
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 30 October 2023
Sec. Evolutionary and Genomic Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1280420
This article is part of the Research Topic Genetics, Genomics, and Breeding of Edible Mushrooms in Asia View all 10 articles
Color variations in cultivated edible mushrooms present novel and potentially valuable alternatives to the research and cultivation industries. We collected, identified, and domesticated a white strain of Auricularia cornea and a white strain of Auricularia heimuer from China. However, due to an unstable phenotype and stricter requirements on environment and management technology, the production and utilization of Auricularia heimuer cv. Bai Muer make slow progress. Outcrossing is an essential means to broaden the intraspecific genetic resources to expand the gene pool and compensate for the limitations of related species hybridization. In this study, interspecies hybridization between Auricularia cornea cv. Yu Muer and Auricularia heimuer cv. Bai Muer was conducted using polyethylene glycol (PEG)-induced double-inactivated protoplast fusion. Apart from the functional complementation of double-inactivated protoplasts, the hybrids were characterized by colony morphology, antagonistic test, primordial morphology, and polymerase chain reaction (PCR) fingerprinting. The results suggested that the hybrids and their parents showed significant differences in their colony morphology. Moreover, positive barrage reactions were observed between each parent and hybrid. Inter-simple sequence repeat (ISSR) and start codon targeted (SCoT) profile analysis of fusants and parents depicted that fusants contained polymorphic bands, which indicated the rearrangement and deletion of deoxyribonucleic acid (DNA) in the fusants. Yellowish-white primordia were obtained from two hybrids. Protoplast fusion may reinforce the genetic potential and provide an ideal alternative for breeding albino Auricularia.
Auricularia Bull. (family Auriculariaceae, order Auriculariales) is an important wood-decaying fungal genus widely distributed worldwide (Wu et al., 2014). Moreover, it is one of the earliest cultivated mushrooms in the world and was first recorded in Tang Materia Medica, written by Gong Su (Yuan et al., 2019). It has traditionally been consumed as food and medicine for over 1,000 years in China (Miao et al., 2020). As the primary producer of cultivated Auricularia in the world, China’s output reached approximately 9.24 million tons in 2021, representing over 90% of the global production (China Edible Fungi Association, 2022). Auricularia cornea Ehrenb. and Auricularia heimuer F. Wu, B.K. Cui, and Y.C. Dai are the main species commercially cultivated in China.
Edible mushrooms with color variation have high research value and commercial value, such as Agaricus bisporus (J.E. Lange) Imbach, Flammulina filiformis (Z.W. Ge, X.B. Liu & Zhu L. Yang) P.M. Wang, Y.C. Dai, E. Horak & Zhu L. Yang, Hypsizygus marmoreus (Peck) H.E. Bigelow (Lee et al., 2008; Liu et al., 2013, 2016). Auricularia cornea cv. Yu Muer is an albino mutant strain of A. cornea with numerous biological activities, such as antidiabetic, antinephritic, antioxidant, anticoagulant, and hepatoprotective effects (Wang et al., 2019; Li et al., 2021). The pigment in the fruiting body of A. cornea was γ-glutaminyl-3,4-dihydroxy-benzoate. In the process of synthesizing pigment, the key enzymes were polyphenol oxidase and 20 other enzyme genes (Ma et al., 2023). In addition to its white color, the popularity of this mushroom is due to its high nutritional content and short production cycle (45–55 days). The mushroom is grown on an industrial scale in many regions of China because it has a high-yielding capacity and low output cost, is adaptable to a different environment, and is resistant to many pathogens (Chen et al., 2021).
In 2019, the white variety of A. heimuer was successfully domesticated and cultivated at Jilin Agricultural University (Li et al., 2019). According to the latest statistics, A. heimuer (ranks second in production) is more popular than A. cornea (ranks seventh in production) in China due to its flavor, slippery texture, and unique taste. However, A. heimuer cv. Bai Muer has a longer production cycle and higher output cost than A. cornea cv. Yu Muer, so it needs stricter requirements on environment and management technology. In addition, the color of the fruiting body is easily affected by light during the cultivation period. Therefore, selecting new strains of Auricularia with good characteristics is of great importance.
There are many ways to breed new strains, for example, artificial selection breeding, cross-breeding, protoplast fusion breeding, mutation breeding, and genetic engineering breeding. A lot of traditional mushroom breeding methods have been carried out intraspecifically. However, due to a lack of basic knowledge of the genetics and breeding system of this crop, advances in research on mushroom breeding and production are very limited compared with other crops. Moreover, the fruiting body of a mushroom is a complex organism with a series of complex characteristics. Many of these characteristics, especially those related to yield, are controlled by multiple genes (Chakravarty, 2011).
Gene transfer using protoplast fusion is a non-conventional method that is used to break down the natural barrier to gene exchange encountered in conventional breeding systems. Protoplast fusion technology can be performed intraspecifically, interspecifically, intergenerically, and even inter-hetero-generically (Dhitaphichit and Pornsuriya, 2005). The course of biological processes can be significantly influenced by protoplast fusion between different species. Through this process, gene control can be deregulated either positively or negatively, and metabolic pathways may be combined to create new metabolites. This can result in high yields, fast spawn runs, tolerance to adverse conditions, utilization of various agricultural waste, unique taste, attractive color, enhanced nutritive value, and medicinal properties in mushrooms (Selvakumar et al., 2015; Raman et al., 2021). Hybrids constructed by protoplast fusion in several mushrooms have been reported (Mallick and Sikdar, 2014). Interfamily hybrid strains with high biological efficiency and cold-tolerant ability have been obtained through protoplast fusion (He et al., 2018). A successful interspecific protoplast fusion has been carried out between the two edible mushroom strains Lentinula edodes (Berk.) Pegler and Coriolus versicolor (L.) Quél. (Kim et al., 1997). Somatic hybrids between Calocybe indica Purkay & A. Chandra and Pleurotus fiorida Singer showed a significant increase in bio-eficiency and γ-linoleic acid content (Chakraborty and Sikdar, 2010). The structural investigation of polysaccharides obtained from somatic hybrid mushrooms through protoplast fusion showed that they are different from the polysaccharides isolated from the fruit bodies of parental strains and exhibited strong immune activation of macrophages, splenocytes, and thymocytes (Patra et al., 2011; Maity et al., 2013; Maji et al., 2013; Sen et al., 2013). Therefore, distant hybridization can introduce important quantitative and qualitative traits, such as high bio-efficiency, good palatability, and a shorter cropping period, from either of the parents into their progeny. Interspecies hybridization between white Auricularia through protoplast fusion can enhance genetic potential and offer an excellent alternative for breeding edible mushrooms.
Thus, considering the beneficial characteristics of the two parents, the present study carried out the protoplast fusion between A. heimuer cv. Bai Muer and A. cornea cv. Yu Muer to obtain new intergeneric strains of albino Auricularia with improved characteristics. In our study, we successfully developed 10 hybrids, which were successfully characterized by microstructure, mycelial morphology, inter-simple sequence repeat (ISSR), and start codon targeted (SCoT) analysis.
The A. cornea cv. Yu Muer strain (MC6), the A. heimuer cv. Bai Muer strain (JAUH-W-591), monokaryotic strains of A. cornea cv. Yu Muer (D-MC6), and A. heimuer cv. Bai Muer (D-JAUH-W-591) were preserved at Jilin Agricultural University (Changchun, China). Vegetative cultures of both strains were maintained on potato dextrose agar (PDA) medium, containing 20 g/L of glucose, 2 g/L of KH2PO4, 2 g/L of MgSO4·7H2O, 1.5 g/L of agar, and 1 L of potato juice (He et al., 2018). Before protoplast isolation, the strains were grown in liquid malt yeast extract glucose (MYG) medium (10 g/L of malt, 4 g/L of yeast extract, and 10 g/L of glucose, pH = 6.2) under stationary conditions for 10 days at 30°C (Chakraborty and Sikdar, 2008; Xu et al., 2012). The same MYG medium supplemented with 0.6 M MgSO4 and 2% agar was used as a regeneration medium.
Monokaryotic mycelia derived from a single spore isolate of each species were incubated for 10 days at 28°C in 100 mL of liquid MYG medium for static culture. Cultures were harvested by the filter (0.22 μm), washed twice with distilled water, and dried with sterile paper. Then, 200–300 mg of mycelium was added to a 1 mL aliquot of lywallzyme solution (2%, purchased from the Guangdong Institute of Microbiology), which contained 0.6 M osmotic stabilizer and was incubated at 30°C for 7 h. The suspension was filtered and centrifuged at 3000 × g for 5 min. The obtained protoplasts were collected and washed twice with a 0.6 M osmotic stabilizer. The total yield was calculated using a hemocytometer (Wang et al., 2017). Finally, purified protoplast pellets were suspended in 200 μL of osmotic stabilizer solution for further use.
The protoplast suspensions of A. heimuer cv. Bai Muer and A. cornea cv. Yu Muer were inactivated by heat and ultraviolet (UV) radiation, respectively. For heat inactivation, the protoplasts were treated at 55, 60, and 65°C for 10, 20, and 30 min, respectively; for UV inactivation, protoplasts were placed 30 cm away under a 15 W UV lamp for 1, 3, 5, 8, and 10 min. After serial dilution, the inactivated protoplasts were plated on the regeneration MYG medium to check the inactivation effect (He et al., 2018). The medium was cultured at 28°C, and the number of regenerated colonies was recorded after 15 days. Protoplasts without inactivation were set as the control group. The inactivated protoplasts were then used for fusion (Zhao et al., 2011).
An equivalent amount of inactive protoplasts of A. heimuer cv. Bai Muer and A. cornea cv. Yu Muer was mixed in a test tube and centrifuged at 1,000 × g for 5 min. The supernatant was rinsed off, and 1 mL of sterilized polyethylene glycol (PEG 4000; 30 g PEG in 100 mL 0.05 M CaCl2·2H2O) was added to the protoplasts in the test tube and incubated at room temperature for 30 min (Moturi and Charya, 2009). During this period, protoplast fusion was followed by observation under the optical microscope (Nikon, Japan). The fused protoplasts were centrifuged at 1,000 × g for 5 min. The supernatant was rinsed off, and protoplasts were washed twice with the osmotic stabilizer and added 1 mL of osmotic stabilizer again. They were then serially diluted, and approximately 0.1 mL from the protoplast suspension was coated in MYG with 0.6 M MgSO4 at 25°C until colonies developed (Zhao and Chang, 1996). Protoplasts from the same parent strains were also fused as controls. Only the progeny that continued growing on the regeneration medium were considered fusion hybrids. The nuclear phase of the putative hybrid stained with 4′,6-diamidino-2-phenylindole (DAPI) dye was observed by fluorescence microscopy. These procedures excluded the possibility of a dual culture.
Hybrid mycelia, on slabs of PDA, were inoculated at a distance of 2 cm, with three in each Petri plate (i.e., the two parent cultures and a single hybrid). The plates were incubated at 25°C for 14 days, after which the point of contact zone was observed.
All hybrids were subjected to a fruiting test in the laboratory. The spawn substrate, which consisted of (w/w) 40% flake hardwood sawdust (4 mm × 6 mm), 37.5% powdered hardwood sawdust, 11% bran, 10% corncob, 1% gypsum, 0.5% lime, pH = 7, and 58–60% water, was autoclaved at 121°C for 120 min. After spawning, when the mycelia showed complete colonization in the substrate, several “V” pores were made all over the surface of the polypropylene packet (approximately 2 cm apart). The temperature was then maintained at 22–28°C, and the relative humidity was adjusted to 85–90%. After pin head emergence through the pores on the polypropylene packets, high humidity was maintained by misting the room. Ventilation and light were required for healthy fruiting body development. If a strain did not form any primordia in all triplicate bags after 25 days, it was considered sterile. The morphology of the fruiting bodies of the hybrids was compared with that of the parents.
Genomic deoxyribonucleic acid (DNA) was isolated from actively growing mycelia using a DNA Extraction Kit (Beijing CoWin Biotech Co., Ltd.). The ISSR primers used in the test are shown in Table 1. The ISSR amplification condition was as follows: 5 min initial denaturation at 94°C; 60 s initial denaturation at 94°C; 35 cycles consisting of 45 s denaturation at 52–58°C; 1 min extension at 72°C; and a final extension for 10 min at 72°C. Reaction termination was conducted at 4°C. ISSR-PCR reaction system (20 μL) was as follows: 10 μL of PCR Master Mix (2 X), 7.5 μL of dd H2O, 1 μL of ISSR primer, and 1.5 μL of DNA. The final PCR products were separated by electrophoresis on a 1.0% (w/v) agarose gel (Chiu et al., 1995). According to Zhao et al. (2013), SCoT amplifications were performed with modifications. The SCoT primers used in the test are shown in Table 2.
Sufficient protoplasts could be obtained by using mycelium aged 10 days under the conditions of an enzymatic hydrolysis temperature of 30°C, an enzymatic hydrolysis solution concentration of 2.0%, and an enzymatic hydrolysis time of 7 h. The protoplast yield was 7.84 × 107 CFU/mL in A. heimuer cv. Bai Muer and 7.36 × 107 CFU/mL in A. cornea cv. Yu Muer. The regeneration percentage of A. heimuer cv. Bai Muer protoplast was found to be 6.6%, while the rate of A. cornea cv. Yu Muer was 6.4%. The process of protoplasts being released from young hyphae was observed under a microscope (Figure 1A). Protoplast regeneration was observed in MYG medium containing 0.6 M MgSO4 after 4 days.
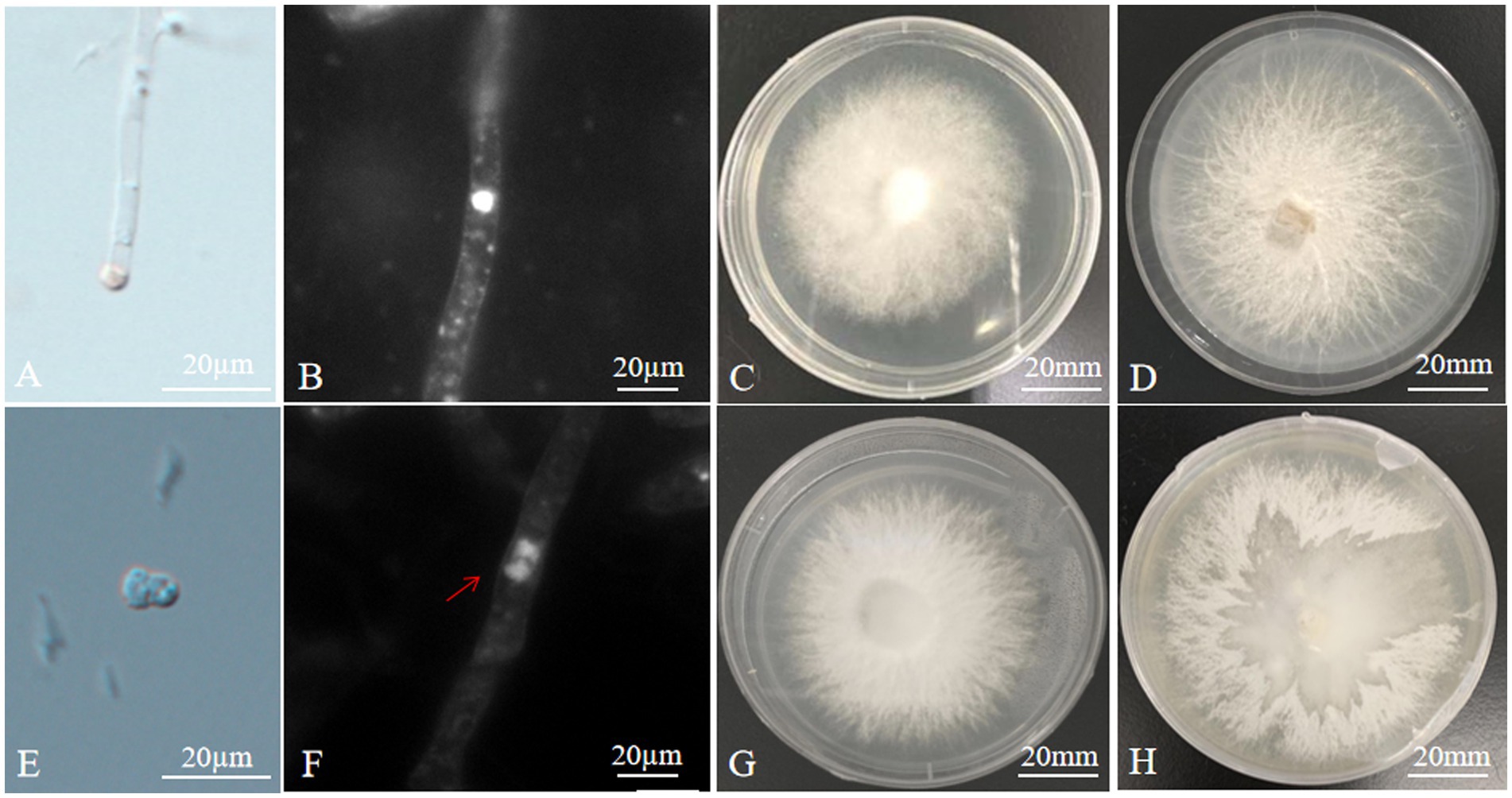
Figure 1. Microstructure and mycelial morphology of parents and hybrids (A) isolation of protoplast; (B) monokaryotic mycelium of A. heimuer cv. Bai Muer; (C) colony morphology of A. heimuer cv. Bai Muer; (D) colony morphology of A. cornea cv. Yu Muer; (E) protoplast fusion; (F) dikaryotic mycelium of hybrid; (G) colony morphology of hybrid strains R2; and (H) separation of one parent from unstable heterokaryons.
Inactivation of A. heimuer CV. Bai Muer protoplasts at 60°C for 20 min yielded good results, as no protoplasts regenerated in the MYG medium with 0.6 M MgSO4 after this treatment. After 3 min of UV inactivation, the inactivation rate of the A. cornea CV. Yu Muer protoplast was 100%, and the regeneration rate was 0.
The contact and fusion of protoplasts induced by PEG were observed under the microscope (Figure 1E). A total of 26 hybrid colonies were regenerated from five fusion experiments. No regenerated colonies were found in the control group. Sectors appearing in the protoplast fusion of distant hybrids are frequently observed when cultured in PDA, as shown in Figure 1H. This phenomenon is generally caused by the discordant division of heterokaryons from different sources, which separates one parent from unstable heterokaryons. Sixteen hybrids exhibited this particular phenomenon in PDA culture. The remaining 10 fusions are confirmed as hybrid strains, renamed R1 ~ R10. There were no single protocols developed from any of the parental protoplasts in this regeneration medium because protoplasts were inactivated. Only hybrid protoplasts could regenerate in a regeneration medium due to the complementation of the parental genome. This confirmed that 10 hybrids had dikaryotic hyphae, while the parent strain had monokaryotic hyphae, as observed under the fluorescence microscope (Figures 1B,F).
The colony morphology of hybrids was different from that of their parents. The colony morphology of A. heimuer cv. Bai Muer showed a whitish colony with linear and centrally radiating mycelia; A. cornea cv. Yu Muer produced a whitish colony with fluffy mycelia. The colony morphology of hybrids had the characteristics of both parents (Figures 1C,D,G).
The antagonistic reaction was a specific example of somatic incompatibility. The antagonist tests were conducted to confirm that the hybrids and parental strains had significant genetic differences. The antagonist test results showed that 10 hybrids and parental strains have a strong degree of antagonism resistance (Figure 2). This indicated that 10 strains generated through protoplast fusion are genetically different from the parental trains.
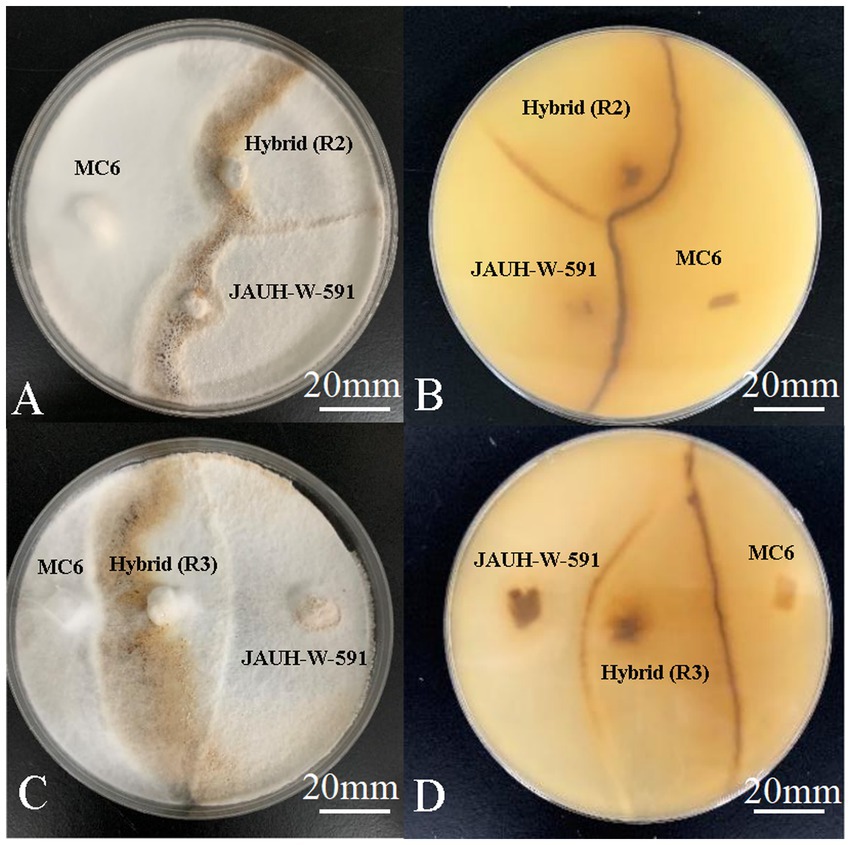
Figure 2. Antagonism of hybrids and parents. (A-B) The upper and reverse colony of JAUH-W-591, MC6, and R2; (C-D) the upper and reverse colony of JAUH-W-591, MC6, and R3.
In the cultivation study, hybrid mycelia grew thickly in a cultivation bag with sawdust as the main material. After 8 days of “V” pores being made, a yellowish-white primordium was observed in hybrid R2. Hybrid R4 required 11 days for primordial initiation, where parent A. heimuer cv. Bai Muer required 9 days and A. cornea cv. Yu Muer required 7 days. The results of lab-scale experiments indicated that all primordia of hybrids fail to differentiate even after 50 days. The fruit bodies of these parents and hybrids are shown in Figure 3.
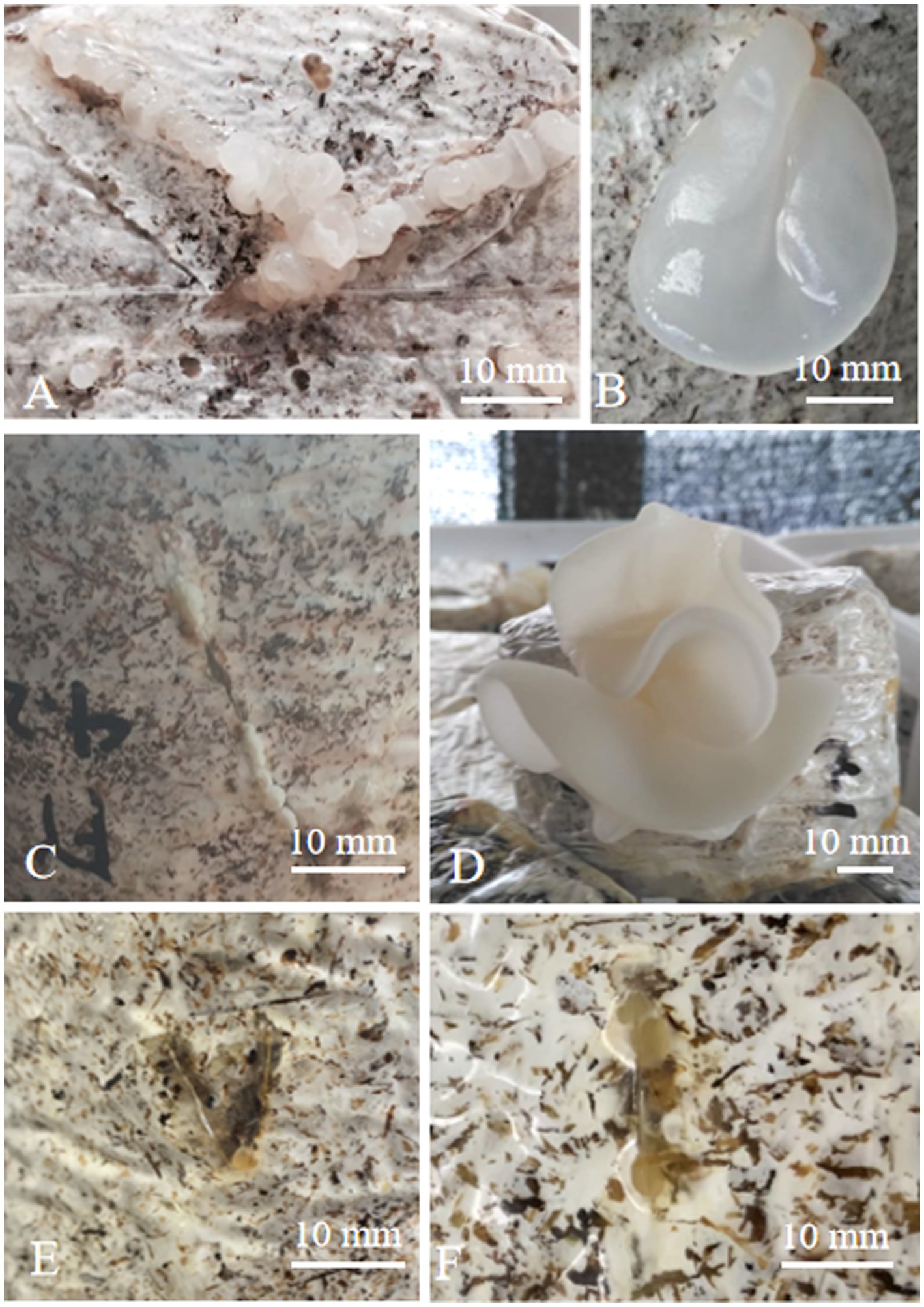
Figure 3. Morphology of fruiting bodies of hybrids (A) primordium of A. heimuer cv. Bai Muer; (B) fruiting body of A. heimuer cv. Bai Muer; (C) primordium of A. cornea cv. Yu Muer; (D) fruiting body of A. cornea cv. Yu Muer; and (E,F) primordium of hybrids.
Genetic variations among parental strains and 10 Auricularia hybrids were determined by using ISSR and SCoT markers. The PCR band profiles of the hybrids were compared with those of the parental strains. If ISSR and SCoT bands are present (or absent) in the possible fusion but not in the parents, they are considered distinct fragments. For example, the ISSR-3 primer amplified and generated new bands of approximately 600 bp in the R2. The ISSR-4 primer amplified a band of approximately 400 bp in A. heimuer cv. Bai Muer, but this band was not present in R2. Similarly, ISSR-6 could generate polymorphic bands in the R2, R3, R5, R6, and R9 strains. However, the band of approximately 250 bp appeared in both parent strains but was not present in the R2 and R6 strains. R3, R5, and R9 strains were similar to A. cornea cv. Yu Muer, but bands of approximately 700 bp and 850 bp appeared in A. cornea cv. Yu Muer were not present in the R3, R5, and R9 strains. SCoT-28 could generate polymorphic bands in all hybrids. This indicated that ISSR and SCoT were efficient in analyzing the genetic diversity of Auricularia. Meanwhile, SCoT markers have high polymorphism, a large amount of information, and a wide evaluation range, which are more suitable for genetic diversity research (Zhao et al., 2013). However, it has not been reported yet whether SCoT molecular markers have been applied to study the genetic diversity of Auricularia. In this study, we applied SCoT molecular markers to the genetic diversity of Auricularia, aiming to provide a reference for the construction of Auricularia molecular fingerprints and the evaluation of strains of Auricularia. The moderated genetic transformation was observed, as shown in Figure 4, and the hybrids obtained were confirmed to be heterokaryotic.
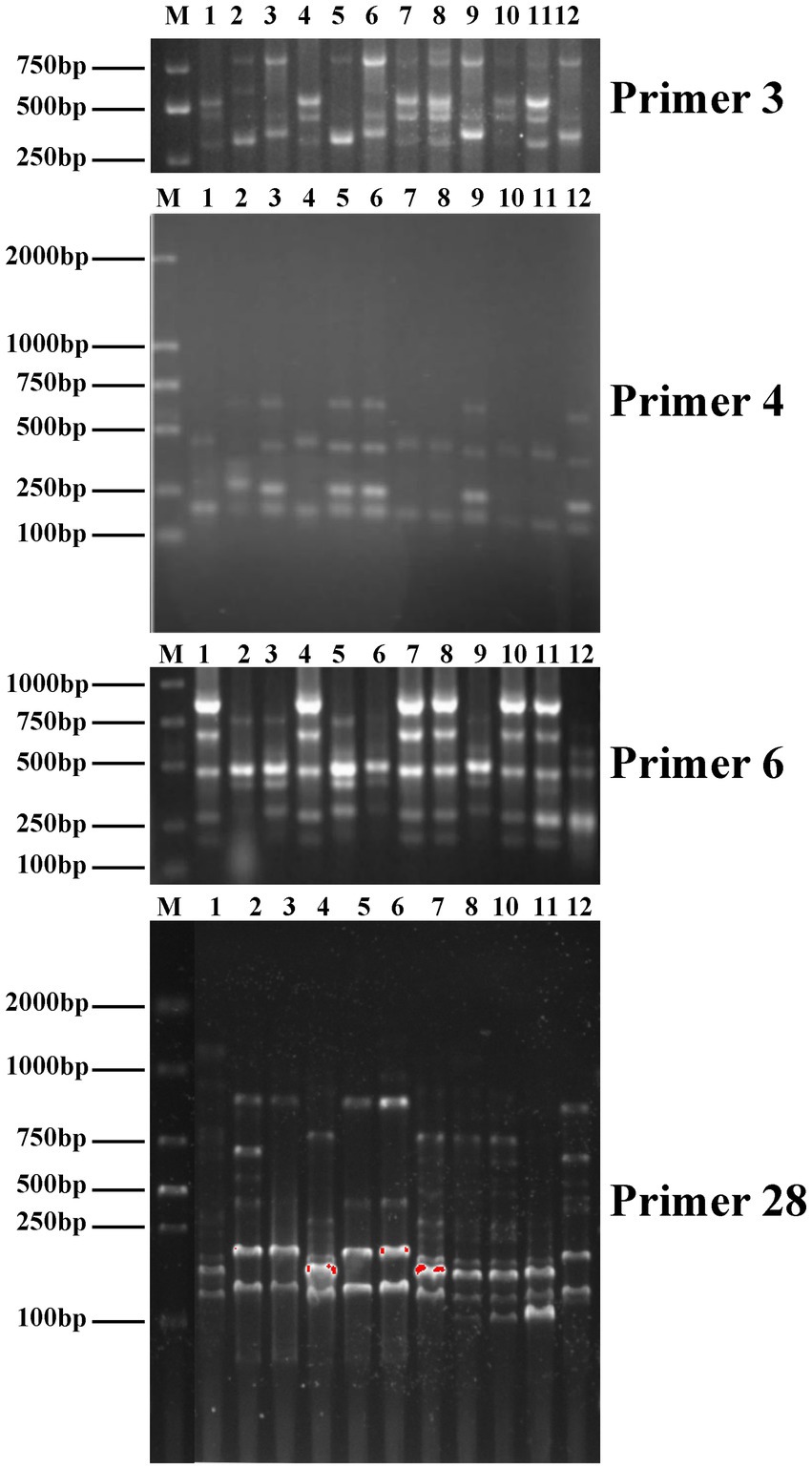
Figure 4. Inter-simple sequence repeat (ISSR) profiles of primers 3, 4, and 6 and start codon targeted (SCoT) profiles of primer 28 (1-R1, 2-R2, 3-R3, 4-R4, 5-R5, 6-R6, 7-R7, 8-R8, 9-R9, 10-R10, 11-A. cornea cv. Yu Muer, and 12-A. heimuer cv. Bai Muer).
Selecting true hybrids was an essential step in breeding, which can directly affect breeding efficiency. The PEG-induced double-inactivated protoplast has been widely applied in protoplast fusion in edible mushrooms (He et al., 2020). PEG is widely used to mediate cell–cell fusion in the production of somatic cell hybrids. PEG can cause changes in electron distribution on the cell surface in the presence of calcium ions. Then, fusion points and recesses form in the plasma membrane, constituting a bridge of protoplasts. Finally, intercellular channels were formed and gradually expanded until protoplast fusion was completed (Zhu et al., 2016). In this study, the protoplast-regenerated mononuclear strain of the parents was used as the starting strain of interspecific fusion, and different inactivation methods were used for marking inactivation to ensure that only fusion products regenerated into colonies. In the regeneration medium, neither the A. heimuer cv. Bai Muer protoplasts (due to heat inactivation) nor the A. cornea cv. Yu Muer protoplasts (due to UV inactivation) will grow. Hybrid protoplasts can grow due to the complementation of the parental genome or nuclear–cytoplasmic interactions (Mallick and Sikdar, 2014). The nuclear phase of the fused hybrid was observed as binucleate hypha under a fluorescence microscope, which showed that the genetic materials of both parents were complementarily repaired during the fusion process, and the heterokaryons were successfully obtained, which ruled out the possibility that the fused strain was the parent dikaryotic strain and monokaryon strain (Chiu et al., 1995).
This is the first time that interspecific protoplast fusion has been carried out among white varieties of Auricularia species. Yellowish-white primordia were obtained from two hybrids. The antagonistic line showed rejection between the fusion strain and its parents. The morphology of hybrids on the PDA medium had the characteristics of their parents. However, it may be due to the special mechanism of heterokaryon development after fusion or the change in environmental requirements of the fusion strain (Eyini et al., 2006); the primordia have not developed into a fruiting body, so it needs to be further domesticated and cultivated.
There are reports of the hybrids exhibiting novel nutrient and biochemical characteristics even though they resembled any of their parents molecularly (Loveleen and Kapoor, 2014; Mallick and Sikdar, 2016). Many different molecular markers, such as simple sequence repeats (SSRs), randomly amplified polymorphic DNA (RAPD), and sequence-characterized amplified region (SCAR), were used to find evidence of gene recombination (Yoo et al., 2002; Su et al., 2008; Mallick et al., 2017). Therefore, it is necessary to establish an accurate and rapid PCR-based diagnostic system for hybrid strains of white Auricularia hybrids. Moreover, the ISSR and SCoT primers are suitable for A. heimuer cv. Bai Muer and A. cornea cv. Yu Muer that are screened to reveal high polymorphism, which helps distinguish individuals at the inter- and/or intra-species level.
Post-fusion incompatibility caused by heterokaryons is common in mushrooms and has been reported in several mushrooms (Peberdy and Fox, 2018). Separating one parent from unstable heteronuclear cells in PDA culture proves this point. This phenomenon of parental separation is caused by the disharmony of heterokaryotic nuclei in distant fusion. Although protoplast fusion can bypass the natural barriers of cytoplasmic fusion and achieve distant hybridization between different species, protoplast fusion cannot eliminate the hybridization barriers caused by post-fusion incompatibility during hybrid development. In our experiment, we observed that the primordia of hybrids failed to differentiate into fruiting bodies. How to maintain the stability of heterokaryons is a crucial problem during the development of distant hybrids (Kim et al., 1997). Regardless of the genetic mechanism, when two distant parents undergo protoplast fusion, the resulting hybrids can offer a range of benefits. These benefits include enhanced biological efficiency, increased fruiting body yield, higher polysaccharide content, enhanced enzyme production, and other improvements (Okamura et al., 2000; Khattab and Mohamed, 2012; Das et al., 2021). This method has been proven to be successfully used in the improvement of naturally incompatible strains (Chakraborty and Sikdar, 2010). In addition, protoplast fusion may result in interactions between nuclear and exonuclear genes, such as mitochondrial genes (Stasz and Harman, 1990; Harman and Stasz, 1991; Harman and Hayes, 1993). Fukuda has reported the successful mitochondrial DNA transmission in interspecific fusion protoplasts of Pleurotus, which increased the genetic variability of economically significant mushrooms (Fukuda et al., 2007). Because mitochondrial genomes may influence the phenotypic characteristics of edible mushrooms, this possibility is useful in mushroom breeding (Zhao and Chang, 1997).
In this study, the double-inactivated method, colony morphology, barrage reaction, ISSR, and SCoT strongly proved their hybrid nature. The somatic hybrids obtained through this study are not end products. Instead, the non-fruit body-generating somatic hybrid could serve as resource material for backcrossing with parents, and other further studies would give us insight into the basic genetics of Basidiomycetes mating-type genes, clamp formation, and mode of sexuality. Moreover, these hybrids could be used for further mushroom improvement programs.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
KQ: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZQ: Conceptualization, Software, Writing – review & editing, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft. AX: Supervision, Project administration, Investigation, Methodology, Writing – review & editing, Conceptualization. BZ: Supervision, Project administration, Investigation, Methodology, Validation, Writing – review & editing. XL: Supervision, Project administration, Validation, Investigation, Writing – review & editing, Funding acquisition, Methodology, Resources. YL: Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by Demonstration and Promotion of Key Agricultural Core Technologies in Jilin Province (Industrial Technology System 202300601), the Jilin Province Science and Technology Development Plan Project (No. 20230202114NC), and the Scientific and Technological Tackling Plan for the Key Fields of Xinjiang Production and Construction Corps (No. 2021AB004).
The authors would like to thank Zong Liu for his kind help in the cultivation studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chakraborty, U., and Sikdar, S. R. (2008). Production and characterization of somatic hybrids raised through protoplast fusion between edible mushroom strains Volvariella volvacea and Pleurotus florida. World J. Microbiol. Biotechnol. 24, 1481–1492. doi: 10.1007/s11274-007-9630-1
Chakraborty, U., and Sikdar, S. R. (2010). Intergeneric protoplast fusion between Calocybe indica (milky mushroom) and Pleurotus florida aids in the qualitative and quantitative improvement of sporophore of the milky mushroom. World J. Microbiol. Biotechnol. 26, 213–225. doi: 10.1007/s11274-009-0162-8
Chakravarty, B. (2011). Trends in mushroom cultivation and breeding. Aust. J. Agric. Eng. 2, 102–109.
Chen, Y. Q., Sossah, F. L., Lv, Z. W., Lv, Y. C., Tian, L., Sun, X. Z., et al. (2021). Effect of wheat bran and maize straw substrates on the agronomic traits and nutritional content of Auricularia cornea cv. Yu Muer. Sci. Hortic. 286:110200. doi: 10.1016/j.scienta.2021.110200
China Edible Fungi Association. (2022). Analysis of the results of the 2021 national edible fungi statistical survey. Available at: https://mp.weixin.qq.com/s/M6ZQN5IYkkMCOmsojfLtUw (Accessed October 18, 2023).
Chiu, S. W., Chen, M., and Chang, S. T. (1995). Differentiating homothallic Volvariella mushrooms by RFLPs and AP-PCR. Mycol. Res. 99, 333–336. doi: 10.1016/s0953-7562(09)80909-x
Das, P., Sikdar, S. R., and Samanta, A. (2021). Nutritional analysis and molecular characterization of hybrid mushrooms developed through intergeneric protoplast fusion between Pleurotus sajor-caju and Calocybe indica with the purpose to achieve improved strains. World J. Microbiol. Biotechnol. 37:69. doi: 10.1007/s11274-021-03032-3
Dhitaphichit, P., and Pornsuriya, C. (2005). Protoplast fusion between Pleurotus ostreatus and P. Djamor. Warasan. Songkhla. Nakharin. 27, 975–982.
Eyini, M., Rajkumar, K., and Balaji, P. (2006). Isolation, regeneration and PEG-induced fusion of protoplasts of Pleurotus pulmonarius and Pleurotus florida. Mycobiology. 34, 73–78. doi: 10.4489/MYCO.2006.34.2.073
Fukuda, M., Wakayama, M., Uchida, M., Fukumasa-Nakai, Y., and Matsumoto, T. (2007). Introduction of mitochondrial DNA from Pleurotus ostreatus into Pleurotus pulmonarius by interspecific protoplast fusion. J. Wood Sci. 53, 339–343. doi: 10.1007/s10086-006-0861-9
Harman, G. E., and Hayes, C. K. (1993). The genetic nature and biocontrol ability of progeny from protoplast fusion in Trichoderma. Biotechnol. Plan. Dis. Control. 42, 237–255.
Harman, G. E., and Stasz, T. E. (1991). Protoplast fusion for the production of superior biocontrol Fungi. Microb. Control. Weeds. 10, 171–186. doi: 10.1007/978-1-4615-9680-6-10
He, B. L., You, L. R., Ye, Z. W., Guo, L. Q., Lin, J. F., Wei, T., et al. (2018). Construction of novel cold-tolerant strains of Volvariella volvacea through protoplast fusion between Volvariella volvacea and Pleurotus eryngii. Sci. Hortic. 230, 161–168. doi: 10.1016/j.scienta.2017.12.003
He, P. X., Yu, M., Wang, K., Cai, Y. L., Li, B., and Liu, W. (2020). Interspecific hybridization between cultivated morels Morchella importuna and Morchella sextelata by PEG-induced double inactivated protoplast fusion. World J. Microbiol. Biotechnol. 36:58. doi: 10.1007/s11274-020-02835-0
Khattab, A. A., and Mohamed, S. A. (2012). Mutation induction and protoplast fusion of Streptomyces spp. for enhanced alkaline protease production. J. Appl. Sci. Res. 8, 807–814.
Kim, C., Choi, E. C., and Kim, B. K. (1997). Protoplast fusion between Lentinula edodes and Coriolus versicolor. Arch. Pharm. Res. 20, 448–453. doi: 10.1007/BF02973938
Lee, Y. L., Jian, S. Y., Lian, P. Y., and Mau, J. L. (2008). Antioxidant properties of extracts from a white mutant of the mushroom Hypsizigus marmoreus. J. Food Compos. Anal. 21, 116–124. doi: 10.1016/j.jfca.2007.09.005
Li, X., Qian, K. Q., and Han, W. W. (2021). Prediction of hyaluronic acid target on sucrase-isomaltase (SI) with reverse docking and molecular dynamics simulations for inhibitors binding to SI. PLoS One 16:e0255351. doi: 10.1371/journal.pone.0255351
Li, X., Qian, K. Q., and Xu, A. R. (2019). Studies on the breeding of albino Auricularia heimuer. Acta Edulis Fungi. 26, 24–30. doi: 10.16488/j.cnki.1005-9873.2019.02.004
Liu, X. B., Feng, B., Li, J., Yan, C., and Yang, Z. L. (2016). Genetic diversity and breeding history of winter mushroom (Flammulina velutipes) in China uncovered by genomic SSR markers. Gene 591, 227–235. doi: 10.1016/j.gene.2016.07.009
Liu, J., Jia, L., Kan, J., and Jin, C. H. (2013). In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 51, 310–316. doi: 10.1016/j.fct.2012.10.014
Loveleen, K., and Kapoor, S. (2014). Protoplast electrofusion for development of somatic hybrids between Pleurotus florida and Pleurotus sajor-caju. Int J Pharm. Bio. Sci. 5, 507–519.
Ma, X., Lu, L., Yao, F., Fang, M., Wang, P., Meng, J., et al. (2023). High-quality genome assembly and multi-omics analysis of pigment synthesis pathway in Auricularia cornea. Front. Microbiol. 14:1211795. doi: 10.3389/fmicb.2023.1211795
Maity, K. K., Patra, S., Dey, B., Bhunia, S. K., Mandal, S., Bahera, B., et al. (2013). A β-glucan from the alkaline extract of a somatic hybrid (PfloVv5FB) of Pleurotus florida and Volvariella volvacea: structural characterization and study of immunoactivation. Carbohydr. Res. 370, 13–18. doi: 10.1016/j.carres.2013.01.016
Maji, P. K., Sen, I. K., Devi, K. S. P., Maiti, T. K., Sikdar, S. R., and Islam, S. S. (2013). Structural elucidation of a biologically active heteroglycan isolated from a hybrid mushroom of Pleurotus florida and Lentinula edodes. Carbohydr. Res. 368, 22–28. doi: 10.1016/j.carres.2012.12.008
Mallick, P., Chattaraj, S., and Sikdar, S. R. (2017). Molecular characterizations of somatic hybrids developed between Pleurotus florida and Lentinus squarrosulus through inter-simple sequence repeat markers and sequencing of ribosomal RNA-ITS gene. Biotech 7:298. doi: 10.1007/s13205-017-0931-2
Mallick, P., and Sikdar, S. R. (2014). Production and molecular characterization of somatic hybrids between Pleurotus florida and Lentinula edodes. World J. Microbiol. Biotechnol. 30, 2283–2293. doi: 10.1007/s11274-014-1652-x
Mallick, P., and Sikdar, S. R. (2016). Restriction fragment length polymorphism and sequence analysis of rRNA-ITS region of somatic hybrids produced between Pleurotus florida and Lentinula edodes. Ann. Microbiol. 66, 389–395. doi: 10.1007/s13213-015-1121-2
Miao, J., Regenstein, J. M., Qiu, J., Zhang, J., Zhang, X., Li, H., et al. (2020). Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-a review. Int. J. Biol. Macromol. 150, 102–113. doi: 10.1016/j.ijbiomac.2020.02.054
Moturi, B., and Charya, M. A. (2009). Strain improvement in dye decolourising mutants of Mucor mucedo by protoplast fusion. Afr. J. Biotechnol. 8, 6908–6912. doi: 10.4314/ajb.v8i24.68774
Okamura, T., Takeno, T., Dohi, M., Yasumasa, I., Hayashi, T., Toyoda, M., et al. (2000). Development of mushrooms for thrombosis prevention by protoplast fusion. J. Biosci. Bioeng. 89, 474–478. doi: 10.1016/s1389-1723(00)89099-4
Patra, S., Maity, K. K., Bhunia, S. K., Dey, B., Mandal, S., Maiti, T. K., et al. (2011). Structural characterization and study of immunoenhancing properties of heteroglycan isolated from a somatic hybrid mushroom (PfloVv1aFB) of Pleurotus florida and Volvariella volvacea. Carbohydr. Res. 346, 1967–1972. doi: 10.1016/j.carres.2011.06.014
Peberdy, J. F., and Fox, H. M. (2018). Protoplast technology and edible mushrooms genetics and breeding of edible mushrooms. UK: Routledge: 125–155.
Raman, J., Jang, K. Y., Oh, Y. L., OH, M., Im, J. H., Lakshmanan, H., et al. (2021). Interspecific hybridization between Ganoderma lingzhi and G. Applanatum through protoplast fusion. World J. Microbiol. Biotechnol. 37, 114–117. doi: 10.1007/s11274-021-03084-5
Selvakumar, P., Rajasekar, S., Babu, A. G., Periasamy, K., Raaman, N., and Reddy, M. S. (2015). Improving biological efficiency of Pleurotus strain through protoplast fusion between P. Ostreatus var. florida and P. Djamor var. roseus. Food Sci. Biotechnol. 24, 1741–1748. doi: 10.1007/s10068-015-0226-5
Sen, I. K., Maji, P. K., Behera, B., Mallick, P., Maiti, T. K., Sikdar, S. R., et al. (2013). Structural characterization of an immunoenhancing heteroglycan of a hybrid mushroom (pfls1h) of Pleurotus florida and Lentinus squarrosulus (Mont.) singer. Carbohydr. Res. 371, 45–51. doi: 10.1016/j.carres.2013.02.004
Stasz, T. E., and Harman, G. E. (1990). Nonparental progeny resulting from protoplast fusion in Trichoderma in the absence of parasexuality. Exp. Mycol. 14, 145–159. doi: 10.1016/0147-5975(90)90073-3
Su, H. Y., Wang, L., Ge, Y. H., Feng, E., Sun, J., and Liu, L. (2008). Development of strain-specific SCAR markers for authentication of Ganoderma lucidum. World J. Microbiol. Biotechnol. 24, 1223–1226. doi: 10.1007/s11274-007-9579-0
Wang, S. H., Duan, M. G., Liu, Y. L., Fan, S., Lin, X. S., and Zhang, Y. (2017). Enhanced production of fructosyltransferase in aspergillus oryzae by genome shuffling. Biotechnol. Lett. 39, 391–396. doi: 10.1007/s10529-016-2254-5
Wang, D., Jiang, X., Teng, S. S., Zhang, Y. Q., Liu, Y., Li, X., et al. (2019). The antidiabetic and antinephritic activities of Auricularia cornea (an albino mutant strain) via modulation of oxidative stress in the db/db mice. Front. Immunol. 10:1039. doi: 10.3389/fimmu.2019.01039
Wu, F., Yuan, Y., Malysheva, V. F., Du, P., and Dai, Y. C. (2014). Species clarification of the most important and cultivated Auricularia mushroom “Heimuer”: evidence from morphological and molecular data. Phytotaxa. 186, 241–253. doi: 10.11646/phytotaxa.186.5.1
Xu, J. Z., Zhang, J. L., Zhang, W. G., and Hu, K. H. (2012). The novel role of fungal intracellular laccase: used to screen hybrids between Hypsizigus marmoreus and Clitocybe maxima by protoplasmic fusion. World J. Microbiol. Biotechnol. 28, 2625–2633. doi: 10.1007/s11274-012-1072-8
Yoo, Y. B., Lee, K. H., and Kim, B. G. (2002). Characterization of somatic hybrids with compatible and incompatible species by protoplast fusion in genera Pleurotus (Fr.) P. Karst. and Ganoderma P. Karst. by RAPD-PCR analysis. Int. J. Med. Mushrooms 4, 147–157. doi: 10.1615/IntJMedMushr.v4.i2.80
Yuan, Y., Wu, F., Si, J., Zhao, Y. F., and Dai, Y. C. (2019). Whole genome sequence of Auricularia heimuer (Basidiomycota, Fungi), the third most important cultivated mushroom worldwide. Genomics 111, 50–58. doi: 10.1016/j.ygeno.2017.12.013
Zhao, J., and Chang, S. T. (1996). Intergeneric hybridization between Pleurotus ostreatus and Schizophyllum commune by PEG-induced protoplast fusion. World J. Microbiol. Biotechnol. 12, 573–578. doi: 10.1007/bf00327717
Zhao, J., and Chang, S. T. (1997). Interspecific hybridization between Volvariella volvacea and V. Bombycina by PEG-induced protoplast fusion. World J. Microbiol. Biotechnol. 13, 145–151. doi: 10.1023/A:1018519827561
Zhao, M. R., Chen, Q., Zhang, J. X., Wu, X. L., and Huang, C. Y. (2013). Comparison studies of genetic diversity of Pleurotus eryngii var. tuoliensis by IGS2-RFLP, SCoT and ISSR markers. Mycosystema 32, 682–689. doi: 10.13346/j.mycosystema.2013.04.002
Zhao, K., Sun, L., Ma, X., Li, X., Wang, X., Ping, W., et al. (2011). Improved taxol production in Nodulisporium sylviforme derived from inactivated protoplast fusion. Afr. J. Biotechnol. 10, 4175–4182.
Keywords: edible mushrooms, color variations, interspecies hybridization, protoplast inactivation, Auricularia
Citation: Qian K, Qi Z, Xu A, Li X, Zhang B and Li Y (2023) Interspecies hybridization between Auricularia cornea cv. Yu Muer and Auricularia heimuer cv. Bai Muer through protoplast fusion. Front. Microbiol. 14:1280420. doi: 10.3389/fmicb.2023.1280420
Received: 20 August 2023; Accepted: 09 October 2023;
Published: 30 October 2023.
Edited by:
Chenyang Huang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Wei Gao, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2023 Qian, Qi, Xu, Li, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Li, bHhtb2d1QDE2My5jb20=; Bo Zhang, emhhbmdib2Z1bmdpQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.