
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 27 October 2023
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1290858
This article is part of the Research Topic New and advanced mechanistic insights into the influences of the infant gut microbiota on human health and disease View all 23 articles
 Tadatsugu Morimoto1*
Tadatsugu Morimoto1* Takaomi Kobayashi1
Takaomi Kobayashi1 Toshihiko Kakiuchi2
Toshihiko Kakiuchi2 Motohiro Esaki3
Motohiro Esaki3 Masatsugu Tsukamoto1
Masatsugu Tsukamoto1 Tomohito Yoshihara1
Tomohito Yoshihara1 Hirohito Hirata1
Hirohito Hirata1 Shoji Yabuki4
Shoji Yabuki4 Masaaki Mawatari1
Masaaki Mawatari1As society ages, the number of patients with spinal degenerative diseases (SDD) is increasing, posing a major socioeconomic problem for patients and their families. SDD refers to a generic term for degenerative diseases of spinal structures, including osteoporosis (bone), facet osteoarthritis (joint), intervertebral disk degeneration (disk), lumbar spinal canal stenosis (yellow ligament), and spinal sarcopenia (muscle). We propose the term “gut-spine axis” for the first time, given the influence of gut microbiota (GM) on the metabolic, immune, and endocrine environment in hosts through various potential mechanisms. A close cross-talk is noted between the aforementioned spinal components and degenerative diseases. This review outlines the nature and role of GM, highlighting GM abnormalities associated with the degeneration of spinal components. It also summarizes the evidence linking GM to various SDD. The gut-spine axis perspective can provide novel insights into the pathogenesis and treatment of SDD.
With an increasing aging population in society, the number of patients with spinal degenerative diseases (SDD) has been on the rise, with a reported annual prevalence of 27.1% for SDD in the United States (Parenteau et al., 2021). SDD refers to a generic term for diseases caused by the degeneration of spinal structures. Examples of combinations of spinal structures and their degenerative diseases include osteoporotic vertebral fractures (OVF) in bones, facet joint osteoarthritis (OA) in cartilages, intervertebral disk degeneration (IVDD) in the intervertebral disk (IVD), lumbar spinal canal stenosis (LSS) in ligaments, and spinal sarcopenia in muscles. Furthermore, osteoporosis, facet joint OA, IVDD, LSS, and spinal sarcopenia tend to coexist, as their incidence increases with age (Parenteau et al., 2021), adversely affecting each other’s pathology and forming a negative spiral cycle.
Several studies have reported risk factors, including aging, heavy labor (mechanical stress), trauma, genetics, obesity, and metabolic syndrome, for SDD (Puenpatom and Victor, 2009; Yoshimura et al., 2012; Imajo et al., 2015; Chen et al., 2019; Guss et al., 2019; Azzini et al., 2020; Kuo et al., 2020; Parenteau et al., 2021; Li et al., 2022). The pain and paralysis caused by SDD have become a major problem that affects the patient’s life and work and imposes a significant economic burden on the patient, family, and society (Imajo et al., 2015). The causes of SDD remain poorly understood, and understanding their causes can be critical for prevention and treatment. The progressive degeneration of spinal structures has been attributed not only to biomechanical injury or stress but also to biochemical stressors that can adversely affect the regular activity of cells and tissues in spinal structures. These risk factors, independently or complexly linked, contribute to the complex interplay between mechanical and biochemical factors that lead to the etiology of SDD.
Metabolic syndrome involves multiple physiological systems that are directly related to the presence of four major clusters: insulin resistance, obesity, vascular pathology, and dyslipidemia (Azzini et al., 2020). Metabolic syndrome induces “meta-inflammation,” characterized by persistent, low-grade systemic inflammation caused by metabolic stress, which places biochemical stress on systemic tissues (Azzini et al., 2020). In particular, in metabolic syndrome, chronic low-level inflammation mediated by macrophages occurs in the liver, visceral fat, pancreas, colon, brain, and musculoskeletal tissues and is implicated in the development of various diseases (Azzini et al., 2020). In addition, chronic low-level persistent tissue inflammation that occurs with aging, also referred to as “inflammaging,” has been reported to be associated with the development of osteoporosis (Benoist, 2003; Ibáñez et al., 2019; Zaiss et al., 2019), IVDD (Benoist, 2003; Franceschi and Campisi, 2014; Sadowska et al., 2018; Li et al., 2022), OA (Berthelot et al., 2019; De Sire et al., 2020; Favazzo et al., 2020; Gracey et al., 2020; Qaiyum et al., 2021; Zaiss et al., 2021; Motta et al., 2023; Romero-Figueroa et al., 2023), and sarcopenia (Picca et al., 2018; Ticinesi et al., 2019; Kuo et al., 2020; Przewłócka et al., 2020). The gut microbiota (GM), a complex of intestinal bacterial populations, is responsible for a series of metabolic, immune, structural, and neurological functions, including metabolic homeostasis, immune system development and maturation, resistance to infection, and neurotransmitter production (Biver et al., 2019). Recent studies have highlighted important regulatory functions of GM in neuroendocrine and immune functions through the activity of microbiome and its metabolites and its involvement in disease processes in various organs inside and outside the gut (such as brain, kidney, liver, heart, musculoskeletal) (Biver et al., 2019). GM is also involved in the development and progression of inflammatory diseases such as obesity and metabolic syndrome. GM disruption has emerged as a hidden risk factor inducing the production of inflammatory cytokines and bacterial metabolites, which may be involved in the pathophysiological mechanisms of musculoskeletal diseases, including SDD (Biver et al., 2019).
Although the gut-brain axis is the best-known term for this cross-talk between GM and the gut and distant organs, data supporting the important role of GM in spinal conditions and its involvement in the onset and progression of SDD have been obtained. A gut-bone axis (Ibáñez et al., 2019; Zaiss et al., 2019), gut-joint axis (Berthelot et al., 2019; De Sire et al., 2020; Favazzo et al., 2020; Gracey et al., 2020; Qaiyum et al., 2021; Zaiss et al., 2021; Romero-Figueroa et al., 2023), gut-disk axis (Li et al., 2022), and gut-muscle axis (Picca et al., 2018; Ticinesi et al., 2019; Kuo et al., 2020; Przewłócka et al., 2020) have also been proposed, with possible association between GM and SDD-related pain (Tonelli Enrico et al., 2022). Furthermore, studies have also reported a relationship between GM and adolescent idiopathic scoliosis (Shen et al., 2019) and ankylosing spondylitis (Guggino et al., 2021). Given the close relationship between GM and SDD based on the common factors of immunity, metabolism, and inflammation, we can hypothesize the involvement of GM in the degeneration of spinal structures, with GM being a potential mechanism for the development of SDD.
We propose the novel and comprehensive concept of the “gut-spine axis” for the first time, as knowledge about the “gut-spine cross-talk,” which summarizes the relationship of GM with spinal structures (bone, cartilage, disks, ligaments, and muscle) and the impact of GM on pain can improve our understanding of the etiology of SDD and contribute to development of treatment strategies for SDD. This review outlines the nature and role of GM and GM abnormalities associated with the degeneration of spinal structures and also summarizes the evidence linking GM to various SDD. For a broader, more flexible, and more comprehensive organization, we employed a narrative review approach and analyzed several important articles regarding the relationship between SDD and GM published in peer-reviewed scientific journals. This study outlines the reports supporting the presence of a gut-spine axis in the etiology of SDD.
Surprisingly, the large intestine contains more than 70% of all the microorganisms in the human body (Jandhyala et al., 2015). GM refers to the diverse collection of microorganisms, including commensal, symbiotic, and pathogenic species, that reside in the intestine (Jandhyala et al., 2015). The intestine, being a multicellular organ acquired at birth, exists as a distinct entity yet has clearly co-evolved with the human genome. It interacts with the host and exerts various influences on it through communication and other mechanisms (Qin et al., 2010). Although there are more than 1,000 species of bacteria and 1014 bacterial cells in the human gut (Chen et al., 2022b), detection of most anaerobic bacteria has been difficult using culture techniques. In recent years, a new sequencing technology, known as high-throughput “next-generation sequencing” (NGS) of microbial DNA, has emerged as a leading approach to better characterize the human microbiota (Chen et al., 2022b). GM samples for analysis are collected from the stool. Microbiome profiling is often performed by sequencing 16S rRNA gene amplicons in a culture-independent method or by shotgun sequencing (metagenomics) of the entire microbiome. As a result, GM has gained significant attention due to advancements in NGS technology. The importance of GM’s diversity, composition, and functional characteristics in shaping human health has been widely acknowledged.
Under normal physiologic conditions, GM plays a fundamental role in various aspects of host physiology. This includes functions such as nutrition and metabolism, maintaining the integrity of the gut barrier to prevent pathogen invasion, and supporting immune responses. In contrast, disruption of these roles may contribute to inflammation, pain, and development of various diseases (Jandhyala et al., 2015).
GM provides the host with vital functions, including the fermentation of dietary fiber and indigestible polysaccharides, leading to the production of biotransformed bile acids that regulate calcium absorption. Additionally, GM is involved in the synthesis of vitamins B and K, as well as the production of essential and nonessential amino acids, short-chain fatty acids (SCFA) (i.e., acetic acid, propionic acid, and butyric acid), and neurotransmitters (including some precursors) (Ibrahim et al., 2022, p. 191; Chen et al., 2022b; Figure 1A). GM is often referred to as an endocrine organ capable of influencing the function of distant organs and systems, given the involvement of GM in the production of metabolites (Clarke G. et al., 2014).
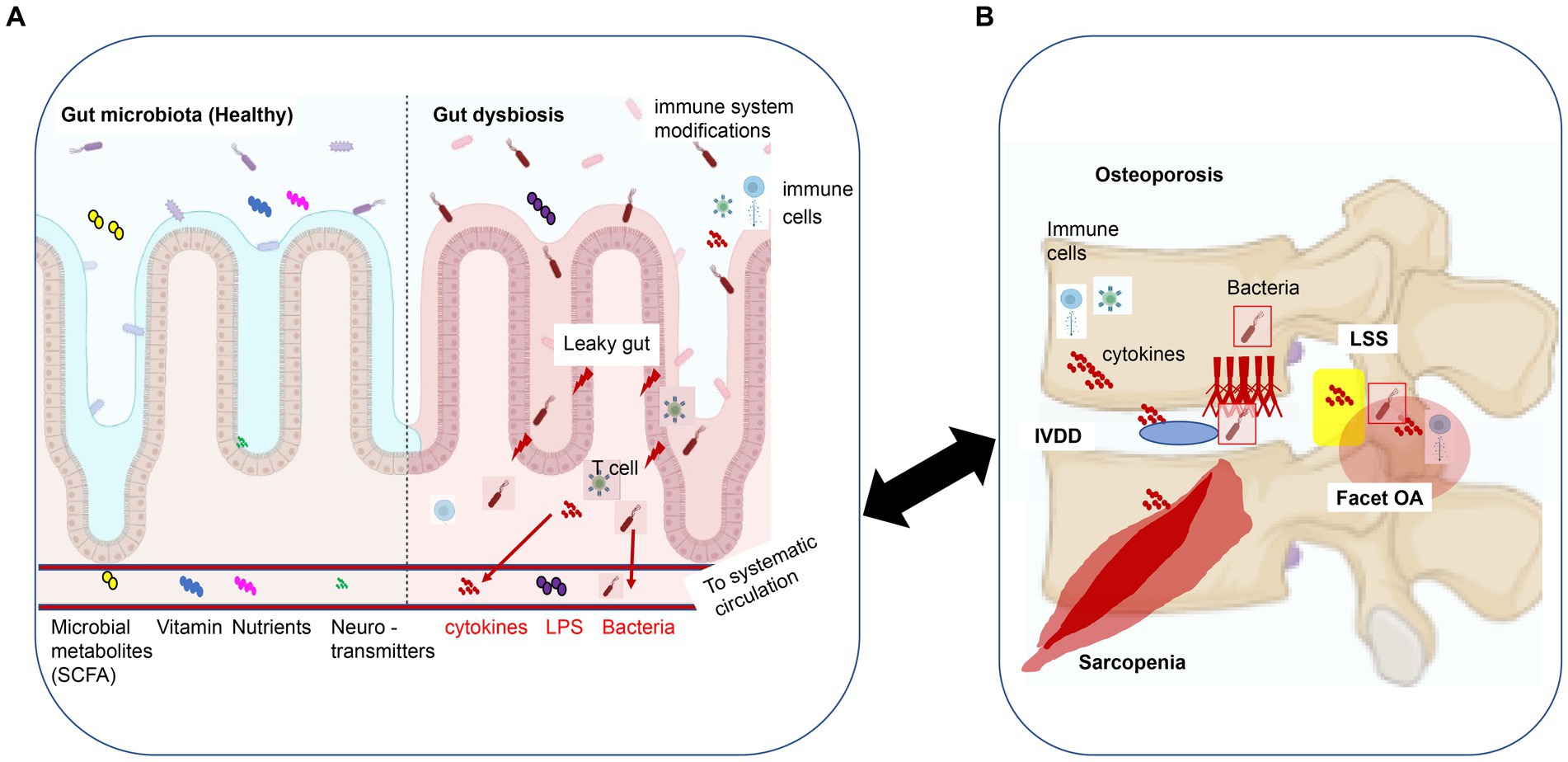
Figure 1. The gut-spine axis: a model of intestinal epithelial damage signaling that may regulate degeneration of spinal structures. (A) Gut microbiota and dysbiosis: GM supplies the host with essential functions such as the synthesis of short-chain fatty acids (SCFA), vitamins, nutrients, and neurotransmitters (including some precursor substances). When leaky gut syndrome occurs, which results in altered GM composition and GM dysbiosis, the intestinal barrier is compromised, altering physiological and metabolic functions and disrupting immune, endocrine, vascular, and nervous system responses, resulting in bacteremia, bacterial-derived compounds (LPS), and cytokines circulating along the bloodstream to systemic tissues. (B) Spinal degenerative diseases (SDD): osteoporosis, IVDD, facet OA, and LSS. GM dysbiosis-derived metabolites, bacteremia, and inflammatory cytokines can result in local and systemic responses that may cause SDD. This figure was adapted and modified from the figure by Li et al. (2022) and was created using BioRender.com. This content is available under Creative Commons Attribution 4.0 International License (https://link.springer.com/article/10.1007/s00586-022-07152-8). IVDD, intervertebral disk degenerative disease; OA, osteoarthritis; LSS, lumbar spinal canal stenosis; SCFA, short-chain fatty acids; LPS, lipopolysaccharide.
Naturally, the absorption of calcium and the production of vitamin K and amino acids, both of which involve GM, are essential for musculoskeletal health (Chen et al., 2022b). In addition, because GM secretes estrogen-regulating enzymes, aberrations in GM, such as low diversity of GM, may lead to a decrease in circulating estrogen level (Baker et al., 2017). Estrogen deficiency at menopause is a major cause of postmenopausal osteoporosis, with excessive osteoclast formation and bone resorption stimulation due to estrogen deficiency at menopause leading to rapid bone loss (D’Amelio et al., 2008). Decreased estrogen levels increase the production of inflammatory and osteoclastogenic cytokines, leading to osteoporosis. There is speculation that postmenopausal women, who lose the immunosuppressive effects of estrogen, may experience greater bone loss due to an increased inflammatory state caused by an unfavorable GM composition (Baker et al., 2017). Thus, the interaction between GM and estrogen influences the composition of GM and the response of host tissues, including bone, to GM (Baker et al., 2017). Thus, the interaction between GM and estrogen is also referred to as the “estrogen-gut microbiome axis” (Baker et al., 2017).
The analgesic, anti-inflammatory, antinociceptive, and neuroprotective effects of the B vitamins have been widely documented. Among the B vitamins, vitamin B12 is known to be effective in reducing back pain and nerve pain (Tonelli Enrico et al., 2022).
SCFA are largely secreted in the colon by GM through anaerobic fermentation of dietary fiber and have a critical role in regulating intestinal inflammation and epithelial barrier function. In addition, SCFA enters the bloodstream and further contributes to the regulation of systemic inflammation (Tonelli Enrico et al., 2022). Besides the immune system controlling inflammation, SCFA influences various aspects of metabolism, including bone metabolism, glucose metabolism, brown adipose tissue activation, liver mitochondrial function regulation, whole body energy homeostasis, appetite, and sleep regulation (Lucas et al., 2018). Neurotransmitters such as dopamine, serotonin, leptin, noradrenaline, glutamate, and GABA can be directly produced by GM or indirectly regulated by GM (Tonelli Enrico et al., 2022).
At least 50% of dopamine is produced in the gut and has regulatory functions in systemic inflammation and chronic pain through its involvement in immunity responses (Vidal and Pacheco, 2020). A previous study has reported that 90% of serotonin is produced in the intestinal tract and that circulating serotonin of intestinal origin inhibits bone formation and decreases bone mass (Yadav et al., 2008). The GM was shown to regulate intestinal chromaffin cells that modulate serotonin production in the gut (Yadav et al., 2008). Serotonin also regulates physiological functions related to pain (Malinova et al., 2018). Leptin, an adipocyte-specific hormone, regulates bone formation, while GM positively regulates systemic levels of leptin and is involved in bone metabolism (Charnay et al., 2000).
Noradrenaline is also responsible for pain and inflammation (Tonelli Enrico et al., 2022). Glutamate and GABA function as major excitatory and inhibitory neurometabolites, respectively, in the central nervous system and play various important physiological roles, including processing and regulation of pain (e.g., inflammation; Tonelli Enrico et al., 2022).
Consecutively, vitamin B, SCFA, and neurotransmitters are involved in the immune response and regulate pain and inflammation. Therefore, GM-related changes in nutrition and metabolites can impact musculoskeletal health, reduce anti-inflammatory effects globally, and lower pain tolerance through the gut-brain axis. These factors may contribute to the development of SDD and SDD-related pain.
Conversely, lipopolysaccharide (LPS) from gram-negative bacteria, a metabolite derived from GM, is an inflammatory metabolite that acts on macrophages, which maintain tissue homeostasis and exert pro-inflammatory effects (Burcelin et al., 2013). LPS enters the systemic circulation through the intestinal tract and bloodstream to induce low-level systemic inflammation. LPS levels are known to be elevated in chronic inflammatory diseases such as obesity (Burcelin et al., 2013), metabolic syndromes (Burcelin et al., 2013), osteoporosis (Wu et al., 2021), OA (Huang and Kraus, 2016), and IVDD (Lisiewski et al., 2022).
Therefore, while some molecules produced by intestinal bacteria are beneficial, others are harmful and can affect endocrine cells in the gut, the enteric nervous system, intestinal permeability, and the immune system (Ohlsson and Sjögren, 2015). Meanwhile, GM composition and function may have beneficial or detrimental effects on the degeneration of spinal structures (such as bones, cartilage, disks, ligaments, and muscles) and pain associated with SDD (Charles et al., 2015; Chen et al., 2022b; Figure 1B).
GM is acquired at birth, derived almost exclusively from the mother (Das and Nair, 2019; Chen et al., 2022a). In the case of healthy people, the characteristic features of GM are a state of dynamic homeostasis defined by the richness and diversity of GM compositions and by its stability and resilience to withstand various types of disturbances (Das and Nair, 2019). The richness and diversity of GM compositions can be altered by environmental factors (such as aging) and lifestyle factors (such as diet, sleep, and exercise; Ohlsson and Sjögren, 2015; Fei et al., 2021; Figure 2).
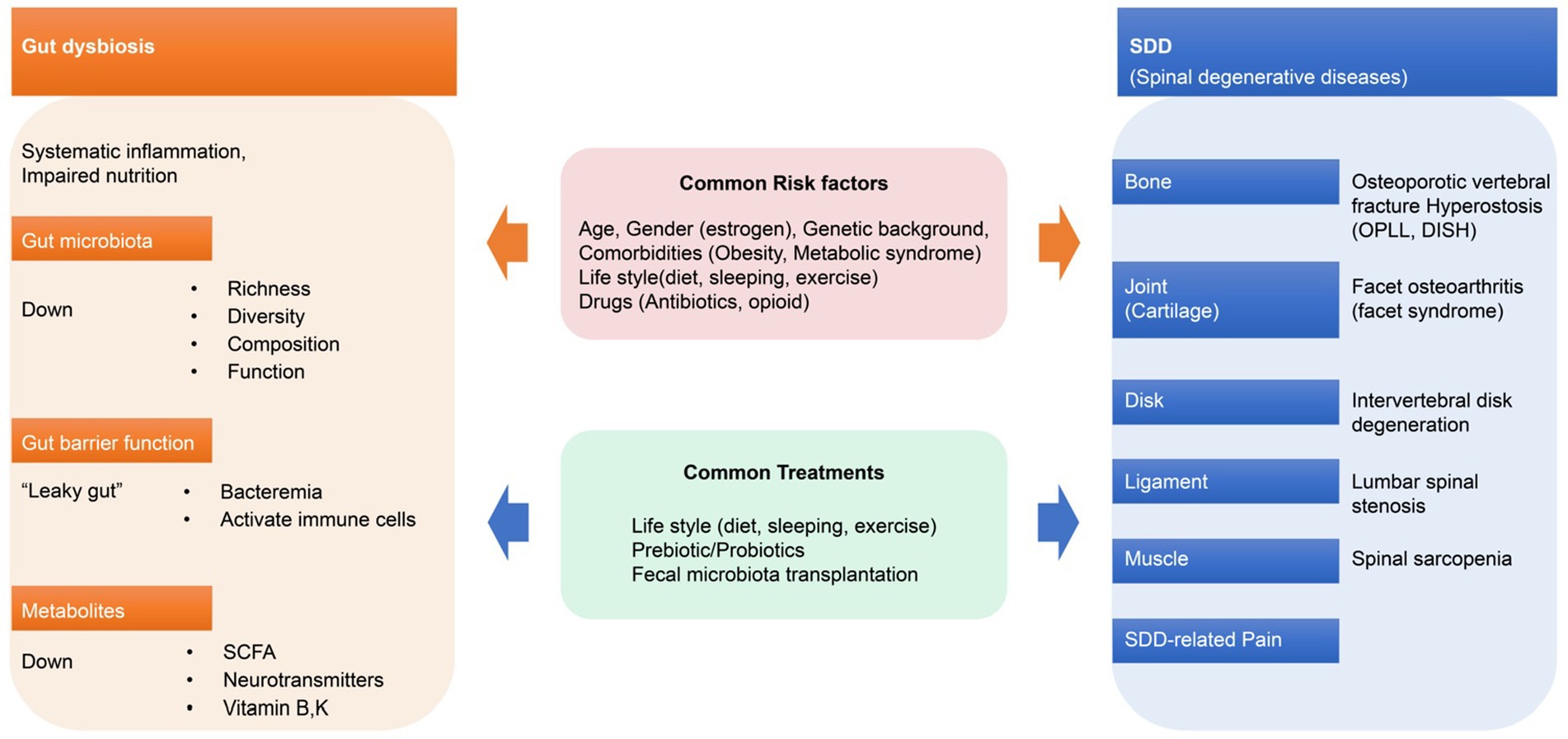
Figure 2. Gut dysbiosis and spinal degenerative diseases. Gut dysbiosis and SDD have common risk factors and treatments that can interact with each other. OPLL, ossification of the posterior longitudinal ligament; DISH, diffuse idiopathic skeletal hyperostosis.
Since GM composition continues to change in an age-dependent pattern from infancy to old age as GM co-evolves with the host (Chen et al., 2022a), organismal aging is inevitably accompanied by changes in GM. GM in older adults is characterized by reduced diversity and stability, decreased expression of genes producing SCFA, reduced ability to degrade glycoconjugates, enhanced proteolytic function, and at the genus level, enrichment of Proteobacteria (including many members of opportunistic pathogenic bacteria), and an increased proportion of the Bacteroidetes and Clostridium genera (Buford, 2017). Notably, reduced diversity of GM may correlate with “frailty index,” an indicator of biological age and a predictor of healthy life expectancy (Ke et al., 2021).
Furthermore, aging not only affects the composition of GM but also is associated with changes in the gut lumen and barrier function, including shrinkage of the protective mucus layer of the gut, loss of gut tight junction proteins, and increased permeability of the epithelial barrier (Elderman et al., 2017). Several lines of evidence in this regard suggest that low-level systemic inflammation has recently been recognized as a phenomenon associated with older adults (Konturek et al., 2015; Borghesan et al., 2020). Meanwhile, in healthy centenarian GM, the anti-inflammatory effect of Faecalibacterium spp. is strong, and blood LPS level is low, indicating that Faecalibacterium spp. may be suppressing host inflammation (Park et al., 2015).
In recent years, a cellular senescence-centric view of the aging process has emerged. Cellular senescence results in senescent cells (SCs), which exhibit age-dependent accumulation in tissues and organs of various mammalian species, including rodents and primates (Sharma, 2022). The surviving senescent cells develop a senescence-associated secretory phenotype (SASP), which secretes various inflammatory cytokines and promotes chronic inflammation and carcinogenesis (Tuttle et al., 2021). Low levels of chronic inflammation occur in older adults, which has been partly attributed to cellular senescence (Herbig et al., 2006). Increased SC burden with aging destroys tissue structure and function and is emerging as an important factor in increasing disease risk and mortality in older adults (Burd et al., 2013). Osteoporosis, sarcopenia, IVDD, and OA have been reported as musculoskeletal diseases associated with SASP (Burd et al., 2013; Khosla et al., 2020; Tuttle et al., 2021).
Increased age-related oxidative/inflammatory stress contributes to SC accumulation, and the application of antioxidants has been shown to inhibit cellular senescence both in vitro and in vivo (Kumar et al., 2019; Varela-Eirín et al., 2020). Therefore, it is conceivable that neutralizing oxidative/inflammatory stressors may attenuate or delay SC development and accumulation. In a landmark study, removing SC load in aging tissues delayed the onset and severity of age-related conditions (Baker et al., 2011). Since then, several natural and synthetic compounds that selectively target SC, called “senolytics,” have been identified. Clinical trials based on senolytics have already shown promising results in countering the harmful effects of aging (Baker et al., 2011; Kumar et al., 2019; Varela-Eirín et al., 2020).
Various metabolites of GM are known to exert strong anti-inflammatory and antioxidant properties and may be useful in the prevention of inflammatory and tumorigenic environments associated with aging (Chang et al., 2014; De Marco et al., 2018; Wang et al., 2018; Riaz Rajoka et al., 2019; Chung et al., 2020; Rossi et al., 2020; Sharma, 2022). In contrast, GM dysbiosis can also cause chronic inflammatory stress throughout the body, which may promote cellular senescence. GM dysbiosis may also affect SDD via cellular senescence. Thus, GM is closely related to human aging, and changes in GM may predict the development and prognosis of SDD.
Diet, sleep, and exercise are three key components of a healthy lifestyle that can affect GM composition and function (Ohlsson and Sjögren, 2015; Fei et al., 2021; Figure 2). Diets can significantly change GM composition depending on nutrients. This can be attributed to the fact that nutrients can alter the GM microenvironment, including GM composition, metabolism, and the host’s immune response (Li et al., 2016).
Sleep length has been demonstrated to have a significant effect on the composition and function of GM (Fei et al., 2021). Short sleep duration promotes GM abnormalities (less diversity of gut bacteria and fewer anti-inflammatory gut bacteria), which may affect chronic inflammation-related diseases (Benedict et al., 2016; Poroyko et al., 2016; Reynolds et al., 2017; Fei et al., 2021).
Sleep disruption has been reported to affect physical, mental, and emotional functioning, in addition to hormonal and metabolic disturbances (Fei et al., 2021). Possible causes include increased inflammatory markers (Leproult et al., 2014), increased sympathetic nervous system activity, abnormal cortisol rhythms, and changes in appetite-regulating hormones and food intake (Spiegel et al., 1999), all of which contribute to the risk of diabetes and obesity. Given the association between diseases associated with sleep disorders and diseases derived from abnormalities in the GM, the adverse health effects observed in sleep disorders may be partly due to gut bacteria (Fei et al., 2021).
Physical activity (including exercise) has been shown to produce positive changes in the qualitative and quantitative composition and metabolic function of GM and provide health benefits to the host in animal models (Li et al., 2016; Poroyko et al., 2016; Reynolds et al., 2017) and humans (Clarke S. F. et al., 2014). Athletes generally have a high diversity of GMs with anti-inflammatory properties and a high capacity for SCFA synthesis compared to sedentary controls (Barton et al., 2018; Ticinesi et al., 2019). In studies including both younger and older adults, the expression of Bifidobacterium spp. and Faecalibacterium prausnitzii involved in SCFA production, which potentiates anti-inflammatory effects, increased after exercise implementation (aerobic and resistance training), and the concentration of butyrate in the stool increased (Allen et al., 2018; Erlandson et al., 2021). Regular exercises have been shown to benefit older adults, especially those who are overweight, by maintaining the stability (composition and function) of the intestinal microbiota (Zhu et al., 2020). The utility of exercise in preventing and treating SDD is not limited to the typical rehabilitative effects, such as improved muscle function, pain relief, and improved range of motion, but also to the synergistic effects of improving GM composition and function with improved health benefits for the host.
Aging, dietary changes, smoking and alcohol consumption, disease, antibiotic treatment, and pathogens can alter GM composition, leading to dysbiosis, defined as detrimental changes in bacterial composition, diversity, and function (Zheng et al., 2020). Dysbiosis can lead to imbalances in metabolic and immune regulatory networks that usually suppress intestinal inflammation, leading to inflammation, tissue destruction, and disease development (Kinashi and Hase, 2021).
The so-called “leaky gut syndrome,” which is characterized by altered permeability of the intestinal wall to antigens, is an example of dysbiosis, leading to systemic inflammation and abnormal immune response (Kinashi and Hase, 2021). When leaky gut syndrome, an example of dysbiosis, occurs, GM loses its protective capacity, the intestinal barrier is compromised, diffusion of bacterial-derived compounds is enhanced, and metabolite and cytokine expression is altered (Ohlsson and Sjögren, 2015; Kinashi and Hase, 2021). Therefore, it is not impossible for dysregulated circulatory inflammatory cytokines to reach spinal structures (bones, cartilage, disks, ligaments, and muscles; Figures 1A,B, 2) and disrupt normal cell signaling and metabolic activity.
In ophthalmology, GM dysbiosis reportedly causes chronic low-grade inflammation, which is characteristic of inflammatory conditions with increased intestinal permeability and increased production of IL-6, IL-1β, TNF-α, and VEGF-A, ultimately leading to exacerbation of pathological angiogenesis in choroidal neovascularization (Andriessen et al., 2016). Similar mechanisms may exist in the pathological angiogenesis of various diseases, including SDD. Dysbiosis, with an altered composition of GM, can alter gut wall permeability and physiological and metabolic functions, and disrupt immune, endocrine, vascular, and nervous system responses, ultimately leading to: diseases characterized by immune dysregulation (allergies, autoimmune diseases, inflammatory; Miyauchi et al., 2023), metabolic diseases (diabetes, metabolic syndrome; Guss et al., 2019), cardiovascular diseases (coronary artery disease; Jie et al., 2017), neurodegenerative diseases (autism; Cryan et al., 2019), cancer (stomach, lung, colon; DeStefano Shields et al., 2021; Sepich-Poore et al., 2021), and even SDD such as osteoporosis, facet joint OA, IVDD, and LSS (Ohlsson and Sjögren, 2015; Azzini et al., 2020; DeStefano Shields et al., 2021; Kinashi and Hase, 2021; Li et al., 2022; Lisiewski et al., 2022; Figure 2).
Furthermore, the reduced number of myeloid progenitor cells in sterile mice suggests that the metabolites of intestinal bacteria affect immune cells in the bone marrow (Khosravi et al., 2014). The human spine has 26 vertebrae that are the source of immune cells. GM dysbiosis can cause abnormal immune cell formation in the spinal bone marrow, leading to systemic inflammation and back pain (Nouh and Eid, 2015). To summarize, the role and relevance of GM in the pathogenesis of a group of age-related chronic inflammation-related diseases is an important emerging phenomenon that should not be overlooked.
Culture-independent approaches such as 16S rRNA sequencing and shotgun metagenomics have expanded our ability to identify all bacteria, confirming the presence of bacteria at sites previously considered sterile (Ozkan and Willcox, 2019). As a result, microbiomes are present in sites outside of joint environments traditionally thought to be sterile, including the reproductive tract, sperm, fetus, breast, and eye (Ardissone et al., 2014; Ozkan and Willcox, 2019).
During the neovascularization phase in OA, there is evidence that bacteria and bacterial products have a greater tendency to enter cartilage and subchondral bone from the blood; this is particularly notable as these areas are typically less vascularized (Pesesse et al., 2011). Certainly, the microbiome is present in knee and hip OA (Dunn et al., 2020). In addition, a systematic review reported the presence of various bacteria in degenerated and Modic change-altered IVD (Granville Smith et al., 2022).
In dysbiosis, due to the impaired barrier function of the intestinal wall and immune system, some GM may migrate from the intestinal tract to the bloodstream and cause transient bacteremia (Fine et al., 2020), allowing some GM to settle directly in the joints or allowing leukocytes and macrophages (Miller et al., 2020) to reach the joints and IVD as “Trojan horses” for the bacteria (Alverdy et al., 2020). This may result in the migration of some GM in the subchondral bone marrow and deep in the cartilage or disks, which may directly contribute to the deformity changes (Chisari et al., 2021; Rajasekaran et al., 2022). Chronic inflammation and adverse immune effects due to GM dysbiosis and bacteremia may also contribute to postoperative infection for SDD.
GM influences host metabolism, immunity, the endocrine environment, and the gut-brain-bone axis. It also affects spinal structures (bone, cartilage, disks, ligaments, and muscles) through a variety of potential mechanisms (Behera et al., 2020; Seely et al., 2021; Figures 1A,B).
GM dysbiosis affects the health of spinal structures through the following three mechanisms: (1) nutrition, including calcium, amino acids, and vitamin K; (2) immune regulation related to estrogen, SCFA, and systemic inflammation; and (3) neurotransmitters such as serotonin and leptin that have been demonstrated to affect bone metabolism, ultimately causing an imbalance between osteoblasts and osteoclasts (Chen et al., 2022b). Additionally, increased inflammatory stress associated with GM dysbiosis promotes the senescence of spinal musculoskeletal cells and the accumulation of various SCs at spinal structures (Borghesan et al., 2020; Sharma, 2022). SCs may cause local and systemic inflammation via SASP and contribute to the development of the following SDD: osteoporosis for senescent osteocytes and osteoblasts, OA for senescent chondrocytes, and IVDD for senescent nucleus pulposus cells (Borghesan et al., 2020; Sharma, 2022). This chapter outlines the nature and role of GM and dysbiosis associated with the degeneration of spinal structures (bone, cartilage, ligaments, disks, and muscles) and contributing to the development and progression of SDD and SDD-derived pain.
SDD related to bone metabolism includes OVF and hyperostotic diseases [i.e., ossification of posterior longitudinal ligament (OPLL) and diffuse idiopathic skeletal hyperostosis (DISH)] (Figure 3). Osteoporosis and hyperostotic diseases have an immune-inflammatory mechanism involved in their pathogenesis (Zaiss et al., 2019; Mader et al., 2021; Kawaguchi, 2022). Bone is an organ that depends on the dynamic balance between osteoblasts and osteoclasts to maintain normal function.
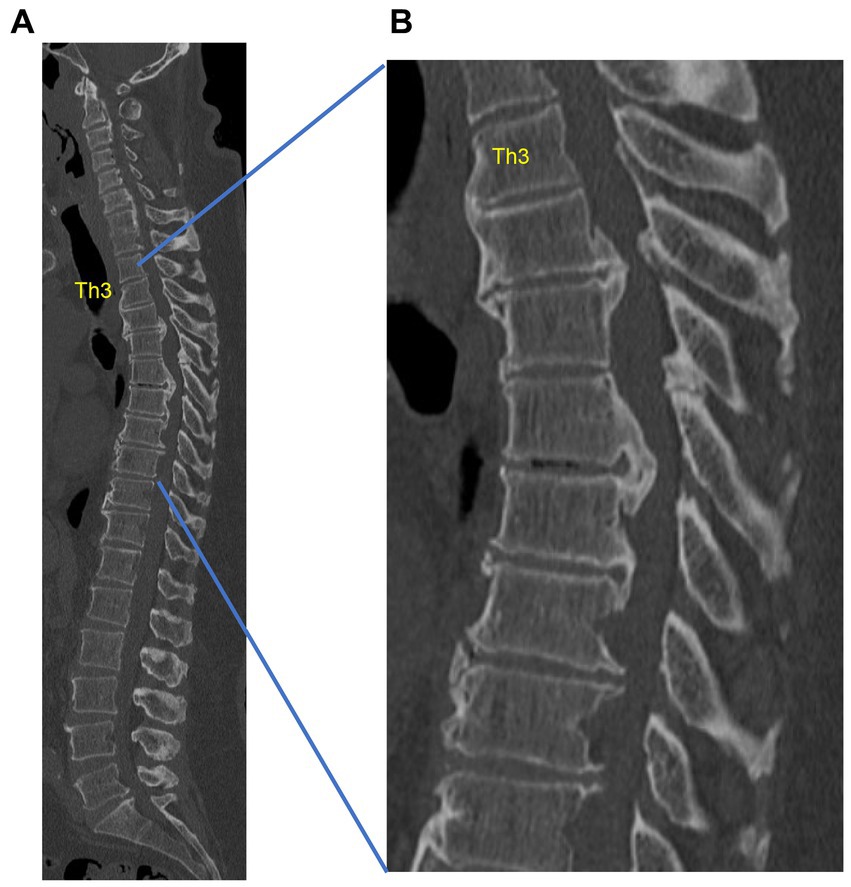
Figure 3. Example of thoracic ossification of the posterior longitudinal ligament (OPLL) in a 53-year-old obese male with type 2 diabetes mellitus. The spinal canal is markedly narrowed by thoracic OPLL, with inability to walk. (A) Sagittal computed tomography (CT) of the whole spine. (B) Sagittal CT of the thoracic spine. This is a case from our institution.
Inflammatory diseases involve several inflammatory cytokines that play a role in regulating osteoblasts and osteoclasts, and immune activity is considered a significant risk factor for osteoporosis (Arron and Choi, 2000). The term “osteoimmunology” describes the close interaction between the immune system and bone metabolism and the role of immune cells or immune-related factors in the regulation of skeletal development (Takayanagi, 2021).
In recent years, the effects of GM on bone tissue have been confirmed in animals lacking GM (Axenic mice), in animals fed GM-modifying antibiotics and diet, and in humans (Ibáñez et al., 2019). In addition, the presence of osteoporosis is associated with the composition and diversity of GM components (Wang et al., 2017; Ling et al., 2021).
Regarding osteoimmunology, GM dysbiosis has been reported to contribute to osteoporosis (Wang et al., 2017; Ibáñez et al., 2019; Ling et al., 2021) but may also affect hyperostosis diseases. Hyperostotic diseases such as OPLL and DISH exhibit a strong association with obesity, type 2 diabetes mellitus, and the complications of metabolic syndrome, which are characterized by systemic low-grade inflammation related to aging (Mader et al., 2021; Kawaguchi, 2022). In addition, OPLL has been reported to be associated with leptin and chronic inflammation (Kawaguchi, 2022). As mentioned, GM dysbiosis affects bone metabolism in association with low levels of chronic inflammation-related diseases and the neurotransmitter leptin, suggesting that it could be a pathological factor not only in osteoporosis but also in hyperostotic diseases such as OPLL. Thus, the term “gut-bone axis” (Zaiss et al., 2019) indicates that GM is associated with bone metabolism via nutrient absorption, inflammation immunity, and neurotransmitters, suggesting a strong association between GM dysbiosis and osteoporosis and hyperostotic diseases such as DISH and OPLL. As our understanding of the gut-bone axis’ pathophysiology improves, GM could emerge as a potential treatment option for bone metabolism disorders and low back pain. However, to the best of our knowledge, no epidemiological studies have reported on the association between GM and bone-related SDD such as osteoporotic spinal vertebral fractures or hyperostotic diseases.
OA caused by cartilage degeneration in facet joints has been proposed as a facet joint syndrome among SDD (Du et al., 2022; Figure 4). The “gut-joint axis” has been established based on the possibility of cross-talk between the gut and joint (Berthelot et al., 2019; De Sire et al., 2020; Chisari et al., 2021; Qaiyum et al., 2021).
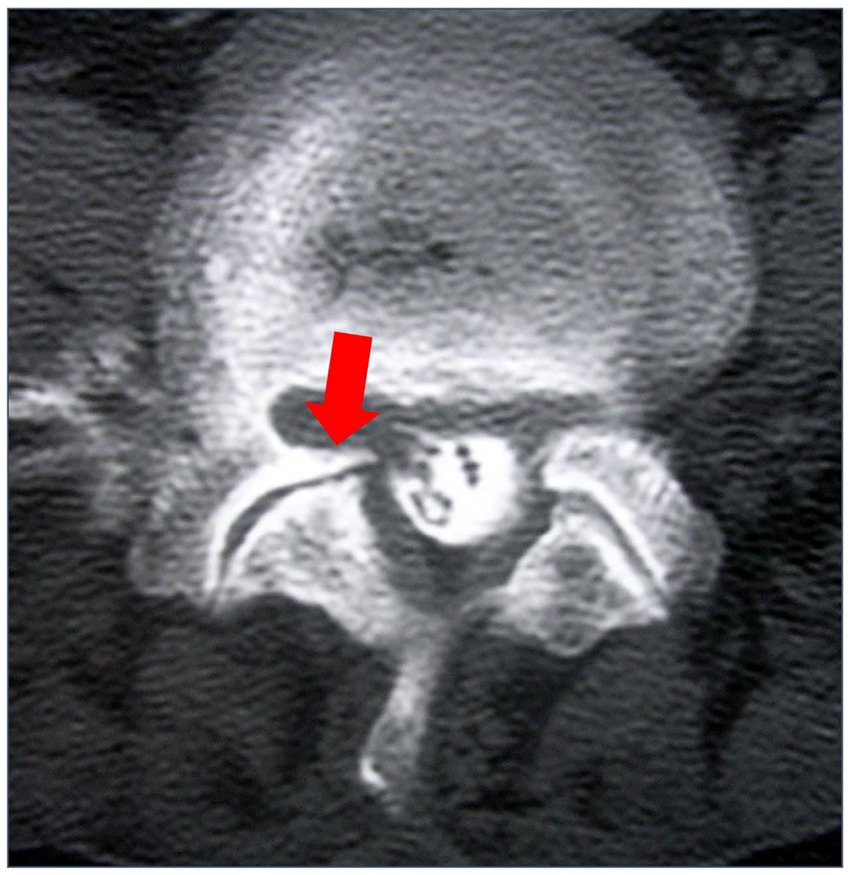
Figure 4. Myelography computed tomography (CT) in lumbar axial view. The red arrow indicates facet degeneration and osteophyte. This is a case from our institution.
Unfortunately, no reports have shown an association between facet joint syndrome and GM. However, based on the correlation between GM and lower-extremity OA, we believe that the association between GM and facet joint syndrome could be analogous. Obesity and metabolic syndrome are risk factors for OA not only in the load-bearing joints of the lower extremities but also in non-load-bearing joints (wrist and temporomandibular joints), indicating that systemic chronic low-level inflammation may play a role in its development (Puenpatom and Victor, 2009; Yoshimura et al., 2012). Although mechanical and genetic factors have classically been shown to play a major role in the development of OA, a growing number of reports indicate that low levels of inflammation play an important role in the development and progression of OA (Puenpatom and Victor, 2009; Yoshimura et al., 2012). Low levels of inflammation have been associated with obesity/metabolic syndrome as well as aging, diet, and postmenopausal women (estrogen deficiency), all of which are strongly associated with both OA and GM dysbiosis (Li et al., 2016).
Based on common factors between OA and GM dysbiosis, it seems reasonable that GM dysbiosis is associated with the development of OA. It has been suggested that OA patients show significant GM dysbiosis, revealing a shift in pathogenic microorganisms associated with OA (Huang and Kraus, 2016; Favazzo et al., 2020). In addition, because microbes are present in knee and hip OA, occult or subclinical bacterial infections resulting from bacteremia due to GM dysbiosis may accelerate OA (Dunn et al., 2020). Thus, the concept of the “gut-joint axis” can be applied to facet OA, a subset of SDD. However, further research is required to establish the relationship between the gut-joint axis and facet OA in both basic and clinical settings.
The IVD comprises three interrelated structures—namely, the central gelatinous nucleus pulposus, the outer annulus fibrosus, and the upper and lower cartilaginous endplates (Li et al., 2022). Vascular invasion into the IVD, which is generally considered the largest non-vascularized structure in the human body, may be detected in IVDD (Li et al., 2022).
While IVDD is multifactorial, chronic uncontrolled low-grade inflammation is progressively implicated in its etiology (Li et al., 2016). The microenvironment of a healthy IVD is vulnerable to immune surveillance functions (playing the role of sentry) because it has a blood-disk barrier, such as the blood–brain barrier in the central nervous system that keeps the IVD immunodominant and provides protection against systemic infection. Hypoxia and a lack of immune surveillance in the IVD create ideal conditions for the growth of anaerobic bacteria in degenerated disks (Wedderkopp et al., 2009). These bacteria growing on the IVD can recruit more inflammatory cells (e.g., T cells, B cells, dendritic cells, macrophages) via the release of inflammatory factors (e.g., IL-6, TNFα; Schirmer et al., 2016). Hence, a damaged IVD can become an ideal site for the growth and proliferation of microbes that evade humoral and cellular immunity, as well as the spread of harmful microbiome metabolites (Li et al., 2022). Therefore, GM dysbiosis has been suggested to possibly cause GM and GM metabolites to migrate into the bloodstream and IVD, causing or exacerbating IVDD (Li et al., 2022).
Rajasekaran et al., 2020 evaluated 24 lumbar IVDs and reported that the microbiome composition of healthy IVD differed from that of degenerative and herniated IVD. Thus, the concept of gut-disk axis (Li et al., 2022) is emerging, which may play an important role in IVDD and low back pain.
In addition to facet joint OA and IVDD, thickening of the yellow lumbar ligament is the leading cause of LSS pathogenesis (Figure 5), and an inflammation-related scar mechanism has been suggested for the thickening of the lumbar ligament (Sairyo et al., 2007). Although an association has been shown between LSS and diabetes, hypertension, and metabolic syndrome, all of which have been closely associated with GM dysbiosis (Fujita, 2021), no reports have shown an association between LSS and GM. However, IVDD and facet OA, which are factors of LSS, have a relationship with GM. Furthermore, the association of LSS with chronic inflammation-related conditions such as diabetes and metabolic syndrome, as well as inflammation observed in ligament thickening among LSS patients, indicates a potential connection between LSS and GM, suggesting the possibility of a gut-ligament axis in LSS.
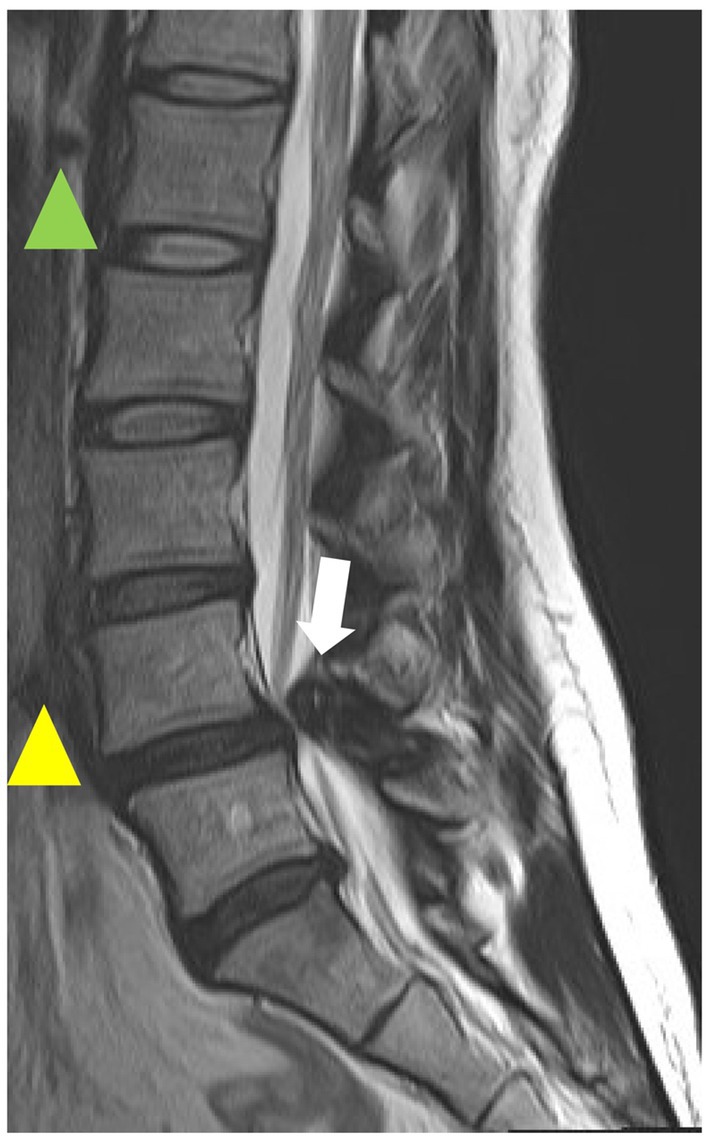
Figure 5. T2-weighted magnetic resonance imaging (MRI) sagittal image of the lumbar spine. The green arrowhead indicates normal intervertebral disk, the yellow arrowhead indicates intervertebral degenerated disk, and the white arrowhead indicates hypertrophic yellow ligament. This is a case from our institution.
Sarcopenia is defined as a progressive and systemic loss of skeletal muscle mass, strength, and function, according to the European Working Group on Sarcopenia in Older People (Cruz-Jentoft et al., 2019). GM dysbiosis can affect muscle mass and function via inflammation, immunity, protein metabolism, SCFA metabolism, and mitochondrial dysfunction, thereby leading to sarcopenia in the spine and ultimately affecting host physiology (Picca et al., 2018). In several studies exploring the correlation between GM and skeletal muscle, significant differences in GM diversity and composition were observed in sarcopenia cases, and a gut-muscle axis has been proposed to indicate cross-talk between the two (Picca et al., 2018; Ticinesi et al., 2019; Kuo et al., 2020; Przewłócka et al., 2020). The number of adult spinal deformities due to spinal sarcopenia (loss of erector spinae) is increasing, and its involvement with GM is an interesting topic of investigation (Kuo et al., 2020).
The main symptoms of SDD include low back pain and pain or numbness in the upper and lower extremities of nerve origin. Since GM is involved in the production of SCFA, neurotransmitters (including some precursors), and vitamins that regulate inflammation and pain (Benedict et al., 2016; Yan and Charles, 2017; Barton et al., 2018; Lucas et al., 2018), it is plausible that it is strongly involved in SDD-derived pain.
GM produces the neurotransmitters involved in pain modulation and analgesia (including some precursors)–namely, dopamine, serotonin, noradrenaline, glutamate, and GABA (Liu et al., 2020). In addition, the analgesic effects of B vitamins produced by GM are explained by their anti-inflammatory, antinociceptive, and neuroprotective effects (Buesing et al., 2019). The association of abnormal GM composition has also been demonstrated in patients with low back pain (Dekker Nitert et al., 2020), fibromyalgia (Minerbi et al., 2019), chronic pain (Freidin et al., 2021), knee OA (Boer et al., 2019), hand OA (Wei et al., 2021), and peripheral neuropathic pain (Ellis et al., 2022), suggesting that abnormal GM composition may be involved in individual differences in pain sensitivity. However, GM composition abnormalities have not been validated for SDD-related pain.
In addition, analgesics may be used in cases of pain of SDD origin; however, GM strongly interacts with certain drugs, affecting their response and effectiveness (Vich Vila et al., 2020). Especially in cases of long-term opioid use, it has been inferred that adverse changes in GM composition (dysbiosis) can occur and cause predisposition to opioid tolerance (Akbarali and Dewey, 2017). Therefore, understanding the cross-talk between GM and SDD-derived pain may be helpful in understanding the pathogenesis of SDD. In addition, understanding the effect of GM on analgesics used for SDD may explain individual differences in analgesic efficacy and prognosis.
Table 1 presents a summary of the association between GM and spinal musculoskeletal diseases, including SDD (Yang et al., 2016; Wang et al., 2017; Wen et al., 2017; Shen et al., 2019; Rajasekaran et al., 2020; Kang et al., 2021; Ling et al., 2021).
As a combination of specific microbiota abundance and SDD, Blautia and osteoporosis (Wang et al., 2017; Ling et al., 2021), Prevotella and Intervertebral disk disease (Rajasekaran et al., 2020), and Adolescent idiopathic scoliosis (Shen et al., 2019), Spondyloarthritis (Wen et al., 2017).
SDD usually first results from degeneration of IVD and facet joints, i.e., IVDD or facet OA develops. As a result, the stabilizing function between intervertebral bodies is impaired, which leads to the formation of bony spurs around intervertebral bodies and facet joints, and the thickening of the yellow ligament, resulting in LSS, including lumbar spondylolisthesis. In addition, spinal sarcopenia and OVF can accelerate the pathology. Moreover, SDD-related pain derived from IVDD, facet OA, LSS, and OVF impairs activity, further aggravating GM dysbiosis, sarcopenia, and osteoporosis. Thus, osteoporosis, IVDD, facet OA, LSS, and spinal sarcopenia coexist, adversely affecting not only SDD but also GM dysbiosis, leading to a negative spiral. With the advent of an aging society, understanding the pathophysiology, prevention, and treatment of SDD will become increasingly important. We have discussed that GM affects all pathologies of SDD and SDD-derived pain. Investigating the profile of GM in the progression of SDD could help identify patients with rapidly progressive SDD and improve our understanding of the pathogenesis of SDD.
Given the worldwide prevalence of SDD, there is a critical need for effective disease-modifying treatment strategies to alleviate symptoms and slow SDD progression.
GM communicates with the distant spinal structures via various axes, such as the gut-bone axis, gut-joint axis, gut-disk axis, gut-ligament axis, and gut-muscle axis. Bacteremia and chronic low-level inflammation caused by GM dysbiosis adversely affect spinal structures (bones, cartilage, disks, ligaments, and muscle) and contribute to the onset and progression of SDD, including osteoporosis, IVDD, and OA. Conversely, restoring GM dysbiosis through therapeutic intervention may restore physiological regulation through various axes and prevent the onset and progression of SDD (Figure 2).
GM has attracted attention as a promising target for therapeutic strategies because it can be modified by lifestyle modifications such as dietary interventions, sleep and exercise, fecal transplants, and future microbiome-targeted therapies (Kolasinski et al., 2020).
Given the association between GM dysbiosis and SDD and chronic inflammation, GM dysbiosis may be involved in the development and progression of SDD. As discussed in Chapter 2 (Li et al., 2022), a healthy lifestyle (including factors such as diet, sleep, and exercise) improves GM composition (increases “good” bacteria) and may improve GM dysbiosis. An improvement in GM dysbiosis may also alleviate inflammation, inhibit the degeneration of spinal structures, and relieve pain via SCFA or neurotransmitters. The interaction between lifestyle (diet, sleep, exercise) and GM has contributed to our understanding of the role of lifestyle in the prevention and treatment of chronic inflammation-related diseases, including SDD, in which GM dysbiosis plays a significant role. Notably, dietary interventions involving prebiotics and probiotics have shown promising effects in managing osteoporosis and OA via GM modulation (Vitetta et al., 2013; Zhang et al., 2022a).
Prebiotics are non-digestible food components such as dietary fiber and oligosaccharides. Prebiotics stimulate the growth and/or activity of beneficial bacteria in the digestive tract in ways that are beneficial to health, induce SCFA synthesis, affect cell growth and differentiation, hormone production, and inflammation regulation, and have a beneficial effect on the host (Vitetta et al., 2013; Zhang et al., 2022a). Probiotics consist of live microorganisms, typically lactic acid bacteria that modulate protease-activated receptor expression in epithelial cells, gastrointestinal smooth muscle cells, and capsaicin-sensitive neurons to regulate gastrointestinal mucosal barrier function and inflammation (Vitetta et al., 2013; Zhang et al., 2022a). As a result, they play an important role in the homeostasis of healthy GM by promoting epithelial barrier function and reducing dysbiosis, stimulating the production of antimicrobial substances and immunoglobulins, and inhibiting the production of bacterial toxins, thereby promoting host immune responses and anti-inflammatory pathways (Vitetta et al., 2013; Zhang et al., 2022a).
Both prebiotics and probiotics have been reported to have anti-inflammatory effects, promote calcium and vitamin D absorption, reduce osteoclast differentiation, and protect the bone and cartilage. They have also shown beneficial effects on osteoporosis and OA (Table 2; Abrams et al., 2005; Jones et al., 2013; Vitetta et al., 2013; Lei et al., 2017; Takimoto et al., 2018; Lyu et al., 2020; Paul et al., 2021; Zhang et al., 2022a). Nevertheless, no reports on their effects on IVD or LSS have yet been published.
Regarding exercise interventions, focusing on the “gut-muscle” axis and spinal sarcopenia, GM has been shown to improve skeletal muscle mass and function, while exercise affects GM composition (Locantore et al., 2020). In addition, the gut-spine axis can be considered as a result of treatment for spinal structures degeneration (bones, cartilage, disks, ligaments, and muscles), in which improvement in exercise level leads to improvement in GM composition. This positive spiral relationship between exercise, GM, and spinal structures could be useful in the prevention and treatment of SDD.
Sleep disturbances promote GM dysbiosis (decreased intestinal bacterial diversity and anti-inflammatory intestinal bacteria), which induces systemic chronic inflammation, which has been reported to be a risk factor for chronic inflammation-related diseases (diabetes and obesity). Chronic inflammation has been associated with the onset and progression of SDD; therefore, the association between sleep disturbances and SDD cannot be ruled out. Conversely, healthy sleep can be expected to contribute to SDD improvement. However, no studies have reported on the effects of sleep on the development or progression of SDD or its preventive effects.
GM also plays an important role in drug metabolism, which may influence drug efficacy (Vich Vila et al., 2020). Hence, GM modulation through lifestyle modification may enhance the efficacy of analgesics and contribute to drug reduction. Drug reduction is a critical issue in SDD patients, mostly older individuals because polypharmacy is also a significant problem. Regulation of GM through lifestyle improvement may also be effective in this regard.
Maintaining a healthy lifestyle is well understood to be based on good nutrition, regular exercise, and adequate sleep. However, only few studies have investigated the correlation between a healthy lifestyle and SDD in the field of spine surgery.
The GM-mediated therapeutic effect of lifestyle interventions is both a new perspective on SDD treatment and effective for SDD. Large-scale clinical trials on lifestyle interventions are required.
FMT is a method of improving certain medical conditions such as GM dysbiosis-related diseases of three types of inflammation: acute inflammation (e.g., Clostridioides difficile infection), chronic inflammation (e.g., chronic Crohn’s disease and ulcerative colitis), and chronic low-grade inflammation by delivering specially prepared stool material from a healthy donor to the patient (i.e., recipient) and restoring the balance of the intestinal bacterial community (Wang et al., 2022; Zhang et al., 2022b).
In contrast to applications targeting single microorganisms, such as probiotics and prebiotics, FMT maintains the integrity of GM and metabolites during the entire process, thus preserving the original function of GM to the maximum extent possible, significantly improving GM-related diseases and restoring gut microenvironmental homeostasis more rapidly and efficiently (Zhang et al., 2022b). While physiological disturbances due to disease can alter the composition and abundance of GM, GM dysbiosis can, on the other hand, induce or exacerbate disease, and FMT can be expected to prevent or alleviate disease conditions.
The potential regulatory mechanisms involving the FMT are to reestablish a normal intestinal environment and to correct disturbances in the intestinal microbiota. It is believed to potentially restore the intestinal mucosal barrier, improve intestinal permeability, restore imbalances in intestinal metabolites (SCFA, indole derivatives, vitamins, cholic acid, polyamines), and regulate immune responses (Zhang et al., 2022b).
FMT has been performed and validated for GM dysbiosis-related diseases in (1) Gastrointestinal diseases: Bacterial intestinal infection (Clostridioides difficile infection), inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome, (2) Liver Diseases: Severe alcoholic hepatitis, primary sclerosing cholangitis, and liver cirrhosis, (3) Brain diseases: Autism spectrum disorder, Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, and epilepsy, (4) Metabolic diseases: Diabetes, obesity, metabolic syndrome, and gout, (5) Cancer: Melanoma, and gastroesophageal cancer, (6) Skin diseases: alopecia and atopic dermatitis (Wang et al., 2022; Zhang et al., 2022b).
Spinal musculoskeletal disorders in which FMT has been reported to be effective are inflammatory, immune, or metabolic diseases: osteoporosis (Zhang et al., 2022b), psoriatic arthritis (Selvanderan et al., 2019), and axial arthritis (Mahajan et al., 2020). For other SDD (OA, IVDD, LSS, spinal sarcopenia) for which the effects of FMT have not yet been reported, GM dysbiosis may increase the risk of development and progression, and further normalization of GM composition and function may contribute to prevention, treatment, and symptom improvement.
The close relationship between GM and spinal structures (bones, cartilage, disks, ligaments, and muscles) due to common factors such as immunity, metabolism, and inflammation has been described by the terms gut-bone axis, gut-joint axis, gut-disk axis, gut-ligament axis, and gut-muscle axis.
Bacteremia and chronic low-level inflammation caused by GM dysbiosis have been found to adversely affect spinal structures and contribute to the development and progression of SDD, including osteoporosis, LSS, IVDD, spinal sarcopenia, and SDD-derived pain. Since GM-derived neurotransmitters may regulate the excitability of neurons in the peripheral nervous system and nociceptors involved in the onset of SDD-derived pain, GM may modulate the pathogenesis and therapeutic effects of SDD. This close association between GM and SDD led us to propose the gut-spine axis.
Scientific progress is often driven by the clear demarcation of research areas; however, to understand the complex and multifaceted nature of SDD, it is necessary to integrate the findings of various disciplines, such as spinal anatomy, immunology, microbiology, aging, and more. The perspective provided by GM research is a good example of such interdisciplinary integration and may not only provide a new framework for understanding these biological systems but may also offer many valuable insights into the development of effective therapies for the treatment of SDD (new disease-modifying therapies that intervene in GM). New biomarkers associated with inflammation and gut dysbiosis may predict the development of SDD and monitor the effectiveness of therapeutic interventions. Chronic inflammation and adverse immune effects due to GM dysbiosis and bacteremia (joint and IVD are not always sterile) may also contribute to postoperative infection of SDD.
However, the literature on the relationship between GM and SDD is scarce due to various issues such as cost, time, and declining participation, suggesting that there is an existing knowledge gap between the two. While a healthy lifestyle (diet, sleep, exercise) and FMT may improve GM dysbiosis and consequently contribute to SDD improvement, few reports exist on this issue, and large-scale studies are therefore needed.
Preventive medicine is important because increasing SDD is an urgent problem in an aging society. Preventive medicine is divided into three categories: primary prevention (health promotion, anti-aging medicine, prevention of diseases), secondary prevention (early detection, early treatment, prevention of aggravation), and tertiary prevention (rehabilitation, prevention of recurrence). GM is strongly related to all these SDD prevention aspects. The Nobel Prize-winning discovery of the bacterium Helicobacter pylori as the primary cause of gastritis and peptic gastritis was a game changer in diagnosis and treatment, radically shifting the management of peptic ulcers from surgical treatment to antibiotics and acid secretion inhibitors (Rajasekaran et al., 2020).
A better understanding of GM could be the catalyst for a new game changer in the prevention, diagnosis, and treatment of SDD, with GM as the axis. The established association between GM and spinal structures (gut-spine axis) supports the feasibility of a new approach to the prevention, diagnosis, and treatment of SDD.
TM: Data curation, Visualization, Writing – original draft, Writing – review & editing. TaK: Investigation, Methodology, Writing – original draft. ToK: Project administration, Resources, Writing – review & editing. ME: Supervision, Writing – review & editing. MT: Formal analysis, Writing – review & editing. TY: Formal analysis, Data curation, Visualization, Writing – review & editing. HH: Resources, Visualization, Writing – review & editing. MM: Supervision, Writing – review & editing. SY: Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Health Labor Sciences Research Grant (22FG2001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DISH, diffuse idiopathic skeletal hyperostosis; GM, gut microbiota; IVD, intervertebral disk; IVDD, intervertebral disk degeneration; LPS, lipopolysaccharide; LSS, lumbar spinal canal stenosis; NGS, next-generation high-throughput microbial DNA sequencing; OA, osteoarthritis; OPLL, ossification of posterior longitudinal ligament; OVF, osteoporotic vertebral fractures; SASP, senescence-associated secretory phenotype; SCFA, short-chain fatty acids; SDD, spinal degenerative diseases.
Abrams, S. A., Griffin, I. J., Hawthorne, K. M., Liang, L., Gunn, S. K., Darlington, G., et al. (2005). A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 82, 471–476. doi: 10.1093/ajcn/82.2.471
Akbarali, H. I., and Dewey, W. L. (2017). The gut-brain interaction in opioid tolerance. Curr. Opin. Pharmacol. 37, 126–130. doi: 10.1016/j.coph.2017.10.012
Allen, J. M., Mailing, L. J., Niemiro, G. M., Moore, R., Cook, M. D., White, B. A., et al. (2018). Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757. doi: 10.1249/MSS.0000000000001495
Alverdy, J. C., Hyman, N., and Gilbert, J. (2020). Re-examining causes of surgical site infections following elective surgery in the era of asepsis. Lancet Infect. Dis. 20, e38–e43. doi: 10.1016/S1473-3099(19)30756-X
Andriessen, E. M., Wilson, A. M., Mawambo, G., Dejda, A., Miloudi, K., Sennlaub, F., et al. (2016). Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol. Med. 8, 1366–1379. doi: 10.15252/emmm.201606531
Ardissone, A. N., De la Cruz, D. M., Davis-Richardson, A. G., Rechcigl, K. T., Li, N., Drew, J. C., et al. (2014). Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One 9:e90784. doi: 10.1371/journal.pone.0090784
Arron, J. R., and Choi, Y. (2000). Bone versus immune system. Nature 408, 535–536. doi: 10.1038/35046196
Azzini, G. O. M., Santos, G. S., Visoni, S. B. C., Azzini, V. O. M., Santos, R. G. D., Huber, S. C., et al. (2020). Metabolic syndrome and subchondral bone alterations: the rise of osteoarthritis - a review. J. Clin. Orthop. Trauma 11, S849–S855. doi: 10.1016/j.jcot.2020.06.021
Baker, D. J., Wijshake, T., Tchkonia, T., LeBrasseur, N. K., Childs, B. G., Van de Sluis, B., et al. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. doi: 10.1038/nature10600
Baker, J. M., Al-Nakkash, L., and Herbst-Kralovetz, M. M. (2017). Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. doi: 10.1016/j.maturitas.2017.06.025
Barton, W., Penney, N. C., Cronin, O., Garcia-Perez, I., Molloy, M. G., Holmes, E., et al. (2018). The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 67, 313627–313633. doi: 10.1136/gutjnl-2016-313627
Behera, J., Ison, J., Tyagi, S. C., and Tyagi, N. (2020). The role of gut microbiota in bone homeostasis. Bone 135:115317. doi: 10.1016/j.bone.2020.115317
Benedict, C., Vogel, H., Jonas, W., Woting, A., Blaut, M., Schürmann, A., et al. (2016). Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 5, 1175–1186. doi: 10.1016/j.molmet.2016.10.003
Benoist, M. (2003). Natural history of the aging spine. Eur. Spine J. 12, S86–S89. doi: 10.1007/s00586-003-0593-0
Berthelot, J. M., Sellam, J., Maugars, Y., and Berenbaum, F. (2019). Cartilage-gut-microbiome axis: a new paradigm for novel therapeutic opportunities in osteoarthritis. RMD Open 5:e001037. doi: 10.1136/rmdopen-2019-001037
Biver, E., Berenbaum, F., Valdes, A. M., Araujo de Carvalho, I., Bindels, L. B., Brandi, M. L., et al. (2019). Gut microbiota and osteoarthritis management: an expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res. Rev. 55:100946. doi: 10.1016/j.arr.2019.100946
Boer, C. G., Radjabzadeh, D., Medina-Gomez, C., Garmaeva, S., Schiphof, D., Arp, P., et al. (2019). Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 10:4881. doi: 10.1038/s41467-019-12873-4
Borghesan, M., Hoogaars, W. M. H., Varela-Eirin, M., Talma, N., and Demaria, M. (2020). A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol. 30, 777–791. doi: 10.1016/j.tcb.2020.07.002
Buesing, S., Costa, M., Schilling, J. M., and Moeller-Bertram, T. (2019). Vitamin B12 as a treatment for pain. Pain Physician 1, E45–E52. doi: 10.36076/ppj/2019.22.E45
Buford, T. W. (2017). (dis)trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5:80. doi: 10.1186/s40168-017-0296-0
Burcelin, R., Serino, M., Chabo, C., Garidou, L., Pomié, C., Courtney, M., et al. (2013). Metagenome and metabolism: the tissue microbiota hypothesis. Diabetes Obes. Metab. 15, 61–70. doi: 10.1111/dom.12157
Burd, C. E., Sorrentino, J. A., Clark, K. S., Darr, D. B., Krishnamurthy, J., Deal, A. M., et al. (2013). Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cells 152, 340–351. doi: 10.1016/j.cell.2012.12.010
Chang, P. V., Hao, L., Offermanns, S., and Medzhitov, R. (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U. S. A. 111, 2247–2252. doi: 10.1073/pnas.1322269111
Charles, J. F., Ermann, J., and Aliprantis, A. O. (2015). The intestinal microbiome and skeletal fitness: connecting bugs and bones. Clin. Immunol. 159, 163–169. doi: 10.1016/j.clim.2015.03.019
Charnay, Y., Cusin, I., Vallet, P. G., Muzzin, P., Rohner-Jeanrenaud, F., and Bouras, C. (2000). Intracerebroventricular infusion of leptin decreases serotonin transporter binding sites in the frontal cortex of the rat. Neurosci. Lett. 283, 89–92. doi: 10.1016/S0304-3940(00)00951-4
Chen, H. F., Mi, J., Zhang, H. H., and Zhao, C. Q. (2019). Pelvic incidence measurement using a computed tomography data-based three-dimensional pelvic model. J. Orthop. Surg. Res. 14:13. doi: 10.1186/s13018-018-1050-4
Chen, Y., Wang, H., Lu, W., Wu, T., Yuan, W., Zhu, J., et al. (2022a). Human gut microbiome aging clocks based on taxonomic and functional signatures through multi-view learning. Gut Microbes 14:2025016. doi: 10.1080/19490976.2021.2025016
Chen, Y., Wang, X., Zhang, C., Liu, Z., Li, C., and Ren, Z. (2022b). Gut microbiota and bone diseases: a growing partnership. Front. Microbiol. 13:877776. doi: 10.3389/fmicb.2022.877776
Chisari, E., Wouthuyzen-Bakker, M., Friedrich, A. W., and Parvizi, J. (2021). The relation between the gut microbiome and osteoarthritis: a systematic review of literature. PLoS One 16:e0261353. doi: 10.1371/journal.pone.0261353
Chung, H. J., Lee, H., Na, G., Jung, H., Kim, D. G., Shin, S. I., et al. (2020). Metabolic and lipidomic profiling of vegetable juices fermented with various probiotics. Biomol. Ther. 10:725. doi: 10.3390/biom10050725
Clarke, G., Stilling, R. M., Kennedy, P. J., Stanton, C., Cryan, J. F., and Dinan, T. G. (2014). Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 28, 1221–1238. doi: 10.1210/me.2014-1108
Clarke, S. F., Murphy, E. F., O'Sullivan, O., Lucey, A. J., Humphreys, M., Hogan, A., et al. (2014). Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63, 1913–1920. doi: 10.1136/gutjnl-2013-306541
Cruz-Jentoft, A. J., Bahat, G., Bauer, J., Boirie, Y., Bruyère, O., Cederholm, T., et al. (2019). Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. doi: 10.1093/ageing/afy169
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
D’Amelio, P., Grimaldi, A., Di Bella, S., Brianza, S. Z. M., Cristofaro, M. A., Tamone, C., et al. (2008). Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 43, 92–100. doi: 10.1016/j.bone.2008.02.017
Das, B., and Nair, G. B. (2019). Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 44:117. doi: 10.1007/s12038-019-9926-y
De Marco, S., Sichetti, M., Muradyan, D., Piccioni, M., Traina, G., Pagiotti, R., et al. (2018). Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018:1756308. doi: 10.1155/2018/1756308
De Sire, A., De Sire, R., Petito, V., Masi, L., Cisari, C., Gasbarrini, A., et al. (2020). Gut-joint axis: the role of physical exercise on gut microbiota modulation in older people with osteoarthritis. Nutrients 12:574. doi: 10.3390/nu12020574
Dekker Nitert, M., Mousa, A., Barrett, H. L., Naderpoor, N., and de Courten, B. (2020). Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front. Endocrinol. 11:605. doi: 10.3389/fendo.2020.00605
DeStefano Shields, C. E., White, J. R., Chung, L., Wenzel, A., Hicks, J. L., Tam, A. J., et al. (2021). Bacterial-driven inflammation and mutant BRAF expression combine to promote murine colon tumorigenesis that is sensitive to immune checkpoint therapy. Cancer Discov. 11, 1792–1807. doi: 10.1158/2159-8290.CD-20-0770
Du, R., Xu, G., Bai, X., and Li, Z. (2022). Facet joint syndrome: pathophysiology, diagnosis, and treatment. J. Pain Res. 15, 3689–3710. doi: 10.2147/JPR.S389602
Dunn, C. M., Velasco, C., Rivas, A., Andrews, M., Garman, C., Jacob, P. B., et al. (2020). Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. 72, 1111–1122. doi: 10.1002/art.41210
Elderman, M., Sovran, B., Hugenholtz, F., Graversen, K., Huijskes, M., Houtsma, E., et al. (2017). The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One 12:e0184274. doi: 10.1371/journal.pone.0184274
Ellis, R. J., Heaton, R. K., Gianella, S., Rahman, G., and Knight, R. (2022). Reduced gut microbiome diversity in people with HIV who have distal neuropathic pain. J. Pain 23, 318–325. doi: 10.1016/j.jpain.2021.08.006
Erlandson, K. M., Liu, J., Johnson, R., Dillon, S., Jankowski, C. M., Kroehl, M., et al. (2021). An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 8:20499361211027067. doi: 10.1177/20499361211027067
Favazzo, L. J., Hendesi, H., Villani, D. A., Soniwala, S., Dar, Q. A., Schott, E. M., et al. (2020). The gut microbiome-joint connection: implications in osteoarthritis. Curr. Opin. Rheumatol. 32, 92–101. doi: 10.1097/BOR.0000000000000681
Fei, N., Choo-Kang, C., Reutrakul, S., Crowley, S. J., Rae, D., Bedu-Addo, K., et al. (2021). Gut microbiota alterations in response to sleep length among African-origin adults. PLoS One 16:e0255323. doi: 10.1371/journal.pone.0255323
Fine, R. L., Manfredo Vieira, S., Gilmore, M. S., and Kriegel, M. A. (2020). Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes 11, 217–230. doi: 10.1080/19490976.2019.1629236
Franceschi, C., and Campisi, J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9. doi: 10.1093/gerona/glu057
Freidin, M. B., Stalteri, M. A., Wells, P. M., Lachance, G., Baleanu, A. F., Bowyer, R. C. E., et al. (2021). An association between chronic widespread pain and the gut microbiome. Rheumatology (Oxford) 60, 3727–3737. doi: 10.1093/rheumatology/keaa847
Fujita, N. (2021). Lumbar spinal canal stenosis from the perspective of locomotive syndrome and metabolic syndrome: a narrative review. Spine Surg. Relat. Res. 5, 61–67. doi: 10.22603/ssrr.2020-0112
Gracey, E., Vereecke, L., McGovern, D., Fröhling, M., Schett, G., Danese, S., et al. (2020). Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 16, 415–433. doi: 10.1038/s41584-020-0454-9
Granville Smith, I., Danckert, N. P., Freidin, M. B., Wells, P., Marchesi, J. R., and Williams, F. M. K. (2022). Evidence for infection in intervertebral disc degeneration: a systematic review. Eur. Spine J. 31, 414–430. doi: 10.1007/s00586-021-07062-1
Guggino, G., Mauro, D., Rizzo, A., Alessandro, R., Raimondo, S., Bergot, A. S., et al. (2021). Inflammasome activation in ankylosing spondylitis is associated with gut dysbiosis. Arthritis Rheumatol. 73, 1189–1199. doi: 10.1002/art.41644
Guss, J. D., Ziemian, S. N., Luna, M., Sandoval, T. N., Holyoak, D. T., Guisado, G. G., et al. (2019). The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthr. Cartil. 27, 129–139. doi: 10.1016/j.joca.2018.07.020
Herbig, U., Ferreira, M., Condel, L., Carey, D., and Sedivy, J. M. (2006). Cellular senescence in aging primates. Science 311:1257. doi: 10.1126/science.1122446
Huang, Z., and Kraus, V. B. (2016). Does lipopolysaccharide-mediated inflammation have a role in OA. Nat. Rev. Rheumatol. 12, 123–129. doi: 10.1038/nrrheum.2015.158
Ibáñez, L., Rouleau, M., Wakkach, A., and Blin-Wakkach, C. (2019). Gut microbiome and bone. Joint Bone Spine 86, 43–47. doi: 10.1016/j.jbspin.2018.02.008
Ibrahim, I., Syamala, S., Ayariga, J. A., Xu, J., Robertson, B. K., Meenakshisundaram, S., et al. (2022). Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites. 12. doi: 10.3390/metabo12121247
Imajo, Y., Taguchi, T., Yone, K., Okawa, A., Otani, K., Ogata, T., et al. (2015). Japanese 2011 nationwide survey on complications from spine surgery. J. Orthop. Sci. 20, 38–54. doi: 10.1007/s00776-014-0656-6
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. doi: 10.3748/wjg.v21.i29.8787
Jie, Z., Xia, H., Zhong, S. L., Feng, Q., Li, S., Liang, S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8:845. doi: 10.1038/s41467-017-00900-1
Jones, M. L., Martoni, C. J., and Prakash, S. (2013). Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 98, 2944–2951. doi: 10.1210/jc.2012-4262
Kang, L., Li, P., Wang, D., Wang, T., Hao, D., and Qu, X. (2021). Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 11:4628. doi: 10.1038/s41598-021-84031-0
Kawaguchi, Y. (2022). Biomarker research approach to the pathogenesis of ossification of the spinal ligament: a review. Spine Surg. Relat. Res. 6, 224–232. doi: 10.22603/ssrr.2021-0229
Ke, S., Mitchell, S. J., MacArthur, M. R., Kane, A. E., Sinclair, D. A., Venable, E. M., et al. (2021). Gut microbiota predicts healthy late-life aging in male mice. Nutrients 13:3290. doi: 10.3390/nu13093290
Khosla, S., Farr, J. N., Tchkonia, T., and Kirkland, J. L. (2020). The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16, 263–275. doi: 10.1038/s41574-020-0335-y
Khosravi, A., Yáñez, A., Price, J. G., Chow, A., Merad, M., Goodridge, H. S., et al. (2014). Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381. doi: 10.1016/j.chom.2014.02.006
Kinashi, Y., and Hase, K. (2021). Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front. Immunol. 12:673708. doi: 10.3389/fimmu.2021.673708
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., Block, J., et al. (2020). 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72, 220–233. doi: 10.1002/art.41142
Konturek, P. C., Haziri, D., Brzozowski, T., Hess, T., Heyman, S., Kwiecien, S., et al. (2015). Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 66, 483–491.
Kumar, R., Sharma, A., Kumari, A., Gulati, A., Padwad, Y., and Sharma, R. (2019). Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 20, 171–189. doi: 10.1007/s10522-018-9785-1
Kuo, Y. K., Lin, Y. C., Lee, C. Y., Chen, C. Y., Tani, J., Huang, T. J., et al. (2020). Novel insights into the pathogenesis of spinal sarcopenia and related therapeutic approaches: a narrative review. Int. J. Mol. Sci. 21:3010. doi: 10.3390/ijms21083010
Lei, M., Guo, C., Wang, D., Zhang, C., and Hua, L. (2017). The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 8, 697–703. doi: 10.3920/BM2016.0207
Leproult, R., Holmbäck, U., and Van Cauter, E. (2014). Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869. doi: 10.2337/db13-1546
Li, W., Lai, K., Chopra, N., Zheng, Z., Das, A., and Diwan, A. D. (2022). Gut-disc axis: a cause of intervertebral disc degeneration and low back pain. Eur. Spine J. 31, 917–925. doi: 10.1007/s00586-022-07152-8
Li, Y., Luo, W., Deng, Z., and Lei, G. (2016). Diet-intestinal microbiota axis in osteoarthritis: a possible role. Mediat. Inflamm. 2016:3495173. doi: 10.1155/2016/3495173
Ling, C. W., Miao, Z., Xiao, M. L., Zhou, H., Jiang, Z., Fu, Y., et al. (2021). The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J. Clin. Endocrinol. Metab. 106, e3852–e3864. doi: 10.1210/clinem/dgab492
Lisiewski, L. E., Jacobsen, H. E., Viola, D. C. M., Kenawy, H. M., Kiridly, D. N., and Chahine, N. O. (2022). Intradiscal inflammatory stimulation induces spinal pain behavior and intervertebral disc degeneration in vivo. bioRxiv 2022:487751. doi: 10.1101/2022.04.11.487751
Liu, J., Xu, F., Nie, Z., and Shao, L. (2020). Gut microbiota approach-a new strategy to treat Parkinson's disease. Front. Cell. Infect. Microbiol. 10:570658. doi: 10.3389/fcimb.2020.570658
Locantore, P., Del Gatto, V., Gelli, S., Paragliola, R. M., and Pontecorvi, A. (2020). The interplay between immune system and microbiota in osteoporosis. Mediat. Inflamm. 2020:3686749. doi: 10.1155/2020/3686749
Lucas, S., Omata, Y., Hofmann, J., Böttcher, M., Iljazovic, A., Sarter, K., et al. (2018). Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 9:55. doi: 10.1038/s41467-017-02490-4
Lyu, J. L., Wang, T. M., Chen, Y. H., Chang, S. T., Wu, M. S., Lin, Y. H., et al. (2020). Oral intake of Streptococcus thermophil us improves knee osteoarthritis degeneration: a randomized, double-blind, placebo-controlled clinical study. Heliyon 6:e03757. doi: 10.1016/j.heliyon.2020.e03757
Mader, R., Pappone, N., Baraliakos, X., Eshed, I., Sarzi-Puttini, P., Atzeni, F., et al. (2021). Diffuse idiopathic skeletal hyperostosis (DISH) and a possible inflammatory component. Curr. Rheumatol. Rep. 23:6. doi: 10.1007/s11926-020-00972-x
Mahajan, R., Midha, V., Singh, A., Mehta, V., Gupta, Y., Kaur, K., et al. (2020). Incidental benefits after fecal microbiota transplant for ulcerative colitis. Intest. Res. 18, 337–340. doi: 10.5217/ir.2019.00108
Malinova, T. S., Dijkstra, C. D., and de Vries, H. E. (2018). Serotonin: a mediator of the gut-brain axis in multiple sclerosis. Mult. Scler. 24, 1144–1150. doi: 10.1177/1352458517739975
Miller, R. J., Malfait, A. M., and Miller, R. E. (2020). The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 28, 562–571. doi: 10.1016/j.joca.2019.11.006
Minerbi, A., Gonzalez, E., Brereton, N. J. B., Anjarkouchian, A., Dewar, K., Fitzcharles, M. A., et al. (2019). Altered microbiome composition in individuals with fibromyalgia. Pain 160, 2589–2602. doi: 10.1097/j.pain.0000000000001640
Miyauchi, E., Shimokawa, C., Steimle, A., Desai, M. S., and Ohno, H. (2023). The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 23, 9–23. doi: 10.1038/s41577-022-00727-y
Motta, F., Barone, E., Sica, A., and Selmi, C. (2023). Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol. 64, 222–238. doi: 10.1007/s12016-022-08941-1
Nouh, M. R., and Eid, A. F. (2015). Magnetic resonance imaging of the spinal marrow: basic understanding of the normal marrow pattern and its variant. World J. Radiol. 7, 448–458. doi: 10.4329/wjr.v7.i12.448
Ohlsson, C., and Sjögren, K. (2015). Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 26, 69–74. doi: 10.1016/j.tem.2014.11.004
Ozkan, J., and Willcox, M. D. (2019). The ocular microbiome: molecular characterisation of a unique and low microbial environment. Curr. Eye Res. 44, 685–694. doi: 10.1080/02713683.2019.1570526
Parenteau, C. S., Lau, E. C., Campbell, I. C., and Courtney, A. (2021). Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci. Rep. 11:5389. doi: 10.1038/s41598-021-84724-6
Park, S. H., Kim, K. A., Ahn, Y. T., Jeong, J. J., Huh, C. S., and Kim, D. H. (2015). Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol. 15:49. doi: 10.1186/s12866-015-0386-8
Paul, A. K., Paul, A., Jahan, R., Jannat, K., Bondhon, T. A., Hasan, A., et al. (2021). Probiotics and amelioration of rheumatoid arthritis: significant roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms 9:1070. doi: 10.3390/microorganisms9051070
Pesesse, L., Sanchez, C., and Henrotin, Y. (2011). Osteochondral plate angiogenesis: a new treatment target in osteoarthritis. Joint Bone Spine 78, 144–149. doi: 10.1016/j.jbspin.2010.07.001
Picca, A., Fanelli, F., Calvani, R., Mulè, G., Pesce, V., Sisto, A., et al. (2018). Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediat. Inflamm. 2018:7026198. doi: 10.1155/2018/7026198
Poroyko, V. A., Carreras, A., Khalyfa, A., Khalyfa, A. A., Leone, V., Peris, E., et al. (2016). Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 6:35405. doi: 10.1038/srep35405
Przewłócka, K., Folwarski, M., Kaźmierczak-Siedlecka, K., Skonieczna-Żydecka, K., and Kaczor, J. J. (2020). Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 12:1451. doi: 10.3390/nu12051451
Puenpatom, R. A., and Victor, T. W. (2009). Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad. Med. 121, 9–20. doi: 10.3810/pgm.2009.11.2073
Qaiyum, Z., Lim, M., and Inman, R. D. (2021). The gut-joint axis in spondyloarthritis: immunological, microbial, and clinical insights. Semin. Immunopathol. 43, 173–192. doi: 10.1007/s00281-021-00845-0
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Rajasekaran, S., Pushpa, B. T., Soundararajan, D. C. R., Sri Vijay Anand, K. S., Murugan, C., Nedunchelian, M., et al. (2022). Are Modic changes 'Primary infective endplatitis'?-insights from multimodal imaging of non-specific low back pain patients and development of a radiological 'Endplate infection probability score'. Eur. Spine J. 31, 2884–2896. doi: 10.1007/s00586-022-07335-3
Rajasekaran, S., Soundararajan, D. C. R., Tangavel, C., Muthurajan, R., Sri Vijay Anand, K. S., Matchado, M. S., et al. (2020). Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur. Spine J. 29, 1621–1640. doi: 10.1007/s00586-020-06446-z
Reynolds, A. C., Paterson, J. L., Ferguson, S. A., Stanley, D., Wright, K. P., and Dawson, D. (2017). The shift work and health research agenda: considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med. Rev. 34, 3–9. doi: 10.1016/j.smrv.2016.06.009
Riaz Rajoka, M. S., Zhao, H., Mehwish, H. M., Li, N., Lu, Y., Lian, Z., et al. (2019). Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res. Int. 123, 286–297. doi: 10.1016/j.foodres.2019.05.002
Romero-Figueroa, M. D. S., Ramírez-Durán, N., Montiel-Jarquín, A. J., and Horta-Baas, G. (2023). Gut-joint axis: gut dysbiosis can contribute to the onset of rheumatoid arthritis via multiple pathways. Front. Cell. Infect. Microbiol. 13:1092118. doi: 10.3389/fcimb.2023.1092118
Rossi, T., Vergara, D., Fanini, F., Maffia, M., Bravaccini, S., and Pirini, F. (2020). Microbiota-derived metabolites in tumor progression and metastasis. Int. J. Mol. Sci. 21:5786. doi: 10.3390/ijms21165786
Sadowska, A., Touli, E., Hitzl, W., Greutert, H., Ferguson, S. J., Wuertz-Kozak, K., et al. (2018). Inflammaging in cervical and lumbar degenerated intervertebral discs: analysis of proinflammatory cytokine and TRP channel expression. Eur. Spine J. 27, 564–577. doi: 10.1007/s00586-017-5360-8
Sairyo, K., Biyani, A., Goel, V. K., Leaman, D. W., Booth, R., Thomas, J., et al. (2007). Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine (Phila Pa 1976) 32, E340–E347. doi: 10.1097/01.brs.0000263407.25009.6e
Schirmer, M., Smeekens, S. P., Vlamakis, H., Jaeger, M., Oosting, M., Franzosa, E. A., et al. (2016). Linking the human gut microbiome to inflammatory cytokine production capacity. Cells 167, 1125–1136.e8. doi: 10.1016/j.cell.2016.10.020
Seely, K. D., Kotelko, C. A., Douglas, H., Bealer, B., and Brooks, A. E. (2021). The human gut microbiota: a key mediator of osteoporosis and osteogenesis. Int. J. Mol. Sci. 22:9452. doi: 10.3390/ijms22179452
Selvanderan, S. P., Goldblatt, F., Nguyen, N. Q., and Costello, S. P. (2019). Faecal microbiota transplantation for Clostridium difficile infection resulting in a decrease in psoriatic arthritis disease activity. Clin. Exp. Rheumatol. 37, 514–515.
Sepich-Poore, G. D., Zitvogel, L., Straussman, R., Hasty, J., Wargo, J. A., and Knight, R. (2021). The microbiome and human cancer. Science 371:eabc4552. doi: 10.1126/science.abc4552
Sharma, R. (2022). Emerging interrelationship between the gut microbiome and cellular senescence in the context of aging and disease: perspectives and therapeutic opportunities. Probiotics Antimicrob. Proteins 14, 648–663. doi: 10.1007/s12602-021-09903-3
Shen, N., Chen, N., Zhou, X., Zhao, B., Huang, R., Liang, J., et al. (2019). Alterations of the gut microbiome and plasma proteome in Chinese patients with adolescent idiopathic scoliosis. Bone 120, 364–370. doi: 10.1016/j.bone.2018.11.017
Spiegel, K., Leproult, R., and Van Cauter, E. (1999). Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439. doi: 10.1016/S0140-6736(99)01376-8
Takayanagi, H. (2021). Osteoimmunology as an intrinsic part of immunology. Int. Immunol. 33, 673–678. doi: 10.1093/intimm/dxab057
Takimoto, T., Hatanaka, M., Hoshino, T., Takara, T., Tanaka, K., Shimizu, A., et al. (2018). Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 37, 87–96. doi: 10.12938/bmfh.18-006
Ticinesi, A., Lauretani, F., Tana, C., Nouvenne, A., Ridolo, E., and Meschi, T. (2019). Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 25, 84–95.
Tonelli Enrico, V., Vo, N., Methe, B., Morris, A., and Sowa, G. (2022). An unexpected connection: a narrative review of the associations between gut microbiome and musculoskeletal pain. Eur. Spine J. 31, 3603–3615. doi: 10.1007/s00586-022-07429-y
Tuttle, C. S. L., Luesken, S. W. M., Waaijer, M. E. C., and Maier, A. B. (2021). Senescence in tissue samples of humans with age-related diseases: a systematic review. Ageing Res. Rev. 68:101334. doi: 10.1016/j.arr.2021.101334
Varela-Eirín, M., Carpintero-Fernández, P., Sánchez-Temprano, A., Varela-Vázquez, A., Paíno, C. L., Casado-Díaz, A., et al. (2020). Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging (Albany NY) 12, 15882–15905. doi: 10.18632/aging.103801
Vich Vila, A., Collij, V., Sanna, S., Sinha, T., Imhann, F., Bourgonje, A. R., et al. (2020). Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11:362. doi: 10.1038/s41467-019-14177-z
Vidal, P. M., and Pacheco, R. (2020). The cross-talk between the dopaminergic and the immune system involved in schizophrenia. Front. Pharmacol. 11:394. doi: 10.3389/fphar.2020.00394
Vitetta, L., Coulson, S., Linnane, A. W., and Butt, H. (2013). The gastrointestinal microbiome and musculoskeletal diseases: a beneficial role for probiotics and prebiotics. Pathogens 2, 606–626. doi: 10.3390/pathogens2040606
Wang, J., Wang, Y., Gao, W., Wang, B., Zhao, H., Zeng, Y., et al. (2017). Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 5:e3450. doi: 10.7717/peerj.3450
Wang, L. C., Pan, T. M., and Tsai, T. Y. (2018). Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. J. Food Drug Anal. 26, 973–984. doi: 10.1016/j.jfda.2017.11.009
Wang, Y., Zhang, S., Borody, T. J., and Zhang, F. (2022). Encyclopedia of fecal microbiota transplantation: a review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 135, 1927–1939. doi: 10.1097/CM9.0000000000002339
Wedderkopp, N., Thomsen, K., Manniche, C., Kolmos, H. J., Secher Jensen, T., and Leboeuf Yde, C. (2009). No evidence for presence of bacteria in modic type I changes. Acta Radiol. 50, 65–70. doi: 10.1080/02841850802524485
Wei, J., Zhang, C., Zhang, Y., Zhang, W., Doherty, M., Yang, T., et al. (2021). Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya osteoarthritis study. Arthritis Rheumatol. 73, 1656–1662. doi: 10.1002/art.41729
Wen, C., Zheng, Z., Shao, T., Liu, L., Xie, Z., Le Chatelier, E., et al. (2017). Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 18:142. doi: 10.1186/s13059-017-1271-6
Wu, X., Zhao, K., Fang, X., Lu, F., Zhang, W., Song, X., et al. (2021). Inhibition of lipopolysaccharide-induced inflammatory bone loss by saikosaponin D is associated with regulation of the RANKL/RANK pathway. Drug Des. Devel. Ther. 15, 4741–4757. doi: 10.2147/DDDT.S334421
Yadav, V. K., Ryu, J. H., Suda, N., Tanaka, K. F., Gingrich, J. A., Schütz, G., et al. (2008). Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cells 135, 825–837. doi: 10.1016/j.cell.2008.09.059
Yan, J., and Charles, J. F. (2017). Gut microbiome and bone: to build, destroy, or both. Curr. Osteoporos. Rep. 15, 376–384. doi: 10.1007/s11914-017-0382-z
Yang, L., Wang, L., Wang, X., Xian, C. J., and Lu, H. (2016). A possible role of intestinal microbiota in the pathogenesis of ankylosing spondylitis. Int. J. Mol. Sci. 17:2126. doi: 10.3390/ijms17122126
Yoshimura, N., Muraki, S., Oka, H., Tanaka, S., Kawaguchi, H., Nakamura, K., et al. (2012). Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthr. Cartil. 20, 1217–1226. doi: 10.1016/j.joca.2012.06.006
Zaiss, M. M., Jones, R. M., Schett, G., and Pacifici, R. (2019). The gut-bone axis: how bacterial metabolites bridge the distance. J. Clin. Invest. 129, 3018–3028. doi: 10.1172/JCI128521
Zaiss, M. M., Joyce Wu, H. J., Mauro, D., Schett, G., and Ciccia, F. (2021). The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 224–237. doi: 10.1038/s41584-021-00585-3
Zhang, Y. W., Cao, M. M., Li, Y. J., Dai, G. C., Lu, P. P., Zhang, M., et al. (2022a). The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: involvement of brain-gut-bone axis. Crit. Rev. Food Sci. Nutr. 63, 7510–7528. doi: 10.1080/10408398.2022.2047005
Zhang, Y. W., Cao, M. M., Li, Y. J., Zhang, R. L., Wu, M. T., Yu, Q., et al. (2022b). Fecal microbiota transplantation as a promising treatment option for osteoporosis. J. Bone Miner. Metab. 40, 874–889. doi: 10.1007/s00774-022-01375-x
Zheng, D., Liwinski, T., and Elinav, E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506. doi: 10.1038/s41422-020-0332-7
Keywords: gut microbiota, gut dysbiosis, spinal degenerative disease, inflammation, gut-spine axis, gut-bone axis
Citation: Morimoto T, Kobayashi T, Kakiuchi T, Esaki M, Tsukamoto M, Yoshihara T, Hirata H, Yabuki S and Mawatari M (2023) Gut-spine axis: a possible correlation between gut microbiota and spinal degenerative diseases. Front. Microbiol. 14:1290858. doi: 10.3389/fmicb.2023.1290858
Received: 08 September 2023; Accepted: 10 October 2023;
Published: 27 October 2023.
Edited by:
Mingsong Kang, Canadian Food Inspection Agency, CanadaReviewed by:
Zhengzhong Zou, Oregon Health and Science University, United StatesCopyright © 2023 Morimoto, Kobayashi, Kakiuchi, Esaki, Tsukamoto, Yoshihara, Hirata, Yabuki and Mawatari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadatsugu Morimoto, bW9yaW1vdDNAY2Muc2FnYS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.