
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 December 2023
Sec. Infectious Agents and Disease
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1283814
This article is part of the Research Topic Zoonotic Diseases: Epidemiology, Multi-omics, and Host-pathogen interactions View all 25 articles
 Abdul Majid1
Abdul Majid1 Mashal M. Almutairi2
Mashal M. Almutairi2 Abdulaziz Alouffi3
Abdulaziz Alouffi3 Tetsuya Tanaka4
Tetsuya Tanaka4 Tsai-Ying Yen5
Tsai-Ying Yen5 Kun-Hsien Tsai5*
Kun-Hsien Tsai5* Abid Ali1*
Abid Ali1*Tick-borne Rickettsia spp. have long been known as causative agents for zoonotic diseases. We have previously characterized Rickettsia spp. in different ticks infesting a broad range of hosts in Pakistan; however, knowledge regarding Rickettsia aeschlimannii in Haemaphysalis and Hyalomma ticks is missing. This study aimed to obtain a better understanding about R. aeschlimannii in Pakistan and update the knowledge about its worldwide epidemiology. Among 369 examined domestic animals, 247 (66%) were infested by 872 ticks. Collected ticks were morphologically delineated into three genera, namely, Rhipicephalus, Hyalomma, and Haemaphysalis. Adult females were the most prevalent (number ₌ 376, 43.1%), followed by nymphs (303, 34.74%) and males (193, 22.13%). Overall, genomic DNA samples of 223 tick were isolated and screened for Rickettsia spp. by the amplification of rickettsial gltA, ompA, and ompB partial genes using conventional PCR. Rickettsial DNA was detected in 8 of 223 (3.58%) ticks including nymphs (5 of 122, 4.0%) and adult females (3 of 86, 3.48%). The rickettsial gltA, ompA, and ompB sequences were detected in Hyalomma turanicum (2 nymphs and 1 adult female), Haemaphysalis bispinosa (1 nymph and 1 adult female), and Haemaphysalis montgomeryi (2 nymphs and 1 adult female). These rickettsial sequences showed 99.71–100% identity with R. aeschlimannii and phylogenetically clustered with the same species. None of the tested Rhipicephalus microplus, Hyalomma isaaci, Hyalomma scupense, Rhipicephalus turanicus, Hyalomma anatolicum, Rhipicephalus haemaphysaloides, Rhipicephalus sanguineus, Haemaphysalis cornupunctata, and Haemaphysalis sulcata ticks were found positive for rickettsial DNA. Comprehensive surveillance studies should be adopted to update the knowledge regarding tick-borne zoonotic Rickettsia species, evaluate their risks to humans and livestock, and investigate the unexamined cases of illness after tick bite among livestock holders in the country.
Ticks are important ectoparasites due to their capacity to feed on animals including humans (De la Fuente et al., 2017) and transmit various pathogens including viruses, bacteria, and protozoans to their hosts (Boulanger et al., 2019). Among bacteria, Rickettsia species are transmitted by ticks, mites, and fleas, causing rickettsioses (Fournier and Raoult, 2009; Labruna, 2009). The main tick genera that are the potential vectors for Rickettsia spp. are Amblyomma, Dermacentor, Ixodes, Hyalomma, Rhipicephalus, and Haemaphysalis (Fournier and Raoult, 2009). Several studies have revealed the extensive diversity of the spotted fever group rickettsiae in different tick species and geographic locations (Socolovschi et al., 2009). Approximately 34 species in the genus Rickettsia have been validated, and several others are yet to be determined to the species level (El Karkouri et al., 2022). Spotted fever group Rickettsia spp. are distributed in Pakistan and have recently been reported in different ticks including Rh. microplus, Rh. haemaphysaloides, Rh. turanicus, H. kashmirensis, and H. cornupunctata infesting different animals (Ali et al., 2021, 2022; Khan et al., 2023).
Hard ticks are important reservoirs for various Rickettsia species including Rickettsia aeschlimannii (Parola et al., 2013). Previously, R. aeschlimannii has been detected in different tick species belonging to different genera such as Rhipicephalus, Hyalomma, Amblyomma, Haemaphysalis, and Ixodes in Morocco, Spain, Senegal, and Bolivia (Beati et al., 1997; Fernández-Soto et al., 2003; Mediannikov et al., 2010; Tomassone et al., 2010). The first human infection by R. aeschlimannii was documented serologically in Morocco (Raoult et al., 2002). Till then, several cases of human infection with tick-borne R. aeschlimannii have been reported (Raoult et al., 2002; Znazen et al., 2006; Mokrani et al., 2008; Germanakis et al., 2013; Igolkina et al., 2022). Molecular identification and genetic characterization of Rickettsia spp. are based on the gltA (citrate synthase gene), ompA (outer membrane protein A), ompB (outer membrane protein B), and sca4 (surface cell antigen 4) genes (Roux et al., 1997; Fournier et al., 2003).
Earlier, we characterized pathogenic and undetermined Rickettsia spp. associated with different ticks parasitizing a wide range of vertebrate hosts in Pakistan (Karim et al., 2017; Ali et al., 2021, 2022, 2023; Numan et al., 2022; Aneela et al., 2023; Shehla et al., 2023; Ullah et al., 2023). However, there is a paucity of information regarding the presence of R. aeschlimannii in different ticks in the country, and its potential risks to the public and animal’s health. Updated knowledge regarding the epidemiology, association with ticks infesting different hosts, and phylogenetic position of spotted fever group (SFG) R. aeschlimannii is essential for effective management of infection. This study aimed to molecularly characterize tick-borne Rickettsia spp. in selected districts of Pakistan and update the information about the global epidemiology of uncharacterized Rickettsia spp.
Tick specimens were collected from seven districts of Khyber Pakhtunkhwa (KP), a province in northwest Pakistan, namely, Mohmand (34.5356°N, 71.2874°E), Bajaur (34.7865°N, 71.5249° E), Swabi (34.0719°N, 72.4732°E), Charsadda (34.1682°N, 71.7504°E), Mardan (34.1986°N, 72.0404°E), Dir-Upper (35.3356°N, 72.0468°E), and Dir-Lower (34.9161°N, 71.8097°E). Data regarding the host, ticks, climate, and location were noted. Climatic information was taken from the website climate-data.org. Geo-coordinates of location sites were obtained from the global positioning system and were entered into a spreadsheet, and the study map was designed in ArcGIS10.8.1.3 (ESRI, Redlands, CA, USA) (Figure 1).
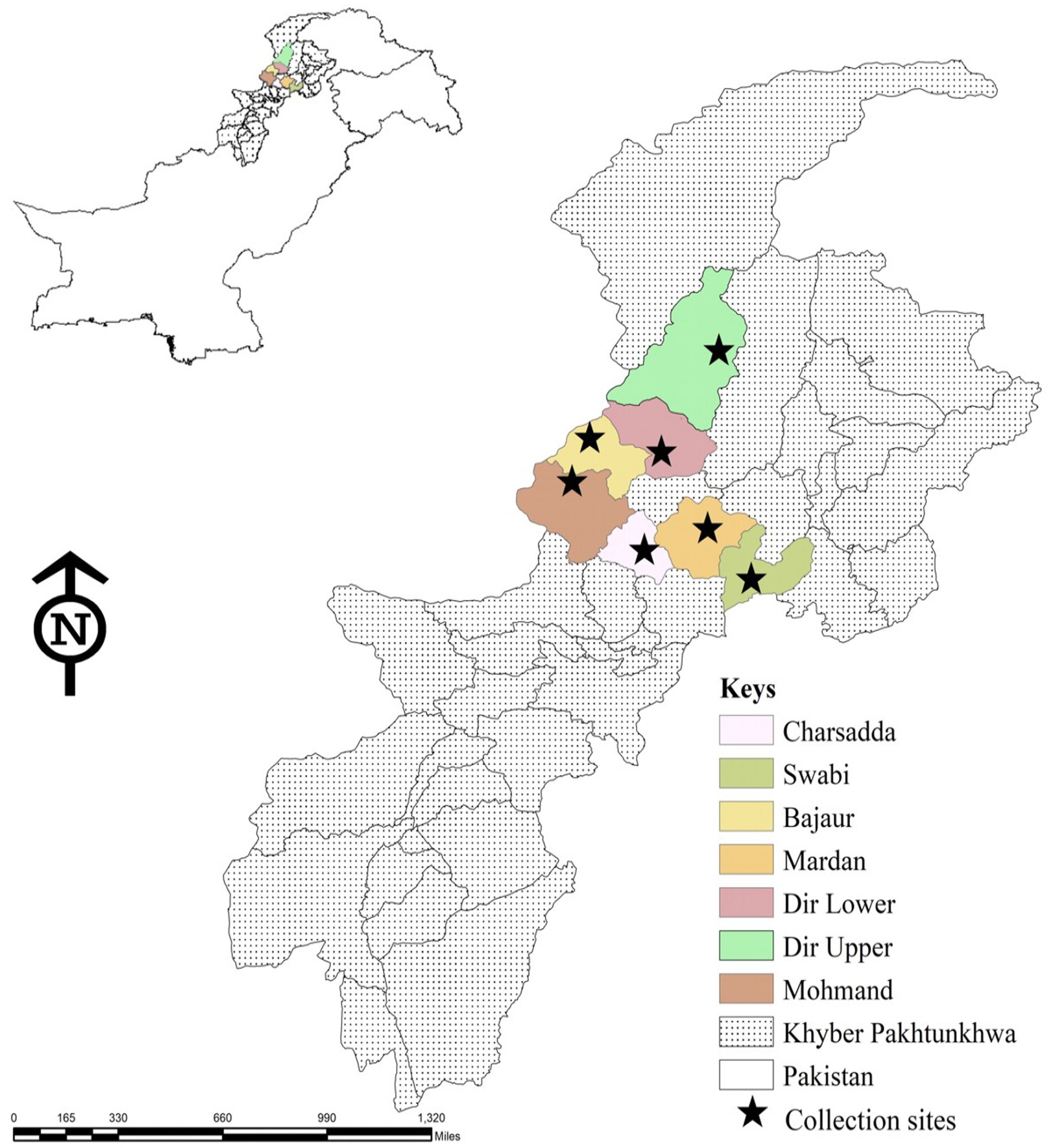
Figure 1. Map showing locations in seven districts of Khyber Pakhtunkhwa, where ticks were collected from domestic animals.
The design of the current study has received approval from the members of the Advanced Study and Research Board (Dir/A&R/AWKUM/2023/0014) and the Faculty of Zoology Department, Abdul Wali Khan University Mardan, Pakistan. Permission was obtained from the owner of the animal before collecting ticks from their animals.
Tick specimens were collected conveniently from March 2020 to February 2021 from domestic animals in seven districts of Khyber Pakhtunkhwa, Pakistan. The collection was opportunistic occurring whenever tick-infested animals were found within the survey regions. The whole body of the animal was examined for ticks. With the help of curved forceps, ticks were collected carefully so that the morphological features of ticks were not damaged. Every sample was tagged with its location of collection, date, and species of the animal.
The collected tick specimens were morphologically identified through a stereo microscope (CM100, China) by using standard taxonomic keys (Hoogstraal, 1962, 1966, 1973; Walker et al., 2000; Apanaskevich et al., 2008; 2010; Geevarghese and Mishra, 2011; Ali et al., 2022).
All ticks were identified morphologically, and genomic DNA was individually extracted from a subset of 223 (122N, 86F, and 15M) ticks. Ticks were cleaned with 70% ethanol, followed by distilled water and phosphate-buffered saline for the elimination of surface contaminants. Each rinsed tick was separately kept in a 1.5 mL tube and subjected to drying within an incubator for 30 min. Using a sterilized scalpel, the samples were cut into pieces inside the Eppendorf tube. The phenol-chloroform method was used for the extraction of genomic DNA from the ticks (Sambrook et al., 1989). The DNA concentration in each extracted sample was quantified with Nanodrop (OPTIZEN, Daejeon, South Korea).
The extracted DNA was used in conventional PCR (Thermo Fisher Scientific, and Walham, MA, USA), to amplify fragments of three genes of Rickettsia, namely, gltA (citrate synthase gene), ompA (outer membrane protein A), and ompB (outer membrane protein B). PCR was conducted in a 25 μL reaction mixture, containing 1 μL of each primer (forward and reverse primers), 8.5 μL of PCR water, 2 μL of template DNA, and 12.5 μL of DreamTaq PCR Master Mix (2×) (Thermo Scientific, Waltham, MA, USA). The primers used in the current study are presented in Table 1, and thermocycling conditions were set as previously used (Regnery et al., 1991; Roux and Raoult 2000; Labruna et al., 2004). Positive and negative control samples were Rickettsia massiliae DNA and “nuclease-free” water, respectively (Shehla et al., 2023). The amplified products of each PCR were observed by electrophoresis on 2% agarose gel and stained with ethidium-bromide, and the results were visualized on the Gel Doc system (BioDoc-It™ Imaging Systems, Upland, CA, USA).
The amplified DNA fragments were purified using the GENECLEAN II Kit (Qbiogene, Illkirch, France) and sequenced using the Sanger sequencing (Macrogen, Inc., Seoul, South Korea) in both forward and reverse directions. Poor-quality sequences were removed by trimming all the obtained sequences using SeqMan v 5.00 (DNASTAR, Inc.), and a consensus sequence was generated. Sequences with the highest identity were selected from GenBank using the Basic Local Alignment Search Tool (BLAST) on the user interface of National Center for Biotechnology Information, and these sequences were aligned with the obtained sequences by BioEdit v. 7.0.5 using CLUSTALW multiple alignments (Thompson et al., 1994), followed by MUSCLE alignment (Edgar, 2004). The Neighbor-Joining method was applied for obtaining phylogenies with 1,000 bootstrap replicates in Molecular Evolutionary Genetic Analysis (MEGA-X) software (Kumar et al., 2018). The sequences that were obtained made up the final positions in the dataset.
The recorded data of tick-infested hosts and tick distribution were described with frequency and percentage using descriptive statistics. Fisher’s exact test was used to determine the association between host, tick species, rickettsial species, and locations in GraphPad Prism software (V 5.0). p-value <0.05 was considered as significant standard.
The literature-based search was carried out by using different databases including ScienceDirect, PubMed, Web of Sciences, and Google Scholar to collect published data regarding Rickettsia aeschlimannii in different tick species, wild and domestic animals, humans, environment, and vegetation. The search was conducted by using some keywords, such as ticks, tick-borne pathogens, domestic animals, small ruminants, zoonosis, livestock, and Rickettsia aeschlimannii. Complete research articles, short communication, review papers, and conference articles were downloaded by using a combination of the above mentioned keywords. Lists of references from downloaded studies were examined to relevant articles (Table 2).
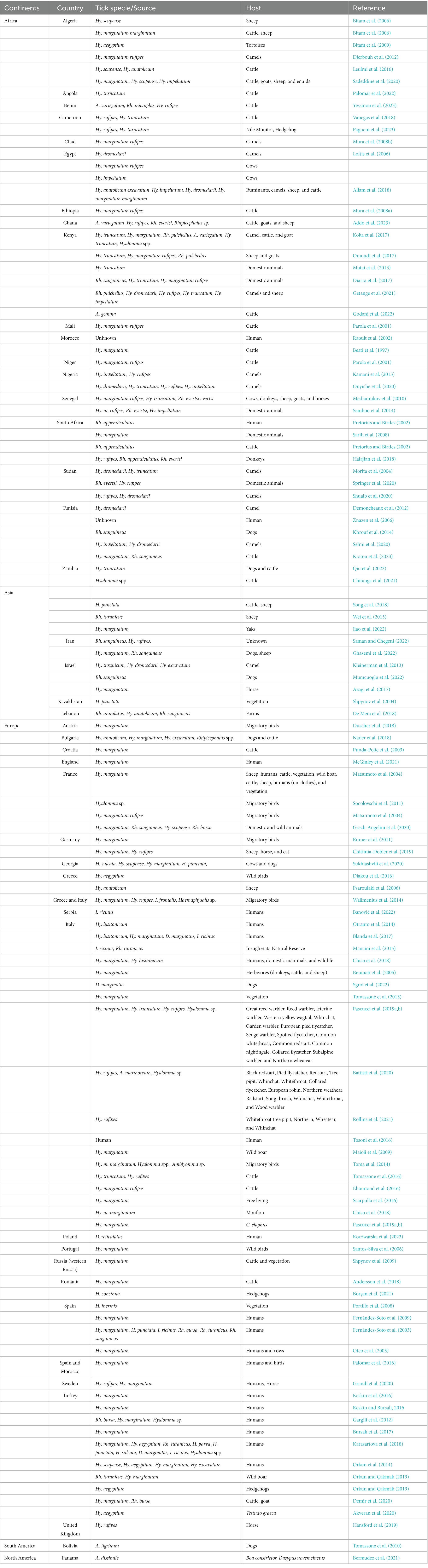
Table 2. Global epidemiology of Rickettsia aeschlimannii detected in different ticks and vertebrate hosts including human.
A total of 369 domestic animals were examined in 7 districts including Mohmand (23 cattle, 14 goats, and 12 sheep), Dir Upper (20 cattle, 15 goats, 12 sheep, and 7 dogs), Dir lower (26 cattle, 17 goats, 13 sheep, and 5 dogs), Bajaur (19 cattle, 16 goats, 13 sheep, and 11 dogs), Charsadda (17 cattle, 15 goats, 13 sheep, and 5 dogs), Mardan (16 cattle, 13 goats, 20 sheep, and 5 dogs), and Swabi (17 cattle, 13 goats, 10 sheep, and 4 dogs) (p = 0.248). Of the examined hosts, 66% (247 of 369) of hosts were parasitized by 872 ticks of various life stages (Table 3), having a mean intensity of 3.5 ticks/infested host while the mean abundance was 2.3 ticks/examined host. The highest tick infestation was found on goats (74 of 103, 71.8%) compared with cattle (99 of 138, 71.7%), sheep (62 of 91, 60%), and dogs (12 of 37, 32.4%) (p < 0.0001). All collected ticks belonged to three different genera of ixodid ticks, namely, Rhipicephalus, Hyalomma, and Haemaphysalis. Adult female including engorged ticks were the most prevalent (376, 43.1%), followed by nymphs (303, 34.74%) and males (193, 22.13%) (Table 3) (p < 0.0001). The highest tick burden was observed on domestic animals in the Bajaur district (19.3%), followed by Mardan (15%), Swabi (14.9%), Charsadda (13.9%), Mohmand (13.3%), Dir Upper (12.7%), and Dir Lower (10.6%) (p < 0.0001). The most dominant species was Rhipicephalus microplus (19.0%), followed by Hyalomma anatolicum (15.7%), Rhipicephalus turanicus (11.5%), Haemaphysalis sulcata (10.6%), Haemaphysalis bispinosa (9.8%), Haemaphysalis montgomeryi (9.1%), Rhipicephalus sanguineus (8.8%), Hyalomma scupense (4.9%), Rhipicephalus haemaphysaloides (3.8%), Haemaphysalis cornupunctata (2.7%) Hyalomma isaaci (1.9%), and Hyalomma turanicum (1.60%) (p < 0.0001).
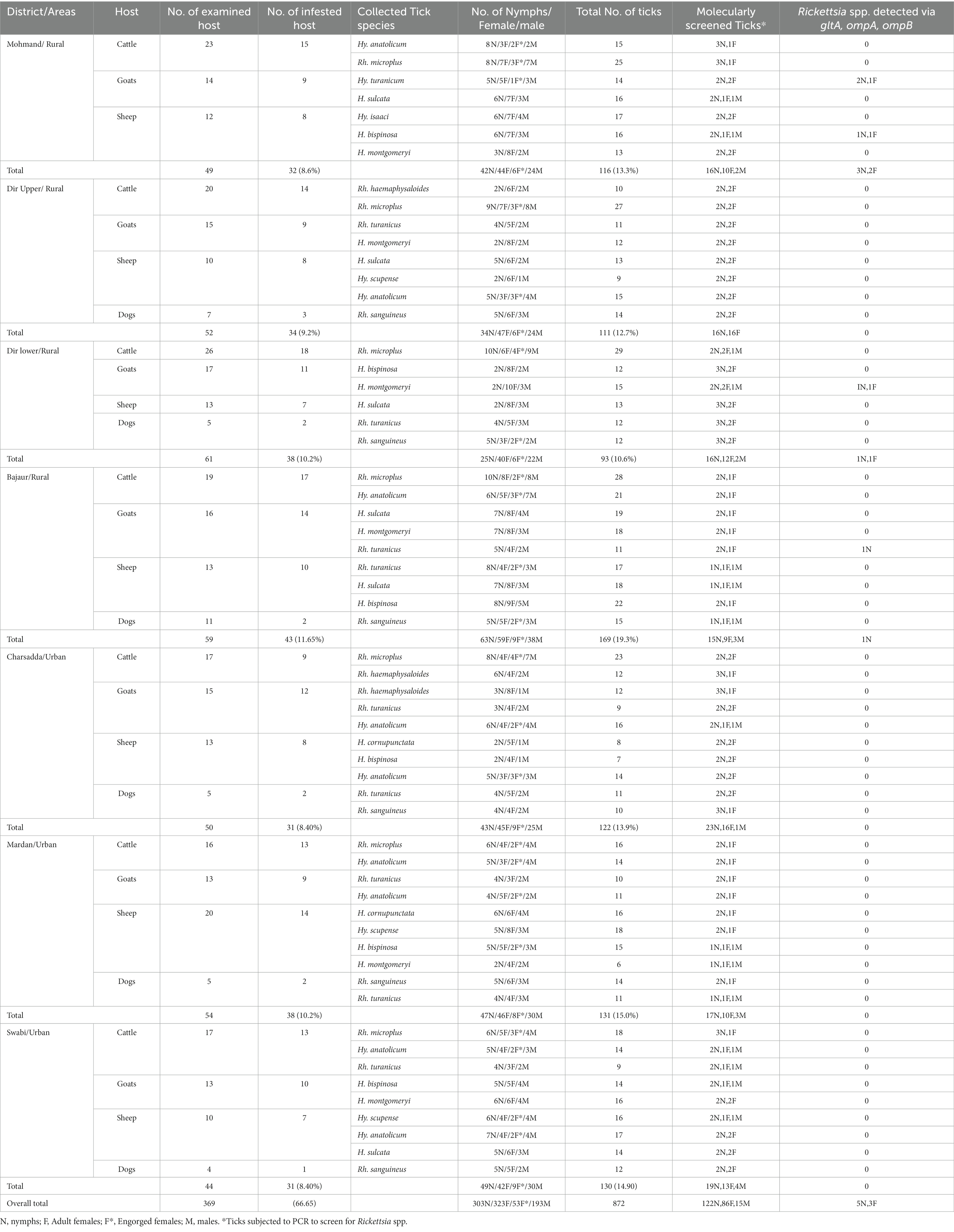
Table 3. Occurrence of ticks infesting various domestic animals and detection of rickettsial DNA associated with ticks.
DNA of a subset of 223 ticks was used for the detection of Rickettsia spp. by the amplification of three rickettsial markers, namely, gltA, ompA, and ompB gene fragments. Positive ticks for rickettsial gltA were also positive when tested with the primers of ompA and ompB. In total, 8 out of 223 (3.58%) ticks including 5 of 28 (17.85%) from Mohmand, 2 of 30 (6.6%) from Dir Lower, and 1 of 27 (3.7%) from Bajaur were found positive for Rickettsia spp. (P, 0.1621). Rickettsial DNA was found in three tick species, namely, H. montgomeryi, H. bispinosa, and H. turanicum, which was collected from sheep and goats. The overall prevalence of Rickettsia spp. was 3.58% (8 of 223). No rickettsial DNA was detected in Rh. microplus (27), Hy. isaaci (4), Hy. scupense (11), Rh. turanicus (33), Hy. anatolicum (33), Rh. haemaphysaloides (12), Rh. sanguineus (23), H. cornupunctata (7), and H. sulcata (23). Information regarding the prevalence of Rickettsia spp. in various ticks is shown in Table 3.
Assembled contigs of direct and reverse sequence reads for each PCR-amplified fragment were analyzed. Due to a single haplotype, consensus sequences were generated for each partial gene. The consensus sequence of gltA (348 bp) showed 100% identity with R. aeschlimannii from Russia, Senegal, and Kazakhstan, followed by 99.71% identity with R. aeschlimannii-type strain from Morocco. Similarly, 100% identity was shown by the obtained ompA (467 bp) consensus sequence with R. aeschlimannii from Russia, Spain, Turkey, and Kazakhstan, followed by 99.79% identity with R. aeschlimannii-type strain. Similarly, the ompB (764 bp) consensus sequence also showed 100% identity with R. aeschlimannii from Kazakhstan, Russia, Italy, and Portugal, followed by 99.74% identity with R. aeschlimannii-type strain. In all cases, the query coverage was 100%. The obtained rickettsial gltA sequence (accession number: OR351959), ompA sequence (accession number: OR351960), and ompB sequence (accession number: OR351961) were submitted to GenBank.
In phylogenetic tree based on rickettsial gltA, R. aeschlimannii clustered with R. aeschlimannii reported from Senegal (HM050283) and Kazakhstan (MW922554) (Figure 2). Rickettsial ompA clustered with R. aeschlimannii reported from Senegal (HM050286), Kazakhstan (MW922585), and Morocco (U43800) (Figure 3), while ompB sequence clustered with R. aeschlimannii reported from China (MF098413), Senegal (HM050278), and Morocco (AF123705) (Figure 4).
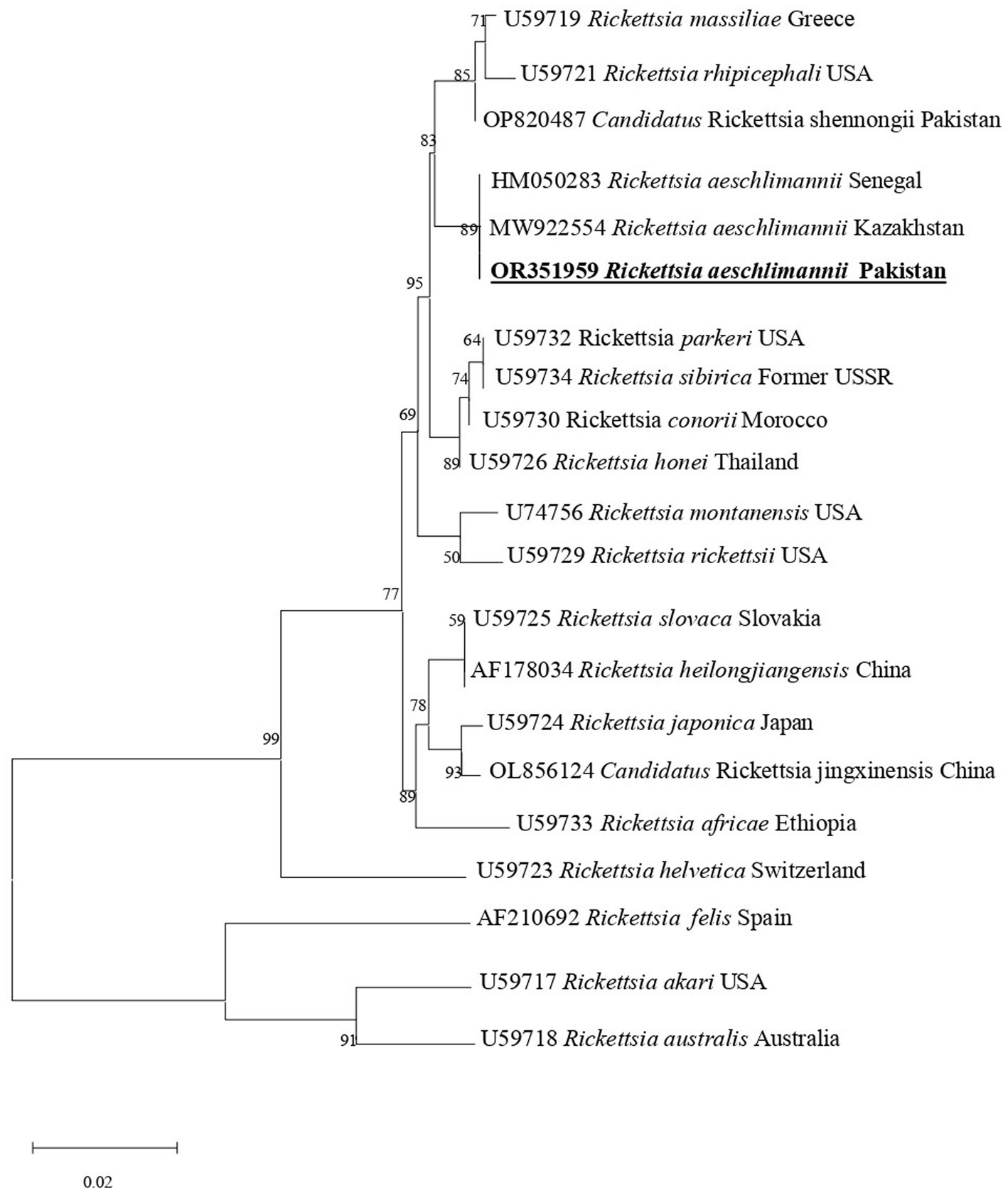
Figure 2. Phylogenetic analysis based on the gltA sequences of Rickettsia aeschlimannii. The obtained sequences of the present study are indicated in bold and underlined fonts. Rickettsia akari and Rickettsia australis were used as out-group.
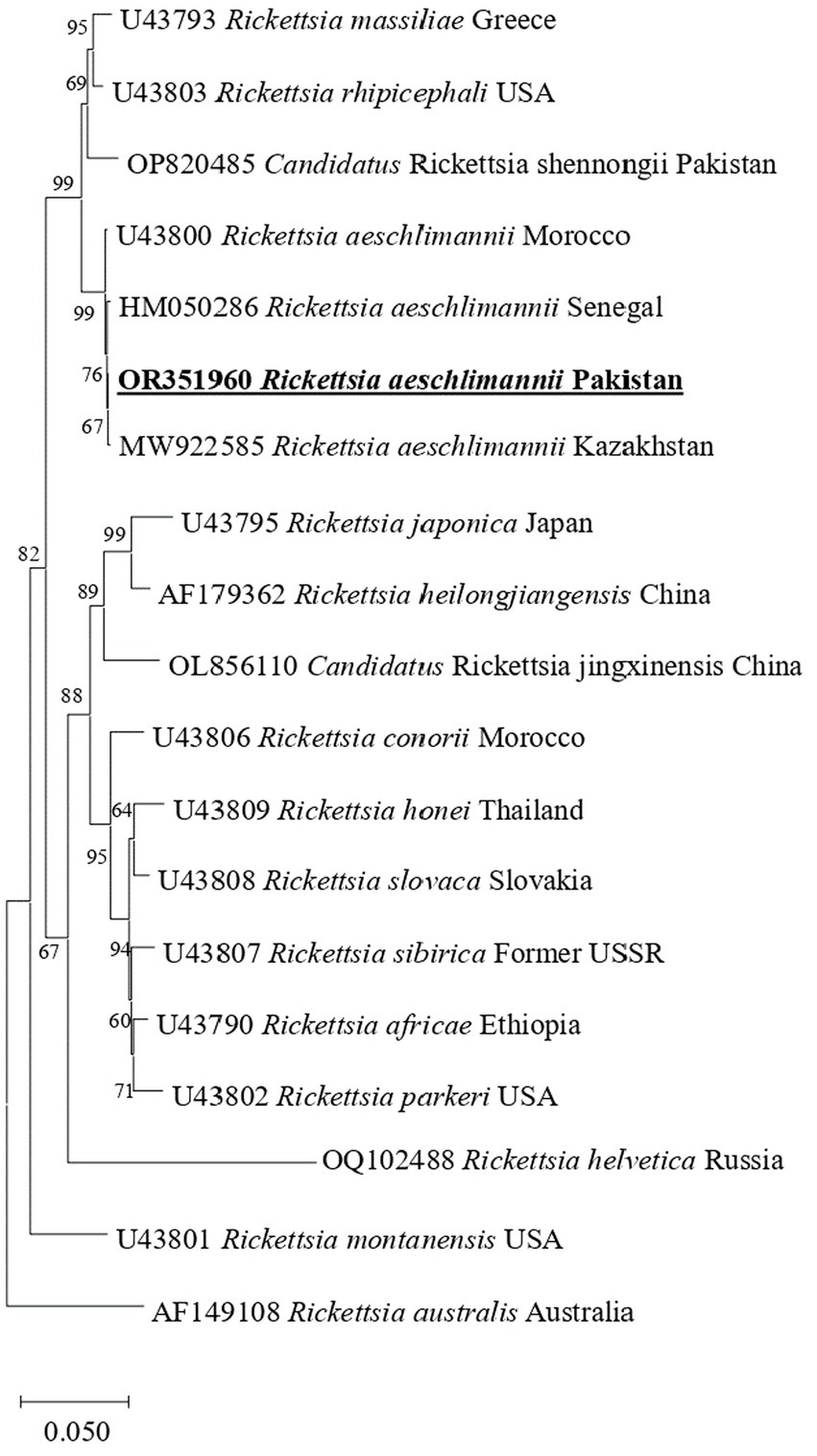
Figure 3. Phylogenetic analysis based on the ompA sequences of R. aeschlimannii. The sequences obtained in this study are indicated in bold and underlined fonts. Rickettsia australis is used as an out-group.
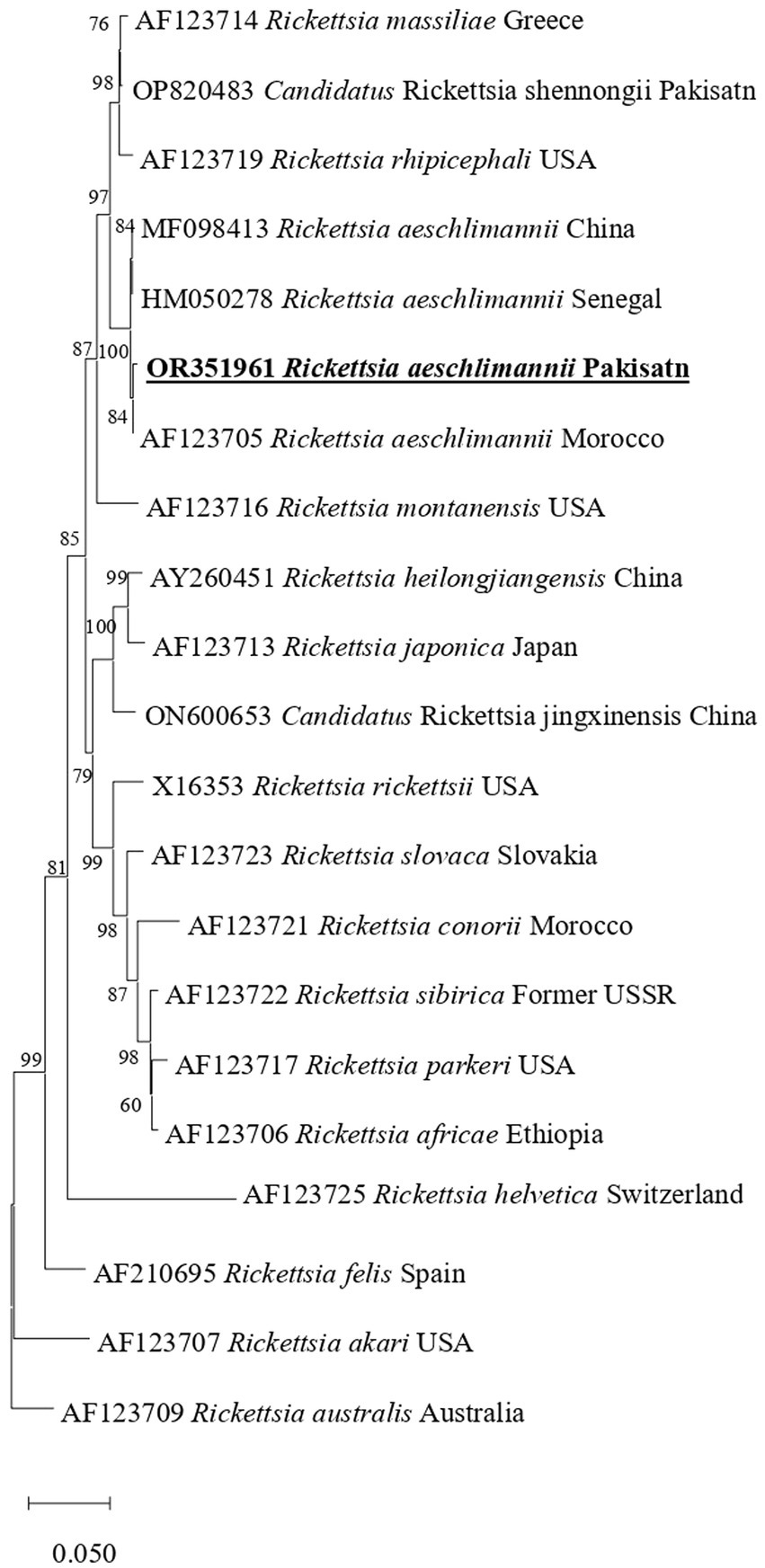
Figure 4. Phylogenetic analysis based on the sequences of the ompB genes of R. aeschlimannii. The obtained sequences in the present study are indicated in bold and underlined fonts. Rickettsia australis used as an out-group.
Previous studies have recorded the presence of Rickettsia spp. in different tick-infesting hosts in Pakistan. However, there was a paucity of information regarding R. aeschlimannii in the region. This study presents the first report on molecular detection of R. aeschlimannii in H. bispinosa, H. montgomeryi, and Hy. turanicum ticks collected from sheep and goats in Pakistan. The obtained sequences showed maximum identity and phylogenetically clustered with Rickettsia aeschlimannii, which confirms the occurrence of Rickettsia aeschlimannii in the regions. Rickettsia aeschlimannii, an emerging pathogen with zoonotic potential, has been observed to cause infections in humans across various countries such as Morocco (Raoult et al., 2002), Tunisia (Znazen et al., 2006), Algeria (Mokrani et al., 2008), Greece (Germanakis et al., 2013), and Russia (Igolkina et al., 2022). The detection of R. aeschlimannii in tick-parasitizing domestic animals suggests a high exposure of livestock holders to this pathogen.
Small ruminants (goats and sheep), domestic dogs, and cattle were found infested by the ticks of genera Rhipicephalus, Hyalomma, and Haemaphysalis. Among the collected ticks, Rh. microplus, Hy. anatolicum, H. bispinosa and H. montgomeryi were the dominant tick species. These findings mirror the pattern observed in other previous studies conducted in same region (Karim et al., 2017; Ali et al., 2019; Alam et al., 2022; Khan Z. et al., 2022; Tila et al., 2023) underscoring the regional significance of these tick species. Furthermore, it emphasizes the need for further research to comprehensively investigate their prevalence, distribution, and potential implications for public health (Ali et al., 2023).
Ticks of different genera have been reported as carriers for various Rickettsia spp. in Pakistan (Ali et al., 2021, 2022; Khan M. et al., 2022; Khan Z. et al., 2022; Numan et al., 2022; Ullah et al., 2023). Herein, Rickettsia aeschlimannii was detected in H. bispinosa, H. montgomeryi, and Hy. turanicum ticks using three genetic markers, namely, gltA, ompA, and ompB. To date, there has been a lack of information regarding the detection of R. aeschlimannii in H. bispinosa and H. montgomeryi tick-infesting domestic animals, such as goats and sheep. There is also a possibility that the rickettsial DNA is detected in the ticks may be due to ingesting rickettsemic host blood. Literature search revealed that R. aeschlimannii is associated with a variety of tick species belonging to six genera of hard ticks, namely, Hyalomma, Rhipicephalus, Haemaphysalis, Amblyomma, Dermacentor, and Ixodes (Parola et al., 2001; Fernández-Soto et al., 2003; Shpynov et al., 2004; Tomassone et al., 2010; Karasartova et al., 2018). Furthermore, there are limited reports, which have detected this pathogen in Asia (Wei et al., 2015; Satjanadumrong et al., 2019). This study found its association with two new ticks, expanding its known host and geographical range. The detection of R. aeschlimannii in Hyalomma and Haemaphysalis ticks suggests a potential threat to livestock holders. Additionally, the rate of R. aeschlimannii was observed highest in Haemaphysalis ticks, which are the primary ticks that infest goats and sheep. This enhances the feasibility of public health risks as these ticks may occasionally infest humans (Guglielmone and Robbins, 2018). Rickettsia aeschlimannii has been detected in all life stages of ticks, such as adult females, males, larvae, and nymphs (Raoult et al., 2002; Shpynov et al., 2009; Germanakis et al., 2013; Orkun et al., 2014; Wallmenius et al., 2014; Tosoni et al., 2016). This study presents the first molecular evidence of R. aeschlimannii in H. bispinosa and H. montgomeryi ticks, which suggests that other tick species could also serve as competent vectors for this pathogen in the region.
Molecular methods are considered faster and more accurate for the genetic characterization and phylogenetic analysis of Rickettsia spp. (Fournier et al., 1998; Roux and Raoult, 2000). The gltA, ompA, ompB, and sca4 DNA sequences have been used as suitable genetic markers to discriminate different Rickettsia spp (Roux and Raoult, 2000; Fournier et al., 2003; Labruna et al., 2004). Herein, these sequences were targeted for molecular characterization and phylogenetic analysis of R. aeschlimannii which revealed the close relatedness with corresponding species of the SFG. We assume that human infection caused by rickettsial agents of SFG maybe underreported due to the lack of epidemiological information among health practitioners and laboratory technicians and the lack of diagnostic procedures in Pakistan, given the relevance of the occurrence of these agents in the region. Further studies in the region should be encouraged to obtain information on zoonotic outcomes due to these infectious agents.
This study for the first time contributes to the neglected knowledge and genetic characterization of tick-borne R. aeschlimannii in H. bispinosa, H. montgomeryi, and Hy. turanicum ticks in Pakistan. The results of this study also indicated that goats and sheep are exposed to R. aeschlimannii. Further molecular studies are important to screen R. aeschlimannii in livestock and livestock holders who have close contact with domestic animals.
We have released your GenBank submissions in OR351959-OR351961. Your sequences will be available for public access within a few days. If you have additional information about your sequences or wish to make further revisions, see: https://www.ncbi.nlm.nih.gov/Genbank/update.html for proper update formats.
The design of the current work has received an approval from the Advanced Study and Research Board members (Dir/A&R/AWKUM/2023/0014) and the Faculty of Zoology department, Abdul Wali Khan University Mardan, Pakistan. Permissions were obtained from the animal’s owner before collecting ticks from their animals. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
AM: Data curation, Investigation, Methodology, Software, Writing – original draft. MA: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. AbdA: Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. TT: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. T-YY: Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. K-HT: Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. AbiA: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing –original draft, Writing – review & editing.
The authors appreciate the financial support from the Pakistan Science Foundation and Higher Education Commission of Pakistan. The researchers supporting project number (RSP2023R494), King Saud University, Riyadh, Saudi Arabia. This research was also partially funded by the National Science and Technology Council, grant number: 112-2327-B-002-008.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Addo, S. O., Bentil, R. E., Baako, B. O. A., Yartey, K. N., Behene, E., Asiamah, B., et al. (2023). Occurrence of Rickettsia spp. and Coxiella burnetii in ixodid ticks in Kassena-Nankana, Ghana. Exp. Appl. Acarol. 90, 137–153. doi: 10.1007/s10493-023-00808-0
Akveran, G. A., Karasartova, D., Keskin, A., Comba, A., Celebi, B., Mumcuoglu, K. Y., et al. (2020). Bacterial and protozoan agents found in Hyalomma aegyptium (L., 1758) (Ixodida: Ixodidae) collected from Testudo graeca L., 1758 (Reptilia: Testudines) in Corum Province of Turkey. Ticks Tick-borne Dis. 11:101458. doi: 10.1016/j.ttbdis.2020.101458
Alam, S., Khan, M., Alouffi, A., Almutairi, M. M., Ullah, S., Numan, M., et al. (2022). Spatio-temporal patterns of ticks and molecular survey of Anaplasma marginale, with notes on their phylogeny. Microorganisms 10:1663. doi: 10.3390/microorganisms10081663
Ali, A., Khan, M.A., Zahid, H., Yaseen, P. M., Qayash Khan, M., Nawab, J., et al. (2019). Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Frontiers in Physiology 10:793. doi: 10.3389/fphys.2019.00793
Ali, A., Ullah, S., Numan, M., Almutairi, M. M., Alouffi, A., and Tanaka, T. (2023). First report on tick-borne pathogens detected in ticks infesting stray dogs near butcher shops. Front. Vet. Sci. 10:1246871. doi: 10.3389/fvets.2023.1246871
Ali, A., Numan, M., Khan, M., Aiman, O., Muñoz-Leal, S., Chitimia-Dobler, L., et al. (2022). Ornithodoros (Pavlovskyella) ticks associated with a Rickettsia sp. in Pakistan. Parasit. Vect. 15, 1–13. doi: 10.1186/s13071-022-05248-0
Ali, A., Zahid, H., Zeb, I., Tufail, M., Khan, S., Haroon, M., et al. (2021). Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit. Vectors 14, 363–312. doi: 10.1186/s13071-021-04836-w
Allam, N., El Moghazy, F. M., and Abdel-Baky, S. (2018). Molecular epidemiological updates on spotted fever rickettsioses in animal species and their hard ticks settling Egyptian desert. J. Adv. Pharm. Educat. Res. 8:65.
Aneela, A., Almutairi, M. M., Alouffi, A., Ahmed, H., Tanaka, T., da Silva Vaz, I., et al. (2023). Molecular detection of Rickettsia hoogstraalii in Hyalomma anatolicum and Haemaphysalis sulcata: updated knowledge on the epidemiology of tick-borne Rickettsia hoogstraalii. Vet. Sci. 10:605. doi: 10.3390/vetsci10100605
Andersson, M. O., Tolf, C., Tamba, P., Stefanache, M., Radbea, G., Frangoulidis, D., et al. (2018). Molecular survey of neglected bacterial pathogens reveals an abundant diversity of species and genotypes in ticks collected from animal hosts across Romania. Parasit. Vectors 11, 1–10. doi: 10.1186/s13071-018-2756-1
Apanaskevich, D. A., Filippova, N. A., and Horak, I. G. (2010). The genus Hyalomma Koch, 1844. X. Redescription of all parasitic stages of Hy. scupense, 1919 (Hy. detritum Schulze) (Acari: Ixodidae) and notes on its biology. Folia Parasitol. 57, 69–78. doi: 10.14411/fp.2010.009
Apanaskevich, D. A., Schuster, A. L., and Horak, I. G. (2008). The genus Hyalomma: VII. Redescription of all parasitic stages of Hy. dromedarii and Hy. schulzei (Acari: Ixodidae). J. Med. Entomol. 45, 817–831. doi: 10.1093/jmedent/45.5.817
Azagi, T., Klement, E., Perlman, G., Lustig, Y., Mumcuoglu, K. Y., Apanaskevich, D. A., et al. (2017). Francisella-like endosymbionts and Rickettsia species in local and imported Hyalomma ticks. Appl. Environ. Microbiol. 83, e01302–e01317. doi: 10.1128/AEM.01302-17
Banović, P., Díaz-Sánchez, A. A., Simin, V., Foucault-Simonin, A., Galon, C., Wu-Chuang, A., et al. (2022). Clinical aspects and detection of emerging rickettsial pathogens: A “One Health” approach study in Serbia, 2020. Frontiers in microbiology 12, p.797399. doi: 10.3389/fmicb.2021.797399
Battisti, E., Urach, K., Hodžić, A., Fusani, L., Hufnagl, P., Felsberger, G., et al. (2020). Zoonotic pathogens in ticks from migratory birds, Italy. Emerg. Infect. Dis. 26, 2986–2988. doi: 10.3201/eid2612.181686
Beati, L., Meskini, M., Thiers, B., and Raoult, D. (1997). Rickettsia aeschlimannii sp. nov., a new spotted fever group Rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Evol. Microbiol. 47, 548–554. doi: 10.1099/00207713-47-2-548
Beninati, T., Genchi, C., Torina, A., Caracappa, S., Bandi, C., and Lo, N. (2005). Rickettsiae in ixodid ticks, Sicily. Emerg. Infect. Dis. 11, 509–511. doi: 10.3201/eid1103.040812
Bermudez, S., Martinez-Mandiche, J., Dominguez, L., Gonzalez, C., Chavarria, O., Moreno, A., et al. (2021). Diversity of Rickettsia in ticks collected from wild animals in Panama. Ticks Tick-borne Dis. 12:101723. doi: 10.1016/j.ttbdis.2021.101723
Bitam, I., Kernif, T., Harrat, Z., Parola, P., and Raoult, D. (2009). First detection of Rickettsia aeschlimannii in Hyalomma aegyptium from Algeria. Clin. Microbiol. Infect. 15, 253–254. doi: 10.1111/j.1469-0691.2008.02274.x
Bitam, I., Parola, P., Matsumoto, K., Rolain, J. M., Baziz, B., Boubidi, S. C., et al. (2006). First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann. N. Y. Acad. Sci. 1078, 368–372. doi: 10.1196/annals.1374.073
Blanda, V., Torina, A., La Russa, F., D’Agostino, R., Randazzo, K., Scimeca, S., et al. (2017). A retrospective study of the characterization of Rickettsia species in ticks collected from humans. Ticks Tick-borne Dis. 8, 610–614. doi: 10.1016/j.ttbdis.2017.04.005
Borşan, S. D., Ionică, A. M., Galon, C., Toma-Naic, A., Peştean, C., Sándor, A. D., et al. (2021). High diversity, prevalence, and co-infection rates of tick-borne pathogens in ticks and wildlife hosts in an urban area in Romania. Front. Microbiol. 12:645002. doi: 10.3389/fmicb.2021.645002
Boulanger, N., Boyer, P., Talagrand-Reboul, E., and Hansmann, Y. (2019). Tick-borne diseases. Medecine et Maladies Infectieuses 49, 87–97. doi: 10.13140/RG.2.2.18223.30881
Brouqui, P., Parola, P., Fournier, P. E., and Raoult, D. (2007). Spotted fever rickettsioses in Southern and Eastern Europe. FEMS 49, 2–12. doi: 10.1111/j.1574-695X.2006.00138.x
Bursalı, A., Keskin, A., Keskin, A., Kul Köprülü, T., and Tekin, Ş. (2017). Investigation of the presence of Rickettsiae in ticks parasitizing humans in Corum Region. Turk. Hij. Tecr. Biyol. Derg. 74, 293–298. doi: 10.5505/TurkHijyen.2017.28291
Chisu, V., Foxi, C., and Masala, G. (2018). First molecular detection of the human pathogen Rickettsia raoultii and other spotted fever group rickettsiae in Ixodid ticks from wild and domestic mammals. Parasitology research, 17, pp. 3421–3429. doi: 10.1007/s00436-018-6036-y
Chitanga, S., Chibesa, K., Sichibalo, K., Mubemba, B., Nalubamba, K. S., Muleya, W., et al. (2021). Molecular detection and characterization of Rickettsia species in Ixodid ticks collected from cattle in Southern Zambia. Front. Vet. Sci. 8:684487. doi: 10.3389/fvets.2021.684487
Chitimia-Dobler, L., Schaper, S., Rieß, R., Bitterwolf, K., Frangoulidis, D., Bestehorn, M., et al. (2019). Imported Hyalomma ticks in Germany in 2018. Parasit. Vectors 12, 134–139. doi: 10.1186/s13071-019-3380-4
Djerbouh, A., Kernif, T., Beneldjouzi, A., Socolovschi, C., Kechemir, N., Parola, P., et al. (2012). The first molecular detection of Rickettsia aeschlimannii in the ticks of camels from Southern Algeria. Ticks Tick Borne Dis. 3, 374–376. doi: 10.1016/j.ttbdis.2012.10.014
De la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7:114. doi: 10.3389/fcimb.2017.00114
Demoncheaux, J. P., Socolovschi, C., Davoust, B., Haddad, S., Raoult, D., and Parola, P. (2012). First detection of Rickettsia aeschlimannii in Hyalomma dromedarii ticks from Tunisia. Ticks Tick Borne Dis. 3, 398–402. doi: 10.1016/j.ttbdis.2012.10.003
de Mera, I. G. F., Blanda, V., Torina, A., Dabaja, M. F., El Romeh, A., Cabezas-Cruz, A., et al. (2018). Identification and molecular characterization of spotted fever group Rickettsiae in ticks collected from farm ruminants in Lebanon. Ticks Tick Borne Dis. 9, 104–108. doi: 10.1016/j.ttbdis.2017.10.001
Demir, S., Erkunt Alak, S., Köseoğlu, A. E., Ün, C., Nalçacı, M., and Can, H. (2020). Molecular investigation of Rickettsia spp. and Francisella tularensis in ticks from three provinces of Turkey. Exp. Appl. Acarol. 81, 239–253. doi: 10.1007/s10493-020-00498-y
Diakou, A., Norte, A. C., Lopes de Carvalho, I., Núncio, S., Nováková, M., Kautman, M., et al. (2016). Ticks and tick-borne pathogens in wild birds in Greece. Parasitol. Res. 115, 2011–2016. doi: 10.1007/s00436-016-4943-3
Diarra, A. Z., Almeras, L., Laroche, M., Berenger, J. M., Koné, A. K., Bocoum, Z., et al. (2017). Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl. Trop. Dis. 11:e0005762. doi: 10.1371/journal.pntd.0005762
Duscher, G. G., Hodžić, A., Hufnagl, P., Wille-Piazzai, W., Schötta, A. M., Markowicz, M. A., et al. (2018). Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Eur. Secur. 23:1800595. doi: 10.2807/1560-7917.ES.2018.23.48.1800595
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1016/j.sbi.2006.04.004
Ehounoud, C. B., Yao, K. P., Dahmani, M., Achi, Y. L., Amanzougaghene, N., Kacou N’Douba, A., et al. (2016). Multiple pathogens including potential new species in tick vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 10:e0004367. doi: 10.1371/journal.pntd.0004367
El Karkouri, K., Ghigo, E., Raoult, D., and Fournier, P. E. (2022). Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 12:3807. doi: 10.1038/s41598-022-07725-z
Fernández-Soto, P., Encinas Grandes, A., and Pérez Sánchez, R. (2003). Rickettsia aeschlimannii in Spain: molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg. Infect. Dis. 9, 889–890. doi: 10.3201/eid0907.030077
Fernández-Soto, P., Díaz Martín, V., Pérez-Sánchez, R., and Encinas-Grandes, A. (2009). Increased prevalence of Rickettsia aeschlimannii in Castilla y Leon, Spain. Eur. J. Clin. Microbiol. Infect. Dis. 28, 693–695. doi: 10.1007/s10096-008-0667-3
Fournier, P. E., and Raoult, D. (2009). Current knowledge on phylogeny and taxonomy of Rickettsia spp. Ann. N. Y. Acad. Sci. 1166, 1–11. doi: 10.1111/j.1749-6632.2009.04528.x
Fournier, P. E., Roux, V., and Raoult, D. (1998). Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA.. International Journal of Systematic and Evolutionary Microbiology 48, 839–849. doi: 10.1099/00207713-48-3-839
Fournier, P. E., Dumler, J. S., Greub, G., Zhang, J., Wu, Y., and Raoult, D. (2003). Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41, 5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003
Gargili, A., Palomar, A. M., Midilli, K., Portillo, A., Kar, S., and Oteo, J. A. (2012). Rickettsia species in ticks removed from humans in Istanbul, Turkey. Vector-Borne Zoonotic Dis. 12, 938–941. doi: 10.1089/vbz.2012.0996
Germanakis, A., Chochlakis, D., Angelakis, E., Tselentis, Y., and Psaroulaki, A. (2013). Rickettsia aeschlimannii infection in a man, Greece. Emerg. Infect. Dis. 19:1176. doi: 10.3201/eid1907.130232
Getange, D., Bargul, J. L., Kanduma, E., Collins, M., Bodha, B., Denge, D., et al. (2021). Ticks and tick-borne pathogens associated with dromedary camels (Camelus dromedarius) in northern Kenya. Microorganisms 9:1414. doi: 10.3390/microorganisms9071414
Ghasemi, A., Latifian, M., Esmaeili, S., Naddaf, S. R., and Mostafavi, E. (2022). Molecular surveillance for Rickettsia spp. and Bartonella spp. in ticks from Northern Iran. PLoS One 17:e0278579. doi: 10.1371/journal.pone.0278579
Godani, S. K., Chengo, M. N., and Muturi, M. W. (2022). Zoonotic pathogens detected in ticks in Kenyan game reserves. Adv. Entomol. 11, 1–9. doi: 10.4236/ae.2023.111001
Grandi, G., Chitimia-Dobler, L., Choklikitumnuey, P., Strube, C., Springer, A., Albihn, A., et al. (2020). First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick-borne Dis. 11:101403. doi: 10.1016/j.ttbdis.2020.101403
Grech-Angelini, S., Stachurski, F., Vayssier-Taussat, M., Devillers, E., Casabianca, F., Lancelot, R., et al. (2020). Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 67, 745–757. doi: 10.1111/tbed.13393
Guglielmone, A.A., and Robbins, R.G., (2018). Hard Ticks (Acari: Ixodida: Ixodidae) Parasitizing Humans. Cham: Springer, 230
Halajian, A., Palomar, A. M., Portillo, A., Heyne, H., Romero, L., and Oteo, J. A. (2018). Detection of zoonotic agents and a new Rickettsia strain in ticks from donkeys from South Africa: implications for travel medicine. Travel Med. Infect. Dis. 26, 43–50. doi: 10.1016/j.tmaid.2018.10.007
Hansford, K. M., Carter, D., Gillingham, E. L., Hernandez-Triana, L. M., Chamberlain, J., Cull, B., et al. (2019). Hyalomma rufipes on an untraveled horse: is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Ticks Tick-borne Dis. 10, 704–708. doi: 10.1016/j.ttbdis.2019.03.003
Hoogstraal, H., El Kammah, K.M., Santana, F.J., and Van Peenen, P.D. (1973). Studies on southeast Asian Haemaphysalis ticks (Ixodoidea: Ixodidae). H.(Kaiseriana) lagrangei Larrousse: identity, distribution, and hosts. The Journal of Parasitology , 1118–1129. doi: 10.2307/3278651
Hoogstraal, H., Trapido, H., and Kohls, G. M. (1996). Studies on southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae). Speciation in the H.(Kaiseriana) obesa group: H. semermis Neumann, H. obesa Larrousse, H. roubaudi Toumanoff, H. montgomeryi Nuttall, and H. hirsuta sp. n.” The Journal of Parasitology 169–191. doi: 10.2307/3276410
Hoogstraal, H., and Varma, M. G. R. (1962). Haemaphysalis cornupunctata sp. n. and H. kashmirensis sp. n. from Kashmir, with Notes on H. sundrai Sharif and H. sewelli Sharif of India and Pakistan (Ixodoidea, Ixodidae). J. Parasitol. 48, 185–194. doi: 10.2307/3275561
Igolkina, Y., Rar, V., Krasnova, E., Filimonova, E., Tikunov, A., Epikhina, T., et al. (2022). Occurrence and clinical manifestations of tick-borne rickettsioses in Western Siberia: first Russian cases of Rickettsia aeschlimannii and Rickettsia slovaca infections. Ticks Tick-borne Diseases 13:101927. doi: 10.1016/j.ttbdis.2022.101927
Jian, Y., Li, J., Adjou Moumouni, P. F., Zhang, X., Tumwebaze, M. A., Wang, G., et al. (2020). Human spotted fever group Rickettsia infecting yaks (Bos grunniens) in the Qinghai-Tibetan plateau area. Pathogens 9:249. doi: 10.1016/j.ttbdis.2020.101466
Jiao, J., Yu, Y., He, P., Wan, W., OuYang, X., Wen, B., et al. (2022). First detection of Rickettsia aeschlimannii in Hyalomma marginatum in tibet, China. Zoonoses
Kamani, J., Baneth, G., Apanaskevich, D. A., Mumcuoglu, K. Y., and Harrus, S. (2015). Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Med. Vet. Entomol. 29, 205–209. doi: 10.1111/mve.12094
Karasartova, D., Gureser, A. S., Gokce, T., Celebi, B., Yapar, D., Keskin, A., et al. (2018). Bacterial and protozoal pathogens found in ticks collected from humans in Corum province of Turkey. PLoS Negl. Trop. Dis. 12:e0006395. doi: 10.1371/journal.pntd.0006395
Karim, S., Budachetri, K., Mukherjee, N., Williams, J., Kausar, A., Hassan, M. J., et al. (2017). A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 11:e0005681. doi: 10.1371/journal.pntd.0005681
Keskin, A., and Bursali, A. (2016). Detection of Rickettsia aeschlimannii and Rickettsia sibirica mongolitimonae in Hyalomma marginatum (Acari: Ixodidae) ticks from Turkey. Acarologia 56, 533–536. doi: 10.1051/acarologia/20164140
Keskin, A., Bursali, A., Keskin, A., and Tekin, S. (2016). Molecular detection of spotted fever group Rickettsiae in ticks removed from humans in Turkey. Ticks Tick-borne Dis. 7, 951–953. doi: 10.1016/j.ttbdis.2016.04.015
Khan, M., Islam, N., Khan, A., Islam, Z. U., Muñoz-Leal, S., Labruna, M. B., et al. (2022). New records of Amblyomma gervaisi from Pakistan, with detection of a reptile-associated Borrelia sp. Ticks Tick-borne Dis. 13:102047. doi: 10.1016/j.ttbdis.2022.102047
Khan, S. M., Khan, M., Alouffi, A., Almutairi, M. M., Numan, M., Ullah, S., et al. (2023). Phylogenetic position of Haemaphysalis kashmirensis and Haemaphysalis cornupunctata, with notes on Rickettsia spp. Genes 14:360. doi: 10.3390/genes14020360
Khan, Z., Shehla, S., Alouffi, A., Kashif Obaid, M., Zeb Khan, A., Almutairi, M. M., et al. (2022). Molecular survey and genetic characterization of Anaplasma marginale in ticks collected from livestock hosts in Pakistan. Animals 12:1708. doi: 10.3390/ani12131708
Khrouf, F., M'Ghirbi, Y., Znazen, A., Ben Jemaa, M., Hammami, A., and Bouattour, A. (2014). Detection of Rickettsia in Rhipicephalus sanguineus ticks and Ctenocephalides felis fleas from southeastern Tunisia by reverse line blot assay. J. Clin. Microbiol. 52, 268–274. doi: 10.1128/JCM.01925-13
Kleinerman, G., Baneth, G., Mumcuoglu, K. Y., van Straten, M., Berlin, D., Apanaskevich, D. A., et al. (2013). Molecular detection of Rickettsia africae, Rickettsia aeschlimannii, and Rickettsia sibirica mongolitimonae in camels and Hyalomma spp. ticks from Israel. Vector Borne Zoonotic Dis 13, 851–856. doi: 10.1089/vbz.2013.1330
Koczwarska, J., Pawełczyk, A., Dunaj-Małyszko, J., Polaczyk, J., and Welc-Falęciak, R. (2023). Rickettsia species in Dermacentor reticulatus ticks feeding on human skin and clinical manifestations of tick-borne infections after tick bite. Sci. Rep. 13:9930. doi: 10.1038/s41598-023-37059-3
Koka, H., Sang, R., Kutima, H. L., and Musila, L. (2017). The detection of spotted fever group Rickettsia DNA in tick samples from pastoral communities in Kenya. J. Med. Entomol. 54, 774–780. doi: 10.1093/jme/tjw238
Kratou, M., Belkahia, H., Selmi, R., Andolsi, R., Dhibi, M., Mhadhbi, M., et al. (2023). Diversity and phylogeny of cattle ixodid ticks and associated spotted fever group Rickettsia spp. in Tunisia. Pathogens 12:552. doi: 10.3390/pathogens12040552
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35:1547. doi: 10.1093/molbev/msy096/4990887
Labruna, M. B. (2009). Ecology of rickettsia in South America. Ann. N. Y. Acad. Sci. 1166, 156–166. doi: 10.1111/j.1749-6632.2009.04516.x
Labruna, M. B., Whitworth, T., Horta, M. C., Bouyer, D. H., McBride, J. W., Pinter, A., et al. (2004). Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 42, 90–98. doi: 10.1128/JCM.42.1.90-98.2004
Leulmi, H., Aouadi, A., Bitam, I., Bessas, A., Benakhla, A., Raoult, D., et al. (2016). Detection of Bartonella tamiae, Coxiella burnetii and Rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasit. Vectors 9, 1–8. doi: 10.1186/s13071-016-1316-9
Loftis, A. D., Reeves, W. K., Szumlas, D. E., Abbassy, M. M., Helmy, I. M., Moriarity, J. R., et al. (2006). Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 40, 67–81. doi: 10.1007/s10493-006-9025-2
Maioli, G., Bonilauri, P., Barbieri, I., Calzolari, M., and Francesco, D.F. And Dottori, M ., Detection of spotted fever group Rickettsia and Anaplasma phagocytophilum in ticks removed from wild animals in northern Italy in 2009 (2009).
Mancini, F., Ciccozzi, M., Lo Presti, A., Cella, E., Giovanetti, M., Di Luca, M., et al. (2015). Characterization of spotted fever group Rickettsiae in ticks from a city park of Rome, Italy. Ann. Ist. Super Sanita 51, 284–290. doi: 10.4415/ANN_15_04_07
Matsumoto, K., Parola, P., Brouqui, P., and Raoult, D. (2004). Rickettsia aeschlimannii in Hyalomma ticks from Corsica. Eur. J. Clin. Microbiol. Infect. Dis. 23, 732–734. doi: 10.13140/RG.2.2.22971.98080
McGinley, L., Hansford, K. M., Cull, B., Gillingham, E. L., Carter, D. P., Chamberlain, J. F., et al. (2021). First report of human exposure to Hyalomma marginatum in England: further evidence of a Hyalomma moulting event in North-Western Europe? Ticks and Tick-borne Diseases 12:101541. doi: 10.1016/j.ttbdis.2020.101541
Mediannikov, O., Diatta, G., Fenollar, F., Sokhna, C., Trape, J. F., and Raoult, D. (2010). Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 4:e821. doi: 10.1371/journal.pntd.0000821
Mokrani, N., Parola, P., Tebbal, S., Dalichaouche, M., Aouati, A., and Raoult, D. (2008). Rickettsia aeschlimannii infection, Algeria. Emerg. Infect. Dis. 14, 1814–1815. doi: 10.3201/eid1411.071221
Morita, C., El Hussein, A. R. M., Matsuda, E., Abdel Gabbar, K. M. A., Muramatsu, Y., Abdel Rahman, M. B., et al. (2004). Spotted fever group Rickettsiae from ticks captured in Sudan. Jpn. J. Infect. Dis. 57, 107–109.
Mumcuoglu, K. Y., Arslan-Akveran, G., Aydogdu, S., Karasartova, D., Koşar, A., Savci, U., et al. (2022). Pathogens in ticks collected in Israel: II. Bacteria and protozoa found in Rhipicephalus sanguineus sensu lato and Rhipicephalus turanicus. Ticks Tick-borne Dis. 13:101986. doi: 10.1016/j.ttbdis.2022.101986
Mura, A., Masala, G., Tola, S., Satta, G., Fois, F., Piras, P., et al. (2008a). First direct detection of rickettsial pathogens and a new rickettsia, ‘Candidatus Rickettsia barbariae’, in ticks from Sardinia, Italy. Clin. Microbiol. Infect. 14, 1028–1033. doi: 10.1111/j.1469-0691.2008.02082.x
Mura, A., Socolovschi, C., Ginesta, J., Lafrance, B., Magnan, S., Rolain, J. M., et al. (2008b). Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans. R. Soc. Trop. Med. Hyg. 102, 945–949. doi: 10.1016/j.trstmh.2008.03.015
Mutai, B. K., Wainaina, J. M., Magiri, C. G., Nganga, J. K., Ithondeka, P. M., Njagi, O. N., et al. (2013). Zoonotic surveillance for Rickettsiae in domestic animals in Kenya. Vector Borne Zoonotic Dis. 13, 360–366. doi: 10.1089/vbz.2012.0977
Nader, J., Król, N., Pfeffer, M., Ohlendorf, V., Marklewitz, M., Drosten, C., et al. (2018). The diversity of tick-borne bacteria and parasites in ticks collected from the Strandja Nature Park in South-Eastern Bulgaria. Parasit. Vectors 11, 165–110. doi: 10.1186/s13071-018-2721-z
Numan, M., Islam, N., Adnan, M., Zaman Safi, S., Chitimia-Dobler, L., Labruna, M. B., et al. (2022). First genetic report of Ixodes kashmiricus and associated Rickettsia sp. Parasit. Vectors 15, 378–312. doi: 10.1186/s13071-022-05509-y
Omondi, D., Masiga, D. K., Fielding, B. C., Kariuki, E., Ajamma, Y. U., Mwamuye, M. M., et al. (2017). Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and Lake Baringo, Kenya. Front. Vet. Sci. 4:73. doi: 10.3389/fvets.2017.00073
Onyiche, T. E., Răileanu, C., Tauchmann, O., Fischer, S., Vasić, A., Schäfer, M., et al. (2020). Prevalence and molecular characterization of ticks and tick-borne pathogens of one-humped camels (Camelus dromedarius) in Nigeria. Parasit. Vectors 13, 428–416. doi: 10.1186/s13071-020-04272-2
Orkun, Ö., and Çakmak, A. (2019, 2019). Molecular identification of tick-borne bacteria in wild animals and their ticks in Central Anatolia, Turkey. Comp. Immunol. Microbiol. Infect. Dis. 63, 58–65. doi: 10.1016/j.cimid.2018.12.007
Orkun, Ö., Karaer, Z., Çakmak, A., and Nalbantoğlu, S. (2014). Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLoS Negl. Trop. Dis. 8:e3067. doi: 10.1371/journal.pntd.0003067
Oteo, J. A., Portillo, A., Blanco, J. R., Ibarra, V., Pérez-Martínez, L., Izco, C., et al. (2005). Low risk of developing human Rickettsia aeschlimannii infection in the north of Spain. Ann. N. Y. Acad. Sci. 1063, 349–351. doi: 10.1196/annals.1355.057
Otranto, D., Dantas-Torres, F., Giannelli, A., Latrofa, M. S., Cascio, A., Cazzin, S., et al. (2014). Ticks infesting humans in Italy and associated pathogens. Parasit. Vectors 7, 1–9. doi: 10.1186/1756-3305-7-3
Paguem, A., Manchang, K., Kamtsap, P., Renz, A., Schaper, S., Dobler, G., et al. (2023). Ticks and rickettsiae associated with wild animals sold in bush meat markets in Cameroon. Pathogens 12:348. doi: 10.3390/pathogens12020348
Palomar, A. M., Molina, I., Bocanegra, C., Portillo, A., Salvador, F., Moreno, M., et al. (2022). Old zoonotic agents and novel variants of tick-borne microorganisms from Benguela (Angola), July 2017. Parasit. Vectors 15:140. doi: 10.1186/s13071-022-05238-2
Palomar, A. M., Portillo, A., Mazuelas, D., Roncero, L., Arizaga, J., Crespo, A., et al. (2016). Molecular analysis of Crimean-Congo hemorrhagic fever virus and Rickettsia in Hyalomma marginatum ticks removed from patients (Spain) and birds (Spain and Morocco), 2009–2015. Ticks Tick-borne Dis. 7, 983–987. doi: 10.1016/j.ttbdis.2016.05.004
Parola, P., Inokuma, H., Camicas, J. L., Brouqui, P., and Raoult, D. (2001). Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7, 1014–1017. doi: 10.3201/eid0706.010616
Parola, P., Paddock, C. D., Socolovschi, C., Labruna, M. B., Mediannikov, O., Kernif, T., et al. (2013). Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 26, 657–702. doi: 10.1128/CMR.00032-13
Pascucci, I., Di Domenico, M., Curini, V., Cocco, A., Averaimo, D., D’Alterio, N., et al. (2019a). Diversity of Rickettsia in ticks collected in Abruzzi and Molise regions (Central Italy). Microorganisms 7:696. doi: 10.3390/microorganisms7120696
Pascucci, I., Di Domenico, M., Dondona, G. C., Di Gennaro, A., Polci, A., Dondona, A. C., et al. (2019b). Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: an Italian experience. Ticks Tick-borne Dis. 10:101272. doi: 10.1016/j.ttbdis.2019.101272
Portillo, A., Santibáñez, P., Santibáñez, S., Pérez-Martínez, L., and Oteo, J. A. (2008). Detection of Rickettsia spp. in Haemaphysalis ticks collected in La Rioja, Spain. Vector-Borne Zoonotic Dis. 8, 653–658. doi: 10.1089/vbz.2007.0272
Pretorius, A. M., and Birtles, R. J. (2002). Rickettsia aeschlimannii: a new pathogenic spotted fever group Rickettsia, South Africa. Emerg. Infect. Dis. 8, 874a–8874a. doi: 10.3201/eid0808.020199
Psaroulaki, A., Ragiadakou, D., Kouris, G., Papadopoulos, B., Chaniotis, B., and Tselentis, Y. (2006). Ticks, tick-borne Rickettsiae, and Coxiella burnetii in the Greek island of Cephalonia. Ann. N. Y. Acad. Sci. 1078, 389–399. doi: 10.1196/annals.1374.077
Punda-Polic, V., Petrovec, M., Trilar, T., Duh, D., Bradaric, N., Klismanic, Z., et al. (2003). “Detection and identification of spotted fever group Rickettsiae in ticks collected in southern Croatia” in Ticks and tick-borne pathogens: Proceedings of the 4th international conference on ticks and tick-borne pathogens the Banff Centre Banff, Alberta, Canada 21–26 July 2002 (Dordrecht: Springer Netherlands), 169–176.
Qiu, Y., Simuunza, M., Kajihara, M., Ndebe, J., Saasa, N., Kapila, P., et al. (2022). Detection of tick-borne bacterial and protozoan pathogens in ticks from the Zambia-Angola border. Pathogens 11:566. doi: 10.3390/pathogens11050566
Raoult, D., Fournier, P. E., Abboud, P., and Caron, F. (2002). First documented human Rickettsia aeschlimannii infection. Emerg. Infect. Dis. 8, 748–749. doi: 10.3201/eid0807.010480
Regnery, R. L., Spruill, C. L., and Plikaytis, B. (1991). Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173, 1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991
Rollins, R. E., Schaper, S., Kahlhofer, C., Frangoulidis, D., Strauß, A. F., Cardinale, M., et al. (2021). Ticks (Acari: Ixodidae) on birds migrating to the island of Ponza, Italy, and the tick-borne pathogens they carry. Ticks Tick-borne Dis. 12:101590. doi: 10.1016/j.ttbdis.2020.101590
Roux, V., and Raoult, D. (2000). Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50, 1449–1455. doi: 10.1099/00207713-50-4-1449
Roux, V., Fournier, P. E., and Raoult, D. (1997). Differentiation of spotted fever group Rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34, 2058–2065. doi: 10.1128/JCM.34.9.2058-2065.1996
Rumer, L., Graser, E., Hillebrand, T., Talaska, T., Dautel, H., Mediannikov, O., et al. (2011). Rickettsia aeschlimannii in Hyalomma marginatum ticks, Germany. Emerging infectious diseases 17, 325–326. doi: 10.3201/eid1702.100308
Sadeddine, R., Diarra, A. Z., Laroche, M., Mediannikov, O., Righi, S., Benakhla, A., et al. (2020). Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from North-Eastern Algeria. Ticks Tick-borne Dis. 11:101330. doi: 10.1016/j.ttbdis.2019.101330
Saman, E. A. G., and Chegeni, A. H. (2022). Hard ticks (Acari: Ixodidae) infected by Rickettsias: the first record of Rickettsia aeschlimannii (Rickettsiales: Rickettsiaceae) in Iran. Syst. Appl. Acarol. 27, 749–762. doi: 10.11158/saa.27.4.7
Sambou, M., Faye, N., Bassène, H., Diatta, G., Raoult, D., and Mediannikov, O. (2014). Identification of Rickettsial pathogens in ixodid ticks in northern Senegal. Ticks Tick-borne Dis. 5, 552–556. doi: 10.1016/j.ttbdis.2014.04.002
Sambrook, J., Fritsch, E.F., and Maniatis, T. Molecular cloning: a laboratory manual, 2nd; Cold Spring Harbor Laboratory Press: New York, NY, USA, (1989)
Santos-Silva, M. M., Sousa, R., Santos, A. S., Melo, P., Encarnação, V., and Bacellar, F. (2006). Ticks parasitizing wild birds in Portugal: detection of Rickettsia aeschlimannii, R. helvetica and R. massiliae. Exp. Appl. Acarol. 39, 331–338. doi: 10.1007/s10493-006-9008-3
Sarih, M., Socolovschi, C., Boudebouch, N., Hassar, M., Raoult, D., and Parola, P. (2008). Spotted fever group Rickettsiae in ticks, Morocco. Emerg. Infect. Dis. 14, 1067–1073. doi: 10.3201/eid1407.070096
Satjanadumrong, J., Robinson, M. T., Hughes, T., and Blacksell, S. D. (2019). Distribution and ecological drivers of spotted fever group Rickettsia in Asia. EcoHealth 16, 611–626. doi: 10.1007/s10393-019-01409-3
Scarpulla, M., Barlozzari, G., Marcario, A., Salvato, L., Blanda, V., De Liberato, C., et al. (2016). Molecular detection and characterization of spotted fever group Rickettsiae in ticks from Central Italy. Ticks Tick-borne Dis. 7, 1052–1056. doi: 10.1016/j.ttbdis.2016.06.003
Selmi, R., Ben Said, M., Ben Yahia, H., Abdelaali, H., and Messadi, L. (2020). Molecular epidemiology and phylogeny of spotted fever group Rickettsia in camels (Camelus dromedarius) and their infesting ticks from Tunisia. Transbound. Emerg. Dis. 67, 733–744. doi: 10.1111/tbed.13392
Sgroi, G., Iatta, R., Lia, R. P., Napoli, E., Buono, F., Bezerra-Santos, M. A., et al. (2022). Tick exposure and risk of tick-borne pathogens infection in hunters and hunting dogs: a citizen science approach. Transbound. Emerg. Dis. 69, e386–e393. doi: 10.1111/tbed.14314
Shehla, S., Ullah, F., Alouffi, A., Almutairi, M. M., Khan, Z., Tanaka, T., et al. (2023). Association of SFG Rickettsia massiliae and Candidatus Rickettsia shennongii with different hard ticks infesting livestock hosts. Pathogens 12:1080. doi: 10.3390/pathogens12091080
Shpynov, S., Fournier, P. E., Rudakov, N., Tankibaev, M., Tarasevich, I., and Raoult, D. (2004). Detection of a rickettsia closely related to Rickettsia aeschlimannii, “Rickettsia heilongjiangensis,” Rickettsia sp. strain RpA4, and Ehrlichia muris in ticks collected in Russia and Kazakhstan. J. Clin. Microbiol. 42, 2221–2223. doi: 10.1128/JCM.42.5.2221-2223.2004
Shpynov, S., Rudakov, N., Tohkov, Y., Matushchenkso, A., Tarasevich, I., Raoult, D., et al. (2009). Detection of Rickettsia aeschlimannii in Hyalomma marginatum ticks in western Russia. Clin. Microbiol. Infect. 15, 315–316. doi: 10.1111/j.1469-0691.2008.02256.x
Shuaib, Y. A., Elhag, A. M. A. W., Brima, Y. A., Abdalla, M. A., Bakiet, A. O., Mohmed-Noor, S. E. T., et al. (2020). Ixodid tick species and two tick-borne pathogens in three areas in the Sudan. Parasitol. Res. 119, 385–394. doi: 10.1007/s00436-019-06458-9
Socolovschi, C., Mediannikov, O., Raoult, D., and Parola, P. (2009). The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet. Res. 40:34. doi: 10.1051/vetres/2009017
Socolovschi, C., Reynaud, P., Raoult, D., and Parola, P. (2011). “Rickettsiae and Borrelia in ticks on migratory birds from the Camargue National Park, France” in Abstract book of the 6th international meeting on Rickettsiae and Rickettsial diseases (Heraklion, Crete, Greece)
Song, S., Chen, C., Yang, M., Zhao, S., Wang, B., Hornok, S., et al. (2018). Diversity of Rickettsia species in border regions of northwestern China. Parasit. Vectors 11, 634–637. doi: 10.1186/s13071-018-3233-6
Springer, A., Shuaib, Y. A., Isaa, M. H., Ezz-Eldin, M. I. E., Osman, A. Y., Yagoub, I. A., et al. (2020). Tick fauna and associated Rickettsia, Theileria, and Babesia spp. in domestic animals in Sudan (North Kordofan and Kassala States). Microorganisms 8:1969. doi: 10.3390/microorganisms8121969
Sukhiashvili, R., Zhgenti, E., Khmaladze, E., Burjanadze, I., Imnadze, P., Jiang, J., et al. (2020). Identification and distribution of nine tick-borne spotted fever group Rickettsiae in the country of Georgia. Ticks Tick-borne Dis. 11:101470. doi: 10.1016/j.ttbdis.2020.101470
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Tila, H., Khan, M., Almutairi, M. M., Alouffi, A., Ahmed, H., Tanaka, T., et al. (2023). First report on detection of Hepatozoon ayorgbor in Rhipicephalus haemaphysaloides and Hepatozoon colubri in Haemaphysalis sulcata and Hyalomma anatolicum: risks of spillover of Hepatozoon spp. from wildlife to domestic animals. Front. Vet. Sci. 10:1255482. doi: 10.3389/fvets.2023.1255482
Toma, L., Mancini, F., Di Luca, M., Cecere, J. G., Bianchi, R., Khoury, C., et al. (2014). Detection of microbial agents in ticks collected from migratory birds in Central Italy. Vector-borne Zoonotic Dis. 14, 199–205. doi: 10.1089/vbz.2013.1458
Tomassone, L., Conte, V., Parrilla, G., and De Meneghi, D. (2010). Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum ticks, Cochabamba department, Bolivia. Vector-Borne and Zoonotic Diseases 10, 953–958. doi: 10.1089/vbz.2009.0126
Tomassone, L., De Meneghi, D., Adakal, H., Rodighiero, P., Pressi, G., and Grego, E. (2016). Detection of Rickettsia aeschlimannii and Rickettsia africae in ixodid ticks from Burkina Faso and Somali Region of Ethiopia by new real-time PCR assays. Ticks Tick-borne Dis. 7, 1082–1088. doi: 10.1016/j.ttbdis.2016.09.005
Tomassone, L., Grego, E., Auricchio, D., Iori, A., Giannini, F., and Rambozzi, L. (2013). Lyme borreliosis spirochetes and spotted fever group Rickettsiae in ixodid ticks from Pianosa island, Tuscany archipelago, Italy. Vector Borne Zoonotic Dis. 13, 84–91. doi: 10.1089/vbz.2012.1046
Tosoni, A., Mirijello, A., Ciervo, A., Mancini, F., Rezza, G., Damiano, F., et al. (2016). Human Rickettsia aeschlimannii infection: first case with acute hepatitis and review. Eur. Rev. Med. Pharmacol. Sci. 20, 2630–2633.
Ullah, S., Alouffi, A., Almutairi, M. M., Islam, N., Rehman, G., Ul Islam, Z., et al. (2023). First report of Rickettsia conorii in Hyalomma kumari ticks. Animals 13:1488. doi: 10.3390/ani13091488
Vanegas, A., Keller, C., Krüger, A., Manchang, T. K., Hagen, R. M., Frickmann, H., et al. (2018). Molecular detection of spotted fever group Rickettsiae in ticks from Cameroon. Ticks Tick Borne Dis. 9, 1049–1056. doi: 10.1016/j.ttbdis.2018.03.022
Walker, J.B., Keirans, J.E., and Horak, I.G. (2000) The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge, NY Cambridge University Press
Koponen, S., and Niemi, R. (2000). Review: Brown ticks of the world. Entomol. Fennica 11:124. doi: 10.33338/ef.84056
Wallmenius, K., Barboutis, C., Fransson, T., Jaenson, T. G., Lindgren, P. E., Nyström, F., et al. (2014). Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vectors 7, 1–20. doi: 10.1186/1756-3305-7-318
Wei, Q. Q., Guo, L. P., Wang, A. D., Mu, L. M., Zhang, K., Chen, C. F., et al. (2015). The first detection of Rickettsia aeschlimannii and Rickettsia massiliae in Rhipicephalus turanicus ticks, in Northwest China. Parasit. Vectors 8, 631–634. doi: 10.1186/s13071-015-1242-2
Yessinou, R. E., Cazan, C. D., Panait, L. C., Mollong, E., Biguezoton, A. S., Bonnet, S. I., et al. (2023). New geographical records for tick-borne pathogens in ticks collected from cattle in Benin and Togo. Vet. Med. Sci. 9, 345–352. doi: 10.1002/vms3.1022
Keywords: ticks, Rickettsia aeschlimannii, Hyalomma turanicum, Haemaphysalis bispinosa, Haemaphysalis montgomeryi
Citation: Majid A, Almutairi MM, Alouffi A, Tanaka T, Yen T-Y, Tsai K-H and Ali A (2023) First report of spotted fever group Rickettsia aeschlimannii in Hyalomma turanicum, Haemaphysalis bispinosa, and Haemaphysalis montgomeryi infesting domestic animals: updates on the epidemiology of tick-borne Rickettsia aeschlimannii. Front. Microbiol. 14:1283814. doi: 10.3389/fmicb.2023.1283814
Received: 27 August 2023; Accepted: 13 November 2023;
Published: 15 December 2023.
Edited by:
Hong Yin, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Valentina Virginia Ebani, University of Pisa, ItalyCopyright © 2023 Majid, Almutairi, Alouffi, Tanaka, Yen, Tsai and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abid Ali, dW9wX2FsaUB5YWhvby5jb20=; Kun-Hsien Tsai, a3VuaHRzYWlAbnR1LmVkdS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.