
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 21 September 2023
Sec. Food Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1276493
This article is part of the Research TopicFermentation of Silage in Cold and Tropical Regions: Effects of Phyllosphere MicrobiotaView all 6 articles
Orychophragmus violaceus is a local Brassicaceae in China, while most of it is directly mowed and discarded after the ornamental period. In order to develop forage resources, this study firstly evaluated the potential preservation of O. violaceus silage. O. violaceus was harvested at full-bloom stage, and ensiled without (CK) or with maize meal (Y5), lactic acid bacteria inoculant (Z) and compound additive (Y5Z) for 60 d. Results of chemical and microbiological analysis showed that a large amount of lactic acid was produced and the final pH value was below 4.1 in silages regardless of additive application. CK silage was well preserved as indicated by the low levels of dry matter loss and butyric acid content, and the predominant genus were identified as Enterococcus and Pediococcus. Y5 silage had potential health risks for humans and animals as seen by frequent occurrence of pathogenic bacteria Clostridium and Achromobacter. Z and Y5Z silages were poorly preserved, resulting in great dry matter loss and butyric acid content. Considering the abundant acetic acid production, the dominant Lactobacillus might possess a heterofermentative pathway in Z and Y5Z silages. In conclusion, O. violaceus has the potential to be long stored as silage because of its sufficient water-soluble carbohydrates, while exogenous lactic acid bacteria and maize meal generally provided little positive effect. In future research, efficient homofermentative Lactobacillus strains were suggested to be screened to further enhance the ensiling process of O. violaceus silage.
Orychophragmus violaceus (Brassicaceae) is a popular ornamental plant with a local flavor and natural charm which is endemic to China (Zhou et al., 2008), with strong environmental adaptability and breeding ability, and is widely distributed in various regions of China (Hang, 2022). Starting from the February of the lunar calendar, the blooming O. violaceus spread from the south to the north of China, creating a spectacular and eye-catching scene. However, the greatest ornamental period of full flowered O. violaceus was only 20 to 30 d. After the ornamental period, a small part of O. violaceus is used as green manure to provide nutrients for the soil (Cao et al., 2014), while most of it is directly mowed and discarded, resulting in a large amount of waste of biomass resources.
O. violaceus is a common wild vegetable that has large tender stems and highly nutritious leaves. In addition, this plant has a long history as a traditional Chinese medicine for anti-tumor, anti-inflammatory, anti-bacterial and liver-protecting properties, as it is rich in bioactive components (Xu et al., 2022). Therefore, we speculated that the application of O. violaceus as feed may be favorable for preserving and improving animal performance. Especially, in the context of the current global shortage of feed resources, expansion of the forage sources will make an important contribution to enhance the level of national food security and to maintain a sustainable and economically viable livestock system (Ni et al., 2017). However, O. violaceus harvest is seasonal with high accumulation, if it is not consumed in a short period of time by animals, it gets spoilage due to the high moisture content.
Ensiling is a practicable technique for long-term preservation of moist crops, for the preparation of well-preserved silage, a rapid development of the lactic acid (LA) fermentation is necessary to reduce the pH and inhibit the growth of inefficient and spoilage microorganisms (Zheng et al., 2018). In addition to various environmental factors, such as method of ensiling, climate, stage of maturation and wilting, lactic acid bacteria (LAB) and water-soluble carbohydrates (WSC) are crucial factors controlling the ensiling process. LAB inoculants have become the most commonly used silage additives even for direct-cut crops, because the LAB that are the essential microflora for spontaneous silage fermentation are orders of magnitude lower in population than other groups of microorganisms on the crop at ensiling (Muck, 2010). Homofermentative Lactobacillus plantarum was often used to improve the ensiling process as it allows for a rapid pH decrease, while usage of heterofermentative L. buchneri could also be beneficial as it produces sufficient acetic acid (AA) to depress aerobic deterioration by inhibiting growth of yeast and fungi (Zhang et al., 2009; Guo et al., 2018). However, to our knowledge, limited information is available on the role of LAB inoculant in O. violaceus silage. Maize meal with wide source and low cost is also commonly used to regulate the raw material properties of wet forage for high quality silage preparation because of its high dry matter (DM) and WSC contents (Niu et al., 2018).
Silage fermentation is a complex biochemical process with the participation of many microorganisms. Considering the fact that microorganisms are the key factors driving ensiling process, monitoring of the ensiling process with respect to changes in the chemical and microbial compositions would be helpful for thoroughly understanding and improving the ensiling process (Zheng et al., 2017; Huang et al., 2023). Therefore, this study first aimed to evaluate the fermentation quality and bacterial community of O. violaceus silages with or without exogenous LAB inoculant and maize meal. The results will provide a scientific theoretical basis for O. violaceus silage preparation.
Orychophragmus violaceus was cultivated under an ash (Fraxinus chinensis) forest (40°9′N, 116°57′E) located at Beijing, China, and harvested artificially at full-bloom stage after the ornamental period. The harvested O. violaceus was chopped to length of 2–3 cm using a forage cutter, mixed manually, and divided randomly into four treatments: (i) addition with an equal volume of distilled water (Control, CK); (ii) addition with maize meal at an application rate 50 g/kg fresh matter (FM)(Y5); (iii) addition with LAB inoculant at a concentration of 106 cfu/g FM (Z); and (iv) addition with maize meal at an application rate 50 g/kg FM and LAB inoculant at a concentration of 106 cfu/g FM (Y5Z). Maize meal used in this study was from a local supermarket, its chemical and microbial compositions were shown in Table 1. The applied LAB inoculant was ZHUANGLEMEI (Sichuan Gao Fu Ji Biological Technology Co., Ltd., Chengdu, China), consisting of L. plantarum and L. buchneri with a ratio of 7:3. O. violaceus material was mixed homogenously with additives, packed manually into plastic film bag silos (Hiryu KN type, 180 × 260 mm; Asahikasei, Tokyo, Japan) and vacuumed tightly. Silos were prepared in triplicate and stored at ambient temperature (20–35°C) for 60 d of ensiling.
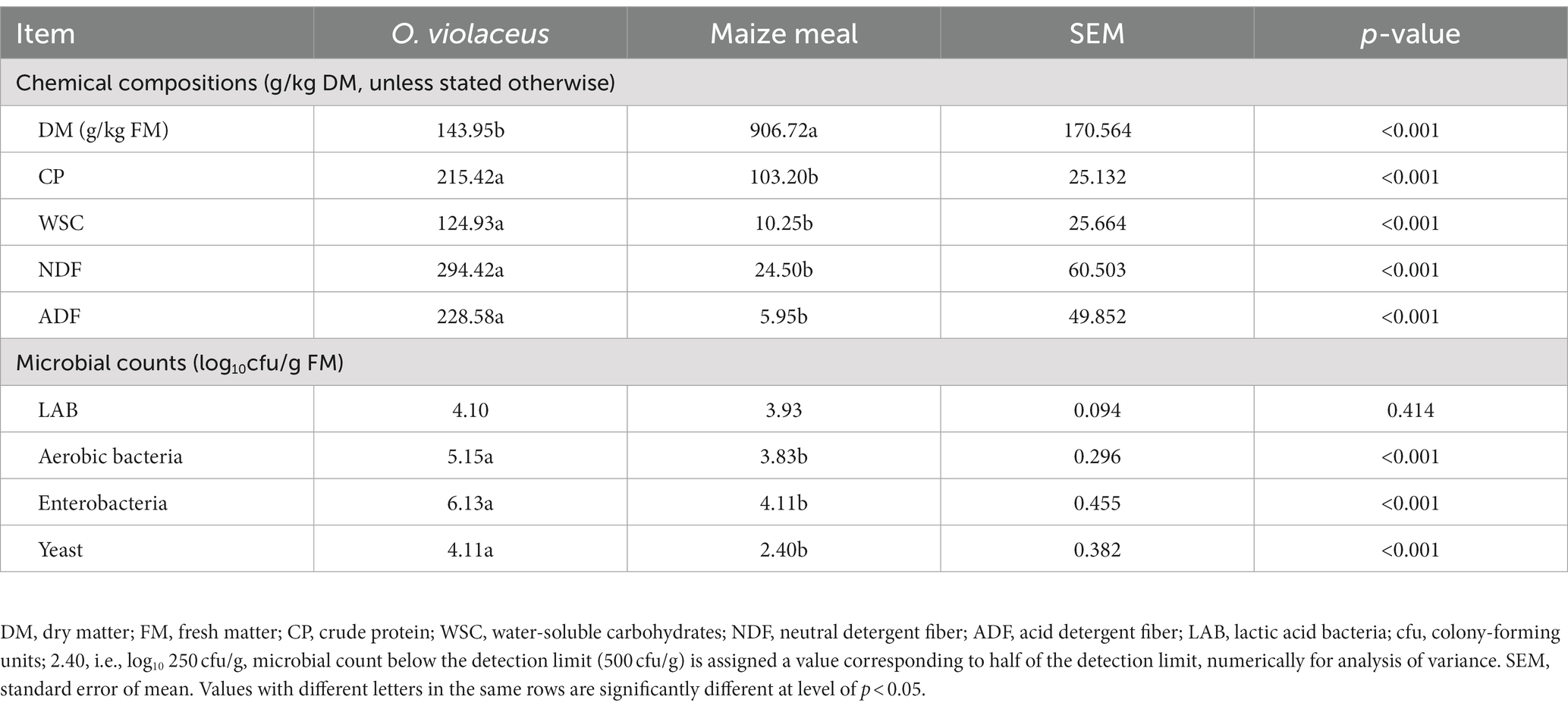
Table 1. Chemical compositions and microbial counts of fresh Orychophragmus violaceus and maize meal.
Fermentation products of silage were determined from cold-water extracts. Wet silage (10 g) was homogenized with 90 mL of sterilized distilled water, and then filtered through four layers of medical gauze and a qualitative filter paper. The pH was measured with a glass electrode pH meter (S20K; Mettler Toledo, Greifensee, Switzerland), and ammonia nitrogen (NH3−N) content was determined by the method of Broderick and Kang (1980). The filtrate was further processed with a dialyser of 0.22 μm to determine organic acids contents as described by Zhang et al. (2020). Briefly, LA was analyzed by ion chromatography (Dionex ICS-2500, Dionex instruments, California, CA) equipped with an InoPac AS11-HC analysis column (4 × 250 mm), an InoPac AS11-HC protect column (4 × 50 mm), and an ASRS ULTRA II 4 mm suppressor. The sampling amount was 25 μL, the column temperature was 30°C, the mobile phase was 50 mmoL/L sodium hydroxide solution at a flow rate of 0.80 mL/min. AA, propionic acid (PA) and butyric acid (BA) contents were determined with GC 3420 gas chromatograph (Agilent Tech Inc., Dionex, FTC, Palo Alto) fitted with HP-INNO wax capillary column (30 m × 0.32 mm).
Silage samples were dried at 65°C for 48 h to determine the DM content, and then ground through 0.20 mm-mesh sieves for analysis of chemical components. The crude protein (CP) was determined by the method of AOAC (1990). Both neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using an ANKOM 2000 fiber analyzer (Ankom Technology, Fairport, NY) by the method of Vansoest et al. (1991). The WSC was determined by the method of Owens et al. (1999).
For microbial population analysis, wet silage samples (10 g) were blended with 90 mL of sterilized water and serially diluted (10-1 to 10-5) in sterilized water. The numbers of LAB were measured by plate count on de Man, Rogosa and Sharpe Agar (Difco Laboratories, Detroit, MI) incubated at 37°C for 48 h under anaerobic condition (Anaerobic box, TE-HER Hard Anaerobox, ANX-1; Hirosawa Ltd., Tokyo, Japan). The microbial counts for aerobic bacteria were determined on Nutrient Agar (Nissui Ltd., Tokyo, Japan) incubated at 37°C for 48 h. The numbers of Enterobacteria were determined on Blue Light Broth Agar (Nissui Ltd) incubated at 37°C for 48 h. Yeasts were counted on Potato Dextrose Agar (Nissui Ltd.) incubated at 30°C for 48 h. The colonies were counted from the plates at appropriate dilutions, and the number of colony forming units is expressed per gram of FM (Wang et al., 2016).
The extraction of total genomic DNA from each silage sample was performed using the FastDNA™ SPIN Kit for Soil and the FastPrep Instrument (MP Biomedicals, Santa Ana, CA) as we described previously (Zheng et al., 2017). The V3–V4 regions of the bacterial 16S rRNA genes were amplified using the primers pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′). TruSeq™ DNA Sample Prep Kit (Illumina, San Diego, CA) was used to construct the sequencing library following manufacturer’s recommendations. DNA libraries were then paired-end sequenced on the Illumina MiSeq PE300 platform (Illumina). Both of PCR amplification of 16S rRNA genes and MiSeq sequencing were carried out by the Majorbio Biopharm Technology Co., Ltd. (Shanghai, China). The sequencing data were submitted to the NCBI Sequence Read Archive database (accession: PRJNA1002122).
The raw data from sequencing were merged using FLASH (version 1.2.11, https://ccb.jhu.edu/software/FLASH/index.shtml), followed by removing low-quality sequences and chimeric sequences using FASTP (version 0.19.6, https://github.com/OpenGene/fastp), respectively. A default similarity level of 97% was used to cluster sequences into individual operational taxonomic units (OTUs) using UPARSE (version 7.0.1090, http://drive5.com/uparse/). The representative sequence from each clustered OTU was used to taxonomic classification in the SILVA database (Release 138, https://www.arb-silva.de/) at a minimum confidence cut-off of 0.7 using the RDP Classifier (version 2.13, https://sourceforge.net/projects/rdp-classifier/). Bacterial alpha diversity indices, including Sobs, Ace, Chao1, Shannon, Simpson and Coverage, were estimated using MOTHUR (version 1.30.2, https://www.mothur.org/wiki/Download_mothur). To explore relationships between the microbial community and fermentation products, Spearman’s rank correlation matrix was generated by calculating the Spearman’s correlation coefficient. The level of high significance was set to p-value of less than 0.05 with |r| > 0.40. The correlation matrix was visualized as a heat map produced at the genus level using the R program (version 3.6.0, https://www.r-project.org) (Li et al., 2020). Bioinformatics analysis was performed on the online platform of Majorbio Cloud Platform.1
All microbial counts were log10 transformed to obtain log-normal distributed data. To calculate averages, the values below the detection level (500 cfu/g) were assigned a value corresponding to half of the detection level (i.e., 250 cfu/g). Data for chemical compositions, microbial counts and bacterial community indices were analyzed by a one-way ANOVA using the GLM procedures of SAS 9.1 (SAS Institute, Inc., Cary, NC), and the Tukey’s test was used for multiple comparisons at 5% significant level.
The microbial and chemical compositions of O. violaceus prior to silage preparation are shown in Table 1. The CP content was as high as 215.42 g/kg DM, which was comparable to that of alfalfa (Zheng et al., 2017), indicating that O. violaceus is a promising source of protein for animal feed. Ensiling fermentation by microbial decomposition of WSC can produce organic acids, thereby lowering the pH value and inhibiting the proliferation of deteriorating bacteria (Huang et al., 2023). In this study, the WSC content of 124.93 g/kg DM of O. violaceus was sufficient (>50 g/kg DM) for LA fermentation. However, the epiphytic LAB of forages do not always reduce pH rapidly because the initial load can be too low or fast acidifying homofermentative species may be absent (Zheng et al., 2018). Therefore, LAB inoculants were used to enhance the ensiling process of O. violaceus based on its insufficient (<105 cfu/g FM) epiphytic LAB counts of 104 cfu/g FM. A lower DM content of 143.95 g/kg FM (<300 g/kg FM) is an additional inhibitory factor to LA fermentation, as high moisture content might dilute WSC concentration resulting in BA fermentation (Pahlow et al., 2003).
For CK silage (Table 2), the DM content (139.26 g/kg FM) was comparable with fresh material and the CP content was similar to that of alfalfa silage (Zheng et al., 2018; Guo et al., 2020), therefore, this study further confirmed the potentiality for O. violaceus silage as protein resource. As expected, the residue WSC content in Z and Y5Z silages was lower (p < 0.05) than that in CK and Y5 silages, so LAB inoculation promoted WSC consumption for acids production (Niu et al., 2018). The lowest (p < 0.05) NDF and ADF contents occurred for Y5 and Y5Z silages in relative to CK. We attributed the lower NDF and ADF contents in maize meal-added silages to a result from acidic hydrolysis and/or fibrinolytic enzyme production by microorganisms during silage fermentation. However, although we could not classify the behind reason clearly, the NDF and ADF contents increased (p < 0.05) when LAB inoculant was added alone in Z silage compared to CK.
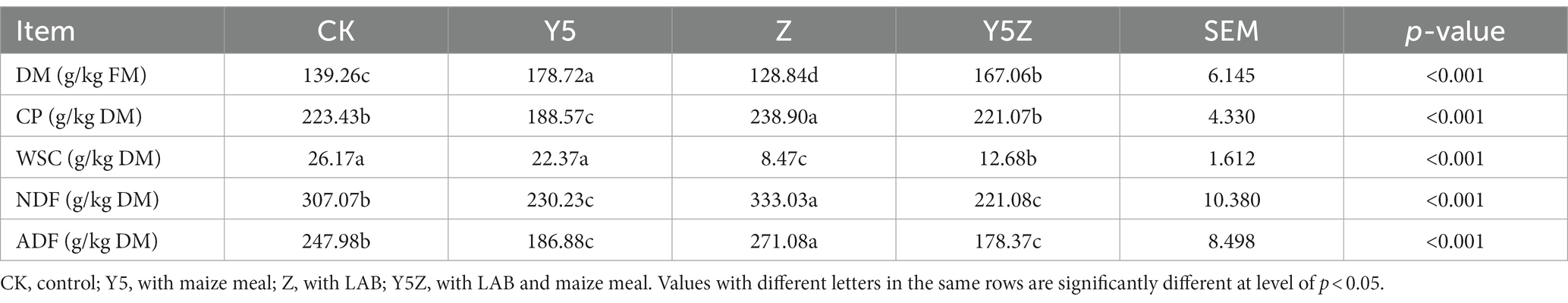
Table 2. Chemical compositions of Orychophragmus violaceus silages with or without LAB and maize meal.
The final pH values in all silage samples were below 4.1 (Table 3), suggesting a potential rapid acidification process of O. violaceus silage. LA production in CK silage was 179.13 g/kg DM, which was higher than that in alfalfa silage of 12.2–82.4 g/kg DM (Zheng et al., 2017) and whole crop maize silage of 80–120 g/kg DM (Wang et al., 2022). The effects of maize meal and LAB inoculant on O. violaceus silage fermentation were unexpected, regarding the lower (p < 0.05) LA concentration in Y5, Z and Y5Z silages compared to CK. The limited effect of maize meal and LAB inoculant on LA production might partly be related to the sufficient fermentable carbohydrates as well as other bioactive compounds favoring the growth of native LAB in O. violaceus. Forage crops used for silage preparation vary in DM content, carbohydrates, buffering capacity and carry a microflora of unknown magnitude and composition, these factors plus the establishing and maintaining a strictly anaerobic environment often determine the conversion of plant sugars to LA (Pahlow et al., 2003).
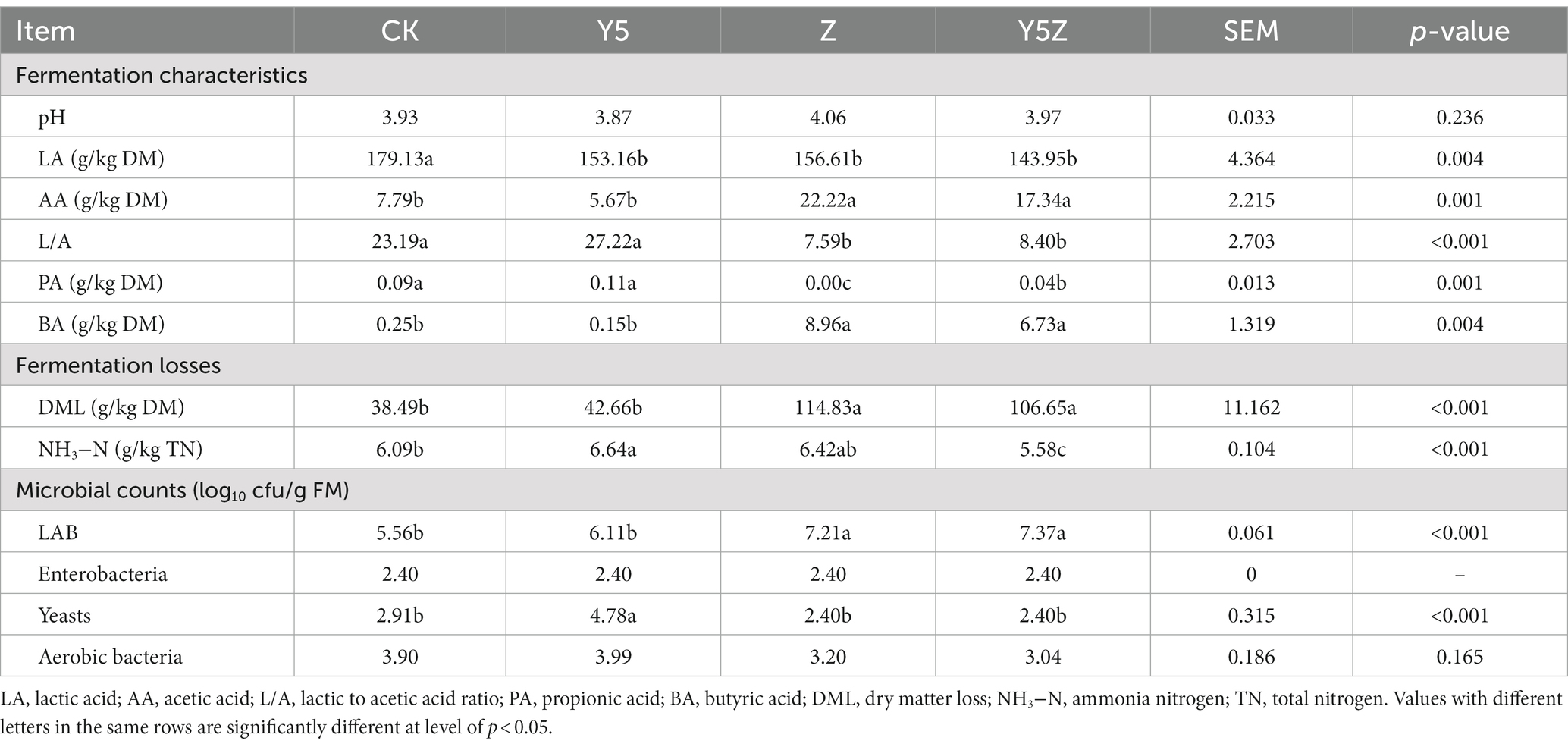
Table 3. Fermentation characteristics, fermentation losses and microbial counts of Orychophragmus violaceus silages with or without LAB and maize meal.
AA (pKa = 4.76) is weaker for silage acidification compared to LA (pKa = 3.86) (Contreras-Govea et al., 2013; Kung et al., 2018). Similar to previous results found in corn silage (Wang et al., 2022), a combination inoculant of L. buchneri and L. plantarum increased (p < 0.05) the AA concentration in Z and Y5Z silages compared to that in CK and Y5 silages. The most common reason for an inoculant not producing an effect is competition from the epiphytic LAB population, therefore, we hypothesized that L. plantarum strain applied in this study could not adapt to the nutritional characteristics of O. violaceus, or they are more sensitive than L. buchneri to the bioactive ingredients of O. violaceus. This shows the importance of the origins of the LAB inoculants when selecting them, and many studies show that the best isolates for a specific crop would come from that crop itself (Wang et al., 2006). Undetectable or traceable PA concentration was observed in all silages, while high BA (>5 g/kg DM) production was detected in Z and Y5Z silages. BA was undesirable in well-fermented silage, as the presence of this acid usually indicates the clostridial fermentation (Zheng et al., 2020). Although we do not have direct evidence, the high BA production in Z and Y5Z silages might be due to the slow acidification process at the initial fermentation stage, resulting from the competition for nutrients between the inoculated L. buchneri and native LAB.
NH3−N is undesirable during ensiling fermentation as it is an important indicator of protein degradation. In this study, NH3−N concentration in all O. violaceus silages ranged from 5.58 to 6.64 g/kg total nitrogen (TN), which was less than normal level of NH3−N (100–150 g/kg TN) in high moisture legume silage (Kung et al., 2018). It is considered that NH3−N accumulation during ensiling was attributed to the growth of undesirable bacteria (such as Enterobacteria, Bacillus and Clostridia) and/or the activities of inherent plant proteolytic enzymes (Li et al., 2022). Therefore, the decreased NH3−N content which indicated an improvement in protein quality in O. violaceus silage might be due to the fact that the lowered pH limited the proteolytic activity of microorganisms and plant proteolytic enzymes (Bao et al., 2023). Whatever strategies are developed, they must be consistent with well accepted practices that minimize silage storage losses (Kung et al., 2018). The dry matter loss (DML) associated with silage fermentation are primarily from carbon dioxide and volatile fatty acids production, and these losses typically are in the range of 20 to 40 g/kg DM (Borreani et al., 2018). In this study, the DML in CK and Y5 silages was similar to the normal level, while the values in Z and Y5Z silages were higher than 106.65 g/kg DM. The amount of DML from fermentation depends on the dominant microbial species and the substrates fermented, and it was reported that DML of 48–328, 170, 511 and 489 g/kg DM could be occurred because of fermentation caused by heterofermentative LAB, Enterobacteria, Clostridia and yeasts, respectively (Borreani et al., 2018).
Due to the high concentration and strong adaptability of LAB inoculants, it was hypothesized that inoculant strains are highly competitive over the epiphytic LAB as well as other epiphytic bacteria on crops, dominating silage fermentation (Muck, 2013). In fact, the LAB counts in Z and Y5Z silages were higher (p < 0.05) than that in CK and Y5 silages. Proper management during silage preparation is an important and efficient way for controlling the development of undesirable microorganisms (Franco et al., 2022). Enterobacteria decreased to below the detectable level in all silages. The yeast numbers also dropped below the threshold of detection in Z and Y5Z silages, might being due to the antifungal inhibition by AA. The aerobic bacteria decreased by an order of magnitude after ensiling fermentation, although the final numbers were still higher than 103 cfu/g FM in all silages. The suppression of detrimental microorganisms in O. violaceus silages with or without additives might partly be related to efficient acidic environment as well as antimicrobial bioactive components found in O. violaceus.
The alpha diversity estimates of bacterial community are presented in Table 4. Coverage index in all silages was above 0.99, indicating that the depth of high-throughput sequencing could represent the profile of bacterial community. As expected, Sobs index was observed lower (p < 0.05) in Z and Y5Z silages compared to that in CK and Y5 silages. Chao 1 and Ace indices were decreased because of LAB or maize meal addition in relative to CK silage, and Y5Z silage presented the lowest (p < 0.05) Chao1 index. During ensiling process, microbes on the plants were mostly inhibited because of the anaerobic-acidic condition and were substituted by LAB (Sun J. J. et al., 2023). Therefore, a noticeable decrease in bacterial abundance was observed due to these transformations. Clear differences in diversity indices (Shannon and Simpson) were observed, as Y5 silage showed the highest (p < 0.05) bacterial diversity, followed by CK and Z silages, while Y5Z silage showed the lowest (p < 0.05) diversity. In agreement with our results, Li et al. (2020) reported that the bacterial diversity decreased in LAB or sucrose treated alfalfa silages due to the increased relative abundance of dominant Lactobacillus.
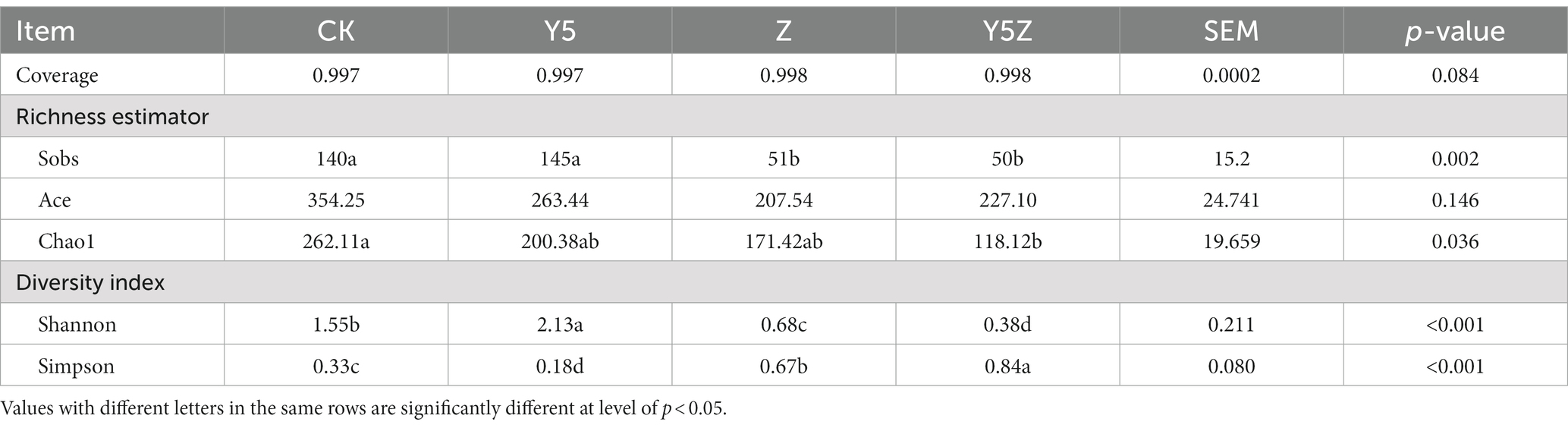
Table 4. Bacterial alpha diversity of Orychophragmus violaceus silages with or without LAB and maize meal.
The top ten dominant bacteria at the family and genus levels in O. violaceus silage are shown in Figures 1, 2. Multiple comparisons indicated that the relative abundance varied across the different treatments. Similar to previous results found in silages prepared from alfalfa (Li et al., 2022; Sun J. J. et al., 2023), barley (Franco et al., 2022) and rice straw (Sun H. et al., 2023), the bacterial community observed in O. violaceus silage was dominated by Lactobacillaceae, mainly members of Lactobacillus and Pediococcus. In addition, the abundance of Lactobacillaceae and Lactobacillus was higher (p < 0.05) in Z and Y5Z silages than that in CK and Y5 silages.
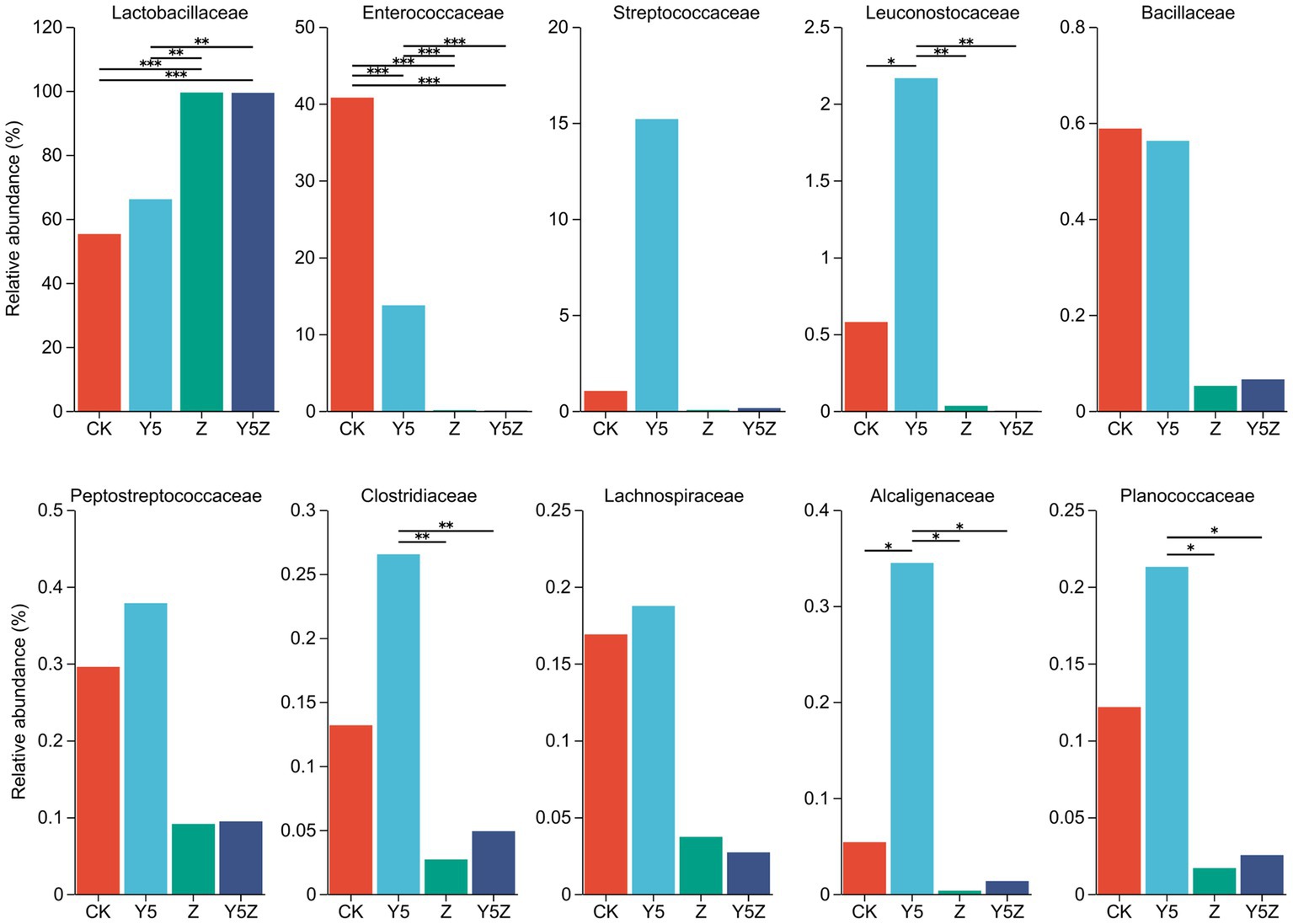
Figure 1. The family level bacterial taxa compositions of Orychophragmus violaceus silages with or without LAB and maize meal. CK, control; Y5, with maize meal; Z, with LAB; Y5Z, with LAB and maize meal. *, **, and *** stand for 0.01 < p < 0.05, 0.001 < p < 0.01, p < 0.001, respectively.
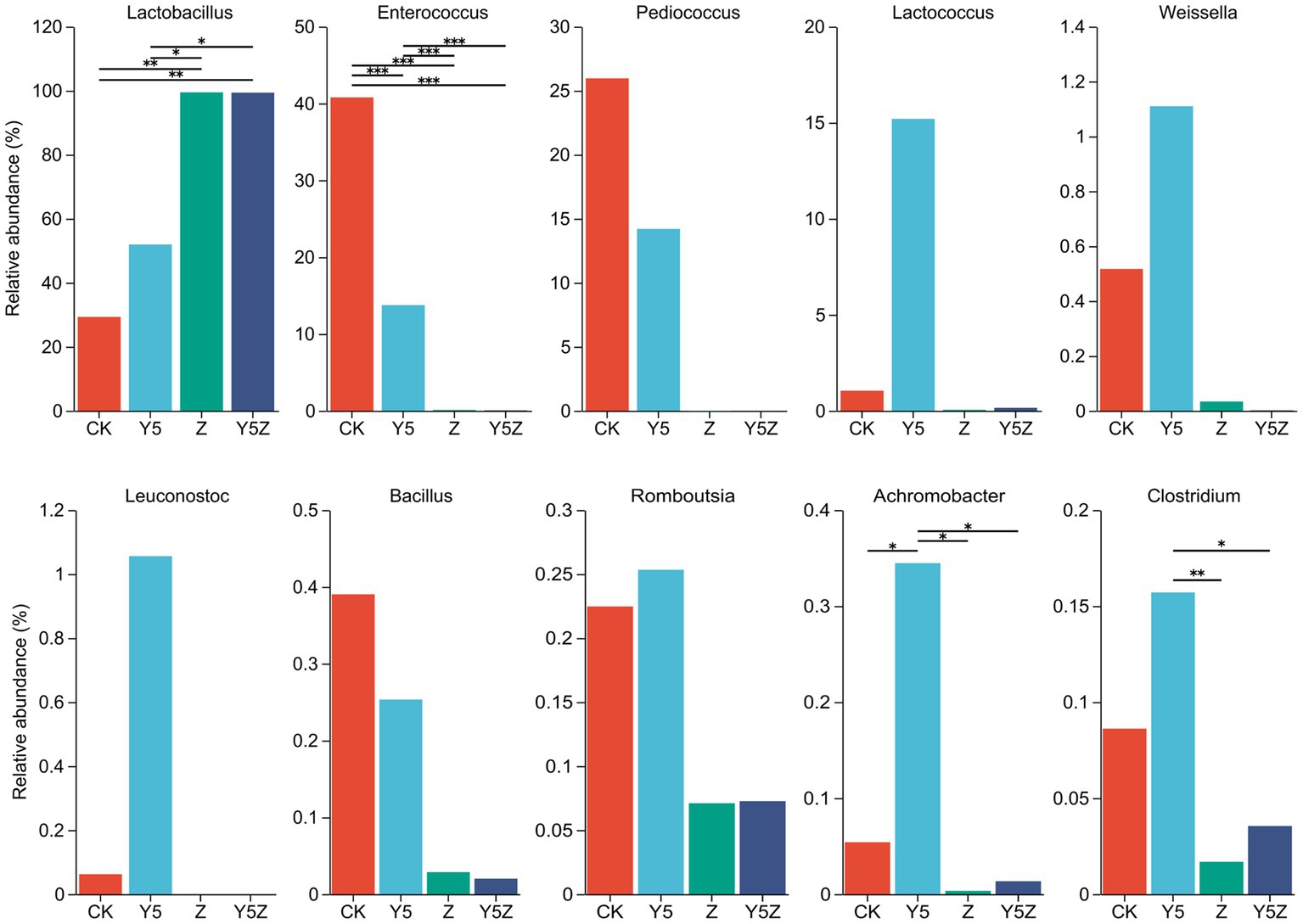
Figure 2. The genus level bacterial taxa compositions of Orychophragmus violaceus silages with or without LAB and maize meal. *, **, and *** stand for 0.01 < p < 0.05, 0.001 < p < 0.01, p < 0.001, respectively.
According to the classical theory of ensiling fermentation, LA-producing cocci (Weissella, Leuconostoc, Pediococcus, Lactococcus, and Enterococcus) initiates LA fermentation at the early stage of ensiling, while LA-producing rod Lactobacillus, with acid tolerance ability, plays an important role in pH reduction at the later stage of ensiling (Jiang et al., 2020). The tendency of Enterococcaceae abundance was CK > Y5 > Z and Y5Z (p < 0.05), and the main genus was Enterococcus. In agreement with results observed in natural fermented soybean silage (Ni et al., 2017), Enterococcus was the dominant genus in CK silage followed by Pediococcus and Lactobacillus. During ensiling fermentation, Enterococcus played a pivotal role in accelerating the LA fermentation and building an anaerobic-acidic circumstance for the development of Lactobacillus (Wang et al., 2020; Dong et al., 2023). Recently, Enterococcus sp., such as E. faecalis and E. faecium, were successfully used as silage inoculants to stimulate ensiling process (Li et al., 2018). Similar tendency to Enterococcus was found for Pediococcus, although no significant difference was observed among treatments.
High relative abundance of LA-producing cocci, including Enterococcus (13.74%), Pediococcus (14.20%), Lactococcus (15.20%), Weissella (1.11%) and Leuconostoc (1.06%), was also observed in Y5 silage, although maize meal contributed to the growth of Lactobacillus (51.95%). The probable reason for the occurrence of abundant LA-producing cocci in CK and Y5 silages was that high moisture content partly compensate for acid sensitivity of LA-producing cocci as well as the limited competitiveness of native homofermentative Lactobacillus in O. violaceus. In addition, based on the concept proposed by Li et al. (2022), an unfinished or incomplete fermentation might occur in CK and Y5 silages as indicated by the high presence of LA-producing cocci. From a practical perspective, when reducing the moisture content of raw materials is not feasible or economical, homofermentative Lactobacillus strains that not only grow fast but also have other competitive advantages over their fellow LAB as well as other epiphytic bacteria were suggested to be applied in O. violaceus silage preparation to accelerate the ensiling process, and then to decrease the nutrient loss.
Clostridium is the main contributor for clostridial fermentation which not only causes reduction in nutritional value but also decreases hygienic quality of legume silage (Zheng et al., 2017). The relative abundance of Clostridium in Y5 silage was numerally higher than that in CK silage, and was higher (p < 0.05) than that in Z and Y5Z silages. The chances of a clostridial fermentation can be minimized by decreasing forages moisture content and inducing a rapid production of LA, because Clostridia are intolerant of both high osmotic pressure and low pH (Kung et al., 2018). In this study, however, maize meal might provide sufficient fermentable substrates for spoilage Clostridia growth in Y5 silage, when low pH alone did not necessarily depress growth of potentially undesired bacteria (Eikmeyer et al., 2013). It was suggested that potential positive effects of ensiling fermentation on inhibition deleterious microorganisms might be due to a direct effect of rapid acidification, reduced availability of water, production of antimicrobial compounds, or a combination of factors (Garde et al., 2014). Achromobacter, the main genus of family Alcaligenaceae, is non-fermentative and potential pathogen bacteria found ubiquitously in environmental reservoirs including rivers, ponds, residential water sources, soil, mud and some plants (Price et al., 2020). Similar to Clostridium, the relative abundance of undesirable Achromobacter was also observed higher (p < 0.05) in Y5 silage than other three groups.
A Spearman correlation was performed to identify the relationship between fermentation parameters and top 15 genera in O. violaceus silage (Figure 3). During desirable ensiling process, epiphytic LAB converts WSC into LA resulting in decreased silage pH, thereby, results of correlation analysis usually reveal that Lactobacillus was positively correlated to LA and negatively correlated to WSC and pH. In this study, Lactobacillus was positively (p < 0.05) correlated to LAB, DML, AA, BA and pH, and negatively (p < 0.05) correlated to WSC, PA, Enterobacteria, LA and lactic to acetic acid ratio. Although the correlation was not always significant, LA-producing cocci was positively correlated to LA, and negatively correlated to pH. These results suggested that most of Lactobacillus in O. violaceus silage possessed a heterofermentative pathway and LA-producing cocci was the main contributor to LA production. Silage with clostridial fermentation is characterized by trace amount of LA and resultantly high levels of pH, BA, NH3−N and amines (Pahlow et al., 2003). In this study, Clostridium was negatively (p < 0.05) correlated to BA although positive correlation was observed for NH3−N production. This was partly consistent with the report of Franco et al. (2022), who found that BA was positively correlated to Leuconostoc and Lactococcus. It was not surprising because different species of the one genus, even if the one species existed in different habitats, contain different phylogenetic and basic characteristics (Huang et al., 2023). Hence, bacterial community in O. violaceus silage could potentially be further studied at species level and metabolic capability.
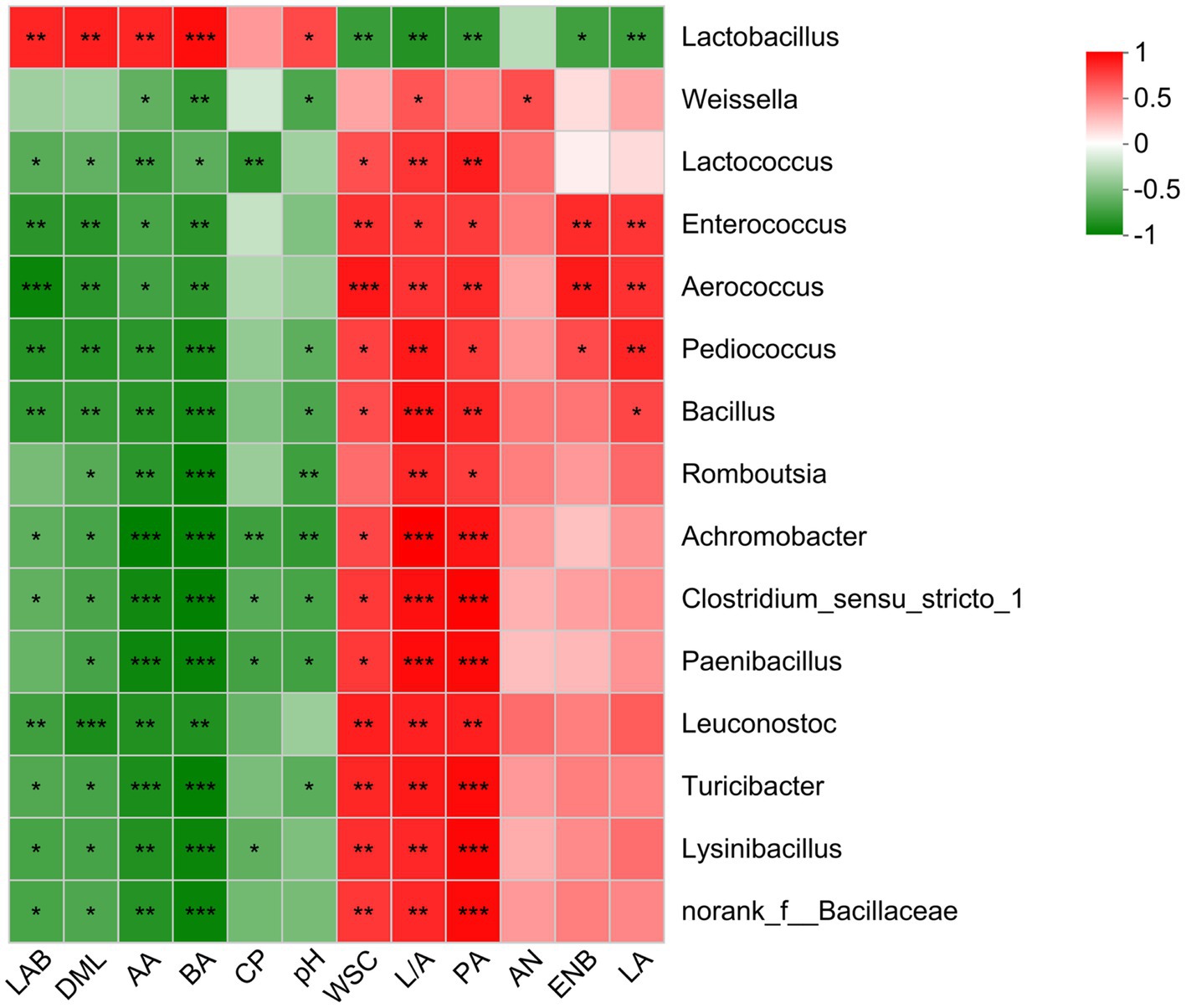
Figure 3. Spearman correlations between bacterial communities and fermentation parameters of Orychophragmus violaceus silages with or without LAB and maize meal. LAB, lactic acid bacteria; DML, dry matter loss; AA, acetic acid; BA, butyric acid; CP, crude protein; WSC, water-soluble carbohydrates; L/A, lactic to acetic acid ratio; PA, propionic acid; AN, ammonia nitrogen; ENB, Enterobacteria; LA, lactic acid. The corresponding value of the right heatmap is the correlation coefficient r, which ranges between −1 and 1. Red squares indicate a positive correlation, and green squares indicate a negative correlation. *, **, and *** stand for 0.01 < p < 0.05, 0.001 < p < 0.01, p < 0.001, respectively.
This study confirmed the potential of ensiling of O. violaceus as silage, the sufficient WSC enabled a large amount of LA production resulting in a rapid reduction in pH value. The growth of LA-producing cocci in CK silage was facilitated for good preservation of silage nutrients with low levels of DML and BA. However, exogenous LAB and maize meal generally provided little positive effect. Y5 silage had potential health risks for humans and animals as seen by frequent occurrence of pathogenic bacteria Clostridium and Achromobacter. AA, BA, and DML were increased dramatically in Z and Y5Z silages, indicating that the dominant Lactobacillus possessed a heterofermentative pathway and the L. plantarum strain composed in LAB inoculant was not suitable for O. violaceus silage. Homofermentative Lactobacillus strains, may be origin from O. violaceus plant, will be screened to further enhance ensiling process of O. violaceus silage.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
MZ: Writing – review & editing, Funding acquisition, Investigation, Writing – original draft. PM: Writing – review & editing, Data curation. XT: Data curation, Writing – review & editing. LM: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32201461) and the Youth Research Fund of BAAFS (QNJJ202310).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AOAC (1990). Official methods of analysis, 15th ed. Association of Official Analytical Chemists, Arlington, VA, USA
Bao, X. Y., Guo, G., Huo, W. J., Li, Q. H., Xu, Q. F., and Chen, L. (2023). Ensiling pretreatment fortified with laccase and microbial inoculants enhances biomass preservation and bioethanol production of alfalfa stems. Sci. Total Environ. 857:159442. doi: 10.1016/j.scitotenv.2022.159442
Borreani, G., Tabacco, E., Schmidt, R. J., Holmes, B. J., and Muck, R. E. (2018). Silage review: factors affecting dry matter and quality losses in silages. J. Dairy Sci. 101, 3952–3979. doi: 10.3168/jds.2017-13837
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino-acids in ruminal fluid and invitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Cao, W. D., Rui, Y. K., and Li, X. G. (2014). Determination of forty-six elements in different organs of Orychophragmus violaceus in agricultural farm. Asian J. Chem. 26, 1038–1040. doi: 10.14233/ajchem.2014.15837
Contreras-Govea, F. E., Muck, R. E., Broderick, G. A., and Weimer, P. J. (2013). Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 179, 61–68. doi: 10.1016/j.anifeedsci.2012.11.008
Dong, T. Y., Liang, Y. C., Shao, T., Wang, W. B., Ma, P. F., Wang, W. K., et al. (2023). Detoxifying mycotoxins and antifungal properties of two rumen-derived Enterococcus species in artificially contaminated corn silages. J. Sci. Food Agric. 103, 5981–5991. doi: 10.1002/jsfa.12670
Eikmeyer, F. G., Koefinger, P., Poschenel, A., Juenemann, S., Zakrzewski, M., Heinl, S., et al. (2013). Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J. Biotechnol. 167, 334–343. doi: 10.1016/j.jbiotec.2013.07.021
Franco, M., Tapio, I., and Rinne, M. (2022). Preservation characteristics and bacterial communities of crimped ensiled barley grains modulated by moisture content and additive application. Front. Microbiol. 13:1092062. doi: 10.3389/fmicb.2022.1092062
Garde, S., Gomez-Torres, N., Hernandez, M., and Avila, M. (2014). Susceptibility of Clostridium perfringens to antimicrobials produced by lactic acid bacteria: Reuterin and nisin. Food Control 44, 22–25. doi: 10.1016/j.foodcont.2014.03.034
Guo, X. S., Ke, W. C., Ding, W. R., Ding, L. M., Xu, D. M., Wang, W. W., et al. (2018). Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 8:357. doi: 10.1038/s41598-017-18348-0
Guo, L. N., Yao, D. D., Li, D. X., Lin, Y. L., Bureenok, S., Ni, K. K., et al. (2020). Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front. Microbiol. 10:2998. doi: 10.3389/fmicb.2019.02998
Hang, H. T. (2022). Analysis of high-throughput transcriptome sequencing of Orychophragmus violaceus seedlings. Pol. J. Environ. Stud. 31, 3561–3571. doi: 10.15244/pjoes/146557
Huang, F. Q., Wang, T. W., Zhang, J. Q., Tahir, M., Sun, J. H., Liu, Y. Y., et al. (2023). Exploring the bacterial community succession and metabolic profiles of Lonicera japonica Thunb. Residues during anaerobic fermentation. Bioresour. Technol. 367:128264. doi: 10.1016/j.biortech.2022.128264
Jiang, D., Li, B., Zheng, M., Niu, D., Zuo, S., and Xu, C. (2020). Effects of Pediococcus pentosaceus on fermentation, aerobic stability and microbial communities during ensiling and aerobic spoilage of total mixed ration silage containing alfalfa (Medicago sativa L.). Grassl. Sci. 66, 215–224. doi: 10.1111/grs.12272
Kung, L. M., Shaver, R. D., Grant, R. J., and Schmidt, R. J. (2018). Silage review: interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 101, 4020–4033. doi: 10.3168/jds.2017-13909
Li, R. R., Jiang, D., Zheng, M. L., Tian, P. J., and Xu, C. C. (2020). Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 10:17782. doi: 10.1038/s41598-020-74958-1
Li, J. F., Yuan, X. J., Desta, S. T., Dong, Z. H., Mugabe, W., and Shao, T. (2018). Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour. Technol. 257, 76–83. doi: 10.1016/j.biortech.2018.02.070
Li, P., Zhao, W. J., Yan, L. J., Chen, L. Y., Chen, Y. L., Gou, W. L., et al. (2022). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan plateau. Bioresour. Technol. 347:126347. doi: 10.1016/j.biortech.2021.126347
Muck, R. E. (2010). Silage microbiology and its control through additives. Rev. Bras. Zootec. 39, 183–191. doi: 10.1590/s1516-35982010001300021
Muck, R. E. (2013). Recent advances in silage microbiology. Agric. Food Sci. 22, 3–15. doi: 10.23986/afsci.6718
Ni, K. K., Wang, F. F., Zhu, B. G., Yang, J. X., Zhou, G. A., Pan, Y., et al. (2017). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. doi: 10.1016/j.biortech.2017.04.055
Niu, D. Z., Zheng, M. L., Zuo, S. S., Jiang, D., and Xu, C. C. (2018). Effects of maize meal and limestone on the fermentation profile and aerobic stability of smooth bromegrass (Bromus inermis Leyss) silage. Grass Forage Sci. 73, 622–629. doi: 10.1111/gfs.12355
Owens, V. N., Albrecht, K. A., Muck, R. E., and Duke, S. H. (1999). Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total nonstructural carbohydrates. Crop Sci. 39, 1873–1880. doi: 10.1046/j.1365-2494.2002.00333.x
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S. J. W. H. O., and Spoelstra, S. F. (2003). “Microbiology of ensiling” in Silage science and technology. eds. D. R. Buxton, R. E. Muck, and J. H. Harrison (Madison, WI: American Society of Agronomy), 31–93.
Price, E. P., Arango, V. S., Kidd, T. J., Fraser, T. A., Nguyen, T. K., and Bell, S. C. (2020). Duplex real-time PCR assay for the simultaneous detection of Achromobacter xylosoxidans and Achromobacter spp. Microb. Genom. 6:000406. doi: 10.1099/mgen.0.000406
Sun, H., Liao, C. S., Chen, L. Y., Cheng, Q. M., Zheng, Y. L., Wang, C. M., et al. (2023). Potential for volatile fatty acid production via anaerobically-fermenting rice straw pretreated with silage effluent and phenyllactic acid. Bioresour. Technol. 369:128355. doi: 10.1016/j.biortech.2022.128355
Sun, J. J., Wang, J., Bai, C. S., Zhao, J. M., Yun, Y., Yu, Z., et al. (2023). Natural fermentation quality, bacteria, and functional profiles of three cuttings of alfalfa silage in a year in Inner Mongolia, China. Front. Microbiol. 14:1083620. doi: 10.3389/fmicb.2023.1083620
Vansoest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, W. B., Cai, X. Y., Shao, T., Yangzong, Z., Wang, W. K., Ma, P. F., et al. (2022). Effects of bacterial inoculants on the microbial community, mycotoxin contamination, and aerobic stability of corn silage infected in the field by toxigenic fungi. Chem. Biol. Technol. Agric. 9:93. doi: 10.1186/s40538-022-00364-6
Wang, X. F., Haruta, S., Wang, P., Ishii, M., Igarashi, Y., and Cui, Z. J. (2006). Diversity of a stable enrichment culture which is useful for silage inoculant and its succession in alfalfa silage. FEMS Microbiol. Ecol. 57, 106–115. doi: 10.1111/j.1574-6941.2006.00099.x
Wang, H. L., Ning, T. T., Hao, W., Zheng, M. L., and Xu, C. C. (2016). Dynamics associated with prolonged ensiling and aerobic deterioration of total mixed ration silage containing whole crop corn. Asian Australas. J. Anim. Sci. 29, 62–72. doi: 10.5713/ajas.15.0319
Wang, S. R., Zhao, J., Dong, Z. H., Li, J. F., Kaka, N. A., and Shao, T. (2020). Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 309:123371. doi: 10.1016/j.biortech.2020.123371
Xu, Z. X., Li, B., Tian, Y., Li, M., Dong, J. X., and Zhang, G. J. (2022). Chemical constituents from the aerial parts of Orychophragmus violaceus. Nat. Prod. Res., 1–9. doi: 10.1080/14786419.2022.2118745
Zhang, T., Li, L., Wang, X. F., Zeng, Z. H., Hu, Y. G., and Cui, Z. J. (2009). Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage. World J. Microbiol. Biotechnol. 25, 965–971. doi: 10.1007/s11274-009-9973-x
Zhang, Y., Liu, Y., Meng, Q., Zhou, Z., and Wu, H. (2020). A mixture of potassium sorbate and sodium benzoate improved fermentation quality of whole-plant corn silage by shifting bacterial communities. J. Appl. Microbiol. 128, 1312–1323. doi: 10.1111/jam.14571
Zheng, M. L., Niu, D. Z., Jiang, D., Li, R. R., Meng, L., and Xu, C. C. (2020). Metagenome analyses reveal the role of Clostridium perfringens in alfalfa silage anaerobic deterioration. FEMS Microbiol. Lett. 367:fnaa052. doi: 10.1093/femsle/fnaa052
Zheng, M. L., Niu, D. Z., Jiang, D., Zuo, S. S., and Xu, C. C. (2017). Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J. Appl. Microbiol. 122, 1456–1470. doi: 10.1111/jam.13456
Zheng, M. L., Niu, D. Z., Zuo, S. S., Mao, P. C., Meng, L., and Xu, C. C. (2018). The effect of cultivar, wilting and storage period on fermentation and the clostridial community of alfalfa silage. Ital. J. Anim. Sci. 17, 336–346. doi: 10.1080/1828051x.2017.1364984
Keywords: Orychophragmus violaceus, silage, lactic acid bacteria, fermentation quality, bacterial community
Citation: Zheng M, Mao P, Tian X and Meng L (2023) Effects of exogenous lactic acid bacteria and maize meal on fermentation quality and microbial community of Orychophragmus violaceus silage. Front. Microbiol. 14:1276493. doi: 10.3389/fmicb.2023.1276493
Received: 21 August 2023; Accepted: 05 September 2023;
Published: 21 September 2023.
Edited by:
Ping Li, Guizhou University, ChinaReviewed by:
Qing Zhang, South China Agricultural University, ChinaCopyright © 2023 Zheng, Mao, Tian and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Meng, bWVuZ2xpbjk1OTlAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.