- 1Laboratory “Health, Systemic, Process” (P2S), UR4129, University Claude Bernard Lyon 1, Lyon, France
- 2Public Health Service, Department of Dentistry, Cheikh Anta Diop University, Dakar, Senegal
- 3Laboratory “Research on Healthcare Performance” (RESHAPE), INSERM U1290, University Claude Bernard Lyon 1, Lyon, France
- 4Hospices Civils of Lyon, Lyon, France
Steroid hormones and the oral microbiota of pregnant women both appear as cumulative risk factors for gingivitis. This cross-sectional study, using real-time PCR, investigated the composition and diversity of the microbiota in interdental spaces of 3 months pregnant women with intact periodontium according the 2018 EFP/AAP classification. Bacteria identified were belonged to the red (Porphyromonas gingivalis Treponema denticola, and Tanerella forsythia), orange (Fusobacterium nucleatum, Prevotella intermedia, Campylobacter rectus, and Parvimonas micra), and green (Eikenella corrodens and A. actinomycetencomitans) Socransky complexes. Approximatively 109.11 bacteria were counted per interdental space in pregnant women. Bacteria from the red complex represented 33.80% versus 62.81% for the orange group versus 3.39% for the green group of the total number spread over the 3 groups. Dietary habits and physical activity did not have a significant impact on interdental microbiota, although a decrease in the median amount of 9 periodontopathogens was observed when fruit and vegetable consumption increased. Pregnant women who brushed their teeth at least twice a day had lower counts of total bacteria and 9 periodontal pathogens than those who brushed less. In 3 months pregnant women at high risk of periodontal disease (>30% bleeding sites), the dendogram revealed 2 clusters of the 9 periodontopathogens. This provides further support for the “key pathogen” hypothesis, among which Porphyromonas gingivalis plays a key role, indicating that specific bacteria in limited quantities can influence the host immune system and convert the microbiota from symbiotic to dysbiotic to induce inflammatory disorder. As a result, this study reported that 3 months pregnant women with healthy periodontium had high levels of interdental bleeding and a dysbiotic microbiota with periodontal pathogens of the Socransky orange and red complexes. These subjects were therefore potentially at increased risk of developing periodontal disease and, consequently, an adverse pregnancy outcome. So, preventive oral prophylaxis measures, in particular individual interdental prophylaxis, should be implemented as soon as pregnancy is established.
1. Introduction
According to the 2018 EFP-AAP classification of periodontal and peri-implant diseases and conditions, clinical gingival conditions can be observed on an intact periodontium, either in a gingival health or as gingivitis (Caton et al., 2018). However, initial clinical changes from healthy to plaque-induced gingivitis may not be detectable (Murakami et al., 2018). In fact, incipient gingivitis can be considered part of a “clinical health” spectrum, with some sites reporting mild inflammation (Murakami et al., 2018). Chronic gingivitis (ICD-10: K05.1) is a non-specific dental plaque-induced inflammatory condition characterized by gingival inflammation on an intact periodontium, or a reduced periodontium in an individual without a history of periodontitis (Chapple et al., 2018). Gingival bleeding is the most easily identifiable clinical sign of gingivitis (Baudet et al., 2020). Current evidence suggests that oral microbial dysbiosis is a primary etiological factor in gingivitis (Alnasser et al., 2023; Morrison et al., 2023), in which bacterial biofilms on the teeth and gingival tissue play a crucial role (Kharitonova et al., 2021). Moreover, environmental and host susceptibility factors may considerably impact the oral microbiote composition and lead to the onset of lifestyle-related disorders (Saadaoui et al., 2021). The 2018 EFP-AAP classification of periodontal diseases applied to gingivitis mentions local risk factors, known as predisposing factors, and systemic risk factors, so-called modifying factors (Chapple et al., 2018).
Adult interdental spaces – in fact the 30 interdental spaces for an individual – with an intact periodontium are among the most representative local risk factors for gingivitis (Carrouel et al., 2016). These ecological niches facilitate the accumulation, maturation and retention of biofilm. They also restrict access to the oral hygiene practices needed to mechanical disorganization of the biofilm. Similarly, reduced salivary flow in the interdental space has the effect of limiting cleaning of dental and gingival surfaces, and promotes increased gingival inflammation. Recent evidence has revealed that the interdental biofilm of young periodontally healthy subjects is composed of pathogens (Streptococcus spp., Streptococcus mutans, Lactobacillus spp., Enterococcus spp., and Candida albicans) that are able to induce interproximal caries (Bourgeois et al., 2017; Inquimbert et al., 2019). Virulent periodontal pathogens such as the bacteria of orange and red complex (Campylobacter rectus (C. rectus), Prevotella intermedia (P. intermedia), Parvimonas micra (P. micra), Fusobacterium nucleatum (F. nucleatum), Porphyromonas gingivalis (P. gingivalis), Tannerella forsythia (T. forsythia), Treponema denticola (T. denticola)) were found in interdental spaces indicating that the periodontal disease process could be initiated (Carrouel et al., 2016; Inquimbert et al., 2019; Kharitonova et al., 2021).
Elevations in sex steroid hormones during pregnancy is considered as a systemic risk factor, negatively influence the immune-inflammatory response to a given dental plaque biofilm burden, resulting in exaggerated or “hyper” inflammation (Chapple et al., 2018). Pregnancy includes a multitude of physiological changes: weight gain, hormonal, and metabolic changes, as well as immune changes (Nuriel-Ohayon et al., 2016). An additional important factor which both influences and is affected by these physiological processes is the oral microbiome (Ye and Kapila, 2021). Gingival tissues contain receptors for the sex hormones, estrogen and progesterone, which are susceptible to hormonal imbalances that occur in women during the pregnancy (Sathish et al., 2022). Increased sensitivity to stimuli occurs in the gingiva during pregnancy, gingival alterations associated to the biofilm formation during pregnancy may increase the severity of gingivitis (Terzic et al., 2021).
Recently, gingivitis was suggested as a new risk factor for systemic inflammation. Numerous correlations have been suggested between periodontal conditions and pregnancy complications and outcomes (Gare et al., 2021; Nannan et al., 2022). Oral microbiome composition can contribute to pregnancy complications (Gare et al., 2021; Saadaoui et al., 2021). The infected periodontal tissues act as a reservoir for bacteria that can transfer from periodontal tissues to the fetal placenta unit and trigger a metastatic infection (Bobetsis et al., 2020; Genco and Sanz, 2020).
Symptoms of gingivitis in pregnancy typically emerge during the first trimester (Giglio et al., 2009). Recent evidence has revealed that gingivitis cases and bleeding were particularly high among 3 months pregnant women. Gare et al., indicated that 93% among 3 months pregnant Senegalese women had at least one site with bleeding and 18.6% had 100% of sites with bleeding. 15% had moderate gingivitis, and 73% had severe gingivitis (Gare et al., 2023). Massoni et al., underlined that progesterone levels in the first trimester are positively correlated with P. gingivalis, and this relevance suggests that progesterone levels during this period stimulates P. gingivalis growth and elevates gingival inflammation (Massoni et al., 2019).
Current knowledge about the subgingival bacterial environment most closely related to periodontal disease in pregnant women is incomplete (Yang et al., 2019). There is limited research on the composition of the oral microbiome throughout pregnancy. There may be alterations in the oral microbiome during pregnancy such as increased relative abundance of pathogenic bacteria (Koerner et al., 2023). The distribution and clustering of oral microbiome for gingivitis among women at 3 months of pregnancy have not been explored and this especially in the interdental spaces. So, to develop better prediction and intervention approaches for adverse pregnancy outcomes, it is critical to understand the oral microbiome changes during pregnancy (Ye and Kapila, 2021).
The recent 2018 EFP/AAP classification, considered as a “precision medicine” approach, provide standardization for determining which patients with intact periodontium would be clinically diagnosed as having gingivitis in terms of prevalence and severity (Chapple et al., 2018). The cartography of bacteria based on the Socransky complexes, which represent a reference framework for the entire scientific community is a key element (Socransky et al., 1998). Six bacteria complexes often encountered in case of periodontal disease were identified and coded as blue, green, yellow, violet, orange, and red. The yellow, green, blue, and purple complexes were mainly associated with periodontal health whereas orange and red complexes were linked to periodontal disease (Socransky et al., 1998; Socransky and Haffajee, 2005). However, as previously described, recent research on bacterial quantification of gingivally healthy adolescents and young adults has shown a significant proportion of orange and red complex bacteria in the interdental spaces (Carrouel et al., 2016; Inquimbert et al., 2019). Thus, in our study, 9 periodontal bacteria (2 from green complex, 4 from the orange complex and the 3 of the red complex) were analyzed qualitatively and quantitatively by real-time PCR.
The objective of this research was to investigate in vivo the composition and diversity of the periodontal subgingival microbiota in interdental spaces of 3 months pregnancy women with an intact periodontium according the 2018 classification standard.
2. Materials and methods
2.1. Study design and setting
This cross-sectional study is a part of the randomized controlled trial OP-PE protocol published by Kanoute et al. (2021) and registered at ClinicalTrials.gov (NCT04989075). The ethical committee of Dakar (Senegal) approved the protocol on 8 June 2021 (000086/MSAS/CNERS/SP).
The multicentre study occurred in 6 National Hospital Center of Dakar (Senegal) and carried out in conformance with the Declaration of Helsinki. Written informed consent was obtained from each participant before enrolment.
This research was performed in accordance with the STROBE guidelines (Supplementary File 1).
2.2. Participants
The population was composed of 100 pregnant women at 3 months who had voluntarily presented at one of the 6 hospitals from Dakar for their first visit for a pregnancy diagnosis between March 2022 and August 2022.
Eligibility was limited to (i) women less than 12 weeks pregnant, (ii) aged 18 to 35 years, (iii) from sub-Saharan Africa, (iv) nulliparous at the time of the obstetric visit, (v) accepting the terms and conditions of the study, and (vi) signing the informed consent form.
The obstetric exclusion criteria concerned pregnant women: (i) with congenital uterine and vaginal anomalies, (ii) who had undergone premature termination of pregnancy for medical reasons, (iii) with fetal distress, and (iv) with infectious or systemic diseases.
The oral exclusion criteria concerned pregnant women: (i) having fewer than 20 natural teeth, except third molars, (ii) having none of the 4 premolar-molar pairs, (iii) having a history or treatment of PD, (iv) undergoing dental or orthodontic treatment, (v) having generalized periodontal lesions (>30% of sites) of stage II, III, IV (PD ≥ 4 mm, and/or CAL ≥ 4 mm), (vi) receiving drugs, including antibiotics, affecting the gingiva and/or oral mucosa, (vii) using dental floss and/or interdental brushes and/or mouthwash regularly, or (viii) unable to provide answers to questions or uncooperative.
2.3. Outcomes
2.3.1. Primary outcomes measures
The primary outcome was to quantify the total bacterial load as well as the 9 periodontal bacteria of the interdental microbiota in 3 months pregnant women.
2.3.2. Secondary outcomes measures
The secondary outcomes were to analyze the relationships between demographics, lifestyle factors, oral hygiene habits, oral clinical health status and periodontal pathogens load of the interdental microbiota in 3 months pregnant women.
2.4. Procedures
The screening of pregnant women was realized during their first prenatal visit. After presentation of the study, pregnant women who met the inclusion criteria and consented to participate were invited to the inclusion visit scheduled at 3 months of pregnancy.
Included pregnant women signed an informed consent form, completed a questionnaire, and underwent obstetrical, oral clinical examinations and interdental sampling. All this information was recorded on an electronic medical record (e-CRF Voozalyon 1.3; Voozanoo, Caluire, France).
2.4.1. Demographic and behavioral features
Sociodemographic characteristics, oral hygiene habits, lifestyle factors, and medication use were obtained using a questionnaire. Pregnant women were considered to have insufficient vegetable and fruit intake if they declared eating less than five portions per day, so at least 400 g (or 5 servings) of vegetables and fruits per day (Nishida et al., 2004; Wang et al., 2014). Pregnant women who performed less than 150 min of moderate-intensity physical activity (600 metabolic equivalents of task (METs)) per week were classified as insufficiently physically active. Inactivity of less than 150 min of moderate physical activity per week (i.e., 30 min a day, 5 days a week) or 75 min of vigorous physical activity (25 min, 3 days a week) is sedentary (Bull et al., 2020).
2.4.2. Prenatal clinical status evaluation
Measurement of weight (kg) and height (cm) using a stadiometer and a calibrated clinical scale were performed during the obstetric clinical examination. Body mass index (BMI) was calculated. Pregnant women were classified as underweight (BMI < 18.5 kg/m2), healthy (18.5 kg/m2 ≤ BMI < 25 kg/m2), overweight (25.0 kg/m2 ≤ BMI < 30 kg/m2), or obese (BMI ≥ 30.0 kg/m2) (CDC, 2022).
2.4.3. Oral clinical examination
Trained and calibrated periodontists performed the clinical examination. The kappa statistic between different examiners was 0.78 (95% CI: 0.14–1.38). Clinical examinations of the whole mouth, including probing depth (PD), clinical attachment level (CAL), gingival index (GI) and plaque index (PI), were carried out by a practitioner in a clinical center using a sterile Williams PDT probe at 20 g pressure (Zila-Pro-Dentec Inc., Batesville, AR, United States) located parallel to the long axis of the tooth (Löe, 1967).
Bleeding on the interdental brushing index (BOIB) corresponds to the bleeding response to horizontal pressure applied in the interdental area by a calibrated interdental brush (IDB) and was performed as described by Bourgeois et al. (2016). A score of 0 corresponds to no bleeding after 30 s and a score of 1 to bleeding after 30 s (Caton and Polson, 1985; Hofer et al., 2011).
Gingivitis on intact periodontium and gingivitis on reduced periodontium in a patient with no history of periodontitis are defined as bleeding sites ≥10% with probing depths ≤3 mm. Localized gingivitis is defined as 10–30% bleeding sites, and generalized gingivitis as >30% bleeding sites (Trombelli et al., 2018).
The diameter of all interproximal spaces of four pairs of teeth (premolars-molars) was assessed using an IAP CURAPROX© colorimetric probe (Curaden, Kriens, Switzerland). Participants were not to brush, drink, eat or practice oral hygiene for 3 h prior to the sampling visit.
2.4.4. Interdental sampling
For all pregnant women, the same four interdental sites (15–16, 25–26, 35–36, and 45–46) were evaluated (100 sites in total). If one of the sites was missing due to the absence of a tooth, the adjacent medial site was sampled as a replacement. The appropriate interdental brushes (Curaden, Kriens, Switzerland) were determined during the clinical assessment of the interdental spaces (Bourgeois et al., 2015). Selected teeth were isolated using sterile cotton rolls. Interdental biofilm was collected using a sterile, calibrated interdental brush (IDB) introduced and then removed from the interdental space (Carrouel et al., 2016). The 4 IDBs from one pregnant woman were pooled into 1 sterile 1.5 mL microcentrifuge tube and stored at 4°C for further processing.
2.4.5. Microbiological analysis
2.4.5.1. DNA extraction
Total DNA was extracted from IDBs using the QIAcube HT Plasticware and Cador Pathogen 96 QIAcube HT Kit (Qiagen, Hilden, Germany), according to manufacturer’s guidelines. Using UV at 260 and 280 nm, the quality and quantity were measured.
2.4.5.2. Quantification of periodontal pathogens from the interdental microbiota
To quantify the total bacterial load (TB) and that of 9 pathogen species (E. corrodens, A. actinomycetemcomitans, C. rectus, P. intermedia, P. micra, F. nucleatum, P. gingivalis, T. forsythia, T. denticola) present in the biofilm interdental samples, the method described previously was used (Carrouel et al., 2016).
2.4.6. Statistical analysis
The statistical analysis consisted of three main steps: producing descriptive summaries of the data, modeling the data using a mixed (linear) model and assessing the correlations between bacterial abundances. SPSS Windows 20.0 (IBM, Chicago, IL, United States) was used for the descriptive statistics median values and interquartile range (IQR) and mean values with SD for the quantitative variables and percentages for categorical variables. Microbiological variables will be presented as total periodontal bacteria counts, frequency of detection of target pathogens, counts of each pathogen studied, and proportions of each pathogen in the total microbiota. Total periodontal counts will be log-transformed to match a normal distribution.
Descriptive bivariate analyses between participant characteristics and the outcome, gingivitis, were evaluated using t-tests and logistic regression for continuous and binary/categorical variables, respectively. Kruskal–Wallis tests were performed to compare the mean counts for the different bacterial species relative to each clinical characteristic. The detailed statistical methods are indicated in the table footnotes. Shapiro–Wilk test was used to check the normality of the distribution. Results will be considered statistically significant at p < 0.05.
Principal component analysis (PCA) was performed to identify clusters of variables highly associated with each other and to visually assess whether groups with and without these outcomes of interest could be distinguished using the variables identified in both univariate and multivariate analyses. Briefly, the dataset was dimensionally reduced into linear variables termed principal components (PCs) with those possessing eigenvalues >1 included in further analyses. Loadings were computed against PCs to determine correlations, which were visualized in PC biplots. Using matplotlib.pyplot (V.3.7.1) in Jupyter Notebooks. A Min-Max normalization was done for the non-(0-1) variables. A correlation circle was used to figure the results. The main idea of these analyses is thus to assess whether pregnant women with and without a given outcome of interest may be regrouped within two different groups or, on the contrary, cannot be distinguished.
3. Results
3.1. Characteristics of pregnant women
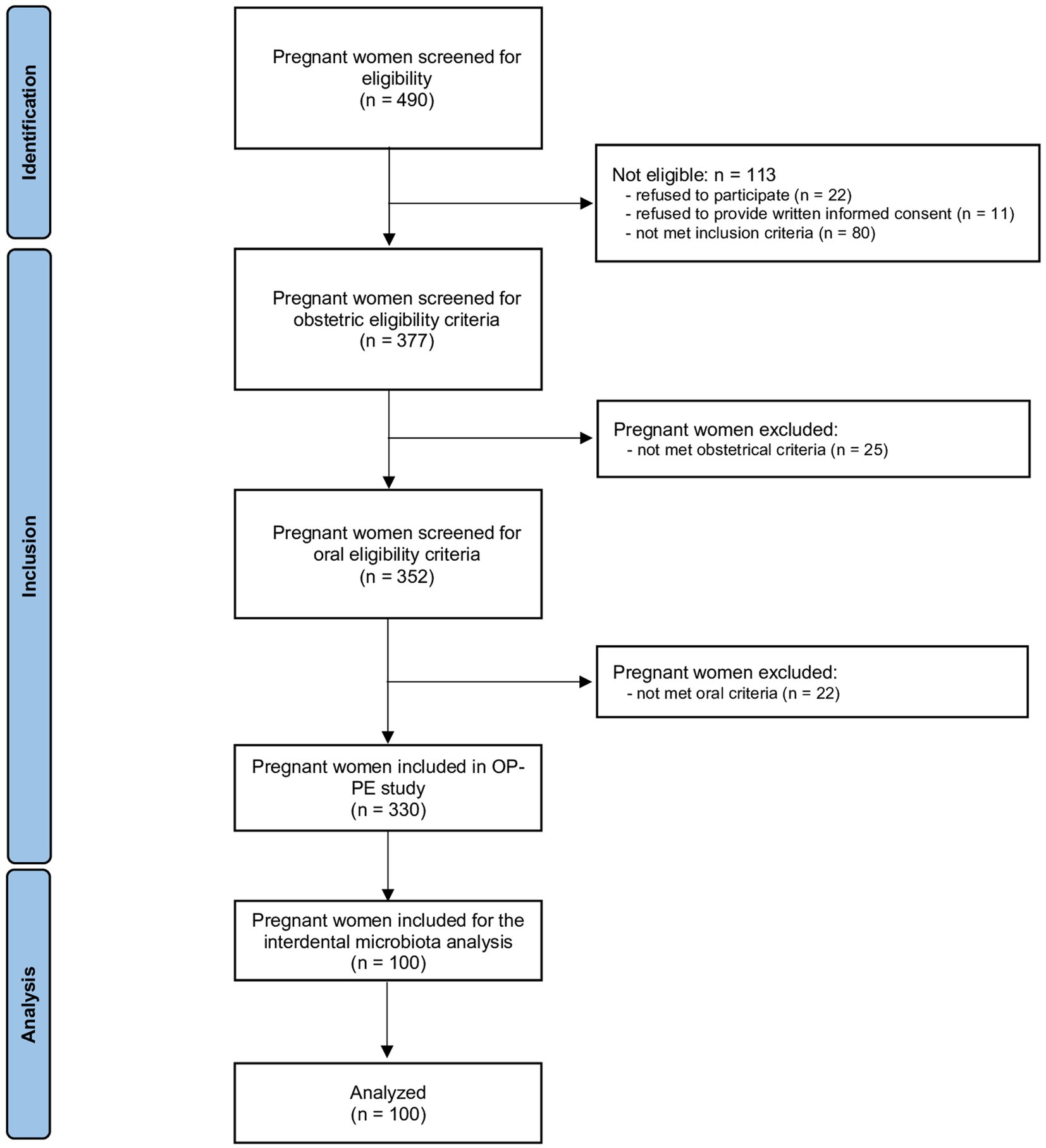
The flow chart is presented in Figure 1. Of the 490 pregnant women who was accessed for eligibility, 113 were ineligible, 25 did not meet obstetrical inclusion criteria and 22 did not meet oral inclusion criteria. Among the 330 women included in OP-PE study, 100 were randomly selected to analyse the interdental microbiota and are included in this study.
3.1.1. Demographic characteristics
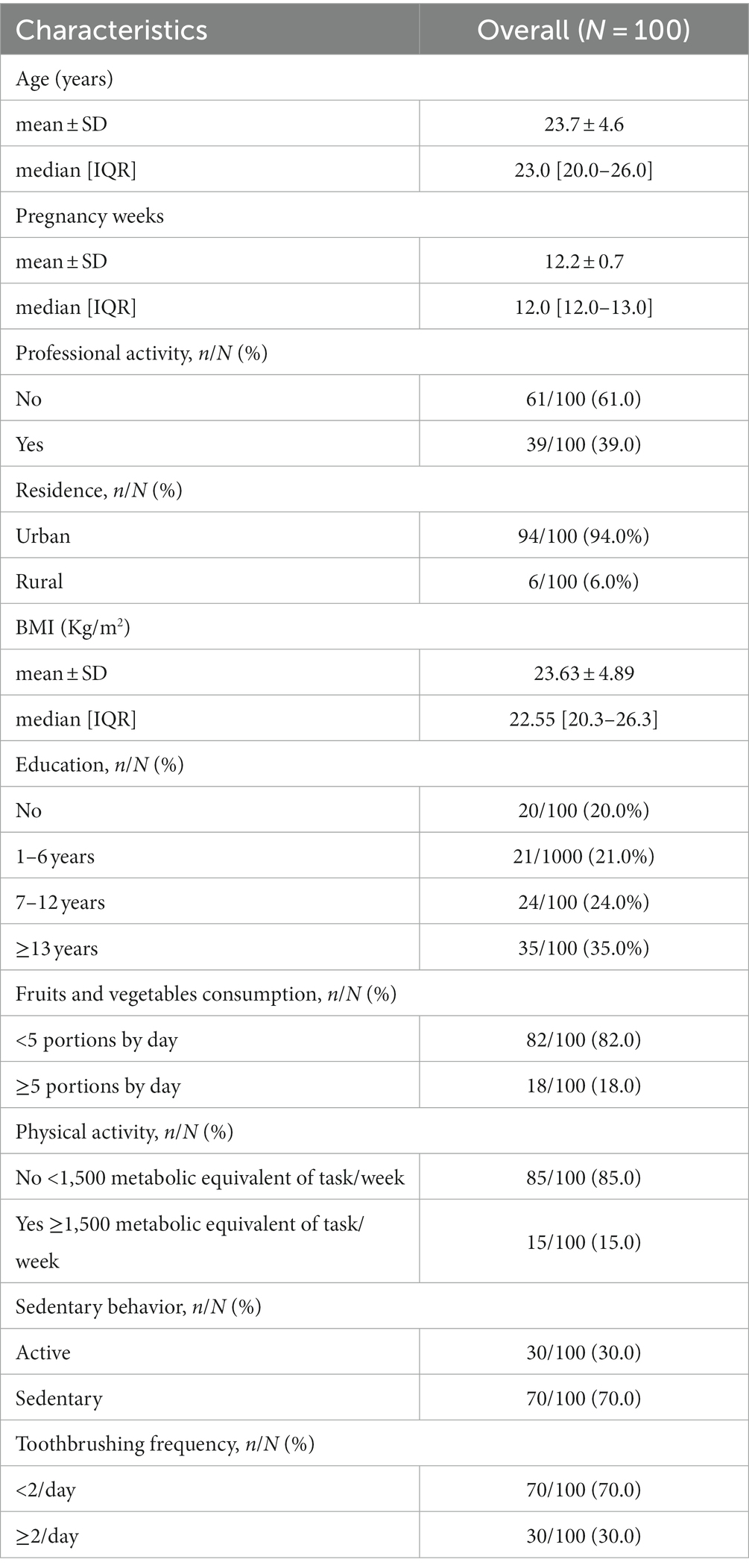
Table 1 presents characteristics of the pregnant women. Among the 100 pregnant women included, the median age was 23.0 [20.0–26.0] years. Of these, 94.0% (n = 94) were urban dwellers, 20.0% (n = 20) had never been to school, and 61.0% (n = 61) had no professional activity. No women were current smokers and 1% (n = 1) reported harmful alcohol use. The pregnant women had a median BMI of 22.55 Kg/m2 (IQR 20.3–26.3). Among them, 12.0% (n = 12) were classified as underweight, 58.0% (n = 58) normal, and 30.0% (n = 30) obese. In addition, 82.0% (n = 82) declared consumed less than 5 fruits and vegetables per day and 70.0% (n = 70) reported a sedentary lifestyle during the first 3 months of pregnancy. In terms of oral hygiene, 30% (n = 30) of participants reported cleaning their teeth at least twice a day with a toothbrush, 87% (n = 87) reported using a toothbrush alone, 1% (n = 1) a dental stick and 12% (n = 12) both.
3.1.2. Clinical characteristics
The clinical parameters of the pregnant women are described in Table 2. In 32.0% of cases, pregnant women had sound teeth (no cavities, no fillings, no missing teeth due to cavities). The DMFT index was very low (<3) for 86.0% (n = 86) of pregnant women. More than 1 decayed tooth was observed for 37.0% (n = 37) of cases and one or more missing teeth was reported for 30% (n = 30) of cases. Conservative care was observed for 9.0% (n = 9) of pregnant women.
Interdental bleeding was described at least once for 94.0% of the pregnant women, and the median was 54.0% (IQR 29.0–82.0). While 7% of the women had 100% of the sites bleeding, 8% had no bleeding on interdental brushing. Gingivitis (gingival bleeding score ≥10%) was present in 87.0% of pregnant women, generalized gingivitis in 14.0%, and localized gingivitis in 73.0%.
3.2. Quantification of the total number of bacteria and 9 periodontopathogens
3.2.1. Quantification of Socransky complexes
The 9 periodontopathogens analyzed in this study were grouped according to the green, orange and red complexes defined by Socransky et al. (1998). The percentages of each of these complexes per pregnant women were analyzed (Figure 2). The main complex was the orange complex, which represents an average of 62.81%, followed by the red complex (33.80%) and the green complex (3.39%). The percentage of each complex depend on the women. One woman did not have bacteria from the red complex whereas for 26 women the red complex represented more than 50% of periodontopathogens tested.
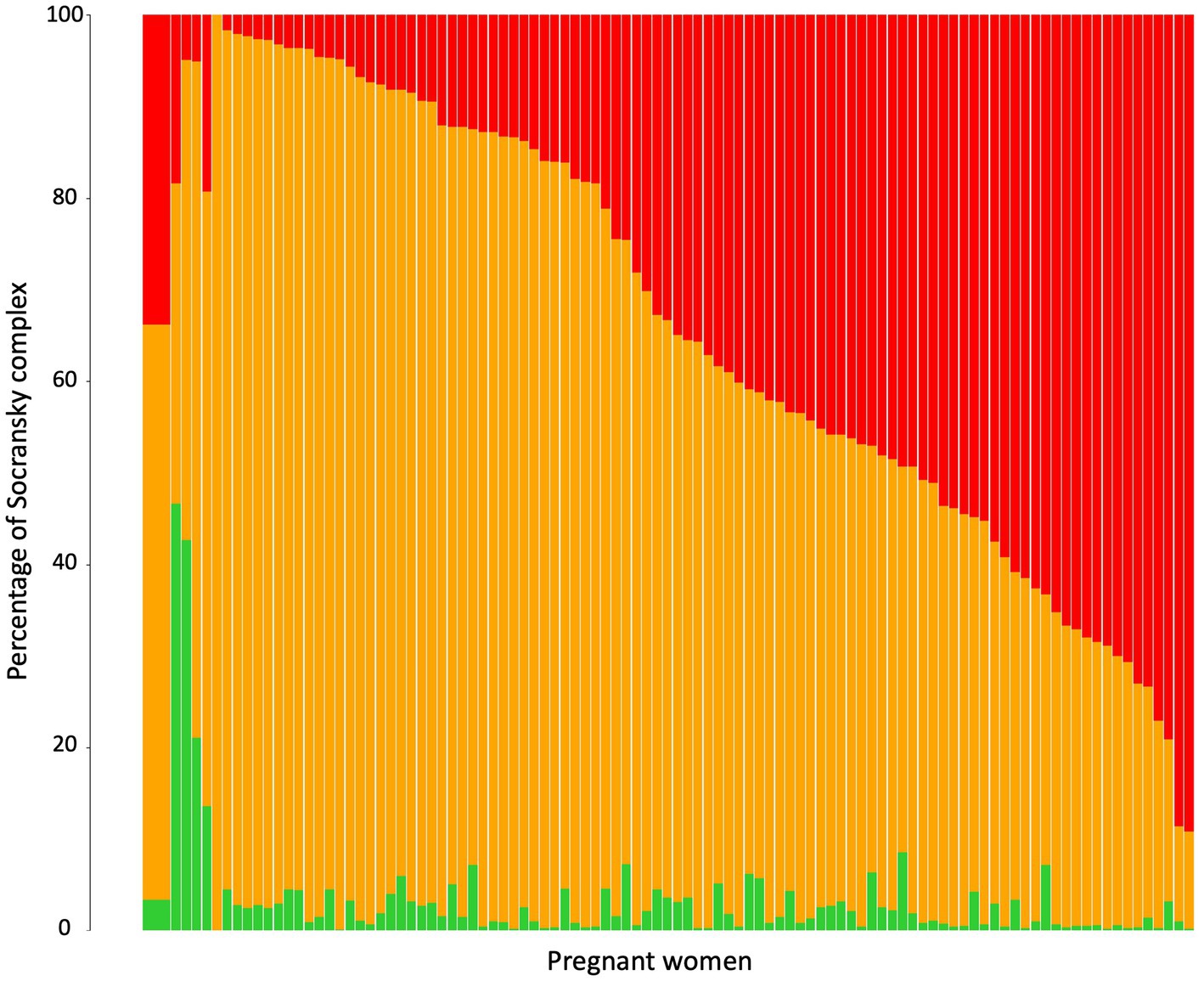
Figure 2. Relative abundance of Socransky complexes among the pregnant women. Percentage of Socransky complex = Quantity of periodontopathogens for one Socransky complex/Quantity of the 9 periodontopathogens. The first bar displays the average proportion of each complex in all pregnant women included. The other bars display the average proportion of each complex in one pregnant woman. The colors refer to the colors of the Socransky complexes: Green = Ec + Aa, Orange = Cg + Cr + Pi + Pm + Fn, and Red = Pg + Tf + Td. Avg, average.
3.2.2. Quantification of periodontopathogens in relation to demographic, behavioral, and clinical parameters
Table 3 shows the mean total bacterial load and that of the 9 periodontopathogens in the interdental microbiota of pregnant women. On average, the microbiota of an interdental site consisted of 109.11 bacteria. Bacteria from the orange and red complexes were present in greater quantities (From 106.38 for C. rectus to 107.35 for P. micra) than those from the green complex. F. nucleatum and C. rectus were present in all the pregnant women whereas P. intermedia was present in 51% of women. No significant effect of age, fruits and vegetables consumption, metabolic equivalent of task/week (MET) or body mass index (BMI) factors was observed.
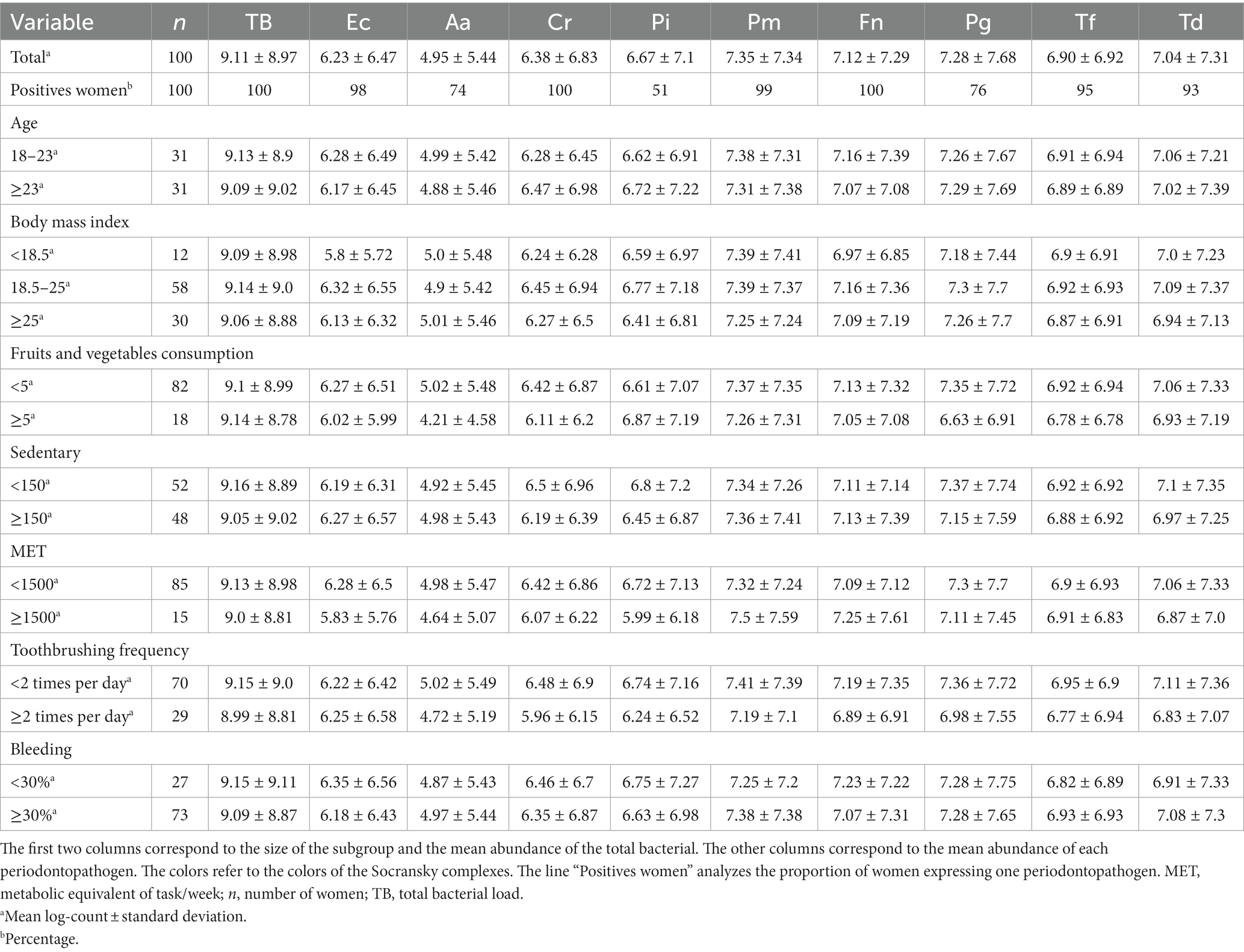
Table 3. Average abundances for total number of bacteria and 9 periodontopathogens in various subgroups of the cohort of pregnant women.
Figure 3 and Supplementary Figure S1 presents the repartition of the abundance of total bacteria and 9 periodontopathogens. Concerning the age, the BMI, fruits, and vegetables consumption, sedentarity and MET did not impact significantly on the quantity of bacteria. However, a decrease of the median quantity of the 9 periodontopathogens with the increase of consumption of fruits and vegetable consumption was observed. The average of TB and P. intermedia significantly higher in sedentary pregnant women than in active women. Pregnant women who report brushing their teeth at least twice a day have a significantly lower number of total bacteria, P. micra, F. nucleatum, and T. denticola. The number of other periodontopathogens was also reduced but not significantly. Concerning the bleeding, pregnant women with more than 30% of bleeding, have a significant lower quantity of the orange complex bacteria and F. nucleatum, but a very significant higher quantity of red complex bacteria, P. gingivalis and T. denticola.
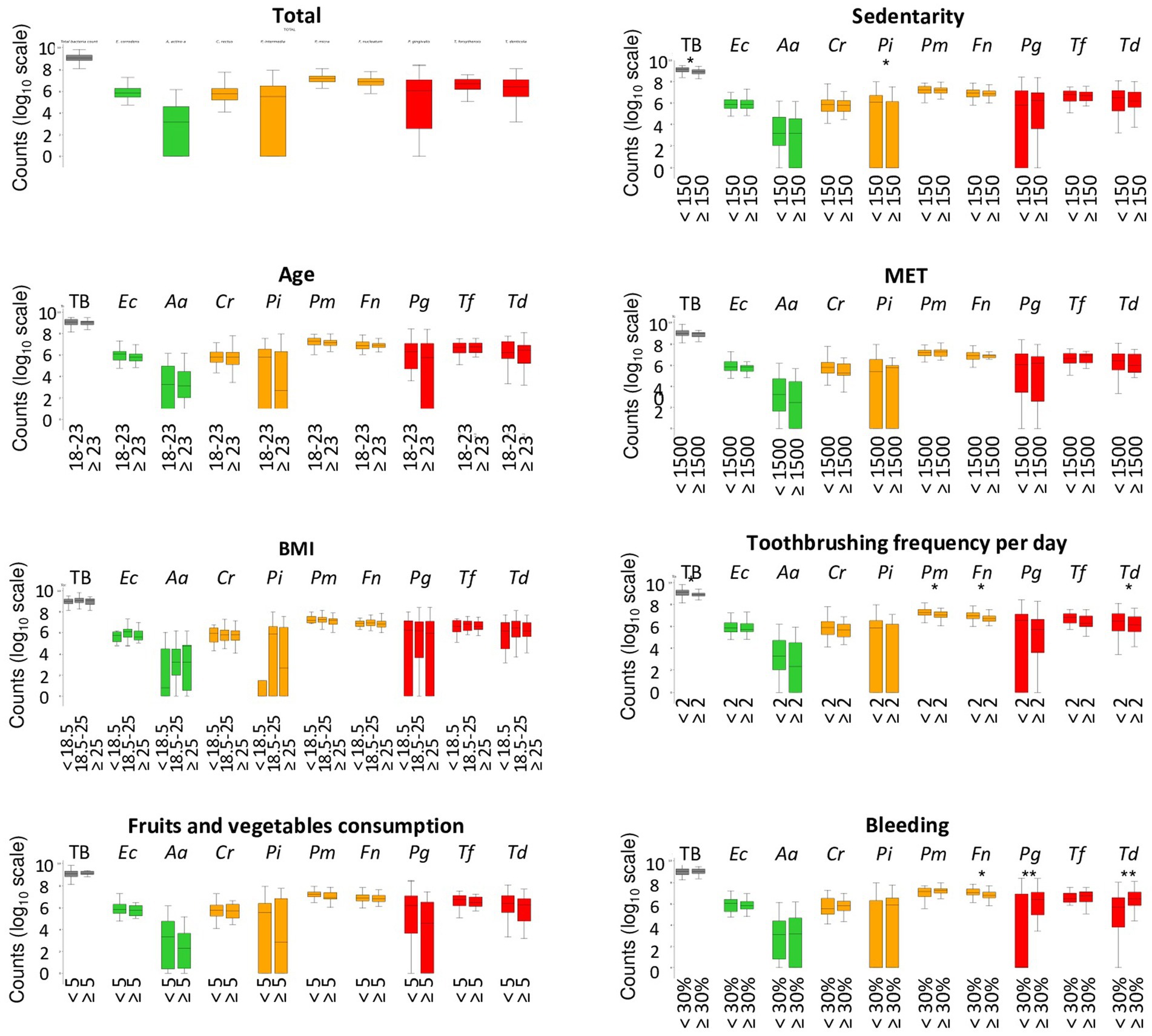
Figure 3. Abondance of periodontopathogens among pregnant woman in relation to demographic, clinical, and behavioral parameters. Systematic significance tests were performed between the periodontopathogen abundances and the tested experimental factors (age, body mass index, fruits, and vegetables consumption, MET, Sedentary, toothbrushing frequency, and bleeding). The stars indicate that the test is significant (*p < 0.05) and highly significant (**p < 0.01). MET, metabolic equivalent of task/week; TB, total bacteria; TG, total of bacteria from the green complex (Ec + Aa); TO, total of bacteria from the orange complex (Cr + Pi + Pm + Fn); TR, total of bacteria from the red complex (Pg + Tf + Td).
3.2.3. Dimension reduction and clustering by PCA in the orange and red periodontopathogens subgroups
Figure 4 presents the correlation plot from the principal component analysis (PCA) applied to demographic, behavioral, clinical parameters, and periodontopathogens from the orange and red complexes. A Bartlett sphericity test was performed and assessed the variables were dependent (value of p < 0.0001). The Kaiser–Meyer–Olkin overall measure of sampling adequacy was 0.51, which indicate a moderate level of sampling adequacy for PCA. The PCA revealed 4 principal components accounted for 55%, 88% of the overall variation of the data produced from the 19 active variables. The first 4 PCs, representing 17,09%, 14,86%, 14,00%, and 9,93% variance respectively, were included in PCA correlations with bleeding percentage; determined using Cattell’s scree plot. Two biplots (correlation circles) were chosen (Figures 4A,B), one according to PC1 and PC2 accounting for 31,95% of the total variations and one according to PC3 and PC4 accounting for 23,93% of the total variations considering a relative low contribution of each component. One third of the vectors in both biplots are of modest size, so the results regarding these variables must be taken with caution.
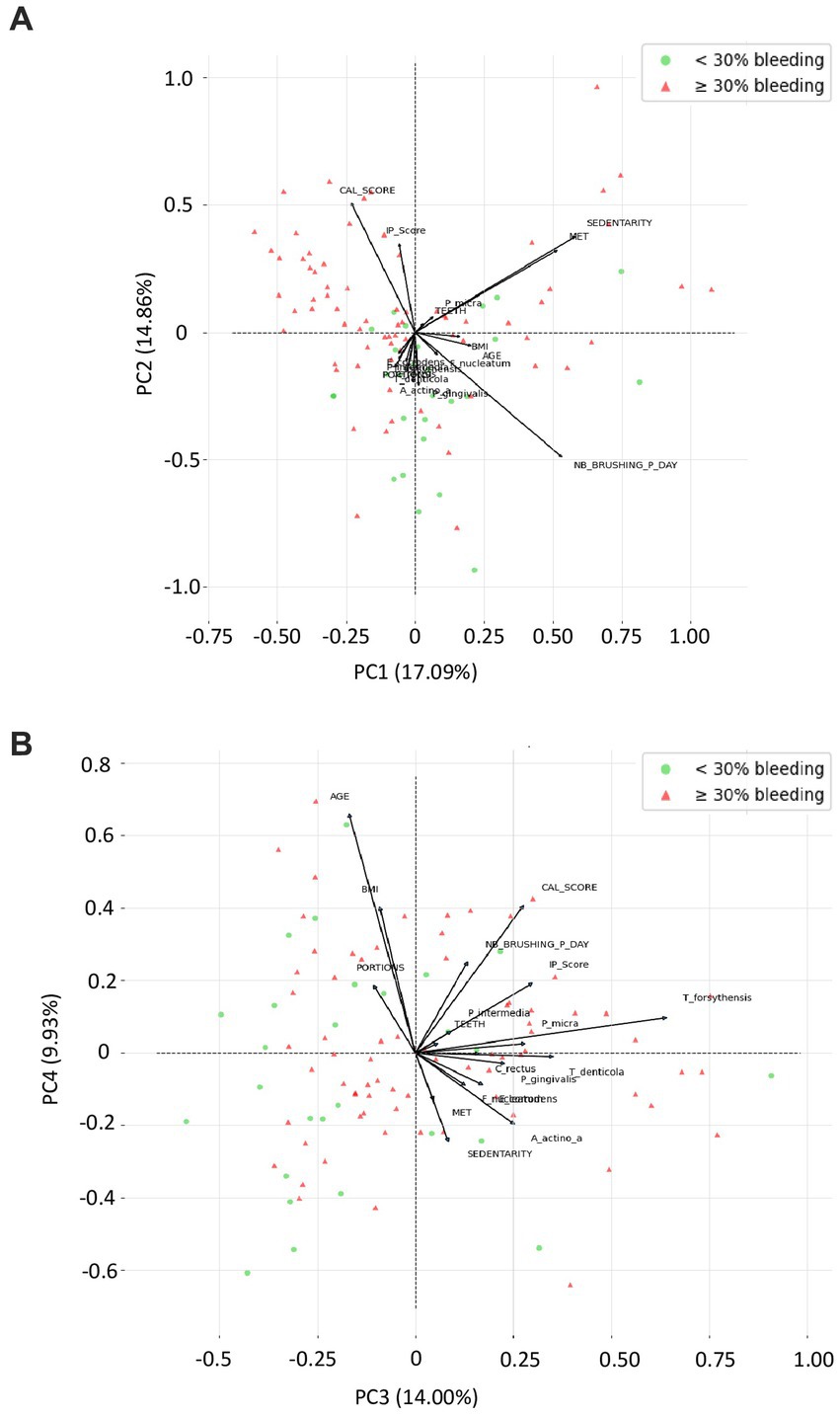
Figure 4. Variable factor map from the principal component analysis (PCA) applied to demographic, behavioral, clinical parameters, and periodontopathogens from the orange and red complexes. These two correlations circles are a visualization displaying how much the original active variables are correlated with the first two principal components. Moreover, bleeding <30% (green dots) and bleeding ≥30% (red dots) are the active observations represented according to the first 2 components computed using principal component analysis of Scikit-Learn. In this PCA, on each correlation circle, active variable is represented by vectors in a 2-dimensional space in such a manner that the closer 2 vectors are, the more women share similar characteristics. If 2 arrows are close, the angle between them is close to 0 and Cos (0°) = 1, so their correlation coefficient is close to 1. If 2 arrows are perpendicular, then the angle between them is 90° and Cos (90°) = 0, so their correlation coefficient is close to 0. BMI, body mass index; CAL_score, clinical attachment loss score; IP_score, plaque index score; MET, metabolic equivalent of task/week; Nb_brushing_p_day, number of toothbrushing per day; Portions, vegetable and fruit intake per day; SEDENTARY, Sedentary behavior; Teeth, number of teeth.
According to Figure 4A, C. rectus, E. corrodens, and A. actinomycetemcomitans are nearly 100% positively correlated with PC1, while, sedentarity, IP score are highly negatively correlate to PC2, and while P. micra, T. forsythensis, F. nucleatum, T. denticola tend to be correlated with a combination of PC1 and PC2. PC1 seem to be highly linked to bacterial identification, as PC2 seem to be more related to pregnant women behavior. BMI, age, and no teeth brushing had low contribution. Bleeding lower than 30% seem to be highly dispersed. E. corrodens and C. rectus seem to be almost collinear, as MET and sedentarity with their respective PC.
According to Figure 4B, CAL score seems to be nearly 100% negatively correlated with PC3, as portions with PC4. Age, BMI and the frequency of tooth brushing per day, tend to be negatively correlated with a combination of PC3 and PC4, as sedentarity and MET tend to be positively correlated with a combination of PC3 and PC4. PC3 seem to be highly linked to pregnant women behavior, as PC4 seem to be more related to pregnant women socio-demographic characteristics. Most bacteria had low contribution. In this projection, bleeding <30% seem to be more frequent when variables are highly correlates with PC3, but we cannot formulate a clear hypothesis about pregnant behavior as involved in bleeding occurrence considering discrepancy with first biplot.
3.2.4. Correlation of periodontopathogens in relation to the bleeding
Figure 5 shows the correlations between the 9 periodontopathogens and the interdental site of the 100 pregnant women according to the percentage of bleeding. The dendogram analyzing all the pregnant women underlines the presence of 2 clusters but with a low correlation between bacteria. The first is composed of bacteria from the orange and red complexes (F. nucleatum, P. micra, T. forsythia and P. gingivalis). The second is composed of the bacteria from the green, orange and red complexes (C. rectus, E. corrodens, T. denticola, A. actinomycetemcomitans, and P. intermedia). The 3 bacteria of the red complex belong to 2 different clusters but are in close contact. For pregnant women presenting less than 30% of bleeding, a cluster composed of 5 highly correlated periodontopathogens (F. nucleatum, C. rectus, T. forsythia, E. corrodens, and T. denticola) appears. For pregnant women presenting more than 30% of bleeding, periodontopathogens are organized in 2 clusters. The first one is composed of bacteria from the orange complex (F. nucleatum, P. micra, and P. intermedia) and the red complex (P; gingivalis and T. forsythia) but the correlation is low between bacteria. The second one is composed of bacteria of the green, orange and red complexes and underlines a high correlation between T. denticola, C. rectus, A. actinomycetemcomitans and a low correlation with T. forsythia.
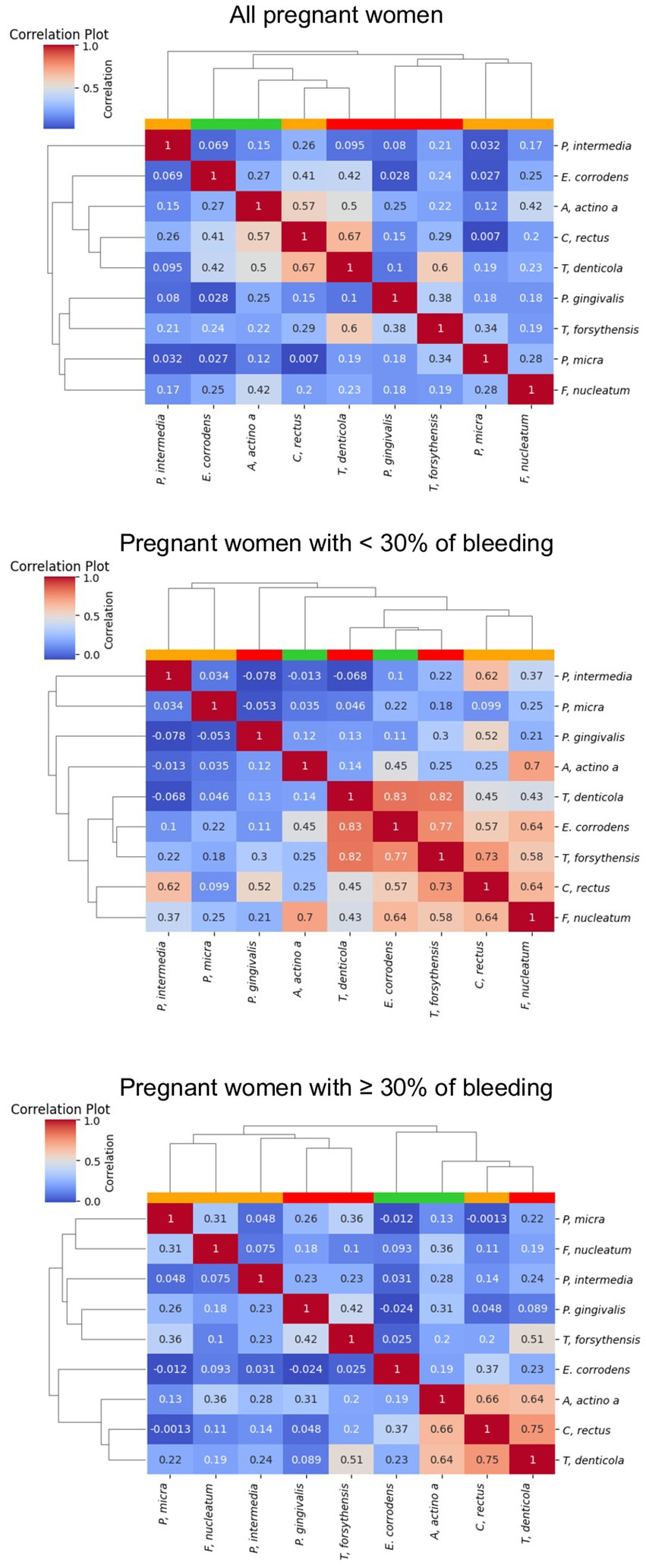
Figure 5. Correlation plot of the abundance of the periodontopathogens. The red, white, blue squares indicate positive, zero, negative correlations, respectively. The colored leaves on the top dendrogram represent the color of Socransky complexes.
4. Discussion
To our knowledge, this study is the first to analyze qualitatively and quantitatively the total number of bacteria and 9 periodontopathogens present in the interdental microbiota of 3 months pregnant women with intact periodontium according the 2018 classification standard (Chapple et al., 2018). A characterization of the oral microbiota of pregnant women with gingivitis could greatly help to establish effective ways to prevent and treat these diseases and decrease risk factors conducing to adverse pregnancy outcomes (Jang et al., 2021). More particularly, it is important to analyze the interdental microbiota of pregnant women because previous studies have demonstrated that the interdental space is a reservoir of periodontopathogens, even in healthy young people without clinical signs of periodontal disease (Carrouel et al., 2016).
Gingivitis – plaque induced – in our study was present in 87.0% of 3 months pregnant women. If classically, epidemiologic data have shown plaque-induced gingivitis to be prevalent at all ages in dentate populations (Mostafa and El-Refai, 2018), we must be aware that additional researches were necessary to obtain standardized information due to the heterogeneity of these studies including different target group and in a context where the definition of gingivitis prior to the 2018 classification was unclear (Gare et al., 2023). Characterizing the oral microbiome of healthy gingival pregnant women is an effective initial stage to understand the role of the microbiome in the progression of pregnancy-induced gingivitis (Balan et al., 2021). As mentioned by Murakami et al. (2018), it must be considerate that the intensification of the inflammatory gingival response, whether due to changes in sex steroid hormone secretions or pathogenic biofilms, represent an individual’s protective responses to systemic and local environments by destroying, attenuating and isolating invasive microorganisms (Murakami et al., 2018).
Our study indicated that one interdental space of pregnant women contains approximatively 109.11 bacteria. For information, in periodontally healthy young adults aged 20–35, an interdental site contained 1010 bacteria (Carrouel et al., 2016) and in periodontally healthy adults aged 35–56, the supragingival biofilm of the interdental space contained 107.56 bacteria (Field et al., 2012).
Periodontopathogens were identified in interdental microbiota of pregnant women. The 9 periodontopathogens are organized into clusters with a similar organization that the complexes of Socransky et al. (1998). The periodontopathogens were present in pregnant women (green complex: E. corrodens in 98% of pregnant women and A. actinomycetencomitans in 74% of pregnant women); orange complex: P. intermedia in 51% of pregnant women; P. micra in 99% of pregnant women; C. rectus and F. nucleatum in 100% of pregnant women; red complex: P. gingivalis in 76% of pregnant women; T. denticola in 93% of pregnant women; T. forsythia in 95% of pregnant women. Pregnant women (23.66 ± 4.62 years old) compared to periodontally healthy young adults (mean age of 26.8 ± 4.6 years old) (Carrouel et al., 2016) have a higher quantity of P. gingivalis (107.28 versus 103 in one ID space), T. denticola (106.9 versus 106 in one ID space), and T. forsythia (107.28 versus 103 in one ID space). The increasing of periodontal bacteria could be explained by the pregnancy. Indeed, during pregnancy, hormonal and metabolic fluctuations have a systemic action on cellular and immunological levels in order to respond and adapt to the demands of the developing fetus. Maternal hormonal changes (estrogen and progesterone) during pregnancy modify the maturation and activation of immune cells against infectious organisms (Gibbs, 2001). A reduced activation of maternal peripheral lymphocytes was observed when subjected to antigens and, compared to non-pregnant or male subjects, altered maternal estrogen and progesterone levels increased and suppressed prostaglandin E2 and interleukin-1β, respectively (Ortiz-Sánchez et al., 2021). Thus, the link between high maternal hormone levels and increased numbers of periodontal pathogenic bacteria, could be influenced directly and indirectly, facilitating the entry and multiplication of pathogenic bacteria (AlSharief and Alabdurubalnabi, 2023). Regarding the direct pathway, Gibbons et al. demonstrated that pathogens, in particular P. intermedia and P. gingivalis, produce vitamin K from these hormones, which is vital for bacterial growth (Gibbons and Macdonald, 1960). With regard to the indirect pathway, increased hormone levels altered gingival clinical parameters with increased probing depth and greater quantity and rate of gingival crevicular fluid, affecting the quality and quantity of marginal gingival keratinization and diminishing the immune response (Lindhe and Brånemark, 1967). In a previous study, Carrillo-de-Albornoz et al. (2010) demonstrated that higher progesterone levels in salivary samples were significantly associated with P. gingivalis bacterial counts (Carrillo-de-Albornoz et al., 2010). In addition, the presence of periodontopathogens of the orange and red complex that are considered as major etiologic agents of periodontal disease (Mohanty et al., 2019) indicates that pregnant women are at risk of adverse outcomes (Gare et al., 2023). This may be explained by the fact that hormonal changes in periodontal tissues lead to changes in connective tissue cell turnover rates, a decrease in vascular response and an increase in gingival vascular permeability. This creates a pathway for periodontal pathogens to infiltrate and invade the circulation, establishing infections in feto-placental units (Wu et al., 2015).
In addition, in case of gingivitis, the 2018 EFP-AAP classification of periodontal diseases mentions local risk factors, known as predisposing factors, and systemic risk factors, so-called modifying factors (Chapple et al., 2018). These environmental and host susceptibility factors can impact on the symbiosis of the oral microbiome and lead to the onset of lifestyle-related disorders (Saadaoui et al., 2021; Sedghi et al., 2021). Regarding the total count of bacteria and the 9 periodontopathogens, our study indicated that demographic parameters, eating habits and physical activity did not impact significantly on the interdental microbiota. However, a decrease of the median quantity of 9 periodontopathogens with the increase of consumption of fruits and vegetable consumption was observed. This data could be correlated to the fact that people adopting a ‘healthy diet’ have a reduced risk of non-communicable diseases (Martinon et al., 2021). Laiola et al. (2020) demonstrated that a Mediterranean diet in a group overweight/obese subjects led to the significant decreasing of periodontal pathogens such as P. gingivalis, P. intermedia, and T. denticola in the saliva (Laiola et al., 2020). Furthermore, the extract of phenolic-rich cranberry (0.1–1.0 mg/mL) demonstrated a significant antibiofilm activity acting on bacterial adhesion in the early stages of biofilm development, as found in F. nucleatum, P. gingivalis, and A. actinomycetemcomitans (Sánchez et al., 2020).
Contrary to previous factors analyzed in this study, the oral hygiene behavior and more particularly the frequency of toothbrushing impact on the quantity of bacteria Socransky’s complexes. The quantity of the total number of bacteria and of the 9 periodontopathogens decreased in pregnant women brushing their teeth at least 2 times per day compared to pregnant women who brushed less. More particularly, the decrease was statistically significant for the total number, P. micra, F. nucleatum, and T. denticola. This is in accordance with the review of Rajwani et al. (2020) who concluded that toothbrushing promote plaque removal and reduce gingivitis (Rajwani et al., 2020). However, as the toothbrush cannot enter in the interdental space, toothbrushing acts on supragingival plaque but not on the interdental microbiota. Thus, it is necessary to implement oral health education program and more particularly, to educate pregnant women to the interdental cleaning using calibrated interdental brushes (Kiger et al., 1991; Bourgeois et al., 2015). Indeed, several studies have demonstrated that daily use of interdental brushes reduces the dysbiosis of the interdental microbiota, the interdental bleeding and therefore the risk of PD and other non-communicable diseases (Bourgeois et al., 2016, 2019). Particularly in pregnant women, it is important to reduce the risk of adverse pregnancy outcomes due to periodontal bacterial and there, public health policies based on individual oral prophylaxis should be implemented.
In terms of clinical signs, in pregnant women, the percentage of bleeding sites was associated with the plaque index that is to say the quantity of bacteria. This is in concordance with the definition of gingivitis caused by plaque (Chapple et al., 2018). Gingival tissue is characterized by redness, swelling, sensitivity, a shiny surface and bleeding on gentle probing. Generally painless and without spontaneous bleeding, gingivitis often goes undiagnosed (Trombelli et al., 2018). However, in our study, 92% of pregnant women suffered of bleeding. Focusing on pregnant women with more than 30% bleeding revealed, via dendogram observation, a different organization of bacterial clusters to that observed for women with less than 30% bleeding. The 2 clusters organization reveals a high risk to develop periodontal disease. In fact, the first cluster was composed of F. nucleatum, P. micra, P. intermedia, P. gingivalis, and T. forsythia and previous studies have demonstrated that co-infection with F. nucleatum and P. gingivalis or T. forsythia induced host immune response and provoked alveolar bone loss (Polak et al., 2009; Settem et al., 2012; Chen et al., 2022). The second cluster was composed of T. denticola, C. rectus, A. actinomycetemcomitans, and T. forsythia which can be correlated with previous studies that concluded that P. gingivalis, C. rectus, F. nucleatum, A. actinomycetemcomitans, and T. denticola was associated with biofilm formation at the bottom of human periodontal pockets, the so-called “plaque-free zone.” In addition, pregnant women with more than 30% of bleeding had higher quantity of bacteria from the green, orange and red complexes except F. nucleatum that was significantly lower. P. gingivalis and T. denticola was very significantly higher compared to pregnant women with less than 30% of bleeding sites. These results support the “Keystone–Pathogen Hypothesis,” of which P. gingivalis plays a key role, indicating that specific bacteria in limited quantities can influence the host immune system and convert the microbiota from symbiotic to dysbiotic to induce inflammatory disorder (Hajishengallis et al., 2012). Specific periodontal pathogens such as the one of the red complex have virulence factors and thus are considered as potential contributors in adverse pregnancy outcomes (Gómez et al., 2020; Radaic and Kapila, 2021). This could be explained because the increase of bleeding periodontal sites would induce hematological dissemination of periodontal pathogens and their products, and subsequently would later lead to an immune/inflammatory reaction in the feto-placental unit (Horliana et al., 2014).
This study has several limitations. Firstly, our research was to focus on 3 months pregnant women with healthy periodontium thus including adults with healthy gums or largely with localized or generalized gingivitis. In fact, this option was guided on the one hand that initial changes from health to plaque-induced gingivitis may not be clinically detectable, raising effective debates about clinical thresholds for defining pathological versus physiological inflammation and by the high prevalence of gingivitis observed in this population (Gare et al., 2023). Secondly, as in any cross-sectional study, our survey of 3 months pregnant women is penalized by the absence of a control group. The results of this research should be considered as a valid report on the status of periodontal pathogens, but limited to the population of pregnant women concerned. The ideal study design would have been to monitor the participating women from pre-pregnancy to 3 months post-pregnancy. Thirdly, saliva gland diseases which could have implications for the microbiota analysis, were not considered. Fourthly, only 9 pathogens according to the green, orange and red Socransky complexes vs. the 5 major complexes were selected (Socransky et al., 1998).
5. Conclusion
This study demonstrated that 3 months pregnant women with healthy periodontium presented interdental bleeding and a dysbiotic microbiota with periodontal pathogens from the orange and red complexes of Socransky. Therefore, these women had a high risk of periodontal disease and, consequently, a risk of an unfavorable pregnancy outcome. Preventive oral prophylaxis measures, particularly individual interdental prophylaxis, must therefore be implemented from the very beginning of pregnancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ethical committee of Dakar (Senegal). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FC: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AK: Investigation, Writing – review & editing. V-EL: Writing – review & editing. DB: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received external funding from Curaden AG (Switzerland).
Acknowledgments
The authors acknowledge all the pregnant women who participated in this study and the teacher–researchers from the Public Health Service, Department of Dentistry, Cheikh Anta Diop University for their participation: A. Dieng, S. Ndame Dieng, M. Diop, L. Mbow, and D. Faye.
Conflict of interest
This work was partially supported by Curaden AG, Kriens, Switzerland, by Hospices Civils of Lyon, France and by Laboratory ‘Systemic Health Care’, UR4129, University of Lyon, France. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All the authors have full access to all the data in the study and had final responsibility for the decision to submit for publication.
The author(s) DB and FC declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1275180/full#supplementary-material
References
Alnasser, B. H., Alkhaldi, N. K., Alghamdi, W. K., and Alghamdi, F. T. (2023). The potential association between periodontal diseases and adverse pregnancy outcomes in pregnant women: a systematic review of randomized clinical trials. Cureus 15:e33216. doi: 10.7759/cureus.33216
AlSharief, M., and Alabdurubalnabi, E. (2023). Periodontal pathogens and adverse pregnancy outcomes: a narrative review. Life 13:1559. doi: 10.3390/life13071559
Balan, P., Brandt, B. W., Chong, Y. S., Crielaard, W., Wong, M. L., Lopez, V., et al. (2021). Subgingival microbiota during healthy pregnancy and pregnancy gingivitis. JDR Clin. Trans. Res. 6, 343–351. doi: 10.1177/2380084420948779
Baudet, A., Veynachter, T., Rousseau, H., Anagnostou, F., Jeanne, S., Orti, V., et al. (2020). Perception of gingival bleeding by people and healthcare professionals: a multicentre study in an adult French population. Int. J. Environ. Res. Public Health 17:5982. doi: 10.3390/ijerph17165982
Bobetsis, Y. A., Graziani, F., Gürsoy, M., and Madianos, P. N. (2020). Periodontal disease and adverse pregnancy outcomes. Periodontol. 83, 154–174. doi: 10.1111/prd.12294
Bourgeois, D., Bravo, M., Llodra, J.-C., Inquimbert, C., Viennot, S., Dussart, C., et al. (2019). Calibrated interdental brushing for the prevention of periodontal pathogens infection in young adults – a randomized controlled clinical trial. Sci. Rep. 9:15127. doi: 10.1038/s41598-019-51938-8
Bourgeois, D., Carrouel, F., Llodra, J. C., Bravo, M., and Viennot, S. (2015). A colorimetric interdental probe as a standard method to evaluate interdental efficiency of interdental brush. Open Dent. J. 9, 431–437. doi: 10.2174/1874210601509010431
Bourgeois, D., David, A., Inquimbert, C., Tramini, P., Molinari, N., and Carrouel, F. (2017). Quantification of carious pathogens in the interdental microbiota of young caries-free adults. PLoS One 12:e0185804. doi: 10.1371/journal.pone.0185804
Bourgeois, D., Saliasi, I., Llodra, J. C., Bravo, M., Viennot, S., and Carrouel, F. (2016). Efficacy of interdental calibrated brushes on bleeding reduction in adults: a 3-month randomized controlled clinical trial. Eur. J. Oral Sci. 124, 566–571. doi: 10.1111/eos.12302
Bull, F. C., Al-Ansari, S. S., Biddle, S., Borodulin, K., Buman, M. P., Cardon, G., et al. (2020). World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462. doi: 10.1136/bjsports-2020-102955
Carrillo-de-Albornoz, A., Figuero, E., Herrera, D., and Bascones-Martínez, A. (2010). Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. J. Clin. Periodontol. 37, 230–240. doi: 10.1111/j.1600-051X.2009.01514.x
Carrouel, F., Viennot, S., Santamaria, J., Veber, P., and Bourgeois, D. (2016). Quantitative molecular detection of 19 major pathogens in the interdental biofilm of periodontally healthy young adults. Front. Microbiol. 7:840. doi: 10.3389/fmicb.2016.00840
Caton, J. G., Armitage, G., Berglundh, T., Chapple, I. L. C., Jepsen, S., Kornman, K. S., et al. (2018). A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J. Clin. Periodontol. 45, S1–S8. doi: 10.1111/jcpe.12935
Caton, J. G., and Polson, A. M. (1985). The interdental bleeding index: a simplified procedure for monitoring gingival health. Compend Contin Educ Dent 6, 90–92.
CDC (2022). Defining adult overweight and obesity. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/obesity/basics/adult-defining.html (Accessed February 10, 2023).
Chapple, I. L. C., Mealey, B. L., Van Dyke, T. E., Bartold, P. M., Dommisch, H., Eickholz, P., et al. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and Peri-implant diseases and conditions. J. Clin. Periodontol. 45, S68–S77. doi: 10.1111/jcpe.12940
Chen, Y., Huang, Z., Tang, Z., Huang, Y., Huang, M., Liu, H., et al. (2022). More than just a periodontal pathogen – the research progress on Fusobacterium nucleatum. Front. Cell. Infect. Microbiol. 12:815318. doi: 10.3389/fcimb.2022.815318
Field, C. A., Gidley, M. D., Preshaw, P. M., and Jakubovics, N. (2012). Investigation and quantification of key periodontal pathogens in patients with type 2 diabetes. J. Periodontal Res. 47, 470–478. doi: 10.1111/j.1600-0765.2011.01455.x
Gare, J., Kanoute, A., Meda, N., Viennot, S., Bourgeois, D., and Carrouel, F. (2021). Periodontal conditions and pathogens associated with pre-eclampsia: a scoping review. Int. J. Environ. Res. Public Health 18:7194. doi: 10.3390/ijerph18137194
Gare, J., Kanoute, A., Orsini, G., Gonçalves, L. S., Ali Alshehri, F., Bourgeois, D., et al. (2023). Prevalence, severity of extension, and risk factors of gingivitis in a 3-month pregnant population: a multicenter cross-sectional study. J. Clin. Med. 12:3349. doi: 10.3390/jcm12093349
Genco, R. J., and Sanz, M. (2020). Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol. 83, 7–13. doi: 10.1111/prd.12344
Gibbons, R. J., and Macdonald, J. B. (1960). Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J. Bacteriol. 80, 164–170. doi: 10.1128/jb.80.2.164-170.1960
Gibbs, R. S. (2001). The relationship between infections and adverse pregnancy outcomes: an overview. Ann. Periodontol. 6, 153–163. doi: 10.1902/annals.2001.6.1.153
Giglio, J. A., Lanni, S. M., Laskin, D. M., and Giglio, N. W. (2009). Oral health care for the pregnant patient. J. Can. Dent. Assoc. 75, 43–48.
Gómez, L. A., De Avila, J., Castillo, D. M., Montenegro, D. A., Trujillo, T. G., Suárez, L. J., et al. (2020). Porphyromonas gingivalis placental atopobiosis and inflammatory responses in women with adverse pregnancy outcomes. Front. Microbiol. 11:591626. doi: 10.3389/fmicb.2020.591626
Hajishengallis, G., Darveau, R. P., and Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725. doi: 10.1038/nrmicro2873
Hofer, D., Sahrmann, P., Attin, T., and Schmidlin, P. R. (2011). Comparison of marginal bleeding using a periodontal probe or an interdental brush as indicators of gingivitis. Int. J. Dent. Hyg. 9, 211–215. doi: 10.1111/j.1601-5037.2010.00483.x
Horliana, A. C. R. T., Chambrone, L., Foz, A. M., Artese, H. P. C., Rabelo, M. D. S., Pannuti, C. M., et al. (2014). Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One 9:e98271. doi: 10.1371/journal.pone.0098271
Inquimbert, C., Bourgeois, D., Bravo, M., Viennot, S., Tramini, P., Llodra, J. C., et al. (2019). The oral bacterial microbiome of interdental surfaces in adolescents according to carious risk. Microorganisms 7:319. doi: 10.3390/microorganisms7090319
Jang, H., Patoine, A., Wu, T. T., Castillo, D. A., and Xiao, J. (2021). Oral microflora and pregnancy: a systematic review and meta-analysis. Sci. Rep. 11:16870. doi: 10.1038/s41598-021-96495-1
Kanoute, A., Gare, J., Meda, N., Viennot, S., Tramini, P., Fraticelli, V., et al. (2021). Effect of oral prophylactic measures on the occurrence of pre-eclampsia (OP-PE) in high-risk pregnant women: A cluster randomized controlled trial. Methods Protoc. 4:61. doi: 10.3390/mps4030061
Kharitonova, M., Vankov, P., Abdrakhmanov, A., Mamaeva, E., Yakovleva, G., and Ilinskaya, O. (2021). The composition of microbial communities in inflammatory periodontal diseases in young adults Tatars. AIMS Microbiol 7, 59–74. doi: 10.3934/microbiol.2021005
Kiger, R. D., Nylund, K., and Feller, R. P. (1991). A comparison of proximal plaque removal using floss and interdental brushes. J. Clin. Periodontol. 18, 681–684. doi: 10.1111/j.1600-051x.1991.tb00109.x
Koerner, R., Prescott, S., Alman, A., Duffy, A., and Groer, M. (2023). The oral microbiome throughout pregnancy: a scoping review. MCN Am. J. Matern. Child Nurs. 48, 200–208. doi: 10.1097/NMC.0000000000000930
Laiola, M., De Filippis, F., Vitaglione, P., and Ercolini, D. (2020). A Mediterranean diet intervention reduces the levels of salivary periodontopathogenic bacteria in overweight and obese subjects. Appl. Environ. Microbiol. 86:20. doi: 10.1128/AEM.00777-20
Lindhe, J., and Brånemark, P. I. (1967). Changes in microcirculation after local application of sex hormones. J. Periodontal Res. 2, 185–193. doi: 10.1111/j.1600-0765.1967.tb01888.x
Löe, H. (1967). The gingival index, the plaque index and the retention index systems. J. Periodontol. 38, 610–616. doi: 10.1902/jop.1967.38.6.610
Martinon, P., Fraticelli, L., Giboreau, A., Dussart, C., Bourgeois, D., and Carrouel, F. (2021). Nutrition as a key modifiable factor for periodontitis and Main chronic diseases. J. Clin. Med. 10:197. doi: 10.3390/jcm10020197
Massoni, R. S. D. S., Aranha, A. M. F., Matos, F. Z., Guedes, O. A., Borges, Á. H., Miotto, M., et al. (2019). Correlation of periodontal and microbiological evaluations, with serum levels of estradiol and progesterone, during different trimesters of gestation. Sci. Rep. 9:11762. doi: 10.1038/s41598-019-48288-w
Mohanty, R., Asopa, S. J., Joseph, M. D., Singh, B., Rajguru, J. P., Saidath, K., et al. (2019). Red complex: polymicrobial conglomerate in oral flora: a review. J Family Med Prim Care 8, 3480–3486. doi: 10.4103/jfmpc.jfmpc_759_19
Morrison, A. G., Sarkar, S., Umar, S., Lee, S. T. M., and Thomas, S. M. (2023). The contribution of the human oral microbiome to oral disease: a review. Microorganisms 11:318. doi: 10.3390/microorganisms11020318
Mostafa, B., and El-Refai, I. (2018). Prevalence of plaque-induced gingivitis in a sample of the adult Egyptian population. Open Access Maced J Med Sci 6, 554–558. doi: 10.3889/oamjms.2018.131
Murakami, S., Mealey, B. L., Mariotti, A., and Chapple, I. L. C. (2018). Dental plaque-induced gingival conditions. J. Clin. Periodontol. 45, S17–S27. doi: 10.1111/jcpe.12937
Nannan, M., Xiaoping, L., and Ying, J. (2022). Periodontal disease in pregnancy and adverse pregnancy outcomes: progress in related mechanisms and management strategies. Front Med 9:963956. doi: 10.3389/fmed.2022.963956
Nishida, C., Uauy, R., Kumanyika, S., and Shetty, P. (2004). The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 7, 245–250. doi: 10.1079/phn2003592
Nuriel-Ohayon, M., Neuman, H., and Koren, O. (2016). Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 7:1031. doi: 10.3389/fmicb.2016.01031
Ortiz-Sánchez, B. J., Legorreta-Herrera, M., and Rodriguez-Sosa, M. (2021). Influence of gestational hormones on the bacteria-induced cytokine response in periodontitis. Mediat. Inflamm. 2021, 1–12. doi: 10.1155/2021/5834608
Polak, D., Wilensky, A., Shapira, L., Halabi, A., Goldstein, D., Weiss, E. I., et al. (2009). Mouse model of experimental periodontitis induced by porphyromonas gingivalis/fusobacterium nucleatum infection: bone loss and host response. J. Clin. Periodontol. 36, 406–410. doi: 10.1111/j.1600-051X.2009.01393.x
Radaic, A., and Kapila, Y. L. (2021). The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 19, 1335–1360. doi: 10.1016/j.csbj.2021.02.010
Rajwani, A. R., Hawes, S. N. D., To, A., Quaranta, A., and Rincon Aguilar, J. C. (2020). Effectiveness of manual toothbrushing techniques on plaque and gingivitis: a systematic review. Oral Health Prev. Dent. 18, 843–854. doi: 10.3290/j.ohpd.a45354
Saadaoui, M., Singh, P., and Al Khodor, S. (2021). Oral microbiome and pregnancy: a bidirectional relationship. J. Reprod. Immunol. 145:103293. doi: 10.1016/j.jri.2021.103293
Sánchez, M. C., Ribeiro-Vidal, H., Bartolomé, B., Figuero, E., Moreno-Arribas, M. V., Sanz, M., et al. (2020). New evidences of antibacterial effects of cranberry against periodontal pathogens. Foods 9:246. doi: 10.3390/foods9020246
Sathish, A. K., Varghese, J., and Fernandes, A. J. (2022). The impact of sex hormones on the periodontium during a woman’s lifetime: a concise-review update. Curr Oral Health Rep 9, 146–156. doi: 10.1007/s40496-022-00321-0
Sedghi, L. M., Bacino, M., and Kapila, Y. L. (2021). Periodontal disease: the good, the bad, and the unknown. Front. Cell. Infect. Microbiol. 11:766944. doi: 10.3389/fcimb.2021.766944
Settem, R. P., El-Hassan, A. T., Honma, K., Stafford, G. P., and Sharma, A. (2012). Fusobacterium nucleatum and tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect. Immun. 80, 2436–2443. doi: 10.1128/IAI.06276-11
Socransky, S. S., and Haffajee, A. D. (2005). Periodontal microbial ecology. Periodontol. 2000, 135–187. doi: 10.1111/j.1600-0757.2005.00107.x
Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C., and Kent, R. L. (1998). Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x
Terzic, M., Aimagambetova, G., Terzic, S., Radunovic, M., Bapayeva, G., and Laganà, A. S. (2021). Periodontal pathogens and preterm birth: current knowledge and further interventions. Pathogens 10:730. doi: 10.3390/pathogens10060730
Trombelli, L., Farina, R., Silva, C. O., and Tatakis, D. N. (2018). Plaque-induced gingivitis: case definition and diagnostic considerations. J. Periodontol. 89, S46–S73. doi: 10.1002/JPER.17-0576
Wang, X., Ouyang, Y., Liu, J., Zhu, M., Zhao, G., Bao, W., et al. (2014). Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349:g4490. doi: 10.1136/bmj.g4490
Wu, M., Chen, S.-W., and Jiang, S.-Y. (2015). Relationship between gingival inflammation and pregnancy. Mediat. Inflamm. 2015:623427. doi: 10.1155/2015/623427
Yang, I., Knight, A. K., Dunlop, A. L., and Corwin, E. J. (2019). Characterizing the subgingival microbiome of pregnant African American women. J. Obstet. Gynecol. Neonatal. Nurs. 48, 140–152. doi: 10.1016/j.jogn.2018.12.003
Keywords: gingivitis, microbiota, biofilm, interdental, dysbiosis, periodontal health, pregnancy, periodontitis
Citation: Carrouel F, Kanoute A, Lvovschi V-E and Bourgeois D (2023) Periodontal pathogens of the interdental microbiota in a 3 months pregnant population with an intact periodontium. Front. Microbiol. 14:1275180. doi: 10.3389/fmicb.2023.1275180
Edited by:
George Grant, University of Aberdeen, United KingdomReviewed by:
Hengyi Xu, The University of Texas at Austin, United StatesZhenting Xiang, University of Pennsylvania, United States
Copyright © 2023 Carrouel, Kanoute, Lvovschi and Bourgeois. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence Carrouel, ZmxvcmVuY2UuY2Fycm91ZWxAdW5pdi1seW9uMS5mcg==
†These authors have contributed equally to this work
 Florence Carrouel
Florence Carrouel Aida Kanoute2†
Aida Kanoute2† Denis Bourgeois
Denis Bourgeois