- 1Department of Gastroenterology, Jinshan Hospital Affiliated to Fudan University, Shanghai, China
- 2Shanghai Mobio Biomedical Technology Co. Ltd., Shanghai, China
Introduction: Constipation-predominant irritable bowel syndrome (IBS-C) is a functional bowel disease that affects 10–20% of the population worldwide. Curcumin (CUR) is widely used in traditional Chinese medicine to treat IBS, but its mechanism of action needs further investigation.
Methods: In this study, we used mosapride (MOS) as a positive control to evaluate the changes in gut microbiota in IBS-C rat models after treatment with CUR or MOS by analyzing 16S rDNA variation. In addition, we used enzyme immunoassay kits and immunohistochemical analysis to investigate whether CUR or MOS influenced serotonin (5-HT), substance P (SP), and vasoactive intestinal peptide (VIP) levels in the serum and colon of IBS-C rats.
Results: The study showed that rats supplemented with CUR showed significantly increased fecal weight, fecal water content, small intestine transit rate and significantly decreased serum levels of 5-HT, VIP and SP compared to the IBS group (p < 0.05). In addition, treatment with CUR changed the relative abundance of Blautia, Sutterella, Acetanaerobacterium and Ruminococcus2 in the gut microbiota.
Discussion: This study showed that the efficacy of CUR on IBS-C was possibly by modulating the microbiota and lowering the serum levels of HT, SP, and VIP.
1 Introduction
Constipation-predominant irritable bowel syndrome (IBS-C) is a functional bowel disorder characterized by disturbed bowel habits and recurrent abdominal pain often associated with defecation (Defrees and Bailey, 2017; Di Rosa et al., 2023). According to global epidemiological studies, IBS affects approximately 7% of the population in Southeast Asia and the Middle East, while the prevalence—in Southern Europe, Africa, and South America ranges from 15% to 21% (Black and Ford, 2020). In addition, women are more likely to develop IBS-C than men (Hadjivasilis et al., 2019), and some symptoms of IBS-C are more prevalent in female patients and in people between the ages of 20–49 years (Kosako et al., 2018). IBS is becoming more common and is evolving into a chronic recurrent condition due to dietary and lifestyle changes (Enck et al., 2016; Di Rosa et al., 2023). Although IBS does not have a significant mortality rate, it causes long-term symptoms in patients, increases the psychological and economic burden of patients, and significantly limits their quality of life (Zhang et al., 2016; Tack et al., 2019).
The etiologic factors of irritable bowel syndrome are complex, and the mechanism is not yet fully understood. Major risk factors include genetics, diet, gut motor dysfunction, gut microbiota imbalance, alterations in gastrointestinal hormones, and psychological factors that may affect the brain-gut axis. In recent years, the role of the gut microbiota in gut motility disorders has been widely studied and discussed. Previous studies have suggested that an imbalance in the gut microbiota may be involved in the development of IBS-C (Ohkusa et al., 2019; Sun et al., 2019; Wen et al., 2020). Gastrointestinal motility is mainly controlled by the enteric nervous system, which regulates gastrointestinal activity, including neurotransmitters such as 5-hydroxytryptamine (5-HT), substance P (SP), and vasoactive intestinal peptide (VIP; Sun et al., 2023). In addition, a clinical study found that the gut microbiota of patients with IBS-C is significantly different from that of healthy individuals, which may influence gut habits (Parthasarathy et al., 2016). Serotonin signaling also plays an important role in the pathogenesis of IBS. Concentrations of 5-HT have been found to be significantly elevated in patients with IBS compared with controls and are associated with intestinal motility (Smith and Koh, 2017; Xi et al., 2019). Recent studies indicate that the gut microbiota is involved in the biosynthesis of 5-HT, while the concentrations of 5-HT in the gut are directly or indirectly regulated by the gut microbiota (Bhattarai et al., 2017; Agus et al., 2018). Studies have also shown that patients with IBS have abnormal levels of gastrointestinal hormones such as 5-HT, SP and VIP (Sun et al., 2023). Most patients also have long-term emotional problems such as depression, anxiety, and tension, which exacerbate functional disorders such as gastrointestinal motility and secretion via the brain-gut axis and further aggravate the condition of IBS patients.
Curcumin (CUR) is the major active constituent of Curcuma longa. Modern studies have demonstrated its potent anti-inflammatory (Kocaadam and Şanlier, 2017), antioxidant (Ahangarpour et al., 2019), and antidepressant (Chang et al., 2016) effects. 5-HT is an important neurotransmitter and is closely related to the regulation of intestinal motility. It has been found that treatment with CUR can modulate 5-HT levels, with treatment with CUR significantly increasing 5-HT levels in the hippocampus, while decreasing 5-HT levels in the colon in IBS models (Yu et al., 2015). SP is a neuropeptide involved in conduction and sensory transmission in the intestine. VIP is a neuropeptide that regulates intestinal smooth muscle relaxation and secretory function (Liu et al., 2015). In addition, CUR has low bioavailability (Cheng et al., 2023), and most of the unmetabolized CUR is excreted in the feces, which means that CUR can interact directly with the gut microbiota. CUR has been shown to positively affect the gut microbiota and modulate microbiota composition and function, further improving gut health (Ng et al., 2018). These studies support the potential therapeutic effect of CUR on IBS-C through modulation of the gut microbiota and neurotransmitters.
Because of the close relationship between the gut microbiota, neurotransmitters, and IBS-C, we hypothesized that treatment with CUR may modulate the gut microbiota and levels of 5-HT, VIP and SP. To test this hypothesis, mosapride (MOS) was used as a positive control for the efficacy on IBS-C in this study, and 16S rDNA variation analysis was used to observe the changes in gut microbiota communities in IBS-C rat models after treatment with CUR.
2 Materials and methods
2.1 Reagent
CUR, MOS, and 0.5% sodium carboxymethylcellulose used in this study were purchased from Sigma Chemical Co. in the United States.
2.2 Animals and experimental design
Twenty-eight specific pathogen free (SPF) male Sprague–Dawley rats weighing 160 to 200 grams and 6 weeks of age were used for this study. They were provided by Shanghai Jishijie Laboratory Animal Co., Ltd. and maintained under standard conditions, including a temperature of 22°C ± 2°C, a relative humidity of 55% ± 5%, and a 12-h light–dark cycle. After 2 weeks of adaptive training, the rats were randomly divided into four groups: normal control group (CTL), IBS model group (IBS), MOS + IBS group (MOS), and CUR + IBS group (CUR). Rats in the IBS model, MOS and CUR groups received 0°C saline (2 mL/day) in the stomach for 2 weeks to induce the IBS-C rat model. Subsequently, rats in the MOS and CUR groups received 2 mL MOS or 0.2 g/kg body weight CUR daily for 2 weeks, whereas rats in the CTL and IBS groups received the corresponding normal saline. Successful model establishment was partially confirmed by the assessment of the physiological characteristics of the rats, including body weight loss, decreased food intake and defecation frequency, dry feces, disheveled hair, and signs of mental fatigue. All rats had free access to food and water throughout the study.
Rats experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The study procedures were approved by the Laboratory Animal Welfare & Ethics Committee of Shanghai Public Health Clinical Center (2023-A026-01) to minimize pain and avoid harm. The experimental procedure is shown in Figure 1A.
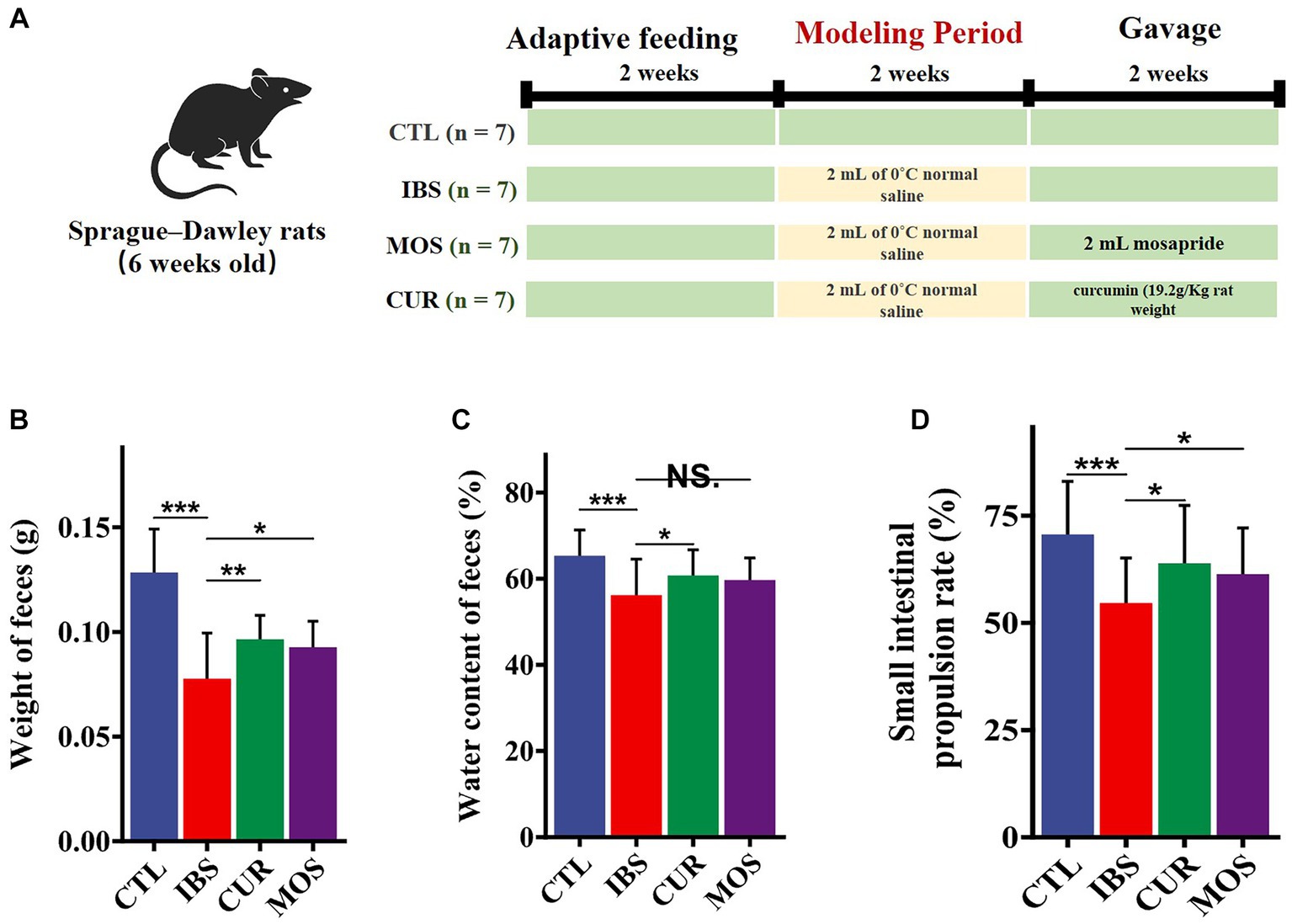
Figure 1. Experimental procedures and the palliative effect of CUR and MOS on constipation in IBS-C rats. Establishment process and intervention methods of IBS-C rat models (A). Effects of CUR and MOS on stool weight (B), stool water content (C), and small intestine transit rate (D) in IBS-C rats. Results are indicated as mean ± SD and comparison between groups were made using the Student t test (*p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant).
2.3 Determination of feces water contents
Each group of rats was placed individually in a cage and observed for a period of 3 h. During this time, the number and weight of excreted feces were recorded for each rat and fecal samples were collected. The fecal samples were then dried in an oven and weighed again. The water content of the feces was calculated using the following formula: % water content = (wet weight of feces (g) – dry weight of feces (g))/wet weight of feces (g) × 100%.
2.4 Small intestine transit rate
After a 12-h fast, all rats received 2 mL of 2% India ink and were euthanized 30 min later. Samples of the small intestine were then collected from the pylorus to the cecum. Small intestinal transit rate was calculated according to the following formula: Small intestine transit rate = length facilitated by ink (cm)/total intestine length (cm) × 100%.
2.5 Histopathological analysis of rat colon
Colonic tissue samples were collected from each rat 6 cm above the anus and 3 cm long. Fixation was performed with 10% paraformaldehyde, followed by dehydration with ethanol, alcohol soaking in xylene, and finally kerosene embedding. The embedded tissues were cut into 5 μm thick sections and stained with Hematoxylin and Eosin (H&E).
2.6 Immunohistochemical analysis
The colon sections were incubated with anti-5-HT (ab6336, Abcam, United States) and anti-VIP (ab30680, Abcam, United States) antibodies (dilution 1:100) for 24 h at 4°C. Subsequently, the immunoreaction was revealed by using HRP-labeled secondary goat anti-rat antibody (ab97057, Abcam, United States, dilution 1:500) for 2 h at room temperature. Then the slides were stained with 3,3′-Diaminobenzidine (DAB) substrate solution, and counterstained with Hematoxylin. The slides were scanned using a microscope (Olympus, Tokyo, Japan). Five high-power fields (×400 magnification) from each slide were randomly selected and analyzed using Image-Pro Plus image analysis software. Integrated option density (IOD) and area value of each image were measured, and then mean density (mean density = IOD/area) was calculated. The mean optical density values were used to plot the relative expression levels of the proteins.
2.7 Serum 5-HT, VIP and SP analysis
Rats were anesthetized with 10% chloral hydrate and sacrificed, and blood samples were collected from the jugular vein. The collected blood samples were placed in centrifuge tubes and centrifuged (e.g., 3,000 rpm for 10 min) to separate plasma or serum. After centrifugation, serum was transferred to a new centrifuge tube and stored at −80°C for subsequent experimental analysis. Finally, serum levels of 5-HT, VIP and SP were measured using enzyme immunoassay kits (Shanghai Mobio Biomedical Technology Co., Ltd.) provided by the manufacturer according to the instructions.
2.8 DNA extraction and 16S rDNA sequencing analysis of rat feces
Twenty-eight rat feces samples (seven from each group respectively) were collected at week 4 and stored frozen at −80°C until DNA extraction. DNA was extracted from the fecal samples using the QIAamp DNA Stool Mini Kit. The V3–V4 region of 16S rRNA was amplified using primers 341F and 805R (Li et al., 2020). All PCR products were purified using the Axy PrepDNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) and quantified by fluorescence using the FTC-3000TM real-time PCR system. Subsequently, the purified DNA samples were homogenized and sequenced using the Illumina Hiseq PE300 platform (Shanghai Mobio Biomedical Technology Co., Ltd.). This data has been uploaded to a repository, the SRA number is PRJNA1003150.
2.9 Bioinformatic analysis and statistical analysis
Reads were spliced using Flash software, and sequence quality was checked using Mothur software (version 1.44.1) to obtain optimized sequences (Schloss et al., 2009). Sequences were then clustered into operational taxonomic units (OTUs) based on 97% similarity. The α-diversity analysis, including ACE, Chao1, Shannon, and Simpson indices, and β-diversity analysis were performed using Mothur software. Based on OTU abundance and distance, R software (version 4.3) was used to visualize bacterial community classification and distribution, e.g., principal coordinate analysis (PCoA). Linear discriminant effect size analysis (LEfSe; Liu et al., 2021) and nonparametric Kruskal-Wallis rank sum test (KW) were used to detect different profiles between groups. Means of each group were compared by one-way comparison (ANOVA), and differences between the 2 groups were determined by Student’s t-test. p-values less than 0.05 were considered statistically significant.
3 Results
3.1 Improved effect of CUR and MOS on constipation in IBS-C rats
To verify the effect of CUR and MOS on constipation in IBS-C rats, we measured the water content and weight of the stool of rats. The results showed a significant decrease in fecal pellet weight in the IBS group compared to the control group (p < 0.05). In addition, fecal water content was also significantly decreased in the IBS-C group compared to the CTL group (p < 0.05). However, rats supplemented with CUR and MOS showed significantly increased fecal weight (Figure 1B) and increased fecal water content (Figure 1C) compared to the IBS group, indicating that CUR and MOS have a good effect on improving constipation.
3.2 Small intestinal transit rate
The results of the test of intestinal transit rate showed that IBS-C rats had significantly lower intestinal transit rate compared with normal controls. However, rats supplemented with CUR and MOS showed significantly increased small intestinal transit rates compared with the IBS group (Figure 1D), suggesting that CUR and MOS can improve the peristaltic capacity of the intestine.
3.3 Histological analysis of the colon
According to the results in Figure 2, there were no obvious differences in the histological characteristics of the colon among the four groups. The structure of the colon tissue was normal in all groups, with intact colon mucosal epithelium and well-organized glandular arrangement. No congestion, edema, ulceration, inflammatory cell infiltration, or other pathologic changes were observed in any group.
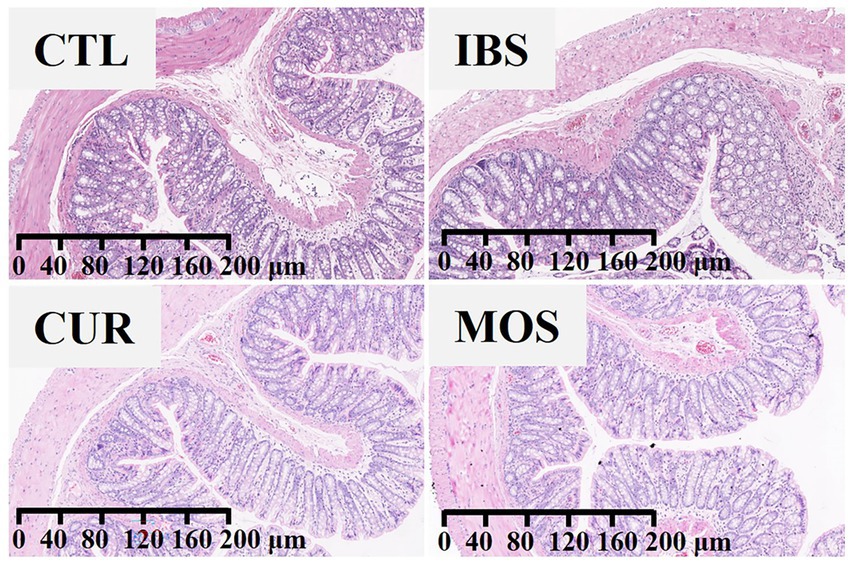
Figure 2. The effect of CUR and MOS on colon tissue using Hematoxylin and Eosin (H&E) staining (viewed with white light).
3.4 Results of histochemical analysis of the colon
The immunohistochemical results showed that the immunoreactivity of 5 HT and VIP was mainly concentrated in the colonic mucosa, circular muscle, and intestinal muscle bundles of rats (Figures 3A,C). Remarkably, we did not detect significant changes in the expression of 5-HT and VIP in rats after treatment with CUR or MOS (Figures 3B,D).
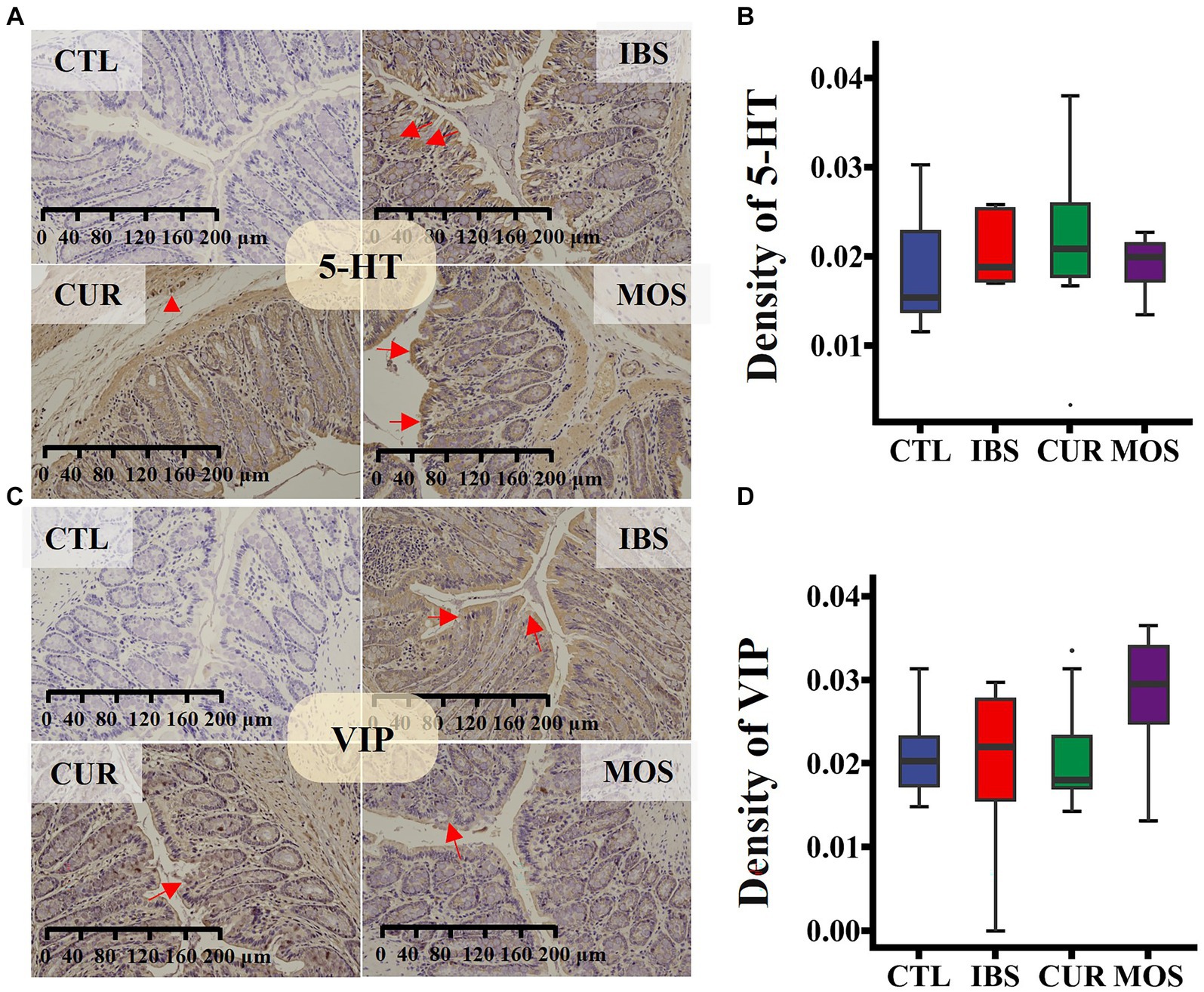
Figure 3. Immunohistochemical staining for 5-HT (A,B) and VIP (C,D) in colon tissue. Specific proteins are brown after 3,3′-Diaminobenzidine staining, and cell nucleus are blue after Hematoxylin counterstaining (viewed with white light). In panels (A,C), red arrows point to the positive cytoplasmic reaction, red triangle shows strong positive staining in enteric neurocyte. In plots (B,D), the transverse lines in boxplot mean the median.
3.5 Effects of CUR and MOS on serum levels of 5-HT, VIP and SP
Since we did not find any significant differences in the concentrations of HT and VIP between the different groups in the colon tissue, we further investigated the serum concentrations of HT, VIP and SP. The results showed that the serum levels of 5-HT, VIP and SP in the IBS group were significantly higher than those in the CTL group (p < 0.05). However, treatment with CUR significantly decreased the serum levels of 5-HT, VIP and SP (Figure 4). Notably, the serum levels of HT, VIP and SP were not significantly different in the MOS group compared to the IBS group (p > 0.05; Figure 4). These results suggest that CUR significantly decreases serum levels of neurotransmitters compared to MOS.
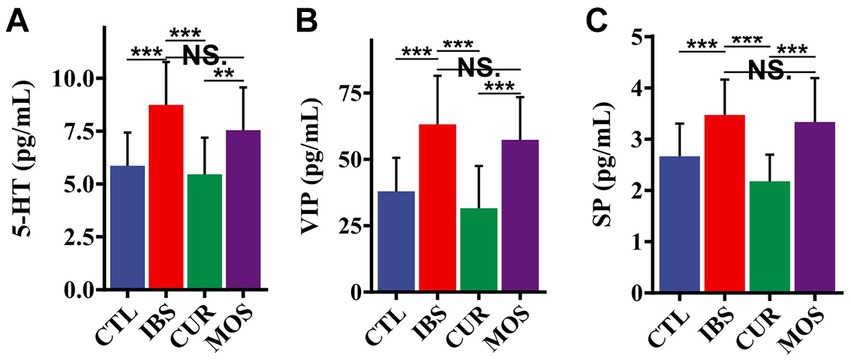
Figure 4. Effect of CUR and MOS on serum levels of 5-HT (A) VIP (B) and SP (C) in IBS-C rats. 5-HT, 5-hydroxytryptamine; VIP, vasoactive intestinal peptide; SP, substance P. Results are indicated as mean ± SD and comparison between groups were made using the Student t test (**p < 0.01, ***p < 0.001, NS, not significant).
3.6 Effects of CUR and MOS on the intestinal microbiota in IBS-C rats
We further investigated the effects of CUR and MOS on the intestinal microbiota of IBS-C rats using 16S rRNA sequencing. The gut microbiota of rats in the IBS group showed increased richness (ACE and Chao1 index) and decreased diversity (Shannon index) compared with the CTL group (Figure 5A). However, both the richness and diversity of the gut microbiota improved in the IBS-C rats after treatment with CUR and MOS. We observed a significant decrease in the ACE index in the CUR group (p < 0.05).
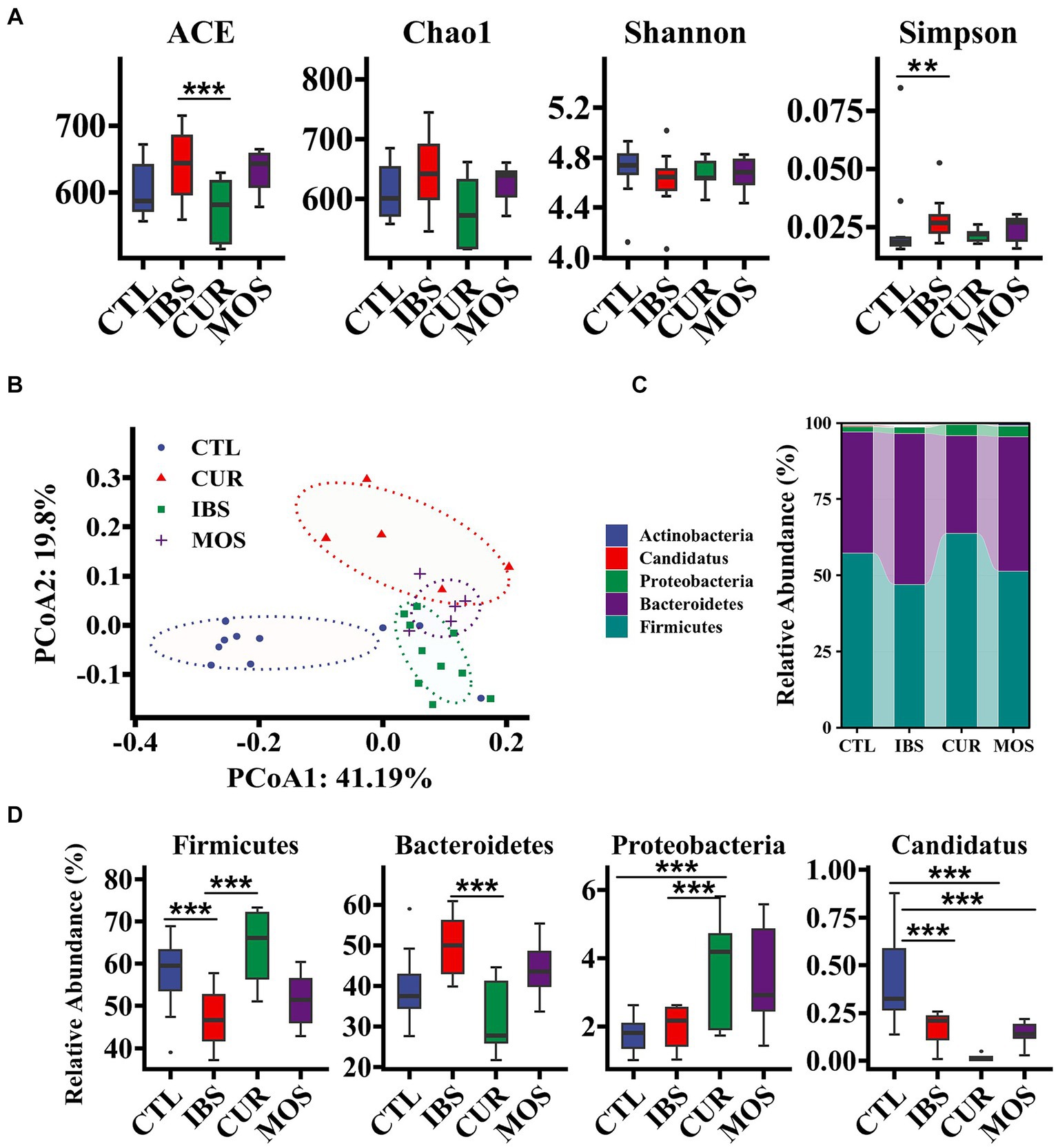
Figure 5. Effect of treatment with CUR or MOS on gut microbiota in IBS-C rats. Panels (A,B) show the effect of treatment with CUR or MOS on the alpha and beta diversity of the gut microbiota in IBS-C rats. Panels (C,D) show the effects of treatment with CUR or MOS on the relative abundance of gut microbiota at the phylum level in IBS-C rats. The transverse lines in boxplot mean the median. *p < 0.05, **p < 0.01, and ***p < 0.001.
Then, we performed multivariate analysis using principal coordinate analysis (PCoA) based on Unifrac distance to analyze the global structural changes in the gut microbiota. The PCoA score showed that the gut microbiota in the IBS group had significant structural changes on the first principal component (PCoA1) and the second principal component (PCoA2) compared with the CTL group (Figure 5B). However, treatment with CUR and MOS significantly reversed this change, with CUR having a more dramatic effect on the gut microbiota (Figure 5B). Thus, the structure of the gut microbiota was altered in IBS-C rats, and treatment with CUR had a greater effect on the gut microbiota.
At the phylum level, the predominant components of the microbiota were Firmicutes and Bacteroidetes, followed by Proteobacteria, which had relative abundances above 99. 9% (Figure 5C). Compared with the CTL group, the IBS group showed a significant decrease in the relative abundance of Firmicutes and Candidatus, whereas Bacteroidetes showed a significant increase. This trend reversed significantly after the intervention of CUR. The relative frequency of Firmicutes was significantly increased in the CUR group compared to the IBS group, while the frequency of Bacteroidetes was significantly decreased. In addition, MOS showed a similar trend, but no significant difference compared with the IBS group. Of note, both the CUR and MOS interventions resulted in an increase in Proteobacteria abundance (Figure 5D).
3.7 LEfSe analysis and correlation analysis
LEfSe analysis showed that Alistipes, Helicobacter, Sporobacter, Christensenella, Hydrogenoanaerobacterium and Neisseria were enriched in the CTL group. Prevotella, Vampirovibrio and Elusimicrobium were increased in the intestine of rats of the IBS-C model, while Blautia, Sutterella, Acetanaerobacterium and Ruminococcus2 were enriched according to CUR. In contrast, treatment with MOS resulted in increased abundance of Allobaculum, Paraprevotella, Facklamia, Staphylococcus, Butyricimonas, Jeotgalicoccus, Holdemania, and Collinsella (Figure 6A). In addition, correlation analysis showed that the enrichment of Ruminococcus2 in the CUR group was negatively correlated with VIP and SP, while the enrichment of Prevotella in the IBS group and Jeotgalicoccus in the MOS group were positively correlated with VIP (Figure 6B). The enrichment of Paraprevotella in the MOS group was positively associated with SP.
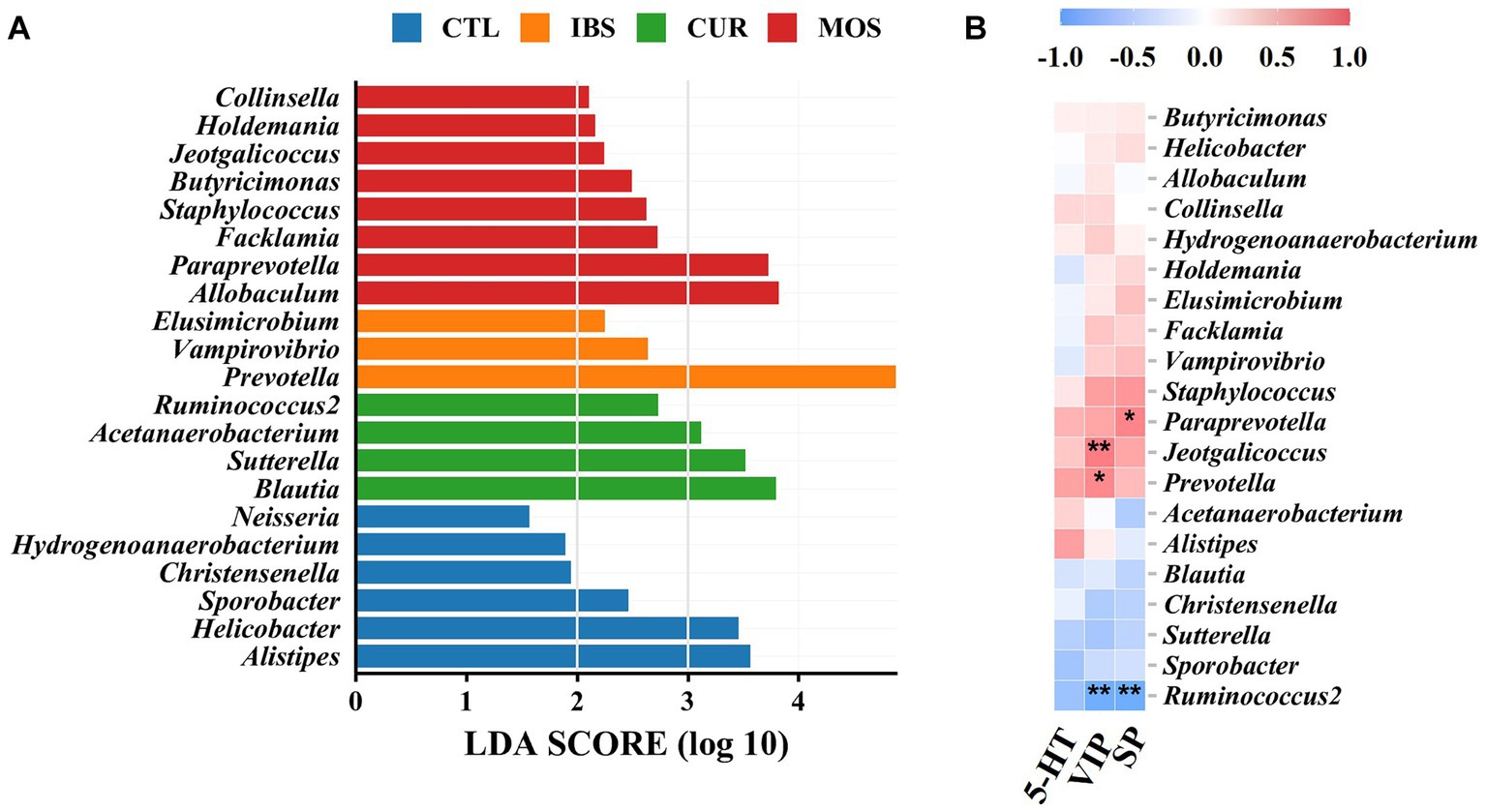
Figure 6. LEfSe analysis and correlation analysis. (A) LEfSe analysis. (B) Heatmap of Pearson correlation analysis, blue-white-red color: negative correlation-no correlation-positive correlation. **p < 0.01, *p < 0.05. 5-HT, 5-hydroxytryptamine; VIP, vasoactive intestinal peptide; SP, substance P.
4 Discussion
In this study, we developed a rat model of IBS with 0°C saline. The results showed that after 2 weeks of modeling, the number of fecal pellets and water content were significantly lower in the IBS group than in the CTL group, indicating that the rats in the IBS group developed constipation symptoms. In addition, H&E staining of the colon tissue showed no significant pathological changes in the structure of the colon tissue of the IBS rats compared with the CTL group, which is consistent with previous findings that IBS-C is not associated with an inflammatory response and damage to the colon epithelial cells (Peters et al., 2017).
5-HT, VIP and SP are ubiquitous peptides in the central and enteric nervous systems that are closely related to the development of IBS and play an important regulatory role in visceral sensation and movement (Liu et al., 2015; Liang et al., 2016). VIP may relax gastrointestinal smooth muscle and inhibit gastrointestinal motility, while SP may enhance gastrointestinal peristalsis, promote gastrointestinal smooth muscle contraction, and support group movement of the colon (Tang et al., 2022; Fan et al., 2023). Visceral hyperalgesia in patients with IBS is associated with an increase in the content of 5-HT in their peripheral tissues (Guo et al., 2022). In this study, immunohistochemical analysis showed that the levels of 5-HT, SP, and VIP in colon tissues were not significantly different between the different groups. However, the serum levels of 5-HT, SP and VIP were significantly increased in rats with irritable bowel syndrome. In contrast, the levels of 5-HT, SP and VIP were significantly decreased in the serum of CUR and MOS rats. These neurotransmitters are closely associated with symptoms such as constipation, bloating, and abdominal pain. In a 30-day randomized trial, in subjects with IBS, abdominal bloating can be successfully reduced with a supplementation with CUR (Giacosa et al., 2022). Previous studies have shown that CUR has a significant effect on reducing the levels of 5-HT in serum and colon (Yu et al., 2019). The intestine is the source of most serotonin in the human body, which is predominantly located in the endocrine cells of the intestinal epithelium (Ge et al., 2018).
In this study, we also investigated changes in the composition of the gut microbiota using 16S rRNA sequencing. Consistent with previous results, PCoA analysis showed distinct distances between groups, suggesting that the diversity of the gut microbiota in IBS-C rats and CUR /MOS-treated rats differs from that of normal rats. In the present study, we observed increased abundance of Bacteroidetes in the IBS rat model, which is consistent with previous studies and shows that Bacteroidetes may be a potentially harmful microbiota in patients with IBS (Ponnusamy et al., 2011; Wang et al., 2018; Pittayanon et al., 2019). In addition, we found high relative abundances of Prevotella, Vampirovibrio, and Elusimicrobium in rats with IBS-C. Previous studies have shown that Prevotella is strongly associated with a high risk of IBS-D and can interact with other members of the gut microbiota and cause visceral allergic reactions that further exacerbate IBS symptoms (Kovatcheva-Datchary et al., 2015; Su et al., 2018). In addition, Prevotella is thought to be associated with gut permeability and proinflammatory function (Larsen, 2017; Guerreiro et al., 2018). Some studies have shown that Allobaculum, a bacterium that produces short-chain fatty acids, can alleviate the symptoms of irritable bowel patients by regulating intestinal transit time (Camilleri, 2015). We observed a significant increase in the frequency of Allobaculum in rats from the MOS group. In addition, the abundance of Ruminococcus2 decreased in CUR group. Interestingly, these changes in the gut microbiota associated with IBS were partially reversed by treatment with CUR, which has been observed in other disease models. Some studies have shown that the gut microbiota was reversed by CUR, including Bacteroidetes, a kind of microbiota potentially harmful in patients with IBS (Huang et al., 2021). Ruminococcus2 is considered a protective microbiota in IBS patients as it maintains the integrity of the intestinal mucosa and reduces IBS symptoms (Rivière et al., 2016). In conclusion, the results of this study show that CUR was able to lower the levels of HT, VIP and SP and modulate the gut microbiota in rats with IBS-C. However, it is worth noting that there is significant heterogeneity among the studies and even with modern technologies, no uniform characteristics of IBS-related gut microbiota has been identified. Further studies are needed to investigate the regulatory mechanism of CUR on gut microbiota in IBS-C rats.
In this study, the effects of CUR and MOS on rats with IBS-C were comprehensively investigated by histological analysis, immunohistochemistry, and 16S rRNA sequencing, and provided important clues for studying the microbiological mechanism of IBS-C. Determination of neurotransmitter levels in serum also improves our understanding of the role of neurotransmitters in disease development and provides clues for therapeutic strategies. However, this study also has several shortcomings, including small sample size, limitations of animal models, and lack of validation of clinical data. To improve the reliability and generalizability of the study, we need to perform more clinical validations and a more comprehensive experimental design. Overall, these results provide valuable information for the treatment and microbiological study of IBS-C and provide a foundation for future research, but more in-depth studies are needed to confirm and refine these results. Future work should more comprehensively investigate neurotransmitter changes and their interactions with the gut microbiota to better understand the role of the brain-gut axis in the treatment of IBS-C (Sun et al., 2023).
5 Conclusion
The results of this study show that CUR is able to decrease the levels of HT, VIP and SP and regulate the gut microbiota in rats with IBS-C, and then exert the therapeutic effect on IBS-C. The results suggest that CUR may represent a treatment option for IBS-C through modulation of the gut microbiota and relevant neurotransmitters. Our results support the possibility of using CUR to treat IBS-C patients.
Data availability statement
The data presented in the study are deposited in the National Center for Biotechnology Information database, accession number PRJNA1003150.
Ethics statement
The animal study was approved by Shanghai Public Health Clinical Center Laboratory Animal Welfare and Ethics. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XT: Writing – original draft, Writing – review & editing. HR: Writing – review & editing, Data curation. SB: Writing – review & editing, Funding acquisition, Investigation, Methodology, Project administration, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Shanghai Science and Technology Commission (grant no. 16411972100).
Acknowledgments
The authors thank Ruifang Li for her help with animal feeding and data collection. We thank Zhonghui Gai for advice on bioinformatics analysis and also thank Manfei Jin and Yao Dong for their advice and help with language polish and image manipulation.
Conflict of interest
HR was employed by Shanghai Mobio Biomedical Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Ahangarpour, A., Sayahi, M., and Sayahi, M. (2019). The antidiabetic and antioxidant properties of some phenolic phytochemicals: a review study. Diabetes Metab. Syndr. 13, 854–857. doi: 10.1016/j.dsx.2018.11.051
Bhattarai, Y., Schmidt, B. A., Linden, D. R., Larson, E. D., Grover, M., Beyder, A., et al. (2017). Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT(3) receptor expression via acetate production. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G80–g87. doi: 10.1152/ajpgi.00448.2016
Black, C. J., and Ford, A. C. (2020). Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 17, 473–486. doi: 10.1038/s41575-020-0286-8
Camilleri, M. (2015). Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin. Gastroenterol. Hepatol. 13:1051. doi: 10.1016/j.cgh.2014.07.020
Chang, X. R., Wang, L., Li, J., and Wu, D. S. (2016). Analysis of anti-depressant potential of curcumin against depression induced male albino wistar rats. Brain Res. 1642, 219–225. doi: 10.1016/j.brainres.2016.03.010
Cheng, H., Zhang, D., Wu, J., Liu, J., Zhou, Y., Tan, Y., et al. (2023). Interactions between gut microbiota and polyphenols: a mechanistic and metabolomic review. Phytomedicine 119:154979. doi: 10.1016/j.phymed.2023.154979
Defrees, D. N., and Bailey, J. (2017). Irritable bowel syndrome: epidemiology, pathophysiology, diagnosis, and treatment. Prim. Care 44, 655–671. doi: 10.1016/j.pop.2017.07.009
Di Rosa, C., Altomare, A., Terrigno, V., Carbone, F., Tack, J., Cicala, M., et al. (2023). Constipation-predominant irritable bowel syndrome (IBS-C): effects of different nutritional patterns on intestinal Dysbiosis and symptoms. Nutrients 15:1647. doi: 10.3390/nu15071647
Enck, P., Aziz, Q., Barbara, G., Farmer, A. D., Fukudo, S., Mayer, E. A., et al. (2016). Irritable bowel syndrome. Nat. Rev. Dis. Primers. 2:16014. doi: 10.1038/nrdp.2016.14
Fan, X., Shi, J., Liu, Y., Zhang, M., Lu, M., and Qu, D. (2023). Cannabidiol-decorated Berberine-loaded microemulsions improve IBS-D therapy through ketogenic diet-induced Cannabidiol receptors overexpression. Int. J. Nanomedicine 18, 2839–2853. doi: 10.2147/IJN.S402871
Ge, X., Pan, J., Liu, Y., Wang, H., Zhou, W., and Wang, X. (2018). Intestinal crosstalk between microbiota and serotonin and its impact on gut motility. Curr. Pharm. Biotechnol. 19, 190–195. doi: 10.2174/1389201019666180528094202
Giacosa, A., Riva, A., Petrangolini, G., Allegrini, P., Fazia, T., Bernardinelli, L., et al. (2022). Beneficial effects on abdominal bloating with an innovative food-grade formulation of Curcuma longa and Boswellia serrata extracts in subjects with irritable bowel syndrome and small bowel Dysbiosis. Nutrients 14:416. doi: 10.3390/nu14030416
Guerreiro, C. S., Calado, Â., Sousa, J., and Fonseca, J. E. (2018). Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front. Med. 5:349. doi: 10.3389/fmed.2018.00349
Guo, J., Chen, L., Wang, Y. H., Song, Y. F., Zhao, Z. H., Zhao, T. T., et al. (2022). Electroacupuncture attenuates post-inflammatory IBS-associated visceral and somatic hypersensitivity and correlates with the regulatory mechanism of Epac1-Piezo2 Axis. Front. Endocrinol. 13:918652. doi: 10.3389/fendo.2022.918652
Hadjivasilis, A., Tsioutis, C., Michalinos, A., Ntourakis, D., Christodoulou, D. K., and Agouridis, A. P. (2019). New insights into irritable bowel syndrome: from pathophysiology to treatment. Ann. Gastroenterol. 32, 554–564. doi: 10.20524/aog.2019.0428
Huang, J., Guan, B., Lin, L., and Wang, Y. (2021). Improvement of intestinal barrier function, gut microbiota, and metabolic endotoxemia in type 2 diabetes rats by curcumin. Bioengineered 12, 11947–11958. doi: 10.1080/21655979.2021.2009322
Kocaadam, B., and Şanlier, N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895. doi: 10.1080/10408398.2015.1077195
Kosako, M., Akiho, H., Miwa, H., Kanazawa, M., and Fukudo, S. J. B. M. (2018). Impact of symptoms by gender and age in Japanese subjects with irritable bowel syndrome with constipation (IBS-C): a large population-based internet survey. Biopsychosoc Med 12:12. doi: 10.1186/s13030-018-0131-2
Kovatcheva-Datchary, P., Nilsson, A., Akrami, R., Lee, Y. S., De Vadder, F., Arora, T., et al. (2015). Dietary Fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982. doi: 10.1016/j.cmet.2015.10.001
Larsen, J. M. (2017). The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151, 363–374. doi: 10.1111/imm.12760
Li, R., Yao, Y., Gao, P., and Bu, S. (2020). The therapeutic efficacy of curcumin vs. metformin in modulating the gut microbiota in NAFLD rats: a comparative study. Front. Microbiol. 11:555293. doi: 10.3389/fmicb.2020.555293
Liang, W. J., Zhang, G., Luo, H. S., Liang, L. X., Huang, D., and Zhang, F. C. (2016). Tryptase and protease-activated receptor 2 expression levels in irritable bowel syndrome. Gut Liver 10, 382–390. doi: 10.5009/gnl14319
Liu, C., Cui, Y., Li, X., and Yao, M. (2021). Microeco: an R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 97:fiaa255. doi: 10.1093/femsec/fiaa255
Liu, J., Li, F., Tang, X. D., Ma, J., Ma, X., Ge, D. Y., et al. (2015). XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement. Altern. Med. 15:387. doi: 10.1186/s12906-015-0913-z
Ng, Q. X., Soh, A. Y. S., Loke, W., Venkatanarayanan, N., Lim, D. Y., and Yeo, W. S. (2018). A Meta-analysis of the clinical use of curcumin for irritable bowel syndrome (IBS). J. Clin. Med. 7:298. doi: 10.3390/jcm7100298
Ohkusa, T., Koido, S., Nishikawa, Y., and Sato, N. (2019). Gut microbiota and chronic constipation: a review and update. Front. Med. 6:19. doi: 10.3389/fmed.2019.00019
Parthasarathy, G., Chen, J., Chen, X., Chia, N., O'Connor, H. M., Wolf, P. G., et al. (2016). Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 150, 367–79.e1. doi: 10.1053/j.gastro.2015.10.005
Peters, S. A., Edogawa, S., Sundt, W. J., Dyer, R. B., Dalenberg, D. A., Mazzone, A., et al. (2017). Constipation-predominant irritable bowel syndrome females have Normal colonic barrier and secretory function. Am. J. Gastroenterol. 112, 913–923. doi: 10.1038/ajg.2017.48
Pittayanon, R., Lau, J. T., Yuan, Y., Leontiadis, G. I., Tse, F., Surette, M., et al. (2019). Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology 157, 97–108. doi: 10.1053/j.gastro.2019.03.049
Ponnusamy, K., Choi, J. N., Kim, J., Lee, S. Y., and Lee, C. H. (2011). Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J. Med. Microbiol. 60, 817–827. doi: 10.1099/jmm.0.028126-0
Rivière, A., Selak, M., Lantin, D., Leroy, F., and De Vuyst, L. (2016). Bifidobacteria and butyrate-producing Colon Bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7:979. doi: 10.3389/fmicb.2016.00979
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Smith, T. K., and Koh, S. D. (2017). A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G1–g14. doi: 10.1152/ajpgi.00337.2016
Su, T., Liu, R., Lee, A., Long, Y., Du, L., Lai, S., et al. (2018). Altered intestinal microbiota with increased abundance of Prevotella is associated with high risk of diarrhea-predominant irritable bowel syndrome. Gastroenterol. Res. Pract. 2018:6961783. doi: 10.1155/2018/6961783
Sun, Q., Jia, Q., Song, L., and Duan, L. (2019). Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: a systematic review and meta-analysis. Medicine 98:e14513. doi: 10.1097/MD.0000000000014513
Sun, Z., Wang, X., Feng, S., Xie, C., Xing, Y., Guo, L., et al. (2023). A review of neuroendocrine immune system abnormalities in IBS based on the brain-gut axis and research progress of acupuncture intervention. Front. Neurosci. 17:934341. doi: 10.3389/fnins.2023.934341
Tack, J., Stanghellini, V., Mearin, F., Yiannakou, Y., Layer, P., Coffin, B., et al. (2019). Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol. 19:69. doi: 10.1186/s12876-019-0985-1
Tang, R., Zhang, J., Nan, H., Lv, R., Chen, X., Liu, Y., et al. (2022). Exploring molecular mechanisms of Aloe barbadmsis miller on Diphenoxylate-induced constipation in mice. Evid. Based Complement. Alternat. Med. 2022:6225758. doi: 10.1155/2022/6225758
Wang, X., Qi, Q., Wang, Y., Wu, H., Jin, X., Yao, H., et al. (2018). Gut microbiota was modulated by moxibustion stimulation in rats with irritable bowel syndrome. Chin. Med. 13:63. doi: 10.1186/s13020-018-0220-y
Wen, Y., Li, J., Long, Q., Yue, C. C., He, B., and Tang, X. G. (2020). The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis based on seventeen randomized controlled trials. Int. J. Surg. 79, 111–119. doi: 10.1016/j.ijsu.2020.04.063
Xi, T. F., Li, D. N., Li, Y. Y., Qin, Y., Wang, H. H., Song, N. N., et al. (2019). Central 5-hydroxytryptamine (5-HT) mediates colonic motility by hypothalamus oxytocin-colonic oxytocin receptor pathway. Biochem. Biophys. Res. Commun. 508, 959–964. doi: 10.1016/j.bbrc.2018.11.122
Yu, Y. C., Li, J., Zhang, M., Pan, J. C., Yu, Y., Zhang, J. B., et al. (2019). Resveratrol improves brain-gut Axis by regulation of 5-HT-dependent signaling in the rat model of irritable bowel syndrome. Front. Cell. Neurosci. 13:30. doi: 10.3389/fncel.2019.00030
Yu, Y., Wu, S., Li, J., Wang, R., Xie, X., Yu, X., et al. (2015). The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: involvement of 5-HT-dependent signaling. Metab. Brain Dis. 30, 47–55. doi: 10.1007/s11011-014-9554-z
Keywords: curcumin, gut microbiota, irritable bowel syndrome, neurotransmitters, constipation
Citation: Tu X, Ren H and Bu S (2023) Therapeutic effects of curcumin on constipation-predominant irritable bowel syndrome is associated with modulating gut microbiota and neurotransmitters. Front. Microbiol. 14:1274559. doi: 10.3389/fmicb.2023.1274559
Edited by:
Qin Xiang Ng, Singapore General Hospital, SingaporeReviewed by:
Valentina Caputi, University College Cork, IrelandStanislav Kotlyarov, Ryazan State Medical University named after academician I.P. Pavlov, Russia
Wuwen Feng, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2023 Tu, Ren and Bu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shurui Bu, YnVzaHVydWlAZnVkYW4uZWR1LmNu
 Xiaoting Tu
Xiaoting Tu Hongyan Ren2
Hongyan Ren2 Shurui Bu
Shurui Bu