- 1Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Clinical Microbiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Clinical Laboratory, Huaihe Hospital of Henan University, Kaifeng, Henan, China
- 4Department of Burn, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most commonly encountered pathogens among burn patients incurring substantial morbidity and mortality. To investigate the epidemiology and features of MRSA in burn wound infections, we conducted a 10-year retrospective study on MRSA isolated from burn patients with burn wound infections from southeast China from 2013 to 2022.
Methods: One hundred MRSA isolates (10 isolates each year) from burn wound infection among burn patients from 2013 to 2022 were randomly selected and enrolled. In addition to the clinical data of the 100 burn patients, MRSA isolates were characterized by antimicrobial susceptibility testing, detection of toxin genes, and molecular typing.
Results: The median time from the onset of burns and admission to MRSA detected was 13 and 5 days, respectively. No MRSA isolate was found resistant to quinupristin/dalfopristin, linezolid, and vancomycin. Toxin gene seg was found most frequently (90%) followed by sea (70%) and eta (64%). CC8 (74%), ST239 (70%), and SCCmec III (72%) were the most common CC, ST, and SCCmec types, respectively.
Conclusion: ST239-III (70%) was the predominant clone found in MRSA from burn wound infection among burn patients in southeast China. ST239-III was less found from 2018 to 2022. A higher diversity of MRSA clones was observed in these recent 5 years than that from 2013 to 2017.
Introduction
Millions of people get burned across the world every year. Burns are one of the most devastating injuries leading to high mortality and morbidity (Mater et al., 2020; Savetamal, 2021). Secondary to the loss of the skin barrier and suppression of the immune system, burn patients are more likely to develop invasive infections, leading to serious complications and even death. As the fourth most common type of trauma across the world, the World Health Organization (WHO) has estimated that ~11 million burn patients require medical attention and result in ~265,000 deaths each year (Mater et al., 2020). In China, approximately 5,000–10,000 people per million suffer burn injuries, and 10% of them require medical intervention (Chen et al., 2018).
Staphylococcus aureus is a serious public health concern and an important clinical pathogen that can cause a variety of infections in the skin and soft tissue, respiratory tract, bloodstream, and catheter (Jiang et al., 2018; Mahmoudi et al., 2019). S. aureus, particularly methicillin-resistant S. aureus (MRSA), remains a leading cause of gram-positive burn wound infections worldwide and has been one of the major contributors to increase the morbidity and mortality rates (Branski et al., 2009). Researchers analyzed 29 articles on multidrug-resistant bacteria outbreaks in burn units, and they found that one of the most frequent bacteria was MRSA (Girerd-Genessay et al., 2016). MRSA burn wound infection can potentially cause a fatal sequence of burn wound sepsis, invasive infection, septicemia, multiple organ failure, and even death (Kalligeros et al., 2019). Moreover, MRSA has been the second most common pathogen only to Pseudomonas aeruginosa among burn patients with bacteremia as reported (Kalligeros et al., 2019).
To learn more about the characteristics of MRSA from burn wound infections, we carried out a 10-year retrospective study of MRSA isolated from burn patients with burn wound infections from 2013 to 2022 in Ruijin Hospital in Shanghai, hosting mostly burn patients from southern China. In addition, we would also explore the dynamics of the epidemiology of MRSA in burn wound infections over these 10 years.
Materials and methods
Study design
This study was carried out in Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, a tertiary teaching hospital in Shanghai, with more than 3,000 beds serving patients from all over China. Department of Burn is one of the best burn units in China providing medical care to burn victims across the country, mainly from southeast China.
Clinical diagnosis of burn wound infections relies on the appearance of the wound, laboratory abnormalities, and wound cultures (D'Abbondanza and Shahrokhi, 2021). Changes in the appearance of the burn wound, including conversion of partial-thickness to full-thickness injury, loss of previously viable skin grafts, rapid cellulitis expansion of healthy tissue surrounding the injury, rapid eschar separation, or tissue necrosis, may indicate an acute infection. This typically manifests as some combination of pain, purulence, edema, malodor, or discoloration of the skin graft or donor site. Systemic alterations and laboratory abnormalities may help to diagnose burn wound infections. Leukocytosis (white blood cell count of >10,000 cells/mm3) and/or multiple protein biomarkers including acute-phase reactants (C-reactive protein/erythrocyte sedimentation rate and sedimentation rate), anticoagulant factors, cytokines, and tissue injury biomarkers (serum lactate) may or may not be associated with burn wound infections. Surface wound swabs and cultures may help identify the predominant pathogen and can be used as surveillance if there is any clinical concern about changes in the burn wound (Church et al., 2006; D'Abbondanza and Shahrokhi, 2021; Ladhani et al., 2021). Diagnosis of burn wound infections can be complicated and requires comprehensive consideration. The burn patients with burn wound infections enrolled in this study were diagnosed by MRSA-positive cultures of wound swabs combined with clinical signs and symptoms and/or laboratory results mentioned above.
This study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, and the review committee removed the requirement of informed consent for this retrospective study, which just focused on bacteria and did not involve patient interventions.
MRSA isolates
From January 2013 to December 2022, 410 non-repetitive MRSA isolates were collected from cultures of wound swabs from burn patients with burn wound infections in Ruijin Hospital, and 100 MRSA isolates were randomly selected and enrolled (10 isolates each year) using the random number generation function of Microsoft Office Excel 2016 (Microsoft Corporation, Redmond, WA, USA). The initial species were identified by MALDI-TOF mass spectrometry (bioMérieux, Marcy-l'Étoile, France).
Antimicrobial susceptibility testing
The broth microdilution method was used for antimicrobial susceptibility testing in accordance with the guidelines of the Clinical and Laboratory Standards Institute issued in 2023 (CLSI 2023) (Institute CaLS., 2023). Antibiotics selected and tested are presented in Table 2. S. aureus ATCC29213 strain was included as a control strain for the antimicrobial susceptibility testing.
Detection of toxin genes
Thirteen significant toxin genes were detected by polymerase chain reaction (PCR) containing lukS/F-PV (encoding Panton-Valentine leukocidin), tst (encoding toxic shock syndrome toxin 1), eta and etb (encoding exfoliative toxin A and B), and sea-see and seg-sej (encoding staphylococcal enterotoxins SEA-SEE and SEG-SEJ) as described previously (Gu et al., 2020).
Molecular typing
Multilocus sequence typing (MLST), spa typing, SCCmec typing, and agr typing were performed by PCR and/or sequencing as described previously (Gu et al., 2016, 2020). mecA detection was performed by PCR to confirm the MRSA strains.
Statistical analysis
The t-test, chi-square test, or Fisher's exact test was performed for statistical analysis as appropriate, and a two-sided P < 0.05 was considered statistically significant. In this study, statistical analysis was performed using the SAS 8.2 software package (SAS Institute Inc., Cary, NC, USA).
Results
Clinical data
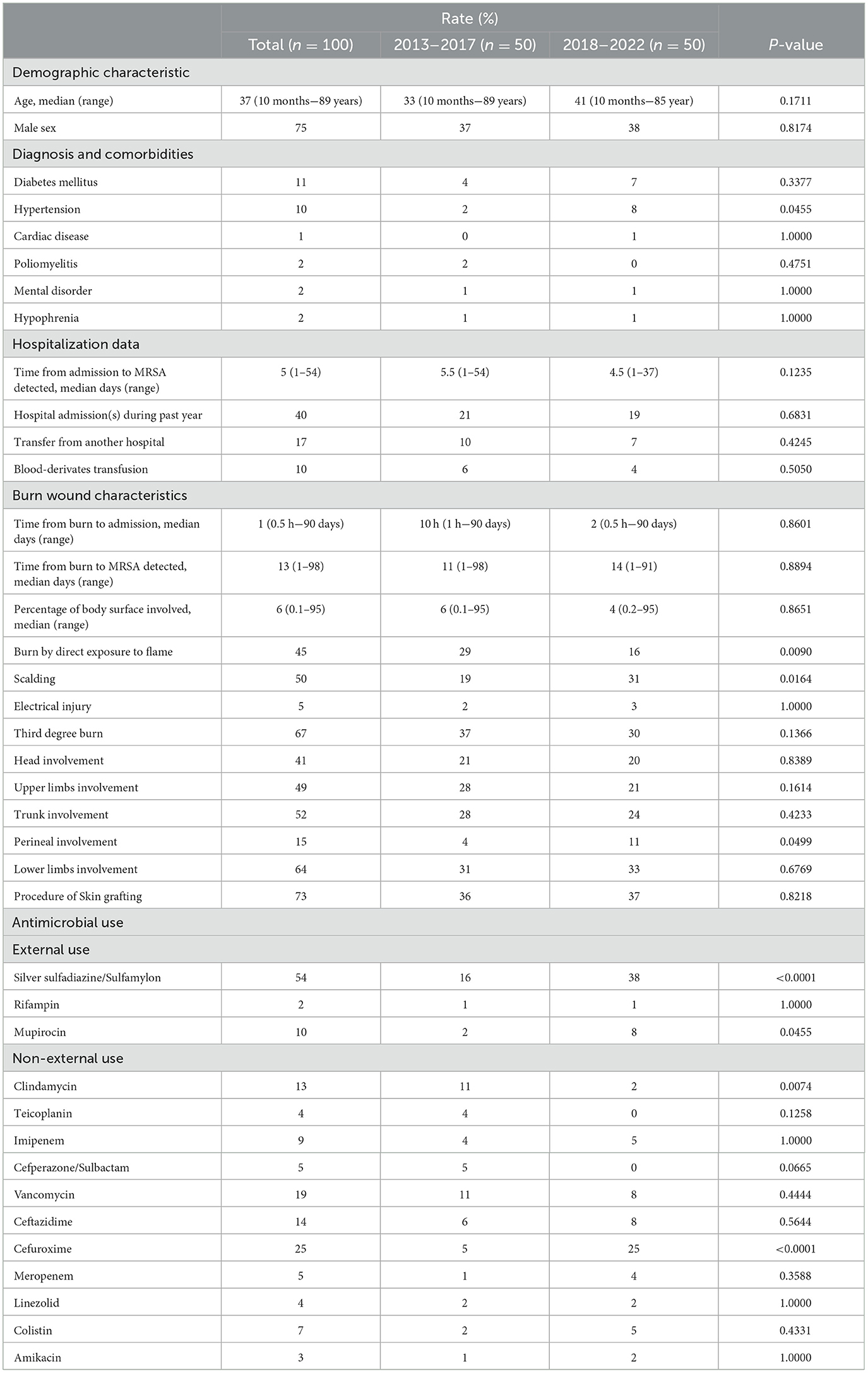
From January 2013 to December 2022, the median age of burn patients with MRSA burn wound infection was 37 years (range: 10 months−89 years; interquartile range: 15–54 years), and the sex distribution (male/female) was 75/25% (P < 0.0001). The median time from the onset of burns to receiving medical attention was 1 day (range: 0.5 h−90 days); the median time from admission to detection of MRSA was 5 days (range: 1–54 days), and the median time from the onset of burns to MRSA detected was 13 days (range: 1–98 days). Burn by direct exposure to flame accounted for 45%, and scalding accounted for 50%. Furthermore, the incidence of burn by direct exposure to flame was higher in 2013–2017 (P = 0.0090), and scalding was higher in 2018–2022 (P = 0.0164). More information about the burn patients with MRSA burn wound infection is presented in Table 1.
Antimicrobial resistance
All MRSA isolates in this study have been mecA-confirmed by PCR. No isolate was found resistant to quinupristin/dalfopristin, linezolid, and vancomycin. In addition to cefoxitin, benzylpenicillin, and oxacillin, all the MRSA isolates were resistant to tigecycline as well. The resistance rates of MRSA isolates to gentamicin, ciprofloxacin, levofloxacin, moxifloxacin, tetracycline, and rifampin were significantly higher in 2013–2017 (P < 0.05), as shown in Table 2. However, inducible clindamycin resistance was higher in 2018–2022 (P = 0.0166).
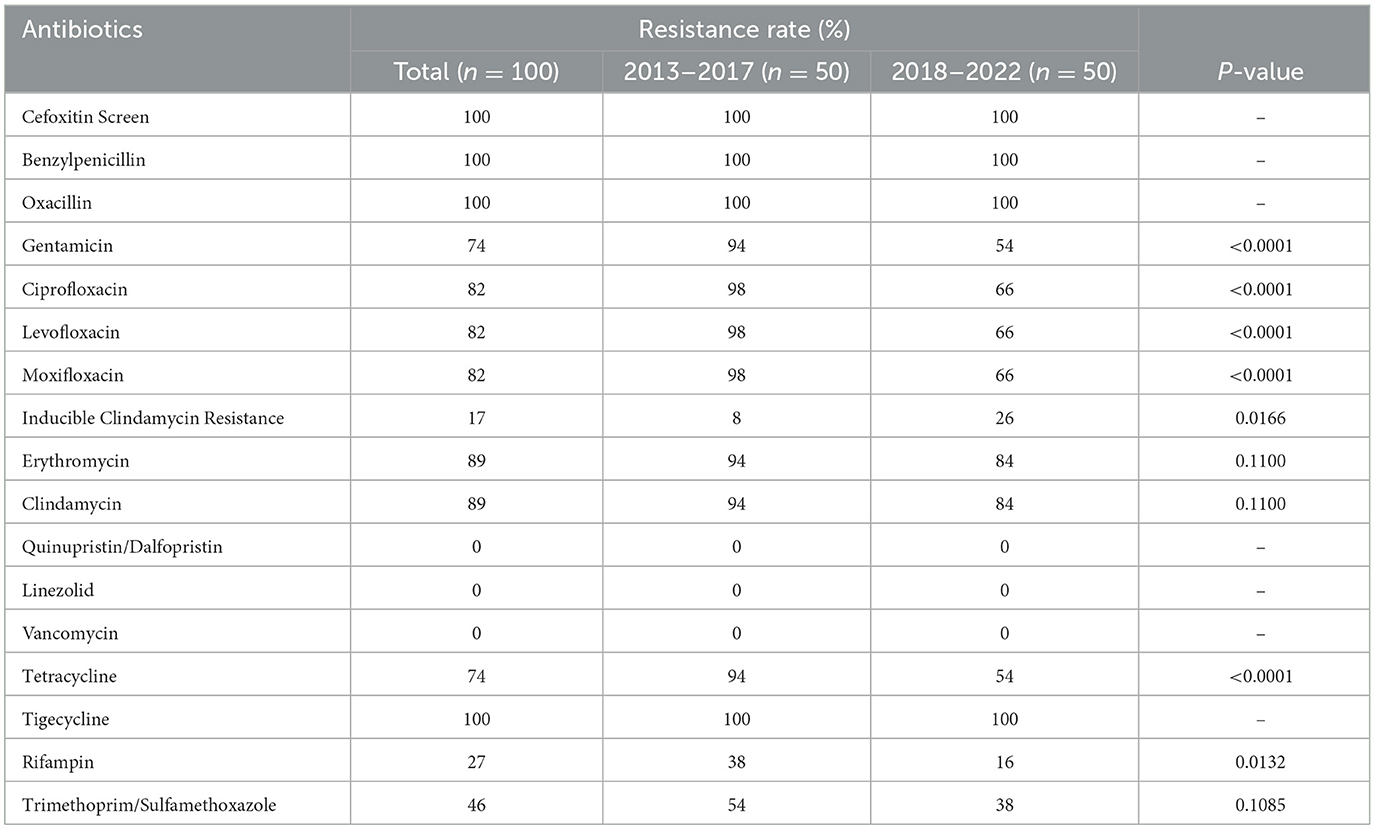
Table 2. The antibiotic resistance rates of MRSA isolated from burn wound infection from 2013 to 2022.
Toxin genes
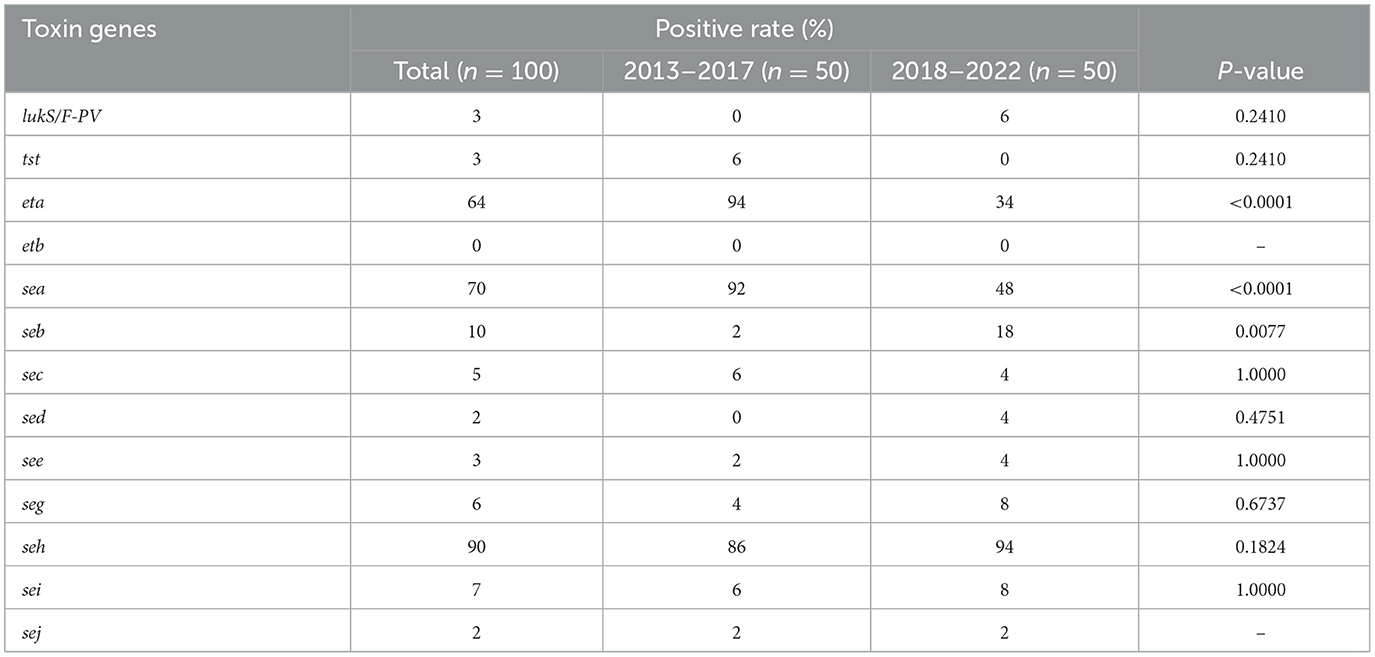
The seg was found most frequently (90%) followed by sea (70%) and eta (64%), as shown in Table 3. The eta and sea were found more frequently among the MRSA isolates in 2013–2017 (P < 0.0001 and P = 0.0001, respectively). However, seb was observed more frequently among the MRSA isolates in 2018–2022 (P = 0.0196). The toxin gene etb has not been discovered in this study.
Molecular types
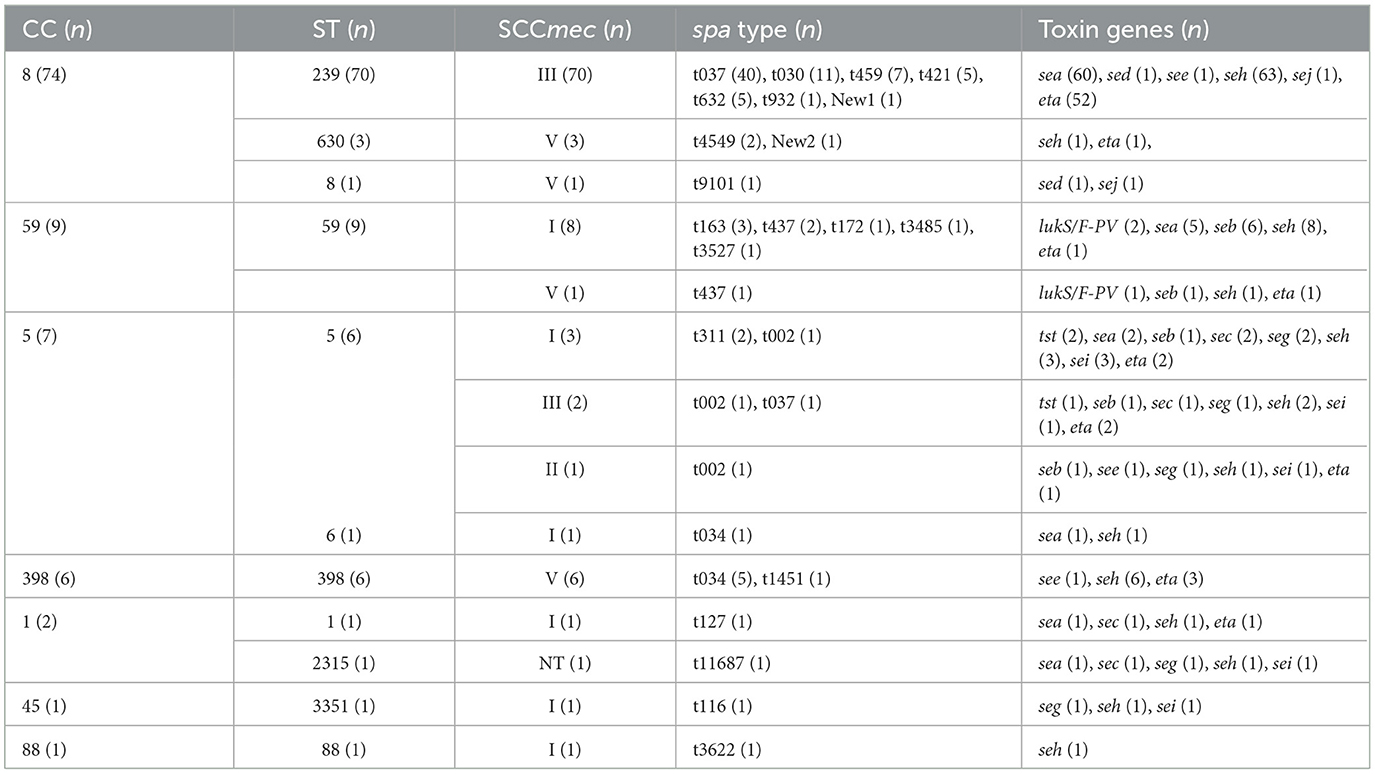
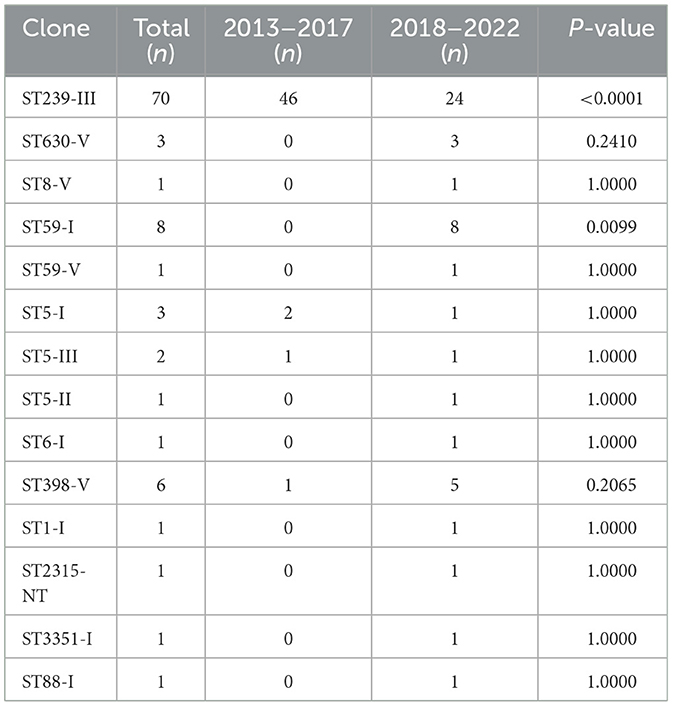
In total, 11 sequence types (STs) and 7 clonal complexes (CCs) were identified, as shown in Table 4. CC8 (74%) and ST239 (70%) were the most common CC and ST, respectively, and CC8 and ST239 came from the same burn units. SCCmec III (72%) was the most frequently found SCCmec type, followed by SCCmec I (15%), SCCmec V (11%), SCCmec III (1%), and SCCmec NT (1%). One MRSA isolate (ST2315-SCCmecNT-t11687) was unable to be SCCmec-typed by PCR. Whole-genome sequencing was performed on this isolate to obtain more information about SCCmec elements, but it still cannot by SCCmec typed for lack of ccr gene. The sequence data have been deposited in GenBank under BioProject ID PRJNA1024061 (http://www.ncbi.nlm.nih.gov/bioproject/1024061). As to spa types, t037 (41%) was the most common type, followed by t030 (11%) and t459 (7%). The agrI (91%) was the most frequent agr group, followed by agrII (6%) and agrIII (3%). The distribution of MRSA clones in 2013–2017 and 2018–2022 is presented in Table 5. ST239-III was the most common clone accounting for 70%, and ST239-III was more found in 2013–2017 (P < 0.0001); however, ST59-I was more found in 2018–2022 (P = 0.0099). In total, 4 and 14 MRSA clones were found in 2013–2017 and 2018–2022, respectively, and the diversity of MRSA clones in 2018–2022 was much greater than that in 2013–2017 (P = 0.0004).
Discussion
Burn is one of the most common and devastating forms of trauma, and patients with severe thermal injuries require immediate specialized medical care to minimize morbidity and mortality (Church et al., 2006). However, burn patients with infections presented more than twice the mortality rate of uninfected burn patients. It has been reported that 42–65% of deaths in burn patients can be attributed to infections over the past decades (D'Abbondanza and Shahrokhi, 2021). The bacteria responsible for infection appear in chronological order, changing with the time since the initial burn happened. So far, one of the most common pathogens involved in burn wound infections remains S. aureus (D'Abbondanza and Shahrokhi, 2021). The prevalence of multidrug-resistant (MDR) bacteria in burn units may lead to a vicious cycle of increasing antibiotic resistance by selecting antibiotics empirically that target MDR bacteria (Lachiewicz et al., 2017). Therefore, MRSA deserves more attention as one of the most common pathogens in burn infections. In addition to the loss of skin barrier function, burns may lead to a certain degree of immune suppression, which, in turn, makes the pathogen MRSA more aggressive (Kaiser et al., 2011).
Our study is a 10-year retrospective of MRSA from burn wound infections from 2013 to 2022, and we split it into two groups by year as 2013–2017 and 2018–2022 to find out how MRSA might change over time. As shown in Table 1, in the last 5 years in 2018–2022, the incidence of burn by direct exposure to flame was lower and instead the incidence of burn by scalding was higher. More notably, topical antibiotics such as silver sulfadiazine/sulfamylon and mupirocin were more frequently used in 2018–2022, which can prevent infections in burn patients. The microbial load on the open burn wounds and the risk of infections could be greatly reduced by the wide application of effective topical antimicrobials. A series of studies have demonstrated the role of topical antimicrobials in reducing morbidity and mortality among burn patients with severe burn injuries (partial-thickness or full-thickness skin involvement), especially before early excision (Church et al., 2006).
Several studies have shown that partial antibiotic resistance rates of S. aureus and MRSA have declined in recent years (Murray et al., 2009; Jiang et al., 2018; Mahmoudi et al., 2019). In our study, the resistance rates of MRSA to gentamicin, ciprofloxacin, tetracycline, rifampin, and other series antibiotics from 2018 to 2022 were significantly lower than that in 2013–2017. However, inducible clindamycin resistance was higher in 2013–2017. It is still unclear what accounts for this trend of MRSA resistance, or if it is simply due to chance. One research studied vancomycin susceptibility trends of MRSA isolated from burn wounds and found that the proportion of MRSA isolates exhibiting higher vancomycin MICs increased significantly (Zorgani et al., 2015). In our study, the 75% vancomycin MIC value of MRSA isolates was 2 μg/ml; the 21% vancomycin MIC value was 1 μg/ml, and only 4% vancomycin MIC value was ≤0.5 μg/ml. Those high MIC values indicate that vancomycin heteroresistance might emerge, although relevant information was not available. However, there is an urgent need to implement infection control measures to prevent the spread of MRSA, especially in burn patients.
In addition to the high positive rates of enterotoxin genes such as seh (90%) and sea (70%), exfoliative toxin A (ETA) gene eta was also frequently detected accounting for 64%, which was much higher than the S. aureus isolates we studied before from bloodstream infections, skin and soft tissue infections, and colonization (Gu et al., 2015, 2016, 2020; Zhang et al., 2015; He et al., 2021). Staphylococcal exfoliative toxins are responsible for staphylococcal scalded skin syndrome, which is characterized by dehydration, detachment of superficial skin layers, and secondary infections (Ahmad-Mansour et al., 2021). The prevalence of ETA in MRSA and methicillin-susceptible (MSSA) strains does not differ considerably as reported; nevertheless, 4% of MSSA strains carry the eta or etb gene, while approximately 10% of MRSA strains possess the eta gene, according to a recent study (Ahmad-Mansour et al., 2021). However, resistant strains such as MRSA with eta-positive may pose a problem nowadays or in future, especially among burn patients.
CC8 (74%) was found as the dominant clonal complex in MRSA burn wound infection among burn patients, and ST239-III was the most common clone with 70%. CC8 or ST239-III is a predominant healthcare-associated MRSA (HA-MRSA) clone across the world including America, Europe, Africa, Middle East, and Asia (Lakhundi and Zhang, 2018). As to burn infections, a study conducted at Jiangxi Burn Center in China reported that SCCmecIII-CC239-t030 was the most common clone (Chen et al., 2018). ST239-SCCmec III/t037 has emerged as the major MRSA clone in burn patients in Iran as reported in 2017 (Goudarzi et al., 2017). It is interesting that ST239-III was less found in recent 5 years in 2018–2022 (46 vs. 24, P < 0.0001), and ST59-I was more found in 2018–2022 (0 vs. 8, P = 0.0099) in our study. The diversity of MRSA clones from 2018 to 2022 was obviously greater than that in 2013–2017 (4 vs. 14, P = 0.0004), as shown in Table 5. It might suggest that besides the predominant clone ST239-III, other MRSA clones should also be watched and monitored among burn patients. In this study, livestock-associated MRSA (LA-MRSA) clone CC398-V occurred in 2017, 2018, 2020, 2021, and 2022 has emerged as a cause of hospital outbreaks, and more discoveries in recent years might indicate its greater ability to spread among patients in hospital along with time. ST398 has been reported as one of the predominant MRSA clones with ST239 in hospitals in India (Patil et al., 2023), and MRSA CC398 has been described in human colonization and infections over these years including our previous studies (Ballhausen et al., 2017; Gu et al., 2020; He et al., 2021; Silva et al., 2022).
MRSA colonization upon admission and during hospitalization among burn patients cannot be ignored, and it could result in multiclonal MRSA outbreaks in the burn units if decolonization protocols are not implemented (Kalligeros et al., 2019; Kim et al., 2019). The implementation of universal decolonization with intranasal mupirocin was effective as reported, and the prevalence of HA-MRSA in burn centers was significantly decreased after the application of the decolonization protocol (Johnson et al., 2016; Kim et al., 2019). Burn patients colonized or infected with MRSA are more likely to be a main reservoir transfer to others; a comprehensive concept to control the spread of all multidrug-resistant pathogens including MRSA is deeply needed in burn units, perhaps temporarily shut down, supplemented by intensive cleaning, are effective measures to stop the transmission events (Baier et al., 2018).
Data availability statement
The original contributions presented in the study are publicly available. This data can be found at: https://www.ncbi.nlm.nih.gov/bioproject; PRJNA1024061.
Author contributions
FG: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. WH: Data curation, Methodology, Writing – original draft. DZ: Investigation, Software, Writing – original draft. PY: Data curation, Writing – review & editing. JS: Conceptualization, Supervision, Writing – review & editing. LH: Formal analysis, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (Grant numbers 82102452). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad-Mansour, N., Loubet, P., Pouget, C., Dunyach-Remy, C., Sotto, A., Lavigne, J. P., et al. (2021). Staphylococcus aureus toxins: an update on their pathogenic properties and potential treatments. Toxins 13:677. doi: 10.3390/toxins13100677
Baier, C., Ipaktchi, R., Ebadi, E., Limbourg, A., Mett, T. R., Vogt, P. M., et al. (2018). A multimodal infection control concept in a burn intensive care unit - lessons learnt from a meticillin-resistant Staphylococcus aureus outbreak. J. Hosp. Infect. 98, 127–133. doi: 10.1016/j.jhin.2017.07.023
Ballhausen, B., Kriegeskorte, A., van Alen, S., Jung, P., Kock, R., Peters, G., et al. (2017). The pathogenicity and host adaptation of livestock-associated MRSA CC398. Vet. Microbiol. 200, 39–45. doi: 10.1016/j.vetmic.2016.05.006
Branski, L. K., Al-Mousawi, A., Rivero, H., Jeschke, M. G., Sanford, A. P., and Herndon, D. N. (2009). Emerging infections in burns. Surg. Infect. 10, 389–397. doi: 10.1089/sur.2009.024
Chen, K., Lin, S., Li, P., Song, Q., Luo, D., Liu, T., et al. (2018). Characterization of Staphylococcus aureus isolated from patients with burns in a regional burn center, Southeastern China. BMC Infect. Dis. 18:51. doi: 10.1186/s12879-018-2955-6
Church, D., Elsayed, S., Reid, O., Winston, B., and Lindsay, R. (2006). Burn wound infections. Clin. Microbiol. Rev. 19, 403–434. doi: 10.1128/CMR.19.2.403-434.2006
D'Abbondanza, J. A., and Shahrokhi, S. (2021). Burn infection and burn sepsis. Surg. Infect. 22, 58–64. doi: 10.1089/sur.2020.102
Girerd-Genessay, I., Benet, T., and Vanhems, P. (2016). Multidrug-resistant bacterial outbreaks in burn units: a synthesis of the literature according to the ORION statement. J. Burn Care Res. 37, 172–180. doi: 10.1097/BCR.0000000000000256
Goudarzi, M., Bahramian, M., Satarzadeh Tabrizi, M., Udo, E. E., Figueiredo, A. M., Fazeli, M., et al. (2017). Genetic diversity of methicillin resistant Staphylococcus aureus strains isolated from burn patients in Iran: ST239-SCCmec III/t037 emerges as the major clone. Microb. Pathog. 105, 1–7. doi: 10.1016/j.micpath.2017.02.004
Gu, F., He, W., Xiao, S., Wang, S., Li, X., Zeng, Q., et al. (2020). Antimicrobial resistance and molecular epidemiology of Staphylococcus aureus causing Bloodstream infections at Ruijin Hospital in Shanghai from 2013 to 2018. Sci. Rep. 10:6019. doi: 10.1038/s41598-020-63248-5
Gu, F. F., Chen, Y., Dong, D. P., Song, Z., Guo, X. K., Ni, Y. X., et al. (2016). Molecular epidemiology of Staphylococcus aureus among patients with skin and soft tissue infections in two chinese hospitals. Chinese Med. J. 129, 2319–2324. doi: 10.4103/0366-6999.190673
Gu, F. F., Hou, Q., Yang, H. H., Zhu, Y. Q., Guo, X. K., Ni, Y. X., et al. (2015). Characterization of Staphylococcus aureus isolated from non-native patients with skin and soft tissue infections in Shanghai. PLoS ONE 10:e0123557. doi: 10.1371/journal.pone.0123557
He, W. P., Gu, F. F., Zhang, J., Li, X. X., Xiao, S. Z., Zeng, Q., et al. (2021). Molecular characteristics and risk factor analysis of Staphylococcus aureus colonization put insight into CC1 colonization in three nursing homes in Shanghai. PLoS ONE 16:e0253858. doi: 10.1371/journal.pone.0253858
Institute CaLS. (2023). Performance Standards for Antimicrobial Susceptibility Testing. CLSI document M100-S29. Wayne, PA: Institute CaLS.
Jiang, B., Yin, S., You, B., Gong, Y., Huang, G., Yang, Z., et al. (2018). Antimicrobial resistance and virulence genes profiling of methicillin-resistant Staphylococcus aureus isolates in a burn center: a 5-year study. Microb. Pathog. 114, 176–179. doi: 10.1016/j.micpath.2017.11.020
Johnson, A. T., Nygaard, R. M., Cohen, E. M., Fey, R. M., and Wagner, A. L. (2016). The impact of a universal decolonization protocol on hospital-acquired methicillin-resistant Staphylococcus aureus in a burn population. J. Burn Care Res. 37, e525–e530. doi: 10.1097/BCR.0000000000000301
Kaiser, M. L., Thompson, D. J., Malinoski, D., Lane, C., and Cinat, M. E. (2011). Epidemiology and risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus among burn patients. J. Burn Care Res. 32, 429–434. doi: 10.1097/BCR.0b013e318217f92d
Kalligeros, M., Shehadeh, F., Karageorgos, S. A., Zacharioudakis, I. M., and Mylonakis, E. (2019). MRSA colonization and acquisition in the burn unit: a systematic review and meta-analysis. Burns 45, 1528–1536. doi: 10.1016/j.burns.2019.05.014
Kim, J. J., Blevins, M. W., Brooks, D. J., Stehle, J. R. Jr, McLouth, C. J., Viviano, J. P., et al. (2019). Successful control of a methicillin-resistant Staphylococcus aureus outbreak in a burn intensive care unit by addition of universal decolonization with intranasal mupirocin to basic infection prevention measures. Am. J. Infect. Control 47, 661–665. doi: 10.1016/j.ajic.2018.11.016
Lachiewicz, A. M., Hauck, C. G., Weber, D. J., Cairns, B. A., and van Duin, D. (2017). Bacterial infections after burn injuries: impact of multidrug resistance. Clin. Infect. Dis. 65, 2130–2136. doi: 10.1093/cid/cix682
Ladhani, H. A., Yowler, C. J., and Claridge, J. A. (2021). Burn wound colonization, infection, and sepsis. Surg. Infect. 22, 44–48. doi: 10.1089/sur.2020.346
Lakhundi, S., and Zhang, K. (2018). Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 31:e00020-18. doi: 10.1128/CMR.00020-18
Mahmoudi, H., Pourhajibagher, M., Chiniforush, N., Soltanian, A. R., Alikhani, M. Y., and Bahador, A. (2019). Biofilm formation and antibiotic resistance in meticillin-resistant and meticillin-sensitive Staphylococcus aureus isolated from burns. J. Wound Care 28, 66–73. doi: 10.12968/jowc.2019.28.2.66
Mater, M. E., Yamani, A. E., Aljuffri, A. A., and Binladen, S. A. (2020). Epidemiology of burn-related infections in the largest burn unit in Saudi Arabia. Saudi Med. J. 41, 726–732. doi: 10.15537/smj.2020.7.25141
Murray, C. K., Holmes, R. L., Ellis, M. W., Mende, K., Wolf, S. E., McDougal, L. K., et al. (2009). Twenty-five year epidemiology of invasive methicillin-resistant Staphylococcus aureus (MRSA) isolates recovered at a burn center. Burns 35, 1112–1117. doi: 10.1016/j.burns.2009.02.013
Patil, S., Dong, S., Sharma, D., Lopes, B. S., Hanafiah, A., Chen, X., et al. (2023). Molecular epidemiology and characterization of multidrug-resistant MRSA ST398 and ST239 in Himachal Pradesh, India. Infect. Drug Resist. 16, 2339–2348. doi: 10.2147/IDR.S409037
Savetamal, A. (2021). Infection in elderly burn patients: what do we know? Surg. Infect. 22, 65–68. doi: 10.1089/sur.2020.322
Silva, V., Monteiro, A., Pereira, J. E., Maltez, L., Igrejas, G., and Poeta, P. (2022). MRSA in humans, pets and livestock in Portugal: where we came from and where we are going. Pathogens 11:1110. doi: 10.3390/pathogens11101110
Zhang, J., Gu, F. F., Zhao, S. Y., Xiao, S. Z., Wang, Y. C., Guo, X. K., et al. (2015). Prevalence and molecular epidemiology of Staphylococcus aureus among residents of seven nursing homes in Shanghai. PLoS ONE 10:e0137593. doi: 10.1371/journal.pone.0137593
Keywords: methicillin-resistant Staphylococcus aureus, Staphylococcus aureus, burn wound infection, burns, epidemiology
Citation: Gu F, He W, Zhu D, Yang P, Sun J and Han L (2023) A 10-year retrospective study of methicillin-resistant Staphylococcus aureus from burn wound infection in southeast China from 2013 to 2022. Front. Microbiol. 14:1301744. doi: 10.3389/fmicb.2023.1301744
Received: 25 September 2023; Accepted: 13 November 2023;
Published: 01 December 2023.
Edited by:
Efthymia Giannitsioti, University General Hospital Attikon, GreeceReviewed by:
Xin Du, University of California, San Diego, United StatesShaimaa Mouftah, Zewail City of Science and Technology, Egypt
Copyright © 2023 Gu, He, Zhu, Yang, Sun and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Gu, Z3VmZWlmZWkxMTA3QDE2My5jb20=
†These authors have contributed equally to this work
 Feifei Gu
Feifei Gu Weiping He
Weiping He Dedong Zhu1,2†
Dedong Zhu1,2† Jingyong Sun
Jingyong Sun Lizhong Han
Lizhong Han