000-5862.2020.01.13
Hoke, A. K., Reynoso, G., Smith, M. R., Gardner, M. I., Lockwood, D. J., Gilbert, N. E., et al. (2021). Genomic signatures of Lake Erie bacteria suggest interaction in the Microcystis phycosphere. PLoS One 16:e0257017. doi: 10.1371/journal.pone.0257017
Kang, H., Kim, H., Bae, S., and Joh, K. (2021). Mucilaginibacter aquatilis sp. nov., Mucilaginibacter arboris sp. nov., and Mucilaginibacter ginkgonis sp. nov., novel bacteria isolated from freshwater and tree bark. Int. J. Syst. Evol. Microbiol. 71:004755. doi: 10.1099/ijsem.0.004755
Kazamia, E., Helliwell, K. E., Purton, S., and Smith, A. G. (2016). How mutualisms arise in phytoplankton communities: building eco-evolutionary principles for aquatic microbes. Ecol. Lett. 19, 810–822. doi: 10.1111/ele.12615
Kim, M., Shin, B., Lee, J., Park, H. Y., and Park, W. (2019). Culture-independent and culture-dependent analyses of the bacterial community in the phycosphere of cyanobloom-forming Microcystis aeruginosa. Sci. Rep. 9:20416. doi: 10.1038/s41598-019-56882-1
Kouzuma, A., and Watanabe, K. (2015). Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 33, 125–129. doi: 10.1016/j.copbio.2015.02.007
Lin, W., Hung, T. C., Kurobe, T., Wang, Y., and Yang, P. (2021). Microcystin-induced immunotoxicity in fishes: a scoping review. Toxins (Basel) 13:765. doi: 10.3390/toxins13110765
Liu, L. P. (1999). Characteristics of blue algal bloom in Dianchi Lake and analysis on its cause. Res. Environ. Sci. 12, 36–37.
Liu, Y., Gao, B., Yue, Q., Guan, Y., Wang, Y., and Huang, L. (2012). Influences of two antibiotic contaminants on the production, release and toxicity of microcystins. Ecotoxicol. Environ. Saf. 77, 79–87. doi: 10.1016/j.ecoenv.2011.10.027
Massouras, A., Hens, K., Gubelmann, C., Uplekar, S., Decouttere, F., Rougemont, J., et al. (2010). Primer-initiated sequence synthesis to detect and assemble structural variants. Nat. Methods 7, 485–486. doi: 10.1038/nmeth.f.308
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P., and Göker, M. (2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60
Minnikin, D. E., Collins, M. D., and Goodfellow, M. (1979). Fatty acid and polar lipid composition in the classification of Cellulomonas, Oerskovia and related taxa. J. Appl. Bacteriol. 47, 87–95. doi: 10.1111/j.1365-2672.1979.tb01172.x
Ndlela, L. L., Oberholster, P. J., Van Wyk, J. H., and Cheng, P. (2018). Bacteria as biological control agents of freshwater cyanobacteria: is it feasible beyond the laboratory? Appl. Microbiol. Biotechnol. 102, 9911–9923. doi: 10.1007/s00253-018-9391-9
Nguyen, L. T., Schmidt, H. A., von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. doi: 10.1093/molbev/msu300
Ostensvik, O., Skulberg, O., Underdal, B., and Hormazabal, V. (1998). Antibacterial properties of extracts from selected planktonic freshwater cyanobacteria – a comparative study of bacterial bioassays. J. Appl. Microbiol. 84, 1117–1124. doi: 10.1046/j.1365-2672.1998.00449.x
Pankratov, T. A., Tindall, B. J., Liesack, W., and Dedysh, S. N. (2007). Mucilaginibacter paludis gen. Nov., sp. nov. and Mucilaginibacter gracilis sp. nov., pectin-, xylan- and laminarin-degrading members of the family Sphingobacteriaceae from acidic Sphagnum peat bog. Int. J. Syst. Evol. Microbiol. 57, 2349–2354. doi: 10.1099/ijs.0.65100-0
Park, M. H., Chung, I. M., Ahmad, A., Kim, B. H., and Hwang, S. J. (2009). Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls. Aquat. Bot. 90, 309–314. doi: 10.1016/j.aquabot.2008.11.007
Parveen, B., Ravet, V., Djediat, C., Mary, I., Quiblier, C., Debroas, D., et al. (2013). Bacterial communities associated with Microcystis colonies differ from free-living communities living in the same ecosystem. Environ. Microbiol. Rep. 5, 716–724. doi: 10.1111/1758-2229.12071
Pérez-Carrascal, O. M., Tromas, N., Terrat, Y., Moreno, E., Giani, A., Marques, L. C. B., et al. (2021). Single-colony sequencing reveals microbe-by-microbiome phylosymbiosis between the cyanobacterium Microcystis and its associated bacteria. Microbiome 9:194. doi: 10.1186/s40168-021-01140-8
Ramanan, R., Kim, B. H., Cho, D. H., Oh, H. M., and Kim, H. S. (2016). Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol. Adv. 34, 14–29. doi: 10.1016/j.biotechadv.2015.12.003
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. doi: 10.1093/oxfordjournals.molbev.a040454
Seemann, T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi: 10.1093/bioinformatics/btu153
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60
Seyedsayamdost, M. R., Case, R. J., Kolter, R., and Clardy, J. (2011). The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 3, 331–335. doi: 10.1038/nchem.1002
Shao, K., Zhang, L., Wang, Y., Yao, X., Tang, X., Qin, B., et al. (2014). The responses of the taxa composition of particle-attached bacterial community to the decomposition of Microcystis blooms. Sci. Total Environ. 488-489, 236–242. doi: 10.1016/j.scitotenv.2014.04.101
Tamaoka, J., Katayama-Fujimura, Y., and Kuraishi, H. (1983). Analysis of bacterial menaquinone mixtures by high performance liquid chromatography. J. Appl. Bacteriol. 54, 31–36. doi: 10.1111/j.1365-2672.1983.tb01297.x
Tamura, K., Stecher, G., and Kumar, S. (2021). MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. doi: 10.1093/molbev/msab120
Urai, M., Aizawa, T., Nakagawa, Y., Nakajima, M., and Sunairi, M. (2008). Mucilaginibacter kameinonensis sp., nov., isolated from garden soil. Int. J. Syst. Evol. Microbiol. 58, 2046–2050. doi: 10.1099/ijs.0.65777-0
Valdor, R., and Aboal, M. (2007). Effects of living cyanobacteria, cyanobacterial extracts and pure microcystins on growth and ultrastructure of microalgae and bacteria. Toxicon 49, 769–779. doi: 10.1016/j.toxicon.2006.11.025
Vasconcelos, V. M., Sivonen, K., Evans, W. R., Carmichael, W. W., and Namikoshi, M. (1996). Hepatotoxic microcystin diversity in cyanobacterial blooms collected in portuguese freshwaters. Water Res. 30, 2377–2384. doi: 10.1016/0043-1354(96)00152-2
Wang, J., Wagner, N. D., Fulton, J. M., and Scott, J. T. (2021). Diazotrophs modulate phycobiliproteins and nitrogen stoichiometry differently than other cyanobacteria in response to light and nitrogen availability. Limnol. Oceanogr. 66, 2333–2345. doi: 10.1002/lno.11757
Won, M., Weon, H., Heo, J., Lee, D., Han, B., Hong, S., et al. (2022). Ferruginibacter albus sp. nov., isolated from a mountain soil, and Mucilaginibacter robiniae sp. nov., isolated from a black locust ffower, Robinia pseudoacacia. Int. J. Syst. Evol. Microbiol. 72:005556. doi: 10.1099/ijsem.0.005556
Xiao, Y., Chen, J., Chen, M., Deng, S., Xiong, Z., Tian, B., et al. (2022b). Mycolicibacterium lacusdiani sp. nov., an attached bacterium of Microcystis aeruginosa. Front. Microbiol. 13:861291. doi: 10.3389/fmicb.2022.861291
Xiao, Y., Chen, M., Chen, J., Mao, L., Peng, Y., Gui, S., et al. (2022a). Microbacterium kunmingensis sp. nov., an attached bacterium of Microcystis aeruginosa. J. Antibiot. 75, 662–670. doi: 10.1038/s41429-022-00568-w
Xiao, Y., Wang, L., Wang, X., Chen, M., Chen, J., Tian, B., et al. (2022c). Nocardioides lacusdianchii sp. nov., an attached bacterium of Microcystis aeruginosa. Antonie Van Leeuwenhoek 115, 141–153. doi: 10.1007/s10482-021-01690-9
Xue, H., Zhang, D., Xu, L., Wang, X., Zhang, A., Huang, J., et al. (2021). Actirhodobacter atriluteus gen. Nov., sp. nov., isolated from the surface water of the Yellow Sea. Antonie Van Leeuwenhoek 114, 1059–1068. doi: 10.1007/s10482-021-01576
Yang, L., Cao, X., Chen, X., Deng, Q., Wan, L., Li, X., et al. (2021). Community composition and functional genes explain different ecological roles of heterotrophic bacteria attached to two bloom-forming cyanobacterial genera. Sci. Total Environ. 758:143850. doi: 10.1016/j.scitotenv.2020.143850
Yang, C., Wang, Q., Simon, P. N., Liu, J., Liu, L., Dai, X., et al. (2017). Distinct network interactions in particle-associated and free-living bacterial communities during a Microcystis aeruginosa bloom in a plateau lake. Front. Microbiol. 8:1202. doi: 10.3389/fmicb.2017.01202
Yang, L., and Xiao, L. (2011). Outburst, jeopardize and control of cyanobacterial bloom in lakes. Science Press, Beijing, pp. 71–212.
Young, A. J. (1991). The photoprotective role of carotenoids in higher plants. Physiol. Plantarum 83, 702–708. doi: 10.1111/j.1399-3054.1991.tb02490.x
Zhang, B., Chen, W., Li, H., Zhou, E., Hu, W., Duan, Y., et al. (2015). An antialgal compound produced by Streptomyces jiujiangensis JXJ 0074T. Appl. Microbiol. Biotechnol. 99, 7673–7683. doi: 10.1007/s00253-015-6584-3
Zhang, B., Ding, Z., Li, H., Mou, X., Zhang, Y., Yang, J., et al. (2016a). Algicidal activity of Streptomyces eurocidicus JXJ-0089 metabolites and their effects on Microcystis physiology. Appl. Environ. Microbiol. 82, 5132–5143. doi: 10.1128/AEM.01198-16
Zhang, B., Salam, N., Cheng, J., Li, H., Yang, J., Zha, D., et al. (2017). Microbacterium lacusdiani sp. nov., a phosphate-solubilizing novel actinobacterium isolated from mucilaginous sheath of Microcystis. J. Antibiot. 70, 147–151. doi: 10.1038/ja.2016.125
Zhang, B., Salam, N., Cheng, J., Li, H., Yang, J., Zha, D., et al. (2016c). Modestobacter lacusdianchii sp. nov., a phosphate-solubilizing actinobacterium with ability to promote Microcystis growth. PLoS One 11:e0161069. doi: 10.1371/journal.pone.0161069
Zhang, B., Salam, N., Cheng, J., Xiao, M., Li, H., Yang, J., et al. (2016b). Citricoccus lacusdiani sp. nov., an actinobacterium promoting Microcystis growth with limited soluble phosphorus. Antonie Van Leeuwenhoek 109, 1457–1465. doi: 10.1007/s10482-016-0745-y
Zhang, J., Zhang, W., Wang, H., Chen, J., and Shen, H. (2019). Progress in the relationships between Microcystis and aquatic bacteria. Acta Hydrobiol. Sin. 43, 448–456. doi: 10.7541/2019.055
Zhao, G., Du, J., Jia, Y., Lv, Y., Han, G., and Tian, X. (2012). The importance of bacteria in promoting algal growth in eutrophic lakes with limited available phosphorus. Ecol. Eng. 42, 107–111. doi: 10.1016/j.ecoleng.2012.02.007
Zhao, J., Li, J., Guan, Z., Xu, L., Pan, C., and Li, P. (2011). Effect of an attached bacterium of alkaline phosphatase producting on the growth of Microcystis aeruginosa. J. Lake Sci. 23, 49–55. doi: 10.18307/2011.0108
Keywords: Mucilaginibacter lacusdianchii, harmful algal bloom, bacterial symbiont, genome, algal-bacterial interactions
Citation: Xiao Y, Du M, Deng Y, Deng Q, Wang X, Yang Y, Zhang B and Zhang Y-Q (2024) Modulation of growth, microcystin production, and algal-bacterial interactions of the bloom-forming algae Microcystis aeruginosa by a novel bacterium recovered from its phycosphere. Front. Microbiol. 15:1295696. doi: 10.3389/fmicb.2024.1295696
Edited by:
Ruichang Gao, Jiangsu University, ChinaCopyright © 2024 Xiao, Du, Deng, Deng, Wang, Yang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Qin Zhang, eXpoYW5nQGltYi5wdW1jLmVkdS5jbg==; Binghuo Zhang, YmluZ2h1b3poQDEyNi5jb20=
†These authors share first authorship
 Yao Xiao1†
Yao Xiao1† Binghuo Zhang
Binghuo Zhang Yu-Qin Zhang
Yu-Qin Zhang