
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 October 2023
Sec. Infectious Agents and Disease
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1266583
This article is part of the Research Topic Zoonotic Diseases: Epidemiology, Multi-omics, and Host-pathogen interactions View all 25 articles
 Shengchun Wu1†
Shengchun Wu1† Jiao Meng1†
Jiao Meng1† Fuxun Yu2
Fuxun Yu2 Caomin Zhou3
Caomin Zhou3 Bin Yang2
Bin Yang2 Xingxing Chen2
Xingxing Chen2 Guanghong Yang4
Guanghong Yang4 Yi Sun5
Yi Sun5 Wuchun Cao5
Wuchun Cao5 Jiafu Jiang5
Jiafu Jiang5 Jiahong Wu1*
Jiahong Wu1* Lin Zhan2*
Lin Zhan2*Piroplasmosis is a zoonotic disease mainly caused by the Babesia and Theileria parasites. Piroplasmosis is often a subclinical infection in dogs and cats that is difficult to detect and is often suspected when clinical signs such as anemia are present. It has been reported to be prevalent in China. However, molecular evidence of the disease has not been reported in pet dogs and cats in Guiyang. In this study, we collected 307 anticoagulated blood samples from an animal hospital in the Wudang District of Guiyang during the period March 2021 to November 2021 and extracted DNA from the samples. The 18S rDNA gene was amplified using PCR, and the positive amplification product was sequenced. The sequences were then analyzed for homology and phylogeny. Of the 307 samples collected, 164 were feline and 143 were canine, with a total of 23 amplifying a target band of approximately 400 bp. The percentage of positives of piroplasms infection in pet cats was 4.27% (7/164), with the pathogens being T. uilenbergi (3) and T. luwenshuni (4). One Colpodella sp. and two undetermined species were also detected in the cat samples. The percentage of positives of piroplasms infection in pet dogs was 7.69% (11/143), with the pathogen being T. uilenbergi (11). One Colpodella sp. was also detected in the dog samples. The results confirmed that T. uilenbergi and T. luwenshuni are prevalent in pet cats and dogs in this area. In addition, the study found a rare zoonotic pathogen, Colpodella sp., in cats and dogs. Therefore, this study is expected to serve as a valuable reference for decision-making regarding animal health management and public health work.
Piroplasms belong to the phylum Apicomplexa, class Piroplasmea, and order Piroplasmida and include the genera Babesia and Theileria. They are an order of protozoan parasites that live in macrophages, lymphocytes and red blood cells. Piroplasmosis is a human-animal infectious disease caused by piroplasms (Zhang and Li, 2010), with the main clinical symptoms being fever, anemia and swollen lymph nodes. Babesia is widely distributed around the world, and its onset often lacks typical symptoms, making it easy to misdiagnose or overlook. In recent years, the number of reported human cases has been increasing, with the United States, Canada and China having the most reported cases (Yang et al., 2021). The Babesia species that most often infect humans are B. microti, B. venatorum, B. duncani, and B. divergens (Ord and Lobo, 2015). Theileria species are mainly pathogenic to animals and cause serious losses in the livestock industry. Liu et al. (2010) detected Theileria sp. in the blood of a hospitalized patient in Suizhou City, Hubei Province (NCBI Accession: HQ844673.1). Although the paper has not yet been published, this finding suggests that Theileria sp. may be infectious to humans. Since there are relatively few studies on human Theileria infections in China, its study should not be neglected. At this time, none of the canine piroplasm species are zoonotic.
As living standards improve, pet ownership is becoming a normal part of life. Piroplasmosis in dogs and cats can be a chronic or subclinical infection, or it can be a severe acute disease in which the death of infected animals may occur (Irwin and Hutchinson, 1991). When piroplasmosis occurs a subclinical infection in dogs and cats, it is difficult to detect. It is often discovered due to clinical signs such as anemia, fever and lethargy. For example, 36 cases of B. gibsoni were seen in a veterinary hospital in Xi’an, and the signs and symptoms were mainly fever, yellow urine and decreased red blood cells and platelets (Feng et al., 2021). Blood transfusions can have an immediate effect, but they are expensive and there is often a shortage of blood available. Therefore, early identification of the pathogen facilitates the diagnosis and treatment of the disease, reducing the animal’s suffering as well as the medical burden.
However, there are no reports of piroplasmosis in dogs or cats in Guiyang, southwestern China. Therefore, this study aims to investigate the percentage of infections in pet cats and dogs in an animal hospital in Guiyang, and to provide a scientific basis for the prevention and control of piroplasms infections in pets in the region.
From March 2021 to November 2021, the anticoagulated blood samples were collected from pet cats and dogs attending an animal hospital in Guiyang, China. The blood samples were stored in EDTA anticoagulation tubes, randomly numbered and then stored at 4°C for later use. For practical reasons, we were unable to obtain basic information about the animals (age, sex, etc.), geographic location of residence, and clinical data information.
This study was approved by the Animal Care Welfare Committee of Guizhou Medical University (Ethical approval number: 2305072). All animals were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China. Informed consent was obtained from pet owners to acquire the anticoagulated blood samples from the animals.
Nucleic acids were extracted from 200 μL of blood using the qEx-DNA/RNA Virus Kit (Xi’an Tianlong Science and Technology, Xi’an, China) according to the instructions, and then stored in a refrigerator at −20°C until PCR.
PCR amplification was performed on all genomic DNA samples using the 18S rDNA gene nesting primers for piroplasm parasites (National Health Commission of the People’s Republic of China, 2017). A total volume of 25 μl was used for PCR amplification, which included 2.5 μl of 10 × PCR buffer, 2 μl of 2.5 mM dNTP Mixture, 0.5 μl of each primer (10 μM/L), 0.125 μl of Taq polymerase (5 U/μl) (Takara Biotechnology, China), 1 μl of extracted genomic DNA, and double-distilled water to fill the remainder. Genomic DNA from B. bigemina stored in our laboratory and sterile double-distilled water were used as positive and negative controls, respectively. The first round of nested PCR reaction procedures included pre-denaturation at 96°C for 2 min, denaturation at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 40 s, a total of 35 cycles, and a final extension at 72°C for 5 min. The reaction conditions for the second round of Nestor PCR were the same as the reaction procedure for the first round, except that the annealing temperature was changed to 57°C. Finally, 5 μL of PCR products from all samples were taken and subjected to 1.5% agarose gels treated with 4S Green nucleotide stain (Sangon Biotech), and the results were observed using a gel imager (Bio-Rad).
The PCR products with amplified target bands were sent to BGI-Chongqing for bi-directional sequencing and sequence splicing. The obtained sequences were aligned with the sequences of registered genes in GenBank using the BLAST tool on the National Center for Biotechnology Information (NCBI) website. After identifying the species, multiple sequence alignments with related genes were conducted using DNAMAN (version 6.0, Lynnon Corporation, Canada) software and representative DNA sequences were taken. The reference sequences were also downloaded from the GenBank database. Representative sequences were selected for evolutionary tree construction, with Plasmodium berghei (NCBI Accession: AZ522148.1) and P. falciparum (NCBI Accession: DK896461.1) as outgroups. MEGA (version 7.0)1 software was used to construct a phylogenetic evolutionary tree using the neighbor-joining method and self-extension test 1,000 times for genetic evolutionary analysis.
A total of 307 anticoagulated blood samples were collected, including 164 pet cat samples and 143 pet dog samples. A total of 11 (6.7%) positive amplifications were obtained from the 164 pet cat blood samples, and 12 (8.4%) positives were obtained from 143 pet dog blood samples.
A total of 22 target amplicon were successfully sequenced with BLAST homology matching, the piroplasms were sequenced as T. uilenbergi (14), T. luwenshuni (4) and Colpodella sp. (2), with the remaining two sequenced as other eukaryotes (Uncultured Baldinia and the Uncultured eukaryote). Specifically, pet cats were found to have T. uilenbergi (3), T. luwenshuni (4), Colpodella sp. (1), Uncultured Baldinia (1), and Uncultured eukaryote (1) (Table 1). All sequences were deposited in GenBank (Table 2). The sequences of T. uilenbergi, T. luwenshuni, Colpodella sp., Uncultured Baldinia, and Uncultured eukaryote genes detected in the cat samples were the closest to T. uilenbergi (NCBI Accession: MG940889.1), T. luwenshuni (NCBI Accession: MK685118.1), Colpodella sp. (NCBI Accession: JX624256.1), Uncultured Baldinia clone (NCBI Accession: MF685332.1), and Uncultured eukaryote (NCBI Accession: KU820642.1) in the GenBank database, respectively, while their sequence identities were 100.00, 100.00, 98.23, 99.26, and 98.69%, respectively. In addition, pet dog blood samples were found to have T. uilenbergi (11) and Colpodella sp. (1). Their gene sequences were closest in the GenBank database to T. uilenbergi (NCBI Accession: MG940889.1) and Colpodella sp. (NCBI Accession: OQ540589.1) with sequence identities of 100.00 and 94.99%, respectively. The sequence identity of the two Colpodella sp. (NCBI Accession: OR226256 and OR226258) was 81.71%, and compared with the reference strain Colpodella sp. ATCC50594 strain (NCBI Accession: AY142075) isolated from soil in the United States, the sequence identity was 81.77 and 83.00%, respectively. The sequence of Colpodella sp. from cat samples (NCBI Accession: OR226256) was only 79.69%∼83.14% when compared with Colpodella sp. strains from suspected clinical cases in China (NCBI Accession: KT364261 and GQ411073), but the sequence identity with another suspected clinical case from China (NCBI Accession: MF594625) was as high as 99.60%. When the sequence of Colpodella sp. from dog samples (NCBI Accession: OR226258) was compared with the sequences of Colpodella sp. strains from suspected clinical cases (NCBI Accession: KT364261, GQ411073, and MF594625), the sequence identity was only 79.01∼84.39%.
The gene sequences of pet cats (NCBI Accession: OR016205) and pet dogs (NCBI Accession: OR016208) formed a clade with high homology to the sequences of T. uilenbergi infecting yaks in the Gansu region, Dermacentor everestianus in the Qinghai region, and Haemaphysalis longicornis in the Beijing region (NCBI Accession: MG799806.1, MG940889.1, and KC601647.1), with 99.73%∼100.00% homology. The gene sequences of pet cats (NCBI Accession: OR016425) were homopolymerized into a clade with 100.00% homology to the sequences of T. luwenshuni infecting sheep in Shandong (NCBI Accession: KJ850935.1) and the United Kingdom (NCBI Accession: KU234526.1). The above Theileria gene sequences were clustered with T. cervi sequences (NCBI Accession: KT863524.1 and KT863529.1) infecting Sika deer in the Jilin region of China, all of which were T. sp. The pet cat gene sequences (NCBI Accession: OR226256) clustered into a single clade with Colpodellidae sp. (NCBI Accession: MF594625.1) detected in the blood of a patient with relapsing fever in China, with 99.60% homology. However, pet cat gene sequences (NCBI Accession: OR226256) were distant from Colpodella sp. gene sequences detected in ticks (NCBI Accession: OQ540589), horses (NCBI Accession: MW261749), cattle (NCBI Accession: OL848461), and tigers (NCBI Accession: MN640809). The pet dog gene sequences (NCBI Accession: OR226258) clustered into a clade with Colpodella sp. (NCBI Accession: OQ540589.1) detected in H. longicornis from Yiyuan County, Shandong, China, with a homology of 94.99%. The pet dog gene sequences (NCBI Accession: OR226258) were distant from Colpodella sp. gene sequences detected in humans (NCBI Accession: MF594625), horses (NCBI Accession: MW261749), cattle (NCBI Accession: OL848461) and tigers (NCBI Accession: MN640809). In addition, the studied sequences (NCBI Accession: OR226257 and OR226255) were clustered separately and not assigned to a eukaryotic group, occupying an evolutionary position between Colpodella sp. and Theileria (Figure 1).
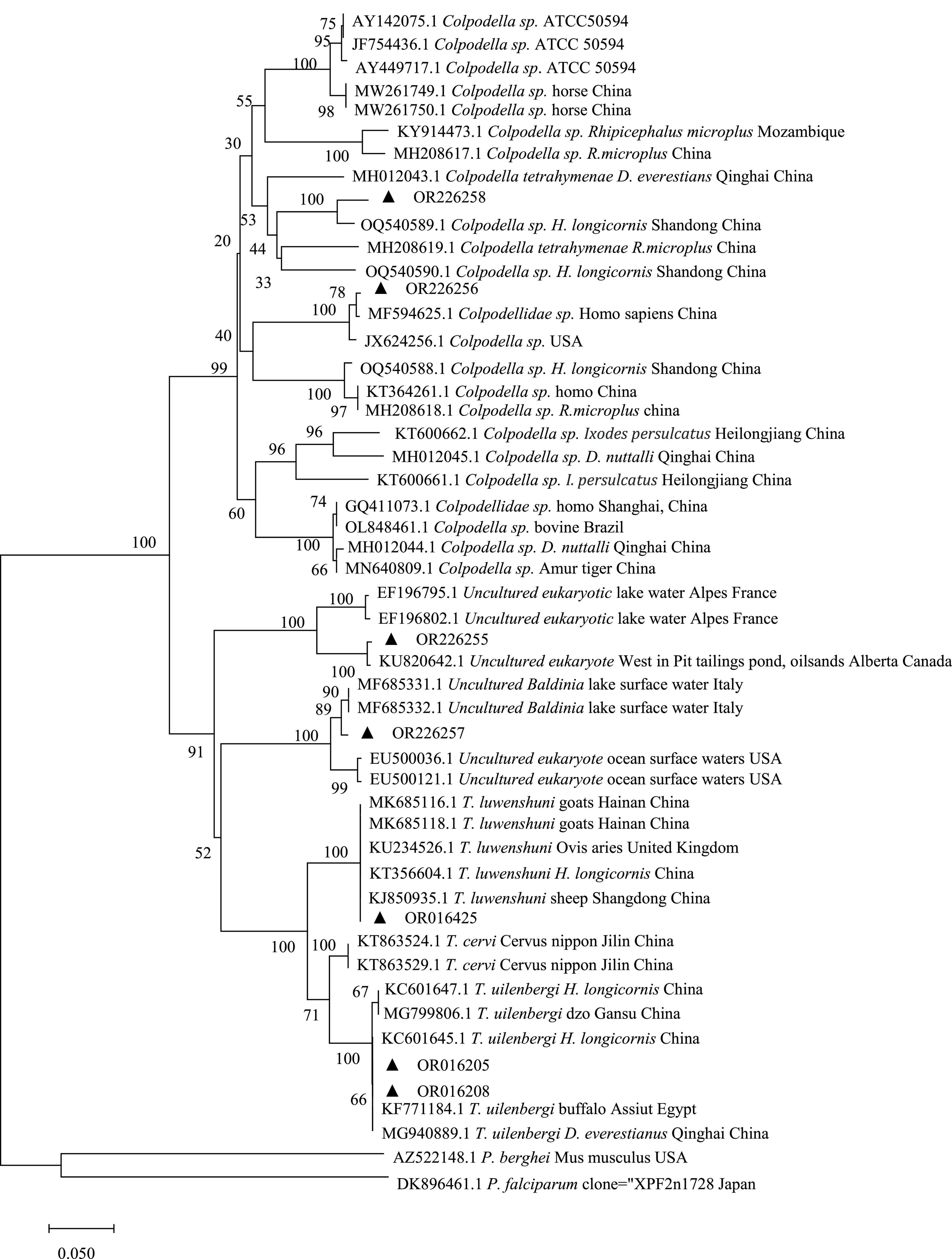
Figure 1. Genetic evolutionary analysis of the 18S rDNA piroplasm gene in pet cats and dogs. The 18S rRNA sequences obtained in this study were indicated with black triangles.
The percentage of positives of piroplasms infection in pet dogs in this study was 7.69% (11/143), which was higher than the prevalence of canine-origin piroplasm infections reported in Türkiye (0.13%, 2/757) (Aktas et al., 2015) and China’s Hunan Province (4.30%, 5/115) (Wang J. et al., 2020). This may be related to the different geographic locations where the samples were collected, the sensitivity of the diagnostic technique used, and the different clinical statuses of the dogs studied. To the best of our knowledge, this study is the first to report the detection of T. uilenbergi in the blood of pet dogs in China. The common hosts of T. uilenbergi are sheep and deer (Mans et al., 2015), and host infections may present with fever, diarrhea, respiratory distress and enlarged lymph nodes. However, there are no reports on the pathology and symptoms of T. uilenbergi in dogs, and the clinical significance of this is not known. The vectors of T. uilenbergi have been confirmed to be H. longicornis and H. qinghaiensis (Li et al., 2009), and Xiang et al. (2022) reported that H. longicornis is the dominant tick species in Guizhou, which increases the likelihood of a T. uilenbergi epidemic in the area. The main piroplasms reported to infect dogs in China and abroad are B. canis, B. vogeli, B. rossi, B. conradae, B. vulpes, and B. gibsoni of the genus Babesia (Irwin, 2010; He et al., 2017; Niu et al., 2017). Although Babesia was not detected in the pet dog samples in this study, Colpodella sp. (NCBI Accession: OR226258) was detected in one dog sample, and its pathogenicity requires further investigation. The sequence from the pet dog (NCBI Accession: OR226258) was in close proximity to Colpodella sp. (NCBI Accession: MH012043, OQ540589, MH208619, and OQ540590) detected in ticks (Figure 1). This result suggests that ticks that may carry Colpodella sp. and could be screened for possible Colpodella sp. at this site in the future. The Wudang District of Guiyang has many natural scenic spots, and there are a number of parks with dense vegetation and rich ecology distributed around this hospital. When pet owners walk their dogs in the park, their dogs may be bitten by ticks when brushing against vegetation and may be infected with pathogens carried by ticks during the biting process, posing a threat to the animals’ health.
The percentage of positives of blood piroplasms in pet cats in this study was 4.27% (7/164), which was lower than the 8.00% (2/25) infection rate reported in Hunan Province (Wang J. et al., 2020). In this study, the pet cat pathogens were more diverse, containing not only T. uilenbergi, but also T. luwenshuni, Colpodella sp. and two unknown species of gene sequences. Reports of piroplasm infections in domestic cats and wild felines include the species such as B. felis, B. cati, B. leo, B. hongkongensis, B. gibsoni, and Cytauxzoon sp. (Hartmann et al., 2013; Palmer et al., 2022; Yin et al., 2022), but there have been no reports on the detection of T. uilenbergi and T. luwenshuni in blood samples from pet cats. This may be related to the fact that piroplasm in cats is often an asymptomatic infection (Wong et al., 2012), resulting in a lack of research and underreporting. T. uilenbergi and T. luwenshuni are common Theileria in China and are the more pathogenic species (Hao, 2020). They are often found in mixed infections. Studies have reported that T. luwenshuni can infect a wide range of host animals, such as goats, sheep, deer and sheepdogs (Ge et al., 2012; Li et al., 2014; Gholami et al., 2016), and it is evident that it may have a wider distribution range. For example, Zhong et al. (2019) and Wang K. L. et al. (2020) investigated the infection of cattle and sheep with T. luwenshuni in Aba Prefecture, Sichuan Province and Linyou County, Shanxi Province and found that the infection rates were as high as 75.00 and 61.20%, respectively. The rare Colpodella sp., a tick-borne pathogen that has been detected in Qinghai Province (Hu et al., 2022), was also detected in the blood samples of pet cats. Colpodella sp. is also a zoonotic pathogen, with the first case of a Colpodella sp.-like pathogen infecting a human found in Kunming, Yuan et al. (2012). Colpodella sp. was also detected in horses from Jingxi and Napo, Guangxi (Xu et al., 2022; Zhou et al., 2022), as well as being recently detected in the blood of Amur tigers (Chiu et al., 2022). Cui (2013) also amplified Colpodella sp. sequences in the blood of febrile patients and in the cerebrospinal fluid of in-patients with neurological symptoms (Jiang et al., 2018). The cat sample Colpodella sp. sequence (NCBI Accession: OR226256) clustered with the reference strain ATCC50594 and was closer to the gene sequences of the currently published patients (NCBI Accession: MF594625), with a higher sequence identity (99.60%). The possibility of zoonotic disease transmission to humans in this region cannot be excluded. In this study, it should be noted that Colpodella is not a piroplasm. Colpodella is a sister group to an apicomplexan clade (Kuvardina et al., 2002). The initial aim of the study was to investigate blood carriage of piroplasms in cats and dogs with 18S rRNA amplification sequencing, but we inadvertently discovered Colpodella, which is a significant finding. Therefore, it is not unusual that Colpodella was amplified unintentionally with these common primers, which is similar to what has been observed in previous studies (Jiang et al., 2018; Xu et al., 2022). The Colpodella sp. pathogen and whether it can infect humans remains controversial and requires further confirmation. The taxonomic statuses of the gene sequences of two unknown species also need to be confirmed.
The PCR molecular biology technique used in this study addresses the morphological difficulty of distinguishing parasites in the blood when the quantity is low. It is able to accurately detect the presence of pathogens. However, there are some limitations to the study, which failed to collect basic information regarding the pet dogs and cats during the sample collection process and failed to analyze the epidemiological characteristics of the hosts in a comprehensive manner. According to the current detection results, the tick-borne pathogens Theileria and Colpodella sp. were found in the blood samples of dogs and cats. The data suggest that Theileria and Colpodella sp. were existent in dogs and cats in Guizhou. The Theileria and Colpodella sp. found in the study are different in their treatments. At the present stage, there is no highly efficient drug for the treatment of piroplasmosis caused by T. uilenbergi and T. luwenshuni, and the more commonly used drugs include Imidocarb, Diminazene Aceturate, Acriflavine, Atovaquone, Azithromycin, Clindamycin, Quinine, etc. (Hao, 2020). Colpodella has been reported in fewer cases in host animals and humans. The paucity of case reports makes it difficult to draw conclusions regarding treatment. For example, the patient reported by Yuan et al. showed some common features with Babesia cases and responded well to treatment with Atovaquone and Azithromycin (Yuan et al., 2012). The patient reported by Jiang et al. (2018) exhibited neurological symptoms and was treated with Doxycycline. Neculicioiu et al. (2021) found that a combination regimen of Ceftriaxone and Metronidazole was effective against urinary contamination due to Colpodella sp.
In conclusion, this study is the first report of pet dogs and cats infected with T. uilenbergi, T. luwenshuni, and Colpodella sp. in Guiyang, southwestern China, which provides scientific data for the diagnosis of the common piroplasmosis in pet dogs as we as decision-making in the management of animal health and public health.
The data presented in the study are deposited in the NCBI repository, accession numbers OR016205, OR016425, OR226256, OR226257, OR226255, OR016208, and OR226258.
The animal studies were approved by the Animal Care Welfare Committee of Guizhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
SW: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review and editing, Project administration. JM: Investigation, Supervision, Writing – review and editing, Project administration. FY: Funding acquisition, Supervision, Writing – review and editing, Project administration. CZ: Supervision, Writing – review and editing, Project administration. BY: Supervision, Writing – review and editing, Project administration. XC: Supervision, Writing – review and editing, Project administration. GY: Supervision, Writing – review and editing. YS: Supervision, Writing – review and editing. WC: Supervision, Writing – review and editing. JJ: Project administration, Supervision, Writing – review and editing, Validation. JW: Funding acquisition, Project administration, Supervision, Writing – review and editing. LZ: Funding acquisition, Investigation, Project administration, Supervision, Writing – review and editing, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by grants from the National Natural Science Foundation of China (Nos. 82160633 and 81760605), and the GZPH-NSFC-2021-17, the High-level and Innovative Talents of Guizhou Province (QKH-GCC [2022] 033-1).
We thank the animal owners for their permission. We also thank the doctors and nurses from the animal hospital in Guizhou for their help in sampling.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aktas, M., Özübek, S., Altay, K., Ipek, N. D., Balkaya, İ, Utuk, A. E., et al. (2015). Molecular detection of tick-borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasit. Vectors 8:157. doi: 10.1186/s13071-015-0763-z
Chiu, H. C., Sun, X., Bao, Y., Fu, W., Lin, K., Chen, T., et al. (2022). Molecular identification of Colpodella sp. of South China tiger Panthera tigris amoyensis (Hilzheimer) in the Meihua Mountains, Fujian, China. Folia Parasitol. 69:2022019. doi: 10.14411/fp.2022.019
Cui, J. R. (2013). Animal infection, morphological and molecular identification of a new piroplasma pathogen. Master’s thesis. Naval: Naval Medical University.
Feng, P., Wang, X., Zhang, X. B., Wei, T., Tian, Q. L., Zhang, J., et al. (2021). Diagnosis and treatment of 36 cases of Babesia gibsonni infected dogs. Progress Vet. Med. 42, 138–141. doi: 10.16437/j.cnki.1007-5038.2021.02.026
Ge, Y., Pan, W., and Yin, H. (2012). Prevalence of Theileria infections in goats and sheep in southeastern China. Vet. Parasitol. 186, 466–469. doi: 10.1016/j.vetpar.2011.11.066
Gholami, S., Laktarashi, B., Shiadeh, M. M., and Spotin, A. (2016). Genetic variability, phylogenetic evaluation and first global report of Theileria luwenshuni, T. buffeli, and T. ovis in sheepdogs in Iran. Parasitol. Res. 115, 2125–2130. doi: 10.1007/s00436-016-5005-6
Hao, G. Y. (2020). Progress in the study of caprine and ovine theileriosis in sheep. Chin. J. Vet. Sci. 40, 424–434. doi: 10.16303/j.cnki.1005-4545.2020.02.34
Hartmann, K., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Frymus, et al. (2013). Babesiosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 15, 643–646. doi: 10.1177/1098612x13489230
He, L., Miao, X., Hu, J., Huang, Y., He, P., He, J., et al. (2017). First Molecular Detection of Babesia gibsoni in Dogs from Wuhan, China. Front. Microbiol. 8:1577. doi: 10.3389/fmicb.2017.01577
Hu, X. Y., He, Y. C., Kang, M., Li, Z. K., Li, J. X., Sun, Y. L., et al. (2022). Information analysis of ticks and tick-borne pathogens in Qinghai. Prog. Vet. Med. 43, 73–79. doi: 10.16437/j.cnki.1007-5038.2022.10.026
Irwin, P. J. (2010). Canine babesiosis. Vet. Clin. North Am. Small Anim. Pract. 40, 1141–1156. doi: 10.1016/j.cvsm.2010.08.001
Irwin, P. J., and Hutchinson, G. W. (1991). Clinical and pathological findings of Babesia infection in dogs. Austral. Vet. J. 68, 204–209. doi: 10.1111/j.1751-0813.1991.tb03194.x
Jiang, J. F., Jiang, R. R., Chang, Q. C., Zheng, Y. C., Jiang, B. G., Sun, Y., et al. (2018). Potential novel tick-borne Colpodella species parasite infection in patient with neurological symptoms. PLoS Neglect. Trop. Dis. 12:e0006546. doi: 10.1371/journal.pntd.0006546
Kuvardina, O. N., Leander, B. S., Aleshin, V. V., Myl’nikov, A. P., Keeling, P. J., and Simdyanov, T. G. (2002). The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J. Eukaryotic Microbiol. 49, 498–504. doi: 10.1111/j.1550-7408.2002.tb00235.x
Li, Y., Chen, Z., Liu, Z., Liu, J., Yang, J., Li, Q., et al. (2014). Molecular identification of Theileria parasites of northwestern Chinese Cervidae. Parasit. Vectors 7:225. doi: 10.1186/1756-3305-7-225
Li, Y., Luo, J., Guan, G., Ma, M., Liu, A., Liu, J., et al. (2009). Experimental transmission of Theileria uilenbergi infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol. Res. 104, 1227–1231. doi: 10.1007/s00436-009-1347-7
Liu, Z., Ma, M., Peng, Y., Wang, Z., Yang, G., Deng, J., et al. (2010). Theileria sp. SZH-human 18S ribosomal RNA gene, complete sequence.
Mans, B. J., Pienaar, R., and Latif, A. A. (2015). A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 4, 104–118. doi: 10.1016/j.ijppaw.2014.12.006
National Health Commission of the People’s Republic of China (2017). Diagnosis of Babesiosis. Beijing: National Health Commission of the People’s Republic of China.
Neculicioiu, V. S., Colosi, I. A., Toc, D. A., Lesan, A., and Costache, C. (2021). When a ciliate meets a flagellate: A rare case of Colpoda spp. and Colpodella spp. isolated from the urine of a human patient. Case report and brief review of literature. Biology 10:476. doi: 10.3390/biology10060476
Niu, Q., Yang, J., Liu, Z., Gao, S., Pan, Y., Guan, G., et al. (2017). First molecular detection of piroplasm infection in pet dogs from Gansu, China. Front. Microbiol. 8:1029. doi: 10.3389/fmicb.2017.01029
Ord, R. L., and Lobo, C. A. (2015). Human babesiosis: Pathogens, prevalence, diagnosis and treatment. Curr. Clin. Microbiol. Rep. 2, 173–181. doi: 10.1007/s40588-015-0025-z
Palmer, J. P., Gazêta, G., André, M., Coelho, A., Corrêa, L., Damasceno, J., et al. (2022). Piroplasm infection in domestic cats in the mountainous region of Rio de Janeiro, Brazil. Pathogens 11:900. doi: 10.3390/pathogens11080900
Wang, J., Wang, X., Sun, H., Lv, Z., Li, Y., Luo, J., et al. (2020). Molecular evidence of piroplasm infection in companion animals in Hunan Province, China. BMC Vet. Res. 16:297. doi: 10.1186/s12917-020-02500-6
Wang, K. L., Zhou, Y. C., Zhao, S. S., Zhang, Y. J., Yan, Y. Q., Jing, J. C., et al. (2020). Investigation on infection and phylogenetic analysis of Theileria luwenshuni in Goats of Linyou, Shanxi. Prog. Vet. Med. 41, 123–126. doi: 10.16437/j.cnki.1007-5038.2020.04.026
Wong, S. S., Poon, R. W., Hui, J. J., and Yuen, K. Y. (2012). Detection of Babesia hongkongensis sp. nov. in a free-roaming Felis catus cat in Hong Kong. J. Clin. Microbiol. 50, 2799–2803. doi: 10.1128/JCM.01300-12
Xiang, Y. L., Zhou, J. Z., Liu, Y., Liu, P. T., Hu, Y., and Liang, W. Q. (2022). An investigation of ticks and tick-borne bacteria in some areas of Guizhou province, China. Chin. J. Vector Biol. Control 33, 148–152.
Xu, M., Hu, Y., Qiu, H., Wang, J., and Jiang, J. (2022). Colpodella sp. (Phylum Apicomplexa) identified in horses shed light on its potential transmission and zoonotic pathogenicity. Front. Microbiol. 13:857752. doi: 10.3389/fmicb.2022.857752
Yang, Y., Christie, J., Köster, L., Du, A., and Yao, C. (2021). Emerging Human Babesiosis with “Ground Zero” in North America. Microorganisms 9:440. doi: 10.3390/microorganisms9020440
Yin, F., Mu, D., Tian, Z., Li, D., Ma, X., Wang, J., et al. (2022). Molecular Detection of Babesia gibsoni in Cats in China. Animals 12:3066. doi: 10.3390/ani12223066
Yuan, C. L., Keeling, P. J., Krause, P. J., Horak, A., Bent, S., Rollend, L., et al. (2012). Colpodella spp.-like parasite infection in woman, China. Emerg. Infect. Dis. 18, 125–127. doi: 10.3201/eid1801.110716
Zhong, W., Du, X. M., Hao, L. L., Yuan, D. B., Guo, L., Hou, W., et al. (2019). Investigation on molecular epidemiology of Piroplasma infection in yaks and Tibetan sheep in aba Tibetan autonomous prefecture of Sichuan Province. Chin. J. Vet. Med. 55, 20–27.
Keywords: dog, cat, phylogenetic studies, piroplasms, Theileria uilenbergi, Theileria luwenshuni, Colpodella, Guizhou
Citation: Wu S, Meng J, Yu F, Zhou C, Yang B, Chen X, Yang G, Sun Y, Cao W, Jiang J, Wu J and Zhan L (2023) Molecular epidemiological investigation of piroplasms carried by pet cats and dogs in an animal hospital in Guiyang, China. Front. Microbiol. 14:1266583. doi: 10.3389/fmicb.2023.1266583
Received: 25 July 2023; Accepted: 25 September 2023;
Published: 12 October 2023.
Edited by:
Hong Yin, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Rocio Checa, Complutense University of Madrid, SpainCopyright © 2023 Wu, Meng, Yu, Zhou, Yang, Chen, Yang, Sun, Cao, Jiang, Wu and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhan, emhhbmxpbjMwMEBob3RtYWlsLmNvbQ==; Jiahong Wu, amlhaG9uZ3dAZ21jLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.