
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 05 January 2024
Sec. Food Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1299917
This article is part of the Research TopicMicrobial Communities of Traditional Fermented Beverages: Physiology, Metabolism and Interactions in Fermentative ProcessesView all 9 articles
 Jing Zhang1,2†
Jing Zhang1,2† Minhui Zhao3†
Minhui Zhao3† Jing Chen1,2†
Jing Chen1,2† Yuanting Zhu3
Yuanting Zhu3 Chen Xiao3
Chen Xiao3 Qi Li3
Qi Li3 Xiaoqi Weng3
Xiaoqi Weng3 Yunxuan Duan3
Yunxuan Duan3 Yong Zuo3*
Yong Zuo3*The quality of Baijiu was largely affected by raw materials, which determine the flavor and taste. In the present study, organic acids, polyphenols, volatile flavor components and microbial community in Hovenia acerba-sorghum co-fermented Baijiu (JP1) and pure sorghum-fermented Baijiu (JP2) were comprehensively analyzed. Organic acids, polyphenols and volatile flavor components in JP1 were more abundant than JP2. The abundance and diversity of bacteria and fungi in JP1 was higher than that in JP2 in the early stage of fermentation, but presented opposite trend in the middle and late stages. Leuconostoc, Lentilactobacillus and Issatchenkia were dominant genera in JP1. Whereas, Cronobacter, Pediococcus and Saccharomyces occupied the main position in JP2. Lentilactobacillus and Issatchenkia were positively related to most of organic acids and polyphenols. Pseudomonas, Rhodococcus, Cronobacter, Pediococcus, Brucella, Lentilactobacillus, Lactobacillus, Saccharomycopsis, Wickerhamomyces, Aspergillus, Thermomyces and unclassified_f—Dipodascaccae were associated with the main volatile flavor components. The main metabolic pathways in two JPs exhibited the variation trend of first decreasing and then increasing, and the metabolism activity in JP1 were higher than that in JP2. The results demonstrated the introduction of Hovenia acerba improved the functional ingredients and volatile flavor components, which is helpful for the quality promotion of Baijiu. This study identified the key microorganisms and discussed their effect on organic acids, polyphenols and volatile flavor components during the fermentation of Baijiu with different raw materials, providing a scientific basis for the development and production of high-quality Baijiu.
Baijiu, fermented from sorghum, corn, and other grains and with a brewing history of more than 2,000 years, fulfills a typical element of Chinese traditional culture (Li et al., 2017; Sha et al., 2017). Due to the differences in raw materials, the nutritional ingredients and volatile flavor components vary, further influencing the taste and quality of Baijiu. With the improvement in living standards, consumers pay more attention to the quality of products, and health has become the focus. However, Baijiu fermented from sole raw material was weak in flavor and nutrition. Additionally, present research on Baijiu mainly focuses on flavor, neglecting the functions of nutritional ingredients and their importance to health (Liu et al., 2012; Xiao et al., 2014; Zhao et al., 2018). Therefore, the development of the Baijiu industry should adhere to the dual orientation of flavor and health.
Hovenia acerba, a kind of tall tree of the Rhamnaceae family, is a source of ingredients including polysaccharides, amino acids, flavonoids, and fatty acids (Wang et al., 2017), providing functions such as liver protection, hypoglycemia, and anti-oxidation (Zhou et al., 2013). As a medicinal and edible plant, Hovenia acerba is widely applied in medicine, healthcare, and the food industry (Ma and Zhou, 2022). In recent years, as a kind of raw material, Hovenia acerba has been used in the production of Baijiu alongside sorghum (Minhui et al., 2023). Although research on raw material treatment and brewing technology optimization has been conducted to improve the quality of Hovenia acerba-sorghum co-fermented Baijiu, systematic discussions of nutritional ingredients and volatile flavor components and their correlation with the microbial community have not been reported.
With the rapid development of modern molecular biology and detection technology, high-throughput sequencing, gas chromatography–mass spectrometry (GC–MS), and high-performance liquid chromatography (HPLC) have been widely applied in the study of microbial diversity and flavor substances during the fermentation of Baijiu, enhancing the understanding of microbial community composition and characteristic flavor (Cai et al., 2021; Qian et al., 2021). The correlation between the microbial community and the flavor substances of strong aromatic Baijiu was studied by Zhang et al. Water and alcohol content had a great impact on the production of flavor compounds such as ethyl lactate, caproic acid, and ethyl caproate. Six bacterial genera and seven fungal genera exhibited a significant influence on caproic acid (Zhang et al., 2021). Based on redundancy analysis and the Mantel test, Lin et al. studied the correlation between microorganisms and metabolic substances during the fermentation of Xiaoqu Baijiu. The content of water, amino acid nitrogen, acids, and reducing sugar were significantly related to bacteria and fungi. Pichia, Rhizopus, Saccharomyces, and Wickerhamomyces were significantly related to the main alcohols, esters, and aldehydes, playing important roles in the formation of the flavor of Xiaoqu Baijiu (Li et al., 2022). The studies mentioned above provide reliable means for us to understand the succession of microbial communities and changes in flavor components in the process of Baijiu brewing. It is helpful for the correlation analysis of the microbial community, flavor substances, and bioactive components during the fermentation of Hovenia acerba-sorghum co-fermented Baijiu and conducive to in-depth research of its brewing mechanism.
In this study, the dynamics of physicochemical properties, organic acids, polyphenols, volatile flavor components, and microbial community during the fermentation of Hovenia acerba-sorghum co-fermented Baijiu and pure sorghum-fermented Baijiu were overall compared by combining physicochemical analysis, GC–MS, HPLC, and high-throughput sequencing. The interaction networks of the microbial community and their relationship with organic acids, polyphenols, and volatile flavor components were also revealed.
Sample preparation: the brewing technology of Hovenia acerba-sorghum co-fermented Baijiu was as follows:
Key points: (1) Hovenia acerba was cut into 1 cm segments using scissors and steamed for 40 min in a steamer. (2) Sorghum was crushed, then soaked in 70°C water for 1 h, and steamed for another hour. After that, sorghum was mixed with 40% sterile water (w/w), blended well, and cooled to room temperature. (3) 20% Hovenia acerba and 80% sorghum (w/w) were mixed with Daqu and transferred into a fermentation barrel for airtight fermentation at 28°C. Pure sorghum Baijiu was prepared using a similar method mentioned above by using sorghum as the sole raw material.
Sample collection: The fermented grain of Hovenia acerba-sorghum co-fermented Baijiu (marked as JP1) and the pure sorghum-fermented Baijiu (marked as JP2) were collected on the 0, 7, 14, 21, 28, and 35th days of fermentation.
The determination of volatile flavor components was carried out according to the report with minor modifications (Yang et al., 2023).
Headspace solid phase microextraction (HS-SPME): A total of 5 mL of sample was added into the headspace bottle with 2.5 g NaCl and 20 μL internal standard (2-octanol, 450 μg/mL). The bottle was heated in a 50°C thermostatic water bath for 5 min. The activated and balanced extraction head (aging at 250°C for 15 min) was inserted into the headspace bottle for 45 min.
GC condition: The chromatographic separation was performed on the DB-WAX Capillary column (60 m × 250 μm × 0.25 μm). Temperature procedure was as follows: (1) the initial temperature was kept at 50°C for 2 min; (2) it was increased to 60°C at a rate of 2°C/min and held for 1 min; (3) it was increased to 105°C at a rate of 3°C/min and held for 3 min; (4) it was increased to 180°C at a rate of 4°C/min and held for 3 min; and (5) it was increased to 230°C at a rate of 6°C/min and held for 1 min. The injection port temperature was 230°C. The carrier gas was highly pure He (99.999%) with a flow rate of 1 mL/min and no split flow.
MS condition: The MS was conducted in an electron ionization (EI) at 70 eV. The ion source temperature was 230°C. The interface temperature was 230°C.
The detection of organic acids was performed according to Zhang’s description (Zhang et al., 2023).
A total of 5 g of fermented grain was added to 20 mL of ultrapure water and then shaken and mixed well. Then, the sample was sonicated for 30 min and centrifuged at 7104 × g at 4°C for 5 min. The supernatant was obtained for further use after passing through the 0.22 μm nylon filter membrane.
HPLC conditions: The chromatographic separation was performed on an Agilent ZORBAX SB-Sq column (4.6 mm × 250 mm, 5 μm). The column temperature was 30°C. The mobile phase consisted of 95% KH2PO4 (0.05 M, pH = 2.54) and 5% methanol with a flow rate of 0.4 mL/min. The injection volume was 20 μL. The detection wavelength was 210 nm.
Qualitative and quantitative analysis: The standard solution of oxalic acid, tartaric acid, pyruvic acid, malic acid, lactic acid, citric acid, acetic acid, succinic acid, and fumaric acid was prepared with KH2PO4 solution. The qualitative analysis of organic acids was conducted by referring to the retention time of standards. The quantitative analysis of organic acids was carried out by calculating the peak area.
Polyphenols were detected by referring to Valero-Cases’s report (Valero-Cases et al., 2017).
The polyphenols were analyzed using high-performance liquid chromatography. 50 mg of fermented grain was added to a 10 mL 70% methanol solution and extracted using ultrasonication for 30 min, followed by centrifugation at 7104 × g for 15 min at 4°C. The supernatant was obtained by passing it through the 0.22 μm filter membrane.
HPLC conditions: The chromatographic separation was performed on an Agilent ZORBAX SB-Sq column (4.6 mm × 250 mm, 5 μm). The column temperature was 30°C. mobile phase A: 1% formic acid. Mobile phase B: 100% acetonitrile. The flow rate was 1 mL/min. The injection volume was 10 μL. The detection wavelength was 280 nm. The gradient elution procedure is shown in Supplementary Table S1.
In total, 10 mg of quercetin, dihydromyricetin, epicatechin, catechin, chlorogenic, vanillic acid, isovanillic acid, syringic acid, p-coumaric acid, rutin, caffeic acid, and ferulic acid were weighed separately and dissolved in 5 mL methanol (70%). The solution was then transferred into a 10-mL volumetric flask.
The standard solution was diluted step by step (50, 75, 100, 150, 250, and 500 μg/mL) and analyzed according to the chromatographic conditions. The standard curve and the regression equation for polyphenols were established by fitting the peak area and concentration of the standard substance. The qualitative analysis of polyphenols was conducted according to the retention time, and the quantitative analysis was carried out according to the peak area.
The community structure of bacteria and fungi in JP1 and JP2 was identified by high-throughput sequencing by referring to Jiang’s report (Jiang et al., 2023). For bacteria, the amplification region was the V3-V4 region with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAA-3′). The amplification region was ITS1 for fungi, and the primers were ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′). The high-throughput sequencing was conducted by Shanghai Meiji Biomedical Technology Co., Ltd., mainly including total DNA extraction, sample quality inspection, PCR amplification, product purification, library construction, and Illumina sequencing.
All experiments were repeated three times, and the results were expressed as mean ± standard deviations. Analysis of variance (ANOVA) was performed using SPSS 20.0 to evaluate the significance level. Pearson’s correlation analysis between microbial composition and biochemical components was performed by Origin2021.
The dynamics of the main physicochemical properties throughout the fermentation process are shown in Figure 1. During the fermentation, the water content in the two JPs modestly increased, possibly caused by the aerobic respiration of mold and yeast. Reducing sugar in JP1 presented an upward trend and reached its maximum on day 7, indicating the saccharification speed was faster than the consumption speed. After that, reducing sugar gradually decreased until the end of fermentation. It is worth noting that reducing sugar in JP1 is higher than that in JP2, due to the rich sugar content in Hovenia acerba (Yang et al., 2023). The initial acidity of JP1 was slightly higher than that of JP2 due to the addition of Hovenia acerba. In the early stage of fermentation, acidity increased rapidly, and pH presented the opposite trend due to the proliferation of acid-producing bacteria, such as Leuconostoc and Pediococcus (Figure 2). From the 7th to the 28th day, acidity and pH tend to be stable owing to the transformation of organic acids to esters through the esterification reaction, leading to the production and consumption of organic acids in dynamic equilibrium (Xu et al., 2017).
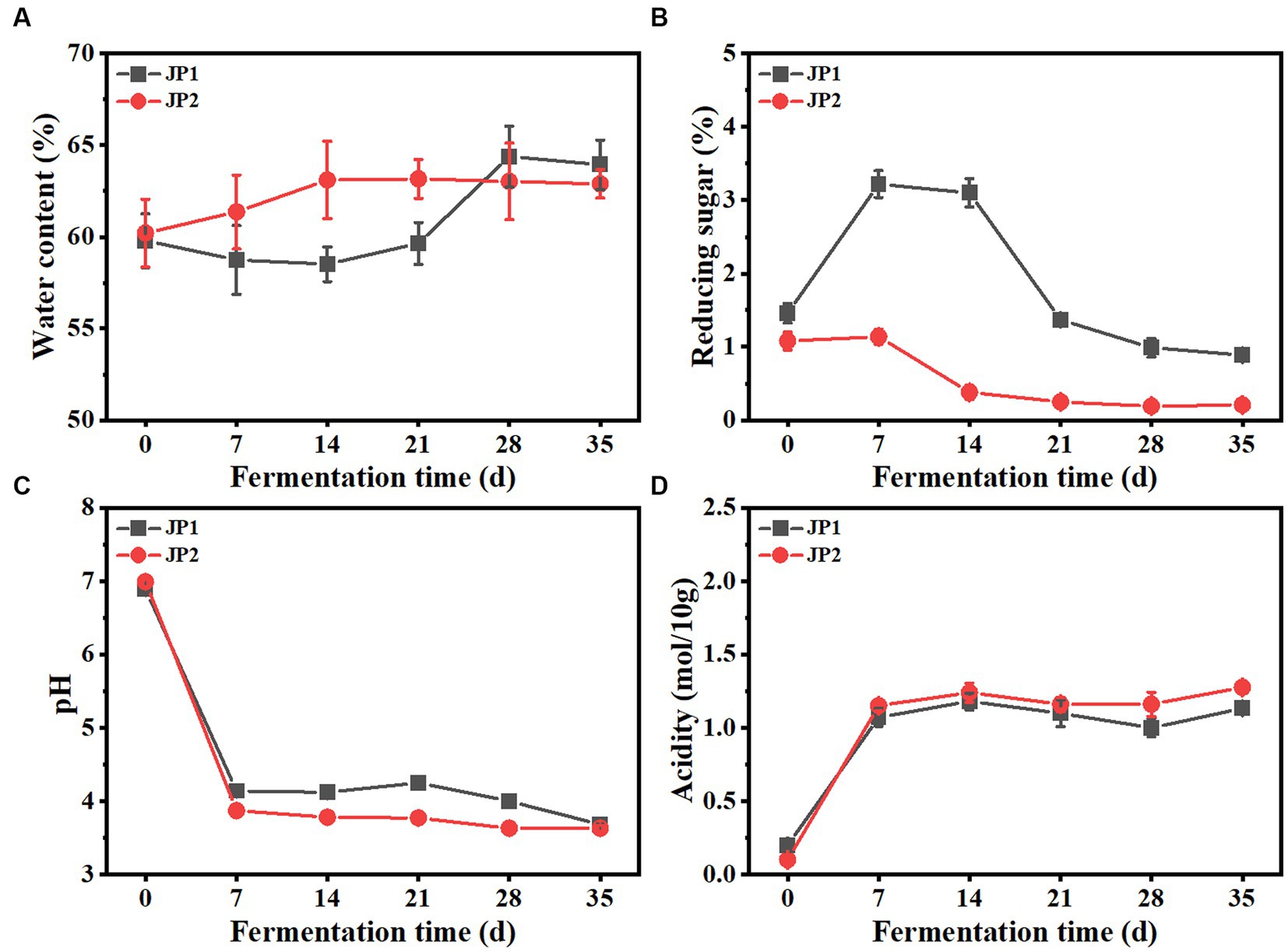
Figure 1. Dynamics of physicochemical properties during the fermentation of JPs. (A) Water content. (B) Reducing sugar. (C) pH. (D) Acidity.
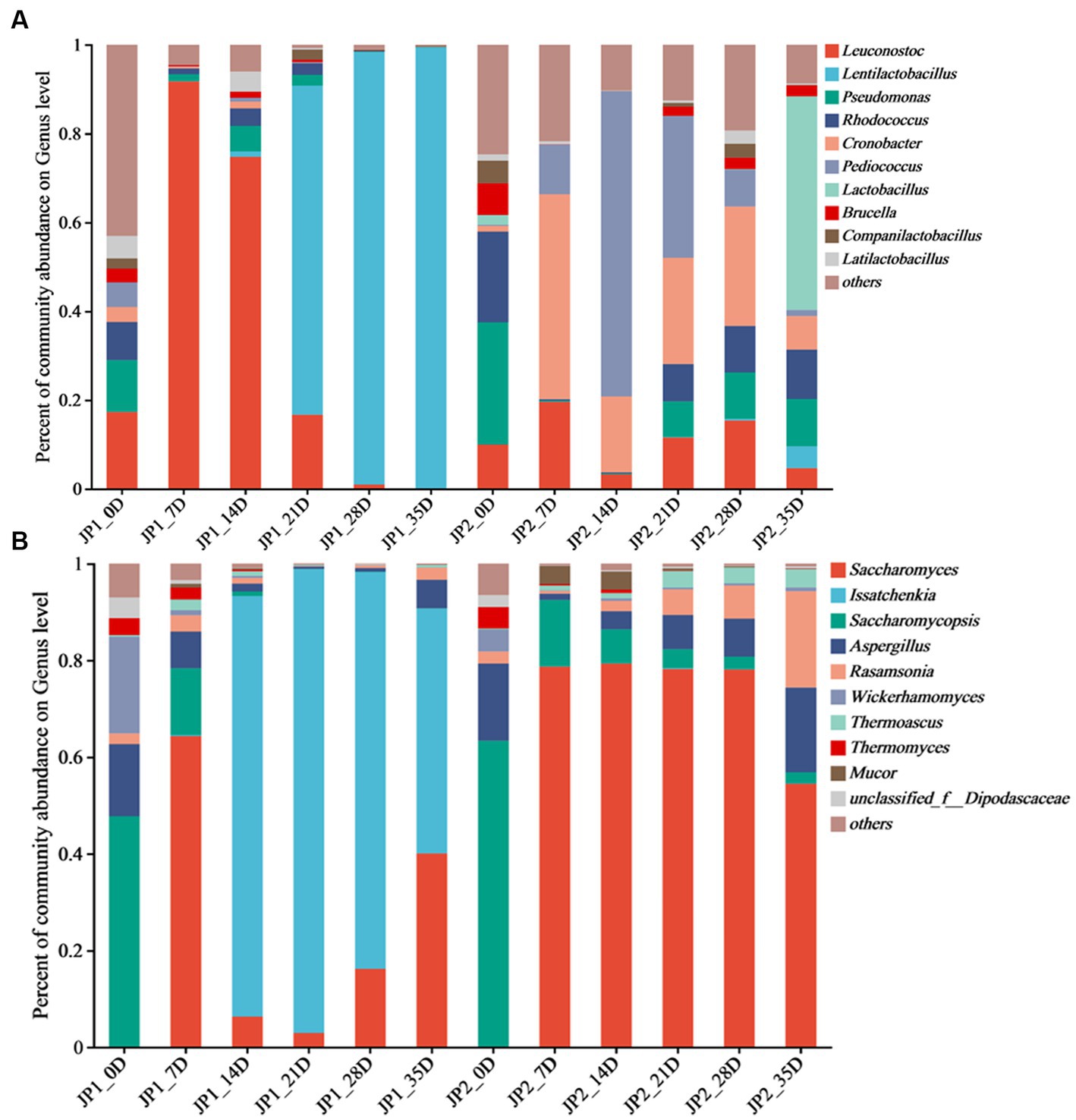
Figure 2. Distribution of microbial community at genus level during the fermentation of JPs. (A) 201 Bacteria. (B) Fungi.
Volatile flavor compounds were detected using HS-SPME-GC–MS. As shown in Figure 3A and Supplementary Figure S1A, a total of 50 kinds of components were observed in all samples, including 14 alcohols, 20 esters, 4 acids, 2 aldehydes, 4 ketones, 3 phenols, and 3 alkenes. The number of volatile flavor compounds in JP1 was higher than that in JP2. At the beginning of fermentation, benzyl alcohol, 1-heptyl acetate, ethyl caprate, isobutyric acid, butyric acid, hydroxyacetone, 2-heptanol, and 2-methoxy-4-vinylphenol were detected only in JP1, which may be derived from Hovenia acerba. As the fermentation proceeded, volatile flavor compounds continuously changed. Alcohols, acids, esters, aldehydes, and ketones transformed into each other, endowing Baijiu with a unique flavor (Xu et al., 2022). Principal component analysis based on the content of volatile flavor compounds showed that the flavor structure of two JPs significantly differentiated during the fermentation (Supplementary Figure S2).
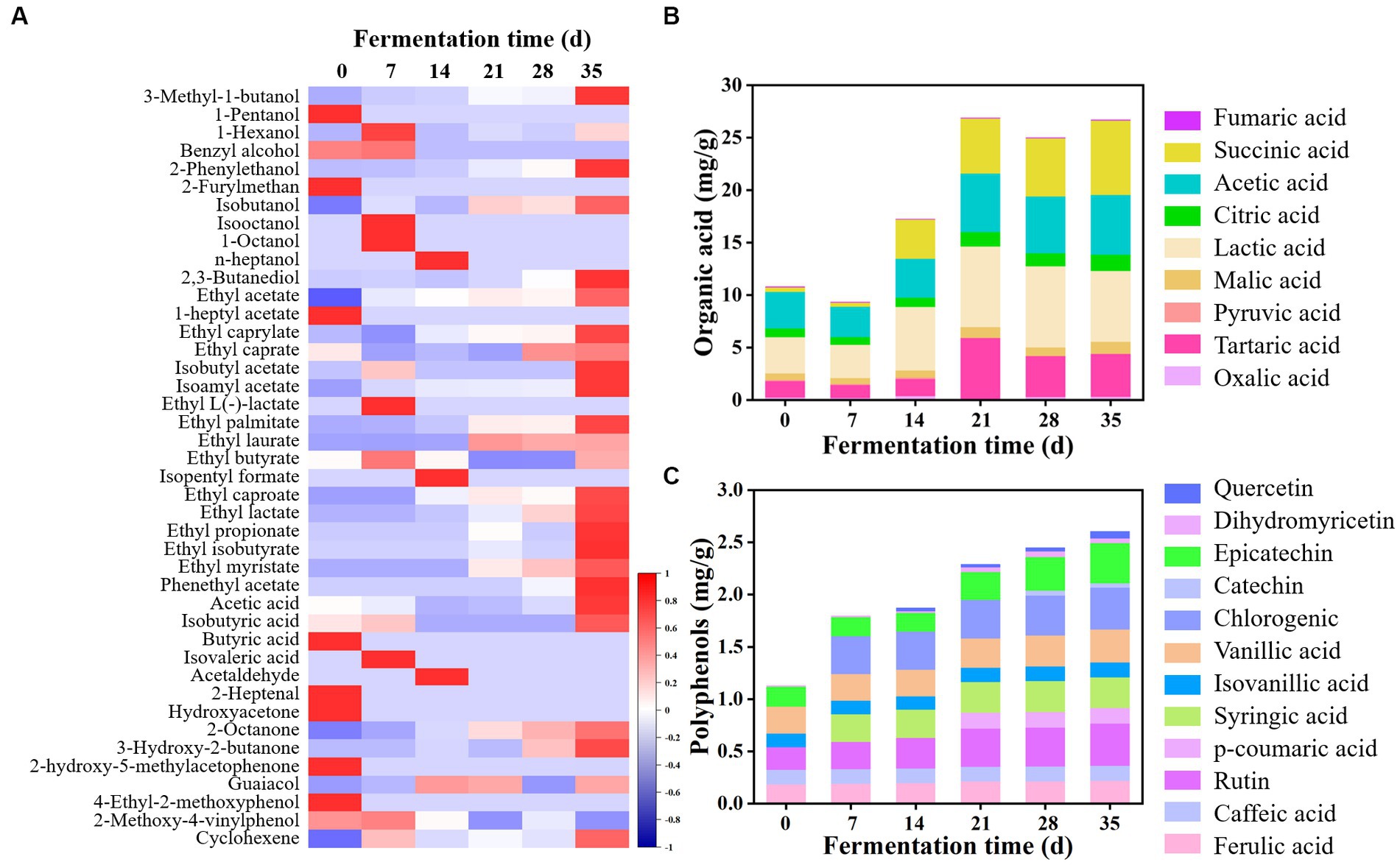
Figure 3. Dynamics of volatile flavor compounds, organic acids, and polyphenols during the fermentation of JP1. (A) Heatmap of volatile flavor compounds. The statistic of volatile flavor compounds had been produced by z-score. (B) Organic acids. (C) Polyphenols.
Esters were the most abundant volatile flavor compounds in two JPs, among which ethyl acetate was the dominant component. In addition, ethyl palmitate, ethyl lactate, ethyl propionate, phenyl ethyl acetate, ethyl isobutyrate, and ethyl caproate were also largely produced in JPs. It was worth noting that the content of ethyl acetate, ethyl butyrate, and ethyl caproate in JP1 was higher than that in JP2, probably because Lentilactobacillus and Issatchenkia largely existed in JP1 (Figure 2), resulting in the formation of large amounts of acids and esters (Qian et al., 2023). Higher alcohols in JP1 were higher than those in JP2, which may be derived from the hydrolysis of pectin, as well as the deamination and decarboxylation of amino acids in Hovenia acerba (Li et al., 2018). Research suggested that certain content of higher alcohols could increase the sense of harmony and fullness of Baijiu (Yang et al., 2014). Acetic acid was the main acid in Baijiu. As for aldehydes and ketones, 2-octanone and 3-hydroxy-2-butanone were relatively high in JP1. Furthermore, guaiacol and 4-ethyl-2-methoxyphenol also existed in both JPs.
Organic acids, one of the most important ingredients in Baijiu, not only endow Baijiu with a unique flavor but also inhibit the growth of undesired microorganisms during fermentation (Wei et al., 2020). As shown in Figure 3B and Supplementary Figure S1B, a total of nine kinds of organic acids were detected in two JPs. Organic acids were partly derived from raw materials. At the beginning of the fermentation, the total content of organic acids in JP1 and JP2 were 10.78 and 3.47 mg/g, respectively, indicating that Hovenia acerba is rich in organic acids. In addition, organic acids could also be produced by the metabolism of yeast and bacteria (Li et al., 2018; Sifeeldein et al., 2019). As the fermentation proceeded, organic acids increased and reached 26.68 and 24.84 mg/g in JP1 and JP2 on day 35, among which lactic acid, succinic acid, acetic acid, and citric acid were the dominant components.
Polyphenols are common bioactive substances in fermented food, exhibiting functions of anti-cancer, anti-inflammation, anti-oxidation, and anti-atherosclerosis (Stuper-Szablewska and Perkowski, 2019). As shown in Figure 3C and Supplementary Figure S1C, a total of eight kinds of polyphenols have been detected in raw materials, including ferulic acid, caffeic acid, rutin, isocyanic acid, vanillic acid, epicatechin, dihydromyricetin, and p-coumaric acid. Notably, dihydromyricetin was a unique component in Hovenia acerba, possessing functions such as reducing blood sugar and blood lipid, inhibiting liver cell deterioration, and preventing alcoholic liver disease (Song et al., 2022). It can also promote the growth of Lactobacillus and Lentilactobacillus (Dong et al., 2021) and inhibit the activity of Staphylococcus aureus, Salmonella, Escherichia coli, and Aspergillus flavus (Cui et al., 2021). During fermentation, polyphenols gradually increased and reached 2.60 and 2.27 mg/g at the end of fermentation, among which epicatechin, chlorogenic, vanillic acid, and rutin were the main components. Some new components were also produced, such as p-coumaric acid and catechin in JP1, and syringic acid and chlorogenic in JP2.
The microbial community structure was explored by high-throughput sequencing. A total of 526 bacterial OTUs and 113 fungal OTUs were obtained according to 97% sequence homology. As the diversity analysis shown in Table 1, the coverage of all samples was above 0.9995, indicating that the sequencing depth was sufficient to fully reveal the microbial community of JPs.
The difference in microbial community structure between two JPs during fermentation was studied using principal coordinate analysis (Supplementary Figure S3). At the beginning of the fermentation, the samples of JP1 and JP2 were close to each other, indicating that the initial microbial community structure of the two JPs was similar. As the fermentation proceeded, the microbial community structure of JP1 and JP2 began to differentiate. In JP1, the bacterial community has mainly changed from days 0 to 7 and 14 to 21. The change in fungi was observed in the early stage of fermentation. In JP2, the bacterial community structure continually changed throughout the fermentation. Fungi remained stable in the middle and late stages of fermentation.
At the phylum level, a total of 25 bacterial phyla were observed, among which Firmicutes, Proteobacteria, and Actinobacteriota presented a relative abundance of more than 1% (Supplementary Figure S4A). Throughout the fermentation process, the diversity of the microbial community declined, and Firmicutes was the dominant phylum (Li et al., 2011).
A total of 315 bacterial genera were found at the genus level. Leuconostoc, Lentilactobacillus, Pseudomonas, Rhodococcus, Cronobacter, Pediococcus, Lactobacillus, Brucella, Companilactobacillus, and Latilactobacillus were the top 10 abundant bacterial genera (Figure 2A). In the early stage of fermentation, the bacterial community structure in the two JPs was similar. Leuconostoc, Pseudomonas, and Rhodococcus were the main genera. Leuconostoc, which commonly exists in fermented foods such as pickles, became the dominant bacterial genera with a relative abundance of 91.8% in JP1 on day 7. As fermentation proceeded, the anaerobic environment gradually formed, and the acidity increased with the accumulation of lactic acid. Lentilactobacillus became the dominant genus in the middle and late stages of fermentation in JP1, with a relative abundance of 99.4% on day 35. Lactic acid and a variety of antibacterial substances were produced by Lentilactobacillus, inhibiting the growth of pathogens and spoilage microbes and leading to a significant reduction of bacterial diversity (Cappello et al., 2017). On day 7, the main bacteria in JP2 were Cronobacter (46.2%), Pediococcus (11.2%), and Leuconostoc (19.6%), whose relative abundance constantly changed during the fermentation. Lactobacillus was the dominant genus in the late stage of fermentation. The significant difference in bacterial community structure between two JPs may be caused by fermentation substrates and conditions. The dihydromyricetin in JP1 was beneficial for the growth and metabolism of Lentilactobacillus and Leuconostoc (Dong et al., 2021).
At the phylum level, six fungal phyla were detected in the two JPs. Ascomycota was the dominant phylum, with a relative abundance of more than 90%, followed by Mucoromycota and Basidiomycota (Supplementary Figure S4B).
A total of 66 fungal genera were observed at the genus level, among which Saccharomyces, Issatchenkia, Saccharomycopsis, Aspergillus, Rasamsonia, Mucor, Wickerhamomyces, Thermoascus, Thermomyces, and unclassified_f__Dipodascaceae were identified as the dominant genera (Figure 2B). At the beginning of the fermentation, Saccharomycopsis was the predominant fungus in the two JPs, accounting for 47.7 and 63.3%, respectively, followed by Aspergillus and Wickerhamomyces. Saccharomycopsis can secrete amylase, protease, and β-glucosidase to promote the saccharification of starch (Chen et al., 2020). Aspergillus, widely distributed in cereals, with strong protein decomposition and saccharification ability, can convert starch into fermentable sugar and further translate fermentable sugar into organic acids, amino acids, and esters (Liu et al., 2021). Wickerhamomyces, often found in fruit skin, can improve the flavor of Baijiu (Sun et al., 2022). According to the research, these fungi are important for the production of secondary metabolites, which contribute to enhancing the flavor of the Baijiu (Huang et al., 2018). Issatchenkia was the dominant fungus in JP1, and Saccharomyces was the most important fungus in JP2 in the middle and late stages of the fermentation. Compared with Saccharomyces, Issatchenkia was more tolerant to low acidity and could degrade malic acid and citric acid. In addition, Issatchenkia could promote the formation of esters and aromatic compounds (Wu et al., 2012).
Correlation analysis of microorganisms and physiochemical properties in Hovenia acerba-sorghum co-fermented Baijiu was conducted using redundancy analysis. As shown in Figure 4A and Supplementary Figure S5A, environmental factors exhibited distinct influences on bacteria and fungi. pH presented a great influence on microbial community distribution in the early stage of fermentation. In the middle stage of fermentation, reducing sugar became the main factor influencing the structure of the microbial community. At the end of fermentation, the microbial community structure was largely affected by water and acidity, which were consistent with the research of Xu (Xu et al., 2022). The bacterial genera Pseudomonas, Rhodococcus, and Latilactobacillus exhibited a positive correlation with pH (Chen et al., 2020). In addition, Lentilactobacillus and Leuconostoc presented a positive correlation between water content and reducing sugar, respectively. For fungi, Saccharomycopsis, Wicherhamomyces, and Aspergillus are located in the same quadrant with pH, indicating a strong correlation between them. Saccharomyces was related to reducing sugar due to its ability to produce various carbohydrate enzymes, especially amylase (Sun et al., 2010).

Figure 4. Correlation analysis of bacterial genera and physicochemical properties, and the co-occurrence network analysis of bacterial genera in JP1. (A) Correlation analysis of bacterial communities and physicochemical properties. (B) Co-occurrence network analysis of bacterial genera. Red and green represent positive and negative correlations, respectively. The thicker the line, the stronger the correlation.
To illustrate the beneficial or antagonistic relationship among microorganisms, the Spearman correlation coefficient and value of p were used to analyze the interaction among microorganisms at the genus level. As the results of the bacteria shown in Figure 4B, Lentilactobacillus, Enterobacter, and Weissella presented a close relationship with other species. Leuconostoc and Pseudomonas exhibited a maximum positive correlation with Acinetobacter and Cronobacter, respectively. Lentilactobacillus showed a maximum negative correlation with Weissella. Leuconostoc can rapidly produce lactic acid and acetic acid in the early stage of fermentation, reducing the pH (Zhou et al., 2023). However, along with the fermentation, acidity and ethanol increased, and the oxygen content decreased, inhibiting the growth of Leuconostoc. Lentilactobacillus was more resistant to acidity, ethanol, and low oxygen, completing the subsequent fermentation process, which was consistent with the fact that the relative abundance change of Leuconostoc and Lentilactobacillus is described in Figure 2A (Wang et al., 2008). For fungi (Supplementary Figure S5B), Wallemia, Saccharomycopsis, Cryptococcus_f_Tremellaceae, Pichia, and Wickerhamomyces possessed more nodes. Cryptococcus_f_Tremellaceae, Geotrichum, and Coniochaeta exhibited a maximum positive correlation with Wallemia, unclassified_f__Dipodascaceae, and Trichosporon. Aspergillus presented a negative relationship with Issatchenkia.
To explore the roles of microorganisms in the formation of organic acids, polyphenols, and volatile flavor compounds during Baijiu fermentation, correlation analysis was conducted.
The relationship of bacteria and fungi with organic acids and polyphenols in JP1 is shown in Figure 5 and Supplementary Figure S6. Lentilactobacillus and Issatchenkia were positively correlated with most organic acids and polyphenols. Lentilactobacillus plays an important role in the formation of organic acids in fermented food, including lactic acid, acetic acid, and citric acid. Issatchenkia might be involved in malic–lactic acid fermentation and transform malic acid and citric acid into lactic acid with a more mellow flavor (Qiu et al., 2022). Pyruvate was positively correlated with most of the bacteria and fungi genera.
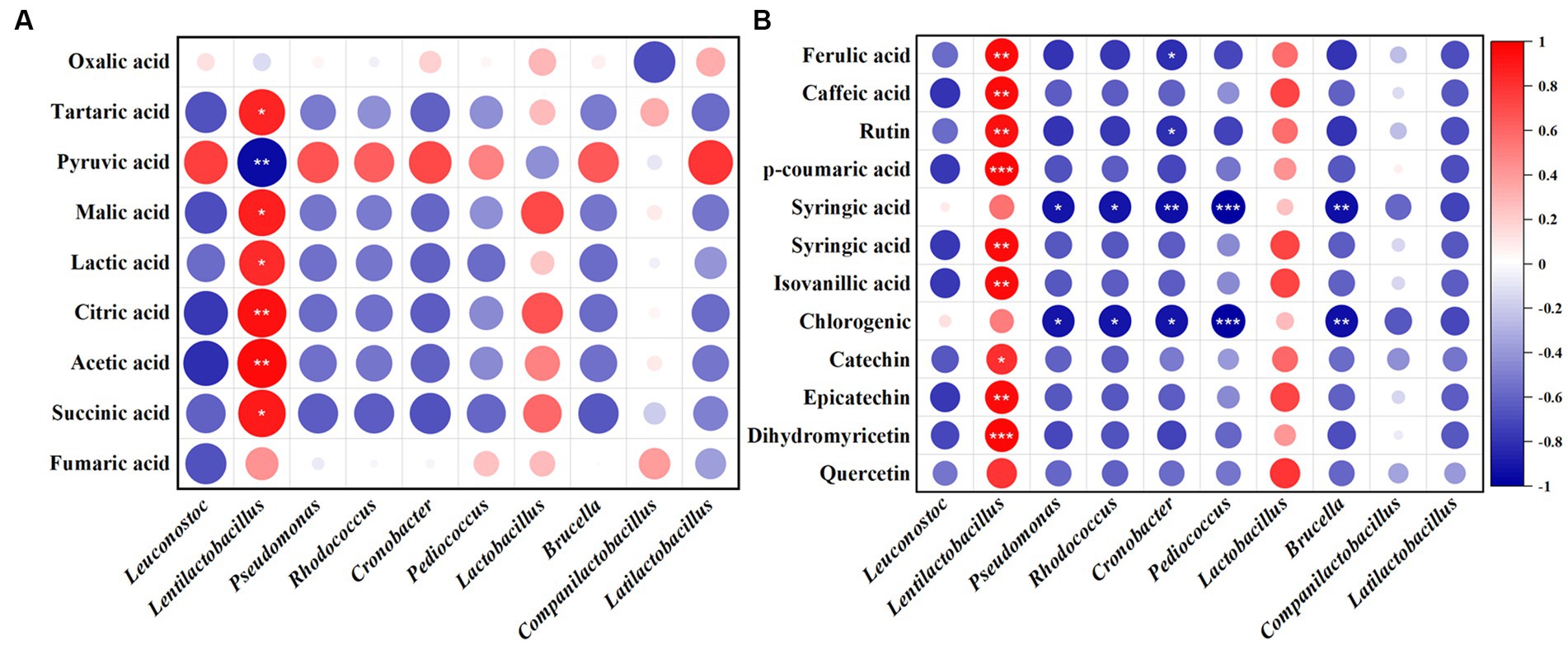
Figure 5. Correlation analysis of bacterial genera with organic acids and polyphenols in JP1. (A) Organic acids. (B) Polyphenols. Red and blue represent positive and negative correlations, respectively.
The correlation analysis of microorganisms and volatile flavor compounds in JP1 is shown in Figure 6 and Supplementary Figure S7. For bacteria, 1-pentanol, 2-furylmethan, 1-heptyl acetate, butyric acid, hydroxyacetone, and 2-heptanol were positively correlated with Pseudomonas, Rhodococcus, Cronobacter, Pediococcus, and Brucella. Ethyl myristate, 4-ethyl-2-methoxyphenol, guaiacol, and 2-octanone presented a positive correlation with Lentilactobacillus. Lactobacillus was positively related to the most volatile flavor compounds.
Fungi also play important roles in the formation of volatile flavor compounds. Saccharomycopsis, Wickerhamomyces, Aspergillus, Thermomyces, and unclassified_f__Dipodascaccae were positively correlated with 1-pentanol, 2-furylmethan, 1-heptyl acetate, butyric acid, hydroxyacetone, and 2-heptanol.
Compared with JP1, microorganisms presented a different influence on organic acids and polyphenols in JP2. As the relationship of microorganisms with organic acids and polyphenols shown in Supplementary Figures S8, S9, Lentilactobacillus and Lactobacillus were positively correlated with most organic acids and polyphenols. Rasamsonia, Thermoascus, and Saccharomyces exhibited a positive relationship with all organic acids and polyphenols, except for oxalic acid, fumaric acid, and catechin. The analysis of microorganisms and volatile flavor compounds in JP2 is shown in Supplementary Figures S10, S11. Pseudomonas, Rhodococcus, Brucella, Companilactobacillus, Saccharomycopsis, Wickerhamomyces, Thermomyces, and unclassified_f__Dipodascaceae presented positive relationships with 1-pentanol, 2-furylmethan, banana oil, 3-hydroxy-2-butanone, and 1-heptene. Thermoascus and Saccharomyces were positively related to most of the volatile flavor compounds.
From the results above, we can conclude that the different raw materials have different physicochemical properties and components. The fermentation conditions and components further affect the growth of microorganisms during the fermentation. Similarly, microorganisms can affect the production of components of organic acids, polyphenols, and volatile flavor compounds.
Finally, we predicted the metabolic function of the bacterial community in the two JPs with different raw materials. As shown in Figure 7, the top 20 third-order metabolic pathways in terms of abundance in the two JPs during the fermentation were screened, among which metabolic pathways, biosynthesis of secondary metabolites, microbial metabolism in diverse environments, biosynthesis of amino acids, and carbon metabolism occupied the main position. These main metabolic pathways exhibited a trend of decreasing in the early stage of fermentation and then increasing until the end of fermentation. Compared with JP2, the abundance of biosynthesis of secondary metabolites, biosynthesis of amino acids, and carbon metabolism was relatively higher than that in JP1, which may be caused by the difference in abundance and diversity of bacteria in two JPs (Figure 2). In JP1, Lentilactobacillus presented a positive relationship with all of the metabolic pathways as Lentilactobacillus was the most aboundance bacteria, which playing vital roles in the term of metabolic. For other bacteria, relatively small contribution was exhibited owing to the low abundance. In JP2, the main metabolic pathways exhibited a positive relationship with almost all bacteria except Leuconostoc, Cronobacter, and Pediococcus (Supplementary Figure S12). That was because the abundance and diversity of bacteria in JP2 were more balanced than in JP1.
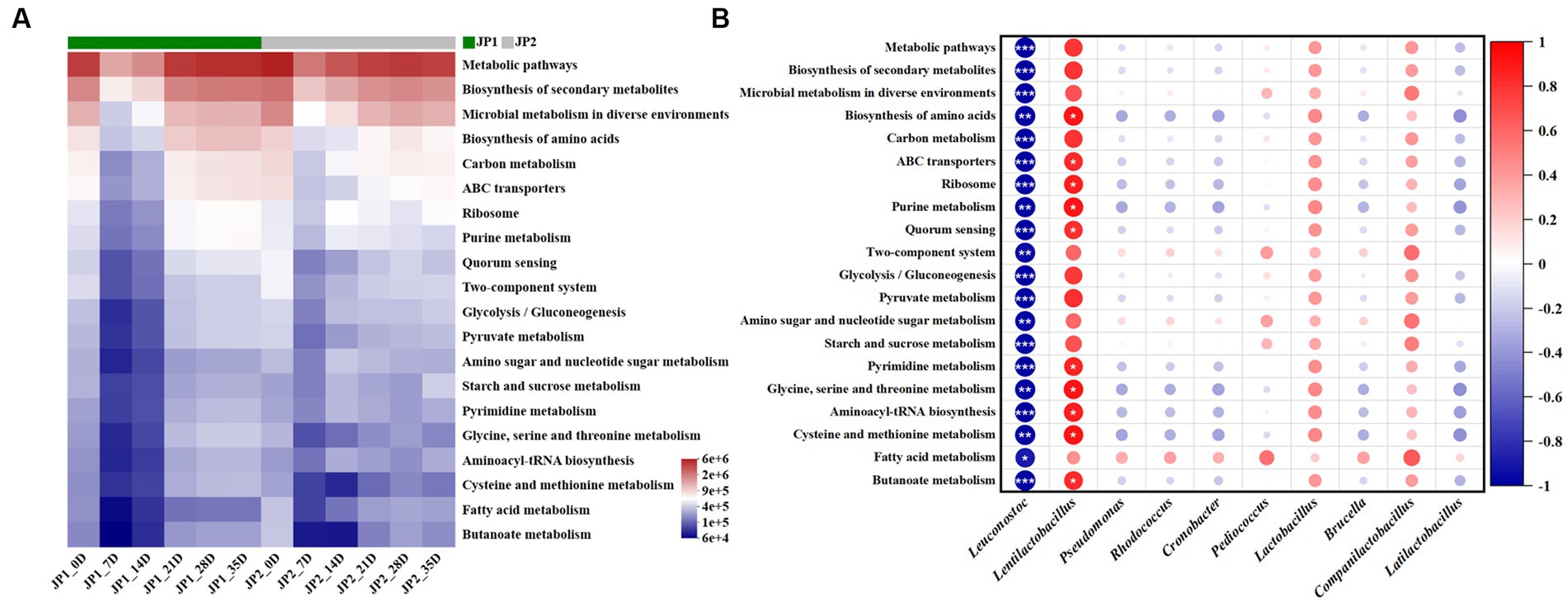
Figure 7. Metabolic functions prediction of the bacterial community. (A) Prediction of the metabolic functions of the bacterial community during the fermentation of JPs. (B) Correlation analysis of the main bacterial genera and metabolic pathways in JP1.
The results above exhibited that the difference in metabolic pathways existed in the two JPs, demonstrating that the different raw materials could promote the growth of specific bacteria, which further led to the difference in metabolism.
In this study, microbial community diversity and its correlation with physicochemical properties, organic acids, polyphenols, and volatile flavor components of Baijiu fermented from different raw materials were comprehensively researched. In Hovenia acerba-sorghum co-fermented Baijiu, organic acids, polyphenols, and volatile flavor components were abundant, which improved the nutritional value and health function of Baijiu. During the fermentation, four kinds of bacterial phyla were annotated, including Firmicutes, Proteobacteria, and Firmicutes. Leuconostoc was the dominant genus at the beginning of fermentation, and Lentilactobacillus was the main genus in the late stage of fermentation in JP1. Cronobacter and Pediococcus were the dominant genera in JP2. Issatchenkia and Saccharomyces were the dominant fungal genera in JP1 and JP2, respectively. Correlation analysis indicated that Lentilactobacillus and Issatchenkia were core functional microorganisms that played a crucial role in the bioconversion of organic acids and polyphenols. Pseudomonas, Rhodococcus, Cronobacter, Pediococcus, Brucella, Lentilactobacillus, Lactobacillus, Saccharomycopsis, Wickerhamomyces, Aspergillus, Thermomyces, and unclassified_f__Dipodascaccae exhibited a positive correlation with the main volatile flavor components. Furthermore, the main metabolic pathways in JP1 were more active than those in JP2. Overall, the understanding of microbial diversity as well as the correlation of microorganisms with physicochemical properties, organic acids, polyphenols, and volatile flavor components can lay the foundation for sustainable, high-quality Hovenia acerba-sorghum co-fermented Baijiu production.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JZ: Conceptualization, Methodology, Writing – original draft. MZ: Conceptualization, Writing – original draft. JC: Methodology, Writing – original draft. YuZ: Methodology, Writing – review & editing. CX: Investigation, Writing – review & editing. QL: Data curation, Writing – review & editing. XW: Conceptualization, Writing – original draft. YD: Supervision, Writing – review & editing. YoZ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Solid-state Fermentation Resource Utilization Key Laboratory of Sichuan Province (No. 2020GTY002), the Key Research and Development Project of Sichuan Province (2022YFN0056), and the Scientific and Technological Achievements Transfer and Transformation Demonstration Project of Sichuan Province (2021ZHCG0075).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1299917/full#supplementary-material
Cai, W., Wang, Y., Ni, H., Liu, Z., Liu, J., Zhong, J. A., et al. (2021). Diversity of microbiota, microbial functions, and flavor in different types of low-temperature Daqu. Food Res. Int. 150:110734. doi: 10.1016/j.foodres.2021.110734
Cappello, M. S., Zapparoli, G., Logrieco, A., and Bartowsky, E. J. (2017). Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 243, 16–27. doi: 10.1016/j.ijfoodmicro.2016.11.025
Chen, C., Liu, Y., Tian, H., Ai, L., and Yu, H. (2020). Metagenomic analysis reveals the impact of JIUYAO microbial diversity on fermentation and the volatile profile of Shaoxing-jiu. Food Microbiol. 86:103326. doi: 10.1016/j.fm.2019.103326
Cui, S.-M., Li, T., Liang, H.-Y., He, K.-K., Zheng, Y.-M., Tang, M., et al. (2021). Antibacterial activities and mechanisms of vine tea extract and 2R, 3R-dihydromyricetin on Escherichia coli. Lwt 146:111393. doi: 10.1016/j.lwt.2021.111393
Dong, S., Zhu, M., Wang, K., Zhao, X., Hu, L., Jing, W., et al. (2021). Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 171:105767. doi: 10.1016/j.phrs.2021.105767
Huang, Z. R., Hong, J. L., Xu, J. X., Li, L., Guo, W. L., Pan, Y. Y., et al. (2018). Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 76, 487–496. doi: 10.1016/j.fm.2018.07.014
Jiang, X., Peng, Z., Zhu, Q., Zheng, T., Liu, X., Yang, J., et al. (2023). Exploration of seasonal fermentation differences and the possibility of flavor substances as regulatory factors in Daqu. Food Res. Int. 168:112686. doi: 10.1016/j.foodres.2023.112686
Li, W., Chen, S.-J., Wang, J.-H., Zhang, C.-Y., Shi, Y., Guo, X.-W., et al. (2018). Genetic engineering to alter carbon flux for various higher alcohol productions by Saccharomyces cerevisiae for Chinese baijiu fermentation. App. Microbiol. Biotechnol. 102, 1783–1795. doi: 10.1007/s00253-017-8715-5
Li, J., Hou, C., Huo, D., Yang, M., Zhang, S., Ma, Y., et al. (2017). A minimalist Chinese liquor identification system based on a colorimetric sensor array with multiple applications. Anal. Meth. 9, 141–148. doi: 10.1039/c6ay02882a
Li, R. Y., Zheng, X. W., Zhang, X., Yan, Z., Wang, X. Y., and Han, B. Z. (2018). Characterization of bacteria and yeasts isolated from traditional fermentation starter (fen-Daqu) through a (1) H NMR-based metabolomics approach. Food Microbiol. 76, 11–20. doi: 10.1016/j.fm.2018.03.015
Li, X. R., Ma, E. B., Yan, L. Z., Meng, H., Du, X. W., Zhang, S. W., et al. (2011). Bacterial and fungal diversity in the traditional Chinese liquor fermentation process. Int. J. Food Microbiol. 146, 31–37. doi: 10.1016/j.ijfoodmicro.2011.01.030
Li, Y., Cheng, Y., Wang, H., Hu, X., Wang, L., and Huang, Y. (2022). Diverse structure and characteristics of the fungal community during the different rounds of Jiang-flavoured baijiu production in Moutai town. Lwt 161:113313. doi: 10.1016/j.lwt.2022.113313
Liu, M., Han, X., Tu, K., Pan, L., Tu, J., Tang, L., et al. (2012). Application of electronic nose in Chinese spirits quality control and flavour assessment. Food Cont. 26, 564–570. doi: 10.1016/j.foodcont.2012.02.024
Liu, X., Bai, W., Zhao, W., Qian, M., and Dong, H. (2021). Correlation analysis of microbial communities and precursor substances of ethyl carbamate (EC) during soy sauce fermentation. Lwt 152:112288. doi: 10.1016/j.lwt.2021.112288
Ma, X., and Zhou, H. (2022). Hovenia acerba Lindl.: an insight into botany, phytochemistry, bioactivity, quality control, and exploitation. J. Food Biochem. 46:e14434. doi: 10.1111/jfbc.14434
Minhui, Z., Yuan, Y., Qiang, Z., Jing, C., and Yong, Z. (2023). Optimization of brewing Technology of Hovenia acerba-Sorghum co-fermented distilled liquor and its characteristic flavor substances and antioxidant activity in vitro. Sci. Technol. Food Ind. 44, 224–232. doi: 10.13386/j.issn1002-0306.2022030118
Qian, M., Ruan, F., Zhao, W., Dong, H., Bai, W., Li, X., et al. (2023). The dynamics of physicochemical properties, microbial community, and flavor metabolites during the fermentation of semi-dry Hakka rice wine and traditional sweet rice wine. Food Chem. 416:135844. doi: 10.1016/j.foodchem.2023.135844
Qian, W., Lu, Z. M., Chai, L. J., Zhang, X. J., Li, Q., Wang, S. T., et al. (2021). Cooperation within the microbial consortia of fermented grains and pit mud drives organic acid synthesis in strong-flavor baijiu production. Food Res. Int. 147:110449. doi: 10.1016/j.foodres.2021.110449
Qiu, S., Chen, K., Liu, C., Wang, Y., Chen, T., Yan, G., et al. (2022). Non-saccharomyces yeasts highly contribute to characterisation of flavour profiles in greengage fermentation. Food Res. Int. 157:111391. doi: 10.1016/j.foodres.2022.111391
Sha, S., Chen, S., Qian, M., Wang, C., and Xu, Y. (2017). Characterization of the typical potent odorants in Chinese roasted sesame-like flavor type liquor by headspace solid phase microextraction-aroma extract dilution analysis, with special emphasis on Sulfur-containing odorants. J. Agric. Food Chem. 65, 123–131. doi: 10.1021/acs.jafc.6b04242
Sifeeldein, A., Wang, S., Li, J., Dong, Z., Chen, L., Kaka, N. A., et al. (2019). Phylogenetic identification of lactic acid bacteria isolates and their effects on the fermentation quality of sweet sorghum (Sorghum bicolor) silage. J. Appl. Microbiol. 126, 718–729. doi: 10.1111/jam.14123
Song, Y., Sun, L., Ma, P., Xu, L., and Xiao, P. (2022). Dihydromyricetin prevents obesity via regulating bile acid metabolism associated with the farnesoid X receptor in Ob/Ob mice. Food Funct. 13, 2491–2503. doi: 10.1039/d1fo03971g
Stuper-Szablewska, K., and Perkowski, J. (2019). Phenolic acids in cereal grain: occurrence, biosynthesis, metabolism and role in living organisms. Crit. Rev. Food Sci. Nutr. 59, 664–675. doi: 10.1080/10408398.2017.1387096
Sun, H., Zhao, P., Ge, X., Xia, Y., Hao, Z., Liu, J., et al. (2010). Recent advances in microbial raw starch degrading enzymes. Appl. Biochem. Biotechnol. 160, 988–1003. doi: 10.1007/s12010-009-8579-y
Sun, N., Gao, Z., Li, S., Chen, X., and Guo, J. (2022). Assessment of chemical constitution and aroma properties of kiwi wines obtained from pure and mixed fermentation with Wickerhamomyces anomalus and Saccharomyces cerevisiae. J. Sci. Food Agric. 102, 175–184. doi: 10.1002/jsfa.11344
Valero-Cases, E., Nuncio-Jauregui, N., and Frutos, M. J. (2017). Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 65, 6488–6496. doi: 10.1021/acs.jafc.6b04854
Wang, H.-Y., Zhang, X.-J., Zhao, L.-P., and Xu, Y. (2008). Analysis and comparison of the bacterial community in fermented grains during the fermentation for two different styles of Chinese liquor. J. Ind. Microbiol. Biotechnol. 35, 603–609. doi: 10.1007/s10295-008-0323-z
Wang, M., Liu, Y., Qiang, M., and Wang, J. (2017). Structural elucidation of a pectin-type polysaccharide from Hovenia dulcis peduncles and its proliferative activity on RAW264.7 cells. Int. J. Biol. Macromol. 104, 1246–1253. doi: 10.1016/j.ijbiomac.2017.07.004
Wei, Y., Zou, W., Shen, C. H., and Yang, J. G. (2020). Basic flavor types and component characteristics of Chinese traditional liquors: a review. J. Food Sci. 85, 4096–4107. doi: 10.1111/1750-3841.15536
Wu, Q., Xu, Y., and Chen, L. (2012). Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavour liquor making. Lett. Appl. Microbiol. 55, 301–307. doi: 10.1111/j.1472-765X.2012.03294.x
Xiao, Z., Yu, D., Niu, Y., Chen, F., Song, S., Zhu, J., et al. (2014). Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 945–946, 92–100. doi: 10.1016/j.jchromb.2013.11.032
Xu, S., Zhang, M., Xu, B., Liu, L., Sun, W., Mu, D., et al. (2022). Microbial communities and flavor formation in the fermentation of Chinese strong-flavor baijiu produced from old and new Zaopei. Food Res. Int. 156:111162. doi: 10.1016/j.foodres.2022.111162
Xu, Y., Sun, B., Fan, G., Teng, C., Xiong, K., Zhu, Y., et al. (2017). The brewing process and microbial diversity of strong flavour Chinese spirits: a review. J. Inst. Brew. 123, 5–12. doi: 10.1002/jib.404
Xu, Y., Zhao, J., Liu, X., Zhang, C., Zhao, Z., Li, X., et al. (2022). Flavor mystery of Chinese traditional fermented baijiu: the great contribution of ester compounds. Food Chem. 369:130920. doi: 10.1016/j.foodchem.2021.130920
Yang, D., Luo, X., and Wang, X. (2014). Characteristics of traditional Chinese shanlan wine fermentation. J. Biosci. Bioeng. 117, 203–207. doi: 10.1016/j.jbiosc.2013.07.010
Yang, L., Chen, R., Liu, C., Chen, L., Yang, F., and Wang, L. (2023). Spatiotemporal accumulation differences of volatile compounds and bacteria metabolizing pickle like odor compounds during stacking fermentation of Maotai-flavor baijiu. Food Chem. 426:136668. doi: 10.1016/j.foodchem.2023.136668
Yang, Z., Chen, H., Lin, C., Sun, J., Wen, W., Zhu, X., et al. (2023). Comprehensive evaluation of quality traits of Hovenia acerba germplasm resources in Fujian Province. Forests 14:204. doi: 10.3390/f14020204
Zhang, J., Zhao, M., Yi, Y., Huang, Y., Yin, Q., and Zuo, Y. (2023). Exploration of microbial community diversity and bioactive substances during fermentation of mulberry Jiaosu, an edible naturally fermented mulberry product. Fermentation 9:910. doi: 10.3390/fermentation9100910
Zhang, M., Wu, X., Mu, D., Xu, B., Xu, X., Chang, Q., et al. (2021). Profiling the influence of physicochemical parameters on the microbial community and flavor substances of zaopei. J. Sci. Food Agric. 101, 6300–6310. doi: 10.1002/jsfa.11299
Zhao, D., Shi, D., Sun, J., Li, A., Sun, B., Zhao, M., et al. (2018). Characterization of key aroma compounds in Gujinggong Chinese baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 105, 616–627. doi: 10.1016/j.foodres.2017.11.074
Zhou, Q., Zheng, Z., Wu, Y., Zhang, X., Jia, Z., Zhong, K., et al. (2023). Unraveling the core bacterial community responsible for quality and flavor improvement of the radish paocai during spontaneous fermentation. Food Biosci. 55:102956. doi: 10.1016/j.fbio.2023.102956
Keywords: microbial community, organic acids, polyphenols, volatile flavor components, correlation analysis
Citation: Zhang J, Zhao M, Chen J, Zhu Y, Xiao C, Li Q, Weng X, Duan Y and Zuo Y (2024) The improvement of Hovenia acerba-sorghum co-fermentation in terms of microbial diversity, functional ingredients, and volatile flavor components during Baijiu fermentation. Front. Microbiol. 14:1299917. doi: 10.3389/fmicb.2023.1299917
Received: 27 September 2023; Accepted: 07 December 2023;
Published: 05 January 2024.
Edited by:
Christian Ariel Lopes, Institute for Research and Development in Process Engineering, Biotechnology and Alternative Energies (CONICET PROBIEN), ArgentinaReviewed by:
Yongsheng Tao, Northwest A&F University, ChinaCopyright © 2024 Zhang, Zhao, Chen, Zhu, Xiao, Li, Weng, Duan and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zuo, c2d6dW95b25nQHRvbS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.