- 1Laboratory of Tissue Engineering, Faculty of Life Science, Northwest University, Xi’an, China
- 2Department of Critical Care Medicine, The 940th Hospital of the Joint Logistics Support Force of People’s Liberation Army (PLA), Lanzhou, Gansu, China
- 3The First Hospital of Northwestern University, Xi’an, China
Background: Although clinical studies have revealed a potential link between Helicobacter pylori (H. pylori) infection and irritable bowel syndrome (IBS), the causal relationship between them remains unknown. The objective of this study was to investigate whether H. pylori infection is causally associated with IBS.
Method: A two-sample Mendelian randomization (MR) analysis using the inverse variance weighted (IVW), weighted mode, weighted median and MR-Egger methods was performed. We used the publicly available summary statistics data sets of genome-wide association studies (GWAS) for H. pylori infection in individuals of European descent (case = 1,058, control = 3,625) as the exposure and a GWAS for non-cancer illness code self-reported: IBS (case = 10,939, control = 451,994) as the outcome.
Results: We selected 10 single nucleotide polymorphisms at genome-wide significance from GWASs on H. pylori infection as the instrumental variables. The IVW, weighted mode, weighted median and MR-Egger methods all provided consistent evidence that suggests a lack of causal association between H. pylori and IBS. MR-Egger regression revealed that directional pleiotropy was unlikely to be biasing the result (intercept = −1e-04; P = 0.831). Cochran’s Q-test and the funnel plot indicated no evidence of heterogeneity and asymmetry, indicating no directional pleiotropy.
Conclusion: The results of MR analysis support that H. pylori infection may not be causally associated with an increased risk of IBS.
1 Introduction
Irritable bowel syndrome (IBS) is a chronic functional disorder characterized by abdominal discomfort and changes in bowel habits (Chey et al., 2015; Sultan and Malhotra, 2017). The Rome Foundation Global Study reported a pooled prevalence rate of 4.1% among 54,127 individuals from 26 countries (Sperber et al., 2021). IBS is known to cause distress, morbidity, and disability, and its impact on individuals’ quality of life and healthcare burden cannot be ignored (Chey et al., 2015; Black and Ford, 2020). Therefore, it is crucial to identify the risk factors of IBS and develop targeted treatments. Recent research has increasingly shown that the gut-brain axis and gut microbiota play roles in the development of IBS, potentially explaining its underlying pathogenesis (Holtmann et al., 2016; Pittayanon et al., 2019).
Helicobacter pylori is an important gastroenterological pathogen that is highly prevalent worldwide. Approximately 60% of the world’s population has been infected with H. pylori (Hooi et al., 2017). Previous studies have reported the associations between H. pylori infection and various intra-gastric and extra-gastric diseases, including peptic ulcers, functional dyspepsia, and cholelithiasis (Zhao et al., 2014; Cen et al., 2018). Some studies have suggested that H. pylori infection may contribute to the development of IBS (Yakoob et al., 2012; Liang et al., 2020). Meta-analyses have also indicated an increased risk of IBS associated with H. pylori infection and that eradication treatment can improve IBS symptoms (Wang et al., 2023). However, other multicenter retrospective studies and meta-analyses have not found evidence supporting this association (Xiong et al., 2016; Ng et al., 2019; Kim et al., 2020; Wang et al., 2022). In addition, we should consider the limitations of these observational studies, which may be subject to biases such as unmeasured or imprecisely measured confounders and reverse causation (Lee et al., 2013).
Mendelian randomization (MR) is an epidemiological analytical technique that utilizes genetic variants as instrumental variables (IVs) to evaluate whether an observational association between the exposure factors and complex disorders is indicative of a causal relationship (Burgess et al., 2015).
In this study, we first conducted a two-sample MR analysis to investigate the potential causal relationship between H. pylori infection and the occurrence of IBS. This analysis was based on genome-wide association study (GWAS) data.
2 Materials and methods
2.1 Mendelian randomization design
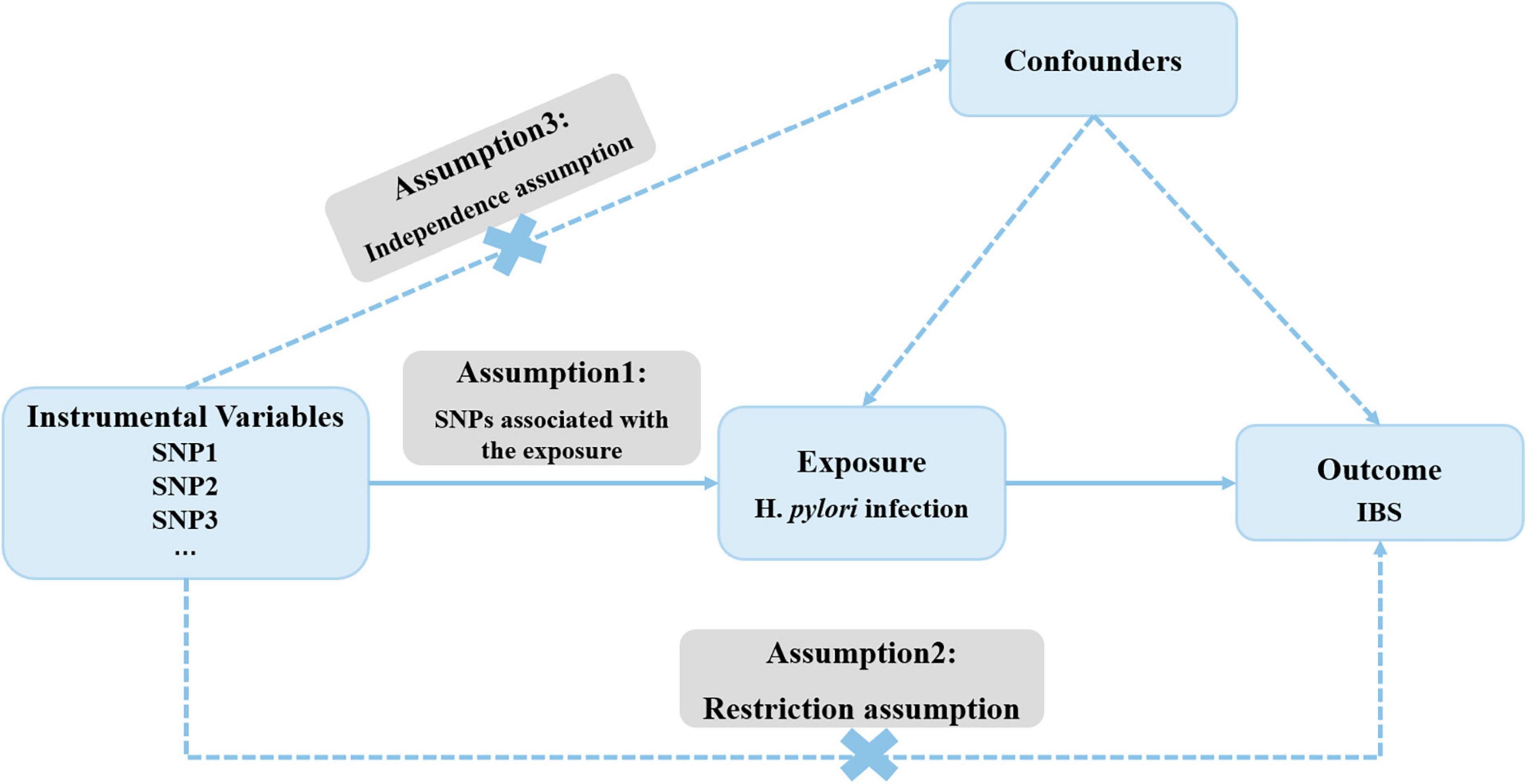
Figure 1 outlines the framework for the current MR study, which utilized genetic variants as IVs. The validity of MR study rests on three core assumptions: (1) the relevance assumption; (2) the independence assumption; and (3) the exclusion-restriction assumption (Emdin et al., 2017).
2.2 Data sources description
The summary data of H. pylori infection in GWAS were collected from the publicly available data compiled by the European Bioinformatics Institute (EBI) database. The dataset consisted of 1,058 cases and 3,625 controls of European ancestry. The genetic association of IBS was also generated from the GWAS data sources on EBI, comprising 10,939 European cases and 451,994 European controls. Supplementary Table 1 provides the detailed information of the GWAS data contained in this study.
2.3 Selection of genetic instrumental variables for H. pylori infection
To obtain the IVs for H. pylori infection, the GWAS summary statistics were utilized. A series of quality control measures were implemented to select eligible genetic IVs that fulfilled the assumptions of MR analysis. Firstly, single nucleotide polymorphisms (SNPs) were required to demonstrate genome-wide significance, with a relaxed correlation threshold of P < 5 × 10–6. Secondly, a linkage disequilibrium (LD) clumping algorithm was used to exclude SNPs in strong LD, with an R2 < 0.001, a window size of 10,000 kb, and a significance level of P < 5 × 10–8. Finally, to ensure that the effect alleles were consistent across the exposure and outcome datasets, SNP harmonization was performed, eliminating SNPs with intermediate allele frequencies and ambiguous SNPs with non-concordant alleles. Following these rigorous selection criteria, these SNPs were used as the IVs for subsequent analysis.
2.4 Statistical analysis
Two-sample Mendelian randomization was used to estimate the potential causal relationship between H. pylori infection and IBS. This analysis was performed using the “TwoSampleMR” packages in R software version 4.0.2. The inverse variance weighted (IVW) method was primarily used for the MR analysis, as it provides a consistent estimates of exposure-outcome associations when the IVs are not pleiotropic (Burgess et al., 2013). Cochran’s Q statistics was applied to assess the heterogeneity across individual SNPs (Hemani et al., 2018). Sensitivity analyses were also conducted to verify the robustness of our results. MR-Egger and weighted-median methods were used to explore and adjust for pleiotropy, which refers to the association of genetic variants with more than one variable (Bowden et al., 2015, 2016; Zhu et al., 2016). The threshold for statistical significance was set at a two-sided P-value ≤ 0.05.
3 Results
3.1 Genetic instrumental variable selection
Based on a genome-wide significant threshold of P < 5 × 10–6, a total of 12 SNPs for H. pylori infection were identified. Subsequently, the screening was rigorous as described previously, resulting in the identification of 10 SNPs (rs12591869, rs2169557, rs35030589, rs41263973, rs55871438, rs72708546, rs73512476, rs74045808, rs77516628, rs78825412) (total R2 of 4.8%) for the associations between H. pylori and IBS. All F-statistic values for the obtained instrumental variables (IVs) were greater than 10, indicating no significant weak IV bias. The information on these genetic variants utilized in the MR analyses is detailed in Supplementary Table 2.
3.2 Effects of genetically proxied H. pylori infection on IBS
Figures 2, 3 and Table 1 display the causal estimate of H. pylori on IBS. Genetically predicted H. pylori infection showed no association with IBS under the IVM (OR = 1.000, 95% CI: 0.998–1.002, P = 0.652) and MR-Egger methods (OR = 1.001, 95% CI: 0.996–1.006, P = 0.7269). The intercept represents the average pleiotropic effect across the genetic variants. An intercept that differs from zero indicates directional pleiotropy. In this study, MR-Egger regression revealed that directional pleiotropy was unlikely to be biasing the result (intercept = −1e-04; P = 0.831). Similar results were obtained when using the weighted median and weighted mode methods (OR = 1.001, 95% CI: 0.998–1.003, P = 0.3131 and OR = 1.002, 95% CI: 0.998–1.005, P = 0.4199, respectively). Taking all of the above into consideration, the results of the MR analysis may not support a potential causal association between H. pylori and IBS.
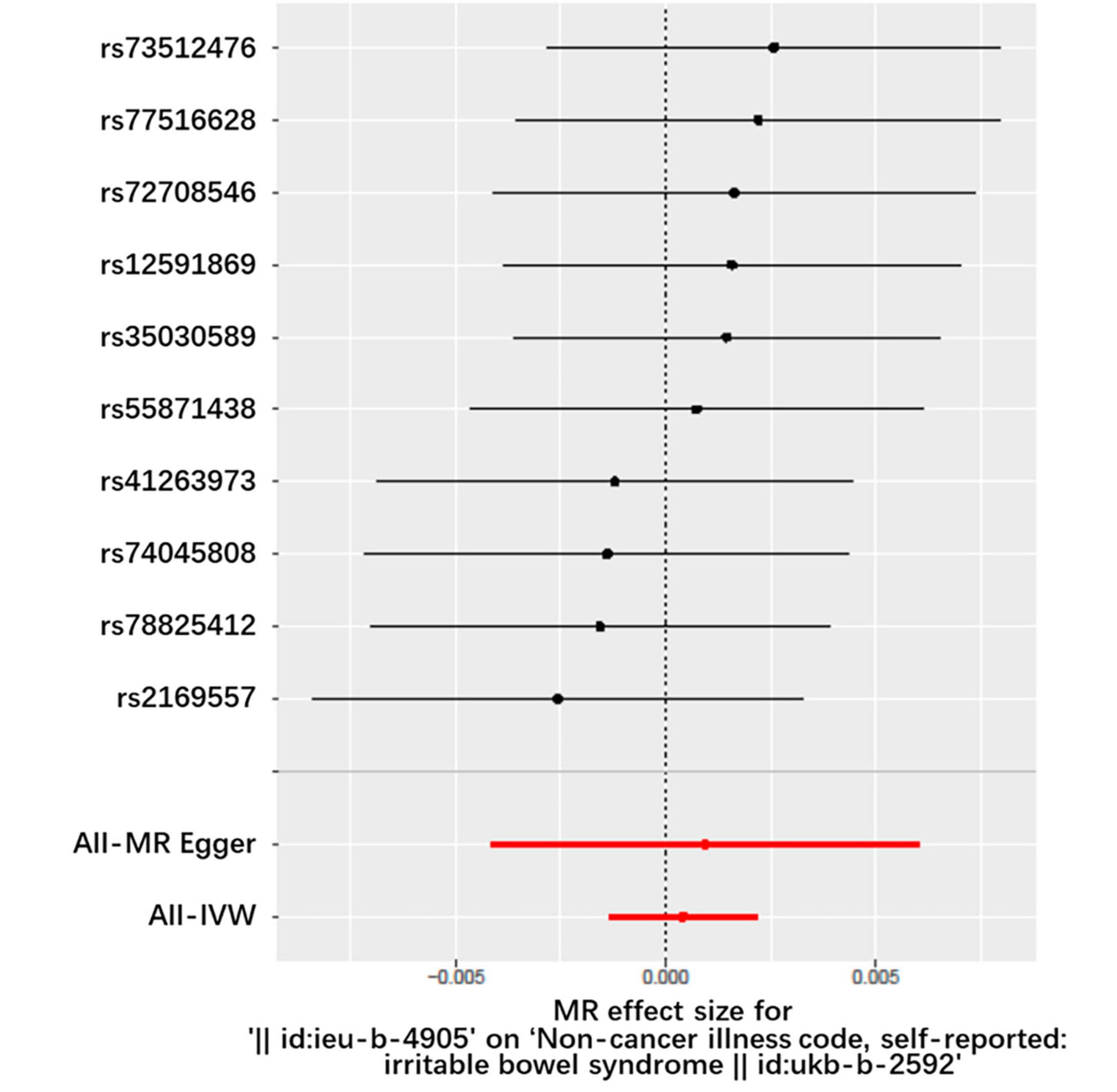
Figure 2. Forest plot of the causal effects of single nucleotide polymorphisms associated with H. pylori infection on IBS. The significance of red lines are MR results of MR-Egger test and IVW method.

Figure 3. Forest plot for the causal effect of H. pylori infection on the risk of IBS. OR, odds ratio; CI, confidence interval.

Table 1. MR estimates from each method of assessing the causal effect of H. pylori on the risk of IBS.
3.3 Heterogeneity and sensitivity test
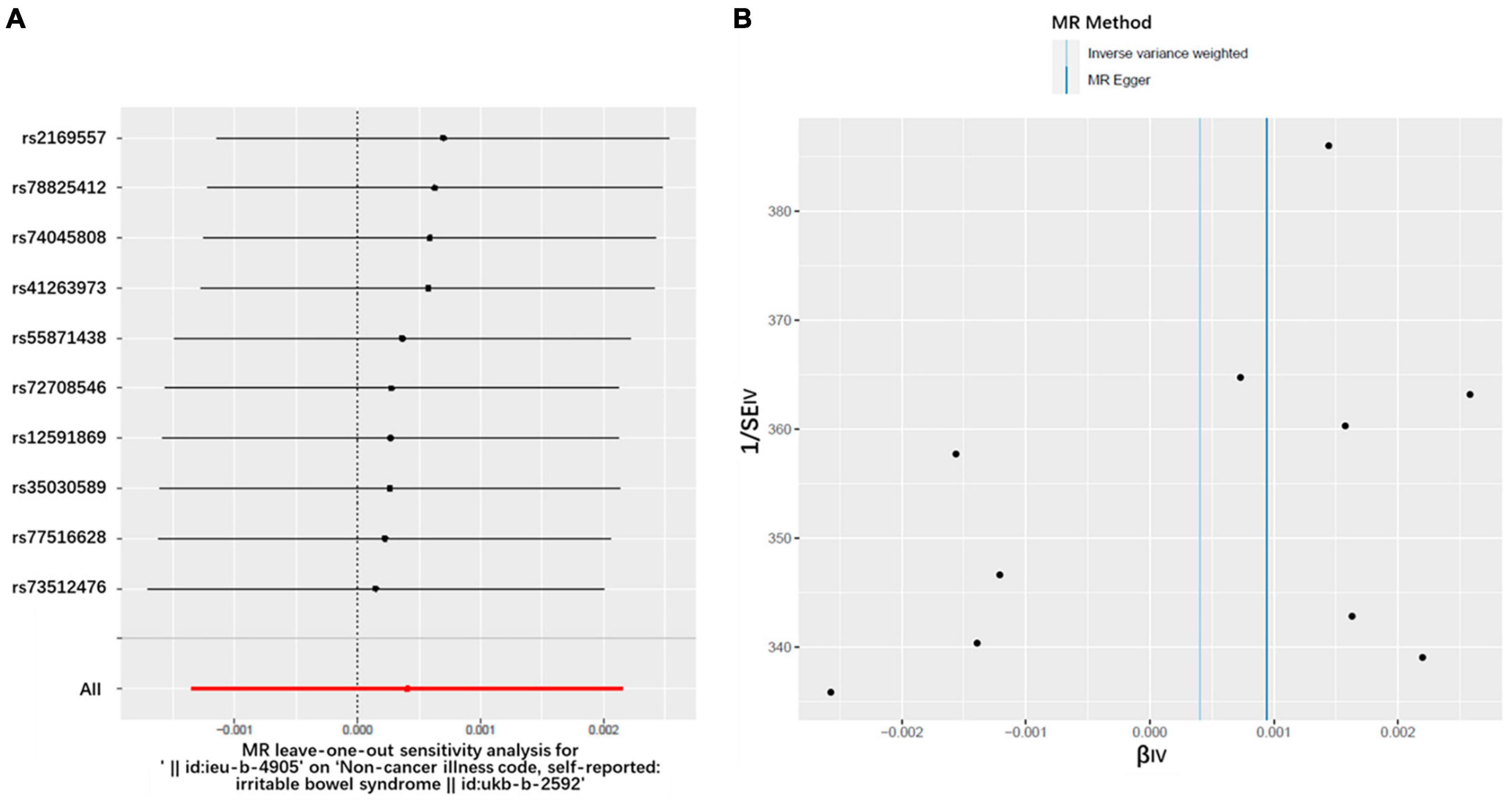
Heterogeneity refers to the variability observed in the causal estimates obtained for each SNP. Low heterogeneity suggests that the results of MR estimates are more reliable. In this study, the Cochran’s Q-test revealed no evidence of heterogeneity among the IV estimates based on the individual variants (Table 1). Furthermore, the “leave-one-out” analysis demonstrated that no single SNP was influential in driving the IVW point estimate (Figure 4A). Additionally, the funnel plot displayed no evidence of asymmetry, indicating the absence of directional horizontal pleiotropy (Figure 4B).
4 Discussion
In this study, we utilized an MR design to investigate the causal association between H. pylori infection and the risk of developing IBS, using publicly available GWAS data. Based on this study, we do not find a significant causal relationship between H. pylori infection and IBS.
There is inconsistent evidence regarding the causal relationship between H. pylori infection and the risk of IBS. One study conducted on a large cohort showed that H. pylori infection was associated with an increased risk of IBS (HR = 4.16; 95% CI: 2.508–6.900), and that eradication therapy can reduce the risk of IBS (Liang et al., 2020; Xiong et al., 2020). A meta-analysis of 31 studies also reached similar results (Wang et al., 2023). However, the relationship between the two has been controversial. Some reports from different countries failed to find the association (Agreus et al., 1995; Locke et al., 2000; Kawamura et al., 2001; Zhao et al., 2010). Additionally, a multicenter retrospective study concluded that H. pylori infection may not be related to IBS, and that the symptoms of IBS patients, such as abdominal pain and stool frequency, cannot be improved after H. pylori eradication (Xiong et al., 2016). Furthermore, a study found that the prevalence of H. pylori infection was lower in the IBS group (Athab, 2017).
The controversy surrounding the relationship between H. pylori infection and IBS can be attributed to several reasons. Firstly, as mentioned earlier, observational studies are prone to biases, which may explain the discrepancies (Lee et al., 2013). Secondly, differences in diagnostic approaches could result in unavoidable bias. Some studies used serum or fetal IgG antibodies to diagnose H. pylori infection, which may lead to false positive results (Kim et al., 2017; Abdel-Razik et al., 2018; Abo-Amer et al., 2020; Alvarez et al., 2020). As everyone knows, the urea breath test (UBT) has been shown to have the best accuracy among non-invasive tests (Liu et al., 2022). Additionally, subgroup analysis revealed opposite correlations between H. pylori infection and IBS when diagnosed using Rome III or non-Rome III criteria (Li et al., 2020). Therefore, it is crucial for the analysis to employ a unified and strict diagnostic criterion. Thirdly, as described in a meta-analysis study (Hooi et al., 2017), the prevalence of H. pylori infection and IBS varies across different geographical regions and races, which may introduce selection bias.
Irritable bowel syndrome (IBS) poses a significant medical challenge to society, and the development of a novel treatment for this disease is hindered by the lack of understanding of its etiology and pathogenesis. This study aims to shed light on the pathogenic factors of IBS from the perspective of systems biology. Our MR study has several strengths. Firstly, to the best of our knowledge, this is the first research to reveal the causal relationship between H. pylori infection and IBS. Secondly, this study has a large sample size and high statistical efficiency. Thirdly, the results were consistent across all approaches. Additionally, we conducted Cochran’s Q-test, MR-Egger intercept test, and various sensitivity analyses, including a leave-one-out analysis and a funnel plot, to test the validity of the conclusion and assess the pleiotropy of IVs. However, this analysis also has several limitations. Firstly, the diagnosis of H. pylori infection was based on serum IgG antibodies testing in the datasets. Secondly, the datasets used primarily include the European population, so the results may not be generalizable to other populations. Thirdly, IBS patients were classified based on non-cancer illness codes, which may introduce information bias. Lastly, in order to incorporate a certain number of SNPs, the P-value limits were adjusted.
In conclusion, this MR study did not identify a causal effect between H. pylori infection and IBS, indicating that IBS patients may not derive any benefits from the prevention or eradication of H. pylori infection. However, due to the presence of these limitations, larger prospective studies and additional MR studies may be necessary to evaluate the correlation.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
CW: Data curation, Software, Writing – original draft. JZ: Writing – original draft. FH: Writing – review & editing. DL: Writing – review & editing. YH: Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by project China Natural Science Foundation Committee, project 82103227.
Acknowledgments
We want to acknowledge the European Bioinformatics Institute for providing related GWAS summary data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1268492/full#supplementary-material
References
Abdel-Razik, A., Mousa, N., Shabana, W., Refaey, M., Elhelaly, R., Elzehery, R., et al. (2018). Helicobacter pylori and non-alcoholic fatty liver disease: A new enigma? Helicobacter 23, e12537. doi: 10.1111/hel.12537
Abo-Amer, Y., Sabal, A., Ahmed, R., Hasan, N., Refaie, R., Mostafa, S., et al. (2020). Relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease (NAFLD) in a developing country: a cross-sectional study. Diabetes Metab. Syndr. Obes. 13, 619–625. doi: 10.2147/DMSO.S237866
Agreus, L., Engstrand, L., Svardsudd, K., Nyren, O., and Tibblin, G. (1995). Helicobacter pylori seropositivity among Swedish adults with and without abdominal symptoms. A population-based epidemiologic study. Scand. J. Gastroenterol. 30, 752–757. doi: 10.3109/00365529509096323
Alvarez, C., Florio, A., Butt, J., Rivera-Andrade, A., Kroker-Lobos, M., Waterboer, T., et al. (2020). Associations between Helicobacter pylori with nonalcoholic fatty liver disease and other metabolic conditions in Guatemala. Helicobacter 25, e12756. doi: 10.1111/hel.12756
Athab, A. (2017). Rate of helicobacter pylori infection among patients with irritable bowel syndrome. Gulf Med. J. 2017:6.
Black, C., and Ford, A. (2020). Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 17, 473–486. doi: 10.1038/s41575-020-0286-8
Bowden, J., Davey, S., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey, S., Haycock, P., and Burgess, S. (2016). Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Burgess, S., Butterworth, A., and Thompson, S. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. doi: 10.1002/gepi.21758
Burgess, S., Daniel, R., Butterworth, A., and Thompson, S. (2015). Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 44, 484–495. doi: 10.1093/ije/dyu176
Cen, L., Pan, J., Zhou, B., Yu, C., Li, Y., Chen, W., et al. (2018). Helicobacter Pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: A systematic review and meta-analysis. Helicobacter 23:12457. doi: 10.1111/hel.12457
Chey, W., Kurlander, J., and Eswaran, S. (2015). Irritable bowel syndrome: a clinical review. JAMA 313, 949–958. doi: 10.1001/jama.2015.0954
Emdin, C. A., Khera, A. V., Kathiresan, S. (2017) Mendelian Randomization. JAMA 318, 1925–1926. doi: 10.1001/jama.2017.17219
Hemani, G., Bowden, J., and Davey, S. (2018). Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27, R195–R208. doi: 10.1093/hmg/ddy163
Holtmann, G., Ford, A., and Talley, N. (2016). Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 1, 133–146. doi: 10.1016/S2468-1253(16)30023-1
Hooi, J., Lai, W., Ng, W., Suen, M., Underwood, F., Tanyingoh, D., et al. (2017). Global Prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153, 420–429. doi: 10.1053/j.gastro.2017.04.022
Kawamura, A., Adachi, K., Takashima, T., Yuki, M., Ono, M., and Kinoshita, Y. (2001). Prevalence of irritable bowel syndrome and its relationship with Helicobacter pylori infection in a Japanese population. Am. J. Gastroenterol. 96:1946.
Kim, T., Sinn, D., Min, Y., Son, H., Kim, J., Chang, Y., et al. (2017). A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J. Gastroenterol. 52, 1201–1210. doi: 10.1007/s00535-017-1337-y
Kim, Y., Cho, Y., and Kwak, S. (2020). The association between Helicobacter pylori infection and irritable bowel syndrome: A meta-analysis. Int. J. Environ. Res. Public Health 17:2524. doi: 10.3390/ijerph17072524
Lee, Y., Bae, S., and Song, G. (2013). Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin. Exp. Rheumatol. 31, 118–121.
Li, C., Shuai, Y., Zhou, X., and Chen, H. (2020). Association between Helicobacter pylori infection and irritable bowel syndrome: A systematic review and meta-analysis. Medicine 99, e22975. doi: 10.1097/MD.0000000000022975
Liang, C., Hsu, C., Chung, C., Chen, C., Wang, L., Hsu, S., et al. (2020). Risk for Irritable Bowel Syndrome in Patients with Helicobacter Pylori Infection: A Nationwide Population-Based Study Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 17:3737. doi: 10.3390/ijerph17103737
Liu, Y., Xu, H., Zhao, Z., Dong, Y., Wang, X., and Niu, J. (2022). No evidence for a causal link between Helicobacter pylori infection and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. Front. Microbiol. 13:1018322.
Locke, C., Talley, N., Nelson, D., Haruma, K., Weaver, A., Zinsmeister, A., et al. (2000). Helicobacter pylori and dyspepsia: a population-based study of the organism and host. Am. J. Gastroenterol. 95, 1906–1913. doi: 10.1111/j.1572-0241.2000.02251.x
Ng, Q., Foo, N., Loke, W., Koh, Y., Seah, V., Soh, A., et al. (2019). Is there an association between Helicobacter pylori infection and irritable bowel syndrome? A meta-analysis. World J. Gastroenterol. 25, 5702–5710. doi: 10.3748/wjg.v25.i37.5702
Pittayanon, R., Lau, J., Yuan, Y., Leontiadis, G., Tse, F., Surette, M., et al. (2019). Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology 157, 97–108. doi: 10.1053/j.gastro.2019.03.049
Sperber, A., Bangdiwala, S., Drossman, D., Ghoshal, U., Simren, M., Tack, J., et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology 160, 99–114. doi: 10.1053/j.gastro.2020.04.014
Sultan, S., and Malhotra, A. (2017). Irritable bowel syndrome. Ann. Intern. Med. 166, ITC81–ITC96. doi: 10.7326/AITC201706060
Wang, C., Yin, Y., Wang, L., Guo, X., Liu, L., and Qi, X. (2023). Association between Helicobacter pylori infection and irritable bowel syndrome: a systematic review and meta-analysis. Postgrad. Med. J. 99, 166–175. doi: 10.1136/postgradmedj-2021-141127
Wang, Z., Liu, Y., Peng, Y., and Peng, L. (2022). Helicobacterpylori infection-a risk factor for irritable bowel syndrome? an updated systematic review and meta-analysis. Medicina 58, 1035. doi: 10.3390/medicina58081035
Xiong, F., Xiong, M., Ma, Z., Huang, S., Li, A., and Liu, S. (2016). Lack of Association Found between Helicobacter pylori Infection and Diarrhea-Predominant Irritable Bowel Syndrome: A Multicenter Retrospective Study. Gastroenterol. Res. Pract. 2016, 3059201. doi: 10.1155/2016/3059201
Xiong, Y., Liu, L., Zhou, X., Wen, Y., and Wang, R. (2020). Anti-Helicobacter pylori treatment can effectively improve the clinical remission rates of irritable bowel syndrome: a controlled clinical trial meta-analysis. Clinics 75, e1857. doi: 10.6061/clinics/2020/e1857
Yakoob, J., Abbas, Z., Naz, S., Islam, M., and Jafri, W. (2012). Virulence markers of Helicobacter pylori in patients with diarrhoea-dominant irritable bowel syndrome. Br. J. Biomed. Sci. 69, 6–10. doi: 10.1080/09674845.2012.11669914
Zhao, B., Zhao, J., Cheng, W., Shi, W., Liu, W., Pan, X., et al. (2014). Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J. Clin. Gastroenterol. 48, 241–247. doi: 10.1097/MCG.0b013e31829f2e25
Zhao, Y., Zou, D., Wang, R., Ma, X., Yan, X., Man, X., et al. (2010). Dyspepsia and irritable bowel syndrome in China: a population-based endoscopy study of prevalence and impact. Aliment. Pharmacol. Ther. 32, 562–572. doi: 10.1111/j.1365-2036.2010.04376.x
Keywords: Helicobacter pylori, Mendelian randomization, irritable bowel syndrome, causality, genome-wide association studies
Citation: Wang C, Zhang J, Han F, Liu D and Han Y (2024) No evidence for a causal link between Helicobacter pylori infection and Irritable bowel syndrome: a Mendelian randomization study. Front. Microbiol. 14:1268492. doi: 10.3389/fmicb.2023.1268492
Received: 28 July 2023; Accepted: 27 December 2023;
Published: 07 February 2024.
Edited by:
George Tsiamis, University of Patras, GreeceReviewed by:
Tian Li, Air Force Medical University, ChinaYanmin Xia, Air Force Medical University, China
Mohsen Norouzinia, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2024 Wang, Zhang, Han, Liu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuying Han, aGFueXV5aW5nQG53dS5lZHUuY24=; Dong Liu, TGl1dHVueUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Chenchen Wang1,2†
Chenchen Wang1,2† Yuying Han
Yuying Han