- 1Fermentation Science and Metabolic Engineering Group, Department of Food Science, University of Wisconsin-Madison, Madison, WI, United States
- 2Biotechnology Program, Department of Chemistry and Chemical Biology, The Roux Institute, Northeastern University, Portland, ME, United States
Although anaerobic digestate contains >90% water, the high nutrient content of digestate makes it economically and technically intractable to treatment by existing wastewater treatment technologies. This study separately assessed the feasibility of nutrient removal from digestate by Rhizopus delemar DSM 905 and a culture of phosphate-accumulating organisms (PAOs). With Rhizopus delemar DSM 905, we investigated concomitant nutrient removal from digestate-supplemented medium and fumaric acid production, as a potentially economical strategy for digestate treatment. Following the cultivation of R. delemar DSM 905 in a fermentation medium containing 25% (v/v) digestate, the concentrations of Al, Cr, Cu, Fe, K, Mg, Mn, Pb, and Zn reduced 40, 12, 74, 96, 12, 26, 23%, ~18, and 28%, respectively. Similarly, the concentrations of total phosphorus, total nitrogen, phosphate (PO4-P), ammonium (NH4-N), nitrate (NO3-N), and sulfur decreased 93, 88, 97, 98, 69, and 13%, respectively. Concomitantly, cultures supplemented with 25 and 15% (v/v) digestate produced comparable titers of fumarate (~11 and ~ 17 g/L, respectively) to the digestate un-supplemented control cultures. With PAOs, we assessed the removal of total phosphorus, total nitrogen, PO4-P, and NH4-N, of which the concentrations reduced 86, 90%, ~99, and 100%, respectively in 60% (v/v) digestate. This study provides additional bases for microbial removal of excess nutrients from anaerobic digestate, with the potential to engender future water recovery from this waste stream that is currently largely recalcitrant to treatment.
1 Introduction
Although anaerobic digestion (AD) represents an excellent technology for treating organic wastes (Fagbohungbe et al., 2017; Kumar et al., 2022), the waste derived from AD [i.e., anaerobic digestate (ADE)] is increasingly becoming a burden on the AD industry and the environment. This is because, ADE produced by large AD facilities outstrip local fertilizer demands. Consequently, ADE is commonly stored in lagoons, a practice that poses considerable hazard to the environment. In addition to contributing to the emission of greenhouse gases (GHGs) due to continued release of methane and CO2 from lagoons in the event of flooding, ADE introduces substantial amounts of ammonium and phosphate into the environment, which engender eutrophication, an increasingly worsening environmental challenge (Paerl and Barnard, 2020; Ralston and Moore, 2020). Transporting heavy, water-replete ADE from where it is produced to where it may be needed as fertilizer is expensive, with the attendant emission of CO2. Depending on the AD feedstock, fresh ADE can contain over 3,000 and 5,000 mg/L ammonium and phosphate, respectively (Paerl and Barnard, 2020; Kumar et al., 2022), whereas municipal sewage contains about 40 and 20 mg/L, respectively. Consequently, ADE is technically and economically intractable to treatment by standard wastewater treatment technologies. Additionally, prolonged/heavy application of ADE on land as fertilizer exerts toxicological effects on soil microbes, which adversely affects vital nitrifying bacteria (Franchino et al., 2016; Bankston et al., 2020). ADE typically contains high levels of sodium. As a result, when extensively applied on agricultural land as fertilizer, ADE increases soil salinity and electrical conductivity thereby damaging the hydraulic properties of soil (Voelkner et al., 2015; Holatko et al., 2021). Similarly, prolonged or heavy application of ADE on arable land has been shown to increase soil hydrophobicity, which impairs seed germination (Wallach, 2010). Asides sodium, land application of ADE increases the overall risk of overloading arable land with metals—including heavy metals—and non-metals (Golovko et al., 2022; Baldasso et al., 2023; Wang et al., 2023). This increases the risk of heavy metal leaching into groundwater and the transfer of heavy metals to humans and/or animals through food crops.
There are growing efforts therefore, to develop cost-effective and environmentally benign approaches for ADE management (Campos et al., 2019; Ujor et al., 2020; Kumar et al., 2022; Okonkwo et al., 2023). With >90% water content, a sustainable ADE management approach should involve water recovery. Increasing impacts of climate change, particularly, mounting pressure on fresh water resources call for deft measures to recover water from water-replete wastes—such as ADE—other than municipal sewage in the future. Although different methods have been investigated for ADE treatment, cost remains a major impediment to real-world application of such methods (Campos et al., 2019; Herbes et al., 2020). In this study, we investigated two approaches for nutrient removal from the liquid fraction of ADE. In the first approach, we assessed supplementation of the liquid fraction of ADE as a source of minerals during fumarate production by R. delemar DSM 905 (hereafter referred to as R. delemar). R. delemar was chosen for this study because of the ability of Rhizopus species to thrive on diverse substrates and environments (Shimada et al., 1983; Pranamuda et al., 1995; Mousavi et al., 2023). Additionally, R. delemar produces fumaric acid, an important industrial chemical building block with numerous applications in synthesizing pharmaceuticals, resins and polyamides, and more recently, as a supplement in cattle feed, that leads to ~70% reduction in methane emission (Das and Brar, 2014; Jiménez-Quero et al., 2016; Karaffa et al., 2021). Therefore, we rationalized that cultivation of R. delemar in ADE-based medium would reduce the ADE nutrient load, particularly ammonium (NH4+), total nitrogen (N), phosphate (PO4-P), total phosphorus (P), sulfur (S), nitrate (NO3-N), copper (Cu), chromium (Cr), and lead (Pb). In the second approach, a culture of phosphate-accumulating organisms (PAOs) was adapted to high phosphate concentrations and then, grown in PO4-P - and NH4+-replete ADE, as a means for reducing the PO4-P and NH4+ concentrations of the ADE.
2 Materials and methods
2.1 Fumarate fermentation coupled to nutrient sequestration from ADE
R. delemar was procured from the German culture collection (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig-Süd, Germany). The City of Madison, Wisconsin (United States) community AD facility kindly provided the ADE used in this study. A flowchart of the various treatments adopted for fumarate production in ADE-supplemented cultures are presented in Figure 1. The liquid fraction of the ADE was obtained by centrifuging the ADE five times at 3,000 × g for 30 min in a Jouan CR 312 centrifuge (Thermo Scientific, Waltham, MA, United States). Afterwards, the resulting liquid fraction of the ADE was incubated at 80°C for 12 h in a Thermo Scientific Heratherm incubator oven (Waltham, MA, United States) to eliminate residual microbial cells. Sterilization at higher temperatures led to precipitation of the ADE constituents. Different fermentation media were formulated to initially determine the ideal rate of ADE supplementation. Subsequently, the predetermined ADE supplementation rates were used in batch fermentation. To determine the optimum ADE supplementation rate in the fumarate fermentation medium, a preliminary experiment was set up with different concentrations of ADE. R. delemar was grown in media containing a final glucose concentration of 60 g/L supplemented with 25, 50, and 75% (v/v) ADE liquid fraction without buffering. The media were aseptically inoculated with mycelial suspension prepared by cutting mycelial-agar plugs from the front of actively spreading mycelia on potato dextrose agar. Approximately 10 mycelial-agar plugs excised with sterile scalpel blade were placed in sterile 50 mL tubes containing 12 mL of sterile distilled water and approximately 2.5 g of sterile glass beads (Fisher Scientific, Waltham MA, United States). The tubes were shaken vigorously for 2–3 min to shred the mycelia mat into a suspension. Afterwards, 100 mL of each medium above was inoculated with 2 mL of mycelial suspension in sterile 250 mL Erlenmeyer flasks plugged with sterile foam bung. The cultures were incubated for 84 h in a MaxQ 4,450 shaker incubator (Fisher Scientific, Waltham MA, United States) at 150 rpm and 30°C. The cultures were not buffered to determine if the ADE provided a degree of buffering. Non-buffered cultures containing glucose (60 g/L) with and without minerals [ZnSO4.7H2O (0.088 g/L), KH2PO4 (0.6) and MgSO4 (0.25)] were inoculated and incubated as the ADE-containing cultures as controls.
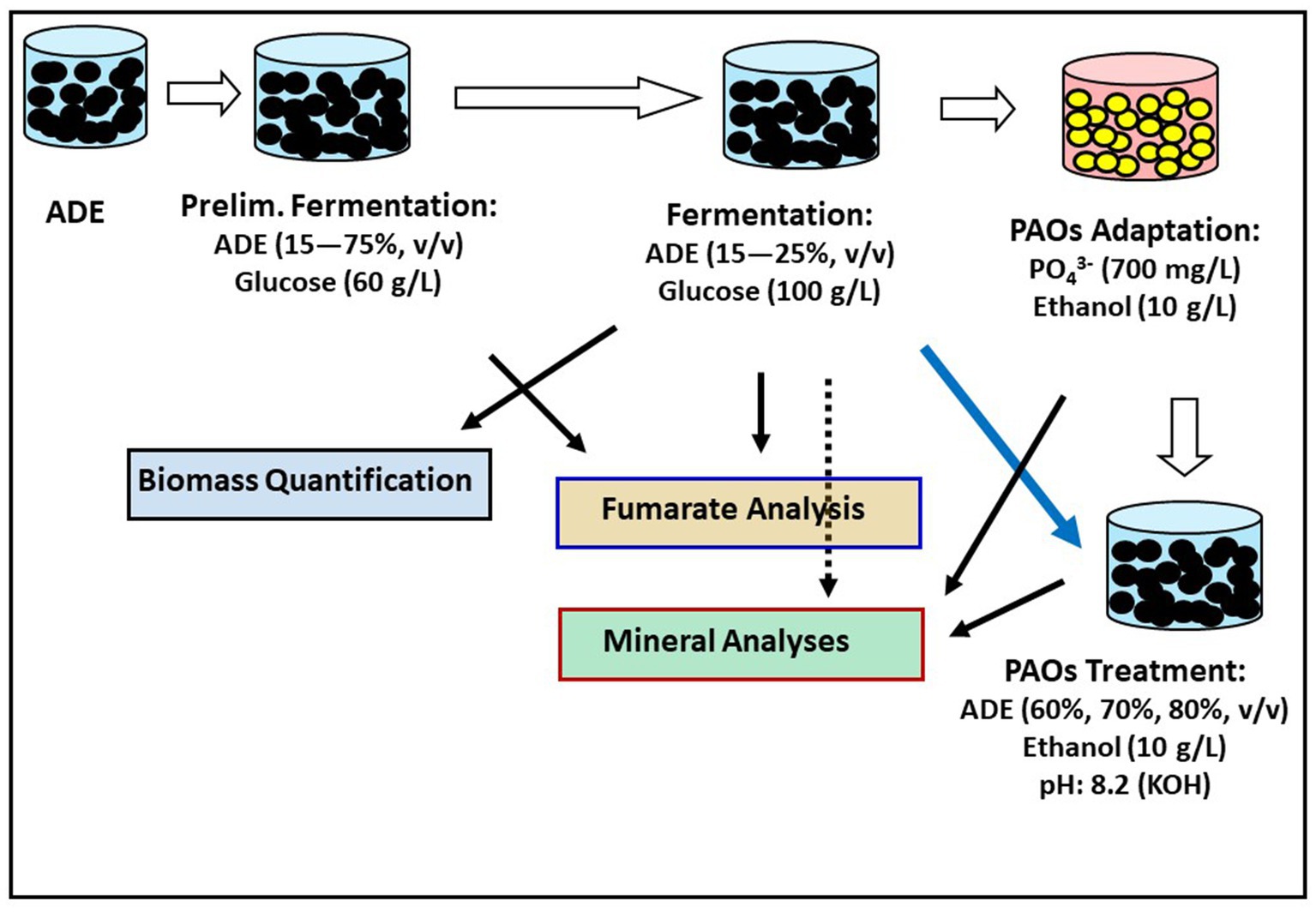
Figure 1. Flowchart of various treatments deployed to assess fumarate production and nutrient removal from ADE-supplemented cultures.
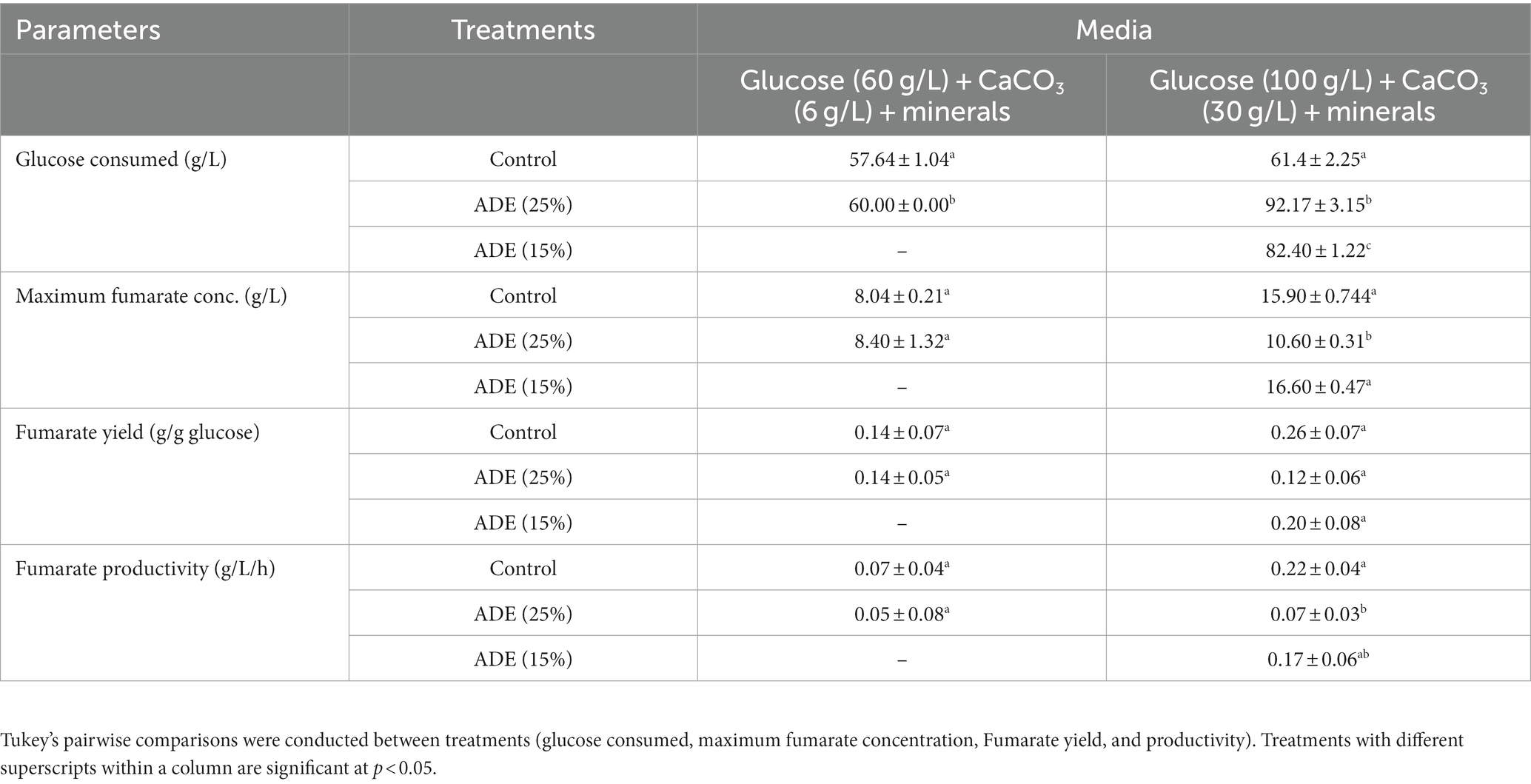
R. delemar cultures supplemented with 25% (v/v) ADE produced the highest amount of fumarate. Therefore, subsequent fermentations were supplemented with 25% (v/v) ADE, except where stated otherwise. For fumarate fermentation, both the test (ADE-supplemented) and control media contained glucose (60 g/L) and yeast extract (1.0 g/L). Subsequent analysis of the mineral constituents of the batch of ADE used during fumarate fermentation revealed a low zinc concentration, and zinc has been shown to play an important role in the growth of R. delemar (Wilson, 2015). Hence, the ADE-containing cultures were supplemented with ZnSO4.7H2O to the same concentration as the control cultures (0.088 g/L). Additionally, our analysis showed that the ADE medium was low on phosphate. Thus, the ADE-containing medium was supplemented with 0.25 g/L KH2PO4 as a source of phosphate. The control medium was prepared according the recipe described by Sebastian et al. (2021), which contained (in g/L): glucose (60), yeast extract (1.0), KH2PO4 (0.6), MgSO4 (0.25), and ZnSO4.7H2O (0.088). Both the control and the test (ADE-containing) media were buffered with CaCO3 (6.0 g/L). Fermentation cultures were aseptically inoculated with the mycelial suspension of R. delemar as described earlier. The cultures were incubated in a MaxQ 4,450 shaker incubator (Fisher Scientific, Waltham MA, United States) at 150 rpm and 30°C. Samples (1.0 mL) were drawn every 6–12 h, spun at 7,000 × g in a Sorvall Legend Micro 21R centrifuge (Thermo Scientific, Waltham, MA, United States) for 5 min and then, the supernatant was stored at 4°C until future analysis. In an attempt to increase fumarate yield, additional experiment was conducted with 15 and 25% (v/v) ADE and the control medium (without ADE) containing 100 g/L glucose and 30 g/L CaCO3.
At the end of fermentation, the cultures were spun down in an Allegra X-30R centrifuge (Beckman-Coulter, Pasadena, CA, United States) at 7,000 × g for 10 min. Subsequently, the supernatants were drained off and the resulting pellets were placed on pre-weighed filter papers, which were then placed in a Thermo Scientific Heratherm incubator oven (Waltham, MA, United States) for 1 week. Afterwards, the filter papers and fungal biomasses were reweighed to estimate the weights of the mycelial pellets. All cultures were grown and analyzed in triplicate.
2.2 PAOs-mediated removal of nutrients from ADE
PAOs were assessed for nutrient sequestration from ADE. A culture of PAOs was obtained from the city of Madison, WI, United States, Sewerage District. For the PAOs experiments, fresh PO4-P-and NH4+-replete ADE was obtained from the City of Madison, Wisconsin (United States) community AD facility. The solid fraction of the ADE was removed by centrifugation as described earlier. First, the PAOs were adapted to higher than normal phosphate concentrations by supplementing the culture with incremental concentrations of phosphate using K2HPO4 (10–700 mg/L). Ethanol (10 g/L) was supplied as carbon source. The cultures were grown in cycles of anaerobic (4 h) and aerobic (5 h) conditions in a MaxQ 4,450 shaker incubator (Fisher Scientific, Waltham MA, USA) at 150 rpm and 25°C. When 700 mg/L phosphate was reached (after 1 week of adaptation to incremental phosphate supply), the adapted culture of PAOs was mixed with ADE at varying concentrations (60, 70, and 80%, v/v ADE). All cultures were supplemented with ethanol (10 g/L) as carbon source. K and Mg ions have been reported to enhance PO4-P uptake, by counteracting the triple negative charge of PO4-P (Pattarkine and Randall, 1999). In addition, pH in the range of 7.5–8.5 has been reported to enhance biological phosphate removal (Schuler and Jenkins, 2002; Serafim et al., 2002). Therefore, the pH of the ADE-supplemented cultures were adjusted to 8.2 using KOH, after mixing with the PAOs suspension. The KOH doubled as a source of K and as a buffer. The cultures were grown over 2 anaerobic-aerobic cycles as described above (18 h). Subsequently, PO4-P, P, NH4+ and N concentrations were analyzed using inductively coupled plasma spectrometry. Cultures were grown and analyzed in triplicate.
2.3 Analytical procedures
Changes in pH during the growth of R. delemar and the PAOs were monitored using Orion Star A214 pH meter (Thermo Fisher Scientific, Waltham, MA, United States). Fumarate concentration was quantified by high performance liquid chromatography (HPLC). The analytical system consisted of a Dionex U3000 HPLC (Thermo Scientific, Sunnyvale, CA, United States), equipped with an autosampler, fluorescence and a diode array detector (Dionex Ultimate 3000 Fluorescence detector). The HPLC column was a Rezex ROA-Organic Acid H+ column (8%) with a dimension of 300 × 7.8 mm (length and internal diameter, respectively; Phenomenex, Terrance, CA, United States). The mobile phase was 0.005 M H2SO4 and the flow rate was 1.0 mL/min. The column and detector were maintained at 60°C, the run time was 12 min, and an injection volume of 10 μL was used. Fumarate was detected at 210 nm.
Glucose concentration was measured with a YSI 2900 Series biochemistry analyzer (YSI, Yellow Springs, OH, United States) equipped with glucose calibrator, and configured with YSI 2357 buffer and the YSI 2365 glucose oxidase enzyme membrane, according to the manufacturer’s instruction. Samples were diluted (10x) in 15% saline and filtered through 0.22 μm sterile PTFE filter with 13 mm diameter (CELLTREAT, Pepperell, MA, United States). Glucose analysis was conducted with a sample volume of 25 μL. The mineral constituents of test (supplemented with 25%, v/v ADE) and control media were quantified by inductive coupled plasma optical emission spectroscopy (ICP-OES) using Agilent 5,110 ICP-OES unit (Agilent Technologies Inc., Wilmington, DE, United States) as previously described (Ujor et al., 2020). Ethanol concentrations in the PAOs cultures were monitored by gas chromatography as previously described (Agyeman-Duah et al., 2022).
2.4 Statistical analysis
Maximum fumarate, glucose consumed, fumarate yield, fumarate productivity, residual metals, and non-metals in 25% ADE cultures were compared to the control cultures using the General Linear Model in Minitab 21 (Minitab Inc., State College, PA). To determine significant differences between the parameters in the 25% ADE and the control cultures, one-way analysis of variance (ANOVA) was performed followed by Tukey’s pairwise comparisons. The confidence level for the analysis was set at 95%, and treatments with a p < 0.05 were considered statistically significant.
3 Results
3.1 Fumarate production in ADE-supplemented media
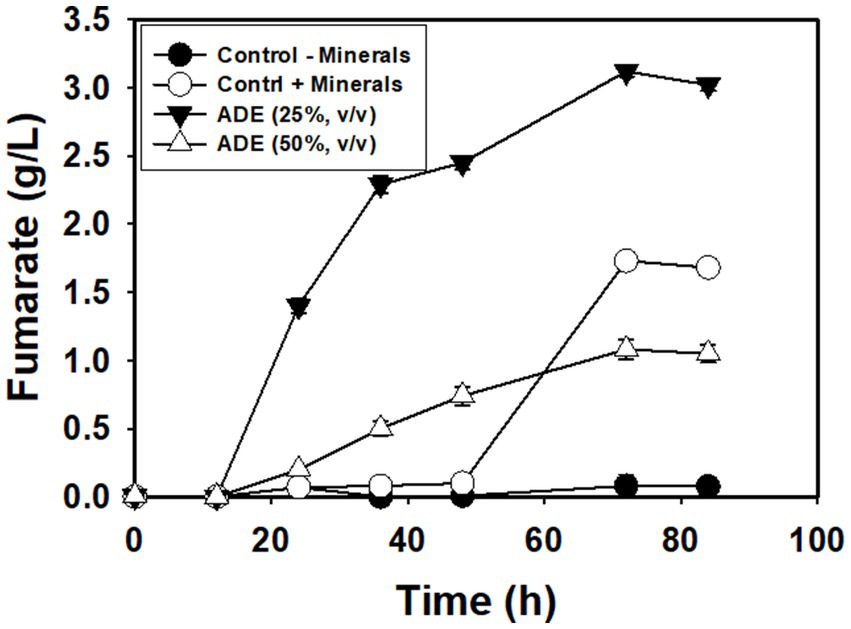
Without buffering, 25% (v/v) ADE produced the highest concentration (3.12 g/L) of fumarate (Figure 2). Non-buffered cultures of R. delemar grown in the control medium with mineral supplementation produced 1.73 g/L fumarate—45% less fumarate than the cultures supplemented with 25% (v/v) ADE. In parallel, poor growth and fumarate production were observed in the 50% (Figure 2) and 75% (v/v) ADE (data not shown). With 60 g/L glucose and 6 g/L CaCO3, the control medium exhibited greater fumarate productivity (0.07 g/L/h) than the 25% (v/v) ADE (0.05 g/L/h) (Table 1). Nonetheless, the ADE (25%, v/v)-supplemented and un-supplemented cultures of R. delemar produced similar maximum concentrations of fumarate (8.4 and 8.04 g/L, respectively) (Figure 3A). Concomitantly, the ADE (25%, v/v)-supplemented cultures produced 27% less biomass than the control cultures (Figure 3B). With 30 g/L CaCO3 and 100 g/L glucose, R. delemar produced a maximum of ~16 g/L fumarate in 72 h (productivity = 0.22 g/L/h) (Figure 4A) in the control medium. On the other hand, 15 and 25% (v/v) ADE produced ~17 and 11 g/L fumarate at 96 and 144 h, respectively, which translate to respective productivities of 0.17 and 0.07 g/L/h. Notably, increasing glucose and CaCO3 concentrations led to ~500% increase in the concentration of R. delemar biomass in cultures supplemented with 25% (v/v) ADE (Figures 3B, 4B). However, substantial increase in the biomass concentration of R. delemar following increases in the concentrations of glucose and CaCO3 led to a 37.5% increase in fumarate titer (Figures 3A, 4A). It is important to note however, that by lowering the ADE concentration from 25 to 15% and increasing glucose and CaCO3 concentrations to 100 and 30 g/L, respectively, R. delemar biomass concentration increased 41 and 58%, respectively, relative the control and the 25% (v/v) ADE (Figures 3B, 4B). Although growth reduced in the 25% (v/v), when compared to the control and the 15% (v/v), glucose utilization was unaffected. In fact, with 25% ADE, glucose consumption increased 12 and 50% relative to the 15% (v/v) ADE and the control, respectively (Table 1).

Figure 3. Fumarate and biomass concentrations in CaCO3-buffered cultures of R. delemar with and without ADE (25%, v/v). (A) Fumarate titer. (B) Biomass concentration. Asterisk denotes statistical significance relative to the control culture.
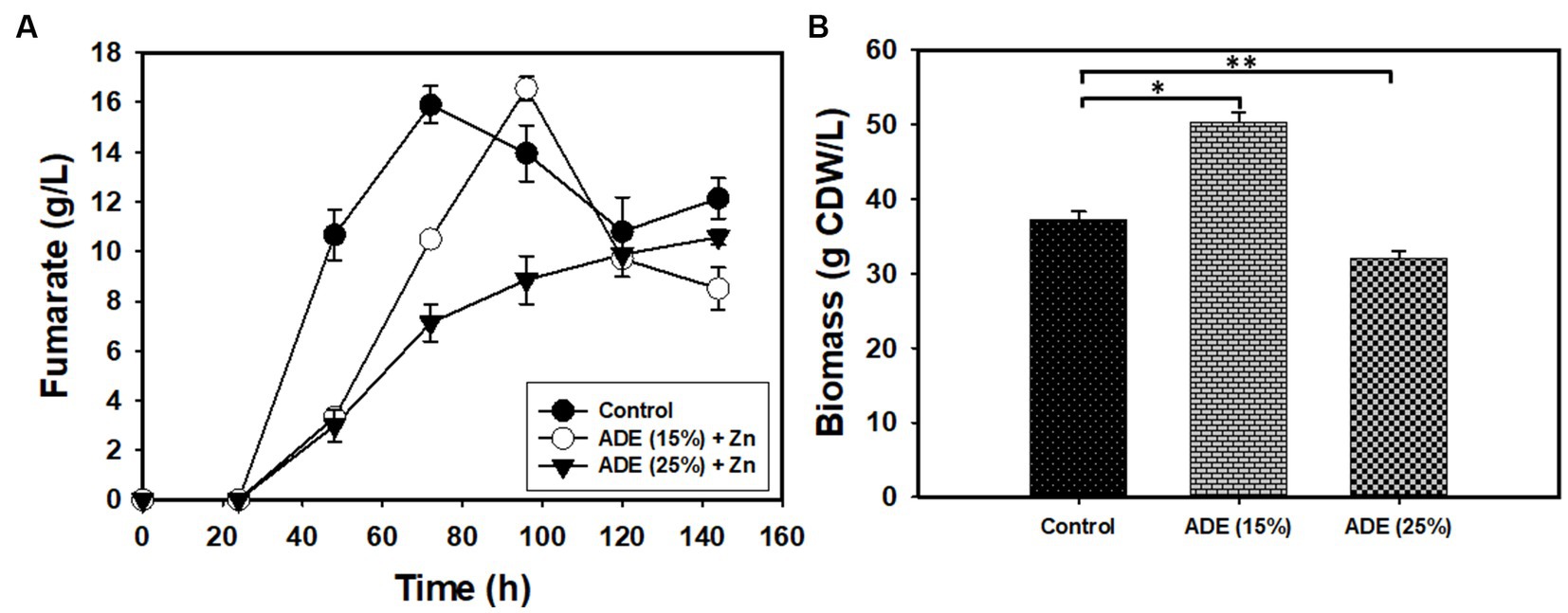
Figure 4. Biomass and fumarate concentrations in cultures of R. delemar grown on 100 g/L glucose, with and without ADE (15 and 25%, v/v) supplementation and buffered with 30 g/L CaCO3. (A) Fumarate concentration. (B) Biomass concentration. Asterisks denote statistical significance in comparison to the control medium.
3.2 Sequestration of ADE-borne minerals during fumarate fermentation
A central thrust of this study was to reduce the concentrations of minerals in ADE-supplemented cultures, such that the resulting effluent would be amenable to conventional wastewater treatment technologies. As depicted in Table 2, ADE-supplemented and un-supplemented cultures of R. delemar showed similar metal concentrations after fumarate fermentation. Interestingly, addition of ADE (25%, v/v) to the fermentation medium did not result in greater metallic content, relative to the un-supplemented medium (Table 2). The concentrations of As, B, Ba, Be, Ca, Cd, Co, Li, Mo, Na, Ni, Sb, Si, Sr., and V did not undergo significant variations during the growth of R. delemar (Table 2), both in the ADE (25%, v/v)-supplemented and un-supplemented cultures. On the other hand, the concentrations of Al, Cr, Cu, Fe, and K decreased in both the ADE (25%, v/v)-supplemented and un-supplemented cultures of R. delemar, albeit to varying degrees (Table 2). Notably, the concentrations of Al, K and Cu reduced 40.20, 12.3, and 74.40%, respectively, in the ADE (25%, v/v)-supplemented culture (Table 2). Conversely, the concentrations of the same metals reduced 25.40%, marginally (2.00%) and 43.00%, respectively, in the control cultures. Similarly, the concentrations of Mg, Mn, Pb, and Zn reduced 26.00, 23, 17.50, and 28.00%, respectively, with ADE supplementation (Table 2; Figure 5). On the contrary, statistically, the concentrations of the same metals barely decreased in the control medium (Table 2).
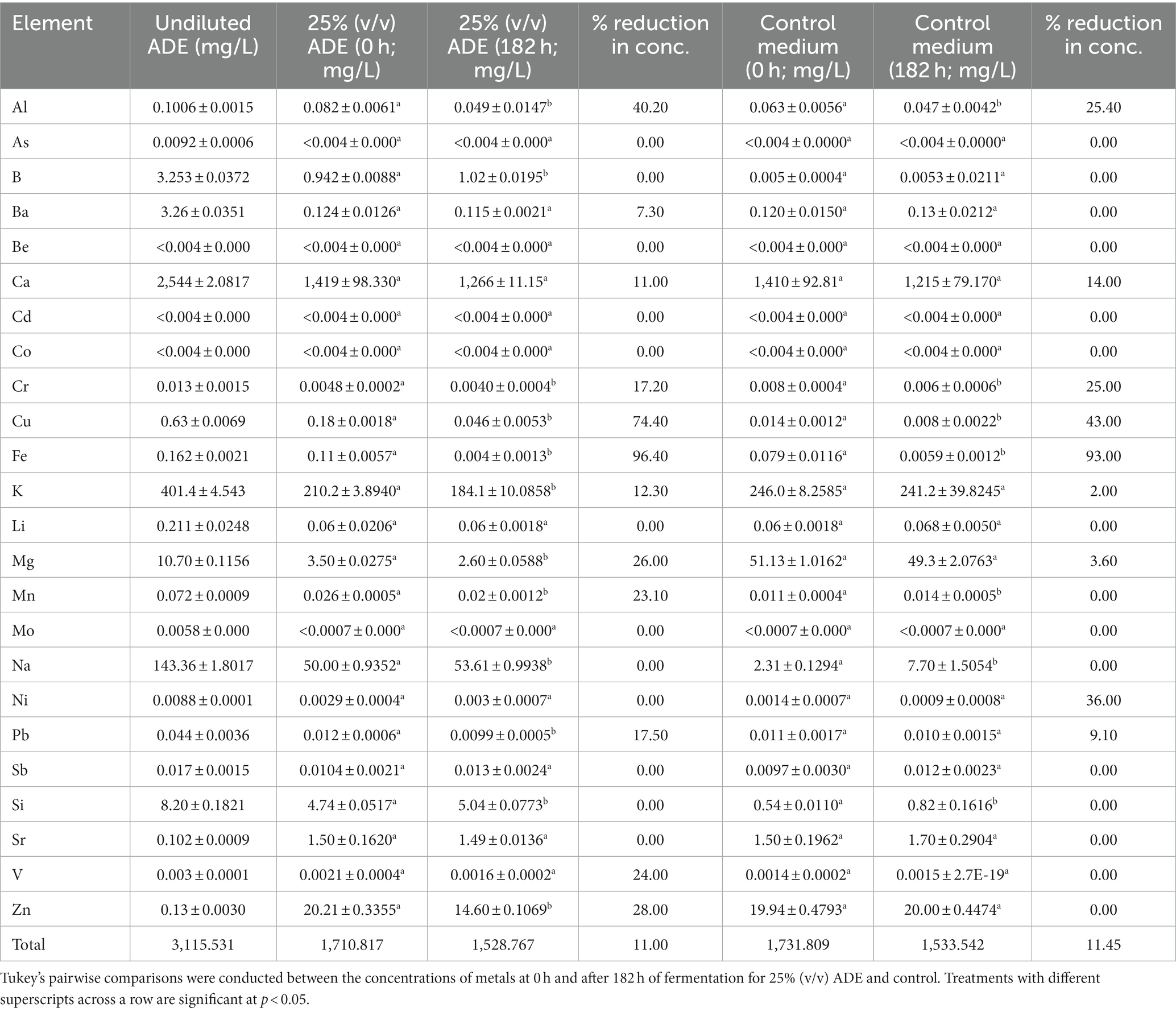
Table 2. The concentrations of metals in ADE-supplemented and un-supplemented cultures of R. delemar during fumarate fermentation.
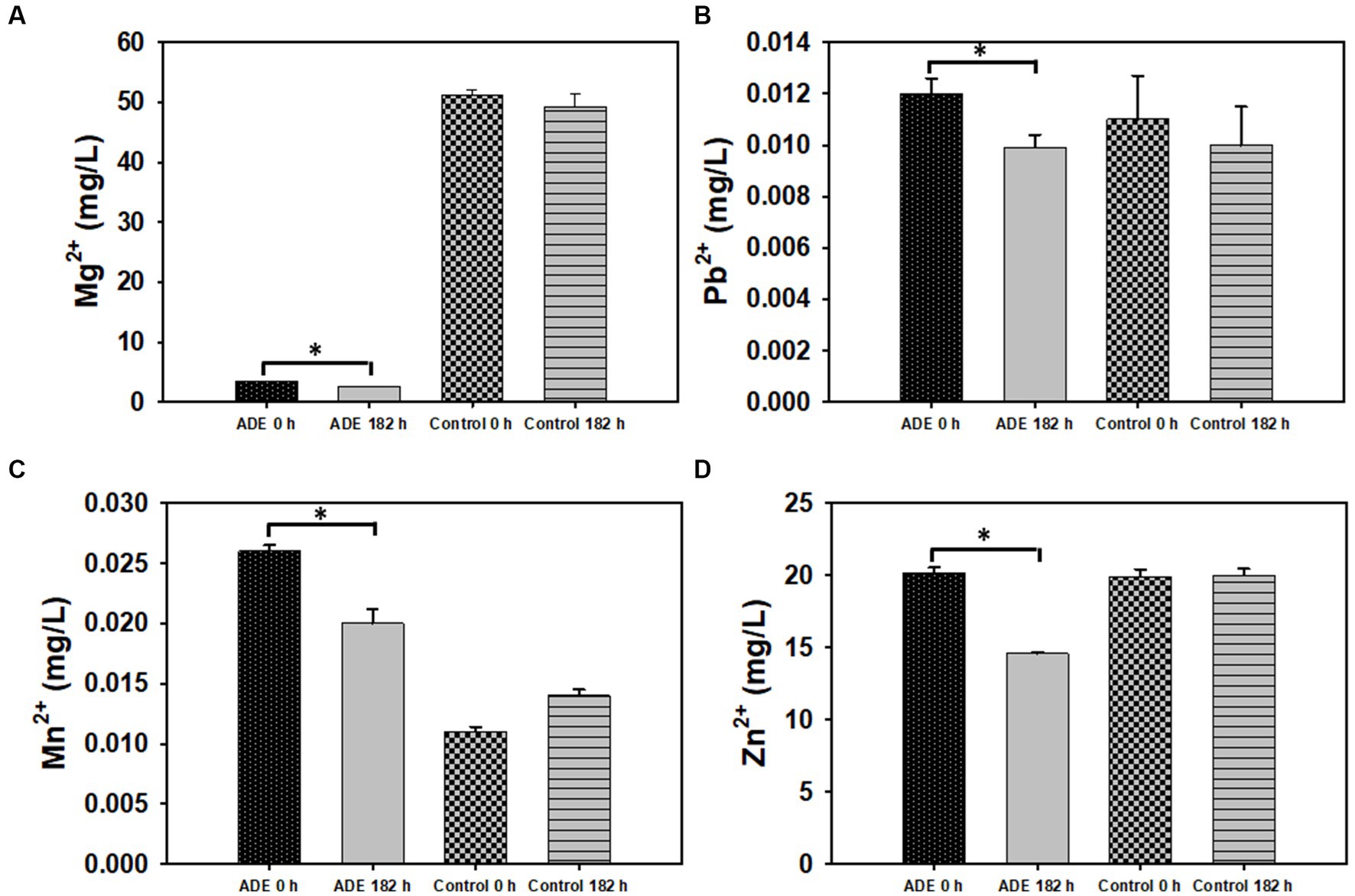
Figure 5. The concentrations of select metals in ADE (25%, v/v)-supplemented and un-supplemented cultures of R. delemar before and after fumarate fermentation. (A) Magnesium (Mg2+) concentration. (B) Lead (Pb2+) concentration. (C) Manganese (Mn2+) concentration. (D) Zinc (Zn2+) concentration. Asterisks denote statistical significance relative to 0 h of fermentation.
Fe concentrations before and after fermentation show strong sequestration of Fe from both the ADE-supplemented and un-supplemented media (Table 2). However, it is worth noting that although a similar percentage of Fe was sequestered from both media, due to 28.2% higher Fe concentration in the ADE-supplemented medium, a higher actual concentration of Fe (0.106 mg/L) was removed from this medium than from the control medium (0.073 mg/L). Because CaCO3 was added to both sets of cultures as a buffer, calcium concentrations were high and similar in both the control (1,410 mg/L) and ADE-supplemented (1,419 mg/L) cultures (Table 2). Calcium concentrations did not undergo under significant decreases in both the ADE-supplemented and un-supplemented cultures.
As shown in Table 3, the concentrations of non-metals and non-metallic ionic species in both the test and cultures decreased significantly, relative to the degrees of reductions observed for metals. Nitrite (NO2-N) and chloride (Cl−) were discernibly detected only at 0 h of fermentation but not at 182 h for both the test and the control cultures. Similarly, both ions could not be distinctively quantified in the undiluted ADE. P and PO4-P concentrations decreased 93.0 and 97.1%, respectively, in the ADE-supplemented cultures (Table 3; Figure 6). On the hand, the concentrations of both P and PO4-P reduced 24.21 and 30.50% in the control cultures. Nonetheless greater concentrations of P and PO4-P (41.0 and 49.0 mg/L, respectively) were removed from the control cultures than from the ADE-supplemented cultures (12.22 and 7.48 mg/L, respectively). This is because; the ADE-supplemented cultures contained 92.2 and 95.2% less P and PO4-P than the control cultures (Table 3; Figures 6A,B). It is important to note that the concentrations of NH4+, P and PO4-P in the batch of ADE used in the fumarate fermentation experiments were significantly lower than the typical concentrations reported in ADE. This is likely because the ADE was stored in the laboratory at room temperature for several months. As a result, NH4+ in the ADE (see results below) possibly underwent both volatilization and oxidation [to nitrite (NO2-N) and nitrate (NO3-N)]. Further, it is likely that continued microbial activity in the unsterilized ADE during storage also led to reduced P and PO4-P concentrations. Storage of the ADE at cold temperatures led to precipitation of the mineral constituents.
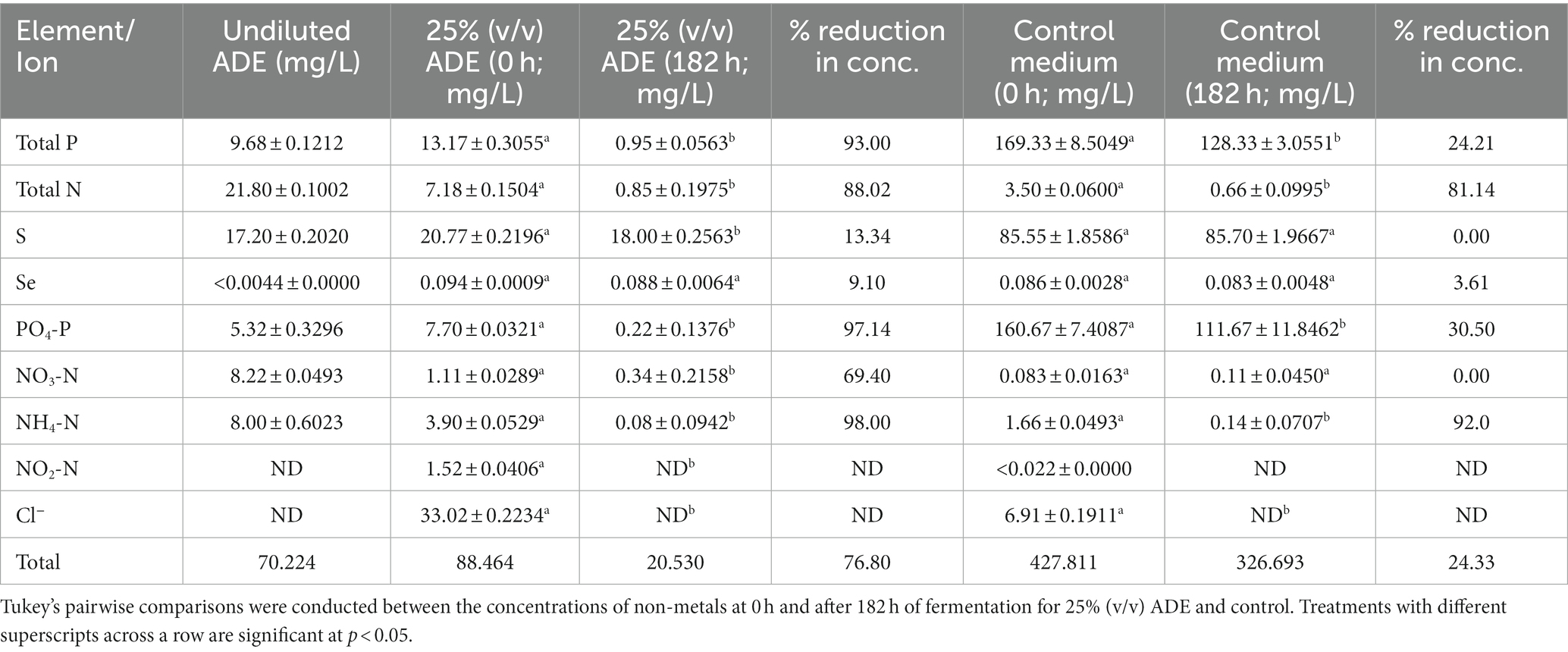
Table 3. The concentrations of non-metals and non-metallic ions in ADE-supplemented and un-supplemented cultures of R. delemar.
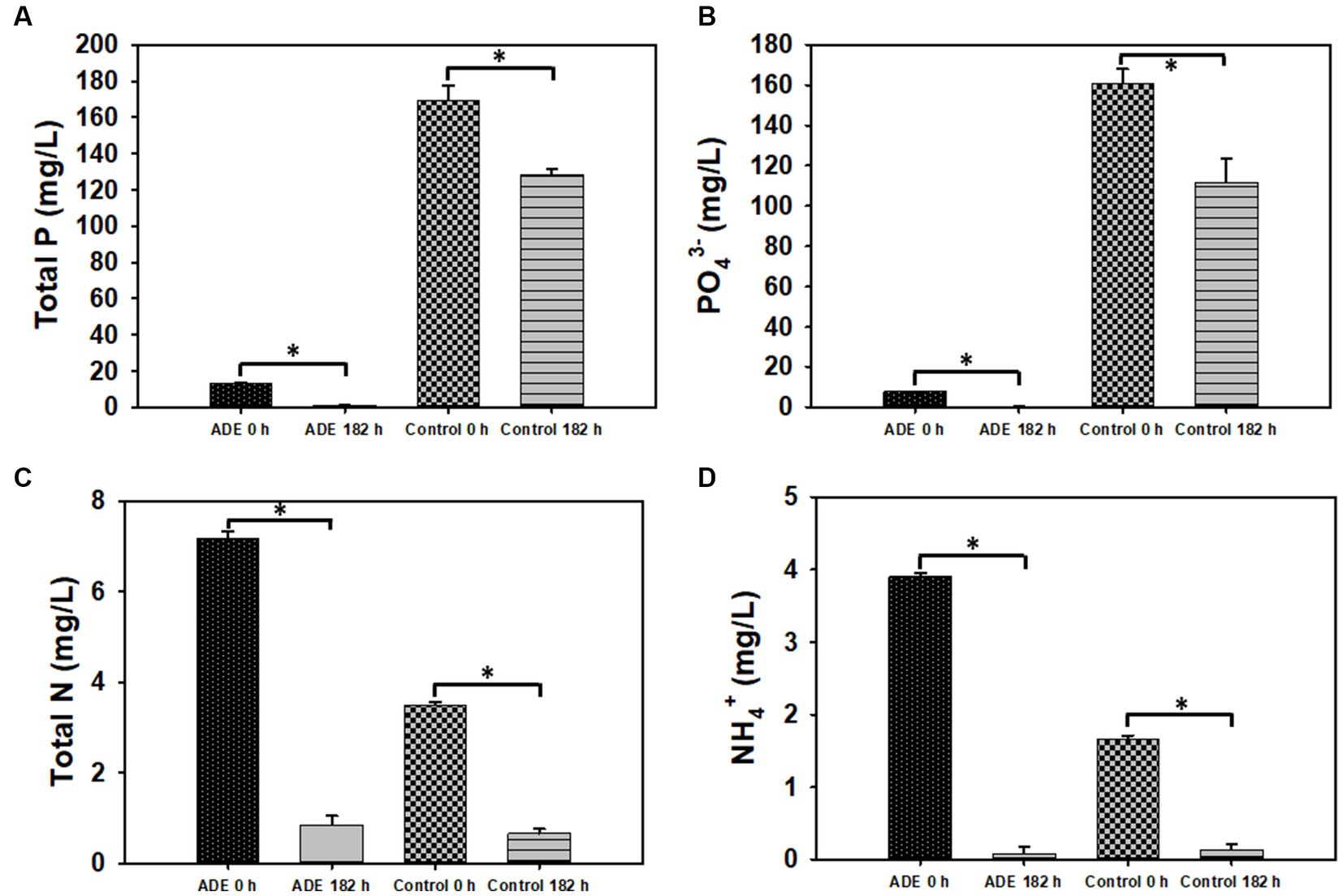
Figure 6. Residual concentrations of select inorganic nutrients following fermentation in ADE (25%v/v)-supplemented and un-supplemented cultures of R. delemar before and after fumarate fermentation. (A) Total phosphorus concentration (P). (B) Phosphate concentration. (C) Total nitrogen (N). (D) Ammonium concentration. Asterisks denote statistical significance relative to 0 h of fermentation.
The ADE-supplemented cultures contained 51.5 and 57.4% greater concentrations of total nitrogen (N) and NH4+, respectively, than the control cultures (Table 3; Figures 6C,D). Although both sets of cultures exhibited similar degrees of reduction for N and NH4+ in percentage, due to higher initial concentrations in the ADE-supplemented cultures, greater actual concentrations of both nutrients were removed from the ADE-supplemented cultures. Specifically, 6.33 and 3.82 mg/L of N and NH4+, respectively, were sequestered from the ADE-supplemented cultures, whereas 2.84 and 1.52 mg/L, respectively, were removed from the control cultures. Notably, a greater concentration of NO3-N was detected in the ADE-supplemented cultures (1.11 mg/L) than in the control cultures (0.083 mg/L). Whereas 69.4% (1.03 mg/L) of NO3-N was removed during fermentation in the ADE-supplemented cultures, the concentration of NO3-N did not change during fermentation in the control medium.
Contrasting patterns of change in concentration were observed for sulfur (S). Although the control cultures contained 75.7% more S than the ADE-supplemented fermentations, the concentration of this element did not reduce in the control cultures after fermentation (Table 3). Conversely, out of the 20.80 mg/L S detected in the ADE-supplemented cultures at 0 h, 2.8 mg/L (15.40%) was removed at the end of fermentation. Overall, greater reductions in the concentrations of non-metals and non-metallic ionic species—particularly, P and PO4-P—were observed in the ADE-supplemented cultures (~77.00%), relative to the control cultures (24.33%). Comparatively, the ADE-supplemented cultures contained 79.4% less non-metals and non-metallic ionic species than the control cultures (Table 3).
3.3 PAOs-mediated sequestration of nutrients from ADE
Nurmiyanto et al. (2017) showed that excessive PO4-P concentration inhibits biological phosphate removal. Thus, in an effort to increase PO4-P removal from PO4-P-replete ADE, the culture of PAOs was pre-adapted to high PO4-P concentration. Following adaptation, cultivation of PAOs in 60, 70, and 80% (v/v) ADE led to varying degrees of reduction in the concentrations of P, PO4-P, N, and NH4+. However, this was more pronounced in the 60% (v/v) ADE. Specifically, in the 60% (v/v) ADE, the concentrations of P, PO4-P, N and NH4+ reduced 86, 90%, ~99, and 100%, respectively (Figure 7). Concomitantly, the ethanol (10 g/L) added to the cultures of PAOs was completely used after 18 h of incubation.
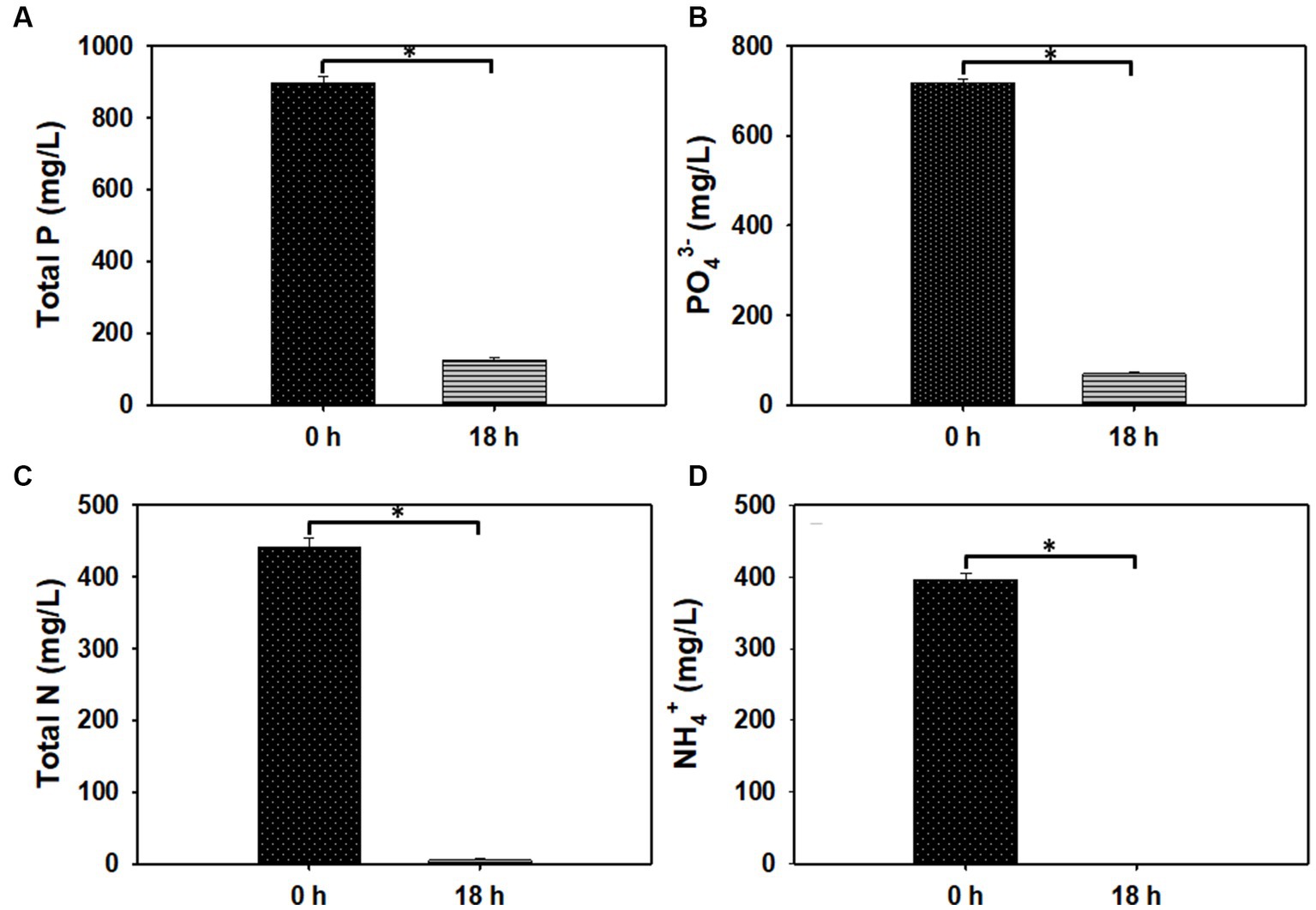
Figure 7. The concentrations of total phosphorus (P), phosphate (PO43−), total nitrogen (N), and ammonium (NH4+) in 60% (v/v) ADE before and after the growth of PAOs. (A) P concentration. (B) PO43-concentration. (C) N concentration. (D) NH4+ concentration. Asterisks denote statistical significance relative to 0 h of fermentation.
4 Discussion
Biological sequestration of ADE-borne nutrients, particularly, PO4-P, and NH4+ has the potential to reduce ADE nutrient loads to levels that may permit economical treatment of the liquid fraction of ADE by existing wastewater treatment technologies. With >90% water content, ADE represents a scarcely researched source of water. Currently, treating ADE is capital intensive relative to municipal sewage, due to high nutrient contents, mainly, PO4-P and NH4+. To determine if biological approaches could be deployed to reduce the nutrient loads of ADE, we evaluated a product (fumarate)-coupled and a non-product coupled approach. A product-coupled approach has the potential to deploy ADE as a source of minerals in biological production of value-added chemicals. Moreover, coupling nutrient removal from ADE to bioproduction of a valuable compound would defray the attendant cost of the sugar used to grow the biological agent—R. delemar in this case.
Both the ADE-supplemented and un-supplemented cultures of R. delemar produced comparable concentrations of fumarate, irrespective of the sugar and CaCO3 concentrations. Apparently, the strain of R. delemar used in this study is not a robust producer of fumarate. Nonetheless, the results indicate that ADE exerted some degree of stress on R. delemar. Notably, with 15% (v/v) ADE (in medium containing 30 g/L CaCO3 and 100 g/L glucose), R. delemar produced 70% more fumarate and ~ 42% more biomass than cultures grown in 25% (v/v) ADE (Figure 4B). Additionally, with 6 or 30 g/L CaCO3, fumarate productivity in the ADE-supplemented cultures was lower, relative to the control cultures (Figures 3A, 4B). It is likely that a factor present in the ADE negatively affected R. delemar. Chemical cleaning agents used in animal facilities are likely to be transferred to the AD process, where animal manure—as in the present study—is a key component of the feedstock. Hence, residual cleaning agents will likely affect fungal/microbial growth during fermentation in ADE-supplemented medium. Hence, careful selection of the source of the ADE is fundamental to potential deployment of this strategy for bioproduction and ADE pretreatment.
Despite the low fumarate yield, the concentrations of Al, Cr, Cu, Fe, K, Mg, Mn, Pb, and Zn reduced significantly (p < 0.05) in ADE-supplemented cultures (Table 2). Conversely, the concentrations of K, Mg, Mn, Pb, and Zn, did not undergo significant reductions in the control cultures. Concomitantly, whereas the concentrations of Al, Cu and Fe decreased in the control cultures, stronger decreases were observed in the ADE-supplemented cultures. Apparently, despite containing similar overall metal concentrations, specific metals were more strongly sequestered from the cultures of R. delemar supplemented with ADE. Although Al is thought to be largely unconnected to biological processes, Cochrane (1948) suggested that trace amounts of Al alongside Zn, Fe, Cr, and Mn were necessary for improved yield of citric acid by Aspergillus niger. While there is no evidence of direct involvement of Al in fumarate production, the concentrations of Al reduced considerably in both the ADE-supplemented and un-supplemented cultures. Thompson and Medve (1984) reported that some ectomycorrhizal fungi were tolerant to Al at 500 mg/L. However, with increasing acidity, Al toxicity increased. Although the Al concentrations in this study were significantly lower than 500 mg/L [0.082 and 0.063 mg/L in the ADE-supplemented and un-supplemented cultures, respectively], acidity amplified considerably with increases in fumarate production, which may likely potentiate Al toxicity, especially in the presence of numerous other metals. This might explain the 40 and 25% reductions in Al concentrations in the ADE-supplemented and un-supplemented cultures, respectively. Stronger sequestration of Al from the ADE-supplemented culture when compared to the control may be ascribed to 30% higher Al concentration in the ADE-supplemented medium, relative to the control. Greater abundance of Al likely triggered increased assimilation, especially if Al-mediated toxicity was prevalent during fumarate production.
Strong Cu removal was observed in both the ADE-supplemented and un-supplemented cultures, albeit more pronounced in the former. Notably, ADE-supplemented cultures contained ~13-fold higher concentration of Cu than the control cultures. We reason that this possibly accounts for the more robust sequestration of Cu from the ADE-supplemented medium. Although Cu is a fungal micronutrient, it is only required at extremely low concentrations, causing severe oxidative damage at relatively low concentrations (Robinson et al., 2021). From an environmental standpoint, robust removal of Cu from the ADE-supplemented medium by R. delemar holds some promise for Cu sequestration from ADE.
Due to marginal supplementation of KH2PO4 and no addition of MgSO4 to the ADE-supplemented cultures, the control cultures contained 14.6 and 93.2% greater K and Mg, respectively, than the ADE-supplemented cultures at 0 h (Table 2; Figure 5A). However, the concentrations of both metals reduced 12.3 and 26.0% in the ADE-supplemented cultures, respectively, while barely reducing in the control cultures during fermentation. Apparently, stronger sequestration of K and Mg in the ADE-supplemented cultures cannot be explained by the presence of greater concentrations of these metals—as this was not the case. On the contrary, lower levels of K and Mg in the ADE-supplemented cultures is the likely underlying driving factor for this trend. Both K and Mg are particularly essential nutrients, more so than Al and Cu. This therefore raises the question as to whether the nutritional and metabolic relevance of K and Mg engendered a stronger uptake response to both metals. However, it is important to highlight that residual 210.2 and 2.6 mg/L K and Mg, respectively, remained in the medium post fermentation (Table 2). A form of ‘metabolic urgency’ to reinforce the uptake of K and Mg should have resulted in significantly lower residual concentrations for both metals after fermentation. Thus, the residual concentrations of both metals suggests that metabolic requirements alone do not account for greater K and Mg sequestration from the ADE-supplemented medium. It appears therefore, that other factor(s) might account for the enhanced sequestration of K and Mg in the ADE-supplemented cultures, relative to the control cultures. Interestingly, the control cultures contained 52.0% less Ni than the ADE-supplemented cultures. However, Ni concentration decreased 36.0 and 0.0% in the control and ADE-supplemented cultures, respectively. Ni was the only metal that occurred at a greater concentration in the ADE-supplemented medium but was more strongly sequestered from the control medium (Table 2). The underlying reason for this trend is unclear. However, we speculate that this might be related to cross-uptake of the divalent metals, Mg and Ni. Ni is taken up by the Mg transport system in most organisms, and often, Mg uptake supersedes Ni uptake (Babich and Stotzky, 1983; Joho et al., 1995). Noticeably, as mentioned above, the ADE-supplemented cultures contained 93.2% less Mg than the control cultures. Thus, it is plausible that the lower concentration of Mg—an essential nutrient in most organisms including fungi (Prasad et al., 2019)—favored a stronger Mg uptake over Ni in the ADE-supplemented cultures.
Although both the ADE-supplemented and the control cultures contained identical concentrations of Zn, a 28.0% reduction in Zn concentration was observed in the ADE-supplemented cultures, as opposed to 0.0% in the control cultures. It is not clear as to why both sets of R. delemar cultures exhibited contrasting profiles of Zn after fermentation, despite containing identical Zn concentrations at the beginning of fermentation (Table 2). Co-import of Zn, Fe and Cu by the divalent metal transporter 1 (DMT1; Mims and Prchal, 2005) might explain this trend. Whereas both the ADE-supplemented and control cultures contained similar total metal concentrations, the concentrations of specific elements such as Fe, Cu, Na, Ni, and Mn were higher in the ADE-supplemented cultures. Among these metals, Cu, Na, Ni, and Mn exert considerable toxicity on microbial cells (Robinson et al., 2021). Thus, it is likely that R. delemar underwent greater metal import in the ADE-supplemented cultures to sequester more toxic metals from the medium, which might account for the drastically lower concentration of Zn—likely due to reduced discrimination among metals imported by DMT1 [encoded by smf genes (Smf1p, Smf2p, Smf3p)]—in the ADE-supplemented cultures post fermentation. As did Zn and Cu—both of which are divalent—the concentrations of Pb and Mn reduced ~18 and 23%, respectively, in ADE-supplemented cultures, while no decreases in concentrations were observed for the same metals in the control cultures (Table 2; Figure 5). These results suggest that a mechanism that improves metal uptake, particularly, divalent metals may have been upregulated in the ADE-grown cultures of R. delemar, in comparison to the control.
Unsurprisingly, Fe was strongly sequestered from the ADE-supplemented and un-supplemented cultures. Although Fe is typically not considered an environmental threat in waste streams such ADE or landfill leachate, it can indirectly exert far-reaching impacts on aquatic environments. This is because; Fe is a particularly essential nutrient/cofactor in biological cells (Philpott, 2006; Caza and Kronstad, 2013). In fact, Fe is a critical micronutrient for algal growth owing to its vital role in diverse metabolic processes such as photosynthetic electron transport, chlorophyll synthesis, respiration, nitrate reduction, and nitrogen fixation (Wang et al., 2014; Ermis et al., 2020; Liu et al., 2021). Thus, asides the removal of more directly toxic heavy metals, there is considerable merit to Fe removal from waste streams that have the potential to introduce Fe into the environment. Ultimately, this can help to starve algae of a vital nutrient. Similarly, excessive release of K-rich waste into the environment negatively impacts the structural stability of soil, leading to impaired hydraulic conductivity (Arienzo et al., 2009; Liang et al., 2021). Likewise, Cu has been reported to exert toxic effects on aquatic life at 6.3 μg/L (Hall et al., 1998), whereas the ADE used in this study contained 180 μg/L Cu—28.6-fold higher than the toxic threshold. Furthermore, Pb and Mn pose a significant threat to both humans and aquatic organisms (Baker et al., 2015; Wani et al., 2015). Indeed, these results highlight the potential for developing a bio-based approach for nutrient sequestration from ADE, and perhaps, similar nutrient-replete waste streams. It is important however, to mention that CaCO3 is an essential buffering agent in fermentative production of fumarate. As a result, this increases the likelihood of introducing high amounts of Ca into the environment post fermentation. Besides increasing soil or water pH, Ca may lead to the mobilization of heavy metals in soil (Fay and Shi, 2012). Therefore, developing a fermentation strategy that limits Ca concentration in the effluent, thereby minimizing the introduction of Ca into the environment is an important research undertaking to consider.
R. delemar-mediated nutrient sequestration from ADE-supplemented cultures was not limited to metals. The concentrations of P, PO4-P, N, NH4+, NO3-N and S reduced significantly following fumarate fermentation in the ADE-supplemented medium. A considerably higher concentration of NO3-N was detected in the ADE-supplemented cultures, when compared to the control. Accordingly, this possibly elicited stronger NO3-N removal from the ADE-supplemented medium, relative to the control. Furthermore, the presence of NO3-N in the ADE used for fumarate fermentation is indication of NH4+ oxidation, most plausibly due to prolonged storage of the ADE in the laboratory. Additionally, NO3-N has been reported to impinge some toxicity on fungi (Zhou et al., 2017). We speculate that this is likely responsible for the enhanced NO3-N removal from the ADE-supplement medium. In fact, the control cultures contained 13.4-fold less NO3-N than the ADE-supplemented cultures (Table 3). Hence, if NO3-N were physiologically essential to R. delemar, the extremely low concentration of NO3-N (0.083 mg/L) in the control cultures would have triggered a strong NO3-N uptake. On the contrary, NO3-N was more strongly sequestered from the ADE-supplemented cultures. In parallel, S concentration showed a greater degree of reduction in the ADE-supplemented cultures, in comparison to the controls. Notably, the control medium contained 4.1-fold greater concentration of S. MgSO4 was added to the control medium, but not to the ADE-supplemented medium. We reason that this accounts for the greater amount of S in the control medium. Further, the lack of reduction in the concentration of S in the control cultures suggests that sulfate-might not be a preferred form of S for R. delemar. Alternatively, ADE contains residual amino acids, vitamins and nucleic acids, some of which contain S and are typically preferred sources of N and S by most microorganisms (Liu et al., 2009; Möller and Müller, 2012). Hence, greater absorption of such nutrients may explain the higher reduction in concentration observed for S in the ADE-supplemented cultures. With significantly lower concentrations of total S, P, and PO4-P, it is plausible that appropriate high-affinity uptake permeases were more strongly expressed during fermentation in the ADE-supplemented medium to scavenge for these nutrients.
Expectedly, NH4+ was strongly sequestered from both the ADE-supplemented and the control cultures (Table 3). The established role of NH4+ as an essential biological nutrient underscores the extent of NH4+ removal from both media. NH4+ is a key ingredient in most fermentative processes—for bio-production of assorted value-added chemicals—and the industrial Haber-Bosch process, the sole source of NH4+ in society, is energy intensive, accounting for 1.8% of global energy consumption and 2–3% of global fossil fuel consumption, while generating >560 million tons of CO2 (3% of global output)/yr (Smil, 2001; Lehnert et al., 2016; The Royal Society, 2020; Dong et al., 2021; Lehnert et al., 2021). Therefore, given the current scale of industrial production of bio-chemicals and the expected growth in this sector, on account of growing efforts to decarbonize the economy, transitioning to a biobased economy will have far-reaching environmental impacts, unless more sustainable sources of NH4+ are identified and harnessed. To this end, fresh NH4+,-rich ADE might be a potential candidate for supplanting or at least, minimizing the demand for synthetic NH4+ in appropriate biomanufacturing processes. Similarly, with increasing concerns over reducing PO4-P reserves (Desmidt et al., 2015), indirect “mining” of PO4-P-rich wastes such as ADE by tethering ADE-borne phosphate to fermentative production of bio-chemicals holds considerable promise for enhancing the long-term sustainability of biomanufacturing. For this approach to be successful, however, it is critical to identify/develop microbial strains capable of efficient growth and target compound production in ADE or ADE-supplemented media.
In addition to coupling nutrient sequestration to bioproduction, we assessed the potential of using PAOs to remove P, PO4-P, N and NH4+ from ADE. For this, 60% (v/v) ADE produced the most promising results. More importantly, we demonstrate that prior adaptation of PAOs to higher PO4-P can improve the capacity for PO4-P sequestration from a PO4-P-rich waste stream. Our analysis showed that ethanol (10 g/L) was completely used by the PAOs, with concomitant removal of high amounts of P, PO4-P, N and NH4+ from the ADE. Notably, nutrient removal from higher ADE concentrations (70 and 80%) was not as efficient, albeit promising (data not shown). It is likely that increasing nutrient concentrations with increase in ADE concentration reduced nutrient sequestration. Prior exposure of the PAOs to a maximum of 700 mg/L PO4-P may account for the results obtained with 60% (v/v) ADE. This is because; the 60% (v/v) ADE contained ~700 mg/L PO4-P. Therefore, adaptation to a greater concentration of PO4-P may prove instructive. It is important to note that despite the technical merits of PAOs-mediated nutrient removal from ADE, in reality, the utility of this approach in a real world setting may prove challenging due to the inherent cost. Thus, it may be useful to combine a product-coupled approach with PAOs-mediated nutrient sequestration. Previously, we reported significant removal of a broad range of ADE-borne nutrients following fermentative cultivation of Saccharomyces cerevisiae in an ADE-based medium (Ujor et al., 2020). This led to significant increase in ethanol production, although with a considerably high residual PO4-P. Therefore, combining both approaches (i.e., growing PAOs in the ADE effluent after growth and ethanol production by S. cerevisiae), will likely help to achieve efficient PO4-P removal, preceded by bioproduction of a value-added compound alongside pre-removal of a wide range of ADE-borne nutrients. This would entail leaving behind some residual ethanol in the ADE-based medium (after ethanol recovery) as a carbon source to fuel PAOs-mediated nutrient sequestration. We will likely explore this strategy in future studies. The ethanol/other appropriate product resulting from the process would help to defray the attendant operational costs.
5 Conclusion
The present study explored the potential of product-coupled and non-product-coupled approaches for the removal of excess nutrients from the liquid fraction of ADE. Our results indicate that both approaches can reduce the ADE nutrient load, particularly, P, PO4-P, N, NH4+, Al, Cu, and Fe. The yield of fumarate by R. delemar in this study suggests that a potent producer of fumarate (or another target product) with the robust capacity to sequester nutrients from ADE, may prove efficacious for product-coupled microbial sequestration of excess nutrients from ADE. The PAOs approach proved particularly successful at 60% (v/v) ADE. Ideally, closer to 100% (v/v) ADE will likely be more attractive. Thus, it would be interesting to determine the extent to which PAOs can be adapted, with a view to sequestering greater amounts of nutrients from ADE. In addition, operating the system for a longer period to assess stability would provide considerable insight. Furthermore, tethering product-coupled nutrient sequestration from ADE to post bioproduction nutrient removal by PAOs looks particularly attractive for robust nutrient removal, while making a value-added compound. With growing stress on water resources due to climate change, such radical measures may prove essential in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EA-D: Investigation, Methodology, Writing – review & editing. CO: Formal analysis, Writing – review & editing. VU: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin-Madison, with funding from the Wisconsin Alumni Research Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agyeman-Duah, E., Kumar, S., Gangwar, B., and Ujor, V. C. (2022). Glycerol utilization as a sole carbon source disrupts the membrane architecture and Solventogenesis in Clostridium beijerinckii NCIMB 8052. Fermentation 8:339. doi: 10.3390/fermentation8070339
Arienzo, M., Christen, E. W., Quayle, W., and Kumar, A. (2009). A review of the fate of potassium in the soil-plant system after land application of wastewaters. J. Hazard. Mater. 164, 415–422. doi: 10.1016/j.jhazmat.2008.08.095
Babich, H., and Stotzky, G. (1983). Nickel toxicity to estuarine/marine fungi and its amelioration by magnesium in seawater. Wat. Air Soil Pollut. 19, 193–202. doi: 10.1007/BF00211805
Baker, M. G., Criswell, S. R., Racette, B. A., Simpson, C. D., Sheppard, L., Checkoway, H., et al. (2015). Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand. J. Work Environ. Health 41, 94–101. doi: 10.5271/sjweh.3466
Baldasso, V., Bonet-Garcia, N., Sayen, S., Guillon, E., Frunzo, L., Gomes, C. A. R., et al. (2023). Trace metal fate in soil after application of digestate originating from the anaerobic digestion of non-source-separated organic fraction of municipal solid waste. Front. Environ. Sci. 10:1007390. doi: 10.3389/fenvs.2022.1007390
Bankston, E., Wang, Q., and Higgins, B. T. (2020). Algae support populations of heterotrophic, nitrifying, and phosphate-accumulating bacteria in the treatment of poultry litter anaerobic digestate. Chem. Engine J. 398:125550. doi: 10.1016/j.cej.2020.125550
Campos, J. L., Crutchik, D., Franchil, Ó., Pavissich, J. P., Belmonte, M., Pedrouso, A., et al. (2019). Nitrogen and phosphorus recovery from anaerobically pretreated agro-food wastes: a review. Front. Sustain. Food Syst. 2:91. doi: 10.3389/fsufs.2018.00091
Caza, M., and Kronstad, J. W. (2013). Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell. Infect. Microbiol. 19:80. doi: 10.3389/fcimb.2013.00080
Cochrane, V. W. (1948). Commercial production of acids by Fungi. Econ. Bot. 2, 145–157. doi: 10.1007/BF02858998
Das, R. K., and Brar, S. K. (2014). Enhanced fumaric acid production from brewery wastewater and insight into the morphology of Rhizopus oryzae 1526. Appl. Biochem. Biotechnol. 172, 2974–2988. doi: 10.1007/s12010-014-0739-z
Desmidt, E., Ghyselbrecht, K., Zhang, Y., Pinoy, L., Van der Bruggen, B., Verstraete, W., et al. (2015). Global phosphorus scarcity and full-scale P-recovery techniques: a review. Crit. Rev. Environ. Sci. Technol. 45, 336–384. doi: 10.1080/10643389.2013.866531
Dong, F., Lee, Y. S., Gaffney, E. M., Grattieri, M., Haddadin, H., Minteer, S. D., et al. (2021). An engineered, non-diazotrophic cyanobacterium and its application in bioelectrochemical nitrogen fixation. Cell Rep. Phys. Sci. 2:100444. doi: 10.1016/j.xcrp.2021.100444
Ermis, H., Guven-Gulhan, U., Cakir, T., and Altinbas, M. (2020). Effect of iron and magnesium addition on population dynamics and high value product of microalgae grown in anaerobic liquid digestate. Sci. Rep. 10:3510. doi: 10.1038/s41598-020-60622-1
Fagbohungbe, M. O., Herbert, B. M., Hurst, L., Ibeto, C. N., Li, H., Usmani, S. Q., et al. (2017). The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 61, 236–249. doi: 10.1016/j.wasman.2016.11.028
Fay, L., and Shi, X. (2012). Environmental impacts of chemicals for snow and ice control: state of the knowledge. Wat Air Soil Poll. 223, 2751–2770. doi: 10.1007/s11270-011-1064-6
Franchino, M., Tigini, V., Varese, G. C., Mussat Sartor, R., and Bona, F. (2016). Microalgae treatment removes nutrients and reduces ecotoxicity of diluted piggery digestate. Sci. Total Environ. 569–570, 40–45. doi: 10.1016/j.scitotenv.2016.06.100
Golovko, O., Ahrens, L., and Schelin, J., Sörengård,M., Bergstrand, K., Asp, H., Hutberg, M., and Wiberg, K. (2022) Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 313,:114997, doi: 10.1016/j.jenvman.2022.114997
Hall, L. W. J., Scott, M. C., and Killen, W. D. (1998). Ecological risk assessment of copper and cadmium in surface waters of Chesapeake Bay watershed. Environ. Toxicol. Chem. 292:292940
Herbes, C., Roth, U., Wulf, S., and Dahlin, J. (2020). Economic assessment of different biogas digestate processing technologies: a scenario-based analysis. J. Clean. Prod. 255:120282. doi: 10.1016/j.jclepro.2020.120282
Holatko, J., Hammerschmiedt, T., Kintl, A., Danish, S., Skarpa, P., Latal, O., et al. (2021). Effect of carbon-enriched digestate on the microbial soil activity. PLoS One 16:e0252262. doi: 10.1371/journal.pone.0252262
Jiménez-Quero, A., Pollet, E., Zhao, M., Marchioni, E., Avérous, L., and Phalip, V. (2016). Itaconic and Fumaric acid production from biomass hydrolysates by aspergillus strains. J. Microbiol. Biotechnol. 26, 1557–1565. doi: 10.4014/jmb.1603.03073
Joho, M., Inouhe, M., Tohoyama, H., and Murayama, T. (1995). Nickel resistance mechanisms in yeasts and other fungi. J. Ind. Microbiol. 14, 164–168. doi: 10.1007/BF01569899
Karaffa, L., Fekete, E., and Kubicek, C. P. (2021). The role of metal ions in fungal organic acid accumulation. Microorganisms 9:1267. doi: 10.3390/microorganisms9061267
Kumar, S., Posmanik, R., Spatari, S., and Ujor, V. C. (2022). Repurposing anaerobic digestate for economical biomanufacturing and water recovery. Appl. Microbiol. Biotechnol. 106, 1419–1434. doi: 10.1007/s00253-022-11804-6
Lehnert, N., Coruzzi, G., Hegg, E., Seefeldt, L., and Stein, L. (2016) NSF workshop report: feeding the world in the 21st century: grand challenges in the nitrogen cycle. National Science Foundation, Arlington, VA.
Lehnert, N., Musselman, B. M., and Seefeldt, L. C. (2021). Grand challenges in the nitrogen cycle. Chem. Soc. Rev. 50, 3640–3646. doi: 10.1039/D0CS00923G
Liang, X., Rengasamy, O., Smernik, R., and Mosley, L. M. (2021). Does the high potassium content in recycled winery wastewater used for irrigation pose risks to soil structural stability? Agric. Water Manag. 343:106422. doi: 10.1016/j.agwat.2020.106422
Liu, J., Qiu, Y., He, L., Luo, K., and Wang, Z. (2021). Effect of iron and phosphorus on the microalgae growth in co-culture. Arch. Microbiol. 203, 733–740. doi: 10.1007/s00203-020-02074-9
Liu, W., Yang, Q., and Du, L. (2009). Soilless cultivation for high-quality vegetables with biogas manure in China: feasibility and benefit analysis. Renew Agric. Food Syst. 24, 300–307. doi: 10.1017/S1742170509990081
Mims, M. P., and Prchal, J. T. (2005). Divalent metal transporter 1. Hematology 10, 339–345. doi: 10.1080/10245330500093419
Möller, K., and Müller, T. (2012). Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng. Life Sc. 12, 242–257. doi: 10.1002/elsc.201100085
Mousavi, S. N., Parchami, M., Ramamoorthy, S. K., Soufiani, A. M., Hakkarainen, M., and Zamani, A. (2023). Bioconversion of carrot pomace to value-added products: Rhizopus delemar fungal biomass and cellulose. Fermentation 9:374. doi: 10.3390/fermentation9040374
Nurmiyanto, A., Kodera, H., Kindaich, I. T., Ozaki, N., Aoi, Y., and Ohashi, A. (2017). Dominant Candidatus accumulibacter phosphatis enriched in response to phosphate concentrations in EBPR process. Microbes Environ. 32, 260–267. doi: 10.1264/jsme2.ME17020
Okonkwo, C. C., Duduyemi, A., Ujor, V. C., Atiyeh, H. K., Iloba, I., Qureshi, N., et al. (2023). From agricultural wastes to fermentation nutrients: a case study of 2,3-Butanediol production. Fermentation 9:36. doi: 10.3390/fermentation9010036
Paerl, H. W., and Barnard, M. A. (2020). Mitigating the global expansion of harmful cyanobacterial blooms: moving targets in a human-and climatically-altered world. Harmful Algae 96:101845. doi: 10.1016/j.hal.2020.101845
Pattarkine, V. M., and Randall, C. W. (1999). The requirement of metal cations for enhanced biological phosphorus removal by activated sludge. Water Sci. Technol. 40, 159–165. doi: 10.2166/wst.1999.0112
Philpott, C. C. (2006). Iron uptake in fungi: a system for every source. Biochim. Biophys. Acta 1763, 636–645. doi: 10.1016/j.bbamcr.2006.05.008
Pranamuda, H., Tokiwa, Y., and Tanaka, H. (1995). Microbial degradation of an aliphatic polyester with a high melting point, poly(tetramethylene succinate). Appl. Environ. Microbiol. 61, 1828–1832. doi: 10.1128/aem.61.5.1828-1832.1995
Prasad, D., Verma, N., Bakshi, M., Narayan, O. P., Singh, A. K., Dua, M., et al. (2019). Functional characterization of a magnesium transporter of root endophytic fungus Piriformospora indica. Front. Microbiol. 9:3231. doi: 10.3389/fmicb.2018.03231
Ralston, D. K., and Moore, S. K. (2020). Modeling harmful algal blooms in a changing climate. Harmful Algae 91:101729. doi: 10.1016/j.hal.2019.101729
Robinson, J. R., Isikhuemhen, O. S., and Anike, F. N. (2021). Fungal-metal interactions: a review of toxicity and homeostasis. J. Fungi 7:225. doi: 10.3390/jof7030225
Schuler, A. J., and Jenkins, D. (2002). Effects of pH on enhanced biological phosphorus removal metabolisms. Water Sci. Technol. 46, 171–178. doi: 10.2166/wst.2002.0579
Sebastian, J., Dominguez, K. V., Brar, S. K., and Rouissi, T. (2021). Fumaric acid production using alternate fermentation mode by immobilized Rhizopus oryzae-a greener production strategy. Chemosphere 281:130858. doi: 10.1016/j.chemosphere.2021.130858
Serafim, L. S., Lemos, P. C., Levantesi, C., Tandoi, V., Santos, H., and Reis, M. A. M. (2002). Methods for detection and visualization of intracellular polymers stored by polyphosphate accumulating microorganisms. J. Microbiol. Methods 51, 1–18. doi: 10.1016/S0167-7012(02)00056-8
Shimada, Y., Tominaga, Y., Iwai, M., and Tsujisaka, Y. (1983). Increase in the activity of Rhizopus delemar lipase on water-soluble esters by its binding with phosphatidylcholine. J. Biochem. 93, 1655–1660. doi: 10.1093/oxfordjournals.jbchem.a134305
Smil, V. (2001) Enriching the earth: Fritz Haber, Carl Bosch and the transformation of world food production. Cambridge. MIT Press.
The Royal Society (2020) Ammonia: zero-carbon fertilizer, fuel and energy store. Policy Briefing. Available at: https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf (Accessed July 27, 2023).
Thompson, G. W., and Medve, R. J. (1984). Effects of aluminum and manganese on the growth of ectomycorrhizal fungi. Appl. Environ. Microbiol. 48, 556–560. doi: 10.1128/aem.48.3.556-560.1984
Ujor, V. C., Okonkwo, C. C., Rush, B. B., McCrea, G. E., and Ezeji, T. C. (2020). Harnessing the residual nutrients in anaerobic Digestate for ethanol fermentation and Digestate remediation using Saccharomyces cerevisiae. Fermentation 6:52. doi: 10.3390/fermentation6020052
Voelkner, A., Ohl, S., Holthusen, D., Hartung, E., Dörner, J., and Horn, R. (2015). Impact of mechanically pre-treated anaerobic digestates on soil properties. J. Soil Sci. Plant Nutr. 15, 882–895. doi: 10.4067/S0718-95162015005000061
Wallach, R. (2010). Effect of soil water repellency on moisture distribution from a subsurface point source. Water Resour. Res. 46:8521. doi: 10.1029/2009WR007774
Wang, W., Chang, J. S., and Lee, D. J. (2023). Anaerobic digestate valorization beyond agricultural application: current status and prospects. Bioresour. Technol. 373:128742. doi: 10.1016/j.biortech.2023.128742
Wang, Y., Wu, M., Yu, J., Zhang, J., Zhang, R., Zhang, L., et al. (2014). Differences in growth, pigment composition and photosynthetic rates of two phenotypes Microcystis aeruginosa strains under high and low iron conditions. Biochem. Syst. Ecol. 55, 112–117. doi: 10.1016/j.bse.2014.02.019
Wani, A. L., Ara, A., and Usmani, J. A. (2015). Lead toxicity: a review. Interdiscip. Toxicol. 8, 55–64. doi: 10.1515/intox-2015-0009
Wilson, D. (2015). An evolutionary perspective on zinc uptake by human fungal pathogens. Metallomics 7, 979–985. doi: 10.1039/C4MT00331D
Keywords: anaerobic digestate, fumaric acid, phosphate-accumulating organisms, microbial nutrient removal, phosphate removal
Citation: Agyeman-Duah E, Okonkwo CC and Ujor VC (2023) Microbial removal of nutrients from anaerobic digestate: assessing product-coupled and non-product-coupled approaches. Front. Microbiol. 14:1299402. doi: 10.3389/fmicb.2023.1299402
Edited by:
Thomas Maskow, Helmholtz Centre for Environmental Research, Helmholtz Association of German Research Centres (HZ), GermanyReviewed by:
Amrik Bhattacharya, Amity University, IndiaBiswanath Patra, M.B. Veterinary College, India
Copyright © 2023 Agyeman-Duah, Okonkwo and Ujor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor C. Ujor, dWpvckB3aXNjLmVkdQ==
 Eric Agyeman-Duah
Eric Agyeman-Duah Christopher C. Okonkwo
Christopher C. Okonkwo Victor C. Ujor
Victor C. Ujor