- 1Medical School, Qingdao University, Qingdao, China
- 2Lishui Second People’s Hospital Affiliated to Wenzhou Medical University, Lishui, China
- 3School of Basic Medicine and Forensics, Key Laboratory of Bio-tech Vaccine of Zhejiang Province, Engineering Research Center of Novel Vaccine of Zhejiang Province, Hangzhou Medical College, Hangzhou, China
- 4Key Laboratory of Biomarkers and In Vitro Diagnosis Translation of Zhejiang province, School of Laboratory Medicine and Bioengineering, Hangzhou Medical College, Hangzhou, China
Acute pancreatitis is caused by trypsinogen activation in acinar cells caused by various injury forms (gallstone, high triglycerides, alcohol, etc.). Viral pancreatitis is a clinically rare disease type, which is easily neglected by clinicians and causes serious adverse consequences. Viral pancreatitis involves the entry of viruses into pancreatic cells, triggering inflammation, immune response activation, and enzymatic autodigestion, leading to tissue damage and potential complications. At present, there are few available reports on viral pancreatitis, most of which are case reports. This review brings attention to clinicians by describing the incidence of viral pancreatitis to enhance clinical understanding and patient care.
Introduction
Acute pancreatitis (AP) is a common acute abdominal disease, and its incidence may vary based on geographical, cultural, and demographic factors. For example, AP is the number one cause of acute hospitalizations of the digestive system in the United States (Fagenholz et al., 2007; Peery et al., 2012). Various forms of injury (gallstone, hypertriglyceride, alcohol, etc.) induce the activation of trypsin in acinus cells, which in turn induces acute pancreatitis (; Crockett et al., 2018). Gallstones are a common trigger for AP. The pancreas and gallbladder share a drainage system through the common bile duct (Garcia et al., 2019; Moody et al., 2019). If a gallstone migrates and obstructs this duct, it can lead to a backup of digestive enzymes within the pancreas, causing inflammation. Other factors can also contribute to AP. For example, chronic and excessive alcohol intake can trigger the release of pancreatic enzymes prematurely within the pancreas, causing inflammation (Maleth et al., 2015). Hypertriglyceridemic pancreatitis (HTGP) is a specific subtype of AP triggered by elevated levels of triglycerides in the blood (Carr et al., 2016; Adiamah et al., 2018). Triglycerides are a type of fat that the body uses for energy storage. When levels become excessively high, particularly above 1,000 mg/dL, they can lead to the development of HTGP. In summary, the etiology of AP involves various factors that can initiate a cascade of events leading to pancreatic injury, inflammation, and potentially systemic complications. The overall clinical mortality rate was close to 10%, and over 30% were from severe necrotizing pancreatitis (IAP/APA evidence-based guidelines for the management of acute pancreatitis, 2013).
Viral infections, bacterial infections and parasitic infections can also trigger pancreatitis by direct invasion, immune response, or obstruction of pancreatic ducts. However, acute pancreatitis directly induced by viral infection, which is relatively rare in clinical practice, is easy to be ignored or confused. Certain viruses can directly or indirectly contribute to the development of pancreatitis. The pancreas is a sensitive organ, and viral infections can impact its structure and function. At present, most of the available studies on viral pancreatitis are case reports, so reliable clinical research is necessary to explore the pathogenesis characteristics of viral pancreatitis. Especially during COVID-19 outbreaks, the risk of viral pancreatitis should be considered and clarified.
Diagnostic criteria
The diagnosis of acute pancreatitis is established by referring to the Revised Atlanta Classification (RAC), and its severity is classified into mild, moderately severe, and severe (Tenner et al., 2013). Acute pancreatitis can be diagnosed if at least two of the following three criteria are fulfilled: abdominal pain (acute onset of persistent and severe epigastric pain, often radiating to the back); serum lipase (or amylase) activity at least three times the upper limit of normal; or characteristic findings of acute pancreatitis on contrast-enhanced CT or, less often, MRI or transabdominal ultrasonography. The diagnosis of viral pancreatitis is mainly based on the discovery of virus in pancreas tissues at autopsy or on biopsy and the exclusion of acute pancreatitis caused by any other etiology. It is important to note that viral pancreatitis cannot be confirmed by laboratory etiology alone.
Viral pathogen
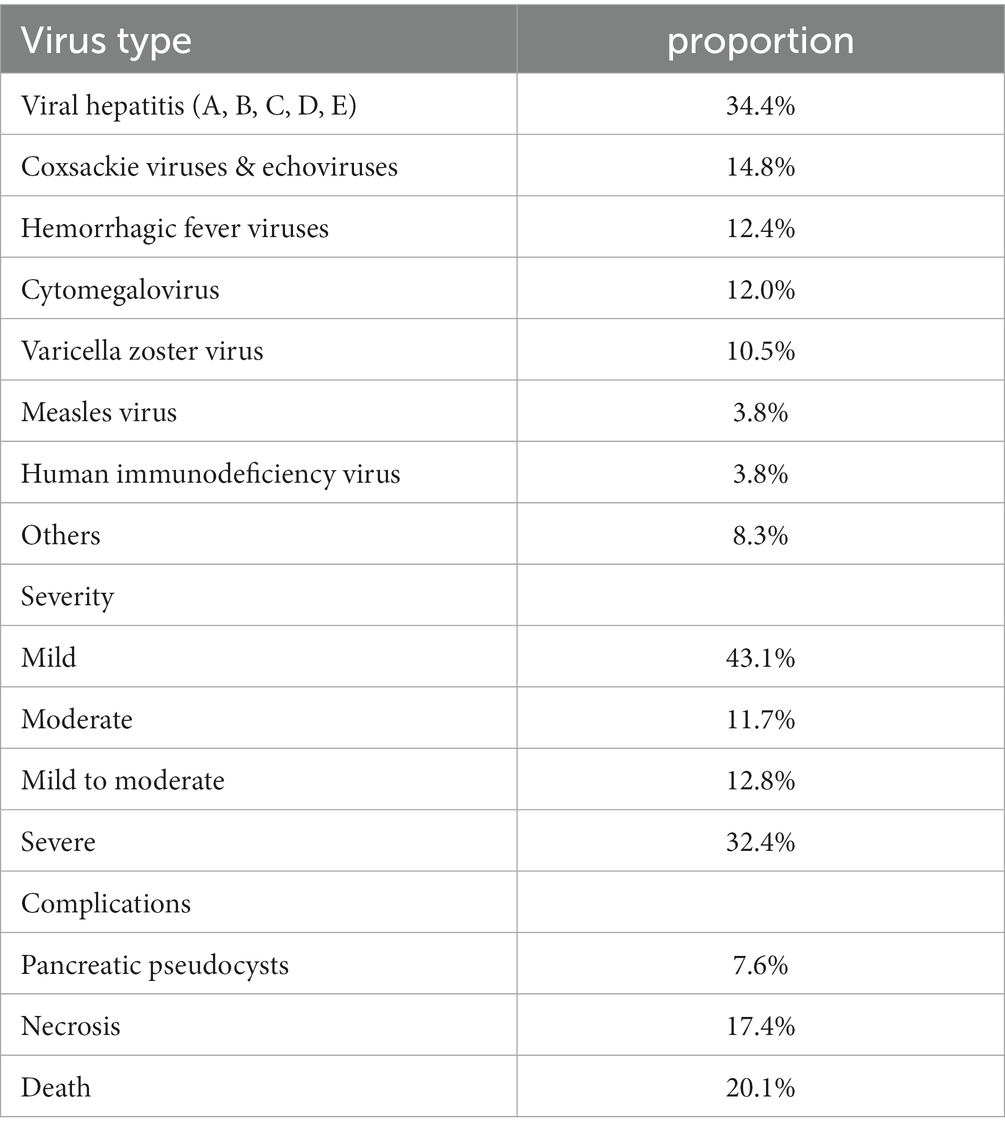
At present, the pathogen of known viral pancreatitis mainly includes mumps virus, epstein–barr virus, hepatitis virus, coxsackie virus, echoviruses, cytomegalovirus, epidemic hemorrhagic fever virus, measles virus, human immunodeficiency virus and so on (Table 1) (Simons-Linares et al., 2021). In addition, as the COVID-19 pandemic continues, more and more evidence shows that novel coronavirus can directly induce acute pancreatitis (Alves et al., 2020; Gupta et al., 2020; Inamdar et al., 2020; Karimzadeh et al., 2020). We hereby name it COVID-19 associated pancreatitis.
Mechanism of viral associated pancreatitis
Mechanisms of cellular injury: When the virus infects acinar cells, it mediates the transfer of the virus into the cells through the action of angiotensin-converting enzyme 2 (ACE2), thereby initiating further damage (Figure 1) (Lakshmanan and Malik, 2020). The triggering mechanism of pancreatitis: When the virus successfully enters the acinar cells, it prevents the cells from playing the role of exocytosis in a certain way, thus forcing the zymogen granules (containing digestive enzymes) to remain in the cell. The zymogen granules combine with intracellular lysosomes in acinar cells to form condensing or autophagic vacuoles containing an admixture of digestive and lysosomal enzymes. The lysosomal enzyme cathepsin B can activate the conversion of trypsinogen to trypsin. Finally, the accumulated intracellular trypsin exerts its own digestive damage through cascade action (Gaisano et al., 2001; Gukovsky et al., 2012; Sendler et al., 2018). This is similar to the process of virus entering acinar cells in the 2003 outbreak, and ACE2 receptor may be the key factor mediating the damage of most virus-infected acinar cells. In addition, inflammatory cells play a significant role in the development and progression of viral pancreatitis (Aghdassi et al., 2018). When a viral infection occurs in the pancreas, immune cells, such as macrophages, dendritic cells, and T lymphocytes, are recruited to the site (Armstrong et al., 2013). These immune cells recognize viral particles and initiate an immune response to neutralize and eliminate the virus. Inflammatory cells release various chemical messengers called cytokines. Cytokines help orchestrate the immune response by activating other immune cells, promoting inflammation, and signaling for tissue repair. However, an excessive release of cytokines can lead to an exaggerated and damaging inflammatory response. Inflammatory cells infiltrate the pancreatic tissue, contributing to the characteristic swelling, redness, and heat associated with inflammation. What’s more, activated immune cells generate reactive oxygen species (ROS) as part of the immune response (Wang et al., 2015; Sarshari et al., 2023). While ROS play a role in eliminating pathogens, excessive ROS production can lead to oxidative stress, damaging cellular components and contributing to tissue injury. The immune response, along with viral infection and other factors, can lead to cellular damage and death. This can result in the release of intracellular contents and further aggravate inflammation, potentially leading to the development of necrotic tissue and even organ failure.
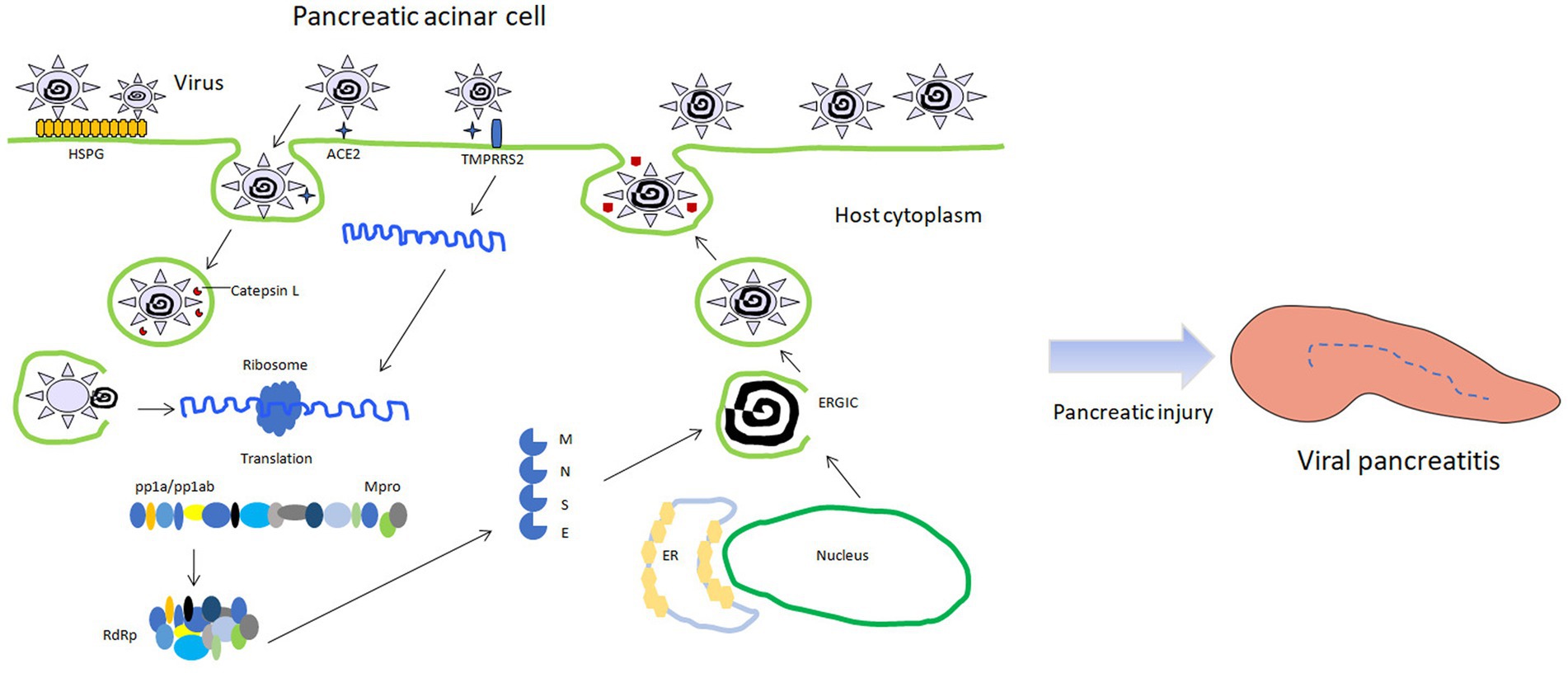
Figure 1. Schematic diagram of viral pancreatitis induced by viral infection of pancreatic acinar cells. HSPG: heparin sulfate proteogylican; TMPRSS2: transmembrane protease serine 2; RdRp: RNA dependent RNA polymerase; Mpro: main protease; ERGIC: endoplasmic reticulum-golgi intermediate compartment; pp1a/pp1ab: polymeric protein 1a/1ab.
Viral pancreatitis is a relatively uncommon manifestation of viral infections, but several viruses have been associated with pancreatitis (Hatch-Vallier et al., 2021; Almutairi et al., 2022; Choden et al., 2023). Viruses may have unique ways of interacting with host cells, so the specific mechanisms of pancreatitis may vary. Some viruses may directly infect pancreatic cells, while others may induce an inflammatory response or trigger immune-mediated damage. The severity and clinical presentation of viral pancreatitis can also differ depending on the underlying virus. It is still uncertain whether all the occurrence of viral pancreatitis is caused by ACE2-mediated cell injury. However, viruses may indeed employ different mechanisms that may lead to viral pancreatitis. Virus may interact with pancreatic cells in distinct ways, resulting in inflammation and damage to the pancreas. For example, Mumps is a well-known cause of viral pancreatitis, especially in children. The virus can directly infect and damage pancreatic cells, leading to inflammation (Simons-Linares et al., 2021). Mumps-related pancreatitis is often a part of systemic involvement in mumps infection. However, the way coronavirus invades cells provides reference and possibility for us to explore virus damage. However, in an in vitro model of acute pancreatitis, ACE2-angiotensin-(1–7)-Mas axis has been observed to protect against AP (Wang et al., 2015). From this point of view, what specific role does ACE2 play in the development and progression of acute pancreatitis still needs further study.
However, the immunocompromised status of patients can indeed play a role in the development and severity of viral-induced acute pancreatitis. Immunocompromised individuals, such as those with weakened immune systems due to conditions like varicella zoster virus/cytomegalovirus, organ transplantation, or immunosuppressive therapy, may be more susceptible to viral infections (Oku et al., 2005; Picod et al., 2017; Ahmad et al., 2021). Viruses that can cause pancreatitis may take advantage of the compromised immune response, leading to more severe and prolonged episodes of inflammation in the pancreas. It’s important to note that the relationship between viral infections, immunocompromised status, and acute pancreatitis is complex, and not all cases of viral-induced pancreatitis occur in immunocompromised individuals. The specific viruses involved and the mechanisms by which they induce pancreatitis can vary.
Clinical characteristics
Pancreatitis induced by different types of hepatitis virus infection accounted for the largest proportion of reported cases, about one third. Coxsackie and echoviruses come next. Abdominal pain is the predominant clinical manifestation of almost all viral pancreatitis and is accompanied by an increase in amylase or lipase. At present, the unique clinical manifestations of viral pancreatitis have not been reported (Simons-Linares et al., 2021). It should also be emphasized that some reported diagnoses of viral pancreatitis are not based on isolation of the virus from pancreatic tissue. It’s just laboratory virus testing on the basis of excluding other causes. This means that these cases may be false-positive or not really viral pancreatitis. Discussing the specific signs and symptoms associated with viral-induced pancreatitis can aid clinicians in prompt diagnosis and intervention. The main complications associated with viral pancreatitis include pancreatic necrosis, pseudocysts, abscess formation, organ failure, sepsis, and in severe cases, the development of chronic pancreatitis. These complications underscore the importance of timely diagnosis and appropriate management to prevent adverse outcomes in individuals with viral pancreatitis.
Therapy and control
Antiviral therapy should be a necessary treatment for viral pancreatitis, even if it does not eradicate the virus. The following treatment of viral pancreatitis should refer to the latest version of clinical treatment guidelines-- revision of the Atlanta classification (RAC), and select individualized treatment plans, including medication, fluid resuscitation, nutritional support, antibiotic use, pain management, complication management, and etiology management, etc. (Tenner et al., 2013). In particular, virus-specific treatment is needed. In addition, ACE2 may provide a new target for the treatment of viral pancreatitis in clinical practice (Yamaya et al., 2020).
Most importantly, once viral pancreatitis is confirmed, quarantine measures and relevant protective measures should be taken as soon as possible to prevent potential viral transmission. Clinicians should explore the characteristics of the virus and the source of infection, and identify the primary organs of virus infection, so as to make timely clinical decisions. In addition, it is equally important to explore preventive measures, such as vaccination strategies against viruses such as mumps. Highlighting the distinctive therapeutic considerations for viral pancreatitis ensures a more nuanced approach to patient management.
In addition, clinicians should distinguish between the following two concepts: acute pancreatitis combined with virus infection and viral pancreatitis. This is necessary because the treatment of patients in the context of the pandemic requires such consideration. Acute pancreatitis combined with virus infection means that the occurrence of the two diseases overlaps in time, but is not cause-and-effect. However, viral pancreatitis indicates that acute pancreatitis is caused by viral infection. It is more convenient for the clinician to make appropriate clinical judgment to distinguish the difference between them.
Conclusion
Viral pancreatitis refers to a specific type of AP that is triggered or influenced by viral infections. It is a rare etiological type in clinical practice, and it is very easy to be missed or misdiagnosed by clinicians, which not only delays the patient’s condition, but also causes the risk of the spread of potential viral infections. Viral pancreatitis can present with symptoms similar to other causes of AP, including severe abdominal pain, nausea, vomiting, and elevated pancreatic enzymes. Diagnosis and management should be conducted by qualified medical professionals. Always refer to authoritative medical sources for accurate and up-to-date information on viral pancreatitis and its associated viruses. Especially in the context of the current COVID-19 pandemic, there is indeed a need for adequate vigilance and attention.
Author contributions
XY: Methodology, Investigation, Formal analysis, Writing – original draft. MW: Writing – original draft. QK: Project administration, Writing – review & editing, Funding acquisition.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents under grant number WJW2021002; Science Foundation of National Health Commission of the PRC under grant number WJW-ZJ-2203; and the Key R&D projects of Zhejiang province under grant number 2022C03109.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adiamah, A., Psaltis, E., Crook, M., and Lobo, D. N. (2018). A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin. Nutr. 37, 1810–1822. doi: 10.1016/j.clnu.2017.09.028
Aghdassi, A. A., John, D. S., Sendler, M., Weiss, F. U., Reinheckel, T., Mayerle, J., et al. (2018). Cathepsin D regulates cathepsin B activation and disease severity predominantly in inflammatory cells during experimental pancreatitis. J. Biol. Chem. 293, 1018–1029. doi: 10.1074/jbc.M117.814772
Ahmad, J., Sayedy, N., Sanivarapu, R., Akella, J., and Iqbal, J. (2021). CMV pancreatitis in an immunocompromised patient. Case Rep Crit Care 2021, 1–4. doi: 10.1155/2021/8811396
Almutairi, F., Rabeie, N., Awais, A., Samannodi, M., Aljehani, N., Tayeb, S., et al. (2022). COVID-19 induced acute pancreatitis after resolution of the infection. J. Infect. Public Health 15, 282–284. doi: 10.1016/j.jiph.2022.01.003
Alves, A. M., Yvamoto, E. Y., Marzinotto, M., Teixeira, A., and Carrilho, F. J. (2020). SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz. J. Infect. Dis. 24, 561–564. doi: 10.1016/j.bjid.2020.08.011
Armstrong, J. A., Cash, N., Soares, P. M., Souza, M. H., Sutton, R., and Criddle, D. N. (2013). Oxidative stress in acute pancreatitis: lost in translation? Free Radic. Res. 47, 917–933. doi: 10.3109/10715762.2013.835046
Carr, R. A., Rejowski, B. J., Cote, G. A., Pitt, H. A., and Zyromski, N. J. (2016). Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology 16, 469–476. doi: 10.1016/j.pan.2016.02.011
Choden, U., Yangzom, S., Pradhan, G., and Wangchuk, P. (2023). Acute pancreatitis following SARS-CoV-2 infection: a case report. SAGE Open Med Case Rep 11, 2050313X2311752–231175288X. doi: 10.1177/2050313X231175288
Crockett, S., Falck-Ytter, Y., Wani, S., and Gardner, T. B. (2018). Acute pancreatitis guideline. Gastroenterology 154:1102. doi: 10.1053/j.gastro.2018.02.029
Fagenholz, P. J., Fernandez-del, C. C., Harris, N. S., Pelletier, A. J., and Camargo, C. J. (2007). Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 35, 302–307. doi: 10.1097/MPA.0b013e3180cac24b
Gaisano, H. Y., Lutz, M. P., Leser, J., Sheu, L., Lynch, G., Tang, L., et al. (2001). Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J. Clin. Invest. 108, 1597–1611. doi: 10.1172/JCI9110
Garcia, D. L. F. M., Garcia, G. D. P. A., Martinez, O. A., Marcos, C. N., Rodriguez, D. S. E., Sanchez, A. R., et al. (2019). Biliary sphincterotomy reduces the risk of acute gallstone pancreatitis recurrence in non-candidates for cholecystectomy. Dig. Liver Dis. 51, 1567–1573. doi: 10.1016/j.dld.2019.05.007
Gukovsky, I., Pandol, S. J., Mareninova, O. A., Shalbueva, N., Jia, W., and Gukovskaya, A. S. (2012). Impaired autophagy and organellar dysfunction in pancreatitis. J. Gastroenterol. Hepatol. 27, 27–32. doi: 10.1111/j.1440-1746.2011.07004.x
Gupta, R., Patnaik, I., and Kumar, A. (2020). Letter to the editor in response to COVID-19 presenting as acute pancreatitis. Pancreatology 20, 1021–1022. doi: 10.1016/j.pan.2020.06.017
Hatch-Vallier, B., Jarodiya, V., Hawa, F., and Daniel, R. (2021). Rare presentation of acute pancreatitis in mild COVID-19. BMJ Case Rep. 14. doi: 10.1136/bcr-2021-246720
IAP/APA evidence-based guidelines for the management of acute pancreatitis (2013). IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 13, e1–e15. doi: 10.1016/j.pan.2013.07.063
Inamdar, S., Benias, P. C., Liu, Y., Sejpal, D. V., Satapathy, S. K., and Trindade, A. J. (2020). Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology 159, 2226–2228.e2. doi: 10.1053/j.gastro.2020.08.044
Karimzadeh, S., Manzuri, A., Ebrahimi, M., and Huy, N. T. (2020). COVID-19 presenting as acute pancreatitis: lessons from a patient in Iran. Pancreatology 20, 1024–1025. doi: 10.1016/j.pan.2020.06.003
Lakshmanan, S., and Malik, A. (2020). Acute pancreatitis in mild COVID-19 infection. Cureus 12:e9886. doi: 10.7759/cureus.9886
Maleth, J., Balazs, A., Pallagi, P., Balla, Z., Kui, B., Katona, M., et al. (2015). Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 148, 427–439.e16. doi: 10.1053/j.gastro.2014.11.002
Moody, N., Adiamah, A., Yanni, F., and Gomez, D. (2019). Meta-analysis of randomized clinical trials of early versus delayed cholecystectomy for mild gallstone pancreatitis. Br. J. Surg. 106, 1442–1451. doi: 10.1002/bjs.11221
Oku, T., Maeda, M., Waga, E., Wada, Y., Nagamachi, Y., Fujita, M., et al. (2005). Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J. Gastroenterol. 40, 987–992. doi: 10.1007/s00535-005-1683-z
Peery, A. F., Dellon, E. S., Lund, J., Crockett, S. D., McGowan, C. E., Bulsiewicz, W. J., et al. (2012). Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002
Picod, A., Corre, E., Maury, E., Duriez, P., Hoyeau, N., and Coppo, P. (2017). Acute pancreatitis in immunocompromised patients: beware of varicella zoster virus primo-infection. Clin Case Rep 5, 1261–1263. doi: 10.1002/ccr3.1053
Sarshari, B., Zareh-Khoshchehreh, R., Keshavarz, M., Dehghan, M. S., SeyedAlinaghi, S., Asadzadeh, A. H., et al. (2023). The possible role of viral infections in acute pancreatitis: a review of literature. Gastroenterol Hepatol Bed Bench 16, 270–281. doi: 10.22037/ghfbb.v16i2.2582
Sendler, M., Weiss, F. U., Golchert, J., Homuth, G., van den Brandt, C., Mahajan, U. M., et al. (2018). Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology 154, 704–718.e10. doi: 10.1053/j.gastro.2017.10.018
Simons-Linares, C. R., Imam, Z., and Chahal, P. (2021). Viral-attributed acute pancreatitis: a systematic review. Dig. Dis. Sci. 66, 2162–2172. doi: 10.1007/s10620-020-06531-9
Tenner, S., Baillie, J., DeWitt, J., and Vege, S. S. (2013). American College of Gastroenterology guideline: management of acute pancreatitis. Am. J. Gastroenterol. 108, 1400–1415. doi: 10.1038/ajg.2013.218
Wang, J., Liu, R., Qi, H., Wang, Y., Cui, L., Wen, Y., et al. (2015). The ACE2-angiotensin-(1-7)-mas axis protects against pancreatic cell damage in cell culture. Pancreas 44, 266–272. doi: 10.1097/MPA.0000000000000247
Keywords: viral pancreatitis, acute pancreatitis, angiotensin-converting enzyme, acinar cells, virus
Citation: Yu X, Wang M and Kong Q (2024) Viral pancreatitis: research advances and mechanisms. Front. Microbiol. 14:1326837. doi: 10.3389/fmicb.2023.1326837
Edited by:
Mohammed Rohaim, Lancaster University, United KingdomReviewed by:
Shallu Tomer, University of California, Los Angeles, United StatesFouzia Sadiq, Shifa Tameer-e-Millat University, Pakistan
Varenka J. Barbero Becerra, South Medical Hospital, Mexico
Ehab M. El-Nahas, Benha University, Egypt
Copyright © 2024 Yu, Wang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingming Kong, cW1rb25nXzEwMjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xianqiang Yu
Xianqiang Yu Minchao Wang2†
Minchao Wang2†