- 1Department of Spine Surgery, Xi’an Jiaotong University Affiliated HongHui Hospital, Xi’an, China
- 2Laboratory of Translational Medicine, School of Medicine, Shihezi University, Shihezi, China
Bone cystic echinococcosis (CE) is one of the most complex and dangerous of all echinococcoses. The lack of typical imaging features and clinical manifestations makes diagnosis and treatment of this disease difficult. X-ray and computed tomography (CT) images of bone CE are similar to those of bone cysts, giant-cell bone tumors, and bone metastases, but magnetic resonance imaging (MRI) shows good diagnostic value due to excellent soft-tissue imaging features. Serological tests cannot be used as a definitive diagnostic method for bone CE due to cross-reactivity, which can lead to false-positive or false-negative results. The development of novel antigens can open new frontiers in the diagnosis of the disease. Currently, views conflict on how to diagnose and treat bone CE. Both surgical and pharmacological treatments can be used, but determining which is appropriate is difficult due to the different sites and clinical manifestations of bone CE. Radical resection is not indicated for large-bone injuries, and Pharmacotherapy becomes important. This article reviews the progress of research into the pathogenesis and clinical manifestations of, and diagnostic strategies and treatment options for, bone CE. We aimed to provide a reference for clinical diagnosis and -treatment options.
1. Introduction
Cystic echinococcosis, commonly called Hydatid disease is caused by the larval form of the parasitic tapeworm, Echinococcus granulosus (E. granulosus). Cystic echinococcosis (CE) is found on all continents except Antarctica and is classified by the World Health Organization (WHO) as one of the most neglected and geographically widespread parasitic diseases (World Health Organization, 2015). The lifecycle of E. granulosus involves two main hosts, one intermediate and one final. Dogs are common final hosts; the adult larvae adhere to their small intestinal mucosa, and eggs are excreted with feces. In intermediate hosts—which are humans and herbivores (cattle, sheep, goats, camels, horses, and pigs)—the eggs hatch in the body and can reach various sites via the circulatory system (Gnanasekaran et al., 2016).
Musculoskeletal involvement is rare, with an incidence of 0.5–4.0% in all CE cases (Neumayr et al., 2013a). CE can parasitize almost any bone in the body, but half of all cases occur in the spine (Loudiye et al., 2003); the incidence in other bones is lower (Schnepper and Johnson, 2004). Spinal CE was seen in all age groups, both sexes can be affected (Altinörs et al., 2000). Musculoskeletal infection of the spine often results in severe disability or even death (Inayat et al., 2019). By contrast, clinical presentations of patients with nonspinal bone CE are often nonspecific, with pain and pathological fractures being the most common (Monge-Maillo et al., 2017). Clinical history as well as laboratory, imaging, and serological tests play crucial roles in diagnosing the disease. Radical surgical resection combined with chemotherapy is the current clinical treatment of choice, but the postsurgical recurrence rate can be as high as 40% (Salman et al., 2018). Patients who experience spinal encopresis due to spinal CE often have a high recurrence rate after surgery (Caglar et al., 2019). The prognosis for patients with bone CE is poor: paraplegia, impaired mobility, postoperative disability, and even death (Gdoura et al., 2010; Arkun and Mete, 2011). Because the pathological mechanism of bone CE is unknown, the current literature consists mostly of case studies rather than systematic, comprehensive reports; therefore, consensus is lacking on the diagnosis and treatment of this disease.
2. Possible pathological mechanism of bone CE: hematogenous pathway and secondary infection
The route of parasitic infection in bone CE remains unclear (Cattaneo et al., 2019). In most cases, the disease is confined to the bones and rarely infects other organs (Torricelli et al., 1990). Protoscoleces (PSCs) invade the body and, via blood circulation, usually parasitize organs other than the liver. Commonly parasitized sites are the lungs; spleen; and multiple locations in brain tissue, bones, lymph nodes, and muscles (Petra et al., 2003). Therefore, both primary hematogenous and secondary infections in other organs can cause the development and progression of bone CE.
CE appears mostly in cancellous bone. Cysts lining cancellous bone can fracture bone tissue by attacking it; the disease can also spread to invade exoskeletal structures (Papanikolaou, 2008). Possible pathological mechanisms are as follows. (1) The growing cysts compress bone tissue, causing bones to atrophy (Jacquier and Piroth, 2018). (2) Cysts invade in multiple directions along less-resistant microstructures such as the bone canal; hydatid tissue erodes and replaces bone trabeculae and then destroys and breaks through the bone cortex (Neumayr et al., 2013a,b). (3) Enlarged echinococcal cysts obstruct the vessels that nourish bone, causing ischemic necrosis (Thomas et al., 1997). (4) CE cysts directly activate the proliferation of osteoclasts, causing physiological osteolysis (Song et al., 2007). (5) Cystic invasion decreases host immunity and causes soft-tissue infiltration and fistula formation, while the resulting inflammatory reaction can lead to bone destruction with neurological and joint infection (Morris et al., 2002). (6) CE lesions can spread directly to adjacent bone tissue and destroy its bony structure (Jacquier and Piroth, 2018).
The rigid structure of bone inhibits cysts from forming an exterior membrane therein (Neumayr et al., 2013a,b). Therefore, in the early stage of bone CE, cysts grow invasively along structures that offer the least resistance, such as the bone canal, and lesions appear as irregular branches (Torricelli et al., 1990). However, late-stage intrabony cysts can break through the bone cortex and involve extraosseous structures, which lack rigidity and therefore cannot restrict cystic proliferation. In addition, soft-tissue intracapsular cysts are often accompanied by plasma exudate that invades surrounding tissues. The periosteum and articular cartilage are resistant to parasitic attack; therefore, cartilage infection is rarely reported in cases of bone CE (Morris et al., 2002).
In the spine, particularly in the thoracolumbar region, due to a dense regional vascular network and rich blood supply, cysts infiltrate vertebral cancellous bone via the vertebral artery and develop along the bone marrow cavity toward the epiphyseal plate and articular cartilage in a swollen honeycomb-like or “soap bubble” shape (Arana Iniquez, 1978). Progressive sclerotic cysts compress the vertebral body, pedicle, and lamina to varying degrees, but most of the infected tissue does not attack the intervertebral disc (IVD) due to the periosteal barrier (Schnepper and Johnson, 2004).
3. Clinical manifestations of three types of bone CE
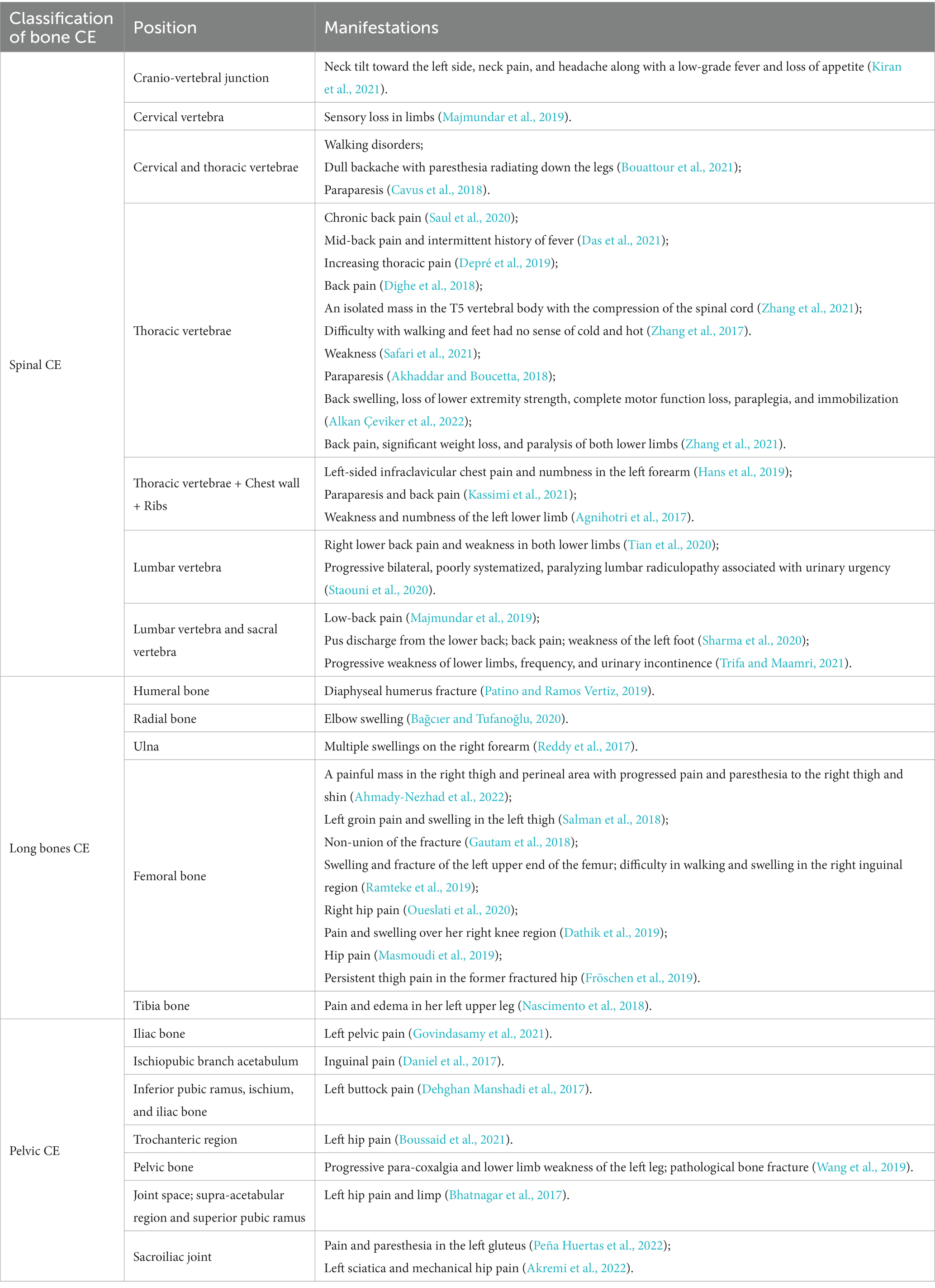
We searched the PubMed database for studies addressing recent treatment and diagnosis of bone CE and found 41 case reports thereof in the last 5 years. As shown in Table 1, the clinical presentation of bone CE is complex, with symptoms depending on the location of the infection, size of the lesion, degree of bone and surrounding-tissue invasion, and complications arising from the cyst and secondary infection (McManus et al., 2012). As shown in Figure 1, cysts can parasitize any bone in the body, but most infect only a single bone (602/721, 83%) (Steinmetz et al., 2014). The results of a European multicenter study showed that 45% of CE cases involved spinal CE and that long bones (femur, 10%; humerus; 2%) were sites of parasitization, while flat bones such as pelvic bones (14%) and ribs (8%) could also be invaded (Cattaneo et al., 2019). Echinococcosis in other parts (such as the skull, sternum, scapula and phalanx) is rare. Therefore, this article focuses on spinal CE, long bone CE and pelvic CE.
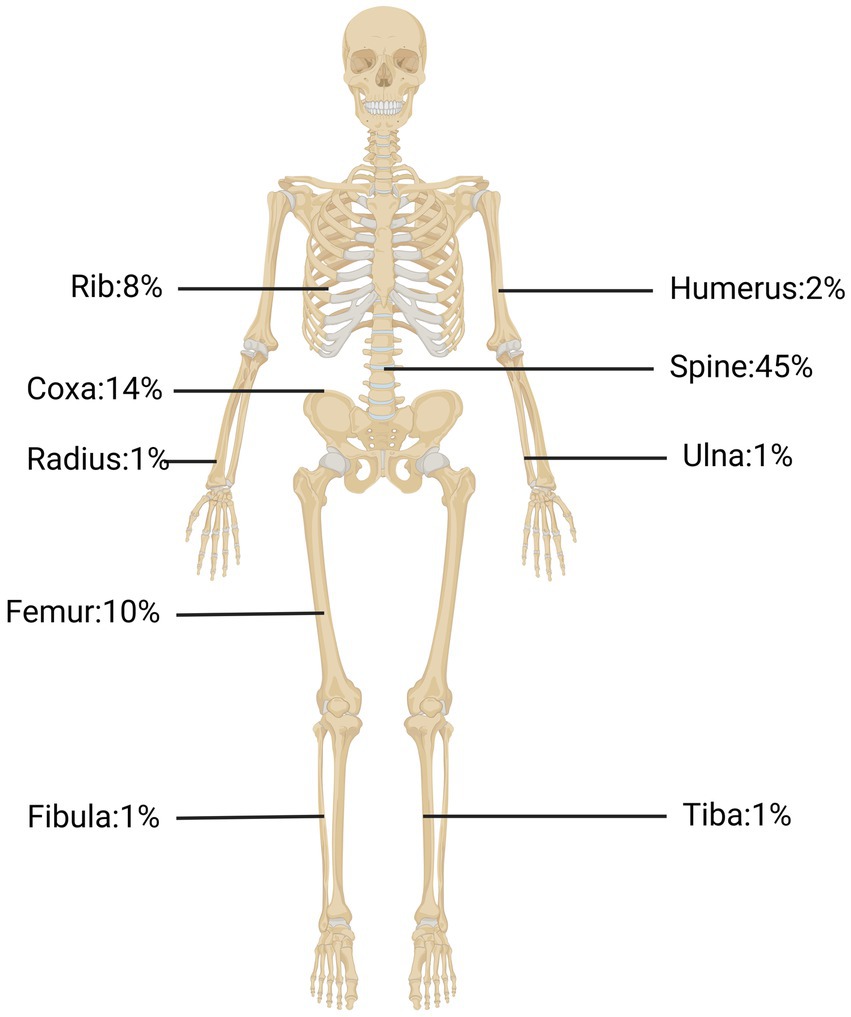
Figure 1. The occurrence rates of bone CE in the spine, pelvic bone, tibia bone, ribs, and other locations.
3.1. Spinal CE
The thoracic segments (46–50%) have the highest infection rate in spinal CE, and the lumbar (20–29%) and sacral (20–23%) disease also occur. The cervical spine is the least susceptible to infection of the spinal CE (Pamir et al., 2002). Eventually, spinal CE patients usually present with symptoms of spinal cord compression, with back pain in 85%, radicular symptoms in 25%, and cauda equina syndrome or even paralysis in 25–77% (Neumayr et al., 2013a). Neurological infection occurs in 20–80% of cases (Sharma et al., 2020). Patients can present with decreased sensation in one or both legs and the perineum, gradually developing signs of neurological damage such as bilateral lower-limb mobility impairments, urinary and fecal dysfunction, and weakness in urination (Sioutis et al., 2021). Ozek et al. reported that rapid-onset neurological disorders are due to inadequate blood supply caused by vascular injury; in such cases, patient recovery is often slow and incomplete (Ozek, 1994). ROBINSON RG’s case report of a female patient with severe neurological symptoms. Despite surgical treatment to remove the cyst, the patient did not have a good prognosis (Robinson, 1959). Paraplegia due to disease recurrence has been reported in as many as 45% of cases (Bhojraj and Shetty, 1999). The recurrence rate of spinal CE is 30–40%, usually due to intraoperative cyst rupture (Johnson and Hobson, 1977). Moreover, the resulting spillage of cyst contents can cause a variety of allergic reactions such as pruritus, urticaria, dyspnea, asthma, vomiting, diarrhea, abdominal cramps, bacterial infection, and even anaphylaxis. This complex clinical presentation poses great difficulties in diagnosis (Pathania et al., 2000).
3.2. Pelvic CE
The incidence of pelvic CE is second only to that of spinal CE. A study included 31 patients with pelvic encopresis from 1991 to 2017, with the ilium being the most common (21/31), followed by the acetabulum (7/31), pubis (6/31), sciatica (5/31), and sacrum (5/31) (Inayat et al., 2019). The pelvic bone is densely packed with cancellous bones and is rich in blood supply, providing highly favorable conditions for parasitization by E. granulosus. Hemipelvic infection has commonly been reported in recent years; the hip joint easily becomes infected, impairing mobility. Pelvic CE can lie latent for several years and gradually become symptomatic as the disease progresses, generally manifesting as symptoms of lumbosacral-nerve compression (Arik et al., 2015). Generally, the first clinical manifestations appear late in the disease’s progression due to the rigid skeletal structure and slow cystic growth. Severe cases are usually associated with late complications such as lumbosacral pain, swelling, fistula formation, and progressive worsening of pain in both legs (Inayat et al., 2019). Although sciatica is often reported as the first symptom of pelvic CE, it must be emphasized that the symptoms of this condition depend on the sizes and locations of cysts.
3.3. Long bones CE
A total of 702 patients with encopresis were included in one study, including 111 patients with long bone encopresis. The highest frequency of infection was in the femur (72/111, 65%), followed by the humerus (11/111, 10%), radius (3/111, 2.7%) and tibia (3/111, 2.7%), with ulna (1/111, 0.9%) and fibula (1/111, 0.9%) cases being rare (Steinmetz et al., 2014). As mentioned above, the femur is the most susceptible to infection. Colonization of these bones by E. granulosus mostly involves the epiphysis in the early stages and can initially be asymptomatic. Extensive bony lesions in later stages can lead to pathological hyperplasia of the infected limb, causing swelling and pain that becomes progressively more intense as the burden of activity increases (Song et al., 2007). Local examination of patients with femoral CE can reveal deep pressure pain in the greater trochanter, accompanied by limitation of hip motion, which can lead to late complications such as pathological fracture and fistula formation in severe cases (Kapoor et al., 2013; Inayat et al., 2019). Patients with humeral CE similarly have no obvious symptoms in the early stages. Bone erosion progresses to an advanced stage of severe bone damage, at which point patients often seek medical attention for severe pain. Although the CE of the long bones does not infect the joint surface, advanced pathological bone destruction, inflammation, and infection of the surrounding soft tissues can affect adjacent joints. Therefore, localized masses, restricted mobility, and severe pain can be clinical features of this type of CE.
4. Imaging examination combined with serological results to diagnose bone CE
Because the disease features of bone CE are often atypical, they often pose a diagnostic challenge to clinicians. Given that bone CE progresses very slowly, intrabony cysts can remain quiescent for long periods, even decades (Cattaneo et al., 2019). Spinal CE takes an average of at least 6 months to be diagnosed even after the onset of symptoms (Khazim et al., 2003). Imaging combined with serological testing is now the mainstay of clinical diagnosis.
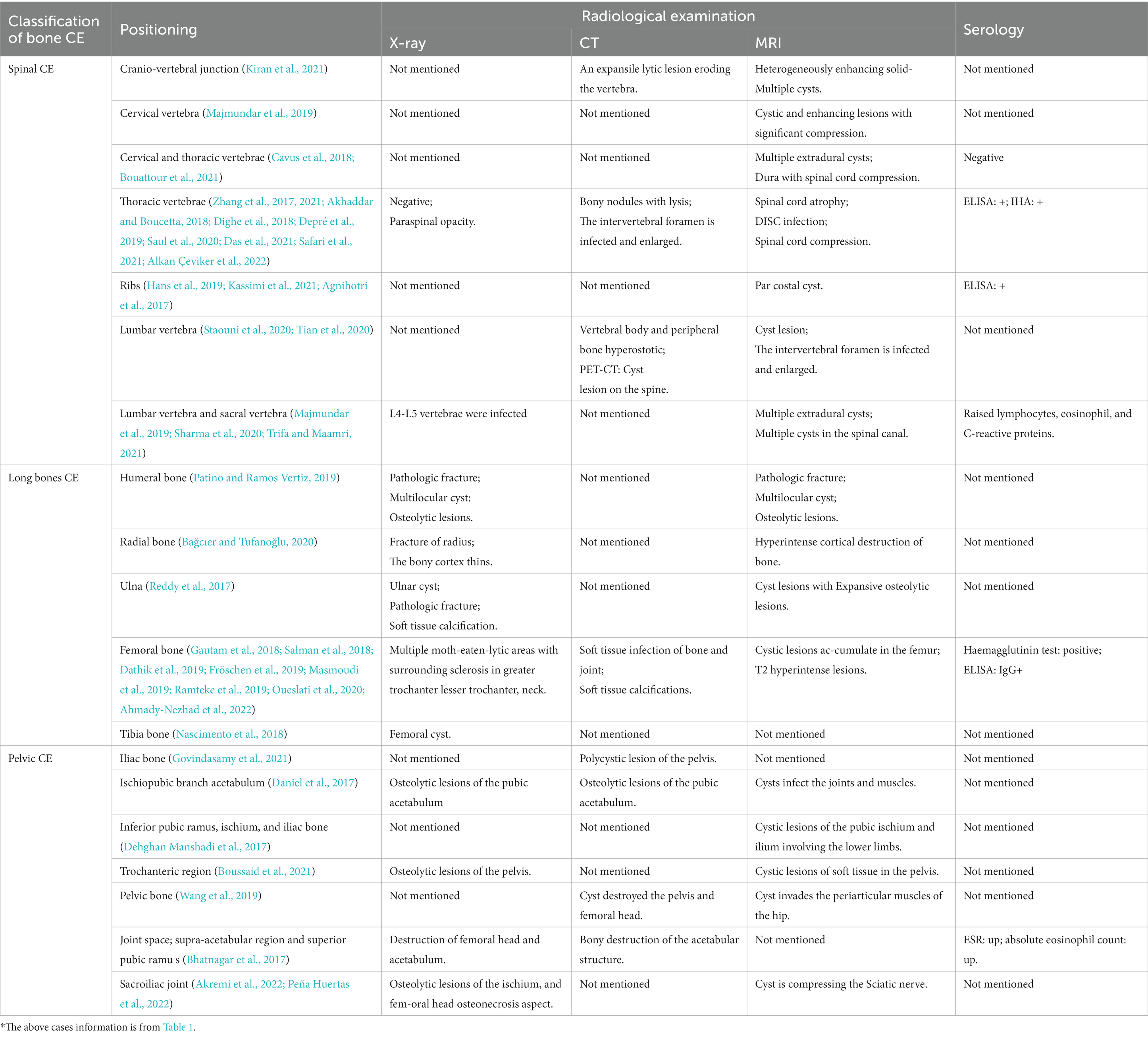
4.1. Radiographic examination
The most commonly used imaging techniques for bone CE are X-ray, computed tomography (CT), and magnetic resonance imaging (MRI). X-ray is the first diagnostic step when patients present with pain, swelling, and other associated symptoms (Ira et al., 2001). While X-rays often do not show typical imaging features of patients with bone CE (Table 2). In addition, the periosteal reaction is usually not visible on X-ray images; if not, this indicates a pathological fracture caused by an attack on the bone cortex (Chen et al., 2020). Bone infections usually show bone destruction and cystic lesions on imaging. Cystic lesions appear as hypodensities on both CT and X-ray, but CT can show more information on these lesions, including size, extent, location, and degree of bone destruction (Tüzün and Hekimoğlu, 2001). MRI is the most relevant of all imaging modalities for the diagnosis of CE; its excellent soft-tissue resolution clearly shows the relationship between the lesion and adjacent tissues (Pamir et al., 2002).
The most common manifestation of spinal CE is one or more round or oval sockets with indistinct borders that cannot be distinguished from chronic osteomyelitis on imaging (Muscolo et al., 2015). In the early stage, the lesion appears in the vertebral body and can spread to all vertebral structures. When the lesion involves the spinal canal, MRI can show the exact number and sizes of cysts, the integrity of the cyst wall, and the degree of spinal-cord compression (Herrera et al., 2005). Berk et al. reviewed the characteristics of spinal CE on MRI: (World Health Organization, 2015) sausage-like appearance with thin-walled, regular, semicircular terminals; (Gnanasekaran et al., 2016) capsular cavities without septa or fragments, occasionally spherical; (Neumayr et al., 2013a) signal intensity of capsule contents similar to that of CSF; and (Loudiye et al., 2003) capsule wall signal equal to or slightly lower than that of cystic contents on T1-weighted (T1W) images (Berk et al., 1998). In vertebral CE, the most common features are uninjured IVD and vertebral body, while the paraspinal area, subperiosteal bone, and adjacent ribs are more commonly infected (Herrera et al., 2005). Destruction of discs in the advanced stages makes spinal CE difficult to distinguish from inflammatory spinal conditions.
In pelvic CE, osteolytic cystic lesions are the single striking feature located in the ilium but can span the hip and sacroiliac joints. Vertebral osteochondral reactions are uncommon. Calcifications and cysts can be found on imaging after adjacent tissues are invaded (Rangheard et al., 2001). In long-bone CE, the primary cyst begins in the epiphysis (Dathik et al., 2019). The lesion, which can be either monocystic or polycystic, is mostly located in the metaphysis and can expand into the diaphysis and form a fan-shaped cortex; however, dilatation, sclerosis, and periosteal reaction seldom occur. Polycystic lesions are more common, presenting as large round or oval ground areas of bone destruction that collect in the epiphysis or metaphysis and greatly expand the extent of bone destruction.
The progression of bone cysts is characterized by two imaging stages: (World Health Organization, 2015) the microcystic-infiltration stage, in which the cyst creates a cluster of “grape”-like changes; and (Gnanasekaran et al., 2016) the secondary-infection stage, in which inflammatory bone disease casts a grape-like shadow of bone proliferation and destruction (Arias, 1946). In advanced stages of bone CE, the inflammatory stimulation of bone proliferation exceeds the osteolytic process, and imaging has limited specificity to distinguish the disease from bone malignancy. A study by Farrokh Saidi found that “a single cyst only,” “lamellar separation,” and “cyst degeneration” are independent predictors of good prognosis in hepatic CE (Fathi et al., 2016). However, no studies have determined whether cystic calcification can also predict prognosis in bone CE. In the author’s opinion, calcified cysts indicate a lower capacity for cystic growth, a lessened ability to invade surrounding tissues, and a tendency to limit the lesion. Nevertheless, a calcified cyst can act as an intrabony occupying lesion, compressing or even blocking the ability of intrabony trophoblastic vessels to support the bone, thereby causing bone ischemia and compressing nerve tissue in some cases.
4.2. Serology
Serological tests can be used to support bone CE diagnosis and as screening tools. Such tests are divided into two categories: (World Health Organization, 2015) antigen detection using encapsulated cystic fluid and PSC larvae; and (Gnanasekaran et al., 2016) detection of antibodies (aBs) in patient serum. Commonly used antigen indicators in the laboratory include anti–E. granulosus cyst fluid (EgCF) antigen, fine-grained echinococcal cestode antigen, epithelial glycoprotein (EGP), semi-purified CE cyst fluid antigen B (AgB), and E2 receptor alpha (Era2) (Siles-Lucas et al., 2017). In antigen-based sensitivity (Sens) and specificity (Sp) experiments, the Sen of antigen detection was 45–92% in both CE patients and healthy populations, while Spc was 70–100%. This means that the surface antigens of both populations contain similar antigenic determinant clusters, which are thought to be prone to cross-reactivity (Carmena et al., 2006). Some newer antigens, including E. granulosus tegumental antigen (EgTeg) and E. granulosus alkaline phosphatase (EgAP), have shown >90% Spc and Sen in experiments (Ortona et al., 2005). Although such results still require support from studies with large samples, they provide important reference values for the diagnosis of CE.
The sensitivity of a diagnostic test for bone CE depends on the integrity, growth viability, and locations of cysts (List et al., 2010). In the early stages, intrabony cysts are positive on serological examination due to their inability to form fibrous membranes or due to cystic rupture, infection, or abscess formation (McManus, 2014). Serological tests are mostly negative in the late stages due to cyst aging or calcification, and false-negative results cannot be avoided. The Casoni and indirect-hemagglutination tests also show good diagnostic potential for bone CE (Wang et al., 2019). Ozdemir et al. reported three cases of spinal CE; two were serologically negative but confirmed to have spinal CE via pathology (Ozdemir et al., 2004). Three problems exist with the immune response to serological diagnostic tests for CE: (1) E. granulosus antigens cross-react with antigens of other parasitic diseases, which can impair test specificity (2) The strength of the patient’s immune system affects serological test results, with both false-positive and -negative results occurring. (3) Test sensitivity decreases to 25–56% in extrahepatic CE (Xiao et al., 2003). Therefore, the serological examination does not provide sufficient evidence for it to be used as the main diagnostic method in bone CE and must hence be combined with other methods for comprehensive analysis.
5. Treatment: radical resection and drug therapy
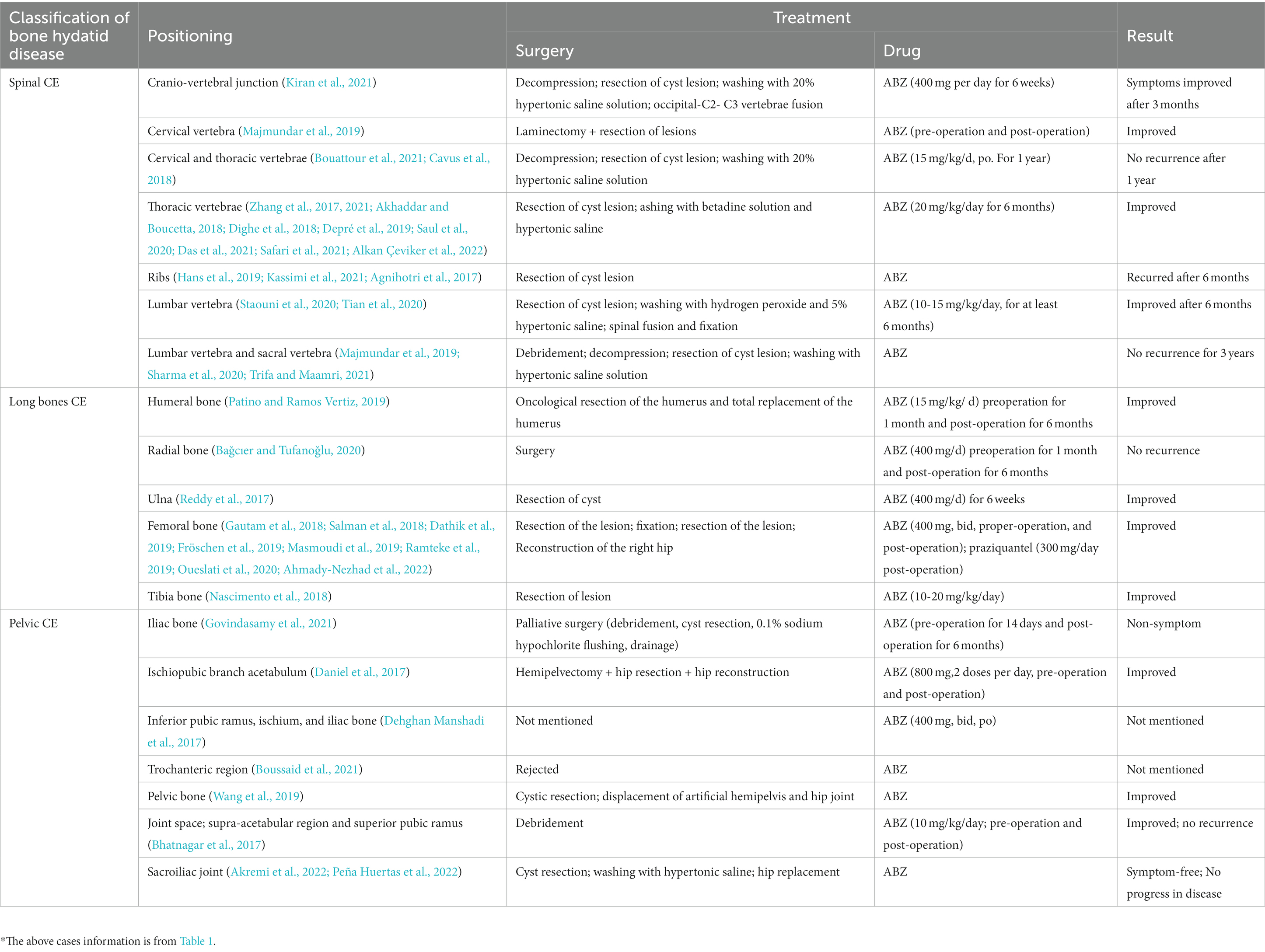
As shown in Table 3, the treatment of bone CE is site dependent. Currently, the most appropriate treatment is radical surgery or resection of all infected bone (Arkun and Mete, 2011). However, radical surgery is difficult to perform and leaves the patient prone to recurrence, especially when the spine, pelvic bones, and ribs are infected. Therefore, surgery is sometimes combined with other treatments (e.g., radiotherapy) to prevent recurrence.
5.1. Surgery
Before the operation, need to determine the locations and sizes of cysts and the degree to which soft tissues surrounding the bone have been invaded. Surgical recommendations for bone CE are as follows. (1) The bones and surrounding soft tissues infected by CE must be exposed (Luan et al., 2022). (2) Integrity of the cyst wall must be ensured during resection of CE cysts (Das et al., 2021). (3) After such resection, 1–2 cm of parasite-free bone must be removed (Ozdemir et al., 2004). (4) During the operation, the surgical area should be cleaned with a short-term insecticide such as hypertonic saline to avoid recurrence caused by remaining E. granulosus (Khazim et al., 2003). (5) Bone grafts can be implanted for functional reconstruction after cyst resection (Thomas et al., 1997). Although many preoperative tests are available to detect osteochondroma-like lesions, bone CE is often found during surgery and confirmed by pathological examination.
Spinal CE should be given higher treatment priority than other types of bone CE. The internationally recognized classification of this disease largely guides the choice of surgical approach (Açikgöz et al., 1996). Complete resection is not possible in extensive intradural CE (Kaen et al., 2009). Intradural CE generally features multiple cysts that can attach to the lumbar-spinal roots, as well as some thin-walled cysts that can easily rupture during surgery (İşlekel et al., 1998). A limited single cyst is associated with better treatment outcomes, and surgery in such cases is considered curative if the cyst is completely removed and does not rupture (Neumayr et al., 2013a). Epidural CE lesions can vary from a single epidural worm cyst to a paravertebral encapsulated cyst to a large, dumbbell-shaped encapsulated cyst with surrounding soft-tissue invasion (Khazim et al., 2003). Patients with these two types of spinal CE are often found to have spinal cord compression. Anterior resection of the cyst is usually performed in these cases; however, if complete resection is not possible, negative-pressure aspiration and partial wall resection are desirable, and drugs with high toxicity should be avoided (Parvaresh et al., 1996). The recurrence rate of epidural CE is high (27%) because multiple cysts cannot be completely excised and the cysts are prone to rupture (Neumayr et al., 2013a).
When bone CE occurs within the vertebral body, microcystic infiltration makes complete resection difficult to achieve. However, surgical intervention can prolong patient survival (Turtas et al., 1980). Complete excision of the cyst with no destruction of the cyst wall is the standard treatment for spinal CE. However, complete cyst removal is difficult in many cases for various reasons: (World Health Organization, 2015) the cyst wall is thin; (Gnanasekaran et al., 2016) the surrounding soft tissue is attached to the cyst wall; Neumayr et al. (2013a) bone CE was not considered preoperatively; (Loudiye et al., 2003) the lesion is extensive, making surgery too invasive for the patient to tolerate; and Schnepper and Johnson (2004) surgery results in bone defects and requires the use of various techniques such as bone grafts, pedicle screw systems, titanium-cage implants, plates, or bone cement to stabilize the spine (Iplikçioğlu et al., 1991). Bone cement might be one of the best options for postoperative vertebral stabilization due to its high-temperature killing effect on PSCs, which reduces postoperative recurrence of bone CE (Yildiz et al., 2001). For large spinal-CE lesions, palliative surgical treatment plus chemotherapy might be more appropriate to limit surgical stress or damage to the patient’s neural tissues (Sudo and Minami, 2010).
Pelvic CE is the second-most widespread type of bone CE, which is difficult to treat, and the outcome and prognosis depend on whether the CE has invaded the sacroiliac or hip joints (Martínez et al., 2001). Surgical attempts to remove the lesion can fail, resulting in severe functional disability when the joint is infected. Currently, common surgical treatments for pelvic CE include simple drainage or debridement, complete resection, total hip arthroplasty, bone grafting, pubic fusion, giant prosthesis, arthroplasty, osteotomy, and hemipelvic resection (Liang et al., 2014). Hemipelvic resection is frequently used in patients with extensive pelvic CE who are infected in multiple sites. However, it is accompanied by high mortality and complications such as sepsis, pressure sores, and loss of function, meaning that patients are often resistant to this procedure. Palliative surgical treatment with long-term oral administration of effective anthelmintics such as albendazole (ABZ) is usually a good option for patients with bone CE accompanied by extensive bone destruction (Sudo and Minami, 2010). Daniel et al. reported a case of pelvic CE extending to the hip. After hip resection combined with total hip arthroplasty supplemented by perioperative medication, the patient had no signs of recurrence or sepsis at 1-year postoperative follow-up, but he required a walker as a mobility aid (Daniel et al., 2017). As can be seen, the outcome of pelvic-joint infection is very serious. Once pelvic CE infects the hip joint, it usually causes weakness in the legs and reduces joint function. Total hip replacement may be considered to restore the function of the joint.
Treatment and prognosis of long-bone CE are better than those of spinal and pelvic CE because the growth of worms is more limited in these bones than in the spine or pelvis. When the infection occurs proximally, femoral CE is more likely to infiltrate the neighboring pelvic bone. When early lesions are limited to a single segment, radical long-bone resection is the treatment of choice. If the lesion is diffusely spread, preserving the limb is not possible; amputation is the only effective treatment (Zlitni et al., 2001). Postoperative bone defects are often treated with different methods, including bone cement filling and bone grafting. Moore et al. reported a case of total femoral-replacement surgery to treat diffuse osteopathy caused by left-femoral CE, using a total femoral prosthesis (MOST Total Femoral System) to reconstruct the defect. The patient’s 1-year postoperative prognosis was good, with femoral function mostly restored (Moore et al., 2015). The use of re-aspiration (PAIR) has shown encouraging results in localized cases where surgical removal is not possible or the patient refuses surgery, relapses postoperatively, or does not respond to pharmacological treatment (Peer et al., 2023).
5.2. Pharmacotherapy
If PSCs infection is localized to the axial bone or if the lesion is too large, radical surgical treatment is not possible; instead, palliative surgery plus long-term medication is often the best option for improving symptoms or even curing the patient. Pharmacological treatment of CE is similar to tumor chemotherapy; ABZ can be used preoperatively to inhibit further growth of CE and even reduce cyst size (Horton, 1989), or postoperatively, either alone or in combination with other antiparasitic drugs, to prevent recurrence (Horton, 1989). However, no drugs yet exist that can effectively prevent PSCs from invading and destroying bone and muscle (Togral et al., 2016).
As shown in Table 3, drugs are an important part of perioperative bone CE management, with dosage and duration depending on the site of parasitism and degree of invasion. The action of ABZ is effective in bone CE; 10–15 mg/kg/day for at least 6 continuous months is required for better prognosis as well as a lower relapse rate. To reduce the risk of cystic-fluid rupture and its potential complications, at least 300 mg/day of praziquantel must be given in combination with ABZ. Although ABZ + praziquantel has been reported to have anti-CE activity in some cases, its efficacy remains to be further investigated in subsequent bone CE trials (Gautam et al., 2018). Postoperative chemotherapy plus surgery, a popular form of bone CE treatment in recent years, can be extended for up to 2 years in complicated cases (Agarwal et al., 1992).
One study reported a drug-loaded nanoemulsion to be similar in efficiency to ABZ in inactivating PSCs in subcutaneous tissue. The investigators concluded that the nanoemulsion had high stability, high water solubility, and greater ability to cross biomembranes, thereby proving more efficacious against lesions that were difficult to reach with ABZ (Ahmadi et al., 2020). However, validation was not performed in animal models of bone CE.
6. Discussion
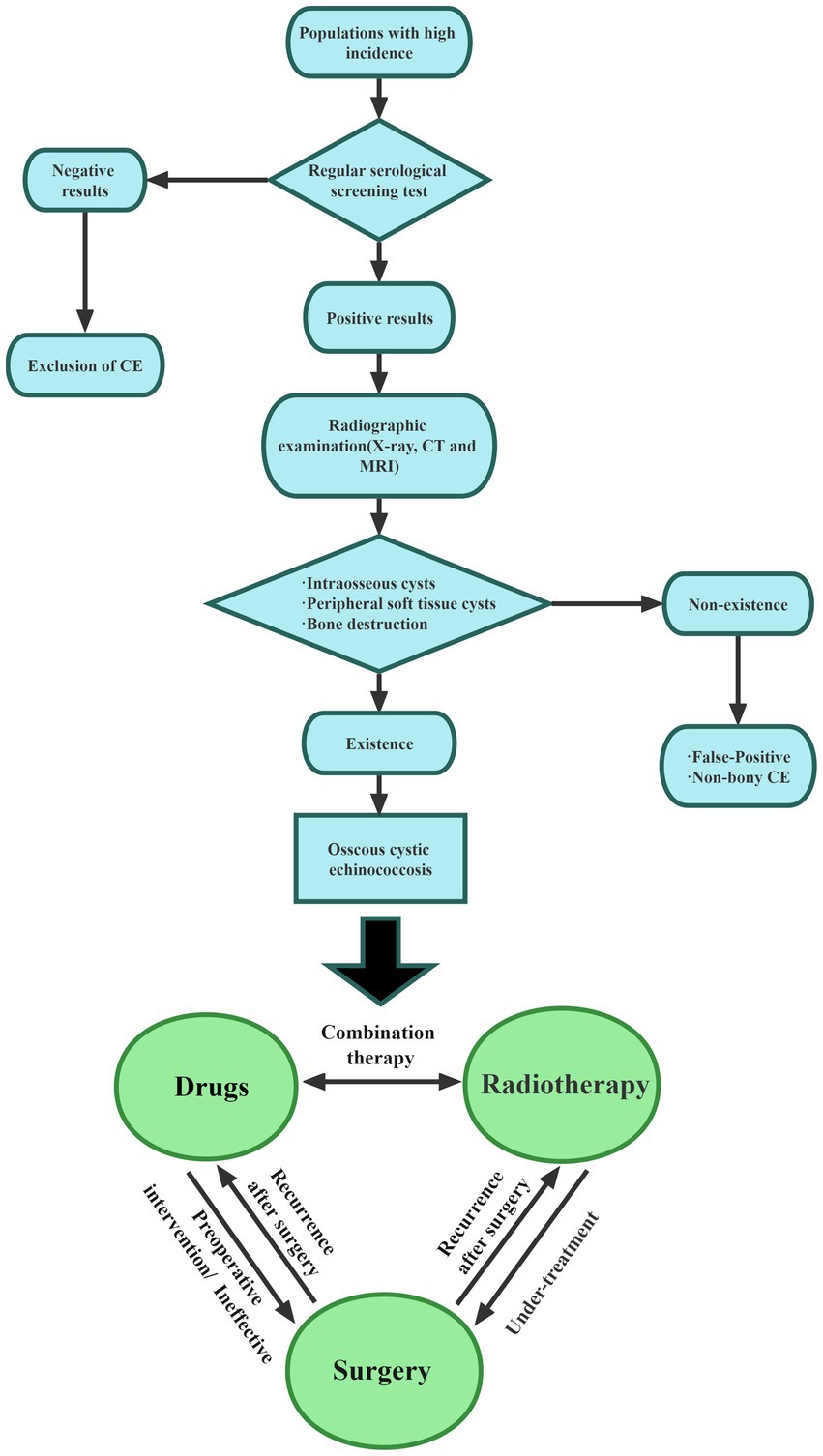
The research reviewed in this paper emphasizes the complexity of diagnosis and treatment of bone echinococcosis. Therefore, to understand the management of bone echinococcosis, the following aspects should be carried out. Bone CE with high rates of recurrence, disability, and paralysis, is a serious parasitic disease that imposes a severe burden on patients and families. Since bone CE mainly exists in pastoral areas, the medical level is not developed, and there is currently no clear consensus on bone CE, how to use convenient and appropriate methods for early diagnosis is undoubtedly the most important. Therefore, special medical examination centers for bone CE should be established to provide regular screening of sensitive populations and to regularly monitor the musculoskeletal conditions of vulnerable individuals. The clinical symptoms of bone CE are less pronounced in the early stages and become apparent in the later stages. Symptoms of bone CE are related to the location of the lesion and its severity. In spinal CE specifically, pain is the earliest symptom and can be accompanied by neurological manifestations. Early diagnosis and treatment are important for improving bone quality and avoiding complications, Figure 2 provides a diagnostic flow chart based on the 2015 Chinese Journal of Surgery expert consensus on the diagnosis and treatment of bone CE, hoping to provide a reference for the management of bone CE (Orthopaedics Professional Committee of Xinjiang Medical, 2015). The use of improved serological methods and new antigen development has undoubtedly improved the specificity and sensitivity of diagnosis, but there is a lack of large sample verification, which needs to be combined with imaging results. MRI is undoubtedly the most suitable imaging examination. The ‘bone window ‘and ‘soft tissue window ‘are the most sensitive for the diagnosis of bone CE. Therefore, new serological tests combined with imaging results can yield greater diagnostic value. Radical surgery combined with filler PMMA as the treatment of choice for bone CE not only repairs bone defects but can also kill PSCs. However, patients with large-bone defects often refuse radical surgery, and the risk of cystic-fluid leakage is high in such procedures due to cyst location, cyst depth, and degree of bone infiltration. Palliative surgical treatment improves patient survival while relieving the symptoms. Surgery combined with antiparasitic drugs (ABZ, praziquantel) can be used for complex manifestations of bone CE, as a chronic disease management, through systematic treatment, control and avoid complications.
Author contributions
SiW and ShW: Conceptualization, Writing – review & editing. HS, YH and YL: Data curation, Writing – review & editing. YM and QR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Açikgöz, B., Sungur, C., Ozgen, T., Camurdanoğlu, M., and Berker, M. (1996). Endoscopic evacuation of sacral hydatid cysts: a case report. Spinal Cord 34, 361–364. doi: 10.1038/sc.1996.66
Agarwal, S., Shah, A., Kadhi, S. K., and Rooney, R. J. (1992). Hydatid bone disease of the pelvis. A report of two cases and review of the literature. Clin. Orthop. Relat. Res. 280, 251–255. doi: 10.1097/00003086-199207000-00034
Agnihotri, M., Goel, N., Shenoy, A., Rai, S., and Goel, A. (2017). Hydatid disease of the spine: a rare case. J. Craniovert. Junct. Spine 8, 159–160. doi: 10.4103/jcvjs.JCVJS_16_17
Ahmadi, A., Moazeni, M., and Shaddel, M. (2020). Subcutaneous hydatid cyst in laboratory mice: is it a suitable method for evaluating therapeutic agents against hydatid cyst? Arch. Razi. Inst. 75, 75–81. doi: 10.22092/ari.2018.123382.1245
Ahmady-Nezhad, M., Rezainasab, R., Khavandegar, A., Rashidi, S., and Mohammad-Zadeh, S. (2022). Perineal and right femoral hydatid cyst in a female with regional paresthesia: a rare case report. BMC Surg. 22:64. doi: 10.1186/s12893-022-01516-z
Akhaddar, A., and Boucetta, M. (2018). A new surgical technique for the removal of extensive spinal epidural hydatid cyst. Surg. Neurol. Int. 9:96. doi: 10.4103/sni.sni_89_18
Akremi, M. S. E., Bellil, M., Souissi, M., Zaibi, M., Balti, W., and Salah, M. B. (2022). Sciatica and femoral head osteonecrosis complicating a gluteal hydatid cyst: a case report. Int. J. Surg. Case Rep. 92:106841. doi: 10.1016/j.ijscr.2022.106841
Alkan Çeviker, S., Yüksel, C., Şener, A., Önder, T., Metineren, M. H., Özel, Ç., et al. (2022). Hydatid cyst of the spine: a rare case report. Turkiye Parazitol. Derg. 46, 78–81. doi: 10.4274/tpd.galenos.2021.29392
Altinörs, N., Bavbek, M., Caner, H. H., and Erdogan, B. (2000). Central nervous system hydatidosis in Turkey: a cooperative study and literature survey analysis of 458 cases. J. Neurosurg. 93, 1–8. doi: 10.3171/jns.2000.93.1.0001
Arana Iniquez, R. E. (1978). “Echinococcus” in Infections of the nervous system. III. Handbook of clinical neurology am sterdam North-Holland. eds. P. J. Vinken and G. W. Bruyn, vol. 35, 175–208.
Arias, B. M. (1946). Osteohydatidosis; its radiological features. Radiology 47, 569–574. doi: 10.1148/47.6.569
Arik, H. O., Arican, M., Cetin, N. K., and Sarp, U. (2015). Primary intraosseous hydatid cyst of the femur. Iran Red Crescent Med J 17:e21070. doi: 10.5812/ircmj.21070
Arkun, R., and Mete, B. D. (2011). Musculoskeletal hydatid disease. Semin. Musculoskelet. Radiol. 15, 527–540. doi: 10.1055/s-0031-1293498
Bağcıer, F., and Tufanoğlu, F. H. (2020). A rare presentation of hydatid cyst: a case with radial bone involvement. Turkiye Parazitol. Derg. 44, 185–186. doi: 10.4274/tpd.galenos.2020.6416
Berk, C., Ciftçi, E., and Erdoğan, A. (1998). MRI in primary intraspinal extradural hydatid disease: a case report. Neuroradiology 40, 390–392. doi: 10.1007/s002340050608
Bhatnagar, N., Kishan, H., Sura, S., Lingaiah, P., and Jaikumar, K. (2017). Pelvic hydatid disease: a case report and review of literature. J. Orthopaedic Case Rep. 7, 25–28. doi: 10.13107/jocr.2250-0685.834
Bhojraj, S. Y., and Shetty, N. R. (1999). Primary hydatid disease of the spine: an unusual cause of progressive paraplegia. Case report and review of the literature. J. Neurosurg. 91, 216–218. doi: 10.3171/spi.1999.91.2.0216
Bouattour, N., Nabli, F., Nagi, S., Saied, Z., Ben Abdelaziz, I., Hentati, F., et al. (2021). Primary spinal Hydatidosis revealed by spinal cord compression. Neurol. Clin. Pract. 11, e37–e39. doi: 10.1212/CPJ.0000000000000763
Boussaid, S., Daldoul, C., Hassayoun, M., Rekik, S., Jammali, S., Sahli, H., et al. (2021). Primitive pelvic bone hydatidosis: what an amazing extension. Clin. Case Rep. 9:e05054. doi: 10.1002/ccr3.5054
Caglar, Y. S., Ozgural, O., Zaimoglu, M., Kilinc, C., Eroglu, U., Dogan, I., et al. (2019). Spinal hydatid cyst disease: challenging surgery – an institutional experience. J. Korean Neurosurg. Soc. 62, 209–216. doi: 10.3340/jkns.2017.0245
Carmena, D., Benito, A., and Eraso, E. (2006). Antigens for the immunodiagnosis of echinococcus granulosus infection: an update. Acta Trop. 98, 74–86. doi: 10.1016/j.actatropica.2006.02.002
Cattaneo, L., Manciulli, T., Cretu, C. M., Giordani, M. T., Angheben, A., Bartoloni, A., et al. (2019). Cystic echinococcosis of the bone: a European Multicenter study. Am. J. Trop. Med. Hyg. 100, 617–621. doi: 10.4269/ajtmh.18-0758
Cavus, G., Acik, V., Bilgin, E., Gezercan, Y., and Okten, A. I. (2018). Endless story of a spinal column hydatid cyst disease: a case report. Acta Orthop. Traumatol. Turc. 52, 397–403. doi: 10.1016/j.aott.2018.03.004
Chen, Y. W., Aikebaier, A., and Zhao, Y. (2020). Evaluation of imaging features of pelvic echinococcosis based on multimodal images. J. Orthop. Surg. Res. 15:493. doi: 10.1186/s13018-020-01912-2
Daniel, C., Matthieu, H., Goulven, R., Jocelyn, M., Valerie, D., and Christophe, H. (2017). Successful pelvic resection for acetabular Hydatidosis. Case Rep. Orthop. 2017, 1–4. doi: 10.1155/2017/9495783
Das, G., Nanda, S. N., Sahu, N. K., Kumar, D. S., and Patro, B. P. (2021). Hydatid cyst of dorsal spine masquerading as tubercular infection: a case report and review of literature. Asian J. Neurosurg. 16, 886–889. doi: 10.4103/ajns.ajns_199_21
Dathik, S., Chopra, R. K., Talwar, J., Pheroz, M., and Prasad, R. (2019). Primary hydatidosis of distal femur masquerading malignancy -A rare case. J Clin Orthop Trauma. 10, 213–220. doi: 10.1016/j.jcot.2017.12.003
Dehghan Manshadi, S. A., Rezahosseini, O., Saberi, S., Salahshour, F., and Mahdavi, I. S. (2017). Collapsed membranes within pelvic cyst: what is the diagnosis? Clin Case Rep. 5, 199–200. doi: 10.1002/ccr3.767
Depré, F., Dittmayer, C., and Schneider, T. (2019). Compression of the spinal cord in echinococcus infection. Int. J. Infect. Dis. 89, 10–11. doi: 10.1016/j.ijid.2019.09.014
Dighe, M. P., Balasubramaniam, S., and Nadkarni, T. D. (2018). Transthoracic approach for ventrally situated paraspinal extradural hydatid cyst of the dorsal spine. J. Craniovertebr. Junct. Spine. 9, 271–273. doi: 10.4103/jcvjs.JCVJS_71_18
Fathi, S., Jalousian, F., Hosseini, S. H., Parsa, H., and Kordafshari, S. (2016). A study of cross-reactivity between recombinant EPC1 antigen of echinococcus granulosus in serum from patients with confirmed cystic echinococcosis infection and other parasitic infections. Am. J. Trop. Med. Hyg. 94, 1313–1317. doi: 10.4269/ajtmh.15-0680
Fröschen, F. S., Fischer, H. P., Hischebeth, G. T., Reiter-Owona, I., Randau, T. M., Gravius, S., et al. (2019). Osseous hydatidosis of the proximal femur: a rare diagnosis in revision total hip arthroplasty. Infection 47, 301–305. doi: 10.1007/s15010-018-1193-6
Gautam, D., Malhotra, R., and Dubey, S. (2018). Combination drug chemotherapy and massive skeletal allograft in the management of hydatid disease of the femur. BMJ Case Rep. 2018, 1–4. doi: 10.1136/bcr-2017-223332
Gdoura, F., Trigui, M., Zribi, W., Ellouze, Z., Bouzidi, R., Ayedi, K., et al. (2010). Pelvic bone hydatidosis. Orthop. Traumatol. Surg. Res. 96, 85–89. doi: 10.1016/j.otsr.2009.04.020
Gnanasekaran, K. K., Prabhu, A. J., and George, S. (2016). Osseous hydatidosis of femur in a patient with fracture non-union: an uncommon entity. J. Clin. Diagn. Res. 10, ED06–ED08. doi: 10.7860/JCDR/2016/23610.9097
Govindasamy, A., Bhattarai, P. R., and John, J. (2021). Lytic parasitic: a case of bone destructing echinococcosis. Ther. Adv. Infect. Dis. 8, 204993612110476–204993612110477. doi: 10.1177/20499361211047664
Hans, B., Gupta, K., Kalra, K., and Suri, J. C. (2019). An unusual case of extrapulmonary hydatid cyst masquerading as a mediastinal tumor. Cureus 11:e5612. doi: 10.7759/cureus.5612
Herrera, A., Martínez, A. A., and Rodríguez, J. (2005). Spinal hydatidosis. Spine (Phila Pa 1976) 30, 2439–2444. doi: 10.1097/01.brs.0000184688.68552.90
Horton, R. J. (1989). Chemotherapy of echinococcus infection in man with albendazole. Trans. R. Soc. Trop. Med. Hyg. 83, 97–102. doi: 10.1016/0035-9203(89)90724-4
Inayat, F., Azam, S., Baig, A. S., Nawaz, G., and Ullah, W. (2019). Presentation patterns, diagnostic modalities, management strategies, and clinical outcomes in patients with hydatid disease of the pelvic bone: a comparative review of 31 cases. Cureus 11:e4178. doi: 10.7759/cureus.4178
Iplikçioğlu, C. A., Kökes, F., Bayar, A., Doğanay, S., and Buharali, Z. (1991). Spinal invasion of pulmonary Hydatidosis: computed tomographic demonstration. Neurosurgery 29, 467–468. doi: 10.1227/00006123-199109000-00026
Ira, B., Wilson, M., Dukes, K., Greenfield, S., Kaplan, S., and Hillman, B. (2001). Patient’s role in the use of radiology testing for common office practice complaints. Arch. Intern. Med. 161, 256–263. doi: 10.1001/archinte.161.2.256
İşlekel, S., Zileli, M., and Erşahin, Y. (1998). Intradural spinal hydatid cysts. Eur. Spine J. 7, 162–164. doi: 10.1007/s005860050048
Jacquier, M., and Piroth, L. (2018). Vertebral Hydatidosis. N. Engl. J. Med. 379:e5. doi: 10.1056/NEJMicm1714206
Johnson, F. W., and Hobson, D. (1977). The effect of penicillin on genital strains of Chlamydia trachomatis in tissue culture. Antimicrobial Chemother. 3, 46–56. doi: 10.1093/jac/3.1.49
Kaen, A., Lagares, A., Perez-Nunez, A., Rivas, J. J., Ramos, A., and Lobato, R. D. (2009). Intradural extramedullary spinal hydatidosis: case report. Neurocirugia 20, 282–287. doi: 10.1016/S1130-1473(09)70169-1
Kapoor, S. K., Kataria, H., Patra, S. R., Bharadwaj, M., Vijay, V., and Kapoor, S. (2013). Multi-organ hydatidosis with extensive involvement of the hemipelvis and ipsilateral femur. Parasitol. Int. 62, 82–85. doi: 10.1016/j.parint.2012.08.006
Kassimi, M., Rami, A., Habi, J., Guerroum, H., El Pardya, T. R., Chikhaoui, N., et al. (2021). Recurrent costovertebral hydatidosis with epidural extension. Radiol Case Rep. 16, 1712–1714. doi: 10.1016/j.radcr.2021.04.016
Khazim, R., Fares, Y., Heras-Palou, C., and Ruiz, B. P. (2003). Posterior decompression of spinal hydatidosis: long-term results. Clin. Neurol. Neurosurg. 105, 209–214. doi: 10.1016/S0303-8467(03)00013-1
Kiran, M., Prasad, C. K., Mrudul, B., and Shishir, K. (2021). An intraoperative surprise! A rare case report of primary craniovertebral junction hydatid disease mimicking a bony tumor. World Neurosurg. 146, 171–176. doi: 10.1016/j.wneu.2020.11.072
Liang, Q., Wen, H., Yunus, A., Tian, Z., Jiang, F., and Song, X. (2014). Treatment experiences of pelvic bone hydatidosis. Int. J. Infect. Dis. 18, 57–61. doi: 10.1016/j.ijid.2013.09.010
List, C., Qi, W., Maag, E., Gottstein, B., Muller, N., and Felger, I. (2010). Serodiagnosis of echinococcus spp. infection: explorative selection of diagnostic antigens by peptide microarray. PLoS Negl. Trop. Dis. 4:e771. doi: 10.1371/journal.pntd.0000771
Loudiye, H., Aktaou, S., Hassikou, H., El-Bardouni, A., El-Manouar, M., Fizazi, M., et al. (2003). Hydatid disease of bone. Review of 11 cases. Joint Bone Spine 70, 352–355. doi: 10.1016/S1297-319X(03)00039-3
Luan, H., Liu, K., Deng, Q., Sheng, W., Maimaiti, M., Guo, H., et al. (2022). Multiple debridement of cavity lesions combined with antiparasitic chemotherapy in the treatment of mid or advanced spinal echinococcosis: a retrospective study of 33 patients. Int. J. Infect. Dis. 114, 261–267. doi: 10.1016/j.ijid.2021.11.014
Majmundar, N., Patel, P. D., Dodson, V., Tran, A., Goldstein, I., and Assina, R. (2019). Parasitic infections of the spine: case series and review of the literature. Neurosurg. Focus. 46:E12. doi: 10.3171/2018.10.FOCUS18472
Martínez, A. A., Herrera, A., Cuenca, J., and Herrero, L. (2001). Hydatidosis of the pelvis and hip. Int. Orthop. 25, 302–304. doi: 10.1007/s002640100278
Masmoudi, K., Ben Maitigue, M., Frikha, R., Mouelhi, T., and Cheikhrouhou, H. (2019). Loosening of a Total hip arthroplasty associated with a periprosthetic femoral fracture caused by recurrent Hydatidosis of the hip: a case report. JBJS Case Connect. 9:e0044. doi: 10.2106/JBJS.CC.19.00044
McManus, D. P. (2014). Immunodiagnosis of sheep infections with echinococcus granulosus: in 35 years where have we come? Parasite Immunol. 36, 125–130. doi: 10.1111/pim.12072
McManus, D. P., Gray, D. J., Zhang, W., and Yang, Y. (2012). Diagnosis, treatment, and management of echinococcosis. BMJ 344:e3866. doi: 10.1136/bmj.e3866
Monge-Maillo, B., Chamorro Tojeiro, S., and Lopez-Velez, R. (2017). Management of osseous cystic echinococcosis. Expert Rev. Anti-Infect. Ther. 15, 1075–1082. doi: 10.1080/14787210.2017.1401466
Moore, D., Baker, K. C., and Les, K. (2015). Hydatid disease of the femur treated with a Total femoral replacement: a case report. JBJS Case Connect. 5:e7. doi: 10.2106/JBJS.CC.M.00279
Morris, B. S., Madiwale, C. V., Garg, A., and Chavhan, G. B. (2002). Hydatid disease of bone: a mimic of other skeletal pathologies. Australas. Radiol. 46, 431–434. doi: 10.1046/j.1440-1673.2002.t01-1-01099.x
Muscolo, D. L., Zaidenberg, E. E., Farfalli, G. L., Aponte-Tinao, L. A., and Ayerza, M. A. (2015). Use of massive allografts to manage hydatid bone disease of the femur. Orthopedics 38, e943–e946. doi: 10.3928/01477447-20151002-92
Nascimento, G., Silva, C., Marques, R., Silva, C., Oliveira, J. F., Santos, J., et al. (2018). Periprosthetic pathologic fracture following tibial echinococcosis: a case report. Int. J. Surg. Case Rep. 51, 231–236. doi: 10.1016/j.ijscr.2018.08.045
Neumayr, A., Tamarozzi, F., Goblirsch, S., Blum, J., and Brunetti, E. (2013a). Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 1. Epidemiology and anatomy. PLoS Negl. Trop. Dis. 7:e2450. doi: 10.1371/journal.pntd.0002450
Neumayr, A., Tamarozzi, F., Goblirsch, S., Blum, J., and Brunetti, E. (2013b). Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 2. Treatment, follow-up, and outcome. PLoS Negl. Trop. Dis. 7:e2458. doi: 10.1371/journal.pntd.0002458
Orthopaedics Professional Committee of Xinjiang Medical (2015). Expert consensus on the diagnosis and treatment of bone hydatid disease. Chin. J. Surg. 53, 922–927. Hydatid Surgery Professional Committee SSB, China Medical Association.
Ortona, E., Margutti, P., Delunardo, F., Nobili, V., Profumo, E., Riganò, R., et al. (2005). Screening of an echinococcus granulosus cDNA library with IgG4 from patients with cystic echinococcosis identifies a new tegumental protein involved in the immune escape. Clin. Exp. Immunol. 142, 528–538. doi: 10.1111/j.1365-2249.2005.02939.x
Oueslati, A., Amri, K., Chefi, M. A., Mallat, Y., Znagui, T., and Nouisri, L. (2020). Hydatid disease of proximal femur treated using a mega prosthesis: a case report. Int. J. Surg. Case Rep. 68, 67–73. doi: 10.1016/j.ijscr.2020.02.002
Ozdemir, H. M., Ogün, T. C., and Tasbas, B. (2004). A lasting solution is hard to achieve in primary hydatid disease of the spine: long-term results and an overview. Spine (Phila Pa 1976) 29, 932–937. doi: 10.1097/00007632-200404150-00022
Ozek, M. M. (1994). Complications of central nervous system hydatid disease. Pediatr. Neurosurg. 20, 84–91. doi: 10.1159/000120770
Pamir, M. N., Ozduman, K., and Elmaci, I. (2002). Spinal hydatid disease. Spinal Cord 40, 153–160. doi: 10.1038/sj.sc.3101214
Papanikolaou, A. (2008). Osseous hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 102, 233–238. doi: 10.1016/j.trstmh.2007.09.012
Parvaresh, M., Moin, H., and Miles, J. B. (1996). Dumbbell hydatid cyst of the spine. Br. J. Neurosurg. 10, 211–214. doi: 10.1080/02688699650040403
Pathania, V. P., Kakkar, S., and Sikdar, J. (2000). Hydatid disease of femur. Med. J. Armed Forces India 56, 75–76. doi: 10.1016/S0377-1237(17)30103-X
Patino, J. M., and Ramos Vertiz, A. J. (2019). Hydatidosis of the complete humerus. Treated with radical resection and endoprosthesis. Case report. Int. J. Surg. Case Rep. 65, 296–300. doi: 10.1016/j.ijscr.2019.10.083
Peer, S., Jabbal, H. S., Singh, P., Ma, P. S., Kakkera, S., and Bhat, P. (2023). A case report of successful percutaneous aspiration, injection, and re-aspiration (PAIR) technique for treatment of retrovesical pelvic hydatid cyst. Radiol. Case Rep. 18, 331–334. doi: 10.1016/j.radcr.2022.10.056
Peña Huertas, M., Zafra Martín, J., Álvarez García de Quesada, I., Díaz Gavela, A. A., Guerrero Gómez, L. L., Sánchez García, S., et al. (2022). IJRadiation therapy for recurrent hydatid cyst of the pelvic bone: a case report. Int. J. Infect. Dis. 115, 168–170. doi: 10.1016/j.ijid.2021.12.313
Petra, K. B., Bardonnet, K., Renner, E., Auer, H., Pawlowski, Z., Ammann, R. W., et al. (2003). European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg. Infect. Dis. 9, 343–349. doi: 10.3201/eid0903.020341
Ramteke, P., Phulware, R. H., Shende, T., Sahoo, B., and Barwad, A. (2019). Hydatid cyst of the femur, radiologically mimicking a sarcoma. Diagn. Cytopathol. 47, 1045–1048. doi: 10.1002/dc.24198
Rangheard, A. S., Vanel, D., Viala, J., Schwaab, G., Casiraghi, O., and Sigal, R. (2001). Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. AJNR Am. J. Neuroradiol. 22, 851–857.
Reddy, I. V., Kumar, A. H. A., Samorekar, B., Babu, B. A., and Mettu, A. K. (2017). Complicated hydatid cyst of ulna- A rare case report. J. Clin. Diagn. Res. 11, RD01–RD03. doi: 10.7860/JCDR/2017/21804.9773
Robinson, R. G. (1959). Hydatid disease of the spine and its neurological complications. Br. J. Surg. 47, 301–306. doi: 10.1002/bjs.18004720324
Safari, H., Mirzavand, S., Rafiei, A., and Beiromvand, M. (2021). Twenty-six years of involvement with cystic echinococcosis: a case report. J. Med. Case Rep. 15:266. doi: 10.1186/s13256-021-02810-9
Salman, F., Khan, M. I., Hussain, I., and Abdullah, H. M. A. (2018). Pathological fracture of the femoral neck in a middle-aged woman: a rare presentation of primary hydatid cyst disease in humans. BMJ Case Rep. 2018. doi: 10.1136/bcr-2017-222980
Saul, D., Seitz, M.-T., Weiser, L., Oberthür, S., Roch, J., Bremmer, F., et al. (2020). Of cestodes and men: surgical treatment of a spinal hydatid cyst. J. Neurol. Surg. A Cent. Eur. Neurosurg. 81, 086–090. doi: 10.1055/s-0039-1693707
Schnepper, G. D., and Johnson, W. D. (2004). Recurrent spinal hydatidosis in North America. Neurosurg. Focus. 17, 1–6. doi: 10.3171/foc.2004.17.6.8
Sharma, J. K., Tandon, V., Marathe, N. A., Agrahari, Y., Mallepally, A. R., and Chhabra, H. S. (2020). Hydatid cyst of the spine: a rare case report and review of literature. J. Orthop. Case Rep. 10, 57–59. doi: 10.13107/jocr.2020.v10.i03.1748
Siles-Lucas, M., Casulli, A., Conraths, F. J., and Müller, N. (2017). Laboratory diagnosis of echinococcus spp. in human patients and infected animals. Adv. Parasitol. 96, 159–257. doi: 10.1016/bs.apar.2016.09.003
Sioutis, S., Reppas, L., Bekos, A., Soulioti, E., Saranteas, T., Koulalis, D., et al. (2021). Echinococcosis of the spine. EFORT Open Rev. 6, 288–296. doi: 10.1302/2058-5241.6.200130
Song, X. H., Ding, L. W., and Wen, H. (2007). Bone hydatid disease. Postgrad. Med. J. 83, 536–542. doi: 10.1136/pgmj.2007.057166
Staouni, I. B., Marzouki, Z., Haloua, M., Lamrani, Y. A., Boubbou, M., Maâroufi, M., et al. (2020). Horse tail syndrome revealing spinal-medullary hydatid disease. Pan Afr. Med. J. 36:225. doi: 10.11604/pamj.2020.36.225.21606
Steinmetz, S., Racloz, G., Stern, R., Dominguez, D., Al-Mayahi, M., Schibler, M., et al. (2014). Treatment challenges associated with bone echinococcosis. J. Antimicrob. Chemother. 69, 821–826. doi: 10.1093/jac/dkt429
Sudo, H., and Minami, A. (2010). Neurological picture. A widespread echinococcosis of the spine. J. Neurol. Neurosurg. Psychiatry 81:892. doi: 10.1136/jnnp.2009.198341
Thomas, M., Keller, M., Schweitzer, J. S., Helfend, L. K., and Chappell, T. (1997). Treatment of progressive cervical spinal instability secondary to hydatid disease. Spine 22, 915–919. doi: 10.1097/00007632-199704150-00016
Tian, Y., Jiang, M., Shi, X., Hao, Y., and Jiang, L. (2020). Case report: huge dumbbell-shaped primary hydatid cyst across the intervertebral foramen. Front. Neurol. 11:592316. doi: 10.3389/fneur.2020.592316
Togral, G., Arikan, S. M., Ekiz, T., Kekec, A. F., and Eksioglu, M. F. (2016). Musculoskeletal hydatid cysts resembling Tumors: a report of five cases. Orthop. Surg. 8, 246–252. doi: 10.1111/os.12246
Torricelli, P., Martinelli, C., Biagini, R., Ruggieri, P., and De Cristofaro, R. (1990). Radiographic and computed tomographic findings in hydatid disease of bone. Skeletal Radio 19, 435–439. doi: 10.1007/BF00241799
Trifa, A., and Maamri, K. (2021). Aggressive behavior and recurrent spinal hydatid cyst. Pan Afr. Med. J. 40:202. doi: 10.11604/pamj.2021.40.202.32389
Turtas, S., Viale, E. S., and Pau, A. (1980). Long-term results of surgery for hydatid disease of the spine. Surg. Neurol. 13, 468–470.
Tüzün, M., and Hekimoğlu, B. (2001). Pictorial essay. Various locations of cystic and alveolar hydatid disease: CT appearances. J. Comput. Assist. Tomogr. 25, 81–87. doi: 10.1097/00004728-200101000-00014
Wang, R., Shi, X.-D., and Cao, Y.-P. (2019). Artificial hemipelvis displacement treatment for bone hydatid disease. Chin. Med. J. 132, 1621–1622. doi: 10.1097/CM9.0000000000000281
World Health Organization (2015). WHO estimates of the global burden of foodborne diseases burden epidemiology reference group 2007–2015. J. Diagn. Res., Ed06–ed08.
Xiao, N., Mamuti, W., Yamasaki, H., Sako, Y., Nakao, M., Nakaya, K., et al. (2003). Evaluation of use of recombinant Em18 and affinity-purified Em18 for serological differentiation of alveolar echinococcosis from cystic echinococcosis and other parasitic infections. J. Clin. Microbiol. 41, 3351–3353. doi: 10.1128/JCM.41.7.3351-3353.2003
Yildiz, Y., Bayrakci, K., Altay, M., and Saglik, Y. (2001). The use of polymethylmethacrylate in the management of hydatid disease of bone. J. Bone Joint Surg. Br. 83-B, 1005–1008. doi: 10.1302/0301-620X.83B7.0831005
Zhang, Z., Fan, J., Dang, Y., Xu, R., and Shen, C. (2017). Primary intramedullary hydatid cyst: a case report and literature review. Eur Spine J. 26, 107–110. doi: 10.1007/s00586-016-4896-3
Zhang, T., Ma, L.-H., Liu, H., and Li, S.-K. (2021). Incurable and refractory spinal cystic echinococcosis: a case report. World J. Clin. Cases 9, 10337–10344. doi: 10.12998/wjcc.v9.i33.10337
Zhang, B., Zhang, L., Zhou, H., Tian, J., and Wang, J. (2021). Progressive compressive myelopathy induced by a rare primary isolated thoracic vertebral hydatid cyst: a case report. Medicine 100:e25177. doi: 10.1097/MD.0000000000025177
Keywords: bone cystic echinococcosis, endemic disease, orthopedic surgery, neglected disease, medical advice
Citation: Meng Y, Ren Q, Xiao J, Sun H, Huang Y, Liu Y, Wang S and Wang S (2023) Progress of research on the diagnosis and treatment of bone cystic echinococcosis. Front. Microbiol. 14:1273870. doi: 10.3389/fmicb.2023.1273870
Edited by:
Hong Yin, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Satya Deo Pandey, University of Louisville, United StatesJayaraman Tharmalingam, University of Wisconsin-Madison, United States
Copyright © 2023 Meng, Ren, Xiao, Sun, Huang, Liu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sibo Wang, d2FuZ3NpYm93YW5nZmVpQDE2My5jb20=; Shan Wang, d2FuZ3NoYW40MzExNDFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yibin Meng1†
Yibin Meng1† Qian Ren
Qian Ren